Abstract
The implications of the recent change in the definition of pulmonary hypertension on epidemiology and outcomes are not known. We sought to determine the percentage of patients with the two most common lung diseases that would be reclassified regarding the presence/absence of pulmonary hypertension with the revised definition. A query of the United Network for Organ Sharing database was performed. The percentage of patients meeting the current and previous definition of pulmonary hypertension was described. Outcomes of patients stratified by the current and previous definitions were compared. There were 15,563 patients with right heart catheterization data analyzed. Pulmonary hypertension was more prevalent in both chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis under the new definition at 52.4% versus 82.4%, and 47.6% versus 73.6%, respectively. “Pre-capillary” pulmonary hypertension by the new definition was lower at 28.1% for chronic obstructive pulmonary disease and 36.8% for idiopathic pulmonary fibrosis. Of the patients with pulmonary hypertension by the old definition, 23.9% of chronic obstructive pulmonary disease patients and 18.7% of idiopathic pulmonary fibrosis patients were not classified as pulmonary hypertension by the new definition. Conversely, 15.9% of chronic obstructive pulmonary disease patients and 15.1% of idiopathic pulmonary fibrosis patients who did not meet diagnostic criteria for pulmonary hypertension by the old definition did have pulmonary hypertension by the new definition. Patients in both disease categories had shorter transplant-free waitlist survival in the presence of pulmonary hypertension by both the new and old definitions. There was a trend toward the new definition of pre-capillary pulmonary hypertension better discerning outcomes compared to the old definition of pulmonary hypertension in idiopathic pulmonary fibrosis patients. Most patients with advanced lung disease who are listed for lung transplantation have pulmonary hypertension, but fewer have pre-capillary pulmonary hypertension than pulmonary hypertension by the old definition. Both the old and new definition of precapillary pulmonary hypertension appear to discern outcomes among the two groups of lung disease analyzed, with some evidence to suggest that the new definition performs slightly better in the idiopathic pulmonary fibrosis population.
Keywords: pulmonary vascular disease, lung transplantation, group 3 pulmonary hypertension
Introduction
The initial definition and classification of pulmonary hypertension (PH) has been standardized through the World Health Symposium which was initially convened under the auspices of the World Health Organization in 1973.1 This conference has been held five times subsequently with serial “tweaking” of the classification system. The 2013 World Symposium on Pulmonary Hypertension (WSPH) reiterated the definition of PH as “a mean pulmonary artery pressure (mPAP) ≥25 mmHg at rest measured by right heart catheterization (RHC)”.2 Pulmonary arterial hypertension (PAH) on the other hand was characterized at this meeting by a mPAP of ≥25 mmHg together with a pulmonary artery wedge pressure (PAWP) of ≤15 mmHg and a pulmonary vascular resistance (PVR) of ≥3 Wood units (WU).2 If the pulmonary capillary wedge pressure is > 15 mmHg, then this is indicative of underlying cardiac disease and the PH would then be categorized according to the World Health Organization under group 2 PH. In the presence of lung disease, the PH might be categorized under group 3 PH, although there is no clear guidance on how much lung disease is permissible in order to qualify as group 1 PAH. This is especially germane to patients with connective tissue disorders who are at risk of both PH and interstitial lung disease.
The 6th WSPH convened in Nice, France in 2018. At this meeting, the task force acknowledged that a mPAP > 20 mmHg should be regarded as abnormal and proposed a change in definitions to better reflect the presence of pulmonary vascular dysfunction (PVD) by incorporating a PVR threshold for not only group 1, but also for other PH groups. The new hemodynamic categories of PH therefore includes: (i) pre-capillary PH (mPAP > 20 mmHg, PAWP ≤15 mmHg, and a PVR ≥3 WU), (ii) post-capillary PH (mPAP > 20 mmHg, PAWP >15 mmHg, and PVR < 3 WU), and (iii) combined pre- and post-capillary PH (mPAP > 20 mmHg, PAWP >15 mmHg, PVR ≥3 WU)3 and finally a group with a mPAP >20 mmHg not having a specific categorization since they neither fulfill criteria for pre- nor post-capillary PH (PVR < 3 WU and PAWP ≤15 mmHg). This hemodynamic profile is generally not associated with structural changes in the small arteries (no PVD) and may be seen in association with conditions such as a high cardiac output (CO) state. One hemodynamic profile not covered in the current definition are those patients with a mPAP < 20 mmHg but whose PVR > 3. Since this category is undefined and a PVR ≥3 is generally regarded as evidence of PVD, we have called this group “PVD with no PH.”
PH complicating the various lung diseases tends to be mild with a histogram distribution of mPAPs typically centered in the 20–30 mmHg range.5 Therefore group 3 patients are potentially the largest group affected by this new definition of pre-capillary PH. Although use of the PVR threshold was previously proposed at the 5th World PH symposium for group 1 PH, it was not implemented for the definition of group 3 PH. Therefore the impact of the new definition on patients with lung disease is yet to be determined. Specifically, how many with PH by the old definition will be recategorized as having pre-capillary PH and how might this impact their management, prognostication, and enrollment in future clinical trials?
We therefore sought to evaluate the impact of the new definition in the context of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF), two of the most common forms of advanced lung disease. We sought to accomplish this through mining of the United Network for Organ Sharing (UNOS) database, the largest database of advanced lung disease patients with available RHC data. The questions we attempted to answer were: (a) What is the prevalence of PH by the prior definition versus the new definition of pre-capillary PH; (b) How many patients “switch” categories based on the new versus old definition; and (c) What is the prognosis of patients with pre-capillary PH by the new definition and does the new definition discriminate outcomes better?
Methods
Study criteria
The UNOS database was queried for all patients aged 18+ listed for lung transplantation between May 2005 and September of 2018. We chose to include only those patients who were listed since the lung allocation system was changed in the USA in 2005, since the likelihood of receiving a transplant was significantly impacted by this alteration in the system. Only patients with IPF and COPD, the two most common indications for lung transplantation were included. Patients with other diagnoses that may lead to lung transplantation including connective tissue disorders, sarcoidosis, cystic fibrosis, and non-cystic fibrosis bronchiectasis were excluded.
Data extracted for all patients included age, gender, ethnicity, listing for type of transplant, blood type, body mass index, pulmonary function testing including percent predicted forced vital capacity, and percent predicted forced expired volume in the first one second. Hemodynamic data collated included the right atrial pressure, mPAP, PAWP, CO, and the PVR.
Within the two disease subgroups, patients were divided into those with PH (mPAP > 20 mmHg) and no PH (mPAP ≤ 20 mmHg). Those with PH were further divided into four groups: pre-capillary PH (mPAP > 20 mmHg, PAWP ≤15 mmHg, PVR ≥ 3 WU), post-capillary PH (mPAP > 20 mmHg, PAWP > 15 mmHg, PVR < 3 WU), combined pre- and post-capillary PH (mPAP > 20 mmHg, PAWP > 15 mmHg, PVR ≥ 3 WU), and an uncategorized group of the remaining patients with PH who did not fit into any of the above categories (mPAP > 20 mmHg, PVR < 3, and pulmonary capillary wedge pressure (PCWP) ≤15 mmHg). The patients without PH were divided into two groups; no PVD (PVR < 3) and no PH but evidence of PVD (PVR ≥3 WU). These subgroups were compared to patients with (mPAP ≥25 mmHg) and without (mPAP < 25 mmHg) PH using the previous definition.
Statistical analysis
Data are presented as mean ± standard deviation or frequency and percent, where appropriate. The primary endpoint was “transplant-free waitlist survival.” Specifically, patients who were transplanted were censored as alive and thus the survival analysis was solely based on patients who died on the waitlist.” Patients not transplanted by time of data abstraction were right censored on 7 September 2018. The Kaplan–Meier method was used to calculate the overall survival rate with the log-rank test used to formally test group differences. A p-value ≤ 0.05 was considered statistically significant. All analyses were performed with SAS (Version 9.4; SAS Inc., Cary, NC).
Results
There were a total of 32,055 patients listed for lung transplantation between January 2005 and September 2017. Of these 15,563 qualified for our analysis, including 6572 with COPD and 8991 with IPF. The patients who did not qualify for inclusion were listed with other diagnoses. The baseline demographics of the groups, their categorization by the presence or absence of PH by the old definition, and their outcomes are shown in Table 1. The disease group categorization by the new definition is shown in Table 2. Fig. 1 provides a histogram distribution of mPAPs for each of the patient subgroups at the time of listing subgrouped by PVR (<3 or ≥3).
Table 1.
Clinical and demographic characteristics (n = 15,563).
| Parameter | COPD (n = 6572) | IPF(n = 8991) |
|---|---|---|
| Age, mean ± SD | 60.4 ± 6.3 | 61.1 ± 8.3 |
| Male, % | 3320 (49.1) | 6349 (70.6) |
| Ethnicity | ||
| White | 5918 (90.0) | 7244 (80.6) |
| Black | 508 (7.7) | 579 (6.4) |
| Hispanic | 87 (1.3) | 830 (9.2) |
| Other | 59 (9.0) | 338 (3.8) |
| FVC%, mean ± SD | 53.1 ± 17.1 | 46.7 ± 16.5 |
| FEV1%, mean ± SD | 21.9 ± 11.1 | 50.4 ± 16.5 |
| Initial LAS, mean ± SD | 32.5 ± 8.5 | 45.9 ± 18.7 |
| BMI, kg/m2, mean ± SD | 24.7 ± 4.2 | 27.2 ± 3.9 |
| CO, L/Min, mean ± SD | 5.2 ± 1.4 | 5.4 ± 1.4 |
| PAWP, mean ± SD | 12.3 ± 5.1 | 9.6 ± 5.3 |
| PAWP ≤ 15 mmHg, n (%) | 5078 (77.3) | 7974 (88.7) |
| PAWP >15 mmHg, n (%) | 1494 (22.7) | 1017 (11.3) |
| PVR, mean ± SD | 2.9 ± 1.8 | 3.4 ± 3.3 |
| PVR < 3 WU, n (%) | 4224 (64.3) | 4997 (55.6) |
| PVR ≥ 3 WU, n (%) | 2348 (35.7) | 3994 (44.4) |
| mPAP, mmHg, mean ± SD | 25.7 ± 7.4 | 25.6 ± 9.7 |
| mPAP < 25 mmHg, n (%) | 3127 (47.6) | 4712 (52.4) |
| mPAP ≥ 25 mmHg, n (%) | 3446 (52.4) | 4279 (47.6) |
| Transplant outcomes | ||
| Died | 318 (4.8) | 793 (8.8) |
| Transplanted | 5028 (76.5) | 7215 (80.2) |
| Still waiting | 391 (5.9) | 223 (2.5) |
| Removed | 835 (12.7) | 760 (8.5) |
Note: values presented are either mean ± SD or frequency (percent), where appropriate.
FVC: forced vital capacity; FEV1: forced expiratory volume; LAS: lung allocation score; CO: cardiac output; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; WU: Wood units; mPAP: mean pulmonary arterial pressure; COPD: chronic obstructive pulmonary disease; IPF: idiopathic pulmonary fibrosis.
Table 2.
Categorization by the new hemodynamic criteria.
| COPD (n = 6572) | IPF (n = 8991) | |
|---|---|---|
| No PHmPAP <20 mmHg | ||
| No PVDPVR <3 | 992 (15.1%) | 2046 (22.8%) |
| PVDPVR >3 | 164 (2.5%) | 327 (3.6%) |
| PH mPAP >20 mmHg | ||
| Uncategorized PHPVR < 3, PCWP <15 mmHg | 2077 (31.6%) | 2291 (25.5%) |
| Pre-capillary PHPVR > 3, PCWP <15 mmHg | 1847 (28.1%) | 3310 (36.8%) |
| Post-capillary PHPVR < 3, PCWP > 15 mmHg | 1155 (17.6%) | 660 (7.3%) |
| Combined pre- and post-capillary PHPVR >3, PCWP > 15 mmHg | 337 (5.1%) | 357 (4%) |
COPD: chronic obstructive pulmonary disease; IPF: idiopathic pulmonary fibrosis; PH: pulmonary hypertension; mPAP: mean pulmonary arterial pressure; PVD: pulmonary vascular dysfunction; PVR: pulmonary vascular resistance.
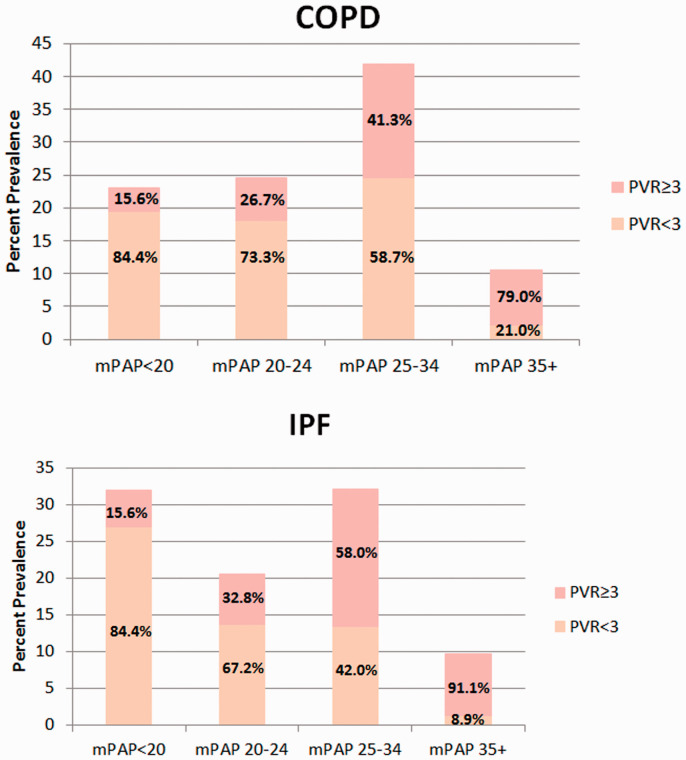
Fig. 1.
Histogram distribution of mean pulmonary artery pressures subgrouped by PVR ≥3 or <3 Wood units.
COPD: chronic obstructive pulmonary disease; IPF: idiopathic pulmonary fibrosis; mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance.
The prevalence of PH at the time of listing by the old definition versus new definition for COPD and IPF was 52.4% versus 82.4% and 47.6% versus 73.6%, respectively. However, the prevalence of pre-capillary PH by the new definition was substantially lower at 28.1% for COPD and 36.8% for IPF.
There were 23.9% of COPD patients and 18.7% of IPF patients with PH by the old definition who did not qualify for any of the three newly defined PH categories, respectively. Specifically, although these patients had PH (mPAP >20 mmHg), they had a PVR < 3 WU and a PAWP ≤15 mmHg. The percentage of patients who crossed over from not having PH by the old definition to qualifying as pre-capillary PH by the new definition was 15.9% for the COPD cohort and 15.1% for the IPF cohort. Of those patients with severe PH (mPAP ≥35 mmHg) by the old definition, 21% of COPD patients and 8.9% of IPF patients did not qualify as having precapillary PH by the new definition (Fig. 1).
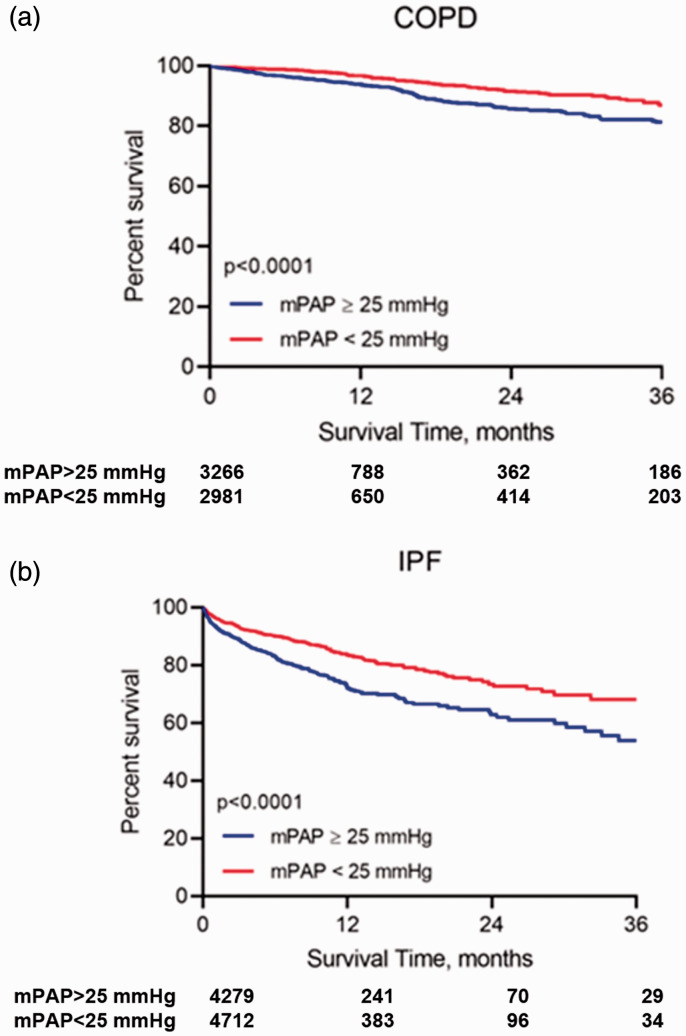
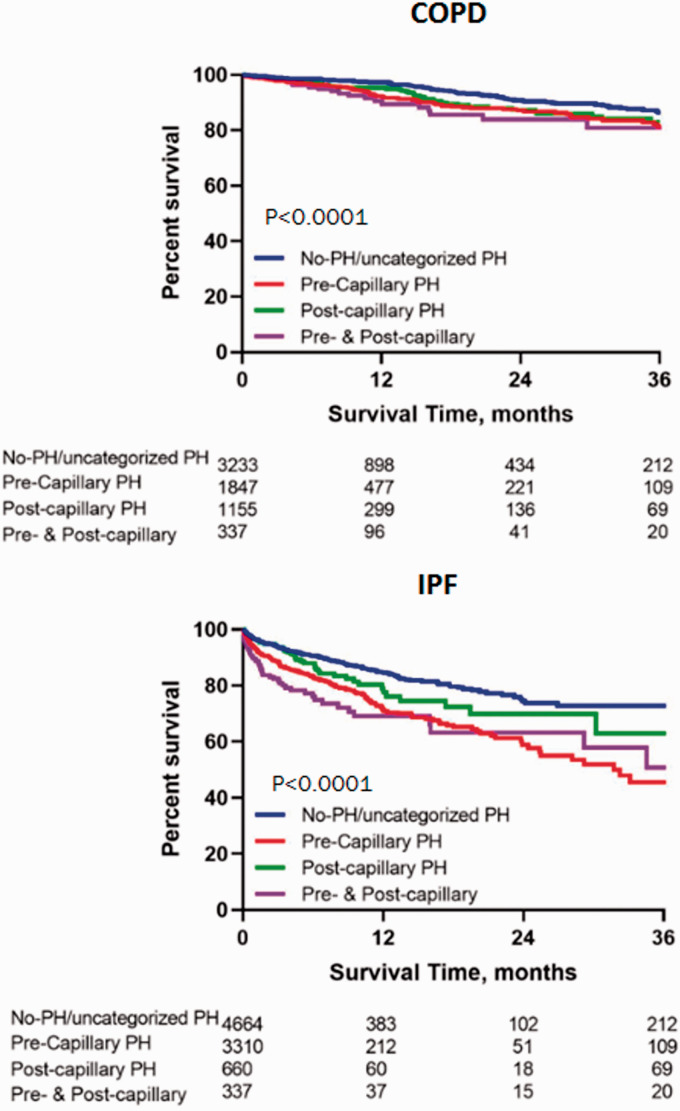
Kaplan–Meier survival curves of both patient groups by the old and new definitions are shown in Figs 2 and 3 respectively. Both the old definition of PH and the new definition of pre-capillary PH discerned outcomes equivalently.
Fig. 2.
Kaplan–Meier survival curves of transplant-free waitlist survival of the two patient groups by the old definition.
COPD: chronic obstructive pulmonary disease; IPF: idiopathic pulmonary fibrosis; mPAP: mean pulmonary artery pressure.
Fig. 3.
Kaplan–Meier survival curves of transplant-free waitlist survival of the two patient groups by the new definition.
COPD: chronic obstructive pulmonary disease; IPF: idiopathic pulmonary fibrosis; PH: pulmonary hypertension.
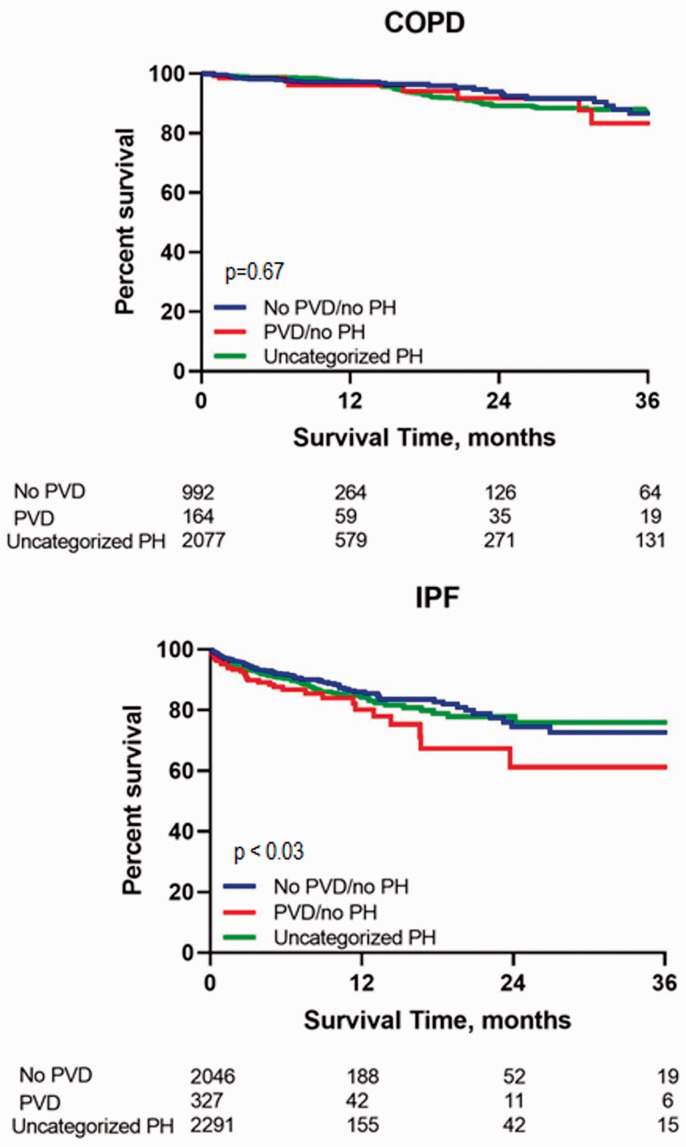
In the uncategorized group of patients with PH based on a mPAP > 20 mmHg, but PAWPs ≤15 mmHg and PVRs < 3, the prevalence was 31.6% for COPD and 25.5% for IPF. Another “neglected” group are those patients with no PH (mPAP ≤20 mmHg), but with PVD as evidenced by a PVR ≥3. In fact, 2.5% of the COPD cohort and 3.6% of the IPF cohort fell into this category. A further survival comparison was therefore performed of the patients with uncategorized PH versus no PH/no PVD versus no PH/+PVD and is shown in Fig. 4. In IPF, the presence of PVD without PH did appear to portend worse outcomes.
Fig. 4.
Transplant-free waitlist survival comparison of the three groups of patients; those with uncategorized PH versus the group with no PH and no pulmonary vascular disease (PVR <3 Wood units) versus those with no PH but evidence of pulmonary vascular disease (PVR ≥3 Wood units).
COPD: chronic obstructive pulmonary disease; PH: pulmonary hypertension; mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance; PAWP: pulmonary artery wedge pressure.
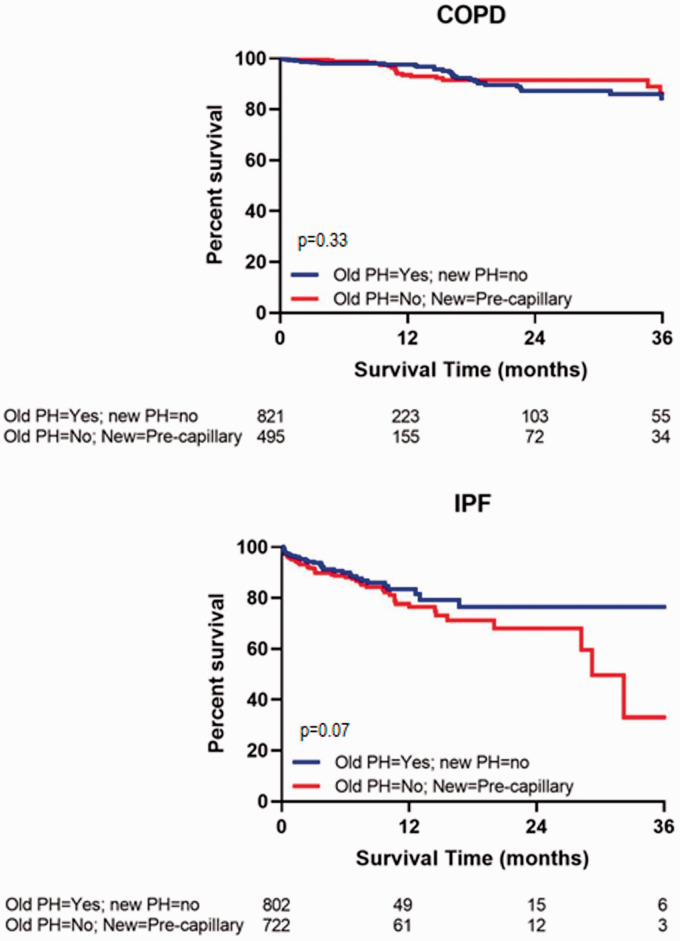
Survival of patients with PH by the old definition, but not the new definition of precapillary PH, was compared to survival of patients who qualify as pre-capillary PH by the new definition but not by the old PH definition. There was no difference between the two hemodynamic groups in the COPD cohort; however, there was a trend for worse outcomes in the IPF populations among those patients who qualify as pre-capillary PH by the new definition, but did not qualify as PH by the old definition (Fig. 5).
Fig. 5.
Transplant-free waitlist survival of patients with PH by the old definition, but not the new definition of precapillary PH compared to survival of patients who qualified as pre-capillary PH by the new definition but did not have PH by the old definition.
IPF: idiopathic pulmonary fibrosis; PH: pulmonary hypertension; mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance; PAWP: pulmonary artery wedge pressure.
Discussion
The significance and added value of the new definition for PH in patients with underlying parenchymal lung disease remain mostly unknown. Not unexpectedly, if one regards all patients with a mPAP > 20 mmHg as having PH, then the prevalence goes up substantially in patients with various forms of advanced lung disease. However, when one incorporates evidence of PVD (PVR ≥3 WU, pre-capillary PH), then the prevalence decreases compared to the old definition of group 3 PH (mPAP ≥25 mmHg). The new definition of pre-capillary PH failed to discern survival better than the old definition, however. Our analysis also highlights the existence of a small population of patients without PH, but who have PVD as evidence by a PVR ≥3. In the IPF population, this small subgroup appeared to have worse outcomes than those without PVD and no PH, as well as those with PH but no PVD. This underscores the importance of the PVR in any patient categorization.
Lung disease and heart disease patients are the two groups who are most likely to contribute the greatest numbers of patients who qualify by the new PH definition. Indeed, there is prior data demonstrating that lower thresholds for mPAP do have prognostic implications for patients with lung disease with thresholds as low as mPAP 17–20 mmHg discerning groups with differing survivals.4,6 This might be viewed as supporting the change in definition, although these data have been known for years and it appears that the impetus and implications for this new change is more for patients with group 1 PAH.
Approximately 20% of patients qualified as having PH by the old definition, but did not meet criteria for any of the new PH categories. On the other end of the spectrum, ∼15% of patients crossed over from not having PH by the old definition to qualifying as pre-capillary PH by the new definition. Our direct comparison of outcomes between patients with PH by the old definition who did not qualify as precapillary PH versus the converse group (those without PH by the old definition, but precapillary PH by the new definition) showed no difference in patients with COPD, but a trend toward worse outcomes in the new precapillary PH groups for IPF. This supports the notion that more high-risk IPF patients are being captured than are being “dropped” by the new definition. Whether this lends support to the new definition providing more accurate prognostic information than the old definition remains uncertain.
There are some limitations to this analysis. There might have been coding or errors of inputting data with coordinators at multiple centers being responsible for the listing information from which this analysis was derived. However, the large number of patients included likely minimized the impact of any data entry errors. Many of the patients in the initial cohort were transplanted which decreased the size of the study population. Also transplanted patients represent a sick group of patients at high risk for death. This should be borne in mind when interpreting our data since our findings do not address how the new and old definitions would perform in discerning outcomes in a population in whom transplantation is not an option. It is likely therefore that our analysis underestimates the impact of PH on mortality given that a large percentage of patients were transplanted. Also, we censored patients who were transplanted as alive, which arguably included the patients at highest risk of mortality. Therefore our survival curves might have underestimated the true survival implications of underlying PH. However, this survival bias was common to all patients in the cohort, and therefore should not have impacted differences noted in any of our analyses. There are inherent biases to this database that precludes generalization of these results to broader patient populations with the same conditions. First, the population studied is rather unique in that it includes only patients with advanced lung disease, specifically IPF and COPD, who are ill enough to be listed for transplant. Although other diagnoses could be included, we elected to focus on the two largest populations. We performed similar analyses of two other groups; sarcoidosis and cystic fibrosis/non-cystic fibrosis bronchiectasis, but found that these smaller cohorts did not alter the primary finding of the study and therefore we elected not to include them. Furthermore, a population listed for transplantation is further biased since patients with significant comorbidities and elderly individuals are less likely to be included. However, this can also be regarded as a strength of this study since other comorbidities may contribute to the development of PH and also potentially influence outcomes. It is also worth bearing in mind that the prevalence of PH by the new or old definition is likely significantly less in patients with milder forms of these diseases. Finally, the relatively high number of patients with evidence of post-capillary PH raises the question as to the reliability/validity of the PAWP measurement in patients with severe lung diseases; indeed, this was a subject of intense debate at the 6th World Symposium.
In summary, we have provided a comprehensive evaluation of the new PH definition in a large group of patients with lung disease. We demonstrate that the prevalence of both PH and pre-capillary PH according to the new definition is high in both IPF and COPD patients who are listed for transplant. Our analysis elicited a signal in the IPF population that the new definition performs slightly better in predicting outcomes compared to the old PH definition. We have also drawn attention to two previously unheralded groups, those with uncategorized PH and patients with PVD, but no PH. This analysis provides a framework for further study into these different hemodynamic categories.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894021999960 for Impact of the new definition for pulmonary hypertension in patients with lung disease: an analysis of the United Network for Organ Sharing database by Steven D. Nathan, Scott D. Barnett, Christopher S. King, Steeve Provencher, Joan A. Barbera, Jean Pastre, Oksana A. Shlobin and Werner Seeger in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_2045894021999960 for Impact of the new definition for pulmonary hypertension in patients with lung disease: an analysis of the United Network for Organ Sharing database by Steven D. Nathan, Scott D. Barnett, Christopher S. King, Steeve Provencher, Joan A. Barbera, Jean Pastre, Oksana A. Shlobin and Werner Seeger in Pulmonary Circulation
Supplemental material, sj-pdf-3-pul-10.1177_2045894021999960 for Impact of the new definition for pulmonary hypertension in patients with lung disease: an analysis of the United Network for Organ Sharing database by Steven D. Nathan, Scott D. Barnett, Christopher S. King, Steeve Provencher, Joan A. Barbera, Jean Pastre, Oksana A. Shlobin and Werner Seeger in Pulmonary Circulation
Footnotes
Author’s contributions: S.D.N.: development of idea, development of figures and tables, and primary author of manuscript; S.D.B.: statistical analysis; C.S.K.: manuscript editing and wrote a portion of manuscript; S.P.: development of idea for manuscript and analysis and manuscript editing; J.B.: development of idea for manuscript and analysis and manuscript editing; O.A.S.: development of idea for manuscript and analysis and manuscript editing; W.S.: development of idea for manuscript and analysis and manuscript editing.
Declaration of conflicting interests: The author(s) declare that there is no conflict of interest.
Ethical approval: No ethical approval, consent to participate required.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Christopher S. King https://orcid.org/0000-0003-3101-319X
Joan A. Barbera https://orcid.org/0000-0003-1469-4990
Supplemental material: Supplemental material for this article is available online.
References
- 1.Luthy E. Proceedings: the epidemic of primary pulmonary hypertension in Europe. Pathol Microbiol (Basel) 1975; 43: 246–247. [PubMed] [Google Scholar]
- 2.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–D50. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med 2018; 197: 509–516. [DOI] [PubMed] [Google Scholar]
- 5.Lettieri CJ, Nathan SD, Barnett S, et al. Prevalence and outcomes of pulmonary arterial hypertension in idiopathic pulmonary fibrosis. Chest 2006; 129: 746–752. [DOI] [PubMed] [Google Scholar]
- 6.Weitzenblum E, Hirth C, Ducolone A, et al. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax 1981; 36: 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894021999960 for Impact of the new definition for pulmonary hypertension in patients with lung disease: an analysis of the United Network for Organ Sharing database by Steven D. Nathan, Scott D. Barnett, Christopher S. King, Steeve Provencher, Joan A. Barbera, Jean Pastre, Oksana A. Shlobin and Werner Seeger in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_2045894021999960 for Impact of the new definition for pulmonary hypertension in patients with lung disease: an analysis of the United Network for Organ Sharing database by Steven D. Nathan, Scott D. Barnett, Christopher S. King, Steeve Provencher, Joan A. Barbera, Jean Pastre, Oksana A. Shlobin and Werner Seeger in Pulmonary Circulation
Supplemental material, sj-pdf-3-pul-10.1177_2045894021999960 for Impact of the new definition for pulmonary hypertension in patients with lung disease: an analysis of the United Network for Organ Sharing database by Steven D. Nathan, Scott D. Barnett, Christopher S. King, Steeve Provencher, Joan A. Barbera, Jean Pastre, Oksana A. Shlobin and Werner Seeger in Pulmonary Circulation