Abstract
Background:
Opioid abuse is a chronic disorder likely involving stable neuroplastic modifications. While a number of molecules contributing to these changes have been identified, the broader spectrum of genes and gene networks that are affected by repeated opioid administration remain understudied.
Methods:
We employed Next-Generation RNA-sequencing (RNA-seq) followed by quantitative chromatin immunoprecipitation to investigate changes in gene expression and their regulation in adult male and female rats’ dorsomedial prefrontal cortex (dmPFC) after a regimen of daily injection of morphine (5.0 mg/kg; 10 days). Ingenuity Pathway Analysis (IPA) was used to analyze affected molecular pathways, gene networks, and associated regulatory factors. A complementary behavioral study evaluated the effects of the same morphine injection regimen on locomotor activity, pain sensitivity, and somatic withdrawal signs.
Results:
Behaviorally, repeated morphine injection induced locomotor hyperactivity and hyperalgesia in both sexes. 90% of differentially expressed genes (DEGs) in morphine-treated rats were upregulated in both males and females, with a 35% overlap between sexes. A substantial number of DEGs play roles in synaptic signaling and neuroplasticity. Chromatin immunoprecipitation revealed enrichment of H3 acetylation, a transcriptionally activating chromatin mark. Although broadly similar, some differences were revealed in the gene ontology networks enriched in females and males.
Conclusions:
Our results cohere with findings from previous studies based on a priori gene selection. Our results also reveal novel genes and molecular pathways that are upregulated by repeated morphine exposure, with some common to males and females and others that are sex-specific.
Keywords: Opioid, prefrontal cortex, RNA-sequencing, addiction, plasticity
1. Introduction
Opioid addiction is a chronic, often lifelong, disorder, characterized by high rates of relapse (Camí and Farré, 2003). The disorder’s enduring nature is attributable to a variety of long-term changes in neuronal structure and neuronal plasticity (Hyman et al., 2006). These, in turn, are likely induced, and subsequently maintained, through alterations in patterns of gene expression (Nestler, 2001). As such, characterizing the molecular mechanisms underlying opioid addiction is essential for developing more effective treatments.
Investigations into the cellular, molecular, and genetic effects of opioid administration have undergone rapid progress (Imperio et al., 2016; Valentino et al., 2020). Nevertheless, while a number of molecular targets that play key roles in opioid addiction have been identified (Browne et al., 2020; Hurd and O’Brien, 2018), the ways in which a broader range of functional pathways and networks interact to produce and/or maintain addictive behavior remain to be determined. Elucidating molecular networks and targets requires assessing global changes in gene expression after opioid exposure. In an earlier effort along these lines, Spijker et al. (2004) assessed transcriptional changes of 159 genes within pre-selected gene networks, including neurotransmitters, neuronal morphology and plasticity, and intracellular signaling. The advent of Next-Generation RNA Sequencing (RNA-seq) technique allows for measurement of expression levels of genes throughout the transcriptome, without a priori selection criteria (Hitzemann et al., 2013). This approach has already yielded critical insights into genomic and downstream regulatory mechanisms underlying cocaine addiction (Walker et al., 2018). The current study applied the same approach to investigate the transcriptional changes following repeated opioid (morphine) exposure.
The role of dysregulated brain reward pathways in addiction has long been recognized (Kreek and Koob, 1998). Both clinical and preclinical studies have implicated alterations in prefrontal cortex function as critically important in producing compulsive drug use, drug seeking, and relapse (Goldstein and Volkow, 2002; Kalivas et al., 2005; Koya et al., 2009; Wilson et al., 2004). Responding to reward or in anticipation of reward in a variety of contexts is a function of the activity of glutamatergic projections from medial prefrontal cortex (mPFC) to the nucleus accumbens (NAc) (Hearing, 2019; Pascoli et al., 2014). Activity in both structures is modulated by dopaminergic inputs from the ventral tegmental area (Hyman et al., 2006). The mPFC is therefore an appropriate locus for investigating changes in gene expression induced by exposure to opioids.
In the current study, we tested global gene expression changes in the dorsal medial PFC (dmPFC) after a regimen of morphine injections (5.0 mg/kg per day over 10 days) that produced locomotor hyperactivity and withdrawal-induced hyperalgesia. Given that our most striking finding was a preponderance (>90%) of upregulation among differentially expressed genes (DEGs; see below) we also conducted a study on biological replicates to see if a selection of upregulated genes showed hyperacetylation of lysine residues in promoter regions in the H3 histone tail. This phenomenon is an epigenetic feature of heroin exposure (Browne et al., 2020) and thus likely contributes to the hypertranscriptional state revealed in the dmPFC after repeated exposure to morphine.
The 24h-timepoint after morphine exposure at which tissue was harvested in this study is the same timepoint at which rats exhibit anhedonia (Rothwell et al., 2009; Swain, et al., 2020). Anhedonia is a core symptom of depression, and transcriptional changes in the mPFC of three mouse models of depression correlate highly with transcriptional changes assayed postmortem in the mPFC of individuals diagnosed with major depressive disorder (MDD) (Scarpa et al., 2020). Therefore, as a preliminary exploration of the translational relevance of post-opioid anhedonia to depression, we conducted a similar comparison of the transcriptional changes induced by repeated morphine exposure in this study to the same MDD dataset._Given that the intensity of morphine withdrawal-induced anhedonia also predicts the severity of subsequent morphine self-administration (Swain et al., 2020), our approach has the potential to identify genes and gene ontology networks that are important in vulnerability to opioid addiction and anhedonia.
In view of differences in vulnerability of males and females to opioid addiction (Becker et al., 2017; Brady and Randall, 1999; Lee and Ho, 2013), and the historical paucity of studies in this area that have included female subjects or been powered sufficiently to measure sex differences (Becker and Koob, 2016), both male and female groups were included. Commonalities and differences between the sexes in the effects of repeated opioid exposure on genomic regulation could therefore be characterized.
2. Materials and Methods
2.1. Animals
Eighty adult Sprague Dawley rats (40 males, 40 females, 65-75 days) were obtained from Envigo (Indianapolis, IN). Of these, 10 rats of each sex were used in the behavioral experiment, 18 animals of each sex were used in the RNA-seq and confirmative RT-qPCR experiment, and 12 animals of each sex were used in the ChIP-qPCR experiment._Animals were same-sex pair-housed in polypropylene cages with ad libitum access to food and water. The colony was maintained on a 12-h light-dark cycle. All procedures were conducted during the light phase of the light-dark cycle. Animals were habituated to the colony for at least 1 week before experimentation. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota.
2.2. Drug treatments
Animals were randomly assigned to morphine or saline conditions, which resulted in 4 groups (Male/Morphine, Male/Saline, Female/Morphine, and Female/Saline). Morphine sulfate (Research Triangle International, NC) was dissolved in 0.9% sterile saline and injected subcutaneously. Animals received daily injections of either morphine (5.0 mg/kg) or an equivalent volume of saline daily over 10 consecutive days. This is similar to the dosing regimen used in previous studies of morphine-induced hyperactivity and hyperalgesia (Paolone et al., 2003; Rothwell et al., 2010; Shippenberg et al., 1996; Tumati et al., 2012).
2.3. Assessment of somatic signs
One hour prior to the 10th daily injection of morphine or saline (i.e., 23 hours after the 9th injection), rats were habituated to opaque plastic circular chambers for 10 min and then videotaped for 10 min. Tapes were later scored for somatic withdrawal signs by a blinded trained observer using a validated checklist (Gellert and Holtzman, 1978; Swain et al., 2020). Total scores were analyzed by a two-way ANOVA with group and sex as between-subjects factors followed by Tukey’s multiple comparisons tests. These and all other behavioral data were analyzed using GraphPad Prism (version 9.0.0), with α set at p < 0.05.
2.4. Nociception test
On Day 10, thermal nociception was assessed immediately before, and again 40 min after injection of morphine or saline (i.e., 20 min prior to locomotor testing) using a plantar test apparatus (Ugo Basile, Varese, Italy) based on Hargreaves et al. (1988). The apparatus consisted of three identical clear plastic cages (22 × 127 × 14 cm) resting on top of a glass floor (2.2 mm thickness). After a 10-min habituation period, latency of hind paw removal in response to a thermal stimulus (Osram halogen bulb; 8 V, 50 W) located beneath the glass floor was measured (to the nearest 0.1 s) and displayed by a digital timer connected to the heat source. The thermal stimulus was adjusted to produce a mean baseline paw removal latency of 20 s and was programmed to automatically shut off after 32.7 s to prevent tissue damage. Results were analyzed by a three-way ANOVA with drug treatment and sex as between-subjects factors and pre/post-test as a within-subjects factor. Data were further analyzed using a two-way (drug treatment x pre/post-test) ANOVA and Tukey’s multiple comparisons tests for between-group comparisons.
2.5. Locomotor activity
Locomotor activity was monitored in clear plastic cages (16.5” x 8” x 7.5”) with corn cob bedding on the floor. Activity was videotaped by surveillance cameras, and travel distances were later analyzed with ANY-maze behavioral tracking software (Version 6.3; Stoelting Co., Wood Dale, IL). Rats were habituated to the apparatus for 90 min one day before the first injection. On the last injection day (Day 10), locomotor activity was measured for 90 min starting 1 h after injection (i.e., 20 min after nociception testing). Results were analyzed by a two-way ANOVA with drug treatment and sex as between-subjects factors followed by Tukey’s multiple comparisons tests, with α set at p < 0.05.
2.6. Tissue collection
Animals were sacrificed 24 hours after the 10th injection. The dmPFC was dissected bilaterally, immediately flash-frozen in liquid nitrogen, and stored at −80°C. Tissue from both hemispheres was used for RNA-seq and ChIP-qPCR assays.
2.7. RNA preparation and sequencing
Samples within each condition for each sex were randomly pooled in groups of three prior to RNA preparation. RNA-seq was conducted as previously described (Barks et al., 2018). Total RNA was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. Isolated RNA was further purified and concentrated using the MinElute Cleanup Kit (Qiagen). Library preparation and RNA-seq were conducted at the University of Minnesota Genomics Center. RNA was quantified using the RiboGreen RNA Assay kit (Invitrogen) and assessed for quality using capillary electrophoresis (Agilent Bio Analyzer 2100; Agilent). Barcoded libraries were constructed for each sample using the TruSeq RNA v2 kit (Illumina). Libraries were size-(200 bp) selected and sequenced (50bp paired-end read, ~20 million reads/library) using Illumina HiSeq 2500.
2.8. RNA-seq analysis
Quality control on raw sequence data was performed with FastQC. Mapping of reads was performed via Hisat2 (version 2.1.0) using the rat genome (rn6) as reference. Differentially expressed genes (DEGs) were identified by gene-wise negative binomial generalized linear models using the EdgeR feature in CLC Genomics Workbench (Qiagen, version 10.1.1). The generated list was filtered based on ≥ 2x absolute fold change and false discovery rate (FDR) corrected p-value (q-value) < 0.05. Principle Component Analysis (PCA) of DEGs was conducted via unsupervised clustering.
2.9. Ingenuity Pathway Analysis
DEGs were annotated by Ingenuity pathway Analysis (IPA; Qiagen) to identify relevant canonical pathways, molecular networks and cellular functions that showed significant alterations in experimental versus control groups as previously described (Barks et al., 2018). Statistical significance p < 0.05; −log(p) > 1.3] was determined by Fisher’s exact test.
2.10. Chromatin immunoprecipitation (ChIP) assay
ChIP experiments were performed as previously described (Tran et al., 2015), with modifications. In brief, chromatin was prepared from dissected dmPFC tissue following the manufacturer’s recommendation (Millipore, Temecula, CA). Tissue was homogenized and cross-linked in 1% formaldehyde solution (Sigma) with intermittent mixing at 37°C (5 min) and room temperature (5 min). Fixed tissue was sonicated (Bioruptor Pico, Diagenode) in lysis buffer [1% SDS, 10 mM EDTA, 50 mM Tris-HCl pH 8.1, 1 mM PMSF, protease inhibitor cocktails (Roche, Indianapolis, IN)] to shear chromatin DNA. Chromatin DNA fragments were validated by agarose gel electrophoresis following a cross-linking reversal (0.2 M NaCl, 65°C overnight). Sonicated lysates were diluted 10-fold with ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl pH 8.1, 167 mM NaCl) and pre-cleared with Protein A agarose (50% slurry, Sigma). Pre-cleared lysate was immunoprecipitated by ChIP Acetyl-Histone H3 antibody (H3Ac; #17-615, Millipore, Temecula, CA). The antibody-histone complex was collected by Protein A agarose slurry with mixing (4° C, ⩾ 1 h). Following washes (per manufacturer’s protocol, Millipore), immune-histone complex was eluted in elution buffer (1% SDS, 0.1 M NaHCO3). Reverse cross-linking was achieved by incubation in NaCl (0.2 M, 65°C overnight). DNA was recovered by protease digestion (20 μg proteinase K, 20 mM EDTA, 100 mM Tris-pH 6.5, 45°C 1 h), and purified using phenol/chloroform extraction and isopropanol precipitation (1/10 volume 3 M sodium acetate pH 5.2, 1 volume isopropanol). Normal IgG was used as a negative control. Levels of enriched Gapdh (active) and Myod1 (inactive) promoter regions were used to validate ChIP experiments.
2.11. Real-time quantitative PCR (RT-qPCR)
For RNA-seq confirmation, RT-qPCR was performed on technical replicates (6/group) as previously described (Barks et al., 2018). RNA was isolated using RNAqueous Total RNA Isolation Kit (Invitrogen). cDNA synthesis was performed using High-Capacity RNA-to-cDNA Kit (Applied Biosystems). RT-qPCR was performed using a TaqMan Universal PCR Master Mix (Applied Biosystem) and TaqMan gene expression assays (ThermoFisher Scientific). Beta actin (Actb) was used as an endogenous control. Samples were run in duplicate, normalized to Actb and averaged to generate fold-changes relative to controls. For analysis of precipitated DNA from ChIP experiments, SYBR-green PCR (Fast SYBR green master mix, ABI) was used to amplify selected gene promoter regions using validated oligonucleotides (Supplementary Material, S.11). Input DNA (10%) was used as a normalizer to account for input amount (ΔCt). Data were expressed as a ratio to saline control (2−ΔΔCt) using one of the samples from the saline group as a calibrator (ΔΔCt). Both RT-qPCR assays were performed on a DNA analyzer (QuantStudio 3, ThermoFisher Scientific). Results were analyzed by two-tailed t-tests in RStudio (version 1.2.5033), with α set at p < 0.05.
2.12. Rank-rank hypergeometric overlap (RRHO)
The RRHO method was used to evaluate overlap in gene signatures between major depressive disorder (MDD) in humans (assayed in mPFC postmortem by RNA-seq) and morphine dependence in rats in this study in a threshold-free manner (Plaisier et al., 2010). FastQ and metadata files from human MDD samples (GSE102556) were obtained from Gene Expression Omnibus. Briefly, we ranked differential gene expression in both datasets based on the signed −log(p-value), with the sign depending upon whether a gene was up or down-regulated. We implemented a sliding window approach to scan through genes iteratively in both gene lists and computed hyper-geometric test p-values for each window. The Benjamini-Yekutieli multiple hypothesis correction was applied to the p-values. The computed p-adj values were converted to −log (p-adj) values and represented as a heat map; color scale ranging from blue to red.
3. Results
3.1. Repeated morphine exposure produced hyperalgesia and locomotor hyperactivity
Morphine withdrawal did not produce significant evidence of overt somatic signs (Drug treatment, p = 0.4017; Sex, p = 0.8876; Drug treatment x Sex, p = 0.1338; Supplementary Material, S.22).
A three-way mixed model (drug treatment x sex x pre/post-test) ANOVA revealed significant main effects of drug treatment [F (1, 32) = 9.226, p = 0.0047] and pre/post-test [F (1, 32) = 31.73, p < 0.0001] but not of sex [F (1, 32) = 1.309, p = 0.2611], and a significant interaction between drug treatment and pre/post-test [F (1, 32) = 36.58, p < 0.0001] on opioid-induced changes in nociception. A follow-up two-way (drug treatment x pre/post-test) ANOVA revealed a significant main effect of drug treatment [F (1, 36) = 9.644, p = 0.0037] and pre/post-test [F (1, 36) = 33.17, p < 0.0001], and a significant interaction between drug treatment and pre/post-test [F (1, 36) = 38.24, p < 0.0001]. Post-hoc Tukey’s tests for multiple comparisons indicated that prior morphine exposure led to hyperalgesia prior to injection on Day 10 (p < 0.0001, Fig. 1A), with a reversal of this effect following morphine treatment (p < 0.0001, Fig. 1A).
Fig. 1.
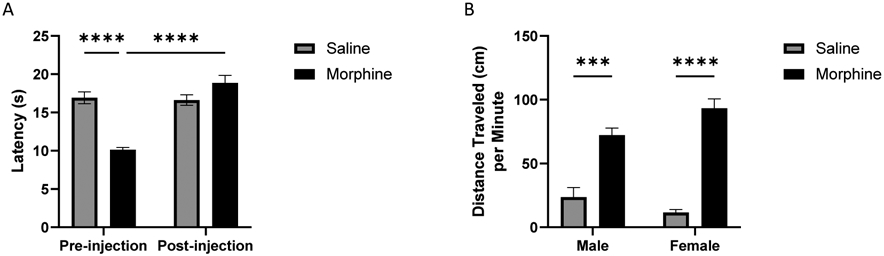
Repeated morphine exposure induced hyperalgesia and locomotor hyperactivity. (A) Nociception test. Nociception was tested prior to the final morphine/saline injection and again 40 min after injection. A three-way (drug treatment x sex x pre/post-test) ANOVA followed by a two-way (drug treatment x pre/post-test) ANOVA and Tukey’s multiple comparisons tests revealed hyperalgesia effects prior to injection on Day 10, and a reversal of this effect following morphine treatment (n = 10/group, **** p < 0.0001). (B) Male and female locomotor activity test. A two-way (drug treatment x sex) ANOVA followed by Tukey’s multiple comparisons tests revealed that the morphine group within each sex showed locomotor hyperactivity 1 hr after their final morphine injection, compared to saline controls (n = 5/group; *** p < 0.001, **** p < 0.0001).
A two-way (drug treatment x sex) ANOVA revealed a significant main effect of drug treatment [F (1, 16) = 114.1, p < 0.0001] but not of sex (p = 0.4663) and a significant interaction between drug treatment and sex [F (1, 16) = 7.403, p = 0.0151] on locomotor activity, measured after the final (10th) drug injection. Tukey’s multiple comparisons tests showed that repeated morphine administration induced hyperactivity in both sexes, as indicated by significantly greater activity 1 h after injection in the morphine-treated compared to controls groups (Male, p = 0.0002; Female, p < 0.0001; Fig. 1B), but no differences between males and females for either treatment condition (Saline, p = 0.5193; Morphine, p = 0.1069).
3.2. Repeated morphine injections altered gene expression in the dmPFC
NGS data were aligned to 17,336 loci in morphine- or saline-treated rats (Figs. 2A, 2B; raw data files available on request). Principal Component Analysis of the 500 most divergent genes showed that samples clustered into four discrete groups based on drug treatment and sex (Supplementary Material, S.32). In male rats, 377 genes were differentially expressed in the morphine-treated relative to the saline-treated group, among which 337 (89%) were up-regulated and 40 (11%) were down-regulated (Supplementary Material, S.42). In female rats, 409 genes were significantly differentially expressed in morphine-treated relative to saline-treated rats, with 370 (90%) up-regulated and 39 (10%) down-regulated (Supplementary Material, S.52). RNA-seq results were verified by RT-qPCR with selected genes that are known to regulate nervous system development and function (Figs. 2C, 2D; Supplementary Material, S.62).
Fig. 2.
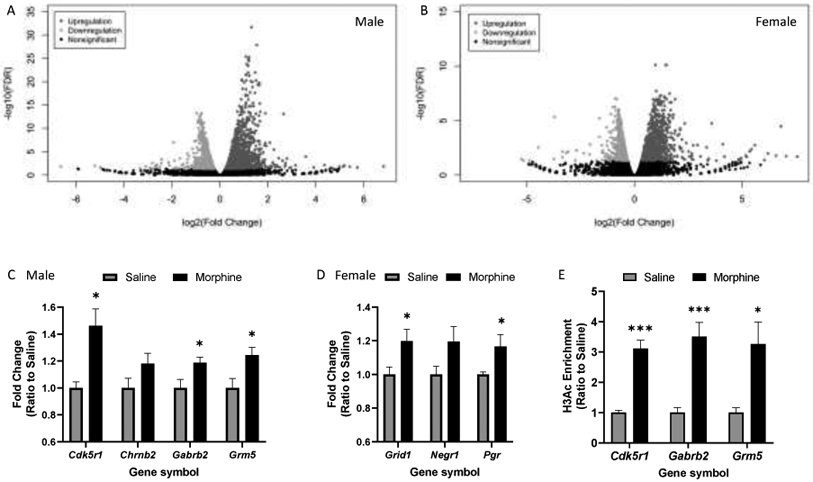
Transcriptional and epigenetic changes in the rat dmPFC following the 10-day morphine/saline injection regimen. (A, B) Volcano plots generated from unfiltered RNA-seq data from males and females, respectively [FDR, False Discovery Rate corrected p-value (q-value)]. (C, D) RT-qPCR validation of male and female RNA-seq datasets (n = 5-6/group; * p < 0.05 compared with the respective saline control group). (E) ChIP-qPCR revealed H3Ac enrichment at upregulated gene promoter regions (n = 4-5/group; * p < 0.05, *** p < 0.001).
3.3. Morphine treatment enriched H3 acetylation in the promoter regions of several upregulated genes.
To investigate long-term effects of repeated morphine exposure on gene expression regulation, we conducted RT-qPCR after ChIP (i.e., ChIP-qPCR) with the H3Ac activation chromatin marker. Targeted gene promoter regions were selected from genes that were significantly upregulated in both RNA-seq and subsequent confirmatory RT-qPCR assays. In males, H3Ac enrichment was found at all three gene (Cdk5r1, Gabrb2, Grm5) promoter regions (Fig. 2E). In contrast, no significant changes were found in the female group (Supplementary Material, S.73).
3.4. Morphine treatment altered gene transcription in neuronal plasticity and intra-/inter-cellular signaling pathways
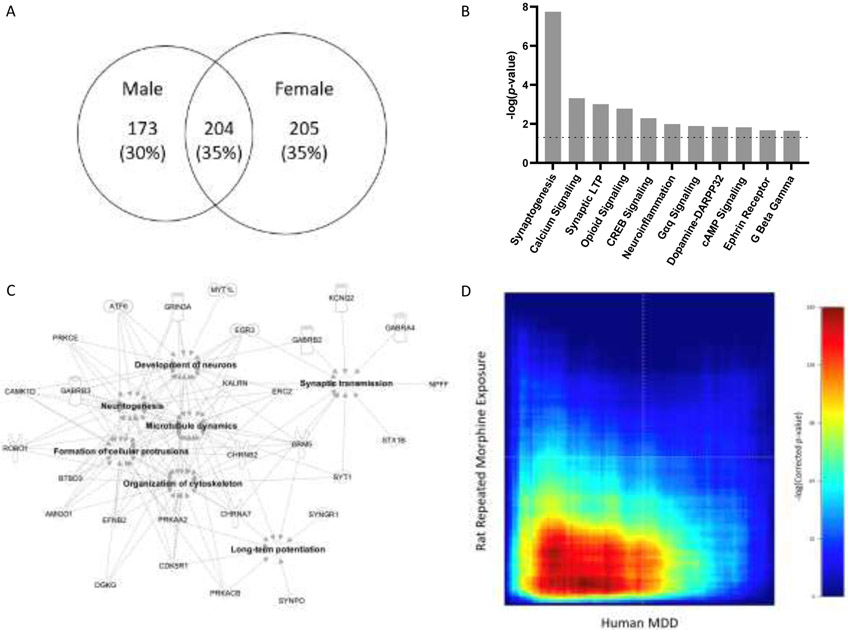
Male and female groups shared a subset of 204 (35%) DEGs (Fig. 3A). This overlapping set of genes was analyzed using IPA to identify non-sex-specific effects. DEGs were enriched in canonical pathways critical for synaptic/intracellular signaling, including synaptogenesis, long-term potentiation, opioid signaling, dopamine-DARPP32 signaling and ephrin receptor signaling pathways (Fig. 3B). All of the top affected pathways were activated (z-score ≥ 2).
Fig. 3.
Overlapping genes, affected canonical pathways and gene networks analyses. (A) Venn diagram of filtered total number of DEGs in males and females. (B) DEGs in both males and females were enriched in cell signaling-, and synaptic growth- and plasticity-related pathways [absolute z-score ≥ 2; p-value < 0.05, −log(p-value) > 1.3, dotted line]. (C) Annotated functions of gene networks in dmPFC affected in all morphine-treated rats (absolute z-score ≥ 2; p-value < 0.05). (D) RRHO comparing differential gene expression in human MDD patients versus controls and morphine-exposed rats versus control rats. The red signal in the bottom left quadrant represents the presence of significantly co-upregulated genes in experimental groups in the two studies (Maximum hypergeometric p-adj < 1 x 10−160).
DEGs that were shared between sexes were also analyzed via IPA to identify enrichment of diseases and biological functions (Fig. 3C). Enriched functional groups of genes included those coding for neurotransmitter receptor subunits (Chrna7, Chrnb2, Gabra4, Gabrb2, Gabrb3, Grin3a, Grm5, Htr2a, Htr5a, Pgr), intra-/inter-cellular signaling regulation (Camk2d, Cdk5r1, Efnb2, Kalrn, Prkaa2, Prkacb, Prkce), and synaptic morphology/function (Stx1b, Syngr1, Synpo, Syt1) (Supplementary Materials, S.4 and S.53). In addition, these DEGs implicated JAK1/2, FEV, and ADORA2A as key upstream regulators of the transcriptional effects of repeated morphine exposure (Supplementary Material, S.83).
Finally, separate analyses of DEGs in each sex showed enrichment of the neuroinflammatory pathway in males but not females (z-scores = 2.53 and 1.13, respectively) and the endocannabinoid and long-term depression pathways in females (z-scores = 2.71 and 2.12) but not males (z-scores = 1.13 and 0.45) (Supplementary Materials, S.9 and S.103).
3.5. Gene dysregulation in morphine-treated rats correlates with dysregulation in the mPFC of MDD patients
Since substantial anhedonia has been found in rats at the same timepoint at which we sacrificed animals in this study (Swain et al., 2020), we performed an unbiased, threshold-free, interspecies RRHO analysis to compare gene expression measured postmortem in the mPFC in human MDD patients versus controls (Labonté et al., 2017; Scarpa et al., 2020) and in morphine-exposed versus control rats in our study. The red signal in the bottom left quadrant represents the presence of significantly co-upregulated genes in the two studies (Fig. 3D; Maximum hypergeometric p-adj < 1 x 10−160). Thus, human MDD and our rat anhedonia model shared similar gene dysregulation patterns.
4. Discussion
We analyzed the transcriptome from the rat dmPFC, a key brain structure implicated in addictive behavior, to identify a posteriori genes and gene networks dysregulated by repeated opioid exposure. Using a regimen of morphine injections that produced robust behavioral changes, the most striking outcome of our transcriptomal analysis at a global level was that 90% of DEGs were upregulated in both sexes. In males, this increase was accompanied by H3 histone tail acetylation, a chromatin modification that maintains the nucleosome in a transcriptionally active state (Shahbazian and Grunstein, 2007). Such H3 hyperacetylation has been reported as a common epigenetic consequence of opioid exposure, although those studies were conducted only on male subjects (Browne et al., 2020). Given that this effect was not seen in females in this study, there is a need for further studies to elucidate alternative epigenetic modifications (e.g., methylation of histone lysine residues) underlying the activational influence of prior opioid exposure on gene expression.
The high degree of upregulation seen here is consistent with the results of a previous RT-qPCR study conducted on the NAc in male rats at the same time point after morphine exposure (Spijker et al., 2004) and implicates widespread transcriptional activation as a regulatory response following chronic opioid exposure. Interestingly, a cocaine self-administration protocol in mice yielded a similarly high proportion of upregulated expression (>90%) at the same time point during withdrawal, exclusively in the mPFC (Walker et al., 2018). The latter finding suggests the intriguing possibility that this phenomenon in the mPFC may be a common counteradaptional transcriptomic signature among addictive drugs having distinct acute pharmacological modes of action (Koob and Le Moal, 1997).
The overlapping upregulated genes common to both sexes participate in canonical pathways broadly associated with synaptogenesis and neuroplasticity, consistent with the idea that recruitment of these cellular processes is a fundamental process in the development of addiction (Lüscher and Malenka, 2011). Altered dmPFC gene expression indicates activation in molecular networks associated with opioid and dopamine signaling, and intracellular Ca2+, G-protein, and cAMP/CREB signaling pathways. These findings support the view that addiction is induced via neuroadaptations and neuroplasticity and concomitant alterations in opioidergic and dopaminergic neurotransmission via G-protein-coupled receptors (Dani et al., 2001; Kauer and Malenka, 2007). For example, the cAMP-dependent pathway is upregulated by chronic morphine treatment (Avidor-Reiss et al., 1996; Nestler and Tallman, 1988; Terwilliger et al., 1991), resulting in the activating phosphorylation of CREB (Haghparast et al., 2014; Morón et al., 2010). These changes in turn likely affect neurotransmitter release and enhance synaptic connectivity (Chavez-Noriega and Stevens, 1994; Weisskopf et al., 1994). It has been further proposed that such upregulation of the cAMP pathway represents a compensatory response to the acute inhibitory effect of opioid administration (Sharma et al., 1977, 1975; Traber et al., 1975), that may play an important role in opioid dependence, tolerance, and withdrawal (Hamdy et al., 2001; Lai et al., 2014; Nestler, 2016).
A number of the genes that were significantly upregulated in both sexes have already been implicated in addiction, validating this study’s approach. These include ionotropic and metabotropic glutamate receptors (Grin3a, Grm5), nicotinic (Chrna7, Chrnb2) and serotonergic (Htr2a, Htr5a) receptor subtypes, and the progesterone receptor (Evans and Foltin, 2006; Jackson et al., 2006; Muneoka et al., 2010; Popik and Wróbel, 2002; Yuan et al., 2013). In addition, our findings reveal potential novel molecular substrates of opioid-induced addiction-related plasticity. These include cyclin dependent kinase 5 (Cdk5) regulatory subunit 1 (Cdk5r1) and ephrin B2 (Efnb2). Ck5r1 encodes a neuronal-specific activator of Cdk5, which is strongly implicated in addictive properties of cocaine and opioids (Bibb et al., 2001; Ferrer-Alcón et al., 2003; Narita et al., 2005). Cdk5r1 upregulation in this study differs from a previous study that found downregulation of Cdk5/Cdk5r1 in morphine-treated rats (Ferrer-Alcón et al., 2003). These disparate findings may reflect differences in the timing of the assay relative to drug exposure (Spijker et al., 2004).
The Ephrin family of tyrosine kinase-related receptors regulates neurogenesis, neuronal migration, synaptic plasticity, axon guidance and neuroadaptation (Ashton et al., 2012; McClelland et al., 2009; Xiao et al., 2006). Ephrins also interact with glutamatergic and dopaminergic pathways (Essmann et al., 2008; Piccinin et al., 2010; Planagumà et al., 2016; Yue et al., 1999). Despite participating in such a highly relevant set of functional domains, the possible role of ephrins in the pathophysiology of mental illnesses, including addiction, has received relatively little attention. Nevertheless, the current finding of increased Efnb2 expression in the dmPFC adds to two previous transcriptomic screens that detected its upregulation in the NAc after repeated morphine exposure (Martínez-Rivera et al., 2019; Spijker et al., 2004). Thus, accumulating evidence implicates Efnb2 in the long-term effects of opioid exposure in the mesocorticolimbic system.
Analysis of upstream regulators from the common DEGs of both sexes suggests increased activity of JAK1/2, FEV, and ADORA2A. Both FEV and ADORA2A are potential hubs in the network of genes involved in opioid addiction. FEV transcription factor (PET-1) is localized in serotonin neurons in the CNS, where it is necessary for serotonin synthesis and release (Liu et al., 2010; Puzerey et al., 2015; Wyler et al., 2016). Our data additionally showed that genes coding the serotonin 5-HT2a and 5-HT5a receptors were upregulated after opioid exposure, further supporting the possibility of interactions between the opioidergic and serotonergic systems in the PFC (Marek and Aghajanian, 1998; Marek et al., 2001). The adenosine 2a receptor (ADORA2A) also interacts with the opioid system (Brown et al., 2009; Yao et al., 2006) and forms heteromeric complexes with other receptors, including the dopaminergic D2 receptor and the glutamatergic mGluR5 receptor (Ferré et al., 2007), which was upregulated in our study. Hence, the emergence of PET-1 and the adenosine 2a receptor as upstream regulators may provide insights into the hubs underlying gene networks critical in the development or maintenance of opioid addiction.
Our identification of molecular networks that were differentially affected by morphine in males and females highlights potential mechanisms underlying the gender-specific effects of vulnerability to opioid addiction. Female rats showed significant enrichment of genes in the long-term depression and endocannabinoid synaptic pathways. The latter likely reflects the interaction between cannabinoid and opioid systems (Ledent et al., 1999; Martin et al., 2000; Wenzel and Cheer, 2018), which occurs in response to opioid drugs (Maldonado and Rodríguez De Fonseca, 2002). Additionally, endocannabinoid receptors participate in estradiol-potentiated cocaine-induced locomotor activity (Peterson et al., 2016). The interactions among endocannabinoid, opioid and hormonal systems may represent female-specific mechanisms underlying addiction vulnerability (Anker and Carroll, 2011; Carroll and Anker, 2009; Chartoff and McHugh, 2016; Lynch et al., 2002). In contrast, male rats showed enrichment of genes in neuroinflammatory pathways, adding to an emerging literature on the role of neuroinflammatory processes in sex differences in the effects of opioids (Averitt et al., 2019; Doyle et al., 2017).
In summary, repeated morphine administration produced substantial upregulation of gene transcription in the dmPFC, particularly affecting genes involved in synaptogenesis, neuroplasticity, and neuronal development. These changes may reflect a compensatory response to repeated stimulation of mu opioid receptors. It is noteworthy that assessment of transcriptional changes 24-h after drug exposure in this study coincides with robust manifestations of opioid withdrawal symptoms (Bechara et al., 1995; Gold et al., 1994; Hand et al., 1988; Rothwell et al., 2009). We have found previously that one of these measures is withdrawal-induced anhedonia (Swain. et al., 2020). The clinical relevance of this finding was supported in the current study in that gene expression changes after repeated morphine exposure correlated highly with those identified postmortem in individuals with a diagnosis of major depression, a condition characterized by anhedonia. Moreover, we have found that withdrawal-induced anhedonia correlates with_the severity of subsequent morphine self-administration. Thus, to the extent that their functional relevance is confirmed, a subset of the genes and gene ontology pathways identified in this study may represent opioid-stimulated biomarkers for long-term vulnerability to anhedonia and opioid addiction.
Supplementary Material
Highlights.
Chronic morphine administration induced hyperactivity and hyperalgesia.
The same regimen altered the transcriptome in rats’ dorsomedial prefrontal cortex.
Gene networks involved in cellular plasticity/signaling were altered in both sexes.
Males and females showed some differences in gene ontology network enrichment.
Acknowledgments
We thank Anna Slukvin and Matthew Steen for help with behavioral assays, Dr. Amanda Barks for help with RT-qPCR and Dr. Juan E. Abrahante Lloréns for help with RNA-seq data analysis.
Role of Funding Source
This work was supported by R21 DA037728, U01 DA051993 and T32 DA007097, and by grants from the Engdahl Family Research Fund and the Office of the Vice President for Research, University of Minnesota, and the Hennepin Healthcare Research Institute Career Development Award.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: https://doi.org/10.1016/j.drugalcdep.2021.108598
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: https://doi.org/10.1016/j.drugalcdep.2021.108598
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: https://doi.org/10.1016/j.drugalcdep.2021.108598
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker JJ, Carroll ME, 2011. Females are more vulnerable to drug abuse than males: Evidence from preclinical studies and the role of ovarian hormones. Curr. Top. Behav. Neurosci 8, 73–96. 10.1007/7854_2010_93 [DOI] [PubMed] [Google Scholar]
- Ashton RS, Conway A, Pangarkar C, Bergen J, Lim K Il, Shah P, Bissell M, Schaffer DV, 2012. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci 15, 1399–1406. 10.1038/nn.3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averitt DL, Eidson LN, Doyle HH, Murphy AZ, 2019. Neuronal and glial factors contributing to sex differences in opioid modulation of pain. Neuropsychopharmacology. 10.1038/s41386-018-0127-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T, Nevo I, Levy R, Pfeuffer T, Vogel Z, 1996. Chronic opioid treatment induces adenylyl cyclase V superactivation. Involvement of G(βγ). J. Biol. Chem 271, 21309–21315. 10.1074/jbc.271.35.21309 [DOI] [PubMed] [Google Scholar]
- Barks A, Fretham SJB, Georgieff MK, Tran PV, 2018. Early-Life Neuronal-Specific Iron Deficiency Alters the Adult Mouse Hippocampal Transcriptome. J. Nutr 10.1093/jn/nxy125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Nader K, van der Kooy D, 1995. Neurobiology of Withdrawal Motivation: Evidence for Two Separate Aversive Effects Produced in Morphine-Naive Versus Morphine-Dependent Rats by Both Naloxone and Spontaneous Withdrawal. Behav. Neurosci 109, 91–105. 10.1037/0735-7044.109.1.91 [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex differences in animal models: Focus on addiction. Pharmacol. Rev 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG, 2017. Sex differences, gender and addiction. J. Neurosci. Res 95, 136–147. 10.1002/jnr.23963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P, 2001. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410, 376–380. 10.1038/35066591 [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL, 1999. GENDER DIFFERENCES IN SUBSTANCE USE DISORDERS. Psychiatr. Clin. North Am 22, 241–252. 10.1016/S0193-953X(05)70074-5 [DOI] [PubMed] [Google Scholar]
- Brown RM, Short JL, Cowen MS, Ledent C, Lawrence AJ, 2009. A differential role for the adenosine A2A receptor in opiate reinforcement vs opiate-seeking behavior. Neuropsychopharmacology 34, 844–856. 10.1038/npp.2008.72 [DOI] [PubMed] [Google Scholar]
- Browne CJ, Godino A, Salery M, Nestler EJ, 2020. Epigenetic Mechanisms of Opioid Addiction. Biol. Psychiatry 10.1016/j.biopsych.2019.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camí J, Farré M, 2003. Mechanisms of disease: Drug addiction. N. Engl. J. Med 10.1056/NEJMra023160 [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, 2009. Sex differences and ovarian hormones in animal models of drug dependence. Horm. Behav 58, 44–56. 10.1016/j.yhbeh.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Chartoff EH, McHugh RK, 2016. Translational studies of sex differences in sensitivity to opioid addiction. Neuropsychopharmacology, 10.1038/npp.2015.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Stevens CF, 1994. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J. Neurosci 14, 310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Ji D, Zhou FM, 2001. Synaptic plasticity and nicotine addiction. Neuron. 10.1016/S0896-6273(01)00379-8 [DOI] [PubMed] [Google Scholar]
- Doyle HH, Eidson LN, Sinkiewicz DM, Murphy AZ, 2017. Sex differences in microglia activity within the periaqueductal gray of the rat: A potential mechanism driving the dimorphic effects of morphine. J. Neurosci 37,3202–3214. 10.1523/JNEUROSCI.2906-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, Acker-Palmer A, 2008. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat. Neurosci 11, 1035–1043. 10.1038/nn.2171 [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW, 2006. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology 31, 659–674. 10.1038/sj.npp.1300887 [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Woods AS, Lluis C, Franco R, 2007. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 10.1016/j.tins.2007.07.001 [DOI] [PubMed] [Google Scholar]
- Ferrer-Alcón M, La Harpe R, Guimón J, Garaa-Sevilla JA, 2003. Downregulation of neuronal cdk5/p35 in opioid addicts and opiate-treated rats: Relation to neurofilament phosphorylation. Neuropsychopharmacology 28, 947–955. 10.1038/sj.npp.1300095 [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG, 1978. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J. Pharmacol. Exp. Ther 205. [PubMed] [Google Scholar]
- Gold LH, Stinus L, Inturrisi CE, Koob GF, 1994. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. Eur. J. Pharmacol 253, 45–51. 10.1016/0014-2999(94)90755-2 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2002. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 10.1176/appi.ajp.159.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghparast A, Fatahi Z, Alamdary SZ, Reisi Z, Khodagholi F, 2014. Changes in the Levels of p-ERK, p-CREB, and c-fos in Rat Mesocorticolimbic Dopaminergic System After Morphine-Induced Conditioned Place Preference: The Role of Acute and Subchronic Stress. Cell. Mol. Neurobiol 34, 277–288. 10.1007/s10571-013-0011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy MM, Mamiya T, Noda Y, Sayed M, Assi AA, Gomaa A, Yamada K, Nabeshima T, 2001. A selective phosphodiesterase IV inhibitor, rolipram blocks both withdrawal behavioral manifestations, and c-Fos protein expression in morphine dependent mice. Behav. Brain Res 118, 85–93. 10.1016/S0166-4328(00)00315-6 [DOI] [PubMed] [Google Scholar]
- Hand TH, Koob GF, Stinus L, Le Moal M, 1988. Aversive properties of opiate receptor blockade: evidence for exclusively central mediation in naive and morphine-dependent rats. Brain Res. 474, 364–368. 10.1016/0006-8993(88)90452-0 [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, & Joris J (1988). A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain, 32(1), 77–88. 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hearing M, 2019. Prefrontal-accumbens opioid plasticity: Implications for relapse and dependence. Pharmacol. Res 10.1016/j.phrs.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzemann R, Bottomly D, Darakjian P, Walter N, Iancu O, Searles R, Wilmot B, Mcweeney S, 2013. Genes, behavior and next-generation RNA sequencing. Genes, Brain Behav. 12, 1–12. 10.1111/gbb.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, O’Brien CP, 2018. Molecular Genetics and New Medication Strategies for Opioid Addiction. Am. J. Psychiatry 175, 935–942. 10.1176/appi.ajp.2018.18030352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ, 2006. NEURAL MECHANISMS OF ADDICTION: The Role of Reward-Related Learning and Memory. Annu. Rev. Neurosci 29, 565–598. 10.1146/annurev.neuro.29.051605.113009 [DOI] [PubMed] [Google Scholar]
- Imperio CG, McFalls AJ, Colechio EM, Masser DR, Vrana KE, Grigson PS, Freeman WM, 2016. Assessment of individual differences in the rat nucleus accumbens transcriptome following taste-heroin extended access. Brain Res. Bull 123, 71–80. 10.1016/j.brainresbull.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB, 2006. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 31, 129–138. 10.1038/sj.npp.1300778 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J, 2005. Unmanageable motivation in addiction: A pathology in prefrontal-accumbens glutamate transmission. Neuron. 10.1016/j.neuron.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC, 2007. Synaptic plasticity and addiction. Nat. Rev. Neurosci 10.1038/nrn2234 [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 1997. Drug abuse: Hedonic homeostatic dysregulation. Science (80-. ). 278, 52–58. 10.1126/science.278.5335.52 [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y, 2009. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56, 177–185. 10.1016/j.neuropharm.2008.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF, 1998. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 51, 23–47. 10.1016/S0376-8716(98)00064-7 [DOI] [PubMed] [Google Scholar]
- Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YHE, Cahill M, Lorsch ZS, Hamilton PJ, Calipari ES, Hodes GE, Issler O, Kronman H, Pfau M, Obradovic ALJ, Dong Y, Neve RL, Russo S, Kazarskis A, Tamminga C, Mechawar N, Turecki G, Zhang B, Shen L, Nestler EJ, 2017. Sex-specific transcriptional signatures in human depression. Nat. Med 23, 1102–1111. 10.1038/nm.4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Zhu H, Sun A, Zhuang D, Fu D, Chen W, Zhang HT, Zhou W, 2014. The phosphodiesterase-4 inhibitor rolipram attenuates heroin-seeking behavior induced by cues or heroin priming in rats. Int. J. Neuropsychopharmacol 17, 1397–1407. 10.1017/S1461145714000595 [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bóhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M, 1999. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science (80-. ). 283, 401–404. 10.1126/science.283.5400.401 [DOI] [PubMed] [Google Scholar]
- Lee CWS, Ho IK, 2013. Sex differences in opioid analgesia and addiction: Interactions among opioid receptors and estrogen receptors. Mol. Pain 10.1186/1744-8069-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES, 2010. Pet-1 is required across different stages of life to regulate serotonergic function. Nat. Neurosci 13, 1190–1198. 10.1038/nn.2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC, 2011. Drug-Evoked Synaptic Plasticity in Addiction: From Molecular Changes to Circuit Remodeling. Neuron. 10.1016/j.neuron.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME, 2002. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl). 164, 121–137. 10.1007/s00213-002-1183-2 [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodríguez De Fonseca F, 2002. Cannabinoid Addiction: Behavioral Models and Neural Correlates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK, 1998. 5-Hydroxytryptamine-induced excitatory postsynaptic currents in neocortical layer V pyramidal cells: Suppression by μ-opiate receptor activation. Neuroscience 86, 485–497. 10.1016/S0306-4522(98)00043-8 [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD, 2001. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience 105, 379–392. 10.1016/S0306-4522(01)00199-3 [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O, 2000. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur. J. Neurosci 12, 4038–4046. 10.1046/j.1460-9568.2000.00287.x [DOI] [PubMed] [Google Scholar]
- Martínez-Rivera FJ, Martínez NA, Martínez M, Ayala-Pagán RN, Silva WI, Barreto-Estrada JL, 2019. Neuroplasticity transcript profile of the ventral striatum in the extinction of opioid-induced conditioned place preference. Neurobiol. Learn. Mem 163, 107031. 10.1016/j.nlm.2019.107031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland AC, Sheffler-Collins SI, Kayser MS, Dalva MB, 2009. Ephrin-B1 and ephrin-B2 mediate EphB-dependent presynaptic development via syntenin-1. Proc. Natl. Acad. Sci. U. S. A 106, 20487–20492. 10.1073/pnas.0811862106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Homayoun H, 2008. Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 10.1038/sj.npp.1301554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morón JA, Gullapalli S, Taylor C, Gupta A, Gomes I, Devi LA, 2010. Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: Conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology 35, 955–966. 10.1038/npp.2009.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Kuwagata M, Ogawa T, Shioda S, 2010. Sex-specific effects of early neonatal progesterone treatment on dopamine and serotonin metabolism in rat striatum and frontal cortex. Life Sci. 87, 738–742. 10.1016/j.lfs.2010.10.016 [DOI] [PubMed] [Google Scholar]
- Narita Minoru, Shibasaki M, Nagumo Y, Narita Michiko, Yajima Y, Suzuki T, 2005. Implication of cyclin-dependent kinase 5 in the development of psychological dependence on and behavioral sensitization to morphine. J. Neurochem 93, 1463–1468. 10.1111/j.1471-4159.2005.03136.x [DOI] [PubMed] [Google Scholar]
- Nestler EJ, 2016. Reflections on: “A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function.” Brain Res. 10.1016/j.brainres.2015.12.039 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, 2001. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci 2, 119–128. 10.1038/35053570 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Tallman JF, 1988. Chronic morphine treatment increases cyclic AMP-dependent protein kinase activity in the rat locus coeruleus. Mol. Pharmacol 33. [PubMed] [Google Scholar]
- Paolone G, Burdino R, Badiani A, 2003. Dissociation in the modulatory effects of environmental novelty on the locomotor, analgesic, and eating response to acute and repeated morphine in the rat. Psychopharmacology (Berl). 166, 146–155. 10.1007/s00213-002-1321-x [DOI] [PubMed] [Google Scholar]
- Park YK, Goda Y, 2016. Integrins in synapse regulation. Nat. Rev. Neurosci 10.1038/nrn.2016.138 [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’connor EC, Lüscher C, 2014. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509, 459–464. 10.1038/nature13257 [DOI] [PubMed] [Google Scholar]
- Peterson BM, Martinez LA, Meisel RL, Mermelstein PG, 2016. Estradiol impacts the endocannabinoid system in female rats to influence behavioral and structural responses to cocaine. Neuropharmacology 110, 118–124. 10.1016/j.neuropharm.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinin S, Cinque C, Calò L, Molinaro G, Battaglia G, Maggi L, Nicoletti F, Melchiorri D, Eusebi F, Massey PV, Bashir ZI, 2010. Interaction between ephrins and mGlu5 metabotropic glutamate receptors in the induction of long-term synaptic depression in the hippocampus. J. Neurosci 30, 2835–2843. 10.1523/JNEUROSCI.4834-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisier SB, Taschereau R, Wong JA, & Graeber TG (2010). Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic acids research, 38(17), e169. 10.1093/nar/gkq636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planagumà J, Haselmann H, Mannara F, Petit- Pedrol M, Grünewald B, Aguilar E, Röpke L, Martín- García E, Titulaer MJ, Jercog P, Graus F, Maldonado R, Geis C, Dalmau J, 2016. Ephrin- B2 prevents N- methyl- D- aspartate receptor antibody effects on memory and neuroplasticity. Ann. Neurol 80, 388–400. 10.1002/ana.24721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Wróbel M, 2002. Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology 43, 1210–1217. 10.1016/S0028-3908(02)00309-X [DOI] [PubMed] [Google Scholar]
- Puzerey PA, Kodama NX, Galán RF, 2015. Abnormal cell-intrinsic and network excitability in the neocortex of serotonin-deficient Pet-1 knockout mice. J. Neurophysiol 115, 813–825. 10.1152/jn.00996.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Gewirtz JC, Thomas MJ, 2010. Episodic withdrawal promotes psychomotor sensitization to morphine. Neuropsychopharmacology 35, 2579–2589. 10.1038/npp.2010.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Thomas MJ, Gewirtz JC, 2009. Distinct profiles of anxiety and dysphoria during spontaneous withdrawal from acute morphine exposure. Neuropsychopharmacology 34, 2285–2295. 10.1038/npp.2009.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa JR, Fatma M, Loh YHE, Traore SR, Stefan T, Chen TH, … Labonté B (2020). Shared Transcriptional Signatures in Major Depressive Disorder and Mouse Chronic Stress Models. Biological Psychiatry, 88(2), 159–168. 10.1016/j.biopsych.2019.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M, 2007. Functions of Site-Specific histone acetylation and deacetylation. Annu. Rev. Biochem 10.1146/annurev.biochem.76.052705.162114 [DOI] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M, 1977. Opiate dependent modulation of adenylate cyclase. Proc. Natl. Acad. Sci. U. S. A 74, 3365–3369. 10.1073/pnas.74.8.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M, 1975. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc. Natl. Acad. Sci. U. S. A 72, 3092–3096. 10.1073/pnas.72.8.3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW, 2011. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc. Natl. Acad. Sci. U. S. A 108, 19407–19412. 10.1073/pnas.1112052108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C, Lefevour A, 1996. Sensitization to the conditioned rewarding effects of morphine: Pharmacology and temporal characteristics. Eur. J. Pharmacol 299, 33–39. 10.1016/0014-2999(95)00852-7 [DOI] [PubMed] [Google Scholar]
- Spijker S, Houtzager SWJ, De Gunst MCM, De Boer WPH, Schoffelmeer ANM, Smit AB, 2004. Morphine exposure and abstinence define specific stages of gene expression in the rat nucleus accumbens. FASEB J. 18, 848–850. 10.1096/fj.03-0612fje [DOI] [PubMed] [Google Scholar]
- Swain Y, Muelken P, Skansberg A, Lanzdorf D, Haave Z, LeSage MG, Gewirtz JC, & Harris AC (2020). Higher anhedonia during withdrawal from initial opioid exposure is protective against subsequent opioid self-administration in rats. Psychopharmacology, 237(8), 2279–2291. 10.1007/s00213-020-05532-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ, 1991. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 548, 100–10. 10.1016/0006-8993(91)91111-d [DOI] [PubMed] [Google Scholar]
- Thompson CM, Wojno H, Greiner E, May EL, Rice KC, Selley DE, 2004. Activation of G-Proteins by Morphine and Codeine Congeners: Insights to the Relevance of O- and N-Demethylated Metabolites at μ- and δ-Opioid Receptors. J. Pharmacol. Exp. Ther 308, 547–554. 10.1124/jpet.103.058602 [DOI] [PubMed] [Google Scholar]
- Traber J, Gullis R, Hamprecht B, 1975. Influence of opiates on the levels of adenosine 3′: 5′-cyclic monophosphate in neuroblastoma x glioma hybrid cells. Life Sci. 16, 1863–1868. 10.1016/0024-3205(75)90292-1 [DOI] [PubMed] [Google Scholar]
- Tran PV, Kennedy BC, Lien YC, Simmons RA, & Georgieff MK (2015). Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. American Journal of Physiology - Regulatory Integrative and Comparative Physiology, Vol. 308, pp. R276–R282. 10.1152/ajpregu.00429.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumati S, Largent-Milnes TM, Keresztes A, Ren J, Roeske WR, Vanderah TW, & Varga EV (2012). Repeated morphine treatment-mediated hyperalgesia, allodynia and spinal glial activation are blocked by co-administration of a selective cannabinoid receptor type-2 agonist. Journal of neuroimmunology, 244(1-2), 23–31. 10.1016/j.jneuroim.2011.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Koroshetz W, Volkow ND, 2020. Neurobiology of the Opioid Epidemic: Basic and Translational Perspectives. Biol. Psychiatry 10.1016/j.biopsych.2019.09.003 [DOI] [PubMed] [Google Scholar]
- Walker DM, Cates HM, Loh YHE, Purushothaman I, Ramakrishnan A, Cahill KM, Lardner CK, Godino A, Kronman HG, Rabkin J, Lorsch ZS, Mews P, Doyle MA, Feng J, Labonté B, Koo JW, Bagot RC, Logan RW, Seney ML, Calipari ES, Shen L, Nestler EJ, 2018. Cocaine Self-administration Alters Transcriptome-wide Responses in the Brain’s Reward Circuitry. Biol. Psychiatry 84, 867–880. 10.1016/j.biopsych.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA, 1994. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science (80-. ). 265, 1878–1882. 10.1126/science.7916482 [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Cheer JF, 2018. Endocannabinoid Regulation of Reward and Reinforcement through Interaction with Dopamine and Endogenous Opioid Signaling. Neuropsychopharmacology, 10.1038/npp.2017.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA, 2004. Prefrontal responses to drug cues: A neurocognitive analysis. Nat. Neurosci 7, 211–214. 10.1038/nn1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler SC, Spencer WC, Green NH, Rood BD, Crawford LT, Craige C, Gresch P, McMahon DG, Beck SG, Deneris E, 2016. Pet-1 switches transcriptional targets postnatally to regulate maturation of serotonin neuron excitability. J. Neurosci 36, 1758–1774. 10.1523/JNEUROSCI.3798-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Miller GM, Jassen A, Westmoreland SV, Pauley D, Madras BK, 2006. Ephrin/Eph receptor expression in brain of adult nonhuman primates: Implications for neuroadaptation. Brain Res. 1067, 67–77. 10.1016/j.brainres.2005.10.073 [DOI] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Ueda T, Diamond I, 2006. Adenosine A2a blockade prevents synergy between μ-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proc. Natl. Acad. Sci. U. S. A 103, 7877–7882. 10.1073/pnas.0602661103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Mameli M, O’Connor EC, Dey PN, Verpelli C, Sala C, Perez-Otano I, Lüscher C, Bellone C, 2013. Expression of Cocaine-Evoked Synaptic Plasticity by GluN3A-Containing NMDA Receptors. Neuron 80, 1025–1038. 10.1016/j.neuron.2013.07.050 [DOI] [PubMed] [Google Scholar]
- Yue Y, Widmer DAJ, Halladay AK, Cerretti DP, Wagner GC, Dreyer JL, Zhou R, 1999. Specification of distinct dopaminergic neural pathways: Roles of the Eph family receptor EphB1 and ligand Ephrin-B2. J. Neurosci 19, 2090–2101. 10.1523/jneurosci.19-06-02090.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.