Abstract
Treatment with highly active antiretroviral therapy (HAART) can prolong a patient's life-span by disrupting pivotal steps in the replication cycle of the human immunodeficiency virus-1 (HIV-1). However, drug resistance is emerging as a major problem worldwide due to the prolonged period of treatment undergone by HIV-1 patients. Since the approval of zidovudine in 1987, over thirty antiretroviral drugs have been categorized into the following six distinct classes based on their biological function and resistance profiles: (1) nucleoside analog reverse-transcriptase inhibitors; (2) non–nucleoside reverse transcriptase inhibitors; (3) integrase strand transferase inhibitors; (4) protease inhibitors; (5) fusion inhibitors; and (6) co-receptor antagonists. Additionally, several antiretroviral drugs have been developed recently, such as a long active drug, humanized antibody and pro-drug metabolized into an active form in the patient's body. Although plenty of antiretroviral drugs are beneficially used to treat patients with HIV-1, the ongoing efforts to develop antiretroviral drugs have overcome the drug resistances, adverse effects, and limited adherence of drugs observed in previous drugs to some extent. Furthermore, studies focused on agents targeting latent HIV-1 reservoirs should be strengthened, as that may lead to eradication of HIV-1.
Keywords: Human immunodeficiency virus, Anti-HIV-1 drugs, HIV-1 Tat
Introduction
In 1981, the United States Centers for Disease Control and Prevention (US CDC) reported the seemingly sudden development of a fatal infectious disease among homosexuals and drug users. This new disease caused a deficiency in the patient's immune state, leading to Kaposi sarcoma, Pneumocystis jirovecii pneumonia, lymphoma and, eventually, death. Because the disease was initially detected mainly in male homosexuals, it was initially called "Gay Related Immunodeficiency Syndrome" (GRID). In 1983, Luc Montagnier and other members of the INSTITUT PASTEUR, France, isolated a retrovirus from the lymph node of a lymphoma patient, which was later identified as a virus called human immunodeficiency virus-1 (HIV-1) [1,2,3,4]. Thereafter, the life cycle of the virus was discovered through many studies, thus enabling the rapid development of therapeutic agents and diagnostic technology. Because the main characteristic of HIV-1 is the reverse transcription process, a therapeutic drug development strategy aimed at blocking this process was applied first of all, and the first HIV-1 treatment drug, zidovudine (ZDV), a nucleoside reverse transcriptase inhibitor, was developed in 1987 [5]. This was followed by the successive development of a non-nucleoside reverse transcriptase inhibitors, protease inhibitors, viral entry inhibitors, and virus integrase strand transferase inhibitors. More recently, continuous progress has been made in improving the effects of existing drugs as well as in developing a new paradigm of HIV-1 drugs. This study aims to review the process of development of these drugs and their mechanisms, along with more recently developed antiviral agents, in order to suggest a developmental direction for HIV/acquired immune deficiency syndrome (AIDS) therapeutic drugs.
The HIV-1 replication steps as the drug target
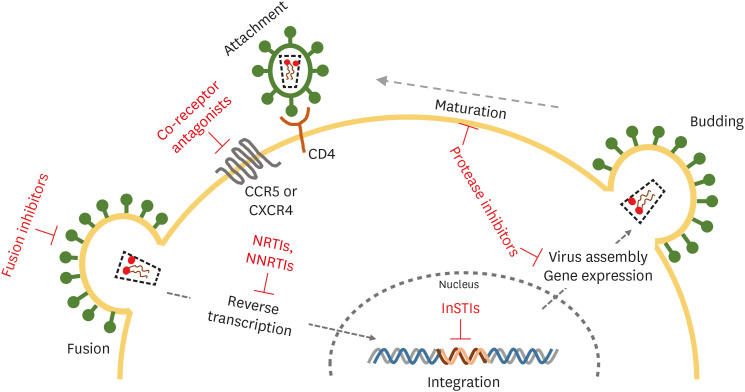
Since the discovery of HIV-1 in 1983, the life cycle of HIV-1 has been revealed and therapeutic drugs that inhibit HIV-1 proliferation have been developed on the basis of the results of studies [6]. HIV-1 binds to the receptor CD4 of CD4+ T cells and co-receptors (CCR5 or CXCR4), and then enters the host cell [7]. After entry, the viral RNA genome is transcribed to DNA by reverse transcriptase, moved to the nucleus, and then integrated into a host chromosome by integrase. The inserted viral DNA (provirus), with the help of the viral transcription factor Tat (Trans-activator of Transcription) and several factors in the host cell, expresses viral mRNA. The full length of the expressed viral mRNA becomes the genome of the progeny virus, and some mRNAs are translated into several proteins necessary for outer membrane proteins (gp120 and gp41) and formation of a virion, which then enclose the host cell membrane and are released out of the cell. At this time, the virion is maturated by viral protease and transformed into a virus with infectivity, which then re-infects the nearby cells so as to begin a new viral life cycle (Fig. 1) [8].
Figure 1. Stages of the HIV-1 life cycle and current target for antiretroviral drugs.
HIV-1, human immunodeficiency virus-1; NRTIs, nucleoside analogue reverse transcriptase inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; InSTIs, integrase strand transferase inhibitors.
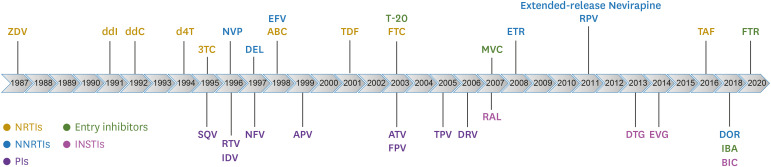
From the appearance of the first reverse transcriptase inhibitor, ZDV, in 1987 to the recent integrase strand transferase inhibitor, bictegravir (BIC), about thirty HIV-1 therapeutic drugs capable of inhibiting each stage have been developed and used in the treatment of HIV-1 patients (Fig. 2) [8,9]. These HIV-1 therapeutic drugs have been classified into five types according to their targeted stage and chemical forms: reverse transcriptase inhibitors (nucleoside type and non-nucleoside type), protease inhibitors, integrase strand transferase inhibitors, CCR5 antagonists, and membrane fusion inhibitors [8,9,10].
Figure 2. Timeline for FDA-approved antiretroviral drugs.
FDA, food and drug administration; ZDV, zidovudine; ddI, didanosine; ddC, zalcitabine; d4T, stavudine; 3TC, lamivudine; SQV, saquinavir; NVP, nevirapine; RTV, ritonavir; IDV, indinavir; DEL, delavirdine; NFV, nelfinavir; EFV, efavirenz; ABC, abacavir; APV, asunaprevir; TDF, tenofovir disoproxil fumarate; T-20, enfuvirtide; FTC, emtricitabine; ATV, atazanavir; FPV, fosamprenavir; TPV, tipranavir; DRV, darunavir; MVC, maraviroc; RAL, raltgravir; ETR, etravirine; RPV, rilpivirine; DTG, dolutegravir; EVG, elvitegravir; TAF, tenofovir alafenamide; DOR, doravirine; IBA, ibalizumab; BIC, bictegravir; ; FTR, fostemsavir; NRTIs, nucleoside reverse-transcriptase inhibitors; NNRTIs, non-nucleoside reverse-transcriptase inhibitors; InSTIs, integrase strand transfer inhibitors; PIs, protease inhibitors.
Reverse transcriptase inhibitors
Since the development of the first HIV-1 therapeutic drug, namely the nucleoside reverse transcriptase inhibitor (NRTI) zidobudine (3ʹ-azido 3ʹ- deoxythymidine, ZDV), in 1987, various nucleoside reverse transcriptase inhibitors have been developed one after another [6,11]. As resistance to nucleoside-like drugs gradually increased, the non-nucleoside reverse transcriptase inhibitor (NNRTI), nevirapine (NVP), was developed first in 1996, followed by the development of several NNRTIs [12].
1. Nucleoside reverse-transcriptase inhibitors (NRTIs)
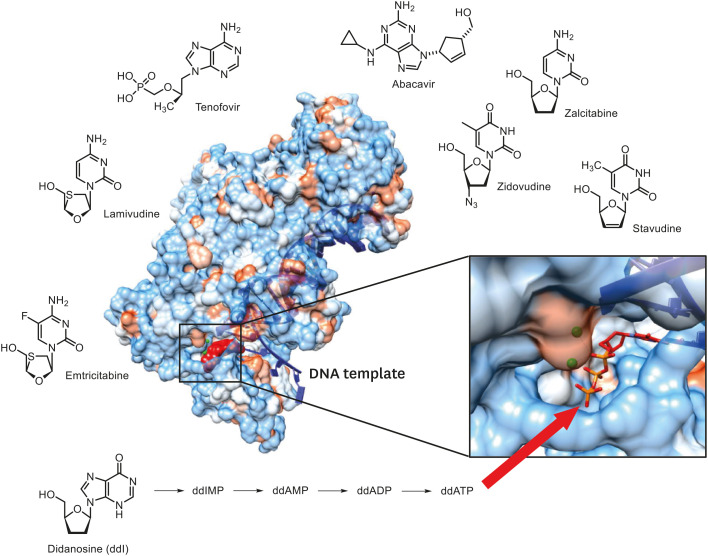
NRTIs constitute a group of anti-HIV-1 drugs that were approved for the first time by the US food and drug administration (FDA). These drugs are mainly administered as precursors, which then enter the host cell and are phosphorylated to the nucleotide form in order to display activity as a drug [13,14]. So far, eight types of NRTIs have been developed; recently, tenofovir alafenamide (TAF), with improved pharmacological activity of tenofovir, was developed and used (Table 1) [15]. These drugs commonly lack a 3-OH (hydroxyl group) on the deoxyribose moiety of the nucleoside, and thus demonstrate an action mechanism during viral DNA synthesis, which terminates viral DNA synthesis, by incorporating themselves into the growing DNA chain and blocking the 3′-5′ phosphodiester bond with the next incoming nucleotide (Fig. 3) [10,16].
Table 1. Nucleos(t)ide reverse-transcriptase inhibitors.
| Generic name | Brand name | FDA approval date |
|---|---|---|
| Zidovudine | Retrovir | 1987 |
| Didanosine | Videx | 1991 |
| zalcitabine | Hivid | 1992 |
| Stavudine | Zerit | 1994 |
| Lamivudine | Epivir | 1995 |
| Abacavir | Ziagen | 1998 |
| Tenofovir disoproxil fumarate | Viread | 2001 |
| Emtricitabine | Emtriva | 2003 |
| Tenofovir alafenamide fumarate | Vemlidy | 2016 |
FDA, food and drug administration.
Figure 3. X-ray crystal structure of HIV-1 RT in complex with viral DNA template/primer and Nucleos(t)ide reverse-transcriptase inhibitors. A nucleoside inhibitor didanosine (ddI) is converted to ddAMP missing OH, following termination of DNA synthesis by the incoming dTTP. The cartoon of the crystal structure has been adapted from data deposited by Huang et al. (1998) [87].
RT, reverse-transcriptase; HIV-1, human immunodeficiency virus-1; DNA, deoxyribonucleic acid; ddAMP, 2′3′-Dideoxyadenosine-5′monophosphate; dTTP, deoxythymidine triphosphate; OH, hydroxyl group.
2. Non-nucleoside reverse-transcriptase inhibitors (NNRTIs)
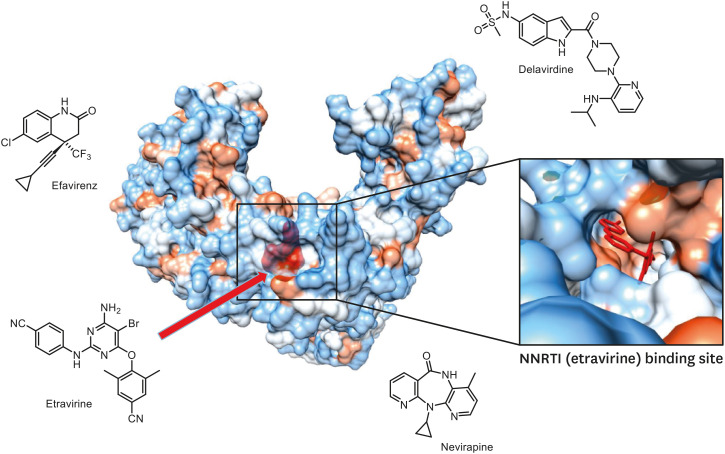
NNTRIs, unlike NRTIs, do not act as a terminator for viral DNA synthesis, but directly bind to the hydrophobic pocket near the active site of the reverse transcriptase to change the enzyme structure so as to inhibit enzymatic action (Fig. 4) [10,16]. As such, they show no cross-resistance with existing NRTIs. Although these high potent NNRTIs were still under development recently, the single administration of these drugs makes HIV-1 vulnerable to the acquisition of resistance and develops cross-resistance to other NNRTIs. Therefore, these drugs are administered in combination with other anti-HIV-1 drugs. So far, seven types of these drugs have been approved (Table 2).
Figure 4. Crystal structure of HIV-1 RT complexed with etravirine and Non-nucleoside reverse-transcriptase inhibitors. The cartoon of the crystal structure has been adapted from data deposited by Lansdon et al. (2010) [88].
HIV-1, human immunodeficiency virus-1; RT, reverse-transcriptase.
Table 2. Non-nucleoside reverse-transcriptase inhibitors.
| Generic name | Brand name | FDA approval date |
|---|---|---|
| Nevirapine | Viramune | 1996 |
| Delavirdine | Rescriptor | 1997 |
| Efavirenz | Sustiva | 1998 |
| Etravirine | Intelence | 2008 |
| Extended-release Nevirapine | Viramune XR | 2011 |
| Rilpivirine | Edurant | 2011 |
| Doravirine | Pifeltro | 2018 |
FDA, food and drug administration.
Protease inhibitors (PIs)
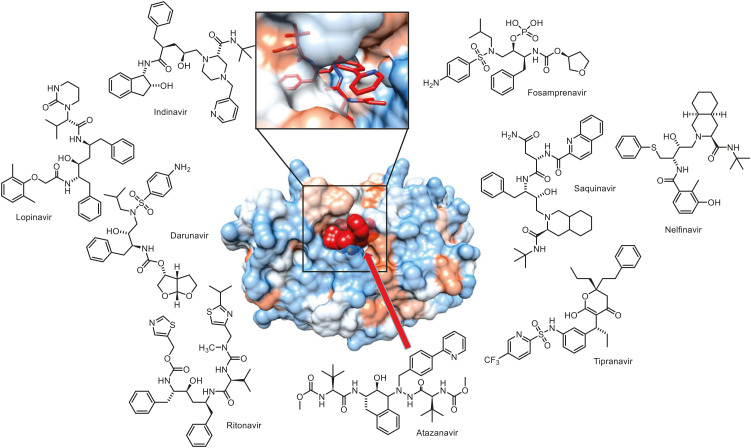
After the mid-1990s, various protease inhibitors (PIs) against HIV-1 were developed (Table 3) [17,18]. HIV-1's protease is an important enzyme that cuts the precursor proteins of GAG (group-specific antigen) and GAG-POL (polymerase) contained in the virion without infectivity and turns them into a matured virus with infectivity [19]. Therefore, protease inhibitors were welcomed with great expectations in the area of novel anti-HIV-1 drugs. During the earlier period, when such protease inhibitors were being developed, they were thought less likely to develop resistance because this enzyme plays a very important role in viral proliferation and is as tiny as 11 kDa [20]. But resistant mutation in patients with PI treatment was observed to develop with very high frequency, and it was found to be vulnerable to cross-resistance - particularly because all PIs have similar chemical structures for binding to the active site of the enzyme [20,21]. Thus, PIs are mainly used in ‘cocktail therapy’ with three types of anti-HIV-1 drugs at present [10]. Also, the long-term use of PIs results in the development of disadvantages such as a change of lipid distribution in the body and abnormal lipid metabolism [22]. Thus, no new PIs have been developed since darunavir in 2006 (Fig. 5) [10].
Table 3. Protease inhibitors.
| Generic name | Brand name | FDA approval date |
|---|---|---|
| Saquinavir | Invirase | 1995 |
| Ritonavir | Norvir | 1996 |
| Indinavir | Crixivan | 1996 |
| Nelfinavi | Viracept | 1997 |
| Amprenavir | Agenerase | 1999 |
| Atazanavir | Reyataz | 2003 |
| Fosamprenavir | Lexiva | 2003 |
| Tipranavir | Aptivus | 2005 |
| Darunavir | Prezista | 2006 |
FDA, food and drug administration.
Figure 5. The crystal structure of HIV-1 protease blocked by atazanavir and protease inhibitors. The cartoon of the crystal structure has been adapted from data by King et al. (2012) [89].
HIV-1, human immunodeficiency virus-1.
Membrane fusion inhibitors
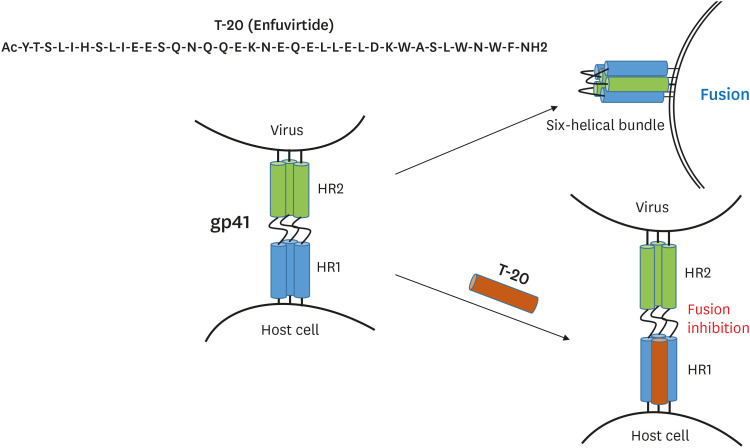
In 1997, a study on crystal structure revealed that glycoprotein 41 (gp41) for viral fusion consisted of two helical repeats, namely, heptad repeat 1 (HR1) and heptad repeat 2 (HR2), which interact with each other to form a six-helix bundle structure from trimeric gp41, after which the viral membrane fuses with the cell membrane; thus, a peptide capable of blocking this process was developed (Fig. 6) [23,24]. Then, it was approved by the US FDA in 2003 as enfuvirtide (T-20, Fuzeon), which has mainly been prescribed as an injection therapy for patients who show multi-drug resistance and/or adverse effects during treatment with existing drugs [25,26,27].
Figure 6. Peptide sequences of T-20 and the mode of action of T-20.
The formation of the gp41 six alpha-helix bundle is blocked by T-20 (enfuvirtide) in the final step of the HIV-1 entry process. The cartoon has been adapted from data by Berkhout et al. (2012) [90].
HIV-1, human immunodeficiency virus-1.
CCR5 antagonists
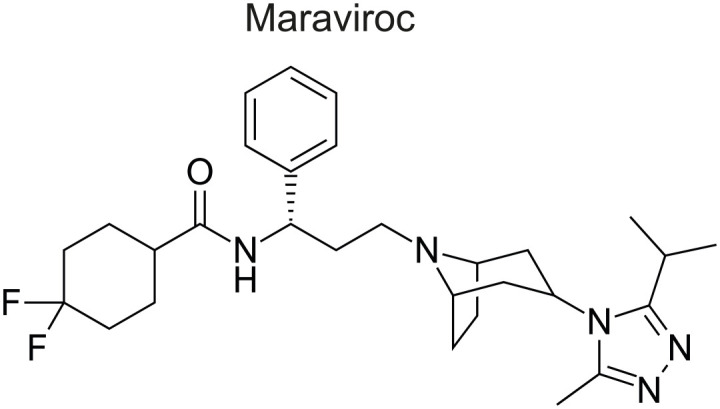
Maraviroc (MVC) is an antagonist of the HIV-1 co-receptor CCR5 and a small-molecule drug developed in 2007 (Fig. 7) [28]. It binds to the hydrophobic pocket of the CCR5 co-receptor and changes its conformation, and interrupts the binding of the V3 loop of the viral membrane protein gp120 to CCR5, in order to prevent viral entry into the host cell [29]. Although this drug targets the co-receptor of the host cell, it has been reported to acquire resistance using a different resistance mechanism, unlike other anti-HIV drugs that directly bind to the viral protein [30,31]. The mechanisms of resistance acquisition for this drug include a change of the infection route to CXCR4 instead of CCR5 of the viral protein gp120, a strengthening of coherence with CCR5 stronger than maraviroc, and the acquisition of penetration ability by recognizing the maraviroc-CCR5 complex [10,32,33].
Figure 7. Chemical structure of the CCR5 Antagonist (Maraviroc) [91].
Integrase strand transferase inhibitors (InSTIs)
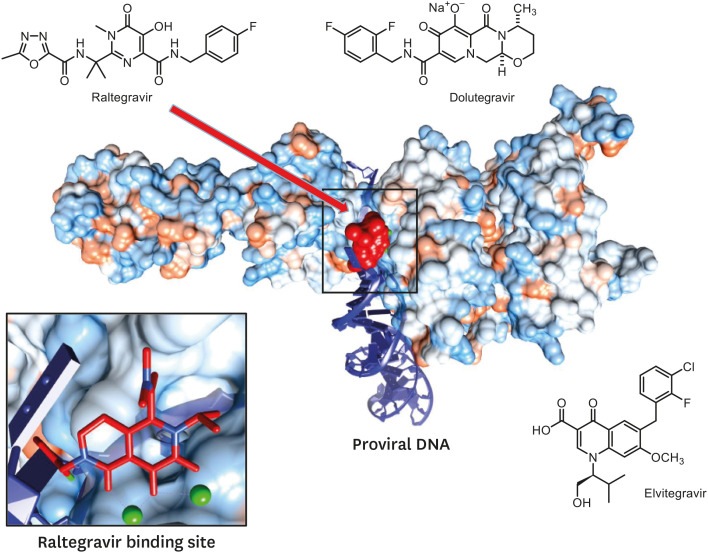
After reverse transcription is terminated, viral DNA is incorporated into the host chromosome to become a provirus. From then on, the virus can express its own genes and produce its progeny [34,35]. As such, integrase is necessary for incorporation of the viral DNA into the host chromosome, which is responsible for the essential stages of the viral life cycle. This integrase is the target of the most recently developed anti-HIV-1 drugs [34,35]. The integrase performs the following action: it cuts off the 3′-OH group and then transfers the viral strand into the host chromosome for integration [36,37]. Integrase inhibitors - called integrase (DNA) strand transferase inhibitors (InSTIs) - have been developed to target the function of viral strand transfer among them. Raltegravir (RAL) was first developed as an integrase inhibitor in 2007, followed by dolutegravir (DTG) in 2013 and elvitegravir (EVG) in 2014 (Table 4) [38,39,40]. These drugs bind to two magnesium ions required for binding the active site of integrase and the viral DNA, and thus block the binding of the integrase active site and the DNA (Fig. 8) [10,41,42,43]. After the phase 3 clinical trial, BIC was approved by the US FDA in 2018 in the form of a triple complex BIC/emtricitabine (FTC)/TAF with 2 types of reverse transcriptase inhibitors [44]. During a clinical study, this triple complex showed inhibitory effects on virus proliferation similar to those of the triple complex DTG/FTC/TAF including DTG, and also showed fewer adverse drug effects [45,46]. Furthermore, BIC develops less resistance and is highly effective against the resistance mutation observed in DTG. Its half-life is 18 hours [47,48,49].
Table 4. Integrase strand transfer.
| Generic name | Brand name | FDA approval date |
|---|---|---|
| Raltgravir | Isentress | 2007 |
| Dolutegravir | Tivicay | 2013 |
| Elvitegraivr | Vitekta | 2014 |
| Bictegravir | Biktarvy (BIC/FTC/TAF) | 2018 |
FDA, food and drug administration; BIC, bictegravir; FTC, emtricitabine; TAF, tenofovir alafenamide.
Figure 8. The crystal structure of virus integrase complexed with raltegravir [41] and integrase strand transfer inhibitors.
Pharmacokinetic booster
Several years ago, cobicistat (COBI) without its own antiviral properties was developed as a booster, which can increase the plasma concentration and absorption rate of antiretroviral agents [50,51,52,53]. Approved by the US FDA in August 2012, COBI, a potent inhibitor of the cytochrome P450 3A enzyme, inhibits intestinal transport proteins following an increase of the overall absorption of several antiretroviral agents (atazanavir, darunavir and tenofovir) [54]. Otherwise, it increases the efficacy of the integrase strand transferase inhibitor (InSTI) by inhibiting liver enzymes hydrolyzing the InSTI [55,56].
Recent progress of new antiretroviral drugs
1. Long-term active drugs: Cabotegravir (CAB) + Rilpivirine (RPV)
Antiviral agents that act for a long time in the patient's body have been developed as long-term active drugs, such as cabotegravir and rilpivirine [57]. The half-life of cabotegravir (CAB, GSK744), an InSTI currently under development, lasts for up to forty days, while that of nanoparticle-type nano-suspension generally lasts for 21 - 50 days, but can last for up to 90 days, and just 1 dosage can effectively inhibit viral proliferation [58,59]. Meanwhile, rilpivirine (RPV) is an already developed NNTRI. The half-life of a 25 mg tablet of rilpivirine is up to 50 days, while the half-life of long-term active nano-suspension may be as much as 90 days [12,60]. According to the results of a recent randomized phase 2b clinical trial, the combined administration of these two drugs maintained the virus concentration in the blood at below 50 copies/mL in 94% of patients taking the drugs at up to 8-week (56 days) intervals, and increased the convenience of patients compared to drugs who were administered them on a daily basis [61]. After a phase 3 clinical trial study, the two-drug regimen was submitted to the US and the EU for approval in 2019. It is expected that the development of long-term active drugs will greatly increase the quality of life for patients who can take drugs once every few months [62,63,64]. Otherwise, the injectable long-acting CAB is being investigated for pre-exposure prophylaxis (PrEP) in HIV-uninfected men under HPTN083 (HIP Prevention Trials Network 083) as of 2020.
2. Doravirine (DOR)
DOR is a next-generation NNRTI approved by the US FDA in August 2018 for the treatment of multi-drug-resistant HIV-1 in a combination tablet, DOR/3TC/TDF (Delstrigo) [65]. It seldom demonstrates a drug-drug interaction when combined with other anti-HIV-1 drugs, and shows a decreased level of LDL compared to other anti-HIV-1 drug groups, while its central nervous system (CNS) side effects are almost non-existent [66,67]. Considerable attention is being paid to the FDA approval depending on the results of a supplementary study [68,69].
3. Fostemsavir (FTR)
FTR is metabolized to temsavir (TMR) in the body and binds to gp120 of the virus, and then inhibits the penetration of HIV-1 into the host cell by blocking binding with the co-receptors CCR5 and CXCR4 [70,71]. Cross-resistance with other types of antiretroviral drugs has not yet been reported [70,71,72,73]. FTR was approved by the US FDA in July 2020 for the treatment of multi-drug-resistant HIV-1.
4. Ibalizumab (IBA)
IBA is a humanized immunoglobulin G4 monoclonal antibody that binds to CD4 of the host cell with high affinity [74,75]. It effectively inhibits the entry of HIV-1 by using the co-receptor CCR5 or CXCR4, into the host cell. In addition, it shows a therapeutic effect in existing multi-drug resistant viruses [74]. It was approved by the US FDA in March 2018 for the treatment of multi-drug-resistant HIV-1, and is used as an intravenous injection every 14 days [76,77]. It has been recommended for possible combination therapy with other anti-retroviral drugs such as InSTI, PI and NRTI/NNRTI, all of which have a mode of action distinct from that of IBA [77,78].
5. Islatravir
Islatravir is being developed as a nucleoside reverse transcriptase translocation inhibitor for HIV-1 prevention and has multiple mechanisms of action [79]. Islatravir is notable for its high potency and long plasma half-life (~120 hours), and has a 4-fold lower IC50 than any other NRTIs and a greater inhibitory effect against NRTI mutations, including M184I/V, K65R and K70E. It is being tested in its phase 3 of development as an HIV-1 treatment and PrEP using a two-drug fixed dose combination of DOR and islatravir [80].
6. GS-6207
GS-6207 is a small molecule that inhibits the HIV-1 capsid protein. It binds tightly at a conserved interface between capsid protein monomers, causing interference in the interaction between the capsid protein and cellular cofactors such as Nup153 and CPSF6, which are essential for multiple phases of the viral replication cycle. In Phase 1 clinical studies, monotherapy with a subcutaneous dose of GS-6207 (450mg) exhibited a mean 2.2 log reduction of the viral load at day 10, and showed sustained anti-viral active concentrations for more than 6 months. Due to the potency of GS-6207, the agent has been proposed as a potent long-acting drug to treat or prevent infection with HIV-1 [81,82].
7. Viral transcription factor Tat as therapeutic target
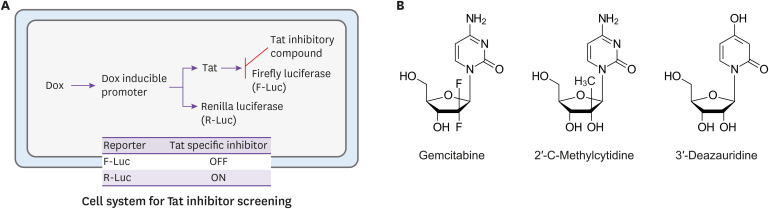
To overcome the resistance to HIV-1 drugs, novel drug targets including viral assembly, viral transcription, and the movement of the pre-integration complex (PIC) into nuclear etc. are being investigated. Of these, the transcription of the HIV-1 long terminal repeat (LTR) is considered a potential target for blocking HIV-1 replication, because this process is entirely dependent on the HIV-1 transcription factor Tat and is distinguishable from cellular transcription [83]; if a drug targeting this is developed, it could be used as a next-generation antiretroviral drug capable of overcoming the existing resistance problem [84]. Accordingly, a screening system that can discover substances which only inhibit the transcriptional activity of Tat by carefully distinguishing between the transcriptional activity of Tat and that of the host cell was established recently (Fig. 9A) [85]. During a drug repositioning study that used this system, anti-HIV-1 substances targeting the HIV-1 transcription factor Tat, gemcitabine and its two analogs (2′-C-methylcyitidine, 3-deazauridine), were discovered (Fig. 9B) [86]. Furthermore, the relevant research has continued to discover novel substances by screening various compound libraries, while a pharmaceuticalization study has been steadily carried out with the aim of using the discovered substances as antiretroviral drugs.
Figure 9. Schematic diagram of discovery of the HIV-1 transcriptional inhibitor (A) and nucleoside analogues of novel HIV-1 transcription-repressing compounds (B) [86].
HIV-1, human immunodeficiency virus-1; DOX, doxycycline; Tat, viral transactivator; OFF, signal-off; ON, signal-on.
Conclusion
After the discovery of HIV-1 in 1983, the very first HIV therapeutic drug, ZDV, was developed in 1987, followed by the development of about thirty anti-HIV-1 drugs over the subsequent thirty years. Once infected, patients cannot be completely cured of HIV-1 and must take medication for the rest of their life. In this way, HIV-1 is continuously exposed to drugs under a situation in which it cannot be completely eliminated, and resistance-mutations accumulate to avoid this, whereupon a new virus that cannot be controlled by the drug is re-created. To fight against HIV-1 with excellent resistance acquisition, researchers have developed a range of therapeutic targets and made efforts to develop drugs to block HIV-1 ever since the development of the NRTI. In the mid-1990s, PIs such as saquinavir were developed to inhibit viral mutation, and NNRTIs such as nevirapine were developed to overcome the high frequency of resistance development and the cross-resistance of NRTIs. As more than two types of therapeutic targets have been developed, combination anti-retroviral therapy (cART), which prescribes more than three types of therapeutic target drugs at the same time in order to minimize the development of resistance, has become the general HIV/AIDS treatment. Nevertheless, the virus has not completely disappeared, and the problem of resistance persists due to exposure to continuous drug treatments, which further develop multiple drug resistance. Since the 2000s, researchers have been striving to find novel therapeutic targets to overcome multi-drug resistance. In 2003, enfuviritide was developed to target the viral penetration process, while maraviroc was developed to competitively inhibit viral attachment to the CCR5 co-receptor in 2007. These drugs also induced various types of resistance, making it necessary to develop even more powerful anti-HIV-1 drugs. Among them, integrase, which incorporates viral nucleic acid into the host chromosome, aroused much interest due to its great potential as a new therapeutic target, and the first integrase inhibitor, RAL, was developed in 2007. This was followed by DTG in 2013, EVG in 2014 and, most recently, BIC and CAB as drug sources for complex medication in 2018 and 2019, respectively. As the battle between HIV-1 and humanity has become increasingly fierce, integrase inhibitor-based long-term active drugs have been developed to reduce the discomfort of taking drugs every day. Also, the humanized antibody IBA, an entirely different type compared to existing drugs, was successfully developed in 2018. Antiretroviral drugs have been developed on a continuous basis, but the latent viral reservoir must be detected and selectively killed if we are to fundamentally and completely cure patients with HIV-1. However, even specific markers for latent viral reservoirs have not yet been discovered. Furthermore, it is expected that another type of resistance could develop soon because InSTIs - currently the most popular type of drugs - were developed on the basis of the chemical structures of existing drugs. Therefore, we should predict new drug-resistance problems with antiretroviral drugs in the future, and therefore must find new therapeutic targets to resolve them. In addition, much greater efforts should be poured into the development of a complete cure technology capable of eliminating the HIV-1 hiding in the host cell from the patient's body, in order to win the final battle between mankind and HIV-1.
ACKNOWLEDGEMENTS
This work was supported by grants from the Korea National Institute of Health (Grant Number: 2019-NI-066 and 2020-NI-020).
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: CHY.
- Data curation: CHY, YHS, CMP.
- Formal analysis: CMP.
- Funding acquisition: CHY.
- Project administration: CHY.
- Supervision: CHY.
- Visualization: CMP.
- Writing - original draft: CHY, YHS, CMP.
- Writing - review & editing: CHY.
References
- 1.Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Gallo RC, Montagnier L. The discovery of HIV as the cause of AIDS. N Engl J Med. 2003;349:2283–2285. doi: 10.1056/NEJMp038194. [DOI] [PubMed] [Google Scholar]
- 3.Gallo RC. HIV-1: a look back from 20 years. DNA Cell Biol. 2004;23:191–192. doi: 10.1089/104454904773819770. [DOI] [PubMed] [Google Scholar]
- 4.Weiss RA. How does HIV cause AIDS? Science. 1993;260:1273–1279. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 5.Yarchoan R, Broder S. Development of antiretroviral therapy for the acquired immunodeficiency syndrome and related disorders. A progress report. N Engl J Med. 1987;316:557–564. doi: 10.1056/NEJM198702263160925. [DOI] [PubMed] [Google Scholar]
- 6.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. 2010;85:1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2:a006866. doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laskey SB, Siliciano RF. A mechanistic theory to explain the efficacy of antiretroviral therapy. Nat Rev Microbiol. 2014;12:772–780. doi: 10.1038/nrmicro3351. [DOI] [PubMed] [Google Scholar]
- 9.Cihlar T, Fordyce M. Current status and prospects of HIV treatment. Curr Opin Virol. 2016;18:50–56. doi: 10.1016/j.coviro.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2:a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, Bolognesi D, Barry DW, Broder S. 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985;82:7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namasivayam V, Vanangamudi M, Kramer VG, Kurup S, Zhan P, Liu X, Kongsted J, Byrareddy SN. The journey of HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) from lab to clinic. J Med Chem. 2019;62:4851–4883. doi: 10.1021/acs.jmedchem.8b00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Clercq E. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): past, present, and future. Chem Biodivers. 2004;1:44–64. doi: 10.1002/cbdv.200490012. [DOI] [PubMed] [Google Scholar]
- 14.De Clercq E, Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wassner C, Bradley N, Lee Y. A Review and clinical understanding of tenofovir: tenofovir disoproxil fumarate versus tenofovir alafenamide. J Int Assoc Provid AIDS Care. 2020;19:2325958220919231. doi: 10.1177/2325958220919231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, De Clercq E, Li G. Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment. Expert Opin Drug Metab Toxicol. 2019;15:813–829. doi: 10.1080/17425255.2019.1673367. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh AK, Osswald HL, Prato G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J Med Chem. 2016;59:5172–5208. doi: 10.1021/acs.jmedchem.5b01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boden D, Markowitz M. Resistance to human immunodeficiency virus type 1 protease inhibitors. Antimicrob Agents Chemother. 1998;42:2775–2783. doi: 10.1128/aac.42.11.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis DA, Soule EE, Davidoff KS, Daniels SI, Naiman NE, Yarchoan R. Activity of human immunodeficiency virus type 1 protease inhibitors against the initial autocleavage in Gag-Pol polyprotein processing. Antimicrob Agents Chemother. 2012;56:3620–3628. doi: 10.1128/AAC.00055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patick AK, Potts KE. Protease inhibitors as antiviral agents. Clin Microbiol Rev. 1998;11:614–627. doi: 10.1128/cmr.11.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali A, Bandaranayake RM, Cai Y, King NM, Kolli M, Mittal S, Murzycki JF, Nalam MN, Nalivaika EA, Ozen A, Prabu-Jeyabalan MM, Thayer K, Schiffer CA. Molecular basis for drug resistance in HIV-1 protease. Viruses. 2010;2:2509–2535. doi: 10.3390/v2112509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Cunha J, Maselli LM, Stern AC, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: Old and new drugs. World J Virol. 2015;4:56–77. doi: 10.5501/wjv.v4.i2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang M, Wang W, De Feo CJ, Vassell R, Weiss CD. Trimeric, coiled-coil extension on peptide fusion inhibitor of HIV-1 influences selection of resistance pathways. J Biol Chem. 2012;287:8297–8309. doi: 10.1074/jbc.M111.324483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, De Feo CJ, Zhuang M, Vassell R, Weiss CD. Selection with a peptide fusion inhibitor corresponding to the first heptad repeat of HIV-1 gp41 identifies two genetic pathways conferring cross-resistance to peptide fusion inhibitors corresponding to the first and second heptad repeats (HR1 and HR2) of gp41. J Virol. 2011;85:12929–12938. doi: 10.1128/JVI.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalezari JP, Henry K, O'Hearn M, Montaner JS, Piliero PJ, Trottier B, Walmsley S, Cohen C, Kuritzkes DR, Eron JJ, Jr, Chung J, DeMasi R, Donatacci L, Drobnes C, Delehanty J, Salgo M TORO 1 Study Group. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 26.Kitchen CM, Nuño M, Kitchen SG, Krogstad P. Enfuvirtide antiretroviral therapy in HIV-1 infection. Ther Clin Risk Manag. 2008;4:433–439. doi: 10.2147/tcrm.s1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huet T, Kerbarh O, Schols D, Clayette P, Gauchet C, Dubreucq G, Vincent L, Bompais H, Mazinghien R, Querolle O, Salvador A, Lemoine J, Lucidi B, Balzarini J, Petitou M. Long-lasting enfuvirtide carrier pentasaccharide conjugates with potent anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2010;54:134–142. doi: 10.1128/AAC.00827-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woollard SM, Kanmogne GD. Maraviroc: a review of its use in HIV infection and beyond. Drug Des Devel Ther. 2015;9:5447–5468. doi: 10.2147/DDDT.S90580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Der Ryst E. Maraviroc - A CCR5 antagonist for the treatment of HIV-1 infection. Front Immunol. 2015;6:277. doi: 10.3389/fimmu.2015.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian K, Morris-Natschke SL, Lee KH. HIV entry inhibitors and their potential in HIV therapy. Med Res Rev. 2009;29:369–393. doi: 10.1002/med.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi S, Nguyen PK, Cabral HJ, Diez-Barroso R, Derry PJ, Kanahara SM, Kumar VA. Development of peptide inhibitors of HIV transmission. Bioact Mater. 2016;1:109–121. doi: 10.1016/j.bioactmat.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondru R, Zhang J, Ji C, Mirzadegan T, Rotstein D, Sankuratri S, Dioszegi M. Molecular interactions of CCR5 with major classes of small-molecule anti-HIV CCR5 antagonists. Mol Pharmacol. 2008;73:789–800. doi: 10.1124/mol.107.042101. [DOI] [PubMed] [Google Scholar]
- 33.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craigie R, Bushman FD. HIV DNA integration. Cold Spring Harb Perspect Med. 2012;2:a006890. doi: 10.1101/cshperspect.a006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hindmarsh P, Leis J. Retroviral DNA integration. Microbiol Mol Biol Rev. 1999;63:836–843. doi: 10.1128/mmbr.63.4.836-843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelman A, Cherepanov P. Retroviral integrase structure and DNA recombination mechanism. Microbiol Spectr. 2014;2:1–22. doi: 10.1128/microbiolspec.MDNA3-0024-2014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee GE, Mauro E, Parissi V, Shin CG, Lesbats P. Structural insights on retroviral DNA integration: learning from foamy viruses. Viruses. 2019;11:770. doi: 10.3390/v11090770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mesplède T, Wainberg MA. Integrase strand transfer inhibitors in HIV therapy. Infect Dis Ther. 2013;2:83–93. doi: 10.1007/s40121-013-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong E, Trustman N, Yalong A. HIV pharmacotherapy: A review of integrase inhibitors. JAAPA. 2016;29:36–40. doi: 10.1097/01.JAA.0000475465.07971.19. [DOI] [PubMed] [Google Scholar]
- 40.Blanco JL, Whitlock G, Milinkovic A, Moyle G. HIV integrase inhibitors: a new era in the treatment of HIV. Expert Opin Pharmacother. 2015;16:1313–1324. doi: 10.1517/14656566.2015.1044436. [DOI] [PubMed] [Google Scholar]
- 41.Hare S, Vos AM, Clayton RF, Thuring JW, Cummings MD, Cherepanov P. Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Proc Natl Acad Sci U S A. 2010;107:20057–20062. doi: 10.1073/pnas.1010246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engelman AN. Multifaceted HIV integrase functionalities and therapeutic strategies for their inhibition. J Biol Chem. 2019;294:15137–15157. doi: 10.1074/jbc.REV119.006901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi E, Mallareddy JR, Lu D, Kolluru S. Recent advances in the discovery of small-molecule inhibitors of HIV-1 integrase. Future Sci OA. 2018;4:FSO338. doi: 10.4155/fsoa-2018-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deeks ED. Bictegravir/emtricitabine/tenofovir alafenamide: A review in HIV-1 infection. Drugs. 2018;78:1817–1828. doi: 10.1007/s40265-018-1010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, Serenata C, Akpomiemie G, Qavi A, Chandiwana N, Norris S, Chersich M, Clayden P, Abrams E, Arulappan N, Vos A, McCann K, Simmons B, Hill A. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381:803–815. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 46.Radford M, Parks DC, Ferrante S, Punekar Y. Comparative efficacy and safety and dolutegravir and lamivudine in treatment naive HIV patients. AIDS. 2019;33:1739–1749. doi: 10.1097/QAD.0000000000002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira M, Ibanescu RI, Anstett K, Mésplède T, Routy JP, Robbins MA, Brenner BG Montreal Primary HIV (PHI) Cohort Study Group. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology. 2018;15:56. doi: 10.1186/s12977-018-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sax PE, Pozniak A, Montes ML, Koenig E, DeJesus E, Stellbrink HJ, Antinori A, Workowski K, Slim J, Reynes J, Garner W, Custodio J, White K, SenGupta D, Cheng A, Quirk E. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390:2073–2082. doi: 10.1016/S0140-6736(17)32340-1. [DOI] [PubMed] [Google Scholar]
- 49.Hill L, Smith SR, Karris MY. Profile of bictegravir/emtricitabine/tenofovir alafenamide fixed dose combination and its potential in the treatment of HIV-1 infection: evidence to date. HIV AIDS (Auckl) 2018;10:203–213. doi: 10.2147/HIV.S145529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu L, Liu H, Murray BP, Callebaut C, Lee MS, Hong A, Strickley RG, Tsai LK, Stray KM, Wang Y, Rhodes GR, Desai MC. Cobicistat (GS-9350): A potent and selective inhibitor of human CYP3A as a novel pharmacoenhancer. ACS Med Chem Lett. 2010;1:209–213. doi: 10.1021/ml1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah BM, Schafer JJ, Priano J, Squires KE. Cobicistat: a new boost for the treatment of human immunodeficiency virus infection. Pharmacotherapy. 2013;33:1107–1116. doi: 10.1002/phar.1237. [DOI] [PubMed] [Google Scholar]
- 52.Crutchley RD, Guduru RC, Cheng AM. Evaluating the role of atazanavir/cobicistat and darunavir/cobicistat fixed-dose combinations for the treatment of HIV-1 infection. HIV AIDS (Auckl) 2016;8:47–65. doi: 10.2147/HIV.S99063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Temesgen Z. Cobicistat, a pharmacoenhancer for HIV treatments. Drugs Today (Barc) 2013;49:233–237. doi: 10.1358/dot.2013.49.4.1947288. [DOI] [PubMed] [Google Scholar]
- 54.von Hentig N. Clinical use of cobicistat as a pharmacoenhancer of human immunodeficiency virus therapy. HIV AIDS (Auckl) 2015;8:1–16. doi: 10.2147/HIV.S70836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lepist EI, Phan TK, Roy A, Tong L, Maclennan K, Murray B, Ray AS. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro . Antimicrob Agents Chemother. 2012;56:5409–5413. doi: 10.1128/AAC.01089-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deeks ED. Darunavir/cobicistat/emtricitabine/tenofovir Alafenamide: A review in HIV-1 infection. Drugs. 2018;78:1013–1024. doi: 10.1007/s40265-018-0934-2. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez C, van Halsema CL. Evaluating cabotegravir/rilpivirine long-acting, injectable in the treatment of HIV infection: emerging data and therapeutic potential. HIV AIDS (Auckl) 2019;11:179–192. doi: 10.2147/HIV.S184642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trezza C, Ford SL, Spreen W, Pan R, Piscitelli S. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS. 2015;10:239–245. doi: 10.1097/COH.0000000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitfield T, Torkington A, van Halsema C. Profile of cabotegravir and its potential in the treatment and prevention of HIV-1 infection: evidence to date. HIV AIDS (Auckl) 2016;8:157–164. doi: 10.2147/HIV.S97920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Usach I, Melis V, Peris JE. Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability. J Int AIDS Soc. 2013;16:1–14. doi: 10.7448/IAS.16.1.18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, Latiff G, Pokrovsky V, Bredeek F, Smith G, Cahn P, Kim YS, Ford SL, Talarico CL, Patel P, Chounta V, Crauwels H, Parys W, Vanveggel S, Mrus J, Huang J, Harrington CM, Hudson KJ, Margolis DA, Smith KY, Williams PE, Spreen WR. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382:1112–1123. doi: 10.1056/NEJMoa1904398. [DOI] [PubMed] [Google Scholar]
- 62.Landovitz RJ, Kofron R, McCauley M. The promise and pitfalls of long-acting injectable agents for HIV prevention. Curr Opin HIV AIDS. 2016;11:122–128. doi: 10.1097/COH.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh K, Sarafianos SG, Sönnerborg A. Long-acting anti-HIV drugs targeting HIV-1 reverse transcriptase and integrase. Pharmaceuticals (Basel) 2019;12:62. doi: 10.3390/ph12020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, Eron JJ, Yazdanpanah Y, Podzamczer D, Lutz T, Angel JB, Richmond GJ, Clotet B, Gutierrez F, Sloan L, Clair MS, Murray M, Ford SL, Mrus J, Patel P, Crauwels H, Griffith SK, Sutton KC, Dorey D, Smith KY, Williams PE, Spreen WR. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390:1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 65.Pham HT, Xiao MA, Principe MA, Wong A, Mesplède T. Pharmaceutical, clinical, and resistance information on doravirine, a novel non-nucleoside reverse transcriptase inhibitor for the treatment of HIV-1 infection. Drugs Context. 2020;9:2019-11-4. doi: 10.7573/dic.2019-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gatell JM, Morales-Ramirez JO, Hagins DP, Thompson M, Keikawus A, Hoffmann C, Rugina S, Osiyemi O, Escoriu S, Dretler R, Harvey C, Xu X, Teppler H. Forty-eight-week efficacy and safety and early CNS tolerability of doravirine (MK-1439), a novel NNRTI, with TDF/FTC in ART-naive HIV-positive patients. J Int AIDS Soc. 2014;17(4 Suppl 3):19532. doi: 10.7448/IAS.17.4.19532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molina JM, Squires K, Sax PE, Cahn P, Lombaard J, DeJesus E, Lai MT, Xu X, Rodgers A, Lupinacci L, Kumar S, Sklar P, Nguyen BY, Hanna GJ, Hwang C DRIVE-FORWARD Study Group. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e211–20. doi: 10.1016/S2352-3018(18)30021-3. [DOI] [PubMed] [Google Scholar]
- 68.Colombier MA, Molina JM. Doravirine: a review. Curr Opin HIV AIDS. 2018;13:308–314. doi: 10.1097/COH.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 69.Wilby KJ, Eissa NA. Clinical pharmacokinetics and drug interactions of doravirine. Eur J Drug Metab Pharmacokinet. 2018;43:637–644. doi: 10.1007/s13318-018-0497-3. [DOI] [PubMed] [Google Scholar]
- 70.Cahn P, Fink V, Patterson P. Fostemsavir: a new CD4 attachment inhibitor. Curr Opin HIV AIDS. 2018;13:341–345. doi: 10.1097/COH.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 71.Kozal M, Aberg J, Pialoux G, Cahn P, Thompson M, Molina JM, Grinsztejn B, Diaz R, Castagna A, Kumar P, Latiff G, DeJesus E, Gummel M, Gartland M, Pierce A, Ackerman P, Llamoso C, Lataillade M BRIGHTE Trial Team. Fostemsavir in adults with multidrug-resistant HIV-1 infection. N Engl J Med. 2020;382:1232–1243. doi: 10.1056/NEJMoa1902493. [DOI] [PubMed] [Google Scholar]
- 72.Chen K, Risatti C, Bultman M, Soumeillant M, Simpson J, Zheng B, Fanfair D, Mahoney M, Mudryk B, Fox RJ, Hsaio Y, Murugesan S, Conlon DA, Buono FG, Eastgate MD. Synthesis of the 6-azaindole containing HIV-1 attachment inhibitor pro-drug, BMS-663068. J Org Chem. 2014;79:8757–8767. doi: 10.1021/jo5016008. [DOI] [PubMed] [Google Scholar]
- 73.Lalezari J, Latiff GH, Brinson C, Echevarria J, Treviño-Pérez S, Bogner JR, Stock D, Joshi SR, Hanna GJ, Lataillade M. Safety profile of HIV-1 attachment inhibitor prodrug BMS-663068 in antiretroviral-experienced subjects: week 24 analysis. J Int AIDS Soc. 2014;17(4 Suppl 3):19530. doi: 10.7448/IAS.17.4.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iacob SA, Iacob DG. Ibalizumab targeting CD4 receptors, an emerging molecule in HIV therapy. Front Microbiol. 2017;8:2323. doi: 10.3389/fmicb.2017.02323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beccari MV, Mogle BT, Sidman EF, Mastro KA, Asiago-Reddy E, Kufel WD. Ibalizumab, a novel monoclonal antibody for the management of multidrug-resistant HIV-1 infection. Antimicrob Agents Chemother. 2019;63:e00110–e00119. doi: 10.1128/AAC.00110-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blair HA. Ibalizumab: A review in multidrug-resistant HIV-1 infection. Drugs. 2020;80:189–196. doi: 10.1007/s40265-020-01258-3. [DOI] [PubMed] [Google Scholar]
- 77.Emu B, Fessel J, Schrader S, Kumar P, Richmond G, Win S, Weinheimer S, Marsolais C, Lewis S. Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. N Engl J Med. 2018;379:645–654. doi: 10.1056/NEJMoa1711460. [DOI] [PubMed] [Google Scholar]
- 78.Rizza SA, Bhatia R, Zeuli J, Temesgen Z. Ibalizumab for the treatment of multidrug-resistant HIV-1 infection. Drugs Today (Barc) 2019;55:25–34. doi: 10.1358/dot.2019.55.1.2895651. [DOI] [PubMed] [Google Scholar]
- 79.Michailidis E, Huber AD, Ryan EM, Ong YT, Leslie MD, Matzek KB, Singh K, Marchand B, Hagedorn AN, Kirby KA, Rohan LC, Kodama EN, Mitsuya H, Parniak MA, Sarafianos SG. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms. J Biol Chem. 2014;289:24533–24548. doi: 10.1074/jbc.M114.562694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collins S. HIV i-Base. HIV pipeline 2020: new drugs in development. [Aceessed 12 March 2020]. Available at: https://i-base.info/htb/37221.
- 81.Link JO, Rhee MS, Tse WC, Zheng J, Somoza JR, Rowe W, Begley R, Chiu A, Mulato A, Hansen D, Singer E, Tsai LK, Bam RA, Chou CH, Canales E, Brizgys G, Zhang JR, Li J, Graupe M, Morganelli P, Liu Q, Wu Q, Halcomb RL, Saito RD, Schroeder SD, Lazerwith SE, Bondy S, Jin D, Hung M, Novikov N, Liu X, Villaseñor AG, Cannizzaro CE, Hu EY, Anderson RL, Appleby TC, Lu B, Mwangi J, Liclican A, Niedziela-Majka A, Papalia GA, Wong MH, Leavitt SA, Xu Y, Koditek D, Stepan GJ, Yu H, Pagratis N, Clancy S, Ahmadyar S, Cai TZ, Sellers S, Wolckenhauer SA, Ling J, Callebaut C, Margot N, Ram RR, Liu YP, Hyland R, Sinclair GI, Ruane PJ, Crofoot GE, McDonald CK, Brainard DM, Lad L, Swaminathan S, Sundquist WI, Sakowicz R, Chester AE, Lee WE, Daar ES, Yant SR, Cihlar T. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature. 2020;584:614–618. doi: 10.1038/s41586-020-2443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bester SM, Wei G, Zhao H, Adu-Ampratwum D, Iqbal N, Courouble VV, Francis AC, Annamalai AS, Singh PK, Shkriabai N, Van Blerkom P, Morrison J, Poeschla EM, Engelman AN, Melikyan GB, Griffin PR, Fuchs JR, Asturias FJ, Kvaratskhelia M. Structural and mechanistic bases for a potent HIV-1 capsid inhibitor. Science. 2020;370:360–364. doi: 10.1126/science.abb4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ott M, Geyer M, Zhou Q. The control of HIV transcription: keeping RNA polymerase II on track. Cell Host Microbe. 2011;10:426–435. doi: 10.1016/j.chom.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mousseau G, Valente S. Strategies to block HIV transcription: Focus on small molecule tat inhibitors. Biology (Basel) 2012;1:668–697. doi: 10.3390/biology1030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin Y, Choi BS, Kim KC, Kang C, Kim K, Yoon CH. Development of a dual reporter screening assay for distinguishing the inhibition of HIV Tat-mediated transcription from off-target effects. J Virol Methods. 2017;249:1–9. doi: 10.1016/j.jviromet.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Shin Y, Kim HG, Park CM, Choi MS, Kim DE, Choi BS, Kim K, Yoon CH. Identification of novel compounds against Tat-mediated human immunodeficiency virus-1 transcription by high-throughput functional screening assay. Biochem Biophys Res Commun. 2020;523:368–374. doi: 10.1016/j.bbrc.2019.12.029. [DOI] [PubMed] [Google Scholar]
- 87.Huang H, Chopra R, Verdine GL, Harrison SC. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 88.Lansdon EB, Brendza KM, Hung M, Wang R, Mukund S, Jin D, Birkus G, Kutty N, Liu X. Crystal structures of HIV-1 reverse transcriptase with etravirine (TMC125) and rilpivirine (TMC278): implications for drug design. J Med Chem. 2010;53:4295–4299. doi: 10.1021/jm1002233. [DOI] [PubMed] [Google Scholar]
- 89.King NM, Prabu-Jeyabalan M, Bandaranayake RM, Nalam MN, Nalivaika EA, Özen A, Haliloğlu T, Yilmaz NK, Schiffer CA. Extreme entropy-enthalpy compensation in a drug-resistant variant of HIV-1 protease. ACS Chem Biol. 2012;7:1536–1546. doi: 10.1021/cb300191k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berkhout B, Eggink D, Sanders RW. Is there a future for antiviral fusion inhibitors? Curr Opin Virol. 2012;2:50–59. doi: 10.1016/j.coviro.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 91.Haqqani AA, Tilton JC. Entry inhibitors and their use in the treatment of HIV-1 infection. Antiviral Res. 2013;98:158–170. doi: 10.1016/j.antiviral.2013.03.017. [DOI] [PubMed] [Google Scholar]