Abstract
Aortic stiffening is a major independent risk factor for cardiovascular diseases, kidney dysfunction and cognitive impairment. Doxorubicin (DOXO) chemotherapy-treated cancer survivors have greater aortic stiffness relative to healthy controls, but the mechanisms by which DOXO induces arterial stiffening are unknown. We tested the hypothesis that DOXO increases aortic stiffness by increasing intrinsic mechanical wall stiffness due to pro-inflammatory signaling-induced adverse structural changes, including collagen deposition (fibrosis), elastin fragmentation and/or formation of advanced glycation end products (AGEs). In vivo aortic stiffness (assessed via aortic pulse-wave velocity [PWV]), aortic intrinsic wall stiffness (ex vivo assessment of elastic modulus [EM]) and potential underlying mechanisms were assessed 4 weeks after administration of DOXO (10 mg/kg) or vehicle (saline) in young adult male C57BL6/J mice. Aortic PWV increased by ~30% following DOXO (Pre: 341±18 vs. Post: 431±28 cm/s, mean±SEM, P=0.001) and aortic EM was ~100% higher following DOXO (5438±445 kPa) vs. vehicle (2659±433 kPa) (P=0.003). These effects of DOXO were associated with an ~3-fold greater formation of AGEs (P=0.01) and an ~50% reduction in elastin (P=0.01), whereas collagen deposition was unaffected. DOXO increased aortic pro-inflammatory cytokines (P=0.03) without a compensatory increase in the anti-inflammatory cytokine interleukin-10. Direct ex vivo exposure of aorta rings to DOXO mimicked the increase in aortic EM observed in vivo with DOXO, whereas tumor necrosis factorα (TNFα) inhibition prevented this response. DOXO induces aortic stiffening in vivo due in part to an increase in intrinsic wall stiffness associated with elastin degradation and AGES formation and mediated by TNFα-dependent vascular inflammation.
Keywords: anthracyclines, Adriamycin, aortic pulse-wave velocity, elastic modulus
Summary
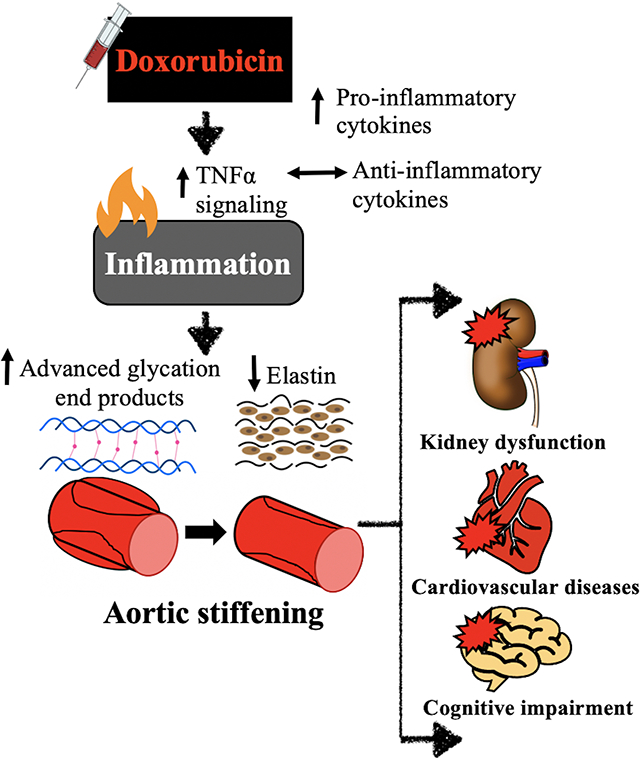
We show that doxorubicin-induced aortic stiffening is mediated by an increase in intrinsic mechanical wall stiffness associated with elastin degradation and accelerated formation of advanced glycation end products linked to tumor necrosis factor alpha-related vascular inflammation. Our results identify several potential therapeutic targets for reducing cardiovascular risk associated with aortic stiffening in cancer patients treated with anthracycline-based chemotherapy.
Graphical Abstract
INTRODUCTION
The National Cancer Institute estimates 2 million new cancer cases and 600,000 cancer-related deaths in the United States in 20211. Approximately 650,000 people undergo chemotherapy treatment annually2. In many cases, these treatments are effective at treating the cancer, but damage the cardiovascular system of the surviving patients3. As a result, cardiovascular diseases (CVD) are a leading cause of later morbidity and mortality among chemotherapy-treated cancer survivors4. A major cause of chemotherapy-associated increases in CVD risk in cancer survivors stems from use of anthracyclines5. Anthracyclines are a class of chemotherapeutics used to treat several common cancers, including leukemias and lymphomas, that elicit particularly toxic effects on the CV system6, 7. Doxorubicin (DOXO) is the most commonly administered anthracycline8, and its use markedly increases the risk of subsequent CVD9.
Much of the CVD-related mortality associated with DOXO is linked to clinical atherosclerotic diseases and stroke10–13, and the major antecedent of these CV disorders is arterial dysfunction14. A key feature of arterial dysfunction is, in turn, aortic stiffening15, 16, which is also a major risk factor for cognitive dysfunction, Alzheimer’s Disease, chronic kidney disease and many other chronic disorders17–19. Aortic stiffness is determined in part by the intrinsic stiffness of the arterial wall, which is influenced by the composition of the extracellular matrix, including the abundance of the main structural proteins -- collagen and elastin -- and the presence or absence of advanced glycation end products (AGEs), which form cross-links between structural proteins20–22. Cellular processes such as chronic low-grade inflammation can stimulate changes in these determinants of arterial stiffness23. DOXO-treated cancer survivors develop greater aortic stiffness compared with age-matched healthy controls, as indicated by higher carotid-femoral pulse wave velocity (PWV)5, 24–26. However, the mechanisms by which DOXO induces aortic stiffening have not been systematically investigated.
Here we use complementary experimental approaches to investigate the cellular and molecular mechanisms underlying DOXO treatment-evoked aortic stiffening. We first determined the temporal development of aortic stiffening following DOXO treatment in vivo by measuring aortic PWV in young adult mice. Using this model, we established that DOXO induces aortic stiffening within 4 weeks of administration compared with vehicle-treated controls. Next, we used an ex vivo stress-strain model to show that the increase in aortic PWV with DOXO is accompanied by a corresponding increase in intrinsic mechanical wall stiffness, and that direct incubation of aorta rings from untreated animals with DOXO induces a similar effect. We then show that these increases in aortic stiffness are associated with changes in the extracellular matrix of the aortic wall featuring elastin degradation and increases in the formation of AGEs. Finally, we demonstrate that the aortas of DOXO-treated mice show increased abundance of pro-inflammatory cytokines, most predominantly tumor necrosis factor α (TNFα), in the absence of compensatory induction of anti-inflammatory cytokines, and that inhibition of TNFα prevents DOXO-stimulated increases in intrinsic mechanical wall stiffness. Overall, our results extend current insight into the pathophysiological mechanisms by which DOXO administration induces aortic stiffening and identify potential therapeutic targets for preventing arterial stiffness in DOXO-treated cancer survivors.
METHODS
All data presented in this article and in the Online Supplement will be made available upon reasonable request to the corresponding author.
Animals
All animal protocols were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee (protocol #2618) and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 61 male C57BL/6 J mice were obtained for these studies from Jackson Laboratories (mice received at 3 months of age). Male mice were selected as male C57BL/6 mice are an established model or arterial stiffness in response to various stressors (e.g. aging27, 28 and Western-style diet29). All mice were housed in a conventional facility on a 12-hour light/dark cycle, given ad libitum access to rodent chow and drinking water, and allowed to acclimate to our facility for 4 weeks before the start of any testing. Mice that were assigned to receive in vivo injections of vehicle or DOXO were single housed for the duration of the study to prevent indirect exposure of DOXO, as DOXO and its primary metabolites are secreted in the urine and feces for up to ~96 hours following injection30. Food (Envigo 7917) and water intake were assessed every other day for the duration of the study and averaged. Energy intake was calculated my multiplying food intake (g) by kcal/g of the diet.
All mice were euthanized by exsanguination while maintained under anesthesia (inhaled isoflurane). The thoracic aorta was excised, dissected free of surrounding tissue, sectioned and stored appropriately for later stress-strain testing to assess aortic intrinsic mechanical stiffness, protein abundance by WES capillary electrophoresis-based immunoblotting (Protein Simple, San Jose, CA) and concentrations of pro-inflammatory cytokines. Investigators were blinded to treatment group for data collection and biochemical analyses. Details on all procedures, antibodies, and kits used are provided in the Online Supplement.
Aortic pulse wave velocity and blood pressure
Aortic PWV was assessed using Doppler ultrasound, as we have previously described27, 28, 31, 32. Briefly, mice were anesthetized via inhaled isoflurane (2.5–3%) and positioned supine on a warmed platform with paws secured to electrocardiogram leads. Doppler probes were placed at the transverse aortic arch and abdominal aorta to detect pulse waves. Three consecutive 2-second recordings were made for each animal and used to determine the time delay between the electrocardiogram R-wave and the foot of the Doppler signal for each site (Δtimeabdominal and Δtimetransverse). Aortic PWV was then calculated as: aortic PWV = (physical distance between the two probes)/(Δtimeabdominal minus Δtimetransverse) and reported in centimeters per second. In vivo blood pressure was measured in the second cohort of mice at baseline and 4 weeks following injection of DOXO or vehicle on three consecutive days using a non-invasive tail-cuff method (CODA; Kent Scientifc, Torrington, CT), as we have previously described27, 28, 31, 32.
DOXO administration
At 4 months of age, mice received a single injection of DOXO (10 mg/kg intraperitoneal injection; n = 14) or vehicle (intraperitoneal injection of saline; n = 10). Groups were matched for baseline body weight and aortic PWV. This method of administration causes cardiac dysfunction in 4-month-old male C57BL/6 J mice33. Estrogen may be protective against DOXO-induced cardiac dysfunction34; thus, only male mice were used in the present study to determine the mechanism by which DOXO causes aortic stiffening without the confounding effects of female sex hormones. All mice were sacrificed 4 weeks following treatment, with the exception of the pilot longitudinal cohort of animals.
Statistical analyses
Detailed descriptions of all statistical analyses performed are provided in the Data Supplement. Data are presented as mean ± SEM in text, figures, and tables unless specified otherwise. Statistical significance was set to α=0.05. All statistical analyses were performed using Prism, version 8 (GraphPad Software, Inc, La Jolla, CA).
RESULTS
Animals
Four-month old C57BL/6J mice were administered DOXO (single 10 mg/kg intraperitoneal injection; in sterile saline) or vehicle (body weight to volume-matched intraperitoneal injection of sterile saline). In C57BL/6J mice, this age is equivalent to ~25 years of age in humans35, which falls within the adolescent and young adult (AYA) age range for cancer (15–39 years of age) – a common age range for diagnosis of lymphoma and leukemia36, 37 and subsequent treatment with DOXO37.
After 4 weeks post injection, mice that received DOXO had lower body mass, energy intake, water intake, and quadricep skeletal muscle and epididymal white adipose mass as observed previously in rodent models38, 39 and similar to the effects of DOXO chemotherapy in humans40, 41. DOXO did not alter spleen weight, aortic diameter or blood pressure (Table S1).
Aortic stiffness
Patients who receive DOXO have higher aortic stiffness (carotid-femoral PWV) relative to age-matched untreated controls24–26, but the underlying mechanisms are unknown. First, we first conducted a pilot study to determine the temporal response of aortic stiffening after DOXO treatment assessed in vivo using serial non-invasive measurements of aortic pulse wave velocity [PWV] and found that aortic PWV peaks 4 weeks post-injection, and remains elevated, in young adult (5 mo) male C57BL/6 J mice [Figure S1]).
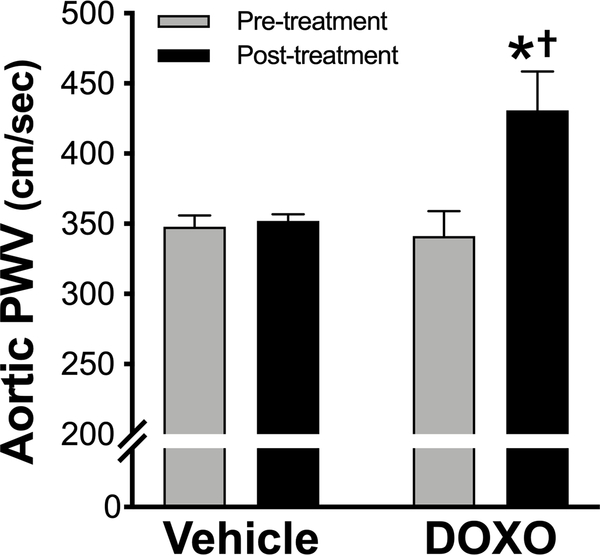
Guided by the results of this pilot study, in a second cohort of young adult (4 months of age) male C57BL/6 J mice, we next determined aortic PWV prior to and 4 weeks following a single injection of DOXO or vehicle and assessed several potential mechanisms of action. Prior to the injections (Pre) there were no differences in aortic PWV between the DOXO- and vehicle-treated groups. However, 4 weeks following the injections (Post), mice that received DOXO had a 30% increase (P = 0.004) in aortic PWV, while mice that received the vehicle showed no change (Figure 1). These data are consistent with observations in humans that DOXO chemotherapy increases carotid-femoral PWV by ~30% at 4 weeks following treatment5.
Figure 1. In vivo aortic pulse-wave velocity (PWV) is increased in young mice four weeks following a single injection of Doxorubicin.
n = 8/group. Data are expressed as mean ± SEM. *P < 0.05 vs. vehicle; †P < 0.05 vs. Pre-treatment within group.
Intrinsic stiffness of the aortic wall
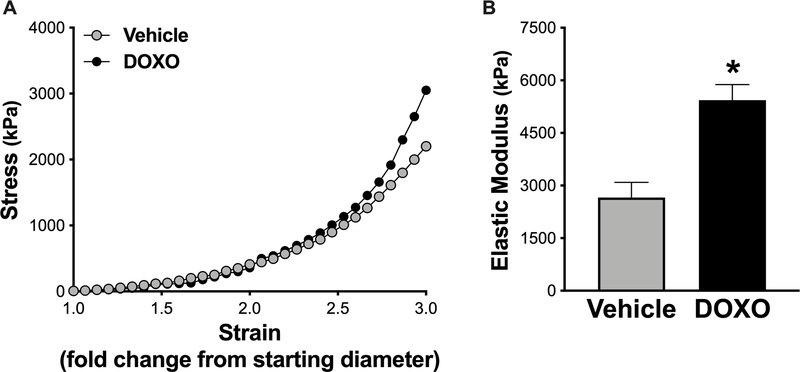
To investigate whether increased aortic PWV caused by DOXO is associated with increased intrinsic mechanical stiffness of aortic wall, we measured the elastic modulus of aorta rings isolated from mice treated with vehicle or DOXO. Elastic modulus is the strain on the arterial wall in response to a given stress (stretch), which is indicative of the intrinsic mechanical stiffness of the aorta27, 28, 31, 32. Figure 2 shows representative stress-strain curves and group average aortic elastic moduli from vehicle- and DOXO-treated mice. The aortic elastic modulus was ~100% greater (P = 0.003) in DOXO- vs vehicle-treated mice, indicating that DOXO increases the intrinsic stiffness of the aortic wall.
Figure 2. Ex vivo aortic elastic modulus (intrinsic mechanical stiffness) is higher in mice treated with Doxorubicin (DOXO).
A) Representative stress-strain curve of an aortic ring from vehicle- and DOXO-treated mice for determination of ex vivo elastic modulus. B) Aortic elastic modulus in vehicle and DOXO-treated mice. n = 8/group. Data are expressed as mean ± SEM. *P < 0.05 vs. vehicle.
Direct effect of DOXO on aortic stiffening
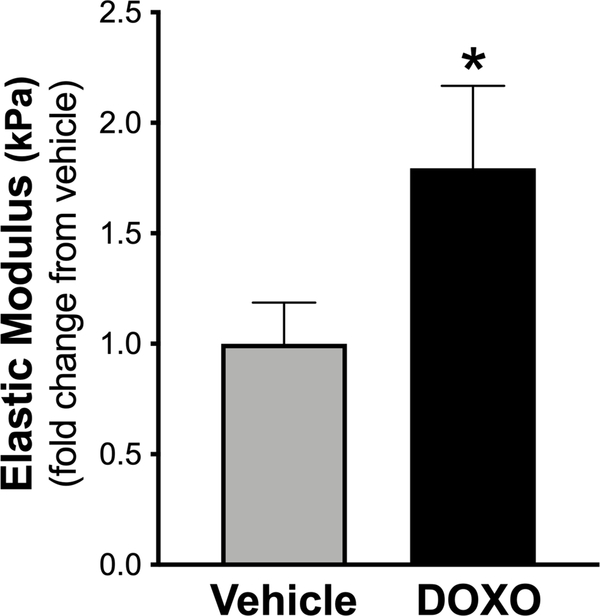
To determine whether DOXO directly affects aortic stiffening, i.e., independent of changes in the circulating plasma and endovascular milieu, thoracic aorta rings (1 mm in length) from young adult (4 months of age) untreated C57BL6/J male mice were incubated with = medium (DMEM + 10% fetal calf serum + 1% penicillin-streptomycin) containing vehicle or DOXO (1 μM) for 72 hours (2 rings for each condition per mouse), an experimental paradigm previously utilized by our laboratory to systemically interrogate mechanisms underlying changes in aortic stiffness in response to various treatments42, 43. 1 μM was previously shown to be the peak concentration of DOXO in plasma following administration in humans44. Intrinsic mechanical wall stiffness was assessed after the 72-hour incubation period. Similar to in vivo treatment with DOXO, ex vivo DOXO exposure increased the aortic elastic modulus by ~2-fold (P = 0.03 vs. vehicle) (Figure 3), suggesting that DOXO directly increases aortic stiffening independent of potential changes in circulating factors or other in vivo processes that might have occurred with in vivo treatment.
Figure 3. Direct exposure of aorta rings with Doxorubicin (DOXO) increases the elastic modulus (intrinsic mechanical stiffness).
Direct exposure of aorta rings with vehicle (DMEM + 10% fetal calf serum + 1% Penicillin-Streptomycin) or 1 μM DOXO (in vehicle). n = 10/condition. Data (mean ± SEM) in the DOXO condition is expressed as fold-change relative to vehicle. *P < 0.05 vs. vehicle.
Effects of DOXO on arterial wall structural proteins and advanced glycation end products
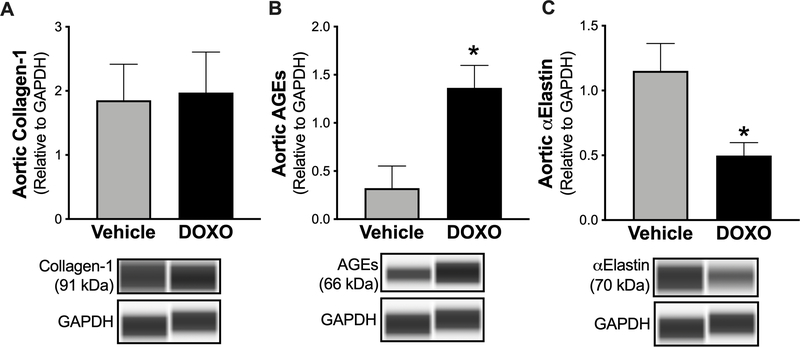
Aortic stiffening is increased by collagen deposition31 and/or cross-linking by advanced glycation end products (AGEs)43, and the latter can occur independent of changes in collagen abundance45. Elastin degradation (reduced elastin abundance) also contributes to aortic stiffening in various settings as this is the structural protein that confers elasticity to the arterial wall46. Therefore, we assessed the abundance of collagen, AGEs and elastin in aortic lysates from DOXO- and vehicle-treated mice by immunoblotting. Aortic collagen was unchanged after DOXO treatment (P = 0.89) (Figure 4A). In contrast, aortic AGEs were ~3-fold higher (P = 0.012) (Figure 4B) and aortic elastin was ~2.5-fold lower (P = 0.011) (Figure 4C) after DOXO treatment. Next, we aimed to determine whether the DOXO-mediated elastin degradation and increased formation of AGEs were associated with the corresponding DOXO-associated increases in aortic PWV and elastic modulus. Linear regression analyses revealed that DOXO-induced aortic elastin degradation was indeed related to the increases in aortic PWV (P = 0.007; Figure S2A) and aortic elastic modulus (P = 0.003, Figure S2C), as were the DOXO-associated increases in aortic AGEs (P = 0.002 vs. change in aortic PWV, Figure S2B; P = 0.0007 vs. change in elastic modulus, Figure S2D). These results suggest that DOXO induces aortic stiffening, at least in part, by degrading elastin and increasing AGEs-related cross-linking.
Figure 4. In vivo administration of Doxorubicin (DOXO) results in higher advanced glycation end products (AGEs), and lower elastin abundance in the aorta.
Aortic abundance of A) collagen-1, B) AGEs, and C) α-elastin in mice treated with vehicle or DOXO. n = 10/group. Data are expressed as mean ± SEM. *P < 0.05 vs. vehicle.
Effect of DOXO on aortic inflammatory cytokines
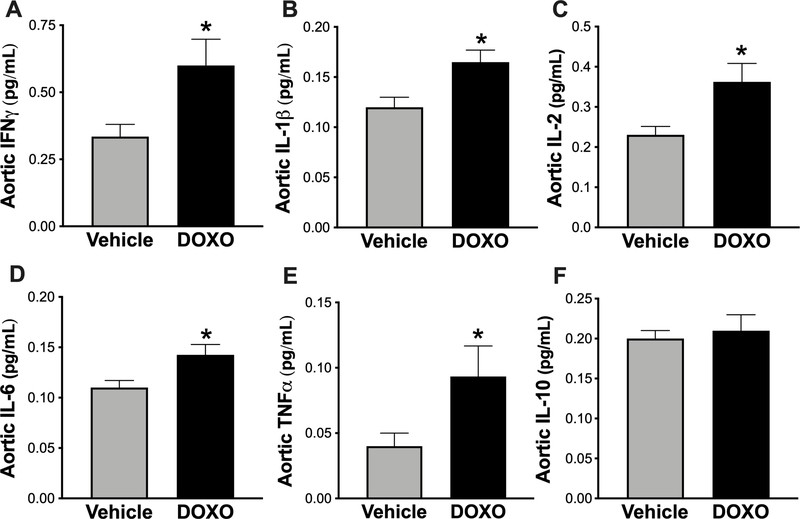
We have previously demonstrated an association between higher aortic inflammatory cytokine abundance and aortic stiffening28, 47, 48. Furthermore, DOXO stimulates inflammation systemically39 and in specific tissues, including CV tissues49. Therefore, we asked if higher levels of inflammatory cytokines are associated with DOXO-induced aortic stiffening. To address this, we assessed pro- and anti-inflammatory cytokines in aortic lysates from DOXO- and vehicle-treated mice by multiplex ELISA. Indeed, aortas from mice that received DOXO had higher protein concentrations of the pro-inflammatory mediators IFNγ (P = 0.04) (Figure 5A), IL-1β (P = 0.03) (Figure 5B), IL-2 (P = 0.03) (Figure 5C), IL-6 (P = 0.04) (Figure 5D), and TNFα (P = 0.04) (Figure 5E), without a compensatory upregulation in the anti-inflammatory cytokine, IL-10 (Figure 5F). Among the pro-inflammatory cytokines, TNFα appeared to be most strongly stimulated by DOXO treatment.
Figure 5. Greater abundance of pro-inflammatory, but not anti-inflammatory cytokines in the aorta of Doxorubicin (DOXO) treated mice.
Aortic abundance of A) interferon-gamma (IFNγ), B) interleukin-1β (IL-1β), C) interleukin-2 (IL2), D) interleukin-6 (IL-6), E) tumor necrosis factorα (TNFα), and F) interleukin-10 (IL10) in mice treated with vehicle or DOXO. n = 8/group. Data are expressed as mean ± SEM. *P < 0.05 vs. vehicle.
Direct effect of TNFα on DOXO-induced arterial stiffening
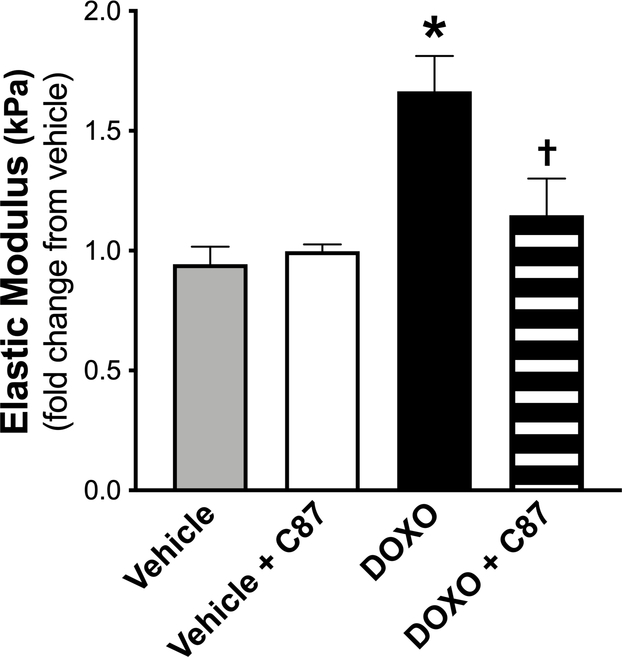
Our interrogation into DOXO-induced changes in aortic inflammation demonstrated that TNFα was most influenced by DOXO. Accordingly, we next asked whether TNFα is a major regulator of aortic stiffening by DOXO. We incubated aorta rings (1 mm in length) from a separate cohort of intervention-naïve young adult (5 mo old) C57BL/6 J male mice in the following conditions: 1) vehicle (in culture medium); 2) vehicle + the TNFα inhibitor C8750, 51 (20 μM); 3) DOXO (1 μM in culture medium); and 4) DOXO + C87. Notably, C87 directly binds to TNF and prevents its interaction with the TNF receptor52. After 72 hours, we assessed the intrinsic mechanical stiffness of the aortic wall as described above. Similar to our previous mouse cohort, DOXO increased (P = 0.004) the elastic modulus by ~2-fold and C87 completely prevented this increase (P = 0.002, DOXO vs. DOXO + C87; P = 0.41 vehicle vs. DOXO + C87). There were no differences (P = 0.41) between vehicle and vehicle + C87, suggesting there were no off-target effects of C87 (Figure 6).
Figure 6. Direct exposure of aorta rings with the tumor necrosis factorα (TNFα) inhibitor C87 prevents Doxorubicin (DOXO)-mediated aortic stiffening.
Direct exposure of aorta rings with vehicle (DMEM + 10% fetal calf serum + 1% Penicillin-Streptomycin); vehicle + C87 (20 μM); DOXO (1 μM DOXO); and DOXO + C87. n = 10/condition. Data are the mean ± SEM expressed as fold-change relative to vehicle. *P < 0.05 vs. vehicle; †P < 0.05 vs. DOXO.
DISCUSSION
Aortic stiffening is a key antecedent to the development of clinical CVD, including hypertension53, atherosclerotic occlusive diseases54 and stroke55, and is an independent predictor of CV-related mortality56. Aortic stiffening is also a major risk factor for chronic kidney disease19 and is strongly implicated in impaired cognitive function and risk of Alzheimer’s disease and other dementias17, 18. DOXO-treated cancer survivors have greater aortic stiffness relative to age-, sex- and CV risk factor-matched healthy individuals and demonstrate higher prevalence of the above-mentioned arterial stiffening-related chronic disorders with advancing age24–26. As such, it is clinically important that we understand the mechanisms of DOXO treatment-associated aortic stiffening in order to identify the processes involved that can be targeted therapeutically. However, to date, the mechanisms underlying DOXO-induced aortic stiffening have not been systemically investigated.
In the present study, we used complementary in vivo and ex vivo translational models of aortic stiffness to gain insight into these issues. Our major findings are that DOXO induces aortic stiffening in vivo at least in part by increasing intrinsic mechanical wall stiffness. The latter is, in turn, associated with a marked increase in AGEs formation and elastin degradation, as well as aortic inflammation in the absence of an obvious compensatory anti-inflammatory response. Finally, the increase in intrinsic wall stiffness with DOXO is prevented by inhibition of TNFα. Overall, these findings provide new evidence regarding the mechanisms by which DOXO increases aortic stiffness and identify several possible therapeutic targets for future treatment strategies.
Implications for management and treatment of aortic stiffening after DOXO chemotherapy
At this time, there are no guidelines in place to monitor aortic stiffness during or following DOXO chemotherapy treatment. However, a recent meta-analysis on the topic of DOXO chemotherapy and aortic stiffness concluded that that non-invasive approaches, including assessment of carotid-femoral PWV, should be used to monitor changes in vascular health during treatment and throughout survival to better understand the risk of CVD and other chronic disorders associated with DOXO25.
The present results address current research gaps by providing experimental evidence for potential targets of future therapies aimed at preventing/treating aortic stiffening with DOXO. Specifically, our results suggest that lifestyle and pharmacological strategies that inhibit AGEs formation, elastin degradation and TNFα signaling-dependent vascular inflammation are among the candidate targets for mitigating DOXO treatment-associated aortic stiffening. Of these therapeutic targets, TNFα inhibitors may be the most promising, as etanercept, adalimumab and infliximab all are presently in clinical use and have shown efficacy for reducing arterial stiffness in other groups, including patients with chronic inflammatory disorders and estrogen-deficient postmenopausal women free of clinical disease57–60. However, it should be noted that in a preclinical model of atherosclerosis, the TNFα inhibitor CNTO5048 was reported to paradoxically exacerbate vascular inflammation61.
Other lifestyle and pharmacological interventions aimed at reducing vascular inflammation, including voluntary aerobic exercise62, energy restriction63, 64, salicylate treatment65, 66, statin therapy67, and dietary supplementation with both mitochondrial antioxidants31, 68 and NAD+ boosting compounds69, 70 reduce arterial stiffness in healthy older adults and also may be effective for mitigating DOXO-mediated aortic stiffening, which may be viewed as a condition of accelerated arterial aging. Inhibition of AGEs formation and crosslinking are effective in reducing arterial stiffness in preclinical models71–73 but these compounds are not used clinically at this time. Taken together, there are currently a variety of lifestyle and pharmacological strategies that may be effective for reducing arterial stiffening in patients that have undergone DOXO chemotherapy treatment. Establishing the efficacy of these approaches represents an important future goal in cardiovascular-oncology research.
The mouse as a translational model of DOXO-induced aortic stiffening
In general, assessment of aortic stiffness using aortic PWV in C57BL/6 mice is a well-established translational model of studies of carotid-femoral PWV in humans27, 74. The results of the present study extend the use of this model to establish DOXO-treated young adult mice as a translational model of DOXO-induced aortic stiffening in clinical settings. Carotid-femoral PWV is reported to be ~30% higher several weeks following DOXO administration in cancer patients5, i.e., the same magnitude of increase we observed in aortic PWV in the present study. Thus, our findings also provide the first evidence supporting the validity of C57BL/6 mice as a translational model for studying the underlying mechanisms of DOXO-induced aortic stiffness clinically. Further refinement of this mouse model to facilitate more clinically relevant translation to cancer patients include the use of a tumor-bearing mice to model human cancer and the administration of DOXO intravenously in smaller consecutive doses over time, as is used medically25.
Perspectives
Here, we demonstrate for the first time that DOXO-induced aortic stiffening is mediated, at least in part, by enhanced TNFα-dependent pro-inflammatory signaling without an obvious compensatory anti-inflammatory response. These events trigger pathophysiological changes to the extracellular matrix of the aortic wall featuring a marked increase in the formation of AGEs and accelerated degradation of elastin in the absence of augmented collagen deposition. Collectively, these changes substantially increase the intrinsic mechanical stiffness of the wall of the aorta and result in a physiologically and clinically meaningful aortic stiffening in vivo, as indicated by an increase in aortic PWV similar to that observed in DOXO-treated cancer patients. Lifestyle strategies or targeted pharmacological therapies that suppress pro-inflammatory signaling in arteries may be effective strategies for preventing and/or treating DOXO chemotherapy-induced aortic stiffening, which could ultimately decrease the risk for CVD, kidney dysfunction and cognitive impairment in this patient population.
Future preclinical studies should consider other experimental approaches to facilitate translation to patient populations including: 1) studying female animals; 2) administering DOXO intravenously in smaller consecutive doses over time to more closely mimic clinical therapy; 3) assessing cardiac function in parallel with aortic stiffness; and 4) extending the age-range to older animals given the common use of DOXO in middle-aged and older patients.
Supplementary Material
Novelty and Significance.
What Is New?
Using complementary in vivo and ex vivo translational models, we systematically identified key mechanisms underlying doxorubicin-mediated aortic stiffening.
We demonstrate a clear role of increased intrinsic wall stiffness associated with remodeling of the extracellular matrix and tumor necrosis factor alpha-related increases in vascular inflammation as key mechanisms driving aortic stiffening with doxorubicin.
What Is Relevant?
Aortic stiffening is a major antecedent and key initiating step in the development of cardiovascular diseases, including hypertension.
Doxorubicin chemotherapy treatment causes aortic stiffening, but the underlying mechanisms have not been systematically investigated.
This study provides evidence for increased tumor necrosis factor alpha signaling as a potentially important upstream mechanism in doxorubicin-induced aortic stiffening that could be targeted to improve vascular health and reduce cardiovascular disease risk in anthracycline-treated cancer survivors.
Acknowledgements:
Authors thank Marissa Burnsed-Torres, Jill Miyamato-Ditmon, Nicholas VanDongen and Nathan Greenberg for assistance with data collection, and Dr. Brad Fleenor for consultations regarding the assessment of aortic elastic modulus.
Sources of Funding: R01 AG055822 (D.R.S.; J.C.; and S.M.); T32 DK007135 (Z.S.C.); and F32 HL151022 (Z.S.C.).
Footnotes
Disclosures: Authors have no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. January 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Halpern MT, Yabroff KR. Prevalence of outpatient cancer treatment in the United States: estimates from the Medical Panel Expenditures Survey (MEPS). Cancer Invest. July 2008;26(6):647–51. doi: 10.1080/07357900801905519 [DOI] [PubMed] [Google Scholar]
- 3.Truong J, Yan AT, Cramarossa G, Chan KK. Chemotherapy-induced cardiotoxicity: detection, prevention, and management. Can J Cardiol. August 2014;30(8):869–78. doi: 10.1016/j.cjca.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 4.Meinardi MT, Gietema JA, van Veldhuisen DJ, van der Graaf WT, de Vries EG, Sleijfer DT. Long-term chemotherapy-related cardiovascular morbidity. Cancer Treat Rev. December 2000;26(6):429–47. doi: 10.1053/ctrv.2000.0175 [DOI] [PubMed] [Google Scholar]
- 5.Drafts BC, Twomley KM, D’Agostino R, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. August 2013;6(8):877–85. doi: 10.1016/j.jcmg.2012.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipshultz SE, Karnik R, Sambatakos P, Franco VI, Ross SW, Miller TL. Anthracycline-related cardiotoxicity in childhood cancer survivors. Curr Opin Cardiol. January 2014;29(1):103–12. doi: 10.1097/HCO.0000000000000034 [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Franco VI, Miller TL, Colan SD, Sallan SE. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med. 2015;66:161–76. doi: 10.1146/annurev-med-070213-054849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaklamani VG, Gradishar WJ. Epirubicin versus doxorubicin: which is the anthracycline of choice for the treatment of breast cancer? Clin Breast Cancer. April 2003;4 Suppl 1:S26–33. [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. November 1979;91(5):710–7. doi: 10.7326/0003-4819-91-5-710 [DOI] [PubMed] [Google Scholar]
- 10.Min SS, Wierzbicki AS. Radiotherapy, chemotherapy and atherosclerosis. Curr Opin Cardiol. July 2017;32(4):441–447. doi: 10.1097/HCO.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 11.Mukai M, Komori K, Oka T. Mechanism and Management of Cancer Chemotherapy-Induced Atherosclerosis. J Atheroscler Thromb. October 2018;25(10):994–1002. doi: 10.5551/jat.RV17027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bruin ML, Dorresteijn LD, van’t Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. July 2009;101(13):928–37. doi: 10.1093/jnci/djp147 [DOI] [PubMed] [Google Scholar]
- 13.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. October 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185 [DOI] [PubMed] [Google Scholar]
- 14.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. January 2003;107(1):139–46. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. February 2010;121(4):505–11. doi: 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adji A, O’Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens. January 2011;24(1):5–17. doi: 10.1038/ajh.2010.192 [DOI] [PubMed] [Google Scholar]
- 17.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. January 2008;51(1):99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674 [DOI] [PubMed] [Google Scholar]
- 18.Pase MP, Beiser A, Himali JJ, et al. Aortic Stiffness and the Risk of Incident Mild Cognitive Impairment and Dementia. Stroke. 09 2016;47(9):2256–61. doi: 10.1161/STROKEAHA.116.013508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. March 2005;45(3):494–501. doi: 10.1053/j.ajkd.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 20.Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis. April 2013;4(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- 21.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. May 2005;25(5):932–43. doi: 10.1161/01.ATV.0000160548.78317.29 [DOI] [PubMed] [Google Scholar]
- 22.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation - a mini-review. Gerontology. 2012;58(3):227–37. doi: 10.1159/000334668 [DOI] [PubMed] [Google Scholar]
- 23.Aging Sun Z., arterial stiffness, and hypertension. Hypertension. February 2015;65(2):252–6. doi: 10.1161/HYPERTENSIONAHA.114.03617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaosuwannakit N, D’Agostino R, Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. January 2010;28(1):166–72. doi: 10.1200/JCO.2009.23.8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parr SK, Liang J, Schadler KL, Gilchrist SC, Steele CC, Ade CJ. Anticancer Therapy-Related Increases in Arterial Stiffness: A Systematic Review and Meta-Analysis. J Am Heart Assoc. July 2020:e015598. doi: 10.1161/JAHA.119.015598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yersal Ö, Eryilmaz U, Akdam H, Meydan N, Barutca S. Arterial Stiffness in Breast Cancer Patients Treated with Anthracycline and Trastuzumab-Based Regimens. Cardiol Res Pract. 2018;2018:5352914. doi: 10.1155/2018/5352914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gioscia-Ryan RA, Clayton ZS, Fleenor BS, et al. Late-life voluntary wheel running reverses age-related aortic stiffness in mice: a translational model for studying mechanisms of exercise-mediated arterial de-stiffening. Geroscience. June 2020;doi: 10.1007/s11357-020-00212-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gioscia-Ryan RA, Clayton ZS, Zigler MC, et al. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress, and inflammation in mice. J Physiol. 599.3. 2021;911–915. doi: 10.1113/JP280607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foote CA, Castorena-Gonzalez JA, Ramirez-Perez FI, et al. Arterial Stiffening in Western Diet-Fed Mice Is Associated with Increased Vascular Elastin, Transforming Growth Factor-β, and Plasma Neuraminidase. Front Physiol. 2016;7:285. doi: 10.3389/fphys.2016.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Asperen J, van Tellingen O, Tijssen F, Schinkel AH, Beijnen JH. Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdr1a P-glycoprotein. Br J Cancer. January 1999;79(1):108–13. doi: 10.1038/sj.bjc.6690019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985). May 2018;124(5):1194–1202. doi: 10.1152/japplphysiol.00670.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunt VE, Gioscia-Ryan RA, Richey JJ, et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol. February 2019;doi: 10.1113/JP277336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demaria M, O’Leary MN, Chang J, et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 02 2017;7(2):165–176. doi: 10.1158/2159-8290.CD-16-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cadeddu Dessalvi C, Pepe A, Penna C, et al. Sex differences in anthracycline-induced cardiotoxicity: the benefits of estrogens. Heart Fail Rev. 11 2019;24(6):915–925. doi: 10.1007/s10741-019-09820-2 [DOI] [PubMed] [Google Scholar]
- 35.Hagan Catherine. When are mice considered old? June 22, 2020, 2017. [Google Scholar]
- 36.Crombie JL, LaCasce AS. Current considerations in AYA Hodgkin lymphoma. Br J Haematol. 01 2019;184(1):72–81. doi: 10.1111/bjh.15640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curran E, Stock W. How I treat acute lymphoblastic leukemia in older adolescents and young adults. Blood. June 2015;125(24):3702–10. doi: 10.1182/blood-2014-11-551481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang K, Liu Y, Tang H, et al. Glabridin Prevents Doxorubicin-Induced Cardiotoxicity Through Gut Microbiota Modulation and Colonic Macrophage Polarization in Mice. Front Pharmacol. 2019;10:107. doi: 10.3389/fphar.2019.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilliam LA, Ferreira LF, Bruton JD, et al. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol (1985). December 2009;107(6):1935–42. doi: 10.1152/japplphysiol.00776.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilliam LA, St Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal. November 2011;15(9):2543–63. doi: 10.1089/ars.2011.3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kutynec CL, McCargar L, Barr SI, Hislop TG. Energy balance in women with breast cancer during adjuvant treatment. J Am Diet Assoc. October 1999;99(10):1222–7. doi: 10.1016/s0002-8223(99)00301-6 [DOI] [PubMed] [Google Scholar]
- 42.LaRocca TJ, Hearon CM, Henson GD, Seals DR. Mitochondrial quality control and age-associated arterial stiffening. Exp Gerontol. October 2014;58:78–82. doi: 10.1016/j.exger.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol. August 2012;47(8):588–94. doi: 10.1016/j.exger.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenner DE, Galloway S, Cooper J, Noone R, Hande KR. Improved high-performance liquid chromatography assay of doxorubicin: detection of circulating aglycones in human plasma and comparison with thin-layer chromatography. Cancer Chemother Pharmacol. 1985;14(2):139–45. doi: 10.1007/BF00434353 [DOI] [PubMed] [Google Scholar]
- 45.Aronson D Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. January 2003;21(1):3–12. doi: 10.1097/00004872-200301000-00002 [DOI] [PubMed] [Google Scholar]
- 46.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res. June 2012;5(3):264–73. doi: 10.1007/s12265-012-9349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sindler AL, Fleenor BS, Calvert JW, et al. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. June 2011;10(3):429–37. doi: 10.1111/j.1474-9726.2011.00679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleenor BS, Sindler AL, Marvi NK, et al. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol. February 2013;48(2):269–76. doi: 10.1016/j.exger.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka R, Umemura M, Narikawa M, et al. Reactive fibrosis precedes doxorubicin-induced heart failure through sterile inflammation. ESC Heart Fail. 04 2020;7(2):588–603. doi: 10.1002/ehf2.12616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma L, Gong H, Zhu H, et al. A novel small-molecule tumor necrosis factor α inhibitor attenuates inflammation in a hepatitis mouse model. J Biol Chem. May 2014;289(18):12457–66. doi: 10.1074/jbc.M113.521708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He MM, Smith AS, Oslob JD, et al. Small-molecule inhibition of TNF-alpha. Science. November 2005;310(5750):1022–5. doi: 10.1126/science.1116304 [DOI] [PubMed] [Google Scholar]
- 52.Steeland S, Libert C, Vandenbroucke RE. A New Venue of TNF Targeting. Int J Mol Sci. May 2018;19(5)doi: 10.3390/ijms19051442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell GF. Arterial stiffness and hypertension. Hypertension. July 2014;64(1):13–8. doi: 10.1161/HYPERTENSIONAHA.114.00921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. February 2001;32(2):454–60. doi: 10.1161/01.str.32.2.454 [DOI] [PubMed] [Google Scholar]
- 55.Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. May 2003;34(5):1203–6. doi: 10.1161/01.STR.0000065428.03209.64 [DOI] [PubMed] [Google Scholar]
- 56.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. May 2001;37(5):1236–41. doi: 10.1161/01.hyp.37.5.1236 [DOI] [PubMed] [Google Scholar]
- 57.Végh E, Kerekes G, Pusztai A, et al. Effects of 1-year anti-TNF-α therapy on vascular function in rheumatoid arthritis and ankylosing spondylitis. Rheumatol Int. March 2020;40(3):427–436. doi: 10.1007/s00296-019-04497-0 [DOI] [PubMed] [Google Scholar]
- 58.Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension. February 2010;55(2):333–8. doi: 10.1161/HYPERTENSIONAHA.109.143982 [DOI] [PubMed] [Google Scholar]
- 59.Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens. June 2012;25(6):644–50. doi: 10.1038/ajh.2012.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis. October 2013;230(2):390–6. doi: 10.1016/j.atherosclerosis.2013.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oberoi R, Vlacil AK, Schuett J, et al. Anti-tumor necrosis factor-α therapy increases plaque burden in a mouse model of experimental atherosclerosis. Atherosclerosis. 10 2018;277:80–89. doi: 10.1016/j.atherosclerosis.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 62.Lesniewski LA, Durrant JR, Connell ML, et al. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol. September 2011;301(3):H1025–32. doi: 10.1152/ajpheart.01276.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donato AJ, Walker AE, Magerko KA, et al. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. October 2013;12(5):772–83. doi: 10.1111/acel.12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dengo AL, Dennis EA, Orr JS, et al. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension. April 2010;55(4):855–61. doi: 10.1161/HYPERTENSIONAHA.109.147850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci. April 2011;66(4):409–18. doi: 10.1093/gerona/glq233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jablonski KL, Donato AJ, Fleenor BS, et al. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor κ B signalling. J Hypertens. December 2015;33(12):2477–82. doi: 10.1097/HJH.0000000000000742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Upala S, Wirunsawanya K, Jaruvongvanich V, Sanguankeo A. Effects of statin therapy on arterial stiffness: A systematic review and meta-analysis of randomized controlled trial. Int J Cardiol. January 2017;227:338–341. doi: 10.1016/j.ijcard.2016.11.073 [DOI] [PubMed] [Google Scholar]
- 68.Rossman MJ, Santos-Parker JR, Steward CAC, et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension. 06 2018;71(6):1056–1063. doi: 10.1161/HYPERTENSIONAHA.117.10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Picciotto NE, Gano LB, Johnson LC, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 06 2016;15(3):522–30. doi: 10.1111/acel.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD. Nat Commun. March 2018;9(1):1286. doi: 10.1038/s41467-018-03421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steppan J, Tran H, Benjo AM, et al. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. August 2012;47(8):565–72. doi: 10.1016/j.exger.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolffenbuttel BH, Boulanger CM, Crijns FR, et al. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci U S A. April 1998;95(8):4630–4. doi: 10.1073/pnas.95.8.4630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaitkevicius PV, Lane M, Spurgeon H, et al. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc Natl Acad Sci U S A. January 2001;98(3):1171–5. doi: 10.1073/pnas.98.3.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butlin M, Tan I, Spronck B, Avolio AP. Measuring Arterial Stiffness in Animal Experimental Studies. Arterioscler Thromb Vasc Biol. 05 2020;40(5):1068–1077. doi: 10.1161/ATVBAHA.119.313861 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.