Abstract
The recent pandemic of COVID-19 has made abundantly clear that Type 2 diabetes (T2D) increases the risk of more frequent and more severe viral infections. At the same time, pro-inflammatory cytokines of an anti-viral Type-I profile promote insulin resistance and form a risk factor for development of T2D. What this illustrates is that there is a reciprocal, detrimental interaction between the immune and endocrine system in the context of T2D. Why these two systems would interact at all long remained unclear. Recent findings indicate that transient changes in systemic metabolism are induced by the immune system as a strategy against viral infection. In people with T2D, this system fails, thereby negatively impacting the antiviral immune response. In addition, immune-mediated changes in systemic metabolism upon infection may aggravate glycemic control in T2D. In this review, we will discuss recent literature that sheds more light on how T2D impairs immune responses to viral infection and how virus-induced activation of the immune system increases risk of development of T2D.
Keywords: Viral infection, COVID-19, Diabetes mellitus type 2, Insulin resistance, Immune system, Immune defects, Immunometabolism, Corona virus, Infection, Antidiabetic drugs, T2D, Diabetes
1. Introduction
In the clinics, immunological dysfunction is seldom recognized as a major comorbidity of Type 2 diabetes (T2D). Until recently, people in developed countries rarely encountered dangerous pathogens. As a result, increased susceptibility to infection was typically only experienced as a cause of minor inconveniences such as recurrent urinary tract infection [1]. However, as the recent outbreak of the COVID-19 pandemic has shown, people with diabetes (both type 1 and 2) have increased risk of severe complications upon infection with lethal pathogens such as SARS-CoV-2 [2], [3]. Moreover, even before the corona pandemic, reduced immune cell function on top of microvascular pathology was well known to exacerbate dangerous complications such as persistent foot ulcers that can lead to gangrene. This observation indicates that diabetes increases susceptibility to a broad range of pathogens and negatively impacts the duration, morbidity and mortality associated with infectious disease. Indeed, people with diabetes are more frequently infected with cytomegalovirus [4] and suffer more often from surgical site infections [1]. Clearly, when the immune system is challenged, its dysfunction in T2D progresses from a minor inconvenience to a major health risk and merits our attention as health care professionals.
A key question that arises from these observations is why impaired blood glucose regulation would reduce the ability of the immune system to fight infection. The metabolism of immune cells, especially after their activation, is predominantly regulated through cytokines and was therefore long thought to be impervious to endocrine control [5]. However, recent insights indicate that changes in systemic metabolism are actively induced by the immune system as a defence mechanism against viral infection [6]. Deregulation of this system in the context of diabetes is therefore thought to be an important underlying cause of increased susceptibility to viruses [7], [8]. Conversely, immune-mediated changes in systemic metabolism appear to be an important underlying cause of insulin resistance (IR) in patients with metabolic disease [9]. Notably, infection and inflammation appear to predispose people to the development of Type 2 diabetes [7] especially in people with pre-existing metabolic dysfunction, such as in patients with pre-diabetes. Recently, some of the underlying molecular mechanisms of these processes have been revealed and provide important new targets for future anti-diabetic therapies.
In this review we will revisit recent literature on immune-endocrine interactions in the context of viral infection. We will explore several ways in which the endocrine system regulates immune cell function under healthy conditions and in the context of metabolic disease. In addition, we will discuss how the activated immune system contributes to the development of T2D. Whereas many aspects that we will discuss here are also relevant for Type 1 diabetes, a focus will be on T2D. Diabetes in context of COVID-19 is discussed as a separate section below.
2. Diabetes as a risk factor for infection
2.1. Epidemiology of diabetes and infection
Patients with T2D are well known to be more prone to infection. In fact, a number of infectious diseases, such as emphysematous pyelonephritis, malignant otitis externa, mucormycosis and Fournier’s gangrene [10] are pathognomonic of T2D. However, in addition to these rare conditions, patients with T2D also acquire common infections more frequently. A landmark prospective study from primary care institutions followed up 6.712 T2D patients and 18,911 controls for one year and investigated susceptibility to infection [1]. The authors showed that T2D patients had a higher risk of lower respiratory tract infections (odds ratio (OR) of 1.3, confidence interval (CI) 95% 1.11–1.52) urinary tract infections (OR 1.21, 95% CI 1.07–1.38), bacterial infections of skin and mucosa (OR 1.32, 95%CI 1.13–1.55) and fungal infection (OR 1.41, 95% CI 1.24–1.61). In 2011, a meta-study of 97 prospective cohort studies was published in which 123.205 cause-specific deaths were reported among 820.900 people [11]. This revealed that T2D is associated with a significant increase of infection-related death when pneumonia was excluded (Relative Risk (RR) of 2.39, CI 95% of 1.95–2.93) and also of pneumonia itself (RR 1.8, CI 95% of 1.71–1.9). Importantly, T2D is not only associated with susceptibility to infection, but also with the course and duration of these diseases and with an increased risk of complications. A study comparing bloodstream infections of 71 patients with and 252 patients without diabetes patients showed that the former group had a longer stay at intensive care (RR 7.1, 95% CI of 2–25), longer mechanical ventilation (RR 8.4, 95% CI 1.2–57) and higher chance of renal or hepatic failure (RR 8.2, 95% CI of 1.6–43) [12]. Similar observations were made for a broad range of other infectious diseases [13]. For example, a systematic review of 13 observational studies showed that people with T2D have an RR of 3.11 (CI 2.27–4.26) to develop tuberculosis compared to healthy controls [14].
Due to the sensitivity of patients with diabetes to infection, the guidelines for their medical care prescribe vaccination of T2D patients for influenza, pneumonia and hepatitis B in addition to routine age-related recommendations for vaccination [15]. When the new vaccines for COVID-19 are released it would therefore be recommended to prioritize inoculation of patients with diabetes.
2.2. Normal endocrine control of immune cell function
To better understand how diabetes affects the immune system, it is important to grasp how the endocrine system regulates immune cell functionality under healthy conditions. The immune system puts a major drain on systemic resources and can use up to 30% of all the body‘s nutrients in circulation upon infection [16], [17]. Whereas the metabolism of immune cells is mostly regulated by cytokines, they are not exempt from endocrine control. Notably, many hormone receptors share intracellular signaling components with those of immune receptors, indicating an overlap in function. Of major importance for endocrine control of immune cells are the hormones leptin and adiponectin produced by adipose tissue. Leptin secretion positively correlates with adipose fat content and it provides the body with signals that suppress satiety and increase energy expenditure [18]. In addition, leptin plays a role in regulation of blood glucose levels in concert with insulin as it lowers glycemia, insulinemia and insulin resistance [19]. Injection of leptin in streptozotocin (STZ) treated, hyperglycemic rats and mice was shown to lower blood glucose levels independently of food intake [20], [21]. In humans, leptin levels positively correlate with IR independently of BMI and patients with T2D often have high levels of leptin in addition to hyperinsulinemia [19]. Moreover, people with T2D also display leptin resistance [22]. Unfortunately, leptin administration is not an effective treatment for T2D [22]. The receptor of leptin is expressed on many cells, including those of the immune system. The leptin receptor signals over the intracellular signaling molecules Jak2 and STAT3, which are also used by the receptor for the pro-inflammatory cytokine IL-6 [23]. Leptin therefore promotes immune cell activation and proliferation. Studies in rodents show that intravenous administration of leptin promotes the increase of granulocytes, monocytes and NK cells in circulation [24]. Stimulation of human dendritic cells with leptin leads to an increase in their production of cytokines, including IL-1, IL-6, IL-12 and TNF [25]. Deficiency of leptin or its receptor results in a strong decrease of T cells, NK cells and dendritic cells in the blood [26] and increases susceptibility of animals to infection with Influenza A [25], [27]. Leptin appears to mediate these effects by stimulating immune cell metabolism. Indeed, Leptin was recently shown to revitalize tumor-infiltrating CD8 T cells by reversing metabolic dormancy of these cells [28].
During starvation, when adipose triglyceride stores are low, fat cells produce more adiponectin to signal nutrient scarcity. Adiponectin shares some functional properties with insulin, as it promotes glucose uptake and impairs hepatic gluconeogenesis. In immune cells, adiponectin inhibits activation of NF-κB and promotes production of the anti-inflammatory mediators IL-10 and IL-1Ra by macrophages. High levels of adiponectin therefore reduce immune cell responsiveness [29]. Human T cells stimulated with adiponectin were shown to have reduced antigen-specific expansion [30]. Animals deficient for this adipokine showed increased T cell activation upon infection with Coxsackie virus [30]. In the immune system, adiponectin therefore functions as an anti-inflammatory cytokine which lowers its energy expenditure.
Recently, insulin itself was identified as a molecule that can directly regulate immune cell function, most notably of T cells. Both CD4 and CD8 T cells express the insulin receptor on their cell surface upon activation [7], [31]. Insulin was shown to increase glucose uptake and promote glycolytic metabolism. Importantly, acute loss of insulin production impairs CD8 T cell responses to infection, whereas injection of basal insulin increases their anti-viral potential [7], [31]. The insulin receptor shares its downstream signaling components with CD28, a key co-stimulatory molecule essential for T cell activation, as these pathways both converge on PI3 kinase. These receptors therefore also have functional overlap, such as the induction of glucose transporters on the cell membrane [32]. Not surprisingly, loss of insulin receptor expression on T cells impairs proliferation and cytokine production of anti-viral T cells [7].
In summary, key endocrine hormones involved in the regulation of metabolism also impact immune cell numbers and function, even in absence of overt infection.
2.3. Molecular basis of anti-viral immune dysfunctions in T2D
Several factors play a role in impaired anti-viral immune cell function in the context of T2D, but hyperglycemia appears to be one of the key mediators. The level of glycated haemoglobin (HbA1c) was shown to positively correlate with the duration and severity of infection with several pathogens. A large retrospective case-control study including > 34.000 patients with pneumonia and > 342.000 controls over an 8-year period revealed a relative risk of 1.23 (CI 1.19–1.28) for patients with T2D. Importantly, the risk was significantly higher for patients with T2D with an HbA1c > 9% (RR 1.60, CI 1.44–1.76) compared to those with HbA1c < 7% (RR 1.22, CI 1.14–1.30) [33]. A similar study recruited 4.748 patients with Type 1 diabetes (T1D) and 12.954 controls. People were divided based on five categories of glycemic control (HbA1c < 7%, 7–7.9%, 8–8.9%, 9–9.9% and > 10%), with a follow-up of 14 years. Also in this study, incidence of infection was significantly higher in patents with T1D compared to controls and the frequency of infection positively correlated with the percentage of HbA1c in the blood [34]. Increased blood glucose levels were shown to impair immune cell function in humans and mice. Hyperglycemia in mice induced by injection of STZ, a model for insulin dependent diabetes, caused a decreased ability of macrophages to be activated in response to infection with Mycobacterium tuberculosis (TB). This impaired recruitment of neutrophils, reduced DC activation and lowered cytokine production by these cells [35]. An increase in blood glucose levels was associated with impaired cytotoxicity and cytokine production of CD4 and CD8 T cells and NK cells in patients with T2D following infection with TB [36]. Importantly, T2D has a profound, negative impact on innate immune cell function. For example, granulocytes isolated from patients with T2D were shown to undergo NET-mediated apoptosis, thus impairing wound healing [37], [38]. In addition, production of pro-inflammatory cytokines such as IL-2 and IL-6 was shown to be impaired in peripheral blood mononuclear cells stimulated under hyperglycemic conditions [39]. Finally, hyperglycemia was shown to be of direct benefit for replication of several pathogens [40], [41], which further impedes the ability of the immune system to fight infection under these conditions.
Whereas much is known about which immunological processes are affected by hyperglycemia, how it induces these on a molecular level is much less clear. In immune cells, metabolism and function are tightly linked. In resting state, immune cells predominantly use oxidative phosphorylation to fulfil their energetic needs. Upon activation, especially CD8 T cells and pro-inflammatory M1 macrophages switch their metabolism to glycolytic metabolism for production of ATP and to shuttle metabolites into the penta-phosphate pathway [5]. If proper metabolic control of immune cells is impaired, this has a major impact on their functionality. For example, CD8 T cells that fail to increase glycolytic metabolism cannot form a proper effector response [42], [43]. Whereas memory cell formation is strongly reduced when these cells cannot activate oxygen-dependent metabolism [44]. One mechanism via which hyperglycemia impairs normal immune cell function is therefore by deregulating immuno-metabolism [45].
Apart from glucose, T2D also causes a shift in the homeostasis of many other carbon-based metabolites which are involved in the defence against infection. We all know that if we become sick, we lose appetite and reduce nutrient intake. This is not a pathology, but a carefully regulated process orchestrated by the immune system. As a result, our body switches to a state of ‘fasting‘ metabolism which increases the use of fatty acids and ketone bodies as sources of nutrients. Many pathogens favor glucose metabolism to fulfil metabolic needs [46]. For example, cytomegalovirus was shown to actively induce glycolytic metabolism in host cells to promote its replication, which was strongly impaired if glucose uptake was prevented in cells [46], [47]. SARS-CoV-2 replication is enhanced in the presence of high levels of glucose [48]. Except for key organs such as the brain, most tissues can operate normally at relatively low levels of glucose, which includes the immune system. Even though the immune system uses a high amount of glucose to function, immune cells strongly upregulate glucose transporters upon activation. As a result, CD8 T cells are fully functional at glucose concentrations as low as 0.5 mM [49]. However, as T2D is defined by a state of hyperglycemia even under fasting conditions, this strategy to starve pathogens of glucose fails. In addition, fasting metabolism was recently shown to prime non-immune cells to activate their innate cellular mechanisms against infection. All nucleated cells are able to produce type I interferons upon infection. This alarm signal potently recruits immune cells to the site of infection. Inborn errors of type-I interferons in humans are therefore typically associated with lethal infections at a very young age [50]. An important reason why SARS-CoV-2 leads to severe pathology in relatively many patients is because it causes a delay in IFN-I production [51]. In response to feeding, most glucose is taken up into muscle and rapidly returned in the form of lactate [52]. Fasting is therefore associated with a reduction in systemic lactate levels. Recently, lactate was shown to impair IFN-I production in response to infection by inhibiting RIG-I proteins which sense viral infection. In addition to hyperglycemia, patients with T2D also have higher lactate levels in the blood [53], [54]. As a result, the innate ability of cells to recruit immune cells following viral infection is reduced.
In addition to blood nutrient levels, hormonal disbalance appears to be an underlying cause of immune cell dysfunction in context of diabetes. People with T2D typically have high levels of insulin and leptin in their blood compared to people without this disease, especially early after diagnosis [55], [56]. Considering the immuno-stimulatory role of these hormones, this would suggest that people with diabetes have enhanced immune cell responsiveness. However, the effectiveness of the immune system depends on its ability to optimize its response to a given pathogen. A specific pathogen causes activation of a particular transcriptional profile in key innate immune cells such as dendritic cells, which are responsible for proper activation of T cells. For example, respiratory syncytial virus (RSV) is associated with high levels of IFN-I and –III, whereas SARS-CoV-2 leads to higher expression of IL-6 [57]. T2D skews dendritic cell differentiation, leading to reduced expression of costimulatory molecules CD80 and CD86 [58], whilst promoting development of plasmacytoid dendritic cells that produce type-I interferons [59]. Various studies have shown that hormonal aberrations may cause a Th1/Th2 imbalance, which impairs the immune response against viruses such as RSV and cytomegalovirus [60], [61]. The immuno-stimulatory effect of insulin and leptin in T2D is therefore not beneficial for the patient, because it results in aberrant skewing and therefore reduced efficiency of the immune response.
Changes in the cytokine environment of patients with T2D are also the result of alterations in the interaction between organs involved in maintenance of metabolic homeostasis and its tissue-resident immune cells. People with T2D tend to have increased amounts of pro-inflammatory cytokines in circulation [62], indicative of chronic low-grade infection. This inflammation is thought to originate in visceral adipose tissue. In obesity, adipocytes experience cellular stress because of excessive fat accumulation. Metabolic stress is detected by cells from the innate immune system, such as NK cells, which perceive it as a viral infection and excrete pro-inflammatory cytokines, most notably IFNγ [63]. This, in turn, polarizes macrophages from an anti-inflammatory M2 to a pro-inflammatory M1 phenotype [64]. Activated adipose tissue macrophages promote local inflammation and further recruitment of immune cells, leading to the chronic leakage of cytokines in circulation. T2D was therefore shown to exacerbate cytokine-induced pathology in response to infection with Influenza and Sars-Cov-2 [65], [66]. Moreover, the pro-inflammatory environment in patients with T2D is associated with abnormal clot formation and hypercoagulation, which is an important underlying cause of increased mortality in COVID-19 [67], [68].
Finally, pathological changes in microvasculature are a hallmark of diabetes and disrupt normal organ function in organs heavily dependent on their microcirculation, such as the kidneys, retina and peripheral nervous system. In addition, changes in the microvasculature negatively impact the ability of people with diabetes to mediate immune responses to lesions in skin, resulting in chronic infection, ulcers and poor wound healing. Diabetic foot ulcers are therefore a common complication of diabetes, and this condition may progress to development of gangrene which requires amputation of the affected limb. However, microvascular complications of T2D are of importance for many types of infection, including viral [69]. A detailed description of how damaged microvasculature affects immune responses in T2D reaches beyond the scope of this review, but has been excellently discussed elsewhere [69].
2.4. Conclusion
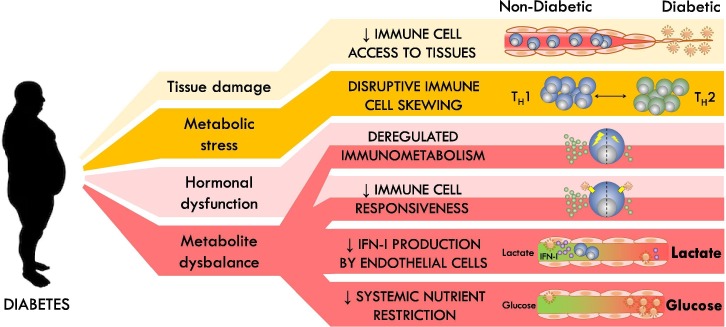
Normal protection against infection is mediated by specialized immune cells, but also by innate abilities of tissues to provide barriers against pathogens and to signal their infection to the immune system. Diabetes impairs the ability to properly respond to infection at almost all these levels of control (Fig. 1 ).
Fig. 1.
Negative impacts of T2D on immunological control of viral infection.
3. Infection as a risk factor for diabetes
3.1. Epidemiology of infection and diabetes
Communication between the endocrine and immune systems is not unidirectional. In response to pathogens such as Influenza A or SARS-CoV-2, we become weak, stop eating, get a temperature and generally feel miserable. This is because the immune system changes normal endocrine regulation of key metabolic processes in our body. Recent data indicates that the physiological changes in metabolism in response to infection may be a trigger for permanent deregulation of blood glucose levels. Many diabetologists will have anecdotal evidence that newly diagnosed patients with T2D had experienced infection in their recent history. In fact, international guidelines recommend screening for infection in newly diagnosed patients, especially if blood glucose levels are very high [70]. Infection was recognized as a cause for increased insulin resistance almost 80 years ago [71]. However, population studies to support this hypothesis have only recently started to emerge.
Because T2D negatively impacts the immune response, it is difficult to determine whether a higher prevalence of infection in patients with T2D is the cause or the result of this disease. Nevertheless, ample evidence is available that infection negatively impacts systemic insulin sensitivity. Infection with viruses such as Influenza A, cytomegalovirus and herpes simplex were all shown to reduce systemic insulin sensitivity [7], [72]. Hyperinsulinemic, euglycemic clamping in patients with a number of respiratory and gastrointestinal infections revealed that insulin resistance was increased in patients, sometimes for more than three months after infection [7], [73].
Whether infection is a risk factor for development of T2D is mostly shown for chronic viruses. A population-based matched case-control cohort study in Korea selected 576 patients infected with cytomegalovirus (CMV), but without T2D and 2.880 matched controls without either condition and followed them for 5 years for development of new onset T2D [61]. The authors showed that the case group had a much higher frequency of new-onset T2D (5.6% vs. 2.2% p < 0.001). Importantly, subgroup analysis revealed that patients with refractory disease had a significantly higher incidence rate (OR 4.01 95% CI 1.76–7.69) than people with non-refractory disease (OR 1.77 95% CI 1.07–2.82) or with non-infected controls (reference population). In addition to CMV, chronic hepatitis C virus was shown to increase the prevalence of T2D, as well as many hepatic manifestations of metabolic syndrome, such as dyslipidemia, non-alcoholic fatty liver disease and hepatocellular carcinoma [74], [75], [76]. Whether acute viruses are also a risk factor for development of T2D remains to be established, though a recent global initiative has started to determine this for COVID-19 [77].
In summary, many infections are able to induce insulin resistance at least transiently, but whether all are a risk factor for development of T2D still requires confirmation.
3.2. Inflammation and diabetes
Whereas the link between infection and diabetes is relatively new, the impact of inflammation on insulin resistance is well explored. Almost 30 years ago it was discovered in preclinical models of diabetes that if animals were deficient for the cytokine TNF they did not develop insulin resistance [78]. Since, many cytokines were shown to have a negative impact on systemic insulin sensitivity, including IL-1β, IFNγ and IL-6 [63], [79]. People with T2D were shown to have a chronic presence of pro-inflammatory cytokines in their circulation, indicative of low-grade inflammation [9]. Neutralization of pro-inflammatory cytokines such as TNF, IL-1β and IL-6 using monoclonal antibody treatment was shown to improve insulin sensitivity in patients with T2D [80], [81], [82]. Notably, patients with T2D have a type-I cytokine profile, which is typically associated with viral infection. The requirement of a specific cytokine environment is also likely the reason that the prevalence of T2D is higher in some inflammatory diseases, such as psoriasis, ulcerative colitis and vasculitis, but not in others such as Crohn’s disease [83].
Pro-inflammatory cytokines mediate insulin resistance at multiple levels. As mentioned, various hormones and cytokines converge on the same signaling cascades, indicating that there is also significant overlap in the molecular mechanisms that are responsible for subsequent shutting down of signaling. For example, IL-6 activates Suppressor of Cytokine Signaling 3 (SOCS3), which is a member of the SOCS family of proteins. SOCS proteins physically bind the intracellular part of activated cytokine and hormone receptors, thus blocking their ability to trigger downstream signaling. This prevents their hyper-stimulation. In addition, SOCS-1 and -3 target the insulin signal transducers IRS1 and IRS2 for ubiquitin-mediated degradation and therefore reduce insulin sensitivity. Cytokines also target other parts of insulin signaling. On myocytes, IFNγ produced by NK cells in response to viral infection was shown to inhibit expression of the insulin receptor, thus reducing insulin sensitivity [7]. TNF inhibits insulin-stimulated tyrosine phosphorylation of both the insulin receptor and IRS-1 [84]. IL-1β causes transcriptional downregulation of IRS-1 [85].
The source of chronic systemic inflammation in the context of T2D long remained elusive but was traced back to visceral adipose tissue (VAT). It is well known that accumulation of subcutaneous fat (the ‘pear shaped’ phenotype) provides a lower risk of developing metabolic disease than an increase of abdominal fat (the ‘apple shaped’ phenotype) [86]. Obese VAT accumulates large numbers of pro-inflammatory immune cells, mostly with an anti-viral Type-I phenotype [87]. As visceral adipocytes accumulate fat and become hypertrophic, they upregulate markers of cellular stress, which mimics a situation induced by viral infection. This form of cellular stress is detected by local, tissue resident NK cells. Activated NK cells produce IFNγ, which polarizes macrophages from an anti-inflammatory M2, to a pro-inflammatory M1 phenotype [63]. These, in turn, produce large amounts of TNF and IL-1β, which activates and recruits more immune cells, such as CD8 T cells and Th1 CD4 T cells [79]. The cytokines generated in this inflammatory environment leak into circulation and contribute to development of systemic insulin resistance. Bariatric surgery, i.e. removal of visceral adipose tissue in obese people, therefore improves systemic insulin sensitivity [63], [88].
In summary, an anti-viral Type-I inflammatory environment promotes the development of insulin resistance. In many organs but most notably in adipose tissue this environment is generated in response to obesity.
3.3. Infection and diabetes
The fact that systemic insulin sensitivity is actively induced by cytokines indicates that IR has an important physiological function in context of infection. However, the physiological benefit of this system has long remained unclear. An important observation is that in absence of metabolic dysfunction, infection does not typically result in a change of either fasting or postprandial blood glucose levels, because IR is compensated by increased insulin output from the pancreas [7], [72], [89]. Hyperinsulinemia therefore appears to be the prime reason of infection-induced IR. Immune cells, most notably activated T cells, express the insulin receptor on their cell surface [7], [31]. Stimulation of the insulin receptor on T cells activates the PI3 kinase signalling pathway, which is also downstream of CD28, a co-stimulatory molecule that is essential for T cell activation. In T cells, CD28 and insulin receptor signalling appear to have overlapping functions in their ability to induce glucose uptake, mediate survival and promote cytokine production [32]. Indeed, mice lacking the ability to produce insulin have a significant reduction in the number of virus-specific CD8 T cells after viral infection [7].
Interferon gamma appears to play a key role in the induction of IR following infection. Viral infection stimulates IFNγ production by NK cells in skeletal muscle. IFNγ induces transcriptional downregulation of the insulin receptor on myocytes, but not hepatocytes, resulting in systemic IR. Deficiency of IFNγ or loss of the IFNγ receptor on myocytes results in a significantly impaired CD8 T cell response following viral infection [7]. Whereas insulin resistance in response to infection was transient in lean mice, it persisted in animals with pre-existing hepatic IR because of diet-induced obesity. Importantly, infection resulted in a permanent loss of blood glucose regulation in these animals [7]. People with pre-diabetes typically display a gradual increase of fasting blood glucose levels over time, which can continue for years or decades. However, development of T2D is typically associated with a strong ‘jump‘ in insulin resistance, which pushes blood glucose levels over diabetes threshold values [90]. It appears likely that infection is responsible for induction of this rapid increase in IR, at least in some patients.
In addition to regulating systemic insulin sensitivity, pancreatic insulin production is also affected by viral infection. Indeed, various viruses have been associated with induction of T1D and destruction of pancreatic beta cells [91]. Recently, expression of ACE2, the receptor through which SARS-CoV-2 enters cells was identified in the human pancreas and virally induced pancreatitis was suggested to have a negative influence on normal blood glucose regulation in patients with COVID-19 [92], [93]. However, glucose sensing and subsequent insulin production are also affected by cytokines produced in response to viral infection. Pancreatic β cells express the receptor for IL-1β and this cytokine was shown to promote postprandial insulin secretion [94]. Infection causes a dramatic increase of IL-1β levels in circulation. Whether IL-1β signalling in pancreatic β cells contributes to the increase of insulin in circulation following viral infection remains to be confirmed.
In summary, viral infection increases systemic insulin resistance and promotes insulin production because of a physiological process orchestrated by the immune system that aims to increase our defences against invading pathogens. This system derails in the context of pre-existing metabolic dysfunction and therefore contributes to the formation of T2D.
4. Diabetes and COVID-19
Currently of very high interest is the susceptibility of people with T2DM for the SARS-CoV-2 virus and the impact of this pathogen on control of blood glucose levels. SARS-CoV-2, which causes COVID-19 was first identified in China in the city of Wuhan in late 2019. It is a highly infectious coronavirus that enters through the airways and from there may spread to other organs, such as the hart, liver and kidney. COVID-19 has a relatively high mortality rate, with an estimated 0.5–1% of patients succumbing to the disease [95]. Patients who develop severe COVID-19 have a delayed IFN-I response [51], which results in rapid spread of the disease and a subsequent cytokine storm, which is associated with hypercoagulation, severe lung edema and neural complications [96], [97].
Initial analysis of patients in Wuhan suggested that T2D is associated with a higher risk of developing severe pneumonia, excessive inflammation and hyper-coagulation upon infection with SARS-CoV-2 [2], [98], [99]. Indeed, retrospective studies from this patient group indicate that poor glycemic control is associated with increased morbidity and mortality of COVID-19 [100]. However, the severity of COVID-19 closely correlates with the age of patients, which is typically also the case for T2D. Initially it was therefore unclear whether T2D was a risk factor beyond age alone. However, follow-up cohort studies in the UK and Italy and various retrospective and meta-analysis studies (summarized in [101]) confirmed that T2D does pose a risk for development of more severe disease and increased mortality following infection with SARS-CoV-2 [98], [102], [103], [104]. Interestingly, a recent multicenter observational study in France amongst both T1D and T2D patients with tracheal intubation and/or death within 7 days after hospital admission as a primary outcome indicated that BMI, but not HbA1c were positively associated in these patient groups [105]. This is in contrast with earlier findings that level of glycemic control is associated with a higher mortality rate [100]. However, it should be noted that the latter study compared patients suffering from very poor glycemic control (glycemic variability exceeding 10.0 mmol/L) with well controlled patients. This may explain why this correlation is not observed within the entire diabetic population.
The underlying molecular mechanism how T2D leads to more severe COVID-19 is currently unclear. One study indicates the involvement of alveolar macrophages [48]. Macrophages increase their glycolytic rate upon activation through a mechanism that involves the transcription factor HIF-1α. SARS-CoV-2 can infect macrophages and benefit from the increased glycolytic rate in these cells. The presence of a hyperglycemic state in patients with T2D was suggested to further facilitate viral replication in these cells and promote disease progression [48]. However, since immune cells highly upregulate glucose transporters upon activation, glucose availability is typically not rate-limiting for their level of metabolism [49]. Additional mechanisms must therefore exist that promote COVID-19 disease severity in the context of T2D.
Conversely, COVID-19 is also associated with reduced glycemic control. Initial studies showed that of 24 patients with T2D hospitalized because of COVID-19, all patients using insulin therapy required to increase their dose, and 9 out of 17 patients using only oral anti-diabetic drugs before infection were started on insulin after infection [2]. This initial finding was confirmed by other studies, which showed that patients with severe COVID-19 needed intensive insulin treatment to control blood glucose levels, with some patients requiring > 200 IU per day [106], [107]. The severity of hyperglycemia was associated with the intensity of the cytokine storm [107], which is a clear indication that immunological triggers are responsible for the changes in blood glucose regulation in the context of severe disease. The underlying molecular mechanisms of this observation are still unclear. In case of critically ill patients, mechanisms of stress hyperglycemia, such as a cortisol-mediated increase of gluconeogenesis, are likely to be activated [8]. Interestingly, an analysis of metabolites in the serum of patients with COVID-19 indicated that blood glucose levels are actually lower in patients with mild disease compared to uninfected control [93]. This may be associated to the physiological mechanism of hyper-insulinemic hypoglycemia that is observed in context of certain infections [108], [109], [110]. It will therefore be interesting to see whether insulin levels in convalescent COVID-19 patients are lower than in patients with acute disease, similar to influenza infection [7].
In summary, COVID-19 illustrates the interaction between the immune and endocrine systems in the context of infection and the risk that deregulation of this interplay causes for people with metabolic syndrome. Further research into the immunological complications of T2D is therefore prudent.
5. Immune-endocrine interactions as a therapeutic target
5.1. Targeting immune-endocrine interactions to reduce IR
Various therapeutic interventions are in development that target the antiviral arm of the immune system to improve systemic insulin sensitivity. A small-scale 13-week study in which patients with T2D received an interleukin-1 receptor antagonist caused a significant decrease in HbA1c levels and a reduction in the ratio of pro-insulin to insulin [111]. A 39-week follow up trial confirmed the sustained effects of treatment [112]. Unfortunately, analysis of the potential complications of prolonged IL-1 inhibition indicated an increased risk of cardiovascular events [113], making this an unattractive therapy for many patients with T2D. Retrospective studies that questioned whether neutralizing antibodies against TNF improve systemic insulin sensitivity suggest a beneficial effect, but to date no prospective studies have been done, nor have cardiovascular risk studies been established [114].
From a practical perspective, it is unlikely that monoclonal antibody treatment will become a feasible solution for patients with T2D. Studies looking at the effectiveness of such therapies typically do this in patients who receive antibody treatment for other, more directly life-threatening conditions [114]. In addition, it is questionable whether therapies targeting individual cytokines are effective in the long run. Pathogens such as CMV, but also SARS-CoV-2 often develop strategies to avoid specific immune responses. As a result, the immune system is designed to be fully functional even when some of its key components are neutralized by a pathogen [115]. Especially in a complex, multi-organ disease such as T2D, it is therefore very difficult to inhibit the detrimental effects of the immune system at large by targeting a single molecule.
How then to exploit our knowledge on immune-endocrine interactions to reduce insulin resistance? It is important to realize that the immune response is a cascade. This cascade starts with the detection of a threat by non-immune cells which activate a small number of tissue-resident innate immune cells. These, in turn, recruit and activate more potent immune cells from circulation such as macrophages, which themselves recruit a second wave of immune cells, like CD8 T cells [79]. Inhibition of an immune response is therefore most effective if you target inflammation upstream in the cascade. Corticosteroids do this, but because of their broad metabolic side effects are a poor choice of therapy in T2D. Previously, we have shown in mouse models that blocking the interaction between VAT-resident NK cells and metabolically stressed adipocytes is an effective way to prevent immune activation in the context of obesity and this prevented development of T2D in mice [63]. Therapies that prevent upstream signals of immune activation are therefore a promising target for future anti-diabetic therapies.
5.2. Enhancing immunity in patients with T2DM
Anti-diabetic treatment does appear to reduce the risk of infection in patients with T2D. Following infection, the severity of disease negatively correlates with the level of glycemic control [33], [116], [117]. Patients with T2D with well controlled blood glucose levels had a significantly lower chance of death than poorly controlled patients following infection with SARS-CoV-2 [100]. In addition, in a cohort study based on a health screening program in Taiwan including 118.645 people with a median follow-up of 8.1 years, it was shown that the level of fasting plasma glucose positively correlated with both the incidence of infection and the risk of infection-associated mortality [118].
Not all anti-diabetic drugs are equal in their ability to reduce the risk of infection in patients with T2D. Whereas insulin has the biggest impact on lowering of blood glucose levels, a large patient-based cohort study of 131.949 patients with T2D in Denmark with a seven year follow up indicated that insulin therapy significantly increased the risk of hospital-treated infection (adjusted hazard ration (HR) or 1.96, 95% CI 1.87–2.07) compared to metformin [119]. Similarly, patients who initiated treatment with sulfonylurea had increased risk of hospitalization for viral infection (HR of 1.7, 95% CI 1.40–2.07), as well as various other infections compared to patients treated with metformin only. The difference between groups was reduced but remained significant even after correction for several confounding effects. Another study in a Swedish population of patients with diabetes showed comparable results [120]. In contrast, the use of metformin has been associated with a reduced risk of surgical site infection, respiratory tract infection and sepsis [121], [122], [123]. There is limited data available on some of the newer types anti-diabetic drugs and their impact on susceptibility to infection. Various trials show no cause-effect association between the use of GLP-1 receptor agonists and more serious infections [124]. Use of SGLT2 inhibitors did not result in a greater risk of developing COVID-19 compared to patients using DPP-4 inhibitors [125]. Whether these drugs may have a beneficial impact on the ability of patients with T2D to fight infection has so far not been studied extensively.
Analysis of the impact of diabetic drugs on susceptibility to infection is complicated by the fact that many of these compounds have a direct impact on the immune system. For example metformin, the first line drug for the treatment of T2D, impairs immune cell effector responses by induction of AMPK and inhibition of mTORC1 signaling [126]. At the same time, this process enhances memory CD8 T cell formation and was shown to promote recall responses in animal models [127]. Thus, further studies are required to segregate the impact of anti-diabetic drugs on glycemia from those on the immune system with regards to susceptibility to infection. Nevertheless, a reduction on blood glucose appears to be overall beneficial.
Despite a potential benefit of reducing glycemia, aggressive treatment of glycemia in the context of (severe) infection should be executed with great caution. Since inflammatory mediators affect regulation of glycemia, viral infection is associated with much greater variability in blood glucose levels [128]. Severe infections are therefore often accompanied with both events of hyper- and hypoglycaemia [8]. Indeed, the NICE-SUGAR prospective study investigating the impact of aggressive control of blood glucose in critically ill patients showed increased mortality because of a larger number of hypoglycemic events [129]. Current guidelines for critical care patients therefore recommend parenteral and enteral feeding over stringent blood glucose regulation [130]. In addition to blood glucose regulation, current guidelines for the treatment of diabetes highly recommend vaccination of patients, in particular for seasonal influenza and hepatitis B [70].
6. Concluding remarks
Proper regulation of metabolism is a key requirement of survival. Not surprisingly, the endocrine system puts limits to the metabolic activity of nutrient intensive cells, such as those of the immune system. At the same time, regulation of metabolism provides a valuable tool in the fight against infection. The immune system therefore modulates this core system to induce a state of systemic preparedness for infection, thus enhancing chances of survival in response to a potentially lethal threat. Not surprisingly, dysregulation of normal metabolic control in the context of T2D strongly impacts the ability of the body to properly respond to infection and leads to a significant increase of morbidity and mortality in response to dangerous pathogens such as SARS-CoV-2. It is therefore important that we do not only acknowledge the pathology of immune-endocrine interactions in diabetes, but also try to understand the physiology behind the processes that regulate insulin sensitivity and blood glucose levels during changes in homeostasis, such as after infection (Fig. 2 ). Only then can we properly target this mechanism to reduce insulin resistance in diabetes and enhance the immune response if a patient with diabetes suffers from infection.
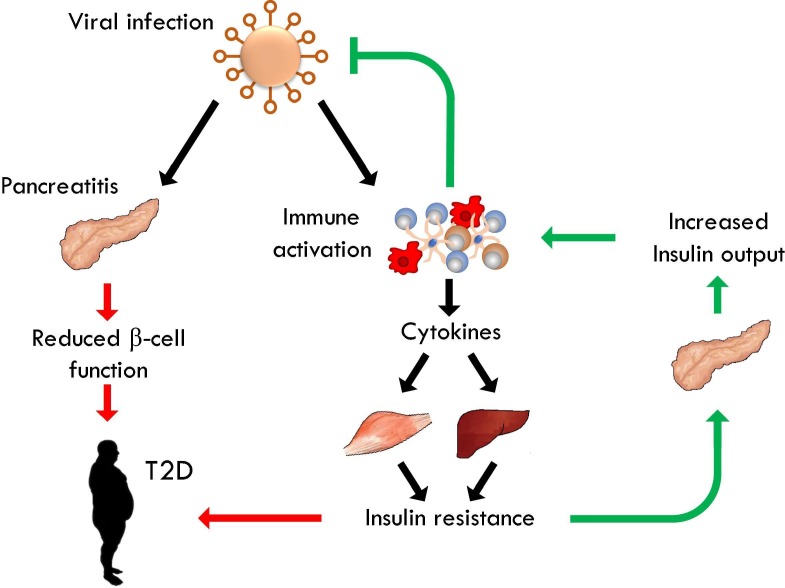
Fig. 2.
Pro-diabetic effects of viral infection. Viral infection activates a Type-I immune response, resulting in the production of cytokines such as TNF, IFNγ and IL-6. These induce transient insulin resistance in muscle and liver. The pancreas compensates IR through increased secretion of insulin, which directly promotes the antiviral immune system. In obesity, cytokine-induced IR can contribute to the formation of IR. In addition, several viruses infect the pancreas, which negatively impacts its ability to produce insulin. This may also contribute to loss of pancreatic β-cell function.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by a University of Rijeka Support grant (19-41-1551) and the Croatian Science Foundation (IP-2016-06-8027, IP-CORONA-2020-04-2045) to FMW and (IP-2020-02-7928) to TTW.
References
- 1.Muller L.M., Gorter K.J., Hak E., Goudzwaard W.L., Schellevis F.G., Hoepelman A.I., et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 2.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev 2020:e3319. [DOI] [PMC free article] [PubMed]
- 3.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts B.W., Cech I. Association of type 2 diabetes mellitus and seroprevalence for cytomegalovirus. South Med J. 2005;98:686–692. doi: 10.1097/01.SMJ.0000163310.12516.2D. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotas M.E., Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sestan M., Marinovic S., Kavazovic I., Cekinovic D., Wueest S., Turk Wensveen T., et al. Virus-induced interferon-gamma causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity. 2018;49(164–77) doi: 10.1016/j.immuni.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Wensveen F.M., Sestan M., Turk Wensveen T., Polic B. 'Beauty and the beast' in infection: How immune-endocrine interactions regulate systemic metabolism in the context of infection. Eur J Immunol. 2019;49:982–995. doi: 10.1002/eji.201847895. [DOI] [PubMed] [Google Scholar]
- 9.Johnson A.R., Milner J.J., Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson-Stuttard J., Blundell S., Harris T., Cook D.G., Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4:148–158. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 11.Emerging Risk Factors C., Seshasai S.R., Kaptoge S., Thompson A., Di Angelantonio E., Gao P., et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoeckle M., Kaech C., Trampuz A., Zimmerli W. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med Wkly. 2008;138:512–519. doi: 10.4414/smw.2008.12228. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S., Koirala J., Khardori R., Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21(617–38):vii. doi: 10.1016/j.idc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Jeon C.Y., Murray M.B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Association A.D. Standards of medical care in diabets - 2017. Diabetes Care. 2017;40:S1–132. [Google Scholar]
- 16.Muehlenbein M.P., Hirschtick J.L., Bonner J.Z., Swartz A.M. Toward quantifying the usage costs of human immunity: Altered metabolic rates and hormone levels during acute immune activation in men. Am J Hum Biol. 2010;22:546–556. doi: 10.1002/ajhb.21045. [DOI] [PubMed] [Google Scholar]
- 17.Straub R.H., Cutolo M., Buttgereit F., Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med. 2010;267:543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Acker H.H., Anguille S., Willemen Y., Van den Bergh J.M., Berneman Z.N., Lion E., et al. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J Hematol Oncol. 2016;9:101. doi: 10.1186/s13045-016-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paz-Filho G., Mastronardi C., Wong M.L., Licinio J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J Endocrinol Metab. 2012;16:S549–S555. doi: 10.4103/2230-8210.105571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X., Park B.H., Wang M.Y., Wang Z.V., Unger R.H. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci U S A. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denroche H.C., Levi J., Wideman R.D., Sequeira R.M., Huynh F.K., Covey S.D., et al. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes. 2011;60:1414–1423. doi: 10.2337/db10-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon H.S., Matarese G., Brennan A.M., Chamberland J.P., Liu X., Fiorenza C.G., et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villanueva E.C., Myers M.G., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes (Lond) 2008;32(Suppl 7):S8–S12. doi: 10.1038/ijo.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Z., Sun R., Wei H., Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- 25.Maurya R., Bhattacharya P., Dey R., Nakhasi H.L. Leptin functions in infectious diseases. Front Immunol. 2018;9:2741. doi: 10.3389/fimmu.2018.02741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Cava A., Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 27.Wieland C.W., Florquin S., Chan E.D., Leemans J.C., Weijer S., Verbon A., et al. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int Immunol. 2005;17:1399–1408. doi: 10.1093/intimm/dxh317. [DOI] [PubMed] [Google Scholar]
- 28.Gerriets V.A., Danzaki K., Kishton R.J., Eisner W., Nichols A.G., Saucillo D.C., et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol. 2016;46:1970–1983. doi: 10.1002/eji.201545861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf A.M., Wolf D., Rumpold H., Enrich B., Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 30.Wilk S., Scheibenbogen C., Bauer S., Jenke A., Rother M., Guerreiro M., et al. Adiponectin is a negative regulator of antigen-activated T cells. Eur J Immunol. 2011;41:2323–2332. doi: 10.1002/eji.201041349. [DOI] [PubMed] [Google Scholar]
- 31.Tsai S., Clemente-Casares X., Zhou A.C., Lei H., Ahn J.J., Chan Y.T., et al. Insulin receptor-mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 2018;28(922–34) doi: 10.1016/j.cmet.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R., et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 33.Kornum J.B., Thomsen R.W., Riis A., Lervang H.H., Schonheyder H.C., Sorensen H.T. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care. 2008;31:1541–1545. doi: 10.2337/dc08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonsen J.R., Harjutsalo V., Jarvinen A., Kirveskari J., Forsblom C., Groop P.H., et al. Bacterial infections in patients with type 1 diabetes: a 14-year follow-up study. BMJ Open Diabetes Res Care. 2015;3 doi: 10.1136/bmjdrc-2014-000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayelign B., Negash M., Genetu M., Wondmagegn T., Shibabaw T. Immunological impacts of diabetes on the susceptibility of mycobacterium tuberculosis. J Immunol Res. 2019;2019:6196532. doi: 10.1155/2019/6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar N.P., Sridhar R., Nair D., Banurekha V.V., Nutman T.B., Babu S. Type 2 diabetes mellitus is associated with altered CD8(+) T and natural killer cell function in pulmonary tuberculosis. Immunology. 2015;144:677–686. doi: 10.1111/imm.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turina M., Fry D.E., Polk H.C., Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624–1633. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 38.Wong S.L., Demers M., Martinod K., Gallant M., Wang Y., Goldfine A.B., et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhold D., Ansorge S., Schleicher E.D. Elevated glucose levels stimulate transforming growth factor-beta 1 (TGF-beta 1), suppress interleukin IL-2, IL-6 and IL-10 production and DNA synthesis in peripheral blood mononuclear cells. Horm Metab Res. 1996;28:267–270. doi: 10.1055/s-2007-979789. [DOI] [PubMed] [Google Scholar]
- 40.Geerlings S.E., Brouwer E.C., Gaastra W., Verhoef J., Hoepelman A.I.M. Effect of glucose and pH on uropathogenic and non-uropathogenic Escherichia coli: studies with urine from diabetic and non-diabetic individuals. J Med Microbiol. 1999;48:535–539. doi: 10.1099/00222615-48-6-535. [DOI] [PubMed] [Google Scholar]
- 41.Geerlings S.E., Hoepelman A.I. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 42.Greiner E.F., Guppy M., Brand K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem. 1994;269:31484–31490. [PubMed] [Google Scholar]
- 43.Gubser P.M., Bantug G.R., Razik L., Fischer M., Dimeloe S., Hoenger G., et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 44.van der Windt G.J., Everts B., Chang C.H., Curtis J.D., Freitas T.C., Amiel E., et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guzik T.J., Cosentino F. Epigenetics and Immunometabolism in Diabetes and Aging. Antioxid Redox Signal. 2018;29:257–274. doi: 10.1089/ars.2017.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landini M.P. Early enhanced glucose uptake in human cytomegalovirus-infected cells. J Gen Virol. 1984;65(Pt 7):1229–1232. doi: 10.1099/0022-1317-65-7-1229. [DOI] [PubMed] [Google Scholar]
- 47.Yu Y., Maguire T.G., Alwine J.C. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. J Virol. 2011;85:1573–1580. doi: 10.1128/JVI.01967-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab. 2020;32(437–46) doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs S.R., Herman C.E., Maciver N.J., Wofford J.A., Wieman H.L., Hammen J.J., et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duncan C.J., Mohamad S.M., Young D.F., Skelton A.J., Leahy T.R., Munday D.C., et al. Human IFNAR2 deficiency: Lessons for antiviral immunity. Sci Transl Med. 2015 doi: 10.1126/scitranslmed.aac4227. 7:307ra154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hui S., Ghergurovich J.M., Morscher R.J., Jang C., Teng X., Lu W., et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y.D., Varasteh B.B., Reaven G.M. Plasma lactate concentration in obesity and type 2 diabetes. Diabete Metab. 1993;19:348–354. [PubMed] [Google Scholar]
- 54.Mongraw-Chaffin M.L., Matsushita K., Brancati F.L., Astor B.C., Coresh J., Crawford S.O., et al. Diabetes medication use and blood lactate level among participants with type 2 diabetes: the atherosclerosis risk in communities carotid MRI study. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saisho Y. Importance of beta cell function for the treatment of type 2 diabetes. J Clin Med. 2014;3:923–943. doi: 10.3390/jcm3030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bandaru P., Shankar A. Association between plasma leptin levels and diabetes mellitus. Metab Syndr Relat Disord. 2011;9:19–23. doi: 10.1089/met.2010.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(1036–45) doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Musilli C., Paccosi S., Pala L., Gerlini G., Ledda F., Mugelli A., et al. Characterization of circulating and monocyte-derived dendritic cells in obese and diabetic patients. Mol Immunol. 2011;49:234–238. doi: 10.1016/j.molimm.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Hannibal T.D., Schmidt-Christensen A., Nilsson J., Fransen-Pettersson N., Hansen L., Holmberg D. Deficiency in plasmacytoid dendritic cells and type I interferon signalling prevents diet-induced obesity and insulin resistance in mice. Diabetologia. 2017;60:2033–2041. doi: 10.1007/s00125-017-4341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinto R.A., Arredondo S.M., Bono M.R., Gaggero A.A., Diaz P.V. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics. 2006;117:e878–e886. doi: 10.1542/peds.2005-2119. [DOI] [PubMed] [Google Scholar]
- 61.Yoo S.G., Han K.D., Lee K.H., La Y., Kwon D.E., Han S.H. Impact of cytomegalovirus disease on new-onset type 2 diabetes mellitus: population-based matched case-control cohort study. Diabetes Metab J. 2019;43:815–829. doi: 10.4093/dmj.2018.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esser N., Legrand-Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Wensveen F.M., Jelencic V., Valentic S., Sestan M., Wensveen T.T., Theurich S., et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16:376–385. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 64.Wensveen F.M., Valentic S., Sestan M., Turk Wensveen T., Polic B. Interactions between adipose tissue and the immune system in health and malnutrition. Semin Immunol. 2015;27:322–333. doi: 10.1016/j.smim.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Chi Y., Ge Y., Wu B., Zhang W., Wu T., Wen T., et al. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J Infect Dis. 2020;222:746–754. doi: 10.1093/infdis/jiaa363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q., Fang P., He R., Li M., Yu H., Zhou L., et al. O-GlcNAc transferase promotes influenza A virus-induced cytokine storm by targeting interferon regulatory factor-5. Sci Adv. 2020 doi: 10.1126/sciadv.aaz7086. 6:eaaz7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Randeria S.N., Thomson G.J.A., Nell T.A., Roberts T., Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc Diabetol. 2019;18:72. doi: 10.1186/s12933-019-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta R., Hussain A., Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020;74:864–870. doi: 10.1038/s41430-020-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abiko Y., Selimovic D. The mechanism of protracted wound healing on oral mucosa in diabetes. Review Bosn J Basic Med Sci. 2010;10:186–191. doi: 10.17305/bjbms.2010.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.American D.A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 71.Greene J.A.K.G. Insulin resistance due to infection in diabetes mellitus in man. JAMA. 1943;121:173–176. [Google Scholar]
- 72.Fernandez-Real J.M., Lopez-Bermejo A., Vendrell J., Ferri M.J., Recasens M., Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058–1064. doi: 10.2337/diacare.2951058. [DOI] [PubMed] [Google Scholar]
- 73.Yki-Jarvinen H., Sammalkorpi K., Koivisto V.A., Nikkila E.A. Severity, duration, and mechanisms of insulin resistance during acute infections. J Clin Endocrinol Metab. 1989;69:317–323. doi: 10.1210/jcem-69-2-317. [DOI] [PubMed] [Google Scholar]
- 74.Kim K.H., Hong S.P., Kim K., Park M.J., Kim K.J., Cheong J. HCV core protein induces hepatic lipid accumulation by activating SREBP1 and PPARgamma. Biochem Biophys Res Commun. 2007;355:883–888. doi: 10.1016/j.bbrc.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 75.Moriya K., Fujie H., Shintani Y., Yotsuyanagi H., Tsutsumi T., Ishibashi K., et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 76.Salmon D., Bani-Sadr F., Loko M.A., Stitou H., Gervais A., Durant J., et al. Insulin resistance is associated with a higher risk of hepatocellular carcinoma in cirrhotic HIV/HCV-co-infected patients: results from ANRS CO13 HEPAVIH. J Hepatol. 2012;56:862–868. doi: 10.1016/j.jhep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Rubino F., Amiel S.A., Zimmet P., Alberti G., Bornstein S., Eckel R.H., et al. New-onset diabetes in covid-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 79.Wensveen F.M., Valentic S., Sestan M., Turk Wensveen T., Polic B. The, “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45:2446–2456. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 80.Schultz O., Oberhauser F., Saech J., Rubbert-Roth A., Hahn M., Krone W., et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kiortsis D.N., Mavridis A.K., Vasakos S., Nikas S.N., Drosos A.A. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765–766. doi: 10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malozowski S., Sahlroot J.T. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;357:302–303. doi: 10.1056/NEJMc071324. author reply 3. [DOI] [PubMed] [Google Scholar]
- 83.Dregan A., Charlton J., Chowienczyk P., Gulliford M.C. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130:837–844. doi: 10.1161/CIRCULATIONAHA.114.009990. [DOI] [PubMed] [Google Scholar]
- 84.Hotamisligil G.S., Murray D.L., Choy L.N., Spiegelman B.M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jager J., Gremeaux T., Cormont M., Le Marchand-Brustel Y., Tanti J.F. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lebovitz H.E. The relationship of obesity to the metabolic syndrome. Int J Clin Pract Suppl. 2003;18–27 [PubMed] [Google Scholar]
- 87.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gumbs A.A., Modlin I.M., Ballantyne G.H. Changes in insulin resistance following bariatric surgery: role of caloric restriction and weight loss. Obes Surg. 2005;15:462–473. doi: 10.1381/0960892053723367. [DOI] [PubMed] [Google Scholar]
- 89.Vafaeimanesh J., Parham M., Seyyedmajidi M., Bagherzadeh M. Helicobacter pylori infection and insulin resistance in diabetic and nondiabetic population. ScientificWorldJournal. 2014;2014 doi: 10.1155/2014/391250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tabak A.G., Jokela M., Akbaraly T.N., Brunner E.J., Kivimaki M., Witte D.R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Filippi C.M., von Herrath M.G. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008;57:2863–2871. doi: 10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18(2128–30) doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182(59–72) doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dror E., Dalmas E., Meier D.T., Wueest S., Thevenet J., Thienel C., et al. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol. 2017;18:283–292. doi: 10.1038/ni.3659. [DOI] [PubMed] [Google Scholar]
- 95.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(1068–77) doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu L., Girgis C.M., Cheung N.W. COVID-19 and diabetes: Insulin requirements parallel illness severity in critically unwell patients. Clin Endocrinol (Oxf) 2020 doi: 10.1111/cen.14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gianchandani R., Esfandiari N.H., Ang L., Iyengar J., Knotts S., Choksi P., et al. Managing hyperglycemia in the COVID-19 inflammatory storm. Diabetes. 2020;69:2048–2053. doi: 10.2337/dbi20-0022. [DOI] [PubMed] [Google Scholar]
- 108.Tucey T.M., Verma J., Harrison P.F., Snelgrove S.L., Lo T.L., Scherer A.K., et al. Glucose homeostasis is important for immune cell viability during candida challenge and host survival of systemic fungal infection. Cell Metab. 2018;27(988–1006) doi: 10.1016/j.cmet.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Teng R.J., Wu T.J., Ho M.M. Mumps infection complicated by transient hyperinsulinemic hypoglycemia. Pediatr Infect Dis J. 1997;16:416–417. doi: 10.1097/00006454-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 110.Freyberg Z., Harvill E.T. Pathogen manipulation of host metabolism: A common strategy for immune evasion. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Larsen C.M., Faulenbach M., Vaag A., Volund A., Ehses J.A., Seifert B., et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 112.Larsen C.M., Faulenbach M., Vaag A., Ehses J.A., Donath M.Y., Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Interleukin 1 Genetics C. Cardiometabolic effects of genetic upregulation of the interleukin 1 receptor antagonist: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol 2015;3:243-53 [DOI] [PMC free article] [PubMed]
- 114.Gupta-Ganguli M., Cox K., Means B., Gerling I., Solomon S.S. Does therapy with anti-TNF-alpha improve glucose tolerance and control in patients with type 2 diabetes? Diabetes Care. 2011;34 doi: 10.2337/dc10-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berry R., Watson G.M., Jonjic S., Degli-Esposti M.A., Rossjohn J. Modulation of innate and adaptive immunity by cytomegaloviruses. Nat Rev Immunol. 2020;20:113–127. doi: 10.1038/s41577-019-0225-5. [DOI] [PubMed] [Google Scholar]
- 116.Wang Z., Du Z., Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ceriello A., Standl E., Catrinoiu D., Itzhak B., Lalic N.M., Rahelic D., et al. Issues of cardiovascular risk management in people with diabetes in the COVID-19 era. Diabetes Care. 2020;43:1427–1432. doi: 10.2337/dc20-0941. [DOI] [PubMed] [Google Scholar]
- 118.Chang CH, Wang JL, Wu LC, Chuang LM, Lin HH. Diabetes, glycemic control, and risk of infection morbidity and mortality: a cohort study. Open Forum Infect Dis. 2019;6:ofz358. [DOI] [PMC free article] [PubMed]
- 119.Mor A., Petersen I., Sorensen H.T., Thomsen R.W. Metformin and other glucose-lowering drug initiation and rates of community-based antibiotic use and hospital-treated infections in patients with type 2 diabetes: a Danish nationwide population-based cohort study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ekstrom N., Schioler L., Svensson A.M., Eeg-Olofsson K., Miao Jonasson J., Zethelius B., et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garnett J.P., Baker E.H., Naik S., Lindsay J.A., Knight G.M., Gill S., et al. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax. 2013;68:835–845. doi: 10.1136/thoraxjnl-2012-203178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duncan A.I., Koch C.G., Xu M., Manlapaz M., Batdorf B., Pitas G., et al. Recent metformin ingestion does not increase in-hospital morbidity or mortality after cardiac surgery. Anesth Analg. 2007;104:42–50. doi: 10.1213/01.ane.0000242532.42656.e7. [DOI] [PubMed] [Google Scholar]
- 123.Shih C.J., Wu Y.L., Chao P.W., Kuo S.C., Yang C.Y., Li S.Y., et al. Association between use of oral anti-diabetic drugs and the risk of sepsis: a nested case-control study. Sci Rep. 2015;5:15260. doi: 10.1038/srep15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Filippatos T.D., Panagiotopoulou T.V., Elisaf M.S. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11:202–230. doi: 10.1900/RDS.2014.11.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sainsbury C., Wang J., Gokhale K., Acosta-Mena D., Dhalla S., Byne N., et al. Sodium-glucose co-transporter-2 inhibitors and susceptibility to COVID-19: A population-based retrospective cohort study. Diabetes Obes Metab. 2020 doi: 10.1111/dom.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schuiveling M., Vazirpanah N., Radstake T., Zimmermann M., Broen J.C.A. Metformin, A new era for an old drug in the treatment of immune mediated disease? Curr Drug Targets. 2018;19:945–959. doi: 10.2174/1389450118666170613081730. [DOI] [PubMed] [Google Scholar]
- 127.Pearce E.L., Walsh M.C., Cejas P.J., Harms G.M., Shen H., Wang L.S., et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hulme K.D., Gallo L.A., Short K.R. Influenza virus and glycemic variability in diabetes: a killer combination? Front Microbiol. 2017;8:861. doi: 10.3389/fmicb.2017.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Investigators N.-S.-S., Finfer S., Chittock D.R., Su S.Y., Blair D., Foster D., et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 130.McClave S.A., Taylor B.E., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]