ABSTRACT
Aims
A recently completed Cochrane review assessed the effectiveness and cost-benefits of Alcoholics Anonymous (AA) and clinically delivered 12-Step Facilitation (TSF) interventions for alcohol use disorder (AUD). This paper summarizes key findings and discusses implications for practice and policy.
Methods
Cochrane review methods were followed. Searches were conducted across all major databases (e.g. Cochrane Drugs and Alcohol Group Specialized Register, PubMed, Embase, PsycINFO and ClinicalTrials.gov) from inception to 2 August 2019 and included non-English language studies. Randomized controlled trials (RCTs) and quasi-experiments that compared AA/TSF with other interventions, such as motivational enhancement therapy (MET) or cognitive behavioral therapy (CBT), TSF treatment variants or no treatment, were included. Healthcare cost offset studies were also included. Studies were categorized by design (RCT/quasi-experimental; nonrandomized; economic), degree of manualization (all interventions manualized versus some/none) and comparison intervention type (i.e. whether AA/TSF was compared to an intervention with a different theoretical orientation or an AA/TSF intervention that varied in style or intensity). Random-effects meta-analyses were used to pool effects where possible using standard mean differences (SMD) for continuous outcomes (e.g. percent days abstinent (PDA)) and the relative risk ratios (RRs) for dichotomous.
Results
A total of 27 studies (21 RCTs/quasi-experiments, 5 nonrandomized and 1 purely economic study) containing 10,565 participants were included. AA/TSF interventions performed at least as well as established active comparison treatments (e.g. CBT) on all outcomes except for abstinence where it often outperformed other treatments. AA/TSF also demonstrated higher health care cost savings than other AUD treatments.
Conclusions
AA/TSF interventions produce similar benefits to other treatments on all drinking-related outcomes except for continuous abstinence and remission, where AA/TSF is superior. AA/TSF also reduces healthcare costs. Clinically implementing one of these proven manualized AA/TSF interventions is likely to enhance outcomes for individuals with AUD while producing health economic benefits.
Data bases up till August 2019 were searched for randomized controlled and other studies in participants with alcohol use disorders that compared the efficacy and costs of treatment that facilitated use of AA versus treatment using other methods such as cognitive behavior therapy and motivational enhancement therapy. The search revealed 27 studies pertaining to 10,565 persons. Meta-analyses showed that AA and facilitating use of AA (‘TSF’) produced similar benefits to other treatments on all drinking-related outcomes except for continuous abstinence and remission, where AA/TSF was superior. Studies analyzing costing found that use of AA/TSF also tended to reduce healthcare costs.
INTRODUCTION
Alcohol use disorder (AUD) is one of the leading preventable causes of premature death and disability globally (Stahre et al., 2014) with 3.3 million attributable deaths each year around the world (World Health Organization, 2014). The economic burden associated with alcohol misuse is also enormous—amounting to approximately USD 250 billion annually in the USA alone due to lost productivity, crime and incarceration and increased healthcare utilization (Sacks et al., 2015). The response to AUD is multipronged and includes a variety of professional services as well as a number of low-cost or free recovery support services (e.g. mutual-help organizations, sober living environments) (White et al., 2012). The oldest and by far the largest of these AUD recovery support services is Alcoholics Anonymous (AA).
AA consists of several million members in 181 countries (Alcoholics Anonymous, 2001; Humphreys, 2004) and is a peer-to-peer support organization intended to help those suffering from AUD to achieve abstinence from alcohol, improve relationships with others and increase quality of life (Alcoholics Anonymous, 2001). In North America, AA is the most commonly sought source of help for AUD (Caetano et al., 1998; Room et al., 2006; Hedden et al., 2015) attracting a diverse membership of women and men from a wide range of racial and ethnic backgrounds (Jilek-Aall, 1981; Hoffman, 2009; Office of the Surgeon General, 2018). It has a significant presence in the UK (Humphreys et al., 1994; Whelan et al., 2009). Today, A.A. states that it is present in some 180 nations with membership estimated at over 2 million. There are believed to be more than 118,000 A.A. groups around the world and its literature is reported in languages as diverse as Afrikaans, Arabic, Hindi, Nepali, Persian, Swahili and Vietnamese, among many others. AA typically operates in local rented accommodation (e.g. churches/synagogues, hospitals, community centers and colleges) with meetings typically lasting 60–90 minutes. During meetings, members share personal narratives of their alcohol addiction and recovery experiences and help one another practice the principles encompassed in a 12-step program that is intended to help participants initiate and sustain AUD remission as well as improve psychological well-being, interpersonal skills and coping with stress (Kelly and McCrady, 2008). Given AUD is prevalent worldwide, especially in middle- and high-income countries, and is susceptible to relapse and recurrence over the long term, AA’s free and widespread availability makes it potentially helpful for a large number of people for extended periods. As such, AA is part of the de facto system of care for AUD in many nations.
The widespread adoption of AA and its influence on the professional treatment industry in some countries has spurred increasing efforts to evaluate its clinical and public health impact. In addition to peer-led AA mutual-help groups themselves, researchers have also evaluated professionally delivered clinical interventions that have adapted the methodology and concepts of AA. These ‘12-Step Facilitation (TSF)’ interventions include extended counseling, adopting some of the techniques and principles of AA and encouraging meeting attendance, as well as brief interventions designed merely to provide a warm handover to community AA groups (Humphreys, 1999). TSF interventions can be delivered clinically in individual or group format.
AA researchers have become increasingly sophisticated at finding methods to rigorously evaluate AA, including in randomized clinical trials. Reviews of this research have been conducted, including a prior Cochrane Review (Ferri et al., 2004, 2006; Kaskutas, 2009; Kelly et al., 2009), but a flurry of additional empirical investigations since these reviews were conducted signified a need for a major update. Consequently, the 2020 Cochrane review (Kelly et al., 2020) was conducted that included the most recent rigorous studies in order to provide the most up to date information about the clinical and public health utility, effectiveness and cost-effectiveness of AA and TSF. Here we summarize the methods and findings of that review and related clinical and policy implications.
METHOD
The protocol for this review is available in the Cochrane Database of Systematic Reviews (Kelly, Humphreys, et al., 2017a). The review (Kelly et al., 2020) was published in accordance with Cochrane methods and guidelines (Higgins and Green, 2019).
Eligibility criteria
We included randomized controlled trials (RCTs), quasi-experimental designs and nonrandomized studies that compared AA or TSF to 1) other interventions (e.g. motivational enhancement therapy (MET) or cognitive behavioral therapy (CBT)), 2) 12-step program variants (e.g. studies comparing different types of 12-step interventions that varied in style or intensity) or 3) no treatment (e.g. waitlist control).
Quasi-experimental studies were ones wherein parallel, simultaneous randomization was not possible due to potential contamination of intervention effects within single sites and that instead used sequential designs (e.g. block randomization or an ‘on/off’ design wherein the intervention was implemented, then not implemented and then reimplemented). Nonrandomized studies used prospective, parallel group designs with intact intervention groups. We also included economic studies that examined formal healthcare cost offsets due to the potential healthcare cost savings of people using freely available AA.
Participants were male and female adults (18 years or older) with AUD, alcohol dependence or alcohol abuse as defined by standardized diagnostic criteria (e.g. the Diagnostic and Statistical Manual of Mental Disorders, 4th and 5th editions (American Psychiatric Association, 1994, 2013). Studies were excluded if participants were coerced to attend AA meetings (e.g. by their employer, court order, etc.).
The primary outcomes were measured through self-report and confirmed via bioassay (when available and appropriate) and included abstinence, measured as the proportion of individuals continuously abstinent, PDA and longest period of abstinence (LPA); drinking intensity, measured as drinks consumed per drinking day (DDD), percentage of days heavy drinking (PDHD) or grams of pure alcohol consumed; alcohol-related consequences, measured as physical, social and psychological sequalae resulting from alcohol (e.g. Short Inventory of Problems (SIP; (Miller et al., 1995), Drinker Inventory of Problems (DRINC; (Miller et al., 1995) etc.); alcohol addiction severity, measured by the Addiction Severity Index (ASI; McLellan et al., 1980) or similar measures. Secondary outcomes included healthcare cost offsets (e.g. mental health-related service utilization, related monetary impacts) assessed by inspection of health care utilization databases.
Study selection
We conducted searches across six bibliographic databases (i.e. Cochrane Drugs and Alcohol Group Specialized Register (CDAG), Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO, Embase Ovid, MEDLINE PubMed and PsycINFO EBSCO) from inception through August 2019 and two trial registries (i.e. WHO International Clinical Trials Registry Platform, ClinicalTrials.gov) from inception through November 2018. We also identified potentially eligible studies through hand-searching (e.g. searching the reference lists of retrieved studies). We placed no restrictions on language or publication year. Two review authors independently scanned the abstract, title or both of every record to determine which studies should be considered for inclusion. Full-text articles were independently evaluated for inclusion and discrepancies resolved through discussion with the third author. Study data (e.g. study design, sample characteristics, study outcomes, etc.) were extracted from included studies by two authors who used a standardized data extraction form. Original study authors were contacted for clarification or additional study details when necessary.
Studies were evaluated using the Cochrane Risk of Bias criteria (Higgins and Green, 2019). Economic studies were evaluated separately for quality using the Evers checklist for economic evaluations (Evers et al., 2005).
Data synthesis
We calculated the relative risk (i.e. risk ratios (RRs)) for dichotomous variables (e.g. proportion of patients completely abstinent) and standardized mean differences (SMDs) for continuous variables (e.g. PDA) using 95% confidence intervals (CIs) to express the uncertainty of the estimate. We used random-effects estimates in RevMan 5.3 (The Cochrane Collaboration, 2014) to account for potential heterogeneity among study interventions. Wherever possible, we pooled and analyzed study effects using random-effects meta-analyses that included statistical estimation of the degree of heterogeneity that was calculated using the Q value and I2 statistic. In cases where pooling was not possible, we described study results in the narrative.
Due to the fact that there is currently no consensus on the proper method for pooling estimates of cost-effectiveness studies (Shemilt et al., 2011; Higgins and Green, 2019), we summarized results from cost-effectiveness studies in the narrative.
To prevent unit of analysis errors (i.e. double counting) that could inflate statistical significance, in cases where one intervention group was compared to two or more comparison groups, we split the intervention group sample in the meta-analysis in accordance with the Cochrane Handbook guidelines (Higgins and Green, 2019).
We conducted sensitivity analyses across three domains: study design (e.g. RCT/quasi-RCT, nonrandomized), degree of manualization (e.g. all study conditions manualized, one or more conditions nonmanualized) and type of comparison intervention (i.e. an intervention based a different theory, such as CBT, or on an intervention based on a TSF-orientation but that varied in TSF style of intensity). We used the GRADE rating system (Schunemann et al., 2013; GRADE Working Group, 2015) to assess the overall certainty of evidence in accordance with Cochrane procedures.
RESULTS
Characteristics of included studies
We included 27 primary studies containing 10,565 participants (see Fig. 1). Results were reported across 36 included published articles (see Table 1). Twenty-one of the 27 studies were RCTs/quasi-experiments; five were nonrandomized and one was purely economic. Twenty-six of these primary studies contributed to the estimate of the effect of AA/TSF and one was used purely for economic purposes (Mundt et al., 2012) and therefore did not contribute to the estimate of the effect of AA/TSF as it did not include a comparison condition. Three of the studies contributing to the estimate of the effectiveness of AA/TSF also included economic studies, for a total of four included economic studies reported across five papers. Most studies were conducted in the USA, with the exception of three conducted in Norway (1 study, Vederhus et al., 2014), the UK (1 study, Manning et al., 2012) and Canada (1 study, Brown et al., 2002). None of the studies included a waitlist control; thus, AA/TSF was always compared to some kind of active treatment. About 13 studies included a bioassay (e.g. breathalyzer, saliva, urinalysis and blood); 13 did not. Due to the time during which data collection was conducted for the included studies, newer bioassays such as phosphatidylethanol (PetH) and ethyl glucuronide (EtG) were not available.
Fig. 1.
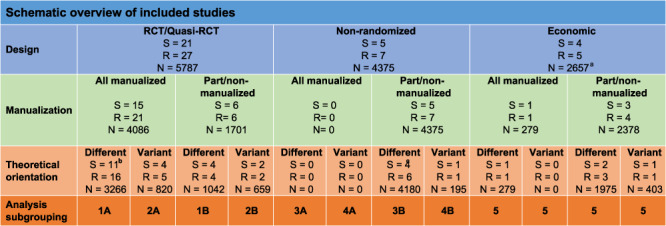
Schematic overview of included studies.
Table 1.
List of included studies
| List of included studies | |
|---|---|
| Blondell 2001 | Blondell RD, Looney SW, Northington AP et al. (2001) Using recovering alcoholics to help hospitalized patients with alcohol problems. J Fam Pract 50: 1–15. |
| Blondell 2011 | Blondell RD, Frydrych LM, Jaanimaqi U et al. (2011) A randomized trial of two behavioral interventions to improve outcomes following inpatient detoxification for alcohol dependence. J Addict Dis 2011 30: 136–48. doi: 10.1080/10550887.2011.554777 |
| Bogenschutz 2014 | Bogenschutz MP, Rice SL, Tonigan JS et al. (2014) 12-step facilitation for the dually diagnosed: A randomized clinical trial. J Subst Abuse Treat 46: 403–111. doi: 10.1016/j.jsat.2013.12.009 |
| Bowen 2014 | Bowen S, Witkiewitz K, Clifasefi SL et al. (2014) Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: A randomized clinical trial. JAMA Psychiatry 71: 547–556. doi: 10.1001/jamapsychiatry.2013.4546 |
| Brooks 2003 | Brooks AJ, Penn PE (2003) Comparing treatments for dual diagnosis: Twelve-step and self-management and recovery training. Am J Drug Alcohol Abuse 29:359–83. doi: 10.1081/ADA-120020519 |
| Brown 2002 | Brown TG, Seraganian P, Tremblay J. et al. (2002) Process and outcome changes with relapse prevention versus 12-step aftercare programs for substance abusers. Addiction 97:677–89. |
| Davis 2002 | Davis WT, Campbell L, Tax J. et al. (2002) A trial of "standard" outpatient alcoholism treatment vs. a minimal treatment control. J Subst Abuse Treat 23:9–19. doi: 10.1016/S0740-5472(02)00227-1 |
| Grant 2018 | Grant KM, Young LB, Tyler KA. et al. (2018) Intensive referral to mutual-help groups: a field trial of adaptations for rural veterans. Patient Educ Couns 101:79–84. doi: 10.1016/j.pec.2017.07.012 |
| Herman 2000 | Herman SE, Frank KA, Mowbray CT. et al. (2000) Longitudinal effects of integrated treatment on alcohol use for persons with serious mental illness and substance use disorders. J Behav Health Serv Res 27:286–302. doi: https://doi.org/10.1007/BF02291740 |
| Humphreys 1996 | Humphreys K, Moos RH. (1996) Reduced substance-abuse-related health care costs among voluntary participants in alcoholics anonymous. Psychiatr Serv 47:709–13. doi: 10.1176/ps.47.7.709 |
| Kahler 2004 | Kahler CW, Read JP, Ramsey SE. et al. (2004) Motivational enhancement for 12-step involvement among patients undergoing alcohol detoxification. J Consult Clin Psychol 72:736–41. doi: 10.1037/0022-006X.72.4.736 |
| Kaskutas, 2009 | Kaskutas LA, Subbaraman MS, Witbrodt J. et al. (2009) Effectiveness of making alcoholics anonymous easier: a group format 12-step facilitation approach. J Subst Abuse Treat 37:228–39. doi: 10.1016/j.jsat.2009.01.004 |
| Kelly 2017b | Kelly JF, Kaminer Y, Kahler CW. et al. (2017) A pilot randomized clinical trial testing integrated 12-step facilitation (iTSF) treatment for adolescent substance use disorder. Addiction 112:2155–66. doi: 10.1111/add.13920 |
| Litt 2007 | *Litt MD, Kadden RM, Kabela-Cormier E. et al. (2007) Changing network support for drinking: initial findings from the network support project. J Consult Clin Psychol 75:542–55. doi: 10.1037/0022-006X.75.4.542 |
| Litt MD, Kadden RM, Kabela-Cormier E. et al. (2009) Changing network support for drinking: network support project 2-year follow-up. J Consult Clin Psychol 77:229–42. doi: 10.1037/a0015252 | |
| Litt 2016 | Litt MD, Kadden RM, Tennen H. et al. (2016) Network support II: randomized controlled trial of network support treatment and cognitive behavioral therapy for alcohol use disorder. Drug Alcohol Depend 165:203–12. doi: 10.1016/j.drugalcdep.2016.06.010 |
| Lydecker 2010 | Lydecker KP, Tate SR, Cummins KM. et al. (2010) Clinical outcomes of an integrated treatment for depression and substance use disorders. Psychol Addict Behav 24:453–65. doi: 10.1037/a0019943 |
| Manning 2012 | Manning V, Best D, Faulkner N. et al. (2012) Does active referral by a doctor or 12-step peer improve 12-step meeting attendance? Results from a pilot randomised control trial. Drug Alcohol Depend 126:131–7. doi: 10.1016/j.drugalcdep.2012.05.004 |
| MATCH 1997 | Holder HD, Cisler RA, Longabaugh R. et al. (2000) Alcoholism treatment and medical care costs from project MATCH. Addiction 95:999–1013. doi: 10.1046/j.1360–0443.2000.9579993.x |
| Longabaugh R, Wirtz PW, Zweben A. et al. (1998) Network support for drinking, alcoholics anonymous and long-term matching effects. Addiction 93:1313–33. doi: 10.1046/j.1360–0443.1998.93913133.x | |
| *Project MATCH Research Group. (1997) Matching alcoholism treatments to client heterogeneity: project MATCH posttreatment drinking outcomes. J Stud Alcohol 58:7–29. | |
| Project MATCH Research Group. (1998a) Matching alcoholism treatments to client heterogeneity: project MATCH three-year drinking outcomes. Alcohol Clin Exp Res 22:1300–11. doi: 10.1111/j.1530–0277.1998.tb03912.x | |
| Project MATCH Research Group. (1998b) Matching alcoholism treatments to client heterogeneity: treatment main effects and matching effects on drinking during treatment. Project MATCH research group. J Stud Alcohol 59:631–9. doi: 10.15288/jsa.1998.59.631 | |
| McCrady 1996 | *Mccrady BS, Epstein EE, Hirsch LS. (1996) Issues in the implementation of a randomized clinical trial that includes alcoholics anonymous: studying AA-related behaviors during treatment. J Stud Alcohol 57:604–12. doi: 10.15288/jsa.1996.57.604 |
| Mccrady BS, Epstein EE, Hirsch LS. (1999) Maintaining change after conjoint behavioral alcohol treatment for men: outcomes at 6 months. Addiction 94:1381–96. doi: 10.1046/j.1360–0443.1999.949138110.x | |
| McCrady BS, Epstein EE, Kahler CW. (2004) Alcoholics Anonymous and relapse prevention as maintenance strategies after conjoint behavioral alcohol treatment for men: 18-Month outcomes. J Consult Clin Psychol 72: 870–8. doi: 10.1037/0022-006X.72.5.870 | |
| Mundt 2012 | Mundt MP, Parthasarathy S, Chi FW. et al. (2012) 12-step participation reduces medical use costs among adolescents with a history of alcohol and other drug treatment. Drug Alcohol Depend 126:124–30. doi: 10.1016/j.drugalcdep.2012.05.002 |
| Oumette 1997 | Humphreys K, Moos R. (2001) Can encouraging substance abuse patients to participate in self-help groups reduce demand for health care? A quasi-experimental study. Alcohol Clin Exp Res 25:711–6. |
| Humphreys K, Moos RH. (2007) Encouraging posttreatment self-help group involvement to reduce demand for continuing care services: two-year clinical and utilization outcomes. Alcohol Clin Exp Res 31:64–8. doi: 10.1111/j.1530-0277.2006.00273.x | |
| *Ouimette PC, Finney JW, Moos RH. (1997) Twelve-step and cognitive–behavioral treatment for substance abuse: a comparison of treatment effectiveness. J Consult Clin Psychol 65:230–40. doi: 10.1037//0022-006X.65.2.230 | |
| Timko 2006 | Timko C, DeBenedetti A. (2007) A randomized controlled trial of intensive referral to 12-step self-help groups: One-year outcomes. Drug Alcohol Depend 90: 270–9. |
| *Timko C, Debenedetti A, Billow R. (2006) Intensive referral to 12-step self-help groups and 6-month substance use disorder outcomes. Addiction 101:678–88. doi: 10.1111/j.1360–0443.2006.01391.x | |
| Timko 2011 | Timko C, Sutkowi A, Cronkite RC. et al. (2011) Intensive referral to 12-step dual-focused mutual-help groups. Drug Alcohol Depend 118:194–201. doi: 10.1016/j.drugalcdep.2011.03.019 |
| Vederhus 2014 | Vederhus JK, Timko C, Kristensen O. et al. (2014) Motivational intervention to enhance post-detoxification 12-step group affiliation: a randomized controlled trial. Addiction 109:766–73. doi: 10.1111/add.12471 |
| Walitzer 2009 | Walitzer KS, Dermen KH, Barrick C (2009) Facilitating involvement in alcoholics anonymous during out-patient treatment: a randomized clinical trial. Addiction 104:391–401. doi: 10.1111/j.1360-0443.2008.02467.x |
| Waliterzer 2015 | Walitzer KS, Deffenbacher JL, Shyhalla K. (2015) Alcohol-adapted anger management treatment: A randomized controlled trial of an innovative therapy for alcohol dependence. J Subst Abuse Treat 59: 83–93. doi: 10.1016/j.jsat.2015.08.003 |
| Zemore 2018 | Zemore SE, Lui C, Mericle A et al. (2018) A longitudinal study of the comparative efficacy of Women for Sobriety, LifeRing, SMART Recovery, and 12-step groups for those with AUD. J Subst Abuse Treat 88: 18–26. doi: 10.1016/j.jsat.2018.02.004 |
Note: In cases where there are multiple papers published from the same study, the primary study is indicated with an asterisk (*).
Across the 27 included studies, the proportion of female participants ranged from 0% (McCrady et al., 1996; Ouimette et al., 1997) to 49.1% (Humphreys and Moos, 1996); the average sample age ranged from 34.2 years old (Brooks and Penn, 2003) to 51.0 (Timko et al., 2011) years old, and the racial composition ranged from 7.3% racial and ethnic minority participants (Litt et al., 2016) to 76.9% (Herman et al., 2000).
Critical appraisal of included studies
The main sources of bias among studies were selection bias and attrition bias. Eleven of the 27 studies were rated as potentially high risk for selection bias (random sequence generation and allocation concealment) because they either used alternation as a nonrandom component in the sequence generation process (6 studies) or were nonrandomized (5 studies). Attrition bias was unclear in approximately half (14) of the studies, high in 9 studies (largely due to moderate (≥20%) attrition rates) and low in the remaining studies. Performance bias (blinding of participants and personnel) was most often high (e.g. if the clinical context precluded participant or personnel blinding) or unclear. Risk of bias arising from the remaining domains (i.e. reporting bias, comparability of cohorts for baseline characteristics and outcome measures, selection of the nonexposed cohort, protection against contamination, detection bias and blinding of outcome assessment) was predominately low or unclear.
Effects of interventions
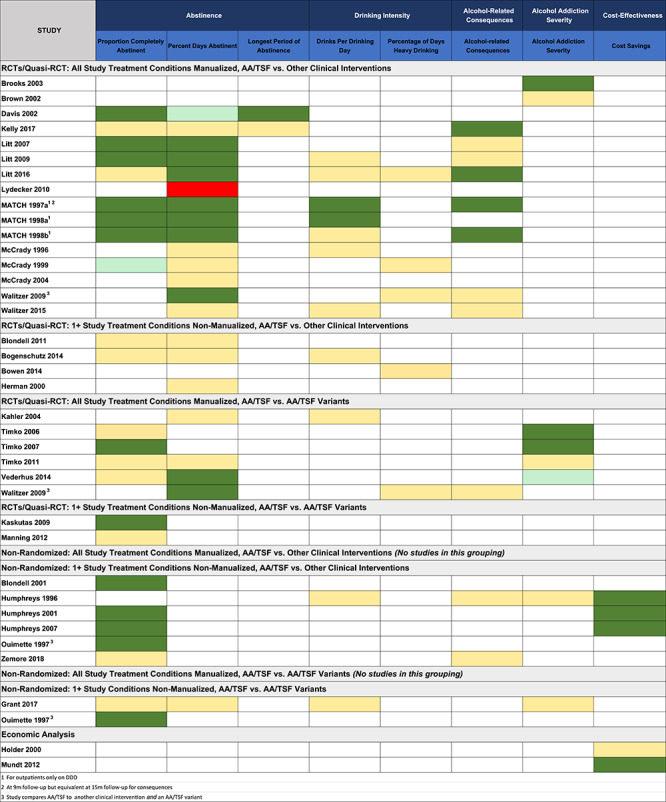
As summarized in Table 2, studies were grouped across the three dimensions noted above (i.e. study design, degree of manualization and type of comparison intervention), creating the nine subgroupings shown. Table 2 shows the number of studies, number of reports and sample sizes within each subcategory of these nine groupings (i.e. 1A—5 in the bottom row of Fig. 1). Table 2 shows the results from these different subgroupings using color coding to enhance easier appraisal of the findings with dark green signifying a significant advantage, pale green signifying a significant trend, yellow signifying similar effectiveness and red signifying a significant disadvantage, for AA/TSF, relative to other active treatments on each given treatment outcome listed across the columns.
Table 2.
Overview of Results
|
|
Key: dark green = statistically significant advantage favoring AA/TSF; light green = statistically significant trend favoring AA/TSF; yellow = no statistically significant difference between AA/TSF and comparison condition; red = statistically significant advantage favoring non-AA/TSF condition.
Overall results summary by outcome domain
Table 2 shows that most studies reported on the proportion of patients completely abstinent or PDA with fewer studies reporting DDD or PDHD, alcohol consequences or addiction severity and only two reporting the longest period of abstinence. Four studies reported economic analyses.
ABSTINENCE
Proportion of patients continuously abstinent
Among the most rigorous studies that employed an RCT/quasi-RCT design with all treatments manualized and AA/TSF compared to a treatment with a different theoretical orientation (e.g. CBT) (n studies = 6; articles = 9), no difference was found for AA/TSF at the end of treatment (RR = 1.07, 95% CI: 0.92–1.25, P = 0.37), but there were advantages for AA/TSF at all of the other follow-up time points (i.e. 6, 12, 24 and 36 months) ranging from a low RR of 1.21 (95% CI: 1.03–1.42, P = 0.02) at 12-month follow-up to a high RR of 1.66 (95% CI: 1.09–2.54, P = 0.02; n = 238) at 6-month follow-up (Project MATCH Research Group, 1997, 1998a, 1998b; McCrady et al., 1999; Davis et al., 2002; Litt et al., 2007, 2009, 2016; Kelly, Kaminer, et al., 2017).
Among studies that employed an RCT/quasi-RCT design where at least one comparison treatment was nonmanualized and AA/TSF was compared to a different type of TSF (i.e. varying in TSF style or intensity), Kaskutas et al. (2009) found that the more intensive AA/TSF intervention had a higher proportion of participants abstinent at the 12-month follow-up compared to the less intensive AA/TSF intervention (RR = 1.15, 95% CI: 1.02–1.29; P = 0.02).
Among studies that employed a nonrandomized design with at least one nonmanualized treatment condition and where AA/TSF was compared to a different theoretical treatment orientation (e.g. CBT), all studies favored the AA/TSF intervention at all three follow-up times: 6 months (RR = 1.50, 95% CI: 1.16–1.92; P = 0.002), 12 months (RR = 1.25, 95% CI: 1.09–1.43; P = 0.002) and 24 months (RR = 1.34, 95% CI: 1.20–1.49; P < 0.001).
For studies that employed a nonrandomized design with at least one nonmanualized treatment condition and where AA/TSF was compared to a different TSF variant (i.e. in varying in TSF style or intensity) (n studies = 2), at a 6-month follow-up Grant (Grant et al., 2018) found no difference (RR = 1.01, 95% CI: 0.86–1.19; P = 0.90), and at the 12-month follow-up Ouimette (Ouimette et al., 1997) found an advantage for the more intensive 12-step treatment (RR = 1.24, 95% CI: 1.05–1.46; P = 0.01).
Percent days abstinent
Among the most rigorous evaluation studies that employed an RCT/quasi-RCT design with all study treatments manualized and in which AA/TSF was compared to a treatment with a different theoretical orientation (e.g. CBT), nine assessed this outcome (McCrady et al., 1996; Project MATCH Research Group, 1997; Davis et al., 2002; Litt et al., 2007, 2016; Walitzer et al., 2009, 2015; Lydecker et al., 2010; Kelly, Kaminer, et al., 2017). AA/TSF showed a small to moderate advantage for this outcome, but only at the 24-month (mean difference (MD) = 12.91, 95% CI: 7.55–18.27; P < 0.001; 2 studies, 302 participants) and 36-month (MD = 6.64, 95% CI: 1.54–11.75; P = 0.01) time points. There was no difference between AA/TSF and comparisons at other time points.
For RCTs in which at least one comparison treatment was nonmanualized and AA/TSF was compared to a treatment with a different theoretical orientation (e.g. CBT), three studies reported this outcome (Herman et al., 2000; Blondell et al., 2011; Bogenschutz et al., 2014), with two observing no difference. At the 9-month follow-up, untransformed data from Bogenschutz et al. (2014) showed a higher PDA for AA/TSF than the comparison (MD = 3.00, 95% CI: 0.31–5.69; P = 0.03). Using a log-transformed days of drinking variable, Herman (Herman et al., 2000) found a slight advantage at 2 months post-treatment (P = 0.03) that favored AA/TSF, but between 2 and 18 months, there was no difference between groups (P = 0.05). The treatment by time interaction was not reported.
Among studies that employed an RCT/quasi-RCT design with all study treatments manualized and in which AA/TSF was compared to a different type of TSF (i.e. varying in TSF style or intensity), two studies reported data for PDA (Kahler et al., 2004; Walitzer et al., 2009). Kahler (Kahler et al., 2004) found no difference, but Walitzer (Walitzer et al., 2009) found an advantage for AA/TSF compared to the comparison at 12-month follow-up (MD = 16.40, 95% CI: 5.12–27.68; P = 0.004; 1 study, 95 participants). Two studies reported on days of alcohol use at the 6-month follow-up (Timko et al., 2011; Vederhus et al., 2014) and found no difference between the more and less intensive AA/TSF interventions studied.
Other outcomes
As shown in Table 2, other outcomes were comparatively rarely reported across included studies. In general, among the most rigorous studies (1A grouping), there were some slight advantages of AA/TSF on alcohol-related consequences and addiction severity; otherwise, AA/TSF performed equally well as comparison treatments.
ECONOMIC ANALYSES
Other noteworthy findings from the review were on economic analyses. Using a 19-item Cochrane-recommended checklist to assess study quality (Evers et al., 2005), studies were judged to be high quality.
Four studies (1 RCT/quasi-RCT, 3 nonrandomized; n = 2,657; n reports = 5; (Humphreys and Moos, 1996, 2001, 2007; Holder et al., 2000; Mundt et al., 2012) assessed AA/TSF cost offsets.
Three studies (4 out of 5 reports; (Humphreys and Moos, 1996, 2001, 2007; Ouimette et al., 1997) found a healthcare cost saving in favor of AA/TSF. Humphreys and Moos (1996) across a 3-year follow-up found AA participants (who were more clinically severe) had alcohol-related outcomes similar to outpatients, yet the alcohol-related health care costs for the AA group were 45% lower (USD 2856, in year-2018-dollars). Humphreys and Moos (2001) found that compared with inpatients in AA/TSF programs, those initially treated in CBT programs had 64% higher annual healthcare costs (USD 7128, in year-2018-dollars; P = 0.001). Psychiatric and substance use outcomes were comparable across treatments, except that AA/TSF participants had higher abstinence rates (45.7% AA/TSF versus 36.2% CBT; P = 0.001). In an analysis of two-year outcomes and healthcare costs, participants treated in AA/TSF programs had 30% lower costs compared with CBT (USD 3678 lower, in year-2018-dollars; P = 0.01).
Mundt et al. (2012) found that for each additional 12-step meeting attended, there was a medical cost reduction of 4.7% during the 7-year follow-up with cost savings of USD 145 for each meeting attended per year (USD 180 in year-2018-dollars). In the MATCH 1997 (Project MATCH Research Group, 1997) study, Holder (Holder et al., 2000) identified medical cost savings during treatment and across follow-up with a significant interaction observing that better cost savings were associated with CBT or TSF, or both, than with MET for participants with poor prognostic characteristics (greater alcohol addiction severity, psychiatric severity and/or social network favoring alcohol use).
DISCUSSION
Despite AA’s international popularity, there had been confusion about its clinical and public health utility and whether it can be subjected to the rigorous evaluation. Addressing this gap in knowledge was the rationale for our 2020 Cochrane review. In sum, we found that AA/TSF was better than other well-established treatments in facilitating continuous abstinence and remission and was at least as effective as other well established treatments in reducing intensity of drinking, alcohol-related consequences and severity of alcohol addiction. AA/TSF also reduced healthcare costs substantially more than other types of treatments.
Given how challenging it is to find differences in clinical outcomes among active psychosocial treatments, the magnitude of the advantage favoring AA/TSF interventions for continuous abstinence was impressive. For instance, in MATCH (Project MATCH Research Group, 1997), the proportion of participants continuously abstinent throughout the first year following treatment among outpatients who were assigned to the AA/TSF intervention was 24%, whereas only 15 and 14% of participants assigned to CBT and MET, respectively, were abstinent during that timeframe. This reflects an absolute advantage of 9% points in favor of AA/TSF, which translates to a relative advantage for AA/TSF compared with CBT of 60% in the number of participants completely abstinent, and a relative advantage of 64%, when compared with MET. This pattern of relative advantage for AA/TSF interventions appeared quite consistent across both RCTs/quasi-experimental and nonrandomized studies. Enhancing rates of continuous abstinence and remission by 60% above what many clinicians might consider to be the current ‘state-of-the art’ intervention (i.e. CBT) are noteworthy, especially given the lethality of AUD. If we were talking about improving remission rates by this degree among a lethal health condition like as cancer, such an improvement in outcome would generate jubilation. Given also that AA/TSF produces these clinical benefits at a greatly reduced health care cost, there may be cause for even greater celebration. Furthermore, because many participants in these studies assigned to non-TSF interventions (e.g. CBT, MET) still elected to attend AA (participation in which is correlated with better outcomes), the positive effects of AA/TSF, where observed, are likely to be conservative.
Although the magnitude was not as large, the average percentage of days on which participants were abstinent (PDA) tended to show an advantage in favor of AA/TSF interventions, especially in the more rigorous manualized RCTs compared to other active treatment orientations (e.g. CBT). Studies involving young people (Kelly, Kaminer, et al., 2017) and couples therapy (McCrady et al., 1996) showed equivalence, but not advantages, for PDA. One study with dual diagnosis participants in the US Veterans Administration healthcare system (Lydecker et al., 2010) found a disadvantage for PDA with AA/TSF. This may be because, although participants met criteria for AUD, the primary problem was mood disorder as opposed to AUD, which may represent a poorer fit with AA (Kelly et al., 2003). That said, a recent meta-analysis by Tonigan (Tonigan et al., 2018) found consistent abstinence benefits from participation in AA by those dually diagnosed. Thus, it is currently unclear why the Lydecker et al. (2010) study did not find benefits, but it may relate to having mood disorder as the major problem (with secondary AUD) versus having AUD as the primary reason for treatment (with a secondary mood disorder). More work is needed to clarify this.
For measures of intensity of drinking, AA/TSF most often performed as well as—and in no case fared worse than—comparison interventions. This is perhaps surprising given that the primary focus of AA/TSF interventions is on complete abstinence, rather than reductions in intensity, which may be a focus in CBT-oriented relapse prevention. Thus, these findings do not support the once-popular theory that by emphasizing the uncontrollability of alcohol consumption (i.e. ‘powerlessness’ over alcohol), AA creates an ‘abstinence violation effect’ that makes the relapses more severe (Marlatt and Donovan, 1985).
When different types of TSF interventions were tested against each other, certain structured TSF interventions (e.g. those that actively prescribed use of AA; monitored attendance; provided personal linkages to existing members) often worked better at improving outcomes than less structured ‘treatment as usual (TAU) TSF’ interventions (e.g. Timko et al., 2006; Kaskutas et al., 2009). For example, in terms of reductions in alcohol-related consequences and alcohol addiction severity, AA/TSF most often did as well as comparison treatments, but in three out of four of the TSF variant studies, there were advantages for the more structured and intensive AA/TSF procedures (Brown et al., 2002; Timko et al., 2006; Vederhus et al., 2014), suggesting that certain specific AA/TSF strategies and techniques may produce better results for these outcomes than others. Thus, although many treatment programs may believe that they ‘already do 12-step’, the effectiveness of those existing methods might potentially be enhanced by implementing more structured and manualized interventions. Some of these strategies could be clinical linkage to existing members using a ‘warm hand-off’ as found by Timko et al. (2006) and Manning et al. (2012), or by being more prescriptive, pro-actively recommending and encouraging attendance (e.g. ‘I’d like you attend three AA meetings this week’) versus leaving it to patients to decide for themselves (e.g. ‘What do you think about going to AA this week?’) whether they want to attend AA (e.g. Walitzer et al., 2009). The UK National Health Service, which has recently been emphasizing the value of ‘social prescribing’ would be an ideal venue to broadly implement these strategies.
The economic analyses found benefits in favor of AA/TSF compared to outpatient treatment and CBT interventions. The magnitude of these benefits was sizeable. For example, the analysis by Ouimette et al. (1997) and Humphreys and Moos (2001, 2007) showed that in addition to increasing abstinence rates, AA/TSF interventions were able to reduce the mental health and substance-related healthcare costs over the next 2 years by over USD 10,000 per patient (when 1994 figures are converted to year-2018-dollars) compared to CBT delivered in residential VA settings. More than 1 million people are treated for AUD in the USA every year, and reducing each of their healthcare costs by this amount would produce an enormous aggregate economic saving (more than USD 10 billion in the USA alone), as well as improving clinical outcomes. A clear policy lesson of these findings is that investments in expanding TSF interventions may well pay for themselves in reduced future health care costs.
In what ways does AA/TSF confer these clinical, public health and economic benefits?
The goal of TSF is to stimulate AA participation during and following treatment, but TSF itself is not AA. The theoretical causal chain that underlies TSF interventions is that AA/TSF leads to higher abstinence rates via its ability to get those with AUD more involved in AA, which is designed as a long-term recovery support service (Longabaugh et al., 1998; MATCH, 2001; Litt et al., 2007; Kelly et al., 2009; Walitzer et al., 2009; Kelly, 2017). Several studies, including two that were included in our Cochrane review, have examined this proposition using appropriate temporally lagged mediational analyses. Findings support this causal chain in which TSF leads to higher AA participation, which subsequently leads to better alcohol outcomes (Litt et al., 2007; Walitzer et al., 2009). The obvious next question is, how then does AA itself confer therapeutic benefits over time?
The original AA intervention is thought to work via its social fellowship and 12-step program (Alcoholics Anonymous, 2001). The social components operate through peer support and role modeling of successful recovery and through providing close mentoring through ‘sponsorship’ (i.e. having a recovery coach/mentor who can serve as a contact and ‘guide’, especially early in recovery). The common suffering of AA members may provide a sense of belonging or ‘universality’ that can help to diminish negative affect, particularly shame, loneliness and guilt, which is similar in principle to the dynamics of professional group psychotherapy (Yalom 2008). Furthermore, the observation of others who are sustaining recovery in AA can instill hope for a better future. AA also provides an arena for members to learn, and model, effective communication and coping skills. Rigorous reviews of the research on the mechanisms of behavior change through which AA enhances recovery have found that AA typically confers benefits by mobilizing multiple therapeutic factors simultaneously—mostly through facilitating adaptive changes in the social networks of participants, but also by boosting members’ recovery coping skills, recovery motivation, abstinence self-efficacy and psychological well-being and by reducing impulsivity and craving (Kelly et al., 2009; Kelly, 2017).
In sum, it seems plausible that AA/TSF often outperforms other treatments at much lower cost because it successfully links people to a free, ubiquitous, easily accessible, long-term recovery support option (i.e. AA) that, in turn, mobilizes other therapeutic mechanisms similar to those mobilized by professional treatment—such as increasing relapse prevention coping skills, abstinence self-efficacy, recovery motivation and reducing craving and impulsivity—and facilitates positive changes in people’s social networks (Kelly et al., 2009; Kelly, 2017).
CONCLUSIONS
Implication for practice
The evidence suggests that compared to other well-established treatments, clinical linkage using well-articulated TSF manualized interventions intended to increase AA participation during and following AUD treatment can lead to enhanced abstinence outcomes over the next months and years. Findings also indicate that AA/TSF may perform as well as other clinical interventions for drinking intensity outcomes. Economic analyses suggest that substantial healthcare cost savings can be obtained when treatment programs proactively and systematically link people with AUD to AA using TSF strategies, such as those used in the studies included in this review. Analyses indicate that the reason for this benefit is due to the ability of the AA/TSF to increase AA participation and thereby increase abstinence rates. Thus, a relatively brief clinical intervention (AA/TSF) can help people with AUD to become engaged in a long-term, freely available, community-based, recovery support resource that can help sustain ongoing remission.
If people with AUD are opposed to attending AA, despite the strong evidence for its potential to aid recovery, clinicians might consider linkage to alternative mutual-help organizations as they may confer benefits at similar levels of engagement. It is plausible, for example that other AUD recovery-supportive, mutual-help organizations, such as Self-Management and Recovery Training (SMART), LifeRing, and Women for Sobriety, may confer similar benefits (Kelly et al., 2009; Kelly and White, 2012). Although these organizations may espouse different theoretical orientations and variations in their approaches to help people attain and maintain recovery from AUD, there may be more similarity than differences in the therapeutic dynamics operating within these groups (Kelly et al., 2009; Kelly, 2017). More research is needed to support this conjecture, but such preliminary results are promising from a public health and long-term recovery management perspective because AUD tends to be highly heterogeneous in its clinical course and impact, and those suffering can often have different preferences as to the kinds of recovery pathways they wish to follow (Kelly et al., 2013).
Another critical clinical implication of the review is the large range of populations in which AA’s benefit has been demonstrated—young and old, racial and ethnic minorities, women and men, religious and nonreligious, people in different settings and indeed different nations. There is no case for concluding on the basis of a patients’ demographic characteristics that they should not give AA a try (Humphreys et al., 1994).
The results also indicate that clinicians who have prejudged AA should give it another look. In a study of NHS workers, Day and colleagues (Day et al., 2005) found that clinicians were highly confident they understand what happens at AA meetings but had never actually visited one. To the extent that such attitudes emerge from a perception that AA is ineffective, we hope the Cochrane Review will prompt a re-evaluation and in turn a greater willingness to help AUD patients test out this remarkable fellowship for themselves.
Funding
J.F.K. has received funding from the US National Institutes of Health and the US Veterans Health Administration to conduct research into AUDs, comorbidities, treatment response and mechanisms of behavior change in AA and Self-Management and Recovery Training (SMART). K.H. has received funding from the US National Institutes of Health and US Veterans Health Administration to evaluate a range of treatment and mutual-help organizations focused on alcohol and other drugs. M.F. and A.A. have no disclosures or conflicts to report.
REFERENCES
- Alcoholics Anonymous (2001) Alcoholics Anonymous: The Story of How Thousands of Men and Women Have Recovered from Alcoholism. New York, NY: Alcoholics Anonymous World Services. [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders 4th Edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders 5th Edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- Blondell RD, Looney SW, Northington AP, Lasch ME, Rhodes SB, McDaniels RL (2001) Using recovering alcoholics to help hospitalized patients with alcohol problems. J Fam Pract 50:1–15. [CRSREF: 12916676]. [PubMed] [Google Scholar]
- Blondell RD, Frydrych LM, Jaanimagi U, et al. (2011) A randomized trial of two behavioral interventions to improve outcomes following inpatient detoxification for alcohol dependence. J Addict Dis 30:136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Rice SL, Tonigan JS, et al. (2014) 12-step facilitation for the dually diagnosed: a randomized clinical trial. J Subst Abuse Treat 46:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, et al. (2014) Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry 71:547–56. [CRSREF: 12916682; doi: 10.1001/jamapsychiatry.2013.4546]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AJ, Penn PE (2003) Comparing treatments for dual diagnosis: Twelve-step and self-management and recovery training. Am J Drug Alcohol Abuse 29:359–83. [DOI] [PubMed] [Google Scholar]
- Brown TG, Seraganian P, Tremblay J, et al. (2002) Process and outcome changes with relapse prevention versus 12-step aftercare programs for substance abusers. Addiction 97:677–89. [DOI] [PubMed] [Google Scholar]
- Caetano R, Clark CL, Greenfield TK (1998) Prevalence, trends, and incidence of alcohol withdrawal symptoms: Analysis of general population and clinical samples. Alcohol Health Res World 22:73–9. [PMC free article] [PubMed] [Google Scholar]
- Davis WT, Campbell L, Tax J, et al. (2002) A trial of "standard" outpatient alcoholism treatment vs. a minimal treatment control. J Subst Abuse Treat 23:9–19. [DOI] [PubMed] [Google Scholar]
- Day E, Gaston RL, Furlong E, et al. (2005) United Kingdom substance misuse treatment workers' attitudes toward 12-step self-help groups. J Subst Abuse Treat 29:321–7. [DOI] [PubMed] [Google Scholar]
- Evers S, Goossens M, De Vet H, et al. (2005) Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 21:240–5. [PubMed] [Google Scholar]
- Ferri M, Amato L, Davoli M (2004) 12-step programmes and alcoholics anonymous for alcohol dependence. Cochrane Database Syst Rev 4:CD005032. doi: 10.1002/14651858.CD005032.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri M, Amato L, Davoli M (2006) Alcoholics anonymous and other 12-step programmes for alcohol dependence. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD005032.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grade Working Group . (2015) GRADEpro GDT 3:CD005032. doi: 10.1002/14651858.CD005032.pub2. [DOI] [Google Scholar]
- Grant KM, Young LB, Tyler KA, et al. (2018) Intensive referral to mutual-help groups: a field trial of adaptations for rural veterans. Patient Educ Couns 101:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden SL, Kennet J, Lipari R. et al. 2015. Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health. In: SAMHSA (ed). www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf: SAMHSA. [Google Scholar]
- Herman SE, Frank KA, Mowbray CT, et al. (2000) Longitudinal effects of integrated treatment on alcohol use for persons with serious mental illness and substance use disorders. J Behav Health Serv Res 27:286–302. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S (eds). (2019) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0, (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org.
- Hoffman F (2009) Cultural adaptations of alcoholics anonymous to serve Hispanic populations. Int J Ad 29:445–60. [DOI] [PubMed] [Google Scholar]
- Holder HD, Cisler RA, Longabaugh R, et al. (2000) Alcoholism treatment and medical care costs from project MATCH. Addiction 95:999–1013. [DOI] [PubMed] [Google Scholar]
- Humphreys K (1999) Professional interventions that facilitate 12-step self-help group involvement. Alcohol Res Health 23:93–8. [PMC free article] [PubMed] [Google Scholar]
- Humphreys K (2004) Circles of Recovery: Self-Help Organizations for Addictions. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Humphreys K, Mavis BE, Stöffelmayr BE (1994) Are twelve step programs appropriate for disenfranchised groups? Evidence from a study of posttreatment mutual help involvement. Prev Hum Serv 11:165–79. [Google Scholar]
- Humphreys K, Moos R (2001) Can encouraging substance abuse patients to participate in self-help groups reduce demand for health care? A quasi-experimental study. Alcohol Clin Exp Res 25:711–6. [PubMed] [Google Scholar]
- Humphreys K, Moos RH (1996) Reduced substance-abuse-related health care costs among voluntary participants in alcoholics anonymous. Psychiatr Serv 47:709–13. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Moos RH (2007) Encouraging posttreatment self-help group involvement to reduce demand for continuing care services: two-year clinical and utilization outcomes. Alcohol Clin Exp Res 31:64–8. [DOI] [PubMed] [Google Scholar]
- Jilek-Aall L (1981) Acculturation, alcoholism and Indian-style alcoholics anonymous. J Stud Alcohol Suppl 9:143–58. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Read JP, Ramsey SE, et al. (2004) Motivational enhancement for 12-step involvement among patients undergoing alcohol detoxification. J Consult Clin Psychol 72:736–41. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA (2009) Alcoholics anonymous effectiveness: Faith meets science. J Addict Dis 28:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskutas LA, Subbaraman MS, Witbrodt J, et al. (2009) Effectiveness of making alcoholics anonymous easier: a group format 12-step facilitation approach. J Subst Abuse Treat 37:228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF (2017) Is alcoholics anonymous religious, spiritual, neither? Findings from 25 years of mechanisms of behavior change research. Addiction 112:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Humphreys K, Ferri M (2017a) Alcoholics anonymous and other 12-step programs for alcohol use disorder [protocol]. Cochrane Database Syst Rev 11:CD012880. doi: 10.1002/14651858.CD012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Humphreys K, Ferri M (2020) Alcoholics anonymous and other 12-step programs for alcohol use disorder. Cochrane Database Syst Rev 3:Cd012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Humphreys K, Yeterian J (2013) Mutual-help groups. In Herie M, Skinner WJW (eds). Fundamentals of Addiction: A Practical Guide for Counsellors. Toronto, ON: Centre on Addiction and Mental Health, 321–48. [Google Scholar]
- Kelly JF, Kaminer Y, Kahler CW, et al. (2017b) A pilot randomized clinical trial testing integrated 12-step facilitation (iTSF) treatment for adolescent substance use disorder. Addiction 112:2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Magill M, Stout RL (2009) How do people recover from alcohol dependence? A systematic review of the research on mechanisms of behavior change in alcoholics anonymous. Addict Res Theory 17:236–59. [Google Scholar]
- Kelly JF, Mccrady BS (2008) Twelve-step facilitation in non-specialty settings. In Kaskutas LA, Galanter M (eds). Recent Developments in Alcoholism: Research on Alcoholics Anonymous and Spirituality in Addiction Recovery. New York, NY: Springer New York, 321–46. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Mckellar JD, Moos R (2003) Major depression in patients with substance use disorders: relationship to 12-step self-help involvement and substance use outcomes. Addiction 98:499–508. [DOI] [PubMed] [Google Scholar]
- Kelly JF, White WL (2012) Broadening the base of addiction mutual help organizations. J Groups Addict Recover 7:82–101. [Google Scholar]
- Litt MD, Kadden RM, Kabela-Cormier E, et al. (2007) Changing network support for drinking: initial findings from the network support project. J Consult Clin Psychol 75:542–55. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Kabela-Cormier E, et al. (2009) Changing network support for drinking: network support project 2-year follow-up. J Consult Clin Psychol 77:229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Tennen H, et al. (2016) Network support II: randomized controlled trial of network support treatment and cognitive behavioral therapy for alcohol use disorder. Drug Alcohol Depend 165:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longabaugh R, Wirtz PW, Zweben A, et al. (1998) Network support for drinking, alcoholics anonymous and long-term matching effects. Addiction 93:1313–33. [DOI] [PubMed] [Google Scholar]
- Lydecker KP, Tate SR, Cummins KM, et al. (2010) Clinical outcomes of an integrated treatment for depression and substance use disorders. Psychol Addict Behav 24:453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning V, Best D, Faulkner N, et al. (2012) Does active referral by a doctor or 12-step peer improve 12-step meeting attendance? Results from a pilot randomised control trial. Drug Alcohol Depend 126:131–7. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Donovan DM (1985) Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors, 2nd edn. New York, NY: The Guilford Press. [Google Scholar]
- Match P (2001) Project MATCH hypotheses: results and causal chain analyses. In Longabaugh RW, Mattson PW, Me Myers JK (eds). National Institute on Alcohol Abuse and Alcoholism Project MATCH Monograph Series Volume 8. Rockville, MD: Department of Health and Human Services, Public Health Service, National Institutes of Health. [Google Scholar]
- McCrady BS, Epstein EE, Hirsch LS (1996) Issues in the implementation of a randomized clinical trial that includes alcoholics anonymous: studying AA-related behaviors during treatment. J Stud Alcohol 57:604–12. [DOI] [PubMed] [Google Scholar]
- McCrady BS, Epstein EE, Hirsch LS (1999) Maintaining change after conjoint behavioral alcohol treatment for men: outcomes at 6 months. Addiction 94:1381–96. [DOI] [PubMed] [Google Scholar]
- McCrady BS, Epstein EE, Kahler CW (2004) Alcoholics Anonymous and relapse prevention as maintenance strategies after conjoint behavioral alcohol treatment for men: 18-Month outcomes. J Consult Clin Psychol 72:870–8. [CRSREF: 12916719; doi: 10.1037/0022-006X.72.5.870]. [DOI] [PubMed] [Google Scholar]
- Mclellan AT, Luborsky L, Woody GE, et al. (1980) An improved diagnostic evaluation instrument for substance abuse patients. The addiction severity index. J Nerv Ment Dis 168:26–33. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R (1995) The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse (Test Manual). Rockville, MD: National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Mundt MP, Parthasarathy S, Chi FW, et al. (2012) 12-step participation reduces medical use costs among adolescents with a history of alcohol and other drug treatment. Drug Alcohol Depend 126:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Surgeon General (2018) Facing Addiction in America: The Surgeon General's Spotlight on Opioids. Facing Addiction in America: The Surgeon General's Spotlight on Opioids. Washington, DC: US Department of Health and Human Services. [PubMed] [Google Scholar]
- Ouimette PC, Finney JW, Moos RH (1997) Twelve-step and cognitive–behavioral treatment for substance abuse: a comparison of treatment effectiveness. J Consult Clin Psychol 65:230–40. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group (1997) Matching alcoholism treatments to client heterogeneity: project MATCH posttreatment drinking outcomes. J Stud Alcohol 58:7–29. [PubMed] [Google Scholar]
- Project MATCH Research Group (1998a) Matching alcoholism treatments to client heterogeneity: project MATCH three-year drinking outcomes. Alcohol Clin Exp Res 22:1300–11. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group (1998b) Matching alcoholism treatments to client heterogeneity: treatment main effects and matching effects on drinking during treatment. Project MATCH research group. J Stud Alcohol 59:631–9. [DOI] [PubMed] [Google Scholar]
- Room R, Makela P, Schmidt L. et al. (2006) Alcohol, Health Disparities and Development. www.robinroom.net/Alchealth.pdf (accessed 9 November 2017).
- Sacks JJ, Gonzales KR, Bouchery EE, et al. (2015) 2010 national and state costs of excessive alcohol consumption. Am J Prev Med 49:e73–9. [DOI] [PubMed] [Google Scholar]
- Schunemann H, Brozek J, Guyatt G, et al. (eds) (2013) Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach, (updated October 2013). GRADE Working Group. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
- Shemilt I, Mugford M, Byford S. et al. 2011. Chapter 15: incorporating economic evidence. In Higgins JP, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. handbook.cochrane.org: The Cochrane Collaboration. [Google Scholar]
- Stahre M, Roeber J, Kanny D, et al. (2014) Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis 11:E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cochrane Collaboration (2014) Review Manager (RevMan) [Computer program] Version 5.3. Copenhagen: The Nordic Cochrane Centre. [Google Scholar]
- Timko C, Debenedetti A, Billow R (2006) Intensive referral to 12-step self-help groups and 6-month substance use disorder outcomes. Addiction 101:678–88. [DOI] [PubMed] [Google Scholar]
- Timko C, DeBenedetti A (2007) A randomized controlled trial of intensive referral to 12-step self-help groups: Oneyear outcomes. Drug Alcohol Depend 90:270–9. [CRSREF: 12916727]. [DOI] [PubMed] [Google Scholar]
- Timko C, Sutkowi A, Cronkite RC, et al. (2011) Intensive referral to 12-step dual-focused mutual-help groups. Drug Alcohol Depend 118:194–201. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Pearson MR, Magill M, et al. (2018) AA attendance and abstinence for dually diagnosed patients: a meta-analytic review. Addiction 113:1970–81. [DOI] [PubMed] [Google Scholar]
- Vederhus JK, Timko C, Kristensen O, et al. (2014) Motivational intervention to enhance post-detoxification 12-step group affiliation: a randomized controlled trial. Addiction 109:766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitzer KS, Deffenbacher JL, Shyhalla K (2015) Alcohol-adapted anger management treatment: a randomized controlled trial of an innovative therapy for alcohol dependence. J Subst Abuse Treat 59:83–93. [CRSREF: 12916736; doi: 10.1016/j.jsat.2015.08.003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitzer KS, Dermen KH, Barrick C (2009) Facilitating involvement in alcoholics anonymous during out-patient treatment: a randomized clinical trial. Addiction 104:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan PJ, Marshall EJ, Ball DM, et al. (2009) The role of AA sponsors: a pilot study. Alcohol Alcohol 44:416–22. [DOI] [PubMed] [Google Scholar]
- White WL, Kelly JF, Roth JD (2012) New addiction-recovery support institutions: mobilizing support beyond professional addiction treatment and recovery mutual aid. J Groups Addict Recover 7:297–317. [Google Scholar]
- World Health Organization. 2014. Global Status Report on Alcohol and Health. In World Health Organization (ed). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Yalom ID, Leszcz M (2008) The Theory and Practice of Group Psychotherapy. 5th edition. New York (NY): Basic Books. [Google Scholar]
- Zemore SE, Lui C, Mericle A, Hemberg J, Kaskutas LA (2018) A longitudinal study of the comparative efficacy of Women for Sobriety, LifeRing, SMART Recovery, and 12-step groups for those with AUD. J Subst Abuse Treat 88:18–26. [CRSREF: 12916738; doi: 10.1016/j.jsat.2018.02.004]. [DOI] [PMC free article] [PubMed] [Google Scholar]