Abstract
Mitochondria are central regulators of cellular metabolism, most known for their role in energy production. They can be “enhanced” by physical activity (including exercise), which increases their integrity, efficiency and dynamic adaptation to stressors, in short “mitochondrial fitness”. Mitochondrial fitness is closely associated with cardiorespiratory fitness and physical activity. Given the importance of mitochondria in immune functions, it is thus not surprising that cardiorespiratory fitness is also an integral determinant of the antiviral host defense and vulnerability to infection.
Here, we first briefly review the role of physical activity in viral infections. We then summarize mitochondrial functions that are relevant for the antiviral immune response with a particular focus on the current Coronavirus Disease (COVID-19) pandemic and on innate immune function. Finally, the modulation of mitochondrial and cardiorespiratory fitness by physical activity, aging and the chronic diseases that represent the most common comorbidities of COVID-19 is discussed.
We conclude that a high mitochondrial - and related cardiorespiratory - fitness should be considered as protective factors for viral infections, including COVID-19. This assumption is corroborated by reduced mitochondrial fitness in many established risk factors of COVID-19, like age, various chronic diseases or obesity. We argue for regular analysis of the cardiorespiratory fitness of COVID-19 patients and the promotion of physical activity – with all its associated health benefits – as preventive measures against viral infection.
Keywords: Physical activity, Exercise, Cardiorespiratory fitness, Mitochondria, COVID, Virus, Immune system
Highlights
-
-
Major COVID-19 risk factors are associated with reduced mitochondrial fitness.
-
-
SARS-coronavirus-2 infection is associated with various mitochondrial dysfunctions, also impairing the innate immune system.
-
-
Physical activity enhances immune functions, mitochondrial fitness and cardiorespiratory fitness.
-
-
Mitochondrial fitness may be an underlying molecular link between established risk factors for COVID-19.
-
-
Increasing mitochondrial and cardiorespiratory fitness, e.g. by appropriate physical activity, consequently may be a powerful preventive measure against viral infection, in particular by SARS-coronavirus-2.
1. Introduction
Controlling the current Coronavirus Disease (COVID-19) pandemic requires the implementation of physical isolation strategies including confinement approaches that potentially lead to reduced physical activity (PA, including exercise, defined as planned, structured, repeated and goal-directed PA [1]) levels with a variety of health consequences [[2], [3], [4]]. Recent calls for a more nuanced and integrated consideration of COVID-19 as a pandemic highlight its impact on a broad array of public health factors, and the importance of targeting these factors beyond “simply controlling epidemic disease or treating individual patients” [5]. This conceptualization includes the need to address also all associated comorbidities, such as hypertension, diabetes, obesity, cardiovascular or respiratory diseases. This should be an opportunity for policy makers to advertise the importance of regular PA [6,7]. It is well established that PA levels are negatively correlated with a number of morbidities and mortality from all causes in both the general population and athletes [8,9]. Several overviews with specific regard to general health benefits of maintained PA and metabolic health even in periods of confinement like during COVID-19 have recently been provided [4,[10], [11], [12]]. Regular PA increases cardiorespiratory fitness or aerobic power (and mitochondrial fitness [13]) in a dose-dependent manner [14], which is thought to mediate the resulting health benefits.
Here we discuss the reasons that render mitochondrial and cardiorespiratory fitness a potential marker for COVID-19 vulnerability and why improving them is an important preventive measure for viral infections in general. In the first section a short overview on general effects of physical exercise on immune functions is provided. We discuss, how PA modulates the function of mitochondria, integral components of eukaryotic cells that are critically involved in energy-production, oxidative stress regulation, and many other processes maintaining cellular functions. They are also importantly involved in the cellular and systemic host immune response to infections. As mitochondria can be “enhanced” (i.e. “trained”) by PA, the combination of their integrity, efficiency and dynamic adaptation to stressors, will be referred to as their “mitochondrial fitness”. Most of the available knowledge on this aspect relates to skeletal muscle tissue but it is becoming increasingly apparent that PA also boosts mitochondrial functions of many other tissues [15,16,[16], [16], [17], [18], [19], [20]]. While this review focuses primarily on the effects of PA on mitochondrial fitness associated with innate immune capacities of mainly tissue-bound cells, equally important effects of exercise - and its regulatory role on metabolism - are expected on the adaptive immune system by improving mitochondrial fitness and immunometabolism (defined as the link of metabolism and immunological state) of its specialized systemic cells. We will, however, outline important developments related to immunometabolism of the adaptive immune system and provide references to more specialized reviews.
We specifically highlight the associations of cardiorespiratory fitness with mitochondrial fitness that could explain parts of the boosted anti-viral host response of well-trained people and possibly reduced risk for viral respiratory infections like COVID-19 on the molecular level. To this end, an overview on mitochondrial functions relevant for this topic is presented. Several considerations that render high cardiorespiratory fitness a protective factor in viral infections such as in COVID-19 are outlined. Especially epidemiological risk factors, including age and chronic diseases that are characterized by reduced cardiorespiratory fitness, point to this possibility. Based on these arguments, evidence on the potential of PA to augment specific mitochondrial functions will be discussed. Despite these accepted associations, the possible role of mitochondrial and cardiorespiratory fitness as potential modulators of COVID-19 infection has been rather neglected so far.
2. PA, the immune system and mitochondria
PA is known to have the capacity to boost immune functions and can strengthen the antiviral host defense, likely due to its potential to stimulate beneficial adaptations, for example to challenges imposed on the organism by exercise [21]. This capacity, however, is heavily modulated by how the PA is performed and depends on factors like volume, frequency and intensity. Regular PA of moderate intensity (approximately 3–6 metabolic equivalents, METs) enhances the efficiency of the immune system but vigorous-intensity PA (>6 METs) may suppress it [[22], [23], [24]]. Moderate PA contributes to a better distribution of leukocytes in the organism [25] and exerts anti-inflammatory effects [26]. If performed regularly, such PA improves the function and action of components of the adaptive immune system, such as tissue macrophages, the activation of neutrophils, natural killer cells, cytotoxic T cells, immature B cells, and further also of immunoglobulins and anti-inflammatory cytokines; it may also induce the downregulation of toll-like receptor (TLR) expression on innate immune cells [27].
High cardiorespiratory fitness, which can be achieved by appropriate regular PA, is generally associated with low inflammation levels [23]. An increased release of myokines and anti-inflammatory cytokines during PA is thought to be involved in the regulation of inflammation [[28], [29]], such as IL-10, IL-37, IL-1 receptor antagonist (IL-1ra).
Accordingly, upper respiratory tract infections (URTI) are generally reduced in subjects engaging in higher amounts of PA [30] by as much as 40–50% [23]. Beneficial effects of PA directly on viral infections have also been reported in some studies. For example, higher PA levels have been shown to reduce mortality in influenza virus [31] and cardiorespiratory fitness protected from herpes virus reactivation [32]. In mice chronic exercise reduced inflammation and protected from disease severity after influenza infection [33].
Conversely, acute intensive exercise seems to exert adverse effects on viral infection; Davis and colleagues [34] studied the effect of exercise on pathological outcomes following respiratory tract infection with herpes simplex type 1 virus in mice. They found that exertion facilitated infection, reduced antiviral resistance of macrophages and increased mortality. In line with this, a large body of evidence in humans shows that prolonged bouts of high-intensity exercise correlate with elevated inflammation, reduced immune system function and increased risk of URTI, in particular after competitions [23]. Vulnerability of athletes to respiratory tract infection thus may be high [35] but certainly depends on training phase, the relative stress levels, various environmental factors, is further modulated by genetic predisposition [36,37]. Paulsen and colleagues [38] discuss in detail the effects of muscle damage on inflammatory responses in dependence of the exercise stimulus and Koelwyn and colleagues [39] vividly visualize the initial increase of inflammation via pro-inflammatory factors after muscle damage that is followed by adaptation and mitigation of inflammation.
Mitochondria are emerging as prominent factors in the regulation of the immune response [40,41] and our knowledge on mitochondrial adaptations to exercise is rapidly expanding [42]. Still, the role of mitochondrial fitness in mediating the divergent outcomes of acute/regular and moderate/intense exercise on the immune system is still poorly understood.
In an excellent recent review, Nieman and Wentz [23] locate the threshold between immunologically beneficial moderate exercise at around 60% of maximal oxygen uptake and heart rate reserve for intensity and at around 60 min for duration. In summary, while regular moderate activity below 60 min may increase immunosurveillance of specialized immune cells and reduce inflammation, high intensity exercise may increase oxidative stress, transiently reduces immune function and elevates inflammation [23].
With regard to COVID-19 - and in line with the discussed benefits of PA – PA and high cardiorespiratory fitness have been suggested to be beneficial by controlling pro-inflammatory responses and potentially by enhancing anti-viral host responses following infection [43,44]. Recently, also epidemiological support for this hypothesis has been reported [45]. In this study, the hospitalization of persons infected by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which is responsible for COVID-19, was correlated to their cardiorespiratory fitness and a clear negative association was found. Similarly, muscle strength was negatively correlated with hospitalization due to COVID-19 [46]. The immune-system enhancing and anti-inflammatory effects of PA, as discussed above, are clearly highly relevant for SARS-CoV-2 host immune defense but also for subsequent cytokine responses that may be inappropriate following SARS-CoV-2 infection [47] resulting in sepsis, a common cause of COVID-19 related death [48]. Sepsis, as a result of dysregulated host response to infection [49], has been directly linked to mitochondrial dysfunction and particularly to reduced energy levels in skeletal muscle biopsies of critically ill septic patients [50].
Here, we propose that mitochondria are crucial factors mediating the beneficial effects of cardiorespiratory fitness on the molecular level. They enable exercise-mediated systemic improvements such as of the cardiovascular system and skeletal muscle, as well as of cellular functions, such as redox control and immune function, both in target tissues of infections (innate immune responses of all cells and of resident immune cells) and in specialized systemic immune cells in blood and lymph/lymphoid organs. Mitochondria thus regulate a number of factors – beside cellular energy provision – that are important not only for cardiorespiratory fitness but also for the anti-viral host defense [51,52]. Despite the recent emergence of hypotheses suggesting a protective role of mitochondrial fitness [13,53] or mitochondrial dysfunction as a risk factor [54], potential drug target [55] and key factor in the pathogenesis in COVID-19 [56,57], no experimental evidence is available yet supporting these notions.
In order to assess the role of mitochondrial fitness in viral infections, some relevant mitochondrial functions involved in the antiviral immune defenses are summarized in the following section.
3. The intimate link of mitochondria and the anti-viral innate immune response
Mitochondria are double-membrane enclosed sub-cellular organelles best known for their role as cellular energy generators by oxidative phosphorylation in eukaryotic cells. In the course of this process mitochondria consume a great part of the organism's inspired oxygen. But mitochondria are also essentially involved in a wide array of other crucial processes for cellular function, comprising - beside ion homeostasis, redox signaling, protein and metabolite synthesis, fatty acid catabolism and the regulation of cell death and survival [58] - essential roles in the immune system and host defense to various pathogens, including viruses [52]. Mitochondria rapidly adapt to environmental stress, by changing metabolism and fuel sources, mitochondrial biogenesis, dynamics, trafficking and quality control (Fig. 1).
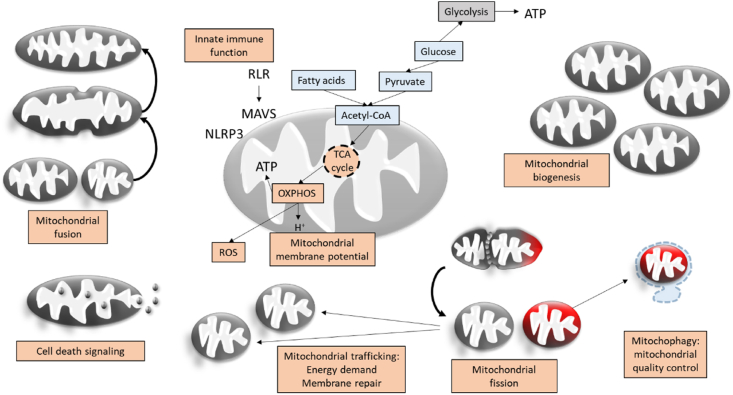
Fig. 1.
Factors of mitochondrial fitness. Nutrients such as glucose or fatty acids enable tricarboxylic acid (TCA) cycle activity and oxidative phosphorylation (OXPHOS). During OXPHOS reactive oxygen species (ROS) are produced and ROS-levels are massively increased, when the OXPHOS system is dysfunctional. Mitochondria are directly involved in the innate immune response and inflammation by RIG-1 like receptor (RLR) activated mitochondrial antiviral signaling (MAVS) system and the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome interaction. Mitochondria can change their morphology by fusion or fission with functional implications, for example on mitochondrial localization, mitochondrial quality control or bioenergetics efficiency. They increase their numbers or density by mitochondrial biogenesis and control cell death and differentiation. An important alternative pathway to OXPHOS to generate energy in the form of adenosine triphosphate (ATP) is glycolysis.
Upon viral infection, the functional, structural and substrate states of mitochondria change and determine the efficiency of the innate immune response [59,60]. As part of the innate immune response cells detect pathogens via pattern recognition receptors (PRRs) including the retinoic acid inducible gene (RIG1)-like receptors (RLR). This detection induces signaling cascades with prominent mitochondrial involvement activating interferons and pro-inflammatory cytokines with direct anti-viral activity or specialized immune cells. RLRs activate the mitochondrial anti-viral signaling complex (MAVS), an outer mitochondrial membrane protein complex that is involved in the anti-viral host defense by inducing for example the transcription of class 1 interferons [61].
As obligate parasites viruses rely on the host cell's molecular machinery and energy [62]. Viruses therefore modulate cellular physiology and metabolism in order to use them for replication but also to evade the host cell's immune response. The central role of mitochondria in anti-viral immune response also renders them a preferential target for viral modulation [63,64], although other organelles are also modulated [62].
Mitochondrial fitness comprises numerous mitochondrial functions that work together to enable an efficient anti-viral host response: the capacity of mitochondria to establish a mitochondrial membrane potential (MMP) and use oxidative phosphorylation, to regulate their numbers and quality by mitochondrial biogenesis and mitophagy, to regulate their localization and metabolic activity by trafficking and dynamics, to maintain their regular metabolic processes, ion and redox homeostasis, as well as signaling functions (including innate immune system signaling) and eventually cell death and survival (Fig. 1).
Some viruses evolved to bypass or counteract the mammalian viral anti-host defense and this seems to also be the case in COVID-19. COVID-19 is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), an enveloped, positive sense single-stranded RNA-virus, with high genetic similarity to SARS-COV-1 the responsible virus for the SARS epidemic in 2002/2003 [65]. The main entry path of SARS-CoV-2 is via angiotensin converting enzyme 2 (ACE2) binding, which is followed by cleavage of the viral spike protein by a protease like TMPRSS2 and endocytosis-mediated import of the virus into the cell triggering the innate and adaptive immune response [66]. A dangerous complication in COVID-19 is the development of a cytokine storm, a surge in pro-inflammatory cytokines in the serum, which may result in pulmonary pathology, sepsis, respiratory failure or multiple organ failure [67].
Similar to what has been described for other RNA-viruses, SARS-CoV-2 RNA is predicted to localize to infected host cells' mitochondria [68] and cause mitochondrial dysfunction [51], such as alterations in mitochondrial respiration, mitochondrial reactive oxygen species (mtROS) production or the regulation of cell death and survival. Some in vitro data support this assumption, demonstrating for example that SARS-CoV-2 binds to components of the mitochondrial membrane (TOM-70) and thereby impairs the host cell's type I interferon response [69]. In another study only small effects of SARS-CoV-2 infection on mitochondrial DNA and MAVS gene expression in cell lines and clinical samples were reported, while nuclear genes of complex I subunits were downregulated [70]. While very few experimental data are available on the effect of mitochondrial fitness on infection with SARS-CoV-2 (e.g. there may be deficits in mitochondrial respiration in peripheral blood mononuclear cells of COVID-19 patients [71]), several pieces of evidence suggest mitochondrial integrity to be crucial for the general anti-viral host defense [51,72]. In the following sections these arguments are discussed for the individual components of mitochondrial fitness.
3.1. Mitochondrial membrane potential
The mitochondrial membrane potential (MMP) denotes the difference of electrical potential across the inner mitochondrial membrane and together with the proton gradient constitutes the proton motive force that constitutes the basis for adenosine triphosphate (ATP) generation via oxidative phosphorylation [73]. It regulates mitochondrial import and export and the fate of mitochondria: alterations in MMP can induce mitophagy or cell death [74]. ROS, calcium fluxes or viral infections can all modulate MMP [64]. Viruses manipulate MMP to either improve cell survival for viral replication and to prevent apoptosis in response to infection or they promote apoptosis, for example for virion release [64,75,76].
The centerpiece of the mitochondrial anti-viral machinery, MAVS, has been demonstrated to depend on MMP [77]. Mitochondria with compromised MMP thus are less efficient in the anti-viral host defense. Supporting this notion, compromised MMP results in diminished cytokine production after infection with various RNA viruses [78]. Accordingly, some viruses (e.g., influenza virus) reduce MMP to inhibit MAVS [79].
Together these data suggest that modulation of MMP by viruses renders mitochondria less capable for antiviral defense and more susceptible for further viral manipulation.
3.2. Oxidative phosphorylation versus glycolysis
The MMP is established by the electron transport system, consisting of the mitochondrial protein complexes I – IV. The resulting chemical potential and the proton gradient between mitochondrial matrix and inter-membrane space is used for ATP-production by ATP-synthase. This process is called oxidative phosphorylation and is the main source of chemical energy in most cells of the human organism. Defects of oxidative phosphorylation, for example complex I [80], are associate with the production of higher levels of ROS that may induce inflammation. Indeed, modulation of inflammatory responses by the electron transport system has recently been shown [81].
MAVS-mediated innate anti-viral signaling also depends on oxidative phosphorylation. This has been demonstrated in cells with oxidative phosphorylation deficits, which are less efficient in mounting anti-viral host responses and induction of interferons and cytokines [82]. Functional mitochondrial respiration is furthermore required to activate specialized immune cells, usually followed by reduced mitochondrial respiration of many of these cells during infection, when glycolytic fluxes are elevated [59,60]. For SARS-CoV-2 several potential direct interactions of viral proteins with the electron transfer system have been reported [83,84] suggesting that oxidative phosphorylation deficits occur in COVID-19.
Viral infection is generally associated with increased reliance of cells on glycolysis for energy production, potentially establishing optimal conditions for viral replication. This phenomenon is for example well described for Dengue [85] and Hepatitis C Virus [86]. On the level of the lung, chronic inhibition of mitochondrial respiration also leads to a metabolic switch to glycolysis, impedes alveolar gas exchanges and ventilator adaptation to match blood oxygen content with oxygen demand [87,88], leading to oxygen desaturation. Resulting low alveolar oxygen pressure leads to inflammatory responses by activation of lung cells and in particular alveolar macrophages [89].
3.3. Mitochondrial antiviral signaling
Viruses have developed a vast repertoire to inhibit MAVS activity on multiple levels, from pathogen detection to interferon signaling [63]. Interferons are an integral cellular weapon against viruses. An inadequate immune reaction has been reported for SARS-CoV-1 [90] and SARS-CoV-2 [47], characterized by low interferon activation, but high levels of pro-inflammatory cytokines. Apart from suggesting this dysregulation to be involved in severe COVID-19 linked cytokine storm formation, it also indicates viral usurpation of host MAVS. For SARS-CoV-1 there is indeed a large body of evidence of mitochondrial antiviral host defense involvement modulation [91,92], including for example the inhibition of RIG-1 and MAVS by viral proteins. Such mechanisms are expected to be at work similarly in COVID-19 [51].
3.4. Oxidative stress
Oxidative phosphorylation is a major source of ROS. Although mitochondrial ROS have important roles in cellular signaling regulating amongst others life-span [93], excessive ROS levels lead to oxidative stress and are linked to damage to mitochondrial DNA and tissue. The proximity of mitochondrial DNA to the sites of oxidative phosphorylation renders it particularly vulnerable to oxidative damage.
Upon infection mitochondria increase ROS production as a defense mechanism or due to damage caused by the infection. Various viruses have developed strategies to modulate mitochondrial ROS generation, for example to promote survival of the host cell and viral replication, and/or to profit from pro-oxidant conditions [62,64]. For viral respiratory infections it has been shown that the endogenous antioxidant systems can be suppressed [94]. While the knowledge on ROS and oxidative stress in SARS-CoV-2 is very limited, several indications of mitochondrial damage and oxidative stress suggest a role of ROS in infection and disease progression: viral RNA deposition in mitochondria [68], interaction of viral proteins with components of the electron transport system [83,84] and oxidized phospholipids in the lungs of SARS patients [95].
3.5. Mitochondrial biogenesis
Mitochondrial biogenesis is the increase of mitochondrial mass from pre-existing mitochondria and is regulated by the co-transcriptional factor peroxisome-proliferator-activated receptor γ co-activator-1α (PGC-1α) and other factors [96,97]. PA or dietary restriction induce several regulators of mitochondrial biogenesis, for example AMP-activated protein kinase (AMPK), which in turn activates PGC-1α. In conjunction with a number of other transcription factors, PGC-1α activates mitochondrial transcription factor A (TFAM), leading to mitochondrial growth and eventually formation of new mitochondria by division [98]. Together with its coactivators, PGC-1α (and also PGC-1β) furthermore are involved in the generation of anti-inflammatory cellular environments following PA [99].
SARS-CoV-1 has been suggested to interact with prohibitin proteins [100] that influence mitochondrial biogenesis and fusion [101]. The regulation of mitochondrial biogenesis by viruses, not to mention SARS-CoV-2, in general is, however, poorly understood.
3.6. Mitophagy
Dysfunctional mitochondria are hazardous for cells as they release toxic substances, including ROS and cell-death regulating factors. They can get targeted to and engulfed by autophagosomes and eventually get degraded by the lysosome in a process referred to as mitophagy, a major cellular mechanism of mitochondrial quality control. Criteria for mitophagic clearance of mitochondria include dysregulated MMP and oxidative phosphorylation or mitochondrial DNA mutations [102].
Of potential relevance for COVID-19 is the observation that hypoxic conditions promote mitophagy [103]. Severe SARS-CoV-2 infection is characterized by reduced oxygen provision and the induction of mitophagy could be beneficial with regard to the clearance of virally compromised mitochondria and to tissue integrity. As discussed below, however, SARS-CoV-2, may promote mitochondrial fusion [104], potentially counteracting hypoxia-induced mitophagy.
3.7. Mitochondrial dynamics and trafficking
“Mitochondrial dynamics” denominates the processes of mitochondrial fusion and fission by division of mitochondria. In contrast to mitochondrial biogenesis and mitophagy, mitochondrial dynamics do not change mitochondrial mass. The main effectors for fusion of the outer mitochondrial membrane are mitofusin 1 (MFN1) and MFN2, for fusion of the inner mitochondrial membrane optic atrophy 1 (OPA1) and for fission dynamin-related protein 1 (DRP1). While fused mitochondria exhibit more efficient oxidative phosphorylation and exchange of metabolites and mitochondrial DNA [105], fission may facilitate shuttling of mitochondria to different cellular locations, for example for cell membrane repair [106], and to clear away damaged mitochondria by mitophagy [103].
Viral infection entails re-modelling of mitochondria [62]. On one hand, mitochondrial dynamics regulate MAVS [107]. Specifically, mitochondrial fusion has been proposed to be beneficial for MAVs activity, which is why elongated mitochondria may be common during viral infection [[108], [109], [110]]. On the other hand, viruses modulate mitochondrial dynamics and interestingly this can promote either fusion or fission depending on the viral species. SARS-CoV-1 has been reported to induce the degradation of the fission factor DRP1 [111], presumably favoring fusion.
Mitochondrial fitness of systemic and resident immune cells is furthermore crucial for the coordinated immune response [112] that is compromised in COVID-19 [113]. While the importance of mitochondrial metabolism (and its coordination with glycolysis-derived energy supply) in specialized immune cells for their activation, differentiation and immune function is now well established, the metabolic differences of specific immune cell types and the mechanisms and associated consequences of immunometabolism regulation are currently under heavy investigation [[114], [115], [116]].
Taken together, disruption of mitochondrial functions in cells infected by RNA-viruses is a common phenomenon. Specific virulence mechanisms but also the immune response of the host cell contribute to mitochondrial dysfunction. Whether this could mean that pre-existing mitochondrial dysfunction aggravates viral infection by for example SARS-CoV-2 and whether improving mitochondrial fitness may confer protective effects, is discussed below.
4. Low cardiorespiratory and mitochondrial fitness as risk factors for COVID-19?
A number of pre-conditions, including cardiovascular diseases, diabetes, chronic obstructive pulmonary disease (COPD) - and maybe obesity - but especially advanced age dramatically increase the risk of severe outcome of SARS-CoV-2 [117,118]. Mitochondrial dysfunction, tightly linked to oxidative stress, reduced immune response and chronic inflammation, and an associated low cardiorespiratory fitness may be mediating this risk. Indeed, regular PA and increased cardiorespiratory fitness beneficially affect mitochondrial fitness, as outlined below.
4.1. The association of cardiorespiratory and mitochondrial fitness
PA determines cardiorespiratory fitness and triggers mitochondrial plasticity that can induce enhanced mitochondrial fitness. The resulting benefits on mitochondrial fitness include mitochondrial biogenesis, mitochondrial respiration, mitochondrial protein synthesis and enzyme activity, better oxidative stress handling and higher mitochondrial oxygen affinity (which denotes the oxygen pressure at the level of mitochondria at which the mitochondrial respiration is 50% of the maximal rate of mitochondrial respiration at saturating oxygen concentrations) [97,[119], [120], [121], [122], [123]]. These effects induce reduced reliance on anaerobic glycolysis, improve fatty acid oxidation and can increase aerobic performance [124]. High mitochondrial fitness furthermore correlates with exercise performance and health [[125], [126], [127]], even at advanced age [128]. Regular exercise induced beneficial mitochondrial changes are especially well known for skeletal muscle [42,97,129] and reversely well-functioning mitochondrial networks are crucial for regulation of skeletal muscle mass and function [130]. Benefits of PA for mitochondria have, however, also been reported for other tissues, such as the heart, kidney and liver [15,16] as well as for the brain [[16], [17], [18]], gonadal tissue and pancreas [19] and the lung [20]. Chronic moderate exercise is believed to exceed muscle adaptations (reviewed for example by Refs. [21,131]) and boost mitochondrial function also in distant organs. This may be possible due to auto-/para-/endocrine skeletal muscle feedback [16] e.g. directly by myokines [132] or exosome/microvesicle-transported “exerkines” [19]. Furthermore, indirect exercise-modulated factors such as systemic temperature or baro- and chemoreceptor sensing may be involved in enabling “reprogramming” also of distant tissues, regulating for example metabolism and immunity [39].
It can thus be assumed that mitochondria – and therefore innate immune responses to SARS-CoV-2 – in many tissues profit from PA. This is particularly relevant in light of increasing evidence of SARS-CoV-2 infection of tissues (including heart, kidney, liver, brain, etc.) other than the respiratory tract [113,133], that remains the primarily affected organ in COVID-19 [134], and associated organ-specific diseases [113] and immune responses [135].
Importantly, remodeling of mitochondrial functions can be controlled by the selection of different types of exercise, duration and intensity [123,136], in theory enabling targeted exercise intervention to modulate specific mitochondrial functions. Granata and colleagues [137] recently provided excellent guidelines for exercise program prescriptions with particular consideration of mitochondrial biogenesis. Conversely, a sedentary lifestyle or prolonged inactivity (i.e., large reduction of PA), such as may occur during quarantining associated with the COVID-19 pandemic [2,3], leads to both reduced mitochondrial content and function [123]. Here we discuss several well described effects of exercise on parameters of mitochondrial fitness (Fig. 2) that may be harnessed to mitigate viral infection risk and severity.
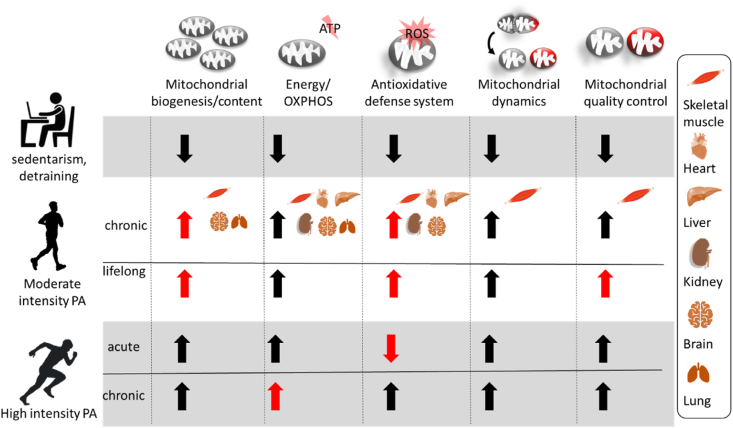
Fig. 2.
Parameters of mitochondrial fitness in different tissues that can be modulated by physical activity (PA). Specific effects on distinct tissues are shown only for chronic moderate intensity PA due to the scarcity of data for other conditions.
Effects that are potentially more pronounced than in other modalities and may represent distinguishing features are highlighted in red. While the indicated effects of PA on mitochondrial functions are established for human skeletal muscle, evidence on mitochondrial benefits in remote tissues are more scarce. While enhanced mitochondrial biogenesis, oxidative phosphorylation (OXPHOS) and anti-oxidant capacities have been convincingly shown in response to PA in various tissues in rodents [16], more research is needed to fully understand exercise effects on mitochondria. However, rodent pathology models support the assumption of the potential of PA to rescue mitochondrial fitness also in other tissues [19] and for other mitochondrial functions, such as mitochondrial dynamics [232]. This aspect has recently been discussed for human brain [233] and the mode of communication of PA effects to other tissues has been outlined recently as well [234]. ROS - reactive oxygen species. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.1.1. Mitochondrial biogenesis and oxidative phosphorylation
Mitochondrial biogenesis and oxidative phosphorylation capacity are the best studied mitochondrial adaptations to exercise. Although they are most likely strongly linked, they do not necessarily depend on each other [122].
Similar metabolic adaptations, including for example PGC-1α upregulation and increased lipid oxidation, were reported for low-volume, high exercise (sprint interval training) and high-volume, moderate intensity endurance training [138]. Higher intensity at similar volumes, however, may have even more pronounced beneficial effects on mitochondrial respiration [139]. The greatest effects on mitochondrial respiration may be induced by a combination of moderate-intensity and high-intensity exercise training [123]. The modulation of training volume, on the other hand, has been suggested to have a more important impact on mitochondrial content/density mediated by mitochondrial biogenesis [123].
Already acute responses to single bouts of high intensity exercise (defined as: including intervals performed above 75% of the maximal power achieved during an 8- to 12-min graded exercise test) can induce metabolic changes resulting in transcriptional upregulation of proteins related to mitochondrial biogenesis, fatty acid oxidation, the Krebs cycle, and oxidative phosphorylation [122]. At the same time nuclear signaling molecules interact with mitochondria and mitochondrial protein synthesis is increased. Repeated bouts of exercise and associated repeated stimulation of mitochondrial protein synthesis and mitochondrial dynamics (especially mitochondrial fusion) results in enhanced mitochondrial biogenesis, function and morphology [122].
Mitochondrial biogenesis effects may be also be increased at higher intensities [122], despite the potential main modulatory effect of volume [123]. Several exercise sessions per day could be superior to single exercise sessions per day (at same total exercise sessions) [122] and combination with resistance training has been shown to render the mitochondrial adaptations, in particular respiration, more robust [140].
4.1.2. Mitochondrial dynamics and quality control
Given the dependence of both mitochondrial biogenesis and oxidative phosphorylation on mitochondrial dynamics, it is not surprising that a single bout of exercise affects mitochondrial morphology. This effect is more subtle in moderate [141] and more pronounced at high intensity exercise bouts [122] and relies mainly on the modulation of mitochondrial fusion, facilitating inter-mitochondrial exchange of metabolites and mitochondrial DNA [97]. Mitochondrial fusion possibly also enhances the immune system by optimizing both respiration and contact with STING-component associated endoplasmic reticulum membranes, resulting in a more effective interferon activation and host response [[107], [108], [109]]. In agreement with this hypothesis, knocking out the pro-fusion factors MFN1 and MFN2 in mice reduced interferon and cytokine induction in response to viral infection with the RNA virus Sendai Virus [77]. Whether enhanced mitochondrial fusion renders mitochondria more resilient during coronavirus infection is however questionable: other SARS-CoVs appear to modulate mitochondrial dynamics by degrading DRP1 [104], thus also favoring fusion. This could mean that acute physical exercise may exacerbate the shift of the mitochondrial dynamics homeostasis by promoting even stronger pre-fusion conditions. The degradation of DRP1 possibly impairs the clearance of dysfunctional – for example viral RNA or protein containing – mitochondria and thus potentially supports survival of the infected cell during viral replication. Most likely the effects of regular PA on the dynamic nature of mitochondrial networks and the stimulation of mitophagy are still reducing COVID-19 infection risk. Intriguingly, both exercise and SARS-CoVs may enhance mitochondrial fusion. How these two pro-fusion effects interact has not been investigated. It is possible that acute exercise-induced mitochondrial fusion supports the manipulation of mitochondrial dynamics by the virus, potentially promoting virulence. This possibility supports existing guidelines recommending to refrain from exercise in case of suspected SARS-CoV-2 infection [142] and reduce PA in dependence of symptom severity [143].
Long-term exercise strongly remodels mitochondria; it enhances fusion by increasing the expression of Opa1 and Mfn2, while also reducing the fission factor Drp1 97 and may induce increased cristae density [144] and modulate supercomplex formation [145]. Lifelong voluntary exercise (free access to running wheels) finally is capable to prevent age-related mitochondrial fragmentation in mice [146].
Both acute and regular exercise furthermore improve mitochondrial quality control and turnover by stimulating mitophagy, facilitating ATP production and resulting in improved lipid metabolism and reduced muscle mass loss and apoptosis 97. These effects have been demonstrated in animal experiments by the quantification of the mitophagic flux 97. In humans, an equivalent effect may be most pronounced with lifelong exercise, while chronic exercise training (4 months) seems to be associated mainly with improved mitochondrial fusion [147].
4.1.3. Antioxidative capacities
Although ROS-signaling is involved in mediating the previously discussed beneficial adaptations to exercise [97,[148], [149], [150]], ROS-induced oxidative stress can have detrimental consequences [151,152]. An improved antioxidative status can be achieved by exercise, especially by regular moderate exercise [151]. High intensity exercise, in particular in repeated bouts, more likely induces oxidative stress-linked damage [153]. Improved anti-oxidative capacities as a consequence of PA [[154], [155], [156]] is supposedly protective by preventing mitochondrial, cellular and tissue damage in conditions of oxidative stress, such as for example in influenza infection [157]. Aging, on the other hand, reduces antioxidant capacities; an effect that, however, can partly be prevented by life-long regular exercise [154]. Other effects of aging on mitochondrial and cardiorespiratory fitness are discussed in the next section.
4.2. Cardiorespiratory and mitochondrial fitness in aging
Aging is associated with a functional decline of various organs; for example oxygen uptake is impaired by compromised lung function [158], an age-related ventilation-perfusion mismatch [159] as well as endothelial and vasculature dysfunction [160] resulting in arterial stiffness. Also, the heart becomes more susceptible to disease [161]. In line with these alterations, also cardiorespiratory fitness decreases massively with age; in the general population cardiorespiratory fitness drops by around 10% per decade after an age of 30 [162]. Regular PA can considerably delay and decrease this decline [163,164].
Supporting the strong correlation with cardiorespiratory fitness, also mitochondrial fitness declines with aging, as evidenced by increased mitochondrial DNA mutations and oxidative stress, as well as less efficient mitochondrial dynamics, biogenesis, MMP and quality control[98,[165], [166], [167], [168], [169]]. Interestingly, mitochondrial ATP-production capacity has been estimated to be reduced by around 8% per decade [170] and age-related mitochondrial fitness decline may also be mainly due to physical inactivity [171], both corresponding well with the decrease in cardiorespiratory fitness. Indeed, beneficial mitochondrial adaptations in response to exercise have been reported to be largely independent of age [140]. On the other hand, a reduced mitochondrial plasticity in age may cause an attenuated mitochondrial response to exercise [97].
Unsurprisingly, the decline in mitochondrial fitness is also associated with a concurrent deterioration of the immune system [172], including impaired interferon responses [173]. Mitochondrial dysfunction [120], oxidative stress [174] and inflammation mediated suppression of immunity are well characterized features of aging [175]. The related term “immunosenescence” [176] denotes a state of an aged immune system that is characterized by reduced efficiency, but as well by an intensified innate immune response leading to increased risk of tissue damage due to the immune response and to a higher risk of inflammatory disease and other associated age- and obesity-related diseases [23,177]. Recent findings on the effects of mitochondrial dysfunction in T-cells, which increased chronic inflammation and accelerate senescence in mice furthermore suggest a causal involvement in mitochondrial dysfunction of immune system components in aging [178]. The role of immunosenescense in COVID-19 and the related potential of targeting immunometabolism has recently been discussed in more detail [179].
In line with the effects of PA on mitochondrial and cardiorespiratory fitness, lifelong exercise has been shown to mitigate age-related increased inflammation [180].
In summary, the presented effects of aging at least partly explain the increased susceptibility and vulnerability to viral infections, including influenza [181,182] and COVID-19 [183,184] in the older population. Importantly, however, these risk factors can be largely reduced by regular PA.
4.3. Cardiorespiratory and mitochondrial fitness and COVID-19 comorbidities
Apart from age, several pre-conditions, notably including cardiovascular and respiratory diseases like COPD increase risk of COVID-19 infection and severe disease progression [117]. Importantly, also these comorbidities of COVID-19 are characterized by reduced cardiorespiratory fitness [185] and associated with mitochondrial dysfunctions.
Epidemiological studies clearly link cardiorespiratory fitness to the risk of developing cardiovascular disease [186] and dying from it [186]. Accordingly, mitochondrial dysfunction is common in cardiovascular diseases as well [187] and includes increased mitochondrial DNA damage [188] and mitochondrial ROS production, as well as deficits in oxidative phosphorylation, mitochondrial morphology and quality control [189].
Also for COPD low cardiorespiratory fitness is a major risk factor [190]. COPD patients exhibit reduced mitochondrial respiration capacities, which may be exacerbated in combination with low body mass index [191]. Specifically, human airway smooth muscle cells from COPD patients are characterized by reduced MMP, reduced mitochondrial respiration capacities, and increased ROS [192]. Viral respiratory infection furthermore is involved in the etiology of - or exacerbates - COPD and several other chronic diseases [193], highlighting the potential long-term effects of SARS-CoV-2 infections on public health.
High cardiorespiratory fitness is an important protective factor for cardiovascular disease [194] and COPD [195] rendering these COVID-19 comorbidities largely amenable to PA. Mitochondrial fitness is a strong candidate to mediate the beneficial effects of regular PA, also by improving immune system function and reducing inflammation [196,197].
Consequently, obesity and diabetes have been discussed to represent further risk factors for COVID-19 severity [[198], [199], [200], [201], [202], [203], [204], [205]]. This is in line with reported negative consequences of obesity on viral infection and immunity that have previously been reported [206,207] and recently been reviewed [205,208,209]. Among the most relevant detrimental consequences of inactivity and elevated body fat content for viral infections are increased oxidative stress and inflammation [210]. The adverse effects especially of unhealthy adipose tissue with regard to COVID-19 have been discussed in more detail elsewhere [12].
Cardiorespiratory fitness until now has been scarcely considered as an independent modifier of COVID-19 [43,45]. This is surprising due to previous findings suggesting that negative consequences of obesity (body mass index or waist circumference) on general mortality may be mediated by cardiorespiratory fitness [211,212].
In summary, compromised immune function and inflammation in aging, sedentary lifestyle and chronic diseases – all interdependently linked to mitochondrial dysfunction – represent pre-dispositions for viral infections including SARS-CoV-2. This implies that avoiding these conditions or preventing their aggravations could strongly mitigate SARS-CoV-2 infection risk and outcome and PA has the potential to do just that.
5. The protective potential of PA in COVID-19
As outlined in this review, regular PA and high mitochondrial and cardiorespiratory fitness can increase the antiviral immune response and reduce basal levels of inflammation and oxidative stress. In addition, PA could counteract COVID-19 related lung, vascular and blood abnormalities and thus improve oxygen supply to enhance mitochondrial fitness, both in specialized immune cells and in target tissues. Acute high-intensity exercise during infection or at risk for infection could, however, be detrimental by negatively impacting on redox status and immune function.
5.1. Blood and vasculature
Adequate oxygen supply relies on a functional vasculature that is capable to control the transport of blood. With age, vascular functions are impaired, mediated in part by impaired endothelial nitric oxide synthase (eNOS) activity [160]. Oxidative stress, lung inflammation and potential direct vascular damage by viral infection also impede blood transport, for example by coagulation dysregulation [213]. Coagulation is a common response to inflammation and low platelet counts and coagulation abnormalities may be associated with adverse outcomes of SARS-CoV-2 infection [213]. PA is essential to maintain vascular integrity. Already five days of reduced PA were sufficient to impair vascular function in young, recreationally active men [214]. This was manifested in artery flow mediated dilation deficits and elevated plasma concentrations of endothelial micro particles indicating activated or apoptotic endothelial cells. A direct association of mitochondrial respiration on immune responses in human blood cells has been recently demonstrated; inhibition of particularly mitochondrial complex IV in this study impaired cytokine induction [81].
5.2. Lung mitochondria
Little information is available on the effect of exercise on mitochondrial dysfunction in the lung following respiratory infection. However, in a rodent study of experimental lung injury by intratracheal instillation of lipopolysaccharide, reduced mitochondrial complex II activity in the lung and formation of lung edema was observed [20]. Two weeks of exercise before the intervention rescued both of these effects. In another study using the same mouse model, but no exercise intervention, the observed severe mitochondrial dysfunction in pulmonary epithelia as well as associated pathologies and mortality were rescued by provision of healthy mitochondria transferred to the lung cells from exogenously applied bone-marrow derived stromal cells [215].
5.3. Exercise recommendations
Given the pronounced PA benefits against viral infection, the question arises, how to best take advantage of this knowledge. Based on the information assembled in section 4.1 and summarized in Fig. 2, some recommendations for suitable forms of exercise to boost mitochondrial fitness with the specific aim to strengthen anti-viral immune defenses is provided in Table 1. Of course, the selection of training programs depends on the immediacy of infection risk. As outlined above, even single high intensity exercise bouts transiently put tremendous stress on mitochondria, increase oxidative stress, reduce immune function and thereby could facilitate viral replication, cell death events and – in the case of COVID-19-induced acute cardiac injury – a proarrhythmic myocardial substrate [216]. Especially eccentric - but also other types of strenuous – exercise furthermore are associated with increased systemic inflammation [38], a dangerous mechanism in the context of COVID-19. Intensive exercise thus should not be performed during infection, suspected infection, persisting illness or symptoms, and not for at least 1–2 weeks after complete symptoms resolution. On the other hand, beneficial systemic and mitochondrial adaptations and anti-inflammatory effects of moderate exercise [28,29], as well as a high cardiorespiratory fitness - that is also associated with low inflammation [23] - resulting from regular exercise are likely important protective factors for COVID-19. For Table 1, high intensity training (HIT) is considered as a training including intervals performed at >75% of the maximal capacity). Moderate intensity training (MIT) is usually performed at around 50–75% of the maximal capacity, often in a continuous fashion (MICT). A detailed review on the effects of exercise intensity on mitochondrial parameters has been published in this journal [123]. Beside intensity, the exercise modality determines mitochondrial outcomes. While mitochondrial benefits, in particular of mitochondrial biogenesis, are very well established for endurance exercise, they are less well understood for resistance exercise. Resistance exercise, however, is an attractive exercise modality also to be performed at home and thus relevant for COVID-19 associated limitations of mobility. Porter et al. [217] reported that 12 weeks of resistance exercise enhanced mitochondrial respiration and mildly increased expression of mitochondrial transcripts and proteins. This indicates that endurance and resistance exercise differentially – and possibly synergistically – boost mitochondrial fitness; a hypothesis that deserves further scientific exploration.
Table 1.
Suggested exercise interventions for infection risk reduction.
| SARS-CoV-2: potential effects on host mitochondria | Absence of infection risk (e.g. no pandemic or complete isolation) | Infection risk (during the pandemic, not isolated) | During infection/recovery |
|---|---|---|---|
| Reduced OXPHOS | 1. regular HIT 2. regular MIT for best effects include resistance training and combine high and low intensity |
regular MIT | avoid exercise during infection and follow published guidelines for re-uptake of exercise after a recovery period of at least 1–2 weeks of convalescence [142,143,216,235,236] |
| Reduced MAVS | 1. regular MIT 2. regular HIT |
regular MIT avoid acute HIT |
|
| Increased oxidative stress | 1. regular MIT 2. regular HIT |
regular MIT avoid acute HIT |
|
| Reduced biogenesis | 1. regular MIT (higher intensity, repeated bouts per day and high volumes may increase effect) 2. regular HIT |
regular MIT | |
| Impaired mitophagy | 1. regular MIT 2. regular HIT |
regular MIT | |
| Impaired dynamics | regular MIT or HIT | regular MIT |
Explanations: HIT – high intensity training, MIT – moderate intensity training, SARS-CoV-2 – Severe Acute Respiratory Syndrome Coronavirus 2, OXPHOS – oxidative phosphorylation, MAVS – mitochondrial anti-viral signaling.
In summary, plasticity of skeletal muscle mitochondria in response to regular exercise [42,97,129] improves mitochondrial fitness parameters, such as energy provision, biogenesis, optimization of mitochondrial dynamics and higher anti-oxidative capacities. Stimulation of skeletal muscle adaptations, including of mitochondria, then allows a metabolic re-programming - possibly improving mitochondrial fitness - also of distal tissues [39], such as of the lung [20], the primarily affected organ by SARS-CoV-2 infection [134]. Improvements of mitochondrial fitness in the lung, as well as in other tissues affected by COVID-19 [113,133] are expected to improve the innate immune function [51] - and therefore reduce infection probability - of these target tissues. The key role of mitochondria in the activation and efficiency of specialized immune roles is also becoming increasingly acknowledged [40,41] and is being discussed more and more in the context of COVID-19 [179,[218], [219], [220]]. In addition, the beneficial effects of regular PA on cardiorespiratory fitness and on inflammation may be protective for the important COVID-19 symptoms hypoxemia [221,222] and cytokine storm [67].
Regular moderate PA via its beneficial effects on mitochondria and consequences on innate immune system of potential target tissues for SARS-CoV-2 infection, oxidative stress, inflammation, adaptive immune and vascular systems possibly reduces viral infection and most likely decreases disease severity and mortality. Acute high intensity exercise, on the other hand may facilitate viral infection via a debated transient reduction of the immune system capacity [37,223]. If performed during infection, it may also aggravate disease progression and mortality. While at risk for infection, or after contraction of SARS-CoV-2, caution is thus necessary for the engagement in PA. Nutritional and pharmacological strategies to enhance mitochondrial involvement in the innate immune function may be preferable alternatives under these conditions [51]. Although the here highlighted preliminary evidence indicates that regular PA-mediated mitochondrial fitness may be protective against COVID-19, many questions remain open. These include the specific effects of exercise intensity, modality and frequency on infection risk, severity of disease and mortality. In addition, the role of tissue-specific mitochondrial fitness in COVID-19 – and generally in infectious diseases – is poorly understood: Will improved fitness of skeletal muscle mitochondria following PA sufficiently boost mitochondrial fitness of distal tissues to protect from COVID-19? Does improved mitochondrial fitness of the respiratory tract reduce the likelihood of viral infection? Does mitochondrial fitness e.g. in heart and brain protect from secondary effects of viral infection on these tissues? Which forms of exercise will best improve the metabolism of specialized immune cells to act against viral infection, and which forms of exercise might even reduce the host immune defense?
6. Conclusions and practical considerations
Beside the well-known roles of mitochondria in energy-production, oxidative stress regulation, ion-homeostasis, metabolite-synthesis, and many others that directly and indirectly contribute to the systemic immune response to infections, direct mitochondrial involvement in innate immunity has emerged as another important function of the organelle. Viral infections, for example by SARS-CoV-2, compromise mitochondrial functions. SARS-CoV-2 strikingly has more adverse effects on population groups characterized by elevated levels of inflammation and mitochondrial dysfunction, such as older or obese people or individuals suffering from cardiovascular or pulmonary diseases. Based on the strong association of mitochondrial and cardiorespiratory fitness, we put forth the hypothesis that enhancing mitochondrial fitness by regular PA is a protective factor in COVID-19. Regular PA and mitochondrial fitness enhance redox and inflammatory status and ameliorate the immune response to infection. In addition, they have clear preventive potential on many chronic diseases that are considered to be risk factors for COVID-19 outcome and counteract several detrimental aging-related processes that may also be associated with higher COVID-19 risk. The necessary political measures to reduce viral spreading, including various lockdown and isolation strategies, for example in the context of contact tracing lead to increases in sedentary lifestyle and inactivity, especially in vulnerable individuals [224,225]. This effect is further aggravated by reduced possibilities to practice sports in gyms, fitness centers and sports clubs. Based on the well-established general benefits of regular PA on chronic diseases and all-cause mortality [8], mental health [3], as well as on incidence and mortality specifically in viral infections, such as influenza [[226], [227], [228]], COVID-19-related reductions in PA are likely to entail massive global health and socio-economic consequences. Importantly, already low volumes of exercise can pronouncedly improve mitochondrial [42] and cardiorespiratory fitness [229], in particular at low baseline fitness levels. Regular exercise is thus an efficient way to promote public health even at low time and cost investment. Conversely, reduced exercise volumes and sedentary lifestyle quickly result in decreased mitochondrial fitness [125]. The benefits of reduced sedentary times have recently been prominently discussed with regard to the World Health Organization 2020 guidelines on physical activity and sedentary behavior [230], the bottom lines of which are that already low levels of PA are beneficial but more PA further improves health outcomes.
With regard to the ongoing COVID-19 pandemic, regular moderate exercise and higher PA levels in summary have the potential to enhance immune system functions via improving mitochondrial fitness. It is thus even more important during isolation and confinement periods to maintain workout-routines and avoid the reduction of PA; e.g. by making sure to reduce sitting times (standing or walking whenever possible during private or professional tasks and interrupt prolonged sitting with periods of PA), walking as much as possible (instead of driving, using elevators, etc.) and as often as possible perform workouts. These workouts may be endurance trainings (e.g. using cycle or rowing ergometers, climbing stairs, etc.), resistance training (e.g. using dumbells or thera bands), dancing, yoga, tai chi or other forms of exercise. Different exercise modalities have overlapping but also differential effects, also on mitochondrial fitness [217], and thus performing workouts sessions of varying exercise modalities may be particularly useful to improve various mitochondrial functions. Importantly, direct mitochondrial improvements after exercise in the skeletal muscle are accompanied by increased mitochondrial fitness of specialized immune cells and other tissues that are relevant for COVID-19 as outlined above. Strenuous exercise should be performed cautiously during the pandemic. As discussed, bouts of intensive exercise (from around >60% of maximal oxygen uptake/heart rate reserve or of durations of >60 min [23]) may transiently decrease immune function and facilitate viral infection. It is possible that this effect is also related to mitochondrial fitness; depletion of energy levels following physical exertion, as well as increased redox signaling/oxidative stress and inflammatory mitochondrial-damage signals first in skeletal muscle and then in the immune system and remote tissues [16,39]. The primary target tissue of SARS-CoV-2, the respiratory tract, might furthermore be directly affected by oxidative stress and mitochondrial dysfunction as a consequence of increased ventilation during high intensity exercise, although this seems not to be the case in healthy subjects as suggested by rodent experiments [231]. Taken together, high intensity exercise is thought to open up an ill-defined window of infection-vulnerability [37] and thus should be avoided whenever there is a risk of SARS-CoV-2 infection.
We conclude that mitochondrial and cardiorespiratory fitness most likely are important - and modifiable - protective factors for COVID-19, possibly representing a link between a number of established risk factors. We argue for regular analysis of COVID-19 patients’ cardiorespiratory fitness that may be a modulator of disease severity and mortality. The promotion of mitochondrial fitness, e.g. by PA, may be a protective measure against viral infection. At the same time the consideration of the potential dangers of high-intensity exercise during or short after the viral phase as facilitator of infection and maybe disease severity is of great importance.
Declaration of competing interest
All authors contributed to writing the manuscript, all read and agreed to submit the final version of it.
The authors declare no conflicts of interest related to the topic of this review.
References
- 1.Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Publ. Health Rep. 1985;100(2):126. [PMC free article] [PubMed] [Google Scholar]
- 2.Hall G., Laddu D.R., Phillips S.A. A tale of two pandemics: how will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog. Cardiovasc. Dis. 2020 doi: 10.1016/j.pcad.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burtscher J., Burtscher M., Millet G.P. (Indoor) isolation, stress and physical inactivity: vicious circles accelerated by Covid-19? Scand. J. Med. Sci. Sports. 2020 doi: 10.1111/sms.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narici M., De Vito G., Franchi M. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2020:1–22. doi: 10.1080/17461391.2020.1761076. [DOI] [PubMed] [Google Scholar]
- 5.Horton R. Offline: COVID-19 is not a pandemic. Lancet (London, England) 2020;396(10255):874. doi: 10.1016/S0140-6736(20)32000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burtscher J., Burtscher M., Millet G.P. Jumping at the opportunity: promoting physical activity after COVID‐19. Scand. J. Med. Sci. Sports. 2020;30(8):1549. doi: 10.1111/sms.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Larochelambert Q., Marc A., Antero J. Covid-19 mortality: a matter of vulnerability among nations facing limited margins of adaptation. Frontiers in Public Health. 2020;8(782) doi: 10.3389/fpubh.2020.604339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burtscher J., Burtscher M. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine; 2019. Run for Your Life: Tweaking the Weekly Physical Activity Volume for Longevity. [DOI] [PubMed] [Google Scholar]
- 9.Antero J., Tanaka H., De Larochelambert Q. Female and male US Olympic athletes live 5 years longer than their general population counterparts: a study of 8124 former US Olympians. Br. J. Sports Med. 2020 doi: 10.1136/bjsports-2019-101696. [DOI] [PubMed] [Google Scholar]
- 10.King A.J., Burke L.M., Halson S.L. The challenge of maintaining metabolic health during a global pandemic. Sports Med. 2020;50(7):1233–1241. doi: 10.1007/s40279-020-01295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwendinger F., Pocecco E. Counteracting physical inactivity during the COVID-19 pandemic: evidence-based recommendations for home-based exercise. Int. J. Environ. Res. Publ. Health. 2020;17(11):3909. doi: 10.3390/ijerph17113909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigro E., Polito R., Alfieri A. Molecular mechanisms involved in the positive effects of physical activity on coping with COVID-19. Eur. J. Appl. Physiol. 2020 doi: 10.1007/s00421-020-04484-5. [published Online First: 2020/09/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burtscher J., Millet G.P., Burtscher M. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine; 2020. Low Cardiorespiratory and Mitochondrial Fitness as Risk Factors in Viral Infections: Implications for COVID-19. [DOI] [PubMed] [Google Scholar]
- 14.Wenger H.A., Bell G.J. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986;3(5):346–356. doi: 10.2165/00007256-198603050-00004. [DOI] [PubMed] [Google Scholar]
- 15.Morris E.M., McCoin C.S., Allen J.A. Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis. J. Physiol. 2017;595(14):4909–4926. doi: 10.1113/JP274281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boveris A., Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic. Biol. Med. 2008;44(2):224–229. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Steiner J.L., Murphy E.A., McClellan J.L. Exercise training increases mitochondrial biogenesis in the brain. J. Appl. Physiol. 2011;111(4):1066–1071. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- 18.Marosi K., Bori Z., Hart N. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience. 2012;226:21–28. doi: 10.1016/j.neuroscience.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Safdar A., Bourgeois J.M., Ogborn D.I. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl. Acad. Sci. Unit. States Am. 2011;108(10):4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Cunha M.J., da Cunha A.A., Scherer E.B. Experimental lung injury promotes alterations in energy metabolism and respiratory mechanics in the lungs of rats: prevention by exercise. Mol. Cell. Biochem. 2014;389(1–2):229–238. doi: 10.1007/s11010-013-1944-8. [published Online First: 2014/01/01] [DOI] [PubMed] [Google Scholar]
- 21.Hawley John A., Hargreaves M., Joyner Michael J. Integrative biology of exercise. Cell. 2014;159(4):738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Shephard R.J., Shek P.N., DiNubile N.A. Exercise, immunity, and susceptibility to infection: a j-shaped relationship? Physician Sportsmed. 1999;27(6):47–71. doi: 10.3810/psm.1999.06.873. [DOI] [PubMed] [Google Scholar]
- 23.Nieman D.C., Wentz L.M. The compelling link between physical activity and the body's defense system. Journal of Sport and Health Science. 2019;8(3):201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bermon S., Castell L.M., Calder P.C. Consensus statement immunonutrition and exercise. Exerc. Immunol. Rev. 2017;23:8–50. [PubMed] [Google Scholar]
- 25.Adams G.R., Zaldivar F.P., Nance D.M. Exercise and leukocyte interchange among central circulation, lung, spleen, and muscle. Brain Behav. Immun. 2011;25(4):658–666. doi: 10.1016/j.bbi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen B.K. Anti‐inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur. J. Clin. Invest. 2017;47(8):600–611. doi: 10.1111/eci.12781. [DOI] [PubMed] [Google Scholar]
- 27.Collao N., Rada I., Francaux M. Anti-inflammatory effect of exercise mediated by toll-like receptor regulation in innate immune cells–A review: anti-inflammatory effect of exercise mediated by toll-like receptor regulation in innate immune cells. Int. Rev. Immunol. 2020;39(2):39–52. doi: 10.1080/08830185.2019.1682569. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 2008 doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes P., de Mendonça Oliveira L., Brüggemann T.R. Physical exercise induces immunoregulation of TREG, M2, and pDCs in a lung allergic inflammation model. Front. Immunol. 2019;10:854. doi: 10.3389/fimmu.2019.00854. [published Online First: 2019/06/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fondell E., Lagerros Y.T., Sundberg C.J. Physical activity, stress, and self-reported upper respiratory tract infection. Med. Sci. Sports Exerc. 2011;43(2) doi: 10.1249/MSS.0b013e3181edf108. [DOI] [PubMed] [Google Scholar]
- 31.Wong C.M., Lai H.K., Ou C.Q. Is exercise protective against influenza-associated mortality? PloS One. 2008;3(5) doi: 10.1371/journal.pone.0002108. [published Online First: 2008/05/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson R.J., Hussain M., Baker F. Cardiorespiratory fitness is associated with better control of latent herpesvirus infections in a large ethnically diverse community sample: evidence from the Texas City Stress and Health Study. Brain Behav. Immun. 2017;66:e35. doi: 10.1016/j.bbi.2017.07.128. [DOI] [Google Scholar]
- 33.Kohut M.L., Sim Y.-J., Yu S. Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J. Infect. Dis. 2009;200(9):1434–1442. doi: 10.1086/606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis J.M., Kohut M.L., Colbert L.H. Exercise, alveolar macrophage function, and susceptibility to respiratory infection. J. Appl. Physiol. 1985;83(5):1461–1466. doi: 10.1152/jappl.1997.83.5.1461. [published Online First: 1998/01/07] [DOI] [PubMed] [Google Scholar]
- 35.Walsh N.P., Oliver S.J. Exercise, immune function and respiratory infection: an update on the influence of training and environmental stress. Immunol. Cell Biol. 2016;94(2):132–139. doi: 10.1038/icb.2015.99. [DOI] [PubMed] [Google Scholar]
- 36.Gleeson M., Pyne D.B., Elkington L.J. Developing a multi-component immune model for evaluating the risk of respiratory illness in athletes. Exerc. Immunol. Rev. 2017;23 [PubMed] [Google Scholar]
- 37.Simpson R.J., Campbell J.P., Gleeson M. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020;26:8–22. [PubMed] [Google Scholar]
- 38.Paulsen G., Ramer Mikkelsen U., Raastad T. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012:18. [PubMed] [Google Scholar]
- 39.Koelwyn G.J., Quail D.F., Zhang X. Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Canc. 2017;17(10):620–632. doi: 10.1038/nrc.2017.78. [published Online First: 2017/09/26] [DOI] [PubMed] [Google Scholar]
- 40.Buck M.D., Sowell R.T., Kaech S.M. Metabolic instruction of immunity. Cell. 2017;169(4):570–586. doi: 10.1016/j.cell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung J., Zeng H., Horng T. Metabolism as a guiding force for immunity. Nat. Cell Biol. 2019;21(1):85–93. doi: 10.1038/s41556-018-0217-x. [DOI] [PubMed] [Google Scholar]
- 42.Granata C., Caruana N.J., Botella J. Multi-omics reveal intricate network of mitochondrial adaptations to training in human skeletal muscle. bioRxiv. 2021 [Google Scholar]
- 43.Zbinden‐Foncea H., Francaux M., Deldicque L. Does high cardiorespiratory fitness confer some protection against pro‐inflammatory responses after infection by SARS‐CoV‐2? Obesity. 2020 doi: 10.1002/oby.22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.da Silveira M.P., da Silva Fagundes K.K., Bizuti M.R. Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin. Exp. Med. 2020:1–14. doi: 10.1007/s10238-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brawner C.A., Ehrman J.K., Bole S. Maximal exercise capacity is inversely related to hospitalization secondary to coronavirus disease 2019. Mayo Clin. Proc. 2020 doi: 10.1016/j.mayocp.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheval B., Sieber S., Maltagliati S. Muscle strength is associated with COVID-19 hospitalization in adults 50 years of age and older. medRxiv. 2021:2021. doi: 10.1101/2021.02.02.21250909. 02.02.21250909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruan Q., Yang K., Wang W. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singer M., Deutschman C.S., Seymour C.W. The third international consensus definitions for sepsis and septic shock (Sepsis-3) J. Am. Med. Assoc. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brealey D., Brand M., Hargreaves I. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 51.Burtscher J., Cappellano G., Omori A. Mitochondria–in the crossfire of SARS-CoV-2 and immunity. iScience. 2020:101631. doi: 10.1016/j.isci.2020.101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiku V., Tan M.-W., Dikic I. Mitochondrial functions in infection and immunity. Trends Cell Biol. 2020 doi: 10.1016/j.tcb.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nunn A.V., Guy G.W., Brysch W. SARS-CoV-2 and mitochondrial health: implications of lifestyle and ageing. Immun. Ageing. 2020;17(1):1–21. doi: 10.1186/s12979-020-00204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno Fernández-Ayala D.J., Navas P., López-Lluch G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp. Gerontol. 2020;142:111147. doi: 10.1016/j.exger.2020.111147. [published Online First: 2020/11/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chernyak B.V., Popova E.N., Zinovkina L.A. Mitochondria as targets for endothelial protection in COVID-19. Front. Physiol. 2020:11. doi: 10.3389/fphys.2020.606170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh K.K., Chaubey G., Chen J.Y. American Physiological Society Rockville; MD: 2020. Decoding SARS-CoV-2 Hijacking of Host Mitochondria in Pathogenesis of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saleh J., Peyssonnaux C., Singh K.K. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roger A.J., Muñoz-Gómez S.A., Kamikawa R. The origin and diversification of mitochondria. Curr. Biol. 2017;27(21):R1177. doi: 10.1016/j.cub.2017.09.015. [published Online First: 2017/11/08] [DOI] [PubMed] [Google Scholar]
- 59.Banoth B., Cassel S.L. Mitochondria in innate immune signaling. Transl. Res. 2018;202:52–68. doi: 10.1016/j.trsl.2018.07.014. [published Online First: 2018/08/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sander L.E., Garaude J. The mitochondrial respiratory chain: a metabolic rheostat of innate immune cell-mediated antibacterial responses. Mitochondrion. 2018;41:28–36. doi: 10.1016/j.mito.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Refolo G., Vescovo T., Piacentini M. Mitochondrial interactome: a focus on antiviral signaling pathways. Front Cell Dev Biol. 2020;8:8. doi: 10.3389/fcell.2020.00008. [published Online First: 2020/03/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glingston R.S., Deb R., Kumar S. Organelle dynamics and viral infections: at cross roads. Microb. Infect. 2019;21(1):20–32. doi: 10.1016/j.micinf.2018.06.002. [published Online First: 2018/06/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frieman M., Heise M., Baric R. SARS coronavirus and innate immunity. Virus Res. 2008;133(1):101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anand S.K., Tikoo S.K. Viruses as modulators of mitochondrial functions. Advances in Virology. 2013;2013:738794. doi: 10.1155/2013/738794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azkur A.K., Akdis M., Azkur D. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020 doi: 10.1111/all.14364. [published Online First: 2020/05/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu K.E., Zou J., Chang H.Y. RNA-GPS predicts SARS-CoV-2 RNA localization to host mitochondria and nucleolus. bioRxiv. 2020 doi: 10.1016/j.cels.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang H-w, Zhang H-n, Meng Q-f. SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell. Mol. Immunol. 2020;17(9):998–1000. doi: 10.1038/s41423-020-0514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller B., Silverstein A., Flores M. Host mitochondrial transcriptome response to SARS-CoV-2 in multiple cell models and clinical samples. Sci. Rep. 2021;11(1):1–10. doi: 10.1038/s41598-020-79552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ajaz S., McPhail M.J., Singh K.K. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of COVID-19 patients. Am. J. Physiol. Cell Physiol. 2020 doi: 10.1152/ajpcell.00426.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shenoy S. Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm. Res. 2020 doi: 10.1007/s00011-020-01389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191(4784):144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 74.Zorova L.D., Popkov V.A., Plotnikov E.Y. Mitochondrial membrane potential. Anal. Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [published Online First: 2017/07/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen C.Y., Ping Y.H., Lee H.C. Open reading frame 8a of the human severe acute respiratory syndrome coronavirus not only promotes viral replication but also induces apoptosis. J. Infect. Dis. 2007;196(3):405–415. doi: 10.1086/519166. [published Online First: 2007/06/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Everett H., McFadden G. Viruses and apoptosis: meddling with mitochondria. Virology. 2001;288(1):1–7. doi: 10.1006/viro.2001.1081. [DOI] [PubMed] [Google Scholar]
- 77.Koshiba T., Yasukawa K., Yanagi Y. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci. Signal. 2011;4(158):ra7. doi: 10.1126/scisignal.2001147. ra7. [DOI] [PubMed] [Google Scholar]
- 78.Ichinohe T., Yamazaki T., Koshiba T. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110(44):17963–17968. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Varga Z.T., Grant A., Manicassamy B. Influenza virus protein PB1-F2 inhibits the induction of type I interferon by binding to MAVS and decreasing mitochondrial membrane potential. J. Virol. 2012;86(16):8359–8366. doi: 10.1128/JVI.01122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balsa E., Perry E.A., Bennett C.F. Defective NADPH production in mitochondrial disease complex I causes inflammation and cell death. Nat. Commun. 2020;11(1):2714. doi: 10.1038/s41467-020-16423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karan K.R., Trumpff C., McGill M.A. Mitochondrial respiratory capacity modulates LPS-induced inflammatory signatures in human blood. Brain, Behavior, & Immunity - Health. 2020;5:100080. doi: 10.1016/j.bbih.2020.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshizumi T., Imamura H., Taku T. RLR-mediated antiviral innate immunity requires oxidative phosphorylation activity. Sci. Rep. 2017;7(1):5379. doi: 10.1038/s41598-017-05808-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gordon D.E., Jang G.M., Bouhaddou M. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gordon D.E., Jang G.M., Bouhaddou M. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. BioRxiv. 2020 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fontaine K.A., Sanchez E.L., Camarda R. Dengue virus induces and requires glycolysis for optimal replication. J. Virol. 2015;89(4):2358–2366. doi: 10.1128/JVI.02309-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ripoli M., D'Aprile A., Quarato G. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1α-mediated glycolytic adaptation. J. Virol. 2010;84(1):647–660. doi: 10.1128/JVI.00769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cloonan S.M., Choi A.M. Mitochondria in lung disease. J. Clin. Invest. 2016;126(3):809–820. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piantadosi C.A., Suliman H.B. Mitochondrial dysfunction in lung pathogenesis. Annu. Rev. Physiol. 2017;79(1):495–515. doi: 10.1146/annurev-physiol-022516-034322. [DOI] [PubMed] [Google Scholar]
- 89.Chen T., Yang C., Li M. Alveolar hypoxia-induced pulmonary inflammation: from local initiation to secondary promotion by activated systemic inflammation. J. Vasc. Res. 2016;53(5–6):317–329. doi: 10.1159/000452800. [DOI] [PubMed] [Google Scholar]
- 90.Channappanavar R., Fehr Anthony R., Vijay R. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Current Opinion in Virology. 2012;2(3):264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lim Y.X., Ng Y.L., Tam J.P. Human coronaviruses: a review of virus-host interactions. Diseases. 2016;4(3) doi: 10.3390/diseases4030026. [published Online First: 2016/07/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molenaars M., Daniels E.G., Meurs A. Mitochondrial cross-compartmental signalling to maintain proteostasis and longevity. Philosophical Transactions of the Royal Society B. 2020;375(1801):20190414. doi: 10.1098/rstb.2019.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Komaravelli N., Casola A. Respiratory viral infections and subversion of cellular antioxidant defenses. J. Pharmacogenomics Pharmacoproteomics. 2014;5(4) doi: 10.4172/2153-0645.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imai Y., Kuba K., Neely G.G. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [published Online First: 2008/04/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Popov L.-D. Mitochondrial biogenesis: an update. J. Cell Mol. Med. 2020;24(9):4892–4899. doi: 10.1111/jcmm.15194. [published Online First: 2020/04/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hood D.A., Memme J.M., Oliveira A.N. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu. Rev. Physiol. 2019;81:19–41. doi: 10.1146/annurev-physiol-020518-114310. [published Online First: 2018/09/15] [DOI] [PubMed] [Google Scholar]
- 98.Jornayvaz F.R., Shulman G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eisele P.S., Furrer R., Beer M. The PGC-1 coactivators promote an anti-inflammatory environment in skeletal muscle in vivo. Biochem. Biophys. Res. Commun. 2015;464(3):692–697. doi: 10.1016/j.bbrc.2015.06.166. [published Online First: 2015/07/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cornillez-Ty C.T., Liao L., Yates J.R. Severe acute respiratory syndrome coronavirus nonstructural protein 2 interacts with a host protein complex involved in mitochondrial biogenesis and intracellular signaling. J. Virol. 2009;83(19):10314–10318. doi: 10.1128/JVI.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Merkwirth C., Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim. Biophys. Acta Mol. Cell Res. 2009;1793(1):27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 102.Ashrafi G., Schwarz T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pickles S., Vigié P., Youle R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018;28(4):R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi C.-S., Qi H.-Y., Boularan C. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 2014;193(6):3080–3089. doi: 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schrepfer E., Scorrano L. Mitofusins, from mitochondria to metabolism. Mol. Cell. 2016;61(5):683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 106.Horn A., Raavicharla S., Shah S. Mitochondrial fragmentation enables localized signaling required for cell repair. JCB (J. Cell Biol.) 2020;219(5) doi: 10.1083/jcb.201909154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Castanier C., Garcin D., Vazquez A. Mitochondrial dynamics regulate the RIG‐I‐like receptor antiviral pathway. EMBO Rep. 2010;11(2):133–138. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pernas L., Scorrano L. Mito-morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu. Rev. Physiol. 2016;78:505–531. doi: 10.1146/annurev-physiol-021115-105011. [DOI] [PubMed] [Google Scholar]
- 109.West A.P., Shadel G.S., Ghosh S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Onoguchi K., Onomoto K., Takamatsu S. Virus-infection or 5′ ppp-RNA activates antiviral signal through redistribution of IPS-1 mediated by MFN1. PLoS Pathog. 2010;6(7) doi: 10.1371/journal.ppat.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shi C.S., Qi H.Y., Boularan C. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 2014;193(6):3080–3089. doi: 10.4049/jimmunol.1303196. [published Online First: 2014/08/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mills E.L., Kelly B., O'Neill L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017;18(5):488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 113.Gupta A., Madhavan M.V., Sehgal K. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 114.Makowski L., Chaib M., Rathmell J.C. Immunometabolism: from basic mechanisms to translation. Immunol. Rev. 2020;295(1):5–14. doi: 10.1111/imr.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lercher A., Baazim H., Bergthaler A. Systemic immunometabolism: challenges and opportunities. Immunity. 2020;53(3):496–509. doi: 10.1016/j.immuni.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang A., Luan H.H., Medzhitov R. An evolutionary perspective on immunometabolism. Science. 2019;363(6423):eaar3932. doi: 10.1126/science.aar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang B., Li R., Lu Z. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (N Y) 2020;12(7):6049. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lippi G., Henry B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir. Med. 2020 doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Larsen F.J., Schiffer T.A., Zinner C. Mitochondrial oxygen affinity increases after sprint interval training and is related to the improvement in peak oxygen uptake. Acta Physiol. 2020 doi: 10.1111/apha.13463. [published Online First: 2020/03/08] [DOI] [PubMed] [Google Scholar]
- 120.Sun N., Youle R.J., Finkel T. The mitochondrial basis of aging. Mol. Cell. 2016;61(5):654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holloszy J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967;242(9):2278–2282. [published Online First: 1967/05/10] [PubMed] [Google Scholar]
- 122.Bishop D.J., Botella J., Genders A.J. High-intensity exercise and mitochondrial biogenesis: current controversies and future research directions. Physiology. 2019;34(1):56–70. doi: 10.1152/physiol.00038.2018. [DOI] [PubMed] [Google Scholar]
- 123.Granata C., Jamnick N.A., Bishop D.J. Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med. 2018;48(8):1809–1828. doi: 10.1007/s40279-018-0936-y. [DOI] [PubMed] [Google Scholar]
- 124.Hood D.A. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 2001;90(3):1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- 125.Granata C., Oliveira R.S., Little J.P. Mitochondrial adaptations to high‐volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. Faseb. J. 2016;30(10):3413–3423. doi: 10.1096/fj.201500100R. [DOI] [PubMed] [Google Scholar]
- 126.Jacobs R.A., Rasmussen P., Siebenmann C. Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. J. Appl. Physiol. 2011 doi: 10.1152/japplphysiol.00625.2011. [DOI] [PubMed] [Google Scholar]
- 127.Jacobs R.A., Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J. Appl. Physiol. 2013;114(3):344–350. doi: 10.1152/japplphysiol.01081.2012. [DOI] [PubMed] [Google Scholar]
- 128.Broskey N.T., Greggio C., Boss A. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J. Clin. Endocrinol. Metabol. 2014;99(5):1852–1861. doi: 10.1210/jc.2013-3983. [DOI] [PubMed] [Google Scholar]
- 129.Huertas J.R., Casuso R.A., Agustín P.H. Stay fit, stay young: mitochondria in movement: the role of exercise in the new mitochondrial paradigm. Oxidative Medicine and Cellular Longevity. 2019;2019:7058350. doi: 10.1155/2019/7058350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Romanello V., Sandri M. The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass. Cell. Mol. Life Sci. 2020 doi: 10.1007/s00018-020-03662-0. [published Online First: 2020/10/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neufer P.D., Bamman M.M., Muoio D.M. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metabol. 2015;22(1):4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 132.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [published Online First: 2012/04/05] [DOI] [PubMed] [Google Scholar]
- 133.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dorward D.A., Russell C.D., Um I.H. Tissue-specific immunopathology in fatal Covid-19. Am. J. Respir. Crit. Care Med. 2021;203(2):192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Egan B., Zierath Juleen R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabol. 2013;17(2):162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 137.Granata C., Jamnick N.A., Bishop D.J. Principles of exercise prescription, and how they influence exercise-induced changes of transcription factors and other regulators of mitochondrial biogenesis. Sports Med. 2018;48(7):1541–1559. doi: 10.1007/s40279-018-0894-4. [DOI] [PubMed] [Google Scholar]
- 138.Burgomaster K.A., Howarth K.R., Phillips S.M. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008;586(1):151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.MacInnis M.J., Zacharewicz E., Martin B.J. Superior mitochondrial adaptations in human skeletal muscle after interval compared to continuous single‐leg cycling matched for total work. J. Physiol. 2017;595(9):2955–2968. doi: 10.1113/JP272570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Irving B.A., Lanza I.R., Henderson G.C. Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. J. Clin. Endocrinol. Metabol. 2015;100(4):1654–1663. doi: 10.1210/jc.2014-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Picard M., Gentil B.J., McManus M.J. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J. Appl. Physiol. 2013;115(10):1562–1571. doi: 10.1152/japplphysiol.00819.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bhatia R.T., Marwaha S., Malhotra A. Exercise in the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) era: a question and answer session with the experts endorsed by the section of sports cardiology & exercise of the European association of preventive cardiology (EAPC) European Journal of Preventive Cardiology. 2020 doi: 10.1177/2047487320930596. 2047487320930596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barker-Davies R.M., O'Sullivan O., Senaratne K.P.P. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020 doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nielsen J., Gejl K.D., Hey‐Mogensen M. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J. Physiol. 2017;595(9):2839–2847. doi: 10.1113/JP273040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greggio C., Jha P., Kulkarni S.S. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metabol. 2017;25(2):301–311. doi: 10.1016/j.cmet.2016.11.004. [published Online First: 2016/12/06] [DOI] [PubMed] [Google Scholar]
- 146.Halling J.F., Ringholm S., Olesen J. Exercise training protects against aging-induced mitochondrial fragmentation in mouse skeletal muscle in a PGC-1α dependent manner. Exp. Gerontol. 2017;96:1–6. doi: 10.1016/j.exger.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 147.Arribat Y., Broskey N.T., Greggio C. Distinct patterns of skeletal muscle mitochondria fusion, fission and mitophagy upon duration of exercise training. Acta Physiol. 2019;225(2) doi: 10.1111/apha.13179. [DOI] [PubMed] [Google Scholar]
- 148.Gomez-Cabrera M.C., Viña J., Olaso-Gonzalez G. Special issue: exercise redox biology from health to performance. Redox Biology. 2020;35:101584. doi: 10.1016/j.redox.2020.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Henriquez-Olguin C., Meneses-Valdes R., Jensen T.E. Compartmentalized muscle redox signals controlling exercise metabolism – current state, future challenges. Redox Biology. 2020;35:101473. doi: 10.1016/j.redox.2020.101473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Margaritelis N.V., Paschalis V., Theodorou A.A. Redox basis of exercise physiology. Redox Biology. 2020;35:101499. doi: 10.1016/j.redox.2020.101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Radak Z., Chung H.Y., Koltai E. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008;7(1):34–42. doi: 10.1016/j.arr.2007.04.004. [published Online First: 2007/09/18] [DOI] [PubMed] [Google Scholar]
- 152.Pesta D., Roden M. The Janus head of oxidative stress in metabolic diseases and during physical exercise. Curr. Diabetes Rep. 2017;17(6):41. doi: 10.1007/s11892-017-0867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Holloway G.P. Nutrition and training influences on the regulation of mitochondrial adenosine diphosphate sensitivity and bioenergetics. Sports Med. 2017;47(Suppl 1):13–21. doi: 10.1007/s40279-017-0693-3. [published Online First: 2017/03/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bouzid M.A., Filaire E., Matran R. Lifelong voluntary exercise modulates age-related changes in oxidative stress. Int. J. Sports Med. 2018;39(1):21–28. doi: 10.1055/s-0043-119882. [published Online First: 2017/11/24] [DOI] [PubMed] [Google Scholar]
- 155.Lancaster G.I., Febbraio M.A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014;35(6):262–269. doi: 10.1016/j.it.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 156.Valacchi G., Virgili F., Cervellati C. OxInflammation: from subclinical condition to pathological biomarker. Front. Physiol. 2018;9:858. doi: 10.3389/fphys.2018.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu M., Chen F., Liu T. The role of oxidative stress in influenza virus infection. Microb. Infect. 2017;19(12):580–586. doi: 10.1016/j.micinf.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 158.Lowery E.M., Brubaker A.L., Kuhlmann E. The aging lung. Clin. Interv. Aging. 2013;8:1489–1496. doi: 10.2147/cia.S51152. [published Online First: 2013/11/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sorbini C., Grassi V., Solinas E. Arterial oxygen tension in relation to age in healthy subjects. Respiration. 1968;25(1):3–13. doi: 10.1159/000192549. [DOI] [PubMed] [Google Scholar]
- 160.Ungvari Z., Tarantini S., Kiss T. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018;15(9):555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lye M., Donnellan C. Heart disease in the elderly. Heart. 2000;84(5):560–566. doi: 10.1136/heart.84.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Capelli C., Rittveger J., Bruseghini P. Maximal aerobic power and anaerobic capacity in cycling across the age spectrum in male master athletes. Eur. J. Appl. Physiol. 2016;116(7):1395–1410. doi: 10.1007/s00421-016-3396-9. [DOI] [PubMed] [Google Scholar]
- 163.Booth F.W., Roberts C.K., Laye M.J. Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology. 2011;2(2):1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Valenzuela P.L., Maffiuletti N.A., Joyner M.J. Lifelong endurance exercise as a countermeasure against age-related VO2max decline: physiological overview and insights from masters athletes. Sports Med. 2020;50(4):703–716. doi: 10.1007/s40279-019-01252-0. [DOI] [PubMed] [Google Scholar]
- 165.Srivastava S. The mitochondrial basis of aging and age-related disorders. Genes. 2017;8(12):398. doi: 10.3390/genes8120398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chistiakov D.A., Sobenin I.A., Revin V.V. Mitochondrial aging and age-related dysfunction of mitochondria. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/238463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Conley K.E., Amara C.E., Jubrias S.A. Mitochondrial function, fibre types and ageing: new insights from human muscle in vivo. Exp. Physiol. 2007;92(2):333–339. doi: 10.1113/expphysiol.2006.034330. [published Online First: 2006/12/16] [DOI] [PubMed] [Google Scholar]
- 168.Liu Y.J., McIntyre R.L., Janssens G.E. Mitochondrial fission and fusion: a dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020;186:111212. doi: 10.1016/j.mad.2020.111212. [DOI] [PubMed] [Google Scholar]
- 169.Montgomery M.K., Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4(1):R1–r15. doi: 10.1530/ec-14-0092. [published Online First: 2014/11/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Short K.R., Bigelow M.L., Kahl J. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. Unit. States Am. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brierley E.J., Johnson M.A., James O.F. Mitochondrial involvement in the ageing process. Facts and controversies. Mol. Cell. Biochem. 1997;174(1–2):325–328. [published Online First: 1997/10/06] [PubMed] [Google Scholar]
- 172.McGuire P.J. Mitochondrial dysfunction and the aging immune system. Biology. 2019;8(2):26. doi: 10.3390/biology8020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Molony R.D., Nguyen J.T., Kong Y. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci. Signal. 2017;10(509) doi: 10.1126/scisignal.aan2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chan D.C. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 175.Salminen A. Activation of immunosuppressive network in the aging process. Ageing Res. Rev. 2020;57:100998. doi: 10.1016/j.arr.2019.100998. [DOI] [PubMed] [Google Scholar]
- 176.Franceschi C., Bonafè M., Valensin S. Inflamm‐aging: an evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908(1):244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 177.Weyand C.M., Goronzy J.J. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13(Suppl 5):S422. doi: 10.1513/AnnalsATS.201602-095AW. [published Online First: 2016/12/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Desdín-Micó G., Soto-Heredero G., Aranda J.F. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020 doi: 10.1126/science.aax0860. [DOI] [PubMed] [Google Scholar]
- 179.Omarjee L., Perrot F., Meilhac O. Immunometabolism at the cornerstone of inflammaging, immunosenescence, and autoimmunity in COVID-19. Aging (N Y) 2020;12(24):26263–26278. doi: 10.18632/aging.202422. [published Online First: 2020/12/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lavin K.M., Perkins R.K., Jemiolo B. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J. Appl. Physiol. 2019;128(1):87–99. doi: 10.1152/japplphysiol.00495.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Simonsen L., Clarke M.J., Schonberger L.B. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J. Infect. Dis. 1998;178(1):53–60. doi: 10.1086/515616. [DOI] [PubMed] [Google Scholar]
- 182.Thompson W.W., Shay D.K., Weintraub E. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 183.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy. Jama. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Steell L., Ho F.K., Sillars A. Dose-response associations of cardiorespiratory fitness with all-cause mortality and incidence and mortality of cancer and cardiovascular and respiratory diseases: the UK Biobank cohort study. Br. J. Sports Med. 2019;53(21):1371–1378. doi: 10.1136/bjsports-2018-099093. [DOI] [PubMed] [Google Scholar]
- 186.Carnethon M.R., Gulati M., Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. Jama. 2005;294(23):2981–2988. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]
- 187.Ballinger S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radic. Biol. Med. 2005;38(10):1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 188.Fetterman J.L., Holbrook M., Westbrook D.G. Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc. Diabetol. 2016;15(1):53. doi: 10.1186/s12933-016-0372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chistiakov D.A., Shkurat T.P., Melnichenko A.A. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann. Med. 2018;50(2):121–127. doi: 10.1080/07853890.2017.1417631. [published Online First: 2017/12/15] [DOI] [PubMed] [Google Scholar]
- 190.Hansen G.M., Marott J.L., Holtermann A. Midlife cardiorespiratory fitness and the long-term risk of chronic obstructive pulmonary disease. Thorax. 2019;74(9):843–848. doi: 10.1136/thoraxjnl-2018-212821. [DOI] [PubMed] [Google Scholar]
- 191.Rabinovich R.A., Bastos R., Ardite E. Mitochondrial dysfunction in COPD patients with low body mass index. Eur. Respir. J. 2007;29(4):643–650. doi: 10.1183/09031936.00086306. [DOI] [PubMed] [Google Scholar]
- 192.Wiegman C.H., Michaeloudes C., Haji G. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015;136(3):769–780. doi: 10.1016/j.jaci.2015.01.046. [published Online First: 2015/04/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Papi A., Bellettato C.M., Braccioni F. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006;173(10):1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 194.Myers J., Prakash M., Froelicher V. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 195.Vogelmeier C.F., Criner G.J., Martinez F.J. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 196.Antunes B., Cayres S., Lira F. Arterial thickness and Immunometabolism: the mediating role of chronic exercise. Curr. Cardiol. Rev. 2016;12(1):47–51. doi: 10.2174/1573403X12666160126115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Karstoft K., Pedersen B.K. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol. Cell Biol. 2016;94(2):146–150. doi: 10.1038/icb.2015.101. [DOI] [PubMed] [Google Scholar]
- 198.Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 Years is a risk factor for COVID-19 hospital admission. Clin. Infect. Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395(10236):1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stefan N., Birkenfeld A.L., Schulze M.B. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cai Q., Chen F., Wang T. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 202.Tartof S.Y., Qian L., Hong V. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann. Intern. Med. 2020 doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ritter A., Kreis N.-N., Louwen F. Obesity and COVID-19: molecular mechanisms linking both pandemics. Int. J. Mol. Sci. 2020;21(16):5793. doi: 10.3390/ijms21165793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Klang E., Kassim G., Soffer S. Morbid obesity as an independent risk factor for COVID‐19 mortality in hospitalized patients younger than 50. Obesity. 2020 doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Drucker D.J. Diabetes, obesity, metabolism and SARS-CoV-2 infection: the end of the beginning. Cell Metabol. 2021 doi: 10.1016/j.cmet.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sheridan P.A., Paich H.A., Handy J. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012;36(8):1072–1077. doi: 10.1038/ijo.2011.208. [published Online First: 2011/10/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Green W.D., Beck M.A. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc. 2017;14(Supplement_5):S406. doi: 10.1513/AnnalsATS.201706-447AW. [published Online First: 2017/11/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Honce R., Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front. Immunol. 2019;10:1071. doi: 10.3389/fimmu.2019.01071. [published Online First: 2019/05/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rojas-Osornio S.A., Cruz-Hernández T.R., Drago-Serrano M.E. Immunity to influenza: impact of obesity. Obes. Res. Clin. Pract. 2019;13(5):419–429. doi: 10.1016/j.orcp.2019.05.003. [published Online First: 2019/09/23] [DOI] [PubMed] [Google Scholar]
- 210.Schaffler A., Muller-Ladner U., Scholmerich J. Role of adipose tissue as an inflammatory organ in human diseases. Endocr. Rev. 2006;27(5):449–467. doi: 10.1210/er.2005-0022. [DOI] [PubMed] [Google Scholar]
- 211.Mayo Clinic Proceedings. Elsevier; 2012. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Katzmarzyk P.T., Church T.S., Janssen I. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 2005;28(2):391–397. doi: 10.2337/diacare.28.2.391. [DOI] [PubMed] [Google Scholar]
- 213.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020:1–8. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Boyle L.J., Credeur D.P., Jenkins N.T. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J. Appl. Physiol. 2013;115(10):1519–1525. doi: 10.1152/japplphysiol.00837.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Islam M.N., Das S.R., Emin M.T. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Phelan D., Kim J.H., Chung E.H. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiology. 2020;5(10):1085–1086. doi: 10.1001/jamacardio.2020.2136. [DOI] [PubMed] [Google Scholar]
- 217.Porter C., Reidy P.T., Bhattarai N. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med. Sci. Sports Exerc. 2015;47(9):1922–1931. doi: 10.1249/mss.0000000000000605. [published Online First: 2014/12/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Berber E., Sumbria D., Rouse B.T. Could targeting immunometabolism be a way to control the burden of COVID-19 infection? Microb. Infect. 2021:104780. doi: 10.1016/j.micinf.2021.104780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kumar S.T., Donzelli S., Chiki A. A simple, versatile and robust centrifugation-based filtration protocol for the isolation and quantification of α-synuclein monomers, oligomers and fibrils: towards improving experimental reproducibility in α-synuclein research. J. Neurochem. 2020:e14955. doi: 10.1111/jnc.14955. e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gleeson L.E., Roche H.M., Sheedy F.J. Obesity, COVID-19 and innate immunometabolism. Br. J. Nutr. 2020:1–5. doi: 10.1017/S0007114520003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Swenson K.E., Ruoss S.J., Swenson E.R. The pathophysiology and dangers of silent hypoxemia in COVID-19 lung injury. Annals of the American Thoracic Society. 2021 doi: 10.1513/AnnalsATS.202011-1376CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Simonson T.S., Baker T.L., Banzett R.B. Silent hypoxaemia in COVID-19 patients. J. Physiol. 2021;599(4):1057–1065. doi: 10.1113/JP280769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Peake J.M., Neubauer O., Walsh N.P. Recovery of the immune system after exercise. J. Appl. Physiol. 2017;122(5):1077–1087. doi: 10.1152/japplphysiol.00622.2016. [DOI] [PubMed] [Google Scholar]
- 224.Jesus I., Vanhee V., Deramaudt T.B. Promising effects of exercise on the cardiovascular, metabolic and immune system during COVID-19 period. J. Hum. Hypertens. 2021;35(1):1–3. doi: 10.1038/s41371-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Burtscher J., Burtscher M. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine; 2020. Run for Your Life: Tweaking the Weekly Physical Activity Volume for Longevity. [DOI] [PubMed] [Google Scholar]
- 226.Wu S., Ma C., Yang Z. Hygiene behaviors associated with influenza-like illness among adults in Beijing, China: a large, population-based survey. PloS One. 2016;11(2) doi: 10.1371/journal.pone.0148448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wong C.-M., Lai H.-K., Ou C.-Q. Is exercise protective against influenza-associated mortality? PloS One. 2008;3(5) doi: 10.1371/journal.pone.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wong C., Chan W., Yang L. Effect of lifestyle factors on risk of mortality associated with influenza in elderly people. Hong Kong Med. J. 2014 [PubMed] [Google Scholar]
- 229.Swain D.P., Franklin B.A. VO2 reserve and the minimal intensity for improving cardiorespiratory fitness. Med. Sci. Sports Exerc. 2002;34(1) doi: 10.1097/00005768-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 230.Bull F.C., Al-Ansari S.S., Biddle S. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020;54(24):1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Caillaud C., Py G., Eydoux N. Antioxidants and mitochondrial respiration in lung, diaphragm, and locomotor muscles: effect of exercise. Free Radic. Biol. Med. 1999;26(9–10):1292–1299. doi: 10.1016/s0891-5849(98)00342-6. [DOI] [PubMed] [Google Scholar]
- 232.Yan Q.-W., Zhao N., Xia J. Effects of treadmill exercise on mitochondrial fusion and fission in the hippocampus of APP/PS1 mice. Neurosci. Lett. 2019;701:84–91. doi: 10.1016/j.neulet.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 233.Memme J.M., Erlich A.T., Phukan G. Exercise and mitochondrial health. J. Physiol. 2021;599(3):803–817. doi: 10.1113/JP278853. [DOI] [PubMed] [Google Scholar]
- 234.Murphy R.M., Watt M.J., Febbraio M.A. Metabolic communication during exercise. Nature Metabolism. 2020;2(9):805–816. doi: 10.1038/s42255-020-0258-x. [DOI] [PubMed] [Google Scholar]
- 235.Nieß A., Bloch W., Friedmann-Bette B. Position stand: return to sport in the current coronavirus pandemic. Dtsch. Z. Sportmed. 2020;71:E1–E2. [Google Scholar]
- 236.Schellhorn P., Klingel K., Burgstahler C. Return to sports after COVID-19 infectionDo we have to worry about myocarditis? Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa448. [DOI] [PMC free article] [PubMed] [Google Scholar]