Abstract
Adiposity may cause adverse health outcomes by increasing oxidative stress and systemic inflammation, which can be reflected by altered telomere length (TL) and mitochondrial DNA copy number (mtCN) in peripheral blood leukocytes. However, little is known about the influence of lifetime adiposity on TL and mtCN in later life. This study was performed to investigate the associations of lifetime adiposity with leukocyte TL and mtCN in 9613 participants from the Nurses’ Health Study. A group-based trajectory modelling approach was used to create trajectories of body shape from age 5 through 60 years, and a genetic risk score (GRS) was created based on 97 known adiposity susceptibility loci. Associations of body shape trajectories and GRS with dichotomized TL and mtCN were assessed by logistic regression models. After adjustment for lifestyle and dietary factors, compared with the lean-stable group, the lean-marked increase group had higher odds of having below-median TL (OR = 1.18, 95% CI: 1.04, 1.35; P = 0.01), and the medium-marked increase group had higher odds of having below-median mtCN (OR = 1.28, 95% CI:1.00,1.64; P = 0.047). There was a suggestive trend toward lower mtCN across the GRS quartiles (P for trend = 0.07). In conclusion, telomere attrition may be accelerated by marked weight gain in middle life, whereas mtCN is likely to be reduced persistently by adiposity over the life course. The findings indicate the importance of lifetime weight management to preserve functional telomeres and mitochondria.
Keywords: Adiposity, Telomere, Mitochondrion, Trajectory analysis, Genetic variants
Introduction
The rising prevalence of overweight and obesity in a number of countries has been considered a global pandemic [1]. Between 1980 and 2013, global prevalence of overweight and obesity combined rose by 27.5% for adults and 47.1% for children [2]. Obesity and overweight increase the risk of major chronic diseases, such as diabetes, cardiovascular disorders, and certain types of cancer [3]. An important pathway through which adiposity contributes to these outcomes is by an increase in oxidative stress and systemic inflammation, leading to progressive cellular damage [4].
Both telomeres and mitochondria serve as critical regulators of the ageing process, and are prone to structural damage caused by excessive oxidative stress [5, 6]. Telomeres are repetitive nucleoprotein complexes that protect the ends of linear chromosomes and maintain genomic stability [7]. Telomeres undergo shortening with cell divisions and this erosion can be increased by oxidative stress [8]. Extremely short and dysfunctional telomeres may elicit chromosomal degradation, end-to-end fusion, and atypical recombination, which have been implicated in disease development [9]. Mitochondria are organelles in the cytoplasm of eukaryotic cells with various functions including energy metabolism, free-radical production, calcium homeostasis, and apoptosis [10]. Mitochondrial DNA (mtDNA) is known to be more sensitive to oxidative damage than nuclear DNA due to its lack of protective histones, introns, and efficient DNA repair mechanisms [11]. Increased oxidative stress may lead to altered abundance and compromised functions of mitochondria. Many epidemiologic studies have linked decreased telomere length (TL) and mitochondrial DNA copy number (mtCN) in peripheral blood leukocytes to elevated risk of obesity-related diseases [12–15]. Therefore, alterations in leukocyte TL and mtCN may reflect the cumulative exposure to oxidative stress and underlie the pathogenesis of obesity-related comorbidities.
To date, studies examining the associations of adiposity with leukocyte TL and mtCN have yielded mixed results. A recent meta-analysis of twelve studies including 8010 participants found non-significantly shorter TL in obese individuals compared to those with normal weight [16]. However, most of the included studies were cross-sectional. Longitudinal studies linking adiposity to TL attrition were sparse with mixed findings [17–20]. More importantly, these studies assessed body mass index (BMI) at only a limited number of time points and were thus unable to capture the long-term effect of adiposity dynamics. For mtCN, epidemiologic evidence about the relationship with adiposity is very limited. Two cross-sectional studies reported an inverse association of mtCN with visceral fat area [21] and waist circumference [22], but not with BMI [22]. In our previous analysis in women from the Nurses’ Health Study (NHS), middle-life BMI was associated with lower mtCN [23].
Given that BMI typically increases with age, and obesity during childhood is associated with the persistence of obesity into adulthood [24], a life course perspective is crucial to better understand the pathophysiological impact of adiposity. In this study, we employed two approaches to assess the association of lifetime adiposity with leukocyte TL and mtCN in women from the Nurses’ Health Study. First, we characterized the trajectories of body shape from age 5 through 60 years using a group-based modeling approach. This approach respects the continuity of body growth throughout life and classifies individuals into distinct, mutually exclusive groups [25]. Second, given the well-established heritability of BMI, we employed genetic proxies for lifetime adiposity by creating a genetic risk score (GRS) based on 97 common genetic variants that have been robustly associated with higher BMI [26]. This genetic approach also helps to establish causality because the genetic information is not confounded by environmental factors. By linking the early-to-middle-life body shape trajectories and GRS with leukocyte TL and mtCN in later life, our study provides the first evidence regarding the influence of lifetime adiposity on telomere and mitochondrial biology.
Materials and methods
Study population
The Nurses’ Health Study (NHS) is a prospective cohort established in 1976 when 121700 female registered nurses aged 30 to 55 years in 11 US states completed and returned a mailed questionnaire. Detailed information on personal characteristics (e.g. lifestyle and dietary factors) and new disease diagnoses has been updated every 2–4 years by questionnaire. From 1989 to 1990, 32826 women provided blood samples and completed a questionnaire (Supplementary Fig. 1, Online Resource). Details of the NHS and blood collection procedure have been described previously [27]. The protocol for this study was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard T.H. Chan School Public Health.
The current analysis was restricted to 9061 participants who had available TL data from previous nested case-control studies for cancer, cardiovascular disorders, rheumatoid arthritis, and cognitive disease [28–34], and 4411 participants with available mtCN data from nested case-control studies for skin, lung, and colorectal cancers [35, 36]. After excluding non-Caucasians (n = 346) and those who had a history of diabetes, cardiovascular disease, and cancer at the time of blood collection (n = 697), a total of 9613 participants (n = 8229 for TL and 4007 for mtCN) were included in the final analysis. These participants were demographically similar to others in the overall NHS cohort in 1990 (Supplementary Table 1, Online Resource).
Assessment of body shape
In 1988, participants were asked to recall their body shape in early and middle life by choosing one of 9 pictorial body diagrams (somatotypes) developed by Stunkard et al [37] that best reflected their body shape at age 5, 10, 20, 30, and 40 (Supplementary Fig. 1, Online Resource). The validity of this measure as a surrogate for adiposity in early life has been assessed among 181 participants aged 71 to 76 in the Third Harvard Growth Study [38]. Comparisons between participants’ recalled somatotypes and their measured BMI at similar ages showed Pearson correlation coefficients of 0.60 for age 5, 0.65 for age 10, and 0.66 for age 20 [38].
Body height and weight were inquired at baseline and updated weight was collected via follow-up questionnaires. Recalled weight at age 18 years was obtained in 1980. We used these data to calculate BMI at age 40, 45, 50, 55, and 60. To minimize random variation, we calculated the average BMI within two years for each age. To convert BMI in later life to the same scale as somatotypes, we built a linear regression model for somatotype and BMI at age 40 among women with available data, and then applied the regression coefficients and BMI to impute the somatotype from age 40 to 60. The imputed somatotype was highly correlated with participants’ reported somatotype at age 40 (r = 0.74) and in 1988 (r = 0.83), indicating good performance of our rescaling method.
Trajectory modeling
A group-based trajectory modeling approach was used to identify subgroups that shared a similar trajectory of body shape from age 5 up to 60. This method represents an application of finite mixture modeling to identify relatively homogeneous clusters of developmental trajectories within the population [39]. It fits longitudinal data as a discrete mixture of multiple latent trajectories via maximum likelihood estimation using SAS Proc Traj [40]. In this study, we used a censored normal model with a polynomial function of age. The optimal number of groups and the shapes of trajectories were selected for the best fit to the data using a two-stage approach, as previously described [41]. Finally, we identified that the five-group model with cubic order function of age fit the data best. We then named the trajectory groups to describe their visual patterns: lean-stable, lean-moderate increase, medium-stable, lean-marked increase, and medium-marked increase.
We calculated the posterior predicted probability for each participant of being a member of each of the trajectories. Using ≥ 0.70 as the recommended criteria [42], our model demonstrated excellent performance in classifying individuals into distinct trajectory groups: the average posterior probability for each trajectory group was 0.93, 0.97, 0.90, 0.91, and 0.96.
Covariate assessment
Potential confounders were derived from the questionnaires administrated prior to the time of blood collection. Waist circumference and waist-hip ratio were obtained from the 1986 questionnaire, and information on menopausal status, postmenopausal hormone use, multivitamin use and pack-years of smoking was derived from the 1990 questionnaire. Average level of physical activity was assessed by questionnaires in 1986 and 1988. Average of a summary dietary score, the Alternate Healthy Eating Index (AHEI), was calculated to represent the overall dietary pattern based on individual food intake from food frequency questionnaires (FFQs) in 1980, 1984, 1986, and 1990. Similarly, we also calculated average consumption of alcohol based on these FFQs. The validity and reproducibility of these measures have been reported elsewhere [43].
Measurement of leukocyte TL and mtCN
Genomic DNA was extracted from peripheral blood leukocytes following the QiAmp (Qiagen, Chatsworth, CA) 96-spin blood protocol. DNA concentrations were determined via pico-green quantitation using a Molecular Devices 96-well spectrophotometer.
Relative TL was measured by use of quantitative polymerase chain reaction (qPCR). The average relative TL was calculated as the telomere repeat copy number / a single gene (36B4) copy number (T/S) exponentiated ratio [44]. Each sample was assayed in triplicate and quality control samples were interspersed on each plate to assess variability. The coefficients of variation (CVs) for the telomere and single gene assays in the quality control (QC) samples were less than 4%. Although this assay provides a relative measurement of telomere length, T/S ratios correlate well with absolute telomere lengths determined by Southern blot (r = 0.68, P < 0.001) [44].
Relative mtCN was determined by using qPCR, and the ratio of mitochondrial ND2 gene copy number to a single gene (aAluYb8) copy number (N/S) was calculated [23]. The average relative mtCN was the exponentiated N/S, which is proportional to the average number of mtCN. Each sample was assayed in triplicate and 10% replicate quality control samples were included. The CVs for ND2 and AluYb8 assays in the QC samples were less than 1%.
Genotyping and GRS calculation
We included 97 SNPs that are confirmed to be associated with BMI [26]. SNP genotyping and imputation have been described in detail elsewhere [45]. All of the SNPs were genotyped or had high imputation quality (r2 ≥ 0.8), as assessed by the MACH software (version 1.0.16, Center for Statistical Genetics, University of Michigan). We calculated a GRS from the 97 SNPs by using an established weighting method. Each SNP genotype was coded as 0, 1, and 2 according to the number of risk alleles (higher BMI-related) and weighted by its relative effect size (β-coefficient) derived from a recent GWAS meta-analysis for BMI [26]. The GRS was calculated by multiplying each β-coefficient by the number of corresponding risk alleles and then summing the products. Each unit of the GRS represented one risk allele, and the GRS could range from 0 to 194, with a higher score indicating greater genetic predisposition to obesity.
Statistical analysis
We calculated z-scores within each of the case-control studies to standardize the distributions of TL and mtCN. Age-adjusted Spearman correlation coefficients (rs) were computed to examine the relationships of TL and mtCN with somatotypes at age 5 and 10; BMI at age 18, 40, 50, and 60; and BMI change between age 18 and 60. We examined the associations of body shape trajectories with median-dichotomized TL and mtCN z-scores using unconditional logistic regression. We adjusted for age at blood collection, as well as other potential confounding factors, including height, menopausal status, postmenopausal hormone use, physical activity, alcohol consumption, pack-years of smoking, AHEI dietary score, and multivitamin use.
Because 5236 participants (54%) were younger than 60 years at the time of blood collection and their body weight at age 60 was assessed after TL/mtCN measurement, we performed a stratified analysis by age at blood collection (<60, ≥60 years) to examine the sensitivity of our findings to inclusion of these individuals. Given the intricate relationship between smoking and body weight dynamics [46], we also stratified the data by smoking status (ever, never). A likelihood ratio test was performed to assess interaction, by comparing the models with and without product terms between the trajectory groups and the stratified variable.
Similar analyses were performed to examine the association of GRS and individual SNPs with TL and mtCN. To account for multiple testing for individual SNP analysis, we calculated the false discovery rate (FDR) using the Benjamini-Hochberg method. All statistical analyses were conducted by SAS Version 9.4 software (SAS Institute Inc, Cary, NC), and P < 0·05 was considered statistically significant.
Results
By using the group-based trajectory modeling approach, we identified five heterogeneous trajectories of body fatness from age 5 up to 60 among 9613 women. Fig. 1 shows the estimated mean body shape levels in the five trajectories at each age: 34% (3275) of women maintained a lean and relatively stable body shape throughout life (lean-stable group); 23% (2178) of women started lean and then experienced a moderate increase in body fatness in middle age (lean-moderate increase group); 17% (1593) of women maintained a medium body shape throughout life (medium-stable group); 17% (1689) of women started lean and then gained a substantial amount of weight (lean-marked increase group); and 9% (878) of women started with a medium body shape and then gained more weight over age (medium-marked increase group).
Fig. 1.
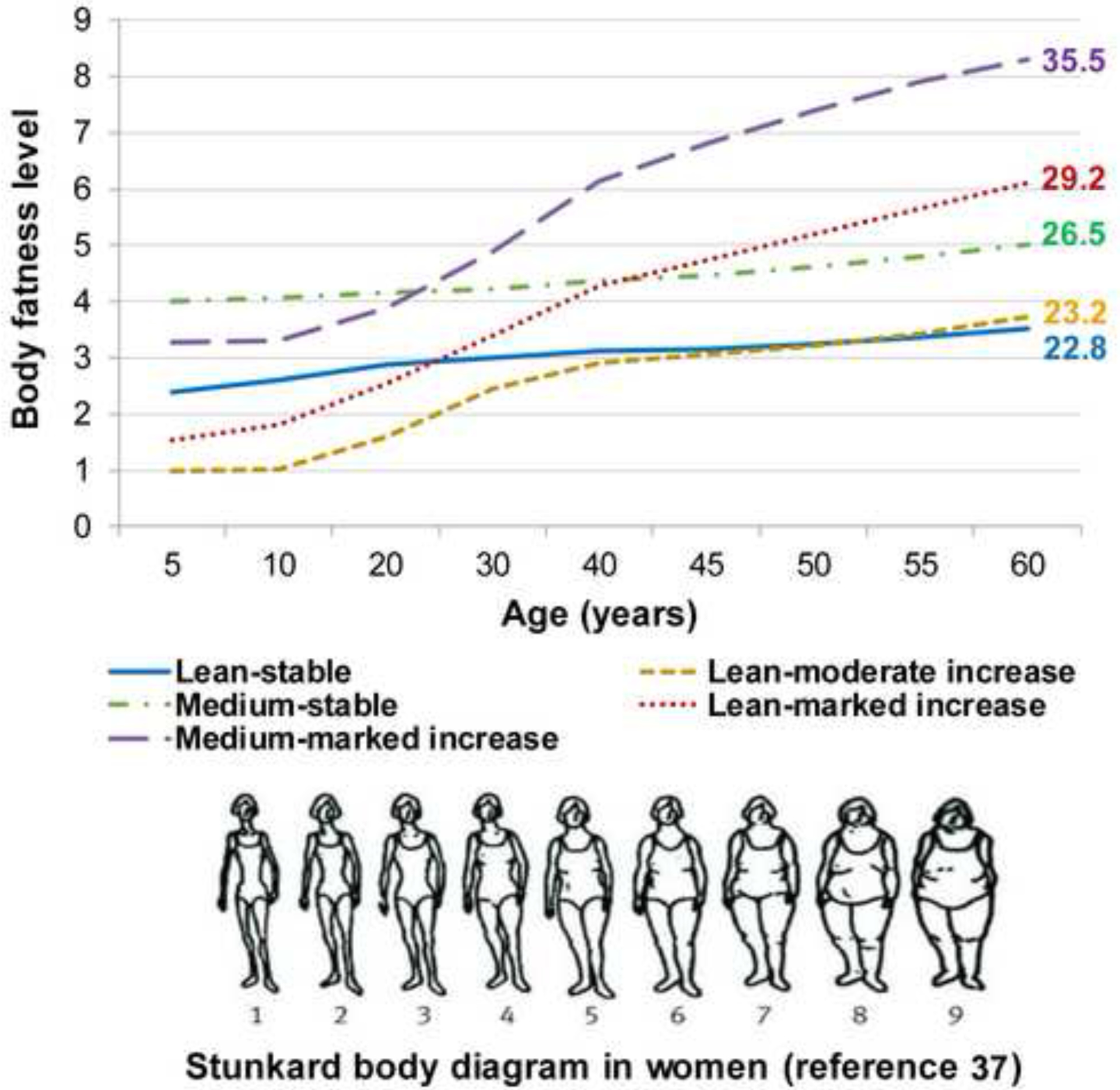
Trajectories of body shape by age among 9613 women in the Nurses’ Health Study.
Mean body mass index at age 60 is shown for each trajectory group
The descriptive characteristics of participants across trajectory groups are shown in Table 1. As expected, the BMI profile throughout adulthood in each group conformed well to the patterns of the identified trajectories, and participants in the heavier trajectories had higher GRS. We also noted that participants in the five trajectories showed distinct lifestyle patterns: those in the lean-stable, lean-moderate increase and medium-stable groups were more physically active and consumed a healthier diet than those in the lean-marked increase and medium-marked increase groups.
Table 1.
Characteristics of 9613 women at blood collection (Nurses’ Health Study) according to body shape trajectories1
| Variable | Lean-stable | Lean-moderate increase | Medium-stable | Lean-marked increase | Medium-marked increase |
|---|---|---|---|---|---|
| Participants, n (%) | 3275(34) | 2178(23) | 1593(17) | 1689(17) | 878(9) |
| Age at blood collection, years | 58.7(6.6) | 59.8(6.3) | 58.4(6.7) | 57.0(6.7) | 55.8(6.7) |
| Genetic risk score2 | 87.3(6.0) | 87.3(5.9) | 89.3(5.8) | 88.5(5.7) | 90.0(5.4) |
| BMI at age 18 (kg/m2) | 20.6(1.9) | 19.4(1.8) | 23.3(2.8) | 21.5(2.4) | 24.6(3.5) |
| BMI at age 40 (kg/m2) | 21.0(1.5) | 20.9(1.8) | 23.9(1.9) | 24.6(1.9) | 30.4(3.6) |
| BMI at age 50 (kg/m2) | 21.9(1.6) | 22.1(1.9) | 25.4(2.1) | 27.2(2.1) | 33.7(3.9) |
| BMI at age 60 (kg/m2) | 22.8(2.1) | 23.2(2.3) | 26.5(2.6) | 29.2(2.5) | 35.5(4.2) |
| Height (inch) | 64.6(2.4) | 64.7(2.3) | 64.6(2.3) | 64.3(2.4) | 64.3(2.4) |
| Waist circumference (cm)3 | 74.0(6.8) | 75.4(7.8) | 81.2(8.1) | 87.2(8.9) | 99.3(10.7) |
| Waist/hip ratio3 | .77(.06) | .78(.09) | .79(.11) | .82(.14) | .84(.11) |
| Postmenopause (%) | 80 | 80 | 82 | 80 | 80 |
| Current hormone use (%)4 | 47 | 47 | 44 | 35 | 27 |
| Physical activity (MET-h/wk)5 | 17.3(19.2) | 16.0(17.5) | 15.0(17.4) | 13.5(16.4) | 10.7(11.1) |
| Alcohol consumption (g/d)5 | 8.2(10.8) | 7.1(9.5) | 6.7(9.9) | 5.7(9.2) | 3.8(7.4) |
| Pack-years of smoking | 14.8(20.6) | 13.3(19.0) | 15.2(20.7) | 13.8(20.8) | 12.8(19.1) |
| Alternative Healthy Eating Index5 | 45.0(9.1) | 43.9(8.8) | 45.7(8.8) | 43.1(8.2) | 41.9(8.2) |
| Multivitamin use (%) | 40 | 39 | 39 | 40 | 37 |
All variables are standardized by age at blood collection. Mean (SD) is presented for continuous variables.
Sample size was 1512 in the lean-stable group, 1054 in the lean-moderate increase group, 749 in the medium-stable group, 861 in the lean-marked increase group, and 483 in the medium-marked increase group.
According to the questionnaire in 1986.
Current hormone use was defined in menopausal women.
Average measurements prior to blood collection.
Abbreviations: BMI, body mass index; MET, metabolic equivalent.
In Table 2, after adjustment for age at blood collection, the Spearman correlation analyses showed a statistically significant inverse association of TL z-score with BMI at age 50 (rs = −0.02) and 60 (rs = −0.03), as well as BMI change between age 18 and 60 (rs = −0.03). In contrast, mtCN z-score was inversely associated with body fatness from age 10 up to 60 years (rs ranged from −0.07 to −0.04). Additionally, we found that age at blood collection was inversely correlated with TL (rs = −0.09, P < 0.01), and age-adjusted pack-years of smoking was inversely correlated with both TL (rs = −0.03, P < 0.01) and mtCN (rs = −0.04, P < 0.01) (data not shown).
Table 2.
Age-adjusted Spearman correlations of leukocyte telomere length and mitochondria DNA copy number with body shape/BMI/waist circumference across the lifespan among women in the Nurses’ Health Study
| Body shape | Body shape | BMI | BMI | BMI | BMI | BMI change | Waist circumference | |
|---|---|---|---|---|---|---|---|---|
| (age 5) | (age 10) | (age 18) | (age 40) | (age 50) | (age 60) | (age 18–60) | (in 1986) | |
| TL z-score | ||||||||
| N | 7908 | 7949 | 7858 | 3441 | 7267 | 8135 | 7782 | 6011 |
| rsa | 0.01 | 0.002 | 0.005 | −0.02 | −.02b | −.03c | −.03c | −.03c |
| mtCN z-score | ||||||||
| N | 3846 | 3868 | 3811 | 1734 | 3556 | 3952 | 3769 | 2885 |
| rsa | −0.02 | −.04b | −.04b | −.07c | −.04b | −.05c | −0.03 | −.05c |
Spearman correlation coefficient adjusted for age at blood collection.
P < 0.05.
P < 0.01.
Abbreviations: BMI, body mass index; TL, telomere length; mtCN, mitochondrial DNA copy number.
We further assessed the associations of body shape trajectories with TL and mtCN in logistic regression models (Table 3). Using the lean-stable group as the reference, the model adjusting for age at blood collection showed that participants in the lean-marked increase group had higher odds of having below-median TL (OR = 1.17, 95% CI: 1.03, 1.33; P = 0.02), and the medium-marked increase group had higher odds of having below-median mtCN (OR = 1.30, 95% CI: 1.02, 1.66; P = 0.03). The observed associations remained statistically significant, after additional adjustment for lifestyle and dietary covariates (OR = 1.18, 95% CI: 1.04, 1.35; P = 0.01 for TL; OR = 1.28, 95% CI: 1.00, 1.64; P = 0.047 for mtCN). However, these associations were attenuated and became statistically nonsignificant after further adjustment for BMI at blood collection (OR = 1.11, 95% CI: 0.94, 1.29; P = 0.21 for TL; OR = 1.04, 95% CI: 0.72, 1.50; P = 0.84 for mtCN).
Table 3.
Associations of body shape trajectories with leukocyte telomere length and mitochondrial DNA copy number among women in the Nurses’ Health Study
| Lean-stable | Lean-moderate increase | Medium-stable | Lean-marked increase | Medium-marked increase | |
|---|---|---|---|---|---|
| TL z-score | |||||
| N | 2784 | 1872 | 1363 | 1459 | 751 |
| Mean (SD)1 | .03(1.02) | .04(1.00) | .01(.97) | −.04(1.02) | .00(1.00) |
| Age-adjusted OR of lower score (95% CI)2 | Ref | .96(.85, 1.08) | 1.01(.89, 1.15) | 1.17(1.03, 1.33) | 1.14(.97, 1.34) |
| P | - | .48 | .88 | .02 | .13 |
| Multivariable-adjusted OR of lower score (95% CI)3 | Ref | .96(.85, 1.08) | 1.02(.89, 1.16) | 1.18(1.04, 1.35) | 1.15(.97, 1.36) |
| P | - | .48 | .80 | .01 | .11 |
| mtCN z-score | |||||
| N | 1358 | 928 | 685 | 695 | 341 |
| Mean (SD)1 | .02(.99) | .04(1.01) | .04(.96) | −.01(1.01) | −.21(.93) |
| Age-adjusted OR of lower score (95% CI)2 | Ref | .86(.73, 1.02) | .96(.80, 1.15) | 1.07(.89, 1.28) | 1.30(1.02, 1.66) |
| P | - | .09 | .63 | .48 | .03 |
| Multivariable-adjusted OR of lower score (95% CI)3 | Ref | .87(.73, 1.03) | .93(.78, 1.13) | 1.06(.88, 1.28) | 1.28(1.00, 1.64) |
| P | - | .10 | .47 | .51 | .047 |
Standardized by age at blood collection.
Unconditional logistic regression models with adjustment for age at blood collection. TL and mtCN z-score outcomes were dichotomized at the median.
Further adjustment for height (continuous), menopausal status (yes or no), postmenopausal hormone use (never, past, current), physical activity (< 5, 5–11.4, 11.5–21.9, ≥ 22 MET-h/wk), alcohol consumption (< 0.15, 0.15–1.9, 2.0–7.4, ≥ 7.5 g/day), pack-years of smoking (0, 1–15, 16–25, 26–45, > 45 years), AHEI dietary score (< 37.8, 38.8–43.5, 43.6–49.9, > 49.9), and multivitamin use (yes or no).
Abbreviations: SD, standard deviation; OR, odds ratio; CI, confidence interval; Ref, reference; TL, telomere length; mtCN, mitochondrial DNA copy number; MET, metabolic equivalent; AHEI, Alternative Healthy Eating Index.
In stratified analyses by age at blood collection (< 60, ≥ 60 years) and smoking history (never, ever), the association between the lean-marked increase group and shorter TL was statistically significant among never smokers (Supplementary Table 2, Online Resource). In contrast, the association between the medium-marked increase group and lower mtCN did not vary by age or smoking status.
Participants in the current analysis comprised women from previous nested case-control studies of various outcomes. To examine the robustness of our results to the inclusion of participants who were identified as cases in those studies, we performed a sensitivity analysis by restricting the analysis to control participants (n = 4780 for TL and 2589 for mtCN). Similar results were observed (Supplementary Table 3, Online Resource).
Table 4 shows the associations of GRS with TL and mtCN. While we did not find any association for TL, participants with higher GRS showed a trend towards lower mtCN (P for trend = 0.07). Compared with the lowest quartile of GRS, the highest quartile tended to associate with below-median mtCN (OR = 1.24, 95% CI: 0.94, 1.64; P = 0.13). The interaction between GRS and age or smoking was statistically nonsignificant (data not shown). In addition, among 97 SNPs in the GRS, 7 and 6 SNPs were nominally associated with TL and mtCN, respectively (P < 0.05, Supplementary Table 4, Online Resource). However, after correction for multiple testing, none of the associations remained statistically significant with FDR values of > 0.05.
Table 4.
Associations of a genetic risk sore for BMI with leukocyte telomere length and mitochondrial DNA copy number among women in the Nurses’ Health Study
| GRS quartile 1 | GRS quartile 2 | GRS quartile 3 | GRS quartile 4 | P for trend | |
|---|---|---|---|---|---|
| TL z-score | |||||
| N | 1035 | 1034 | 1037 | 1049 | |
| Mean (SD)1 | −.04(.99) | −.01(.95) | −.05(1.03) | .04(1.00) | |
| OR of lower score (95% CI)2 | Ref | 1.01(.85, 1.20) | .98(.82, 1.17) | .91(.77, 1.08) | .26 |
| P2 | - | .93 | .82 | .28 | |
| mtCN z-score | |||||
| N | 405 | 392 | 412 | 387 | |
| Mean (SD)1 | .01(.93) | .06(.97) | −.02(1.00) | −.03(.94) | |
| OR of lower score (95% CI)2 | Ref | .90(.68, 1.19) | 1.08(.82, 1.43) | 1.24(.94, 1.64) | .07 |
| P2 | - | .46 | .57 | .13 |
Standardized by age at blood collection.
Unconditional logistic regression models with adjustment for age at blood collection. TL and mtCN z-score outcomes were dichotomized at the median.
Abbreviations: BMI, body mass index; GRS, genetic risk score; SD, standard deviation; OR, odds ratio; CI, confidence interval; Ref, reference; TL, telomere length; mtCN, mitochondrial DNA copy number.
Discussion
To our knowledge, this is the first study to examine the associations of lifetime adiposity with leucocyte TL and mtCN in later life. We used a trajectory-based approach to identify distinct trajectories of body shape in 9613 women from the Nurse’s Health study. Compared to participants who were lean throughout the lifetime, those who gained substantial weight (the lean-marked increase group) had shorter TL, and those with persistently higher body fatness (medium-marked increase group) had lower mtCN. To gain insight into causality, we also created a GRS using adult BMI-related genetic variants and, consistent with the trajectory findings, we found an inverse association between GRS and mtCN. Our findings suggest that TL and mtCN reduction may be accelerated by adiposity in different patterns: TL attrition may be predominantly driven by marked weight gain in middle life, whereas mtCN is likely to be reduced persistently by adiposity across the lifespan.
Oxidative stress and systemic inflammation have been implicated in the associations of obesity with TL and mtCN reduction [47, 48]. In childhood, expanding body fat depots induce leptin production, endothelial dysfunction, and glucose impairment, all of which can lead to excessive production of reactive oxygen species (ROS) [49]. With cumulative weight gain in adulthood, systemic oxidative stress would be aggravated, due to insulin resistance and increased production of adipokines and pro-inflammatory cytokines (e.g. IL-6 and TNF-α) [50]. Telomeres and mitochondria are both sensitive to excessive oxidative stress, and reduced TL and mtCN by oxidative damage may contribute to the risk of obesity-related diseases [13, 51]. In fact, tight regulation of TL and mtCN are essential for normal cell function. Even a small but long-term effect on TL or mtCN may lead to severe consequences. Our study demonstrated the associations of adiposity with TL and mtCN reduction from a life course perspective, supporting the axis of adiposity – excessive oxidative stress – TL and mtCN reduction. Moreover, by examining TL and mtCN simultaneously, our study revealed the different patterns in which adiposity influences telomeres and mitochondria.
Multiple studies have investigated the relationship between adiposity and leukocyte TL; however, the results remain elusive. For instance, a cohort study of 622 Finnish men showed the longitudinal association between midlife BMI and TL in later life [17]. Similarly, in an analysis of 7008 U.S. residents from the National Health and Nutrition Examination Survey, higher BMI at age 25 predicted shorter TL at age 30–40 [18]. Nevertheless, a Danish study of 4576 Caucasians and a cohort including 3600 older Germans reported null association of weight change in middle and late life with TL attrition [19, 20]. In addition, the Health and Retirement Study of 2749 middle-aged and older adults found a positive association between baseline BMI and TL 17 years later [52]. Although the reasons for these discrepancies are unclear, studies differ in population characteristics and adiposity measures. Unlike previous studies assessing adiposity at select time points individually, we applied a group-based modeling approach for identification of distinct trajectories of body shape across the lifespan, thus being able to evaluate long-term effects of adiposity. Our data indicate that BMI at older ages was inversely correlated with TL, and marked weight gain in middle life (lean-marked increase group) may expedite telomere attrition. Consistent with our findings, it has been observed that TL is relatively stable from childhood through young adulthood, and TL shortening predominantly occurs at older ages [53], implying that telomeres are inherently more vulnerable in later life. Moreover, although severe oxidative stress is detrimental to telomeres by causing complete uncapping of telomeric DNA, mild levels of ROS seem to be protective via a pathway increasing telomere repeat additions [54, 55]. Therefore, a delicate balance may be needed to maximize the beneficial effects of certain amount of ROS on telomere maintenance. Individuals who started lean and then gained substantial amount of weight may have progressively increased levels of ROS that may ultimately perturb the balance and induce detrimental effects on telomeres, leading to shorter TL as shown in our study. In addition, we observed that the association between lean-marked increase and TL was stronger among nonsmokers than ever smokers. However, the exact reason remains unknown and future studies are necessary to validate the result.
Previous cross-sectional studies reported that visceral fat area and waist circumference were inversely associated with mtCN [21, 22]. Jokinen et al.’s study with a 12-month follow up suggested that for weight regainers, weight loss may increase mtCN in subcutaneous adipose tissue, while weight regain may reduce mtCN [56]. Our findings extend the knowledge and suggest that higher body fatness over the life course may confer a persistent effect on mtCN reduction. Mitochondria are located in close proximity to sites where ROS are routinely generated, rendering mtDNA particularly susceptible to oxidative attack [57]. Furthermore, unlike nuclear DNA, mtDNA is not protected by histone proteins, introns, and efficient DNA repair mechanisms [11]. At the initial stage when mtDNA was injured by oxidative stress, healthy mitochondria may increase their copy number, to a limited extent, as a feedback mechanism to counterbalance the metabolic defects in impaired mitochondria [48]. Consistently, we observed that participants in the lean-moderate increase (0.04 ± 1.01) and medium-stable (0.04 ± 0.96) groups had almost doubled mtCN than those in the lean-stable (0.02 ± 0.99). However, escalated oxidative stress in the progression of obesity may exhaust the feedback mechanisms and increase the incidence of severe mtDNA damage. To prevent excessive accumulation of oxidative damages, mtDNA degradation would be elicited by a physiologically relevant enzyme system, resulting in the loss of mtCN [58]. This mechanism helps to explain our observation of significantly lower mtCN in women who started with a medium body shape and then gained more weight over age. Of note, we found that adjustment for BMI at blood collection substantially attenuated the association of body shape trajectories with mtCN, whereas the association with TL was minimally affected. This finding further supports the cumulative effect of adiposity on mtCN reduction and the endpoint BMI-independent effect of weight gain on TL attrition.
Our genetic findings provided further support for the influence of lifetime adiposity on mtCN. Many of the variants included in the GRS are within or close to genes that are highly expressed in the central nervous system and may have a persistent effect on the regulation of body mass throughout the life course [26]. Moreover, genetic variants are generally unaffected by environmental factors, and TL and mtCN are unlikely to affect genotypes, thus enabling us to test whether higher BMI precede TL and mtCN reduction. We found no evidence for a causal relationship between genetic obesity variants and TL. This finding is not incompatible with the observed association between the lean-marked increase trajectory and shorter TL, because marked weight gain in middle life is likely to be mainly driven by environmental factors and previous studies did not support a strong association between genetic variants of adiposity susceptibility and middle-life weight gain [59, 60]. On the other hand, we observed that the higher GRS tended to associate with lower mtCN. Because individuals with more adiposity risk alleles are more likely to be overweight in early life and also to gain further weight as they age [61], the genetic association with mitochondria supports the relationship between the medium-marked increase trajectory and lower mtCN.
The strengths of our study include the prospective design, large sample size, and application of a life course analytic method to examine patterns of body shape across the lifespan, which represents a significant advantage over previous studies that examined body fatness at certain ages. Furthermore, we collected detailed data on lifestyle and dietary factors that allowed us to control for potential confounding. Meanwhile, we created a genetic risk score, a proxy for adiposity exposure independent of environmental confounders, to further investigate the influence of lifetime obesity on telomere and mitochondria. Nevertheless, several limitations need to be acknowledged. First, we assessed TL and mtCN at a single time point, which precluded examination of the changes of TL and mtCN over time. Second, the recalled body shape and self-reported BMI data were subject to measurement error; however, given the prospective study design, any error would likely have attenuated the observed associations. In addition, other obesity indicators such as waist circumference and body fat percentage across the lifespan are useful to confirm our conclusions. Third, the homogeneity of study population limits the generalizability of our findings, although it minimizes the likelihood of residual confounding.
In conclusion, our study demonstrates inverse associations of adiposity with leukocytes TL and mtCN from a life course perspective. Substantial weight gain in middle life may increase late-life TL attrition, whereas higher body fatness and genetic obesity susceptibility may exert a persistent effect on mtCN reduction during the lifetime. These findings highlight the importance of maintaining normal weight over the life course for preservation of functional telomeres and mitochondria.
Supplementary Material
Acknowledgements
We would like to thank the participants and staff of the Nurses’ Health Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding
This work was supported by the National Institutes of Health (UM1 CA186107, P01 CA87969, R01 CA49449, R01 HL034594, and R01 HL088521) and by the American Cancer Society Mentored Research Scholar Grant (MRSG-17-220-01 - NEC to M.S.).
The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Reference
- 1.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heymsfield SB, Wadden TA Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med. 2017;376(3):254–266. [DOI] [PubMed] [Google Scholar]
- 4.Rani V, Deep G, Singh RK, Palle K, Yadav UC Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. [DOI] [PubMed] [Google Scholar]
- 5.Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44(3):235–246. [DOI] [PubMed] [Google Scholar]
- 6.Liu CS, Tsai CS, Kuo CL, Chen HW, Lii CK, Ma YS, Wei YH Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic Res. 2003;37(12):1307–1317. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn EH Structure and function of telomeres. Nature. 1991;350(6319):569–573. [DOI] [PubMed] [Google Scholar]
- 8.von Zglinicki T Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. [DOI] [PubMed] [Google Scholar]
- 9.Armanios M, Blackburn EH The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsson CM, Falkenberg M, Larsson NG Maintenance and Expression of Mammalian Mitochondrial DNA. Annu Rev Biochem. 2016;85:133–160. [DOI] [PubMed] [Google Scholar]
- 11.Yakes FM, Van Houten B Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94(2):514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong CM, Lee XW, Wang X Telomere shortening in human diseases. FEBS J. 2013;280(14):3180–3193. [DOI] [PubMed] [Google Scholar]
- 14.Blake R, Trounce IA Mitochondrial dysfunction and complications associated with diabetes. Biochim Biophys Acta. 2014;1840(4):1404–1412. [DOI] [PubMed] [Google Scholar]
- 15.Generation Yu M., function and diagnostic value of mitochondrial DNA copy number alterations in human cancers. Life Sci. 2011;89(3–4):65–71. [DOI] [PubMed] [Google Scholar]
- 16.Mundstock E, Sarria EE, Zatti H, Mattos Louzada F, Kich Grun L, Herbert Jones M, Guma FT, Mazzola In Memoriam J, Epifanio M, Stein RT, Barbe-Tuana FM, Mattiello R Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity (Silver Spring). 2015;23(11):2165–2174. [DOI] [PubMed] [Google Scholar]
- 17.Strandberg TE, Saijonmaa O, Tilvis RS, Pitkala KH, Strandberg AY, Miettinen TA, Fyhrquist F Association of telomere length in older men with mortality and midlife body mass index and smoking. J Gerontol A Biol Sci Med Sci. 2011;66(7):815–820. [DOI] [PubMed] [Google Scholar]
- 18.Wulaningsih W, Watkins J, Matsuguchi T, Hardy R Investigating the associations between adiposity, life course overweight trajectories, and telomere length. Aging (Albany NY). 2016;8(11):2689–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weischer M, Bojesen SE, Nordestgaard BG Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS Genet. 2014;10(3):e1004191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muezzinler A, Mons U, Dieffenbach AK, Butterbach K, Saum KU, Schick M, Stammer H, Boukamp P, Holleczek B, Stegmaier C, Brenner H Body mass index and leukocyte telomere length dynamics among older adults: Results from the ESTHER cohort. Exp Gerontol. 2016;74:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Lee DC, Im JA, Lee JW Mitochondrial DNA copy number in peripheral blood is independently associated with visceral fat accumulation in healthy young adults. Int J Endocrinol. 2014;2014:586017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J, Sidore C, Butler TJ, Wing MK, Qian Y, Meirelles O, Busonero F, Tsoi LC, Maschio A, Angius A, Kang HM, Nagaraja R, Cucca F, Abecasis GR, Schlessinger D Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of ~2,000 Sardinians Using Tailored Sequencing Analysis Tools. PLoS Genet. 2015;11(7):e1005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng S, Wu S, Liang L, Liang G, Giovannucci E, De Vivo I, Nan H Leukocyte mitochondrial DNA copy number, anthropometric indices, and weight change in US women. Oncotarget. 2016;7(37):60676–60686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–488. [DOI] [PubMed] [Google Scholar]
- 25.Song M, Hu FB, Wu K, Must A, Chan AT, Willett WC, Giovannucci EL Trajectory of body shape in early and middle life and all cause and cause specific mortality: results from two prospective US cohort studies. BMJ. 2016;353:i2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Magi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman AK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stancakova A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Arnlov J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Bluher M, Bohringer S, Bonnycastle LL, Bottcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Grassler J, Gronberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson A, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindstrom J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Muller G, Muller-Nurasyid M, Musk AW, Nagaraja R, Nothen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundstrom J, Swertz MA, Swift AJ, Syvanen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, LifeLines Cohort Study, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gadin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van ‘t Hooft FM, Vinkhuyzen AAE, Westra HJ, Zheng W, Zondervan KT, Consortium A. DIPOGen, Group Agen-Bmi Working, Consortium C. ARDIOGRAMplusC4D, Consortium C. KDGen, Glgc, Icbp, Investigators Magic, Mu Ther Consortium, Consortium M. IGen, Consortium Page, ReproGen Consortium, Consortium Genie, International Endogene Consortium, Heath AC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrieres J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hypponen E, Illig T, Jacobs KB, Jarvelin MR, Jockel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimaki T, Lyssenko V, Mannisto S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tonjes A, Tregouet DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Volker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, Marz W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njolstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Perusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colditz GA, Manson JE, Hankinson SE The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 28.De Vivo I, Prescott J, Wong JY, Kraft P, Hankinson SE, Hunter DJ A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J, Qureshi AA, Prescott J, Guo Q, Ye L, Hunter DJ, De Vivo I A prospective study of telomere length and the risk of skin cancer. J Invest Dermatol. 2009;129(2):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prescott J, McGrath M, Lee IM, Buring JE, De Vivo I Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer. 2010;116(18):4275–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page JH, Ma J, Rexrode KM, Rifai N, Manson JE, Hankinson SE Plasma dehydroepiandrosterone and risk of myocardial infarction in women. Clin Chem. 2008;54(7):1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schurks M, Prescott J, Dushkes R, De Vivo I, Rexrode KM Telomere length and ischaemic stroke in women: a nested case-control study. Eur J Neurol. 2013;20(7):1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prescott J, Karlson EW, Orr EH, Zee RY, De Vivo I, Costenbader KH A Prospective Study Investigating Prediagnostic Leukocyte Telomere Length and Risk of Developing Rheumatoid Arthritis in Women. J Rheumatol. 2016;43(2):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devore EE, Prescott J, De Vivo I, Grodstein F Relative telomere length and cognitive decline in the Nurses’ Health Study. Neurosci Lett. 2011;492(1):15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng S, De Vivo I, Liang L, Hu Z, Christiani DC, Giovannucci E, Han J Pre-diagnostic leukocyte mitochondrial DNA copy number and risk of lung cancer. Oncotarget. 2016;7(19):27307–27312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng S, De Vivo I, Liang L, Giovannucci E, Tang JY, Han J Pre-diagnostic leukocyte mitochondrial DNA copy number and skin cancer risk. Carcinogenesis. 2016;37(9):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stunkard AJ, Sorensen T, Schulsinger F Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis. 1983;60:115–120. [PubMed] [Google Scholar]
- 38.Must A, Willett WC, Dietz WH Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138(1):56–64. [DOI] [PubMed] [Google Scholar]
- 39.Nagin Daniel S Group-based trajectory modeling: an overview. Ann Nutr Metab. 2014;65(2–3):205–210. [DOI] [PubMed] [Google Scholar]
- 40.Jones Bobby L, Nagin Daniel S Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–571. [Google Scholar]
- 41.Song M, Willett WC, Hu FB, Spiegelman D, Must A, Wu K, Chan AT, Giovannucci EL Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagin Daniel S Group-based modeling of development. Cambridge: Harvard University Press. 2005. [Google Scholar]
- 43.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87(1):43–47. [PubMed] [Google Scholar]
- 44.Cawthon RM Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A, Wang J, Yu K, Chatterjee N, Orr N, Willett WC, Colditz GA, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Hayes RB, Tucker M, Gerhard DS, Fraumeni JF Jr., Hoover RN, Thomas G, Chanock SJ A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39(7):870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song M, Giovannucci E Estimating the Influence of Obesity on Cancer Risk: Stratification by Smoking Is Critical. J Clin Oncol. 2016;34(27):3237–3239. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, Durante-Montiel I, Sanchez-Rivera G, Valadez-Vega C, Morales-Gonzalez JA Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee HC, Wei YH Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37(4):822–834. [DOI] [PubMed] [Google Scholar]
- 49.Vincent HK, Innes KE, Vincent KR Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9(6):813–839. [DOI] [PubMed] [Google Scholar]
- 50.Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, Arrigo T Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16(1):378–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akhmedov AT, Marin-Garcia J Mitochondrial DNA maintenance: an appraisal. Mol Cell Biochem. 2015;409(1–2):283–305. [DOI] [PubMed] [Google Scholar]
- 52.An R, Yan H Body weight status and telomere length in U.S. middle-aged and older adults. Obes Res Clin Pract. 2017;11(1):51–62. [DOI] [PubMed] [Google Scholar]
- 53.Oeseburg H, de Boer RA, van Gilst WH, van der Harst P Telomere biology in healthy aging and disease. Pflugers Arch. 2010;459(2):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mishra S, Kumar R, Malhotra N, Singh N, Dada R Mild oxidative stress is beneficial for sperm telomere length maintenance. World J Methodol. 2016;6(2):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jokinen R, Rinnankoski-Tuikka R, Kaye S, Saarinen L, Heinonen S, Myohanen M, Rappou E, Jukarainen S, Rissanen A, Pessia A, Velagapudi V, Virtanen KA, Pirinen E, Pietilainen KH Adipose tissue mitochondrial capacity associates with long-term weight loss success. Int J Obes (Lond). 2017. [DOI] [PubMed] [Google Scholar]
- 57.Larsen NB, Rasmussen M, Rasmussen LJ Nuclear and mitochondrial DNA repair: similar pathways? Mitochondrion. 2005;5(2):89–108. [DOI] [PubMed] [Google Scholar]
- 58.Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009;37(8):2539–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandholt CH, Allin KH, Toft U, Borglykke A, Ribel-Madsen R, Sparso T, Justesen JM, Harder MN, Jorgensen T, Hansen T, Pedersen O The effect of GWAS identified BMI loci on changes in body weight among middle-aged Danes during a five-year period. Obesity (Silver Spring). 2014;22(3):901–908. [DOI] [PubMed] [Google Scholar]
- 60.Hertel JK, Johansson S, Sonestedt E, Jonsson A, Lie RT, Platou CG, Nilsson PM, Rukh G, Midthjell K, Hveem K, Melander O, Groop L, Lyssenko V, Molven A, Orho-Melander M, Njolstad PR FTO, type 2 diabetes, and weight gain throughout adult life: a meta-analysis of 41,504 subjects from the Scandinavian HUNT, MDC, and MPP studies. Diabetes. 2011;60(5):1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belsky DW, Moffitt TE, Houts R, Bennett GG, Biddle AK, Blumenthal JA, Evans JP, Harrington H, Sugden K, Williams B, Poulton R, Caspi A Polygenic risk, rapid childhood growth, and the development of obesity: evidence from a 4-decade longitudinal study. Arch Pediatr Adolesc Med. 2012;166(6):515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


