Abstract
Monitoring veterinary antimicrobial use is part of the global strategy to tackle antimicrobial resistance. The purpose of this study was to quantify veterinary antimicrobials imported into Timor-Leste between 2016 and 2019 and describe the antimicrobial import profile of importers. Data were obtained from import applications received by the Ministry of Agriculture and Fisheries (MAF) of Timor-Leste. Import quantities were analysed by antimicrobial class, importance for human medicine, recommended route of administration and type of importer. An average of 57.4 kg (s.d. 31.0 kg) and 0.55 mg/kg (s.d. 0.27 mg/kg) animal biomass of antimicrobials was imported per year. Tetracyclines (35.5%), penicillins (23.7%), and macrolides (15.9%) were the commonly imported antimicrobial classes. Antimicrobials imported for parenteral administration were most common (60.1%). MAF was the largest importer (52.4%). Most of the critically important antimicrobials for human medicine were imported by poultry farms for oral administration and use for growth promotion could not be ruled out. In conclusion, the use of antimicrobials in animals in Timor-Leste is very low, in keeping with its predominantly subsistence agriculture system. Farmer education, development of treatment guidelines, and strengthening of the veterinary service is important for addressing the potential future misuse of antimicrobials especially in the commercial poultry industry.
Keywords: antimicrobial use, antimicrobial resistance (AMR), Timor-Leste, antibiotic, antimicrobial, veterinary, prudent use, critically important antimicrobials, growth promotion, poultry
1. Introduction
The emergence of antimicrobial resistance is a major global health threat for the 21st century [1]. It is also a One Health challenge that requires coordinated action as transmission of resistant bacteria can occur between humans, animals, plants and the environment [2,3,4]. This emergence has been rapid and is linked to the overuse and misuse of antimicrobials in humans and animals [5,6]. Despite this, it is projected that the use of antimicrobials in humans and animals will continue to rise over the next decade [7,8]. In particular, the use of antimicrobials in food producing animals has received attention due to high levels of use globally for disease prevention and growth promotion [9,10]. While some developed countries have demonstrated a reduction in usage levels [11,12,13,14,15], usage in many developing countries have risen due to farm intensification and demand for animal-based protein associated with rising incomes [16,17,18]. This puts low- and middle-income countries at a higher risk for emergence of resistance.
Antimicrobial resistance limits the effectiveness of antimicrobial therapy which has a greater impact in low and middle-income countries due to their weaker health systems, higher prevalence of infectious diseases and limited access to more expensive treatment alternatives [19,20]. To preserve the effectiveness of antimicrobials, a global strategy has been developed to tackle antimicrobial resistance [21]. This strategy is wide-ranging and multi-sectoral and includes initiatives to strengthen monitoring of antimicrobial use in animals [21].
Monitoring of antimicrobial use in animals at the national level enables a country to identify trends of use over time and assess the impact of policy measures to promote prudent use in animals [22]. When analysed in conjunction with data on antimicrobial resistance in animal and humans, it can also identify potential associations between antimicrobial use and resistance patterns [23,24]. To harmonize antimicrobial use data collection, the World Organisation for Animal Health (OIE) has published guidelines for monitoring the use of antimicrobials in food producing animals [25]. The guidelines acknowledge that antimicrobial use data can be obtained from different levels such as import, manufacturing, sales, dispensing records or from end-use sources [25]. While many higher income countries have been collecting data for many years [26,27], some low to middle income countries in Africa and Asia-Pacific are still facing challenges such as a lack of regulation, under-reporting and unreliable data when monitoring antimicrobial use in animals [10,28,29].
Timor-Leste is a lower-middle income country [30] located in the south-east portion of the Malay Archipelago with a population of 1.3 million [31]. Subsistence farming is the main livelihood for most of the rural population [32,33], with a high proportion of households owning livestock [34]. Chicken and pigs are the two most commonly reared species in the country [35]. Commercial animal farming is uncommon [36,37] but may increase with rising income levels [33]. Currently, there are two large commercial layer farms [38] and a growing number of commercial broiler farms. There are no major commercial livestock farms for other species. There is no local manufacture of antimicrobials, and all antimicrobials are imported into the country. All applications to import veterinary medicines into the country must be submitted to the Ministry of Agriculture and Fisheries (MAF) and there is no re-export of veterinary antimicrobials.
The aims of this study were to quantify veterinary antimicrobial imports into Timor-Leste between 2016 to 2019; and to describe these imports based on antimicrobial class, importance for human medicine, recommended route of administration and type of importer. The findings can help improve monitoring and control of veterinary antimicrobial use in Timor-Leste.
2. Materials and Methods
2.1. Data Collection for Antimicrobial Imports
All applications to import veterinary medicines into Timor-Leste submitted to MAF between January 2016 to December 2019 were screened to identify veterinary antimicrobials using OIE’s list of antimicrobials of veterinary importance [39]. Data on the date of application, name of importer, brand name, quantity imported, name of active ingredient, concentration of active ingredient, route of administration and target species was extracted for each veterinary antimicrobial. Any missing details on the name of active ingredient, concentration of active ingredient and route of administration was obtained from the technical product sheets. Data collection was performed by two MAF staff who received training on recording antimicrobial import data from received import applications through three workshops and ongoing side-by-side mentorship [40]. The data was stored on an Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA). Data accuracy was checked independently by three researchers from Menzies School of Health Research between November and December 2020.
2.2. Data Categorisation for Antimicrobial Imports
Using the name of the active ingredient, each antimicrobial was classified into an antimicrobial class/subclass based on OIE guidelines [41]. The name of the active ingredient was also used to classify antimicrobials as a critically important antimicrobial (CIA), highly important antimicrobial (HIA) or an important antimicrobial (IA) using the World Health Organization (WHO) List of Critically Important Antimicrobials for Human Medicine [42]. The importer name was used to classify importers into 6 types to understand their individual import patterns: “MAF”, “agriculture shops”, “veterinary clinics”, “layer farms”, “broiler farms”, and “education institutions”. Layer and broiler farms were placed in separate categories because they may have different antimicrobial use patterns. In Timor-Leste, agriculture shops are enterprises where veterinary medicines can be procured without a prescription.
2.3. Animal Biomass Calculation
Data for biomass calculation (i.e., number of live animals, number of animals slaughtered and meat product quantity) were obtained from the Food and Agricultural Organization Global Statistical Database (FAOSTAT) [43,44]. Common animal species in Timor-Leste (buffalo, cattle, chicken, goats, horse, pigs, and sheep) [34] were included in the biomass calculation. Ducks, rabbits, dogs, and cats were excluded because data were not available. Total animal biomass was calculated for each year between 2016 and 2019 using an OIE method [25] except for bovine biomass because the proportion of animals in different age groups are not known. The data for total animal biomass calculation and estimates for annual biomass can be found in Supplementary Table S1.
2.4. Data Analysis
The weight of active ingredient in one unit of imported product per pharmaceutical form (e.g., bottle, bag, or tube) was estimated by multiplying the strength of the antimicrobial active ingredient by the volume or weight. All weights were expressed in kilograms (kg). Conversion factors based on OIE guidelines was used to mathematically convert international units (IU) into kilograms [29].
The weight of each active ingredient imported between 2016 to 2019 was calculated by multiplying the weight of active ingredient in one unit of product by the quantity imported. Adjustment for animal biomass was achieved by dividing the total weight of active ingredient by the total animal biomass. The result was expressed in milligram (mg) of active ingredient per kilogram (kg) of animal biomass.
Annual and total imports were calculated for each active ingredient, antimicrobial class, WHO class of importance in human medicine, route of administration and type of importer. Total annual imports of all antimicrobials by weight and weight adjusted for biomass were summarized as mean ± s.d. Spearman’s rank correlation coefficient (rs) was used to test the hypothesis of a monotonic (increasing or decreasing) trend in imports by total weight, total weight adjusted for biomass, individual active ingredient and type of importer. Data analysis was performed using Stata 15 software (StataCorp, College Station, TX, USA)
2.5. Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by Human Research Ethics Committee of the Northern Territory (NT) Department of Health and Menzies School of Health Research (2020-3841) and Institute Nacional de Saude in Timor-Leste (MS-INS/DE/IX/2020/1411).
3. Results
3.1. Import Quantities and Trends
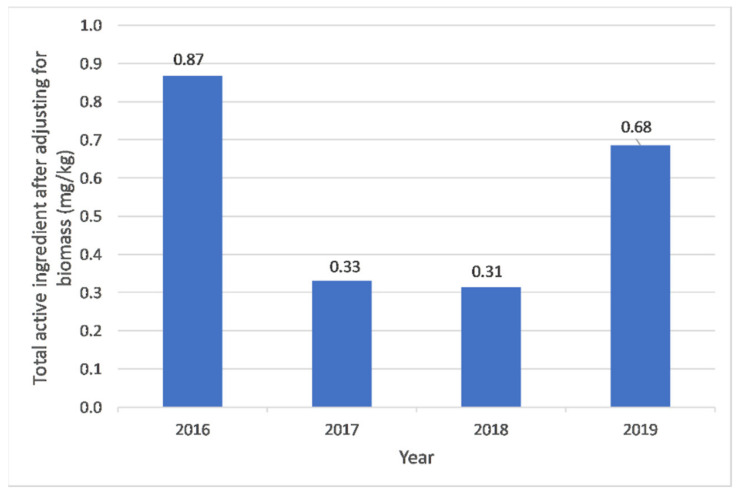
Between 2016 to 2019, a total of 229.8 kg of active ingredients of veterinary antimicrobials were imported into Timor-Leste (mean: 57.4 ± 31.0 kg per year). Import quantities were lower in 2017 and 2018 compared to 2016 and 2019 (see Table 1). After adjusting for animal biomass, the average amount of imported antimicrobials was 0.55 ± 0.27 mg/kg biomass per year. There was no evidence of a significant monotonic trend in antimicrobial imports based on total weight (rs: −0.40, p value: 0.60) or weight adjusted by biomass (rs: −0.40, p value: 0.60) (see Figure 1).
Table 1.
Weight of veterinary antimicrobials imported into Timor-Leste between 2016 and 2019, by year and overall for individual antimicrobial and antimicrobial class.
| Antimicrobial Class | Antimicrobial (WHO Classification 1) | Kilogram of Active Ingredient (%) | rs (p Value) 2 |
Kilogram for all Years (%) | Kilogram for all Years for Each Class (%) | |||
|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | |||||
| Aminoglycosides | Neomycin (CIA) |
0 | 2.5×10−4 (<0.01) | 0.01 (0.05) | 0.01 (0.02) | 0.95 (0.05) | 0.03 (0.01) | 25.85 (11.25) |
| Dihydrostreptomycin (CIA) | 4.64 (4.83) | 6.20 (18.56) | 5.80 (18.5) | 9.18 (13.32) | 0.80 (0.20) | 25.82 (11.24) | ||
| Cephalosporins (3rd/4th gen) | Cefotaxime (CIA) | 0 | 0.01 (0.03) | 0 | 0 | −0.26 (0.74) | 0.01 (<0.00) | 0.01 (<0.00) |
| Fluroquinolones | Norfloxacin (CIA) | 0.20 (0.21) | 0.20 (0.60) | 0 | 0 | −0.89 (0.11) | 0.40 (0.17) | 6.01 (2.62) |
| Enrofloxacin (CIA) | 3.61 (3.76) | 0 | 0 | 2.00 (2.90) | −0.32 (0.68) | 5.61 (2.44) | ||
| Macrolides | Tylosin (CIA) | 25.10 (26.12) | 0.10 (0.30) | 0 | 0 | −0.95 (0.05) | 25.20 (10.97) | 36.45 (15.86) |
| Tilmicosin (CIA) | 0 | 0 | 0 | 11.25 (16.33) | 0.77 (0.23) | 11.25 (4.9) | ||
| Penicillins | Ampicillin (CIA) | 0 | 0 | 3.27 (10.41) | 0.63 (0.91) | 0.74 (0.26) | 3.89 (1.69) | 54.39 (23.67) |
| Benzylpenicillin (HIA) | 2.78 (2.90) | 3.72 (11.13) | 3.48 (11.10) | 5.70 (8.28) | 0.80 (0.20) | 15.69 (6.83) | ||
| Amoxicillin (CIA) | 20.31 (21.13) | 0 | 0 | 14.51 (21.05) | −0.32 (0.68) | 34.82 (15.15) | ||
| Polypeptides | Bacitracin (IA) | 0 | 0 | 0.02 (0.06) | 0 | 0.26 (0.74) | 0.02 (0.01) | 10.93 (4.76) |
| Colistin (CIA) | 10.04 (10.45) | 0 | 0.31 (0.98) | 0.57 (0.82) | −0.20 (0.80) | 10.91 (4.75) | ||
| Polymyxin B (CIA) | 0 | 9.5 × 10−5 (<0.01) | 0 | 0 | −0.26 (0.74) | 9.5 × 10−5 (<0.01) | ||
| Sulfonamides | Sulfamonomethoxine (HIA) | 0.40 (0.42) | 0.20 (0.60) | 0 | 0 | −0.95 (0.05) | 0.60 (0.26) | 14.46 (6.29) |
| Sulfaquinoxaline (HIA) | 0.33 (0.34) | 0 | 0 | 0 | −0.77 (0.23) | 0.33 (0.14) | ||
| Sulfadoxine (HIA) | 0 | 0 | 0 | 0.04 (0.06) | 0.77 (0.23) | 0.04 (0.02) | ||
| Sulfamerazine (HIA) | 0 | 0.08 (0.23) | 0.02 (0.06) | 0.78 (1.14) | 0.80 (0.20) | 0.88 (0.38) | ||
| Sulfadiazine (HIA) | 2.09 (2.17) | 1.48 (4.42) | 1.59 (5.06) | 1.57 (2.27) | −0.40 (0.60) | 6.72 (2.92) | ||
| Sulfadimidine (HIA) | 1.67 (1.74) | 0.58 (1.73) | 0.80 (2.56) | 2.35 (3.41) | 0.40 (0.60) | 5.40 (2.35) | ||
| Trimethoprim (HIA) | 0.20 (0.21) | 0.28 (0.84) | 0 | 0.01 (0.01) | −0.06 (0.40) | 0.49 (0.21) | ||
| Tetracyclines | Oxytetracycline (HIA) | 24.73 (25.73) | 20.56 (61.55) | 16.07 (51.23) | 20.32 (29.48) | −0.80 (0.20) | 81.67 (35.54) | 81.67 (35.54) |
| Overall | 96.1 (100) | 33.41 (100) | 31.36 (100) | 68.91 (100) | −0.40 (0.60) | 229.77 (100) | 229.77 (100) | |
1 CIA refers to critically important antimicrobials; HIA refers to highly important antimicrobials; IA refers to important antimicrobials.2 Spearman rank-order correlation coefficient (rs) and p-value assessing the strength and direction of possible monotonic trends in the quantities of antimicrobials imported over time.
Figure 1.
Antimicrobial import weight (mg) adjusted by animal biomass (kg) into Timor-Leste between 2016 and 2019.
A total of 21 antimicrobial active ingredients belonging to 8 classes of antimicrobials were imported during the study period. The import quantities of different antimicrobials between 2016 and 2019 can be found in Table 1. The active ingredients imported in the largest quantities were oxytetracyline (81.7 kg; 35.5%), amoxicillin (34.8 kg; 15.2%), tylosin (25.2 kg; 11.0%) and dihydrostreptomycin (25.8 kg; 11.2%). The classes of antimicrobials imported in the largest quantities were tetracyclines (81.7 kg; 35.5%), penicillins (54.4 kg; 23.7%), macrolides (36.5 kg; 15.9%) and aminoglycosides (25.8 kg; 11.3%). There was some evidence of monotonic increase in imports of neomycin (rs: 0.95, p value: 0.05) but quantities imported each year were extremely small. There was also some evidence of a monotonic decrease in imports of tylosin (rs: −0.95, p value: 0.05) driven by a relatively large import in 2016 and sulfamonomethoxine (rs: −0.95, p value: 0.05) although quantities imported each year were extremely small. There was no strong evidence of a monotonic trend in the import of any of the other individual antimicrobials (see Table 1). Based on WHO classification, most of the imported veterinary antimicrobials were CIAs (117.9 kg; 51.3%) followed by HIAs (111.8 kg; 48.7%).
3.2. Import Pattern by Recommended Route of Administration
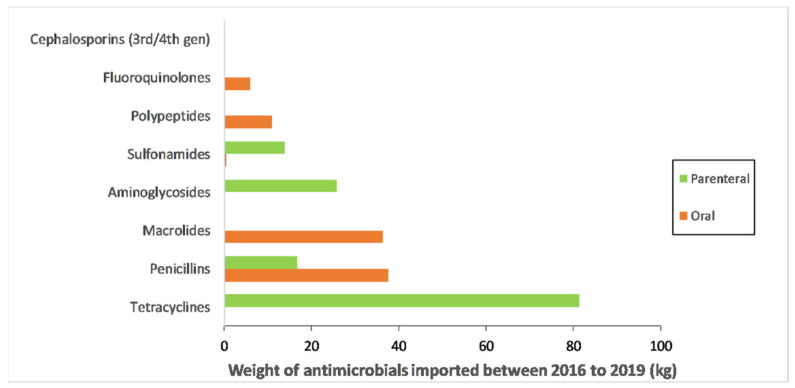
Recommended routes of administration for imported veterinary antimicrobials during the study period were parenteral (138.0 kg; 60.1%), oral (91.5 kg; 39.8%), and topical (0.3 kg; 0.1%). The majority of tetracyclines (81.3 kg; 99.6%), aminoglycosides (25.8 kg; 99.9%), sulphonamides (13.4 kg; 96.2%), and cephalosporins (0.01 kg; 100%) were for parenteral administration, while the majority of penicillins (37.6 kg; 69.2%), macrolides (36.3 kg; 99.5%), polypeptides (10.9 kg; 100%), and fluoroquinolones (6.0 kg; 100%) were for oral administration. The quantities of different antimicrobial classes for parenteral, oral and topical administration are shown in Figure 2. The weight of antimicrobial classes recommended for administration through different routes for each year over the study period can be found in Supplementary Table S2.
Figure 2.
Total weight of veterinary antimicrobials imported into Timor-Leste between 2016 and 2019, by route of administration and antimicrobial class. Antimicrobials for administration via the topical route represented less than 0.3 kg (0.1%) of total imports and were therefore not included in the diagram.
3.3. Import Pattern by Importer Type
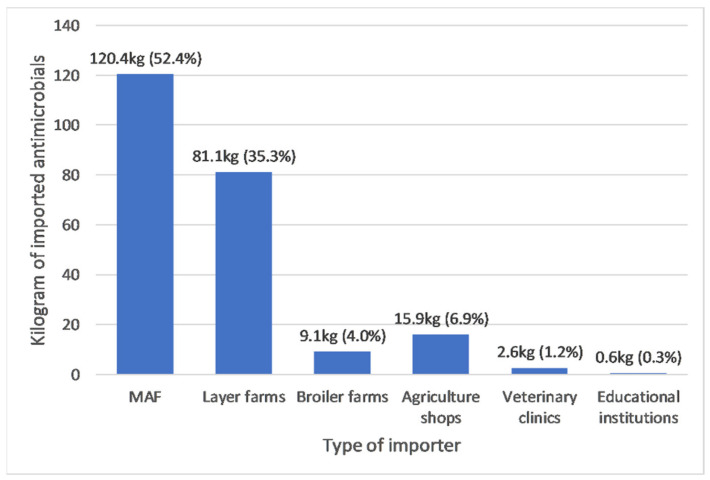
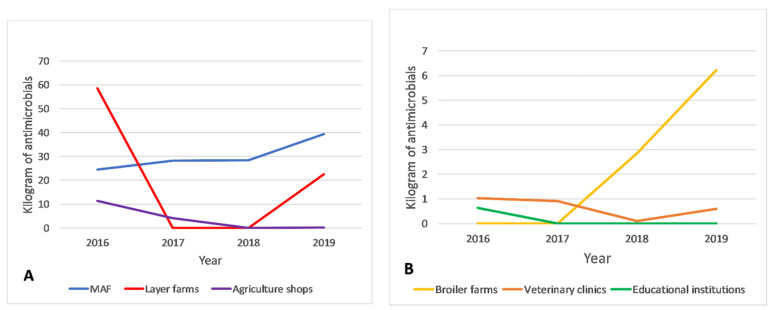
Between 2016 and 2019, the biggest importers of antimicrobials were MAF (120.4 kg; 52.4%), followed by layers farms (81.1 kg; 35.3%) and agriculture shops (15.9 kg; 6.9%) (See Figure 3). There was very strong evidence of a monotonic increase in antimicrobial imports by MAF (rs: 1.0, p value: <0.001) and evidence of a monotonic increase in antimicrobial imports by broiler farms (rs: 0.95, p value: 0.05) but no evidence of a monotonic trend in antimicrobial import patterns for other types of importers (see Figure 4A,B). The pattern of imports by layer farms was unique as imports were high in 2016 (58.6 kg) and 2019 (22.5 kg) but negligible between those years (see Figure 4A). Educational institutions imported a relatively small amount (0.6 kg) of antimicrobials once in 2016. Colistin, neomycin and enrofloxacin were only imported by layer or broiler farms. Cephalosporins were only imported by veterinary clinics. The weights of individual antimicrobials and antimicrobial classes imported by different type of importers for each year during the study period can be found in Supplementary Table S3.
Figure 3.
Total active ingredients imported between 2016 and 2019, by type of importer.
Figure 4.
(A,B): Trend of antimicrobials imported by different importers between 2016 and 2019. Bigger importers are represented in (B) and smaller importers in (B), thus y-axes differ between diagrams.
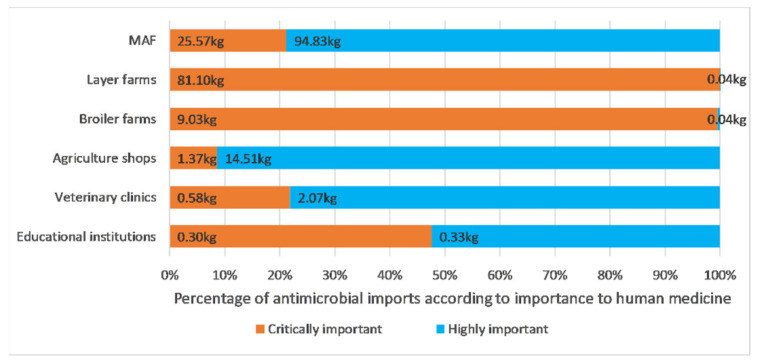
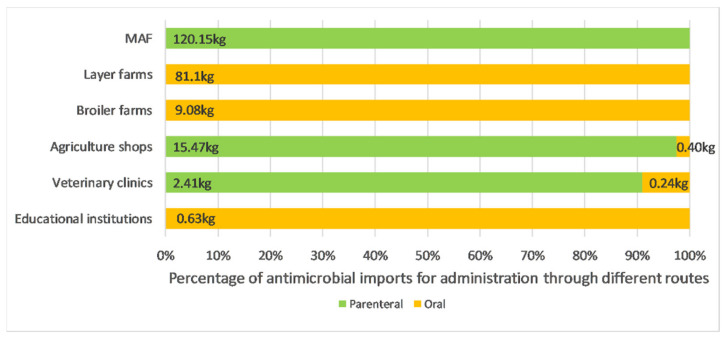
The biggest importers of CIAs were layer farms (81.1 kg), MAF (25.6 kg) and broiler farms (9.0 kg). Layer and broiler farms imported CIAs almost exclusively; while CIAs accounted for less than a quarter of imports by MAF, agriculture shops and veterinary clinics (see Figure 5). Almost all antimicrobial imports by layer and broiler farms were for oral administration; while almost all imports by MAF and agriculture shops were for parenteral administration (see Figure 6).
Figure 5.
Profile of antimicrobial imports of different importer types by WHO classification of importance to human medicine. Important antimicrobials for human medicine represented less than 0.02 kg (0.01%) of total imports and were therefore not included in the diagram.
Figure 6.
Profile of antimicrobial imports of different importer types, by recommended route of administration. Antimicrobials for administration via the topical route represented less than 0.3 kg (0.1%) of total imports and were therefore not included in the diagram.
4. Discussion
4.1. Strengths of the Study
This is the first study to describe veterinary antimicrobial imports into Timor-Leste. It showed a very low level of antimicrobial use in animals. Future studies of a similar nature will enable analysis of long-term trends and identification of changes in import patterns arising from interventions. Import data is a reasonable proxy for actual antimicrobial use for Timor-Leste since there is no local manufacture of veterinary antimicrobials and no re-export of antimicrobials. The data collection method was implemented consistently as it was performed by trained personnel using a written protocol. The accuracy of data was checked rigorously by authors to minimise data entry errors, and calculations were done with methods aligned with international guidelines. The training during data collection strengthened the capacity of MAF personnel to record antimicrobial import data and facilitated the timely reporting of results to the OIE, which is often a challenge in developing countries.
4.2. Quantity of Antimicrobial Import
The quantity of antimicrobials imported for use in animals in Timor-Leste after adjusting for biomass (0.55 mg/kg biomass) is very low compared to the global average of 144.39 mg/kg and regional average (Asia, Far East, and Oceania) of 237.72 mg/kg in 2016 [29]. The use of veterinary antimicrobials in Timor-Leste was even lower than countries such as New Zealand, Norway, and Iceland which are known to have some of the lowest use levels in the world [45,46]. The low level of use is likely due to the subsistence agriculture system in Timor-Leste [33,47] where there is poor access to veterinary services and medicines. The low level of use is also consistent with another study in Timor-Leste which showed that only 1% of backyard chicken farmers used commercial medicines in their animals [48]. It would be interesting to compare the results from Timor-Leste to other countries with a similar agriculture background but similar studies from such countries could not be found [10]. Although antimicrobial use levels are currently low, use may increase in the future with farming intensification, as seen in other developing countries [49,50]. In this study, there is already evidence of increasing use in the broiler industry, with import levels rising by 119% between 2018 and 2019.
4.3. Trend of Antimicrobial Import
Trends in antimicrobial imports over the study period can be explained by looking individually at each importer. For MAF, the rise in antimicrobial imports during the study period represented increased procurement following annual feedback that government employed animal health professionals (e.g., veterinary and livestock technicians) faced shortages for field use [51].
For layer farms, it is likely that the import quantities were inconsistent between years because this group included only two large commercial layer farms that import antimicrobials in bulk quantities for use over a few years. For broiler farms, antimicrobial imports occurred only after 2018 following the import of day-old chicks from Indonesia after the lifting of avian-influenza related import restrictions [52]. The easing of restrictions was followed by a government effort to promote the growth of the broiler industry. The use of antimicrobials may also reflect the lack of resources to implement farm biosecurity and vaccination programmes on these farms [53,54]. Use of antimicrobials on broiler farms could be expected to rise in the future, mimicking the trends seen in neighbouring Indonesia where there was a rise of antimicrobial use due to industry growth, lack of alternative disease control options and a relatively low cost of antibiotics [55]. Therefore, farmer education programmes to improve knowledge on good animal husbandry practices and biosecurity could be useful [56]. The availability of quality vaccines would provide further options for disease prevention and control [57].
For agriculture shops, the reason for a decrease in imports during the study period was unclear but could be partially attributed to non-adherence to the MAF import application process resulting in data not being captured. For veterinary clinics, the low quantities imported reflect the small size of the industry—there were only four veterinary practices operating in Timor-Leste during the study period. The closure of one veterinary clinic in 2018 coincided with a drop in antimicrobial imports by veterinary clinics that year. For education institutions, there was only a once off import of antimicrobials by an agriculture school in 2016. There were no direct imports of antimicrobials by other types of commercial livestock farms apart from poultry, but animals on these farms could still receive antimicrobials imported by MAF or agriculture shops.
4.4. Antimicrobial Class and Importance for Human Medicine
The common antimicrobial classes in Timor-Leste (tetracycline, penicillin, and macrolide) are consistent with global and regional (Asia, Far East, and Oceania) usage patterns [29]. The most imported antimicrobials in Timor-Leste (oxytetracycline, amoxicillin, tylosin, and dihydrostreptomycin) were consistent with antimicrobials used in poultry and pig production in developing countries in Asia and Africa [18,50,58,59]. Oxytetracycline is popular because of its broad-spectrum action, low cost, and availability in long-acting formulations [60,61] and it is likely that similar reasons underpin its popularity in Timor-Leste. Amoxicillin and tylosin were imported almost exclusively in oral formulation by commercial poultry farms, and the popularity of these antimicrobials in small scale poultry farms were also reported in other studies in other countries [50,62]. Dihydrostreptomycin was commonly imported in formulations with benzylpenicillin by MAF due to the combination’s broad-spectrum action across a wide range of livestock species. It was positive that colistin, which is an antibiotic of last resort for human medicine that is commonly used in developing countries [63,64] contributed to less than 5% of imports to Timor-Leste with the majority imported in 2016. However, the broiler industry has been importing colistin albeit in small quantities in recent years and this should be closely monitored.
There has been a strong push towards reducing the use of medically important antimicrobials in livestock globally [65]. The almost exclusive imports of CIAs by commercial poultry farms could be attributed to the lack of awareness on antimicrobial resistance and its impact on public health, which has been observed in studies elsewhere [66,67]. On the other hand, the low proportion of CIA imports by MAF (21.2%) and veterinary clinics (21.7%) puts the professional veterinary service in positive light in terms of preserving critically important antimicrobials for use in human health. Of important concern is the import of fluroquinolones, polymyxins, and 3rd and 4th generation cephalosporins which are highest priority critically important antimicrobials for human medicine. Although the combined quantity of these classes contributed to less than 8% of total imports, future import and distribution of these antimicrobials should be closely monitored because of the potential risk of the development and transmission of antimicrobial resistance from livestock to humans [68,69]. To address the high proportion of CIA usage in the commercial poultry sector, a jointly developed antimicrobial treatment guideline between government and industry preferencing the use of non-CIA antibiotics may be effective [70].
4.5. Route of Administration
In this study, antimicrobials recommended for oral administration (39.8%) were less common than reported in some countries [12,14,71]. Antimicrobials imported by MAF and agriculture shops were mostly for parenteral administration. This is likely to be because they were mainly for use in species such as pigs and cattle that are reared extensively on small-holder livestock farms. On the other hand, commercial poultry farms probably imported mainly antimicrobials for oral administration because they are convenient for mass administration in poultry reared in semi-intensive or intensive production environments. The use of orally administered antimicrobials should be monitored in Timor-Leste as it has been demonstrated elsewhere that this route is more prone to misuse from inappropriate dosing and promotes the development of antimicrobial resistance [72].
4.6. Use of Antimicrobials for Growth Promotion
The import of antimicrobials intended for oral administration raises the concern of use of antimicrobials for growth promotion. Ideally antimicrobials should not be used for growth promotion without a public health risk assessment and any use should be phased out especially for critically important antimicrobials [21,65]. According to MAF, antimicrobials are not known to be used for growth promotion in the country. However, oral bacitracin and tylosin that were imported by poultry farms have been used for growth promotion worldwide [28,73]. In addition, the technical fact sheet of some antimicrobials indicated that the products could be administered for growth promotion. Therefore, it is possible that commercial farmers are administering antimicrobials at low doses, as recommended for growth promotion, without being aware. This has also been reported in another study [49]. The possible use of antimicrobials for growth promotion in Timor-Leste should be further investigated.
4.7. Use of Antimicrobials in Aquaculture
Although antimicrobial use is common practice in aquaculture systems worldwide and regionally [74,75,76], there were no aquaculture importers in this study and no products were indicated for use in aquatic animals. The absence of antimicrobial use in this sector is likely due to the relatively small and underdeveloped aquaculture sector [77].
4.8. Limitations
Timor-Leste is not immune to the challenges and limitations of monitoring antimicrobial use. The study only included import data after 2016 because of the five-year holding limit of hardcopy applications in the MAF office and the lack of digital record keeping. Thus, only data from 2016 to 2020 were available. The study excluded data from 2020 because the calendar year of 2020 had not yet ended at the point of data collection. This short study period limited the power of the study to detect trends in import quantities. However, digital record keeping was initiated as part of the study which will enable future studies to cover a longer time period.
It is likely that the study provided an under-estimation of the total amount of antimicrobials used in Timor-Leste due to the non-submission of import applications by some importers as reported in another study [71]. The possible reasons for non-submission include an importer’s desire to avoid waiting times for approval, a weak regulatory framework and the lack of enforcement. Antimicrobials intended for human use could have also been administered to animals although elsewhere this is usually limited to companion animals [78]. The authors predict that the underestimation would not result in more than a doubling in the total amount of imported antimicrobials during the study period. Even if this happened, Timor-Leste would still demonstrate one of the lowest use rates compared to other countries that have reported usage data.
There may be a small degree of inaccuracy for the animal biomass estimation because the data obtained from FAOSTAT was based on extrapolations. This source of data was used because annual census data was unavailable during the study period. The OIE method for biomass calculation involved the use of European conversion coefficients and breeding cycles that may be different to Timor-Leste. However, these default parameters were used as no suitable alternative for a Timor-Leste context was found. The exclusion of minor species such as ducks, rabbits, dogs, and cats from the biomass calculation is likely to have only a marginal impact on the result since the population is relatively small [25].
It was not possible to quantify the antimicrobials that were administered to different animal species based on the import data due to the multi-species indication for many of the antimicrobials. However, a rough estimation of the division of antimicrobial use between livestock and companion animals could be estimated by assuming that antimicrobials imported by veterinary clinics were administered exclusively to companion animals, and antimicrobials imported by all other importers were administered exclusively to livestock.
4.9. Future Directions
Although the use of antimicrobials in animals is Timor-Leste is very low, there is potential for future misuse and overuse with farming intensification. Future studies investigating the knowledge, attitudes and practices of animal health professionals and farmers on antimicrobial use would be useful for identify strategies for promoting prudent use of antimicrobials in animals as identified in other studies [79,80,81]. Even in the absence of such studies, early action can be informed by studies conducted in other developing countries [55,58,82]. In addition to farmer education, which was mentioned previously, improving farmers access to animal health professionals [64], and training of animal health professionals to engage with farmers on prudent antimicrobial use has been shown to be effective elsewhere [82]. The strengthening of laboratory capacity in bacterial culture and antimicrobial susceptibility testing will also facilitate better decision making on antimicrobial use [65].
To improve the quality of data collected, MAF is engaging with importers such as agriculture shops to understand their reservations on submitting import applications and exploring legislative tools to improve compliance on import application submission. Future monitoring could focus on collecting data more proximal to the site of usage such as at end-user level to elucidate species and production type usage patterns [83,84].
5. Conclusions
This baseline study demonstrated very low levels of antimicrobial use in animals in Timor-Leste consistent with its subsistence agriculture system. Antimicrobial classes imported in the largest quantities were tetracyclines, penicillins, and macrolides. This is very similar to usage patterns in other countries globally and regionally. Import of CIAs for administration via the oral route was high in the poultry industry, and antimicrobial use for growth promotion could not be ruled out. Antimicrobial use in the poultry industry is expected to rise due to industry growth and the limited alternative disease control strategies. Education of farmers, development of antimicrobial treatment guidelines and improving access to veterinary services can help to ensure good antimicrobial stewardship in the animal health sector. Through this study, in-country capacity to monitor antimicrobial imports according to OIE reporting requirements was developed.
Acknowledgments
We acknowledge the staff from the Ministry of Agriculture and Fisheries of Timor-Leste for their support in this study. We thank Winnie Chen (Menzies School of Health Research) and Agnes Agunos (Public Health Agency of Canada) for critically reviewing the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10040426/s1, Table S1: Animal biomass for buffalo, cattle, chicken, goats, horses, pigs and sheep in Timor-Leste between 2016 and 2019. Table S2: Import weight of various veterinary antimicrobial classes by route of administration between 2016 and 2019. Table S3: Total weight of active ingredients of different classes of veterinary antimicrobials imported into Timor-Leste, by type of importer between 2016 and 2019.
Author Contributions
Conceptualization, S.T., J.Y., J.R.F. and J.B.d.C.J.; Methodology, S.T., A.P., A.d.J.A. and S.D.; Software, S.T.; Investigation and data curation, S.T., A.P., A.d.J.A., S.D., C.d.C.S., S.F., F.J.S., O.d.C.H. and J.B.d.C.J.; Formal analysis, S.T., A.P., A.d.J.A., S.D. and T.S.B.; Writing—original draft preparation, S.T., A.P., A.d.J.A. and S.D.; Writing—review and editing, S.T., A.P., A.d.J.A., S.D., C.d.C.S., S.F., F.J.S., O.d.C.H., J.Y., J.R.F., T.S.B. and J.B.d.C.J.; Visualization, S.T., A.P., A.d.J.A., S.D. and T.S.B.; Supervision, S.T.; J.Y. and J.R.F.; Project administration, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Fleming Fund Country Grant for Timor-Leste (FF/17/233). The Fleming Fund is a UK aid investment programme to tackle antimicrobial resistance in low- and middle-income countries around the world and is managed by the UK Department of Health and Social Care.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved Human Research Ethics Committee of the Northern Territory (NT) Department of Health and Menzies School of Health Research (2020-3841) and Institute Nacional de Saude in Timor-Leste (MS-INS/DE/IX/2020/1411).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robinson T.P., Bu D.P., Carrique-Mas J., Fèvre E.M., Gilbert M., Grace D., Hay S.I., Jiwakanon J., Kakkar M., Kariuki S., et al. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop Med. Hyg. 2016;110:377–380. doi: 10.1093/trstmh/trw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Léger A., Lambraki I., Graells T., Cousins M., Henriksson P.J.G., Harbarth S., Carson C., Majowicz S., Troell M., Parmley E.J., et al. AMR-Intervene: A social–ecological framework to capture the diversity of actions to tackle antimicrobial resistance from a One Health perspective. J. Antimicrob. Chemother. 2021;76:1–21. doi: 10.1093/jac/dkaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wall B.A., Mateus A.L.P., Marshall L., Pfeiffer D.U., Lubroth J., Ormel H.J., Otto P., Patriarchi A. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production. [(accessed on 16 February 2021)]; Available online: http://www.fao.org/feed-safety/resources/resources-details/en/c/452608/
- 4.Lim K., Chee D., Ting S., Choo E., Tan W.L., Lin Y.N. Singapore’s National Action Plan on Antimicrobial Resistance. Asian Fish. Sci. 2020;33:107–111. doi: 10.33997/j.afs.2020.33.S1.015. [DOI] [Google Scholar]
- 5.Tang K.L., Caffrey N.P., Nóbrega D.B., Cork S.C., Ronksley P.E., Barkema H.W., Polachek A.J., Ganshorn H., Sharma N., Kellner J.D., et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health. 2017;1:e316–e327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventola C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 7.Klein E., Boeckel T., Martinez E., Pant S., Gandra S., Levin S., Goossens H., Laxminarayan R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA. 2018;115:201717295. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Animal Production ScienceVan Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Boeckel T.P., Glennon E.E., Chen D., Gilbert M., Robinson T.P., Grenfell B.T., Levin S.A., Bonhoeffer S., Laxminarayan R. Reducing antimicrobial use in food animals. Science. 2017;357:1350. doi: 10.1126/science.aao1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiseo K., Huber L., Gilbert M., Robinson T.P., Van Boeckel T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics (Basel) 2020;9:918. doi: 10.3390/antibiotics9120918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.BelVet-SAC Belgium Veterinary Surveillance of Antimicrobial Consumption: National Consumption Report 2019. [(accessed on 21 February 2021)]; Available online: https://belvetsac.ugent.be/BelvetSac_report_2019.pdf.
- 12.ANSES-ANMV Sales Survey of Veterinary Medicinal Products Containing Antimicrobials in France in 2019. [(accessed on 21 February 2021)]; Available online: https://www.anses.fr/en/system/files/ANMV-Ra-Antibiotiques2019EN.pdf.
- 13.Swedres-Svarm Sales of Antibiotics and Occurrence of Resistance in Sweden 2019. [(accessed on 21 February 2021)]; Available online: https://www.folkhalsomyndigheten.se/contentassets/fb80663bc7c94d678be785e3360917d1/swedres-svarm-2019.pdf.
- 14.UK-VARSS Veterinary Antibiotic Resistance and Sales Surveillance Report (UK-VARSS 2019) [(accessed on 20 February 2021)]; Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/950126/UK-VARSS_2019_Report__2020-TPaccessible.pdf.
- 15.National Food Institute. Statens Serum Institut DANMAP 2019—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. [(accessed on 21 February 2021)]; Available online: https://www.danmap.org/-/media/Sites/danmap/Downloads/Reports/2019/DANMAP_2019.ashx?la=da&hash=AA1939EB449203EF0684440AC1477FFCE2156BA5.
- 16.Van Boeckel T.P., Pires J., Silvester R., Zhao C., Song J., Criscuolo N.G., Gilbert M., Bonhoeffer S., Laxminarayan R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365:1944. doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- 17.Goutard F.L., Bordier M., Calba C., Erlacher-Vindel E., Góchez D., de Balogh K., Benigno C., Kalpravidh W., Roger F., Vong S. Antimicrobial policy interventions in food animal production in South East Asia. BMJ. 2017;358:j3544. doi: 10.1136/bmj.j3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ström G., Halje M., Karlsson D., Jiwakanon J., Pringle M., Fernström L.L., Magnusson U. Antimicrobial use and antimicrobial susceptibility in Escherichia coli on small- and medium-scale pig farms in north-eastern Thailand. Antimicrob. Resist. Infect. Control. 2017;6:75. doi: 10.1186/s13756-017-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Government of the United Kingdom; London, UK: 2016. [Google Scholar]
- 20.Laxminarayan R.P., Duse A.M.D., Wattal C.M.D., Zaidi A.K.M.M.D., Wertheim H.F.L.M.D., Sumpradit N.P., Vlieghe E.M.D., Hara G.L.P., Gould I.M.M., Goossens H.P., et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . Global Action Plan on Antimicrobial Resistance. World Health Organization; Geneva, Switzerland: 2015. [DOI] [PubMed] [Google Scholar]
- 22.World Organisation for Animal Health (OIE) Terrestrial Animal Health Code: Chapter 6.9 Monitoring of the Quantities and Usage Patterns of Antimicrobial Agents Used in Food-Producing Animals. [(accessed on 16 February 2021)]; Available online: https://www.oie.int/index.php?id=169&L=0&htmfile=chapitre_antibio_monitoring.htm.
- 23.Chantziaras I., Boyen F., Callens B., Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014;69:827–834. doi: 10.1093/jac/dkt443. [DOI] [PubMed] [Google Scholar]
- 24.Callens B., Cargnel M., Sarrazin S., Dewulf J., Hoet B., Vermeersch K., Wattiau P., Welby S. Associations between a decreased veterinary antimicrobial use and resistance in commensal Escherichia coli from Belgian livestock species (2011–2015) Prev. Vet. Med. 2018;157:50–58. doi: 10.1016/j.prevetmed.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Góchez D., Raicek M., Pinto Ferreira J., Jeannin M., Moulin G., Erlacher-Vindel E. OIE Annual Report on Antimicrobial Agents Intended for Use in Animals: Methods Used. Front. Vet. Sci. 2019;6 doi: 10.3389/fvets.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grave K., Torren-Edo J., Mackay D. Comparison of the sales of veterinary antibacterial agents between 10 European countries. J. Antimicrob. Chemother. 2010;65:2037–2040. doi: 10.1093/jac/dkq247. [DOI] [PubMed] [Google Scholar]
- 27.Hosoi Y., Asai T., Koike R., Tsuyuki M., Sugiura K. Use of veterinary antimicrobial agents from 2005 to 2010 in Japan. Int. J. Antimicrob. Agents. 2013;41:489–490. doi: 10.1016/j.ijantimicag.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Page S.W., Gautier P. Use of antimicrobial agents in livestock. Rev. Sci. Tech. OIE. 2012;31:145–188. doi: 10.20506/rst.31.1.2106. [DOI] [PubMed] [Google Scholar]
- 29.World Organisation for Animal Health (OIE) OIE Annual Report on Antimicrobial Agents Intended for Use in Animals. Fourth report. [(accessed on 16 February 2021)]; Available online: https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_Fourth_Annual_Report_AMR.pdf.
- 30.The World Bank World Bank Country and Lending Groups. [(accessed on 16 February 2021)]; Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519.
- 31.The World Bank Population, Total—Timor-Leste. [(accessed on 16 February 2021)]; Available online: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=TL.
- 32.General Directorate of Statistics—Ministry of Finance (Timor Leste) and ICF . Timor-Leste Demographic and Health Survey 2016. GDS and ICF; Dili, Timor-Leste: 2018. [Google Scholar]
- 33.Lundahl M., Sjöholm F. Improving the Lot of the Farmer: Development Challenges in Timor-Leste during the Second Decade of Independence. Asian Econ. Pap. 2013;12:71–96. doi: 10.1162/ASEP_a_00211. [DOI] [Google Scholar]
- 34.Bettencourt E., Tilman M., Narciso V., Da Silva Carvalho M.L., Henriques P. The Livestock Roles in the Wellbeing of Rural Communities of Timor-Leste. Revista de Economia e Sociologia Rural. 2015;53:63–80. doi: 10.1590/1234-56781806-94790053s01005. [DOI] [Google Scholar]
- 35.General Directorate of Statistics—Ministry of Finance (Timor Leste) Food and Agriculture Organization of the United Nations (FAO) United Nations Population Fund (UNFPA) Timor-Leste Population and Housing Census 2015: Thematic Report Volume 12. Government of Timor-Leste; Canberra, Australia: 2018. [Google Scholar]
- 36.Spencer P.R., Sanders K.A., Judge D.S. Rural Livelihood Variation and its Effects on Child Growth in Timor-Leste. Hum. Ecol. 2018;46:787–799. doi: 10.1007/s10745-018-0027-6. [DOI] [Google Scholar]
- 37.de Correia V.P., Rola-Rubzen M.F. Breaking the poverty cycle through linking farmers to markets in Timor Leste: The World Vision income generation project. Hum. Dev. Capacit. Build. Asia Pac. Trends Chall. Prospect. Future. 2016 doi: 10.4324/9781315637488. [DOI] [Google Scholar]
- 38.The Poultry Site International Egg and Poultry Review: Timor-Leste. [(accessed on 18 February 2021)]; Available online: https://www.thepoultrysite.com/news/2011/06/international-egg-and-poultry-review-timorleste.
- 39.World Organisation for Animal Health (OIE) OIE List of Antimicrobial Agents of Veterinary Importance (July 2019) [(accessed on 19 February 2021)]; Available online: https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_OIE_List_antimicrobials_July2019.pdf.
- 40.Jong J.B.D.C. Multi-Capacity Building for Antimicrobial Use (AMU) Data Collection: Improving the Recording of Antimicrobial Agents Intended for Use in Animals in Timor-Leste. [(accessed on 22 February 2021)]; Available online: https://rr-asia.oie.int/en/projects/antimicrobial-resistance/good-practices-addressing-amr-in-asia-and-the-pacific-region/multi-capacity-building-for-antimicrobial-use-amu-data-collection/
- 41.World Organisation for Animal Health (OIE) Guidance for completing the OIE Template for the Collection of Data on Antimicrobial Agents Intended for Use in Animals (Version 1_Sept 2020) [(accessed on 19 February 2021)]; Available online: https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/2020/ENG_AMUse_Guidance_Final_2020.pdf.
- 42.World Health Organization . Critically Important Antimicrobials for Human Medicine 6th Revision. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 43.Food and Agriculture Organization of the United Nations Live Animals in Timor-Leste: Stocks. [(accessed on 10 January 2021)]; Available online: http://www.fao.org/faostat/en/#data/QA.
- 44.Food and Agriculture Organization of the United Nations Livestock Primary in Timor-Leste: Producing animals/slaughtered. [(accessed on 10 January 2021)]; Available online: http://www.fao.org/faostat/en/#data/QL.
- 45.Hillerton J.E., Irvine C.R., Bryan M.A., Scott D., Merchant S.C. Use of antimicrobials for animals in New Zealand, and in comparison with other countries. N. Z. Vet. J. 2017;65:71–77. doi: 10.1080/00480169.2016.1171736. [DOI] [PubMed] [Google Scholar]
- 46.European Medicines Agency—European Surveillance of Veterinary Antimicrobial Consumption Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018. [(accessed on 16 February 2021)]; Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf.
- 47.Alders R.G., Ali S.N., Ameri A.A., Bagnol B., Cooper T.L., Gozali A., Hidayat M.M., Rukambile E., Wong J.T., Catley A. Participatory Epidemiology: Principles, Practice, Utility, and Lessons Learnt. Front. Vet. Sci. 2020;7:532763. doi: 10.3389/fvets.2020.532763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serrao E.A. PhD Thesis. University of Queensland; Queensland, Australia: 2012. Constraints to Production of Village Chickens in Timor-Leste. [Google Scholar]
- 49.Om C., McLaws M.-L. Antibiotics: Practice and opinions of Cambodian commercial farmers, animal feed retailers and veterinarians. Antimicrob. Resist. Infect. Control. 2016;5:42. doi: 10.1186/s13756-016-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiambi S., Mwanza R., Sirma A., Czerniak C., Kimani T., Kabali E., Dorado-Garcia A., Eckford S., Price C., Gikonyo S., et al. Understanding Antimicrobial Use Contexts in the Poultry Sector: Challenges for Small-Scale Layer Farms in Kenya. Antibiotics (Basel) 2021;10:106. doi: 10.3390/antibiotics10020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jong J.B.D.C. ((National Director of Veterinary Directorate, Ministry of Agriculture and Fisheries. Timor-Leste)). Personal Communication. 2021.
- 52.Democratic Republic of Timor-Leste Decree-Law No. 3/2018 of March 14: About the Import Ban on Poultry and Poultry Products. [(accessed on 19 February 2021)]; Available online: http://extwprlegs1.fao.org/docs/pdf/tim187132.pdf.
- 53.Jong J.B.D.C. Scavenging for protein and micronutrients: Village poultry in Timor-Leste. [(accessed on 22 February 2021)]; Available online: https://www.crawfordfund.org/wp-content/uploads/2017/04/CF-2016-Conference-Proceedings-Jong.pdf.
- 54.Conan A., Goutard F.L., Sorn S., Vong S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012;8:240. doi: 10.1186/1746-6148-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coyne L., Patrick I., Arief R., Benigno C., Kalpravidh W., McGrane J., Schoonman L., Sukarno A.H., Rushton J. The Costs, Benefits and Human Behaviours for Antimicrobial Use in Small Commercial Broiler Chicken Systems in Indonesia. Antibiotics (Basel) 2020;9:154. doi: 10.3390/antibiotics9040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimera Z.I., Frumence G., Mboera L.E.G., Rweyemamu M., Mshana S.E., Matee M.I.N. Assessment of Drivers of Antimicrobial Use and Resistance in Poultry and Domestic Pig Farming in the Msimbazi River Basin in Tanzania. Antibiotics (Basel) 2020;9:838. doi: 10.3390/antibiotics9120838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lhermie G., Gröhn Y.T., Raboisson D. Addressing Antimicrobial Resistance: An Overview of Priority Actions to Prevent Suboptimal Antimicrobial Use in Food-Animal Production. Front. Microbiol. 2017;7:2114. doi: 10.3389/fmicb.2016.02114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ström G., Boqvist S., Albihn A., Fernström L.L., Andersson Djurfeldt A., Sokerya S., Sothyra T., Magnusson U. Antimicrobials in small-scale urban pig farming in a lower middle-income country—Arbitrary use and high resistance levels. Antimicrob. Resist. Infect. Control. 2018;7:35. doi: 10.1186/s13756-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nhung N.T., Cuong N.V., Thwaites G., Carrique-Mas J. Antimicrobial Usage and Antimicrobial Resistance in Animal Production in Southeast Asia: A Review. Antibiotics (Basel) 2016;5:37. doi: 10.3390/antibiotics5040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olatoye O., Afisu B. Antibiotic Usage and Oxytetracycline Residue in African Catfish (Clarias gariepinus in Ibadan, Nigeria) World J. Fish. Mar. Sci. 2013;5:302–309. doi: 10.5829/idosi.wjfms.2013.05.03.71214. [DOI] [Google Scholar]
- 61.Aktas I., Yarsan E. Pharmacokinetics of Conventional and Long-Acting Oxytetracycline Preparations in Kilis Goat. Front. Vet. Sci. 2017;4:229. doi: 10.3389/fvets.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu J., Sangthong R., McNeil E., Tang R., Chongsuvivatwong V. Antibiotic use in chicken farms in northwestern China. Antimicrob. Resist. Infect. Control. 2020;9:10. doi: 10.1186/s13756-019-0672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carrique-Mas J.J., Trung N.V., Hoa N.T., Mai H.H., Thanh T.H., Campbell J.I., Wagenaar J.A., Hardon A., Hieu T.Q., Schultsz C. Antimicrobial usage in chicken production in the Mekong Delta of Vietnam. Zoonoses Public Health. 2015;62:70–78. doi: 10.1111/zph.12165. [DOI] [PubMed] [Google Scholar]
- 64.Lekagul A., Tangcharoensathien V., Mills A., Rushton J., Yeung S. How antibiotics are used in pig farming: A mixed-methods study of pig farmers, feed mills and veterinarians in Thailand. BMJ Glob. Health. 2020;5:e001918. doi: 10.1136/bmjgh-2019-001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aidara-Kane A., Angulo F.J., Conly J.M., Minato Y., Silbergeld E.K., McEwen S.A., Collignon P.J., Group W.H.O.G.D. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control. 2018;7:7. doi: 10.1186/s13756-017-0294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sommanustweechai A., Chanvatik S., Sermsinsiri V., Sivilaikul S., Patcharanarumol W., Yeung S., Tangcharoensathien V. Antibiotic distribution channels in Thailand: Results of key-informant interviews, reviews of drug regulations and database searches. Bull. World Health Organ. 2018;96:101–109. doi: 10.2471/BLT.17.199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tufa T.B., Gurmu F., Beyi A.F., Hogeveen H., Beyene T.J., Ayana D., Woldemariyam F.T., Hailemariam E., Gutema F.D., Stegeman J.A. Veterinary medicinal product usage among food animal producers and its health implications in Central Ethiopia. BMC Vet. Res. 2018;14:409. doi: 10.1186/s12917-018-1737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carson C., Li X.-Z., Agunos A., Loest D., Chapman B., Finley R., Mehrotra M., Sherk L.M., Gaumond R., Irwin R. Ceftiofur-resistant Salmonella enterica serovar Heidelberg of poultry origin—A risk profile using the Codex framework. Epidemiol. Infect. 2019;147:e296. doi: 10.1017/S0950268819001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sproston E.L., Wimalarathna H.M.L., Sheppard S.K. Trends in fluoroquinolone resistance in Campylobacter. Microb. Genom. 2018;4:e000198. doi: 10.1099/mgen.0.000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Speksnijder D.C., Mevius D.J., Bruschke C.J., Wagenaar J.A. Reduction of veterinary antimicrobial use in the Netherlands. The Dutch success model. Zoonoses Public Health. 2015;62:79–87. doi: 10.1111/zph.12167. [DOI] [PubMed] [Google Scholar]
- 71.Mouiche M.M.M., Moffo F., Betsama J.D.B., Mapiefou N.P., Mbah C.K., Mpouam S.E., Penda R.E., Ciake S.A.C., Feussom J.M.K., Kamnga Z.F., et al. Challenges of antimicrobial consumption surveillance in food-producing animals in sub-Saharan African countries: Patterns of antimicrobials imported in Cameroon from 2014 to 2019. J. Glob. Antimicrob. Resist. 2020;22:771–778. doi: 10.1016/j.jgar.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Simoneit C., Burow E., Tenhagen B.A., Käsbohrer A. Oral administration of antimicrobials increase antimicrobial resistance in E. coli from chicken—A systematic review. Prev. Vet. Med. 2015;118:1–7. doi: 10.1016/j.prevetmed.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Van Cuong N., Nhung N.T., Nghia N.H., Mai Hoa N.T., Trung N.V., Thwaites G., Carrique-Mas J. Antimicrobial Consumption in Medicated Feeds in Vietnamese Pig and Poultry Production. Ecohealth. 2016;13:490–498. doi: 10.1007/s10393-016-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schar D., Klein E.Y., Laxminarayan R., Gilbert M., Van Boeckel T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020;10:21878. doi: 10.1038/s41598-020-78849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park Y.H., Hwang S.Y., Hong M.K., Kwon K.H. Use of antimicrobial agents in aquaculture. Rev. Sci. Tech. 2012;31:189–197. doi: 10.20506/rst.31.1.2105. [DOI] [PubMed] [Google Scholar]
- 76.Zellweger R.M., Carrique-Mas J., Limmathurotsakul D., Day N.P.J., Thwaites G.E., Baker S., Southeast Asia Antimicrobial Resistance Network A current perspective on antimicrobial resistance in Southeast Asia. J. Antimicrob. Chemother. 2017;72:2963–2972. doi: 10.1093/jac/dkx260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.López-Angarita J., Hunnam K.J., Pereira M.M.D., Pant J., Teoh S.J., Eriksson H., Amaral L., Tilley A. Fisheries and Aquaculture of Timor-Leste in 2019: Current Knowledge and Opportunities. WorldFish; Penang, Malaysia: 2019. [Google Scholar]
- 78.Food and Drug Administration 2017 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. [(accessed on 22 February 2021)]; Available online: https://www.fda.gov/media/119332/download.
- 79.Speksnijder D.C., Wagenaar J.A. Reducing antimicrobial use in farm animals: How to support behavioral change of veterinarians and farmers. Anim. Front. 2018;8:4–9. doi: 10.1093/af/vfy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caudell M.A., Dorado-Garcia A., Eckford S., Creese C., Byarugaba D.K., Afakye K., Chansa-Kabali T., Fasina F.O., Kabali E., Kiambi S., et al. Towards a bottom-up understanding of antimicrobial use and resistance on the farm: A knowledge, attitudes, and practices survey across livestock systems in five African countries. PLoS ONE. 2020;15:e0220274. doi: 10.1371/journal.pone.0220274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vijay D., Bedi J.S., Dhaka P., Singh R., Singh J., Arora A.K., Gill J.P.S. Knowledge, Attitude, and Practices (KAP) Survey among Veterinarians, and Risk Factors Relating to Antimicrobial Use and Treatment Failure in Dairy Herds of India. Antibiotics (Basel) 2021;10:216. doi: 10.3390/antibiotics10020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Truong D.B., Doan H.P., Doan Tran V.K., Nguyen V.C., Bach T.K., Rueanghiran C., Binot A., Goutard F.L., Thwaites G., Carrique-Mas J., et al. Assessment of Drivers of Antimicrobial Usage in Poultry Farms in the Mekong Delta of Vietnam: A Combined Participatory Epidemiology and Q-Sorting Approach. Front. Vet. Sci. 2019;6 doi: 10.3389/fvets.2019.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schar D., Sommanustweechai A., Laxminarayan R., Tangcharoensathien V. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med. 2018;15:e1002521. doi: 10.1371/journal.pmed.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lekagul A., Tangcharoensathien V., Yeung S. The use of antimicrobials in global pig production: A systematic review of methods for quantification. Prev. Vet. Med. 2018;160:85–98. doi: 10.1016/j.prevetmed.2018.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.