Abstract
Long-term treatment of Parkinson’s disease (PD) by levodopa leads to motor complication “wearing-off”. Zonisamide is a nondopaminergic antiparkinsonian drug that can improve “wearing-off” although response to the treatment varies between individuals. To clarify the genetic basis of zonisamide responsiveness, we conducted a genome-wide association study (GWAS) on 200 PD patients from a placebo-controlled clinical trial, including 67 responders whose “off” time decreased ≥1.5 h after 12 weeks of zonisamide treatment and 133 poor responders. We genotyped and evaluated the association between 611,492 single nucleotide polymorphisms (SNPs) and “off” time reduction. We also performed whole-genome imputation, gene- and pathway-based analyses of GWAS data. For promising SNPs, we examined single-tissue expression quantitative trait loci (eQTL) data in the GTEx database. SNP rs16854023 (Mouse double minute 4, MDM4) showed genome-wide significant association with reduced “off” time (PAdjusted = 4.85 × 10−9). Carriers of responsive genotype showed >7-fold decrease in mean “off” time compared to noncarriers (1.42 h vs 0.19 h; P = 2.71 × 10−7). In silico eQTL data indicated that zonisamide sensitivity is associated with higher MDM4 expression. Among the 37 pathways significantly influencing “off” time, calcium and glutamate signaling have also been associated with anti-epileptic effect of zonisamide. MDM4 encodes a negative regulator of p53. The association between improved motor fluctuation and MDM4 upregulation implies that p53 inhibition may prevent dopaminergic neuron loss and consequent motor symptoms. This is the first genome-wide pharmacogenetics study on antiparkinsonian drug. The findings provide a basis for improved management of “wearing-off” in PD by genotype-guided zonisamide treatment.
Subject terms: Genome-wide association studies, Genetics research
Introduction
Parkinson’s disease (PD) is a chronic progressive movement disorder in which loss of dopaminergic neurons in the substantia nigra of the midbrain leads to motor symptoms including tremor, bradykinesia, rigidity, and postural instability [1]. Previous studies suggest that the neurodegeneration results from the interplay of intrinsic as well as extrinsic factors including genetic mutations, altered activity patterns of ion channels such as l-type calcium channels and ATP-sensitive potassium channels, reduced neurotransmission of dopaminergic neurons, lysosomal and mitochondrial dysfunction, α-synuclein accumulation, and neurotoxic stress [2].
Current clinical management of PD is largely confined to symptomatic treatment, with levodopa being the most effective drug. Conversion of levodopa to dopamine compensates the loss of dopamine resulting from degeneration of dopaminergic neurons in the substantia nigra of PD patients. However, due to the short half-life of levodopa and the continuous loss of dopaminergic neurons following disease progression, beneficial effect of each dose of the drug gets shorter. As a result, motor and nonmotor symptoms re-emerge in PD patients before the next dose of levodopa is being taken. This phenomenon, which termed “wearing-off”, normally appears after several years of levodopa treatment and can significantly affect quality of life of PD patients [3]. “Wearing-off” is often managed by altering the dosing, timing, and formulation of levodopa to prolong the effect of the drug. Co-administration of other drugs such as monoamine oxidase (MAO)-B inhibitors, catechol-O-methyl transferase inhibitors, and dopamine agonists that have longer half-life has also been shown to be useful for extending the effect of levodopa [4].
Zonisamide is a sulfonamide anti-epileptic drug that improves “wearing-off” in PD patients without increasing dyskinesia [5]. In Japan, zonisamide was approved as an adjunctive treatment for PD in 2009. The efficacy of zonisamide in improving motor symptoms and reducing “off” time in PD patients has been evaluated in a series of double-blind placebo-controlled clinical trials [5–7]. Owing to good safety profiles and limited interactions with other drugs, zonisamide has been used as an anti-convulsant since 1989 and has demonstrated beneficial effects in various neurological and psychiatric diseases [8].
Previous studies suggest that zonisamide may exert its anti-epileptic and antiparkinsonian effects through inhibition of sodium and T-type calcium channels, and MAO-B activity, as well as regulation of striatal delta1-receptor-associated gamma aminobutyric acid (GABA)ergic neurotransmission [9–11]. More recently, an investigation of the antiparkinsonian effect of zonisamide revealed a potential role against oxidative stress and dopaminergic neurodegeneration [12, 13].
Although inter-individual variation in the response to zonisamide treatment has been documented in clinical settings [14], the genetic basis for this observation has yet to be explored. We have previously identified common variants associated with PD through a genome-wide association study (GWAS) [15]. Here, we report a GWAS that investigates the association between genetic variations and response to zonisamide treatment in Japanese PD patients.
Subjects/materials and methods
Subjects
Subjects were Japanese PD patients who took part in a multicenter, randomized, double-blind, placebo-controlled study that aimed to determine the efficacy of zonisamide in treating “wearing-off” [7]. The subjects of the clinical trial have mean daily “off” time of at least 2 h for the last 7 days of the run-in period. These patients have been treated with any combination drugs of levodopa and dopa decarboxylase inhibitor for at least 6 consecutive months and had responded to levodopa during the first few years of levodopa treatment. Among the 373 clinical trial participants whose DNA samples were available for the current study, 119 who received a placebo were excluded along with 32 subjects who lacked a record on dosing information, one who lacked UPDRS part III total score or “off” time values at week 12, and 19 who did not complete the clinical trial either due to adverse events, withdrawal from the study, investigator’s discretion, or worsening of PD. The GWAS was conducted for 202 subjects who received either 25 or 50 mg zonisamide per day and who had a complete record of changes in “off” time from baseline after 12 weeks of treatment. A flowchart detailing the selection of samples is shown as Supplementary Fig. 1. All participants have provided written informed consent to participate in the current pharmacogenetic study when provided informed consent to take part in the clinical trial in accordance with the process approved by Ethical Committee at each of the medical institution where the patients were enrolled.
Definition of zonisamide responsiveness
Responsiveness to zonisamide was evaluated as efficacy of the drug in reducing “off” time of PD patients. Patients who showed a decrease of at least 1.5 h from baseline in “off” time after 12 weeks of zonisamide treatment were defined as responders; whereas all the remaining subjects were classified as poor responders. Briefly, all subjects were instructed to record in a diary their “on” and “off” states as well as the occurrence of dyskinesia for every 30 min that they were awake. The “on” stage was defined as the effective time of the medication when symptoms were well controlled, whereas the “off” stage referred to the stage when the medication was no longer effective, and symptoms re-emerged. “Off” time was calculated based on information in the diary, considering the last 7 days before each hospital visit (except for the screening visit). When “off” time data for fewer than 5 days were available, the data obtained at the visit were handled as missing. Further details regarding clinical assessment of patients are described in the paper detailing the clinical trial [7]. Demographic and clinical characteristics of subjects included in the final analysis are summarized in Table 1.
Table 1.
Characteristics of responders and poor responders defined on change in “off” time from baseline
| Variables | Responsiveness defined based on change in “off” time from baseline | ||
|---|---|---|---|
| Responders, R (n = 67) | Poor responders, PR (n = 133) | P value (R vs PR)* | |
| Female sex (%) | 41/67 (61.2%) | 78/133 (58.6%) | |
| Age (Year, Mean ± SD) | 64.6 (7.2) | 63.2 (7.6) | 0.5073 |
| BMI (Mean ± SD) | 22.6 (3.8) | 22.7 (3.7) | 0.5527 |
| Onset age of PD (Year, Mean ± SD) | 56.6 (9.0) | 54.8 (8.3) | 0.3886 |
| Duration from onset of PD (Year, Mean ± SD) | 8.0 (4.7) | 8.4 (4.3) | 0.5809 |
| Onset age of “wearing-off” (Year, Mean ± SD) | 62.2 (8.3) | 60.6 (8.2) | 0.3520 |
| Duration from onset of “wearing-off” (Year, Mean ± SD) | 2.4 (3.0) | 2.6 (3.0) | 0.4675 |
| MMSE (Mean ± SD) | 28.3 (2.0) | 28.5 (2.0) | 0.8391 |
| Modified Hoehn & Yahr score (ON) (Mean ± SD) | 2.2 (0.8) | 2.3 (0.7) | 0.9305 |
| Modified Hoehn & Yahr score (OFF) (Mean ± SD) | 3.2 (0.8) | 3.4 (0.8) | 0.2974 |
| Dose of levodopa (mg/day, Mean ± SD) | 1781.7 (639.0) | 1907.9 (708.3) | 0.0819 |
| LEDDa (mg/day, Mean ± SD) | 469.9 (164.3) | 498.0 (170.9) | 0.1114 |
| Number of concomitant drugs (Mean ± SD) | 3.0 (1.1) | 3.3 (1.1) | 0.7516 |
| UPDRS part III total score at week 12 (Mean ± SD) | 13.1 (9.6) | 16.0 (10.8) | 0.5177 |
| UPDRS part III total score at baseline (Mean ± SD) | 16.8 (10.3) | 19.1 (11.4) | 0.9439 |
| Change in UPDRS part III total score from baseline (Mean ± SD) | −3.8 (5.8) | −3.1 (6.5) | 0.2230 |
| Average “Off” time at week 12b (Hour, Mean ± SD) | 3.8 (2.3) | 6.7 (2.6) | 0.0010 |
| Average “Off” time at baselineb (Hour, Mean ± SD) | 6.5 (2.4) | 6.2 (2.1) | 0.7917 |
| Change in “off” time from baseline (Hour, Mean ± SD) | −2.8 (1.3) | 0.5 (1.5) | 5.95 × 10−7 |
SD standrad deviation, BMI body mass index, PD Parkinson’s disease, MMSE Mini–Mental State Examination, LEDD levodopa-equivalent daily dose
*P values were determined by using two-tailed unpaired t-test with equal variance
aConversion factor for LEDD: bromocriptine mesilate, ×10; cabergoline, ×70; pergolide mesilate, ×100; pramipexole hydrochloride hydrate, ×60
ropinirole hydrochloride, ×16.67; talipexole hydrochloride, ×60
b“Off” time was calculated using patients’ diary information for the last 7 days before each visit (excluding screening visit)
Genotyping and quality controls (QCs)
GWAS was performed by using Illumina Infinium HumanOmniExpressExome-8_v1.2 BeadChip (Illumina, San Diego, CA, USA). The 202 PD patients were genotyped for 964,193 SNPs (of which 273,246 were exonic markers). As QC, 11 subjects were genotyped twice to assess concordance of genotyping. SNPs with a call rate <99%, deviating from the Hardy–Weinberg equilibrium (P ≤ 1 × 10−6), and those that were monomorphic or nonautosomal were excluded from the analysis. Population stratification was evaluated by principal component analysis (PCA) using EIGENSOFT v.5.0.2 software [16], with the four HapMap [17] populations—namely, Europeans, Africans, and East Asians (Japanese and Han Chinese)—serving as reference groups. Degree of relatedness between samples was examined with the “--genome” function of PLINK v.1.07 [18].
Statistical analysis
Associations between SNPs and responsiveness to zonisamide were assessed with the Cochran–Armitage trend test and multivariate logistic regression analysis (PLINK v.1.07). For logistic regression analysis, daily dose of zonisamide administered by patients and baseline “off” time of patients were included as additional covariates to predict zonisamide responsiveness. Three genetic models, namely additive, dominant, and recessive models, were examined in multivariate logistic regression analysis, with the minor allele found in the responders was considered as reference allele. Model producing the smallest P value was used to determine the mode of inheritance of the SNPs. The significance threshold for the GWAS was set at 5 × 10−8. A Manhattan plot was generated using Haploview v.4.1 [19] based on the P value from the trend test. Genomic inflation factor (λgc) was determined and a quantile–quantile (QQ) plot was generated using R statistical software v.3.13 (http://www.R-project.org/). A regional association plot generated using LocusZoom [20]. Box and whisker plots (R statistical software v.3.13) were used to graphically illustrate the association between clinical variables (including changes in “off” time) and SNP genotypes. The Kruskal–Wallis and Mann–Whitney U tests (R statistical software v.3.13) were used to assess differences in the distribution of clinical variables based on SNP genotypes. Single-tissue eQTL data of SNP were retrieved from the GTEx portal (https://www.gtexportal.org).
Whole-genome imputation (WGI)
WGI was conducted with data for 286 East Asians in 1000 Genome [21] (Phase Iv3 2010-11, data freeze on 20120314). Markers with minor allele frequencies <1% were excluded from the WGI analysis, which was performed using minimac2 [22] the subsequent association analysis was performed using mach2dat (http://www.unc.edu/~yunmli/software.html) with the daily dose of zonisamide included as a covariate. Markers with a quality score of R2 < 0.9 were excluded from the association analysis. Genome-wide significance thresholds were set at P = 8.61 × 10−9 for WGI analysis considering the markers investigated.
Gene-based association analysis
Gene-based association analysis was performed using Versatile Gene-based Association Study 2 [23]. Briefly, after assigning SNPs to genes based on hg19 genomic coordinates, gene-based P values were calculated using a simulation method that considers linkage disequilibrium (LD) between markers in a gene. Data from the Asian population in the 1000 Genomes Project were used for LD estimation. Gene boundary was defined as ±50 kb for SNP selection, and only the top 10% of associated SNPs within each gene were included in the analysis. Genome-wide significance thresholds were set at 2.11 × 10−6 for gene-based association analysis considering 23,735 genes investigated.
Pathway-based analysis
Pathway-based analysis was carried out using Gene Set Analysis-SNP [24]. Briefly, SNPs were assigned to the gene if they were located within ±20 kb of the gene locus. In the case where more than one SNP was mapped to a gene, the P-value of the gene was represented by the second most significant P value of all SNPs mapped to the gene to avoid false positive association arising from random associations as discussed in Nam et al. [24]. Pathways analyzed in this study were downloaded from the Molecular Signatures Database v.5.0 [25]. The analyzed pathways include 1330 gene sets in the canonical pathways (Biocarta, Kyoto Encyclopedia of Genes and Genomes, and Reactome) and 1434 gene ontology (GO) terms (including biological processes, cellular components, and molecular functions). The significance of the association between gene sets and reduction in “off” time was determined using the Bonferroni-corrected P value. Genome-wide significance thresholds were set at 3.76 × 10−5 and 3.44 × 10−5 for canonical pathways and GO terms, respectively.
Association between SNPs and improvement in motor symptoms measured by Unified PD Rating Scale (UPDRS) Part III total score
Responsiveness to zonisamide was measured as a reduction in UPDRS Part III total score, which measures motor symptoms of PD patients. Patients who showed a decrease in UPDRS Part III total score of at least 5 points from baseline after 12 weeks of zonisamide treatment were defined as responders; and all remaining subjects were considered as poor responders. SNP genotyping and SNP-, gene-, and pathway-based association analyses were performed as described above.
Results
Quality controls (QCs) and GWAS
The overall genotyping rate for subjects and genotyping consistency for all duplicates exceeded 99.8%. PCA with HapMap populations as reference groups confirmed that all subjects were of East Asian origin. A subsequent PCA analysis limited to the subjects of the present study revealed two outliers (Supplementary Fig. 2a, b) that were excluded from subsequent analyses. All subject pairs showed a low degree of relatedness, with a PI_HAT value <0.05. A genomic inflation factor (λgc) of 1.068 in the QQ plot (Supplementary Fig. 3) indicated negligible population sub-stratification among subjects. After QC, we identified 611,492 SNPs in 200 PD patients who received zonisamide at 25 or 50 mg/day (n = 100 each) and were available for the analysis (Supplementary Fig. 1).
Among the 200 PD patients analyzed, 67 who responded very well to zonisamide by showing a decrease of at least 1.5 h in “off” time from baseline after 12 weeks of treatment were designated as responders; whereas the remaining 133 subjects were classified as poor responders. As summarized in Table 1, responders achieved a mean decrease in “off” time of 2.8 h; whereas poor-responders showed an average increase in “off” time of 0.5 h after 12 weeks of zonisamide treatment. Responders and poor responders were matched in terms of demographic and clinical characteristics. Importantly, the two groups exhibited no obvious difference in baseline “off” time value or onset age and duration from onset of “wearing-off”.
Based on the Manhattan plot of the GWAS data, SNP rs16854023 (chromosome 1q32.1) showed a genome-wide significant association (PTrend = 4.23 × 10−8) with a reduction in “off” time (Supplementary Fig. 4). A strong association between this SNP and a reduction in “off” time was observed in patients administered zonisamide at 25 mg/day (P = 2.44 × 10−4) and 50 mg/day (P = 3.00 × 10−5) (Table 2). This was confirmed by a logistic regression analysis in which daily dose of zonisamide and baseline “off” time were covariates (PAdjusted = 4.85 × 10−9). Allele C is detected as minor allele in responders and is associated with lower drug responsiveness (OR = 0.133). The GWAS identified 13 markers that showed suggestive associations (PTrend < 10−5) with the responsiveness to zonisamide treatment (Supplementary Table 1). A further association analysis after accounting for the effects of the marker SNP did not reveal any additional associations that achieved genome-wide significance.
Table 2.
SNP showing genome-wide significant association with reduction in “off” time in PD patients administered zonisamide
| Chr. | SNP | Location | A1/A2 | Zonisamide | Responders | Poor-responders | P-value* | Logistic regression analysisa | Nearest | Location | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dose (mg/day) | CC | CT | TT | MAF | CC | CT | TT | MAF | Pmin | OR | Model | gene | ||||||
| 1 | rs16854023 | 204551830 | C/T | 50 | 4 | 11 | 22 | 0.26 | 15 | 40 | 8 | 0.56 | 3.00 × 10−5 | MDM4 | Intron | |||
| 25 | 2 | 9 | 19 | 0.22 | 16 | 38 | 16 | 0.50 | 2.44 × 10−4 | |||||||||
| All | 6 | 20 | 41 | 0.24 | 31 | 78 | 24 | 0.53 | 4.23 × 10−8 | 4.85 × 10−9 | 0.133 | DOM | ||||||
Chr. Chromosome, SNP single nucleotide polymorphism, A1 Allele 1 (minor allele detected in Responders); A2 Allele 2 (major allele detectedin Responders), MAF minor allele frequency; Pmin Minimum P value, OR odds ratio, DOM dominant model
*P value of Armitage Trend test
aLogistic regression analysis considering daily dose of zonisamide as well as baseline “off” time value as covariates; three models of inheritance, namely allelic,dominant, and recessive were considered. Genetic model that gave the minimum P value was shown
Whole-genome imputation (WGI)
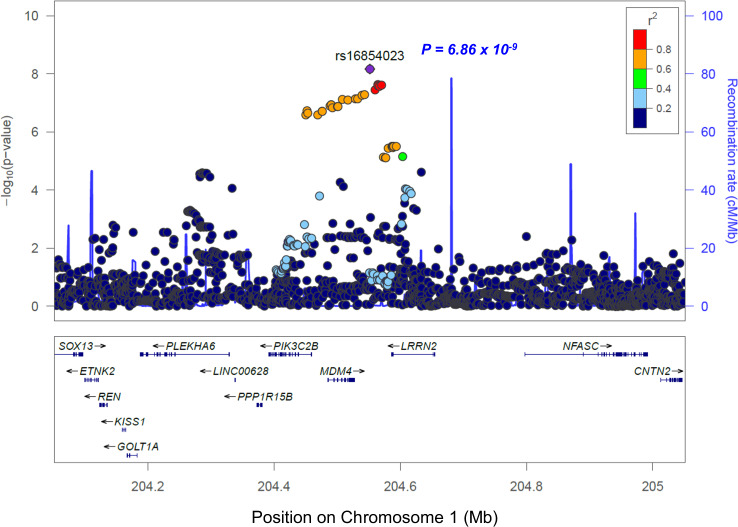
To increase the study resolution, we performed WGI to infer the genotypes of SNPs that were not investigated in the GWAS. Associations between changes from baseline in “off” time after 12 weeks of treatment and the 5,805,599 markers with imputation quality score of R2 ≥ 0.9 were examined. A total of 42 markers with P < 10−6 were identified (Supplementary Table 2). Two markers (including an SNP) located on chromosome 1q32 achieved a genome-wide significance threshold of P = 8.61 × 10−9. A regional plot (Fig. 1) based on WGI data indicated that the strongly associated region encompassed two genes, namely mouse double minute 4 (MDM4) and leucine-rich repeat transmembrane neuronal 2 (LRRN2).
Fig. 1.
Regional plot for the most associated region on Chromosome 1q32.1. Regional plot was generated based on the association data from whole-genome imputation analysis by using 1000 Genome data of the 286 East Asians. cM centiMorgan, Mb Megabases
Correspondence between changes in “off” time, genotypes of SNPs and eQTL data
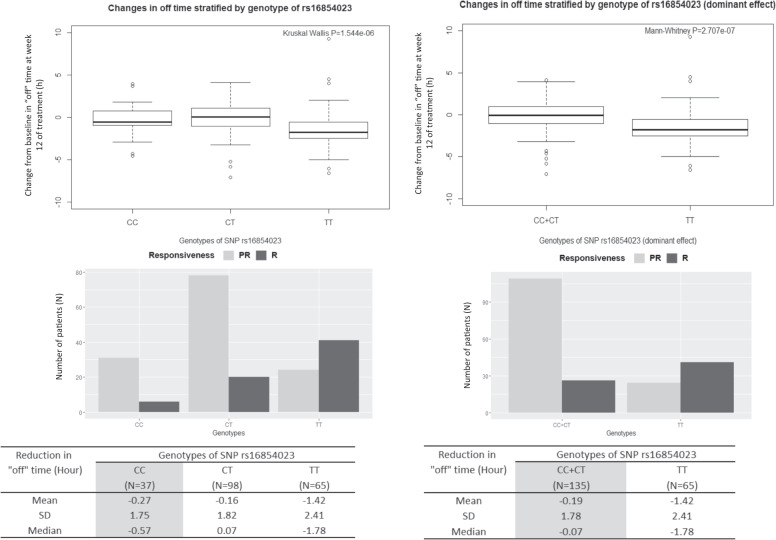
We generated box and whisker plots to visualize the correspondence between changes in “off” time and genotypes of the marker SNP rs16854023 (Fig. 2). More than 60% of TT carriers were responders to zonisamide. On average, TT carriers showed a 1.42 h decrease in “off” time as compared to 0.19 h in carriers of the CT and CC genotypes. To determine the reason for the increased responsiveness to the drug among TT carriers, we used the GTEx portal to examine eQTL data for the SNP. Although the marker SNP has been reported to influence the expression of the phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2β gene in esophageal mucosa (Supplementary Fig. 5), expression data in neurons and nerve tissue are lacking. We therefore investigated other SNPs on 1q32.1 that showed a suggestive association (PTrend < 10−5) with reduction in “off” time in the GWAS (Supplementary Table 1) for which eQTL data in neuron and nerve tissue were available. Two SNPs (rs10900597 and rs2290854) with GWAS PTrend = 7.62 × 10−6 that were in complete LD (R2 = 1) but had an R2 = 0.24 with the marker SNP (Supplementary Fig. 6) influenced MDM4 expression in tibial nerve (Supplementary Fig. 7a, b). A box and whisker plot illustrating changes in “off” time based on the genotypes of SNP rs10900597 (Supplementary Fig. 8) indicated that carriers of the CC responsive genotype showed an average decrease in “off” time of 2.19 h as compared to 0.09 h for carriers of the homozygous TT genotype. The associations between SNP (rs16854023 and rs10900597) genotypes and other clinical variables such as changes in UPDRS Part III total score after 12 weeks of treatment, plasma concentration of zonisamide at week 4 of treatment, age of PD/“wearing-off” onset, and duration from onset of PD/“wearing-off” were nonsignificant (Supplementary Fig. 9a, b).
Fig. 2.
Distribution of “changes in “off” time from baseline at week 12” stratified by the genotypes of SNP rs16854023 as demonstrated by the Box and Whisker plot, histogram and table. N Number of subjects; PR poor responders; R responders; SD standard deviation; SNP single nucleotide polymorphism
Gene- and pathway-based analysis
To further increase the coverage of our study by detecting combined effects of weaker signals from GWAS that may have been overlooked in conventional SNP-based GWAS, we carried out gene- and pathway-based association analyses. The former examined the associations between 23,735 genes and responsiveness to zonisamide. A Manhattan plot for gene-based GWAS (Supplementary Fig. 10) indicated that only the MDM4 gene (Pgene = 2.0 × 10−6) was significantly associated with a decrease in “off” time.
For pathway-based analysis, 16 of the 1330 canonical pathways examined showed significant associations with a reduction in “off” time (PBonferroni-corrected < 3.76 × 10−5). On the other hand, of the 1454 GO terms examined, 21 showed a significant association (PBonferroni-corrected < 3.44 × 10−5) (Table 3). These pathways included calcium signaling pathway, potassium channels, transmembrane receptor protein phosphatase activity, extracellular matrix-related pathways, and Ras GTPase binding, among others.
Table 3.
Pathways showing significant association with a reduction in “off” time after Bonferroni correction
| MsigDB category | Pathways | Gene count | Set size | z-score | P value | |
| C2 canonical pathways | KEGG_CALCIUM_SIGNALING_PATHWAY | 166 | 178 | 5.964 | 1.23 × 10−9 | |
| REACTOME_TRANSMISSION_ACROSS_CHEMICAL_SYNAPSES | 174 | 186 | 5.816 | 3.01 × 10−9 | ||
| KEGG_ABC_TRANSPORTERS | 42 | 44 | 5.015 | 2.66 × 10−7 | ||
| NABA_COLLAGENS | 41 | 44 | 4.774 | 9.02 × 10−7 | ||
| REACTOME_ABC_FAMILY_PROTEINS_MEDIATED_TRANSPORT | 32 | 34 | 4.734 | 1.10 × 10−6 | ||
| NABA_BASEMENT_MEMBRANES | 38 | 40 | 4.685 | 1.40 × 10−6 | ||
| REACTOME_TRANSPORT_OF_INORGANIC_CATIONS_ANIONS_AND_AMINO_ACIDS_OLIGOPEPTIDES | 88 | 94 | 4.591 | 2.20 × 10−6 | ||
| PID_REELIN_PATHWAY | 29 | 29 | 4.463 | 4.05 × 10−6 | ||
| KEGG_ECM_RECEPTOR_INTERACTION | 83 | 84 | 4.439 | 4.52 × 10−6 | ||
| REACTOME_TANDEM_PORE_DOMAIN_POTASSIUM_CHANNELS | 12 | 12 | 4.395 | 5.53 × 10−6 | ||
| REACTOME_EFFECTS_OF_PIP2_HYDROLYSIS | 22 | 25 | 4.304 | 8.38 × 10−6 | ||
| REACTOME_NEUROTRANSMITTER_RECEPTOR_BINDING_AND_DOWNSTREAM_TRANSMISSION_IN_THE_POSTSYNAPTIC_CELL | 127 | 137 | 4.135 | 1.78 × 10−5 | ||
| NABA_ECM_GLYCOPROTEINS | 182 | 196 | 4.134 | 1.79 × 10−5 | ||
| REACTOME_SIGNALING_BY_RHO_GTPASES | 100 | 113 | 4.094 | 2.12 × 10−5 | ||
| KEGG_LONG_TERM_POTENTIATION | 65 | 70 | 4.039 | 2.68 × 10−5 | ||
| REACTOME_PHOSPHOLIPID_METABOLISM | 184 | 198 | 3.957 | 3.79 × 10−5 | ||
| C5 GO terms | TRANSMEMBRANE_RECEPTOR_PROTEIN_PHOSPHATASE_ACTIVITY | 19 | 19 | 5.837 | 2.65 × 10−9 | |
| COLLAGEN | 22 | 23 | 4.932 | 4.08 × 10−7 | ||
| METAL_ION_TRANSMEMBRANE_TRANSPORTER_ACTIVITY | 138 | 147 | 4.754 | 9.97 × 10−7 | ||
| VOLTAGE_GATED_CHANNEL_ACTIVITY | 69 | 73 | 4.730 | 1.12 × 10−6 | ||
| GLUTAMATE_RECEPTOR_ACTIVITY | 20 | 20 | 4.714 | 1.21 × 10−6 | ||
| CELL_RECOGNITION | 18 | 19 | 4.680 | 1.43 × 10−6 | ||
| GLUTAMATE_SIGNALING_PATHWAY | 16 | 17 | 4.678 | 1.45 × 10−6 | ||
| EMBRYONIC_MORPHOGENESIS | 17 | 17 | 4.627 | 1.86 × 10−6 | ||
| VOLTAGE_GATED_CALCIUM_CHANNEL_ACTIVITY | 17 | 18 | 4.615 | 1.97 × 10−6 | ||
| ACTIVE_TRANSMEMBRANE_TRANSPORTER_ACTIVITY | 114 | 122 | 4.542 | 2.78 × 10−6 | ||
| VOLTAGE_GATED_CATION_CHANNEL_ACTIVITY | 63 | 66 | 4.509 | 3.26 × 10−6 | ||
| HYDROLASE_ACTIVITY_ACTING_ON_ACID_ANHYDRIDESCATALYZING_TRANSMEMBRANE_MOVEMENT_OF_SUBSTANCES | 35 | 39 | 4.328 | 7.52 × 10−6 | ||
| ATPASE_ACTIVITY_COUPLED_TO_MOVEMENT_OF_SUBSTANCES | 36 | 40 | 4.303 | 8.41 × 10−6 | ||
| EXTRACELLULAR_MATRIX_PART | 53 | 57 | 4.302 | 8.45 × 10−6 | ||
| EXTRACELLULAR_MATRIX | 95 | 100 | 4.296 | 8.70 × 10−6 | ||
| RAS_GTPASE_BINDING | 25 | 25 | 4.222 | 1.21 × 10−5 | ||
| PROTEINACEOUS_EXTRACELLULAR_MATRIX | 93 | 98 | 4.142 | 1.72 × 10−5 | ||
| SMALL_GTPASE_BINDING | 32 | 33 | 4.087 | 2.19 × 10−5 | ||
| PRIMARY_ACTIVE_TRANSMEMBRANE_TRANSPORTER_ACTIVITY | 36 | 40 | 4.034 | 2.74 × 10−5 | ||
| GTPASE_BINDING | 33 | 34 | 4.022 | 2.88 × 10−5 | ||
| CATION_CHANNEL_ACTIVITY | 113 | 119 | 3.990 | 3.31 × 10−5 | ||
Pathways written in bold are those have been previously reported to be associated with mechanisms of action of zonisamide
Association between SNPs and improvement in motor symptoms measured by Unified Parkinson’s Disease Rating Scale (UPDRS) Part III total score
Given that zonisamide has been reported to improve motor symptoms of PD patients [6, 26] a GWAS was performed to detect variants that are significantly associated with this effect, as reflected by a reduction in UPDRS Part III total score. On average, poor responders did not exhibit any improvement in the score after 12 weeks of zonisamide treatment, whereas responders achieved a mean decrease of 9.9 (P = .73 × 10−8) (Supplementary Table 3). The two patient groups had similar demographic and clinical characteristics except for baseline UPDRS Part III total score, which was higher in responders than in poor responders (23.6 ± 10.0 vs 15.7 ± 10.6, P = 0.0113).
None of the SNPs or genes investigated in the GWAS showed a genome-wide significant association with a reduction in UPDRS Part III total score (Supplementary Figs. 11–13). The four SNPs showing a suggestive association (P < 1 × 10−5) with this parameter are listed in Supplementary Table 4. However, in the subsequent pathway-based analysis, six canonical pathways and 12 GO terms (e.g., transmission across chemical synapses, axonogenesis, and neurogenesis) showed a genome-wide significant association (Supplementary Table 5). In addition, processes associated with calcium signaling also showed a suggestive association with improvement in the motor symptoms of PD patients.
Discussion
To our knowledge, this is the first GWAS to investigate the genetic basis of the response to an antiparkinsonian drug. SNP-, gene-, and pathway-based association analyses revealed that chromosome 1q32.1 and several pathways were significantly associated with a reduction in “off” time by zonisamide treatment in PD patients. This association was independent of the baseline “off” time value and the daily dose of zonisamide administered to patients.
The SNP (rs16854023) showing the strongest association with a reduction in “off” time in the GWAS is located between the MDM4 and LRRN2 genes. More than 60% of patients with the TT genotype of this SNP showed a reduction in “off” time of over 1.5 h, and responsive TT carriers showed a greater than sevenfold decrease in mean “off” time compared to carriers of alternative genotypes (1.42-h vs 0.19-h). Evidence from the eQTL data suggests that several SNPs on 1q32.1 that showed a suggestive association in the GWAS influenced MDM4 expression in tibial nerve tissue. For instance, homozygous responsive allele (C) carriers at SNP rs10900597 showed an over 24-fold decrease in “off” time compared to homozygous carriers of the alternative allele (T) in our study (Supplementary Fig. 8); and according to eQTL data from GTEx, subjects with the CC genotype had higher expression of MDM4 in the tibial nerve (Supplementary Fig. 7a).
On the other hand, the lack of association between these SNPs and other clinical variables (Supplementary Fig. 9a, b) implies that the association between these SNPs and the reduction in “off” time are not influenced by the severity of PD and “wearing-off”. In addition, these polymorphisms do not appear to alter zonisamide metabolism; in fact, our data indicate that the reduction in “off” time is independent of the zonisamide dosage. Interestingly, the lack of association between these SNPs and changes in the UPDRS part III total score of PD patients suggests that zonisamide may act via different mechanisms to improve motor symptoms of PD patients. This finding is supported by results from the GWAS of UPDRS Part III total score (Supplementary Tables 4 and 5) that revealed sets of SNPs and pathways distinct from those identified in the GWAS of “off” time. Due to the characteristic of our study subjects who were typical “wearing-off” patients with good “on” levels (i.e., who responded well to levodopa and other standard drugs) but experienced a shorter drug effect [7], we were unable to identify variants significantly associated with improvement in motor symptoms, as reflected by a reduction in UPDRS Part III total score.
The MDM4 gene encodes MDMX, an ubiquitin ligase that is a negative regulator of the tumor suppressor gene p53 [27]. Although the role of MDMX in PD is unclear, it is known to be critical for neuronal survival [28, 29], which may be related to its inhibition of p53. While increased p53 activity in PD promoted neuronal apoptosis [30, 31], dopaminergic neuron-specific deletion and pharmacological inhibition of p53 reduced the loss of dopaminergic neuron [31] and motor deficits [32, 33], respectively. In our study, carriers of genotypes associated with higher MDM4 expression (GTEx portal) responded more favorably to zonisamide treatment and achieved a greater reduction in “off” time. Since motor fluctuations in advanced PD may result from the progressive loss of dopaminergic presynaptic terminals, which decreases dopamine storage capacity [34], our finding supports a potential neuroprotective effect of zonisamide in reducing dopaminergic neuron loss through a p53-mediated mechanism, although the details thereof require clarification through functional studies.
The results of our pathway-based analysis are in accordance with those of previous studies and suggest that calcium-, glutamate-, and potassium-related pathways mediate the antiparkinsonian effects of zonisamide [35–39], although other novel pathways may also be involved. For instance, GTPases including Ras GTPase identified in the GWAS of “off” time have been previously reported to play a role in PD pathogenesis and are potential therapeutic targets [40]. Similarly, pathways associated with axonogenesis and neurogenesis identified in the GWAS of UPDRS strongly support a neuroprotective role for zonisamide. However, validation of our findings in independent subjects and follow-up functional analysis of the MDM4 gene are necessary to translate our findings into better management of “wearing-off” in PD. The latter would be facilitated in clinical settings by determining of patient genotype and predicting their response to zonisamide in order to identify those who are most likely to benefit from this drug treatment.
Most pharmacogenetic studies on the response to antiparkinsonian drugs have been limited to investigations of candidate genes [41, 42]. Ours is the first hypothesis-free study examining genes on a genome-wide scale. Additional studies of this nature should provide more clues for clinicians to identify patients who are more likely to respond well to a drug so that the most appropriate intervention can be prescribed. This would lead to better management of “wearing-off” in PD through precision treatment.
Supplementary information
Acknowledgements
The authors thank all investigators included in The Japan Zonisamide on PD Study Group who participated in the clinical study [7]. This study was funded and supported by Sumitomo Dainippon Pharma Co., Ltd, of which the roles were the collection of DNA samples in the clinical trial and the approval of the study design; AMED, under Grant Numbers JP17km0405206 to TT and 17ek0109207h0001 to WS; and JSPS under Grant Number 17H04056 to WS.
Author contributions
PCC analyzed the data and wrote the manuscript with inputs from all authors. PCC, WS, MM, and TT interpreted the data. WS, YAK, and KY conducted and supervised SNP genotyping. MM collected the samples. MM and TT conceived the original idea. WS and TT obtained funding. TT supervised the study.
Compliance with ethical standards
Conflict of interest
WS receives honoraria from Sumitomo Dainippon Pharma Co., Ltd, Otsuka Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Takeda Pharmaceutical Co., Ltd, and AbbVie GK. MM receives honoraria from Sumitomo Dainippon Pharma Co., Ltd, Otsuka Pharmaceutical Co., Ltd, Nihon Medi-Physics Co., Ltd, Nippon Boehringer Ingelheim Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Hisamitsu Pharmaceutical Co., Inc., FUJIFILM Pharma Co., Ltd, and AbbVie GK. TT receives honoraria from Sumitomo Dainippon Pharma Co., Ltd, Otsuka Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Takeda Pharmaceutical Co., Ltd, Eisai Co., Ltd, FP Pharmaceutical Co., Biogen Japan Ltd, Daiichi-Sankyo Co., Ltd, and AbbVie GK.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s10038-020-0760-8) contains supplementary material, which is available to authorized users.
References
- 1.Arias-Carrion O, Yamada E, Freundlieb N, Djufri M, Maurer L, Hermanns G, et al. Neurogenesis in substantia nigra of parkinsonian brains? J Neural Transm Suppl. 2009;73:279–85. doi: 10.1007/978-3-211-92660-4_23. [DOI] [PubMed] [Google Scholar]
- 2.Duda J, Potschke C, Liss B. Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson’s disease. J Neurochem. 2016;139(Suppl 1):156–78.. doi: 10.1111/jnc.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2008;4:743–57. doi: 10.2147/NDT.S2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rascol O, Perez-Lloret S, Ferreira JJ. New treatments for levodopa-induced motor complications. Mov Disord. 2015;30:1451–60. doi: 10.1002/mds.26362. [DOI] [PubMed] [Google Scholar]
- 5.Murata M, Horiuchi E, Kanazawa I. Zonisamide has beneficial effects on Parkinson’s disease patients. Neurosci Res. 2001;41:397–9. doi: 10.1016/S0168-0102(01)00298-X. [DOI] [PubMed] [Google Scholar]
- 6.Murata M, Hasegawa K, Kanazawa I. Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology. 2007;68:45–50. doi: 10.1212/01.wnl.0000250236.75053.16. [DOI] [PubMed] [Google Scholar]
- 7.Murata M, Hasegawa K, Kanazawa I, Fukasaka J, Kochi K, Shimazu R. Zonisamide improves wearing-off in Parkinson’s disease: a randomized, double-blind study. Mov Disord. 2015;30:1343–50. doi: 10.1002/mds.26286. [DOI] [PubMed] [Google Scholar]
- 8.Rosler TW, Arias-Carrion O, Hoglinger GU. Zonisamide: aspects in neuroprotection. Exp Neurol. 2010;224:336–9. doi: 10.1016/j.expneurol.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Wilfong AA, Willmore LJ. Zonisamide—a review of experience and use in partial seizures. Neuropsychiatr Dis Treat. 2006;2:269–80. doi: 10.2147/nedt.2006.2.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonsalla PK, Wong LY, Winnik B, Buckley B. The antiepileptic drug zonisamide inhibits MAO-B and attenuates MPTP toxicity in mice: clinical relevance. Exp Neurol. 2010;221:329–34. doi: 10.1016/j.expneurol.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamura S, Ohoyama K, Nagase H, Okada M. Zonisamide enhances delta receptor-associated neurotransmitter release in striato-pallidal pathway. Neuropharmacology. 2009;57:322–31. doi: 10.1016/j.neuropharm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Asanuma M, Miyazaki I, Diaz-Corrales FJ, Kimoto N, Kikkawa Y, Takeshima M, et al. Neuroprotective effects of zonisamide target astrocyte. Ann Neurol. 2010;67:239–49. doi: 10.1002/ana.21885. [DOI] [PubMed] [Google Scholar]
- 13.Sano H, Murata M, Nambu A. Zonisamide reduces nigrostriatal dopaminergic neurodegeneration in a mouse genetic model of Parkinson’s disease. J Neurochem. 2015;134:371–81.. doi: 10.1111/jnc.13116. [DOI] [PubMed] [Google Scholar]
- 14.Murata M. [The discovery of an antiparkinsonian drug, zonisamide] Rinsho Shinkeigaku. 2010;50:67–73. doi: 10.5692/clinicalneurol.50.67. [DOI] [PubMed] [Google Scholar]
- 15.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41:1303–7. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 16.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 17.International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–96. [DOI] [PubMed]
- 18.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 20.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–7. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchsberger C, Abecasis GR, Hinds DA. minimac2: faster genotype imputation. Bioinformatics. 2015;31:782–4. doi: 10.1093/bioinformatics/btu704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra A, Macgregor S. VEGAS2: software for more flexible gene-based testing. Twin Res Hum Genet. 2015;18:86–91. doi: 10.1017/thg.2014.79. [DOI] [PubMed] [Google Scholar]
- 24.Nam D, Kim J, Kim SY, Kim S. GSA-SNP: a general approach for gene set analysis of polymorphisms. Nucleic Acids Res. 2010;38:W749–54. [DOI] [PMC free article] [PubMed]
- 25.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata M, Hasegawa K, Kanazawa I, Shirakura K, Kochi K, Shimazu R. Randomized placebo-controlled trial of zonisamide in patients with Parkinson’s disease. Neurol Clin Neurosci. 2015;4:10–5. doi: 10.1111/ncn3.12026. [DOI] [Google Scholar]
- 27.Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. Embo J. 1996;15:5349–57. doi: 10.1002/j.1460-2075.1996.tb00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migliorini D, Denchi EL, Danovi D, Jochemsen A, Capillo M, Gobbi A, et al. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol Cell Biol. 2002;22:5527–38. doi: 10.1128/MCB.22.15.5527-5538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benosman S, Gross I, Clarke N, Jochemsen AG, Okamoto K, Loeffler JP, et al. Multiple neurotoxic stresses converge on MDMX proteolysis to cause neuronal apoptosis. Cell Death Differ. 2007;14:2047–57. doi: 10.1038/sj.cdd.4402216. [DOI] [PubMed] [Google Scholar]
- 30.Alves da Costa C, Checler F. Apoptosis in Parkinson’s disease: is p53 the missing link between genetic and sporadic Parkinsonism? Cell Signal. 2011;23:963–8. doi: 10.1016/j.cellsig.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Lu T, Kim P, Luo Y. Tp53 gene mediates distinct dopaminergic neuronal damage in different dopaminergic neurotoxicant models. Neural Regen Res. 2017;12:1413–7. doi: 10.4103/1673-5374.215243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi X, Davis B, Chiang YH, Filichia E, Barnett A, Greig NH, et al. Dopaminergic neuron-specific deletion of p53 gene is neuroprotective in an experimental Parkinson’s disease model. J Neurochem. 2016;138:746–57. doi: 10.1111/jnc.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, et al. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- 34.Melamed E, Ziv I, Djaldetti R. Management of motor complications in advanced Parkinson’s disease. Mov Disord. 2007;22(Suppl 17):S379–84. doi: 10.1002/mds.21680. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki S, Kawakami K, Nishimura S, Watanabe Y, Yagi K, Seino M, et al. Zonisamide blocks T-type calcium channel in cultured neurons of rat cerebral cortex. Epilepsy Res. 1992;12:21–7. doi: 10.1016/0920-1211(92)90087-A. [DOI] [PubMed] [Google Scholar]
- 36.Okada M, Kawata Y, Mizuno K, Wada K, Kondo T, Kaneko S. Interaction between Ca2+, K+, carbamazepine and zonisamide on hippocampal extracellular glutamate monitored with a microdialysis electrode. Br J Pharmacol. 1998;124:1277–85. doi: 10.1038/sj.bjp.0701941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawata Y, Okada M, Murakami T, Mizuno K, Wada K, Kondo T, et al. Effects of zonisamide on K+ and Ca2+ evoked release of monoamine as well as K+ evoked intracellular Ca2+ mobilization in rat hippocampus. Epilepsy Res. 1999;35:173–82. doi: 10.1016/S0920-1211(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 38.Huang CW, Huang CC, Wu SN. Activation by zonisamide, a newer antiepileptic drug, of large-conductance calcium-activated potassium channel in differentiated hippocampal neuron-derived H19-7 cells. J Pharm Exp Ther. 2007;321:98–106. doi: 10.1124/jpet.106.116954. [DOI] [PubMed] [Google Scholar]
- 39.Ueda Y, Doi T, Tokumaru J, Willmore LJ. Effect of zonisamide on molecular regulation of glutamate and GABA transporter proteins during epileptogenesis in rats with hippocampal seizures. Brain Res Mol Brain Res. 2003;116:1–6. doi: 10.1016/S0169-328X(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 40.Hong L, Sklar LA. Targeting GTPases in Parkinson’s disease: comparison to the historic path of kinase drug discovery and perspectives. Front Mol Neurosci. 2014;7:52. doi: 10.3389/fnmol.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreau C, Meguig S, Corvol JC, Labreuche J, Vasseur F, Duhamel A, et al. Polymorphism of the dopamine transporter type 1 gene modifies the treatment response in Parkinson’s disease. Brain. 2015;138:1271–83. doi: 10.1093/brain/awv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masellis M, Collinson S, Freeman N, Tampakeras M, Levy J, Tchelet A, et al. Dopamine D2 receptor gene variants and response to rasagiline in early Parkinson’s disease: a pharmacogenetic study. Brain. 2016;139:2050–62. doi: 10.1093/brain/aww109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.