Abstract
Background
Atypical haemolytic uraemic syndrome (aHUS) is a rare disorder characterised by thrombocytopenia, microangiopathic haemolytic anaemia, and acute kidney injury. The condition is primarily caused by inherited or acquired dysregulation of complement regulatory proteins with ~40% of those affected aged < 18 years. Historically, kidney failure and death were common outcomes, however, improved understanding of the condition has led to discovery of novel therapies.
Objectives
To evaluate the benefits and harms of interventions for aHUS.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies for randomised controlled studies (RCTs) up to 3 September 2020 using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov. MEDLINE(OVID) 1946 to 27 July 2020 and EMBASE (OVID) 1974 to 27 July 2020 were searched for non‐RCTs.
Selection criteria
All randomised and non‐randomised clinical trials comparing an intervention with placebo, an intervention with supportive therapy, or two or more interventions for aHUS were included. Given the rare nature of the condition in question, prospective single‐arm studies of any intervention for aHUS were also included.
Data collection and analysis
Two authors independently extracted pre‐specified data from eligible studies and evaluated risk of bias using a newly developed tool based on existing Cochrane criteria. As statistical meta‐analysis was not appropriate, qualitative analysis of data was then performed.
Main results
We included five single‐arm studies, all of which evaluated terminal complement inhibition for the treatment of aHUS. Four studies evaluated the short‐acting C5 inhibitor eculizumab and one study evaluated the longer‐acting C5 inhibitor ravulizumab. All included studies within the review were of non‐randomised, single‐arm design. Thus, risk of bias is high, and it is challenging to draw firm conclusions from this low‐quality evidence. One hundred patients were included within three primary studies evaluating eculizumab, with further data reported from 37 patients in a secondary study. Fifty‐eight patients were included in the ravulizumab study. After 26 weeks of eculizumab therapy there were no deaths and a 70% reduction in the number of patients requiring dialysis. Complete thrombotic microangiopathic (TMA) response was observed in 60% of patients at 26 weeks and 65% at two years. After 26 weeks of ravulizumab therapy four patients had died (7%) and complete TMA response was observed in 54% of patients. Substantial improvements were seen in estimated glomerular filtration rate and health‐related quality of life in both eculizumab and ravulizumab studies. Serious adverse events occurred in 42% of patients, and meningococcal infection occurred in two patients, both treated with eculizumab.
Authors' conclusions
When compared with historical data, terminal complement inhibition appears to offer favourable outcomes in patients with aHUS, based upon very low‐quality evidence drawn from five single‐arm studies. It is unlikely that an RCT will be conducted in aHUS and therefore careful consideration of future single‐arm data as well as longer term follow‐up data will be required to better understand treatment duration, adverse outcomes and risk of disease recurrence associated with terminal complement inhibition.
Plain language summary
What is the most effective treatment for atypical haemolytic uraemic syndrome?
What is the issue?
Haemolytic uraemic syndrome (HUS) is a condition involving blockages in small blood vessels leading to destruction of blood cells and dysfunction of several organs, most notably the kidneys. It is commonly caused by infections, often E.Coli, and can be associated with diarrhoea. A rare form of HUS known as atypical HUS (aHUS) is a more aggressive form of the disease caused by inherited or acquired abnormalities of proteins involved in controlling an aspect of our immune system known as “complement”. Almost half of cases involve patients aged less than 18 years. In the past a diagnosis of aHUS was associated with a poor prognosis with patients often progressing to kidney failure and death. More recently, an improved understanding of the condition has led to better treatments. This review aims to evaluate the usefulness of these treatments by systematically examining the available evidence in order to find out the most effective treatments available for aHUS.
What did we do?
We searched the literature extensively and found five studies which tested therapies for aHUS. In four studies the treatment used was eculizumab and in one study the treatment was ravulizumab. Both of these recently developed drugs act in a similar way and have shown promise in other medical conditions.
What did we find?
The included studies demonstrated that the majority of patients treated with either eculizumab or ravulizumab showed improvements in kidney function with a large proportion improving enough to stop dialysis treatment. Markers of disease activity in the blood also improved significantly. Over the course of 26 weeks of treatment, no patients given eculizumab died, although two patients did contract meningococcal infections, a likely consequence of the treatment. Although four patients treated with ravulizumab died, none of these deaths were thought to be caused by the drug. The quality of life of patients treated with both drugs was improved significantly.
Conclusions
aHUS is an extremely rare condition and without treatment is often fatal. For this reason, we found no studies which were able to compare one treatment with another, or one treatment with no treatment. Instead, the included studies gave all participants either eculizumab or ravulizumab, with results only comparable with historical data obtained before these drugs were available. This introduces substantial bias into the review, and therefore limits the confidence of any recommendations which stem from it. Nevertheless, the best available evidence suggests that treatment with either eculizumab or ravulizumab is effective in patients with aHUS and appears superior to previous therapies.
Summary of findings
Background
Description of the condition
Haemolytic uraemic syndrome (HUS) is a form of thrombotic microangiopathy (TMA) which affects adults and children and is characterised by thrombocytopenia, microangiopathic haemolytic anaemia and acute kidney injury (AKI) (Noris 2009). The term HUS encompasses several different disease processes which can be broadly divided into infection‐induced HUS, HUS secondary to a pre‐existing condition, or atypical HUS (aHUS) (Loirat 2016).
Around 90% of cases of HUS are considered infection‐induced and are most typically associated with either Shiga toxin‐producing Escherichia coli (STEC) or Streptococcus pneumoniae (Ariceta 2009). STEC‐associated cases are often (but not always) preceded by the onset of bloody diarrhoea (Besbas 2006). Pre‐existing conditions leading to the development of HUS include autoimmune diseases, stem cell or solid organ transplantation, and certain malignancies. This category of HUS can also be associated with certain drugs and with pregnancy (Fakhouri 2017).
The remainder of cases fall into the category of aHUS. Classically this term has been used to describe HUS associated with dysregulation of the alternative complement pathway due to either inherited or acquired dysfunction of complement regulatory proteins (Noris 2009). Further subdivision of this group is now possible, including recognition of those with anti‐factor H autoantibodies (a subgroup which accounts for ~10% of paediatric aHUS) (Fremeaux‐Bacchi 2013). More recently the definition of aHUS has been broadened to include other rare forms of HUS including diacylglycerol kinase E and Cobalamin C deficiency, as well as HUS with no clear precipitant (Loirat 2016).
The incidence of aHUS based on this broader classification is 0.23 to 0.42 cases/million population/year, with around 35% to 42% of cases occurring in children under the age of 18 (Fremeaux‐Bacchi 2013; Sheerin 2016).
Diagnosis is based on the presence of thrombocytopenia, microangiopathic haemolysis, and kidney injury and the exclusion of other forms of HUS or thrombotic thrombocytopenic purpura (TTP). A genetic or acquired abnormality of complement regulation can be identified in around 50% of cases, however therapeutic interventions are often required before this information is available (Sheerin 2016).
Historically up to 25% of people die during the acute illness (Kaplan 2014). Of those surviving, 50% require acute kidney replacement therapy (KRT) of whom a significant proportion never recover native kidney function and require long‐term KRT (Constantinescu 2004; Noris 2009).
Description of the intervention
Plasma exchange and plasma infusion have been the main treatments for aHUS since the early 1990s and work by replacing absent or removing abnormal complement proteins within the body. Although death rates decreased significantly following the introduction of plasma therapies a proportion of patients struggle to tolerate regular plasma therapy and relapse following discontinuation of treatment (Lara 1999; Noris 2005). Various other agents including corticosteroids, antiplatelet agents and thrombolytics have been studied with varying results. Liver transplantation has also been used as a treatment for aHUS in a small number of patients with known genetic complement factor deficiencies (Saland 2009). As certain complement factors such as factor H and factor I are produced in the liver successful transplantation, either alone or with combined kidney transplant, has the potential to cure aHUS in this subset of patients (Coppo 2016). However, this intervention is not without significant risk of morbidity and death and is not available in all centres (Remuzzi 2005).
Eculizumab, a humanised monoclonal antibody, targets complement component C5 in an attempt to halt the dysregulated activation of the complement pathway, reducing endothelial injury and subsequent organ dysfunction. Eculizumab is now recognised as the treatment of choice for paroxysmal nocturnal haemoglobinuria (PNH), a condition which also involves dysregulated terminal complement activation. Several studies have shown it to be effective for this indication with an acceptable safety profile (Hillmen 2013; Kanakura 2011). More recently, a longer‐acting C5 inhibitor, ravulizumab, has demonstrated non‐inferiority to eculizumab for the treatment of PNH with the additional benefit of reduced dosing frequency (Lee 2019; Kulasekararaj 2019). Both eculizumab and ravulizumab may therefore be superior to plasma therapy in the treatment of aHUS due to the ability to “switch off” abnormal complement activity.
Why it is important to do this review
A previous Cochrane Review has identified and evaluated interventions for HUS but this was not specific to aHUS (Michael 2009). Importantly, this review was conducted prior to the widespread use of eculizumab for the treatment of aHUS. We therefore considered it important to carry out this review in order to identify and evaluate emerging interventions for aHUS given the recent advances in our understanding of disease pathogenesis and directed treatment.
Objectives
This review aims to evaluate the benefits and harms of interventions for aHUS.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs), quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) and non‐randomised studies which compare an intervention with placebo, an intervention with supportive therapy, or two or more interventions for aHUS were included. Given the rare nature of the condition in question, prospective single‐arm studies of any intervention for aHUS were also included.
Types of participants
Inclusion criteria
Studies including patients of all ages with a confirmed diagnosis of aHUS. This diagnosis is defined as all three of:
Evidence of kidney impairment (raised serum creatinine (SCr) or equivalent biomarker which meets local criteria for AKI)
Evidence of thrombocytopenia (platelet count < 150 x 109/L)
Evidence of haemolysis (lactate dehydrogenase (LDH) above upper limit of normal, haptoglobin count below the lower limit of normal, evidence of fragmented red blood cells in a peripheral blood smear, or presence of schistocytes)
Exclusion criteria
The following patient groups were excluded.
Patients with evidence of STEC infection
Patients with evidence of Streptococcus pneumoniae infection
Patients with evidence of ADAMTS‐13 deficiency (level at or below 10% in plasma)
Patients with evidence of HUS as a consequence of a pre‐existing condition or disease including malignancy, haematopoietic stem cell transplantation, solid organ transplantation, infections, autoimmune conditions, malignant hypertension, and drug‐induced HUS
Patients with pregnancy‐associated HUS.
Types of interventions
Any intervention for aHUS was considered including, but not limited to, eculizumab, ravulizumab, plasma exchange, plasma infusion, anti‐platelet agents, thrombolytics, immunosuppressants, corticosteroids, and liver transplantation.
Types of outcome measures
Primary outcomes
Death (any cause)
Requirement for KRT
Successful remission as defined independently by each study. This may involve normalisation or stabilisation of platelet count, resolution of haemolysis, or resolution of AKI.
Secondary outcomes
Change in SCr or glomerular filtration rate (GFR)
Persistent requirement for plasma therapy
Degree of proteinuria: as evidenced by urinalysis, urine protein:creatinine ratio (UPCR), or 24‐hour urine protein measurement
Presence of hypertension: as evidenced by the need for, and number of, antihypertensive agents
Kidney biopsy changes: as there is no standardised system for reporting the varied biopsy changes associated with aHUS, we planned to report subjective scales as presented in each paper individually, e.g. mild/moderate/severe, an estimation of severity such as presence/percentage of crescents, an estimation of acute activity if provided‐ for example by endothelial cell swelling, lumen narrowing or thrombi formation in the interlobular arteries, arterioles and glomerular capillaries as well as information on chronicity by evaluating the extent of glomerulosclerosis, tubular atrophy and interstitial fibrosis present on the biopsy
Health‐related quality of life (HRQoL): as no disease specific tools exist, currently validated tools such as questionnaires like the 36 Item Short‐Form Survey, EuroQoL 5 Domain tool, COnsensus‐based Standards for the selection of health status Measurement INstruments (COSMIN) and the Evaluating Measures of Patient‐Reported Outcomes (EMPRO) are appropriate
Adverse events
Meningococcal infection.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 3 September 2020 through contact with the Information Specialist using search terms relevant to this review. The register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals, and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website under CKT Register of Studies.
MEDLINE(OVID) 1946 to 27 July 2020 and EMBASE (OVID) 1974 to 27 July 2020 were searched for non‐RCTs.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that included relevant data or information on studies were retained initially. Two authors then independently assessed retrieved abstracts and, if necessary, the full text of these studies, to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standardised data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses.
Assessment of risk of bias in included studies
Three independent risk of bias assessment tools were assigned to analyse risk of bias in RCTs, non‐randomised studies and single‐arm studies. For each included study, two authors independently assessed risk of bias using the assigned tool. It was our intention, where possible, to evaluate risk of bias in RCTs using the Cochrane risk of bias tool (Appendix 2), in non‐RCTs (where there was direct comparison between two or more treatment arms, or with a historical or external comparator) using the ROBINS‐I tool (Sterne 2016), and in single‐arm studies using a newly developed tool designed specifically for this purpose (Appendix 3).
The recently designed tool for assessing risk of bias in single‐arm studies considered the following items.
Selection bias
Lead time bias/immortal time bias
Confounding by indication
Misclassification bias/information bias
Bias from natural recovery/regression to the mean
Bias due to adjunctive therapies
Attrition bias
Selective reporting of outcomes.
Measures of treatment effect
For dichotomous outcomes results were to be expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment the mean difference (MD) was to be used, or the standardised mean difference (SMD) if different scales were used. Due to the nature of the included studies these analyses were not possible.
Unit of analysis issues
There were no unit of analysis issues.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author). Evaluation of important numerical data such number of patients screened, randomised as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
If suitable studies were identified, we planned to first assess heterogeneity by visual inspection of the forest plot and subsequently quantify statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). Due to the nature of the included studies no such assessment was possible. Due to the inclusion of non‐randomised and single‐arm studies, it was anticipated that a greater degree of heterogeneity would be apparent among included studies and this was taken into account when considering potential sources of bias.
Assessment of reporting biases
If possible, funnel plots were to be used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Unadjusted data from included RCTs were to be pooled using the random‐effects model or the fixed‐effect model however this was not possible. It was our intention to combine data from non‐randomised studies for meta‐analysis only when such data were considered to be significantly free from bias and heterogeneity as assessed independently by two authors, however again this was not possible. In such circumstances, consideration would be given to analyse adjusted, rather than unadjusted, effect estimates as an inverse‐variance weighted average. Where pooling of data was not appropriate then qualitative data synthesis only was performed.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was to be used to explore possible sources of heterogeneity (e.g. participants or interventions). Heterogeneity among participants could be related to their age, whether they were being treated for a first presentation or relapse of aHUS, specific complement mutations identified, whether they required acute KRT, or whether or not they had developed aHUS after a kidney transplant. Heterogeneity in interventions could be related to the dosage and duration of therapies used, as well as co‐interventions and previous treatments.
Sensitivity analysis
If suitable data were acquired, we planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
Summary of findings and assessment of the certainty of the evidence
We planned to present the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We planned to present the following outcomes in the 'Summary of findings' tables.
Death (any cause)
Requirement for KRT
Disease remission
Persistent elevation of SCr and/or GFR of < 60 mL/min/1.73 m²
Persistent requirement for plasma therapy
Adverse events due to treatment.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
We performed an initial search of Cochrane Kidney and Transplant's Specialised Register (including CENTRAL, MEDLINE and EMBASE) in November 2017. From this initial search we identified 81 records. Further searches were conducted in February 2019 (yielding a further six records) and September 2020 (yielding a further 20 records) providing a total of 107 records. Two records were identified through additional searching of record references. After screening titles and abstracts and full‐text review, five studies (36 reports) were included, 59 studies (67 reports) were excluded, and six ongoing studies were identified (EUCTR2017‐001082‐24; EudraCT2014‐001032‐11; NCT01757431; NCT03131219; NCT03205995; UMIN000014869) These six studies and will be assessed in a future update of this review (Figure 1).
1.
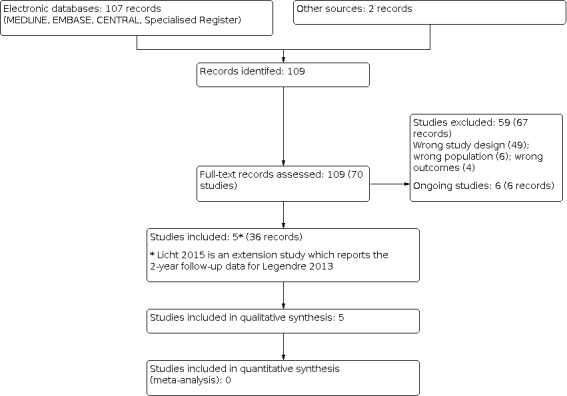
Study flow diagram.
Included studies
All five included studies were of non‐randomised, single‐arm design and evaluated terminal complement inhibition in patients with aHUS without a comparator group. Four studies evaluated eculizumab, a short‐acting C5 inhibitor (Fakhouri 2016; Greenbaum 2016; Legendre 2013; Licht 2015), and one study evaluated ravulizumab, a longer‐acting C5 inhibitor (Rondeau 2020). More information is presented in the Characteristics of included studies section. Licht 2015 reported two‐year extension data from Legendre 2013 and thus describes the same patient population. It has therefore been described as a secondary study, with the remainder of the included studies described as primary studies. All included studies were sponsored by Alexion Pharmaceuticals who are responsible for manufacturing both eculizumab and ravulizumab.
Given the rare nature of the condition in question all four primary studies recruited patients from multiple sites. Two studies (Fakhouri 2016; Legendre 2013) included patients from Europe and North America. Greenbaum 2016 included patients from Europe, North America, and Australia, and Rondeau 2020 included patients from Europe, North America, Australia, and Asia.
All included patients had a diagnosis of aHUS with similar diagnostic criteria used within each study (Characteristics of included studies). Legendre 2013 subdivided participants into two sub‐studies; those with progressive TMA with resistance to plasma therapy, and those with a chronic aHUS phenotype receiving maintenance plasma therapy. In all other primary studies there was no such subdivision. Of note, Rondeau 2020 was the only study to exclude patients requiring chronic haemodialysis at baseline.
Greenbaum 2016 focused on the paediatric aHUS population, including patients aged one month to 18 years. Legendre 2013 included adolescent and adult patients (≥12 years) in both sub‐studies, and Fakhouri 2016 and Rondeau 2020 included only adult patients (≥ 18 years). As Licht 2015 is an extension of Legendre 2013, this study also involved adolescent and adult patients.
There were 158 patients included in the four primary studies with further data reported from 37 patients in the secondary study. The age range was five months to 80 years with 28 of the 158 included primary patients under the age of 18 years (18%). Total duration of therapy was 26 weeks in all four primary studies. Two studies (Fakhouri 2016; Legendre 2013) used a dosing regimen of intravenous eculizumab 900 mg once/week for 4 weeks, 1,200 mg at week 5, and then 1,200 mg every 2 weeks. Paediatric dosing of eculizumab (Greenbaum 2016) was based upon weight with dosing sufficient to ensure that > 95% of patients had complete and sustained terminal complement inhibition and to provide peak plasma concentrations within a target range of 50 to 700 mg/mL. Ravulizumab dosing was based upon weight with an initial intravenous loading dose of 2400 to 3000 mg followed by maintenance doses of 3000 to 3600 mg on day 15 and every 8 weeks thereafter.
Plasma therapy and/or plasma exchange was used as additional therapy in the eculizumab studies where required (Fakhouri 2016; Greenbaum 2016; Legendre 2013; Licht 2015), however was not permitted in the ravulizumab study (Rondeau 2020).
Excluded studies
We excluded 59 studies (67 records). Reasons for exclusion included retrospective study design, incorrect patient population, duplicate data, and incorrect study outcomes.
We contacted two authors to enquire about unpublished data discussed in abstracts, and to seek clarification where data discussed in papers appeared to represent a subgroup of a larger published study. One author confirmed that the data represented analysis of patient cohorts published elsewhere and the study was grouped accordingly (Van De Kar 2014). The other author confirmed that the study did not meet our inclusion criteria (Khandelwal 2016).
Risk of bias in included studies
All included studies within the review were of non‐randomised, single‐arm design. Thus, the overall risk of bias is high, limiting the confidence in the results of this review. Despite this, some single‐arm studies are subject to higher degrees of bias than others, and certain forms of bias can largely be eliminated with effective single‐arm study design. The following assessment was compiled using the Cochrane risk of bias in single‐arm studies assessment tool (Appendix 3).
As all included studies were non‐randomised, single‐arm studies, there was no blinding of participants or investigators. It was therefore clear which patients were receiving either eculizumab or ravulizumab (all included patients) and this will have influenced results.
Eligibility criteria were clearly described and were similar in all studies thus reducing the possibility of selection bias. However, as all included studies were of single‐arm design it was not possible to compare eligibility criteria with studies with comparator groups. The process of patient recruitment was not clearly defined in any of the included studies. Given the rarity of the condition it is likely that all eligible participants encountered during the recruitment period were recruited, but this is not specifically reported.
In three of the four primary studies (Fakhouri 2016; Greenbaum 2016; Rondeau 2020) the time between recruitment and initiation of therapy was minimal. In Legendre 2013 participants in the first sub‐study were treated within 3 days, however those in the second sub‐study underwent an eight‐week observation period following recruitment and prior to initiation of treatment which may have increased the potential for lead time bias.
Eculizumab and ravulizumab dosing and outcome measures were clearly defined at the outset in all studies reducing the potential for misclassification or information bias.
Outcome variables were generally measured at outset and at end‐point in all studies (e.g. requirement for dialysis, degree of kidney impairment, requirement for plasma therapy). Previous studies of aHUS have demonstrated that prior to the advent of effective therapies the natural course of the condition if untreated is likely to be death. It is likely, therefore, that bias from natural recovery was negligible in all studies.
The requirement for plasma therapy (the main adjunctive therapy used) both before, during and after the treatment period was well described in all studies where it was permitted.
All four primary studies clearly documented reasons for participant withdrawal, which was uncommon. Three primary studies were analysed based on an intention to treat basis (Fakhouri 2016; Greenbaum 2016; Legendre 2013). Rondeau 2020 included all initially enrolled and treated participants in their safety analysis, however, excluded two participants from their full analysis on the basis of ineligibility.
There was no evidence of selective reporting in any of the included studies.
Effects of interventions
Summary of findings 1. Eculizumab versus placebo or alternative treatment for children and adults with atypical haemolytic uraemic syndrome (aHUS).
| Eculizumab versus placebo or alternative treatment for children and adults aHUS | ||||||
|
Patient or population: children and adults with aHUS Settings: inpatient Intervention: eculizumab Comparison: placebo or alternative treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with placebo or alternative treatment | Risk with eculizumab treatment | |||||
| Death | N/A | 1/100 | N/A | 100 (4 single‐arm studies) | ⊕⊝⊝⊝ very low | All 100 patients were alive at 26 weeks. There was 1 death in the 37 patients who were subsequently followed up over 2 years |
| Requirement for KRT | N/A | N/A | N/A | 100 (4 single‐arm studies) | ⊕⊝⊝⊝ very low | 37/100 were undergoing dialysis at initiation of eculizumab therapy. Of these patients, 11 (30%) continued to require regular dialysis after 26 weeks of treatment representing a 70% reduction in dialysis requirement. At 2 years, 3/37 patients included within the secondary study remained dialysis‐dependent compared with 7 at baseline (57% reduction) |
| Disease remission | N/A | 60/100 | N/A | 100 (4 single‐arm studies) | ⊕⊝⊝⊝ very low | 60/100 patients treated with eculizumab achieved complete TMA response after 26 weeks of treatment. Median time to complete TMA response was 56 ‐ 60 days. In a cohort of patients followed up for 2 years, 65% maintained complete TMA response at this time point |
| Change in eGFR | N/A | N/A | N/A | 88 (3 single‐arm studies) | ⊕⊝⊝⊝ very low | In patients treated with eculizumab, mean change in eGFR over 26 weeks was 33 ± 34 mL/min/1.73 m². At 2 years, eGFR had improved by ≥ 15 mL/min/1.73 m² in 37 patients (49%) |
| HRQoL | N/A | N/A | N/A | 100 (4 single‐arm studies) | ⊕⊝⊝⊝ very low | In 49 patients aged ≥ 12 years treated with eculizumab, 67% demonstrated a clinically significant improvement in EQ‐5D score. In 22 paediatric patients treated with eculizumab the mean improvement in FACIT‐F score was 19.7 (improvement of > 4.7 considered clinically meaningful) |
| Adverse events | N/A | 100/100 | N/A | 100 (4 single‐arm studies) | ⊕⊝⊝⊝ very low | Adverse events occurred in 100% of patients treated with eculizumab. Serious adverse events occurred in 37% of patients. The most commonly reported events included diarrhoea (23%), fever (21%), headache (19%), upper respiratory tract infection (19%), cough (17%) and urinary tract infection (10%) |
| Meningococcal infection | N/A | 2/100 | N/A | 100 (4 single‐arm studies) | ⊕⊝⊝⊝ very low | Meningococcal infection occurred in 2 patients (2%) treated with eculizumab |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; N/A: not applicable; KRT: Kidney replacement therapy; TMA: Thrombotic microangiopathy; eGFR: Estimated glomerular filtration rate; HRQoL: health‐related quality of life; FACIT‐F: Functional assessment of chronic illness therapy ‐ fatigue. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Ravulizumab versus placebo or alternative treatment for adults with atypical haemolytic uraemic syndrome (aHUS).
| Ravulizumab versus placebo or alternative treatment for adults with aHUS | ||||||
|
Patient or population: adults with aHUS Settings: inpatient Intervention: ravulizumab Comparison: placebo or alternative treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with placebo or alternative treatment | Risk with ravulizumab treatment | |||||
| Death | N/A | 4/58 | N/A | 58 (1 single‐arm study) | ⊕⊝⊝⊝ very low | Four deaths occurred, including one in a patient ultimately excluded based on eligibility criteria but who had received one dose of ravulizumab. No deaths were considered treatment‐related by the study investigators |
| Requirement for KRT | N/A | N/A | N/A | 56 (1 single‐arm study) | ⊕⊝⊝⊝ very low | 29/56 were undergoing dialysis at initiation of ravulizumab therapy. Of these patients, 12 (41%) continued to require regular dialysis after 26 weeks of treatment representing a 59% reduction in dialysis requirement |
| Disease remission | N/A | 30/56 | N/A | 56 (1 single‐arm study) | ⊕⊝⊝⊝ very low | 30/56 patients treated with ravulizumab achieved complete TMA response after 26 weeks of treatment. Median time to complete TMA response was 86 days |
| Change in eGFR | N/A | N/A | N/A | 56 (1 single‐arm study) | ⊕⊝⊝⊝ very low | In patients treated with ravulizumab, mean change in eGFR over 26 weeks was 35 ± 35 mL/min/1.73 m² |
| HRQoL | N/A | N/A | N/A | 44 (1 single‐arm study) | ⊕⊝⊝⊝ very low | A clinically meaningful improvement in FACIT‐F score (≥ 3‐point increase) was observed in 84% of patients treated with ravulizumab |
| Adverse events | N/A | 58/58 | N/A | 58 (1 single‐arm study) | ⊕⊝⊝⊝ very low | Adverse events occurred in 100% of patients treated with ravulizumab. Serious adverse events occurred in 52% of patients. The most commonly reported events included headache (36%), diarrhoea (31%), vomiting (26), hypertension (22%), nausea (22%) and urinary tract infection (17%) |
| Meningococcal infection | N/A | 0/58 | N/A | 58 (1 single‐arm study) | ⊕⊝⊝⊝ very low | No patients treated with ravulizumab developed meningococcal infection |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; N/A: not applicable; KRT: Kidney replacement therapy; TMA: Thrombotic microangiopathy; eGFR: Estimated glomerular filtration rate; HRQoL: Health‐related quality of life; FACIT‐F: Functional assessment of chronic illness therapy ‐ fatigue. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Either eculizumab or ravulizumab therapy was the sole intervention in all five included studies with no comparator groups. Meta‐analysis was therefore not feasible, and a descriptive analysis was performed.
Death (any cause)
Eculizumab
All 100 patients included within the three primary studies were alive at 26 weeks (completion of initial eculizumab treatment period). Of the 37 patients included in Licht 2015, one patient (3%) had died at two years. This was as a result of complications of intestinal haemorrhage which were thought to be unrelated to eculizumab therapy.
Ravulizumab
Of the 58 patients included within the safety analysis, four patients (7%) had died by 26 weeks. None of these deaths were thought to be related to the study drug and were caused by septic shock (2), intracerebral haemorrhage, and cerebral artery thrombosis.
Requirement for kidney replacement therapy
Eculizumab
There were 37/100 patients included in the primary studies who were undergoing dialysis at initiation of eculizumab therapy. Of these patients, 26 discontinued regular dialysis after 26 weeks of treatment representing a 70% reduction in dialysis requirement. At two years, 3/37 patients included within the secondary study remained dialysis‐dependent compared with 7/37 at baseline (57% reduction) (Licht 2015).
Ravulizumab
Unlike the eculizumab studies, patients undergoing chronic haemodialysis for established end‐stage kidney disease (ESKD) at baseline were excluded from Rondeau 2020, although patients undergoing haemodialysis due to AKI as a result of aHUS were included. Dialysis was discontinued in 17/29 patients who required dialysis at baseline (59%).
Disease remission
Eculizumab
Disease remission, or complete TMA response, was defined similarly by each of the three included primary eculizumab studies. In all three studies the definition included haematological normalisation (platelet count maintained ≥ 150 x 109/L and LDH maintained below the upper limit of normal on two separate measurement ≥ four weeks apart) and either improvement (≥ 25% reduction in SCr from baseline) (Greenbaum 2016; Legendre 2013) or preservation (< 25% increase in SCr from baseline) (Fakhouri 2016) of kidney function. Sixty percent of patients achieved complete TMA response after 26 weeks of eculizumab treatment. Median time to complete TMA response was 56 days in one study involving adult patients (Fakhouri 2016) and 60 days in a study involving patients < 18 years (Greenbaum 2016). Follow‐up data from Licht 2015 showed that 65% of patients (37) had achieved complete TMA response at two years.
Ravulizumab
Complete TMA response was defined by Rondeau 2020 as platelet count normalisation (≥ 150 x 109/L), LDH normalisation (< 246 U/L), and ≥ 25% improvement in SCr from baseline at two separate assessments obtained at least 28 days apart. Fifty‐four percent of patients achieved complete TMA response during the 26‐week treatment period. The median time to complete TMA response was 86 days.
Kidney function
Eculizumab
Mean increase in eGFR over 26 weeks was 33 ± 34 mL/min/1.73 m²for patients in the three primary studies (88 patients). Baseline eGFR was not reported. eGFR improved by ≥15 mL/min/1.73 m² in 50% of patients at 26 weeks. At two years, eGFR had improved by ≥15 mL/min/1.73 m² in 37 patients (49%) (Licht 2015).
Ravulizumab
Mean increase in eGFR over 26 weeks in those treated with ravulizumab was 35 ± 35 mL/min/1.73 m² (56 patients). eGFR improved by at least one eGFR category in 47 patients with available data (68%).
Requirement for plasma therapy
Eculizumab
Prior to the widespread use of eculizumab, treatment of aHUS largely depended upon regular plasma therapy (including plasma exchange). At initiation of eculizumab 81 patients (81%) were receiving plasma therapy. After 26 weeks of treatment, two patients (2%) remained reliant upon plasma therapy, representing a 98% reduction in requirement for this time‐ and labour‐intensive treatment.
Ravulizumab
Plasma therapy was not permitted as an adjunctive treatment in the ravulizumab study.
Proteinuria
Eculizumab
Change in degree of proteinuria was only assessed by one primary study (Legendre 2013) and by the secondary study (Licht 2015). At baseline, 26 patients (70%) had ≥ 1+ proteinuria on dipstick urinalysis. Of this group, 18 patients (69%) had a reduction of ≥ 1 proteinuria ‘grade’ (as assessed by dipstick urinalysis) after 26 weeks. This figure increased to 85% at two years (Licht 2015). Baseline UPCR was 2.46 ±1.74 g/mmol with a mean reduction in UPCR of 0.74 ±0.75 g/mmol after 26 weeks (37 patients).
Ravulizumab
Proteinuria was not assessed by Rondeau 2020.
Blood pressure
This outcome was not reported by any of the included studies.
Renal biopsy change
This outcome was not reported by any of the included studies.
Health‐related quality of life
Eculizumab
HRQoL was measured in all studies using a variety of tools including EQ‐5D, 36‐Item Short From Health Survey (SF‐36), and Functional Assessment of Chronic Illness – Fatigue (FACIT‐F) (adult and paediatric versions). Of the adult and adolescent population (age ≥ 12 years), 67% demonstrated a clinically meaningful improvement (> 0.06) in EQ‐5D score (49 patients). Greenbaum 2016 used a paediatric FACIT‐F tool to demonstrate a mean improvement in score of 19.7, with an improvement of > 4.7 considered clinically meaningful (22 patients) (Lai 2007).
Ravulizumab
Rondeau 2020 assessed HRQoL using the adult FACIT‐F score at baseline and at 26 weeks. A clinically meaningful improvement (≥ 3‐point increase) was observed in 44 patients with available data (84%).
Adverse events
Eculizumab
Serious adverse events occurred in 37% of patients included within the primary eculizumab studies. Adverse events occurred in 100% of patients. The most commonly reported events included diarrhoea (23%), fever (21%), headache (19%), upper respiratory tract infection (19%), cough (17%), and urinary tract infection (10%). Reporting of adverse events was incomplete in two of three primary studies (Fakhouri 2016; Greenbaum 2016). Fakhouri 2016 reported all serious adverse events and adverse events occurring in > 15% of patients. Greenbaum 2016 reported all adverse events but only serious adverse events occurring in ≥ 2 patients. All serious adverse events and adverse events were clearly reported by Legendre 2013.
Ravulizumab
Although only 56 patients with confirmed eligibility were included within the complete analysis for this study, all patients (58) treated with at least one dose of ravulizumab were included within the safety analysis. At least one adverse event occurred in all patients, and serious adverse events occurred in 52%. The most frequent adverse events observed were headache (36%), diarrhoea (31%), vomiting (26), hypertension (22%), nausea (22%), and urinary tract infection (17%). The most common serious adverse events (occurring in ≥ 3% of patients) included malignant hypertension (3%) and infections including pneumonia (5%), and septic shock (3%).
Meningococcal infection
Eculizumab
Meningococcal infection occurred in two patients (2%) in the primary studies. One patient developed meningococcal meningitis which led to permanent discontinuation of eculizumab therapy. A second patient developed meningococcal sepsis and was hospitalised but was able to continue eculizumab. In both cases the patients recovered. Both patients had received meningococcal vaccination against serogroups A, C, W, and Y but had not been prescribed long‐term antibiotics.
Ravulizumab
No episodes of meningococcal infection were reported within the ravulizumab study.
Discussion
Summary of main results
This review evaluates the best available evidence for current interventions for aHUS. In four single‐arm studies, terminal complement inhibition (either eculizumab or ravulizumab) was used to treat 158 patients over a period of 26 weeks with no comparator groups. A fifth study described two‐year follow‐up data from a subset patients treated with eculizumab.
Death at 26 weeks was zero in each of the eculizumab studies, and of the selected 37 patients which formed the two‐year follow‐up only one died. In the ravulizumab study death was 7% at 26 weeks. Plasma exchange/infusion has been the historical standard of care for aHUS prior to the introduction of C5 inhibitors. Observational data of patients with aHUS treated with plasma exchange/infusion describe death rates of up to 8% at first presentation and 11% at three years in one series (Noris 2010), and 4% after median follow‐up of 45 months in another (Fremeaux‐Bacchi 2013). While longer term data are lacking, reported death rates for eculizumab in particular are notably improved.
Substantial improvements were seen in kidney function, haematological parameters, and quality of life after 26 weeks of therapy in all studies. All studies showed an overall reduction in the number of patients with a requirement for KRT following treatment, ranging from a reduction of 57% (Legendre 2013) to 82% (Greenbaum 2016). At 26 weeks, 35% of patients who were KRT‐dependent at baseline and treated with either eculizumab or ravulizumab remained KRT‐dependent. Historically, patients treated with plasma therapies have demonstrated rates of KRT dependency of up to 67% (Noris 2010). Haematological remission was achieved in 58% of patients at 26 weeks. Longer term data are limited, but effects with eculizumab appear to be maintained at two years with 65% of patients in the secondary study achieving complete TMA response by this time point. Again, this compares favourably with observational data of patients treated with plasma therapies with one series reporting partial or complete response in ~50% of those treated with plasma exchange or infusion, but with fewer patients maintaining this response in the long term (Noris 2010).
Adverse events were common in all included studies. Licht 2015 identified four patients (11% of that study group) undergoing extended therapy who suffered serious adverse events (accelerated hypertension, asymptomatic bacteriuria, and hypertension). Less serious adverse events were common, and most often occurred during the first six months of treatment. Legendre 2013 reported that, while there were no “infection related” events, all patients had at least one serious adverse event in their first sub‐trial, while 50% had serious adverse events in their second sub‐trial. While the authors do not ascribe all of these events directly to eculizumab, they included hypertension, peritonitis, influenza, and a venous disorder not otherwise specified. All events resolved within 26 weeks of treatment and did not necessitate interruption to the treatment regime. Greenbaum 2016 reported similarly high levels of treatment‐emergent adverse events in 20/22 patients, the most common being fever, cough, abdominal pain, diarrhoea, and upper respiratory tract infection. The most serious events occurred in an infant and child less than 12 years of age – viral induced bone marrow suppression (parainfluenza type 3), wrist fracture and acute respiratory failure. Fifty nine percent of patients reported one or more serious adverse event, one of which led to treatment discontinuation. All patients included within the safety analysis of Rondeau 2020 suffered at least one adverse event and > 50% suffered a serious adverse event. Three patients discontinued ravulizumab because of a serious adverse event. Despite this, the authors report that none of the serious adverse events observed were considered 'unexpected'. All patients were vaccinated against meningococcal infection. Despite this, two patients treated with eculizumab developed meningococcal infections, both of whom recovered.
The included studies offer little evidence to guide discontinuation of C5 inhibitor therapy. Eculizumab and ravulizumab are both expensive, and thus guidance on when, if at all, these drugs can be safely withdrawn in patients with aHUS without risking relapse is an important consideration.
Thirty‐four patients included within the four primary studies had previously undergone kidney transplantation with recurrence of aHUS post‐transplant and outcomes following treatment with either eculizumab or ravulizumab in this setting suggest efficacy. Several retrospective case‐series have suggested positive outcomes with eculizumab following kidney transplantation in patients with aHUS as a measure to maintain remission, rather than treat recurrent disease (Alpay 2019; Bhalla 2019), but this was not assessed in this review. Similarly, our search strategy did not identify any studies which prospectively evaluated liver transplantation (either combined or in associated with kidney transplantation) as a treatment for aHUS. Although this treatment is likely to decline further following the widespread adoption of C5 inhibitor therapy as the mainstay of aHUS treatment, case report data suggests there may still be a role for liver transplantation in select cases where treatment with C5 inhibitors has failed (Coppo 2016).
Overall completeness and applicability of evidence
All studies were well conducted and data were largely complete. However, follow‐up is limited and kidney biopsy data, which would have been a useful supplementary measure of kidney outcomes, is absent. We assessed the risk of bias as being high due to the single‐arm nature of the studies and as a result the certainty of the evidence is low. This is primarily due to study limitation biases detected with the risk of bias in single‐arm studies assessment tool (Appendix 3).
Quality of the evidence
We conducted this review according to the processes described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), however, deviated when assessing risk of bias in single‐arm studies, instead using a new tool developed by Cochrane for this purpose.
This review is limited by the sole inclusion of single‐arm studies which presents a number of important confounders. The lack of an internal control leaves all studies open to bias and misinterpretation making any inference about intervention effect much less powerful, with conclusions drawn based upon comparisons with historical registry data of aHUS outcomes.
Potential biases in the review process
While this review was conducted according to methods developed by the Cochrane Collaboration, some bias may be present in the review process. We searched for all relevant studies using sensitive and validated strategies in major medical databases and grey literature sources. However, it is possible that some studies (such as unpublished data and studies with negative or no effects) were not identified. There is extensive multicentre collaboration in the field and a number of patient cohorts reappear in multiple abstracts and papers. Every effort was taken to ensure no patients have been included more than once or inadvertently excluded.
Agreements and disagreements with other studies or reviews
No previous Cochrane reviews have specifically evaluated interventions for aHUS. Michael 2009 previously published Interventions for haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura, however this was prior to the advent of C5 inhibitor therapy for aHUS and did not include non‐randomised or single‐arm studies. It therefore did not include any studies examining interventions for aHUS.
To our knowledge, this is the only systematic review of interventions for aHUS to have been published since the majority of the landmark C5 inhibitor studies were published. A previous review by Rathbone 2013 was conducted in 2013 and includes results from Legendre 2013 as well as several retrospective studies. Our review is largely in agreement with this review.
Authors' conclusions
Implications for practice.
In patients with aHUS, terminal complement inhibition with either eculizumab or ravulizumab appears to offer favourable outcomes in terms of death, disease remission, requirement for KRT and quality of life when compared with historical data obtained prior to the use of these treatments. This is based upon data from five single‐arm, non‐comparative studies which represents very low‐quality evidence. The lack of any RCT impacts significantly upon the quality of evidence generated and limits the strength of any conclusions drawn from this review. Adverse events are common with both eculizumab and ravulizumab, but rarely necessitate drug discontinuation. It seems unlikely that an RCT will be conducted given the apparent improvement in outcomes associated with C5 inhibitor use, and so careful consideration of future single‐arm data and longer term follow‐up data will be required to better understand treatment duration, adverse outcomes, and risk of disease recurrence.
All of the included studies were conducted in highly specialist centres with strict inclusion criteria which should be taken into account when considering the logistics of treatment and patient selection and extrapolating results to other patient populations.
Implications for research.
Despite the very low‐quality evidence presented here, an RCT investigating the efficacy of terminal complement inhibitor therapy for aHUS is unlikely to be feasible or warranted. Any future single‐arm studies of interventions for aHUS should consider the potential sources of bias outlined in this review in order to produce evidence of as high a quality as possible (specifically, dealing with selection bias, lead time bias, selective reporting of outcomes and attrition bias). It would be important for future studies to have a thoughtfully designed comparison group. It might be useful to compare the estimated risk/effect size with a historical or external comparator. Alternatively, a comparative study could be designed (i.e. measuring symptoms in a single group of individuals before and after intervention).
The authors are aware of several studies of other novel therapies for aHUS currently under investigation, the results of which should be compared to both historical data and eculizumab/ravulizumab where possible (EUCTR2017‐001082‐24; EudraCT2014‐001032‐11; NCT01757431; NCT03131219; NCT03205995; UMIN000014869).
History
Protocol first published: Issue 11, 2017 Review first published: Issue 3, 2021
Acknowledgements
The authors wish to acknowledge the help of all at Cochrane Kidney and Transplant who assisted with all aspects of this review.
The authors are grateful to the following peer reviewers for their time and comments: Professor Lesley Rees MD FRCPCH (Consultant Paediatric Nephrologist AT Gt Ormond St Hospital; Professor of Paediatric Nephrology at Institute of Child Health, UCL); Mini Michael, MD (Associate Professor, Department of Pediatrics‐Renal Section, Baylor College of Medicine, Houston, Texas, USA).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 3. Risk of bias in single arm studies assessment tool
| Domain | Signalling Questions | Yes | No | Can't tell / not reported / not applicable | Quote or reason for judgement |
| Selection bias | 1. Was the selection of participants either consecutive, or randomly selected from the population? | ||||
| 2a. Were the eligibility criteria clearly described? | |||||
| 2b. If yes, were the eligibility criteria similar to the other studies in the review that had a control group? | |||||
| Lead time bias / immortal time bias | 3. Was the time between starting follow‐up for outcomes (recruitment) and starting the intervention of an appropriately short duration? | ||||
| 4. Was it similar to other studies in the review that had a control group? | |||||
| Confounding by indication | 5. Are those in the study at a similar stage/severity of their disease and have similar prognostic factors to other studies in the review that had a control group? | ||||
| Misclassification bias / information bias | 6. Was dose (or other details) of intervention, both planned and given, clearly described? | ||||
| 7. Was measurement of outcome made by a reliable and valid method (e.g. objective measure)? | |||||
| Bias from natural recovery / regression to the mean | 8. Were outcome variables measured pre‐intervention (i.e. interrupted time series design with multiple measurements, of before‐after design)? | ||||
| Bias due to adjunctive therapies | 9. Is there adequate reporting of adjunctive therapies both before and during the study period? | ||||
| Attrition bias | See Cochrane RoB tool | ||||
| Selective reporting of outcomes | See Cochrane RoB tool | ||||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fakhouri 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | All subjects treated with eculizumab. Given the rarity of the condition it is likely that all eligible participants encountered during the recruitment period were recruited, but this is not specifically reported. There is no reporting of how participants were selected for the study. Eligibility criteria clearly described. |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Little or no missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes published |
| Other bias | Low risk | See below for single‐arm study bias assessment |
| Lead time bias / immortal time bias | Low risk | The time between recruitment and initiation of therapy was minimal (maximum seven days) |
| Confounding by indication | Low risk | Given the rarity of the condition participants at all stages of the disease were included with similar prognostic factors as compared with other studies |
| Misclassification bias / information bias | Low risk | Clear dosing regimen and outcome reporting |
| Bias from natural recovery / regression to the mean | Low risk | Given the severe nature of the condition and historical data it is unlikely that there was no significant bias from natural recovery observed |
| Bias due to adjunctive therapies | Low risk | The need for plasma therapy (the main adjunctive therapy) was well described |
Greenbaum 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | All subjects treated with eculizumab. Given the rarity of the condition it is likely that all eligible participants encountered during the recruitment period were recruited, but this is not specifically reported. There is no reporting of how participants were selected for the study. Eligibility criteria clearly described. |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very little missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes published |
| Other bias | Low risk | See below for single‐arm study bias assessment |
| Lead time bias / immortal time bias | Low risk | The time between recruitment and initiation of therapy was minimal (maximum seven days) |
| Confounding by indication | Low risk | Given the rarity of the condition participants at all stages of the disease were included with similar prognostic factors as compared with other studies |
| Misclassification bias / information bias | Low risk | Clear dosing regimen and outcome reporting |
| Bias from natural recovery / regression to the mean | Low risk | Given the severe nature of the condition and historical data it is unlikely that there was no significant bias from natural recovery observed |
| Bias due to adjunctive therapies | Low risk | The need for plasma therapy (the main adjunctive therapy) was well described |
Legendre 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | All subjects treated with eculizumab. Given the rarity of the condition it is likely that all eligible participants encountered during the recruitment period were recruited, but this is not specifically reported. There is no reporting of how participants were selected for the study. Eligibility criteria clearly described. |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very little missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes published |
| Other bias | Low risk | See below for single‐arm study bias assessment |
| Lead time bias / immortal time bias | Unclear risk | Participants in the first sub‐study were treated within 3 days, however those in the second sub‐study underwent an eight‐week observation period following recruitment and prior to initiation of treatment which may have increased the potential for lead time bias |
| Confounding by indication | Low risk | Given the rarity of the condition participants at all stages of the disease were included with similar prognostic factors as compared with other studies |
| Misclassification bias / information bias | Low risk | Clear dosing regimen and outcome reporting |
| Bias from natural recovery / regression to the mean | Low risk | Given the severe nature of the condition and historical data it is unlikely that there was no significant bias from natural recovery observed |
| Bias due to adjunctive therapies | Low risk | The need for plasma therapy (the main adjunctive therapy) was well described |
Licht 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention group
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | All subjects treated with eculizumab. Given the rarity of the condition it is likely that all eligible participants encountered during the recruitment period were recruited, but this is not specifically reported. There is no reporting of how participants were selected for the study. Eligibility criteria clearly described. |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very little missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes published |
| Other bias | Low risk | See below for single‐arm study bias assessment |
| Lead time bias / immortal time bias | Unclear risk | Participants in the first sub‐study were treated within 3 days, however those in the second sub‐study underwent an eight‐week observation period following recruitment and prior to initiation of treatment which may have increased the potential for lead time bias. |
| Confounding by indication | Low risk | Given the rarity of the condition participants at all stages of the disease were included with similar prognostic factors as compared with other studies |
| Misclassification bias / information bias | Low risk | Clear dosing regimen and outcome reporting |
| Bias from natural recovery / regression to the mean | Low risk | Given the severe nature of the condition and historical data it is unlikely that there was no significant bias from natural recovery observed |
| Bias due to adjunctive therapies | Unclear risk | The need for plasma therapy (the main adjunctive therapy) was well described |
Rondeau 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention group
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | All subjects treated with ravulizumab. Given the rarity of the condition it is likely that all eligible participants encountered during the recruitment period were recruited, but this is not specifically reported. There is no reporting of how participants were selected for the study. Eligibility criteria clearly described. |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very little missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes published |
| Other bias | Low risk | See below for single‐arm study bias assessment |
| Lead time bias / immortal time bias | Low risk | The time between first disease symptom and initiation of therapy was minimal (median 0.28 months) |
| Confounding by indication | Low risk | Given the rarity of the condition participants at all stages of the disease were included with similar prognostic factors as compared with other studies |
| Misclassification bias / information bias | Low risk | Clear dosing regimen and outcome reporting |
| Bias from natural recovery / regression to the mean | Low risk | Given the severe nature of the condition and historical data it is unlikely that there was no significant bias from natural recovery observed |
| Bias due to adjunctive therapies | Low risk | Plasma therapy (the main adjunctive treatment used in the other included studies) was not permitted in this study |
CKD ‐ chronic kidney disease; CNI ‐ calcineurin inhibitor; ESKD ‐ end‐stage kidney disease; (e)GFR ‐ (estimated) glomerular filtration rate; (a)HUS ‐ (atypical) haemolytic uraemic syndrome; Hb ‐ haemoglobin; HRQoL ‐ health‐related QoL; IV ‐ intravenous/ly; LDH ‐ lactate dehydrogenase; mTORi ‐ mammalian target of rapamycin inhibitor; QoL ‐ quality of life; PCR ‐ protein:creatinine ratio; PE/PI ‐ plasma exchange/plasma infusion; RBC ‐ red blood cell; STEC ‐ Shiga toxin–producing E. coli infection; TMA ‐ thrombotic microangiopathy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aigner 2018 | Wrong study design: observational |
| Aigner 2020 | Wrong study design: retrospective analysis |
| Al‐Akash 2012 | Wrong study design: subgroup analysis |
| Alpay 2019 | Wrong study design: retrospective analysis |
| Alsakran 2019 | Wrong study design: retrospective analysis |
| Bhalla 2019 | Wrong study design: retrospective analysis |
| Bohl 2016 | Wrong study population: haematopoietic stem cell transplantation‐associated TMA |
| Cao 2018 | Wrong study design: retrospective analysis |
| Cataland 2014 | Wrong study design: subgroup analysis |
| Cofiell 2015 | Wrong study outcomes: study designed to monitor the effect of eculizumab on multiple physiologic pathways affected by complement dysregulation in aHUS but does not measure the clinical outcomes included within this review |
| Escribano 2017 | Wrong study design: retrospective analysis |
| Favi 2018 | Wrong study population: transplant recipients |
| Fremeaux‐Bacchi 2015 | Wrong study design: review |
| Frykman 2016 | Wrong study design: observational |
| Galbusera 2019 | Wrong study design and outcomes: case‐series evaluating the efficacy of an ex‐vivo test for disease activity in aHUS during treatment |
| Gavriilaki 2015 | Wrong study design and outcomes: pre‐clinical study designed to develop a novel cell assay to distinguish aHUS from other TMA |
| Gomez‐Alvarez 2016 | Wrong study design: retrospective analysis |
| Gruppo 2011 | Wrong study design: retrospective analysis |
| Han 2019 | Wrong study design: case report |
| Hanes 2019 | Wrong patient population: healthy volunteers |
| Ito 2019 | Wrong study design: observational |
| Jozsi 2007 | Wrong study design and outcomes: pre‐clinical study examining the role of factor‐H autoantibodies in aHUS |
| Kant 2020 | Wrong study design: retrospective analysis |
| Kato 2019 | Wrong study design: observational |
| Khandelwal 2016 | Wrong study design: retrospective analysis |
| Khandelwal 2019 | Wrong study design: retrospective analysis |
| Khursigara 2014 | Wrong study design: retrospective analysis |
| Kumar 2019 | Wrong study design: retrospective analysis |
| Lappegard 2015 | Wrong study design: review |
| Legault 2009 | Wrong study design: case study |
| Levi 2016 | Wrong patient population: transplant recipients |
| Mazo 2017 | Wrong study design: retrospective analysis |
| Menne 2019 | Wrong study design and patient population: observational study reporting long‐term outcomes for patients previously treated with eculizumab in prospective studies. Participants not required to fulfil TMA criteria at enrolment in this trial |
| Monet‐Didailler 2020 | Wrong patient population: Shiga toxin‐producing E. coli HUS |
| Nayer 2016 | Wrong study design: review |
| Nester 2013 | Wrong study design: review |
| Nilsson 2007 | Wrong study design and outcomes: observational study designed to understand the role of factor I in complement regulation in patients with aHUS |
| Noris 2014 | Wrong study outcomes: designed to test new assays of complement activation and identify a tool for eculizumab titration |
| Pape 2016 | Wrong study design and patient population: observational study reporting long‐term outcomes for patients previously treated with eculizumab in prospective study. Participants not required to fulfil TMA criteria at enrolment in this trial |
| Pape 2017 | Wrong study design and patient population: observational study reporting long‐term outcomes for patients previously treated with eculizumab in prospective study. Participants not required to fulfil TMA criteria at enrolment in this trial |
| Picard 2015 | Wrong study design: review |
| Raina 2018 | Wrong study design: retrospective analysis |
| Siedlecki 2019 | Wrong study design: retrospective analysis |
| Simonetti 2011 | Wrong study design: retrospective analysis |
| Socie 2019 | Wrong study design: retrospective analysis |
| Taylor 2010 | Wrong study design: guideline |
| Ubago 2014 | Wrong study design: review |
| Uriol 2018 | Wrong study design: retrospective analysis |
| Vakiti 2019 | Wrong study design and patient population: case series; drug induced aHUS |
| Vilalta 2012 | Wrong study design: retrospective analysis |
| Vivarelli 2014 | Wrong study design: review |
| Volokhina 2016 | Wrong study outcomes: pharmacodynamics |
| Volokhina 2016a | Wrong study outcomes: pharmacodynamics |
| Walle 2017 | Wrong study design: observational |
| Walle 2017a | Wrong study design: retrospective analysis of pooled data |
| Weston‐Davies 2013 | Wrong patient population: healthy volunteers |
| Wijnsma 2016 | Wrong study design: case series |
| Wong 2015 | Wrong study design: review |
| Wuhl 2020 | Wrong study design: retrospective analysis |
aHUS ‐ atypical haemolytic uraemic syndrome; TMA ‐ thrombotic microangiopathy
Characteristics of ongoing studies [ordered by study ID]
EUCTR2017‐001082‐24.
| Study name | A phase 2, open label, multicenter study of ALN‐CC5 administered subcutaneously in adult patients with atypical hemolytic uremic syndrome |
| Methods | Open‐label multicentre study of investigational medicinal product |
| Participants | Aged >18 years with diagnosis of aHUS |
| Interventions | Cemdisiran |
| Outcomes | To evaluate the effect of ALN‐CC5 on platelet response in adult patients with aHUS |
| Starting date | 20/06/2017 |
| Contact information | clinicaltrials@alnylam.com |
| Notes | Trial terminated prematurely |
EudraCT2014‐001032‐11.
| Study name | A Phase 2, uncontrolled, three‐stage, dose‐escalation cohort study to evaluate the safety, pharmacokinetics, pharmacodynamics, immunogenicity, and clinical activity of OMS721 in adults with thrombotic microangiopathies |
| Methods | Open‐label ascending‐dose‐escalation study |
| Participants | Age >18 years and diagnosis of aHUS, HSCT‐associated TMA, or TTP |
| Interventions | OMS721 |
| Outcomes | Primary end points:
|
| Starting date | 02/06/2014 |
| Contact information | Omeros Corporation, Pawel.Szczesniak@psi‐cro.com |
| Notes |
NCT01757431.
| Study name | The safety and efficacy of eculizumab in Japanese patients with atypical hemolytic uremic syndrome (aHUS) |
| Methods | Open‐label single‐arm study |
| Participants | Age > 1 month with confirmed diagnosis of aHUS |
| Interventions | Eculizumab |
| Outcomes | Adverse events and serious adverse events and their severity and relationship to the drug |
| Starting date | 31/12/2012 |
| Contact information | Alexion Pharmaceuticals |
| Notes |
NCT03131219.
| Study name | A phase 3, open‐label, multicenter study of ALXN1210 in children and adolescents with atypical hemolytic uremic syndrome (aHUS) |
| Methods | Open‐label, single‐arm |
| Participants | Age < 18 years with diagnosis of aHUS |
| Interventions | ALXN1210 |
| Outcomes | Complete TMA response |
| Starting date | 27/04/2017 |
| Contact information | Alexion Pharmaceuticals |
| Notes |
NCT03205995.
| Study name | A phase 3 study to evaluate the safety and efficacy of OMS721 for the treatment of atypical hemolytic uremic syndrome (aHUS) in adults and adolescents |
| Methods | Open‐label, single‐arm |
| Participants | Age > 12 years with diagnosis of aHUS |
| Interventions | OMS721 |
| Outcomes | The effect of OMS721 as measured by platelet count change from baseline |
| Starting date | 02/07/2017 |
| Contact information | Omeros Corporation |
| Notes |
UMIN000014869.
| Study name | Observational study of atypical hemolytic uremic syndrome in Japan |
| Methods | Observation study |
| Participants | All patients with diagnosis of aHUS |
| Interventions | Nil |
| Outcomes | To recognize the morbidity, genetic abnormalities, prognosis, and outcomes of treatment in aHUS |
| Starting date | 01/09/2014 |
| Contact information | mnangaku‐tky@umin.ac.jp |
| Notes |
AHUS ‐ atypical haemolytic uraemic syndrome; HSCT ‐ haematopoietic stem cell transplant; TMA ‐ thrombotic microangiopathy; TTP ‐ thrombotic thrombocytopenic purpura
Differences between protocol and review
Removal of secondary outcome "Persistent requirement for, and duration of, RRT" as duplication of primary outcome "Requirement for RRT". Removal of secondary outcome "Extra‐renal manifestations of disease" as considered too vague.
Rather than using the standard risk of bias assessment tool we adopted a newly created risk of bias tool developed specifically for single‐arm studies (Appendix 3). This allowed a more accurate assessment of the risk of bias in included studies.
Contributions of authors
Draft the protocol: DP, EDO
Study selection: DP, EDO
Extract data from studies: DP, EDO
Enter data into RevMan: DP, EDO
Carry out the analysis: EDO, FAID, PM
Interpret the analysis: EDO, FAID, PM
Draft the final review: DP, EDO, FAID, PM, DK
Disagreement resolution: DK, PM
Update the review: DP, EDO
Declarations of interest
D Pugh has declared that they have no conflict of interest
ED O'Sullivan has declared that they have no conflict of interest
FAI Duthie has declared that they have no conflict of interest
P Masson has declared that they have no conflict of interest
D Kavanagh: Newcastle University has received funding from Biomarin, Gyroscope Therapeutics and Alexion Pharmaceuticals for consultancy work undertaken by DK. DK is scientific advisor to Gyroscope Therapeutics, London.
New
References
References to studies included in this review
Fakhouri 2016 {published data only}
- Fakhouri F, Bedrosian C, Ogawa M, Kincaid J, Loirat C. Eculizumab safety and efficacy in adult patients with aHUS, with or without baseline dialysis [abstract no: SA-PO507]. Journal of the American Society of Nephrology 2014;25(Abstracts):751A. [Google Scholar]
- Fakhouri F, Hourmant M, Campistol JM, Cataland S, Espinosa M, Gaber AO, et al. Eculizumab inhibits thrombotic microangiopathy, and improves renal function in adult atypical hemolytic uremic syndrome patients: 1-year update [abstract no: SA-PO508]. Journal of the American Society of Nephrology 2014;25(Abstracts):751A. [Google Scholar]
- Fakhouri F, Hourmant M, Campistol JM, Cataland SR, Espinosa M, Gaber AO, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. American Journal of Kidney Diseases 2016;68(1):84-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fakhouri F, Hourmant M, Cataland S, Espinosa M, Gaber AO, Menne J, et al. Eculizumab (ECU) inhibits thrombotic microangiopathy (TMA) and improves renal function in adult patients (pts) with atypical hemolytic uremic syndrome (aHUS) [abstract no: 2179]. Blood 2013;122(21). [EMBASE: 71265761] [Google Scholar]
- Loirat C, Legendre C, Ogawa M, Bedrosian C, Kincaid J, Fakhouri F. Safety and efficacy of eculizumab in adult aHUS patients, with or without a history of renal transplant [abstract no: SA-PO511]. Journal of the American Society of Nephrology 2014;25(Abstracts):752A. [Google Scholar]
- Minetti E, Legendre C, Hourmant M, Campistol JM, Espinosa M, Gaber AO, et al. Inhibition of complement-mediated thrombotic microangiopathy with eculizumab improves hematological and renal outcomes in adult patients with atypical hemolytic uremic syndrome [abstract no: S1371]. Haematologica 2014;99(Suppl 1):537. [EMBASE: 71700845] [Google Scholar]
- Weekers LE, Campistol JM, Espinosa M, Gaber AO, Menne J, Minetti E, et al. Eculizumab treatment of atypical haemolytic uraemic syndrome: Results from the largest prospective clinical trial to date [abstract]. Critical Care 2014;18:S37. [EMBASE: 71506822] [Google Scholar]
- Weekers LE, Minetti EE, Fakhouri F, Hourmant M, Campistol JM, Cataland SR, et al. Eculizumab for the treatment of atypical haemolytic uraemic syndrome: Results from the largest prospective clinical trial to date in adults [abstract]. Acta Clinica Belgica 2015;70:S2. [EMBASE: 612330483] [Google Scholar]
Greenbaum 2016 {published data only}
- Ariceta G, Greenbaum LA, Wang J, Kincaid J, Licht C. Safety of eculizumab in pediatric patients with atypical hemolytic uremic syndrome [abstract no: TH-PO460]. Journal of the American Society of Nephrology 2015;26(Abstracts):191A. [Google Scholar]
- Greenbaum L, Fila M, Ardissino G, Al-Akash S, Evans J, Henning P, et al. Eculizumab (ECU) inhibits Thrombotic Microangiopathy (TMA) and improves renal function in pediatric patients (Pts) with atypical Hemolytic Uremic Syndrome (aHUS) [abstract]. Blood 2013;122(21). [EMBASE: 71265773] [Google Scholar]
- Greenbaum L, Fila M, Ardissino G, Al-Akash S, Evans J, Lieberman K, et al. Eculizumab inhibits Thrombotic Microangiopathy and improves renal function in pediatric patients with Atypical Hemolytic Uremic Syndrome: 1-year update [abstract no: 4986]. Blood 2014;124(21). [EMBASE: 71756929] [Google Scholar]
- Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney International 2016;89(3):701-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Van De Kar N, Greenbaum LA, Fila M, Tsimaratos M, Ardissino G, Al-Akash SI, et al. Evaluation of eculizumab treatment of paediatric patients with atypical haemolytic uraemic syndrome: a prospective clinical trial [abstract]. Pediatric Nephrology 2014;29(9):1670-1. [EMBASE: 71662350] [Google Scholar]
- Vande Walle J, Greenbaum LA, Bedrosian C, Ogawa M, Kincaid J, Loirat C. Safety and efficacy of eculizumab in pediatric patients with aHUS, with or without baseline dialysis [abstract no: SA-PO546]. Journal of the American Society of Nephrology 2014;25(Abstracts):761A. [Google Scholar]
Legendre 2013 {published data only}
- Ariceta G, Greenbaum LA, Wang J, Kincaid J, Licht C. Safety of eculizumab in pediatric patients with atypical hemolytic uremic syndrome [abstract no: TH-PO460]. Journal of the American Society of Nephrology 2015;26(Abstracts):191A. [Google Scholar]
- Bedrosian C, Babu S, Furman R, Sheerin N, Cohen D, Gaber O, et al. Eculizumab is effective in patients resistant to plasma exchange/infusion with atypical hemolytic uremic syndrome (AHUS) [abstract no: P-WE-419]. Journal of Thrombosis & Haemostasis 2011;9(Suppl 2):651-2. [EMBASE: 70614363] [Google Scholar]
- Davin JC, Sheerin N, Legendre C, Greenbaum L, Furman R, Gaber AO, et al. Long-term improvements in outcomes with eculizumab in aHUS patients with progressing TMA [abstract]. Acta Clinica Belgica 2013;68(4):318. [EMBASE: 71518311] [Google Scholar]
- Goodship T, Smith RJ, Legendre C, Muus P, Rodig N, Al-Akash SI, et al. Eculizumab (ECU) is effective in patients (pts) with atypical hemolytic uremic syndrome (aHUS) regardless of underlying genetic mutations or complement factor H (CFH) auto-antibodies [abstract no: TH-PO442]. Journal of the American Society of Nephrology 2012;23(Abstracts):199A. [Google Scholar]
- Goodship TH, Muus P, Legendre C, Douglas K, Hourmant M, Delmas Y, et al. Interim analysis of phase II efficacy and safety data for eculizumab in patients with atypical hemolytic uremic syndrome (aHUS) receiving chronic plasma exchange/infusion [abstract]. Molecular Immunology 2011;48(14):1712. [EMBASE: 70969332] [Google Scholar]
- Greenbaum L, Legendre CM, Babu S, Furman RR, Sheerin N, Cohen D, et al. Eculizumab (ECU) in atypical hemolytic uremic syndrome (AHUS) patients with progressing thrombotic microangiopathy (TMA): 2-year data [abstract no: 2084]. Blood 2012;120(21). [EMBASE: 70964821] [Google Scholar]
- Greenbaum LA, Babu S, Furman R, Sheerin N, Cohen D, Gaber AO, et al. Continued improvements in renal function with sustained eculizumab (ECU) in patients (Pts) with atypical Hemolytic Uremic Syndrome (aHUS) resistant to plasma exchange/infusion (PE/PI) [abstract no: TH-PO366]. Journal of the American Society of Nephrology 2011;22(Abstracts):197A. [Google Scholar]
- Legendre C, Babu S, Furman R. Safety & efficacy of eculizamab in aHUS patients resistant to plasma therapy: interim analysis from a phase II trial [abstract]. Journal of the American Society of Nephrology 2011;22(Abstracts):201. [Google Scholar]
- Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. New England Journal of Medicine 2013;368(23):2169-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Licht C, Muus P, Legendre C, Douglas K, Hourmant M, Delmas Y, et al. A phase II study of eculizumab in adolescent/adult patients with atypical hemolytic uremic syndrome (aHUS) receiving chronic plasma exchange/infusion: interim efficacy and safety analysis [abstract no: PS2-FRI-272]. Pediatric Nephrology 2011;26(9):1660. [Google Scholar]
- Licht C, Muus P, Legendre C, Douglas K, Hourmant M, Delmas Y, et al. A phase II study of eculizumab in patients with atypical hemolytic uremic syndrome (AHUS) receiving chronic plasma exchange/infusion: interim analysis [abstract no: P-WE-418]. Journal of Thrombosis & Haemostasis 2011;9(Suppl 2):651. [EMBASE: 70614362] [Google Scholar]
- Licht C, Muus P, Legendre C, Douglas K, Hourmant M, Delmas Y, et al. Ph II study of eculizumab (ECU) in patients (pts) with atypical hemolytic uremic syndrome (aHUS) receiving chronic plasma exchange/iinfusion (PE/PI) [abstract no: TH-PO366]. Journal of the American Society of Nephrology 2011;22(Abstracts):197A. [Google Scholar]
- Loirat C, Babu S, Furman R, Sheerin N, Cohen D, Gaber O, et al. Eculizumab in adolescents/adult patients with atypical hemolytic uremic syndrome resistant to plasma exchange/infusion: a phase II efficacy and safety study [abstract no: PS2-FRI-273]. Pediatric Nephrology 2011;26(9):1660-1. [Google Scholar]
- Muus P, Legendre C, Douglas K, Hourmant M, Delmas Y, Herthelius M, et al. Safety & efficacy of eculizumab in aHUS patients on chronic plasma therapy: interim analysis of a phase II trial [abstract no: F-PO1274]. Journal of the American Society of Nephrology 2010;21(Abstracts):402A. [Google Scholar]
Licht 2015 {published data only}
- Ariceta G, Greenbaum LA, Wang J, Kincaid J, Licht C. Safety of eculizumab in pediatric patients with atypical hemolytic uremic syndrome [abstract no: TH-PO460]. Journal of the American Society of Nephrology 2015;26(Abstracts):191A. [Google Scholar]
- Douglas K, Sheerin N, Legendre C, Greenbaum L, Furman R, Cohen D, et al. Progressing thrombotic microangiopathy in atypical haemolytic uraemic syndrome patients: long term improvements in outcomes with eculizumab [abstract no: P212]. Haematologica 2013;98(Suppl 1):91-2. [EMBASE: 71696065] [Google Scholar]
- Greenbaum L, Delmas Y, Fakhouri F, Kincaid J, Licht C, Minetti E, et al. Eculizumab prevents thrombotic microangiopathy: long-term follow-up study of patients with atypical hemolytic uremic syndrome [abstract no: 2252]. Blood 2016;126(23):2252. [EMBASE: 72172548] [Google Scholar]
- Legendre C, Greenbaum LA, Babu S, Furman R, Sheerin N, Cohen D, et al. Eculizumab (ECU) in atypical hemolytic uremic syndrome (aHUS) patients (pts) with progressing TMA: continued improvements at 2-year follow-up [abstract no: SA-OR101]. Journal of the American Society of Nephrology 2012;23(Abstracts):88-9. [Google Scholar]
- Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney International 2015;87(5):1061-73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht C, Muus P, Legendre C, Delmas Y, Herthelius M, Bedrosian CL, et al. Time to hematologic and renal improvements in atypical hemolytic uremic syndrome patients with long disease duration and chronic kidney disease (CKD) treated with eculizumab [abstract]. Blood 2013;122(21). [EMBASE: 71265768] [Google Scholar]
- Licht C, Muus P, Legendre C, Douglas K, Hourmant M, Delmas Y, et al. Eculizumab (ECU) is effective in atypical hemolytic uremic syndrome (aHUS) patients (pts) with a long disease duration and CKD: 2-year data [abstract no: SA-OR103]. Journal of the American Society of Nephrology 2012;23(Abstracts):89A. [Google Scholar]
- Licht C, Muus P, Legendre CM, Douglas KW, Hourmant M, Delmas Y, et al. Eculizumab (ECU) safety and efficacy in atypical hemolytic uremic syndrome (aHUS) patients with long disease duration and chronic kidney disease (CKD): 2-year results [abstract no: 985]. Blood 2012;120(21). [EMBASE: 70962893] [Google Scholar]
- Sheerin N, Legendre C, Greenbaum L, Furman R, Cohen D, Gaber AO, et al. Long-term improvements in outcomes with eculizumab in patients with atypical haemolytic uraemic syndrome and progressing TMA [abstract no: MP036]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i310. [EMBASE: 71075948] [Google Scholar]
Rondeau 2020 {published data only}
- Rondeau E, Scully M, Ariceta G, Barbour T, Cataland S, Heyne N, et al. The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naive to complement inhibitor treatment [Erratum in: Kidney Int. 2020 Dec;98(6):1621; PMID: 33276869]. Kidney International 2020;97(6):1287-96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aigner 2018 {published data only}
- Aigner C, Bohmig G, Eskandary F, Gaggl M, Kain R, Sunder-Plassmann R, et al. Preemptive plasma therapy and eculizumab (ECU) rescue for atypical hemolytic uremic syndrome (AHUS) relapse following kidney transplantation (KTX) [abstract no: C76]. American Journal of Transplantation 2018;18(Suppl 4):793-4. [EMBASE: 622280126] [Google Scholar]
- Aigner C, Bohmig G, Eskandary F, Gaggl M, Kain R, Sunder-Plassmann R, et al. Preemptive plasma therapy and eculizumab rescue for atypical hemolytic uremic syndrome relapse following kidney transplantation [abstract no: SP730]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i592-3. [EMBASE: 622606849] [Google Scholar]
Aigner 2020 {published data only}
- Aigner C, Bohmig GA, Eskandary F, Herkner H, Prohaszka Z, Csuka D, et al. Preemptive plasma therapy prevents atypical hemolytic uremic syndrome relapse in kidney transplant recipients. European Journal of Internal Medicine 2020;73:51-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Al‐Akash 2012 {published data only}
- Al-Akash SI, Goodship TH, Smith RJ, Legendre CM, Licht C, Muus P, et al. Eculizumab is an effective treatment for atypical hemolytic uremic syndrome in patients with or without identified genetic complement mutations or complement factor H auto-antibodies [abstract no: 2085]. Blood 2012;120(21):2085. [EMBASE: 70964820] [Google Scholar]
Alpay 2019 {published data only}
- Alpay N, Ozcelik U. Renal transplantation in patients with atypical hemolytic uremic syndrome: a single center experience. Transplantation Proceedings 2019;51(7):2295-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alsakran 2019 {published data only}
- Alsakran A, Ibrahim A, Alshareef T, Saadeh S, Alsabban E. Eculizumab for the treatment of atypical hemolytic uremic syndrome in pediatric patients, a tertiary care hospital experience [abstract]. Nephrology Dialysis Transplantation 2019;34(Suppl 1). [EMBASE: 631305535] [Google Scholar]
Bhalla 2019 {published data only}
- Bhalla A, Alasfar S, Alachkar N. Eculizumab in kidney transplant recipients with atypical hemolytic uremic syndrome-single center experience [abstract no: A232]. American Journal of Transplantation 2019;19(Suppl 3):682-3. [EMBASE: 628454026] [Google Scholar]
Bohl 2016 {published data only}
- Bohl SR, Harsdorf S, Schoensteiner S, Schwarzwaelder P, Scholl K, Wais V, et al. Eculizumab therapy of adult TA-TMA: A high response rate is associated with a high infection-related mortality [abstract no: 2255]. Blood 2016;128(22). [EMBASE: 614309788] [Google Scholar]
Cao 2018 {published data only}
- Cao M, Leite BN, Ferreiro T, Calvo M, Fernandez C, Alonso A, et al. Eculizumab modifies outcomes in adults with atypical hemolytic uremic syndrome with acute kidney injury. American Journal of Nephrology 2018;48(3):225-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cataland 2014 {published data only}
- Cataland SR, Feldkamp T, Bedrosian CL, Kincaid J, Minetti EE. Eculizumab Is an effective treatment for atypical hemolytic uremic syndrome in pediatric and adult patients with or without identified genetic complement mutations or complement factor H autoantibodies [abstract no: 2789]. Blood 2014;124(21). [EMBASE: 71760434] [Google Scholar]
Cofiell 2015 {published data only}
- Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle A, Ogawa M, et al. Biomarkers of complement and endothelial activation, inflammation, thrombosis and renal injury in patients (pts) with aHUS treated with eculizumab (ECU) [abstract no: 2184]. Blood 2013;122(21). [EMBASE: 71265766] [Google Scholar]
- Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle A, Ogawa M, et al. Eculizumab reduces terminal complement (TC) and complement alternative pathway (CAP) activation, inflammation, endothelial damage, thrombosis and renal injury in atypical hemolytic uremic syndrome (aHUS) patients [abstract no: MP035]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii339-40. [EMBASE: 71492439] [Google Scholar]
- Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle AP, Ogawa M, et al. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood 2015;125(21):3253-62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofiell R, Kukreja A, Bedard K, Yan Y. Eculizumab reduces terminal complement, complement alternative pathway activation, inflammation, endothelial damage, thrombosis and renal injury markers in patients with atypical hemolytic uremic syndrome [abstract no: SA-PO506]. Journal of the American Society of Nephrology 2014;25(Abstracts):750A. [Google Scholar]
Escribano 2017 {published data only}
- Escribano TC, Praga M, De Cordoba SR. Eculizumab in secondary atypical hemolytic uremic syndrome [abstract]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii58. [EMBASE: 617291197] [Google Scholar]
Favi 2018 {published data only}
- Favi E, Ardissino G, Cresseri D, Brocca J, Testa S, Tel F, et al. Thirty-six consecutive kidney transplants in recipients with atypical hemolytic uremic syndrome: a single-centre experience [abstract]. American Journal of Transplantation 2018;18(Suppl 4):286. [EMBASE: 622279700] [Google Scholar]
Fremeaux‐Bacchi 2015 {published data only}
- Fremeaux-Bacchi V, Legendre CM. The emerging role of complement inhibitors in transplantation. Kidney International 2015;88(5):967-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frykman 2016 {published data only}
- Frykman A, Swerkersson S, Westphal Ladfors S, Hansson S. Long-term outcome after hemolytic uremic syndrome in childhood [abstract no: PO-332]. Pediatric Nephrology 2016;31(10):1871. [EMBASE: 612479657] [Google Scholar]
Galbusera 2019 {published data only}
- Galbusera M, Noris M, Gastoldi S, Bresin E, Mele C, Breno M, et al. An ex vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. American Journal of Kidney Diseases 2019;74(1):56-72. [MEDLINE: 30851964] [DOI] [PubMed] [Google Scholar]
Gavriilaki 2015 {published data only}
- Gavriilaki E, Yuan X, Ye Z, Ambinder AJ, Shanbhag SP, Streiff MB, et al. Modified ham test for atypical hemolytic uremic syndrome. Blood 2015;125(23):3637-46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomez‐Alvarez 2016 {published data only}
- Gomez-Alvarez S, Llopis-Salvia P, Avila-Bernabeu A, Pallardo-Mateu L, Climente-Marti M. Effectiveness and safety of eculizumab in atypical haemolytic uraemic syndrome and thrombotic microangiopathy [abstract]. European Journal of Hospital Pharmacy 2016;23(Suppl 1):A88. [EMBASE: 614325739] [Google Scholar]
Gruppo 2011 {published data only}
- Gruppo R , Rodig N, Vilalta R, Hernandez J, Camacho Diaz J, Lapeyraque A-L, et al. Eculizumab (ECU) therapy for atypical hemolytic uremic syndrome (AHUS) in pediatric patients: efficacy and safety outcomes from a retrospective study [abstract no: P-WE-444]. Journal of Thrombosis & Haemostasis 2011;9(Suppl 2):660. [EMBASE: 70614387] [Google Scholar]
Han 2019 {published data only}
- Han H, Alagusundaramoorthy S, Swanson K, Gardezi AI, Chan MR. Acute candida albicans peritonitis in a patient with atypical hemolytic uremic syndrome treated with eculizumab. Peritoneal Dialysis International 2019;39(6):575-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hanes 2019 {published data only}
- Hanes V, Pan J, Mytych DT, Chien D, Chow V. Relationship between pharmacokinetics and antidrug antibody status of ABP 959, a biosimilar candidate to eculizumab: results from a pharmacokinetic similarity study [abstract no: 128621]. Blood 2019;134(Suppl 1). [EMBASE: 630317971] [Google Scholar]
Ito 2019 {published data only}
- Ito S, Hidaka Y, Inoue N, Kaname S, Kato H, Matsumoto M, et al. Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clinical & Experimental Nephrology 2019;23(1):112-21. [EMBASE: 623239057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jozsi 2007 {published data only}
- Jozsi M, Strobel S, Dahse HM, Liu WS, Hoyer PF, Oppermann M, et al. Anti factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood 2007;110(5):1516-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kant 2020 {published data only}
- Kant S, Bhalla A, Alasfar S, Alachkar N. Ten-year outcome of eculizumab in kidney transplant recipients with atypical hemolytic uremic syndrome- a single center experience. BMC Nephrology 2020;21(1):189. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 2019 {published data only}
- Kato H, Miyakawa Y, Hidaka Y, Inoue N, Ito S, Kagami S, et al. Safety and effectiveness of eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clinical & Experimental Nephrology 2019;23(1):65-75. [EMBASE: 622799582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khandelwal 2016 {published data only}
- Khandelwal P, Sinha A, Hari P, Bagga A. Multicenter cohort of atypical hemolytic uremic syndrome (aHUS) in Indian children [abstract no: PO-378]. Pediatric Nephrology 2016;31(10):1886. [EMBASE: 612479699] [Google Scholar]
Khandelwal 2019 {published data only}
- Khandelwal P, Thomas CC, Rathi BS, Hari P, Tiwari AN, Sinha A, et al. Membrane-filtration based plasma exchanges for atypical hemolytic uremic syndrome: audit of efficacy and safety. Journal of Clinical Apheresis 2019;34(5):555-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khursigara 2014 {published data only}
- Khursigara G, Johnson S, Harvey E, Kincaid J, Bedrosian C. Time to end-stage renal disease in patients with atypical hemolytic uremic syndrome receiving supportive care and eculizumab [abstract no: SA-PO509]. Journal of the American Society of Nephrology 2014;25(Abstracts):752A. [Google Scholar]
Kumar 2019 {published data only}
- Kumar G, Al-Masri O, Alismaili Z, Tawfik E, Al-Ghabra MK, Ilyas SH, et al. Eculizumab in paediatric atypical haemolytic uraemic syndrome: lessons learned from a single-centre experience in the United Arab Emirates. Journal of Paediatrics & Child Health 2019;55(10):1237-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lappegard 2015 {published data only}
- Lappegard KT, Bjerre A, Tjonnfjord GE, Mollnes TE. Therapeutic complement inhibition - from experimental to clinical medicine. Tidsskrift for Den Norske Laegeforening 2015;135(19):1745-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Legault 2009 {published data only}
- Legault DJ, Boelkins MR. Successful treatment of aHUS recurrence and arrest of plasma exchange resistant TMA post-renal transplantation with the terminal complement inhibitor eculizumab [abstract no: 2421]. Blood 2009;114(22). [EMBASE: 70247442] [Google Scholar]
Levi 2016 {published data only}
- Levi C, Rabant M, Fremeaux-Bacchi V, Scemla A, Zuber J, Legendre C, et al. Outcome after eculizumab therapy to prevent recurrence of atypical hemolytic uremic syndrome: experience in twelve renal transplant recipients [abstract no: 550.9]. Transplantation 2016;100(7 Suppl 1):S325. [EMBASE: 613004527] [Google Scholar]
Mazo 2017 {published data only}
- Mazo A, Ananin P, Pushkov A, Savostianov K, Zrobok O, Tsygin A. Efficacy of treatment with eculizumab in children with aHUS [abstract no: P-338]. Pediatric Nephrology 2017;32(9):1788. [EMBASE: 618119670] [Google Scholar]
Menne 2019 {published data only}
- Menne DJ, Delmas Y, Kincaid JF, Licht C, Minetti EE, Mix C, et al. Ongoing eculizumab (ECU) prevents thrombotic microangiopathy (TMA) in patients (pts) with atypical hemolytic uremic syndrome (aHUS): final long-term observational study data [abstract no: FR-PO136]. Journal of the American Society of Nephrology 2017;28(Abstracts):435. [EMBASE: 633699768] [Google Scholar]
- Menne J, Delmas Y, Fakhouri F, Kincaid JF, Licht C, Minetti EE, et al. Eculizumab prevents thrombotic microangiopathy in patients with atypical haemolytic uraemic syndrome in a long-term observational study. Clinical Kidney Journal 2019;12(2):196-205. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne J, Delmas Y, Fakhouri F, Licht C, Lommelé Å, Minetti EE, et al. Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrology 2019;20(1):125. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne J, Delmas Y, Rondeau E, Licht C, Wang J, Mix C, et al. Eculizumab prevents thrombotic microangiopathy in atypical hemolytic uremic syndrome patients: long-term follow-up [abstract no: FR-PO446]. Journal of the American Society of Nephrology 2015;26(Abstracts):458A. [Google Scholar]
- Menne J, Greenbaum L, Licht C, Mix C, Kincaid J, Wang J, et al. Long-term safety and effectiveness of eculizumab for patients with atypical haemolytic uraemic syndrome: Outcomes from a prospective observational clinical trial [abstract]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii572-3. [EMBASE: 617290186] [Google Scholar]
Monet‐Didailler 2020 {published data only}
- Monet-Didailler C, Chevallier A, Godron-Dubrasquet A, Allard L, Delmas Y, Contin-Bordes C, et al. Outcome of children with Shiga toxin-associated haemolytic uraemic syndrome treated with eculizumab: a matched cohort study. Nephrology Dialysis Transplantation 2020;35(12):2147-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nayer 2016 {published data only}
- Nayer A, Asif A. Atypical hemolytic-uremic syndrome: a clinical review. American Journal of Therapeutics 2016;23(1):e151-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nester 2013 {published data only}
- Nester CM, Brophy PD. Eculizumab in the treatment of atypical haemolytic uraemic syndrome and other complement-mediated renal diseases. Current Opinion in Pediatrics 2013;25(2):225-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nilsson 2007 {published data only}
- Nilsson SC, Karpman D, Vaziri-Sani F, Kristoffersson AC, Salomon R, Provot F, et al. A mutation in factor I that is associated with atypical hemolytic uremic syndrome does not affect the function of factor I in complement regulation. Molecular Immunology 2007;44(8):1835-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noris 2014 {published data only}
- Noris M, Galbusera M, Gastoldi S, Macor P, Banterla F, Bresin E, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood 2014;124(11):1715-26. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pape 2016 {published data only}
- Pape L, Mix C, Wang J, Greenbaum L, Lapeyraque A-L. Outcomes from an observational clinical trial evaluating the long-term safety and effectiveness of eculizumab use in paediatric patients with atypical haemolytic uraemic syndrome (aHUS) [abstract no: FP-S30-2]. Pediatric Nephrology 2016;31(10):1757. [EMBASE: 612479810] [Google Scholar]
Pape 2017 {published data only}
- Pape L, Mix C, Wang J, Greenbaum L, Lapeyraque A-L. Improved outcomes for paediatric patients with atypical haemolytic uraemic syndrome (AHUS) receiving long-term eculizumab treatment during on-treatment periods compared with off-treatment periods [Abstract no: O-46]. Pediatric Nephrology 2017;32(9):1662. [EMBASE: 618120141] [Google Scholar]
Picard 2015 {published data only}
- Picard C, Burtey S, Bornet C, Curti C, Montana M, Vanelle P. Pathophysiology and treatment of typical and atypical hemolytic uremic syndrome. Pathologie Biologie 2015;63(3):136-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raina 2018 {published data only}
- Raina R, Chauvin A, Fox K, Kesav N, Ascha M, Vachharajani TJ, et al. Effect of immunosuppressive therapy on the occurrence of atypical hemolytic uremic syndrome in renal transplant recipients. Annals of Transplantation 2018;23:631-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siedlecki 2019 {published data only}
- Siedlecki AM, Isbel N, Vande Walle J, James Eggleston J, Cohen DJ, Global aHUS Registry. Eculizumab use for kidney transplantation in patients with a diagnosis of atypical hemolytic uremic syndrome. KI Reports 2019;4(3):434-46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simonetti 2011 {published data only}
- Simonetti G, Gruppo R , Rodig N, Hernandez J, Lapeyraque A, Sherwinter J, et al. Eculizumab therapy for atypical hemolytic uremic syndrome (aHUS) in pediatric patients: efficacy and safety outcomes from a retrospective study [abstract no: PS2-FRI-288]. Pediatric Nephrology 2011;26(9):1663. [EMBASE: 70530739] [Google Scholar]
Socie 2019 {published data only}
- Socie G, Caby-Tosi MP, Marantz JL, Cole A, Bedrosian CL, Gasteyger C, et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. British Journal of Haematology 2019;185(2):297-310. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2010 {published data only}
- Taylor CM, Machin S, Wigmore SJ, Goodship TH, Working Party from the Renal Association the British Committee for Standards in Haematology and the British Transplantation Society. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. British Journal of Haematology 2010;148(1):37-47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ubago 2014 {published data only}
- Ubago Perez R, Castillo Munoz MA, Galvan Banqueri M, Abdel-Kader L, Vega Coca MD, Romero Tabares A, et al. Efficacy of eculizumab in adult patients with atypical hemolytic uremic syndrome resistant to plasma treatment [abstract]. European Journal of Hospital Pharmacy 2014;21(Suppl 1):A99. [EMBASE: 611784993] [Google Scholar]
Uriol 2018 {published data only}
- Uriol M, Cabello S, Allende N, Ballester C, Mas A, Perez A, et al. Efficacy and safety of the eculizumab on haemolytic uremic syndrome [abstract]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i97. [EMBASE: 622605278] [Google Scholar]
Vakiti 2019 {published data only}
- Vakiti A, Singh D, Pilla R, Alhaj-Moustafa M, Fitzpatrick KW. Bevacizumab-induced atypical hemolytic uremic syndrome and treatment with eculizumab. Journal of Oncology Pharmacy Practice 2019;25(4):1011-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vilalta 2012 {published data only}
- Vilalta R, Al-Akash S, Davin J, Diaz J, Gruppo R , Hernandez J, et al. Eculizumab therapy for pediatric patients with atypical hemolytic uremic syndrome: Efficacy and safety outcomes of a retrospective study [abstract]. Haematologica 2012;97(Suppl 1):479. [EMBASE: 71724689] [Google Scholar]
Vivarelli 2014 {published data only}
- Vivarelli M, Emma F. Treatment of C3 glomerulopathy with complement blockers. Seminars in Thrombosis & Hemostasis 2014;40(4):472-7. [EMBASE: 53127391] [DOI] [PubMed] [Google Scholar]
Volokhina 2016 {published data only}
- Volokhina E, Wijnsma K, Sweep F, Bruggemann R, Wetzels J, Van De Kar N, et al. Pharmacokinetics and pharmacodynamics of eculizumab in individualized treatment of atypical hemolytic uremic syndrome [abstract]. Immunobiology 2016;31(10):1141. [EMBASE: 613695985] [Google Scholar]
Volokhina 2016a {published data only}
- Volokhina E, Wijnsma K, Sweep F, Bruggemann R, Wetzels J, Van De Kar N, et al. Pharmacokinetics and pharmacodynamics of eculizumab in individualized treatment of atypical HUS [abstract no: FP-S30-1]. Pediatric Nephrology 2016;31(10):1757. [EMBASE: 612479791] [Google Scholar]
Walle 2017 {published data only}
- Walle JV, Siedlecki A, Isbel N, Kupelian V, Cohen D. Timing of eculizumab initiation and the need for dialysis in patients with atypical haemolytic uraemic syndrome undergoing kidney transplantation [abstract]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii85. [EMBASE: 617289751] [Google Scholar]
Walle 2017a {published data only}
- Walle JV, Delmas Y, Ardissino G, Wang J, Kincaid JF, Haller H. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of eculizumab treatment. Journal of Nephrology 2017;30(1):127-34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weston‐Davies 2013 {published data only}
- Weston-Davies W, Westwood JP, Nunn M. Phase 1 clinical trial of novel complement C5 inhibitor coversin [abstract]. Molecular Immunology 2013;56(3):264. [EMBASE: 71684209] [Google Scholar]
Wijnsma 2016 {published data only}
- Wijnsma KL, Volokhina E, Van Den Heuvel LP, Wetzels JF, de Kar NC. Safety and effectiveness of restricted eculizumab regimen in atypical HUS [abstract no: PO-371]. Pediatric Nephrology 2016;31(10):1883-4. [EMBASE: 612479611] [Google Scholar]
Wong 2015 {published data only}
- Wong EK, Kavanagh D. Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Translational Research: The Journal Of Laboratory & Clinical Medicine 2015;165(2):306-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wuhl 2020 {published data only}
- Wuhl E, Adams B, Cheong HI, Constantinescu A, Denker A, Dixon B. The long-acting complement inhibitor ravulizumab in children with atypical hemolytic uremic syndrome (interim analysis) [abstract]. Nieren und Hochdruckkrankheiten 2020;40(3):112. [EMBASE: 631397518] [Google Scholar]
References to ongoing studies
EUCTR2017‐001082‐24 {published data only}
- EUCTR2017-001082-24. An international study at different study sites providing a drug called cemdisiran (ALN-CC5) to patients with atypical hemolytic uremic syndrome by a subcutaneous injection. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2017-001082-24 2017.
EudraCT2014‐001032‐11 {published data only}
- EudraCT2014-001032-11. A study to investigate the safety, pharmacokinetics, pharmacodynamics, immunogenicity, and clinical activity of study drug OMS721 in adults with thrombotic microangiopathies. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2014-001032-11 (first received 25 June 2014).
NCT01757431 {published data only}
- NCT01757431. The safety and efficacy of eculizumab in Japanese patients with atypical hemolytic uremic syndrome (aHUS). www.clinicaltrials.gov/show/NCT01757431 (first received 31 December 2012).
NCT03131219 {published data only}
- NCT03131219. Study of ravulizumab in children and adolescents with atypical hemolytic uremic syndrome (aHUS). www.clinicaltrials.gov/show/NCT03131219 (first received 27 April 2017).
NCT03205995 {published data only}
- Leifke E. Safety and efficacy study of OMS721 in patients with atypical hemolytic uremic syndrome (aHUS). www.clinicaltrials.gov/show/NCT03205995 (first received 2 July 2017).
UMIN000014869 {published data only}
- Nangaku M. Observational study of atypical hemolytic uremic syndrome in Japan. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000017280 (first received 1 September 2014).
Additional references
Ariceta 2009
- Ariceta G, Besbas N, Johnson S, Karpman D, Landau D, Licht C, et al. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatric Nephrology 2009;24(4):687–96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Besbas 2006
- Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, et al. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney International 2006;70(3):423-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Constantinescu 2004
- Constantinescu AR, Bitzan M, Weiss LS, Christen E, Kaplan BS, Cnaan A, et al. Non-enteropathic hemolytic uremic syndrome: causes and short-term course. American Journal of Kidney Diseases 2004;43(6):976-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coppo 2016
- Coppo R, Bonaudo R, Peruzzi RL, Amore A, Brunati A, Romagnoli R, et al. Liver transplantation for aHUS: still needed in the eculizumab era? Pediatric Nephrology 2016;31(5):759-68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fakhouri 2017
- Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome [Erratum in: Lancet. 2017 Aug 12;390(10095):648; PMID: 28438379]. Lancet 2017;390(10095):681-96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fremeaux‐Bacchi 2013
- Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey MA, Ngo S, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clinical Journal of The American Society of Nephrology: CJASN 2013;8(4):554-62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hillmen 2013
- Hillmen P, Muus P, Röth A, Elebute MO, Risitano AM, Schrezenmeier H, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. British Journal of Haematology 2013;162(1):62–73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanakura 2011
- Kanakura Y, Ohyashiki K, Shichishima T, Okamoto S, Ando K, Ninomiya H, et al. Safety and efficacy of the terminal complement inhibitor eculizumab in Japanese patients with paroxysmal nocturnal hemoglobinuria: the AEGIS clinical trial. International Journal of Hematology 2011;93(1):36-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kaplan 2014
- Kaplan BS, Ruebner RL, Spinale JM, Copelovitch L. Current treatment of atypical hemolytic uremic syndrome. Intractable & Rare Diseases Research 2014;3(2):34–45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulasekararaj 2019
- Kulasekararaj AG, Hill A, Rottinghaus ST, Langemeijer S, Wells R, Gonzalez-Fernandez FA, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood 2019;133(6):540-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2007
- Lai JS, Cella D, Kupst MJ, Holm S, Kelly ME, Bode RK, et al. Measuring fatigue for children with cancer: development and validation of the pediatric Functional Assessment of Chronic Illness Therapy-Fatigue (pedsFACIT-F). Journal of Pediatric Hematology/Oncology 2007;29(7):471-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lara 1999
- Lara PN Jr, Coe TL, Zhou H, Fernando L, Holland PV, Wun T. Improved survival with plasma exchange in patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. American Journal of Medicine 1999;107(6):573-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2019
- Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, Pessoa V, Gualandro S, Fürender W, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood 2019;133(6):530-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loirat 2016
- Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatric Nephrology 2016;31(1):15-39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Michael 2009
- Michael M, Elliott EJ, Ridley GF, Hodson EM, Craig JC. Interventions for haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD003595. [DOI: 10.1002/14651858.CD003595.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noris 2005
- Noris M, Remuzzi G. Hemolytic uremic syndrome. Journal of the American Society of Nephrology 2005;16(4):1035-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noris 2009
- Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. New England Journal of Medicine 2009;361(17):1676-87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noris 2010
- Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clinical Journal of The American Society of Nephrology: CJASN 2010;5(10):1844-59. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathbone 2013
- Rathbone J, Kaltenthaler E, Richards A, Tappenden P, Bessey A, Cantrell A. A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS). BMJ Open 2013;3(11):e003573. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Remuzzi 2005
- Remuzzi G, Ruggenenti P, Colledan M, Gridelli B, Bertani A, Bettinaglio P, et al. Hemolytic uremic syndrome: a fatal outcome after kidney and liver transplantation performed to correct factor H gene mutation. American Journal of Transplantation 2005;5(5):1146-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saland 2009
- Saland JM, Ruggenenti P, Remuzzi G, Consensus Study Group. Liver-kidney transplantation to cure atypical hemolytic uremic syndrome. Journal of the American Society of Nephrology 2009;20(5):940-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Sheerin 2016
- Sheerin NS, Kavanagh D, Goodship TH, Johnson S. A national specialized service in England for atypical haemolytic uraemic syndrome-the first year’s experience. QJM 2016;109(1):27-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van De Kar 2014
- Van De Kar N, Greenbaum LA, Fila M, Tsimaratos M, Ardissino G, Al-Akash SI, et al. Evaluation of eculizumab treatment of paediatric patients with atypical haemolytic uraemic syndrome: a prospective clinical trial [abstract]. Pediatric Nephrology 2014;29(9):1670-1. [EMBASE: 71662350] [Google Scholar]
References to other published versions of this review
Pugh 2017
- Pugh D, O'Sullivan ED, Duthie FAI, Masson P, Kavanagh D. Interventions for atypical haemolytic uraemic syndrome. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012862. [DOI: 10.1002/14651858.CD012862] [DOI] [PMC free article] [PubMed] [Google Scholar]


