Abstract
Development of novel multimodality radiotherapy treatments in metastatic breast cancer, especially in the most aggressive triple negative (TNBC) subtype, is of significant clinical interest. Here we show that a novel inhibitor of Polo-Like Kinase 4 (PLK4), CFI-400945, in combination with radiation, exhibits a synergistic anti-cancer effect in TNBC cell lines and patient-derived organoids in vitro and leads to a significant increase in survival to tumor endpoint in xenograft models in vivo, compared to control or single-agent treatment. Further preclinical and proof-of-concept clinical studies are warranted to characterize molecular mechanisms of action of this combination and its potential applications in clinical practice.
Keywords: Triple-negative breast cancer, Radiotherapy, Polo-like kinase 4, CFI-400945, Radiosensitization, Patient-derived organoids
Highlights
-
•
PLK4 inhibitor CFI-400945, combined with radiation, shows synergistic antiproliferative activity in immortalized breast cancer cell lines.
-
•
CFI-400945 in combination with radiation shows synergistic antiproliferative activity in breast cancer patient-derived organoids.
-
•
In MDA-MB-231 xenograft mice, CFI-400945 sensitizes to radiation and significantly improves survival to the tumour endpoint.
Abbreviations
- TNBC
triple negative breast cancer
- BME
basement membrane extract
- CIN
chromosomal instability
- CDK4/6
cyclin-dependent kinase 4/6
- PLK4
Polo-Like Kinase 4
- PDO
patient-derived organoids
- PDXO
patient-derived organoids established from a xenograft
- NOD/SCID
Nonobese diabetic/severe combined immunodeficiency
Introduction
Local disease control at primary or metastatic sites in patients with metastatic or inoperable breast cancer is commonly addressed with radiotherapy. However, outcomes remain poor, and progression in irradiated areas is not uncommon [[1], [2], [3], [4]]. Development of novel combined modality radiotherapy treatments in metastatic or inoperable disease, especially in the most aggressive triple negative breast cancer (TNBC) subtype, is therefore of significant clinical interest. With the development of more selective chemical inhibitors, there has been a renewed interest in agents targeting regulators of genomic stability and cell cycle which can further exacerbate numerical chromosomal instability (CIN) and lead to cellular lethality [5,6]. Ionizing radiation induces genotoxic stress and aneuploidy and may thus synergize with agents targeting cell cycle checkpoints [[7], [8], [9]]. CIN itself mediates susceptibility to radiation [10,11], and therefore therapies that induce CIN may increase radiosensitivity. Polo-like kinase 4 (PLK4) is a key regulator of the cell cycle and centriole duplication that is aberrantly expressed in breast cancer and is associated with CIN [12,13]. PLK4 was identified as a promising anticancer therapeutic target in TNBC, and a first-in-class inhibitor, CFI-400945, has been recently characterized [14,15]. CFI-400945 exacerbates CIN, has been shown to have antitumor activity in preclinical models, including TNBC, and is being evaluated in clinical trials in patients with metastatic breast cancer, and other cancer types [14,[16], [17], [18], [19], [20]]. We therefore assessed whether radiation and CFI-400945 exhibit combined anticancer effects in breast cancer cell lines and patient-derived organoids (PDO).
Methods
Colony formation assays were performed for breast cancer cell lines and organoids using previously described methodologies [21,22]. In vitro radiation was performed using an X-RAD 320 irradiator (Accela), where cells were treated with various doses of radiation. The media was replaced and supplemented with varying concentrations of CFI-400945 or vehicle control. Synergy of dose-response matrix data was assessed by Bliss synergy score using SynergyFinder Software [23]. All patient samples (Supplementary Table 1) were obtained after participants’ consent and used in accordance with a research ethics board-approved protocol (REB#159481). Organoids were generated and propagated as previously described [24]. Organoid cancer cells were plated (2000 cells/well) in Basement Membrane Extract, Type II (Bio-Techne) in 48-well plates. Animal experiments were performed under an institutionally approved protocol (#1113). MDA-MB-231 cells (2 × 106) were injected into the mammary fat pad of NOD/SCID mice. When the xenograft volume reached 100–150 mm3 (Day 0), mice were randomized to no treatment (N = 6), CFI-400945 only (7.5 mg/kg daily, N = 6), radiation only (8 Gy single dose by targeted radiation to the xenograft, N = 6) or combination treatment of CFI-400945 and radiotherapy (N = 7). CFI-400945 was initiated on Day 0 in the respective groups. On Day 7, mice in the assigned groups received targeted 8 Gy radiation to the tumor site using an XRAD 225Cx (Precision X-RAY) micro-IGRT delivery system. On Day 30, CFI-400945 was stopped and monitoring of the tumor volume continued until tumor endpoint was reached, at which point the animal was sacrificed, and survival time was recorded. Kaplan-Meier survival analysis was used to assess survival times. P-values were calculated using a log-rank test (Prism 8, GraphPad Software).
Results
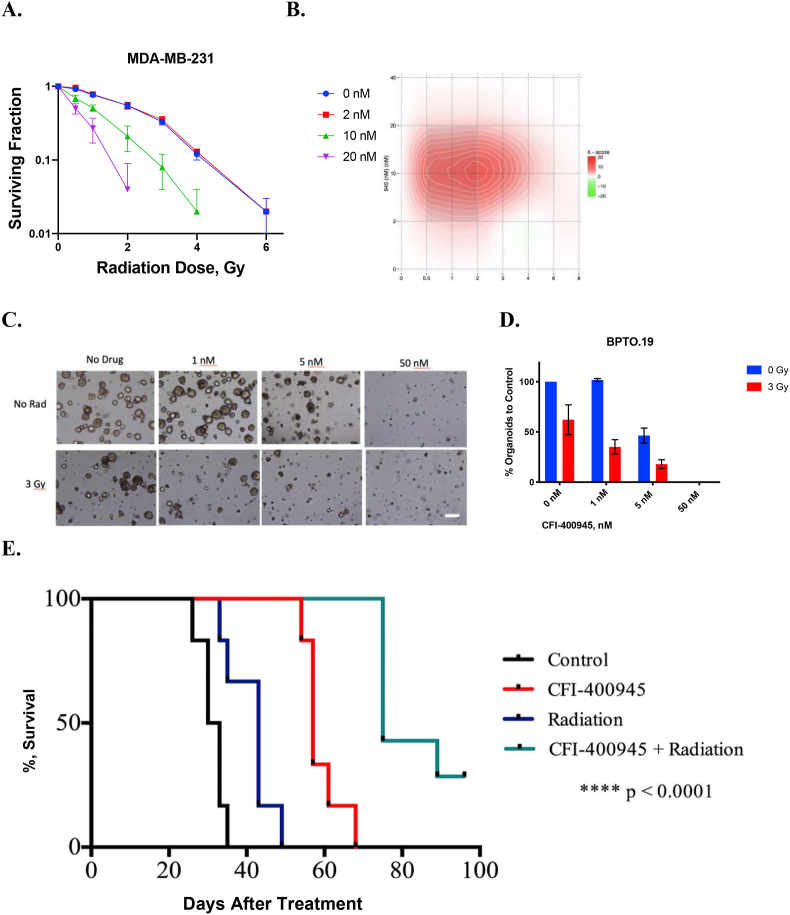
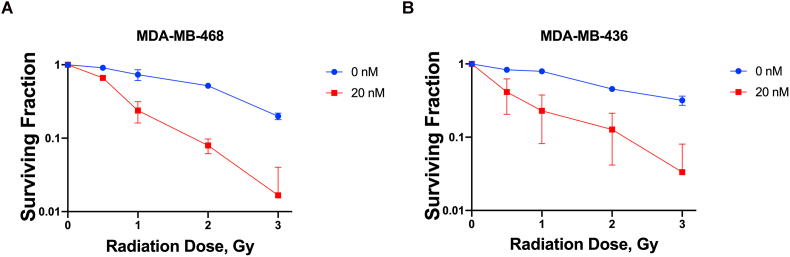
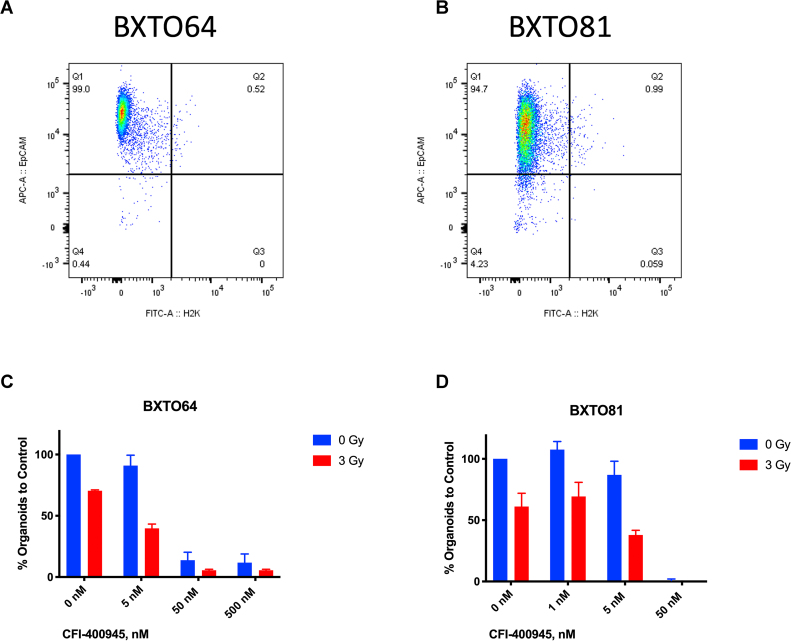
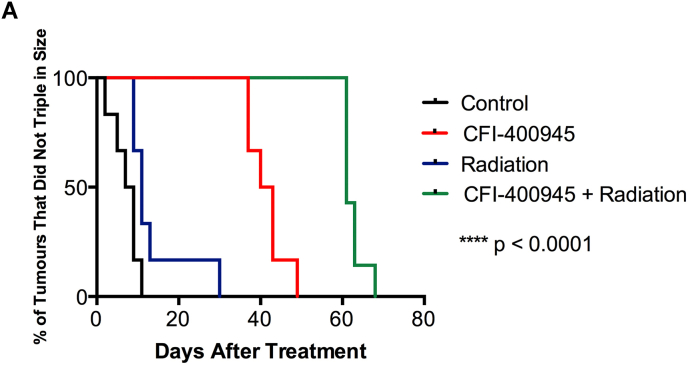
In the MDA-MB-231 TNBC cell line (Fig. 1A), synergy of combined treatment was observed at 10 and 20 nM of CFI-400945 (Fig. 1A) and was greatest at 10 nM of CFI-400945 and 2 Gy of radiation (Fig. 1B). Similar synergistic relationships were observed in two other TNBC lines, MDA-MB-468 and MDA-MB-436 (Supplementary Fig 1). In the TNBC PDO model, BPTO19, 1 nM of CFI-400945 had no significant effect on organoid formation, while at 50 nM no organoid formation was observed (Fig. 1C and D). At 5 nM concentration, CFI-400945 alone caused 2.1 times decrease in colony formation. Irradiation with 3 Gy reduced the number of organoids by 1.6 times, compared to no-radiation control. When combined with CFI-400945 at 1 or 5 nM, irradiation resulted in an average 2.8- and 5.6-fold decrease in organoid formation respectively, compared to no treatment control. Similar effects were observed in 2 other organoid models, BXTO81 and BXTO64 (Supplementary Fig 2). We then assessed the combination of orally delivered CFI-400945 with a single fraction of targeted radiation to MDA-MB-231 xenografts in NOD/SCID mice (Fig. 1E). In the control arm, median survival to tumor humane endpoint was 32 days, which was increased to 43, 57 and 75 days for radiation-only, CFI-400945-only and combination treatment respectively (Fig. 1E). Statistically significant (p < 0.0005) improvements in survival comparing combination versus single treatments or control groups were observed. Similarly, analysis of the median time to tumor volume tripling showed that combination treatment resulted in a significantly prolonged tumor volume tripling time, compared to control, drug-only or radiation-only arms (Supplementary Fig 3). No significant effects on animal general health and weight were observed.
Fig. 1.
Effects of CFI-400945 and Radiation in Triple Negative Breast Cancer In Vitro and In Vivo Models. A. Combined CFI-400945 and radiation resulted in dose-dependent reductions in colony formation in MDA-MB-231. The number of colonies in each treatment arm were normalized to the respective control (no-radiation, no-drug). B. Synergy of combined CFI-400945 and radiation was observed across several dose levels. Bliss synergy scores, calculated with SynergyFinder, are displayed in the heatmap, where intensity of red indicates higher degree of synergy. C. Organoid formation assay using various concentrations of CFI-400945 alone (top panel) or in combination with 3 Gy radiation (bottom panel) in BPTO19, generated from a chest wall metastasis of a TNBC patient. Bright-field microscopy (at 4× magnification) images were taken 28 days following treatment. The number of organoids was counted by 2 independent observers in at least 3 random fields per each well. The counts were normalized to respective unirradiated controls in each group. Bar represents 100 μm. D. Effect of CFI-400945 and radiation in BPTO19. Average number of organoids was normalized to that of control (no-radiation, no-drug). Averages and standard deviations (SD, bars) of replicate experiments are presented. E. In Vivo effects of CFI-400945 and Radiation on MDA-MB-231 Xenografts in NOD/SCID mice. Tumor endpoint survival curve (left) using Kaplan-Meier analysis. P-value was calculated using log-rank test. No significant effects on animal general health and weight were observed, and no detectable metastases (liver and lungs) were seen on the autopsy in all study arms. Significantly smaller proportion of mice developed tumor ulceration in the combination treatment arm compared to other treatment arms.
Discussion
Augmenting the effects of radiation with chemotherapeutic agents or targeted therapy is an attractive strategy to improve survival and quality of life of cancer patients, through achieving synergistic antitumor activity and minimization of the overlapping toxic effects [25,26]. This strategy has not been incorporated into the standard clinical management of breast cancer, though chemoradiation is sometimes used, and targeted combinations are being explored in clinical trials [27]. Our results demonstrate combination synergy and radiosensitizing effects of CFI-400945 across multiple preclinical TNBC models. We show that the combined delivery of these treatments in vivo results in significant prolongation of survival and delay in growth of tumors. CFI-400945 has been shown to be safe and well-tolerated in humans and is being tested in Phase 2 clinical trials in patients with advanced/metastatic breast cancer [19,20]. Emerging data suggest synergistic effects of combining radiotherapy with other novel therapeutic compounds, particularly those that affect cell cycle control and exacerbate CIN. In patients with breast cancer brain metastases receiving CDK4/6 inhibitors, delivery of radiotherapy has been reported to confer prolonged survival outcomes [28]. Ongoing clinical trials are evaluating CDK4/6 inhibitors in combination with radiotherapy in patients with bone metastases and oligometastatic breast cancer [29,30]. Similar to our results, an inhibitor of the spindle assembly checkpoint control protein TTK has been shown in preclinical studies to confer radiosensitization effects and substantially improved tumor control in TNBC [31]. Our findings support development of further preclinical studies of this combination and its molecular mechanisms, as well as early clinical trials to evaluate safety/toxicity in patients with metastatic breast cancer.
Declaration of competing interest
D.W.C serves as a consultant for Agendia, Dynamo Therapeutics, AstraZeneca, Exact Sciences, GSK, Merck, Novartis, Pfizer, Puma and Roche; receives research support (to institution) from GSK, Merck and Pfizer and holds intellectual property as co-investigator on a patent related to biomarkers for TTK inhibitors. The other authors declare no potential conflicts of interests.
CFI-400945 was developed by the Therapeutics group at the authors’ institution (University Health Network, Toronto, ON), and has been licensed to Treadwell Therapeutics.
Acknowledgments
This research was conducted with the support of the Terry Fox Research Institute (New Frontiers Research Program PPG-1064, to D.W.C) and Stand Up To Cancer Canada – Canadian Cancer Society Breast Cancer Dream Team Research Funding, with supplemental support of the Ontario Institute for Cancer Research through funding provided by the Government of Ontario (Funding Award Number: SU2C-AACR-DT-18-15). Stand Up To Cancer Canada is a program of the Entertainment Industry Foundation Canada. Research funding is administered by the American Association for Cancer Research International – Canada, the Scientific Partner of SU2C Canada. This research was also supported by the Young Investigator Startup Grant, Department of Surgery, Western University and the London Regional Cancer Program Catalyst Grant for Translational Cancer Research, Western University (London, ON) to A.P.
A. P. was supported by the Strategic Training in Transdisciplinary Radiation Science for the 21st (STARS21, Terry Fox Foundation, Princess Margaret Radiation Medicine Program and Cancer Centre), Princess Margaret Cancer Centre Scholarship (Toronto, ON); Department of Surgery, University of Toronto (ON); the Clinician Scientist Award, Department of Surgery, Western University and the Academic Medical Organization of Southwestern Ontario (AMOSO) Opportunities Fund (London, ON).
V. B. was supported by Western Postdoctoral Fellowship (Western University, London, ON) and the Breast Cancer Society of Canada Postdoctoral Fellowship. S.P was supported by the Translational Breast Cancer Research Unit Trainee Scholarship (Source: Breast Cancer Society of Canada), London, ON.
We thank Drs Kelsie L. Thu and Isabel Soria-Bretones for input on experimental methodologies. PDO and PDXO models were developed in collaboration with the Princess Margaret Living Biobank (PMLB, Toronto, ON).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.03.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Effects of CFI-400945 and Radiation in Breast Cancer Cell Lines. Combined CFI-400945 and radiation resulted in dose-dependent reductions in colony formation in MDA-MB-468 (A.) and MDA-MB-436 (B.). The number of colonies in each treatment arm were normalized to the respective control (no-radiation, no-drug). Averages and standard deviations (SD, bars) of replicate experiments are presented.
Fig. S2.
Effects of CFI-400945 and Radiation on Organoid Formation in Breast Cancer Patient-Derived Organoids. Quality assurance for mouse cell contamination in BXTO64 (A.) and BXTO81 (B.) organoid models established from patient-derived mouse xenograft (PDX) tissue (see Supplementary Table 1). Established BXTO64 and BXTO81 organoids were stained with mouse anti-H2K-FITC and anti-human EpCAM-APC and analyzed by flow cytometry for mouse and human cell content, respectively, showing that at least ∼95% of the preparation contains human cancer cells as shown. Effect of CFI-400945 and radiation in BXTO64 (C.) and BXTO81 (D.) models. Average number of organoids was normalized to that of control (no-radiation, no-drug). Averages and standard deviations (SD, bars) of duplicate experiments are presented.
Fig. S3.
Effects of In Vivo CFI-400945 Treatment and Targeted Radiation on MDA-MB-231 Xenografts. Tumor tripling time using Kaplan-Meier analysis. Tumor tripling time was calculated based on the time (in days) that a tumor took to triple in volume during treatment period. The median time to tumor volume tripling in the control arm was 8 days. After a single-dose 8 Gy dose of targeted radiation, xenograft volume tripling time increased to 11 days (p = 0.03). CFI-400945 monotherapy (administered for 30 days) increased tumor volume tripling time to 42 days (p = 0.0006). Combination treatment resulted in a significantly increased tumor volume tripling time to 61 days, compared to control, drug-only or radiation-only arms (p = 0.0002). P-values are calculated using log-rank test.
References
- 1.Rades D., Fischer D., Veninga T., Stalpers L.J., Schild S.E. Prognostic factors for survival and intracerebral control after irradiation for brain metastases from gynecological cancer. Gynecol Oncol. 2009;114:506–508. doi: 10.1016/j.ygyno.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Milano M.T., Katz A.W., Zhang H., Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 3.Onal C., Guler O.C., Yildirim B.A. Treatment outcomes of breast cancer liver metastasis treated with stereotactic body radiotherapy. Breast. 2018;42:150–156. doi: 10.1016/j.breast.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Dellas K. Does radiotherapy have curative potential in metastatic patients? The concept of local therapy in oligometastatic breast cancer. Breast Care (Basel) 2011;6:363–368. doi: 10.1159/000333115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez-Brauer C., Thu K.L., Mason J.M., Blaser H., Bray M.R., Mak T.W. Targeting mitosis in cancer: emerging strategies. Mol Cell. 2015;60:524–536. doi: 10.1016/j.molcel.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Thu K.L., Soria-Bretones I., Mak T.W., Cescon D.W. Targeting the cell cycle in breast cancer: towards the next phase. Cell Cycle. 2018;17:1871–1885. doi: 10.1080/15384101.2018.1502567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citrin D.E., Mitchell J.B. Mechanisms of normal tissue injury from irradiation. Semin Radiat Oncol. 2017;27:316–324. doi: 10.1016/j.semradonc.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho Y.H., Kim S.Y., Woo H.D., Kim Y.J., Ha S.W., Chung H.W. Delayed numerical chromosome aberrations in human fibroblasts by low dose of radiation. Int J Environ Res Publ Health. 2015;12:15162–15172. doi: 10.3390/ijerph121214979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu N., Qin Y., Fridley B.L., Hou J., Kalari K.R., Zhu M. Radiation pharmacogenomics: a genome-wide association approach to identify radiation response biomarkers using human lymphoblastoid cell lines. Genome Res. 2010;20:1482–1492. doi: 10.1101/gr.107672.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakhoum S.F., Kabeche L., Wood M.D., Laucius C.D., Qu D., Laughney A.M. Numerical chromosomal instability mediates susceptibility to radiation treatment. Nat Commun. 2015;6:5990. doi: 10.1038/ncomms6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao D., Gu P., Wang Y., Luo W., Chi H., Ge J. Inhibiting polo-like kinase 1 enhances radiosensitization via modulating DNA repair proteins in non-small-cell lung cancer. Biochem Cell Biol. 2018;96:317–325. doi: 10.1139/bcb-2017-0063. [DOI] [PubMed] [Google Scholar]
- 12.Denu R.A., Zasadil L.M., Kanugh C., Laffin J., Weaver B.A., Burkard M.E. Centrosome amplification induces high grade features and is prognostic of worse outcomes in breast cancer. BMC Canc. 2016;16:47. doi: 10.1186/s12885-016-2083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marina M., Saavedra H.I. Nek2 and Plk4: prognostic markers, drivers of breast tumorigenesis and drug resistance. Front Biosci (Landmark Ed) 2014;19:352–365. doi: 10.2741/4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason J.M., Lin D.C., Wei X., Che Y., Yao Y., Kiarash R. Functional characterization of CFI-400945, a Polo-like kinase 4 inhibitor, as a potential anticancer agent. Canc Cell. 2014;26:163–176. doi: 10.1016/j.ccr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Sampson P.B., Liu Y., Forrest B., Cumming G., Li S.W., Patel N.K. The discovery of Polo-like kinase 4 inhibitors: identification of (1R,2S).2-(3-((E).4-(((cis).2,6-dimethylmorpholino)methyl)styryl). 1H.indazol-6-yl)-5’-methoxyspiro[cyclopropane-1,3’-indolin]-2’-one (CFI-400945) as a potent, orally active antitumor agent. J Med Chem. 2015;58:147–169. [PubMed] [Google Scholar]
- 16.Kawakami M., Mustachio L.M., Zheng L., Chen Y., Rodriguez-Canales J., Mino B. Polo-like kinase 4 inhibition produces polyploidy and apoptotic death of lung cancers. Proc Natl Acad Sci U S A. 2018;115:1913–1918. doi: 10.1073/pnas.1719760115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohse I., Mason J., Cao P.M., Pintilie M., Bray M., Hedley D.W. Activity of the novel polo-like kinase 4 inhibitor CFI-400945 in pancreatic cancer patient-derived xenografts. Oncotarget. 2017;8:3064–3071. doi: 10.18632/oncotarget.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sredni S.T., Bailey A.W., Suri A., Hashizume R., He X., Louis N. Inhibition of polo-like kinase 4 (PLK4): a new therapeutic option for rhabdoid tumors and pediatric medulloblastoma. Oncotarget. 2017;8:111190–111212. doi: 10.18632/oncotarget.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veitch Z.W., Cescon D.W., Denny T., Yonemoto L.M., Fletcher G., Brokx R. Safety and tolerability of CFI-400945, a first-in-class, selective PLK4 inhibitor in advanced solid tumours: a phase 1 dose-escalation trial. Br J Canc. 2019;121:318–324. doi: 10.1038/s41416-019-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cescon D, Pezo R. CFI-400945 in patients with advanced/metastatic breast cancer. In: ClinicalTrials.gov, editor.Accessed February 25, 2021.
- 21.Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 22.Yao Y., Xu X., Yang L., Zhu J., Wan J., Shen L. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 2020;26:17–26 e6. doi: 10.1016/j.stem.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Ianevski A., He L., Aittokallio T., Tang J. SynergyFinder: a web application for analyzing drug combination dose-response matrix data. Bioinformatics. 2017;33:2413–2415. doi: 10.1093/bioinformatics/btx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs N., de Ligt J., Kopper O., Gogola E., Bounova G., Weeber F. A living Biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373–386 e10. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Rosenzweig K.E., Gomez J.E. Concurrent chemotherapy and radiation therapy for inoperable locally advanced non-small-cell lung cancer. J Clin Oncol. 2017;35:6–10. doi: 10.1200/JCO.2016.69.9678. [DOI] [PubMed] [Google Scholar]
- 26.Sharma R.A., Plummer R., Stock J.K., Greenhalgh T.A., Ataman O., Kelly S. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol. 2016;13:627–642. doi: 10.1038/nrclinonc.2016.79. [DOI] [PubMed] [Google Scholar]
- 27.Loap P., Loirat D., Berger F., Ricci F., Vincent-Salomon A., Ezzili C. Combination of olaparib and radiation therapy for triple negative breast cancer: preliminary results of the RADIOPARP phase 1 trial. Int J Radiat Oncol Biol Phys. 2021;109:436–440. doi: 10.1016/j.ijrobp.2020.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Figura N.B., Potluri T.K., Mohammadi H., Oliver D.E., Arrington J.A., Robinson T.J. CDK 4/6 inhibitors and stereotactic radiation in the management of hormone receptor positive breast cancer brain metastases. J Neuro Oncol. 2019;144:583–589. doi: 10.1007/s11060-019-03260-6. [DOI] [PubMed] [Google Scholar]
- 29.Jeong J. Local treatment in ER-positive/HER2-negative oligo-metastatic breast cancer (CLEAR) ClinicalTrials.gov; November. 2018;23 [Google Scholar]
- 30.Torres M. October 1, 2018. Radiation Therapy, Palbociclib, and Hormone Therapy in Treating Breast Cancer Patients with Bone Metastasis (ASPIRE), NCT03691493.ClinicalTrials.gov [Google Scholar]
- 31.Chandler B.C., Moubadder L., Ritter C.L., Liu M., Cameron M., Wilder-Romans K. TTK inhibition radiosensitizes basal-like breast cancer through impaired homologous recombination. J Clin Invest. 2020;130:958–973. doi: 10.1172/JCI130435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.