Abstract
Vitamin D, an important hormone with a central role in calcium and phosphate homeostasis, is required for bone and muscle development as well as preservation of musculoskeletal function. The most abundant vitamin D metabolite is 25-hydroxyvitamin D [25(OH)D], which is currently considered the best marker to evaluate overall vitamin D status. 25(OH)D is therefore the most commonly measured metabolite in clinical practice. However, several other metabolites, although not broadly measured, are useful in certain clinical situations. Vitamin D and all its metabolites are circulating in blood bound to vitamin D binding protein, (VDBP). This highly polymorphic protein is not only the major transport protein which, along with albumin, binds over 99% of the circulating vitamin D metabolites, but also participates in the transport of the 25(OH)D into the cell via a megalin/cubilin complex.
The accurate measurement of 25(OH)D has proved a difficult task. Although a reference method and standardization program are available for 25(OH)D, the other vitamin D metabolites still lack this. Interpretation of results, creation of clinical supplementation, and generation of therapeutic guidelines require not only accurate measurements of vitamin D metabolites, but also the accurate measurements of several other “molecules” related with bone metabolism.
IFCC understood this priority and a committee has been established with the task to support and continue the standardization processes of vitamin D metabolites along with other bone-related biomarkers.
In this review, we present the position of this IFCC Committee on Bone Metabolism on the latest developments concerning the measurement and standardization of vitamin D metabolites and its binding protein, as well as clinical indications for their measurement and interpretation of the results.
Keywords: Vitamin D; 25-hydroxyvitamin D; Liquid chromatography; Mass spectrometry; Immunoassays; Standardization; Vitamin D Standardization Program; Vitamin D binding protein; 1α,25-dihydroxyvitamin D; 24,25-dihydroxyvitamin D
1. Introduction
Vitamin D is an important hormone required for bone and muscle development as well as the preservation of musculoskeletal function. Due to its central role in calcium and phosphate homeostasis, it plays an important role in bone metabolism.[1] Moreover, a number of non-skeletal diseases have been associated with a vitamin D deficiency, including cancer, cardiovascular disease, diabetes, immune dysfunction, etc.[2,3] Although genetic, molecular, and animal studies suggest that vitamin D signaling has many extraskeletal effects, and observational studies in human subjects, also suggest that poor vitamin D status is associated with nearly all diseases, results of randomized controlled trials and Mendelian randomization studies are mixed. Well designed basic and clinical studies are needed with larger numbers of patients as well as well-designed randomized clinical trials, with baseline vitamin D determination and accurate monitoring to establish a cause-effect relationship between vitamin D deficiency and some diseases.[4,5]
1.1. Sources and production of vitamin D
Vitamin D is a fat-soluble secosteroid that is extensively metabolized in the human body. Over the last 40 years, its synthesis and metabolism have been elucidated and more than 50 metabolites of vitamin D have been discovered.[6-8] However, to date, researchers have been able to develop measurement procedures for only a few of them (Table 1).
Table 1.
Vitamin D compounds and metabolites.
| Vitamin D Metabolite Name | Alternative names | Chemical formula | Synthesis / Location | Units | Clinical Utility Measurement Method |
|---|---|---|---|---|---|
| Vitamin D2 | Ergocalciferol, Calciferol, Viosterol | 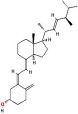 |
Form of vitamin D synthesized by yeast and fungi in the presence of UV light | – | Provides information of ingestion of vitamin D2. • Not measured routinely only in research studies • Measured by LC-MS/MS and RIA. |
| Formula: C28H44O MW: 396.6 g/mol |
|||||
| Vitamin D3 | Cholecalciferol, Calciol | 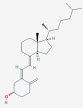 |
Produced in the skin when exposed to ultraviolet light or obtained from dietary sources | – | Provides information of synthesized in the skin and/or ingested vitamin D3 • Not measured routinely only in research studies • Measured by LC-MS/MS and RIA. |
| Formula: C27H44O MW: 384.6 g/mol |
|||||
| 25-Hydroxyvitamin D3 [25(OH)D3] | 25-Hydroxycholecalciferol, Calcidiol, Calcifediol |  |
Major circulating metabolite of vitamin D3. Produced in the liver | nmol/L ng/mL |
Its measurement is useful in combination with D2 (see below) • Immunoassays cannot distinguish D2 from D3. • Can be selectively quantitated only by HPLC and LC-MS/MS. |
| Formula: C27H44O2 MW: 400.6 g/mol |
|||||
| 25-Hydroxyvitamin D2 [25(OH)D2] | 25-Hydroxyergocalciferol, Ercalcidiol, 25-Hydroxyergocalciferol, 25-Hydroxycalciferol |  |
Major circulating metabolite of vitamin D2. Produced in the liver. | nmol/L ng/mL |
Its measurement is useful in combination with D3 (see below) • Immunoassays cannot distinguish D2 from D3. • Can be selectively quantitated only by HPLC and LC-MS/MS. |
| Formula: C28H44O2 MW: 412.6 g/mol |
|||||
| Total 25-Hydroxyvitamin D [total 25(OH)D] | – | Represents the sum of 25(OH)D2 and 25(OH)D3. | nmol/L ng/mL |
Best indicator of the body’s vitamin D stores. • Immunoassays can only quantitate total 25(OH)D. • HPLC and LC-MS/MS can separate and quantitate both D2 and D3. |
|
| 1α,25-Dihydroxyvitamin D3 [1α,25(OH)2D3] | Calcitriol, 1-Alpha,25-Dihydroxyvitamin D3, 1-Alpha, 25-Dihydroxycholecalciferol, Dihydroxyvitamin D3 |  |
Synthesis takes place in the kidney and in extrarenal tissues. Active metabolite, Endocrine, Autocrine and paracrine actions |
pmol/L pg/mL |
Measurement is useful in the investigation of • calcium disorders • calcipenic rickets/osteomalacia • differentiation between FGF23 and non-FGF23 phosphopenic rickets • Can be measured by LC-MS/MS and immunoassays. • Reduced in kidney disease |
| Formula: C27H44O3 MW: 416.6 g/mol |
|||||
| 1α,25-Dihydroxyvitamin D2 [1α,25(OH)2D2] | 1-Alpha, 25-Dihydroxyergocalciferol |  |
Synthesis takes place in the kidney and in extrarenal tissues. Active metabolite, Endocrine, Autocrine and paracrine actions |
pmo/L pg/mL |
|
| Formula: C28H44O3 MW: 428.6 g/mol |
|||||
| 24R,25-Dihydroxyvitamin D3 [24R,25(OH)2D3] | 24,25-dihydroxyvitamin D3, secalciferol, 24,25-dihydroxycholecalciferol, | 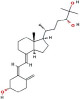 |
Produced in the kidney and is the first product in a five step catabolic process leading to either calcitroic acid or 25(OH)2D-23,26-lactone Can be subject of 1α-hydroxylation |
nmol/L ng/mL |
Measurement is useful is the investigation of 24-Hydroxylase inactivating mutations, • Can be measured by LC-MS/MS • Reduced in kidney disease |
| 24R,25-Dihydroxyvitamin D2 [24R,25(OH)2D2] | 24,25-dihydroxyergocalciferol | 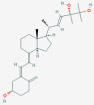 |
Produced in the kidney and is the first product in a five step catabolic process leading to either calcitroic acid or 25(OH)2D-23,26-lactone Can be subject of 1α-hydroxylation |
nmol/L ng/mL |
|
| Formula: C28H44O3 MW: 428,6 g/mol |
|||||
| C3-Epimers of vitamin D metabolites | 3-Epi-25-Hydroxy Vitamin D3; 25-Hydroxy-3-epi-vitamin D3; [C3-epi-25(OH)D3] | 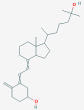 |
C3-epi-25(OH)D3 is the most prevalent epimer of 25(OH)D | ng/ml | it usually presents in measurable quantities in neonates and recently was also found in adults.it is potential interferent in the measurement of 25(OH)D.the separation and quantification is not recommended in adults however it should be considered in pediatric samples • separation and quantification can be achieved only with LC-MS/MS |
| Formula: C27H44O2 MW: 400,6 g/mol |
|||||
| 3-epi-25-Hydroxy Vitamin D2; 25-Hydroxy-3-epi-vitamin D2 [C3-epi-25(OH)D2] | 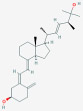 |
ng/mL | |||
| Formula: C28H44O2 MW:412.6 g/mol |
|||||
| Vitamin D binding protein | VDBP, DBP, Gc-globulin | 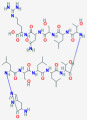 |
is primarily expressed in the liver and to a lesser extent in the kidney. serves as a carrier protein for vitamin D metabolites in the circulation |
ng/mL | its measurement is useful in the calculation of free vitamin D • Can be measured with immunoassays and LC-MS/MS • Immunoassays using polyclonal antdibodies and LC-MS/MS assays are not biased by genotype |
| Formula: C54H95N17O17 MW:~58 kDa |
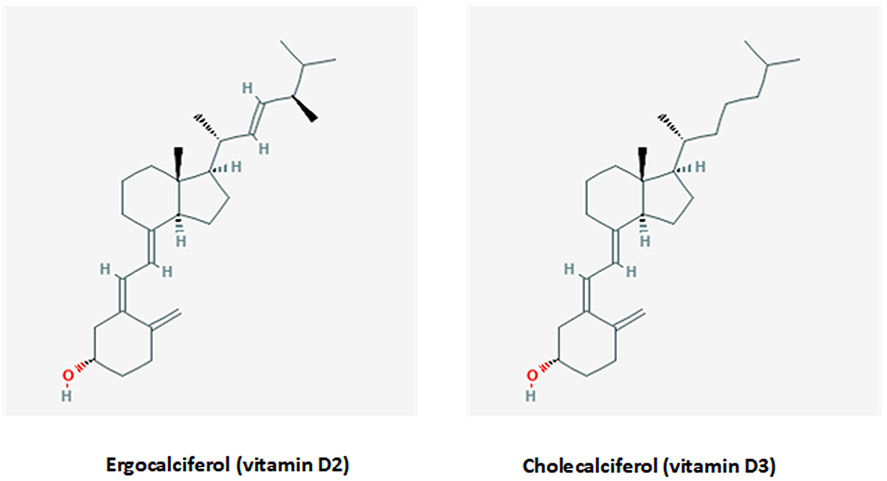
Vitamin D exists in two major forms, vitamin D2 (or ergocalciferol) and vitamin D3 (or cholecalciferol), which exhibit only minor differences in their structure. (Fig. 1). As a consequence, vitamin D2 and D3 have different molecular weights of 396.65 g/mol and 384.64 g/mol, respectively.[9] These differences in the chemical structure of vitamin D2 contribute to its lower affinity for vitamin D binding protein (VDBP), thus resulting in faster clearance from blood, a limited conversion to 25 hydroxyvitamin D [25(OH)D], and an altered catabolism by 24-hydroxyase (CYP24A1).[10-12] A recent meta-analysis found that vitamin D3 is more potent at raising serum 25(OH)D concentrations than is vitamin D2. Hence, vitamin D3 could potentially become the preferred choice for supplementation.[13]
Fig. 1.
Two forms of vitamin D: ergocalciferol (left) and cholecalciferol (right). The chemical structures are taken from PubChem (https://pubchem.ncbi.nlm.nih.gov).
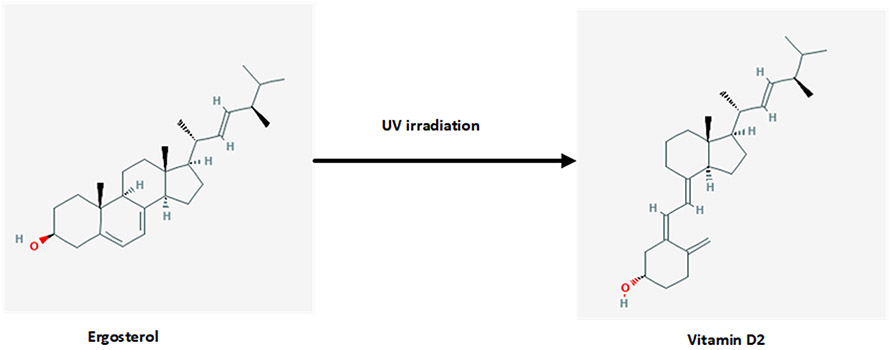
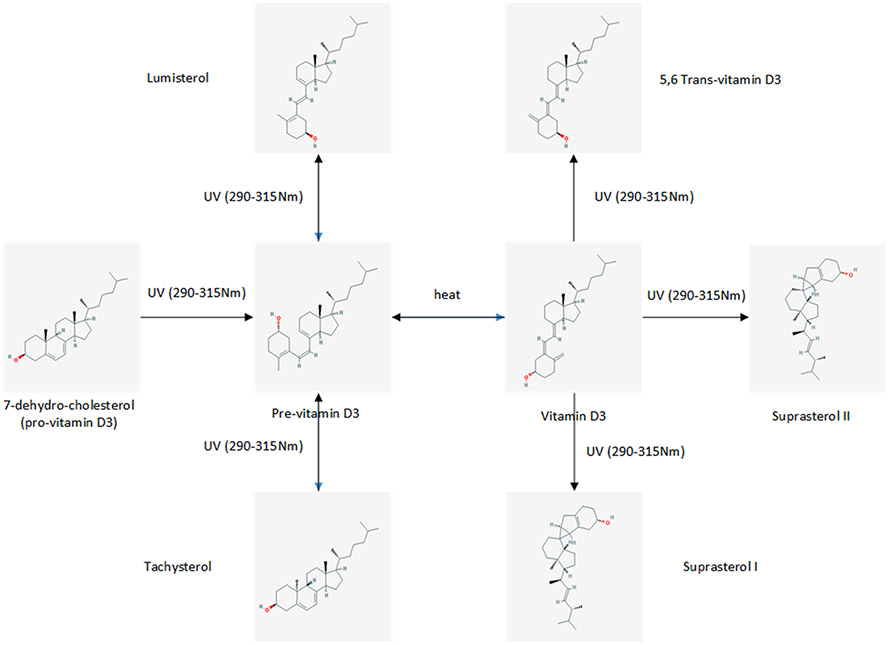
Vitamin D3 is synthesized from 7-dehydrocholesterol (7-DHC) in the skin by UVB radiation while vitamin D2 is derived from plant/yeast by irradiation of ergosterol (Figs. 2 and 3).[14,15] In humans the main sources of vitamin D (e.g., D2 and/or D3), are sunlight, diet, and supplements. However, most foods (except for fatty fish) contain low levels of vitamin D unless fortified (Table 2). Exposure of human skin to solar UVB radiation (wavelengths 290–315 nm) leads to the conversion of 7-DHC to pre-vitamin D (pre-D) in the skin, which isomerizes to D3 in a non-catalytic, thermo-sensitive process.[16] Vitamin D3 production depends on the intensity of UV irradiation, which varies with season, latitude and altitude.[17] Skin pigmentation, sunscreen use, and clothing have been reported to affect the conversion of 7-DHC to vitamin D3.[18-20] Melanin in the skin blocks UVB from converting 7-DHC, thus limiting D3 production, as does extensive covering of the body with clothes and the use of sun-screen. A recent meta-analysis concluded that pigmented skin has less effective photoproduction of vitamin D and 25(OH)D. The quantity of sun exposure needed for dark-skinned, compared with light-skinned, people to achieve vitamin D sufficiency however remains uncertain.[21] However this view has been debated lately; Bogh et al., in an elegant study show that baseline vitamin D levels and total cholesterol levels are more important factors than skin pigmentation.[22,23]
Fig. 2.
Production of vitamin D2 from ergosterol. Ultraviolet (UV) radiation in the 290–315 nm wavelength range cleaves the B ring of ergosterol, yielding ergocalciferol. The irradiation of milk and yeast is a commercial means of producing D2 from ergosterol. Dihydrotachysterol (DHT) is a synthetic analog of vitamin D2. The chemical structures are taken from PubChem (https://pubchem.ncbi.nlm.nih.gov).
Fig. 3.
When the skin is exposed to UV radiation in the 290–315 nm wavelength range, 7-dehydrocholesterol absorbs this energy, which causes chemical bonds within the molecule to break and rearrange, resulting in the formation of pre-vitamin D. In the skin, pre-vitamin D undergoes rapid, thermally-induced, isomerization to produce vitamin D. Once formed, pre-vitamin D and vitamin D continue to absorb UV. Prolonged exposure to UV radiation results in the breakdown of these molecules into biologically inactive photoproducts. For this reason, during prolonged irradiation, a steady state is reached when only 10–15% of 7-dehydrocholesterol is simultaneously converted to pre-vitamin D3. This ensures that no toxic levels of vitamin D are synthesized under excessive sun exposure conditions. (The chemical structures are taken from PubChem: https://pubchem.ncbi.nlm.nih.gov).
Table 2.
| Source | Vitamin D content |
|---|---|
| Natural Sources | |
| Cod liver oil | ~400–100 IU vitamin D3 / teaspoon |
| Egg yolk | ~20 IU vitamin D2 or D3 / yolk |
| Mackerel canned | ~ 250 IU vitamin D3 /100 gr |
| Salmon fresh farmed | ~ 150–250 IU vitamin D3 /100 gr |
| Salmon canned | ~ 300–600 IU vitamin D3 /100 gr |
| Sardines canned | ~ 300 IU vitamin D3 / 100 gr |
| Tunafish canned | ~ 235 IU vitamin D3 / 100 gr |
| mushrooms fresh | ~ 100 IU vitamin D2 / 100 gr |
| mushrooms dried | ~ 1600 IU vitamin D2 / 100gr |
| sunlight/ exposure to UVB radiation | variable D3 depending on several factors |
| Fortified food | |
| Cereals | ~ 100 IU vitamin D3 / per serving |
| Butter | ~56 IU vitamin D3 / 100 gr |
| Cheese - milk - yogurt | ~ 100 IU vitamin D3 / 250 gr |
| Margarine | ~ 400 IU vitamin D3 / 100 gr |
| Infant milk-formula | ~ 100 IU vitamin D3 / 250 gr |
| Juices | ~ 100 IU vitamin D3 / 250 gr |
| Supplements | |
| Multivitamins | from 400 to 1000 IU |
| Vitamin D3 supplements | from 400 to 50000 IU |
1.2. Vitamin D metabolism
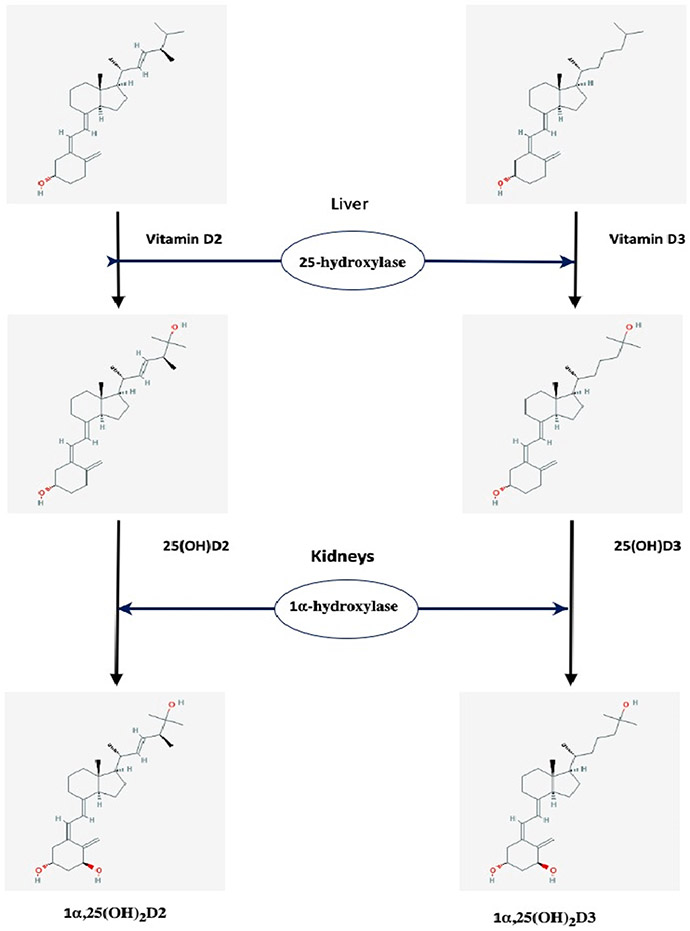
Vitamin D synthesized in the skin diffuses into the bloodstream where it is transported by vitamin D binding protein (VDBP) to the liver. Vitamin D from the diet is absorbed in the small intestine, incorporated into chylomicrons, which are released into the lymphatic system, and enters the venous blood where it binds to VDBP and lipoproteins before being transported to the liver. Vitamin D is essentially biologically inactive and must be converted to hydroxylated metabolites to gain hormonal activity. Its activation involves two hydroxylation steps (Fig. 4).[24]
Fig. 4.
The two steps of vitamin D activation. (The chemical structures are taken from PubChem: https://pubchem.ncbi.nlm.nih.gov.
The first step occurs predominantly in the liver where vitamin D is hydroxylated at the C25 position by the cytochrome p450 enzyme CYP2R1 (also called 25-hydroxylase) to 25-hydroxyvitamin D [25(OH) D]. There are several other CYP enzymes that are capable of 25-hydroxylation, namely CYP27A1, CYP3A4, CYP2D25, and perhaps others, but the CYP2R1 is emerging as the most critical enzyme for 25-hydroxylation.[25-27] This step is poorly regulated by any feedback mechanism in the context of the vitamin D endocrine system and it seems to be dependent primarily on the concentration of vitamin D [28]. Hence, 25(OH)D levels increase in proportion to vitamin D intake and, plasma 25(OH)D levels are a good indicator of vitamin D status. The 25(OH)D produced in the liver is returned to circulation. Severe liver failure affects the function of the CYP2R1 enzyme. Moreover, loss-of-function mutations for the same enzyme are responsible for vitamin D-dependent rickets (VDDR), type 1B (VDDR-1B) [29] as shown in Table 3.
Table 3.
Calcipenic Rickets due to nutritional cause or mutations and genetic disorders of vitamin D action, modified from Ref:[330] Abbreviations are as follows: 1α,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, cholecalciferol; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; ALP, alkaline phosphatase; Ca, serum levels of calcium; FGF23, fibroblast growth factor 23; N, not applicable; PO4, serum levels of phosphate; PTH, parathyroid hormone; TmP/GFR, maximum rate of renal tubular reabsorption of phosphate per glomerular filtration rate; U-Ca, urinary calcium excretion; U-PO4, urinary phosphate excretion; VDR, vitamin D receptor.
| Disorder | Gene | Ca | PO4 | ALP | U- Ca |
U- PO4 |
TmP/ GFR |
FGF23 | PTH | 25 (OH)D |
1α,25 (OH)2D |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcipenic Rickets | ||||||||||||
| Nutritional rickets | N, ↓ | N, ↓ | ↑↑↑ | ↓ | Varies | ↓ | N | ↑↑↑ | N, ↓↓ | Varies | Vitamin D deficiency | |
| Vitamin D dependent rickets 1A (VDDR1A) |
CYP27B1 (12q14.1) |
↓ | N, ↓ | ↑↑↑ | ↓ | Varies | ↓ | N, ↓ | ↑↑↑ | N | ↓ | Impaired synthesis of 1α,25(OH)2D |
| Vitamin D dependent rickets 1B (VDDR1B) |
CYP2R1 (11p15.2) |
↓ | N, ↓ | ↑↑↑ | ↓ | Varies | ↓ | N | ↑↑↑ | ↓↓ | Varies | Impaired synthesis of 25(OH)D |
| Vitamin D dependent rickets 2A (VDDR2A |
VDR (12q13.11) |
↓ | N, ↓ | ↑↑↑ | ↓ | Varies | ↓ | N, ↓ | ↑↑↑ | N | ↑↑ | Impaired VDR signaling |
| Vitamin D dependent rickets 2B (VDDR2B) |
HNRNPC (14q11.2) |
↓ | N, ↓ | ↑↑↑ | ↓ | Varies | ↓ | N | ↑↑↑ | N | ↑↑ | Impaired VDR signaling |
| Vitamin D dependent rickets 3 (VDDR3) |
CYP3A4 (7q12.11) |
↓ | ↓ | ↑↑↑ | ↓ | Varies | ↓ | ?? | ↑↑↑ | ↓ | ↓ | overexpression / increased inactivation of 1α,25(OH)2D |
The second step in vitamin D activation is the formation of 1α,25-dihydroxy vitamin D [or calcitriol, 1α,25(OH)2D]. It occurs, under physiological conditions, mainly in the kidney by another CYP450 enzyme, CYP27B1 or 1α-hydroxylase. This second hydroxylation takes place at the carbon in the C1 position. Calcitriol binds again to VBDP and re-enters the systemic circulation. It is now recognized that 1α-hydroxylase is expressed in many other extrarenal tissues, including skin, brain, and colon and serves as an autocrine/paracrine factor with cell specific functions (Table 4 and Fig. 5).[9,30-34].
Table 4.
Tissues and cells outside kidney expressing 1α-hydroxylase (CYP27b1).
| epithelia | placenta | bone | immune system | endocrine glands | other |
|---|---|---|---|---|---|
| Epidermis and hair follicles (keratinocytes) | Decidua | Osteoblasts | Macrophages | Parathyroid gland | brain |
| Prostate | Trophoblasts | Osteoclasts | Monocytes | Pancreatic islets | liver |
| Colon epithelium | Osteocytes | Dendritic cells | Thyroid gland | endothelia | |
| Mammary epithelium | Chondrocytes | T cells | Adrenal medulla | ||
| Uterus (endometrium) | B cells | Testes | |||
| Corneal epithelium | Ovary | ||||
| Retinal pigment epithelium | |||||
| Ciliary body epithelium |
Fig. 5.
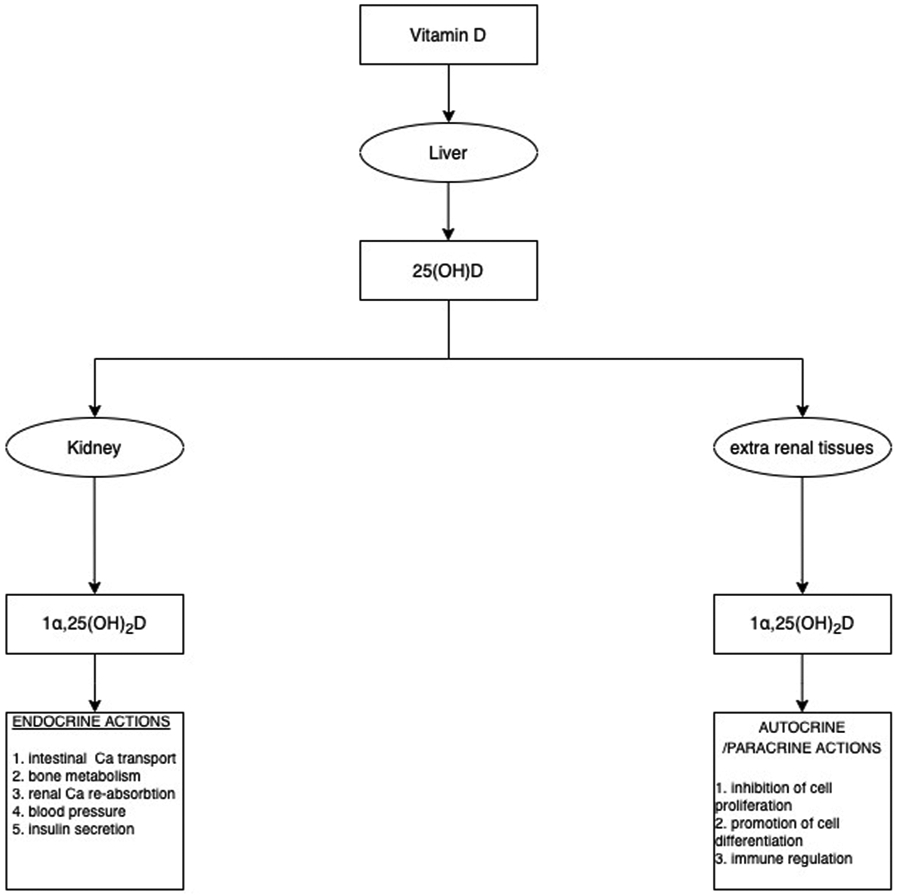
Renal and extra renal calcitriol production serves an endocrine, autocrine, and paracrine function (original figure.
The activity of renal 1α-hydroxylase is tightly controlled by 1α,25(OH)2D itself, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23) and serum concentrations of calcium (Ca) and phosphate (). Under normal conditions, FGF23 acts on kidney proximal tubular cells regulating phosphate excretion in order to maintain systemic phosphate homeostasis. However, FGF23 can also influence the synthesis of calcitriol in the proximal tubular cells by suppressing the expression of 1α-hydroxylase and increasing the expression of 24-hydroxylase.[30,35] The activity of extrarenal 1α-hydroxylase is not regulated by the same factors controlling its renal synthesis.[30] The kidney is the major source for circulating 1α,25(OH)2D. Only in certain granulomatous diseases such as sarcoidosis does the extrarenal tissue produces sufficient 1,25(OH)2D to contribute to the circulating levels, which is generally associated with hypercalcemia.[36] Inactivating mutations of this enzyme are responsible for vitamin D-dependent rickets (VDDR) type 1A [VDDR-1A] [28,32,33,37] as shown in Table 3.
1.3. Catabolism
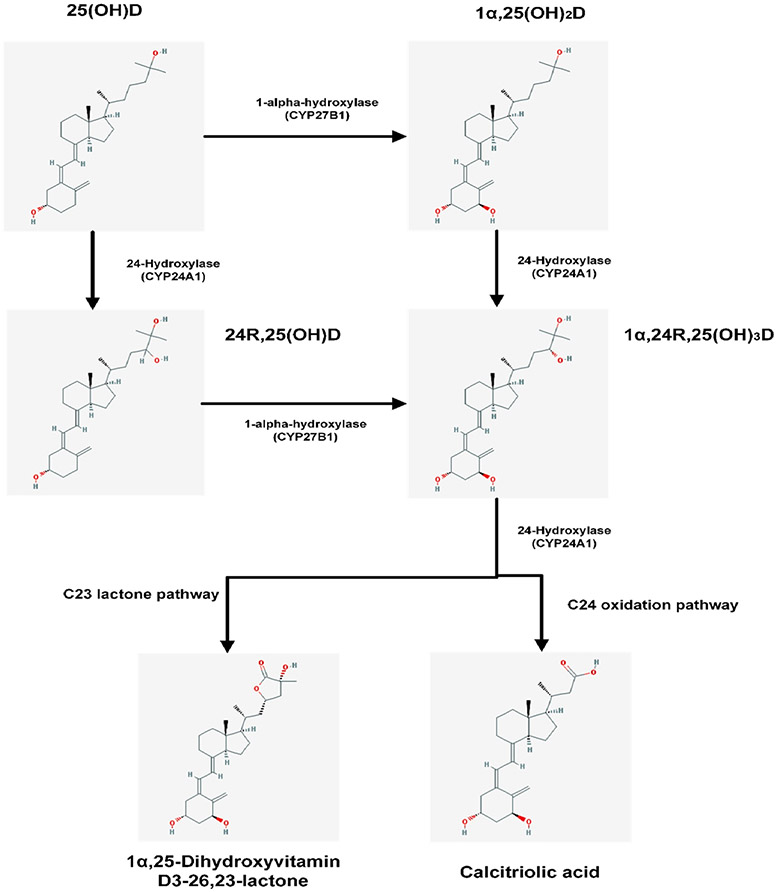
To retain calcitriol levels within the strict boundaries required for appropriate calcium homeostasis and bone metabolism, both 1α,25(OH)2D and 25(OH)D may undergo further hydroxylation by renal CYP24A1 (24-hydroxylase), leading to 1,24,25-trihydroxyvitamin D [1,24,25(OH)3D] and 24R,25-dihydroxyvitamin D [24,25(OH)2D], respectively (Fig. 6). Thus the main function of 24-hydroxylase is vitamin D inactivation, since [1] it limits the amount of 1α,25(OH)2D3 in target tissues both by accelerating its catabolism to 1,24,25(OH)3D3 and ultimately in calcitroic acid or [2] by producing 24,25(OH)2D3 and thus decreasing the pool of 25(OH)D3 available for 1 hydroxylation.[38]
Fig. 6.
Schematic representation of catabolic pathways of vitamin D: CYP24A1 catalyzes the C24-oxidation pathway that leads to 1α,25(OH)2D degradation that ultimately limits the amount of calcitriol in target tissues by accelerating its catabolism. This pathway comprises of 5 enzymatic steps that lead to the production of calcitroic acid that is excreted in bile. When the initial substrate is 25(OH)D the end product is again calcitroic acid.[332,333] CYP24A1 can also catalyze the C23-oxidation pathway which creates the biologically active 1α,25(OH)2D-26,23 lactone from 1α,25(OH)2D, and 25(OH)D-26,23 lactone from 25(OH)D.[7,33,333] The biological activity of the C23-oxidation pathway is not clear however there have been claims that the 1α,25(OH)2D derived end product, 1α,25(OH)2D-26,23 lactone, may act as a VDR antagonist.[334,335] (The chemical structures are taken from PubChem: https://pubchem.ncbi.nlm.nih.gov).
CYP24A1 has been found in many tissues that express the vitamin D receptor. In the kidney, it is found in the proximal and distal tubules. [39,40] The CYP24A1 gene is highly inducible by 1α,25(OH)2D in all tissues in which it is found and it acts as a control mechanism to prevent intoxication from 1α,25(OH)2D. [41] The importance of this feedback mechanism was demonstrated when inactivating mutations of CYP24A1 reported in children and adults with hypercalcemia.[29,42]
Another enzyme, CYP3A4, also plays a role in vitamin D catabolism. [43] This enzyme is involved in drug metabolism, and is located in the liver and the intestine. Recently, a gain-of-function mutation in CYP3A4 was described that leads to rickets with decreased serum calcium and phosphate and elevated PTH and alkaline phosphatase (Table 3).[44] This is a distinct form of vitamin D dependent rickets (named type 3 vitamin D-dependent rickets or VDDR3) since it does not involve a defect in synthesis of vitamin D metabolites but rather is due to accelerated inactivation of vitamin D metabolites as CYP3A4 was found to inactivate both 25(OH)D3 and 1,25(OH)2D, leading to vitamin D deficiency through accelerated vitamin D metabolite inactivation (Table 3). [24,45] It is well known that CYP3A4 is induced by certain drugs, such as rifampicin.[46,47] Thus, the induction of CYP3A4 gene expression by certain drugs may enhance 25OHD and 1α,25(OH)2D3 catabolism.[43] and hence modulate vitamin D effects in the body and could present as an alternative therapeutic strategy to reduce serum levels of vitamin D metabolites in cases of patients with inactivating mutations of CYP24A1. [48]
2. Measurement of vitamin D metabolites
Today, more than 50 vitamin D metabolites have been described and characterized, with some of them exhibiting biological activity [6]. However, methods for measurement have only been developed for five of them (vitamin D, 25(OH)D2 and 25(OH)D3, 1α,25(OH)2D, 24R,25(OH)2D, and C3-epi-25(OH)D) as shown in Table 1. These metabolites are present in serum at concentrations that allow for their measurement with these methods.[49]
The above metabolites differ significantly in their biological activity. For example, 1α,25(OH)2D is five times more potent than vitamin D in its ability to regulate calcium allowing for its extraction from the intestines and mobilization in bones. [50] A significant factor that determines the biological activity of a metabolite is its affinity to the VDR. Experimental studies have shown that 1α,25(OH)2D exhibits the highest affinity to the VDR among all vitamin D metabolites [51], while the affinity of the rest of the metabolites is significantly lower. For instance, 25OHD and 24,25(OH)2D exhibit approximately 900 and 5000 times lower affinity to the VDR, respectively, compared to that of 1α,25(OH)2D.[52]
2.1. Pre-analytical considerations
Five types of pre-analytical variability will be examined in detail in this section:
sample collection and handling
factors that relate to the individual (i.e., age, sex, ethnicity, and lifestyle)
environmental factors
disease factors and pregnancy
genetic factors
2.2. Sample collection and handling
Sample types and collection tubes:
Serum and plasma (heparin and EDTA) can be used for the measurement of vitamin D and its metabolites.[53] Serum is the preferred matrix since it has the advantage of being free of anticoagulants used for plasma collection such as EDTA, heparin, or citrate, which may interfere with their measurement, especially in immunoassays. However even when serum is used, significant interferences can be observed with certain commercial assays, and even with High Performance Liquid Chromatography (HPLC) and Liquid Chromatography tandem Mass Spectrometry (LC-MS/MS) methods when tubes with serum clot activator and/or gel are used.[54-57] However, this type of interference does not seem to apply to all commercial assays and methods as well as all commercial collection tubes. [58] Vitamin D metabolites have been also detected and measured in several body fluids and tissues including breast milk, urine, semen, cerebrospinal fluid, synovial fluid, hair and in skin and muscle biopsies. However, these matrices may require specific preanalytical protocols which are not standardized. Moreover, standardization of analytical techniques and External Quality Assurance Schemes for the measurement of vitamin D metabolites in these matrices are lacking.[59] Saliva has also been explored in several studies and with assays based on different principles with often inconsistent results. More recently, the technological advances in LC-MS/MS have made it possible to measure 25(OH)D in dried blood spots (DBSs).[60] The utility of the measurement of vitamin D metabolites in these matrices is limited to research setting.[59]
Sample stability:
The stability of Vitamin D metabolites in clinical samples is a key aspect for a reliable assessment of the results from epidemiological studies where the samples usually are not tested on the day of collection. The majority of studies evaluating the influence of storage conditions only studied 25(OH)D while the other metabolites have been ignored or insufficiently studied.[53,61-67] These studies have shown that 25(OH)D is stable in plasma and serum when samples are stored at room temperature (24 °C), 4 °C, or frozen, as long as metabolites are not separated from their binding protein. There are studies that claim that 25(OH)D can be stored without any significant loss in concentration for several days at room temperature and for up to 3 years at −20 °C. In addition, no special precautions are necessary during the transport of samples to the laboratory. In stored samples, repeated cycles of freeze–thaw don’t seem to have any significant effect on 25(OH)D levels.[68] Attention is only needed when the samples have been already pre-treated and vitamin D has been separated from its binding protein. Then, samples should be kept in dark vials to avoid exposure to light and should be stored at < −70 °C. [53,61,69,70] One study that examined the stability of 1α,25(OH)2D and 24,25(OH)2D concluded that these two metabolites exhibit a lower stability in comparison to 25(OH) D upon storage, with significantly decreased levels after 3 freeze–thaw cycles.[66] We must note here that these stability studies present several limitations (i.e., a limited number of specimens examined, chosen time intervals for storage, and lack of uniform definition of instability).
2.3. Environmental factors
Effect of season on 25(OH)D levels:
UVB sunlight exposure, rather than diet, has been reported as the main source of 25(OH)D for majority of the population.[71] Therefore, levels of vitamin D are directly dependent upon exposure to UVB irradiation from the sun. Several environmental factors such as latitude, altitude, season, and prevailing weather conditions determine whether sunlight of a sufficient strength is available to stimulate the conversion of 7-DHC in the skin to cholecalciferol (vitamin D3). This results in a 25(OH)D seasonal variation and an effect based on the geographical location where the person lives (distance from equator and altitude).[72,73] Generally, people that live in the northern hemisphere present the highest levels of 25(OH)D during the summer and autumn with lower levels during winter and spring.[74-77]
2.4. Factors that relate to the individual
Age, sex, body fat, and lifestyle do have a, often small, effect on 25(OH)D levels.[78]
Age:
It is known that age affects calcium and vitamin D metabolism. [1.] Calcium absorption is reduced with age [2.] Intestinal resistance of calcium absorption to circulating 1,25(OH)2D increases with age. [3.] The ability of the older skin to produce vitamin D is reduced [4.] VDR expression is also reduced with age. [5.] The ageing kidneys are less able to produce 1α,25(OH)2D compared to younger kidneys. [6.] Substrate deficiency of vitamin D increases with age.[79-82] Finally, older people are more home-bound and therefore less exposed to sunshine and to outdoors activities compared to younger people.[83] Recent studies, however, have shown that the effect of age on 25(OH)D levels is small. [75,84] These studies included only subjects less than 75 years of age, which might explain the lack of association between 25(OH)D levels and age.
Body mass index (BMI).
There is a consistent association in literature between increasing BMI and lower serum 25(OH)D concentrations. Several studies have reported an association between obesity (BMI greater than 30) and low serum 25(OH)D, 1α,25(OH)2D concentrations, and high PTH concentrations.[85-88]
Adipose tissue might play a role in the low vitamin D levels observed in people with obesity.[89-91] However, this relationship between obesity and low 25(OH)D levels, has not been elucidated completely. Different mechanisms have been proposed to explain this inverse association using behavioral factors such as a reduced exposure to sunlight due to less outdoor physical activity and a low dietary intake of vitamin D enriched food.[92,93] Moreover, decreased intestinal absorption, impaired hydroxylation in adipose tissue, and 25(OH)D accumulation in fat have been proposed to explain the hypovitaminosis in obesity.[91] The fact that vitamin D is a fat soluble molecule led to the hypothesis that vitamin D is sequestered in body fat depots, resulting in a lower bioavailability in the obese state.[91,94] On the other hand, some studies have speculated that vitamin D deficiency itself could cause obesity or even prevent weight loss.[91,94] Despite the well-established association between obesity and vitamin D deficiency, few experimental studies have investigated the biological bases involved in vitamin D metabolism in adipose tissue, with those studies that have investigated this demonstrating inconsistent results. [95]
Sex.
Some studies have shown that men have higher levels of 25(OH) D, which are independent of age, season, and race.[96-100] This could be explained by the fact that women have relatively more body fat than men and store more fat in the gluteal-femoral region, while men typically store more fat in the visceral (abdominal) depot.[101] On average, men have 10–15% less fat content than women with the same BMI, thus having a smaller reservoir to sequestrate vitamin D.[102-104] These differences in body fat amongst genders might be an explanation for the difference between men and women in 25(OH)D concentrations. However, these sex differences do not seem to be universal, as in several large studies, women showed either no significant differences or had higher levels of vitamin D compared to men.[84,105-112]
Lifestyle.
Depending on the time of the day, duration of exposure, season, latitude, and skin pigmentation, daily exposure of the skin to sunlight (i.e. arms or legs for 5–30 min) can promote adequate endogenous synthesis of vitamin D3.[113] Outdoors activities allowing for more exposure to sunlight,[114-118] and dressing habits (e.g., coverage of the body and even the type of clothes), affect 25(OH)D levels.[119-121] Sunscreen use seems to have less effect on 25(OH)D levels than earlier thought, although high SPF sunscreens have not been studied. [122]
Ethnicity.
Although most studies are conducted in subjects of European descent, there are studies that have shown that the levels of 25(OH)D differ according to ethnicity and skin color. This seems logical since a darker skin color protects from exposure to UV irradiation and increases the risk of vitamin D deficiency.[18,123] Vitamin D synthesis is highly dependent on the concentration of melanin in the skin as melanin absorbs and takes care of ultraviolet radiation (UVR), resulting in a less efficient conversion of 7-DHC to provitamin D3.[124-127] Therefore, darker-skinned individuals will experience a slower vitamin D synthesis than lighter-skinned individuals. This is more obvious and important at higher latitudes where the intensity and duration of sunlight is limited. Metabolic differences based on race/ethnicity may provide an additional explanation.[128]
2.5. Effect of disease and pregnancy
Effect of liver and kidney disease:
The liver and the kidneys are the two most important organs involved in the metabolism of vitamin D.
The liver is the organ where 25-hydroxylation of vitamin D occurs and the majority of VDBP is synthesized.[129-132] In patients with liver disease, the prevalence of insufficiency and deficiency ranges between 64 and 92%, which is much higher than in the general population. Serum 25(OH)D is inversely related to the severity of liver disease.[133-135] The high prevalence of vitamin D deficiency in this populations occurs regardless of the etiology of liver disease.[136]
Synthetic liver dysfunction is not entirely responsible, as vitamin D deficiency is still highly prevalent in those with non-cirrhotic liver disease.[133] 25(OH)D levels “normalize” after oral or parenteral administration of vitamin D in patients with cirrhosis, indicating that 25-hydroxylation is preserved in this patient population.[137] A recent study showed that in patients with liver disease, 25-hydroxylase activity, although low compared to subjects without liver disease, was relatively well-preserved and did not affect serum 25(OH)D concentrations.[138]
Low vitamin D levels in chronic liver disease (CLD) may result from a variety of reasons and mechanisms including: [1] reduced sun exposure and dietary intake, [2] intestinal malabsorption of dietary vitamin D, [3] reduced endogenous production of VDBP and albumin in the liver, which are both impaired in CLD and in the presence of cirrhosis, [4] decreased hepatic hydroxylation of vitamin D to 25(OH)D and finally [5] increased catabolic removal of 25(OH)D.[139,140] Hence, when catabolism is increased, there will be less 25(OH)D available for production of the active hormone.[139] Low total 25(OH)D levels do not seem to disrupt its biological activity as long as unbound vitamin D levels are maintained within a normal range.[141]
As VDBP has a single sterol-binding site and only 5% of the total circulating VDBP is actually bound to a vitamin D metabolite at any time [142], liver function would have to be severely impaired in order for low VDBP levels to have a significant role in 25(OH)D deficiency in CLD. [143] However, although total 25(OH)D levels decrease as the severity of CLD increases, PTH levels are not associated with total 25(OH)D levels.[144] Patients with end stage liver disease and low total 25(OH)D levels maintain a normal serum corrected calcium concentration and do not develop secondary hyperparathyroidism.[145]
The kidneys are essential not only for the conversion of 25(OH)D to 1α,25(OH)2D, but also for the re-absorption of 25(OH)D from renal ultra filtrate for its recycling into circulation. Normal renal function is also essential to maintain the endocrine actions of calcitriol, which by itself contributes to maintaining the VDR in target tissues since it protects the receptor from degradation by binding.[146]
In chronic kidney disease (CKD), less 1α,25(OH)2D is produced. The mechanisms involved in the reduced calcitriol production during the course of CKD have been discussed in detail elsewhere. [147] Impaired uptake of 25(OH)D by the kidneys seems to be the main cause of 1α,25(OH)2D deficiency.[148] Decreased kidney function and calcitriol deficiency lead to hypocalcemia and are key contributors to secondary hyperparathyroidism (SHPT).[148]
This is more obvious among patients with end-stage renal disease where 1α,25(OH)2D is almost undetectable. CKD is also characterized by low serum 25(OH)D levels. The main causes and risk factors for vitamin D deficiency among CKD patients have also been discussed in detail elsewhere.[148] The 25(OH)D levels for CKD patients are suggested to be progressively low as renal function deteriorates. However, not all studies show that 25(OH)D insufficiency or deficiency in CKD patients is greater than in the general population.[149,150] For CKD patients, vitamin D deficiency is a strong predictor of accelerated renal disease and death. In addition, since the kidney is not the only site of calcitriol production, the maintenance of sufficient 25(OH)D levels could be a possible objective.[151] However, the best treatment approach and the best biomarker for follow-up in CKD patients is still a debate.[152,153]
Kidney disease also disrupts vitamin D catabolism. Within the kidneys, 1α-hydroxylase and 24-hydroxylase are under hormonal regulation of FGF23 and PTH. FGF23 is responsible for the reduced expression of 1α-hydroxylase in renal tubular cells and induces the expression of 24-hydroxylase, which is responsible for the catabolism of vitamin D. PTH seems to increase the expression of 1α-hydroxylase in renal tubular cells.[40,154]. CKD is characterized by high levels of FGF23 and phosphorus. These increased phosphate levels have been correlated with low concentrations of 1α,25(OH)2D, but it is not clear whether this correlation is direct, induced by FGF23, or confounded with other factors. [40] Moreover, other metabolic disturbances that are observed in patients with CKD such as diabetes, metabolic acidosis, and uremia are able to reduce the expression of CYP27B1.[155-157] In the end, this results in the reduced production of 1α,25(OH)2D in patients with CKD. Also, 24R,25(OH)2D seems to be lower in patients with CKD compared to healthy subjects.[158] The net effect of FGF23 and PTH on vitamin D catabolism in CKD is however still debated.
Systemic inflammatory response (SIR):
As vitamin D is often linked to acute and chronic inflammatory disease it is important to realize that 25(OH)D can act as a negative acute phase reactant.[159] This was clearly shown in a study by Waldron et al, where 25(OH)D concentrations decreased after an elective orthopedic surgery leading to a systemic inflammatory response with increased CRP levels. Also VDBP decreases after SIR however cannot explain all of the decrease in 25(OH)D.
Pregnancy:
Special attention must be given to pregnancy since several studies report low levels of 25(OH)D in pregnant women. In a recent meta-analysis, it was reported that 54% of pregnant women had levels of vitamin D below 50 nmol/L.[160] Moreover, several studies have suggested that low levels of 25(OH)D during pregnancy are associated with an increased risk of pre-eclampsia, gestational diabetes, and other pregnancy complications.[161-164] However, the results from these studies that relate low levels of 25(OH)D during pregnancy with adverse outcomes are conflicting.[165-167] These conflicting results are not only due to possible methodological problems related to the study design, but also with the methods used for 25(OH)D quantitation. In pregnancy, VDBP is known to be increased and when 25(OH)D is measured with an immunoassay, its levels can be underestimated due to incomplete dissociation of 25(OH)D from its binding protein. On the other hand, when a HPLC or a LC-MS/MS method is used, the dissociation of 25(OH)D from its binding protein is more complete due to the use of strong chemical solvents during sample preparation.[168-170] These analytical problems cause significant assay variation and the results from meta-analyses may be subject to error, especially when results are included from studies based on certain immunoassay measurements or from unstandardized assays.
2.6. Genetic factors
Gene-environment interactions that could have an impact on various vitamin D-related disorders have recently drawn the attention of several researchers.[171,172] For example, it has been suggested that hypovitaminosis D occurs in the presence of specific gene variations related to vitamin D metabolism. Therefore, individuals with specific vitamin D-related genotypes may require specific personalized advice to optimize their vitamin D status. Data from twin and family-based studies have demonstrated that circulating vitamin D concentrations can be partially determined by genetic factors.[173,174] Moreover, it has been shown that genetic variants (e.g., mutation) and alterations (e.g., deletion, amplification, and inversion) in genes involved in the metabolism, catabolism, transport, or even binding of vitamin D to its receptor might have an effect on vitamin D levels.[175] However, the underlying genetic determinants of 25(OH)D plasma levels have not been fully elucidated. Furthermore, the association between epigenetic modifications such as DNA methylation and vitamin D levels have now been reported in several studies.[175]
Linkage studies, studies involving candidate genes in the vitamin D metabolism pathway, as well as genome wide association studies (GWAS) have shown human genetic variants to be related to vitamin D status.
Single nucleotide polymorphisms:
Candidate gene studies and GWAS have shown that certain gene single nucleotide polymorphisms (SNP) involved in vitamin D metabolism pathways (e.g., CYP2R1, CYP27B1, CYP24A1, DHCR7, the VDR, and GC) have an effect on vitamin D levels as shown in Ref [175]. Vitamin D binding protein (VDBP) is discussed in detail further down in this article, but, briefly, VDBP has two common SNPs (rs7041 and rs4588), which results in three VDBP isotypes (Gc1f, Gc1s, and Gc2). These isotypes show different binding affinity constants to 25(OH)D. This means that persons with different SNPs have different total 25(OH)D concentrations while they might have the same concentration of free 25(OH)D. These polymorphisms are distributed differently based on ethnicity as shown in several studies and might affect the way we interpret the total 25(OH)D concentration.[176] The effect of these SNPs on the levels of circulating 25(OH)D only account for 5% of its variability and is considered small compared to other environmental factors that have a more significant effect on circulating 25(OH)D levels.[32,177,178]. Therefore, their presence does not seem to have significant clinical value in everyday practice if we consider that most laboratory assays present an analytical variability of approximately 10%.
3. The measurement of 25(OH)D
3.1. Clinical relevance
The measurement of 25(OH)D is performed mainly for two reasons: [1] to determine the nutritional status of vitamin D, and [2] to monitor the efficacy of supplementation. As previously mentioned, vitamin D exists in two different forms and in order to adequately monitor therapy, both types of vitamin D need to be accurately quantitated. In fact, the accurate measurement of 25(OH)D for the assessment of vitamin D status has always been a major goal of all clinical laboratories involved in the measurement of vitamin D metabolites. 25(OH)D is the metabolite of choice to determine vitamin D status for several reasons:
25(OH)D levels in the blood are higher than those of any other vitamin D metabolite. The serum concentration of 25(OH)D is in the range of 25–200 nmol/L, which is 1000 times higher than that of 1α,25(OH)2D, whose concentration is in the range of 50–150 pmol/L. Majority of 25(OH)D is found in the systemic circulation, with limited distribution in less accessible tissues (e.g., fat) [179].
It is well accepted that adequate levels of vitamin D are required to prevent nutritional rickets and osteomalacia, both of which are characterized by low levels of 25(OH)D as shown in Table 3. [9]
Several clinical studies have demonstrated that there is an association between serum levels of 25(OH)D and several clinical outcomes such as bone mineralization, fracture risk, fall risk, cancer, diabetes, and cardiovascular events. Meta-analyses and randomized control trials demonstrated a positive dose–response relationship between vitamin D supplementation and fracture prevention, which could partly be attributed to fall reduction.[180,181]
25(OH)D has a relatively long half-life (2–3 weeks) compared to that of 1α,25(OH)2D (approximately 4–6 h), and, therefore, serum levels vary little within short periods of time [41,182].
The hydroxylase enzymes that metabolize vitamin D to 25(OH)D in vivo behave according to first-order reaction kinetics. This means that its rate of production is dependent on vitamin D levels and, therefore, its level in systemic circulation is the best indicator of vitamin D nutritional status [183].
Furthermore, 25(OH)D represents the sum of vitamin D intake and dermal production [184].
Serum levels of 25(OH)D are relatively stable and not affected by diet (i.e., calcium intake) and lifestyle (i.e., sedative life or regular physical exercise), whereas 1α,25(OH)2D levels are affected by all the latter [179,182].
Serum levels of 25(OH)D can determine if there is enough 25(OH)D for the extrarenal tissues to produce 1α,25(OH)2D for autocrine or paracrine action. Recent data have revealed that many of these tissues also contain CYP27B1, which is responsible for converting 25(OH)D to 1,25(OH)2D. Regulation of CYP27B1 in these non-renal tissues is generally different from that in the kidney and may be more substrate-dependent. This finding has led to the concept that the maintenance of adequate 25OHD levels in the blood is required for vitamin D regulation of a large number of physiologic functions beyond those of the classic actions involved in bone mineral metabolism. Measurement of 1α,25(OH)2D does not provide this information since its extrarenal production does not contribute much to the systemic load [52,185].
3.2. Methods of measurement
25(OH)D can be measured using various methods including immunoassays, which are mainly used, protein-binding assays, HPLC-UV, or LC-MS/MS.[59,183,186-188]
3.3. Analytical variability and the standardization of the 25(OH)D assays
Serum total 25(OH)D, [the sum of 25(OH)D2 and 25(OH)D3], is considered to be the best biological marker of an individual’s vitamin D status as described above. However, in clinical guidelines, differences exist in the definition deficiency, insufficiency, and sufficiency, creating a great deal of controversy [189,190]. The most critical factor that confounds efforts to develop consensus clinical and nutritional public health guidelines for interpreting serum 25(OH)D concentrations is the substantial variability that existed (and still exists) in many assays that have been used over the years to measure 25(OH)D in clinical research studies [191]. The lack of assay standardization is the main source of bias, making it impossible to pool research results in order to develop consensus cut-off points [192].
To overcome these problems, the Vitamin D Standardization Program (VDSP) was established in 2010 by the Office of Dietary Supplements, National Institutes of Health, as an international collaborative effort with the National Institute of Standards and Technology (NIST), the Centers for Disease Control and Prevention (CDC), Ghent University in Belgium, the American Association for Clinical Chemistry (AACC), the IFCC, and national health and nutrition surveys from Australia, Canada, Germany, Ireland, Mexico, South Korea, United Kingdom, and the USA [193]
A reference measurement procedure (RMP) is now available. [194,195] NIST first developed a RMP based on ID-LC-MS/MS for the determination of 25(OH)D2 and 25(OH)D3 in human serum [195]. This method is now recognized by the Joint Committee for Traceability in Laboratory Medicine (JCTLM) as a RMP. Later, Stepman et al. (at Ghent University, Belgium) also described an ID-LC-MS/MS method for the determination of 25(OH)D2 and 25(OH)D3, which was also recognized as an RMP for 25(OH)D by the JCTLM [196]. Finally, the CDC developed an ID-LC-MS/MS method that was subsequently recognized by the JCTLM as an RMP for 25(OH)D [197]. These three methods are currently the only JCTLM-recognized RMPs for the determination of 25(OH)D2 and 25(OH)D3and make it possible to standardize 25(OH)D measurements. As standardization is crucial as cut-off values for deficiency, insufficiency, and sufficiency are used internationally, the Centers for Disease Control (CDC) started an international Vitamin D standardization certification program (VDSCP).[193] This led to an impressive improvement in the number of standardized 25(OH)D assays.
The achievements of VDSP and of the institutions that supported the objectives of the VDSP were significant: it resulted in a reference measurement system that is the backbone for standardizing 25(OH)D measurements in current and future assay systems [198,199]. The components of this reference measurement system include: [1] the gold standard RMPs, [2] NIST Standard Reference Materials (SRMs), [3] the VDSCP (developed, implemented and executed by the CDC), [4] the accuracy-based performance testing or external quality assessment schemes (PT/EQA) conducted by the College of American Pathologists (CAP) and the Vitamin D External Quality Assessment Scheme (DEQAS) [195,200,5] methods for retrospective standardization of studies completed in the past, and [6] a set of laboratory performance guidelines for both reference laboratories (those that govern the RMPs) and for routine laboratories developed by the VDSP [200].
The VDSP adopted the assay performance criteria for 25(OH)D that were developed by Stockl et al. for different types of labs and assays [200]. According to these criteria, for RMPs, the limits for total CV and mean bias should be less than or equal to 5% and less than or equal to 1.7%, respectively. For routine clinical laboratories, the limits for total CV and mean bias are not so strict and should be less than or equal to 10% and 5%, respectively.
However even today, several immunoassays suffer from other analytical issues that lead to continuing problems with the quality of 25(OH)D measurements. These immunoassays show patient or matrix dependent deviations, for instance in pregnant women, patients on intensive care, hemodialysis patients, osteoporotic patients, and patients with liver failure. Sera from these patient groups behave differently in these immunoassays than in LC-MS/MS assays. [168,201-205] One of the causes is a vitamin D binding protein (DBP) dependency in the immunoassay, but other causes are still unknown. Furthermore, immunoassays often demonstrate difficulties with the measurement of 25(OH)D3 and 25(OH)D2.[204,206,207] Where LC-MS/MS methods can separate 25(OH)D3 and 25(OH)D2, immunoassays make use of antibodies with a different affinity for 25(OH)D3 and 25(OH)D2 and therefore often over- or underestimate measured 25(OH)D depending on whether the subject uses 25(OH)D2. In addition, some immunoassays suffer from cross reactivity with other vitamin D metabolites such as 24,25(OH)2D. [208,209] On the other hand, LC-MS/MS assays are not always designed to separate the epimer of 25(OH)D. This can lead to falsely high 25(OH)D concentrations, especially in young children.[209-211] Most immunoassays do not show cross reactivity with the epimer.
The above-mentioned issues, including the different affinities for 25(OH)D3 and 25(OH)D2, cross reactivity with 24,25(OH)2D, and matrix and/or patient-dependent biological variations make these currently available immunoassays difficult for standardization and accurate measurements.
3.4. Recommendations
1. Preanalytical recommendations:
25(OH)D is a very stable analyte, which can be measured in serum and plasma.
There is no clear evidence that other prenalytical factors should be taken into account with regard to blood withdrawal. However they have impact on the measured value of 25(OH)D and the results should be interpreted accordingly
2. Analytical recommendations:
All clinical and research laboratories are encouraged to participate in an accuracy based external quality assessment scheme (i.e., CAP or DEQAS). The providers of these schemes should regularly perform commutability studies to ensure that the results they provide correspond to the clinical results obtained with the different assays
Manufacturers as well as research and reference laboratories are encouraged to participate in the Vitamin D Standardization-Certification Program (VDSCP)
Many immunoassays suffer from patient and pregnancy dependent deviations (due to patient related specific changes in the composition of the serum which influence the assays) and manufacturers should improve these assays. Well-characterized and standardized LC-MS/MS methods are currently the only methods which are able to measure 25(OH)D in all serum samples, regardless of the nature of the sample.
In published studies the absolute levels of total 25(OH)D should be interpreted with caution and the standardization status of the assay used should be taken into account. In meta-analyses only studies that have been used standardized assays should be included and those that a retrospective standardization has been performed according to VDSP methods.
3. Post-analytical recommendations:
For results reporting of total 25(OH) preference should be given to SI units (nmol/L) as opposed to mass units (ng/mL).
Further research:
Revision of the assay performance criteria
Is 25(OH) the optimal marker for determining vitamin D status?
Find consensus on the reference values (or target values) to report with clinical samples
4. The measurement of 1,25(OH)2D
4.1. Clinical relevance
Although 1α,25(OH)2D is the active form of vitamin D, its measurement does not provide any additional value in determining an individual’s vitamin D status. Its measurement thus, should be limited in serious clinical conditions such as hypo- or hypercalcemia.[179,212]
Three types of conditions can influence 1α,25(OH)2D disorders such as [1] disorders regarding CYP27B1, [2] disorders in the VDR, and [3] disorders in the extrarenal production of 1α,25(OH)2D. For example, vitamin D dependent rickets type 1 or pseudo-vitamin D deficiency rickets is a genetic disorder leading to a CYP27B1 deficiency, which causes hypocalcemia and early onset rickets.[213]
Also X-linked hypophosphatemia, autosomal dominant hypophosphatemic rickets, autosomal recessive hypophosphatemic rickets 1 – 3, tumor induced osteomalacia, and other rare disorders leading to FGF23-mediated hypophosphatemia all led to inhibition of the CYP27B1 gene, abnormally low 1α,25(OH)2D concentrations, and eventually osteomalacia or rickets [see Table 5 and for detailed review see references [214,215]]. Additionally, there are some rare disorders that may manifest as FGF23-mediated hypophosphatemia. These include, osteoglophonic dysplasia, McCune–Albright syndrome, epidermal nevus syndrome, neurofibromatosis, hypophosphatemic rickets with hyperparathyroidism, and Jansen metaphyseal chondrodysplasia. High FGF23 levels result in inhibition of CYP27B1 and therefore, abnormally low 1α,25(OH)2D concentrations (Table 5).
Table 5.
Characteristics and laboratory findings of inherited and acquired causes of phosphopenic rickets, modified from Ref:[330,331] Abbreviations are as follows: 1α,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, cholecalciferol; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; ALP, alkaline phosphatase; Ca, serum levels of calcium; FGF23, fibroblast growth factor 23; N, not applicable; PO4, serum levels of phosphate; PTH, parathyroid hormone; TmP/GFR, maximum rate of renal tubular reabsorption of phosphate per glomerular filtration rate; U-Ca, urinary calcium excretion; U-PO4, urinary phosphate excretion; VDR, vitamin D receptor.
| Disorder | Gene | Ca | PO4 | ALP | U-Ca | U- PO4 |
TmP/ GFR |
FGF23 | PTH | 25 (OH) D |
1α,25 (OH)2D |
pathogenesis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FGF23-mediated hypophosphatemia (phosphopenic rickets and /or osteomalacia due to elevated FGF23 and / or signaling) | ||||||||||||
| X-linked hypophosphatemia (XLH) |
PHEX (Xp22.1) |
N | ↓ | ↑,↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑ | N | ↓,N(*) | ↑ FGF23 expression in bone |
| Autosomal dominant hypophosphatemic rickets (ADHR) |
FGF23 (12p.13.3) |
N | ↓ | ↑,↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑ | N | ↓,N(*) | FGF23 protein resistant to degradation |
| Autosomal recessive hypophosphatemic rickets 1 (ARHR1) |
DMP1 (4q22.1) |
N | ↓ | ↑,↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑ | N | ↓,N(*) | ↑ FGF23 expression in bone |
| Autosomal recessive hypophosphatemic rickets 2 (ARHR2) | ENPP1 (6q23.2) |
N | ↓ | ↑,↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑ | N | ↓,N(*) | ↑ FGF23 expression in bone |
| McCune-albright syndrome /fibrous dysplasia |
GNAS (20q13.3) |
N,↓ | ↓ | ↑,↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑ | N | ↓,N(*) | ↑ FGF23 expression in bone |
| Raine syndrome related hypophosphatemia (ARHR3) |
FAM20C (7q22.3) |
N | ↓ | ↑,↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑ | N | ↓,N(*) | ↑ FGF23 expression in bone |
| Tumor induced osteomalacia (TIO) | NA | N,↓ | ↓ | ↑,↑↑ | ↓ | ↑ | ↓ | N,↑ | N,↑ | N | ↓,N(*) | ↑ FGF23 expression in tutor cells |
| Osteoglophonic dysplasia (OGD) |
FGFR1 (8p11.23) |
N | ↓ | ↑,N | ↓ | ↑ | ↓ | N,↑ | N,↑ | N | ↓,N(*) | ↑ FGF23 expression in bone |
| Cutaneous skeletal hypophosphatemia syndrome (SFM) |
RAS (1p13.2) |
↓ | ↑,↑↑ | ↓ | ↑ | ↓ | N | N,↑ | N | N | Unknown | |
|
KLOTHO (13q13.1) |
↓ | ↑,↑↑ | ↓ | ↑ | ↓ | ↑ | ↑↑ | N | N | |||
| Non FGF23-mediated hypophosphatemia (phosphopenic rickets and /or osteomalacia due to primary tubular phosphate wasting) | ||||||||||||
| Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) | SLC34A3 | N | ↓ | ↑,↑↑ | ↓ | N,↑ | ↑ | ↓ | ↓ | N,↓ | ↑↑ | Loss of function of NaPi2c in the proximal tubule |
| x-linked recessive hypophosphatemic rickets | CLCN5 | N | ↓ | ↑,↑↑ | N,↑ | ↑ | ↓ | Varies | Varies | N | ↑ | Loss of function of CLCN5 in the proximal tubule |
| Hypophosphatemia and nephrocalciniosis and Fanconi reno-tubula syndrome 2 | SLC34A1 | N | ↓ | ↑,↑↑ | ↑ | ↑ | ↓ | ↓ | Varies | N | ↑ | Loss of function of NaPi2c in the proximal tubule |
| Hereditary forms of Fanconi syndrome | CTNS | N,↓ | ↓ | ↑,↑↑ | N,↑ | ↑ | N,↑ | N,↑ | N,↑ | N | N | Cysteine accumulation in the proximal tubule |
| Iatrogenic proximal tubulopathy | – | N | ↓ | ↑,↑↑ | Varies | ↑ | ↓ | ↓ | Varies | N | ↑ | Drug toxicity |
Inappropriately normal relative to decreased serum phosphate concentration
High 1α,25(OH)2D concentrations can be seen in disorders associated with the VDR such as hereditary vitamin D resistant rickets (or vitamin D dependent rickets type 2). Despite high 1α,25(OH)2D concentrations, hypocalcemia and rickets appear as the VDR does not detect vitamin D (see Table 3). [216] Also, in diseases with excessive and uncontrolled extrarenal production of active vitamin D, high concentrations of 1α,25(OH)2D are detected. Examples of these disorder types include sarcoidosis, tuberculosis, rheumatoid arthritis, inflammatory bowel disease, and lymphoproliferative disorders (Table 6). These disorders often result in a low bone mineral density/osteomalacia.[36,217-219] This extrarenal 1α-hydroxylation by local CYP27B1 is not controlled by PTH, FGFG23, phosphate, or 1α,25(OH)2D itself, but is rather regulated by local factors such as IFN-γ and IL15, and is dependent on the availability of substrate.[36,220] When the locally produced 1α,25(OH)2D concentration is too high, it may escape the confinements of the intracellular space, spill over to the systemic circulation, and raise blood concentrations to abnormally high levels.[219] In the case of hypo- or hypercalcemia or hypophosphatemia, the measurement of 1α,25(OH)2D plays an important role in the verification of absence or onset of these conditions.
Table 6.
Common causes of vitamin D mediated hypercalcemia (modified from Ref:[42]).
| Exogenous Vitamin D Toxicity | |
|---|---|
| Administration of excessive amounts of vitamin D3 or D2 | |
| Administration of excessive amounts of 25(OH)D3 | |
| Administration of excessive amounts of 1α,25(OH)2D3, or other 1α-hydroxylated vitamin D analogs [i.e., 1α(OH)D3, paricalcitol, and doxercalciferol] in the context of CKD, end-stage kidney disease, and hemodialysis therapy | |
| Excessive Production of Vitamin D Metabolites | |
| Congenital disorders: | excessive production of 25(OH)D and 1α,25(OH) 2D3, in Williams-Beuren syndrome with mutations of the Williams Syndrome Transcription Factor |
| Granulomatous disease: | excessive production of 1α,25(OH)2D3 in: sarcoidosis, tuberculosis, leprosy, histoplasmosis, coccidioidomycosis, paracoccidioidomycosis, candidiasis, cat-scratch disease, Pneumocystis jiroveci or P. carinii pneumonia, Mycobacterium avium complex, Wegener’s granulomatosis, Crohn’s disease, infantile sc fat necrosis, giant cell polymyositis, berylliosis, silicone-induced granuloma, paraffin-induced granulomatosis, talc granuloma. |
| Lymphomas and malignant lymphoproliferative disease: | excessive production of 1,25(OH)2D3: lymphoma, non-Hodgkin lymphoma, lymphomatoid, granulomatosis, inflammatory myofibroblastic tumor, dysgerminoma |
| Mutations in Enzymes Associated With Vitamin D Metabolite Degradation | |
| Mutations of the CYP24A1 gene: | reduced degradation of 1,25(OH)2D3: infantile and adult hypercalcemia |
4.2. Analytical methods
As serum 1,25(OH)2D concentrations are very low (pmol/L), it is very difficult to measure this analyte. Over the few past decades 1,25(OH)2D was often measured using manual competitive protein binding assays or radioimmunoassays. Many of these radioimmunoassays encountered problems with specificity, as cross-reactivity with other vitamin D metabolites influenced the results.[221] Recently automated immunoassays became commercially available, which appear to perform better regarding cross reactivity. However, these methods cannot separate 1,25(OH)2D2 from 1,25(OH)2D3, which might be problematic in countries with D2 supplementation.[222,223] In addition, over the last few years, several laboratories have developed LC-MS/MS methods to measure 1,25(OH)2D. The sensitivity issue with these LC-MS/MS methods can be resolved by 2D chromatography, derivatization of the vitamin D molecule, and/or immunopurification. [212] This last option is not only advantageous for the sensitivity, but also regarding specificity. LC-MS/MS methods without immunopurification might suffer from isobaric interferences as it is difficult to separate 1β-25-dihydroxyvitamin D3 from its epimer.[224] Therefore, not all LC-MS/MS methods fully agree in method comparison studies. [225]
Unfortunately, no reference methods exist for measuring 1,25(OH)2D. Without reference method standardization, an evaluation of the quality of currently available methods is not possible. Absent of standardization, the determination of reference values is subjective to the method used.
4.3. Recommendations
- Measurement of 1α,25(OH)2D is recommended in the investigation of
- Hypercalcemia
- Calcipenic rickets/osteomalacia
- Differentiation of phosphopenic rickets between those that are/are not FGF23 mediated
Measurement of 1α,25(OH)2D is not recommended for the monitoring of chronic kidney disease patients
Further research is needed to ensure the development of a reference method for 1α,25(OH)2D, standardization of currently available methods, and development of reference values in adults and children traceable to the reference method
5. The measurement of 24,25(OH)2D
The dihydroxylated metabolite of vitamin D, 24R,25(OH)2D, has attracted much attention recently. It is the main product and first step in the process of vitamin D catabolism. This process is mediated by the enzyme 24-hydroxylase (CYP24A1). This metabolite exists in two molecular forms: 24R,25(OH)2D and 24S,25(OH)2D. They are named R or S depending on the spatial position of the carbon C24 and hydroxyl group. As the 24S isomer is not present in humans, this review focuses on the 24R isomer, and from now on when we refer to 24,25(OH)2D, we mean the R isomer.[32,226]
5.1. Clinical relevance
24,25(OH)2D circulates in a concentration range of nmol/L and has a half-life of about 7 days. These characteristics make this metabolite attractive for quantitation and a candidate biomarker of vitamin D catabolism.[158,227] The amount of circulating 24,25(OH)2D depends on the amount of its predecessor 25(OH)D and the activity of CYP24A1. The expression of CYP24A1 is upregulated by 1α,25(OH)2D, and FGF23 and is downregulated by PTH. Moreover, it is partly regulated by VDR activity.[228,229]
Consequently, if there are sufficient levels of biologically active vitamin D and the expression of CYP24A1 is adequate, then calculating the ratio 25(OH)D/24,25(OH)2D (also called vitamin D metabolite ratio or VMR) is a good indication of the catabolic clearance by CYP24A. If we also take into account that the production of 24,25(OH)2D is dependent on 25(OH)D and on the expression of CYP24A1, then the absolute concentration of 24,25(OH)2D or the VMR may be a better indicator of vitamin D sufficiency than 25(OH)D alone since it is not affected by race. [230] In addition, several studies have suggested that 24,25(OH)2D has effects of its own [231-235], and that human bone cells and human mesenchymal stem cells (hMSCs) metabolize 25(OH)D3 into both 1α,25(OH)2D3 and 24R,25(OH)2D3.[236-238] These results demonstrate the ability of bone cells to convert 25(OH)D3 in vitro, indicate the importance of systemic and tissue-specific 24,25(OH)2D3 actions, suggest a role in osteoblastic differentiation, and enhance the concept that the hydroxylation of 25(OH)D3 leads to 2 bioactive forms of vitamin D3, 24,25(OH)2D3 and 1α,25(OH)2D3, each with its own unique functions. [239] Moreover these studies demonstrated that 24,25(OH)2D3 is an active form of vitamin D3 with an essential role in osteoblast maturation, Ca2+ mineralization, gene expression, and the regulation of cytochrome P450 expression, resulting in decreased 1α,25(OH)2D3 biosynthesis.[239] These data suggest a direct role in bone cells—in particular, in osteoblasts. It should also be noted that 24-hydroxylation is the first step of a degradation cascade. Therefore, the biologically-active levels of 24,25D3 or 1,24,25D3 fully depend on the velocity of the subsequent steps in the degradation pathway.[240] It is of no surprise the biological significance of 24-hydroxylase has been continuously discussed because of its dual role first as a catalytic enzyme initiating the side chain catabolism of both 25(OH)D3 and more importantly 1α,25(OH)2D3 in target tissues and second as an enzyme with a synthetic capacity since, in some situations, it is activated to produce 24,25(OH)2D3.[241]
Chronic kidney disease (CKD) is characterized by a state of active vitamin D deficiency. In contrast to the focus placed on the decreased renal production of 1α,25(OH)2D3, relatively little attention has been paid to the potential role of altered vitamin D catabolism in CKD. In healthy people, the concentrations of vitamin D metabolites in blood and target tissues represent a balance of production and catabolism. CYP24A1 is the primary enzyme responsible for the multistep catabolism of both 25(OH)D and 1α,25(OH)2D3. CYP24A1 is present in most tissues in the body and is rapidly induced by 1α,25(OH)2D3. In the kidney, CYP24A1 is also induced by FGF23 and suppressed by PTH. In CKD, the net effects of declining kidney function with increasing FGF23 and PTH concentrations on vitamin D catabolism are not clear. [40,158,229]
The measurement of 24,25(OH)2D3 also seems to be useful in the detection of loss-of-activity mutations of the gene that encodes for CYP24A1.[242,243] Recent studies have shown that loss-of-function mutations for 24-hydroxylase are associated with a clinical phenotype/condition that is characterized by low PTH levels, increased 1α,25(OH)2D, hypercalcemia, hypercalciuria, and/or the presence of kidney stones (Tables 6 and 7).[242,244-246]
Table 7.
The typical laboratory profile of CYP24A1 mutations is, variable degrees of hypercalcemia, low PTH, and inappropriate 1α,25(OH)2D concentration (at the upper normal limits or higher). Low serum 24,25(OH)2D3, an elevated 25(OH)D3:24,25(OH)2D3 ratio, and undetectable 1,24,25(OH)3D3 are useful in identifying patients with CYP24A1 mutations. [Ref: [330]] Abbreviations are as follows: 1α,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, cholecalciferol; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; ALP, alkaline phosphatase; Ca, serum levels of calcium; FGF23, fibroblast growth factor 23; N, not applicable; PO4, serum levels of phosphate; PTH, parathyroid hormone; TmP/GFR, maximum rate of renal tubular reabsorption of phosphate per glomerular filtration rate; U-Ca, urinary calcium excretion; U-PO4, urinary phosphate excretion; VDR, vitamin D receptor.
| disorder | Gene | Ca | PO4 | ALP | U- Ca |
U- PO4 |
FGF23 | PTH | 25 (OH)D |
1α,25 (OH)2D |
24R,25 (OH)2D |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D mediated hypercalcemia due to CYP24A1 mutations | ||||||||||||
| Vitamin D mediated hypercalcemia |
CYP24A1 (20q13.2) |
↑↑or high N | N | ? | ↑↑ | N | ↓ | ↓ | ↑↑ | ↑↑ | ↓↓↓ | inactivating mutation (children and adults) |
For the evaluation and the diagnosis of patients with inactivating mutations of CYP24A1, it has been proposed that if a 25(OH)D/24,25(OH)2D ratio is greater than 80, the presence of a homozygous mutation is very probable. If the ratio is between 25 and 80, the presence of a mutation is probable. However, confirmation with molecular testing is always necessary.[242,247] We must note here that the use of ratios for measured analytes is open to criticism. Indeed, there is not yet standardization of the expression of the ratio, which can lead to added criticism. In the literature, the VMR is expressed as the ratio of 24,25(OH)2D to 25(OH)D, the ratio of 25(OH)D to (R)-24,25(OH)2D, or even as a percentage (24,25(OH)2D/(25(OH)D) × 100). Finally, in patients with a CYP24A1 mutation, 24,25(OH)2D may not be quantifiable, making the calculation of the VMR impossible as the concentration of 24,25D approaches zero.[230,248-250] Cashman et al. described the presence of 24,25(OH)2D in human serum as a “double-edged sword—an interferent for some immunoassays, yet potentially informative of nutritional status”.[208]
In summary, the molar ratio of 25(OH)D to 24,25(OH)2D (or the VMR) has been recently used as an index of vitamin D deficiency and catabolism in healthy individuals, as well as in individuals with genetic mutations in the CYP24A1 gene such as individuals with idiopathic infantile hypercalcemia (IIH) and to evaluate the efficacy of supplementation. [208,251]
5.2. Assays for the measurement of 24,25(OH)2D
The measurement of 24,25(OH)2D presents difficulties since the molecule is encountered in relatively low concentration in serum and is challenging to ionize in LC-MS/MS methods. The LC-MS/MS methods developed to measure 24,25(OH)2D ensure a high sensitivity and specificity, but unfortunately, are not available in all clinical labs. [208,242,252,253] LC-MS/MS methods present several advantages. The pretreatment of the samples prior to LC-MS/MS analysis ensures the removal of binding proteins and the excellent chromatographic separation of various metabolites. As a result, LC-MS/MS methods often allow for the simultaneous quantitation of several vitamin D metabolites.[247,252-254]
Concerning reference intervals, one study showed that 24,25(OH)2D levels in the general population are between 1.1 and 13.5 nmol/L.[252] Moreover, in this study, the investigators showed that levels greater than 4.2 nmol/L were indicative of 25(OH)D sufficiency. A more recent study calculated a slightly lower reference interval (0.4–8.9 nmol/L) using a method that was standardized using the recently developed NIST reference material.[255] In these two studies, the authors also calculated the ratio of 25(OH)D/24,25(OH)2D with similar results.
5.3. Standardization of the measurements
VDSP has expanded its interest beyond 25(OH)D and to other vitamin D metabolites such as dihydroxyvitamin D metabolites and mainly 24,25(OH)2D. Recently, NIST developed a candidate RMP based on ID-LC-MS/MS for the determination 24,25(OH)2D. This method was published recently and recognized as a RMP by JCTLM in 2017.[256-258] Although several researchers have published methods based on ID-LC-MS/MS for the determination of 24,25(OH)2D3 in human serum, the NIST method is the only RMP for this metabolite, and as such, it represents a key component in VDSP efforts to move toward the standardization of measurements for this metabolite. This method was used recently to assign values for 24,25(OH)2D3 in two SRMs (SRM972a SRM2971) and in critical study samples.[256-259] DEQAS is offering an accuracy based external quality assessment scheme for 24,25(OH)2D.
5.4. Recommendations
The measurement of 24,25(OH)2D is useful and recommended for the detection of loss-of-activity mutations for the gene that encodes for CYP24A1
-
Further research is needed to
2.1. clarify if the absolute concentration of 24,25(OH)2D or the VMR may be a better indicator of vitamin D sufficiency compared to 25(OH)D
2.2. determine the role of the VMR in the diagnosis of IIH
2.3. determine the role of the VMR in patients with recurrent kidney stones
2.4. develop cutoff values for the VMR
promote the standardization of currently used 24,25(OH)2D methods
Laboratories that measure 24,25(OH)2D, should participate in an external quality assessment scheme like DEQAS.
6. The epimers of vitamin D
6.1. Metabolism and clinical relevance
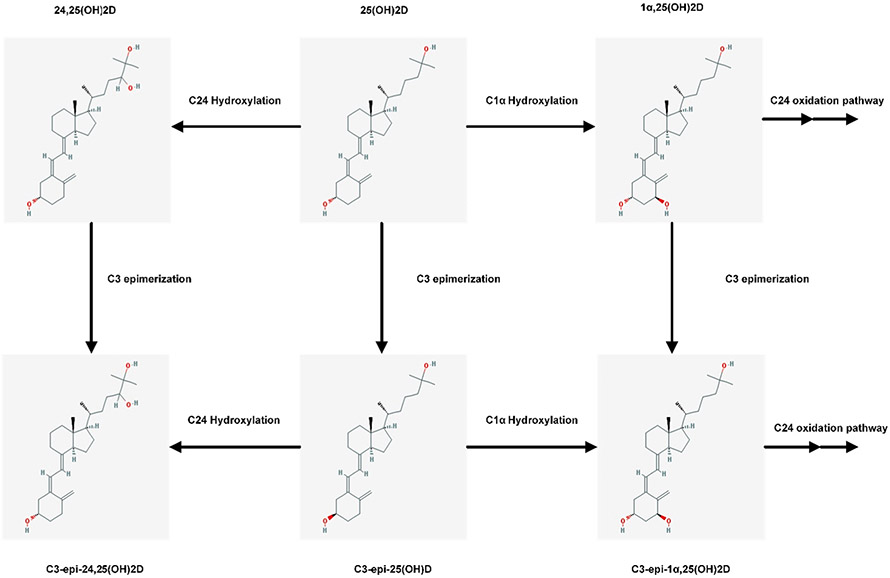
In addition to the primary pathway of vitamin D metabolism, there are also a number of minor metabolic pathways. It was recently discovered that vitamin D can alternatively be metabolized through the a C3-epimerization pathway that parallels the standard metabolic pathway.[260] This pathway creates the vitamin D epimers - a certain group of metabolites that has attracted much attention (Fig. 7). The C3 epimerization pathway leads to the conversion of the configuration of the hydroxyl group at C3 of the A-ring. In the C3 epimerization pathway, the hydroxyl group at position C3 of the A-ring is inverted from the β position to its diastereomer (α) while the other chiral centers remain unchanged. Therefore, epimers are molecules with an identical structure, but different a stereochemical configuration (diastereoisomers) at one chiral center.[261,262] Both vitamin D2 and D3 can be epimerized. The C3-epimers of 25(OH)D are produced by 25(OH)D3-C3-epimerase. This enzyme is present in the endoplasmic reticulum of a range of cells/tissues including liver, bone and skin, but not kidney. The gene responsible for encoding this enzyme has not yet been identified.[8] The process of epimerization is irreversible. Epimerase enzymes can also carry out the epimerization process of 1α,25(OH)2D3 and 24,25(OH)2D although it is not performed at the same rate as 25(OH)D.[263] Microsomes containing the epimerase have been reported to act on 1α,25(OH)2D3 and 24R,25(OH)2D3, producing 3α-epimers.[264]
Fig. 7.
Schematic representation of the epimerization pathways of vitamin D (The chemical structures are taken from PubChem: https://pubchem.ncbi.nlm.nih.gov).
The C3-epimer of 25(OH)D [C3-epi-25(OH)D] is the most abundant epimer found in systemic circulation.[265] The C3-epi-25(OH)D can also undergo 1α hydroxylation to give C3-epi-1α,25(OH)2D, and 24 hydroxylation to give C3-epi-24,25(OH)2D.[262] Subsequently, C3-epi-1α,25(OH)2D3 is metabolized to three polar compounds, C3-epi-1α,24 (R),25(OH)3D3, C3-epi-24-oxo-1α,25(OH)2D3, and C3-epi-24-oxo-1α,23(S),25-trihydroxyvitamin D3. Therefore, CYP27B1 and CYP24 are able to perform C-1α and C-24 hydroxylation irrespective of the stereochemistry of the C3 hydroxyl group.[262-265]
At present, the source of the C3-epimer from diet, supplements, or endogenous metabolism and factors underlying the epimerization of vitamin D metabolites are still unclear. Also, their physiological importance is not known very well and is under debate.[265] From in vitro studies and experiments with rodent models, we have learned that C3-epi-25(OH)D3 and the epimeric form of calcitriol (C3-epi-1α,25(OH)2D3) can bind to VDBP at 36–46% and to vitamin D receptor (VDR) at 2–3% as compared to their non-epimeric forms.[262] Nevertheless, C3-epi-1α,25(OH)2D3 appears nearly as potent as calcitriol in suppressing PTH, although it has significantly reduced calcemic effects and a reduced ability to stimulate rat Cyp24a1 gene expression.[8,266] Detailed reviews of the metabolic pathway and physiological functions of the C3-epimer forms of vitamin D can be found elsewhere. [263,265,267]
The C3-epi-25(OH)D was initially found in neonates. In a 2006 publication by Singh et al., it was reported that the C3-epimer was detectable at a significantly high percentage (up to 60%) in neonates and children up to 1 years of age.[268] In another publication, C3-epi-25(OH)D3 was found to be present in all neonatal samples, but contributed less than 10% of the total 25(OH)D concentration, which was considered by the authors as unlikely to be clinically significant.[269]
Although recently significant concentrations (ranging from 0.1 to 24 ng/mL) have also been reported in adults, highly variable concentrations have been reported by several groups, which makes it difficult to evaluate the real importance of this metabolite in patient samples. [260,270-272] Detectable levels of the epimer range from 0 to 100% in the adults tested and it is estimated that on average, adults have a median concentration range for the epimer of 1.72 (0–9.01) ng/mL and that the epimer makes up to 6.1% (0–47.0%) of total 25(OH)D.[265]
6.2. Measurement of the C3-epimers
Currently there are several assays that can quantitate the C3 epimer. These assays all implement LC-MS/MS and usually are applications that measure multiple metabolites of vitamin D.[49,265,273,274]
We must note here that the C3-epimer can also interfere with the measurement of 25(OH)D. Although the antibodies used in immunoassays are (supposedly) selective and do not cross-react with the epimer, commercial assays are reported to have variable degrees of cross reactivity (ranging from 0.07% to 91%) with the epimer.[57] However, one study showed that the C3-epimer cross-reactivity as calculated from exogenous 3-epi-25(OH)D3 recovery is different from the cross-reactivity determined from real neonatal samples with endogenous 3-epi-25(OH)D3. This study concluded that the cross recovery based on spiking experiments should be regarded as an in vitro anomaly. [275,276] We might therefore conclude that immunoassays seem to be free from C3-epimer interferences.[57]
Interference with the epimers is also a problem for HPLC and some LC-MS/MS applications. The differentiation of the epimers with LC-MS/MS methods is not routine in clinical laboratories, as many laboratory specialists choose not to distinguish these compounds. This is primarily because the epimers display similar MS/MS spectra and their separation requires extended chromatography times or specialty columns. Although numerous LC methods capable of chromatographic resolution have been published in the last 5 years, time of analysis generally exceeds 10–20 min per sample. A more rapid method based on ion mobility spectrometry (IMS) has also been recently described.[277] This interference with the epimers might be of clinical importance when we measure pediatric samples. However, in adult populations the epimers do not seem to have a significant impact.[278,279]
Currently, there is no reference measurement procedure (RMP) for the determination of 3-epi-25(OH)D3; however, NIST measures the epimer using a method similar to the RMPs, i.e., an ID-LC-MS/MS method that separates 25(OH)D3 and 3-epi-25(OH)D3 using isotopically-labeled 3-epi-25(OH)D3 as an internal standard.
6.3. Recommendations
The biological origin of these C3-epimers has not yet been clearly identified, and more clinical research is required.
Existing methods of vitamin D analysis, especially LC-MS/MS methods, can lead to a variable overestimation of vitamin D levels due to interfering epimers, especially among infant groups and pregnant women.
Until more studies are published and the measurements are standardized, it is not recommended to routinely report the value of C3-epi-25(OH)D in adults.
Significant serum concentrations of 3-epi- 25-OHD are commonly found in infants. Although the biological consequences of this phenomenon remains uncertain, in clinical practice, it can lead to the overestimation of serum 25-OHD levels. Because the calcemic effects of the active downstream metabolite 3-epi-1,25-OHD are low, this might result in an inappropriate reduction or omission of 25-OHD treatment in some children.
Serum 25(OH)D in children below the age of one should be measured with an assay that either does not cross-react with C3-epi-25(OH)D or allows unequivocal separation of C3-epi-25(OH)D from 25(OH)D.
6.4. Vitamin D binding protein – Free vitamin D
Steroid hormones are highly lipophilic and need a serum carrier protein to ensure effective delivery to target cells. Given the abundance of proteins in serum, some of this transport will be non-specific. However most of their trafficking is accomplished with ligand-specific serum carriers.[280] The majority of circulating 25(OH)D and 1α25(OH)2D is tightly bound to VDBP and less tightly bound to albumin, with less than 1% circulating in an unbound form.[132] Consequently factors affecting VDBP levels alter the interpretation of 25(OH)D levels. Moreover, the free hormone hypothesis postulates that protein-bound hormones are biologically inactive and only unbound hormones (or free) are able to exert their physiologic activity.[281] This hypothesis has been proposed as a universal mechanism for the cellular uptake of steroid hormones mainly because these are highly lipophilic molecules such as 25(OH)D, which have the potential to passively diffuse across cell membranes.[282]
Vitamin D binding protein (VDBP) was discovered with Immunoelectrophoresis in 1959, but it was not until 1975 that its function as a carrier protein of vitamin D metabolites was established.[283,284] VDBP is a protein (alpha globulin) that was initially known as Gc-globulin (group-specific component of serum) and subsequently named VDBP because of its ability to bind the majority of (>85%) circulating 25(OH)D. VDBP is the ligand-specific carrier protein for 25(OH)D and 1α,25(OH)2D, and is a member of the same family of proteins that include albumin.[285,286] VDBP has actions beyond vitamin D binding.[286-288] As only 1–2% of its sterol binding sites are utilized, multiple metabolic roles beyond the transportation of vitamin D metabolites have been described for VDBP. Actin scavenging, modulation of inflammatory processes and innate immunity, binding of fatty acids, and influencing of bone metabolism are some.[285,288,289]
In 1985, the VDBP cDNA was cloned from an adult liver library. The gene that encodes VDBP in humans is located on chromosome 4 at the 4q12-q13 position. It is 35 kb in length and comprised of 13 exons encoding a 474 amino acids peptide, including a 16 amino acid leader sequence that is cleaved before release.[281] The mature VDBP molecule in humans is approximately 58 kDa in size, although differences in glycosylation of the protein for different alleles may alter the actual size, it has 458 amino acids, and possess a highly conserved number of cysteines and disulfide bridges.[281] It shares 25% and 19% sequence identity with albumin and alpha-fetoprotein, respectively. The VDBP molecule is comprised of 3 structurally similar domains. The disulfide bridges are responsible for the formation of three double loops in the three domains. The first domain (between aa 35 and aa 45) is the binding site of all vitamin D metabolites.[281] Differences in glycosylation of the protein for different alleles may alter the actual size. The half-life of VDBP in human plasma is about 1.7 days (markedly shorter than that of 25(OH)D, which is 215 days) and the estimated daily production in an adult person is about 700 – 900 mg/day.[132] Compared with albumin, its concentration is 300 times lower. About 40% of VDBP is intravascular and the remaining 60% is distributed in the interstitial space of various organs, similar to albumin.[132]
Several tissues can express VDBP, but the main site of its production is the liver.[290] The clearing site of VDBP is not fully understood. It is known that VDBP is filtered by the glomerulus and reabsorbed in the epithelial cells of the proximal tubules by a carrier receptor mechanism (megalin) where it is degraded intracellularly.[132] VDBP is produced at stable levels through life. However, its expression is increased by estrogen, as it was found to be elevated in pregnancy and upon oral contraceptive administration.[291-294] The exact mechanism for this induction is not clear, as a response element for the estrogen receptor in the VDBP promoter has not been identified.[281]
Androgens, on the other hand, do not appear to affect VDBP levels.[295] Glucocorticoids and certain cytokines increase VDBP production. Alternatively, primary hyperparathyroidism is associated with a reduction in VDBP levels, contributing to the lower total 25(OH)D levels seen in these patients as the free 25(OH)D levels are not reduced.[296] Vitamin D itself or any of its metabolites do not regulate VDBP production.[297] A more detailed presentation of the factors and conditions that affect the VDBP levels can be found in the article by Jassil et al. [295]
VDBP is a highly polymorphic protein. Gene sequencing has revealed many variations in the VDBP gene. However, the genetic effects of VDBP on25(OH)D are complicated and have not been elucidated completely. VDBP was originally characterized in humans by serum electrophoresis as the product of two autosomal, codominant alleles GC1 and GC2. Isoelectric focusing (IEF) was subsequently used and allowed for the further characterization of two subtypes of the GC1, the “fast” GC1F and the “slow” GC1S, resulting in six common phenotypes.
In addition to the three common alleles, more than 120 variants have been described in humans. However, three major genotypes account for the majority of VDBP variants. The geographic distribution of these variants often correspond to patterns of human migrations and thus are of anthropological interest. GC1F is most abundant among those of African ancestry, whereas GC1S is most abundant in the European populations, with Asians exhibiting intermediate frequencies of both GC1 forms. The GC2 form is exceedingly rare in the black ethnic groups and found in similar frequencies in people of Asian and European ancestry. These VDBP variants exhibit differences in their affinity to 25(OH)D and 1α,25(OH)2D, as well as in their serum concentration with the hierarchy in both cases being GC1F > GC1S > GC2.[298,299] The existence of the different forms of VDBP with different affinities to vitamin D metabolites could be explained by the differences in skin pigmentation of populations living in geographic locations with different UV exposures, which results in different cutaneous vitamin D synthesis. As such, people groups that migrated to northern latitudes were exposed to less UV, resulting in “selective pressure” for lighter skin tones and lower affinity GC1S and GC2 forms, which allowed for greater free vitamin D levels. Conversely, in darker-skinned individuals, the combination of dark skin and the VDBP variant was ideal in geographical areas with latitudes that were closer to equator. However, upon immigration to northern latitudes, a disadvantage may have been present because of a diminished cutaneous vitamin D synthesis imposed by darker skin and reduced free vitamin D levels due to higher affinity GC1F VDBP. Thus, these individuals may require greater vitamin D supplementation to compensate for these genetic and environmental factors.[288]
6.5. The free hormone hypothesis
The mechanism by which the ligand is released from its binding protein and acquired by the target cell is crucial to hormone signaling pathways. This is very important for vitamin D since there is evidence for an extrarenal, intracrine, conversion of 25(OH)D to 1α,25(OH)2D. Factors affecting this conversion include the tissue specific expression of 1α-hydroxylase, the nuclear receptor VDR, and the availability of 25(OH)D.
Although greater than 99% of 25(OH)D circulates in the serum bound to VDBP or other carrier proteins, the general assumption is that biological activity involves the unbound or free fractions, even though this component is very small.[141,300] The free hormone hypothesis has been proposed as a universal mechanism for the cellular uptake of steroid hormones mainly because these molecules are highly lipophilic and have the potential to diffuse passively and rapidly across cell membranes.[282] However, it is still unknown whether the free hormone hypothesis is applicable to 25(OH)D as other free steroid hormones are feedback regulated whereas in case of vitamin D, the active vitamin D 1α,25(OH)2D is feedback regulated and not 25(OH)D.[301] Moreover, this hypothesis has been under “debate” because of the discrepancy between the likely available amounts of free hormone for passive diffusion and the levels required to efficiently occupy the intracellular receptors.[141,280,302,303] A second reservation concerning the free hormone hypothesis is the megalin-dependent uptake of the VDBP-25(OH)D complex into cells. (the role of megalin in proximal tubule protein reabsorption has been reviewed in detail elsewhere: [304,305] Although this type of uptake has a clear role in the renal proximal tubular cell uptake of 25(OH)D, it is not clear whether this mechanism is utilized by other target tissues of vitamin D. Outside the kidney, megalin is expressed by several tissues, including the placenta, mammary gland, parathyroid, and thyroid glands, which also exhibit 1α-hydroxylase activity so that a VDBP-megalin interaction can be used for vitamin D internalization similar to that described for the kidney. Therefore, it is possible that some extrarenal tissues employ the megalin-mediated receptor uptake of 25(OH)D bound to VDBP similar to that found in the proximal tubular cells of the kidney. Most of the extrarenal tissues that do not appear to express megalin or its associated coreceptors are more likely to acquire free or bioavailable 25(OH)D.[303]
6.6. Bound, free and bioavailable vitamin D
In serum, vitamin D metabolites mainly circulate bound to VDBP (85–90%), but they are also known to associate with serum albumin (10–15%). The affinities of 25OHD and 1,25(OH)2D for VDBP are Ka = 6 × 105 M−1 and Ka = 5.4 × 104 M−1, respectively. For albumin, the affinities are substantially lower (Ka = 7 × 108 M−1 and Ka = 4 × 107 M−1, respectively). However, because of the relative abundance of albumin in serum (650 μM) compared to VDBP (5 μM), it seems logical that some vitamin D metabolites are transported in circulation by albumin. Moreover, the vast majority of VDBP in serum circulates unbound because its concentration is much higher than the concentrations of vitamin D metabolites found in circulation. Therefore, it seems likely that most circulating vitamin D metabolites are bound to a carrier protein of some sort. Moreover, at any given time, a small proportion of vitamin D metabolites will not be bound to VDBP or albumin, but will instead be unbound or ‘free’.[280] In the case of 25OHD, it is estimated that less than 0.1% of the total circulating levels of this metabolite are ‘free’.[306] An alternative term, ‘bioavailable’ 25OHD, has also been proposed.[307] Bioavailable 25OHD refers to all the circulating 25OHD that is not bound to VDBP, in other words the sum of free plus that bound to albumin. Calculation of bioavailable 25OHD has been used as an alternative to free 25OHD in some clinical studies.
6.7. The direct measurement and the calculation of the free-25(OH)D
It is obvious that the variation observed in serum VDBP concentrations under certain conditions results in higher or lower free 25(OH)D levels. These observations have provoked an interest in the measurement of free 25(OH)D since it was hypothesized that it may be a better marker of vitamin D activity than the classical measurement of total 25(OH)D. Free 25(OH)D can be measured either directly or estimated through calculations, based on the measurements of total 25(OH)D, VDBP, and albumin serum levels. In principle, both methods should give similar results, but this not always the case. The direct measurement of free 25(OH)D can be performed either by centrifugal ultrafiltration or by a recently-developed commercially-available ELISA.[170,303] Centrifugal ultrafiltration was a method introduced in the 1980s that did not become commonly used due to its high costs and technical difficulties in application although it was proved to be quite accurate.[141,308,309]
In the early 2010s, a two-step ELISA was developed using monoclonal antibodies that is now commercially available. In this assay, an anti-vitamin D antibody is coated on a microtiter plate. Serum samples and calibrators are pipetted into the wells of the microtiter plate. Free 25(OH)D is captured by the antibody during a first incubation. After washing, a biotin-labeled 25(OH)D analog is allowed to react with the unoccupied antibody binding sites in a second incubation. After a second washing step and incubation with a streptavidin-peroxidase conjugate, bound enzyme is quantitated using a colorimetric reaction. Intensity of the signal is inversely proportional to the level of free 25(OH)D in the sample. The limit of detection of this assay is 2.8 pg/mL. However, the antibody used in the current assay does not equally recognize 25(OH)D2 and 25(OH)D3 (77% of the 25(OH)D3 value). Therefore, it underestimates free 25(OH)D2. However, under most situations where the predominant vitamin D metabolite is 25(OH)D3, this issue is not a major concern. Data from both normal subjects and from subjects with variations in VDBP levels (e.g., cirrhotic patients and pregnant women) correlate well to that obtained from similar populations using the centrifugal ultrafiltration assay.[281,310] However, the accuracy of this assay cannot be determined as no reference method is currently available.
The calculation of the free portion of 25(OH)D requires the measurements of total 25(OH)D, albumin, and VDBP as well as a mathematical equation that will take into account the affinity of both binding proteins.[308,311] According to the theory of protein–ligand binding kinetics, total 25(OH) vitamin D can be defined as follows:[312,313]
Free 25(OH)D can be assessed by the following formula, first suggested by Bikle et al. in 1986 [311] and subsequently used in several studies:
where, Free25(OH)D is the calculated concentration of free 25(OH)D in mol/L, Kalb is the affinity constant between albumin and 25(OH)D in mol−1, Kvdbp is the affinity constant between VDBP and 25(OH)D in mol−1, Alb is the concentration of albumin in serum in mol/L, and VDBP is the concentration of total VDBP in serum in mol/L. After the calculation, the value of Free25(OH)D is converted to pmol/L.
After determining the concentration of free 25(OH)D, the bioavailable amount can be determined using the following formula:
where Free25(OH)D is the concentration of free 25(OH)D in mol/L, Kalb is the affinity constant between albumin and 25(OH)D in mol−1, and Alb is the concentration of albumin in serum in mol/L. After this calculation, the value of bioavailable 25(OH)D is converted to nmol/L.
The direct measurement of free vitamin D shows various correlation coefficients for the calculated free vitamin D concentration (e.g., R2 = 0.69 in a transgender population; R2 = 0.13 in a combined group of pregnant women, patients with liver failure, and control group). [314,315] These correlations are difficult to interpret, as many variables are present (i.e., CV% of the direct free vitamin D, 25(OH)D, VDBP, and albumin measurement) and the quality/accuracy of all these measurements should be taken into account.
6.8. The measurement of VDBP – Laboratory and clinical implications
It is obvious that in order to use these formulas, one requires an accurate measurement of VDBP, albumin, and the vitamin D metabolite of interest. Moreover, the calculation depends on an assumption that the affinity constants are invariant.
25(OH)D immunoassays, especially automated immunoassays, are limited by their possibilities in displacement of vitamin D from the VDBP [168]. Therefore, the VDBP concentration can influence the vitamin D levels reported using these assays. If immunoassays are used to calculate free 25(OH)D, different results will be generated compared to calculated free 25(OH)D levels determined using LC-MS/MS methods for 25(OH)D.
In initial studies using normal serum, in which VDBP levels were measured with polyclonal assays, the calculated values correlated well with the directly measured values using the centrifugal ultrafiltration method.[141,311] However, commercially-developed VDBP assays that use monoclonal antibodies appear to differ in their ability to detect the different VDBP alleles compared with the polyclonal antibody assays [316,317], to determine the apparent change in the VDBP affinities for the vitamin D metabolites under different physiologic/pathologic conditions [141,309,315], and to calculate values diverged substantially from the directly measured levels.[315]
For the measurement of VDBP, several immunological methods have been used, which included assays using polyclonal antibodies (e.g., radial immunodiffusion, nephelometry, turbidimetry, rocket immunodiffusion, and radioimmunoassay) or commercial ELISA assays that employed either polyclonal or monoclonal antibodies. Given the polymorphic nature of human VDBP and the isotype-specific post-translational modifications (mainly glycosylations), the assays should be able to equally detect all isoforms of VDBP. However, this is not always the case.
In 2013, Powe et al.[128] reported that black Americans compared with white Americans had similar levels of calculated bioavailable 25OHD despite lower levels of total 25OHD. Similar results came from other studies that used the same assay and all seemed to conclude that the best biomarker to test vitamin D status was free vitamin D rather than total 25(OH)D. However, the VDBP assay they used was a monoclonal antibody assay which resulted in lower VDBP levels and therefore an increased bioavailable fraction in subjects of African-American descent (with the GC1F allele) Previous studies with assays using polyclonal antibodies did not find racial differences in serum levels of VDBP. [318,319] In studies that followed using assays that employed polyclonal antibodies or LC-MS/MS methods demonstrated that the monoclonal antibody-based assay discriminated against the GC1F variant and that the differences in VDBP levels between black and white Americans were minimal when VDBP was measured with polyclonal assays. [316,317,320,321] The results of the polyclonal assays were confirmed by direct measurement of free 25(OH)D using a commercial ELISA assay and by an LC-MS/MS method. Thus, although it is not clear whether the different VDBP alleles have different affinities for the vitamin D metabolites, the alleles do affect the results of immunoassays when a monoclonal antibody is used.[320-322] Polyclonal antibody techniques do not exhibit this kind of bias, but the absolute concentration of VDBP differs according to the assay technique.[301,322]
LC-MS/MS methods may avoid the potential bias observed in antibody-based assays because such methods allow for the measuring of peptides from a region of VDBP that is common to all genetic variants and separate from genotype-specific sequences.[320] However, there are other sources of error in this type of assay that may also lead to poor results if not carefully controlled.[323] Aside from the investigation of optimal digestion conditions, the quantitation of different isoforms should take into account the various degrees and sites of glycosylation. Therefore, assay standardization is needed, and it is among the priorities of the IFCC Committee of Bone metabolism, which is in close collaboration with NIST, CDC VDSCP, VDSP, and research laboratories. NIST recently developed a candidate reference method that is able to measure all three major isoforms of VDBP and this method has been used to assign values to reference materials.[323]
In order to have an accurate calculation of free vitamin D metabolites, we need valid estimations of the affinity constants of these metabolites with VDBP at 37 °C. It is understood that 25(OH)D has a high affinity for VDBP (i.e., the Ka is in the nmol/L range, approx. 1.5 × 108 M−1) and that the affinity of calcitriol is somewhat 10 to 100 times lower.[132] Whether the different isoforms of VDBP have different affinities is still a matter of debate. This also needs to be further researched since the affinity constant is incorporated into the equations that calculate free-25(OH)D and affect the accuracy of the calculations.
Clinical implications:
Powe et al. concluded that measuring total 25(OH)D does not reveal genetic differences in free-25(OH)D. The authors instead suggested that estimated free vitamin D might be a better marker of vitamin D status and correlate better with various health outcomes.[128]
However, calculated free 25(OH)D in African-Americans gave contradictory results depending on the nature of antibodies (monoclonal vs. polyclonal) and it is now well accepted that assays that use monoclonal antibodies overestimate free 25(OH)D in these populations.
Moreover, contradictory results were also observed in studies that used the assay that directly measured free 25(OH)D. Some researchers found lower levels of directly measured free-25(OH)D in African-Americans while others (also using the same assay) found no difference between blacks and whites. The question about the racial or genetic differences in levels of measured free-25(OH)D remains unanswered. There is also another question that needs to be addressed: whether the measurement of free vitamin D metabolites in serum generates better clinical endpoints than the measurement of their total concentrations.
6.9. Recommendations
For the measurement of VDBP, we recommend the use immunoassays that employ polyclonal antibodies over assays that employ monoclonal antibodies as these are suspected to show genotype specific reactivity.
Standardization of VDBP is a priority (after adoption of a reference measurement procedure and development of international reference materials for the calibration of commercial assays for VDBP).
The genetic influence on the affinity of various vitamin D metabolites should be investigated as this affects the accuracy of equations that calculate the levels of free and bioavailable metabolites. We propose a standardization effort to focus on the development of a reference method for free 25(OH)D.
We do not recommend the use of equations to estimate the free-vitamin D in routine, as its calculation is based on the measurement of non-standardized analytes. Also we do not recommend the measurement direct measurement of free-vitamin D in routine as long as a reference method is not available.
Studies should investigate if the levels of free vitamin D metabolites better reflect clinical end points than their total concentrations.
7. Conclusion
Major developments have taken place in the measurement of vitamin D metabolites during the last 10 years. Standardization of 25(OH)D measurements has contributed significantly to the progress of the assays that measure this analyte. Although LC-MS/MS methods continue to serve as the gold standard for the measurement of 25(OH)D, these are not immune from interferences.[324] The performance of immunoassays still needs improvement and manufacturers are therefore encouraged to continue their work on standardizing serum 25(OH)D assays. [325] Participation in accuracy based external quality assessment schemes (CAP or DEQAS) has made an important contribution to improving analytical performance in clinical laboratories. The routine measurement of 1α,25(OH)2D is recommended only for the investigation of inherited or acquired disorders of vitamin D metabolism. Further research is needed to investigate whether the measurement of free-vitamin D and 24,25(OH)2D will offer any further insight to vitamin D status. Improvement of analytical performance criteria and support of the standardization efforts for VDBP, 1α,25(OH)2D and free vitamin D are priorities for the future
8.
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service and the US Department of Health and Human Services.
The opinions, recommendations, findings, and conclusions in this report do not necessarily reflect the views or policies of NIST or the United States Government.
Abbreviations:
- 7-DHC
dehydrocholesterol or provitamin D3
- 24,25(OH)2D3
24,25-dihydroxyvitamin D3
- 25(OH)D3
calcidiol or 25-hydroxyvitamin D3
- 1α,25(OH)2D3
calcitriol or 1,25-dihydroxyvitamin D3
- C3-epi-25(OH)D
C3-epimer of the 25(OH)D
- CYP27B1
25(OH)D-1α-hydroxylase
- CYP24A1
24-hydroxylase
- PTH
parathyroid hormone
- FGF-23
fibroblast growth factor-23
- VDBP
vitamin D binding protein
- VDDR
vitamin D dependent rickets
- VDR
vitamin D receptor
- VDSP
Vitamin D Standardization Program
- VDSCP
Vitamin D standardization certification program
- VMR
vitamin D metabolite ratio
- CLD
chronic liver disease
- CKD
chronic kidney disease
- SHPT
secondary hyperparathyroidism
- UV
ultraviolet
- HPLC
high-performance liquid chromatography
- LC-MS/MS
liquid chromatography coupled with mass spectrometry
- CPBA
competitive protein binding assays
- RIA
radioimmunoassays
- ELISA
enzyme-linked immunosorbent assays
- CLIA
chemiluminescent assays
- RMP
reference measurement procedure
- PRM
Primary Reference Material
- NIST
National Institute for Standards and Technology
- IFCC
International Federation of Clinical Chemistry
- JCTLM
Joint Committee for Traceability in Laboratory Medicine
- AACC
American Association for Clinical Chemistry
- DEQAS
Vitamin D External Quality Assessment Scheme
- CAP
College of American Pathologists
- CDC
Center for Disease Control
- EQA
External Quality Assessment
Footnotes
Declaration of Competing Interest
Konstantinos Makris, Harjit P Bhattoa, Karen Phinney, Christopher T Sempos, Candice Z. Ulmer, Samuel D Vasikaran, Hubert Vesper, and Annemieke C Heijboer declare no conflict of interest. Etienne Cavalier is a consultant for DiaSorin, IDS, Fujirebio, bioMérieux, Nittobo, and Menarini.
References
- [1].Bikle DD, Vitamin D and bone, Curr. Osteoporos. Rep 10 (2) (2012) 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Glendenning P, Inderjeeth CA, Screening for vitamin D deficiency: defining vitamin D deficiency, target thresholds of treatment and estimating the benefits of treatment, Pathology 44 (2) (2012) 160–165. [DOI] [PubMed] [Google Scholar]
- [3].Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, Bischoff-Ferrari HA, Cavalier E, Ebeling PR, Fardellone P, Gandini S, Gruson D, Guerin AP, Heickendorff L, Hollis BW, Ish-Shalom S, Jean G, von Landenberg P, Largura A, Olsson T, Pierrot-Deseilligny C, Pilz S, Tincani A, Valcour A, Zittermann A, Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice, Autoimmun. Rev 9 (11) (2010) 709–715. [DOI] [PubMed] [Google Scholar]
- [4].Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, Lips P, Munns CF, Lazaretti-Castro M, Giustina A, Bilezikian J, Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions, Endocr. Rev 40 (4) (2019) 1109–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marino R, Misra M, Extra-skeletal effects of vitamin D, Nutrients 11 (7) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zerwekh JE, Blood biomarkers of vitamin D status, Am. J. Clin. Nutr 87 (4) (2008) 1087S–1091S. [DOI] [PubMed] [Google Scholar]
- [7].Jenkinson C, The vitamin D metabolome: an update on analysis and function, Cell Biochem. Funct 37 (6) (2019) 408–423. [DOI] [PubMed] [Google Scholar]
- [8].Tuckey RC, Cheng CYS, Slominski AT, The serum vitamin D metabolome: What we know and what is still to discover, J. Steroid Biochem. Mol. Biol 186 (2019) 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bikle DD, Vitamin D metabolism, mechanism of action, and clinical applications, Chem. Biol 21 (3) (2014) 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Houghton LA, Vieth R, The case against ergocalciferol (vitamin D2) as a vitamin supplement, Am. J. Clin. Nutr 84 (4) (2006) 694–697. [DOI] [PubMed] [Google Scholar]
- [11].Hollis BW, Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites, J. Steroid Biochem 21 (1) (1984) 81–86. [DOI] [PubMed] [Google Scholar]
- [12].Horst RL, Reinhardt TA, Ramberg CF, Koszewski NJ, Napoli JL, 24-Hydroxylation of 1,25-dihydroxyergocalciferol. An unambiguous deactivation process, J. Biol. Chem 261 (20) (1986) 9250–9256. [PubMed] [Google Scholar]
- [13].Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R, Lanham-New S, Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis, Am. J. Clin. Nutr 95 (6) (2012) 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Holick MF, Environmental factors that influence the cutaneous production of vitamin D, Am. J. Clin. Nutr 61 (3 Suppl) (1995) 638S–645S. [DOI] [PubMed] [Google Scholar]
- [15].Bouillon R, Suda T, Vitamin D: calcium and bone homeostasis during evolution, Bonekey Rep. 3 (2014) 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Holick MF, Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health, Anticancer Res. 36 (3) (2016) 1345–1356. [PubMed] [Google Scholar]
- [17].Holick MF, The photobiology of vitamin D and its consequences for humans, Ann. N. Y. Acad. Sci 453 (1985) 1–13. [DOI] [PubMed] [Google Scholar]
- [18].Clemens TL, Adams JS, Henderson SL, Holick MF, Increased skin pigment reduces the capacity of skin to synthesise vitamin D3, Lancet 1 (8263) (1982) 74–76. [DOI] [PubMed] [Google Scholar]
- [19].Libon F, Cavalier E, Nikkels AF, Skin color is relevant to vitamin D synthesis, Dermatology (Basel, Switzerland) 227 (3) (2013) 250–254. [DOI] [PubMed] [Google Scholar]
- [20].Holick MF, Chapter 4 - Photobiology of vitamin D, in: Feldman D (Ed.), Vitamin D, fourth ed., Academic Press, 2018, pp. 45–55. [Google Scholar]
- [21].Xiang F, Lucas R, de Gruijl F, Norval M, A systematic review of the influence of skin pigmentation on changes in the concentrations of vitamin D and 25-hydroxyvitamin D in plasma/serum following experimental UV irradiation, Photochem. Photobiol. Sci.: Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol 14 (12) (2015) 2138–2146. [DOI] [PubMed] [Google Scholar]
- [22].Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC, Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation, J. Invest. Dermatol 130 (2) (2010) 546–553. [DOI] [PubMed] [Google Scholar]
- [23].Young AR, Some light on the photobiology of vitamin D, J. Invest. Dermatol 130 (2) (2010) 346–348. [DOI] [PubMed] [Google Scholar]
- [24].Christakos S, Li S, De La Cruz J, Bikle DD, New developments in our understanding of vitamin metabolism, action and treatment, Metabolism 98 (2019) 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF, CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo, Proc. Natl. Acad. Sci. U S A 110 (39) (2013) 15650–15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW, Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase, Proc. Natl. Acad. Sci. U S A 101 (20) (2004) 7711–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thacher TD, Fischer PR, Singh RJ, Roizen J, Levine MA, CYP2R1 mutations impair generation of 25-hydroxyvitamin D and cause an atypical form of vitamin D deficiency, J. Clin. Endocrinol. Metab 100 (7) (2015) E1005–E1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Henry HL, Regulation of vitamin D metabolism, Best Pract. Res. Clin. Endocrinol. Metab 25 (4) (2011) 531–541. [DOI] [PubMed] [Google Scholar]
- [29].Jones G, Kottler ML, Schlingmann KP, Genetic diseases of vitamin D metabolizing enzymes, Endocrinol. Metab. Clin. North Am 46 (4) (2017) 1095–1117. [DOI] [PubMed] [Google Scholar]
- [30].Larner DP, Adams JS, Hewison M, Chapter 8 - Regulation of renal and extrarenal 1α-hydroxylase, in: Feldman D (Ed.), Vitamin D (Fourth Edition), Academic Press, 2018, pp. 117–137. [Google Scholar]
- [31].Plum LA, DeLuca HF, The functional metabolism and molecular biology of vitamin D action, Clin. Rev. Bone Mineral Metab 7 (1) (2009) 20–41. [Google Scholar]
- [32].Jones G, Prosser DE, Kaufmann M, Cytochrome P450-mediated metabolism of vitamin D, J. Lipid Res 55 (1) (2014) 13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G, Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects, Physiol. Rev 96 (1) (2016) 365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pike JW, Christakos S, Biology and mechanisms of action of the vitamin D hormone, Endocrinol. Metab. Clin. North Am 46 (4) (2017) 815–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Prie D, Friedlander G, Reciprocal control of 1,25-dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system, Clin. J. Am. Soc. Nephrol 5 (9) (2010) 1717–1722. [DOI] [PubMed] [Google Scholar]
- [36].Adams JS, Hewison M, Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase, Arch. Biochem. Biophys 523 (1) (2012) 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Takeda E, Yamamoto H, Taketani Y, Miyamoto K, Vitamin D-dependent rickets type I and type II, Acta Paediatr. Jpn 39 (4) (1997) 508–513. [DOI] [PubMed] [Google Scholar]
- [38].Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ, Vitamin D: metabolism, Endocrinol. Metab. Clin. North Am 39(2) (2010) 243–53, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jones G, Strugnell SA, DeLuca HF, Current understanding of the molecular actions of vitamin D, Physiol. Rev 78 (4) (1998) 1193–1231. [DOI] [PubMed] [Google Scholar]
- [40].Bosworth C, de Boer IH, Impaired vitamin D metabolism in CKD, Semin. Nephrol 33 (2) (2013) 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jones G, Pharmacokinetics of vitamin D toxicity, Am. J. Clin. Nutr 88 (2) (2008) 582S–586S. [DOI] [PubMed] [Google Scholar]
- [42].Tebben PJ, Singh RJ, Kumar R, Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment, Endocr. Rev 37 (5) (2016) 521–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M, Dickmann LJ, Nelson SD, Baillie TA, Hebert MF, Blough D, Davis CL, Thummel KE, An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway, Mol. Pharmacol 81 (4) (2012) 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Roizen JD, Li D, O’Lear L, Javaid MK, Shaw NJ, Ebeling PR, Nguyen HH, Rodda CP, Thummel KE, Thacher TD, Hakonarson H, Levine MA, CYP3A4 mutation causes vitamin D-dependent rickets type 3, J. Clin. Investig 128 (5) (2018) 1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Demay MB, The good and the bad of vitamin D inactivation, J. Clin. Invest 128 (9) (2018) 3736–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhou SF, Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4, Curr. Drug Metab 9 (4) (2008) 310–322. [DOI] [PubMed] [Google Scholar]
- [47].Gonzalez FJ, CYP3A4 and pregnane X receptor humanized mice, J. Biochem. Mol. Toxicol 21 (4) (2007) 158–162. [DOI] [PubMed] [Google Scholar]
- [48].Hawkes CP, Li D, Hakonarson H, Meyers KE, Thummel KE, Levine MA, CYP3A4 induction by rifampin: an alternative pathway for vitamin D inactivation in patients with CYP24A1 mutations, J. Clin. Endocrinol. Metab 102 (5) (2017) 1440–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Baecher S, Leinenbach A, Wright JA, Pongratz S, Kobold U, Thiele R, Simultaneous quantification of four vitamin D metabolites in human serum using high performance liquid chromatography tandem mass spectrometry for vitamin D profiling, Clin. Biochem 45 (16–17) (2012) 1491–1496. [DOI] [PubMed] [Google Scholar]
- [50].Wong RG, Myrtle JF, Tsai HC, Norman AW, Studies on calciferol metabolism. V. The occurrence and biological activity of 1,25-dihydroxy-vitamin D 3 in bone, J. Biol. Chem 247 (18) (1972) 5728–5735. [PubMed] [Google Scholar]
- [51].Norman AW, Okamura WH, Friedlander EJ, Henry HL, Johnson RL, Mitra MN, Proscal DA, Wecksler WW, Current concepts of the chemical conformation, metabolism, and interaction of the steroid, vitamin D, with the endocrine system for calcium homeostasis, Calcif. Tissue Res 21 (Suppl) (1976) 153–159. [PubMed] [Google Scholar]
- [52].Herrmann M, Farrell CL, Pusceddu I, Fabregat-Cabello N, Cavalier E, Assessment of vitamin D status - a changing landscape, Clin. Chem. Lab. Med 55 (1) (2017) 3–26. [DOI] [PubMed] [Google Scholar]
- [53].Colak A, Toprak B, Dogan N, Ustuner F, Effect of sample type, centrifugation and storage conditions on vitamin D concentration, Biochem. Med. (Zagreb) 23 (3) (2013) 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK, HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays, Clin. Chem 52 (6) (2006) 1120–1126. [DOI] [PubMed] [Google Scholar]
- [55].Elder PA, Lewis JG, King RI, Florkowski CM, An anomalous result from gel tubes for vitamin D, Clin. Chim. Acta 410 (1–2) (2009) 95. [DOI] [PubMed] [Google Scholar]
- [56].Yu S, Zhou W, Cheng X, Fang H, Zhang R, Cheng Q, Han J, Su W, Xia L, Qiu L, Blood collection tubes and storage temperature should be evaluated when using the siemens ADVIA centaur XP for measuring 25-hydroxyvitamin D, PLoS ONE 11 (11) (2016), e0166327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Heureux N, Vitamin D testing-where are we and what is on the horizon? Adv. Clin. Chem 78 (2017) 59–101. [DOI] [PubMed] [Google Scholar]
- [58].Borai A, Bahijri S, Livingstone C, Nawajha M, Bawazeer A, Baarmah Z, Shanaa A, Kadam I, Abdelaal M, Assessment of Becton Dickinson plain and serum separator tubes in measurement of 25-hydroxyvitamin D3 (25OHD3) by HPLC and immunoassay methods, J. Clin. Lab. Anal 30 (1) (2016) 32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fraser WD, Tang JCY, Dutton JJ, Schoenmakers I, Vitamin D measurement, the debates continue, new analytes have emerged, developments have variable outcomes, Calcif. Tissue Int 106 (1) (2020) 3–13. [DOI] [PubMed] [Google Scholar]
- [60].Makowski AJ, Rathmacher JA, Horst RL, Sempos CT, Simplified 25-hydroxyvitamin D standardization and optimization in dried blood spots by LC-MS/MS, J. AOAC Int 100 (5) (2017) 1328–1336. [DOI] [PubMed] [Google Scholar]
- [61].Lissner D, Mason RS, Posen S, Stability of vitamin D metabolites in human blood serum and plasma, Clin. Chem 27 (5) (1981) 773. [PubMed] [Google Scholar]
- [62].Wielders JP, Wijnberg FA, Preanalytical stability of 25(OH)-vitamin D3 in human blood or serum at room temperature: solid as a rock, Clin. Chem 55 (8) (2009) 1584–1585. [DOI] [PubMed] [Google Scholar]
- [63].Agborsangaya C, Toriola AT, Grankvist K, Surcel HM, Holl K, Parkkila S, Tuohimaa P, Lukanova A, Lehtinen M, The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione, Nutr. Cancer 62 (1) (2010) 51–57. [DOI] [PubMed] [Google Scholar]
- [64].Hayden Y, Pillay T, Marx G, de Lange W, Kuyl JM, Pre-analytical stability of 25(OH)-vitamin D in primary collection tubes, Clin. Chem. Lab. Med 53 (3) (2015) e55–e57. [DOI] [PubMed] [Google Scholar]
- [65].Mena-Bravo A, Priego-Capote F, Luque de Castro MD, Study of blood collection and sample preparation for analysis of vitamin D and its metabolites by liquid chromatography-tandem mass spectrometry, Anal. Chim. Acta 879 (2015) 69–76. [DOI] [PubMed] [Google Scholar]
- [66].Mena-Bravo A, Calderon-Santiago M, Luque de Castro MD, Priego-Capote F, Evaluation of short-term storage prior to analysis of vitamin D3 and metabolites in human serum by liquid chromatography coupled to tandem mass spectrometry, Talanta 198 (2019) 344–349. [DOI] [PubMed] [Google Scholar]
- [67].Borai A, Khalil H, Alghamdi B, Alhamdi R, Ali N, Bahijri S, Ferns G, The pre-analytical stability of 25-hydroxyvitamin D: Storage and mixing effects, J. Clin. Lab. Anal 34 (2) (2020) e23037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Antoniucci DM, Black DM, Sellmeyer DE, Serum 25-hydroxyvitamin D is unaffected by multiple freeze-thaw cycles, Clin. Chem 51 (1) (2005) 258–261. [DOI] [PubMed] [Google Scholar]
- [69].Wielders JPM, Wijnberg FA, Preanalytical stability of 25(OH)–vitamin D3 in human blood or serum at room temperature: solid as a rock, Clin. Chem 55 (8) (2009) 1584. [DOI] [PubMed] [Google Scholar]
- [70].Hayden Y, Pillay T, Marx G, de Lange W, Kuyl Johannes M, Pre analytical stability of 25(OH)-vitamin D in primary collection tubes, Clin. Chem. Lab. Med. (CCLM) (2015), e55. [DOI] [PubMed] [Google Scholar]
- [71].Calvo MS, Whiting SJ, Barton CN, Vitamin D intake: a global perspective of current status, J. Nutrit 135 (2) (2005) 310–316. [DOI] [PubMed] [Google Scholar]
- [72].Maxwell JD, Seasonal variation in vitamin D, Proc. Nutr. Soc 53 (3) (1994) 533–543. [DOI] [PubMed] [Google Scholar]
- [73].Spiro A, Buttriss JL, Vitamin D: an overview of vitamin D status and intake in Europe, Nutrit. Bull 39 (4) (2014) 322–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Webb AR, Kline L, Holick MF, Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin, J. Clin. Endocrinol. Metab 67 (2) (1988) 373–378. [DOI] [PubMed] [Google Scholar]
- [75].Vuistiner P, Rousson V, Henry H, Lescuyer P, Boulat O, Gaspoz JM, Mooser V, Vollenweider P, Waeber G, Cornuz J, Paccaud F, Bochud M, Guessous I, A population-based model to consider the effect of seasonal variation on serum 25(OH)D and vitamin D status, Biomed. Res. Int 2015 (2015), 168189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Krzywanski J, Mikulski T, Krysztofiak H, Mlynczak M, Gaczynska E, Ziemba A, Seasonal vitamin D status in polish elite athletes in relation to sun exposure and oral supplementation, PLoS ONE 11 (10) (2016), e0164395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].O’Neill CM, Kazantzidis A, Ryan MJ, Barber N, Sempos CT, Durazo-Arvizu RA, Jorde R, Grimnes G, Eiriksdottir G, Gudnason V, Cotch MF, Kiely M, Webb AR, Cashman KD, Seasonal changes in vitamin D-effective UVB availability in europe and associations with population serum 25-hydroxyvitamin D, Nutrients 8 (9) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR, The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency, Am. J. Clin. Nutr 86 (4) (2007) 959–964. [DOI] [PubMed] [Google Scholar]
- [79].Holick MF, Matsuoka LY, Wortsman J, Age, vitamin D, and solar ultraviolet, Lancet 2 (8671) (1989) 1104–1105. [DOI] [PubMed] [Google Scholar]
- [80].Gallagher JC, Vitamin D and aging, Endocrinol. Metab. Clin. North Am 42 (2) (2013) 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Meehan M, Penckofer S, The role of vitamin D in the aging adult, J. Aging Gerontol 2 (2) (2014) 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S, Vitamin D, calcium homeostasis and aging, Bone Res. 4 (2016) 16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gloth FM 3rd, Gundberg CM, Hollis BW, Haddad JG Jr., Tobin JD, Vitamin D deficiency in homebound elderly persons, JAMA 274 (21) (1995) 1683–1686. [DOI] [PubMed] [Google Scholar]
- [84].Guessous I, Dudler V, Glatz N, Theler JM, Zoller O, Paccaud F, Burnier M, Bochud M, Swiss G Survey on Salt, Vitamin D levels and associated factors: a population-based study in Switzerland, Swiss Med Wkly 142 (2012). [DOI] [PubMed] [Google Scholar]
- [85].Arunabh S, Pollack S, Yeh J, Aloia JF, Body fat content and 25-hydroxyvitamin D levels in healthy women, J. Clin. Endocrinol. Metab 88 (1) (2003) 157–161. [DOI] [PubMed] [Google Scholar]
- [86].Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA, The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults, J. Clin. Endocrinol. Metab 89 (3) (2004) 1196–1199. [DOI] [PubMed] [Google Scholar]
- [87].Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J, The dependency of vitamin D status on body mass index, gender, age and season, Anticancer Res. 29 (9) (2009) 3713–3720. [PubMed] [Google Scholar]
- [88].Zhang Y, Zhang X, Wang F, Zhang W, Wang C, Yu C, Zhao J, Gao L, Xu J, The relationship between obesity indices and serum vitamin D levels in Chinese adults from urban settings, Asia Pac J Clin Nutr 25 (2) (2016) 333–339. [DOI] [PubMed] [Google Scholar]
- [89].Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB, Obesity and vitamin D deficiency: a systematic review and meta-analysis, Obes. Rev 16 (4) (2015) 341–349. [DOI] [PubMed] [Google Scholar]
- [90].Yao Y, Zhu L, He L, Duan Y, Liang W, Nie Z, Jin Y, Wu X, Fang Y, A meta-analysis of the relationship between vitamin D deficiency and obesity, Int. J. Clin. Exp. Med 8 (9) (2015) 14977–14984. [PMC free article] [PubMed] [Google Scholar]
- [91].Migliaccio S, Di Nisio A, Mele C, Scappaticcio L, Savastano S, Colao A, E.R. Obesity Programs of nutrition, G. Assessment, Obesity and hypovitaminosis D: causality or casualty?, Int. J. Obes. Suppl 9(1) (2019) 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hyppönen E, Power C, Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors, Am. J. Clin. Nutr 85 (3) (2007) 860–868. [DOI] [PubMed] [Google Scholar]
- [93].Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR, Vitamin D status and bone histomorphometry in gross obesity, Am. J. Clin. Nutr 34 (11) (1981) 2359–2363. [DOI] [PubMed] [Google Scholar]
- [94].Vanlint S, Vitamin D and obesity, Nutrients 5 (3) (2013) 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Abboud M, Gordon-Thomson C, Hoy AJ, Balaban S, Rybchyn MS, Cole L, Su Y, Brennan-Speranza TC, Fraser DR, Mason RS, Uptake of 25-hydroxyvitamin D by muscle and fat cells, J. Steroid. Biochem. Mol. Biol 144 (Pt A) (2014) 232–236. [DOI] [PubMed] [Google Scholar]
- [96].Muscogiuri G, Barrea L, Somma CD, Laudisio D, Salzano C, Pugliese G, de Alteriis G, Colao A, Savastano S, Sex differences of vitamin D status across BMI classes: an observational prospective cohort study, Nutrients 11 (12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Verdoia M, Schaffer A, Barbieri L, Di Giovine G, Marino P, Suryapranata H, De Luca G, G. Novara Atherosclerosis Study, Impact of gender difference on vitamin D status and its relationship with the extent of coronary artery disease, Nutr. Metab. Cardiovasc. Dis 25 (5) (2015) 464–470. [DOI] [PubMed] [Google Scholar]
- [98].Yan X, Zhang N, Cheng S, Wang Z, Qin Y, Gender differences in vitamin D status in China, Med. Sci. Monit 25 (2019) 7094–7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yoshimura N, Muraki S, Oka H, Morita M, Yamada H, Tanaka S, Kawaguchi H, Nakamura K, Akune T, Profiles of vitamin D insufficiency and deficiency in Japanese men and women: association with biological, environmental, and nutritional factors and coexisting disorders: the ROAD study, Osteoporos. Int 24 (11) (2013) 2775–2787. [DOI] [PubMed] [Google Scholar]
- [100].Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR, Determinants of vitamin D status in older men living in a subtropical climate, Osteoporos. Int 17 (12) (2006) 1742–1748. [DOI] [PubMed] [Google Scholar]
- [101].Blaak E, Gender differences in fat metabolism, Curr. Opin. Clin. Nutrit. Metabolic Care 4 (6) (2001). [DOI] [PubMed] [Google Scholar]
- [102].Gurrici S, Hartriyanti Y, Hautvast J, Deurenberg P, Relationship between body fat and body mass index: differences between Indonesians and Dutch Caucasians, Eur. J. Clin. Nutr 52 (11) (1998) 779–783. [DOI] [PubMed] [Google Scholar]
- [103].Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y, Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index, Am. J. Clin. Nutrit 72 (3) (2000) 694–701. [DOI] [PubMed] [Google Scholar]
- [104].Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF, Decreased bioavailability of vitamin D in obesity, Am. J. Clin. Nutr 72 (3) (2000) 690–693. [DOI] [PubMed] [Google Scholar]
- [105].Rabenberg M, Scheidt-Nave C, Busch MA, Rieckmann N, Hintzpeter B, Mensink GB, Vitamin D status among adults in Germany–results from the German Health Interview and Examination Survey for Adults (DEGS1), BMC Public Health 15 (2015) 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Janssen HC, Emmelot-Vonk MH, Verhaar HJ, van der Schouw YT, Determinants of vitamin D status in healthy men and women aged 40–80 years, Maturitas 74 (1) (2013) 79–83. [DOI] [PubMed] [Google Scholar]
- [107].Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, Vestergaard P, Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis, Osteoporos. Int 20 (1) (2009) 133–140. [DOI] [PubMed] [Google Scholar]
- [108].Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J, I.O.F.C.o.S. A. N.W. Group, Global vitamin D status and determinants of hypovitaminosis D, Osteoporos. Int 20 (11) (2009) 1807–1820. [DOI] [PubMed] [Google Scholar]
- [109].Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K, A systematic review of vitamin D status in populations worldwide, Br. J. Nutr 111 (1) (2014) 23–45. [DOI] [PubMed] [Google Scholar]
- [110].Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT, Taylor CL, Durazo-Arvizu RA, Maw KL, Chaudhary-Webb M, Johnson CL, Pfeiffer CM, National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US pop during 2007–2010, J. Nutrit 146 (5) (2016) 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sarafin K, Durazo-Arvizu R, Tian L, Phinney KW, Tai S, Camara JE, Merkel J, Green E, Sempos CT, Brooks SPJ, Standardizing 25-hydroxyvitamin D values from the Canadian Health Measures Survey, Am. J. Clin. Nutrit 102 (5) (2015) 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Cashman KD, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtuena J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Molgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GB, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Kiely M, Vitamin D deficiency in Europe: pandemic? Am. J. Clin. Nutr 103 (4) (2016) 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Holick MF, Vitamin D deficiency in 2010: health benefits of vitamin D and sunlight: a D-bate, Nat. Rev. Endocrinol 7 (2) (2011) 73–75. [DOI] [PubMed] [Google Scholar]
- [114].Manios Y, Moschonis G, Lambrinou CP, Mavrogianni C, Tsirigoti L, Hoeller U, Roos FF, Bendik I, Eggersdorfer M, Celis-Morales C, Livingstone KM, Marsaux CFM, Macready AL, Fallaize R, O’Donovan CB, Woolhead C, Forster H, Walsh MC, Navas-Carretero S, San-Cristobal R, Kolossa S, Hallmann J, Jarosz M, Surwillo A, Traczyk I, Drevon CA, van Ommen B, Grimaldi K, Matthews JNS, Daniel H, Martinez JA, Lovegrove JA, Gibney ER, Brennan L, Saris WHM, Gibney M, Mathers JC, S. Food4Me, Associations of vitamin D status with dietary intakes and physical activity levels among adults from seven European countries: the Food4Me study, Eur. J. Nutr 57 (4) (2018) 1357–1368. [DOI] [PubMed] [Google Scholar]
- [115].Touvier M, Deschasaux M, Montourcy M, Sutton A, Charnaux N, Kesse-Guyot E, Assmann KE, Fezeu L, Latino-Martel P, Druesne-Pecollo N, Guinot C, Latreille J, Malvy D, Galan P, Hercberg S, Le Clerc S, Souberbielle JC, Ezzedine K, Determinants of vitamin D status in Caucasian adults: influence of sun exposure, dietary intake, sociodemographic, lifestyle, anthropometric, and genetic factors, J. Invest. Dermatol 135 (2) (2015) 378–388. [DOI] [PubMed] [Google Scholar]
- [116].Sato Y, Iwamoto J, Kanoko T, Satoh K, Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer’s disease: a randomized controlled trial, J. Bone Miner. Res 20 (8) (2005)1327–1333. [DOI] [PubMed] [Google Scholar]
- [117].Jones G, Dwyer T, Bone mass in prepubertal children: gender differences and the role of physical activity and sunlight exposure, J. Clin. Endocrinol. Metab 83 (12) (1998) 4274–4279. [DOI] [PubMed] [Google Scholar]
- [118].Thuesen B, Husemoen L, Fenger M, Jakobsen J, Schwarz P, Toft U, Ovesen L, Jorgensen T, Linneberg A, Determinants of vitamin D status in a general population of Danish adults, Bone 50 (3) (2012) 605–610. [DOI] [PubMed] [Google Scholar]
- [119].Guzel R, Kozanoglu E, Guler-Uysal F, Soyupak S, Sarpel T, Vitamin D status and bone mineral density of veiled and unveiled Turkish women, J. Women’s Health Gender-Based Med 10 (8) (2001) 765–770. [DOI] [PubMed] [Google Scholar]
- [120].Mishal AA, Effects of different dress styles on vitamin D levels in healthy young Jordanian women, Osteoporos. Int 12 (11) (2001) 931–935. [DOI] [PubMed] [Google Scholar]
- [121].Chakhtoura M, Rahme M, Chamoun N, El-Hajj Fuleihan G, Vitamin D in the Middle East and North Africa, Bone Rep. 8 (2018) 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Neale RE, Khan SR, Lucas RM, Waterhouse M, Whiteman DC, Olsen CM, The effect of sunscreen on vitamin D: a review, Brit. J. Dermatol 181 (5) (2019) 907–915. [DOI] [PubMed] [Google Scholar]
- [123].Kritchevsky SB, Tooze JA, Neiberg RH, Schwartz GG, Hausman DB, Johnson MA, Bauer DC, Cauley JA, Shea MK, Cawthon PM, Harris TB, Rubin SM, Tylavsky FA, Houston DK, A.B.C.S. Health, 25-Hydroxy vitamin D, parathyroid hormone, and mortality in black and white older adults: the health ABC study, J. Clin. Endocrinol. Metab 97 (11) (2012) 4156–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Neer RM, The evolutionary significance of vitamin D, skin pigment, and ultraviolet light, Am. J. Phys. Anthropol 43 (3) (1975) 409–416. [DOI] [PubMed] [Google Scholar]
- [125].Jablonski NG, The evolution of human skin and skin color, Ann. Rev. Anthropol 33 (1) (2004) 585–623. [Google Scholar]
- [126].Webb AR, Who, what, where and when-influences on cutaneous vitamin D synthesis, Prog. Biophys. Mol. Biol 92 (1) (2006) 17–25. [DOI] [PubMed] [Google Scholar]
- [127].Bonilla C, Ness AR, Wills AK, Lawlor DA, Lewis SJ, Davey Smith G, Skin pigmentation, sun exposure and vitamin D levels in children of the Avon Longitudinal Study of Parents and Children, BMC Public Health 14 (2014) 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R, Vitamin D-binding protein and vitamin D status of black Americans and white Americans, N. Engl. J. Med 369 (21) (2013) 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ponchon G, DeLuca HF, The role of the liver in the metabolism of vitamin D, J. Clin. Invest 48 (7) (1969) 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ponchon G, Kennan AL, DeLuca HF, “Activation” of vitamin D by the liver, J. Clin. Invest 48 (11) (1969) 2032–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Bouillon R, Pauwels S, The vitamin D-binding protein, in: Feldman D, Pike WJ, Bouillon R, Giovannucci E, Goltzman D, Hewison M (Eds.), Vitamin D, Volume1: Biochemistry, Physiology and Diagnostics, Elsevier, 2018. [Google Scholar]
- [132].Bouillon R, Schuit F, Antonio L, Rastinejad F, Vitamin D binding protein: a historic overview, Front. Endocrinol. (Lausanne) 10 (2019) 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Fisher L, Fisher A, Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease, Clin. Gastroenterol. Hepatol 5 (4) (2007) 513–520. [DOI] [PubMed] [Google Scholar]
- [134].Arteh J, Narra S, Nair S, Prevalence of vitamin D deficiency in chronic liver disease, Dig. Dis. Sci 55 (9) (2010) 2624–2628. [DOI] [PubMed] [Google Scholar]
- [135].Konstantakis C, Tselekouni P, Kalafateli M, Triantos C, Vitamin D deficiency in patients with liver cirrhosis, Ann Gastroenterol 29 (3) (2016) 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Malham M, Jorgensen SP, Ott P, Agnholt J, Vilstrup H, Borre M, Dahlerup JF, Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology, World J. Gastroenterol 17 (7) (2011) 922–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Heaf JG, Hepatic Osteodystrophy, Scand. J. Gastroenterol 20 (9) (1985) 1035–1040. [DOI] [PubMed] [Google Scholar]
- [138].Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, Silecchia G, Leonetti F, Fraioli A, Picardi A, Morini S, Cavallo MG, Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus, Hepatology 56 (6) (2012) 2180–2187. [DOI] [PubMed] [Google Scholar]
- [139].Stokes CS, Volmer DA, Grunhage F, Lammert F, Vitamin D in chronic liver disease, Liver Int 33 (3) (2013) 338–352. [DOI] [PubMed] [Google Scholar]
- [140].Costa Silva M, Erotides Silva T, de Alentar ML, Honorio Coelho MS, Wildner LM, Bazzo ML, Gonzalez-Chica DA, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon Lde L, Factors associated with 25-hydroxyvitamin D levels in patients with liver cirrhosis, Ann. Hepatol 14 (1) (2015) 99–107. [PubMed] [Google Scholar]
- [141].Bikle DD, Gee E, Halloran B, Haddad JG, Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease, J. Clin. Invest 74 (6) (1984) 1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].White P, Cooke N, The multifunctional properties and characteristics of vitamin D-binding protein, Trends Endocrinol. Metab 11 (8) (2000) 320–327. [DOI] [PubMed] [Google Scholar]
- [143].Kitson MT, Roberts SK, D-livering the message: the importance of vitamin D status in chronic liver disease, J. Hepatol 57 (4) (2012) 897–909. [DOI] [PubMed] [Google Scholar]
- [144].Miroliaee A, Nasiri-Toosi M, Khalilzadeh O, Esteghamati A, Abdollahi A, Mazloumi M, Disturbances of parathyroid hormone-vitamin D axis in noncholestatic chronic liver disease: a cross-sectional study, Hepatol. Int 4 (3) (2010) 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Corey RL, Whitaker MD, Crowell MD, Keddis MT, Aqel B, Balan V, Byrne T, Carey E, Douglas DD, Harrison ME, Vargas HE, Rakela J, Vitamin D deficiency, parathyroid hormone levels, and bone disease among patients with end-stage liver disease and normal serum creatinine awaiting liver transplantation, Clin. Transplant 28 (5) (2014) 579–584. [DOI] [PubMed] [Google Scholar]
- [146].Wiese RJ, Uhland-Smith A, Ross TK, Prahl JM, DeLuca HF, Up-regulation of the vitamin D receptor in response to 1,25-dihydroxyvitamin D3 results from ligand-induced stabilization, J. Biol. Chem 267 (28) (1992) 20082–20086. [PubMed] [Google Scholar]
- [147].Dusso AS, Tokumoto M, Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease, Kidney Int. 79 (7) (2011) 715–729. [DOI] [PubMed] [Google Scholar]
- [148].Jean G, Souberbielle JC, Chazot C, Vitamin D in chronic kidney disease and dialysis patients, Nutrients 9 (4) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Guessous I, McClellan W, Kleinbaum D, Vaccarino V, Zoller O, Theler JM, Paccaud F, Burnier M, Bochud M, G. Swiss Survey on Salt, Comparisons of serum vitamin D levels, status, and determinants in populations with and without chronic kidney disease not requiring renal dialysis: a 24-hour urine collection population-based study, J. Ren. Nutr 24 (5) (2014) 303–312. [DOI] [PubMed] [Google Scholar]
- [150].Kim SM, Choi HJ, Lee JP, Kim DK, Oh YK, Kim YS, Lim CS, Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease, J. Ren. Nutr 24 (1) (2014) 20–25. [DOI] [PubMed] [Google Scholar]
- [151].Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P, 25-hydroxyvitamin D levels, race, and the progression of kidney disease, J. Am. Soc. Nephrol 20 (12) (2009) 2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Agarwal R, Georgianos PI, Con: Nutritional vitamin D replacement in chronic kidney disease and end-stage renal disease, Nephrol. Dial. Transplant 31 (5) (2016) 706–713. [DOI] [PubMed] [Google Scholar]
- [153].Goldsmith DJ, Pro: Should we correct vitamin D deficiency/insufficiency in chronic kidney disease patients with inactive forms of vitamin D or just treat them with active vitamin D forms? Nephrol. Dial. Transplant 31 (5) (2016) 698–705. [DOI] [PubMed] [Google Scholar]
- [154].Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL, Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease, Kidney Int. 71 (1) (2007) 31–38. [DOI] [PubMed] [Google Scholar]
- [155].Lee SW, Russell J, Avioli LV, 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol: conversion impaired by systemic metabolic acidosis, Science 195 (4282) (1977) 994–996. [DOI] [PubMed] [Google Scholar]
- [156].Hsu CH, Patel SR, Young EW, Vanholder R, Effects of purine derivatives on calcitriol metabolism in rats, Am. J. Physiol 260 (4 Pt 2) (1991) F596–F601. [DOI] [PubMed] [Google Scholar]
- [157].Vanholder R, Patel S, Hsu CH, Effect of uric acid on plasma levels of 1,25(OH) 2D in renal failure, J. Am. Soc. Nephrol 4 (4) (1993) 1035–1038. [DOI] [PubMed] [Google Scholar]
- [158].Bosworth CR, Levin G, Robinson-Cohen C, Hoofnagle AN, Ruzinski J, Young B, Schwartz SM, Himmelfarb J, Kestenbaum B, de Boer IH, The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease, Kidney Int. 82 (6) (2012) 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Waldron JL, Ashby HL, Cornes MP, Bechervaise J, Razavi C, Thomas OL, Chugh S, Deshpande S, Ford C, Gama R, Vitamin D: a negative acute phase reactant, J. Clin. Pathol 66 (7) (2013) 620–622. [DOI] [PubMed] [Google Scholar]
- [160].Saraf R, Morton SM, Camargo CA Jr., Grant CC, Global summary of maternal and newborn vitamin D status - a systematic review, Matern. Child Nutr 12 (4) (2016) 647–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Christesen HT, Elvander C, Lamont RF, Jorgensen JS, The impact of vitamin D in pregnancy on extraskeletal health in children: a systematic review, Acta Obstet. Gynecol. Scand 91 (12) (2012) 1368–1380. [DOI] [PubMed] [Google Scholar]
- [162].Christesen HT, Falkenberg T, Lamont RF, Jorgensen JS, The impact of vitamin D on pregnancy: a systematic review, Acta Obstet. Gynecol. Scand 91 (12) (2012) 1357–1367. [DOI] [PubMed] [Google Scholar]
- [163].Urrutia RP, Thorp JM, Vitamin D in pregnancy: current concepts, Curr. Opin. Obstet. Gynecol 24 (2) (2012) 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM, Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies, BMJ 346 (2013), f1169. [DOI] [PubMed] [Google Scholar]
- [165].Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, Cole Z, Tinati T, Godfrey K, Dennison E, Bishop NJ, Baird J, Cooper C, Vitamin D supplementation in pregnancy: a systematic review, Health Technol. Assess 18 (45) (2014) 1–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Amegah AK, Klevor MK, Wagner CL, Maternal vitamin D insufficiency and risk of adverse pregnancy and birth outcomes: a systematic review and meta-analysis of longitudinal studies, PLoS ONE 12 (3) (2017), e0173605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Curtis EM, Moon RJ, Harvey NC, Cooper C, Maternal vitamin D supplementation during pregnancy, Br. Med. Bull 126 (1) (2018) 57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Heijboer AC, Blankenstein MA, Kema IP, Buijs MM, Accuracy of 6 routine 25-hydroxyvitamin D assays: influence of vitamin D binding protein concentration, Clin. Chem 58 (3) (2012) 543–548. [DOI] [PubMed] [Google Scholar]
- [169].Tsuprykov O, Buse C, Skoblo R, Haq A, Hocher B, Reference intervals for measured and calculated free 25-hydroxyvitamin D in normal pregnancy, J. Steroid Biochem. Mol. Biol 181 (2018) 80–87. [DOI] [PubMed] [Google Scholar]
- [170].Tsuprykov O, Chen X, Hocher CF, Skoblo R, Lianghong Y, Hocher B, Why should we measure free 25(OH) vitamin D? J. Steroid Biochem. Mol. Biol 180 (2018) 87–104. [DOI] [PubMed] [Google Scholar]
- [171].Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D, Genome-wide association study of circulating vitamin D levels, Hum. Mol. Genet 19 (13) (2010) 2739–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Dickinson JL, Perera DI, van der Mei AF, Ponsonby AL, Polanowski AM, Thomson RJ, Taylor BV, McKay JD, Stankovich J, Dwyer T, Past environmental sun exposure and risk of multiple sclerosis: a role for the Cdx-2 Vitamin D receptor variant in this interaction, Mult. Scler 15 (5) (2009) 563–570. [DOI] [PubMed] [Google Scholar]
- [173].Orton SM, Morris AP, Herrera BM, Ramagopalan SV, Lincoln MR, Chao MJ, Vieth R, Sadovnick AD, Ebers GC, Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis, Am. J. Clin. Nutr 88 (2) (2008) 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D’Agostino RB Sr., Ordovas JM, O’Donnell CJ, Dawson-Hughes B, Vasan RS, Booth SL, Genetic and non-genetic correlates of vitamins K and D, Eur. J. Clin. Nutr 63 (4) (2009) 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Bahrami A, Sadeghnia HR, Tabatabaeizadeh SA, Bahrami-Taghanaki H, Behboodi N, Esmaeili H, Ferns GA, Mobarhan MG, Avan A, Genetic and epigenetic factors influencing vitamin D status, J. Cell. Physiol 233 (5) (2018) 4033–4043. [DOI] [PubMed] [Google Scholar]
- [176].Braithwaite VS, Jones KS, Schoenmakers I, Silver M, Prentice A, Hennig BJ, Vitamin D binding protein genotype is associated with plasma 25OHD concentration in West African children, Bone 74 (2015) 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Prosser DE, Jones G, Enzymes involved in the activation and inactivation of vitamin D, Trends Biochem. Sci 29 (12) (2004) 664–673. [DOI] [PubMed] [Google Scholar]
- [178].Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Roller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD, Common genetic determinants of vitamin D insufficiency: a genome-wide association study, Lancet 376 (9736) (2010) 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Lips P, Relative value of 25(OH)D and 1,25(OH)2D measurements, J. Bone Miner. Res 22 (11) (2007) 1668–1671. [DOI] [PubMed] [Google Scholar]
- [180].Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J, Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials, Arch. Intern. Med 169 (6) (2009) 551–561. [DOI] [PubMed] [Google Scholar]
- [181].Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, Almandoz JP, Mullan RJ, Lane MA, Liu H, Erwin PJ, Hensrud DD, Montori VM, Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis, J. Clin. Endocrinol. Metab 96 (10) (2011) 2997–3006. [DOI] [PubMed] [Google Scholar]
- [182].Su Z, Narla SN, Zhu Y, 25-Hydroxyvitamin D: analysis and clinical application, Clin. Chim. Acta 433 (2014) 200–205. [DOI] [PubMed] [Google Scholar]
- [183].Bikle DD, Vitamin D Assays, Front. Horm. Res 50 (2018) 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Holick MF, Vitamin D status: measurement, interpretation, and clinical application, Ann. Epidemiol 19 (2) (2009) 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Gil A, Plaza-Diaz J, Mesa MD, Vitamin D: classic and novel actions, Ann. Nutr. Metab 72 (2) (2018) 87–95. [DOI] [PubMed] [Google Scholar]
- [186].Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M, Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations, Steroids 75 (7) (2010) 477–488. [DOI] [PubMed] [Google Scholar]
- [187].Altieri B, Cavalier E, Bhattoa HP, Perez-Lopez FR, Lopez-Baena MT, Perez-Roncero GR, Chedraui P, Annweiler C, Della Casa S, Zelzer S, Herrmann M, Faggiano A, Colao A, Holick MF, Vitamin D testing: advantages and limits of the current assays, Eur. J. Clin. Nutr 74 (2) (2020) 231–247. [DOI] [PubMed] [Google Scholar]
- [188].Rezayi M, Ghayour-Mobarhan M, Tavakoly Sany SB, Fani M, Avan A, Pasdar Z, Ferns GA, Abouzari-Lotf E, Amiri IS, A comparison of analytical methods for measuring concentrations of 25-hydroxy vitamin D in biological samples, Anal. Methods 10 (47) (2018) 5599–5612. [Google Scholar]
- [189].Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited, J. Clin. Endocrinol. Metab 97 (4) (2012) 1153–1158. [DOI] [PubMed] [Google Scholar]
- [190].Medicine I.o., Dietary Reference Intakes for Calcium and Vitamin D, The National Academies Press, Washington, DC, 2011. [PubMed] [Google Scholar]
- [191].Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK, Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization, J. Clin. Endocrinol. Metab 89 (7) (2004) 3152–3157. [DOI] [PubMed] [Google Scholar]
- [192].Sempos CT, Durazo-Arvizu RA, Binkley N, Jones J, Merkel JM, Carter GD, Developing vitamin D dietary guidelines and the lack of 25-hydroxyvitamin D assay standardization: The ever-present past, J. Steroid Biochem. Mol. Biol 164 (2016) 115–119. [DOI] [PubMed] [Google Scholar]
- [193].Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, Vitamin DSP, Vitamin D status as an international issue: national surveys and the problem of standardization, Scand. J. Clin. Lab. Invest. Suppl 243 (2012) 32–40. [DOI] [PubMed] [Google Scholar]
- [194].Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM, Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry, Clin. Chem 57 (3) (2011) 441–448. [DOI] [PubMed] [Google Scholar]
- [195].Tai SS, Bedner M, Phinney KW, Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry, Anal. Chem 82 (5) (2010) 1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Stepman HCM, Vanderroost A, Van Uytfanghe K, Thienpont LM, Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry, Clin. Chem 57 (3) (2011) 441–448. [DOI] [PubMed] [Google Scholar]
- [197].Mineva EM, Schleicher RL, Chaudhary-Webb M, Maw KL, Botelho JC, Vesper HW, Pfeiffer CM, A candidate reference measurement procedure for quantifying serum concentrations of 25-hydroxyvitamin D(3) and 25-hydroxyvitamin D(2) using isotope-dilution liquid chromatography-tandem mass spectrometry, Anal. Bioanal. Chem 407 (19) (2015) 5615–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Binkley N, Sempos CT, Vitamin DSP, Standardizing vitamin D assays: the way forward, J. Bone Miner. Res 29 (8) (2014) 1709–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Sempos CT, Betz JM, Camara JE, Carter GD, Cavalier E, Clarke MW, Dowling KG, Durazo-Arvizu RA, Hoofnagle AN, Liu A, Phinney KW, Sarafin K, Wise SA, Coates PM, General steps to standardize the laboratory measurement of serum total 25-hydroxyvitamin D, J. AOAC Int 100 (5) (2017) 1230–1233. [DOI] [PubMed] [Google Scholar]
- [200].Stockl D, Sluss PM, Thienpont LM, Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis, Clin. Chim. Acta 408 (1–2) (2009) 8–13. [DOI] [PubMed] [Google Scholar]
- [201].Depreter B, Heijboer AC, Langlois MR, Accuracy of three automated 25-hydroxyvitamin D assays in hemodialysis patients, Clin. Chim. Acta 415 (2013) 255–260. [DOI] [PubMed] [Google Scholar]
- [202].Cavalier E, Lukas P, Bekaert AC, Carlisi A, Le Goff C, Delanaye P, Souberbielle JC, Analytical and clinical validation of the new Abbot Architect 25(OH)D assay: fit for purpose? Clin. Chem. Lab. Med 55 (3) (2017) 378–384. [DOI] [PubMed] [Google Scholar]
- [203].Cavalier E, Lukas P, Crine Y, Peeters S, Carlisi A, Le Goff C, Gadisseur R, Delanaye P, Souberbielle JC, Evaluation of automated immunoassays for 25(OH)-vitamin D determination in different critical populations before and after standardization of the assays, Clin. Chim. Acta 431 (2014) 60–65. [DOI] [PubMed] [Google Scholar]
- [204].Moreau E, Bacher S, Mery S, Le Goff C, Piga N, Vogeser M, Hausmann M, Cavalier E, Performance characteristics of the VIDAS(R) 25-OH Vitamin D Total assay - comparison with four immunoassays and two liquid chromatography-tandem mass spectrometry methods in a multicentric study, Clin. Chem. Lab. Med 54 (1) (2016) 45–53. [DOI] [PubMed] [Google Scholar]
- [205].Rousseau AF, Damas P, Janssens M, Kalin S, Ledoux D, Le Goff C, Gadisseur R, Delanaye P, Cavalier E, Critical care and vitamin D status assessment: what about immunoassays and calculated free 25OH-D? Clin. Chim. Acta 437 (2014) 43–47. [DOI] [PubMed] [Google Scholar]
- [206].Shu I, Pina-Oviedo S, Quiroga-Garza G, Meng QH, Wang P, Influence of vitamin D2 percentage on accuracy of 4 commercial total 25-hydroxyvitamin D assays, Clin. Chem 59 (8) (2013) 1273–1275. [DOI] [PubMed] [Google Scholar]
- [207].Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, Drezner MK, Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults, J. Clin. Endocrinol. Metab 96 (4) (2011) 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M, Hoofnagle AN, Carter GD, Durazo-Arvizu RA, Sempos CT, Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin. Chem 61 (4) (2015) 636–645. [DOI] [PubMed] [Google Scholar]
- [209].Carter GD, Jones JC, Shannon J, Williams EL, Jones G, Kaufmann M, Sempos C, 25-Hydroxyvitamin D assays: potential interference from other circulating vitamin D metabolites, J. Steroid Biochem. Mol. Biol 164 (2016) 134–138. [DOI] [PubMed] [Google Scholar]
- [210].Phinney KW, Sempos CT, Tai SS, Camara JE, Wise SA, Eckfeldt JH, Hoofnagle AN, Carter GD, Jones J, Myers GL, Durazo-Arvizu R, Miller WG, Bachmann LM, Young IS, Pettit J, Caldwell G, Liu A, Brooks SPJ, Sarafin K, Thamm M, Mensink GBM, Busch M, Rabenberg M, Cashman KD, Kiely M, Galvin K, Zhang JY, Kinsella M, Oh K, Lee SW, Jung CL, Cox L, Goldberg G, Guberg K, Meadows S, Prentice A, Tian L, Brannon PM, Lucas RM, Crump PM, Cavalier E, Merkel J, Betz JM, Baseline assessment of 25-hydroxyvitamin D reference material and proficiency testing/external quality assurance material commutability: a vitamin D standardization program study, J. AOAC Int 100 (5) (2017) 1288–1293. [DOI] [PubMed] [Google Scholar]
- [211].Wise SA, Phinney KW, Tai SS, Camara JE, Myers GL, Durazo-Arvizu R, Tian L, Hoofnagle AN, Bachmann LM, Young IS, Pettit J, Caldwell G, Liu A, Brooks SPJ, Sarafin K, Thamm M, Mensink GBM, Busch M, Rabenberg M, Cashman KD, Kiely M, Kinsella M, Galvin K, Zhang JY, Oh K, Lee SW, Jung CL, Cox L, Goldberg G, Guberg K, Prentice A, Carter GD, Jones J, Brannon PM, Lucas RM, Crump PM, Cavalier E, Merkel J, Betz JM, Sempos CT, Baseline assessment of 25-hydroxyvitamin D assay performance: a vitamin D standardization program (VDSP) interlaboratory comparison study, J. AOAC Int 100 (5) (2017) 1244–1252. [DOI] [PubMed] [Google Scholar]
- [212].Dirks NF, Ackermans MT, Lips P, de Jongh RT, Vervloet MG, de Jonge R, Heijboer AC, The when, what & how of measuring vitamin D metabolism in clinical medicine, Nutrients 10 (4) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Zalewski A, Ma NS, Legeza B, Renthal N, Fluck CE, Pandey AV, Vitamin D-dependent rickets type 1 caused by mutations in CYP27B1 affecting protein interactions with adrenodoxin, J. Clin. Endocrinol. Metab 101 (9) (2016) 3409–3418. [DOI] [PubMed] [Google Scholar]
- [214].A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium, Nat Genet 11(2) (1995) 130–6. [DOI] [PubMed] [Google Scholar]
- [215].Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ, Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations, J. Clin. Endocrinol. Metab 95 (4) (2010) 1846–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Malloy PJ, Feldman D, Genetic disorders and defects in vitamin d action, Endocrinol. Metab. Clin. North Am 39(2) (2010) 333–46, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, Chen S, Zehnder D, Lin YC, Yang H, Hewison M, Adams JS, Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density, Gut 53 (8) (2004) 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Karakelides H, Geller JL, Schroeter AL, Chen H, Behn PS, Adams JS, Hewison M, Wermers RA, Vitamin D-mediated hypercalcemia in slack skin disease: evidence for involvement of extrarenal 25-hydroxyvitamin D 1alpha-hydroxylase, J. Bone Miner. Res 21 (9) (2006) 1496–1499. [DOI] [PubMed] [Google Scholar]
- [219].Donovan PJ, Sundae L, Pretorius CJ, d’Emden MC, McLeod DS, Calcitriol-mediated hypercalcemia: causes and course in 101 patients, J. Clin. Endocrinol. Metab 98 (10) (2013) 4023–4029. [DOI] [PubMed] [Google Scholar]
- [220].Adams JS, Rafison B, Witzel S, Reyes RE, Shieh A, Chun R, Zavala K, Hewison M, Liu PT, Regulation of the extrarenal CYP27B1-hydroxylase, J. Steroid Biochemi. Mol. Biol 144 (2014) 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Hawkes CP, Schnellbacher S, Singh RJ, Levine MA, 25-Hydroxyvitamin D can interfere with a common assay for 1,25-dihydroxyvitamin D in vitamin D intoxication, J. Clin. Endocrinol. Metab 100 (8) (2015) 2883–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Zittermann A, Ernst JB, Becker T, Dreier J, Knabbe C, Gummert JF, Kuhn J, Measurement of circulating 1,25-dihydroxyvitamin D: comparison of an automated method with a liquid chromatography tandem mass spectrometry method, Int. J. Anal. Chem 2016 (2016) 8501435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Valcour A, Zierold C, Podgorski AL, Olson GT, Wall JV, DeLuca HF, Bonelli F, A novel, fully-automated, chemiluminescent assay for the detection of 1,25-dihydroxyvitamin D in biological samples, J. Steroid Biochem. Mol. Biol 164 (2016) 120–126. [DOI] [PubMed] [Google Scholar]
- [224].Pauwels S, Jans I, Billen J, Heijboer A, Verstuyf A, Carmeliet G, Mathieu C, Maestro M, Waelkens E, Evenepoel P, Bouillon R, Vanderschueren D, Vermeersch P, 1beta,25-Dihydroxyvitamin D3: a new vitamin D metabolite in human serum, J. Steroid Biochem. Mol. Biol 173 (2017) 341–348. [DOI] [PubMed] [Google Scholar]
- [225].Dirks NF, Martens F, Vanderschueren D, Billen J, Pauwels S, Ackermans MT, Endert E, Heijer MD, Blankenstein MA, Heijboer AC, Determination of human reference values for serum total 1,25-dihydroxyvitamin D using an extensively validated 2D ID-UPLC-MS/MS method, J. Steroid Biochem. Mol. Biol 164 (2016) 127–133. [DOI] [PubMed] [Google Scholar]
- [226].Petkovich M, Jones G, CYP24A1 and kidney disease, Curr. Opin. Nephrol. Hypertens 20 (4) (2011) 337–344. [DOI] [PubMed] [Google Scholar]
- [227].Leeuwenkamp OR, van der Wiel HE, Lips P, van der Vijgh WJ, Barto R, Greuter H, Netelenbos JC, Human pharmacokinetics of orally administered (24 R)-hydroxycalcidiol, Eur. J. Clin. Chem. Clin. Biochem 31 (7) (1993) 419–426. [DOI] [PubMed] [Google Scholar]
- [228].Pike JW, Meyer MB, Regulation of mouse Cyp24a1 expression via promoter-proximal and downstream-distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D(3) action, Arch. Biochem. Biophys 523 (1) (2012) 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].de Boer IH, Sachs MC, Chonchol M, Himmelfarb J, Hoofnagle AN, Ix JH, Kremsdorf RA, Lin YS, Mehrotra R, Robinson-Cohen C, Siscovick DS, Steffes MW, Thummel KE, Tracy RP, Wang Z, Kestenbaum B, Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: a participant-level analysis of 5 cohort studies and clinical trials, Am. J. Kidney Dis 64 (2) (2014) 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Berg AH, Powe CE, Evans MK, Wenger J, Ortiz G, Zonderman AB, Suntharalingam P, Lucchesi K, Powe NR, Karumanchi SA, Thadhani RI, 24,25-Dihydroxyvitamin d3 and vitamin D status of community-dwelling black and white Americans, Clin. Chem 61 (6) (2015) 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Endo H, Kiyoki M, Kawashima K, Naruchi T, Hashimoto Y, Vitamin D3 metabolites and PTH synergistically stimulate bone formation of chick embryonic femur in vitro, Nature 286 (5770) (1980) 262–264. [DOI] [PubMed] [Google Scholar]
- [232].van Driel M, Koedam M, Buurman CJ, Roelse M, Weyts F, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP, Evidence that both 1alpha,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization, J. Cell. Biochem 99 (3) (2006) 922–935. [DOI] [PubMed] [Google Scholar]
- [233].Ornoy A, Goodwin D, Noff D, Edelstein S, 24, 25-dihydroxyvitamin D is a metabolite of vitamin D essential for bone formation, Nature 276 (5687) (1978) 517–519. [DOI] [PubMed] [Google Scholar]
- [234].Norman AW, Henry HL, Malluche HH, 24R,25-Dihydroxyvitamin D3 and 1alpha,25-dihydroxyvitamin D3 are both indispensable for calcium and phosphorus homeostasis, Life Sci. 27 (3) (1980) 229–237. [DOI] [PubMed] [Google Scholar]
- [235].Norman AW, Okamura WH, Bishop JE, Henry HL, Update on biological actions of 1 alpha,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3, Mol. Cell. Endocrinol 197 (1–2) (2002) 1–13. [DOI] [PubMed] [Google Scholar]
- [236].Howard GA, Turner RT, Sherrard DJ, Baylink DJ, Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3, J. Biol. Chem 256 (15) (1981) 7738–7740. [PubMed] [Google Scholar]
- [237].Zhou S, LeBoff MS, Glowacki J, Vitamin D metabolism and action in human bone marrow stromal cells, Endocrinology 151 (1) (2010) 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Geng S, Zhou S, Glowacki J, Effects of 25-hydroxyvitamin D(3) on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase, J. Bone Miner. Res 26 (5) (2011) 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Curtis KM, Aenlle KK, Roos BA, Howard GA, 24R,25-dihydroxyvitamin D3 promotes the osteoblastic differentiation of human mesenchymal stem cells, Mol. Endocrinol 28 (5) (2014) 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].van Driel M, van Leeuwen JPTM, Vitamin D endocrine system and osteoblasts, Bonekey Rep 3 (2014) 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Henry HL, The 25(OH)D(3)/1alpha,25(OH)(2)D(3)-24R-hydroxylase: a catabolic or biosynthetic enzyme? Steroids 66 (3–5) (2001) 391–398. [DOI] [PubMed] [Google Scholar]
- [242].Ketha H, Kumar R, Singh RJ, LC-MS/MS for identifying patients with CYP24A1 mutations, Clin. Chem 62 (1) (2016) 236–242. [DOI] [PubMed] [Google Scholar]
- [243].Carpenter TO, CYP24A1 loss of function: clinical phenotype of monoallelic and biallelic mutations, J. Steroid Biochem. Mol. Biol 173 (2017) 337–340. [DOI] [PubMed] [Google Scholar]
- [244].Tebben PJ, Milliner DS, Horst RL, Harris PC, Singh RJ, Wu Y, Foreman JW, Chelminski PR, Kumar R, Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy, J. Clin. Endocrinol. Metab 97 (3) (2012) E423–E427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, Fehrenbach H, Wingen AM, Güran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M, Mutations in CYP24A1 and idiopathic infantile hypercalcemia, N. Engl. J. Med 365 (5) (2011) 410–421. [DOI] [PubMed] [Google Scholar]
- [246].Jacobs TP, Kaufman M, Jones G, Kumar R, Schlingmann K-P, Shapses S, Bilezikian JP, A lifetime of hypercalcemia and hypercalciuria, finally explained, J. Clin. Endocrinol. Metab 99 (3) (2014) 708–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Kaufmann M, Gallagher JC, Peacock M, Schlingmann K-P, Konrad M, DeLuca HF, Sigueiro R, Lopez B, Mourino A, Maestro M, St-Arnaud R, Finkelstein JS, Cooper DP, Jones G, Clinical Utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD, J. Clin. Endocrinol. Metab 99 (7) (2014) 2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Ketha H, Thacher TD, Oberhelman SS, Fischer PR, Singh RJ, Kumar R, Comparison of the effect of daily versus bolus dose maternal vitamin D(3) supplementation on the 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) ratio, Bone 110 (2018) 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Molin A, Baudoin R, Kaufmann M, Souberbielle JC, Ryckewaert A, Vantyghem MC, Eckart P, Bacchetta J, Deschenes G, Kesler-Roussey G, Coudray N, Richard N, Wraich M, Bonafiglia Q, Tiulpakov A, Jones G, Kottler M-L, CYP24A1 mutations in a cohort of hypercalcemic patients: evidence for a recessive trait, J. Clin. Endocrinol. Metab 100 (10) (2015) E1343–E1352. [DOI] [PubMed] [Google Scholar]
- [250].Selamet U, Katz R, Ginsberg C, Rifkin DE, Fried LF, Kritchevsky SB, Hoofhagle AN, Bibbins-Domingo K, Drew D, Harris T, Newman A, Gutiérrez OM, Sarnak MJ, Shlipak MG, Ix JH, Serum Calcitriol Concentrations and Kidney Function Decline, Heart Failure, and Mortality in Elderly Community-Living Adults: The Health, Aging, and Body Composition Study, American journal of kidney diseases : the official journal of the National Kidney Foundation 72 (3) (2018) 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, Sidhom G, Rousseau D, Cole DE, Vieth R, The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation, J. Steroid Biochem. Mol. Biol 126 (3–5) (2011) 72–77. [DOI] [PubMed] [Google Scholar]
- [252].Tang JCY, Nicholls H, Piec I, Washbourne CJ, Dutton JJ, Jackson S, Greeves J, Fraser WD, Reference intervals for serum 24,25-dihydroxyvitamin D and the ratio with 25-hydroxyvitamin D established using a newly developed LC-MS/MS method, J. Nutr. Biochem 46 (2017) 21–29. [DOI] [PubMed] [Google Scholar]
- [253].Fabregat-Cabello N, Farre-Segura J, Huyghebaert L, Peeters S, Le Goff C, Souberbielle JC, Cavalier É, A fast and simple method for simultaneous measurements of 25(OH)D, 24,25(OH)2D and the Vitamin D Metabolite Ratio (VMR) in serum samples by LC-MS/MS, Clin. Chim. Acta 473 (2017) 116–123. [DOI] [PubMed] [Google Scholar]
- [254].Jones G, Kaufmann M, Vitamin D metabolite profiling using liquid chromatography–tandem mass spectrometry (LC–MS/MS), J. Steroid Biochem. Mole. Biol 164 (2016) 110–114. [DOI] [PubMed] [Google Scholar]
- [255].Dirks NF, Ackermans MT, de Jonge R, Heijboer AC, Reference values for 24,25-dihydroxyvitamin D and the 25-hydroxyvitamin D/24,25-dihydroxyvitamin D ratio, Clin. Chem. Lab. Med (2019). [DOI] [PubMed] [Google Scholar]
- [256].Tai SS, Nelson MA, Candidate reference measurement procedure for the determination of (24R),25-dihydroxyvitamin D3 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry, Anal. Chem 87 (15) (2015) 7964–7970. [DOI] [PubMed] [Google Scholar]
- [257].Wise SA, Tai SS, Burdette CQ, Camara JE, Bedner M, Lippa KA, Nelson MA, Nalin F, Phinney KW, Sander LC, Betz JM, Sempos CT, Coates PM, Role of the national institute of standards and technology (NIST) in support of the vitamin D initiative of the national institutes of health, office of dietary supplements, J. AOAC Int 100 (5) (2017) 1260–1276. [DOI] [PubMed] [Google Scholar]
- [258].Wise SA, Tai SS, Nelson MA, Burdette CQ, Camara JE, Hoofnagle AN, Laha TJ, Carter GD, Jones J, Williams EL, Barclay ZJ, Jones G, Kaufmann M, Binkley N, Kapoor A, Ziegler T, Cashman KD, Dowling KG, Sempos CT, Interlaboratory comparison for the determination of 24,25-dihydroxyvitamin D (3) in human serum using liquid chromatography with tandem mass spectrometry, J. AOAC Int 100 (5) (2017) 1308–1317. [DOI] [PubMed] [Google Scholar]
- [259].Phinney KW, Bedner M, Tai SS, Vamathevan VV, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb M, Pfeiffer CM, Betz JM, Coates PM, Picciano MF, Development and certification of a standard reference material for vitamin D metabolites in human serum, Anal. Chem 84 (2) (2012) 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Reddy GS, Muralidharan KR, Okamura WH, Tserng KY, McLane JA, Metabolism of 1alpha,25-dihydroxyvitamin D(3) and its C-3 epimer 1alpha,25-dihydroxy-3-epi-vitamin D(3) in neonatal human keratinocytes, Steroids 66 (3–5) (2001)441–450. [DOI] [PubMed] [Google Scholar]
- [261].Kamao M, Tatematsu S, Reddy GS, Hatakeyama S, Sugiura M, Ohashi N, Kubodera N, Okano T, Isolation, identification and biological activity of 24R,25-dihydroxy-3-epi-vitamin D3: a novel metabolite of 24R,25-dihydroxyvitamin D3 produced in rat osteosarcoma cells (UMR 106), J. Nutr. Sci. Vitaminol. (Tokyo) 47 (2) (2001) 108–115. [DOI] [PubMed] [Google Scholar]
- [262].Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Ozono K, Kubodera N, Reddy GS, Okano T, C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation, J. Biol. Chem 279 (16) (2004) 15897–15907. [DOI] [PubMed] [Google Scholar]
- [263].Al-Zohily B, Al-Menhali A, Gariballa S, Haq A, Shah I, Epimers of vitamin D: a review, Int. J. Mol. Sci 21 (2) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Kamao M, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Kubodera N, Reddy GS, Okano T, Measurement and characterization of C-3 epimerization activity toward vitamin D3, Arch. Biochem. Biophys 436 (1) (2005) 196–205. [DOI] [PubMed] [Google Scholar]
- [265].Bailey D, Veljkovic K, Yazdanpanah M, Adeli K, Analytical measurement and clinical relevance of vitamin D(3) C3-epimer, Clin. Biochem 46 (3) (2013) 190–196. [DOI] [PubMed] [Google Scholar]
- [266].Brown AJ, Ritter CS, Weiskopf AS, Vouros P, Sasso GJ, Uskokovic MR, Wang G, Reddy GS, Isolation and identification of 1alpha-hydroxy-3-epi-vitamin D3, a potent suppressor of parathyroid hormone secretion, J. Cell. Biochem 96 (3) (2005) 569–578. [DOI] [PubMed] [Google Scholar]
- [267].Molnár F, Sigüeiro R, Sato Y, Araujo C, Schuster I, Antony P, Peluso J, Muller C, Mouriño A, Moras D, Rochel N, 1α,25(OH)2–3-Epi-vitamin D3, a natural physiological metabolite of vitamin D3: its synthesis, biological activity and crystal structure with its receptor, PLoS ONE 6 (3) (2011), e18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Singh RJ, Taylor RL, Reddy GS, Grebe SK, C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status, J. Clin. Endocrinol. Metab 91 (8) (2006) 3055–3061. [DOI] [PubMed] [Google Scholar]
- [269].Cooke DJ, Cooke BR, Bell DA, Vasikaran SD, Glendenning P, 25-Hydroxyvitamin D C3-epimer is universally present in neonatal Western Australian samples but is unlikely to contribute to diagnostic misclassification, Ann. Clin. Biochem 53 (Pt 5) (2016) 593–598. [DOI] [PubMed] [Google Scholar]
- [270].Stepman HC, Vanderroost A, Stockl D, Thienpont LM, Full-scan mass spectral evidence for 3-epi-25-hydroxyvitamin D(3) in serum of infants and adults, Clin. Chem. Lab. Med 49 (2) (2011) 253–256. [DOI] [PubMed] [Google Scholar]
- [271].Lensmeyer G, Poquette M, Wiebe D, Binkley N, The C-3 epimer of 25-hydroxyvitamin D(3) is present in adult serum, J. Clin. Endocrinol. Metab 97 (1) (2012) 163–168. [DOI] [PubMed] [Google Scholar]
- [272].Cashman KD, Kinsella M, Walton J, Flynn A, Hayes A, Lucey AJ, Seamans KM, Kiely M, The 3 epimer of 25-hydroxycholecalciferol is present in the circulation of the majority of adults in a nationally representative sample and has endogenous origins, J. Nutrit 144 (7) (2014) 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Glendenning P, Issues of standardization and assay-specific clinical decision limits for the measurement of 25-hydroxyvitamin D, Am. J. Clin. Nutr 77 (2) (2003) 522–523. [DOI] [PubMed] [Google Scholar]
- [274].Roth HJ, Schmidt-Gayk H, Weber H, Niederau C, Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference, Ann. Clin. Biochem 45 (Pt 2) (2008) 153–159. [DOI] [PubMed] [Google Scholar]
- [275].van den Ouweland JM, Beijers AM, van Daal H, Overestimation of 25-hydroxyvitamin D3 by increased ionisation efficiency of 3-epi-25-hydroxyvitamin D3 in LC-MS/MS methods not separating both metabolites as determined by an LC-MS/MS method for separate quantification of 25-hydroxyvitamin D3, 3-epi-25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum, J. Chromatogr. B, Anal. Technol. Biomed. Life Sci 967 (2014) 195–202. [DOI] [PubMed] [Google Scholar]
- [276].van den Ouweland JM, Beijers AM, van Daal H, Elisen MG, Steen G, Wielders JP, Evaluation of 3-epi-25-hydroxyvitamin D3 cross-reactivity in the Roche Elecsys Vitamin D Total protein binding assay, Clin. Chem. Lab. Med 52 (3) (2014) 373–380. [DOI] [PubMed] [Google Scholar]
- [277].Chouinard CD, Cruzeiro VWD, Beekman CR, Roitberg AE, Yost RA, Investigating differences in gas-phase conformations of 25-hydroxyvitamin D3 sodiated epimers using ion mobility-mass spectrometry and theoretical modeling, J. Am. Soc. Mass Spectrom 28 (8) (2017) 1497–1505. [DOI] [PubMed] [Google Scholar]
- [278].Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M, State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods, Clin. Chem 58 (3) (2012) 531–542. [DOI] [PubMed] [Google Scholar]
- [279].Farrell C, Soldo J, Williams P, Herrmann M, 25-Hydroxyvitamin D testing: challenging the performance of current automated immunoassays, Clin. Chem. Lab. Med 50 (11) (2012) 1953–1963. [DOI] [PubMed] [Google Scholar]
- [280].Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M, Vitamin D and DBP: the free hormone hypothesis revisited, J. Steroid Biochem. Mol. Biol 144 Pt A (2014) 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Bikle DD, Schwartz J, Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions, Front. Endocrinol 10 (2019) 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Mendel CM, The free hormone hypothesis: a physiologically based mathematical model, Endocr. Rev 10 (3) (1989) 232–274. [DOI] [PubMed] [Google Scholar]
- [283].Hirschfeld J, Immune-electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins, Acta Pathol. Microbiol. Scand 47 (1959) 160–168. [DOI] [PubMed] [Google Scholar]
- [284].Daiger SP, Schanfield MS, Cavalli-Sforza LL, Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D, Proc. Natl. Acad. Sci. U S A 72 (6) (1975) 2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Speeckaert M, Huang G, Delanghe JR, Taes YE, Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism, Clin. Chim. Acta 372 (1–2) (2006) 33–42. [DOI] [PubMed] [Google Scholar]
- [286].Bhan I, Vitamin d binding protein and bone health, Int. J. Endocrinol 2014 (2014), 561214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Adebanjo OA, Moonga BS, Haddad JG, Huang CL, Zaidi M, A possible new role for vitamin D-binding protein in osteoclast control: inhibition of extracellular Ca2+ sensing at low physiological concentrations, Biochem. Biophys. Res. Commun 249 (3) (1998) 668–671. [DOI] [PubMed] [Google Scholar]
- [288].Chun RF, New perspectives on the vitamin D binding protein, Cell Biochem. Funct 30 (6) (2012) 445–456. [DOI] [PubMed] [Google Scholar]
- [289].Delanghe JR, Speeckaert R, Speeckaert MM, Behind the scenes of vitamin D binding protein: more than vitamin D binding, Best Pract. Res. Clin. Endocrinol. Metab 29 (5) (2015) 773–786. [DOI] [PubMed] [Google Scholar]
- [290].Cooke NE, Haddad JG, Vitamin D binding protein (Gc-globulin), Endocr. Rev 10 (3) (1989) 294–307. [DOI] [PubMed] [Google Scholar]
- [291].Hagenfeldt Y, Carlström K, Berlin T, Stege R, Effects of orchidectomy and different modes of high dose estrogen treatment on circulating “free” and total 1,25-dihydroxyvitamin D in patients with prostatic cancer, J. Steroid Biochem. Mol. Biol 39 (2) (1991) 155–159. [DOI] [PubMed] [Google Scholar]
- [292].Møller UK, Streym S, Heickendorff L, Mosekilde L, Rejnmark L, Effects of 25OHD concentrations on chances of pregnancy and pregnancy outcomes: a cohort study in healthy Danish women, Eur. J. Clin. Nutr 66 (7) (2012) 862–868. [DOI] [PubMed] [Google Scholar]
- [293].Zhang JY, Lucey AJ, Horgan R, Kenny LC, Kiely M, Impact of pregnancy on vitamin D status: a longitudinal study, Br. J. Nutr 112 (7) (2014) 1081–1087. [DOI] [PubMed] [Google Scholar]
- [294].Moller UK, Streym S, Jensen LT, Mosekilde L, Schoenmakers I, Nigdikar S, Rejnmark L, Increased plasma concentrations of vitamin D metabolites and vitamin D binding protein in women using hormonal contraceptives: a cross-sectional study, Nutrients 5 (9) (2013) 3470–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Jassil NK, Sharma A, Bikle D, Wang X, Vitamin D binding protein and 25-hydroxyvitamin D levels: emerging clinical applications, Endocr. Pract 23 (5) (2017) 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Wang X, Shapses SA, Al-Hraishawi H, Free and bioavailable 25-hydroxyvitamin D levels in patients with primary hyperparathyroidism, Endocr. Pract 23 (1) (2017) 66–71. [DOI] [PubMed] [Google Scholar]
- [297].Bjorkhem-Bergman L, Torefalk E, Ekstrom L, Bergman P, Vitamin D binding protein is not affected by high-dose vitamin D supplementation: a post hoc analysis of a randomised, placebo-controlled study, BMC Res. Notes 11 (1) (2018) 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Arnaud J, Constans J, Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP), Hum. Genet 92 (2) (1993) 183–188. [DOI] [PubMed] [Google Scholar]
- [299].Lauridsen AL, Vestergaard P, Nexo E, Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women, Clin. Chem 47 (4) (2001) 753–756. [PubMed] [Google Scholar]
- [300].Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P, Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration, J. Clin. Invest 67 (3) (1981) 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Bouillon R, Free or Total 25OHD as marker for vitamin D status? J. Bone Miner. Res 31 (6) (2016) 1124–1127. [DOI] [PubMed] [Google Scholar]
- [302].Vieth R, Simple method for determining specific binding capacity of vitamin D-binding protein and its use to calculate the concentration of “free” 1,25-dihydroxyvitamin D, Clin. Chem 40 (3) (1994) 435–441. [PubMed] [Google Scholar]
- [303].Chun RF, Nielson CM, Free vitamin D: concepts, assays, outcomes and prospects, in: Feldman D, Wesley Pike J, Bouillon R, Giovannucci E, Goltzman D, Hewison M (Eds.), Vitamin D volume 1: biochemistry, Physiology and Diagnosis, Elsevier, 2017. [Google Scholar]
- [304].Christensen EI, Birn H, Megalin and cubilin: multifunctional endocytic receptors, Nat. Rev. Mol. Cell Biol 3 (4) (2002) 256–266. [DOI] [PubMed] [Google Scholar]
- [305].Nielsen R, Christensen EI, Birn H, Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease, Kidney Int. 89 (1) (2016) 58–67. [DOI] [PubMed] [Google Scholar]
- [306].Schwartz JB, Lai J, Lizaola B, Kane L, Weyland P, Terrault NA, Stotland N, Bikle D, Variability in free 25(OH) vitamin D levels in clinical populations, J. Steroid Biochem. Mol. Biol 144 Pt A (2014) 156–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI, Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients, Kidney Int. 82 (1) (2012) 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Bikle DD, Siiteri PK, Ryzen E, Haddad JG, Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels, J. Clin. Endocrinol. Metab 61 (5) (1985) 969–975. [DOI] [PubMed] [Google Scholar]
- [309].Bikle DD, Halloran BP, Gee E, Ryzen E, Haddad JG, Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels, J. Clin. Invest 78 (3) (1986) 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Heureux N, Lindhout E, Swinkels L, A direct assay for measuring free 25-hydroxyvitamin D, J. AOAC Int 100 (5) (2017) 1318–1322. [DOI] [PubMed] [Google Scholar]
- [311].Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG, Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein, J. Clin. Endocrinol. Metab 63 (4) (1986) 954–959. [DOI] [PubMed] [Google Scholar]
- [312].Feldman H, Rodbard D, Levine D, Mathematical theory of cross-reactive radioimmunoassay and ligand-binding systems of equilibrium, Anal. Biochem 45 (2) (1972) 530–556. [DOI] [PubMed] [Google Scholar]
- [313].Dunn JF, Computer simulation of vitamin D transport, Ann. N. Y. Acad. Sci 538 (1988) 69–76. [DOI] [PubMed] [Google Scholar]
- [314].Chen H, Wiepjes CM, van Schoor NM, Heijboer AC, de Jongh RT, den Heijer M, Lips P, Changes of vitamin D-binding protein, and total, bioavailable, and free 25-hydroxyvitamin D in transgender people, J. Clin. Endocrinol. Metab 104 (7) (2019) 2728–2734. [DOI] [PubMed] [Google Scholar]
- [315].Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, Terrault NA, Stotland N, Bikle D, A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations, J. Clin. Endocrinol. Metab 99 (5) (2014) 1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Nielson CM, Jones KS, Bouillon R, G. Osteoporotic Fractures in Men Research, Chun RF, Jacobs J, Wang Y, Hewison M, Adams JS, Swanson CM, Lee CG, Vanderschueren D, Pauwels S, Prentice A, Smith RD, Shi T, Gao Y, Zmuda JM, Lapidus J, Cauley JA, Schoenmakers I, Orwoll ES, Role of assay type in determining free 25-hydroxyvitamin D levels in diverse populations, N. Engl. J. Med 374(17) (2016) 1695–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Nielson CM, Jones KS, Chun RF, Jacobs JM, Wang Y, Hewison M, Adams JS, Swanson CM, Lee CG, Vanderschueren D, Pauwels S, Prentice A, Smith RD, Shi T, Gao Y, Schepmoes AA, Zmuda JM, Lapidus J, Cauley JA, Bouillon R, Schoenmakers I, Orwoll ES, G. Osteoporotic Fractures in Men Research, Free 25-hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations, J. Clin. Endocrinol. Metab 101(5) (2016) 2226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].M’Buyamba-Kabangu JR, Fagard R, Lijnen P, Bouillon R, Lissens W, Amery A, Calcium, vitamin D-endocrine system, and parathyroid hormone in black and white males, Calcif. Tissue Int 41 (2) (1987) 70–74. [DOI] [PubMed] [Google Scholar]
- [319].Winters SJ, Chennubhatla R, Wang C, Miller JJ, Influence of obesity on vitamin D-binding protein and 25-hydroxy vitamin D levels in African American and white women, Metabolism 58 (4) (2009) 438–442. [DOI] [PubMed] [Google Scholar]
- [320].Henderson CM, Lutsey PL, Misialek JR, Laha TJ, Selvin E, Eckfeldt JH, Hoofnagle AN, Measurement by a novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D-binding protein in blacks and whites, Clin. Chem 62 (1) (2016) 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Hoofnagle AN, Eckfeldt JH, Lutsey PL, Vitamin D-binding protein concentrations quantified by mass spectrometry, N. Engl. J. Med 373 (15) (2015) 1480–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ, Durazo-Arvizu R, Whitehead K, Feldman HI, Leonard MB, i. Chronic Renal Insufficiency Cohort study, Comparison of Two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes, J. Bone Miner. Res 31(6) (2016) 1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Kilpatrick LE, Phinney KW, Quantification of total vitamin-D-binding protein and the glycosylated isoforms by liquid chromatography-isotope dilution mass spectrometry, J. Proteome Res 16 (11) (2017) 4185–4195. [DOI] [PubMed] [Google Scholar]
- [324].Volmer DA, Mendes LR, Stokes CS, Analysis of vitamin D metabolic markers by mass spectrometry: current techniques, limitations of the “gold standard” method, and anticipated future directions, Mass Spectrom. Rev 34 (1) (2015) 2–23. [DOI] [PubMed] [Google Scholar]
- [325].Bjerg LN, Halgreen JR, Hansen SH, Morris HA, Jorgensen NR, An evaluation of total 25-hydroxyvitamin D assay standardization: Where are we today? J. Steroid Biochem. Mol. Biol 190 (2019) 224–233. [DOI] [PubMed] [Google Scholar]
- [326].Holick MF, Vitamin D deficiency, N. Engl. J. Med 357 (3) (2007) 266–281. [DOI] [PubMed] [Google Scholar]
- [327].Holick MF, Resurrection of vitamin D deficiency and rickets, J. Clin. Investig 116 (8) (2006) 2062–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Jäpelt RB, Jakobsen J, Vitamin D in plants: a review of occurrence, analysis, and biosynthesis, Front. Plant Sci 4 (2013) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Schmid A, Walther B, Natural vitamin D content in animal products, Adv. Nutrit. (Bethesda Md.) 4 (4) (2013) 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D, Wicart P, Bockenhauer D, Santos F, Levtchenko E, Harvengt P, Kirchhoff M, Di Rocco F, Chaussain C, Brandi ML, Savendahl L, Briot K, Kamenicky P, Rejnmark L, Linglart A, Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia, Nat. Rev. Nephrol 15 (7) (2019) 435–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Beck-Nielsen SS, Mughal Z, Haffner D, Nilsson O, Levtchenko E, Ariceta G, de Lucas Collantes C, Schnabel D, Jandhyala R, Makitie O, FGF23 and its role in X-linked hypophosphatemia-related morbidity, Orphanet. J. Rare Dis 14 (1) (2019) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Yu OB, Arnold LA, Calcitroic acid-a review, ACS Chem. Biol 11 (10) (2016) 2665–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].St-Arnaud R, Jones G, Chapter 6 - CYP24A1: Structure, Function, And Physiological Role, in: Feldman D (Ed.), Vitamin D (Fourth Edition), Academic Press, 2018, pp. 81–95. [Google Scholar]
- [334].Toell A, Gonzalez MM, Ruf D, Steinmeyer A, Ishizuka S, Carlberg C, Different molecular mechanisms of vitamin D(3) receptor antagonists, Mol. Pharmacol 59 (6) (2001) 1478–1485. [DOI] [PubMed] [Google Scholar]
- [335].Ishizuka S, Norman AW, Metabolic pathways from 1 alpha,25-dihydroxyvitamin D3 to 1 alpha,25-dihydroxyvitamin D3–26,23-lactone. Stereoretained and stereo-selective lactonization, J. Biol. Chem 262 (15) (1987) 7165–7170. [PubMed] [Google Scholar]