Abstract
Background:
Though immune-mediated diseases (IMD) including inflammatory bowel diseases (IBD) are known to cluster, to what extent this is due to common environmental influences is unknown.
Aim:
To examine the incidence of IBD in individuals with another IMD.
Methods:
We used data from the prospective Nurses Health Study II cohort (1995–2017) to examine the effect of a diagnosis of one of several common IMDs on subsequent risk of Crohn’s disease (CD) or ulcerative colitis (UC) using Cox proportional hazards models, adjusting for detailed diet and lifestyle confounders.
Results:
We documented 132 cases of CD and 186 cases of UC over 2,016,163 person-years of follow-up (median age at IBD diagnosis 50 years). Compared to participants with no history of IMD, the HRs of CD for those with 1 and ≥ 2 IMDs were 2.57 (95% CI 1.77–3.74) and 2.74 (95% CI 1.36 to 5.49), respectively (Ptrend < 0.0001). This association was only modestly attenuated by adjustment for environmental risk factors (HR 2.35 and 2.46, respectively). The risk of UC was not increased, with multivariable-adjusted HRs of 1.22 (95% CI 0.85 to 1.76) and 1.33 (95% CI 0.67 to 2.65) for those with 1 and ≥ 2 IMDs, respectively, compared to those with none (Ptrend 0.16) (Pheterogeneity comparing CD and UC 0.037). Asthma, atopic dermatitis, psoriasis, and rosacea were individually associated with higher risk of CD (HR ranging from 2.15 to 3.39) but not UC.
Conclusions:
Individuals with one or more IMDs are at an increased risk for CD but not UC.
Keywords: inflammatory bowel disease, Crohn’s disease, ulcerative colitis, immune-mediated disease, autoimmune disease
INTRODUCTION
Immune-mediated diseases (IMDs), represented by autoimmune and allergic diseases, are a group of disabling medical conditions characterized by acute or chronic inflammation affecting a wide array of organ systems. Increasing in incidence over the past few decades1, they affect up to 10% of the Western population2 and often have protracted courses impairing quality of life and posing significant burden on the individual and the healthcare system3. While each IMD differs in the specific underlying pathophysiologic disruption, broadly they arise at the intersection of genetic predisposition and a dysregulated immune response, often to antigens in the external environment or the internal microenvironment, namely the microbiome4,5. Genome-wide association studies demonstrate sharing of suspceptibility loci across many IMDs. Such common genetic pathways may account for the clustering of these diseases6–8. Supporting this is data from observational studies that suggest frequent co-occurrence of multiple disorders within individuals and families9–11. However, an important limitation of the prior studies is the lack of ability to adjust for potential shared environmental influences that could also account for the clustering of IMDs. For example, smoking and obesity are associated with increased risk of Crohn’s disease12,13, asthma14,15, psoriasis16,17, and rheumatoid arthritis (RA)18,19. Thus, smoking behavior and body composition could potentially explain, at least in part, the co-occurrence of these diseases.
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC). These chronic inflammatory disorders of the gut affect an estimated 7 million individuals worldwide20. They are characterized by typical onset in early adulthood and progressive bowel damage. Prior studies examining the co-occurrence of CD or UC with other IMDs have been limited by a cross-sectional or case-control design6–8, and thus unable to present the magnitude of long-term risk of CD or UC in individuals with other IMDs. By relying on administrative health data, many of these studies are subject to ascertainment bias and reliance on billing codes that may lack sufficient accuracy to identify incident disease. More importantly, they were also limited by inability to adjust for key health behaviors that may be important confounders, such as smoking and diet.
To overcome limitations of these prior studies and to robustly provide an estimate of the absolute incidence of CD or UC in the presence of another IMD, we utilized data from an ongoing prospective cohort study of women with detailed information on diet and lifestyle to (1) examine the effect of a concurrent IMD diagnosis on the incidence of CD or UC after robust adjustment for environmental risk factors; and (2) define the magnitude of change in risk for several common IMDs.
METHODS
Study population
The Nurses’ Health Study (NHS) II, established in 1989, is an ongoing prospective cohort of 116,429 female registered nurses in the United States who were between the ages of 25 and 42 years at the time of enrollment. Self-reported questionnaires were mailed to each participant at baseline and every 2 years thereafter to obtain detailed information regarding demographics, lifestyle factors, and medical history. Diet was assessed using validated semi-quantitative food frequency questionnaires (FFQs) beginning in 1991 and updated every 4 years. The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
Ascertainment of immune-mediated disease
Our primary exposure of interest was the presence of a concurrent IMD prior to the diagnosis of CD or UC. The IMDs of interest included asthma, atopic dermatitis (AD), psoriasis, rosacea, type I diabetes mellitus (T1D), Guillain-Barré syndrome (GBS), multiple sclerosis (MS), RA, and SLE. Supplemental Table 1 presents the details of ascertainment for each individual IMD. In biennial questionnaires, participants were asked to report a diagnosis of asthma (biennially beginning in 1991), AD (2013), psoriasis (2005, 2009, 2013), rosacea (2005), diabetes mellitus (biennially beginning in 1989), MS (biennially beginning in 1991), RA (biennially beginning in 1991 except 1995) and SLE (biennially beginning in 1993 except 1995). For each disease, participants were sent a detailed supplemental questionnaire regarding symptoms, diagnostic testing, and medications. For most diseases, either medical records were reviewed, or strict algorithms were developed for a subset of patients to establish high validity of self-reported diagnosis (Supplementary Table 1). Information on type of diabetes (T1D vs T2D) was confirmed through supplemental questionnaire and medical record review. GBS was not inquired specifically in the biennial questionnaires and therefore all reports were of participants who wrote in their diagnosis. All write-in diagnoses were reviewed and assigned an ICD code. We identified GBS cases with ICD-8 code 354.0.
Ascertainment of inflammatory bowel disease
We have previously detailed our methods for defining cases of CD and UC21. Briefly, participants were asked to self-report a physician diagnosis of CD or UC in biennial questionnaires. For those who reported ever having been diagnosed with CD or UC, we obtained permission to review their medical records. A detailed supplemental questionnaire was also sent to these participants inquiring type of IBD, date of diagnosis, disease complications, and treatment. From participants who provided consent, medical records were obtained and independently reviewed by 2 board-certified gastroenterologists (H.K., A.N.A., P.L., E.W.L., K.E.B., J.M.R.) blinded to exposure and outcome. A diagnosis of CD or UC was made based on accepted clinical criteria incorporating symptoms, endoscopic, histologic, radiographic, or operative findings22,23. Disagreements on case definition were infrequent and resolved through consensus.
Assessment of covariates
In the baseline and biennial follow-up questionnaires, we assessed participants’ demographic and lifestyle factors including race, smoking, body mass index (BMI), physical activity, and medication use including nonsteroidal anti-inflammatory drug (NSAID), oral contraceptive pill, and menopausal hormone therapy. Physical activity was measured by multiplying the typical intensity expressed in metabolic equivalent of task (MET) by the reported hours spent per week. Region of residence was obtained using geocoded residential addresses to the street or ZIP code level and updated every 2 years beginning in 1989. Median family income was determined from the US Census data for the census tract of residence updated every 2 years. In the 2013 questionnaire, participants were asked to indicate if their biological siblings, offspring, or parents have ever had CD, UC, asthma, MS, or RA. Family history of IBD was defined by the presence of first degree relative with reported CD or UC while family history of other IMDs indicated the presence of asthma, MS, or RA in family members. Dietary information was assessed every 4 years using semi-quantitative validated FFQs24. As a covariate in our multivariable model, we adjusted for total fiber intake which has been previously reported to modify risk of CD25.
Statistical analysis
We established 1995 as the study baseline since this was the earliest year in which data on all prevalent IMDs were available. Individuals who were diagnosed with CD, UC, or cancer (except non-melanoma skin cancer) prior to 1995 were excluded from the analysis, while those diagnosed with any IMD prior to baseline remained in the study. Person-years were accrued from the return date of baseline questionnaire until a diagnosis of CD or UC, death, or the end of follow-up (June 1, 2017), whichever occurred first. Cox proportional hazards models were used to estimate the age-adjusted and multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) stratified by age and time period. All multivariable models were adjusted for race, area of residence, median family income, family history of IBD, family history of IMD, smoking, BMI, physical activity, total fiber intake, regular NSAID use, oral contraceptive pill, and menopausal hormone therapy.
IMDs of interest included asthma, AD, psoriasis, rosacea, T1D, GBS, MS, RA, and SLE. Status for all diseases was time-updated throughout the course of follow-up. For our main analysis, we examined the association between number of IMDs and risk of subsequent CD and UC. We grouped participants into 3 categories: 0, 1, and ≥ 2 IMDs. Tests for linear trend were conducted by modeling the number of diseases (range 0–7) as a continuous variable in the regression models. Test for heterogeneity of association comparing CD and UC was conducted using a likelihood ratio test, comparing a model that allows separate associations for the 2 IBD subtypes with a model that assumes a common association. We also examined the individual associations of incident CD or UC with 4 common IMDs–asthma, AD, psoriasis, rosacea. As the numbers of participants with each of the other IMDs was small, we pooled together participants with any of the following less frequent diagnoses: RA, SLE, T1D, MS, and GBS. To minimize competing effects from multiple IMDs, we censored participants at the time of diagnosis of other IMDs in the main analysis. In a sensitivity analysis, we allowed these individuals to contribute person-years to the reference group.
We performed a sensitivity analysis by adjusting for Mediterranean dietary pattern quantified using the aMED score as this pattern has been linked to risk of IBD in some studies26. We also repeated the analysis adjusting for intake of vitamin D which has been associated inversely with various IMDs27. In addition, we excluded those with family history of IBD and tested the heterogeneity of the association between number of IMDs and the 2 IBD subtypes. In a stratified analysis, we examined the association of any IMDs with CD and UC according to family history of other IMDs. Test for interaction was calculated by including a cross-product interaction term in the model and estimating the significance using Wald test. All analyses were conducted using the SAS software (SAS Institute, Inc., Version 9.4, Cary, NC). All statistical analyses were two-sided with a P value less than 0.05 indicating statistical significance.
Patient and public involvement
This research was done without public involvement. Participants were not invited to comment on the study design and were not consulted to develop relevant outcomes or interpret the results. Participants were not invited to contribute to the writing or editing of this paper for readability or accuracy.
Data availability
The data that support the findings of this study are available on request at https://www.nurseshealthstudy.org/researchers (email: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/. The data are not publicly available due to ethical restrictions.
RESULTS
Our study included 101,019 women from the NHS II among whom we documented 132 cases of CD (incidence 6.5/100,000 person-years) and 186 cases of UC (incidence 9.2/100,000 person-years) over 2,016,163 person-years of follow-up. The median age of IBD diagnosis was 50 years (range 33–69 years); 89% of the diagnoses were before the age of 60 years. A total of 20,710 women (20.5%) had one IMD, 4,613 (4.6%) had two, and 940 (0.9%) had more than two IMDs (Supplementary Figure 1). Among those with one IMD, the most common IMDs were asthma (31.7%), rosacea (18.9%), and AD (16.1%). A total of 39.4% of CD patients and 26.6% of UC patients had at least one IMD prior to IBD diagnosis. The most commonly diagnosed IMD in these patients was asthma, followed by atopic dermatitis and rosacea (Figure 1). The age-standardized baseline characteristics according to number of IMDs are summarized in Table 1. Participants with greater number of IMDs were more likely to have family history of IBD as well as other IMDs. Higher BMI and regular NSAID use were more prevalent among those with higher burden of IMDs.
Figure 1. Immune-mediated diseases among participants with Crohn’s disease and ulcerative colitis in the Nurses’ Health Study II (1995–2017).
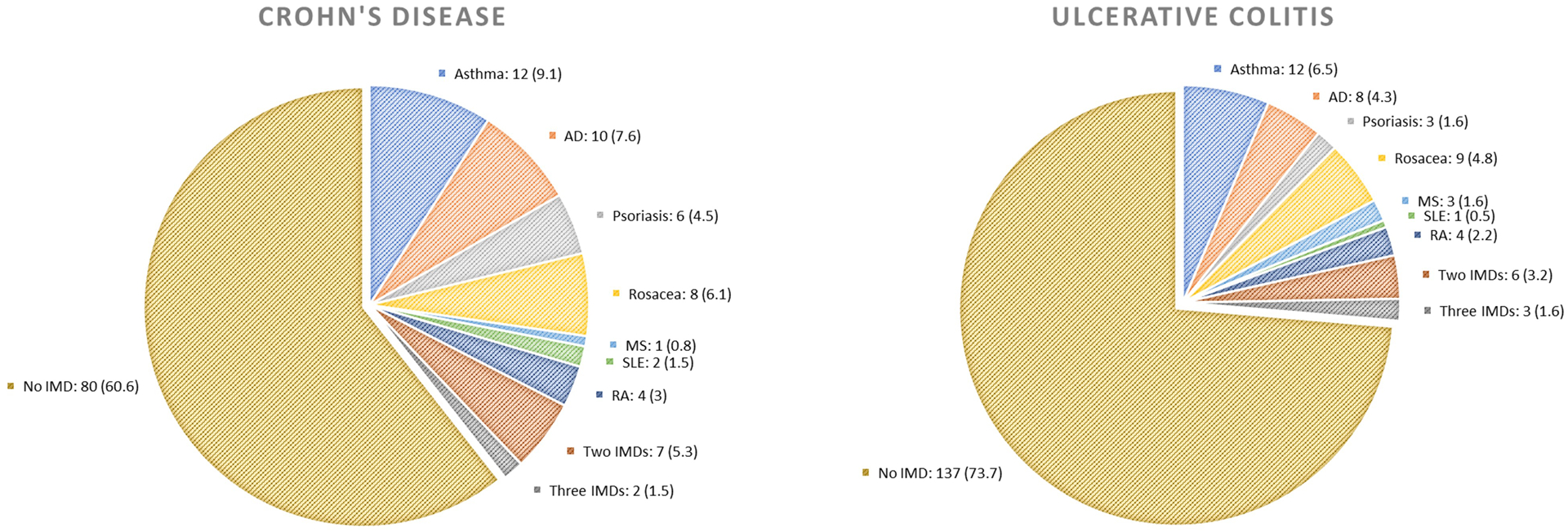
This figure shows the number and proportion of participants who were diagnosed with immune-mediated disease at the time of diagnosis of Crohn’s disease (n =132) and ulcerative colitis (n =186).
Table 1.
Age-standardized characteristics of study participants according to history of immune-mediated disease in the Nurses’ Health Study II (1995–2017)a
| Number of immune-mediated diseasesb | |||
|---|---|---|---|
| Variablec | 0 | 1 | ≥ 2 |
| Person-years | 1579394 | 359363 | 66140 |
| Age at baseline, year, mean (SD) | 40.1 (4.7) | 40.6 (4.6) | 40.9 (4.5) |
| Race, % | |||
| Southern European/Mediterranean | 18 | 17 | 18 |
| Scandinavian | 8 | 8 | 8 |
| Other Caucasian | 66 | 68 | 68 |
| Asian, Hispanic, African or others | 8 | 7 | 6 |
| Region of residence, % | |||
| New England | 5 | 6 | 6 |
| Mid-Atlantic | 28 | 29 | 30 |
| Midwest | 35 | 34 | 32 |
| Southeast | 9 | 8 | 8 |
| West | 5 | 6 | 6 |
| Annual family income, US dollar, mean (SD) | 64850 (24000) | 65443 (23848) | 65985 (23654) |
| Family history of inflammatory bowel disease, % | 4 | 6 | 7 |
| Family history of immune-mediated disease, % | 18 | 31 | 42 |
| Smoking, % | |||
| Never smoker | 66 | 65 | 64 |
| Past smoker | 26 | 28 | 29 |
| Current smoker | 8 | 7 | 7 |
| Body mass index, kg/m2, mean (SD) | 26.6 (6.0) | 27.7 (6.7) | 28.7 (7.4) |
| Physical activity, MET-hr/wk, mean (SD) | 22.7 (24.5) | 21.2 (21.8) | 21.0 (21.5) |
| Vitamin D intake, IU/d, mean (SD) | 418.7 (224.6) | 438.0 (231.2) | 450.3 (234.1) |
| Total fiber intake, g/d, mean (SD) | 19.3 (4.9) | 19.5 (5.0) | 19.7 (5.0) |
| Nonsteroidal anti-inflammatory drug use, % | 34 | 42 | 46 |
| Oral contraceptive use, % | |||
| Never user | 19 | 14 | 13 |
| Past user | 76 | 81 | 82 |
| Current user | 5 | 5 | 5 |
| Menopausal hormone therapy, % | |||
| Premenopausal | 58 | 55 | 54 |
| Postmenopausal never user | 20 | 19 | 18 |
| Postmenopausal past user | 11 | 13 | 14 |
| Postmenopausal current user | 11 | 13 | 13 |
Abbreviations: SD, standard deviation; IQR, interquartile range; MET, metabolic equivalent of task.
The basic characteristics are presented by number of immune-mediated disease. All variables are standardized to the age distribution of the study population, except for age. Mean (SD) is presented for continuous variables, median (IQR) for ordinal variables, and percentage of participants for categorical variables.
Immune-mediated diseases include asthma, atopic dermatitis, psoriasis, rosacea, rheumatoid arthritis, systemic lupus erythematosus, type I diabetes mellitus, multiple sclerosis, and Guillain-Barré syndrome.
All variables are presented across person-time except for age.
In the age-adjusted model, compared to participants with no history of IMD (incidence 5.1/100,000 person-years), those with one (incidence 12.0/100,000 person-years) and two or more IMDs (incidence 12.0/100,000 person-years) had an elevated risk of CD (HR 2.57; 95% CI 1.77–3.74 and HR 2.74; 95% CI 1.36–5.49, respectively; Ptrend < 0.0001) (Table 2). Adjusting for key environmental risk factors for IBD only modestly attenuated the increase in risk of CD associated with one (HR 2.35; 95% CI 1.60–3.45) and two or more IMDs (HR 2.46; 95% CI 1.21–5.01). More granular characterization of women with 2 or more IMDs demonstrated that the highest numerical risk of CD was among those with three or more IMDs (incidence 17.8/100,000 person-years; HR 3.40; 95% CI 0.81–14.21) while the HRs for those with one and two IMDs were numerically similar (2.35 and 2.29) (Supplementary Table 2).
Table 2.
Age-adjusted and multivariable associations of immune-mediated disease with risk of Crohn’s disease and ulcerative colitis
| Number of immune-mediated diseasesa | |||||
|---|---|---|---|---|---|
| 0 | 1 | ≥ 2 | Ptrende | Pheterogeneityf | |
| Crohn’s disease | |||||
| Case | 80 | 43 | 9 | ||
| Person-year | 1579394 | 359363 | 77406 | ||
| Crude incidence rateb | 5.1 | 12.0 | 11.6 | ||
| Age-adjusted HR (95% CI)c | 1 (reference) | 2.57 (1.77–3.74) | 2.74 (1.36–5.49) | <0.0001 | |
| Multivariable-adjusted HR (95% CI)d | 1 (reference) | 2.35 (1.60–3.45) | 2.46 (1.21–5.01) | <0.0001 | |
| Ulcerative colitis | |||||
| Case | 137 | 40 | 9 | ||
| Person-year | 1579394 | 359363 | 77406 | ||
| Crude incidence rateb | 8.7 | 11.1 | 11.6 | ||
| Age-adjusted HR (95% CI)c | 1 (reference) | 1.36 (0.95–1.94) | 1.55 (0.79–3.07) | 0.03 | |
| Multivariable-adjusted HR (95% CI)d | 1 (reference) | 1.22 (0.85–1.76) | 1.33 (0.67–2.65) | 0.16 | 0.037 |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; IBD, inflammatory bowel disease; MET, metabolic equivalent of task; NSAID, nonsteroidal anti-inflammatory drug.
Immune-mediated diseases include asthma, atopic dermatitis, psoriasis, rosacea, rheumatoid arthritis, systemic lupus erythematosus, type I diabetes mellitus, multiple sclerosis, and Guillain-Barré syndrome.
Per 100,000 person-years.
Cox proportional hazards regression model stratified by age (continuous, month) and study period (in 2-year intervals).
Further adjusted for race (Southern European/Mediterranean, Scandinavian, other Caucasian, other races), area of residence (New England, Mid-Atlantic, Midwest, Southeast, West), median family income (in quintiles, US dollar), family history of inflammatory bowel disease (no, yes), family history of immune-mediated disease (no, yes), smoking (never smoker, past smoker with 1–19 pack-years, past smoker with ≥20 pack-years, current smoker with 1–19 pack-years, current smoker with ≥20 pack-years), BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2), physical activity (in quintiles, MET-hr/wk), total fiber intake (in quintiles, g/d), NSAID use (no, yes), oral contraceptive pill (never user, past user, current user), menopausal hormone therapy (premenopausal, postmenopausal never user, postmenopausal past user, postmenopausal current user).
P for trend was calculated using the number of immune-mediated disease (range 0–7) as a continuous variable.
P for heterogeneity was calculated using a likelihood ratio test, comparing a model that allows separate associations for the 2 IBD subtypes with a model that assumes a common association.
Dichotomous classification of IMDs showed that compared to women with no IMDs, those with one or more IMDs had a 2.6-fold increase in CD risk in the age-adjusted model (HR 2.60; 95% CI 1.82–3.70), which remained largely unchanged upon adjusting for environmental factors (HR 2.36; 95% CI 1.64–3.41). Among the individual IMDs, a statistically significant elevated risk was individually noted with asthma (HR 2.49, 95% CI 1.26–4.90), AD (HR 3.58, 95% CI 1.84–6.94), psoriasis (HR 3.67; 95% CI 1.59–8.47), and rosacea (HR 2.53; 95% CI 1.21–5.30). Adjusting for environmental factors resulted in only a modest attenuation compared to the age-adjusted associations for each of these diseases. A history of less common IMDs, including RA, SLE, T1D, MS, and GBS, as a joint exposure was also associated with a trend towards an increased risk of CD (HR 1.96; 95% CI 0.89–4.34).
In contrast to the association with CD, we found a weaker association between a history of IMD and risk of UC. Compared to women with no IMDs (incidence 8.7/100,000 person-years), those with one (incidence 11.1/100,000 person-years) and two or more IMDs (incidence 11.6/100,000 person-years) had no increase in risk of UC in the age-adjusted model (HR 1.36; 95% CI 0.95–1.94 and HR 1.55; 95% CI 0.79–3.07, respectively), though a weak trend across strata was noted (Ptrend 0.03) (Table 2). Similar to CD, adjustment for relevant environmental factors did not modify this association. The difference in the observed association between CD and UC was statistically significant (Pheterogeneity 0.037), suggesting the stronger clustering of IMDs with CD than UC. Multivariable model demonstrated that a history of any IMD was not associated with higher risk of UC (HR 1.24, 95% CI 0.88–1.74) (Table 4). None of the individual IMDs demonstrated a statistically significant association with UC, with HRs of individual IMD ranging from 0.67 to 1.61 (all P > 0.05).
Table 4.
Age-adjusted and multivariable associations of immune-mediated disease with risk of ulcerative colitis
| Case | Person-year | Crude incidence ratec | Age-adjusted HR (95% CI)d | Multivariable-adjusted HR (95% CI)e |
|
|---|---|---|---|---|---|
| Any immune-mediated diseasea | |||||
| No | 137 | 1579394 | 8.7 | 1 (reference) | 1 (reference) |
| Yes | 49 | 436769 | 11.2 | 1.39 (1.00–1.93) | 1.24 (0.88–1.74) |
| Asthma | |||||
| No | 112 | 987817 | 11.3 | 1 (reference) | 1 (reference) |
| Yes | 6 | 66664 | 9.0 | 0.82 (0.36–1.87) | 0.67 (0.29–1.55) |
| Atopic dermatitis | |||||
| No | 137 | 1579394 | 8.7 | 1 (reference) | 1 (reference) |
| Yes | 8 | 59902 | 13.4 | 1.65 (0.81–3.38) | 1.44 (0.70–2.97) |
| Psoriasis | |||||
| No | 137 | 1579394 | 8.7 | 1 (reference) | 1 (reference) |
| Yes | 3 | 36768 | 8.2 | 0.98 (0.31–3.08) | 0.91 (0.29–2.88) |
| Rosacea | |||||
| No | 137 | 1579394 | 8.7 | 1 (reference) | 1 (reference) |
| Yes | 9 | 66057 | 13.6 | 1.74 (0.88–3.43) | 1.61 (0.81–3.19) |
| Other immune-mediated diseasesb | |||||
| No | 137 | 1579394 | 8.7 | 1 (reference) | 1 (reference) |
| Yes | 9 | 65276 | 13.8 | 1.68 (0.85–3.31) | 1.58 (0.79–3.13) |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; MET, metabolic equivalent of task; NSAID, nonsteroidal anti-inflammatory drug.
Immune-mediated diseases include asthma, atopic dermatitis, psoriasis, rosacea, rheumatoid arthritis, systemic lupus erythematosus, type I diabetes mellitus, multiple sclerosis, and Guillain-Barré syndrome.
Other immune-mediated diseases include rheumatoid arthritis, systemic lupus erythematosus, type I diabetes mellitus, multiple sclerosis, and Guillain-Barré syndrome.
Per 100,000 person-years.
Cox proportional hazards regression model stratified by age (continuous, month) and study period (in 2-year intervals).
Further adjusted for race (Southern European/Mediterranean, Scandinavian, other Caucasian, other races), area of residence (New England, Mid-Atlantic, Midwest, Southeast, West), median family income (in quintiles, US dollar), family history of inflammatory bowel disease (no, yes), family history of immune-mediated disease (no, yes), smoking (never smoker, past smoker with 1–19 pack-years, past smoker with ≥20 pack-years, current smoker with 1–19 pack-years, current smoker with ≥20 pack-years), BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2), physical activity (in quintiles, MET-hr/wk), total fiber intake (in quintiles, g/d), NSAID use (no, yes), oral contraceptive pill (never user, past user, current user), menopausal hormone therapy (premenopausal, postmenopausal never user, postmenopausal past user, postmenopausal current user).
We then performed various sensitivity and subgroup analyses. Allowing participants diagnosed with other IMDs to continue contributing person-years to the reference group after diagnosis demonstrated a moderate attenuation in all the tested associations, with the exception of AD and psoriasis and risk of CD, which remained strongly associated (Supplementary Table 3 & Supplementary Table 4). The results remained unchanged upon additional adjustment for Mediterranean diet (HR1 IMD 2.34; 95% CI 1.60–3.44; HR≥ 2 IMDs 2.46; 95% CI 1.21–5.01) or vitamin D intake (HR1 IMD 2.32; 95% CI 1.58– 3.40; HR≥ 2 IMDs 2.41; 95% CI 1.19–4.91). The increase in risk of CD associated with a history of an IMD was similar among those who had a family history of IMD and those without (Pinteraction 0.63) (Supplementary Table 5). An increase in risk of CD with one and two or more IMDs was also noted in those who had no family history of IBD (Supplementary Table 6).
DISCUSSION
Epidemiologic studies have demonstrated a clustering of IMDs within individuals with IBD and their families. In this large prospective cohort of US women, we demonstrate a 2-fold increase in risk of CD (but not UC) among women with other IMDs. This risk was dose-dependent with a greater number of IMDs being associated with a higher incidence of CD. Specifically, asthma, AD, psoriasis, and rosacea were all individually associated with increased risk of CD. Importantly, we also demonstrate that these findings were only modestly attenuated after adjustment for environmental factors, suggesting that common diet and lifestyle risk factors do not fully explain the increased co-occurrence of these diseases.
Several observational studies have suggested that individuals with a pre-existing IMD may be more likely to go on to develop IBD. In a population-based cohort in Alberta, Canada, Kuenzig et al. reported a 50% increase in risk of CD among those with asthma28. Summarized in a meta-analysis from the same group that included 18 observational studies, asthma was associated with an increase in risk of CD but not UC29. Using health insurance data, another study similarly showed that there is a modest increase in risk of both CD and UC among those with AD30. We, and others, have previously demonstrated an increase in risk of CD among those with psoriasis31,32 and rosacea33,34. In a meta-analysis of cohort studies, the association between psoriasis and CD was stronger than noted for UC31, similar to our observations..
Our study overcomes some important weaknesses of prior studies. First, a critical limitation of prior literature is the inability to adjust for environmental determinants of disease. The clustering of IMDs may be explained through contributions from shared genetics, environmental influences, and gut microbiome. For example, smoking is a common risk factor among various IMDs and has been associated with CD12, asthma14, psoriasis16, RA18, and SLE35. Socioeconomic status, as part of the “hygiene hypothesis”, may also underpin the occurrence of several of these IMDs36. There have been reports of a positive correlation between gross national product and incidence of asthma, T1D and MS in Europe37 and CD in Manitoba, Canada38. Being able to adjust for key confounders is critical to our understanding of the biologic basis of the clustering of IMDs. The persistence of our findings after detailed adjustment for environmental determinants suggest that perhaps shared genetics and immunologic pathways play a stronger role in the clustering of these IMDs39,40. Second, many prior studies have relied on administrative codes rather than confirmed cases to identify occurrence of IBD or other IMDs. Reliance on administrative data introduces bias when patients with an IMD come into contact with the healthcare system more frequently than those without. This may result in more frequent IBD diagnosis due to greater rates of diagnostic testing or more frequent administrative coding from heightened suspicion even in the absence of a confirmed diagnosis. In addition, cross-sectional studies cannot determine sequence of diagnosis of IMDs. Case-control studies are also limited by their inability to provide absolute incidence rates and a lifetime risk. This absolute incidence is an important parameter to estimate the utility of either at-risk screening or interventions for primary prevention. In addition, most prior studies focused on a single IMD and much less is known about the impact of multiple IMDs on the risk of CD and UC. Here, we demonstrate that the highest numerical increase in risk and absolute incidence of CD was noted in individuals with three or more IMDs.
Large genome-wide association study (GWAS) meta-analyses of CD and UC41,42 identified over 200 confirmed IBD risk loci, many of which are shared between CD and UC. A number of these risk loci have been implicated in other IMDs. In AD, two established susceptibility loci (IL18R1/IL18RAP, TNFRSF6B) reported in GWAS43 conferred risk of CD with an effect in the same direction44,45. Similarly, psoriasis and IBD share several genetic susceptibility loci such as 1p31.1 (IL23R) and 5q33.1 (IL12B), both of which have been identified in the pathogenesis of psoriasis and IBD46,47. Another common compartment likely influenced by both genetics and the environment is the gut microbiome. One study48 demonstrated that the gut microbiome of psoriatic arthritis patients was characterized by a reduction in Akkermansia and Ruminococcus, a profile similar to that previously described in patients with CD49–51. An increase in secretory IgA levels in these patients suggested an increased immune activation in the intestine that accompanied the dysbiosis. Another study reported an increase in the abundance of Collinsella in RA patients52, as noted similarly in stool samples of patients with fistulizing phenotype of CD53. Collinsella may increase gut permeability by lowering the expression of tight junction protein in epithelial cells and inducing the expression of interleukin IL-17 network cytokines52, both of which has been implicated in the development of IBD54,55.
There are several strengths to our study. The prospective design, long-term follow-up, large sample size, and high rates of completed follow-up allow for robust estimation of the magnitude of association as well as a clearer delineation of the temporal relationship between the development of IMDs and the diagnosis of CD or UC. The availability of detailed and validated information on covariates allowed us to control for various shared environmental risk factors between IMDs that may have confounded the associations. In addition, our IBD cases were confirmed through detailed medial record review by board-certified gastroenterologists blinded to exposure information. The elevated risk of CD associated with other IMDs suggest that there should be a heightened suspicion for CD in such individuals and early referral for evaluation. Given the low absolute risk, it is likely that most individuals with an IMD who have co-existing gastrointestinal symptoms may have an alternate etiology. However, in the right clinical circumstance, one may need to consider a lower threshold for non-invasive blood tests and assessment of fecal inflammatory markers such as calprotectin in individuals with unexplained gastrointestinal symptoms in the setting of other IMDs.
We readily acknowledge several limitations. First, the ascertainment of IMDs was based on self-report. While some misclassification bias is possible, the effect of this should be modest as our study participants were healthcare professionals and high validity of self-report has been established previously (Supplementary Table 1). Similarly, while we identified the occurrence of various IMDs, this may not have been comprehensive, particularly for the rarer IMDs. As above, such misclassification would introduce a bias towards the null, thereby strengthening our findings. Second, we were able to adjust for various environmental risk factors. However, as with all observational studies, the possibility of unmeasured or residual confounding could not be excluded. Third, the median age of IBD diagnosis is higher than other population-based cohorts due to the age structure of our cohorts. Fourth, we did not have information on medications use to treat the underlying IMDs including use of biologics that may also have treated subclinical IBD. However, we do not expect this to significantly alter our findings for several reasons. Our associations were significant even for various IMDs that do not share common long-term maintenance treatments with IBD. Also, the effect of such treatments, if there is a biologic impact, would be to decrease risk of overt IBD in that population, biasing towards the null. This would only strengthen the magnitude of our findings. Finally, our study population consists of female registered nurses who were mostly white. Therefore, extrapolating our findings to men or individuals of other ethnicities should be performed with caution. However, prior studies have not identified gender to significantly alter genetic risk or the impact of environmental determinants of disease.
In conclusion, we demonstrate that individuals with one or more IMDs are at a higher lifetime risk of CD or UC due to shared genetics or early-life exposures rather than diet or lifestyle. While there may not be a role for routine screening, those with suspicious symptoms may benefit from a lower threshold for early evaluation of suspected IBD.
Supplementary Material
Table 3.
Age-adjusted and multivariable associations of immune-mediated disease with risk of Crohn’s disease
| Case | Person-year | Crude incidence ratec | Age-adjusted HR (95% CI)d | Multivariable-adjusted HR (95% CI)e | |
|---|---|---|---|---|---|
| Any immune-mediated diseasea | |||||
| No | 80 | 1579394 | 5.1 | 1 (reference) | 1 (reference) |
| Yes | 52 | 436769 | 11.9 | 2.60 (1.82–3.70) | 2.36 (1.64–3.41) |
| Asthma | |||||
| No | 58 | 987817 | 5.9 | 1 (reference) | 1 (reference) |
| Yes | 10 | 66664 | 15.0 | 2.49 (1.26–4.90) | 2.15 (1.07–4.33) |
| Atopic dermatitis | |||||
| No | 80 | 1579394 | 5.1 | 1 (reference) | 1 (reference) |
| Yes | 10 | 59902 | 16.7 | 3.58 (1.84–6.94) | 3.39 (1.73–6.65) |
| Psoriasis | |||||
| No | 80 | 1579394 | 5.1 | 1 (reference) | 1 (reference) |
| Yes | 6 | 36768 | 16.3 | 3.67 (1.59–8.47) | 2.88 (1.22–6.79) |
| Rosacea | |||||
| No | 80 | 1579394 | 5.1 | 1 (reference) | 1 (reference) |
| Yes | 8 | 66057 | 12.1 | 2.53 (1.21–5.30) | 2.34 (1.10–4.96) |
| Other immune-mediated diseasesb | |||||
| No | 80 | 1579394 | 5.1 | 1 (reference) | 1 (reference) |
| Yes | 7 | 65276 | 10.7 | 2.26 (1.04–4.93) | 1.96 (0.89–4.34) |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; MET, metabolic equivalent of task; NSAID, nonsteroidal anti-inflammatory drug.
Immune-mediated diseases include asthma, atopic dermatitis, psoriasis, rosacea, rheumatoid arthritis, systemic lupus erythematosus, type I diabetes mellitus, multiple sclerosis, and Guillain-Barré syndrome.
Other immune-mediated diseases include rheumatoid arthritis, systemic lupus erythematosus, type I diabetes mellitus, multiple sclerosis, and Guillain-Barré syndrome.
Per 100,000 person-years.
Cox proportional hazards regression model stratified by age (continuous, month) and study period (in 2-year intervals).
Further adjusted for race (Southern European/Mediterranean, Scandinavian, other Caucasian, other races), area of residence (New England, Mid-Atlantic, Midwest, Southeast, West), median family income (in quintiles, US dollar), family history of inflammatory bowel disease (no, yes), family history of immune-mediated disease (no, yes), smoking (never smoker, past smoker with 1–19 pack-years, past smoker with ≥20 pack-years, current smoker with 1–19 pack-years, current smoker with ≥20 pack-years), BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2), physical activity (in quintiles, MET-hr/wk), total fiber intake (in quintiles, g/d), NSAID use (no, yes), oral contraceptive pill (never user, past user, current user), menopausal hormone therapy (premenopausal, postmenopausal never user, postmenopausal past user, postmenopausal current user).
ACKNOWLEDGEMENTS
We would like to thank the participants and staff of the Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Grant Support:
This work was supported by the U.S. National Institutes of Health (U01 CA176726; R00 CA215314 to M.S.; K24 DK098311 to A.T.C.), the Crohn’s and Colitis Foundation (to H.K., P.L., A.T.C., A.N.A.), the Beker Foundation (to H.K.), and the Chleck Family Foundation (to A.N.A.). A.T.C. is a Stuart and Suzanne Steele MGH Research Scholar. The funders had no role in the design and conduct of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Guarantor:
Ashwin N Ananthakrishnan accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Competing interests:
H.K. receives grant funding from Takeda and Pfizer and has received consulting fees from Abbvie and Takeda. J.M.R. is a consultant to Policy Analysis Inc. and Takeda Pharmaceuticals. A.T.C. serves as a consultant for Pfizer, Bayer Pharma AG, and Boehringer Ingelheim for work unrelated to the topic of this manuscript. A.N.A. has served as a Scientific Advisory Board member for Abbvie, Gilead, Kyn therapeutics and received research grants from Pfizer and Merck. No other conflict of interest exists.
Abbreviations:
- AD
atopic dermatitis
- BMI
body mass index
- CI
confidence interval
- CD
Crohn’s disease
- DcR3
decoy receptor 3
- FFQ
food frequency questionnaire
- GWAS
genome-wide association study
- GBS
Guillain-Barré syndrome
- HR
hazard ratio
- IMD
immune-mediated disease
- IBD
inflammatory bowel disease
- IL
interleukin
- MET
metabolic equivalent of task
- MS
multiple sclerosis
- NSAID
nonsteroidal anti-inflammatory drug
- NHS
Nurses’ Health Study
- Th17
T-helper 17
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- T1D
Type I diabetes mellitus
- UC
ulcerative colitis
Footnotes
Ethics approval: The study was approved by the Institutional Review Board of Partners Healthcare (2001P001128).
REFERENCE
- 1.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmunity Reviews 2003;2:119–25. [DOI] [PubMed] [Google Scholar]
- 2.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med 2001;345:340–50. [DOI] [PubMed] [Google Scholar]
- 3.Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health 2000;90:1463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014;157:121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MF, Wang H. Environmental exposures and autoimmune diseases: contribution of gut microbiome. Front Immunol 2020;10:3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baranzini SE. The genetics of autoimmune diseases: a networked perspective. Current Opinion in Immunology 2009;21:596–605. [DOI] [PubMed] [Google Scholar]
- 7.Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet 2009;10:43–55. [DOI] [PubMed] [Google Scholar]
- 8.Cotsapas C, Voight BF, Rossin E, et al. Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet 2011;7:e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weng X, Liu L, Barcellos LF, et al. Clustering of inflammatory bowel disease with immune mediated diseases among members of a Northern California-managed care organization. Am J Gastroenterology 2007;102:1429–35. [DOI] [PubMed] [Google Scholar]
- 10.Cohen R, Robinson D, Paramore C, et al. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001–2002: Inflammatory Bowel Diseases 2008;14:738–43. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology 2005;129:827–36. [DOI] [PubMed] [Google Scholar]
- 12.Mahid SS, Minor KS, Soto RE, et al. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clinic Proceedings 2006;81:1462–71. [DOI] [PubMed] [Google Scholar]
- 13.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Measures of obesity and risk of Crohnʼs disease and ulcerative colitis: Inflammatory Bowel Diseases 2015;21:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 2012;129:735–44. [DOI] [PubMed] [Google Scholar]
- 15.Lang JE, Bunnell HT, Hossain MJ, et al. Being overweight or obese and the development of asthma. Pediatrics 2018;142:e20182119. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol 2014;170:304–14. [DOI] [PubMed] [Google Scholar]
- 17.Budu-Aggrey A, Brumpton B, Tyrrell J, et al. Evidence of a causal relationship between body mass index and psoriasis: a mendelian randomization study. PLoS Med 2019;16:e1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costenbader KH, Feskanich D, Mandl LA, et al. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. The American Journal of Medicine 2006;119:503.e1–503.e9. [DOI] [PubMed] [Google Scholar]
- 19.Qin B, Yang M, Fu H, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther 2015;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504–17. [DOI] [PubMed] [Google Scholar]
- 21.Khalili H, Huang ES, Ananthakrishnan AN, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut 2012;61:1686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 23.Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 24.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 25.Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013;145:970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalili H, Håkansson N, Chan SS, et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: results from two large prospective cohort studies. Gut 2020;:gutjnl-2019–319505. [DOI] [PubMed] [Google Scholar]
- 27.Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Current Opinion in Pharmacology 2010;10:482–96. [DOI] [PubMed] [Google Scholar]
- 28.Kuenzig ME, Barnabe C, Seow CH, et al. Asthma is associated with subsequent development of inflammatory bowel disease: a population-based case–control study. Clinical Gastroenterology and Hepatology 2017;15:1405–1412.e3. [DOI] [PubMed] [Google Scholar]
- 29.Kuenzig ME, Bishay K, Leigh R, et al. Co-occurrence of asthma and the inflammatory bowel diseases: a systematic review and meta-analysis. Clinical and Translational Gastroenterology 2018;9:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt J, Schwarz K, Baurecht H, et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. Journal of Allergy and Clinical Immunology 2016;137:130–6. [DOI] [PubMed] [Google Scholar]
- 31.Fu Y, Lee C-H, Chi C-C. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol 2018;154:1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W-Q, Han J-L, Chan AT, et al. Psoriasis, psoriatic arthritis and increased risk of incident Crohn’s disease in US women. Ann Rheum Dis 2013;72:1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W-Q, Cho E, Khalili H, et al. Rosacea, use of tetracycline, and risk of incident inflammatory bowel disease in women. Clinical Gastroenterology and Hepatology 2016;14:220–225.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F-Y, Chi C-C. Association of rosacea with inflammatory bowel disease: a MOOSE-compliant meta-analysis. Medicine 2019;98:e16448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costenbader KH, Kim DJ, Peerzada J, et al. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis & Rheumatism 2004;50:849–57. [DOI] [PubMed] [Google Scholar]
- 36.Okada H, Kuhn C, Feillet H, et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clinical & Experimental Immunology 2010;160:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach J-F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–20. [DOI] [PubMed] [Google Scholar]
- 38.Blanchard JF. Small-area variations and sociodemographic correlates for the incidence of Crohn’s disease and ulcerative colitis. American Journal of Epidemiology 2001;154:328–35. [DOI] [PubMed] [Google Scholar]
- 39.Li YR, Zhao SD, Li J, et al. Genetic sharing and heritability of paediatric age of onset autoimmune diseases. Nat Commun 2015;6:8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li YR, Li J, Zhao SD, et al. Meta-analysis of shared genetic architecture across ten pediatric autoimmune diseases. Nat Med 2015;21:1018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The International IBD Genetics Consortium (IIBDGC), Jostins L, Ripke S, et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.International Multiple Sclerosis Genetics Consortium, International IBD Genetics Consortium, Liu JZ, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellinghaus D, Baurecht H, Esparza-Gordillo J, et al. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat Genet 2013;45:808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franke A, McGovern DPB, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 2010;42:1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franke A, Balschun T, Sina C, et al. Genome-wide association study for ulcerative colitis identifies risk loci at 7q22 and 22q13 (IL17REL). Nat Genet 2010;42:292–4. [DOI] [PubMed] [Google Scholar]
- 46.Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. The American Journal of Human Genetics 2007;80:273–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 2008;8:458–66. [DOI] [PubMed] [Google Scholar]
- 48.Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis & Rheumatology 2015;67:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Png CW, Lindén SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. American Journal of Gastroenterology 2010;105:2420–8. [DOI] [PubMed] [Google Scholar]
- 50.Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 2012;13:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 2010;139:1844–1854.e1. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Wright K, Davis JM, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med 2016;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. The Lancet 2017;389:1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujino S Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003;52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luissint A-C, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte–epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016;151:616–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request at https://www.nurseshealthstudy.org/researchers (email: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/. The data are not publicly available due to ethical restrictions.


