Abstract
While stress may be a potential mechanism by which childhood threat and deprivation influence mental health, few studies have considered specific stress‐related white matter pathways, such as the stria terminalis (ST) and medial forebrain bundle (MFB). Our goal was to examine the relationships between childhood adversity and ST and MFB structural integrity and whether these pathways may provide a link between childhood adversity and affective symptoms and disorders. Participants were young adults (n = 100) with a full distribution of maltreatment history and affective symptom severity. Threat was determined by measures of childhood abuse and repeated traumatic events. Socioeconomic deprivation (SED) was determined by a measure of childhood socioeconomic status (parental education). Participants underwent diffusion spectrum imaging. Human Connectome Project data was used to perform ST and MFB tractography; these tracts were used as ROIs to extract generalized fractional anisotropy (gFA) from each participant. Childhood threat was associated with ST gFA, such that greater threat was associated with less ST gFA. SED was also associated with ST gFA, however, conversely to threat, greater SED was associated with greater ST gFA. Additionally, threat was negatively associated with MFB gFA, and MFB gFA was negatively associated with post‐traumatic stress symptoms. Our results suggest that childhood threat and deprivation have opposing influences on ST structural integrity, providing new evidence that the context of childhood adversity may have an important influence on its neurobiological effects, even on the same structure. Further, the MFB may provide a novel link between childhood threat and affective symptoms.
Keywords: child abuse, socioeconomic factors, psychosocial deprivation, early‐life stress, medial forebrain bundle, diffusion magnetic resonance imaging, affective symptoms
This study provides the first evidence that childhood threat and deprivation have opposing influences on stria terminalis generalized fractional anisotropy (gFA), and novel evidence that different dimensions of childhood adversity may have differential influences on structural integrity or underlying microstructure, even within the same region. Further, our results demonstrate that the medial forebrain bundle may provide a novel link between childhood threat and affective symptoms. Examining how these stress‐related, visceral circuits link childhood adversity to affective symptoms could improve our understanding of mechanisms underlying affective disorders and provide novel targets for intervention.
1. INTRODUCTION
Childhood adversity was recently posited to be psychiatry's greatest public health challenge (Sara & Lappin, 2017), as it is highly prevalent (Felitti et al., 1998; Kessler et al., 2010; Merrick, Ford, Ports, & Guinn, 2018) and a major predictor of affective disorders (Danese et al., 2009; Silverman, Reinherz, & Giaconia, 1996). Longitudinal studies indicate that childhood adversity predicts 24% and 37% of mood and anxiety/trauma‐related disorders in early adulthood, respectively (Danese et al., 2009; Silverman et al., 1996), as well as worse prognosis in terms of severity and chronicity of illness (Nanni, Uher, & Danese, 2012).
The type and timing of childhood adversity are important factors for neural development (Baker et al., 2013; Dunn, Nishimi, Gomez, Powers, & Bradley, 2018; Teicher & Samson, 2016; Tottenham & Galván, 2016; Tottenham & Sheridan, 2010). McLaughlin et al. proposed a novel conceptual framework distinguishing two dimensions of childhood adversity—threat and deprivation, in which deprivation is “the absence of expected environmental inputs and complexity” while threat is “the presence of experiences that represent a threat to one's physical integrity” (McLaughlin, Sheridan, & Lambert, 2014; Sheridan & McLaughlin, 2014). Deprivation is a continuum of experiences that includes institutionalization/institutional rearing, neglect, poverty, and low socioeconomic status (SES), all of which are characterized by environments with diminished social and cognitive stimuli. In contrast, experiences of threat include traumatic events (actual or threatened death, serious injury, sexual violation, or harm to one's physical integrity), physical and sexual abuse, and domestic or community violence. Much of the childhood adversity literature has focused on the stress or allostatic load model involving alterations to the HPA axis, neural effects of glucocorticoids, and related excitotoxic neural damage. The authors propose considering neural mechanisms beyond this model, in which deprivation may shape neural development via activity‐dependent plasticity leading to over‐pruning of synaptic connections, particularly within association cortices. Threat, however, may influence emotional or fear learning networks, with structural and functional alterations within the hippocampus, amygdala, and medial prefrontal cortex. The goal of the present manuscript is to examine relationships of threat and deprivation with visceral white matter structural integrity. We hypothesize that while social and cognitive deprivation may yield less neural complexity, particularly in cortical areas, the pervasive stress of childhood socioeconomic deprivation (SED) may yield repeated firing of, and thus, strengthening of visceral, stress‐related circuits.
It is well known that childhood threat and/or deprivation are associated with brain white matter differences (Choi, Jeong, Polcari, Rohan, & Teicher, 2011; Choi, Jeong, Rohan, Polcari, & Teicher, 2009; Frodl et al., 2012; Gianaros, Marsland, Sheu, Erickson, & Verstynen, 2012; Lu et al., 2013; Ugwu, Amico, Carballedo, Fagan, & Frodl, 2014), particularly within large bundles such as the cingulum and uncinate fasciculus. Studies have typically found that childhood adversity is associated with less fractional anisotropy (FA) within the uncinate fasciculus (Eluvathingal et al., 2006; Govindan, Behen, Helder, Makki, & Chugani, 2009; Kumar et al., 2013) and cingulum (Hanson et al., 2013; Kumar et al., 2013). Childhood adversity has been linked to dysregulated neuroendocrine and autonomic stress reactivity in childhood and later in life (Carpenter et al., 2007; Carpenter, Shattuck, Tyrka, Geracioti, & Price, 2011; Chen, Langer, Raphaelson, & Matthews, 2004; Gunnar, Frenn, Wewerka, & Van Ryzin, 2009; Hackman, Betancourt, Brodsky, Hurt, & Farah, 2012; Heim et al., 2000; Heim, Newport, Bonsall, Miller, & Nemeroff, 2001; Koopman et al., 2004; Lovallo, Farag, Sorocco, Cohoon, & Vincent, 2011). However, research has not yet revealed how childhood threat and deprivation impact specific, proximally stress‐responsive white matter pathways.
Visceral neural circuits, comprised of descending preautonomic/visceromotor and ascending viscerosensory circuits, may be influenced by childhood adversity and may contribute importantly to differences in stress reactivity and affective symptoms. Visceral circuits have been implicated in affective processes (e.g., conditioned and unconditioned fear (Schweimer, Fendt, & Schnitzler, 2005), phasic and sustained responses to threat (Avery, Clauss, & Blackford, 2016; Lebow & Chen, 2016)), and are critical in the control of stress responses (Rinaman, Banihashemi, & Koehnle, 2011). Descending preautonomic circuits directly control autonomic outflow to target organs, including stressor‐evoked changes, while viscerosensory circuits have important modulatory influences on stress responses (Banihashemi & Rinaman, 2006; Clayton & Williams, 2000; Pacak, Palkovits, Kopin, & Goldstein, 1995). Both descending preautonomic and ascending viscerosensory projections course through the white matter of the medial forebrain bundle (MFB) (Coenen, Panksepp, Hurwitz, Urbach, & Mädler, 2012; Nieuwenhuys, Geeraedts, & Veening, 1982).
These visceral circuits converge on several regions that play a role in stress regulation, the paraventricular nucleus of the hypothalamus (PVN), bed nucleus of the stria terminalis (BST), and the amygdala. The PVN is critical in the control of stress responses, controlling both autonomic and neuroendocrine responses (Herman, Cullinan, Ziegler, & Tasker, 2002; Luiten, Ter Horst, Karst, & Steffens, 1985). The BST and amygdala are also preautonomic, preparasympathetic structures (Rinaman, Levitt, & Card, 2000), innervating the brainstem nucleus of the solitary tract (NST) (Card et al., 1990; Westerhaus & Loewy, 2001). The NST receives viscerosensory information from the vagus (Kalia & Sullivan, 1982) and relays it to both the ventral BST and PVN (Banihashemi & Rinaman, 2006; Card & Sved, 2011; Ter Horst, De Boer, Luiten, & Van Willigen, 1989). The BST is an important stress‐control hub with direct access (Dong, Petrovich, Watts, & Swanson, 2001) and influence over stress‐related PVN activity and physiological stress responses (Choi et al., 2007; Crane, Buller, & Day, 2003; Gray & Piechowski, 1993). The BST also heavily interconnects with the amygdala (Dong, Petrovich, & Swanson, 2001) and is connected to the PVN and amygdala via the white matter of the stria terminalis (ST) (Dong, Petrovich, & Swanson, 2001).
Research on both rodents and humans indicates a link between childhood adversity and visceral, stress‐related circuits. In rodents, manipulations of early experience that alter stress reactivity later in life have been shown to change the neonatal synaptic assembly of preautonomic circuits originating within the PVN, BST, and central nucleus of the amygdala (Card, Levitt, Gluhovsky, & Rinaman, 2005), as well as stressor‐evoked activation of the PVN, BST, and NST later in life (Banihashemi, O'Neill, & Rinaman, 2011). In humans, previous research in healthy adults demonstrated relationships between childhood threat (i.e., physical abuse) and stressor‐evoked activity within the preautonomic hypothalamus (including PVN), BST, and amygdala (Banihashemi, Sheu, Midei, & Gianaros, 2015). These findings suggest that these visceral brain regions are sensitive to levels of abuse that fall below the severe‐to‐extreme range, characteristic of a healthy sample.
The current study builds on this previous work, examining childhood adversity‐related differences in the white matter connecting visceral, stress‐related regions in a mixed (continuous and transdiagnostic) mental health sample with a full range of childhood adversity experiences and affective symptoms. Guided by the threat and deprivation framework, our goal was to examine how threat and deprivation may influence specific stress‐related, visceral white matter pathways, the ST and MFB. We hypothesized that proximal, familial threats like abuse may diminish structural integrity, while more distal, environmental stressors of low SES or SED may strengthen these pathways (via repeated, coordinated activation of this visceral, stress‐control network). Several studies have mapped the human MFB (Anthofer et al., 2015; Bracht et al., 2014; Coenen et al., 2012; Coenen et al., 2018; Hana, Hana, Dooms, Boecher‐Schwarz, & Hertel, 2015). The ST has been carefully mapped in non‐human primates using neuroanatomical and neuroimaging techniques (Oler et al., 2017). Several studies have examined and/or mapped the ST in humans (Avery et al., 2014; Dzafic, Oestreich, Martin, Mowry, & Burianová, 2019; Eluvathingal et al., 2006; Folloni et al., 2019; Kamali et al., 2015; Koller, Hatton, Rogers, & Rafal, 2019; Krüger, Shiozawa, Kreifelts, Scheffler, & Ethofer, 2015; Kwon, Byun, Ahn, Son, & Jang, 2011; Tromp et al., 2019) and Rafal et al. have examined the ST in humans and non‐human primates (Rafal et al., 2015). The majority of these human ST and MFB studies have used standard diffusion tensor imaging (DTI); thus, detailed tractography of the ST and MFB using high‐resolution diffusion weighted imaging (DWI) (e.g., multi‐shell DWI and/or diffusion spectrum imaging) in human brain is of interest. To our knowledge, this is the first study to examine these specific structures using high‐resolution diffusion spectrum imaging (DSI) in the context of childhood threat and deprivation.
2. METHODS AND MATERIALS
2.1. Participants
Participants were recruited from the community using a variety of methods, including referrals from research studies and online and city bus advertisements. Out of 1,020 contacts made, 111 (18.5%) were consented and enrolled in the study. Of those consented, 100 (90%) participants completed all study procedures. Participants included 59 female and 41 male young adults (n = 100, mean age = 27.28, SD = 3.99). Of these 100 participants, 45% self‐reported their race as White, 36% as Black or African American, 13% as Asian, 4% as multiracial, and 2% as biracial. Within this sample, 7 individuals reported their ethnicity as Hispanic or Latino. All participants provided informed consent after receiving an explanation of study protocols and were examined with the approval of the University of Pittsburgh Institutional Review Board.
Following initial contact, respondents were screened and excluded for: MRI contraindications (e.g., claustrophobia, metal in the body, severe visual or auditory impairment), pregnancy, left‐handedness, cardiovascular disease and diabetes, neurological disorders (including seizure disorders, migraine disorder, traumatic brain injury, or neurodegenerative disorders), psychotropic medications or any medications affecting cardiovascular or neural function, suicidality or marked functional impairment, and current psychiatric disorders (bipolar, psychotic disorders, substance abuse, or dependence) except for depression, anxiety, or trauma‐related disorders.
Screening included five childhood physical abuse items derived from the Childhood Trauma Questionnaire (CTQ) in order to recruit a relatively even distribution of participants across four physical abuse severity levels defined by the CTQ (Bernstein et al., 1994). The following distribution of physical abuse severity was achieved in the final sample (n = 100): 29% None‐Minimal, 23% Low‐Moderate, 21% Moderate–Severe, and 27% Severe‐Extreme. A relatively even distribution across childhood SES (as assessed by maximum parental education level), 31% Low (GED—some college, no degree), 34% Middle (Associate or Bachelor's), and 35% High (Master's or Doctorate), was also achieved. All participants had at least one parent with a GED or higher education level.
2.2. Study protocol & measures
The study involved two visits completed within 1 month (mean number of days between visits: 14.39 ± 10.96), an intake visit followed by an MRI scan visit at the University of Pittsburgh Magnetic Resonance Research Center. During the first visit, eligibility was assessed more rigorously via questionnaires regarding medical history, 2‐week medication history, current substance use, and traumatic brain injury. Participants were excluded if deemed ineligible by these additional measures.
2.2.1. Childhood threat
Childhood maltreatment was assessed using the Childhood Trauma Questionnaire (CTQ), a 28‐item Likert‐type scale that measures five subscales of maltreatment: physical, emotional and sexual abuse, and physical and emotional neglect (Bernstein et al., 1994). Each subscale contains five items, presented as a mix of both objective (e.g., “I was punished with a belt, a board, a cord, or some other hard object”) and subjective statements (e.g., “I believe that I was emotionally abused”), with scores ranging from 1—Never to 5—Very Often True. Scores for each subscale range from 5 to 25, with 5 indicating no maltreatment. A sum of the abuse subscales represented our CTQ Threat variable. (A sum of the neglect subscales represented our CTQ Deprivation variable, which was used in secondary analyses. See Variable Selection below).
Additional forms of childhood threat were assessed using the Trauma History Questionnaire (THQ). The THQ is a 24‐item questionnaire that assesses both the occurrence and recurrence of traumatic events throughout an individual's lifetime (Stamm, 1996). We used an adapted version of the THQ in which participants responded “yes” or “no” to indicate whether a particular event occurred, then indicated the frequency that an event was experienced (either “Once” or “Two or more times”) at three possible age ranges: 0–11, 12–17, and >18 (Insana, Kolko, & Germain, 2012). Traumatic events included experiences with crime, environmental disasters, injury or death, as well as physical or sexual abuse.
2.2.2. Childhood socioeconomic deprivation
A sociodemographic inventory was used to collect data on childhood and adulthood socioeconomic status (SES). Maximum parental education level was used to determine childhood SES; the participants' own educational level determined adulthood SES; both were presented as a 9‐point education level scale (0—No high school diploma, 1—GED, 2—High school diploma, 3—Technical training, 4—Some college, no degree, 5—Associate degree, 6—Bachelor's degree, 7—Master's degree, 8—MD/PhD/JD/PharmD). Measures such as low SES, socioeconomic disadvantage or neighborhood deprivation in early life are considered forms of childhood deprivation (Berti & Pivetti, 2019; McLaughlin et al., 2014; Morris, Berk, Maes, Carvalho, & Puri, 2019; Webb et al., 2017). Further, education level is often used as an index of SES and has been shown to identify mental health inequalities (Reiss, 2013), is associated with physiological measures of stress (Ursache, Merz, Melvin, Meyer, & Noble, 2017) and is a strong predictor of physical health, namely cardiovascular disease risk (Winkleby, Jatulis, Frank, & Fortmann, 1992). Thus, we used maximum parental education level (reverse coded) as our primary measure of childhood SED. Adulthood SES was used as a covariate.
2.2.3. Negative life events
The 24‐item Life Events List assesses major life events experienced by the participant within the past 12 months; these include experiences such as moving, separation or divorce and death of someone close (Cohen, Tyrrell, & Smith, 1991). Participants respond whether or not they have experienced a particular event in the past year with possible follow up questions assessing emotional valence and/or details if affirmative. This inventory was used to assess the total number of negative life events, which was used as a covariate.
2.2.4. Affective symptom severity
Participants completed questionnaires to assess depression and post‐traumatic stress symptom severity. Beck's Depression Inventory (BDI‐II) was used to assess presence and severity of depression within the past 2 weeks. The BDI‐II is a 21‐item questionnaire that asks participants whether they have experienced a thought or behavior related to depressive symptoms, such as feelings of hopelessness or sleep problems, on a scale of 0 to 3 (Beck, Steer, Ball, & Ranieri, 1996). The PTSD Checklist—Civilian Version (PCL‐C) assessed post‐traumatic stress symptom severity in the last month on a 5‐point Likert scale ranging from not at all (1) to extremely (5). It is a valid and reliable, 20‐item measure that includes assessment of all re‐experiencing, avoidance and arousal symptoms, as well as negative cognitions (Wilkins, Lang, & Norman, 2011).
2.2.5. Diagnostic assessment
Psychiatric diagnoses of mood, anxiety, or trauma‐related disorders were evaluated and confirmed via in‐person interview using the Structured Clinical Interview for DSM‐IV Axis I Disorders by a trained interviewer. Of the 100 participants who completed the study, 29% were healthy controls, whereas 71% had a history of affective diagnosis. Of those with a diagnostic history, 30 had a trauma‐related disorder, 24 had a depressive disorder, and 17 had an anxiety disorder, as their primary lifetime diagnosis. The most frequent diagnosis in the sample was post‐traumatic stress disorder (30% of the sample) and the next most common disorder was major depressive disorder (15% of the sample). Also, 37% had comorbid lifetime mood and anxiety/trauma‐related disorders.
2.2.6. Sample characterization
Participants also completed questionnaires to characterize the sample, including the Perceived Stress Scale (PSS, 10‐item version) to assess frequency of stress‐related feelings (Cohen, Kamarck, & Mermelstein, 1983), the State Trait Anxiety Inventory (STAI‐Y2) to assess presence and severity of trait anxiety (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) and the NEO Five‐Factor Inventory‐3 (NEO‐FFI‐3, 60 items) to assess five domains of personality (neuroticism, extraversion, openness, agreeableness, and conscientiousness) (McCrae & Costa Jr, 2007). See Table 1 for Participant Characteristics.
TABLE 1.
Participant characteristics (n = 100)
| Characteristic | Mean | SD | Range |
|---|---|---|---|
| Age (years) | 27.28 | 3.99 | 21–35 |
| CTQ threat | 31.25 | 13.59 | 15–69 |
| CTQ deprivation | 21.14 | 8.79 | 10–46 |
| CTQ total score | 52.39 | 21.11 | 25–100 |
| THQ (age 0–11) | .94 | 1.46 | 0–7 |
| THQ (age 12–17) | 1.17 | 1.89 | 0–11 |
| Parental education level | 5.29 | 2.04 | 1–8 |
| Education level | 5.19 | 1.56 | 1–8 |
| BDI II total score | 11.64 | 10.79 | 0–49 |
| PCL‐C total score | 32.35 | 13.92 | 17–75 |
| Perceived stress | 16.97 | 8.75 | 2–37 |
| STAI‐T | 41.76 | 13.33 | 20–77 |
| NEO: Neuroticism (%) | 36.86 | 10.18 | 13–59 |
| NEO: Extraversion (%) | 39.54 | 7.96 | 12–56 |
| NEO: Openness (%) | 33.58 | 4.73 | 19–42 |
| NEO: Agreeableness (%) | 44.10 | 4.96 | 28–55 |
| NEO: Conscientiousness (%) | 43.88 | 7.42 | 20–59 |
2.2.7. MRI protocol and data acquisition
MRI data were collected on a 3‐Tesla Trio TIM whole‐body MRI scanner (Siemens, Erlangen, Germany), equipped with a 32‐channel head coil. Diffusion spectrum imaging (DSI) data were acquired using a 19‐minute, 271‐direction scan (including 20 reference images) using a twice‐refocused spin‐echo EPI sequence and multiple q values (TR = 4,250 ms, TE = 150 ms, voxel size = 2.4 × 2.4 × 2.4 mm3, FoV = 230 × 230 mm, b‐max = 4,000 s/mm2). We also included structural imaging for anatomical comparisons using a 4.8‐min T1‐weighted sagittal MPRAGE sequence (TR = 1,500 ms, TE = 3.19 ms, flip angle = 8°, 176 slices, FoV = 256 × 256 mm2, voxel size = 1 × 1 × 1.0 mm3). DSI data were reconstructed using a Q‐space diffeomorphic reconstruction (QSDR) approach (Yeh & Tseng, 2011), using a 1.25 diffusion sampling length ratio. Spatial normalization was conducted using an SPM‐like normalization with 14‐18‐14 basis functions and an output resolution of 2 mm isotropic. The generalized fractional anisotropy (gFA) (Tuch, 2004) of each participant was calculated in native space and then nonlinearly warped to MNI152 space (Yeh & Tseng, 2011). [Fractional anisotropy is canonically interpreted as an overall index of structural integrity, however, can be influenced by other factors (e.g., underlying microstructural properties of white matter and support tissue) (Hagmann et al., 2006; Pierpaoli et al., 2001).] Four participants were excluded from hierarchical regression analyses due to excessive movement and/or distortion (analytic n = 96). The average group template of 96 participants (created for visualization purposes) revealed high quality DSI data with crisp endpoint terminations and few wandering fibers (Figure 1).
FIGURE 1.
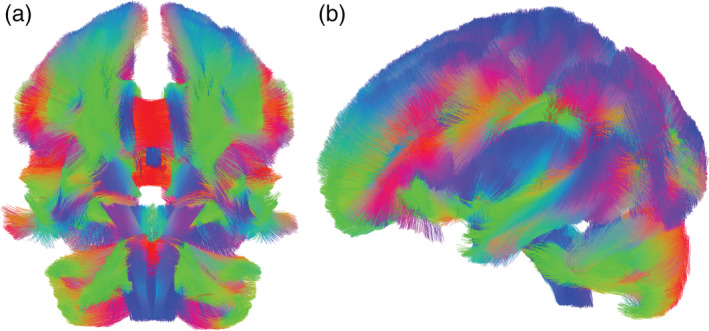
Average group template (n = 96) demonstrated high quality diffusion spectrum imaging (DSI) data showed in ventral (a) and sagittal views (b)
2.3. HCP image acquisition, reconstruction and tractography: Creating ST and MFB ROIs using HCP data
Human connectome project (HCP) data were used to perform ST and MFB tractography (https://www.humanconnectome.org/, n = 488); these ST and MFB tracts from the HCP group template were then used as regions of interest (ROIs) to extract ST and MFB gFA from the voxelwise reconstructed spin distribution function (SDF, a normalized orientation distribution function) of each participant in the current study (n = 96). ST and MFB tractography was performed using a single ROI‐based (not an ROI‐to‐ROI) approach; dilated regions shown in figures (Figures 2 and 3, and Figures S3 & S4) are for the purposes of visualization and descriptive anatomy. Please see Data S1 for details on HCP image acquisition and reconstruction, and tractography and analysis (Figures S1–S4 and Table S1).
FIGURE 2.
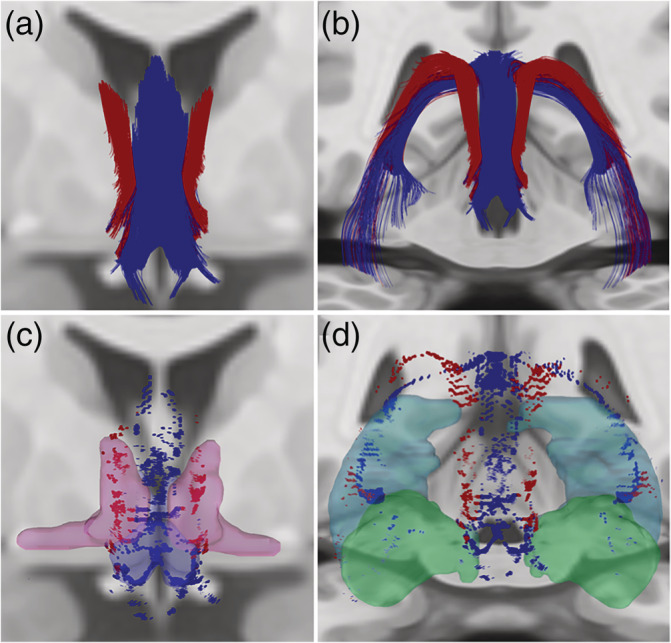
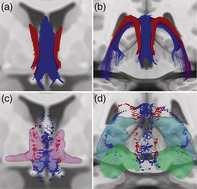
Fornix and Stria Terminalis Human Connectome Project Group Tractography. (a,b). We observed fornix fibers (blue) extending from the hippocampus, arcing through the crura, forming the body and dividing into the columns. The stria terminalis (red) curved along the medial aspect of the caudate and extended toward the anterior commissure. Stria terminalis endpoints (red) were localized primarily within the dorsal and ventral BST (c). (c,d) Fornix endpoints (blue) were located within the subiculum and Cornu Ammonis (primarily CA3, d), and within the medial preoptic and paraventricular hypothalamus (c). Dilated regions represent BST (magenta, c) and paraventricular/preautonomic hypothalamus (blue, c) ROIs used previously (Banihashemi et al., 2015), and hippocampus (light blue, d) and amygdala (aqua green, d) ROIs from the AAL atlas
FIGURE 3.
Medial Forebrain Bundle Human Connectome Project Group Tractography. (a) MFB tractography demonstrating fibers coursing through the mid‐brain tegmentum toward the BST. (b) Endpoints from the above tractography localized within the BST. Dilated regions represent the BST (yellow, a&b) ROI used previously (Banihashemi et al., 2015) and the dorsal brainstem seed ROI (blue rectangular prism, a&b) used for tractography
2.4. Variable selection
Preliminary data analyses revealed that our childhood threat and deprivation measures were correlated with one another (Table 2); however, among the potential deprivation measures, maximum parental education level was the least correlated with the threat measures (Pearson r = 0.232 to 0.403, Table 2). CTQ Threat (abuse) and CTQ Deprivation (neglect) were strongly correlated (r = 0.756). Because of the apparent lack of differentiation between these constructs from this measure, CTQ Deprivation was considered only in secondary analyses (Table S5 and S6) and maximum parental education level (reverse coded) was used as the primary measure of socioeconomic deprivation (SED), such that higher values reflected greater levels of deprivation. As early childhood experiences are key for neural development (Tottenham & Sheridan, 2010) and white matter is shaped by repeated events (Fields, 2008; Fields, 2010), we focused our primary analyses of trauma on early, repeated traumatic events (THQ 0–11, events that occurred two or more times; exploratory analyses examining later, repeated traumatic events analyses, THQ 12–17, are included in Data S1, Table S4). Because CTQ Threat and THQ 0–11 are strongly correlated (r = 0.647), these threat measures were considered in separate models. Doing so allowed examination of abuse (CTQ Threat) and broader traumatic events (THQ 0–11) separately. Because the threat measures co‐occur with SED and are not completely independent from one another, we considered each threat measure along with SED in the same step of our hierarchical regression models (see Data Analysis). Thus, we examined the additive effects (Fahrmeir, Kneib, Lang, & Marx, 2013) of threat and deprivation similar to Lawson et al. (Lawson et al., 2017).
TABLE 2.
Correlations between childhood threat and deprivation measures
| CTQ threat | THQ 0–11 | THQ 12–17 | CTQ deprivation | SED | ||
|---|---|---|---|---|---|---|
| CTQ threat (abuse) | Pearson r | — | .647 ** | .653 ** | .756 ** | .403 ** |
| p (2‐tailed) | — | .000 | .000 | .000 | .000 | |
| THQ 0–11 | Pearson r | .647 ** | — | .770 ** | .454 ** | .232 * |
| p (2‐tailed) | .000 | — | .000 | .000 | .023 | |
| THQ 12–17 | Pearson r | .653 ** | .770 ** | — | .551 ** | .236 * |
| p (2‐tailed) | .000 | .000 | — | .000 | .020 | |
| CTQ deprivation (neglect) | Pearson r | .756 ** | .454 ** | .551 ** | — | .399 ** |
| p (2‐tailed) | .000 | .000 | .000 | — | .000 | |
| Socioeconomic deprivation (SED) | Pearson r | .403 ** | .232 * | .236 * | .399 ** | — |
| p (2‐tailed) | .000 | .023 | .020 | .000 | — |
Correlation is significant at the 0.05 level (2‐tailed);
Correlation is significant at the 0.01 level (2‐tailed).
2.5. Data analysis
2.5.1. Childhood adversity and visceral white matter
We examined whether childhood threat and SED variables were associated with ST or MFB gFA. All hierarchical regression models covaried for age, sex, and race in Step 1 and examined the additive effects of childhood threat (abuse or early repeated trauma) and SED together in Step 2. We also evaluated whether our findings remained after multiple comparison correction (FDR <0.05, for four tests, CTQ Threat and THQ 0–11 models for each tract, ST and MFB) (Benjamini & Hochberg, 1995) and after adjusting for adulthood trauma (all traumatic events occurring after age 18), adulthood SES (education level), and negative life events within the past year (Life Events List); these variables were entered together in Step 3. Where a significant relationship was found between CTQ Threat and visceral white matter, post‐hoc analyses were performed substituting each abuse subscale in the model to examine which type of abuse may be driving the effects.
2.5.2. Visceral white matter and affective symptoms/disorders
We also examined whether ST and MFB gFA were associated with depressive or post‐traumatic stress symptom severity or the number of lifetime diagnoses. Hierarchical regression models covaried for age, sex, and race in Step 1 and examined the effect of either ST gFA or MFB gFA in Step 2 in two separate models. We also evaluated whether our findings remained after multiple comparison correction (FDR <0.05, for three tests, one for each measure of affect) and after adjusting for adulthood trauma (all traumatic events occurring after age 18), adulthood SES (education level) and negative life events within the past year (Life Events List); these variables were entered together in Step 3.
3. RESULTS
3.1. Human connectome project ST and MFB tractography
Our observed fornix and ST tractography was largely consistent with literature describing ex vivo histology (Nieuwenhuys, Voogd, & van Huijzen, 2008), with fornix fibers extending from the hippocampus, arcing through the crura, forming the body and dividing into the columns (Figure 2a,b). The ST curved along the medial aspect of the caudate and extended toward the anterior commissure (Figure 2a,b). ST endpoints were localized primarily within the dorsal and ventral BST (Figure 2c). Fornix endpoints were located within the subiculum and Cornu Ammonis (primarily CA3, Figure 2d), and within the medial preoptic and paraventricular hypothalamus (Figure 2c). The MFB coursed through the mid‐brain tegmentum toward the BST (Figure 3a), with some endpoints localized within the BST (Figure 3b).
3.2. Childhood threat, deprivation, and visceral white matter
3.2.1. Stria terminalis
Our analyses from both models [abuse (CTQ Threat) and early repeated trauma (THQ 0–11)] revealed that threat and socioeconomic deprivation (SED, maximum parental education level reverse coded) had significant, yet opposing effects on ST gFA (Figure 4a–c and Tables 3 and 4, left). In the CTQ Threat and SED model, CTQ Threat had a negative effect (ß = −0.337; p = .003, Figure 4a), while SED had a positive effect, on ST gFA (ß = 0.317; p = .005, Figure 4b and Table 3, left); both survived multiple comparison correction (adjusted p values: CTQ Threat, p = .006, SED, p = .020) and remained significant when adulthood trauma, adulthood SES and negative life events were added to the model (Table 3, left).
FIGURE 4.
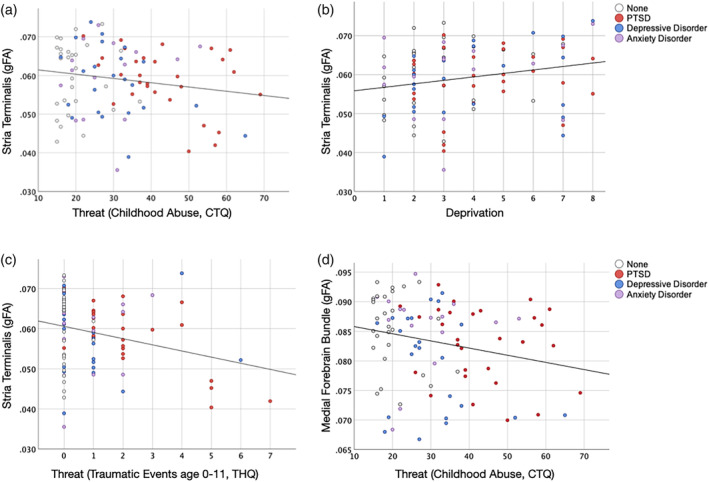
Relationships between threat, socioeconomic deprivation and visceral white matter. (a,b) Opposing relationships of threat and socioeconomic deprivation (SED) on Stria Terminalis generalized fractional anisotropy (gFA); (a) CTQ Threat (abuse) had a negative effect (ß = −0.337; p = .003) on ST gFA. (b) SED (maximum parental education level, reverse coded) had a positive effect on ST gFA (ß = 0.317; p = .005). (c) Similar to abuse (CTQ Threat), early repeated traumatic events (THQ 0–11) had a negative effect (ß = −0.332; p = .001) on ST gFA. (d) CTQ Threat (abuse) had a negative effect on MFB gFA (ß = −0.269; p = .020). Scatterplots indicate primary lifetime diagnosis from the SCID‐IV (white—no history of affective diagnosis, red—post‐traumatic stress disorder [PTSD], blue—depressive disorder, purple—anxiety disorder)
TABLE 3.
Regression results: Childhood threat (abuse), socioeconomic deprivation and visceral white matter analyses
| Stria Terminalis gFA | Medial forebrain bundle gFA | ||||||
|---|---|---|---|---|---|---|---|
| Step | Variable | St. Beta | t | p | St. Beta | t | p |
| 1 | Age | .060 | .571 | .570 | .087 | .835 | .406 |
| Sex | −.146 | −1.411 | .162 | .062 | .602 | .548 | |
| Race | −.062 | −.597 | .552 | −.126 | −1.211 | .229 | |
| 2 | Age | .058 | .564 | .574 | .134 | 1.252 | .214 |
| Sex | −.165 | −1.650 | .102 | .024 | .228 | .820 | |
| Race | .005 | .046 | .964 | −.086 | −.832 | .408 | |
| CTQ threat | −.337 | −3.071 | .003 * | −.269 | −2.371 | .020 * | |
| Socioeconomic deprivation | .317 | 2.891 | .005 * | .070 | .616 | .540 | |
| 3 | Age | .056 | .479 | .633 | .146 | 1.201 | .233 |
| Sex | −.203 | −1.911 | .059 | .025 | .223 | .824 | |
| Race | −.013 | −.132 | .895 | −.096 | −.908 | .367 | |
| CTQ threat | −.316 | −2.792 | .006 * | −.260 | −2.188 | .031 * | |
| Socioeconomic deprivation | .279 | 2.341 | .021 | .041 | .330 | .742 | |
| THQ >18 | .099 | .813 | .419 | −.015 | −.118 | .907 | |
| Adulthood SES | −.122 | −1.149 | .254 | −.077 | −.694 | .490 | |
| Negative life events | −.146 | −1.218 | .227 | −.002 | −.014 | .989 | |
Note: Bold values indicate significance at p < .05.
Survival of FDR correction (0.05) for four tests.
TABLE 4.
Regression results: Childhood threat (repeated traumatic events, age 0–11), socioeconomic deprivation and visceral white matter analyses
| Stria Terminalis gFA | Medial forebrain bundle gFA | ||||||
|---|---|---|---|---|---|---|---|
| Step | Variable | St. Beta | t | p | St. Beta | t | p |
| 1 | Age | .060 | .571 | .570 | .087 | .835 | .406 |
| Sex | −.146 | −1.411 | .162 | .062 | .602 | .548 | |
| Race | −.062 | −.597 | .552 | −.126 | −1.211 | .229 | |
| 2 | Age | .023 | .230 | .819 | .107 | 1.011 | .315 |
| Sex | −.126 | −1.289 | .201 | .054 | .531 | .597 | |
| Race | .009 | .087 | .931 | −.082 | −.794 | .429 | |
| THQ 0–11 | −.332 | −3.292 | .001 * | −.271 | −2.601 | .011 * | |
| Socioeconomic deprivation | .271 | 2.611 | .011 * | .035 | .325 | .746 | |
| 3 | Age | −.007 | −.060 | .952 | .093 | .756 | .452 |
| Sex | −.149 | −1.403 | .164 | .071 | .638 | .525 | |
| Race | −.009 | −.089 | .929 | −.090 | −.855 | .395 | |
| THQ 0–11 | −.326 | −2.919 | .004 * | −.283 | −2.424 | .017 * | |
| Socioeconomic deprivation | .229 | 2.003 | .048 | .002 | .020 | .984 | |
| THQ >18 | .135 | 1.096 | .276 | .019 | .144 | .886 | |
| Adulthood SES | −.097 | −.910 | .366 | −.054 | −.485 | .629 | |
| Negative life events | −.095 | −.773 | .442 | .045 | .348 | .728 | |
Note: Bold values indicate significance at p < .05.
Survival of FDR correction (0.05) for four tests.
Post‐hoc analyses examining CTQ Threat (abuse) subscales revealed that emotional (ß = −0.249; p = .024), physical (ß = −0.362; p = .002), and sexual (ß = −0.215; p = .043) abuse were each negatively associated with ST gFA; all of these survived multiple comparison correction for three tests (one for each abuse type) and remained significant with the additional adulthood covariates. In each of these CTQ Threat subscale analyses, SED had a significant, opposing effect on ST gFA (see Data S1 for abbreviated results, Table S2).
Similar effects were seen with the THQ 0–11 and SED model, in which both THQ 0–11 and SED had significant, opposing effects on ST gFA; THQ 0–11 had a negative effect (ß = −0.332; p = .001, Figure 4c), while SED had a positive effect (ß = 0.271; p = .011) on ST gFA (Table 4, left). Both survived multiple comparison correction (adjusted p values: THQ 0–11, p = .004, SED, p = .022) and remained significant with the additional adulthood covariates (Table 4, left).
3.2.2. Medial forebrain bundle
In the CTQ Threat and SED model, CTQ Threat had a negative effect on MFB gFA (ß = −0.269; p = .020, Figure 4d). This effect survived multiple comparison correction (adjusted p value: CTQ Threat, p = .020) and remained significant with the additional adulthood covariates (Table 3, right). SED did not have a significant effect on MFB gFA.
Post‐hoc analyses revealed that physical abuse (ß = −0.281; p = .018) was negatively associated with ST gFA; this finding did not survive multiple comparison correction, but did remain significant with the additional adulthood covariates (ß = −0.277; p = .031). SED did not have a significant effect on MFB gFA in any subscale model (see Data S1 for abbreviated results, Table S3).
Similar to the CTQ Threat model, THQ 0–11 had a significant negative effect on MFB gFA (ß = −0.271; p = .011, Figure S5). This effect survived multiple comparison correction (adjusted p value: THQ 0–11, p = .015) and remained significant with the additional adulthood covariates (Table 4, right). SED did not have a significant effect on MFB gFA (Table 4, right). Removing one outlier from the THQ 0–11 and MFB gFA model makes this finding more robust. THQ 0–11 had a significant negative effect on MFB gFA (ß = −0.340; p = .001). This effect also survived multiple comparison correction (adjusted p value: THQ 0–11, p = .025) and remained significant with the additional adulthood covariates (ß = −0.332; p = .004). [See Data S1 for secondary analyses examining later, repeated traumatic events, THQ 12–17 (Table S4), CTQ Deprivation (neglect) as an alternative measure of deprivation (Tables S5 and S6), and regression analyses stratified by sex (Tables S7 and S8)].
3.3. Visceral white matter and affective symptoms
Regression analyses revealed that ST gFA was not significantly associated with depression symptoms (ß = −0.066; p = .524), post‐traumatic stress symptoms (ß = −0.069; p = .507) or the number of lifetime diagnoses (ß = −0.141; p = .145).
Regression analyses revealed that MFB gFA was negatively associated with depression (ß = −0.218; p = .033) and post‐traumatic stress symptoms (ß = −0.210; p = .043), as well as the number of lifetime affective diagnoses (ß = −0.198; p = .040). The relationships between MFB gFA and depression (ß = −0.202; p = .040) and the number of lifetime diagnoses (ß = −0.180; p = .044) remained significant with the additional adulthood covariates, however, only the relationship between MFB gFA and post‐traumatic stress symptoms survived multiple comparison correction (adjusted p value: MFB gFA, p = .043) (see Data S1 for relationships between childhood adversity variables and affective symptoms/diagnoses, Table S9).
4. DISCUSSION
Childhood threat and deprivation are dimensions of childhood adversity thought to have different influences on the brain via distinct mechanisms (McLaughlin et al., 2014; Sheridan & McLaughlin, 2014). How threat and deprivation may differentially influence stress‐related, visceral neural circuits is unknown. This study examined effects of childhood threat and deprivation on stress‐related, visceral white matter. We hypothesized that threat would be associated with less structural integrity, while socioeconomic deprivation (SED, low SES/parental education level) would be associated with greater structural integrity of visceral white matter. Our primary finding was that childhood threat and SED have opposing relationships with ST gFA, in which greater threat (both abuse and early repeated trauma) was associated with less, while greater SED was associated with higher ST gFA. To our knowledge, this is the first study to show opposing effects of threat and deprivation on visceral white matter. Further, threat (both abuse and early repeated trauma) also had a negative relationship with MFB gFA, while SED did not have an effect. Interestingly, only MFB gFA was associated with affective symptoms and disorders, suggesting it may be a novel link between childhood threat and affect.
4.1. Potential neural mechanisms underlying relationships of threat and deprivation with visceral white matter
4.1.1. Opposing relationships of threat and deprivation within stria terminalis
Childhood threat experiences may shape neural development via effects of stress on the brain, such as neural effects of glucocorticoids and related excitotoxic damage (Tottenham & Sheridan, 2010), along with specific changes in fear learning circuits (prefrontal cortex, amygdala, and hippocampus) (McLaughlin et al., 2014); while deprivation may shape neural development via synaptic pruning (McLaughlin et al., 2014) or activity‐dependent plasticity, in which activity strengthens synapses that repeatedly fire one another and eliminates those which do not. The notion that deprivation influences neural development via activity‐dependent plasticity is likened to other studies of sensory deprivation. For example, in the visual system, early life monocular deprivation leads to loss of cortical space devoted to the deprived eye as terminals from the visual thalamic nucleus of the non‐deprived eye expand (Hubel, Wiesel, & LeVay, 1977). Similar processes may contribute to our findings; for example, proximal, familial threats like abuse may diminish structural integrity via excitotoxic effects of glucocorticoids on the brain, while more distal, environmental stressors of SED/low SES may strengthen these pathways via repeated, coordinated activation (Rinaman et al., 2011). Differences in the chronicity of these dimensions of childhood adversity could also contribute to the opposing findings seen here. For example, abuse and repeated traumatic events may be phasic occurrences while deprivation may be more tonic or sustained.
4.1.2. Threat and visceral white matter
Greater childhood threat was also associated with less MFB gFA potentially via similar mechanisms, or diminished structural integrity due to excitotoxic damage from glucocorticoids. These effects of childhood threat on MFB gFA appear to be driven by physical abuse, which has been shown to dysregulate stress reactivity (Carpenter et al., 2011) and predict affective symptoms (Springer, Sheridan, Kuo, & Carnes, 2007). Physical abuse is also associated with greater stress reactivity within stress‐related, visceral regions, PVN, BST, amygdala and subgenual anterior cingulate cortex (sgACC), while emotional abuse was associated with greater stress reactivity in regions more distal to the stress response, sgACC and amygdala (Banihashemi et al., 2015). Thus, individuals with a developmental history of physical abuse may be engaging more of this visceral, stress‐control network to a greater extent, eliciting greater excitotoxic damage to the MFB.
Effects of threat on ST and MFB may also be mediated by inflammatory processes. Greater inflammation is linked to diminished white matter integrity (Favrais et al., 2011; Walker et al., 2018). In humans, individuals that transitioned from low to high systemic inflammation during midlife had the least white matter structural integrity compared to those that maintained low systemic inflammation (Walker et al., 2018). Further, unpredictable chronic stress increases central proinflammatory cytokines and decreases oligodendrocyte number in rats (Yang et al., 2015). Unpredictable chronic stress may be similar to the potentially more proximal, phasic experiences of childhood threat, however, Gianaros et al. found that inflammatory pathways partially mediated the relationship between socioeconomic position and white matter integrity (Gianaros et al., 2012). Thus, it is possible that both childhood threat and deprivation influence these stress‐related neural circuits via distinct (e.g., glucocorticoid excitoxicity and activity‐dependent plasticity) and similar mechanisms (e.g., inflammatory pathways) to produce a net effect that reflects the more dominant (frequent/chronic/severe) dimension of childhood adversity.
4.2. Neuroendocrine and autonomic implications of threat and deprivation findings
Childhood adversity is associated with dysregulated (heightened or diminished) stress reactivity in childhood and later in life, with alterations in both neuroendocrine and autonomic physiology and stress reactivity (Carpenter et al., 2007; Carpenter et al., 2011; Chen et al., 2004; Gunnar et al., 2009; Hackman et al., 2012; Heim et al., 2000; Heim et al., 2001; Koopman et al., 2004; Lovallo et al., 2011). There are inconsistencies in the literature regarding directionality depending on age and sample composition, however, there is evidence that threat (i.e., abuse/maltreatment) blunts (Bernard, Frost, Bennett, & Lindhiem, 2017; Carpenter et al., 2007; Carpenter et al., 2011; Doom, Cicchetti, & Rogosch, 2014; Peckins, Susman, Negriff, Noll, & Trickett, 2015), while deprivation (i.e., low SES) heightens basal levels of catecholamines and cortisol, as well as cortisol reactivity (Chen, Cohen, & Miller, 2009; Cohen, Doyle, & Baum, 2006; Lê‐Scherban et al., 2018; Lupien, King, Meaney, & McEwen, 2001). Longitudinal research shows that maltreated children with higher initial cortisol levels display blunted cortisol levels over time, perhaps related to the hypothalamic–pituitary–adrenal (HPA) axis being overburdened and suppressed by chronic stress (Doom et al., 2014). Longitudinal research in low SES children shows the opposite trend; low SES children display greater 2‐year increases in daily cortisol compared to high SES children (Chen et al., 2009). This may be due to stress associated with low SES children appraising ambiguous situations as threatening, as opposed to the stress of directly experiencing/witnessing threats (Chen et al., 2004; Chen et al., 2009). As these visceral white matter structures connect regions involved in the regulation of stress responses (Banihashemi & Rinaman, 2006; Choi et al., 2007; Rinaman et al., 2011), our threat, deprivation and visceral white matter findings may be linked to specific neuroendocrine and autonomic profiles.
4.3. Functional implications of deprivation‐related enhanced stria terminalis structural integrity: A potential neural adaptation?
To further interpret how these differences in ST microstructure may relate to functional outcomes, two recent studies provide insights. Koller et al. showed that greater ST FA is associated with greater orienting bias toward threat in a saccadic decision task (Koller et al., 2019). Another study by Dzafic et al. showed that greater ST microstructure was associated with faster recognition of anger following an anger cue during a dynamic emotional perception task (Dzafic et al., 2019). Their findings suggest that individuals with greater ST microstructure have more efficient emotion processing under threat (Dzafic et al., 2019). In the context of our findings, in which greater SED is associated with greater ST structural integrity, these studies suggest a potential neural adaptation in which greater ability to orient to threat or recognize emotion may yield enhanced situational awareness of threat, therefore enhancing likelihood of survival. Further investigation will be necessary to determine to what extent these opposing relationships of threat and deprivation with ST structural integrity are adaptive, however, our findings may have broad implications for orienting responses to threatening stimuli, emotion processing and social behavior. Further, these findings may challenge the notion that early life stress creates neural changes that indicate maladaptive damage, but that neural differences in the context of childhood adversity may be adaptive for the relevant context (for review see Champagne et al., 2008; Teicher & Samson, 2016; Teicher, Samson, Anderson, & Ohashi, 2016).
4.4. Visceral white matter and affective symptoms: The medial forebrain bundle as a novel link between threat and affect
Despite evidence that ST structural integrity and related neural circuits are associated with anxiety and post‐traumatic stress (Avery et al., 2016; Harnett, Ference, Knight, & Knight, 2020; Kim, Kim, Kiu Choi, & Lee, 2017), in our sample, recruited along a continuum of physical abuse and affective symptom severity, we did not find significant relationships with affective measures. Our findings revealed that only MFB gFA was associated with affective symptoms and disorders, suggesting it may be a novel link between childhood threat and affect. Greater MFB gFA was linked to fewer depressive and post‐traumatic stress symptoms. Interestingly, vagal nerve stimulation (targeting brainstem NST) alleviates treatment‐resistant depression, as does deep brain stimulation of the MFB, suggesting altered visceral circuit signaling in mood disorders (Berry et al., 2013; Fenoy et al., 2016). Repeated coordinated activation between preautonomic and viscerosensory pathways (Rinaman et al., 2011) may act to strengthen this pathway, contributing to a greater capacity to regulate affective responses to stress.
4.5. Limitations
A limitation of this work is the cross‐sectional nature of the study focused on young adults. A novel continuous and transdiagnostic design was implemented in which a relatively even distribution across physical abuse severity was achieved. This is an advantage over work done in healthy samples in which threat severity distributions can be heavily skewed with the majority of the sample having none‐to‐minimal childhood threat. However, we have not captured the developmental trajectory of these visceral white matter tracts during presumably narrow sensitive periods in childhood, as indicated by related structures (Andersen et al., 2008; Pechtel, Lyons‐Ruth, Anderson, & Teicher, 2014; Teicher et al., 2018; Zhu et al., 2019). The developmental trajectory of these visceral white matter circuits is unclear in humans; available evidence suggests that these tracts are present in neonates (Chen et al., 2011) and reach peak structural integrity earlier than most major white matter bundles (Dubois et al., 2008; Lebel et al., 2012; Westlye et al., 2010). At this time, our goal was to investigate these circuits in their mature state to examine potential neural outcomes of childhood threat and deprivation experiences. We were able to examine traumatic events retrospectively using the broad age ranges of 0–11 and 12–17 and saw similar effect sizes of traumatic events during both age ranges on ST gFA, while earlier traumatic events had more robust effects on MFB gFA (Table 4 and Table S4). Future work will be necessary to examine the dynamic relationships of threat and deprivation with the ST and MFB using a longitudinal approach and to examine the role of timing in the opposing relationships shown with ST here.
This study also used retrospective assessments of childhood abuse and traumatic events; concerns with such reports are recall bias and false positives, however, agreement between sibling retrospective reports of abuse, and prospectively collected records suggests reliability of such reports (Bifulco, Brown, & Harris, 1994; Bifulco, Brown, Lillie, & Jarvis, 1997; Hardt & Rutter, 2004). Further, Scott et al. found no differences between prospective and retrospective reports of maltreatment in the strength of the relationships between maltreatment history and affective disorders (Scott, McLaughlin, Smith, & Ellis, 2012).
Measures of HPA or autonomic function were not collected in this study, which limits our ability to interpret how the relationships of threat, deprivation and visceral white matter may be linked to neuroendocrine and physiological function. An important area of future work will be to examine how threat, deprivation and visceral white matter alterations relate to measures of neuroendocrine, autonomic and neuroimmune signaling.
Another limitation may be that the tractography templates for ST and MFB may vary across individuals or may vary depending on an individual's diagnostic history or state. However, for the scope of the current work, using the HCP data as a template with high‐resolution DSI was a robust method of analysis mitigating the inconsistencies of individual tractography. It will be important for future work to isolate components of the tract that are related to childhood threat and/or deprivation and to further investigate potential sources of microstructural differences (e.g., myelination, axonal degeneration, extent of axonal dispersion). Future work will also consider additional pathways that link these visceral regions of interest, such as the amygdalofugal pathway (Folloni et al., 2019).
In future analyses, it will also be important to test whether the effects of childhood threat and/or deprivation on affective symptoms are mediated by visceral white matter in a larger sample that is sufficiently powered to detect such effects (Fritz & MacKinnon, 2007). Further, using SED, or low SES based on maximum parental education level as our primary index of deprivation may also be considered a limitation. Although secondary analyses using CTQ Threat and CTQ Deprivation (neglect) also show similar trends toward opposing effects on ST gFA (Table S5), suggesting that our findings may be robust to the specific measure of deprivation. Future work will be necessary to investigate other components of deprivation that integrate cognitive deprivation and/or more comprehensive measures of SES (e.g., neighborhood deprivation as derived from census tract data) (Sumner, Colich, Uddin, Armstrong, & McLaughlin, 2019).
5. SUMMARY AND CONCLUSIONS
This study provides the first evidence that childhood threat and deprivation have opposing influences on ST gFA, and novel evidence that different dimensions of childhood adversity may have differential influences on structural integrity or underlying microstructure, even within the same region. Further, our results demonstrate that the MFB may provide a novel link between childhood threat and affective symptoms. Examining how these stress‐related, visceral circuits link childhood adversity to affective symptoms could improve our understanding of mechanisms underlying affective disorders and provide novel targets for intervention.
CONFLICT OF INTEREST
The authors declare no competing financial interests. Anne Germain, Ph.D., has no conflict of interest related to this work; she serves as CEO and holds equity in Rehat, LLC and has also served as a consultant for Jazz Pharmaceuticals, Inc.
Supporting information
DATA S1 Supporting information
ACKNOWLEDGMENTS
This work was funded by National Institute of Mental Health Grant K01 MH102406 to L.B. For Stria Terminalis & Medial Forebrain Bundle Tractography: Data were provided by the Human Connectome Project, WU‐Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. Many thanks to Noelle Rode for providing database construction, Mark Jones for providing clinical interview supervision and Drs. Hoby Hetherington, Claudiu Schirda and Tae Kim of the Magnetic Resonance Research Center (MRRC) for providing pilot time and sequence optimization.
Banihashemi L, Peng CW, Verstynen T, et al. Opposing relationships of childhood threat and deprivation with stria terminalis white matter. Hum Brain Mapp. 2021;42:2445–2460. 10.1002/hbm.25378
Funding information National Institute of Mental Health, Grant/Award Number: K01 MH102406
DATA AVAILABILITY STATEMENT
Research data are not shared at this time. The data that support the findings of this study may be made available from the corresponding author upon reasonable request in the future.
REFERENCES
- Andersen, S. L. , Tomada, A. , Vincow, E. S. , Valente, E. , Polcari, A. , & Teicher, M. H. (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of Neuropsychiatry and Clinical Neurosciences, 20(3), 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthofer, J. M. , Steib, K. , Fellner, C. , Lange, M. , Brawanski, A. , & Schlaier, J. (2015). DTI‐based deterministic fibre tracking of the medial forebrain bundle. Acta Neurochirurgica, 157(3), 469–477. [DOI] [PubMed] [Google Scholar]
- Avery, S. N. , Clauss, J. A. , & Blackford, J. U. (2016). The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology, 41(1), 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, S. N. , Clauss, J. A. , Winder, D. G. , Woodward, N. , Heckers, S. , & Blackford, J. U. (2014). BNST neurocircuitry in humans. NeuroImage, 91, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, L. M. , Williams, L. M. , Korgaonkar, M. S. , Cohen, R. A. , Heaps, J. M. , & Paul, R. H. (2013). Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging and Behavior, 7(2), 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi, L. , O'Neill, E. J. , & Rinaman, L. (2011). Central neural responses to restraint stress are altered in rats with an early life history of repeated brief maternal separation. Neuroscience, 192, 413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi, L. , & Rinaman, L. (2006). Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic‐pituitary‐adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. The Journal of Neuroscience, 26(44), 11442–11453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi, L. , Sheu, L. K. , Midei, A. J. , & Gianaros, P. J. (2015). Childhood physical abuse predicts stressor‐evoked activity within central visceral control regions. Social Cognitive and Affective Neuroscience, 10(4), 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , Ball, R. , & Ranieri, W. F. (1996). Comparison of Beck depression inventories‐IA and ‐II in psychiatric outpatients. Journal of Personality Assessment, 67(3), 588–597. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B: Methodological, 57(1), 289–300. [Google Scholar]
- Bernard, K. , Frost, A. , Bennett, C. B. , & Lindhiem, O. (2017). Maltreatment and diurnal cortisol regulation: A meta‐analysis. Psychoneuroendocrinology, 78, 57–67. [DOI] [PubMed] [Google Scholar]
- Bernstein, D. P. , Fink, L. , Handelsman, L. , Foote, J. , Lovejoy, M. , Wenzel, K. , … Ruggiero, J. (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry, 151(8), 1132–1136. [DOI] [PubMed] [Google Scholar]
- Berry, S. M. , Broglio, K. , Bunker, M. , Jayewardene, A. , Olin, B. , & Rush, A. J. (2013). A patient‐level meta‐analysis of studies evaluating vagus nerve stimulation therapy for treatment‐resistant depression. Medical Devices (Auckland, NZ), 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti, C. , & Pivetti, M. (2019). Childhood economic disadvantage and antisocial behavior: Intervening factors and pathways. Children and Youth Services Review, 97, 120–126. [Google Scholar]
- Bifulco, A. , Brown, G. , Lillie, A. , & Jarvis, J. (1997). Memories of childhood neglect and abuse: Corroboration in a series of sisters. Journal of Child Psychology and Psychiatry, 38(3), 365–374. [DOI] [PubMed] [Google Scholar]
- Bifulco, A. , Brown, G. W. , & Harris, T. (1994). Childhood Experience of Care and Abuse (CECA): A retrospective interview measure. Journal of Child Psychology and Psychiatry, 35(8), 1419–1435. [DOI] [PubMed] [Google Scholar]
- Bracht, T. , Horn, H. , Strik, W. , Federspiel, A. , Schnell, S. , Höfle, O. , … Walther, S. (2014). White matter microstructure alterations of the medial forebrain bundle in melancholic depression. Journal of Affective Disorders, 155, 186–193. [DOI] [PubMed] [Google Scholar]
- Card, J. P. , Levitt, P. , Gluhovsky, M. , & Rinaman, L. (2005). Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. The Journal of Neuroscience, 25(40), 9102–9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card, J. P. , Rinaman, L. , Schwaber, J. S. , Miselis, R. R. , Whealy, M. E. , Robbins, A. K. , & Enquist, L. W. (1990). Neurotropic properties of pseudorabies virus: Uptake and transneuronal passage in the rat central nervous system. The Journal of Neuroscience, 10(6), 1974–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card, J. P. , & Sved, A. F. (2011). Central autonomic pathways. In Llewellyn‐Smith I. J. & Verberne A. J. (Eds.), Central regulation of autonomic functions (pp. 3–22). New York, NY: Oxford University Press, Inc.. [Google Scholar]
- Carpenter, L. , Shattuck, T. , Tyrka, A. , Geracioti, T. , & Price, L. (2011). Effect of childhood physical abuse on cortisol stress response. Psychopharmacology, 214(1), 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, L. L. , Carvalho, J. P. , Tyrka, A. R. , Wier, L. M. , Mello, A. F. , Mello, M. F. , … Price, L. H. (2007). Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry, 62(10), 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne, D. L. , Bagot, R. C. , van Hasselt, F. , Ramakers, G. , Meaney, M. J. , de Kloet, E. R. , … Krugers, H. (2008). Maternal care and hippocampal plasticity: Evidence for experience‐dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. The Journal of Neuroscience, 28(23), 6037–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, E. , Cohen, S. , & Miller, G. E. (2009). How low socioeconomic status affects 2‐year hormonal trajectories in children. Psychological Science, 21(1), 31–37. [DOI] [PubMed] [Google Scholar]
- Chen, E. , Langer, D. A. , Raphaelson, Y. E. , & Matthews, K. A. (2004). Socioeconomic status and health in adolescents: The role of stress interpretations. Child Development, 75(4), 1039–1052. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , An, H. , Zhu, H. , Jewells, V. , Armao, D. , Shen, D. , … Lin, W. (2011). Longitudinal regression analysis of spatial–temporal growth patterns of geometrical diffusion measures in early postnatal brain development with diffusion tensor imaging. NeuroImage, 58(4), 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, D. C. , Furay, A. R. , Evanson, N. K. , Ostrander, M. M. , Ulrich‐Lai, Y. M. , & Herman, J. P. (2007). Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic‐pituitary‐adrenal axis activity: Implications for the integration of limbic inputs. The Journal of Neuroscience, 27(8), 2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. , Jeong, B. , Polcari, A. , Rohan, M. L. , & Teicher, M. H. (2011). Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. NeuroImage, 59(2), 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. , Jeong, B. , Rohan, M. L. , Polcari, A. M. , & Teicher, M. H. (2009). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry, 65(3), 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, E. C. , & Williams, C. L. (2000). Adrenergic activation of the nucleus tractus solitarius potentiates amygdala norepinephrine release and enhances retention performance in emotionally arousing and spatial memory tasks. Behavioural Brain Research, 112, 151–158. [DOI] [PubMed] [Google Scholar]
- Coenen, V. A. , Panksepp, J. , Hurwitz, T. A. , Urbach, H. , & Mädler, B. (2012). Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): Imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. The Journal of Neuropsychiatry and Clinical Neurosciences, 24(2), 223–236. [DOI] [PubMed] [Google Scholar]
- Coenen, V. A. , Schumacher, L. V. , Kaller, C. , Schlaepfer, T. E. , Reinacher, P. C. , Egger, K. , … Reisert, M. (2018). The anatomy of the human medial forebrain bundle: Ventral tegmental area connections to reward‐associated subcortical and frontal lobe regions. NeuroImage, 18, 770–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S. , Doyle, W. J. , & Baum, A. (2006). Socioeconomic status is associated with stress hormones. Psychosomatic Medicine, 68(3), 414–420. [DOI] [PubMed] [Google Scholar]
- Cohen, S. , Kamarck, T. , & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Cohen, S. , Tyrrell, D. A. J. , & Smith, A. P. (1991). Psychological stress and susceptibility to the common cold. New England Journal of Medicine, 325(9), 606–612. [DOI] [PubMed] [Google Scholar]
- Crane, J. W. , Buller, K. M. , & Day, T. A. (2003). Evidence that the bed nucleus of the stria terminalis contributes to the modulation of hypophysiotropic corticotropin‐releasing factor cell responses to systemic interleukin‐1beta. The Journal of Comparative Neurology, 467, 232–242. [DOI] [PubMed] [Google Scholar]
- Danese, A. , Moffitt, T. E. , Harrington, H. L. , Milne, B. J. , Polanczyk, G. , Pariante, C. M. , … Caspi, A. (2009). Adverse childhood experiences and adult risk factors for age‐related disease: Depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics and Adolescent Medicine, 163(12), 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, H.‐W. , Petrovich, G. D. , & Swanson, L. W. (2001). Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research Reviews, 38, 192–246. [DOI] [PubMed] [Google Scholar]
- Dong, H.‐W. , Petrovich, G. D. , Watts, A. G. , & Swanson, L. W. (2001). Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of Comparative Neurology, 436, 430–455. [DOI] [PubMed] [Google Scholar]
- Doom, J. R. , Cicchetti, D. , & Rogosch, F. A. (2014). Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. Journal of the American Academy of Child & Adolescent Psychiatry, 53(11), 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois, J. , Dehaene‐Lambertz, G. , Perrin, M. , Mangin, J.‐F. , Cointepas, Y. , Duchesnay, E. , … Hertz‐Pannier, L. (2008). Asynchrony of the early maturation of white matter bundles in healthy infants: Quantitative landmarks revealed noninvasively by diffusion tensor imaging. Human Brain Mapping, 29(1), 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, E. C. , Nishimi, K. , Gomez, S. H. , Powers, A. , & Bradley, B. (2018). Developmental timing of trauma exposure and emotion dysregulation in adulthood: Are there sensitive periods when trauma is most harmful? Journal of Affective Disorders, 227, 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzafic, I. , Oestreich, L. , Martin, A. K. , Mowry, B. , & Burianová, H. (2019). Stria terminalis, amygdala, and temporoparietal junction networks facilitate efficient emotion processing under expectations. Human Brain Mapping, 40(18), 5382–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal, T. J. , Chugani, H. T. , Behen, M. E. , Juhász, C. , Muzik, O. , Maqbool, M. , … Makki, M. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics, 117(6), 2093–2100. [DOI] [PubMed] [Google Scholar]
- Fahrmeir, L. , Kneib, T. , Lang, S. , & Marx, B. (2013). Regression Models. In Fahrmeir L., Kneib T., Lang S., & Marx B. (Eds.), Regression: Models, methods and applications (pp. 21–72). Berlin/Heidelberg, Germany: Springer Berlin Heidelberg. [Google Scholar]
- Favrais, G. , van de Looij, Y. , Fleiss, B. , Ramanantsoa, N. , Bonnin, P. , Stoltenburg‐Didinger, G. , … Gressens, P. (2011). Systemic inflammation disrupts the developmental program of white matter. Annals of Neurology, 70(4), 550–565. [DOI] [PubMed] [Google Scholar]
- Felitti, V. J. , Anda, R. F. , Nordenberg, D. , Williamson, D. F. , Spitz, A. M. , Edwards, V. , … Marks, J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Fenoy, A. J. , Schulz, P. , Selvaraj, S. , Burrows, C. , Spiker, D. , Cao, B. , … Soares, J. (2016). Deep brain stimulation of the medial forebrain bundle: Distinctive responses in resistant depression. Journal of Affective Disorders, 203, 143–151. [DOI] [PubMed] [Google Scholar]
- Fields, R. D. (2008). White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences, 31(7), 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, R. D. (2010). Change in the brain's white matter. Science, 330(6005), 768–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folloni, D. , Sallet, J. , Khrapitchev, A. A. , Sibson, N. , Verhagen, L. , & Mars, R. B. (2019). Dichotomous organization of amygdala/temporal‐prefrontal bundles in both humans and monkeys. eLife, 8, e47175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz, M. S. , & MacKinnon, D. P. (2007). Required sample size to detect the mediated effect. Psychological Science, 18(3), 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl, T. , Carballedo, A. , Fagan, A. J. , Lisiecka, D. , Ferguson, Y. , & Meaney, J. F. (2012). Effects of early‐life adversity on white matter diffusivity changes in patients at risk for major depression. Journal of Psychiatry & Neuroscience, 37(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros, P. J. , Marsland, A. L. , Sheu, L. K. , Erickson, K. I. , & Verstynen, T. D. (2012). Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cerebral Cortex, 23(9), 2058–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan, R. M. , Behen, M. E. , Helder, E. , Makki, M. I. , & Chugani, H. T. (2009). Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract‐based spatial statistics (TBSS). Cerebral Cortex, 20(3), 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, T. S. , & Piechowski, R. A. (1993). Ibotenic acid lesions of the bed nucleus of the stria terminalis attenuate conditioned stress‐induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology, 57, 517–524. [DOI] [PubMed] [Google Scholar]
- Gunnar, M. R. , Frenn, K. , Wewerka, S. S. , & Van Ryzin, M. J. (2009). Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12‐year‐old children. Psychoneuroendocrinology, 34(1), 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman, D. A. , Betancourt, L. M. , Brodsky, N. L. , Hurt, H. , & Farah, M. J. (2012). Neighborhood disadvantage and adolescent stress reactivity. Frontiers in Human Neuroscience, 6, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann, P. , Jonasson, L. , Maeder, P. , Thiran, J. P. , Wedeen, V. J. , & Meuli, R. (2006). Understanding diffusion MR imaging techniques: From scalar diffusion‐weighted imaging to diffusion tensor imaging and beyond. Radiographics, 26(Suppl 1), S205–S223. [DOI] [PubMed] [Google Scholar]
- Hana, A. , Hana, A. , Dooms, G. , Boecher‐Schwarz, H. , & Hertel, F. (2015). Visualization of the medial forebrain bundle using diffusion tensor imaging. Frontiers in Neuroanatomy, 9, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Adluru, N. , Chung, M. K. , Alexander, A. L. , Davidson, R. J. , & Pollak, S. D. (2013). Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development, 84(5), 1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt, J. , & Rutter, M. (2004). Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology and Psychiatry, 45(2), 260–273. [DOI] [PubMed] [Google Scholar]
- Harnett, N. G. , Ference, E. W. , Knight, A. J. , & Knight, D. C. (2020). White matter microstructure varies with post‐traumatic stress severity following medical trauma. Brain Imaging and Behavior, 14(4), 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim, C. , Newport, D. J. , Bonsall, R. , Miller, A. H. , & Nemeroff, C. B. (2001). Altered pituitary‐adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry, 158, 575–581. [DOI] [PubMed] [Google Scholar]
- Heim, C. , Newport, D. J. , Heit, S. , Graham, Y. P. , Wilcox, M. , Bonsall, R. , … Nemeroff, C. B. (2000). Pituitary‐adrenal and autonomic responses to stress in woman after sexual and physical abuse in childhood. JAMA, 284(5), 592–597. [DOI] [PubMed] [Google Scholar]
- Herman, J. P. , Cullinan, W. E. , Ziegler, D. R. , & Tasker, J. G. (2002). Role of the paraventricular nucleus microenvironment in stress integration. European Journal of Neuroscience, 16(3), 381–385. [DOI] [PubMed] [Google Scholar]
- Hubel, D. H. , Wiesel, T. N. , & LeVay, S. (1977). Plasticity of ocular dominance columns in monkey striate cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 278(961), 377–409. [DOI] [PubMed] [Google Scholar]
- Insana, S. P. , Kolko, D. J. , & Germain, A. (2012). Early‐life trauma is associated with rapid eye movement sleep fragmentation among military veterans. Biological Psychology, 89, 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia, M. , & Sullivan, J. M. (1982). Brainstem projections of sensory and motor components of the vagus nerve in the rat. The Journal of Comparative Neurology, 211, 248–264. [DOI] [PubMed] [Google Scholar]
- Kamali, A. , Yousem, D. M. , Lin, D. D. , Sair, H. I. , Jasti, S. P. , Keser, Z. , … Hasan, K. M. (2015). Mapping the trajectory of the stria terminalis of the human limbic system using high spatial resolution diffusion tensor tractography. Neuroscience Letters, 608, 45–50. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , McLaughlin, K. A. , Green, J. G. , Gruber, M. J. , Sampson, N. A. , Zaslavsky, A. M. , … Angermeyer, M. (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry, 197(5), 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M.‐K. , Kim, B. , Kiu Choi, T. , & Lee, S.‐H. (2017). White matter correlates of anxiety sensitivity in panic disorder. Journal of Affective Disorders, 207, 148–156. [DOI] [PubMed] [Google Scholar]
- Koller, K. , Hatton, C. M. , Rogers, R. D. , & Rafal, R. D. (2019). Stria terminalis microstructure in humans predicts variability in orienting towards threat. European Journal of Neuroscience, 50(11), 3804–3813. [DOI] [PubMed] [Google Scholar]
- Koopman, C. , Carrion, V. , Butler, L. D. , Sudhakar, S. , Palmer, L. , & Steiner, H. (2004). Relationships of dissociation and childhood abuse and neglect with heart rate in delinquent adolescents. Journal of Traumatic Stress, 17(1), 47–54. [DOI] [PubMed] [Google Scholar]
- Krüger, O. , Shiozawa, T. , Kreifelts, B. , Scheffler, K. , & Ethofer, T. (2015). Three distinct fiber pathways of the bed nucleus of the stria terminalis to the amygdala and prefrontal cortex. Cortex, 66, 60–68. [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Behen, M. E. , Singsoonsud, P. , Veenstra, A. L. , Wolfe‐Christensen, C. , Helder, E. , & Chugani, H. T. (2013). Microstructural abnormalities in language and limbic pathways in orphanage‐reared children: A diffusion tensor imaging study. Journal of Child Neurology, 29(3), 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, H. G. , Byun, W. M. , Ahn, S. H. , Son, S. M. , & Jang, S. H. (2011). The anatomical characteristics of the stria terminalis in the human brain: A diffusion tensor tractography study. Neuroscience Letters, 500(2), 99–102. [DOI] [PubMed] [Google Scholar]
- Lawson, G. M. , Camins, J. S. , Wisse, L. , Wu, J. , Duda, J. T. , Cook, P. A. , … Farah, M. J. (2017). Childhood socioeconomic status and childhood maltreatment: Distinct associations with brain structure. PLoS One, 12(4), e0175690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , Gee, M. , Camicioli, R. , Wieler, M. , Martin, W. , & Beaulieu, C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60(1), 340–352. [DOI] [PubMed] [Google Scholar]
- Lebow, M. A. , & Chen, A. (2016). Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê‐Scherban, F. , Brenner, A. B. , Hicken, M. T. , Needham, B. L. , Seeman, T. , Sloan, R. P. , … Diez Roux, A. V. (2018). Child and adult socioeconomic status and the cortisol response to acute stress: Evidence from the multi‐ethnic study of atherosclerosis. Psychosomatic Medicine, 80(2), 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo, W. R. , Farag, N. H. , Sorocco, K. H. , Cohoon, A. J. , & Vincent, A. S. (2011). Lifetime adversity leads to blunted stress Axis reactivity: Studies from the Oklahoma Family Health Patterns Project. Biological Psychiatry, 71(4), 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S. , Wei, Z. , Gao, W. , Wu, W. , Liao, M. , Zhang, Y. , … Li, L. (2013). White matter integrity alterations in young healthy adults reporting childhood trauma: A diffusion tensor imaging study. Australian and New Zealand Journal of Psychiatry, 47(12), 1183–1190. [DOI] [PubMed] [Google Scholar]
- Luiten, P. G. M. , Ter Horst, G. J. , Karst, H. , & Steffens, A. B. (1985). The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Research, 329(1–2), 374–378. [DOI] [PubMed] [Google Scholar]
- Lupien, S. J. , King, S. , Meaney, M. J. , & McEwen, B. S. (2001). Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology, 13(3), 653–676. [DOI] [PubMed] [Google Scholar]
- McCrae, R. R. , & Costa, P. T., Jr. (2007). Brief versions of the NEO‐PI‐3. Journal of Individual Differences, 28(3), 116–128. [Google Scholar]
- McLaughlin, K. A. , Sheridan, M. A. , & Lambert, H. K. (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick, M. T. , Ford, D. C. , Ports, K. A. , & Guinn, A. S. (2018). Prevalence of adverse childhood experiences from the 2011‐2014 behavioral risk factor surveillance system in 23 states. JAMA Pediatrics, 172(11), 1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, G. , Berk, M. , Maes, M. , Carvalho, A. F. , & Puri, B. K. (2019). Socioeconomic deprivation, adverse childhood experiences and medical disorders in adulthood: Mechanisms and associations. Molecular Neurobiology, 56(8), 5866–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni, V. , Uher, R. , & Danese, A. (2012). Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta‐analysis. American Journal of Psychiatry, 169(2), 141–151. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys, R. , Geeraedts, L. M. G. , & Veening, J. G. (1982). The medial forebrain bundle of the rat. I. General introduction. The Journal of Comparative Neurology, 206(1), 49–81. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys, R. , Voogd, J. , & van Huijzen, C. (2008). The human central nervous system: A synopsis and atlas. Fourth Edition, Berlin/Heidelberg, Germany: Springer. [Google Scholar]
- Oler, J. A. , Tromp, D. P. M. , Fox, A. S. , Kovner, R. , Davidson, R. J. , Alexander, A. L. , … de Campo, D. M. (2017). Connectivity between the central nucleus of the amygdala and the bed nucleus of the stria terminalis in the non‐human primate: Neuronal tract tracing and developmental neuroimaging studies. Brain Structure and Function, 222(1), 21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak, K. , Palkovits, M. , Kopin, I. J. , & Goldstein, D. S. (1995). Stress‐induced norepinephrine release in hypothalamic paraventricular nucleus and pituitary‐adrenocortical and sympathoadrenal activity: In vivo microdialysis studies. Frontiers in Neuroendocrinology, 16, 89–150. [DOI] [PubMed] [Google Scholar]
- Pechtel, P. , Lyons‐Ruth, K. , Anderson, C. M. , & Teicher, M. H. (2014). Sensitive periods of amygdala development: The role of maltreatment in preadolescence. NeuroImage, 97, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckins, M. K. , Susman, E. J. , Negriff, S. , Noll, J. , & Trickett, P. K. (2015). Cortisol profiles: A test for adaptive calibration of the stress response system in maltreated and nonmaltreated youth. Development and Psychopathology, 27(4pt2), 1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli, C. , Barnett, A. , Pajevic, S. , Chen, R. , Penix, L. , Virta, A. , & Basser, P. (2001). Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage, 13(6), 1174–1185. [DOI] [PubMed] [Google Scholar]
- Rafal, R. D. , Koller, K. , Bultitude, J. H. , Mullins, P. , Ward, R. , Mitchell, A. S. , & Bell, A. H. (2015). Connectivity between the superior colliculus and the amygdala in humans and macaque monkeys: Virtual dissection with probabilistic DTI tractography. Journal of Neurophysiology, 114(3), 1947–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss, F. (2013). Socioeconomic inequalities and mental health problems in children and adolescents: A systematic review. Social Science & Medicine, 90, 24–31. [DOI] [PubMed] [Google Scholar]
- Rinaman, L. , Banihashemi, L. , & Koehnle, T. J. (2011). Early life experience shapes the functional organization of stress‐responsive visceral circuits. Physiology & Behavior, 104, 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman, L. , Levitt, P. , & Card, J. P. (2000). Progressive postnatal assembly of limbic‐autonomic circuits revealed by central transneuronal transport of pseudorabies virus. The Journal of Neuroscience, 20(7), 2731–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara, G. , & Lappin, J. (2017). Childhood trauma: psychiatry's greatest public health challenge? The Lancet Public Health, 2(7), e300–e301. [DOI] [PubMed] [Google Scholar]
- Schweimer, J. , Fendt, M. , & Schnitzler, H.‐U. (2005). Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. European Journal of Pharmacology, 507, 117–124. [DOI] [PubMed] [Google Scholar]
- Scott, K. M. , McLaughlin, K. A. , Smith, D. A. R. , & Ellis, P. M. (2012). Childhood maltreatment and DSM‐IV adult mental disorders: Comparison of prospective and retrospective findings. The British Journal of Psychiatry, 200(6), 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan, M. A. , & McLaughlin, K. A. (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18(11), 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, A. B. , Reinherz, H. Z. , & Giaconia, R. M. (1996). The long‐term sequelae of child and adolescent abuse: A longitudinal community study. Child Abuse & Neglect, 20(8), 709–723. [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the state‐trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Springer, K. W. , Sheridan, J. , Kuo, D. , & Carnes, M. (2007). Long‐term physical and mental health consequences of childhood physical abuse: Results from a large population‐based sample of men and women. Child Abuse & Neglect, 31(5), 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm, B. H. (1996). Measurement of stress, trauma, and adaptation. Baltimore, MD: The Sidran Press. [Google Scholar]
- Sumner, J. A. , Colich, N. L. , Uddin, M. , Armstrong, D. , & McLaughlin, K. A. (2019). Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry, 85(3), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , Anderson, C. M. , Ohashi, K. , Khan, A. , McGreenery, C. E. , Bolger, E. A. , … Vitaliano, G. D. (2018). Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. NeuroImage, 169, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , & Samson, J. A. (2016). Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, 57(3), 241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , Samson, J. A. , Anderson, C. M. , & Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. [DOI] [PubMed] [Google Scholar]
- Ter Horst, G. J. , De Boer, P. , Luiten, P. G. M. , & Van Willigen, J. D. (1989). Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience, 31(3), 785–797. [DOI] [PubMed] [Google Scholar]
- Tottenham, N. , & Galván, A. (2016). Stress and the adolescent brain: Amygdala‐prefrontal cortex circuitry and ventral striatum as developmental targets. Neuroscience & Biobehavioral Reviews, 70, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham, N. , & Sheridan, M. (2010). A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience, 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp, D. P. M. , Williams, L. E. , Fox, A. S. , Oler, J. A. , Roseboom, P. H. , Rogers, G. M. , … Kalin, N. H. (2019). Altered Uncinate fasciculus microstructure in childhood anxiety disorders in boys but not girls. American Journal of Psychiatry, 176(3), 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch, D. S. (2004). Q‐ball imaging. Magnetic Resonance in Medicine, 52(6), 1358–1372. [DOI] [PubMed] [Google Scholar]
- Ugwu, I. D. , Amico, F. , Carballedo, A. , Fagan, A. J. , & Frodl, T. (2014). Childhood adversity, depression, age and gender effects on white matter microstructure: A DTI study. Brain Structure and Function, 220(4), 1997–2009. [DOI] [PubMed] [Google Scholar]
- Ursache, A. , Merz, E. C. , Melvin, S. , Meyer, J. , & Noble, K. G. (2017). Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology, 78, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, K. A. , Windham, B. G. , Power, M. C. , Hoogeveen, R. C. , Folsom, A. R. , Ballantyne, C. M. , … Gottesman, R. F. (2018). The association of mid‐to late‐life systemic inflammation with white matter structure in older adults: The atherosclerosis risk in communities study. Neurobiology of Aging, 68, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, S. , Janus, M. , Duku, E. , Raos, R. , Brownell, M. , Forer, B. , … Muhajarine, N. (2017). Neighbourhood socioeconomic status indices and early childhood development. SSM ‐ Population Health, 3, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhaus, M. J. , & Loewy, A. D. (2001). Central representation of the sympathetic nervous system in the cerebral cortex. Brain Research, 903(1–2), 117–127. [DOI] [PubMed] [Google Scholar]
- Westlye, L. T. , Walhovd, K. B. , Dale, A. M. , Bjørnerud, A. , Due‐Tønnessen, P. , Engvig, A. , … Fjell, A. M. (2010). Life‐span changes of the human brain white matter: Diffusion tensor imaging (DTI) and Volumetry. Cerebral Cortex, 20(9), 2055–2068. [DOI] [PubMed] [Google Scholar]
- Wilkins, K. C. , Lang, A. J. , & Norman, S. B. (2011). Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depression and Anxiety, 28(7), 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkleby, M. A. , Jatulis, D. E. , Frank, E. , & Fortmann, S. P. (1992). Socioeconomic status and health: How education, income, and occupation contribute to risk factors for cardiovascular disease. American Journal of Public Health, 82(6), 816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, P. , Gao, Z. , Zhang, H. , Fang, Z. , Wu, C. , Xu, H. , & Huang, Q.‐J. (2015). Changes in proinflammatory cytokines and white matter in chronically stressed rats. Neuropsychiatric Disease and Treatment, 11, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F.‐C. , & Tseng, W.‐Y. I. (2011). NTU‐90: A high angular resolution brain atlas constructed by q‐space diffeomorphic reconstruction. NeuroImage, 58(1), 91–99. [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Lowen, S. B. , Anderson, C. M. , Ohashi, K. , Khan, A. , & Teicher, M. H. (2019). Association of prepubertal and postpubertal exposure to childhood maltreatment with adult amygdala function. JAMA Psychiatry, 76(8), 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DATA S1 Supporting information
Data Availability Statement
Research data are not shared at this time. The data that support the findings of this study may be made available from the corresponding author upon reasonable request in the future.


