Abstract
With an exception of few reports, the plasma concentration of ouabain and marinobufagenin, mostly studied cardiotonic steroids (CTS) assessed by immunoassay techniques, is less than 1 nM. During the last 3 decades, the implication of these endogenous CTS in the pathogenesis of hypertension and other volume-expanded disorders is widely disputed. The threshold for inhibition by CTS of human and rodent α1-Na,K-ATPase is ∼1 and 1000 nM, respectively, that rules out the functioning of endogenous CTS (ECTS) as natriuretic hormones and regulators of cell adhesion, cell-to-cell communication, gene transcription and translation, which are mediated by dissipation of the transmembrane gradients of monovalent cations. In several types of cells ouabain and marinobufagenin at concentrations corresponding to its plasma level activate Na,K-ATPase, decrease the [Na+]i/[K+]i-ratio and increase cell proliferation. Possible physiological significance and mechanism of non-canonical Na+i/K+i-dependent and Na+i/K+i-independent cell responses to CTS are discussed.
Keywords: Cell proliferation; Cellular signaling; Endogenous cardiotonic steroids; Na+,K+-ATPase; Transcription; Translation
Introduction
A half century ago, De Wardener and co-workers reported that in dogs natriuresis triggered by intravenous saline administration occurs even in the absence of significant changes in renal perfusion pressure and glomerular filtration rate. This observation suggesting an implication in renal salt handling new unknown system termed as a Third Factor.1 Twenty years later, it was shown that natriuretic effect of the Third Factor might be at least partially explained by augmented production of atrial and brain natriuretic peptides (ANP and BNP) which inhibit in renal epithelial cells the basolateral Na,K-ATPase via their interaction with G protein-coupled receptors and activation of cGMP-mediated signaling.2 At the same time, several research teams demonstrated that low molecular compounds distinct of ANP and BNP can also contribute to this phenomenon. Thus, Buckalew and co-workers found that serosal application of plasma ultrafiltrate from salt-loaded dogs to the frog skin lacking ANP and BNP receptors decreases the transepithelial potential difference and short current thus suggesting an inhibition of the basolateral Na+ transport3 (for historical details, see4,5).
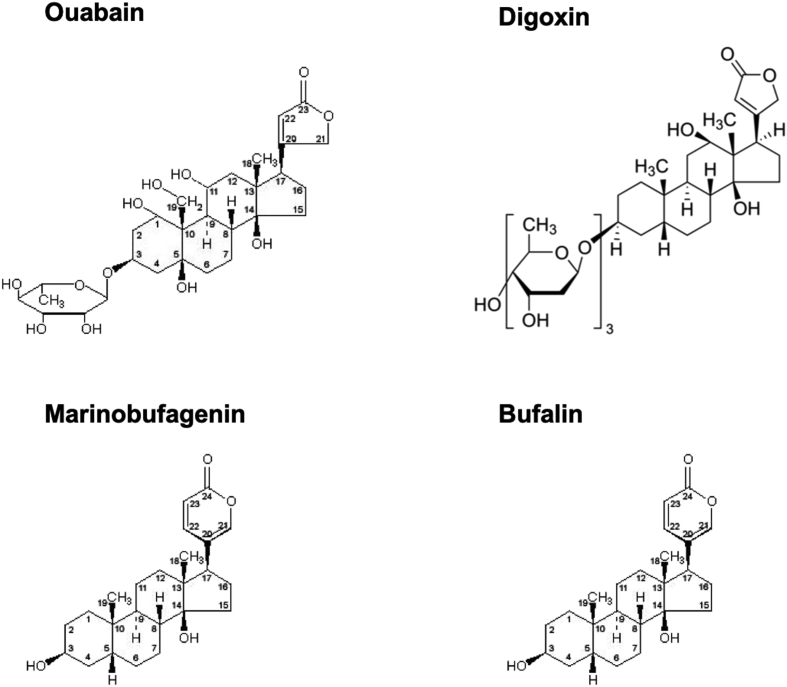
The beneficial effect of digitalis in the therapy of heart failure, described more than 200 years ago, led to the identification of numerous plant-derived cardenolides, including ouabain, i.e. the most hydrophilic steroid used in an overwhelming number of in vitro studies. Other members of the cardiotonic steroid (CTS) superfamily, bufadienolides, were isolated from amphibians.6 A long-lasting search for endogenous CTS (ECTS) resulted in the purification from mammalian species compounds identical to ouabain,7, 8, 9 digoxin,10 bufalin,11 marinobufagenin (MBG),12,13 telocinobufagin14 and marinobufotoxin15 (structure of some endogenous cardiotonic steroids are presented in Fig. 1) Data on association of cardiovascular, renal and neuronal diseases with increased ECTS level and preventive actions of passive immunization by anti-ECTS antibodies allowed researchers to propose an implication of ECTS in the pathogenesis of these and other volume-expanded disorders (for comprehensive review, see16, 17, 18, 19, 20, 21).
Figure 1.
Structure of 4 cardiotonic steroids: cardenolides (ouabain and digoxin) having 5-membering lactone ring in 17-th position of steroid core; bufadienolides (marinobafagenin and bufalin) with 6-membering lactone ring in this position.
Starting from early 90th the focus of investigations has been directed to the mechanisms by which ECTS may regulate various cellular functions. Since the seminal publication of Dr. J. Skou on the complete inhibition, by digitalis, of Mg2+-dependent (Na++K+)-stimulated adenosine triphosphatase (NKA, EC 7.2.2.13),22 this enzyme remains the only known target of CTS. As predicted, NKA inhibition by CTS sharply affects cellular functions that are directly linked to transmembrane gradients of monovalent cations, such as maintenance of electrical membrane potential (Em), cell volume, intracellular concentration of Ca2+, H+, Cl−, inorganic phosphate and low molecular weight organic compounds by Na+- and K+-coupled transporters. More recently, it was shown that side-by-side with dissipation of transmembrane gradients of monovalent cations ECTS trigger transcriptomic and proteomic changes and affect cell adhesion, proliferation and death. Several research teams proposed that these non-canonical cellular responses are mediated by Na+i,K+i-independent signaling and contribute to physiological and pathophysiological actions of ECTS.16,17,20,23, 24, 25 In this review, we examine the potential role of ECTS in the triggering of canonical and non-canonical cellular responses by comparative analysis of their plasma content and dose-dependent actions of ECTS on NKA activity, intracellular content of monovalent cations, intracellular signaling pathways and cellular responses affecting cell proliferation and gene expression.
The content of circulating ECTS
Data on the content of ECTS in the extracellular fluids were mainly obtained by ELISA, RIA, DELFIA and other immunoassay approaches. Table 1 displays that with few exceptions the plasma concentration of immunoreactive ouabain and MBG in mammalian species is less than 1 nM. The huge variability of the plasma content of ouabain (from 0.05 to 1 nM) and MBG (from 0.2 to 0.6 nM) in healthy patients reported by different laboratories (Table 1) can be explained by numerous features of self-made reagents employed in these investigations. This comment becomes important because 3 research teams failed to detect any immunoreactive ouabain in the human plasma after its high-performance liquid chromatography separation.26, 27, 28 More recently, the negative results were also obtained by newly developed ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). This sophisticated technique having a lower limit of quantification of 0.002 nM failed to detect ouabain in the plasma of healthy subjects as well as patients with heart failure.29 Keeping these data in mind it might be proposed that immunoreactive ouabain-like substance(s) rather than authentic ouabain per se has been detected in part of the studies listed in Table 1.
Table 1.
Plasma levels of CTS in hypertension and states associated with sodium loading and/or plasma volume expansion and estimated by immunoassay technique.
| CTS and groups under investigation | Values, nM | References |
|---|---|---|
| Ouabain | ||
| Humans EH/PA/control | 3.39 ± 0.57/4.09 ± 1.12/0.53 ± 0.10 | 110 |
| Humans CHF/control | 0.030–8.3/0.16–0.77 | 111 |
| Humans CHF/control before HPLC | 0.25–1.6/0.13–0.56 | 28 |
| Humans CHF/control after HPLC | ND/ND | 28 |
| MHS/MNS | 0.076 ± 0.029/0.027 ± 0.014 | 112 |
| NaCl-loaded rats/control | 1.43 ± 0.06/1.14 ± 0.05 | 113 |
| ACTH-treated subjects/control | 0.87 ± 0.25/0.64 ± 0.17 | 114 |
| ACTH-treated rats/control | 0.09 ± 0.01/0.10 ± 0.04 | 115 |
| Humans: CRF/EH/PA/control | 0.14 ± 0.02/0.13 ± 0.05/0.10 ± 0.03/0.09 ± 0.02 | 116 |
| Humans: preeclampsia/control | 0.70 ± 0.16/0.32 ± 0.07 | 117 |
| 3rd trimester of pregnancy/control | 0.024 ± 0.004/0.009 ± 0.001 | 118 |
| Mild EH/control | 0.039 ± 0.024/0.029 ± 0.018 | 119 |
| DS, high/low-NaCl diet | 0.12 ± 0.02/0.10 ± 0.02 | 120,121 |
| Rats, high/normal NaCl intake | 0.28 ± 0.04/0.34 ± 0.06 | 122 |
| Humans: volume expansion/control | 0.21 ± 0.04/0.09 ± 0.02 | 121 |
| Dogs: controls | 0.138 ± 0.043 | 7 |
| Humans: controls | 0.037 ± 0.007 | 7 |
| Humans: nephrectomy/control | 0.20 ± 0.06/0.12 ± 0.06 | 123 |
| Humans: mild hypertension/control | 1.34 ± 0.91/0.38 ± 0.31 | 124 |
| Humans: control | 0.152 ± 0.067 | 125 |
| Humans: low-renin EH/control | 0.94 ± 0.22/0.37 ± 0.04 | 126 |
| Marinobufagenin | ||
| ACTH-treated rats/control | 0.44 ± 0.06/0.21 ± 0.05 | 115 |
| Patients with CRF/EH/PA/control | 16.6 ± 5.3/1.7 ± 0.7/13.5 ± 12.9/0.26 ± 0.05 | 116 |
| Patients with preeclampsia/control | 2.63 ± 0.10/0.63 ± 0.07 | 117 |
| DS, high/low-NaCl diet | 1.24 ± 0.0.27/0.38 ± 0.04 | 120,121 |
| Patients with CHF stage IV/stage I | 1.90 ± 0.04/0.60 ± 0.14 | 127 |
| Rats, high/normal-NaCl intake | 1.14 ± 0.12/0.55 ± 0.06 | 122 |
| Patients with AMI/control | 1.9 ± 0.38/0.38 ± 0.01 | 13 |
| Rats with volume expansion/control | 0.49 ± 0.05/0.20 ± 0.06 | 121 |
| Patients with nephrectomy/control | 0.57 ± 0.04/0.36 ± 0.02 | 128 |
| Patients: 24-hr low/high salt diet | 0.16–0.30/0.18–0.37 | 108 |
Abbreviations: AMI – acute myocardial ischemia; CHF – congestive heart failure; CRF – chronic renal failure; DS – Dahl salt-sensitive rats; EH – essential hypertension; HPLC – high-performance liquid chromatography; MHS and MNS – Milan hypertensive and normotensive strains, respectively; ND – non detectable; PA - primary aldosteronism.
Do ECTS inhibit Na,K-ATPase?
Na,K-ATPase is a complex of proteins integrated into plasma membrane, it is found in all types of animal cells. The enzyme is composed of α- and β-subunits. Larger α-subunit (∼110 kDa) hydrolyses ATP, this results in the phosphorylation of Asp369 residue located in enzyme active site. After that the enzyme undergoes E1-E2 conformational change that, in turn, leads to enzyme dephosphorylation. As consequence of these events Na,K-ATPase provides electrogenic ion transport (3Na+ vs 2K+) with a rate of 60–80 phosphorylation–dephosphorylation cycles per sec. Three other NKA α-subunit isoforms were found by screening cDNA libraries in addition to the ubiquitous α1-isoform. These isoforms are highly expressed in astrocytes, neuronal cells (α3 and α2), heart, skeletal muscle (α2), and testis (α4). In majority of tissues (possibly, with an exception of kidney epithelial cells) at least two different isoforms are expressed: the ubiquitous α1-NKA is supplied by another (usually regulated) isoform (for review, see30,31). The mechanism of CTS inhibiting effect on NKA was mostly investigated with ouabain originated from Strophanthus gratus and having much higher water solubility in comparison with other CTS. Lingrel with coauthors demonstrated that at least 10 amino acid residues in H1, H5 and H7 α-subunit transmembrane segments as well as in H1–H2, H5–H6 and H7–H8 extracellular loops exert an influence on ouabain affinity of NKA.31 Their important role in CTS binding was also approved by comparative analysis of NKA derived from different species. Ouabain affinity to rat and mouse NKA α1-subunit (CTS-resistant α1R-NKA) is 3 orders of magnitude lower in comparison with other mammalian species (CTS-sensitive α1S-NKA). It was shown that the replacement of neutral Gln111 and Asn122 in α1-subunit by charged Arg and Asp amino acids resulted in ∼1000-fold decrease of ouabain affinity to it. The same amino acid replacement significantly decreased α2- and α3-NKA affinity for ouabain. Unlike ubiquitous α1-isozyme, the affinity for CTS of α2- α4-NKA in rodents and other mammalian species are about the same.32
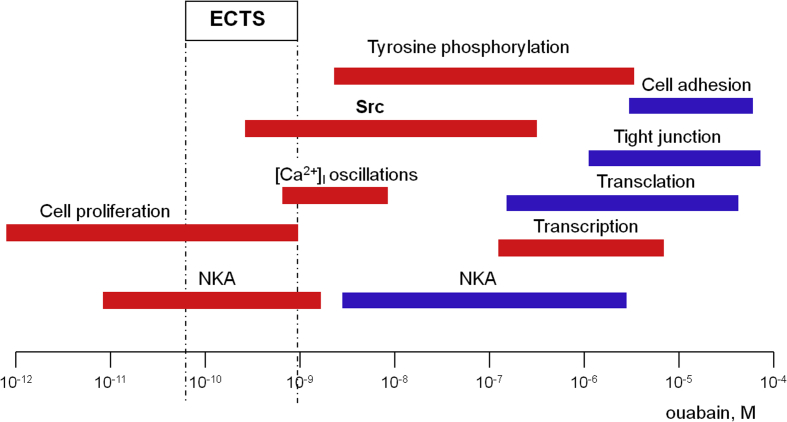
In the early 1980s, M.P. Blaustein proposed that augmented ECTS production partially normalizes renal function by inhibiting the Na-pump located in basolateral membranes of renal epithelial cells but elevates total peripheral resistance via suppression of this enzyme in vascular smooth muscle cells.33 It should be noted, however, that the treatment of congestive heart failure with commercially available cardenolides occurs in the absence of significant natriuresis.34 The hypothesis on the implication of ECTS via NKA inhibition also contradicts to the comparative analysis of ECTS concentration detected in the extracellular fluids and their dose-dependent action on NKA activity (Fig. 2). Indeed, at concentrations less than 100 nM ouabain had no significant impact on NKA activity in rat renal epithelial cells and vascular smooth muscle cells isolated form rat aorta. In both cases, half-maximal modulation of intracellular cation content was observed at 500–1000 μM of ouabain35 that was at least 3 orders of magnitude higher than the plasma content of immunoreactive ouabain and MBG. Data on the inhibitory action of ouabain and MBG on the activity of human α1S-NKA as well as CTS-sensitive α2-, α3-isozymes at concentrations less than 1 nM are limited to few publications (Table 2). To the best of our knowledge there is no report showing dissipation of transmembrane gradients of monovalent cations by ECTS at the range corresponding to their concentrations in plasma.
Figure 2.
Plasma concentration of ECTS detected by immunoassay technique and dose-dependent cellular actions of ouabain in non-rodent mammalian cells. Activatory and inhibitory actions of ouabain are shown by red and blue, respectively. For original data, see text and Table 1, Table 2, Table 3
Table 2.
Thresh-old and half-maximal concentration (IC50) of ouabain (OU) and marinobufagenin (MBG) triggered Na,K-ATPase and K+/86Rb uptake inhibition.
| Cell type | Tested CTS | Thresh-old, nM | Half-maximal effect, nM | References |
|---|---|---|---|---|
| Na,K-ATPase | ||||
| c-kidney | OU | 1 | 20 | 129 |
| r-kidney | OU | 5 × 103 | 105 | 130 |
| m-kidney | OU | 105 | 106 | 131 |
| r-sarcolemma from aorta | OU | 10 | 50 | 36 |
| r-kidney medulla | OU | 104 | >105 | 132 |
| r-intravascular nerve ending | OU | 1 | 2.6 | 36 |
| h-rings from mesenteric artery | OU | 1 | 14 | 12 |
| r-sarcolemma from left ventricle | OU | 10 | 2.5 × 103 | 133 |
| h-erythrocytes | MBG | 1 | 70 | 134 |
| r-sarcolemma from aorta | MBG | 1 | 2.1 | 36 |
| r-intravascular nerve ending | MBG | 100 | 140 | 36 |
| r-sarcolemma from left ventricle | MBG | 1 | 5.5 × 103 | 133 |
| r-kidney medulla | MBG | 0.5 | 10 | 132 |
| r-kidney medulla | MBG | 0.1 | 50 | 120 |
| h-sarcolemma from mesenteric artery | MBG | 1 | 50–60 | 117,135 |
| h-rings from mesenteric artery | MBG | 1 | 50 | 136 |
| h-sarcolemma from pulmonary artery | MBG | 0.1 | 50 | 137 |
| Sf-9 cells transfected withr-α1β1 | OU | 43,000 | 138 | |
| Sf-9 cells transfected withr-α2β1 | OU | 170 | 138 | |
| Sf-9 cells transfected withr-α1β1 | OU | 31 | 138 | |
| K+/86Rb uptake | ||||
| h-erythrocytes | OU | 1–2 | 50 | 139,140 |
| r-erythrocytes | OU | >103 | 2 × 105 | 141 |
| h-leukocytes | OU | 0.1 | 0.56 | 142 |
| c-renal epithelial cells | OU | >3 | 50 | 37 |
| r-fetal brain | OU | <10 | 20 | 143 |
| r-renal epithelial cells | OU | 103 | 5 × 104 | 79 |
| r-renal epithelial cells | OU | 104 | 3 × 105 | 129 |
| gp-carotid artery | OU | <1 | 10 | 144 |
| c-renal epithelial cells | MBG | 30 | 80 | 37 |
Abbreviations: c – canine; gp – guinea pig; h – human; m - mouse; r – rat.
Data obtained in rodents appear in italics.
Comparative analysis of the dose-dependent actions of ouabain and MBG on NKA activity in membrane fractions enriched by vascular smooth muscle sarcolemma and perivascular nerve endings (neuronal plasmalemma) containing predominantly α1- and α3-isozymes respectively, demonstrated that affinity for ouabain and MBG of α1- and α3-isozymes are sharply different (IC50 for inhibition of α1-NKA by ouabain and MBG are 50 and 2 nM, respectively, vs 3 and 140 nM in the case of α3-NKA).36 These data contradict to 5-fold elevation of the affinity for ouabain compared to MBG in Madin–Darby canine kidney cells abundant with α1-NKA37 and about the same affinity demonstrated in α1-NKA purified from duck salt glands.38 Possible mechanisms underlying this discrepancy should be examined further.
It is known that in some cell types (neurons, glia, vascular smooth muscle myocytes) α2- and α3-isozymes of NKA are located in microdomains of plasma membrane that are in close proximity to the underlying endoplasmic reticulum. Keeping in mind this finding and the extremely low affinity of rodent α1R-NKA for CTS, Blaustein and co-workers proposed that ECTS evoked an increase of vascular tone via elevation of [Na+]i in the space-limited plasma membrane-junctional endoplasmic reticulum compartment (plasmerosomes) abundant with ubiquitous isoform of the Na+/Ca2+ exchanger (NCX1) and with α2-, α3-NKA.39 A key role of NCX1 in the development of salt-sensitive hypertension was demonstrated by using a selective inhibitor of this carrier, compound SEA0400. Moreover, it was shown that DOCA-salt-induced increment of blood pressure was absent in NCX1-deficient Slc8a1+/− mice but was increased in transgenic mice expressing canine Ncx1.3 driven by the smooth muscle-specific promoter.40 To the best of our knowledge, the elevation of Na+ concentration in cytoplasm or plasmerosome compartments by ECTS at concentrations detected in the extracellular fluids has not been demonstrated.
Do ECTS trigger non-canonical Na+i/K+i-mediated cellular responses?
In this section, we briefly summarized the data on non-canonical cellular responses triggered by CTS and mediated by elevation of the [Na+]i/[K+]i-ratio.
Gene expression
We observed that long-term inhibition of NKA in rat vascular smooth muscle cells (RVSMC) by ouabain results in sharp elevation of RNA synthesis41 and appearance of hundreds of newly synthesized proteins.42 Later on we employed Affymetrix technology for identification of cell-type specific and ubiquitous set of Na+i,K+i-sensitive transcripts by comparative analysis of the action of ouabain and K+-free medium on transcriptomic changes in HUVEC, RVSMC and HeLa cell line.43 In this study, we detected changes in expression levels of hundreds of genes that were highly correlated between two treatments thus demonstrating a key role of Na+i/K+i-mediated mechanism of excitation-transcription coupling. Importantly, about 80 Na+i/K+i-sensitive transcripts were found in all types of cells. This set of ubiquitous Na+i/K+i-sensitive transcripts was highly abundant with early response genes (ERG) and other genes involved in transcription regulation.43,44
Data on the time- and dose-dependent actions of ouabain and MBG on intracellular Na+ and K+ content and gene expression in human endothelial cells strongly suggest that both ECTS affect excitation-transcription coupling via NKA inhibition and [Na+]i elevation.45 This study demonstrated that 4-fold elevation of c-Fos mRNA occurs within 30 min after the addition of ouabain. At that moment [Na+]i was increased by 5-fold whereas [K+]i was declined by less than 15%.46
After Lubin and Ennis47 pioneer observation, a number of laboratories have shown K+ requirement for protein synthesis thus assuming the bimodal effect of CTS on gene expression via activation and inhibition of transcription and translation, respectively (for review, see48,49). In reticulocytes [Na+]i increase reduces the efficiency of K+i-dependent regulation of protein synthesis probably through the competition for one binding site within hypothetical K+i-sensor.50 As another hypothesis it might be suggested that elevation of [Na+]i reduces the elongation factors transcription. Moreover, we discovered that 6-hr incubation of HUVEC in the presence of ouabain resulted in 3-fold decrease of mRNA encoding eukaryotic translation initiation factor 5 (eIF5).51 This factor plays common role in protein synthesis by inducing mRNA translation and GTP hydrolysis.52,53 The molecular origin of intracellular Na+ and K+ sensors involved in activation of gene transcription and translation remains unknown.
Tight junctions and cell adhesion
Gupta and co-workers have shown that discrepancies in dose-dependent decrease the attachment of human and monkey cells possessing CTS-sensitive α1S-NKA by ouabain, vs mouse and hamster cells, having CTS-resistant α1R-NKA, positively correlate with discrepancies in dose-dependent suppression of 86Rb influx.54 At large doses, ouabain severely decreased the adhesion of COS-7 55 and human retinal pigment epithelial cells56 and blocked tight junctions in Madin–Darby canine kidney (MDCK) cells,57 RVSMC58,59 and HeLa cells.58 Importantly, tight junction and adhesion breakdown in cells with α1R- and α1S-NKA was observed at concentration of ouabain ∼1000 and 1 μM, respectively,55,57, 58, 59, 60 i.e. in the range of these enzymes’ complete inhibition. It should be noted that these ouabain effects were revoked in the medium without Na+ and were imitated by NKA inhibition in K+-depleted medium.56,57,60,61 Viewed collectively, these data assume that Na+ and K+ transmembrane gradient maintenance is an obligatory factor for the establishment the adhesion and cell-to-cell communications. The relative contribution the gain of Na+i and loss of K+i in this phenomenon remains a matter of speculations.62
Do ECTS activate Na,K-ATPase?
Numerous research team reported that low doses of CTS activate rather than inhibit Na,K-ATPase. Thus, it was demonstrated that Na-pump providing ion current in single cardiac myocytes from dog, human hearts, and guinea pig hearts63 is augmented with 10 nM and less ouabain concentration. At this low ouabain doses Na+i concentration in guinea pig atria64,65 was decreased. At 0.1 nM ouabain an activation of 86Rb uptake was seen in human erythrocytes,66 whereas at 10 nM and 10 pM ouabain augmented 86Rb uptake was found in opossum and human kidney proximal tubule cells, respectively.67,68 Stimulation of Na-pump and increment of NKA activity by ouabain and MBG were also documented in hippocampal slice cultures and human mesenteric arteries12,69 as well as microsomal fractions from mammalian kidney and duck salt glands.51,70 We observed that prolonged incubation of HUVEC with ouabain (1 and 3 nM) decreased [Na+]i and increased [K+]i resulting in [Na+]i/[K+]i-ratio attenuation by 30–50%. It should be noted that low doses of ouabain increased the rate of 86Rb influx suggesting that elevation the [Na+]i/[K+]i-ratio is caused by NKA activation.51 Considered the data on the plasma content of ECTS obtained by immunoassay technique (Table 1, Fig. 2) it might be assumed that their actions in vivo documented using anti-CTS antibodies16,17,25 are at least partially mediated by NKA activation.
The mechanism of bimodal actions of CTS on NKA activity (activation and inhibition at low and high concentration, respectively) remains poorly understood.71 Keeping in mind data on NKA functioning within the plasma membrane as dimer (αβ) and tetramer ((αβ)2)72 we proposed that binding of CTS at low doses with αβ activates enzyme whereas the occupation of (αβ)2 dimer binding sites with higher CTS concentrations suppresses the enzyme activity.73 This suggestion is now investigated in our laboratory.
Do ECTS trigger cell proliferation?
Proliferation of cultured human and canine VSMC,74,75 proximal tubule cells from opossum kidney,67 HUVEC75,76 and human polycystic kidney cells77 having α1S-NKA increased by 20–30% after the addition of ouabain at concentrations less than 1 nM, that is in the range corresponding to its plasma concentrations (Table 1). At doses lower than 1 nM ouabain also increased growth of rat proximal tubule cells,75 rat astrocytes78 and rat VSMC75 expressing α1R-NKA. As noted above at these concentrations ouabain did not inhibit NKA67,74,76,77,79 thus indicating that CTS proliferation action is mediated by Na+i,K+i-independent signaling induced by [Na+]i/[K+]i-ratio elevation. Nevertheless, it should be noted that the lack of ouabain effect on NKA documented in above-cited studies might be explained by the variety of incubation times used in these measurements. Indeed, cells were placed in the medium with ouabain for more than 24 h in order to estimate proliferation, whereas to assess 86Rb influx rate and NKA activity67,74,76,77,79,80 15–30 min of incubation were used. This is important because of long–time interaction of NKA with CTS at their low concentrations documented in human lymphocytes81 and HUVEC.51 Thus, in HUVEC indeed, half-maximal elevation of [Na+]i by 100 nM ouabain was detected in 6 h, whereas in 24 and 48 h the same increment was detected at ouabain concentrations of 30- and 10 nM, respectively.51 We observed that 48–72 h exposure of HUVEC to low nanomolar to picomolar concentrations of ouabain increased cell growth of by 20–40%.51 Importantly, prolonged exposure to 1 and 3 nM ouabain increased [K+]i and decreased [Na+]i resulting in attenuation of the [Na+]i/[K+]i-ratio by 30–50%. At these concentrations, ouabain increased the rate of 86Rb influx suggesting that side-by-side with Na+i,K+i-independent signaling augmented cell proliferation might be caused by NKA activation and elevation the [Na+]i/[K+]i-ratio.51 Data on the inhibitory actions of CTS at concentrations 3–4 order of magnitude higher than their plasma level on cell proliferation, oncosis and apoptosis is out of scope of our review and considered elsewhere73,82, 83, 84
Do ECTS evoke Na+i/K+i-independent signals?
Xie and Askari were probably the first to propose that side-by-side with monovalent ions transmembrane gradient dissipation CTS affect cellular function by triggering Na+i,K+i-independent signals.85 Table 3 displays the early data on dose-dependent ouabain effects on intracellular signaling intermediates. Recent studies considering the comparative contribution of Na+i,K+i-mediated and -independent signaling are briefly described below.
Table 3.
Major signaling pathways triggered by ouabain.
| Cellular response | Type of cells/ouabain concentration, nM | References |
|---|---|---|
| [Ca2+]i oscillations & elevation | r-PTC, 10–100 | 100 |
| h-EC, 1–10 | 76 | |
| r-PTC, >5 × 104 | 100,145 | |
| r-CM, 105 | 87,146 | |
| ERK phosphorylation & activation | r-PTC - 0.1–10** | 79 |
| h-PSMC, 0.1*** | 147 | |
| c-VSMC, 1*** | 74 | |
| h-EC, 1–10 | 76 | |
| h-HeLa, 106 | 148 | |
| r-CM, 105 | 149 | |
| p-LLC-PK1, >102 | 88 | |
| r-A7r5, 104 | 88 | |
| gp-heart, 103 | 150 | |
| r-heart, 5 × 104 | 150 | |
| h-breast cancer cells, 102 | 151 | |
| h-SkMC, 102 | 152 | |
| c-VSMC, 1 | 74 | |
| Src activation | p-LLC-PK1, 103 | 88 |
| r-A7r5, 106 | 88 | |
| r-CM, 105 | 87 | |
| h-breast cancer cells, 102 | 151 | |
| h-SkMC, 102 | 152 | |
| Protein tyrosine phosphorylation | r-CM, 105 | 87 |
| h-HeLa cells, 103 | 87 | |
| c-REC, 104 | 153 | |
| c-VSMC, 1 | 74 | |
| Akt phosphorylation | o-kidney PTC, 10* | 67 |
Abbreviations: c – canine, f – fish, gp – guinea pig, h – human, m – mouse, mn – monkey; o – opossum; p – pig, r – rat; CGC – cerebellar granule cells; CM – cardiomyocytes; CytD – cytochalasin D, EC – endothelial cells, EGFR – epidermal growth factor receptor, PKC – protein kinase C; PLC – phospholipase C; PSMC - prostate smooth muscle cells, PTC – proximal tubule cells, SkMC – skeletal muscle cells; VSMC – vascular smooth muscle cells.
Data obtained in rodents appear in italics.
* P < 0.05; ** P < 0.01; *** P ≤ 0.001.
Src-kinase
First evidence supporting membrane-associated nonreceptor tyrosine kinase Src activation came from experiments that demonstrated time- and dose-dependent tyrosine phosphorylation in cells treated by CTS.86 Hence, exposure of cardiac myocytes, HeLa, L929, and A7r5cells to ouabain led to fast activation of Src, its epidermal growth factor receptor (EGFR) and several proteins tyrosine phosphorylation that was removed by Src kinase inhibitors PP2 and herbymicin A.87,88 It was demonstrated that ouabain activates ERK MAPK and Src in transfected pig renal epithelial cells (PY-17) having α1-but not α2-NKA.89 Detailed mapping of α1-NKA nucleotide binding domain reveled 20-amino acid peptide (Ser-415 to Gln-434, NaKtide). This chemically synthesized NaKtide inhibited Scr (IC50 = 70 nM). Positively charged analogs of NaKtide entered into LLC-PK1 cells and suppressed ouabain-induced Src and ERK MAPK activation.90 Using FRET technology it was shown that ouabain induces Src kinase domain dissociation from α1-NKA nucleotide binding domain that leads to Src tyrosine phosphorylation and activation91 in LLC-PK1 cells. Lately, however, Gable et al. re-examined this effect and reported that Src-418 phosphorylation as the measure of Src activation is increased in cell-free systems not only by ouabain but also by two other NKA inhibitors (oligomycin and vanadate). They concluded that decrease of Src phosphorylation is primary result of ATP-sparing effect and cannot serve as an evidence of NKA and Src interaction triggered by CTS binding.92 Further investigations were carried by Yu et al. using native and mutant forms of α2-NKA.93 Native α2-isoform is known to lack putative Src-binding sites and fail to carry on Src-dependent signaling. Authors introduced key amino acid residues of the two Src-interacting domains that are on α1-but not α2-sequence into the α2-polypeptide and generate stable cell lines expressing this mutant. Comparison Src-signaling properties of cells expressing this mutant demonstrated that in contrast to wild-type α2, the mutant cells gained α1-like signaling function.
It has been proposed that signaling cascades triggered by NKA interaction with Src does not depend on the change of intracellular Na+, K+ and Ca2+ concentrations.85,86 Indeed, initial publications reported about increased EGFR and several other proteins tyrosine phosphorylation at ouabain concentrations that have no considerably influence on intracellular Na+ content and 86Rb influx.74,79,94 It should be noted, however, that cells loaded with fluorescent dye possessing low Na+/K+-selectivity was employed in this study. Using Na+/K+-selective isotope technique we found that MAPK phosphorylation in HUVEC occurs at ouabain concentrations leading to elevation of the [Na+]i/[K+]i-ratio.51 This observation is consisting with other reports showing Src-mediated signaling at CTS concentrations that inhibit NKA88,91,95, 96, 97, 98 and data on the complete dissociation of Src from α1S-NKA in the presence of 1 μM ouabain, i.e. at concentration resulted in the full-scale Na+-pump inhibition.91 Importantly, the augmented tyrosine phosphorylation was mimicked by NKA inhibition in K+-depleted medium.87 Viewed collectively, these data strongly suggest that in CTS-treated cells raised [Na+]i/[K+]i-ratio contributes to Src-mediated signaling triggering/progression.
PI3K-Akt
Liu et al.99 reported about activation of serine/threonine-specific protein kinase B also known as Akt in the presence of 50 μM ouabain that was abolished by phosphatidylinositol 3-kinase (PI3K) inhibitors in cultured neonatal rat cardiac myocytes. They also detected that ouabain induces phosphatidylinositol 3,4,5-triphosphate (PIP3) content increase and leads to co-immunoprecipitation of Na,K-ATPase α-subunit and p85 subunit PI3K (class IA). To reveal Src role in PI3K/Akt signal inducing, Wu and co-workers studied mouse fibroblasts without Src (SYF cells) and control Src+++ cells. They discovered that ouabain triggers PIP3 accumulation, PI3KIA and Akt activation, and p85 subunit of PI3KIA and NKA co-immunoprecipitation in both cell types that was insensitive to Src inhibitor PP2. These data allowed to suggest that Akt activation is triggered by interaction of NKA α-subunit proline-rich domain with SH3 domain of PI3KIA p85 subunit induced by CTS.97 Unfortunately, the role of elevated [Na+]i/[K+]i-ratio connected with NKA inhibition in PI3K/Akt-mediated signaling has not been studied yet.
Ca2+i-oscillations
Aperia and co-workers reported that in rat proximal tubule cells partial NKA inhibition by 50–250 μM ouabain was accompanied by increased amplitude of low-frequency [Ca2+]i oscillation which were abolished by L-type Ca2+ channel blocker nifedipine.100 It is well-documented that [Ca2+]i oscillations activate transcription factors NF-kB and CREB.101,102 In fact, ouabain-induced [Ca2+]i oscillations blockade that eliminated NF-kB and CREB activation was provided by their enter into the nucleus and phosphorylation, respectively.80,100 [Ca2+]i oscillations in human COS-7 cells were found in the presence of 100 nM ouabain that induces 10% 86Rb influx inhibition.103 Similar oscillations were also observed in the presence of 100 nM MBG and digoxin.104
Importantly, unlike modest ouabain concentrations, complete suppression of NKA by 2 mM of ouabain did not produce [Ca2+]i oscillations but resulted in continuous [Ca2+]i increase. It was also demonstrated that [K+]o decrease from 4.0 to 0.5 mM led to the same [Na+]i increase as 250 μM of ouabain. Inversely to ouabain [K+]o decrease abolished [Ca2+]i oscillation rather than enhanced them. Based on these observations, authors suggested that [Ca2+]i oscillations found in ouabain-treated cells are not primary result of NKA inhibition.100 Additional investigations should be accomplished to reveal the role of Na+i,K+i-independent signaling and dissipation of monovalent cations transmembrane gradient in [Ca2+]i oscillations produced by interaction of NKA and InsP3 receptor interaction.
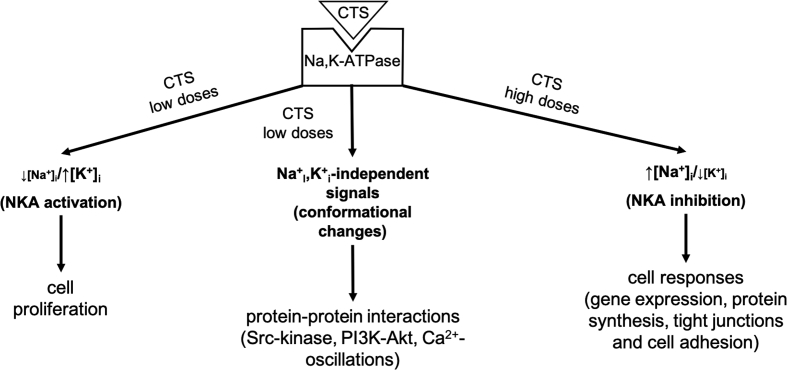
Conclusion and unresolved issues
Scheme illustrating data considered in this review are presented on Fig. 3. Results we examined demonstrate that with exception of few reports the plasma concentration of ouabain and MBG, i.e. two mostly studied CTS assessed by immunoassay techniques, is less than 1 nM. The threshold for inhibition by CTS of human and rodent α1-NKA, i.e. the only isoform detected in renal epithelial cells, is ∼1 and 1000 nM, respectively, that rules out the functioning of ECTS as natriuretic hormones (at least in rodents). As predicted, at concentrations <1 nM CTS have no impact on non-canonical cellular responses, including cell adhesion, cell-to-cell communication via tight junction, gene transcription and translation, which are mediated by dissipation of the transmembrane gradients of monovalent cations (for review see73). It should be noted, however, that local ECTS concentration might be essentially higher than that detected in plasma. In addition, NKA sensitivity to CTS is augmented by diverse stimuli, increasing its content in the E2∼P state, including attenuation of [K+]o and elevation of [Na+]i. Importantly, baseline [K+]o in CSF and tubular fluid delivered to distal nephrons is decreased by ∼2-fold compared to plasma.105,106 In neurons, short periods of synaptic activity produce increases of [Na+]i, from ∼10 to 30 and 100 mM in apical dendrites and dendritic spines, respectively.107
Figure 3.
Effects of CTS binding to Na,K-ATPase: role of [Na+]i/[K+]i -ratio changes (increase and decrease) that are produced by Na,K-ATPase inhibition and activation, respectively, and triggering cell signaling due to Na,K-ATPase conformational changes and protein–protein interaction.
At concentrations less than 1 nM ouabain increases by 20–30% proliferation of several cell types (cultured human and canine VSMC,74,75 proximal tubule cells from opossum kidney,67 HUVEC75,76 and human polycystic kidney cells77) having α1S-NKA. Because many authors reported that ouabain within this range (0.1–1 nM) activates NKA by about 25% (for review see73) we may suggest that cell proliferation is due to NKA activation and elevation the [Na+]i/[K+]i-ratio.
So, more experiments should be performed to investigate ECTS role in the triggering of Na+i/K+i-mediated cellular responses. What is [Na+]i- and [K+]i-sensors molecular origin participating in regulation of gene transcription, translation and other non-canonical cellular responses triggered by NKA inhibition and elevation of the [Na+]i/[K+]i-ratio? What is the mechanism of NKA activation by low doses of CTS? Does this mechanism contribute to proliferative effects and activation of several signaling pathways documented in cells subjected to chronic exposure to low doses of CTS? Do these actions provide a link between the augmented content of ECTS and pathogenesis of volume-expanded disorders proposed by several research teams?16, 17, 18, 19, 20, 21,53,71,108,109 We address these questions to forthcoming studies.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgements
This work was supported by grants from the Russian Science Foundation #19-75-10009 – E.A.K.; the Russian Foundation for Basic Research (#18-04-00063 - S.N.O., #18-34-00308 - A.M.T.); the National Institutes of Health Award 1R56HL127395 (N.O.D.) and National Center For Advancing Translational Sciences of the National Institutes of Health Award UL1TR000430 (N.O.D.).
In memorium: Sergei N. Orlov (1947–2019) Paper “Na+,K+-ATPase as a target for endogenous cardiotonic steroids: what’s the evidence?” was the last one written by our dear friend and colleague professor Sergei N. Orlov. He passed away on 13 October 2019. Sergei N. Orlov was born on 6 December 1947 in small town Kashira (Russia) that is located on the pictorial bank of the Oka. He graduated from middle school here and in 1966 was accepted at Lomonosov Moscow State University, Faculty of Biology. He graduated from the university in 1971 with outstanding academic achievement and entered a PhD program at Moscow State University in the specialty ”biophysics”. His PhD thesis was related to the study of free radical oxidation of higher fatty acids in phospholipids of biological membranes. Since 1975 he stated to work in Central scientific research laboratory of Ministry of Health under the guidance of chief forensic pathologist professor Yu.V. Posnov. S.N. Orlov and Yu.V. Postnov collected a group of young scientists and started to study the peculiarities of transport of monovalent cations through the cell membranes of hypertensive animals and patients. In 1983 they established discovery: “Phenomenon of propagated disturbances of cation transport through plasma membrane in essential hypertension”. In 1993 Sergei Orlov was awarded by International Society on Hypertension (Pfizer Award) and became a recipient of professorship in Montreal University. Since 1993 up to 2013 he conducted his research in Research Centre of Montreal University. Main problem that was interested professor S.N. Orlov during this time was effect of monovalent cations fluxes on physiological state of different animal cells and especially on gene expression. In 2013 professor S.N. Orlov returned to Moscow State University where he continued research studies up to the fall of 2019. S.N. Orlov is an author of about 350 scientific papers, 7 books and 6 patents. He was a member of editorial teams of 9 scientific journals. Last years of life S.N. Orlov devoted to the search of sensors of monovalent cations, which were considered him as second messengers. Being seriously ill Sergei continued to work until last day of his life. His grave is on the local cemetery of his lovely town Kashira.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.de Wardener H.E., Mills I.H., Clapham W.F., Hayter C.J. Studies on the efferent mechanism of the sodium diuresis which follows the administration of intravenous saline in the dog. Clin Sci. 1961;21:249–258. [PubMed] [Google Scholar]
- 2.de Bold A.J., Borenstein H.B., Veress A.T., Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extracts in rats. Life Sci. 1981;28(1):89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 3.Buckalew V.M., Martinez F.J., Green W.E. The effect of dialysates and ultrafiltrates of plasma of saline-loaded dogs on toad bladder sodium transport. J Clin Invest. 1970;49(5):926–935. doi: 10.1172/JCI106312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckalew V.M. Endogenous digitalis-like factors: an overview of the history. Front Endocrinol. 2015;6 doi: 10.3389/fendo.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamlyn J.M. Natriuretic hormones, endogenous ouabain, and related sodium transport inhibitors. Front Endocrinol. 2014;5 doi: 10.3389/fendo.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krenn L., Kopp B. Bufadienolides from animal and plant sources. Phytochemistry. 1998;48(1):1–29. doi: 10.1016/s0031-9422(97)00426-3. [DOI] [PubMed] [Google Scholar]
- 7.Hamlyn J.M., Blaustein M.P., Bova S. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991;88(14):6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawamura A., Guo J., Itagaki Y. On the structure of endogenous ouabain. Proc Natl Acad Sci USA. 1999;96(12):6654–6659. doi: 10.1073/pnas.96.12.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider R., Wray V., Nimtz M. Bovine adrenals contain, in addition to ouabain, a second inhibitor of the sodium pump. J Biol Chem. 1998;273(2):784–792. doi: 10.1074/jbc.273.2.784. [DOI] [PubMed] [Google Scholar]
- 10.Goto A., Ishiguro T., Yamada K. Isolation of an urinary digitalis-like factor indistinguishable from digoxin. Biochem Biophys Res Commun. 1990;173(3):1093–1101. doi: 10.1016/s0006-291x(05)80898-8. [DOI] [PubMed] [Google Scholar]
- 11.Lichtstein D., Gati I., Samuelov S. Identification of digitalis-like compounds in human cataractous lenses. Eur J Biochem. 1993;216(1):261–268. doi: 10.1111/j.1432-1033.1993.tb18141.x. [DOI] [PubMed] [Google Scholar]
- 12.Bagrov A.Y., Fedorova O.V. Effects of two putative endogenous digitalis-like factors, marinobufagenin and ouabain, on the Na+,K+-pump in human mesenteric arteries. J Hypertens. 1998;16(12 Pt 2):1953–1958. doi: 10.1097/00004872-199816121-00015. [DOI] [PubMed] [Google Scholar]
- 13.Bagrov A.Y., Fedorova O.V., Dmitrieva R.I. Characterization of a urinary bufodielnolide Na,K-ATPase inhibitor in patients after acute myocardial infarction. Hypertension. 1998;31(5):1097–1103. doi: 10.1161/01.hyp.31.5.1097. [DOI] [PubMed] [Google Scholar]
- 14.Komiyama Y., Dong X.H., Hishimura N. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin Biochem. 2005;38(1):36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Yoshika M., Komiyama Y., Konishi M. Novel digitalis-like factor, marinobufotoxin, isolated from cultured Y-1 cells, and its hypertensive effect in rats. Hypertension. 2007;49(1):209–214. doi: 10.1161/01.HYP.0000250433.64202.78. [DOI] [PubMed] [Google Scholar]
- 16.Schoner W., Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their role in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293(2):C509–C536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 17.Bagrov A.Y., Shapiro J.I., Fedorova O.V. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61(1):9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leenen F.H.H. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta. 2010;1802(12):1132–1139. doi: 10.1016/j.bbadis.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Hodes A., Lichtstein D. Natriuretic hormones in brain function. Front Endocrinol. 2014;5:201. doi: 10.3389/fendo.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalaf F.K., Dube P., Mohamed A. Cardiotonic steroids and the sodium trade balance: new insights into trade-off mechanisms mediated by the Na+,K+-ATPase. Int J Mol Sci. 2018;19(9) doi: 10.3390/ijms19092576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaustein M.P., Chen L., Hamlyn J.M. Pivotal role od a2 Na+ pumps and their high affinity ouabain binding site in cardiovascular health and disease. J Physiol. 2016;594(21):6079–6103. doi: 10.1113/JP272419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skou J.C. Further investigation on a Mg2+ + Na+-activated adenosinetriphosphatase possibly related to the active transport of Na+ and K+ across the nerve cell membrane. Biochim Biophys Acta. 1960;42:6–23. [Google Scholar]
- 23.Dvela M., Rosen H., Feldmann T., Nesher M., Lichtstein D. Diverse biological responses of different cardiotonic steroids. Pathophysiology. 2007;14(3–4):159–166. doi: 10.1016/j.pathophys.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Xie Z., Xie J. The Na/K-ATPase-mediated signal transduction as a target for new drug development. Front Biosci. 2005;10:3100–3109. doi: 10.2741/1766. [DOI] [PubMed] [Google Scholar]
- 25.Hamlyn J.M., Blaustein M.P. Endogenous ouabain: recent advances and controversies. Hypertension. 2016;68(3):526–532. doi: 10.1161/HYPERTENSIONAHA.116.06599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doris P.A., Jenkins L.A., Stocco D.M. Is ouabain an authentic endogenous mammalian substance derived from the adrenal? Hypertension. 1994;23(5):632–638. doi: 10.1161/01.hyp.23.5.632. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Sanchez E.P., Poecking M.F., Sellers D., Gomez-Sanches C.E. Is the circulation ouabain-like compound ouabain? Am J Hypertens. 1994;7(7 Pt 1):647–650. doi: 10.1093/ajh/7.7.647. [DOI] [PubMed] [Google Scholar]
- 28.Lewis L.K., Yandle T.G., Lewis J.G. Ouabain is not detectable in human plasma. Hypertension. 2019;24(5):549–555. doi: 10.1161/01.hyp.24.5.549. [DOI] [PubMed] [Google Scholar]
- 29.Baecher S., Kroiss M., Fassnacht M., Vogeser M. No endogenous ouabain is detectable in human plasma by ultra-sensitive UPLC-MS/MS. Clin Chim Acta. 2014;431:87–92. doi: 10.1016/j.cca.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Clausen M., Hilbers F., Poulsen H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lingrel J.B., Croyle M.L., Woo A.L., Argьello J.M. Ligand binding sites of Na,K-ATPase. Acta Physiol Scand. 1998;163(suppl. 643):69–77. [PubMed] [Google Scholar]
- 32.Lingrel J.B. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaustein M. Sodium transport in hypertension. Where are we going? Hypertension. 1984;6(4):445–453. doi: 10.1161/01.hyp.6.4.445. [DOI] [PubMed] [Google Scholar]
- 34.Hauptman P.J., Garg R., Kelly R.A. Cardiac glycosides in the next millennium. Prog Cardiovasc Dis. 1999;41(4):247–254. doi: 10.1053/pcad.1999.0410247. [DOI] [PubMed] [Google Scholar]
- 35.Orlov S.N., Taurin S., Hamet P. The α1-Na/K pump does not mediate the involvement of ouabain in the development of hypertension in rats. Blood Pres. 2002;11(1):56–62. doi: 10.1080/080370502753543972. [DOI] [PubMed] [Google Scholar]
- 36.Fedorova O.V., Bagrov A.Y. Inhibition of Na/K ATPase from rat aorta by two Na/K pump inhibitors, ouabain and marinobufagenin: evidence of interaction with different alpha-subunit isoform. Am J Hypertens. 1997;10(8):929–935. doi: 10.1016/s0895-7061(97)00096-4. [DOI] [PubMed] [Google Scholar]
- 37.Akimova O.A., Bagrov A.Y., Lopina O.D. Cardiotonic steroids differentially affect intracellular Na+ and [Na+]i/[K+]i-independent signaling in C7-MDCK cells. J Biol Chem. 2005;280(1):832–839. doi: 10.1074/jbc.M411011200. [DOI] [PubMed] [Google Scholar]
- 38.Klimanova E.A., Petrushenko I.Y., Mitkevich V.A. Binding of ouabain and marinobufagenin leads to different structural changes in Na,K-ATPase and depends on the enzyme conformation. FEBS Lett. 2015;589(19 Pt B):2668–2674. doi: 10.1016/j.febslet.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Blaustein M.P., Zhang J., Chen L., Hamilton B.P. How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R514–R523. doi: 10.1152/ajpregu.00819.2005. [DOI] [PubMed] [Google Scholar]
- 40.Iwamoto T., Kita S., Zhang J. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle cells. Nat Med. 2004;10(11):1193–1199. doi: 10.1038/nm1118. [DOI] [PubMed] [Google Scholar]
- 41.Orlov S.N., Taurin S., Tremblay J., Hamet P. Inhibition of Na+,K+ pump affects nucleic acid synthesis and smooth muscle cell proliferation via elevation of the [Na+]i/[K+]i ratio: possible implication in vascular remodeling. J Hypertens. 2001;19(9):1559–1565. doi: 10.1097/00004872-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Taurin S., Seyrantepe V., Orlov S.N. Proteome analysis and functional expression identify mortalin as an anti-apoptotic gene induced by elevation of [Na+]i/[K+]i ratio in cultured vascular smooth muscle cells. Circ Res. 2002;91(10):915–922. doi: 10.1161/01.res.0000043020.45534.3e. [DOI] [PubMed] [Google Scholar]
- 43.Koltsova S.V., Trushina Y., Haloui M. Ubiquitous [Na+]i/[K+]i-sensitive transcriptome in mammalian cells: evidence for Ca2+i-independent excitation-transcription coupling. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klimanova E.A., Sidorenko S.V., Smolyaninova L.V. Ubiquitous and cell type-specific transcriptomic changes triggered by dissipation of monovalent cation gradients in rodent cells: physiological and pathophysiological implications. Curr Top Membr. 2019;83:107–150. doi: 10.1016/bs.ctm.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Klimanova E.A., Tverskoi A.M., Koltsova S.V. Time- and dose-dependent actions of cardiotonic steroids on transcriptome and intracellular content of Na+ and K+: a comparative analysis. Sci Rep. 2017;7 doi: 10.1038/srep45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taurin S., Dulin N.O., Pchejetski D. c-Fos expression in ouabain-treated vascular smooth muscle cells from rat aorta: evidence for an intracellular-sodium-mediated, calcium-independent mechanism. J Physiol. 2002;543(Pt 3):835–847. doi: 10.1113/jphysiol.2002.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lubin M., Ennis H.L. On the role of intracellular potassium in protein synthesis. Biochim Biophys Acta. 1964;80:614–631. doi: 10.1016/0926-6550(64)90306-8. [DOI] [PubMed] [Google Scholar]
- 48.Orlov S.N., Hamet P. Intracellular monovalent ions as second messengers. J Membr Biol. 2006;210(3):161–172. doi: 10.1007/s00232-006-0857-9. [DOI] [PubMed] [Google Scholar]
- 49.Orlov S.N., Hamet P. Salt and gene expression: evidence for Na+i,K+i-mediated signaling pathways. Pflueg Arch Eur J Physiol. 2015;467(3):489–498. doi: 10.1007/s00424-014-1650-8. [DOI] [PubMed] [Google Scholar]
- 50.Cahn F., Lubin M. Inhibition of elongation steps of protein synthesis at reduced potassium concentrations in reticulocytes and reticulocyte lysate. J Biol Chem. 1978;253(21):7798–7803. [PubMed] [Google Scholar]
- 51.Tverskoi A.M., Sidorenko S.V., Klimanova E.A. Effects of ouabain on proliferation of human endothelial cells correlate with Na+,K+-ATPase activity and intracellular ratio of Na+ and K+ Biochemistry (Mosc) 2016;81(8):876–883. doi: 10.1134/S0006297916080083. [DOI] [PubMed] [Google Scholar]
- 52.Das S., Maitra U. Mutational analysis of mammalian translation initiation factor 5 (eIF5): role of interaction between β subunit of eIF2 and eIF5 in eIF5 function in vitro and in vivo. Mol Cell Biol. 2000;20(11):3942–3950. doi: 10.1128/mcb.20.11.3942-3950.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jennings M.D., Pavitt G.D. eIF5 is a dual function GAP and GDI for eukaryotic translational control. Small GTPases. 2010;1(2):118–123. doi: 10.4161/sgtp.1.2.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta R.S., Chora A., Stetsko D.K. Cellular basis for the species differences in sensitivity to cardiac glycosides (digitalis) J Cell Physiol. 1986;127(2):197–206. doi: 10.1002/jcp.1041270202. [DOI] [PubMed] [Google Scholar]
- 55.Belusa R., Aizman O., Andersson R.M., Aperia A. Changes in Na+-K+-ATPase activity influence cell attachment to fibronectin. Am J Physiol Cell Physiol. 2001;282(2):C302–C309. doi: 10.1152/ajpcell.00117.2001. [DOI] [PubMed] [Google Scholar]
- 56.Rajasekaran S.A., Hu J., Gopal J. Na,K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am J Physiol Cell Physiol. 2003;284(6):C1497–C1507. doi: 10.1152/ajpcell.00355.2002. [DOI] [PubMed] [Google Scholar]
- 57.Rajasekaran S.A., Palmer L.G., Moon S.Y. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell. 2001;12(12):3717–3732. doi: 10.1091/mbc.12.12.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin P.E., Hill N.S., Kristensen B., Errington R.J., Griffith T.M. Ouabain exerts biphasic effects on connexin functionality and expression in vascular smooth muscle cells. Br J Pharmacol. 2003;140(7):1261–1271. doi: 10.1038/sj.bjp.0705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matchkov V.V., Gustafsson H., Rahman A. Interaction between Na+/K+-pump and Na+/Ca2+-exchanger modulates intercellular communication. Circ Res. 2007;100(7):1026–1035. doi: 10.1161/01.RES.0000262659.09293.56. [DOI] [PubMed] [Google Scholar]
- 60.Violette M.I., Madan P., Watson A.J. Na+/K+-ATPase regulates tight junction formation and function during mouse preimplantation development. Dev Biol. 2006;289(2):406–419. doi: 10.1016/j.ydbio.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Lee J.-M., Grabb M.C., Zipfel G.J., Choi D.W. Brain tissue responses to ischemia. J Clin Invest. 2000;106(6):723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajasekaran A.K., Rajasekaran S.A. Role of Na-K-ATPase in the assembly of tight junctions. Am J Physiol Ren Physiol. 2003;285(3):F388–F396. doi: 10.1152/ajprenal.00439.2002. [DOI] [PubMed] [Google Scholar]
- 63.Gao J., Wymore R.S., Wang Y. Isoform-specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J Gen Physiol. 2002;119(4):297–312. doi: 10.1085/jgp.20028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J., Zelenin S., Aperia A., Aizman O. Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-kappaB. J Am Soc Nephrol. 2006;17(7):1848–1857. doi: 10.1681/ASN.2005080894. [DOI] [PubMed] [Google Scholar]
- 65.Ghysel-Burton J., Godfraind T. Stimulation and inhibition of the sodium pump by cardiotonic steroids in relation to their binding sites and ionotropic effect. Br J Pharmacol. 1979;66(2):175–184. doi: 10.1111/j.1476-5381.1979.tb13662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balzan S., D'Urso G., Nicolini G., Forini F., Pellegrino M., Montali U. Erythrocyte sodium pump stimulation by ouabain and an endogenous ouabain-like factor. Cell Biochem Funct. 2007;25(3):297–303. doi: 10.1002/cbf.1387. [DOI] [PubMed] [Google Scholar]
- 67.Khundmiri S.J., Metzler M.A., Ameen M., Amin V., Rane M.J., Delamere N.A. Ouabain induces cell proliferation through calcium dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol. 2006;291(6):C1247–C1257. doi: 10.1152/ajpcell.00593.2005. [DOI] [PubMed] [Google Scholar]
- 68.Khundmiri S.J., Salyer S.A., Farmer B. Structural determinants for te ouabain-stimulated increase in Na-K ATPase activity. Biochim Biophys Acta. 2014;1843(6):1089–1102. doi: 10.1016/j.bbamcr.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Oselkin M., Tian D., Bergold P.J. Low-dose cardiotonic steroids increase sodium-potassium ATPase activity that protects hippocampal slice cultures from experimental ischemia. Neurosci Lett. 2010;473(2):67–71. doi: 10.1016/j.neulet.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 70.Holthouser K.A., Mandal A., Merchant M.L. Ouabain stimulates Na-K-ATPase through a sodium/hydrogen exchanger-1 (NHE1)-dependent mechanism in human kidney proximal tubule cells. Am J Physiol Ren Physiol. 2010;299(1):F77–F90. doi: 10.1152/ajprenal.00581.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khundmiri S.J. Advances in understanding the role of cardiac glycosides in control of sodium transport in renal tubules. J Endocrinol. 2014;222(1):R11–R24. doi: 10.1530/JOE-13-0613. [DOI] [PubMed] [Google Scholar]
- 72.Askari A. Na+,K+-ATPase: on the number of the ATP sites of the functional unit. J Bioenerg Biomembr. 1987;19(4):359–374. doi: 10.1007/BF00768539. [DOI] [PubMed] [Google Scholar]
- 73.Orlov S.N., Klimanova E.A., Tverskoi A.M., Vladychenskaya E.A., Smolyaninova L.V., Lopina O.D. Na+i,K+i-dependent and -independent signaling triggered by cardiotonic steroids: facts and artifacts. Molecules. 2017;22(4) doi: 10.3390/molecules22040635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aydemir-Koksoy A., Abramowitz J., Allen J.C. Ouabain-induced signaling and vascular smooth muscle cell proliferation. J Biol Chem. 2001;276(49):46605–46611. doi: 10.1074/jbc.M106178200. [DOI] [PubMed] [Google Scholar]
- 75.Abramowitz J., Dai C., Hirschi K.K. Ouabain- and marinobufagenin-induced proliferation of human umbilical vein smooth muscle cells and a rat vascular smooth muscle cell line, A7r5. Circulation. 2003;108(24):1049–1054. doi: 10.1161/01.CIR.0000101919.00548.86. [DOI] [PubMed] [Google Scholar]
- 76.Saunders R., Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur J Biochem. 2004;271(5):1054–1062. doi: 10.1111/j.1432-1033.2004.04012.x. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen A.N., Wallace D.P., Blanco G. Ouabain binds with high affinity to the Na+,K+-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol. 2007;18(1):46–57. doi: 10.1681/ASN.2006010086. [DOI] [PubMed] [Google Scholar]
- 78.Murata Y., Matsuda T., Tamada K. Ouabain-induced cell proliferation in cultured rat astrocytes. Jpn J Pharmacol. 1996;72(4):347–353. doi: 10.1254/jjp.72.347. [DOI] [PubMed] [Google Scholar]
- 79.Dmitrieva R.I., Doris P.A. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J Biol Chem. 2004;278(30):28160–28166. doi: 10.1074/jbc.M303768200. [DOI] [PubMed] [Google Scholar]
- 80.Desfrere L., Karlsson M., Hiyoshi H. Na,K-ATPase signal transduction triggers CREB activation and dendritic growth. Proc Natl Acad Sci USA. 2009;106(7):2212–2217. doi: 10.1073/pnas.0809253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Segel G.B., Lichtman M.A. The apparent discrepancy of ouabain inhibition of cation transport and lymphocyte proliferation is explained by time-dependency of ouabain binding. J Cell Physiol. 1980;104(1):21–26. doi: 10.1002/jcp.1041040104. [DOI] [PubMed] [Google Scholar]
- 82.Orlov S.N., Hamet P. Apoptosis vs oncosis: role of cell volume and intracellular monovalent cations. Adv Exp Med Biol. 2004;559:219–233. doi: 10.1007/0-387-23752-6_21. [DOI] [PubMed] [Google Scholar]
- 83.Akimova O.A., Platonova A.A., Koltsova S.V. Cell death triggered by cardiotonic steroids: role of cell volume perturbations and α1-Na+,K+-ATPase subunit. Siberian Med Bull. 2013;12:24. [Google Scholar]
- 84.Akimova O.A., Tverskoi A.M., Smolyaninova L.V. Critical role of the a1-Na+,K+-ATPase subunit in insensitivity of rodent cells to cytotoxic action of ouabain. Apoptosis. 2015;20(9):1200–1210. doi: 10.1007/s10495-015-1144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie Z., Askari A. Na+/K+-ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 86.Liu J., Xie Z. The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomains in regulation of transporter trafficking. Biochim Biophys Acta. 2010;1802(12):1237–1245. doi: 10.1016/j.bbadis.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haas M., Askari A., Xie Z. Involvement of Src and epidermal growth factor receptor in the signal transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275(36):27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 88.Haas M., Wang H., Tian J., Xie Z. Src-mediated inter-receptor cross-talk between the Na+,K+-ATPase and epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277(21):18694–18702. doi: 10.1074/jbc.M111357200. [DOI] [PubMed] [Google Scholar]
- 89.Xie J., Ye Q., Cui X. Expression of rat Na-K-ATPase a2 enables ion pumping but not ouabain-induced signaling in a1-deficient porcine renal epithelial cells. Am J Physiol Cell Physiol. 2015;309(6):C373–C382. doi: 10.1152/ajpcell.00103.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Z., Cai T., Tian J. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J Biol Chem. 2009;284(31):21066–21076. doi: 10.1074/jbc.M109.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian J., Cai T., Yuan Z. Binding of Src to Na+,K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gable M.E., Abdallah S.L., Najjar S.M., Liu L., Askari A. Digitalis-induced cell signaling by the sodium pump: on the relation of Src and Na+,K+-ATPase. Biochem Biophys Res Commun. 2014;446(4):1151–1154. doi: 10.1016/j.bbrc.2014.03.071. [DOI] [PubMed] [Google Scholar]
- 93.Yu H., Cui X., Zhang J. Heterogeneity of signal transduction by Na-K-ATPase α-isoforms: role of SRC interaction. Am J Physiol Cell Physiol. 2018;314(2):C202–C210. doi: 10.1152/ajpcell.00124.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu J., Tian J., Haas M., Shapiro J.I., Askari A., Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascade independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275(36):27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 95.Tian J., Gong X., Xie Z. Signal-transducing function of Na+,K+-ATPase is essential for ouabain's effect on [Ca2+]i in rat cardiac myocytes. Am J Physiol. 2001;281(5):H1899–H1907. doi: 10.1152/ajpheart.2001.281.5.H1899. [DOI] [PubMed] [Google Scholar]
- 96.Kulikov A., Eva A., Kirch U., Boldyrev A., Scheiner-Bobis G. Ouabain activates signaling pathways associated with cell death in human neuroblastoma. Biochim Biophys Acta. 2007;1768(7):1691–1702. doi: 10.1016/j.bbamem.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 97.Wu J., Akkuratov E.E., Bai Y., Gaskill C.M., Askari A., Liu L. Cell signaling associated with Na+/K+-ATPase; activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochem. 2013;52(50):9059–9067. doi: 10.1021/bi4011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu L., Abramowitz J., Askari A., Allen J.C. Role of caveolae in ouabain-induced proliferation of cultured vascular smooth muscle cells of the synthetic phenotype. Am J Physiol Heart Circ Physiol. 2004;287(5):H2173–H2182. doi: 10.1152/ajpheart.00352.2004. [DOI] [PubMed] [Google Scholar]
- 99.Liu L., Zhao X., Pierre S.V., Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293(5):C1489–C1497. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 100.Aizman O., Uhlen P., Lal M., Brismar H., Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci USA. 2001;98(23):13420–13424. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dolmetsch R.E., Xu K., Lewis R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392(6679):933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 102.Lonze B.E. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 103.Zhang S., Maimersjo S., Li J. Distinct role of the N-terminal tail of the Na,K-ATPase catalytic subunit as a signal transducer. J Biol Chem. 2006;281(31):21954–21962. doi: 10.1074/jbc.M601578200. [DOI] [PubMed] [Google Scholar]
- 104.Fontana J.M., Burlaka I., Khodus G., Brismar H., Aperia A. Calcium oscillations triggered by cardiotonic steroids. FEBS J. 2013;280(21):5450–5455. doi: 10.1111/febs.12448. [DOI] [PubMed] [Google Scholar]
- 105.Somjen G.G. Oxford University Press; New York: 2004. Ions in the Brain: Normal Functions, Seizures, and Stroke. [Google Scholar]
- 106.Vander A.J. Renal Physiology. 5 ed. McGraw-Hill, Inc.; New York: 1991. [Google Scholar]
- 107.Rose C.R., Konnerth A. NMDA-receptor-mediated Na+ signals in spines and dendrites. J Neurosci. 2001;21(12):4207–4214. doi: 10.1523/JNEUROSCI.21-12-04207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fedorova O.V., Lakatta E.G., Bagrov A.Y., Melander O. Plasma level of the endogenous sodium pump ligand marinobufagenin is related to the the salt-sensitivity. J Hypertens. 2015;33(3):533–541. doi: 10.1097/HJH.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schoner W., Scheiner-Bobis G. Role of endogenous cardiotonic steroids in sodium homeostasis. Nephrol Dial Transplant. 2008;23(9):2723–2729. doi: 10.1093/ndt/gfn325. [DOI] [PubMed] [Google Scholar]
- 110.Rossi G., Manunta P., Hamlyn J.M. Immunoreactive endogenous ouabain in primary aldosteronism and essential hypertension: relationship with plasma renin, aldosterone and blood pressure levels. J Hypertens. 1995;13(10):1181–1191. doi: 10.1097/00004872-199510000-00013. [DOI] [PubMed] [Google Scholar]
- 111.Gottlieb S.S., Rogowski A.S., Weinberg M., Krichten C.M., Hamilton B.P., Hamlyn J.M. Elevated concentration of endogenous ouabain in patients with congestive heart failure. Circulation. 1992;86(2):420–425. doi: 10.1161/01.cir.86.2.420. [DOI] [PubMed] [Google Scholar]
- 112.van Horck F.P., Ahmadian M.R., Haeussler L.C., Moolenaar W.H., Kranenburg O. Characterization of p190RhoCEF, a Rho-specific guanine nucleotide exchange factor that interacts with microtubule. J Biol Chem. 2001;276(7):4948–4956. doi: 10.1074/jbc.M003839200. [DOI] [PubMed] [Google Scholar]
- 113.Butt A.N., Semra Y.K., Ho C.S., Swaminathan R. Effect of high salt intake on plasma and tissue concentration of endogenous ouabain-like substances in the rat. Life Sci. 1997;61(24):2367–2373. doi: 10.1016/s0024-3205(97)00953-3. [DOI] [PubMed] [Google Scholar]
- 114.Butt A.N., Semra Y.K., Lane S.J., Lee T., Swaminathan R. Endogenous ouabain secretion in man is not regulated by ACTH. J Steroid Biochem Mol Biol. 1998;66(3):151–157. doi: 10.1016/s0960-0760(98)00047-8. [DOI] [PubMed] [Google Scholar]
- 115.Fedorova O.V., Anderson D.E., Bagrov A.Y. Plasma marinobufagenin-like and ouabain-like immunoreactivity in adrenocorticotropin-treated rats. Am J Hypertens. 1998;11(7):796–802. doi: 10.1016/s0895-7061(98)00042-9. [DOI] [PubMed] [Google Scholar]
- 116.Gonick H.C., Ding Y., Vaziri N.D., Bagrov A.Y., Fedorova O.V. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease state. Clin Exp Hypertens. 1998;20(5–6):617–627. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- 117.Lopatin D.A., Ailmazian E.K., Dmitrieva R.I. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17(8):1179–1187. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 118.Vakkuri O., Arnason S.S., Pouta A., Vuolteenaho O., Leppaluoto J. Radioimmunoassay of plasma ouabain in healthy and pregnant individuals. J Endocrinol. 2000;165(3):669–677. doi: 10.1677/joe.0.1650669. [DOI] [PubMed] [Google Scholar]
- 119.Balzan S., Neglia D., Ghione S. Increased circulating level of ouabain-like factor in patients with asymptomatic left ventricular dysfunction. Eur J Heart Fail. 2001;3(2):165–171. doi: 10.1016/s1388-9842(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 120.Fedorova O.V., Kolodkin N.I., Agalakova N.I., Lakatta E.G., Bagrov A.Y. Marinobufagenin, an endogenous α-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension. 2001;37(2 Pt 2):462–466. doi: 10.1161/01.hyp.37.2.462. [DOI] [PubMed] [Google Scholar]
- 121.Fedorova O.V., Talan M.I., Agalakova N.I., Lakatta E.G., Bagrov A.Y. Endogenous ligand of α1 sodium pump, marinobufagenin, is a novel mediator of sodium chloride-dependent hypertension. Circulation. 2002;105(9):1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 122.Fedorova O.V., Kolodkin N.I., Agalakova N.I. Antibody to marinobufagenin lowers blood pressure in pregnant rats on high NaCl intake. J Hypertens. 2005;23(4):835–842. doi: 10.1097/01.hjh.0000163153.27954.33. [DOI] [PubMed] [Google Scholar]
- 123.Harwood S., Mullen A.M., McMahon A.C., Dawnay A. Plasma OLS is elevated in mild experimental uremia but is not associated with hypertension. Am J Hypertens. 2001;14(11 Pt 1):1112–1115. doi: 10.1016/s0895-7061(01)02219-1. [DOI] [PubMed] [Google Scholar]
- 124.Berendes E., Cullen P., van Aken H. Endogenous glycosides in critically ill patients. Crit Care Med. 2003;31(5):1331–1337. doi: 10.1097/01.CCM.0000059721.57219.C3. [DOI] [PubMed] [Google Scholar]
- 125.Wang J.G., Staessen J.A., Messangio E. Salt, endogenous ouabain and blood pressure interactions in general population. J Hypertens. 2003;21(8):1475–1481. doi: 10.1097/00004872-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 126.Balzan S., Nicolini G., Iervasi A., Di Cecco P., Fommei E. Endogenous ouabain and acute salt loading in low-renin hypertension. Am J Hypertens. 2005;18(7):906–909. doi: 10.1016/j.amjhyper.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 127.Fridman A.I., Matveev S.A., Agalakova N.I., Fedorova O.V., Lakatta E.G., Bagrov A.Y. Marinobufagenin, an endogenous ligand of α-1 Na/K-ATPase, is a marker of congestive heart failure severity. J Hypertens. 2002;20(6):1189–1194. doi: 10.1097/00004872-200206000-00032. [DOI] [PubMed] [Google Scholar]
- 128.Kennedy D.J., Vetteth S., Periyasamy S.M. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47(3):488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 129.Ferrari P., Torielli L., Ferrandi M. PST2238: a new antihypertensive compound that antagonizes the long-term pressor effect of ouabain. J Pharmacol Exp Therapeut. 1998;285(1):83–94. [PubMed] [Google Scholar]
- 130.Lee K., Jung J., Kim M., Guidotti G. Interaction of the α subunit of Na,K-ATPase with cofilin. Biochem J. 2001;353(Pt 2):377–385. doi: 10.1042/0264-6021:3530377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dostanic I., Schultz J.E.J., Lorenz J.N., Lingrel J.B. The α1 isoform of Na,K-ATPase regulates contractility and functionally interacts and co-localizes with the Na/Ca-exchanger in heart. J Biol Chem. 2004;279(52):54053–54061. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- 132.Fedorova O.V., Lakatta E.G., Bagrov A.Y. Differential effects of acute NaCl loading on endogenous ouabain-like and marinobufagenin-like ligands of the sodium pump in Dahl hypertensive rats. Circulation. 2000;102:3009–3014. doi: 10.1161/01.cir.102.24.3009. [DOI] [PubMed] [Google Scholar]
- 133.Fedorova O.V., Talan M.I., Agalakova N.I., Lakatta E.G., Bagrov A.Y. Coordinated shifts in Na/K-ATPase isoforms and their endogenous ligands during cardiac hypertrophy and failure in NaCl-sensitive hypertension. J Hypertens. 2004;22(2):1–9. doi: 10.1097/00004872-200402000-00025. [DOI] [PubMed] [Google Scholar]
- 134.Dmitrieva R.I., Georgiev I.Y., Shpen V.M., Bagrov A.Y. Bufadienolide nature of an endogenous inhibitor of sodium-potassium adenosine triphosphatase in humans. J Evol Biochem Physiol. 1997;33(3):355–363. [PubMed] [Google Scholar]
- 135.Fedorova O.V., Dorofeeva N.A., Lopatin D.A., Lakatta E.G., Bagrov A.Y. Phorbol diacetate potentiates Na+,K+-ATPase inhibition by a putative endogenous ligand, marinobufagenin. Hypertension. 2002;39(2):298–302. doi: 10.1161/hy0202.104344. [DOI] [PubMed] [Google Scholar]
- 136.Sceinin M., Koulu M., Laurikainen E., Allonen H. Hypokalemia and other non-bronchial effects of inhaled fenoterol and salbutamol: a placebo controlled dose-response study in healthy volunteers. Br J Clin Pharmacol. 1987;24(5):645–653. doi: 10.1111/j.1365-2125.1987.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bagrov A.Y., Dmitrieva R.I., Fedorova O.V., Kazakov G.P., Roukoyatkina N.I., Shpen V.M. Endogenous marinobufagenin-like immunoreactive substance: a possible endogenous Na,K-ATPase inhibitor with vasoconstrictor activity. Am J Hypertens. 1996;9(10 Pt 1):982–990. doi: 10.1016/0895-7061(96)00148-3. [DOI] [PubMed] [Google Scholar]
- 138.Blanco G., Sanchez G., Mercer R.W. Comparison of the enzymatic properties of the Na,K-ATPase α3/β1 and α3/β2 isozymes. Biochem. 1995;34(31):9897–9903. doi: 10.1021/bi00031a011. [DOI] [PubMed] [Google Scholar]
- 139.Brownlee A.A., Johnsosn P., Mills I.H. Actions of bufalin and cinobufotalin, two bufadienolides respectively more active and less active than ouabain, on ouabain binding and 86Rb uptake by human erythrocytes. Clin Sci. 1990;78(2):169–174. doi: 10.1042/cs0780169. [DOI] [PubMed] [Google Scholar]
- 140.Flier J.S. Ouabain-like activity in toad skin and its implication for endogenous regulation of ion transport. Nature. 1978;274(5668):285–286. doi: 10.1038/274285a0. [DOI] [PubMed] [Google Scholar]
- 141.Senn N., Lelievre L.G., Braquet P., Garay R. High sensitivity of the Na+,K+-pump of human red blood cells to genins of cardiac glycosides. Br J Pharmacol. 1988;93(4):803–810. doi: 10.1111/j.1476-5381.1988.tb11465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moller B., Vaag A., Johansen T. Ouabain inhibition of the sodium-potassium pump: estimation of ED50 in different types of human leucocytes in vitro. Br J Clin Pharmacol. 1990;29(1):93–100. doi: 10.1111/j.1365-2125.1990.tb03607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tao Q.F., Hollenberg N.K., Price D.A., Graves S.V. Sodium pump isoform specificity for digitalis-like factor isolated from human peritoneal dialysate. Hypertension. 1996;29(3):815–821. doi: 10.1161/01.hyp.29.3.815. [DOI] [PubMed] [Google Scholar]
- 144.Rodriguez-Manas L., Pareja A., Sanchez-Ferrer C.F., Casado M.A., Salacies M.J. Endothelial role in ouabain-induced contraction of Guinea pig carotid arteries. Hypertension. 1992;20(5):674–681. doi: 10.1161/01.hyp.20.5.674. [DOI] [PubMed] [Google Scholar]
- 145.Miakawa-Naito A., Uhlйn P., Lal M. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-triphosphate receptor generates calcium oscillations. J Biol Chem. 2003;278(50):50355–50361. doi: 10.1074/jbc.M305378200. [DOI] [PubMed] [Google Scholar]
- 146.Peng M., Huang L., Xie Z., Huang W.-H., Askari A. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expression of early-response genes in cardiac myocytes. J Biol Chem. 1996;271(17):10372–10378. doi: 10.1074/jbc.271.17.10372. [DOI] [PubMed] [Google Scholar]
- 147.Chueh S.C., Guh J.H., Chen J., Lai M.K., Teng C.M. Dual effect of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J Urol. 2001;166(1):347–353. [PubMed] [Google Scholar]
- 148.Li S., Wattenberg E.V. Differential activation of mitogen-activated protein kinases by palytoxin and ouabain, two ligands for the Na+,K+-ATPase. Toxicol Appl Pharmacol. 1998;151(2):377–384. doi: 10.1006/taap.1998.8471. [DOI] [PubMed] [Google Scholar]
- 149.Kometiani P., Li J., Gnudi L., Kahn B.B., Askari A., Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes: the roles of ras and mitogen-activated protein kinases. J Biol Chem. 1998;273(24):15249–15256. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- 150.Mohammadi K., Liu L., Tian J., Kometiani P., Askari A. Positive ionotropic effect of ouabain on isolated heart is accompanied by activation of signal pathways that link Na+/K+-ATPase to ERK1/2. J Cardiovasc Pharmacol. 2003;41(4):609–614. doi: 10.1097/00005344-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 151.Kometiani P., Liu L., Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol. 2005;67(3):929–936. doi: 10.1124/mol.104.007302. [DOI] [PubMed] [Google Scholar]
- 152.Kotova O., Al-Khalili L., Hooke C., Fedorova O.V., Bagrov A.Y., Chibalin A.V. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J Biol Chem. 2006;281(29):20085–20094. doi: 10.1074/jbc.M601577200. [DOI] [PubMed] [Google Scholar]
- 153.Valente R.C., Capella L.S., Monteiro R.Q., Rumjanek V.M., Lopes A.G., Capella M.A.M. Mechanisms of ouabain toxicity. Faseb J. 2003;17(12):1700–1702. doi: 10.1096/fj.02-0937fje. [DOI] [PubMed] [Google Scholar]