Abstract
Background
Eczema and food allergy are common health conditions that usually begin in early childhood and often occur together in the same people. They can be associated with an impaired skin barrier in early infancy. It is unclear whether trying to prevent or reverse an impaired skin barrier soon after birth is effective in preventing eczema or food allergy.
Objectives
Primary objective
To assess effects of skin care interventions, such as emollients, for primary prevention of eczema and food allergy in infants
Secondary objective
To identify features of study populations such as age, hereditary risk, and adherence to interventions that are associated with the greatest treatment benefit or harm for both eczema and food allergy.
Search methods
We searched the following databases up to July 2020: Cochrane Skin Specialised Register, CENTRAL, MEDLINE, and Embase. We searched two trials registers and checked reference lists of included studies and relevant systematic reviews for further references to relevant randomised controlled trials (RCTs). We contacted field experts to identify planned trials and to seek information about unpublished or incomplete trials.
Selection criteria
RCTs of skin care interventions that could potentially enhance skin barrier function, reduce dryness, or reduce subclinical inflammation in healthy term (> 37 weeks) infants (0 to 12 months) without pre‐existing diagnosis of eczema, food allergy, or other skin condition were included. Comparison was standard care in the locality or no treatment. Types of skin care interventions included moisturisers/emollients; bathing products; advice regarding reducing soap exposure and bathing frequency; and use of water softeners. No minimum follow‐up was required.
Data collection and analysis
This is a prospective individual participant data (IPD) meta‐analysis. We used standard Cochrane methodological procedures, and primary analyses used the IPD dataset. Primary outcomes were cumulative incidence of eczema and cumulative incidence of immunoglobulin (Ig)E‐mediated food allergy by one to three years, both measured by the closest available time point to two years. Secondary outcomes included adverse events during the intervention period; eczema severity (clinician‐assessed); parent report of eczema severity; time to onset of eczema; parent report of immediate food allergy; and allergic sensitisation to food or inhalant allergen.
Main results
This review identified 33 RCTs, comprising 25,827 participants. A total of 17 studies, randomising 5823 participants, reported information on one or more outcomes specified in this review. Eleven studies randomising 5217 participants, with 10 of these studies providing IPD, were included in one or more meta‐analysis (range 2 to 9 studies per individual meta‐analysis).
Most studies were conducted at children's hospitals. All interventions were compared against no skin care intervention or local standard care. Of the 17 studies that reported our outcomes, 13 assessed emollients. Twenty‐five studies, including all those contributing data to meta‐analyses, randomised newborns up to age three weeks to receive a skin care intervention or standard infant skin care. Eight of the 11 studies contributing to meta‐analyses recruited infants at high risk of developing eczema or food allergy, although definition of high risk varied between studies. Durations of intervention and follow‐up ranged from 24 hours to two years.
We assessed most of this review's evidence as low certainty or had some concerns of risk of bias. A rating of some concerns was most often due to lack of blinding of outcome assessors or significant missing data, which could have impacted outcome measurement but was judged unlikely to have done so. Evidence for the primary food allergy outcome was rated as high risk of bias due to inclusion of only one trial where findings varied when different assumptions were made about missing data.
Skin care interventions during infancy probably do not change risk of eczema by one to two years of age (risk ratio (RR) 1.03, 95% confidence interval (CI) 0.81 to 1.31; moderate‐certainty evidence; 3075 participants, 7 trials) nor time to onset of eczema (hazard ratio 0.86, 95% CI 0.65 to 1.14; moderate‐certainty evidence; 3349 participants, 9 trials). It is unclear whether skin care interventions during infancy change risk of IgE‐mediated food allergy by one to two years of age (RR 2.53, 95% CI 0.99 to 6.47; 996 participants, 1 trial) or allergic sensitisation to a food allergen at age one to two years (RR 0.86, 95% CI 0.28 to 2.69; 1055 participants, 2 trials) due to very low‐certainty evidence for these outcomes. Skin care interventions during infancy may slightly increase risk of parent report of immediate reaction to a common food allergen at two years (RR 1.27, 95% CI 1.00 to 1.61; low‐certainty evidence; 1171 participants, 1 trial). However, this was only seen for cow’s milk, and may be unreliable due to significant over‐reporting of cow’s milk allergy in infants. Skin care interventions during infancy probably increase risk of skin infection over the intervention period (RR 1.34, 95% CI 1.02 to 1.77; moderate‐certainty evidence; 2728 participants, 6 trials) and may increase risk of infant slippage over the intervention period (RR 1.42, 95% CI 0.67 to 2.99; low‐certainty evidence; 2538 participants, 4 trials) or stinging/allergic reactions to moisturisers (RR 2.24, 95% 0.67 to 7.43; low‐certainty evidence; 343 participants, 4 trials), although confidence intervals for slippages and stinging/allergic reactions are wide and include the possibility of no effect or reduced risk.
Preplanned subgroup analyses show that effects of interventions were not influenced by age, duration of intervention, hereditary risk, FLG mutation, or classification of intervention type for risk of developing eczema. We could not evaluate these effects on risk of food allergy. Evidence was insufficient to show whether adherence to interventions influenced the relationship between skin care interventions and risk of developing eczema or food allergy.
Authors' conclusions
Skin care interventions such as emollients during the first year of life in healthy infants are probably not effective for preventing eczema, and probably increase risk of skin infection. Effects of skin care interventions on risk of food allergy are uncertain.
Further work is needed to understand whether different approaches to infant skin care might promote or prevent eczema and to evaluate effects on food allergy based on robust outcome assessments.
Plain language summary
Skin care interventions for preventing eczema and food allergy
Does moisturising baby skin prevent eczema or food allergies?
Key messages
Skin care treatments in babies, such as using moisturisers on the skin during the first year of life, probably do not stop them from developing eczema, and probably increase the chance of skin infection.
We are uncertain how skin care treatments might affect the chances of developing a food allergy. We need evidence from well‐conducted studies to determine effects of skin care on food allergies in babies.
What are allergies?
An immune response is how the body recognises and defends itself against substances that appear harmful. An allergy is a reaction of the body's immune system to a particular food or substance (an allergen) that is usually harmless. Different allergies affect different parts of the body, and their effects can be mild or serious.
Food allergies and eczema
Eczema is a common skin allergy that causes dry, itchy, cracked skin. Eczema is common in children, often developing before their first birthday. It is sometimes a long‐lasting condition, but it may improve or clear as a child gets older.
Allergies to food can cause itching in the mouth, a raised itchy red rash, swelling of the face, stomach symptoms or difficulty breathing. They usually happen within 2 hours after a food is eaten.
People with food allergies often have other allergic conditions, such as asthma, hay fever, and eczema.
Why we did this Cochrane Review
We wanted to learn how skin care affects the risk of a baby developing eczema or food allergies. Skin care treatments included:
• putting moisturisers on a baby's skin;
• bathing babies with water containing moisturisers or moisturising oils;
• advising parents to use less soap, or to bathe their child less often; and
• using water softeners.
We also wanted to know if these skin care treatments cause any unwanted effects.
What did we do?
We searched for studies of different types of skin care for healthy babies (aged up to one year) with no previous food allergy, eczema, or other skin condition.
Search date: we included evidence published up to July 2020.
We were interested in studies that reported:
• how many children developed eczema, or food allergy, by age one to three years;
• how severe the eczema was (assessed by a researcher and by parents);
• how long it took for eczema to develop;
• parents' reports of immediate (under two hours) reactions to a food allergen;
• how many children developed sensitivity to a particular food allergen; and
• any unwanted effects.
We assessed the strengths and weaknesses of each study to determine how reliable the results might be. We then combined the results of all relevant studies and looked at overall effects.
What we found
We found 33 studies involving 25,827 babies. These studies took place in Europe, Australia, Japan, and the USA, most often at children's hospitals. Skin care was compared against no skin care or care as usual (standard care). Treatment and follow‐up times ranged from 24 hours to two years. Many studies (13) tested the use of moisturisers; others mainly tested the use of bathing and cleansing products and how often they were used.
We combined the results of 11 studies; eight included babies thought to have high risk of developing eczema or a food allergy.
What are the main results of our review?
Compared to no skin care or standard care, moisturisers:
• probably do not change the risk of developing eczema by the age of one to two years (evidence from 7 studies in 3075 babies) nor the time needed for eczema to develop (9 studies; 3349 babies);
• may slightly increase the number of immediate reactions to a common food allergen at two years, as reported by parents (1 study; 1171 babies);
• probably cause more skin infections (6 studies; 2728 babies);
• may increase unwanted effects, such as a stinging feeling or an allergic reaction to moisturisers (4 studies; 343 babies); and
• may increase the chance of babies slipping (4 studies; 2538 babies).
We are uncertain whether skin care treatments affect the chance of developing a food allergy as assessed by a researcher (1 study; 996 babies) or sensitivity to food allergens (2 studies; 1055 babies) at age one to two years.
Confidence in our results
We are moderately confident in our results for developing eczema and the time needed to develop eczema. These results might change if more evidence becomes available. We are less confident about our results for food allergy or sensitivity, which are based on small numbers of studies with widely varied results. These results are likely to change when more evidence is available. Our confidence in our findings for skin infections is moderate but is low for stinging or allergic reactions and slipping.
Summary of findings
Summary of findings 1. Skin care intervention compared to standard skin care or no skin care intervention for the prevention of eczema and food allergy.
|
Patient or population: infants age 12 months or younger Setting: prevention Intervention: skin care intervention Comparison: standard skin care or no skin care intervention |
|||||||
| Assumed risk | Corresponding risk | ||||||
| Outcome | Standard care | Skin care intervention | Relative effect (95% CI) | No. participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Eczema diagnosis by 1 to 2 years | 150 per 1000 | 155 per 1000 (122 to 197) | RR 1.03 (0.81 to 1.31) | 3075 (7) | MODERATEa | In sensitivity analysis that included studies that measured eczema using Hanifin and Rajka, or UK Working Party methods only, total N = 2919(6), the pooled treatment effect for eczema by 1 to 2 years was RR 1.02, 95% CI 0.78 to 1.34. In a separate sensitivity analysis including studies rated as low risk of bias only, total N = 1739(3), the pooled treatment effect for eczema by 1 to 2 years was RR 0.97, 95% CI 0.81 to 1.17 | |
| IgE‐mediated food allergy (oral food challenge) by 1 to 2 years | 50 per 1000 | 127 per 1000 (50 to 335) | RR 2.53 (0.99 to 6.47) | 996 (1) | VERY LOWb | In a sensitivity analysis that examined IgE‐mediated food allergy as measured by oral food challenge or based upon a panel assessment of clinical history and/or allergic sensitisation by 1 to 2 years, total N = 1115 (1), was RR = 1.46, 95% CI 0.91 to 2.34. For parent report of a doctor diagnosis of food allergy at 1 to 2 years, total N = 1614 (3), and the pooled treatment effect was RR 1.02, 95% CI 0.80 to 1.31. No low risk of bias sensitivity analysis was possible | |
| Slippages (over the intervention period) | 20 per 1000 | 29 per 1000 (14 to 87) | RR 1.42 (0.67 to 2.99) | 2538 (4) | LOWc | ‐ | |
| Skin infection (over the intervention period) | 50 per 1000 | 67 per 1000 (51 to 89) | RR 1.34 (1.02 to 1.77) | 2728 (6) | MODERATEd | ‐ | |
| Stinging/allergic reactions to moisturisers (over the intervention period) | 40 per 1000 | 90 per 1000 (27 to 298) | RR 2.24 (0.67 to 7.43) | 343 (4) | LOWc | ‐ | |
| Time to onset of eczema | 24 months | 27.9 months (21.1 to 36.9 months) | HR 0.86 (0.65 to 1.14) | 3349 (9) | MODERATEe | ‐ | |
| Parent report of immediate reaction to common food allergen (at 2 years) | 160 per 1000 | 204 per 1000 (160 to 258) | RR 1.27 (1.00 to 1.61) | 1171 (1) | LOWf | ‐ | |
| Allergic sensitisation to a food allergen (at 1 to 2 years) | 90 per 1000 | 78 per 1000 (26 to 242) | RR 0.86 (0.28 to 2.69) | 1055 (2) | VERY LOWg | ‐ | |
aDowngraded one level for heterogeneity driven by one trial contributing 21.8% of the weight of the analysis, for which the review authors were unable to identify a plausible explanation.
bDowngraded one level for overall high risk of bias due to missing data (29%), and two levels for imprecision due to small numbers of events from a single study, with wide confidence intervals, which include both a harmful effect and no effect.
cDowngraded by two levels for imprecision due to small numbers of events, with wide confidence intervals, which include both a harmful effect and a beneficial effect.
dDowngraded by one level for imprecision due to wide confidence intervals, which include both a harmful effect and no effect.
eDowngraded one level for heterogeneity driven by more than one trial, for which review authors were unable to identify a plausible explanation.
fDowngraded two levels for imprecision due to small numbers of events from a single study, with wide confidence intervals, which include both a harmful effect and no effect.
gDowngraded one level for heterogeneity, for which the review authors were unable to identify a plausible explanation, and two levels for imprecision due to wide confidence intervals, which include both a harmful and a beneficial effect.
Background
Please see Table 2 for explanations of specific terms used in this review.
1. Glossary of terms.
| Term | Definition |
| Adolescence | A period in development, roughly between ages 10 and 19 years, between onset of puberty and acceptance of adult identity and behaviour |
| Allergic (atopic) march | Typical pattern of onset of allergic disease from eczema, to food allergy, to asthma and allergic rhinitis |
| Allergic rhinitis | Rhinitis is a group of symptoms affecting the nose, typically by sneezing, itching, or congestion. Allergic rhinitis occurs when these symptoms are due to environmental allergies |
| Allergic sensitisation | Demonstrated by a positive skin prick test of specific IgE to a known allergen |
| Anaphylaxis | Acute, potentially life‐threatening immediate reaction to an allergen |
| Angioedema | Pronounced swelling of the deep dermis, subcutaneous or submucosal tissue |
| Atopic dermatitis (atopic eczema) |
Eczema with IgE sensitisation, either by IgE antibody or by skin prick test, is classified as atopic eczema |
| Atopy | Genetic predisposition to develop allergic diseases such as eczema, food allergy, asthma, and allergic rhinitis, often associated with production of IgE antibodies |
| Ceramides | Lipid (fatty) molecules found in the lipid bilayer of the intercellular matrix |
| Eczema | Complex chronic skin condition characterised by itch, a form of dermatitis |
| Filaggrin gene (FLG) | Gene encoding for filaggrin, which is a filament‐binding protein in the skin |
| Flare | In eczema, a period of worsening of signs and symptoms of eczema |
| Food allergy | Adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food. Can be IgE‐mediated or non‐IgE‐mediated |
| Food sensitisation | Production of IgE to a food, in the form of a positive skin prick test or immunoglobulin E; may not equate to food allergy |
| Humectant | Substance or product that draws water towards it |
| Immunoglobulin E (IgE) | Class of antibody that plays a key role in allergic disease. Signs and symptoms of IgE‐mediated disease include urticaria, angioedema, wheeze, anaphylaxis |
| Infant | A baby in the first year of life |
| Inhalant allergen | Allergen that typically enters the immune system via the respiratory tract and is airborne, such as house dust mite or pollen |
| Mast cell | Granular basophil cell present in connective tissue that releases histamine and other mediators in allergic reactions |
| Neonate | A baby in the first 28 days of life |
| Phenotype | Observable characteristics from an interaction between genes and the environment |
| Prevalence | In statistics, refers to the number of cases of a disease, present in a particular population at a given time |
| Quality of life | Defined by WHO as individuals' perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns |
| Transepidermal water loss (TEWL) | Non‐invasive measurement of water loss across the epidermis used as a measure of skin barrier function |
| Urticaria | Rash that is a transient erythematous itchy swelling of skin |
Description of the condition
Allergic diseases such as eczema and food allergy are some of the most common long‐term health conditions in children and young people (Bai 2017; Van Cleave 2010). There is no definitive cure for allergic disease, although treatments can be used to alleviate symptoms. The burden of allergic disease on the individual, the family, and society is significant (Gupta 2004; Pawankar 2014). The prevalence of allergic disease appears to have increased; traditionally, higher prevalence was seen in high‐income countries, but prevalence of allergic disease is now increasing in urban cities of low‐ and middle‐income countries (Deckers 2012; Prescott 2013).
Eczema is a chronic inflammatory skin disorder, diagnosed clinically based on a collection of symptoms, primarily including itch. Its aetiology is complex and involves interaction between genes, environment, the immune system, and impairment of the skin barrier (Leung 2004). Eczema with immunoglobulin (Ig)E sensitisation, either by IgE antibody or by skin prick test, is classified as atopic eczema (Johansson 2003). This review is focused on prevention of eczema and food allergy in infants and children and does not address adult‐onset eczema, which has different associations from childhood atopic eczema (Abuabara 2019). Likewise this review does not address adult‐onset food allergy, which accounts for a small proportion of food allergy among adults, resulting in loss of tolerance to a food that the adult previously tolerated (Ramesh 2017).
Atopic eczema (atopic dermatitis) is most often associated with other atopic diseases and typically presents in younger children; it may be the first step along the so called 'allergic march' (Leung 2004). Eczema often occurs in families with atopic diseases such as asthma, allergic rhinitis/hay fever (and food allergy), and atopic eczema. These diseases share a common pathogenesis and are frequently present together in the same individual and family. The word 'atopy' refers to the genetic tendency to produce IgE antibodies in response to small quantities of common environmental proteins such as pollen, house dust mites, and food allergens (Stone 2002; Thomsen 2015). Around 30% of people with eczema develop asthma, and 35% develop allergic rhinitis (Luoma 1983). However, it is known that atopy does not concurrently occur in all people with atopic eczema. In view of this, it has been proposed that the term 'eczema' should be used to define people both with and without atopy. In agreement with the 'Revised nomenclature for allergy for global use' (Johansson 2003), and similar to other Cochrane Reviews evaluating eczema therapies (Van Zuuren 2017), we will therefore use the term 'eczema' throughout the review.
The main mechanism of this disease is the combination of an epidermal barrier function defect and cutaneous inflammation. Barrier dysfunction can be attributed in part to a genetic susceptibility, such as a mutation in the filaggrin gene (FLG). Cutaneous inflammation is demonstrated by inflammatory cell infiltration of the dermis, predominantly by Th2 cells (Weidinger 2016).
Eczema is diagnosed clinically by its appearance and its predilection for certain skin sites, which is age‐dependent (Spergel 2003). In a research setting, the most commonly used diagnostic criteria are the UK Working Party Diagnostic Criteria for Atopic Dermatitis (Williams 1994). Prevalence of eczema is reported at up to 20% in children and may be increasing (Flohr 2014). Eczema has a significant impact on the patient and the family. In childhood, eczema is often associated with sleep disturbance and behavioural difficulties. Eczema also significantly impacts the quality of life of parents of affected children. Partaking in their children's treatment can take up to two hours per day, their own sleep is often disturbed along with their child's, and this exacerbates the distress experienced (Carroll 2005). The impact of moderate to severe eczema on family dynamics is comparable to that of other chronic health conditions such as type 1 diabetes (Su 1997). The financial cost of childhood eczema incorporates both the direct cost of the child's care and the indirect costs of parental time off work and decreased productivity due to decreased sleep and increased stress. The total cost of eczema care in the USA has been estimated at over USD 5 billion per annum (Drucker 2017).
Eczema often improves during childhood, with more than 50% of childhood eczema resolving by adolescence (Williams 1998). Recent studies suggest that some aspects of skin barrier and immune dysfunction may persist into adulthood (Abuabara 2018). Adult eczema is estimated at approximately 5% in the USA and 2% in Japan (Barbarot 2018). Adults with eczema have significantly decreased social functioning and greater psychological distress than both the general population and adults with some other long‐term conditions (Carroll 2005). In a recent systematic review, a positive association was seen between eczema and suicidal ideation in adults and adolescents. It was proposed that chronic itch, sleep disturbance, and the social stigma of a visible disease contribute to mental health effects (Ronnstad 2018).
As is seen in most disease prevalence studies, reported prevalence of eczema may vary depending on the location of the trial and variation in measurements used for classification and diagnosis. Using consistent measurements, the International Study of Asthma and Allergies in Childhood (ISAAC) has shown an increase in reporting of eczema across different settings and in different populations apart from those with already high prevalence (Asher 2006). Admittedly, the youngest children in this cohort were six to seven years old ‐ not preschool age, at which eczema prevalence can be higher. This variation in reported prevalence between different regions and over time suggests that environmental influences may contribute significantly to disease prevalence. Eczema has been associated with smaller families, higher social class, and urban living. Children of immigrants moving from a country with low eczema prevalence to a country with higher eczema prevalence have a relatively higher prevalence of eczema, supporting a role for environmental factors acting during early life (Martin 2013). Family history of eczema, that is, genetics, is the strongest determinant of eczema, and it cannot be modified (Apfelbacher 2011). However interaction of genes with environmental factors may be influenced by skin barrier interventions.
Food allergy has been defined as an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food (Boyce 2010). Food allergy can be further classified into IgE‐mediated, non‐IgE‐mediated, and mixed types. IgE‐mediated food allergy typically occurs within two hours of exposure to the offending food, and symptoms are well characterised, ranging from minor oral or gastrointestinal symptoms, urticaria, or angioedema to more severe symptoms such as anaphylaxis, which can occasionally result in death (Boyce 2010). IgE‐mediated reactions involve degranulation of mast cells, and the condition is diagnosed by a clinical history supported by skin prick or serum‐specific IgE testing. A positive test alone indicates sensitisation to the food but does not always predict clinical reactivity. The titre of IgE or the size of the skin prick test wheal is a predictor of clinical reactivity, although not an indication of the severity of a reaction. Oral food challenges ‐ either open or blinded placebo‐controlled challenges ‐ are used to confirm the diagnosis in cases where the clinical history and test results are inconclusive (Bock 1988). Non‐IgE‐mediated food allergy and mixed food allergies have a slower onset and less specific symptoms. Diagnosis is more difficult and relies on clinical history supported by exclusion or reintroduction of suspected foods, or both (Johansson 2003). It is unclear whether non‐IgE‐mediated allergies have the same association with skin barrier function and eczema; therefore we will not consider non‐IgE‐mediated food allergies in this review.
Exact prevalence rates for food allergy are difficult to ascertain and are largely dependent on the method used to diagnose food allergy and the population studied. Self‐reported food allergy rates are generally higher than those confirmed by specific allergy testing (Woods 2002). Previous population‐based studies have suggested that IgE‐mediated food allergy affects around 3% to 10% of children (Kelleher 2016; Osbourne 2011; Venter 2008). For some people, food allergy can resolve spontaneously during childhood, particularly for foods such as milk and egg. However a recent US survey study identified a history suggestive of IgE‐mediated food allergy in over 10% of adults, demonstrating that it is not just a disease of childhood (Gupta 2019). Like eczema, food allergy is thought to have increased in prevalence in recent decades, although epidemiological data from the 1990s onwards in England and Australia suggest that food allergy prevalence in young children may be stable (Peters 2018 ; Prescott 2013; Sicherer 2003; Venter 2008). Food allergy also varies in prevalence across different regions, with lower prevalence in areas with lower overall rates of allergic disease, such as rural settings in Asia and Africa (Botha 2019; Prescott 2013).
Food allergy is a considerable burden on the individual, the family, and wider society. Acute reactions can cause significant anxiety and when severe may rarely result in a fatal outcome within minutes of food ingestion (Umasunthar 2013). The continuous vigilance required to avoid potential triggers has an adverse impact on quality of life of allergic children and adults and their families (Knibb 2010). People with food allergy and their carers report a negative impact of dietary restrictions, limitations to social activities, and an emotional and financial burden of living with food allergy. For example, in the USA, the financial cost of food allergy for affected families and healthcare providers has been estimated as at least USD 25 billion per annum (Gupta 2013). In recent decades, numbers of hospital admissions for food‐related anaphylaxis have increased. It is unclear however whether this represents a true increase in incidence or greater recognition of the potential for acute food allergy as a cause of symptoms, as there has not been a concomitant increase in fatal anaphylaxis (Jerschow 2014; Poulos 2007; Turner 2015).
Eczema and food allergy are closely associated. Both conditions typically begin during the first year of life. Genetic variations that damage skin barrier function are associated with both eczema and food allergy (Palmer 2006; Van den Oord 2009). In particular, FLG mutation ‐ a mutation in the gene encoding for filaggrin binding protein in the epidermis ‐ has been the most widely studied of the genes associated with atopy. Those with a mutation have significantly increased prevalence of eczema and food allergy (Irvine 2011). Animal studies demonstrate that exposure to food allergen across a damaged skin barrier predisposes to food sensitisation (Strid 2004; Strid 2005). Human observational studies support an onset timing and severity‐dependent relationship between childhood eczema and risk of food allergy. In Martin 2015, over 50% of infants who needed prescription topical steroids before three months of age for treatment of eczema were IgE‐sensitised to one or more of egg white, peanut, or sesame. This study was included in a systematic review, which demonstrated a strong dose‐dependent relationship between eczema food sensitisation and food allergy, and suggested that eczema may be an important cause of food allergy (Tsakok 2016).
With regards to primary prevention of eczema, some studies have suggested that maternal supplementation with a probiotic supplement during pregnancy and breastfeeding may reduce risk of eczema (Garcia‐Larsen 2018). However the mechanism of action of such an intervention is unclear, findings are inconsistent between trials, and few of the relevant studies have published protocols that confirm the absence of selective reporting.
With regards to primary prevention of food allergy, it has been shown that early introduction of allergenic foods such as egg and peanut can decrease the risk of allergy to those foods (Du Toit 2015; Ierodiakonou 2016; Natsume 2017; Perkin 2016). However, it is unclear whether this approach will reduce the prevalence of food allergy at a population level because applying the intervention to multiple foods is likely to be too onerous for some parents (Voorheis 2019), and some children already have allergy to the food before the age when complementary foods are usually introduced (Du Toit 2015).
New approaches are therefore required for prevention of eczema and food allergy; simple interventions designed to promote skin barrier function represent one potential approach.
Description of the intervention
In this review we included all interventions designed to improve the skin barrier in infants, either by enhancement or by promotion of the barrier through hydration via directly applied topical products such as emollients or moisturisers or through reduction of potential damage to the skin barrier and consequent dryness through various means such as avoiding soaps or reducing water hardness. We expected promotion of the skin barrier and skin hydration through topical emollients would be the most widely used intervention. Emollients are described as mainly lipid‐based products that smooth the skin, whereas moisturisers give water and moisture to the skin (Penzer 2012). However, sometimes 'emollient' is referred to as an ingredient of 'moisturisers' (Lodén 2012). There is not yet a clear nomenclature for topical preparations for the skin. The terms 'moisturiser' and 'emollient' are used interchangeably in different settings to describe directly applied topical products. Several different 'classes' or 'formulations' of emollients and moisturisers are available, including oil‐in‐water creams, water‐in‐oil creams, ointments, lotions, oils, gels, sprays, and emulsions (Van Zuuren 2017). However these may not reflect accurately the format, ingredient, and effects of the product. Further complicating this is the fact that many skin care products are classed as cosmetics and therefore are not subjected to the same regulations as medicines. A recently proposed classification includes considering the vehicle, the formulation, and the active ingredients (Surber 2017).
Emollients themselves may be categorised by their mode of use, as leave‐on emollients that are directly applied to the skin and allowed to dry in; as soap substitutes whereby an emollient may be used instead of a soap to clean; and as bath oils or emollients by which a product is added to the bath water (Van Zuuren 2017). In this review we expect most intervention trials to use leave‐on emollients, although the characteristics of emollients may vary.
As part of treatment for established eczema, emollients are recommended to be applied two to three times a day, at up to 150 g to 200 g per week in young children and up to 500 g in adults (Eichenfield 2014; Ring 2012). Overall, emollients are regarded as safe, with few adverse effects. However, daily application of sufficient emollient can be time‐consuming and unpleasant, having a negative impact on the child and the family (Carroll 2005). Certain emollients can cause stinging, especially to skin with established eczema (Oakley 2016). There is concern that emollients can actively sensitise to their individual components, leading to cutaneous reactions (Danby 2011), and even systemic allergic reactions (Voskamp 2014). Slippage of infants covered in emollient from the hands of carers is a stated potential adverse reaction in emollient prevention studies such as the BEEP study (Chalmers 2017).
Protection of the skin barrier could also be achieved by limiting water loss across the skin, or by limiting skin contact with potentially harmful substances or irritants. Activities and substances that may harm the skin barrier, at least in people with established eczema, include excessive bathing, wash products, and hard water (Cork 2002). Thus, ameliorating any of these factors in the first months of life may potentially improve hydration and skin barrier function, thereby reducing subsequent eczema prevalence.
Neonatal skin is different from the skin of children and adults, as it takes time to adjust to the dry extrauterine environment during the postnatal period (Cooke 2018). Postnatal maturation of skin structure and physiology can take up to a year, with regional differences in maturation, with cheek skin maturing more slowly than skin at other sites (McAleer 2018). However, very early neonatal skin has decreased water permeability compared to the skin of older children and adults, along with decreased surface pH and stratum corneum formation, demonstrating an effective skin barrier in the first two days of life, which changes rapidly (Yosipovitch 2000). It was previously thought that infant skin beyond the first few weeks following birth was structurally and functionally equivalent to the skin of adults; however skin undergoes a maturation process that can last for several years after birth (Chiou 2004; Stamatas 2011; Visscher 2017). This process involves higher keratinocyte proliferation and desquamation rates with impaired keratinocyte differentiation compared to adults (Liu 2018; Stamatas 2010). The increased keratinocyte cell turnover results in smaller corneocytes and a thinner stratum corneum (Stamatas 2010). These changes in the stratum corneum create a shorter path for penetration of irritants and allergens through the skin of normal babies. The increased permeability of a baby's stratum corneum compared to that of an adult is reflected in higher transepidermal water loss (TEWL) rates (Nikolovski 2008). This higher stratum corneum permeability is likely to be an important factor in the development of eczema early in life. Infants, with their thinner skin and an increased body surface area‐to‐volume ratio compared with adults, may be more susceptible to percutaneous uptake of any potentially harmful substances (Mancini 2008).
Standard care for neonatal and infant skin differs internationally and is affected by cultural factors. The World Health Organization recommends not bathing newborn infants in the first 24 hours after birth but does not recommend any specific method of infant skin care beyond this time (WHO 2015). In the UK, standard skin care advice given to parents of newborns is to wash in plain water for the first month and to use a mild non‐perfumed soap if one is required. What constitutes a 'mild soap' is not described, and there is no set recommendation for bathing frequency or use of moisturisers (NICE 2006). Few emollient studies have included term infants; most have incorporated premature infants, whose skin is different from the skin of term infants (Irvin 2015). Application of an emollient or oil to the skin of newborn infants is practised in some regions and cultures for a variety of reasons often unrelated to allergy prevention (Amare 2015).
Timing of the first bath in neonates may be important. In some areas of the world, infants are washed immediately after birth, but the World Health Organization recommends leaving the vernix caseosa intact and allowing it to wear off with normal handling (WHO 2015). When modes of washing were compared, a comparison of infant bathing with water versus washing with a cotton wash cloth did not demonstrate a significant difference in skin barrier properties after four weeks but did show regional differences in skin barrier properties and demonstrated dynamic adaption of the skin barrier over the first four weeks of life (Garcia Bartels 2009). Among neonates bathed twice weekly, those washed in age‐appropriate liquid cleanser with added cream had lower transepidermal water loss (TEWL) than those washed with water only, whereas stratum corneum hydration was similar. Whether this shows improvement in the skin barrier is unclear (Garcia Bartels 2010). Although specific wash products or moisturiser ingredients such as sodium laureth sulfate are thought to be harmful, plain water or wash products without known skin irritants are thought to be safe, other than the risk of slippages with oil‐based products (Blume‐Peytavi 2016). Some groups recommend pharmaceutical‐grade oils or specially formulated baby skin products over locally produced oils that are traditionally used in many parts of the world (Blume‐Peytavi 2016). However, such recommendations sometimes come from industry‐funded groups, and there is little direct evidence to suggest that traditional local oils are inferior to commercial products. Frequency and timing of infant bathing may vary by culture and region, and although excessively frequent infant bathing is thought to harm skin barrier function and physiology, the optimal frequency of infant washing or bathing is not known.
Hard water is relatively rich in calcium and magnesium, and water hardness varies depending on geographical location. Water of a certain hardness will cause limescale and may corrode pipes (Ewence 2011). Hard water is associated with increased eczema prevalence (Engebretsen 2017). It is thought that the skin barrier disruption associated with hard water is due to the interaction between surfactants in wash products and hard water itself (Danby 2018).
This review covers all potential skin care interventions designed to promote, or reduce damage to, the skin barrier and to enhance skin hydration for the primary prevention of eczema and food allergy.
How the intervention might work
Emollients, as one intervention, are the mainstay of treatment for those with already established eczema, as detailed in a Cochrane Review (Van Zuuren 2017), because dry skin (xerosis) is a key feature of eczema, and topical moisturisers have an integral role in the standard treatment of eczema of all severities (Eichenfield 2014). Emollients can decrease water loss across the skin (TEWL), increase stratum corneum hydration, improve comfort, and reduce itch when used on skin that already has active eczema (Lodén 2012; Rawlings 2004), and therefore are a key component in treatment of eczema (Ring 2012). They may be more effective than interventions such as less frequent bathing or use of water softeners for eczema prevention.
All moisturisers contain varying amounts of active ingredients such as humectant or ceramide, as well as excipient ingredients such as emulsifiers (Lodén 2012). Humectants, such as glycerol or urea, aid retention and attraction of water by the stratum corneum. Ceramides are intracellular lipids found in the stratum corneum that are reduced in lesional eczematous skin (Meckfessel 2014). Occlusives such as petrolatum form a layer on the skin surface that may prevent TEWL across the stratum corneum and can soften the skin (Eichenfield 2014; Rawlings 2004). Moisturisers can be hydrophilic or lipophilic. Hydrophilic moisturisers attract water and are important for skin hydration, whereas lipophilic moisturisers tend to stay on the surface to aid the skin barrier (Caussin 2009).
Van Zuuren 2017 showed that regular use of emollients for those with eczema can prolong time to eczema flare, can reduce the number of flares, and can reduce the need for topical corticosteroids. In infants, skin barrier dysfunction is seen before the development of clinical eczema (Danby 2011; Flohr 2010). Therefore, applying moisturisers before eczema is noted may offer a route for primary prevention of eczema. Three published pilot studies suggest that applying moisturisers to infant skin might reduce the prevalence of eczema during the application period (Horimukai 2014; Lowe 2018a; Simpson 2014). These pilot studies were small‐scale studies testing the feasibility of the intervention or looking for signals of a preventative effect, or both. They were insufficiently powered for confirming a preventative effect. It is not known whether applying moisturisers could lead to a programming effect on the skin, causing longer‐term effects on skin physiology, immunology, or clinical manifestations of eczema.
The strong association between eczema and food allergy would suggest that reduced clinical manifestations of eczema could potentially reduce the risk of food allergy, even if it were just to delay the onset of eczema from early infancy, where the association with development of food allergy is strongest (Martin 2015). In a small pilot study of a ceramide‐dominant emollient with an action described as a lipid replacement, evidence suggests reduced allergic sensitisation to foods in the per‐protocol analysis of the intervention group (Lowe 2018a).
Mechanistic studies within the clinical trials suggest that emollients can increase stratum corneum hydration when used in healthy infants, but trials have not consistently identified changes in skin pH or TEWL (Yonezawa 2018). It is unclear whether this increase in stratum corneum hydration will lead to reduced skin inflammation and associated allergic sensitisation.
Why it is important to do this review
Preliminary data suggest that variations in infant skin care protection interventions, such as application of emollients, might influence the risk of eczema or food sensitisation, at least during the intervention period (Horimukai 2014; Lowe 2018a; Simpson 2014). This raises the possibility of a relatively simple, cheap, and safe intervention for primary prevention of two common and burdensome conditions. This review is important and timely because larger clinical trials are now formally testing the hypothesis that variations in infant skin care can influence the risk of eczema or food allergy (Chalmers 2020; Skjerven 2020).
At the time this systematic review was initiated, two major ongoing interventional trials were assessing whether skin care interventions in the first year of life will reduce the prevalence of eczema or food allergy; these have both been published. The National Institute for Health Research‐Health Technology Assessment (NIHR‐HTA)‐funded Barrier Enhancement for Eczema Prevention (BEEP) study was designed to assess whether daily application of emollients for the first year of life would reduce the prevalence of eczema or allergic disease in the first five years of life (Chalmers 2017; Chalmers 2020; ISRCTN 21528841). Preventing Atopic Dermatitis and Allergies in Children ‐ the PreventADALL study ‐ is a large, prospective, mother‐child birth cohort study incorporating a randomised controlled 2 x 2 factorial‐designed intervention strategy (skin care and early complementary food introduction) to prevent eczema and food allergy (Lødrup 2018; NCT02449850; Skjerven 2020). This study will report food allergy outcomes after all assessments at age three years have been completed.
The BEEP study was powered to detect a difference in eczema during the second year. However, statistical power within this sample size was limited for other outcomes such as food allergy, and for subgroup analyses. For example, BEEP had 80% power at two‐sided alpha of 0.05 for detecting a 50% reduction in food allergy. BEEP was a pragmatic study, which could further limit statistical power if compliance with recommended skin care advice in that setting was low.
PreventADALL was powered to detect a difference in eczema during the second year. PreventADALL also had limited statistical power for other outcomes such as food allergy, and for subgroup analyses. Several other smaller studies of primary prevention of eczema or food allergy were ongoing at the time this systematic review was initiated ‐ in Australia (ACTRN12613000472774), Germany (NCT03376243), Japan,(JPRN‐UMIN000004544; JPRN‐UMIN000010838; JPRN‐UMIN000013260), and the USA (NCT01375205). Many have now published and are included in this review (Dissanayake 2019; Lowe 2018a; McClanahan 2019; Yonezawa 2018).
This systematic review aimed to determine whether infant skin care interventions influence eczema or food allergy prevalence. We undertook an individual participant data (IPD) meta‐analysis. This type of meta‐analysis is considered the gold standard for systematic reviews. Database and analysis errors in individual trials can be identified and potentially corrected. IPD meta‐analysis also allows (i) fitting of a consistent analysis model to all trial data sets for each outcome to ensure that we are comparing treatment effects that are adjusted for the same covariates across trials; (ii) obtaining more reliable and powerful subgroup analyses; and (iii) better evaluating the relationship between compliance with the intervention and outcomes of interest (Stewart 2002). This systematic review also incorporated a prospectively planned component whereby two of the main studies aligned outcomes and details of the meta‐analysis were planned before the results of each trial were known. Prospectively Planned Meta‐Analysis (PPMA) aims to reduce bias related to knowledge of existing trial outcomes. Sharing clinical trial data is encouraged as best practice in clinical trials, and sharing of individual participant data maximises knowledge gained through the efforts of trial participants (Taichman 2017).
Objectives
Primary objective
To assess effects of skin care interventions, such as emollients, for primary prevention of eczema and food allergy in infants.
Secondary objective
To identify features of study populations such as age, hereditary risk and adherence to interventions that are associated with the greatest treatment benefit or harm for both eczema and food allergy.
Methods
Criteria for considering studies for this review
Types of studies
Parallel‐group or factorial randomised controlled trials (RCTs), including both individual and cluster‐randomised trials. Quasi‐RCTs and controlled clinical trials were excluded. Cross‐over trials were also excluded, as the design is inappropriate to the clinical context.
Types of participants
Infants (age 12 months or younger). As this is a primary prevention review, we did not include studies on infants who already had diagnosed eczema or food allergy at the time of randomisation. We excluded study populations defined by a pre‐existing health state in the infant, such as preterm birth (less than 37 weeks' gestation) or congenital skin conditions, because findings in these populations may not be generalisable.
We attempted to obtain individual participant data for all included studies. If individual participant data were not available, we obtained aggregate data instead. For studies with only aggregate data available, we excluded the whole study if some participants were not eligible, unless ineligible participants made up an insignificant proportion of the total group, that is, less than 5%. In trials with individual participant data, we planned to include only the data on participants who meet our eligibility criteria; however no exclusions were necessary, as all obtained individual participant data were eligible.
Types of interventions
All skin care interventions that could potentially enhance skin barrier function, reduce dryness, or reduce subclinical inflammation. These include:
moisturisers/emollients;
bathing products (these may include oils or emollients);
advice regarding reducing soap exposure and bathing frequency; and
use of water softeners.
Interventions could be simple single interventions; others could be complex interventions that utilise a combination of measures to protect or promote skin barrier function and hydration or to reduce subclinical inflammation. The comparators were no treatment intervention or advice, or standard care, in the study setting. We excluded multi‐faceted interventions, whereby the skin care component was only a small part of the study if the skin care component was likely trivial or irrelevant to the outcome. We also planned to assess separately those interventions that primarily aim to enhance the skin barrier through direct application of emollient or moisturiser (skin care intervention A) and those that aim to protect the skin barrier from irritation, that is, through use of water softeners (skin care intervention B). However, we did not find any eligible studies for type B.
Types of outcome measures
No minimum follow‐up was required. However, we separately analysed outcomes that related to symptoms during the intervention period and outcomes that occurred and were reported after the intervention period, when appropriate and feasible.
Primary outcomes
Eczema. When multiple measures were reported, the hierarchy of diagnosis was investigator assessment as described by the Hanifin and Rajka criteria in their original form (Hanifin 1980), or by the UK Working Party refinement of them (Williams 1994), other modifications of the Hanifin and Rajka criteria, doctor diagnosis of eczema, then patient or parent report of eczema
Food allergy. When multiple measures of food allergy were reported, the hierarchy of diagnosis was confirmed IgE‐mediated food allergy diagnosed via oral food challenge, with eligibility for oral food challenge decided as per study protocol, although ideally based on current recommendations (Grabenhenrich 2017). If oral food challenge was not available, then food allergy was as diagnosed by investigator assessment using a combination of clinical history and allergy testing: skin prick testing and serum‐specific IgE. We defined IgE sensitisation as skin test to a food of 3 mm or more, or specific IgE of 0.35 kUa/L or higher. The primary foods of interest were milk, egg, and peanut; however we collected data on any foods that were available from each study
The time point for all food allergy and eczema outcome analyses was by age one to three years using the closest available time point to two years, from each included trial. Adverse event outcomes were measured during the intervention period only. When pooling data from different trials, we considered the relationship between timing of the intervention and timing of the outcome measure, for example, we pooled separately measures of eczema taken during the intervention period and measures of eczema taken after the intervention period has ceased.
As we identified multiple measures of eczema across trials, we conducted sensitivity analysis to look separately at eczema measured using the Hanifin and Rajka criteria in their original form (Hanifin 1980), or the UK Working Party refinement of them (Williams 1994), and other modifications of the Hanifin and Rajka criteria only. We had planned to separately look at food allergy measured using secure diagnosis of food allergy by oral food challenge in a sensitivity analysis, if necessary.
Secondary outcomes
Adverse events, including skin infection during the intervention period; stinging or allergic reactions to moisturisers; or slippage accidents around the time of bathing or application of emollient. We will report all serious adverse events
Eczema severity: clinician‐assessed using EASI (Eczema Area and Severity Index) or a similarly validated method (Hanifin 2001)
Parent‐reported eczema severity using POEM (Patient Orientated Eczema Measure) or a similarly validated patient‐reported measure (Charman 2004)
Time to onset of eczema
Parent report of immediate (less than two hours) reaction to a known food allergen: milk, soya, wheat, fish, seafood, peanut, tree nut, egg, or local common food allergen
Allergic sensitisation to foods and inhalants via skin prick test (or, if not available, via serum‐specific IgE)
When available, from each trial, we analysed any relevant core outcomes identified as part of the Cochrane Skin COUSIN and HOME initiatives (www.homeforeczema.org). Relevant HOME domains include clinician signs measured using the EASI instrument, patient‐reported symptoms using the POEM instrument, long‐term disease control, and quality of life. These outcomes were designed for trials involving those with established eczema. There is not yet a set of core outcomes for defining eczema or food allergy in prevention studies; however, for eczema, a modified version of the UK Hanifin and Rajka criteria has been proposed to differentiate between an incident diagnosis of eczema and transient eczematous rashes of infancy (Simpson 2012). When feasible, we contacted trial authors early in the design or set‐up of their trial to encourage sharing of outcome assessment methods, instruments used, and timing. We did not include long‐term disease control or quality of life outcomes in this review.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 14 July 2020, using strategies based on the draft strategy for MEDLINE in our published protocol (Kelleher 2020).
Cochrane Skin Specialised Register, using the search strategy in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 7), in the Cochrane Library, using the strategy in Appendix 2.
MEDLINE via Ovid (from 1946 onwards), using the strategy in Appendix 3.
Embase via Ovid (from 1974 onwards), using the strategy in Appendix 4.
Trials registers
We (MK, SC, and LT) searched the following trials registers on 25 October 2019, and to update, again on 23 July 2020.
ClinicalTrials.gov (www.clinicaltrials.gov), using the draft search strategy in Appendix 5.
World Health Organization International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/), using the draft strategy in Appendix 6.
Searching other resources
Conference proceedings: we reviewed the proceedings of Asia Pacific Association of Pediatric Allergy, Respirology & Immunology Conferences (APAPARI) for 2018, 2019, and 2020.
Searching reference lists: we checked the bibliographies of included trials and identified relevant systematic reviews for further references to relevant RCTs.
Adverse effects: we did not perform a separate search for adverse effects of interventions used for prevention of eczema and food allergy. We considered only adverse effects described in included trials.
Data collection and analysis
This systematic review was undertaken according to the methods recommended by Cochrane, including the updated Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Stewart 2019), with special attention to Chapter 26, 'Individual Patient Data'. A summary record of prospectively planned components of the meta‐analysis was registered on PROSPERO (reference 42017056965; registered 10 February 2017) (Boyle 2017).
Selection of studies
Two review authors (from MK, SC, and LT) independently carried out title, abstract, and full‐text screening, with arbitration by a third review author (RJB) when necessary. In this systematic review, we combined both retrospective and prospectively acquired data in the meta‐analysis. Retrospective data are outcome data acquired, analysed, unblinded, and known to the trial Chief Investigator before registration of the systematic review protocol (PROSPERO reference 42017056965; registered 10 February 2017; Boyle 2017). Prospectively acquired data are those data known to the trial Chief Investigator, in analysed and unblinded form, before 10 February 2017. This systematic review used participant‐level data from all trials when possible. We invited the authors of each included trial to collaborate in accordance with Section 26.2 of the updated Cochrane Handbook for Systematic Reviews of Interventions (Stewart 2019). We asked all trial authors to provide individual participant data. One review author (from LT and MK) sent a data request email to the first and corresponding authors of the associated trial listing the variables required for the analysis (Appendix 7). Following completion of a data sharing agreement, selected variables, or full data sets when appropriate permissions were obtained, were exchanged between researchers along with a data dictionary. If study authors were unable to provide participant‐level data, we accepted appropriate summary data.
Data extraction and management
We conducted data collection and handling in accordance with guidance provided in Chapter 26.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Stewart 2019). For each of the included trials, descriptive data on trial setting, methods, participants, interventions, comparator, length of follow‐up, instruments used for measuring outcomes, funding source, and conflicts of interest were extracted. Two review authors (MK, SC, or LT) independently extracted data using a standardised data collection form, discussing disagreements to reach resolution. When unsuccessful, a third review author (RJB) was consulted. We requested that trial authors who agreed to provide information or data beyond those available in the public sphere share protocol and statistical analysis plan details, along with details of available data fields.
All IPD data used in the systematic review were de‐identified. The list of variables that we requested from each trial is provided in Appendix 7. We transferred specific data fields and then cleaned and coded data for analysis for those trials willing to provide individual participant data. Data sources from previously published trials were provided as anonymised whole databases when trial authors preferred. We carried out range and consistency checks for all data. Any missing data, obvious errors, inconsistencies between variables, or extreme values were queried and rectified with individual trial authors as necessary. We also cross‐checked summaries of provided data with those in published reports of the trial and contacted original trial authors to resolve identified inconsistencies. A secure record was kept of all correspondence, agreements and data transfers with trial authors, and the systematic review database.
For included trials that were unable to provide individual participant data, we recorded the reason for data unavailability and requested aggregate data on our outcomes. If aggregate data could not be obtained directly from the trial authors, two review authors assessed whether any relevant appropriate aggregate‐level data were available in the trial publication or other sources (e.g. clinical trials registry). We recorded aggregate data on a standardised data extraction form. Two review authors (MK, SC) independently extracted data. Any disagreements on extracted aggregate data were discussed and resolved by consensus, and it did not prove necessary for a third review author to arbitrate over data extraction.
The detailed statistical analysis plan for this review was written when data to be collected for the trials providing IPD were known, but before any grouped outcome data from prospective trials had been evaluated (Cro 2020a). The statistical analysis plan was therefore written with consideration of the nature and limitations of the data recorded in trials known to be eligible for inclusion, and the statistician remained blind to intervention and control group outcomes for each data field, so that bias was not introduced by exploring the possible impact of different data analyses and coding decisions on findings.
Assessment of risk of bias in included studies
We assessed risk of bias using the Cochrane 'Risk of bias' 2 tool (Higgins 2018; Higgins 2020b). This tool is designed specifically for RCTs and assesses bias from five domains.
Bias arising from the randomisation process.
Bias due to deviations from intended interventions.
Bias due to missing outcome data.
Bias in measurement of the outcome.
Bias in selection of the reported result.
We assessed the risk of bias separately for eczema (by age one to three years using the closest time point to two years), food allergy (by age one to three years using the closest time point to two years), slippage accidents (during the intervention period), skin infection (during the intervention period), allergic reactions (during the intervention period), time to onset of eczema, parent report of food allergy reaction (at age one to three years using the closest time point to two years), and allergic sensitisation (at age one to three years using the closest time point to two years). The RoB 2 tool is outcome‐specific, and we rated each domain as 'low risk of bias', 'some concerns' or 'high risk of bias'. For bias due to deviations from intended interventions, we were interested in effects of assignment to the interventions at baseline, regardless of whether interventions were received as intended, by an intention‐to‐treat analysis that included all randomised participants. Bias in selection of the reported result was low risk for all prospectively identified studies, as we obtained the full data set for these trials. Risk of bias assessments were not performed for qualitative narrative information.
At the time of writing of this review, the RoB 2 tool for cluster RCTs was under development. For cluster‐RCTs, we therefore similarly assessed risk of bias using the Cochrane 'Risk of bias' tool 2 (Higgins 2018) as outlined above but including an additional domain specific for cluster‐RCTs from the archived version of the RoB 2 tool for cluster‐RCTs (Eldridge 2016) ‐ 'Domain 1b ‐ Bias arising from the timing and identification and recruitment of participants'.
To reach an overall 'risk of bias' judgement for a specific outcome, we used the following criteria.
Overall low risk of bias: all domains considered at low risk for the specific result.
Some concerns: some concerns have been raised in at least one domain for the specific result, but no domains are considered at high risk of bias.
High risk of bias: at least one domain is considered at high risk for the specific result, or there are some concerns for multiple domains, which substantially lowers confidence in the result.
Two review authors (MK and SC) independently conducted 'Risk of bias' assessments with any disagreements resolved via discussion or through arbitration with a third review author (RJB). For a trial for which MK and RJB were investigators (Chalmers 2020), SC and VC independently conducted risk of bias assessments.
Measures of treatment effect
For binary outcomes when meta‐analysis was considered appropriate, we calculated risk ratios (RRs). For continuous outcomes when trials used the same measurement scale, we calculated mean differences (MDs); when trials used different measurement scales, we calculated standardised mean differences (SMDs). For time‐to‐event outcomes, we expressed the intervention effect as a hazard ratio (HR). We computed a 95% confidence interval (CI) for each outcome.
Unit of analysis issues
This review included RCTs only. As elaborated on further below (see Data synthesis), we adopted a two‐stage approach for this IPD meta‐analysis. In stage 1, we separately estimated the treatment effect of interest for each included trial. In stage 2, we pooled treatment effects using methods for meta‐analyses of aggregate data.
Factorial RCTs and cluster‐RCTs were included. For factorial randomised trials, if we noted a significant interaction between the two active interventions with respect to our primary outcome, we included only the arms ‘skin care intervention/control’ versus ‘control/control’. Sensitivity analysis explored the impact of including data from all arms of factorial trials when an interaction was present, with adjustment for non‐skin care interventions.
For other trials with more than two treatment arms (excluding factorial trials, which were handled as described above), which could have multiple intervention groups in a particular meta‐analysis, we combined all relevant intervention groups into a single intervention group and all relevant control groups into a single control group.
For all stage 1 analyses for cluster‐RCTs providing individual participant data, we used mixed models that allow analysis at the level of the individual while accounting for clustering in the data. Treatment effects from cluster‐RCTs were therefore appropriately adjusted for correlation within clusters, before inclusion in the stage 2 (pooled) analysis, following recommendations for analysis of cluster‐RCTs (Higgins 2020a).
For cluster‐RCTs providing non‐individual participant data, we planned to extract data from trial reports that had taken into account the clustering in these data, and to then analyse the data using the generic‐inverse variance method in Review Manager 5 (RevMan 5; Review Manager 2014). If data were not adjusted for clustering, we planned to attempt to estimate the intervention effect by calculating an intracluster correlation coefficient (ICC) while following the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020a).
Dealing with missing data
We dealt with missing data according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019). To resolve missing information about methodological properties of identified trials, we contacted authors of the included trials. When trial authors were unable to provide the required information, we rated the relevant 'Risk of bias' criterion using Cochrane 'Risk of bias 2' (Higgins 2018). We did not anticipate substantial quantities of missing data for the primary outcomes. For trials providing individual participant data, we naturally handled missing participant data under the assumption of missing‐at‐random within each trial analysis.
For trials that did not provide individual participant data and reported an MD but no standard deviation (SD) or other statistic that could be used to derive the SD, we planned to use imputation (Furlan 2009). Specifically, we planned to impute SDs for each outcome by using the pooled SD across all other trials within the same meta‐analysis by treatment group. This is an appropriate method of analysis if a majority of the trials do not have missing SDs in the meta‐analysis. If a large proportion of trials (e.g. ≥ 20%) were missing data on parameter variability for a particular outcome, imputation would not be appropriate, and we planned to conduct analysis using only trials providing complete data and to discuss the implications of this alongside results. However such imputation did not prove necessary.
In risk of bias assessments, to address the impact of non‐negligible missing data (≥ 5%) on individual trial outcomes, we conducted sensitivity analyses using individual participant data and best case/worst case scenarios, that is, we conducted analysis by imputing a best case scenario of response in both treatment groups, followed by analysis under a worst case scenario of no response in both treatment groups. Results of sensitivity analyses under these scenarios were compared to primary complete case analyses (conducted under the missing‐at‐random assumption) to assess the risk of bias due to missing data.
In meta‐analysis, we included trials with substantial quantities of missing data (e.g. rated as high risk of bias or some concerns due to missing data), but to investigate the robustness of pooled results, we performed sensitivity analysis while excluding trials rated overall at high risk of bias or with some concerns, which included excluding trials rated at high risk of bias or some concerns due to missing data.
Assessment of heterogeneity
We examined both clinical and statistical heterogeneity and combined data in meta‐analysis only when we judged that evaluation would yield a meaningful summary. We assessed clinical heterogeneity by examining characteristics of included participants, types of interventions, primary and secondary outcomes, and the follow‐up period. We used the I² statistic and the Chi2 test to quantify the degree of statistical heterogeneity of trials judged as clinically homogeneous (Higgins 2003). For interpretation, we considered an I² of 0% to 40% might not be important heterogeneity; 30 to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and I² greater than 75% indicative of considerable heterogeneity (Deeks 2019). The observed I2 value was judged against this guide in combination with its 95% confidence interval, the P value from the Chi2 test, and the magnitude and direction of effect. When the magnitude and direction of effects and the strength of evidence for heterogeneity based on the P value from the Chi2 confidence intervals for I² revealed heterogeneity, or if we observed considerable heterogeneity, we explored reasons for heterogeneity and when appropriate conducted sensitivity analysis while excluding any trials identified as outlying.
Assessment of reporting biases
By including as many prospective trials as possible in this review, as well as individual participant data, risks of reporting bias and publication bias should be reduced. However, if at least 10 trials were included in the meta‐analysis, we planned to formally assess reporting bias using funnel plots to explore the likelihood of any reporting bias or small‐study effects. We planned to assess funnel plot asymmetry visually and to use formal tests for funnel plot asymmetry. For continuous outcomes, we planned to use the test proposed by Egger 1997. For dichotomous outcomes, we planned to use the test proposed by Rucker when estimated between‐study heterogeneity variance of log odds ratios, tau², is greater than 0.1 (Rucker 2008). Otherwise, when the heterogeneity variance tau² was less than 0.1, we planned to use one of the tests proposed in Harbord 2006. If asymmetry was detected in any of these tests or was suggested by a visual assessment, we planned to explore and discuss possible explanations. However, we did not conduct a meta‐analysis with 10 or more trials included; therefore we did not undertake any formal assessment of reporting bias.
Data synthesis
We conducted an IPD meta‐analysis of both prospective and retrospectively acquired data. Primary meta‐analysis used individual participant data only. Aggregate data were not used in the primary meta‐analysis when individual participant data could not be provided, as the total proportion of participants that made up aggregate data was less than 10% of the overall number of participants across all trials (i.e. total aggregate data represented a negligible proportion of the data set). We performed a sensitivity analysis by adding in the aggregate data, as described further below, to explore the impact of data availability bias. We undertook a prospectively planned meta‐analysis (PPMA) of a more limited number of trials, as a sensitivity analysis. PPMA was limited to those trials in which trial authors were not aware of trial outcomes at the time of PPMA protocol registration on PROSPERO (Boyle 2017).
The main analyses estimated the effect of being assigned to receive the intervention, according to the intention‐to‐treat principle. We retained all eligible participants in the treatment group to which they were originally assigned who had an outcome, irrespective of the treatment they actually received. To understand the effect of compliance, we included pre‐planned secondary supplementary analysis to estimate the complier average causal effect.
We planned to perform all analyses stratified by type of intervention group. Planned comparisons were therefore:
skincare intervention versus no treatment or standard care;
skincare intervention 'A' versus no treatment or standard care; and
skincare intervention 'B' versus no treatment or standard care.
We planned to consider interventions in two broad categories: A interventions promoting hydration and skin barrier mainly through emollients, and B interventions that would protect from harm, such as water softeners or avoidance of irritants. Because our search did not reveal any eligible trials of B‐type skin care interventions, it did not prove necessary to stratify comparisons by type of skin care intervention; therefore, we undertook only comparisons of type A. For each outcome, when we judged a sufficient number of trials (two or more) to be clinically similar, we pooled results in a meta‐analysis. When we did not undertake meta‐analyses owing to clinical heterogeneity or to insufficient data, we narratively discuss the results from individual trials.
We adopted a two‐stage approach to analysis for all primary and secondary analyses. In the first stage, we derived individual trial treatment effect estimates from individual participant data. For analyses of binary outcomes, including both primary outcomes (eczema and food allergy), the stage 1 model, fitted to each trial providing individual participant data separately, was a binomial regression model. For analyses of continuous outcomes, the stage 1 model fitted to each trial providing individual participant data was a linear regression model. For time‐to‐event outcomes, the stage 1 model fitted to each trial providing individual participant data was a binomial regression model with a complementary log‐log link, where follow‐up time was split into appropriate intervals for the obtained data (3 months, 6 months, 12 months, 18 months, and 24 months). This model was appropriate for time‐to‐event data of a discrete nature. In addition to the treatment group variable indicating use of a skin care intervention, we included the important prognostic factors of sex and family history of atopic disease within the stage 1 models.
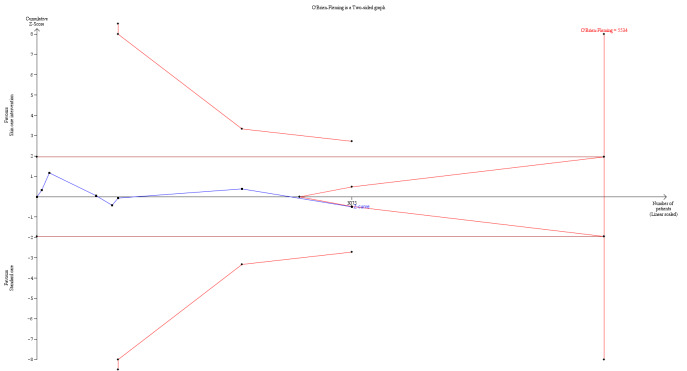
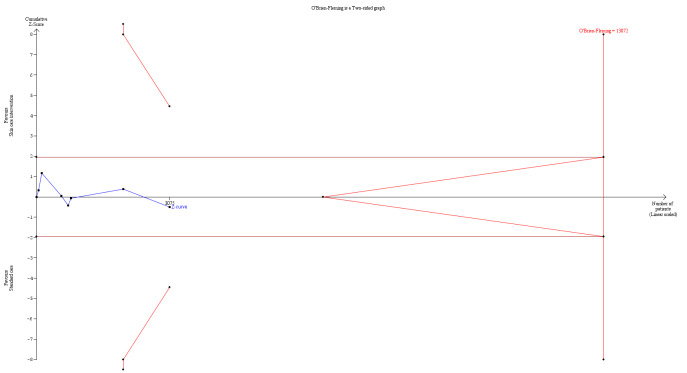
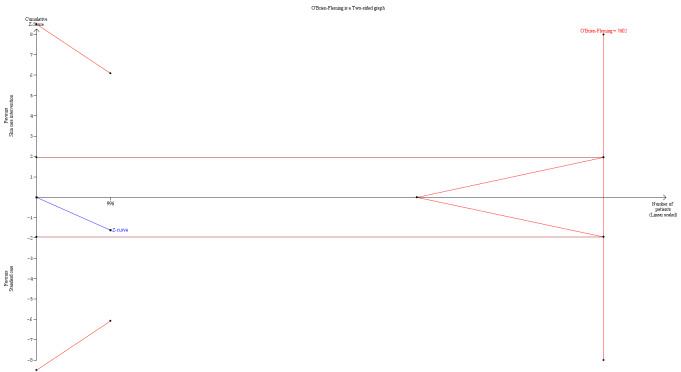
In the second stage, we combined derived treatment effects using methods for meta‐analyses of aggregate data. We used random‐effects models in stage 2 to derive the pooled treatment effect (DerSimonian 1986). We planned to use random‐effects models because we anticipated some level of variability across trials, for example, by types of interventions, length of follow‐up, and methods of measurement. A random‐effects model incorporates heterogeneity among trials and allows the true treatment effect to be different in each trial. In sensitivity analysis, the second stage also included aggregate data from trials whose authors did not provide individual patient data.
We performed residual analysis for all IPD meta‐analyses and PPMAs to assess model assumptions and fit. Meta‐analyses also included trial sequential analysis, using two‐sided 5% significance and 80% power to estimate optimum heterogeneity‐adjusted information sizes needed to identify relative risk reductions of 20% and 30% (Wetterslev 2008). We estimated control event rates using random‐effects meta‐analyses of pooled proportions from the largest trials included in the meta‐analyses and compared them with event rates from large population‐based studies. Trial sequential analysis was used to identify when the optimum information size or futility boundaries for pre‐defined effect sizes in relation to primary outcomes will be reached. We performed stage 1 of the IPD meta‐analysis in Stata 15 or above (Stata), with summary results of these analyses added into RevMan Web (revman.cochrane.org).
To explore the impact of compliance, we estimated the effect of complying with the intended intervention. For the subgroup of trials providing compliance data, we estimated the complier average causal effect (CACE) for each primary outcome. As in the primary analysis, we followed a two‐stage approach to analysis. For each trial, we estimated the CACE using instrumental variable (IV) analysis. We used randomisation as an instrumental variable for intervention received, and we estimated the CACE using a two‐stage residual inclusion estimator approach (2SRI) (Cook 2018). Randomisation meets the criteria for an adequate instrument in that (i) randomisation predicts the treatment receipt, (ii) randomisation is unconfounded with the outcome, and (iii) we assume no direct effect of randomisation on the outcome (other than via treatment receipt): 'the exclusion restriction'. Here, we initially defined a ‘complier’ as an individual who used the prescribed intervention for three or more days a week over the intervention period. When interventions and the quality of compliance data were sufficiently comparable, we used random‐effects models in stage 2 to derive the pooled CACE effect. We repeated the primary analysis for each of the trials in the subgroup of trials with compliance data to compare pooled CACE estimates against the primary treatment effect (RR) while estimating the effect of being assigned to the intervention for the subgroup of trials for which compliance data were available. Subsequently, we explored the impact of different threshold values for defining compliance (≥ 5 days a week over the intervention period, 7 days over the intervention period, ≥ 3 days a week over the first 3 months of the intervention period, ≥ 5 days a week over the first 3 months of the intervention period, and 7 days a week over the first 3 months of the intervention period).
The detailed statistical analysis plan, which set out all comparisons to be made and the precise model forms and fitting strategy to be used, can be consulted for additional information (Cro 2020a). For trials providing only narrative information, or incomplete measures of effect (i.e. no denominators available) when meta‐analysis could not be performed, we summarised available effect estimates or narrative informative alongside meta‐analyses for the same groupings of populations, interventions, outcomes, and study design as were used for the quantitative meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Subgroups of interest that were identified a priori for analysis were as follows.
-
By participant‐level characteristics.
Comparing effects of the intervention on 'high' or 'not high' risk for atopy based on filaggrin genotype.
Comparing effects of the intervention on 'high' or 'not high' risk for atopy based on family history of allergic disease.
-
By study‐level characteristics.
Comparing effects of interventions aimed at preventing damage to the skin (e.g. reduced exposure to soaps, wipes, bathing, hard water) versus interventions aimed at promoting skin hydration or barrier function (e.g. emollient cream, lotion, ointment, oil) versus combined treatment.
Intervention timing: comparing effects of intervention on participants advised to commence the skin care intervention within the first four weeks of life versus those who commenced intervention after four weeks.
Intervention duration: comparing duration of intended treatment, when 'short' is regarded as up to six months of treatment compared to 'longer' treatment durations ‐ six months' duration or longer. When feasible, we planned to undertake modelling to assess the relationship between study outcome and timing or duration of intervention.
We calculated subgroup effects for participant‐level characteristics on the two primary outcomes by first estimating treatment by covariate interaction terms within studies using individual participant data. We then combined interaction terms across studies in the same way as for the main intervention effects, using a random‐effects meta‐analysis. For study‐level characteristics, we pooled treatment effects separately for each characteristic, and we performed a test for subgroup differences using a Chi² test.
Sensitivity analysis
We conducted the following a priori planned sensitivity analyses for the co‐primary outcomes when relevant.
By overall risk of bias: in primary analysis, we included all trials regardless of overall risk of bias, and we undertook a sensitivity analysis of trial outcomes assessed as having an overall low risk of bias. The 'low risk of bias' sensitivity analysis excluded trial outcomes at overall high risk or those with some concerns, assessed via the Cochrane 'Risk of bias' tool 2 (Higgins 2018). This included omitting trial outcomes with high risk of bias and those with some concerns due to missing data, because inclusion would have led to the trial receiving an overall rating that was not low risk of bias.
By outcome measures: we explored the impact of using different definitions of outcome measures by undertaking sensitivity analyses of outcomes that had previously been validated. For the primary outcome eczema, in the absence of agreed core outcomes, we undertook sensitivity analysis of eczema evaluated using only the UK Working Party Criteria (Williams 1994), or other variations of the Hanifin and Rajka criteria (Hanifin 1980). For the primary outcome food allergy, we undertook sensitivity analysis for secure diagnosis of food allergy by oral food challenge or investigator decision using an algorithm developed for the BEEP study.
Including aggregate data from trials that do not provide individual participant data: as aggregate data made up less than 10% of the total number of participants across all trials, our primary analysis included individual participant data only, and we conducted a sensitivity analysis including aggregate data from trials that did not provide individual participant data.
Excluding any data that are not prospectively acquired: prospectively acquired data are data that were not known to the study Chief Investigator, in analysed and unblinded form, before 10 February 2017. PPMA reduces bias related to knowledge of existing trial outcomes, which might influence trial selection in a retrospective study, because trials are included without any knowledge of outcome. Additionally, outcomes across prospectively planned trials were more closely aligned due to awareness of being included in this IPD meta‐analysis. Sensitivity analysis of prospectively acquired data was conducted using the same approach to the primary analysis (i.e. using individual participant data only; see Data synthesis section).
To explore heterogeneity: when considerable statistical heterogeneity was observed (I² > 75%), we explored reasons for heterogeneity and when appropriate conducted sensitivity analysis while excluding any trials identified as outlying. Outlying trials are those with very different trial findings from others reporting comparable interventions/outcomes. Outliers were identified from inspection of individual trial treatment estimates and 95% CIs in forest plots.
Including data from all arms of factorial trials with a significant interaction: for factorial trials, when there was a significant interaction between the two active interventions with respect to our primary outcome, we included only the arms ‘skin care intervention/control’ versus ‘control/control’. In such scenarios, an additional sensitivity analysis explored the impact of including data from all arms of factorial trials, with adjustment for the non‐skin barrier intervention in stage 1 of the analysis.
Summary of findings and assessment of the certainty of the evidence
We planned our 'Summary of findings' tables to include the following.
-
'Summary of findings' table 1. Skin care intervention versus no treatment or standard care only.
This table includes primary estimates of treatment effects in addition to key sensitivity analyses for primary outcomes.
-
'Summary of findings' table 2. Skin care intervention A versus no treatment or standard care only. Intervention A = skin care interventions that aim to promote hydration or barrier function.
This table would have included subgroup analyses of low risk of eczema and food allergy versus high risk of eczema and food allergy, either by FLG mutation or by family history of allergic disease.
-
'Summary of findings' table 3. Skin care intervention B versus no treatment or standard care only. Intervention B = skin care interventions that aim to prevent damage.
This table would have included subgroup analyses of low risk of eczema and food allergy versus high risk of eczema and food allergy, either by FLG mutation or by family history of allergic disease.
It did not prove necessary to stratify comparisons by types of skin care interventions because we did not identify any eligible trials of skin care interventions type B. Therefore, our 'Summary of findings' tables include only Table 1.
Outcomes for 'Summary of findings' tables
Primary outcomes
Eczema diagnosis
IgE‐mediated food allergy
Key secondary outcomes
Adverse events during the intervention period, such as slippage, skin infection, stinging or allergic reaction to moisturiser
Time to onset of eczema
Parental report of immediate reaction to a common food allergen
Allergic sensitisation to a food allergen
We included outcomes for sensitivity analyses by method of outcome assessment and by risk of bias in the 'Comments' in Table 1.
Quality of the evidence
We applied the GRADE approach to our main comparisons listed above (Andrews 2013; Schünemann 2020). The outcomes that we included in our 'Summary of findings' tables are the primary outcomes of eczema and food allergy and adverse events during the intervention period, along with key secondary outcomes of time to onset of eczema, parental report of immediate food allergy, and allergic sensitisation to a food allergen. Two review authors (MK, SC, or RJB) independently assessed each outcome for risk of bias, imprecision, inconsistency, indirectness, and publication bias, and downgraded when appropriate. We graded each outcome as high, moderate, low, or very low quality.
Results
Description of studies
We included 33 RCTs in the review.
Results of the search
Searches of the four databases and two trials registers (see Electronic searches) yielded 7046 records. Our searches of other resources identified no further relevant records. Once duplicates had been removed, we had a total of 6133 records. We excluded 5891 records based on titles and abstracts. We obtained the full text of the remaining 242 records. We excluded 150 studies reported in 152 articles (see Characteristics of excluded studies). We identified eight ongoing studies reported in 11 articles (see Characteristics of ongoing studies), and three studies reported in four articles are awaiting classification (see Characteristics of studies awaiting classification). We included 33 studies reported in 75 articles (see Characteristics of included studies). For a further description of our screening process, see the study flow diagram (Figure 1).
1.
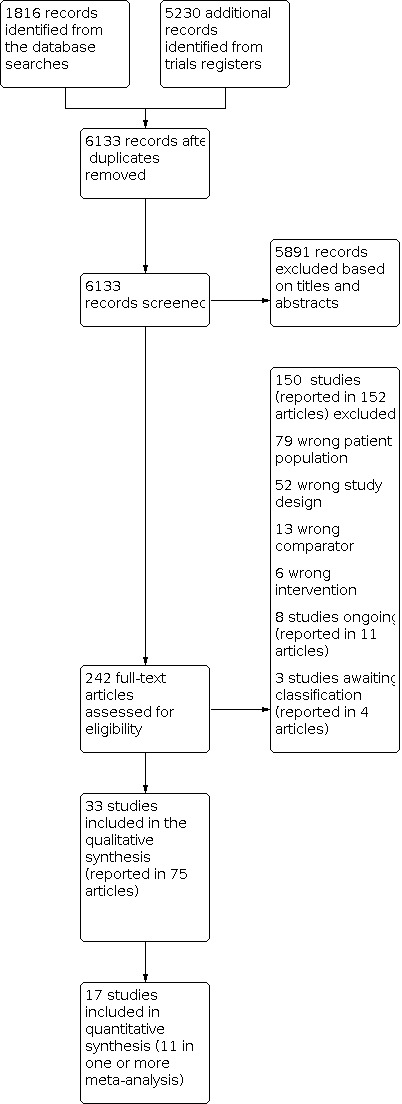
Study flow diagram.
Included studies
Thirty‐three studies with 25,827 participants were included. Full details for each trial are summarised in the Characteristics of included studies tables. Of the included studies, only 17 trials, randomising 5823 participants, had outcome data relevant to eczema, food allergy, or the adverse events of interest; these data were IPD, either aggregate or narrative.
The 16 included studies that did not have any outcome data relevant to the review were Abraham 2019; Baldwin 2001; Duan 2019; Garcia Bartels 2010; Garcia Bartels 2011; Garcia Bartels 2012; Garcia Bartels 2014; Lavender 2011; Lavender 2012; Lavender 2013; Lund 2020; Raisi Dehkordi 2010; Rush 1986; Sankaranarayanan 2005; Tielsch 2007; and Zhao 2005. Ten of these studies assessed the impact of short‐term application of skin care products in term infants in the first few weeks of life on physiological skin outcomes (Abraham 2019; Duan 2019; Garcia Bartels 2010; Garcia Bartels 2012; Garcia Bartels 2014; Lavender 2011; Lavender 2012; Lavender 2013; Lund 2020; Raisi Dehkordi 2010). Rush 1986 evaluated the impact of daily bathing on Staphylococcus aureus colonisation levels. Baldwin 2001 assessed diaper products to prevent diaper dermatitis. Garcia Bartels 2011 looked at effects of swimming and lotion on infant skin, with Zhao 2005 evaluating the impact of swimming alone on neonatal skin barrier. Tielsch 2007 was a cluster‐randomised trial including more than 17,000 participants, evaluating effects of chlorhexidine wipes on neonatal mortality and infection in rural Nepal, and Sankaranarayanan 2005 evaluated effects of coconut and mineral oil on weight velocity. For these trials, the longest follow‐up period was four weeks, and outcomes were physiological skin measures or non‐skin‐related outcomes. These trials met the criteria for inclusion; however, they did not include eczema or food allergy outcomes, nor usable adverse event outcomes.
Participant Characteristics
For studies included in the meta‐analysis, almost all participants were enrolled in the study before 14 days of age.
Female sex ranged from 43% of participants in Cooke 2015 to 56% in NCT03376243. Vaginal delivery ranged from 26% in Simpson 2014 to 83% in Cooke 2015.
Design
As per the inclusion criteria, all trials were randomised controlled trials of a skin barrier intervention versus standard care or no skin care intervention. Most (25/33) trials recruited infants before one month of age and randomised them to a "control" group, which provided standard care for infant skin in the locality, or an "intervention" group. Both intervention and control groups were then followed up at specified intervals for assessment of outcomes. Because the most common intervention was the application of emollients or changes to skin care, in almost all studies, participants were not blinded to their allocation status. All trials were individually randomised, apart from Skjerven 2020 and Tielsch 2007.
Two studies were factorial RCTs: Skjerven 2020 was a cluster‐randomised trial evaluating both a skin barrier intervention and early introduction of solid foods. Due to significant interaction between interventions, only the skin care and control arms of the study were used for IPD analysis. Dissanayake 2019 was an individually randomised factorial trial evaluating both a skin barrier intervention and an oral synbiotic. The following studies included more than two arms but included a control arm: Abraham 2019; Cooke 2015; Dizon 2010; Duan 2019; Garcia Bartels 2010; Garcia Bartels 2014; Raisi Dehkordi 2010; Sankaranarayanan 2005.
Simpson 2014 is the pilot study for Chalmers 2020. Lowe 2018a is the pilot study for an ongoing study, Lowe 2019.
Sample sizes
The three largest studies contributing to data analysis were Skjerven 2020 (n = 2397), Chalmers 2020 (n = 1394), and Dissanayake 2019 (n = 549). Eight studies enrolled between 100 and 250 participants (Bellemere 2018; Cooke 2015; Da Cunha 2008; Dizon 2010; Horimukai 2014; McClanahan 2019; Simpson 2014; Yonezawa 2018), and six studies included fewer than 100 participants (Amer 2017; Kataoka 2010; Lowe 2018a; Migacheva 2018; NCT03376243; Thitthiwong 2019).
Setting
In most trials, women were approached during pregnancy or in the first few weeks after birth and were offered participation. Most studies were conducted from tertiary referral hospitals. All studies that contributed to the meta‐analysis were conducted in well‐resourced settings. Visits mainly took place at children's hospitals, apart from Chalmers 2020, which was a pragmatic study, and a majority of end of study assessments were conducted in participants' homes. Chalmers 2020 recruited from multiple sites across England. Skjerven 2020 recruited mainly in Oslo, Norway, but also in Sweden. Four studies were based in Japan (Dissanayake 2019; Horimukai 2014; Kataoka 2010; Yonezawa 2018). Remaining trials were based in Australia, France, Germany, and the United States.
Participants
All trials set their own inclusion and exclusion criteria. The meta‐analysis protocol set inclusion criteria as infants under one year old; however a vast majority of studies in the meta‐ analysis that contributed data to meta‐analyses enrolled newborns up to three weeks of age. The only exceptions to this were Garcia Bartels 2011 (infants 3 to 6 months), Dizon 2010 (mean age approximately 5.4 months), Duan 2019 (mean age 3 months), and Garcia Bartels 2014 (infants enrolled at 9 months of age). As this was a primary prevention review, infants who already had eczema diagnosed were excluded, along with infants with a known skin condition. Studies that focused on a pre‐specified population such as preterm infants were excluded, as their findings may not be generalisable. Most studies contributing to meta‐analyses enrolled participants with a family history of allergy, although this was defined in different ways across studies. Horimukai 2014 included infants with high risk of atopic dermatitis from family history, and Kataoka 2010 included infants with "family history of AD in second degree of kinship". Four studies included infants with at least one first‐degree relative with eczema, hay fever, or asthma (Chalmers 2020; McClanahan 2019; NCT03376243; Simpson 2014). Lowe 2018a and Thitthiwong 2019 included infants with a self‐reported family history in parent or sibling of any allergic disease, whereas Bellemere 2018 required two atopic first‐degree relatives. Dissanayake 2019, Skjerven 2020, and Yonezawa 2018 did not require family history of atopy for enrolment. Key baseline characteristics of participants included in the meta‐analyses are summarised in Table 3 and Table 4.
2. Baseline characteristics of participants included in meta‐analysis.
| Characteristic | Chalmers 2020 | Cooke 2015 | Dissanayake 2019 | Horimukai 2014 | Lowe 2018a | Migacheva 2018 | McClanahan 2019 | Simpson 2014 | Skjerven 2020 |
| Gestational age: mean (SD) | 40 (1.3) | 40 (1.3) | 39 (1.1) | 39.1 (1) | 39.3 (1.6) | 40.3 (1.3) | 39.3 (1.6) | ||
| Female: n (%) | 661 (47) | 49 (43) | 275 (50) | 50 (42) | 41 (51) | 29 (48) | 49 (49) | 41 (53) | 1134 (47) |
| Birth weight: mean g (SD) | 3405 (452) | 3048 (322) | 3054 (363) | 3356 (442) | 3577 (480) | ||||
| Vaginal delivery: n (%) | 954 (68) | 95 (83) | 420 (77) | 89 (75) | 31 (41) | 80 (80) | 32 (26) | 1999 (83) | |
| Age intervention began: mean days (SD) | 13 (10) | All < 3 | 2 (2) | 12 (7) | 13 (14) | 14 (6) | |||
| Family history atopy: n (%) | 1394 (100) | 37 (79) | 457 (83) | 118 (100) | 80 (100) | 100 (100) | 124 (100) | 1814 (78) | |
| FLG (1/2 mutations): n (%) | 125 (15) | 5 (9) | 17 (27) | ||||||
| Sample size | 1394 | 115 | 549 | 118 | 80 | 60 | 100 | 124 | 2396 |
Please note that a blank cell indicates that the study report did not provide relevant information.
3. Baseline characteristics of participants included in meta‐analysis (continued).
| Characteristic | NCT03376243 | Yonezawa 2018 |
| Gestational age: mean (SD) | 39.4 (1.3) | |
| Female: n (%) | 30 (56) | 98 (43) |
| Birth weight: mean g (SD) | 3547 (497) | 3017 (362) |
| Vaginal delivery: n (%) | 34 (63) | 194 (85) |
| Age intervention began: mean days (SD) | 6 (2) | |
| Family history atopy: n (%) | 54 (100) | 62 (27) |
| FLG (1/2 mutations): n (%) | ||
| Sample Size | 54 | 227 |
Interventions
Interventions were any skin barrier interventions that could alter the skin barrier in the infant. We did not identify any completed trials of interventions to reduce exposure to substances that might damage the skin barrier, but we did identify one ongoing trial of a water softener with this aim (Jabbar‐Lopez 2019). Some included trials used a single intervention, others used a package of skin care interventions, and two were factorial trials. The intervention was compared to standard infant care in the country of the study setting.
i. Emollients
The most common intervention, used in 13 of 33 trials, was emollient with standard care. The type of emollient and the treatment regimen varied.
Five trials compared a commercial daily emollient for a treatment duration of three to six months with standard care (Bellemere 2018; Kataoka 2010; Lowe 2018a; Simpson 2014; Yonezawa 2018). Bellemere 2018 compared the use of a "French cosmetic brand" emollient in neonates, twice daily for the first six months of life, versus control, with outcomes measured at six months of age. Kataoka 2010 randomised newborn infants to an unspecified daily emollient "more than once a day", or to control, for six months, with outcome measured at six months of age. Lowe 2018a randomised 80 newborn infants to a ceramide dominant emollient (EpiCeram™; PuraCap Pharmaceutical LLC, South Plainfield, NJ, USA) or to control (no intervention); participants were advised to apply the emollient all over twice a day for six months. Outcomes were measured at 12 months of age. Simpson 2014 was the pilot study for Chalmers 2020 and randomised 124 infants to once‐daily all‐over emollient or standard care for six months, starting within three weeks of birth, with outcomes measured at 24 weeks. The emollient was chosen from sunflower oil, Doublebase gel, or paraffin in UK‐based participants, and from sunflower oil, Aquaphor, or Cetaphil in US‐based participants. In Yonezawa 2018, newborn infants were also randomised to an emollient one or more times per day and reduced bathing to every second day or standard care for the control group. Soap for washing was provided by the team. The intervention period lasted from week 1 to week 12 after birth; outcomes were measured at three months of age.
Five trials compared the use of commercial emollients with standard care over a longer intervention period, between 32 weeks and 12 months (Chalmers 2020; Horimukai 2014; McClanahan 2019; NCT03376243; Thitthiwong 2019). Chalmers 2020 was a pragmatic randomised controlled trial of all‐over body, once‐daily emollient from enrolment to one year, with outcome assessment one year after treatment ended. This trial used Doublebase Gel or Diprobase Cream, and participants were able to choose between them and to swap during the study. In Horimukai 2014, infants younger than one week were randomised to an all‐over, once‐daily emulsion‐type emollient (2e (Douhet) Emulsion; Shiseido, Tokyo, Japan) or to control for 32 weeks. Participants in the control group were allowed to use petroleum jelly if they wished. Outcomes were measured at 32 weeks. McClanahan 2019 randomised 100 infants under three weeks of age to Cetaphil Restoraderm emollient or to an emollient of choice on an as‐needed basis. The intervention group was advised to apply the emollient all over the baby once daily until 12 months of age, with outcomes recorded at two years of age. Thitthiwong 2019 randomised 54 infants less than 10 weeks old to once‐daily, all‐over body cold cream or control. The skin outcome was assessed at nine months of age; however, it is unclear when the intervention period was completed. NCT03376243 randomised newborn infants to once‐daily all‐over Lipikaur Baume or control. Both groups received general skin care advice for infants. The intervention was given for 12 months and outcome was assessed at two years of age.
Skjerven 2020 evaluated skin care intervention and early introduction of allergenic food. As Skjerven 2020 reported a significant interaction between interventions for the primary eczema outcome, we used only data from the skin care and control arms for our primary analysis, where infants were bathed using an oil emulsion and had cream applied to the face once daily from age two weeks to eight months. Eczema outcome was measured at 12 months of age, and food allergy was assessed at three years of age.
Two trials compared the use of emollient and synbiotic with standard care (Dissanayake 2019; Migacheva 2018). Dissanayake 2019 was a factorial trial of skin care intervention plus a synbiotic versus normal care for prevention of eczema in infants. The skin care intervention was the use of a lipid‐based emollient, which was advised to be put on the cheeks twice daily and on the body if wished. The synbiotic was a combination of 0.5 g (7 × 109 colony‐forming units (CFUs)/g) of Bifidobacterium bifidum and fructo‐oligosaccharides twice a day. The intervention period lasted from birth to six, months with outcome assessment at 12 months of age. One study compared an emollient and an oral synbiotic versus control. Migacheva 2018 randomised 63 infants younger than three weeks of age to twice‐daily all‐over emollient for six months and two supplements of synbiotic at three and six months, or control.
ii. Topical oils
Cooke 2015 was a three‐armed trial of the effect of sunflower oil or olive oil versus control on neonatal skin. Parents were advised to apply 4 drops of oil to their baby's left forearm, left thigh, and abdomen, twice a day. All groups were advised not to use any other skin care products, and the intervention period lasted four weeks, with outcomes measured at four weeks. Raisi Dehkordi 2010 randomised 120 infants who were 10 to 15 days old to massage with sunflower oil, sesame oil, or no oil. Mothers were advised to massage the oil into infants twice daily for 28 days, with outcomes measured at the end of the 28 days. Sankaranarayanan 2005 randomised 224 babies to coconut oil, mineral oil, or control with four times daily oil massage from birth until 31 days of age; outcomes were measured at 31 days of age.
iii. Bathing products and frequency
In Abraham 2019, 102 children were randomly assigned to bathing with one of chlorhexidine, saline, or standard bath, with outcomes assessed up to 24 hours after the intervention. In Dizon 2010, children younger than one year were randomly assigned to three groups to be bathed for two weeks with Group I ‐ Johnson's Baby Top‐to‐Toe Wash, Group II ‐ Sebamed Baby Liquid Cleanser, or Group III ‐ clear water. Assessment was done at one and two weeks of treatment. In Duan 2019, 150 infants were randomised to Group I commercial baby wash (Johnson's Baby Wash) and commercial baby lotion (Johnson's Baby Lotion), and Group II to water wash and commercial baby lotion, or water only. Parents were asked to wash their infant daily in the wash and apply the lotion, or to wash their infant daily with water and apply the lotion daily, or to wash their infant daily with water only. This intervention was given for 12 weeks, with outcomes assessed at the end of the 12‐week period. In Garcia Bartels 2010, 64 newborn infants were randomised to twice‐weekly washing for the first eight weeks of life in one of four groups: Group WG, bathing with wash gel (Top‐To‐Toe Baby Gel Penaten, Johnson & Johnson); Group C, bathing with clear water and afterwards topical cream (Baby Caring Facial & Body Cream Penaten, Johnson & Johnson); Group WG + C, bathing with wash gel and topical cream; or Group C, bathing with wash only (i.e. control). Outcome assessment was conducted at eight weeks of age. Lavender 2011 randomised newborn infants to be washed with Johnson's Baby Top‐To‐Toe or water, at least three times a week, for the first eight weeks of life. Skin was assessed at four and eight weeks, the first during and the second at the end of the intervention period. This was a pilot trial; the full trial ‐ Lavender 2013 ‐ randomised 307 infants to washing with commercial product or water alone at least three times a week. The intervention period lasted for four weeks only, with outcome assessment at four weeks ‐ the end of the intervention period. Lund 2020 randomised 100 newborn infants to be washed with Johnson's Baby Top‐To‐Toe or water, in the first hours of life. Skin was assessed before and "after" this bath. Rush 1986 randomised healthy term newborn infants to daily washing with soap and water versus dry skin care and no bath. The outcome was measured on day 4 of bathing, or immediately before discharge.
iv. Combined skin interventions
Amer 2017 compared detailed instructions to use a combined skin intervention for parents of newborn infants involving delaying the first bath and using daily baby oil on scalp and body or chlorhexidine wash for umbilical cord bathing twice per week versus study shampoo versus standard care. The cream, wash, shampoo, and wipes used were provided to parents for a four‐week interval, with outcomes measured at the end of the treatment period. Skjerven 2020 was a factorial randomised trial of 2396 infants evaluating effects of the skin barrier intervention of emollient and bath oil and early introduction of allergenic food on the impact of developing eczema and food allergy. Those randomised to skin barrier intervention were advised to bathe daily with bath oil and to apply emollient to the infant's face from two weeks to eight months of age. Outcomes were assessed at 12 months to three years of age. Garcia Bartels 2011 evaluated effects of infant swimming and applying an emollient after swimming versus swimming alone in three‐ to six‐month‐old infants. Both groups participated in the swimming classes once a week for four weeks. The intervention group was instructed to apply a simple emollient all over after swimming. The final outcome was assessed at one week after the end of the intervention period. In another swimming‐related intervention, Zhao 2005 evaluated the impact of daily infant swimming in the maternity hospital compared to control. Infants in the swimming group had twice‐daily, 10‐ to 15‐minute swimming sessions whilst in the maternity hospital. Outcomes were measured on discharge.
v. Diapers
Baldwin 2001 compared a new zinc oxide diaper to a commercially available diaper for prevention of diaper dermatitis. Previously well children without diaper dermatitis were randomly assigned to control diaper or zinc oxide‐based diaper for four weeks. The outcome of diaper dermatitis was measured at the end of the treatment period.
vi. Cleansing products
In Da Cunha 2008, newborn infants were randomised to receive chlorhexidine liquid soap bath versus control liquid soap. Outcome was measured at 24 hours after bath. In Garcia Bartels 2012, newborn infants were randomised to washing of the diaper area with commercial wipes or water‐moistened cloths (control) for four weeks. Skin was assessed at four weeks ‐ the end of the intervention period. In Lavender 2012, 280 newborn infants were also randomised to washing of the diaper area with a commercial wipe (Johnson's Baby Skincare Fragrance Free Wipe) or cotton wool and water for four weeks post birth, with outcome assessed at four weeks ‐ the end of the intervention period. Tielsch 2007 was a cluster‐randomised study of 17,530 infants in rural Nepal, which compared all‐over cleansing at birth with a chlorhexidine wipe versus a wipe without chlorhexidine. This was a one‐time intervention provided at birth. The outcome was measured at 28 days.
Follow‐up
Our specified primary and secondary outcomes were measured from one to three years, apart from adverse events, which were measured during the intervention period. Studies with emollients and Skjerven 2020 were the only studies with follow‐up long enough to meet this outcome timing. Most of the other studies followed up with these infants to a maximum of four weeks, which was too soon to assess whether eczema or food allergy was present.
Comparators
All trials included a comparison arm that provided "routine" skin care for their country. We did not specify what the comparator was, as daily practices on how to treat infant skin vary between countries and within countries according to cultural norms. We excluded any trial that had an active comparator such as an emollient.
Outcomes
Of 33 studies, 17 studies provided data on one or more outcomes relevant to this review, of which 11 could be included in the meta‐analysis. Ten trials provided IPD, including nine studies that provided IPD for the primary outcome eczema or food allergy. Cooke 2015 provided IPD for adverse events only. Pre‐specified outcome timings were one to three years for both primary and secondary outcomes related to eczema and food allergy. Adverse events were measured during the intervention period.
Eczema data were measured between one and three years of age in eight trials (Chalmers 2020; Dissanayake 2019; Lowe 2018a; McClanahan 2019; Migacheva 2018; NCT03376243; Skjerven 2020; Yonezawa 2018). Two trials had eczema outcomes before one year (Horimukai 2014; Simpson 2014). Four further trials recorded some data on eczema outcomes, which were not usable in the meta‐analysis (Amer 2017; Bellemere 2018, Kataoka 2010 Thitthiwong 2019), but these data were included in narrative format in the results. The primary outcome of eczema was measured by Hanifin and Rajka, or by UK Working Party methods, in all trials except one, which used parental report of a doctor diagnosis of eczema (Yonezawa 2018). Three studies measured eczema severity between one and three years by clinician assessment, including Chalmers 2020 and NCT03376243 using the EASI at two years and one year, respectively, and Lowe 2018a using objective SCORAD (clinical tool used to assess the extent and severity of eczema) at one year. Only Chalmers 2020 recorded parental report of eczema severity based on the POEM at two years. Nine studies measured time to onset of eczema (Chalmers 2020; Dissanayake 2019; Horimukai 2014; Lowe 2018a; McClanahan 2019; NCT03376243; Simpson 2014; Skjerven 2020; Yonezawa 2018).
The primary outcome of IgE‐mediated food allergy diagnosed by oral food challenge between one and three years was measured in Chalmers 2020 only. Three trials provided data on parental report of doctor diagnosis of food allergy between one and three years: Dissanayake 2019 (by one year), Yonezawa 2018 (by two years), and Chalmers 2020 (by two years). Two trials provided data on parental report of an immediate (< 2 hours) reaction to a common food allergen: Chalmers 2020 (at two years) and NCT03376243 (by one year). Data on allergic sensitisation to foods between one and three years were provided in Chalmers 2020 (at two years) and Lowe 2018a (at one year). Two trials had data on allergic sensitisation to foods at eight months ‐ Horimukai 2014 ‐ and at nine months ‐ Dissanayake 2019 ‐ which were used in sensitivity analysis only. Kataoka 2010 and Thitthiwong 2019 provided narrative information on food allergy that could not be used in the meta‐analysis but was included in narrative format in the results.
Adverse event data for the pre‐specified adverse events of interest were provided by 10 studies (Amer 2017; Chalmers 2020; Cooke 2015; Da Cunha 2008; Dizon 2010; Lowe 2018a; McClanahan 2019; NCT03376243; Simpson 2014; Skjerven 2020). Nine studies contributed some narrative data on non‐specific adverse events, which were not included in the meta‐analysis but are presented descriptively in the effects of interventions section (Dissanayake 2019; Garcia Bartels 2011; Horimukai 2014; Lavender 2012; Migacheva 2018; Raisi Dehkordi 2010; Sankaranarayanan 2005; Thitthiwong 2019; Tielsch 2007).
Funding
Of the 11 trials contributing to one or more meta‐analyses, two did not specify funding (McClanahan 2019; Migacheva 2018); the other nine contributing trials were funded through higher‐level institutions.
Of the six trials that contributed aggregate data that were not relevant for inclusion in one or more meta‐analyses, three studies did not specify funding, two were supported by local hospitals, and one was commercially sponsored.
Of the 16 trials that did not contribute any data on outcomes, two did not report on funding, two were sponsored by local hospitals, one was sponsored by a local hospital and the Gates Foundation, and the other 11 were commercially sponsored.
Excluded studies
We excluded 178 studies (see Characteristics of excluded studies). We excluded 93 for including the "wrong patient population". The aim of this review was to evaluate prevention of eczema and food allergy in infants; therefore any population already diagnosed with eczema was excluded, along with participants over the age of 12 months. We also excluded any studies that were primarily looking at preterm infants, as these infants did not have a "normal" neonatal course, with most cared for in neonatal intensive care units, where skin care practices are inherently different. We excluded 63 studies for using the "wrong study design" ‐ mainly if they were not randomised controlled trials. We excluded 14 studies as using a "wrong comparator", as there was no standard control in the study design. Finally, we excluded eight studies as they provided the "wrong intervention" ‐ some including oral probiotics.
Studies awaiting classification
We marked three trials as "awaiting classification" (Studies awaiting classification).
ISRCTN38965585 is a World Health Organization (WHO)‐sponsored cluster‐randomised trial including over 40,000 infants and assessing effects of newborn massage with cold pressed olive oil on newborn survival in rural India. This study was registered initially in 2014 and is marked as "recruitment complete". This study could have contributed only to adverse events; no results are recorded at the clinical trial registry or in our search. We received no response from study authors when we contacted them via the email addresses provided.
JPRN‐UMIN000026877 is a small trial of 50 infants evaluating the efficacy of a foam body cleanser and "lotion" on infant skin; it was sponsored by a Japanese cosmetics company and is marked as "complete" at the clinical trial register. We received no response from study authors when we contacted them via the email addresses provided.
NCT03640897 is a commercial trial of a wipe for infants. It does meet our criteria; however, it could contribute only to adverse events. This study has been marked as "complete" at the trial register since March 2019. No data have been published. We received no response from study authors when we contacted them through their website at www.labogilbert.com/.
Ongoing studies
We classified eight studies as "ongoing studies" (Characteristics of ongoing studies), which could provide further useful information in an update to this review in the future. Specifically for prevention of eczema in infants, NCT02906475 is an RCT of 160 infants undertaken by HIPP Pharmaceuticals to evaluate the impact of use of daily milk lotion in infants for prevention of eczema. Jabbar‐Lopez 2019 is a pilot RCT in Kings College London of 80 infants that is evaluating whether families would be willing to be randomised to have domestic ion exchange water softener installed, with a secondary outcome of prevention of eczema in infants. NCT03808532 is an RCT by MYOR Corporation of 290 infants that is evaluating the effect of daily emollient for prevention of eczema. Eichner 2020 is an RCT of 1250 infants conducted in the USA to evaluate the impact of daily lipid‐rich emollient from birth to two years on the cumulative incidence of eczema at 24 months. For prevention of eczema and food allergy, NCT03871998 is an RCT in Ireland of 242 infants evaluating the effect of twice‐daily all‐over emollient in the first two months of life on incidence of eczema at 12 months and of IgE‐mediated food allergy at 24 months. Lowe 2019 is an RCT of 760 infants undertaken in Melbourne, Australia, to evaluate the impact of a twice‐daily ceramide‐dominant emollient for prevention of eczema and food allergy. NCT04398758 is an RCT that is under way in Germany to evaluate twice‐daily paraffin‐based cream on infants with a family history of diagnosed eczema. The primary outcome is eczema at six months, with food sensitisation at 12 months ‐ one of its secondary outcomes. For evaluation of the skin barrier in infants, NCT03142984 is a study of 160 infants that is exploring effects on the skin barrier of a new baby wash and baby lotion.
Trialists leading the following ongoing trials are all involved in the IPD collaboration and will be involved in an update of this review when planned: NCT02906475, NCT03142984, Jabbar‐Lopez 2019, NCT03871998, Lowe 2019, and Eichner 2020.
Risk of bias in included studies
We assessed risk of bias using the Cochrane 'Risk of bias' tool 2 (Higgins 2018) for trials providing outcome data on one or more of eczema, food allergy, slippage accidents, skin infections, stinging or allergic reactions, serious adverse events, time to eczema onset, parent report of immediate reaction to a common allergenic food, and allergic sensitisation. Detailed risk of bias assessment data, with consensus responses to each RoB 2 signalling question, are available at Cro 2020b. Risk of bias assessments by analysis are also summarised in the results level RoB 2 tables. Risk of bias assessments were not performed for trials providing qualitative narrative information.
Risk of bias summary by domain
Most studies (12/17) were at low risk of bias for the randomisation process; we rated five studies as some concerns for risk of bias, as insufficient information on allocation concealment or balance in baseline characteristics was provided (Amer 2017; Bellemere 2018; Dizon 2010; Kataoka 2010; Migacheva 2018).
Most studies (12/17) providing outcome data were judged to be at low risk of bias for deviations from intended interventions for all outcomes, as whilst it was not possible to blind participants or carers in the individual studies due to the nature of the intervention under study, no evidence indicates that deviations arose because of the trial context. Control group rates of skin care application were consistent with those of other trials and observational studies, which have reported that up to 75% of individuals apply a skin care intervention (Rendell 2011). Additionally, analyses were appropriately performed according to the intention‐to‐treat principle to identify the effect of assignment to the intervention.
Three studies were rated to be at some concerns of bias for deviations from intended interventions for all outcomes (Amer 2017; Migacheva 2018; Thitthiwong 2019), as they provided no information for assessment of whether deviations arose because of trial context. For two other studies, analysis populations were unclear, and we consequently rated these as having high risk of bias (Bellemere 2018; Kataoka 2010).
For missing data on our outcomes of interest, studies were predominantly at low risk of bias or at some concerns if they included a non‐negligible quantity of missing data, and if sensitivity analysis using the individual participant revealed that trial conclusions changed (point estimate changed by at least 20% of the complete case estimate). However across most outcomes, whilst missingness could have depended on the true value, it was rated unlikely that missingness in the outcome depended on its true value due to trial circumstances and the fact that rates of missingness did not vary considerably by intervention groups. The one exception to this was a single trial that provided outcome data on food allergy as assessed by oral food challenge and was rated having high risk of bias for missing data (see risk of bias for analysis table for Analysis 1.29) (Chalmers 2020). Data were missing for 398/1394 (29%) randomised participants. Results varied in missing data sensitivity analyses performed using the IPD, and it was judged potentially likely that missingness depending on the value of the outcome (because there was a difference between treatment groups in the proportion of participants who underwent oral food challenge) and recorded reasons for missingness included decline in oral food challenge and unwillingness to participate, which could have depended on the outcome.
1.29. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 29: Food allergy by 1‐3 years
For measurement of the outcome, outcomes reported by carers (e.g. adverse events, report of reaction, POEM, eczema for one study) were judged at some concerns for risk of bias, as carers were unblinded due to the nature of the intervention. However, whilst knowledge of the intervention could have influenced the measurement, it was judged unlikely to have impacted measurement. For other outcome measurements judged at low risk of bias, if no information was available on the blinding status of assessors, these were rated as some concerns. One trial supplied no information on how eczema was measured and by whom and was rated as having high risk of bias for this outcome.
Selection of the reported result
Of the 17 studies providing data on one or more of our outcomes assessed for risk of bias, 10 were rated at low risk of bias for selection of reported results; these supplied individual participant data (Chalmers 2020; Cooke 2015; Dissanayake 2019; Horimukai 2014; Lowe 2018a; McClanahan 2019; Simpson 2014; Skjerven 2020; NCT03376243; Yonezawa 2018). For each of these trials, for each outcome, we performed the required analysis using the supplied trial data set in keeping with a pre‐specified statistical analysis plan that was finalised before unblinded outcome data were available for analysis (Cro 2020a).
Seven trials contributing some aggregated outcome data were rated as some concerns for selection of reported results (Amer 2017; Bellemere 2018; Da Cunha 2008; Dizon 2010; Kataoka 2010; Migacheva 2018; Thitthiwong 2019). Only Migacheva 2018 was included in the meta‐analysis. All seven provided no information on whether a pre‐specified statical analysis plan, finalised before unblinding, was followed.
Overall risk of bias
In summary, most of the evidence included in this review was assessed as low risk or some concern of risk of bias. Over all RoB 2 domains, 3/17 studies were rated as low risk of bias across all included outcomes (Cooke 2015; Dissanayake 2019; Horimukai 2014); eight were rated as some concerns for risk of bias across all included outcomes (Da Cunha 2008; Dizon 2010; McClanahan 2019; Migacheva 2018; NCT03376243; Simpson 2014; Thitthiwong 2019; Yonezawa 2018), and one was at high risk of bias for its sole included outcome (Bellemere 2018). Two trials were predominantly rated as some concerns of risk of bias across all included outcomes due to measurement of the outcome or missing data (4/5 and 4/6 outcomes for Skjerven 2020 and Lowe 2018a, respectively) but also included outcomes at low risk of bias (1/5 and 2/6 outcomes for Skjerven 2020 and Lowe 2018a, respectively). One study was at high risk of bias for eczema and of some concerns for risk of bias for the skin infection outcome overall (Amer 2017). Another study was at high risk of bias for allergic sensitisation and of some concerns for risk of bias for the eczema outcome overall (Kataoka 2010). Finally, one study was at low risk of bias for 2/7 outcomes, some concerns for 4/7 outcomes due to unblinded measurement, and at high risk for food allergy due to missing data (Chalmers 2020).
Effects of interventions
See: Table 1
Primary outcomes
Primary and secondary outcome results are presented for comparison 1: skin care intervention versus no treatment or standard care only. Comparison 2 ‐ "skin care intervention A versus no treatment or standard care only" and "skin care intervention B versus no treatment or standard care only" ‐ was not conducted because our search did not identify any eligible studies of skin care interventions type B. Results are summarised in Table 1.
Eczema
Individual participant data on eczema diagnosis by age one to three years were available from seven studies including 4800 randomised participants (Chalmers 2020; Dissanayake 2019; Lowe 2018a; McClanahan 2019; NCT03376243; Skjerven 2020; Yonezawa 2018). Eczema was measured using the Hanifin and Rajka criteria (Hanifin 1980), or the UK Working Party refinement of these criteria (Williams 1994), or other modifications of the Hanifin and Rajka criteria for six trials (Chalmers 2020; Dissanayake 2019; Lowe 2018a; McClanahan 2019; NCT03376243; Skjerven 2020), and eczema was doctor diagnosed for one trial (Yonezawa 2018). Eczema was diagnosed cumulatively up to 12 months for four trials ‐ Dissanayake 2019; Lowe 2018a; NCT03376243; Skjerven 2020 ‐ and up to 24 months for three studies ‐ Chalmers 2020; McClanahan 2019; Yonezawa 2018. Pooled individual patient data available from 3075 participants in these studies show no benefit of skin care intervention (risk ratio (RR) 1.03, 95% confidence interval (CI) 0.81 to 1.31; Analysis 1.1). However, the 95% CI for the pooled RR indicates that we cannot rule out potential benefit or harm, extending from 0.81 to 1.31. Results show moderate statistical heterogeneity between studies for this outcome (I2 = 41%, P = 0.12). This is driven by one trial with an RR favouring standard care (RR 1.57, 95% CI 1.10 to 2.23) (Skjerven 2020). This heterogeneity may be driven by the type of intervention (bathing with oil and emollient applied to the face only) and/or by timing of intervention initiation (initiated from two weeks). When this study was excluded, pooled individual patient data available from 2004 participants in the remaining six studies continued to show no benefit of skin care intervention (RR 0.95, 95% CI 0.81 to 1.12; I2 = 0%; Analysis 1.12).
1.1. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 1: Eczema by 1‐3 years
1.12. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 12: Participant level treatment interaction: Eczema by 1‐3 years for treatment initiation < 4 days versus ≥ 4 days of age
A series of pre‐planned sensitivity analyses were conducted (see Table 5). Sensitivity analysis including aggregate data from one additional study that did not supply individual participant data involving 63 randomised participants, of which 60 completed the study (Migacheva 2018), did not change the pooled result (RR 0.97, 95% CI 0.75 to 1.25; Analysis 1.3). When only the six studies that used the Hanifin and Rajka criteria (Hanifin 1980), or the UK Working Party refinement of them (Williams 1994), or other modifications of the Hanifin and Rajka criteria to assess eczema were included, the pooled result remained consistent (RR 1.02, 95% CI 0.78 to 1.34; Analysis 1.3). The pooled result also remained consistent in sensitivity analyses that included data from all four arms of Skjerven 2020 (RR 1.03, 95% CI 0.81 to 1.31; Analysis 1.5); included only studies at low risk of bias (RR 0.97, 95% CI 0.81 to 1.17; Analysis 1.6); excluded non‐prospectively acquired data (RR 1.08, 95% CI 0.84 to 1.37; Analysis 1.7); incorporated studies assessing eczema by six months to three years (RR 0.89, 95% CI 0.70 to 1.14; Analysis 1.8); and considered eczema only after the intervention period (i.e. at one year or beyond) (RR 1.06, 95% CI 0.77 to 1.47; Analysis 1.8).
4. Sensitivity analysis for eczema .
| Analysis | N trials | Nintervention |
N control |
Pooled riskratio | 95% CI |
|
Primary: Eczema by1 to 2years |
7a | 1489 | 1586 | 1.03 | 0.81 to 1.31 |
| Sensitivity: Eczema by 1 to 2 years including aggregate data |
8b | 1518 | 1617 | 0.97 | 0.75 to 1.25 |
| Eczema by 1 to 2 years using UK Working Party Criteria only | 6c | 1420 | 1499 | 1.02 | 0.78 to 1.34 |
| Eczema by 1 to 2 years including all 4 arms from the PreventADALL trial | 7a | 1993 | 2183 | 1.03 | 0.81 to 1.31 |
| Eczema by 1 to 2 years, low risk of bias data only | 3d | 868 | 871 | 0.97 | 0.81 to 1.17 |
| Eczema by 1 to 2 years, excluding non‐prospectively acquired data | 6e | 1451 | 1550 | 1.08 | 0.84 to 1.37 |
| Eczema by 6 months to 2 years | 9f | 1549 | 1688 | 0.89 | 0.70 to 1.14 |
aChalmers 2020; Dissanayake 2019; Lowe 2018a; McClanahan 2019; Skjerven 2020; NCT03376243; Yonezawa 2018. bChalmers 2020; Dissanayake 2019; Lowe 2018a; McClanahan 2019; Migacheva 2018; Skjerven 2020; NCT03376243; Yonezawa 2018. cChalmers 2020; Dissanayake 2019; Lowe 2018a; McClanahan 2019; Skjerven 2020; NCT03376243. dChalmers 2020; Dissanayake 2019; Lowe 2018a. eChalmers 2020; Dissanayake 2019; McClanahan 2019; Skjerven 2020; NCT03376243; Yonezawa 2018. fChalmers 2020; Dissanayake 2019; Horimukai 2014; Lowe 2018a; McClanahan 2019; Simpson 2014; Skjerven 2020; NCT03376243; Yonezawa 2018.
1.3. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 3: Sensitivity analysis: Eczema by 1‐3 years (UKWP only)
1.5. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 5: Sensitivity analysis: Eczema by 1‐3 years ‐ low risk of bias
1.6. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 6: Sensitivity analysis: Eczema by 1‐3 years ‐ excluding non‐prospectively acquired data
1.7. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 7: Sensitivity analysis: Eczema by 6 months‐3 years
1.8. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 8: Sensitivity analysis: Eczema after the intervention period (at 1 year or beyond ‐ up to 2 years)
Four additional studies randomising a total of 314 participants provided aggregate data on eczema at four weeks ‐ Amer 2017 ‐ six months ‐ Bellemere 2018; Kataoka 2010 ‐ and nine months ‐ Thitthiwong 2019 that were not eligible for meta‐analysis due to not supplying individual participant data nor data from the short follow‐up period. Amer 2017 reported eczema in 2/35 (5.7%) participants randomised to standard care and 0/35 (0%) randomised to skin care intervention over a four‐week follow‐up period. At six months, Bellemere 2018 reported eczema in 9.8% of the intervention arm versus 18.3% of the control arm (60 participants were randomised to each treatment group, but the analysis population was not clear); after follow‐up of 24 months, seven new cases of atopic dermatitis (AD) were observed in the intervention group and six new cases in the control group. Kataoka 2010 reported 5/35 (14%) versus 6/32 (19%) cases of eczema in the intervention and control arms, respectively. At nine months, Thitthiwong 2019 reported 0/26 (0%) versus 4/27 (15%) cases of eczema in the intervention and control arms, respectively.
Subgroup analysis did not suggest any differences in treatment effects by intervention type or intervention duration. For basic emollients (Chalmers 2020; McClanahan 2019; Skjerven 2020), there was a pooled RR of 1.04 (95% CI 0.66 to 1.65), and for complex emollients (Dissanayake 2019; Lowe 2018a; NCT03376243; Yonezawa 2018), there was a pooled RR of 1.01 (95% CI 0.75 to 1.37) (Analysis 1.10). Only one study reported a prescribed intervention period < 6 months (Yonezawa 2018), with an RR of 1.01 (95% CI 0.45 to 2.27). For the six studies with an intervention period ≥ 6 months (Chalmers 2020; Dissanayake 2019; Lowe 2018a; McClanahan 2019; NCT03376243; Skjerven 2020), there was a pooled RR of 1.02 (95% CI 0.78 to 1.34) (Analysis 1.11).
1.10. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 10: Subgroup analysis (study level): Eczema by 1‐3 years by prescribed intervention duration
1.11. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 11: Subgroup analysis (study level): Eczema by 1‐3 years, by prescribed intervention timing
The interaction effect between treatments and actual age of treatment initiation (< 4 days, ≥ 4days) for eczema by one to three years could be estimated for two trials (Chalmers 2020; Lowe 2018a), which randomised a total of 1474 participants. Pooled individual patient data available from 1284 participants in these two studies show no impact of age of treatment initiation on treatment effect (RR 1.05, 95% CI 0.64 to 1.73; Analysis 1.12). In one additional study randomising 118 participants (Horimukai 2014), the interaction between treatment and actual age of treatment initiation for eczema by eight months could be estimated for 99 participants. When data from this study are incorporated, pooled individual patient data available from 1383 participants continue to show no impact of age of treatment initiation (RR 1.59, 95% CI 0.56 to 4.51; Analysis 1.13).
1.13. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 13: Participant level treatment interaction: Eczema by 6 months‐3 years for treatment initiation < 4 days versus ≥ 4 days of age
The interaction effect between treatment and FLG genotype on eczema by one to three years could be estimated for one trial (Chalmers 2020), which randomised 1394 participants. Based on available data from 816 participants in Chalmers 2020, the RR of eczema by two years for having 1/2 FLG mutations in the skin care intervention group versus zero mutations was RR 1.22 (95% CI 0.71 to 2.11) (Analysis 1.14). The large width of the 95% confidence interval indicates uncertainty surrounding the direction and magnitude of any effect of FLG on treatment effect from this one study. In one additional study randomising 124 participants (Simpson 2014), the interaction between treatment and FLG mutations for eczema by six months could be estimated for 63 participants. When data from this study are incorporated, pooled individual patient data available from 879 participants continue to show uncertainty for the impact of FLG mutations on treatment effect (RR 1.03, 95% CI 0.42 to 2.51; Analysis 1.15).
1.14. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 14: Participant level treatment interaction: Eczema by 1‐3 years by FLG genotype (0 mutations versus 1/2 mutations)
1.15. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 15: Participant level treatment interaction: Eczema by 6months‐3 years by FLG genotype (0 mutations versus 1/2 mutations)
The interaction effect between treatment and family history of atopic disease on eczema by one to three years could be estimated for three trials (Dissanayake 2019; Skjerven 2020; Yonezawa 2018), which randomised 3172 participants. Based on available data from 1663 participants, the pooled RR of eczema by two years for having ≥ 1 family member in the skin care intervention group versus 0 did not indicate a notable interaction (RR 0.95, 95% CI 0.35 to 2.61; Analysis 1.16).
1.16. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 16: Participant level treatment interaction: Eczema by 1‐3 years by ≥ 1 first degree relative with history of allergic disease
The complier average causal effect (CACE) for an individual using skin care intervention for three or more days a week could be estimated for three trials providing adequate compliance and eczema outcome data (Chalmers 2020; Lowe 2018a; Yonezawa 2018). The pooled CACE for an individual using skin care intervention for three or more days a week from 1440 participants in these studies was on average more in favour of skin care intervention (RR 0.65, 95% CI 0.29 to 1.45; Analysis 1.17) in comparison to the pooled intention‐to‐treat effect for these same three studies (RR 0.93, 95% CI 0.77 to 1.12; Analysis 1.23). However the 95% CI for the CACE estimate is considerably wider than for the intention‐to‐treat effect and is consistent with the intention‐to‐treat effect of no difference. Therefore, we cannot infer any difference in treatment effects for a complier. Additional CACE estimates for alternative definitions of a complier are displayed in Table 6 and similarly do not reveal an impact of compliance on treatment effect.
1.17. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 17: CACE: Eczema by 1‐3 years for use over intervention period ≥ 3 days a week
1.23. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 23: Sensitivity analysis: Eczema by 1‐3 years for studies included in CACE for use over intervention period ≥ 3 days a week
5. Complier average causal effect analyses for eczema.
| Complier | N trials | N intervention | N control | CACE | Intention‐to‐treat | ||
| Pooled RR | 95% CI | Pooled RR | 95% CI | ||||
|
Primary CACE: ≥ 3 days of use over interventionperiod |
3a | 705 | 735 | 0.65 | 0.29 to 1.45 | 0.93 | 0.77 to 1.12 |
| Sensitivity CACE: ≥ 5 days of use over intervention period |
2b | 667 | 699 | 0.74 | 0.26 to 2.09 | 0.95 | 0.79 to 1.15 |
| 7 days use over intervention period | 3c | 689 | 699 | 0.78 | 0.23 to 2.71 | 0.95 | 0.79 to 1.15 |
| ≥ 3 days of use over first 3 months of intervention period | 2b | 667 | 669 | 1.02 | 0.79 to 1.31 | 0.95 | 0.79 to 1.15 |
| ≥ 5 days of use over first 3 months of intervention period | 2b | 667 | 669 | 0.84 | 0.46 to 1.52 | 0.95 | 0.79 to 1.15 |
| 7 days use over first 3 months of intervention period | 3c | 689 | 726 | 0.83 | 0.34 to 2.03 | 0.95 | 0.79 to 1.15 |
Trial sequential analysis shows that a target sample size of 5534 would be necessary to demonstrate a minimum relative risk reduction of 30% (assuming a control rate of 15% versus skin care intervention 10.5%) with 90% power (Figure 2). However, based on the data accumulated to date and included within the primary eczema meta‐analysis (3075 participants), the Z value falls on the boundary of the inner wedge and is well within the two‐sided significance testing boundaries, indicating that a conclusion can be made: the intervention effect is not greater than 30%. A target sample size of 13,072 would be necessary to demonstrate a minimum relative risk reduction of 20% (assuming a control rate of 15% versus skin care intervention 12%) with 90% power (Figure 3). Currently, the meta‐analysis is inconclusive for an effect size of 20 because it has not yet crossed the upper or lower boundary for statistical significance or non‐superiority.
2.
Trial sequential analysis for eczema (RR of 30%).
3.
Trial sequential analysis for eczema (RR of 20%).
Food allergy
Individual participant data on IgE‐mediated food allergy, confirmed by oral food challenge, by age one to three years were recorded in one study that included 1394 randomised participants (Chalmers 2020). Data on oral food challenge, conducted at two years, was available for 996 participants and favoured standard care (RR 2.53, 95% CI 0.99 to 6.47; Analysis 1.29). The 95% CI for the RR is wide, indicating that we cannot rule out no difference nor small to large harm of skin care intervention based on this one study.
In sensitivity analysis (see Table 7) for IgE‐mediated food allergy confirmed by oral food challenge or via an investigator assessment based on clinical history and/or skin prick tests, data were available for 1115 participants from one study and favoured standard care (RR 1.46, 95% CI 0.91 to 2.34; Analysis 1.30) (Chalmers 2020). The width of the 95% CI for the RR is reduced but it is still wide, indicating that we cannot rule out no difference nor small to large harm of skin care intervention based on this one study.
6. Sensitivity analysis for food allergy.
| Analysis | N trials | Nintervention |
N control |
Pooled riskratio | 95% CI |
|
Primary: Food allergy (oral food challenge) by1 to 2years |
1a | 491 | 505 | 2.53 | 0.99 to 6.47 |
| Sensitivity: Food allergy (oral food challenge + panel assessment) by 1 to 2 years |
1a | 547 | 568 | 1.46 | 0.91 to 2.34 |
| Food allergy (parent report of doctor diagnosis) by 1 to 2 years | 3b | 798 | 816 | 1.02 | 0.80 to 1.31 |
1.30. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 30: Sensitivity analysis: Food allergy by 1‐3 years (diagnosed by oral food challenge or investigator assessment)
For food allergy, as measured by a parental report of a doctor diagnosis, individual participant data were available for three studies including 2170 randomised participants (Chalmers 2020; Dissanayake 2019; Yonezawa 2018). Food allergy diagnosis was cumulative up to 12 months for one trial (Dissanayake 2019), and up to 24 months for two trials (Chalmers 2020; Yonezawa 2018). Pooled individual patient data available from 1614 participants in these studies showed no effect of skin care intervention (RR 1.02, 95% CI 0.80 to 1.31; I2 = 0; Analysis 1.31). But uncertainty exists around the true effect value because the 95% CI includes benefit or harm of skin care intervention.
1.31. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 31: Sensitivity analysis: Food allergy by 1‐3 years (parent report of doctor diagnosis)
One additional trial randomising a total of 53 participants reported some narrative information on food allergy for only the control group that was not suitable for inclusion in meta‐analysis (Thitthiwong 2019). It was narratively reported that "none of the 4 IAD infants developed cow’s milk protein allergy or any other food allergy".
As only one study reported the primary food allergy outcome, we did not conduct planned subgroup analysis at the study level. Access to Individual participant data did allow us to assess the interaction between actual age of treatment initiation and treatment. Based on data available for 996 participants in Chalmers 2020, the RR of food allergy for starting skin care treatment at ≥ 4 days of age, in comparison to initiation of skin care treatment < 4 days, was 0.51 (95% CI 0.07 to 3.53). The large width of the 95% confidence interval indicates uncertainty surrounding the direction and magnitude of any effect of age of treatment initiation.
The CACE for an individual using a skin care intervention for three or more days a week could be estimated for one study providing adequate compliance and food allergy outcome data (Chalmers 2020). The CACE on food allergy assessed via an oral food challenge for an individual using a skin care intervention for three or more days a week from 996 participants was accompanied by considerable uncertainty, as reflected by a very wide 95% CI (RR 31.19, 95% CI 0.43 to 2236.62; Analysis 1.32). Whilst the point estimate for the RR was more in favour of standard care in comparison to the intention‐to‐treat effect of RR 2.53 (95% CI 0.99 to 6.47) (Analysis 1.29), the 95% CI for the CACE estimate is considerably wider than for the intention‐to‐treat effect and is consistent with the intention‐to‐treat effect of no difference. Therefore we cannot infer any difference in treatment effect for a complier. Additional CACE estimates for alternative definitions of a complier are displayed in Table 8 and similarly do not demonstrate an impact of compliance on the treatment effect due to a large amount of uncertainty in estimation.
1.32. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 32: CACE: Food allergy by 1‐3 years for use over intervention period ≥ 3 days a week
7. Complier average causal effect analyses for food allergy.
| Complier | N trials | N intervention | N control | CACE | Intention‐to‐treat | ||
| Pooled RR | 95% CI | Pooled RR | 95% CI | ||||
|
Primary CACE: ≥ 3 days of use over interventionperiod |
1a | 491 | 505 | 31.19 | 0.43 to 2236.62 | 2.53 | 0.99 to 6.47 |
| Sensitivity: ≥ 5 days of use over intervention period |
1a | 491 | 505 | 47.49 | 0.09 to 24643.91 | 2.53 | 0.99 to 6.47 |
| 7 days use over intervention period | 1a | 491 | 505 | 125.21 | 0.00 to 315317.50 | 2.53 | 0.99 to 6.47 |
| ≥ 3 days of use over first 3 months of intervention period | 1a | 491 | 505 | 7.39 | 0.79 to 69.02 | 2.53 | 0.99 to 6.47 |
| ≥ 5 days of use over first 3 months of intervention period | 1a | 491 | 505 | 8.08 | 0.56 to 116.23 | 2.53 | 0.99 to 6.47 |
| 7 days use over first 3 months of intervention period | 1a | 491 | 505 | 19.11 | 0.11 to 3310.01 | 2.53 | 0.99 to 6.47 |
aOne study is included across all presented analyses: Chalmers 2020.
Trial sequential analysis shows that a target sample size of 7602 would be necessary to demonstrate a minimum relative risk reduction of 30% (assuming a control rate of 5% versus skin care intervention 3.5%) with 90% power (Figure 4). A target sample size of 18,063 would be necessary to demonstrate a minimum relative risk reduction of 20% (assuming a control rate of 5% versus skin care intervention 4%) with 90% power (Figure 5). Based on the data accumulated to date included within the primary food allergy meta‐analysis (996 participants), the meta‐analysis is inconclusive for a relative effect size of 30 or smaller, because it has not yet crossed the upper or lower boundary for statistical significance or non‐superiority.
4.
Trial sequential analysis for food allergy (RR of 30%).
5.
Trial sequential analysis for food allergy (RR of 20%).
Secondary outcomes
Adverse effects
Our adverse events of interest for which we separately report meta‐analysis below are skin infections, stinging or allergic reactions, slippages, and serious adverse events recorded over the study intervention period. Ten trials reported some data on one or more of these specific adverse events, including seven trials that could be included in one or more meta‐analyses.
An additional nine trials randomising 18,869 participants (one trial randomised 17,530 participants) provided a short general qualitative narrative on adverse events that was not eligible for inclusion in the meta‐analysis (Dissanayake 2019; Garcia Bartels 2011; Horimukai 2014; Lavender 2012; Migacheva 2018; Raisi Dehkordi 2010; Sankaranarayanan 2005; Thitthiwong 2019; Tielsch 2007). Of these nine trials, six trials randomising 18,593 participants (9412 control, 9181 intervention) narratively reported no intervention‐related adverse events (Dissanayake 2019; Horimukai 2014; Lavender 2012; Migacheva 2018; Thitthiwong 2019; Tielsch 2007). Of the remaining three trials, Sankaranarayanan 2005 (randomised 112 term babies to groups of 38 coconut oil, 37 mineral oil, and 37 placebo) reported, "3 in the coconut oil group, 3 in the mineral oil group and 2 in the placebo group developed mild rash that did not require discontinuation of application”; Garcia Bartels 2011 (randomised 44; 20 lotion and 24 no lotion) reported, "the overall occurrence of adverse events (AEs) was lower in group L (lotion) (n = 18) than in group WL (without lotion) (n = 33, Table 3)"; and Raisi Dehkordi 2010 (randomised 120) reported, "some adverse events in the oil massage groups; however they were mild rash, which required no cessation”.
Skin infections
Individual participant data on skin infections were available from six studies (Chalmers 2020; Cooke 2015; Lowe 2018a; McClanahan 2019; Simpson 2014; Skjerven 2020) including a total of 4209 randomised participants. Pooled individual participant data available from 2728 participants in these studies were in favour of standard care (RR 1.34, 95% CI 1.02 to 1.77; Analysis 1.38) with I2 = 0 (P = 0.99).
1.38. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 38: Adverse event: skin infection
One additional trial that randomised 70 participants (35 to each group) reported weekly infection rates over four weeks of 0 (0%), 3 (8.6%), 7 (20%), and 5 (14.3%) in the control group versus 0 (0%), 0 (0%), 1 (2.9%), and 0 (0%) in the skin care group (Amer 2017).
Stinging or allergic reactions
Individual participant data on stinging or allergic reactions to moisturisers were available from four studies (Cooke 2015; Lowe 2018a; McClanahan 2019; NCT03376243) including a total of 349 randomised participants. Pooled individual patient data available from 343 participants in these studies were in favour of standard care (RR 2.24, 95% CI 0.67 to 7.43; Analysis 1.39) with I2 = 0 (P = 0.99) but with some uncertainty about the true effect, with the 95% CI including no difference and a benefit of skin care intervention.
1.39. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 39: Adverse event: stinging or allergic reaction to moisturisers
One study randomising 120 participants to Johnson's Baby Top‐To‐Toe wash (group 1, n = 60), Sebamed Baby Liquid Cleanser (group 2, n = 60), and lukewarm tap water (group 3, n = 60) reported, "in group I, 1/60 subjects had mild rashes and redness on the neck and arms that appeared around 4 days after use. The irritation appeared 2‐3 hours after bathing and lasted for a few minutes. In group II, 2/ 60 subjects had irritation in the first week. These were mild rashes on the back and leg and lasted for 1‐ 2 days. For group II, 1/60 subjects had mild rashes and dryness 3 days after starting use of the product. No irritation was noted with any of the three compounds in the second week" (Dizon 2010). It was also reported how "no statistically significant irritation was visible to the clinician for all three groups of the study".
One additional study randomised 93 participants to receive the first bath with chlorhexidine (n = 44) or neutral liquid soap in the control group (n = 49) (Da Cunha 2008). No adverse effects were reported for the use of chlorhexidine , including skin irritation.
Slippage accidents
Individual participant data on slippage accidents were available from four studies (Chalmers 2020; Lowe 2018a; Simpson 2014; Skjerven 2020) including a total of 3994 randomised participants. Two studies recorded no slippage events in both treatment groups (Simpson 2014; Skjerven 2020 ). Pooled individual patient data available from 1242 participants in two studies where the treatment effect could be estimated (Chalmers 2020; Lowe 2018a) favoured standard care (RR 1.42, 95% CI 0.67 to 2.99; Analysis 1.40) with I2 = 0 (P = 0.68) but with some uncertainty about the true effect, with the 95% CI including no difference and a benefit of skin care intervention.
1.40. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 40: Adverse event: slippage accidents
Skjerven 2020 provided data on slippage accidents and was a 2 x 2 factorial trial. Because significant interaction was noted between the two interventions in this trial (food intervention and skin care intervention), we included in our analysis only data from the skin care intervention group versus the control group, in line with our pre‐specified statistical analysis plan (Cro 2020a). One accident connected with bathing was reported for a participant in the food and skin care intervention group (1/583, 0.2%).
Serious adverse events
Individual participant data on serious adverse events were available from three studies including a total of 2591 randomised participants (Cooke 2015; Lowe 2018a; Skjerven 2020). Pooled individual patient data available from 1367 participants in these studies favoured standard care (RR 1.80, 95% CI 0.45 to 7.18; Analysis 1.41) but with some uncertainty about the true effect, with the 95% CI including no difference and a benefit of skin care intervention. Evidence shows some heterogeneity (I2 = 51, P = 0.13); however only three trials were included in this comparison, each of which had wide confidence intervals for the effect size, including no difference and a benefit of standard care and a benefit of skin care intervention due to small numbers of events. See Table 9 for a description of reported serious adverse events.
1.41. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 41: Serious Adverse Events
8. Serious adverse events.
| Trial | Serious adverse event |
Skin care (N events) |
Control (N events) |
Total (N events) |
| Cooke 2015 | Jaundice | 3 | 0 | 3 |
| Viral lung infection | 1 | 0 | 1 | |
| Seizure (benign myoclonic jerks) | 1 | 0 | 1 | |
| Total events | 5 | 0 | 5 | |
| Lowe 2018a | Bronchiolitis | 3 | 1 | 4 |
| Fever infection | 1 | 0 | 1 | |
| Respiratory distress | 1 | 0 | 1 | |
| Total events | 5 | 1 | 6 | |
| Skjerven 2020 | Allergic reaction | 1 | 2 | 3 |
| Seizure (non‐febrile) | 1 | 1 | 2 | |
| Other | 13 | 18 | 31 | |
| Bronchiolitis (RS virus or other) | 9 | 6 | 15 | |
| Croup | 0 | 1 | 1 | |
| Foreign body aspiration | 0 | 1 | 1 | |
| Influenza | 0 | 1 | 1 | |
| Surgery (operation) | 1 | 5 | 6 | |
| Pneumonia | 2 | 1 | 3 | |
| Flu/diarrhoea | 3 | 1 | 4 | |
| Injury or accident | 3 | 4 | 7 | |
| Urinary infection | 2 | 3 | 5 | |
| Unspecified reaction | 6 | 10 | 16 | |
| Total events | 41 | 54 | 95 |
Eczema severity
Individual participant data on eczema severity as assessed by a clinician at one to three years were available from three studies including 1528 randomised participants (Chalmers 2020; Lowe 2018a; NCT03376243). Eczema severity was measured using the EASI at 24 months for Chalmers 2020; the EASI at 12 months for NCT03376243; and the objective SCORAD at 12 months for Lowe 2018a. When no eczema was present, two studies had recorded an eczema severity rating of 0 (Chalmers 2020; NCT03376243). For one study, we imputed an eczema severity rating of 0 for the purpose of analysis when no eczema was reported (Lowe 2018a).
We first assessed the risk of moderate/severe/very severe eczema versus clear/mild eczema. Two studies did not record any incidences of moderate/severe/very severe eczema in either treatment group amongst a total of 108 participants (58 skin care intervention versus 50 standard care) (Lowe 2018a; NCT03376243). In the third study (Chalmers 2020), data on eczema severity, measured by a clinician at two years, were available for 1120 participants and showed no difference between treatment groups (RR 0.92, 95% CI 0.37 to 2.27; Analysis 1.42). The 95% CI for the RR is wide, indicating no difference or small to large harm, and benefit of the skin care intervention for moderate/severe/very severe eczema cannot be ruled out based on this one study.
1.42. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 42: Clinician‐assessed eczema severity at 1‐3 years (clear/mild versus moderate/severe/very severe)
We subsequently assessed the pooled standardised mean treatment group difference for clinician‐assessed eczema severity. Pooled individual participant data available from 1228 participants in the three studies providing participant data on clinician‐assessed eczema severity showed no difference between treatment groups (SMD ‐0.02, 95% CI ‐0.17 to 0.12; Analysis 1.43) with I2 = 7% (P = 0.34) (Chalmers 2020; Lowe 2018a; NCT03376243). When the SMD is re‐expressed on the EASI Scale, this is equivalent to an MD of ‐0.035 (95% CI ‐0.296 to 0.209), using the pooled SD of 1.74 for EASI as observed in the largest study (Chalmers 2020).
1.43. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 43: Clinician‐assessed eczema severity at 1‐3 years (standardised mean difference)
An additional study randomising 63 participants (31 intervention, 32 control) that did not provide individual participant data reported narratively that 60 infants completed the study (29 intervention, 31 control), and that the severity of eczema measured using the SCORAD in the control group was significantly greater than in the intervention group "(22.6 +/‐ 12.9 vs 17.6 +/‐ 5.3 respectively, U = 348, P < 0.058)" (Migacheva 2018). Bellemere 2018 reported that amongst 120 randomised participants (60 intervention, 60 control), the frequency of AD during the first six months of life was 9.8% in the prevention group, 18.3% in the control group, and 6.7% in the no‐risk group.....Mean SCORAD scores were 24.1 and 23.3 in the at‐risk groups".
Parent‐assessed eczema severity
Individual participant data on eczema severity as assessed by a parent were available from one study including 1394 randomised participants (Chalmers 2020). Eczema severity was measured using the POEM at 24 months. We first assessed risk of moderate/severe/very severe eczema versus clear/mild eczema as assessed by the parent. Data on eczema severity, as assessed by a parent at two years, were available for 1171 participants and showed no difference between treatment groups (RR 1.17, 95% CI 0.82 to 1.67; Analysis 1.44 ). The width of the 95% CI for the RR indicates that we cannot rule out no difference or small to large favouring of standard care or skin care intervention for moderate/severe/very severe eczema based on this one study.
1.44. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 44: Parent‐reported eczema severity at 1‐3 years (clear/mild versus moderate/severe/very severe)
We subsequently assessed the mean difference for parent‐assessed eczema severity for the only study with available data (Chalmers 2020). Individual participant data available from 1171 participants showed no difference between treatment groups (MD 0.07, 95% CI ‐0.38 to 0.52; Analysis 1.45).
1.45. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 45: Parent‐reported eczema severity at 1‐3 years (mean difference)
Time to onset of eczema
Individual participant data on time to eczema onset were available from nine studies, including 5042 randomised participants (Chalmers 2020; Dissanayake 2019; Horimukai 2014; Lowe 2018a; McClanahan 2019; Simpson 2014; Skjerven 2020; NCT03376243; Yonezawa 2018). Eczema was measured using the Hanifin and Rajka criteria (Hanifin 1980), or the UK Working Party refinement of them (Williams 1994), or other modifications of the Hanifin and Rajka criteria for six studies (Dissanayake 2019; Horimukai 2014; Lowe 2018a; McClanahan 2019; NCT03376243; Skjerven 2020), was doctor diagnosed for two studies (Simpson 2014; Yonezawa 2018), and was assessed by first parent report of a clinical diagnosis for one study (Chalmers 2020). Eczema onset was assessed up to six months for one study (Simpson 2014); up to 8 months (32 weeks) for one study (Horimukai 2014); up to 12 months for four studies (Dissanayake 2019; Lowe 2018a; NCT03376243; Skjerven 2020); and up to 24 months for three studies (Chalmers 2020; McClanahan 2019; Yonezawa 2018). For each trial, data were censored at the trial‐specific last measured time point for individuals not experiencing eczema.
Pooled individual patient data available from 3349 participants in these nine studies showed no benefit of skin care intervention (hazard ratio 0.86, 95% CI 0.65 to 1.14; Analysis 1.46). However the 95% CI for the pooled HR indicates that we cannot rule out potential benefit or harm, extending from 0.65 to 1.14. The median time to eczema onset across included studies in the control group was six months. The pooled hazard ratio corresponds to a median time to eczema onset of 6.98 months (95% CI.26 to 9.23 months) in the skin care intervention group. Results show moderate statistical heterogeneity between studies for this outcome (I2 = 53%, P = 0.03). Post‐hoc subgroup analysis, conducted to explore heterogeneity, showed a significant interaction effect (P = 0.03) of trial follow‐up (< 1 year versus ≥ 1 year). The pooled HR from the two studies with follow‐up less than a year was 0.55 (95% CI 0.35 to 0.87) (I2 = 0%, P = 0.67) (Analysis 1.48). However, as this result is based upon a total of only 224 participants from two studies that followed up participants for only six months or eight months, there is uncertainty as to whether the skin care intervention delays eczema. The pooled HR from the seven studies with follow‐up of at least one year was 1.00 (95% CI 0.75 to 1.33) (I2 = 44%, P = 0.10) (Analysis 1.48). Remaining heterogeneity amongst studies with follow‐up of at least one year is driven by one trial (Skjerven 2020). When Skjerven 2020 was further excluded, the pooled HR with follow‐up of at least one year was 0.91 (95% CI 0.78 to 1.07) (I2 = 0%, P = 0.67), which continues to showing no benefit of skin care intervention. This heterogeneity may be driven by type of intervention (bathing and oil and emollient applied to the face only) and/or by timing of intervention initiation (from two weeks).
1.46. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 46: Time to onset of eczema
1.48. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 48: Parent report of immediate (< 2 hours) reaction to a known common food allergen
One additional study that did not provide individual participant data with a follow‐up period of nine months did not identify any diagnoses of eczema in the skin care intervention group and reported four (14.8%) AD diagnoses in the control group, for whom "the mean age of the 4 infants at the onset of IAD was 5.5 ± 0.55 months" (Thitthiwong 2019).
Parent report of immediate reaction to food allergen
Individual participant data on parental report of an immediate reaction (within two hours) to a known common food allergen at one to three years were available from two studies, including 1448 randomised participants (Chalmers 2020; NCT03376243). In one study, no immediate reactions were reported in either treatment group from a total of 41/54 participants who were followed up to one year (NCT03376243). In the the other study, data were available for 1171/1394 participants (Chalmers 2020), who were followed up to two years; reactions were reported for 118/574 (21%) of the skin care intervention group and for 96/597 (16%) of the standard care group, favouring standard care (RR 1.27, 95% CI 1.00 to 1.61; Analysis 1.48).
For one trial (Chalmers 2020), we were able to examine parental reports of an immediate reaction (within two hours) separately for milk, egg, and peanut. Reactions to milk were reported for 61/575 (11%) of the skin care intervention group and for 46/598 (8%) of the standard care group, favouring standard care on average but with some uncertainty about the true effect, with the 95% CI including no difference (RR 1.38, 95% CI 0.95 to 2.00; Analysis 1.49). Reactions to egg were reported for 44/575 (8%) of the skin care intervention group and for 41/598 (7%) of the standard care group, favouring standard care on average but with some uncertainty about the true effect, with the 95% CI including no difference and a benefit of skin care intervention (RR 1.12, 95% CI 0.74 to 1.68; Analysis 1.50). Reactions to peanuts were reported for 8/574 (1.4%) of the skin care intervention group and for 10/598 (2%) of the standard care group, favouring skin care intervention on average but with some uncertainty about the true effect, with the 95% CI including no difference and a benefit of standard care (RR 0.84, 95% CI 0.33 to 2.10; Analysis 1.51).
1.49. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 49: Parent report of immediate (< 2 hours) reaction to milk
1.50. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 50: Parent report of immediate (< 2 hours) reaction to egg
1.51. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 51: Parent report of immediate (< 2 hours) reaction to peanut
Allergic sensitisation to foods or inhalants
Allergic sensitisation data on foods or inhalants at one to three years were available from two studies, including 1474 randomised participants (Chalmers 2020; Lowe 2018a). In one study (Chalmers 2020), data were available for 988/1394 participants who were followed up at two years; sensitization was reported for 88/490 (18%) of the skin care intervention group and for 74/498 (15%) of the standard care group. In the second study (Lowe 2018a), data were available for 70/80 participants who were followed up at one year; sensitisation was reported for 6/34 (18%) of the skin care intervention group and for 8/36 (22%) of the standard care group. Pooled individual patient data available from 1058 participants in these studies favoured standard care but there is uncertainty about the true effect, with the 95% CI including a benefit of skin care intervention and no treatment difference (RR 1.09, 95% CI 0.72 to 1.66) (I2 = 24%; Analysis 1.52).
1.52. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 52: Allergic sensitisation to common foods or inhalants at 1‐3 years
For allergic sensitisation to food only, data were available for 985/1394 participants in Chalmers 2020 who were followed up at two years; sensitisation was reported for 58/487 (12%) of the skin care intervention group and for 44/498 (9%) of the standard care group. In the second study (Lowe 2018a), data were available for 70/80 participants who were followed up at one year; sensitisation was reported for 3/34 (9%) of the skin care intervention group and for 7/36 (19%) of the standard care group. Pooled individual patient data available from 1055 participants in these two studies showed reduced food sensitisation in the skin care intervention groups but there is uncertainty about the true effect, with the 95% CI including benefit or harm of skin care intervention and no treatment difference (RR 0.86, 95% CI 0.28 to 2.69) (I2 = 70%; Analysis 1.53). There was a high level of statistical heterogeneity across the two included trials (I2 = 70%, P = 0.07), which could not be explained. We separately pooled results for milk, egg, and peanut across the two trials providing allergic sensitisation data on foods or inhalants at one to two years. Pooled results for milk were RR 1.16 (95% CI 0.55 to 2.43) (Analysis 1.54), for egg RR 0.75 (95% CI 0.18 to 3.08) (Analysis 1.55), and for peanut RR 1.03 (95% CI 0.53 to 2.01) (Analysis 1.56). Each separate food analysis also showed a high level of heterogeneity across the two included trials.
1.53. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 53: Allergic sensitisation to common foods at 1‐3 years
1.54. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 54: Allergic sensitisation to milk at 1‐3 years
1.55. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 55: Allergic sensitisation to egg at 1‐3 years
1.56. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 56: Allergic sensitisation to peanut at 1‐3 years
Two additional studies reported allergic sensitisation to foods at eight months and at nine months (Dissanayake 2019; Horimukai 2014). When data from these two studies were included in sensitivity analyses, the pooled treatment effect was RR 1.08 (95% CI 0.87 to 1.33), with I2 = 35% (P = 0.20) (Analysis 1.58). Pooled results for milk were RR 0.84 (95% CI 0.59 to 1.21) (Analysis 1.59), for egg RR 1.09 (95% CI 0.88 to 1.37) (Analysis 1.60), and for peanut RR 1.03 (95% CI 0.53 to 2.01) (Analysis 1.61). Again, each separate analysis continued to show a high level of heterogeneity across included trials.
1.58. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 58: Sensitivity analysis: Allergic sensitisation to common foods at 6 months‐3 years
1.59. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 59: Sensitivity analysis: Allergic sensitisation to milk at 6 months‐3 years
1.60. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 60: Sensitivity analysis: Allergic sensitisation to egg at 6 months‐3 years
1.61. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 61: Sensitivity analysis: Allergic sensitisation to peanut at 6 months‐3 years
One additional study that did not provide individual participant data with a follow‐up period of six months reported eczema onset for "5/35 of the intervention group and 6/32 of the control group. And eczema onset ratio among infants who reacted positive to a prick test were 5/1 vs. 6/8" (Kataoka 2010).
Discussion
Summary of main results
A summary of the main results is shown in Table 1.
Our key results, presented here, are based on 10 trials that provided individual participant data (IPD). These studies assessed skin care interventions that aim to promote hydration or barrier function: eight assessed emollients; one assessed combined skin interventions, including emollients; and one assessed topical oils, although this study provided IPD only for adverse events.
For our primary outcome of eczema, this review identified data from 3075 participants in seven randomised controlled trials and found that skin care interventions probably do not influence the development of eczema by age one to two years in healthy term infants when compared with standard care. The certainty of this evidence was moderate. One trial ‐ Skjerven 2020 ‐ showed an increase in eczema in the intervention group, leading to some statistical heterogeneity in the main eczema analysis. This was a factorial randomised trial with skin care interventions and early allergenic food introduction. Due to significant interaction between interventions, only the skin care and control arms of the trial could be utilised in the primary analysis. The skin intervention was a combination of daily facial emollient with daily baths with paraffin‐based bath oil, which is a somewhat different intervention than was provided in the other trials providing data towards the primary analysis. This difference in intervention may explain the statistical heterogeneity seen in the main eczema analysis, with daily baths potentially having an adverse effect on skin barrier function and risk of eczema development compared with direct emollient application. Our pre‐planned subgroup analyses show that the following did not influence the effect of intervention on risk of developing eczema: family history of atopy or FLG mutation, classification of intervention type, duration of intervention, and age. We could not evaluate these effects on risk of food allergy. We also found that the skin care interventions used probably do not change time to onset of eczema when compared with standard care (based on 3349 participants in nine trials; moderate‐certainty evidence). This is thought to be important in the interaction between eczema and food allergy because increased length of time with eczema is associated with increased likelihood of food sensitisation (Tsilochristou 2019). Overall, evidence from this review demonstrates with moderate certainty that the skin care interventions used in these randomised controlled trials (RCTs) do not impact the development of eczema (Chalmers 2020; Dissanayake 2019; Horimukai 2014; Lowe 2018a; McClanahan 2019; NCT03376243; Simpson 2014; Skjerven 2020; Yonezawa 2018).
For our co‐primary outcome of food allergy, we are uncertain whether skin care interventions influence development of immunoglobulin (Ig)E‐mediated food allergy when compared with standard care. Unfortunately, due to delays in the progress of trial outcome assessments related to the COVID‐19 pandemic, food allergy outcomes from Skjerven 2020 were not available for inclusion in this review. Only one trial with 996 participants had food allergy diagnosed by oral food challenge as an outcome (Chalmers 2020). The certainty of this evidence is very low, and we are thus uncertain whether skin care interventions influence risk of food allergy by age one to two years, compared with standard care. A sensitivity analysis for data from the same trial, using an alternative method for food allergy outcome assessment (combination of oral food challenge and investigator assessment using clinical history and sensitisation data), also favoured standard care (1115 participants, one trial), suggesting that skin care interventions may increase risk of food allergy. The width of the 95% confidence interval (CI) for the risk ratio (RR) is reduced, but it is still wide, indicating that we cannot rule out no difference nor small to large harm of skin care intervention based on this one study.
Data from the same trial (1171 participants) suggest that again when compared with standard care, skin care interventions may slightly increase the risk of parent‐reported immediate reaction to a common food allergen at two years, but this association was seen only for parent‐reported immediate reaction to cow's milk, which is thought to be an unreliable measure of IgE‐mediated cow's milk allergy due to commercially influenced over‐reporting of cow's milk allergy in infants (Munblit 2020). Hence, the certainty of this evidence was considered low. Evidence for allergic sensitisation to food was of very low certainty due to statistical heterogeneity and severe imprecision, with increased allergic sensitisation to food in the intervention group in one trial (Chalmers 2020), as well as decreased allergic sensitisation to food in the intervention group in a second trial (Lowe 2018a); both studies compared skin care interventions against standard care, with 1055 participants included in this analysis.
Complier average causal effect (CACE) analysis was utilised to assess whether compliance with the assigned intervention influenced outcomes. For both > 3 days per week and > 5 days per week, although the CACE favoured the intervention for the eczema outcome, the confidence interval ranged from large benefit to moderate harm; therefore, we were unable to demonstrate whether increased adherence to assigned interventions was associated with different outcomes. Insufficient evidence meant that we were unable to ascertain whether treatment adherence affected risk of developing food allergy.
For adverse events, the key adverse events of interest were local infection, stinging or allergic reactions to moisturisers, and slippage accidents. We found that compared to standard care, skin care interventions probably increase the risk of skin infection in an analysis that included IPD from 2728 infants participating in six trials (moderate‐certainty evidence). In terms of stinging and allergic reactions (four trials including 343 participants) or slippage accidents (four trials including 2538 participants), there may be an increase in these adverse events with the intervention; however, the certainty of these findings was low due to very small numbers of events, yielding very wide confidence intervals which include the possibility of no effect or reduced risk.
Overall completeness and applicability of evidence
Although 33 studies were included in the review, only 17 reported outcomes relevant to this review. Of these 17 studies, eight reported our primary outcome cumulative incidence of eczema by one to three years, and three of these studies measured eczema severity between one and three years by clinician assessment, with one of these studies also reporting parent report of eczema severity. Nine studies measured time to onset of eczema. Two studies reported parent report of immediate food allergy, and two studies reported allergic sensitisation to food or inhalant allergen. Ten studies reported adverse event data for our pre‐specified adverse events of interest.
Only 10 trials provided IPD analysis. Most of the other identified trials (SCiPAD ‐ Skin Barrier Interventions for Prevention of Allergic Disease) included types of skin barrier interventions other than emollients, such as change in bath routine, but they did not provide follow‐up long enough after intervention to identify our primary outcomes, and they were limited to contributing some narrative data on safety outcomes. Therefore, the impact of non‐emollient interventions on the development of eczema or food allergy, rather than their short‐term impact on skin barrier physiology or safety outcomes, is unknown.
All of the trials reporting eczema as an outcome used an emollient on its own as the intervention, or within a combination of skin care interventions, and commenced within the first month of life. Eczema was largely evaluated by a blinded trained assessor using widely accepted tools such as Hanifin and Rajka or UK Working Party criteria for diagnosis. Therefore, we can be confident in the diagnosis of eczema in these studies. Interventions mainly focused on emollients, with each study using a different emollient with different constituents. Trials also had variable regimens and durations of emollient therapy. The emollients used were a combination of so called simple emollients and more complex emollients that contain lipids. Emollient regimens varied in intensity from twice‐daily all‐over body emollient to once daily on cheeks in combination with other skin care interventions. Skin care for infants varies by country and culture, and there is no standard recommendation. Thus, our comparison of control (standard skin care or no skin care intervention) may have varied between studies.
We analysed data based on assignment to intervention rather than on adherence to intervention. Compliance with daily emollient, when reported, was low but may have been higher in smaller pilot studies, in which potentially greater input from the research team could have aided compliance. Complier average causal effect was utilised to assess whether compliance with the assigned intervention influenced outcomes. We were, however, unable to identify whether or not adherence to skin care interventions influences risk of eczema.
The prospective portion of this meta‐analysis, planned in 2017, ensured that the two largest trials in the series had aligned outcomes and used similar methods of outcome evaluation (Chalmers 2020; Skjerven 2020). Meta‐analysis of these two studies and another five studies providing IPD on the development of eczema by one to three years allowed us to assess both study factors and individual participant factors that may influence results. When we evaluated study factors such as type of emollient used and duration of intervention and individual factors such as age, FLG genotype, or family history of atopy, we found no evidence for an interaction between these factors and the intervention on eczema risk.
Ongoing studies are randomising newborn healthy infants to a skin care intervention; half are assessing emollients. NCT03871998 is designed to address whether the emollient intervention applied from day one or two of life for a short (two month) time period would influence eczema and IgE‐mediated food allergy development. Lowe 2019 and Eichner 2020 are evaluating daily complex emollients and are expected to report in 2022. It is possible that the use of more complex emollient interventions may have different effects on risk of eczema.
Sixteen studies that were eligible for the review did not have relevant outcome measures (i.e. they did not record eczema or food allergy at one to three years or at any time, and they did not comment on adverse events. Infants in these trials were followed up to a maximum of four weeks, which was too soon to assess whether eczema or food allergy was present. These trials mainly involved different timings and mechanisms of bathing infants, along with different types of bathing products. Their follow‐up was four weeks or less, and outcomes were skin physiology, such as transepidermal water loss. It is possible that had these infants been followed up for longer, and had eczema outcome been measured, these skin care practices would have an effect on eczema. Furthermore, although "skin care intervention type B ‐ any skin care interventions that aimed to protect the skin barrier from irritation such as water softeners" were pre‐specified in our search, they could not be assessed, as no suitable trials were found. A trial of water softeners is currently ongoing (Jabbar‐Lopez 2019).
Evidence for effects of skin care interventions in infants on risk of food allergy is incomplete. Only one trial reported food allergy outcomes using oral food challenge, within the time period set out in the protocol (Chalmers 2020); however, these results were inconclusive, as the quality of evidence for food allergy in this study was low or very low due to high risk of bias and imprecision of the result, with wide confidence intervals. Evidently as only one trial had food allergy outcomes, we could not look at the effects of planned subgroup analyses on food allergy. Skjerven 2020 intended to contribute food allergy outcomes to this review; however the clinical trial team experienced delay with final data collection due to the COVID‐19 pandemic, which means that these data were not complete in time for this review. It will be important to update this review when food allergy outcomes are available from more trials, including Skjerven 2020. Food allergy is a more difficult outcome to measure reliably than eczema, and it is a less common condition than eczema. Oral food challenge is the currently accepted standard for food allergy diagnosis, but a European initiative is currently working on development of core outcome measures for food allergy in clinical trials (COMFA 2020).
Quality of the evidence
Our confidence in the findings for eczema was moderate. Sensitivity analysis including only studies with low risk of bias was consistent with the primary analysis. We downgraded the certainty of evidence for eczema due to statistical heterogeneity that could not be explained. For the primary eczema outcome, this heterogeneity was due to one trial (Skjerven 2020), which found increased eczema in the intervention group compared with the control group. One potential cause of this heterogeneity is the nature of the intervention. Skjerven 2020 used a bathing intervention, and other trials contributing to the primary eczema analysis used direct application of a moisturiser to the infant's skin. However, for the outcome time to onset of eczema, we downgraded the certainty of evidence due to statistical heterogeneity, and heterogeneity in this analysis was due to more than one trial.
Our confidence in the findings for food allergy was low or very low. We downgraded the certainty of evidence for food allergy due to severe imprecision, with small numbers of studies and events and wide confidence intervals encompassing both a harmful effect and no effect. For the primary food allergy outcome, we also downgraded due to risk of bias related to missing outcome data. In the only trial contributing to this analysis (Chalmers 2020), participants who were invited for an oral food challenge due to suspected food allergy frequently declined to attend an oral food challenge, and the proportion that did attend differed between intervention group (29%) and control group (17%), suggesting high risk of bias related to missing outcome data. Although the findings are similar if all those with missing outcome data are assumed to not have food allergy, they differ if patients are assumed to have food allergy. Findings also differ in sensitivity analysis when investigator assessment was used for categorising participants with no available oral food challenge data (Analysis 1.30). For the secondary food allergy outcome, allergic sensitisation to a food allergen, we also downgraded due to statistical heterogeneity that could not be explained.
Our confidence in the findings for adverse events was moderate or low. We downgraded the certainty of evidence for skin infection due to imprecision, with wide confidence intervals, which included a harmful effect and no effect. We downgraded the certainty of evidence for slippages and stinging/allergic reactions to moisturisers due to severe imprecision, with small numbers of events and wide confidence intervals, which included both a harmful effect and a beneficial effect. All studies included in the adverse events analyses, except one, were rated at some concerns for risk of bias for the specific adverse events assessed, but this was due to the nature of adverse events being self‐recalled by carers, who could not be blinded due to the type of intervention under study. Otherwise there were no concerns for risk of bias for adverse events.
Potential biases in the review process
The meta‐analysis collaboration group for this review (SCiPAD ‐ Skin Barrier Interventions for Prevention of Allergic Disease) was formed in 2017 through collaboration between the two largest trials ‐ Chalmers 2020 and Skjerven 2020. The purpose of the prospective collaboration was to increase the alignment of outcomes measured in the two individual trials and to enhance the power of individual trials to identify effects of the interventions on the outcome of food allergy, which is less prevalent than eczema. Other trial groups were invited to join the collaboration between 2017 and 2020, and their data formed the basis of the prospective portion of the meta‐analysis. Prospective meta‐analysis allows for alignment of outcomes before completion of the trial, so that comparisons and analysis are more readily conducted between trials. Within this collaborative group, trials mainly involved emollients as an intervention and eczema as an outcome. We attempted to reduce any availability bias by conducting a sensitive search, which identified over 6000 potentially eligible studies, and by contacting authors of eligible studies to request their collaboration in the IPD meta‐analysis, but follow‐up for most studies was too short for inclusion.
We classified collaborating trials when trial outcome data were not analysed and known to study authors before February 2017 as contributing 'prospectively acquired data'. Data from some of these trials became known to the investigators or to the public before the statistical analysis plan (SAP) for this meta‐analysis was locked, so that in theory, findings could have influenced the design of the statistical analysis plan. Development of the SAP was led by a statistician (SC) with no detailed knowledge of eligible trial publications at the time of finalising the SAP, and the principles of the SAP and the review protocol were aligned with the February 2017 PROSPERO registration of the prospective meta‐analysis (Boyle 2017). The SAP was signed off before any unblinding of individual participant data sets received from individual trials. Due to our close collaboration with trial investigators, they were involved in protocol and SAP development and in interpretation of meta‐analysis findings. A strength of this approach was that collaborating trialists had the opportunity to review our analyses of their data sets before stage 2 of the IPD meta‐analysis and to correct any misinterpretation of data coding. MK and RB were quite involved with the Chalmers 2020 trial, with RB the principal investigator for food allergy, and MK involved in food allergy diagnosis. Therefore, they were not involved in data extraction nor risk of bias assessment for this trial, other than as investigators commenting on meta‐analysis findings.
Use of IPD in this review allowed us to (i) fit a consistent analysis model to trial data sets for each outcome to ensure that we compared treatment effects adjusted for the same covariates across trials; (ii) fully explore the risk of bias due to missing data by conducting additional sensitivity analyses that had not previously been reported; (iii) obtain more reliable and powerful subgroup analyses including estimation of treatment interactions with participant characteristics that had not previously been reported; and (iv) evaluate the relationship between compliance with the intervention and outcomes of interest. However, the method of sharing IPD can result in availability bias. We attempted to limit this by offering all trials administrative assistance in working with their individual institutions for data agreement development and sign‐off and for data sharing and extraction. Overall, trials with relevant outcomes from which we did not manage to access IPD were generally small (fewer than 100 participants) or were industry‐funded and researchers were concerned about the commercial impact of data sharing. Only one trial, with 60 participants, had primary outcome data eligible for inclusion in the meta‐analysis and did not supply IPD; sensitivity analysis including aggregate data from this one trial showed similar findings to the main analysis. One industry‐funded trial was involved in the SCiPAD collaboration (NCT02906475 ); however, this trial is ongoing, and results are expected in 2022.
Overall, the close relationship between trialists and systematic reviewers in this project carries risk of availability bias and academic bias towards individual clinical trial findings, which is mitigated only in part by the prospectively planned nature of the meta‐analysis for most studies contributing data.
The interventions used in included trials varied in their composition, and there is no standard classification system for emollients (Surber 2017). This led to difficulty in classification of the intervention for the purpose of subgroup analysis, as different emollients have overlapping constituents, which may have differential effects on the skin barrier or on skin health. We took advice from a member of our collaboration (MC) with expertise in emollient formulation and effects on skin barrier, who classified the interventions used before seeing the meta‐analysis results. These were used for the subgroup analysis of 'basic' versus 'complex' skin care interventions, which showed no evidence of an interaction. We acknowledge that other groups may have classified the interventions differently. Similarly, although we planned to stratify interventions as A ‐ "those that primarily aim to enhance the skin barrier through direct application of emollient or moisturiser" ‐ and B ‐ "those that aim to protect the skin barrier from damage" ‐ we did not find any trial results that had used intervention B. Jabbar‐Lopez 2019 would be classified as an intervention B but is still ongoing. Similarly, there is no standard recommended skin care for infants, with practices varying by country and by culture; therefore, the comparison of control, taken as standard skin care or no skin care intervention, may have digressed between studies.
Several trials did not contribute data to the meta‐analysis, or contributed only narrative data on non‐specific adverse events. There were some difficulties in deciding whether some of these trials should be included based on the type of intervention and the type of comparator evaluated. For example, we excluded trials that compared one way of bathing to another (Bryanton 2004), but we included a trial that was using a different product in the bath compared to the control group bath, because this might influence skin barrier function (Lund 2020). We concentrated on a normal healthy term population so that these findings were generalisable and were not impacted by the unique structure of preterm skin and the different skin care practices to which preterm infants are exposed.
Agreements and disagreements with other studies or reviews
This is the first Cochrane Review of skin barrier intervention in term infants. A previous Cochrane Review looked at topical emollients for preventing infection in preterm infants (Cleminson 2016). This review concluded that there was no evidence that emollients prevent invasive infection in preterm infants in high‐, middle‐, or low‐income countries. This review also looked at topical oils ‐ mainly vegetable oils ‐ and showed that there was some evidence that those treated with topical vegetable oils had increased growth compared to those given control treatment, although long‐term growth was not measured. This review had invasive infection as a primary outcome. It did not discuss or report on cutaneous infection, so it is unclear whether this was not seen as an adverse effect of the interventions studied, or if it just was not measured. If skin care interventions increase risk of cutaneous infection in term infants, but not in preterm infants, the reason for this difference is unclear. Preterm infants have more permeable skin than term infants and have specific vulnerability to serious bacterial infection. It is possible that hand hygiene for those who apply emollients or oils to the skin of preterm infants may be more thorough than hand hygiene for those who apply emollients or oils to the skin of healthy term infants. If this were the case, then differences in effects on risk of cutaneous infection could potentially be explained by differences in hand hygiene practice.
A previous Cochrane overview of interventions for primary prevention of eczema in children identified systematic reviews of trials evaluating dietary interventions such as maternal dietary antigen avoidance, exclusive breastfeeding for a defined period of time, omega‐3 and omega‐6 fatty acid supplementation, hydrolysed protein formula, soya formula, and pre‐biotics and probiotics. The overview did not identify trials of skin care interventions and did not identify an intervention that effectively prevents the onset of eczema (Foisy 2011).
A systematic review of interventions for prevention of food allergy was undertaken by a European Academy of Allergy and Clinical Immunology committee to inform an update of their guidance on food allergy prevention (DaSilva 2020). This systematic review included Dissanayake 2019 in its section on emollients for food allergy prevention, as other studies reporting eligible food allergy outcomes had not been published at the time this systematic review was conducted. Review authors concluded that emollients may not reduce risk of food allergy (low‐certainty evidence). Our review included the larger and more recently published trial using oral food challenge for outcome assessment ‐ Chalmers 2020 ‐ and did not include food challenge outcomes from Dissanayake 2019 in the primary food allergy analysis because oral food challenge was not used for food allergy assessment in this trial. We rated the evidence for this outcome as very low certainty and could not therefore make a conclusion about effects of emollients on risk of food allergy.
Two pilot studies ‐ Simpson 2014 and Horimukai 2014 ‐ were reported as showing significant reductions in eczema risk. Larger, more definitive trials published subsequently found no reduction in eczema, and our meta‐analysis results are consistent with the findings from these larger trials (Chalmers 2020; Skjerven 2020). The reason for the difference in findings between small pilot studies and larger trials is not clear, but differences in adherence to treatment, in methods and timing of outcome assessment, and in study population may all be relevant.
Finally, emollients as treatment for already established eczema has been the topic of a previous Cochrane Review (Van Zuuren 2017). This review concluded that although evidence was weak, emollients reduce disease severity compared to no treatment. However, moisturisers reduce flares, prolong time between flares, and decrease the need for topical corticosteroids. Our new review concerns the primary prevention of eczema and does not directly impact the well‐established and well‐accepted intervention of emollients for people who already have eczema.
Authors' conclusions
Implications for practice.
This review found that skin care interventions such as emollients probably do not influence the development or time to onset of eczema in healthy term infants by age one to two years and probably increase the risk of skin infection (moderate‐certainty evidence). This suggests that regular application of emollients or other skin care interventions probably is not necessary for healthy infants, unless there are other specific reasons for using such products. This information should be taken into account by guideline developers in this field. Given the probable increase in local skin infection risk, it may be important for carers to practise appropriate hygiene measures when applying emollients to the skin of infants.
This review could not draw conclusions about the impact of skin care interventions on IgE‐mediated food allergy by age one to two years (very low‐certainty evidence); only one study had food allergy diagnosed by oral food challenges, and in this study, only 29% of eligible participants attended for oral food challenge (OFC). Low‐certainty evidence from one trial suggests that skin care intervention may slightly increase parent reports of immediate food allergy (to a common allergen) at two years. However, this outcome was only detected in cow’s milk, which may be unreliable as a measure due to the commercially influenced over‐reporting of cow's milk allergy in infants. Evidence was insufficient to detect effects of skin care interventions on food sensitisation at age one to two years (very low‐certainty evidence). The gold standard for diagnosing food allergy is an OFC; however, these are costly and time consuming for participants and trialists. Alternative modes of diagnosis of food allergy, by standardised questionnaires and documented sensitisation, or even by more complex methods such as basophil activation test, could be considered in further trials.
Infant slippages and stinging/allergic reactions to moisturisers may increase with the use of skin care interventions during infancy (low‐certainty evidence), although confidence intervals for slippages and stinging/allergic reactions are wide and include the possibility of no effect or reduced risk. All results presented here are in comparison to standard care.
Subgroup analysis showed that age, hereditary risk, FLG mutation, duration of intervention, and classification of intervention type did not have an impact on the risk of developing eczema. We could not evaluate these effects for food allergy risk. We do not know if adherence to treatment effects the relationship between skin care interventions and risk of developing eczema or food allergy.
The common clinical practice of applying emollients to the skin of people who already have eczema is not directly affected by our findings.
Implications for research.
In this review, the trials with eczema as an outcome were mainly emollient trials. Other methods of skin barrier intervention in this review had very short follow‐up and did not measure eczema as an outcome, so their impact on eczema remains unclear. Potential future studies on bathing practices should have longer follow‐up of clinical outcomes that use standard methods of eczema measurement. Trialists may wish to consider using novel interventions that impact skin barrier function, rather than those that have already been evaluated in these trials.
We were unable to identify whether skin care interventions such as emollients have an impact on risk of developing food allergy. More research is needed to identify whether food allergy risk is influenced by early skin care practices. Future trials should measure food allergy using a robust outcome assessment (Asai 2020), and researchers may wish to consider applying published algorithms to evaluate food allergy outcomes in participants who do not undergo oral food challenge (Kelleher 2020b). The paucity of oral food challenge‐diagnosed food allergy outcomes in this meta‐analysis infers that oral food challenges are difficult to conduct and are infrequently attended in prevention studies. We would suggest that future studies incorporate Core Outcome Measures for Food Allergy. An update of this review with food allergy outcomes from Skjerven 2020 and potentially the other ongoing trials with foods allergy outcomes will be needed to fully address the hypothesis that skin care interventions may impact the risk of food allergy (Lowe 2019; NCT03871998; NCT03808532).
Conclusions on adherence to intervention could not be made, and we would suggest that future studies carefully document adherence and compliance with interventions. Also, collaboration between groups regarding future potential studies may allow for larger numbers with less imprecision.
This review focused on primary prevention of eczema and food allergy, preventing the diagnosis of eczema and food allergy in infants. Given the strong links between early‐onset eczema and food allergy, another body of work has begun on secondary prevention of food allergy among infants already diagnosed with eczema. These trials ‐ NCT03742414 and UMIN000028043 ‐ include infants younger than 13 weeks with diagnosed eczema and randomise them to active eczema management from onset with emollient and topical corticosteroids. Both studies have IgE‐mediated food allergy as a primary outcome and are ongoing.
History
Protocol first published: Issue 2, 2020 Review first published: Issue 2, 2021
Risk of bias
Risk of bias for analysis 1.1 Eczema by 1‐3 years.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | 74/80 = 93% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.61 [0.26 to 1.40] or if all assumed to have eczema, RR=0.67 [0.34 to 1.33]. | Low risk of bias | UK working party criteria and blinded assessor used. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias for all 5 domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011.). | Some concerns | Loss to follow‐up was 40% for eczema by 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all missing are assumed to not have eczema: RR=0.53 [0.20, 1.35], but conclusions do change if all missing were assumed to have eczema RR=0.93 [0.65, 1.34] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 49/54=91% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.73, 95% 0.24 to 2.23 but conclusions do change when all missing are assumed to have eczema RR=1.24, 95% CI 0.55 to 2.78 (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Quote: "assessor‐blind." UKWP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=1.42 [1.00 to 2.02] but conclusions change if all missing are assumed to have eczema, RR=2.03 [1.56 to 2.63] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Used UK Working party criteria and HF at 12 months. Assessors were blinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | 156/227 = 69% have outcome. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to not have eczema: RR=0.68 [0.29, 1.61] or if all missing are assumed to have eczema RR=1.40 [0.98, 2.00] (point estimate of RR >20% of the complete case estimate). | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
Risk of bias for analysis 1.2 Sensitivity analysis: Eczema by 1‐3 years including aggregate trial data.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | 74/80 = 93% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.61 [0.26 to 1.40] or if all assumed to have eczema, RR=0.67 [0.34 to 1.33]. | Low risk of bias | UK working party criteria and blinded assessor used. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias for all 5 domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011.). | Some concerns | Loss to follow‐up was 40% for eczema by 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all missing are assumed to not have eczema: RR=0.53 [0.20, 1.35], but conclusions do change if all missing were assumed to have eczema RR=0.93 [0.65, 1.34] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Migacheva 2018 | Some concerns | No information given on randomization process.Baseline characteristics (russian language table) do not suggest a problem with with imbalance in a small number of key baseline characteristics, but only limited baseline aggregate data provided. | Some concerns | Trial not registered so intended intervention unclear and it's not clear whether the patients were analysed as randomised. | Some concerns | It's unclear how many participants were included in the analysis. No information available on the number of participants with missing data. The circumstances of the trial make it unlikely that missingness depends on outcome. | Some concerns | Eczema assessed by a trained investigator but no information on blinding status. | Some concerns | Not a registered trial, therefore unclear if outcomes are reported as initially planned. | Some concerns | This study was unregistered and reported in an abstract only, not clear regarding blinding of outcomes or missing data and analysis population. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 49/54=91% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.73, 95% 0.24 to 2.23 but conclusions do change when all missing are assumed to have eczema RR=1.24, 95% CI 0.55 to 2.78 (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Quote: "assessor‐blind." UKWP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=1.42 [1.00 to 2.02] but conclusions change if all missing are assumed to have eczema, RR=2.03 [1.56 to 2.63] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Used UK Working party criteria and HF at 12 months. Assessors were blinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | 156/227 = 69% have outcome. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to not have eczema: RR=0.68 [0.29, 1.61] or if all missing are assumed to have eczema RR=1.40 [0.98, 2.00] (point estimate of RR >20% of the complete case estimate). | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
Risk of bias for analysis 1.3 Sensitivity analysis: Eczema by 1‐3 years (UKWP only).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | 74/80 = 93% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.61 [0.26 to 1.40] or if all assumed to have eczema, RR=0.67 [0.34 to 1.33]. | Low risk of bias | UK working party criteria and blinded assessor used. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias for all 5 domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011.). | Some concerns | Loss to follow‐up was 40% for eczema by 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all missing are assumed to not have eczema: RR=0.53 [0.20, 1.35], but conclusions do change if all missing were assumed to have eczema RR=0.93 [0.65, 1.34] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 49/54=91% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.73, 95% 0.24 to 2.23 but conclusions do change when all missing are assumed to have eczema RR=1.24, 95% CI 0.55 to 2.78 (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Quote: "assessor‐blind." UKWP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=1.42 [1.00 to 2.02] but conclusions change if all missing are assumed to have eczema, RR=2.03 [1.56 to 2.63] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Used UK Working party criteria and HF at 12 months. Assessors were blinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
Risk of bias for analysis 1.4 Sensitivity analysis: Eczema by 1‐3 years (including data from all 4 arms of PreventADALL).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | 74/80 = 93% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.61 [0.26 to 1.40] or if all assumed to have eczema, RR=0.67 [0.34 to 1.33]. | Low risk of bias | UK working party criteria and blinded assessor used. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias for all 5 domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011.). | Some concerns | Loss to follow‐up was 40% for eczema by 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all missing are assumed to not have eczema: RR=0.53 [0.20, 1.35], but conclusions do change if all missing were assumed to have eczema RR=0.93 [0.65, 1.34] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 49/54=91% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.73, 95% 0.24 to 2.23 but conclusions do change when all missing are assumed to have eczema RR=1.24, 95% CI 0.55 to 2.78 (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Quote: "assessor‐blind." UKWP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=1.42 [1.00 to 2.02] but conclusions change if all missing are assumed to have eczema, RR=2.03 [1.56 to 2.63] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Used UK Working party criteria and HF at 12 months. Assessors were blinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | 156/227 = 69% have outcome. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to not have eczema: RR=0.68 [0.29, 1.61] or if all missing are assumed to have eczema RR=1.40 [0.98, 2.00] (point estimate of RR >20% of the complete case estimate). | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
Risk of bias for analysis 1.5 Sensitivity analysis: Eczema by 1‐3 years ‐ low risk of bias.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | 74/80 = 93% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.61 [0.26 to 1.40] or if all assumed to have eczema, RR=0.67 [0.34 to 1.33]. | Low risk of bias | UK working party criteria and blinded assessor used. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias for all 5 domains. |
Risk of bias for analysis 1.6 Sensitivity analysis: Eczema by 1‐3 years ‐ excluding non‐prospectively acquired data.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011.). | Some concerns | Loss to follow‐up was 40% for eczema by 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all missing are assumed to not have eczema: RR=0.53 [0.20, 1.35], but conclusions do change if all missing were assumed to have eczema RR=0.93 [0.65, 1.34] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 49/54=91% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.73, 95% 0.24 to 2.23 but conclusions do change when all missing are assumed to have eczema RR=1.24, 95% CI 0.55 to 2.78 (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Quote: "assessor‐blind." UKWP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=1.42 [1.00 to 2.02] but conclusions change if all missing are assumed to have eczema, RR=2.03 [1.56 to 2.63] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Used UK Working party criteria and HF at 12 months. Assessors were blinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | 156/227 = 69% have outcome. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to not have eczema: RR=0.68 [0.29, 1.61] or if all missing are assumed to have eczema RR=1.40 [0.98, 2.00] (point estimate of RR >20% of the complete case estimate). | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
Risk of bias for analysis 1.7 Sensitivity analysis: Eczema by 6 months‐3 years.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Horimukai 2014 | Low risk of bias | Randomised trial, no information provided on allocation concealment. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Control rates of skin care in line with those observed in other trials and observational studies (e.g. up to ~75 % in Rendell at al. 2011). Quote: “Because the IRB recommended permitting application of petroleum jelly when the parents thought it necessary, we calculated the amount of these 2 types of moisturizers used by each group based on their diaries. The mean daily amount of emulsion‐type moisturizer used by the intervention group was 7.86 6 4.34 g (0 g for the control group, excluding the 2 infants placed in the wrong group). The mean daily amount of petroleum jelly applied to the control group was 0.101 6 0.286 g (mean frequency of use, 0.235 d/wk). Petroleum jelly (20 g per bottle) was prescribed to all neonates born at the NCCHD, but we had no information about how much was used by the intervention group. Nevertheless, only a few of the parents occasionally used a small, almost ignorable, amount of the jelly on their infants.” | Low risk of bias | 79/118=84% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.66 [0.42, 1.05], or if everyone missing is assumed to have eczema RR=0.79, [0.63, 0.99]. | Low risk of bias | Quote: "The primary outcome measure was the cumulative rate of incidence of AD, eczema, or both by temporal observation. The diagnostic criteria for infantile eczema, AD, or both (AD/eczema) were developed based on a modification of the United Kingdom Working Party’s criteria and were applied by a dermatology specialist" | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | 74/80 = 93% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.61 [0.26 to 1.40] or if all assumed to have eczema, RR=0.67 [0.34 to 1.33]. | Low risk of bias | UK working party criteria and blinded assessor used. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias for all 5 domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011.). | Some concerns | Loss to follow‐up was 40% for eczema by 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all missing are assumed to not have eczema: RR=0.53 [0.20, 1.35], but conclusions do change if all missing were assumed to have eczema RR=0.93 [0.65, 1.34] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 49/54=91% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.73, 95% 0.24 to 2.23 but conclusions do change when all missing are assumed to have eczema RR=1.24, 95% CI 0.55 to 2.78 (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Quote: "assessor‐blind." UKWP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Simpson 2014 | Low risk of bias | Quote: "Infants were randomized at a 1:1 ratio using random block sizes to either the intervention or control group with a central, Web‐based, computer generated, Internet randomization service." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote “Eight (13.3%) parents in the control group reported using emollients in a way that mirrored the intervention (ie, regular generalized application of emollient for reasons other than the treatment of cradle cap, nappy rash, or eczema).” The IPD shows regular use of emollient (≥ 3 days a week) by 50/55 intervention individuals and only 3/53 control participants with eczema outcome recorded). | Some concerns | 108/124=87% have outcome. Sensitivity analysis using the IPD reveals findings do not change significantly if everyone missing is assumed to not have eczema RR=0.53 [0.29 to 0.99] but or if all missing are assumed to have eczema RR= 0.71 [0.46 to 1.10] (point estimate of RR >20% of the complete case estimate and upper bound of 95% CI shifted above 1). | Low risk of bias | Eczema was determined by an investigator. Outcome assessors were blind to treatment allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=1.42 [1.00 to 2.02] but conclusions change if all missing are assumed to have eczema, RR=2.03 [1.56 to 2.63] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Used UK Working party criteria and HF at 12 months. Assessors were blinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | 156/227 = 69% have outcome. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to not have eczema: RR=0.68 [0.29, 1.61] or if all missing are assumed to have eczema RR=1.40 [0.98, 2.00] (point estimate of RR >20% of the complete case estimate). | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
Risk of bias for analysis 1.9 Subgroup analysis (study level): Eczema by 1‐3 years by intervention type.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.9.1 Basic emolient | ||||||||||||
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011.). | Some concerns | Loss to follow‐up was 40% for eczema by 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all missing are assumed to not have eczema: RR=0.53 [0.20, 1.35], but conclusions do change if all missing were assumed to have eczema RR=0.93 [0.65, 1.34] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=1.42 [1.00 to 2.02] but conclusions change if all missing are assumed to have eczema, RR=2.03 [1.56 to 2.63] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Used UK Working party criteria and HF at 12 months. Assessors were blinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Subgroup 1.9.2 Complex emolient | ||||||||||||
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | 74/80 = 93% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.61 [0.26 to 1.40] or if all assumed to have eczema, RR=0.67 [0.34 to 1.33]. | Low risk of bias | UK working party criteria and blinded assessor used. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias for all 5 domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 49/54=91% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.73, 95% 0.24 to 2.23 but conclusions do change when all missing are assumed to have eczema RR=1.24, 95% CI 0.55 to 2.78 (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Quote: "assessor‐blind." UKWP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | 156/227 = 69% have outcome. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to not have eczema: RR=0.68 [0.29, 1.61] or if all missing are assumed to have eczema RR=1.40 [0.98, 2.00] (point estimate of RR >20% of the complete case estimate). | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
Risk of bias for analysis 1.10 Subgroup analysis (study level): Eczema by 1‐3 years by prescribed intervention duration .
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.10.1 Intervention prescribed for <6 months | ||||||||||||
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | 156/227 = 69% have outcome. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to not have eczema: RR=0.68 [0.29, 1.61] or if all missing are assumed to have eczema RR=1.40 [0.98, 2.00] (point estimate of RR >20% of the complete case estimate). | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Subgroup 1.10.2 Intervention prescribed for ≥6 months | ||||||||||||
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | 74/80 = 93% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.61 [0.26 to 1.40] or if all assumed to have eczema, RR=0.67 [0.34 to 1.33]. | Low risk of bias | UK working party criteria and blinded assessor used. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias for all 5 domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011.). | Some concerns | Loss to follow‐up was 40% for eczema by 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all missing are assumed to not have eczema: RR=0.53 [0.20, 1.35], but conclusions do change if all missing were assumed to have eczema RR=0.93 [0.65, 1.34] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 49/54=91% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.73, 95% 0.24 to 2.23 but conclusions do change when all missing are assumed to have eczema RR=1.24, 95% CI 0.55 to 2.78 (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Quote: "assessor‐blind." UKWP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=1.42 [1.00 to 2.02] but conclusions change if all missing are assumed to have eczema, RR=2.03 [1.56 to 2.63] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Used UK Working party criteria and HF at 12 months. Assessors were blinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
Risk of bias for analysis 1.11 Subgroup analysis (study level): Eczema by 1‐3 years, by prescribed intervention timing.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.11.1 Intervention prescribed to start in first week of life | ||||||||||||
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | Low risk of bias | 1210/1394 = 87% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.93, 95% 0.76 to 1.15, or if all assumed to have eczema, RR=0.99, 95% CI 0.86 to 1.15. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial data set provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | 455/549=83% have outcome. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema (RR 1.24 95% CI[0.84, 1.83]) or they do have eczema (RR 1.00 95% CI[0.79, 1.27]). | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | 74/80 = 93% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.61 [0.26 to 1.40] or if all assumed to have eczema, RR=0.67 [0.34 to 1.33]. | Low risk of bias | UK working party criteria and blinded assessor used. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias for all 5 domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011.). | Some concerns | Loss to follow‐up was 40% for eczema by 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all missing are assumed to not have eczema: RR=0.53 [0.20, 1.35], but conclusions do change if all missing were assumed to have eczema RR=0.93 [0.65, 1.34] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 49/54=91% have outcome. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=0.73, 95% 0.24 to 2.23 but conclusions do change when all missing are assumed to have eczema RR=1.24, 95% CI 0.55 to 2.78 (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Quote: "assessor‐blind." UKWP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | 156/227 = 69% have outcome. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to not have eczema: RR=0.68 [0.29, 1.61] or if all missing are assumed to have eczema RR=1.40 [0.98, 2.00] (point estimate of RR >20% of the complete case estimate). | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
| Subgroup 1.11.2 Intervention prescribed to start after first week of life | ||||||||||||
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, RR=1.42 [1.00 to 2.02] but conclusions change if all missing are assumed to have eczema, RR=2.03 [1.56 to 2.63] (point estimate of RR >20% of the complete case estimate). | Low risk of bias | Used UK Working party criteria and HF at 12 months. Assessors were blinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing outcome data (results varied in sensitivity analysis), low risk of bias for all other domains. |
Risk of bias for analysis 1.29 Food allergy by 1‐3 years.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months” | High risk of bias | 996/1394 have outcome =71% of randomised participants. Sensitivity analysis using the IPD data shows conclusions do not change significantly if all missing are assumed to not have food allergy, RR=2.46, 95% CI 0.96 to 6.30. But conclusions vary when all missing are assumed to have food allergy RR=1.09, 95% CI 0.93 to 1.28. The overall proportion of missing data is similar in the two treatment groups. However there is a difference between treatment groups in the proportion of participants who underwent oral food challenge. Of those invited to oral food challenge due to suspected food allergy, only 39 of 133 (29%) in the intervention group and 21 of 125 (17%) in the control group underwent oral food challenge. This suggests that missingness is likely to have depended on the outcome. | Low risk of bias | Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | High risk of bias | High risk of bias due to missing data (29% and results varied in sensitivity analysis) which could have depended on the outcome, but low risk of bias in all other domains. |
Risk of bias for analysis 1.30 Sensitivity analysis: Food allergy by 1‐3 years (diagnosed by oral food challenge or investigator assessment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months.” | Some concerns | 1115/1394 have outcome = 80% of randomised participants. Sensitivity analysis using the IPD data shows conclusions do not change if all missing are assumed to not have food allergy, RR=1.42, 95% CI 0.90 to 2.26, but when all missing are assumed to have food allergy RR=1.17, 95% CI 0.97 to 1.40 (Point estimate varies by ≥20% complete case estimate.) Missingness could have depended on outcome, but not likely as panel assessment based on skin prick testing and history incorporated. | Low risk of bias | Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (results varied in sensitivity analysis). Missingness could have depended on outcome, but not likely as panel assessment based on skin prick testing and history incorporated. Low risk of bias in all other domains. |
Risk of bias for analysis 1.38 Adverse event: skin infection.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months.” | Low risk of bias | Skin infections were prompted for at all visits. | Some concerns | Skin infections were reported by parents on the 3, 6 and 12 month questionnaires. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| Cooke 2015 | Low risk of bias | Quote: "Randomization was 1:1:1 via a central telephone‐based service" and "Allocation was concealed from the participant and independent research midwife until the point of allocation". Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote “The weekly ranges of adherence for treatment use were 79% to 93% of participants for the olive oil group, 83% to 94% for the sunflower oil group and 100% for the no oil group” | Low risk of bias | Adverse events collected for all participants. | Low risk of bias | Quote: “All measurements were taken by the investigator who remained blind to the treatment allocation. Changes in the skin were observed and recorded by the investigator at baseline and follow‐up (erythema, dryness and scaling, need for medical products/attention) between 48 h and 4 weeks.” | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all 5 domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | Adverse events prompted for at visits and an event recorded for 74/80=92.5%. | Some concerns | Adverse events prompted for at visits (week 6, month 6 and month 12). Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context.: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011). | Low risk of bias | Adverse events self‐recalled by all participants and prompted for at visits. | Some concerns | Adverse events self‐recalled by all participants and prompted for at visits (2, 6 and 12mo) and telephone follow‐up calls (18 and 24 mo). Outcome assessors were reporters who were aware of the allocation (parents). | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| Simpson 2014 | Low risk of bias | Quote: "Infants were randomized at a 1:1 ratio using random block sizes to either the intervention or control group with a central, Web‐based, computer generated, Internet randomization service." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote “Eight (13.3%) parents in the control group reported using emollients in a way that mirrored the intervention (ie, regular generalized application of emollient for reasons other than the treatment of cradle cap, nappy rash, or eczema).” The IPD shows regular use of emollient (≥ 3 days a week) by 50/55 intervention individuals and only 3/53 control participants with eczema outcome recorded). | Low risk of bias | Skin infections were prompted for at all patient visits. | Some concerns | Skin infections were prompted for at 10days, 6 weeks, 12 weeks, 18 weeks and 24 weeks. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Low risk of bias | Adverse events collected for all participants. | Some concerns | Quote: "Adverse events were recorded in weekly electronic diaries up to week 26, in electronic questionnaires every 3 months, and in specific forms by personnel at the discretion of the study personnell." Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
Risk of bias for analysis 1.39 Adverse event: stinging or allergic reaction to moisturisers.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Cooke 2015 | Low risk of bias | Quote: "Randomization was 1:1:1 via a central telephone‐based service" and "Allocation was concealed from the participant and independent research midwife until the point of allocation". Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote “The weekly ranges of adherence for treatment use were 79% to 93% of participants for the olive oil group, 83% to 94% for the sunflower oil group and 100% for the no oil group." | Low risk of bias | Adverse events collected for all participants. | Low risk of bias | Quote: " All measurements were taken by the investigator who remained blind to the treatment allocation. Changes in the skin were observed and recorded by the investigator at baseline and follow‐up (erythema, dryness and scaling, need for medical products/ attention) between 48 h and 4 weeks." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | Adverse events prompted for at visits and an event recorded for 74/80=92.5%. | Some concerns | Adverse events prompted for at visits (week 6, month 6 and month 12). Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arise because of the trial context: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011). | Low risk of bias | Adverse events self‐recalled by all participants and prompted for at visits. | Some concerns | Adverse events self‐recalled by all participants and prompted for at visits (2, 6 and 12mo) and telephone follow‐up calls (18 and 24 mo). Outcome assessors were reporters who were aware of the allocation (parents). | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Low risk of bias | Skin reactions prompted for at all visits and when participants left the study early (e.g. due to eczema). Note: a number of participants dropped out of the trial early due to loss to follow‐up (n=5, 9%) or when they had eczema (n=10, 18%, with median follow‐up 18 weeks, IQR 12 to 30 weeks). | Some concerns | Skin reactions prompted for at all visits and when participants left the study early (e.g. due to eczema). Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
Risk of bias for analysis 1.40 Adverse event: slippage accidents.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months.” | Low risk of bias | Slippage accidents were prompted for at all visits. | Some concerns | Slippage incidents and skin infections were reported by parents on the 3, 6 and 12 month questionnaires. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arise because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | Adverse events prompted for at visits and an event recorded for 74/80=92.5%. | Some concerns | Adverse events prompted for at visits (week 6, month 6 and month 12). Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| Simpson 2014 | Low risk of bias | Quote: "Infants were randomized at a 1:1 ratio using random block sizes to either the intervention or control group with a central, Web‐based, computer generated, Internet randomization service." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote “Eight (13.3%) parents in the control group reported using emollients in a way that mirrored the intervention (ie, regular generalized application of emollient for reasons other than the treatment of cradle cap, nappy rash, or eczema).” The IPD shows regular use of emollient (≥ 3 days a week) by 50/55 intervention individuals and only 3/53 control participants with eczema outcome recorded). | Low risk of bias | AE's were prompted for at all patient visits, including accidents. | Some concerns | AE's were prompted for at all follow‐ups (10days, 6 weeks, 12 weeks, 18 weeks and 24 weeks), including accidents. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Low risk of bias | Adverse events collected for all participants. | Some concerns | Quote: "Adverse events were recorded in weekly electronic diaries up to week 26, in electronic questionnaires every 3 months, and in specific forms by personnel at the discretion of the study personnell." Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
Risk of bias for analysis 1.41 Serious Adverse Events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Cooke 2015 | Low risk of bias | Quote: "Randomization was 1:1:1 via a central telephone‐based service" and "Allocation was concealed from the participant and independent research midwife until the point of allocation". Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote “The weekly ranges of adherence for treatment use were 79% to 93% of participants for the olive oil group, 83% to 94% for the sunflower oil group and 100% for the no oil group.” | Low risk of bias | Adverse events collected for all participants. | Low risk of bias | Quote: " All measurements were taken by the investigator who remained blind to the treatment allocation. Changes in the skin were observed and recorded by the investigator at baseline and follow‐up (erythema, dryness and scaling, need for medical products/attention) between 48 h and 4 weeks." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | Adverse events prompted for at visits and an event recorded for 74/80=92.5%. | Low risk of bias | Outcome assessors were reporters who were aware of the allocation, but assessment of serious adverse events not likely to be influenced by knowledge of intervention received. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Low risk of bias | Adverse events collected for all participants. | Low risk of bias | Outcome assessors were reporters who were aware of the allocation, but assessment of serious adverse events not likely to be influenced by knowledge of intervention received. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 1.46 Time to onset of eczema.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months.” | Low risk of bias | 1236/1394 =89% have outcome. Sensitivity analysis using the IPD data shows conclusions do not change if all missing are assumed to have eczema, HR=0.94, 95% 0.79 to 1.12 or when all missing are assumed to have eczema, HR=0.92, 95% CI 0.79 to 1.11. | Some concerns | Outcome was parent report of doctor diagnosis of eczema and parents unblinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | Outcome missing for 21.5% (not all people with eczema outcome have time of eczema onset). Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema by 12months HR=1.27 [0.83 to 1.95], or to have eczema onsets by 12 months: HR= 1.16 [0.76 to 1.78]. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly" | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Horimukai 2014 | Low risk of bias | Randomised trial, no information provided on allocation concealment. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Control rates of skin care in line with those observed in other trials and observational studies (e.g. up to ~75 % in Rendell at al. 2011). Quote: “Because the IRB recommended permitting application of petroleum jelly when the parents thought it necessary, we calculated the amount of these 2 types of moisturizers used by each group based on their diaries. The mean daily amount of emulsion‐type moisturizer used by the intervention group was 7.86 6 4.34 g (0 g for the control group, excluding the 2 infants placed in the wrong group). The mean daily amount of petroleum jelly applied to the control group was 0.101 6 0.286 g (mean frequency of use, 0.235 d/wk). Petroleum jelly (20 g per bottle) was prescribed to all neonates born at the NCCHD, but we had no information about how much was used by the intervention group. Nevertheless, only a few of the parents occasionally used a small, almost ignorable, amount of the jelly on their infants.” | Low risk of bias | Data on time to eczema available for 116/118=98%, assuming censoring at random for 37/118=31% participants who dropped out of the trial prior to 9 months with no eczema outcome recorded prior to drop out. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone censored is assumed to not have eczema by 9 months, HR=0.59 [0.33, 1.07], or if everyone missing is assumed to have eczema by 9 months 0.61, [0.39, 0.97]. | Low risk of bias | Modification of the UKWP criteria used to record eczema up to 32 weeks and blinded assessment. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | Outcome available for 79/80=99%. | Low risk of bias | UK working party criteria used by blinded assessor. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context.: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011). | Some concerns | Data on time to eczema available for 100% (assuming censoring at random for 40/100 participants who dropped out of the trial prior to 2 years with no eczema outcome recorded prior to drop out). Loss to follow‐up was 40% for eczema at 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all censored before two years are assumed to not have eczema, 0.49 [0.17 to 1.40]. But conclusions do change if all censored before two years are assumed to have eczema, 0.91 [0.52 to 1.57] by the end of trial follow‐up (point estimate of RR HR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (40% censored before 2 years and results varied in sensitivity analysis) but low risk of bias in all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 5/54 (9%) participants are censored prior to 12 months due to loss to follow‐up/eczema status unknown. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to have eczema, HR=1.27, 0.46 to 3.53 (point estimate of HR >20% of the complete case estimate). Missingness could have depended on outcome, but this is not likely. | Low risk of bias | Quote: "assessor‐blind." UK WP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (9% and result varied in sensitivity analysis) but low risk of bias for all other domains. |
| Simpson 2014 | Low risk of bias | Quote: "Infants were randomized at a 1:1 ratio using random block sizes to either the intervention or control group with a central, Web‐based, computer generated, Internet randomization service." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote “Eight (13.3%) parents in the control group reported using emollients in a way that mirrored the intervention (ie, regular generalized application of emollient for reasons other than the treatment of cradle cap, nappy rash, or eczema).” The IPD shows regular use of emollient (≥ 3 days a week) by 50/55 intervention individuals and only 3/53 control participants with eczema outcome recorded). | Some concerns | Outcome is missing for 13% of randomised participants. Sensitivity analysis using the IPD reveals findings do not change significantly if everyone missing is assumed to not have eczema HR 0.48 [0.23 to 0.98] but if all are assumed to have eczema at 3 or 6 months HR = 0.65 [0.36 to 1.15] (point estimate of HR >20% of the complete case estimate). Missingness could have depended on outcome, but this is not likely. | Low risk of bias | Eczema was determined by an investigator. Outcome assessors were blind to treatment allocation. This only failed in 4% of cases. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (13% and results varied in sensitivity analysis), but low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, HR=1.46 [1.00 to 2.12] but conclusions change if all missing are assumed to have eczema, HR=2.20 [1.66 to 2.93] (point estimate of HR >20% of the complete case estimate). Missingness could have depended on outcome, but this is not likely. | Low risk of bias | Used UK WP +Hanifin and Rajka criteria and blinded assessor. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (10% and results varied in sensitivity analysis) but low risk of bias in all other domains. |
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | Eczema outcome available for 156/227 randomised = 69% participants. Sensitivity analysis of IPD reveals conclusions change if all missing do not have eczema: 0.63 [0.23, 1.73] or if all missing are assumed to have eczema at 12 or 24 months: 1.60 [1.03 to 2.47] (point estimate of HR >20% of the complete case estimate). Missingness could have depended on the outcome, but not likely. | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (31% and results varied in sensitivity analysis) and how outcome reported by unblinded assessors (parents) but low risk of bias for other domains. |
Risk of bias for analysis 1.47 Subgroup analysis: Time to onset of eczema (< 1 year follow‐up versus ≥ 1 year follow‐up).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.47.1 Follow‐up ≥ 1 year | ||||||||||||
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months.” | Low risk of bias | 1236/1394 =89% have outcome. Sensitivity analysis using the IPD data shows conclusions do not change if all missing are assumed to have eczema, HR=0.94, 95% 0.79 to 1.12 or when all missing are assumed to have eczema, HR=0.92, 95% CI 0.79 to 1.11. | Some concerns | Outcome was parent report of doctor diagnosis of eczema and parents unblinded. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | Outcome missing for 21.5% (not all people with eczema outcome have time of eczema onset). Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing eczema outcome are assumed to not have eczema by 12months HR=1.27 [0.83 to 1.95], or to have eczema onsets by 12 months: HR= 1.16 [0.76 to 1.78]. | Low risk of bias | UK working party criteria and blinded assessor used. Quote: "AD diagnosis was further confirmed blindly" | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Low risk of bias | Outcome available for 79/80=99%. | Low risk of bias | UK working party criteria used by blinded assessor. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| McClanahan 2019 | Low risk of bias | Quote: "Randomization was completed via a computer‐generated randomization list issued by a statistician with block sizes of 10 and concealed from the recruitment coordinator until randomization." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context.: "At 2, 6 and 12 months, the intervention group had 87%, 72.4% and 66.7% high adherence, respectively, vs. 45.2%, 45.8% and 45.5% in the control group, respectively." The control group rates are high but similar to those reported in another study by the same group (e.g. up to ~75 % in Rendell et al. 2011). | Some concerns | Data on time to eczema available for 100% (assuming censoring at random for 40/100 participants who dropped out of the trial prior to 2 years with no eczema outcome recorded prior to drop out). Loss to follow‐up was 40% for eczema at 2 years and incident eczema was captured through voluntary contacting of investigators. Sensitivity analysis of IPD reveals conclusions do not change if all censored before two years are assumed to not have eczema, 0.49 [0.17 to 1.40]. But conclusions do change if all censored before two years are assumed to have eczema, 0.91 [0.52 to 1.57] by the end of trial follow‐up (point estimate of RR HR >20% of the complete case estimate). | Low risk of bias | U.K. Working Party Criteria adapted to identify incident cases, some as young as 2 weeks. Some uncertainty about validity of this tool at this age. Incident cases not followed further after first diagnosis. Low risk of bias as this is our pre‐defined preferred tool. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (40% censored before 2 years and results varied in sensitivity analysis) but low risk of bias in all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | 5/54 (9%) participants are censored prior to 12 months due to loss to follow‐up/eczema status unknown. Sensitivity analysis of IPD reveals conclusions change if all missing are assumed to have eczema, HR=1.27, 0.46 to 3.53 (point estimate of HR >20% of the complete case estimate). Missingness could have depended on outcome, but this is not likely. | Low risk of bias | Quote: "assessor‐blind." UK WP criteria used. Patients were followed up until they had eczema or 12 months or were lost to follow‐up. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (9% and result varied in sensitivity analysis) but low risk of bias for all other domains. |
| Skjerven 2020 | Low risk of bias | Quote: “we used computer‐generated cluster randomisation based on 92 geographical living area blocks as well as eight 3‐month time blocks. All infants born in the same 3‐month period and belonging to the same postal code or city area were allocated to the same intervention group”. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The IPD shows regular use of emollient (ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant and 262/599 = 44% intervention arm. | Some concerns | Outcome available for 89.8%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have eczema, HR=1.46 [1.00 to 2.12] but conclusions change if all missing are assumed to have eczema, HR=2.20 [1.66 to 2.93] (point estimate of HR >20% of the complete case estimate). Missingness could have depended on outcome, but this is not likely. | Low risk of bias | Used UK WP +Hanifin and Rajka criteria and blinded assessor. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (10% and results varied in sensitivity analysis) but low risk of bias in all other domains. |
| Yonezawa 2018 | Low risk of bias | Quote: "Randomization was performed individually according to central registration after measuring the skin barrier function at baseline.” Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participant carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to ~75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 63/69 (91%) intervention participants 52/87 (60%) control participants with outcomes recorded). Quote: “The frequency of bathing (mean +/‐ standard deviation [SD], 0.81 +/‐ 0.22 vs 0.99 +/‐ 0.06 times/day, P < 0.001) and soap use was lower (mean +/‐ SD, 0.50 +/‐ 0.09 vs 0.98 +/‐ 0.05 times/ day, P < 0.001), and that of lotion use was higher (1.30 +/‐ 0.67 vs 0.48 +/‐ 0.39 times/day, P < 0.001) in the intervention group than in the control group during the study period.” | Some concerns | Eczema outcome available for 156/227 randomised = 69% participants. Sensitivity analysis of IPD reveals conclusions change if all missing do not have eczema: 0.63 [0.23, 1.73] or if all missing are assumed to have eczema at 12 or 24 months: 1.60 [1.03 to 2.47] (point estimate of HR >20% of the complete case estimate). Missingness could have depended on the outcome, but not likely. | Some concerns | Eczema was collected from parents using a self‐reported questionnaire. Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (31% and results varied in sensitivity analysis) and how outcome reported by unblinded assessors (parents) but low risk of bias for other domains. |
| Subgroup 1.47.2 Follow‐up < 1 year | ||||||||||||
| Horimukai 2014 | Low risk of bias | Randomised trial, no information provided on allocation concealment. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Control rates of skin care in line with those observed in other trials and observational studies (e.g. up to ~75 % in Rendell at al. 2011). Quote: “Because the IRB recommended permitting application of petroleum jelly when the parents thought it necessary, we calculated the amount of these 2 types of moisturizers used by each group based on their diaries. The mean daily amount of emulsion‐type moisturizer used by the intervention group was 7.86 6 4.34 g (0 g for the control group, excluding the 2 infants placed in the wrong group). The mean daily amount of petroleum jelly applied to the control group was 0.101 6 0.286 g (mean frequency of use, 0.235 d/wk). Petroleum jelly (20 g per bottle) was prescribed to all neonates born at the NCCHD, but we had no information about how much was used by the intervention group. Nevertheless, only a few of the parents occasionally used a small, almost ignorable, amount of the jelly on their infants.” | Low risk of bias | Data on time to eczema available for 116/118=98%, assuming censoring at random for 37/118=31% participants who dropped out of the trial prior to 9 months with no eczema outcome recorded prior to drop out. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone censored is assumed to not have eczema by 9 months, HR=0.59 [0.33, 1.07], or if everyone missing is assumed to have eczema by 9 months 0.61, [0.39, 0.97]. | Low risk of bias | Modification of the UKWP criteria used to record eczema up to 32 weeks and blinded assessment. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Simpson 2014 | Low risk of bias | Quote: "Infants were randomized at a 1:1 ratio using random block sizes to either the intervention or control group with a central, Web‐based, computer generated, Internet randomization service." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote “Eight (13.3%) parents in the control group reported using emollients in a way that mirrored the intervention (ie, regular generalized application of emollient for reasons other than the treatment of cradle cap, nappy rash, or eczema).” The IPD shows regular use of emollient (≥ 3 days a week) by 50/55 intervention individuals and only 3/53 control participants with eczema outcome recorded). | Some concerns | Outcome is missing for 13% of randomised participants. Sensitivity analysis using the IPD reveals findings do not change significantly if everyone missing is assumed to not have eczema HR 0.48 [0.23 to 0.98] but if all are assumed to have eczema at 3 or 6 months HR = 0.65 [0.36 to 1.15] (point estimate of HR >20% of the complete case estimate). Missingness could have depended on outcome, but this is not likely. | Low risk of bias | Eczema was determined by an investigator. Outcome assessors were blind to treatment allocation. This only failed in 4% of cases. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (13% and results varied in sensitivity analysis), but low risk of bias for all other domains. |
Risk of bias for analysis 1.48 Parent report of immediate (< 2 hours) reaction to a known common food allergen.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months.” | Low risk of bias | Outcome available for 1171/1394=84%. Sensitivity analysis using the IPD data shows conclusions do not change if all missing are assumed to assumed not to have reaction: RR = 1.24 [0.97 to 1.59] or if everyone missing is assumed to have reaction RR=1.20 [1.02 to 1.40]. | Some concerns | Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Outcome assessors were reporters who were aware of the allocation. Low risk of bias for all other domains. |
| NCT03376243 | Low risk of bias | Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. e.g. up to ~75 % in Rendell et al. 2011. The IPD shows daily use of emollient over intervention period by 7/27 (26%) control and 19/22 (86%) intervention participants with eczema outcomes recorded. | Some concerns | Data available for 41/54 = 76%. Zero events observed, therefore results differ in sensitivity analysis. Missingness could depend on outcome but not likely. | Some concerns | Outcome assessors were reporters who were aware of the allocation. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (24%) and how outcome reporters (parents) were unblinded, but low risk of bias for other domains. |
Risk of bias for analysis 1.52 Allergic sensitisation to common foods or inhalants at 1‐3 years.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months.” | Low risk of bias | Outcome available for 988/1394=71%. Sensitivity analysis using the IPD data shows conclusions do not change if all missing are assumed to not have any allergic sensitisation, RR=1.21, 95% CI 0.90 to 1.61, or if everyone missing is assumed to have any allergic sensitisation, RR=1.06, 95% CI 0.94 to 1.21. | Low risk of bias | Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Some concerns | Outcome available for 70/80=88% of randomised participants at 12 months. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have allergic sensitisation, 0.68 [0.27 to 1.71] but conclusions change if all missing the outcome are assumed to have allergic sensitisation, 1.04 [0.53 to 2.01] (point estimate of RR >20% of the complete case estimate). Missingness could have depended on outcome, but not likely. | Low risk of bias | Skin prick tests used by blinded assessor. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (12% and result varied in sensitivity analysis) but low risk of bias in all other domains. |
Risk of bias for analysis 1.53 Allergic sensitisation to common foods at 1‐3 years.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months.” | Low risk of bias | Sensitivity analysis using the IPD data shows conclusions do not change if all missing are assumed to not have allergic sensitisation to any foods, RR=1.34, 95% 0.92 to 1.96, or if all missing are assumed to have allergic sensitisation to foods, RR=1.08, 95% CI 0.94 to 1.24. | Low risk of bias | Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Some concerns | Outcome available for 70/80=88% of randomised participants at 12 months. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have allergic sensitisation to any food inhalant, 0.40 [0.11 to 1.38] but conclusions change if all missing the outcome are assumed to have allergic sensitisation, 0.89 [0.42 to 1.90] (point estimate of RR >20% of the complete case estimate). Missingness could have depended on outcome, but not likely. | Low risk of bias | Skin prick tests used by blinded assessor. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (12% and results varied in sensitivity analysis) but low risk of bias in all other domains. |
Risk of bias for analysis 1.58 Sensitivity analysis: Allergic sensitisation to common foods at 6 months‐3 years.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chalmers 2020 | Low risk of bias | Quote: "The randomisation schedule was created by the CTU using computer‐generated pseudo‐random code with permuted blocks of randomly varying size. The sequence was known only to the programmer until database lock." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available) | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies (e.g. up to 75 % in Rendell et al. 2011). Quote: “Of families in the emollient group with complete questionnaire data on adherence at each time point, 466 (88%) of 532 had satisfactory adherence at 3 months, 427 (82%) of 519 at 6 months, and 375 (74%) of 506 at 12 months.” “No emollient was supplied to the control group, but self‐directed use of emollients at least three times per week to most of the body (contamination) occurred in 18% (82 of 457) at 3 months, 17% (62 of 372) at 6 months, and 15% (49 of 324) at 12 months.” | Low risk of bias | Sensitivity analysis using the IPD data shows conclusions do not change if all missing are assumed to not have allergic sensitisation to any foods, RR=1.34, 95% 0.92 to 1.96, or if all missing are assumed to have allergic sensitisation to foods, RR=1.08, 95% CI 0.94 to 1.24. | Low risk of bias | Quote: "Research nurses doing skin examinations, skin prick testing, food challenges, or making food allergy decisions, and the statistician, were masked to treatment allocation during the study." | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Dissanayake 2019 | Low risk of bias | Randomisation performed using minimization at a central location. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Quote: “we did not prohibit the application of moisturizers in the no‐intervention group. As this could mask the effect of the moisturizer used as an intervention, we looked at the effect of any emollient (irrespective of the formal intervention assigned) on the development of AD. We did not observe any difference in incidence between children who received any type of emollient vs. those who did not (data not shown).” “Approximately 80% of the parents/guardians in the emollient groups reported that they applied the emollient at least twice a day. Adjusting for emollient application rate did not lead to a significant difference in the rate of AD development among the groups. The effect of emollient application was also investigated in all babies who received emollient (groups 1 and 3) versus those who did not (groups 2 and 4), but this too did not show any difference in the incidence of AD.” | Low risk of bias | Outcome is available for 458/549 randomised individuals = 83%. Sensitivity analysis using the IPD shows conclusions do not change significantly if all individuals missing allergic sensitisation outcome are assumed to not have allergic sensitisation to food RR=1.19 [0.99, 1.42] or to all have allergic sensitisation to food, RR=1.05 [0.93, 1.20]. | Low risk of bias | An objective method of measurement used: IgE. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk of bias in all domains. |
| Horimukai 2014 | Low risk of bias | Randomised trial, no information provided on allocation concealment. Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. Control rates of skin care in line with those observed in other trials and observational studies (e.g. up to ~75 % in Rendell at al. 2011). Quote: “Because the IRB recommended permitting application of petroleum jelly when the parents thought it necessary, we calculated the amount of these 2 types of moisturizers used by each group based on their diaries. The mean daily amount of emulsion‐type moisturizer used by the intervention group was 7.86 6 4.34 g (0 g for the control group, excluding the 2 infants placed in the wrong group). The mean daily amount of petroleum jelly applied to the control group was 0.101 6 0.286 g (mean frequency of use, 0.235 d/wk). Petroleum jelly (20 g per bottle) was prescribed to all neonates born at the NCCHD, but we had no information about how much was used by the intervention group. Nevertheless, only a few of the parents occasionally used a small, almost ignorable, amount of the jelly on their infants.” | Low risk of bias | Data for any allergic sensitisation is available for 92/118 randomised = 78%. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have sensitisation for any food RR=1.00, 95% CI 0.66 to 1.50. If all missing are assumed to have sensitisation for any food RR=0.91, 95% CI 0.70 to 1.17. | Low risk of bias | Outcome obtained using an objective method of measurement and blinded assessment: IgE levels using a blood test ('DLC chip' ). | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Low risk of bias | Low risk fo bias in all domains. |
| Lowe 2018a | Low risk of bias | Quote from trial protocol: "A computer generated random allocation list in blocks of variable length (4‐12) will be used. This list will be held by The RCH Pharmacy Department, which will be independent from the participant recruitment or testing. At all times, the allocation list will be concealed from the study coordinator and the other study investigators, who will manage participant recruitment." Baseline variables by treatment group do not suggest a problem with randomisation, characteristics well balanced (IPD available). | Low risk of bias | Not possible to blind participants’ carers, but there is no evidence that deviations arised because of the trial context. The control group rates of skin care application were consistent with other trials and observational studies e.g. up to 75 % in Rendell et al. 2011. The IPD shows regular use of emollient (≥ 3 days a week) by 11/36 (31%) control participants and 30/38 (79%) with eczema outcome recorded). | Some concerns | Outcome available for 70/80=88% of randomised participants at 12 months. Sensitivity analysis using the IPD reveals conclusions do not change significantly if everyone missing is assumed to not have allergic sensitisation to any food inhalant, 0.40 [0.11 to 1.38] but conclusions change if all missing the outcome are assumed to have allergic sensitisation, 0.89 [0.42 to 1.90] (point estimate of RR >20% of the complete case estimate). Missingness could have depended on outcome, but not likely. | Low risk of bias | Skin prick tests used by blinded assessor. | Low risk of bias | Full trial dataset provided by investigators and IPD meta‐analysis SAP followed. | Some concerns | Some concerns due to missing data (12% and results varied in sensitivity analysis) but low risk of bias in all other domains. |
Acknowledgements
We are grateful to Emma Thomas, Boaz Gaventas, Alexa Baracaia, and the Centre of Evidence Based Dermatology patient panel for feedback on prioritisation of outcomes and outcome measures for this systematic review.
The draft search strategy for the World Health Organization International Clinical Trials Registry Platform was developed with advice from Douglas Grindlay, Information Specialist at the Centre of Evidence Based Dermatology, University of Nottingham, Nottingham, UK. We are extremely grateful to Liz Doney, Business Manager and Information Specialist at Nottingham University, who ran the search of the Cochrane Skin Specialised Register, the CENTRAL database, MEDLINE, and Embase in October 2019 and for the update in July 2020.
We gratefully acknowledge all members of the wider SCiPAD group, especially those who contributed to discussion and input at the annual meetings in Munich 2018, and Lisbon 2019, and at the online results meeting in 2020, including Sarah Brown, Carsten Flohr, Elisabeth Harberl, Jonathan Hourihane, Michael Perkin, Jochen Schmidt, and Stephan Weidinger.
This systematic review is supported by an award of an NIHR Transitional Research Fellowship to MK hosted by Imperial College London, and an award of an NIHR Research for Patient Benefit grant to Nottingham University Hospitals NHS Trust.
The Cochrane Skin editorial base wishes to thank Robert Dellavalle, Cochrane Dermatology Editor for this review; Jennifer Hilgart, Cochrane MOSS Network Associate Editor, who provided methodological peer review for this review; Simon Turner, who provided statistical peer review through the Cochrane Methods Support Unit; Helen A Brough and Masaki Futamura, clinical referees; Amanda Roberts, consumer referee; Dolores Matthews who copy‐edited the review; and Carolyn Hughes who wrote the plain language summary.
We are also indebted to all participants of the individual studies whose contributions have furthered our knowledge on skin care in infants.
Appendices
Appendix 1. Search strategy for Cochrane Skin Specialised Register (CRSW)
(Emollient* or moisturis* or moisturiz* or cream*):ti,ab AND INREGISTER
(Petrolatum or emulsion* or lubrica* or ointment* or lotion* or oil or oils or gel or gels or paste or pastes or salve* or unguent*):ti,ab AND INREGISTER
(Bath or baths or bathing or bathe* or soap* or water soften* or hard water or water hardness or skin care):ti,ab AND INREGISTER
#1 OR #2 OR #3
MESH DESCRIPTOR infant EXPLODE ALL AND INREGISTER
MESH DESCRIPTOR infant newborn EXPLODE ALL AND INREGISTER
(new next born*):ti,ab AND INREGISTER
(newly next born*):ti,ab AND INREGISTER
(neo next nat*):ti,ab AND INREGISTER
(Infant* or infancy or newborn* or perinat* or neonat* or baby* or babies):ti,ab,kw AND INREGISTER
#5 OR #6 OR #7 OR #8 OR #9 OR #10
#4 AND #11
Appendix 2. Search strategy for CENTRAL (Cochrane Library)
#1 MeSH descriptor: [Emollients] explode all trees #2 emollient*:ti,ab,kw #3 moisturis*:ti,ab,kw #4 moisturiz*:ti,ab,kw #5 MeSH descriptor: [Skin Cream] explode all trees #6 cream*:ti,ab,kw #7 {OR #1‐#6} #8 MeSH descriptor: [Petrolatum] explode all trees #9 petrolatum:ti,ab,kw #10 MeSH descriptor: [Emulsions] explode all trees #11 emulsion*:ti,ab,kw #12 MeSH descriptor: [Lubricants] explode all trees #13 lubrica*:ti,ab,kw #14 MeSH descriptor: [Ointments] explode all trees #15 ointment*:ti,ab,kw #16 lotion*:ti,ab,kw #17 MeSH descriptor: [Oils] explode all trees #18 (oil or oils):ti,ab,kw #19 (gel or gels):ti,ab,kw #20 (paste or pastes or salve* or unguent*):ti,ab,kw #21 {OR #8‐#20} #22 skin:ti,ab,kw #23 MeSH descriptor: [Skin] explode all trees #24 #22 or #23 #25 #21 and #24 #26 (bath? or bathe? or bathing):ti,ab,kw #27 MeSH descriptor: [Baths] explode all trees #28 MeSH descriptor: [Soaps] explode all trees #29 soap*:ti,ab,kw #30 MeSH descriptor: [Water Softening] explode all trees #31 water soften*:ti,ab,kw #32 (hard water or water hardness):ti,ab,kw #33 MeSH descriptor: [Skin Care] explode all trees #34 {OR #26‐#33} #35 #7 or #25 or #34 #36 MeSH descriptor: [Infant] explode all trees #37 MeSH descriptor: [Infant, Newborn] explode all trees #38 (Infant? or infancy or newborn* or perinat* or neonat* or baby* or babies or new next born* or newly next born* or neo next nat*):ti,ab #39 #36 or #37 or #38 #40 #35 and #39
Appendix 3. Search strategy for MEDLINE (Ovid)
1. exp Emollients/ 2. emollient$.ti,ab. 3. moisturis$.ti,ab. 4. moisturiz$.ti,ab. 5. exp Skin Cream/ 6. cream$.ti,ab. 7. or/1‐6 8. exp Petrolatum/ 9. petrolatum.ti,ab. 10. Emulsions/ 11. emulsion$.ti,ab. 12. exp Lubricants/ 13. lubrica$.ti,ab. 14. exp Ointments/ 15. ointment$.ti,ab. 16. lotion$.ti,ab. 17. exp Oils/ 18. oil$1.ti,ab. 19. (gel or gels).ti,ab. 20. (paste$1 or salve$ or unguent$).ti,ab. 21. or/8‐20 22. skin.mp. 23. exp Skin/ 24. or/22‐23 25. 21 and 24 26. bath$3.ti,ab. 27. exp Baths/ 28. exp Soaps/ 29. soap$.ti,ab. 30. exp Water Softening/ 31. water soften$.ti,ab. 32. (hard water or water hardness).ti,ab. 33. exp Skin Care/ 34. or/26‐33 35. 7 or 25 or 34 36. randomized controlled trial.pt. 37. controlled clinical trial.pt. 38. randomized.ab. 39. placebo.ab. 40. clinical trials as topic.sh. 41. randomly.ab. 42. trial.ti. 43. 36 or 37 or 38 or 39 or 40 or 41 or 42 44. exp animals/ not humans.sh. 45. 43 not 44 46. exp infant/ or exp infant, newborn/ 47. (Infan$ or newborn$ or new next born$ or newly next born$ or perinat$ or neonat$ or neo next nat$ or baby$ or babies).mp. 48. 46 or 47 49. 35 and 45 and 48
[ Lines 36‐45: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format, from section 3.6.1 in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook ]
Appendix 4. Search strategy for Embase (Ovid)
1. exp emollient agent/ 2. emollient$.ti,ab. 3. moisturis$.mp. 4. moisturiz$.mp. 5. skin cream/ 6. cream$.ti,ab. 7. or/1‐6 8. exp petrolatum/ 9. petrolatum.ti,ab. 10. exp emulsion/ 11. emulsion$.ti,ab. 12. exp lubricating agent/ 13. lubrica$.ti,ab. 14. exp ointment/ 15. ointment$.ti,ab. 16. exp lotion/ 17. lotion$.mp. 18. oil$1.ti,ab. 19. (gel or gels).mp. 20. (paste$1 or salve$ or unguent$).ti,ab. 21. exp paste/ 22. exp salve/ 23. or/8‐22 24. exp skin/ 25. skin.mp. 26. 24 or 25 27. 23 and 26 28. exp bath/ 29. bath$3.ti,ab. 30. exp soap/ 31. soap$.ti,ab. 32. water soften$.ti,ab. 33. exp skin care/ 34. exp water hardness/ 35. (hard water or water hardness).ti,ab. 36. or/28‐35 37. 7 or 27 or 36 38. crossover procedure.sh. 39. double‐blind procedure.sh. 40. single‐blind procedure.sh. 41. (crossover$ or cross over$).tw. 42. placebo$.tw. 43. (doubl$ adj blind$).tw. 44. allocat$.tw. 45. trial.ti. 46. randomized controlled trial.sh. 47. random$.tw. 48. or/38‐47 49. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 50. human/ or normal human/ 51. 49 and 50 52. 49 not 51 53. 48 not 52 54. infant/ or baby/ or exp newborn/ 55. (Infan$ or newborn$ or new next born$ or newly next born$ or perinat$ or neonat$ or neo next nat$ or baby$ or babies).mp. 56. 54 or 55 57. 37 and 53 and 56
[Lines 38‐53: Based on terms suggested for identifying RCTs in Embase (section 3.6.2) in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Appendix 5. Search strategy for clinicaltrials.gov register
emollient OR emollients OR moisturiser OR moisturisers OR moisturizer OR moisturizers OR barrier OR skin OR skincare OR bath OR bathing OR water softener OR water softeners OR water treatment
Appendix 6. Search strategy for WHO ICTRP trials register
emollient OR emollients OR moisturiser OR moisturisers OR moisturizer OR moisturizers OR barrier OR skin OR skincare OR bath OR bathing OR water softener OR water softeners OR water treatment
Appendix 7. Variables requested from trials for the individual participant data meta‐analysis (IPDMA)
| Patient identifiers for analysis inclusion |
| 1. Unique patient ID (anonymous ‐ or please give a new SCiPAD ID and keep log of the corresponding trial ID) 2. Randomised treatment allocation 3. Date of randomisation 4. Received randomised treatment (yes/no) 5. Included in the trials' primary analysis (yes/no) |
| Primary outcomes |
| 6. Eczema (at all time points collected and using all recorded measures of eczema or eczema symptoms, e.g. UK Working Party definition and investigator‐assessed – please send all eczema measures used and additional variables on skin condition (itch etc) pre formal eczema diagnosis and time point) 7. Food allergy (at all time points collected and using all recorded measures, e.g. using oral food challenge and investigator‐assessed – please send all food allergy measures used) |
| Secondary outcomes |
| 8. Slippage accidents around the time of bathing or application of emollienta 9. Skin infections during the intervention perioda 10. Stinging or allergic reactions to moisturisersa 11. Serious adverse eventsa 12. Time of eczema onset (first report of a diagnosis of eczema as a specific date or first visit date eczema recorded) 13. Eczema severity ‐ clinician‐assessed: EASI or similar validated measure (at all time points collected) 14. Eczema severity ‐ parent‐assessed: POEM or similar validated measure (at all time points collected) 15. Parent‐reported of immediate (< 2 hours) reaction to a known food allergen: milk, soya, wheat, fish, seafood, peanut, tree nut, egg, or local common food allergen (at all time points collected and for each food allergen recorded) 16. Allergic sensitisation to foods and inhalants via skin prick test (at all time points collected and for each food and inhalant recorded) |
| Infant baseline characteristics |
| 17. Gestational age at birth 18. Sexb 19. Birth weight 20. Pre‐existing health state in the infant, such as very preterm birth (less than 32 weeks' gestation) or congenital skin condition 21. Infant already diagnosed with eczema at the time of randomisation 22. Infant already diagnosed with food allergy at the time of randomisation 23. Age intervention began (e.g. number of days between birth and randomisation) 24. FLG genotype (method of analysis and what FLG mutations were genotyped) 25. Ethnicity 26. Mode of delivery (e.g. caesarean, vaginal) 27. Method of feeding (e.g. breastfeeding at all time points recorded) 28. Any additional trial randomisation stratification factors |
| Family baseline characteristics |
| 29. Age of mother at randomisation or enrolment 30. Age of father at randomisation or enrolment 31. Ethnicity of mother 32. Ethnicity of father 33. Educational status of mother 34. Educational status of father 35. Socioeconomic group 36. Singleton or multiple pregnancy 37. Number of other children living at home (without new child – or indicate if this includes the new child) 38. Whether any cats living in the household/living environment? 39. Whether any dogs living in the household/living environment? 40. Mother took any antibiotics during pregnancy? 41. Mother took any regular probiotic supplements during pregnancy? 42. Smoking status of mother 43. Smoking status of father |
| Family history of atopic disease |
| 44. Number of first‐degree relatives with atopic disease (0, 1, 2, or more)b [Please indicate how atopic disease is defined] 45. Number of first‐degree relatives with eczema (0, 1, 2, or more) 46. Number of first‐degree relatives with food allergy (0, 1, 2, or more) 47. Number of first‐degree relatives with asthma (0, 1, 2, or more) 48. Number of first‐degree relatives with rhinitis/hay fever (0, 1, 2, or more) |
| Compliance data |
| 49. Data on compliance with intervention, including measures such as grams per day and total number of grams of product dispensed over the study 50. Duration of treatment 51. Dates of treatment withdrawal and reason(s) for treatment withdrawal |
| Non‐assigned skin care |
| 52. Frequency of bathing 53. Product used for bathing (if not part of intervention) 54. Prescribed topical treatment use 55. Any other skin treatments |
| For cluster‐randomised trials |
| 56. Cluster‐randomisation factors |
| Food introduction |
| 57. Any data on the time/age when allergenic foods were introduced 58. Any data on the time/age when solid foods were introduced |
| EASI: Eczema Area and Severity Index; FLG: filaggrin gene; POEM: Patient‐Oriented Eczema Measure |
aAdverse events of interest. All adverse events may be sent if trials do not have these separated out. bCritical baseline variables required for covariate adjustment within primary and secondary analyses.
Data and analyses
Comparison 1. Skin care intervention versus standard skin care or no skin care intervention.
1.2. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 2: Sensitivity analysis: Eczema by 1‐3 years including aggregate trial data
1.4. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 4: Sensitivity analysis: Eczema by 1‐3 years (including data from all 4 arms of PreventADALL)
1.9. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 9: Subgroup analysis (study level): Eczema by 1‐3 years by intervention type
1.18. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 18: CACE Sensitivity: Eczema by 1‐3 years for use over intervention period ≥ 5 days a week
1.19. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 19: CACE Sensitivity: Eczema by 1‐3 years for use over intervention period 7 days a week
1.20. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 20: CACE Sensitivity: Eczema by 1‐3 years for use over first 3 months ≥ 3 days a week
1.21. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 21: CACE Sensitivity: Eczema by 1‐3 years for use over first 3 months ≥ 5 days a week
1.22. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 22: CACE Sensitivity: Eczema by 1‐3 years for use over first 3 months 7 days a week
1.24. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 24: Sensitivity analysis: Eczema by 1‐3 years for studies included in CACE for use over intervention period ≥ 5 days a week
1.25. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 25: Sensitivity analysis: Eczema by 1‐3 years for studies included in CACE for use over intervention period 7 days a week
1.26. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 26: Sensitivity analysis: Eczema by 1‐3 years for studies included in CACE for use over first 3 months ≥ 3 days a week
1.27. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 27: Sensitivity analysis: Eczema by 1‐3 years for studies included in CACE for use over first 3 months ≥ 5 days a week
1.28. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 28: Sensitivity analysis: Eczema by 1‐3 years for studies included in CACE for use over first 3 months 7 days a week
1.33. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 33: CACE Sensitivity: Food allergy by 1‐3 years for use over intervention period ≥ 5 days a week
1.34. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 34: CACE Sensitivity: Food allergy by 1‐3 years for use over intervention period 7 days a week
1.35. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 35: CACE Sensitivity: Food allergy by 1‐3 years for use over first 3 months ≥ 3 days a week
1.36. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 36: CACE Sensitivity: Food allergy by 1‐3 years for use over first 3 months ≥ 5 days a week
1.37. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 37: CACE Sensitivity: Food allergy by 1‐3 years for use over first 3 months 7 days a week
1.47. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 47: Subgroup analysis: Time to onset of eczema (< 1 year follow‐up versus ≥ 1 year follow‐up)
1.57. Analysis.

Comparison 1: Skin care intervention versus standard skin care or no skin care intervention, Outcome 57: Allergic sensitisation to inhalants at 1‐3 years
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abraham 2019.
| Study characteristics | ||
| Methods |
Study Design: randomised controlled trial Study conducted: not reported Treatment arms: 3 Follow‐up: 24 hours |
|
| Participants |
Randomised: N = 102 (chlorhexidine n = 34, saline n = 34, standard n = 34) Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions |
Intervention: participants were bathed according to treatment group in either chlorhexidine or saline; concentrations were not reported Comparator: standard bath of soap and water |
|
| Outcomes |
Primary outcome: skin health status of all participants before and 2 hours and 24 hours after the intervention by an individual who is blinded to the intervention using neonatal skin assessment score Adverse events: not reported |
|
| Identification |
Country: India Setting: tertiary care hospital, Vellore Sponsorship Source: Institutional Review Board, Christian Medical College, Vellore |
|
| Declarations of interest | Nil | |
| Notes | ||
Amer 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study conducted: April 2014 and September 2014 Treatment arms: 2 AD follow‐up: 4 weeks |
|
| Participants |
Randomised: N = 70 (group A underwent care n = 35, group B did not undergo care n = 35) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: Caregivers/mothers were instructed to provide a specific skin care regimen Caregivers (if possible) were instructed to gently dry the baby immediately after birth and to gently remove any blood or meconium and to not rub off the vernix (leave it as intact as possible to absorb into the skin) During the 4 weeks of the study, neonates were bathed at variable frequencies by mothers most often 1 to 2 times per week using shampoo as a cleanser; sometimes baby wipes were used as an alternative to bathing. The first bath was given only when the temperature of the newborn was stabilised, instead of considering only the number of hours after birth, and usually during the first week. Mothers were instructed to apply oil to the skin and the scalp 3 to 4 times per week and after bath time, and that this should be applied daily when signs of dryness (flaking/scaling) were presented. Mothers were instructed to keep the umbilical cord clean and dry by applying chlorhexidine in the first 10 days of life until the cord falls off and 2 days after, and allowing it to be exposed to air as frequently as possible. Mothers were instructed to use the best quality nappy available, to change soiled nappies frequently, to cleanse the nappy area with plain water or unperfumed, alcohol‐free baby wipes, to expose the nappy area as often as possible, and to consider using a thin layer of barrier ointment or cream with nappy changes. Mothers were instructed to care for the neonatal intertrigo by keeping it clean and dry. A colourful and informative booklet had been designed for the mothers, which clarifies instructions about care of neonatal skin and benign transient neonatal skin disorders for the reassurance of parents Comparator: group B: did not undergo care; no specific intervention Shampoo, baby oil, wipes, and cream ingredients: Baby shampoo, which is composed of sodium lauroamphoacetate, sodium laureth sulfate, coco glucoside, polyquaternium‐10, and sodium benzoate. Baby oil is a mineral oil that contains paraffinum liquidum, isopropyl palmitate, and parfum, which are safe. It is used as a moisturiser and for massage. Baby wipes consist of a non‐woven carrier soaked with an emulsion‐type watery or oily lotion. Baby cream consists of zinc oxide and olive oil |
|
| Outcomes |
Primary outcomes: optimal skin function, mothers' visual skin assessment questionnaire to evaluate the presence of neonatal skin for erythema and dryness. Clinical examination for skin assessment for appearance of erythema, dryness, and infection or any skin disorders or adverse effects on a weekly basis Adverse events: adverse events were recorded when the baby's skin was assessed during weekly clinical examination The only adverse effect of this study was a case of milaria using emollient because the mother turned on the heater all night, which turns the atmosphere hot and humid; this conclusion is in agreement with a study by Rocha et al., which found that emollient may cause acne, folliculitis, and prickly heat, and may aggravate pruritus when used in extremely hot and humid areas |
|
| Identification |
Country: Egypt Setting: outpatient clinics at the Dermatology and Venereology Department, Obstetric Department, and Pediatric Department, Faculty of Medicine, Zagazig University Hospitals Sponsorship source: not reported |
|
| Declarations of interest | Not reported | |
| Notes | ||
Baldwin 2001.
| Study characteristics | ||
| Methods |
Study design: randomised clinical trial, double‐blinded, parallel‐group comparison trial Study conducted: no date of recruitment or start/end date of trial reported in the study Treatment arms: 2 (Study C) AD follow‐up: 4 weeks (2 visits every week) |
|
| Participants |
Randomised: 304 children (evaluated skin erythema and diaper rash in 268 infants over a 4‐week usage period) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Parents of all eligible children were given a 1‐week supply of the control product and were instructed to use only this diaper until the next scheduled visit 6 days later (‘washout’ period). At completion of the ‘washout’ period, 304 children were randomly allocated to 1 of 2 treatment groups after a baseline dermatological examination of the diaper area. Randomisation was by gender and diaper rash grade All parents were instructed to diaper their child exclusively with the product assigned to him/her, and to avoid the use of any ointments, creams, powders, or other diaper rash or skin care products on the diapered area of their children for the entire duration of the study. Parents were allowed to maintain normal bathing and hygiene routines for their children except that they were asked to use a standard disposable infant wipe, which was supplied to them in lieu of their usual wipe or wash cloth for diaper changing needs Parents were instructed to change their child into a clean diaper approximately 2 hours before each subsequent scheduled visit to the clinical site. Children returned to the clinical site twice per week (Monday/Thursday or Tuesday/Friday) over the next 4 weeks, for a total of 8 post‐baseline visits. At each of these visits, the skin in the diaper area of each child was examined for the presence of rash and erythema Intervention: Group 2 was assigned to use the test diaper Comparator: Group 1 was assigned to continue on the control product Diaper and wipes: control diaper used was a commercially available, premium quality product containing a super‐absorbent(AGM)/cellulose core and a breathable outer cover, which was obtained directly from our manufacturing lines (Procter & Gamble Co., Cincinnati, OH, USA) The test diaper was identical in every respect to the control except for the inclusion of a top sheet (inner layer) impregnated with a proprietary formulation containing primarily petrolatum, stearyl alcohol, and zinc oxide in combination (ZnO/Pet). The wipes used were Pampers Baby Fresh™, Proctor & Gamble Co. |
|
| Outcomes | Severity of diaper dermatitis (skin erythema and rash), scoring given by Table 1 (Baldwin 2001) Adverse events: not reported |
|
| Identification |
Country: USA Sponsorship source: Hill Top Laboratories (Cincinnati, OH, USA, and Winnipeg, Canada), for its collaboration in the conduct of the clinical studies |
|
| Declarations of interest | Not reported | |
| Notes | ||
Bellemere 2018.
| Study characteristics | ||
| Methods |
Study design: randomised clinical study Study conducted: no date of recruitment or start/end date of trial reported in study Treatment arms: 2 AD follow‐up: 6 months |
|
| Participants |
Randomised: N = 120 (n = 60 in the intervention arm, n = 60 in the control arm) Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions | (D0, D30, D90, D120, D180). In parallel, 60 newborns with no familial history of atopy have been followed. Swabs were taken on forearms, face, and AD lesions in 45 children: quantitative PCR for SA and SE and LC/UV + LC/MS for NMF and ceramides Intervention: use balm twice a day, cleansing cream and bath oil twice a week from the same brand for 6 months Comparator: control group, no specific intervention Moisturiser/Emollient: "a French cosmetic brand" dedicated to children's skin (no other details of formulation reported) |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Adverse effects: none reported in conference abstracts |
|
| Identification |
Country: not reported Setting: not reported Sponsorship source: none reported |
|
| Declarations of interest | Not reported | |
| Notes | ||
Chalmers 2020.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, pragmatic, parallel‐group, randomised controlled trial Recruitment: 19 Nov 2014 and 18 Nov 2016 Treatment arms: 2 AD follow‐up: participant follow‐up was 2 years, including follow‐up at 2 weeks (by telephone), and at 3, 6, 12, and 18 months (online or postal questionnaire), and at a 2‐year face‐to‐face appointment FA and inhalants follow‐up: 2 years |
|
| Participants |
Randomised: N = 1394 (emollient n = 693, control n = 701) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Both groups received advice on general skin care in booklet and video formats at the time of randomisation (Appendix pp 11 to 21). The skin care guidance provided advice to use mild cleansers and shampoos specifically formulated for infants, and to avoid soap, bubble bath, and baby wipes. Infants were randomly assigned to a group within a maximum of 21 days after delivery, and randomisation was stratified by recruiting centre and number of first‐degree relatives with atopic disease (1, 2, or > 2) Intervention: adherence was captured at each questionnaire time point during year 1 (3, 6, and 12 months) by asking parents about emollient use since the last questionnaire (Appendix p 4), and was defined in the protocol as satisfactory in the intervention group if emollients were applied at least 3 to 4 times per week to most of the child’s body (defined as at least 2 of face and neck, arms and legs, or trunk). A similar definition was used for contamination in the control group Parents were advised to apply emollient to the whole body of their child at least once daily (excluding the scalp) until the child reached 1 year of age. They were also advised to apply emollient after every bath, even if they had already applied the emollient that day. Daily application was advised to encourage regular use of emollient several times a week, but because the study was designed to reflect how the intervention might be delivered in normal practice, no prompts or reminders were sent to parents The guidance given to those in the emollient group also showed parents how to apply emollients correctly by dotting over the skin and using gentle downward strokes rather than rubbing in, and contained warnings about the skin being slippery after application and the need to clean up spillages from the floor to avoid slipping Comparator: best practice skin care advice only Moisturiser/Emollient: Doublebase Gel (Dermal Laboratories, Herts, UK) or Diprobase Cream (Bayer, Berks, UK) |
|
| Outcomes |
Primary outcome: diagnosis of eczema over the past year (defined by the UK Working Party refinement of Hanifin and Rajka diagnostic criteria for eczema) assessed by research nurses masked to treatment allocation at age 2 years Secondary outcomes: other eczema definitions ‐ i.e. presence of eczema between birth and 2 years of age (assessed by any parental report of a clinical diagnosis of eczema (up to 2 years) and parent completion of UK Working Party criteria at 1 and 2 years); presence of visible eczema at 2 years recorded by a nurse who was masked to treatment allocation; time to onset of eczema (based on first parent report of clinician diagnosis and time of first topical corticosteroid or immunosuppressant prescription); clinician‐reported and patient‐reported severity of eczema (Eczema Area and Severity Index (EASI) at 2 years and Patient‐Oriented Eczema Measure [POEM] at 1 and 2 years). Other secondary outcomes were presence of other allergic diseases (i.e. parent‐reported wheezing and allergic rhinitis (between 1 and 2 years); allergic sensitisation (masked skin prick tests) to milk, egg, peanut, cat dander, grass pollen, or dust mite at 2 years; parent‐reported food allergy and parental report of clinical diagnosis of food allergy at 1 and 2 years; and allergy to milk, egg, or peanut at 2 years confirmed by oral food challenge; for cases in which no oral food challenge was done, an expert allergy panel was asked to allocate treatment Adverse events: safety outcomes were parent‐reported skin infections (parents were asked what the doctor called the infection) and emollient‐related infant slippages during the intervention period (year 1). Skin infections and slippages were collected via 3, 6, and 12 month questionnaires. No other adverse event information was collected |
|
| Identification |
Country: UK Setting: 12 hospitals and 4 primary care sites across the UK Sponsorship source: this study was sponsored by the University of Nottingham, was co‐ordinated by the Nottingham Clinical Trials Unit (CTU), and was funded by the UK National Institute for Health Research (NIHR) Health Technology Assessment Programme |
|
| Declarations of interest | The main funder (NIHR Health Technology Assessment) was involved in refining the trial design through the funding peer review process but had no role in data collection, data analysis, data interpretation, or writing of the report. Funders of the food allergy outcomes and skin prick tests (Goldman Sachs Gives and Sheffield Children’s Hospital Research Fund) had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript HCW, AAM, and LEB had full access to all data in the study, and HCW had final responsibility for the decision to submit for publication | |
| Notes | ||
Cooke 2015.
| Study characteristics | ||
| Methods |
Study design: pilot, assessor‐blinded RCT Study data collected: between September 2013 and July 2014 Treatment arms: 3 AD follow‐up: 4 weeks |
|
| Participants |
Randomised: N = 115 (olive oil n = 38, sunflower oil n = 38, no oil n = 39) Inclusion criteria: participants with or without a family history of AE Exclusion criteria:
|
|
| Interventions |
Intervention: randomization took place within 72 hours of birth, parents were instructed from the day after initial assessment to apply 4 drops of oil to their baby's left forearm, left thigh, and abdomen, twice a day. Parents in all groups were asked not to use any other skin care products at the 3 study sites; water only was advocated. Intervention period was 4 weeks
Comparator: the control group was provided no oil and was asked not to use any other skin care products at the 3 study sites; water only was advocated |
|
| Outcomes |
Primary outcome: the change in structure of the lipid lamellae, a determinant of SC permeability (measured using ATR‐FTIR spectroscopy) and TEWL (measured via Biox Aquaflux Model AF200) Secondary outcome: stratum corneum hydration (measured via a Corneometer® Model CM825); skin surface pH (measured by skin pH meter® Model PH 905); clinical observations around changes in the skin using a modified Neonatal Skin Condition Score; and erythema (measured by a Mexameter® Model MX18 probe) Adverse effects: adverse events, including skin infections, skin reactions, and serious adverse events, were prompted for and collected for all participants |
|
| Identification |
Country: England, UK Setting: St. Mary's Hospital, Manchester Sponsorship source: AC was funded by the NIHR, and this paper was independent research arising from the Doctoral Research Fellowship, supported by NIHR |
|
| Declarations of interest | None reported | |
| Notes | ||
Da Cunha 2008.
| Study characteristics | ||
| Methods |
Study design: randomised clinical trial Recruitment date: infants delivered between 13 September 2005 and 14 March 2006 Treatment arms: 2 AD follow‐up: time points: first bath (first collection), 30 minutes after bath (second collection), and 24 hours after bath (third collection) |
|
| Participants |
Randomised: N = 112 (chlorhexidine, experimental group n = 56, neutral liquid soap control group n = 56) Inclusion criteria: normal term newborns with gestational age 37 and 42 weeks Exclusion criteria:
|
|
| Interventions |
Intervention: chlorhexidine liquid soap bath, admission bath between 1 and 1.5 hours after birth Comparator: neutral liquid soap bath, admission bath same as above Moisturiser/Emollient: neutral liquid soap (ingredients: Texapon SBN (detergent), Dehyton KB (cocamide), Plantaren 2000 (detergent), Glycerin (emollient), Coperlan KDB (thickener), citric acid, deionized water, pH = 7) Chlorhexidine liquid soap bath (chlorhexidine digluconate liquid soap at 0.4%, resulting from dilution of 10 mL of chlorhexidine digluconate liquid soap at 4% in 90 mL of warm water that released 0.25% chlorhexidine) |
|
| Outcomes |
Staphylococcus aureus skin colonisation Adverse effects: none reported |
|
| Identification |
Country: Brazil Setting: Obstetric Centre of Hospital de Clinicas de Porto Alegre Sponsorship source: this study was supported by the Fundo de Incentivo à Pesquisa (Research Incentive Fund) of Hospital de Clínicas de Porto Alegre (FIPE/HCPA), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) |
|
| Declarations of interest | None reported in publication | |
| Notes | ||
Dissanayake 2019.
| Study characteristics | ||
| Methods |
Study design: 2 × 2 factorial, randomised, non‐treatment controlled trial Recruitment: October 2012 and March 2014 Treatment arms: 4 AD follow‐up: 1, 6, and 9 months |
|
| Participants |
Participants: N = 549 (skin care + synbiotics n = 137; synbiotics n = 137; skin care n = 138; no intervention n = 137) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: parents/caregivers were advised on how interventions should be applied at home. Group 1 received a combination of synbiotics and skin care, group 2 received synbiotics only, group 3 received skin care only, and group 4 received no intervention. Interventions were carried out from birth to 6 months of age, and further observation was made for an additional 6 months. Parents/guardian maintained a diary for 6 months of the intervention to record the number of times that the emollient was applied, any illnesses contracted during this period, and the use of antibiotics during this time. Parents/guardians were instructed to apply emollient 2 to 3 times/d, after a bath or on clean skin, particularly on the cheeks and the peri‐oral area. Parents/guardians were allowed to apply the emollient to other parts of the body at their discretion and were not advised for or against it Comparator: the control group was not prevented from applying emollients for ethical reasons. A diary was maintained to record the number of times and the amount of emollient that was applied each month Moisturiser/Emollient: all participants receiving skin care (groups 1 and 3) were given Locobase® REPAIR Cream (Daiichi Sankyo, Japan), which contains ceramide, cholesterol, and free fatty acids Synbiotics: groups that were given synbiotics (groups 1 and 2) received a combination of 0.5 g (7 × 109 CFU/g) of Bifidobacterium bifidum OLB6378 (Meiji Holdings Co. Ltd., Japan) combined with 0.5 g of fructo‐oligosaccharides (Meiji Food Materia Co. Ltd., Japan) twice a day |
|
| Outcomes |
Primary outcomes: The primary outcome assessed was development of AD by 1 year of age. AD was diagnosed according to the criteria of the Japanese Dermatological Association, when an itchy rash lasting 2 months or longer was reported in questionnaires returned at 1, 6, and 9 months and at 1 year of the baby’s age. In addition, AD was diagnosed using the UK Working Party’s diagnostic criteria included in the questionnaire at 1 year Secondary outcomes: Prevalence of FA, as reported in the questionnaires at 1 year. Sensitisation to food and/or inhalant allergens – total and allergen‐specific IgE levels were determined in blood sampled at 9 months of age. EASI score – babies were examined by Dr. YuT (paediatrician) at 9 months of age. If AD was diagnosed, severity was determined using the EASI score and photography of the body. AD diagnosis was further confirmed blindly by YaT (dermatologist) and NS (paediatrician). AD was diagnosed based on criteria of the Japanese Dermatological Association Thymus‐ and activation‐regulated chemokine (TARC) score – blood samples obtained at 9 months were used for evaluating TARC levels of all participants Adverse effects: no adverse effects of the interventions were reported during the study period |
|
| Identification |
Country: Japan Setting: antenatal clinic at the Japanese Red Cross Katsushika Maternity Sponsorship source: this study was supported by the Environmental Restoration and Conservation Agency of Japan in fiscal years 2014 to 2016 and by grants from the Japan Agency for Medical Research and Development (AMED‐CREST) (15652274) |
|
| Declarations of interest | Study authors have no conflicts of interest to disclose | |
| Notes | ||
Dizon 2010.
| Study characteristics | ||
| Methods |
Study design: parallel, randomised, controlled trial Recruitment date: not reported Treatment arms: 3 Follow‐up: assessment was done at baseline, and after 1 week and 2 weeks of using the products |
|
| Participants |
Randomised: N = 180 Filipino infants (< 1 year) (JTT n = 60; SEBAMED n = 60; water only n = 60) Inclusion criteria: Filipino infants (age 1 day to < 1 year) in good health with normal skin Exclusion criteria: prematurely born infants and those with congenital problems |
|
| Interventions |
Intervention:
The above products (Group I) were used on the skin of subjects as whole body cleansers at least twice a week for 2 weeks Assessment was done at baseline and after 1 week and 2 weeks of use: (i) clinically by a dermatologist, (ii) instrumentally, and (iii) by the consumer (parent of the participant) Comparator: Group III ‐ lukewarm tap water |
|
| Outcomes | Clinical assessment (erythema, oedema, dryness, and scaling); skin moisture content; skin surface pH; transepidermal water loss; skin oxyhaemoglobin and deoxyhaemoglobin; and consumer satisfaction were the outcome measures Adverse effects: parents did not report any side effects |
|
| Identification |
Sponsorship source: Johnson and Johnson. Country: Philippines |
|
| Declarations of interest | Not reported | |
| Notes | ||
Duan 2019.
| Study characteristics | ||
| Methods |
Study design: randomised, single‐centre, parallel‐assignment trial Study date: June 2013 to November 2013 Treatment arms: 3 AD follow‐up: 6 weeks and 12 weeks |
|
| Participants |
Randomised: N = 150 (wash + lotion n = 44; water + lotion n = 43; water only n = 43) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: Group 1: Johnson's Baby Top‐To‐Toe Wash (ideally 7 times a week, at least 5 times a week) + Johnson's Baby Lotion (at least once a day). Products/treatments were used for 3 months, and products were placed on the skin with attention to applying them to the arms, legs, and torso Group 2: water (in lieu of bathing products) + Johnson's Baby Lotion (at least once a day). Products/treatments were used for 3 months, and products were placed on the skin with attention to applying the products to the arms, legs, and torso Comparator: water (in lieu of bathing products) only. Products/treatments were used for 3 months. Products were placed on skin with attention to applying products to the arms, legs, and torso Moisturiser/Emollient: Johnson’s® Baby Top‐To‐Toe® Wash and Johnson’s® Baby Lotion, Johnson & Johnson Consumer Inc., Skillman, NJ; wash + lotion |
|
| Outcomes |
Primary outcome: skin surface moisture [Time Frame: 3 months] Skin surface moisture content via capacitance measurements Secondary outcome:
Adverse effects: none reported |
|
| Identification |
Country: China Setting: Beijing Children’s Hospital, Xicheng District Sponsorship source: this study was funded by Johnson & Johnson International Pte. Ltd (Singapore) |
|
| Declarations of interest | YYD, CG, and F‐QK were employees of Johnson & Johnson at the time this study was conducted. YYD and F‐QK are no longer employed at Johnson & Johnson. C‐PS and LM received research support in association with this study. Study authors report no other conflicts of interests in this work | |
| Notes | ||
Garcia Bartels 2010.
| Study characteristics | ||
| Methods |
Study design: monocentric, prospective, randomised study Study conducted: October 2006 to May 2007 Treatment arms: 4 AD follow‐up: day 2; weeks 2, 4, and 8 of life |
|
| Participants |
Randomised: N = 64 (WG, bathing with wash gel n = 16; C, bathing and cream n = 16; WG + C, bathing with wash gel plus cream n = 16; B, bathing with water n = 16) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: full‐term neonates (32 girls, 32 boys) aged ≤ 48 hours were randomly assigned to 4 groups (including one comparator group, each n = 16) receiving treatment twice weekly from day 7 until week 8 of life.
All neonates were washed 3 times with a cotton wash cloth, moistened with water, until day 7 Bathing lasted about 5 minutes using tap water at temperature 37 to 38°C, pH 7.9 to 8.2, hardness 13.4 dH (range 7 to 25 dH). Diapers from Pampers Baby Dry for Newborns were provided. Parents were instructed to avoid treating skin with any other skin care products. Topical products were allowed on areas of skin trauma or diaper dermatitis, including: triclosan 1% cream, octenidin ⁄ phenoxyethanol solution, zinc paste (optional with nystatin). To remove meconium: oil and vaseline were allowed. Comparator: Group B, bathing with clear water |
|
| Outcomes |
Adverse effects: no adverse events reported |
|
| Identification |
Country: Germany Setting: Department of Dermatology, Clinic for Neonatology CCM at Charité‐Universitätsmedizin Berlin, and Department of Gynaecology, Clinic Dahme‐Spreewald Sponsorship source: the work of Franziska Prosch was supported by an unrestricted medical grant from Johnson & Johnson |
|
| Declarations of interest | The funders had no input regarding study design or conduct, data analysis or interpretation, manuscript preparation, or the decision to submit the results for publication | |
| Notes | ||
Garcia Bartels 2011.
| Study characteristics | ||
| Methods |
Study design: monocentric, prospective, randomised, clinical non‐pharmaceutical study Study conducted: September to December 2009 Treatment arms: 2 AD follow‐up: 4 weeks |
|
| Participants |
Randomised: N = 44 (Group L using lotion n = 20; Group WL without lotion n = 24) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Both groups went swimming weekly for 4 weeks for 25 to 40 minutes at the Charité‐Universitätsmedizin Berlin physiotherapy facilities. Both groups received a standard skin care regimen: weekly bathing in tap water, diaper care with water and cotton cloth or Bübchen® Comfort sensitive wipes. No skin care was performed 12 hours before evaluations Intervention: in group L (lotion group), baby skin care lotion was applied to the entire body once weekly after swimming and the skin was dried with a towel Comparator: no lotion or other skin care product was applied in Group WL (without lotion) Moisturiser/Emollient ingredients: Bübchen® Pflege Lotion: aqua, Helianthus annuus seed oil, isopropyl palmitate, dicaprylyl ether, ethylhexyl stearate, polyglyceryl‐3 polyricinoleate, glycerin, butylene glycol, octyldodecanol, polyglyceryl‐3 diisostearate, parfum, zinc stearate, chamomilla‐recutita extract, tocopheryl acetate, glyceryloleate, magnesium sulfate, tocopherol |
|
| Outcomes |
Measured on 4 anatomical test areas (forehead, abdomen, buttock, thigh), using the non‐invasive Multi‐Probe Adapter System MPA® (Courage & Khazaka Electronic, GmbH, Cologne, Germany) at 6 study visits. Baseline visit (V0) was within 4 weeks before the first swimming session. After baseline, visits were performed weekly before swimming sessions (V1 to V4). No swimming took place at follow‐up, 1 week after the last swimming session (V5) Adverse events: method of collection of adverse events is not recorded "Neonatal skin condition score (NSCS) was found to be mainly normal (score 3). A mildly elevated NSCS (4) was found in < 7.5% of infants per visit, and depending on area, an NSCS of 5 was found only once at baseline visit on the thigh (data not shown). NSCS was statistically comparable at all test regions in both groups throughout the study period (n = 44). The overall occurrence of adverse events (AEs) was lower in group L (n = 18) than in group WL(n = 33, Table 3). The occurrence of diaper dermatitis, however, was similar in both groups (n = 9). After dichotomisation of subjects into the two groups “no AE at all” vs “at least one AE”, χ² test showed significantly less occurrence of AEs in group L compared to group WL (55.0% vs 83.3%; P = 0.04; Figure 8)" |
|
| Identification |
Country: Germany Setting: Charité‐Universitätsmedizin Berlin, Germany, physiotherapy facilities Sponsorship source: the clinical study was sponsored by Bübchen Deutschland |
|
| Declarations of interest | Prof. Blume‐Peytavi has received presentation fees from Bübchen Deutschland, which sponsored the study | |
| Notes | ||
Garcia Bartels 2012.
| Study characteristics | ||
| Methods |
Study design: monocentric, prospective, randomised pilot study Study conducted: May 2007 to October 2007 Treatment arms: 2 AD follow‐up: baseline second day of life at neonatal ward, followed by 14th and 28th days of life |
|
| Participants |
Randomised: N = 44 (skin care with baby wipes n = 21; water‐moistened washcloth n = 23 at each diaper change) Inclusion criteria: healthy full‐term newborns with 37 completed weeks of gestation Exclusion criteria:
|
|
| Interventions | Treatments were applied approximately 8 times per 24 hours by parents over 4 weeks Both groups obtained a standard skin care regimen, with twice‐weekly bathing in clear tap water without use of a cleanser as described. No additional skin care was given, except for areas of skin trauma or diaper dermatitis Intervention: skin care with baby wipes (BW). Infants were cleansed with baby wipes during each diaper change Comparator: water‐moistened washcloth (cotton washcloth moistened with tap water) Wipes and diaper ingredients: skin care with baby wipes (BW), Penaten (Procter & Gamble Manufacturing GmbH, Euskirchen, Germany), baby wet wipes with aloe vera (aqua, myristyl alcohol, stearyl alcohol, propylene glycol, epilobium angustifolium extract, aloe barbadensis, PEG‐4 laurate, tocopherol, citric acid, lactic acid, tetrasodium‐EDTA phenoxyethanol, iodopropyynyl butylcarbamate, parfum, Johnson & Johnson GmbH, Duesseldorf, Germany), Pampers Diapers ‘‘newborn’’ size, cotton washcloths provided. Tap water pH 7.9 to 8.2 |
|
| Outcomes |
Primary outcome: transepidermal water loss Secondary outcomes: skin pH, SCH, epidermal desquamation, Neonatal Skin Condition Score (NSCS), IL‐1 alpha level Adverse effects: not reported |
|
| Identification |
Country: Germany Setting: Charité ‐Universitätsmedizin Berlin Sponsorship source: Lida Massoudy’s work was supported by an unrestricted medical grant from Johnson & Johnson GmbH. We thank Dr. Gaelle Bellemere (Johnson & Johnson, Research and Development, France) for support in the IL‐1a analysis and in the D‐Squame technique of blinded samples |
|
| Declarations of interest | Not reported | |
| Notes | ||
Garcia Bartels 2014.
| Study characteristics | ||
| Methods |
Study design: single‐centre, prospective, randomised trial Study conducted: November 2010 to April 2012 Treatment arms: 3 Follow‐up: 4 weeks and 8 weeks |
|
| Participants |
Randomised: N = 89 (group 1 n = 30, group 2 n = 28, group 3 n = 31) Inclusion criteria: Healthy infants aged 9 months (±8 weeks) Exclusion criteria:
|
|
| Interventions |
Intervention: Daily use of wet wipes or water‐moistened washcloths and diaper cream in the diaper area. Group 2 received cleansing with water‐moistened washcloths and diaper cream twice per day. Group 3 received cleansing with wet wipes (Penaten Baby‐Lotion Tucher, Johnson & Johnson) at each diaper change and diaper cream twice per day In addition, all groups were advised a twice‐weekly skin care regimen, which includes bathing with a baby cleanser (Pentanen Baby Bad & Shampoo, Johnson & Johnson) and applying baby lotion after bathing (Penaten Baby Intensive Lotion, Johnson & Johnson) except for the diaper region Parents advised to wash the water‐moistened washcloths at 60°C in the washing machine without fabric softener Clinical measurements were performed at inclusion (week 0 (W0)), week 4 (W4), and week 8 (W8) at the CRC. Diapers were removed 10 minutes before measurements. The minimum duration between last diaper change and measurements was 1 hour; bathing or skin care was allowed 12 hours before measurements. A minimum of 4 diaper changes every 24 hours was required, and if DD occurred, a topical therapy was allowed on affected areas as needed. Ambient conditions were standardised (temperature 22°C to 26°C, relative humidity 40% to 60%). During the study period, DD occurred in the perianal and genital areas but not in investigational areas, that is, the outer upper quadrant of the buttock (diapered area) and the upper leg (non‐diapered area). When DD occurred, extra measurements were taken in the affected area Skin condition was evaluated using a modified NSCS 2, 3, 11, and in the diaper area using a modified DRG 1 (7‐point scale; none = 0, severe = 3). Clinically relevant DD was defined as DRG of 1.5 or greater Parents were advised to document in a diary any changes in skin care and the health status of the infant, which they were given and was explained at the inclusion visit. The investigator verified diary entries at each visit Comparator: Group 1 received cleansing with water‐moistened washcloths at each diaper change |
|
| Outcomes |
Primary outcome: transepidermal water loss Secondary outcomes:
Adverse events: no adverse events reported |
|
| Identification |
Country: Germany Setting: Clinical Research Center for Hair and Skin Science (CRC), Charité‐Universitätsmedizin Berlin Sponsorship source: the trial was sponsored by Johnson & Johnson Consumer EMEA. The sponsor had input into the study design and blinded analysis of interleukin. The sponsor had no influence on conduct of the trial, collection of data, or statistical evaluations |
|
| Declarations of interest | U. Blume‐Peytavi is a consultant to Johnson & Johnson GmbH. N. Garcia Bartels has been speaker for Johnson & Johnson Consumer GmbH | |
| Notes | ||
Horimukai 2014.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled, parallel‐group, investigator‐blinded trial Recruitment date: November 2010 to November 2013 Treatment arms: 2 AD follow‐up: 4 weeks, 12 weeks, 24 weeks, 32 weeks FA follow‐up: sensitisation to food and inhalants at 32 weeks |
|
| Participants |
Randomised: N = 118 (intervention n = 59; control n = 59) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: emollient was applied each day for 32 weeks. Hanifin‐Rajka criteria were used to diagnose AD Comparator: control group applied petroleum jelly if desired Moisturiser/Emollient: emulsion‐type emollient (2e (Douhet) emulsion) from the first week of life; petroleum jelly was prescribed to each infant in both groups on request by the institutional review board |
|
| Outcomes |
Primary outcomes: the cumulative rate of incidence of AD, eczema, or both by temporal observation. Modified UKWP criteria were applied by a dermatology specialist Secondary outcomes:
Adverse events: “the dermatology specialist stopped giving the emollient to 3 infants whose skin lesions seemed to be the result of urticaria or contact dermatitis caused by emulsion‐type emollients (related adverse events). After several days, however, the doctor judged that these skin lesions were not adverse events because they disappeared rapidly, and similar lesions were not seen when the same emollients were used again. These 3 infants did not have AD/eczema or skin rash when they were followed for 32 weeks. There were no infants from families that withdrew consent who had skin lesions. In summary, adverse events caused by this emulsion‐type emollient were not observed during this RCT” No IPD are available on adverse events |
|
| Identification |
Sponsorship source: supported in part by Health and Labour Sciences Research Grants for Research on Allergic Diseases and Immunology from the Ministry of Health, Labour and Welfare of Japan (H22‐Men'eki‐Ippan‐002 to H.S.; H25‐Nanchito‐Ippan‐001 to M.A. and H.S. as principal investigators) and grants from the National Center for Child Health and Development (20S‐1 to Y.O. and 23S‐3 to H.S.) Country: Japan Setting: National Center for Child Health and Development, the only national hospital for mothers and children in Tokyo |
|
| Declarations of interest | Extensive list in the trial publication | |
| Notes | ||
Kataoka 2010.
| Study characteristics | ||
| Methods |
Study design: randomised trial Recruitment date: not reported Treatment arms: 2 Follow‐up: 6 months for AD, 6 months for food allergen sensitivity |
|
| Participants |
Randomised: N = 71 Inclusion criteria: family history of AD in second degree of kinship Exclusion criteria: none reported |
|
| Interventions |
Intervention: apply prescribed emollient more than once a day and do not wash infant's face with any other detergent Comparator: parent preference in skin care ("do what they like") |
|
| Outcomes |
Adverse events: adverse effects not reported |
|
| Identification |
Country: Japan Sponsorship source: not reported |
|
| Declarations of interest | Not reported | |
| Notes | ||
Lavender 2011.
| Study characteristics | ||
| Methods |
Study design: a pilot randomised, assessor‐blinded controlled trial Recruitment date: November 2008 to November 2009 Treatment arms: 2 AD follow‐up: 4 weeks and 8 weeks |
|
| Participants |
Randomised: N = 80 (recruit a sample of babies with family history of atopic eczema (n = 30) and a sample of babies without this family history (n = 50)) Inclusion criteria:
Exclusion criteria:
For the purposes of this study, the following normal variations were not considered skin disorders: erythema neonatorum, erythema toxicum, and milia |
|
| Interventions | All participating parents were supplied with written guidance on baby bathing. These instructions included guidance on regularity of bathing and the non‐use of other products (e.g. oils, sponges, flannels, baby wipes). Participating women were requested to bathe their baby a minimum of 3 times per week. The number of times babies were bathed was recorded by the women. They were also instructed to avoid any rubbing of the baby's skin and were asked not to use any additional products A baseline assessment was made before maternal transfer into the community and before the first bath. A second assessment was made at 4 weeks and at 8 weeks post birth. Measurements were taken on the upper abdomen (above nappy area), upper leg, and forearm Intervention: for those allocated to the wash product (experimental) arm, parents were provided with sufficient baby wash and were advised to use the product as per instructions Comparator: for those allocated to the water only (control) arm, parents were not provided with any products and were advised to bathe their baby with water and cotton wool only Moisturiser/Emollient: bathed in water only or bathed with the baby wash product. The wash product was the commercially available Johnson's® Baby Top‐To‐Toe™ Wash (Johnson & Johnson Consumer Companies, Inc.). This wash is a soap‐free liquid cleanser specifically designed for newborns' skin. It is sodium lauryl sulphate‐free and consists of a proprietary blend of non‐ionic and amphoteric surfactants that, when combined, result in large, gentle‐cleansing micelles. The formula contains only strictly necessary levels of well‐tolerated preservatives and a very low level of fragrance; it is pH‐adjusted (around 5.5) and hypoallergenic. The INCI list comprised aqua, coco glucoside, cocamidopropyl betaine, citric acid, acrylates/C10‐30 alkyl acrylate crosspolymer, sodium chloride, glyceryl oleate, p‐Anisic acid, sodium hydroxide, phenoxyethanol, sodium benzoate, and parfum |
|
| Outcomes |
Adverse effects: the skin was observed and recorded by the assessing midwife, at 4 and 8 weeks post birth using a validated rating scale that records erythema, dryness, scaling, and the need for medical products/attention. Any skin treatments were recorded by the mother |
|
| Identification |
Country: UK Setting: teaching hospital in the North West of England Sponsorship source: this study was funded by Johnson and Johnson. However, the study was investigator led. TL, CB, and MC previously acted as temporary advisors to J & J |
|
| Declarations of interest | As above | |
| Notes | ||
Lavender 2012.
| Study characteristics | ||
| Methods |
Study design: prospective, assessor‐blinded, randomised, controlled trial Recruitment date: February and October 2010 Treatment arms: 2 AD follow‐up: 4 weeks |
|
| Participants |
Randomised: N = 280 (napkin area cleansed with an alcohol‐free baby wipe n = 140 babies; cotton wool and water n = 140 babies) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: napkin cleansing regimen using a specific type of baby wipe. Participating mothers were given a cleansing demonstration by a healthcare assistant. All mothers were advised to use nappies that were supplied by researchers for the duration of the study to ensure similar absorbency ‐ a factor likely to influence skin hydration. Mothers were also advised to avoid using napkin cream, other than that supplied by the research team as a rescue treatment. Parents were provided with cotton wool or baby wipes, according to their allocated trial arm Comparator: napkin cleansing regimen using cotton wool and water Wipe and emollient: Johnson's Baby Skincare Fragrance Free Wipe (Johnson & Johnson Ltd., Maidenhead SL6 3UG, UK). The emollients contained glycerin and glyceryl oleate. The baby wipes also contained citric acid, which can have dual functionality as pH adjuster and chelator. Additionally, it was important to have a wipe with a pH close to the skin pH (around 4.9 in this case); if the pH is too low, this could be an irritant; if too high, this would increase protease activity and inhibit lipid lamellar synthesis in the skin barrier. Wipes contained 97% water and were free of alcohol, fragrance, essential oils, soap, and other harsh detergents; they were appropriately preserved to prevent growth of micro‐organisms. Cloth material of the wipes was a rayon viscose and polyester non‐woven fibre blend, entangled in a matrix of trough water jets without chemical binders. This is designed to reduce friction when wiped across the skin surface |
|
| Outcomes |
Primary outcome: change in stratum corneum hydration scores on the buttocks from first assessment (within 48 hours of birth) to 4 weeks post birth, using a Corneometer Secondary outcomes: change in erythema measurements using a Mexameter (W MX 18) (27); change in transepidermal water loss (TEWL) using an Aquaflux (AF200) (28); change in skin surface pH (using a pH meter). Measurements were taken on the babies’ buttocks at first assessment (within 48 hours of birth) and 4 weeks post birth Adverse effects: study group found no evidence of any adverse effects of using these wipes |
|
| Identification |
Country: UK Setting: North West of England |
|
| Declarations of interest | None reported | |
| Notes | ||
Lavender 2013.
| Study characteristics | ||
| Methods |
Study design: assessor‐blinded, randomised, controlled, non‐inferiority trial Recruitment date: between February 2010 and March 2011 Treatment arms: 2 AD follow‐up: 2 weeks and 4 weeks for AD |
|
| Participants |
Randomised: N = 307 (wash product n = 159; bathing with water alone n = 148) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Bathing regimen using a newborn wash product or water alone before the first bath. Participating mothers were instructed to bathe their neonate at least 3 times per week and to avoid rubbing the skin. On the day of assessment, mothers were requested to delay bathing their neonate until measurements had been taken Intervention: those allocated to the wash product (experimental) were provided with sufficient newborn wash and were advised to dilute the product at a ratio of 3 squirts per bath Comparator: control group used water alone; parents were not provided with any products. If these mothers wished to use shampoo on their neonates’ hair, they were requested to do this outside of the bath, and to ensure that the neonate’s body was wrapped in a towel to prevent contact with the skin Cleanser: Johnson’s Baby Top‐To‐Toe Bath (Johnson & Johnson Limited, Maidenhead SL6 3UG, UK) is a soap‐free liquid cleanser designed for newborns’ skin. It is sodium lauryl sulphate‐free and consists of a proprietary blend of non‐ionic and amphoteric surfactants that when combined result in large micelles that clean via dispersal of fats without disrupting the skin barrier. The formula contains well‐tolerated preservatives and a low level of fragrance; it is pH‐adjusted (around 5.5) and hypoallergenic. The International Nomenclature Cosmetic Ingredients list comprised aqua, coco glucoside, coca midopropyl betaine, citric acid, acrylates/C10‐30 alkyl acrylate crosspolymer, sodium chloride, glyceryl oleate, p‐Anisic acid, sodium hydroxide, phenoxyethanol, sodium benzoate, and parfum |
|
| Outcomes |
Primary outcomes: the average of TEWL measurements, using a closed chamber system, over
3 sites (outer forearm, midpoint between wrist and elbow; front of thigh, midpoint between knee and groin; abdomen, midpoint between umbilicus and sternum) at 14 days following birth using AquaFlux Model AF200 (Biox Systems Ltd, London, UK) Secondary outcomes: TEWL at 4 weeks post birth, skin surface pH using Courage + Khazaka Skin‐pH‐MeterR PH 900, and stratum corneum hydration scores using CorneometerR CM 820 (Courage + Khazaka Electronic GmbH, Cologne, Germany) from baseline (within 48 hours of birth). Because of the sensitivity of the neonate’s skin in the early weeks following birth, this is an ideal time to investigate the effects of wash products. Any differences in these outcomes are likely to be greater than later in an infant’s life, when the skin barrier is more stable Adverse events: not reported |
|
| Identification |
Country: UK Setting: teaching hospital in the North West of England Sponsorship source: funded by Johnson & Johnson Consumer Companies, Inc. |
|
| Declarations of interest | None reported | |
| Notes | ||
Lowe 2018a.
| Study characteristics | ||
| Methods |
Study design: a pilot randomised, parallel, single‐blinded (outcome assessor), controlled trial Recruitment date: 1 May 2013 to 2 July 2014 Treatment arms: 2 AD follow‐up: 6 weeks, 6 months, and 12 months of age for AD FA follow‐up: 6 months and 12 months |
|
| Participants |
Randomised: N = 80 (treatment group n = 41; control group n = 39) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: parents of infants were shown how to apply the emollient to the full skin surface of their child twice a day for the first 6 months of life. Treatment commenced within the first 3 weeks of life (neonatal period). Approximately 6 grams per application
Comparator: control group ‐ no other skin care instructions were provided Emollient: EpiCeram is a ceramide‐dominant emollient cream |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Identification |
Country: Australia Setting: Royal Women's Hospital and Frances Perry House (recruitment) Murdoch Children's Research Institute (assessment and storage of biological samples) University of Melbourne (data storage) Sponsorship source: this trial was supported by the Financial Markets Foundation for Children and the Asthma Foundation of Victoria. Additional support was obtained via an NHMRC equipment grant to purchase instruments used to measure biophysical aspects of skin. PuraCap, the manufacturer of EpiCeram at the time, provided the study with the interventional product free of charge |
|
| Declarations of interest | None declared | |
| Notes | ||
Lund 2020.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled trial Recruitment date: September 2012 to May 2013 Treatment arms: 2 Follow‐up: 1 post‐bath measurement |
|
| Participants |
Randomisation: N = 100 (vaginal delivery n = 50, caesarean section (C/S) n = 50) (intervention n = 49, control n = 51); randomised according to a balanced block design and stratified according to delivery mode Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | All participants were bathed according to the study protocol: immersion bath with water temperature 101°F, depth 5 inches(12.7 cm), which has been shown to be safe even with the umbilical cord in place and swaddle technique to reduce infant distress. The infant was stabilised under a radiant warmer with the 2 study sites ‐ volar forearm and beneath the sternum ‐ exposed for 10 minutes Intervention: bathed using cleanser Comparator: bathed with water only Cleanser ingredients: Johnson & Johnson's Head‐To‐Toe™ was used, as this product was used at the time at this facility. It is a soap‐free liquid cleanser designed for newborn and infant skin; it is sodium lauryl sulfate‐free and pH‐adjusted |
|
| Outcomes |
Primary outcome: skin barrier function, measured by skin surface pH Secondaryoutcomes: transepidermal water loss (TEWL), hydration of the stratum corneum (SCH) Adverse events: not reported |
|
| Identification |
Country: USA Setting: UCSF Benioff Children’s Hospital Oakland, Oakland, CA, USA Sponsorship Source: this study was supported by a grant from Johnson & Johnson Consumer Co. Inc., and by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF‐CTSI Grant Number UL1 TR000004 |
|
| Declarations of interest | This study was supported by a grant from Johnson & Johnson Consumer Co. Inc., and by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF‐CTSI Grant Number UL1 TR000004 | |
| Notes | ||
McClanahan 2019.
| Study characteristics | ||
| Methods |
Study design: single‐centred, investigator‐blinded RCT Recruitment dates: June 2011 and January 2014 Treatment arms: 2 AD follow‐up: study visits occurred at 2, 6, and 12 months. Two phone calls were performed at 18 to 24 months to discuss development of AD, provide education on emollient use, and supply additional product |
|
| Participants |
Randomisation: N = 100 (intervention n = 54, control n = 46) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group: instructed to apply moisturiser daily to all body surfaces excluding the scalp and diaper area and to use the cleanser only as needed during bathing Comparator: control group was given no specific instructions regarding use of emollients except to use emollients of their choice on an as needed basis Moisturiser/Emollient: Cetaphil Restoraderm (Galderma, Baie d’Urfé, Montreal, Canada); key ingredients include shea butter as a lipid source, pseudoceramide‐5, and 2 FLG breakdown products. A cleanser was also provided. No bathing frequency instructions were provided. Both products were to be used within 21 days of birth |
|
| Outcomes |
Primary outcomes: cumulative incidence of AD at 12 months diagnosed by a blinded investigator (‘investigator‐confirmed AD’). UK Working Party Criteria adapted to identify incident cases of AD were used rather than a 12‐month period of prevalence Secondary outcomes: a post‐hoc secondary analysis of the primary outcome was also performed: cumulative incidence of AD defined as AD diagnosed by an investigator and/or an outside paediatrician or chart review within 12 or 24 months (any AD) Adverse events: intervention group vs control group: bacterial skin infections (7.4% vs 6.5%); hypersensitivity reactions including irritant contact dermatitis and urticaria (14.8% vs 8.7%). No serious adverse events were reported in either group |
|
| Identification |
Country: Oregon, USA Setting: maternal hospital wards Sponsorship source: all funding sources supported the work |
|
| Declarations of interest | Dr. Simpson has received consulting fees from Galderma, which supplied emollient for this study | |
| Notes | ||
Migacheva 2018.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled trial Treatment arms: 2 AD follow‐up: 12 months |
|
| Participants |
Randomised: N = 63 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: infants in the intervention group received full‐body emollient therapy (cream) twice a day starting within 3 weeks of birth in combination with supplementation of synbiotic containing LGG and fructo‐oligosaccharides at the age of 3 to 6 months Comparator: parents in the control group were asked to use no emollients and no pro/prebiotics during the study period Adherence to the application came in at 95.2% by the end of the study, which reassured that the process was feasible |
|
| Outcomes | Cumulative incidence of AD at 12 months, as assessed by a trained investigator Adverse events: no intervention‐related adverse events occurred |
|
| Identification |
Country: Russia Sponsorship source: not reported |
|
| Declarations of interest | Not reported | |
| Notes | ||
NCT03376243.
| Study characteristics | ||
| Methods |
Study design: pragmatic, parallel‐group, assessor‐blind, randomised, open‐label, prospective study Study start date: 1 February 2017 Treatment arms: 2 Follow‐up: patients were followed up until they had eczema or 12 months or were lost to follow‐up |
|
| Participants |
Participants: N = 54 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: in the experimental arm, daily application of Lipikar Baume AP+ emollient and structured parent education Comparator: control group had no emollient intervention ‐ only structured parent education |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Adverse events: skin reactions with study product prompted for at follow‐up visits (month 1, month 3, month 6, and month 12) |
|
| Identification |
Country: Germany Sponsorship source: University of Schleswig‐Holstein |
|
| Declarations of interest | Not reported | |
| Notes | ||
Raisi Dehkordi 2010.
| Study characteristics | ||
| Methods |
Study design: triple‐blind clinical trial Recruitment start dates: 9 April 2010 and 23 Aug 2010 Treatment arms: 2 Follow‐up: weekly up to 4 weeks |
|
| Participants |
Randomised: 120 infants who were 10 to 15 days old, full‐term, single, exclusively breastfed, and with no history of hospitalisation Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Mothers administered 15 minutes of massage to their infants twice per day (morning and afternoon) for 28 days. Times of crying and sleep were measured by parents’ information forms at baseline, and at the end of the first, second, third, and fourth weeks of the study Intervention: sunflower oil massage or sesame oil massage Comparator: massage with no oil |
|
| Outcomes |
Main outcomes: crying time and sleeping time Adverse events: none reported |
|
| Identification |
Country: Iran Sponsorship Source: Vice Chancellor, Tehran University for Medical Sciences |
|
| Declarations of interest | None reported | |
| Notes | ||
Rush 1986.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Recruitment date: 19 March to 7 May 1984 Treatment arm: 2 Follow‐up: not reported |
|
| Participants |
Randomised: N = 186 (Group I n = 99, Group II n = 87) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: washed daily with soap and water Comparator: no bath, dry skin care |
|
| Outcomes |
Staphylococcus aureus colonisation rate Adverse events: infection rate reported but no details re: adverse events |
|
| Identification |
Country: Canada Setting: Maternity Unit Sponsorship source: not reported |
|
| Declarations of interest | Not reported | |
| Notes | ||
Sankaranarayanan 2005.
| Study characteristics | ||
| Methods |
Study design: open randomised, controlled trial Recruitment date: 1 August 2003 to 31 January 2004 Treatment arms: 3 Follow‐up: 31 days, daily during hospital stay, weekly thereafter |
|
| Participants |
Randomised: a total of 224 babies (112 preterm and 112 term babies) were enrolled. In each gestation stratum, coconut oil n = 38, mineral oil n = 37, placebo n = 37 Inclusion criteria: full‐term neonates weighing 2500 grams or more were included if they fulfilled the following inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Sessions began an hour after a feed. The total duration of each session was 5 minutes, and sessions were done 4 times a day. Term infants were massaged in a draught‐free room. Massage was given in prone and supine positions to include head, neck, trunk, and extremities. At completion of the massage, kinaesthetic stimulation was provided in the supine position by passive flexion and extension movements of the limbs at each large joint (shoulder, elbow, hip, knee, and ankle) as 5 events of 2 seconds. Massage was given by a trained person from day 2 of life until discharge, and thereafter by the mother until 31 days of age, 4 times a day. Babies were followed up daily until discharge and every week after discharge for anthropometry Intervention: coconut oil or mineral oil massage Comparator: massage using baby powder; methods of application and monitoring the same as in the oil groups Moisturiser/Emollient: coconut oil, mineral oil, and baby powder. No details of ingredients reported |
|
| Outcomes |
Primary outcome: weight gain velocity over first 31 days of life Secondary outcomes: length gain velocity, head growth, neurobehavioural outcome, incidence of adverse events Adverse events: in the preterm group, adverse events occurred in 6 babies, 2 each in the coconut oil, mineral oil, and placebo groups. All adverse events were mild rash and did not require discontinuation of application. Among term babies, 3 in the coconut oil group, 3 in the mineral oil group, and 2 in the placebo group developed mild rash that did not require discontinuation of application |
|
| Identification |
Country: India Setting: premature unit and postnatal wards of a major tertiary care centre in a metropolitan city in Mumbai Sponsorship source: Marico Industries Ltd. provided oils and placebo for the study |
|
| Declarations of interest | Marico Industries Ltd. is involved in the production of coconut oil. BM, AM, and RS Mohile are employees of Marico Industries. None of the authors from Sion Hospital have any shares in the company | |
| Notes | ||
Simpson 2014.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, multi‐national, assessor‐blind, randomised (1:1), controlled pilot trial (6 months) Recruitment date: May 2010 and May 2011 Treatment arms: 2 Follow‐up: 6 months; research nurse contacted parents by telephone at 10 days and 6 weeks, with a face‐to‐face visit at 12 weeks (usually at home in the UK and as a clinic visit in the USA). A further telephone call was made at 18 weeks, and the final contact was a clinic visit at 24 weeks |
|
| Participants |
Randomised: N = 124 (intervention n = 64, control n = 60) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | All parents were given a skin care advice booklet, which reflected current guidelines. Parents were advised to:
Intervention: parents were offered a choice of 3 emollients of different viscosities (an oil, a cream/gel, or an ointment)
Preferred emollient used in the intervention group: cream/gel formulations (67.2%), oil (23.4%), ointment (9.4%). Parents were asked to apply the emollient to the baby's entire body surface, except for the scalp, starting as soon as possible after birth (within a maximum of 3 weeks) and continuing until the infant was 6 months of age Comparator: control arm was asked to use no emollients and was given the infant skin care advice booklet Oil/Moisturiser/Emollient ingredients: sunflower seed oil (a high ratio of linoleic/oleic acid, William Hodgson and Co., Congleton, UK), Doublebase Gel (Dermal Laboratories, Hitchin, United Kingdom), liquid paraffin 50% in white soft paraffin (Cetaphil Cream, Galderma Laboratories, Fort Worth, Texas, USA), Aquaphor Healing Ointment (Beiersdorf, Chester, OH, USA) |
|
| Outcomes | Age of onset of eczema and proportion of transient cases
Incidence of emollient‐related adverse events
Cumulative incidence of eczema at 6 months, as determined by an investigator Adverse events: adverse events, including accidents, infections, and reactions, were prompted for at all patient visits. Three superficial cutaneous infections occurred in each group; all were considered mild in nature. There were no reports of irritant or allergic contact dermatitis (p 821). There were no emollient‐related adverse events and no differences in adverse events between groups (Results section, Abstract). IPD contains 2 participants who had skin infections |
|
| Identification |
Country: UK and the USA Setting: UK: acute NHS hospital trusts (Nottingham University Hospitals, Derby Hospitals, and United Lincolnshire Hospitals) and 1 GP surgery (Surgery @Wheatbridge, Chesterfield) USA: Oregon Health & Science University Hospital and Clinics (Portland, Oregon) Sponsorship source: National Institute for Health Research (NIHR) under its programme grants for Applied Research Programme (RP‐PG‐0407‐10177). United States–based contributions were made possible with funding from a Mentored Patient‐oriented Research Career Development Award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (5K23AR057486). Support was also obtained from the Oregon Clinical and Translational Research Institute (OCTRI) and by grant number 5 KL2 RR024141‐04 from the National Center for Research Resources (NCRR; 5 KL2 RR024141‐04), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Research in the McLean Laboratory is funded by the Wellcome Trust (Programme Grant 092530/Z/10/Z and Strategic Award 098439/Z/12/Z). SJB holds a Wellcome Trust Intermediate Clinical Fellowship ‐ WT086398MA |
|
| Declarations of interest | This study was funded by the National Institute for Health Research (RPPG‐0407‐10177). MJ Cork has received compensation from Almirall Pharmaceuticals for membership on its advisory board; has received or has grants pending from Almirall Pharmaceuticals; and has received payment for delivering lectures, as well as compensation for travel and other meeting‐related expenses, from Almirall, Astellas Pharma, and Steifel (a GlaxoSmithKline company). WHI McLean’s institution has received funding from the Wellcome Trust (WT086398MA), as has that of SJ Brown, who also received an honorarium for speaking at the AAAAI Annual Meeting in 2012 and 2013. Remaining study authors declare that they have no relevant conflicts of interest | |
| Notes | ||
Skjerven 2020.
| Study characteristics | ||
| Methods |
Study design: population‐based, 2 x 2 factorial, cluster‐randomised clinical trial Recruitment date: 9 December 2014 and 31 October 2016 Treatment arms: 4 Follow‐up: 12 months ‐ UK Working Party diagnostic criteria used at 3 month, 6 month, and 12 month follow‐up investigations, with additional use of Hanifin and Rajka diagnostic criteria at age 12 months |
|
| Participants |
Randomised: N = 2396 (no intervention group n = 5967 infants, skin intervention group n = 575, food intervention group n = 642, combined intervention group n = 583) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention characteristics:
Adherence: bath oil additive was used at least 4 days per week in 497 (43%) of 1158 infants assigned to a skin intervention and facial cream on at least 4 days per week in 514 (44%); 316 (27%) were fully protocol adherent for use of both emollients. Between age 13 weeks and 18 weeks, peanut butter was introduced to 966 (79%) of 1225 infants assigned to food intervention, cow’s milk to 838 (68%), wheat to 820 (67%), and egg to 677 (55%). 431 (35%) were fully protocol adherent up to week 26 for peanut butter, 530 (43%) for cow’s milk, 543 (44%) for wheat, and 289 (24%) for egg. Full protocol adherence to the overall food intervention was reported in 387 (32%) Comparator characteristics: No intervention group: no specific advice on feeding practices or skin care was given to parents of infants except regular advice from well‐baby clinics and national guidelines for infant nutrition. Exclusive breastfeeding is generally recommended until age 6 months Adherence: IPD show regular use of emollient (Ceridal cream) (≥ 3 days a week averaged over intervention period) by only 1 control participant Cream/Oil/ Ingredients: cream (Ceridal; GlaxoSmithKline Consumer Healthcare, Philadelphia, PA, USA), bath oil (paraffinum liquidum and trilaureth‐4‐phosphate only were produced specifically for the PreventADALL trial by Pharmatech (Østfold, Norway)) |
|
| Outcomes | The main outcome of food allergy will first be assessed at 36 months of age. Rationale: due to recent reports, published after the PreventADALL study was well in progress, we reassessed the appropriateness of time of the first report for the main outcomes. The LEAP46 and EAT47 studies, both assessing introduction of allergenic foods from infancy in high‐risk and general populations, recruited babies, respectively, published in the NEJM in 2015 and 2016. Both studies assessed food allergy at 36 months of age, in part because natural tolerance development is likely in some children up to this age. The PreventADALL is important and unique in terms of food introduction as well as skin care in infancy in a large mother‐child general cohort; we therefore believe that assessing food allergy at the same time as these 2 pivotal studies will improve the likelihood of solid evidence as to the effectiveness of preventing food allergy by 3 years of age, albeit with different strategies Likewise, the main outcome of atopic dermatitis (AD) will be assessed first at 12 months of age. Rationale: 2 clinical trials of daily emollients from shortly after birth assessed their main outcomes in high‐risk babies first at 6 months or 32 weeks of age, in line with the end of their intervention. With the PreventADALL study being the first large study in a general population birth cohort, we find it important to have the first main outcome endpoint after the intervention has stopped (9 months). Infants are investigated at 12 months of age, with detailed skin examination. Although for factorially designed interventions, both intervention outcomes should ideally be assessed at the same time, we find it ethically challenging to delay outcome assessment for 2 years, in case of significant improvement of the intervention. It is unlikely that reporting the effect of primary prevention use of specifically produced bath oil in infancy will affect behaviour of mothers of 2‐ to 3‐year‐old children in terms of skin care, in a way that interferes with assessment at 3 years of age. Also, we have detailed blinded assessments of skin disease at 6, 12, 24, and 36 months of age to adjust for potential behaviour modification Adverse events: adverse events: recorded in weekly electronic diaries up to week 26, in electronic questionnaires every 3 months, and in specific forms by personnel at the discretion of study personnel |
|
| Identification |
Country: Sweden Setting: Oslo University Hospital and Østfold Hospital Trust, Norway, and Karolinska University Hospital, Stockholm Sponsorship source: this study was funded by several public and private funding bodies: Regional Health Board South East, Norwegian Research Council, Health and Rehabilitation Norway, Foundation for Healthcare and Allergy Research in Sweden‐Vårdalstiftelsen, Swedish Asthma and Allergy Association’s Research Foundation, Swedish Research Council—Initiative for Clinical Therapy Research, Swedish Heart‐Lung Foundation, SFO‐V at the Karolinska Institute, Freemason Child House Foundation in Stockholm, Swedish Research Council for Health, Working Life and Welfare—FORTE, Oslo University Hospital, University of Oslo, and Østfold Hospital Trust |
|
| Declarations of interest | EMR has received honoraria for presentations from Sanofi Genzyme, Novartis, MEDA, and Omega Pharma. KCLC has received honoraria for presentation from Thermo‐Fisher Scientific. All other study authors declare no competing interests | |
| Notes | ||
Thitthiwong 2019.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, controlled trial Recruitment date: January 2016 to April 2017 Treatment arms: 2 Follow‐up: clinic visits at 2, 4, 6, and 9 months old |
|
| Participants |
Randomised: N = 53 (intervention n = 26; control n = 27) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Verbal advice for good skin care practice were repeatedly given to all caregivers (in both groups) during every clinic visit. This comprised bathing for 5 to 10 minutes with tap or lukewarm water, bathing not more than twice daily, and using only a minimal amount of gentle liquid baby cleansers of any manufacturers. Bath oil, bubble bath, or any bath additives were not allowed to be used in both groups Intervention: cold cream applied all over the infant's body except peri‐orbital and peri‐oral areas at least once daily shortly within 3 to 5 minutes after bathing and padding dry baby skin Comparator: control group was asked not to apply any skin care products to the baby's skin except to use gentle liquid cleansers during bathing and barrier ointment or cream on diaper areas as needed. This group also received good skin care advice during every visit Moisturiser/Emollient: emollient called cold cream: white petrolatum, stearyl alcohol, propylene glycol, and glycerin |
|
| Outcomes |
Primary outcomes:
Study endpoints were defined when infants developed AD, or when infants were 9 months old Food allergy outcomes are not mentioned as part of the outcomes, but in the results, study authors reported, "none of the 4 IAD infants developed cows' milk protein allergy or any other food allergy' Secondary outcome: mean onset of AD, adverse reaction to cold cream application, factors associated with developing AD Adverse events: no ADs were reported by caregivers |
|
| Identification |
Country: Thailand Setting: Paediatric Outpatient Department of Phramongkutklao Hospital in Bangkok Sponsorship source: Phramongkutklao Hospital |
|
| Declarations of interest | No conflict of interest reported | |
| Notes | ||
Tielsch 2007.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised, placebo‐controlled, community‐based trial Recruitment date: between 1 September 2002 and 8 March 2005 Treatment arms: 2 Follow‐up: 28 days. Assessed at 2, 3, 4, 6, 8, 10, 12, 14, 21, and 28 days since birth (not eczema or AD related but for assessment of infant vital status and morbidity) |
|
| Participants |
Randomised: N = 17,530 (intervention n = 8650, control n = 8880) Inclusion criteria: all liveborn infants born in the study area Exclusion criteria: newborn infants who died before study staff arrived to conduct interventions |
|
| Interventions |
Intervention: 1‐time skin cleansing of newborn infants, wiping of the total body excluding eyes and ears with Pampers Infant Wipes (Procter and Gamble Co., Cincinnati, OH, USA) that released a solution that contained 0.25% free chlorhexidine (equivalent to 0.44% chlorhexidine digluconate) Newborn skin cleansing occurred soon after delivery at a median time of 5.8 hours after birth (interquartile range 2.1 to 11.8 hours); 91.4% of infants were cleansed within the first 24 hours Comparator: wiping with the same infant wipes that lacked chlorhexidine (placebo) Wipes: all wipes were alcohol‐free, produced by Procter and Gamble Co., and were packaged in sterile plastic sachets that contained 6 wipes. Pampers Infant Wipes (Procter and Gamble Co., Cincinnati, OH, USA) that released a solution that contained 0.25% free chlorhexidine (equivalent to 0.44% chlorhexidine digluconate) |
|
| Outcomes | All‐cause mortality by 28 days Adverse events: none reported |
|
| Identification |
Country: Nepal Setting: Sarlahi District in south‐central Nepal (> 95% of births delivered in the home) Sponsorship source: this study was conducted by the Department of International Health, Bloomberg School of Public Health, Johns Hopkins University (Baltimore, MD, USA), under grants HD 44004 and HD 38753 from the National Institutes of Health (Bethesda, MD, USA); grant 810 −2054 from the Bill and Melinda Gates Foundation (Seattle, WA, USA); and Cooperative Agreements HRN‐A‐00−97−00015−00 and GHS‐A‐00−03−000019−00 between Johns Hopkins University and the Office of Health and Nutrition, US Agency for International Development (Washington, DC, USA). Commodity support was provided by Procter and Gamble Co. (Cincinnati, OH, USA) |
|
| Declarations of interest | Financial supporters and the commodity supplier played no role in design, conduct, management, analysis, or interpretation of results, nor in preparation, review, or approval of this article | |
| Notes | ||
Yonezawa 2018.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel, controlled trial Study conducted: March 2014 and June 2015 Treatment arms: 2 Follow‐up: 24 months (eczema, AD, and food allergy) |
|
| Participants |
Randomised: N = 227 (intervention n = 113, control n = 114) Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions | Each group performed skin care from week 1 to week 12 after birth Intervention: moisturising skin care (bathing every 2 days and using lotion daily). The intervention group performed moisturising skin care as follows: (i) routine bathing every 2 days; and (ii) use of a moisturiser 1 or more times per day. If parents were resistant to reducing the frequency of bathing, they were allowed to bathe their newborn daily, but they could use soap only every other day. The researcher provided soap. Parents were also allowed to choose a moisturiser of their choice Comparator: the control group performed the skin care regimen commonly used in Japan as follows: (i) routine bathing daily; and (ii) no moisturiser. Midwives recommended that all mothers routinely bathe their newborn daily. The researcher provided soap. The control group was allowed to apply a moisturiser to their newborn if they wanted to |
|
| Outcomes |
Primary outcomes: 3‐month outcomes: skin barrier function, by measuring values of transepidermal water loss Secondary outcomes: 3‐month outcomes: skin problems and skin conditions in the diaper area, face, and body recorded in parents'/infants' skin diaries Skin conditions assessed in terms of redness, erythema, dryness, and breakdown Presence of diaper dermatitis assessed using the diaper rash and erythema scoring scale, which rates diaper dermatitis on 7 levels from none to severe Skin problems on the face or the body were assessed by an original score scale that refers to the Neonatal skin Condition Score, which rates a skin condition between 3 and 9 points Infants with skin problems for at least 1 day were considered to have skin problems (p 25). Two year outcomes: parent report of diagnosis of eczema and parent report of diagnosis of food allergy Adverse events: not formally collected |
|
| Identification |
Country: Japan Setting: Tokyo‐Kita Medical Center Sponsorship source: this study was supported by the Mitsubishi Foundation (Grants for Social Welfare Activities on 2013) and the Mishima Kaiun Memorial Foundation |
|
| Declarations of interest | None declared | |
| Notes | ||
Zhao 2005.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Recruitment date: October 2002 and February 2003 (babies delivered between these dates) Treatment arms: 2 Follow‐up: from delivery to discharge; no further details reported |
|
| Participants |
Randomised: N = 377 (Group 1 was the swimming (study) group, comprising a total of 223 newborns including 127 babies delivered after spontaneous vaginal delivery and 96 babies after Cesarean section. Group 2 was the bathing (control) group, comprising 154 newborns including 109 babies delivered after spontaneous vaginal delivery and 45 babies after Cesarean section) Inclusion criteria: no details reported Exclusion criteria: no details reported |
|
| Interventions |
Intervention: the study group (swimming) included 223 cases (127 babies delivered after spontaneous vaginal delivery and 96 babies after Cesarean section). During hospitalisation (from delivery to discharge), newborns in the study group swam twice a day for 10 to 15 minutes each time Comparator: bathing |
|
| Outcomes | Outcomes not relevant to SCiPAD | |
| Identification |
Sponsorship source: not reported Country: China Setting: Guangdong Provincial Maternal and Child Health Hospital |
|
| Declarations of interest | Not reported | |
| Notes | ||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12607000466448 | Wrong study design |
| Ahmed 2007 | Wrong patient population |
| Alonso 2013 | Wrong patient population |
| Baer 2006 | Wrong study design |
| Barria 2004 | Wrong comparator |
| Baudouin 2014 | Wrong patient population |
| Baudouin 2014a | Wrong patient population |
| Berger 2009 | Wrong patient population |
| Bhakoo 1969 | Wrong study design |
| Blume Peytavi 2010 | Wrong study design |
| Blume Peytavi 2012 | Wrong study design |
| Blume Peytavi 2014 | Wrong study design |
| Blume Peytavi 2016 | Wrong study design |
| Brandon 2010 | Wrong patient population |
| Bryanton 2004 | Wrong comparator |
| Chaithirayanon 2016 | Wrong comparator |
| Chen 2009 | Wrong patient population |
| Cleminson 2015 | Wrong study design |
| Cleminson 2016 | Wrong study design |
| Conner 2004 | Wrong study design |
| Cooke 2014 | Wrong study design |
| Cooke 2018 | Wrong study design |
| Cowan 1986 | Wrong comparator |
| CTRI201208002876 | Wrong study design |
| CTRI201709009890 | Wrong patient population |
| Da Cunha 2005 | Wrong patient population |
| Da Cunha 2005a | Wrong patient population |
| Darmstadt 2004 | Wrong patient population |
| Darmstadt 2005 | Wrong patient population |
| Darmstadt 2005a | Wrong patient population |
| Darmstadt 2007 | Wrong patient population |
| Darmstadt 2008 | Wrong patient population |
| Darmstadt 2014 | Wrong patient population |
| Duggan 2015 | Wrong study design |
| Erdemir 2015 | Wrong patient population |
| Ernest 1995 | Wrong intervention |
| EUCTR2005‐001269‐32‐AT | Wrong patient population |
| Fernandez 2018 | Wrong study design |
| Field 2008 | Wrong intervention |
| Fluhr 2012 | Wrong study design |
| Foisy 2011 | Wrong intervention |
| Franck 2000 | Wrong patient population |
| Friscia 2019 | Wrong patient population |
| Gao 2008 | Wrong patient population |
| Garcia Bartels 2009 | Wrong intervention |
| Gezon 1964 | Wrong comparator |
| Gfatter 1997 | Wrong patient population |
| Gleiss 1965 | Wrong comparator |
| Gunt 2018 | Wrong patient population |
| Hauk 2008 | Wrong study design |
| Hawkins 2017 | Wrong patient population |
| Hnatko 1977 | Wrong comparator |
| Horimukai 2016 | Wrong study design |
| Hu 2014 | Wrong patient population |
| IRCT201306164617N | Wrong patient population |
| IRCT201306164617N7 | Wrong patient population |
| IRCT2013090814594N1 | Wrong patient population |
| IRCT2016111530903N | Wrong patient population |
| IRCT20170911036118N1 | Wrong patient population |
| ISRCTN71423189 | Wrong patient population |
| ISRCTN89579779 | Wrong patient population |
| Jabraeile 2016 | Wrong patient population |
| Jensen 1971 | Wrong intervention |
| JPRN UMIN000005158 | Wrong patient population |
| JPRN‐UMIN000018110 | Wrong study design |
| JPRN‐UMIN000025302 | Wrong study design |
| JPRN UMIN000032181 | Wrong study design |
| JPRN UMIN000032798 | Wrong study design |
| JPRN UMIN000035357 | Wrong study design |
| JPRN UMIN000035412 | Wrong study design |
| Kadar 1974 | Wrong study design |
| Kanti 2014 | Wrong patient population |
| Kanti 2017 | Wrong comparator |
| Kiechl Kohlendorfer 2008 | Wrong patient population |
| Konar 2019 | Wrong patient population |
| Koplin 2019 | Wrong study design |
| Kottner 2017 | Wrong patient population |
| Kvenshagen 2014 | Wrong patient population |
| Lane 1993 | Wrong patient population |
| Larson 2005 | Wrong study design |
| Lee 2018 | Wrong patient population |
| LeFevre 2010 | Wrong patient population |
| Leung 2015 | Wrong study design |
| Li 2016 | Wrong study design |
| Ling 2011 | Wrong patient population |
| Linnamaa 2010 | Wrong patient population |
| Lowe 2012 | Wrong study design |
| Lowe 2018b | Wrong study design |
| Lund 2001 | Wrong study design |
| Lund 2001a | Wrong study design |
| Marenholz 2015 | Wrong study design |
| Muggli 2009 | Wrong comparator |
| Nangia 2015 | Wrong patient population |
| Natsume 2018 | Wrong study design |
| NCT00162747 | Wrong patient population |
| NCT00257569 | Wrong patient population |
| NCT00806221 | Wrong study design |
| NCT00917085 | Wrong intervention |
| NCT01131403 | Wrong study design |
| NCT01177111 | Wrong comparator |
| NCT01364948 | Wrong patient population |
| NCT01396642 | Wrong patient population |
| NCT01758068 | Wrong patient population |
| NCT02120833 | Wrong patient population |
| NCT02403999 | Wrong comparator |
| NCT02404493 | Wrong patient population |
| NCT02557698 | Wrong patient population |
| NCT02614248 | Wrong patient population |
| NCT02857062 | Wrong patient population |
| NCT03089476 | Wrong study design |
| NCT03112876 | Wrong study design |
| NCT03143504 | Wrong study design |
| NCT03719742 | Wrong study design |
| NCT03738163 | Wrong study design |
| NCT03742414 | Wrong patient population |
| NCT03813472 | Wrong patient population |
| NCT04001855 | Wrong patient population |
| NCT04099602 | Wrong patient population |
| Nesmiyanov 2018 | Wrong comparator |
| Nopper 1996 | Wrong patient population |
| Noviello 2005 | Wrong study design |
| Pupala 2017 | Wrong study design |
| Pupala 2018 | Wrong patient population |
| Pupala 2019 | Wrong study design |
| Qiu 2008 | Wrong patient population |
| Quinn 2005 | Wrong patient population |
| RBR 93996y | Wrong patient population |
| Rehbinder 2018 | Wrong study design |
| Rosenstock 2007 | Wrong patient population |
| Sach 2019 | Wrong study design |
| Salam 2013 | Wrong study design |
| Salam 2015 | Wrong patient population |
| Sarkar 2010 | Wrong study design |
| Sawatzky 2016 | Wrong patient population |
| Solanki 2005 | Wrong patient population |
| Soll 2000 | Wrong study design |
| Soriano 2000 | Wrong patient population |
| Summers 2017 | Wrong comparator |
| TCTR20161209001 | Wrong patient population |
| Thomas 1979 | Wrong study design |
| Vaivre Douret 2009 | Wrong patient population |
| Visscher 2009 | Wrong patient population |
| Wananukul 2001 | Wrong patient population |
| Wananukul 2002 | Wrong patient population |
| Wang 2009 | Wrong patient population |
| Waserman 2016 | Wrong study design |
| Xatzopoulou 2010 | Wrong patient population |
| Xiao 2009 | Wrong patient population |
| Yamamoto 1996 | Wrong patient population |
| Zhang 2016 | Wrong patient population |
Characteristics of studies awaiting classification [ordered by study ID]
ISRCTN38965585.
| Methods | Single‐centre cluster‐randomised controlled trial |
| Participants | Aim: 41,072 newborns from 276 clusters |
| Interventions |
Intervention: 1. The product, cold‐pressed sunflower seed oil 2. Directions for newborn massage. The directions for newborn massage further consist of the following aspects 2.1. Dosage of SSO, comprising frequency of use, quantity per use, and duration of use 2.1.1. Dose: 10 g per application, applied 3x daily 2.1.2. Duration: 0 to 27 days of life 2.2. Improvements in overall massage practice: 2.2.1. Encourage handwashing before massage 2.2.2. Encourage gently massaging the vernix into the newborn skin, rather than forcefully removing it 2.2.3. Promote gentle massage of newborns 2.2.4. Delay use of mustard oil and skin‐scrubbing substances such as bukwa (coarse‐grained paste made of mustard/wheat seeds along with additives) past the newborn period 2.2.5. Ensure that the newborn is kept warm during and after massage Comparator: control group, which will continue following the same traditional massage practices, including the type of oil used. No further information |
| Outcomes |
Primary outcomes were infant mortality rate
SCiPAD outcome of interests in this study is: 1. Infections and hospitalisation: signs and symptoms of infection during the newborn period, along with episodes of hospitalisation, would be recorded through parent recall. These will include local infections such as pyoderma and umbilical cord infection Skin barrier function: barrier property of stratum corneum (assessed as transepidermal water loss or TEWL) Adherence to intervention: information on continued oil use and adherence to massage technique would be obtained from families (mothers). This would also be applicable to a subsample (5%) of the population |
| Notes | No response from study author, emailed on 14.01.2020. Link to trial: http://www.isrctn.com/ISRCTN38965585 |
JPRN‐UMIN000026877.
| Methods | Factorial randomised |
| Participants | Target sample size: 50 |
| Interventions | Use the foaming cleanser and lotion every single day for 4 weeks Use the foaming cleanser and cream every single day for 4 weeks |
| Outcomes | Change in skin symptoms by dermatological diagnosis before and after topical application of 4 weeks Change in water content, transepidermal water loss, skin pH, and composition of stratum corneum before and after topical application for 4 weeks |
| Notes | Clinical trial link: https://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000026877 |
NCT03640897.
| Methods | Monocentric, prospective, randomised, comparator‐controlled, parallel‐group study |
| Participants | 133 participants |
| Interventions |
Experimental: Liniderm Oleocalcareous liniment Liniderm: The product will be applied on the diaper area by parents/caregivers at each diaper change Active comparator: wipes Free‐fragrance baby wipes. The product will be used on the diaper area by parents/caregivers at each diaper change Active comparator: water Water and cotton pads. The product will be used on the diaper area by parents/caregivers at each diaper change |
| Outcomes |
Primary outcomes: Number of infants who had at least 1 episode of diaper rash [Time Frame: 28 days] Every day, in a daily log, parents/caregivers will report the presence or not of a diaper rash At the end of follow‐up, the investigator must identify infants who have had at least 1 episode of diaper rash Secondary outcomes:
|
| Notes |
Supported by Johnson & Johnson Consumer Inc.
Characteristics of ongoing studies [ordered by study ID]
Eichner 2020.
| Study name | A Community‐based Assessment of Skin Care, Allergies, and Eczema (CASCADE) |
| Methods | Pragmatic, multi‐site, randomised, community‐based trial |
| Participants | Estimated enrolment: 1250 |
| Interventions | Experimental: daily emollient Parents assigned to the intervention arm will receive a lipid‐rich emollient and educational materials promoting once‐daily full‐body emollient use until the infant is 24 months old. Parents will select 1 of 5 emollients to be mailed to the dyad's home at enrolment and approximately every 6 months for the duration of the study. These emollients include (1) CeraVe Healing Ointment, (2) Vaseline, (3) Cetaphil Cream, (4) CeraVe Cream, and (5) Vanicream Intervention: other: participant choice of over‐the‐counter emollients: Vaseline, Vanicream, CeraVe Healing Ointment, CeraVe Cream, Cetaphil Cream Comparator: no Intervention, natural skin Parents assigned to the control arm will receive educational materials promoting general infant skin care guidelines only and will be asked to refrain from emollient use unless dry skin develops (current standard of care guidelines) |
| Outcomes |
Primary outcome Cumulative incidence of AD [Time Frame: 24 months] Secondary outcomes
|
| Starting date | 3 July 2018 |
| Contact information | LeAnn Michaels ‐ michaell@ohsu.edu Clara Stemwedel ‐ stemwedc@ohsu.edu |
| Notes |
Jabbar‐Lopez 2019.
| Study name | Softened Water for Eczema Prevention Pilot Trial (SOFTER) |
| Methods | Assessor‐blinded pilot randomised controlled trial |
| Participants | N = 80 estimated enrolment |
| Interventions | Intervention group will have a domestic ion‐exchange water softener installed before birth Comparator: usual hard water supply; control group will receive the usual domestic water supply |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | 12 February 2018 to June 2019 |
| Contact information | Carsten Flohr ‐ carsten.flohr@kcl.ac.uk |
| Notes |
Lowe 2019.
| Study name | PEBBLES study protocol: a randomised controlled trial to prevent atopic dermatitis, food allergy and sensitisation in infants with a family history of allergic disease using a skin barrier improvement strategy |
| Methods | Multi‐centre, phase III, outcome assessor‐blinded, randomised, controlled trial |
| Participants | Aim: to recruit 760 |
| Interventions |
Intervention: 2 times per day treatment with EpiCeram (intervention group). EpiCeram has been approved for use by patients with AD or eczema by the Food and Drug Administration (FDA) in the USA but does not yet have Australian Therapeutic Goods Administration (TGA) approval and is not currently available in Australia. Parents will be instructed to apply approximately 6 g of EpiCeram per application 2 times per day from birth until the infant is 6 months of age Comparator: standard skin care advice (control group) Parents of children in the control group will be managed as per existing practice and will not be given any emollients. For ethical reasons, parents of children in the control group will not be told to withhold skin care from their infant, and information related to use of emollients will be collected from all participants Compliance: a weekly diary will be completed online by parents, who will document the frequency of EpiCeram application and use of any other creams |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | October 2005 |
| Contact information | Adrian Lowe ‐ lowea@unimelb.edu.au |
| Notes |
NCT02906475.
| Study name | Atopic Dermatitis in Atopy Predisposed Infants (ADAPI) |
| Methods | Randomised, pragmatic, parallel‐group trial |
| Participants | Aim: N = 160 |
| Interventions | Compilation: standardised skin care regimen
Intervention: milk lotion will be applied once daily on the total body including the face by parents or caregivers at home. If bathing is needed, the bathing addendum is used in addition to water Comparator: for the control group, no predetermined or standardised skin care regimen is prescribed |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | Study start date: October 2016 Estimated study completion date: December 2020 |
| Contact information | Stephanie Meyer ‐ stephanie.meyer@hipp.de] |
| Notes |
NCT03142984.
| Study name | Randomized Controlled Trial of Gentle Touch/Early Massage With a New Wash and Lotion Regimen for Improved Skin Barrier Strength, Parental Bonding, and Physical Development in Newborn Babies: The Barrier Optimizing Skincare for Newborn Development (BOND) Trial |
| Methods | Randomised, single‐group assignment |
| Participants | 150 participants |
| Interventions | Experimental: phase 1 An open‐use test in a cohort of newborn babies to confirm tolerability and evaluate acceptability of a new Baby Wash & Shampoo product and Baby Lotion Baby Wash & Shampoo (F# 13217‐070), Baby Lotion (F# 13217‐071) Experimental: phase 2 An evaluator‐blind, randomised, controlled trial to determine whether a wash and lotion regimen used for 12 weeks can strengthen the skin barrier in newborns when compared to standard skin care practices without massage Baby Wash & Shampoo (F# 13217‐070), Baby Lotion (F# 13217‐071), Alternative Baby Wash & Shampoo (GTIN/UPC # 5011451106260) |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | 4 July 2017 to 17 March 2021 |
| Contact information | No contact details on clinical trials |
| Notes | https://clinicaltrials.gov/ct2/show/NCT03142984 |
NCT03808532.
| Study name | Moisturizer to Prevent Atopic Dermatitis (ACE‐AD) |
| Methods | Randomised parallel assignment |
| Participants | 290 participants |
| Interventions | Experimental: high‐risk with moisturiser No intervention: high‐risk without moisturiser |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | June 2020 |
| Contact information | Michael Brandwein ‐ michael@myor.me |
| Notes |
NCT03871998.
| Study name | Short‐term Topical Application to Prevent Atopic Dermatitis (STOP AD) |
| Methods | Single‐centre, randomised, open‐label, controlled study |
| Participants | 242 participants |
| Interventions |
Experimental: intervention arm Skin barrier protection in the first 2 months of life No intervention: control arm Standard skin care advice. No moisturiser in the first 2 months |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 12 March 2019 |
| Contact information | Carol Ní Chaoimh ‐ cnichaoimh@ucc.ie Mairead Murray ‐ mairead.murray@ucc.ie |
| Notes |
NCT04398758.
| Study name | Moisturizer Mediated Prevention of Symptoms of Atopic Dermatitis in Early Childhood (MOPAD) |
| Methods | Randomised controlled trial |
| Participants | Healthy newborns, < 3 weeks of age, one first‐degree relative with medically diagnosed AD N = 360 |
| Interventions | "SanaCutan Basiscreme" applied all over body twice daily for 6 months (main phase) or 12 months (follow‐up phase) |
| Outcomes | Cumulative incidence of children with atopic dermatitis at 6 months of age |
| Starting date | 21 May 2020 |
| Contact information | Dr Wehran ‐ studien@infectopharm.com PI Dr Kristen Beyer, Charité University, Berlin, Germany |
| Notes |
Differences between protocol and review
Objectives: We clarified our Objectives, which included removing some redundant text.
Types of outcome measures: We clarified that the time point for all food allergy and eczema outcome analyses (not just the primary outcomes) was by age one to three years, using the closest available time point to two years from each included trial.
Sensitivity and subgroup analysis
We pre‐specified sensitivity analysis by outcome measures for the co‐primary outcomes of eczema and food allergy by one to three years. This included sensitivity analysis for secure diagnosis of food allergy by oral food challenge or investigator decision using an algorithm developed for the BEEP study. Since only one trial could be included in the primary outcome and in this pre‐specified sensitivity analysis for food allergy, but three trials reported data on food allergy as measured by parental report of a doctor diagnosis, we conducted sensitivity analysis using this additional measure of food allergy ‐ parental report of a doctor diagnosis ‐ to utilise the obtained data.
In subgroup analysis, for co‐primary outcomes, we planned that we would compare the effects of intervention on participants advised to commence the skin care intervention within the first four weeks of life compared to those who commenced intervention after four weeks. Since all identified trials advised skin care commencement during the first four weeks of life, we compared the effects of intervention on participants advised to commence skin care intervention within the first week of life compared to those who commenced intervention after the first week of life. We were able to utilise the obtained IPD and to explore the interaction between treatment effects of skin care intervention and actual age at treatment initiation as < 4 days versus ≥ 4 days. We conducted unplanned sensitivity analysis including outcomes measured at earlier time points for the primary outcome of eczema (6 months to 3 years) and the secondary outcome of allergic sensitisation (8 months to 3 years) to fully utilise the obtained individual participant data and to fully explore the implications of excluding from the main analysis data on early‐onset eczema or allergic sensitisation.
Complier average causal effect analysis
For the complier average causal effect analysis, we were unable to provide thresholds for defining compliance in the protocol, as it was unknown exactly what interventions and data fields would be available across trials. As pre‐specified in the statistical analysis plan (Cro 2020a), before commencement of any meta‐analysis, we held a SCiPAD Investigators meeting to establish alternative thresholds for defining compliance based on available data fields. The primary definition of a complier was use of skin care intervention ≥ 3 days a week over the intervention period, which corresponded to the definition used in the largest trial reporting compliance data (Chalmers 2020). Secondary definitions of a complier included use ≥ 5 days a week, 7 days a week over the intervention period, and ≥ 3 days a week, ≥ 5 days a week, and 7 days a week over the first three months of the intervention period.
Contributions of authors
MK was the contact person with the editorial base. RJB and HW conceived of the meta‐analysis. SC and VC wrote the Statistical Analysis Plan; RJB, MK, and AL contributed to this; and all authors reviewed and contributed to the final version of the SAP. LA and LD provided advice and expertise on conducting a prospective individual participant data (IPD) meta‐analysis. RJB, SC, MK, and EVV co‐ordinated contributions from the co‐authors and wrote the final draft of the review. SC, MK, LT, and RJB screened papers against eligibility criteria; EVV and MK screened grey literature. SC, MK, and LT obtained data on ongoing and unpublished studies. KLC, HS, ER, AL, ED, NS, KY, YO, KYH, KM, JS, ES, DM, MC, AC, JC, SW, and HW provided IPD from their individual trials, reviewed and contributed to the protocol, reviewed and contributed to the SAP, and reviewed the final version of the review. RJB, SC, and MK appraised the quality of papers. SC, MK, and LT extracted data for the review and sought additional information about papers. SC and LT entered data into RevMan. SC analysed and interpreted data. RJB and MK reviewed and commented on data analyses, conducted GRADE evaluations, and drafted the 'Summary of findings' table. RJB, SC, and MK wrote the text of the review and responded to feedback from other authors and peer reviewers. SC and VC responded to the methods and statistics comments of the referees. MC provided expert advice on formulation of topical emollients. CS provided expert advice on classification of topical skin interventions. EA developed the methods section with SC and VC and reviewed the protocol and review and 'Summary of findings' tables to ensure alignment with Cochrane requirements. EVV reviewed the full report to ensure that it corresponded to MECIR standards, and co‐ordinated the final review with all co‐authors.
Disclaimers
This publication presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
The NIHR also supported this project via Cochrane Infrastructure funding to Cochrane Skin. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK
The NIHR, UK, is the largest single funder of Cochrane Skin.
-
The National Institute for Health Research (NIHR), UK
MK was supported by a Transitional Research Fellowship. This funding provides for her salary, University support (University of Nottingham and Imperial College London) and statistical support from authors EA and VC.
-
The National Institute for Health Research (NIHR), UK
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0317‐20028). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The grant was awarded to author RJB (Nottingham University Hospitals NHS Trust) and provided support for SC and LT.
Declarations of interest
MaeveKelleher: I have received honoraria (personal payment) for speaking at educational conferences organised by Allergy UK, University of Oslo, and Nutricia, which does not manufacture/market any of the interventions or potential comparators in this review.
Suzie Cro: None known.
Victoria Cornelius: None known.
Emma Axon: None known.
Karin C Lodrup Carlsen: My institution received money from multiple sources: the Regional Health Board South East, the Norwegian Research Council, Oslo University Hospital, the University of Oslo, Health and Rehabilitation Norway, Østfold Hospital Trust, Norwegian Association of Asthma and Allergy, the Kloster Foundation, Norwegian Society of Dermatology and Venereology, Arne Ingels' legacy, Fürst Medical Laboratory, the Foundation for Healthcare and Allergy Research (in Sweden), the Vårdal Foundation, Swedish Asthma and Allergy Association’s Research Foundation, Swedish Research Council, the Initiative for Clinical Therapy Research, the Swedish Heart‐Lung Foundation, SFO‐V Karolinska Institutet, Hesselman Research Foundation, and Thermo‐Fisher (Uppsala, Sweden). My institution received an honorarium and travel expenses from Thermo‐Fisher (Uppsala, Sweden) for a lecture at the European Academy of Allergy and Clinical Immunology (EAACI) Congress 2018.
Håvard Ove Skjerven: My institution received money for the PreventADALL study from the two largest governmental grant agencies in Norway, The South‐Eastern Norway Regional Health Authority and the Norwegian Research Council, which are not commercial sponsors (Lødrup 2018).
Eva Maria Rehbinder: I declare no real or perceived conflict of interest for the present review; however, I have personally received honoraria in the last 36 months for presentations on atopic dermatitis and psoriasis from Sanofi Genzyme, Perrigo, MEDA, Novartis, and Norwegian patient organisations for atopic dermatitis and psoriasis.
Adrian Lowe: My institution has received National Health and Medical Research Council grant and fellowship funding to undertake a skin barrier intervention study. I also declare that Primus Pharmaceuticals and PuraCap Pharmaceuticals have donated EpiCream (a skin barrier treatment) for use in these studies, free of charge.
Eishika Dissanayake: None known.
Naoki Shimojo: My institution has received grants from the Japan Agency for Medical Research and Development (AMED‐CREST) (15652274). I have received personal payment for the development of educational presentations from AstraZeneca, Maruho, Novartis, Torii, and Miyarisan. Funding and payments for educational lectures from these pharmaceutical companies do not influence any activities related to this review article.
Kaori Yonezawa: My institution has received grants from the Mitsubishi Foundation (this grant supported the following published research: doi: 10.1111/1346‐8138.14080) and the Mishima Kaiun Memorial Foundation (which supported the following published research: doi: 10.1186/s13223‐019‐0385‐7), as well as from Hoyu Science Foundation and JSPS KAKENHI Grant Number 17K17676 12288;20H03995.
YukihiroOhya: I have received honorarium for lectures from AbbVie, Kao, Kyorin Pharmaceutical, Maruho, Mylan, Natural science, Sanofi, Taiho Pharma, and Torii pharmaceutical. I received payment for consultancy from Maruho for opening a forum and a grant from Yakult. All were personal payments.
Kiwako Yamamoto‐Hanada: My institution has received grants from The Japan Society for the Promotion of Science (JSPS) KAKENHI, Japan Health Foundation, the Environmental Restoration and Conservation Agency (ERCA), the National Center for Child Health and development, and the Japanese Society of Allergology. I have personally received travel/accommodations/meeting expenses unrelated to activities listed from Thermo‐Fisher Scientific.
Kumiko Morita: Outside this work, I have personally received speakers' honoraria from Maruho, Japan, and Astellas Pharma, Japan.
Christian Surber: I have personally received money for consultancy, lectures, and development of educational presentations from LEO Pharma (Switzerland, Germany, & Denmark), for explaining galenical concepts including supersaturation; and for lectures and development of educational presentations for explaining galenical concepts including nano emulsions, from Almirall, Germany.
Michael Cork: My institution received a grant from Hyphens Pharma for ‘An investigation of the effects of a barrier repair cream on the structure and function of the skin (BARRIER)’ (clinical trial).
My institution received a grant from L'Oreal (La Roche Possay) for ‘An investigation of the skin barrier restoring effects of a cream containing ceramides in a multi vesicular emulsion in people with dry, eczema‐prone, skin (RESTORE)’ (clinical trial).
My institution received a grant from Johnson & Johnson for ‘A randomised controlled trial of a specially designed wash product and lotion for the maintenance of healthy skin and mind in babies (BOND)’.
My institution and I personally received payment for study design and steering, for travel to meetings for study or other purposes, and for participation in review activities (such as data monitoring boards, statistical analysis, end point committees) from Hyphens Pharma, L'Oreal (La Roche Possay), and Johnson & Johnson.
I received personal payment for consultancy, travel/accommodations/meeting expenses unrelated to activities pre‐mentioned, and lectures from Regeneron in collaboration with Sanofi Genzyme with regard to biologic drug trials for atopic eczema; Pfizer with regard to biologic and topical drug trials for atopic eczema; and Galapagos and Kymab with regard to biologic drug trials for atopic eczema.
My institution received grants/grants pending from Regeneron in collaboration with Sanofi Genzyme with regard to biologic drug trials for atopic eczema; Pfizer with regard to biologic and topical drug trials for atopic eczema; and Galapagos and Kymab with regard to biologic drug trials for atopic eczema.
My institution and I personally received payment for review preparation from Regeneron in collaboration with Sanofi Genzyme with regard to biologic drug trials for atopic eczema; Pfizer with regard to biologic and topical drug trials for atopic eczema; and Galapagos and Kymab with regard to Biologic drug trials for atopic eczema.
My institution received payment for development of educational presentations from Regeneron in collaboration with Sanofi Genzyme with regard to biologic drug trials for atopic eczema.
I am or have been an Investigator and Consultant for the following organisations: Astellas, Boots, Dermavant, Galapagos, Galderma, Hyphens, Johnson & Johnson, Kymab, LEO Pharma, L’Oreal, Menlo, Novartis, Oxagen, Pfizer, Procter & Gamble, Reckitt Benckiser, Regeneron, and Sanofi Genzyme.
Alison Cooke: I was funded by a National Institute for Health Research Doctoral Research Fellowship paid to my institution for the OBSeRvE (Oil in Baby Skincare) study (Cooke 2015). This work was independent research supported by the National Institute for Health Research (Doctoral Research Fellowship DRF‐2012‐05‐160). The views expressed in any Cochrane publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. I was an invited expert to an advisory panel on infant skin care; my consultancy fee from Johnson and Johnson was paid to my institution. I was an invited expert speaker at a neonatal skin care symposium at the Royal College of Midwives Annual Conference and at the European Midwives Association Conference, for which I received personal payment from Johnson and Johnson.
Danielle McClanahan: None known.
Lien Tran: None known.
Lisa M Askie: None known.
LeliaDuley: None known.
Eleanor Van Vogt: None known.
Jochen Schmitt: I have acted as a consultant for Lilly and Sanofi (personal payment), and received institutional grants for investigator‐initiated trials outside the submitted work from Novartis, LaRoche, Sanofi, ALK, and Pfizer.
Eric Simpson: I have received personal payment for the following: board membership (from TARGET‐DERM, LEO, and Pfizer); consultancy (from AbbVie, Eli Lilly, and Pfizer); travel/accommodations/meeting expenses unrelated to activities listed (from Lilly, LEO Pharmaceutical, and Sanofi Genzyme); and review preparation (from Regeneron, Dermira, and Sanofi Genzyme). My institution received grants/grants pending from Eli Lilly, Incyte, and Kyowa Hakko Kirin. My institution and I personally received payment for lectures including service on speakers bureaus from Forte Bio Rx, Incyte, and Sanofi Genzyme. For more than 17 years, I have been actively involved in epidemiological, clinical and translational research centered on atopic dermatitis (AD). My experience leading multi‐centre AD clinical trials and directing our clinical research center for over 10 years shows my commitment to AD research and specifically disease prevention, clinical trial quality, and team science regardless of any previous financial support and activities.
Stephan Weidinger: My institution received grants from La Roche Posay for investigator‐initiated study NCT03376243 and from LEO Pharma for investigator‐initiated study 2019‐000598‐22. I received personal payment for advisory board membership from Sanofi Genzyme, Regeneron, LEO Pharma, AbbVie, Pfizer, Kymab, and Eli Lilly. I received personal payment for lectures from Sanofi Genzyme, Regeneron, LEO Pharma, AbbVie, Eli Lilly, and Novartis, as well as a personal payment for development of educational presentations from Sanofi Genzyme. I am an investigator in a number of clinical trials in atopic dermatitis and psoriasis sponsored by a wide range of pharmaceutical companies. I have received institutional research grants from Sanofi Genzyme, LEO Pharma, and L'Oreal. I have acted as a consultant for Sanofi Genzyme, Regeneron, LEO Pharma, Eli Lilly, AbbVie, Pfizer, Kymab, and Novartis. I have lectured at educational events sponsored by Sanofi Genzyme, Regeneron, LEO Pharma, AbbVie, and Galderma, outside of the submitted work.
Joanne R Chalmers: My institution received money from NIHR for a research for patient benefit grant to conduct this IPD, on which I am a co‐applicant. I am co‐applicant on the BEEP trial and the BEEP pilot trial, both of which are included in this review (Chalmers 2020).
Hywel C Williams: I was director of the NIHR Health Technology Assessment (HTA) Programme until 1 October 2020. HTA is part of the NIHR, which also supports the NIHR systematic reviews programme from which this work is funded. I am chief investigator for the BEEP study (Chalmers 2020), which was funded by NIHR HTA and is included in this review. Funds go to my University (Nottingham) from the National Institute for Health Research (public funds) as a result of open competition.
Robert J Boyle: I have received personal payment for participating in advisory boards for DBV Technologies and Prota Therapeutics, which develop allergy diagnostics or treatments; have received payment for designing a clinical trial for Dairy Goat Co‐operative; and have received personal payment for providing expert testimony in a class action related to an infant formula health claim concerning the development of allergic conditions.
Reports an NIHR RfPB grant awarded to Nottingham University Hospitals Trust. My employing institution, Imperial College London, has a formal research and innovation partnership with Nestle. This partnership does not directly involve me, and I do not think it is relevant to this review, but Nestle do have skin care products and products for prevention or treatment of allergic conditions within their portfolio.
These authors contributed equally to this work.
These authors contributed equally to this work.
During the course of the review, Prof. Shimojo retired from Department of Pediatrics, Graduate School of Medicine, and now works at the Center for Preventive Medical Sciences, Chiba University.
New
References
References to studies included in this review
Abraham 2019 {published data only (unpublished sought but not used)}
- Abraham D, Ravindran V, Joseph M. Effectiveness of chlorhexidine bath, saline bath and standard bath on skin health status. Indian Journal of Public Health Research and Development 2019;10(8):563-568. [DOI: 10.37506/ijphrd.v10i8.7446] [DOI] [Google Scholar]
Amer 2017 {published data only}
- Amer M, Amer A. Neonatal skin care. A four-week follow up randomized controlled trial. European Journal of Pediatric Dermatology 2015;25(2):93-99. [DOI: 10.26326/2281-9649.25.2.1106] [DOI] [Google Scholar]
- Amer M, Diab N, Soliman M, Amer A. Neonatal skin care: what should we do? A four-week follow-up randomized controlled trial at Zagazig University Hospitals. International Journal of Dermatology 2017;56(11):1198‐1203. [DOI: 10.1111/ijd.13735] [DOI] [PubMed] [Google Scholar]
Baldwin 2001 {published data only}
- Baldwin S, Odio MR, Haines SL, O'Connor RJ, Englehart JS, Lane AT. Skin benefits from continuous topical administration of a zinc oxide/petrolatum formulation by a novel disposable diaper. Journal of the European Academy of Dermatology and Venereology: JEADV 2001;15(s1):5-11. [DOI: 10.1046/j.0926-9959.2001.00002.x] [DOI] [PubMed] [Google Scholar]
Bellemere 2018 {published data only}
- Bellemere G, Boyer G, De Belilovsky C, Baudouin C. Prevention of atopic dermatitis using emollients for 6 months - Follow-up for 24 months. Journal of Investigative Dermatology 2019;139(5):S97. [DOI: 10.1016/j.jid.2019.03.639] [DOI] [Google Scholar]
- Bellemere G, Boyer G, De Belilovsky C, Baudouin C. Prevention of atopic dermatitis using emollients for 6 months-follow-up for 24 months. Pediatric Dermatology 2019;36:S9. [DOI: 10.1111/pde.13846] [DOI] [Google Scholar]
- Bellemere G, Boyer G, De Belilovsky C, Moga A, Fontanie M, Baudouin C. Early atopic dermatitis: prevention biological data. Pediatric Dermatology 2017;34:S106-S107. [DOI: 10.1111/pde.13196] [DOI] [Google Scholar]
- Bellemere G, Boyer G, De Belilovsky C, Moga A, Fontanie M, Baudouin C. Early atopic dermatitis: prevention study. Pediatric Dermatology 2018;35:S4‐S5. [DOI: 10.1111/pde.13502] [DOI] [Google Scholar]
- Bellemere G, Boyer G, de Belilovsky C, Moga A, Fontaine M. Early atopic dermatitis: prevention biologic data. Journal of the American Academy of Dermatology 2018;79(3):AB115. [DOI: 10.1016/j.jaad.2018.05.487] [DOI] [Google Scholar]
Chalmers 2020 {published and unpublished data}
- Chalmers JR, Haines RH, Bradshaw L, et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet 2020;395(10228):962-972. [DOI: 10.1016/S0140-6736(19)32984-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers JR, Haines RH, Mitchell EJ, Thomas KS, Brown SJ, Ridd M, et al. Effectiveness and cost-effectiveness of daily all-over-body application of emollient during the first year of life for preventing atopic eczema in high-risk children (the BEEP trial): protocol for a randomised controlled trial. Trials 2017;18(1):343. [DOI: 10.1186/s13063-017-2031-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers JR, Thomas KS, Montgomery A, Sach T, Boyle RJ, Ridd MJ, et al. A protocol for a randomized controlled trial to determine whether application of emollient from birth can prevent eczema in high-risk children (BEEP Trial) (PP01). British Journal of Dermatology 2014;170(6):e32. [DOI: 10.1111/bjd.13064] [DOI] [Google Scholar]
- ISRCTN21528841. Barrier enhancement for eczema prevention. www.isrctn.com/ISRCTN21528841 (first received 25 July 2014). [DOI: 10.1186/ISRCTN21528841] [DOI]
Cooke 2015 {published and unpublished data}
- Cooke A, Cork M, Victor S, Campbell M, Danby S, Chittock J, et al. A pilot, assessor-blinded, randomized controlled trial of topical oils for neonatal skin. British Journal of Dermatology 2015;173:153. [DOI: 10.1111/bjd.13819] [DOI] [Google Scholar]
- Cooke A, Cork MJ, Victor S, Campbell M, Danby S, Chittock J, et al. Olive oil, sunflower oil or no oil for baby dry skin or massage: a pilot, assessor-blinded, randomized controlled trial (the Oil in Baby SkincaRE [OBSeRvE] Study). Acta Dermato-Venereologica 2016;96(3):323-30. [DOI: 10.2340/00015555-2279] [DOI] [PubMed] [Google Scholar]
- ISRCTN37373893. The use of oil in baby skincare trial. www.isrctn.com/ISRCTN37373893 (first received 22 August 2013). [DOI: 10.1186/ISRCTN37373893] [DOI]
Da Cunha 2008 {published data only}
- Da Cunha M, Procianoy R, Franceschini D, De Oliveira L, Cunha ML. Effect of the first bath with chlorhexidine on skin colonization with Staphylococcus aureus in normal healthy term newborns. Scandinavian Journal of Infectious Diseases 2008;40(8):615-20. [DOI: 10.1080/00365540801932447] [DOI] [PubMed] [Google Scholar]
Dissanayake 2019 {published and unpublished data}
- Dissanayake E, Tani Y, Nagai K, Sahara M, Mitsuishi C, Togawa Y, et al. Skin care and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: a 2 x 2 factorial, randomized, non-treatment controlled trial. International Archives of Allergy & Immunology 2019;180(3):202-11. [DOI: 10.1159/000501636] [DOI] [PubMed] [Google Scholar]
- Dissanayake E, Tani Y, Sahara M, Mitsuishi C, Nagai K, Sato Y, et al. Skincare and synbiotics for the prevention of atopic dermatitis or food allergy in newborn infants: a 2 x 2 factorial randomized non-treatment controlled trial. Allergy: European Journal of Allergy and Clinical Immunology 2018;73(105):692-3. [DOI: 10.1111/all.13539] [DOI] [PubMed] [Google Scholar]
- JPRN-UMIN000010838. Skin care and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: a 2 x 2 factorial, randomized, non-treatment controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000010838 (first received 31 May 2013).
Dizon 2010 {published data only}
- Dizon MV, Galzote C, Estanislao R, Mathew N, Sarkar R. Tolerance of baby cleansers in infants: a randomized controlled trial. Indian Pediatrics 2010;47(11):959‐63. [DOI: 10.1007/s13312-010-0161-8] [DOI] [PubMed] [Google Scholar]
Duan 2019 {published data only}
- Duan Y, Ma L, Galzote C, Kong FQ, Shen CP. A randomized pilot clinical assessment of three skincare regimens on skin conditions in infants. Clinical, Cosmetic and Investigational Dermatology 2019;12:895-909. [DOI: 10.2147/CCID.S204216] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02981056. A clinical assessment of the efficacy and effects of different skincare regimens on infants. clinicaltrials.gov/ct2/show/NCT02981056 (first received 2 December 2016).
Garcia Bartels 2010 {published data only}
- Garcia Bartels N, Scheufele R, Prosch F, Schink T, Proquitté H, Wauer RR, et al. Effect of standardized skin care regimens on neonatal skin barrier function in different body areas. Pediatric Dermatology 2010;27(1):1‐8. [DOI: 10.1111/j.1525-1470.2009.01068.x] [DOI] [PubMed] [Google Scholar]
Garcia Bartels 2011 {published data only}
- Garcia Bartels N, Rosler S, Martus P, Stroux A, Lonnfors S, Reisshauer A, et al. Effect of baby swimming and baby lotion on the skin barrier of infants aged 3-6 months. Journal of the German Society of Dermatology 2011;9(12):1018‐26. [DOI: 10.1111/j.1610-0387.2011.07710.x] [DOI] [PubMed] [Google Scholar]
Garcia Bartels 2012 {published data only}
- Garcia Bartels N, Massoudy L, Scheufele R, Dietz E, Proquitté H, Wauer R, et al. Standardized diaper care regimen: a prospective, randomized pilot study on skin barrier function and epidermal IL-1α in newborns. Pediatric Dermatology 2012;29(3):270‐6. [DOI: 10.1111/j.1525-1470.2011.01590.x] [DOI] [PubMed] [Google Scholar]
- NCT01131403. Clinical analysis of the influence of using the skin care products on the diaper area in comparison with using a cotton wool cloth, moistened with clear water on the skin physiology of the newborns from the 1st day to the 4th week of life. clinicaltrials.gov/ct2/show/NCT01131403 (first received 27 May 2010).
Garcia Bartels 2014 {published data only}
- Garcia Bartels N, Lunnemann L, Stroux A, Kottner J, Serrano J, Blume-Peytavi U. Effect of diaper cream and wet wipes on skin barrier properties in infants: a prospective randomized controlled trial. Pediatric Dermatology 2014;31(6):683-91. [DOI: 10.1111/pde.12370] [DOI] [PubMed] [Google Scholar]
Horimukai 2014 {published and unpublished data}
- Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. Journal of Allergy and Clinical Immunology 2014;134(4):824‐30.e6. [DOI: 10.1016/j.jaci.2014.07.060] [DOI] [PubMed] [Google Scholar]
- JPRN-UMIN000004544. Effect of emollients on the prevention of infantile eczema and atopic dermatitis. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000004544 (first received 1 November 2010).
- Morita K. Application of moisturizer to neonates prevents development of atopic dermatitis. World Allergy Organization Journal 2014;8(Suppl 1):764. [DOI: 10.1186/1939-4551-8-S1-A252] [DOI] [PubMed] [Google Scholar]
- Ohya Y, Morita K, Narita M, Futamura M, Kondo M, Kitazawa H, et al. Primary prevention of atopic dermatitis by skin care with emollient: a randomized controlled study (OP02). British Journal of Dermatology 2014;170(6):e9. [DOI: 10.1111/bjd.13064] [DOI] [Google Scholar]
Kataoka 2010 {published data only}
- Kataoka Y, Yoshida N, Nishino H, Maeda N, Sarumaru T, Kijima A, et al. Can skin care from neonatal period prevent the onset of atopic dermatitis? Allergo Journal 2010;19:338-9. [DOI: 10.1007/s40629-019-00114-5] [DOI] [Google Scholar]
Lavender 2011 {published data only}
- ISRCTN72285670. Baby Skin Care Trial: a study comparing an infant skin-cleansing product with water. www.isrctn.com/ISRCTN72285670 (first received 17 March 2009). [DOI: 10.1186/ISRCTN72285670] [DOI]
- Lavender T, Bedwell C, O'Brien E, Cork MJ, Turner M, Hart A. Infant skin-cleansing product versus water: a pilot randomized, assessor-blinded controlled trial. BMC Pediatrics 2011;11:35. [DOI: 10.1186/1471-2431-11-35] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavender 2012 {published data only}
- ISRCTN86207019. Baby skin care research programme: Baby Wipes study. www.isrctn.com/ISRCTN86207019 (first received 17 February 2010). [DOI: 10.1186/ISRCTN86207019] [DOI]
- Lavender T, Furber C, Campbell M, Victor S, Roberts I, Bedwell C, et al. Effect on skin hydration of using baby wipes to clean the napkin area of newborn babies: assessor-blinded randomised controlled equivalence trial. BMC Pediatrics 2012;12:59. [DOI: 10.1186/1471-2431-12-59] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender T, Furber C, Cork M, Campbell M, Victor S. Baby skin care research programme: assessor-blinded randomised controlled trial comparing impregnated cleansing wipes with water in infants. Archives of Disease in Childhood: Fetal and Neonatal Edition 2011;1:Fa43-4. [DOI: 10.1136/archdischild.2011.300164.91] [DOI] [Google Scholar]
Lavender 2013 {published data only}
- Lavender T, Bedwell C, Roberts SA, Hart A, Turner MA, Carter LA, et al. Randomized, controlled trial evaluating a baby wash product on skin barrier function in healthy, term neonates. Journal of Obstetric, Gynaecologic, and Neonatal Nursing 2013;42(2):203‐14. [DOI: 10.1111/1552-6909.12015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2018a {published and unpublished data}
- ACTRN12609000727246. Prevention of eczema by a barrier lipid equilibrium strategy (PEBBLES) pilot study - Testing the compliance and safety of a strategy for improving infant skin function. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000727246 (first received 19 August 2009).
- ACTRN12613000472774. The PEBBLES study: Prevention of Eczema By a Barrier Lipid Equilibrium Strategy. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364115 (first received 23 April 2013).
- ACTRN12617001380381. The PEBBLES study – testing a strategy for preventing eczema and food allergy in high risk infants. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373506 (first received 20 September 2017).
- Lowe A, Su J, Allen K Abramson M, Cranswick N, Robertson C, et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: the pebbles pilot study NCD. Pediatric Dermatology 2017;34(S1):S35-6. [DOI: 10.1111/pde.13195] [DOI] [PubMed] [Google Scholar]
- Lowe A. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitisation: the PEBBLES pilot study NCD. Journal of Investigative Dermatology 2017;137:S72. [DOI: 10.1016/j.jid.2017.02.436] [DOI] [PubMed] [Google Scholar]
- Lowe AJ, Su JC, Allen KJ, Abramson MJ, Cranswick N, Robertson CF, et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: the PEBBLES pilot study. British Journal of Dermatology 2018;178(1):e19‐e21. [DOI: 10.1111/bjd.15747] [DOI] [PubMed] [Google Scholar]
Lund 2020 {published data only}
- Lund C, Kuller J, Durand DJ. Baby's first bath: changes in skin barrier function after bathing full-term newborns with water vs liquid baby cleanser. Pediatric Dermatology 2019;37:115-9. [DOI: 10.1111/pde.14037] [DOI] [PubMed] [Google Scholar]
McClanahan 2019 {published and unpublished data}
- McClanahan D, Wong A, Kezic S, Samrao A, Hajar T, Hill E, et al. A randomized controlled trial of an emollient with ceramide and filaggrin-associated amino acids for the primary prevention of atopic dermatitis in high-risk infants. Journal of the European Academy of Dermatology & Venereology 2019;33(11):2807-94. [DOI: 10.1111/jdv.15786] [DOI] [PubMed] [Google Scholar]
- NCT01375205. Comparing Cetaphil Restoraderm System versus standard skin care in infants. clinicaltrials.gov/ct2/show/NCT01375205 (first received 17 June 2011).
Migacheva 2018 {published data only}
- Migacheva N, Zhestkov A. Combined approach to primary prevention of atopic dermatitis. Allergy 2018;73:331. [DOI: 10.1111/all.13537] [DOI] [Google Scholar]
NCT03376243 {unpublished data only}
- NCT03376243. EARLYEMOLLIENT - Feasibility of early emollient use in children with atopic eczema. clinicaltrials.gov/ct2/show/NCT03376243 (first received 18 December 2017).
Raisi Dehkordi 2010 {published data only}
- IRCT201102265912N1. The effect of massage with sunflower oil and sesame oil. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201102265912N1 (first received 8 January 2012).
- Raisi Dehkordi Z. A comparative study of the effect of massage with sunflower oil or sesame oil on infants' crying and sleep times: a randomized control trial. European Psychiatry 2014;29(Suppl 1):1. [DOI: 10.1016/S0924-9338(14)78247-1] [DOI] [Google Scholar]
Rush 1986 {published data only}
- Rush J. Does routine newborn bathing reduce Staphylococcus aureus colonization rates? A randomized controlled trial. Birth 1986;13(3):176‐80. [DOI] [PubMed] [Google Scholar]
Sankaranarayanan 2005 {published data only}
- Sankaranarayanan K, Mondkar JA, Chauhan MM, Mascarenhas BM, Mainkar AR, Salvi RY. Oil massage in neonates: an open randomized controlled study of coconut versus mineral oil. Indian Pediatrics 2005;42(9):877‐84. [PMID: ] [PubMed] [Google Scholar]
Simpson 2014 {published and unpublished data}
- Chalmers JR, Simpson EL, Chen YY, Cork MJ, Brown SJ, Thomas KS, et al. Feasibility study of barrier enhancement for eczema prevention (BEEP). British Journal of Dermatology 2014;170:e8-e9. [DOI: 10.1186/ISRCTN84854178] [DOI] [Google Scholar]
- Glatz M, Jo J, Kennedy EA, Polley EC, Simpson EL, Kong HH. Emollient therapy alters barrier function and skin microbes in infants at risk for developing atopic dermatitis. Allergy 2017;72:311. [DOI: 10.1111/all.13251] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz M, Polley EC, Schmid-Grendelmeier P, Simpson EL, Kong HH. Emollient therapy alters barrier function and skin microbes in infants at risk for developing atopic dermatitis. Swiss Medical Weekly 2017;147(Suppl 228):53S. [Google Scholar]
- Glatz M, Polley EC, Simpson EL, Kong HH. Emollient therapy alters skin barrier and microbes in infants at risk for developing atopic dermatitis. Journal of Investigative Dermatology 2015;135:S31. [DOI: 10.1038/jid.2015.69] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz M, Jo JH, Kennedy EA, Polley EC, Segre JA, Simpson EL, et al. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS ONE 2018;13(2):0192443. [DOI: 10.1371/journal.pone.0192443] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN84854178. Feasibility study of barrier enhancement for eczema prevention. www.isrctn.com/ISRCTN84854178 (first received 8 January 2010).
- NCT01291940. The effects of emollient therapy on the skin barrier. clinicaltrials.gov/ct2/show/NCT01291940 (first received 9 February 2011).
- Simpson E, Chalmers JR, Irvine AD, Cork MJ, McLean WHI, Williams HC. Barrier enhancement for eczema prevention; the BEEP feasibility study. Allergo Journal 2010;19(5):340. [DOI: 10.1007/BF03362342] [DOI] [Google Scholar]
- Simpson EL, Chalmers JR, Hanifin JM, French ME, Lubianski T, Samrao A, et al. Barrier enhancement for eczema prevention - the BEEP feasibility study. Journal of Investigative Dermatology 2012;132(Suppl 1):S90. [DOI: 10.1038/jid.2012.86] [DOI] [Google Scholar]
- Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. Journal of Allergy and Clinical Immunology 2014;134(4):818‐823. [DOI: 10.1016/j.jaci.2014.08.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skjerven 2020 {published and unpublished data}
- Carlsen L, Rehbinder EM, Skjerven HO, Hauger Carlsen M, Aspelund Fatnes T, Fugelli P, et al. Preventing Atopic Dermatitis and ALLergies in Children—the PreventADALL study. Allergy 2018;73(10):2063-70. [DOI: 10.1111/all.13468] [DOI] [PubMed] [Google Scholar]
- Skjerven HO, Rehbinder EM, Vettukattil R, LeBlanc M, Granum B, Haugen G, et al. Department of Error: Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster-randomised trial (The Lancet (2020) 395(10228) (951-61) (S0140673619329836)). Lancet 2020;395(10228):e53. [DOI: 10.1016/S0140-6736(20)30422-0] [DOI] [PubMed] [Google Scholar]
- Skjerven HO, Rehbinder EM, Vettukattil R, LeBlanc M, Granum B, Haugen G, et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster-randomised trial. Lancet 2020;395(10228):951‐961. [DOI: 10.1016/S0140-6736(19)32983-6] [DOI] [PubMed] [Google Scholar]
Thitthiwong 2019 {published data only}
- Koopitakkajorn T, Thitthiwong P. The good skin care practices and emollient using since early infancy as the primary prevention of infantile atopic dermatitis in infants at risk: a randomized controlled trial. Pediatric Dermatology 2017;34(S1):S35. [DOI: 10.1111/pde.13195] [DOI] [Google Scholar]
- TCTR20161208001. The good skin care practices and emollient using since early infancy as the primary prevention of infantile atopic dermatitis in infants at risk: a randomized controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20161208001 (first received 4 December 2016).
Tielsch 2007 {published data only}
- NCT00109616. Community trial of newborn skin and umbilical cord cleansing on neonatal mortality in Nepal. clinicaltrials.gov/ct2/show/NCT00109616 (first received 2 May 2005).
- Tielsch JM, Darmstadt GL, Mullany LC, Khatry SK, Katz J, LeClerq SC, et al. Impact of newborn skin-cleansing with chlorhexidine on neonatal mortality in Southern Nepal: a community-based, cluster-randomized trial. Pediatrics 2007;119(2):e330-40. [DOI: 10.1542/peds.2006-1192] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yonezawa 2018 {published and unpublished data}
- JPRN-UMIN000013260. Effects of moisturizing skin care from the neonatal stage for improving skin barrier function and preventing skin trouble. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000013260 (first received 1 March 2014).
- Yonezawa K, Haruna M, Matsuzaki M, Shiraishi M, Kojima R. Effects of moisturizing skincare on skin barrier function and the prevention of skin problems in 3-month-old infants: a randomized controlled trial. Journal of Dermatology 2018;45(1):24‐30. [DOI: 10.1111/1346-8138.14080] [DOI] [PubMed] [Google Scholar]
- Yonezawa K, Haruna M. Short-term skin problems in infants aged 0-3 months affect food allergies or atopic dermatitis until 2 years of age, among infants of the general population. Allergy, Asthma & Clinical Immunology 2019;15(74):1552. [DOI: 10.1186/s13223-019-0385-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2005 {published data only}
- Zhao S, Xie L, Hu H, Xia J, Zhang W, Ye N, et al. A study of neonatal swimming (water therapy) applied in clinical obstetrics. Journal of Maternal-Fetal & Neonatal Medicine 2005;17(1):59‐62. [DOI: 10.1080/14767050400028782] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12607000466448 {published data only}
- ACTRN12607000466448. An intervention to reduce the prevalence and impact of asthma and food allergies occurring in association with atopic dermatitis through improved skin care in infants and young children. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82278 (first received 11 September 2007).
Ahmed 2007 {published data only}
- Ahmed AS, Saha SK, Chowdhury MA, Law PA, Black RE, Santosham M, et al. Acceptability of massage with skin barrier-enhancing emollients in young neonates in Bangladesh. Journal of Health, Population, and Nutrition 2007;25(2):236‐40. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Alonso 2013 {published data only}
- Alonso C, Larburu I, Bon E, Gonzalez MM, Iglesias MT, Urreta I, et al. Efficacy of petrolatum jelly for the prevention of diaper rash: a randomized clinical trial. Journal for Specialists in Pediatric Nursing 2013;18(2):123-32. [DOI: 10.1111/jspn.12022] [DOI] [PubMed] [Google Scholar]
- ISRCTN00356649. Efficacy of petroleum jelly in the prevention of irritant diaper dermatitis. www.isrctn.com/ISRCTN00356649 (first received 8 October 2010).
Baer 2006 {published data only}
- Baer EL, Davies MW, Easterbrook K. Disposable nappies for preventing napkin dermatitis in infants. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD004262. [DOI: 10.1002/14651858.CD004262.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barria 2004 {published data only}
- Barría K, Muñoz C, Pérez‐Cotapos ML, Fehlmann E, Brockmann P, Tapia JL. Comparison of 2 models for the initial skin care in the healthy full term newborn infant. Pediatric Dermatology 2004;21:317-18. [DOI: 10.1111/j.0736-8046.2004.21332.x] [DOI] [Google Scholar]
Baudouin 2014 {published data only}
- Baudouin C, De Belilovsky C, Lachmann N, Bredif S, Menu F, Msika P. Tolerance assessment of baby's skin products: Innovative approaches using stinging test and infant epidermis model. Journal of Investigative Dermatology 2014;2:S51. [Google Scholar]
Baudouin 2014a {published data only}
- Baudouin C, De Belilovsky C, Menu F, Lachmann N, Msika P, Bredif S. Tolerance assessment of cosmetic products dedicated to baby's skin: innovative approaches using stinging test and infant epidermis model. Journal of the American Academy of Dermatology 2014;1:AB149. [DOI: 10.1016/j.jaad.2014.01.619] [DOI] [Google Scholar]
Berger 2009 {published data only}
- Berger C, Inzinger R. Study of skin care in premature and newborn infants. Kinderkrankenschwester 2009;28(3):116-25. [PubMed] [Google Scholar]
Bhakoo 1969 {published data only}
- Bhakoo ON, Lall JC, Agarwal KC. Prevention of hospital infections in neonates: an evaluation of no bath regimen. Indian Pediatrics 1969;6(1):697‐700. [PubMed] [Google Scholar]
Blume Peytavi 2010 {published data only}
- Blume-Peytavi U, Garcia Bartels N. Neonatal skin care influence of standardized skin care regimen on skin barrier function. Aktuelle Dermatologie 2010;36(6):214-6. [Google Scholar]
Blume Peytavi 2012 {published data only}
- Blume-Peytavi U, Hauser M, Stamatas GN, Pathirana D, Garcia Bartels N. Skin care practices for newborns and infants: Review of the clinical evidence for best practices. Pediatric Dermatology 2012;29:1-14. [DOI] [PubMed] [Google Scholar]
Blume Peytavi 2014 {published data only}
- Blume-Peytavi U, Hauser M, Lunnemann L, Stamatas GN, Kottner J, Garcia Bartels N. Prevention of diaper dermatitis in infants--a literature review. Pediatric Dermatology 2014;31(4):413-29. [DOI] [PubMed] [Google Scholar]
Blume Peytavi 2016 {published data only}
- Blume-Peytavi U. Improved understanding of infant skin physiology, maturation and skin care. European Journal of Pediatric Dermatology 2016;26(3):166. [Google Scholar]
Brandon 2010 {published data only}
- Brandon DH, Coe K, Hudson-Barr D, Oliver T, Landerman LR. Effectiveness of no-sting skin protectant and Aquaphor on water loss and skin integrity in premature infants. Journal of Perinatology 2010;30(6):414‐9. [DOI: 10.1038/jp.2009.174] [DOI] [PubMed] [Google Scholar]
Bryanton 2004 {published data only}
- Bryanton J, Walsh D, Barrett M, Gaudet D. Tub bathing versus traditional sponge bathing for the newborn. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2004;33(6):704‐12. [DOI: 10.1177/0884217504270651] [DOI] [PubMed] [Google Scholar]
Chaithirayanon 2016 {published data only}
- Chaithirayanon S. Comparative study between talcum and zinc oxide cream for the prevention of irritant contact diaper dermatitis in infants. Journal of the Medical Association of Thailand 2016;99(Suppl 8):S1‐S6. [PMID: ] [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen HY. The clinic effect of Chinese medicine bath and massage on pathological jaundice of newborn. China Foreign Medical Treatment 2009;28(18):128. [Google Scholar]
Cleminson 2015 {published data only}
- Cleminson J, McGuire W. Topical emollient for prevention of infection in preterm infants: a systematic review. Lancet 2015;385:S31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cleminson 2016 {published data only}
- Cleminson J, McGuire W. Topical emollient for preventing infection in preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No: CD001150. [DOI: 10.1002/14651858.CD001150.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conner 2004 {published data only}
- Conner JM, Soll RF, Edwards WH. Topical ointment for preventing infection in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD001150. [DOI: 10.1002/14651858.CD001150.pub2] [DOI] [PubMed] [Google Scholar]
Cooke 2014 {published data only}
- Cooke A, Victor S, Cork M, Lavender T. Topical oils for the prevention or treatment of dry skin in term infants. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD011100. [DOI: 10.1002/14651858.CD011100] [DOI] [Google Scholar]
Cooke 2018 {published data only}
- Cooke A, Bedwell C, Campbell M, McGowan L, Ersser SJ, Lavender T. Skin care for healthy babies at term: a systematic review of the evidence. Midwifery 2018;56:29-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cowan 1986 {published data only}
- Cowan ME, Frost MR. A comparison between a detergent baby bath additive and baby soap on the skin flora of neonates. Journal of Hospital Infection 1986;7(1):91‐5. [DOI: 10.1016/0195-6701(86)90033-2] [DOI] [PubMed] [Google Scholar]
CTRI201208002876 {unpublished data only}
- CTRI/2012/08/002876. A prospective, open label clinical trial to evaluate the efficacy and safety of Tubby Nutririch lotion for the treatment of dry skin with or without atopic tendency in infants and children. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=5054 (first received 9 August 2012).
CTRI201709009890 {published data only}
- CTRI/2017/09/009890. Comparing the effect of three different skin antiseptic preparations in neonates (first 28 days of life) - randomized trial. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=19272 (first received 22 September 2017).
Da Cunha 2005 {published data only}
- da Cunha ML, Procianoy RS. Effect of bathing on skin flora of preterm newborns. Journal of Perinatology 2005;25(6):375-9. [DOI: 10.1038/sj.jp.7211332] [DOI] [PubMed] [Google Scholar]
Da Cunha 2005a {published data only}
- Da Cunha ML, Procianoy RS. Skin microflora and bathing of preterm newborn infants. In: Pediatric Academic Societies Annual Meeting. Washington, DC, 2005.
Darmstadt 2004 {published data only}
- Darmstadt GL, Badrawi N, Law PA, Ahmed S, Bashir M, Iskander I, et al. Topically applied sunflower seed oil prevents invasive bacterial infections in preterm infants in Egypt: a randomized, controlled clinical trial. Pediatric Infectious Disease Journal 2004;23(8):719-25. [DOI: 10.1097/01.inf.0000133047.50836.6f] [DOI] [PubMed] [Google Scholar]
Darmstadt 2005 {published data only}
- Darmstadt GL, Saha SK, Nawshad Uddin Ahmed ASM, Azad Chowdhury MAK, Law PA, Ahmed S, et al. Skin barrier-enhancing emollients prevent nosocomial infections in preterm infants in Bangladesh. In: Pediatric Academic Societies Annual Meeting. Washington DC, 2005.
Darmstadt 2005a {published data only}
- Darmstadt GL, Saha SK, Ahmed AS, Chowdhury MA, Law PA, Ahmed S, et al. Effect of topical treatment with skin barrier-enhancing emollients on nosocomial infections in preterm infants in Bangladesh: a randomised controlled trial. Lancet 2005;365(9464):1039‐45. [DOI: 10.1016/S0140-6736(05)71140-5] [DOI] [PubMed] [Google Scholar]
Darmstadt 2007 {published data only}
- Darmstadt GL, Saha SK, Ahmed AS, Choi Y, Chowdhury MA, Islam M, et al. Effect of topical emollient treatment of preterm neonates in Bangladesh on invasion of pathogens into the bloodstream. Pediatric Research 2007;61(5 Pt 1):588‐93. [DOI: 10.1203/pdr.0b013e3180459f75] [DOI] [PubMed] [Google Scholar]
Darmstadt 2008 {published data only}
- Darmstadt GL, Saha SK, Ahmed AS, Ahmed S, Chowdhury MA, Law PA, et al. Effect of skin barrier therapy on neonatal mortality rates in preterm infants in Bangladesh: a randomized, controlled, clinical trial. Pediatrics 2008;121(3):522-9. [DOI: 10.1542/peds.2007-0213] [DOI] [PubMed] [Google Scholar]
Darmstadt 2014 {published data only}
- Darmstadt GL, Ahmed S, Ahmed AS, Saha SK. Mechanism for prevention of infection in preterm neonates by topical emollients: a randomized, controlled clinical trial. Pediatric Infectious Disease Journal 2014;33(11):1124‐7. [DOI: 10.1097/INF.0000000000000423] [DOI] [PubMed] [Google Scholar]
Duggan 2015 {published data only}
- Duggan C. Eczema on trial. Journal of Family Health 2015;25(3):10-12. [PMID: ] [PubMed] [Google Scholar]
Erdemir 2015 {published data only}
- Erdemir A, Kahramaner Z, Yuksel Y, Cosar H, Turkoglu E, Sutcuoglu S, et al. The effect of topical ointment on neonatal sepsis in preterm infants. Journal of Maternal-Fetal & Neonatal Medicine 2015;28(1):33‐6. [DOI: 10.3109/14767058.2014.900037] [DOI] [PubMed] [Google Scholar]
Ernest 1995 {published data only}
- Ernest E. Complementary therapies, the baby, and the bath water. British Journal of Rheumatology 1995;34(5):479‐80. [DOI: 10.1093/rheumatology/34.5.479-a] [DOI] [PubMed] [Google Scholar]
EUCTR2005‐001269‐32‐AT {published data only}
- EUCTR2005-001269-32-AT. Clinical trial on skin care in neonatology. A comparison between two skin care products and no skin care. www.clinicaltrialsregister.eu/ctr-search/search?query=2005-001269-32 (first received 22 November 2005).
Fernandez 2018 {published data only}
- Fernandez D, Antolin-Rodriguez R. Bathing a premature infant in the intensive care unit: a systematic review. Journal of Pediatric Nursing 2018;42:e52-7. [DOI: 10.1016/j.pedn.2018.05.002] [DOI] [PubMed] [Google Scholar]
Field 2008 {published data only}
- Field T, Field T, Cullen C, Largie S, Diego M, Schanberg S, et al. Lavender bath oil reduces stress and crying and enhances sleep in very young infants. Early Human Development 2008;84(6):399‐401. [DOI] [PubMed] [Google Scholar]
Fluhr 2012 {published data only}
- Fluhr JW, Darlenski R, Lachmann N, Baudouin C, Msika P, De Belilovsky C, et al. Infant epidermal skin physiology: adaptation after birth. British Journal of Dermatology 2012;166(3):483‐90. [DOI: 10.1111/j.1365-2133.2011.10659.x] [DOI] [PubMed] [Google Scholar]
Foisy 2011 {published data only}
- Foisy M, Boyle RJ, Chalmers JR, Simpson EL, Williams HC. Overview of reviews: the prevention of eczema in infants and children: an overview of Cochrane and non-Cochrane reviews. Evidence-Based Child Health: A Cochrane Review Journal 2011;6(5):1322-39. [DOI: 10.1002/ebch.827] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franck 2000 {published data only}
- Franck LS, Quinn D, Zahr L. Effect of less frequent bathing of preterm infants on skin flora and pathogen colonization. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2000;29(6):584‐9. [DOI] [PubMed] [Google Scholar]
Friscia 2019 {published data only (unpublished sought but not used)}
- Friscia D, Telofski L, Nikolovski J. Effects of emollient use on the developing infant skin microbiome. Journal of the American Academy of Dermatology 2019;4 Suppl 1:AB4422019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2008 {published data only}
- Gao Y. Effectiveness observation on moisturizer for infant eczema. Chinese Journal of Leprosy and Skin Disease 2008;24(4):294. [Google Scholar]
Garcia Bartels 2009 {published data only}
- Garcia Bartels N, Mleczko A, Schink T, Proquitte H, Wauer RR, Blume-Peytavi U. Influence of bathing or washing on skin barrier function in newborns during the first four weeks of life. Skin Pharmacology & Physiology 2009;22(5):248-57. [DOI] [PubMed] [Google Scholar]
Gezon 1964 {published data only}
- Gezon HM, Thompson DJ, Rogers KD, Hatch TF, Taylor PM. Hexachlorophene bathing in early infancy. New England Journal of Medicine 1964;270:379‐86. [DOI] [PubMed] [Google Scholar]
Gfatter 1997 {published data only}
- Gfatter R, Hackl P, Braun F. Effects of soap and detergents on skin surface pH, stratum corneum hydration and fat content in infants. Dermatology 1997;195(3):258-62. [DOI: 10.1159/000245955] [DOI] [PubMed] [Google Scholar]
Gleiss 1965 {published data only}
- Gleiss J, Sommerkamp B. Comparative study of soapless skin cleansing of infants. Archiv fur Kinderheilkunde 1965;172(2):154-60. [PubMed] [Google Scholar]
Gunt 2018 {published data only}
- Gunt HB, Levy SB, Lutrario CA. A natural cream-to-powder formulation developed for the prevention of diaper dermatitis in diaper-wearing infants and children: barrier property and in-use tolerance studies. Journal of Drugs in Dermatology 2018;17(5):566‐70. [PubMed] [Google Scholar]
Hauk 2008 {published data only}
- Hauk PJ. The role of food allergy in atopic dermatitis. Current Allergy and Asthma Reports 2008;8(3):188-94. [DOI] [PubMed] [Google Scholar]
Hawkins 2017 {published data only}
- Hawkins S, Edmunds M, Foy V, Vincent C, Cox E, O'Shea S, et al. Superior skin care for infants with a new nourishing regimen containing stearic acid, petrolatum, and fatty acids: tip-to-toe-wash and lotion. Pediatric Dermatology 2017;34(S1):S34. [DOI: 10.1111/pde.13195] [DOI] [Google Scholar]
Hnatko 1977 {published data only}
- Hnatko SI. Alternatives to hexachlorophene bathing of newborn infants. Canadian Medical Association Journal 1977;117(3):223‐6. [PMC free article] [PubMed] [Google Scholar]
Horimukai 2016 {published data only}
- Horimukai K, Morita K, Narita M, Kondo M, Kabashima S, Inoue E, et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergology International 2016;65(1):103-8. [DOI] [PubMed] [Google Scholar]
Hu 2014 {published data only}
- Hu X, Zhang Y. Effect of topically applied sunflower seed oil in preterm infants. Pediatric Critical Care Medicine 2014;15(4 Suppl 1):144‐5. [DOI: 10.1097/01.pcc.0000449366.67893.84] [DOI] [Google Scholar]
IRCT201306164617N {published data only}
- IRCT201306164617N. Effect of bath on clinical outcomes in preterm infants: a randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201306164617N7 (first received 28 June 2013).
IRCT201306164617N7 {published data only}
- IRCT201306164617N7. Effect of bath on clinical outcomes in preterm infants: a randomized controlled trial. en.irct.ir/trial/4950 (first received 28 June 2013).
IRCT2013090814594N1 {published data only}
- IRCT2013090814594N1. A comparison between the effects of swaddled bathing and conventional bathing on physiological and behavioral parameters among the preterm neonates. en.irct.ir/trial/14191 (first received 3 January 2014).
IRCT2016111530903N {published data only}
- IRCT2016111530903N. The efficacy of Chamomile oil on infantile colic. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016111530903N1 (first received 4 April 2017).
IRCT20170911036118N1 {published data only}
- IRCT20170911036118N1. Effect of massage and washing in physiological jaundice. en.irct.ir/trial/27093 (first received 17 January 2018).
ISRCTN71423189 {published data only}
- ISRCTN71423189. Multi-centre randomised controlled trial of ion-exchange water softeners for the treatment of atopic eczema in children (Softened Water Eczema Trial). www.isrctn.com/ISRCTN71423189 (first received 8 January 2007). [DOI: 10.1186/ISRCTN71423189] [DOI]
ISRCTN89579779 {published data only}
- ISRCTN89579779. The use of Aquaphor to prevent transepidermal water loss and to maintain electrolyte balance in preterm newborn infants in the first week of life. www.isrctn.com/ISRCTN89579779 (first received 30 September 2004). [DOI: 10.1186/ISRCTN89579779] [DOI]
Jabraeile 2016 {published data only}
- Jabraeile M, Rasooly AS, Farshi MR, Malakouti J. Effect of olive oil massage on weight gain in preterm infants: a randomized controlled clinical trial. Nigerian Medical Journal 2016;57(3):160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 1971 {published data only}
- Jensen JP. Transcutaneous absorption of boron from a baby ointment used prophylactically against diaper dermatitis. Nordisk Medicin 1971;86(48):1425‐9. [PubMed] [Google Scholar]
JPRN UMIN000005158 {published data only}
- JPRN-UMIN000005158. Randomized controlled trial to evaluate clinical efficacy of once- or twice-daily skincare with emollient for prevention of atopic dermatitis exacerbation. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000005803 (first received 3 July 2011).
JPRN‐UMIN000018110 {published data only}
- JPRN-UMIN000018110. Preliminary study on the skin cleaning method without cleaning agent during bathing in the NICU - Comparison with the case of using a skin cleaning agent. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000018110 (first received 26 June 2015).
JPRN‐UMIN000025302 {published data only}
- JPRN-UMIN000025302. Open test of skin care products for newborns. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000025302 (first received 16 November 2017).
JPRN UMIN000032181 {published data only}
- JPRN-UMIN000032181. Study of neonates on the intestinal bacterial flora and skin conditions. upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_view.cgi?recptno=R000036709 (first received 10 April 2018).
JPRN UMIN000032798 {published data only}
- JPRN-UMIN000032798. Baby diaper study (No.2016-001). upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000037416 (first received 31 May 2018).
JPRN UMIN000035357 {published data only}
- JPRN-UMIN000035357. Effect of moisturizer application on perianal area in neonates; comparison of effects on erythema and skin barrier function at 1-month checkup. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000040287 (first received 25 December 2018).
JPRN UMIN000035412 {published data only}
- JPRN-UMIN000035412. Effect of change in bathing and skin care in infancy on the incidence of eczema at 6 months of age. rctportal.niph.go.jp/en/detail?trial_id=UMIN000035412 (first received 7 January 2019).
Kadar 1974 {published data only}
- Kadar V, Osbourn R. The mildness of soaps in the care of infants' skin: a comparative study. Current Therapeutic Research - Clinical and Experimental 1974;16(5):452-6. [PMID: ] [PubMed] [Google Scholar]
Kanti 2014 {published data only}
- Kanti V, Grande C, Stroux A, Buhrer C, Blume-Peytavi U, Garcia Bartels N. Influence of sunflower seed oil on the skin barrier function of preterm infants: a randomized controlled trial. Dermatology 2014;229(3):230-9. [DOI] [PubMed] [Google Scholar]
Kanti 2017 {published data only}
- Kanti V, Gunther M, Stroux A, Sawatzky S, Henrich W, Abou-Dakn M, et al. Influence of sunflower seed oil or baby lotion on the skin barrier function of newborns: a pilot study. Journal of Cosmetic Dermatology 2017;16(4):500-7. [DOI] [PubMed] [Google Scholar]
Kiechl Kohlendorfer 2008 {published data only}
- Kiechl-Kohlendorfer U, Berger C, Inzinger R. The effect of daily treatment with an olive oil/lanolin emollient on skin integrity in preterm infants: a randomized controlled trial. Pediatric Dermatology 2008;25(2):174-8. [DOI: 10.1111/j.1525-1470.2008.00627.x] [DOI] [PubMed] [Google Scholar]
Konar 2019 {published data only}
- Konar MC, Islam K, Roy A, Ghosh T. Effect of virgin coconut oil application on the skin of preterm newborns: a randomized controlled trial. Journal of Tropical Pediatrics 2019;66:129-35. [DOI: 10.1093/tropej/fmz041] [DOI] [PubMed] [Google Scholar]
Koplin 2019 {published data only}
- Koplin JJ, Allen KJ, Tang MLK. Important risk factors for the development of food allergy and potential options for prevention. Expert Review of Clinical Immunology 2019;15(2):147-52. [DOI] [PubMed] [Google Scholar]
Kottner 2017 {published data only}
- Kottner J, Kanti V, Dobos G, Hahnel E, Lichterfeld-Kottner A, Richter C, et al. The effectiveness of using a bath oil to reduce signs of dry skin: a randomized controlled pragmatic study. International Journal of Nursing Studies 2017;65:17-24. [DOI] [PubMed] [Google Scholar]
Kvenshagen 2014 {published data only}
- Kvenshagen BK, Mowinckel P, Carlsen KH, Carlsen KL. Intensive oil baths from six weeks of age reduce xerosis and possibly atopic eczema in infancy. European Respiratory Journal 2012;40(Suppl 56):424s. [Google Scholar]
- Kvenshagen BK, Carlsen KH, Mowinckel P, Berents TL, Carlsen KC. Can early skin care normalise dry skin and possibly prevent atopic eczema? A pilot study in young infants. Allergologia et Immunopathologia 2014;42(6):539‐543. [DOI: 10.1016/j.aller.2014.06.003] [DOI] [PubMed] [Google Scholar]
Lane 1993 {published data only}
- Lane AT, Drost SS. Effects of repeated application of emollient cream to premature neonates' skin. Pediatrics 1993;92(3):415‐9. [PubMed] [Google Scholar]
Larson 2005 {published data only}
- Larson AA, Dinulos JG. Cutaneous bacterial infections in the newborn. Current Opinion in Pediatrics 2005;17(4):481-5. [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee JC, Lee Y, Park HR. Effects of bathing interval on skin condition and axillary bacterial colonization in preterm infants. Applied Nursing Research 2018;40:34‐8. [DOI: 10.1016/j.apnr.2017.12.012] [DOI] [PubMed] [Google Scholar]
LeFevre 2010 {published data only}
- LeFevre A, Shillcutt SD, Saha SK, Ahmed AS, Ahmed S, Chowdhury MA, et al. Cost-effectiveness of skin-barrier-enhancing emollients among preterm infants in Bangladesh. Bulletin of the World Health Organization 2010;88(2):104‐12. [DOI: 10.2471/BLT.08.058230] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2015 {published data only}
- Leung DYM. Prophylactic emollient use beginning at birth prevents atopic dermatitis. Journal of Pediatrics 2015;166(3):777-8. [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li X, Zhong Q, Tang L. A meta-analysis of the efficacy and safety of using oil massage to promote infant growth. Journal of Pediatric Nursing 2016;31(5):e313-22. [DOI] [PubMed] [Google Scholar]
Ling 2011 {published data only}
- Ling L, Bi XY. Swimming and bathing with Chinese herbal medicine in treatment of neonatal jaundice: clinical analysis of 50 cases. Journal of Qilu Nursing 2011;17(1):42‐3. [Google Scholar]
Linnamaa 2010 {published data only}
- Linnamaa P, Savolainen J, Koulu L, Tuomasjukka S, Kallio H, Yang B, et al. Blackcurrant seed oil for prevention of atopic dermatitis in newborns: a randomized, double-blind, placebo-controlled trial. Clinical and Experimental Allergy 2010;40(8):1247‐55. [DOI: 10.1111/j.1365-2222.2010.03540.x] [DOI] [PubMed] [Google Scholar]
Lowe 2012 {published data only}
- Lowe AJ, Tang MLK, Dharmage SC, Varigos G, Forster D, Gurrin LC, et al. A phase I study of daily treatment with a ceramide-dominant triple lipid mixture commencing in neonates. BMC Dermatology 2012;12(3):no pagination. [DOI: 10.1186/1471-5945-12-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2018b {published data only}
- Lowe AJ, Leung DYM, Tang MLK, Su JC, Allen KJ. The skin as a target for prevention of the atopic march. Annals of Allergy, Asthma, & Immunology 2018;120(2):145-51. [DOI] [PubMed] [Google Scholar]
Lund 2001 {published data only}
- Lund CH, Kuller J, Lane AT, Lott JW, Raines DA, Thomas KK. Neonatal skin care: evaluation of the AWHONN/NANN research-based practice project on knowledge and skin care practices. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2001;30(1):30‐40. [PubMed] [Google Scholar]
Lund 2001a {published data only}
- Lund CH, Osborne JW, Kuller J, Lane AT, Lott JW, Raines DA. Neonatal skin care: clinical outcomes of the AWHONN/NANN evidence-based clinical practice guideline. Association of Women's Health, Obstetric and Neonatal Nurses and the National Association of Neonatal Nurses. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2001;30(1):41‐51. [PubMed] [Google Scholar]
Marenholz 2015 {published data only}
- Marenholz I, Esparza-Gordillo J, Lee YA. The genetics of the skin barrier in eczema and other allergic disorders. Current Opinion in Allergy & Clinical Immunology 2015;15(5):426-34. [DOI] [PubMed] [Google Scholar]
Muggli 2009 {published data only}
- Muggli R. Natural management of napkin rash. European Journal of Pediatric Dermatology 2009;19(1):43‐6. [Google Scholar]
Nangia 2015 {published data only}
- Nangia S, Paul VK, Deorari AK, Sreenivas V, Agarwal R, Chawla D. Topical oil application and trans-epidermal water loss in preterm very low birth weight infants-a randomized trial. Journal of Tropical Pediatrics 2015;61(6):414-20. [DOI: 10.1093/tropej/fmv049] [DOI] [PubMed] [Google Scholar]
Natsume 2018 {published data only}
- Natsume O, Ohya Y. Recent advancement to prevent the development of allergy and allergic diseases and therapeutic strategy in the perspective of barrier dysfunction. Allergology International 2018;67(1):24-31. [DOI] [PubMed] [Google Scholar]
NCT00162747 {published data only}
- NCT00162747. Topical therapy for prevention of infections in preterm infants. clinicaltrials.gov/ct2/show/NCT00162747 (first received 13 September 2005).
NCT00257569 {published data only}
- NCT00257569. Study of atopic dermatitis in pediatrics. clinicaltrials.gov/ct2/show/NCT00257569 (first received 23 November 2005).
NCT00806221 {published data only}
- NCT00806221. An open-label study investigating the effects of early skin barrier protection on the development of atopic dermatitis. clinicaltrials.gov/ct2/show/NCT00806221 (first received 10 December 2008).
NCT00917085 {published data only}
- NCT00917085. Is skin-to-skin care helpful for preterm infants and their mothers after birth? clinicaltrials.gov/ct2/show/NCT00917085 (first received 10 June 2009).
NCT01131403 {published data only}
- NCT01131403. Clinical analysis of the influence of using the skin care products on the diaper area in comparison with using a cotton wool cloth, moistened with clear water on the skin physiology of the newborns from the 1st day to the 4th week of life. https://clinicaltrials.gov/ct2/show/NCT01131403 (first received 27 May 2010).
NCT01177111 {published data only}
- NCT01177111. Impact of sunflower seed oil massage on neonatal mortality and morbidity in Nepal. clinicaltrials.gov/show/NCT01177111 (first received 6 August 2010).
NCT01364948 {published data only}
- NCT01364948. Effect of coconut oil application in reducing water loss from skin of premature babies in first week of life (TEWL) (TopOilTewl). clinicaltrials.gov/ct2/show/NCT01364948 (first received 3 June 2011).
NCT01396642 {published data only}
- NCT01396642. Topical emollient therapy. clinicaltrials.gov/ct2/show/NCT01396642 (first received 19 July 2011).
NCT01758068 {published data only}
- NCT01758068. Effect of twice daily application of coconut oil in reducing water loss from skin of premature babies in first week of life. clinicaltrials.gov/ct2/show/NCT01758068 (first received 31 December 2012).
NCT02120833 {published data only}
- NCT02120833. A test to determine the usefulness and safety of a cream used on babies with dry itchy skin. www.clinicaltrials.gov/ct2/show/NCT02120833 (first received 7 September 2015).
NCT02403999 {published data only}
- NCT02403999. A tolerability assessment study of three wash products in infants. clinicaltrials.gov/ct2/show/NCT02403999 (first received 31 March 2015).
NCT02404493 {published data only}
- NCT02404493. Test to determine the effectiveness of moisturizing balm used on babies with dry, itchy skin. clinicaltrials.gov/ct2/show/NCT02404493 (first received 31 March 2015).
NCT02557698 {published data only}
- NCT02557698. Effectiveness of using an oil bath additive. clinicaltrials.gov/ct2/show/NCT02557698 (first received 23 September 2015).
NCT02614248 {published data only}
- NCT02614248. The use of coconut oil for the prevention and treatment of diaper dermatitis in the NICU population. clinicaltrials.gov/ct2/show/NCT02614248 (first received 25 November 2015).
NCT02857062 {published data only}
- To evaluate the in use tolerance of E45 eczema repair emollient in babies and children with (very(dry/atopic skin. clinicaltrials.gov/ct2/show/NCT02857062 (first received 5 August 2016).
NCT03089476 {published data only}
- NCT03089476. Evaluating skin barrier dysfunction in infants at high risk of atopy. clinicaltrials.gov/ct2/show/NCT03089476 (first received 24 March 2017).
NCT03112876 {published data only}
- NCT03112876. Sebum collection and skin barrier function analysis (Sebum). clinicaltrials.gov/ct2/show/NCT03112876 (first received 13 April 2017).
NCT03143504 {published data only}
- NCT03143504. A longitudinal investigation of skin barrier development from birth and the validation of early predictors of atopic eczema risk: the Skin Testing for Atopic eczema Risk (STAR) Study (STAR). clinicaltrials.gov/ct2/show/NCT03143504 (first received 8 May 2017).
NCT03719742 {published data only}
- NCT03719742. A clinical study to evaluate the safety and efficacy of a baby cleanser and a moisturizer. clinicaltrials.gov/ct2/show/NCT03719742 (first received 25 October 2018).
NCT03738163 {published data only}
- NCT03738163. A clinical investigation with Epaderm® cream (PD-539878). clinicaltrials.gov/ct2/show/NCT03738163 (first received 13 November 2018).
NCT03742414 {published data only}
- NCT03742414. Seal, stopping atopic dermatitis and allergy study. clinicaltrials.gov/ct2/show/NCT03742414 (first received 15 November 2018).
NCT03813472 {published data only}
- NCT03813472. Hydration sensor for atopic dermatitis. clinicaltrials.gov/ct2/show/results/NCT03813472 (first received 23 January 2019).
NCT04001855 {published data only}
- NCT04001855. Evaluating the effect of bathing additives in atopic dermatitis. clinicaltrials.gov/ct2/show/NCT04001855 (first received 28 June 2019).
NCT04099602 {published data only}
- NCT04099602. The effect of massage on bilirubin level in infants. clinicaltrials.gov/ct2/show/NCT04099602 (first received 23 September 2019).
Nesmiyanov 2018 {published data only}
- Nesmiyanov P, Gutov M, Strygin A, Tolkachev B, Morkovin E, Dotsenko A. M. vaccae-based formulation for the primary prevention of atopic dermatitis. Allergy 2018;73:107. [DOI: 10.1111/all.13535] [DOI] [Google Scholar]
Nopper 1996 {published data only}
- Nopper AJ, Horii KA, Sookdeo-Drost S, Wang TH, Mancini AJ, Lane AT. Topical ointment therapy benefits premature infants. Journal of Pediatrics 1996;128(5 Pt 1):660‐9. [DOI: 10.1016/s0022-3476(96)80132-6] [DOI] [PubMed] [Google Scholar]
Noviello 2005 {published data only}
- Noviello MR. Effects after daily use of washing products on infants aged 0-52 weeks. Minerva Pediatrica 2005;57(6):411‐8. [PubMed] [Google Scholar]
Pupala 2017 {published data only}
- Pupala S, Rao S, Strunk T, Patole S. Topical application of coconut oil to the skin of preterm infants-a systematic review. Journal of Paediatrics and Child Health 2017;53 Suppl 2:82. [DOI] [PubMed] [Google Scholar]
Pupala 2018 {published data only}
- Pupala S, Strunk T, Patole S, Doherty D, Hibbert J. Topical coconut oil to improve skin condition in very preterm infants: a pilot randomised clinical trial. Journal of Paediatrics and Child Health 2018;54:103‐4. [DOI: 10.1111/jpc.13882_280] [DOI] [Google Scholar]
Pupala 2019 {published data only}
- Pupala SS, Rao S, Strunk T, Patole S. Topical application of coconut oil to the skin of preterm infants: a systematic review. European Journal of Pediatrics 2019;178(9):1317-24. [DOI] [PubMed] [Google Scholar]
Qiu 2008 {published data only}
- Qiu HM, Zhou L. The clinic effect of Chinese medicine bath therapy on 132 neonates with hyperbilirubinemia. Shaanxi Journal of Traditional Chinese Medicine 2008;29(11):1479‐80. [Google Scholar]
Quinn 2005 {published data only}
- Quinn D, Newton N, Piecuch R. Effect of less frequent bathing on premature infant skin. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2005;34(6):741‐6. [DOI: 10.1177/0884217505282021] [DOI] [PubMed] [Google Scholar]
RBR 93996y {published data only}
- RBR-93996y. Effect of liquid and bar soap in healthy term newborns. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-93996y (first received 13 July 2018).
Rehbinder 2018 {published data only}
- Rehbinder EM, Winger AJ, Landro L, Carlsen KH, Carlsen MH, Fatnes TA, et al. Is dry skin in infants 3 months of age associated with increased transepidermal water loss? British Journal of Dermatology 2018;179(1):e59. [DOI: 10.1111/bjd.16718] [DOI] [Google Scholar]
Rosenstock 2007 {published data only}
- Rosenstock. The impact of emollient therapy during the neonatal period on neurodevelopmental outcomes. Pediatric Academic Society 2007;8315:no pagination. [Google Scholar]
Sach 2019 {published data only}
- Sach TH, McManus E, Levell NJ. Understanding economic evidence for the prevention and treatment of atopic eczema. British Journal of Dermatology 2019;181(4):707-16. [DOI: 10.1111/bjd.17696] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salam 2013 {published data only}
- Salam RA, Das JK, Darmstadt GL, Bhutta ZA. Emollient therapy for preterm newborn infants - evidence from the developing world. BMC Public Health 2013;13 Suppl 3:S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salam 2015 {published data only}
- Salam RA, Darmstadt GL, Bhutta ZA. Effect of emollient therapy on clinical outcomes in preterm neonates in Pakistan: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(3):F210‐5. [DOI: 10.1136/archdischild-2014-307157] [DOI] [PubMed] [Google Scholar]
Sarkar 2010 {published data only}
- Sarkar R, Basu S, Agrawal RK, Gupta P. Skin care for the newborn. Indian Pediatrics 2010;47(7):593-8. [DOI] [PubMed] [Google Scholar]
Sawatzky 2016 {published data only}
- Sawatzky S, Schario M, Stroux A, Lunnemann L, Zuberbier T, Blume-Peytavi U, et al. Children with dry skin and atopic predisposition: outcome measurement with validated scores for atopic dermatitis. Skin Pharmacology & Physiology 2016;29(3):148-56. [DOI] [PubMed] [Google Scholar]
Solanki 2005 {published data only}
- Solanki K, Matnani M, Kale M, Joshi K, Bavdekar A, Bhave S, et al. Transcutaneous absorption of topically massaged oil in neonates. Indian Pediatrics 2005;42(10):998‐1005. [PubMed] [Google Scholar]
Soll 2000 {published data only}
- Soll RF, Edwards WH. Emollient ointment for preventing infection in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD001150. [DOI: 10.1002/14651858.CD001150] [DOI] [PubMed] [Google Scholar]
Soriano 2000 {published data only}
- Soriano CR, Martinez FE, Jorge SM. Cutaneous application of vegetable oil as a coadjutant in the nutritional management of preterm infants. Journal of Pediatric Gastroenterology and Nutrition 2000;31(4):387-90. [DOI] [PubMed] [Google Scholar]
Summers 2017 {published data only}
- Summers A, Visscher M, Khatry S, Sherchand J, LeClerq S, Katz J, et al. Neonatal skin integrity: impact of mustard seed versus sunflower oil massage among newborns in rural Nepal. Pediatric Dermatology 2017;34:S85. [DOI: 10.1111/pde.13195] [DOI] [Google Scholar]
TCTR20161209001 {published data only}
- TCTR20161209001. Efficacy of breast milk application on skin condition in preterm infants: a randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20161209001 (first received 9 December 2016).
Thomas 1979 {published data only}
- Thomas WR. The prevention of superficial infection in neonates. Journal of Antimicrobial Chemotherapy 1979;5(2):235‐6. [DOI: 10.1093/jac/5.2.235] [DOI] [PubMed] [Google Scholar]
Vaivre Douret 2009 {published data only}
- Vaivre-Douret L, Oriot D, Blossier P, Py A, Kasolter-Pere M, Zwang J. The effect of multimodal stimulation and cutaneous application of vegetable oils on neonatal development in preterm infants: a randomized controlled trial. Child: Care, Health & Development 2009;35(1):96-105. [DOI] [PubMed] [Google Scholar]
Visscher 2009 {published data only}
- Visscher M, Odio M, Taylor T, White T, Sargent S, Sluder L, et al. Skin care in the NICU patient: effects of wipes versus cloth and water on stratum corneum integrity. Neonatology 2009;96(4):226-34. [DOI] [PubMed] [Google Scholar]
Wananukul 2001 {published data only}
- Wananukul S, Praisuwanna P, Kesorncam K. Effects of clear topical ointment on transepidermal water loss in jaundiced preterm infants receiving phototherapy. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 2001;84(6):837‐41. [PubMed] [Google Scholar]
Wananukul 2002 {published data only}
- Wananukul S, Praisuwanna P. Clear topical ointment decreases transepidermal water loss in jaundiced preterm infants receiving phototherapy. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 2002;85(1):102‐6. [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang Y L, Tan P, Wu SY, Gao GE, Chen B. The effect of Chinese medicine bath therapy on the levels of neonatal bilirubin. China Pharmacist 2009;12(11):1622‐3. [Google Scholar]
Waserman 2016 {published data only}
- Waserman S. Doctor, can we prevent food allergy and eczema in our baby? Current Opinion in Allergy & Clinical Immunology 2016;16(3):265-71. [DOI] [PubMed] [Google Scholar]
Xatzopoulou 2010 {published data only}
- Xatzopoulou S, Liosis G, Giannouli O, Grigoriadou D, Kyriacou M. Olive oil as a potential prebiotic in infants' skin. Early Human Development 2010;1:S20. [Google Scholar]
Xiao 2009 {published data only}
- Xiao DM, Wu CL, Liu QL. Traditional Chinese medicine bath with the sun on the prevention of neonatal jaundice. Chinese Medicine Modern Distance Education of China 2009;7(3):19. [Google Scholar]
Yamamoto 1996 {published data only}
- Yamamoto K. Soaps and detergents in children. Clinics in Dermatology 1996;14(1):81-4. [DOI] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang SZ, Xu ZH, Zhao LH. Observation on the effect of Shule liniment for neonatal touch combined with acupoint massage in the treatment of neonatal rash. Guangming Journal of Chinese Medicine [Guang Ming Zhong Yi Za Zhi] 2016;31(1):63‐4. [Google Scholar]
References to studies awaiting assessment
ISRCTN38965585 {published data only}
- CTRI/2014/12/005282. Can an intervention involving improvements in community-based newborn massage practice with promotion of cold-pressed sunflower oil as preferred emollient improve newborn survival in rural North India? www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/12/005282 (first received 11 December 2014).
- ISRCTN38965585. Can an intervention involving improvements in community-based newborn massage practice with promotion of cold-pressed sunflower oil as preferred emollient improve newborn survival in rural North India? www.isrctn.com/ISRCTN38965585 (first received 8 July 2014). [DOI: 10.1186/ISRCTN38965585] [DOI]
JPRN‐UMIN000026877 {published data only}
- JPRN-UMIN000026877. Use test of skin care goods. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000026877 (first received 5 April 2017).
NCT03640897 {published data only}
- NCT03640897. Evaluation of the safety and performance of LiNiDERM® in the prevention of infant diaper rash. clinicaltrials.gov/ct2/show/NCT03640897 (first received 21 August 2018).
References to ongoing studies
Eichner 2020 {published data only}
- Eichner B, Michaels LAC, Branca K, Ramsey K, Mitchell J, Morris CD, et al. A Community-based Assessment of Skin Care, Allergies, and Eczema (CASCADE): an atopic dermatitis primary prevention study using emollients-protocol for a randomized controlled trial. Trials 2020;21(1):243. [DOI: 10.1186/s13063-020-4150-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03409367. A Community-based Assessment of Skin Care, Allergies, and Eczema (CASCADE). clinicaltrials.gov/ct2/show/NCT03409367 (first received 24 January 2018).
Jabbar‐Lopez 2019 {published data only}
- Jabbar-Lopez ZK, Gurung N, Greenblatt D, Briley A, Chalmers JR, Thomas KS, et al. Protocol for an outcome assessor-blinded pilot randomised controlled trial of an ion-exchange water softener for the prevention of atopic eczema in neonates, with an embedded mechanistic study: the Softened Water for Eczema Prevention (SOFTER) trial. BMJ Open 2019;9(8):e027168. [DOI: 10.1136/bmjopen-2018-027168] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03270566. Softened water for eczema prevention pilot trial. clinicaltrials.gov/ct2/show/NCT03270566 (first received 1 September 2017).
Lowe 2019 {published data only}
- ACTRN12617001380381. The PEBBLES Study - testing a strategy for preventing eczema and food allergy in high risk infants. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001380381 (first received 28 September 2017).
- Lowe A, Su J, Tang M, Lodge CJ, Matheson M, Allen KJ, et al. PEBBLES study protocol: a randomised controlled trial to prevent atopic dermatitis, food allergy and sensitisation in infants with a family history of allergic disease using a skin barrier improvement strategy. BMJ Open 2019;9(3):e024594. [DOI: 10.1136/bmjopen-2018-024594] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02906475 {published data only}
- NCT02906475. Atopic dermatitis in atopy predisposed infants. clinicaltrials.gov/ct2/show/NCT02906475 (first received 20 September 2016).
NCT03142984 {published data only}
- NCT03142984. UK BABY study using a baby wash and lotion regimen (BOND). clinicaltrials.gov/ct2/show/NCT03142984 (first received 8 May 2017).
NCT03808532 {published data only}
- NCT03808532. Moisturizer to prevent atopic dermatitis (ACE-AD). clinicaltrials.gov/ct2/show/NCT03808532 (first received 17 January 2019).
NCT03871998 {published data only}
- NCT03871998. Short-term topical application to prevent atopic dermatitis (STOP AD). clinicaltrials.gov/ct2/show/NCT03871998 (first received 12 March 2019).
NCT04398758 {unpublished data only}
- NCT04398758. Moisturizer mediated Prevention of symptoms of Atopic Dermatitis in early childhood (MOPAD). clinicaltrials.gov/ct2/show/NCT04398758 (first received 21 May 2020).
Additional references
Abuabara 2018
- Abuabara K, Yu AM, Okhovat JP, Allen IE, Langan SM. The prevalence of atopic dermatitis beyond childhood: a systematic review and meta-analysis of longitudinal studies. Allergy 2018;73(3):696-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abuabara 2019
- Abuabara K, Ye M, McCulloch CE, Sullivan A, Margolis DJ, Strachan DP, et al. Clinical onset of atopic eczema: results from 2 nationally representative British birth cohorts followed through midlife. Journal of Allergy and Clinical Immunology 2019;144(3):710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12613000472774
- ACTRN12613000472774. The PEBBLES study: prevention of eczema by a barrier lipid equilibrium strategy [Does twice daily application of a ceramide dominant cream (EpiCeram) for the first six months of life reduce the incidence of eczema by six months of age, when compared to standard skin care, in infants who have a family history of allergic disease]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364115 (first received 26 April 2013).
Amare 2015
- Amare Y, Shamba DD, Manzi F, Bee MH, Omotara BA, Iganus RB, et al. Current neonatal skin care practices in four African sites. Journal of Tropical Pediatrics 2015;61(6):428-34. [DOI: 10.1093/tropej/fmv053] [DOI] [PubMed] [Google Scholar]
Andrews 2013
- Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. Journal of Clinical Epidemiology 2013;66(7):726-35. [DOI: 10.1016/j.jclinepi.2013.02.003] [DOI] [PubMed] [Google Scholar]
Apfelbacher 2011
- Apfelbacher CJ, Diepgen TL, Schmitt J. Determinants of eczema: population-based cross-sectional study in Germany. Allergy 2011;66(2):206-13. [DOI] [PubMed] [Google Scholar]
Asai 2020
- Asai J, Martino D, Eiwegger T, Nadeau K, Koppelman GH, Clarke AE, et al. Phenotype consensus is required to enable large‐scale genetic consortium studies of food allergy. Allergy 2020;75:2383-7. [DOI: 10.1111/all.14333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asher 2006
- Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006;368(9537):733-43. [DOI] [PubMed] [Google Scholar]
Bai 2017
- Bai G, Herten MH, Landgraf JM, Korfage IJ, Raat H. Childhood chronic conditions and health-related quality of life: findings from a large population-based study. PloS One 2017;12(6):e0178539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbarot 2018
- Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 2018;73(6):1284-93. [DOI] [PubMed] [Google Scholar]
Blume‐Peytavi 2016
- Blume-Peytavi U, Lavender T, Jenerowicz D, Ryumina I, Stalder JF, Torrelo A, et al. Recommendations from a European roundtable meeting on best practice healthy infant skin care. Pediatric Dermatology 2016;33(3):311-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bock 1988
- Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. Journal of Allergy and Clinical Immunology 1988;82(6):986-97. [DOI] [PubMed] [Google Scholar]
Botha 2019
- Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, Ramjith J, et al. Rural and urban food allergy prevalence from the South African Food Allergy (SAFFA) study. Journal of Allergy & Clinical Immunology 2019;143(2):662-8. [DOI: 10.1016/j.jaci.2018.07.023] [DOI] [PubMed] [Google Scholar]
Boyce 2010
- Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. Journal of Allergy and Clinical Immunology 2010;126(6):S1-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2017
- Boyle R, Williams H, Askie L, Lodrup-Carlsen K, Montgomery A, Chalmers J, et al. Prospectively planned meta-analysis of skin barrier studies for the prevention of eczema and associated health conditions. www.crd.york.ac.uk/prospero/display_record.php?RecordID=56965 (first received 10 February 2017).
Carroll 2005
- Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatric Dermatology 2005;22(3):192-9. [DOI] [PubMed] [Google Scholar]
Caussin 2009
- Caussin J, Rozema E, Gooris GS, Wiechers JW, Pavel S, Bouwstra JA. Hydrophilic and lipophilic moisturizers have similar penetration profiles but different effects on SC water distribution in vivo. Experimental Dermatology 2009;18(11):954-61. [DOI] [PubMed] [Google Scholar]
Chalmers 2017
- Chalmers JR, Haines RH, Mitchell EJ, Thomas KS, Brown SJ, Ridd M, et al. Effectiveness and cost-effectiveness of daily all-over-body application of emollient during the first year of life for preventing atopic eczema in high-risk children (the BEEP trial): protocol for a randomised controlled trial. Trials 2017;18(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charman 2004
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Archives of Dermatology 2004;140(12):1513-9. [DOI] [PubMed] [Google Scholar]
Chiou 2004
- Chiou YB, Blume-Peytavi U. Stratum corneum maturation. A review of neonatal skin function. Skin Pharmacology & Physiology 2004;17(2):57-66. [DOI: 10.1159/000076015] [DOI] [PubMed] [Google Scholar]
COMFA 2020
- Core Outcome Measures for Food Allergy. www.cost.eu/actions/CA18227/#tabs|Name:overview (accessed prior to 16 September 2020).
Cook 2018
- Cook JA, MacLennan GS, Palmer T, Lois N, Emsley R. Instrumental variable methods for a binary outcome were used to informatively address noncompliance in a randomized trial in surgery. Journal of Clinical Epidemiology 2018;96:126-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cork 2002
- Cork MJ, Murphy R, Carr J, Buttle D, Ward S, Båvik C, et al. The rising prevalence of atopic eczema and environmental trauma to the skin. Dermatology in Practice 2002;10(3):22-6. [Google Scholar]
Cro 2020a
- Cro S, Boyle R, Kelleher M, Tran L, Cornelius V. Skin care interventions for preventing eczema and food allergy: a statistical analysis plan for a systematic review and individual participant data meta-analysis. zenodo.org/record/3610604#.XiGKU8j7SUk (accessed 16 January 2020). [DOI: 10.5281/zenodo.3610604] [DOI]
Cro 2020b
- Cro S, Kelleher M, Cornelius V, Boyle R. Skin care interventions for preventing eczema and food allergy: RoB 2 assessments (Version 1) [Data set]. Zenodo. [DOI: ]
Danby 2011
- Danby SG, Al-Enezi T, Sultan A, Chittock J, Kennedy K, Cork MJ. The effect of aqueous cream BP on the skin barrier in volunteers with a previous history of atopic dermatitis. British Journal of Dermatology 2011;165(2):329-34. [DOI] [PubMed] [Google Scholar]
Danby 2018
- Danby SG, Brown K, Wigley AM, Chittock J, Pyae PK, Flohr C, et al. The effect of water hardness on surfactant deposition after washing and subsequent skin irritation in atopic dermatitis patients and healthy control subjects. Journal of Investigative Dermatology 2018;138(1):68-77. [DOI] [PubMed] [Google Scholar]
DaSilva 2020
- De Silva D, Halken S, Singh C, Muraro A, Angier E, Arasi S, et al. Preventing food allergy in infancy and childhood: Systematic review of randomised controlled trials. Paediatric Allergy and Immunology 2020;0:1-14. [DOI: 10.1111/pai.13273] [DOI] [PubMed] [Google Scholar]
Deckers 2012
- Deckers IA, McLean S, Linssen S, Mommers M, Van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PloS One 2012;7(7):e39803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Drucker 2017
- Drucker AM, Wang AR, Li W-Q, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. Journal of Investigative Dermatology 2017;137(1):26-30. [DOI] [PubMed] [Google Scholar]
Du Toit 2015
- Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. New England Journal of Medicine 2015;372(9):803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eichenfield 2014
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: Part 2: Management and treatment of atopic dermatitis with topical therapies. Journal of the American Academy of Dermatology 2014;71(1):116-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eldridge 2016
- Eldridge S, Campbell M, Campbell M, Drahota A, Giraudeau B, Higgins J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. www.riskofbias.info/welcome/rob-2-0-tool/archive-rob-2-0-cluster-randomized-trials-2016 (accessed 14 October 2020).
Engebretsen 2017
- Engebretsen KA, Bager P, Wohlfahrt J, Skov L, Zachariae C, Nybo Andersen AM, et al. Prevalence of atopic dermatitis in infants by domestic water hardness and season of birth: cohort study. Journal of Allergy and Clinical Immunology 2017;139(5):1568-74.e1. [DOI] [PubMed] [Google Scholar]
Ewence 2011
- Ewence A, Rumsby P, Danby S, Cork MJ, Williams HC. A review of skin irritation and tap water. www.dwi.defra.gov.uk/research/completed-research/reports/dwi70-2-257.pdf (accessed prior to 24 January 2019).
Flohr 2010
- Flohr C, England K, Radulovic S, McLean WH, Campbell LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. British Journal of Dermatology 2010;163(6):1333-6. [DOI] [PubMed] [Google Scholar]
Flohr 2014
- Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014;69(1):3-16. [DOI] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Van Tulder M, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929-41. [DOI] [PubMed] [Google Scholar]
Garcia‐Larsen 2018
- Garcia-Larsen V, Ierodiakonou D, Jarrold K, Cunha S, Chivinge J, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PloS Medicine 2018;15(2):e1002507. [DOI: 10.1371/journal.pmed.1002507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grabenhenrich 2017
- Grabenhenrich LB, Reich A, Bellach J, Trendelenburg V, Sprikkelman AB, Roberts G, et al. A new framework for the documentation and interpretation of oral food challenges in population-based and clinical research. Allergy 2017;72(3):453-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2004
- Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clinical & Experimental Allergy 2004;34(4):520-6. [DOI] [PubMed] [Google Scholar]
Gupta 2013
- Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatrics 2013;167(11):1026-31. [DOI] [PubMed] [Google Scholar]
Gupta 2019
- Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and severity of food allergies among US adults. JAMA Network Open 2019;2(1):e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanifin 1980
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermato-Venereologica 1980;60 Suppl 92:44-7. [Google Scholar]
Hanifin 2001
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Experimental Dermatology 2001;10(1):11-8. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2018
- Higgins JP, Savovic J, Page MJ, Sterne JA, ROB2 development group. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed 5 February 2019).
Higgins 2020a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Higgins 2020b
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Ierodiakonou 2016
- Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA 2016;316(11):1181-92. [DOI: ] [DOI] [PubMed] [Google Scholar]
Irvin 2015
- Irvin EJ, Miller HD. Emollient use in the term newborn: a literature review. Neonatal Network 2015;34(4):227-30. [DOI] [PubMed] [Google Scholar]
Irvine 2011
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. New England Journal of Medicine 2011;365(14):1315-27. [DOI] [PubMed] [Google Scholar]
ISRCTN 21528841
- ISRCTN 21528841. Barrier enhancement for eczema prevention. www.isrctn.com/ISRCTN21528841 (first received 25 July 2014).
Jerschow 2014
- Jerschow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. Journal of Allergy and Clinical Immunology 2014;134(6):1318-28 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansson 2003
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization. Journal of Allergy and Clinical Immunology 2003;113(5):832-6. [DOI] [PubMed] [Google Scholar]
JPRN‐UMIN000004544
- JPRN-UMIN000004544. Effect of emollients on the prevention of infantile eczema and atopic dermatitis. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000005429 (first received 11 November 2010).
JPRN‐UMIN000010838
- JPRN-UMIN000010838. Skin care and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: a 2 x 2 factorial, randomized, non-treatment controlled trial. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000012665 (first received 30 May 2013).
JPRN‐UMIN000013260
- JPRN-UMIN000013260. Effects of moisturizing skin care from the neonatal stage for improving skin barrier function and preventing skin trouble. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014285 (first received 25 February 2014).
Kelleher 2016
- Kelleher MM, Dunn-Galvin A, Gray C, Murray DM, Kiely M, Kenny L, et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. Journal of Allergy and Clinical Immunology 2016;137(4):1111-6.e8. [DOI] [PubMed] [Google Scholar]
Kelleher 2020b
- Kelleher MM, Jay N, Perkin MR, Haines RH, Batt R, Bradshaw LE, et al. An algorithm for diagnosing IgE-mediated food allergy in study participants who do not undergo food challenge. Clinical and Experimental Allergy 2020;50:334-42. [DOI] [PubMed] [Google Scholar]
Knibb 2010
- Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy 2010;65:933-45. [DOI] [PubMed] [Google Scholar]
Leung 2004
- Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. Journal of Clinical Investigation 2004;113(5):651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2018
- Liu Q, Zhang Y, Danby SG, Cork MJ, Stamatas GN. Infant skin barrier, structure, and enzymatic activity differ from those of adult in an East Asian cohort. BioMed Research International 2018;12:1-8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lodén 2012
- Lodén M. Effect of moisturizers on epidermal barrier function. Clinics in Dermatology 2012;30(3):286-96. [DOI] [PubMed] [Google Scholar]
Luoma 1983
- Luoma R, Koivikko A, Viander M. Development of asthma, allergic rhinitis and atopic dermatitis by the age of five years. A prospective study of 543 newborns. Allergy 1983;38(5):339-46. [DOI] [PubMed] [Google Scholar]
Lødrup 2018
- Lødrup Carlsen KC, Rehbinder EM, Skjerven HO, Carlsen MH, Fatnes TA, Fugelli P, et al. Preventing atopic dermatitis and allergies in children—the PreventADALL study. Allergy 2018;73(10):2063-70. [DOI] [PubMed] [Google Scholar]
Mancini 2008
- Mancini A, Lawley L. Structure and function of newborn skin. In: Neonatal Dermatology. 2 edition. Philadelphia: Elsevier Saunders, 2008:19-31. [Google Scholar]
Martin 2013
- Martin PE, Koplin JJ, Eckert JK, Lowe AJ, Ponsonby AL, Osborne NJ, et al. The prevalence and socio-demographic risk factors of clinical eczema in infancy: a population-based observational study. Clinical and Experimental Allergy 2013;43(6):642-51. [DOI] [PubMed] [Google Scholar]
Martin 2015
- Martin PE, Eckert JK, Koplin JJ, Lowe AJ, Gurrin LC, Dharmage SC, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clinical and Experimental Allergy 2015;45(1):255-64. [DOI] [PubMed] [Google Scholar]
McAleer 2018
- McAleer MA, Jakasa I, Raj N, O'Donnell CP, Lane ME, Rawlings AV, et al. Early-life regional and temporal variation in filaggrin-derived natural moisturizing factor, filaggrin-processing enzyme activity, corneocyte phenotypes and plasmin activity: implications for atopic dermatitis. British Journal of Dermatology 2018;179(2):431-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meckfessel 2014
- Meckfessel MH, Brandt S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. Journal of the American Academy of Dermatology 2014;71(1):177-84. [DOI] [PubMed] [Google Scholar]
Munblit 2020
- Munblit D, Perkin MR, Palmer DJ, Allen KJ, Boyle RJ. Assessment of evidence about common infant symptoms and cow's milk allergy. JAMA Pediatrics 2020;174(6):599-608. [DOI: 10.1001/jamapediatrics.2020.0153] [PMID: ] [DOI] [PubMed] [Google Scholar]
Natsume 2017
- Natsume O, Kabashima S, Nakazato J, Yamamoto-Hanada K, Narita M, Kondo M, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet 2017;389(10066):276-86. [DOI: 10.1016/S0140-6736(16)31418-0] [DOI] [PubMed] [Google Scholar]
NCT01375205
- NCT01375205. Comparing cetaphil restoraderm system versus standard skin care in infants. clinicaltrials.gov/ct2/show/results/NCT01375205 (first received 17 June 2011).
NCT02449850
- NCT02449850. Preventing atopic dermatitis and allergies in children (PreventADALL). clinicaltrials.gov/ct2/show/NCT02449850 (first received 20 May 2015).
NICE 2006
- NICE. Postnatal care up to 8 weeks after birth. www.nice.org.uk/guidance/cg37 (accessed prior to 24 January 2019).
Nikolovski 2008
- Nikolovski J, Stamatas GN, Kollias N, Wiegand BC. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. Journal of Investigative Dermatology 2008;128(7):1728-36. [DOI: 10.1038/sj.jid.5701239] [DOI] [PubMed] [Google Scholar]
Oakley 2016
- Oakley R, Lawton S. Views on unwanted effects of leave-on emollients and experiences surrounding their incidence. Dermatological Nursing 2016;15(4):38-43. [Google Scholar]
Osbourne 2011
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. Journal of Allergy and Clinical Immunology 2011;127(3):668-76 e1-2. [DOI] [PubMed] [Google Scholar]
Palmer 2006
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature Genetics 2006;38(4):441-6. [DOI] [PubMed] [Google Scholar]
Pawankar 2014
- Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organization Journal 2014;7(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penzer 2012
- Penzer R. Best practice in emollient therapy: a statement for healthcare professionals December 2012. Dermatological Nursing 2012;11(4):60-79. [Google Scholar]
Perkin 2016
- Perkin K, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. New England Journal of Medicine 2016;374:1733-43. [DOI: 10.1056/NEJMoa1514210] [DOI] [PubMed] [Google Scholar]
Peters 2018
- Peters RL, Koplin JJ, Allen KJ, Lowe AJ, Lodge CJ, Tang ML, et al. The prevalence of food sensitization appears not to have changed between 2 Melbourne cohorts of high-risk infants recruited 15 years apart. Journal of Allergy & Clinical Immunology In Practice 2018;6(2):440-8. [DOI: 10.1016/j.jaip.2017.11.018] [DOI] [PubMed] [Google Scholar]
Poulos 2007
- Poulos LM, Waters A-M, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. Journal of Allergy and Clinical Immunology 2007;120(4):878-84. [DOI] [PubMed] [Google Scholar]
Prescott 2013
- Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn JKh, Fiocchi A, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organization Journal 2013;6(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramesh 2017
- Ramesh M, Lieberman J. Adult-onset food allergies. Annals of Allergy, Asthma & Immunology 2017;119(2):111-9. [DOI: 10.1016/j.anai.2017.05.014] [DOI] [PubMed] [Google Scholar]
Rawlings 2004
- Rawlings AV, Canestrari DA, Dobkowski B. Moisturizer technology versus clinical performance. Dermatologic Therapy 2004;17(Suppl 1):49-56. [DOI] [PubMed] [Google Scholar]
Rendell 2011
- Rendell ME, Baig-Lewis SF, Berry TM, Denny ME, Simpson BM, Brown PA, et al. Do early skin care practices alter the risk of atopic dermatitis? A case-control study. Pediatric Dermatology 2011;28(5):593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ring 2012
- Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. Journal of the European Academy of Dermatology and Venereology 2012;26(8):1045-60. [DOI] [PubMed] [Google Scholar]
Ronnstad 2018
- Rønnstad AT, Halling-Overgaard A-S, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. Journal of the American Academy of Dermatology 2018;79(3):448-56.e30. [DOI] [PubMed] [Google Scholar]
Rucker 2008
- Rucker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Statistics in Medicine 2008;27(5):746-63. [DOI] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann 20, Higgins JPT, Vist GE, Glasziou et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. [Google Scholar]
Sicherer 2003
- Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. Journal of Allergy and Clinical Immunology 2003;112(6):1203-7. [DOI] [PubMed] [Google Scholar]
Simpson 2012
- Simpson EL, Keck LE, Chalmers JR, Williams HC. How should an incident case of atopic dermatitis be defined? A systematic review of primary prevention studies. Journal of Allergy and Clinical Immunology 2012;130(1):137-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spergel 2003
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. Journal of Allergy and Clinical Immunology 2003;112(6 Suppl):S118-27. [DOI] [PubMed] [Google Scholar]
Stamatas 2010
- Stamatas GN, Nikolovski J, Luedtke MA, Kollias N, Wiegand BC. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatric Dermatology 2010;27(2):125-31. [DOI: 10.1111/j.1525-1470.2009.00973.x] [DOI] [PubMed] [Google Scholar]
Stamatas 2011
- Stamatas GN, Nikolovski J, Mack MC, Kollias N. Infant skin physiology and development during the first years of life: a review of recent findings based on in vivo studies. International Journal of Cosmetic Science 2011;33(1):17-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stata [Computer program]
- Stata. Version 15. College Station, TX, USA: StataCorp, 2017. Available at www.stata.com.
Stewart 2002
- Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Evaluation & the Health Professions 2002;25(1):76-97. [DOI: 10.1177/0163278702025001006] [DOI] [PubMed] [Google Scholar]
Stewart 2019
- Tierney JF, Stewart LA, Clarke M. Chapter 26, Individual patient data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Stone 2002
- Stone KD. Atopic diseases of childhood. Current Opinion in Pediatrics 2002;145(5):634-46. [DOI] [PubMed] [Google Scholar]
Strid 2004
- Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. European Journal of Immunology 2004;34(8):2100-9. [DOI] [PubMed] [Google Scholar]
Strid 2005
- Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clinical and Experimental Allergy 2005;35(6):757-66. [DOI] [PubMed] [Google Scholar]
Su 1997
- Su JC, Kemp AS, Varigos GA, Nolan TM. Atopic eczema: its impact on the family and financial cost. Archives of Disease in Childhood 1997;76(2):159-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Surber 2017
- Surber C, Kottner J. Skin care products: what do they promise, what do they deliver. Journal of Tissue Viability 2017;26(1):29-36. [DOI] [PubMed] [Google Scholar]
Taichman 2017
- Taichman DB, Sahni P, Pinborg A, Peiperl L, Laine C, James A, et al. Data sharing statements for clinical trials — a requirement of the International Committee of Medical Journal Editors. New England Journal of Medicine 2017;376(23):2277-9. [DOI] [PubMed] [Google Scholar]
Thomsen 2015
- Thomsen SF. Epidemiology and natural history of atopic diseases. European Clinical Respiratory Journal 2015;2(1):24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsakok 2016
- Tsakok T, Marrs T, Mohsin M, Baron S, du Toit G, Till S, et al. Does atopic dermatitis cause food allergy? A systematic review. Journal of Allergy and Clinical Immunology 2016;137(4):1071-8. [DOI] [PubMed] [Google Scholar]
Tsilochristou 2019
- Tsilochristou O, du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, et al. Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. Journal of Allergy & Clinical Immunology 2019;144(2):494-503. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2015
- Turner PJ, Gowland MH, Sharma V, Ierodiakonou D, Harper N, Garcez T, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. Journal of Allergy and Clinical Immunology 2015;135(4):956-63.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Umasunthar 2013
- Umasunthar T, Leonardi-Bee J, Hodes M, Turner PJ, Gore C, Habibi P, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clinical and Experimental Allergy 2013;43(12):1333-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
UMIN000028043
- UMIN000028043. Early aggressive intervention for infantile atopic dermatitis to prevent development of food allergy: a multicenter, investigator-blinded, randomized, parallel group controlled trial. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031912 (first received 4 July 2017).
Van Cleave 2010
- Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA 2010;303(7):623-30. [DOI] [PubMed] [Google Scholar]
Van den Oord 2009
- Van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ 2009;339:b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Zuuren 2017
- Van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen AP, Arents BW. Emollients and moisturisers for eczema. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD012119. [DOI: 10.1002/14651858.CD012119.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venter 2008
- Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy 2008;63(3):354-9. [DOI] [PubMed] [Google Scholar]
Visscher 2017
- Visscher MO, Burkes SA, Adams DM, Hammill AM, Wickett RR. Infant skin maturation: Preliminary outcomes for color and biomechanical properties. Skin Research & Technology 2017;23(4):545-51. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Voorheis 2019
- Voorheis P, Bell S, Cornelsen L. Challenges experienced with early introduction and sustained consumption of allergenic foods in the Enquiring About Tolerance (EAT) study: A qualitative analysis. Journal of Allergy & Clinical Immunology 2019;144(6):1615-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Voskamp 2014
- Voskamp AL, Zubrinich CM, Abramovitch JB, Rolland JM, O’Hehir RE. Goat's cheese anaphylaxis after cutaneous sensitization by moisturizer that contained goat's milk. Journal of Allergy and Clinical Immunology 2014;2(5):629-30. [DOI] [PubMed] [Google Scholar]
Weidinger 2016
- Weidinger S, Novak N. Atopic Dermatitis. Lancet 2016;387(10023):1109-22. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI] [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. Pregnancy, childbirth, postpartum and newborn care: a guide for essential practice. www.ncbi.nlm.nih.gov/books/NBK326678/ (accessed prior to 11 March 2019). [PubMed]
Williams 1994
- Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The UK Working Party's diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. British Journal of Dermatology 1994;131(3):383-96. [DOI] [PubMed] [Google Scholar]
Williams 1998
- Williams HC, Strachan DP. The natural history of childhood eczema: observations from the British 1958 birth cohort study. British Journal of Dermatology 1998;139(5):834-9. [DOI] [PubMed] [Google Scholar]
Woods 2002
- Woods RK, Stoney RM, Raven J, Walters EH, Abramson M, Thien FC. Reported adverse food reactions overestimate true food allergy in the community. European Journal Of Clinical Nutrition 2002;56(1):31-6. [DOI] [PubMed] [Google Scholar]
Yosipovitch 2000
- Yosipovitch G, Maayan-Metzger A, Merlob P, Sirota L. Skin barrier properties in different body areas in neonates. Pediatrics 2000;106(1):105-8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kelleher 2020
- Kelleher MM, Cro S, Cornelius V, Axon E, Lodrup Carlsen KC, Skjerven HO. Skincare interventions in infants for preventing eczema and food allergy. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD013534. [DOI: 10.1002/14651858.CD013534] [DOI] [PMC free article] [PubMed] [Google Scholar]