Abstract
Wildfire smoke is a growing public health concern in the United States. Numerous studies have documented associations between ambient smoke exposure and severe patient outcomes for single‐fire seasons or limited geographic regions. However, there are few national‐scale health studies of wildfire smoke in the United States, few studies investigating Intensive Care Unit (ICU) admissions as an outcome, and few specifically framed around hospital operations. This study retrospectively examined the associations between ambient wildfire‐related PM2.5 at a hospital ZIP code with total hospital ICU admissions using a national‐scale hospitalization data set. Wildfire smoke was characterized using a combination of kriged PM2.5 monitor observations and satellite‐derived plume polygons from National Oceanic and Atmospheric Administration's Hazard Mapping System. ICU admissions data were acquired from Premier, Inc. and encompass 15%–20% of all U.S. ICU admissions during the study period. Associations were estimated using a distributed‐lag conditional Poisson model under a time‐stratified case‐crossover design. We found that a 10 μg/m3 increase in daily wildfire PM2.5 was associated with a 2.7% (95% CI: 1.3, 4.1; p = 0.00018) increase in ICU admissions 5 days later. Under stratification, positive associations were found among patients aged 0–20 and 60+, patients living in the Midwest Census Region, patients admitted in the years 2013–2015, and non‐Black patients, though other results were mixed. Following a simulated severe 7‐day 120 μg/m3 smoke event, our results predict ICU bed utilization peaking at 131% (95% CI: 43, 239; p < 10−5) over baseline. Our work suggests that hospitals may need to preposition vital critical care resources when severe smoke events are forecast.
Keywords: critical care, Intensive Care Unit, particulate matter, smoke, wildfire
Key Points
Associations between smoke PM2.5 and ICU admissions were estimated using a national‐scale cohort
Smoke PM2.5 was associated with modest changes in ICU admissions
A simulated smoke wave was found to impose severe strain on ICU resources
1. Introduction
The frequency of and acreage burned by large wildfires in the western United States (U.S.) and Alaska has increased since the 1980s and is projected to further increase under the influence of climate change (Abatzoglou & Williams, 2016; Dennison et al., 2014; Ford et al., 2018; Luber et al., 2014; Westerling, 2016; Westerling et al., 2006; Wuebbles et al., 2017) under both low and high emission scenarios (Brey et al., 2021; Stavros et al., 2014; Yue et al., 2013). Each year, an average of 339,000 premature deaths worldwide are estimated to result from wildfire smoke (F. H. Johnston et al., 2012), and emissions of smoke are projected to double by the end of the century in California (Hurteau et al., 2014) as well as Idaho, the southeast, and the Great Lakes region of the U.S. (Ford et al., 2018). Simultaneously, the number of people who experience adverse health effects from wildfires will also likely increase over the course of the century (Liu et al., 2017; Smith et al., 2014). For example, while 0.70% of total deaths were attributable to fire‐related PM2.5 during the early 21st century, that percentage increases to 1.1% and 1.8% by the end of the century under moderate (RCP4.5) and high (RCP8.5) emissions scenarios, respectively (Ford et al., 2018).
Wildfire emissions are a significant contributor to ambient particulate matter (PM) and gas‐phase air pollutants (McClure & Jaffe, 2018; Seager & Vecchi, 2010), with annual yearly emissions of wildfire PM2.5 (airborne PM with aerometric diameter less than 2.5 µm) ranging between 118,000 and 986,000 metric tons (French et al., 2016). The gas‐phase emissions include ozone precursors, which have been shown to lead to local enhancements in ozone far from the source of burning (Brey & Fischer, 2016; Dreessen et al., 2016; Jaffe & Wigder, 2012). Additionally, wildfire smoke contains a mixture of particle‐ and vapor‐phase chemical species that are relevant to human health, determined by factors unique to the environment burned and burn conditions (O'Dell et al., 2020; Urbanski, 2013). Examples include gas‐phase hazardous air pollutants (O'Dell et al., 2020), polycyclic aromatic hydrocarbons, known to have negative health effects in animal toxicity studies (Black et al., 2017), pesticides, herbicides, fire‐retardants, and other human‐made products, many with unknown toxicologic profiles under high‐heat conditions (Carratt et al., 2017; Romagnoli et al., 2014).
Evidence to date demonstrates that exposure to wildfire smoke has direct effects on human health (Liu et al., 2015; Reid et al., 2016). Firefighters and residents in the surrounding areas are at risk of burns, injuries, mental health effects, and death due to exposure to flames or radiant heat (Finlay et al., 2012). Simultaneously, heavy ambient smoke can lead to visual impairment and increase the risk of motor vehicle accidents (Finlay et al., 2012). Wildfire smoke can worsen ambient air pollution as far as 1,000 km from the fire. Associated short‐ and long‐term negative health effects have been found to be worse than comparable increases in ambient air pollution from purely urban sources (DeFlorio‐Barker et al., 2019). Inhaled particles can react with neural receptors resulting in alterations of the autonomic nervous system. Exposures can likewise generate oxidative stress in alveolar‐capillary cells, resulting in local and systemic inflammation and can cross the alveolar membrane, resulting in endothelial injury and prothrombic changes to blood proteins (Newby et al., 2015). Ultimately, these pathophysiologic changes can aggravate normal cardiopulmonary function, exacerbate disease, and lead to premature death in vulnerable populations (Bell et al., 2018; Jerrett et al., 2005). Previous epidemiologic studies have found consistent evidence of negative respiratory and cardiopulmonary health effects (Black et al., 2017; DeFlorio‐Barker et al., 2019; Gan et al., 2017, 2020; Lipner et al., 2019; Rappold et al., 2011; Reid et al., 2016; Wettstein et al., 2018) and increases in all‐cause mortality (Cascio, 2018; Faustini et al., 2015; F. Johnston et al., 2011; Linares et al., 2015) associated with wildfire smoke exposure. The literature supporting associations between smoke exposure and cardiovascular (Faustini et al., 2015; F. Johnston et al., 2011; Morgan et al., 2010; Nunes et al., 2013) and cerebrovascular disease (F. Johnston et al., 2011; Morgan et al., 2010; Wettstein et al., 2018) is less conclusive at this time, potentially due to the heterogeneity of wildfire smoke, the demographics of the population exposed, and/or the air quality variable investigated (Black et al., 2017).
What is clear is that wildfires result in regional increases in healthcare utilization (Black et al., 2017) and expenditures (Fann et al., 2018; Knowlton et al., 2011; Parthum et al., 2017; Rappold et al., 2014). Regional smoke exposure has been shown to increase emergency rooms visits (Alman et al., 2016; Dohrenwend et al., 2013; Haikerwal et al., 2015; Wettstein et al., 2018), hospitalizations (DeFlorio‐Barker et al., 2019; Henderson et al., 2011; Liu et al., 2017; Martin et al., 2013; Reid et al., 2016), and outpatient physician visits (Gan et al., 2020; Henderson et al., 2011; Yao et al., 2016). Wildfire smoke can travel great distances and affect communities and hospitals thousands of miles downwind (Brey et al., 2018; Sapkota et al., 2005; Val Martin et al., 2013), surging demand for emergent health services (Hoyt & Gerhart, 2004) in regions far from its origin.
The specific impact of wildfires on the demand for critical care services is currently unknown; however, studies suggest that regional care protocols are needed to improve the ability of surrounding hospitals to handle surges in patient loads (R. Xu et al., 2020). Intensive Care Units (ICUs) care for the most seriously ill patients and require copious resources, uniquely trained staff, and use of specialized equipment such as mechanical ventilators and dialysis machines. Unexpected spikes in demand for these limited resources may divert resources from other critically ill patients, resulting in shortages of essential personnel, supplies, and equipment, leading to cascading detrimental effects on patient care. To quantify this impact, we investigated the association between wildfire‐related PM2.5 at a hospital's ZIP code and ICU admissions at that hospital, then predict the utilization surge associated with a hypothetical week‐long severe smoke episode. By employing data sets that cover large geographic ranges and time periods, we sought to account for the heterogeneity of wildfire smoke composition and community level factors that limit single‐fire studies. Such information would assist regional public health officials, hospital managers, and emergency planners to predict and prepare for surges in demand for critical care resources that may accompany wildfire smoke exposure using real‐time, readily available air quality information.
2. Methods
2.1. Intensive Care Unit Data
Data on ICU admissions in the years 2006–2015 were acquired from a national‐scale proprietary database owned by Premier, Inc., which comprises approximately 15%–20% of all hospital stays in the U.S. during our study period. Details on the database can be found in the company's white paper (Premier Applied Sciences® PI, 2020). Premier's partner hospitals range in size from <100 beds (27% of all hospitals) to >500 beds (13%). A substantial minority are rural (27%) and/or teaching (29%) hospitals. Thirty‐two percent of patients in the database are covered by Medicare, 19% by Medicaid, and 6% are covered by charity, workers' compensation claims, or indigent care. However, for privacy reasons, we were not able to access detailed information on individual partner hospitals or patients. Similar ICU data from Premier were used in a recent epidemiology study of dust storms (Rublee et al., 2020).
The data provided by Premier included admissions to general, surgical, medical, cardiovascular, and coronary ICUs, including both isolation and nonisolation ICUs of these types. The data did not include neonatal, transplant, psychiatric, burn, and trauma ICUs. Pediatric ICUs other than neonatal ICUs were not included either, though these tend to be found only at specialized children's hospitals. Thus, the data set acquired from Premier included pediatric patients admitted to other types of ICUs, which tend not to be age specific.
Premier provided researchers with counts of ICU stays categorized by hospital admission day and hospital ZIP code, with accompanying ICU counts representing different races, genders, and age groups. Additionally, researchers were provided with patient counts broken down by U.S. census region and year.
2.2. Environmental Data
2.2.1. Determining Wildfire‐Related PM2.5
The understanding of the health impact of wildfire‐related air pollution is hindered by the challenge of estimating exposure to air pollution specifically attributable to wildfires (Liu et al., 2017). Therefore, to characterize wildfire smoke PM2.5 levels at each hospital's ZIP code, we combined satellite‐derived smoke plume polygons from the National Oceanic and Atmospheric Administration's (NOAA) Hazard Mapping System (HMS) (Ruminski et al., 2006; Schroeder et al., 2008) with ambient daily PM2.5 monitoring from the U.S. Environmental Protection Agency (EPA) Air Quality System (AQS) using methods previously described (Lassman et al., 2017; O'Dell et al., 2019) and used in epidemiology studies (Abdo et al., 2019; Lipner et al., 2019). This technique creates a continuous PM2.5 map with approximately 15 × 15 km horizontal grid spacing for each day across the U.S. for the years 2005–2015.
In order to differentiate smoke PM2.5 from other nonfire‐related sources of particulate air pollution, we undertook the following multistep approach. First, we kriged the full set of daily PM2.5 monitor observations to the same grid to get a daily estimate of total PM2.5 at each grid cell. Next, a seasonal nonsmoke background was calculated as the median of nonsmoke PM2.5 estimates (defined as days and grid cells without an overlapping HMS smoke plume) within each season and grid cell. (The seasons were defined as follows: winter: December–February; spring: March–May; summer: June–August; fall: September–November). Then, we subtracted off the seasonal nonsmoke background PM2.5 concentrations from each daily total PM2.5 concentration (setting negative differences to zero) to compute our estimate of the daily component of PM2.5 contributed by wildfire smoke. The remaining component of daily PM2.5 (equal to the seasonal background except where the background was higher than the full daily PM2.5 concentration) was then defined to be the daily nonsmoke PM2.5 concentration. Thus, the daily smoke and nonsmoke PM2.5 concentrations at each grid cell always summed to the total PM2.5 mass concentration at that grid cell.
From the resulting daily concentration grids for smoke PM2.5 and nonsmoke PM2.5 covering the contiguous U.S., we used population‐weighted daily means to estimate the pollutant concentrations at each ZIP code for which a hospital in our cohort was located. Specifically, we took the mean of the PM2.5 concentration in the grid cells overlapping that ZIP code, weighted by both the population of each grid cell and the amount of areal overlap between the grid cell and the ZIP code.
2.2.2. Ozone and PM10
Wildfire smoke contains PM10 (though the PM10/PM2.5 ratio tends to drop the further the plume travels from the fire) as well as nitrogen oxides and volatile organic compounds that can affect downwind ozone formation (Brey & Fischer, 2016; DeBell et al., 2004; Juancosa Calahorrano et al., 2021; Lindaas et al., 2017). However, the present work is focused on the smoke component of PM2.5 as the primary exposure due to that pollutant's greater impact on human health. That said, PM10 and ozone may confound our primary smoke PM2.5 association because these copollutants are correlated with smoke PM2.5 in our data set, are known to affect health, and are causally influenced by wildfires (along with many other factors). Therefore, to reduce bias, we include PM10 and ozone in our primary epidemiologic models, though we also present sensitivity analyses without these variables. Future work will characterize the wildfire‐specific contribution to these other pollutants.
Daily ambient 8‐h maximum ozone mixing ratios and 24‐h mean PM10 (PM with aerometric diameter <10 μm) concentrations were obtained from the U.S. EPA AQS ground‐based monitor system. Daily ozone mixing ratios and PM10 concentrations at each hospital ZIP code were calculated by taking the median mixing values from those monitors located either within the hospital ZIP code boundary or within a 30‐km buffer around the ZIP code centroid. Hospital ZIP codes that did not contain an ozone or PM10 monitor and were not located within 30 km of an ozone or PM10 monitor were assigned a missing value for that variable. These ZIP codes were dropped from regression models in which ozone or PM10 served as a predictor. Alternative buffer distances were explored in a sensitivity analysis.
2.2.3. Meteorological Data
Daily meteorological variables, such as temperature, have been shown to impact health conditions that may then impact ICU admissions (Gronlund et al., 2014; Mares, 2013; Z. Xu et al., 2018). Meteorological data were obtained from the NOAA National Climate Data Center (ftp://ftp.ncdc.noaa.gov/pub/data/). Daily mean temperature and dew point temperature at each hospital ZIP code were calculated by taking the median value from those monitors located either within the ZIP code boundary or within a 30‐km buffer around the ZIP code centroid. Hospital ZIP codes that did not contain and were not located within 30 km of a weather monitor were assigned a missing value for mean temperature and dew point temperature and were dropped from those regression models including meteorological predictors. Alternative buffer distances were explored in a sensitivity analysis.
2.3. Statistical Methods
2.3.1. Exposure Data Preparation
Daily air pollution and meteorological exposure data were determined at each ZIP code on each day of our study period. Lagged values of these variables out to lag Day 5 were also included. Within each ZIP code, dates were categorized by quarter (Q1 2006 = January–March 2006, Q2 2006 = April–June 2006, etc.) to facilitate the creation of the time‐stratified case‐crossover strata below. However, due to the large number of ZIP code‐quarter combinations with little or no smoke, only those ZIP code‐quarters that included at least 1 day with smoke PM2.5 above 15 μg/m3 were retained.
Within each ZIP code‐quarter, all days with nonzero smoke PM2.5 were considered smoke days, and all days falling in 28‐day increments before or after a smoke day (but still in the same quarter) were considered referent days. Thus, each smoke day had 2 or 3 referent days. The 28‐day increment was selected to give adequate time for a patient to return to the risk pool and for smoke events to pass (Gan et al., 2017).
Only smoke days, referent days, and the 5 days following each could contribute to estimating the main association under the conditional Poisson model for wildfire smoke. Thus, only this set of ZIP code‐days was forwarded to Premier, Inc. for merging with ICU data.
2.3.2. Exposure and ICU Data Merging
ICU admission counts and exposure data were merged by Premier, Inc. by hospital ZIP code and date, then returned to the researchers. To protect patient and healthcare provider privacy, individual patient and hospital identifiers were withheld from the researchers. Hospital location (except U.S. census region) and patient residence location were also withheld, and the date was reported only as the relative date within the ZIP code‐quarter (i.e., the day number counting first day of the quarter). The identifiers for the ZIP code‐quarters were also randomized and ZIP code was deleted. Merged, deidentified data sets are available at https://osf.io/62dvr/ (Crooks, 2021).
2.3.3. Case‐Crossover Stratification
A stratum was defined as the set of days in a ZIP code‐quarter spaced 28 days apart containing at least 1 (potentially lagged) smoke day. Thus, a ZIP code‐quarter with a single smoke day would yield six strata, one for each lag day (lags 0–5) and its respective referent days. For example, a ZIP code‐quarter with a single smoke day on July 29 would yield one stratum consisting of the smoke day and referent days July 1, August 26, and September 23; a second stratum consisting of the lag 1 smoke day July 30 and referent days July 2, August 27, and September 24; and, similarly, a stratum for each of the other 4 lag days. Moreover, a ZIP code‐quarter with multiple smoke days could have more than six strata, up to a maximum of 28. This stratification approach automatically controlled for day‐of‐week as well as time‐invariant confounders such as hospital location or individual patient demographic features.
2.3.4. Primary Statistical Analysis
We used distributed‐lag conditional Poisson models under a time‐stratified case‐crossover design to estimate associations between ICU stays and ambient smoke PM2.5 at the hospital ZIP Code. The conditional Poisson models controlled for daily mean temperature, dew point temperature, and copollutant concentrations (ozone, PM10, and nonsmoke PM2.5). To estimate the potentially delayed impact of ambient smoke on ICU stays, smoke PM2.5 and copollutant concentrations were modeled using a distributed‐lag form with daily lag parameters ranging from 0 to 5 days (lags 0–5). These individual single‐day associations were then averaged to find the mean associations over lags 0–2, lags 3–5, and lags 0–5. The nonlinear and potentially delayed relationship between temperature and ICU risk was modeled using a spline with three degrees of freedom on daily mean temperature on both the day of exposure (lag 0) and the day prior (lag 1) to a given ICU stay. The additional impact of dew point temperature on risk on the day of the ICU admission was also accounted for using a spline with three degrees of freedom. Residual seasonal trends in ICU admissions occurring on time scales less than 3 months were modeled using a spline with eight degrees of freedom over the calendar year.
All analyses were performed in R version 3.3.2 (R Core Team, 2017). Conditional Poisson models (Armstrong et al., 2014) were fitted using the gnm function in the gnm package (Turner & Firth, 2020). Associations were considered statistically significant if their 95% confidence interval failed to include the null value.
2.3.5. Stratified Analysis
To investigate whether our results varied between demographic groups, hospital locations, or time periods, we ran five additional models, each of which included category‐specific smoke PM2.5 parameters. These five models stratified by age group (0–20, 21–40, 41–60, 61–80, and 80+), race (Caucasian, African American, and Other), gender (Female, Male), U.S. census region (Midwest, South, and West) (U.S. Census Bureau, 2013), and time period (2006–2009, 2010–2012, and 2013–2015). The Other race category included individuals who identified as Hispanic (included as a race category until June 30, 2011), American Indian or Native Alaskan, and Asian/Pacific Islander (which are 2010 U.S. census race categories), and those whose race was unable to be determined.
2.3.6. Sensitivity Analysis
Two sensitivity analyses were performed. First, we varied the buffer distance that was used to define which ozone, PM10, and meteorological monitors were collected to estimate median values at the hospital ZIP code. Higher buffer distances led to calculating the median over a larger number of monitors, which yields fewer missing values (and thus more complete cases) but potentially greater exposure misclassification, and vice versa. We tested alternative buffer distances of 15, 20, and 50 km in addition to the main 30‐km buffer. Second, to understand the robustness of our results to our modeling choices, we varied the covariate model by dropping the copollutants (nonsmoke PM2.5, ozone, and PM10), the nonlinear time‐of‐year trend, or both. This yielded seven confounder models in addition to our main model that included the time‐of‐year‐trend and all three copollutants.
2.3.7. Simulation of a Smoke Wave Event
We simulated the impact of a week‐long extreme smoke event on ICU admissions and ICU bed utilization. We assumed wildfire smoke concentrations of 120 μg/m3 on each smoke day (Days 1–7), which is characteristic of the recent extreme smoke waves in the U.S. (e.g., northern California in September 2020). We then predicted the percent increase in daily ICU admissions above baseline using our main distributed‐lag model results out to Day 12, 5 days after the end of the smoke wave.
To estimate the impact on overall ICU bed utilization, we then assumed that the length of each ICU stay was negative binomially distributed with a mean of 3.4 days and a size parameter of 1.306. The parameters of the distribution were chosen to exactly match the mean and median and closely match the tail quantiles of the ICU stay lengths reported in the literature for U.S. Medicare patients (Moitra et al., 2016). Given the estimated increase in ICU admissions on each day and 100,000 replicates from the distribution of ICU stay lengths, we simulated the percent increase over baseline in the number of ICU beds in use out through Day 15. As a sensitivity analysis, we reran the simulation replacing the daily distributed‐lag results with the 3‐ and 6‐day averages of the associations over lags 0–2, 3–5, and 0–5.
3. Results
3.1. Demographics
Our data set included 309,293 ICU stays between 2006 and 2015 in the continental U.S., representing four major U.S. census divisions and including patients of all ages. The descriptive statistics of our study population are summarized in Table 1. The majority of ICU stays represented patients visiting hospitals in the Midwest region (35.6%), with only 11.1% from the West region, which has had the most severe smoke episodes. This imbalance is in part due to the fact that only 18% of Premier, Inc.'s provider partners are located in the West region (Premier, 2018). Large shifts in the number of ICU admissions by year reflects both year‐to‐year differences in wildfire intensity and year‐to‐year changes in Premier's healthcare provider partners. The small number of patients aged 0–20 is due to the exclusion of neonatal and pediatric ICU admissions from our sample.
Table 1.
Descriptive Statistics of the Study Population
| Group | N | % | |
|---|---|---|---|
| Total ICU stays | 730,222 | 100 | |
| Age group | 0–20 | 15,728 | 3.2 |
| 21–40 | 67,247 | 13.2 | |
| 41–60 | 202,738 | 33.8 | |
| 61–80 | 320,289 | 38.3 | |
| 81+ | 124,220 | 11.4 | |
| Gender | Female | 341,590 | 63.1 |
| Male | 388,632 | 36.9 | |
| Race | Caucasian | 483,543 | 59.6 |
| African American | 100,296 | 20.4 | |
| Other | 146,383 | 20.1 | |
| Census region | West | 80,515 | 11.1 |
| Midwest | 260,302 | 35.6 | |
| South | 208,026 | 28.5 | |
| Northeast | 181,379 | 24.8 | |
| Year | 2006 | 35,976 | 4.9 |
| 2007 | 128,229 | 17.6 | |
| 2008 | 63,513 | 8.7 | |
| 2009 | 25,174 | 3.4 | |
| 2010 | 53,881 | 7.4 | |
| 2011 | 148,728 | 20.4 | |
| 2012 | 83,394 | 11.4 | |
| 2013 | 61,437 | 8.4 | |
| 2014 | 20,198 | 2.8 | |
| 2015 | 109,692 | 15.0 |
3.2. Meteorology and Air Pollution
Table 2 summarizes the average meteorological conditions and exposures to smoke PM2.5, nonsmoke PM2.5, ozone, and PM10 at study hospital ZIP codes weighted by the number of ICU stays at the reference ZIP code. Smoke PM2.5 ranged as high as 123 μg/m3, while nonsmoke PM2.5 ranged as high as 132 μg/m3. Ozone and PM10 had maximum values of 123 ppb and 589 μg/m3, respectively. Pearson correlations between these variables are shown in Table S1. All correlations are below 0.45 except that between mean temperature and dew point temperature, which has a correlation of 0.766. All correlations have p‐values less than 10−10 except the correlation between PM10 and dew point temperature, which has a p‐value of 0.0031.
Table 2.
Summary Statistics for Meteorological Conditions and Air Pollution Exposures at Hospital ZIP Codes Weighted by the Number of ICU Admissions in That ZIP Code
| Variable | Mean | SD | Min | Max | 2.5% | 25% | 50% | 75% | 97.5% |
|---|---|---|---|---|---|---|---|---|---|
| Mean temperature (°F) | 70.6 | 10.5 | 0.6 | 105 | 45.9 | 64.8 | 72.4 | 78.1 | 86.1 |
| Dew point temperature (°F) | 56.7 | 12.5 | −6.2 | 80.2 | 26.4 | 49.7 | 59.0 | 66.4 | 73.8 |
| Smoke PM2.5 (μg/m3) | 1.47 | 4.65 | 0.0 | 123 | 0.0 | 0.0 | 0.0 | 0.0 | 15.8 |
| Nonsmoke PM2.5 (μg/m3) | 10.2 | 5.76 | 0.0 | 132 | 2.94 | 6.39 | 8.99 | 12.6 | 24.9 |
| PM10 (μg/m3) | 25.6 | 15.3 | 0.0 | 589 | 7.0 | 15.5 | 22.5 | 32.0 | 63.0 |
| Ozone (ppb) | 45.8 | 13.7 | 0.0 | 123 | 21.0 | 36.0 | 45.5 | 55.0 | 74.0 |
Figure S1 shows the smoke PM2.5 concentrations in 12 wildfire‐affected metropolitan areas in the western U.S. estimated using the method described above and averaged over all ZIP codes in the metro area. Among these cities, the largest smoke PM2.5 peaks estimated during our study period occurred in Sacramento, CA and Boise City, ID. However, due to fact that the HMS data may not capture all smoke plumes, some smoke PM2.5 may be misclassified as nonsmoke PM2.5. For example, the spikes in nonsmoke PM2.5 in Boise, ID in 2007 and 2013 may be misclassified.
3.3. Primary Statistical Analysis
Results of our main model are shown in Figure 1. We found that a 10 μg/m3 increase in smoke PM2.5 was associated with a −1.62% (95% CI: −2.97, −0.26; p = 0.020) change in the odds of ICU admission at lag 0 but a 2.66% (95% CI: 1.26, 4.07; p = 0.00018) increase at lag 5. Three of the four remaining individual lag associations had positive point estimates, which yielded a positive average association over lags 3–5 of 0.67% per 10 μg/m3 increase in PM2.5 (95% CI: 0.07, 1.28; p = 0.030) and a positive but nonsignificant average association of 0.25% per 10 μg/m3 increase in PM2.5 (95% CI: −0.08, 0.59; p = 0.14) over lags 0–5.
Figure 1.
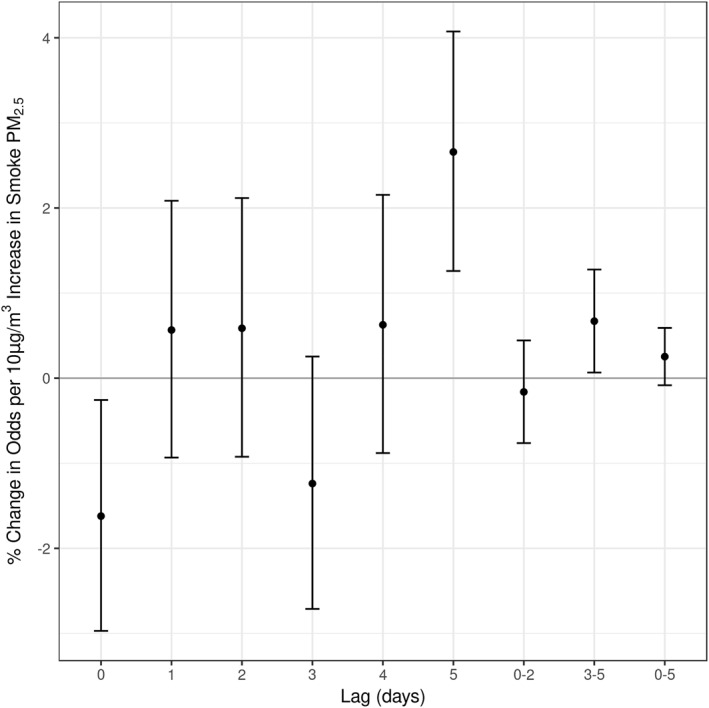
Results of main distributed‐lag conditional Poisson models.
3.4. Stratified Analysis
We investigated possible effect modification by age, gender, and race as well as by U.S. census region and time period (2006–2009, 2010–2012, and 2013–2015). The results of these models are shown in Figures S2 and S3. Categories in which statistically significant associations were found tended to be the categories with highest or among the highest ICU counts (Table 1).
Daily and average lag effects for the oldest age groups were most similar to our main results in Figure 1, with daily associations generally increasing at higher lags. However, the pattern was reversed for the youngest age group, which had a strong positive association at lag 0 and negative association at lag 4. Results for Caucasians were similar to our main results. No associations were found among African Americans, but among people of other races the mean 0–5‐day association was found to be positive. Associations were similar between males and females, with males showing the closest similarity to our main results. Among census regions, the Midwest had a positive association at lag 5 and positive mean associations at lags 3–5 and 0–5, the South had a negative association at lag 3, and the West had a negative association at lag 0 and a positive association at lag 5. Among time periods, no associations were found over 2005–2009, a negative association at lag 0 was found over 2010–2012, and positive associations were found over 2013–2015 at lag 5, lags 3–5, and lags 0–5.
3.5. Sensitivity Analysis
Sensitivity results by buffer distance and confounder model are shown in Figure S4. Our main result of a positive association at lag 5 was robust to all changes. The negative association at lag 0 and positive mean association over lags 3–5 were only apparent for the fuller models and only under the 30‐ and 50‐km buffers. A negative association at lag 4 was present under all buffer distances for models without copollutants.
3.6. Simulation of a Smoke Wave Event
Results of our 7‐day smoke wave (120 μg/m3 each day for 7 days) simulation are shown in Figure 2. Due to the negative daily association at lag 0, ICU admissions were lower than baseline on the first day of the smoke wave (Day 1). However, due to the compounding effects of the four positive lagged associations, by Day 8 the percent change in admissions over baseline was positive and remains positive through Day 12, with point estimates ranging from a 25% to a 50% increase (Figure 2, left panel). The highest daily increase in admissions over baseline was found to be 47.7% (95% CI: 21.6, 79.3) on Day 11, 4 days after the end of the smoke wave.
Figure 2.
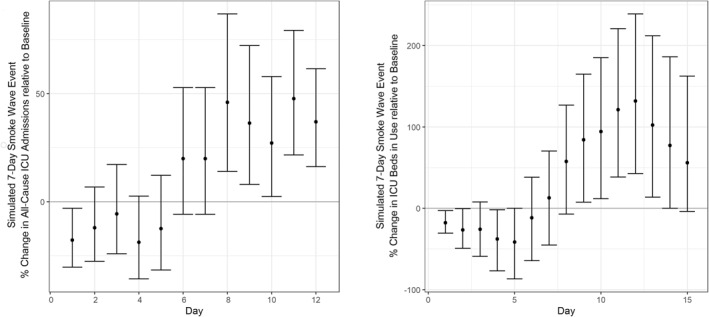
Predicted surge in ICU admissions (left) and ICU bed use (right) during and immediately following a simulated week‐long smoke wave with daily wildfire smoke concentrations of 120 μg/m3. ICU, Intensive Care Unit.
The change in ICU bed utilization over baseline (Figure 2, right panel) was also found to be negative early in the smoke wave (Days 1 and 4) but positive following the smoke wave (Days 9–13). The largest change in utilization over baseline was found to be 131.7% (95% CI: 42.7, 238.8) on Day 12. All 8 days between Days 8 and 15 had point estimates greater than 50%, and Days 11–13 had point estimates greater than 100%.
Results using multiday average lag associations are shown in Figure S5. In general, these were attenuated in magnitude compared to the results using the lag‐specific associations and predicted earlier peaks in admission/utilization. Using the 3‐day average associations for ICU admissions, there were no decreases in ICU admissions on any day, but there were increases on Days 9–12 with the largest increase being 27.2% (95% CI: 2.4, 57.9) on Day 10. This yielded increases in ICU bed utilization on Days 8–13, with the largest increase over baseline (81.3% [95% CI: 22.1, 156.6]) occurring on Day 10, 2 days earlier than under the lag‐specific association simulations.
Using the 6‐day average association for ICU admissions yielded no statistically significant increases in admissions in either direction, though point estimates on all days were positive, achieving a maximum value of 20.0% (95% CI: −5.8, 52.8) on Days 6 and 7. These consistently positive point estimates produced statistically significant increases in ICU bed utilization on Days 3–12, with a maximum increase of 63.0% (95% CI: 12.3, 128.7) occurring on Day 8, 4 days earlier than under the lag‐specific association simulations.
4. Discussion
In this study, encompassing approximately 15%–20% of all U.S. hospital admissions between 2006 and 2015, we found that each 10 μg/m3 increase in wildfire‐related PM2.5 at a hospital's ZIP code was associated with an increase in odds of an ICU admission of 2.66% (95% CI: 1.26, 4.07; p = 0.00018) 5 days later. This finding was robust to changes in the confounder model and the buffer distance used to calculate temperature, dew point temperature, PM10, and ozone at hospital ZIP codes from ground‐based monitors. A negative association at lag 0 and a positive average association over lags 3–5 were also observed but were not as robust to these changes.
Among age‐specific subgroups, we found immediate effects in younger patients but more delayed effects in older patients. This may reflect an age‐dependent difference in chronic health conditions: a higher fraction of ICU visits being asthma related in younger patients who may therefore respond rapidly to air pollution exposure and a higher fraction being related to cardiovascular disease in older patients who may therefore respond after a delay. We did not find notable differences by patient sex. Results stratified by race, region, and time period appeared to be sensitive to sample size differences between groups.
While our main distributed‐lag results were modest, under a severe but not unprecedented smoke wave the estimated changes in admission can have a substantial impact on ICU operations. Given a week‐long smoke wave with daily concentrations of 120 μg/m3, we found that ICU admissions were predicted to increase by 25%–50% over baseline on each of 5 consecutive days. We further found that these consistent increases in admissions had a profound impact on ICU bed utilization, with bed utilization more than doubling baseline utilization for 3 days. Interestingly, both the surge in admission and the surge in bed utilization occurred after the smoke wave had ended, though this would not be the case for a longer smoke wave.
Our stratified results indicate that many age groups, genders, and races have an increased risk of ICU admission following exposure to smoke PM2.5, in particular among adults aged 60–80 and patients whose race was neither Caucasian nor African American. However, African Americans, children (age 0–20), and the elderly (age 81+) had the smallest sample sizes (or nearly so) among all such groups. Thus, our findings in these groups may reflect lower power rather than the lack of a true effect. Previous work has found stronger associations in such vulnerable populations (Liu et al., 2016; Mills et al., 2018; Rappold et al., 2017).
Additionally, we found the relationship between smoke PM2.5 and ICU admissions to be more pronounced in recent years compared to earlier in our study period, possibly indicating that the U.S. population is becoming more sensitive to wildfire smoke, perhaps due to repeated exposure. However, other explanations are possible. For example, since the set of hospitals in the Premier database shifts over time, the patients represented in the database may have shifted toward populations living in older housing stock, populations more likely to work outside, or vulnerable subgroups.
Finally, we found that the associations were most consistently positive in the Midwest region, which had the largest population. Results were more mixed in the West region, which has experienced the greatest increase in wildfire frequency and duration over the past decades (Dennison et al., 2014) and is among the regions with the greatest expected increase by 2050 (Yue et al., 2013). However, because people may be located close to fires in the West, smoke concentrations may change greatly throughout the day (narrow, fresh plumes that may shift day–night meteorological patterns and fire behavior), this region may be more susceptible to exposure misclassification compared to the Midwest (where large, easily identifiable plumes have traveled from the West to the Midwest). People living in the West may also be more aware of the dangers of exposure and do more to mitigate their exposure. Any exposure misclassification could weaken our derived smoke–health relationships (Gan et al., 2017).
This study provides a broad view of health and healthcare system impacts, utilizing a large sample size, wide geographic area, and long study period. One potential limitation is that we did not analyze ICU admissions disaggregated by medical cause. However, the benefit of using aggregated data is that we were able to capture all potential pathways by which smoke could acutely and severely impact health, which allows us to speak to the overall impact on hospital resource use and operations. This study was also limited by a lack of detailed information on patient sociodemographic factors (such as income, insurance status, and location of residence) and on hospital vulnerability factors (such as size and resources). Incorporating this type of information into future studies would help to identify vulnerable individuals within communities as well as hospital‐based factors which contribute to vulnerability. The study also lacked information on when individual hospitals entered or left Premier's network, information which might have helped explain the observed effect modification by year. Additionally, the our simulation assumed that the distribution of ICU stay durations in the general population was similar to the distribution in the Medicare population (Moitra et al., 2016), which may not be correct, though patients aged 61–80 are disproportionately represented in our ICU data set. Finally, this study did not attempt to separate out the wildfire smoke contribution to the air pollutant confounder variables, PM10 and ozone, or characterize the multipollutant composition of the smoke mixture. However, characterization of smoke‐specific daily PM10 and ozone fields is the goal of ongoing modeling efforts by the authors.
Improving health outcomes during wildfire events is an emerging public health priority given the recent increase in the frequency, duration, and burned acreage of wildfires (Dennison et al., 2014; Hurteau et al., 2014; Westerling, 2016) in the U.S. and the projected future increased risk of wildfires from climate change under both high and moderate emission scenarios (Abatzoglou & Williams, 2016; Ford et al., 2018; Luber et al., 2014; Stavros et al., 2014; Wuebbles et al., 2017; Yue et al., 2013). The 2017 wildfire season in the U.S. was historically destructive, burning 9.8 million acres and killing at least 44 people (National Centers for Environmental Information, 2017), and in 2018 the Camp Fire in northern California became the deadliest single wildfire in U.S. history, with at least 85 fatalities (Chan & Sterling, 2019). The 2020 fire season was also extreme, with California alone losing over 4 million acres (Associated Press, 2020) and Colorado experiencing the two largest fires in state history (Blumhardt, 2020).
In addition to direct trauma and immediate deaths associated with fires, smoke exposure aggravates respiratory, cardiovascular, and cerebrovascular disease and causes premature mortality (Black et al., 2017; DeFlorio‐Barker et al., 2019; Liu et al., 2017; Reid et al., 2016; Wettstein et al., 2018). As air pollution concentrations overall have declined in response to tightening regulations, wildfire smoke is anticipated to become a major source of ambient air pollution (Ford et al., 2018; Kim, 2016). Currently, wildfires are estimated to contribute to approximately 20% of the total atmospheric primary PM2.5 emissions in the U.S. (Phuleria et al., 2005; U.S. Environmental Protection Agency, 2018). However, on days exceeding National Ambient Air Quality Standards (NAAQS) for daily PM2.5 (35 μg/m3), over 71% of total PM2.5 in the western U.S. is attributable to wildfires (Liu et al., 2016). Improving health outcomes during wildfire events is an emerging public health priority given this recent increase in the frequency, duration, and burned acreage of wildfires (Dennison et al., 2014; Hurteau et al., 2014; Westerling, 2016) in the U.S. and the projected future increased risk of wildfires from climate change under both high and moderate emission scenarios (Abatzoglou & Williams, 2016; Brey et al., 2021; Ford et al., 2018; Luber et al., 2014; Stavros et al., 2014; Wuebbles et al., 2017; Yue et al., 2013).
The impact on hospitals of surges of critically ill patients depends upon several factors, including the preexisting ICU census, the hospital's physical capacity, staffing availability, geographic location, and the underlying robustness of resources. For example, small hospitals in rural areas may suffer high impacts due to lack of trained staff (ICU patients often require one‐to‐one nurse–patient ratios), lack of necessary equipment such as ventilators, and limited numbers of hospital beds. For these hospitals, a 20% increase in patients requiring ICU‐level care would likely overwhelm resources, necessitating critical care transport (also a limited resource) to another hospital. Larger hospitals in more urban environments may have more robust physical and human resources, which could prevent patient transfers. However, depending on preexisting ICU census, surges in ICU admissions can still overwhelm these systems, resulting in prolonged emergency department boarding, which can worsen patient outcomes due to delays in definitive care. Furthermore, pediatric ICU capacity is frequently far more scarce, and often these patients must be transferred by ground or air crews to regional centers, which concentrates impact of surges. This is especially relevant as immediate health impacts were found to be most prominent in the pediatric populations.
Provision of critical care services are incredibly costly both to patients and healthcare systems. ICU patients account for 13% of all hospital costs and $121–265 billion in annual healthcare expenditures in the U.S. (Halpern & Pastores, 2015). Each ICU patient costs on average 2.5 (Barrett et al., 2006) to 3 (Halpern & Pastores, 2015) times that of non‐ICU patient. Caring for critically ill, ICU‐level patients are also resource‐intensive, and surges in demand for specialized medical services can overwhelm hospital capabilities, especially in smaller hospital systems. Prolonged boarding times in emergency departments, unsafe nurse–patient ratios, systems and informatics limitations, and depletion of essential supplies, pharmaceuticals, and trained‐personnel can compound to yield poor patient outcomes (Hick et al., 2014). In order to mitigate these stressors, hospital systems within wildfire exposure zones need timely air quality information and system‐wide procedural strategies to accommodate surges in demand for ICU and other resources.
Currently, the tools exist to combine satellite observations, meteorological data, and information regarding fire emissions to forecast smoke PM2.5 at fine spatial and temporal resolutions up to 48 h prior to plume arrival (Rappold et al., 2014). Combining predictive wildfire‐plume models with published exposures–response functions for ED visits, hospitalizations and ICU admissions can form the basis of forecasting systems for public health and healthcare systems (Gan, 2018; Morrison et al., 2016). Lead time to the arrival of a smoke plume would permit the prepositioning of vital resources into at‐risk areas, such as skilled personal and equipment. Such forecast‐based interventions have the potential to reduce the health burden of wildfires, especially in areas far from burn sites that may not be aware of potential smoke exposure.
Climate change is currently driving rising seasonal temperatures and arid conditions in North America, which, in turn, fuel more frequent and prolonged wildfire events (Brey et al., 2021; Wuebbles et al., 2017). In recent years, we have seen a drastic increase in fires across a wide range of ecologic, geographic, and climatic zones (Dennison et al., 2014). More than half of these fires, and the PM they produce, are attributable to anthropogenic climate change (Abatzoglou & Williams, 2016). Under the current carbon emission pathway (RCP8.5), average wildfire‐related PM2.5 levels in the continental U.S. are estimated to increase 190% from 2000–2010 to 2090–2099, nearly offsetting projected reductions from other sources and doubling the number of premature deaths attributable to fire‐related PM2.5 between 2000 and 2100 (Ford et al., 2018). Further research is needed to understand the exposure–impact relationship between wildfire emissions and health outcomes in order to predict potential health and healthcare impacts and develop critical public health interventions.
Since 1988, average nationwide PM2.5 trends have been decreasing; however, the 98th percentile of daily PM2.5 concentrations are currently rising again in western states due to recent increases in wildfire activity (McClure & Jaffe, 2018) while wildfires in this region have also prevented expected reduction in the summertime‐average PM2.5 concentrations (O'Dell et al., 2019). While PM2.5 emissions from sources such as vehicles or power plants can be regulated, wildfire frequency and intensity are driven by complex synergistic interactions between ecological conditions, climatic conditions, and land use decisions. Furthermore, recent studies suggest that wildfire smoke is more lethal than urban air pollution (R. Xu et al., 2020). Therefore, climate mitigation is urgently needed to prevent costly and dangerous wildfire seasons in the years to come.
To our knowledge, this is the first study to analyze the impact of wildfire‐specific PM2.5 on total ICU admissions over multiple years and at a large geographic scale. We find that extreme or long‐lasting smoke events have the potential to place severe strain on hospital resources. Climate models predict that wildfires, and associated health impacts, will increase throughout the century with tens of millions of U.S. citizens negatively affected. Given this emerging public health threat and the high resource demand of ICU care, integrating smoke forecasts into hospital operations decisions has the potential to allow for prepositioning of vital critical care resources, which may translate to improved operations and patient outcomes during wildfire smoke events.
Conflict of Interest
The authors declare they have no actual or potential competing financial interests.
Supporting information
Supporting Information S1
Acknowledgments
Support for J. L. Crooks was provided by the Boettcher Foundation through the project “Wildfire Smoke and Pediatric Asthma.” Support for E. V. Fischer and S. J. Brey was provided by the Environmental Protection Agency award number 83588401. Support for K. O'Dell, B. Ford, E. V. Fischer, and J. R. Pierce was provided by NASA Applied Sciences Program award number NNX15AG35G. We thank Ryan Gan and Sheryl Magzamen. This paper is considered a contribution by the University of Colorado Consortium for Climate Change and Health.
Sorensen, C. , House, J. A. , O'Dell, K. , Brey, S. J. , Ford, B. , Pierce, J. R. , et al. (2021). Associations between wildfire‐related PM2.5 and Intensive Care Unit admissions in the United States, 2006–2015. GeoHealth, 5, e2021GH000385. 10.1029/2021GH000385
Data Availability Statement
Data supporting our conclusions can be obtained from the Open Science Framework website at https://osf.io/62dvr/ (Crooks, 2021).
References
- Abatzoglou, J. T. , & Williams, A. P. (2016). Impact of anthropogenic climate change on wildfire across western US forests. Proceedings of the National Academy of Sciences of the United States of America, 113, 11770–11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdo, M. , Ward, I. , O'Dell, K. , Ford, B. , Pierce, J. R. , Fischer, E. V. , et al. (2019). Impact of wildfire smoke on adverse pregnancy outcomes in Colorado, 2007–2015. International Journal of Environmental Research and Public Health, 16(19), 3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alman, B. L. , Pfister, G. , Hao, H. , Stowell, J. , Hu, X. , Liu, Y. , et al. (2016). The association of wildfire smoke with respiratory and cardiovascular emergency department visits in Colorado in 2012: A case crossover study. Environmental Health, 15, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, B. G. , Gasparrini, A. , & Tobias, A. (2014). Conditional Poisson models: A flexible alternative to conditional logistic case cross‐over analysis. BMC Medical Research Methodology, 14, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Associated Press . (2020). Epic scale of California wildfires continues to grow. Associate Press. [Google Scholar]
- Barrett, M. L. , Smith, M. W. , Elixhauser, A. , Honigman, L. S. , & Pines, J. M. (2006). Utilization of intensive care services, 2011: Statistical brief #185. Rockville, MD: Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]
- Bell, J. E. , Brown, C. L. , Conlon, K. , Herring, S. , Kunkel, K. E. , Lawrimore, J. , et al. (2018). Changes in extreme events and the potential impacts on human health. Journal of the Air & Waste Management Association, 68, 265–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, C. , Tesfaigzi, Y. , Bassein, J. A. , & Miller, L. A. (2017). Wildfire smoke exposure and human health: Significant gaps in research for a growing public health issue. Environmental Toxicology and Pharmacology, 55, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumhardt, M. (2020). Two largest wildfires in Colorado history are burning at the same time, 10 miles apart. USA Today. [Google Scholar]
- Brey, S. J. , Barnes, E. A. , Pierce, J. R. , Swann, A. L. S. , & Fischer, E. V. (2021). Past variance and future projections of the environmental conditions driving western U.S. summertime wildfire burn area. Earth's Future, 9, e2020EF001645. 10.1029/2020EF001645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey, S. J. , & Fischer, E. V. (2016). Smoke in the city: How often and where does smoke impact summertime ozone in the United States? Environmental Science & Technology, 50, 1288–1294. [DOI] [PubMed] [Google Scholar]
- Brey, S. J. , Ruminski, M. , Atwood, S. A. , & Fischer, E. V. (2018). Connecting smoke plumes to sources using Hazard Mapping System (HMS) smoke and fire location data over North America. Atmospheric Chemistry and Physics, 18, 1745–1761. [Google Scholar]
- Carratt, S. A. , Flayer, C. H. , Kossack, M. E. , & Last, J. A. (2017). Pesticides, wildfire suppression chemicals, and California wildfires: A human health perspective. [Google Scholar]
- Cascio, W. E. (2018). Wildland fire smoke and human health. The Science of the Total Environment, 624, 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S. , & Sterling, J. (2019). Death toll in camp fire revised down by one to 85. CNN. [Google Scholar]
- Crooks, J. (2021). Archived data for “associations between wildfire‐related PM2.5 and Intensive Care Unit admissions in the United States, 2006‐2015”. Open Science Framework. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBell, L. J. , Talbot, R. W. , Dibb, J. E. , Munger, J. W. , Fischer, E. V. , & Frolking, S. E. (2004). A major regional air pollution event in the northeastern United States caused by extensive forest fires in Quebec, Canada. Journal of Geophysical Research, 109, D19305. 10.1029/2004JD004840 [DOI] [Google Scholar]
- DeFlorio‐Barker, S. , Crooks, J. , Reyes, J. , & Rappold, A. G. (2019). Cardiopulmonary effects of fine particulate matter exposure among older adults, during wildfire and non‐wildfire periods, in the United States 2008–2010. Environmental Health Perspectives, 127, 037006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison, P. E. , Brewer, S. C. , Arnold, J. D. , & Moritz, M. A. (2014). Large wildfire trends in the western United States, 1984–2011. Geophysical Research Letters, 41, 2928–2933. 10.1002/2014GL059576 [DOI] [Google Scholar]
- Dohrenwend, P. , Le, M. , Bush, J. , & Thomas, C. (2013). The impact on emergency department visits for respiratory illness during the Southern California wildfires. The Western Journal of Emergency Medicine, 14, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreessen, J. , Sullivan, J. , & Delgado, R. (2016). Observations and impacts of transported Canadian wildfire smoke on ozone and aerosol air quality in the Maryland region on June 9–12, 2015. Journal of the Air & Waste Management Association, 66, 842–862. [DOI] [PubMed] [Google Scholar]
- Fann, N. , Alman, B. , Broome, R. A. , Morgan, G. G. , Johnston, F. H. , Pouliot, G. , & Rappold, A. G. (2018). The health impacts and economic value of wildland fire episodes in the U.S.2008–2012. The Science of the Total Environment, 610–611, 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustini, A. , Alessandrini, E. R. , Pey, J. , Perez, N. , Samoli, E. , Querol, X. , et al. (2015). Short‐term effects of particulate matter on mortality during forest fires in southern Europe: Results of the med‐particles project. Occupational and Environmental Medicine, 72, 323–329. [DOI] [PubMed] [Google Scholar]
- Finlay, S. E. , Moffat, A. , Gazzard, R. , Baker, D. , & Murray, V. (2012). Health impacts of wildfires. PLoS Currents, 4, e4f959951cce2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, B. , Val Martin, M. , Zelasky, S. E. , Fischer, E. V. , Anenberg, S. C. , Heald, C. L. , & Pierce, J. R. (2018). Future fire impacts on smoke concentrations, visibility, and health in the contiguous united states. GeoHealth, 2, 229–247. 10.1029/2018GH000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, N. H. , McKenzie, D. , Erickson, T. , Koziol, B. , Billmire, M. , Endsley, K. A. , et al. (2016). Annual wildland fire emissions (WFEIS v0. 5) for Conterminous US and Alaska, 2001–2013. ORNL DAAC. [Google Scholar]
- Gan, R. W. (2018). Smoke Health Impact Assessment (HIA) forecaster (beta). Retrieved from http://rgan.atmos.colostate.edu/smoke_forecaster/ [Google Scholar]
- Gan, R. W. , Ford, B. , Lassman, W. , Pfister, G. , Vaidyanathan, A. , Fischer, E. , et al. (2017). Comparison of wildfire smoke estimation methods and associations with cardiopulmonary‐related hospital admissions. GeoHealth, 1, 122–136. 10.1002/2017GH000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, R. W. , Liu, J. , Ford, B. , O'Dell, K. , Vaidyanathan, A. , Wilson, A. , et al. (2020). The association between wildfire smoke exposure and asthma‐specific medical care utilization in Oregon during the 2013 wildfire season. Journal of Exposure Science and Environmental Epidemiology, 30, 618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronlund, C. J. , Zanobetti, A. , Schwartz, J. D. , Wellenius, G. A. , & O'Neill, M. S. (2014). Heat, heat waves, and hospital admissions among the elderly in the United States, 1992–2006. Environmental Health Perspectives, 122, 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikerwal, A. , Akram, M. , Del Monaco, A. , Smith, K. , Sim, M. R. , Meyer, M. , et al. (2015). Impact of fine particulate matter (PM2.5) exposure during wildfires on cardiovascular health outcomes. Journal of the American Heart Association, 4, e001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern, N. A. , & Pastores, S. M. (2015). Critical care medicine beds, use, occupancy, and costs in the United States. Critical Care Medicine, 43, 2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, S. B. , Brauer, M. , MacNab, Y. C. , & Kennedy, S. M. (2011). Three measures of forest fire smoke exposure and their associations with respiratory and cardiovascular health outcomes in a population‐based cohort. Environmental Health Perspectives, 119, 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hick, J. L. , Einav, S. , Hanfling, D. , Kissoon, N. , Dichter, J. R. , Devereaux, A. V. , & Christian, M. D. (2014). Surge capacity principles. Chest, 146, e1S–e16S. [DOI] [PubMed] [Google Scholar]
- Hoyt, K. S. , & Gerhart, A. E. (2004). The San Diego county wildfires: Perspectives of healthcare. Disaster Management & Response, 2, 46–52. [DOI] [PubMed] [Google Scholar]
- Hurteau, M. D. , Westerling, A. L. , Wiedinmyer, C. , & Bryant, B. P. (2014). Projected effects of climate and development on California wildfire emissions through 2100. Environmental Science & Technology, 48, 2298. [DOI] [PubMed] [Google Scholar]
- Jaffe, D. A. , & Wigder, N. L. (2012). Ozone production from wildfires: A critical review. Atmospheric Environment, 51, 1. [Google Scholar]
- Jerrett, M. , Burnett, R. T. , Ma, R. , Pope, C. A., III , Krewski, D. , Newbold, K. B. , et al. (2005). Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology, 16, 727–736. [DOI] [PubMed] [Google Scholar]
- Johnston, F. , Hanigan, I. , Henderson, S. , Morgan, G. , & Bowman, D. (2011). Extreme air pollution events from bushfires and dust storms and their association with mortality in Sydney, Australia 1994–2007. Environmental Research, 111, 811–816. [DOI] [PubMed] [Google Scholar]
- Johnston, F. H. , Henderson, S. B. , Chen, Y. , Randerson, J. T. , Marlier, M. , Defries, R. S. , et al. (2012). Estimated global mortality attributable to smoke from landscape fires. Environmental Health Perspectives, 120, 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juancosa Calahorrano, J. , Lindaas, J. , O'Dell, K. , Palm, B. B. , Peng, Q. , Flocke, F. , et al. (2021). Daytime oxidized reactive nitrogen partitioning in western U.S. wildfire smoke plumes. Journal of Geophysical Research: Atmospheres, 126, e2020JD033484. 10.1029/2020JD033484 [DOI] [Google Scholar]
- Kim, E. J. (2016). The impacts of climate change on human health in the United States: A scientific assessment (312 pp.), by US Global Change Research Program. Washington, DC: Author. Retrieved from https://health2016.Globalchange.Gov/downloads. Taylor & Francis; [Google Scholar]
- Knowlton, K. , Rotkin‐Ellman, M. , Geballe, L. , Max, W. , & Solomon, G. M. (2011). Six climate change‐related events in the United States accounted for about $14 billion in lost lives and health costs. Health Affairs, 30, 2167–2176. [DOI] [PubMed] [Google Scholar]
- Lassman, W. , Ford, B. , Gan, R. W. , Pfister, G. , Magzamen, S. , Fischer, E. V. , & Pierce, J. R. (2017). Spatial and temporal estimates of population exposure to wildfire smoke during the Washington state 2012 wildfire season using blended model, satellite, and in situ data. GeoHealth, 1, 106–121. 10.1002/2017GH000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares, C. , Carmona, R. , Tobías, A. , Mirón, I. J. , & Díaz, J. (2015). Influence of advections of particulate matter from biomass combustion on specific‐cause mortality in Madrid in the period 2004–2009. Environmental Science & Pollution Research, 22, 7012–7019. [DOI] [PubMed] [Google Scholar]
- Lindaas, J. , Farmer, D. K. , Pollack, I. B. , Abeleira, A. , Flocke, F. , Roscioli, R. , et al. (2017). Changes in ozone and precursors during two aged wildfire smoke events in the Colorado front range in summer 2015. Atmospheric Chemistry and Physics, 17, 10691–10707. [Google Scholar]
- Lipner, E. M. , O'Dell, K. , Brey, S. J. , Ford, B. , Pierce, J. R. , Fischer, E. V. , & Crooks, J. L. (2019). The associations between clinical respiratory outcomes and ambient wildfire smoke exposure among pediatric asthma patients at national Jewish health, 2012–2015. GeoHealth, 3, 146–159. 10.1029/2018GH000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. C. , Mickley, L. J. , Sulprizio, M. P. , Dominici, F. , Yue, X. , Ebisu, K. , et al. (2016). Particulate air pollution from wildfires in the western US under climate change. Climatic Change, 138, 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. C. , Pereira, G. , Uhl, S. A. , Bravo, M. A. , & Bell, M. L. (2015). A systematic review of the physical health impacts from non‐occupational exposure to wildfire smoke. Environmental Research, 136, 120–132. 10.1016/j.envres.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. C. , Wilson, A. , Mickley, L. J. , Dominici, F. , Ebisu, K. , Wang, Y. , et al. (2017). Wildfire‐specific fine particulate matter and risk of hospital admissions in urban and rural counties. Epidemiology, 28, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber, G. , Knowlton, K. , Balbus, J. , Frumkin, H. , Hayden, M. , Hess, J. , et al. (2014). Human health. Climate change impacts in the United States: The third National Climate Assessment (pp. 220–256). [Google Scholar]
- Mares, D. (2013). Climate change and crime: Monthly temperature and precipitation anomalies and crime rates in St. Louis, MO 1990–2009. Crime, Law and Social Change, 59(2), 185–208. [Google Scholar]
- Martin, K. L. , Hanigan, I. C. , Morgan, G. G. , Henderson, S. B. , & Johnston, F. H. (2013). Air pollution from bushfires and their association with hospital admissions in Sydney, Newcastle and Wollongong, Australia 1994–2007. Australian and New Zealand Journal of Public Health, 37, 238–243. [DOI] [PubMed] [Google Scholar]
- McClure, C. D. , & Jaffe, D. A. (2018). US particulate matter air quality improves except in wildfire‐prone areas. Proceedings of the National Academy of Sciences of the United States of America, 115, 7901–7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, D. , Jones, R. , Wobus, C. , Ekstrom, J. , Jantarasami, L. , St Juliana, A. , et al. (2018). Projecting age‐stratified risk of exposure to inland flooding and wildfire smoke in the united states under two climate scenarios. Environmental Health Perspectives, 126, 047007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra, V. K. , Guerra, C. , Linde‐Zwirble, W. T. , & Wunsch, H. (2016). Relationship between ICU length of stay and long‐term mortality for elderly ICU survivors. Critical Care Medicine, 44, 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, G. , Sheppeard, V. , Khalaj, B. , Ayyar, A. , Lincoln, D. , Jalaludin, B. , et al. (2010). Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology, 21, 47. [DOI] [PubMed] [Google Scholar]
- Morrison, K. T. , Shaddick, G. , Henderson, S. B. , & Buckeridge, D. L. (2016). A latent process model for forecasting multiple time series in environmental public health surveillance. [DOI] [PubMed] [Google Scholar]
- National Centers for Environmental Information . (2017). Assessing the U.S. climate in 2017. Retrieved from https://www.ncei.noaa.gov/news/national-climate-201712 [Google Scholar]
- Newby, D. E. , Mannucci, P. M. , Tell, G. S. , Baccarelli, A. A. , Brook, R. D. , Donaldson, K. , et al. (2015). Expert position paper on air pollution and cardiovascular disease. European Heart Journal, 36, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, K. V. R. , Ignotti, E. , & Hacon, S. de S. (2013). Circulatory disease mortality rates in the elderly and exposure to PM2.5 generated by biomass burning in the Brazilian Amazon in 2005. Cadernos de Saúde Pública, 29, 589–598. [DOI] [PubMed] [Google Scholar]
- O'Dell, K. , Ford, B. , Fischer, E. V. , & Pierce, J. R. (2019). The contribution of wildland‐fire smoke to US PM2.5 and its influence on recent trends. Environmental Science & Technology. [DOI] [PubMed] [Google Scholar]
- O'Dell, K. , Hornbrook, R. S. , Permar, W. , Levin, E. J. T. , Garofalo, L. A. , Apel, E. C. , et al. (2020). Hazardous air pollutants in fresh and aged western US wildfire smoke and implications for long‐term exposure. Environmental Science & Technology, 54, 11838–11847. [DOI] [PubMed] [Google Scholar]
- Parthum, B. , Pindilli, E. , & Hogan, D. (2017). Benefits of the fire mitigation ecosystem service in the great dismal swamp national wildlife refuge, Virginia, USA. Journal of Environmental Management, 203, 375–382. [DOI] [PubMed] [Google Scholar]
- Phuleria, H. C. , Fine, P. M. , Zhu, Y. , & Sioutas, C. (2005). Air quality impacts of the October 2003 Southern California wildfires. Journal of Geophysical Research, 110, D07S20. 10.1029/2004JD004626 [DOI] [Google Scholar]
- Premier Applied Sciences® PI . (2020). Premier healthcare database white paper: Data the informs and performs. Premier Applied Sciences®, Premier Inc. [Google Scholar]
- Rappold, A. G. , Fann, N. L. , Crooks, J. , Huang, J. , Cascio, W. E. , Devlin, R. B. , & Diaz‐Sanchez, D. (2014). Forecast‐based interventions can reduce the health and economic burden of wildfires. Environmental Science & Technology, 48, 10571–10579. [DOI] [PubMed] [Google Scholar]
- Rappold, A. G. , Reyes, J. , Pouliot, G. , Cascio, W. E. , & Diaz‐Sanchez, D. (2017). Community vulnerability to health impacts of wildland fire smoke exposure. Environmental Science & Technology, 51, 6674–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold, A. G. , Stone, S. L. , Cascio, W. E. , Neas, L. M. , Kilaru, V. J. , Carraway, M. S. , et al. (2011). Peat bog wildfire smoke exposure in rural North Carolina is associated with cardiopulmonary emergency department visits assessed through syndromic surveillance. Environmental Health Perspectives, 119, 1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical computing. Retrieved from https://www.R-project.org/ [Google Scholar]
- Reid, C. E. , Brauer, M. , Johnston, F. H. , Jerrett, M. , Balmes, J. R. , & Elliott, C. T. (2016). Critical review of health impacts of wildfire smoke exposure. Environmental Health Perspectives, 124, 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli, E. , Barboni, T. , Santoni, P.‐A. , & Chiaramonti, N. (2014). Quantification of volatile organic compounds in smoke from prescribed burning and comparison with occupational exposure limits. Natural Hazards and Earth System Sciences, 14, 1049–1057. [Google Scholar]
- Rublee, C. S. , Sorensen, C. J. , Lemery, J. , Wade, T. J. , Sams, E. A. , Hilborn, E. D. , et al. (2020). Associations between dust storms and Intensive Care Unit admissions in the United States, 2000–2015. GeoHealth, 4, e2020GH000260. 10.1029/2020GH000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruminski, M. , Kondragunta, S. , Draxler, R. , & Zeng, J. (2006). Recent changes to the Hazard Mapping System. Paper presented at 15th International Emission Inventory Conference, New Orleans: Louisiana. [Google Scholar]
- Sapkota, A. , Symons, J. M. , Kleissl, J. , Wang, L. , Parlange, M. B. , Ondov, J. , et al. (2005). Impact of the 2002 Canadian forest fires on particulate matter air quality in Baltimore city. Environmental Science & Technology, 39, 24–32. [DOI] [PubMed] [Google Scholar]
- Schroeder, W. , Ruminski, M. , Csiszar, I. , Giglio, L. , Prins, E. , Schmidt, C. , & Morisette, J. (2008). Validation analyses of an operational fire monitoring product: The Hazard Mapping System. International Journal of Remote Sensing, 29, 6059. [Google Scholar]
- Seager, R. , & Vecchi, G. A. (2010). Greenhouse warming and the 21st century hydroclimate of southwestern North America. Proceedings of the National Academy of Sciences, 107, 21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. , Woodward, A. , Campbell‐Lendrum, D. , Chadee, D. , Honda, Y. , Liu, Q. , et al. (2014). Human health: Impacts, adaptation, and co‐benefits. In Field C. B., et al. (Eds.), Climate change. [Google Scholar]
- Stavros, E. N. , Abatzoglou, J. T. , McKenzie, D. , & Larkin, N. K. (2014). Regional projections of the likelihood of very large wildland fires under a changing climate in the contiguous western United States. Climatic Change, 126, 455–468. [Google Scholar]
- Turner, H. , & Firth, D. (2020). Generalized nonlinear models in R: An overview of the gnm package. [Google Scholar]
- Urbanski, S. P. (2013). Combustion efficiency and emission factors for wildfire‐season fires in mixed conifer forests of the northern Rocky Mountains, US. Atmospheric Chemistry and Physics, 13, 7241–7262. [Google Scholar]
- U.S. Census Bureau . (2013). Census regions and divisions of the United States. Retrieved from https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf [Google Scholar]
- U.S. Environmental Protection Agency . (2018). 2011 National Emissions Inventory (NEI) data. Retrieved from https://www.epa.gov/air-emissions-inventories/2011-national-emissions-inventory-nei-data
- Val Martin, M. , Heald, C. L. , Ford, B. , Prenni, A. J. , & Wiedinmyer, C. (2013). A decadal satellite analysis of the origins and impacts of smoke in Colorado. Atmospheric Chemistry and Physics, 13, 7429–7439. [Google Scholar]
- Westerling, A. L. (2016). Increasing western us forest wildfire activity: Sensitivity to changes in the timing of spring. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1696), 20150178. 10.1098/rstb.2015.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerling, A. L. , Hidalgo, H. G. , Cayan, D. R. , & Swetnam, T. W. (2006). Warming and earlier spring increase western U.S. forest wildfire activity. Science, 313(5789), 940–943. 10.1126/science.1128834 [DOI] [PubMed] [Google Scholar]
- Wettstein, Z. S. , Hoshiko, S. , Fahimi, J. , Harrison, R. J. , Cascio, W. E. , & Rappold, A. G. (2018). Cardiovascular and cerebrovascular emergency department visits associated with wildfire smoke exposure in California in 2015. Journal of the American Heart Association, 7(8), e007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuebbles, D. , Fahey, D. , Hibbard, K. , Dokken, B. , Stewart, B. , & Maycock, T. (2017). Climate science special report: Fourth national climate assessment (Vol. I, 470 pp.). Washington, DC. [Google Scholar]
- Xu, R. , Yu, P. , Abramson, M. J. , Johnston, F. H. , Samet, J. M. , Bell, M. L. , et al. (2020). Wildfires, global climate change, and human health. New England Journal of Medicine, 383, 2173–2181. [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Crooks, J. L. , Davies, J. M. , Khan, A. F. , Hu, W. , & Tong, S. (2018). The association between ambient temperature and childhood asthma: A systematic review. International Journal of Biometeorology, 62, 471–481. [DOI] [PubMed] [Google Scholar]
- Yao, J. , Eyamie, J. , & Henderson, S. B. (2016). Evaluation of a spatially resolved forest fire smoke model for population‐based epidemiologic exposure assessment. Journal of Exposure Science and Environmental Epidemiology, 26, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, X. , Mickley, L. J. , Logan, J. A. , Kaplan, J. O. (2013). Ensemble projections of wildfire activity and carbonaceous aerosol concentrations over the western United States in the mid‐21st century. Atmospheric Environment, 77, 767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data supporting our conclusions can be obtained from the Open Science Framework website at https://osf.io/62dvr/ (Crooks, 2021).


