Abstract
The balance between pro- and antioxidant molecules has been established as an important driving force in the pathogenesis of cardiovascular disease. Chronic heart failure is associated with oxidative stress in the myocardium and globally. Redox balance in the heart and brain is controlled, in part, by antioxidant proteins regulated by the transcription factor Nuclear factor erythroid 2-related factor 2 (Nrf2), which is reduced in the heart failure state. Nrf2 can, in turn, be regulated by a variety of mechanisms including circulating microRNAs (miRNAs) encapsulated in extracellular vesicles (EVs) derived from multiple cell types in the heart. Here, we review the role of the Nrf2 and antioxidant enzyme signaling pathway in mediating redox balance in the myocardium and the brain in the heart failure state. This review focuses on Nrf2 and antioxidant protein regulation in the heart and brain by miRNA-enriched EVs in the setting of heart failure. We discuss EV-mediated intra- and inter-organ communications especially, communication between the heart and brain via an EV pathway that mediates cardiac function and sympatho-excitation in heart failure. Importantly, we speculate how engineered EVs with specific miRNAs or antagomirs may be used in a therapeutic manner in heart failure.
Graphical Abstract
1. Introduction
While this review will focus on the role of extracellular vesicles (EVs) in the regulation of Nuclear factor erythroid 2-related factor 2 (Nrf2), antioxidant enzymes and oxidative stress in heart failure, it is necessary to also discuss the role of non-coding RNA in the regulation of Nrf2 in tissues outside of the heart. We will therefore, provide an overview of these mechanisms prior to a discussion of the role played by EVs, including exosomes and microvesicles in inter and intra organ communication.
Chronic heart failure (CHF) is a major cause of mortality and morbidity worldwide, and is a growing public health concern due, in part, to aging and obesity in the contemporary population [1, 2] and the growing incidence of Type 2 diabetes [3]. Based on data from NHANES (National Health and Nutrition Examination Survey; 2011 to 2014), an estimated 6.5 million Americans ≥20 years of age suffer from CHF. Projections show that the prevalence of CHF will increase by 46% by 2030, resulting in >8 million people ≥18 years of age suffering from some form of CHF. These data also predict that the total cost of CHF will increase almost 127%, to $69.7 billion from 2012, amounting to ≈$244 for every adult in the United States [4, 5].
Therapy for CHF has changed only modestly over the last 30 years. While surgical and device therapies have grown, there have not been any major changes in medical treatment since drugs were developed and used to block the renin-angiotensin-aldosterone system (RAAS) and the autonomic nervous system (e.g. beta-1 adrenergic blockers); both of which have clearly improved long-term survival [6]. However, 5-year survival for CHF is still only approximately 50%. These are sobering statistics that behooves the medical and scientific community to understand the complexity of this syndrome in all organ systems in order to identify new and effective therapies.
The mechanisms by which cardiovascular (CV) function spirals downward in the heart failure state are multifactorial, involving almost every organ system. It has been well established that oxidative stress is a major contributor to the pathogenesis of heart failure [7-9]. An imbalance between the synthesis of oxygen free radicals and their elimination by antioxidant defense mechanisms results in macromolecular damage and disruption of cellular redox signaling [10, 11], thus affecting both cardiac structure and function [12-14] as well as remote targets. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a highly conserved transcription factor regulating antioxidant enzymes, phase II detoxifying enzymes and other detoxification proteins by direct binding to the antioxidant response elements (AREs) in their promoter regions [15, 16]. The critical roles of the Nrf2/ARE signaling pathway in various physiological and pathophysiological conditions through both anti-inflammatory and anti-oxidant mechanisms has been well-documented [17]. In addition, many Nrf2-regulated enzymes have been implicated in the pathogenesis of CV diseases and are strongly associated with oxidative stress-mediated cardiac remodeling and heart failure [18-20]. Nrf2-regulated enzymes may also serve as sensitive and specific markers reflecting ventricular function in heart failure patients [21].
Recently, systemic Nrf2 activation has been shown to be beneficial in CV disease [22, 23], while Nrf2 know-out mice have no effects on growth or development and on cardiac structure and function under normal conditions [24, 25]. Global deficiency of Nrf2 in mice facilitates transverse aortic constriction (TAC)-induced cardiac dysfunction, including cardiac hypertrophy, fibrosis and apoptosis, overt heart failure, and increased mortality [24]. These mice are susceptible to angiotensin II-induced cardiac hypertrophy [26]. Many of these events can be attributed to elevated myocardial oxidative stress. Cardiac-specific overexpression of Nrf2 in mice is cardio-protective resulting in the smaller infarct size, less cardiac fibrosis and inflammation induced, in part, by stabilizing the Keap1-Nrf2 complex and enhancing Nrf2 nuclear translocation and subsequent inhibition of pro-oxidant pathways [27]. Because sympatho-excitation is a hallmark of the heart failure state the role of oxidative stress and Nrf2 in the brainstem is of high relevance. Selective deletion of Nrf2 in areas of the brain that modulate sympathetic outflow results in sustained hypertension in normal mice [28]. Selective activation or overexpression of Nrf2 in the brain reduces sympatho-excitation in the setting of heart failure [29, 30]. Overall, these studies suggest that the Nrf2/ARE signaling pathway plays a critical role in maintaining redox homeostasis in the heart and brain in the heart failure state. Thus, it is important to understand the regulation of Nrf2 in heart failure in order to develop novel therapeutic strategies in the treatment of this disease.
Increasing evidence suggest that the regulation of the Nrf2/ARE signaling pathway in the progression of heart failure is multi-faced, involving protein-protein interactions and epigenetic regulation. Interestingly, EVs, generally classified into exosomes, microvesicles or apoptotic bodies, are found to serve as carriers of biological messages in various pathophysiological settings, including heart failure [31-33]. The focus of this review will also be on the role of cardiac-derived EVs and their cargo (microRNAs, proteins, small molecules) in the CHF state with particular emphasis on the regulation of Nrf2 and oxidant stress in the heart and remote tissues.
2. Regulation of Nrf2/Antioxidant Response Element (ARE) signaling by protein-protein interactions.
The Kelch-like-ECH-associated protein 1 (Keap1)-Nrf2 system is a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Keap1-Nrf2 forms a major node of cellular and organismal defense against oxidative and electrophilic stresses of both exogenous and endogenous origins and plays an important role in the progression of disease [34]. Under normal conditions (i.e. low levels of oxidant stress), Nrf2 is bound in the cytoplasm by Keap1 and Cullin 3, working together to regulate the ubiquitination of Nrf2 [35]. Oxidative stress can directly target critical cysteine residues in Keap1, resulting in degradation of the Keap1-Cullin 3 ubiquitination system. In addition, p62 physically interacts with Keap1 [36, 37] through its STGE motif, similar with that of Nrf2 to mediate autophagic degradation of Keap1, resulting in the dissociation of Nrf2 from Keap1 and formation of unbound/free Nrf2 [38]. Oxidative stress-mediated activation of Nrf2 has been attributed to the phosphorylation of the p62 STGE motif at Ser349 by mTORC1 enhancing the binding of p62 to Keap1 and subsequent Keap1 autophagic degradation indirectly increasing Nrf2 [39-42]. Once Nrf2 is released from Keap1, it translocates to the nucleus where it either binds to AREs in the promoters of target genes thereby activating their expression [43, 44] or directly binds its own ARE-like elements located in the proximal region of its promoter to auto-regulate its own transcription and subsequently enhance the expression of downstream target genes [16]. Thus, Nrf2 can be considered an “amplifier” of anti-oxidant pathways.
In addition to Keap1-dependent ubiquitination and proteasomal degradation of Nrf2, an increasing body of literature has demonstrated alternative mechanisms of Nrf2 regulation, including the phosphorylation of Nrf2 by various protein kinases [45-49], direct acetylation of Nrf2 by histone acetyltransferases (HATs) [50] and de-glycation by Fructosamine-3-Kinase (FN3K) [51]. These mechanisms enhance the nuclear retention and ARE binding of Nrf2 [52-54], and its interaction with other protein partners [51, 55-61]}. These are potentially important determinants of Nrf2 activity and function, and therefore contribute to the maintenance of cellular redox homeostasis in heart failure (as illustrated in figure 1).
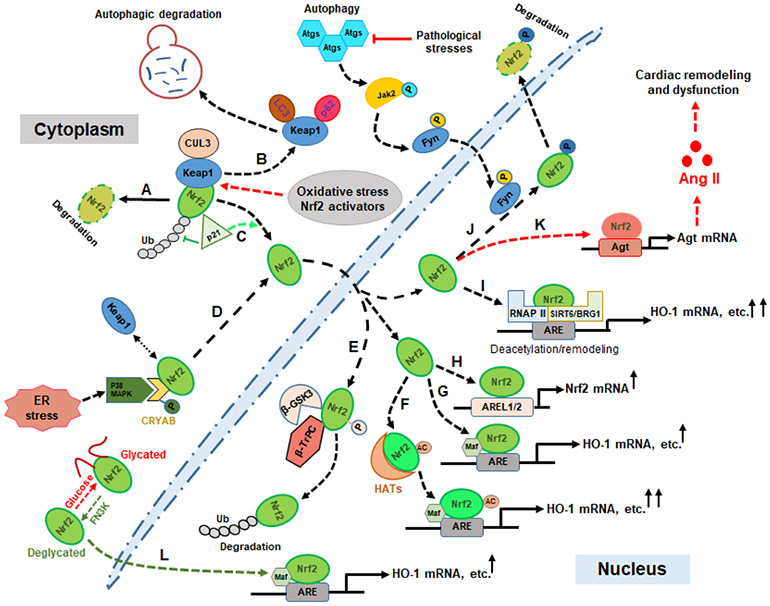
Figure 1. Schematic diagram of potential mechanisms by which Nrf2 signaling is regulated via protein interplay as a therapeutic process and in the pathogenesis of heart failure.
Keap1-dependent Nrf2 degradation in the normal condition (A); Autophagic degradation of Keap1 (B); p21 (Cip1/WAF1)-mediated inhibition of Nrf2 ubiquitination (C); ER stress-mediated Nrf2 activation by the p38 MAPK-CRYAB axis (D); β-GSK3-β-TrPC-mediated Nrf2 ubiquitination and nuclear degradation (E); HAT-mediated Nrf2 acetylation and enhanced transcriptional activation of downstream targets (F); the Nrf2 canonical activation pathway (G); Nrf2 acts as an “Amplifier” via self-activation (H); Transcriptional enhancements of Nrf2 targets by Nrf2-SIRT6/BRG1-RNAPII complex-mediated ARE deacetylation or chromatin remodeling (I); Fine regulation of Nrf2 nuclear accumulation by autophagic signaling (J); Autophagic impairment initiates Nrf2 detrimental effects by activating angiotensinogen expression and subsequent pathophysiological alterations (K); The de-glycation of Nrf2 protein by FN3K enhances its stability and binding to small MAF protein and subsequent transcriptional activation (L). Abbreviations: Histone NAD(+)-dependent deacylase 6 (SIRT6); Heme oxygenase 1 (HO-1); α-crystallin B (CRYAB); Glycogen synthase kinase-3 (GSK-3β); RNA polymerase II (RNAP II); Brahma-related gene 1 (BRG1); Endoplasmic reticulum (ER); Histone acetyltransferases (HATs); Antioxidant response element (ARE); Autophagy-related genes (Atg); Ubiquitination (Ub); Angiotensinogen (Agt); Angiotensin II (Ang II); Autophagy-related genes (Atgs); ARE-like sequence 1/2 (AREL1/2); Cullin 3 (CUL3); Fructosamine-3-kinase (FN3K).
It has been reported that the small heat-shock protein α-crystallin B (CRYAB) acts as a molecular switch in determining endoplasmic reticulum (ER) stress-induced cardiomyocyte apoptosis following myocardial infarction (MI) [58]. A mechanistic study from the same laboratory showed that as a direct target of p38 MAPK, the deactivation of CRYAB changed in parallel with Nrf2 and was observed in p38 MAPK inhibitor-treated MI rats [62], suggesting p38 MAPK-mediated cardio-protective effects may rely on the interplay between Nrf2 and CRYAB. Interestingly, the pathogenesis of heart failure has also been associated with some inheritable mutations in CRYAB, such as R120GCryAB causing protein aggregation cardiomyopathy (PAC) [63]. Further mechanistic study revealed that this mutation causes increased ROS and protein aggregation contributing to the dissociation of Keap1 from Nrf2 and constitutive activation of Nrf2, promoting “reductive stress (RS)” due to the dysregulation of glutathione homeostasis. Increasing evidence suggests RS can cause endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) [64]. UPR signaling can not only trigger ROS generation from the ER and the mitochondria, which will sulfenylate a cysteine of IRE-1 (Inositol-requiring enzyme 1, ER stress sensor) [65], but can also activate Nrf2 signaling by phosphorylation mediated by the IRE1-TRAF2-ASK1-JNK pathway [66]. These novel findings suggest that chronic RS caused by constitutive Nrf2 activation may contribute to the pathogenesis of heart failure, and is indicative of the “dark side” of Nrf2 which must be taken account when we develop antioxidant-based therapeutic strategies.
3. Epigenetic regulation of Nrf2/ARE signaling pathway in heart failure
In addition to genetic regulation, epigenetic regulation plays an important role in gene expression. This includes DNA methylation, histone methylation and acetylation, non-coding RNAs, and chromatin remodeling [67, 68]. Relevant to this review, epigenetic mechanisms have been involved in oxidative stress during mutagenesis [69-71]. Numerous Nrf2 chemical activators have also been identified to activate downstream gene expression by epigenetic mechanisms [72, 73]. Mounting evidence suggests that epigenetic regulation plays a critical role in the pathogenesis of heart failure [74-77]. As a master regulator of redox homeostasis, the idea that Nrf2/ARE signaling is genetically regulated in the pathophysiological progression of heart failure is attracting much attention, increasing evidence suggests that this epigenetic regulation of the Nrf2/ARE signaling pathway are also involved in heart failure [23, 60, 78-81] (see Table 1).
Table 1:
Epigenetic regulation of Nrf2/ARE signaling pathway in heart failure
| Target | Epigenetic type |
Experimental Model | Mechanisms and outcomes |
References |
|---|---|---|---|---|
| Keap1 promoter | Demethylation of CpG island | Diabetic cardiomyopathy patients | Failure of Nrf2 mediated antioxidant system | [78] |
| Nrf2 promoter | Demethylation of CpG island | Cardiomyocytes; TAC mouse model | Nrf2 transcriptional activation | [79, 83, 85] |
| Nrf2 protein | Potential acetylation of Nrf2 | MI rat model | Enhanced binding of Nrf2 to CBP/p300 | [23] |
| Histone | Deacetylation of histone | DOX-induced cardiotoxicity in mice | Activation of Nrf2 and SIRT2 to inhibit myocardial apoptosis and oxidative stress | [103] |
| Histone | Deacetylation of histone | Cardiomyocytes in vitro | Enhanced activation of Nrf2 signaling by SIRT6 | [60] |
| Nrf2 mRNA | miNRA-27a, -28a and -34a | MI rat model and Cardiomyocytes in vitro | Inhibition of Nrf2 translation in response to cardiac stress | [129, 260] |
| Bach1 mRNA | miRNA-155-5p | Human Coronary Arteryc Endothelial Cells in vitro | Down-regulation of Bach1 potentially enhancing the cytoprotective response and preventing oxidative injury within infarct hearts | [132] |
| Keap1 mRNA | miRNA-7 | Patients with MI and heart failure; human neuroblastoma cells | Inhibition of Keap1 expression and enhanced Nrf2 activity | [133, 134] |
| Keap1 mRNA | miRNA-200a | Human cardiomyocytes; ischemic myocardial tissues; MI mouse model | Increased the nuclear translocation of Nrf2, and downstream target expressions | [135, 136] |
| Nrf2 promoter | lncRNA Sarrah | Acute MI mouse model; Rat cardiomyocytes in vitro | Nrf2 activation mediating the cardioprotective effects of Sarrah, which involves the recruitment of CRIP2 and CBP/p300 to Nrf2 promoter | [155] |
| Nrf2 mRNA | LncRNA LINC00261 | Rats I/R myocardial tissues and H/R-induced cardiomyocytes | LINC00261 acts as a spong of miRNA-23b-3p to positively regulates Nrf2 expression in cardiomyocyte | [159] |
Abbreviations: Transverse aortic constriction (TAC); Myocardial infarction (MI); Ischemia/reperfusion (I/R); hypoxia/reoxygenation (H/R); cysteine-rich protein 2 (CRIP2)
3.1. DNA methylation of Nrf2/ARE signaling in heart failure.
Recent studies in diabetic animals have identified cardiomyocyte dysfunction as an important mediator of heart failure, which was attributed to the demethylation of CpG islands within the Keap1 promoter, and subsequent overexpression of Keap1 and proteosomal degradation of Nrf2 as well as alterations of redox hemostasis [78]. Conversely, the demethylation of CpG islands in the promoter of Nrf2 is required for Nrf2 transcriptional activation. Tanshinone IIA (TIIA), a bioactive compound isolated from Salvia miltiorrhiza, has been reported to have a cyto-protective function against oxidative stress, myocardial ischemic injury and inflammation [82-86]. Molecular studies suggest that the cyto-protective effects of TIIA can be attributed to Nrf2 signaling activation and its downstream target gene expression [82][79]. Further evidence indicates that TIIA induces the degradation of Keap1 protein without effects on Keap1 transcription and directly inhibits the activity of DNA methyltransferases (DNMTs) resulting in the upregulation of Nrf2 and downstream genes in ventricular myocytes and subsequent inhibition of myocardial apoptosis [79]. These studies suggest that methylation/demethylation regulation of the Keap1/Nrf2 signaling axis may be potential targets for heart failure therapy.
3.2. Histone modifications of Nrf2/ARE signaling in heart failure.
Alterations in histone modifications also impact DNA accessibility to transcriptional machinery and other regulatory factors in human diseases [87, 88]. It has been well-documented that histone acetyltransferases (HATs) and deacetylases (HDACs) can control Nrf2 activity and ARE-dependent gene transcription by direct acetylation/deacetylation of either Nrf2 protein or in the promoter regions [52-54, 89, 90]. In addition to direct histone modifications of Nrf2 and ARE elements, other components of the Nrf2/ARE signaling pathway, such as Keap1 [91, 92], HO-1 [89], SOD2 [93] and Gclc [94], can also be regulated by histone acetylation and methylation. While histone modifications of Nrf2/ARE have been well-documented and studied in various diseases, these modifications of Nrf2/ARE signaling in CV diseases, especially heart failure remain to be further elucidated. Epigenetic studies on humans with heart failure and in animal models have recently been reviewed [95], suggesting that histone acetylation/deacetylation are closely tied to cardiac hypertrophy. Interestingly, in a recent study from this laboratory we demonstrated that curcumin, a Nrf2 activator, enhances exercise capacity in heart failure by activating Nrf2 signaling and subsequently upregulating antioxidant defenses in skeletal muscle [29]. It has been reported that curcumin can prevent both the acetylation of histone (H3 and H4) and GATA4 DNA-binding activity by inhibiting CBP/p300 activity which is a well-known HAT and is upregulated in the myocardium in decompensated heart failure. Curcumin also prevents the interaction between CBP/P300 and GATA4 in cardiomyocytes, thus contributing to the prevention of cardiac hypertrophy and the development of chronic heart failure [80, 96]. In a recent study from our laboratory we showed that systemic administration of bardoxolone methyl (CDDO-Me), another pharmacological activator of Nrf2, improves cardiac function in a rodent model of post MI chronic heart failure. Mechanistic studies revealed that CDDO-Me enhances the competitive binding of Nrf2 to CBP/P300 with NF-κB by inducing both transcriptional and translational increase of Nrf2, resulting in the selective increase of Nrf2 targets and the attenuation of myocardial inflammation [23].
It has been well-documented that the Sirtuin family of proteins, including Sirtuin 1-7, which are class III histone deacetylases (HDACs) and are distributed in different subcellular compartments (Sirt 1, 2 in cytoplasm and nucleus; Sirt 3, 4, 5 in mitochondria and Sirt 6, 7 exclusively in the nucleus). The Sirtuins play important roles in protecting the heart against oxidative stress and myocardial inflammation in cardiovascular disease, including heart failure [97-101]. Recently, adiponectin, a unique adipocyte-derived cytokine has been shown to be cardio-protective [102]. The adiponectin agonist, ADP355 has been demonstrated to possess cardio-protective effects in Doxorubicin (DOX)-induced cardiotoxicity by activating Nrf2 and Sirt 2 signaling pathways to inhibit myocardial apoptosis and oxidative stress [103]. This suggests that Sirt 2-mediated deacetylation of histones may enhance the binding of Nrf2 to ARE and the expression of downstream target genes. Sirt 6 plays an important role in the maintenance of cardiovascular homeostasis, especially in the preservation of cardiac function in response to stress [98, 101, 104]. Although Sirt 6-mediated cardio-protection depends on its deacetylase activity, Pan, et al. reported that Sirt 6 acts as a Nrf2 coactivator together with RNA polymerase II to transactivate Nrf2-regulated antioxidant genes, indicative of the importance of histone deacetylation for Nrf2 ARE-binding [59]. In addition, the Sirt6/Nrf2 axis is involved in the Forkhead box protein O6 (FOXO6)-regulated oxidative stress following MI. Down-regulation of FOXO6 can prevent hypoxic injury of cardiomyocytes through upregulating Sirt 6 to indirectly enhance Nrf2 activation [60]. Certainly, the regulatory functions of Sirt 6 on Nrf2/ARE signaling may be multi-faceted. Sirt 6 can also potentially promote Nrf2 activation by antagonizing the functions of Keap1 and Bach1, both Nrf2 natural inhibitors [104-106].
3.3. Non-coding RNA regulation of Nrf2/ARE signaling in heart failure.
Non-coding RNAs (ncRNAs) including microRNAs (miRNAs), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) are emerging as key regulators of cellular homeostasis, and have high relevance to various human diseases, including CV disease. They have become promising therapeutic targets [107-112]. The regulation and functional roles of these ncRNAs in heart failure has been recently reviewed by Gomes, et al. [113-115]. While the regulatory roles of miRNAs, lncRNAs and circRNAs in Nrf2 signaling have been well-documented in human disease [116-122], their regulatory functions in heart failure remains to be determined.
In 2009, Matkovich, et al. performed a miRNA profile analysis of human myocardial specimens from heart failure patients with or without left ventricular assist devices (LVAD) compared to normal heart specimens. Myocardial miRNA profiles suggested that 28 miRNAs were significantly up-regulated in failing hearts [123]. Interestingly, 4 out of 28 heart failure miRNAs, including miRNA-24, -26, -27, and -103, have been implicated in cellular hypoxic responses [124], and 1 out of the 4 heart failure miRNAs, miRNA-27a, was identified in the post-transcriptional control of Nrf2 expression and redox homeostasis in neuro-pathologies [125], diabetes [126] and liver diseases [127, 128]. This miRNA was recently found by our laboratory to be upregulated in an animal model of chronic heart failure (post MI) and contributing to the dysregulation of Nrf2 protein [129]. In addition to miR-27a, we also demonstrated that miRNA-28a and -34a were dysregulated in the ischemic myocardium, further contributing to the down-regulation of Nrf2 protein through translational inhibition.
MiRNAs can also indirectly target other components of the Nrf2/ARE signaling pathway, such as Keap1 and Bach1 (BTB and CNC homolog1, DJ-1 (PAPK7, Parkinson protein 7). It has been well-documented that miRNAs, including miRNA let-7, miRNA-98 and miRNA-196, enhance Nrf2-target gene expression, such as HO-1 in hepatocytes [130, 131], through the down-regulation of Bach1. Using Core-Shell Polymer-Based Nanoparticle technology, Antunes, et al. recently suggested that miR-155-5p can be delivered to endothelial cells and down-regulate Bach1 expression, potentially enhancing the cytoprotective response and preventing oxidative injury in infarcted hearts [132]. In addition, Xu, et al. performed a functional network analysis to investigate dysregulated miRNAs in myocardial infarction and heart failure. They showed that miR-7 plays a critical role in maintaining the dynamic balance of protein interactions in heart failure [133]. In a related study it was shown that miRNA-7 negatively regulates Keap1 expression resulting in enhanced Nrf2 activity [134]. In addition, miRNA-200a exerts protective effects on oxidative stress-induced cell death and inflammation in both the MI state and in diabetes by targeting Keap1-Nrf2 signaling [135, 136]. MiRNA-122 also plays a central role in various CV diseases, including heart failure, hypertension, MI, atherosclerosis and atrial fibrillation [137]. The potential mechanisms involve the indirect deactivation of Nrf2 by miRNA-122 targeting Sirt1 and Sirt 6, which enhance Nrf2 transcription by epigenetic regulation [59, 60, 138] (as illustrated in figure 2).
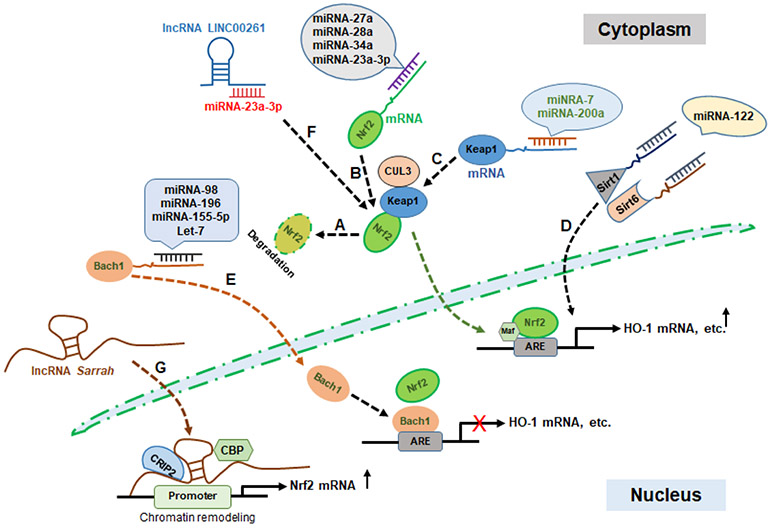
Figure 2. Schematic diagram of epigenetic regulations of Nrf2 signaling by noncoding RNAs in the pathogenesis of heart failure.
Keap1-dependent Nrf2 degradation in the normal condition (A); miRNAs inhibit Nrf2 signaling by directly inhibiting Nrf2 translation (B); miRNAs enhance Nrf2 signaling by indirectly inhibiting Keap1 translation (C); miRNA impacts Nrf2 signaling by inhibiting the translation of Nrf2 transcriptional enhancers (Sirt1/Sirt6) (D); miRNAs enhance Nrf2 signaling by inhibiting the translation of Nrf2 binding competitor (Bach1) (E); lncRNA LINC00261 enhances Nrf2 signaling by sponging miRNA-23a-3p (F); The recruitment of the cardiac transcription factor cysteine-rich protein 2 (CRIP2) and CBP/p300 by lncRNA Sarrah to the Nrf2 promoter for chromatin remodeling and Nrf2 transcriptional activation (G).
Long noncoding RNA (lncRNA, >200 bp) dysregulation has recently emerged as a mechanism for the modulation of Nrf2 signaling (as reviewed in [139]), while Nrf2 as a transcriptional factor also regulates the expression of several lncRNAs contributing to its cytoprotective effects as either transcriptional activators [140-143] or repressors [144]. These lncRNAs regulate Nrf2 signaling by different mechanisms including acting as miRNA sponges [145-149], histone acetylation [150], targeting Nrf2 inhibitors [151, 152] and physical interaction with Nrf2 [153] as well as serving as a functional pseudogene to regulate Nrf2 targets [143]. While these lncRNAs have been involved in Nrf2 regulation in neurologic diseases [148, 154], reproductive diseases [150], hepatic ischemia/reperfusion injury [147] and cancer [121, 143, 152, 153], little research has focused on their regulation in heart failure.
Recent studies suggest that lncRNAs also contribute to the pathophysiology of heart failure through targeting Nrf2 signaling. Trembinski, et al. [155] recently identified an aging-regulated lncRNA, designated as lncRNA Sarrah, which can protect cardiomyocytes from apoptosis after acute myocardial infarction. Using lncRNA Sarrah gain- and loss-of function strategies, this group observed that both transcriptional and translational regulation of Nrf2 were reduced after knockdown of lncRNA Sarrah by siRNAs. They further showed that Sarrah can directly bind to the promoter of genes that regulate chromatin remodeling by forming RNA-DNA-DNA triplex and subsequent gene activation and that Nrf2 was one of the targets mediating the cardioprotective effects of lncRNA Sarrah. This lncRNA also involves the recruitment of the cardiac transcription factor cysteine-rich protein 2 (CRIP2) and CBP/p300 to the Nrf2 promoter, suggesting the contribution of the lncRNA Sarrah-Nrf2 axis to cardiomyocyte survival following MI. Another lncRNA, LINC00261 has been reported to suppress tumor progression by either sponging miRNAs or inhibiting the demethylation of the oncogene promoter [156-158]. Recently, this lncRNA was shown to be involved in ischemia/reperfusion (I/R)-mediated acute MI. Zhang, et al. [159] demonstrated that the lncRNA LINC00261 protects cardiomyocytes from hypoxia/reoxygenation (H/R)-induced apoptosis by sponging miRNA-23a-3p to positively regulate Nrf2 expression. The potential roles of some non-coding RNAs in the regulating of the Nrf2/ARE signaling pathway in heart failure is illustrated in figure 2. However, their functions in heart failure still remain to be further elucidated.
The above discussion is preamble to the following assessment of the role played by myocardial derived extracellular vesicles (EVs) in transporting regulatory non-coding RNAs, proteins and other molecules that regulate oxidative stress through the Nrf2/Keap1 pathway in the heart and in remote tissues.
4. Extracellular vesicles, oxidative stress and chronic heart failure
Extracellular Vesicles have long been thought to be cell garbage, but are now emerging as important mediators of intercellular and intra-organ communication involved in various physiological and pathological processes in human diseases [160-163]. Based on their biogenic mechanisms, size range and vesicle components, EVs have been classified into two main categories, including ectosomes and exosomes. Ectosomes are further divided into different categories, including microvesicles, microparticles and large vesicles in the size range of 50-1000 nm in diameter. Exosomes are one type of endosomal small EV with a 40-150 nm size range. Exosomes can be secreted by most cell types and classified into classical- (CD63+, CD81+ and CD9+ expressing) and non-classic (CD63−, CD81− and CD9−) exosomes (as reviewed in [164-166]). It has been well-known that proteins, genetic material, lipids and metabolites can be actively and selectively incorporated into EVs [167, 168]. These EVs mediate intra- and inter-organ communication under normal and pathological conditions by shuttling their cargoes from donor to recipient cells and tissues.
4.1. Extracellular vesicles and cardiac remodeling in heart failure
While several soluble factors secreted by cardiac tissue including cytokines, chemokines and growth factors have been directly implicated in local inflammatory responses, oxidative stress and cardiac repair following MI in a growing number of studies have demonstrated that EVs have been shown to impact the pathogenesis of heart failure through local and remote communication mechanisms [169, 170]. For instance, circulating angiotensin receptor 1 (AT1R)-enriched EVs released from the heart under cardiac stress likely modulate vascular responses to neurohormonal stimulation [171]. Myocardial infarction can significantly increase cardiac EV generation which, in turn, drive cardiac inflammation and remodeling by stimulating the innate immune system and cardiac monocyte infiltration. Recent studies suggest that large EVs, mainly secreted from cardiomyocytes and endothelial cells in the ischemic myocardium, were taken up by infiltrating monocytes resulting in an increase of pro-inflammatory cytokines and chemokines [169]. Interestingly, pro-inflammatory macrophages after MI also secrete EVs containing pro-inflammatory cytokines, including IL-1α and IL-1β which, in turn, contribute to cardiomyocyte toxicity and cardiac dysfunction through TLR4-dependent NF-κB activation [172]. These events suggest a pathological role of EV proteins in heart failure.
It has been well-documented that circulating miRNAs are highly stable and actively transported by either binding to RNA-binding proteins resistant to nuclease degradation or entrapped in EVs [173-176]. Cardiac miRNA-enriched EVs secreted from cardiac fibroblasts have become a paracrine signaling mediator of cardiomyocyte hypertrophy through EV communication [177, 178]. In addition to cardiac hypertrophy, EV components, such as non-coding RNAs, are involved in cardiac fibrosis following MI [179]. For example, EV-enriched miRNA-21 secreted by cardiomyocytes in response to cardiac stress, contributes to cardiac fibrosis after myocardial ischemia through the activation of the AKT signaling pathway [180]. In addition, miRNA-30d has been used as a biomarker to predict left ventricular remodeling and clinical outcome in heart failure [181-183]. Recent mechanistic studies showed that miRNA-30d can not only inhibit autophagy-promoted ferroptosis in cardiomyocytes via binding to ATG5 after MI [184], but is also selectively overexpressed in cardiomyocytes in response to acute hypoxic stress and protects cells against MI-induced apoptosis through targeting mitogen-associate protein kinase (MA4K4). Interestingly, miRNA-30d can be incorporated into cardiomyocyte-secreted EVs, further contributing to cardiac fibrosis by directly targeting integrin-5α [185]. Recently, lncRNA-enriched EVs secreted by cardiomyocytes during cardiac hypoxia have also been found to contribute to cardiac fibrosis after MI [186]. These studies suggest a role for diagnostic, prognostic and therapeutic applications of EVs in heart failure.
4.2. Extracellular vesicles and Nrf2/ARE signaling in heart failure
A critical role for EVs has been demonstrated in redox-related processes by either producing ROS or eliminating ROS machinery [187]. The biogenesis of EVs can also be regulated by ROS [188, 189]. Extracellular vesicles can carry ROS generating and ROS scavenging molecules to recipient cells to regulate the redox status of remote targets. For example, functional NADPH oxidase 2 (NOX2)-enriched EVs released from cytokine-recruited inflammatory macrophages mediate the regeneration of injured axons by ROS generation [190], and Nox2-enriched EVs can prevent immune processes associated with chronic inflammation and autoimmune diseases by inhibiting the proliferation CD4+ T cells once secreted by CD8+ regulatory T cells [191, 192]. In addition to membrane bound Nox2, mitochondrial proteins, mitochondrial DNA and even free mitochondria which accumulates DAMPs such as TNF-α and ROS can mediate intercellular communications by EVs in human diseases, including cancer [193] and vascular inflammatory disease [194]. On the other hand, increasing evidence suggests that antioxidant proteins and small molecule ROS scavengers as well as the reductive power of NAPDH can also be transferred by EVs to protect recipient cells against oxidative stress [195-198].
Important for this discussion, EVs are emerging as critical mediators for redox modulation in heart failure. For instance, circulating vesicular redox sensitive-miRNAs have been demonstrated to have a diagnostic, prognostic and therapeutic potential in heart failure patients [199]. It has been reported that circulating miRNA-1 is significantly increased in heart failure [123, 200, 201], contributing to cardiac oxidative stress by directly targeting SOD1, Gclc, and G6PD to increase ROS levels and susceptibility to oxidative stress in the myocardium [202]. In addition to miRNA-1, 27 miRNAs (miR-15a, -16, -22, -24, -26a, -26b, -27a, -29a, -29b, -30a-5p, -30b, -30c, -30d, -103, -125b, -126, -130a, -133b, -143, -195, -199a-3p, -378, -499, let-7f, let-7g, let-7i) have been identified to be significantly increased in heart failure patients. Importantly, these miRNA signatures in heart failure are reversed by left ventricular assist device (LVAD) implantation [123]. Bioinformatic analysis (TargetScanHuman 7.2) suggests that miRNA-27 including miRNA-27a-3p and miRNA-27b-3p, can directly target the 3’ UTR of Nrf2 to inhibit the translation of Nrf2 mRNA. This is consistent with the above clinical findings in heart failure patients and has been validated in infarcted hearts of rats [129]. In addition, other miRNAs, including miR-155-5p [132], miRNA-7 [133, 134], miRNA-200a [135, 136], miRNA-28a and miRNA-34a [129] have been directly or indirectly involved in the regulation of Nrf2 translation in the pathogenesis of heart failure. Interestingly, studies from our laboratory demonstrated that three of these miRNAs, including miRNA-27a, -28-3p, and -34a, were highly expressed in the left ventricle of infarcted hearts. In vitro data suggest that these miRNAs can be preferentially incorporated into EVs in response to TNF-α stimulation, and secreted from cardiac cells to dysregulate Nrf2 translation in recipient cells by EV-mediated intercellular communication [129]. MiRNA-200a has been reported to epigenetically inhibit Keap1 expression, indirectly activating Nrf2/ARE signaling to prevent hepatic fibrosis primarily due to a reduction in oxidative stress [203]. Interestingly, down-regulated miRNA-200a in EVs released from aged mesenchymal stem cells (MSC) contributes to the impairment of young MSC-EV-induced cardio-protective effects post MI, [204] probably through restoration of Keap1 expression to significantly abrogate Nrf2 signaling-mediated cardio-protective effects.
Recently, miRNA-155-5p has been reported to induce vascular protection. Nanoparticle-loaded miRNA-155-5p was found to be cytoprotective in endothelial cells (ECs) and decreased injury in the infarcted heart [132]. Further mechanistic studies show that miRNA155-5p can be incorporated into EVs and secreted from fibroblasts and contribute to the proliferative inhibition of vascular smooth muscle cells by suppressing ACE expression [205]. Moreover, EV-miRNA-155 derived from macrophages is a paracrine regulator of fibroblast proliferation and inflammation in MI by targeting the Son of Sevenless 1 (Sos1) and Suppressor of Cytokine Signaling 1 (SOCS1) [206]. These studies suggest that EV-enriched miRNA-155 mediated intercellular communication contribute to cardio-protection through inhibition of Bach1 translation to indirectly enhance the Nrf2/HO-1 signaling pathway following MI. Interestingly, a recent study by Li, et al. [207] suggested that EV Nrf2 protein secreted from Nrf2-overexpressing adipose-derived stem cells protects endothelial progenitor cells against high glucose-induced ROS generation and inflammatory cytokine expression. The latter promotes vascularization in a diabetic foot ulcer rat model, suggesting that this strategy may be therapeutic.
In addition to the involvement of EVs in the dysregulation of oxidative stress through Nrf2 signaling in the infarcted and failing heart through intercellular communication within the heart, EV-mediated inter-organ communications, especially heart-brain communication, may contribute to the pathogenesis of heart failure via regulating Nrf2 signaling. Mounting evidence has demonstrated a reciprocal crosstalk between the heart and brain [208, 209]. On the one hand, brain injuries, such as stroke, may contribute to cardiac dysfunction by several mechanisms, including neurohormonal regulation, immune responses and inflammation [208]. On the other hand, a reciprocal relationship exists between the heart and brain in heart failure resulting in functional and anatomic abnormalities known as the cardio-cerebral syndrome [210-213]. While increased sympathetic outflow to the heart has been well established in heart failure [48, 214-216], and this communication pathway is mediated primarily by discrete neural connections [217], increasing evidence suggest that cardiac-derived EVs following MI play important roles in brain injury. Sun et al. [209] reported that MI causes neuronal microtubular damage in the hippocampus through increased cardiac-derived miRNA-1 which circulates to the brain and targets brain-specific tubulin polymerization promoting protein (TPPP/p25). Our previous studies have demonstrated that sympatho-excitation in heart failure was attributed, in part, to central oxidative stress and reduced Nrf2 and antioxidant enzyme expression in the RVLM [30]. Selective deletion of Nrf2 in the RVLM mimicked this pathophysiological phenotype including elevated blood pressure, increased sympathetic outflow and impaired arterial baroreflex function [28]. Conversely, selective overexpression of Nrf2 in the RVLM attenuated sympatho-excitation in mice with chronic heart failure [30]. These studies suggest an important role for Nrf2 as a central target for autonomic modulation in heart failure, and suggest a heart-brain communication that involves Nrf2. In a recent study we showed that cardiac cells including cardiomyocytes and fibroblasts, can secrete Nrf2-targeting miRNA-enriched EVs in response to cardiac stress, and that these miRNAs contribute to dysregulation of Nrf2 signaling once taken up by recipient cells and contribute to pathological alterations, such as cardiomyocyte hypertrophy [129]. Given that Nrf2-targeting miRNA-enriched EVs are secreted into the extracellular space and circulate to sympatho-regulatory areas of the brain in diseases such as chronic heart failure, they are likely to contribute to the dysregulation of Nrf2 signaling in structures such as the RVLM and modulate oxidative stress-mediated sympatho-excitation (Figure 3). Thus, it remains to be further investigated if these cardiac-derived EV-enriched Nrf2-targeting miRNAs or other potential components contribute to the heart-brain crosstalk in the oxidative regulation of sympathetic outflow through targeting the Nrf2/Antioxidant signaling pathway.
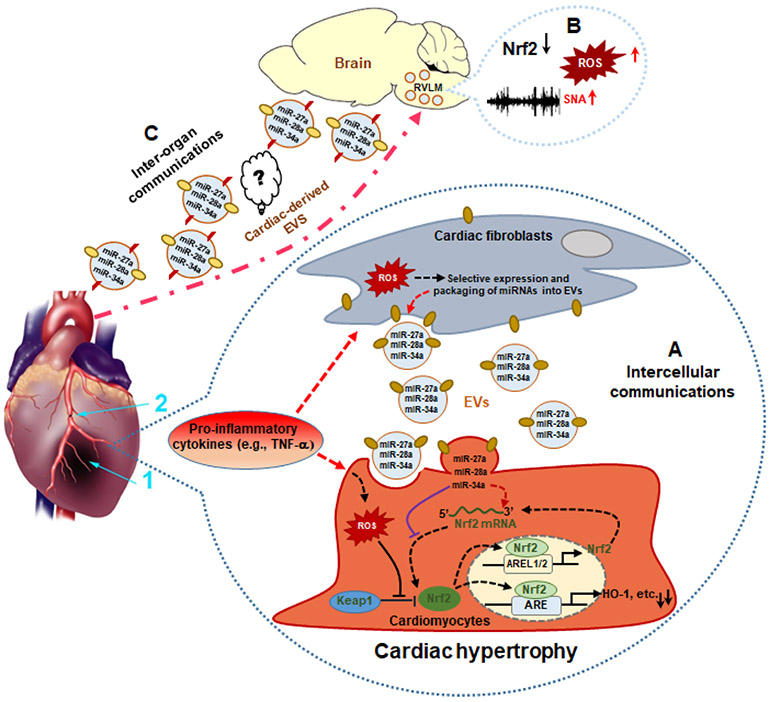
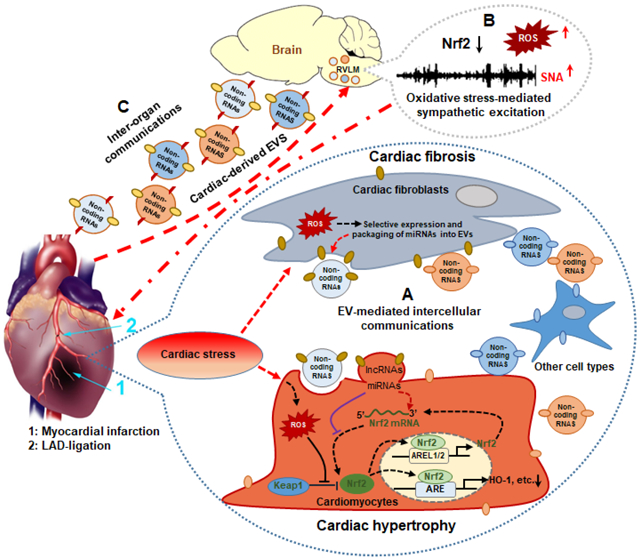
Figure 3. MI-induced cardiac-derived EVs potentially regulate myocardial and brain Nrf2 signaling pathways by intra- and inter-organ communication.
Under cardiac stress, fibroblast-derived EVs with miRNAs targeting Nrf2 mRNA inhibits Nrf2 translation in cardiomyocytes contributing to the dysregulation of antioxidant genes during MI (A); Nrf2 signaling was dysregulated in the rostral ventrolateral medulla (RVLM) in rats with heart failure, contributing to the increased ROS and sympathetic nerve activity (SNA) (B); Circulating cardiac-derived EVs may potentially mediate heart-brain communication through targeting brain the Nrf2 signaling pathway in heart failure (C); 1: Myocardial Infarction (MI); 2: Ligation of Left Anterior Descending (LAD) coronary artery
5. Potential clinical applications and future perspectives
Given the potent effects of Nrf2 in cardiovascular disease, it is important to develop novel prognostic, diagnostic and therapeutic strategies for heart failure that rely on activation of this transcription factor. It has been well documented that the central renin angiotensin aldosterone system (RAAS) mediates, in part, sympatho-excitation in the setting of heart failure [218-220]. Recent studies from our laboratory suggest that selective activation of Nrf2 by sulforaphane (a natural isothiocyanate derived from cruciferous vegetables) in the RVLM can attenuate the central Ang II-induced pressor response by upregulating Nrf2-downstream antioxidant enzymes [221]. Activation of Nrf2 in skeletal muscle of mice with heart failure with low ejection fraction (HFrEF) by curcumin improves exercise capacity in heart failure [29]. These findings suggest a promising therapeutic strategy impacting exercise tolerance and the quality of life in HFrEF patients. Furthermore, mechanistic studies have shown that suforaphane not only promotes the dissociation of Nrf2 from Keap1 by modifying the sulfhydryl groups of Keap1 [222], but also enhances Nrf2 expression by regulating the methylation and acetylation status at the Nrf2 promoter (reviewed in [119]). In addition, curcumin-induced Nrf2 signaling activation has been attributed to the demethylation of CpG islands in the Nrf2 promoter by inhibiting the activity of DNA methyltransferases [223].
Recently, we investigated the effects of bardoxolone methyl, a pharmacological activator of Nrf2, in a rodent model of heart failure and observed that short-term systemic administration of bardoxolone methyl exhibited beneficial effects on cardiac function, including increased cardiac output and stroke volume and decreased left ventricle end-diastolic pressure. Molecular studies further suggest that bardoxolone methyl regulates Nrf2 signaling by either modifying Keap1 to release Nrf2 and translocation to the nucleus where Nrf2 competitively binds to the Creb binding protein (CBP) with NF-κB, or directly inhibits IkKβ activity resulting in a reduction of IκB phosphorylation and subsequent cytosolic sequestration of NF-κB, both contributing to enhanced antioxidant enzyme transcription and attenuated myocardial inflammation in injured myocardium [23]. These studies suggest that small molecule Nrf2 activators provide a promising therapeutic strategy for the treatment of heart failure. It has been well-documented that Nrf2 activation mediated by small molecule activators contributes to cardio-protection by multiple mechanisms including disruption of Keap1-mediated Nrf2 ubquitination, promoting autophagic degradation of Keap1, and metabolic regulation [224-226]. Pharmacological activators of Nrf2 have been used clinically for the treatment of oxidative stress-related chronic inflammatory diseases including diabetes, neurodegenerative disorders, cardiovascular diseases and renal disease [227]. The cardio-protective effects of Nrf2 signaling appears to be dependent on the integrity of myocardial autophagy during cardiac remodeling. Qin, et al. reported that functional autophagy switches on Jak2/Fyn signaling to mediate Nrf2 nuclear export and subsequent degradation. Once cell autophagic function is impaired during pressure overload, abnormal nuclear accumulation of Nrf2 contributes to maladaptive cardiac remodeling and heart failure through direct Nrf2-mediated overexpression of angiotensinogen [49]. This may explain why patients with diabetic renal disease clinically treated with bardoxolone methyl are prone to heart failure. This finding directly led to the early termination of clinical trials of bardoxolone methyl [228, 229]. It is likely that small molecule Nrf2 activators have broad applications in the clinical treatment of heart failure, but not without potential complications. Importantly, the concept of reductive stress (RS) is also attracting attention by the scientific community. RS is defined as excessive antioxidant production in the presence of an intact redox system. Chronic RS can induce oxidative stress, which in turn, feeds forward to amplify RS [230, 231]. Increasing evidence [63, 231-233] shows that constitutive activation of Nrf2 signaling can cause RS which will drive pathological cardiomyopathy with diastolic dysfunction, leading to heart failure. Nrf2 deficiency will attenuate the RS-induced pathological phenotypes. These studies suggest that Nrf2 activity needs to be maintained at optimal levels so that abnormal activation of Nrf2 does not contribute to either oxidative stress or reductive stress contributing to the pathogenesis of heart failure.
Circulating miRNAs are emerging as promising biomarkers for various diseases, including heart failure. Clinical and animal studies have demonstrated the possibility that altered circulating miRNA profiling can serve as a diagnostic and prognostic test in end-stage cardiomyopathy and heart failure patients [123, 234, 235]. Oxidative stress associated miRNAs have been linked to the progression of heart failure and show therapeutic potential for heart failure (reviewed in [199]). Importantly, some miRNAs that target the Nrf2 signaling pathway, also show significant alteration in the plasma of patients with coronary artery disease. These include miRNA-155 [236] and miRNA-27a [237]. Increased levels of circulating miRNA-27a has also been used to predict clinical outcomes from acute MI [238], consistent with the alterations of miRNA-27a seen in patients with failing hearts [123]. Accumulating evidence supports the view that EVs are major vehicles for circulating miRNAs protected from circulating ribonuclease [239]. Previous studies from this laboratory also demonstrated EV-mediated intercellular communication by which miRNAs that target Nrf2 dysregulate Nrf2 expression in heart failure [129], supporting the possibility that EV-enriched Nrf2-targeting miRNAs may be used as prognostic and diagnostic markers for MI and heart failure.
In addition to EV-mediated intercellular communications within heart, different tissues also communicate with each other by various signals, (e.g. bioactive molecules) delivered by EVs to maintain homeostasis and adapt to external conditions [240, 241]. Heart failure is associated with brain injury/dysfunction including anatomic and physiological alterations (reviewed in [213]). It has recently been demonstrated that cardiac dysfunction contributes to brain structural changes by altering gray matter density (GMD) [211]. These investigators concluded that ejection fraction is positively correlated with regional GMD. Although neurohormonal regulation is believed to contribute to heart-brain crosstalk in the setting of heart failure, mounting evidence suggests that EVs and their cargoes, can act as mediators of this communication pathway. For example, cardiac-derived miRNA-1 has been implicated in MI-induced neuronal damage in the mouse hippocampus [209]. Transgenic overexpression of miRNA-1 in the mouse heart attenuates hippocampal synaptic vesicle exocytosis through miRNA-1-enriched EV-mediated translational inhibition of SNAP-25, whereas stereotaxic injection of a miRNA inhibitor into the hippocampus significantly ameliorates MI-induced deficits in hippocampal long-term potentiation (LTP) [242, 243]. Given that MI can induce Nrf2-targeting miRNA-enriched EV secretion from cardiac cells, and Nrf2 signaling dysregulation and sympatho-excitation were observed in sympathetic regulatory areas of the brain in heart failure, it will be of interest to determine if cardiac-derived miRNA-enriched EVs mediate dysregulation of Nrf2 signaling and subsequent oxidative stress, inflammation and sympatho-excitation in heart failure patients. Certainly, important concerns about the role of miRNAs contained in EVs in heart-brain interaction still remain to be addressed. These include the following questions: How do alterations in oxidative stress-associated miRNAs, especially those related to the Nrf2 signaling pathway, in the heart affect autonomic function? Can these Nrf2 signaling-related microRNAs be used to differentiate preserved (HFpEF) from reduced ejection fraction heart failure (HFrEF)? While circulating miRNA signatures have shown the potential to improve the diagnosis of heart failure and to differentiate between HFpEF and HFrEF [244, 245], the combination of circulating miRNAs including EV-enriched miRNAs and natriuretic peptides, will warrant the maximal diagnostic accuracy [246, 247]. How do cardiac-derived EVs distribute to specific brain regions? Does the expression profile of circulating and cardiac-derived EVs correspond to disease stages in heart failure? Because Nrf2 signaling also has anti-inflammatory functions, do cardiac-derived EV-enriched miRNAs affect central function and structure and infiltrating inflammatory cells indirectly secreted from brain EVs and their cargoes? Further studies are warranted to elucidate the role of intercellular communication in facilitating interaction between the heart and brain in the progression of heart failure.
Moreover, circulating miRNA-enriched EVs are not only used as prognostic and diagnostic biomarkers for heart failure, but may also be developed into new therapeutic strategies for heart failure. In particular, EVs as therapeutic tools may have potential to regulate the Nrf2 signaling pathway. Overexpressing Nrf2 in adipose-derived stem cells (ADSCs) may increase Nrf2-enriched EV secretion, which can promote increased vascularization, decrease in inflammation and oxidative stress in the progression of wound healing [207]. Similarly, platelet-derived exosomes also promote wound healing through YAP activation [248]. The interactions between Nrf2, Pix2 and YAP have been proposed to maintain the antioxidant response during cardiac repair after injury [249], suggesting that Nrf2 may also be involved in the platelet-derived EVs mediated protective effects. Small molecule Nrf2 activators, such as curcumin, have been encapsulated into EVs enhancing tissue bioavailability and efficacy [250, 251], and has been used for the treatment of oxidative stress-related and inflammatory diseases [252, 253]. In a recent preclinical study Cai et al. demonstrated the therapeutic potential of EVs carrying miRNA inhibitors (antagomirs) for ischemia-reperfusion-induced lung injury [254], suggesting the possibility of restoring Nrf2 signaling by EV-enriched antagomirs targeting Nrf2-related miRNAs. The use of EVs as delivery tools to treat heart failure still faces some challenges, especially insufficient targeting capabilities. Recent research in engineering modifications of EVs provide a new direction for EV-mediated therapeutic strategies in heart failure. Extracellular vesicle membrane surfaces engineered with either directly embedded tissue-specific antibodies or homing peptides ex vivo can enhance vesicle uptake in cells of interest [255]. For instance, EVs engineered with cyclo (Arg-Gly-Asp-D-Tyr-Lys) peptide (c(RGDyK)) peptide efficiently target lesions in the ischemic brain [256]. Extracellular vesicles engineered with rabies viral glycoprotein (RVG) peptide specifically bind in the central nervous system using the acetylcholine receptor [257]. Ischemic myocardial-targeting peptide CSTSMLKAC (IMTP) and cardiac homing peptide (CHP) were found to enhance the specificity of EV targeting ischemic myocardium as a therapeutic for MI [258, 259]. Strategies of this type can be used to target the Nrf2 signaling pathway in CV diseases.
Highlights.
Cardiac and central oxidative stress is characterized by reductions in Nrf2 following cardiac injury.
miRNAs targeting Nrf2 mRNA are highly expressed in the left ventricle of infarcted hearts and can be secreted into extracellular space by extracellular vesicles contributing to Nrf2 dysregulation.
Intra- and inter-organ communications are mediated by extravesicular transport of specific miRNAs that target Nrf2 translation in the heart failure state.
Delivery of miRNAs or antigomirs by the extracellular vesicles that target the Nrf2/Keap1 system can be developed as therapeutics for the heart failure syndrome.
Acknowledgements
Some of the work described here was supported by the National Institution of Health Grant P01 HL62222 to IHZ and American Heart Association (AHA) Career Development Award (19CDA34520004) to CT. IHZ was supported, in part, by the Theodore F. Hubbard Foundation.
Abbreviations
- Ang II
Angiotensin II
- Agt
Angiotensinogen
- ARE
Antioxidant response element
- AO
Antioxidant enzymes
- AREL1/2
ARE-like sequence 1/2
- Atgs
Autophagy-related genes
- BRG1
Brahma-related gene 1
- CNS
Central nervous system
- CHF
Chronic heart failure
- CBP
CREB-binding protein
- CUL3
Cullin 3
- CRIP2
Cysteine-rich protein 2
- CRYAB
α-crystallin B
- DNMTs
DNA methyltransferases
- ER
Endoplasmic reticulum
- EVs
Extracellular vesicles
- FN3K
Fructosamine-3-kinase
- GSK-3β
Glycogen synthase kinase-3
- HO-1
Heme oxygenase 1
- HATs
Histone acetyltransferases
- HDACs
Histone deacetylases
- H/R
Hypoxia/Reoxygenation
- I/R
Ischemia/reperfusion
- Keap1
Kelch Like ECH Associated Protein 1
- lncRNAs
Long non-coding RNAs
- KAT8
Lysine Acetyltransferase 8
- MI
Myocardial infarction
- Nox2
NADPH Oxidase
- NQO-1
NADPH Oxidase Quinone 1
- ncRNAs
Non-coding RNAs
- NLRP
NOD-, LRR- and pyrin domain-containing protein
- Nrf2
Nuclear factor erythroid 2-related factor 2
- ROS
Reactive oxygen species
- RAAS
Renin angiotensin aldosterone system
- RNAP II
RNA polymerase II
- RVLM
Rostral ventrolateral medulla
- SIRT6
Sirtuin 6
- SNA
Sympathetic nerve activity
- TAC
Transverse aortic constriction
- Ub
Ubiquitination
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ziaeian B, Fonarow GC, Epidemiology and aetiology of heart failure, Nat Rev Cardiol 13(6) (2016) 368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dharmarajan K, Rich MW, Epidemiology, Pathophysiology, and Prognosis of Heart Failure in Older Adults, Heart Fail Clin 13(3) (2017) 417–426. [DOI] [PubMed] [Google Scholar]
- [3].Chen L, Magliano DJ, Zimmet PZ, The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives, Nat Rev Endocrinol 8(4) (2011) 228–36. [DOI] [PubMed] [Google Scholar]
- [4].Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG, Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association, Circ Heart Fail 6(3) (2013) 606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association, Circulation 137(12) (2018) e67–e492. [DOI] [PubMed] [Google Scholar]
- [6].McMurray JJ, Pfeffer MA, Heart failure, Lancet 365(9474) (2005) 1877–89. [DOI] [PubMed] [Google Scholar]
- [7].Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A, Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells, Circ Res 99(9) (2006) 924–32. [DOI] [PubMed] [Google Scholar]
- [8].Takimoto E, Kass DA, Role of oxidative stress in cardiac hypertrophy and remodeling, Hypertension 49(2) (2007) 241–8. [DOI] [PubMed] [Google Scholar]
- [9].Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM, Redox signaling in cardiac myocytes, Free Radic Biol Med 50(7) (2011) 777–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tsutsui H, Ide T, Kinugawa S, Mitochondrial oxidative stress, DNA damage, and heart failure, Antioxid Redox Signal 8(9-10) (2006) 1737–44. [DOI] [PubMed] [Google Scholar]
- [11].Canton M, Menazza S, Sheeran FL, Polverino de Laureto P, Di Lisa F, Pepe S, Oxidation of myofibrillar proteins in human heart failure, J Am Coll Cardiol 57(3) (2011) 300–9. [DOI] [PubMed] [Google Scholar]
- [12].Siwik DA, Tzortzis JD, Pimental DR, Chang DL, Pagano PJ, Singh K, Sawyer DB, Colucci WS, Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro, Circ Res 85(2) (1999) 147–53. [DOI] [PubMed] [Google Scholar]
- [13].Siwik DA, Pagano PJ, Colucci WS, Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts, Am J Physiol Cell Physiol 280(1) (2001) C53–60. [DOI] [PubMed] [Google Scholar]
- [14].Costa S, Reina-Couto M, Albino-Teixeira A, Sousa T, Statins and oxidative stress in chronic heart failure, Rev Port Cardiol 35(1) (2016) 41–57. [DOI] [PubMed] [Google Scholar]
- [15].Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F, Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection, Progress in neurobiology 100 (2013) 30–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kwak MK, Itoh K, Yamamoto M, Kensler TW, Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter, Mol Cell Biol 22(9) (2002) 2883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tu W, Wang H, Li S, Liu Q, Sha H, The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases, Aging Dis 10(3) (2019) 637–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hu CM, Chen YH, Chiang MT, Chau LY, Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo, Circulation 110(3) (2004) 309–16. [DOI] [PubMed] [Google Scholar]
- [19].Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y, Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction, Hypertension 51(1) (2008) 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ashrafian H, Czibik G, Bellahcene M, Aksentijevic D, Smith AC, Mitchell SJ, Dodd MS, Kirwan J, Byrne JJ, Ludwig C, Isackson H, Yavari A, Stottrup NB, Contractor H, Cahill TJ, Sahgal N, Ball DR, Birkler RI, Hargreaves I, Tennant DA, Land J, Lygate CA, Johannsen M, Kharbanda RK, Neubauer S, Redwood C, de Cabo R, Ahmet I, Talan M, Gunther UL, Robinson AJ, Viant MR, Pollard PJ, Tyler DJ, Watkins H, Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway, Cell Metab 15(3) (2012) 361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Andrukhova O, Salama M, Rosenhek R, Gmeiner M, Perkmann T, Steindl J, Aharinejad S, Serum glutathione S-transferase P1 1 in prediction of cardiac function, J Card Fail 18(3) (2012) 253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Satta S, Mahmoud AM, Wilkinson FL, Yvonne Alexander M, White SJ, The Role of Nrf2 in Cardiovascular Function and Disease, Oxid Med Cell Longev 2017 (2017) 9237263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tian C, Gao L, Zhang A, Hackfort BT, Zucker IH, Therapeutic Effects of Nrf2 Activation by Bardoxolone Methyl in Chronic Heart Failure, J Pharmacol Exp Ther 371(3) (2019) 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T, Nrf2 protects against maladaptive cardiac responses to hemodynamic stress, Arterioscler Thromb Vasc Biol 29(11) (2009) 1843–50. [DOI] [PubMed] [Google Scholar]
- [25].Chan K, Lu R, Chang JC, Kan YW, NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development, Proc Natl Acad Sci U S A 93(24) (1996) 13943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li J, Zhang C, Xing Y, Janicki JS, Yamamoto M, Wang XL, Tang DQ, Cui T, Up-regulation of p27(kip1) contributes to Nrf2-mediated protection against angiotensin II-induced cardiac hypertrophy, Cardiovasc Res 90(2) (2011) 315–24. [DOI] [PubMed] [Google Scholar]
- [27].Shanmugam G, Challa AK, Litovsky SH, Devarajan A, Wang D, Jones DP, Darley-Usmar VM, Rajasekaran NS, Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice, Redox biology (2019) 101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gao L, Zimmerman MC, Biswal S, Zucker IH, Selective Nrf2 Gene Deletion in the Rostral Ventrolateral Medulla Evokes Hypertension and Sympathoexcitation in Mice, Hypertension 69(6) (2017) 1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wafi AM, Hong J, Rudebush TL, Yu L, Hackfort B, Wang H, Schultz HD, Zucker IH, Gao L, Curcumin improves exercise performance of mice with coronary artery ligation-induced HFrEF: Nrf2 and antioxidant mechanisms in skeletal muscle, Journal of applied physiology (Bethesda, Md. : 1985) 126(2) (2019) 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ma A, Hong J, Shanks J, Rudebush T, Yu L, Hackfort BT, Wang H, Zucker IH, Gao L, Upregulating Nrf2 in the RVLM ameliorates sympatho-excitation in mice with chronic heart failure, Free Radic Biol Med 141 (2019) 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen P, Wang L, Fan X, Ning X, Yu B, Ou C, Chen M, Targeted delivery of extracellular vesicles in heart injury, Theranostics 11(5) (2021) 2263–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Loyer X, Vion AC, Tedgui A, Boulanger CM, Microvesicles as cell-cell messengers in cardiovascular diseases, Circ Res 114(2) (2014) 345–53. [DOI] [PubMed] [Google Scholar]
- [33].Voukalis C, Shantsila E, Lip GYH, Microparticles and cardiovascular diseases, Ann Med 51(3-4) (2019) 193–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yamamoto M, Kensler TW, Motohashi H, The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis, Physiol Rev 98(3) (2018) 1169–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Itoh K, Ishii T, Wakabayashi N, Yamamoto M, Regulatory mechanisms of cellular response to oxidative stress, Free radical research 31(4) (1999) 319–24. [DOI] [PubMed] [Google Scholar]
- [36].Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD, A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62, Mol Cell Biol 30(13) (2010) 3275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M, The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1, Nat Cell Biol 12(3) (2010) 213–23. [DOI] [PubMed] [Google Scholar]
- [38].Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD, p62 links autophagy and Nrf2 signaling, Free Radic Biol Med 88(Pt B) (2015) 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Saito T, Ichimura Y, Taguchi K, Suzuki T, Mizushima T, Takagi K, Hirose Y, Nagahashi M, Iso T, Fukutomi T, Ohishi M, Endo K, Uemura T, Nishito Y, Okuda S, Obata M, Kouno T, Imamura R, Tada Y, Obata R, Yasuda D, Takahashi K, Fujimura T, Pi J, Lee MS, Ueno T, Ohe T, Mashino T, Wakai T, Kojima H, Okabe T, Nagano T, Motohashi H, Waguri S, Soga T, Yamamoto M, Tanaka K, Komatsu M, p62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent metabolic reprogramming, Nat Commun 7 (2016) 12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM, mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery, Cell 110(2) (2002) 163–75. [DOI] [PubMed] [Google Scholar]
- [41].Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M, Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy, Mol Cell 51(5) (2013) 618–31. [DOI] [PubMed] [Google Scholar]
- [42].Hay N, Sonenberg N, Upstream and downstream of mTOR, Genes Dev 18(16) (2004) 1926–45. [DOI] [PubMed] [Google Scholar]
- [43].Kaspar JW, Niture SK, Jaiswal AK, Nrf2:INrf2 (Keap1) signaling in oxidative stress, Free Radic Biol Med 47(9) (2009) 1304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA, Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers, Proc Natl Acad Sci U S A 103(3) (2006) 768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A, SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner, Mol Cell Biol 31(6) (2011) 1121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kaspar JW, Jaiswal AK, Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression, Faseb j 25(3) (2011) 1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jain AK, Jaiswal AK, GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2, J Biol Chem 282(22) (2007) 16502–10. [DOI] [PubMed] [Google Scholar]
- [48].Du ZX, Yan Y, Zhang HY, Liu BQ, Gao YY, Niu XF, Meng X, Wang HQ, Proteasome inhibition induces a p38 MAPK pathway-dependent antiapoptotic program via Nrf2 in thyroid cancer cells, J Clin Endocrinol Metab 96(5) (2011) E763–71. [DOI] [PubMed] [Google Scholar]
- [49].Qin Q, Qu C, Niu T, Zang H, Qi L, Lyu L, Wang X, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T, Nrf2-Mediated Cardiac Maladaptive Remodeling and Dysfunction in a Setting of Autophagy Insufficiency, Hypertension 67(1) (2016) 107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen H-H, Chang H-H, Chang J-Y, Tang Y-C, Cheng Y-C, Lin L-M, Cheng S-Y, Huang C-H, Sun M-W, Chen C-T, Kuo C-C, Enhanced B-Raf-mediated NRF2 gene transcription and HATs-mediated NRF2 protein acetylation contributes to ABCC1-mediated chemoresistance and glutathione-mediated survival in acquired topoisomerase II poison-resistant cancer cells, Free Radical Biology and Medicine 113 (2017) 505–518. [DOI] [PubMed] [Google Scholar]
- [51].Sanghvi VR, Leibold J, Mina M, Mohan P, Berishaj M, Li Z, Miele MM, Lailler N, Zhao C, de Stanchina E, Viale A, Akkari L, Lowe SW, Ciriello G, Hendrickson RC, Wendel HG, The Oncogenic Action of NRF2 Depends on De-glycation by Fructosamine-3-Kinase, Cell 178(4) (2019) 807–819.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sun Z, Chin YE, Zhang DD, Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response, Mol Cell Biol 29(10) (2009) 2658–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kawai Y, Garduño L, Theodore M, Yang J, Arinze IJ, Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization, J Biol Chem 286(9) (2011) 7629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen Z, Ye X, Tang N, Shen S, Li Z, Niu X, Lu S, Xu L, The histone acetylranseferase hMOF acetylates Nrf2 and regulates anti-drug responses in human non-small cell lung cancer, Br J Pharmacol 171(13) (2014) 3196–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang J, Ohta T, Maruyama A, Hosoya T, Nishikawa K, Maher JM, Shibahara S, Itoh K, Yamamoto M, BRG1 interacts with Nrf2 to selectively mediate HO-1 induction in response to oxidative stress, Mol Cell Biol 26(21) (2006) 7942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rojo AI, Medina-Campos ON, Rada P, Zuniga-Toala A, Lopez-Gazcon A, Espada S, Pedraza-Chaverri J, Cuadrado A, Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: Role of glycogen synthase kinase-3, Free Radic Biol Med 52(2) (2012) 473–87. [DOI] [PubMed] [Google Scholar]
- [57].Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD, Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response, Mol Cell 34(6) (2009) 663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mitra A, Basak T, Datta K, Naskar S, Sengupta S, Sarkar S, Role of α-crystallin B as a regulatory switch in modulating cardiomyocyte apoptosis by mitochondria or endoplasmic reticulum during cardiac hypertrophy and myocardial infarction, Cell Death Dis 4(4) (2013) e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pan H, Guan D, Liu X, Li J, Wang L, Wu J, Zhou J, Zhang W, Ren R, Zhang W, Li Y, Yang J, Hao Y, Yuan T, Yuan G, Wang H, Ju Z, Mao Z, Li J, Qu J, Tang F, Liu GH, SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2, Cell Res 26(2) (2016) 190–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jin A, Zhang Q, Li S, Li B, Downregulation of FOXO6 alleviates hypoxia-induced apoptosis and oxidative stress in cardiomyocytes by enhancing Nrf2 activation via upregulation of SIRT6, Journal of bioenergetics and biomembranes 52(6) (2020) 409–419. [DOI] [PubMed] [Google Scholar]
- [61].Yang Y, Tian T, Wang Y, Li Z, Xing K, Tian G, SIRT6 protects vascular endothelial cells from angiotensin II-induced apoptosis and oxidative stress by promoting the activation of Nrf2/ARE signaling, Eur J Pharmacol 859 (2019) 172516. [DOI] [PubMed] [Google Scholar]
- [62].Mitra A, Ray A, Datta R, Sengupta S, Sarkar S, Cardioprotective role of P38 MAPK during myocardial infarction via parallel activation of α-crystallin B and Nrf2, J Cell Physiol 229(9) (2014) 1272–82. [DOI] [PubMed] [Google Scholar]
- [63].Rajasekaran NS, Varadharaj S, Khanderao GD, Davidson CJ, Kannan S, Firpo MA, Zweier JL, Benjamin IJ, Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice, Antioxid Redox Signal 14(6) (2011) 957–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].KK SN, Devarajan A, Karan G, Sundaram S, Wang Q, van Groen T, Monte FD, Rajasekaran NS, Reductive stress promotes protein aggregation and impairs neurogenesis, Redox biology 37 (2020) 101739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hourihan JM, Moronetti Mazzeo LE, Fernández-Cárdenas LP, Blackwell TK, Cysteine Sulfenylation Directs IRE-1 to Activate the SKN-1/Nrf2 Antioxidant Response, Mol Cell 63(4) (2016) 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D, Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1, Science 287(5453) (2000) 664–6. [DOI] [PubMed] [Google Scholar]
- [67].Jaenisch R, Bird A, Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals, Nat Genet 33 Suppl (2003) 245–54. [DOI] [PubMed] [Google Scholar]
- [68].Wolffe AP, Matzke MA, Epigenetics: regulation through repression, Science 286(5439) (1999) 481–6. [DOI] [PubMed] [Google Scholar]
- [69].Shen L, Song CX, He C, Zhang Y, Mechanism and function of oxidative reversal of DNA and RNA methylation, Annu Rev Biochem 83 (2014) 585–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Niu Y, DesMarais TL, Tong Z, Yao Y, Costa M, Oxidative stress alters global histone modification and DNA methylation, Free Radic Biol Med 82 (2015) 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI, Oxidative stress, DNA methylation and carcinogenesis, Cancer Lett 266(1) (2008) 6–11. [DOI] [PubMed] [Google Scholar]
- [72].Su ZY, Shu L, Khor TO, Lee JH, Fuentes F, Kong AN, A perspective on dietary phytochemicals and cancer chemoprevention: oxidative stress, nrf2, and epigenomics, Top Curr Chem 329 (2013) 133–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Belanger AS, Tojcic J, Harvey M, Guillemette C, Regulation of UGT1A1 and HNF1 transcription factor gene expression by DNA methylation in colon cancer cells, BMC Mol Biol 11 (2010) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kim SY, Morales CR, Gillette TG, Hill JA, Epigenetic regulation in heart failure, Curr Opin Cardiol 31(3) (2016) 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Marín-García J, Akhmedov AT, Epigenetics of the failing heart, Heart Fail Rev 20(4) (2015) 435–59. [DOI] [PubMed] [Google Scholar]
- [76].Stratton MS, McKinsey TA, Epigenetic regulation of cardiac fibrosis, J Mol Cell Cardiol 92 (2016) 206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cao DJ, Epigenetic regulation and heart failure, Expert Rev Cardiovasc Ther 12(9) (2014) 1087–98. [DOI] [PubMed] [Google Scholar]
- [78].Liu ZZ, Zhao XZ, Zhang XS, Zhang M, Promoter DNA demethylation of Keap1 gene in diabetic cardiomyopathy, Int J Clin Exp Pathol 7(12) (2014) 8756–62. [PMC free article] [PubMed] [Google Scholar]
- [79].Yan SH, Zhao NW, Geng ZR, Shen JY, Liu FM, Yan D, Zhou J, Nie C, Huang CC, Fang ZY, Modulations of Keap1-Nrf2 signaling axis by TIIA ameliorated the oxidative stress-induced myocardial apoptosis, Free Radic Biol Med 115 (2018) 191–201. [DOI] [PubMed] [Google Scholar]
- [80].Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, Hasegawa K, The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats, J Clin Invest 118(3) (2008) 868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Qian M, Peng L, Liu Z, Tang X, Wang Z, Liu B, SIRT6 as a transcriptional coactivator of GATA4 prevents doxorubicin cardiotoxicity independently of its deacylase activity, bioRxiv (2019) 725044. [Google Scholar]
- [82].Zhang HS, Wang SQ, Nrf2 is involved in the effect of tanshinone IIA on intracellular redox status in human aortic smooth muscle cells, Biochem Pharmacol 73(9) (2007) 1358–66. [DOI] [PubMed] [Google Scholar]
- [83].Gu Y, Liang Z, Wang H, Jin J, Zhang S, Xue S, Chen J, He H, Duan K, Wang J, Chang X, Qiu C, Tanshinone IIA protects H9c2 cells from oxidative stress-induced cell death via microRNA-133 upregulation and Akt activation, Exp Ther Med 12(2) (2016) 1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cao FL, Xu M, Wang Y, Gong KR, Zhang JT, Tanshinone IIA attenuates neuropathic pain via inhibiting glial activation and immune response, Pharmacol Biochem Behav 128 (2015) 1–7. [DOI] [PubMed] [Google Scholar]
- [85].Jin HJ, Xie XL, Ye JM, Li CG, TanshinoneIIA and cryptotanshinone protect against hypoxia-induced mitochondrial apoptosis in H9c2 cells, PLoS One 8(1) (2013) e51720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li YI, Elmer G, Leboeuf RC, Tanshinone IIA reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidase, Life Sci 83(15-16) (2008) 557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Biancotto C, Frigè G, Minucci S, Histone modification therapy of cancer, Adv Genet 70 (2010) 341–86. [DOI] [PubMed] [Google Scholar]
- [88].Greer EL, Shi Y, Histone methylation: a dynamic mark in health, disease and inheritance, Nat Rev Genet 13(5) (2012) 343–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Liu GH, Qu J, Shen X, NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK, Biochim Biophys Acta 1783(5) (2008) 713–27. [DOI] [PubMed] [Google Scholar]
- [90].Mercado N, Thimmulappa R, Thomas CM, Fenwick PS, Chana KK, Donnelly LE, Biswal S, Ito K, Barnes PJ, Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress, Biochem Biophys Res Commun 406(2) (2011) 292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mishra M, Zhong Q, Kowluru RA, Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy, Invest Ophthalmol Vis Sci 55(11) (2014) 7256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang B, Zhu X, Kim Y, Li J, Huang S, Saleem S, Li RC, Xu Y, Dore S, Cao W, Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage, Free Radic Biol Med 52(5) (2012) 928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhong Q, Kowluru RA, Epigenetic modification of Sod2 in the development of diabetic retinopathy and in the metabolic memory: role of histone methylation, Invest Ophthalmol Vis Sci 54(1) (2013) 244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mishra M, Zhong Q, Kowluru RA, Epigenetic modifications of Nrf2-mediated glutamate-cysteine ligase: implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression, Free Radic Biol Med 75 (2014) 129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Liu CF, Tang WHW, Epigenetics in Cardiac Hypertrophy and Heart Failure, JACC Basic Transl Sci 4(8) (2019) 976–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yanazume T, Hasegawa K, Morimoto T, Kawamura T, Wada H, Matsumori A, Kawase Y, Hirai M, Kita T, Cardiac p300 is involved in myocyte growth with decompensated heart failure, Mol Cell Biol 23(10) (2003) 3593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Matsushima S, Sadoshima J, The role of sirtuins in cardiac disease, Am J Physiol Heart Circ Physiol 309(9) (2015) H1375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].D’Onofrio N, Servillo L, Balestrieri ML, SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection, Antioxid Redox Signal 28(8) (2018) 711–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Winnik S, Auwerx J, Sinclair DA, Matter CM, Protective effects of sirtuins in cardiovascular diseases: from bench to bedside, Eur Heart J 36(48) (2015) 3404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Saiyang X, Deng W, Qizhu T, Sirtuin 6: A potential therapeutic target for cardiovascular diseases, Pharmacol Res (2020) 105214. [DOI] [PubMed] [Google Scholar]
- [101].Yepuri G, Ramasamy R, Significance and Mechanistic Relevance of SIRT6-Mediated Endothelial Dysfunction in Cardiovascular Disease Progression, Circ Res 124(10) (2019) 1408–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Maeda N, Funahashi T, Matsuzawa Y, Shimomura I, Adiponectin, a unique adipocyte-derived factor beyond hormones, Atherosclerosis 292 (2020) 1–9. [DOI] [PubMed] [Google Scholar]
- [103].Zhao D, Xue C, Li J, Feng K, Zeng P, Chen Y, Duan Y, Zhang S, Li X, Han J, Yang X, Adiponectin agonist ADP355 ameliorates doxorubicin-induced cardiotoxicity by decreasing cardiomyocyte apoptosis and oxidative stress, Biochem Biophys Res Commun 533(3) (2020) 304–312. [DOI] [PubMed] [Google Scholar]
- [104].Kanwal A, Pillai VB, Samant S, Gupta M, Gupta MP, The nuclear and mitochondrial sirtuins, Sirt6 and Sirt3, regulate each other’s activity and protect the heart from developing obesity-mediated diabetic cardiomyopathy, Faseb j 33(10) (2019) 10872–10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yu J, Sun W, Song Y, Liu J, Xue F, Gong K, Yang X, Kang Q, SIRT6 protects retinal ganglion cells against hydrogen peroxide-induced apoptosis and oxidative stress by promoting Nrf2/ARE signaling via inhibition of Bach1, Chem Biol Interact 300 (2019) 151–158. [DOI] [PubMed] [Google Scholar]
- [106].Ka SO, Bang IH, Bae EJ, Park BH, Hepatocyte-specific sirtuin 6 deletion predisposes to nonalcoholic steatohepatitis by up-regulation of Bach1, an Nrf2 repressor, Faseb j 31(9) (2017) 3999–4010. [DOI] [PubMed] [Google Scholar]
- [107].Esteller M, Non-coding RNAs in human disease, Nat Rev Genet 12(12) (2011) 861–74. [DOI] [PubMed] [Google Scholar]
- [108].Lu D, Thum T, RNA-based diagnostic and therapeutic strategies for cardiovascular disease, Nature Reviews Cardiology 16(11) (2019) 661–674. [DOI] [PubMed] [Google Scholar]
- [109].Qureshi IA, Mehler MF, Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease, Nature Reviews Neuroscience 13(8) (2012) 528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Goodall GJ, Wickramasinghe VO, RNA in cancer, Nature Reviews Cancer (2020). [DOI] [PubMed] [Google Scholar]
- [111].Aufiero S, Reckman YJ, Pinto YM, Creemers EE, Circular RNAs open a new chapter in cardiovascular biology, Nature Reviews Cardiology 16(8) (2019) 503–514. [DOI] [PubMed] [Google Scholar]
- [112].Tang Y, Zhou T, Yu X, Xue Z, Shen N, The role of long non-coding RNAs in rheumatic diseases, Nature Reviews Rheumatology 13(11) (2017) 657–669. [DOI] [PubMed] [Google Scholar]
- [113].Devaux Y, Creemers EE, Boon RA, Werfel S, Thum T, Engelhardt S, Dimmeler S, Squire I, Circular RNAs in heart failure, Eur J Heart Fail 19(6) (2017) 701–709. [DOI] [PubMed] [Google Scholar]
- [114].Altesha MA, Ni T, Khan A, Liu K, Zheng X, Circular RNA in cardiovascular disease, J Cell Physiol 234(5) (2019) 5588–5600. [DOI] [PubMed] [Google Scholar]
- [115].Gomes CPC, Schroen B, Kuster GM, Robinson EL, Ford K, Squire IB, Heymans S, Martelli F, Emanueli C, Devaux Y, Regulatory RNAs in Heart Failure, Circulation 141(4) (2020) 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cheng X, Ku CH, Siow RC, Regulation of the Nrf2 antioxidant pathway by microRNAs: New players in micromanaging redox homeostasis, Free Radic Biol Med 64 (2013) 4–11. [DOI] [PubMed] [Google Scholar]
- [117].Guo Y, Yu S, Zhang C, Kong AN, Epigenetic regulation of Keap1-Nrf2 signaling, Free Radic Biol Med 88(Pt B) (2015) 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Cai LJ, Tu L, Huang XM, Huang J, Qiu N, Xie GH, Liao JX, Du W, Zhang YY, Tian JY, LncRNA MALAT1 facilitates inflammasome activation via epigenetic suppression of Nrf2 in Parkinson’s disease, Mol Brain 13(1) (2020) 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Bhattacharjee S, Dashwood RH, Epigenetic Regulation of NRF2/KEAP1 by Phytochemicals, Antioxidants (Basel) 9(9) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gong W, Li J, Zhu G, Wang Y, Zheng G, Kan Q, Chlorogenic acid relieved oxidative stress injury in retinal ganglion cells through IncRNA-TUG1/Nrf2, Cell Cycle 18(14) (2019) 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Joo MS, Shin SB, Kim EJ, Koo JH, Yim H, Kim SG, Nrf2-lncRNA controls cell fate by modulating p53-dependent Nrf2 activation as an miRNA sponge for Plk2 and p21(cip1), Faseb j 33(7) (2019) 7953–7969. [DOI] [PubMed] [Google Scholar]
- [122].Zhang RJ, Li Y, Liu Q, Gao YJ, Du J, Ma J, Sun SG, Wang L, Differential Expression Profiles and Functional Prediction of Circular RNAs and Long Non-coding RNAs in the Hippocampus of Nrf2-Knockout Mice, Front Mol Neurosci 12 (2019) 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Matkovich SJ, Van Booven DJ, Youker KA, Torre-Amione G, Diwan A, Eschenbacher WH, Dorn LE, Watson MA, Margulies KB, Dorn GW 2nd, Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support, Circulation 119(9) (2009) 1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu C-G, Croce CM, Negrini M, Calin GA, Ivan M, A MicroRNA Signature of Hypoxia, Molecular and Cellular Biology 27(5) (2007) 1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, Mahimainathan L, Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells, PLoS One 7(12) (2012) e51111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ebrahimpour S, Shahidi SB, Abbasi M, Tavakoli Z, Esmaeili A, Quercetin-conjugated superparamagnetic iron oxide nanoparticles (QCSPIONs) increases Nrf2 expression via miR-27a mediation to prevent memory dysfunction in diabetic rats, Sci Rep 10(1) (2020) 15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yang H, Li TW, Zhou Y, Peng H, Liu T, Zandi E, Martínez-Chantar ML, Mato JM, Lu SC, Activation of a novel c-Myc-miR27-prohibitin 1 circuitry in cholestatic liver injury inhibits glutathione synthesis in mice, Antioxid Redox Signal 22(3) (2015) 259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Teimouri M, Hosseini H, Shabani M, Koushki M, Noorbakhsh F, Meshkani R, Inhibiting miR-27a and miR-142-5p attenuate nonalcoholic fatty liver disease by regulating Nrf2 signaling pathway, IUBMB Life 72(3) (2020) 361–372. [DOI] [PubMed] [Google Scholar]
- [129].Tian C, Gao L, Zimmerman MC, Zucker IH, Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure, Am J Physiol Heart Circ Physiol 314(5) (2018) H928–h939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hou W, Tian Q, Zheng J, Bonkovsky HL, MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins, Hepatology 51(5) (2010) 1494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hou W, Tian Q, Steuerwald NM, Schrum LW, Bonkovsky HL, The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes, Biochim Biophys Acta 1819(11-12) (2012) 1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Antunes JC, Benarroch L, Moraes FC, Juenet M, Gross MS, Aubart M, Boileau C, Caligiuri G, Nicoletti A, Ollivier V, Chaubet F, Letourneur D, Chauvierre C, Core-Shell Polymer-Based Nanoparticles Deliver miR-155-5p to Endothelial Cells, Mol Ther Nucleic Acids 17 (2019) 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Xu Y, Zhu W, Sun Y, Wang Z, Yuan W, Du Z, Functional Network Analysis Reveals Versatile MicroRNAs in Human Heart, Cell Physiol Biochem 36(4) (2015) 1628–43. [DOI] [PubMed] [Google Scholar]
- [134].Kabaria S, Choi DC, Chaudhuri AD, Jain MR, Li H, Junn E, MicroRNA-7 activates Nrf2 pathway by targeting Keap1 expression, Free Radic Biol Med 89 (2015) 548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sun X, Zuo H, Liu C, Yang Y, Overexpression of miR-200a protects cardiomyocytes against hypoxia-induced apoptosis by modulating the kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2 signaling axis, Int J Mol Med 38(4) (2016) 1303–11. [DOI] [PubMed] [Google Scholar]
- [136].Ma Y, Pan C, Tang X, Zhang M, Shi H, Wang T, Zhang Y, MicroRNA-200a represses myocardial infarction-related cell death and inflammation by targeting the Keap1/Nrf2 and β-catenin pathways, Hellenic J Cardiol (2020). [DOI] [PubMed] [Google Scholar]
- [137].Liu Y, Song JW, Lin JY, Miao R, Zhong JC, Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases, Cardiovasc Toxicol 20(5) (2020) 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Zhang B, Zhai M, Li B, Liu Z, Li K, Jiang L, Zhang M, Yi W, Yang J, Yi D, Liang H, Jin Z, Duan W, Yu S, Honokiol Ameliorates Myocardial Ischemia/Reperfusion Injury in Type 1 Diabetic Rats by Reducing Oxidative Stress and Apoptosis through Activating the SIRT1-Nrf2 Signaling Pathway, Oxid Med Cell Longev 2018 (2018) 3159801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Bhattacharjee S, Li J, Dashwood RH, Emerging crosstalk between long non-coding RNAs and Nrf2 signaling, Cancer Lett 490 (2020) 154–164. [DOI] [PubMed] [Google Scholar]
- [140].Sun Z, Huang G, Cheng H, Transcription factor Nrf2 induces the up-regulation of lncRNA TUG1 to promote progression and adriamycin resistance in urothelial carcinoma of the bladder, Cancer Manag Res 11 (2019) 6079–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gao M, Zhao B, Chen M, Liu Y, Xu M, Wang Z, Liu S, Zhang C, Nrf-2-driven long noncoding RNA ODRUL contributes to modulating silver nanoparticle-induced effects on erythroid cells, Biomaterials 130 (2017) 14–27. [DOI] [PubMed] [Google Scholar]
- [142].Moreno Leon L, Gautier M, Allan R, Ilié M, Nottet N, Pons N, Paquet A, Lebrigand K, Truchi M, Fassy J, Magnone V, Kinnebrew G, Radovich M, Cheok MH, Barbry P, Vassaux G, Marquette CH, Ponzio G, Ivan M, Pottier N, Hofman P, Mari B, Rezzonico R, The nuclear hypoxia-regulated NLUCAT1 long noncoding RNA contributes to an aggressive phenotype in lung adenocarcinoma through regulation of oxidative stress, Oncogene 38(46) (2019) 7146–7165. [DOI] [PubMed] [Google Scholar]
- [143].Johnson GS, Li J, Beaver LM, Dashwood WM, Sun D, Rajendran P, Williams DE, Ho E, Dashwood RH, A functional pseudogene, NMRAL2P, is regulated by Nrf2 and serves as a coactivator of NQO1 in sulforaphane-treated colon cancer cells, Mol Nutr Food Res 61(4) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Zhang Y, Xia J, Li Q, Yao Y, Eades G, Gernapudi R, Duru N, Kensler TW, Zhou Q, NRF2/long noncoding RNA ROR signaling regulates mammary stem cell expansion and protects against estrogen genotoxicity, J Biol Chem 289(45) (2014) 31310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gao M, Chen M, Li C, Xu M, Liu Y, Cong M, Sang N, Liu S, Long non-coding RNA MT1DP shunts the cellular defense to cytotoxicity through crosstalk with MT1H and RhoC in cadmium stress, Cell Discov 4 (2018) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wu LL, Cai WP, Lei X, Shi KQ, Lin XY, Shi L, NRAL mediates cisplatin resistance in hepatocellular carcinoma via miR-340-5p/Nrf2 axis, J Cell Commun Signal 13(1) (2019) 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Huang X, Gao Y, Qin J, Lu S, The mechanism of long non-coding RNA MEG3 for hepatic ischemia-reperfusion: Mediated by miR-34a/Nrf2 signaling pathway, J Cell Biochem 119(1) (2018) 1163–1172. [DOI] [PubMed] [Google Scholar]
- [148].Geng JF, Liu X, Zhao HB, Fan WF, Geng JJ, Liu XZ, LncRNA UCA1 inhibits epilepsy and seizure-induced brain injury by regulating miR-495/Nrf2-ARE signal pathway, Int J Biochem Cell Biol 99 (2018) 133–139. [DOI] [PubMed] [Google Scholar]
- [149].Ye W, Ma J, Wang F, Wu T, He M, Li J, Pei R, Zhang L, Wang Y, Zhou J, LncRNA MALAT1 Regulates miR-144-3p to Facilitate Epithelial-Mesenchymal Transition of Lens Epithelial Cells via the ROS/NRF2/Notch1/Snail Pathway, Oxid Med Cell Longev 2020 (2020) 8184314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhang L, Liu Z, Li X, Zhang P, Wang J, Zhu D, Chen X, Ye L, Low long noncoding RNA HOTAIR expression is associated with down-regulation of Nrf2 in the spermatozoa of patients with asthenozoospermia or oligoasthenozoospermia, Int J Clin Exp Pathol 8(11) (2015) 14198–205. [PMC free article] [PubMed] [Google Scholar]
- [151].Zeng R, Zhang R, Song X, Ni L, Lai Z, Liu C, Ye W, The long non-coding RNA MALAT1 activates Nrf2 signaling to protect human umbilical vein endothelial cells from hydrogen peroxide, Biochem Biophys Res Commun 495(4) (2018) 2532–2538. [DOI] [PubMed] [Google Scholar]
- [152].Amodio N, Stamato MA, Juli G, Morelli E, Fulciniti M, Manzoni M, Taiana E, Agnelli L, Cantafio MEG, Romeo E, Raimondi L, Caracciolo D, Zuccalà V, Rossi M, Neri A, Munshi NC, Tagliaferri P, Tassone P, Drugging the lncRNA MALAT1 via LNA gapmeR ASO inhibits gene expression of proteasome subunits and triggers anti-multiple myeloma activity, Leukemia 32(9) (2018) 1948–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Zhang Z, Xiong R, Li C, Xu M, Guo M, LncRNA TUG1 promotes cisplatin resistance in esophageal squamous cell carcinoma cells by regulating Nrf2, Acta Biochim Biophys Sin (Shanghai) 51(8) (2019) 826–833. [DOI] [PubMed] [Google Scholar]
- [154].Wang L, Yang H, Wang Q, Zhang Q, Wang Z, Zhang Q, Wu S, Li H, Paraquat and MPTP induce alteration in the expression profile of long noncoding RNAs in the substantia nigra of mice: Role of the transcription factor Nrf2, Toxicology letters 291 (2018) 11–28. [DOI] [PubMed] [Google Scholar]
- [155].Trembinski DJ, Bink DI, Theodorou K, Sommer J, Fischer A, van Bergen A, Kuo CC, Costa IG, Schürmann C, Leisegang MS, Brandes RP, Alekseeva T, Brill B, Wietelmann A, Johnson CN, Spring-Connell A, Kaulich M, Werfel S, Engelhardt S, Hirt MN, Yorgan K, Eschenhagen T, Kirchhof L, Hofmann P, Jaé N, Wittig I, Hamdani N, Bischof C, Krishnan J, Houtkooper RH, Dimmeler S, Boon RA, Aging-regulated anti-apoptotic long non-coding RNA Sarrah augments recovery from acute myocardial infarction, Nat Commun 11(1) (2020) 2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Yan D, Liu W, Liu Y, Luo M, LINC00261 suppresses human colon cancer progression via sponging miR-324-3p and inactivating the Wnt/β-catenin pathway, J Cell Physiol 234(12) (2019) 22648–22656. [DOI] [PubMed] [Google Scholar]
- [157].Chen T, Lei S, Zeng Z, Zhang J, Xue Y, Sun Y, Lan J, Xu S, Mao D, Guo B, Linc00261 inhibits metastasis and the WNT signaling pathway of pancreatic cancer by regulating a miR-552-5p/FOXO3 axis, Oncol Rep 43(3) (2020) 930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Liu S, Zheng Y, Zhang Y, Zhang J, Xie F, Guo S, Gu J, Yang J, Zheng P, Lai J, Yin L, Wang H, Methylation-mediated LINC00261 suppresses pancreatic cancer progression by epigenetically inhibiting c-Myc transcription, Theranostics 10(23) (2020) 10634–10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zhang R, Li Y, Liu X, Qin S, Guo B, Chang L, Huang L, Liu S, FOXO3a-mediated long non-coding RNA LINC00261 resists cardiomyocyte hypoxia/reoxygenation injury via targeting miR23b-3p/NRF2 axis, J Cell Mol Med 24(15) (2020) 8368–8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Thompson AG, Gray E, Heman-Ackah SM, Mäger I, Talbot K, Andaloussi SE, Wood MJ, Turner MR, Extracellular vesicles in neurodegenerative disease - pathogenesis to biomarkers, Nat Rev Neurol 12(6) (2016) 346–57. [DOI] [PubMed] [Google Scholar]
- [161].Boulanger CM, Loyer X, Rautou PE, Amabile N, Extracellular vesicles in coronary artery disease, Nat Rev Cardiol 14(5) (2017) 259–272. [DOI] [PubMed] [Google Scholar]
- [162].Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F, Extracellular Vesicles in Angiogenesis, Circ Res 120(10) (2017) 1658–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Shah R, Patel T, Freedman JE, Circulating Extracellular Vesicles in Human Disease, N Engl J Med 379(10) (2018) 958–966. [DOI] [PubMed] [Google Scholar]
- [164].Kalluri R, LeBleu VS, The biology, function, and biomedical applications of exosomes, Science 367(6478) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wu P, Zhang B, Ocansey DKW, Xu W, Qian H, Extracellular vesicles: A bright star of nanomedicine, Biomaterials (2020) 120467. [DOI] [PubMed] [Google Scholar]
- [166].Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ, Reassessment of Exosome Composition, Cell 177(2) (2019) 428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Colombo M, Raposo G, Théry C, Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles, Annu Rev Cell Dev Biol 30 (2014) 255–89. [DOI] [PubMed] [Google Scholar]
- [168].van Niel G, D’Angelo G, Raposo G, Shedding light on the cell biology of extracellular vesicles, Nat Rev Mol Cell Biol 19(4) (2018) 213–228. [DOI] [PubMed] [Google Scholar]
- [169].Loyer X, Zlatanova I, Devue C, Yin M, Howangyin KY, Klaihmon P, Guerin CL, Kheloufi M, Vilar J, Zannis K, Fleischmann BK, Hwang DW, Park J, Lee H, Menasché P, Silvestre JS, Boulanger CM, Intra-Cardiac Release of Extracellular Vesicles Shapes Inflammation Following Myocardial Infarction, Circ Res 123(1) (2018) 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Sahoo S, Losordo DW, Exosomes and cardiac repair after myocardial infarction, Circ Res 114(2) (2014) 333–44. [DOI] [PubMed] [Google Scholar]
- [171].Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA, Circulating Exosomes Induced by Cardiac Pressure Overload Contain Functional Angiotensin II Type 1 Receptors, Circulation 131(24) (2015) 2120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Biemmi V, Milano G, Ciullo A, Cervio E, Burrello J, Dei Cas M, Paroni R, Tallone T, Moccetti T, Pedrazzini G, Longnus S, Vassalli G, Barile L, Inflammatory extracellular vesicles prompt heart dysfunction via TRL4-dependent NF-κB activation, Theranostics 10(6) (2020) 2773–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells, Nat Cell Biol 9(6) (2007) 654–9. [DOI] [PubMed] [Google Scholar]
- [174].Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD, Nana-Sinkam SP, Jarjoura D, Marsh CB, Detection of microRNA expression in human peripheral blood microvesicles, PLoS One 3(11) (2008) e3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM, Functional delivery of viral miRNAs via exosomes, Proc Natl Acad Sci U S A 107(14) (2010) 6328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Turchinovich A, Weiz L, Langheinz A, Burwinkel B, Characterization of extracellular circulating microRNA, Nucleic Acids Res 39(16) (2011) 7223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T, Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy, J Clin Invest 124(5) (2014) 2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Tian C, Hu G, Gao L, Hackfort BT, Zucker IH, Extracellular vesicular MicroRNA-27a* contributes to cardiac hypertrophy in chronic heart failure, J Mol Cell Cardiol 143 (2020) 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Videira RF, da Costa Martins PA, Non-coding RNAs in Cardiac Intercellular Communication, Frontiers in Physiology 11(738) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Xia YW, Wang SB, Microvesicles containing microRNA-21 induce myocardial fibrosis via AKT pathway, Eur Rev Med Pharmacol Sci 22(14) (2018) 4634–4641. [DOI] [PubMed] [Google Scholar]
- [181].Melman YF, Shah R, Danielson K, Xiao J, Simonson B, Barth A, Chakir K, Lewis GD, Lavender Z, Truong QA, Kleber A, Das R, Rosenzweig A, Wang Y, Kass D, Singh JP, Das S, Circulating MicroRNA-30d Is Associated With Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study, Circulation 131(25) (2015) 2202–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Xiao J, Gao R, Bei Y, Zhou Q, Zhou Y, Zhang H, Jin M, Wei S, Wang K, Xu X, Yao W, Xu D, Zhou F, Jiang J, Li X, Das S, Circulating miR-30d Predicts Survival in Patients with Acute Heart Failure, Cell Physiol Biochem 41(3) (2017) 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Jia K, Shi P, Han X, Chen T, Tang H, Wang J, Diagnostic value of miR-30d-5p and miR-125b-5p in acute myocardial infarction, Mol Med Rep 14(1) (2016) 184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Tang S, Wang Y, Ma T, Lu S, Huang K, Li Q, Wu M, Yang H, Zhong J, MiR-30d inhibits cardiomyocytes autophagy promoting ferroptosis after myocardial infarction, Panminerva Med (2020). [DOI] [PubMed] [Google Scholar]
- [185].Li J, Salvador AM, Li G, Valkov N, Ziegler O, Yeri AS, Xiao CY, Meechoovet B, Alsop E, Rodosthenous RS, Kundu P, Huan T, Levy D, Tigges JC, Pico AR, Ghiran I, Silverman MG, Meng X, Kitchen R, Xu J, Van Keuren-Jensen K, Shah RV, Xiao J, Das S, Mir-30d Regulates Cardiac Remodeling by Intracellular And Paracrine Signaling, Circ Res (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Kenneweg F, Bang C, Xiao K, Boulanger CM, Loyer X, Mazlan S, Schroen B, Hermans-Beijnsberger S, Foinquinos A, Hirt MN, Eschenhagen T, Funcke S, Stojanovic S, Genschel C, Schimmel K, Just A, Pfanne A, Scherf K, Dehmel S, Raemon-Buettner SM, Fiedler J, Thum T, Long Noncoding RNA-Enriched Vesicles Secreted by Hypoxic Cardiomyocytes Drive Cardiac Fibrosis, Mol Ther Nucleic Acids 18 (2019) 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Bodega G, Alique M, Puebla L, Carracedo J, Ramírez RM, Microvesicles: ROS scavengers and ROS producers, J Extracell Vesicles 8(1) (2019) 1626654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Benedikter BJ, Weseler AR, Wouters EFM, Savelkoul PHM, Rohde GGU, Stassen FRM, Redox-dependent thiol modifications: implications for the release of extracellular vesicles, Cell Mol Life Sci 75(13) (2018) 2321–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Thom SR, Bhopale VM, Yang M, Neutrophils generate microparticles during exposure to inert gases due to cytoskeletal oxidative stress, J Biol Chem 289(27) (2014) 18831–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Hervera A, De Virgiliis F, Palmisano I, Zhou L, Tantardini E, Kong G, Hutson T, Danzi MC, Perry RB, Santos CXC, Kapustin AN, Fleck RA, Del Río JA, Carroll T, Lemmon V, Bixby JL, Shah AM, Fainzilber M, Di Giovanni S, Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons, Nat Cell Biol 20(3) (2018) 307–319. [DOI] [PubMed] [Google Scholar]
- [191].Collison J, Vasculitis syndromes: Dysfunctional CD8 TREG cells implicated in GCA, Nat Rev Rheumatol 12(6) (2016) 314. [DOI] [PubMed] [Google Scholar]
- [192].Wen Z, Shimojima Y, Shirai T, Li Y, Ju J, Yang Z, Tian L, Goronzy JJ, Weyand CM, NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs, J Clin Invest 126(5) (2016) 1953–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, Galkin A, Thakur BK, Soplop N, Uryu K, Hoshino A, Norton L, Bonafé M, Cricca M, Gasparre G, Lyden D, Bromberg J, Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer, Proc Natl Acad Sci U S A 114(43) (2017) E9066–e9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Coly PM, Boulanger CM, Extracellular Mitochondria and Vesicles, Circ Res 125(1) (2019) 53–54. [DOI] [PubMed] [Google Scholar]
- [195].Mondola P, Ruggiero G, Serù R, Damiano S, Grimaldi S, Garbi C, Monda M, Greco D, Santillo M, The Cu,Zn superoxide dismutase in neuroblastoma SK-N-BE cells is exported by a microvesicles dependent pathway, Brain Res Mol Brain Res 110(1) (2003) 45–51. [DOI] [PubMed] [Google Scholar]
- [196].Soleti R, Lauret E, Andriantsitohaina R, Carmen Martínez M, Internalization and induction of antioxidant messages by microvesicles contribute to the antiapoptotic effects on human endothelial cells, Free Radic Biol Med 53(11) (2012) 2159–70. [DOI] [PubMed] [Google Scholar]
- [197].Nóbrega-Pereira S, Fernandez-Marcos PJ, Brioche T, Gomez-Cabrera MC, Salvador-Pascual A, Flores JM, Viña J, Serrano M, G6PD protects from oxidative damage and improves healthspan in mice, Nat Commun 7 (2016) 10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Bodega G, Alique M, Bohórquez L, Morán M, Magro L, Puebla L, Ciordia S, Mena MC, Arza E, Ramírez MR, Young and Especially Senescent Endothelial Microvesicles Produce NADPH: The Fuel for Their Antioxidant Machinery, Oxid Med Cell Longev 2018 (2018) 3183794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Ali Sheikh MS, Salma U, Zhang B, Chen J, Zhuang J, Ping Z, Diagnostic, Prognostic, and Therapeutic Value of Circulating miRNAs in Heart Failure Patients Associated with Oxidative Stress, Oxid Med Cell Longev 2016 (2016) 5893064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, Li K, Yu B, Li Z, Wang R, Wang L, Li Q, Wang N, Shan H, Li Z, Yang B, Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction, Biochem Biophys Res Commun 391(1) (2010) 73–7. [DOI] [PubMed] [Google Scholar]
- [201].Long G, Wang F, Duan Q, Chen F, Yang S, Gong W, Wang Y, Chen C, Wang DW, Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction, Int J Biol Sci 8(6) (2012) 811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Wang L, Yuan Y, Li J, Ren H, Cai Q, Chen X, Liang H, Shan H, Fu ZD, Gao X, Lv Y, Yang B, Zhang Y, MicroRNA-1 aggravates cardiac oxidative stress by post-transcriptional modification of the antioxidant network, Cell Stress Chaperones 20(3) (2015) 411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Yang JJ, Tao H, Hu W, Liu LP, Shi KH, Deng ZY, Li J, MicroRNA-200a controls Nrf2 activation by target Keap1 in hepatic stellate cell proliferation and fibrosis, Cell Signal 26(11) (2014) 2381–9. [DOI] [PubMed] [Google Scholar]
- [204].Sun L, Zhu W, Zhao P, Zhang J, Lu Y, Zhu Y, Zhao W, Liu Y, Chen Q, Zhang F, Down-Regulated Exosomal MicroRNA-221 – 3p Derived From Senescent Mesenchymal Stem Cells Impairs Heart Repair, Frontiers in Cell and Developmental Biology 8(263) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Ren XS, Tong Y, Qiu Y, Ye C, Wu N, Xiong XQ, Wang JJ, Han Y, Zhou YB, Zhang F, Sun HJ, Gao XY, Chen Q, Li YH, Kang YM, Zhu GQ, MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression, J Extracell Vesicles 9(1) (2020) 1698795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Wang C, Zhang C, Liu L, Chen XA,B, Li Y, Du J, Macrophage-Derived mir-155-Containing Exosomes Suppress Fibroblast Proliferation and Promote Fibroblast Inflammation during Cardiac Injury, Mol Ther 25(1) (2017) 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M, Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model, Exp Mol Med 50(4) (2018) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J, Brain-Heart Interaction: Cardiac Complications After Stroke, Circ Res 121(4) (2017) 451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Sun LL, Duan MJ, Ma JC, Xu L, Mao M, Biddyut D, Wang Q, Yang C, Zhang S, Xu Y, Yang L, Tian Y, Liu Y, Xia SN, Li KX, Jin Z, Xiong Q, Ai J, Myocardial infarction-induced hippocampal microtubule damage by cardiac originating microRNA-1 in mice, J Mol Cell Cardiol (2018). [DOI] [PubMed] [Google Scholar]
- [210].Scherbakov N, Doehner W, Heart-brain Interactions in Heart Failure, Card Fail Rev 4(2) (2018) 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Mueller K, Thiel F, Beutner F, Teren A, Frisch S, Ballarini T, Möller HE, Ihle K, Thiery J, Schuler G, Villringer A, Schroeter ML, Brain Damage With Heart Failure: Cardiac Biomarker Alterations and Gray Matter Decline, Circ Res 126(6) (2020) 750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Ogren JA, Fonarow GC, Woo MA, Cerebral impairment in heart failure, Curr Heart Fail Rep 11(3) (2014) 321–9. [DOI] [PubMed] [Google Scholar]
- [213].Havakuk O, King KS, Grazette L, Yoon AJ, Fong M, Bregman N, Elkayam U, Kloner RA, Heart Failure-Induced Brain Injury, J Am Coll Cardiol 69(12) (2017) 1609–1616. [DOI] [PubMed] [Google Scholar]
- [214].Gao L, Wang W, Liu D, Zucker IH, Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure, Circulation 115(24) (2007) 3095–102. [DOI] [PubMed] [Google Scholar]
- [215].Gao L, Wang WZ, Wang W, Zucker IH, Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure, Hypertension 52(4) (2008) 708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Esler M, Kaye D, Is very high sympathetic tone in heart failure a result of keeping bad company?, Hypertension 42(5) (2003) 870–2. [DOI] [PubMed] [Google Scholar]
- [217].Zucker IH, Patel KP, Schultz HD, Neurohumoral stimulation, Heart Fail Clin 8(1) (2012) 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP, The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide, Prog Biophys Mol Biol 84(2-3) (2004) 217–32. [DOI] [PubMed] [Google Scholar]
- [219].Huang BS, Leenen FH, The brain renin-angiotensin-aldosterone system: a major mechanism for sympathetic hyperactivity and left ventricular remodeling and dysfunction after myocardial infarction, Curr Heart Fail Rep 6(2) (2009) 81–8. [DOI] [PubMed] [Google Scholar]
- [220].Francis J, Wei SG, Weiss RM, Felder RB, Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure, Am J Physiol Heart Circ Physiol 287(5) (2004) H2138–46. [DOI] [PubMed] [Google Scholar]
- [221].Ma A, Gao L, Wafi AM, Yu L, Rudebush T, Zhou W, Zucker IH, Overexpression of Central ACE2 (Angiotensin-Converting Enzyme 2) Attenuates the Pressor Response to Chronic Central Infusion of Ang II (Angiotensin II): A Potential Role for Nrf2 (Nuclear Factor [Erythroid-Derived 2]-Like 2), Hypertension 76(5) (2020) 1514–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P, Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants, Proc Natl Acad Sci U S A 99(18) (2002) 11908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Khor TO, Huang Y, Wu TY, Shu L, Lee J, Kong AN, Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation, Biochem Pharmacol 82(9) (2011) 1073–8. [DOI] [PubMed] [Google Scholar]
- [224].Ge ZD, Lian Q, Mao X, Xia Z, Current Status and Challenges of NRF2 as a Potential Therapeutic Target for Diabetic Cardiomyopathy, Int Heart J 60(3) (2019) 512–520. [DOI] [PubMed] [Google Scholar]
- [225].da Costa RM, Rodrigues D, Pereira CA, Silva JF, Alves JV, Lobato NS, Tostes RC, Nrf2 as a Potential Mediator of Cardiovascular Risk in Metabolic Diseases, Front Pharmacol 10 (2019) 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Chen QM, Maltagliati AJ, Nrf2 at the heart of oxidative stress and cardiac protection, Physiol Genomics 50(2) (2018) 77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Robledinos-Antón N, Fernández-Ginés R, Manda G, Cuadrado A, Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development, Oxid Med Cell Longev 2019 (2019) 9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM, Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease, N Engl J Med 369(26) (2013) 2492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Chin MP, Wrolstad D, Bakris GL, Chertow GM, de Zeeuw D, Goldsberry A, Linde PG, McCullough PA, McMurray JJ, Wittes J, Meyer CJ, Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl, J Card Fail 20(12) (2014) 953–8. [DOI] [PubMed] [Google Scholar]
- [230].Singh F, Charles AL, Schlagowski AI, Bouitbir J, Bonifacio A, Piquard F, Krähenbühl S, Geny B, Zoll J, Reductive stress impairs myoblasts mitochondrial function and triggers mitochondrial hormesis, Biochim Biophys Acta 1853(7) (2015) 1574–85. [DOI] [PubMed] [Google Scholar]
- [231].Shanmugam G, Wang D, Gounder SS, Fernandes J, Litovsky SH, Whitehead K, Radhakrishnan RK, Franklin S, Hoidal JR, Kensler TW, Dell’Italia L, Darley-Usmar V, Abel ED, Jones DP, Ping P, Rajasekaran NS, Reductive Stress Causes Pathological Cardiac Remodeling and Diastolic Dysfunction, Antioxid Redox Signal 32(18) (2020) 1293–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Brewer AC, Mustafi SB, Murray TV, Rajasekaran NS, Benjamin IJ, Reductive stress linked to small HSPs, G6PD, and Nrf2 pathways in heart disease, Antioxid Redox Signal 18(9) (2013) 1114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Kannan S, Muthusamy VR, Whitehead KJ, Wang L, Gomes AV, Litwin SE, Kensler TW, Abel ED, Hoidal JR, Rajasekaran NS, Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy, Cardiovasc Res 100(1) (2013) 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN, A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure, Proc Natl Acad Sci U S A 103(48) (2006) 18255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].D’Alessandra Y, Chiesa M, Carena MC, Beltrami AP, Rizzo P, Buzzetti M, Ricci V, Ferrari R, Fucili A, Livi U, Aleksova A, Pompilio G, Colombo GI, Differential Role of Circulating microRNAs to Track Progression and Pre-Symptomatic Stage of Chronic Heart Failure: A Pilot Study, Biomedicines 8(12) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S, Circulating microRNAs in patients with coronary artery disease, Circ Res 107(5) (2010) 677–84. [DOI] [PubMed] [Google Scholar]
- [237].Roncarati R, Viviani Anselmi C, Losi MA, Papa L, Cavarretta E, Da Costa Martins P, Contaldi C, Saccani Jotti G, Franzone A, Galastri L, Latronico MV, Imbriaco M, Esposito G, De Windt L, Betocchi S, Condorelli G, Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy, J Am Coll Cardiol 63(9) (2014) 920–7. [DOI] [PubMed] [Google Scholar]
- [238].Devaux Y, Vausort M, McCann GP, Kelly D, Collignon O, Ng LL, Wagner DR, Squire IB, A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction, PLoS One 8(8) (2013) e70644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR, Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease, Cell Metab 30(4) (2019) 656–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Castillo-Armengol J, Fajas L, Lopez-Mejia IC, Inter-organ communication: a gatekeeper for metabolic health, EMBO Rep 20(9) (2019) e47903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Zhao Y, Zhao M-F, Jiang S, Wu J, Liu J, Yuan X-W, Shen D, Zhang J-Z, Zhou N, He J, Fang L, Sun X-T, Xue B, Li C-J, Liver governs adipose remodelling via extracellular vesicles in response to lipid overload, Nature Communications 11(1) (2020) 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Duan MJ, Yan ML, Wang Q, Mao M, Su D, Sun LL, Li KX, Qu Y, Sun Q, Zhang XY, Huang SY, Ma JC, Ban T, Ai J, Overexpression of miR-1 in the heart attenuates hippocampal synaptic vesicle exocytosis by the posttranscriptional regulation of SNAP-25 through the transportation of exosomes, Cell communication and signaling : CCS 16(1) (2018) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Ma JC, Duan MJ, Li KX, Biddyut D, Zhang S, Yan ML, Yang L, Jin Z, Zhao HM, Huang SY, Sun Q, Su D, Xu Y, Pan YH, Ai J, Knockdown of MicroRNA-1 in the Hippocampus Ameliorates Myocardial Infarction Induced Impairment of Long-Term Potentiation, Cell Physiol Biochem 50(4) (2018) 1601–1616. [DOI] [PubMed] [Google Scholar]
- [244].Wong LL, Armugam A, Sepramaniam S, Karolina DS, Lim KY, Lim JY, Chong JP, Ng JY, Chen YT, Chan MM, Chen Z, Yeo PS, Ng TP, Ling LH, Sim D, Leong KT, Ong HY, Jaufeerally F, Wong R, Chai P, Low AF, Lam CS, Jeyaseelan K, Richards AM, Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction, Eur J Heart Fail 17(4) (2015) 393–404. [DOI] [PubMed] [Google Scholar]
- [245].Watson CJ, Gupta SK, O’Connell E, Thum S, Glezeva N, Fendrich J, Gallagher J, Ledwidge M, Grote-Levi L, McDonald K, Thum T, MicroRNA signatures differentiate preserved from reduced ejection fraction heart failure, Eur J Heart Fail 17(4) (2015) 405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Chen YT, Wong LL, Liew OW, Richards AM, Heart Failure with Reduced Ejection Fraction (HFrEF) and Preserved Ejection Fraction (HFpEF): The Diagnostic Value of Circulating MicroRNAs, Cells 8(12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Schmitter D, Voors AA, van der Harst P, HFpEF vs. HFrEF: can microRNAs advance the diagnosis?, Eur J Heart Fail 17(4) (2015) 351–4. [DOI] [PubMed] [Google Scholar]
- [248].Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ, Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model, Theranostics 7(1) (2017) 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Tao G, Kahr PC, Morikawa Y, Zhang M, Rahmani M, Heallen TR, Li L, Sun Z, Olson EN, Amendt BA, Martin JF, Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury, Nature 534(7605) (2016) 119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG, A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes, Mol Ther 18(9) (2010) 1606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Oskouie MN, Aghili Moghaddam NS, Butler AE, Zamani P, Sahebkar A, Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes, J Cell Physiol 234(6) (2019) 8182–8191. [DOI] [PubMed] [Google Scholar]
- [252].Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N, Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia, Life Sci 107(1–2) (2014) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Wang H, Sui H, Zheng Y, Jiang Y, Shi Y, Liang J, Zhao L, Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway, Nanoscale 11(15) (2019) 7481–7496. [DOI] [PubMed] [Google Scholar]
- [254].Cai J, Gehrau R, Tu Z, Leroy V, Su G, Shang J, Mas VR, Emtiazjoo A, Pelaez A, Atkinson C, Machuca T, Upchurch GR Jr., Sharma AK, MicroRNA-206 antagomiR–enriched extracellular vesicles attenuate lung ischemia–reperfusion injury through CXCL1 regulation in alveolar epithelial cells, J Heart Lung Transplant 39(12) (2020) 1476–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Antes TJ, Middleton RC, Luther KM, Ijichi T, Peck KA, Liu WJ, Valle J, Echavez AK, Marbán E, Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display, J Nanobiotechnology 16(1) (2018) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J, Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy, Biomaterials 150 (2018) 137–149. [DOI] [PubMed] [Google Scholar]
- [257].Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ, Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes, Nat Biotechnol 29(4) (2011) 341–5. [DOI] [PubMed] [Google Scholar]
- [258].Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, Yang Z, Chen Y, Li J, Ma T, Liu H, Li Z, Yang J, Shen Z, Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction, J Am Heart Assoc 7(15) (2018) e008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG, Qian L, Cheng K, Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide, Theranostics 8(7) (2018) 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Weng CF, Wu CF, Kao SH, Chen JC, Lin HH, Down-Regulation of miR-34a-5p Potentiates Protective Effect of Adipose-Derived Mesenchymal Stem Cells Against Ischemic Myocardial Infarction by Stimulating the Expression of C1q/Tumor Necrosis Factor-Related Protein-9, Front Physiol 10 (2019) 1445. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]