Abstract
Chemical tools capable of detecting ferrous iron with oxidation-state specificity have only recently become available. Coincident with this development in chemical biology has been increased study and appreciation for the importance of ferrous iron during infection and more generally in host-pathogen interaction. Some of the recent findings are surprising and challenge long- standing assumptions about bacterial iron homeostasis and the innate immune response to infection. Here we review these recent developments and their implications for antibacterial therapy.
Keywords: Gram-negative bacteria, host-pathogen interaction, iron metabolism, iron speciation, labile iron pool, activity-based probes, reactivity-based probes, anti-bacterials, anti-infectives
Background
Antibiotics were the formative products of the early pharmaceutical industry, and their importance to human health has only increased in recent years [1]. The antibiotic ‘golden age’ of the mid 20th century saw unprecedented productivity in terms of numbers of new agents brought to market, but the focus on natural product scaffolds for which resistance mechanisms were already extant in bacterial populations, combined with rampant overuse of some agents, has led to the current situation where ~70% of bacterial pathogens exhibit resistance to the most widely used antibiotics [2,3]. It is estimated that by 2050, if no corrective action is taken, these infections will cause over 10 million deaths per year, with economic costs of 100 trillion USD in lost global production [4].
The dearth of effective treatment options is especially concerning for multidrug-resistant (MDR) Gram-negative bacteria such as carbapenem-resistant Acinetobacter, Pseudomonas and Enterobacterales [5]. These MDR pathogens possess efflux transporters and outer membrane permeability barriers, which act synergistically to increase their resistance to diverse antibacterial classes [6]. Horizontal gene transfer furthermore enables these pathogens to rapidly acquire new resistance mechanisms that further limit antibiotic efficacy [7]. This situation has led to calls for novel targets and new approaches that could lead to a more robust and less vulnerable antibiotic pipeline for the new century [8]. We argue here that increasing understanding and appreciation for the role of low-molecular mass ferrous iron species (‘labile iron’) in bacterial growth, pathogenicity, and host interaction could provide inroads into novel antibiotic strategies. The recent development of chemical probes of labile iron should aid such efforts but have to date been employed primarily in studies of eukaryotic iron homeostasis and speciation. Here we review the emerging biology surrounding ferrous iron in bacterial growth and pathogenicity and describe the new chemical biology tools and therapeutic approaches being explored to exploit ferrous iron at the interface of infection and immunity.
Bacterial Iron Homeostasis
Transition metals such as iron, manganese, copper, and zinc, are essential micronutrients for most pathogenic bacteria, with an estimated 30% of proteins in the bacterial proteome using metal cofactors to enable their cellular functions. Bacterial life evolved in a reducing, anoxic environment where reduced forms of iron were prevalent and were apparently co-opted by early life for the redox chemistry they enabled. The ‘great oxidation’ that coincided with the evolution of photosynthesis presented early bacterial life with an existential challenge, since ferrous iron readily converts oxygen to toxic reactive oxygen species, whilst yielding water insoluble ferric hydroxides. Continued utilization of iron thus required the evolution of iron chaperones (siderophores) that could bind and solubilize the ferric ion, as well as new biochemical pathways to detoxify reactive oxygen species. While the study of bacterial iron homeostasis has historically focused on transport and storage of the ferric ion, there has been increasing study and appreciation over the past decade that bacteria have not lost the means to utilize ferrous iron [9], enabled by biochemistry that is evolutionarily ancient [10].
The ‘labile iron pool’ (LIP) of a bacterial cell consists of low molecular mass ferrous (Fe2+) iron species bound by still poorly defined cellular ligands (Figure 1). Mössbauer and electron paramagnetic resonance (EPR) spectroscopy of live E. coli puts the concentration of LIP in the low-mid micromolar range [11], but LIP levels can be significantly higher than this during exponential-stage growth as detailed below [12]. Bacteria employ various iron regulatory proteins to maintain the LIP and can utilize this system to sense iron depletion as a marker of host tissue during infection [13]. These proteins modulate LIP by activating the expression of metal-acquisition systems under iron limiting conditions or by the expression of efflux transporters and storage proteins under iron replete conditions [14]. The regulation of metal homeostasis involves both transcriptional and post-transcriptional control, as recently reviewed [15]. The Fur (Ferric uptake regulator) family of iron regulatory proteins are ubiquitous in bacteria and regulate the binding of Fe2+ (or mismetallation with Mn2+ in some instances) from the LIP in order to initiate the transcription of iron-regulatory genes [16]. Genetic knockouts of the fur gene in several bacterial species have shown variable and species-specific effects including decreased virulence [17] and impairment of the growth of bacterial colonies [18]. New players in bacterial iron homeostasis continue to be identified, including a family of Fe2+ transporters (IroT/MavN) residing within a specialized vacuole in the bacteria Legionella pneumophilia [19].
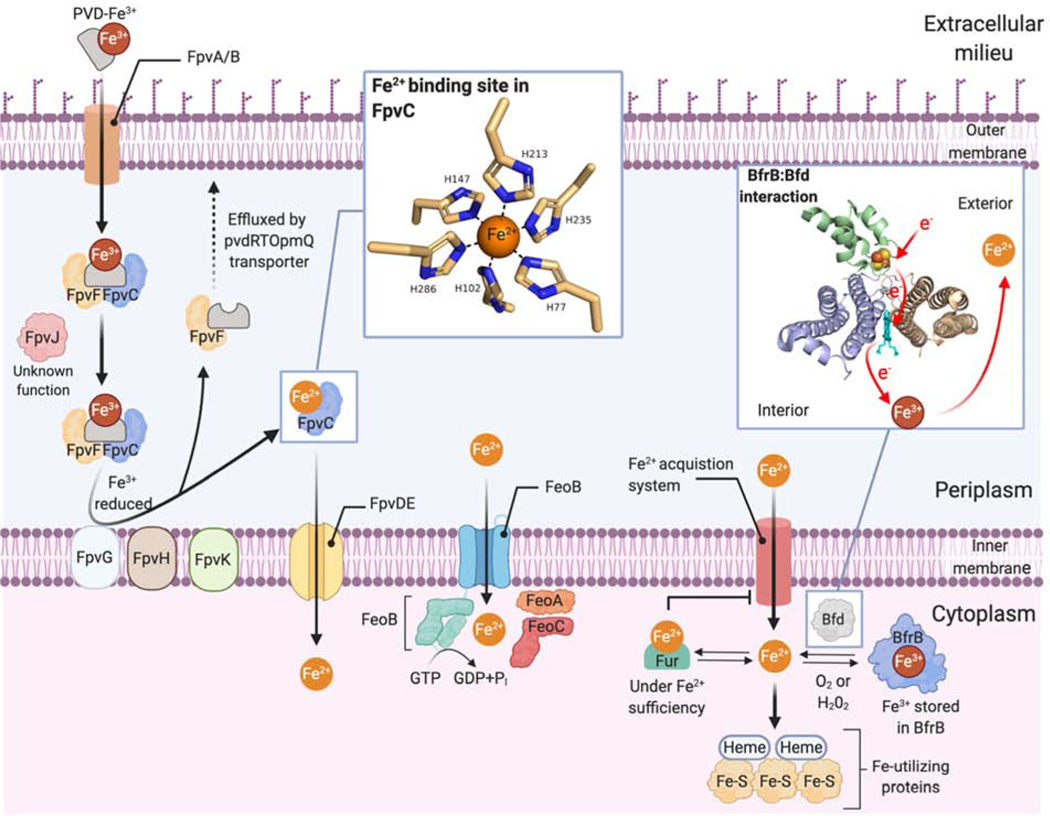
Figure 1.
Overview of iron acquisition, storage, and transport in Gram-negative bacteria with an emphasis on mechanisms of ferrous iron (Fe2+) uptake and mobilization from ferric (Fe3+) forms. The figure summarizes recent findings from studies conducted in more than one species of Gram-negative bacteria.
Metal-sensing regulatory elements called riboswitches have recently been shown to modulate bacterial gene expression in response to different metal ions including Ni2+/Co2+ [20], Mn2+ [21–23], Mg2+ [24] and Fe2+ [25]. The riboswitch czcD (NiCO) employed by the human gut microbe Erysipelotrichaceae bacterium, was shown to preferentially bind Fe2+ (and to a lesser extent Mn2+) under physiological conditions [25]. Previous studies of czcD performed under aerobic conditions had erroneously suggested it was unresponsive to iron, highlighting the importance of considering oxygen tension when studying an oxidation-prone analyte like iron. Studies in the Gram-negative pathogen Treponema denticola revealed the presence of a cytoplasmic oxygen-binding di-iron (ODP) metalloprotein that functions as a chemoreceptor to sense both oxygen and ferrous iron for various bacterial signal transduction pathways [26].
Bacteria possess two ferritin-like iron storage proteins, the bacterial ferritin (FtnA) and bacterioferritin (Bfr) as recently reviewed [27]. In P. aeruginosa under iron limiting conditions, cytosolic BfrB binds to the bacterioferritin-associated ferredoxin (Bfd) and accepts electrons from its [2Fe-2S] cluster, causing Fe3+ stored within BfrB to be reduced and mobilized as Fe2+ [28]. The essentiality of the BfrB:Bfd interaction for P. aeruginosa has been demonstrated by genetic deletion of the bfd gene [28] and with small-molecule inhibitors [29]. However, other recent studies have questioned whether these bacterial proteins serve the same essential iron storage function as eukaryotic ferritins. Using Mössbauer and EPR spectroscopy, Lindahl and co-workers found significant elevation of LIP concentrations (as high as 500 μM!) in E. coli during exponential growth and conversely found little spectroscopic evidence for FtnA or Bfr bound iron [12]. Moreover, the major ferric signature observed during stationary growth was present even in FtnA or Bfr deletion strains, and ascribed to Fe3+ oxyhydroxide nanoparticles. Based on these findings, it was suggested that a ‘respiratory shield’ comprising membrane-bound enzymes of the electron transfer chain serve to maintain a microaerobic cytoplasm in which even high-μM concentrations of LIP can persist during exponential growth without causing cellular damage. If correct, these findings imply that bacterial cells should be easily distinguishable from eukaryotic cells in their iron speciation and invites strategies to target bacteria on this basis, as will be discussed later.
Ferrous Iron at the Interface of Host-Pathogen Interaction
The concept of “nutritional immunity” [30] – the limitation of systemic iron and other nutrient resources from a pathogen – lies at the heart of the innate immune response as has been well reviewed [9,31,32]. During the mammalian response to bacterial infection, interleukin-6 (IL-6) acts as the main pro-inflammatory cytokine, mediating the release of the peptide hormone hepcidin from the liver. Direct binding of hepcidin to the cellular iron exporter ferroportin then leads to its internalization and degradation, promoting iron accumulation in phagocytic cells [33]. The expression of mammalian iron-binding proteins such as ferritin, transferrin and lactoferrin also plays a crucial role in restricting the iron resources available to invading pathogens [32]. The host protein lipocalin 2 is another effector of innate immunity, recognizing bacterial siderophore-bound iron (primarily enterobactin) [34]. In addition to withholding of Fe and Mn to limit bacterial proliferation, recent evidence has also suggested that copper and zinc can be weaponized to poison intracellular bacteria via phagocyte-mediated delivery to the phagosome [35–38].
To overcome iron withholding by the host, pathogenic bacteria employ diverse tactics to acquire the iron they need for growth and virulence. Best studied among these strategies is the secretion of Fe3+-chelating small molecules called siderophores (Figure 2a). Upon binding of the ferric ion, a soluble Fe3+-siderophore complex is formed that can be transported into a bacterium and unloaded in either the periplasm or cytoplasm depending on the specific siderophores and transporters involved [39,40]. Recent studies in P. aeruginosa have identified complex networks of interacting proteins and transporters that enable, for example, the reduction of ferric iron bound to pyoveridine (PVD, Figure 1). This reduction step takes place in the bacterial periplasm and liberates Fe2+ from the PVD-Fe3+ complex, where it can bind to chaperone proteins and be shepherded into the cytoplasm [40–43]. Siderophores such as PVD in P. aeruginosa, have also been shown to function as signaling molecules in the production of bacterial virulence factors [44,45]. Proteomics experiments investigating the iron starvation response in P. aeruginosa have revealed a variety of novel compensatory responses made by the pathogen in order to survive iron-limitation, which includes the upregulation of proteins required for iron-sulfur cluster biogenesis [46].
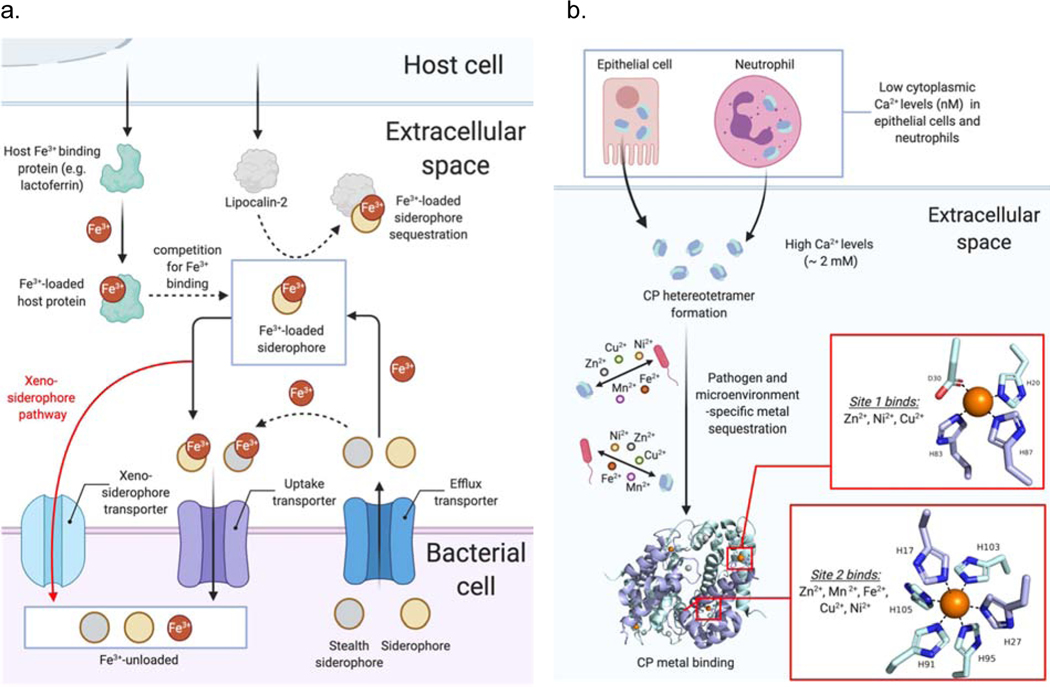
Figure 2.
Iron and other metal ions during host-pathogen interaction. (a) Competition for Fe3+ resources is centered around bacterial siderophores and mammalian Fe3+ and siderophore-binding proteins. (b) Calprotectin (CP) is a general metal sequestering protein the binds Fe2+ and other divalent metal ions in the extracellular environment.
The host protein calprotectin (CP) is a metal-sequestering protein released from host neutrophils as part of the metal-withholding innate immune response (Figure 2b). While the ability of CP to bind Zn2+ and Mn2+ has been recognized for some time, work from Nolan and co-workers over the past decade has revealed a high affinity of the His6 site in CP for Fe2+ [47], and this has uncovered a hitherto unappreciated role for CP in limiting Fe2+ [48] during host-pathogen interaction, as recently reviewed [49]. The high affinity of the CP His6 site for the Fe2+ ion can even shift the iron redox equilibrium in favor of the ferrous ion under aerobic conditions, an effect that is further facilitated by pyocyanins, redox-cycling phenazines produced by P. aeruginosa [50,51]. Further emphasizing the complex and multifaceted role of CP in innate immunity is a recent report [52] that CP can inhibit the growth of Borrelia burgdorferi, the pathogen responsible for Lyme disease, by physical interaction with the bacterium, absent metal sequestration.
Under conditions where the Fe2+ ion predominates over Fe3+ (e.g., in highly acidic, reducing and/or anaerobic conditions), bacteria utilize the ferrous iron transport system FeO (Figure 1) as the primary means to import Fe2+ into the cytoplasm [53]. Like PVD siderophores, the FeO system has been identified as a major contributor to the virulence of P. aeruginosa, helping the bacterium colonize hypoxic environments [54] such as in the lungs of cystic fibrosis (CF) patients [55,56]. The production of aforementioned pyocyanin metabolites by P. aeruginosa is implicated in chronic CF infection where these redox-cycling small molecules are proposed to liberate Fe2+ from host proteins in the extracellular environment [57], leading to Fe2+ uptake via the transporter FeoB [58]. Phenazine production has also been reported to promote biofilm formation [55,57] and to increase tolerance to clinically relevant antibiotics [59].
The First Reactivity-Based Probes of Ferrous Iron
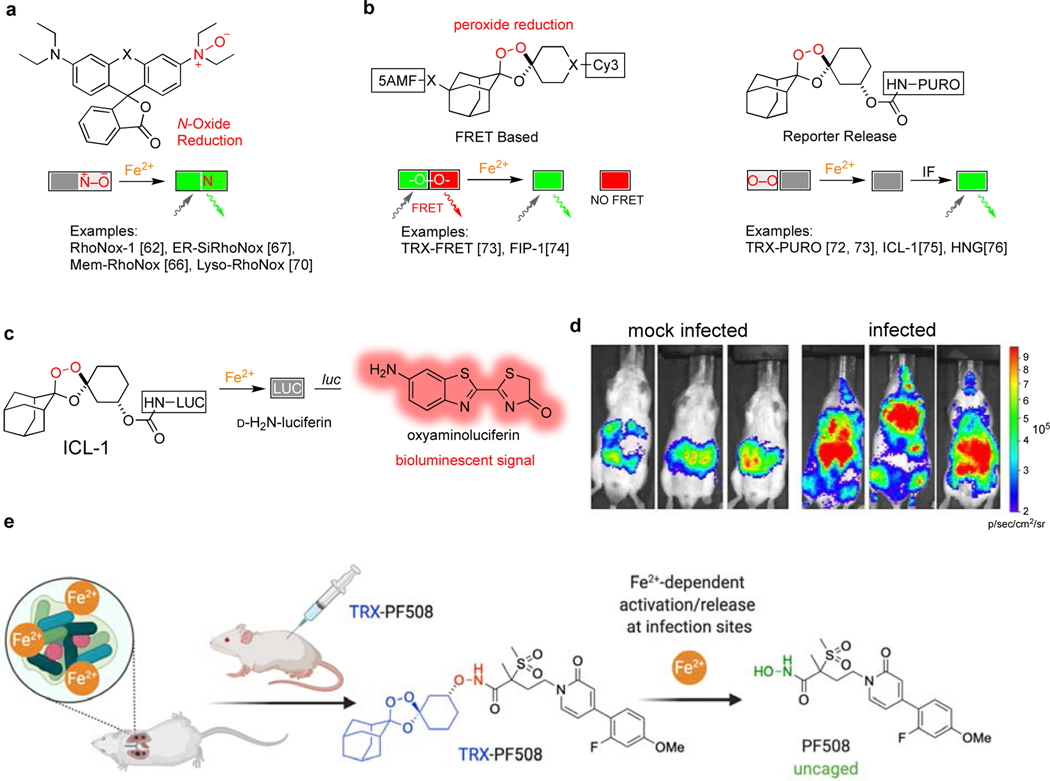
Increased appreciation for the role of Fe2+ in bacterial growth and innate immunity, as described in the prior sections, has coincided with the development of new chemical probes to study iron with oxidation-stage specificity. While chelation of the electron-poor ferric iron is dominated by σ-donation from electron-rich ligands (e.g., catechol and hydroxamate siderophores), the more electron-rich ferrous ion can engage in back-donation from metal d-orbitals to heterocyclic ligands such as the imidazole ring of histidine (e.g., as in the His6 site in CP). While some measure of selectivity can be achieved in chelation-based iron probes [60], the true breakthrough in oxidation-state specificity has been realized through reactivity-based sensing, as recently reviewed [61]. First generation reactivity-based probes Rho-Nox1 [62] and IP-1 [63] gave early indications that this approach might improve oxidation-state selectivity as compared to chelation-based probes like PhenGreen SK [64]. Further development of the RhoNox-family of probes by Hirayama and co-workers (Figure 3a) has yielded new tools to study Fe2+ in the endosome-lysosome system [65], at the plasma membrane [66], in hypoxic tumor spheroids [67], in cellular models of the blood-brain barrier [68] and neural vascular barrier [69], and in mammalian cells undergoing ferroptosis [70], a form of non-apoptotic, iron(II)-dependent cell death [71]. A separate class of reactivity-based probes inspired by the antimalarial trioxolane (TRX) arterolane was described by Renslo and co-workers [72,73] and further developed by Chang [74,75] and Zhang [76] (Figure 3b). The TRX-based probes TRX-PURO [72] and FIP-1 [74] were able to detect an increase in LIP in cancer cell lines and revealed a previously unappreciated link between GPCR signaling and epigenetic regulation by mononuclear Fe2+-dependent TET enzymes [77,78].
Figure 3.
New tools to study ferrous iron in vitro and in vivo. (a) RhoNox-family probes are activated by reduction of an N-oxide bond; (b) TRX-based probes are activated by reduction of a hindered peroxide bond; (c) The probe ICL-1 releases D-aminoluciferin and can be used in luciferin-expressing cells and animals; (d) mice infected with A. baumannii show elevated Fe2+ as detected with ICL-1; (e) the drug conjugate TRX-PF508 releases an LpxC inhibitor upon sensing Fe2+ in vivo and is efficacious in an acute P. aeruginosa lung infection model.
These new tools have only just begun to be applied to the study of Fe2+ in bacterial iron-homeostasis and in bacterial infection. Thus, after first establishing that ICL-1 (Figure 3c) was highly selective for reaction with the Fe2+ ion over other biologically relevant metals and reductants, this caged form of luciferin was used to detect the mobilization of Fe2+ in luciferin-expressing mice (FVB-luc+) infected with the Gram-negative pathogen A. baumannii [75]. Whereas ICL-1 treatment of mock-infected control animals showed only weak bioluminescence near the intra-peritoneal site of ICL-1 administration, infected mice treated administered ICL-1 showed more dramatic bioluminescence in organs that were later shown by ex vivo analysis to also be major sites of infection (Figure 3d). Analysis of total iron load by ICP-MS in the infected tissues showed a substantial elevation of total iron only in liver, an observation consistent with canonical nutritional immunity and iron sequestration by that organ. The bioluminescent signal in the other infected tissues was not correlated with total iron load however, and thus indicates that Fe2+ specifically is elevated during infection. This mobilization of Fe2+ might reflect LIP (Fe2+) expansion by a pathogen undergoing exponential growth, as described by Lindhal in the spectroscopic studies described above [12]. Alternatively, these changes might reflect the pathogen’s utilization of extracellular Fe2+ in the infection microenvironment [53,55], or the canonical unloading of Fe2+ from siderophore–Fe3+ within the pathogen. Further studies will be required to address the questions posed by these recent studies and new tools, which are challenging some long-held assumptions about iron utilization and sequestration during infection.
Antibiotic therapy exploiting iron has been well explored for siderophore-antibiotic conjugates (‘sideromycins’) designed to undergo active uptake via bacterial Fe3+-siderophore transporters [79,80]. More recent findings concerning Fe2+ in infection, as detailed herein, invite study of a new therapeutic approach comprising the reactivity-based activation of antibiotics selectively at infection sites, in response to Fe2+. To explore this notion, our laboratory recently described [81] the design and synthesis of an Fe2+-activatable form of the LpxC inhibitor PF-5081090 (i.e., TRX-PF508) and its study in P. aeruginosa infection models (Figure 3e). Whereas our previously described Fe2+ sensors [73,82] were designed to cage amine-bearing payloads, the TRX sensor employed in TRX-PF508 enables caging of hydroxamate-based metalloenzyme inhibitors, potentially mitigating the well-known [83] toxicological and pharmacokinetic liabilities of the hydroxamate function. Intriguingly, whereas TRX-PF508 showed only weak activity in MIC experiments, the compound was highly efficacious in an acute P. aeruginosa lung infection model, reducing bacterial CFU counts in a dose-dependent fashion and being well tolerated even at the highest dose of 64 mg/kg. By contrast, mice administered the parent drug PF-5081090 showed only a modest (non-significant) reduction in lung CFU counts at a dose equimolar to the lowest effective dose of TRX-PF508 (16 mg/kg). Overall, the findings of this study were consistent with a drug concentrating effect of TRX-PF508 in the lung, presumably resulting from selective activation by Fe2+ in this (infected) tissue.
Here we have summarized recent studies of bacterial acquisition and utilization of ferrous iron during infection and in the context of host-pathogen interaction. New chemical probes of ferrous iron emerging contemporaneously with these microbiological studies invite future opportunities to apply these new tools to advance a more nuanced understanding or iron acquisition and specification, both in vitro and in vivo. Early studies of this type include the imaging of Fe2+ in a murine infection model with ICL-1 and the development of antibiotics conjugates like TRX-PF508 that are activated in the infection microenvironment by reactivity-based sensing of Fe2+. These new tools and emerging biology suggest a bright future for the field of chemical (micro)biology.
Acknowledgements
This work was supported by US National Institutes of Health R01 Grant AI105106 to AR Renslo.
Footnotes
Declaration of competing interest
AR Renslo is a founder of Tatara Therapeutics, Inc.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, Goossens H, Laxminarayan R: Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115:E3463–E3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson N, Czaplewski L, Piddock LJV: Discovery and development of new antibacterial drugs: learning from experience? J. Antimicrob. Chemother 2018, 73:1452–1459. [DOI] [PubMed] [Google Scholar]
- 3.Bérdy J: Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot 2012, 65:385–395. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill J: Tackling Drug-resistant Infections Globally: Final Report and Recommendations, The Review on Antimicrobial Resistance, London, UK, HM Government and the Wellcome Trust. [Internet]. Wellcome Trust; 2016. [Google Scholar]
- 5.Centers for Disease Control and Prevention (U.S.): Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threatsreport/2019-ar-threats-report-508.pdf (last accessed 5th October 2020). National Center for Emerging Zoonotic and Infectious Diseases (U.S.); 2019. [Google Scholar]
- 6.Li X-Z, Plésiat P, Nikaido H: The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev 2015, 28:337–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Wintersdorff CJH, Penders J, van Niekerk JM, Mills ND, Majumder S, van Alphen LB, Savelkoul PHM, Wolffs PFG: Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol 2016, 7:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.Theuretzbacher U, Bush K, Harbarth S, Paul M, Rex JH, Tacconelli E, Thwaites GE: Critical analysis of antibacterial agents in clinical development. Nat. Rev. Microbiol 2020, 18:286–298.A current take from thought leaders on the current status of antibacterial development and a call to arms for the exploration of new targets and innovative approaches.
- 9.Zhang Y, Sen S, Giedroc DP: Iron Acquisition by Bacterial Pathogens: Beyond Tris-Catecholate Complexes. Chembiochem 2020, 21:1955–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hantke K: Is the bacterial ferrous iron transporter FeoB a living fossil? Trends Microbiol. 2003, 11:192–195. [DOI] [PubMed] [Google Scholar]
- 11.Keyer K, Imlay JA: Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 1996, 93:13635–13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Wofford JD, Bolaji N, Dziuba N, Outten FW, Lindahl PA: Evidence that a respiratory shield in Escherichia coli protects a low-molecular-mass FeII pool from O2-dependent oxidation. J. Biol. Chem 2019, 294:50–62.A rigorous spectroscopic investigation of iron speciation in live E. coli under stationary and exponential growth conditions. Presents a dramatically new and contrarion view of bacterial iron homeostasis that if correct will inspire new therapeutic approaches to distiguish host cell from pathogen by dramatically elevated ferrous iron concentrations in the latter.
- 13.Skaar EP: The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010, 6:e1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandrangsu P, Rensing C, Helmann JD: Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol 2017, 15:338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Jordan MR, Wang J, Capdevila DA, Giedroc DP: Multi-metal nutrient restriction and crosstalk in metallostasis systems in microbial pathogens. Curr. Opin. Microbiol 2020, 55:17–25.Contemporary review of metal ion sensing and ‘metallostasis’.
- 16.Pinochet-Barros A, Helmann JD: Redox sensing by fe2+ in bacterial fur family metalloregulators. Antioxid. Redox Signal 2018, 29:1858–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porcheron G, Dozois CM: Interplay between iron homeostasis and virulence: Fur and RyhB as major regulators of bacterial pathogenicity. Vet. Microbiol 2015, 179:2–14. [DOI] [PubMed] [Google Scholar]
- 18.Pasqua M, Visaggio D, Lo Sciuto A, Genah S, Banin E, Visca P, Imperi F: Ferric Uptake Regulator Fur Is Conditionally Essential in Pseudomonas aeruginosa. J. Bacteriol 2017, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abeyrathna SS, Abeyrathna NS, Thai NK, Sarkar P, D’Arcy S, Meloni G: IroT/MavN Is a Legionella Transmembrane Fe(II) Transporter: Metal Selectivity and Translocation Kinetics Revealed by in Vitro Real-Time Transport. Biochemistry 2019, 58:4337–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furukawa K, Ramesh A, Zhou Z, Weinberg Z, Vallery T, Winkler WC, Breaker RR: Bacterial riboswitches cooperatively bind Ni(2+) or Co(2+) ions and control expression of heavy metal transporters. Mol. Cell 2015, 57:1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dambach M, Sandoval M, Updegrove TB, Anantharaman V, Aravind L, Waters LS, Storz G: The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Mol. Cell 2015, 57:1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price IR, Gaballa A, Ding F, Helmann JD, Ke A: Mn(2+)-sensing mechanisms of yybP-ykoY orphan riboswitches. Mol. Cell 2015, 57:1110–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin JE, Le MT, Bhattarai N, Capdevila DA, Shen J, Winkler ME, Giedroc DP: A Mn-sensing riboswitch activates expression of a Mn2+/Ca2+ ATPase transporter in Streptococcus. Nucleic Acids Res. 2019, 47:6885–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dann CE, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC: Structure and mechanism of a metal-sensing regulatory RNA. Cell 2007, 130:878–892. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Cotruvo JA: The czcD (NiCo) Riboswitch Responds to Iron(II). Biochemistry 2020, 59:1508–1516. [DOI] [PubMed] [Google Scholar]
- 26.Muok AR, Deng Y, Gumerov VM, Chong JE, DeRosa JR, Kurniyati K, Coleman RE, Lancaster KM, Li C, Zhulin IB, et al. : A di-iron protein recruited as an Fe[II] and oxygen sensor for bacterial chemotaxis functions by stabilizing an iron-peroxy species. Proc. Natl. Acad. Sci. USA 2019, 116:14955–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivera M: Bacterioferritin: Structure, Dynamics, and Protein-Protein Interactions at Play in Iron Storage and Mobilization. Acc. Chem. Res 2017, 50:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eshelman K, Yao H, Punchi Hewage AND, Deay JJ, Chandler JR, Rivera M: Inhibiting the BfrB:Bfd interaction in Pseudomonas aeruginosa causes irreversible iron accumulation in bacterioferritin and iron deficiency in the bacterial cytosol. Metallomics 2017, 9:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewage Punchi AND, Yao H, Nammalwar B, Gnanasekaran KK, Lovell S, Bunce RA, Eshelman K, Phaniraj SM, Lee MM, Peterson BR, et al. : Small Molecule Inhibitors of the BfrB-Bfd Interaction Decrease Pseudomonas aeruginosa Fitness and Potentiate Fluoroquinolone Activity. J. Am. Chem. Soc 2019, 141:8171–8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg ED: Nutritional immunity: host’s attempt to withhold iron from microbial invaders. Jama 1975, 231:39–41. [DOI] [PubMed] [Google Scholar]
- *31.Palmer LD, Skaar EP: Transition metals and virulence in bacteria. Annu. Rev. Genet 2016, 50:67–91.Conemporary review of transition metals in host-pathogen interaction.
- 32.Carver PL: The Battle for Iron between Humans and Microbes. Curr. Med. Chem 2018, 25:85–96. [DOI] [PubMed] [Google Scholar]
- 33.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T: IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest 2004, 113:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao X, Yeoh BS, Vijay-Kumar M: Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu. Rev. Nutr 2017, 37:103–130. [DOI] [PubMed] [Google Scholar]
- 35.Sheldon JR, Skaar EP: Metals as phagocyte antimicrobial effectors. Curr. Opin. Immunol 2019, 60:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White C, Lee J, Kambe T, Fritsche K, Petris MJ: A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem 2009, 284:33949–33956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladomersky E, Khan A, Shanbhag V, Cavet JS, Chan J, Weisman GA, Petris MJ: Host and Pathogen Copper-Transporting P-Type ATPases Function Antagonistically during Salmonella Infection. Infect. Immun 2017, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong C-LY, Berking O, Walker MJ, McEwan AG: New Insights into the Role of Zinc Acquisition and Zinc Tolerance in Group A Streptococcal Infection. Infect. Immun 2018, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schalk IJ, Mislin GLA, Brillet K: Structure, function and binding selectivity and stereoselectivity of siderophore-iron outer membrane transporters. Curr Top Membr 2012, 69:37–66. [DOI] [PubMed] [Google Scholar]
- 40.Moynié L, Milenkovic S, Mislin GLA, Gasser V, Malloci G, Baco E, McCaughan RP, Page MGP, Schalk IJ, Ceccarelli M, et al. : The complex of ferric-enterobactin with its transporter from Pseudomonas aeruginosa suggests a two-site model. Nat. Commun 2019, 10:3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganne G, Brillet K, Basta B, Roche B, Hoegy F, Gasser V, Schalk IJ: Iron Release from the Siderophore Pyoverdine in Pseudomonas aeruginosa Involves Three New Actors: FpvC, FpvG, and FpvH. ACS Chem. Biol 2017, 12:1056–1065. [DOI] [PubMed] [Google Scholar]
- 42.Bonneau A, Roche B, Schalk IJ: Iron acquisition in Pseudomonas aeruginosa by the siderophore pyoverdine: an intricate interacting network including periplasmic and membrane proteins. Sci. Rep 2020, 10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vigouroux A, Aumont-Nicaise M, Boussac A, Marty L, Lo Bello L, Legrand P, Brillet K, Schalk IJ, Moréra S: A unique ferrous iron binding mode is associated with large conformational changes for the transport protein FpvC of Pseudomonas aeruginosa. FEBS J. 2020, 287:295–309. [DOI] [PubMed] [Google Scholar]
- 44.Lamont IL, Beare PA, Ochsner U, Vasil AI, Vasil ML: Siderophore-mediated signaling regulates virulence factor production in Pseudomonasaeruginosa. Proc. Natl. Acad. Sci. USA 2002, 99:7072–7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilderman PJ, Vasil AI, Johnson Z, Wilson MJ, Cunliffe HE, Lamont IL, Vasil ML: Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect. Immun 2001, 69:5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson CE, Huang W, Brewer LK, Nguyen AT, Kane MA, Wilks A, Oglesby-Sherrouse AG: Proteomic Analysis of the Pseudomonas aeruginosa Iron Starvation Response Reveals PrrF Small Regulatory RNA-Dependent Iron Regulation of Twitching Motility, Amino Acid Metabolism, and Zinc Homeostasis Proteins. J. Bacteriol 2019, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **47.Nakashige TG, Zhang B, Krebs C, Nolan EM: Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol 2015, 11:765–771.Reports a fundamentally important finding that the host immune response involves sequestration of ferrous iron in addition to the much better studied and well recognized host responses to sequester ferric iron and limit total available iron via the ferroportin/hepcidin axis.
- 48.Wang J, Lonergan ZR, Gonzalez-Gutierrez G, Nairn BL, Maxwell CN, Zhang Y, Andreini C, Karty JA, Chazin WJ, Trinidad JC, et al. : Multi-metal Restriction by Calprotectin Impacts De Novo Flavin Biosynthesis in Acinetobacter baumannii. Cell Chem. Biol 2019, 26:745–755.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Zygiel EM, Nolan EM: Exploring iron withholding by the innate immune protein human calprotectin. Acc. Chem. Res 2019, 52:2301–2308.Contemporary review of recent studies concerning the role of caprotectin in the innate immune response.
- 50.Nakashige TG, Nolan EM: Human calprotectin affects the redox speciation of iron. Metallomics 2017, 9:1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zygiel EM, Nelson CE, Brewer LK, Oglesby-Sherrouse AG, Nolan EM: The human innate immune protein calprotectin induces iron starvation responses in Pseudomonas aeruginosa. J. Biol. Chem 2019, 294:3549–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Besold AN, Culbertson EM, Nam L, Hobbs RP, Boyko A, Maxwell CN, Chazin WJ, Marques AR, Culotta VC: Antimicrobial action of calprotectin that does not involve metal withholding. Metallomics 2018, 10:1728–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sestok AE, Linkous RO, Smith AT: Toward a mechanistic understanding of Feo-mediated ferrous iron uptake. Metallomics 2018, 10:887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minandri F, Imperi F, Frangipani E, Bonchi C, Visaggio D, Facchini M, Pasquali P, Bragonzi A, Visca P: Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection. Infect. Immun 2016, 84:2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, Malfroot A, Cornelis P, Newman DK: Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. MBio 2013, 4.Clinically significant finding that also presaged more recent work concerning the utilization of ferrous iron by pathogens during infection.
- 56.Tyrrell J, Callaghan M: Iron acquisition in the cystic fibrosis lung and potential for novel therapeutic strategies. Microbiology (Reading, Engl.) 2016, 162:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, Newman DK: Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J. Bacteriol 2011, 193:3606–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.Konings AF, Martin LW, Sharples KJ, Roddam LF, Latham R, Reid DW, Lamont IL: Pseudomonas aeruginosa uses multiple pathways to acquire iron during chronic infection in cystic fibrosis lungs. Infect. Immun 2013, 81:2697–2704.Early paper describing the diverse iron acquisition mechanisms of P. aeruginosa, including acquisition and utilization of ferrous iron.
- 59.Schiessl KT, Hu F, Jo J, Nazia SZ, Wang B, Price-Whelan A, Min W, Dietrich LEP: Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat. Commun 2019, 10:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carter KP, Young AM, Palmer AE: Fluorescent sensors for measuring metal ions in living systems. Chem. Rev 2014, 114:4564–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *61.Aron AT, Reeves AG, Chang CJ: Activity-based sensing fluorescent probes for iron in biological systems. Curr. Opin. Chem. Biol 2018, 43:113–118.Review of the foundational developments in activity-bsed sensing of iron in cellular contexts.
- 62.Hirayama T, Okuda K, Nagasawa H: A highly selective turn-on fluorescent probe for iron(ii) to visualize labile iron in living cells. Chem. Sci 2013, 4:1250. [Google Scholar]
- 63.Au-Yeung HY, Chan J, Chantarojsiri T, Chang CJ: Molecular imaging of labile iron(II) pools in living cells with a turn-on fluorescent probe. J. Am. Chem. Soc 2013, 135:15165–15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrat F, Rauen U, de Groot H: Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology 1999, 29:1171–1179. [DOI] [PubMed] [Google Scholar]
- 65.Niwa M, Hirayama T, Okuda K, Nagasawa H: A new class of high-contrast Fe(II) selective fluorescent probes based on spirocyclized scaffolds for visualization of intracellular labile iron delivered by transferrin. Org. Biomol. Chem 2014, 12:6590–6597. [DOI] [PubMed] [Google Scholar]
- 66.Niwa M, Hirayama T, Oomoto I, Wang DO, Nagasawa H: Fe(II) Ion Release during Endocytotic Uptake of Iron Visualized by a Membrane-Anchoring Fe(II) Fluorescent Probe. ACS Chem. Biol 2018, 13:1853–1861. [DOI] [PubMed] [Google Scholar]
- 67.Hirayama T, Tsuboi H, Niwa M, Miki A, Kadota S, Ikeshita Y, Okuda K, Nagasawa H: A universal fluorogenic switch for Fe(ii) ion based on N-oxide chemistry permits the visualization of intracellular redox equilibrium shift towards labile iron in hypoxic tumor cells. Chem. Sci 2017, 8:4858–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imai T, Iwata S, Hirayama T, Nagasawa H, Nakamura S, Shimazawa M, Hara H: Intracellular Fe2+ accumulation in endothelial cells and pericytes induces blood-brain barrier dysfunction in secondary brain injury after brain hemorrhage. Sci. Rep 2019, 9:6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui D, Arima M, Hirayama T, Ikeda E: Hypoxia-induced disruption of neural vascular barrier is mediated by the intracellular induction of Fe(II) ion. Exp. Cell Res 2019, 379:166–171. [DOI] [PubMed] [Google Scholar]
- 70.Hirayama T, Miki A, Nagasawa H: Organelle-specific analysis of labile Fe(ii) during ferroptosis by using a cocktail of various colour organelle-targeted fluorescent probes. Metallomics 2019, 11:111–117. [DOI] [PubMed] [Google Scholar]
- 71.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. : Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012, 149:1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spangler B, Morgan CW, Fontaine SD, Vander Wal MN, Chang CJ, Wells JA, Renslo AR: A reactivity-based probe of the intracellular labile ferrous iron pool. Nat. Chem. Biol 2016, 12:680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fontaine SD, Spangler B, Gut J, Lauterwasser EMW, Rosenthal PJ, Renslo AR: Drug delivery to the malaria parasite using an arterolane-like scaffold. ChemMedChem 2015, 10:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aron AT, Loehr MO, Bogena J, Chang CJ: An Endoperoxide Reactivity-Based FRET Probe for Ratiometric Fluorescence Imaging of Labile Iron Pools in Living Cells. J. Am. Chem. Soc 2016, 138:14338–14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **75.Aron AT, Heffern MC, Lonergan ZR, Vander Wal MN, Blank BR, Spangler B, Zhang Y, Park HM, Stahl A, Renslo AR, et al. : In vivo bioluminescence imaging of labile iron accumulation in a murine model of Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2017, 114:12669–12674.Describes the probe ICL-1 and its use in live mice to reveal elevation of ferrous iron in infected tissues, running counter to contervailing views abour iron limitation during infection.
- 76.Xu S, Liu H-W, Chen L, Yuan J, Liu Y, Teng L, Huan S-Y, Yuan L, Zhang X-B, Tan W: Learning from Artemisinin: Bioinspired Design of a Reaction-Based Fluorescent Probe for the Selective Sensing of Labile Heme in Complex Biosystems. J. Am. Chem. Soc 2020, 142:2129–2133. [DOI] [PubMed] [Google Scholar]
- 77.Camarena V, Sant DW, Huff TC, Mustafi S, Muir RK, Aron AT, Chang CJ, Renslo AR, Monje PV, Wang G: cAMP signaling regulates DNA hydroxymethylation by augmenting the intracellular labile ferrous iron pool. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huff TC, Camarena V, Sant DW, Wilkes Z, Van Booven D, Aron AT, Muir RK, Renslo AR, Chang CJ, Monje PV, et al. : Oscillatory cAMP signaling rapidly alters H3K4 methylation. Life Sci. Alliance 2020, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schalk IJ: Siderophore-antibiotic conjugates: exploiting iron uptake to deliver drugs into bacteria. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2018, 24:801. [DOI] [PubMed] [Google Scholar]
- 80.Lin Y-M, Ghosh M, Miller PA, Möllmann U, Miller MJ: Synthetic sideromycins (skepticism and optimism): selective generation of either broad or narrow spectrum Gram-negative antibiotics. Biometals 2019, 32:425–451. [DOI] [PubMed] [Google Scholar]
- *81.Blank BR, Talukder P, Muir RK, Green ER, Skaar EP, Renslo AR: Targeting Mobilization of Ferrous Iron in Pseudomonas aeruginosa Infection with an Iron(II)-Caged LpxC Inhibitor. ACS Infect. Dis 2019, 5:1366–1375.First report of ferrous iron targeted antibacterial chemotherapy in animals, demonstrating enhanced efficacy for iron(II)-targeted form of a LpxC inhibitor.
- 82.Fontaine SD, DiPasquale AG, Renslo AR: Efficient and stereocontrolled synthesis of 1,2,4-trioxolanes useful for ferrous iron-dependent drug delivery. Org. Lett 2014, 16:5776–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen S, Kozikowski AP: Why Hydroxamates May Not Be the Best Histone Deacetylase Inhibitors--What Some May Have Forgotten or Would Rather Forget? ChemMedChem 2016, 11:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]