Abstract
BACKGROUND
The use of naltrexone plus bupropion to treat methamphetamine use disorder has not been well studied.
METHODS
We conducted this multisite, double-blind, two-stage, placebo-controlled trial with the use of a sequential parallel comparison design to evaluate the efficacy and safety of extended-release injectable naltrexone (380 mg every 3 weeks) plus oral extended-release bupropion (450 mg per day) in adults with moderate or severe methamphetamine use disorder. In the first stage of the trial, participants were randomly assigned in a 0.26:0.74 ratio to receive naltrexone–bupropion or matching injectable and oral placebo for 6 weeks. Those in the placebo group who did not have a response in stage 1 underwent rerandomization in stage 2 and were assigned in a 1:1 ratio to receive naltrexone–bupropion or placebo for an additional 6 weeks. Urine samples were obtained from participants twice weekly. The primary outcome was a response, defined as at least three methamphetamine-negative urine samples out of four samples obtained at the end of stage 1 or stage 2, and the weighted average of the responses in the two stages is reported. The treatment effect was defined as the between-group difference in the overall weighted responses.
RESULTS
A total of 403 participants were enrolled in stage 1, and 225 in stage 2. In the first stage, 18 of 109 participants (16.5%) in the naltrexone–bupropion group and 10 of 294 (3.4%) in the placebo group had a response. In the second stage, 13 of 114 (11.4%) in the naltrexone–bupropion group and 2 of 111 (1.8%) in the placebo group had a response. The weighted average response across the two stages was 13.6% with naltrexone–bupropion and 2.5% with placebo, for an overall treatment effect of 11.1 percentage points (Wald z-test statistic, 4.53; P<0.001). Adverse events with naltrexone–bupropion included gastrointestinal disorders, tremor, malaise, hyperhidrosis, and anorexia. Serious adverse events occurred in 8 of 223 participants (3.6%) who received naltrexone–bupropion during the trial.
CONCLUSIONS
Among adults with methamphetamine use disorder, the response over a period of 12 weeks among participants who received extended-release injectable naltrexone plus oral extended-release bupropion was low but was higher than that among participants who received placebo. (Funded by the National Institute on Drug Abuse and others; ADAPT-2 ClinicalTrials.gov number, NCT03078075.)
THERE HAS BEEN A RISE IN METHAMphetamine use disorder in the United States, particularly in the Midwest and West, where methamphetamine is a leading cause of overdose deaths.1,2 There is no medication approved by the Food and Drug Administration for the treatment of methamphetamine use disorder, and effective treatment has been identified as an essential public health goal.3,4
Bupropion5–7 and naltrexone8–10 used individually have shown some positive evidence of efficacy in clinical trials for the treatment of methamphetamine use disorder.11–13 Bupropion is a stimulant-like antidepressant that acts through the norepinephrine and dopamine systems and might ameliorate the dysphoria associated with methamphetamine withdrawal that drives continued use.14,15 Naltrexone is an opioid-receptor antagonist that is effective for the treatment of opioid use disorder. In some trials, it has also been shown to have a modest effect in preventing relapse of alcohol use,16 perhaps by attenuating the reinforcing effects of substances or cue-induced cravings.10,17,18 The results of a small, open-label pilot trial suggested that naltrexone plus bupropion might be effective for the treatment of severe methamphetamine use disorder.19 These findings supported the development of the current trial (Accelerated Development of Additive Treatment for Methamphetamine Disorder [ADAPT-2]), which assessed the efficacy of combining these agents for the treatment of methamphetamine use disorder.
METHODS
TRIAL DESIGN AND CONDUCT
This randomized, double-blind trial, which used a sequential parallel comparison design,20,21 was conducted at eight sites from May 23, 2017, to July 25, 2019. It evaluated the efficacy and safety of extended-release injectable naltrexone (380 mg every 3 weeks) combined with once-daily oral extended-release bupropion (450 mg per day) as compared with placebo in adult outpatients with moderate or severe methamphetamine use disorder.
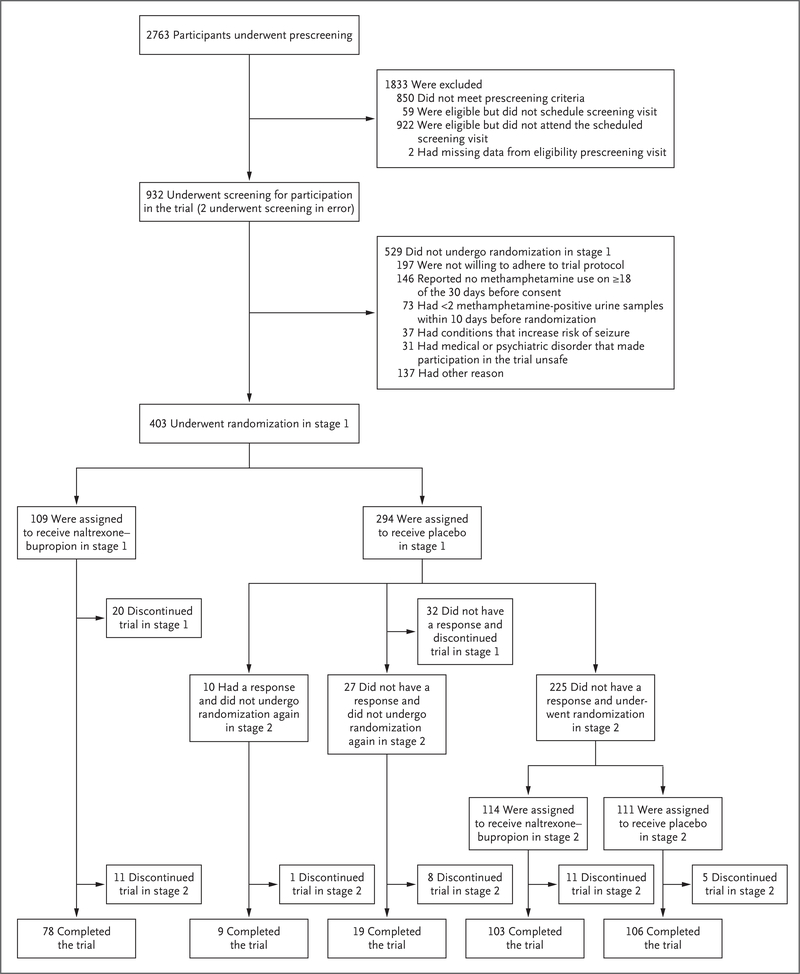
This 12-week trial was conducted in two stages consisting of 6 weeks each. Participants initially underwent randomization in a 0.26:0.74 ratio to receive naltrexone–bupropion or placebo during the first 6-week stage; participants in the placebo group who did not have a response in the first stage underwent randomization again in a 1:1 ratio in the second 6-week stage (Fig. 1). The ratios used for randomization were chosen on the basis of established practices in sequential parallel design trials and are described in the Statistical Analysis section and in the statistical analysis plan, included in the protocol (available with the full text of this article at NEJM.org).22 The purpose of rerandomization was to enrich the sample in the second stage with participants who were unlikely to have a response to placebo. The results from both stages were combined for analysis as described in the statistical analysis plan.
Figure 1. (facing page). Screening and Randomization.
Participants may have had more than one reason for not undergoing randomization in stage 1. The analysis of the primary outcome was performed in the intention-to-treat population, which included all participants who underwent randomization in stage 1 and all participants who underwent randomization again in stage 2.
Participants visited the clinic twice a week for drug screening of urine samples (for a potential total of 24 urine samples per participant [12 in each stage]), for safety monitoring, and for assessments. Additional safety and outcome assessments were performed at week 6 and week 12. The integrity of urine samples was determined with the use of an embedded temperature strip on the collection cup (valid samples were considered to be those with a temperature of 32° to 38°C [90° to 100°F]) and a negative test for adulterants. Valid samples were tested for 10 drugs with the use of a point-of-care urine drug test card in accordance with the regulations of the Clinical Laboratory Improvement Amendments of 1988.
Extended-release naltrexone was supplied in standard single-use intramuscular injection kits, each containing one 380-mg vial of naltrexone microspheres. In each stage, injections of naltrexone or placebo were administered by trial clinicians on the day of randomization (or rerandomization) and in the third week of each stage. Naltrexone was administered every 3 weeks to mitigate the lower naltrexone blood levels that would most likely occur with a 4-week injection schedule, according to the product labeling.
Extended-release bupropion (in 150-mg tablets) or placebo was provided weekly in matching blister cards. Beginning on the day of randomization or rerandomization, the dose was raised over the course of 3 days to a total daily dose of 450 mg. If appropriate, doses could be reduced before week 13 to 300 mg per day to alleviate adverse effects; clinicians were encouraged to attempt to raise the dose back up to the target dose. At the end of the trial (week 13), the dose was tapered over a period of 4 days, at which point it was discontinued.
Adherence to the assigned regimen was determined by participant-reported tablet ingestion (confirmed on the basis of tablet count) and by documentation by the trial staff who administered the injections. To encourage adherence, participants were asked to use a smartphone-based application to track tablet ingestion. Trial clinicians, who were unaware of group assignments, met weekly with participants to manage adverse events, assess and encourage adherence to the oral regimen, address participant concerns, and provide counseling for reducing substance use.
TRIAL OVERSIGHT
The trial was conducted in accordance with the principles of the Declaration of Helsinki. The protocol was approved by the data and safety monitoring board of the National Institute on Drug Abuse (NIDA) Clinical Trials Network, by a central institutional review board, and by institutional review boards at four sites. The data and safety monitoring board monitored trial progress and safety, reviewed a one-time sample-size reestimation and interim efficacy analysis, and appraised the final outcome and safety results. The data analysis was performed by the fifth, sixth, and eighth authors. The first draft of the manuscript was written by the second author. All authors vouch for the adherence of the trial to the protocol, the completeness and accuracy of the data, and the complete reporting of adverse events. Alkermes donated naltrexone in the form of extended-release injectable suspension and matched injectable placebo for this trial under a written agreement with NIDA (the sponsor). AiCure (New York) provided the smartphone-based application for tracking adherence to the oral regimen under a paid subcontract. Neither company had a role in the collection or analysis of the data or the writing of the manuscript. There were no confidentiality agreements between the investigators and the commercial entities.
PARTICIPANTS
Adults 18 to 65 years of age who wanted to quit or reduce methamphetamine use were recruited from communities near the trial sites with the use of advertisements (e.g., print, Web, radio, and television advertising) and through direct referrals (e.g., by participants who were already enrolled in the trial, medical clinics, and social-service agencies). Eligible participants met the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), for moderate or severe stimulant use disorder (methamphetamine type); reported methamphetamine use on at least 18 of the 30 days before consent; had two or more methamphetamine-positive urine samples (obtained ≥2 days apart) within 10 days before randomization; and were opioid-free at the time of randomization. Participants were excluded if they were undergoing concurrent treatment for substance use disorder, had an expected need for opioid-containing medications (e.g., planned surgery) during the trial, or did not meet additional criteria that would ensure that participation would be safe (e.g., participants would not be eligible if they had conditions that increased the risk of seizure or were taking medications that were contraindicated). Participants who had received a diagnosis of a specific medical or psychiatric disorder were not routinely excluded and were evaluated on a case-by-case basis to determine whether it was safe for them to participate.
Persons who were interested in participation completed a brief telephone prescreening, and, if appropriate, a visit was scheduled so that participants could learn about trial procedures and the potential benefits and risks of participation, have the opportunity to ask questions, and provide written informed consent. After consent was obtained, a screening period of 4 to 21 days was begun to evaluate eligibility criteria. Eligible participants were then randomly assigned to receive naltrexone–bupropion or placebo. Participants were compensated for participation in the trial. Details on eligibility criteria and compensation are provided in the protocol.
OUTCOMES
The primary outcome was a response to the trial regimen, defined as at least three methamphetamine-negative urine tests out of a possible four obtained at the end of stage 1 (during week 5 through week 6) or at the end of stage 2 (during week 11 through week 12). A response was included in the analysis only in the stage in which it first occurred. Participants who had two or more missing results of urine drug screenings or who discontinued the trial were recorded as not having had a response. To combine results across the two trial stages, the weighted average of the responses across the two stages was calculated for each trial group. The overall treatment effect was defined as the between-group difference in the weighted responses.
Secondary outcomes that were evaluated in each stage were the percentage of methamphetamine-negative urine samples (i.e., the number of methamphetamine-negative urine samples per stage divided by 12, which was the total number of samples expected in each stage); the most severe methamphetamine craving during the previous week,23 assessed weekly with the use of a visual analogue scale (values range from 0 to 100, with higher values indicating greater cravings); depressive symptoms, assessed weekly with the use of the Patient Health Questionnaire 9 (PHQ-9; each of nine items is given a score of 0 to 3, with a score of 0 indicating the absence of depressive symptoms and a score of 3 indicating the presence of depressive symptoms nearly every day; total scores range from 0 to 27, with higher scores indicating greater depressive symptoms); and results of the Treatment Effectiveness Assessment at week 6 and week 12, which assesses reduced substance use and improvements in lifestyle, health, and community and interpersonal interactions according to participant report24,25 (total scores range from 4 to 40, with higher scores indicating greater improvement in these factors).
Safety outcomes were assessed at each visit and included participant-reported adverse events and assessment of vital signs, liver-function tests, injection-site reactions, results on electrocardiograms, and suicidality.26 Adverse events were classified according to the preferred term and system organ class of the Medical Dictionary for Regulatory Activities, version 22.1. Site investigators, who were unaware of trial-group assignments, determined whether an event was a serious adverse event and evaluated the severity and cause of the event. Serious adverse events were adjudicated by a medical monitor assigned by the sponsor.
STATISTICAL ANALYSIS
According to the sample-size calculation,22 we determined that 370 participants would give the trial 90% power to detect a weighted difference between the two trial groups under the assumption that 24% of participants in the naltrexone–bupropion group and 15% in the placebo group would have a response in stage 1, and 24% in the naltrexone–bupropion group and 10% in the placebo group would have a response in stage 2. The assumption that 24% of participants in the naltrexone–bupropion group would have a response was determined on the basis of a small pilot study.19 Because the goal of stage 2 was to enrich the sample by including only participants in the placebo group who did not have a response in stage 1, we expected a smaller number of participants in the placebo group to have a response in stage 2 than in stage 1. The prespecified sample-size reestimation analysis was performed with data from the first 185 participants who underwent randomization. Investigators were not informed of the results of the reestimation analysis. The data and safety monitoring board recommended increasing the sample size to 400 to maintain 90% power to detect a difference in response between the two groups. This recommendation was approved by the sponsor on August 13, 2018.
The trial used a two-stage, sequential parallel comparison design.20,21 This design requires two parameters: a randomization fraction and a weight. Each value was chosen to maximize the power of the test in accordance with the sample-size calculation, resulting in a randomization ratio in stage 1 of 0.26:0.74 to naltrexone–bupropion or placebo. The overall treatment effect with this design was defined as the average response in the naltrexone–bupropion group minus the average response in the placebo group, calculated with the use of a weight of 0.43 in stage 1 and a weight of 0.57 in stage 2. Additional information regarding the formula used to calculate the size of the treatment effect, h = [w(p1) + (1 − w) p2] − [w(q1) + (1 − w)(q2)] (with h indicating the overall treatment effect, w indicating the weight, p1 indicating the response in the naltrexone–bupropion group in stage 1, p2 indicating the response in the naltrexone–bupropion group in stage 2, q1 indicating the response in the placebo group in stage 1, and q2 indicating the response in the placebo group in stage 2), is provided in the statistical analysis plan.
The analyses were performed in the intention-to-treat population, which included all participants who underwent randomization in stage 1 and all participants who underwent randomization again in stage 2 (Fig. S1 in the Supplementary Appendix, available at NEJM.org). The primary outcome was evaluated with the use of a one-sided Wald z-test statistic22 with a one-sided type I error rate of 0.025, corresponding to a two-sided test with an alpha level of 0.05. The standard error of h accounted for the inclusion of some participants from stage 1 in stage 2. To determine the sensitivity of these results, we repeated the primary outcome analysis with the use of a prespecified complete-case approach (a complete case was defined as four urine samples obtained during the final 2 weeks of each stage). We conducted an additional prespecified sensitivity analysis that assumed equal weight for each stage. Subgroup effects according to trial site, sex, race, ethnic group, and age were assessed with the use of generalized linear mixed models and a forest plot presenting the treatment effect with 95% confidence intervals.
Secondary outcomes were analyzed with the use of the Doros method27 for repeated measures of a continuous outcome. Because there was no prespecified plan for adjustment of confidence intervals for multiple comparisons of secondary outcomes, no clinical conclusions can be drawn from these results. Adverse events were compared between groups in stage 1 and stage 2 with Fisher’s exact tests. All analyses were conducted with SAS software, version 9.4 (SAS Institute).
RESULTS
PARTICIPANTS
A total of 403 participants underwent randomization in stage 1: 109 participants (27.0%) were assigned to receive naltrexone–bupropion, and 294 (73.0%) to receive placebo (Fig. 1). Of the 225 participants in the placebo group who did not have a response in stage 1 and underwent randomization again in stage 2, a total of 114 (50.7%) were assigned to receive naltrexone–bupropion and 111 (49.3%) to receive placebo. The 403 participants who underwent randomization in stage 1 were assessed for the primary outcome at the end of the trial. Table 1 shows demographic and clinical characteristics of all participants according to group assignment. The average age of participants was 41 years, 68.7% were male, 71.2% were White, and 38.7% were employed. On average, participants used methamphetamine on 27 of the 30 days before consent was provided.
Table 1.
Baseline Characteristics of the Participants in the Intention-to-Treat Population.*
| Characteristic | All Participants | Stage 1 | Stage 2 | ||
|---|---|---|---|---|---|
| Total† (N = 403) | Naltrexone–Bupropion (N = 109) | Placebo (N = 294) | Naltrexone–Bupropion (N = 114) | Placebo (N = 111) | |
| Demographic characteristics | |||||
| Male — no. (%) | 277 (68.7) | 78 (71.6) | 199 (67.7) | 78 (68.4) | 79 (71.2) |
| Age — yr | 41.0±10.1 | 41.0±10.6 | 41.0±10.0 | 41.0±10.5 | 42.0±9.6 |
| Hispanic or Latino ethnic group — no. (%)‡ | 55 (13.6) | 13 (11.9) | 42 (14.3) | 20 (17.5) | 18 (16.2) |
| Race or ethnic group — no. (%)‡ | |||||
| White | 287 (71.2) | 82 (75.2) | 205 (69.7) | 84 (73.7) | 69 (62.2) |
| Black | 48 (11.9) | 10 (9.2) | 38 (12.9) | 8 (7.0) | 22 (19.8) |
| Other | 68 (16.9) | 17 (15.6) | 51 (17.3) | 22 (19.3) | 20 (18.0) |
| High school diploma, GED, or lower education level — no. (%) | 142 (35.2) | 39 (35.8) | 103 (35.0) | 36 (31.6) | 33 (29.7) |
| Marital status — no. (%) | |||||
| Married or living with partner | 93 (23.1) | 26 (23.9) | 67 (22.8) | 25 (21.9) | 25 (22.5) |
| Never married | 204 (50.6) | 49 (45.0) | 155 (52.7) | 60 (52.6) | 59 (53.2) |
| Divorced, separated, widowed, or unknown — no. (%) | 106 (26.3) | 34 (31.2) | 72 (24.5) | 29 (25.4) | 27 (24.3) |
| Employed — no. (%)§ | 156 (38.7) | 43 (39.4) | 113 (38.4) | 46 (40.4) | 44 (39.6) |
| Methamphetamine use | |||||
| No. of days that methamphetamine was used in the 30 days before consent¶ | 26.7±4.1 | 27.0±3.9 | 26.5±4.2 | 26.7±4.1 | 26.1±4.3 |
| Most frequent route of methamphetamine use — no. (%) | |||||
| Smoking | 293 (72.7) | 80 (73.4) | 213 (72.4) | 83 (72.8) | 79 (71.2) |
| Intravenous | 77 (19.1) | 23 (21.1) | 54 (18.4) | 21 (18.4) | 22 (19.8) |
| Nasal or oral | 33 (8.2) | 6 (5.5) | 27 (9.2) | 10 (8.8) | 10 (9.0) |
| Participants reporting intravenous methamphetamine use ≥1 days in the 30 days before consent — no. (%) | 135 (33.5) | 39 (35.8) | 96 (32.7) | 38 (33.3) | 36 (32.4) |
| Intensity of methamphetamine craving∥ | 66.1±22.3 | 65.7±22.2 | 65.8±21.6 | 66.7±21.3 | 63.7±21.9 |
| Age of first methamphetamine use — yr | 24.8±9.9 | 24.7±10.7 | 24.8±9.6 | 25.5±10.9 | 24.8±9.1 |
| Other characteristics | |||||
| Coexisting cocaine use disorder according to DSM-5 criteria — no./total no. (%) | 31/365 (8.5) | 9/97 (9.3) | 22/268 (8.2) | 9/104 (8.7) | 9/100 (9.0) |
| Coexisting opioid use disorder according to DSM-5 criteria — no./total no. (%) | 27/370 (7.3) | 7/93 (7.5) | 20/277 (7.2) | 7/109 (6.4) | 7/104 (6.7) |
| Coexisting alcohol use disorder according to DSM-5 criteria — no./total no. (%) | 94/293 (32.1) | 25/77 (32.5) | 69/216 (31.9) | 23/85 (27.1) | 27/75 (36.0) |
| Coexisting cannabis use disorder according to DSM-5 criteria — no./total no. (%) | 116/318 (36.5) | 29/89 (32.6) | 87/229 (38.0) | 33/86 (38.4) | 33/85 (38.8) |
| Daily nicotine cigarette use — no./total no. (%) | 238/337 (70.6) | 66/99 (66.7) | 172/238 (72.3) | 73/89 (82.0) | 56/89 (62.9) |
| Score on PHQ-9 depression scale** | 19.9±6.5 | 19.4±6.5 | 20.0±6.5 | 20.1±6.9 | 19.5±5.9 |
| Score on Treatment Effectiveness Assessment†† | 18.3±7.2 | 16.7±7.0 | 18.6±7.3 | 18.4±7.5 | 19.2±7.1 |
| HIV-positive status — no./total no. (%) | 90/356 (25.3) | 24/92 (26.1) | 66/264 (25.0) | 24/96 (25.0) | 33/105 (31.4) |
Plus–minus values are means ±SD. The intention-to-treat population included all participants who underwent randomization in stage 1 and the participants who underwent randomization again in stage 2. DSM-5 denotes Diagnostic and Statistical Manual of Mental Disorders, fifth edition, and GED General Educational Development diploma. Percentages may not total 100 because of rounding.
The total number reflects all participants who underwent randomization in stage 1.
Race and ethnic group were reported by the participants. The “other” category included American Indian or Alaska Native (6 participants), Asian (11 participants), Native Hawaiian or Pacific Islander (2 participants), other (20 participants), multiracial (16 participants), don’t know (10 participants), and declined to answer (3 participants).
The remaining participants were unemployed, disabled, or retired; were keeping house; were students; or had other status.
The number of days of methamphetamine use at baseline was assessed for the 30 days before informed consent. One participant had only 24 days of assessment. Eligibility required a minimum of 18 days of methamphetamine use at baseline.
Methamphetamine craving (the intensity of the worst craving over the previous week) was assessed at each screening or baseline visit on a visual analogue scale; ratings on the scale, which range from 0 to 100, were averaged to determine a baseline craving, with 0 indicating no craving at all and 100 indicating the most intense craving possible.
Depressive symptoms were assessed weekly with the use of the Patient Health Questionnaire 9 (PHQ-9); scores range from 0 to 27, with higher scores indicating greater depressive symptoms.
Life satisfaction was reported by the participants and was assessed with the use of the Treatment Effectiveness Assessment, which consists of four items; the scores for each item are summed, and total scores range from 4 to 40, with higher scores indicating greater satisfaction.
In stage 1, adherence to the assigned regimen was 75.1% in the naltrexone–bupropion group (63.9% to the oral regimen and 86.2% to the injection) and 83.5% in the placebo group (74.1% and 92.7%, respectively). In stage 2, adherence was 77.4% in the naltrexone–bupropion group (68.8% to the oral regimen and 86.4% to the injection) and 82.0% in the placebo group (75.1% and 89.2%, respectively).
PRIMARY OUTCOME
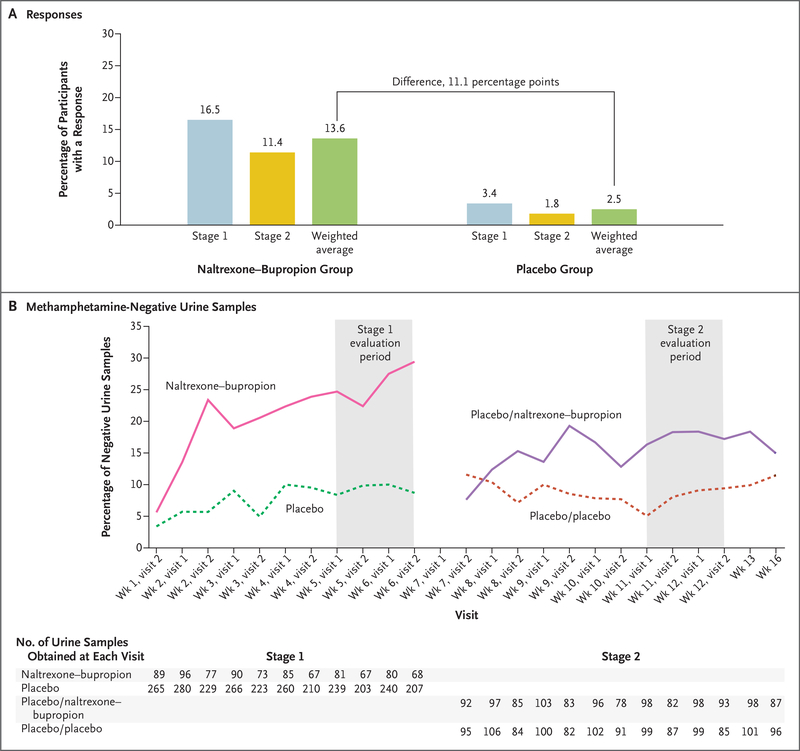
The primary outcome was a response, defined as at least three methamphetamine-negative urine samples out of a possible four samples obtained at the end of stage 1 (during week 5 through week 6) or the end of stage 2 (during week 11 through week 12). At the end of stage 1, a total of 16.5% (18 of 109 participants) in the naltrexone–bupropion group and 3.4% (10 of 294 participants) in the placebo group had a response. At the end of stage 2, a total of 11.4% (13 of 114 participants) in the naltrexone–bupropion group and 1.8% (2 of 111 participants) in the placebo group had a response (Table 2). After weighting and combining the percentage of responses across the stages, we calculated that the overall weighted response was 13.6% in the naltrexone–bupropion group and 2.5% in the placebo group (Fig. 2A). The treatment effect, defined as the between-group difference in the overall weighted response, was 11.1 percentage points (lower boundary of the 95% confidence interval [CI], 6.3; Wald z-test statistic, 4.53; P<0.001) (Table 2 and Fig. 2A). Figure 2B shows methamphetamine-negative urine results across trial visits; these results are consistent with those of the primary outcome. The overall treatment effects according to age, ethnic group, race, sex, and trial site are shown in Figure S2.
Table 2.
Primary and Secondary Outcomes in the Intention-to-Treat Population.*
| Outcome | Stage 1 | Stage 2 | Treatment Effect | |||
|---|---|---|---|---|---|---|
| Naltrexone–Bupropion (N = 109) | Placebo (N = 294) | Naltrexone–Bupropion (N = 114) | Placebo (N = 111) | Weighted Difference | 95% CI | |
| Primary outcome — no. of participants (%)† | 18 (16.5) | 10 (3.4) | 13 (11.4) | 2 (1.8) | 11.1±2.5 | — |
| Secondary outcomes | ||||||
| Methamphetamine-negative urine samples — %‡ | 20.4±2.2 | 12.3±1.6 | 19.2±2.6 | 13.4±1.5 | 6.8±1.7 | 3.5 to 10.1 |
| Change in methamphetamine craving according to visual analogue scale§ | −30.0±3.2 | −22.3±1.8 | −31.8±3.2 | −20.5±1.7 | −9.7±2.1 | −13.8 to −5.6 |
| Change in score on PHQ-9 depression scale§ | −4.8±0.7 | −3.3±0.3 | −4.4±0.6 | −3.7±0.4 | −1.1±0.4 | −1.9 to −0.2 |
| Change in score on Treatment Effectiveness Assessment§¶ | 6.5±1.5 | 2.2±1.0 | 6.2±1.5 | 2.5±1.1 | 4.0±0.9 | 2.3 to 5.7 |
Plus–minus values are means ±SE unless otherwise noted. The total number in stage 1 reflects the number of participants who underwent randomization. The total number in stage 2 reflects the number of participants in the placebo group who did not have a response in stage 1 and therefore underwent randomization again in stage 2. No clinical conclusions can be drawn from secondary outcomes because confidence intervals were not adjusted for multiple comparisons.
The primary outcome was a response, defined as at least three methamphetamine-negative samples out of four obtained at the end of stage 1 or stage 2. The overall treatment effect was defined as the weighted average of the responses in the naltrexone–bupropion group minus the responses in the placebo group, reported in percentage points ±SE, determined with the use of a weight of 0.43 in stage 1 and a weight of 0.57 in stage 2. The formula for this calculation is provided in the statistical analysis plan, available with the protocol at NEJM.org. The Wald z-test statistic for the primary outcome was 4.530 (P<0.001).
The percentage of methamphetamine-negative urine samples per participant was calculated by dividing the number of methamphetamine-negative urine samples obtained per stage by 12 (the number of expected samples per stage). The treatment effect is the between-group difference in the weighted average of negative urine samples, reported as percentage points ±SE.
The changes in stage 1 reflect the change from baseline, and the changes in stage 2 reflect the change from the end of stage 1. The treatment effect is the between-group difference in the weighted average change in scores, reported as the difference in points ±SE.
Data were available for 306 participants in stage 1 (74 in the naltrexone–bupropion group and 232 in the placebo group) and for 196 in stage 2 (98 in the naltrexone–bupropion group and 98 in the placebo group).
Figure 2. Responses and Methamphetamine-Negative Urine Samples.
The primary outcome was a response, defined as at least three methamphetamine-negative urine samples out of a possible four obtained at the end of stage 1 (during weeks 5 through 6) or at the end of stage 2 (during weeks 11 through 12). We calculated the weighted average of the responses in each stage, and the difference between these results was used to determine the overall treatment effect. Panel A shows the percentage of participants with a response and the weighted average of the response in each trial group in the intention-to-treat population, which included all participants who underwent randomization in stage 1 and all participants who underwent randomization again in stage 2. Panel B shows the percentage of methamphetamine-negative urine samples according to stage and trial group in the intention-to-treat population. Placebo/naltrexone–bupropion refers to participants in the placebo group who did not have a response in stage 1 and were assigned to the naltrexone–bupropion group in stage 2. Placebo/placebo refers to participants in the placebo group who did not have a response in stage 1 and were assigned to the placebo group in stage 2. During the 12-week intervention period, participants visited the clinic twice per week, after which they had a visit at week 13 and week 16. The evaluation period was the last 2 weeks of each stage (each evaluation stage is shown in the shaded areas). The number of urine samples obtained indicates the number of urine drug screening results available according to trial group at each visit for all participants in the intention-to-treat population. Results of urine drug screenings obtained at the first visit during week 1 (the day of randomization) are not shown. Results of drug screenings obtained on or before the rerandomization date of each participant in stage 2 are not shown because these samples were obtained when participants were still receiving the regimen assigned in stage 1.
In the prespecified sensitivity analysis that included participants who provided all four of the expected urine samples in the last 2 weeks of each stage, 28.8% (15 of 52 participants) in the naltrexone–bupropion group and 5.1% (9 of 177 participants) in the placebo group had a response in stage 1; 16.2% (13 of 80 participants) in the naltrexone–bupropion group and 1.3% (1 of 75 participants) in the placebo group had a response in stage 2 (Table S1). The overall treatment effect in this population was an 18.7-percentage-point difference in response (95% CI, 11.6 to 25.8). In the prespecified sensitivity analysis that assumed equal weight for each stage, 16.5% (18 of 109 participants) in the naltrexone–bupropion group and 3.4% (10 of 294 participants) in the placebo group had a response in stage 1; 11.4% (13 of 114 participants) in the naltrexone–bupropion group and 1.8% (2 of 111 participants) in the placebo group had a response in stage 2. The overall treatment effect, under the assumption of equal weight for each stage, was an 11.4-percentage-point between-group difference in response (95% CI, 6.5 to 16.2).
SECONDARY OUTCOMES
The percentage of participants with methamphetamine-negative urine samples was 20.4% in the naltrexone–bupropion group and 12.3% in the placebo group in stage 1 and 19.2% in the naltrexone–bupropion group and 13.4% in the placebo group in stage 2. The weighted difference between the two groups in the percentage of participants with methamphetamine-negative urine samples was 6.8 percentage points (Table 2). The weighted difference between the naltrexone–bupropion group and the placebo group in weekly methamphetamine craving scores on the visual analogue scale was −9.7 points. The weighted difference between the naltrexone–bupropion group and the placebo group in weekly PHQ-9 scores was −1.1 points. The weighted difference between the naltrexone–bupropion group and the placebo group in participant-reported scores on the Treatment Effectiveness Assessment was 4.0 points. There was no prespecified plan for adjustments of confidence intervals for multiple comparisons of secondary outcomes, and no definite conclusions can be drawn from these results.
SAFETY
Across both stages, 17 disparate serious adverse events occurred during the trial in the safety population (all participants who underwent randomization): 8 in the naltrexone–bupropion group and 9 in the placebo group. Table 3 includes 13 events that occurred in the intention-to-treat population. The other 4 events (3 in the naltrexone–bupropion group and 1 in the placebo group) occurred during stage 2 in the participants who did not undergo rerandomization. Adverse events were mostly mild or moderate (Table S2). Adverse events that occurred more frequently (P<0.05) with naltrexone–bupropion than with placebo were nausea (37.6% vs. 15.3% in stage 1 and 28.1% vs. 7.2% in stage 2), vomiting (11.9% vs. 2.0% in stage 1 and 10.5% vs. 2.7% in stage 2), constipation (9.2% vs. 2.4% in stage 1), dry mouth (8.3% vs. 1.7% in stage 1), upper abdominal pain (4.6% vs. 0.3% in stage 1), dizziness (10.1% vs. 2.7% in stage 1), tremor (4.6% vs. 0.3% in stage 1), feeling jittery (3.7% vs. 0.7% in stage 1), malaise (3.7% vs. 0.3% in stage 1), hyperhidrosis (7.3% vs. 1.0% in stage 1), and decreased appetite (7.3% vs. 2.0% in stage 1). Complete reports of adverse events are provided in Table 3 and Table S2.
Table 3.
Adverse Events That Occurred during the Trial Period.*
| Event | Stage 1 | Stage 2 | ||||
|---|---|---|---|---|---|---|
| Naltrexone–Bupropion (N = 109) | Placebo (N = 294) | P Value | Naltrexone–Bupropion (N = 114) | Placebo (N = 111) | P Value | |
| Participants with at least one serious adverse event — no. (%)† | 1 (0.9) | 4 (1.4) | 1.00 | 3 (2.6) | 4 (3.6) | 0.72 |
| Total no. of serious adverse events‡ | 1 | 4 | 4 | 4 | ||
| Any adverse event — no. (%) | 99 (90.8) | 245 (83.3) | 0.08 | 88 (77.2) | 77 (69.4) | 0.23 |
| Adverse events — no. (%)§ | ||||||
| Gastrointestinal events | ||||||
| Nausea | 41 (37.6) | 45 (15.3) | <0.001 | 32 (28.1) | 8 (7.2) | <0.001 |
| Diarrhea | 7 (6.4) | 18 (6.1) | 1.00 | 6 (5.3) | 5 (4.5) | 1.00 |
| Vomiting | 13 (11.9) | 6 (2.0) | <0.001 | 12 (10.5) | 3 (2.7) | 0.03 |
| Constipation | 10 (9.2) | 7 (2.4) | 0.005 | 2 (1.8) | 3 (2.7) | 0.68 |
| Dry mouth | 9 (8.3) | 5 (1.7) | 0.003 | 1 (0.9) | 2 (1.8) | 0.62 |
| Upper abdominal pain | 5 (4.6) | 1 (0.3) | 0.006 | 6 (5.3) | 3 (2.7) | 0.50 |
| Abdominal discomfort | 4 (3.7) | 5 (1.7) | 0.26 | 1 (0.9) | 2 (1.8) | 0.62 |
| Nervous system symptoms and disorders | ||||||
| Headache | 13 (11.9) | 68 (23.1) | 0.01 | 11 (9.6) | 6 (5.4) | 0.31 |
| Dizziness | 11 (10.1) | 8 (2.7) | 0.006 | 7 (6.1) | 1 (0.9) | 0.07 |
| Somnolence | 3 (2.8) | 10 (3.4) | 1.00 | 0 | 1 (0.9) | 0.49 |
| Tremor | 5 (4.6) | 1 (0.3) | 0.006 | 3 (2.6) | 0 | 0.25 |
| Psychiatric symptoms and disorders | ||||||
| Irritability | 6 (5.5) | 19 (6.5) | 0.82 | 5 (4.4) | 4 (3.6) | 1.00 |
| Anxiety | 10 (9.2) | 14 (4.8) | 0.10 | 1 (0.9) | 1 (0.9) | 1.00 |
| Insomnia | 6 (5.5) | 12 (4.1) | 0.59 | 3 (2.6) | 1 (0.9) | 0.62 |
| Libido decreased | 4 (3.7) | 5 (1.7) | 0.26 | 1 (0.9) | 0 | 1.00 |
| Lability affected | 4 (3.7) | 4 (1.4) | 0.22 | 2 (1.8) | 1 (0.9) | 1.00 |
| Depression | 2 (1.8) | 6 (2.0) | 1.00 | 4 (3.5) | 4 (3.6) | 1.00 |
| General disorders and injection-site reactions | ||||||
| Fatigue | 8 (7.3) | 33 (11.2) | 0.35 | 7 (6.1) | 8 (7.2) | 0.80 |
| Feeling jittery | 4 (3.7) | 2 (0.7) | 0.05 | 1 (0.9) | 0 | 1.00 |
| Malaise | 4 (3.7) | 1 (0.3) | 0.02 | 1 (0.9) | 0 | 1.00 |
| Metabolism and nutrition disorders | ||||||
| Decreased appetite | 8 (7.3) | 6 (2.0) | 0.03 | 3 (2.6) | 3 (2.7) | 1.00 |
| Musculoskeletal and connective tissue disorders | ||||||
| Arthralgia | 4 (3.7) | 6 (2.0) | 0.47 | 2 (1.8) | 0 | 0.50 |
| Injury, poisoning, and procedural complications | ||||||
| Contusion | 3 (2.8) | 5 (1.7) | 0.45 | 0 | 5 (4.5) | 0.03 |
| Skin and subcutaneous tissue disorders | ||||||
| Hyperhidrosis | 8 (7.3) | 3 (1.0) | 0.002 | 2 (1.8) | 0 | 0.50 |
Events shown for stage 1 include events that occurred before the start of stage 2 in the safety population (all participants who underwent randomization). Events shown for stage 2 include those that occurred on or after the date of rerandomization in participants in the intention-to-treat population (participants who underwent randomization again in stage 2). Adverse events were classified according to the preferred term and system organ class of the Medical Dictionary for Regulatory Activities, version 22.1.
Of the 17 serious adverse events that occurred in the safety population (all participants who gave informed consent), 13 occurred in the intention-to-treat population and are reported in this table. Of the 13 serious adverse events, all except an event of seizure were recorded as serious because they resulted in either inpatient hospitalization or prolongation of an existing hospitalization. The additional 4 serious adverse events occurred after consent was given but before randomization; the events were hypertensive crisis (in 1 participant), genito-urinary chlamydia infection (in 1 participant), neurosyphilis (in 1 participant), and appendicitis (in 1 participant). Four additional adverse events occurred in stage 2 in participants who did not undergo rerandomization (3 events in the naltrexone–bupropion group and 1 in the placebo group).
The serious adverse events in stage 1 were substance-induced psychosis, paranoia, pancreatitis, and seizure (in 1 participant each) in the placebo group, and gastroenteritis in 1 participant in the naltrexone–bupropion group. The serious adverse events in stage 2 were gastroenteritis shigella, pneumonia, urosepsis, and being the victim of a crime (in 1 participant each) in the placebo group, and homicidal ideation, cellulitis, neck pain, and hyperglycemia (in 1 participant each) in the naltrexone–bupropion group.
The adverse events reported here are events of interest that occurred in 3% or more of participants in either stage in the naltrexone–bupropion group and events that had a P value of ≤0.05 for any pairwise comparison. Table S2 lists all adverse events that occurred during the trial in the safety population.
DISCUSSION
The goal of this trial was to assess the effectiveness of the combination of naltrexone and extended-release bupropion in treating methamphetamine use disorder. The primary outcome was a response, defined as at least three methamphetamine-negative urine samples out of four samples obtained in the last 2 weeks of each stage. The response in each group was calculated by combining the weighted average of the responses in the two stages of the trial. The overall weighted response was 13.6% in the naltrexone–bupropion group and 2.5% in the placebo group. The results of the analyses of secondary outcomes, including the assessment of craving for methamphetamine and improvements in social functioning, were generally in the same direction as those of the primary outcome, but no definite conclusions can be drawn from these data because of the lack of a prespecified plan for multiplicity adjustment of confidence intervals for the point estimates of differences between the two trial groups.
Methamphetamine use disorder is a serious illness and is associated with medical conditions and mental health issues, marked functional impairment, and frequent relapses.28,29 The participants in our trial were severely affected by methamphetamine use disorder, with almost daily use before entry into the trial. Our definition of a response included valid negative urine samples obtained after only 4 to 6 weeks in each stage of the trial. The percentage of participants who had a response in each stage of the trial was low; however, there was a significant difference in the weighted response (11.1 percentage points) between the naltrexone–bupropion group and the placebo group. The number needed to treat in order for one patient to have a response under the assumptions in this trial is 9.
The strengths of this trial include low attrition, high adherence to the trial regimen, a prospective evaluation to establish illness severity, and an objective primary outcome assessed on the basis of valid urine samples. However, the low attrition and high adherence may limit generalizability to clinical practice. Other limitations include the relatively low representation of women, although the male-to-female ratio in this trial is consistent with the difference in incidences of amphetamine use disorder between men and women in the United States. Adherence to the oral regimen was determined on the basis of participant report and cannot be confirmed because ingestion was not observed by trial clinicians. The results of the trial may be difficult to explain to patients and practitioners because of the sequential parallel comparison design, which included enrichment of the stage 2 sample with the random reassignment of participants in the placebo group who did not have a response in stage 1 and the use of a weighted combination to analyze the response in each stage. This method was intended to enhance the likelihood of detecting efficacy of the combination treatment. Replication of our trial results in a more naturalistic effectiveness design could be a next step. An additional consideration in interpreting our trial results is the possible continuation of the trial, although the results of the interim sample-size reestimation analysis performed by the data and safety monitoring board showed a significant difference in outcomes between trial groups, and no adjustment was made in the significance level of the test of the primary outcome. The 12-week duration of a trial of a substance use disorder requires consideration of how the treatment can be adapted to practice.
In persons with moderate or severe methamphetamine use disorder, treatment with the combination of extended-release injectable naltrexone and daily oral extended-release bupropion over a period of 12 weeks resulted in a higher response than placebo.
Supplementary Material
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Alkermes.
Supported by awards (UG1DA020024 [to Dr. Trivedi], UG1DA013035 [to Dr. Nunes], UG1DA040316, UG1DA013727, and UG1DA015815) from the National Institute on Drug Abuse (NIDA) of the National Institutes of Health; and by the Department of Health and Human Services under contract numbers HHSN271201500065C (Clinical Coordinating Center, the Emmes Company) and HHSN271201400028C (Data and Statistics Center, the Emmes Company). Alkermes provided Vivitrol (naltrexone for extended-release injectable suspension) and matched placebo free of charge for use in this trial under a written agreement with NIDA.
Dr. Trivedi reports receiving consulting fees from Academy-Health, Acadia, Alkermes, Allergan, Alto Neuroscience, Axsome Therapeutics, Boehringer Ingelheim, Vital Signs GreenLight, Janssen, Jazz Pharmaceuticals, Lundbeck, Medscape, Merck Sharp and Dohme, MSI Methylation Sciences, Myriad Neuroscience, Navitor, Otsuka, Oxford PharmaGenesis, Perception Neuroscience Holdings, Pharmerit International, Sage Therapeutics, Signant Health, and Takeda Pharmaceuticals America, fees for serving on a data and safety monitoring board from Applied Clinical Intelligence, fees for serving on an endpoint review committee from Engage Health Media, and grant support, paid to his institution, from Janssen Research and Development, Johnson & Johnson Health Care Systems, and the Patient-Centered Outcomes Research Institute; Dr. Walker, receiving donated drugs for use in a trial from Alkermes; Dr. Ling, receiving consulting fees from Indivior; Dr. Carmody, owning stock in CRISPR Therapeutics and Vertex; Dr. Rush, receiving consulting fees from COMPASS Pathways, Holmusk, Janssen Global Services, LivaNova, Neurocrine Biosciences, Otsuka America, and the Emmes Company; Dr. Nunes, receiving grant support, paid to his institution, from Brainsway USA and Reckitt Benckiser, donated drugs for use in a trial from Pear Therapeutics, and serving on advisory boards for Alkermes. No other potential conflict of interest relevant to this article was reported.
We thank the trial participants; the staff at the participating trial sites for their work in conducting the trial (Behavioral Health Services of Pickens County [Pickens, SC]; CODA [Portland, OR]; Hennepin County Medical Center–Berman Center for Research [Minneapolis]; New York State Psychiatric Institute–Substance Use Research Center [New York]; the Substance Use Research Unit at the San Francisco Department of Public Health [San Francisco]; the University of California, Los Angeles, Center for Behavioral Addiction Medicine [Los Angeles]; the Peter O’Donnell Jr. Brain Institute at the University of Texas Southwestern Medical Center [Dallas]; and the University of Texas Southwestern Medical Center [Dallas]); the trial collaborators (Matthew Wright and Eve Jelstrom [the Clinical Coordinating Center at the Emmes Company]; Catherine Mudrick, Ashley Case, and Jacquie King [the Data and Statistics Center at the Emmes Company]; Brien Hawley, Laura Schafner, and Gordon Kessler [AiCure]; and Angela Casey-Willingham, who helped monitor the implementation of the trial); Jennifer Sharpe Potter, Nora Volkow, and Taryn Mayes for providing input on earlier versions of the manuscript; and Georganna Carlock and Taryn Mayes for administrative assistance.
Footnotes
Contributor Information
M.H. Trivedi, Peter O’Donnell Jr. Brain Institute at the University of Texas Southwestern Medical Center
R. Walker, University of Texas Southwestern Medical Center, Dallas
W. Ling, University of California, Los Angeles, Los Angeles
A. dela Cruz, University of Texas Southwestern Medical Center, Dallas.
G. Sharma, Emmes Company, Rockville, Maryland
T. Carmody, University of Texas Southwestern Medical Center, Dallas
U.E. Ghitza, National Institute on Drug Abuse Center for the Clinical Trials Network, Maryland
A. Wahle, Emmes Company, Rockville, Maryland
M. Kim, University of Texas Southwestern Medical Center, Dallas
K. Shores-Wilson, University of Texas Southwestern Medical Center, Dallas
S. Sparenborg, National Institute on Drug Abuse Center for the Clinical Trials Network, Maryland
P. Coffin, San Francisco Department of Public Health and the University of California, San Francisco, San Francisco
J. Schmitz, University of Texas Health Science Center at Houston, Houston
K. Wiest, CODA, Portland, OR
G. Bart, Hennepin Healthcare, University of Minnesota, Minneapolis
S.C. Sonne, Medical University of South Carolina, Charleston
S. Wakhlu, University of Texas Southwestern Medical Center, Dallas
A.J. Rush, Texas Tech University, Permian Basin, Odessa Duke–National University of Singapore, Singapore; Duke Medical School, Durham, NC.
E.V. Nunes, Columbia University, New York
S. Shoptaw, University of California, Los Angeles, Los Angeles
REFERENCES
- 1.Hedegaard H, Bastian BA, Trinidad JP, Spencer MR, Warner M. Regional differences in the drugs most frequently involved in drug overdose deaths: United States, 2017. Natl Vital Stat Rep 2019;68:1–16. [PubMed] [Google Scholar]
- 2.Ellis MS, Kasper ZA, Cicero TJ. Twin epidemics: the surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend 2018;193:14–20. [DOI] [PubMed] [Google Scholar]
- 3.Ashok AH, Mizuno Y, Volkow ND, Howes OD. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry 2017;74:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soares E, Pereira FC. Pharmacotherapeutic strategies for methamphetamine use disorder: mind the subgroups. Expert Opin Pharmacother 2019;20:2273–93. [DOI] [PubMed] [Google Scholar]
- 5.Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology 2008;33:1162–70. [DOI] [PubMed] [Google Scholar]
- 6.Heinzerling KG, Swanson A-N, Hall TM, Yi Y, Wu Y, Shoptaw SJ. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction 2014;109:1878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson AL, Li S-H, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 2015;150:170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Runarsdottir V, Hansdottir I, Tyrfingsson T, et al. Extended-release injectable naltrexone (XR-NTX) with intensive psychosocial therapy for amphetamine-dependent persons seeking treatment: a placebo-controlled trial. J Addict Med 2017;11:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayaram-Lindström N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry 2008;165:1442–8. [DOI] [PubMed] [Google Scholar]
- 10.Ray LA, Bujarski S, Courtney KE, et al. The effects of naltrexone on subjective response to methamphetamine in a clinical sample: a double-blind, placebo-controlled laboratory study. Neuropsychopharmacology 2015;40:2347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol 2017;10:305–14. [DOI] [PubMed] [Google Scholar]
- 12.Chan B, Freeman M, Kondo K, et al. Pharmacotherapy for methamphetamine/amphetamine use disorder — a systematic review and meta-analysis. Addiction 2019;114:2122–36. [DOI] [PubMed] [Google Scholar]
- 13.Siefried KJ, Acheson LS, Lintzeris N, Ezard N. Pharmacological treatment of methamphetamine/amphetamine dependence: a systematic review. CNS Drugs 2020;34:337–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks ML, Smith DA, Blough BE. Methamphetamine-like discriminative stimulus effects of bupropion and its two hydroxy metabolites in male rhesus monkeys. Behav Pharmacol 2016;27:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton TF, Roache JD, De La Garza R II, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology 2006; 31:1537–44. [DOI] [PubMed] [Google Scholar]
- 16.Aboujaoude E, Salame WO. Naltrex-one: a pan-addiction treatment? CNS Drugs 2016;30:719–33. [DOI] [PubMed] [Google Scholar]
- 17.Hendershot CS, Wardell JD, Samokhvalov AV, Rehm J. Effects of naltrexone on alcohol self-administration and craving: meta-analysis of human laboratory studies. Addict Biol 2017;22:1515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche DJO, Worley MJ, Courtney KE, et al. Naltrexone moderates the relationship between cue-induced craving and subjective response to methamphetamine in individuals with methamphetamine use disorder. Psychopharmacology (Berl) 2017;234:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mooney LJ, Hillhouse MP, Thomas C, et al. Utilizing a two-stage design to investigate the safety and potential efficacy of monthly naltrexone plus once-daily bupropion as a treatment for methamphetamine use disorder. J Addict Med 2016;10:236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fava M, Evins AE, Dorer DJ, Schoen-feld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom 2003;72:115–27. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y-F, Yang Y, Hung HMJ, Wang S-J. Evaluation of performance of some enrichment designs dealing with high placebo response in psychiatric clinical trials. Contemp Clin Trials 2011;32:592–604. [DOI] [PubMed] [Google Scholar]
- 22.Tamura RN, Huang X. An examination of the efficiency of the sequential parallel design in psychiatric clinical trials. Clin Trials 2007;4:309–17. [DOI] [PubMed] [Google Scholar]
- 23.McHugh RK, Fitzmaurice GM, Carroll KM, et al. Assessing craving and its relationship to subsequent prescription opioid use among treatment-seeking prescription opioid dependent patients. Drug Alcohol Depend 2014;145:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling W, Farabee D, Liepa D, Wu L-T. The Treatment Effectiveness Assessment (TEA): an efficient, patient-centered instrument for evaluating progress in recovery from addiction. Subst Abuse Rehabil 2012;3:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trivedi MH, Wisniewski SR, Morris DW, et al. Concise Associated Symptoms Tracking Scale: a brief self-report and clinician rating of symptoms associated with suicidality. J Clin Psychiatry 2011;72:765–74. [DOI] [PubMed] [Google Scholar]
- 27.Doros G, Pencina M, Rybin D, Meisner A, Fava M. A repeated measures model for analysis of continuous outcomes in sequential parallel comparison design studies. Stat Med 2013;32:2767–89. [DOI] [PubMed] [Google Scholar]
- 28.Courtney KE, Ray LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend 2014;143:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panenka WJ, Procyshyn RM, Lecomte T, et al. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend 2013;129:167–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.