Abstract
Since the last century, advances in healthcare, housing, and education have led to an increase in life expectancy. Longevity is accompanied by a higher prevalence of age-related diseases, such as cancer, autoimmunity, diabetes, and infection, and part of this increase in disease incidence relates to the significant changes that aging brings about in the immune system. The eye is not spared by aging either, presenting with age-related disorders of its own, and interestingly, many of these diseases have immune pathophysiology. Being delicate organs that must be exposed to the environment in order to capture light, the eyes are endowed with a mucosal environment that protects them, the so-called ocular surface. As in other mucosal sites, immune responses at the ocular surface need to be swift and potent to eliminate threats but are at the same time tightly controlled to prevent excessive inflammation and bystander damage. This review will detail how aging affects the mucosal immune response of the ocular surface as a whole and how this process relates to the higher incidence of ocular surface disease in the elderly.
Keywords: aging, immune cells, tear film, T regulatory t cells, lacrimal gland, goblet cells
I. Introduction
As sensory organs, the eyes are exposed to the environment to be able to capture and encode light into neural signals and thus initiate the process of sight. At the same time, the delicate ocular structures require protection from numerous environmental threats in order to fulfill their role. First and foremost is the cornea, the most exposed of the refractive media of the eye. The proper refraction of light onto the retina requires a clear, smooth corneal surface, which is maintained so by the tear film. The ocular surface is a mucosal site that has evolved to keep the cornea wet and safe. The ocular surface functional unit encompasses the cornea itself and its adjacent protective structures: the limbus, the conjunctiva, the eyelids and their meibomian glands, the lacrimal glands, and the nerves [1, 2]. As every mucosal site, the ocular surface is equipped with a full-fledged immune system capable of mounting defensive responses against dangerous pathogens [3]. However, since inflammation comes at a cost for the ocular tissues, a set of regulatory mechanisms has also evolved to keep the immune response at bay, thus preventing unwanted or excessive reactions against harmless foreign antigens [4, 5]. Remarkably, the dysfunction of immune regulation is at the core of many ocular surface disorders [4].
The aging process does not spare the ocular surface. The United Nations Organization estimates that the percentage of subjects aged 65 years of old will more than double in 2050 compared to 2020 numbers [6] (https://population.un.org/wpp/). The precise physiology of aging is not entirely understood, and several theories have been proposed that involve programmed cell death, genetic mutations, the epigenetic clock, wear-and-tear, and free radical imbalances [7–12]. Current mechanisms of aging at the cellular/metabolic levels include increased oxidative stress and DNA damage, accumulation of senescent cells, altered DNA repair, telomere shortening, decreased proteasome function, inflammation, and immune senescence [13–20].
A typical feature of aging is a chronic, low-grade inflammatory status named inflammaging [21], characterized by a general increase in the production of proinflammatory cytokines. This pro-inflammatory state in aging is relevant because increased serum inflammatory mediators are associated with age-related diseases [21–24]. For example, increased anti-inflammatory transforming growth factor (TGF)-β serum levels in centenarians are considered a biomarker of good health [25], while increased serum levels of pro-inflammatory interleukin (IL)-6 and tumor necrosis factor (TNF)-α cytokines are predictors of disability and mortality in octogenarians and centenarians [23, 26, 27]. Inflammaging is detrimental because it decreases the resilience and reserve of aged organisms to external/internal stressors [28].
With advanced age, the prevalence of diseases that accompany aging is also expected to rise. Age-related diseases such as cardiovascular diseases, type 2 diabetes, hypertension, and Alzheimer’s have a significant burden on the individual and society, affecting not only the quality of life but also shortening the life span. The eye is an organ that is affected by aging, as diseases such as cataracts, age-related macular degeneration, glaucoma, and dry eye are very prevalent in the aged population. These diseases not only affect the quality of life and compromise independence but can also lead to blindness. Recent evidence from the literature has shown that not only the ocular mucosa suffers from age-related alterations in the ocular surface, but also age-related changes in the immune system can modulate ocular surface health. This review will focus on the different aspects of the aged ocular surface and its immunoregulatory mechanisms.
II. The Ocular Immune System during Aging
The ocular mucosal immune system is endowed with most, if not all, the cell populations that have been described in other mucosal sites. There are, of course, certain elements that are unique to the eye, such as the tear film and the meibomian and lacrimal glands, but the unifying approach to mucosal immunology still applies. Schematically, the initiation and maintenance of a mucosal immune response require multiple steps that have been thoroughly characterized for the gut and the airways, and to a lesser extent, for the ocular surface (Figure 1). For clarity, each step is centered around the concept of an antigen, the molecule or molecular structure against which the adaptive immune system reacts. Antigens can derive from pathogens, foreign substances, or even self-tissues. It should be noted that the immune system is continuously interacting with various antigens simultaneously. These fundamental processes are:
Interaction of the antigen with the mucosal epithelial lining, which in the eye is influenced by the ocular microbiome, the tear film (including the meibomian and lacrimal glands), the actual epithelial barrier, and goblet cells.
Detection of the pattern- and damage-associated molecular patterns associated with an antigen by the innate immune system, which results in its early containmentand also leads to the fine-tuning and priming of the subsequent adaptive immune response. The nervous system also contributes to this last task.
Antigen capture and processing by mucosal antigen-presenting cells (APCs) and subsequent presentation to T cells, which occurs in the draining lymph node for the initiation of the adaptive immune response and also locally for maintenance of an already initiated adaptive immune response.
Polarization, amplification, migration, and activation of effector T cells (proinflammatory) or regulatory T cells (tolerogenic).
Generation of humoral immune responses (antibody-mediated, involving B cells and plasma cells)
In addition, extensive crosstalk between the innate and adaptive branches of the mucosal immune system and also with the nervous system influences each of the previous steps.
We will follow this schematic approach for reviewing the literature on ocular surface immunology over these aspects of the mucosal immune response and how they are affected by aging.
Figure 1: Schematic mucosal immune response of the ocular surface.
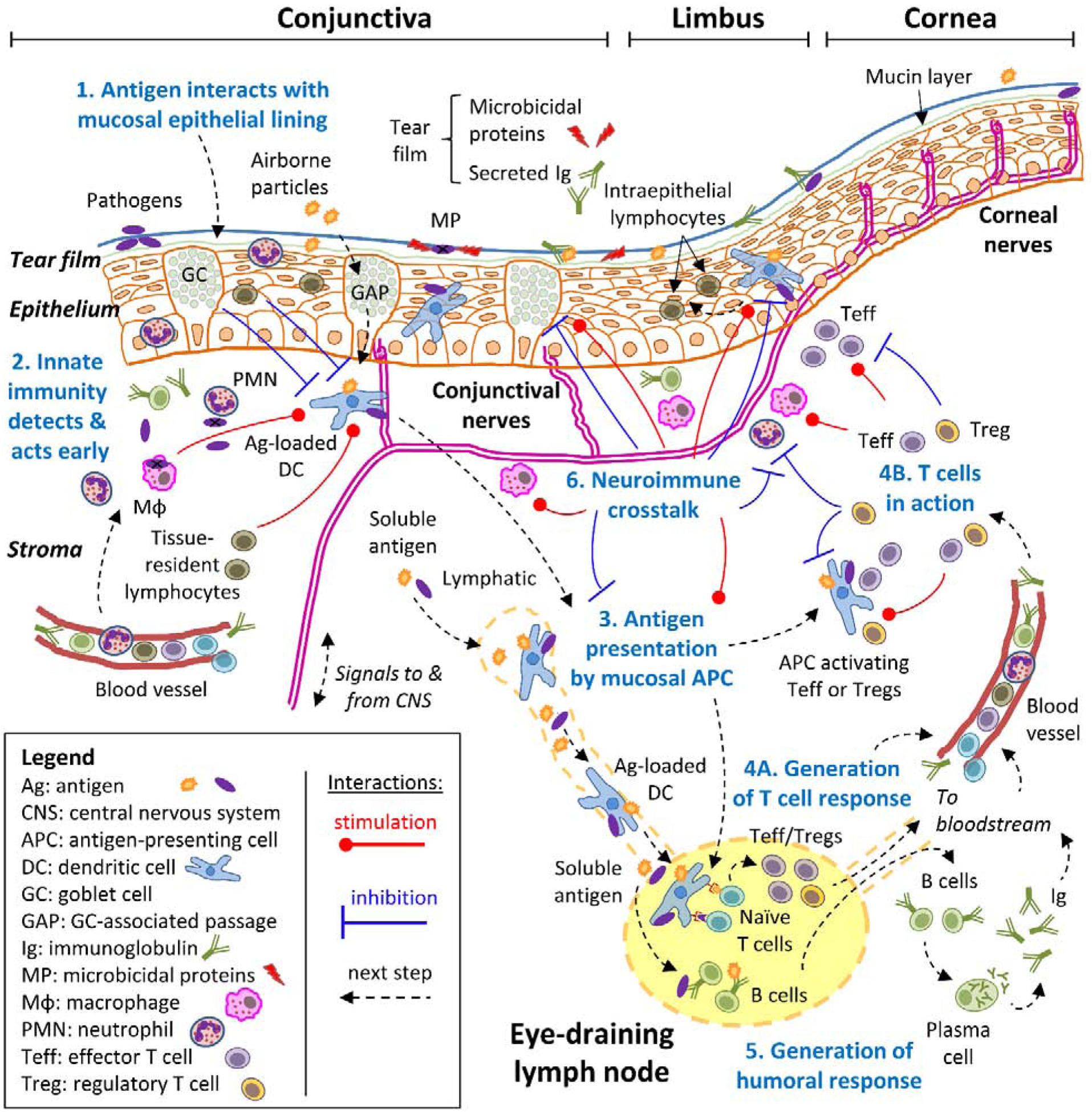
A simplified approach to the mucosal immune response to diverse antigens, consisting of 6 consecutive steps (numbered in blue), is exemplified and adopted throughout this review. For clarity, all steps revolve around the concept of an antigen (the molecule or molecular structure against which the adaptive immune system reacts), which can derive from pathogens, foreign substances, or even self-tissues. Step 1: the antigen interacts with the mucosal epithelial lining, which acts as a barrier aided by blinking action and the tears. Step 2: antigens may breach through the epithelial barrier, setting off the innate immune system’s detection systems because receptors on diverse cell types recognize molecular patterns that are associated with potentially dangerous entities. This triggers a swift defense that results in early containment and that influences the next step at the same time. Step 3: specialized antigen-presenting cells (APC) that reside in the ocular surface capture antigens and migrate to the eye-draining lymph nodes, where they process them and present antigen-derived peptides on their surface. Step 4: naïve T cells that continuously recirculate through lymph nodes recognize specific peptides on APC, become activated, and then expand and differentiate to either effector (Teff) or regulatory (Treg) T cells depending on additional signals that they pick up during antigen presentation. These antigen-experienced T cells leave the lymph node and through the bloodstream, reach the ocular surface where they can exert their many functions in the immune response. Step 5: concomitantly with step 3 and 4, soluble antigens reach the lymph node from the ocular surface, where B cells might interact with them, become activated, and then expand and differentiate into plasma cells that migrate to other tissues through the bloodstream, mainly the bone marrow. Once at their final destination, plasma cells secrete large amounts of immunoglobulin (Ig) that reach the ocular surface and the lacrimal glands through the bloodstream. Step 6: the nervous system can also sense signals associated with some antigens and interacts with every cell type of the immune response in a bidirectional process (neuroimmune regulation). Although most of the immune response takes place at the ocular surface, it should be noted that critical steps 3, 4, and 5 begin in the draining lymph node. Also, the immune system is continuously interacting with various antigens, and thus, these processes take place simultaneously.
II.A. Aging and the Microbiome
The ocular surface is an exposed mucosa that is in constant contact with the environment and it is a site rich in potent antimicrobial defenses [lactoferrin, lysozyme, defensins, antimicrobial peptides, immunoglobulin (Ig)A] that limit bacterial colonization [29–32]. Recent advances and low costs in sequencing bacterial genes have led to an expansion of our knowledge about the bacterial communities that inhabit our body, now known as the microbiome.
Although bacterial colonization and commensalism are widespread in most mucosal linings, results from the Human Microbiome Project identified site-specific microbiomes. [33]. In the past, the eye was considered a sterile site as traditional cultures of conjunctival swabs yielded viable colonies in less than 50% of the cases [34, 35]. The advent of next-generation sequencing using ribosomal 16S [which sequences variable regions (V1-V9) of the 16S ribosomal RNA] or whole-shotgun sequencing (which sequences the whole genome) demonstrated that the ocular surface is not sterile. However, compared to other mucosal sites in the body, it is very paucibacterial [36, 37]. Because of its low mass, proper identification of the ocular microbiome using metagenomic techniques varies with the method of collection, region that was sampled, depth of sequencing, and if the collection was performed upon awakening [38–40]. Furthermore, diseases and conditions that affect the ocular surface (such as graft-versus-host-disease, Sjögren Syndrome, dry eye, blepharitis, meibomian gland dysfunction and use of contact lenses) alter the ocular microbiome [40–48]. There is still controversy about the composition of the core conjunctival microbiome [46–50]. The microbiome in the eyelids closely resembles the microbiome of periocular skin. However, because DNA is very long-lived, identification of bacterial DNA does neither equal to viable microbes nor indicates commensalism, and further investigations using molecular probes or dyes to identify live bacteria have been proposed [51]. At least two studies addressed the temporal stability of the conjunctiva microbiome, where the authors concluded that at least for a few months, there is a stable microbiome [49].
Age is a significant factor that shapes the microbiome, with dysbiosis (or altered microbial composition) being postulated as another hallmark of aging [52]. The most remarkable changes in the microbiome happen after birth: the intestinal microbiome of infants changes rapidly within the first 3 years of life, reaching a relatively steady-state afterward. Middle-aged and elderly C57BL/6 mice [53] have intestinal dysbiosis [54], and certain therapies (such as calorie restriction) or oral gavage with specific commensal bacteria (e.g., Akkermansia muciniphila) can modulate changes in the immune compartment, decrease age-related insulin resistance [55, 56], and improve overall health. It has been postulated that increased colon permeability, reduced mucin layer, fewer goblet cells, and increased cytokines such as TNF-α participate in the maintenance of a vicious circle of inflammation and dysbiosis in the colon [52, 56, 57]. It has become clear that the intestinal microbiome can also influence ocular health, with some studies showing that it can have a protective effect on the ocular surface. For instance, mice that are born and raised in a germ-free environment have increased frequency of activated dendritic cells (DCs) and spontaneously develop a Sjögren syndrome-like phenotype [58]. Also, mice that received a cocktail of oral antibiotics before and after exposure to desiccating stress showed greater corneal barrier disruption and lower goblet cell density than mice that drank normal water and had a complex microbiome [46]. CD25−/− mice, which develop a severe dry eye phenotype, have worsened ocular surface disease and lacrimal gland inflammation when raised in germ-free environment or after antibiotics-induced dysbiosis [59]. Remarkably, the effects of a germ-free environment (worsening or ameliorating disease) are tissue specific. For example, female NOD mice do not develop dacryoadenitis unless they are reared in a germ-free environment [60]. Specific studies investigating the microbiome and the ocular surface during aging are lacking.
The conjunctival microbiome in children differs from the microbiome in adults [50]. Careful evaluation of conjunctival microbiome using whole-shotgun sequencing demonstrated that older subjects (mean age 67 years) have a different microbial diversity compared to young, healthy subjects (mean age 27 years), with other differences observed between aged female and male subjects [61]. This is also true for the meibum microbiome and periocular eyelid microbiome [62]. Interestingly, a recent study evaluated the conjunctival microbiome composition in three different cities in China and observed that subjects from a particular city had significant differences in their microbiome from healthy subjects from another city. Furthermore, traveling from one city to the other for at least two weeks changed the conjunctival microbiome in two-thirds of the cases [62].
Functional studies in mice have demonstrated that commensal bacteria that inhabit the conjunctiva can participate in active defense from Pseudomonas aeruginosa and Candida albicans keratitis [63, 64]. Lipopolysaccharide (LPS) is a potent inducer of inflammation after topical administration on the ocular surface. Administration of oral antibiotics, which causes microbial imbalance (or dysbiosis), increases the expression of CD86 in DCs in draining lymph nodes while increasing inflammatory markers on the corneal epithelium and in the conjunctiva [58].
These findings indicate that the ocular surface is not a sterile site but has a very low bacterial biomass. A proper, stable, ocular commensal microbiome has yet to be shown, although we are still just beginning to understand the relationship of ocular microbes and their influence on the ocular health. The effects of aging on the intestinal microbiome and how it relates to the eye and the effects of aging on the conjunctival microbiome warrant further investigation.
II.B. Aging, the Tear Film, the Meibomian and Lacrimal Glands
The ocular surface is kept moist (a prerequisite for proper sight) by the tear film, a complex fluid composed of water, lipids, mucins, cytokines, growth factors, and antimicrobial peptides. Traditionally, the tear film is described as composed of three different layers (mucins, lipids, and water), although this classical view has been recently challenged [65]. Any disease that affects the goblet cells (that secrete mucins), meibomian glands (that secrete lipids), and lacrimal glands (that secrete water and growth factors) can alter the quality and stability of the tear film and lead to ocular surface damage. The tear film provides the ocular surface with lubrication and defense from pathogens, and the production of its components is regulated through parasympathetic and sympathetic innervation [66–69].
II.B.1. Aging and the Tear Film
Tear volume is maintained mainly by the aqueous portion of the tear film (produced by the lacrimal glands), and aqueous-tear-deficient dry eye causes the greatest damage to the ocular surface. The secretion of basal and reflex tears is an intricate mechanism controlled by the ocular surface nerves that, together with the lacrimal gland and the ocular surface epithelia, constitute the lacrimal gland functional unit [70]. The association of advanced age in humans and low tear volume has been noted since the 1940s [71–75]. Other age-related alterations are also present, such as a decrease in tear flow, tear meniscus height, and lipid layer thickness [72–82]. In aged rodents, it has been reported either no change, a decrease, or an increase in tear volume. The discrepancy may be related to the mouse strain used and also if normalization of tear volume by body mass was performed and the sex of the animals [77, 83–86].
Tear composition also changes with aging [83, 84, 87, 88]. In vitro studies have shown that protein content after agonist stimulation decreases in aged rat lacrimal glands[68, 69, 89–91]. A decrease in IgG and IgA in aged rats [84, 88] has been reported, while other studies have shown an increase in some Igs [87]. IgG1 is a prototypical Ig secreted by B cells after T helper (Th) 2 cooperation, while IgG2a, IgG2b, and IgG3 are hallmarks of Th1-B cell cooperation [92]. Tears are collected for analysis at the eyelid meniscus and represent a mix of lacrimal gland secretion and mucins and lipids from the goblet and meibomian gland secretions. Because IgM is not commonly found in tear fluid, the presence of elevated tear levels of IgM [93] might indicate exudation from vessels and leakage through conjunctival epithelium or increased secretion by the lacrimal gland. Tears from young (1–5 months of age) and aged C57BL/6 (24–26 months) mice showed significantly higher levels of IgG1, IgG2a, IgG2b, IgG3, IgA, and IgM in the aged group (Figure 2A). However, tear levels of these Igs were not normalized by tear protein content.
Figure. 2. Tear film and lacrimal gland changes during aging.
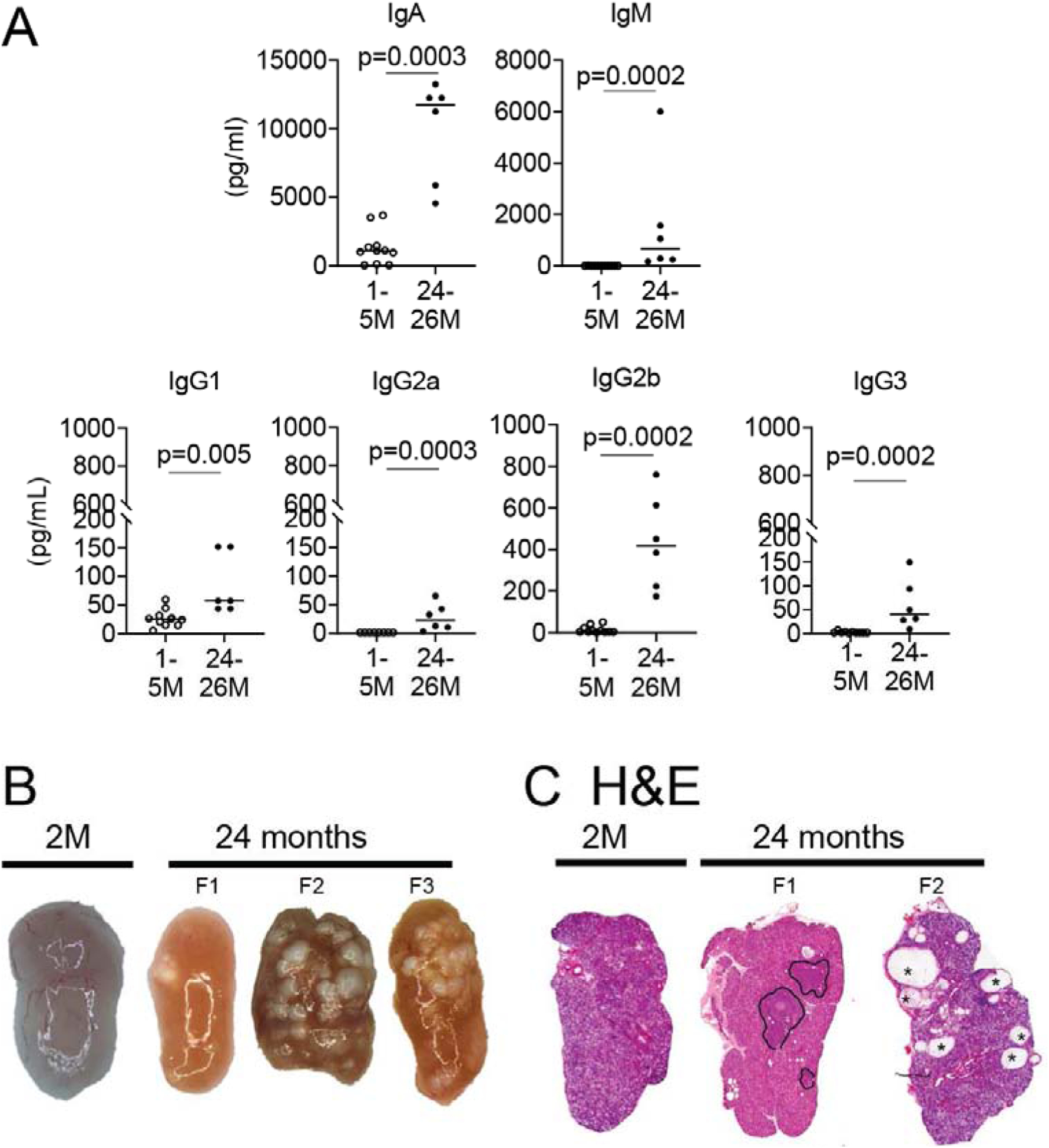
A. Tear washings from young and aged mice of both sexes were collected, and immunoglobulins were measured using Luminex assay. Each dot corresponds to tear washings from 10 animals (20 eyes). B. Representative macro images of young and aged female lacrimal glands. Note the yellowish-tan appearance of aged lacrimal gland and presence of cysts (present in 20–30% of aged lacrimal glands, F2 and F3). C. Representative scans of lacrimal gland sections stained with H&E from young and aged B6 female mice. Areas of lymphocytic infiltration are demarcated aged glands (F1), and areas of dilated ducts/cysts are easily identified (F2, asterisks). 2M = 2 months; F = female.
Compared to the multitude of articles that investigated tear biomarkers and tear composition in patients with dry eye disease, only a few studies are investigating the alterations of the tear film in healthy aging without dry eye disease. Recent publications have shown that inflammatory cytokines such as IL-6, IL-8, and TNF-α are increased in tears of aged subjects, while some growth factors are decreased [94–99].
II.B.2. Aging and the Meibomian Glands
The lipid portion of the tear film is produced by the meibomian glands: holocrine glands that are located in the tarsal plate of both superior and inferior eyelids. The lipids secreted by the meibomian glands are collectively referred to as meibum, encompassing a complex mix of bioactive lipids with different biological characteristics. Overall, meibum aids in decreasing tear evaporation and maintaining a stable tear film with each blinking [100, 101].
Aging brings significant changes in the meibomian glands that impact their function and promote tear film alterations. In addition to meibomian dropout, orifice plugging, cystic dilation, hyperkeratinization of the duct, and alterations of the meibum have been described [102–110]. Meibomian gland dysfunction is highly prevalent in the aged population [102, 104, 110], and it is thought that it participates in the vicious circle of dry eye by increasing tear instability and hyperosmolarity [111]. While meibomian gland dysfunction is frequent in both sexes, elderly male subjects have more abnormal eyelid vascularity [102, 103], and there are sex-specific gene expression patterns [112].
Clinically, meibomian dropout visualized by transillumination techniques has been used as a pathognomonic sign of meibomian gland dysfunction since the 1980s [113]. It is well established that loss of gland acini accompanies clinical findings of poor expressibility, altered meibum composition, and orifice telangiectasia. Human biopsies showed that older adults had a decrease in Ki67+ cells (a marker of proliferating cells) and a change in peroxisome proliferator-activated receptor gamma (from cytoplasmic to predominantly nuclear), a critical regulator of meibocytes and lipid production [114].
Aged C57BL/6 mice develop age-related meibomian gland dysfunction and gene alterations, with significant sex dimorphism in specific genes [115]. 3D reconstruction of murine eyelids and meibomian glands showed that 2-year-old mice have a 27% decrease in meibomian gland volume and a 54% decrease in lipid volume [116]. This was accompanied by an increase in CD45+ (bone marrow-derived) cells around the acini [117]. Furthermore, another study using 3D reconstruction by immunofluorescent computed tomography of young and aged eyelids showed gland atrophy but no hyperkeratinization of the ducts in 2-year-old meibomian glands, suggesting that processes other than hyperkeratinization participate in the aging phenotype [118]. Another possibility is stem cell senescence as a mechanism for age-related meibomian gland dysfunction [119]. A study of eyelids of young (<40) and older (>65 years) subjects using in vivo laser scanning confocal microscope also showed meibomian gland atrophy and no obstructive signs in the older group [120].
The functional consequence of meibomian gland dysfunction is an abnormal lipid layer, which is linked to a higher evaporation rate of the tear film. This, in turn, may cause insufficient lubrication, which leads to excessive attrition (i.e. wear) of corneal epithelial cells and nerves with each blink [121]. Attrition is higher when the epithelial layer is thinner, as it occurs in dry eye [122] and aging [123]. Also, increased evaporation can lead to hyperosmolar tears, which favors epithelial thinning per se [124] and can initiate inflammatory cascades in ocular surface epithelial cells. In rodents, administration of hyperosmolar eye drops is sufficient to activate NF-κB and mitogen-activated protein kinase pathways [125] in epithelial cells, disrupt immune homeostasis, and also affect corneal nerve morphology and function [126]. Interestingly, some of these changes have been reported in aged mice as well [85, 127, 128], although a causal link between them and age-associated meibomian gland dysfunction has not been demonstrated. Overall, considering the influence that meibomian glands have on corneal homeostasis, it is likely that their dysfunction contributes to the ocular surface’s immune dysregulation that comes with aging.
II.B.3. Aging and the Lacrimal Glands
The lacrimal gland is a tubulo-acinar gland where acinar and ductal cells secrete water and electrolytes (acinar, ductal cells) while myoepithelial cells are responsible for contraction and secretion of tear fluid [129]. Lacrimal glands also secrete Ig, growth factors, and antimicrobial agents (such as lysozyme, lactoferrin, and SA100 peptides) into the tears [130].
The lacrimal gland and its excretory ducts are endowed with IgA+ plasma cells and other immune cells [131, 132] and also express the secretory component [133], thus contributing to IgA secretion in tears. The lacrimal gland is commonly accepted as the primary source of Ig in tears, although this is still a matter of debate [133]. Lacrimal glands of mice and humans exhibit sexual dimorphism, with male glands showing larger acini than female glands [91, 134–137].
Several pathological processes have been observed in aged lacrimal glands, not only in acinar cells but also in the ducts and immune resident cells. It is well accepted that there is an age-related decrease in lacrimal gland function. A reduction in protein output in response to adrenaline and acetylcholine and basal peroxidase secretion has been described in dissociated acinar cells from aged rodents [68, 69, 90, 91, 138]. However, other anatomical changes also accompany aging. In rodents, a significant decrease in lacrimal gland mass has been observed in aged rats and mice compared to young animals [84, 87, 138, 139]. Elegant studies showed a decrease in lacrimal gland innervation in aged Balb/c mice and rats as early as 12 months of age, with a significant decrease by 24 months of age [138, 140].
In rodents, the main lacrimal gland is extraorbital, and it is located in front of the ear canal. Accessory lacrimal glands in rodents are the intraorbital and the Harderian gland, which are not covered in this review. Lacrimal glands from young animals have a pink color, while aged glands have a distinctive yellowish-tan appearance. In aged glands, dilated “cysts” can be observed macroscopically in about 20–30% of the cases. These cysts correspond to areas of ductal enlargement under microscopic evaluation (Figure 2B) [87, 141]. It has been postulated that these cysts are caused by blocked ducts [141]. In a study that evaluated 99 human cadaveric lacrimal glands, with a mean age of 62±17 years (range 7–93), Damato and colleagues observed the formation of “cysts” in about 20% of cases, and this occurred more significantly in older subjects. Various degrees of fibrosis and acinar atrophy were also observed, and while no sex bias was present, these findings were more frequent in the older subjects [141].
Histologic and electron-microscopical studies showed that age-related changes range from mild lymphocytic infiltration to severe dacryoadenitis, increased glandular apoptosis, and fibrosis and tissue destruction [90, 138, 141–144]. The acinar vesicles also change from serous to mucoid-like with aging [90]. The frequency of these changes is associated with increasing age [138].
Lymphocytic infiltration in lacrimal glands is a feature most commonly associated with autoimmune diseases such as Sjögren Syndrome [145–148], but it is a frequent finding in aged glands (Figure 2C) [138, 141, 148]. Similar to Sjögren Syndrome, the infiltration is periductal and tends to organize in areas resembling germinal centers; age-related infiltrates have almost the same histologic features, albeit at decreased grade [141, 148]. These infiltrates are a mix of CD4, CD8, B, and mast cells [83, 86, 140]. Because of its high prevalence in aged tissues, there is still controversy in the literature on whether finding lymphocytic infiltration in aged lacrimal glands constitutes evidence of autoimmunity. Some authors defend this is part of natural aging, while others opine that this is low-grade autoimmunity [141, 148]. There is also a question if lymphocytic infiltration follows or precedes acinar changes. For example, animal models have shown that anticholinergic blockade or post-ganglionic denervation of lacrimal gland secretion can induce immune infiltrates, inflammation and impair the lacrimal gland secretion [149, 150]. Also, constitutive epithelial activation of NF-κB in keratin-14+ cells can induce gland infiltration [151, 152].
A greater understanding of pathological aspects of aging, including inflammaging and senescence [153–155], and new therapies that can modulate aging to a certain extent [155] (calorie restriction, exercise, senolytics) have challenged the view of aging being an unpreventable event [156–162]. It is possible that aging might represent a low-grade autoimmunity state and that therapies aimed at aging could also benefit the overall health of the gland. In support of this, calorie restriction studies for 6 months significantly improved tear production in aged rats [161].
Any imbalance in the redox system caused by either decreased antioxidants or increased reactive oxygen species leads to oxidative stress. Lipid, proteins, and mitochondria are susceptible to oxidative stress, which has been linked to aging processes in many organs, including the eye [163]. One by-product of oxidative stress is the accumulation of oxidized lipids and proteins and metals, which sometimes form lipofuscin granules. Autofluorescence caused by deposits such as lipofuscin can be seen at different wavelengths [87, 164–167]. Accumulation of lipofuscin is considered one of the hallmarks of aging [165, 168], and it might indicate autophagy failure [169]. With aging, it has been shown that the aged lacrimal gland develops oxidative stress with deleterious effects [170–172]. Aged lacrimal glands exhibit an increased frequency of lipofuscin/ceroid structures [87, 138]: this can be observed as early as 12 months of age in Balb/c mice [138].
While it is evident that several age-related changes take place in the lacrimal gland, it remains unclear if these modifications are part of normal aging or if they are part of immune and inflammatory changes that accompany aging. If the latter is true, then therapies aimed at controlling inflammation and ameliorating aging will be successful in improving these alterations.
II.C. Aging and the Epithelial Barrier
The ocular mucosal epithelial lining, the actual physical barrier of the ocular surface, is directly exposed to the environment, which puts it at risk of desiccation and in contact with pathogens and potential allergens. Thus, the epithelial barrier function constitutes the first line of defense and is paramount to ocular surface immune homeostasis [173]. This barrier function is diminished in aged humans [174] and rabbits [175], but the underlying mechanisms that are affected remain to be identified. The ocular surface epithelial barrier depends on the protection afforded by the tear film (reviewed in the previous section) and comprises the glycocalyx, the intercellular junctions, and the epithelial basement membrane.
The glycocalyx is the dense, glycan-rich coating that decorates the numerous microfolds on the apical membrane of the foremost epithelial cells of the ocular surface; it is mostly mucins, transmembrane proteins with large glycosylated extracellular domain [176]. In humans, ocular surface stratified epithelial cells express four membrane-associated mucins (MUC1, MUC4, MUC16, MUC20) [176, 177], and ocular surface goblet cells secrete the soluble mucins MUC2 and MUC5AC [178]. These mucins, especially MUC16, contribute directly to the epithelial barrier function as they interact with galectin-3, a highly expressed soluble lectin, and form a lattice within the glycocalyx [177]. The ocular surface glycocalyx is altered in dry eye and ocular allergy, and some of its components are cleaved by proteases and released into the tear film [179]. So far, no studies have directly addressed the effect of aging on the ocular surface glycocalyx. By contrast, at least one study has shown that aging is associated with diminished endothelial glycocalyx in systemic small blood vessels and that this is caused in part by decreased expression of a glycocalyx synthesizing enzyme in endothelial cells [180].
Among intercellular connections, tight junctions represent the most significant component of the paracellular pathway in the ocular surface epithelial barrier function [181]; these junctions are dynamic and made up of different proteins, namely occludin, zonula occludens-1 and −2, and claudins [181]. Aged murine cornea and conjunctival whole-mounts show loss of occludin expression; this is accompanied by a decrease in the apical cell area (Figure 3A) [182] consistent with increased apical desquamation. [183]. Other aged tissues may also have a break in the epithelial barrier. In the gut, an age-induced reduction in epithelial tight junction proteins in old baboons and rats may account for the increased intestinal permeability [184, 185]. In mice, the systemic inflammation and microbial dysbiosis that come with age increase intestinal permeability in a TNF-α dependent fashion, driving more inflammation [52].
Figure 3: Ocular surface changes during aging.
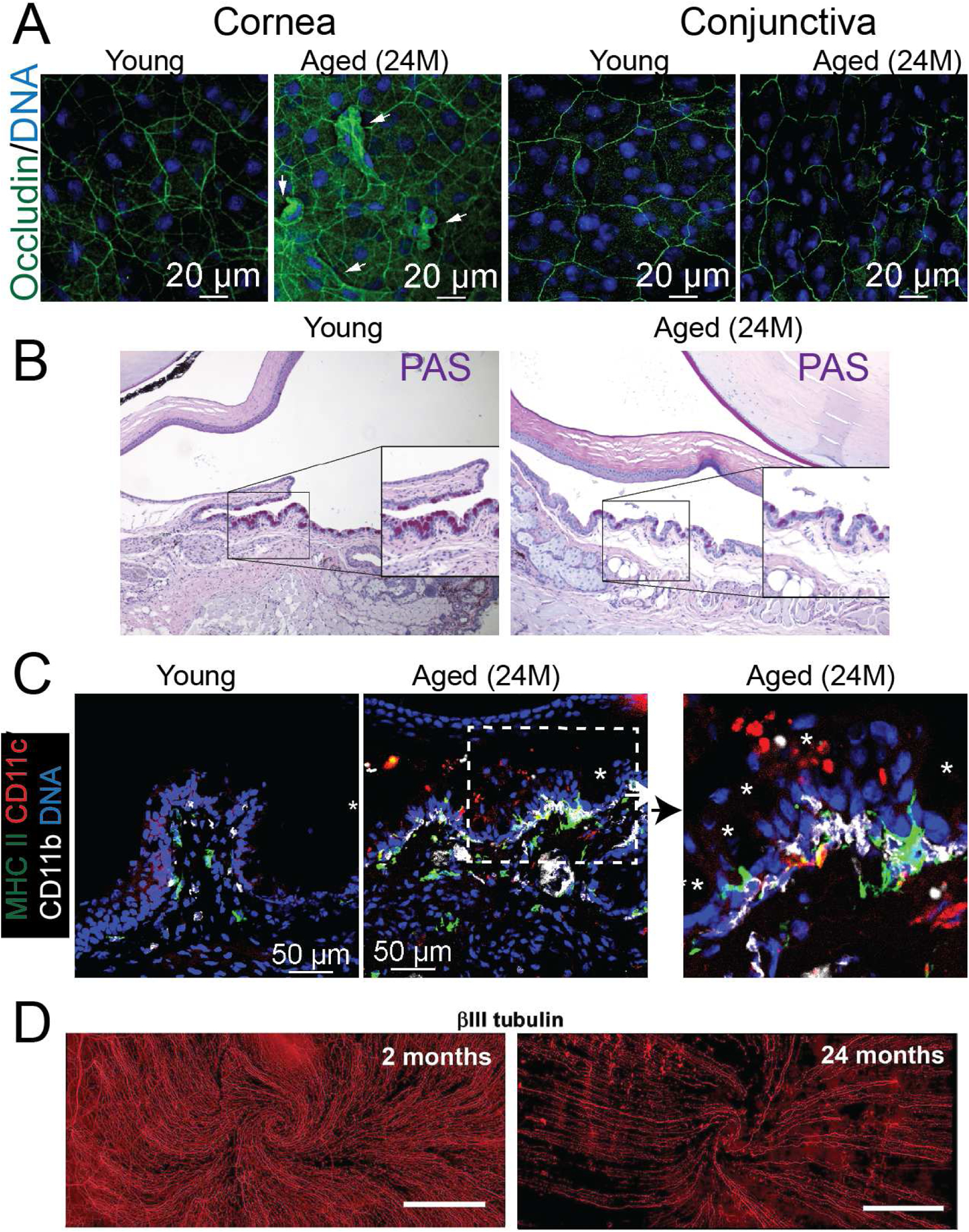
A. Representative laser scanning confocal microscope images of whole mounts of cornea and conjunctiva stained with occludin (green) and Hoechst 33342 DNA staining (blue). Arrows indicate desquamating cells. B. Representative bright-field images of conjunctiva sections showing PAS+ cells (purple-magenta). Insets are high magnification of a demarcated area. C. Representative laser scanning confocal microscope images of conjunctiva cryosections stained with MHC II (green), CD11c (red) and CD11b (white), and Hoechst 33342 nuclear staining (blue). Asterisks indicate goblet cells; the dotted area is magnified on the right. D. Representative whole-mount images of corneas stained with β-III tubulin antibody ([421], published under a Creative Commons CC BY 4.0 license). 24M = 24 months
Matrix metalloproteinases regulate these junctions through proteolytic cleavage, leading to a decrease in barrier function in dry eye as a consequence of the increased enzymatic activity [186]. Of note, levels of matrix metalloproteinases increase with age in human tears [95, 99]. Peripheral basal epithelial cell density in the cornea also decreases with aging [187], which could be related to the reported reduction in limbal precursors [188]. It remains to be established whether any of these changes have any bearing on the decreased ocular surface epithelial barrier function observed with aging [174, 175].
II.D. The Aging Conjunctival Mucosal Niche
The conjunctiva is a wet mucosa, with a specialized epithelium, goblet cells, and several immune components. Unlike the cornea, which is an avascular tissue, the conjunctiva is rich in vessels and lymphatics. Also, although the cornea is endowed with DCs and macrophages that act as APCs, these and other types of resident immune cells (CD4+ and CD8+T cells, natural killer (NK) and NKT cells [189–192]) are more abundant in the conjunctiva, and in both tissues they participate in immune surveillance. The effects of aging on corneal and conjunctival immune cells are discussed in other sections of this review. Here we will focus on two features that are exclusive to the conjunctiva: goblet cells and conjunctiva-associated lymphoid tissue.
II.D.1. Goblet cells
Among the epithelial components, goblet cells are probably the most relevant for immune homeostasis. These mucin-laden cells have a species-specific distribution in the conjunctiva, found mostly in clusters in the eyelid area of mice and isolated or in clusters mostly in the nasal bulbar and the lid wiper areas in humans [193]. Goblet cells contribute the gel-soluble mucins MUC2, MUC5AC, and MUC5B to the tear film; in addition to their protective function, mucins exert immunoregulatory effects in part by promoting tolerogenic antigen presentation [194]. Goblet cells also produce TGF-β2 [195, 196] and retinoic acid [197], two soluble factors that induce tolerogenic DC function. Goblet cell homeostasis and secretion are under the control of specific T helper (Th) cytokines: IL-13 has a homeostatic role, while interferon (IFN)-γ blocks goblet cell secretion and induces conjunctival metaplasia, endoplasmic reticulum stress, and misfolded protein response [198–201].
Another immune function of goblet cell is their aid in antigen uptake: goblet cell-associated passages have been described in the gut [202] and the ocular surface [203]. These structures facilitate transepithelial antigen delivery to the underlying stromal DCs, most likely with the aforementioned tolerogenic signals. For a thorough review of the immunobiology of these cells, refer to Alam et al. [204] and Swamynathan et al. [178].
Evaluation of goblet cell density in humans has been performed more commonly by periodic acid-Schiff or mucin-specific staining in epithelial sheets obtained from impression cytology [205]. Impression cytology of the conjunctiva has also been used to investigate gene expression in the conjunctiva in aged [206] and dry eye patients; however, impression cytology collects not only the superficial layers of the epithelium but also the associated intraepithelial lymphocytes and DCs [207, 208]. Recently, in vivo confocal microscopy has also been used [110, 209, 210].
In 1975, Ralph proposed that goblet cell loss is a universal feature of ocular surface diseases such as Sjögren Syndrome, Stevens-Johnson Syndrome, and cicatricial pemphigoid [211]. It has also been observed that goblet cell density inversely correlates with dry eye severity [212, 213]. However, the effect of aging (without dry eye) on goblet cells is controversial in the literature, with some studies showing no correlation with aging, while others showed a significant impact [110, 214, 215]. Abnormal goblet cells, containing a high proportion of neutral rather than acidic mucopolysaccharides, were reported to increase with age [214]. In another study, a reduction in the goblet cell population was observed in subjects over 80 years [215], which was sometimes associated with the presence of hyaline bodies (Periodic acid–Schiff-positive structures that possibly represent a degenerative form of goblet cells). In a more recent study, the goblet cell density obtained by impression cytology from the superior part of the bulbar conjunctiva in normal subjects showed no correlation with age, tests of lacrimal function, or tear-break-up-time but negatively correlated with rose bengal scores [216]. Doughty also found no correlation with age and goblet cell density [217]. The differences in these studies may relate to the techniques used to identify goblet cells but also the presence of undiagnosed dry eye disease, as this information is missing in some publications.
Aged C57BL/6 mice spontaneously develop dry eye disease [83, 218]. One striking feature of these mice is the early loss of conjunctival goblet cells, observed as early at 9 months of age (Figure 3B) [83]. Interestingly, the deletion of IFN-γ partially rescued age-related goblet cell loss [218], indicating that some of the mechanisms that cause goblet cell loss in young mice (such as IFN-γ) are also implicated in aging processes.
These findings indicate that goblet cells are not just mucin-producing cells but highly specialized cells with a direct impact on immune tolerance and surveillance on the eye. Therapies that can preserve goblet cells may promote overall ocular health by decreasing the immune tone in addition to providing lubrication.
II.D.2. Conjunctiva-Associated Lymphoid Tissue
The conjunctiva, as other mucosal linings, is equipped with lymphoid tissue that increases in density from the limbus to the lid margin, reaching its maximal expression in the tarsal area. [219]. This lymphoid tissue is present in a diffuse form throughout the conjunctival lamina propria, rich in IgA+ plasma cells, and as follicles in the palpebral conjunctiva, with their corresponding B-cell and T-cell areas, follicle-associated epithelium, and high endothelial venules [219]. The full conjunctival epithelial lining expresses the secretory component [133] that is crucial for translocation of IgA and IgM to the tear film. The same applies to the lacrimal drainage system [133, 219], and nasal mucosal uptake of drained antigen applied onto the ocular surface leads to strong IgA responses in tears. However, the nasal mucosa-associated lymphoid tissue is dispensable for tear IgA production, as has been shown by surgical closure of the nasolacrimal duct in rats [220]. Irrespective of its source, tear IgA contributes to ocular mucosal homeostasis by specifically neutralizing its cognate antigens, thus preventing pathogen adhesion and invasion [221]. Moreover, secreted Ig could play a role in shaping the ocular microbiome, as it does in other mucosal sites [222].
The conjunctival lymphoid tissue in humans is known to increase in size until adulthood, and then undergoes involution [223]. Tear levels of IgA, however, are maintained or even increased with age in humans [224, 225] and rodents [87, 226]. Also, lacrimal glands undergo significant anatomical and functional changes with age (for a thorough review, see de Souza et al. [87], and the composition of tears shifts towards an inflammatory profile with increased concentration of IgM [87, 95, 99]. However, much remains to be established about the humoral immune response at the ocular surface and its contribution to ocular mucosal homeostasis to try to ascertain any effect of aging.
II.E. Aging innate immunity
As in the rest of the body, the innate immune system of the ocular surface comprises the elements that participate early in the defense against pathogens [227]. This system comprises the ocular surface epithelium, with its defenses, its threat detection and alarm subsystems, and its immune control center; and the ocular surface populations of granulocytes, monocytes/macrophages, and ILCs. In addition to its defensive function, the innate immune system also has critical regulatory roles both in preserving ocular surface homeostasis and amplifying and instructing the adaptive immune response that follows [228].
II.E.1. Aging and Ocular Surface Epithelial Immunity
Although ocular surface epithelial cells are traditionally thought to only contribute passively to ocular surface immunity by acting as a physical barrier, they play the lead role in the mucosal immune response [229]. They do so by means of their defenses, their alarm systems, and their immunoregulatory effects.
Both corneal and conjunctival epithelial cells secrete antimicrobial peptides: β-defensins 1–3 and the cathelicidin LL-37 [29, 230]. Antimicrobial peptides are released constitutively to the tear film, but their production increases upon epithelial injury or detection of bacterial products like lipopolysaccharide or peptidoglycan [230, 231]. These highly charged peptides kill microbes by disrupting their cell membranes. For an excellent review of this subject, see [230]. Of note, aging does not seem to affect constitutive antimicrobial peptide secretion at the ocular surface, as no difference in cathelicidin levels were observed in young and aged tear samples [95]. Although the effect of aging on all ocular antimicrobial peptides was not explored directly, this is in line with reports of preservation of systemic antimicrobial peptide production with age [232, 233]. In addition to these antimicrobial peptides, ocular surface epithelial cells constitutively express other proteins and peptides that are released upon activation or cell damage and have multiple effects on other immune cells: the so-called alarmins [234]. At least some of these alarmins (S100A8 and S100A9) are known to increase in tears with aging [95], although it is unclear whether they derive from ocular epithelial cells or neutrophils [235]. Interestingly, they are also increased in patients with inflammatory ocular surface disorders such as pterygium and dry eye [235], yet another evidence of the dysregulated aged immune response. Also, it remains to be established whether aging affects the release of the epithelial alarmins IL-25, IL-33, and thymic stromal lymphopoietin, which influence the type 2 immune responses that underlie allergy [236].
In addition to sounding the alarm after being damaged, ocular epithelial cells can detect threats by sensing microbial products like a bacterial membrane or flagellum constituents, and viral double-stranded RNA; and also by detecting other compounds that are released by tissue damage [237, 238]. They do so through a wide assortment of pattern recognition receptors. Of these, the two most studies families are transmembrane or intracellular Toll-like receptors (TLRs) that bind pathogen-associated molecular patterns [237] and intracellular nucleotide-binding and oligomerization domain-like receptors (NLRs) for damage-associated molecular patterns [238]. Both families of receptors activate signaling cascades that ultimately converge and initiate inflammatory responses, in part by releasing alarmins [237, 238]. Interestingly, epithelial membrane-associated mucins decrease the activation of TLRs [239], whereas desiccating stress upregulates their expression [240, 241] and their functional response [242]. Mice deficient in the downstream signaling cascade triggered by TLRs do not develop ocular surface epithelial damage under desiccating stress, suggesting a pivotal role for pattern recognition receptors in dry eye [243]. Dry eye and corneal alkali burns are also linked to increased NLR expression or signaling [238, 244, 245]. Although the effect of aging on the ocular surface’s pattern recognition receptor system has not been studied so far, the systemic response to several TLR agonists is impaired in older subjects [246, 247]. Also, the expression of NLRs in the oral mucosa in non-human primates changes with age, and this could relate to aging-associated oral mucosal inflammation [248]. Polymorphisms in one of the NLRs are strongly linked to inflammatory bowel disease [249], more evidence to support the role of the epithelial pattern recognition receptors in mucosal pathophysiology. After activation by their ligands, the intracellular NLRs assemble with caspase-1 and other adaptor proteins to generate inflammasomes, which catalyze IL-1β and IL-18 production and amplify the inflammatory response [250]. Inflammasome activation is involved in dry eye pathogenesis [251–253], and interestingly, dysregulated NLRP3 inflammasome activity is also linked to inflammaging [254], as its NLR binds ligands that are increasingly generated by physiological aging and metabolic stress.
Within the ocular surface epithelial lining, different signaling pathways converge, thus integrating the input information from the danger detection systems and other receptors; in turn, the corresponding epithelial output has a decisive impact on the outcome of the mucosal immune response [229, 255]. The nuclear factor (NF)-κB pathway serves as the central integrator of the epithelial outcome: low activity is associated with anti-inflammatory, tolerogenic immune responses, whereas increased activity results in a pro-inflammatory immune response [256, 257]. Tolerogenic immune responses in the ocular surface are lost in mice exposed to eye drop preservatives or desiccating stress after increased epithelial NF-κB signaling; conversely, topical NF-κB inhibition prevents the immune disruption and disease development [258–260]. Also, constitutive activation of NF-κB causes a hyperinflammatory phenotype in mice, which can be rescued by a TNF-α receptor deficiency; quite interestingly, as these mice age, they still develop dry eye signs despite the absent TNF-α signaling [261]. Human tear proteomic analysis shows increased NF-κB activity with aging [95], and enhanced NF-κB activity is a hallmark of inflammaging [262, 263]. It is thus evident that dysregulated epithelial NF-κB signaling is one of the changes in the ocular surface immune response brought about by inflammation. However, it has been shown that aging itself is accompanied by NF-κB activation and that conditional genetic deletions of pharmacological therapies aimed at NF-κB can decrease or reverse pathological aging in tissues and change gene expression signatures from old to young [264–268].
In addition to the more conventional immune pathways of mucosal epithelia described above, the ocular surface epithelium exhibits other immunoregulatory mechanisms. Corneal and conjunctival epithelial cells synthesize thrombospondin-1, an extracellular matrix protein that is involved in the activation of latent TGF-β, a highly immunoinhibitory cytokine that is also required for the development of Th17 responses [269]. Goblet cells (section II.D.1) represent a significant source of TGF-β2 in the ocular surface. Intriguingly, there is evidence of a newly appreciated role of thrombospondin-1 in age-related extraocular diseases [270]. Corneal epithelial cells also express Fas Ligand, which induces lymphocyte apoptosis by activating their Fas receptor [271]. It remains to be determined whether aging affects these specific immunoregulatory pathways. Finally, corneal epithelial cells express major histocompatibility complex (MHC) II molecules in diverse inflammatory settings and may act as antigen-presenting cells [272]. Also, increased expression of conjunctival epithelial MHC II has been reported in dry eye patients, although the pathophysiological significance of this finding is unknown [273–276].
In summary, given the key role of the epithelial lining in the mucosal immune response and the numerous changes in epithelial immunity that come with aging, it is very likely that immune dysregulation in the aged ocular surface is partly caused by epithelial dysfunction.
II.E.2. Aging and Neutrophils
Neutrophils are most commonly associated with pathology, i.e., acute inflammation, as they represent the first line of defense against bacterial infections [277]. Neutrophils are fully equipped to sense microbial threats [278] and, in response, secrete proinflammatory cytokines [279], trigger an oxidative burst to enhance bacterial killing [280], and release DNA-rich extracellular traps [281]. However, they have regulatory and homeostatic roles as well, and this is readily evident in the ocular surface. Prolonged eye closure during sleep reduces the spread of the tears, favors hypoxia, and decreases epithelial barrier function [282]. This diurnal tear cycle leads to subclinical ocular surface inflammation, which is accompanied by an influx of neutrophils every night [283].
Interestingly, C5a levels in tears, a product of complement activation and a potent recruiter of neutrophils, increase over daylight hours, and reach the highest point at night [284]. This probably reflects the accumulated exposure of the ocular surface to airborne particles and microorganisms. The recruited neutrophils participate in the clearance and containment of these threats during eye closure, as the proinflammatory microenvironment favors neutrophil degranulation. Upon eye-opening, the change in the ocular surface towards a normoxic environment promotes a shift in the functional profile of neutrophils, some of which undergo death and give rise to DNA-rich extracellular traps, which in turn enclose and clear inflammation-associated debris. The result of this process is the resolution of ocular surface inflammation and the formation of eye rheum [284]. Also, limbal neutrophils play a regulatory role in the ocular surface and are specifically downregulated in female mice under desiccating stress, thus contributing to sex susceptibility to the disease [285]. The number of circulating neutrophils in basal conditions or inflammatory states does not seem to be affected by aging, but their ability to be recruited to the infected site and kill microbes is indeed impaired [286]. Conjunctival imprint levels of IL-8, a proinflammatory cytokine that is released by neutrophil and epithelial cells, increase with aging [287]. In addition to the increased susceptibility to ocular infections in the elderly, dysregulated neutrophil function might contribute to the pro-inflammatory changes that come with aging, as these cells have a considerable homeostatic role in the ocular surface.
II.E.3. Aging and Complement
The complement system is a unique component of the innate immune system and, at the same time, a pillar of the humoral immune response [288]. Complement comprises a group of soluble factors that are activated by a cascade of enzymatic reactions; the consequences of its activation are diverse but uniformly pro-inflammatory [289]. For detailed complement physiology, we refer the reader to any of the excellent reviews [290]. A functional complement set is found in tears, and its factors probably arrive from leaky conjunctival blood vessels or released by ocular surface epithelial cells and infiltrating neutrophils [291]. Their concentration is higher in closed-eye tears than in open-eye tears, and more importantly, closed-eye tears have more factors in the activated state, evidence of complement activation in the ocular surface during sleep [288]. The complement’s role in recruiting neutrophils to the ocular surface at night was discussed in the previous section. Dysregulated complement activation can lead to tissue destruction, and therefore, complement activation is tightly controlled by several regulatory factors [288, 289]. Corneal epithelial cells express membrane-bound inhibitory factors [292]. Also, lactoferrin in tears dampens complement activation [293].
Interestingly, some bacteria release phospholipases that cleave membrane-bound complement regulators, thus making the corneal and conjunctival epithelial cells more susceptible to complement-mediated damage and perhaps facilitating bacterial adhesion [294]. In herpetic keratopathy in mice, complement and T cells mediate corneal nerve damage [295]. Remarkably, herpetic infection increases the local synthesis of complement activating factors in the cornea, and although it is unclear whether this is done by infiltrating immune cells or by corneal epithelial cells, it probably favors pathogenic complement activation [295]. In murine dry eye, complement contributes to ocular surface inflammation induced by autoantibodies [296].
Interestingly, increased complement factors 3 and 4 levels in diverse tissues and serum are associated with aging [297–299]. As complement dysregulation has been implicated in inflammaging and renal disease [300] and age-related macular degeneration [301], it might also contribute to the ocular mucosal immune dysfunction that comes with aging. A local shift in the balance of activating and inhibitory complement factors towards the first, as has been reported for herpetic keratopathy [295] and in the aged brain [299], might be implicated. Sex is another variable that influences serum complement levels in humans and should be taken into account [289].
II.E.4. Aging and Monocytes and Macrophages
Tissue-resident macrophages are phagocytes that play an essential part in tissue homeostasis, participating in the clearance of dead cells and wound healing, and also in the immune response: they can present antigen to and activate CD4+T cells, and also phagocytose targets recognized by their receptors or marked by the adaptive immune response [302, 303]. Corneal macrophages, as in the rest of the body, can have two origins: some reach the tissue during embryogenesis and self-renew in situ indefinitely [C-C chemokine receptor (CCR) 2−], whereas others differentiate from blood-borne monocytes (CCR2+) [304]. Upon injury, monocyte-derived CCR2+ corneal macrophages rapidly increase and then return to normal levels after 24 hours, whereas CCR2− macrophage numbers fall immediately after injury and then recover to even higher levels after 24–36 hours. Interestingly, depletion of CCR2+ corneal macrophages reduces the injury-induced inflammatory response, suggesting they have an M1 pro-inflammatory profile, whereas depletion of CCR2− corneal macrophages enhances the inflammatory response, suggesting the opposite M2 anti-inflammatory profile. Both subsets, however, are required for appropriate wound healing [304], highlighting the macrophage’s crucial role in tissue homeostasis. Although conjunctival macrophages have not been studied in such detail so far, they do have many of the properties observed in tissue-resident macrophages elsewhere. As in the other tissues, conjunctival macrophages change their functional profiles according to the microenvironment [305]: under desiccating stress, their number does not vary significantly, but they become more pro-inflammatory [303]. Also, conjunctival macrophages polarize to an M2 profile (which also involves pro-allergic Th2 cytokine production) in a murine model of allergic conjunctivitis and thus amplify the allergic response [306].
Although there are no specific studies on aged ocular surface macrophages, there is ample evidence that age does affect monocytes and macrophages in other tissues [307]. Aged skin macrophages are partially responsible for the reduced delayed-type hypersensitivity responses in older individuals because they release less TNF-α (deficient immunosurveillance) and thus do not amplify the local inflammatory reaction, which prevents blood vessel endothelial activation and extravasation of memory T cells [308]. Conversely, aging increases the proportion of proinflammatory M1 macrophages in adipose tissue, which is related to inflammaging and has profound metabolic implications [309]. Macrophages are crucial for the resolution of inflammation, contributing to the cleaning of inflammatory debris and dead cells and their transition to an M2 anti-inflammatory profile. However, both of these macrophage functions are impaired by aging [310]. It remains to be established how much of this age-induced macrophage dysregulation takes place in the ocular surface and how relevant it is for ocular surface disorders.
II.E.5. Aging, ILCs and other Tissue-Resident Lymphocytes
Mucosal linings are rich in tissue-resident lymphocytes, which include ILCs, unconventional T cells (NKT cells, mucosal-associated invariant T cells, and γδ T cells), and tissue-resident memory T cells [189, 311]. The defining feature of tissue-resident lymphocytes is their distinct migration pattern: as opposed to naïve and central memory T lymphocytes, tissue-resident lymphocytes do not travel between secondary lymphoid organs [312]. Since they are already posted at the mucosal site, tissue-resident lymphocytes are uniquely poised to react early to threats and thus influence the initial phases of the immune response.
II.E.5.a. ILCs
ILCs share a common lymphoid progenitor with B and T cells, but unlike them, do not activate their recombination activation genes (RAG1/2) and thus do not rearrange their antigen receptors [313]. In other words, they only express innate receptors for identifying threats and exerting their function. ILCs are found in mucosal linings and other tissues, and they can be classified into three groups akin to the Th profiles of T cells: group 1, which produce IFN-γ and include NK cells; group 2, which produce Th2 cytokines; and group 3, which produce IL-17 and/or IL-22 [313]. ILCs in mucosal sites are advantageously posed to rapidly react to threats, amplify the inflammatory response, and direct the type of adaptive immune response that will eventually supersede them [314]. This has been well established in the gut, where group 3 ILCs are responsible for trophic IL-22 secretion, thus maintaining an effective epithelial barrier along with neutrophils and Th17 T cells [315]. In the ocular surface, NK cells respond early to desiccation-induced epithelial signals with a burst of IFN-γ, thus setting the stage for the subsequent Th1 adaptive immune response that drives dry eye [316].
Although the literature on ILCs in the ocular surface is scarce, it should be considered that the current concept of ILCs is relatively recent, and thus some of the previous reports on ocular surface NK cells might today be interpreted as ILCs [317]. For example, CD161 was previously considered an NK cell marker, but nowadays is known to be expressed on all ILCs, and the NK cell denomination is best reserved for those group 1 ILCs that have cytotoxic capacity [313]. Therefore, the second most abundant intraepithelial lymphocyte population in the murine conjunctiva (10% of isolated live cells are γδ T cells, and 5% are CD3−CD161+, previously considered NK cells) would be regarded as ILCs today [189], and their contribution of IL-17 in a desiccating stress model of dry eye might be considered the result of activation of group 3 ILCs. Also, the reported increase of IFN-γ-producing CD3−CD161+NK cells in the conjunctiva of mice under desiccating stress [318] would probably be interpreted today as an expansion of group 1 ILCs. In both reports, further characterization might elucidate whether there are more blood-borne NK cells arriving to the conjunctiva or if there is increased local proliferation of other group 1 and group 3 ILCs. Comparably, the homeostatic effect on conjunctival goblet cells reported for IL-13 derived from conjunctival CD161+ NK cells might be ascribed today to group 2 ILCs [198].
There is direct evidence for an IL-33/group 2 ILCs axis in the ocular surface: transgenic mice with ocular surface epithelial overexpression of IL-33 (keratin 14-driven) have activated group 2 ILCs in the ocular surface and develop an atopic keratoconjunctivitis/blepharitis phenotype [319]. Corneal limbal tissue-resident macrophages (CCR2−) also contribute to this axis by secreting IL-33, and the responding group 2 ILCs favor corneal epithelial regeneration [320]. The human conjunctiva harbors an IL-22 producing ILC population, presumably group 3, although this was not confirmed in the study [321]. However, in the human conjunctiva, conventional T cells (αβ) are the predominant IL-22 source, whereas, in the murine conjunctiva, IL-22+ γδ T cells are more abundant [321]. Finally, very few studies have addressed how ILCs change with aging, but at least one report shows a compartmentalized effect of age: increased bone marrow generation of ILC precursors but reduced settling in the lung [322]. Interestingly, the adoptive transfer of group 2 ILCs from young mice to aged hosts increased their resistance to influenza infection. It would be interesting to study how ILCs in the aged ocular surface influence its inflammatory predisposition.
II.E.5.b. γδ T cells and Tissue-resident CD8+T cells
Although they are not innate immune cells because they do rearrange their T cell receptor genes, γδ T cells share some functional characteristics with ILCs that set them apart from the conventional αβ T cells. γδ T cells are present in the conjunctiva and cornea and even constitute the predominant tissue-resident lymphocyte population in the murine conjunctiva [189]. CCR6+ γδ T cells contribute to corneal epithelial wound healing in mice as they are recruited by epithelial C-C chemokine ligand (CCL) 20 to the wound edge, where they contribute IL-22, a critical epithelial growth factor, and exert microbicidal effects through IL-17 and the recruitment of neutrophils [323]. Conjunctival γδ T cells also respond to ocular commensal bacteria in mice by recognizing some of their antigens and producing IL-17 in response [63]. Interestingly, this interaction keeps the ocular surface’s immune response primed for action, as a bactericidal treatment increases the susceptibility to fungal and other bacterial infections. Since γδ T cells are impaired or skewed by aging [324, 325], it is tempting to speculate how the increased susceptibility to ocular infections in the elderly might relate to impaired γδ T cell function.
Intraepithelial CD8+T cells are another tissue-resident lymphocyte population that has been observed in the conjunctiva of mice [189] and humans [191]. Most of these cells in both species are CD103+ [326, 327], a marker associated with a regulatory phenotype in mice [328]. Antibody-based depletion of CD8+ cells during desiccating stress worsens dry eye [327]. Interestingly, CD8+T cells do not increase with age in the human conjunctiva, but CD4+ T cells do; thus, their ratio decreases and probably their regulatory influence as well [191]. Aging also decreases the number of both CD8+ and γδ T cells in the gut while activated αβ T cells increase [329]. Whether conjunctival-resident CD8+T cells are implicated in the functional immune changes brought about by aging to the ocular surface is unknown.
II.F. Aging, Antigen-presenting cells, and Antigen Presentation
APCs are a heterogeneous family of cells that participate in immune surveillance and are critical players in tolerance and immune responses. They are named so because of their ability to activate T cells, which can only recognize their cognate antigens when they are presented within the MHC molecules that are found on the surface of APCs. All cells express MHC class I, which is required by CD8+T cells to become activated. By contrast, professional APCs are those that express the MHC class II that is required to activate CD4+T cells and encompass DCs, macrophages, and B cells. DCs received their name due to their dendriform appearance that resembles neurons. Their highly elongated dendrites intersperse among mucosal epithelial cells [197, 203] and participate in tolerance induction by sampling the exterior (in the case of the eye) or the gut lumen. Epithelial cells can also express MHC class II and activate CD4+T cells under some settings [208, 273, 330], but by far, the most potent and relevant professional APCs are DCs. For the remainder of this section, we will focus on DCs as the prototypical APCs.
Immature DCs reside in peripheral tissues, where they sense the microenvironment and capture antigens. Diverse signals trigger DCs to mature and migrate to the lymph nodes, where they interact with naive CD4+T cells and polarize them into specific cell lineages (Figure 1). Tissue-resident DCs respond to signals from the microenvironment through their pattern and danger recognition receptors, which recognize molecular patterns from microorganisms and self-derived molecules like DNA. Among these pattern recognition receptors, TLRs and NLRs are also expressed by other cells that take part in the ocular surface innate immune response, and they were described above in section II.E.1.
As important decision-making cells (attack or tolerate an antigen), APCs receive many clues from the environment. Horev and colleagues showed that mucosal APCs are more affected by an aged microenvironment than skin APCs [331]. An aged inflammatory milieu rich in TNF-α, IL-17, CCC2, CCL20 was described in the gingiva [331], whereas the aged conjunctiva milieu is rich in CXC chemokine receptor ligand (CXCL) 13, IL-1β, MHC II, IL-12, and IFN-γ [182]. By contrast, the aged skin had minimal alterations [331]. DCs and epithelial cells are in close proximity, and there is epithelial-DC crosstalk. While it is well established that epithelial cells can activate DCs by secreting cytokines, studies have also shown that supernatant from aged DCs can activate epithelial cells, promote release of cytokines, and decrease epithelial barrier function [332]. TNF-α secreted by aged DCs was critical in epithelial activation [332]. These findings demonstrate that aging affects mucosal tissues differently from other sites [182, 331] and that epithelial-DC crosstalk is bidirectional.
The ocular surface harbors many APCs in the conjunctiva, limbus, and in the cornea. Interestingly, many of the cornea APCs are MHC II-negative [333, 334]. The conjunctiva is an APC-rich tissue, and many different subtypes of APCs co-exist dispersed in the stroma and among the epithelial layers, including monocytes, macrophages, and CD11c+CD103+ DCs [189, 199, 335]. In aged mice, CD11b+, CD11c+, and MHC II+ cells move from the stroma into the epithelium and accumulate in the goblet cell-rich area (Figure 3C and [182]). These APCs express higher levels of CD86 than young APCs and are very phagocytic [182].
Important APC-modulating molecules are TGF-β and retinoic acid (RA), frequently found in high concentration in mucosal tissues of the gut and the eye [195, 197, 336]. TGF-β2 maintains the MHC II-negative status of corneal APCs and decreases activation of conjunctival goblet cells [195, 197]. Vitamin A (retinol) from the diet needs to be metabolized into RA by an orchestrated sequence of alcohol dehydrogenases and aldehyde dehydrogenases. RA is a critical tolerance-promoting factor through the generation of regulatory T cells [337]. Deficiency in vitamin A has been linked to colitis, night blindness, and severe keratoconjunctivitis, with corneal opacification and corneal neovascularization [338, 339]. In addition to the gut, the conjunctival epithelium and goblet cells express alcohol dehydrogenases and aldehyde dehydrogenases [197, 336]. Conjunctival goblet cell-conditioned media has high levels of RA, and it is equally potent as RA in reducing the activation, release of pro-inflammatory cytokines (such as IL-12), and induction of IFN-γ -producing T cells by DCs stimulated with LPS. This effect was mediated through suppressor of cytokine signaling-3 (Socs3) in vitro [197]. Topical administration of conjunctival goblet-cell conditioned media to mice devoid of conjunctival goblet cells blunted the inflammatory response to LPS and significantly decreased production of IL-12 by CD11b+CD11c+ cells [340]. In vitro, human RA-stimulated DCs that were co-cultured with CD4+ cells were defective in generating regulatory T cells (Tregs), leading to decreased IL-10 production and higher levels of IFN-γ by CD4+ T cells [341]. In aged mice, the aldehyde activity in the conjunctiva [182] and aldehyde dehydrogenase family 1, subfamily A2 (aldh1a2) gene expression is decreased in conjunctiva [182] and gut mucosa [342], suggesting that altered RA signaling in the aging may contribute to ocular immunoregulation.
In aged mice, aldehyde dehydrogenase activity and aldehyde dehydrogenase family 1, subfamily A2 (aldh1a2) gene expression is decreased in the conjunctiva [182], suggesting that altered RA signaling may contribute to age-associated immune-dysregulation in the ocular mucosa. Consistently, the expression of the same aldehyde dehydrogenase is also reduced in the aged gut mucosa [342].
Antigen presentation involves several steps, namely, uptake and processing of antigens followed by the actual presentation of the resulting epitopes (the fragments of the original antigens that are recognized by T cells) on MHC class II molecules. DCs, process (catabolize) putative antigens into smaller peptides in their acidic vesicles (e.g., lysosomes). Proteases such as cathepsins digest antigens and remove the preloaded CD74 (invariant li) from the MHC II groove, thus facilitating contact of the peptide binding region of the MHC molecule with the newly processed peptides and the consequent MHC loading. After processing, the newly formed peptide-MHC II complexes are shuttled to the surface of DCs, where they can interact with CD4+T cells through the T cell receptor. However, a second signal is necessary to activate T cells, and this second signal comes from co-stimulatory molecules such as the ones from the B7 family (CD80 and CD86) that bind to CD28 on T cells. Other costimulatory molecules are CD40-CD40 ligand, inducible T-cell costimulator (ICOS)-ICOS ligand, and adhesion molecules ICAM-1-LFA1 (intercellular adhesion molecule and leukocyte function-associated antigen 1, respectively). The need for a second signal prevents that self-proteins that are processed through autophagy and that bind to MHC II enlist a CD4+T cell response to self. Interestingly, aged tissue-resident APCs have increased levels of co-stimulatory CD86 [182, 329].
In the thymus, APCs play a critical role in central tolerance, participating in the selection of self-reactive T cells that are marked for deletion and do not reach the periphery. The protease cathepsin L is expressed preferentially by thymocytes, while DCs rely more on cathepsin S [343–349]. The abundance of an antigen and the duration of presentation are factors that influence the outcome of antigen presentation, as prolonged MHC II presentation in the periphery has been associated with autoimmunity [350–352]. Consistently, cathepsin S, one of the proteases involved in the MHC II presentation, [343–345, 350], is increased during aging [353–356]. Recently, increased levels of cathepsin S in tears have been proposed as a novel biomarker for Sjögren Syndrome and dry eye [357–360]. Interestingly, HLA-DR expression in conjunctival epithelial cells has been reported in patients with dry eye and Sjögren Syndrome [273–276], but the significance of this expression in the aged population has not been thoroughly investigated.
DCs, as sentinels, also secrete large quantities of cytokines in response to specific stimuli that can polarize naive T cells into specific subsets, a critical step in differentiating the immune self from the immune non-self and thus preventing autoimmunity. For example, IL-12 secreted by DCs can polarize T cells into Th1 cells (IFN-γ-producing cells) while IL4 and IL5 can polarize into Th2 (IL-13 and IL-4-producing cells). IL-12+ cells were found in the conjunctiva of aged C57BL/6 mice and germ-free mice, two models that have high levels of IFN-γ [58, 182]. DCs migrate from the peripheral tissues (e.g., the ocular surface) to the local lymph node to present the captured antigen to naïve T cells (Figure 1, step 3). Interestingly, antigen uptake after ocular eye drop administration or fluorochrome painting in oral mucosa and the subsequent migration to draining nodes was augmented in aged compared to young mice [182, 331]. However, studies in vitro with APCs isolated from spleens and mesenteric lymph nodes showed a decrease in migration to a gradient of CCL19 and decreased expression of CCR7 [342]. The discrepancies in the migratory capabilities of aged APCs shed light on the vital role of the microenvironment in influencing APC behavior as studies using tissue-resident APCs, or lymph node-APCs have opposite results [182, 331, 342].
In vitro antigen-presentation assays showed no quantitative effect of aging, as aged APCs can present just as efficiently as young APCs. There is, however, a qualitative effect: aged splenic APCs preferentially prime Th1 and Th2 cells and induce fewer regulatory T cells [329]. This age-induced change is more pronounced in mucosa-draining lymph nodes [329] than in the spleen. Using regional APCs obtained from the eye-draining nodes from 24-month old C57BL/6 mice in OVA antigen-presenting assay, Bian and colleagues showed that aged APCs primed a greater percentage and number of Th1 cells and a decrease in the number of CD4+Foxp3+cells [182].
In summary, age-related changes abound in DCs and antigen presentation in general. Overall defects in priming of regulatory T cells and increased production of cytokines and activation markers are characteristics of aged APCs, which likely contribute to immune dysregulation. However, there are still many unanswered questions about the role of the microenvironment and DC activation in the aged population.
II.G. Aging and T cells
T cells are the cornerstone of adaptive immunity and thus crucial in the control of infections and tumors. During their development in the thymus, T cells undergo random recombination of their T cell receptor genes, which allows them to react to a wide range of potential antigens. This unique feat, which B cells also perform, confers the evolutionary advantage of defense against new pathogens to the host, but also comes with the threat of targeting the host’s tissue antigens. To prevent this, there are mechanisms in place during the development of T and B cells in the primary lymphoid organs (thymus and bone marrow, respectively). Only the T cell precursors that can interact with the host’s antigen presentation molecules but do not recognize the self-antigens that are presented by thymic epithelial cells are selected to become naïve T cells. The precursors that do react with the autoantigens available in the thymus become Tregs (natural or thymic T cells, see subsection II.G.2). Thus, only the T cells showing no reactivity towards the self-antigens that are available during their development may exit the thymus and patrol the lymph nodes and spleen as naïve T cells, awaiting their opportunity to become effector or memory T cells upon encountering an APC presenting their cognate antigen. This section will discuss the role of conventional (i.e., non-regulatory) CD4+ and CD8+ T cells and Tregs. Tissue-resident CD8+ T cells, although a subset of conventional T cells, were covered in the section II.E.5.b.
II.G.1. Conventional T cells
Conventional T cells encompass CD4+ and CD8+ T cells, both naïve and antigen-experienced CD4+ T cells provide help to a variety of cells. These cells are therefore named T helper [Th] cells: follicular helper cells (Tfh) aid B cells in antibody class switching whereas others secrete cytokines and usher in the appropriate immune response to intracellular bacteria and virus (Th1), extracellular parasites (Th2), and extracellular pathogens and fungi (Th17). Thus, CD4+ T cells act as gatekeepers of the adaptive immune response, allowing and guiding the appropriate cascade of immune events to follow. It has become apparent that in disease states, specific Th subtypes outnumber others. An example is asthma and allergic diseases that require IgE antibodies and are characterized by strong Th2 responses. CD8+ cells have cytotoxic activity and are very relevant in viral infections and tumor immunity.
Profound changes in the immune system accompany aging in each cell compartment. It has been postulated that the immune system in the elderly suffers from both immunosenescence and inflamm-aging [153, 361, 362]. Immunosenescence refers to the aging of the immune system, where thymus involution plays a central role in decreasing tolerance and generation of naive cells. At the same time, there is an accumulation of experienced memory T cells in the periphery [329, 363–366].
With aging, CD4+ and CD8+T cells accumulate in many tissues [52, 83, 367–370]. They also become more activated, secrete greater amounts of cytokines, and participate in tissue pathology either directly or indirectly by inducing bystander damage [86, 182, 364]. Aged T cells are also more responsive to a non-antigenic stimulation than young cells in vitro [371] and are resistant to regulatory T cell suppression [372]. The aged T cell pool is more heterogeneous, with increased expression of exhaustion and activation markers such as PD-L1 and Eomes [370, 372]. Also, there is a well-documented increase in memory CD4+T cell pool and a decrease in naive cells in the blood of aged subjects [366].
Using flow cytometry of cells obtained with impression cytology of human conjunctiva, Williams and colleagues showed that lymphocytes constituted the majority of resident cells in human conjunctiva, followed by monocytes and neutrophils. Among the lymphocytes, the CD8a/b was the most predominant subtype, followed by CD4+T cells. They also showed that in healthy subjects younger than 35 or older than 65 years, the older group exhibited an increase in percentage and absolute number of IFN-γ-producing CD4+T cells and no change in IL-17 or IL-10 production [191]. The lacrimal gland infiltrates in aged mice are a mix of conventional (CD4+ and CD8+) and regulatory T cells and B cells [83, 86, 182]. Aged C57BL/6 mice (24-months or older) have increased infiltration of CD4+T cells in the conjunctiva: these cells express the CXC chemokine receptor (CXCR) 3 and secrete high amounts of IFN-γ, showing there is an increased local (conjunctiva and lacrimal gland) and regional (eye-draining nodes) Th1 response with aging [182]. 12–14 month-old mice display stronger Th17 responses upon restimulation with desiccating conditions than young mice [373]. The skewed Th phenotype in the aged ocular surface is relevant since IFN-γ-producing T cells can directly induce apoptosis of epithelial and acinar cells, decrease goblet cells, and promote conjunctival metaplasia, whereas IL-17-producing T cells participate in corneal barrier disruption [199, 373–380].
In the young, any infiltration of organs by T cells is considered “autoimmunity.” Because tissue infiltration by T cells during aging is so prevalent, many have come to accept that the infiltration of T cells accompanies a normal aging process. However, others have defended that aging is similar to autoimmunity in many aspects, and the presence of infiltrates should not be considered normal [141]. There is mounting evidence that aged T cells may be more pathogenic than young T cells, and they may escape control by other cells. For example, aged mice have increased antinuclear antibodies (autoimmune), and this increase was MHC II-dependent, demonstrating that CD4+T cells contribute to this aspect of immune dysfunction [364]. In support of the increased pathogenicity of aged CD4+T cells in vivo, the adoptive transfer of CD4+T cells from aged mice but not from young mice is sufficient to induce decreased goblet cell density and cause lacrimal gland infiltration in immunodeficient mice [83, 381]. Also, the adoptive transfer of young and aged CD4+T cells into young and aged congenic hosts showed that intrinsic factors of the aged hosts decreased the CD4+T cell response after active immunization [364, 382].
Overall, the adoptive transfer experiments demonstrate that T cells in aged individuals have both intrinsically and extrinsically derived impairment. While several cell-specific defects have been identified, the aged environment also modulates T cell function. Conversely, excessively activated T cells can modify the environment by secreting cytokines such as IFN-γ and TNF-α, which have a bystander effect and a deleterious impact on the ocular surface. The interplay of the aged microenvironment and aged T cells warrants further investigation.
II.G.2. Regulatory T cells
Regulatory T (Tregs) cells are a specialized T cell population that can suppress the response of other lymphocytes and promote tolerance to self-antigens and also to harmless foreign (non-self) antigens [4]. Tolerance refers to the immune system’s ability to not react (“tolerate”) against an antigen that can elicit an immune response in another organism. Self-antigens must be tolerated in order to prevent autoimmunity, but this also applies to harmless foreign antigens like those derived from food or other non-threatening organisms that may come in contact with the body. Tregs are instrumental in immune tolerance, just as effector T cells are critical to immune responses. Most Tregs can be identified by their CD4+CD25+Foxp3+ phenotype, but other markers have also been proposed (GITR, CD127 low). Tregs are mainly produced by the thymus but can also be induced in the periphery from naïve T cells. Thus, thymic Tregs are considered a part of central tolerance, whereas extra-thymic or induced Tregs contribute to peripheral tolerance. The mechanisms by which Tregs suppress immune responses include secretion of anti-inflammatory cytokines, interaction with APCs, and blocking effector T cell activation [383].
The importance of Tregs can be appreciated in experimental models that develop autoimmunity. For example, neonatal thymectomy in mice prevents the formation of thymic Treg and leads to autoreactive CD4+T cell expansion and autoimmunity [364, 384]; this autoimmunity can be prevented by adoptive transfer of Tregs from naive mice. Interestingly, salivary and lacrimal glands are among the organs that are early affected after thymectomy [384]. Furthermore, mice that are defective in either CD25 (CD25−/−) or Foxp3 (scurfy) often develop fatal, lymphoproliferative disorders that affect mucosa tissues, such as the colon, eyes, and lacrimal glands [385–389].
There is a growing body of evidence that shows an increase in Tregs accompanies aging despite age-related thymus involution [390]. This increase in Tregs is paradoxical, as increased Treg frequently accompanies increased tissue pathology; therefore, it has been postulated that aging induces dysfunctional Tregs. Despite extensive investigation, the literature remains controversial regarding a decreased Treg suppressive capacity in aging, with studies reporting normal or decreased function of these cells [381, 391–394]. Factors such as sex of animals, strain, Treg isolation technique (CD25 isolation using beads or sorting on Foxp3+GFP+ cells), age (varying from 18–24 months), and specific assays to evaluate suppressive function (mixed lymphocytic reactions with CD2-CD28 coated beads, in vivo co-adoptive transfer, ex-vivo expansion of Tregs, anti-tumor activity) might contribute to the discrepancy seen regarding Treg suppression capacity. Another complicating factor is the use of splenic Tregs as surrogate Tregs, since systemic Tregs may not accurately represent the suppressor capacity of organ-specific infiltrating Tregs.
Similar to CD4+ T cells, aged Tregs assume a memory/exhausted phenotype and can secrete cytokines such as IFN-γ [372, 381]. Elegant single-cell RNA seq experiments comparing young and aged splenic cells identified greater heterogeneity of aged T cells, both in the non-Treg and Treg compartment (cells were divided into cytotoxic, T effector memory, T exhausted, activated Tregs, resting T regs and naive) [372]. The authors showed that Treg cells acquired an activated phenotype with some markers of chronically exhausted cells.
While studies have also shown an increased/altered Th1/Treg or Th17/Treg ratio with aging [366, 395], many questions remain about the suppressive status of Tregs with aging.
II.H. Aging, B cells, and the Humoral Immune Response
Another component of the immune system that is affected by aging is the B cell population and its ability to produce antibodies and function as APCs. B cell responses are essential for health: the production of antibodies is critical in controlling viral and bacterial infections throughout the life span. B cell activation is an orchestrated sequence of events that leads to the transcription and translation of Ig receptor genes, leading to receptor reorganization and Ig isotype switching (from IgM to IgG, IgA, or IgE). Antibody responses can be thymus-dependent or independent. Antigens that elicit thymus-independent responses do not involve T helper cells and can directly activate B cells to produce IgM antibodies. Thymus-independent responses are decreased in aged mice [396]. Thymus-dependent antibody synthesis happens after T cell-B cell interaction in secondary organs, such as the spleen, lymph nodes, Peyer’s patches, and other mucosa-associated lymphoid tissue like adenoids and tonsils. IgA is an Ig secreted in large quantities in the mucosal surfaces of the eye and the gut. Please see section II-A for a more complete description of IgA on the eye.
Aging is a paradoxical state, where there is a high incidence of cancer, autoimmunity, but a decrease in humoral responses to vaccinations and increased susceptibility to infections [362, 397]. The response to both new and previously encountered antigens is decreased in the elderly population; mechanisms include decreased generation of neutralizing antibodies, decreased affinity, and delayed response compared to young subjects [397, 398]. Other alterations in the B compartment have been described [399]. With aging, there is an increase in the frequency of memory B cells in the blood; however, the pool of naive B cells is impaired [362, 400, Kline, 1999 #4189]. It has been shown that in aged rodents, the population of mature B cells is maintained despite a decrease in bone marrow B cell progenitors [401].
A significant body of work has demonstrated that aging is accompanied by an increase of Ig in serum; this is true for mice and humans [362, 364, 371, 399, 402–404]. This may indicate the presence of long-lived memory B cells [362, 397, 400, 405]. However, the specific role of augmented age-related Igs is still a matter of debate. It has become clear that some of these antibodies are anti-single and double-stranded DNA and anti-nuclear antibodies (ANAs) [364, 400], and they are strain-dependent in mice. [406]. Similarly to humans, genetic predisposition influences the generation of autoantibodies [407]. Non-immune mouse strains, when immunized, produce the same kind of autoantibodies produced in autoimmune mice, such as (NZB X NZW) F1 mice [408]. Nusser and colleagues showed that aged mice had increased production of autoantibodies (ANAs) and IgM deposition in kidneys [364]. Most of the animals with ANA+ IgG also had lymphocytic infiltration in salivary glands. Increased production of autoantibodies was MHC II-dependent, as aged MHC II−/− mice did not show age-related increase in ANA+ antibodies. However, the precise mechanisms that led to T-cell dependency and how tolerance was broken was not fully explored [364]. It has been also postulated that generation of autoantibodies during aging is partially dependent on TLR7 and NF-κB pathways [405, 409, 410].
It has been demonstrated that in chronic inflammation and autoimmunity, ectopic lymphoid structures (or tertiary lymphoid organs) can also develop [411–413]. The ectopic lymphoid formation is highly dependent on CXCL13 and CCL21 chemokines, which attract CXCR5+B cells and CCR7+ T cells. In Sjögren Syndrome, CXCL13 and CCL21 are critical chemokines that participate in germinal center formation in exocrine glands [414, 415]. A recent report showed that aged C57BL/6 mice also develop ectopic lymphoid structures in the bladder. These aggregates were TNF-α dependent, but microbiota independent [370].
B cells are also potent APCs. With aging, there is an accumulation in the spleen of age-associated B cells (named “ABCs”) that are B220+ but CD21 and CD23 negative cells. These ABCs express T-bet and CD11c+, among other markers (such as CD11b+, B220+, CD19+, IgM+, and express CD80, CD86, and MHC II in high levels), are potent APCs, respond to TLR7 agonists, and produce high amounts of autoantibodies. These cells accumulate in the spleen of aged female mice and also increase in autoimmune lupus-prone mice at the time that autoantibodies rise [410, 416, 417]. The precise mechanism that leads to the increase of ABCs with aging is poorly understood.
Overall, aging affects numerous aspects of the systemic humoral immune response. How much of this change applies to the ocular mucosal surface is unknown, but it is tempting to speculate that the aged humoral response contributes to the immune dysfunction observed in the elderly given the weight of Igs in ocular surface homeostasis.
II.I. Aging and the Neuroimmune Regulation of the Ocular Mucosal Surface
The peripheral nervous system is at a vantage point for rapidly detecting threats to the ocular surface and not only inducing defensive behavior but also coordinating the immune response, as is the case for other mucosal sites [418]. Neuroregulation plays an essential role in ocular surface immune homeostasis, and this is in line with the dense innervation of the cornea and the intimate contact between corneal epithelial cells and intraepithelial nerves [419]. Sensory nerve fibers in the cornea express polymodal nociceptors, i.e., ion channels, that react to mechanical forces (e.g., Piezo2), heat, diverse chemical stimuli [e.g. transient receptor potential (TRP)V1 and TRPA1], and cold (e.g. TRPM8) [420]. Interestingly, aging is associated with a decrease in corneal nerve density (Figure 3D) and sensitivity to mechanical and nociceptive stimuli in mice, rats, and humans [421–424]. With aging also comes a reduction in basal tearing [82, 83], which is regulated by sensing of ocular wetness by TRPM8 cold thermoreceptors in the cornea [425]. In the conjunctiva, the innervation pattern is different: heat-sensitive fibers predominate, and mechanical and cold-sensing fibers are almost absent; also, there is a unique itch-mediating fiber population [426]. Also, the conjunctiva has an intriguing cholinergic chemosensory epithelial cell population with unknown properties, as reported for other mucosal surfaces [427].
Neuropeptides and neurotransmitters are the actual effectors through which the nervous system exerts its regulation. In addition to neurotrophic stimuli, sensory intraepithelial corneal nerves and unmyelinated conjunctival nerve fibers contribute neuropeptides substance P and calcitonin gene-related peptide to neuroimmune crosstalk at the ocular surface [428]. Also, parasympathetic nerves secrete vasoactive intestinal peptide, and sympathetic nerves secrete neuropeptide Y [429]. Regarding their trophic function, substance P and calcitonin gene-related peptide modulate E- and N-cadherin and zonula occludens-1 expression and favor mitotic activity and migration of corneal epithelial cells. Thus, these neuropeptides regulate corneal barrier function and wound healing. As a parasympathetic mediator, vasoactive intestinal peptide regulates the secretion of water and electrolytes in the lacrimal gland and of mucin by goblet cells [428]. By contrast, the function of neuropeptide Y is less understood. For a thorough review of neuropeptide biology in the ocular surface, see Sabatino et al. [428]. At least in mice, cholinergic (parasympathetic) signaling favors corneal epithelial wound healing and inhibits neutrophil recruitment, whereas noradrenergic (sympathetic) signaling promotes the latter [430]. Interestingly, these opposite effects seem to be mediated by different corneal limbal macrophage M1 and M2 populations that are either sensitive to norepinephrine or acetylcholine and secrete pro- or anti-inflammatory mediators, respectively [430].
The four main neuropeptides of the ocular surface coordinate the immune response by exerting effects on almost every cell type [428]. Although over-simplistic, it could be said that substance P is mostly a pro-inflammatory mediator whereas the other three (calcitonin gene-related peptide, vasoactive intestinal peptide, neuropeptide Y) are mainly anti-inflammatory. For instance, substance P promotes DC maturation and effector T cell differentiation, whereas vasoactive intestinal peptide favors tolerogenic DC condition, and Treg expansion and calcitonin gene-related peptide inhibit DC and macrophage activation [428]. The local coordination of the immune response by the nervous system is termed neurogenic inflammation, and it encompasses all the previously described effects of neuropeptide and neurotransmitter release upon nervous sensing of appropriate signals. In humans, tear levels of substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide increase after allergen challenge [431].
Interestingly, severing corneal nerve endings as it necessarily occurs in a corneal transplant leads to substance P release in the cornea and the anterior chamber of the eye; the consequence of this is the abrogation of immune privilege [432]. A similar effect was described for retinal laser burns, also involving substance P [433]. Intriguingly, upon unilateral damage to one eye, substance P release takes place in both eyes concomitantly through trigeminal signaling to the brainstem, which could be considered evidence of an ocular-trigeminal neurogenic inflammatory axis [434]. Although not immune-privileged, the ocular surface also displays a comparable bilateral neurogenic inflammatory reflex: it involves TRPV1 nociceptor signaling after injury of corneal and conjunctival nerve endings in the afferent arm and substance P release in the efferent arm [435]. For a thorough review of the bilateral immune reactions of the ocular surface, see Yamaguchi et al. [436].
On the one hand, there is a significant body of evidence in the literature that neuroregulation of the immune response plays a crucial role in ocular surface homeostasis and also in the pathophysiology of dry eye, allergic reactions, and corneal allograft rejection. On the other hand, there is also published data on the effect of aging on ocular surface nerve morphology and function. However, how aging might affect ocular surface neuroimmune regulation has not been addressed yet. Interestingly, substance P levels in the ocular surface decrease with aging, and topical supplementation of substance P can reverse some of the age-related changes in corneal nerve density and sensitivity [423]. It is tempting to speculate that aging could negatively impact the neurogenic inflammatory capacity of the ocular surface, and thus contribute to the increased susceptibility to ocular infections in the elderly.
III. Diseases of Aging that relate to the Ocular Surface or Effect of Aging on Ocular Surface Diseases?
As detailed in the previous sections, biological aging brings about changes to almost every aspect of the eye’s mucosal immune system (Figure 4). Although these alterations are diverse, the balance is tipped towards dysregulation of the immune response and more pro-inflammatory than anti-inflammatory effects. Paradoxically, some of these changes are also associated with dysfunctional immune reactions, so there is also the issue of the reduced efficacy of the immune responses with aging. Thus, it is interesting to consider the effect of aging on the ocular mucosal immune system when studying diseases that are more common in the elderly.
Figure 4: Schematic mucosal immune response of the aged ocular surface.
Summary of the most relevant age-related changes presented in this review. As for figure 1, a simplified, 6-step approach to the mucosal immune response to diverse antigens is shown in the context of aging. Age-related changes are described in red italics, affecting steps 1 (antigen interaction with the mucosal lining), 2 (the innate immune response), 3 (antigen presentation), 4 (generation of a T cell response), 5 (generation of a humoral response), and 6 (neuroimmune interactions). See Figure 1 for a more detailed description of each step.
Although dry eye disease is now observed in young adults, [437] its prevalence increases with age in each decade of life after 40 years of age [437–442]. Dry eye disease is one of the most common reasons to seek eye care and carries a significant economic burden to individuals and society [443–446]. Dry eye disease is characterized by tear film abnormalities, inflammation, and nerve alterations [65]. Clinical signs of the disease include decreased tear stability, decreased tear production, increased epitheliopathy, and loss of goblet cells and symptoms that can vary from very mild to very debilitating. Dry eye disease is an umbrella term for very heterogeneous disease manifestations, and that may have different underlying pathogenesis: from meibomian gland dysfunction to LASIK-induced dry eye to Sjögren’s Syndrome to age-related dry eye. There is a well-established disconnection between signs and symptoms in dry eye disease, although some studies have suggested that aged subjects have less corneal epitheliopathy and fewer dryness symptoms but shorter tear-break-up-time and low Schirmer test results than young subjects [447–449].
Because dry eye is so prevalent in the middle-aged and elderly population, it is challenging to separate aging from age-related dry eye [71]. In a very elegant study, Whaley and colleagues investigated the prevalence of dry eye (diagnosed by low Schirmer II test and corneal staining) in four age groups (5–15, 16–30, 31–80, and 81–93 years) in non-ophthalmologic populations of both sexes. These subjects were recruited from either orthopic clinics, orthopedic outpatient surgeries, or general clinics, and they were classified into definitive dry eye (if both low Schirmer and presence of epitheliopathy) or probable dry eye (if only low Schirmer tests were present). They showed that there was no dry eye in the two young groups, and the prevalence of dry eye (combining possible and definitive) was close to 63% in the geriatric population [71]. This is a landmark study in dry eye prevalence because it investigated not only a general population, but ophthalmic evaluations were performed. However, natural history studies of dry eye and the effects of aging on the severity of the disease lack in the literature, although cross-sectional studies have been attempted [442].
Increased chronological age is a confounding factor in the diagnosis of Sjögren syndrome. Similar to dry eye, dry mouth prevalence increases with age [450]. It has been reported that “elderly onset Sjögren syndrome” patients (65 years or older) may have similar manifestations than patients younger than 65 years; however, some studies showed milder symptoms and/or low frequency of antibodies [451–455]. The higher frequency of histologic alterations in minor salivary gland biopsies in young, healthy, and elderly subjects further complicates the diagnosis of Sjögren syndrome in the elderly [456]. As discussed above, histologic evaluations of cadaveric lacrimal glands also showed lymphocytic infiltration in a high percentage of the cases, suggesting an underappreciated role of these lymphocytes in the pathogenesis of age-related dry eye [141, 148].
Finally, the effect of polypharmacy in the elderly should not be discarded as a risk factor for dry eye, as many drugs have unwanted drying effects on mucosal surfaces, such as antihistamines (see review by Fraunfelder and colleagues [457] and also the National Eye Drug registry (http://www.eyedrugregistry.com/). All of these confounders should be taken into account when studying the aged ocular surface.
IV. Conclusions
In conclusion, the ocular surface functional unit, which consists of the cornea, limbus, conjunctiva, eyelids, meibomian and lacrimal glands, and nerves, is not only subjected to aging but also to the inflammation that comes with aging. The ocular surface immune system undergoes dysregulatory changes with aging as well, which could explain the aforementioned inflammatory increase. There are, however, several aspects of the ocular mucosal immune response delineated in this review that have not been considered under the light of aging. Moreover, in many instances it is difficult to separate the effect of aging from those of inflammatory dry eye, a highly frequent disease whose prevalence increases with age. Thus, further studies are needed to increase our knowledge of the biology of aging and how it applies to the ocular surface. In this regard, there are many lessons to be learned from the aged immune system as a whole and its impact on the eye. It is possible that therapies aimed at improving the aged immune system systemically might also have a beneficial effect on the ocular surface.
Acknowledgments
We are very grateful to Zhiyuan Yu and Leiqi Zhang for expert technical assistance and management of mice colonies, and Sarah Amra for the histology preparation.
Support: This work was supported by NIH EY026893 and EY030447 (CSDP); NIH/NEI EY002520 (Core Grant for Vision Research Department of Ophthalmology); NIH Pathology Core (P30CA125123), Baylor Cytometry and Cell Sorting Core [CPRIT Core Facility Support Award (CPRIT-RP180672), P30 Cancer Center Support Grant (NCI-CA125123), NIH-RR024574, and NIH S10 OD025251 (Union BioMetrica BioSorter), Research to Prevent Blindness (unrestricted grant to the Dept. Of Ophthalmology), The Hamill Foundation, and The Sid Richardson Foundation. Further research support was provided by ARVO Roche Collaborative Research Fellowship (JGG), Agencia Nacional de Promoción Científica y Técnica de Argentina (PICT 2013-1436, 2015-0971, PICT 2018-02911).
Abbreviations
- ABC
age-associated B cells
- ANAs
anti-nuclear antibodies
- APC
antigen-presenting cell
- CALT
conjunctiva-associated lymphoid tissue
- CCL
C-C chemokine ligand (CCL)
- CCR
C-C chemokine receptor
- CXCL
CXC chemokine ligand
- CXCR
CXC chemokine receptor
- DC
dendritic cell
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- ILC
innate lymphoid cell
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- NF
nuclear factor
- NLR
nucleotide-binding and oligomerization domain-like receptor
- NK
natural killer
- RA
retinoic acid
- TGF
transforming growth factor
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- Treg
regulatory T cell
- TRP
transient receptor potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: No financial interests to disclose.
References
- [1].Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. A unified theory of the role of the ocular surface in dry eye. AdvExpMedBiol. 1998;438:643–51. [DOI] [PubMed] [Google Scholar]
- [2].Cher I Ocular surface concepts: Development and citation. Ocular Surface. 2014;12:10–3. [DOI] [PubMed] [Google Scholar]
- [3].Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. JAnat. 2005;206:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Galletti JG, Guzman M, Giordano MN. Mucosal immune tolerance at the ocular surface in health and disease. Immunology. 2017;150:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Foulsham W, Coco G, Amouzegar A, Chauhan SK, Dana R. When Clarity Is Crucial: Regulating Ocular Surface Immunity. Trends in immunology. 2018;39:288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nations U. World Population Prospects 2019. In: Affairs DoEaS, editor. https://population.un.org/wpp2019. [Google Scholar]
- [7].Medawar PB. An Unsolved Problem of Biology: An Inaugural Lecture Delivered at University College, London, 6 December, 1951: H.K. Lewis and Company; 1952. [Google Scholar]
- [8].Harman D The aging process. Proc Natl Acad Sci U S A. 1981;78:7124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kyryakov P, Gomez-Perez A, Glebov A, Asbah N, Bruno L, Meunier C, et al. Empirical verification of evolutionary theories of aging. Aging (Albany NY). 2016;8:2568–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Skulachev VP. Aging is a specific biological function rather than the result of a disorder in complex living systems: biochemical evidence in support of Weismann’s hypothesis. Biochemistry (Mosc). 1997;62:1191–5. [PubMed] [Google Scholar]
- [11].Skulachev MV, Skulachev VP. Programmed Aging of Mammals: Proof of Concept and Prospects of Biochemical Approaches for Anti-aging Therapy. Biochemistry (Mosc). 2017;82:1403–22. [DOI] [PubMed] [Google Scholar]
- [12].Harman D Nutritional implications of the free-radical theory of aging. J Am Coll Nutr. 1982;1:27–34. [DOI] [PubMed] [Google Scholar]
- [13].Franceschi C, Valensin S, Fagnoni F, Barbi C, BonaFe M. Biomarkers of immunosenescence within an evolutionary perspective: the challenge of heterogeneity and the role of antigenic load. ExpGerontol. 1999;34:911–21. [DOI] [PubMed] [Google Scholar]
- [14].Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–9. [DOI] [PubMed] [Google Scholar]
- [15].Troen BR. The biology of aging. MtSinai JMed. 2003;70:3–22. [PubMed] [Google Scholar]
- [16].De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15:3003–26. [DOI] [PubMed] [Google Scholar]
- [17].Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011;90:830–40. [DOI] [PubMed] [Google Scholar]
- [18].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Loaiza N, Demaria M. Cellular senescence and tumor promotion: Is aging the key? Biochim Biophys Acta. 2016;1865:155–67. [DOI] [PubMed] [Google Scholar]
- [20].Aunan JR, Watson MM, Hagland HR, Soreide K. Molecular and biological hallmarks of ageing. Br J Surg. 2016;103:e29–46. [DOI] [PubMed] [Google Scholar]
- [21].Franceschi C, BonaFe M, Valensin S, Olivieri F, De LM, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. AnnNYAcadSci. 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- [22].Gerli R, Monti D, Bistoni O, Mazzone AM, Peri G, Cossarizza A, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. MechAgeing Dev. 2000;121:37–46. [DOI] [PubMed] [Google Scholar]
- [23].Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. CurrOpinHematol. 2001;8:131–6. [DOI] [PubMed] [Google Scholar]
- [24].Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. [DOI] [PubMed] [Google Scholar]
- [25].Carrieri G, Marzi E, Olivieri F, Marchegiani F, Cavallone L, Cardelli M, et al. The G/C915 polymorphism of transforming growth factor beta1 is associated with human longevity: a study in Italian centenarians. Aging cell. 2004;3:443–8. [DOI] [PubMed] [Google Scholar]
- [26].Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. JAmGeriatrSoc. 1999;47:639–46. [DOI] [PubMed] [Google Scholar]
- [27].Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. AmJMed. 2003;115:278–83. [DOI] [PubMed] [Google Scholar]
- [28].Borras C, Ingles M, Mas-Bargues C, Dromant M, Sanz-Ros J, Román-Domínguez A, et al. Centenarians: An excellent example of resilience for successful ageing. Mech Ageing Dev. 2020;186:111199. [DOI] [PubMed] [Google Scholar]
- [29].Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. BrJ Ophthalmol. 1999;83:737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhou L, Huang LQ, Beuerman RW, Grigg ME, Li SF, Chew FT, et al. Proteomic analysis of human tears: defensin expression after ocular surface surgery. J ProteomeRes. 2004;3:410–6. [DOI] [PubMed] [Google Scholar]
- [31].Vinding T, Eriksen JS, Nielsen NV. The concentration of lysozyme and secretory IgA in tears from healthy persons with and without contact lens use. Acta Ophthalmol (Copenh). 1987;65:23–6. [DOI] [PubMed] [Google Scholar]
- [32].Jensen OL, Gluud BS, Birgens HS. The concentration of lactoferrin in tears of normals and of diabetics. Acta Ophthalmol(Copenh). 1986;64:83–7. [DOI] [PubMed] [Google Scholar]
- [33].Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nolan J Evaluation of conjunctival and nasal bacterial cultures before intra-ocular operations. Br J Ophthalmol. 1967;51:483–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fahmy JA, Moller S, Bentzon MW. Bacterial flora in relation to cataract extraction. II. Peroperative flora. Acta Ophthalmol (Copenh). 1975;53:476–94. [DOI] [PubMed] [Google Scholar]
- [36].Doan T, Akileswaran L, Andersen D, Johnson B, Ko N, Shrestha A, et al. Paucibacterial Microbiome and Resident DNA Virome of the Healthy Conjunctiva. Invest Ophthalmol Vis Sci. 2016;57:5116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Doan T, Akileswaran L, Andersen D, Ko N, Shrestha A, Lee CS, et al. Human Ocular Surface Microbiome Composition Revealed By Next-Generation Sequencing. Invest Ophthalmol Vis Sci. 2015;56:4067-. [Google Scholar]
- [38].Ozkan J, Willcox M, Wemheuer B, Wilcsek G, Coroneo M, Thomas T. Biogeography of the human ocular microbiota. The ocular surface. 2019;17:111–8. [DOI] [PubMed] [Google Scholar]
- [39].Ozkan J, Willcox MD. The Ocular Microbiome: Molecular Characterisation of a Unique and Low Microbial Environment. Curr Eye Res. 2019;44:685–94. [DOI] [PubMed] [Google Scholar]
- [40].Willis KA, Postnikoff CK, Freeman A, Rezonzew G, Nichols K, Gaggar A, et al. The closed eye harbours a unique microbiome in dry eye disease. Sci Rep. 2020;10:12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Z, Gong Y, Chen S, Li S, Zhang Y, Zhong H, et al. Comparative portrayal of ocular surface microbe with and without dry eye. J Microbiol. 2019. [DOI] [PubMed] [Google Scholar]
- [42].Lee SHOD, Jung JY, Kim JC, Jeon CO. Comparative Ocular Microbial Communities in Humans with and without Blepharitis. Invest Ophthalmol Vis Sci 2012;53:5585–93. [DOI] [PubMed] [Google Scholar]
- [43].Miller D, Iovieno A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol. 2009;9:466–70. [DOI] [PubMed] [Google Scholar]
- [44].Zhang H, Zhao F, Hutchinson DS, Sun W, Ajami NJ, Lai S, et al. Conjunctival Microbiome Changes Associated With Soft Contact Lens and Orthokeratology Lens Wearing. Invest Ophthalmol Vis Sci. 2017;58:128–36. [DOI] [PubMed] [Google Scholar]
- [45].Watters GA, Turnbull PR, Swift S, Petty A, Craig JP. Ocular surface microbiome in meibomian gland dysfunction in Auckland, New Zealand. Clin Exp Ophthalmol. 2016. [DOI] [PubMed] [Google Scholar]
- [46].de Paiva CS, Jones DB, Stern ME, Bian F, Moore QL, Corbiere S, et al. Altered Mucosal Microbiome Diversity and Disease Severity in Sjogren Syndrome. Sci Rep. 2016;6:23561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Andersson J, Vogt JK, Dalgaard MD, Pedersen O, Holmgaard K, Heegaard S. Ocular surface microbiota in patients with aqueous tear-deficient dry eye. The ocular surface. 2020. [DOI] [PubMed] [Google Scholar]
- [48].Zilliox MJ, Gange WS, Kuffel G, Mores CR, Joyce C, de Bustros P, et al. Assessing the ocular surface microbiome in severe ocular surface diseases. The ocular surface. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ozkan J, Nielsen S, Diez-Vives C, Coroneo M, Thomas T, Willcox M. Temporal Stability and Composition of the Ocular Surface Microbiome. Sci Rep. 2017;7:9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cavuoto KM, Banerjee S, Miller D, Galor A. Composition and Comparison of the Ocular Surface Microbiome in Infants and Older Children. Translational Vision Science & Technology. 2018;7:16-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wan SJ, Sullivan AB, Shieh P, Metruccio MME, Evans DJ, Bertozzi CR, et al. IL-1R and MyD88 Contribute to the Absence of a Bacterial Microbiome on the Healthy Murine Cornea. Front Microbiol. 2018;9:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell host & microbe. 2017;21:455–66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sovran B, Hugenholtz F, Elderman M, Van Beek AA, Graversen K, Huijskes M, et al. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Sci Rep. 2019;9:1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bodogai M, O’Connell J, Kim K, Kim Y, Moritoh K, Chen C, et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci Transl Med. 2018;10:eaat4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cho SY, Kim J, Lee JH, Sim JH, Cho DH, Bae IH, et al. Modulation of gut microbiota and delayed immunosenescence as a result of syringaresinol consumption in middle-aged mice. Sci Rep. 2016;6:39026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Elderman M, Sovran B, Hugenholtz F, Graversen K, Huijskes M, Houtsma E, et al. The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLoS One. 2017;12:e0184274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang C, Zaheer M, Bian F, Quach D, Swennes AG, Britton RA, et al. Sjogren-Like Lacrimal Keratoconjunctivitis in Germ-Free Mice. Int J Mol Sci. 2018;19:pii: E565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zaheer M, Wang C, Bian F, Yu Z, Hernandez H, de Souza RG, et al. Protective role of commensal bacteria in Sjogren Syndrome. J Autoimmun. 2018;pii: S0896–8411:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hansen CH, Yurkovetskiy LA, Chervonsky AV. Cutting Edge: Commensal Microbiota Has Disparate Effects on Manifestations of Polyglandular Autoimmune Inflammation. J Immunol. 2016;197:701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wen X, Miao L, Deng Y, Bible PW, Hu X, Zou Y, et al. The Influence of Age and Sex on Ocular Surface Microbiota in Healthy Adults. Invest Ophthalmol Vis Sci. 2017;58:6030–7. [DOI] [PubMed] [Google Scholar]
- [62].Suzuki T, Sutani T, Nakai H, Shirahige K, Kinoshita S. The Microbiome of the Meibum and Ocular Surface in Healthy Subjects. Invest Ophthalmol Vis Sci. 2020;61:18-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].St Leger AJ, Desai JV, Drummond RA, Kugadas A, Almaghrabi F, Silver P, et al. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal gammadelta T Cells. Immunity. 2017;47:148–58.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kugadas A, Christiansen SH, Sankaranarayanan S, Surana NK, Gauguet S, Kunz R, et al. Impact of Microbiota on Resistance to Ocular Pseudomonas aeruginosa-Induced Keratitis. PLoS Pathog. 2016;12:e1005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. The ocular surface. 2017. [DOI] [PubMed] [Google Scholar]
- [66].Yoshino K, Monroy D, Pflugfelder SC. Cholinergic stimulation of lactoferrin and epidermal growth factor secretion by the human lacrimal gland. Cornea. 1996;15:617–21. [PubMed] [Google Scholar]
- [67].Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bromberg BB, Cripps MM, Welch MH. Sympathomimetic protein secretion by young and aged lacrimal gland. Curr Eye Res. 1986;5:217–23. [DOI] [PubMed] [Google Scholar]
- [69].Bromberg BB, Welch MH. Lacrimal protein secretion: comparison of young and old rats. Exp Eye Res. 1985;40:313–20. [DOI] [PubMed] [Google Scholar]
- [70].Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–9. [DOI] [PubMed] [Google Scholar]
- [71].Whaley K, Williamson J, Wilson T, McGavin DD, Hughes GR, Hughes H, et al. Sjogren’s syndrome and autoimmunity in a geriatric population. Age Ageing. 1972;1:197–206. [DOI] [PubMed] [Google Scholar]
- [72].Wright JC, Meger GE. A review of the Schirmer test for tear production. Arch Ophthalmol. 1962;67:564–5. [DOI] [PubMed] [Google Scholar]
- [73].Norn MS. Tear secretion in normal eyes. Estimated by a new method: the lacrimal streak dilution test. Acta Ophthalmol (Copenh). 1965;43:567–73. [DOI] [PubMed] [Google Scholar]
- [74].de Rotth A On the hypofunction of the lacrimal gland. Am J Ophthalmology 1941;24,:20–5. [Google Scholar]
- [75].Henderson JW, Prough WA. Influence of age and sex on flow of tears. Arch Ophthal. 1950;43:224–31. [DOI] [PubMed] [Google Scholar]
- [76].Mathers WD, Lane JA, Zimmerman MB. Tear film changes associated with normal aging. Cornea. 1996;15:229–34. [DOI] [PubMed] [Google Scholar]
- [77].Shikama Y, Kurosawa M, Furukawa M, Ishimaru N, Matsushita K. Involvement of adiponectin in age-related increases in tear production in mice. Aging (Albany NY). 2019;11:8329–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hamano T, Mitsunaga S, Kotani S, Hamano T, Hamano K, Hamano H, et al. Tear volume in relation to contact lens wear and age. CLAO J. 1990;16:57–61. [PubMed] [Google Scholar]
- [79].Patel S, Wallace I. Tear meniscus height, lower punctum lacrimale, and the tear lipid layer in normal aging. OptomVisSci. 2006;83:731–9. [DOI] [PubMed] [Google Scholar]
- [80].Ozdemir M, Temizdemir H. Age- and gender-related tear function changes in normal population. Eye (Lond). 2010;24:79–83. [DOI] [PubMed] [Google Scholar]
- [81].Cui L, Shen M, Wang J, Jiang J, Li M, Chen D, et al. Age-related changes in tear menisci imaged by optical coherence tomography. OptomVisSci. 2011;88:1214–9. [DOI] [PubMed] [Google Scholar]
- [82].Furukawa RE, Polse KA. Changes in tear flow accompanying aging. AmJOptomPhysiol Opt. 1978;55:69–74. [DOI] [PubMed] [Google Scholar]
- [83].McClellan AJ, Volpe EA, Zhang X, Darlington GJ, Li DQ, Pflugfelder SC, et al. Ocular Surface Disease and Dacryoadenitis in Aging C57BL/6 Mice. Am J Pathol. 2014;184:631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sullivan DA, Hann LE, Yee L, Allansmith MR. Age- and gender-related influence on the lacrimal gland and tears. Acta Ophthalmol(Copenh). 1990;68:188–94. [DOI] [PubMed] [Google Scholar]
- [85].De Silva MEH, Hill LJ, Downie LE, Chinnery HR. The Effects of Aging on Corneal and Ocular Surface Homeostasis in Mice. Invest Ophthalmol Vis Sci. 2019;60:2705–15. [DOI] [PubMed] [Google Scholar]
- [86].Yoon CHR JS; Hwang HS; Kim MK. Comparative Analysis of Age-Related Changes in Lacrimal Glands and Meibomian Glands of a C57BL/6 Male Mouse Model. Int J Mol Sci 2020. 2020;21:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].de Souza RG, de Paiva CS, Alves MR. Age-related Autoimmune Changes in Lacrimal Glands. Immune Netw. 2019;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ebersole JL, Steffen MJ, Pappo J. Secretory immune responses in ageing rats. II. Phenotype distribution of lymphocytes in secretory and lymphoid tissues. Immunology. 1988;64:289–94. [PMC free article] [PubMed] [Google Scholar]
- [89].Draper CE, Singh J, Adeghate E. Effects of age on morphology, protein synthesis and secretagogue-evoked secretory responses in the rat lacrimal gland. Mol Cell Biochem. 2003;248:7–16. [DOI] [PubMed] [Google Scholar]
- [90].Draper CE, Adeghate EA, Singh J, Pallot DJ. Evidence to suggest morphological and physiological alterations of lacrimal gland acini with ageing. Exp Eye Res. 1999;68:265–76. [DOI] [PubMed] [Google Scholar]
- [91].Draper CE, Adeghate E, Lawrence PA, Pallot DJ, Garner A, Singh J. Age-related changes in morphology and secretory responses of male rat lacrimal gland. J Auton Nerv Syst. 1998;69:173–83. [DOI] [PubMed] [Google Scholar]
- [92].Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, et al. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995;25:823–9. [DOI] [PubMed] [Google Scholar]
- [93].You IC, Bian F, Volpe EA, de Paiva CS, Pflugfelder SC. Age-related conjunctival disease in the C57BL/6.NOD-Aec1Aec2 Mouse Model of Sjogren Syndrome develops independent of lacrimal dysfunction. Invest Ophthalmol Vis Sci. 2015;56:2224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ananthi S, Santhosh RS, Nila MV, Prajna NV, Lalitha P, Dharmalingam K. Comparative proteomics of human male and female tears by two-dimensional electrophoresis. Exp Eye Res. 2011;92:454–63. [DOI] [PubMed] [Google Scholar]
- [95].Nattinen J, Jylha A, Aapola U, Makinen P, Beuerman R, Pietila J, et al. Age-associated changes in human tear proteome. Clin Proteomics. 2019;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gagliano C, A R; Pizzo Alessandra; Pezzino S;Rusciano D Age-related differential efficacy of dry eye of lactobionic-acid based eye drops. Paripex-indian journal of research. 2018;VII:523–6. [Google Scholar]
- [97].Patel R, Zhu M, Robertson DM. Shifting the IGF-axis: An age-related decline in human tear IGF-1 correlates with clinical signs of dry eye. Growth Horm IGF Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Micera A, Stampachiacchiere B, Di Zazzo A, Sgrulletta R, Cortes M, Normando EM, et al. NGF Modulates trkANGFR/p75NTR in alphaSMA-Expressing Conjunctival Fibroblasts from Human Ocular Cicatricial Pemphigoid (OCP). PLoS One. 2015;10:e0142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Micera A, Di Zazzo A, Esposito G, Longo R, Foulsham W, Sacco R, et al. Age-Related Changes to Human Tear Composition. Invest Ophthalmol Vis Sci. 2018;59:2024–31. [DOI] [PubMed] [Google Scholar]
- [100].Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006;25:651–5. [DOI] [PubMed] [Google Scholar]
- [103].Alghamdi YA, Mercado C, McClellan AL, Batawi H, Karp CL, Galor A. Epidemiology of Meibomian Gland Dysfunction in an Elderly Population. Cornea. 2016;35:731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11:334–42. [DOI] [PubMed] [Google Scholar]
- [105].Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21:S70–4. [DOI] [PubMed] [Google Scholar]
- [106].Robin JB, Jester JV, Nobe J, Nicolaides N, Smith RE. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction. Ophthalmology. 1985;92:1423–6. [DOI] [PubMed] [Google Scholar]
- [107].Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. AmJOphthalmol. 1982;94:383–7. [DOI] [PubMed] [Google Scholar]
- [108].Hom MM, Martinson JR, Knapp LL, Paugh JR. Prevalence of meibomian gland dysfunction. Optom& VisSci. 1990;67:710–2. [DOI] [PubMed] [Google Scholar]
- [109].Mathers WD, Shields WJ, Sachdev MS, Petroll WM, Jester JV. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10:277–85. [DOI] [PubMed] [Google Scholar]
- [110].Wei A, Hong J, Sun X, Xu J. Evaluation of age-related changes in human palpebral conjunctiva and meibomian glands by in vivo confocal microscopy. Cornea. 2011;30:1007–12. [DOI] [PubMed] [Google Scholar]
- [111].Farina AR, Coppa A, Tiberio A, Tacconelli A, Turco A, Colletta G, et al. Transforming growth factor-á1 enhances the invasiveness of human MDA-MB-231 breast cancer cells by up-regulating urokinase activity. IntJCancer. 1998;75:721–30. [DOI] [PubMed] [Google Scholar]
- [112].Richards SM, Yamagami H, Schirra F, Suzuki T, Jensen RV, Sullivan DA. Sex-related effect on gene expression in the mouse meibomian gland. Curr Eye Res. 2006;31:119–28. [DOI] [PubMed] [Google Scholar]
- [113].Jester JV, Rife L, Nii D, Luttrull JK, Wilson L, Smith RE. In vivo biomicrocropy of meibomian glands in a rabbit model of meibomian gland dysfunction. InvestOphthalmolVisSci. 1982;22:660–7. [PubMed] [Google Scholar]
- [114].Nien CJ, Massei S, Lin G, Nabavi C, Tao J, Brown DJ, et al. Effects of age and dysfunction on human meibomian glands. Arch Ophthalmol. 2011;129:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Parfitt GJ, Brown DJ, Jester JV. Transcriptome analysis of aging mouse meibomian glands. Mol Vis. 2016;22:518–27. [PMC free article] [PubMed] [Google Scholar]
- [116].Jester BE, Nien CJ, Winkler M, Brown DJ, Jester JV. Volumetric reconstruction of the mouse meibomian gland using high-resolution nonlinear optical imaging. Anatomical record (Hoboken, NJ : 2007). 2011;294:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Nien CJ, Paugh JR, Massei S, Wahlert AJ, Kao WW, Jester JV. Age-related changes in the meibomian gland. Exp Eye Res. 2009;89:1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Parfitt GJ, Xie Y, Geyfman M, Brown DJ, Jester JV. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD). Aging (Albany NY). 2013;5:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Parfitt GJ, Lewis PN, Young RD, Richardson A, Lyons JG, Di Girolamo N, et al. Renewal of the Holocrine Meibomian Glands by Label-Retaining, Unipotent Epithelial Progenitors. Stem Cell Reports. 2016;7:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Villani E, Canton V, Magnani F, Viola F, Nucci P, Ratiglia R. The aging Meibomian gland: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2013;54:4735–40. [DOI] [PubMed] [Google Scholar]
- [121].van Setten GB. Impact of Attrition, Intercellular Shear in Dry Eye Disease: When Cells are Challenged and Neurons are Triggered. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lee OL, Tepelus TC, Huang J, Irvine AG, Irvine C, Chiu GB, et al. Evaluation of the corneal epithelium in non-Sjögren’s and Sjögren’s dry eyes: an in vivo confocal microscopy study using HRT III RCM. BMC Ophthalmol. 2018;18:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yang Y, Hong J, Deng SX, Xu J. Age-related changes in human corneal epithelial thickness measured with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:5032–8. [DOI] [PubMed] [Google Scholar]
- [124].Deinema LA, Vingrys AJ, Chinnery HR, Downie LE. Optical Coherence Tomography Reveals Changes to Corneal Reflectivity and Thickness in Individuals with Tear Hyperosmolarity. Transl Vis Sci Technol. 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye & Contact Lens. 2005;31(5)::186–93. [DOI] [PubMed] [Google Scholar]
- [126].Guzmán M, Miglio M, Keitelman I, Shiromizu CM, Sabbione F, Fuentes F, et al. Transient tear hyperosmolarity disrupts the neuroimmune homeostasis of the ocular surface and facilitates dry eye onset. Immunology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Stepp MA, Pal-Ghosh S, Tadvalkar G, Williams AR, Pflugfelder SC, de Paiva CS. Reduced Corneal Innervation in the CD25 Null Model of Sjogren Syndrome. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Inomata T, Mashaghi A, Hong J, Nakao T, Dana R. Scaling and maintenance of corneal thickness during aging. PLoS One. 2017;12:e0185694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Makarenkova HP, Dartt DA. Myoepithelial Cells: Their Origin and Function in Lacrimal Gland Morphogenesis, Homeostasis, and Repair. Curr Mol Biol Rep. 2015;1:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Nguyen DH, Beuerman RW, Thompson HW, DiLoreto DA. Growth factor and neurotrophic factor mRNA in human lacrimal gland. Cornea. 1997;16:192–9. [PubMed] [Google Scholar]
- [131].Sullivan DA, Yee L, Conner AS, Hann LE, Olivier M, Allansmith MR. Influence of ocular surface antigen on the postnatal accumulation of immunoglobulin-containing cells in the rat lacrimal gland. Immunology. 1990;71:573–80. [PMC free article] [PubMed] [Google Scholar]
- [132].Gudmundsson OG, Sullivan DA, Bloch KJ, Allansmith MR. The ocular secretory immune system of the rat. Exp Eye Res. 1985;40:231–8. [DOI] [PubMed] [Google Scholar]
- [133].Knop E, Knop N, Claus P. Local production of secretory IgA in the eye-associated lymphoid tissue (EALT) of the normal human ocular surface. Invest Ophthalmol Vis Sci. 2008;49:2322–9. [DOI] [PubMed] [Google Scholar]
- [134].Gudmundsson OG, Bjornsson J, Olafsdottir K, Bloch KJ, Allansmith MR, Sullivan DA. T cell populations in the lacrimal gland during aging. Acta Ophthalmol (Copenh). 1988;66:490–7. [DOI] [PubMed] [Google Scholar]
- [135].Sashima M, Hatakeyama S, Satoh M, Suzuki A. Harderianization is another sexual dimorphism of rat exorbital lacrimal gland. Acta Anat (Basel). 1989;135:303–6. [DOI] [PubMed] [Google Scholar]
- [136].Ferrara D, Monteforte R, Baccari GC, Minucci S, Chieffi G. Androgen and estrogen receptors expression in the rat exorbital lacrimal gland in relation to “harderianization”. J Exp Zool A Comp Exp Biol. 2004;301:297–306. [DOI] [PubMed] [Google Scholar]
- [137].Cornell-Bell AH, Sullivan DA, Allansmith MR. Gender-related differences in the morphology of the lacrimal gland. Invest Ophthalmol Vis Sci. 1985;26:1170–5. [PubMed] [Google Scholar]
- [138].Rios JD, Horikawa Y, Chen LL, Kublin CL, Hodges RR, Dartt DA, et al. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. ExpEye Res. 2005;80:477–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Batista TM, Tomiyoshi LM, Dias AC, Roma LP, Modulo CM, Malki LT, et al. Age-dependent changes in rat lacrimal gland anti-oxidant and vesicular related protein expression profiles. MolVis. 2012;18:194–202. [PMC free article] [PubMed] [Google Scholar]
- [140].Williams RM, Singh J, Sharkey KA. Innervation and mast cells of the rat exorbital lacrimal gland: the effects of age. J Auton Nerv Syst. 1994;47:95–108. [DOI] [PubMed] [Google Scholar]
- [141].Damato BE, Allan D, Murray SB, Lee WR. Senile atrophy of the human lacrimal gland: the contribution of chronic inflammatory disease. Br J Ophthalmol. 1984;68:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Segerberg-Konttinen M Focal adenitis in lacrimal and salivary glands. A post-mortem study. Scand J Rheumatol. 1988;17:379–85. [DOI] [PubMed] [Google Scholar]
- [143].El-Fadaly AB, El-Shaarawy EA, Rizk AA, Nasralla MM, Shuaib DM. Age-related alterations in the lacrimal gland of adult albino rat: a light and electron microscopic study. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2014;196:336–51. [DOI] [PubMed] [Google Scholar]
- [144].Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging. Ophthalmology. 1995;102:678–86. [DOI] [PubMed] [Google Scholar]
- [145].Xu KP, Katagiri S, Takeuchi T, Tsubota K. Biopsy of labial salivary glands and lacrimal glands in the diagnosis of Sjögren’s syndrome. J Rheumatol. 1996;23:76–82. [PubMed] [Google Scholar]
- [146].Williamson J, Gibson AA, Wilson T, Forrester JV, Whaley K, Dick WC. Histology of the lacrimal gland in keratoconjunctivitis sicca. Br J Ophthalmol. 1973;57:852–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ. Sjogren syndrome: a clinical, pathological, and serological study of sixty-two cases. Medicine. 1965;44:187–231. [PubMed] [Google Scholar]
- [148].Catoggio LJ, Skinner RP, Smith G, Maddison PJ. Systemic lupus erythematosus in the elderly: clinical and serological characteristics. J Rheumatol. 1984;11:175–81. [PubMed] [Google Scholar]
- [149].Jin K, Imada T, Hisamura R, Ito M, Toriumi H, Tanaka KF, et al. Identification of Lacrimal Gland Postganglionic Innervation and Its Regulation of Tear Secretion. Am J Pathol. 2020;190:1068–79. [DOI] [PubMed] [Google Scholar]
- [150].Pitcher J III, de Paiva CS, Pelegrino F, McClellan A, Raince J, Pangelinan S, et al. Pharmacological cholinergic blockade stimulates inflammatory cytokine production and lymphocytic infiltration in the mouse lacrimal gland. Invest OphthalmolVisSci. 2011;52:3221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Das T, Chen Z, Hendriks RW, Kool M. A20/Tumor Necrosis Factor α-Induced Protein 3 in Immune Cells Controls Development of Autoinflammation and Autoimmunity: Lessons from Mouse Models. Front Immunol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Okuma A, Hoshino K, Ohba T, Fukushi S, Aiba S, Akira S, et al. Enhanced apoptosis by disruption of the STAT3-IkappaB-zeta signaling pathway in epithelial cells induces Sjogren’s syndrome-like autoimmune disease. Immunity. 2013;38:450–60. [DOI] [PubMed] [Google Scholar]
- [153].Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol. 2017;8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Lima-Costa MF, Melo Mambrini JV, Lima Torres KC, Peixoto SV, de Oliveira C, Tarazona-Santos E, et al. Predictive value of multiple cytokines and chemokines for mortality in an admixed population: 15-year follow-up of the Bambui-Epigen (Brazil) cohort study of aging. Exp Gerontol. 2017;98:47–53. [DOI] [PubMed] [Google Scholar]
- [155].Figueira I, Fernandes A, Mladenovic Djordjevic A, Lopez-Contreras A, Henriques CM, Selman C, et al. Interventions for age-related diseases: Shifting the paradigm. Mech Ageing Dev. 2016;160:69–92. [DOI] [PubMed] [Google Scholar]
- [156].Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1:S4–9. [DOI] [PubMed] [Google Scholar]
- [157].Sharma R, Kapila R, Kapasiya M, Saliganti V, Dass G, Kapila S. Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr Res. 2014;34:968–81. [DOI] [PubMed] [Google Scholar]
- [158].Franceschi C, BonaFe M. Centenarians as a model for healthy aging. BiochemSocTrans. 2003;31:457–61. [DOI] [PubMed] [Google Scholar]
- [159].Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature communications. 2014;5:3557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Tsubota K, Kawashima M, Inaba T, Dogru M, Matsumoto Y, Ishida R, et al. The antiaging approach for the treatment of dry eye. Cornea. 2012;31 Suppl 1:S3–S8. [DOI] [PubMed] [Google Scholar]
- [161].Kawashima M, Tsubota K. Effect of calorie restriction on change in lacrimal gland with age. Cornea. 2011;30 Suppl 1:S29–33. [DOI] [PubMed] [Google Scholar]
- [162].Turner JE, Brum PC. Does Regular Exercise Counter T Cell Immunosenescence Reducing the Risk of Developing Cancer and Promoting Successful Treatment of Malignancies? Oxid Med Cell Longev. 2017;2017:4234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Sacca SC, Cutolo CA, Ferrari D, Corazza P, Traverso CE. The Eye, Oxidative Damage and Polyunsaturated Fatty Acids. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Seehafer SS, Pearce DA. You say lipofuscin, we say ceroid: defining autofluorescent storage material. Neurobiol Aging. 2006;27:576–88. [DOI] [PubMed] [Google Scholar]
- [165].Rattan SI, Keeler KD, Buchanan JH, Holliday R. Autofluorescence as an index of ageing in human fibroblasts in culture. Biosci Rep. 1982;2:561–7. [DOI] [PubMed] [Google Scholar]
- [166].Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Di Guardo G Lipofuscin, lipofuscin-like pigments and autofluorescence. Eur J Histochem. 2015;59:2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Terman A, Brunk UT. Lipofuscin. Int J Biochem Cell Biol. 2004;36:1400–4. [DOI] [PubMed] [Google Scholar]
- [169].Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci. 2007;1119:97–111. [DOI] [PubMed] [Google Scholar]
- [170].Kojima T, Dogru M, Ibrahim OM, Wakamatsu TH, Ito M, Igarashi A, et al. Effects of Oxidative Stress on the Conjunctiva in Cu, Zn-Superoxide Dismutase-1 (Sod1)-Knockout Mice. Invest Ophthalmol Vis Sci. 2015;56:8382–91. [DOI] [PubMed] [Google Scholar]
- [171].Kojima T, Dogru M, Higuchi A, Nagata T, Ibrahim OM, Inaba T, et al. The effect of Nrf2 knockout on ocular surface protection from acute tobacco smoke exposure: evidence from Nrf2 knockout mice. Am J Pathol. 2015;185:776–85. [DOI] [PubMed] [Google Scholar]
- [172].Dogru M, Kojima T, Simsek C, Tsubota K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Invest Ophthalmol Vis Sci. 2018;59:DES163–DES8. [DOI] [PubMed] [Google Scholar]
- [173].Mantelli F, Mauris J, Argueso P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol. 2013;13:563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Chang SW, Hu FR. Changes in corneal autofluorescence and corneal epithelial barrier function with aging. Cornea. 1993;12:493–9. [DOI] [PubMed] [Google Scholar]
- [175].Ke TL, Clark AF, Gracy RW. Age-related permeability changes in rabbit corneas. J Ocul Pharmacol Ther. 1999;15:513–23. [DOI] [PubMed] [Google Scholar]
- [176].Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. CurrOpinAllergy ClinImmunol. 2008;8:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Uchino Y The Ocular Surface Glycocalyx and its Alteration in Dry Eye Disease: A Review. Invest Ophthalmol Vis Sci. 2018;59:DES157–DES62. [DOI] [PubMed] [Google Scholar]
- [178].Swamynathan SK, Wells A. Conjunctival goblet cells: Ocular surface functions, disorders that affect them, and the potential for their regeneration. Ocul Surf. 2020;18:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Uchino Y, Mauris J, Woodward AM, Dieckow J, Amparo F, Dana R, et al. Alteration of galectin-3 in tears of patients with dry eye disease. Am J Ophthalmol. 2015;159:1027–35.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Machin DR, Bloom SI, Campbell RA, Phuong TTT, Gates PE, Lesniewski LA, et al. Advanced age results in a diminished endothelial glycocalyx. Am J Physiol Heart Circ Physiol. 2018;315:H531–H9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Leong YY, Tong L. Barrier function in the ocular surface: from conventional paradigms to new opportunities. The ocular surface. 2015;13:103–9. [DOI] [PubMed] [Google Scholar]
- [182].Bian F, Xiao Y, Barbosa FL, de Souza RG, Hernandez H, Yu Z, et al. Age-associated antigen-presenting cell alterations promote dry-eye inducing Th1 cells. Mucosal Immunol. 2019;12:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Beardsley RM, de Paiva CS, Power DF, Pflugfelder SC. Desiccating stress decreases apical corneal epithelial cell size--modulation by the metalloproteinase inhibitor doxycycline. Cornea. 2008;27:935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68:1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Ren WY, Wu KF, Li X, Luo M, Liu HC, Zhang SC, et al. Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin Exp Res. 2014;26:183–91. [DOI] [PubMed] [Google Scholar]
- [186].Pflugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, Olmos LC, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Zheng T, Le Q, Hong J, Xu J. Comparison of human corneal cell density by age and corneal location: an in vivo confocal microscopy study. BMC Ophthalmol. 2016;16:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Zheng T, Xu J. Age-related changes of human limbus on in vivo confocal microscopy. Cornea. 2008;27:782–6. [DOI] [PubMed] [Google Scholar]
- [189].Zhang X, Volpe EA, Gandhi NB, Schaumburg CS, Siemasko KF, Pangelinan SB, et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoSOne. 2012;7:e36822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Bose T, Lee R, Hou A, Tong L, Chandy KG. Tissue resident memory T cells in the human conjunctiva and immune signatures in human dry eye disease. Sci Rep. 2017;7:45312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Williams GP, Denniston AK, Oswal KS, Tomlins PJ, Barry RJ, Rauz S, et al. The dominant human conjunctival epithelial CD8alphabeta+ T cell population is maintained with age but the number of CD4+ T cells increases. Age (Dordr). 2012;34:1517–28. Epub 2011 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Allansmith MR, Greiner JV, Baird RS. Number of inflammatory cells in the normal conjunctiva. Am J Ophthalmol. 1978;86:250–9. [DOI] [PubMed] [Google Scholar]
- [193].Knop N, Korb DR, Blackie CA, Knop E. The lid wiper contains goblet cells and goblet cell crypts for ocular surface lubrication during the blink. Cornea. 2012;31:668–79. [DOI] [PubMed] [Google Scholar]
- [194].Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One. 2015;10:e0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Pflugfelder SC, de Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27:64–9. [DOI] [PubMed] [Google Scholar]
- [197].Xiao Y, de Paiva CS, Yu Z, de Souza RG, Li DQ, Pflugfelder SC. Goblet cell-produced retinoic acid suppresses CD86 expression and IL-12 production in bone marrow-derived cells. Int Immunol. 2018;30:457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].de Paiva CS, Raince JK, McClellan AJ, Shanmugam KP, Pangelinan SB, Volpe EA, et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011;4:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].de Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, et al. Dry Eye-Induced Conjunctival Epithelial Squamous Metaplasia Is Modulated by Interferon-{gamma}. Invest Ophthalmol Vis Sci. 2007;48:2553–60. [DOI] [PubMed] [Google Scholar]
- [200].Garcia-Posadas L, Hodges RR, Li D, Shatos MA, Storr-Paulsen T, Diebold Y, et al. Interaction of IFN-gamma with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol. 2016;9:206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Coursey TG, Henriksson JT, Barbosa FL, de Paiva CS, Pflugfelder SC. Interferon-gamma-Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjogren Syndrome. Am J Pathol. 2016;186:1547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Barbosa FL, Xiao Y, Bian F, Coursey TG, Ko BY, Clevers H, et al. Goblet Cells Contribute to Ocular Surface Immune Tolerance-Implications for Dry Eye Disease. Int J Mol Sci. 2017;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Alam J, de Paiva CS, Pflugfelder SC. Immune - Goblet cell interaction in the conjunctiva. The ocular surface. 2020;18:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Nelson JD, Wright JC. Conjunctival goblet cell densities in ocular surface disorders. ArchOphthalmol. 1984;102:1049–51. [DOI] [PubMed] [Google Scholar]
- [206].Giebel J, Woenckhaus C, Fabian M, Tost F. Age-related differential expression of apoptosis-related genes in conjunctival epithelial cells. Acta Ophthalmol Scand. 2005;83:471–6. [DOI] [PubMed] [Google Scholar]
- [207].Sheppard JD Jr., Singh R, McClellan AJ, Weikert MP, Scoper SV, Joly TJ, et al. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca: A Randomized Double-Blind Clinical Trial. Cornea. 2013;32:1297–304. [DOI] [PubMed] [Google Scholar]
- [208].Jirsova K, Seidler Stangova P, Palos M, Mahelkova G, Kalasova S, Rybickova I, et al. Aberrant HLA-DR expression in the conjunctival epithelium after autologous serum treatment in patients with graft-versus-host disease or Sjögren’s syndrome. PLoS One. 2020;15:e0231473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Doughty MJ. On the in vivo assessment of goblet cells of the human bulbar conjunctiva by confocal microscopy – A review. Contact Lens and Anterior Eye. 2020. [DOI] [PubMed] [Google Scholar]
- [210].Villani E, Beretta S, Galimberti D, Viola F, Ratiglia R. In vivo confocal microscopy of conjunctival roundish bright objects: young, older, and Sjogren subjects. Invest Ophthalmol Vis Sci. 2011;52:4829–32. [DOI] [PubMed] [Google Scholar]
- [211].Ralph RA. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol. 1975;14:299–302. [PubMed] [Google Scholar]
- [212].Murube J, Rivas L. Impression cytology on conjunctiva and cornea in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol. 2003;13:115–27. [DOI] [PubMed] [Google Scholar]
- [213].Pflugfelder SC, Tseng SCG, Yoshino K, Monroy D, Felix C, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104:223–35. [DOI] [PubMed] [Google Scholar]
- [214].Kessing SV. Mucous gland system of the conjunctiva: A quantitative normal anatomical study. Acta Ophthalmol(Copenh). 1968;95 (Suppl):25–60. [PubMed] [Google Scholar]
- [215].Abdel-Khalek LM, Williamson J, Lee WR. Morphological changes in the human conjunctival epithelium. I. In the normal elderly population. Br J Ophthalmol. 1978;62:792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Paschides CA, Petroutsos G, Psilas K. Correlation of conjunctival impression cytology results with lacrimal function and age. Acta Ophthalmol (Copenh). 1991;69:422–5. [DOI] [PubMed] [Google Scholar]
- [217].Doughty MJ. Goblet Cells of the Normal Human Bulbar Conjunctiva and Their Assessment by Impression Cytology Sampling. The ocular surface. 2012;10:149–69. [DOI] [PubMed] [Google Scholar]
- [218].Volpe EA, Henriksson JT, Wang C, Barbosa FL, Zaheer M, Zhang X, et al. Interferon-gamma deficiency protects against aging-related goblet cell loss. Oncotarget. 2016;7:64605–6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Knop N, Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest OphthalmolVisSci. 2000;41:1270–9. [PubMed] [Google Scholar]
- [220].Gill RF, Pirockinaite G, O’Sullivan NL, Montgomery PC. Nasal-associated lymphoid tissue is not an absolute requirement for the induction of rat tear IgA antibody responses. Curr Eye Res. 2010;35:1–8. [DOI] [PubMed] [Google Scholar]
- [221].Cerutti A, Chen K, Chorny A. Immunoglobulin Responses at the Mucosal Interface. Annu Rev Immunol. 2011;29:273–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Steven P, Gebert A. Conjunctiva-associated lymphoid tissue - current knowledge, animal models and experimental prospects. Ophthalmic Res. 2009;42:2–8. [DOI] [PubMed] [Google Scholar]
- [224].Sen DK, Sarin GS, Mani K, Saha K. Immunoglobulins in tears of normal Indian people. Br J Ophthalmol. 1976;60:302–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Sen DK, Sarin GS, Mathur GP, Saha K. Biological variation of immunoglobulin concentrations in normal human tears related to age and sex. Acta Ophthalmol (Copenh). 1978;56:439–44. [DOI] [PubMed] [Google Scholar]
- [226].Sullivan DA, Allansmith MR. The effect of aging on the secretory immune system of the eye. Immunology. 1988;63:403–10. [PMC free article] [PubMed] [Google Scholar]
- [227].Dwivedy A, Aich P. Importance of innate mucosal immunity and the promises it holds. Int J Gen Med. 2011;4:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Kurashima Y, Goto Y, Kiyono H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur J Immunol. 2013;43:3108–15. [DOI] [PubMed] [Google Scholar]
- [229].Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].McDermott AM. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res. 2009;41:60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Redfern RL, Reins RY, McDermott AM. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. ExpEye Res. 2011;92:209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Castaneda-Delgado JE, Miranda-Castro NY, Gonzalez-Amaro R, Gonzalez-Curiel I, Montoya-Rosales A, Rivas-Calderon B, et al. Production of antimicrobial peptides is preserved in aging. Clin Immunol. 2013;148:198–205. [DOI] [PubMed] [Google Scholar]
- [233].Castaneda-Delgado JE, Frausto-Lujan I, Gonzalez-Curiel I, Montoya-Rosales A, Serrano CJ, Torres-Juarez F, et al. Differences in Cytokine Production during Aging and Its Relationship with Antimicrobial Peptides Production. Immunol Invest. 2017;46:48–58. [DOI] [PubMed] [Google Scholar]
- [234].Yang, Han Z, Oppenheim JJ. Alarmins and immunity. Immunol Rev. 2017;280:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Tong L, Lan W, Lim RR, Chaurasia SS. S100A proteins as molecular targets in the ocular surface inflammatory diseases. Ocul Surf. 2014;12:23–31. [DOI] [PubMed] [Google Scholar]
- [236].Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129:1441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Pearlman E, Johnson A, Adhikary G, Sun Y, Chinnery HR, Fox T, et al. Toll-like receptors at the ocular surface. Ocul Surf. 2008;6:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Kim YH, Li Z, Cui L, Li Y, Yoon HJ, Choi W, et al. Expression of Nod-like Receptors and Clinical Correlations in Patients With Dry Eye Disease. Am J Ophthalmol. 2019;200:150–60. [DOI] [PubMed] [Google Scholar]
- [239].Menon BB, Kaiser-Marko C, Spurr-Michaud S, Tisdale AS, Gipson IK. Suppression of Toll-like receptor-mediated innate immune responses at the ocular surface by the membrane-associated mucins MUC1 and MUC16. Mucosal Immunol. 2015;8:1000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Redfern RL, Patel N, Hanlon S, Farley W, Gondo M, Pflugfelder SC, et al. Toll-like receptor expression and activation in mice with experimental dry eye. Invest OphthalmolVisSci. 2013;54:1554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Redfern RL, Barabino S, Baxter J, Lema C, McDermott AM. Dry eye modulates the expression of toll-like receptors on the ocular surface. Exp Eye Res. 2015;134:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Simmons KT, Xiao Y, Pflugfelder SC, de Paiva CS. Inflammatory Response to Lipopolysaccharide on the Ocular Surface in a Murine Dry Eye Model. Invest Ophthalmol Vis Sci. 2016;57:2443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Reins RY, Lema C, Courson J, Kunnen CME, Redfern RL. MyD88 Deficiency Protects Against Dry Eye-Induced Damage. Invest Ophthalmol Vis Sci. 2018;59:2967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Li Y, Jin R, Li L, Yoon HJ, Choi JH, Park JH, et al. Expression and Role of Nucleotide-Binding Oligomerization Domain 2 (NOD2) in the Ocular Surface of Murine Dry Eye. Invest Ophthalmol Vis Sci. 2019;60:2641–9. [DOI] [PubMed] [Google Scholar]
- [245].Bian F, Xiao Y, Zaheer M, Volpe EA, Pflugfelder SC, Li DQ, et al. Inhibition of NLRP3 Inflammasome Pathway by Butyrate Improves Corneal Wound Healing in Corneal Alkali Burn. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Bailey KL, Smith LM, Heires AJ, Katafiasz DM, Romberger DJ, LeVan TD. Aging leads to dysfunctional innate immune responses to TLR2 and TLR4 agonists. Aging Clin Exp Res. 2019;31:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Shaw AC, Panda A, Joshi SR, Qian F, Allore HG, Montgomery RR. Dysregulation of human Toll-like receptor function in aging. Ageing research reviews. 2011;10:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Ebersole JL, Kirakodu S, Novak MJ, Exposto CR, Stromberg AJ, Shen S, et al. Effects of aging in the expression of NOD-like receptors and inflammasome-related genes in oral mucosa. Mol Oral Microbiol. 2016;31:18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Strober W, Asano N, Fuss I, Kitani A, Watanabe T. Cellular and molecular mechanisms underlying NOD2 risk-associated polymorphisms in Crohn’s disease. Immunol Rev. 2014;260:249–60. [DOI] [PubMed] [Google Scholar]
- [250].Santana PT, Martel J, Lai HC, Perfettini JL, Kanellopoulos JM, Young JD, et al. Is the inflammasome relevant for epithelial cell function? Microbes Infect. 2016;18:93–101. [DOI] [PubMed] [Google Scholar]
- [251].Zheng Q, Ren Y, Reinach PS, Xiao B, Lu H, Zhu Y, et al. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp Eye Res. 2015;134:133–40. [DOI] [PubMed] [Google Scholar]
- [252].Niu L, Zhang S, Wu J, Chen L, Wang Y. Upregulation of NLRP3 Inflammasome in the Tears and Ocular Surface of Dry Eye Patients. PLoS One. 2015;10:e0126277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Zheng Q, Ren Y, Reinach PS, She Y, Xiao B, Hua S, et al. Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye. Exp Eye Res. 2014;125:1–8. [DOI] [PubMed] [Google Scholar]
- [254].Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73. [DOI] [PubMed] [Google Scholar]
- [255].Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Wullaert A, Bonnet MC, Pasparakis M. NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Lan W, Petznick A, Heryati S, Rifada M, Tong L. Nuclear Factor-kappaB: central regulator in ocular surface inflammation and diseases. Ocul Surf. 2012;10:137–48. [DOI] [PubMed] [Google Scholar]
- [258].Guzman M, Sabbione F, Gabelloni ML, Vanzulli S, Trevani AS, Giordano MN, et al. Restoring conjunctival tolerance by topical nuclear factor-kappaB inhibitors reduces preservative-facilitated allergic conjunctivitis in mice. Invest Ophthalmol Vis Sci. 2014;55:6116–26. [DOI] [PubMed] [Google Scholar]
- [259].Guzman M, Keitelman I, Sabbione F, Trevani AS, Giordano MN, Galletti JG. Mucosal tolerance disruption favors disease progression in an extraorbital lacrimal gland excision model of murine dry eye. Exp Eye Res. 2016;151:19–22. [DOI] [PubMed] [Google Scholar]
- [260].Guzman M, Keitelman I, Sabbione F, Trevani AS, Giordano MN, Galletti JG. Desiccating stress-induced disruption of ocular surface immune tolerance drives dry eye disease. Clin Exp Immunol. 2016;184:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Dong J, Jimi E, Zeiss C, Hayden MS, Ghosh S. Constitutively active NF-kappaB triggers systemic TNFalpha-dependent inflammation and localized TNFalpha-independent inflammatory disease. Genes Dev. 2010;24:1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. [DOI] [PubMed] [Google Scholar]
- [263].Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kappaB in Aging and Disease. Aging Dis. 2011;2:449–65. [PMC free article] [PubMed] [Google Scholar]
- [264].Helenius M, Hänninen M, Lehtinen SK, Salminen A. Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-kB transcription factor in mouse cardiac muscle. J Mol Cell Cardiol. 1996;28:487–98. [DOI] [PubMed] [Google Scholar]
- [265].Helenius M, Hänninen M, Lehtinen SK, Salminen A. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J. 1996;318 (Pt 2):603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nature communications. 2014;2:4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Adler AS, Kawahara TL, Segal E, Chang HY. Reversal of aging by NFkappaB blockade. Cell cycle (Georgetown, Tex). 2008;7:556–9. [DOI] [PubMed] [Google Scholar]
- [268].Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21:3244–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Foulsham W, Dohlman TH, Mittal SK, Taketani Y, Singh RB, Masli S, et al. Thrombospondin-1 in ocular surface health and disease. Ocul Surf. 2019;17:374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Isenberg JS, Roberts DD. Thrombospondin-1 in maladaptive aging responses: a concept whose time has come. Am J Physiol Cell Physiol. 2020;319:C45–C63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. ProgRetinEye Res. 1999;18:293–309. [DOI] [PubMed] [Google Scholar]
- [272].Royer DJ, Elliott MH, Le YZ, Carr DJJ. Corneal Epithelial Cells Exhibit Myeloid Characteristics and Present Antigen via MHC Class II. Invest Ophthalmol Vis Sci. 2018;59:1512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Barabino S, Montaldo E, Solignani F, Valente C, Mingari MC, Rolando M. Immune response in the conjunctival epithelium of patients with dry eye. Exp Eye Res. 2010;91:524–9. [DOI] [PubMed] [Google Scholar]
- [274].Franco A, Valesini G, Barnaba V, Silvagni C, Tiberti A, Balsano F. Class II MHC antigen expression on epithelial cells of salivary glands from patients with Sjögren’s syndrome. Clin Exp Rheumatol. 1987;5:199–203. [PubMed] [Google Scholar]
- [275].Pflugfelder SC, Bian F, Gumus K, Farley W, Stern ME, De Paiva CS. Severity of Sjogren’s Syndrome Keratoconjunctivitis Sicca Increases with Increased Percentage of Conjunctival Antigen-Presenting Cells. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Stern ME, Gao J, Schwalb TA, Ngo M, Tieu DD, Chan CC, et al. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–14. [PubMed] [Google Scholar]
- [277].Iula L, Keitelman IA, Sabbione F, Fuentes F, Guzman M, Galletti JG, et al. Autophagy Mediates Interleukin-1beta Secretion in Human Neutrophils. Front Immunol. 2018;9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Fuxman Bass JI, Alvarez ME, Gabelloni ML, Vermeulen ME, Amaral MM, Geffner JR, et al. GM-CSF enhances a CpG-independent pathway of neutrophil activation triggered by bacterial DNA. Mol Immunol. 2008;46:37–44. [DOI] [PubMed] [Google Scholar]
- [279].Keitelman IA, Sabbione F, Shiromizu CM, Giai C, Fuentes F, Rosso D, et al. Short-Term Fever-Range Hyperthermia Accelerates NETosis and Reduces Pro-inflammatory Cytokine Secretion by Human Neutrophils. Front Immunol. 2019;10:2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Gabelloni ML, Sabbione F, Jancic C, Fuxman Bass J, Keitelman I, Iula L, et al. NADPH oxidase derived reactive oxygen species are involved in human neutrophil IL-1beta secretion but not in inflammasome activation. Eur J Immunol. 2013;43:3324–35. [DOI] [PubMed] [Google Scholar]
- [281].Sabbione F, Keitelman IA, Iula L, Ferrero M, Giordano MN, Baldi P, et al. Neutrophil Extracellular Traps Stimulate Proinflammatory Responses in Human Airway Epithelial Cells. J Innate Immun. 2017;9:387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Sack RA, Beaton A, Sathe S, Morris C, Willcox M, Bogart B. Towards a closed eye model of the pre-ocular tear layer. ProgRetinEye Res. 2000;19:649–68. [DOI] [PubMed] [Google Scholar]
- [283].Sakata M, Sack RA, Sathe S, Holden B, Beaton AR. Polymorphonuclear leukocyte cells and elastase in tears. Curr Eye Res. 1997;16:810–9. [DOI] [PubMed] [Google Scholar]
- [284].Mahajan A, Gruneboom A, Petru L, Podolska MJ, Kling L, Maueroder C, et al. Frontline Science: Aggregated neutrophil extracellular traps prevent inflammation on the neutrophil-rich ocular surface. J Leukoc Biol. 2019;105:1087–98. [DOI] [PubMed] [Google Scholar]
- [285].Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. Female-Specific Downregulation of Tissue Polymorphonuclear Neutrophils Drives Impaired Regulatory T Cell and Amplified Effector T Cell Responses in Autoimmune Dry Eye Disease. J Immunol. 2015;195:3086–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Tseng CW, Liu GY. Expanding roles of neutrophils in aging hosts. Curr Opin Immunol. 2014;29:43–8. [DOI] [PubMed] [Google Scholar]
- [287].Di Zazzo A, Micera A, Coassin M, Varacalli G, Foulsham W, De Piano M, et al. InflammAging at Ocular Surface: Clinical and Biomolecular Analyses in Healthy Volunteers. Invest Ophthalmol Vis Sci. 2019;60:1769–75. [DOI] [PubMed] [Google Scholar]
- [288].Willcox MD, Morris CA, Thakur A, Sack RA, Wickson J, Boey W. Complement and complement regulatory proteins in human tears. Invest Ophthalmol Vis Sci. 1997;38:1–8. [PubMed] [Google Scholar]
- [289].Gaya da Costa M, Poppelaars F, van Kooten C, Mollnes TE, Tedesco F, Wurzner R, et al. Age and Sex-Associated Changes of Complement Activity and Complement Levels in a Healthy Caucasian Population. Front Immunol. 2018;9:2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nat Immunol. 2017;18:1288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].McDermott AM. Antimicrobial compounds in tears. Exp Eye Res. 2013;117:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kaplan HJ. Differential expression of the complement regulatory proteins in the human eye. Invest Ophthalmol Vis Sci. 1993;34:3579–84. [PubMed] [Google Scholar]
- [293].Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91:35–43. [DOI] [PubMed] [Google Scholar]
- [294].Cocuzzi E, Guidubaldi J, Bardenstein DS, Chen R, Jacobs MR, Medof EM. Release of complement regulatory proteins from ocular surface cells in infections. Curr Eye Res. 2000;21:856–66. [DOI] [PubMed] [Google Scholar]
- [295].Royer DJ, Echegaray-Mendez J, Lin L, Gmyrek GB, Mathew R, Saban DR, et al. Complement and CD4(+) T cells drive context-specific corneal sensory neuropathy. eLife. 2019;8:e48378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Stern ME, Schaumburg CS, Siemasko KF, Gao J, Wheeler LA, Grupe DA, et al. Autoantibodies contribute to the immunopathogenesis of experimental dry eye disease. Invest OphthalmolVisSci. 2012;53:2062–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Johnson AA, Shokhirev MN, Wyss-Coray T, Lehallier B. Systematic review and analysis of human proteomics aging studies unveils a novel proteomic aging clock and identifies key processes that change with age. Ageing research reviews. 2020;60:101070. [DOI] [PubMed] [Google Scholar]
- [298].Nagaki K, Hiramatsu S, Inai S, Sasaki A. The effect of aging on complement activity (CH50) and complement protein levels. J Clin Lab Immunol. 1980;3:45–50. [PubMed] [Google Scholar]
- [299].Reichwald J, Danner S, Wiederhold KH, Staufenbiel M. Expression of complement system components during aging and amyloid deposition in APP transgenic mice. J Neuroinflammation. 2009;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Franzin R, Stasi A, Fiorentino M, Stallone G, Cantaluppi V, Gesualdo L, et al. Inflammaging and Complement System: A Link Between Acute Kidney Injury and Chronic Graft Damage. Front Immunol. 2020;11:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Rogińska D, Kawa MP, Pius-Sadowska E, Lejkowska R, Łuczkowska K, Wiszniewska B, et al. Depletion of the Third Complement Component Ameliorates Age-Dependent Oxidative Stress and Positively Modulates Autophagic Activity in Aged Retinas in a Mouse Model. Oxid Med Cell Longev. 2017;2017:5306790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Davies LC, Taylor PR. Tissue-resident macrophages: then and now. Immunology. 2015;144:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].You IC, Coursey TG, Bian F, Barbosa FL, de Paiva CS, Pflugfelder SC. Macrophage Phenotype in the Ocular Surface of Experimental Murine Dry Eye Disease. Arch Immunol Ther Exp (Warsz). 2015;63:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Liu J, Xue Y, Dong D, Xiao C, Lin C, Wang H, et al. CCR2(−) and CCR2(+) corneal macrophages exhibit distinct characteristics and balance inflammatory responses after epithelial abrasion. Mucosal Immunol. 2017;10:1145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–9. [DOI] [PubMed] [Google Scholar]
- [306].Deng R, Chen X, Zhang Y, Bian F, Gao N, Hu J, et al. Short ragweed pollen promotes M2 macrophage polarization via TSLP/TSLPR/OX40L signaling in allergic inflammation. Mucosal Immunol. 2019;12:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Albright JM, Dunn RC, Shults JA, Boe DM, Afshar M, Kovacs EJ. Advanced Age Alters Monocyte and Macrophage Responses. Antioxid Redox Signal. 2016;25:805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou A-P, Hall S, et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. The Journal of experimental medicine. 2009;206:1929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Sendama W The effect of ageing on the resolution of inflammation. Ageing Res Rev. 2020;57:101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Sun H, Sun C, Xiao W, Sun R. Tissue-resident lymphocytes: from adaptive to innate immunity. Cell Mol Immunol. 2019;16:205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Chou C, Li MO. Tissue-Resident Lymphocytes Across Innate and Adaptive Lineages. Front Immunol. 2018;9:2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. [DOI] [PubMed] [Google Scholar]
- [314].Kotas ME, Locksley RM. Why Innate Lymphoid Cells? Immunity. 2018;48:1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Zenewicz LA. IL-22: There Is a Gap in Our Knowledge. Immunohorizons. 2018;2:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Coursey TG, Bohat R, Barbosa FL, Pflugfelder SC, de Paiva CS. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-gamma in experimental dry eye. J Immunol. 2014;193:5264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].O’Sullivan TE. Dazed and Confused: NK Cells. Front Immunol. 2019;10:2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon-{gamma}-secreting NK cells promote induction of dry eye disease. J Leukoc Biol. 2011;89:965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Imai Y, Hosotani Y, Ishikawa H, Yasuda K, Nagai M, Jitsukawa O, et al. Expression of IL-33 in ocular surface epithelium induces atopic keratoconjunctivitis with activation of group 2 innate lymphoid cells in mice. Sci Rep. 2017;7:10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Liu J, Xiao C, Wang H, Xue Y, Dong D, Lin C, et al. Local Group 2 Innate Lymphoid Cells Promote Corneal Regeneration after Epithelial Abrasion. Am J Pathol. 2017;187:1313–26. [DOI] [PubMed] [Google Scholar]
- [321].Yoon CH, Lee D, Jeong HJ, Ryu JS, Kim MK. Distribution of Interleukin-22-secreting Immune Cells in Conjunctival Associated Lymphoid Tissue. Korean J Ophthalmol. 2018;32:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].D’Souza SS, Shen X, Fung ITH, Ye L, Kuentzel M, Chittur SV, et al. Compartmentalized effects of aging on group 2 innate lymphoid cell development and function. Aging Cell. 2019;18:e13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Li Z, Burns AR, Miller SB, Smith CW. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011;25:2659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Colonna-Romano G, Aquino A, Bulati M, Lio D, Candore G, Oddo G, et al. Impairment of gamma/delta T lymphocytes in elderly: implications for immunosenescence. Exp Gerontol. 2004;39:1439–46. [DOI] [PubMed] [Google Scholar]
- [325].Xu W, Lau ZWX, Fulop T, Larbi A. The Aging of gammadelta T Cells. Cells. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Williams GP, Pachnio A, Long HM, Rauz S, Curnow SJ. Cytokine production and antigen recognition by human mucosal homing conjunctival effector memory CD8+ T cells. Invest Ophthalmol Vis Sci. 2014;55:8523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Zhang X, Schaumburg CS, Coursey TG, Siemasko KF, Volpe EA, Gandhi NB, et al. CD8(+) cells regulate the T helper-17 response in an experimental murine model of Sjogren syndrome. Mucosal Immunol. 2014;7:417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. JImmunol. 2006;177:2775–83. [DOI] [PubMed] [Google Scholar]
- [329].Santiago AF, Alves AC, Oliveira RP, Fernandes RM, Paula-Silva J, Assis FA, et al. Aging correlates with reduction in regulatory-type cytokines and T cells in the gut mucosa. Immunobiology. 2011;216:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Tsubota K, Fukagawa K, Fujihara T, Shimmura S, Saito I, Saito K, et al. Regulation of human leukocyte antigen expression in human conjunctival epithelium. Invest OphthalmolVisSci. 1999;40:28–34. [PubMed] [Google Scholar]
- [331].Horev Y, Salameh R, Nassar M, Capucha T, Saba Y, Barel O, et al. Niche rather than origin dysregulates mucosal Langerhans cells development in aged mice. Mucosal Immunol. 2020. [DOI] [PubMed] [Google Scholar]
- [332].Prakash S, Agrawal S, Vahed H, Ngyuen M, BenMohamed L, Gupta S, et al. Dendritic cells from aged subjects contribute to chronic airway inflammation by activating bronchial epithelial cells under steady state. Mucosal Immunol. 2014;7:1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Shen L, Barabino S, Taylor AW, Dana MR. Effect of the ocular microenvironment in regulating corneal dendritic cell maturation. ArchOphthalmol. 2007;125:908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–8. [DOI] [PubMed] [Google Scholar]
- [335].Khandelwal P, Blanco-Mezquita T, Emami P, Lee HS, Reyes NJ, Mathew R, et al. Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses. PLoS One. 2013;8:e64193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Ahadome SD, Mathew R, Reyes NJ, Mettu PS, Cousins SW, Calder VL, et al. Classical dendritic cells mediate fibrosis directly via the retinoic acid pathway in severe eye allergy. JCI insight. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Tseng SCG, Farazdaghi M, Rider AA. Conjunctival transdifferentiation induced by systemic vitamin A deficiency in vascularized rabbit corneas. InvestOphthalmolVisSci. 1987;28:1497–504. [PubMed] [Google Scholar]
- [339].Tseng SC, Hatchell D, Tierney N, Huang AJ, Sun TT. Expression of specific keratin markers by rabbit corneal, conjunctival, and esophageal epithelia during vitamin A deficiency. JCell Biol. 1984;99:2279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Ko BY, Xiao Y, Barbosa FL, de Paiva CS, Pflugfelder SC. Goblet cell loss abrogates ocular surface immune tolerance. JCI insight. 2018;3: 98222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Agrawal S, Ganguly S, Tran A, Sundaram P, Agrawal A. Retinoic acid treated human dendritic cells induce T regulatory cells via the expression of CD141 and GARP which is impaired with age. Aging (Albany NY). 2016;8:1223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Park J, Miyakawa T, Shiokawa A, Nakajima-Adachi H, Tanokura M, Hachimura S. Attenuation of migration properties of CD4+ T cells from aged mice correlates with decrease in chemokine receptor expression, response to retinoic acid, and RALDH expression compared to young mice. Biosci Biotechnol Biochem. 2014;78:976–80. [DOI] [PubMed] [Google Scholar]
- [343].Bania J, Gatti E, Lelouard H, David A, Cappello F, Weber E, et al. Human cathepsin S, but not cathepsin L, degrades efficiently MHC class II-associated invariant chain in nonprofessional APCs. Proc Natl Acad Sci U S A. 2003;100:6664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Beers C, Burich A, Kleijmeer MJ, Griffith JM, Wong P, Rudensky AY. Cathepsin S controls MHC class II-mediated antigen presentation by epithelial cells in vivo. J Immunol. 2005;174:1205–12. [DOI] [PubMed] [Google Scholar]
- [345].Riese RJ, Mitchell RN, Villadangos JA, Shi GP, Palmer JT, Karp ER, et al. Cathepsin S activity regulates antigen presentation and immunity. J Clin Invest. 1998;101:2351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10:197–206. [DOI] [PubMed] [Google Scholar]
- [347].Hsieh CS, deRoos P, Honey K, Beers C, Rudensky AY. A role for cathepsin L and cathepsin S in peptide generation for MHC class II presentation. J Immunol. 2002;168:2618–25. [DOI] [PubMed] [Google Scholar]
- [348].Hsing LC, Kirk EA, McMillen TS, Hsiao SH, Caldwell M, Houston B, et al. Roles for cathepsins S, L, and B in insulitis and diabetes in the NOD mouse. J Autoimmun. 2010;34:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, et al. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–3. [DOI] [PubMed] [Google Scholar]
- [350].Saegusa K, Ishimaru N, Yanagi K, Arakaki R, Ogawa K, Saito I, et al. Cathepsin S inhibitor prevents autoantigen presentation and autoimmunity. J Clin Invest. 2002;110:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Tato M, Kumar SV, Liu Y, Mulay SR, Moll S, Popper B, et al. Cathepsin S inhibition combines control of systemic and peripheral pathomechanisms of autoimmune tissue injury. Sci Rep. 2017;7:2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Thanei S, Theron M, Silva AP, Reis B, Branco L, Schirmbeck L, et al. Cathepsin S inhibition suppresses autoimmune-triggered inflammatory responses in macrophages. Biochem Pharmacol. 2017;146:151–64. [DOI] [PubMed] [Google Scholar]
- [353].Ogawa T, Boylan SA, Oltjen SL, Hjelmeland LM. Changes in the spatial expression of genes with aging in the mouse RPE/choroid. Mol Vis. 2005;11:380–6. [PubMed] [Google Scholar]
- [354].Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: identification of aging biomarkers and impact of dietary antioxidants. Aging cell. 2009;8:484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. NeurobiolAging. 2007;28:1507–21. [DOI] [PubMed] [Google Scholar]
- [356].Wendt W, Lubbert H, Stichel CC. Upregulation of cathepsin S in the aging and pathological nervous system of mice. Brain Res. 2008;1232:7–20. [DOI] [PubMed] [Google Scholar]
- [357].Hamm-Alvarez SF, Janga SR, Edman MC, Madrigal S, Shah M, Frousiakis SE, et al. Tear cathepsin S as a candidate biomarker for Sjogren’s syndrome. Arthritis & rheumatology (Hoboken, NJ). 2014;66:1872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Li X, Wu K, Edman M, Schenke-Layland K, MacVeigh-Aloni M, Janga SR, et al. Increased expression of cathepsins and obesity-induced proinflammatory cytokines in lacrimal glands of male NOD mouse. Invest Ophthalmol Vis Sci. 2010;51:5019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [359].Shah M, Edman MC, Janga SR, Shi P, Dhandhukia J, Liu S, et al. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjogren’s syndrome. J Control Release. 2013;171:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Edman MC, Janga SR, Meng Z, Bechtold M, Chen AF, Kim C, et al. Increased Cathepsin S activity associated with decreased protease inhibitory capacity contributes to altered tear proteins in Sjogren’s Syndrome patients. Sci Rep. 2018;8:11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Salminen A Activation of immunosuppressive network in the aging process. Ageing research reviews. 2020;57:100998. [DOI] [PubMed] [Google Scholar]
- [362].Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012;24:342–9. [DOI] [PubMed] [Google Scholar]
- [363].Goronzy J, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Nusser A, Nuber N, Wirz OF, Rolink H, Andersson J, Rolink A. The development of autoimmune features in aging mice is closely associated with alterations of the peripheral CD4(+) T-cell compartment. Eur J Immunol. 2014;44:2893–902. [DOI] [PubMed] [Google Scholar]
- [365].Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly : scientific basis and clinical implications. Drugs Aging. 2005;22:589–603. [DOI] [PubMed] [Google Scholar]
- [366].van der Geest KS, Abdulahad WH, Tete SM, Lorencetti PG, Horst G, Bos NA, et al. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp Gerontol. 2014;60:190–6. [DOI] [PubMed] [Google Scholar]
- [367].Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, et al. Activated T Lymphocytes are Essential Drivers of Pathological Remodeling in Ischemic Heart Failure. Circ Heart Fail. 2017;10:e003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Yu Q, Watson RR, Marchalonis JJ, Larson DF. A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol. 2005;289:H643–51. [DOI] [PubMed] [Google Scholar]
- [369].Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis ResTher. 2003;5:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Ligon MM, Wang C, DeJong EN, Schulz C, Bowdish DME, Mysorekar IU. Single cell and tissue-transcriptomic analysis of murine bladders reveals age- and TNFα-dependent but microbiota-independent tertiary lymphoid tissue formation. Mucosal Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Speziali E, Santiago AF, Fernandes RM, Vaz NM, Menezes JS, Faria AM. Specific immune responses but not basal functions of B and T cells are impaired in aged mice. Cell Immunol. 2009;256:1–5. [DOI] [PubMed] [Google Scholar]
- [372].Elyahu Y, Hekselman I, Eizenberg-Magar I, Berner O, Strominger I, Schiller M, et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci Adv. 2019;5:eaaw8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Foulsham W, Mittal SK, Taketani Y, Chen Y, Nakao T, Chauhan SK, et al. Aged mice exhibit severe exacerbations of dry eye disease with an amplified memory Th17 cell response. Am J Pathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Chauhan SK, El AJ, Ecoiffier T, Goyal S, Zhang Q, Saban DR, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. JImmunol. 2009;182:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Chen Y, Chauhan SK, Shao C, Omoto M, Inomata T, Dana R. IFN-gamma-Expressing Th17 Cells Are Required for Development of Severe Ocular Surface Autoimmunity. J Immunol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].de Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD III, Fang B, Zheng X, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Zhang X, Chen W, de Paiva CS, Corrales RM, Volpe EA, McClellan AJ, et al. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci. 2011;52:6279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. InvestOphthalmolVisSci. 1983;24:1442–3. [PubMed] [Google Scholar]
- [379].Bukhari AA, Basheer NA, Joharjy HI. Age, gender, and interracial variability of normal lacrimal gland volume using MRI. Ophthal Plast Reconstr Surg. 2014;30:388–91. [DOI] [PubMed] [Google Scholar]
- [380].Seo KY, Kitamura K, Han SJ, Kelsall B. T(H)17 cells mediate inflammation in a novel model of spontaneous experimental autoimmune lacrimal keratoconjunctivitis with neural damage. The Journal of allergy and clinical immunology. 2018;142:96–108.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Coursey TG, Bian F, Zaheer M, Pflugfelder SC, Volpe EA, de Paiva CS. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunol. 2017;10:743–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, Haynes L. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging cell. 2012;11:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. [DOI] [PubMed] [Google Scholar]
- [384].Bagavant H, Tung KSK. Failure of CD25+T Cells from Lupus-Prone Mice to Suppress Lupus Glomerulonephritis and Sialoadenitis. The Journal of Immunology. 2005;175:944. [DOI] [PubMed] [Google Scholar]
- [385].de Paiva CS, Hwang CS, Pitcher JD III, Pangelinan SB, Rahimy E, Chen W, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology. 2010;49:246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Rahimy E, Pitcher JD III, Pangelinan SB, Chen W, Farley WJ, Niederkorn JY, et al. Spontaneous autoimmune dacryoadenitis in aged CD25KO mice. Am J Pathol. 2010;177:744–53 Epub 2010 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Suzuki T, Murakami S, Biswal SS, Sakaguchi S, Harigae H, Yamamoto M, et al. Systemic Activation of NRF2 Alleviates Lethal Autoimmune Inflammation in Scurfy Mice. Mol Cell Biol. 2017;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Sharma R, Zheng L, Guo X, Fu SM, Ju ST, Jarjour WN. Novel animal models for Sjogren’s syndrome: expression and transfer of salivary gland dysfunction from regulatory T cell-deficient mice. J Autoimmun. 2006;27:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Zheng L, Sharma R, Gaskin F, Fu SM, Ju ST. A novel role of IL-2 in organ-specific autoimmune inflammation beyond regulatory T cell checkpoint: both IL-2 knockout and Fas mutation prolong lifespan of Scurfy mice but by different mechanisms. J Immunol. 2007;179:8035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Carpentier M, Chappert P, Kuhn C, Lalfer M, Flament H, Burlen-Defranoux O, et al. Extrathymic induction of Foxp3(+) regulatory T cells declines with age in a T-cell intrinsic manner. Eur J Immunol. 2013;43:2598–604. [DOI] [PubMed] [Google Scholar]
- [391].Tsaknaridis L, Spencer L, Culbertson N, Hicks K, LaTocha D, Chou YK, et al. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. [DOI] [PubMed] [Google Scholar]
- [392].Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–93. [DOI] [PubMed] [Google Scholar]
- [393].Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–55. [DOI] [PubMed] [Google Scholar]
- [394].Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013;48:1379–86. [DOI] [PubMed] [Google Scholar]
- [396].Turner VM, Mabbott NA. Ageing adversely affects the migration and function of marginal zone B cells. Immunology. 2017;151:349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [398].Roukens AH, Soonawala D, Joosten SA, de Visser AW, Jiang X, Dirksen K, et al. Elderly subjects have a delayed antibody response and prolonged viraemia following yellow fever vaccination: a prospective controlled cohort study. PLoS One. 2011;6:e27753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [399].Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing research reviews. 2011;10:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Johnson SA, Rozzo SJ, Cambier JC. Aging-dependent exclusion of antigen-inexperienced cells from the peripheral B cell repertoire. J Immunol. 2002;168:5014–23. [DOI] [PubMed] [Google Scholar]
- [401].Kline GH, Hayden TA, Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. JImmunol. 1999;162:3342–9. [PubMed] [Google Scholar]
- [402].Sage PT, Tan CL, Freeman GJ, Haigis M, Sharpe AH. Defective TFH Cell Function and Increased TFR Cells Contribute to Defective Antibody Production in Aging. Cell reports. 2015;12:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Rowley MJ, Buchanan H, Mackay IR. Reciprocal change with age in antibody to extrinsic and intrinsic antigens. Lancet. 1968;2:24–6. [DOI] [PubMed] [Google Scholar]
- [404].Teague PO, Friou GJ, Myers LL. Anti-Nuclear Antibodies in Mice. The Journal of Immunology. 1968;101:791. [PubMed] [Google Scholar]
- [405].de Valle E, Grigoriadis G, O’Reilly LA, Willis SN, Maxwell MJ, Corcoran LM, et al. NFkappaB1 is essential to prevent the development of multiorgan autoimmunity by limiting IL-6 production in follicular B cells. J Exp Med. 2016;213:621–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [406].Fuchs S, Mozes E, Stollar BD. The Nature of Murine Immune Response to Nucleic Acids. The Journal of Immunology. 1975;114:1287. [PubMed] [Google Scholar]
- [407].Desai DD, Marion TN. Induction of anti-DNA antibody with DNA-peptide complexes. Int Immunol. 2000;12:1569–78. [DOI] [PubMed] [Google Scholar]
- [408].Desai DD, Krishnan MR, Swindle JT, Marion TN. Antigen-specific induction of antibodies against native mammalian DNA in nonautoimmune mice. The Journal of Immunology. 1993;151:1614. [PubMed] [Google Scholar]
- [409].Rubtsova K, Rubtsov AV, Halemano K, Li SX, Kappler JW, Santiago ML, et al. T Cell Production of IFNγ in Response to TLR7/IL-12 Stimulates Optimal B Cell Responses to Viruses. PLoS One. 2016;11:e0166322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [410].Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Nerviani A, Pitzalis C. Role of chemokines in ectopic lymphoid structures formation in autoimmunity and cancer. J Leukoc Biol. 2018;104:333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [412].Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14:447–62. [DOI] [PubMed] [Google Scholar]
- [413].Bombardieri M, Barone F, Lucchesi D, Nayar S, van den Berg WB, Proctor G, et al. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol. 2012;189:3767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [414].Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren’s syndrome. J Clin Invest. 1998;102:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [415].Gatumu MK, Skarstein K, Papandile A, Browning JL, Fava RA, Bolstad AI. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjögren’s syndrome in salivary glands of non-obese diabetic mice. Arthritis Res Ther. 2009;11:R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [416].Rubtsova K, Rubtsov AV, Cancro MP, Marrack P. Age-Associated B Cells: A T-bet-Dependent Effector with Roles in Protective and Pathogenic Immunity. J Immunol. 2015;195:1933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [417].Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J Immunol. 2015;195:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [418].Basso L, Serhan N, Tauber M, Gaudenzio N. Peripheral neurons: Master regulators of skin and mucosal immune response. Eur J Immunol. 2019;49:1984–97. [DOI] [PubMed] [Google Scholar]
- [419].Stepp MA, Tadvalkar G, Hakh R, Pal-Ghosh S. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia. 2017;65:851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [420].Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What Causes Eye Pain? Curr Ophthalmol Rep. 2015;3:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [421].Stepp MA, Pal-Ghosh S, Tadvalkar G, Williams A, Pflugfelder SC, de Paiva CS. Reduced intraepithelial corneal nerve density and sensitivity accompany desiccating stress and aging in C57BL/6 mice. Exp Eye Res. 2018;169:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [422].Reichard M, Weiss H, Poletti E, Ruggeri A, Guthoff RF, Stachs O, et al. Age-Related Changes in Murine Corneal Nerves. Curr Eye Res. 2016;41:1021–8. [DOI] [PubMed] [Google Scholar]
- [423].Marco B, Alessandro R, Philippe F, Fabio B, Paolo R, Giulio F. The Effect of Aging on Nerve Morphology and Substance P Expression in Mouse and Human Corneas. Invest Ophthalmol Vis Sci. 2018;59:5329–35. [DOI] [PubMed] [Google Scholar]
- [424].Tavakoli M, Ferdousi M, Petropoulos IN, Morris J, Pritchard N, Zhivov A, et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care. 2015;38:838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [425].Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16:1396–9. [DOI] [PubMed] [Google Scholar]
- [426].Huang CC, Yang W, Guo C, Jiang H, Li F, Xiao M, et al. Anatomical and functional dichotomy of ocular itch and pain. Nat Med. 2018;24:1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [427].Wiederhold S, Papadakis T, Chubanov V, Gudermann T, Krasteva-Christ G, Kummer W. A novel cholinergic epithelial cell with chemosensory traits in the murine conjunctiva. Int Immunopharmacol. 2015;29:45–50. [DOI] [PubMed] [Google Scholar]
- [428].Sabatino F, Di Zazzo A, De Simone L, Bonini S. The Intriguing Role of Neuropeptides at the Ocular Surface. Ocul Surf. 2017;15:2–14. [DOI] [PubMed] [Google Scholar]
- [429].Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp Eye Res. 1998;66:421–35. [DOI] [PubMed] [Google Scholar]
- [430].Xue Y, He J, Xiao C, Guo Y, Fu T, Liu J, et al. The mouse autonomic nervous system modulates inflammation and epithelial renewal after corneal abrasion through the activation of distinct local macrophages. Mucosal Immunol. 2018;11:1496–511. [DOI] [PubMed] [Google Scholar]
- [431].Sacchetti M, Micera A, Lambiase A, Speranza S, Mantelli F, Petrachi G, et al. Tear levels of neuropeptides increase after specific allergen challenge in allergic conjunctivitis. Mol Vis. 2011;17:47–52. [PMC free article] [PubMed] [Google Scholar]
- [432].Paunicka KJ, Mellon J, Robertson D, Petroll M, Brown JR, Niederkorn JY. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant. 2015;15:1490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [433].Lucas K, Karamichos D, Mathew R, Zieske JD, Stein-Streilein J. Retinal laser burn-induced neuropathy leads to substance P-dependent loss of ocular immune privilege. J Immunol. 2012;189:1237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [434].Ferrari G, Bignami F, Giacomini C, Capitolo E, Comi G, Chaabane L, et al. Ocular surface injury induces inflammation in the brain: in vivo and ex vivo evidence of a corneal-trigeminal axis. Invest Ophthalmol Vis Sci. 2014;55:6289–300. [DOI] [PubMed] [Google Scholar]
- [435].Guzman M, Miglio MS, Zgajnar NR, Colado A, Almejun MB, Keitelman IA, et al. The mucosal surfaces of both eyes are immunologically linked by a neurogenic inflammatory reflex involving TRPV1 and substance P. Mucosal Immunol. 2018. [DOI] [PubMed] [Google Scholar]
- [436].Yamaguchi T, Hamrah P, Shimazaki J. Bilateral Alterations in Corneal Nerves, Dendritic Cells, and Tear Cytokine Levels in Ocular Surface Disease. Cornea. 2016;35 Suppl 1:S65–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [437].Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol. 2017;182:90–8. [DOI] [PubMed] [Google Scholar]
- [438].Schein OD, Hochberg MC, Munoz B, Tielsch JM, Bandeen-Roche K, Provost T, et al. Dry eye and dry mouth in the elderly: a population-based assessment. Arch Intern Med. 1999;159:1359–63. [DOI] [PubMed] [Google Scholar]
- [439].Terry MA. Dry eye in the elderly. Drugs Aging. 2001;18:101–7. [DOI] [PubMed] [Google Scholar]
- [440].Moss SEKR, Klein BE. Long-term Incidence of Dry Eye in an Older Population. Optom Vis Sci 2008;85:668–74. [DOI] [PubMed] [Google Scholar]
- [441].Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–26. [DOI] [PubMed] [Google Scholar]
- [442].Wang MTM, Muntz A, Lim J, Kim JS, Lacerda L, Arora A, et al. Ageing and the natural history of dry eye disease: A prospective registry-based cross-sectional study. The ocular surface. 2020. [DOI] [PubMed] [Google Scholar]
- [443].Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–9. [DOI] [PubMed] [Google Scholar]
- [444].Buchholz P, Steeds CS, Stern LS, Wiederkehr DP, Doyle JJ, Katz LM, et al. Utility assessment to measure the impact of dry eye disease. OculSurf. 2006;4:155–61. [DOI] [PubMed] [Google Scholar]
- [445].Barber L, Khodai O, Croley T, Lievens C, Montaquila S, Ziemanski J, et al. Dry eye symptoms and impact on vision-related function across International Task Force guidelines severity levels in the United States. BMC Ophthalmol. 2018;18:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [446].Bradley JL, Ozer Stillman I, Pivneva I, Guerin A, Evans AM, Dana R. Dry eye disease ranking among common reasons for seeking eye care in a large US claims database. Clin Ophthalmol. 2019;13:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [447].Han SB, Hyon JY, Woo SJ, Lee JJ, Kim TH, Kim KW. Prevalence of dry eye disease in an elderly Korean population. ArchOphthalmol. 2011;129:633–8. [DOI] [PubMed] [Google Scholar]
- [448].Lu P, Chen X, Liu X, Yu L, Kang Y, Xie Q, et al. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea. 2008;27:545–51. [DOI] [PubMed] [Google Scholar]
- [449].Ayaki M, N K; Kawashima M; Uchino M; Kaido M; Tsubota K. Age Is a Determining Factor of Dry Eye-Related Signs and Symptoms. Diagnostics 2020;10,:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [450].Johansson AK, Johansson A, Unell L, Ekbäck G, Ordell S, Carlsson GE. Self-reported dry mouth in Swedish population samples aged 50, 65 and 75 years. Gerodontology. 2012;29:e107–15. [DOI] [PubMed] [Google Scholar]
- [451].García-Carrasco M, Cervera R, Rosas J, Ramos-Casals M, Morlà RM, Sisó A, et al. Primary Sjögren’s syndrome in the elderly: clinical and immunological characteristics. Lupus. 1999;8:20–3. [DOI] [PubMed] [Google Scholar]
- [452].Haga HJ, Jonsson R. The influence of age on disease manifestations and serological characteristics in primary Sjögren’s syndrome. Scand J Rheumatol. 1999;28:227–32. [DOI] [PubMed] [Google Scholar]
- [453].Tishler M, Yaron I, Shirazi I, Yaron M. Clinical and immunological characteristics of elderly onset Sjögren’s syndrome: a comparison with younger onset disease. J Rheumatol. 2001;28:795–7. [PubMed] [Google Scholar]
- [454].Botsios C, Furlan A, Ostuni P, Sfriso P, Andretta M, Ometto F, et al. Elderly onset of primary Sjögren’s syndrome: clinical manifestations, serological features and oral/ocular diagnostic tests. Comparison with adult and young onset of the disease in a cohort of 336 Italian patients. Joint Bone Spine. 2011;78:171–4. [DOI] [PubMed] [Google Scholar]
- [455].Drosos AA, Andonopoulos AP, Costopoulos JS, Papadimitriou CS, Moutsopoulos HM. Prevalence of primary Sjögren’s syndrome in an elderly population. Br J Rheumatol. 1988;27:123–7. [DOI] [PubMed] [Google Scholar]
- [456].Radfar L, Kleiner DE, Fox PC, Pillemer SR. Prevalence and clinical significance of lymphocytic foci in minor salivary glands of healthy volunteers. Arthritis Rheum. 2002;47:520–4. [DOI] [PubMed] [Google Scholar]
- [457].Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. Journal of ophthalmology. 2012;2012:285851–29. [DOI] [PMC free article] [PubMed] [Google Scholar]


