Abstract
Background:
In youth and young adults with autism spectrum disorder (ASD), executive function (EF) deficits may be a promising treatment target with potential impact on everyday functioning.
Objective:
To conduct a pilot randomized, double-blind, parallel, controlled trial evaluating repetitive transcranial magnetic stimulation (rTMS) for EF deficits in ASD.
Method:
In Toronto, Ontario (November 2014 to June 2017), a 20-session, 4-week course of 20 Hz rTMS targeting dorsolateral prefrontal cortex (DLPFC) (90%RMT) was compared to sham stimulation in 16—35 year-olds with ASD (28 male/12 female), without intellectual disability, who had impaired everyday EF performance (n = 20 active/n = 20 sham). Outcome measures evaluated protocol feasibility and clinical effects of active vs. sham rTMS on EF performance. The moderating effect of baseline functioning was explored.
Results:
Of eligible participants, 95% were enrolled and 95% of randomized participants completed the protocol. Adverse events across treatment arms were mild-to-moderate. There was no significant difference between active vs. sham rTMS on EF performance. Baseline adaptive functioning moderated the effect of rTMS, such that participants with lower baseline functioning experienced significant EF improvement in the active vs. sham group.
Conclusions:
Our pilot RCT demonstrated the feasibility and acceptability of using high frequency rTMS targeting DLPFC in youth and young adults with autism. No evidence for efficacy of active versus sham rTMS on EF performance was found. However, we found promising preliminary evidence of EF performance improvement following active versus sham rTMS in participants with ASD with more severe adaptive functioning deficits. Future work could focus on examining efficacy of rTMS in this higher-need population.
Clinical trial registration:
Repetitive Transcranial Magnetic Stimulation (rTMS) for Executive Function Deficits in Autism Spectrum Disorder and Effects on Brain Structure: A Pilot Study; https://clinicaltrials.gov/ct2/show/NCT02311751?term=ameis&rank=1; NCT02311751. The trial was funded by: an American Academy of Child and Adolescent Psychiatry (AACAP) Pilot Research Award, the Innovation Fund from the Alternate Funding Plan of the Academic Health Sciences Centres of Ontario, and an Ontario Mental Health Foundation (OMHF) Project A Grant and New Investigator Fellowship.
Keywords: Autism, Clinical trial, Repetitive transcranial magnetic, stimulation, Intervention, Executive functioning, Youth
Introduction
Autism spectrum disorder (ASD) affects 1—2% of the population worldwide [1]. The majority of individuals diagnosed with ASD, including those without intellectual disability (ID), feature prominent functional impairment and require substantial support [2–4]. Evidence-based treatment options are particularly lacking for 13-30 year-olds with ASD where no intervention has been shown to improve long-term outcomes [5]. The heterogeneity that is inherent to ASD and the absence of consistent biological markers are key challenges for treatment innovation [6].
Executive functions (EF) are high-order cognitive functions necessary for shifting flexibly from one focus to another (set-shifting), controlling/regulating behavior (response inhibition), and maintaining and manipulating information over a short period of time (working memory) [7]. A recent meta-analysis characterized the presence and stability across development of impaired EF performance (with a moderate effect size) in ASD versus age and IQ-matched control groups across multiple domains (e.g., planning, working memory, mental flexibility) [8]. As EF performance is a strong predictor of adaptive (everyday) functioning and mental health in ASD [9,10], interventions that target EF deficits could have a clinically meaningful impact on functional outcomes.
Repetitive transcranial magnetic stimulation (rTMS) involves stimulation of the superficial cortex by a train of magnetic field pulses at typical frequencies between 1 and 20Hz [11]. Based on favorable safety and efficacy, high frequency rTMS to left dorsolateral prefrontal cortex (DLPFC) is an approved treatment for depression [12]. Improvement on secondary neuropsychological outcomes in efficacy studies for depression has sparked interest in developing TMS interventions for cognition [13]. A meta-analysis examining studies that included adults with depression, schizophrenia, Alzheimer’s dementia or unimpaired controls, indicated that protocols using repeated sessions of high frequency rTMS to DLPFC were most promising for improvement of EF outcomes [13]. Preliminary RCTs (n = 17—36) exploring rTMS effects on specific domains have shown improved verbal memory recall [14], facial affect recognition [15] and working memory [16] in participants with chronic schizophrenia following active rTMS to DLPFC versus sham stimulation with large effect sizes reported [15,16].
Several smaller studies provide preliminary support for the feasibility and acceptability of rTMS as an intervention for individuals with ASD [17]. Two open-label studies (n = 40 and 54, respectively) showed improved performance on a selective attention task following 12—18 weeks of once-weekly, 1 Hz rTMS delivered at 90% resting motor threshold (RMT), stimulating left-only or bilateral DLPFC in 9—21 year-olds with ASD without ID, compared to a waitlist control group [18,19]. Only one controlled study of daily rTMS sessions has been published in ASD [20]. That RCT showed a reduction in self-reported social relating symptoms measured on the Ritvo Autism-Asperger Diagnostic Scale in adults with ASD that received two weeks of weekday 5 Hz rTMS at 90% RMT to bilateral dorsomedial prefrontal cortex versus sham stimulation (n = 28). No study that we are aware of has examined whether rTMS can enhance EF in persons with ASD [20].
The fronto-parietal network (comprised of DLPFC, and parietal cortex along the intraparietal sulcus) is hypothesized to support engagement and flexible integration of distributed brain networks, supporting high-order cognitive ability, including EF [21]. A number of neuroimaging studies have found evidence of altered functional MRI-measured DLPFC activation or altered distributed frontoparietal network connectivity during spatial working memory (SWM) performance in children [22] or adults with ASD versus controls [23–25]. Children and adults with ASD commonly have impaired SWM performance [26] and these deficits have been linked to adaptive functioning [27]. Hence, the DLPFC may be a promising biological target for modifying SWM performance in autism through augmentation of local activation or frontoparietal connectivity with the potential for impact on everyday functioning.
In this pilot RCT, we administered the same high frequency rTMS protocol targeting bilateral DLPFC demonstrated to be safe, feasibly implemented and shown to improve working memory deficits in individuals with chronic schizophrenia [16], in a group of autistic youth and young adults. Individuals with ASD are often treated with medications similar to those used in schizophrenia (e.g., antipsychotics, antidepressants) and similar cognitive and functional impairments have been found across young adults with either primary clinical diagnosis [28]. Parameters selected (i.e., intensity, frequency, duration) were also based on previously published data demonstrating neurophysiological changes to the cortex in control participants [29,30] and in line with intensity used in previously published rTMS trials in ASD. Our primary objectives were to: (i) investigate the feasibility and acceptability of conducting a future definitive trial of rTMS for EF deficits in ASD based on recruitment, retention, adverse events, and (ii) examine for preliminary evidence of efficacy of rTMS on EF performance. Given the heterogeneity inherent to ASD, we anticipated that certain variables might influence efficacy of rTMS. Exploratory sub-group analyses were conducted to examine the moderating effects of baseline adaptive functioning and gender.
Methods and materials
Design:
A randomized, blinded, parallel, sham-controlled design, comparing active versus sham rTMS administered 5 days/week for 4 weeks was conducted at the Centre for Addiction and Mental Health (CAMH, Toronto, Canada). Clinical and cognitive assessments and MRI scanning were completed in clinical trial participants within one week of the first and following the last rTMS session. Clinical and cognitive assessments were repeated 4 weeks following the last rTMS session. The study was approved by the CAMH research ethics board (protocol reference #119—2013), and registered with Clinicaltrials.gov (NCT02311751) [6].
Participants:
Study participants were recruited from clinics at CAMH (a large academic mental health hospital), local community clinics, and through local and online advertisement. Participants were initially screened over telephone. An eligibility visit was subsequently scheduled with research staff and a study clinician. No study data were acquired until participants signed informed consent. The MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) was used to ensure all participants were competent to consent to study participation [31]. Inclusion criteria were: 16—35 years of age, DSM-IV autistic disorder, Asperger’s disorder or pervasive developmental disorder-not otherwise specified or DSM-5 autism spectrum disorder, based on prior clinical assessment and corroborated by assessment using the Autism Diagnostic Observation Schedule-2 (ADOS-2), Module 4 (administered by SHA) [32]. Further inclusion was based on IQ ≥ 70 on the General Abilities Index of the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) [33], and the presence of clinically significant EF impairment [T score>65 on any subscale of the Behavioral Rating Inventory for Executive Function (BRIEF)-Self Report (SR) Version or BRIEF-Adult (self report version for participants ≥18 years] with informant input where available [34]. All psychotropic medications were continued during the trial (i.e., no medications were held at any point during trial participation). No changes were allowed within 4 weeks of randomization to treatment end. Exclusion criteria included: any prior history of seizures (including febrile seizures), pregnant or potential for pregnancy, taking benzodiazepines (≥2 mg lorazepam equivalent) or anticonvulsants, history of major medical or neurological illness. See (6) for full inclusion/exclusion details.
Intervention:
A B65-type figure-of-eight coil connected to a MagPro x100 stimulator (Magventure Inc.) was used. Resting motor threshold (RMT) was determined by a physician not involved in the current study and was blind to treatment assignment [35]. RMT was determined according to standard methods and was not repeated during the treatment course. Active treatment was delivered at 90%RMT intensity. Stimulation was administered at 20 Hz with 25 stimulation trains of 30 stimuli each with a 30-s inter-train interval at equivalent stimulation parameters (750 pulses/hemisphere = 1500 pulses total/session). Stimulation was administered every session starting with either left or right DLPFC, based on random sequence assignment, followed by immediate subsequent stimulation to the contralateral hemisphere. Left/right hemisphere sequence was fixed across sessions. Target localization: a T1 anatomical MRI (Sagital BRAVO, TR = 6.736 ms, TE = 3 ms, flip angle = 8, voxel size 0.9 mm isotropic) on a 3 T GE MR750 (General Electric, Milwaukee, WI) research-dedicated scanner at CAMH with fiducial markers in place for later co-registration was acquired. Individualized neuronavigation using ascension MINIBIRD/MRIcro/MRIreg to target the predetermined stimulation site, BA9 and superior section BA46, [x,y,z = −50, 30, 36 mm (left), +50, 30, 36 mm (right), MNI coordinates] was applied to each participant’s baseline T1-weighted MRI following registration with a standard template [36]. See supplement for additional description of methods used for DLPFC target localization. Sham rTMS consisted of a single-wing tilt position using the same parameters and site to produce tactile and auditory stimulation with minimal direct brain effects.
Outcomes:
A priori thresholds for feasibility and acceptability, were based on: (i) ability to enroll 60% of eligible participants who were approached, (ii) ability to retain 80% of randomized participants, (iii) completion of the full rTMS protocol in 80% of those that began the trial, (iv) the absence of severe or serious adverse events. Preliminary efficacy was examined by comparing clinical effects of rTMS on The Cambridge Neuropsychological Test Automated Battery (CANTAB) SWM total errors and BRIEF Metacognition Index (BRIEF-MCI) score between active and sham groups. The main outcome measure of interest was change in CANTAB SWM total errors (pre-to-post rTMS score differences). Exploratory subgroup analyses were performed examining whether baseline adaptive function or gender moderated change in EF performance in the active vs. sham groups.
Clinical and cognitive assessments
Administered at baseline only:
the Vineland Adaptive Behavior Scale-II (VABS-II) [37], a standardized measure of daily functioning elicited from self-report (with informant/parent input when available) provided a composite score of overall functioning (Adaptive Behavior Composite) and standard scores for Communication, Daily Living and Socialization domains. The Mini International Neuropsychiatric Interview (MINI) (≥18 years) or MINI for Children and Adolescents (MINI-KID) (<18 years) was used to assess for the presence of co-occurring psychiatric disorders [38]. The Keel Transcranial Magnetic Stimulation Adult Safety Screen was used at baseline to identify potential safety problems related to TMS [39].
Administered at baseline, post-rTMS, & one-month follow-up:
The BRIEF-SR or BRIEF-A provided an ecologically sensitive measure of EF performance in everyday (school/home/work) environments. The scale yields eight subscale scores, behavioral regulation and metacognition indices and an overall executive composite score. The BRIEF-MCI is computed using working memory, plan/organize, organization of materials, and monitor subscale scores. BRIEF T scores >65 on any subscale are indicative of clinically significant EF impairment [34]. The CANTAB SWM task (www.cambridgecognition.com) is a self-ordered search task where participants search for tokens hidden inside colored squares and must remember which boxes have already been searched. The number of items to be searched increases from 2 to 8, reflecting increased working memory load. Performance measures include the number of search strategies used in 6- and 8-box trials and the total number of errors in 4-, 6-, and 8-box trials.
Adverse event assessment:
A semi-structured interview was administered following each treatment session and recorded in a treatment log by the rTMS technician. The standardized interview included the open-ended query for any adverse event or physical discomfort experienced. A standardized adverse event assessment form was completed if adverse events or physical discomfort were reported. Using this form, detailed descriptions of the adverse events experienced were recorded, including rating the event as mild (no impairment), moderate (some impairment or need for intervention to prevent impairment), severe (evidence of impairment and need for intervention) or serious (need for hospitalization or major threat to health/well-being). See supplement for treatment log and adverse event form.
Sample size:
The effect size of the mean change difference in working memory performance between active and sham groups from the prior schizophrenia study was Cohen’s d = 0.9 (16). A similar effect size on open-label studies finding improved selective attention (n = 25, 38) in autism following rTMS has been shown [18,19]. These studies were used to guide sample size estimation for the current study. Based on guidance for pilot sample planning, our study was powered (90% power and two-sided 5% significance) to detect a moderate (0.5)-to-large (0.8) standardized effect of treatment [40].
Randomization & Blinding:
For participant allocation, a computer-generated randomization list with 1:1 allocation based on a permuted block method with a random number generator was used. A research assistant (independent of recruitment) concealed the allocation sequence using sealed, opaque envelopes. After consent, eligibility determination and baseline assessment completion, the corresponding envelope for a given participant ID was given to the technician prior to the first rTMS session. The rTMS technician was aware of group assignment, but technicians were not involved in allocation or clinical/cognitive assessments. Study investigators, clinical/cognitive raters and participants remained blind to treatment condition until the last participant completed the full rTMS protocol. An independent research statistician completed all statistical analysis following trial completion. Clinical trial participants were asked whether they believed they received active stimulation following the first and last rTMS session to evaluate participant blinding.
Analysis:
Demographic and clinical variables at baseline were compared between active (n = 20) and sham (n = 20) groups using nonparametric Mann-Whitney U tests for continuous variables and Fisher’s Exact tests for categorical variables. A Fisher’s exact test was used to examine differences between groups in adverse events using an intent-to-treat (ITT) approach. A Poisson regression was used to examine the rate of adverse events per visit, where the outcome was the number of adverse events, and number of visits was included as an exposure variable. An ITT approach with last observation carried forward (LOCF) for missing data was used to explore clinical effects of rTMS. Linear mixed-effect regression models to examine the predictive effects of treatment-group, time, and treatment-by-time interaction on [: SWM total errors and [] BRIEF-MCI scores, including baseline, post-rTMS, and one-month follow-up time-points in the model, were used. Subjects were treated as random effects. Baseline IQ and baseline performance score on the outcome measure of interest were entered as covariates. Within this linear model, effect sizes and 95% confidence intervals were calculated for the mean change difference in CANTAB spatial working memory scores (i.e., pre/post-score differences) between groups (i.e., primary outcome measure of interest) and for change in BRIEF MCI scores.
Exploratory Subgroup Analyses:
To explore whether baseline VABS-II Adaptive Behavior Composite score or gender moderated rTMS effects, two additional regression models (as described above) were run examining the three-way interaction effects of treatment-by-time-by-adaptive functioning and treatment-by-time-by-gender on EF outcome measures. Cohen’s f2 was used to determine clinical effect of between group differences, as appropriate within a multiple regression model with continuous independent and dependent variables [41]. Cohen’s f2≥0.02, f2≥0.15, f2≥0.35 represent small, medium and large effect sizes, respectively [41].
All analyses were conducted using the MIXED Procedure from SAS/STAT 14.1 software, Version 9.4 of SAS System. Graphs were created using ggplot2 [42] in R software (https://www.R-project.org/).
Results
Feasibility and Acceptability:
Participant enrollment took place between November 2014 and June 2017 (when trial recruitment target reached). See CONSORT flow diagram (Fig. 1). Ninety-five percent of eligible participants were enrolled. Two participants allocated to active treatment (5%) dropped out after 1—2 rTMS sessions. Thirty-eight participants remained in the trial until treatment end (95% retention). See Table 1 and Supplementary Table 1 for participant demographic and clinical characteristics at baseline. In the final sample, a larger number of females were enrolled in the study than expected based on the updated prevalence literature (i.e., male-to-female ratio in our ASD sample = 2.3:1 versus 3.3:1 expected ratio) [43]. See Supplementary Table 2 for baseline clinical/cognitive characteristics by gender. While mean standard score on the VABS-II Adaptive Behavior Composite was more than two standard deviations below mean IQ score across the sample, within-subject scores ranged considerably (score range = 43—104, categorized as low to adequate functioning). A total of 9/40 (~23%) clinical trial participants had clinically significant attention-deficit/hyperactivity disorder (ADHD), which was defined as being treated with a stimulant medication at the time of trial participation. EF deficits are prominent in individuals with ADHD [44,45] and may be more pronounced in individuals with ASD and comorbid ADHD. A descriptive comparison in the current sample indicated that EF performance overlapped across trial participants with ASD plus ADHD compared to all other trial participants, without clear indication of a distinction between groups (see Supplementary Fig. 2). Treatment groups did not differ on any demographic or clinical features. No between-group difference was found for RMT expressed as percentage of maximum stimulator output [Active: left DLPFC = 31—67% (mean = 48.5 ± 8.6), right DLPFC = 32—68% (mean = 49.2 ± 8.8); Sham: left DLPFC = 34—76%, mean = 53.8 ± 11.8, right DLPFC = 38—70% (mean = 51.8 ± 10.1)] or for percentage of participants believing they had received active rTMS (active = 84%, sham = 80%), indicating integrity of the blind was upheld. Safety: A total of 8/20 (0.4) and 5/20 (0.25) participants in the active and sham groups, respectively, experienced any adverse event across treatment sessions (risk difference = 0.15; effect size, calculated as relative risk of adverse event in sham/active group = 0.63). Adverse events experienced across groups included: headache, pain, nausea, nose bleed, congestion, laceration. The rate of adverse events in the active group was 1.37 times the rate in the sham group (rate: active = 7%, sham = ~2.6%, p-value = 0.24; p = 0.9, after exclusion of one participant that reported 15 adverse events, see Supplementary Fig. 3 & Supplementary Table 3). All adverse events were rated as mild or moderate (confined to the need for acetaminophen or ibuprofen following stimulation to resolve headache/localized pain). See Table 2 for total number, odds of adverse event by type/group, and number needed to harm.
Fig. 1.
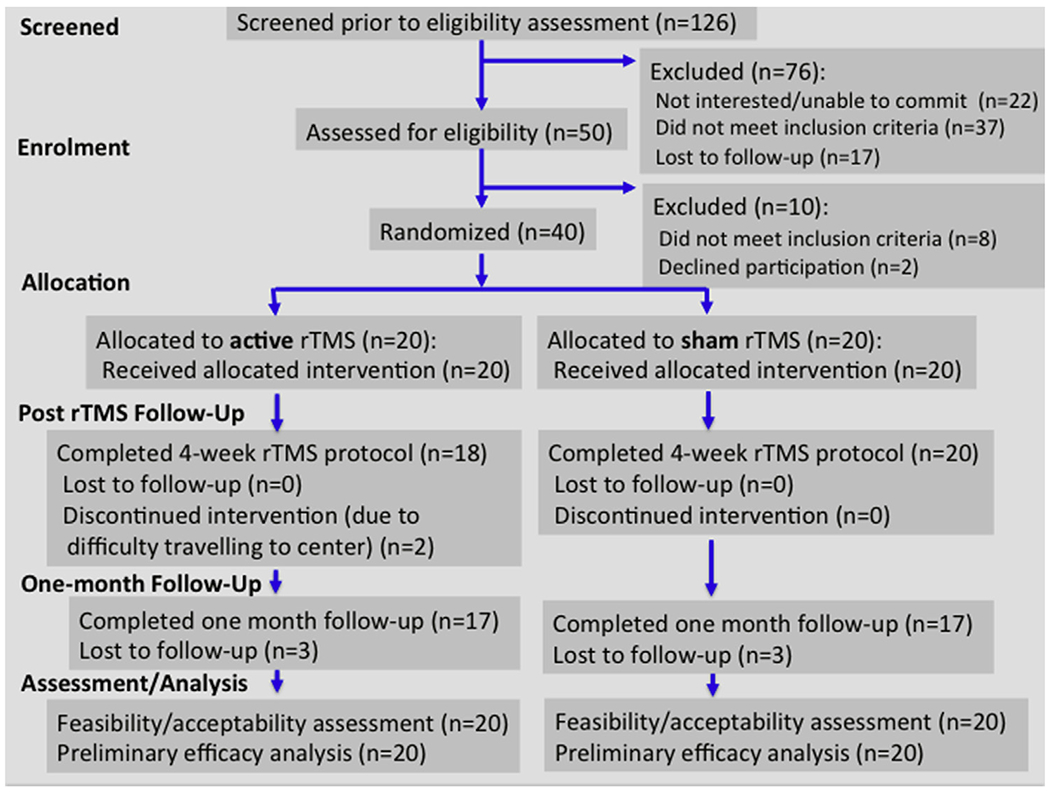
CONSORT flow diagram.
Table 1.
Baseline participant demographic and clinical characteristics.
| Total Sample (n = 40) Mean ± SD | Sham (n = 20) Mean ± SD | Active (n = 20) Mean ± SD | |
|---|---|---|---|
| Age in years | 22.58 ± 4.5 | 21.65 ± 4.6 | 23.50 ± 4.2 |
| Gender | 28 M (70%) | 14 M (70%) | 14 M (70%) |
| Handedness | 35 Right | 15 Right | 20 Right |
| Psychotropic Medications | 26 (65%) | 10 (50%) | 16 (80%) |
| GAI (Inclusion IQ) | 109.7 ± 17.6a | 109.3 ± 17.3 | 110.2 ± 18.2 |
| FSIQ | 100.68 ± 16.3 | 100.45 ± 17.0 | 100.90 ± 15.9 |
| Comorbidity on MINI | 25 (62.5%) | 11 (55%) | 14 (70%) |
| Years of Completed Educationa | 13.8 ± 2.8 | 12.8 ± 2.3 | 15 ± 2.9 |
| VABS-II | |||
| Adaptive Composite | 74.5 ± 10.6 | 76 ± 10.1 | 73 ± 11.1 |
| Communication | 76.4 ± 15.9 | 76.05 ± 18.7 | 76.80 ± 13.1 |
| Social | 78.3 ± 12.9 | 79.70 ± 11.6 | 76.65 ± 14.3 |
| Daily Living | 79.4 ± 10.9 | 81.20 ± 10.6 | 77.60 ± 11.2 |
| BRIEF | |||
| Metacognition Index | 71.7 ± 9.0 | 70.8 ± 10.2 | 72.6 ± 7.9 |
| Global Executive Composite | 69.4 ± 9.1 | 68.90 ± 9.6 | 69.75 ± 9.0 |
| CANTAB | |||
| SWM (total errors) | 23.2 ± 19.8 | 23.6 ± 18.4 | 22.9 ± 21.6 |
| SWM (strategy) | 29.9 ± 6.99 | 30.55 ± 6.7 | 29.25 ± 7.4 |
GAI = General Abilities Index of the Wechsler Adult Intelligence Scale-Fourth Edition, FSIQ = full scale IQ, MINI = The Mini International Neuropsychiatric Interview, VABS-II= Vineland Adaptive Behavior Scale-II, BRIEF= Behavioral Rating Inventory for Executive Function, CANTAB=The Cambridge Neuropsychological Test Automated Battery, SWM = spatial working memory
Years of completed education calculated as total number of education years completed starting from grade 1 (e.g., currently in second year undergraduate university degree = 13, grades 1−12 + 1 additional undergraduate year).
Table 2.
Total events by type presented per case reported and number needed to harm. NNH = number needed to harm, calculated for events that were more likely to occur with active compared to sham rTMS as: 1/Absolute Risk Increase (Absolute Risk Increase = Active rTMS event rate – Sham rTMS event rate).
| Adverse Event |
Sham |
Active |
NNH | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Headache | 4 | 20% | 4 | 20% | – |
| Pain at application site | 1 | 5% | 1 | 5% | – |
| Neck pain | 0 | 0% | 1 | 5% | 20 |
| Nose bleed | 1 | 5% | 0 | 0% | – |
| Nausea | 0 | 0% | 1 | 5% | 20 |
| Laceration | 1 | 5% | 0 | 0% | – |
| Congestion | 0 | 0% | 1 | 5% | 20 |
| Other | 1 | 5% | 1 | 5% | – |
Preliminary Efficacy:
No significant treatment-by-time interaction effect for either SWM total errors (F2,67 = 0.04, p = 0.97) or BRIEF-MCI score (F2,68 = 0.47, p = 0.63) was found (Figs. 2 and 3). Reduction in SWM total errors from baseline to follow-up visit was 17% in the active group and 13% in the sham group. Between-group mean change difference for SWM total errors (t2,67 = 0.24, p = 0.8. effect size = 0.04, 95%CI = −5.8-7.3) and BRIEF-MCI (t2,68 = 0.96, p = 0.3, effect size = 0.28, 95%CI = −2.7-7.7) from baseline to endpoint are detailed in Table 3. A significant main effect of time was found for SWM total errors (F2,67 = 5.17, p = 0.008) and BRIEF-MCI score (F2,68 = 28.4, p < 0.0001), indicating that mean group performance improved across participants over the trial period. Main effects for baseline SWM total errors (F2,67 = 283.2, p < 0.0001) and BRIEF-MCI scores (F2,68 = 105, p < 0.0001) were also found, indicating that baseline score predicted outcome score. No clear pattern of differential response was seen in the participant subgroup with ASD plus ADHD (see Supplementary Fig. 4).
Fig. 2.
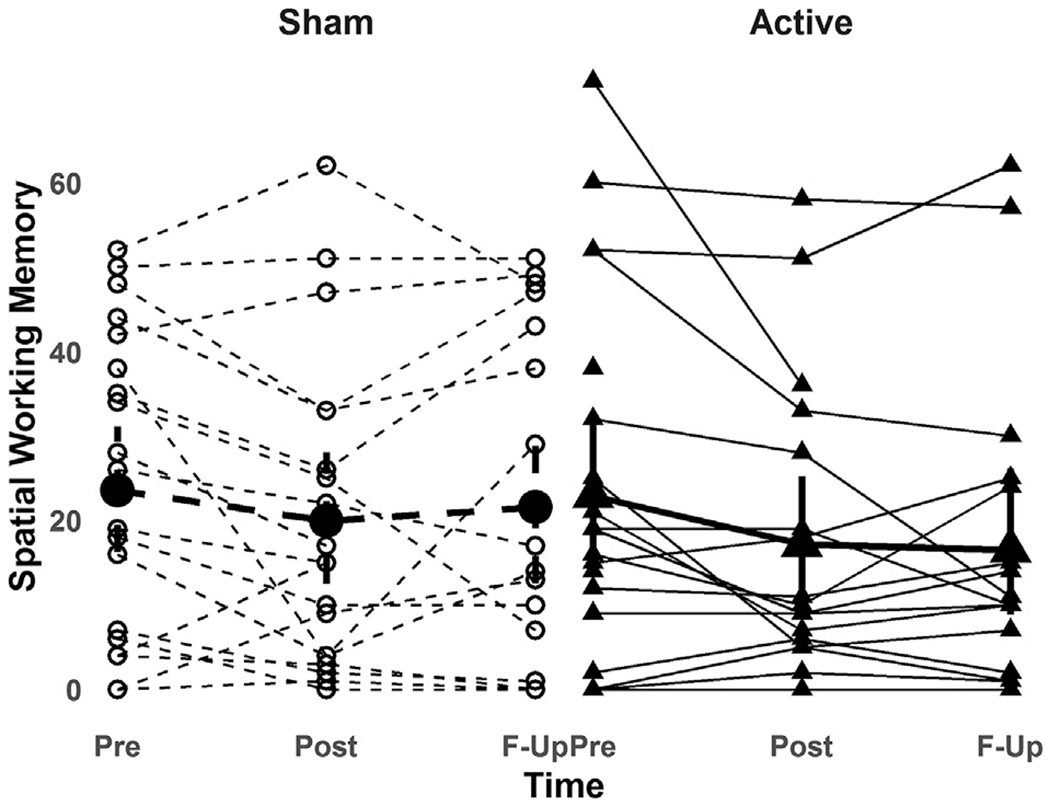
Change in spatial working memory performance in active and sham rTMS groups. across baseline (Pre: n = 20 active, n = 20 sham), post rTMS (Post: n = 18 active, n = 20 sham) and one-month follow-up (F-Up: n = 17 active, n = 17 sham) time-points.
Spatial working memory = total number of errors on the spatial working memory test from the Cambridge Neuropsychological Test Automated Battery (CANTAB).
Fig. 3.
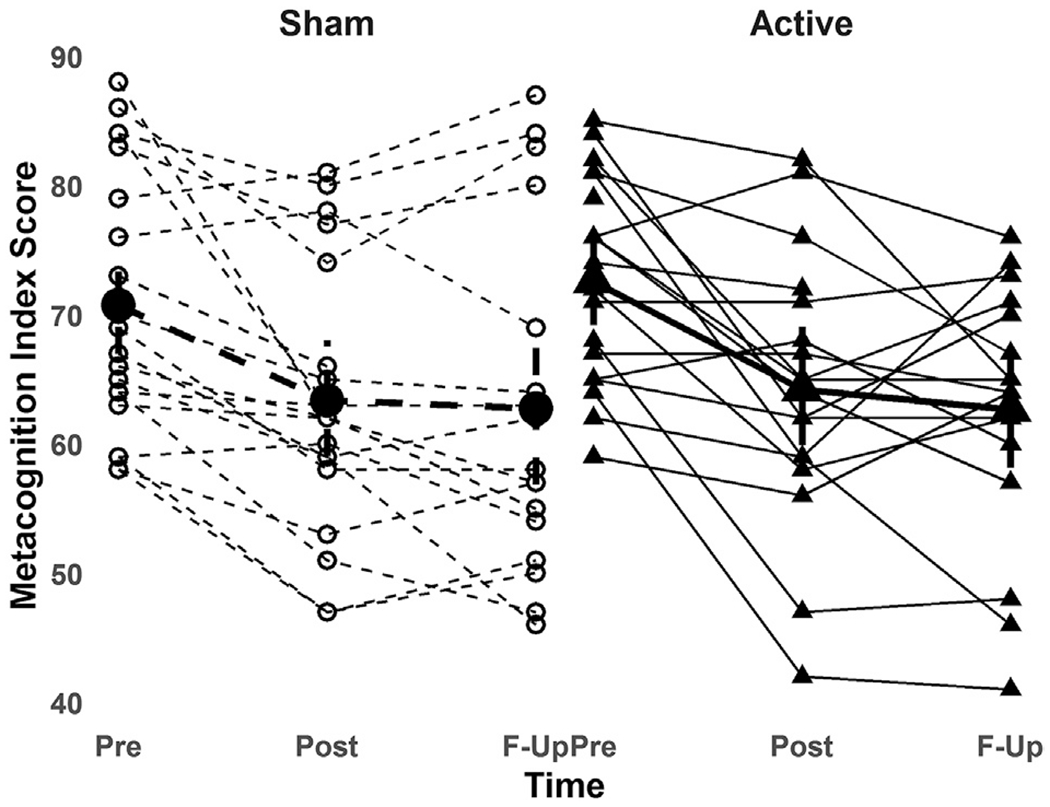
Change in BRIEF Metacognition Index in active and sham rTMS groupsacross baseline (Pre: n = 20 active, n = 20 sham), post rTMS (Post: n = 18 active, n = 20 sham) and one-month follow-up (F-Up: n = 17 active, n = 17 sham) time-points.
Metacognition Index = Behavioral Rating Inventory for Executive Function Metacognition Index (higher scores denote more real-world executive function impairment).
Table 3.
Change in executive function outcomes from baseline to endpoint (one-month follow-up) in active and sham rTMS groups.
| Group | Baseline | Endpoint | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Active (n = 20) (95%CI) | Sham (n = 20) (95%CI) | Active (n = 18) (95%CI) | Sham (n = 20) (95%CI) | MCDb | t | p | 95%CI | ESc | |
| SWM Errorsa | 23 ± 1.7 (19.6–26.5) | 23 ± 1.7 (19.6–26.6) | 19.3 ± 1.9 (15.6–23.1) | 20.2 ± 1.9 (16.4–23.9) | 0.79 | 2,67 = 0.24 | 0.8 | −5.8-7.3 | 0.04 |
| BRIEF MCIa | 71.7 ± 1.4 (68.8–74.6) | 71.5 ± 1.4 (68.6–74.4) | 61.8 ± 1.6 (58.6–64.9) | 64 ± 1.6 (68.6–74.4) | 2.5 | 2,68 = 0.96 | 0.3 | −2.7-7.7 | 0.28 |
BRIEF-MCI = Behavioral Rating Inventory for Executive Function Metacognition Index, SWM Errors = spatial working memory total errors score, MCD = Mean Change Difference, ES = Effect Size, CI=Confidence Interval.
Values presented as Mean ± SE are adjusted for baseline score and IQ.
Difference in mean change from baseline to endpoint were calculated by subtracting the adjusted mean score at follow-up from the adjusted mean score at baseline for each group (active-sham).
Cohen’s d effect sizes were calculated by subtracting the adjusted mean score at follow-up from the adjusted mean score at baseline for each group, then dividing the result by the pooled baseline SDs.
Exploratory Subgroup Analyses:
A significant three-way interaction between treatment group, time and VABS-II Adaptive Behavior Composite score was found for SWM total errors (F2,64 = 3.15, p = 0.049, Cohen’s f2 = 0.06, Fig. 4), driven by a significant decrease in SWM total errors in ASD participants with lower baseline adaptive functioning following active but not sham stimulation. A significant interaction effect between treatment group, time and gender on BRIEF-MCI score (F2,64 = 3.59, p = 0.03, Cohen’s f2 = 0.07) and trend-level interaction between these variables on SWM total errors (F2,63 = 2.75, p = 0.07, Cohen’s f2 = 0.05) was also found, driven by greater reductions in BRIEF-MCI-measured deficits and SWM total errors in females following active versus sham treatment (see Fig. 5).
Fig. 4.
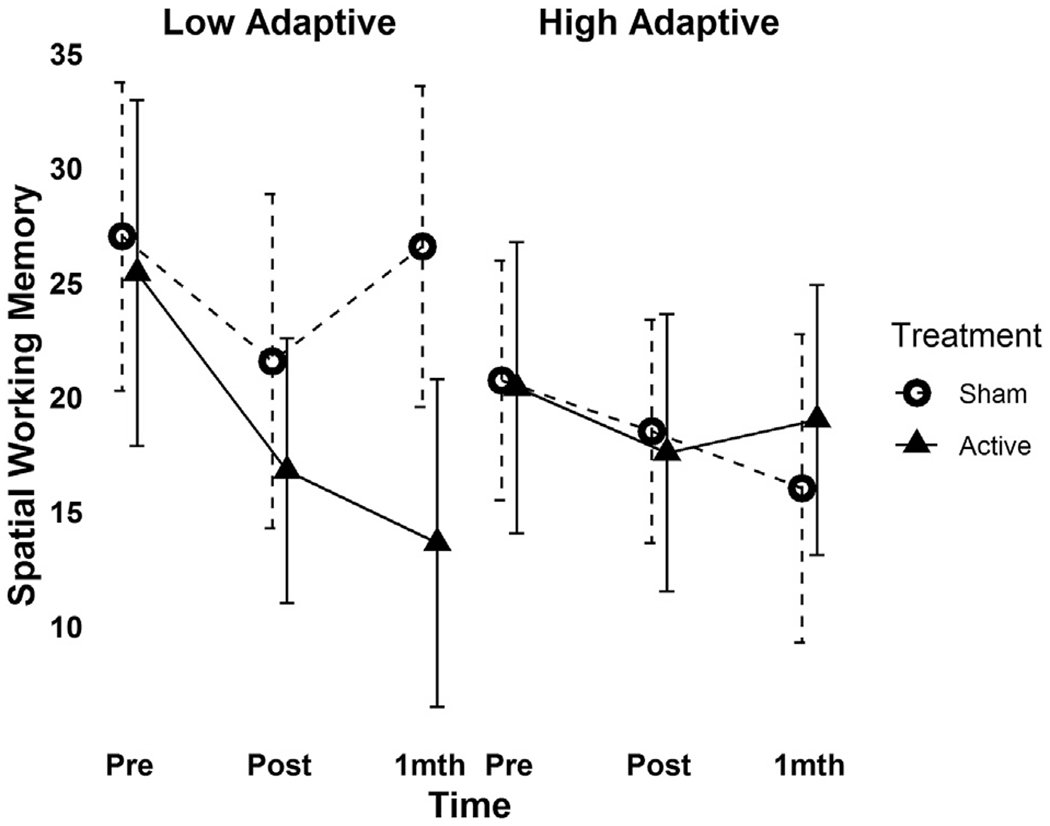
Moderating effect of baseline adaptive functioning on change in spatial working memory performance following rTMS across baseline (Pre: n = 20 active, n = 20 sham), post rTMS (Post: n = 18 active, n = 20 sham) and one-month follow-up (F-Up: n = 17 active, n = 17 sham) time-points.
The significant group-by-time-by-VABS-II (Vineland Adaptive Behavior Scale-II) adaptive composite score is presented in participants following a median split to depict differences in response in participants with low versus high baseline adaptive functioning. Error bars indicate the 95%CI for mean spatial working memory score (in the original scale of the outcome) at a given time point.
Fig. 5.
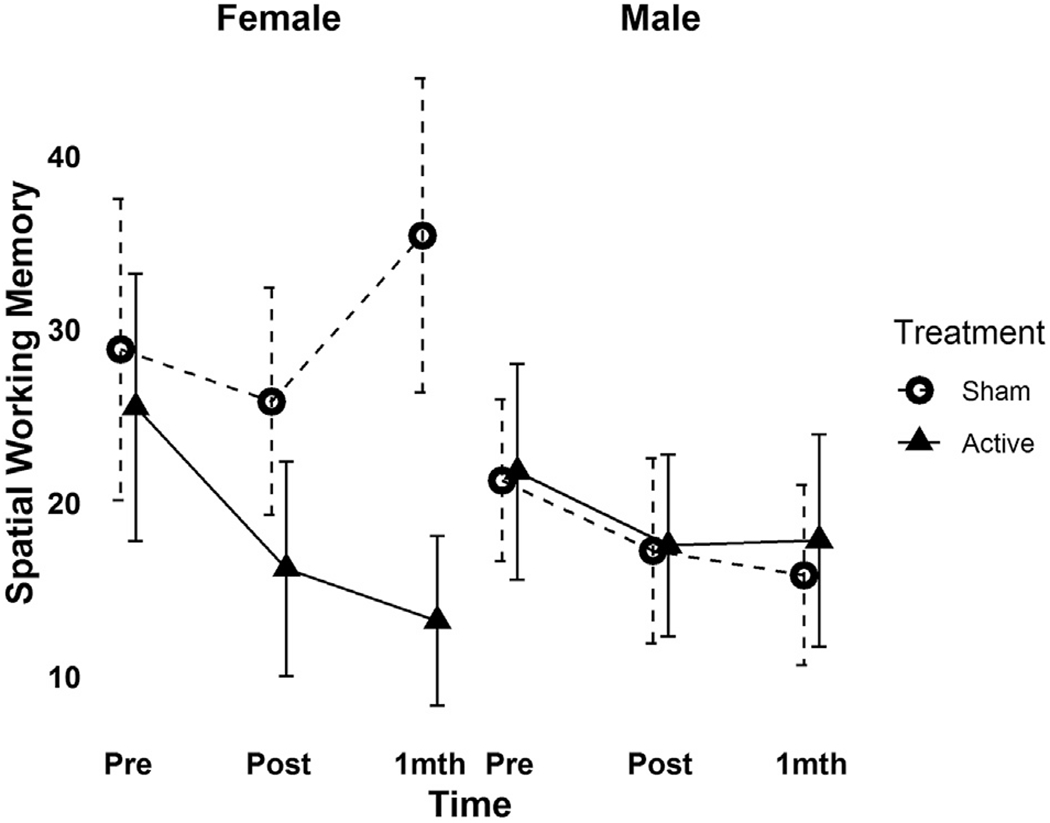
Moderating effect of gender on change in spatial working memory performance following rTMS across baseline (Pre: n = 20 active, n = 20 sham), post rTMS (Post: n = 18 active, n = 20 sham) and one-month follow-up (F-Up: n = 17 active, n = 17 sham) time-points.
Spatial working memory = total number of errors on the spatial working memory test from the Cambridge Neuropsychological Test Automated Battery (CANTAB). Error bars indicate the 95%CI for mean spatial working memory score (in the original scale of the outcome) at a given time point.
Discussion
The current pilot trial is the first to examine the feasibility, acceptability and clinical effects of rTMS for EF deficits in an autism sample. Our pilot study demonstrated good feasibility and acceptability of a 20-session course of 20 Hz rTMS to DLPFC applied to youth and young adults with ASD. We did not find preliminary evidence for efficacy for active vs. sham rTMS in relation to improving EF performance across the sample. A small effect (effect size = 0.28) favoring active compared to sham rTMS on BRIEF-MCI score is potentially clinically relevant. We were powered to detect a moderate to large effect size, somewhat limiting our ability to detect significant effects of this size. Our exploratory analyses implied that baseline adaptive functioning and gender possibly moderated effects of rTMS treatment on EF performance outcomes. Such exploration of clinical effects in our pilot RCT enables evaluation of a number of aspects of our clinical trial design for planning future efficacy studies.
In relation to our aim to determine feasibility and acceptability of our protocol targeting EF deficits, we completed our pilot RCT in 2.5 years with outcomes exceeding a priori thresholds for determining favorable feasibility/acceptability of the implemented protocol. As far as we are aware, only one published double-blind pilot RCT examining rTMS effects in ASD is available [20]. Adverse events in that trial were mild and transient. In our trial, no drop-out was due to adverse events and events experienced were transient, with mild-to-moderate severity. These results are consistent with safety findings from over 40 clinical trials and 15 meta-analyses examining rTMS as a treatment intervention for a variety of patient populations [46], the majority of these testing high-frequency rTMS to DLPFC to modulate cortical excitability [47]. Therefore, one of the key findings from the current pilot trial is that repeated sessions of high-frequency rTMS to DLPFC in youth and young adults with ASD has a similar, favorable safety profile as in other psychiatric populations.
In relation to our examination for preliminary efficacy of rTMS for EF deficits in autism, no effect of rTMS on CANTAB SWM performance was seen. Of note, our ability to detect differences in performance following active versus sham stimulation was diminished by improved EF performance found over time across the trial sample. Similar practice/learning effects have been found on prior small positive rTMS studies for cognitive deficits in schizophrenia [14,15]. A small effect favoring active over sham rTMS on mean change in BRIEF-MCI was found. Although additional research is needed to define a minimum clinically important difference (MCID) on the BRIEF, given that the BRIEF measures EF performance in everyday settings, any change could be clinically meaningful [48]. Evaluation of the 95% confidence interval for the effects estimate of rTMS on BRIEF-MCI in the current study indicates that possible mean change difference may range from a two-point worsening to a seven-point improvement between active versus sham rTMS in individuals with ASD. Given the confidence interval found, it is not evident that an adequately powered large-scale trial using the same protocol would detect a clinically meaningful difference between groups favoring active treatment on the BRIEF [49]. Nonetheless, the upper limit of the confidence interval suggests that further development of rTMS targeting EF deficits in ASD is warranted.
Here, we used the same stimulation protocol as the prior positive pilot RCT that found a large effect of active over sham rTMS on working memory in persons with schizophrenia [16]. An important difference between the prior trial in schizophrenia and the current study is that baseline cognitive deficit on a neuropsychological battery was incorporated as part of the previous trial’s entry criterion. Variable EF impairment and ceiling effects in our ASD sample, in addition to the presence of practice effects, may all contribute to a diminished ability to detect rTMS effects on EF performance. Although large-scale clinical trials of direct effects of rTMS on cognition have yet to be published, one larger study (n = 77 active/79 sham) failed to find significant active over sham rTMS to DLPFC effects on secondary EF outcomes in persons with schizophrenia and prominent negative symptoms [50]. In that study, practice effects across groups were postulated as one factor contributing to the lack of active-sham group difference on EF performance. Interestingly, age, duration and severity of illness also ranged broadly in the larger-scale rTMS study in schizophrenia (50), while participant samples may have been more severely impaired overall (i.e. older age, longer duration of illness, inpatients only) in prior smaller studies finding positive rTMS effects on cognitive outcomes [14–16,51].
Although clinically significant functional impairment in social, occupational or other important areas is part of the diagnostic criteria for ASD, severity of functional impairment varies widely across the autism spectrum [52]. As in the general ASD literature [4,53], a significant gap between IQ and baseline adaptive functioning was found in our sample, but baseline functional ability ranged from low to adequate. We anticipated that individuals with greater baseline functional impairment may exhibit more impaired EF performance and might respond more to active rTMS, relative to participants with higher baseline functioning. Indeed, baseline adaptive functioning may have influenced the clinical effect of rTMS in our sample, as our exploratory analysis revealed a small favorable effect of rTMS on EF performance in participants with lower baseline adaptive functioning. It may be that when functional impairment is more pronounced, there is more room for improvement, pointing to features of an ASD subgroup where the beneficial effect of active rTMS on EF performance may be stronger and perhaps more clinically meaningful. Adaptive functioning has recently been linked to white matter microstructure in children and youth with ASD, where lower functioning predicted reduced fractional anisotropy_ENREF_[34] [54]. This relationship extended to a number of major white matter tracts, including the corpus callosum, connecting homologous DLPFC regions. Functional connectivity of the frontoparietal control network (including the DLPFC) was also found to predict change in adaptive functioning over time in a sample of young people with ASD of similar age and functional level as those in our study [55]. Although speculative, more pronounced alterations at the brain level, involving the DLPFC, may contribute to both EF and general functional impairments.
Interpretation of the influence of gender on clinical effects of rTMS in our study is limited by the small subset of our sample that was female (n = 6/group). Baseline adaptive functioning did not differ between males and females in our sample. However, interpreting the improved effect in females compared to males is complicated by numerically worse baseline EF performance in ASD females compared to males that may have reduced ceiling effects overall, revealing stronger clinical effects of active over sham treatment in a more impaired sample, regardless of gender. Nevertheless, the behavioral presentation of ASD and related cognitive (including EF) characteristics differ in part in females vs. males [56,57] and etiological or neurobiological alterations may vary by sex [58], implying the need for future intervention research to consider sex/gender-stratified approaches. A prior study in schizophrenia also found improved rTMS response on cognitive outcomes in females versus males, though worse baseline performance in females complicated interpretation of this effect as well [59].
Additional considerations for future efficacy study planning include evaluation of: different EF outcome measures that may be less susceptible to practice effects [60], whether the current trial was under-dosed in terms of the intensity and number of pulses per session for targeting cognitive outcomes and the use of an active/sham coil for improved blinding of treatment allocation. Our pilot trial was not powered to determine efficacy and findings from our moderator analyses would not survive stringent multiple comparison correction. Moderator analyses are presented as exploratory to help guide refinement of the approach used for future research. Future research in this area should account for the common presence of co-occurring ADHD in ASD [61] and treatment with medications with the potential to influence EF abilities.
In conclusion, the current pilot, double-blind RCT demonstrates for the first time that repeated sessions of high frequency rTMS is feasible and well tolerated in youth and young adults with ASD and a rigorous clinical trial design can be implemented to study the effects of rTMS in this population. The favorable profile of rTMS highlights the clear opportunity to harness this biological tool for developing targeted treatments that may improve clinical symptoms and functioning in people with ASD. However, the clinical and biological heterogeneity of ASD continues to be a challenge to be overcome. Our findings suggest that any further development of rTMS intervention research in ASD will benefit from careful consideration of which regions of the brain should be targeted, using what stimulation parameters and dosing, for what indication, and in which well-defined clinical subgroup. Our results indicate that a future efficacy trial of rTMS to DLPFC on EF deficits in ASD is warranted in the subgroup of individuals without ID that feature prominent impairment in EF ability and adaptive functioning.
Supplementary Material
Acknowledgements
This publication was made possible through the American Academy of Child and Adolescent Psychiatry (AACAP) Pilot Research Award for Child and Adolescent Psychiatry Residents and Junior Faculty (to SHA), supported by CFAK; its contents are the responsibility of the authors and do not necessarily reflect the official views of AACAP. This research was also supported by: the University of Toronto, Faculty of Medicine, Dean’s Fund New Staff Grant, the Innovation Fund from the Alternate Funding Plan of the Academic Health Sciences Centres of Ontario, an Ontario Mental Health Foundation (OMHF) Project A Grant and New Investigator Fellowship (to SHA). SHA receives support from the Canadian Institutes of Health Research (CIHR), and the National Institutes of Mental Health (NIMH). SHA and M-CL are supported by the O’Brien Scholars Program within the Child and Youth Mental Health Collaborative at the Centre for Addiction and Mental Health (CAMH), The Hospital for Sick Children and University of Toronto, the Slaight Family Child and Youth Mental Health Innovation Fund and The Catherine and Maxwell Meighen Foundation (both via CAMH Foundation). This work was also supported in part by an Academic Scholars Award from the Department of Psychiatry, University of Toroto (to SHA and M-CL). M-CL is also supported by the Ontario Brain Institute via the Province of Ontario Neurodevelopmental Disorders (POND) Network. DMB receives research support from CIHR, NIH, Brain Canada and the Temerty Family Foundation through the CAMH Foundation and the Campbell Family Mental Health Research Institute. ZJD was supported by the OMHF, CIHR, NIMH and the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health Foundation and the Campbell Institute. PEC was supported by R01 MHH3700. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. PS is supported by the Patsy and Jamie Anderson in Child and Youth Mental Health. PD is currently supported by Scottish Rite Charitable Foundation of Canada Research Grant and Caskey/Francis Clinical Research Award.
Disclosures
DMB received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd., and he is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He received in-kind equipment support from Magventure for investigator-initiated research. He received medication supplies for an investigator-initiated trial from Indivior. He has participated in an advisory board for Janssen. PEC has received research grant support from Pfizer, Inc.; equipment support from Neuronetics, Inc.; and received supplies and genotyping services from Assurex Health, Inc. for investigator-initiated studies. He is the primary investigator for a multicenter study funded by Neuronetics, Inc. and a site primary investigator for a study funded by NeoSync, Inc. He is a paid consultant for Procter & Gamble Company. ZJD received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. He has also received in-kind equipment support from Magventure for investigator-initiated research. PS, SHA, MC-L, DJM, PD report no biomedical financial interests or potential conflicts of interest.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2020.01.007.
References
- [1].Lazoff T, Zhong L, Piperni T, Fombonne E. Prevalence of pervasive developmental disorders among children at the English Montreal School Board. Can J Psychiatr 2010. November;55(11):715–20. [DOI] [PubMed] [Google Scholar]
- [2].Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. JCPP (J Child Psychol Psychiatry) 2004. February;45(2):212–29. [DOI] [PubMed] [Google Scholar]
- [3].Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatr 2012. May;57(5):275–83. [DOI] [PubMed] [Google Scholar]
- [4].Tillmann J, San Jose Caceres A, Chatham CH, Crawley D, Holt R, Oakley B, et al. Investigating the factors underlying adaptive functioning in autism in the EU-AIMS Longitudinal European Autism Project. Autism Res 2019. April;12(4): 645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lounds Taylor J, Dove D, Veenstra-VanderWeele J, Sathe NA, McPheeters ML, Jerome RN, et al. 2012. August [PubMed] [Google Scholar]
- [6].Ameis SH, Daskalakis ZJ, Blumberger DM, Desarkar P, Drmic I, Mabbott DJ, et al. Repetitive transcranial magnetic stimulation for the treatment of executive function deficits in autism spectrum disorder: clinical trial approach. J Child Adolesc Psychopharmacol 2017. June;27(5):413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pellicano E The development of executive function in autism. Autism Res Treat 2012;2012:146132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Demetriou EA, Lampit A, Quintana DS, Naismith SL, Song YJC, Pye JE, et al. Autism spectrum disorders: a meta-analysis of executive function. Mol Psychiatry 2018. May;23(5):1198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gilotty L, Kenworthy L, Sirian L, Black DO, Wagner AE. Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychol 2002. December;8(4):241–8. [DOI] [PubMed] [Google Scholar]
- [10].Szatmari P, Bartolucci G, Bremner R, Bond S, Rich S. A follow-up study of high-functioning autistic children. J Autism Dev Disord 1989. June;19(2):213–25. [DOI] [PubMed] [Google Scholar]
- [11].George MS, Padberg F, Schlaepfer TE, O’Reardon JP, Fitzgerald PB, Nahas ZH, et al. Controversy: repetitive transcranial magnetic stimulation or transcranial direct current stimulation shows efficacy in treating psychiatric diseases (depression, mania, schizophrenia, obsessive-complusive disorder, panic, posttraumatic stress disorder). Brain Stimul 2009. January;2(1):14–21. [DOI] [PubMed] [Google Scholar]
- [12].Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burstversus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 2018. April 28;391(10131):1683–92. [DOI] [PubMed] [Google Scholar]
- [13].Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm 2010. January;117(1):105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mogg A, Purvis R, Eranti S, Contell F, Taylor JP, Nicholson T, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res 2007. July;93(1–3):221–8. [DOI] [PubMed] [Google Scholar]
- [15].Wolwer W, Lowe A, Brinkmeyer J, Streit M, Habakuck M, Agelink MW, et al. Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimul 2014. Jul-Aug;7(4):559–63. [DOI] [PubMed] [Google Scholar]
- [16].Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry 2013. March 15;73(6):510–7. [DOI] [PubMed] [Google Scholar]
- [17].Barahona-Correa JB, Velosa A, Chainho A, Lopes R, Oliveira-Maia AJ. Repetitive transcranial magnetic stimulation for treatment of autism spectrum disorder: a systematic review and meta-analysis. Front Integr Neurosci 2018;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sokhadze EM, Baruth JM, Sears L, Sokhadze GE, El-Baz AS, Casanova MF. Prefrontal neuromodulation using rTMS improves error monitoring and correction function in autism. Appl Psychophysiol Biofeedback 2012. June;37(2):91–102. [DOI] [PubMed] [Google Scholar]
- [19].Sokhadze EM, El-Baz AS, Sears LL, Opris I, Casanova MF. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci 2014;8: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Enticott PG, Fitzgibbon BM, Kennedy HA, Arnold SL, Elliot D, Peachey A, et al. A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimul 2014. Mar-Apr;7(2):206–11. [DOI] [PubMed] [Google Scholar]
- [21].Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 2013. September;16(9):1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vogan VM, Morgan BR, Lee W, Powell TL, Smith ML, Taylor MJ. The neural correlates of visuo-spatial working memory in children with autism spectrum disorder: effects of cognitive load. J Neurodev Disord 2014;6(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lynch CJ, Breeden AL, You X, Ludlum R, Gaillard WD, Kenworthy L, et al. Executive dysfunction in autism spectrum disorder is associated with a failure to modulate frontoparietal-insular hub architecture. Biol Psychiatr Cogn Neurosci Neuroimag 2017. September;2(6):537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, et al. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology 2002. September 24;59(6):834–40. [DOI] [PubMed] [Google Scholar]
- [25].Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage 2005. February 1;24(3):810–21. [DOI] [PubMed] [Google Scholar]
- [26].Wang Y, Zhang YB, Liu LL, Cui JF, Wang J, Shum DH, et al. A meta-analysis of working memory impairments in autism spectrum disorders. Neuropsychol Rev 2017. March;27(1):46–61. [DOI] [PubMed] [Google Scholar]
- [27].Rosa M, Puig O, Lazaro L, Valles V, Lera S, Sanchez-Gistau V, et al. Broad cognitive profile in children and Adolescents with HF-ASD and in their siblings: widespread underperformance and its clinical and adaptive correlates. J Autism Dev Disord 2017July;47(7):2153–62. [DOI] [PubMed] [Google Scholar]
- [28].Pinkham AE, Morrison KE, Penn DL, Harvey PD, Kelsven S, Ludwig K, et al. Comprehensive comparison of social cognitive performance in autism spectrum disorder and schizophrenia. Psychol Med 2019. October 2:1–9. [DOI] [PubMed] [Google Scholar]
- [29].Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res 2006. October;174(3):403–12. [DOI] [PubMed] [Google Scholar]
- [30].de Jesus DR, Favalli GPS, Hoppenbrouwers SS, Barr MS, Chen R, Fitzgerald PB, et al. Determining optimal rTMS parameters through changes in cortical inhibition. Clin Neurophysiol 2014. April;125(4):755–62. [DOI] [PubMed] [Google Scholar]
- [31].Kim SY, Appelbaum PS, Swan J, Stroup TS, McEvoy JP, Goff DC, et al. Determining when impairment constitutes incapacity for informed consent in schizophrenia research. Br J Psychiatry 2007. July;191:38–43. [DOI] [PubMed] [Google Scholar]
- [32].Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000. June;30(3):205–23. [PubMed] [Google Scholar]
- [33].Benson N, Hulac DM, Kranzler JH. Independent examination of the wechsler adult intelligence scale-fourth edition (WAIS-IV): what does the WAIS-IV measure? Psychol Assess 2010. March;22(1):121–30. [DOI] [PubMed] [Google Scholar]
- [34].Rosenthal M, Wallace GL, Lawson R, Wills MC, Dixon E, Yerys BE, et al. Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology 2013. January;27(1): 13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry 2018. Jan-Feb;79(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, et al. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp 2010. November;31(11):1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sparrow SS, Cicchetti DV. Diagnostic uses of the Vineland adaptive behavior scales. J Pediatr Psychol 1985. June;10(2):215–25. [DOI] [PubMed] [Google Scholar]
- [38].Duncan L, Georgiades K, Wang L, Van Lieshout RJ, MacMillan HL, Ferro MA, et al. Psychometric evaluation of the Mini international neuropsychiatric interview for children and Adolescents (MINI-KID). Psychol Assess 2018. July;30(7):916–28. [DOI] [PubMed] [Google Scholar]
- [39].Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 2001. April;112(4):720. [DOI] [PubMed] [Google Scholar]
- [40].Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 2016. June;25(3):1057–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A practical guide to calculating cohen’s f(2), a measure of local effect size, from PROC MIXED. Front Psychol 2012;3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wickham H ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- [43].Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2017. June;56(6):466–74. [DOI] [PubMed] [Google Scholar]
- [44].Craig F, Margari F, Legrottaglie AR, Palumbi R, de Giambattista C, Margari L. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatric Dis Treat 2016;12: 1191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dajani DR, Llabre MM, Nebel MB, Mostofsky SH, Uddin LQ. Heterogeneity of executive functions among comorbid neurodevelopmental disorders. Sci Rep 2016. November 9;6:36566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009. December;120(12):2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 2006. December;117(12):2584–96. [DOI] [PubMed] [Google Scholar]
- [48].Chatham CH, Taylor KI, Charman T, Liogier D’ardhuy X, Eule E, Fedele A, et al. Adaptive behavior in autism: minimal clinically important differences on the Vineland-II. Autism Res 2018. February;11(2):270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee EC, Whitehead AL, Jacques RM, Julious SA. The statistical interpretation of pilot trials: should significance thresholds be reconsidered? BMC Med Res Methodol 2014. March 20;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hasan A, Guse B, Cordes J, Wolwer W, Winterer G, Gaebel W, et al. Cognitive effects of high-frequency rTMS in schizophrenia patients with predominant negative symptoms: results from a multicenter randomized sham-controlled trial. Schizophr Bull 2016. May;42(3):608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dlabac-de Lange JJ, Bais L, van Es FD, Visser BG, Reinink E, Bakker B, et al. Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: results of a multicenter double-blind randomized controlled trial. Psychol Med 2015. April;45(6):1263–75. [DOI] [PubMed] [Google Scholar]
- [52].Szatmari P, Georgiades S, Duku E, Bennett TA, Bryson S, Fombonne E, et al. Developmental trajectories of symptom severity and adaptive functioning in an inception cohort of preschool children with autism spectrum disorder. JAMA Psychiatr 2015. March;72(3):276–83. [DOI] [PubMed] [Google Scholar]
- [53].Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, Saulnier CA. The role of adaptive behavior in autism spectrum disorders: implications for functional outcome. J Autism Dev Disord 2011. August;41(8):1007–18. [DOI] [PubMed] [Google Scholar]
- [54].Ameis SH, Lerch JP, Taylor MJ, Lee W, Viviano JD, Pipitone J, et al. A diffusion tensor imaging study in children with ADHD, autism spectrum disorder, OCD, and matched controls: distinct and non-distinct white matter disruption and dimensional brain-behavior relationships. Am J Psychiatry 2016. December 1;173(12):1213–22. [DOI] [PubMed] [Google Scholar]
- [55].Plitt M, Barnes KA, Wallace GL, Kenworthy L, Martin A. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc Natl Acad Sci U S A 2015. December 1;112(48): E6699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hull L, Mandy W, Petrides KV. Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism 2017. August;21(6):706–27. [DOI] [PubMed] [Google Scholar]
- [57].Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry 2015. January;54(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lai MC, Lerch JP, Floris DL, Ruigrok AN, Pohl A, Lombardo MV, et al. Imaging sex/gender and autism in the brain: etiological implications. J Neurosci Res 2017. January 2;95(1–2):380–97. [DOI] [PubMed] [Google Scholar]
- [59].Huber TJ, Schneider U, Rollnik J. Gender differences in the effect of repetitive transcranial magnetic stimulation in schizophrenia. Psychiatry Res 2003. August 30;120(1):103–5. [DOI] [PubMed] [Google Scholar]
- [60].McConachie H, Parr JR, Glod M, Hanratty J, Livingstone N, Oono IP, et al. Systematic review of tools to measure outcomes for young children with autism spectrum disorder. Health Technol Assess 2015. June;19(41): 1–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatr 2019. October;6(10):819–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


