Abstract
Background
Diet may be a modifiable factor for reducing the risk of Alzheimer's disease (AD). Western‐style dietary patterns are considered to increase the risk, whereas Mediterranean‐style dietary patterns are considered to reduce the risk. An association between diet and AD‐related biomarkers have been suggested, but studies are limited.
Aim
To investigate potential relations between dietary patterns and cerebrospinal fluid (CSF) biomarkers for AD among dementia‐free older adults.
Methods
Data were derived from the population‐based Gothenburg H70 Birth Cohort Studies, Sweden. A total of 269 dementia‐free 70‐year‐olds with dietary and cerebrospinal fluid (CSF) amyloid beta (Aβ42 and Aβ40), total tau (t‐tau), and phosphorylated tau (p‐tau) data were investigated. Dietary intake was determined by the diet history method, and four dietary patterns were derived by principal component analysis. A Western dietary pattern, a Mediterranean/prudent dietary pattern, a high‐protein and alcohol pattern, and a high‐total and saturated fat pattern. Logistic regression models, with CSF biomarker pathology (yes/no) as dependent variables, and linear regression models with continuous CSF biomarker levels as dependent variables were performed. The analyses were adjusted for sex, energy intake, body mass index (BMI), educational level, and physical activity level.
Results
The odds ratio for having total tau pathology (odds ratio [OR] 1.43; 95% confidence interval [CI] 1.02 to 2.01) and preclinical AD (Aβ42 and tau pathology; OR 1.79; 95% CI 1.03 to 3.10) was higher among those with a higher adherence to a Western dietary pattern. There were no other associations between the dietary patterns and CSF biomarkers that remained significant in both unadjusted and adjusted models.
Discussion
Our findings suggest that higher adherence to a Western dietary pattern may be associated with pathological levels of AD biomarkers in the preclinical phase of AD. These findings can be added to the increasing amount of evidence linking diet with AD and may be useful for future intervention studies investigating dietary intake in relation to AD.
Keywords: Alzheimer's disease, amyloid, biomarkers, diet, dietary patterns, tau
1. INTRODUCTION
Dementia is the fifth leading cause of death globally. 1 Alzheimer's disease (AD) is the most common form of dementia, and the risk of developing AD increases with age. 2 There is presently no cure for AD and pharmacological treatments are limited. 1 Identifying preventive strategies that may reduce the risk or slow down the disease progression is therefore essential.
Diet, as well as other modifiable lifestyle factors such as physical and cognitive activity, smoking, and body weight, have been associated with the risk of developing AD. 3 , 4 , 5 , 6 Dietary risk factors associated with cardiovascular diseases (CVDs), inflammation, and oxidative stress are suggested to be risk factors also for AD. 4 , 7 , 8 , 9 Western‐style dietary patterns, high in saturated and trans‐ fatty acids, red meat, processed foods, refined cereals, sweets, and high‐sugar beverages, are considered to increase the risk. On the other hand, healthier dietary patterns, such as the Mediterranean and prudent diets, high in mono‐ and polyunsaturated fatty acids, vegetables, fruits, nuts, seeds, low‐fat dairy products, fish and wholegrain products, are considered protective. 10 , 11 There is no consensus regarding the impact of alcohol on the risk of developing AD, but a low to moderate intake has been suggested as protective and a higher intake is a risk factor. 12
There is often a long and relatively symptom‐free preclinical phase before AD is clinically manifested, and biochemical changes related to the disease can appear decades before symptoms of the disease are present. 13 , 14 These changes are reflected in cerebrospinal fluid (CSF), even in the symptom‐free phase of AD progression. 13 , 15 CSF biomarkers that reflect key elements of AD pathophysiology, which are used for screening and diagnostics, are amyloid beta (Aβ42 and Aβ42/Aβ40 ratio) total tau (t‐tau), and phosphorylated tau (p‐tau). 15 , 16 T‐tau and p‐tau reflect tau secretion and phosphorylation, which eventually translates into neurodegeneration and tau tangle formation, and Aβ42 and Aβ40 reflect the metabolism of Aβ and formation of plaques in the brain. 17 Preclinical AD is considered when pathological biomarker levels are present in asymptomatic individuals. 18 Results from a study in the Gothenburg H70 birth cohort studies showed that the prevalence of pathologic AD biomarkers was common (46%) among cognitively normal 70‐year‐olds. 19
Studying single nutrients or foods can provide information about influential dietary components and biological mechanisms underlying risk or protective effects associated with dietary intake. 4 However, effects of interactions between different nutrients and foods are complex and can be missed if dietary patterns are not investigated. A systematic review and meta‐analysis of diet and biomarkers related to AD showed that dietary patterns with a high glycemic load and high in saturated fat were associated with increased Aβ burden, and that adherence to the Mediterranean diet was associated with a reduction (Aβ and tau burden). 20 In the same study, nutrients related to a higher intake of fruit, vegetables, whole grains, fish, and low‐fat dairies, and lower intake of sweets, fried potatoes, high‐fat dairies, processed meat, and butter, were associated with lower cerebral Aβ burden. Another study showed an increased Aβ burden related to a “junk food” dietary pattern, but no relation with either the Mediterranean diet or with a high‐fat or low‐fat diet. 21 However, knowledge about the relation between dietary patterns and CSF biomarkers related to AD is still limited. 20
The aim of this study was to investigate if there is an association between dietary patterns and AD‐specific CSF biomarkers Aβ (Aβ42 and Aβ42/Aβ40), t‐tau, and p‐tau among dementia‐free 70‐year‐olds born 1944.
2. METHODS
2.1. Study design and sample
This cross‐sectional study is part of the ongoing Gothenburg H70 Birth Cohort Studies that started in 1971. 22 The Gothenburg H70 Birth Cohort Studies are multidisciplinary epidemiological studies examining representative birth cohorts of older populations in Gothenburg, Sweden. The study comprised several examinations such as sampling of blood and CSF, psychiatric, cognitive, and physical health examinations, examinations of genetics, social factors, physical fitness and activity, body composition, diet, as well as a close informant interview, described previously in detail. 23 All 70‐year‐olds registered as residents in Gothenburg and born in 1944 on birth dates ending with 0, 2, 5, or 8 were selected as potential participants. Participant flowchart is displayed in Figure 1. All study participants were invited to take part in CSF sampling via a lumbar puncture (LP). A total of 430 individuals consented (response rate 36%), but there were pharmacological contraindications for 108 persons (eg, anticoagulant therapy, immune modulation, cancer therapy), leaving 322 participants (166 men, 156 women). All participants were invited to take part of a dietary examination. A total of 861 (387 men and 474 women) participated in the dietary examination (72%). 23 , 24 There were 273 participants with Aβ42, t‐tau, p‐tau, and dietary data (139 men, 134 women). Four participants were excluded due to dementia diagnosis (one man, three women), leaving 269 participants with Aβ42, t‐tau, p‐tau, and dietary data (138 men, 131 women). The ratio of Aβ42/Aβ40 was calculated for 266 participants (136 men, 130 women); data for three participants were missing due to insufficient CSF volume. The study was approved by the Regional Ethics Review Board in Gothenburg. The participants gave written consent to participate in the examinations according to the Declaration of Helsinki.
FIGURE 1.
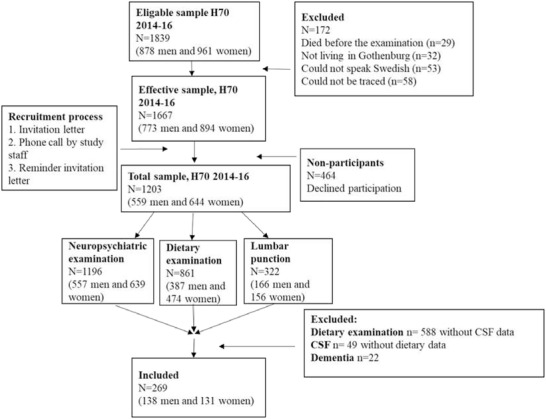
Sample flowchart
2.2. Cerebrospinal fluid (CSF) sampling and variables
All assays are included in the panel of clinical routine analyses at the Mölndal Clinical Neurochemistry laboratory. Analytic runs had to pass quality control criteria for the calibrators, and internal quality control samples had to be approved. CSF total tau (t‐tau) and tau phosphorylated at threonine 181 (p‐tau) were determined with a sandwich enzyme‐linked immunosorbent assay (INNOTEST htau Ag and PHOSPHO_TAU [181P], Fujirebio [formerly Innogenetics], Ghent, Belgium). CSF Aβ42 was measured with a sandwich enzyme‐linked immunosorbent assay (INNOTEST Aβ1‐42) specifically constructed to measure Aβ starting at amino acid 1 and ending at amino acid 42. For the Aβ42/Aβ40 ratio, the V‐PLEX Aβ Peptide Panel 1 (6E10) Kit (Meso Scale Discovery, Rockville, MD) was used. The methods have been described in detail previously. 19 , 25 CSF biomarkers Aβ42, t‐tau, and p‐tau were divided into binary categories (pathology, yes/no), as suggested by the A/T/N classification scheme. 26 Cut‐point values for pathology were ≤530 pg/mL for Aβ42, ≥80 pg/mL for p‐tau, and ≥350 pg/mL for t‐tau. 27 , 28 , 29 The cut‐point for pathological Aβ42/Aβ40 ratio levels was ≤0.082, determined by the bimodal cut‐point of the data (n = 318). To further investigate the relation between dietary patterns and preclinical AD, individuals with Aβ42 and t‐tau and/or p‐tau pathology were divided into a fifth category (pathology, yes/no). 18
HGHLIGHTS
Results indicate links between diet and Alzheimer's disease (AD)–related biomarkers.
Adherence to a Western dietary pattern was associated with pathological total tau levels.
Higher adherence to a Western dietary pattern was associated with preclinical AD.
A Mediterranean‐style dietary pattern was not associated with AD‐related biomarkers.
RESEARCH IN CONTEXT
Systematic review: The literature was reviewed by searches of diet/nutrition/dietary patterns, Alzheimer's disease (AD) and biomarkers/tau/amyloid, mainly in PubMed and Scopus. We found that previous studies are limited, that methodological approaches in examining dietary intake differ in those studies, and that few studies have examined both tau and amyloid beta (Aβ) status. Our study stands out by investigating different dietary patterns in relation to both tau and Aβ status.
Interpretation: We found that higher adherence to a Western‐style dietary pattern (eg, red/processed meat, refined grains, sweets, and high‐sugar beverages) was associated with pathological total tau levels and preclinical AD (pathological tau and Aβ42 levels). These findings support the notion that diet may play a role in the preclinical phase of AD.
Future directions: Longitudinal/intervention studies investigating associations between dietary patterns and cerebrospinal fluid (CSF) biomarkers related to AD could confirm the results.
2.3. Dietary examination procedure
The diet history (DH) method used in this study was a semi‐structured face‐to‐face interview estimating habitual food intake during the preceding 3 months. The interviews were performed by trained registered dietitians. The interviews lasted for ≈1 to 1 ½ hours and were performed either at the participants own home or at the clinic. The protocols for the interviews consist of a meal‐pattern interview, accompanied by a food list with questions on usual frequencies and portion sizes of foods. 24 Pictures of foods from the Swedish National Food Agency (NFA) were used during the interviews to estimate individual portion sizes. Dietary intake was registered as gram of food items usually consumed per day/week/month in a customized (for the H70‐studies) version of the nutritional calculation computer program Dietist Net Pro, containing the NFAs nutrient database of 2015. The method has been validated and described in detail previously. 24
2.4. Dietary variables
Data collected by the DH interview were used to calculate mean daily energy, nutrient, and food intake based on the NFAs nutrient database, as described previously. 24
Reported food intake were placed into 22 food groups based on similarity of nutritional properties and biological classifications (Table S1) and transformed with Box‐Cox transformation to normalize the distribution. Principal component analysis (PCA) 30 was performed to reduce the data into factors representing dietary patterns. The Kaiser‐Meyer‐Olkin Measure of Sampling Adequacy was considered acceptable with a value of 0.63. The breakpoint in the scree plot was used to determine the number of main latent dietary patterns (eigenvalues >1.2 and varimax rotation), shown in Figure S1. Four factors from the PCA were translated into dietary patterns based on factor loadings ≤−0.20 and ≥0.20, shown in Table 1. Factor 1 loaded high on red and processed meat, potatoes, milk products, butter and margarine, refined cereal products, sweets, fast food, and savory bakery and soda, and loaded low on fish and shellfish/seafood. The composition of this dietary patterns resembled less healthy Western‐style dietary patterns and was therefore labeled the “Western” dietary pattern. 31 Factor 2 loaded high on fish and shellfish/seafood, vegetables and pulses, fruits and berries, milk products, nuts and seeds, and low on coffee, alcohol, and red and processed meat. This pattern resembled healthier Mediterranean/prudent‐style dietary patterns and was therefore labeled the “Mediterranean/prudent” dietary pattern. 31 Factor 3 loaded high on protein‐rich foods such as eggs, fish and shellfish/seafood, meat and processed meat, and high on potatoes, sauces and condiments, juice, and alcohol. Based on this food composition, we labeled the pattern the “high‐protein and alcohol” dietary pattern. Factor 4 loaded high on high‐fat dairy products such as cream, crème fraiche, and cheese, and high on refined cereal products and coffee and lower on sauces and condiments and potatoes. Based on this food composition, we labeled the pattern the “high‐total and saturated fat” dietary pattern.
TABLE 1.
Dietary patterns derived from principal component analysis
| Food groups a | ||||
|---|---|---|---|---|
| Factor b | 1 | 2 | 3 | 4 |
| Dietary patterns | Western | Mediterranean/prudent | High‐protein and alcohol | High‐total and saturated fat |
| Eigenvalue | 2.5 | 1.7 | 1.5 | 1.3 |
| Variance explained 32% | 11% | 8% | 7% | 6% |
| Fish and shellfish | –0.27 | 0.29 | 0.52 | |
| Red meat and processed red meat | 0.35 | –0.33 | 0.40 | |
| Poultry | ||||
| Eggs | 0.36 | 0.44 | ||
| Potatoes | 0.39 | 0.45 | –0.28 | |
| Vegetables and pulses | 0.58 | |||
| Fruits and berries | 0.64 | |||
| Milk products | 0.42 | 0.21 | ||
| Cream and crème fraiche | 0.44 | |||
| Cheese | 0.62 | |||
| Sauces, dressings and condiments | 0.44 | −0.27 | ||
| Butter and margarine | 0.49 | |||
| Nuts and seeds | 0.44 | |||
| Whole grain cereal products | 0.33 | |||
| Refined cereal products | 0.53 | 0.25 | ||
| Sweets | 0.60 | |||
| Fast food and savory bakery | 0.50 | |||
| Juice | 0.22 | 0.36 | ||
| Soda | 0.42 | |||
| Coffee | −0.21 | 0.36 | ||
| Tea | 0.61 | |||
| Alcoholic beverages | −0.20 | −0.21 | 0.66 | |
Table S1 contains detailed information about food group contents.
Factors were interpreted into dietary patterns based on food group loadings ≤−0.20 and ≥ 0.20.
2.5. Dementia diagnosis
Dementia was diagnosed following the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM‐III‐R) criteria using information from neuropsychiatric examinations and information from key informants. 23 Cognitive function was rated in accordance with a Swedish version of the Mini‐Mental State Examination (MMSE). 32
2.6. Genotype data
Blood samples were collected and genotyping of the single nucleotide polymorphisms (SNPs) rs7412 and rs429358 in apolipoprotein (APOE; gene map locus 19q13.2) was performed using a KASPar PCR SNP genotyping system (LGC Genomics, Hoddesdon, Herts, UK). 19 , 33 Genotype data for these two SNPs were used to define ε2, ε3, and ε4 alleles. APOE genotype was divided into ε4 carriers (ε4/ε2, ε4/ε3, or ε4/ ε4) and ε4 non‐carriers (ε2/ε2, ε3/ε3, or ε3 /ε2).
2.7. Statistical analyses
CSF biomarkers t‐tau and p‐tau and the ratio p‐tau/Aβ42 were log transformed (natural logarithm) to normalize the distribution before analyses.
Student's t tests, Mann‐Whitney U, and chi‐square tests were performed to compare characteristics, and adherence to each dietary pattern between the dementia‐free participants with dietary and CSF data and those with only dietary data.
Spearman correlation coefficient analyses were performed to investigate which dietary pattern correlated strongest with mean gram carbohydrate, fiber, protein, fat (total, saturated, monounsaturated, polyunsaturated), and alcohol intake/day.
Linear and binary logistic regression analyses were performed, with CSF biomarkers as dependent variables (continuous and binary outcomes) and dietary patterns (continuous factor values derived from the PC analysis) as independent variables. In the linear regression models, CSF biomarker levels for Aβ42, the ratio of Aβ42/Aβ40, t‐tau, p‐tau, and the ratio of p‐tau/Aβ42 were investigated in relation to the four dietary patterns (Western, Mediterranean/prudent, high‐protein and alcohol, and high‐total and saturated fat) separately. Descriptive scatter plots with regression lines between the dietary pattern scores and CSF biomarker levels were performed (Figure S2). In the logistic regression models, the binary outcome was preclinical AD, yes/no (ie, Aβ42 and p‐tau, and/or t‐ tau pathology) and pathology, yes/no of CSF biomarkers Aβ42, the ratio of Aβ42/Aβ40, t‐tau, and p‐tau.
Potential confounders included in the adjusted models were sex, energy intake (kcal), educational level, body mass index (BMI, kg/m2), and physical activity level. An updated version of the Saltin‐Grimby Physical Activity Level Scale was used to determine the level of physical activity. 34 , 35 Educational level was dichotomized into compulsory primary education (≤9 years) versus more than that (>9 years). Confounders were selected a priori based on theory of potential associations with exposure and outcome variables. 36 , 37 The chosen confounders were similar to those included in previous studies investigating dietary patterns and CSF biomarkers related to AD. 20 One participant was excluded in the adjusted models due to missing physical activity data.
Subgroup analyses, stratified by sex, and by genetic risk (APOE ε4 carrier, yes/no) and sex, were performed for the linear regression models, and descriptive scatter plots with regression lines between the dietary patterns and CSF biomarker levels were created if an association was found (Figure S3). Five participants were excluded due to missing APOE data in the analyses that were stratified by sex and APOE ε4 status.
The statistical analyses were performed using IBM SPSS STATISTICS 24. The statistical tests were two‐tailed and P‐values of <.05 were considered statistically significant.
3. RESULTS
3.1. Sample characteristics
Characteristics regarding APOE ε4 allele status, CSF biomarker levels, MMSE score, BMI, energy intake, physical activity, and educational level of the dementia‐free participants with both dietary and CSF data, are shown in Table 2. Mean values of CSF biomarkers Aβ42, p‐tau, t‐tau, Aβ42/Aβ40 ratio, and p‐tau/Aβ42 are shown in Table 2.
TABLE 2.
Characteristics of the dementia‐free participants with both dietary and CSF data
| Participants with dietary and CSF data | Total (n = 269) | Men (n = 138) | Women (n = 131) |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| BMI | 25.8 (4.3) | 26.2 (4.0) | 25.4 (4.6) |
| Energy intake (kcal) | 2221 (542) | 2386 (517) | 2048 (513) |
| Aβ42 (pg/mL) | 723 (220) | 712 (223) | 735 (218) |
| Aβ42/Aβ40 ratio | 0.088 (0.021) | 0.087 (0.022) | 0.089 (0.020) |
| T‐tau (pg/mL) | 326 (128) | 328 (127) | 325 (130) |
| P‐tau (pg/mL) | 49 (17) | 49 (17) | 49 (17) |
| P‐tau/Aβ42 ratio | 0.079 (0.057) | 0.081 (0.063) | 0.076 (0.061) |
| Dietary patterns | |||
| Western | 0.031 (0.945) | 0.210 (0.975) | −0.157 (0.878) |
| Mediterranean/prudent | 0.0233 (0.999) | −0.150 (1.038) | 0.206 (0.925) |
| High‐protein and alcohol | 0.057 (0.990) | 0.508 (0.861) | −0.418 (0.893) |
| High‐total and saturated fat | 0.054 (1.037) | 0.033 (1.011) | 0.075 (1.067) |
| Median (Min/Max) | Median (Min/Max) | Median (Min/Max) | |
| MMSE score a | 29 (23/30) | 29 (23/30) | 29 (24/30) |
| % (cases/total case) | % (cases/total cases) | % (cases/total cases) | |
| APOE ε4 c arrier | 37 (97/264) | 43 (59/138) | 30 (38/126) |
| Physical activity | |||
| Physically inactive | 3 (8/268) | 2 (3/138) | 4 (5/130) |
| Some light physical activity | 12 (31/268) | 11 (15/138) | 12 (16/130) |
| Regular physical activity and training | 83 (222/268) | 84 (116/138) | 82 (106/130) |
| Regular hard physical training | 2 (7/268) | 3 (4/138) | 2 (3/130) |
| Education b | |||
| > Primary school | 88 (238/269) | 86 (119/138) | 91 (119/131) |
Mini‐Mental State Examination (MMSE) has a maximum score of 30. The only participant with an MMSE score of 23 was the one participant in this sample who could not answer a question due to disability.
Compulsory primary school is 9 years.
There were no differences in characteristics between the dementia‐free participants (stratified by sex) with dietary and CSF data compared to those with only dietary data, except for APOE ε4 allele status among men, that was higher in the CSF group (43% vs 31% ε4 carriers P = .02), shown in Table S2. There was no difference between the dementia‐free participants with and without CSF data regarding adherence to the four dietary patterns, shown in Table S2.
Correlations between macronutrients (mean gram/day) and dietary patterns (factors 1‐4) among the dementia‐free participants with CSF data (n = 269) are shown in Table S3. The Western dietary pattern (factor 1) correlated strongest with carbohydrate intake (r = 0.68), the Mediterranean/prudent pattern (factor 2) with fiber (r = 0.63) and polyunsaturated fat (r = 0.28) intake, the high‐protein and alcohol pattern (factor 3) with protein (r = 0.41) and alcohol (r = 0.58) intake, and the high‐total and saturated fat pattern (factor 4) with total fat (r = 0.46), monounsaturated fat (0.41), and saturated fat (r = 0.52) intake (P = .01).
3.2. Dietary patterns and CSF biomarkers
A higher adherence to the Western dietary pattern (factor 1) increased the odds ratio (OR) of having t‐tau pathology (OR 1.36; 95% CI 1.02 to 1.80) and preclinical AD (ie, Aβ42 and t‐tau and/or p‐tau pathology; OR 1.81; 95% CI 1.14 to 2.86) in the unadjusted binary logistic regression models. The findings remained significant in the adjusted models for both t‐tau (OR 1.43; 95% CI 1.02 to 2.01) and preclinical AD (OR 1.79; 95% CI 1.03 to 3.10), shown in Table 3. A higher adherence to the high‐protein and alcohol pattern decreased the OR of having t‐tau pathology in the adjusted model (OR 0.71; 95% CI 0.51 to 0.98), but not in the unadjusted model (OR 0.92; 95% CI 0.71 to 1.20). There were no other associations between the dietary patterns and CSF biomarkers in the binary logistic regression models (Table 3).
TABLE 3.
Associations between dietary patterns and CSF biomarker pathology
| Model 1 a | Model 2 a | |||||||
|---|---|---|---|---|---|---|---|---|
| Aβ42 (60/209) d (60/208) e | OR | 95% CI | S.E | P‐value | OR | 95% CI | S.E | P‐value |
| Western | 1.30 | 0.95;1.78 | 0.16 | .10 | 1.30 | 0.90;1.89 | 0.19 | .17 |
| Mediterranean/prudent | 0.99 | 0.74;1.32 | 0.15 | .94 | 0.89 | 0.65;1.23 | 0.16 | .49 |
| High‐protein and alcohol | 0.98 | 0.73;1.31 | 0.15 | .88 | 0.84 | 0.59;1.19 | 0.18 | .33 |
| High‐total and saturated fat | 0.98 | 0.74;1.29 | 0.14 | .87 | 0.89 | 0.66;1.20 | 0.15 | .44 |
| OR | 95% CI | S.E | P‐value | OR | 95% CI | S.E | P‐value | |
|---|---|---|---|---|---|---|---|---|
| Western | 1.22 | 0.92;1.64 | 0.15 | .17 | 1.25 | 0.88;1.76 | 0.18 | .21 |
| Mediterranean/prudent | 1.06 | 0.81;1.38 | 0.14 | .67 | 0.90 | 0.67;1.21 | 0.15 | .48 |
| High‐protein and alcohol | 0.92 | 0.70;1.20 | 0.14 | .53 | 0.80 | 0.58;1.12 | 0.17 | .19 |
| High‐total and saturated fat | 0.96 | 0.74;1.24 | 0.13 | .76 | 0.90 | 0.68;1.19 | 0.14 | .45 |
| Aβ42+Tau c (27/242) d (27/241) e | OR | 95% CI | S.E | P‐value | OR | 95% CI | S.E | P‐value |
|---|---|---|---|---|---|---|---|---|
| Western | 1.81 | 1.14;2.86 | 0.23 | .01 | 1.79 | 1.03;3.10 | 0.28 | .04 |
| Mediterranean/prudent | 1.01 | 0.68;1.15 | 0.20 | .95 | 0.92 | 0.60;1.41 | 0.22 | .70 |
| High‐protein and alcohol | 0.96 | 0.64;1.44 | 0.21 | .86 | 0.68 | 0.42;1.12 | 0.25 | .13 |
| High‐total and saturated fat | 1.15 | 0.79;1.68 | 0.19 | .47 | 1.03 | 0.68;1.56 | 0.21 | .88 |
| T‐tau (84/185) d (84/184) e | OR | 95% CI | S.E | P‐value | OR | 95% CI | S.E | P‐value |
|---|---|---|---|---|---|---|---|---|
| Western | 1.36 | 1.02;1.80 | 0.14 | .04 | 1.43 | 1.02;2.01 | 0.17 | .04 |
| Mediterranean/prudent | 0.97 | 0.75;1.26 | 0.13 | .83 | 0.94 | 0.71;1.26 | 0.15 | .68 |
| High‐protein and alcohol | 0.92 | 0.71;1;20 | 0.13 | .54 | 0.71 | 0.51;0.98 | 0.17 | .04 |
| High‐total and saturated fat | 1.11 | 0.87;1.43 | 0.13 | .40 | 1.10 | 0.84;1.45 | 0.14 | .50 |
| P‐tau (14/255) d (14/254) e | OR | 95% CI | S.E | P‐value | OR | 95% CI | S.E | P‐value |
|---|---|---|---|---|---|---|---|---|
| Western | 0.89 | 0.51;1.57 | 0.29 | .69 | 0.87 | 0.44;1.73 | 0.35 | .87 |
| Mediterranean/prudent | 0.92 | 0.54;1.59 | 0.28 | .78 | 0.74 | 0.40;1.36 | 0.31 | .33 |
| High‐protein and alcohol | 0.97 | 0.56;1.67 | 0.28 | .90 | 0.93 | 0.49;1.76 | 0.33 | .82 |
| High‐total and saturated fat | 1.42 | 0.86;2.34 | 0.26 | .17 | 1.40 | 0.79;2.48 | 0.29 | .26 |
Analyses in model 1 is unadjusted for confounders. Potential confounders that have been included in model 2 are: BMI, energy intake, sex, physical activity level, and educational level. One participant had missing physical activity level data.
Three participants had missing Aβ42/40 ratio values.
Aβ42+Tau stands for Aβ42 + total tau and/or phosphorylated tau pathology (n = 27). There were 22 participants with pathological Aβ42+total tau levels. The participants with pathological Aβ42+phosphorylated tau (n = 5) levels all had pathological total tau levels as well.
Number of participants with CSF biomarker pathology/number of participants who did not have pathological levels in the unadjusted model.
Number of participants with CSF biomarker pathology/number of participants who did not have pathological levels in the adjusted model.
There were borderline associations between higher adherence to the Western dietary pattern and lower Aβ42/Aβ40 ratio (B −0.002; 95% CI −0.005 to 0.000), higher t‐tau levels (B 0.04; 95% CI −0.01 to 0.09), higher p‐tau levels (B 0.04; 95% CI −0.01 to 0.08), and higher p‐tau/Aβ42 ratio (B 0.063; 95% CI 0.001 to 0.125) in the unadjusted linear regression models, but not in the adjusted models (Table 4). There was a borderline association between higher adherence to the high‐total and saturated fat dietary pattern and higher p‐tau levels in the unadjusted model, but not in the adjusted model (Table 4). No associations were found between the other dietary patterns and CSF biomarkers in the linear regression models shown in Table 4. Descriptive scatter plots with regression lines between the dietary pattern scores and CSF biomarker levels are shown in Figure S2.
TABLE 4.
Associations between dietary patterns and CSF biomarker levels
| Total n = 269 | Total n = 268 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 a | Model 2 a | |||||||||
| Aβ42 | B | 95% CI | S.E | r2 | P‐value | B | 95% CI | S.E | Adj. r² | P‐value |
| Western | –11.96 | –39.96;16.05 | 14.22 | 0.003 | .40 | –3.31 | –36.23;29.60 | 16.72 | 0.000 | .84 |
| Mediterranean/prudent | –14.41 | –40.88;12.07 | 13.44 | 0.004 | .29 | –6.81 | –35.86;22.23 | 14.75 | 0.001 | .65 |
| High‐protein and alcohol | 6.04 | –20.71;32.80 | 13.59 | 0.001 | .66 | 23.79 | –7.37;54.95 | 15.82 | 0.009 | .13 |
| High‐total and saturated fat | 5.18 | –20.38;30.72 | 12.98 | 0.001 | .69 | 15.29 | –12.57;43.16 | 14.15 | 0.005 | .28 |
| Aβ42/ Aβ40 b | B | 95% CI | S.E | r2 | P‐value | B | 95% CI | S.E | Adj. r² | P‐value |
|---|---|---|---|---|---|---|---|---|---|---|
| Western | –0.002 | –0.005;0.000 | 0.001 | 0.011 | .09 | –0.002 | –0.005;0.001 | 0.002 | 0.018 | .16 |
| Mediterranean/ prudent | –0.001 | –0.004;0.001 | 0.001 | 0.004 | .28 | 0.000 | –0.003;0.002 | 0.001 | 0.010 | .83 |
| High‐protein and alcohol | 0.001 | –0.002;0.004 | 0.001 | 0.002 | .44 | 0.003 | 0.000;0.006 | 0.001 | 0.022 | .08 |
| High‐total and saturated fat | 0.001 | –0.002;0.003 | 0.001 | 0.001 | .67 | 0.001 | –0.001;0.004 | 0.001 | 0.014 | .29 |
| T‐tau | B | 95% CI | S.E | r2 | P‐value | B | 95% CI | S.E | Adj. r² | P‐value |
|---|---|---|---|---|---|---|---|---|---|---|
| Western | 0.04 | –0.01;0.09 | 0.02 | 0.012 | .08 | 0.05 | –0.10:0.10 | 0.03 | 0.006 | .11 |
| Mediterranean/ prudent | –0.02 | –0.07;0.02 | 0.02 | 0.004 | .30 | –0.04 | –0.09;0.01 | 0.02 | 0.008 | .08 |
| High‐protein and alcohol | –0.01 | –0.05;0.04 | 0.02 | 0.000 | .79 | –0.02 | –0.08;0.03 | 0.03 | 0.000 | .38 |
| High‐total and saturated fat | 0.03 | –0.01;0.07 | 0.02 | 0.008 | .15 | 0.02 | –0.02;0.07 | 0.02 | 0.000 | .31 |
| P‐tau | B | 95% CI | S.E | r2 | P‐value | B | 95% CI | S.E | Adj. r² | P‐value |
|---|---|---|---|---|---|---|---|---|---|---|
| Western | 0.04 | –0.01;0.08 | 0.02 | 0.011 | .09 | 0.03 | –0.02;0.08 | 0.03 | 0.015 | .21 |
| Mediterranean/prudent | –0.00 | –0.04;0.04 | 0.02 | 0.000 | .97 | –0.02 | –0.07;0.02 | 0.02 | 0.012 | .32 |
| High‐protein and alcohol | 0.01 | –0.03;0.05 | 0.02 | 0.001 | .59 | 0.00 | –0.05;0.05 | 0.02 | 0.009 | .97 |
| High‐total and saturated fat | 0.04 | –0.01;0.08 | 0.02 | 0.014 | .06 | 0.03 | –0.02;0.07 | 0.02 | 0.014 | .22 |
| P‐tau/ Aβ42 | B | 95% CI | S.E | r2 | P‐value | B | 95% CI | S.E | Adj. r² | P‐value |
|---|---|---|---|---|---|---|---|---|---|---|
| Western | 0.063 | 0.001;0.125 | 0.031 | 0.015 | .05 | 0.042 | –0.030;0.114 | 0.036 | 0.038 | .25 |
| Mediterranean/prudent | 0.030 | –0.029;0.088 | 0.030 | 0.004 | .32 | –0.006 | –0.069;0.058 | 0.032 | 0.034 | .86 |
| High‐protein and alcohol | 0.001 | –0.059;0.060 | 0.030 | 0.000 | .98 | –0.041 | –0.109;0.027 | 0.035 | 0.039 | .24 |
| High‐total and saturated fat | 0.030 | –0.027;0.086 | 0.029 | 0.004 | .30 | –0.002 | –0.063;0.059 | 0.031 | 0.033 | .96 |
Analyses in model 1 is unadjusted for confounders. Potential confounders included in model 2 are: BMI, energy intake, sex, physical activity level, educational level. One participant had missing physical activity level data. T‐tau, p‐tau, and the p‐tau/Aβ42 ratio were log‐transformed to normalize the distribution.
Three participants had missing Aβ42/40 values.
In the unadjusted subgroup analyses (stratified by sex and APOE ε4), there were associations between higher adherence to the Western dietary pattern and higher t‐tau (men and women) (B 0.08; 95% CI 0.01 to 0.14, P = .03) (B 0.10; 95% CI 0.02 to 0.18, P = .01) and higher p‐tau (women only) levels among APOE ε4 non‐carriers (B 0.09; 95% CI 0.02 to 0.16, P = .02). A higher adherence to the high‐total and saturated fat pattern was associated with higher t‐tau levels (men only) among APOE ε4 carriers (B 0.14; 95% CI 0.02 to 0.26, P = .03). Descriptive scatter plots with correlation lines are shown in Figure S3. In the adjusted models, the associations remained between higher adherence to the Western dietary pattern and higher t‐tau and p‐tau levels among women that were APOE ε4 non‐carriers (B 0.15; 95% CI 0.05 to 0.24, P = .003) (B 0.12; 95% CI 0.03 to 0.21, P = .01). No other associations were found between the dietary patterns and CSF biomarkers stratified by sex, or by sex and APOE ε4 status in the linear regression models.
4. DISCUSSION
In this study we found that higher adherence to a Western dietary pattern was associated with preclinical AD (ie, having both Aβ and tau pathology) and pathological t‐tau (a marker of neurodegeneration) levels in a population‐based study on cognitively healthy 70‐year‐olds. However, there were no associations with amyloid pathology only and no associations between CSF biomarkers and a Mediterranean‐style dietary pattern, a high‐protein and alcohol pattern, or a high‐total and saturated fat pattern, in both unadjusted and adjusted models.
Previous studies have chosen several different approaches when studying CSF biomarkers in relation to diet. The Australian Women's Health Aging Project (mean age 70 years) showed a relation between increased cerebral Aβ burden measured by positron emission tomography (PET) scanning and a “junk food” diet containing sweets and fast food in healthy women, 38 but, similar to our study, no association with the Mediterranean diet (MeDi), as derived both by PCA and MeDi scores. 21 , 38 However, others report an association between a decreased Aβ burden (measured with PET) and higher adherence to MeDi in cognitively normal individuals (middle‐aged or >70 years). 39 , 40 , 41 , 42 One study examined both Aβ and tau burden (PET) in relation to MeDi adherence. 43 They found that higher adherence was related to a lower Aβ and tau burden among dementia‐free individuals with either subjective memory complaints or mild cognitive impairment (mean age 63). Two other studies among cognitively normal individuals (mean age 54) showed similar relations, where higher adherence to nutrient patterns (eg, macro and micronutrients) based on higher intake of fresh fruit and vegetables, whole grains, fish and low‐fat dairies, and lower intake of sweets, fried potatoes, high‐fat dairies, processed meat, and butter, was associated with lower Aβ burden (PET). 44 , 45
A study among cognitively normal older adults (>65 years) found that adherence to a high glycemic load and high carbohydrate dietary pattern was associated with elevated Aβ burden measured with PET, 46 indicating a possible link between the Western‐style pattern and biomarkers related to preclinical AD seen in our study. An intervention study that investigated relations between CSF Aβ42 and tau levels in relation to high and low glycemic index and saturated fat dietary patterns showed contradictory results. 47 The low glycemic index and low saturated fat dietary pattern was associated with higher CSF Aβ42 levels among individuals with mild cognitive impairment (mean age 68 years). However, the results turned into an opposite direction among cognitively normal individuals (mean age 69 years). Furthermore, they found no associations between diet and CSF Aβ40, t‐tau, or p‐tau levels. In this study, we found an association between higher adherence to the high‐protein and alcohol dietary pattern and decreasing odds ratio for pathological t‐tau levels in the adjusted model but not in the unadjusted model, indicating effect‐modifying properties of the confounders on this association. The high‐protein and alcohol pattern loaded high on foods associated with both healthy (eg, fish) and less healthy (eg, red processed meat) dietary patterns, and contained foods that are suggested to have both risk and protective qualities (eg, juice and alcoholic beverages). 12 , 20 , 31 The combined effects of both risk and protective factors in foods on the development of cognitive decline could be an explanation for lack of associations with the other CSF biomarkers. 10 We found no associations between the dietary patterns and CSF biomarkers in the subgroup analyses based on stratification by sex only. However, in the subgroup analyses based on stratification by sex and APOE ε4 status, we found an association between higher adherence to the Western dietary pattern and higher t‐tau and p‐tau levels in both the unadjusted and adjusted linear regression models among women who were APOE ε4 non‐carriers, indicating a potential effect modification by APOE ε4 status.
There were several differences between the above‐mentioned studies, such as study design, AD‐biomarkers included (Aβ measured with PET most examined), cognitive status, and age, and sample sizes were generally small. This could possibly explain why results differ between studies, and why associations might not be detected. Methodological differences in dietary examination methods and dietary pattern evaluations (eg, dietary index scores or PCA) could also be an issue when comparing results between studies. 48 However, the overall trend in both ours and other studies seem to support the hypothesis that less healthy Western‐style dietary patterns might increase the risk of AD, whereas healthier Mediterranean‐style patterns are protective.
The mechanisms behind the relation between diet and tau and Aβ accumulation are unclear, but it has been suggested that processes involved in disrupted lipid and glucose metabolism, 49 and inflammatory processes (oxidative stress, neuroinflammation) could be of importance. 50 The role of lipid metabolism and glucose‐energy metabolism in the pathogenesis of AD needs to be investigated further, but a disruption of the homeostasis of lipid and glucose metabolism could affect the production and clearance of Aβ and tau phosphorylation and induce neurodegeneration. 49 The role of diet in neuroinflammatory processes is also unclear, but it has been suggested that polyphenols, antioxidant vitamins, and omega‐3 fatty acids could be involved in inhibiting free radicals and cytokine production in microglia cells 50 and potentially reduce the risk of Aβ accumulation. 51 , 52 Components of MeDi and closely related dietary patterns such as DASH (Dietary Approaches to Stop Hypertension) and MIND (Mediterranean‐DASH diet intervention for neurodegeneration delay) 53 are suggested to reduce neuroinflammatory processes, while these processes are promoted by Western‐style dietary patterns.
Strengths with this study are the representative population‐based sample, the comprehensive examinations, and the relatively high response rate for lumbar puncture that has provided us with a sample size larger than similar studies. 20 To our knowledge, this is also one of few studies that has investigated dietary patterns in relation to both Aβ and tau levels in a cognitively healthy sample. Another strength was the use of PCA, which allowed us to identify existing dietary patterns in this population and describe interpersonal differences in dietary intake and combinations of foods consumed.
The study also has limitations. Recall and over‐/under‐reporting of dietary intake is a limitation in most studies examining dietary intake. 54 This is most likely true for our study as well, even though the face‐to‐face interview by a trained dietitian for 1 to 2 hours would have improved this methodological issue. 24 Even though the total number of individuals with CSF data is larger than in several other studies, the number included in some of the analyses is low in terms of statistical power. The results are not corrected for multiple testing and therefore the reported associations should be interpreted with caution. The cross‐sectional design is another limitation, since potential influences of lifetime changes in dietary patterns on disease progression will not be detected. Further studies are needed to confirm the results, preferably with a longitudinal approach. The individuals examined were all cognitively healthy 70‐year‐olds, and the results can therefore not be generalized to other age groups.
5. CONCLUSIONS
The results from this study indicate that a Western‐style dietary pattern may influence the evolution of preclinical AD. This finding can be added to the increasing amount of evidence linking diet with AD and may be useful for future intervention studies investigating dietary intake in relation to AD.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Conception and design of the study: Jessica Samuelsson, Anna Zettergren. Acquisition of data: Ingmar Skoog, Henrik Zetterberg, Kaj Blennow, Elisabet Rothenberg, Silke Kern. Analysis and interpretation of data: Jessica Samuelsson, Ola Wallengren, Anna Zettergren, Ingmar Skoog. Drafting the article: Jessica Samuelsson. Revising the article critically for important intellectual content: Anna Zettergren, Ingmar Skoog, Ola Wallengren, Elisabet Rothenberg, Silke Kern, Henrik Zetterberg, Kaj Blennow. All authors read and approved the final manuscript.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors thank the participants of the Gothenburg H70 birth cohort study.
Samuelsson J, Kern S, Zetterberg H, et al. A Western‐style dietary pattern is associated with cerebrospinal fluid biomarker levels for preclinical Alzheimer's disease—A population‐based cross‐sectional study among 70‐year‐olds. Alzheimer's Dement. 2021;7:e12183. 10.1002/trc2.12183
Ingmar Skoog and Anna Zettergren are joint senior authors.
REFERENCES
- 1. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387‐403. [DOI] [PubMed] [Google Scholar]
- 3. Najar J, Ostling S, Gudmundsson P, et al. Cognitive and physical activity and dementia: a 44‐year longitudinal population study of women. Neurology. 2019;92:e1322‐e1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solfrizzi V, Custodero C, Lozupone M, et al. Relationships of dietary patterns, foods, and micro‐ and macronutrients with Alzheimer's disease and late‐life cognitive disorders: a systematic review. J Alzheimers Dis. 2017;59:815‐849. [DOI] [PubMed] [Google Scholar]
- 5. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653‐666. [DOI] [PubMed] [Google Scholar]
- 6. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England). 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gardener SL, Rainey‐Smith SR, Martins RN. Diet and inflammation in Alzheimer's disease and related chronic diseases: a review. J Alzheimers Dis. 2016;50:301‐334. [DOI] [PubMed] [Google Scholar]
- 8. Freund‐Levi Y, Vedin I, Hjorth E, et al. Effects of supplementation with omega‐3 fatty acids on oxidative stress and inflammation in patients with Alzheimer's disease: the OmegAD study. J Alzheimers Dis. 2014;42:823‐831. [DOI] [PubMed] [Google Scholar]
- 9. Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911‐916.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shakersain B, Santoni G, et al. Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimers Dement. 2016;12:100‐109. [DOI] [PubMed] [Google Scholar]
- 11. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7:889‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rehm J, Hasan OSM, Black SE, Shield KD, Schwarzinger M. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med. 2018;284:643‐663. [DOI] [PubMed] [Google Scholar]
- 16. Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's disease. Alzheimers Res Ther. 2019;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bloom GS. Amyloid‐beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505‐508. [DOI] [PubMed] [Google Scholar]
- 18. Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kern S, Zetterberg H, Kern J, et al. Prevalence of preclinical Alzheimer disease: comparison of current classification systems. Neurology. 2018;90:e1682‐e1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hill E, Goodwill MA, Gorelik A, Szoeke C. Diet and biomarkers of Alzheimer's disease: a systematic review and meta‐analysis. Neurobiol Aging. 2019;76:45‐52. [DOI] [PubMed] [Google Scholar]
- 21. Hill E, Szoeke C, Dennerstein L, Campbell S, Clifton P. Adherence to the Mediterranean diet is not related to beta‐amyloid deposition: data from the Women's Healthy Ageing Project. J Prev Alzheimers Dis. 2018;5:137‐141. [DOI] [PubMed] [Google Scholar]
- 22. Rinder L, Roupe S, Steen B, Svanborg A. Seventy‐year‐old people in Gothenburg. A population study in an industrialized Swedish city. Acta Med Scand. 1975;198:397‐407. [DOI] [PubMed] [Google Scholar]
- 23. Rydberg Sterner T, Ahlner F, Blennow K, et al. The Gothenburg H70 Birth cohort study 2014‐16: design, methods and study population. Eur J Epidemiol. 2019;34:191‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samuelsson J, Rothenberg E, Lissner L, Eiben G, Zettergren A, Skoog I. Time trends in nutrient intake and dietary patterns among five birth cohorts of 70‐year‐olds examined 1971‐2016: results from the Gothenburg H70 birth cohort studies, Sweden. Nutr J. 2019;18:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andreasson U, Kuhlmann J, Pannee J, et al. Commutability of the certified reference materials for the standardization of β‐amyloid 1‐42 assay in human cerebrospinal fluid: lessons for tau and β‐amyloid 1‐40 measurements. Clin Chem Lab Med. 2018;56:2058‐2066. [DOI] [PubMed] [Google Scholar]
- 26. Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow‐up study. Lancet Neurol. 2006;5:228‐234. [DOI] [PubMed] [Google Scholar]
- 28. Vanmechelen E, Vanderstichele H, Davidsson P, et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett. 2000;285:49‐52. [DOI] [PubMed] [Google Scholar]
- 29. Zetterberg H, Wahlund LO, Blennow K. Cerebrospinal fluid markers for prediction of Alzheimer's disease. Neurosci Lett. 2003;352:67‐69. [DOI] [PubMed] [Google Scholar]
- 30. Thorpe MG, Milte CM, Crawford D, McNaughton SA. A comparison of the dietary patterns derived by principal component analysis and cluster analysis in older Australians. Int J Behav Nutr Phys Act. 2016;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Samadi M, Moradi S, Moradinazar M, Mostafai R, Pasdar Y. Dietary pattern in relation to the risk of Alzheimer's disease: a systematic review. Neurol Sci. 2019;40:2031‐2043. [DOI] [PubMed] [Google Scholar]
- 32. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 33. Rydberg Sterner T, Ahlner F, Blennow K, et al. The Gothenburg H70 Birth cohort study 2014‐16: design, methods and study population. Eur J Epidemiol. 2019;34:191‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saltin B, Grimby G. Physiological analysis of middle‐aged and old former athletes. Comparison with still active athletes of the same ages. Circulation. 1968;38:1104‐1115. [DOI] [PubMed] [Google Scholar]
- 35. Rodjer L, Jonsdottir IH, Rosengren A, et al. Self‐reported leisure time physical activity: a useful assessment tool in everyday health care. BMC Public Health. 2012;12:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lederer DJ, Bell SC, Branson RD, et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann Am Thorac Soc. 2019;16:22‐28. [DOI] [PubMed] [Google Scholar]
- 37. Zeraatkar D, Cheung K, Milio K, et al. Methods for the selection of covariates in nutritional epidemiology studies: a meta‐epidemiological review. Curr Dev Nutr. 2019;3:nzz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hill E, Clifton P, Goodwill AM, Dennerstein L, Campbell S, Szoeke C. Dietary patterns and beta‐amyloid deposition in aging Australian women. Alzheimers Dement (N Y). 2018;4:535‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berti V, Walters M, Sterling J, et al. Mediterranean diet and 3‐year Alzheimer brain biomarker changes in middle‐aged adults. Neurology. 2018;90:e1789‐e1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matthews DC, Davies M, Murray J, et al. Physical activity, mediterranean diet and biomarkers‐Assessed risk of Alzheimer's: a multi‐modality brain imaging study. Adv J Mol Imaging. 2014;4:43‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rainey‐Smith SR, Gu Y, Gardener SL, et al. Mediterranean diet adherence and rate of cerebral Aβ‐amyloid accumulation: data from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Transl Psychiatry. 2018;8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vassilaki M, Aakre JA, Syrjanen JA, et al. Mediterranean diet, its components, and amyloid imaging biomarkers. J Alzheimers Dis. 2018;64:281‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Merrill DA, Siddarth P, Raji CA, et al. Modifiable risk factors and brain positron emission tomography measures of amyloid and tau in nondemented adults with memory complaints. Am J Geriatr Psychiatry. 2016;24:729‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berti V, Murray J, Davies M, et al. Nutrient patterns and brain biomarkers of Alzheimer's disease in cognitively normal individuals. J Nutr Health Aging. 2015;19:413‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mosconi L, Murray J, Davies M, et al. Nutrient intake and brain biomarkers of Alzheimer's disease in at‐risk cognitively normal individuals: a cross‐sectional neuroimaging pilot study. BMJ open. 2014;4:e004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high‐glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106:1463‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bayer‐Carter JL, Green PS, Montine TJ, et al. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68:743‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367:31‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sato N, Morishita R. The roles of lipid and glucose metabolism in modulation of beta‐amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front Aging Neurosci. 2015;7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McGrattan AM, McGuinness B, McKinley MC, et al. Diet and inflammation in cognitive ageing and Alzheimer's disease. Curr Nutr Rep. 2019;8:53‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mohammadzadeh Honarvar N, Saedisomeolia A, Abdolahi M, et al. Molecular anti‐inflammatory mechanisms of retinoids and carotenoids in Alzheimer's disease: a review of current evidence. J Mol Neurosci. 2017;61:289‐304. [DOI] [PubMed] [Google Scholar]
- 52. Román GC, Jackson RE, Gadhia R, Román AN, Reis J. Mediterranean diet: the role of long‐chain ω‐3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age‐related cognitive decline, and Alzheimer disease. Rev Neurol (Paris). 2019;175:724‐741. [DOI] [PubMed] [Google Scholar]
- 53. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11:1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hornell A, Berg C, Forsum E, et al. An extension of the STROBE statement for observational studies in nutritional epidemiology (STROBE‐nut): Explanation and elaboration 2017. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information


