Abstract
This guideline provides updated recommendations on the role of preprocedure testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) in individuals undergoing endoscopy in the post-vaccination period and replaces the prior guideline from the American Gastroenterological Association (AGA) (released July 29, 2020). Since the start of the pandemic, our increased understanding of transmission has facilitated the implementation of practices to promote patient and health care worker (HCW) safety. Simultaneously, there has been increasing recognition of the potential harm associated with delays in patient care, as well as inefficiency of endoscopy units. With widespread vaccination of HCWs and the general population, a re-evaluation of AGA’s prior recommendations was warranted. In order to update the role of preprocedure testing for SARS-CoV2, the AGA guideline panel reviewed the evidence on prevalence of asymptomatic SARS-CoV2 infections in individuals undergoing endoscopy; patient and HCW risk of infections that may be acquired immediately before, during, or after endoscopy; effectiveness of COVID-19 vaccine in reducing risk of infections and transmission; patient and HCW anxiety; patient delays in care and potential impact on cancer burden; and endoscopy volumes. The panel considered the certainty of the evidence, weighed the benefits and harms of routine preprocedure testing, and considered burden, equity, and cost using the Grading of Recommendations Assessment, Development and Evaluation framework. Based on very low certainty evidence, the panel made a conditional recommendation against routine preprocedure testing for SARS-CoV2 in patients scheduled to undergo endoscopy. The panel placed a high value on minimizing additional delays in patient care, acknowledging the reduced endoscopy volumes, downstream impact on delayed cancer diagnoses, and burden of testing on patients.
Keywords: COVID-19, Diagnostic Test, SARS-CoV2, Gastrointestinal Endoscopy
Abbreviations used in this paper: CDC, Centers for Disease Control and Prevention; CI, confidence interval; CRC, colorectal cancer; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HCW, health care worker; IDS, Infectious Disease Society of America; NAAT, nucleic acid amplification test; PPE, personal protective equipment; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV2, severe acute respiratory syndrome coronavirus 2
On December 11, 2020, the first vaccine to prevent COVID-19 received emergency use authorization in the United States, thereby signifying the start of the road to recovery from this devastating pandemic.1 As of March 2021, 52% of HCWs had been vaccinated, with population-wide vaccination strategies well underway and, with expanding eligibility, vaccinations rates are expected to rise over time.2 In light of our increased understanding of the effectiveness and availability of vaccinations, there is a need for updated guidance on the role of testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) in asymptomatic individuals before endoscopy. This guideline replaces the prior set of recommendations released on July 29, 2020 and provides updated recommendations on the role of preprocedure testing in the post-vaccination period.3 A summary of the recommendations is outlined in Table 1 .
Table 1.
Executive Summary of Recommendations
| Summarized below are the recommendations with comments related to the role of testing in endoscopy. The strength of a recommendation is expressed as strong or conditional, based on the GRADE methodology and has the following interpretation: Strong recommendation: All centers should follow the recommended course of action, and only a small minority may not. Conditional recommendation: The majority of centers in this situation should follow the suggested course of action but many would not; different choices may be appropriate. These recommendations assume that: 1. All centers have access to PPE, including face shield, eye protection, and surgical mask or N95 (or N99, powered air-purifying respirators) 2. All centers have implemented universal screening of patients for COVID-19 symptoms, using a screening checklist and have implemented universal precautions. including physical distancing, masks, and hand hygiene in the endoscopy unit |
|
Recommendation 1: The AGA suggests against routine preprocedure testing for SARS-CoV2 in patients undergoing upper endoscopy or lower endoscopy (the terms upper and lower endoscopy include all related gastrointestinal procedures, eg, endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, and flexible sigmoidoscopy) irrespective of the vaccination status of patients.Conditional recommendation, very low certainty evidence Remarks: Centers that prioritize the small potential benefit (staff and patient reassurance, detection of asymptomatic positive cases) over the harms (burden of testing on patients, downstream consequences of false positives, potential delays in care and decreased endoscopy efficiency) may choose to implement a pre-procedure testing strategy as outlined in Recommendation 2. |
|
Recommendation 2: In endoscopy centers that implement a preprocedure testing strategy, the AGA suggests using standard nucleic acid testing (laboratory-based NAAT or rapid RT-PCR)arather than a rapid isothermal test or antigen tests, in patients undergoing upper endoscopy or lower endoscopy (the terms upper and lower endoscopy include all related gastrointestinal procedures, eg, endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, and flexible sigmoidoscopy) irrespective of the vaccination status of patients. Conditional recommendation, very low certainty evidence Remarks: Rapid RT-PCR tests that can be easily performed on the day of endoscopy (results within 1 h) are preferable as they pose less burden to patients. In the preprocedure setting, the utility of rapid isothermal tests or antigen tests is limited due to concerns of assay sensitivity. There is no role of antibody tests for preprocedure testing. |
Standard NAATs include laboratory-based NAAT and rapid RT-PCR tests that detect viral RNA and have the best diagnostic test accuracy. Rapid RT-PCR tests are defined as tests that provide results in 1 h. Rapid isothermal tests detect viral RNA. Antigen tests detect viral proteins, with the vast majority of tests detecting nucleocapsid antigen. Most antigen tests are rapid, providing results within 15 min.
Scope and Purpose
We summarize the available data on the diagnostic test characteristics of tests for SARS-CoV2 infection and provide evidence-based clinical guidance on the role of pretesting before endoscopic procedures in the setting of ongoing vaccinations of health care workers (HCWs) and patients. This rapid review and guideline was commissioned and approved by the American Gastroenterological Association (AGA) Governing Board to provide timely, methodologically rigorous guidance on a topic of high clinical importance to the public, HCWs and the AGA membership at large.
Target Audience
The target audience of these guidelines includes gastroenterologists, advanced practice providers, nurses, and other health care professionals in academic centers and in private practice settings across various geographic locations in the United States. Patients, as well as policy makers, may also benefit from these guidelines. These guidelines are not intended to impose a standard of care for individual institutions, health care systems, or countries. They provide the basis for rational informed decisions for clinicians, patients, and other health care professionals. However, decisions may be constrained by local health system–level or state-level policies, as well as availability of resources.
How to Use This Guideline
Recommendations are accompanied by qualifying remarks, which serve to facilitate more accurate implementation. They should never be omitted when recommendations from these guidelines are quoted or translated. A summary of the recommendations is provided in Table 1 with a more detailed rationale for each recommendation in the Results section. The implementation considerations section in this guideline will help clinicians implement these recommendations.
Methods
This guideline was developed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Given the need for guidance during a major public health crisis, the methodological approach was modified according to the Guidelines International Network/McMaster checklist for the development of rapid recommendations.4 For one of the recommendations, we used a process called GRADE-ADOLOPMENT, which allows for adaptation or modification of existing guideline recommendation (see below).5
Panel Composition
The guideline panel included gastroenterologists, an infectious disease expert, and guideline methodologists. A preliminary draft of the recommendations was shared with anesthesiologists at one panel member’s institution and the final draft was reviewed by a patient for feedback.
Guideline Funding and Conflict of Interest Management
Development of the guideline was funded by the AGA and no panel members received any payments. Panel members disclosed all financial, intellectual, or other potential conflicts of interest according to the AGA Institute policy. These are available from the AGA Clinical Guideline Committee staff liaison.
Perspective
These recommendations assume a patient or population perspective. Although the majority of HCWs have been, or will be, vaccinated against SARS-CoV2, the panel acknowledged that a subgroup of HCWs have declined vaccinations. Furthermore, the panel assumed that all endoscopy centers follow universal precautions and that staff have access to personal protective equipment (PPE).
Clinical Questions
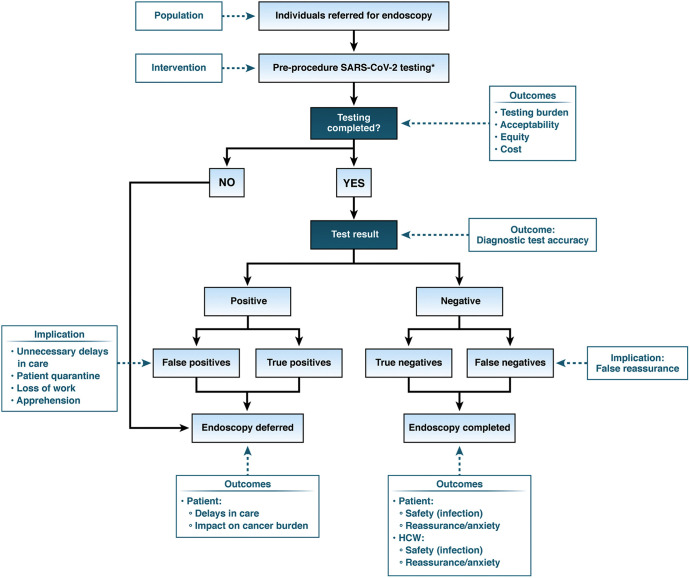
Using a PICO (population, intervention, comparison, outcomes) format, the panel created an analytical framework. See Figure 1 for analytic framework for preprocedural testing and outcomes. Panel members prioritized the following patient-important outcomes for decision-making: patient safety (COVID-19 infection), patient reassurance or anxiety, patient delays in care and impact on cancer burden, HCW safety (COVID-19 infection), HCW reassurance or anxiety, test burden (feasibility, acceptability), cost, and health equity. Patient delays in care and impact on cancer burden were deemed critical outcomes for decision making.
Figure 1.
Analytic framework for preprocedural testing and outcomes. Analytic framework of downstream consequences of preprocedure testing. This framework is based on the assumption that the majority of endoscopy centers are conducting preprocedure testing during the pandemic. ∗Pre-procedure SARS-CoV2 testing in conjunction with universal symptom screening per CDC guidelines. False positive, individuals who test positive for SARS-CoV2 but do not have the infection; false negative, individuals who test negative for SARS-CoV2 but do have the infection.
Search Strategy
Information sources and literature search
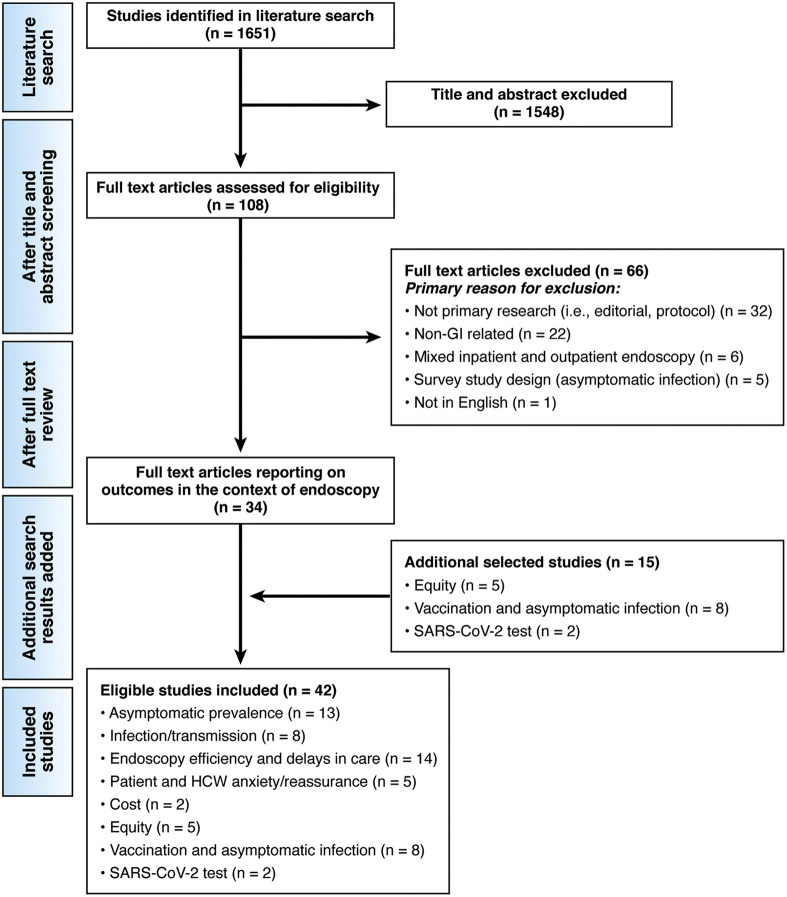
We conducted a systematic literature search to identify all published studies that could be considered eligible for our review with no restrictions on languages. To capture relevant published articles, we electronically searched OVID Medline and Embase from inception to May 1, 2021 using the Medical Subject Heading term developed for COVID-19. A systematic review of the literature identified 1651 references, of which 42 informed the evidence base for these recommendations. See Figure 2 for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses. (PRISMA) flow diagram. PRISMA diagram of included studies and reasons for exclusion. Note that the number of total studies is lower than the sum of each category, as some studies reported on more than 1 outcome. There were no studies reporting directly on cost or vaccine effectiveness in the context of endoscopy. We therefore used existing reviews from the CDC in nonendoscopy settings with an updated search to indirectly inform our guidance as outlined in this document.
Study Selection
Six reviewers (O.A., P.D., J.F., S, Sultan, S. Singh, and S.M.S.) independently screened titles and abstracts, as well as eligible full-text studies. Disagreements were resolved by discussion to reach consensus. Studies were included if they reported data on preprocedure testing and SARS-CoV2 infection among patients and HCWs exposed to endoscopy, patient and HCW anxiety/reassurance, endoscopy volumes, patient delays in care, and impact on cancer burden (colorectal, esophageal, and gastric). We excluded studies that reported on preprocedure tests in nonendoscopy settings and survey studies of infections. With rapidly evolving aspects of effectiveness of COVID-19 vaccines in decreasing risk of infection and SARS-CoV2 transmission, we relied on updated documents published by the Centers for Disease Control and Prevention (CDC) and preprint servers. For equity considerations, because no studies reported specifically on preprocedure testing, we highlighted select articles that reported on equity issues more broadly. For information about diagnostic test performance, the Infectious Disease Society of America (IDSA) living rapid guideline was used to inform diagnostic test accuracy for laboratory-based reverse transcription polymerase chain reaction (RT-PCR) nucleic acid amplification test (NAAT), rapid RT-PCR, antigen tests, and antibody tests.6
Data Collection and Analysis
Reviewers (O.A., P.D., J.F., S, Sultan, S. Singh, and S.M.S.) extracted relevant information into a standardized data extraction form, which included study characteristics (authors, publication year, study dates, country, and study design), endoscopy volumes, preprocedure screening and testing, type of masks, infection rates in HCW and patients, prevalence of positive and negative tests, anxiety/reassurance in HCWs and patients, and numbers of observed or expected colorectal, esophageal, or gastric cancers. For studies on vaccine effectiveness, we extracted data on population vaccinated, type and timing of vaccine, asymptomatic/symptomatic infection, vaccine effectiveness or risk reduction. Because of the heterogeneity of studies and indirect evidence, the evidence was summarized narratively, and no formal meta-analysis was performed.
Certainty of Evidence
The GRADE framework was used to assess overall certainty by evaluating the evidence for each outcome on the following domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias.7 The GRADE summary of findings table and evidence profile was generated using the GRADEpro Guideline Development Tool (Evidence Prime; available at gradepro.org).
Evidence to Recommendations
The panel evaluated the certainty of evidence, balance between benefits and harms, and burden of testing on patients (acceptability, feasibility), cost, and equity. For all recommendations, the panel reached consensus. As per GRADE methodology, recommendations are labeled as “strong” or “conditional.” The phrase “we recommend” indicates strong recommendations and the phrase “we suggest” indicates conditional recommendations.
For one of the recommendations, we used a process called GRADE-ADOLOPMENT, which allows for adaptation or modification of existing guideline recommendation.5 Briefly, the process of adaptation involves identifying the pertinent health care questions, searching for existing guidelines that addressed those questions, critically appraising them, and deciding whether to accept or modify all or selected recommendations. The adapted recommendation may have a change in the specific population, intervention, comparator than the original recommendation and a different certainty in the evidence. This decision also requires considering whether recommendations are credible, up to date, acceptable, applicable, and feasible to implement to one’s organizational context. For this guideline, the panel adapted the recommendation for asymptomatic testing as it applied to the pre-endoscopy setting.
Results
A summary of all of the recommendations is provided in Table 1.
Recommendation 1: The AGA suggests against routine preprocedure testing for SARS-CoV2 in patients undergoing upper endoscopy or lower endoscopy (the terms upper and lower endoscopy include all related gastrointestinal procedures, eg, endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, and flexible sigmoidoscopy), irrespective of the vaccination status of patients. Conditional recommendation, very low certainty evidence
Remarks: Centers that prioritize the small potential benefit (staff and patient reassurance, detection of asymptomatic positive cases) over the harms (burden of testing on patients, downstream consequences of false positives, delays in care, and decreased endoscopy efficiency) may choose to implement a preprocedure testing strategy as outlined in Recommendation 2.
Rationale
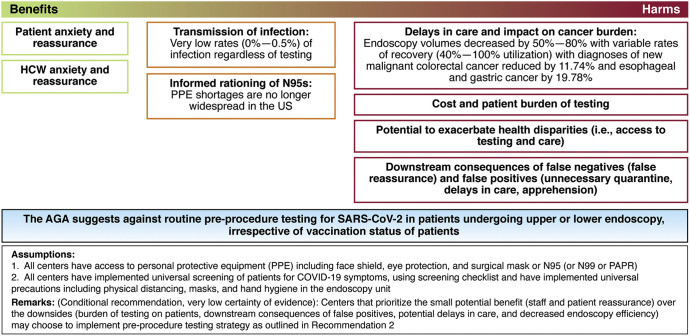
The panel reviewed the evidence on prevalence of asymptomatic infections in individuals undergoing endoscopy; patient and HCW infections after endoscopy; effectiveness of the vaccine on reducing infections; patient and HCW anxiety; and patient delays in care (endoscopy volumes) and impact on cancer burden. The panel then evaluated the certainty of the evidence, weighed the benefits and harms of preprocedure testing, and considered burden, equity, and cost. The panel acknowledged the small potential benefit of preprocedure testing with respect to patient and staff reassurance, but no benefit with regard to infections because the risk of infection was extremely low (with symptom screening, adequate PPE, and protection from infection [both asymptomatic and symptomatic] due to vaccination). The panel also evaluated the yield of testing (rates of positive tests among asymptomatic individuals ranged from 0%–0.5%) and the significant delays in care (reduced numbers of procedures across endoscopy centers with incomplete recovery of volumes) and reduced numbers of diagnoses of colorectal, esophageal, and gastric cancers (compared with expected numbers from historical data). Based on low certainty evidence, the panel made a conditional recommendation against preprocedure testing for SARS-CoV2. The panel placed a high value on minimizing additional delays in patient care, acknowledging the reduced endoscopy volumes, downstream impact on delayed cancer diagnoses, and additional burden of testing on patients. See Figure 3 for implementation of a pre-endoscopic testing strategy.
Figure 3.
Implementation of a pre-endoscopic testing strategy. The AGA suggests against routine preprocedure testing for SARS-CoV2 in patients undergoing upper or lower endoscopy, irrespective of vaccination status of patients. Assumptions are that: 1. All centers have access to PPE, including face shield, eye protection, and surgical mask or N95 (or N99 or powered air-purifying respirators). 2. All centers have implemented universal screening of patients for COVID-19 symptoms, using screening checklist and have implemented universal precautions, including physical distancing, masks, and hand hygiene in the endoscopy unit. Remarks: (Conditional recommendation, very low certainty of evidence): Centers that prioritize the small potential benefit (staff and patient reassurance) over the downsides {burden of testing on patients, downstream consequences of false positives, potential delays in care, and decreased endoscopy efficiency) may choose to implement preprocedure testing strategy as outlined in Recommendation 2.
Summary of the Evidence
The evidence is summarized in Table 2 . We found no studies that provided comparative evidence of preprocedure testing in combination with symptom screening vs symptom screening alone on the following outcomes of interest: patient and HCW infections; patient and HCW reassurance or anxiety, and patient delays in care and impact on cancer burden. We found indirect evidence to inform these outcomes as outlined below.
Table 2.
Summary of Findings Table
| Outcomes | No. of participants (studies), follow-up | Certainty of the evidence (GRADE) |
Impact |
|---|---|---|---|
| Patient safety (infections) | Infection rates (2 prospective and 5 retrospective studies) Asymptomatic prevalence (13 cohort studies) Vaccination (9 cohort studies) |
⊕◯◯◯ VERY LOWa,e,b,f,g,h,i |
Based on very low certainty evidence, there were little to no infections in the health care settings and high effectiveness of protection from infection after vaccination. Rates of asymptomatic infection and potential transmission were also low. There is no direct evidence from RCTs and comparative cohort studies on infection rates in patients and HCWs with and without preprocedure testing strategy. We evaluated direct evidence from single-arm cohort studies that reported on rates of infection and also reviewed indirect evidence from asymptomatic prevalence and protection from vaccination. Infection rates: Based on 2 prospective14,15 and 6 retrospective10,20, 21, 22, 23, 24 cohort studies, the rates of infection in patients ranged from 0% to 0.4% and in HCWs ranged from 0% to 4.0%. Five studies reported use of a pretesting strategy and 3 did not. Asymptomatic prevalence: Based on 13 cohort studies, asymptomatic prevalence ranged from 0.0% to 1.5% but most studies reported a range from 0% to 0.5% regardless of local surges of COVID-19 case counts. Protection from vaccination: Based on an existing CDC review and 6 additional prospective cohort studies (US, UK, Israel, Sweden, Qatar) among HCWs and the general population, large risk reductions in SARS-CoV2 infection were reported ranging from 80% to 94% (7–14 d after the second shot of Pfizer-BioNTech or Moderna). |
| HCW safety (infections) | ⊕◯◯◯ VERY LOWa,e,b,f,g,h,i |
||
| Patient reassurance or anxiety | (2 observational studies) | ⊕◯◯◯ VERY LOWa,b,c |
Based on very low certainty evidence from 2 studies, reporting on patients' attitude and anxiety regarding having gastrointestinal procedures during the COVID-19 pandemic showed mixed results. There is no direct evidence from RCTs and comparative cohort studies reporting on patient anxiety with preprocedural SARS-CoV2 testing vs no testing in the post-vaccination setting. Study 1: In 1 survey study (early in the pandemic), 81% of patients valued testing staff for COVID-19 and 66% felt that on-site patient testing was important but despite testing, they did not feel reassured.36 Study 2: In hospitalized and ambulatory individuals, 83% reported feeling safer because of the testing strategy.35 |
| HCW reassurance or anxiety | (3 observational studies) | ⊕◯◯◯ VERY LOWa,d |
Based on very low certainty evidence from 3 cross-sectional studies, implementation of a pretesting strategy was associated with moderate reduction in anxiety. There is no direct evidence from RCTs and comparative cohort studies reporting on patient anxiety with preprocedural SARS-CoV2 testing vs no testing in the post-vaccination setting. Study 1: Survey study of 47 endoscopy unit personnel regarding preprocedural testing implementation.39 Anxiety regarding contracting infection decreased from 58.1% pre- to 44.7% post-implementation. Anxiety regarding infecting family members decreased from 88.4% pre- to 68.4% post-implementation of testing and self-isolation (living in a separate room from the family) decreased from 21.3% pre- to 10.8% post-implementation of testing). Study 2: Survey of 407 gastroenterologists evaluated psychological symptoms impacting the HCW, but there was no preprocedural testing data.37 Eighty-one percent (330 of 407) reported some sort of psychological symptoms, 74 of 407 (18%) had a concern of being infected with COVID-19 at work, and 145 of 470 (35%) reported a high level of concern about infecting family members. Study 3: In a survey study of 106 providers, 4 measures were ranked as important or critical by 90% of respondents: patients wear surgical masks at all times, patients are screened for fever, COVID-19 symptoms, and COVID-19 exposure.38 Universal preprocedure testing was ranked among the 3 most important measures. With the proposed institution of these measures, the proportion of providers who were very or somewhat concerned decreased from 66% to 35%. |
| Delays in patient care and cancer burden | (16 observational studies) | ⊕◯◯◯ VERY LOWa,j,k |
There was very low certainty evidence demonstrating reduced rates of endoscopy volumes in the early phase of the pandemic (decreased by 50%–80%) and variable rates of recovery (40%–100% utilization) in the late phase of the pandemic. No increased colonoscopy utilization noted. It is unclear how much preprocedural testing directly impacted endoscopy volumes. There was very low certainty evidence of moderate reductions in cancer diagnoses (based on 2019 expected numbers) for colorectal cancer, esophageal cancer, and gastric cancer. No comparative evidence from RCTs or observational studies reporting on preprocedure testing and its impact on endoscopy volumes and cancer burden was found. We identified indirect evidence from reports on endoscopy volumes throughout different periods of the pandemic and database modeling studies on reduction in cancer diagnoses based on 2019 expected numbers. Endoscopy volumes: 14 studies (1 survey study and 13 cohort studies mostly based on administrative datasets) reported on endoscopy volumes from the United States, United Kingdom, The Netherlands, Canada, China, Spain, Japan, and Taiwan. Initial phase of pandemic: across studies, on average, the total number of upper endoscopies decreased by 51%–72% and colonoscopies decreased by 59%–85% compared with the same time in prior years, with the majority of endoscopy centers not reaching pre-COVID endoscopy volumes over the ensuing 3–4 mo. Late phase of the pandemic: based on 4 studies from the United Kingdom, Spain, and United States (VAHCS and TriNetX), the reported endoscopy utilization was 40%–70% in the United States, 100% in the United Kingdom, and 70% of expected volumes in the VA. No studies were identified reporting on endoscopy volumes in the post-vaccination period. Cancer burden: 9 studies (United States, United Kingdom, The Netherlands, Asia) reported on the impact of COVID-19 on the following gastrointestinal cancers: esophageal, gastric and colorectal cancer.41,42,44,48,51,52 Most studies estimated the reduction in cancer diagnoses based on 2019 expected numbers using administrative datasets. In the early phase of the pandemic: Endoscopic cancer detection of CRC reduced by 31%–71.1% Endoscopic cancer detection of esophageal cancer was reduced by 27%–37.1% Endoscopic cancer detection of gastric cancer was reduced by 27%–52.3% In the late phase of the pandemic: Diagnoses of new malignant CRC was reduced by 11.74%, Esophageal and gastric cancer was reduced by 19.78%. One Japanese study (in the late pandemic period) of 123 patients with CRC who underwent surgery, during COVID-19, more patients needed emergency admission, more had obstructive CRC (39% vs 15%), more had partial or complete obstructions (67% vs 19%–42%), and patients were more likely to present with advanced CRC.46 |
RCT, randomized controlled trial; VAHCS, Veterans Affairs Healthcare System.
Serious risk of bias: no comparison group, selection bias (some studies did not include all patients undergoing procedures, just the ones that had PCR test) and recall bias.
Serious indirectness on the level of population and no data on the post-vaccination period.
The mixed results most likely to be explained by serious inconsistency due to different study periods.
Serious indirectness on the level of intervention, as one of the studies did not include data on preprocedure testing.
Residual confounding: could not clearly distinguish between community-acquired infections or health care–acquired infections.
Although most studies reported on testing for patient cohorts undergoing gastrointestinal (GI) procedures only, a few studies that reported on larger cohorts included both GI and non-GI cases.
Asymptomatic prevalence was used as an indirect marker for infection rates.
Serious inconsistency across study results possibly attributable to differences across study time period. Two studies reported time points with asymptomatic prevalence >1% with the highest being 1.27% during the month of May in New York City. One study reporting 4% (42/968) HCWs was from Italy during the first wave of the pandemic (January through March 2020).
Although there were not many events, there were few large studies with several thousands of patients, thus we did not rate down for imprecision
Serious indirectness: on the level of intervention (no studies reporting on preprocedural testing); outcome no studies are reporting on patient important outcomes, such as increase in cancer-related mortality; and presentation at more advanced stages.
Serious inconsistency across study results possibly attributable to differences across study time period and study populations (different countries and health care systems), different baseline risk.
Prevalence of asymptomatic infection
We found 13 studies that reported on asymptomatic SARS-CoV2 among patients referred for endoscopic procedures who underwent testing.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Across these 13 studies, asymptomatic prevalence ranged from 0.0% to 1.5%, but most studies reported a range from 0% to 0.5% regardless of local surges of COVID-19 cases. A notable example of this is highlighted in 2 UK studies, conducted by the same authors at different time periods and surges; during the first time period from May to June 2020, when local prevalence was low, the asymptomatic prevalence was 0.11% (n = 2611) and during a surge in December 2020, the asymptomatic prevalence remained low (0.37% [9 of 2449]).14 , 15 The authors emphasized the role of symptom screening in maintaining low rates of SARS-CoV2 positivity in the endoscopy setting. Similarly, a large dataset from the Veterans Affairs Healthcare System in the United States showed a low prevalence of 0.1%; 46 PCR-positive out of 47,980 individuals that screened negative for symptoms screening before endoscopy (Jason Dominitz and Andrew Gawron, personal communication, February 2021). Finally, it is noteworthy that the few studies that reported on symptom screening results showed that symptom screening was higher yield than a pre-endoscopic testing strategy. Further information on reported rates of prevalence compared with local prevalence across studies is shown in Supplementary Table 1.
Patients’ and health care workers’ infections after endoscopy
We found 8 studies (2 prospective and 6 retrospective cohort studies) that reported rates of infection among HCWs and patients undergoing endoscopy.10 , 14 , 15 , 20, 21, 22, 23, 24 Of these studies, 5 were in the context of a preprocedure testing strategy,10 , 14 , 15 , 21 , 23 and 3 did not have an explicit preprocedure testing strategy.20 , 22 , 24 Among patients who underwent endoscopy, the rates of infection ranged from 0% to 0.4%. Among HCWs, the rates of infection ranged from 0% to 4.0%. The study reporting 4% (42 of 968) was from Italy during the first wave of the pandemic (January through March 2020).22 A notable limitation is the lack of robust contact tracing in included studies; the cases of COVID-19 were attributed to endoscopy exposure if there was no other known exposure. However, this would bias in favor of overestimating infection and transmission and, despite this, cases of reported transmission are rare (see Table 3 ).
Table 3.
Included Studies Reporting on Infections After Endoscopy
| First author, design, and dates | Preprocedure testing | PPE, masks | Patient infections (data source) | HCW infections (data source) | Total endoscopic cases. n |
|---|---|---|---|---|---|
| Hayee (prospective cohort)14 12/14/2020 to 12/31/2020 after emergence of UK variant |
Universal symptom screeninga and preprocedure testing | BSG guidanceb | 3 (post-endoscopy symptom screening d7 and d14 and testing as indicated) |
0 (reporting by local endoscopy centers) |
2440 |
| Hayee (prospective cohort)15 4/30/2020 to 6/30/2020 |
Universal symptom screeninga Some endoscopy units with PCR testing (n = 2611) |
BSG guidanceb | 0 (post-endoscopy symptom screening d7 and d14) |
0 (reporting by local endoscopy centers) |
6208 |
| Huang (retrospective cohort)21 2/1/2020 to 3/31/2020 |
Universal symptom screeninga PCR testing if symptom screening positive |
N95 or medical surgical masks | 0 (post-endoscopy follow-up) |
0/33 (symptom screening, temperature monitoring, PCR testing) |
1808 |
| D’Ovidio24 (retrospective cohort) 3/9/2020 to 5/4/2020 |
Universal symptom screeninga | NR | 0 (post-endoscopy follow-up) |
0 (PCR and serologic testing) |
60 |
| Pena-Rey (retrospective cohort)20 3/13/2020 to 5/11/2020 |
Universal symptom screeninga | NR | 0 NR |
0 “No cases associated with endoscopy” unclear if this included HCWs NR |
3310 |
| Repici (retrospective cohort)22 1/27/2020 to 3/13/2020 |
Screening/triage protocols evolved during this time | Active rationing of N95s; mix of N95s and surgical masks | 1 (post-endoscopy follow-up at 2 wk) |
42/968c (HCW survey) |
802 |
| Jagannath (retrospective cohort)23 4/2/2020 to 5/31/2020 |
Universal symptom screeninga PCR testing if symptom screening positive |
N95s | 6c | 4/74 (0.26%/100 endoscopies) | 1549 |
| Casper (retrospective cohort)10 3/23/2020 to 5/10/2020 |
Universal symptom screeninga PCR testing |
BSG guidanceb | 0 (NR) |
0 (weekly testing of HCWs) |
313 |
BSG, British Society of Gastroenterology; NR, not reported.
Universal symptom screening includes both patient’s symptoms as well as screening for high-risk exposures (travel/sick contacts).
BSG guidance recommends the following: if COVID negative: surgical masks for all cases; if COVID status unknown but symptom screening negative: N95 for upper endoscopy and surgical masks for lower endoscopy.
Of note, all 6 cases in Jagannath et al23 occurred within 48 h after endoscopy (unlikely that endoscopy was the source). Also, it is unclear whether the 42 HCW cases in this study were related to endoscopy or other exposures (contact tracing was not done) and the majority of the cases, 85.7%, were recorded before implementation of stringent preventive measures, including PPE.
Vaccination effectiveness against infection
There were no studies reporting on rates of infection in the context of endoscopy after vaccination of patients or HCWs. However, we used data from an existing CDC review and found an additional 6 prospective cohort studies that reported vaccine effectiveness against symptomatic or asymptomatic infection.25, 26, 27, 28 See Table 4 . Based on these studies, vaccine effectiveness for Pfizer/Moderna against asymptomatic SARS-CoV2 infection and transmission at 7–14 days after the second dose ranges from 80% to 94%. In addition, studies reported that the absolute risk of testing positive for SARS-CoV2 after vaccination among HCWs ranged from 0.5% to 1.19%. It is worthwhile to note that the CDC no longer requires quarantine after known COVID-19 exposure for vaccinated individuals, which include the majority of HCWs.29
Table 4.
Included Studies on Vaccine Effectiveness Against SARS-CoV2 Infection
| First author, year, country | Population (HCWs vs general, n) | Vaccine(s) | Timing | Outcome | Vaccine effectiveness (%) or risk reduction |
|---|---|---|---|---|---|
| Tande, 202127,a United States |
General adult population | Pfizer-BioNTech or Moderna | 0 d after second dose | Asymptomatic infection | 80 |
| Levine-Tiefenbrun, 2021a Israel30 |
General adult population | Pfizer-BioNTech | 14 d after second dose | Asymptomatic infection | 94 |
| Hall, 202125 United Kingdom |
HCWs; n = 25,661 | Pfizer-BioNTech | 7 d after second dose | Asymptomatic infection | 86 |
| Thompson, 202126 United States |
HCWs and other frontline workers; n = 3950 |
Pfizer-BioNTech or Moderna | 14 d or more after second dose | Asymptomatic infection | 90 |
| Keehner, 202131 United States |
HCWs; n = 36,659 | Pfizer-BioNTech or Moderna | 14 d or more after second dose | Asymptomatic infection | SARS-CoV2 positivity rate: 0.05% |
| Jacobson, 202132 United States |
HCWs; n = 22,729 | Pfizer-BioNTech or Moderna | 14 d or more after second dose | SARS-CoV2 infection | COVID-19 positivity rate: 0.11% |
| Zaqout, 202133 Qatar |
General adult population; n = 199,219 | Pfizer-BioNTech (35% with 2 doses) | 28 d or more after second dose (or first in patients who had received only 1 dose) | SARS-CoV2 infection | Incidence rate ratio (vs test positivity within 7 d of vaccination), 0.15 (95% CI, 0.13–0.18) |
| Björk, 202134 Sweden | General adult population; n = 26,587 | Pfizer-BioNTech | 7 d or more after second dose | SARS-CoV2 infection | 86 |
NOTE. Studies reported in this table are limited to cohorts that received US emergency use authorization–approved vaccines (Pfizer BioNTech and Moderna; no reported data on Johnson & Johnson). If a study reported multiple rates at different time points, only the last time point after complete vaccination was reported here. Studies reporting on effectiveness for non-emergency use authorization–approved vaccines were excluded.
These data were extracted from the CDC.28
Patients’ and health care workers’ attitudes and anxiety before endoscopy
We identified 2 studies that reported on patients' attitude and anxiety regarding endoscopy during the early phases of the pandemic.35 , 36 In 1 survey study, patients felt that on-site testing was important but, despite testing, they did not feel reassured. In another study of hospitalized and ambulatory individuals, 83% reported feeling safer because of the testing strategy. Three cross-sectional survey studies reported on preprocedural testing and HCW anxiety during the pandemic and reported a reduction in anxiety about acquiring infection and infecting family members after implementation of a pretesting strategy.37, 38, 39 There were no studies on anxiety in the postvaccination setting.
Patient delays in care and endoscopy volumes
Fourteen studies (1 survey study and 13 cohort studies, mostly based on administrative datasets) reported on endoscopy volumes from the United States, United Kingdom, The Netherlands, Canada, China, Spain, Japan, and Taiwan during the initial 3–4 months of the pandemic.20 , 21 , 37 , 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Four studies report on the later period of the pandemic. Across studies, in the early phases of the pandemic, the total number of upper endoscopies and colonoscopies decreased by 51%–72% and 59%–85%, respectively. This was compared with the same time period from prior years. During the most “COVID-19 impacted” phase (April 2020), the decrease in upper endoscopy and colonoscopy was 78%–87% and 92%–95%. Four studies, 1 from the United Kingdom, 1 from Spain, and 2 from the United States (Veterans Affairs Healthcare System and TriNetX database) reported on endoscopy volumes in the late stages of the pandemic.20 , 41 , 47 , 50 The reported endoscopy utilization was between 40% and 70% in the United States, between 40% and 100% in Europe, and approximately 70% of expected volumes in the Veterans Affairs study. A modeling study from Canada estimated that it will take 41 months to complete all of the backlog of colonoscopies. They also suggest that changing low-yield colonoscopies to fecal immunochemical test would reduce recovery time.49 No studies were identified reporting on endoscopy volumes in the post-vaccination period.
Patient delays in care and impact on cancer burden
We identified 9 studies (United States, United Kingdom, Netherlands, Japan, and Hong Kong) that reported on the impact of COVID-19 on cancer diagnoses.41 , 42 , 44, 45, 46, 47, 48 , 51 , 52 We included studies that focused on the following gastrointestinal cancers: esophageal, gastric and colorectal (gastrointestinal cancers that we perform screening and surveillance for or that are diagnosed endoscopically) and excluded studies reporting on pancreatic and liver cancers. Most studies estimated the reduction in cancer diagnoses based on 2019 expected numbers using administrative datasets. The authors estimated that endoscopic cancer detection was reduced by 31%–71% for colorectal cancer (CRC), by 27%–37% for esophageal cancer, and 27% to 52% for gastric cancer during the early phases of the pandemic. During the late phase of the pandemic, the decline in new diagnoses of malignant CRC was 12% and for esophageal and gastric cancer was 20%. In 1 Japanese single-center retrospective study of 123 CRC patients who underwent surgery during COVID-19, patients were more likely to present with advanced CRC and more patients required emergency admission for obstructive CRC (39% vs 15%).46 See details of studies in Supplementary Table 2. It is important to note that none of these studies specifically reported on whether implementation of preprocedure testing additionally contributed to delays in endoscopy. However, it is possible that preprocedure testing would impose additional burden on patients and may promote procedure cancellation. This is particularly problematic when the testing windows are short and turnaround times for results are prolonged.
Benefits and Harms
In making a recommendation, the panel weighed the potential benefits of a pretesting strategy in the post-vaccination setting against the downsides of testing. The panel acknowledged the small potential benefit of preprocedure testing with respect to patient and staff reassurance, but no benefit with regard to infections. Based on the evidence, there were few to no cases of infections reported among HCWs (performing endoscopy) and patients. Among the few reported cases, the authors could not clearly distinguish between community-acquired infections or health care–acquired infections. Furthermore, with symptom screening, adequate PPE, and the significant protection from infection (both asymptomatic and symptomatic) due to vaccination, the risk of infection was felt to be negligible. The panel also evaluated the yield of testing (rates of positive tests among asymptomatic individuals ranged from 0% to 0.5%) and the significant delays in care (reduced numbers of procedures across endoscopy centers with incomplete recovery of volumes) and reduced numbers of diagnoses of CRC, esophageal, and gastric cancers (compared with expected numbers from historical data). The panel placed a high value on minimizing additional delays in care in light of the downstream impact on cancer diagnoses. See Supplementary Table 3.
Certainty of Evidence
The overall certainty of evidence was very low across outcomes as detailed in Table 2. We rated down for risk of bias (observational studies with many limitations), indirectness (no studies in the post-vaccination period), and inconsistency across the various outcomes. We acknowledged limitations of this body of evidence, including the lack of evidence comparing the impact of a pretesting strategy (combined with screening) vs screening alone on relevant clinical outcomes. Studies reporting on HCW and patient infections did not perform adequate contact tracing and we could not determine whether infections were community-acquired or health care–acquired. No studies directly informed us about the role of preprocedure testing in providing reassurance or reducing anxiety (for patients or HCWs) in the post-vaccination setting. No studies reported on endoscopy volumes in the post-vaccination period, and it is unclear how much preprocedural testing led to reduced endoscopy volumes and whether endoscopy centers are now at 100% capacity and efficiency.
Recommendation 2: In endoscopy centers that implement a preprocedure testing strategy, the AGA suggests using standard nucleic acid testing (rapid RT-PCR or laboratory-based NAAT) rather than a rapid isothermal test or antigen tests in patients undergoing upper endoscopy or lower endoscopy (the terms upper and lower endoscopy include all related gastrointestinal procedures, eg, endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, and flexible sigmoidoscopy) irrespective of the vaccination status of patients. Conditional recommendation, very low certainty evidence
Remarks: Rapid RT-PCR tests that can be easily performed on the day of endoscopy (results within 1 hour), are preferable as they pose less burden to patients. In the preprocedure setting, the utility of rapid isothermal tests or antigen tests is limited due to concerns of assay sensitivity. There is no role of antibody tests for preprocedure testing.
Rationale
Diagnostic test accuracy has important downstream implications on clinical practice. Using tests with the best sensitivity and specificity allows providers to reduce the numbers of false positives (ie, individuals who test positive for SARS-CoV2 but do not have the infection) and false negatives (ie, individuals who test negative for SARS-CoV2 but do have the infection). In a patient who tests negative for SARS-CoV2 infection (false negative) and a surgical mask is used for upper endoscopy, there can be a potential (albeit small) increased risk of infection to the endoscopy staff and false reassurance to the individual. In a patient who tests positive for SARS-CoV2 who does not have infection (false positive), implications for the patient include cancellation of the procedure, self-isolation for 14 days, apprehension, and loss of work.3
Summary of the Evidence
Evidence on the diagnostic test accuracy of available tests in the United States was obtained from the recent IDSA guidelines on SARS-CoV2 infection.6 Six studies evaluated the diagnostic test performance of laboratory-based RT-PCR tests, rapid RT-PCR tests, and rapid isothermal NAATs compared with a composite reference standard of multiple laboratory-based NAATs. The studies included 672 patients. Laboratory-based and rapid RT-PCR tests had comparable sensitivity (0.99; 95% confidence interval [CI], 0.96–0.99 vs 0.98; 95% CI, 0.95–1.00, respectively) and specificity (0.98; 95% CI, 0.94–0.99 vs 0.97; 95% CI, 0.89–0.99). Rapid isothermal NAATs had a lower sensitivity (0.81; 95% CI, 0.75–0.86), but comparable specificity (0.99; 95% CI, 0.96–1.00).53 The IDSA also identified 5 studies comprised of 6946 patients that evaluated the diagnostic test performance of rapid antigen tests in adult asymptomatic patients. The pooled sensitivity of rapid antigen tests was 0.52 (95% CI, 0.42–0.62) and pooled specificity was 1.00 (95% CI, 0.99–1.00).6 The IDSA guideline and review on SARS-CoV2 antibodies tests included 12 studies that evaluated the sensitivity of IgM antibodies in week 1 after symptoms onset, 13 studies of IgG antibodies in week 1 after symptom onset, and 16 studies of IgM and IgG antibodies in weeks 2 after symptom onset. They also identified 21 studies that evaluated the specificity of IgM antibodies and 25 studies of IgG antibodies. The pooled sensitivity in week 1 after symptom onset ranged from 0.23 to 0.33 and in week 2 was 0.68 to 0.73, and the specificity was 0.98 to 0.99 (Table 5 ).54
Table 5.
Summary of Findings Table of Laboratory-Based RT-PCR, Rapid RT-PCR, Rapid Isothermal NAAT, Rapid Antigen Tests, and Antibody Tests
| Test | Laboratory-based RT-PCRa | Rapid RT-PCRa | Rapid Isothermal NAATa | Rapid Antigen Testsb | IgM Antibodiesc,d | IgG Antibodiesc,d |
|---|---|---|---|---|---|---|
| Assuming 1% prevalence: Effect per 1000 patients tested | ||||||
| Sensitivity | 0.99 (0.96–0.99) | 0.98 (0.95–1.00) | 0.81 (0.75–0.86) | 0.52 (0.42–0.62) | 0.33 (0.25–0.41)c 0.73 (0.66–0.78)d |
0.23 (0.16–0.32)c 0.68 (0.62–0.73)d |
| No. of studies (no. of patients) |
6 studies (376 patients) | 4 studies (230 patients) | 4 studies (288 patients) | 5 studies (271 patients) | 12 studies (919 specimens)c 16 studies (2309 specimens)d |
13 studies (1343 specimens)c 16 studies (2708 specimens)d |
| True positives (patients with SARS-CoV2) |
10 (10 to 10) | 10 (10 to 10) | 8 (8 to 8) | 5 (4 to 6) | 3 (3 to 4)c 7 (7 to 8)d |
2 (2 to 3)c 7 (6 to 7)d |
| False negatives (patients incorrectly classified as not having SARS-CoV2) |
0 (0 to 0) | 0 (0 to 0) | 2 (1 to 2) | 5 (4 to 6) | 7 (6 to 7)c 3 (2 to 3)d |
8 (7 to 8)c 3 (3 to 4)d |
| Test accuracy Certainty of evidence |
⊕⊕⊕◯e | ⊕⊕⊕◯e | ⊕⊕⊕◯e | ⊕⊕◯◯f,g | ⊕◯◯◯e,g,h | ⊕◯◯◯e,g,h |
| Specificity | 0.98 (0.94–0.99) | 0.97 (0.89–0.99) | 0.99 (0.96–1.00) | 1.00 (0.99–1.00) | 0.98 (0.97–0.99) | 0.99 (0.99–0.99) |
| No. of studies (no. of patients) |
6 studies (296 patients) | 4 studies (164 patients) | 4 studies (209 patients) | 5 studies (6675 patients) | 21 studies (7165 specimens) | 25 studies (11,887 specimens) |
| True negatives (patients without SARS-CoV2) |
970 (931 to 980) | 960 (881 to 980) | 980 (950 to 990) | 990 (980 to 990) | 970 (960 to 980) | 980 (980 to 980) |
| False positives (patients incorrectly classified as having SARS-CoV2) |
20 (10 to 59) | 30 (10 to 109) | 10 (0 to 40) | 0 (0 to 10) | 20 (10 to 30) | 10 (10 to 10) |
| Test accuracy Certainty of evidence | ⊕⊕⊕◯e | ⊕⊕⊕◯e | ⊕⊕⊕◯e | ⊕⊕⊕◯f | ⊕◯◯◯i,j,k | ⊕◯◯◯i,j,k |
| Considerations | Most patients were symptomatic | Most patients were symptomatic | Most patients were symptomatic | Most patients were asymptomatic; suboptimal reference standard | Case-control studies; suboptimal reference standard | Case-control studies; suboptimal reference standard |
NOTE. These data do not represent comparative differences between tests.
⊕⊕⊕⊕, high certainty; ⊕⊕⊕◯, moderate certainty; ⊕⊕◯◯, low certainty; ◯◯◯◯, very low certainty.
Compared with a composite reference of multiple laboratory-based RT-PCR tests in symptomatic individuals.
Compared with rapid or laboratory-based RT-PCR reference standard in asymptomatic adults.
Compared with rapid or laboratory-based PCR reference in wk 1 after symptom onset.
Compared with rapid or laboratory-based PCR reference in wk 2 after symptom onset.
Rated down for serious indirectness, as the studies included mainly symptomatic individuals.
Rated down for serious risk of bias as the reference was single RT-PCR tests (rapid or laboratory-based).
Rated down for observed serious unexplained inconsistency with considerably variable sensitivity.
Rated down for very serious risk of bias as most of the studies had case-control design, reported results per specimens rather than individual patients, and the reference was single RT-PCR tests (rapid or laboratory-based).
Rated down for very serious risk of bias as most of the studies had case-control design and reported results per specimens rather than individual patients.
Rated down for observed serious unexplained inconsistency with considerably variable specificity.
Rated down for serious indirectness as the many of the studies included stored specimens from time periods before the COVID-19 pandemic.
Benefits and Harms
In making this recommendation, the panel weighed the potential benefits of the tests (true positives and true negatives) against the downsides of the test (false positives and false negatives) in addition to the logistics of testing (delays from test collection to test results). The panel acknowledged that a small minority of endoscopy centers may still choose to implement a pretesting strategy. In this setting, the SARS-CoV2 test should be a NAAT-based test (which has the best sensitivity and specificity based on moderate certainty evidence) or ideally a rapid RT-PCR that can be performed at the endoscopy center on the day of procedure (to reduce the patient burden of needing to get tested before the procedure). Availability and access to tests is an important consideration. The panel deliberated over the utility of the rapid antigen tests in the preprocedure setting, but had concerns about the false-negative rates, which may provide false reassurance. In addition, the lower sensitivity of the rapid isothermal test, would lead to an increase in false-negative results compared with rapid RT-PCR tests; the rapid isothermal test referred to in this document is IDNOW (Abbott). Finally, antibody tests have no role in detection of asymptomatic infection. See Supplementary Table 3.
Certainty of Evidence
The overall certainty of evidence was moderate to very low across the various tests. For the RT-PCR and isothermal tests, the studies included mainly symptomatic patients, thus, the certainty of evidence was rated down to moderate for serious indirectness. For the antigen tests, the studies used single laboratory-based or rapid RT-PCR tests as reference standards and there was considerable variability in the sensitivity in the included studies, thus the certainty of evidence was rated down for serious risk of bias and serious inconsistency. Finally, for the antibody tests, the certainty of evidence was very low due to very serious risk of bias, and serious inconsistency and indirectness.
Other Evidence to Decision Considerations
The panel also evaluated the burden of testing, whether access to testing may magnify any health inequities, and whether there were any cost-effectiveness studies. The panel identified 1 study in which authors reported that 3228 of 5881 patients did not receive preprocedural/presurgical testing; 30.5% were not tested due to inability to reach the patient and the remaining patients (69.5%) declined.13 The most common reasons for declining were lack of interest in testing (19.2%), distance from testing facility (19.0%), and perception of not being at risk due to self-isolation (9.8%). About 4.1% reported that they did not get tested due to lack of transportation and 1.1% reported fear of going to a testing center. See Supplementary Table 3.
Cost-effectiveness of a preprocedure testing strategy
We identified 2 modeling studies reporting on cost-effectiveness of a pre-endoscopic testing strategy. One single-center retrospective study used baseline data from the first week of reopening during the pandemic in March 2020 to simulate costs and concluded that implementing PCR testing is a cost-effective strategy to resume endoscopy.55 However, the following assumptions used in this modeling study were not relevant for our guideline and they did not account for vaccinations: PPE rationing is no longer widespread; asymptomatic prevalence is very low; use of preprocedure symptom screening is not discussed; and assumptions about HCW infections were higher than reported and did not take into account vaccination status or the need to no longer quarantine, per new CDC guidance.29
A second modeling study concluded that testing is most cost-effective when there is a high prevalence of COVID-19 and high-risk PPE is used.56 However, this study did not take into account diagnostic accuracy of testing; as the prevalence rises, false positives also increase, which have additional economic downstream consequences, such as quarantining individuals away from work or school unnecessarily. Similarly, this study did not take into account symptom screening as preprocedure protocol. Despite these studies’ limitations, they highlight the importance of accounting for potential costs of using high-risk PPE for patients with unknown COVID status.
Equity
Our search did not yield any direct evidence on equity issues in the context of preprocedure testing. However, our guideline panel acknowledges the widespread indirect data supporting health disparities in access to testing, clinical care, and vaccines during the COVID-19 pandemic.57, 58, 59, 60, 61 Given this, our guideline panel discussed and acknowledged the potential for testing to serve as an additional barrier to care for underserved populations who may already have disparities in care.
Implementation Considerations
Additional considerations are outlined below:
-
1.
These recommendations are based on high efficacy and real-world effectiveness of COVID-19 vaccine against prevalent variants of SARS-CoV2. If new variants of the virus, which are resistant to the vaccine, dominate in the coming months, then safety of HCWs and patients and risk of asymptomatic transmission may be prioritized by endoscopy centers.
-
2.
The guideline was developed with the intent to be implemented across all different practice settings, including academic and private practices, and hospital-based and ambulatory surgical centers performing elective endoscopy.
-
3.
The guidelines apply to all upper endoscopic and lower endoscopic procedures. Although the majority of the procedures in the included studies were esophagogastroduodenoscopies and colonoscopies, a few studies included endoscopic ultrasound and endoscopic retrograde cholangiopancreatography procedures. Data on implementation of these recommendations for motility procedures (eg, esophageal manometry) are unknown, as studies did not include data on esophageal manometry. However, indirect evidence from endoscopic procedures would provide a similar recommendation suggesting against preprocedure testing for motility procedures. We were unable to specifically address whether preprocedure testing may be appropriate for patients undergoing endotracheal intubation as part of their endoscopic procedure; endotracheal intubation generates a larger volume of aerosols (than endoscopy) and may pose a higher risk of asymptomatic transmission if patients were infected with SARS-CoV2; however, assuming that HCWs have appropriate PPE and are vaccinated, the risk of infection in this setting is likely low.
-
4.
All patients should undergo preprocedure screening for symptoms suggestive of COVID-19 before endoscopy. The CDC provides an updated symptom-based screening questionnaire that can be used by centers.62 Unfortunately, the majority of symptoms have poor diagnostic accuracy to rule in or rule out COVID-19. In a recent Cochrane review, presence of fever and cough has a sensitivity of 64%–67%; isolated diarrhea had a sensitivity of 11%. Patients who are positive on symptom screen should be referred for preprocedure testing with standard NAAT tests.63
-
5.
The recommendations are contingent upon access to, and proper use of, PPE, including face shield, eye protection, and surgical mask or N95 (N99, powered air-purifying respirators) by HCW during endoscopic procedures. Endoscopy centers would continue to take steps to minimize risk of transmission through adequate physical distancing measures and use of facemasks by all patients.
-
6.
In centers that choose to perform preprocedure testing, a rapid RT-PCR (with test result within 1 hour) on the day of the endoscopy is preferred to reduce patient burden. Furthermore, if this strategy is adopted, patient scheduling, patient arrival time, and testing-related logistics must be considered.
-
7.
The evidence base does not support limiting testing to certain subgroups of individuals, such as those who are unvaccinated or elderly. There were no reported subgroups of populations at higher risk for becoming infected in the context of endoscopy. Theoretically, immunocompromised individuals may remain at higher risk despite vaccination. Our review outlines very low rates of asymptomatic prevalence and even lower rates of potential transmission during endoscopy to patients or staff; infections associated with endoscopy were a rare event.
-
8.
In (nonimmunocompromised) symptomatic individuals who test positive for SARS-CoV2, it is estimated that 88%–95% of their specimens no longer yield replication-competent virus 10–15 days after symptom onset (as per CDC).64 Also, recovered individuals may continue to have SARS-CoV2 detected for up to 12 weeks after symptom onset. Based on this information, asymptomatic SARS-CoV2 individuals are also unlikely to have replication-competent virus that is associated with increased risk of infection, and these individuals can probably undergo elective endoscopy after 15 days without the need for repeat testing.
Plans for Updating
In order for guidelines to remain useful, they must be updated as new conclusive information accumulates. This document will be updated or will expire in 12 months.
Research Gaps
In reviewing the existing evidence and developing these guidelines, we identified the following important research gaps.
-
1.
Although delays in patient care have been observed universally in the course of the pandemic, the exact contribution of preprocedure testing, typically performed with standard laboratory-based NAAT tests, to delay in endoscopy was unclear; however, it was assumed to be a barrier to endoscopy.
-
2.
There is paucity of data on patient and HCW values and preferences for preprocedure testing in the post-vaccination period.
-
3.
The aerosol-generation potential of different endoscopic procedures and the risk of asymptomatic SARS-CoV2 transmission is uncertain and warrants further study. There are also very limited data on the impact of room turnover time or number of air exchanges and risk of transmission of SARS-CoV2.
-
4.
Better evidence is needed to understand the downstream impact on cancer diagnoses among different ethnic and racial groups.
Discussion
Since the original release of the AGA guidelines on preprocedure testing (July 29, 2020), our knowledge and understanding of disease transmission, infection risk from endoscopy, and most recently protection from vaccinations, has increased drastically. This accumulation of evidence underscored the need to provide an updated guideline focused on SARS-CoV2 testing and endoscopy in a post-vaccination setting. Unlike the previous guideline, when our limited understanding of transmission risks associated with endoscopy and resources constraints (related to PPE and tests) prompted the panel to place a high value on HCW and patient safety, in this updated guideline, the panel prioritized patient outcomes, specifically patient delays in care from a population perspective.
Early in the pandemic, many centers and patients were forced to reduce endoscopy volumes, resulting in delays in care and implemented preprocedure testing in efforts to safely resume endoscopy. Based on published studies of preprocedure testing, asymptomatic infections in patients undergoing endoscopy throughout the pandemic, including times of COVID surges, remained low (nearly 0.5%) after a negative screening questionnaire. In light of the very low prevalence of SARS-CoV2 in asymptomatic patients, the extremely low risk of infection among vaccinated individuals and the significant delays in endoscopy, the panel advises that the majority of centers should not perform preprocedure testing routinely (conditional recommendation against). Multiple modeling studies have assessed the impact of delays in colonoscopy (for CRC screening/surveillance) related to the pandemic and these delays are projected to lead to a substantial increase in cancer-related mortality through 2050.
Forgoing preprocedure testing allows patients to undergo endoscopic procedures with fewer obstacles, allows for improved access to care, reduces inequalities related to the ability to obtain preprocedure testing, and allows for endoscopy centers to optimize their procedure volumes. The recommendations were developed with a number of assumptions, including that centers having adequate PPE, follow universal precautions, and use a screening checklist before endoscopy.
Nonetheless, the panel acknowledges that a small minority of centers may still choose to continue preprocedure testing, despite the increased burden of testing on patients, downstream consequences of false positives, delays in care, and decreased endoscopy efficiency. If testing is performed, it is important that centers use a nucleic acid test rather than a rapid isothermal test or antigen test. The performance of these tests has downstream implications on clinical practice related to false positives resulting in inappropriate cancellations of patient procedures, and inappropriate patient anxiety and harms from requiring them to self-quarantine and conduct contact tracing. Finally, the panel also acknowledges that local, state, and health system policies may dictate decisions about PPE use and requirements for preprocedural testing of asymptomatic patients.
Acknowledgments
The authors acknowledge Dr Jason Dominitz, MD, MHS and Dr Andrew Gawron, MD, PhD for sharing data on preprocedure testing before endoscopy from the Veterans Affairs Healthcare System and Christoph Meyer-Grimberg for his perspective as a patient representative.
This document represents the official recommendations of the American Gastroenterological Association (AGA) Institute and was developed by select members of the Clinical Guideline Committee and Clinical Practice Update Committee and approved by members of the AGA Governing Board.
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2021.05.039.
Supplementary Material
Narrative Summary of Included Studies on Infections
Patient and Health Care Worker Infections After Endoscopy
We found 8 studies that provided insight on the risk of transmission during endoscopy, with reported rates of infections in HCWs or patients after the endoscopy.1, 2, 3, 4, 5, 6, 7, 8
There were no randomized controlled trials informing transmission of COVID-19 during endoscopy. There were 2 prospective cohort studies2 , 3 and 6 retrospective cohort studies.1 , 4, 8 Of these studies, 5 were in the context of a preprocedure testing strategy1, 2, 3 , 5 , 7and 3 did not have an explicit preprocedure testing strategy.4 , 6 , 8
There were 2 multicenter prospective studies conducted by Hayee et al in the United Kingdom. 2 , 3The first was conducted in the United Kingdom from April 30 to June 30, 2020 and reported 0 transmissions to patients or staff across 18 centers in the United Kingdom (n = 6208 procedures). These transmission data were based on follow-up telephone screening of patients at 7 and 14 days. All patients (n = 6208) underwent preprocedure screening using the SCOTS (symptoms, infectious contacts, occupational risk, travel risk, and shielding status) criteria and some patients (n = 2611) also underwent preprocedure nasopharyngeal PCR testing, which was dependent on the endoscopy unit practices. Of note, the asymptomatic prevalence in this subpopulation was 0.11% (3 of 2611). PPE was not detailed in this study, but there was acknowledgement of British Society of Gastroenterology guidance, which supports the use of surgical masks for COVID-19–negative patients, or N95 for upper endoscopy and surgical masks for lower endoscopy.15 The second study was conducted in December 2020, after the emergence of the UK variant, with rising local prevalence of COVID-19.2In this study, the asymptomatic prevalence was overall still low 0.37% (9 of 2449). Similar to the other study, telephone screening of patient was performed at 7 and 14 days. Patients who screened positive underwent PCR testing, and contact tracing, which entailed the following: patient had to have developed symptoms of COVID-19 within 10 days of endoscopy with no other more likely source identified on direct questioning by the local care team. There were 3 patient cases of PCR-confirmed COVID-19 that were presumed to be attributed to endoscopy after thorough questioning and no other exposures were identified. Of note, there were 15 patients (0.61%) who were COVID-19–positive on post-procedure follow-up calls; however, they were attributed to other known exposures. It is important to note that 0 HCWs were infected despite these potential exposures.
Retrospective study data reported infections in patients after endoscopy ranged from 0%–0.4% and 0%–4% in HCWs, with the caveat that this includes data during early stages of the pandemic, before implementation of COVID-19 precautions and PPE. The study reporting 4% rates of infection was from Italy during the first wave of the pandemic during January to March 2020.6These data were collected based on a HCW web-based survey and it is unclear how many of the 42 reported cases were actually transmitted during endoscopy. The average infection rate among HCWs during that time in Italy was still higher at around 10%. It is notable, however, that despite this, the rate of transmission to patients was quite low in this study (0.12%). This study was limited by a lack of robust contact tracing and unclear attribution of COVID-19 cases to the hospital or community settings.
However, the majority of data show low rates of infection to both patients and staff. Four retrospective studies reported 0 cases of transmission to patients and staff (total number of cases was 310, 60, 1808, and 3310, respectively).1 , 4 , 5 , 8PPE was not explicitly reported, and preprocedure testing was not performed routinely in these 3 studies. Finally, a retrospective cross-sectional questionnaire of 5 endoscopy centers in India during April through May 2020 also found a low rate of infections in HCWs and patients across 1549 endoscopies.7In this study, N95s were worn by HCWs, and 1 of 5 centers conducted preprocedure testing (total endoscopies with testing not reported). This study reported that 0.4% (6 of 1549) patients tested positive for COVID-19 within 72 hours of the procedure, but 0 of 1549 tested positive between 72 hours and 14 days of the procedure, indicating that the early positive cases may not be procedure-related. Of note, the community prevalence of COVID-19 during this time was 4.5%–5.1% and was considered a surge phase. For HCWs, survey data reported 3 of 74 (4%) endoscopy personnel who were COVID-19–positive and concluded that the risk of HCWs getting COVID-19–positive was 0.26% per 100 endoscopies. Limitations to this study include unclear description of whether symptom screening, lack of contact tracing for both patients and HCWs.
Supplementary Table 1.
Included Studies on Prevalence of Asymptomatic Infection
| First author, year, design, setting, dates | Local prevalence | Symptom screening |
Testing strategy |
||||
|---|---|---|---|---|---|---|---|
| Type of screening | Positive screening | Type of test,timing of test | Positive asymptomatic cases, n | Total cases, n | Asymptomatic prevalence, % | ||
| Bowyer, 20219 Retrospective United States (IL) 5/22/20–6/28/20 |
8.4% local positivity rate for Winnebago County, IL between 9/1/20 and 9/9/20 | ASGE preprocedure risk screening questionnaire | 122/1000 = 12.2% positive Of those, 3 of 122 (2.46%) were SARS-CoV–positive |
NP swab Roche COBAS 6800/8800 RT-PCR 72 h prior |
5 | 878 | 0.57 |
| Casper, 202010 Retrospective Germany 3/23/20–5/10/20 |
Cumulative incidence in Saarland: 279 per 100,000 During study period, 2514 tested positive in Saarland |
Symptom screeninga | NR | NP swab RT-PCR Testing for all outpatients starting April 2020 <5 d prior |
0 | 313 | 0 |
| Haidar, 202113 Prospective United States (PA, NY) Period 1: April 21, 2020–June 11, 2020 Period 2: June 12, 2020–September 10, 2020 Period 3: September 11, 2020–December 15, 2020 (universal testing deimplemented) |
Total new cases in Allegheny County: Period 1: 1056 Period 2: 10,014 Period 3: 30,524 |
Symptom screening: fever, cough, and shortness of breath, and asked to self-report other symptoms <7 d of procedure, 1–4 d prior, and day of procedure | 16/817 (1.9%) | NP swab Cepheid Xpert Xpress RT-PCR 1–4 d preprocedure with results in <24 h with a subset of negative patients randomly selected for repeat testing on the same day of the procedure (with results <1 h) |
Period 1: 10 Period 2: 54 Period 3: 101 |
Period 1: 10,539 Period 2: 34,948 (“summer surge”) Period 3: 24,741 (“fall surge”) |
Period 1: 0.10 Period 2: 0.15 Period 3: 0.41 |
| Hernandez Camba, 202116 Retrospective Spain 4/27/20–6/15/20 |
NR | Screening questionnaire (fever, cough, sore throat, or breathing problems, known exposure, and loss of smell or taste) 3 d prior and 14 d after |
0/211: 0% | SARS-CoV2 antibody test followed by RT-PCR if positive only within 48 h | 0a | 211 | 0 |
| Lewis, 202117,b Retrospective United States (NC) 3/31/2020–4/20/2020 |
NR | Symptom screeningc | NR | 5 in-house tests: CDC’s 2019-nCOV RT-PCR diagnostic panel, Diasorin Simplexa COVID-19 direct assay, Cepheid Xpert Xpress, Abbott real-time SARS-CoV02 assay, Abbott ID NOW 72 h prior |
6 | 1580 | 0.4 |
| Tworek, 202119 Retrospective United States (MI) 4/15/20–5/15/20 |
NR | Symptom screeningc 2 d prior |
NR | ID NOW RT-PCR (gold standard) |
ID NOW: 0 RT-PCR: 3 |
386 | IDNow: 0 RT-PCR 0.77 |
| Hayee, 202115 Multicenter prospective study United Kingdom 4/30/2020–6/30/2020 |
NR | Telephone screening (SCOTS: questions on symptoms, infectious contacts, occupational risk, travel, shielding status |
NR | NP swab Type of test NR |
3 | 2611 | 0.11 |
| Hayee, 202114 Multicenter prospective study United Kingdom |
Rising incidence after emergence of new UK variant: at least 800 cases per 100,000 population per week |
Telephone screening (SCOTS: questions on symptoms, infectious contacts, occupational risk, travel, shielding status |
NR | NP swab Type of test NR |
9 | 2449 | 0.37 |
| Mays, 202018 Cross-sectional United States (University of Washington) 4/13/20 |
2-5% region prevalence |
Symptom screeninga | 137/133 6 (10.3%) |
PCR testing (DiaSorin Simplexa SARS-COV-2, Hologic Panther Fusion, or Roche COBAS) prior admission or surgical procedure | 5 | 787 | 0.6 |
| Albendin-Iglesias, 20208 Prospective observational cross-sectional Spain 4/15/20–5/15/20 |
3.34/100,000 between May 1 and May 15, 2020 | NR | NR | NP or OP with Allplex 2019-nCoV Assay (Seegene, Seoul, South Korea) | 21 | 363 | 0.27 |
| Dolinger, 202011 Retrospective single center study United States (NY City) 5/1/20–6/30/20 |
May NY State prevalence 5.34% and NY City 6.27% June NY State prevalence 1.20% and NY City 1.43% |
NR | NR | PCR testing 48–72 h before procedures | 6 | 623 | 0.96 |
| Gawron, 2021 (personal communication) Retrospective multicenter VAHCS United States 3/18/20–12/31/20 |
NR | Symptom screening (flu, cough, fever), travel history, known exposure 7 d before endoscopy |
2497/57,892 869 PCR-positive (1.5%) |
RT-PCR within 7 d of procedure | 106 | 129,410 92,030 |
0.1 |
| Forde, 202012 Retrospective single center study United States (FL) |
Miami-Dade County: 12.7%. In system’s catchment area: 5.4%–9.5% |
Symptom screening (fever, conjunctivitis, cough, sore throat, difficulty breathing, diarrhea, body aches, or lack of smell or taste in the last 3 d), travel history, known exposure 7–9 d prior, and on day of procedure |
NR | CE-IVD kit Gene-Finder COVID-19 Plus RealAmp Kit, QIAstat-Dx Respiratory 2019-nCoV- 2, and Cepheid Xpert Xpress SARS-CoV-2 |
1 | 396 | 0.25% |
ASGE, American Society for Gastrointestinal Endoscopy; NP, nasopharyngeal; OP, oropharyngeal; VAHCS, Veterans Affairs Health Care System.
In this study, antibody testing was used instead of RT-PCR. If IgM-positive, SARS-CoV-2 PCR testing was conducted. If positive, endoscopy was postponed. If negative, PCR was repeated and if negative again, endoscopy was performed. In this study, 1.9% (4 of 211) patients (95% CI, 0.07%–4.8%) were positive for SARS-CoV-2 antibodies, which does not indicate active infection; asymptomatic prevalence was 0% (RT-PCR was negative in the 4 patients with positive antibody testing)
In the study, pre-procedure testing included all ambulatory procedures, encompassing endoscopy. If cases were urgent, providers wore N95s/PAPRs if COVID status was unknown.
Symptom screening indicates that authors reported using symptom screening but provided no details as to the type of screening tool or questions.
Supplementary Table 2.
Included Studies on Patient Delays in Care
| First author, year, design, dates | Country/setting/preprocedure testing | Endoscopy volume | Impact on cancer burden |
|---|---|---|---|
| Moraveji, 202037 Cross-sectional survey study May 2020 |
Unites States 407 respondents 276 centers (51% academic or university) Preprocedure testing: NR |
Procedure volumes 81% of centers reported >60% reduction for upper endoscopy 82% of centers reported >60% reduction for colonoscopy 71% of centers reported >60% reduction for deep enteroscopy |
NR |
| Lui, 202044 Multicenter, retrospective cohort, and modeling data October 1, 2019–March 31, 2020 |
Hong Kong All public hospitals Comparing procedure burden and cancer detection within similar time periods from 2017 to 2019 Preprocedure testing: NR |
Mean no. of upper endoscopies performed per week dropped by 51.0% (from 1813 to 887) Mean no. of lower endoscopies performed per week dropped 58.8% (from 1190 to 491) |
Mean gastric cancer and CRC diagnosed per week fell by 46.2% (from 22.9 to 12.3) and 37.0% (from 92.1 to 58), respectively. Based on the Markov model prediction: 4.6% of patients with gastric cancer and 6.4% of patients with CRC would have higher stage shifting at 6 mo. The proportion of stage IV cancers increased (gastric: 30.5% to 32.4%; and colorectal: 23.5% to 26.8%). |
| Markar, 202045 Multicenter retrospective cohort January 1, 2020–April 30, 2020 |
United Kingdom All hospital trusts (n = 122) Comparing procedures and cancer diagnoses for similar time period from 2019 Preprocedure testing: NR |
No. of diagnostic endoscopies was around 28% lower than in the same period in 2019 (149,043 vs 208,212) | Estimated no. of undiagnosed esophageal and gastric cancers was 750 across England, with a median of 47. 3 (IQR, 35. 7–57. 5) across cancer vanguards, or regions Estimated no. of undiagnosed esophageal and gastric cancers that would have been treated curatively was 213 across England, with a median of 11. 0 (IQR, 6.3–14.4) across cancer vanguards, or regions |
| Morris, 202147 Administrative population-based datasets January 2020–October 2020 |
United Kingdom NHS dataset Comparing colonoscopies, CRC diagnoses, and operations for 2 time periods from 2019 Preprocedure testing: NR |
No. of colonoscopies: April 2020: 92% reduction (95% CI 89- 95) from a mean of 46,441 colonoscopies to 3,484 colonoscopies. October 2020: 46,295 colonoscopies; similar to 2019 monthly average |
No. of confirmed CRC diagnoses: April 2020: 22% reduction (95% CI, 8–24) From 2781 individuals to 2158 individuals October 2020 similar to 2019 No. of monthly CRC operations: April 2020: 31% reduction (95% CI, 19–42) from mean of 2003 to 1378 October 2020: remained below 2019 monthly average Estimated that from April to October 2020, >3500 fewer people diagnosed and treated for CRC in England than would have been expected |
| London, 202051 Administrative population-based datasets January 1, 2020–April 30, 2020 Monthly data reported |
TriNetX Research Network UK and US institutions Comparing no. of patients with CRC for similar time period from 2019 Preprocedure testing: NR |
NR | No. of patients with CRC-related encounters: January 2020 −2.5% February 2020 −7.1% March 2020 −18.4% April 2020 −39.9% No. of patients with new CRC diagnoses: January 2020 7.8% February 2020 –6.7% March 2020 −16.3% April 2020 −54.2% |
| Lantinga, 202142 Retrospective cohort study March 15, 2020–May 15, 2020 (n = 9776) Compared with March 15, 2019–May 15, 2019 (n = 19,296) |
The Netherlands 20 Dutch hospitals (3 academic, 17 nonacademic) Comparing no. of endoscopies and cancers for a similar time period from 2019 Preprocedure testing: NR |
Endoscopic volume: EGD decreased by 57% (7846 to 4467), Colonoscopy decreased by 45% (12,219 to 5609) Stable endoscopic volume: ERCP (578 to 522) |
Endoscopy results identifying cancer decreased (524 to 340). Likelihood of detecting cancer during endoscopy increased from 2.7% in 2019 (95% CI, 2.5–3.0) vs 3.5% in 2020 (95% CI, 3.1–3.9; P < .001). |
| Pena-Rey, 202120 Retrospective observational study May 5, 2020–October 31, 2020 (n = 3310 colonoscopies) |
Spain Galician Programme for the Early Detection of Colon Cancer: Primary Care program affiliated with 7 hospitals performing endoscopy No preprocedure testing; only symptom screening |
Decrease in colonoscopy volume: 3310 in 2020 compared with 7491 in 2019 | NR |
| Rutter, 202148 Administrative population-based datasets (1) Pre-COVID period: January 6, 2020–March 15, 2020; n = 35,478 (2) Transition period: March 16, 2020–March 22, 2020; n = 4315 (3) COVID-impacted period: March 23, 2020–May 31, 2020; n = 6974 |
United Kingdom NHS dataset Comparing referrals of colonoscopies, CRC diagnosis, and operations for 2 time periods with pre-COVID period Preprocedure testing: NR |
(1) Pre-COVID 35,478 endoscopies performed per week (by 3007 endoscopists; mean 12 procedures per endoscopist) (2) Transition week: fell by one-third, to 23,827. (3) COVID-impacted period: 12% of the pre-COVID volume of procedures were conducted (average 4315/wk, performed by 922 endoscopists; mean 4 procedures per endoscopist. At its low point, by the end of March, <1800 procedures/wk were being conducted: 5% of pre-COVID activity. In the end of the period 2 week of May, start recovering on average 6974 but still only 20% of pre-COVID activity. |
CRC detected per week Pre- COVID 394 (1.97 per 100 procedures) COVID impacted 112 (5.77 per 100 procedures) Missing CRC in COVID impacted period 2828 (71.7%) Esophageal cancers detected per week Pre-COVID 205 (1.37 per 100 procedures) COVID impacted 129 (6.16 per 100 procedures) Missing esophageal cancers in COVID-impacted period 759 (37.1%) Gastric cancers detected per week Pre-COVID 61 (0.41 per 100 procedures) COVID impacted 29 (1.40 per 100 procedures) Missing gastric cancers in COVID impacted period 320 (52.3%) |
| Maringe, 202052 Modeling study March 16, 2020 modeled to March 15, 2021 Compared with 1-y period January 1, 2010–December 31, 2010, with follow-up data until December 31, 2014 n = 24,975 colorectal cancer n = 6744 esophageal cancer |
United Kingdom Modeling study that estimate the impact of delays in diagnostic pathways due to pandemic lockdown on cancer survival Preprocedure testing: NR |
NR | Estimate a for CRC, 1445 additional deaths (15.3%–16.6% increase); estimate for esophageal cancer, 330 additional deaths, (5.8%–6.0% increase) up to 5 y after diagnosis. |
| Cheng, 202140 Single-center observational cohort study December 2019–April 20 20; n = 6392 |
Taiwan Comparing trends in compliance to diagnostic colonoscopy in FIT-positive patients with corresponding periods in the last 3 years Preprocedure testing: NR |
Screening uptake during COVID-19 was 88.8% compared with 91.2%–92.7% in the prior 3 y Colonoscopy rate during COVID-19 was 66.1% compared with 70.2%–77.5% in the prior 3 y Rescheduling/cancellation was increased to 10.9% during COVID-19 50% of FIT-positive patients declined diagnostic colonoscopy or rescheduled due to fears of being infected from COVID-19 |
NR |
| Tinmouth, 202149 Retrospective population-based modeling study March 2020–August 2020 |
Canadian Comparing colonoscopy procedures performed pre COVID and in COVID period and estimated hospital-based outpatient colonoscopy volume and time to recovery Preprocedure testing: NR |
Predicted backlog colonoscopies for screening estimated to take 41 mo to complete all the backlog of colonoscopies Changing low-yield colonoscopies to FIT would reduce recovery time: 25% reduction to FIT reduces backlog to 28 mo 50% reduction to FIT reduces backlog to 22 mo 75% reduction to FIT reduces backlog to 19 mo |
NR |
| Mizuno, 202046 Single-center retrospective cohort study (1) Period 1: December 18, 2018–April 16, 2019 (2) Period 2: April 17, 2019–August 14, 2019 (3) Period 3: December 19, 2019–April 16, 2020 (4) Period 4:April 17, 2020–August 14, 2020 |
Japan Analyses of 123 of CRC patients who underwent CRC surgery in 4 different periods Preprocedure testing: NR |
Colonoscopies decreased starting in March 2020 until May 21, 2020 with assumption that this led to fewer diagnoses of CRC | No significant change in no. of CRC patients who underwent surgery During COVID-19 period more patients needed emergency admission and more had obstructive CRC (39% vs 15%) Partial/complete obstructions were also increased (67% vs 19%–42%). Increased patients with advanced CRC in period 4 |
| Leeds, 202143 Retrospective cohort study (1) Period 1: pre-lockdown: March 9, 2020–March 22, 2020 (2) Period 2: national lockdown: March 2, 2020–April 19, 2020 (3) Period 3: early recovery phase: April 20, 2020–May 29, 2020 |
United Kingdom Chart review via endoscopy reporting system Comparing no. of procedures, and key performance indicators for 3 time periods with 2019 Preprocedure testing performed |
Period 2 (“lockdown”): 13.3% of expected activity (187 procedures compared with 1402 expected) Period 3 recovery: 28.9% of the expected activity (644 procedures compared with 2154) During period 3: only 84.2% of colonoscopy slots were filled. 25.8% not filled due to patient cancellation or reluctance to attend |
NR |
| Gawron, 202050 Retrospective population-based study (1) March 2020 and April 2020 (2) September 2020 |
United States VA National Database Comparing number of procedures, in COVID impacted period and recovery period Describes the outcomes from the pre-endoscopy PCR testing and reports around 25% procedure cancelation rate, but cannot distinguish how many were from testing strategy |
Decrease in EGD volume compared with a historical average: March 2020 33% April 2020 78% Decrease in colonoscopy volume compared with a historical average: March 2020 42% April 2020 93% Recovery phase September 2020: Overall recover to 70% from the historical average EGD recover to 86% Colonoscopy recover to 61% |
NR |
| Huang, 202021 Single-center retrospective cohort February 1, 2020–May 31, 2020 |
China Comparing no. of procedures, and endoscopic diagnosis in the COVID period with same period in 2019 Preprocedure testing: NR |
Study period: a total of 1808 endoscopic operations compared with 5903 in the same period in 2019; 30.63% of the expected activity | NR |
| Khan, 202141 Retrospective population-based study (1) Early pandemic: March 15, 2020 and July 15, 2020 (2) Later in the pandemic: July 2020 to November 2020 |
United States TriNetX database Comparing no. of procedures, and diagnosis of new GI cancer in the early COVID-19 pandemic period with same period in 2019 Preprocedure testing: NR |
(1) Early pandemic: Estimated decline in patients who underwent endoscopy (71.84%), colonoscopy (84.66%). (2) Later in the pandemic: Estimated decline in patients who underwent endoscopy (64.74%), colonoscopy (61.64%). |
(1) Early pandemic: Decline in new diagnoses of malignant colorectal (30.91%), esophageal and gastric (26.96%) cancers per 100,000 patients (2) Later in the pandemic: Decline in new diagnoses of malignant colorectal (11.74%), esophageal and gastric (19.78%) |
EGD, esophagogastroduodenoscopy; ERCP, endoscopic retrograde cholangiopancreatography; FIT, fecal immunochemical test; GI, gastrointestinal; IQR, interquartile range; NHS, National Health Service; NR, not reported.
References
- 1.Coronavirus disease 2019 (COVID-19): COVID-19 vaccines. US Food and Drug Administration. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-COVID-19/COVID-19-vaccines Accessed May 10, 2021. Available at:
- 2.Kirzinger A., Kearney A., Hamel L. KFF and Washington Post frontline health care workers survey. The Washington Post/KFF Survey Project. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-washington-post-health-care-workers/ Published April 6, 2021. Accessed June 17, 2021. Available at:
- 3.Sultan S., Siddique S.M., Altayar O. AGA Institute rapid review and recommendations on the role of pre-procedure SARS-CoV-2 testing and endoscopy. Gastroenterology. 2020;159:1935–1948 e5. doi: 10.1053/j.gastro.2020.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schunemann H.J., Wiercioch W., Etxeandia I. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186:E123–E142. doi: 10.1503/cmaj.131237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schunemann H.J., Wiercioch W., Brozek J. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–110. doi: 10.1016/j.jclinepi.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 6.IDSA guidelines on the diagnosis of COVID-19. Infectious Diseases Society of America. https://www.idsociety.org/practice-guideline/COVID-19-guideline-diagnostics/ Accessed May 12, 2021. Available at:
- 7.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albendin-Iglesias H., Mira-Bleda E., Roura-Piloto A.E. Usefulness of the epidemiological survey and RT-PCR test in pre-surgical patients for assessing the risk of COVID-19. J Hosp Infect. 2020;105:773–775. doi: 10.1016/j.jhin.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowyer B., Thukral C., Patel S. Outcomes of symptom screening and universal COVID-19 reverse transcriptase polymerase chain reaction testing before endoscopy in a community-based ambulatory surgery center. Gastrointest Endosc. 2021;93:1060–1064.e1. doi: 10.1016/j.gie.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casper M, Reichert M, Rissland J, et al. Pre-endoscopy SARS-CoV-2 testing strategy during COVID-19 pandemic: the care must go on. Preprint. Posted online October 27, 2020. medRxiv 2020:2020.10.22.20217885. [DOI] [PMC free article] [PubMed]
- 11.Dolinger M.T., Kumta N.A., Greenwald D.A. Outcomes of universal preprocedure coronavirus disease 2019 testing before endoscopy in a tertiary care center in New York City. Gastroenterology. 2020;159:1962–1964. doi: 10.1053/j.gastro.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forde J.J., Goldberg D., Sussman D. Yield and Implications of pre-procedural COVID-19 polymerase chain reaction testing on routine endoscopic practice. Gastroenterology. 2020;159:1538–1540. doi: 10.1053/j.gastro.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haidar G., Ayres A., King W.C. Preprocedural SARS-CoV-2 testing to sustain medically needed health care delivery during the COVID-19 pandemic: a prospective observational study. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayee B, SCOTS II Project Group, Bhandari P, et al. COVID-19 transmission following outpatient endoscopy during pandemic acceleration phase involving SARS-CoV-2 VOC 202012/01 variant in UK [published online ahead of print March 24, 2021]. Gut 10.1136/gutjnl-2021-324354 [DOI] [PubMed]
- 15.Hayee B., SCOTS Project Group. East J. Multicentre prospective study of COVID-19 transmission following outpatient GI endoscopy in the UK. Gut. 2021;70:825–828. doi: 10.1136/gutjnl-2020-322730. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez Camba A., Marcelino Reyes R., Hernandez-Guerra M. Pre-procedural antibody testing for SARS-CoV-2 in the routine endoscopic practice. Rev Esp Enferm Dig. 2021;113:116–118. doi: 10.17235/reed.2020.7434/2020. [DOI] [PubMed] [Google Scholar]
- 17.Lewis S.S., Smith B.A., Akinboyo I.C. Early experience with universal preprocedural testing for SARS-CoV-2 in a relatively low-prevalence area. Infect Control Hosp Epidemiol. 2021;42:341–343. doi: 10.1017/ice.2020.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mays J.A., Greninger A.L., Jerome K.R. Preprocedural surveillance testing for SARS-CoV-2 in an asymptomatic population in the Seattle region shows low rates of positivity. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01193-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tworek J.A., Khan F., Sekedat M.D. The utility of rapid nucleic acid amplification testing to triage symptomatic patients and to screen asymptomatic preprocedure patients for SARS-CoV-2. Open Forum Infect Dis. 2021;8:ofaa607. doi: 10.1093/ofid/ofaa607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pena-Rey I., Almazan R., Rodriguez-Camacho E. Resumption of endoscopy in the Galician colorectal cancer screening programme after the COVID-19 lock down: patient safety results. Rev Esp Enferm Dig. 2021;113:119–121. doi: 10.17235/reed.2020.7647/2020. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q., Liu G., Wang J. Control measures to prevent coronavirus disease 2019 pandemic in endoscopy centers: multi-center study. Dig Endosc. 2020;32:914–920. doi: 10.1111/den.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Repici A., Aragona G., Cengia G. Low risk of COVID-19 transmission in GI endoscopy. Gut. 2020;69:1925–1927. doi: 10.1136/gutjnl-2020-321341. [DOI] [PubMed] [Google Scholar]
- 23.Jagannath S, Agarwal A, Gunjan D, et al. Mandatory preprocedure testing for SARS-CoV-2 for all-comers may not be required for resuming endoscopic services amidst the ongoing COVID-19 pandemic [published online ahead of print November 6, 2020]. Gut 10.1136/gutjnl-2020-323154 [DOI] [PubMed]
- 24.D'Ovidio V., Lucidi C., Bruno G. Impact of COVID-19 pandemic on colorectal cancer screening program. Clin Colorectal Cancer. 2021;20:e5–e11. doi: 10.1016/j.clcc.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall V.J., Foulkes S., Saei A. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson M.G., Burgess J.L., Naleway A.L. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight U.S. locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tande AJ, Pollock BD, Shah ND, et al. Impact of the COVID-19 vaccine on asymptomatic infection among patients undergoing pre-procedural COVID-19 molecular screening [published online ahead of print March 10, 2021]. Clin Infect Dis 10.1093/cid/ciab229 [DOI] [PMC free article] [PubMed]
- 28.Science brief: background rationale and evidence for public health recommendations for fully vaccinated people. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html Accessed May 12, 2021. Available at:
- 29.Interim public health recommendations for fully vaccinated people. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html Available at:
- 30.Levine-Tiefenbrun M., Yelin I., Katz R. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27:790–792. doi: 10.1038/s41591-021-01316-7. [DOI] [PubMed] [Google Scholar]
- 31.Keehner J., Horton L.E., Pfeffer M.A. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384:1774–1775. doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson KB, Pinsky BA, Rath MEM, et al. Post-vaccination SARS-CoV-2 infections and incidence of the B.1.427/B.1.429 variant among healthcare personnel at a northern California academic medical center. Preprint. Posted online April 24, 2021. medRxiv 2021.04.14.21255431. 10.1101/2021.04.14.21255431 [DOI]
- 33.Zaqout A, Daghfal J, Alaqad I, et al. The initial impact of a national BNT162b2 mRNA COVID-19 vaccine rollout. Preprint. Posted online April 28, 2021. medRxiv 2021.04.26.21256087; 10.1101/2021.04.26.21256087 [DOI] [PMC free article] [PubMed]
- 34.Björk J, Inghammar M, Moghaddassi M, et al. Effectiveness of the BNT162b2 vaccine in preventing COVID-19 in the working age population—first results from a cohort study in Southern Sweden. Preprint. Posted online April 21, 2021. medRxiv 2021.04.20.21254636; 10.1101/2021.04.20.21254636 [DOI]
- 35.Khorrami Minaei S, Garrido Duran C, Garcia Hernandez M, et al. Poor agreement between clinical screening and universal pre-procedure SARS-CoV-2 PCR testing prior to endoscopy [published online ahead of print February 16, 2021]. Rev Esp Enferm Dig 10.17235/reed.2021.7612/2020 [DOI] [PubMed]
- 36.Rex D.K., Vemulapalli K.C., Kane M.J. Most patients are willing to undergo elective endoscopic procedures during the reopening period of the coronavirus 2019 pandemic. Gastroenterology. 2020;159:1173–1175.e4. doi: 10.1053/j.gastro.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moraveji S., Thaker A.M., Muthusamy V.R. Protocols, personal protective equipment use, and psychological/financial stressors in endoscopy units during the COVID-19 pandemic: a large survey of hospital-based and ambulatory endoscopy centers in the United States. Gastroenterology. 2020;159:1568–1570.e5. doi: 10.1053/j.gastro.2020.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rex D.K., Vemulapalli K.C., Lahr R.E. Endoscopy staff are concerned about acquiring coronavirus disease 2019 infection when resuming elective endoscopy. Gastroenterology. 2020;159:1167–1169.e3. doi: 10.1053/j.gastro.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podboy A., Cholankeril G., Cianfichi L. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159:1586–1588.e4. doi: 10.1053/j.gastro.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng S.Y., Chen C.F., He H.C. Impact of COVID-19 pandemic on fecal immunochemical test screening uptake and compliance to diagnostic colonoscopy. J Gastroenterol Hepatol. 2021;36:1614–1619. doi: 10.1111/jgh.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan A., Bilal M., Morrow V. Impact of the coronavirus disease 2019 pandemic on gastrointestinal procedures and cancers in the united states: a multicenter research network study. Gastroenterology. 2021;160:2602–2604.e5. doi: 10.1053/j.gastro.2021.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lantinga M.A., Theunissen F., Ter Borg P.C.J. Impact of the COVID-19 pandemic on gastrointestinal endoscopy in the Netherlands: analysis of a prospective endoscopy database. Endoscopy. 2021;53:166–170. doi: 10.1055/a-1272-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leeds J.S., Awadelkarim B., Dipper C. Effect of the SARS-CoV2 pandemic on endoscopy provision—the impact of compliance with national guidance. Expert Rev Gastroenterol Hepatol. 2021;15:459–464. doi: 10.1080/17474124.2021.1857239. [DOI] [PubMed] [Google Scholar]
- 44.Lui T.K.L., Leung K., Guo C.G. Impacts of the coronavirus 2019 pandemic on gastrointestinal endoscopy volume and diagnosis of gastric and colorectal cancers: a population-based study. Gastroenterology. 2020;159:1164–1166.e3. doi: 10.1053/j.gastro.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markar S.R., Clarke J., Kinross J. Practice patterns of diagnostic upper gastrointestinal endoscopy during the initial COVID-19 outbreak in England. Lancet Gastroenterol Hepatol. 2020;5:804–805. doi: 10.1016/S2468-1253(20)30236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizuno R., Ganeko R., Takeuchi G. The number of obstructive colorectal cancers in Japan has increased during the COVID-19 pandemic: a retrospective single-center cohort study. Ann Med Surg (Lond) 2020;60:675–679. doi: 10.1016/j.amsu.2020.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris E.J.A., Goldacre R., Spata E. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6:199–208. doi: 10.1016/S2468-1253(21)00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutter M.D., Brookes M., Lee T.J. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2021;70:537–543. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 49.Tinmouth J., Dong S., Stogios C. Estimating the backlog of colonoscopy due to coronavirus disease 2019 and comparing strategies to recover in Ontario, Canada. Gastroenterology. 2021;160:1400–1402.e1. doi: 10.1053/j.gastro.2020.11.048. [DOI] [PubMed] [Google Scholar]
- 50.Gawron A.J., Kaltenbach T., Dominitz J.A. The impact of the coronavirus disease-19 pandemic on access to endoscopy procedures in the VA healthcare system. Gastroenterology. 2020;159:1216–1220.e1. doi: 10.1053/j.gastro.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.London J.W., Fazio-Eynullayeva E., Palchuk M.B. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maringe C., Spicer J., Morris M. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanson KE, Caliendo AM, Arias CA, et al. The Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: molecular diagnostic testing [published online ahead of print January 22, 2021]. Clin Infect Dis 10.1093/cid/ciab048 [DOI] [PMC free article] [PubMed]
- 54.Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: serologic testing [published online ahead of print September 12, 2020]. Clin Infect Dis 10.1093/cid/ciaa1343 [DOI] [PMC free article] [PubMed]
- 55.Corral J.E., Hoogenboom S.A., Kroner P.T. COVID-19 polymerase chain reaction testing before endoscopy: an economic analysis. Gastrointest Endosc. 2020;92:524–534.e6. doi: 10.1016/j.gie.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebigbo A., Rommele C., Bartenschlager C. Cost-effectiveness analysis of SARS-CoV-2 infection prevention strategies including pre-endoscopic virus testing and use of high risk personal protective equipment. Endoscopy. 2021;53:156–161. doi: 10.1055/a-1294-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balzora S., Issaka R.B., Anyane-Yeboa A. Impact of COVID-19 on colorectal cancer disparities and the way forward. Gastrointest Endosc. 2020;92:946–950. doi: 10.1016/j.gie.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Millett G.A., Jones A.T., Benkeser D. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44. doi: 10.1016/j.annepidem.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wadhera R.K., Wadhera P., Gaba P. Variation in COVID-19 hospitalizations and deaths across New York City Boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webb Hooper M., Napoles A.M., Perez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubin-Miller L., Alban C., Artiga S. COVID-19 racial disparities in testing, infection, hospitalization, and death: analysis of epic patient data. KFF. https://www.kff.org/coronavirus-COVID-19/issue-brief/COVID-19-racial-disparities-testing-infection-hospitalization-death-analysis-epic-patient-data/ Accessed May 12, 2021. Available at:
- 62.CDC facilities COVID-19 screening. Centers for Disease Control and Prevention. https://www.cdc.gov/screening/index.html Accessed May 12, 2021. Available at:
- 63.Struyf T., Deeks J.J., Dinnes J. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst Rev. 2020;7:CD013665. doi: 10.1002/14651858.CD013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Interim guidance on ending isolation and precautions for adults with COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html Updated March 16, 2021. Accessed May 12, 2021. Available at:
Supplementary References
- 1.Casper M., Reichert M., Rissland J. Pre-endoscopy SARS-CoV-2 testing strategy during COVID-19 pandemic: The care must go on. medRxiv. 2020;2020 doi: 10.1186/s40001-022-00672-5. 10.22.20217885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayee B., group S.I.P., Bhandari P. COVID-19 transmission following outpatient endoscopy during pandemic acceleration phase involving SARS-CoV-2 VOC 202012/01 variant in UK. Gut. 2021 doi: 10.1136/gutjnl-2021-324354. [DOI] [PubMed] [Google Scholar]
- 3.Hayee B., Sp group, East J. Multicentre prospective study of COVID-19 transmission following outpatient GI endoscopy in the UK. Gut. 2021;70:825–828. doi: 10.1136/gutjnl-2020-322730. [DOI] [PubMed] [Google Scholar]
- 4.Pena-Rey I., Almazan R., Rodriguez-Camacho E. Resumption of endoscopy in the Galician colorectal cancer screening programme after the COVID-19 lock down: patient safety results. Rev Esp Enferm Dig. 2021;113:119–121. doi: 10.17235/reed.2020.7647/2020. [DOI] [PubMed] [Google Scholar]
- 5.Huang Q., Liu G., Wang J. Control measures to prevent Coronavirus disease 2019 pandemic in endoscopy centers: Multi-center study. Dig Endosc. 2020;32:914–920. doi: 10.1111/den.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Repici A., Aragona G., Cengia G. Low risk of COVID-19 transmission in GI endoscopy. Gut. 2020;69:1925–1927. doi: 10.1136/gutjnl-2020-321341. [DOI] [PubMed] [Google Scholar]
- 7.Jagannath S., Agarwal A., Gunjan D. Mandatory preprocedure testing for SARS- CoV-2 for all-comers may not be required for resuming endoscopic services amidst the ongoing COVID-19 pandemic. Gut. 2020 doi: 10.1136/gutjnl-2020-323154. [DOI] [PubMed] [Google Scholar]
- 8.D'Ovidio V., Lucidi C., Bruno G. Impact of COVID-19 Pandemic on Colorectal Cancer Screening Program. Clin Colorectal Cancer. 2021;20:e5–e11. doi: 10.1016/j.clcc.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.