Abstract
Axons in the adult mammalian nervous system can extend over formidable distances, up to one meter or more in humans. During development, axonal and dendritic growth requires continuous addition of new membrane. Of the three major kinds of membrane lipids, phospholipids are the most abundant in all cell membranes, including neurons. Not only immature axons, but also severed axons in the adult require large amounts of lipids for axon regeneration to occur. Lipids also serve as energy storage, signaling molecules and they contribute to tissue physiology, as demonstrated by a variety of metabolic disorders in which harmful amounts of lipids accumulate in various tissues through the body. Detrimental changes in lipid metabolism and excess accumulation of lipids contribute to a lack of axon regeneration, poor neurological outcome and complications after a variety of central nervous system (CNS) trauma including brain and spinal cord injury. Recent evidence indicates that rewiring lipid metabolism can be manipulated for therapeutic gain, as it favors conditions for axon regeneration and CNS repair. Here, we review the role of lipids, lipid metabolism and ectopic lipid accumulation in axon growth, regeneration and CNS repair. In addition, we outline molecular and pharmacological strategies to fine-tune lipid composition and energy metabolism in neurons and non-neuronal cells that can be exploited to improve neurological recovery after CNS trauma and disease.
Keywords: lipids, axon growth and regeneration, mitochondria transport, myelin formation, adipose tissue, CNS trauma and disease
1. Introduction
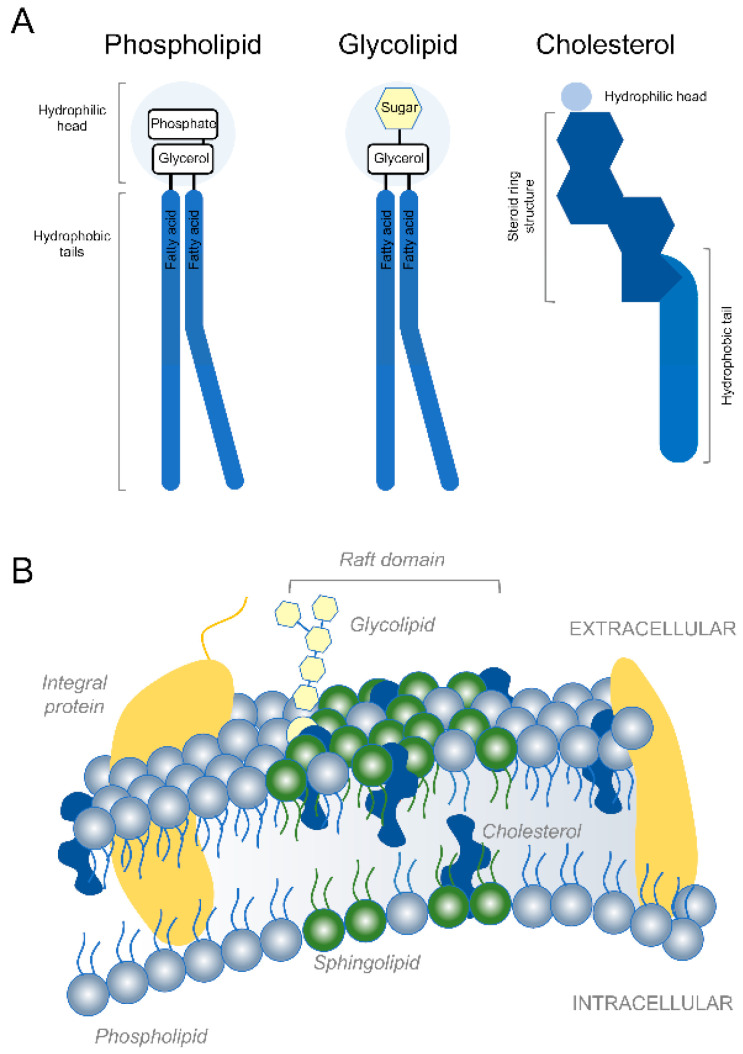
Developing axons in the mammalian nervous system can extend very long distances [1]. After reaching their targets, axons integrated into functional circuits continue to extend via mechanisms of stretch growth as the body continues to grow [2,3]. Continuous addition of new axonal membrane is necessary to fuel axon growth during development as well as long distance axon regeneration in adulthood [4,5,6]. Similarly, complex dendritic growth and myelin formation also require large amounts of membrane. Phospholipids, glycolipids and cholesterol constitute the major kinds of membrane lipids [7] (Figure 1). Phospholipids are the most abundant and are generally composed of two fatty acids that can differ in length and a phosphate group attached to a glycerol backbone [8]. Due to their shape and amphipathic nature, phospholipids spontaneously aggregate to form bilayers in aqueous environments. Made of a hydrophobic lipid tail and one or more sugar groups linked by a glycosidic bond, glycolipids are found on the outer leaflet of eukaryotic cell membranes where they confer membrane stability and facilitate cell–cell communication and signal transduction. By making the lipid bilayer less deformable and less fluid, cholesterol integration alters the permeability-barrier properties of the cellular membranes. Membrane proteins tend to accumulate within specialized microdomains of the plasma membrane called lipid rafts that are rich in cholesterol and sphingolipids. Whereas axons in the mammalian peripheral nervous system spontaneously regenerate over long distances [9,10], axons in the central nervous system (CNS) fail to mount a successful regenerative response [11,12,13]. Axon regeneration failure causes long term structural and functional impairment after a variety of CNS trauma including brain and spinal cord injury (SCI) [14,15]. Previous work has demonstrated that accumulation of certain lipids and dysregulation of lipid metabolism not only contribute to developmental disorders [16,17], axon growth and regeneration failure after CNS trauma [6,18], but also neurodegenerative diseases [19,20]. Lipids are also implicated in myelin formation and serve as bioactive molecules and energy substrates [21,22]. Here, we discuss classical and novel roles of lipids, lipid metabolism and ectopic lipid accumulation in axon growth/regeneration and CNS repair. In addition, we outline molecular and pharmacological strategies that fine tune lipid composition and metabolism in neurons and non-neuronal cells to favor neurological recovery after CNS trauma and disease.
Figure 1.
Lipids and plasma membrane. (A) Schematic representation of the three major kinds of membrane lipids. (B) Illustration of the typical lipid bilayer of the plasma membrane.
2. Membrane Expansion
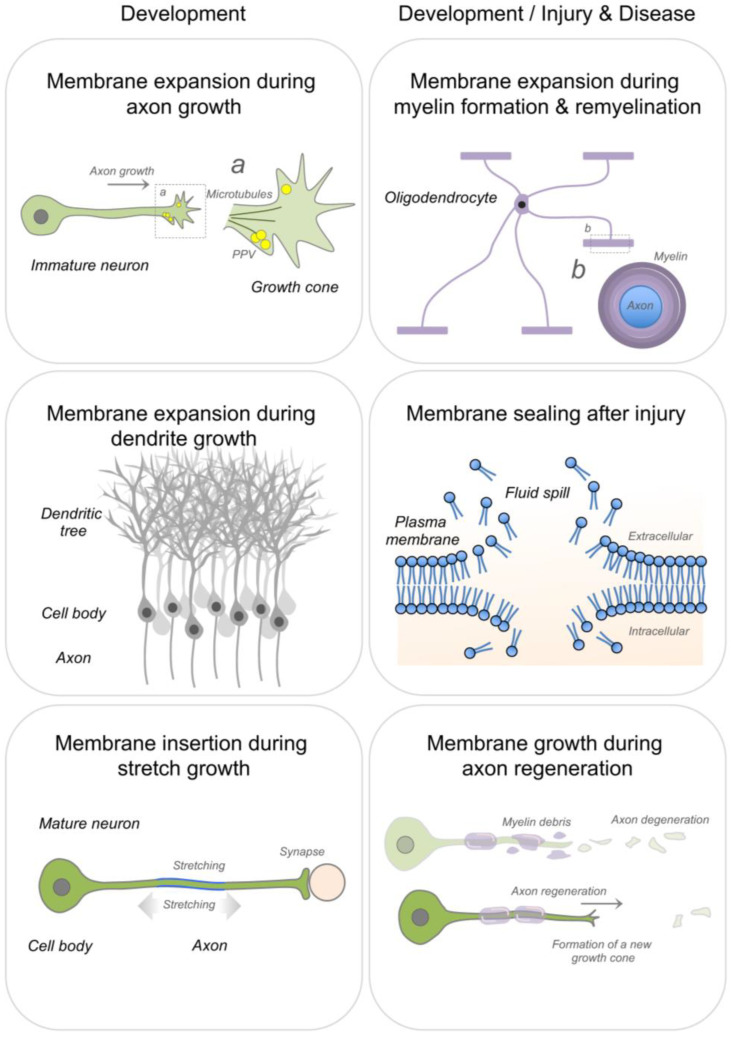
Continuous addition of new membrane is required during axon and dendrite growth [23,24], stretch growth of integrated axons [3] and axon regeneration [6,25,26,27] (Figure 2). Newly synthesized lipids for membrane expansion are anterogradely transported to the distal axonal compartment via lipid containing vesicles, enabling rapid elongation of growing axons [5,23,27,28,29]. In addition, axons can locally synthesize lipids, such as the major membrane phospholipid phosphatidylcholine [27]. Cholesterol can also be supplied via uptake of lipoprotein particles during rapid membrane biogenesis. Along this line, low-density lipoprotein receptors have been found to densely cluster at the tip of peripheral axons during regeneration [30]. Large amounts of lipid supply are also necessary during myelin growth and repair [31,32,33,34] (Figure 2).
Figure 2.
Membrane expansion during nervous system development and repair.
A schematic representation of membrane expansion during axonal and dendritic growth, stretch growth of integrated axons, myelin formation and repair, sealing of membrane ruptures and axon regeneration. PPV: plasmalemmal precursor vesicles.
2.1. Axon Growth & Regeneration
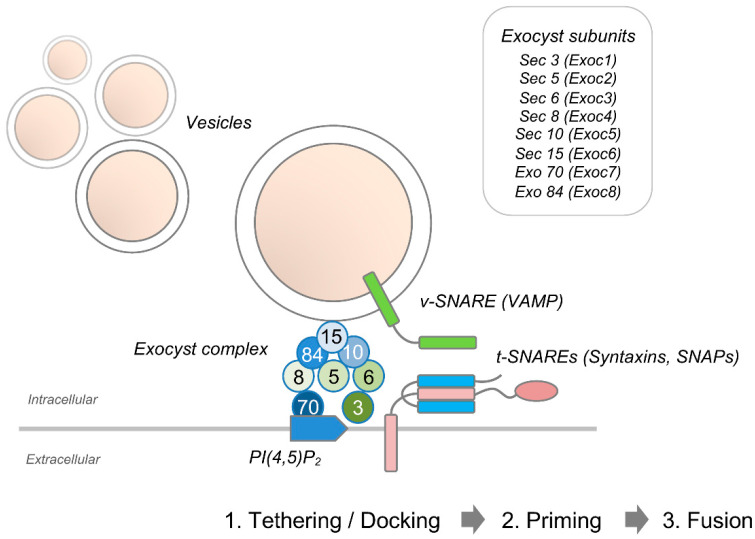
Membrane expansion in developing axons is mediated by polarized exocytosis of plasmalemmal precursor vesicles that are anterogradely transported to the growth cone [35,36]. New membrane insertion may also be added at the cell body and along the neurites [37]. Specific effector complexes including the evolutionarily conserved exocyst complex that tethers post-Golgi secretory vesicles to the plasma membrane mark sites of exocytosis [38]. The octameric exocyst complex is comprised of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 [39,40] (Figure 3). These units are targeted to sites of exocytosis via different mechanisms. Whereas Sec3 and Exo70 are recruited to the plasma membrane upon binding to phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) [41], the remaining exocyst components directly associate with exocytic vesicles [42,43]. Spatial and temporal regulation of the exocyst complex not only allow restricting its function to specific areas of membrane expansion such as the neuronal growth cone, but also to correctly integrating extracellular cues that cause changes in growth rate and guidance. The small cdc42-like GTPase TC10 (also known as RhoQ) controls translocation of Exoc3, Sec8, and Exo70 to the plasma membrane [44,45]. Intra-axonal synthesis of TC10 is required for exocyst function. Specifically, phosphoinositide 3-kinase (PI3K)-dependent activation of the Rheb-mTOR pathway has been shown to trigger local translation of TC10 during axon outgrowth in dorsal root ganglion (DRG) neurons [46]. Upon tethering, plasmalemmal precursor vesicles fuse with the acceptor membrane, leading to expansion of the plasma membrane. During neuronal polarization of cultured hippocampal pyramidal neurons, Dupraz et al. demonstrated that insulin-like growth factor 1-mediated activation of TC10 triggers translocation of exo70 to the plasma membrane in the distal axon and growth cone [47].
Figure 3.
The exocyst protein complex.
A schematic representation of the assembly of the exocyst octameric protein complex that controls the tethering of secretory vesicles to the plasma membrane prior to SNARE-mediated fusion.
Soluble N-ethylmaleamide attachment proteins (SNAP), vesicle associated membrane proteins (VAMP) and syntaxis form the core machinery of vesicle fusion also known as the SNARE (soluble N-ethylmaleamide-sensitive factor attachment protein receptors) complex [48]. Whereas SNAP and syntaxin are associated with the target membrane, VAMP associates with the vesicle membrane. In yeast, mutations that block vesicle tethering prevent the assembly of the SNARE complex [49]. Both syntaxin, a membrane-integrated protein that plays an instrumental role in exocytosis [50], and Sec1, a Sec1/Munc18 family protein that binds to SNAREs and stimulates membrane fusion [51], were shown to co-immunoprecipitate with the exocyst complex [38,39,52].
After axotomy, rapid sealing of disrupted membranes is necessary to restore boundary integrity of the axonal compartment, to equilibrate ion concentration and to counteract osmotic stress [53]. These steps are required to mount a successful axon regeneration response. After small (<1 nm) membrane injuries, lipids in the bilayer spontaneously rearrange to eliminate the free edge with water during membrane resealing. In contrast, active repair mechanisms like those requiring calcium-dependent exocytosis are needed for larger injuries [54,55]. Vesicles accumulate to form multivesicular structures that fuse with the ruptured plasma membrane [56]. Hence, calcium-dependent membrane resealing involves vesicle delivery, docking, and fusion, similar to neurotransmitter exocytosis [57].
Formation of a new growth cone at the axonal stump also requires insertion of new membrane into the neurolemma [58,59]. A proteomics and lipidomics analysis of growth cone pathways during optic nerve regeneration has recently provided new insights into changes in lipid metabolic processes and lipid composition of the growth cone plasmalemma across different developmental ages [60].
Another recent study in adult CNS neurons has shed light on the specific changes in lipid metabolism that contribute to axon regeneration. After axotomy, expression of the phosphatidic acid phosphatase enzyme lipin 1 increase in retinal ganglion cells [6]. Mechanistically, lipin 1 catalyzes the conversion of phosphatidic acid to diglycerides [61]. Instead of producing phospholipids necessary for membrane expansion, increased lipin 1 favors the production of triglyceride storage lipids and therefore contributes to axon regeneration failure in the adult mammalian CNS [6]. Neuronal depletion of lipin 1 promotes axon regeneration after optic nerve injury in mice by regulating glycerolipid metabolism and, more specifically, triglyceride hydrolysis and phospholipid synthesis [6]. This suggests that triglycerides may provide lipid precursors for phospholipid synthesis that is necessary for membrane biosynthesis during axon regeneration.
During early stages of development, immature neurons exhibit an extraordinary axon growth and regenerative capacity [15,62,63]. As neurons mature, however, a development dependent decline in the expression of genes controlling axon growth is, at least in part, responsible for axon regeneration failure in the adult [64,65]. In turn, the identification of positive regulators and molecular mechanisms controlling axon growth and regeneration has been the subject of intensive investigation [12,25,66,67,68,69,70]. In exploring the mechanisms that control intrinsic axon growth, a recent study has discovered that the stemness-associated gene Prom1, which encodes the membrane glycoprotein Prominin-1, acts as a positive regulator of peripheral nerve regeneration in adulthood [71]. Prom1 forced expression enhances axon regeneration via Smad2-dependent signaling and inhibits the expression of genes related to cholesterol biosynthesis [71].
In the adult CNS, the vast majority of cholesterol is present in myelin sheaths and plasma membranes. After SCI, disruption of cellular membranes and myelin sheaths cause an excess of cholesterol to accumulate in the extracellular space. Of interest, cholesterol depletion has been shown to promote axon growth in vitro and regeneration of peripheral nerves in vivo [72]. By lowering cholesterol synthesis, molecular and pharmacological strategies have proven effective in promoting survival and CNS regeneration of retinal ganglion cells after optic nerve injury [73]. Work done in mice deficient for CNS myelin and myelin basic protein (also known as shiverer mice [74]) has demonstrated the inhibitory action of CNS myelin lipids, in particular cholesterol and sphingomyelin, to axon regeneration after SCI [75]. Shiverer mice, but also mice administered 2-hydroxypropyl-β-cyclodextrin to scavenge lipids at the injury site, showed increased regeneration of dorsal column sensory axons after SCI [75].
The neuronal cell bodies in sensory, sympathetic and parasympathetic ganglia are wrapped by satellite glial cells [76]. The importance of satellite glia in nervous system function and dysfunction has attracted attention lately [77]. A recent study has demonstrated that fatty acid synthesis in satellite glial cells contributes to axon elongation during regeneration of peripheral nerves [25]. Hence, satellite glia may facilitate peripheral nerve regeneration by paracrine lipid transfer via lipoprotein particles to DRG neurons.
Together, experimental evidence suggests that rewiring lipid metabolism can be manipulated for therapeutic gain as it favors axon regeneration both in the central and peripheral nervous systems. Whether pharmacological and molecular strategies lowering cholesterol synthesis promote neurological recovery after SCI is unknown and deserves further attention in future investigations.
2.2. Dendritic Growth
The development of complex dendritic branches for sampling and processing synaptic inputs also requires a large lipid supply. Recent studies in Drosophila have shown that dendrite expansion in larva neurons relies on cell-autonomous production of lipids [24,78]. Specifically, dendrite formation in dendritic arborization neurons requires sterol regulatory element binding protein (SREBP), as shown by the presence of dendrites lacking complex morphology in neurons from srebp mutant. SREBP acts as a basic helix–loop–helix leucine zipper transcription factor that binds to sterol regulatory elements to control the expression of genes that encode key enzymes of both fatty acid and cholesterol metabolism [79,80,81]. Interestingly, structural changes in dendrite morphology are accompanied with hypersensitivity to noxious stimuli in srebp mutant larvae. In turn, cell-autonomous production of fatty acids is necessary for correct dendritic development and function. A genetic screen in dendritic arborization sensory neurons of the Drosophila larval peripheral nervous system has identified the easily shocked (eas) gene, which encodes the ethanolamine kinase (also known as the first enzyme of the Kennedy pathway involved in the de novo biosynthesis of the phospholipid phosphotidylethanolamine [82]), as a central regulator of dendritic morphogenesis [78]. Total phosphotidylethanolamine is decreased in eas mutants [83], causing SREBP to translocate to the nucleus where it activates the expression of lipogenic genes [84]. Eas mutant flies experience seizures and show defects in dendrite morphogenesis characterized by a decrease in the number of branches and total dendrite length in dendritic arborization sensory neurons [78]. Defects in these mutants are associated with increased lipogenesis with altered membrane phospholipid composition and increased calcium influx [78]. Forcing SREBP transcriptional activity by expressing a constitutively active form of SREBP leads to a decrease in the number of neurite branches and total length [78], further suggesting SREBP-mediated transcription is crucial during dendrite morphogenesis [85,86]. Thus, accumulating evidence suggests that lipid production must be fine-tuned during development as lipid levels that are too low or too high can lead to structural and functional impairment in dendritic structures.
2.3. Myelin Formation and Repair
Myelin is constituted by multiple overlapping layers of a specialized membrane spiraling around axons [87]. Myelin not only increases axonal resistance, but also enables high density sodium channel clustering at nodes of Ranvier, thereby enabling high speed propagation of electrical signals [88,89,90,91]. CNS myelination is crucial for metabolic support and key for functional recovery after injury and disease [92,93,94,95]. Myelin basic protein and proteolipid proteins represent the two major CNS myelin-specific proteins [96,97,98]. In contrast, all major classes of lipids are present in myelin membranes, suggesting myelin does not contain specific lipids. When compared to other membranes, however, the lipid:protein ratio is much higher in myelin membranes, with lipids contributing 70–80% of the dry weight of myelin [8,99,100]. Myelin membranes contain high levels of cholesterol (>25% by weight) and glycosphingolipids, specifically galactosylceramide and ether-glycerophospholipids, whose deficiency impairs myelin formation [101,102,103]. Cholesterol and lipid accumulation accounts for myelin membrane expansion during CNS development [31,32,33,34]. Large amounts of cholesterol may also contribute to myelin membrane curvature and fluidity [104,105]. Once incorporated into the myelin membrane, cholesterol turnover is very slow [106]. Transcriptional and posttranscriptional mechanisms controlling sterol homeostasis have been identified in eukaryotes [107]. There may be potential mechanisms to remove free cholesterol from oligodendrocytes via secretion of cholesterol-rich exosomes into the extracellular milieu [108].
During normal brain development, astrocyte-derived fatty acids constitute a significant portion of lipids incorporated into CNS myelin [109]. Remarkably, oligodendrocytes are capable of bypassing inhibition of lipid synthesis in astrocytes by incorporating circulating lipids into myelin membranes [109]. This suggests that personalized lipid-enriched diets may rescue, at least in part, myelination defects. Nevertheless, accumulation of ceramides, the precursor of sphingolipids, and its direct product sphingosine in the spinal cord of a rat experimental model of demyelinating disorders causes apoptosis of oligodendrocytes [110].
As result of CNS trauma and disease, myelin sheaths along the axon can be severely damaged. De novo fatty acid synthesis via fatty acid synthase is critical for CNS myelin formation after demyelination injury. Genetic depletion of fatty acid synthase from OPC has no effect on proliferation and differentiation along the oligodendrocyte lineage, yet is necessary for accurate CNS myelination [111]. Treatment with cholesterol lowering drugs such as statins that target HMG-CoA reductase [112], the rate-limiting enzyme of cholesterol biosynthesis, negatively impacts myelin formation and repair after SCI [113,114,115].
In mammals, the ability to regenerate and repair the CNS declines with age [116,117]. In old mice, large quantities of cholesterol-rich myelin debris hijack phagocyte-mediated clearance [118]. Under such pathological conditions, free cholesterol starts to form crystals that cause lysosomal rupture with consequent inflammasome stimulation [118]. In this context, cholesterol lowering drugs used to treat hypercholesterolemia may be repurposed for regenerative medicine applications.
Recent evidence suggests that a delicate balance in lipid synthesis and homeostasis is crucial during myelin formation and remyelination during CNS development, as well as after injury or disease. Strategies targeting lipid composition of the myelin membrane may exert contradictory responses where a lipid-enriched diet may rescue myelination defects while excess lipids trigger oligodendrocyte death and inflammation. Learning how to fine tune lipid composition in myelin membranes via extracellular lipid supply, lowering cholesterol levels or promoting its clearance with spatial precision without causing adverse effects represent important areas of future investigation.
3. Energy Metabolism and Mitochondrial Transport
As discussed above, lipids are necessary for proper sealing of the plasma membrane, formation of a new growth cone and regeneration after axonal injury. These energy demanding processes require optimal mitochondrial transport and adenosine triphosphate (ATP) production [58,119,120,121,122,123]. During glucose metabolism, most ATP is produced by oxidative phosphorylation in mitochondria. Under pathological conditions, however, high levels of fatty acids impair several processes involved in oxidative phosphorylation during ATP synthesis. Accumulated fatty acids depolarize mitochondrial membranes and bind to electron transport chain complexes to generate peroxide and hydroxyl radicals [124,125]. Both neurons and astrocytes slowly convert fatty acids into energy substrates [126]. Fatty acid oxidation, however, cannot guarantee rapid ATP generation during fast and sustained neuronal firing [124]. In response to neuronal activity, astrocytes utilize fatty acids stored in lipid droplets via mitochondrial β-oxidation and activate a detoxification program to protect neurons from fatty acid-mediated toxicity during sustained neuron firing [127]. Using experimental models of amyotrophic lateral sclerosis, recent studies in rats have showed that excess accumulation of lipid droplets, particularly cholesteryl esters linked to polyunsaturated fatty acids, in spinal cord astrocytes is associated with cellular stress and inflammation [128,129]. Therefore, elevated fatty acid levels plus their use as an energy substrate following CNS trauma and disease not only impair neuronal function, but also promote oxidative stress and neurodegeneration.
As axons reach their target field during late stages of development, formation of collateral branches and synaptic contacts allow neurons to establish complex connectivity patterns [130,131,132]. The liver kinase B1 (LKB1) links cellular metabolism and energy homeostasis to cell polarity and growth [133,134,135,136]. The LKB1-NUAK1 kinase pathway promotes cortical axon branching by inducing mitochondria immobilization, including at nascent presynaptic sites [137]. LKB1 phosphorylates AMP-activated protein kinase [138] that acts as a crucial regulator of axonal regenerative signaling [139] and lipid and glucose metabolism in all eukaryotic cells [140]. LKB1 forced expression in adult corticospinal neurons promotes regeneration of corticospinal axons after murine SCI [141]. Regeneration of serotonergic and tyrosine hydroxylase-positive axons is also achieved in mice with systemic overexpression of LKB1 [141]. By inhibiting ATP-consuming biosynthetic pathways, AMP-activated protein kinase restores ATP levels via simultaneous activation of signaling pathways that regenerate ATP from the breakdown of macromolecules [140]. It is also important to note that AMP-activated protein kinase can also activate transcription factors that act as master regulators of metabolism [142,143].
Mitochondria trafficking and anchoring along the axons needs to be tightly regulated to respond to altered energy requirements [144]. To meet energy and calcium buffering needs, mitochondria movement through the dense axoplasm cannot rely on passive diffusion rates. Instead, ATP-hydrolyzing motor proteins of the kinesin-1 family control anterograde transport of mitochondria from the soma to the distal axon [145,146]. In addition, dynein motors control retrograde transport back to the soma [147,148]. The mitochondrial Rho GTPase 1 (Miro 1) associates with milton (TRAK1/2) and the motor proteins kinesin and dynein to form the mitochondria motor/adaptor complex [149]. Axonal microtubules are necessary for long distance fast axonal transport of mitochondria [150].
Mitochondrial positioning along the axon is important as it enables local energy supply [151]. Accumulating evidence indicates that proper mitochondrial transport and high energy supply are necessary to fuel axon growth and regeneration. When actively growing, chick sympathetic neurons preferentially localize mitochondria toward the growth cone [152]. Analysis of mitochondria behavior in these axons indicates that mitochondrial movement is balanced so that net transport is anterograde in growing axons [152]. After axonal injury, acute depolarization of mitochondria causes an energy crisis along injured axons [123]. Depolarized mitochondria not only fail to produce adequate levels of ATP, but also contribute to axonal degeneration by spilling byproducts of mitochondrial metabolism such as toxic reactive oxygen species [153]. Overexpression of Miro 1 or silencing of the mitochondria-anchoring protein syntaphilin enhances mitochondria transport and restores energy balance, thereby promoting axon regrowth [123]. Additional evidence indicates that boosting mitochondrial transport effectively promotes neuron survival and axon regeneration after axotomy both in worms and mice [119,122].
How does cellular metabolism contribute to neuron repair after axotomy? By linking glucose metabolism to the hexosamine biosynthetic pathway, O-linked β-N-acetylglucosamine (O-GlcNAc) post-translational modification of serine and threonine residues of proteins acts as a nutrient sensor and metabolic mediator [154]. One day after laser axotomy in worms, a decrease in O-GlcNAc levels promotes axon regeneration by adopting glycolysis as the primary source of energy [154]. Whilst mutant worms with decreased O-GlcNAc levels fail to regenerate after blocking glucose transport or inhibiting glycolysis, increasing O-GlcNAc levels act on mitochondrial function and enhance axon regeneration in Caenorhabditis elegans through FOXO/DAF-16–dependent mechanisms [154]. The fact that O-GlcNAc levels drive distinct branches of the insulin pathway to promote regeneration in worms may help explain these seemingly contradictory results.
As glial cells are metabolically coupled to axons, they may provide energy metabolites to support axon survival and function [94,155,156,157]. In fact, glia metabolic functions can be reprogrammed in favor of glycolysis to promote anatomical and functional regeneration after CNS injury in both flies and mice [158].
Together, the above examples underscore the importance of achieving optimal mitochondrial transport and glial metabolism as they are necessary for energy production to fuel axon growth and regeneration. Whether mitochondrial transport may be linked to nutrient and energy sensing is not known. It is also not clear whether mitochondria have memories, allowing them to respond rapidly to changes in environmental nutrient and energy levels. The extent to which boosting energy metabolism and mitochondria trafficking may be sufficient to promote functionally relevant regeneration and CNS repair under different experimental conditions awaits confirmation.
4. Energy Balance, Lipid Accumulation and Adipose Tissue
After CNS trauma and disease, altered lipid metabolism and lipid accumulation in neurons and various tissues in the body can be harmful, as it causes permanent cellular damage [159]. To understand the molecular mechanisms that cause or contribute to lipid accumulation, one must understand the concept of energy balance and, in particular, energy intake and expenditure. When the energy intake exceeds the physiological demand, excess energy is stored as fat [160]. Energy can be expended via increasing basal metabolism or physical activity (for example, thermogenesis) [161]. Basal metabolism and thermogenesis refer to the metabolic processes involved in the maintenance of body functions and energy production in response to cold or food intake, respectively. To maintain a healthy energy balance, food intake and energy expenditure have to be correctly regulated through the body. Detrimental changes in either of these can alter the energy balance, thereby causing adipose tissue deposition and increased fatty acid release into the circulation.
4.1. Lipolysis, Adaptive Thermogenesis and Innervation of Adipose Tissue
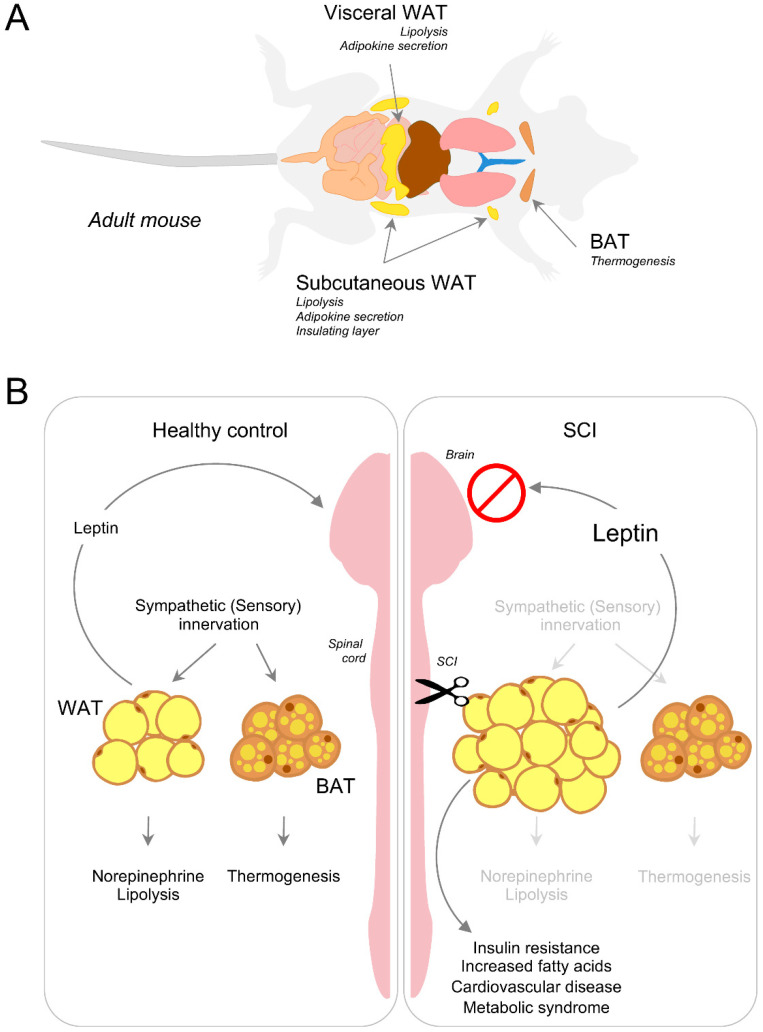
Under normal physiological conditions, excess energy is stored in depots of white adipose tissue (WAT) in the form of triacylglycerides. WAT depots include abdominal and subcutaneous fat [162,163] (Figure 4). To mobilize stored energy during exercise or fasting, WAT releases fatty acids via lipolysis, a biochemical pathway through which triacylglycerol stored in lipid droplets is hydrolyzed into a glycerol and three fatty acids for usage by other organs [164,165]. While it is present in all tissues and cell types, lipolysis occurs mainly in the adipose tissue [166]. Non-esterified fatty acids are not only used as energy substrates but also as precursors for membrane synthesis. The inter-organ demand and utilization of fat breakdown products are therefore necessary for survival. When, however, the energy balance shifts to excess lipid storage, the demand-driven supply chain breaks down leading to ectopic assimilation of lipids in non-adipose organs altering the whole-body energy balance.
Figure 4.
Adipose tissue depots, energy balance and leptin sensing. (A) Representation of adipose depots located under the skin and within the abdomen in adult mice. Whereas WAT can be subdivided into subcutaneous, bone marrow and visceral depots, BAT mainly localizes in the interscapular region. (B) Under normal physiological conditions, sympathetic innervation regulates WAT lipolysis and BAT thermogenesis. The contribution of sensory innervation is far less understood. Leptin action on the neurons located in the hypothalamus is critical for homeostatic regulation of energy balance. After SCI, interruption of adipose tissue innervation causes a breakdown in energy homeostasis leading to adipose tissue accumulation and ectopic lipid spillover to vital organs. If protracted, such detrimental conditions contribute to the development of insulin resistance, increased fatty acids, cardiovascular complications and metabolic syndrome.
Generation of body heat by external stimuli like temperature or food is called adaptive thermogenesis and is further classified as either shivering or non-shivering thermogenesis. By controlling the non-shivering thermogenic function, brown adipose tissue (BAT) plays a crucial role in maintaining whole-body energy balance. BAT’s unique ability to produce heat relies on the mitochondrial uncoupling protein 1 (UCP1), which disengages oxidative phosphorylation from the electron transport chain to produce heat instead of energy in the form of ATP [167]. Increase in BAT volume is associated with increased lipolysis, free fatty acid cycling and oxidation, as well as adipose tissue insulin sensitivity [168].
Recent evidence suggests that innervation of adipose tissue may play a critical role in lipid mobilization and endocrine signaling [169,170,171]. After CNS trauma and neurodegenerative disease, disruption of adipose tissue innervation can lead to deleterious consequences including insulin resistance and cardiovascular disease [172,173] (Figure 4). Despite having a ‘normal’ body mass index, SCI individuals often develop severe adiposity. Given that the majority of these individuals has moderate to normal feeding behavior without hyperphagia [174], the energy imbalance owing to the rise in energy intake cannot explain the substantial increase in adiposity. Reduced physical activity may contribute to this phenomenon. However, adiposity in age-matched healthy individuals with the same activity profile is considerably lower when compared to SCI individuals. Pioneering work by Bartness et al. demonstrated that Siberian hamsters experience seasonal variation in adiposity and that such variation can be reproduced in the laboratory by altering the photoperiod from long to short days [175]. The authors tested photoperiod inducible melatonin and its circulating intermediaries (for example, glucocorticoids and thyroid hormone) as ‘effector’ molecules that can drive the intra-abdominal fat mobilization in hamsters. Surprisingly, experimental evidence has shown that these molecules do not account for the observed changes [175], suggesting other mechanisms may be responsible for adipose mobilization.
More than 100 years ago, Dogiel reported neurons of unknown origin innervate WAT [176]. Decades later, Corell et al. corroborated this observation by demonstrating that electrical stimulation of WAT causes the release of fat breakdown products, suggesting a neuronal mechanism may be controlling lipolysis [177]. Building on this seminal contribution, numerous studies have demonstrated that sympathetic nerve endings connect in the WAT parenchyma and that WAT innervation is required for lipolysis [178,179,180]. Using retrograde tracing techniques, postganglionic sympathetic neurons were found to innervate WAT. The majority of the neurons innervating WAT originate from thoracic (T)12–lumbar (L)1 spinal ganglia [181,182]. New viral and genetic tools allow precise localization of preganglionic and postganglionic neurons innervating adipose tissue. Transneuronal tracing using pseudorabies virus injected in epididymal WAT has demonstrated that sympathetic innervation of WAT connects to several regions in the brain such as the hypothalamus, which controls feeding behavior to maintain energy balance and homeostasis [183]. Disruption of this system could contribute to adiposity after injury and disease.
Sensory neurons also innervate WAT. In fact, T13-L3 neuronal cell bodies can be visualized upon retrograde tracing in WAT [184]. Similarly, herpes simplex viral injection in the inguinal fat pad has revealed that WAT efferent neurons connect with DRG neurons in the lower thoracic dorsal horn and upper lumbar spinal cord [185]. Interestingly, the sensory circuitry innervating WAT is sensitive to leptin, an adipose tissue hormone that sends feeding signals to the hypothalamus [186,187] (Figure 4). While leptin injection in WAT induces c-Fos expression in DRG neurons, leptin intraperitoneal injection fails to elicit such a response, thereby suggesting a functional role of sensory innervation in WAT [188].
Unlike WAT, BAT segments bilaterally [189]. As norepinephrine released by the sympathetic fibers innervating BAT activates mitochondrial UCP1, denervation of the ipsilateral side of BAT lowered UCP1, norepinephrine and the overall BAT mass compared to the non-innervated contralateral side [190]; norepinephrine treatment reverses the loss of BAT activity. Sympathetic projections that innervate BAT arise from the four anterior nerve bundles of the intercostal muscles [190]. A recent study showed that sympathetic post-ganglionic neurons that innervate BAT connect the stellate ganglia along with T1-T5 spinal ganglia [169,191]. Similar to WATs, sympathetic neurons that innervate brown fat connect several key hypothalamic centers [192]. Taken together, this shows that adipose tissue connects with the CNS and the spinal cord via the sympathetic nervous system. Disruption of innervation due to CNS trauma may therefore alter systemic energy balance.
4.2. Disruption of Adipose Tissue Innervation and Energy Metabolism after Injury
Lipolysis and adaptive thermogenesis are necessary to control a healthy energy balance. Neurons that connect the hypothalamic regions and innervate WAT and BAT form a ‘leptin sensitive’ closed loop. The arcuate nucleus in the hypothalamus is one such CNS region that critically senses leptin levels [193] to promote sympathetic innervation of adipose tissue [170]. The ability to sense leptin, as well as leptin’s function in adipose tissue, can be compromised after CNS trauma. In turn, interruption of adipose innervation negatively impacts both areas of energy balance [194]. A rise of leptin in the serum of SCI individuals positively correlates with an increase in adiposity following SCI [195,196,197,198]. Leptin promotes UCP1 expression in BAT to trigger BAT-dependent thermogenesis. Despite having high basal levels of leptin, SCI individuals have a reduced energy expenditure profile [199]. Two possible explanations may help understand this phenomenon. First, local leptin activity on brown fat requires sympathetic innervation of BAT which is reduced after SCI. Second, BAT may become insensitive to leptin and no longer be able to respond to it. Work done in obese mice has shown that central versus peripheral administration of leptin promotes sympathetic outflow to BAT and promotes thermogenesis [200], suggesting that leptin’s action in the brain supports a positive energy balance. In turn, impaired sympathetic function can compromise leptin action on BAT to lower thermogenesis after SCI, causing a reduction in energy expenditure (Figure 4). Whether leptin exerts a direct effect on BAT sympathetic innervation is currently unknown. WAT lipolysis strongly depends on leptin action as well. Along this line, treating rat fat pads ex-vivo with leptin leads to an increase in the release of fatty acids in a dose- and time-dependent manner [201]. A recent study demonstrated that leptin’s lipolytic role is dependent on sympathetic innervation of WAT [202]. Furthermore, intraperitoneal leptin administration reverses defective metabolism in ob/ob mice (e.g., leptin deficient mice) by promoting sympathetic innervation of WAT [170]. Severed adipose innervation following a CNS trauma therefore deregulates leptin sensing, causing adipose accumulation and an overall increase in circulating fatty acids (Figure 4). Future work should aim at understanding how CNS trauma and neurodegenerative diseases impair ‘leptin sensitive’ circuits. Progress along this line may help in the development of therapeutic strategies aimed at lowering the risk of adipose spillover in visceral organs, preventing organ failure. Normalization of the energy metabolism can lower excess fatty acid buildup in the brain and spinal cord and lower the risk of neuronal comorbidities associated with CNS trauma and disease.
4.3. Adiposity and CNS Injury
Individuals that sustained an injury to the brain or spinal cord may have lower life expectancy due to chronic secondary complications that develop months and years after injury. Bladder and bowel dysfunction can cause anxiety, social isolation and depression after SCI [203]. Sympathetic hypoactivity leads to low systemic arterial pressure [204,205]. Moreover, disconnection of the excitatory drive from the brainstem leads to impaired autonomic functions and low sympathetic tone [206]. Sympathetic innervation of the adipose tissue is crucial to maintain the balance in energy storage and expenditure. Loss of sympathetic innervation promotes adiposity and contributes to devastating chronic complications like insulin resistance and cardiac dysfunction. Notably, type 2 diabetes (for example, insulin resistance) and cardiac arrest are the leading cause of death in SCI individuals [207]. For simplicity, we will refer to ‘adiposity’ as the excess lipid accumulation within adipose tissue and ectopic deposition in non-adipose organs.
It is important to note that adiposity in SCI individuals and healthy individuals is not exactly the same. Within a year after injury, SCI individuals lose bodyweight, mostly in lean muscle mass [208]. The drastic loss of muscle mass destabilizes the typical energy expenditure profile and contributes to adipose tissue accumulation in ectopic depots. Of note, the overall increase in adiposity does not affect the body mass index due to the low muscle mass in SCI individuals [209]. The body mass index cutoff range used to determine obesity in non-SCI individuals underestimates adiposity in SCI individuals [210], and therefore needs to be adjusted [211,212].
At least half of individuals with SCI has >30% of their total body mass as adipose tissue, indicating SCI individuals are at high risk of developing metabolic syndrome [213] (Figure 4). Adipose tissue preferentially accumulates in specific locations within the body. Using imaging methods like MRI and dual-energy X-ray absorptiometry to map regional adiposity in SCI individuals has confirmed an increase of ~50% in subcutaneous and visceral adipose tissue [213,214,215]. SCI individuals not only experience increased adiposity in these two common regions of the body, but also a rise in bone marrow adipose tissue [197]. Recent studies on obesity have demonstrated that a rise in bone marrow adiposity promotes osteoporosis and impairs several immune-associated functions [216,217]. An increase in bone marrow adiposity can have an impact on bone marrow stem cell lineage differentiation towards myelopoiesis. As such, newly differentiated myeloid cells can infiltrate into various tissues and promote pro-inflammatory conditions that further exacerbate metabolic syndrome [218,219,220]. Individuals with motor complete SCI develop 2 to 3 times more bone marrow adiposity than age-matched controls [221] and therefore, are at risk of systemic inflammation induced metabolic syndrome. Accumulating evidence suggests that a rise in adiposity in non-adipose tissues including the liver, heart and skeletal muscle is caused by neurological deficits related to SCI [209] and consequently, is independent of physical activity [222]. For example, cervical and midthoracic SCI in rats leads to excess lipid accumulation in the liver, augmented proinflammatory gene expression and increased CD68 positive macrophage infiltration [223]; these are hallmarks of chronic liver injury similar to those of fatty liver disease.
4.4. Ectopic Liver Fat Accumulation after SCI
A rise in triglycerides in the liver may be caused by increased storage after de novo synthesis, reduced breakdown, or impaired secretion in the form of very-low-density lipoproteins (VLDL). Under pathological conditions like obesity, fatty acids are converted into triglycerides. These are stored as neutral lipid droplets as they no longer get converted into energy substrates by oxidation. With more incoming fatty acids adding to the pool of stored lipid droplets in the liver, the de novo synthesis of lipids increases, thereby causing lipid accumulation within the liver [224,225]. As the supply of fat exceeds the demand for energy homeostasis, the liver releases lipids in a controlled manner in the form of VLDL. Several factors regulate this process, but insulin-mediated secretion of VLDL is by far the most crucial [226]. When insulin is high, VLDL secretion from the liver increases. During fasting, insulin is lowered and VLDL secretion halts to (i) prevent detrimental accumulation of triglycerides in circulation and (ii) to maintain steady energy homeostasis in the liver.
Proper insulin signaling in the liver orchestrates the critical steps of VLDL secretion [226]. However, a pathological rise in adiposity promotes hyperinsulinemia and insulin resistance causing a detrimental increase in VLDL in the circulation. Interestingly, SCI individuals with paraplegia and complete sensorimotor injuries (for example, loss of sensation or motor function below the injury) have high serum VLDL levels [227]. With age, high de novo lipogenesis and lipid-laden liver progress towards non-alcoholic fatty liver disease. A clinical study has found that 50% of SCI individuals experience liver adiposity induced non-alcoholic fatty liver disease ~1 year after injury [228]. Accumulation of lipids in the liver at chronic stages after SCI has been reported in rodents and humans [223,228], indicating animal models are extremely valuable as they effectively reproduce mechanisms of injury and disease found in humans [229].
More than half of the triglycerides deposited in the liver originates from circulating fatty acids and ~25% of the triglycerides comes from de novo lipogenesis [230]. Given that excess lipids in the liver depend on circulating fatty acids derived from adipose tissue, a rise in adiposity may precede a breakdown in liver homeostasis. The inflammatory response that develops acutely after SCI exacerbates this condition [231,232]. As systemic inflammation persists well beyond the acute phase of SCI [231], liver adiposity may convert to a more severe pathology known as non-alcoholic steatohepatitis. Increased adiposity followed by a rise in leptin level activates T cells [233,234] and polarizes T cell differentiation towards a T helper cell type 1 phenotype that produces pro-inflammatory cytokines like TNF-alpha and IFNγ [228,235]. When coupled with systemic inflammation at chronic stages of SCI, liver adiposity may lead to tissue fibrosis and non-alcoholic steatohepatitis if left untreated [196,228].
A gradual rise in whole-body adiposity compromises the balance between fatty acid uptake and secretion with negative impacts on the liver metabolic profile. Despite recent progress, a thorough mechanistic understanding of liver pathology after SCI is still lacking. As the liver represents the primary site for drug metabolism, reducing hepatic metabolic dysfunction may boost efficacy of SCI medications and repair strategies.
4.5. Glucose Intolerance, Insulin Resistance and Cardiovascular Complications
As circulating free fatty acid levels increase due to adiposity, the glucose-fatty acid cycle, also known as the ‘Randle’ cycle [236], destabilizes. A rise in plasma-free fatty acids blocks glucose oxidation and impairs glucose uptake in the liver and skeletal muscle [237,238]. The low glucose utilization in muscle and liver increases glucose intolerance and insulin resistance, a chronic condition that develops in individuals with SCI [239]. Individuals with chronic SCI display low plasma glucose clearance [240] with significantly high insulin levels [239]. Both adiposity and muscle wasting could contribute to insulin resistance. In addition, adiposity leads to cardiac lipotoxicity induced damage after SCI.
Cardiovascular complications represent the leading cause of death in chronic SCI individuals [241,242,243]. Partial central deafferentation of spinal neurons causes atrophy of the spinal sympathetic pre-ganglionic neurons [206]. Because these neurons innervate the heart, their severe atrophy may lead to cardiac arrest. However, case report studies in humans suggest cardiac arrest at chronic stages is not due to atrophy of sympathetic pre-ganglionic neurons, as these neurons can regain normal function [206]. With a reduced sympathetic tone and low lipolysis, adipose tissue accrues lipids [202]. SCI individuals with paraplegia have elevated LDL-cholesterol and serum triglyceride levels [244]. Due to increased expression of the fatty acid transporter protein CD36, the cardiac tissue readily takes up low-density lipoproteins and fatty acids [245,246]. With increased fatty acid load, mitochondrial energy metabolism reverts to an embryonic metabolic phenotype that produces ATP by utilizing glucose [247]. Lowering β-oxidation and increasing glycolysis changes the expression of key metabolic enzymes that maintain a proper glycolytic state. Impaired β-oxidation and lipotoxicity increase myocardial triglyceride content and augment the risk of cardiac failure [247,248]. Whether these mechanisms are the primary cause for the development of cardiovascular dysfunction after SCI remains to be evaluated.
5. Conclusions
We have discussed evidence suggesting that reprogramming lipid metabolism, boosting mitochondrial transport and neuron-glia metabolic coupling promote survival and regeneration of injured axons. Whereas large amounts of lipids are necessary during development to grow axons, dendrites and myelin sheaths, dysregulation of lipid metabolism, impaired mitochondrial functions and excess lipid accumulation through the body including in non-adipose organs can be harmful as they cause lipotoxicity and increased levels of fatty acids. Not only do such detrimental conditions contribute to axon regeneration failure, but also to insulin resistance, cardiovascular disease and metabolic syndrome, thus leading to poor neurological outcomes and a diminished quality of life. In addition, spatial and temporal alteration of circulating fatty acids may compromise CNS function and repair under pathological conditions including CNS trauma and neurodegenerative diseases. Correct positioning of organelles for membrane trafficking, lipid exchange and energy production requires a complex interplay between various organelles and molecular motors [249]. The fabrication of new inducible and reversible systems may allow us to gain spatiotemporal control over this process [250], enabling CNS repair with precision. Moreover, a thorough mechanistic understanding of adipose tissue remodeling following CNS trauma and disease is lacking. With the development and optimization of non-invasive three-dimensional imaging of glucose uptake and neuronal tracing strategies, we will begin to understand the importance of adipose tissue innervation, in particular sensory innervation, endocrine function and inter-organ lipid homeostasis. Regaining metabolic control and energy balance may be necessary to promote neuron repair and full body recovery after CNS trauma and disease.
Acknowledgments
We thank all members of the laboratory for providing insightful comments on the manuscript.
Funding
This research was funded by the National Institute of Neurological Disorders and Stroke (R01NS110681 and R21NS109787) and Chronic Brain Injury Discovery Theme at The Ohio State University.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polleux F., Snider W. Initiating and growing an axon. Cold Spring Harb. Perspect Biol. 2010;2:a001925. doi: 10.1101/cshperspect.a001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He Z., Jin Y. Intrinsic Control of Axon Regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Pfister B.J., Iwata A., Meaney D.F., Smith D.H. Extreme stretch growth of integrated axons. J. Neurosci. 2004;24:7978–7983. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu P., Wang Y., Graham L., McHale K., Gao M., Wu D., Brock J., Blesch A., Rosenzweig E.S., Havton L.A., et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfenninger K.H. Plasma membrane expansion: A neuron’s Herculean task. Nat. Rev. Neurosci. 2009;10:251–261. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- 6.Yang C., Wang X., Wang J., Wang X., Chen W., Lu N., Siniossoglou S., Yao Z., Liu K. Rewiring Neuronal Glycerolipid Metabolism Determines the Extent of Axon Regeneration. Neuron. 2020;105:276–292.e5. doi: 10.1016/j.neuron.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harayama T., Riezman H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018;19:281–296. doi: 10.1038/nrm.2017.138. [DOI] [PubMed] [Google Scholar]
- 9.Dun X.P., Parkinson D.B. Visualizing peripheral nerve regeneration by whole mount staining. PLoS ONE. 2015;10:e0119168. doi: 10.1371/journal.pone.0119168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheib J., Hoke A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 11.Liu K., Lu Y., Lee J.K., Samara R., Willenberg R., Sears-Kraxberger I., Tedeschi A., Park K.K., Jin D., Cai B., et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahar M., Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018;19:323–337. doi: 10.1038/s41583-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tedeschi A., Bradke F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr. Opin. Neurobiol. 2017;42:118–127. doi: 10.1016/j.conb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Sofroniew M.V. Dissecting spinal cord regeneration. Nature. 2018;557:343–350. doi: 10.1038/s41586-018-0068-4. [DOI] [PubMed] [Google Scholar]
- 15.Sun W., Larson M.J., Kiyoshi C.M., Annett A.J., Stalker W.A., Peng J., Tedeschi A. Gabapentinoid treatment promotes corticospinal plasticity and regeneration following murine spinal cord injury. J. Clin. Investig. 2020;130:345–358. doi: 10.1172/JCI130391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchovecky C.M., Turley S.D., Brown H.M., Kyle S.M., McDonald J.G., Liu B., Pieper A.A., Huang W., Katz D.M., Russell D.W., et al. A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome. Nat. Genet. 2013;45:1013–1020. doi: 10.1038/ng.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tint G.S., Irons M., Elias E.R., Batta A.K., Frieden R., Chen T.S., Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N. Engl. J. Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 18.Ewan E.E., Avraham O., Carlin D., Goncalves T.M., Zhao G., Cavalli V. Ascending dorsal column sensory neurons respond to spinal cord injury and downregulate genes related to lipid metabolism. Sci. Rep. 2021;11:374. doi: 10.1038/s41598-020-79624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupuis L., Corcia P., Fergani A., Gonzalez De Aguilar J.L., Bonnefont-Rousselot D., Bittar R., Seilhean D., Hauw J.J., Lacomblez L., Loeffler J.P., et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70:1004–1009. doi: 10.1212/01.wnl.0000285080.70324.27. [DOI] [PubMed] [Google Scholar]
- 20.Snowden S.G., Ebshiana A.A., Hye A., An Y., Pletnikova O., O’Brien R., Troncoso J., Legido-Quigley C., Thambisetty M. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med. 2017;14:e1002266. doi: 10.1371/journal.pmed.1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chrast R., Saher G., Nave K.A., Verheijen M.H. Lipid metabolism in myelinating glial cells: Lessons from human inherited disorders and mouse models. J. Lipid. Res. 2011;52:419–434. doi: 10.1194/jlr.R009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neely J.R., Morgan H.E. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu. Rev. Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 23.Quiroga S., Bisbal M., Caceres A. Regulation of plasma membrane expansion during axon formation. Dev. Neurobiol. 2018;78:170–180. doi: 10.1002/dneu.22553. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler A.B., Thiele C., Tenedini F., Richard M., Leyendecker P., Hoermann A., Soba P., Tavosanis G. Cell-autonomous control of neuronal dendrite expansion via the fatty acid synthesis regulator SREBP. Cell Rep. 2017;21:3346–3353. doi: 10.1016/j.celrep.2017.11.069. [DOI] [PubMed] [Google Scholar]
- 25.Avraham O., Deng P.Y., Jones S., Kuruvilla R., Semenkovich C.F., Klyachko V.A., Cavalli V. Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun. 2020;11:4891. doi: 10.1038/s41467-020-18642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Chaves E.I., Rusinol A.E., Vance D.E., Campenot R.B., Vance J.E. Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J. Biol. Chem. 1997;272:30766–30773. doi: 10.1074/jbc.272.49.30766. [DOI] [PubMed] [Google Scholar]
- 27.Vance J.E., Campenot R.B., Vance D.E. The synthesis and transport of lipids for axonal growth and nerve regeneration. Biochim. Biophys. Acta. 2000;1486:84–96. doi: 10.1016/S1388-1981(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 28.Lockerbie R.O., Miller V.E., Pfenninger K.H. Regulated plasmalemmal expansion in nerve growth cones. J. Cell Biol. 1991;112:1215–1227. doi: 10.1083/jcb.112.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maday S., Twelvetrees A.E., Moughamian A.J., Holzbaur E.L. Axonal transport: Cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyles J.K., Zoellner C.D., Anderson L.J., Kosik L.M., Pitas R.E., Weisgraber K.H., Hui D.Y., Mahley R.W., Gebicke-Haerter P.J., Ignatius M.J., et al. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J. Clin. Investig. 1989;83:1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muse E.D., Jurevics H., Toews A.D., Matsushima G.K., Morell P. Parameters related to lipid metabolism as markers of myelination in mouse brain. J. Neurochem. 2001;76:77–86. doi: 10.1046/j.1471-4159.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- 32.Saher G., Brugger B., Lappe-Siefke C., Mobius W., Tozawa R., Wehr M.C., Wieland F., Ishibashi S., Nave K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- 33.Sherman D.L., Brophy P.J. Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 34.Simons M., Trotter J. Wrapping it up: The cell biology of myelination. Curr. Opin. Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Craig A.M., Wyborski R.J., Banker G. Preferential addition of newly synthesized membrane protein at axonal growth cones. Nature. 1995;375:592–594. doi: 10.1038/375592a0. [DOI] [PubMed] [Google Scholar]
- 36.Erez H., Malkinson G., Prager-Khoutorsky M., De Zeeuw C.I., Hoogenraad C.C., Spira M.E. Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J. Cell Biol. 2007;176:497–507. doi: 10.1083/jcb.200607098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popov S., Brown A., Poo M.M. Forward plasma membrane flow in growing nerve processes. Science. 1993;259:244–246. doi: 10.1126/science.7678471. [DOI] [PubMed] [Google Scholar]
- 38.He B., Guo W. The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu S.C., Ting A.E., Hazuka C.D., Davanger S., Kenny J.W., Kee Y., Scheller R.H. The mammalian brain rsec6/8 complex. Neuron. 1996;17:1209–1219. doi: 10.1016/S0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 40.TerBush D.R., Maurice T., Roth D., Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. doi: 10.1002/j.1460-2075.1996.tb01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He B., Xi F., Zhang X., Zhang J., Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007;26:4053–4065. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyd C., Hughes T., Pypaert M., Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 2004;167:889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X., Zajac A., Zhang J., Wang P., Li M., Murray J., TerBush D., Guo W. The critical role of Exo84p in the organization and polarized localization of the exocyst complex. J. Biol. Chem. 2005;280:20356–20364. doi: 10.1074/jbc.M500511200. [DOI] [PubMed] [Google Scholar]
- 44.Inoue M., Chang L., Hwang J., Chiang S.H., Saltiel A.R. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 45.Inoue M., Chiang S.H., Chang L., Chen X.W., Saltiel A.R. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol. Biol. Cell. 2006;17:2303–2311. doi: 10.1091/mbc.e06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gracias N.G., Shirkey-Son N.J., Hengst U. Local translation of TC10 is required for membrane expansion during axon outgrowth. Nat. Commun. 2014;5:3506. doi: 10.1038/ncomms4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupraz S., Grassi D., Bernis M.E., Sosa L., Bisbal M., Gastaldi L., Jausoro I., Caceres A., Pfenninger K.H., Quiroga S. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J. Neurosci. 2009;29:13292–13301. doi: 10.1523/JNEUROSCI.3907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutton R.B., Fasshauer D., Jahn R., Brunger A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 49.Grote E., Carr C.M., Novick P.J. Ordering the final events in yeast exocytosis. J. Cell Biol. 2000;151:439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett M.K., Calakos N., Scheller R.H. Syntaxin: A synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 51.Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiederkehr A., De Craene J.O., Ferro-Novick S., Novick P. Functional specialization within a vesicle tethering complex: Bypass of a subset of exocyst deletion mutants by Sec1p or Sec4p. J. Cell Biol. 2004;167:875–887. doi: 10.1083/jcb.200408001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper S.T., McNeil P.L. Membrane Repair: Mechanisms and Pathophysiology. Physiol. Rev. 2015;95:1205–1240. doi: 10.1152/physrev.00037.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bi G.Q., Alderton J.M., Steinhardt R.A. Calcium-regulated exocytosis is required for cell membrane resealing. Pt 2J. Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terasaki M., Miyake K., McNeil P.L. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J. Cell Biol. 1997;139:63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reddy A., Caler E.V., Andrews N.W. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/S0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 57.Steinhardt R.A., Bi G., Alderton J.M. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- 58.Bradke F., Fawcett J.W., Spira M.E. Assembly of a new growth cone after axotomy: The precursor to axon regeneration. Nat. Rev. Neurosci. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- 59.Hur E.M., Saijilafu, Zhou F.Q. Growing the growth cone: Remodeling the cytoskeleton to promote axon regeneration. Trends. Neurosci. 2012;35:164–174. doi: 10.1016/j.tins.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chauhan M.Z., Arcuri J., Park K.K., Zafar M.K., Fatmi R., Hackam A.S., Yin Y., Benowitz L., Goldberg J.L., Samarah M., et al. Multi-omic analyses of growth cones at different developmental stages provides insight into pathways in adult Neuroregeneration. iScience. 2020;23:100836. doi: 10.1016/j.isci.2020.100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carman G.M., Han G.S. Fat-regulating phosphatidic acid phosphatase: A review of its roles and regulation in lipid homeostasis. J. Lipid. Res. 2019;60:2–6. doi: 10.1194/jlr.S087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y., He X., Kawaguchi R., Zhang Y., Wang Q., Monavarfeshani A., Yang Z., Chen B., Shi Z., Meng H., et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature. 2020;587:613–618. doi: 10.1038/s41586-020-2795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poplawski G.H.D., Kawaguchi R., Van Niekerk E., Lu P., Mehta N., Canete P., Lie R., Dragatsis I., Meves J.M., Zheng B., et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature. 2020;581:77–82. doi: 10.1038/s41586-020-2200-5. [DOI] [PubMed] [Google Scholar]
- 64.Tedeschi A., Dupraz S., Laskowski C.J., Xue J., Ulas T., Beyer M., Schultze J.L., Bradke F. The calcium channel subunit alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron. 2016;92:419–434. doi: 10.1016/j.neuron.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 65.Venkatesh I., Mehra V., Wang Z., Califf B., Blackmore M.G. Developmental chromatin restriction of pro-growth gene networks acts as an epigenetic barrier to axon regeneration in cortical neurons. Dev. Neurobiol. 2018;78:960–977. doi: 10.1002/dneu.22605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang K.C., Bian M., Xia X., Madaan A., Sun C., Wang Q., Li L., Nahmou M., Noro T., Yokota S., et al. Posttranslational modification of Sox11 regulates RGC survival and axon regeneration. eNeuro. 2021;8 doi: 10.1523/ENEURO.0358-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindborg J.A., Tran N.M., Chenette D.M., DeLuca K., Foli Y., Kannan R., Sekine Y., Wang X., Wollan M., Kim I.J., et al. Optic nerve regeneration screen identifies multiple genes restricting adult neural repair. Cell Rep. 2021;34:108777. doi: 10.1016/j.celrep.2021.108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Omura T., Omura K., Tedeschi A., Riva P., Painter M.W., Rojas L., Martin J., Lisi V., Huebner E.A., Latremoliere A., et al. Robust axonal regeneration occurs in the injured CAST/Ei mouse CNS. Neuron. 2015;86:1215–1227. doi: 10.1016/j.neuron.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puttagunta R., Tedeschi A., Soria M.G., Hervera A., Lindner R., Rathore K.I., Gaub P., Joshi Y., Nguyen T., Schmandke A., et al. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nat. Commun. 2014;5:3527. doi: 10.1038/ncomms4527. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z., Reynolds A., Kirry A., Nienhaus C., Blackmore M.G. Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci. 2015;35:3139–3145. doi: 10.1523/JNEUROSCI.2832-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J., Shin J.E., Lee B., Kim H., Jeon Y., Ahn S.H., Chi S.W., Cho Y. The stem cell marker Prom1 promotes axon regeneration by down-regulating cholesterol synthesis via Smad signaling. Proc. Natl. Acad. Sci. USA. 2020;117:15955–15966. doi: 10.1073/pnas.1920829117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosello-Busquets C., de la Oliva N., Martinez-Marmol R., Hernaiz-Llorens M., Pascual M., Muhaisen A., Navarro X., Del Valle J., Soriano E. Cholesterol depletion regulates axonal growth and enhances central and peripheral nerve regeneration. Front. Cell Neurosci. 2019;13:40. doi: 10.3389/fncel.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shabanzadeh A.P., Charish J., Tassew N.G., Farhani N., Feng J., Qin X., Sugita S., Mothe A.J., Walchli T., Koeberle P.D., et al. Cholesterol synthesis inhibition promotes axonal regeneration in the injured central nervous system. Neurobiol. Dis. 2021;150:105259. doi: 10.1016/j.nbd.2021.105259. [DOI] [PubMed] [Google Scholar]
- 74.Chernoff G.F. Shiverer: An autosomal recessive mutant mouse with myelin deficiency. J. Hered. 1981;72:128. doi: 10.1093/oxfordjournals.jhered.a109442. [DOI] [PubMed] [Google Scholar]
- 75.Mar F.M., da Silva T.F., Morgado M.M., Rodrigues L.G., Rodrigues D., Pereira M.I.L., Marques A., Sousa V.F., Coentro J., Sa-Miranda C., et al. Myelin lipids inhibit axon regeneration following spinal cord injury: A novel perspective for therapy. Mol. Neurobiol. 2016;53:1052–1064. doi: 10.1007/s12035-014-9072-3. [DOI] [PubMed] [Google Scholar]
- 76.Hanani M. Satellite glial cells in sensory ganglia: From form to function. Brain. Res. Brain. Res. Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 77.Hanani M., Spray D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020;21:485–498. doi: 10.1038/s41583-020-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meltzer S., Bagley J.A., Perez G.L., O’Brien C.E., DeVault L., Guo Y., Jan L.Y., Jan Y.N. Phospholipid homeostasis regulates dendrite morphogenesis in drosophila sensory neurons. Cell Rep. 2017;21:859–866. doi: 10.1016/j.celrep.2017.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hua X., Yokoyama C., Wu J., Briggs M.R., Brown M.S., Goldstein J.L., Wang X. SREBP-2, a second basic-helix-loop-helix-leucine zipper protein that stimulates transcription by binding to a sterol regulatory element. Proc. Natl. Acad. Sci. USA. 1993;90:11603–11607. doi: 10.1073/pnas.90.24.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tontonoz P., Kim J.B., Graves R.A., Spiegelman B.M. ADD1: A novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell Biol. 1993;13:4753–4759. doi: 10.1128/MCB.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yokoyama C., Wang X., Briggs M.R., Admon A., Wu J., Hua X., Goldstein J.L., Brown M.S. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. doi: 10.1016/S0092-8674(05)80095-9. [DOI] [PubMed] [Google Scholar]
- 82.Kennedy E.P., Weiss S.B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 1956;222:193–214. doi: 10.1016/S0021-9258(19)50785-2. [DOI] [PubMed] [Google Scholar]
- 83.Pavlidis P., Ramaswami M., Tanouye M.A. The Drosophila easily shocked gene: A mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 1994;79:23–33. doi: 10.1016/0092-8674(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 84.Rawson R.B. The SREBP pathway--insights from Insigs and insects. Nat. Rev. Mol. Cell Biol. 2003;4:631–640. doi: 10.1038/nrm1174. [DOI] [PubMed] [Google Scholar]
- 85.Dobrosotskaya I.Y., Seegmiller A.C., Brown M.S., Goldstein J.L., Rawson R.B. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science. 2002;296:879–883. doi: 10.1126/science.1071124. [DOI] [PubMed] [Google Scholar]
- 86.Lim H.Y., Wang W., Wessells R.J., Ocorr K., Bodmer R. Phospholipid homeostasis regulates lipid metabolism and cardiac function through SREBP signaling in Drosophila. Genes. Dev. 2011;25:189–200. doi: 10.1101/gad.1992411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aggarwal S., Yurlova L., Simons M. Central nervous system myelin: Structure, synthesis and assembly. Trends. Cell Biol. 2011;21:585–593. doi: 10.1016/j.tcb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Barres B.A. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 89.Caldwell J.H., Schaller K.L., Lasher R.S., Peles E., Levinson S.R. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc. Natl. Acad. Sci. USA. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hille B. Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys. J. 1978;22:283–294. doi: 10.1016/S0006-3495(78)85489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waxman S.G. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- 92.Bei F., Lee H.H.C., Liu X., Gunner G., Jin H., Ma L., Wang C., Hou L., Hensch T.K., Frank E., et al. Restoration of visual function by enhancing conduction in regenerated axons. Cell. 2016;164:219–232. doi: 10.1016/j.cell.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Funfschilling U., Supplie L.M., Mahad D., Boretius S., Saab A.S., Edgar J., Brinkmann B.G., Kassmann C.M., Tzvetanova I.D., Mobius W., et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee Y., Morrison B.M., Li Y., Lengacher S., Farah M.H., Hoffman P.N., Liu Y., Tsingalia A., Jin L., Zhang P.W., et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saab A.S., Nave K.A. Myelin dynamics: Protecting and shaping neuronal functions. Curr. Opin. Neurobiol. 2017;47:104–112. doi: 10.1016/j.conb.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 96.Harauz G., Ishiyama N., Hill C.M., Bates I.R., Libich D.S., Fares C. Myelin basic protein-diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron. 2004;35:503–542. doi: 10.1016/j.micron.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 97.Weimbs T., Stoffel W. Proteolipid protein (PLP) of CNS myelin: Positions of free, disulfide-bonded, and fatty acid thioester-linked cysteine residues and implications for the membrane topology of PLP. Biochemistry. 1992;31:12289–12296. doi: 10.1021/bi00164a002. [DOI] [PubMed] [Google Scholar]
- 98.Jahn O., Siems S.B., Kusch K., Hesse D., Jung R.B., Liepold T., Uecker M., Sun T., Werner H.B. The CNS Myelin Proteome: Deep Profile and Persistence After Post-mortem Delay. Front. Cell Neurosci. 2020;14:239. doi: 10.3389/fncel.2020.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Norton W.T., Poduslo S.E. Myelination in rat brain: Method of myelin isolation. J. Neurochem. 1973;21:749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- 100.O’Brien J.S., Sampson E.L. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. J. Lipid. Res. 1965;6:537–544. doi: 10.1016/S0022-2275(20)39619-X. [DOI] [PubMed] [Google Scholar]
- 101.Norton W.T., Poduslo S.E. Myelination in rat brain: Changes in myelin composition during brain maturation. J. Neurochem. 1973;21:759–773. doi: 10.1111/j.1471-4159.1973.tb07520.x. [DOI] [PubMed] [Google Scholar]
- 102.Saher G., Simons M. Cholesterol and myelin biogenesis. Subcell. Biochem. 2010;51:489–508. doi: 10.1007/978-90-481-8622-8_18. [DOI] [PubMed] [Google Scholar]
- 103.Malheiro A.R., Correia B., Ferreira da Silva T., Bessa-Neto D., Van Veldhoven P.P., Brites P. Leukodystrophy caused by plasmalogen deficiency rescued by glyceryl 1-myristyl ether treatment. Brain. Pathol. 2019;29:622–639. doi: 10.1111/bpa.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huttner W.B., Zimmerberg J. Implications of lipid microdomains for membrane curvature, budding and fission. Curr. Opin. Cell Biol. 2001;13:478–484. doi: 10.1016/S0955-0674(00)00239-8. [DOI] [PubMed] [Google Scholar]
- 105.McMahon H.T., Boucrot E. Membrane curvature at a glance. J. Cell Sci. 2015;128:1065–1070. doi: 10.1242/jcs.114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith M.E. The turnover of myelin in the adult rat. Biochim. Biophys. Acta. 1968;164:285–293. doi: 10.1016/0005-2760(68)90154-9. [DOI] [PubMed] [Google Scholar]
- 107.Espenshade P.J., Hughes A.L. Regulation of sterol synthesis in eukaryotes. Annu. Rev. Genet. 2007;41:401–427. doi: 10.1146/annurev.genet.41.110306.130315. [DOI] [PubMed] [Google Scholar]
- 108.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 109.Camargo N., Goudriaan A., van Deijk A.F., Otte W.M., Brouwers J.F., Lodder H., Gutmann D.H., Nave K.A., Dijkhuizen R.M., Mansvelder H.D., et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017;15:e1002605. doi: 10.1371/journal.pbio.1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller L.G., Jr., Young J.A., Ray S.K., Wang G., Purohit S., Banik N.L., Dasgupta S. Sphingosine Toxicity in EAE and MS: Evidence for Ceramide Generation via Serine-Palmitoyltransferase Activation. Neurochem. Res. 2017;42:2755–2768. doi: 10.1007/s11064-017-2280-2. [DOI] [PubMed] [Google Scholar]
- 111.Dimas P., Montani L., Pereira J.A., Moreno D., Trotzmuller M., Gerber J., Semenkovich C.F., Kofeler H.C., Suter U. CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. Elife. 2019;8:8. doi: 10.7554/eLife.44702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Istvan E.S., Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 113.Klopfleisch S., Merkler D., Schmitz M., Kloppner S., Schedensack M., Jeserich G., Althaus H.H., Bruck W. Negative impact of statins on oligodendrocytes and myelin formation in vitro and in vivo. J. Neurosci. 2008;28:13609–13614. doi: 10.1523/JNEUROSCI.2765-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miron V.E., Zehntner S.P., Kuhlmann T., Ludwin S.K., Owens T., Kennedy T.E., Bedell B.J., Antel J.P. Statin therapy inhibits remyelination in the central nervous system. Am. J. Pathol. 2009;174:1880–1890. doi: 10.2353/ajpath.2009.080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Triplet E.M., Scarisbrick I.A. Statin use is associated with reduced motor recovery after spinal cord injury. Spinal Cord Ser. Cases. 2021;7:8. doi: 10.1038/s41394-020-00378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Geoffroy C.G., Hilton B.J., Tetzlaff W., Zheng B. Evidence for an age-dependent decline in axon regeneration in the adult mammalian central nervous system. Cell Rep. 2016;15:238–246. doi: 10.1016/j.celrep.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sutherland T.C., Geoffroy C.G. The influence of neuron-extrinsic factors and aging on injury progression and axonal repair in the central nervous system. Front. Cell Dev. Biol. 2020;8:190. doi: 10.3389/fcell.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cantuti-Castelvetri L., Fitzner D., Bosch-Queralt M., Weil M.T., Su M., Sen P., Ruhwedel T., Mitkovski M., Trendelenburg G., Lutjohann D., et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018;359:684–688. doi: 10.1126/science.aan4183. [DOI] [PubMed] [Google Scholar]
- 119.Cartoni R., Norsworthy M.W., Bei F., Wang C., Li S., Zhang Y., Gabel C.V., Schwarz T.L., He Z. The mammalian-specific protein armcx1 regulates mitochondrial transport during axon regeneration. Neuron. 2016;92:1294–1307. doi: 10.1016/j.neuron.2016.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gallo G. The bioenergetics of neuronal morphogenesis and regeneration: Frontiers beyond the mitochondrion. Dev. Neurobiol. 2020;80:263–276. doi: 10.1002/dneu.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han Q., Xie Y., Ordaz J.D., Huh A.J., Huang N., Wu W., Liu N., Chamberlain K.A., Sheng Z.H., Xu X.M. Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metab. 2020;31:623–641.e8. doi: 10.1016/j.cmet.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Han S.M., Baig H.S., Hammarlund M. Mitochondria localize to injured axons to support regeneration. Neuron. 2016;92:1308–1323. doi: 10.1016/j.neuron.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou B., Yu P., Lin M.Y., Sun T., Chen Y., Sheng Z.H. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J. Cell Biol. 2016;214:103–119. doi: 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schonfeld P., Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J. Cereb. Blood Flow Metab. 2013;33:1493–1499. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wojtczak L., Schonfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim. Biophys. Acta. 1993;1183:41–57. doi: 10.1016/0005-2728(93)90004-Y. [DOI] [PubMed] [Google Scholar]
- 126.Yang S.Y., He X.Y., Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J. Biol. Chem. 1987;262:13027–13032. doi: 10.1016/S0021-9258(18)45161-7. [DOI] [PubMed] [Google Scholar]
- 127.Ioannou M.S., Jackson J., Sheu S.H., Chang C.L., Weigel A.V., Liu H., Pasolli H.A., Xu C.S., Pang S., Matthies D., et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177:1522–1535.e14. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 128.Chaves-Filho A.B., Pinto I.F.D., Dantas L.S., Xavier A.M., Inague A., Faria R.L., Medeiros M.H.G., Glezer I., Yoshinaga M.Y., Miyamoto S. Alterations in lipid metabolism of spinal cord linked to amyotrophic lateral sclerosis. Sci. Rep. 2019;9:11642. doi: 10.1038/s41598-019-48059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jimenez-Riani M., Diaz-Amarilla P., Isasi E., Casanova G., Barbeito L., Olivera-Bravo S. Ultrastructural features of aberrant glial cells isolated from the spinal cord of paralytic rats expressing the amyotrophic lateral sclerosis-linked SOD1G93A mutation. Cell Tissue Res. 2017;370:391–401. doi: 10.1007/s00441-017-2681-1. [DOI] [PubMed] [Google Scholar]
- 130.Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev. Neurobiol. 2011;71:201–220. doi: 10.1002/dneu.20852. [DOI] [PubMed] [Google Scholar]
- 131.Jeanneteau F., Deinhardt K., Miyoshi G., Bennett A.M., Chao M.V. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat. Neurosci. 2010;13:1373–1379. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kiyoshi C., Tedeschi A. Axon growth and synaptic function: A balancing act for axonal regeneration and neuronal circuit formation in CNS trauma and disease. Dev. Neurobiol. 2020;80:277–301. doi: 10.1002/dneu.22780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barnes A.P., Lilley B.N., Pan Y.A., Plummer L.J., Powell A.W., Raines A.N., Sanes J.R., Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 134.Hardie D.G. AMPK: Positive and negative regulation, and its role in whole-body energy homeostasis. Curr. Opin. Cell Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 135.Shackelford D.B., Shaw R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shelly M., Cancedda L., Heilshorn S., Sumbre G., Poo M.M. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 137.Courchet J., Lewis T.L., Jr., Lee S., Courchet V., Liou D.Y., Aizawa S., Polleux F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153:1510–1525. doi: 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lizcano J.M., Goransson O., Toth R., Deak M., Morrice N.A., Boudeau J., Hawley S.A., Udd L., Makela T.P., Hardie D.G., et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kong G., Zhou L., Serger E., Palmisano I., De Virgiliis F., Hutson T.H., McLachlan E., Freiwald A., La Montanara P., Shkura K., et al. AMPK controls the axonal regenerative ability of dorsal root ganglia sensory neurons after spinal cord injury. Nat. Metab. 2020;2:918–933. doi: 10.1038/s42255-020-0252-3. [DOI] [PubMed] [Google Scholar]
- 140.Garcia D., Shaw R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell. 2017;66:789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ohtake Y., Sami A., Jiang X., Horiuchi M., Slattery K., Ma L., Smith G.M., Selzer M.E., Muramatsu S.I., Li S. Promoting axon regeneration in adult cns by targeting liver kinase B1. Mol. Ther. 2019;27:102–117. doi: 10.1016/j.ymthe.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Desvergne B., Michalik L., Wahli W. Transcriptional regulation of metabolism. Physiol. Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 143.Mouchiroud L., Eichner L.J., Shaw R.J., Auwerx J. Transcriptional coregulators: Fine-tuning metabolism. Cell Metab. 2014;20:26–40. doi: 10.1016/j.cmet.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chamberlain K.A., Sheng Z.H. Mechanisms for the maintenance and regulation of axonal energy supply. J. Neurosci. Res. 2019;97:897–913. doi: 10.1002/jnr.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Glater E.E., Megeath L.J., Stowers R.S., Schwarz T.L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hollenbeck P.J., Saxton W.M. The axonal transport of mitochondria. Pt 23J. Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin M.Y., Sheng Z.H. Regulation of mitochondrial transport in neurons. Exp. Cell Res. 2015;334:35–44. doi: 10.1016/j.yexcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saxton W.M., Hollenbeck P.J. The axonal transport of mitochondria. Pt 9J. Cell Sci. 2012;125:2095–2104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schwarz T.L. Mitochondrial trafficking in neurons. Cold. Spring Harb. Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baas P.W. Microtubule transport in the axon. Int. Rev. Cytol. 2002;212:41–62. doi: 10.1016/s0074-7696(01)12003-6. [DOI] [PubMed] [Google Scholar]
- 151.Kang J.S., Tian J.H., Pan P.Y., Zald P., Li C., Deng C., Sheng Z.H. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morris R.L., Hollenbeck P.J. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. Pt 3J. Cell Sci. 1993;104:917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- 153.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Taub D.G., Awal M.R., Gabel C.V. O-GlcNAc signaling orchestrates the regenerative response to neuronal injury in caenorhabditis elegans. Cell Rep. 2018;24:1931–1938.e3. doi: 10.1016/j.celrep.2018.07.078. [DOI] [PubMed] [Google Scholar]
- 155.Beirowski B., Babetto E., Golden J.P., Chen Y.J., Yang K., Gross R.W., Patti G.J., Milbrandt J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat. Neurosci. 2014;17:1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saab A.S., Tzvetavona I.D., Trevisiol A., Baltan S., Dibaj P., Kusch K., Mobius W., Goetze B., Jahn H.M., Huang W., et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron. 2016;91:119–132. doi: 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Babetto E., Wong K.M., Beirowski B. A glycolytic shift in Schwann cells supports injured axons. Nat. Neurosci. 2020;23:1215–1228. doi: 10.1038/s41593-020-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li F., Sami A., Noristani H.N., Slattery K., Qiu J., Groves T., Wang S., Veerasammy K., Chen Y.X., Morales J., et al. Glial metabolic rewiring promotes axon regeneration and functional recovery in the central nervous system. Cell Metab. 2020;32:767–785.e7. doi: 10.1016/j.cmet.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wenk M.R. The emerging field of lipidomics. Nat. Rev. Drug Discov. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 160.Spiegelman B.M., Flier J.S. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/S0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 161.Lowell B.B., Spiegelman B.M. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 162.Bagchi D.P., Forss I., Mandrup S., MacDougald O.A. SnapShot: Niche determines adipocyte character I. Cell Metab. 2018;27:266. doi: 10.1016/j.cmet.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cinti S. The adipose organ: Morphological perspectives of adipose tissues. Proc. Nutr. Soc. 2001;60:319–328. doi: 10.1079/PNS200192. [DOI] [PubMed] [Google Scholar]
- 164.Lass A., Zimmermann R., Oberer M., Zechner R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid. Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nielsen T.S., Jessen N., Jorgensen J.O., Moller N., Lund S. Dissecting adipose tissue lipolysis: Molecular regulation and implications for metabolic disease. J. Mol. Endocrinol. 2014;52:R199–R222. doi: 10.1530/JME-13-0277. [DOI] [PubMed] [Google Scholar]
- 166.Yu Y.H., Ginsberg H.N. Adipocyte signaling and lipid homeostasis: Sequelae of insulin-resistant adipose tissue. Circ. Res. 2005;96:1042–1052. doi: 10.1161/01.RES.0000165803.47776.38. [DOI] [PubMed] [Google Scholar]
- 167.Rousset S., Alves-Guerra M.C., Mozo J., Miroux B., Cassard-Doulcier A.M., Bouillaud F., Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl. 1):S130–S135. doi: 10.2337/diabetes.53.2007.S130. [DOI] [PubMed] [Google Scholar]
- 168.Chondronikola M., Volpi E., Borsheim E., Porter C., Saraf M.K., Annamalai P., Yfanti C., Chao T., Wong D., Shinoda K., et al. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab. 2016;23:1200–1206. doi: 10.1016/j.cmet.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Francois M., Qualls-Creekmore E., Berthoud H.R., Munzberg H., Yu S. Genetics-based manipulation of adipose tissue sympathetic innervation. Physiol. Behav. 2018;190:21–27. doi: 10.1016/j.physbeh.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang P., Loh K.H., Wu M., Morgan D.A., Schneeberger M., Yu X., Chi J., Kosse C., Kim D., Rahmouni K., et al. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature. 2020;583:839–844. doi: 10.1038/s41586-020-2527-y. [DOI] [PubMed] [Google Scholar]
- 171.Wolf Y., Boura-Halfon S., Cortese N., Haimon Z., Sar Shalom H., Kuperman Y., Kalchenko V., Brandis A., David E., Segal-Hayoun Y., et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 2017;18:665–674. doi: 10.1038/ni.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Eric Nyam T.T., Ho C.H., Chio C.C., Lim S.W., Wang J.J., Chang C.H., Kuo J.R., Wang C.C. Traumatic brain injury increases the risk of major adverse cardiovascular and cerebrovascular events: A 13-year, population-based study. World Neurosurg. 2019;122:e740–e753. doi: 10.1016/j.wneu.2018.10.130. [DOI] [PubMed] [Google Scholar]
- 173.Karelina K., Sarac B., Freeman L.M., Gaier K.R., Weil Z.M. Traumatic brain injury and obesity induce persistent central insulin resistance. Eur. J. Neurosci. 2016;43:1034–1043. doi: 10.1111/ejn.13194. [DOI] [PubMed] [Google Scholar]
- 174.Silveira S.L., Winter L.L., Clark R., Ledoux T., Robinson-Whelen S. Baseline dietary intake of individuals with spinal cord injury who are overweight or obese. J. Acad. Nutr. Diet. 2019;119:301–309. doi: 10.1016/j.jand.2018.08.153. [DOI] [PubMed] [Google Scholar]
- 175.Bartness T.J., Liu Y., Shrestha Y.B., Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol. 2014;35:473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dogiel A. Die sensiblen nervenendigungen im herzen und in den blutgefässen der säugethiere. Archiv. Für Mikroskopische Anatomie. 1898;52:44–70. doi: 10.1007/BF02976209. [DOI] [Google Scholar]
- 177.Correll J.W. Adipose tissue: Ability to respond to nerve stimulation in vitro. Science. 1963;140:387–388. doi: 10.1126/science.140.3565.387. [DOI] [PubMed] [Google Scholar]
- 178.Blaszkiewicz M., Willows J.W., Dubois A.L., Waible S., DiBello K., Lyons L.L., Johnson C.P., Paradie E., Banks N., Motyl K., et al. Neuropathy and neural plasticity in the subcutaneous white adipose depot. PLoS ONE. 2019;14:e0221766. doi: 10.1371/journal.pone.0221766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Foster M.T., Bartness T.J. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R1630–R1637. doi: 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- 180.Slavin B.G., Ballard K.W. Morphological studies on the adrenergic innervation of white adipose tissue. Anat. Rec. 1978;191:377–389. doi: 10.1002/ar.1091910310. [DOI] [PubMed] [Google Scholar]
- 181.Huesing C., Qualls-Creekmore E., Lee N., Francois M., Torres H., Zhang R., Burk D.H., Yu S., Morrison C.D., Berthoud H.R., et al. Sympathetic innervation of inguinal white adipose tissue in the mouse. J. Comp. Neurol. 2020 doi: 10.1002/cne.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wiedmann N.M., Stefanidis A., Oldfield B.J. Characterization of the central neural projections to brown, white, and beige adipose tissue. FASEB J. 2017;31:4879–4890. doi: 10.1096/fj.201700433R. [DOI] [PubMed] [Google Scholar]
- 183.Song C.K., Bartness T.J. CNS sympathetic outflow neurons to white fat that express MEL receptors may mediate seasonal adiposity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R666–R672. doi: 10.1152/ajpregu.2001.281.2.R666. [DOI] [PubMed] [Google Scholar]
- 184.Fishman R.B., Dark J. Sensory innervation of white adipose tissue. Pt 2Am. J. Physiol. 1987;253:R942–R944. doi: 10.1152/ajpregu.1987.253.6.R942. [DOI] [PubMed] [Google Scholar]
- 185.Song C.K., Schwartz G.J., Bartness T.J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R501–R511. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Caron A., Lee S., Elmquist J.K., Gautron L. Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 2018;19:153–165. doi: 10.1038/nrn.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 188.Murphy K.T., Schwartz G.J., Nguyen N.L., Mendez J.M., Ryu V., Bartness T.J. Leptin-sensitive sensory nerves innervate white fat. Am. J. Physiol. Endocrinol. Metab. 2013;304:E1338–E1347. doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bagchi D.P., MacDougald O.A. Identification and dissection of diverse mouse adipose depots. J. Vis. Exp. 2019 doi: 10.3791/59499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Foster D.O., Depocas F., Zaror-Behrens G. Unilaterality of the sympathetic innervation of each pad of rat interscapular brown adipose tissue. Can. J. Physiol. Pharmacol. 1982;60:107–113. doi: 10.1139/y82-018. [DOI] [PubMed] [Google Scholar]
- 191.Francois M., Torres H., Huesing C., Zhang R., Saurage C., Lee N., Qualls-Creekmore E., Yu S., Morrison C.D., Burk D., et al. Sympathetic innervation of the interscapular brown adipose tissue in mouse. Ann. N. Y. Acad. Sci. 2019;1454:3–13. doi: 10.1111/nyas.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Contreras C., Nogueiras R., Dieguez C., Rahmouni K., Lopez M. Traveling from the hypothalamus to the adipose tissue: The thermogenic pathway. Redox Biol. 2017;12:854–863. doi: 10.1016/j.redox.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Harlan S.M., Morgan D.A., Agassandian K., Guo D.F., Cassell M.D., Sigmund C.D., Mark A.L., Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ. Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guilherme A., Henriques F., Bedard A.H., Czech M.P. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2019;15:207–225. doi: 10.1038/s41574-019-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bauman W.A., Spungen A.M., Zhong Y.G., Mobbs C.V. Plasma leptin is directly related to body adiposity in subjects with spinal cord injury. Horm. Metab. Res. 1996;28:732–736. doi: 10.1055/s-2007-979889. [DOI] [PubMed] [Google Scholar]
- 196.Latifi S., Koushki D., Norouzi Javidan A., Matin M., Sabour H. Changes of leptin concentration in plasma in patients with spinal cord injury: A meta-analysis. Spinal Cord. 2013;51:728–731. doi: 10.1038/sc.2013.82. [DOI] [PubMed] [Google Scholar]
- 197.Wang L., Liu L., Pan Z., Zeng Y. Serum leptin, bone mineral density and the healing of long bone fractures in men with spinal cord injury. Bosn. J. Basic Med. Sci. 2015;15:69–74. doi: 10.17305/bjbms.2015.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang L., Tang X., Zhang H., Yuan J., Ding H., Wei Y. Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture. J. Spinal Cord Med. 2011;34:501–509. doi: 10.1179/2045772311Y.0000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Buchholz A.C., Pencharz P.B. Energy expenditure in chronic spinal cord injury. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:635–639. doi: 10.1097/00075197-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 200.Commins S.P., Watson P.M., Levin N., Beiler R.J., Gettys T.W. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different beta-adrenoceptor subtypes. J. Biol. Chem. 2000;275:33059–33067. doi: 10.1074/jbc.M006328200. [DOI] [PubMed] [Google Scholar]
- 201.Siegrist-Kaiser C.A., Pauli V., Juge-Aubry C.E., Boss O., Pernin A., Chin W.W., Cusin I., Rohner-Jeanrenaud F., Burger A.G., Zapf J., et al. Direct effects of leptin on brown and white adipose tissue. J. Clin. Investig. 1997;100:2858–2864. doi: 10.1172/JCI119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zeng W., Pirzgalska R.M., Pereira M.M., Kubasova N., Barateiro A., Seixas E., Lu Y.H., Kozlova A., Voss H., Martins G.G., et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163:84–94. doi: 10.1016/j.cell.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 203.Westgren N., Levi R. Quality of life and traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 1998;79:1433–1439. doi: 10.1016/S0003-9993(98)90240-4. [DOI] [PubMed] [Google Scholar]
- 204.Claydon V.E., Steeves J.D., Krassioukov A. Orthostatic hypotension following spinal cord injury: Understanding clinical pathophysiology. Spinal Cord. 2006;44:341–351. doi: 10.1038/sj.sc.3101855. [DOI] [PubMed] [Google Scholar]
- 205.Furlan J.C., Fehlings M.G. Cardiovascular complications after acute spinal cord injury: Pathophysiology, diagnosis, and management. Neurosurg. Focus. 2008;25:E13. doi: 10.3171/FOC.2008.25.11.E13. [DOI] [PubMed] [Google Scholar]
- 206.Krassioukov A.V., Bunge R.P., Pucket W.R., Bygrave M.A. The changes in human spinal sympathetic preganglionic neurons after spinal cord injury. Spinal Cord. 1999;37:6–13. doi: 10.1038/sj.sc.3100718. [DOI] [PubMed] [Google Scholar]
- 207.Gorgey A.S., Gater D.R., Jr. Prevalence of obesity after spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2007;12:1–7. doi: 10.1310/sci1204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Spungen A.M., Adkins R.H., Stewart C.A., Wang J., Pierson R.N., Jr., Waters R.L., Bauman W.A. Factors influencing body composition in persons with spinal cord injury: A cross-sectional study. J. Appl. Physiol. 2003;95:2398–2407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- 209.Gorgey A.S., Wells K.M., Austin T.L. Adiposity and spinal cord injury. World J. Orthop. 2015;6:567–576. doi: 10.5312/wjo.v6.i8.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jones L.M., Legge M., Goulding A. Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch. Phys. Med. Rehabil. 2003;84:1068–1071. doi: 10.1016/S0003-9993(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 211.Laughton G.E., Buchholz A.C., Martin Ginis K.A., Goy R.E., Group S.S.R. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47:757–762. doi: 10.1038/sc.2009.33. [DOI] [PubMed] [Google Scholar]
- 212.Yarar-Fisher C., Chen Y., Jackson A.B., Hunter G.R. Body mass index underestimates adiposity in women with spinal cord injury. Obesity. 2013;21:1223–1225. doi: 10.1002/oby.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gorgey A.S., Mather K.J., Gater D.R. Central adiposity associations to carbohydrate and lipid metabolism in individuals with complete motor spinal cord injury. Metabolism. 2011;60:843–851. doi: 10.1016/j.metabol.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 214.Modlesky C.M., Bickel C.S., Slade J.M., Meyer R.A., Cureton K.J., Dudley G.A. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J. Appl. Physiol. 2004;96:561–565. doi: 10.1152/japplphysiol.00207.2003. [DOI] [PubMed] [Google Scholar]
- 215.Gorgey A.S., Mather K.J., Poarch H.J., Gater D.R. Influence of motor complete spinal cord injury on visceral and subcutaneous adipose tissue measured by multi-axial magnetic resonance imaging. J. Spinal Cord Med. 2011;34:99–109. doi: 10.1179/107902610X12911165975106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Benova A., Tencerova M. Obesity-induced changes in bone marrow homeostasis. Front. Endocrinol. 2020;11:294. doi: 10.3389/fendo.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pierce J.L., Begun D.L., Westendorf J.J., McGee-Lawrence M.E. Defining osteoblast and adipocyte lineages in the bone marrow. Bone. 2019;118:2–7. doi: 10.1016/j.bone.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Boroumand P., Klip A. Bone marrow adipose cells—Cellular interactions and changes with obesity. J. Cell Sci. 2020;133 doi: 10.1242/jcs.238394. [DOI] [PubMed] [Google Scholar]
- 219.Fink L.N., Costford S.R., Lee Y.S., Jensen T.E., Bilan P.J., Oberbach A., Bluher M., Olefsky J.M., Sams A., Klip A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity. 2014;22:747–757. doi: 10.1002/oby.20615. [DOI] [PubMed] [Google Scholar]
- 220.Singer K., DelProposto J., Morris D.L., Zamarron B., Mergian T., Maley N., Cho K.W., Geletka L., Subbaiah P., Muir L., et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol. Metab. 2014;3:664–675. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gorgey A.S., Poarch H.J., Adler R.A., Khalil R.E., Gater D.R. Femoral bone marrow adiposity and cortical bone cross-sectional areas in men with motor complete spinal cord injury. PM R. 2013;5:939–948. doi: 10.1016/j.pmrj.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 222.de Groot S., Adriaansen J.J., Tepper M., Snoek G.J., van der Woude L.H., Post M.W. Metabolic syndrome in people with a long-standing spinal cord injury: Associations with physical activity and capacity. Appl. Physiol. Nutr. Metab. 2016;41:1190–1196. doi: 10.1139/apnm-2016-0269. [DOI] [PubMed] [Google Scholar]
- 223.Sauerbeck A.D., Laws J.L., Bandaru V.V., Popovich P.G., Haughey N.J., McTigue D.M. Spinal cord injury causes chronic liver pathology in rats. J. Neurotrauma. 2015;32:159–169. doi: 10.1089/neu.2014.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Alves-Bezerra M., Cohen D.E. Triglyceride metabolism in the liver. Compr. Physiol. 2017;8:1–8. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Walther T.C., Chung J., Farese R.V., Jr. Lipid droplet biogenesis. Annu. Rev. Cell Dev. Biol. 2017;33:491–510. doi: 10.1146/annurev-cellbio-100616-060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kumar M., Ojha S., Rai P., Joshi A., Kamat S.S., Mallik R. Insulin activates intracellular transport of lipid droplets to release triglycerides from the liver. J. Cell Biol. 2019;218:3697–3713. doi: 10.1083/jcb.201903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.La Fountaine M.F., Cirnigliaro C.M., Emmons R.R., Kirshblum S.C., Galea M., Spungen A.M., Bauman W.A. Lipoprotein heterogeneity in persons with Spinal Cord Injury: A model of prolonged sitting and restricted physical activity. Lipids Health Dis. 2015;14:81. doi: 10.1186/s12944-015-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rankin K.C., O’Brien L.C., Segal L., Khan M.R., Gorgey A.S. Liver Adiposity and Metabolic Profile in Individuals with Chronic Spinal Cord Injury. Biomed. Res. Int. 2017;2017:1364818. doi: 10.1155/2017/1364818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.van der Worp H.B., Howells D.W., Sena E.S., Porritt M.J., Rewell S., O’Collins V., Macleod M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Goodus M.T., McTigue D.M. Hepatic dysfunction after spinal cord injury: A vicious cycle of central and peripheral pathology? Exp. Neurol. 2020;325:113160. doi: 10.1016/j.expneurol.2019.113160. [DOI] [PubMed] [Google Scholar]
- 232.Popovich P.G., Wei P., Stokes B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997;377:443–464. doi: 10.1002/(SICI)1096-9861(19970120)377:3<443::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 233.Abella V., Scotece M., Conde J., Pino J., Gonzalez-Gay M.A., Gomez-Reino J.J., Mera A., Lago F., Gomez R., Gualillo O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017;13:100–109. doi: 10.1038/nrrheum.2016.209. [DOI] [PubMed] [Google Scholar]
- 234.Fernandez-Riejos P., Najib S., Santos-Alvarez J., Martin-Romero C., Perez-Perez A., Gonzalez-Yanes C., Sanchez-Margalet V. Role of leptin in the activation of immune cells. Mediators. Inflamm. 2010;2010:568343. doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Otero M., Lago R., Gomez R., Dieguez C., Lago F., Gomez-Reino J., Gualillo O. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology. 2006;45:944–950. doi: 10.1093/rheumatology/kel157. [DOI] [PubMed] [Google Scholar]
- 236.Hue L., Taegtmeyer H. The Randle cycle revisited: A new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kim J.K., Wi J.K., Youn J.H. Plasma free fatty acids decrease insulin-stimulated skeletal muscle glucose uptake by suppressing glycolysis in conscious rats. Diabetes. 1996;45:446–453. doi: 10.2337/diab.45.4.446. [DOI] [PubMed] [Google Scholar]
- 238.Martins A.R., Nachbar R.T., Gorjao R., Vinolo M.A., Festuccia W.T., Lambertucci R.H., Cury-Boaventura M.F., Silveira L.R., Curi R., Hirabara S.M. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: Importance of the mitochondrial function. Lipids Health Dis. 2012;11:30. doi: 10.1186/1476-511X-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Duckworth W.C., Solomon S.S., Jallepalli P., Heckemeyer C., Finnern J., Powers A. Glucose intolerance due to insulin resistance in patients with spinal cord injuries. Diabetes. 1980;29:906–910. doi: 10.2337/diab.29.11.906. [DOI] [PubMed] [Google Scholar]
- 240.Bauman W.A., Spungen A.M. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: A model of premature aging. Metabolism. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 241.Libin A., Tinsley E.A., Nash M.S., Mendez A.J., Burns P., Elrod M., Hamm L.F., Groah S.L. Cardiometabolic risk clustering in spinal cord injury: Results of exploratory factor analysis. Top. Spinal Cord Inj. Rehabil. 2013;19:183–194. doi: 10.1310/sci1903-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Selassie A., Snipe L., Focht K.L., Welldaregay W. Baseline prevalence of heart diseases, hypertension, diabetes, and obesity in persons with acute traumatic spinal cord injury: Potential threats in the recovery trajectory. Top. Spinal Cord Inj. Rehabil. 2013;19:172–182. doi: 10.1310/sci1903-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Soden R.J., Walsh J., Middleton J.W., Craven M.L., Rutkowski S.B., Yeo J.D. Causes of death after spinal cord injury. Spinal Cord. 2000;38:604–610. doi: 10.1038/sj.sc.3101080. [DOI] [PubMed] [Google Scholar]
- 244.Flank P., Fahlstrom M., Bostrom C., Lewis J.E., Levi R., Wahman K. Self-reported physical activity and risk markers for cardiovascular disease after spinal cord injury. J. Rehabil. Med. 2014;46:886–890. doi: 10.2340/16501977-1857. [DOI] [PubMed] [Google Scholar]
- 245.Angin Y., Steinbusch L.K., Simons P.J., Greulich S., Hoebers N.T., Douma K., van Zandvoort M.A., Coumans W.A., Wijnen W., Diamant M., et al. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Biochem. J. 2012;448:43–53. doi: 10.1042/BJ20120060. [DOI] [PubMed] [Google Scholar]
- 246.Greenwalt D.E., Scheck S.H., Rhinehart-Jones T. Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. J. Clin. Investig. 1995;96:1382–1388. doi: 10.1172/JCI118173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Neubauer S. The failing heart--an engine out of fuel. N. Engl. J. Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 248.Fillmore N., Mori J., Lopaschuk G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 2014;171:2080–2090. doi: 10.1111/bph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Petrova V., Nieuwenhuis B., Fawcett J.W., Eva R. Axonal organelles as molecular platforms for axon growth and regeneration after injury. Int. J. Mol. Sci. 2021;22:1798. doi: 10.3390/ijms22041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Passmore J.B., Nijenhuis W., Kapitein L.C. From observing to controlling: Inducible control of organelle dynamics and interactions. Curr. Opin. Cell Biol. 2021;71:69–76. doi: 10.1016/j.ceb.2021.02.002. [DOI] [PubMed] [Google Scholar]