Abstract
Coenzyme Q10 (CoQ10) is an essential cofactor in oxidative phosphorylation (OXPHOS), present in mitochondria and cell membranes in reduced and oxidized forms. Acting as an energy transfer molecule, it occurs in particularly high levels in the liver, heart, and kidneys. CoQ10 is also an anti-inflammatory and antioxidant agent able to prevent the damage induced by free radicals and the activation of inflammatory signaling pathways. In this context, several studies have shown the possible inverse correlation between the blood levels of CoQ10 and some disease conditions. Interestingly, beyond cardiovascular diseases, CoQ10 is involved also in neuronal and muscular degenerative diseases, in migraine and in cancer; therefore, the supplementation with CoQ10 could represent a viable option to prevent these and in some cases might be used as an adjuvant to conventional treatments. This review is aimed to summarize the clinical applications regarding the use of CoQ10 in migraine, neurodegenerative diseases (including Parkinson and Alzheimer diseases), cancer, or degenerative muscle disorders (such as multiple sclerosis and chronic fatigue syndrome), analyzing its effect on patients’ health and quality of life.
Keywords: coenzyme Q10, ubiquinone, neuronal and muscular degenerative disorders, cancer, prevention, supplementation
1. Introduction
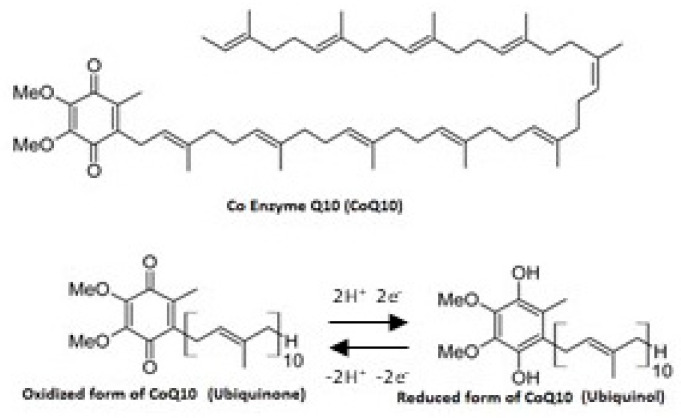
Coenzyme Q10 (CoQ10) is an organic molecule similar to a vitamin and it can even be synthesized by human cells. It consists of a benzoquinone group with a poly-isoprenoid side chain (10 units in humans) and it is generally present in cell membranes and especially in the mitochondria in both reduced (ubiquinol) and oxidized (ubiquinone) forms (Figure 1) [1]. The levels of CoQ10 are particularly high in the liver, kidney, and heart, organs with high metabolic activity (Table 1) [2]. Indeed, CoQ10 plays a key role in supplying energy to all cells and, especially, taking part in redox reactions within the electron transport chain at the mitochondrial level. In fact, this molecule is an excellent electron carrier able to support continuous oxidation-reduction cycles. Specifically, CoQ10 facilitates the production of adenosine triphosphate (ATP), carrying the electrons from complexes I and II to complex III of the mitochondrial respiratory chain [3,4]. In addition, CoQ10 is the major lipid-soluble antioxidant and it protects cell membranes and lipoproteins from oxidative damage [3]. The antioxidant activity of CoQ10 is linked to its reduced form, the ubiquinol, able to reduce oxidative stress, lipid peroxidation, and regenerate also vitamins C and E back to their active, fully reduced forms [5]. Last but not least, some in vitro studies have shown the ability of CoQ10 to reduce the inflammatory markers, suggesting that this molecule might have anti-inflammatory action through the regulation of gene expression [6,7]. However, some factors, such as aging, drugs (e.g., statins), genetic factors, neurodegenerative diseases, and degenerative muscle disorders, are well-known to be associated with reduced plasma concentrations of CoQ10 [5], resulting in exacerbation of oxidative stress and inflammatory processes. This takes place through the upregulation of nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) gene expression and chronic activation of immune inflammatory responses [8].
Figure 1.
Chemical structure of CoQ10.
Table 1.
Ubiquinone and ubiquinol distribution in human tissues.
| Organ | Ubiquinone Concentration (µg/g) |
Ubiquinol Concentration (µg/g) |
References |
|---|---|---|---|
| Heart | 132.0 | 61.0 | [14,15] |
| Kidneys | 77.0 | 75.0 | |
| Liver | 63.6 | 95.0 | |
| Muscle | 39.7 | 65.0 | |
| Brain | 13.4 | 23.0 | |
| Pancreas | 32.7 | ||
| Spleen | 24.6 | ||
| Lung | 7.9 | 25.0 | |
| Thyroid | 24.7 | ||
| Testis | 10.5 | ||
| Intestine | 11.5 | 95.0 | |
| Colon | 10.7 | ||
| Ventricle | 11.8 | ||
| Plasma (µmol/mL) | 1.1 | 96.0 |
The largest percentage CoQ10 is synthetized in the cell (4-hydroxybenzoate is the precursor of a quinone ring, while an isoprenoid tail is derived from the mevalonate pathway), although the pathways involved are not yet completely known. It is a complex multistage process (governed by at least 13 genes), requiring a number of vitamins, amino acids, trace element precursors, and cofactors, and a deficiency of any of which can adversely affect the normal CoQ10 production [9,10].
Furthermore, CoQ10 can be derived from the diet (about 5 mg/day for a Mediterranean diet). In particular it is present in fatty fishes, soja, nuts, and spinach [11], however, its intake may not be sufficient to counteract physiological or pathological deficiencies [12]. For this reason, nutritional supplementation with this nutraceutical could help to maintain adequate levels within the body.
For its important role in the organ function, a deficit of plasma levels of CoQ10 is associated with many degenerative states and several diseases such as cardiovascular and cerebrovascular diseases (e.g., heart failure, myocardial infarction, migraine, chronic kidney disease, and hypertension), Alzheimer (AD) and Parkinson diseases (PD), muscular dystrophy, and others [13]. However, data concerning CoQ10 supplementation beyond the cardiovascular field is still limited and often conflicting. Therefore, this review aims to discuss the potential applications of CoQ10 in various conditions with an analysis of its impact on patients’ health and quality of life.
2. Bioavailability of CoQ10
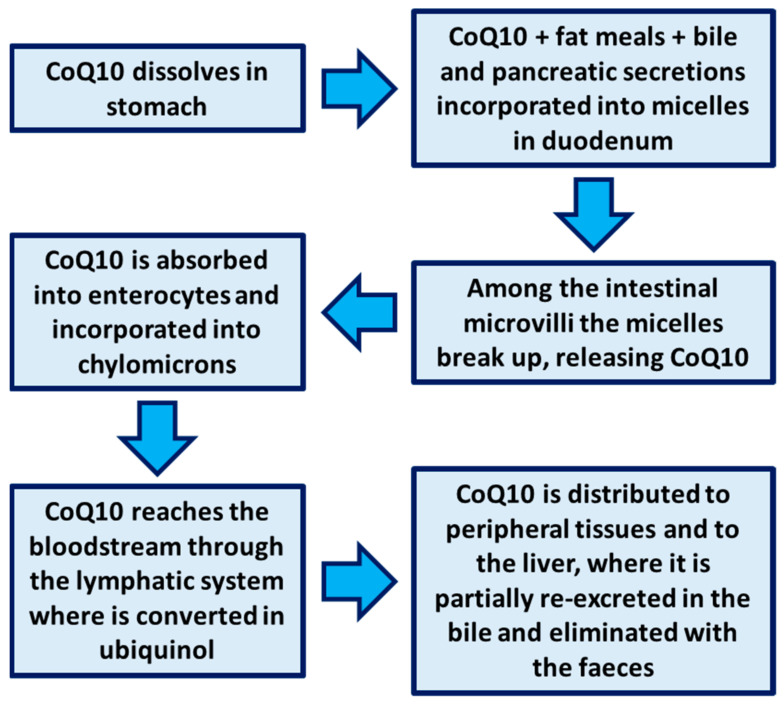
CoQ10 has a very low and variable oral bioavailability, depending on many factors such as the dosage of administration, the type of formulation, the release method, and the mode of administration (e.g., before, during or after meals) [16]. Indeed, if administered in fed state, CoQ10 arrives in the intestinal lumen with exogenous cholesterol, then is taken up from the mixed micelles, together with meal fats, bile, and pancreatic secretions, which facilitate its solubilization and the entrance into enterocytes via the “simple passive facilitated diffusion” (“passive” because energy consumption is not required and “facilitated” because a lipid carrier, usually a monoglyceride fat, makes possible the intestinal transport). CoQ10, incorporated in the chylomicrons in the enterocytes, subsequently reaches the blood through the lymphatic system. From the bloodstream, CoQ10 is distributed to peripheral tissues and to the liver, where it is partially re-excreted in the bile and eliminated with the feces (Figure 2) [17]. The main limiting factor of low bioavailability of CoQ10 (molecular weight = 863) is its poor solubility in gastrointestinal fluids. For this reason, various formulations have been proposed in the market in the form of tablets, capsules, chewable tablets, and gels containing oily suspensions to improve CoQ10 bioaccessibility and bioavailability. The reduction of particle size (e.g., the use of nanoparticles) and the setup of biopharmaceutical strategies like the use of the β-cyclodextrin complex, liposomes, emulsions, nanostructured lipid carriers, and micelles have already been demonstrated to improve the CoQ10 bioavailability with satisfactory results [18,19].
Figure 2.
Coenzyme Q10 physiology.
3. Methods
The literature search has been performed using the most relevant databases recognized for medical scientific literature, including PubMed, MEDLINE (National Library of Medicine, Bethesda, Maryland, MD, USA; January 1970 to February 2021), and the Cochrane Register of Controlled Trials (The Cochrane Collaboration, Oxford, UK) with access to Scopus, EMBASE and ClinicalTrials.gov. The terms used for the electronic search strategy were “coenzyme Q10,” “ubiquinol,” “ubiquinone,” “dietary supplement,” “human,” and “clinical trial.” The eligible papers must be published in English. The selected references were further screened for applications beyond the cardiovascular field. Randomized clinical trials were preferred and included wherever possible, although in some minor conditions open-label studies were considered due to the lack of controlled studies. The work is characterized by a first general introduction regarding the pharmacodynamic profile and the properties of CoQ10 followed by a brief description of the mechanism of action for each potential therapeutic area, the effects observed in clinical studies, and the respective tolerability notes. The Declaration of Interest forms related to real or potential sources of conflicts of interest were compiled by the drafting and review groups.
4. Results
4.1. CoQ10 and Migraine
Migraine shows a prevalence of 11.6% in the world. Acute attacks of unilateral throbbing headache (lasting for 4–72 h), photophobia, phonophobia, osmophobia, nausea, vomiting, cranial allodynia, and movement sensitivity are more frequently referred and, inevitably, associated to worsening of life quality. Of note, a deficiency of CoQ10 is associated with the pathogenesis of migraine, especially in the pediatric and adolescent populations. Indeed the American Academy of Neurology guidelines recommended a supplementation with CoQ10 in migraine prevention (level of evidence C) [20].
In this regard, a randomized double-blind placebo controlled clinical trial, carried out on 45 patients enrolled in two arms: 22 treated with placebo and 23 treated with CoQ10 (400 mg/day), highlighted a significant prophilactic effect of the supplementation on migraine attacks after 3 months. Interestingly, less severe, shorter, and less frequent attacks were reported. Moreover, higher levels of CoQ10 and lower levels of tumor necrosis factor α (TNFα) and calcitonin gene-related peptide (GCPR) were measured in the serum of patients enrolled in the CoQ10-arm, suggesting an effect through the mitigation of inflammatory processes [21].
Such results have been confirmed in a systematic review and meta-analysis in which four randomized clinical trials were analyzed. CoQ10 supplementation significantly reduced the frequency of migraine attacks, although no significant effect on severity and duration has been reported [22]. Unlike, other studies observed a not significant reduction of frequency and severity of attacks; however, a considerable heterogeneity of the dose of CoQ10 and the clinical condition of patients could represent an important limitation [23].
Encouraging results emerge when CoQ10 (400 mg/day) is associated with other nutraceuticals typically used for the prophylaxis of migraine, such as curcumin, magnesium, and Tanacetum parthenium L. or riboflavin [24,25].
4.2. CoQ10 and Fatigue
Fatigue is a generic term which refers to multiple aspects of human physiology. Acute fatigue has been described as “reversible motor weakness and whole-body tiredness that were predominantly brought on by muscular exertion and was relieved by rest.” Chronic fatigue is a consequence of acute fatigue accumulation, and in some cases, it may be irreversible [26]. Another classification of fatigue concerns “physical” and “mental” fatigue [27]. Moreover, it can also be felt as a pervasive sensation in several diseases such as fibromyalgia, cancer, chronic fatigue syndrome (CFS), anemia, human immunodeficiency virus (HIV), and multiple sclerosis (MS) [28]. Patients reported a lack of energy to perform their daily work and fatigue during rest [29]. Considering this, CoQ10 could be a valid nutraceutical supplement for its multiple functions, starting from the key role in energy metabolism, but also for its antioxidant properties [30]. In general, as highlighted by the meta-analysis of RCTs by Mehrabani et al., the therapeutic effects of CoQ10 were better in patients with statin-related fatigue and fibromyalgia when compared with the other disease-related fatigue [28]. However, more clinical trials with sufficient follow-up periods and with adequate sample sizes are required. The potential use of CoQ10 in the treatment of different aspects of fatigue is described below.
4.2.1. CoQ10 in Patients with Chronic Fatigue Syndrome
CFS is a severe, complex, and highly weakening chronic condition. Today its causes are unknown, as well as diagnostic tests and effective treatments, currently, are focused on the control of disease symptoms. It is characterized by protracted, debilitating, and relapsing fatigue, often accompanied by other symptoms, with significant disability for at least 6 months and often for years [31]. Recent studies revealed that oxidative stress and mitochondrial dysfunction can be associated with its pathogenesis and a reduced rate of ATP synthesis has been reported [32,33,34]. Moreover, clinical reports advise an important role for mitochondrial dysfunction-dependent events and oxidative damage at the cellular level [35]. As CoQ10 and nicotinamide adenine dinucleotide (NADH) increase cellular ATP production through mitochondrial OXPHOS, their supplementation in fatigue, as well as in other symptoms in CFS, is considered a new alternative and complementary therapy [36,37].
Castro-Marrero’s group designed a clinical trial on 73 Spanish subjects (NCT02063126) in which a significant fatigue improvement, measured as decrease in fatigue impact scale (FIS) total score, was observed in the group treated with CoQ10 (200 mg/day) + NADH (20 mg/day) versus placebo. Moreover, a recovery of the biochemical parameters was also reported. In particular, CoQ10, NAD+/NADH ratio, citrate synthase activity, and ATP levels were significantly improved, and the cellular indicators of oxidative stress lipoperoxides, were markedly reduced in mononuclear blood cells of the treated group. Then, the combination of CoQ10 + NADH caused a significant reduction in fatigue, oxidative damage, enhancement of mitochondrial function and improvement of energy [34], leading to the possibility that oral supplementation with CoQ10 + NADH could be beneficial to treat fatigue and act on biochemical parameters in CFS.
More recently, the same authors carried out a further RCT on 80 CFS patients, showing that CoQ10 + NADH supplementation was well tolerated and safe and reduced maximum heart rate significantly at week 8 versus baseline during a cycle ergometer test, supposing that at the basis of this there was an improvement of the endothelial function. Perception of fatigue also revealed a decrease during all follow-up visits in active group versus placebo, but no change in pain and sleep was observed [38].
Finally, Fukuda et al. did not support the efficacy of CoQ10 supplementation (150 mg/day) for 8 weeks in patients with CFS; indeed, no improvement of clinical symptoms was observed. However, the authors reported that a 12-weeks treatment with CoQ10 (150 mg/day) was beneficial for improving sleep quality and autonomic nervous dysfunction as well as better performance on the arithmetic task when compared with the placebo group. Interestingly, the decreases in fatigue levels and depression level were strictly correlated with the increases in ubiquinol concentrations [39].
4.2.2. CoQ10 in Patients with Fibromyalgia
The etiology of fibromyalgia is described by different pathophysiological processes such as bioenergetics alteration, mitochondrial dysfunction, oxidative stress, and inflammatory cascades.
AMP-activated protein kinase (AMPK) plays a key regulatory function in all cascades [40,41]. AMPK is an enzyme that contributes to keeping correct cellular energy homeostasis and is down regulated in fibromyalgia patients [42,43]. AMPK activation was induced by CoQ10, resulting in an improvement in clinical symptoms in fibromyalgia patients [44,45].
Four RCTs and one quasi-experimental study have examined the effect of CoQ10 supplementation in people with fibromyalgia. A quasi-experimental study conducted by Cordero et al. 2013 on 35 females treated with CoQ10 at a dose of 300 mg/day or placebo for 3 months showed a significant reduction in fibromyalgia fatigue (assessed by Fibromyalgia Impact Questionnaire (FIQ) and Visual Analogue Scale (VAS) [46]. Similar results were obtained by the same research group in a RCT of 20 female patients treated for 40 days with 300 mg/day of CoQ10 (Cordero et al., 2013). Even Miyamae et al. showed an improvement of juvenile fibromyalgia in patients after 3 months of treatment with 100 mg/day ubiquinol (Chalder’s Fatigue Scale significantly reduced if compared to the control) [47]. Finally, significant improvements in FIQ and VAS were obtained in two other studies including female patients with fibromyalgia (dosages of CoQ10 300–400 mg/day) [40,41].
4.2.3. CoQ10 in Patients with Statin-Associated Myopathy
The mechanisms involved in statin-associated myopathy are still unclear, but presumably involve an increase in intracellular lipid production and lipid myopathy and myocellular phytosterols, decrease in sarcolemmal cholesterol, reduction in mitochondrial CoQ10 and in small guanosine triphosphate-binding proteins [48]. Statin drugs act by inhibiting hydroxyl-methylglutaryl coenzyme A (HMG-CoA) reductase. This enzyme controls not only the synthesis of cholesterol but also the synthesis of farnesyl pyrophosphate, that is necessary for CoQ10 production and could explain the link between statins and CoQ10 deficit [49].
Only two clinical trials have examined the impact of CoQ10 supplementation against fatigue in statin-associated myopathy patients. Fedacko et al. highlighted a marked improvement of VAS in patients with statin-associated myopathy treated with 200 mg/day CoQ10 for 3 months compared to the control [50]. Another study conducted on 50 patients who followed a discontinuous therapy with statin and supplemented with 240 mg/day CoQ10 for 22 months, showed that the incidence of fatigue decreased from 84%, observed at the beginning of the treatment, to 16% at the end of the study [45].
4.2.4. CoQ10 and Fatigue in Healthy Volunteers
The action CoQ10 supplementation had on fatigue was examined in healthy subjects in 4 clinical trials. In the RCT by Mizuno et al., 17 healthy volunteers were randomized to receive CoQ10 at a dosage of 100 or 300 mg/day or placebo during 8 days of activity-induced fatigue. The measurement of subjective fatigue was performed through VAS. The analysis carried out with this tool revealed a significant fatigue reduction in the group treated with the highest dosage of CoQ10 (300 mg/day) when compared with the control group [27]. However, in 3 other studies regarding on 16 soccer players [51], 51 obese subjects [52] and 15 sedentary man [53], CoQ10 administration (100–300 mg/day for period of 1 to 3 months) failed to show any beneficial effects on fatigue reduction during exercise or perceived fatigue. One potential motive for the inefficiency of CoQ10 supplementation against fatigue in healthy subjects might be due to the baseline and change percentage of CoQ10 plasma levels. In particular, to increase the plasma ubiquinol level and exert a beneficial effect on fatigue, at least 300 mg/day of CoQ10, seems to be necessary [27,54].
4.2.5. CoQ10 and Elite Athletes
Athletes subjected to repeated intense physical training are more susceptible to a condition of chronic fatigue, including overtraining syndrome and infectious diseases, thus compromising their performance. In fact, it is well-known how intense exercise stimulates inflammatory responses, moreover reactive oxygen species (ROS) increases, leading to oxidative stress [55]. Inflammation and oxidative stress can contribute to overtraining syndrome and chronic fatigue in athletes. Moreover, it is well-known that intensive physical exercise may also cause muscular injury [56].
Therefore, the use of antioxidant supplementations is considered as a strategy to strengthen the defenses during training and competition.
Several studies have found that, after exercise in trained athletes and untrained individuals, supplementation with CoQ10 improved the aerobic power, anaerobic threshold, exercise performance, and/or recovery [57]. Conversely, other studies have shown no ergogenic benefit in untrained and trained individuals on maximal or submaximal exercise capacity [58].
In a 22-days double-blind study, 120 mg/day of CoQ10 also increased the anaerobic work, but no differences was reported between groups in running maximal oxygen consumption (VO2max) or heart rate and rate of perceived exertion during submaximal work rates. However, the CoQ10 group showed significantly higher levels of creatine kinase activity, possibly for increasing free radical formation. Therefore, the authors of the paper do not recommend the use of CoQ10 in the athletes [59].
On the other hand, a blinded study demonstrated that 18 male adolescent swimming athletes supplemented for 28 days with CoQ10 (100 mg/day), showed significantly elevated levels of it in the blood and a positive correlation with VO2max [60]. Similar results have been obtained on endurance athletes (male road cyclists and triathletes) [61].
Greenwood et al., observed that a supplementation for 2 weeks with 200 mg/day of CoQ10 increased the total CoQ10 plasma concentration. Moreover, acute supplementation with CoQ10 resulted in higher CoQ10 concentration in skeletal muscles and lower serum oxidative stress during and following exercise; this effect was probably due to a combination of enhanced OXPHOS within the mitochondria and/or enhanced antioxidant protection [30].
Kendo exercise is also highly intense and then may cause an increase in oxidative stress and cellular damage [62,63]. Interestingly, 300 mg/day of CoQ10 supplementation, for 2 weeks, in 18 elite athletes enrolled in a randomized clinical trial, showed increased CoQ10 levels in the blood, lower creatine kinase activity and myoglobin concentration when compared with the placebo group, suggesting that it could be useful for reducing exercise-induced muscle damage [63].
Recently, a double-blind 2-weeks long clinical trial carried out on 18 male elite kendo athletes demonstrated that 300 mg/day of CoQ10 contributed to down-regulate the expression of Toll-like receptor 4 (TLR4), considered as an event downstream the ROS production and responsible for immune system regulation. Therefore, in athletes undergoing intensive exercise, continuous CoQ10 supplementation is recommended 14 weeks before starting the training in order to inhibit exercise-induced inflammation and immune deficiency [64].
Moreover, 14-day CoQ10 supplementation (5 mg/Kg/day), significantly improved antioxidant and anti-inflammatory defenses following training such as running (i.e., competitive 3000 m) [65].
Furthermore, a previous study on 25 skiers highlighted higher levels of CoQ10 in the blood of athletes treated with this supplementation after 6 weeks of training [54].
Very recently, Emami et al., reported that in 36 healthy elite swimmers, CoQ10 supplementation prevented changes in mediators of inflammatory cytokines following heavy swimming trainings and acute recording bout [66].
4.2.6. CoQ10 in Patients with Other Fatigue-Related Diseases
Patients awaiting cardiac transplantation with end-stage heart failure experienced fatigue in carrying out daily activities, such as dressing or teeth brushing. The treatment for 3 months with CoQ10 at a dose of 60 mg/day has been reported to significantly reduce fatigue symptoms [67].
Nevertheless, the RCT by Peel et al., showed that the administration of 100 mg/day CoQ10 for 2 months failed to alleviate fatigue in the late-onset sequelae of 101 patients with poliomyelitis [68]. Similarly, in the last RCT, CoQ10 supplementation did not show any significant efficacy in fatigue reduction on newly diagnosed patients (236 females) with breast cancer [69].
4.3. CoQ10 and Neurodegenerative Diseases
4.3.1. CoQ10 and Parkinson Disease
The neurodegenerative diseases (ND) are related to a persistence of neuroinflammation processes and free-radicals generation which leads to cellular oxidative stress mainly due to mitochondrial dysfunction. In this panorama, the recognized role of CoQ10 as an antioxidant agent endowed with anti-inflammatory properties has been suggested in last years as a promising strategy to be associated with the therapy of ND [5]. Concerning PD, in in vitro and in vivo pre-clinical studies, CoQ10 demonstrated a neuroprotective activity on nigrostriatal dopaminergic neurons [70], while clinical evidences highlighted low levels of CoQ10 in mitochondria of parkinsonian patients [71,72]. On this basis, several clinical trials have been carried out on CoQ10 supplementation in PD. Müller et al., in a placebo-controlled double-blind trial on 28 patients treated for four weeks with 360 mg/day of CoQ10, observed a significant mild symptomatic benefit in CoQ10-treated patients compared with placebo-treated parkinsonian patients [73]. In contrast, Shults and colleagues, in a 16-months-long randomized, placebo-controlled, double-blind trial on 80 patients receiving several doses of CoQ10 (300, 600, and 1200 mg/day), found that a supplementation of CoQ10 1200 mg once a day was able to reduce the worsening in early PD, with great improvements in patients daily routine activities such as feeding, bathing, or dressing. Moreover, the same trial recorded also an improvement in NADH-cytochrome c reductase activity and an increase in CoQ10 plasmatic levels [74]. High doses were reported also by Horstink et al., in an open label trial on a limited number of patients (n = 12) who received 1000 mg/day of CoQ10 for three months and then 1500 mg/day of CoQ10 for other three months. At the end of the trial, lower but statistically significant benefits on motor performance in patients who received 1500 mg/day vs. placebo were recorded [75]. In order to assess tolerability of high doses, in 2004, Shults et al., performed an open label study on 17 patients who received growing CoQ10 dosages from 1200 mg to 3000 mg/day over 8 weeks. The results were that 13 of those patients tolerated the highest dose but their plasma levels reached a plateau at 2400 mg/day, indicating the futility to administer higher doses [76]. At the same time, a multicenter, randomized, placebo-controlled, double-blind study was carried out by Storch and colleagues to establish if the CoQ10 effects on PD may be neuroprotective or symptomatic. For this purpose, 106 parkinsonian patients with no motor complications and stratified by treatment with levodopa, received 300 mg/day of CoQ10 for three months. The study had, as its primary endpoint, changes in Unified Parkinson’s Diseases Rating Scale (UPDRS), but despite that serum levels of CoQ10 were increased in all patients in a way similar to the administration of 1200 mg/day performed by Shults et al., in 2002, no significant changes in UPDRS were observed, suggesting that in a mid-stage of PD, the CoQ10 supplementation does not induce symptomatic effects [77]. On the basis of these studies, more recently, the Parkinson Study Group QE3 investigators carried out a phase III RCT on 600 patients from North America, who received placebo, 1200 mg/day or 2400 mg/day of CoQ10, as well as all the patients received 1200 IU/day of vitamin E (vit E). After 16 months of treatment, the primary outcome was the total UPDRS score was analyzed but no significant differences were found between patients treated with placebo or the two doses of CoQ10 [78]. A following interesting study focuses attention on the preclinical models which seem to suggest that the reduced form of CoQ10 (ubiquinol-10) was more effective in a mouse model of PD than the oxidized form of CoQ10. Moreover, higher plasma concentrations of CoQ10 have been recorded after ingestion of the reduced form vs. the oxidized form [79]. According to this hypothesis, a randomized, double-blind, placebo-controlled, parallel-group pilot trial was carried out on Japanese patients to assess the efficacy of 300 mg/day of ubiquinol-10 or placebo for 48 weeks in patients experiencing wearing off, or 96 weeks in early PD patients not treated with levodopa. Results indicated that ubiquinol-10 induced a significant decrease in UPDR scores, showing the ability to improve PD patients experiencing wearing off, but it was not able to induce an improvement in early PD patients [80].
4.3.2. CoQ10 and Huntington Disease
The history of research about a possible role of CoQ10 in Huntington disease (HD) therapy/supplementation started with a small pilot trial in 1996 on 10 patients. During these 6 months long open-label trial, patients receiving from 600 to 1200 mg/day of CoQ10 were evaluated according to the HD Rating Scale (HDRS), the HD Functional Capacity Scale (HDFCS), and standardized neuropsychological measures. The study highlighted a good tolerability of CoQ10, but no significant improvement of HD according to the above clinical scores was recorded. A wider study, the CARE-HD trial, was carried out by the Huntington Study Group on 174 patients with early HD. During this randomized, placebo-controlled, multicenter, double-blind trial, the patients received CoQ10 300 mg twice daily or remacemide 600 mg daily for 30 months, but no significant changes in total functional capacity (TFC) were observed both for CoQ10 and for remacemide treated patients [81]. More recently, Mc Garry and colleagues performed a multicenter randomized, double-blind, placebo-controlled trial on 609 early HD patients from the United States, Canada, and Australia. HD patients received placebo or CoQ10 2400 mg/day for 60 months. Variations in total functional capacity score from baseline to 60th (for patients who survived), combined with time to death (for patients who died), was used as the primary outcome variable in using a joint-rank analysis approach. Even in this study no significant changes in TFC were recorded, leading the authors to assert that on the bases of their results, use of CoQ10 is not justified in a therapy focused on the slowing down of functional decline in HD [82].
4.3.3. CoQ10 and Alzheimer Disease
Data about a possible role of CoQ10 in the treatment of AD is still lacking and the only clinical trial reported about this topic is a double-blind, placebo-controlled trail, carried out on 78 patients with mild/moderate AD. The patients were randomized into three groups receiving a 16-week long treatment with 400 mg of CoQ10 three times/day or an anti-oxidant cocktail composed of 800 IU/day of vit E + 500 mg/day of vitamin C + 900 mg/day of α-lipoic acid (E/C/ALA) or placebo. The main outcomes were represented by changes from the baseline values of cerebrospinal fluids (CSF) biomarkers related to AD and oxidative stress, of cognitive parameters (through the Mini-mental state examination, MMSE) and functional ability (through the Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale). The results of the study reported that CSF F2-isoprostane levels and oxidative stress biomarkers decreased by about 19% in the E/C/ALA group but at the same time an accelerated decline in MMSE scores was recorded for the same E/C/ALA group, while no significant changes occurred in the CoQ10 group both for CSF biomarkers and in MMSE scores. Finally, no significant changes in CSF Aβ42, tau, and P-tau (181) levels were observed in all the three groups [83].
4.3.4. CoQ10 and MS
A degenerative condition in which fatigue is a typical symptom, besides depression, is MS [84]. MS-related fatigue appears when heat intensifies during daytime [85]. Although the exact etiology of fatigue is still unknown, multifactorial causes such as endocrine and autoimmune disorders are involved. Furthermore, there is no connection with the degree of impairment of the nervous system, disability, or MS [86]. In particular, inflammatory processes seem to be involved in these symptoms; indeed, pro-inflammatory cytokines, such as TNF-α, are associated with anorexia, weight loss, locomotors retardation, anxiety, and decreased social exploration [87]. On the other hand, major depression has a strong relationship with inflammation, showing an increase also in oxidative stress markers such as malondialdehyde (MDA) [88]. Regarding a possible role played by CoQ10, a unique paper has been published in 2015 by Sanoobar and colleagues. They carried out a randomized, double-blind, placebo-controlled clinical trial on 48 patients with relapsing-remitting MS in order to evaluate the effect of a CoQ10 supplementation (500 mg/day). After 12 weeks, depression and fatigue were improved. In particular, fatigue was measured by FSS and depression was evaluated through the Beck Depression Inventory (BDI). The results of this study highlighted a significant decrease of FSS and BDI in the CoQ10 group compared to the placebo group, demonstrating a possible beneficial effect of CoQ10 supplementation on fatigue and depression associated with MS [89]. Moreover, the authors found that the levels of inflammatory markers TNF-α, interleukin 6 (IL-6), and matrix metallo-proteinase 9 (MMP-9) were significantly decreased in the CoQ10-treated group, while the supplementation did not affect the levels of the anti-inflammatory markers interleukin 4 (IL-4) and tissue growth factor β (TGF-β), hypothesizing that at the basis of these beneficial effects there are antioxidant and anti-inflammatory activities [89].
4.4. CoQ10 and Neuropathy
4.4.1. CoQ10 and Diabetic Neuropathy
As concerns neuropathy, a 12-week RCT focused on diabetic neuropathy was carried out on 70 patients with type 2 diabetes who received 200 mg/day of CoQ10 or placebo for 12 weeks. At the end of the trial hemoglobin glycate (HbA1c), fasting blood glucose and lipid profiles did not show significant differences between the two groups. On the other hand, in the group treated with CoQ10, a significant increase of the mean insulin sensitivity and the total antioxidant capacity concentration was recorded. Moreover, high sensible C-reactive protein (hsCRP) levels were found significantly decreased in the CoQ10 group compared to placebo. Despite a clear improvement of the biochemical markers, the evaluation of neuropathic symptoms and electromyography measurements did not highlight significant differences between the two groups after the trial [90].
4.4.2. CoQ10 and Glaucoma
Glaucoma is a progressive optic neuropathy which may benefit from a neuroprotective strategy. In this context, 43 open-angle glaucoma patients were treated for 12 months with CoQ10 in association with vit E eye drops formulation in addition to β-blockers or with β-blockers monotherapy. Interestingly, patients who received 100 mg CoQ10 + 500 mg vit E + β-blockers, compared to those only treated with β-blockers, showed a beneficial effect on inner retinal function, electroretinogram improvement and enhancement of the visual cortical responses (measured by visual-evoked potential enhancement) [91]. Moreover, in a prospective randomized clinical study on pseudo-exfoliative glaucoma, 64 patients underwent phacoemulsification and intraocular lens implantation surgery, which was carried out while administering topically 100 mg CoQ10 + 500 mg vit E TPGS eye drops + prostaglandin agent twice daily for one month in a pre-operative phase or only the prostaglandin agent. As results of this study, the authors recorded aqueous humor-superoxide dismutase levels significantly lower in the group treated with the addition of CoQ10 + VitE than in the group receiving only the prostaglandin agent, while no significant differences were observed in malondialdehyde levels between the groups [92].
4.5. CoQ10 and Cancer
4.5.1. CoQ10 and Breast Cancer
One of the first studies on the supplementation with CoQ10 in breast cancer was a randomized clinical trial on 84 patients who received one dosage per day of a complex named “CoRN,” composed of 100 mg of CoQ10, 10 mg of riboflavin, and 50 mg of niacin in addition to 10 mg of tamoxifen twice a day. To evaluate the efficacy of the treatment, as primary outcomes, two markers of circulating breast cancer, carcinoembryonic antigen (CEA) and carbohydrate antigen 15–3 (CA 15–3), were recorded. Moreover, serum cytokine levels (IL-1β, IL-6, IL-8, TNF-α) and vascular endothelial growth factor (VEGF) were also recorded. The levels of the two markers and of cytokine were found elevated in untreated patients and significantly reduced in the patients who received tamoxifen for more than 1 year. Interestingly, CEA, CA 15–3, and serum cytokine were further reduced in patients who, in addition to tamoxifen, received CoRN for 45 days or 90 days demonstrating that CoRN supplement could help chemotherapy to control the risk of metastases [93]. In the same study a significant increase in DNA repair enzymes (poly-ADP-ribose polymerase levels), a disappearance of DNA methylation patterns (RASSF1A DNA methylation pattern), and a reduction of pro-angiogenic marker levels were observed, suggesting an improvement of the prognosis [94,95].
More recent studies focused on a particular aspect concomitant with breast cancer that is fatigue associated with this pathology. In a first randomized, double-blind, placebo-controlled study, 236 women newly diagnosed and who planned adjuvant chemotherapy received CoQ10 300 mg or placebo, each associated with 300IU vit E in three daily doses, for 24 weeks. At the end of the study, the authors found that, despite the increase of CoQ10 levels, no significant differences were found in scores obtained through the Profile of Mood States-Fatigue questionnaire, Functional Assessment of Chronic Illness Therapy-Fatigue tool, Functional Assessment of Cancer Therapy-Breast Cancer instrument, or Center for Epidemiologic Studies-Depression scale [96]. On the other hand, a randomized clinical trial carried out in Japan on 57 breast cancer patients with cancer-related fatigue (CRF) undergoing chemotherapy, receiving the amino acid jelly Inner Power(®) (IP), an oral dietary supplement containing branched-chain amino acids (2500 mg), CoQ10 (30 mg), and l-carnitine (50 mg), or regular care for 21 days, reported significant differences between the two arms in the scores of worst level of fatigue, global fatigue scores, and current feeling of fatigue. While non-significant value were recorded with regard to Hospital Anxiety and, Depression Scale and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30, concluding that IP may control moderate-severe cancer-related fatigue in breast cancer [97].
4.5.2. CoQ10 and Hepatocellular Carcinoma (HCC)
HCC is characterized by higher levels of oxidative stress and inflammation and this condition persists also during its progression after surgery. On this basis Liu and colleagues performed a 12 weeks single-blinded, randomized, parallel, placebo-controlled study on 41 patients diagnosed with primary HCC after surgery consisting of tumor resection, biopsy, and classification of their tumor as primary HCC according to the International Classification of Diseases 9, code 155.0. After this diagnosis, the enrolled patients were treated randomly with placebo or CoQ10 300 mg/day. At the end of the study it was found that oxidative stress and inflammatory markers (hs-CRP and IL-6) were significantly decreased with a contemporary increase in the activity of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) in HCC patients after surgery [96].
4.5.3. CoQ10 and Prostatic Carcinoma
A randomized clinical trial on 80 patients with hormonally untreated prostatic carcinoma was carried out to assess the effect of a daily supplementation containing CoQ10 (2 capsules 2 × 100 mg/day), vitamin C 750 mg, selenium 200 μg, and vit E 350 mg. During the 21 weeks of the study the treated arm was compared with placebo and serum levels of prostatic specific antigen (PSA) and were assessed at baseline and after 6, 13, 19, 20, and 21 weeks. Moreover, also the mean changes in serum level of testosterone, dihydrotestosterone (DHT), luteinizing hormone (LH), and sex hormone binding globulin (SHBG) were recorded. At the end of the study, in the patients who received the supplementation, plasma levels of CoQ10, vit E, and selenium were significantly increased. However, no significant differences were found in serum levels of PSA, testosterone, DHT, LH, and SHBG between the two arms, concluding that in this condition the supplement did not affect the main parameters used to control the progression of prostatic cancer [98].
4.5.4. CoQ10 and Melanoma
Currently, early surgery remains the best therapy for melanoma and there is no standard adjuvant therapy for this type of cancer. However, low serum concentration of CoQ10 was found in melanoma patients [99]. On this basis, Rusciani and colleagues carried out a 3-year trial during which 81 patients with stage I and II melanoma and surgically removed lesions received low doses of recombinant interferon α-2b (9 000 000 000 IU weekly) only, or a combination of interferon α-2b (9 000 000 000 IU weekly) and CoQ10 (400 mg/day). The main outcome was the incidence of recurrence at 5 years and, despite that a survival study could not be undertaken due to the small number of patients and the short duration, the authors observed a significant decrease in rates of recurrence and negligible adverse effects in the arms which received the supplementation of CoQ10 in addition to low doses of interferon α-2 [100].
4.6. CoQ10 and Fertility
Infertility is caused by several factors in about 30–50% of couples, and in this regard a growing body of evidence points toward oxidative stress as a deleterious aspect on spermatozoa, because of peroxidation and DNA damage [101]. It is noteworthy that CoQ10 is found to be involved in energy production in spermatozoa and in the defense from free radical production.
Indeed, Eroglu et al., showed that CoQ10 supplementation had a positive effect on sperm morphology but not on its concentration and life-span [102]. Lower levels of CoQ10 have been correlated with abnormal spermatozoa. In contrast, previous results failed to find a correlation between seminal levels of CoQ10 and sperm motility. Balercia et al., in a randomized clinical trial, reported that 200 mg/day of supplementation for 6 months in infertile men with idiopathic asthenozoospermia showed an increased sperm motility by the end of therapy [103].
Likewise, Sefarinejad and colleagues reported that sperm morphology, motility, and density were improved in men with oligoasthenoteratozoospermia following 26 weeks of treatment with CoQ10 (200 mg/day) [104]. On the contrary, another randomized study demonstrated a non-significant effect on sperm morphology, motility, and concentration [105].
Previously, considering that varicocele can be a main cause of stress-oxidative-mediated infertility, also in men with normozoospermia [106], the exogenous administration of CoQ10 (100 mg/day) for 3 months improved the sperm density and motility together with antioxidant function [107]. In summary, although several studies have been performed on men in order to evaluate the role of CoQ10 supplementation on testosterone, at present the evidence is unclear and inconclusive.
Besides male infertility, a condition that predisposes infertility in women is represented by polycystic ovarian syndrome (PCOS), that is affecting 6–20% of reproductive-aged women [108].
In this condition women generally present hyperandrogenism (in particular acne, hirsutism and alopecia), dysmenorrhea until amenorrhea, polycystic ovarian morphology, but also insulin resistance, obesity, and lipid disorders. Indeed, they are more susceptible to metabolic diseases and inflammation. On the basis of Rotterdam criteria the presence of two or three of these characteristics are necessary for a diagnosis of PCOS [109,110,111]. In this context CoQ10, by virtue of its antioxidant properties, is viewed as a protective agent able to improve the typical metabolic and endocrine disequilibrium.
Notable, 12 weeks of treatment with CoQ10 supplementation (100 mg/day) downregulated the expression of low-density lipoprotein (LDL) receptors, stimulated the AMPK, and reduced the ROS-induced endothelial damage, confirming the positive role on metabolic alterations [44].
Samimi et al., in a RCT on 60 women, confirmed the beneficial effects on cholesterol levels but also on glucose metabolism, with a supplementation with CoQ10 at a dose of 100 mg/day for 12 weeks [112]. Then, another RCT was developed by the same research group on 40 women suffering from PCOS, in which they received CoQ10 (100 mg/day) for 12 weeks. Together with the beneficial effects on glucose and lipid levels, the authors reported also a down-regulated gene expression of oxidized LDL receptor 1 and an up-regulation of peroxisome proliferator-activated receptor-γ (PPAR-γ) gene expression in PBMCs. Furthermore, CoQ10 supplementation in subjects with PCOS down-regulated the gene expression of IL-1, IL-8, and TNF-α in PBMCs if compared to the placebo group [113]. Izadi et al., obtained comparable results in a RCT of 85 PCOS women treated with CoQ10 and/or vit E or placebo. Specially, treatment with CoQ10 alone reduced LH and testosterone levels and improved the insulin resistance. Interestingly, the co-administration of CoQ10 and α-tocopherol showed a more pronounced activity and promoted the SHBG release, justifying the enhancement of insulin tolerance, which is a condition associated with a reduced hepatic synthesis of SHBG. Then, CoQ10, through an improvement of mitochondrial function and a restoration of energy production at mitochondrial level, can stimulate the biosynthesis of steroid hormone and normal reproductive function, like oocyte maturation, fertilization, and embryonic development [114].
4.7. CoQ10 and Dupuytren’s Disease (DD)
DD is defined as a benign progressive fibroproliferative condition of the palmar and digital fascia of the hands, resulting in irreversible flexion deformities of these. Anti-inflammatory drugs are among the most used remedies, as increased cytokine levels, especially TNF-α, have been found in patients with DD [115].
Currently, there are not any indications for supplementation with CoQ10 in this condition. Very recently a case report, in which a patient has been supplemented with CoQ10 (300 mg/day for 3 years), has been published. Indeed, the typical contracture at the hands were resolved, suggesting that CoQ10, by virtue of its anti-inflammatory profile, could be a valid nutritional remedy [116].
4.8. CoQ10 and Schizophrenia
A growing body of evidence suggests that mitochondrial dysfunction and reduced energy are at the basis of the negative and cognitive symptoms in schizophrenia, such as fatigue, isolation, and cognitive impairments. Indeed, a depletion of ATP and an increase of oxidative stress and lactate levels have been found in schizophrenic patients [117,118,119]. In addition, low levels of CoQ10 have been observed in the erythrocytes of patients with schizophrenia [120], therefore, a supplementation with CoQ10 may have a therapeutic value, but to our knowledge no clinical studies have been carried out [121].
5. Discussion
CoQ10 is an ergogenic supplement present in foods, with an excellent safety profile even with chronic exposure to 900 mg/day [122] and in frail patients, like elderly and chronic kidney disease patients, without any known pharmacological interactions [2]. Conversely, ts deficiency is associated with several pathological conditions, including, first of all, cardiovascular diseases, and its supplementation tends to improve the quality of life and in some cases, hard outcomes as well [17]. In this regard, the Q-SYMBIO study, a multicenter randomized placebo-controlled trial, showed that a daily intake of 300 mg of CoQ10 (n = 202) or placebo (n = 218) in patients affected by moderate or severe heart failure (HF) and treated with the conventional gold standard therapy (n = 420), have benefited from a significant reduction in major adverse cardiac events rate, cardiovascular mortality, all-cause mortality, and incidence of hospital stays for HF after 2 years in comparison with the placebo [123]. Despite abundant and encouraging results in the cardiovascular field (although the need for further information exists), data regarding other pathological disorders, such as neurodegenerative diseases, muscular disorders, and cancer (Table 2, Table 3, Table 4 and Table 5), are still limited and sometimes contrasting, making it difficult to arrive at definitive conclusions on the efficacy of CoQ10 in several diseases. However, this review aimed at examining the literature on clinical studies in which CoQ10 has been considered to counter non-cardiovascular pathological conditions, and aimed to highlight further possible and interesting therapeutic uses.
Table 2.
Coenzyme Q10 and migraine.
| Study Design | Daily Doses | Effects on Symptoms | Effects on Lab. or Instrumental Parameters |
Effects on Hard Outcomes | |
|---|---|---|---|---|---|
| Migraine | RCTs | 100–400 mg/day | ↓ duration and severity of attacks | ↓ TNFα and GCPR levels | Not investigated |
↓ = it is indicative of a reduction of a marker or a symptom.
Table 3.
Coenzyme Q10 and muscle-related diseases.
| Study Design |
Daily Doses | Effects on Symptoms | Effects on Lab or Instrumental Parameters |
Effects on Hard Outcomes | |
|---|---|---|---|---|---|
| Fatigue | RCTs | 200 mg/day, in association with NADH (20 mg/day) | ↓ FIS total score (CFS) | ↑ NAD+/NADH ratio and CoQ10, ATP, citrate synthase levels | Not investigated |
| RCTs | 300–400 mg | ↓ FIS total score | - | Not investigated | |
| Fibromyalgia | RCTs | 100–400 mg | ↓ fatigue (FIQ, VAS) | - | Not investigated |
| Statin-associated myopathy | Meta-analysis of RCTs | ≥200 mg | ↓ fatigue (VAS) | - | Not investigated |
| Other fatigue-related diseases | RCTs | 60–500 mg | ↓ fatigue (FSS) only in multiple sclerosis and in patients awaiting cardiac transplantation with end-stage heart failure | - | Not investigated |
FIQ = Fibromyalgia Impact Questionnaire, FSS = Fatigue Severity Scale, RCTs = randomized clinical trials, VAS = Visual Analog Scale. ↓= it is indicative of a reduction of a marker or a symptom. ↑ = it is indicative of an increase of a marker or a symptom.
Table 4.
Coenzyme Q10 and degenerative diseases.
| Study Design |
Daily Doses | Effects on Symptoms | Effects on Lab or Instrumental Parameters |
Effects on Hard Outcomes |
|
|---|---|---|---|---|---|
| PD | RCTs | 300–2400 mg | ↑significant mild symptomatic benefit, ↑ great improvements of patients everyday activities such as feeding, bathing, or dressing, ↑ effects on motor performance, = no significant changes in UPDRS | ↑ improvement in NADH-cytochrome c reductase activity, ↑ increase in CoQ10 plasmatic levels | Not investigated |
| HD | RCTs | 600–2400 mg | = no significant changes in: HDRS, in HDFCS, standardized neuropsychological measures and TFC scores | Not recorded | Not investigated |
| AD | RCTs | 400 mg | = MMSE scores and functional ability | = not significant differences in: CSF F-2-isoprostane levels, oxidative biomarkers, CSF Aβ42, tau, and P-tau (181) levels | Not investigated |
| MS | RCTs | 500 mg | ↑reduction of fatigue and depression | ↓ inflammatory markers TNF-α, IL-6 and MMP-9, = IL-4 and TGF-β levels | Not investigated |
| Glaucoma | RCTs | 100 mg | Not evaluated | ↑ inner retinal function, electroretinogram and visual cortical responses, ↓superoxide dismutase, = malondialdehyde levels | Not investigated |
| Neuropathy | RCTs | 200 mg | No significant improvement of neuropathic symptoms | = no significant differences on HbA1c, fasting blood glucose or lipid profile, ↑mean insulin sensitivity, ↑ total antioxidant capacity concentration, ↓C-protein level, = electromyography measurements | Not investigated |
CSF = cerebrospinal fluids, HDRS = Huntington’s Disease Rating Scale, HDFCS = Huntington’s Disease Functional Capacity Scale, MMSE = mini-mental state examination, NADH = nicotinamide adenine dinucleotide, RCTs = randomized clinical trials, TFC = total functional capacity, UPDRS = Unified Parkinson’s Diseases Rating Scale. ↓ = it is indicative of a reduction of a marker or a symptom. ↑ = it is indicative of an increase of a marker or a symptom.
Table 5.
Coenzyme Q10 and cancer.
| Study Design | Daily Doses | Effects on symptoms | Effects on Lab or Instrumental Parameters | Effects on Hard Outcomes | |
|---|---|---|---|---|---|
| Breast cancer | RCTs | 300–2400 mg | ↓ moderate-severe cancer-related fatigue (30 mg) | ↓ CEA, CA 15-3, IL-1β, IL-6, IL-8, TNF-α, vascular endothelial growth factor, pro-angiogenic marker levels, ↑ DNA repair enzymes (poly-ADP-ribose polymerase levels), a disappearance of DNA methylation patterns (RASSF1A DNA methylation pattern) | Not investigated |
| HCC | RCTs | 300 mg | Not investigated | ↓ hs-CRP, IL-6 ↑ SOD, CAT, GPx | Not investigated |
| Prostatic carcinoma | RCTs | 300 mg | Not investigated | ↑ CoQ10, vit E, selenium = PSA, testosterone, DHT, LH, SBHG | Not investigated |
| Melanoma | RCTs | 400 mg | Not investigated | Not investigated | ↓ rates of recurrence at 5 years |
CAT = catalase, CEA = carcinoembryonic antigen, CA 15-3 = carbohydrate antigen 15-3, DHT = dihydrotestosterone, GPx = glutathione peroxidase, hs-CRP = high sensitivity C-reactive protein, IL-1β = interleukin-1beta, IL-6 = interleukin-6, IL-8 = interleukin-8, SOD = superoxide dismutase, PSA = prostatic specific antigen, RCTs = randomized clinical trials, TNF-α = tumor necrosis factor-alpha. ↓ = it is indicative of a reduction of a marker or a symptom. ↑ = it is indicative of an increase of a marker or a symptom.
Indeed, CoQ10 can exert many mild positive effects on different tissues and metabolism through a reduction of systemic oxidative stress and inflammation, as observed in several RCTs. In this regard, a growing body of evidence suggests that CoQ10 exhibits significant effects on the prophylaxis of migraine, in particular when it is associated to other nutraceuticals, including curcumin, magnesium, and Tanacetum parthenium L. or riboflavin. Indeed the guidelines recommend it at a dose of 300 mg/day.
CoQ10 has been also observed to improve the symptoms of CFS and other diseases characterized by fatigue (i.e., fibromyalgia), and also in healthy athletes submitted to intensive exercise. CoQ10 results generally effective in this context, although sometimes rather than parameters related to the perception of fatigue, markers of inflammation or oxidative stress are measured. Of note, in RCTs carried out on patients with CFS, CoQ10 has been often associated with NADH supplementation.
On the basis of the recognized role of CoQ10 as an antioxidant and anti-inflammatory agent, several clinical trials have been carried out on patients with ND. In MS, CoQ10 controls the symptoms associated to the neurological disease, such as fatigue and depression, but seems to be limited to relapsing-remitting of the MS.
The evaluation of a potential approach with CoQ10 supplementation in oncologic patients highlighted a general improvement in inflammatory, oxidative, and specific biochemical markers, but unfortunately these studies are highly heterogenous, both concerning the dosages and the cocktail of drugs or supplements which oncologic patients received together with CoQ10. This lack of homogeneity makes it challenging to identify the real impact of CoQ10 supplementation on the several types of cancer and further studies should be performed to clarify its full role.
A different evaluation could be done as concerns the use of CoQ10 supplementation in HD. Indeed in the last 25 years, different well-designed clinical trials have been carried out with the administration of high doses (from 300 to 2400 mg/day) of CoQ10 alone, in long chronic treatments (from 6 to 60 months) and trying to select patients in early stages of HD, but despite the appreciable quality of these studies, in all of them the results confirmed that no significant improvements have been recorded in HD after the administration of CoQ10 supplementation. This attests to the lack of encouraging findings which could suggest a CoQ10 application in HD therapy. Moreover, several clinical studies considered a supplementation with CoQ10 to improve fertility in men and women. In men, evidence is unclear and inconclusive, while in women (in particular suffering from PCOS) the supplementation with CoQ10 demonstrated to improve the metabolic disorder, typically associated to the disease.
Finally data on schizophrenia, glaucoma, and DD disease are still preliminary and often inconsistent, therefore further clinical investigations are required. The reasons of the inconsistency of the data could be due to a multiplicity of factors: RCTs are frequently undersized and the period is too short to evaluate the effect on hard outcomes. Moreover, the applied methodology is generally of low-quality with an insufficient standardization of patient characteristics at the baseline. Often there is no quantification of CoQ10 intake with diet (even if this is usually very low) and the tested dosage is not titrated based on the blood CoQ10 level. Furthermore, the use of real endpoints instead of biochemical surrogated outcomes might particularly be useful in order to understand the actual value of a supplementation with CoQ10.
6. Conclusions
If, on the one hand, clinical evidence support supplementation with bioavailable-CoQ10 (≥200 mg/day) in the cardiovascular field, and in particular in patients affected by HF and coronary heart disease to preserve heart health. On the other hand data regarding its supplementation in people with ND, cancer, or other conditions, including glaucoma, fertility, migraine, and fatigue, are often encouraging, but need to be strengthened by long-term high-quality RCTs. In conclusion, this review paves the way for speculating on the possible supplementation with CoQ10 in non-cardiovascular fields, in which an intervention with such a type of nutraceuticals could furnish positive benefits.
Author Contributions
Conceptualization, A.F.G.C. and A.C.; literature search and revision, A.M., L.T. and A.C.; writing—original draft preparation, A.M., L.F., L.T. and A.C.; writing—review and editing, A.F.G.C.; supervision, L.T., A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bentinger M., Brismar K., Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7:S41–S50. doi: 10.1016/j.mito.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Saini R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011;3:466–467. doi: 10.4103/0975-7406.84471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crane F.L. Biochemical Functions of Coenzyme Q10. J. Am. Coll. Nutr. 2001;20:591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- 4.Haas R.H. The evidence basis for coenzyme Q therapy in oxidative phosphorylation disease. Mitochondrion. 2007;7:S136–S145. doi: 10.1016/j.mito.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez-Mariscal F.M., Yubero-Serrano E.M., Villalba J.M., Lopez-Miranda J. Coenzyme Q10: From bench to clinic in aging diseases, a translational review. Crit. Rev. Food Sci. Nutr. 2019;59:2240–2257. doi: 10.1080/10408398.2018.1442316. [DOI] [PubMed] [Google Scholar]
- 6.Schmelzer C., Lindner I., Rimbach G., Niklowitz P., Menke T., Döring F. Functions of coenzyme Q10in inflammation and gene expression. BioFactors. 2008;32:179–183. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 7.Yubero-Serrano E.M., Gonzalez-Guardia L., Rangel-Zuñiga O., Delgado-Lista J., Gutierrez-Mariscal F.M., Perez-Martinez P., Delgado-Casado N., Cruz-Teno C., Tinahones F.J., Villalba J.M., et al. Mediterranean Diet Supplemented With Coenzyme Q10 Modifies the Expression of Proinflammatory and Endoplasmic Reticulum Stress-Related Genes in Elderly Men and Women. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2011;67:3–10. doi: 10.1093/gerona/glr167. [DOI] [PubMed] [Google Scholar]
- 8.Shukla S., Dubey K.K. CoQ10 a super-vitamin: Review on application and biosynthesis. 3 Biotech. 2018;8:1–11. doi: 10.1007/s13205-018-1271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szkopińska A. Ubiquinone. Biosynthesis of quinone ring and its isoprenoid side chain. Intracellular localization. Acta Biochim. Pol. 2000;47:469–480. doi: 10.18388/abp.2000_4027. [DOI] [PubMed] [Google Scholar]
- 10.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta (BBA) Biomembr. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Weber C., Bysted A., Hłlmer G. The coenzyme Q10 content of the average Danish diet. Int. J. Vitam. Nutr. Res. 1997;67:123–129. [PubMed] [Google Scholar]
- 12.Zhang Y., Aberg F., Appelkvist E.L., Dallner G., Ernster L. Uptake of dietary coenzyme Q supplement is limited in rats. J. Nutr. 1995;125:446–453. doi: 10.1093/jn/125.3.446. [DOI] [PubMed] [Google Scholar]
- 13.Garrido-Maraver J., Cordero M.D., Oropesa-Ávila M., Vega A.F., De La Mata M., Pavón A.D., De Miguel M., Calero C.P., Paz M.V., Cotán D., et al. Coenzyme q10 therapy. Mol. Syndr. 2014;5:187–197. doi: 10.1159/000360101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Åberg F., Appelkvist E.-L., Dallner G., Ernster L. Distribution and redox state of ubiquinones in rat and human tissues. Arch. Biochem. Biophys. 1992;295:230–234. doi: 10.1016/0003-9861(92)90511-T. [DOI] [PubMed] [Google Scholar]
- 15.Miles M.V., Horn P.S., Morrison J.A., Tang P.H., Degrauw T., Pesce A.J. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin. Chim. Acta. 2003;332:123–132. doi: 10.1016/S0009-8981(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 16.Weis M., Mortensen S., Rassing M., Møller-Sonnergaard J., Poulsen G., Rasmussen S. Bioavailability of four oral Coenzyme Q10 formulations in healthy volunteers. Mol. Asp. Med. 1994;15:s273–s280. doi: 10.1016/0098-2997(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 17.Martelli A., Testai L., Colletti A., Cicero A.F.G. Coenzyme Q10: Clinical Applications in Cardiovascular Diseases. Antioxidants. 2020;9:341. doi: 10.3390/antiox9040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miles M.V., Horn P., Miles L., Tang P., Steele P., Degrauw T. Bioequivalence of coenzyme Q10 from over-the-counter supplements. Nutr. Res. 2002;22:919–929. doi: 10.1016/S0271-5317(02)00402-5. [DOI] [Google Scholar]
- 19.Kumar S., Rao R., Kumar A., Mahant S., Nanda S. Novel Carriers for Coenzyme Q10 Delivery. Curr. Drug Deliv. 2016;13:1184–1204. doi: 10.2174/1567201813666160104130631. [DOI] [PubMed] [Google Scholar]
- 20.Hershey A.D., Powers S.W., Vockell A.-L.B., LeCates S.L., Ellinor P.L., Segers A., Burdine D., Manning P., Kabbouche M.A. Coenzyme Q10 Deficiency and Response to Supplementation in Pediatric and Adolescent Migraine. Headache J. Head Face Pain. 2007;47:73–80. doi: 10.1111/j.1526-4610.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- 21.Dahri M., Tarighat-Esfanjani A., Asghari-Jafarabadi M., Hashemilar M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr. Neurosci. 2018;22:607–615. doi: 10.1080/1028415X.2017.1421039. [DOI] [PubMed] [Google Scholar]
- 22.Parohan M., Sarraf P., Javanbakht M.H., Ranji-Burachaloo S., Djalali M. Effect of coenzyme Q10 supplementation on clinical features of migraine: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Neurosci. 2019;23:868–875. doi: 10.1080/1028415X.2019.1572940. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Z., Li Y., Lu S., Huang W., Di W. Efficacy of CoQ10 as supplementation for migraine: A meta-analysis. Acta Neurol. Scand. 2018;139:284–293. doi: 10.1111/ane.13051. [DOI] [PubMed] [Google Scholar]
- 24.Gaul C., Diener H.C., Danesch U. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain. 2015;16:1–8. doi: 10.1186/s10194-015-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parohan M., Sarraf P., Javanbakht M.H., Foroushani A.R., Ranji-Burachaloo S., Djalali M. The synergistic effects of nano-curcumin and coenzyme Q10 supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Nutr. Neurosci. 2021;24:317–326. doi: 10.1080/1028415X.2019.1627770. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno K., Tanaka M., Yamaguti K., Kajimoto O., Kuratsune H., Watanabe Y. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav. Brain Funct. 2011;7:1–17. doi: 10.1186/1744-9081-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuno K., Tanaka M., Nozaki S., Mizuma H., Ataka S., Tahara T., Sugino T., Shirai T., Kajimoto Y., Kuratsune H., et al. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition. 2008;24:293–299. doi: 10.1016/j.nut.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Mehrabani S., Askari G., Miraghajani M., Tavakoly R., Arab A. Effect of coenzyme Q10 supplementation on fatigue: A systematic review of interventional studies. Complement. Ther. Med. 2019;43:181–187. doi: 10.1016/j.ctim.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Yancey J.R., Thomas S.M. Chronic fatigue syndrome: Diagnosis and treatment. Am. Fam. Physician. 2012;86:741–746. [PubMed] [Google Scholar]
- 30.Cooke M., Iosia M., Buford T., Shelmadine B., Hudson G., Kerksick C., Rasmussen C., Greenwood M., Leutholtz B., Willoughby D., et al. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J. Int. Soc. Sports Nutr. 2008;5:1–14. doi: 10.1186/1550-2783-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjørklund G., Dadar M., Pen J.J., Chirumbolo S., Aaseth J. Chronic fatigue syndrome (CFS): Suggestions for a nutritional treatment in the therapeutic approach. Biomed. Pharmacother. 2019;109:1000–1007. doi: 10.1016/j.biopha.2018.10.076. [DOI] [PubMed] [Google Scholar]
- 32.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol. Lett. 2009;30:470–476. [PubMed] [Google Scholar]
- 33.Booth N.E., Myhill S., McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Int. J. Clin. Exp. Med. 2012;5:208–220. [PMC free article] [PubMed] [Google Scholar]
- 34.Castro-Marrero J., Cordero M.D., Segundo M.J., Sáez-Francàs N., Calvo N., Román-Malo L., Aliste L., De Sevilla T.F., Alegre J. Does Oral Coenzyme Q10 Plus NADH Supplementation Improve Fatigue and Biochemical Parameters in Chronic Fatigue Syndrome? Antioxid. Redox Signal. 2015;22:679–685. doi: 10.1089/ars.2014.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris G., Maes M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metab. Brain Dis. 2012;28:523–540. doi: 10.1007/s11011-012-9324-8. [DOI] [PubMed] [Google Scholar]
- 36.Nicolson G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Integr. Med. Clin. J. 2014;13:35. [PMC free article] [PubMed] [Google Scholar]
- 37.Alegre J., Rosés J., Javierre C., Ruiz-Baqués A., Segundo M., De Sevilla T.F. Nicotinamida adenina dinucleótido (NADH) en pacientes con síndrome de fatiga crónica. Clín. Esp. 2010;210:284–288. doi: 10.1016/j.rce.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Castro-Marrero J., Sáez-Francàs N., Segundo M.J., Calvo N., Faro M., Aliste L., de Sevilla T.F., Alegre J. Effect of coenzyme Q10 plus nicotinamide adenine dinucleotide supplementation on maximum heart rate after exercise testing in chronic fatigue syndrome: A randomized, controlled, double-blind trial. Clin. Nutr. 2016;35:826–834. doi: 10.1016/j.clnu.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda S., Nojima J., Kajimoto O., Yamaguti K., Nakatomi Y., Kuratsune H., Watanabe Y. Ubiquinol-10 supplementation improves autonomic nervous function and cognitive function in chronic fatigue syndrome. BioFactors. 2016;42:431–440. doi: 10.1002/biof.1293. [DOI] [PubMed] [Google Scholar]
- 40.Di Pierro F., Rossi A., Consensi A., Giacomelli C., Bazzichi L. Role for a water-soluble form of CoQ10 in female subjects affected by fibromyalgia. A preliminary study. Clin. Exp. Rheumatol. 2016;35:20–27. [PubMed] [Google Scholar]
- 41.Cordero M.D., Santos-García R., Bermejo-Jover D., Sánchez-Domínguez B., Jaramillo-Santos M.R., Bullon P. Coenzyme Q10 in salivary cells correlate with blood cells in Fibromyalgia: Improvement in clinical and biochemical parameter after oral treatment. Clin. Biochem. 2012;45:509–511. doi: 10.1016/j.clinbiochem.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Cordero M.D., Alcocer-Gómez E., De Miguel M., Culic O., Carrión A.M., Alvarez-Suarez J.M., Bullón P., Battino M., Fernández-Rodríguez A., Sánchez-Alcazar J.A. Can Coenzyme Q10 Improve Clinical and Molecular Parameters in Fibromyalgia? Antioxid. Redox Signal. 2013;19:1356–1361. doi: 10.1089/ars.2013.5260. [DOI] [PubMed] [Google Scholar]
- 43.Hardie D.G., Ross F.A., Hawley S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai K.L., Chen L.H., Chiou S.H., Chiou G.Y., Chen Y.C., Chou H.Y., Chen L.K., Chen H.Y., Chiu T.H., Tsai C.S., et al. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol. Nutr. Food Res. 2011;55:S227–S240. doi: 10.1002/mnfr.201100147. [DOI] [PubMed] [Google Scholar]
- 45.Langsjoen P.H., Langsjoen J.O., Langsjoen A.M., Lucas L.A. Treatment of statin adverse effects with supplemental Coenzyme Q10 and statin drug discontinuation. BioFactors. 2005;25:147–152. doi: 10.1002/biof.5520250116. [DOI] [PubMed] [Google Scholar]
- 46.Cordero M.D., Cano-García F.J., Alcocer-Gómez E., De Miguel M., Sánchez-Alcázar J.A. Oxidative Stress Correlates with Headache Symptoms in Fibromyalgia: Coenzyme Q10 Effect on Clinical Improvement. PLoS ONE. 2012;7:e35677. doi: 10.1371/journal.pone.0035677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyamae T., Seki M., Naga T., Uchino S., Asazuma H., Yoshida T., Iizuka Y., Kikuchi M., Imagawa T., Natsumeda Y., et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013;18:12–19. doi: 10.1179/1351000212Y.0000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corsini A. Statin-Related Muscle Complaints: An Underestimated Risk. Cardiovasc. Drugs Ther. 2005;19:379–381. doi: 10.1007/s10557-005-6352-1. [DOI] [PubMed] [Google Scholar]
- 49.Deichmann R., Lavie C., Andrews S. Coenzyme Q10 and Statin-Induced Mitochondrial Dysfunction. Ochsner J. 2010;10:16–21. [PMC free article] [PubMed] [Google Scholar]
- 50.Fedacko J., Pella D., Fedackova P., Hänninen O., Tuomainen P., Jarcuska P., Lopuchovsky T., Jedlickova L., Merkovska L., Littarru G.P. Coenzyme Q10and selenium in statin-associated myopathy treatment. Can. J. Physiol. Pharmacol. 2013;91:165–170. doi: 10.1139/cjpp-2012-0118. [DOI] [PubMed] [Google Scholar]
- 51.Gharahdaghi N., Shabkhiz F., Azarboo E., Keyhanian A. The effects of daily coenzyme Q10 supplementation on VO2max, vVO2max and Intermittent Exercise performance in soccer players. Life Sci. J. 2013;10:22–28. [Google Scholar]
- 52.Lee Y.J., Cho W.J., Kim J.K., Lee D.C. Effects of Coenzyme Q10 on Arterial Stiffness, Metabolic Parameters, and Fatigue in Obese Subjects: A Double-Blind Randomized Controlled Study. J. Med. Food. 2011;14:386–390. doi: 10.1089/jmf.2010.1202. [DOI] [PubMed] [Google Scholar]
- 53.Gökbel H., Gül I., Belviranl M., Okudan N. The Effects Of Coenzyme Q10 Supplementation on Performance During Repeated Bouts of Supramaximal Exercise in Sedentary Men. J. Strength Cond. Res. 2010;24:97–102. doi: 10.1519/JSC.0b013e3181a61a50. [DOI] [PubMed] [Google Scholar]
- 54.Ylikoski T., Piirainen J., Hanninen O., Penttinen J. The effect of coenzyme Q10 on the exercise performance of cross-country skiers. Mol. Asp. Med. 1997;18:283–290. doi: 10.1016/S0098-2997(97)00038-1. [DOI] [PubMed] [Google Scholar]
- 55.Macdonald J., Galley H., Webster N. Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 2003;90:221–232. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- 56.Kreher J.B., Schwartz J.B. Overtraining Syndrome. Sports Health. 2012;4:128–138. doi: 10.1177/1941738111434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonetti A., Solito F., Carmosino G., Bargossi A.M., Fiorella P.L. Effect of ubidecarenone oral treatment on aerobic power in middle-aged trained subjects. J. Sports Med. Phys. Fit. 2000;40:51. [PubMed] [Google Scholar]
- 58.Malm C., Svensson M., Ekblom B., Sjödin B. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol. Scand. 1997;161:379–384. doi: 10.1046/j.1365-201X.1997.00198.x. [DOI] [PubMed] [Google Scholar]
- 59.Stear S.J., Castell L.M., Burke L.M., Jeacocke N., Ekblom B., Shing C., Calder P.C., Lewis N. A-Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance—part 10. Br. J. Sports Med. 2010;44:688–690. doi: 10.1136/bjsm.2010.075218. [DOI] [PubMed] [Google Scholar]
- 60.Liao P., Zhang Y., Liao Y., Zheng N.-J., Zhang X. Effects of coenzyme Q10 supplementation on liver mitochondrial function and aerobic capacity in adolescent athletes. J. Appl. Physiol. 2007;23:491–494. [PubMed] [Google Scholar]
- 61.Weston S.B., Zhou S., Weatherby R.P., Robson S.J. Does Exogenous Coenzyme Q10 Affect Aerobic Capacity in Endurance Athletes? Int. J. Sport Nutr. 1997;7:197–206. doi: 10.1123/ijsn.7.3.197. [DOI] [PubMed] [Google Scholar]
- 62.Imai H., Hayashi T., Negawa T., Nakamura K., Tomida M., Koda K., Tajima T., Koda Y., Suda K., Era S. Strenuous Exercise-Induced Change in Redox State of Human Serum Albumin during Intensive Kendo Training. Jpn. J. Physiol. 2002;52:135–140. doi: 10.2170/jjphysiol.52.135. [DOI] [PubMed] [Google Scholar]
- 63.Kon M., Tanabe K., Akimoto T., Kimura F., Tanimura Y., Shimizu K., Okamoto T., Kono I. Reducing exercise-induced muscular injury in kendo athletes with supplementation of coenzyme Q10. Br. J. Nutr. 2008;100:903–909. doi: 10.1017/S0007114508926544. [DOI] [PubMed] [Google Scholar]
- 64.Shimizu K., Kon M., Tanimura Y., Hanaoka Y., Kimura F., Akama T., Kono I. Coenzyme Q10 supplementation downregulates the increase of monocytes expressing toll-like receptor 4 in response to 6-day intensive training in kendo athletes. Appl. Physiol. Nutr. Metab. 2015;40:575–581. doi: 10.1139/apnm-2014-0556. [DOI] [PubMed] [Google Scholar]
- 65.Armanfar M., Jafari A., Dehghan G.R. Effect of coenzyme Q10 Supplementation on Exercise-Induced Response of Oxidative Stress and Muscle Damage Indicators in Male Runners. Med. J. Islam. Repub. Iran. 2015;17:202. doi: 10.17795/zjrms1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emami A. The Impact of Pre-Cooling and CoQ10Supplementation on Mediators of Inflammatory Cytokines in Elite Swimmers. Nutr. Cancer. 2020;72:41–51. doi: 10.1080/01635581.2019.1614200. [DOI] [PubMed] [Google Scholar]
- 67.Berman M., Erman A., Ben-Gal T., Dvir D., Georghiou G.P., Stamler A., Vered Y., Vidne B.A., Aravot D. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: A randomized, placebo-controlled study. Clin. Cardiol. 2004;27:295–299. doi: 10.1002/clc.4960270512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peel M.M., Cooke M., Lewis-Peel H.J., Lea R.A., Moyle W. A randomized controlled trial of coenzyme Q10 for fatigue in the late-onset sequelae of poliomyelitis. Complement. Ther. Med. 2015;23:789–793. doi: 10.1016/j.ctim.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Lesser G.J., Case D., Stark N., Williford S., Giguere J., Garino L.A., Naughton M.J., Vitolins M.Z., Lively M.O., Shaw E.G. A Randomized, Double-Blind, Placebo-Controlled Study of Oral Coenzyme Q10 to Relieve Self-Reported Treatment-Related Fatigue in Newly Diagnosed Patients with Breast Cancer. J. Support. Oncol. 2012;11:31–42. doi: 10.1016/j.suponc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J., Wang L.N., Zhan S.Y., Xia Y. Coenzyme Q10 for Parkinson’s disease. Cochrane Database Syst. Rev. 2012:CD008150. doi: 10.1002/14651858.CD008150.pub3. [DOI] [PubMed] [Google Scholar]
- 71.Shults C.W., Haas R.H., Passov D., Beal M.F. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann. Neurol. 1997;42:261–264. doi: 10.1002/ana.410420221. [DOI] [PubMed] [Google Scholar]
- 72.Matsubara T. Serum coenzyme Q-10 level in parkinson syndrome. In: Folkers K., Littarru G.P., Yamagami T., editors. Biomedical and Clinical Aspects of Coenzyme Q. Elsevier Science Publishers; New York, NY, USA: 1991. pp. 159–166. [Google Scholar]
- 73.Müller T., Büttner T., Gholipour A.-F., Kuhn W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci. Lett. 2003;341:201–204. doi: 10.1016/S0304-3940(03)00185-X. [DOI] [PubMed] [Google Scholar]
- 74.Shults C.W., Oakes D., Kieburtz K., Beal M.F., Haas R., Plumb S., Juncos J.L., Nutt J., Shoulson I., Carter J., et al. Effects of Coenzyme Q10 in Early Parkinson Disease. Arch. Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 75.Horstink M.W.I.M., Van Engelen B.G. The effect of coenzyme Q10 therapy in Parkinson disease could be symptomatic. Arch. Neurol. 2003;60:1170–1172. doi: 10.1001/archneur.60.8.1170-b. [DOI] [PubMed] [Google Scholar]
- 76.Shults C.W., Beal M.F., Song D., Fontaine D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson’s disease. Exp. Neurol. 2004;188:491–494. doi: 10.1016/j.expneurol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Storch A., Jost W.H., Vieregge P., Spiegel J., Greulich W., Durner J., Müller T., Kupsch A., Henningsen H., Oertel W.H., et al. Randomized, Double-blind, Placebo-Controlled Trial on Symptomatic Effects of Coenzyme Q10 in Parkinson Disease. Arch. Neurol. 2007;64:938–944. doi: 10.1001/archneur.64.7.nct60005. [DOI] [PubMed] [Google Scholar]
- 78.Beal M.F., Oakes D., Shoulson I., Henchcliffe C., Galpern W.R., Haas R., Juncos J.L., Nutt J.G., Voss T.S., Ravina B., et al. A Randomized Clinical Trial of High-Dosage Coenzyme Q10 in Early Parkinson Disease. JAMA Neurol. 2014;71:543–552. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 79.Cleren C., Yang L., Lorenzo B., Calingasan N.Y., Schomer A., Sireci A., Wille E.J., Beal M.F. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J. Neurochem. 2007;104:1613–1621. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- 80.Yoritaka A., Kawajiri S., Yamamoto Y., Nakahara T., Ando M., Hashimoto K., Nagase M., Saito Y., Hattori N. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson’s disease. Park. Relat. Disord. 2015;21:911–916. doi: 10.1016/j.parkreldis.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 81.The Huntington Study Group A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57:397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- 82.McGarry A., McDermott M., Kieburtz K., De Blieck E.A., Beal F., Marder K., Ross C., Shoulson I., Gilbert P., Mallonee W.M., et al. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Neurology. 2017;88:152–159. doi: 10.1212/WNL.0000000000003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galasko D.R., Peskind E., Clark C.M., Quinn J.F., Ringman J.M., Jicha G.A., Cotman C., Cottrell B., Montine T.J., Thomas R.G., et al. Antioxidants for Alzheimer Disease. Arch. Neurol. 2012;69:836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janardhan V., Bakshi R. Quality of life in patients with multiple sclerosis. J. Neurol. Sci. 2002;205:51–58. doi: 10.1016/S0022-510X(02)00312-X. [DOI] [PubMed] [Google Scholar]
- 85.Kos D., Kerckhofs E., Nagels G., D’Hooghe M.B., Ilsbroukx S. Origin of Fatigue in Multiple Sclerosis: Review of the Literature. Neurorehabilit. Neural Repair. 2007;22:91–100. doi: 10.1177/1545968306298934. [DOI] [PubMed] [Google Scholar]
- 86.Vercoulen J.H.M.M., Hommes O.R., Swanink C.M.A., Jongen P.J.H., Fennis J.F.M., Galama J.M.D., Van Der Meer J.W.M., Bleijenberg G. The Measurement of Fatigue in Patients with Multiple Sclerosis. Arch. Neurol. 1996;53:642–649. doi: 10.1001/archneur.1996.00550070080014. [DOI] [PubMed] [Google Scholar]
- 87.Gold S.M., Irwin M.R. Depression and Immunity: Inflammation and Depressive Symptoms in Multiple Sclerosis. Neurol. Clin. 2006;24:507–519. doi: 10.1016/j.ncl.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Bilici M., Efe H., Köroğlu M., Uydu H.A., Bekaroğlu M., Değer O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J. Affect. Disord. 2001;64:43–51. doi: 10.1016/S0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 89.Sanoobar M., Dehghan P., Khalili M., Azimi A., Seifar F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutr. Neurosci. 2015;19:138–143. doi: 10.1179/1476830515Y.0000000002. [DOI] [PubMed] [Google Scholar]
- 90.Fakhrabadi M.A., Ghotrom A.Z., Mozaffari-Khosravi H., Nodoushan H.H., Nadjarzadeh A., Information R. Effect of Coenzyme Q10 on Oxidative Stress, Glycemic Control and Inflammation in Diabetic Neuropathy: A Double Blind Randomized Clinical Trial. Int. J. Vitam. Nutr. Res. 2014;84:252–260. doi: 10.1024/0300-9831/a000211. [DOI] [PubMed] [Google Scholar]
- 91.Parisi V., Centofanti M., Gandolfi S., Marangoni D., Rossetti L., Tanga L., Tardini M., Traina S., Ungaro N., Vetrugno M., et al. Effects of Coenzyme Q10 in Conjunction With Vitamin E on Retinal-evoked and Cortical-evoked Responses in Patients With Open-angle Glaucoma. J. Glaucoma. 2014;23:391–404. doi: 10.1097/IJG.0b013e318279b836. [DOI] [PubMed] [Google Scholar]
- 92.Ozates S., Elgin K.U., Yilmaz N.S., Demirel O.O., Sen E., Yilmazbas P. Evaluation of oxidative stress in pseudo-exfoliative glaucoma patients treated with and without topical coenzyme Q10 and vitamin E. Eur. J. Ophthalmol. 2019;29:196–201. doi: 10.1177/1120672118779486. [DOI] [PubMed] [Google Scholar]
- 93.Premkumar V.G., Yuvaraj S., Vijayasarathy K., Gangadaran S.G.D., Sachdanandam P. Effect of Coenzyme Q10, Riboflavin and Niacin on Serum CEA and CA 15-3 Levels in Breast Cancer Patients Undergoing Tamoxifen Therapy. Biol. Pharm. Bull. 2007;30:367–370. doi: 10.1248/bpb.30.367. [DOI] [PubMed] [Google Scholar]
- 94.Premkumar V.G., Yuvaraj S., Shanthi P., Sachdanandam P. Co-enzyme Q10, riboflavin and niacin supplementation on alteration of DNA repair enzyme and DNA methylation in breast cancer patients undergoing tamoxifen therapy. Br. J. Nutr. 2008;100:1179–1182. doi: 10.1017/S0007114508968276. [DOI] [PubMed] [Google Scholar]
- 95.Premkumar V.G., Yuvaraj S., Sathish S., Shanthi P., Sachdanandam P. Anti-angiogenic potential of CoenzymeQ10, riboflavin and niacin in breast cancer patients undergoing tamoxifen therapy. Vasc. Pharmacol. 2008;48:191–201. doi: 10.1016/j.vph.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 96.Liu H.T., Huang Y.C., Cheng S.B., Huang Y.T., Lin P.T. Effects of coenzyme Q10 supplementation on antioxidant capacity and inflammation in hepatocellular carcinoma patients after surgery: A randomized, placebo-controlled trial. Nutr. J. 2015;15:1–9. doi: 10.1186/s12937-016-0205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iwase S., Kawaguchi T., Yotsumoto D., Doi T., Miyara K., Odagiri H., Kitamura K., Ariyoshi K., Miyaji T., Ishiki H., et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and l-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: A multi-institutional, randomized, exploratory trial (JORTC-CAM01) Support. Care Cancer. 2015;24:637–646. doi: 10.1007/s00520-015-2824-4. [DOI] [PubMed] [Google Scholar]
- 98.Hoenjet K., Dagnelie P., Delaere K., Wijckmans N., Zambon J., Oosterhof G. Effect of a Nutritional Supplement Containing Vitamin E, Selenium, Vitamin C and Coenzyme Q10 on Serum PSA in Patients with Hormonally Untreated Carcinoma of the Prostate: A Randomised Placebo-Controlled Study. Eur. Urol. 2005;47:433–440. doi: 10.1016/j.eururo.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 99.Rusciani L., Proietti I., Rusciani A., Paradisi A., Sbordoni G., Alfano C., Panunzi S., De Gaetano A., Lippa S. Low plasma coenzyme Q10 levels as an independent prognostic factor for melanoma progression. J. Am. Acad. Dermatol. 2006;54:234–241. doi: 10.1016/j.jaad.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 100.Rusciani L., Proietti I., Paradisi A., Rusciani A., Guerriero G., Mammone A., De Gaetano A., Lippa S. Recombinant interferon α-2b and coenzyme Q10 as a postsurgical adjuvant therapy for melanoma: A 3-year trial with recombinant interferon-α and 5-year follow-up. Melanoma Res. 2007;17:177–183. doi: 10.1097/CMR.0b013e32818867a0. [DOI] [PubMed] [Google Scholar]
- 101.Sharlip I.D., Jarow J.P., Belker A.M., Lipshultz L.I., Sigman M., Thomas A.J., Schlegel P.N., Howards S.S., Nehra A., Damewood M.D., et al. Best practice policies for male infertility. Fertil. Steril. 2002;77:873–882. doi: 10.1016/S0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 102.Eroglu M., Sahin S., Durukan B., Ozakpinar O.B., Erdinc N., Turkgeldi L., Sofuoglu K., Karateke A. Blood Serum and Seminal Plasma Selenium, Total Antioxidant Capacity and Coenzyme Q10 Levels in Relation to Semen Parameters in Men with Idiopathic Infertility. Biol. Trace Elem. Res. 2014;159:46–51. doi: 10.1007/s12011-014-9978-7. [DOI] [PubMed] [Google Scholar]
- 103.Balercia G., Buldreghini E., Vignini A., Tiano L., Paggi F., Amoroso S., Ricciardo-Lamonica G., Boscaro M., Lenzi A., Littarru G. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: A placebo-controlled, double-blind randomized trial. Fertil. Steril. 2009;91:1785–1792. doi: 10.1016/j.fertnstert.2008.02.119. [DOI] [PubMed] [Google Scholar]
- 104.Safarinejad M.R., Safarinejad S., Shafiei N., Safarinejad S. Effects of the Reduced Form of Coenzyme Q 10 (Ubiquinol) on Semen Parameters in Men with Idiopathic Infertility: A Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 2012;188:526–531. doi: 10.1016/j.juro.2012.03.131. [DOI] [PubMed] [Google Scholar]
- 105.Nadjarzadeh A., Sadeghi M.R., Amirjannati N., Vafa M.R., Motevalian S.A., Gohari M.R., Akhondi M.A., Yavari P., Shidfar F. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: A randomized double-blind, placebo controlled trial. J. Endocrinol. Investig. 2011;34:e224–e228. doi: 10.3275/7572. [DOI] [PubMed] [Google Scholar]
- 106.Pasqualotto F.F., Sundaram A., Sharma R.K., Borges E., Pasqualotto E.B., Agarwal A. Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil. Steril. 2008;89:602–607. doi: 10.1016/j.fertnstert.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 107.Festa R., Giacchi E., Raimondo S., Tiano L., Zuccarelli P., Silvestrini A., Meucci E., Littarru G.P., Mancini A. Coenzyme Q10supplementation in infertile men with low-grade varicocele: An open, uncontrolled pilot study. Andrologia. 2013;46:805–807. doi: 10.1111/and.12152. [DOI] [PubMed] [Google Scholar]
- 108.Ibáñez L., Oberfield S.E., Witchel S.F., Auchus R.J., Chang R.J., Codner E., Dabadghao P., Darendeliler F., Elbarbary N.S., Gambineri A., et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm. Res. Paediatr. 2017;88:371–395. doi: 10.1159/000479371. [DOI] [PubMed] [Google Scholar]
- 109.Agapova S.E., Cameo T., Sopher A.B., Oberfield S.E. Diagnosis and Challenges of Polycystic Ovary Syndrome in Adolescence. Semin. Reprod. Med. 2014;32:194–201. doi: 10.1055/s-0034-1371091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Azziz R. Diagnosis of Polycystic Ovarian Syndrome: The Rotterdam Criteria Are Premature. J. Clin. Endocrinol. Metab. 2006;91:781–785. doi: 10.1210/jc.2005-2153. [DOI] [PubMed] [Google Scholar]
- 111.Banihani S.A. Effect of Coenzyme Q10 Supplementation on Testosterone. Biomolecules. 2018;8:172. doi: 10.3390/biom8040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Samimi M., Mehrizi M.Z., Foroozanfard F., Akbari H., Jamilian M., Ahmadi S., Asemi Z. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2017;86:560–566. doi: 10.1111/cen.13288. [DOI] [PubMed] [Google Scholar]
- 113.Rahmani E., Jamilian M., Samimi M., Mehrizi M.Z., Aghadavod E., Akbari E., Tamtaji O.R., Asemi Z. The effects of coenzyme Q10 supplementation on gene expression related to insulin, lipid and inflammation in patients with polycystic ovary syndrome. Gynecol. Endocrinol. 2017;34:217–222. doi: 10.1080/09513590.2017.1381680. [DOI] [PubMed] [Google Scholar]
- 114.Izadi A., Shirazi S., Taghizadeh S., Gargari B.P. Independent and Additive Effects of Coenzyme Q10 and Vitamin E on Cardiometabolic Outcomes and Visceral Adiposity in Women with Polycystic Ovary Syndrome. Arch. Med Res. 2019;50:1–10. doi: 10.1016/j.arcmed.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 115.Verjee L.S., Verhoekx J.S.N., Chan J.K.K., Krausgruber T., Nicolaidou V., Izadi D., Davidson D., Feldmann M., Midwood K.S., Nanchahal J. Unraveling the signaling pathways promoting fibrosis in Dupuytren’s disease reveals TNF as a therapeutic target. Proc. Natl. Acad. Sci. USA. 2013;110:E928–E937. doi: 10.1073/pnas.1301100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lourens W. Could coenzyme Q10 be the treatment for Dupuytren’s disease? BMJ Case Rep. 2019;12:e226419. doi: 10.1136/bcr-2018-226419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rowland L.M., Pradhan S., Korenic S., Wijtenburg S., Hong L.E., Edden R.A., Barker P.B. Elevated brain lactate in schizophrenia: A 7 T magnetic resonance spectroscopy study. Transl. Psychiatry. 2016;6:e967. doi: 10.1038/tp.2016.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Do K.Q., Cabungcal J.H., Frank A., Steullet P., Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 119.Bergman O., Ben-Shachar D. Mitochondrial Oxidative Phosphorylation System (OXPHOS) Deficits in Schizophrenia. Can. J. Psychiatry. 2016;61:457–469. doi: 10.1177/0706743716648290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Imagawa M. Low Erythrocyte Coenzyme Q10Level in Schizophrenic Patients. Psychiatry Clin. Neurosci. 1989;43:143–145. doi: 10.1111/j.1440-1819.1989.tb02562.x. [DOI] [PubMed] [Google Scholar]
- 121.Maguire Á., Hargreaves A., Gill M. Coenzyme Q10 and neuropsychiatric and neurological disorders: Relevance for schizophrenia. Nutr. Neurosci. 2018;23:756–769. doi: 10.1080/1028415x.2018.1556481. [DOI] [PubMed] [Google Scholar]
- 122.Kikkawa K., Takehara I., Miyakoshi T., Miyawaki H. Safety of high dose supplementation of coenzyme Q10 in healthy human adults. Jpn. J. Food Chem. Saf. 2007;14:76–81. doi: 10.18891/jjfcs.14.2_76. [DOI] [Google Scholar]
- 123.Mortensen A.L., Rosenfeldt F., Filipiak K.J. Effect of coenzyme Q10 in Europeans with chronic heart failure: A sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol. J. 2013;26:147–156. doi: 10.5603/CJ.a2019.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]