Abstract
Chronic kidney disease (CKD) is a common comorbidity among patients taking direct-acting oral anticoagulants (DOACs). Herein, we evaluate the influence of kidney function on Stroke/SEE, hemorrhage and composite endpoints (Stroke/SEE/hemorrhage/death and Stroke/SEE/death) among patients on DOACs and warfarin. Baseline kidney function was categorized as GFR≥60 (reference), 45-59 and <45ml/min/1.73m2 for participants in the RE-LY (n=18,049), ARISTOTLE (n=18,187), and ENGAGE AF (n=20,798) trials. Incidence of events was compared across GFR categories. Hazard ratios for events was estimated using Cox regression using intention-to-treat analysis adjusting for known predictors. A large proportion of participants had GFR<60 (25-29% had GFR ≥45<60 and 9.5 to 12.6% with GFR <45). Compared to patients with GFR≥60, warfarin users across the trials with GFR≥45-59 and GFR<45 had a higher incidence of hemorrhage (p-values<0.0001) and warfarin users in the ARISTOTLE and ENGAGE trials had higher incidence of stroke/SEE (p-values ≤0.05). Compared to patients with GFR≥60, dabigatran users with GFR≥45-59 and GFR<45 had a higher incidence of stroke/SEE (p≤0.02), hemorrhage (p<0.001) and both composite endpoints (p<0.0001). Compared to patients with GFR≥60, apixaban and edoxaban users with GFR≥45-59 and GFR<45 had a higher incidence of hemorrhage (p-values≤0.05) and composite endpoints (p-values≤0.05). After adjustment, compared to patients with GFR≥60, warfarin users with GFR<60 in the ARISTOTLE and RELY trials had a higher risk of hemorrhage (p<0.05), as did dabigatran (p<0.001) and edoxaban (p≤0.005) users, while apixaban users did not exhibit an increased risk (p=0.08 GFR≥45-59; p=0.71 GFR<45). Kidney function significantly influences the safety and efficacy of oral anticoagulants.
Keywords: chronic kidney disease, kidney function, kidney impairment, representation, warfarin, rivaroxaban, apixaban, dabigatran, edoxaban, hemorrhage, thromboembolism, net benefit
Introduction
Chronic kidney disease (CKD) is a public health problem affecting 8–16% people worldwide and 13-15% (estimated 31 million people) in the US.1,2 Among patients with cardiovascular disease (CVD), the prevalence of CKD is as high as 30–35%.3 It is widely recognized that CKD and CVD influence outcomes related to the other, and their co-management remains challenging. CVD is the leading cause of death for patients with CKD, and patients with moderate CKD are more likely to die of CVD than to progress to kidney failure.4 Despite the need for high-quality evidence for CVD interventions, systematic reviews demonstrate a clear and persistent under-representation of patients with CKD in randomized clinical trials (RCTs).5-7 This renders extrapolation of trial results to guide the treatment of complex patients across the spectrum of kidney function seen in real-world clinical practice tenuous.
Consider atrial fibrillation (AF) for instance. Both AF and CKD are increasingly prevalent in the general population and share common risk factors such as older age, hypertension and diabetes mellitus. The presence of CKD increases the risk of incident AF, and, likewise, AF increases the risk of CKD development and progression. Both conditions are associated with substantial thromboembolic risk and patients with CKD exhibit a paradoxical increase in bleeding risk.8-11 These findings have important implications for the use of oral anticoagulants (OACs) and expected cardiovascular risk reduction with treatment.
Direct-acting oral anticoagulants (DoACs; dabigatran, rivaroxaban, apixaban, edoxaban) offer several key advantages including superior or equal efficacy, favorable bleeding risk profile (especially ICH), fewer drug interactions and lack of monitoring requirements.12-16 The increasing use of DOAC (over OACs) among patients with AF17-20 is expected to expand further with the new American Heart Association and European Society of Cardiology guidelines recommending the use of DOACs over warfarin.21,22 Therefore, given the high prevalence of CKD in patients qualifying for anticoagulation understanding the efficacy and safety of DOACs in patients across the spectrum of CKD is vital.
We explore data from the DoAC clinical trials to highlight the representation of patients across the CKD spectrum and evaluate the influence of kidney function on the endpoints of stroke or systemic embolism (Stroke/SEE; primary efficacy) and hemorrhage (primary safety) for warfarin and the DOACs. We also assess evaluate the influence of kidney function on two composite endpoints; Stroke/SEE/ hemorrhage/death and Stroke/SEE/ death.
Methods:
Data sources:
To examine the relationship between GFR and clinical outcome in a post-hoc exploratory analysis, subject-level data were obtained for patients with atrial fibrillation treated with warfarin or a DoAC. Data from three large RCTs; Randomized Evaluation of Long-term Anticoagulant Therapy (RE-LY),17 Apixaban for Reduction in Stroke and Other Thromboembolic Events (ARISTOTLE),19 and The Effective Anticoagulation with Factor Xa Next Generation in AF-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) were analyzed.20 These trials supported approval of dabigatran (Pradaxa), apixaban (Eliquis), and edoxaban (Savaysa), respectively. Data from the study supporting approval of rivaroxaban (Xarelto) were not included because of concerns about the accuracy of the point-of-care International Normalized Ratio (INR) devices used in the study.18,23
Efficacy and Safety outcomes:
Each of the trials assessed the efficacy of the DoAC against warfarin dosed to maintain an INR of 2-3 among patients with non-valvular AF in a non-inferiority design. The primary efficacy outcome was stroke or systemic embolism (Stroke/SEE), uniformly defined across the trials.17-20 The key secondary efficacy outcome was death from any cause. The primary safety endpoint was major bleeding defined according to the ISTH criteria24 as clinically overt bleeding accompanied by a decrease in the hemoglobin level of at least 2 g per deciliter or transfusion of at least two units of packed red cells, occurring at a critical site, or resulting in death. The trials also presented composite safety and efficacy endpoints as measures of net benefit. For each trial we assessed, two composite endpoints the first including Stroke/SEE/ hemorrhage/death and the second including stroke/SEE/death (without major bleeding).
Kidney function:
We categorize CKD based on baseline glomerular filtration rate (GFR)25 following the Kidney Disease- Improving Global Outcomes (KDIGO) recommendations.1,26
We used the four-variable MDRD Study equation27 to calculate the GFR at baseline and categorized patients kidney function into five groups: more than 90 ml/min/1.73m2 (stage 1), 60–89 ml/min/1.73m2 (stage 2), 45-59 ml/min/1.73m2 (Stage 3a) and 30-44 ml/min/1.73m2 (Stage 3b) and <30 ml/min/1.73m2 (stage 4, 5). Patients with severely impaired kidney function, CrCl<25 ml/min (apixaban) 19 or <30 ml/min (dabigatran, rivaroxaban, and edoxaban) were excluded from the trials.17,18,20 However, our analysis included a few patients with severely impaired kidney function. We assume that at the time of enrollment, patients met inclusion criteria related to kidney function. Consider the following as an example. A patient with a previous laboratory result reporting a CrCL of 31ml/min meets inclusion criteria, and is considered enrolled into the trial. However, after the patient has initiated study treatment, results of labs drawn at the baseline study visit report a CrCL of 29ml/min. We retained such patients in our analysis.
Statistical Methods:
Group differences were assessed using analysis of variance for continuous variables and χ2 test of independence for categorical variables.
Absolute risk:
For each individual patient, person years of follow-up (p-years) were calculated by dividing the days from first dose (DOAC or warfarin) to event or end of follow-up by 365.25 days. Incidence rate for Stroke/SEE, hemorrhage and composite stroke/SEE/bleeding/death endpoint within each kidney function category was calculated by dividing the number of events experienced by total follow-up time (p-years) accrued among patients within the kidney function category.
We computed a moving average (Supplementary Figure 1) of the Stroke/SEE, major bleeding and composite stroke/SEE/bleeding/death endpoint incidence by GFR for each treatment arm to depict the relationship between event rate and kidney function. Informed by the KDIGO, we grouped patients in RE-LY (Supplementary Table 1a), ARISTOTLE (Supplementary Table 2a), and ENGAGE (Supplementary Table 3a), into five-kidney function categories. With GFR>90ml/min/1.73m2 as the reference group, we calculated the incidence rate ratio (IRR) for patients with 60–89 ml/min/1.73m2 (stage 2), 45-59 ml/min/1.73m2 (Stage 3a) and 30-44 ml/min/1.73m2 (Stage 3b) and <30 ml/min/1.73m2 (stage 4, 5). The incidence rates for Stroke/SEE and major bleeding are similar for patients with GFR≥90 and GFR≥60-89. Similarly, given the limited number of patients with GFR<30, recognition that stage 3b CKD (GFR≥30-44) reflects progression of CKD and a similar increase risk of events, patients with GFR<30 and GFR≥30-44 were combined for subsequent analyses. Therefore, we present the incidence and IRR for Stroke/SEE and major bleeding and the composite stroke/SEE/bleeding/death endpoint for patients with GFR≥60 (reference group), 45-59 ml/min/1.73m2 (Stage 3a) and <45 ml/min/1.73m2 (Stage 3b, 4, 5) for warfarin and each of the DOACs across trials.
Relative Risk
To assess the risk of clinical (thromboembolic and hemorrhagic) events, the hazard ratios (HR) and 95% CI were calculated using Cox regression in an intention-to-treat analysis. The relative risk of Stroke/SEE, major bleeding and stroke, Stroke/SEE and major bleeding, and death across the spectrum of kidney impairment, are presented within each treatment arm after adjustment for known risk factors (e.g. age, hypertension, etc). All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) at a non-directional alpha level of 0.05.
Results:
Charateristics of 57,934 patients with AF from the RE-LY (n=18,049), ARISTOTLE (n=18,187), and ENGAGE (20,798) trials categorized by baseline GFR are presented in Table 1.
Table 1:
Demographics, AF phenotype and key risk factors at baseline by level of kidney function (glomerular filtration rate; GFR) impairment
| GFR (ml/min/1.73m2) | ≥90 | ≥60-89 | ≥45-59 | ≥30-44 | < 30 | p- value |
|
|---|---|---|---|---|---|---|---|
| RELY (n=18,049) | N=1,293 | N=9,487 | N=5,091 | N=2,022 | N=156 | ||
| Dabigatran 110 | 400 (30.9%) | 3,209 (33.8%) | 1,660 (32.6%) | 672 (33.2%) | 52 (33.3%) | ||
| Dabigatran 150 | 450 (34.8%) | 3,133 (33.0%) | 1,710 (33.6%) | 708 (35.0%) | 57 (36.5%) | ||
| Warfarin | 443 (34.3%) | 3,145 (33.2%) | 1,721 (33.8%) | 642 (31.8%) | 47 (30.1%) | ||
| Age (years) | 67.8 ± 10.7 | 70.1 ± 8.5 | 73.7 ± 8.0 | 74.7 ± 7.3 | 72.0 ± 8.3 | <0.001 | |
| Weight (kg) | 81.0 ± 23.5 | 83.2 ± 19.9 | 82.0 ± 18.5 | 82.6 ± 18.3 | 88.1 ± 19.5 | <0.001 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Female | 333 (25.8%) | 3,136 (33.1%) | 2,005 (39.4%) | 1,011 (50.0%) | 84 (53%) | <0.001 | |
| Race | White | 756 (58.5%) | 6,626 (69.8%) | 3,684 (72.4%) | 1,450 (71.7%) | 108 (69.2%) | <0.001 |
| Black | 21 (1.6%) | 105 (1.1%) | 31 (0.6%) | 15 (0.7%) | 3 (1.9%) | ||
| Asian | 376 (29.1%) | 1,658 (17.5%) | 602 (11.8%) | 216 (10.7%) | 18 (11.5%) | ||
| Paroxysmal AF | 426 (33.0%) | 3,167 (33.4%) | 1,630 (32%) | 633 (31.3%) | 58 (37.2%) | 0.18 | |
| Permanent/Persistent AF | 867 (67.0%) | 6,316 (66.6%) | 3,461 (68.0%) | 1,388 (68.7%) | 98 (62.8%) | ||
| CHADS2 Score | |||||||
| 0-1 | 474 (36.7%) | 3,523 (37.1%) | 1,360 (26.7%) | 383 (18.9%) | 26 (16.7%) | <0.001 | |
| 2 | 472 (36.5%) | 3,300 (34.8%) | 1,896 (37.3%) | 723 (35.8%) | 46 (29.5%) | ||
| 3 | 231 (17.9%) | 1,732 (18.3%) | 1,112 (21.8%) | 508 (25.1%) | 50 (32.1%) | ||
| 4 | 88 (6.8%) | 703 (7.4%) | 524 (10.3%) | 267 (13.2%) | 26 (16.7%) | ||
| 5 to 6 | 29 (2.2%) | 229 (2.4%) | 199 (3.9%) | 141 (7.0%) | 8 (5.1%) | ||
| Hypertension | 912 (70.5%) | 7,270 (76.6%) | 4,151 (81.5%) | 1,756 (86.8%) | 139 (89.1%) | <0.001 | |
| History of Stroke/ TIA | 176 (13.6%) | 1,191 (12.6%) | 610 (12%) | 273 (13.5%) | 19 (12.2%) | 0.31 | |
| Heart failure | 416 (32.2%) | 2,786 (29.4%) | 1,683 (33.1%) | 803 (39.7%) | 85 (54.5%) | <0.001 | |
| Diabetes Mellitus | 297 (23%) | 2,018 (21.3%) | 1,190 (23.4%) | 636 (31.5%) | 64 (41%) | <0.001 | |
| Antiplatelet | 496 (38.4%) | 3,741 (39.0%) | 2,154 (42.3%) | 926 (45.8%) | 70 (44.9%) | <0.001 | |
| ARISTOTLE (n=18,187) | N=1,872 | N=9,960 | N=4,598 | N=1,582 | N=175 | ||
| Apixaban | 941 (51.3%) | 4,975 (50.0%) | 2,304 (50.1%) | 809 (50.1%) | 84 (48.0%) | ||
| Warfarin | 931 (49.7%) | 4,985 (50.0%) | 2,294 (49.9%) | 773 (48.9%) | 91 (52.0%) | ||
| Age (years) | 63.2 ± 10.9 | 68.0 ± 9.4 | 71.8 ± 8.8 | 74.5 ± 8.1 | 73.3 ± 8.0 | <0.001 | |
| Weight (kg) | 85.6 ± 23.8 | 84.6 ± 20.6 | 83.2 ± 20.0 | 81.4 ± 19.2 | 82.3 ± 17.2 | <0.001 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Female | 513 (27.4%) | 3,137 (31.5%) | 1,895 (41.2%) | 779 (49.2%) | 88 (50.3%) | <0.001 | |
| Race | White | 1,403 (75%) | 8,142 (81.8%) | 3,946 (85.8%) | 1,382 (87.4%) | 154 (88%) | <0.001 |
| Black | 47 (2.5%) | 122 (1.22%) | 42 (0.91%) | 14 (0.88%) | 2 (1.1%) | ||
| Asian | 369 (19.7%) | 1,515 (15.2%) | 556 (12.1%) | 172 (10.9%) | 18 (10.3%) | ||
| Paroxysmal AF | 282 (15.1%) | 1,558 (15.6%) | 689 (15%) | 240 (15.2%) | 22 (12.6%) | 0.68 | |
| Permanent/Persistent AF | 1,589 (84.9%) | 8,401 (84.4%) | 3,908 (85%) | 1,342 (84.8%) | 153 (87.4%) | ||
| CHADS2 Score | <0.001 | ||||||
| 0-1 | 815 (43.5%) | 3,796 (38.1%) | 1,276 (27.8%) | 267 (16.9%) | 29 (16.6%) | ||
| 2 | 662 (35.4%) | 3,501 (35.2%) | 1,686 (36.7%) | 607 (38.4%) | 50 (28.6%) | ||
| 3 | 266 (14.2%) | 1,624 (16.3%) | 937 (20.4%) | 387 (24.5%) | 62 (35.4%) | ||
| 4 | 103 (5.5%) | 768 (7.7%) | 486 (10.6%) | 200 (12.6%) | 24 (13.7%) | ||
| 5 to 6 | 26 (1.4%) | 271 (2.7%) | 213 (4.6%) | 121 (7.6%) | 10 (5.7%) | ||
| Hypertension | 1,568 (83.8%) | 8,611 (86.5%) | 4,107 (89.3%) | 1,454 (91.9%) | 166 (94.9%) | <0.001 | |
| History of Stroke/ TIA | 288 (15.4%) | 1,784 (17.9%) | 928 (20.2%) | 345 (21.8%) | 34 (19.4%) | <0.001 | |
| Heart failure | 624 (33.3%) | 3,331 (33.4%) | 1,720 (37.4%) | 677 (42.8%) | 90 (51.4%) | <0.001 | |
| Diabetes Mellitus | 523 (27.9%) | 2,325 (23.3%) | 1,138 (24.8%) | 492 (31.1%) | 63 (36%) | <0.001 | |
| Antiplatelet | 613 (32.8%) | 3,117 (31.3%) | 1,525 (33.2%) | 571 (36.1%) | 73 (41.7%) | ||
| ENGAGE (n=20,798) | N=1,929 | N=10,805 | N=5,682 | N=2,139 | N=243 | ||
| Edoxaban 30mg | 649 (33.6%) | 3,591 (33.2%) | 1,888 (33.2%) | 717 (33.5%) | 81 (33.3%) | ||
| Edoxaban 60mg | 663 (34.4%) | 3,578 (33.1%) | 1,895 (33.4%) | 718 (33.6%) | 78 (32.1%) | ||
| Warfarin | 617 (32.0%) | 3,636 (33.7%) | 1,899 (33.4%) | 704 (32.9%) | 84 (34.6%) | ||
| Age (years) | 65.1 ± 10.4 | 69.0 ± 9.3 | 73.0 ± 8.4 | 74.7 ± 7.9 | 73.3 ± 8.2 | <0.001 | |
| Weight (kg) | 85.8 ± 23.0 | 84.4 ± 21.0 | 82.8 ± 19.7 | 83.8 ± 19.4 | 89.3 ± 20.6 | <0.001 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Female | 493 (25.6%) | 3,606 (33.4%) | 2,589 (45.6%) | 1,090 (51.0%) | 134 (55.1%) | <0.001 | |
| Race | White | 1,457 (75.5%) | 8,634 (79.9%) | 4,698 (82.7%) | 1,810 (84.6%) | 213 (87.7%) | <0.001 |
| Black | 51 (2.6%) | 143 (1.3%) | 57 (1.0%) | 19 (0.9%) | 4 (1.7%) | ||
| Asian | 345 (17.9%) | 1,587 (14.7%) | 700 (12.3%) | 230 (10.8%) | 14 (5.8%) | ||
| Paroxysmal AF | 500 (25.9%) | 2,708 (25.1%) | 1,494 (26.3%) | 523 (24.5%) | 61 (25.1%) | 0.33 | |
| Permanent/Persistent AF | 1,429 (74.1%) | 8,095 (74.9%) | 4,188 (73.7%) | 1,615 (75.5%) | 182 (74.8%) | ||
| CHADS2 Score | |||||||
| 0-1 | excluded | excluded | excluded | excluded | excluded | ||
| 2 | 1,084 (56.2%) | 5,545 (51.3%) | 2,537 (44.7%) | 785 (32.10%) | 78 (32.1%) | <0.001 | |
| 3 | 520 (27.0%) | 3,141 (29.1%) | 1778 (31.3%) | 754 (35.3%) | 97 (39.9%) | ||
| 4 | 250(13.0%) | 1,542 (14.3%) | 894 (15.7%) | 399 (18.7%) | 44 (18.1%) | ||
| 5 to 6 | 75 (3.9%) | 577 (5.4%) | 473 (8.3%) | 201 (9.4%) | 21 (9.9%) | ||
| Hypertension | 1,756 (91.0%) | 10,030 (92.8%) | 5,393 (94.9%) | 2,053 (96.0%) | 233 (95.9%) | <0.001 | |
| History of Stroke/ TIA | 569 (29.5%) | 3,351 (31.0%) | 1,708 (30.0%) | 395 (28.6%) | 55 (22.6%) | <0.001 | |
| Heart failure | 1,127 (58.4%) | 6,086 (56.3%) | 3,261 (57.4%) | 1,320 (61.7%) | 168 (69.1%) | <0.001 | |
| Diabetes Mellitus | 789 (40.9%) | 3,805 (35.2%) | 1,976 (34.8%) | 822 (38.4%) | 129 (53.1%) | <0.001 | |
| Antiplatelet | 609 (31.6%) | 3,337 (30.9%) | 1,820 (32.0%) | 720 (33.7%) | 89 (36.6%) | 0.04 |
Patients of other race are not included in this table (edoxaban n=835; apixaban 302)
CHADS2 denotes a cumulative score assigning one point each for congestive heart failure, hypertension, age >75, Diabetes and two points for a prior stroke/TIA
Missing data for Type of AF (RELY n=5; ARISTOTLE n=3; ENGAGE n=4), History of Stroke/ TIA (ENGAGE n=1)
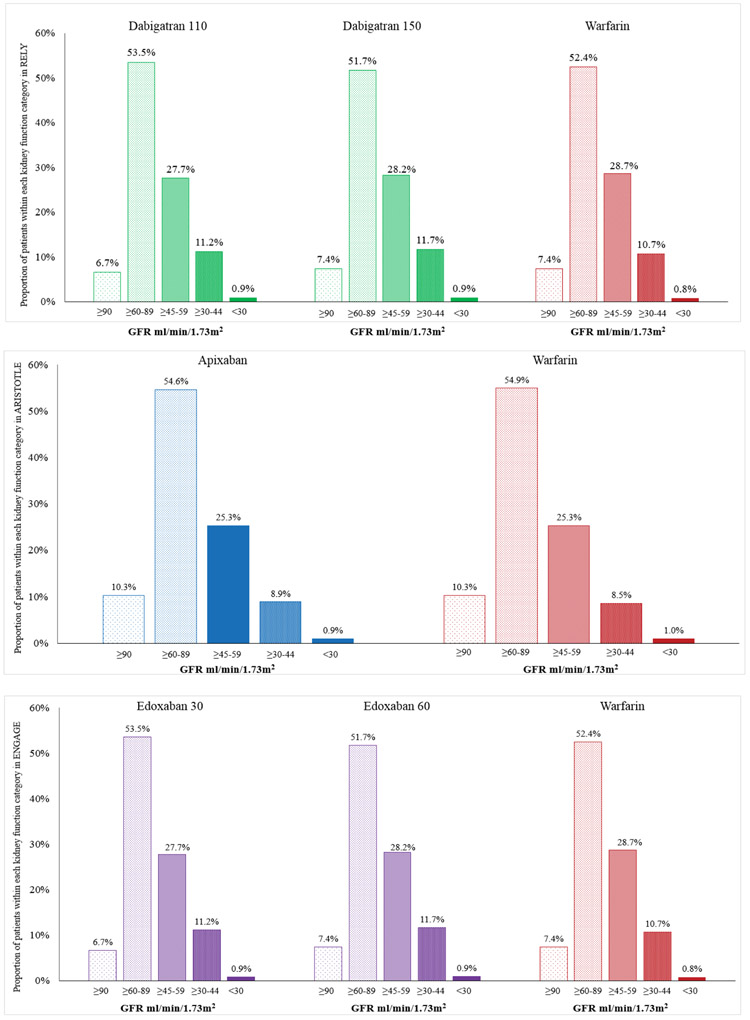
Within each of the trials, the proportional distribution of patients with varying degrees of kidney function was similar in the DOAC and warfarin arms. Of note, 35 to 43% of patients in these trials have impaired kidney function (GFR<60 ml/min/1.73m2) with 25-29% with GFR ≥45<60 ml/min/1.73m2and 9.5 to 12.6% with GFR <45 ml/min/1.73m2 (Figure 1).
Figure 1:
Representation of patients with compromised kidney function (based on baseline glomerular filtration rate; GFR) in DOAC trials
Across the trials, patients with impaired kidney function were older (p<0.001), and more likely to be female (p<0.001; Supplementary Figure 2a). Whites (60-88%) and Asians (6-30%) constituted a majority of patients, with Blacks constituting a minority (1-2.5%; Supplementary Figure 2b). Additionally, the prevalence of permanent or persistent AF was higher (range 63-75%) than that of paroxysmal AF (25-37%; Supplementary Figure 2c). As expected, the prevalence of hypertension (p<0.001), diabetes (p<0.001), and heart failure (p<0.001) was higher among patients with impaired kidney function (Table 1) contributing to higher CHADS2 score in these patients (Supplementary Figure 2d, p<0.001). Parallel to the increase in comorbidities, concomitant antiplatelet use was also higher in patients with impaired kidney function (Supplementary Figure 2e).
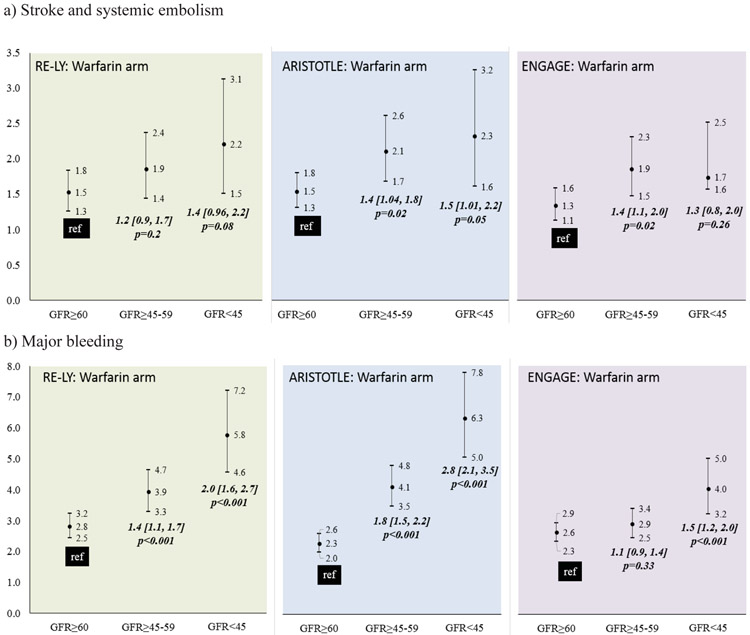
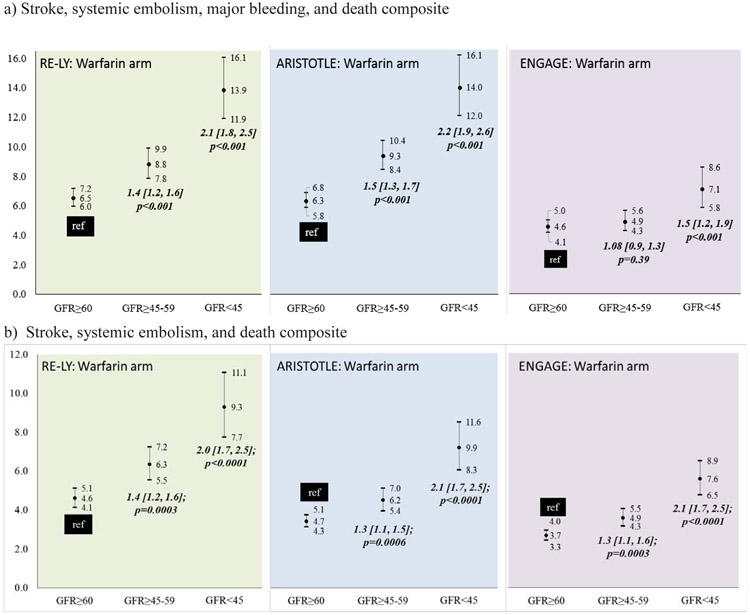
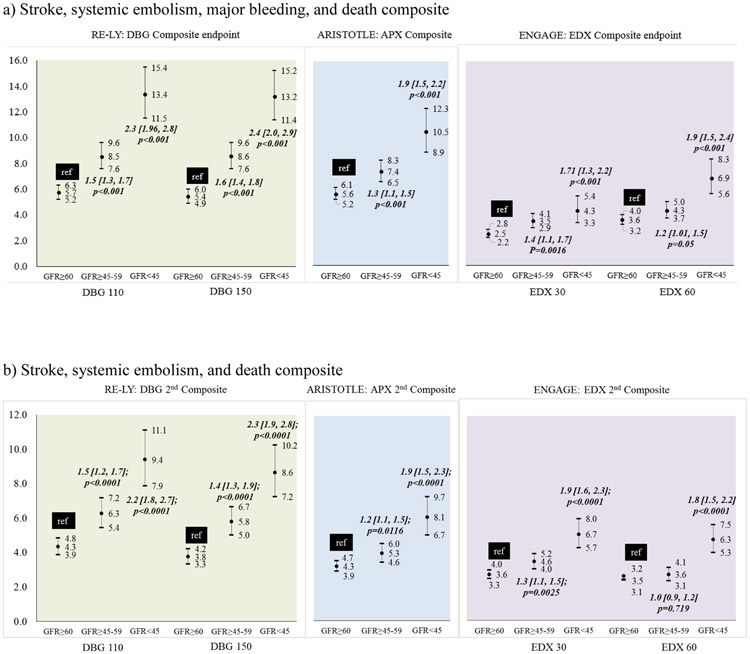
Outcomes among warfarin-treated patients across the DOAC trials displayed a consistent trend. Patients with impaired kidney function had poorer outcomes (Figure 2). In the RELY trial (Supplementary Table 1b), among warfarin treated patients, the incidence of stroke/SEE did not differ significantly by kidney function (Figure 2a-left). However, compared to patients with GFR≥60, those with GFR≥45-59 and GFR<45 had a higher incidence of major bleeding (Figure 2b-left) and composite stroke/SEE/ death endpoint with bleeding (Figure 3a-left) and without bleeding (Figure 3b-left). In the ARISTOTLE trial (Supplementary Table 2b), among warfarin treated patients, compared to patients with GFR≥60, those with GFR≥45-59 and GFR<45 had a higher incidence of stroke/SEE (Figure 2a-center), major bleeding (Figure 2b-center) composite stroke/SEE/ death endpoint with bleeding (Figure 3a-center) and without bleeding (Figure 3b-center). In the ENGAGE trial (Supplementary Table 3b), compared to patients with GFR≥60, those with GFR≥45-59, but not GFR<45 had a higher incidence of stroke/SEE (Figure 2a-right). The risk of major bleeding (Figure 2b-right) and composite stroke/SEE/ death endpoint with bleeding (Figure 3a-right) and without bleeding (Figure 3b-right) was higher in patients with GFR<45.
Figure 2:
Incidence of a) stroke and systemic embolism, b) major bleeding among warfarin treated patients in the DOAC trials
Figure 3:
Incidence of composite endpoint of a) Stroke/SEE/ hemorrhage/death and b) Stroke/SEE/ death among warfarin treated patients in the DOAC trials
Outcomes among DOAC treated patients:
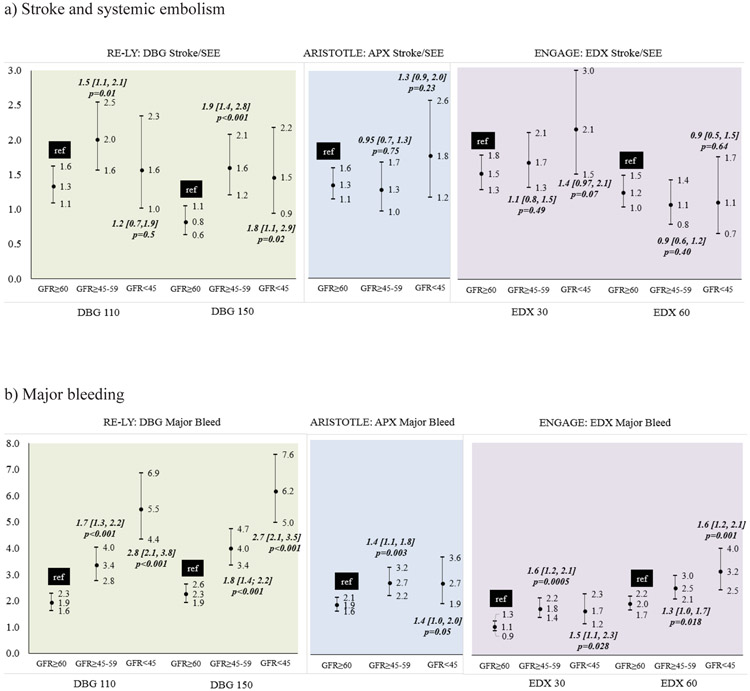
Poorer outcomes were observed among patients with impaired kidney function who were treated with dabigatran (Figure 4). In the RELY trial (Supplementary Table 1b), among patients receiving DBG, compared to patients with GFR≥60, those with GFR≥45-59 and GFR<45 had a higher incidence of stroke/SEE (Figure 4a-left), bleeding (Figure 4b-left) and composite stroke/SEE/ death endpoint with bleeding (Figure 5a-left) and without bleeding (Figure 5b-left). Among patients with impared kidney function treated with apixaban (Figure 4a-center) or edoxaban (Figure 4a-right), stroke SEE/rates were not significantly different. However, similar to dabigatran, a higher incidence of major bleeds and the composite stroke/SEE/ death endpoint with bleeding and without bleeding was observed in patients treated with apixaban (Figure 4b, 5a, 5b center) or edoxaban (Figure 4b, 5a, 5b right) who had impaired kidney funcition.
Figure 4:
Incidence of a) stroke and systemic embolism, b) major bleeding among DOAC treated patients in RELY, ARISTOTLE and ENGAGE trials
Figure 5:
Incidence of composite endpoint of a) Stroke/SEE/ hemorrhage/death and b) Stroke/SEE/ death among DOAC treated patients in RELY, ARISTOTLE and ENGAGE trials
Accounting for other predictors:
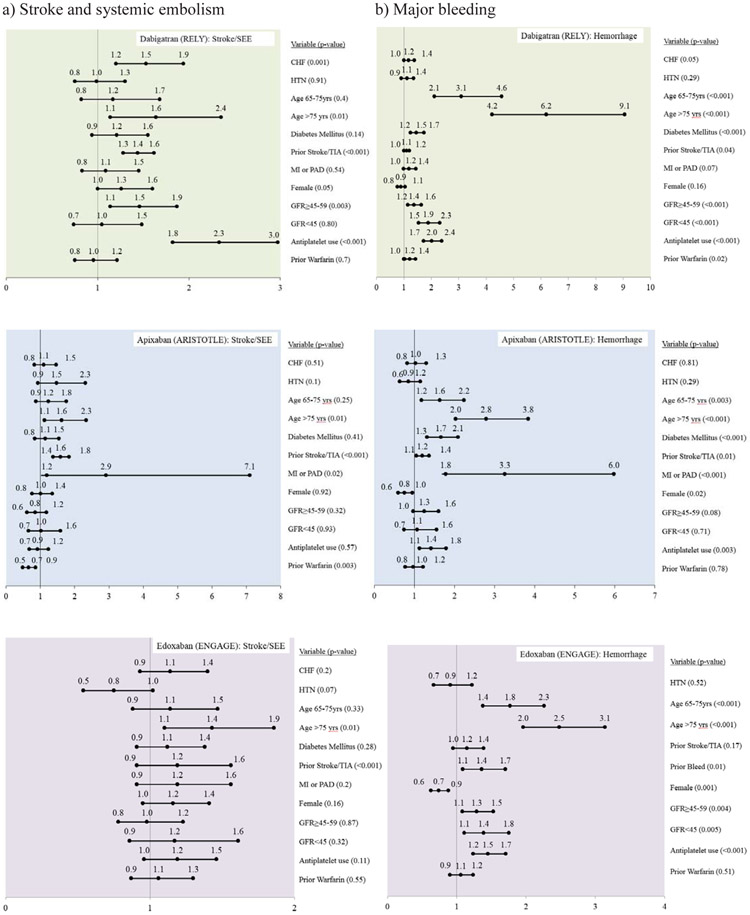
Among warfarin users across the DOAC trials (Supplementary Tables 4,5,6), after accounting for known stroke/SEE factors, there were no differences in risk of stroke/SEE across kidney function. Predictors associated with an increased risk of stroke/SEE included heart failure (RELY), older age (age>75 years RELY, >65 years ARISTOTLE and ENGAGE), prior stroke or TIA (all trials) diabetes (RELY), female gender (RELY) and antiplatelet use (RELY and ENGAGE).
Among warfarin users, after accounting for other predictors, compared with GFR≥60, the risk of hemorrhage was higher among patients with GFR≥45-59 and those with GFR<45 (25% and 69%, respectively in RELY; 51% and 200% respectively in ARISTOTLE). Among warfarin users in the ENGAGE trial, the hemorrhage risk was not significantly influenced by kidney function. Age>65 years and antiplatelet use were associated with an increased risk of hemorrhage across trials.
Among DBG users (Figure 6, Supplementary Table 4), compared to patients with GFR≥60, those with GFR≥45-59 had a higher risk of stroke/SEE (HR 1.46, 95% CI1.14-1.87, p=0.003) and major hemorrhage (HR 1.37, 95%CI 1.15-1.63, p<0.001). Patients with GFR<45 had a higher risk of hemorrhage (HR 1.89, 95% CI 1.54-2.31, p<0.001). Among DBG users, heart failure, age>75 years, prior stroke or TIA, female gender and prior antiplatelet use were associated with higher risk of Stroke/SEE and heart failure, age>65 years, diabetes, and prior antiplatelet and prior warfarin use were associated with higher risk of hemorrhage.
Figure 6:
Adjusted models for a) stroke and systemic embolism, b) major bleeding among DOAC treated patients in RELY, ARISTOTLE and ENGAGE trials
In the ARISTOTLE trial (Figure 6, Supplementary Table 5), among APIXABAN users, the risk of stroke/SEE or hemorrhage did not differ by kidney function. Among APIXABAN users, heart failure, age>75 years, prior stroke or TIA, PAD or MI, and prior warfarin use were associated with higher risk of Stroke/SEE and age>65 years, diabetes, prior stroke or TIA, PAD or MI, female gender, and prior antiplatelet use were associated with higher risk of hemorrhage.
In the ENGAGE trial (Figure 6, Supplementary Table 6), among edoxaban users, the risk of stroke/SEE did not differ by kidney function. However, compared with GFR≥60, the risk of hemorrhage was higher among edoxaban users with GFR≥45-59 (HR 1.29, 95%CI 1.08-1.53, p=0.004) and GFR<45 (HR 1.39, 95%CI 1.11-1.75, p=0.005). Age>75 years and prior stroke were associated higher risk of Stroke/SEE and age>65 years, and prior antiplatelet use were associated higher risk of hemorrhage.
Discussion
The underrepresentation of vulnerable patient subgroups in clinical trials creates an evidence gap, rendering management of complex patients seen in clinical practice challenging. The persistent underrepresentation and exclusion of patients with kidney disease from clinical trials 5,6,28-30 is particularly concerning given the increased risk of CVD in patients with CKD, and the significantly poor outcomes in patients with co-existent CKD-CVD. Recognizing this knowledge gap, we evaluated individual level data from the DoAC clinical trials to demonstrate the representation of CKD and assess its influence on the primary efficacy and safety endpoints in each of the DoAC trials.
To our knowledge, this is the first publication detailing the representation of patients across the kidney function spectrum and addressing key knowledge gaps by presenting the primary safety and efficacy endpoints by degree of kidney impairment. To date, post-hoc analysis of trial have categorized kidney function based on creatinine clearance (CrCL) estimated using the Cockcroft and Gault equation. However, we use GFR to categorize kidney function based on KDIGO recommendations.1,26 Concordant with the literature, the correlation between CrCL (using the Cockcroft and Gault equation),31 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)32 GFR and the MDRD GFR27 were lower (range for Pearson correlation coefficients 0.69 to 0.71 and 0.64 to 0.66, respectively) than that between the MDRD-GFR and CKD-EPI-GFR (range 0.96 to 0.98).33-35 Despite exclusion of patients with severe or end-stage renal disease (CrCl<25 ml/min (apixaban) 19 or <30 ml/min (dabigatran, rivaroxaban, and edoxaban), we demonstrate that clinical trials afford a unique opportunity to assess efficacy and safety of therapeutic interventions across the kidney function spectrum. A large proportion of trial participants had GFR between 45 and 60 (25-29%) or GFR <45 (9.5 to 12.6%), creating robust subgroups for analyses.
We evaluated of stroke/SEE risk, hemorrhage and a combined outcome across the kidney function spectrum for the DOACs. Given the differences in patient characteristics across trials and the lack of head to head comparisons for the DOACs, we refrain from drawing comparisons between trial results, but rather present the risk differences across kidney function within each treatment arm of the trials. Among trial participants, the stroke/SEE risk reduction (efficacy) afforded by warfarin (ARISTOTLE and ENGAGE trials) and dabigatran (RE-LY) was lower among patients with compromised kidney function. Importantly, our results demonstrate a significantly increased risk of major hemorrhage among warfarin and DOAC treated patients with compromised kidney function. This effect was consistent, with increased risk of hemorrhage observed for dabigatran, apixaban and edoxaban and for warfarin treated patients across the DOAC trials. The increased risk of hemorrhage among patients with compromised kidney function significantly lowers treatment net-benefit as demonstrated by the analysis of composite stroke/SEE/bleeding/death endpoint. While this composite endpoint presentation follows the precedent set by clinical trials allowing the reader to draw comparison with the original trial reports, we recognize that combining bleeding with thromboembolic endpoints is counterintuitive. Therefore we also report the composite endpoint without bleeding.
Renal elimination plays a prominent role in DOAC clearance, accounting, on average, 80% for dabigatran, 36% for rivaroxaban, 27% for apixaban and 50% for edoxaban elimination.36-41 Impaired kidney function is associated with longer half-lives and greater exposure (area under the curve) 42and therefore, likely has a greater impact on DOAC response. Current guidelines recommend reduction in DOAC doses based on kidney function. 43 Although, ARISTOTLE included a limited cohort (n=427) receiving reduced apixaban doses (2.5mg bid),19 the small sample size and limited number of events (12 stroke/systemic embolism, 20 major bleed events), did not allow for a separate assessment of events within the reduced dose group. We could not assess the influence of antiplatelet dose or therapy combinations on outcomes as data was not available. Finally, although gastroprotective therapies have been shown to decrease bleeding risk, as data on H2blocker and PPI use was not uniformly available, we could not evaluate their influence on bleeding risk.
Collectively, these results demonstrate that for the large proportion of oral anticoagulant users with compromised kidney function,8 determination of the net-benefit of OAC therapy is challenging.44 These findings have significant implications for assessment of bleeding risk at the time of DOAC initiation for a significant portion of patients with AF.45 Commonly used clinical risk prediction rules such as the HASBLED and ATRIA risk scores assign risk points for severe CKD/ESRD (e.g. GFR<30).46,47 Concordant with ORBIT bleeding score,48 our results demonstrate that the risk of bleeding is also higher for patients with GFR<60, indicating that kidney function has an impact on a significant proportion (42% of AF patients at our institution, data not shown) of patients on OACs. A recent report from the Geisinger health care system supports our findings; the risk of hemorrhage (minor and major) increases with decline in kidney function, for both warfarin and DOACs.49 Additionally, we demonstrate an incremental increase in risk as kidney function decreases, highlighting the need for incorporating a graded risk across the kidney function spectrum (e.g. GFR 45-59, 30-44, <30) to facilitate more nuanced decisions around bleeding risk and net benefit.
This analysis provided a unique opportunity to highlight the effect of kidney function on the efficacy and safety of warfarin, a drug with predominant non-renal clearance. The higher risks of hemorrhage among warfarin users across the trials support reports from our group and others. 49 We have reported on the influence of kidney function on warfarin response, showing 50-53 that patients with GFR<45 have a higher risk of hemorrhage even after adjustment for age, race, gender, INR at the time of the event, CYP2C9 and VKORC1 genotypes and concurrent antiplatelet therapy.52 Compared to patients with eGFR≥ 60 ml/min/1.73m2, those with eGFR <45 ml/min/1.73 m2 are at a 2-fold higher risk of hemorrhage and those with eGFR< 30 ml/min are at a 5.6-fold higher risk.
Given the current American Heart Association and European Cardiology Society recommendations on the preferential use of DOACs over warfarin in patients with AF,21,22 the use of DOACs is expected to continue to rise. The increasing prevalence of CKD and its co-prevalence with CVD calls for comparative effectiveness research to assess risk/ benefit tradeoff and enable personalized treatment decisions in patients with compromised kidney function.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
Patients on oral anticoagulants with impaired kidney function experience lower benefit and a higher risk of adverse effects. The effect of severely impaired kidney function (on drug response is recognized. However, the influence (and effect size) on risk/ benefit in patients with moderate impairment needs to be elucidated.
What question did this study address?
We present the influence and impact of kidney function on Stroke/SEE, hemorrhage and composite endpoints (Stroke/SEE/hemorrhage/death and Stroke/SEE/death) among patients on direct acting oral anticoagulants and warfarin. Importantly kidney function–event associations are presented across the spectrum of impairment.
What does this study add to our knowledge?
The study demonstrates and quantifies the influence of kidney function on the safety and efficacy of oral anticoagulants.
How might this change clinical pharmacology or translational science?
Kidney function should be considered in estimating individual risk-benefit to inform oral anticoagulant treatment. Clinical trials should include appropriate representation of patients with impaired kidney function and report medication safety and efficacy across the spectrum of kidney function at thresholds that can inform decision making.
Acknowledgments
Funding: This work was partially supported by grants from NIH R01HL092173 and K24HL133373 (NAL) and the FDA’s ORISE clinical fellows program.
Footnotes
COI: J.S., N.S., M.P., and J.F. are employed by the Food and Drug Administration (FDA). All other authors declared no competing interests for this work.
FDA Disclaimer: The opinions expressed in this manuscript are those of the authors and should not be interpreted as the position of the U.S. Food and Drug Administration.
References:
- 1.Levey AS, Coresh J. Chronic kidney disease. Lancet 2012;379:165–80. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- 3.Sarnak MJ. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108:2154–69. [DOI] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–52. [DOI] [PubMed] [Google Scholar]
- 5.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. Jama 2006;296:1377–84. [DOI] [PubMed] [Google Scholar]
- 6.Maini R, Wong DB, Addison D, Chiang E, Weisbord SD, Jneid H. Persistent Underrepresentation of Kidney Disease in Randomized, Controlled Trials of Cardiovascular Disease in the Contemporary Era. J Am Soc Nephrol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishida JH, Johansen KL. Exclusion of Patients With Kidney Disease From Cardiovascular Trials. JAMA Intern Med 2016;176:124–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gargiulo R, Suhail F, Lerma EV. Cardiovascular disease and chronic kidney disease. Disease-a-month : DM 2015;61:403–13. [DOI] [PubMed] [Google Scholar]
- 9.SYSTEM USRD. Cardiovascular Disease in Patients With CKD. Accessed Jan 27, 2019 https://www.usrds.org/2016/view/v1_04.aspx. [Google Scholar]
- 10.Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012;367:625–35. [DOI] [PubMed] [Google Scholar]
- 11.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet 2017;389:1238–52. [DOI] [PubMed] [Google Scholar]
- 12.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med 2015;128:1300–5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huisman MV, Rothman KJ, Paquette M, et al. The Changing Landscape for Stroke Prevention in AF. Findings From the GLORIA-AF Registry Phase 2 2017;69:777–85. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and Variation in Oral Anticoagulant Choice in Patients with Atrial Fibrillation, 2010-2017. Pharmacotherapy 2018;38:907–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urbaniak AM, Strom BO, Krontveit R, Svanqvist KH. Prescription Patterns of Non-Vitamin K Oral Anticoagulants Across Indications and Factors Associated with Their Increased Prescribing in Atrial Fibrillation Between 2012-2015: A Study from the Norwegian Prescription Database. Drugs Aging 2017;34:635–45. [DOI] [PubMed] [Google Scholar]
- 16.The Top 300 Drugs of 2019. https://clincalc.com/DrugStats/Top300Drugs.aspx. Accessed 9 Jan 2019. 2019. [Google Scholar]
- 17.Connolly S, Ezekowitz M, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139 – 51. [DOI] [PubMed] [Google Scholar]
- 18.Patel M, Mahaffey K, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883 – 91. [DOI] [PubMed] [Google Scholar]
- 19.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 20.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. Epub 2013 Nov 19. [DOI] [PubMed] [Google Scholar]
- 21.January CT, Wann LS, Calkins H, et al. 2019. AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation;0:CIR.0000000000000665. [Google Scholar]
- 22.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2020. [DOI] [PubMed] [Google Scholar]
- 23.Patel MR, Hellkamp AS, Fox KA. Point-of-Care Warfarin Monitoring in the ROCKET AF Trial. N Engl J Med 2016;374:785–8. [DOI] [PubMed] [Google Scholar]
- 24.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Inker LA. Assessment of Glomerular Filtration Rate in Health and Disease: A State of the Art Review. Clin Pharmacol Ther 2017;102:405–19. [DOI] [PubMed] [Google Scholar]
- 26.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. [DOI] [PubMed] [Google Scholar]
- 27.Levey A, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54. [DOI] [PubMed] [Google Scholar]
- 28.Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int 2006;70:2021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konstantinidis I, Nadkarni GN, Yacoub R, et al. Representation of Patients With Kidney Disease in Trials of Cardiovascular Interventions: An Updated Systematic Review. JAMA Intern Med 2016;176:121–4. [DOI] [PubMed] [Google Scholar]
- 30.Konstantinidis I, Patel S, Camargo M, et al. Representation and reporting of kidney disease in cerebrovascular disease: A systematic review of randomized controlled trials. PLoS One 2017;12:e0176145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rostoker G, Andrivet P, Pham I, Griuncelli M, Adnot S. A modified Cockcroft-Gault formula taking into account the body surface area gives a more accurate estimation of the glomerular filtration rate. J Nephrol 2007;20:576–85. [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Prado R, Castillo-Rodriguez E, Velez-Arribas FJ, Gracia-Iguacel C, Ortiz A. Creatinine Clearance Is Not Equal to Glomerular Filtration Rate and Cockcroft-Gault Equation Is Not Equal to CKD-EPI Collaboration Equation. Am J Med 2016;129:1259–63. [DOI] [PubMed] [Google Scholar]
- 34.Schwandt A, Denkinger M, Fasching P, et al. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. Journal of diabetes and its complications 2017;31:1376–83. [DOI] [PubMed] [Google Scholar]
- 35.Rivera-Caravaca JM, Ruiz-Nodar JM, Tello-Montoliu A, et al. Disparities in the Estimation of Glomerular Filtration Rate According to Cockcroft-Gault, Modification of Diet in Renal Disease-4, and Chronic Kidney Disease Epidemiology Collaboration Equations and Relation With Outcomes in Patients With Acute Coronary Syndrome. Journal of the American Heart Association 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009;15 Suppl 1:9S–16S. [DOI] [PubMed] [Google Scholar]
- 37.Stangier J, Rathgen K, Stahle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010;49:259–68. [DOI] [PubMed] [Google Scholar]
- 38.Kubisz P, Stanciakova L, Dobrotova M, Samos M, Mokan M, Stasko J. Apixaban - Metabolism, Pharmacologic Properties and Drug Interactions. Curr Drug Metab 2017;18:609–21. [DOI] [PubMed] [Google Scholar]
- 39.Kvasnicka T, Malikova I, Zenahlikova Z, et al. Rivaroxaban - Metabolism, Pharmacologic Properties and Drug Interactions. Curr Drug Metab 2017. [DOI] [PubMed] [Google Scholar]
- 40.Lip GY, Agnelli G. Edoxaban: a focused review of its clinical pharmacology. Eur Heart J 2014;35:1844–55. [DOI] [PubMed] [Google Scholar]
- 41.Potpara TS, Ferro CJ, Lip GYH. Use of oral anticoagulants in patients with atrial fibrillation and renal dysfunction. Nat Rev Nephrol 2018;14:337–51. [DOI] [PubMed] [Google Scholar]
- 42.Padrini R Clinical Pharmacokinetics and Pharmacodynamics of Direct Oral Anticoagulants in Patients with Renal Failure. Eur J Drug Metab Pharmacokinet 2019;44:1–12. [DOI] [PubMed] [Google Scholar]
- 43.Aursulesei V, Costache II. Anticoagulation in chronic kidney disease: from guidelines to clinical practice. Clinical cardiology 2019;42:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massicotte-Azarniouch D, Sood MM. Uncertainty surrounds anticoagulation risks and benefits in patients with chronic kidney disease with atrial fibrillation. BMJ evidence-based medicine 2019;24:35–6. [DOI] [PubMed] [Google Scholar]
- 45.Ravera M, Bussalino E, Paoletti E, Bellasi A, Di Lullo L, Fusaro M. Haemorragic and thromboembolic risk in CKD patients with non valvular atrial fibrillation: Do we need a novel risk score calculator? Int J Cardiol 2019;274:179–85. [DOI] [PubMed] [Google Scholar]
- 46.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 47.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011;58:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freeman JV, Simon DN, Go AS, et al. Association Between Atrial Fibrillation Symptoms, Quality of Life, and Patient Outcomes: Results From the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes 2015;8:393–402. [DOI] [PubMed] [Google Scholar]
- 49.Shin JI, Secora A, Alexander GC, et al. Risks and Benefits of Direct Oral Anticoagulants across the Spectrum of GFR among Incident and Prevalent Patients with Atrial Fibrillation. Clin J Am Soc Nephrol 2018;13:1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limdi NA, Beasley TM, Baird MF, et al. Kidney Function Influences Warfarin Responsiveness and Hemorrhagic Complications. J Am Soc Nephrol 2009;20:912–21. PMC2663833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Limdi NA, Limdi MA, Cavallari L, et al. Warfarin Dosing in Patients With Impaired Kidney Function. Am J Kidney Dis 2010;56:823–31. PMC2963672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Limdi MA, Crowley MR, Beasley TM, Limdi NA, Allon M. Influence of kidney function on risk of hemorrhage among patients taking warfarin: a cohort study. Am J Kidney Dis 2013;61:354–7. PMC3654383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Limdi NA, Nolin TD, Booth SL, et al. Influence of kidney function on risk of supratherapeutic international normalized ratio-related hemorrhage in warfarin users: a prospective cohort study. Am J Kidney Dis 2015;65:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.