Abstract
Movement disorders are a heterogeneous group of clinical syndromes in humans and animals characterized by involuntary movements without changes in consciousness. Canine movement disorders broadly include tremors, peripheral nerve hyperexcitability disorders, paroxysmal dyskinesia, and dystonia. Of these, canine paroxysmal dyskinesias remain one of the more difficult to identify and characterize in dogs. Canine paroxysmal dyskinesias include an array of movement disorders in which there is a recurrent episode of abnormal, involuntary, movement. In this consensus statement, we recommend standard terminology for describing the various movement disorders with an emphasis on paroxysmal dyskinesia, as well as a preliminary classification and clinical approach to reporting cases. In the clinical approach to movement disorders, we recommend categorizing movements into hyperkinetic vs hypokinetic, paroxysmal vs persistent, exercise‐induced vs not related to exercise, using a detailed description of movements using the recommended terminology presented here, differentiating movement disorders vs other differential diagnoses, and then finally, determining whether the paroxysmal dyskinesia is due to either inherited or acquired etiologies. This consensus statement represents a starting point for consistent reporting of clinical descriptions and terminology associated with canine movement disorders, with additional focus on paroxysmal dyskinesia. With consistent reporting and identification of additional genetic mutations responsible for these disorders, our understanding of the phenotype, genotype, and pathophysiology will continue to develop and inform further modification of these recommendations.
Keywords: dystonia, movement disorder, myoclonus, myokymia, myotonia, paroxysmal dyskinesia
Abbreviations
- BN
basal nuclei
- cPxDs
canine paroxysmal dyskinesias
- EEG
electroencephalogram
- EMG
electromyogram
- EPN
endopeduncular nucleus
- GABA
gamma‐aminobutyric acid
- GP
globus pallidus
- GTCS
generalized tonic‐clonic seizures
- PNH
peripheral nerve hyperexcitability
- PNKD
paroxysmal nonkinesigenic dyskinesia
- PPTN
pedunculopontine tegmental nucleus
- PxD
paroxysmal dyskinesia
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulata
- VGKCs
voltage‐gated potassium channels
1. INTRODUCTION
Movement disorders are characterized by involuntary movements without changes in consciousness, and include tremors, peripheral nerve hyperexcitability (PNH) (fasciculations, myokymia, neuromyotonia, myotonia, cramps, tetanus/tetany), myoclonus, paroxysmal dyskinesia (PxD), and dystonic movements. Canine paroxysmal dyskinesias (cPxDs) are a subtype of movement disorder in which there is a recurrent episode of abnormal, involuntary movement. Dyskinesias exhibit either a paucity (hypokinetic) or an excess (hyperkinetic) of movement, with or without changes in muscle tone (hypertonic or hypotonic). Normal, voluntary movements are often impaired during these episodes, and may be overridden by involuntary movements.
Despite increasing recognition of their occurrence, clinical descriptions of canine movement disorders inconsistently, and in some cases inaccurately, adopt human terminology or classification schemes to describe movements. The use of human terminology and classification schemes may not be appropriate for veterinary patients due to variation in phenomenology and anatomy between species, and differences in precipitating factors.
Some efforts to classify cPxDs have been attempted, 1 , 2 but a comprehensive consensus remains lacking. As these disorders are becoming more recognized and studied more frequently in veterinary patients, accurate and standardized veterinary terminology and descriptions are necessary. This international task force was established to develop a consensus on veterinary terminology and classification for canine movement disorders.
The collective aim of this task force is to categorize previously described breed‐related movement disorders using the terminology presented herein, categorizing these disorders by their core movement into either hyperkinetic or hypokinetic disorders, and subsequently into the following subcategories: tremors, fasciculations, myokymia, neuromyotonia, myotonia, cramps, tetanus/tetany, myoclonus, cPxDs, and dystonia. Recommended terminology to describe the specific movements observed has been included. This has been done to accomplish the following:
Highlight the overlapping aspects of various breed‐related diseases, to encourage further investigation into pathophysiologic and genetic relationships, and treatment recommendations
Evaluate similarities, where present, between veterinary and human conditions to encourage the use of dogs/cats as large‐animal models of human movement disorders in comparative studies for mutual benefit.
Encourage the use of accurate and standard terminology to describe these conditions, to facilitate clearer discussion between and among clinicians and researchers.
Due to the vastness of the topic of movement disorders, the emphasis of this article will be on cPxDs, while still presenting recommended terminology and classification for all categories of movement disorders.
2. NEUROANATOMY AND NEUROPHYSIOLOGY OF MOVEMENT CONTROL
The basal nuclei (BN) are located subcortically, in the basal portion of the telencephalon, mainly separated from the diencephalon by the internal capsule. 3 , 4 The literature is not consistent which brain nuclei are part of BN. 4 In veterinary texts, the term BN generally includes caudate nucleus, putamen, endopeduncular nucleus (EPN), globus pallidus (GP), claustrum, and amygdala. 3 , 4 In rodent models and human medicine, other subcortical connected nuclei such as the substantia nigra, consisting of the substantia nigra pars compacta (SNc) and substantia nigra pars reticulata (SNr), subthalamic nucleus, the pedunculopontine tegmental nucleus (PPTN) and the red nucleus are considered part of BN. 4 , 5 Basal nuclei are part of a complex integrated neuronal circuitry. It is difficult to assign a certain function to a specific nucleus. Basal nuclei should be more seen, as a complex interplay of its individual parts connected through feedback and reciprocal loops with the thalamus and the cortex, playing a major role in the gating of movement. 4 The cortical connections with the BN are through functionally segregated parallel circuits. 6 Five cortico‐BN‐thalamo‐cortical loops have been characterized: (a) motor, (b) oculomotor, (c) associative, (d) limbic, and (e) orbitofrontal. Basal nuclei also closely communicate with brain stem nuclei such as the reticular formation, the raphe nuclei and the locus ceruleus, but it is thought to be of minor importance. 4 , 5
In general, the dopaminergic projections to the caudate and putamen are the overriding modulatory control of the BN and their associated circuits. 4 , 5 Activation of the D1 dopamine receptors leads to increased glutamatergic activity, and D2 dopamine receptor activation leads to decreased glutamatergic activity. The so called “motor circuit” is somatotopically organized and considered most relevant to the pathophysiology of movement disorders. 5 The motor cortex as well as the primary somatosensory cortex is regulated by nigrostriatal dopaminergic neurons and is excitatory (glutamatergic) to the caudate and putamen, activating mainly GABAergic medium spiny neurons. The GABAergic medium spiny neurons connect directly to the main output structures of the BN, the EPN (the internal portion of the GP in humans), and the SNr. There are 2 important striato‐EPN/SNr circuits to be noted, the direct and indirect pathway (Figure 1). The “direct pathway” is a monosynaptic, inhibitory neuronal connection from the putamen to EPN/SNr, containing mainly D1 dopamine receptors and coexpressing the peptides substance P and dynorphin. 7 Striatal neurons in the “indirect pathway” express mainly D2 dopamine receptors. The “indirect pathway” starts with medium spiny neurones in the caudate nucleus and putamen sending their GABAergic axons to the GP (external portion in humans), which sends GABAergic axons to the subthalamic nucleus, which then sends excitatory axons (glutamate) to the EPN/SNr. Endopeduncular nucleus/substantia nigra pars reticulata in turn inhibit the ventrolateral thalamic nucleus and the PPTN. The EPN/SNr neuronal output is under control of both, the indirect and direct pathways. Direct pathway stimulation leads to an inhibition or reduction of neuronal firing rate in the EPN/SNr, which then consequently results to a disinhibition of their projection. In contrast, a stimulation of the indirect pathway results in an increased excitation of the subthalamic nucleus, which then leads to an increased inhibition from the EPN/SNr onto their projection. Dopamine promotes movement because it excites medium spiny neurons in the direct pathway through the D1 receptor, but inhibits them in the indirect pathway through the D2 receptor. Thus, loss of dopaminergic innervation in Parkinson's disease causes a hypokinetic movement disorder while diseases affecting the indirect pathway lead to hyperkinetic disorders.
FIGURE 1.
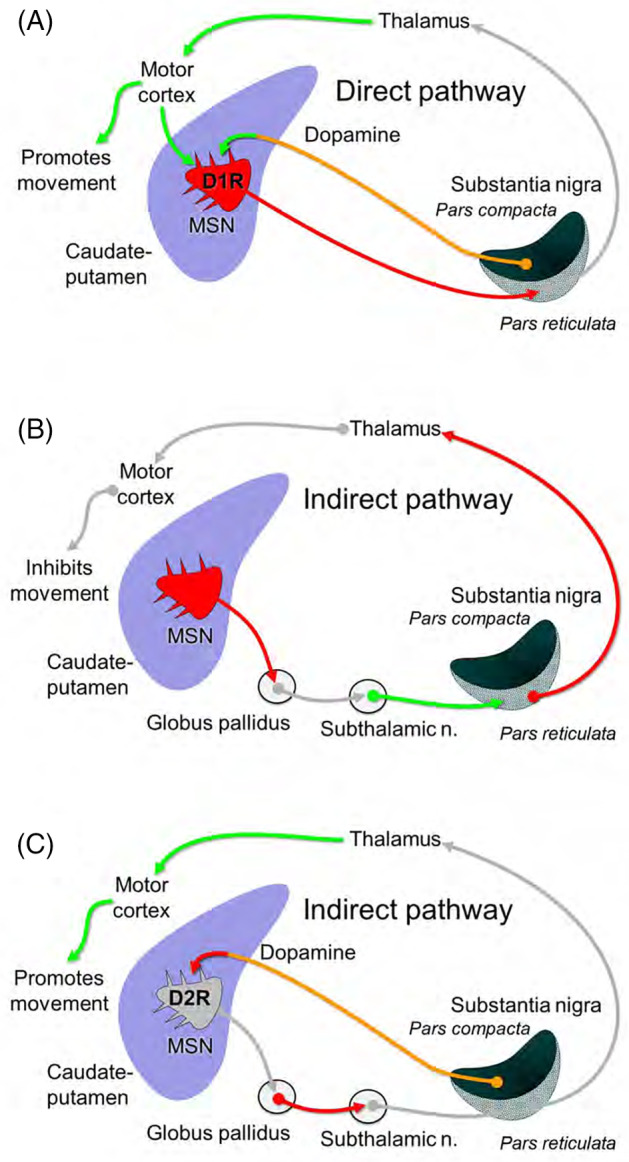
Thalamo‐cortico‐basal ganglia circuits involved in movement disorders. In the direct pathway of the basal nuclei (A), dopaminergic innervation of the caudate/putamen from the SNc increases inhibition of the SNr (and EPN not shown) by activating D1R on GABAergic MSN. This releases the thalamus from tonic inhibition of these nuclei. Loss of this inhibition increases the excitatory feedback from the thalamus to the motor cortex and caudate/putamen, thus promoting movement. The indirect pathway (B) has the opposite effect. MSN inhibit GABAergic neurons in the GP. This releases the subthalamic nucleus from tonic inhibition, increasing excitation of the SNr/EPN, and in turn inhibition of the thalamus. Decreased thalamic excitation of the motor cortex and caudate/putamen inhibits movement. Dopamine inhibits MSN in the indirect pathway through the D2R (C). Thus dopamine promotes movement by increasing activity of the direct pathway (A) and decreasing activity in the indirect pathway (C). D1R, D1 dopamine receptor; D2R, D2 dopamine receptor; EPN, endopeduncular nucleus; GP, globus pallidus; MSN, medium spiny neurons; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata
In addition to the direct and indirect pathways described above, there is some evidence that the cerebellum has a role in the pathophysiology of hyperkinetic disorders through the cerebello‐thalamo‐BN pathway, particularly for dystonic movements. 8 , 9 This pathway connects the lateral cerebellar nucleus to the striatum of the BN via the intralaminar nucleus of the thalamus, and dystonic movements are thought to result a sensorimotor mismatch in which abnormal high‐frequency bursts of cerebellar Purkinje cells and the deep cerebellar nuclei cause dystonia.
3. CURRENT TERMINOLOGY AND ADAPTATION TO VETERINARY MEDICINE
Attempts to apply the terminology used to describe movement disorders in humans to nonprimate species can be problematic. The organization of the nervous system and function of the limbs in a quadruped vs a bipedal species with highly dexterous upper limbs is quite different. While it aids in comparison of veterinary and human disease to use the same terms, in some cases, the terminology may not be appropriate. This section will review the human terminology compared to the usage in veterinary medicine and discuss the pros and cons of different approaches. Specific definitions of terminology can be found in Table 1.
TABLE 1.
Terminology used to describe clinical signs commonly associated with paroxysmal dyskinesia, and adaptations for veterinary use
| Athetosis | Prolonged slow involuntary contraction of the trunk muscles resulting in nonrhythmic bending/sinuous/writhing movements that preclude maintenance of a stable posture. Greek origin is defined as “without position or place”. Athetosis is often seen with concurrent choreatic movements (ie, choreoathetosis). Athetosis typically involves distal limb muscles (less frequently trunk, face, neck). Whereas individual choreatic movements may be distinguished from one another as discrete movements, athetotic movements generally seem to “flow” from one to another and are more difficult to separate from each other. May occur at rest or may be worsened or precipitated by movement. Due to differences in anatomy and degrees of freedom of joint motion between humans and canines (with canines lacking complex movement of the fingers/hands/wrist), athetosis is difficult to distinguish from chorea and ballism, and should be described simply as dyskinesia. |
| Ballism | An abrupt involuntary contraction of proximal limb muscles (eg, shoulders) resulting in large‐amplitude flailing/flinging movements of the limb(s); typically unilateral. May be indistinguishable from chorea/athetosis in animals. |
| Chorea | An abrupt (ie, jerky), irregular (ie, nonpatterned), and unsustained low‐amplitude contraction of muscle groups, particularly distal muscles (+/− shoulders, hips, face less frequently) resulting in 1 or more discrete movements. Similar in distribution to athetosis but faster and larger amplitude. From the Latin word “choreus” (ie, dance). May appear similar to movements associated with restlessness. Not characterized by an inserted posture (as in dystonia) but rather an inserted movement. Similar to athetosis, chorea in dogs is difficult to distinguish from athetosis and ballism and should be described simply as dyskinesia. |
| Cramp | A sudden, severe, and involuntary muscle contraction or over‐shortening that is generally temporary and benign. 10 Can cause mild‐to‐excruciating pain, and immobility of the affected muscle(s). Onset is usually sudden, and resolves on its own over a period of several seconds, minutes, or hours. |
| Dystonia | A sustained, slow, involuntary contraction of agonist and antagonist muscles of a body region producing abnormal postures and/or involuntary movement of portions of the body along a longitudinal axis. May appear as a twisted posture of the limbs, trunk, or neck. Dystonia may create new movement or may inhibit it by inserting an abnormal posture in place of the intended movement (see below for further classification). Associated with varying durations of muscle contracture (arrhythmic). May be prolonged/sustained (as in neuromyotonia) or transient. Typically does not involve rapidly alternating contraction/relaxation, as occur in tremor and myoclonus, although these movements may occur coincidentally. Postures adopted vary, but are largely stereotypical (ie, predictable/patterned) for each individual. Frequently triggered by movement, standing, or by adopting particular postures. Only occurs in wakeful state. Does not necessitate concurrent hypertonia; may only produce sufficient contraction of muscles to allow resistance to gravity (Video S1). |
| Fasciculations | A brief spontaneous contraction resulting from the spontaneous activation of a small number of muscle fibers, often causing a flicker/vermicular movement under the skin. 10 |
| Myoclonic tremor | Likely replaced by term rhythmic myoclonus: brief shock‐like myoclonic movements occurring with a defined unidirectional fast phase (positive or negative) and a slower recovery phase. |
| Myoclonus | A sequence of repeated, variably rhythmic, brief shock‐like jerks resulting from the sudden involuntary contraction or relaxation of 1 or more muscles. Generates movement of the affected body part (ie, overall limb/head movement), whereas tremors, myokymia, neuromyotonia do not. 11 Differs from a startle response in that myoclonic movements frequently occur independent of a sudden stimulatory input and occur repetitively. May be precipitated or worsened by movement, stress. May occur during sleep. |
| Myokymia | A focal or generalized continuous contraction of facial or limb myofibers, often exhibiting an undulation/vermicular movement of the skin overlying the affected muscle (ie, as if worms were crawling under the skin) (Video S2). |
| Myotonia | A disturbance in muscle relaxation after voluntary contraction or percussion. Is most noticeable after a period of rest, and improves with continued activity. Frequently results in noticeable hypertrophy of the affected muscles and may lead to diminished joint flexion when walking (ie, “stiffness” in the limbs) (Video S3). |
| Neuromyotonia | A more severe form of myokymia, seen as persistent muscle stiffness and delayed muscle relaxation due to abnormal electrical discharges of motor nerves. Distinguished from myokymia in that it results in generalized muscle stiffness with delayed relaxation, often resulting in collapse. Both may occur simultaneously. Clinically results in noticeable muscle rippling. May be precipitated by stress and/or excitement (Video S4). |
| Overflow movement | “Spread” of movement beyond an area of (unintended) movement to a nearby/adjacent area, due to presumed local spread of motor command. |
| Tetanus | Severe, sustained muscle contraction resulting from impairment of glycine release within the ventral gray matter of the spinal cord resulting from exotoxin release associated with Clostridium tetani infections. |
| Tetany | Sustained muscle contraction, usually involving extensors muscles. No relaxation is noted. May be accompanied by sensory changes (eg, hypocalcemia‐induced tetany). |
| Tremor | Involuntary, rhythmic oscillatory movements of a body part with symmetric velocity in both directions of movement (i.e. sinusoidal), around a joint axis. Frequently caused by rhythmic alternating contractions of agonist and antagonist muscles. May be present alone or concurrently with other movements. Tremors can be subdivided as follows: rest tremor, postural tremor, action tremor; intention tremor. See below for further classification. |
Broadly speaking, movement disorders can be divided into 2 categories: hyperkinetic and hypokinetic disorders. The former is further subdivided into disorders characterized by involuntary active movements (dyskinesia) and sustained muscle contractions (dystonia), without impairment in consciousness. These excessive movements may be abnormal, normal, or a combination of both. The term dyskinesia is used to describe self‐limiting, episodic, involuntary movements in animals. One difficulty in animals is determining if a movement is involuntary, or an abnormal voluntary movement such as hypermetria. Usually, careful observation of the patient or videos can determine whether or not the animal can control the movements, though some involuntary movements can co‐occur with voluntary movements to allow the animal to function despite the involuntary components (eg, soft‐coated Wheaten terriers with cPxD will walk even as their limbs are moving involuntarily). It is useful to differentiate dyskinesia, where the movements are fragmented and random, from stereotypies, where the animal engages in complex repetitive movements, such “mouse pouncing” or some circling behaviors, that are well organized but repetitive, and not directed toward accomplishing a goal.
Human dyskinesias are further described as athetosis, chorea, or ballism though there is often considerable overlap between these categories. All are characterized by fragmented movements that flow continuously from 1 movement to the next, though they can occur as paroxysms with periods of normal movements between. Because of the differences in the organization of motor control and degrees of freedom of limb movement in animals vs humans, with animals unable to perform complex compound movements of the distal extremities, movements that fit the description of athetosis and chorea in humans are not commonly seen in animals. The limb movements seen in cPxD would better fit in the category of ballism because they are more irregular flexion and extension movements of the limbs.
Identification in animal species of mutations in genes known to cause choreoathetosis in humans may help to clarify the differences and similarities between the movements in different species. Meanwhile, this committee recommends avoiding the terms athetosis, chorea, and ballism, and defining the disorder simply as a dyskinesia and providing a description of the movements (as described in detail later in this consensus statement).
Dystonic movements may also be a component of cPxD. Dystonia in human medicine is defined as sustained involuntary contraction of muscles producing abnormal posture or twisting movement. 1 It can be generalized, segmental, or focal. Physiologically, it is characterized by cocontraction of agonist and antagonist muscles with spread from the origin to extraneous muscles. Electromyographic (EMG) recording during an episode would be needed to demonstrate this feature. Dystonic movements described in animals involve flexion of the limbs such as canine epileptoid cramping syndrome or Chinook dyskinesia, or other postures such as the “deer stalking” in Cavalier King Charles spaniels (Video S1). The term dystonia should be used to describe involuntary postures, and combined with a detailed description of the posture. Dystonia needs to be distinguished from spasticity due to loss of upper motor neuron inhibition, muscle spasm due to abnormal lower motor neuron firing, or abnormal posturing due to pain.
Hypokinetic disorders in human medicine are characterized by a slowness and paucity of voluntary movement. The classic hypokinetic diseases being Parkinson's disease and related disorders such as multiple system atrophy. While the term bradykinesia is used to describe movements in these diseases, the character of the movement is not a “slow motion” movement. Rather there is a delay in the initiation of movement and difficulty in performing fast, repetitive movements. The parkinsonism best characterized in veterinary medicine is seen in hereditary multiple system degeneration in Kerry Blue terriers and Chinese Crested dogs. In the terminal stages of disease, affected dogs may clearly show attempts to move but be unable to initiate the movement. They adopt a kyphotic posture with their center of gravity over the thoracic limbs that resembles the hunched posture of Parkinson's disease. They also demonstrate the postural instability characteristic of parkinsonism in humans where they are unable to make corrections when they lose balance and fall frequently. When still ambulatory, they may demonstrate a festinating gait characterized by difficulty initiating stepping and then fast, small steps when they finally do move. In human medicine, the term parkinsonism is used to describe the signs associated with Parkinson's disease in other conditions such as multiple system atrophy. It is worth noting that the resting tremor, which is a hallmark of Parkinson's disease, is only seen in primate models of parkinsonism, and not in any quadruped species.
4. DESCRIPTIONS OF VETERINARY MOVEMENT DISORDERS
4.1. Tremors
Tremors are the most common movement disorder encountered in humans yet there is no diagnostic standard to distinguish among common types of tremor, which can make the evaluation challenging. 2 , 12 They can be focal (eg, affecting just 1 limb or the head) or generalized. They should only be observed when awake and cease during sleep. When a tremor is recorded on EMG, it is characterized by rhythmic bursts of motor neuron activity occurring in opposing muscle groups creating a biphasic character with a range of frequencies, varying from 1 to 2 Hz up to 12 Hz or higher.
4.1.1. Identification
Identification of tremor can be difficult. The keyword in identification is “rhythmicity,” that is, cycles of regular muscle contractions. Care should be taken not to mistake frequency and amplitude, as many tremors may have variable amplitude (eg, intention tremors) despite a regular frequency. 2 It is this rhythmical character that distinguishes tremor from other involuntary muscle contractions.
4.1.2. Classification
Tremors can be classified by their age of onset, distribution, frequency of occurrence, activating conditions (eg, whether the problem occurs at rest, with posture, on action or with intention), and frequency (Hz) (Figure 2). 12 , 13 This allows flexibility for detailed identification of groups or overlapping occurrences in future. The following definitions of activation conditions have been applied in veterinary medicine:
Rest tremor is observed when the affected body part is not being actively supported against gravity (eg, laying down) and is not voluntarily activated.
- Action tremor is observed when the muscles are active, either supporting the body against gravity or making a movement, and has the following subtypes:
- Postural or orthostatic tremor is a tremor of the limbs that occurs when standing (ie, during voluntary maintenance of a position against gravity), and dissipates on activity or upon lying down.
- Kinetic tremor occurs during any aspect of voluntary movement. It can be present when the movement begins, during the course of movement, and as the target is reached (intention tremor). Typically, they result from cerebellar disease with or without other neuroanatomical components.
FIGURE 2.
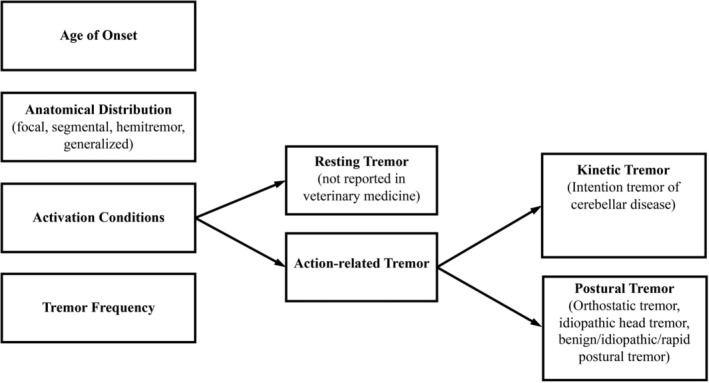
Classification algorithm of tremor in veterinary medicine, adapted from the 2‐Axis approach developed by the International Task Force for tremor in humans. 12 The column on the left lists all the components of the clinical description of tremor in patients. The flowchart extending to the right provides additional details to help describe those criteria on the left. In dogs and cats, idiopathic generalized tremor syndrome is classified as a kinetic tremor despite often being seen at rest
4.2. Peripheral nerve hyperexcitability
This term refers to the dysfunction of the motor nerve causing hyperexcitability, with variable manifestations in the muscle. This hyperexcitability can be caused by a wide variety of central and peripheral nervous system disorders, but is particularly related to ion channel dysfunction. Clinical signs resulting from PNH include fasciculations, myokymia, neuromyotonia, cramps, tetany, and tetanus (defined further in Table 1). These clinical signs share similar features, and some are difficult to distinguish clinically, with fasciculations representing a mild manifestation of PNH through myokymia to neuromyotonia which can be more severe.
4.2.1. Identification
Peripheral nerve hyperexcitability may be focal or generalized. It is differentiated from other syndromes by sustained twitching or muscle contractions of variable frequency and amplitude (making them distinct to tremors that have uniform frequency) that do not result in significant movement in the affected body segment (making them distinct to myoclonus). See Table 1 for individual definitions.
-
Fasciculations
May be benign or indicative of underlying motor neuron pathology. In humans there is evidence suggesting benign fasciculations more frequently occur proximally, while those resulting from chronic partial denervation more frequently occur distally. 14
-
Myokymia
Myokymic discharges are bursts of single motor unit potentials firing at rates of 5 to 150 Hz believed to result from abnormal potassium channel function. The bursts may appear as doublets, triplets, or multiplets. Electromyogram segregates myokymia from neuromyotonia due to the higher frequency discharges of neuromyotonia and their waning nature. 15 May be benign (eg, stress related) or indicative of underlying pathology. In veterinary patients, focal myokymia affecting the muscles of the head is often related to an identifiable brain disorder, 16 , 17 , 18 whereas generalized myokymia is most commonly seen in Jack Russell terriers 19 although other breeds are reported. 20 , 21
-
Neuromyotonia
Neuromyotonic discharges are high‐frequency (150‐300 Hz) bursts of decrementing discharges of motor unit potentials that originate in motor axons and have an abrupt onset and offset. They can be spontaneous or may be initiated by needle movement, voluntary contraction of the muscle, or percussion of the nerve. 15 Neuromyotonic and myokymic activity is unique in that it persists during sleep and general anesthesia although can be blocked by neuromuscular blocking agents.
-
Cramps
Such a phenomenon has only been reported in 2 dogs with hypoadrenocorticism. 22 Proving their existence is difficult without the ability of the patient to describe the phenomena. However, if a muscle contraction appears painful, cramps should be considered. 10 Electromyogram recordings show repetitive firing of motor unit action potential at high rates (up to 150 Hz). 10 The number of motor units activated and the frequency of discharges increase gradually during the cramp and then subside gradually with an irregular firing pattern toward the end. Cramps usually arise from abnormal discharges of nerve terminals in a muscle. Electrically silent muscle cramps occur with strenuous or ischemic exercise in metabolic myopathies associated with defects of glycolysis or glycogenolysis. They can result in myoglobinuria if severe or widespread.
-
Tetanus and tetany
Tetanus and tetany both refer to the clinical sign of sustained muscle contraction without relaxation. 23 The degree of extensor rigidity is variable and results from disinhibition of the extensor motor neurons. Although both terms refer to clinical signs, tetanus is commonly used to describe the disease caused by the production of the neurotoxin tetanospasm, usually within an anaerobic wound; and tetany refers to increased neuronal excitability most commonly associated with hypocalcaemia. 23 Tetany and myoclonus need to be differentiated. Both can be generalized or focal. If generalized tetany is present, one would expect to see other signs suggesting that the dog was infected by the clostridial toxin; for example, a risus sardonicus facial expression. Additionally, movements with myoclonus are sudden contractions of the muscles producing a quick jerk followed by relaxation, whereas tetany or tetanus should involve a persistent state of extensor rigidity within the affected body segments without obvious movement. 13
4.2.2. Classification
A veterinary classification of PNH was proposed based on pathophysiological mechanisms (Table 2). 24 These mechanisms center around the activity of voltage‐gated potassium channels (VGKCs). A brief mention is made regarding acute toxicities, as historically these were considered to result in tremors. However, this group's consensus is that toxicities tend to produce twitches rather than tremors, clinically differentiated by their irregular frequency, and as such they are considered to be a manifestation of PNH in the form of fasciculation or tetany.
TABLE 2.
Pathophysiological classification of generalized peripheral nerve hyperexcitability syndromes
| Classification | Mechanism |
|---|---|
| Hereditary channelopathies | VGKC mutation |
| Immune‐mediated channelopathies | VGKC‐complex antibodies |
| Paraneoplastic causes | VGKC‐complex antibodies |
| Polyneuropathies | Demyelination leading to paranodal reorganization of VGKC and VGKC‐complex proteins |
| Motor neuron disease | Ion channel dysfunction via various mechanisms |
| Neurodegenerative disease | VGKC dysfunction |
| Metabolic disease | Prolonged depolarization of the action potential relating to electrolyte disturbance or endocrinopathy |
| Benign causes | Stress, exercise |
| Toxicities | Stress, exercise |
Abbreviation: VGKC, voltage‐gated potassium channel.
4.3. Myoclonus
Myoclonus can be focal, multifocal, or generalized. Associated movements are typically positive (caused by muscle contraction), but can sometimes be negative (due to brief loss or inhibition of muscular tonus). 25
There are movements that fall within the definition of myoclonus that are considered by some to be distinct to this clinical sign, for example, startle responses (epileptic and nonepileptic) and hemifacial spasm. 11 Strictly speaking, such movements are myoclonic in nature, and so they are considered under the terminology of myoclonus.
4.3.1. Identification
Myoclonus is best likened to the effect seen after stimulating a nerve supplying a muscle with a single electric shock (or with a train of shocks, because myoclonic jerks can occur repetitively within the same muscle). Diagnosis of myoclonus therefore relies on the identification of “shock‐like” movements. When myoclonus occurs in series, the resulting jerks may be synchronous (ie, involving the coordinated repeated contraction of a specific group of muscles/generalized), spreading (contraction of different groups of muscles in a patterned sequence), or moderately asynchronous (multifocal). Sometimes rhythmic myoclonus can be mistaken for tremor but the former has a more abrupt and shock‐like character than a tremor, which has more of a sinusoidal nature. 11 Tetanus and tetany can also be difficult to distinguish on occasion though other clinical signs may prevail to determine that tetanus is present. However, gross appendicular movement should not be induced by tetanus or tetany, and contractions are more refined with these than those encountered with myoclonic contractions.
4.3.2. Classification
Myoclonus may be physiological (eg, hiccups) or pathological. A common method for classifying pathological myoclonus is by its neuroanatomical localization. Accordingly, myoclonus is subdivided into cortical, subcortical (brainstem and spinal cord), and peripheral types. However, since this classification is not always intuitive, classification according to its association with epilepsy has been proposed. 26 Specifically, the additional presence of generalized tonic‐clonic seizures (GTCS) classifies the condition as more likely epileptic myoclonus and therefore cortical myoclonus (Table 3). The absence of associated epileptic seizures is more difficult to interpret, but designates the condition as possibly nonepileptic myoclonus, although the problem may still be cortical in origin and GTCS may have not yet developed. Obtaining an electroencephalogram (EEG) evaluation at the time of occurrence of myoclonic movements facilitates classification.
TABLE 3.
Clinical classification of myoclonus
| Epileptic myoclonus (progressive myoclonic epilepsies) |
Lafora disease Neuronal ceroid lipofuscinosis Feline audiogenic reflex seizures (FARS) Juvenile myoclonic epilepsy in Rhodesian Ridgebacks |
| Nonepileptic myoclonus |
Canine distemper virus Startle disease Hemifacial spasm |
4.4. Paroxysmal dyskinesia
Paroxysmal dyskinesias are a group of conditions characterized by episodes of abnormal self‐limiting movement. 27 The term paroxysmal dyskinesia is used to describe the clinical disease. Terms such as chorea, athetosis, ballism, and dystonia are used to describe the dominant features of the PxD in humans, but are difficult to distinguish in dogs. As such, the more general terms dyskinesia and dystonic movements should be used instead. The infrequent occurrence of cPxD within an individual and their abrupt nature has meant they appear to have historically been underdiagnosed or misdiagnosed as focal seizures. The catalyst for increased awareness of these conditions has been the popularity of the smart phone. Observing these episodes in real time has allowed greater recognition of these conditions and in turn we are gaining more insight into what they actually represent.
4.4.1. Identification
Paroxysmal dyskinesia means episodic “bad movement” and causes intermittent impairment of voluntary movements. Episodes most commonly occur spontaneously at rest (paroxysmal nonkinesigenic dyskinesia [PNKD]) but can be triggered by sudden movement (PKD). A paroxysmal exertion‐induced dyskinesia is recognized in humans but there are no reports in veterinary medicine of this syndrome. Clinical signs associated with cPxD include, but are not limited to, dyskinesia, dystonic movements, and associated tremors (Table 1, Video S1). Diagnosis is limited to observation of an episode. Episodes may be prolonged (minutes to hours); autonomic signs are absent, consciousness is not impaired, and behavior typically seen after epileptic seizure is not observed. These features distinguish cPxD from epileptic seizures. Neurological examination is typically normal in between episodes.
4.4.2. Classification
Clinically, the cPxDs all look very similar and so cannot be separated by clinical signs alone. Classification is summarized in Table 4. The majority are classified as primary in that they are believed to be hereditary. 27 The preferred terminology for this group of cPxDs is inherited (rather than primary). A list of inherited cPxDs reported in the literature is presented in Table 5. Acquired (secondary) dyskinesia occurs in dogs resulting from drug‐administration (eg, propofol and phenobarbital), and structural intracranial lesions. Acquired PxD are identifiable in that they tend to be accompanied by additional neurological signs that are persistent between episodes.
TABLE 4.
Etiological classification of canine paroxysmal dyskinesia
| Inherited or presumed inherited (primary) paroxysmal dyskinesia |
Episodic hypertonicity in Cavalier King Charles Spaniels Paroxysmal dyskinesia in Border terriers Scotty cramp Paroxysmal dyskinesia in the Soft Coated Wheaton Terrier Paroxysmal dyskinesia in Chinooks Dancing Dobermann disease Paroxysmal kinesigenic dyskinesia in German Short‐Haired Pointers Paroxysmal Dyskinesias in Labradors and Jack Russell terriers |
| Acquired (secondary) paroxysmal dyskinesia |
Drug‐administration (eg, propofol and phenobarbital) and structural intracranial lesions Paroxysmal gluten‐sensitive dyskinesia in Border terriers |
TABLE 5.
Breed‐related paroxysmal dyskinesias
| Colloquial name(s) | Primary affected breed | Mode of inheritance | Trigger | Duration (min) | Progression | Similarity with human disorder(s) |
|---|---|---|---|---|---|---|
| Labrador hypertonicity syndrome | Labrador retriever 28 | Unknown | N/A | Constant | Stabilize as adults | Stiff Man Syndrome |
| Chinook seizures | Chinook dog 29 | Unknown | No consistent triggers | 1‐60 | Not reported | PNKD |
| Episodic falling syndrome | Cavalier King Charles spaniel 30 | Autosomal recessive | Exercise, excitement, stress | <1 to several minutes | Improve/stabilize with age | Paroxysmal dyskinesia |
| Canine epileptoid cramping syndrome, Spike disease | Border terrier 31 | Unknown | Waking, excitement, stress, heat/cold | <1‐150 | Not reported (gluten‐free diet may ameliorate) | PNKD |
| Scottie cramps | Scottish terrier 32 , 33 | Autosomal recessive | Excitement, stress, exercise, | 5‐20 | Improve with age | PNKD |
| Paroxysmal dyskinesia | Labrador retriever 34 | Unknown | Excitement, stress, startling | 10 | Improve with age | PKD |
| Paroxysmal dyskinesia | Jack Russell terrier 34 | Unknown | Stress, temperature changes | 10 | Improve with age | PNKD |
| Paroxysmal dyskinesia | Boxer 35 | Unknown | Excitement | 1‐5 | Improve with age | Paroxysmal dyskinesia |
| Paroxysmal dyskinesia | German shorthaired pointer 36 | Unknown | Excitement, exercise | 10‐30 typically, up to 180 | Not reported | Paroxysmal hyperkinetic disorder |
| Paroxysmal dyskinesia | Maltese 37 | Unknown | No consistent trigger | 1‐90 (median 4.5) | Not reported (gluten‐free diet may ameliorate) | Paroxysmal dyskinesia |
| Paroxysmal dyskinesia | Soft‐coated wheaten terrier 38 | Autosomal recessive | No consistent trigger | 1‐240 | Not reported | PNKD |
| Paroxysmal dyskinesia | Shetland sheepdog 39 | Autosomal dominant | Stress, excitement, exercise | Minutes to hours | Not reported (high tryptophan diet may ameliorate) | PKD |
| Paroxysmal dyskinesia | Norwich terrier 40 | Unknown | No consistent trigger | Not reported | Stable | PNKD |
Note: Isolated case reports are purposefully not represented here, as a consistent association within the breed is not yet established.
Abbreviations: PD, paroxysmal dyskinesia; PKD, paroxysmal kinesigenic dyskinesia; PNKD, paroxysmal nonkinesigenic dyskinesia.
4.5. Dystonic movements (dystonia)
Dystonia is a description of a clinical sign, not a disease. Dystonia comes from Greek, “dys‐” (word‐forming element meaning “bad/ill/abnormal”) and “‐tonia” (from tonos, meaning “tension”). Dystonia is a common clinical sign in animals; however, there is very little knowledge about it in veterinary medicine, and few relevant veterinary studies available. However, the resemblance between human and veterinary neurological disorders allows some extrapolation. 41 In order to recognize dystonic movements in dogs, it is important to have knowledge of the appearance and mechanisms that cause them.
4.5.1. Identification
Dystonia is a hyperkinetic movement disorder characterized by sustained or intermittent muscle contractions causing abnormal (often repetitive) movements, postures, or both (Video S1). Dystonic movements are typically patterned, twisting and may be tremulous. Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation. 42 In dystonia, there is a recognizable repetitive recurrence to the movements in the affected body parts that is why dystonic movements are called “patterned.” Typical of dystonia is a cocontraction of agonists and antagonists. This suggests a breakdown of the normal pattern of reciprocal innervation between opposing muscles, and it produces the sustained quality of dystonic movements. There is a wide variety in speed of the dystonic movements. They differ from slow (athetotic dystonia) to shock‐like (myoclonic dystonia). The dystonic movements can be very short (dystonic spasms), sustained for several seconds (dystonic movements), or last minutes to hours (dystonic postures). 43 Dystonic movements occur at many different locations. Because of these broad variations, misdiagnosis is quite frequent. 44
Clinical characteristics describe the phenomenology of dystonic movements in a given patient. The clinical characteristics that should be included in a canine dystonia assessment include age at onset, body distribution, temporal pattern, coexistence of other movement disorders, and other neurological manifestations. The principal aim of this classification is facilitating clinical recognition, diagnosis, treatment and determining prognosis. 1
4.5.2. Classification
Many forms of dystonia lack a fully understood etiology. 1 As such, classification remains difficult. There are 2 characteristics that are assumed to be useful for classification in humans: identifiable anatomical changes and pattern of inheritance. Investigation of anatomical causes can be done using brain imaging or by pathology. Differentiating inherited from acquired forms requires metabolic, genetic, or other tests, the latter of which is not yet available in dogs. 1 Anatomical changes and pattern of inheritance should not be considered 2 separate characteristics for etiological classification. For instance, brain imaging can be helpful for both purposes. 1
5. RECOMMENDED CLINICAL APPROACH AND DESCRIPTIONS FOR VETERINARY CASES
5.1. Hyperkinetic vs hypokinetic
General definition: A range of neurological conditions in which there is an involuntary alteration in movement, either a paucity (usually associated with rigidity), or an excess of movement
Hyperkinetic state: conditions resulting in excess movement, either constant (nonjerky) or brief (jerky). These may be immediately preceded and/or precipitated by voluntary movements, but are not voluntarily generated (involuntary). They are presumed to result from functional abnormalities in the basal nuclei, cerebellum, and cerebral cortex, spinal cord, or from abnormalities at the level of the peripheral nervous system and/or muscles. Examples: dyskinesia, dystonia, myoclonus, tremors.
Hypokinetic state: neurological conditions characterized by a paucity of movement and/or abnormally reduced speed of movements. The classic hypokinetic movement disorder in humans is Parkinson's disease and the constellation of clinical signs are sometimes called parkinsonism when seen in other diseases. Bradykinesia describes the slow movement of these human patients, but the sign is not so much speed of movement as difficulty initiating movement or performing repetitive or sequential movements. 45 This hypokinesia leads to the other devastating sign of parkinsonism, postural instability. The patient is unable to quickly correct slight perturbations of balance resulting in frequent falls. Difficulty initiating movement and postural instability are seen in the only well‐characterized hypokinetic disorder of dogs and cats, multiple system degeneration of Kerry blue terriers and Chinese crested dogs. These signs would be attributed to the degeneration of the substantia nigra as well as caudate nucleus. 46 , 47 , 48
5.2. Paroxysmal vs persistent
Occurring in paroxysms, that is, occurring as “a sudden onset of symptoms…with recurrent manifestation.” 49
5.3. Exercise induced vs not
Exercise induced: movement disorders beginning while the animal is active (ie, while walking, running, playing). This need not be strenuous exercise. Example: “Scottie cramps.”
- Nonexercise induced: movement disorders beginning while the animal is at rest (ie, recumbent or standing). These may also variably begin while the animal is active (eg, walking), but do not require activity to generate the abnormal movement.
- Neuromyotonia, myoclonus, PNKDs
5.4. Detailed clinical description of movements observed
Descriptions should include whether the movement is bilateral or unilateral (if bilateral, note whether the limb movements appear coordinated), proximal or distal, rhythmic or irregular, fragmented or complex, purposeful or not (if possible to determine), and any triggers (if identified). Where dystonic movements occur, include a detailed description of the abnormal posture and position of the spine and limbs as well as facial muscles. Specific terminology presented in this statement may also be included as an assessment of the movements observed; however, a detailed description of movements as described here should also be included.
5.5. Movement disorder or not
Movement disorders (ie, dyskinesias) are neurological conditions characterized by abnormal and involuntary muscle contraction/relaxation generated by abnormalities in the central or peripheral nervous system. These movements appear are painless (ie, not traditionally defined “cramping” movements) and may be episodic or may occur continuously (eg, myoclonus following canine distemper virus infection).
Epileptic seizures are defined as, “Manifestation(s) of excessive synchronous, usually self‐limiting epileptic activity of neurons in the brain. This results in transient occurrence of signs which may be characterized by short episodes with convulsions of focal motor, autonomic, or behavioral features and due to abnormal excessive of synchronous epileptic neuronal activity in the brain.” 50 Epileptic seizures often cause involuntary movements generated by the central nervous system. This may help us to collectively recognize the difficult gray zone between epileptic seizures and movement disorders. This difficulty is compounded by the absence of EEG readings at the time of occurrence of these abnormal movements in the majority of cases.
5.6. Inherited or acquired
Similar to many neurological conditions, cPxD may be inherited (primary) or acquired (secondary). Acquired cPxD most commonly occur in association with drug administration, based on current veterinary literature (Table 4). In the human literature, PxD may additionally occur secondary to multiple sclerosis, hypoxia, encephalitis, stroke, endocrinopathies, trauma, and psychogenic. 51 As such, consideration of other intracranial and extracranial causes, in addition to drug‐induced, should be considered in veterinary patients.
6. FUTURE DIRECTIONS
Grouping the various breed‐specific movement disorders into a consistent and reliable classification scheme is difficult, as little is known about the genetic etiology, pathophysiology, triggering events, treatment responses, and correlation to human movement disorders. Adhering to consensus terminology will help provide consistency and promote future grouping based on clinical phenomenology. Ultimately, genetic studies will help inform appropriate grouping of disorders based on common pathophysiologic etiologies, as well as comparative assessments between dogs and humans. For example, is it appropriate to apply human terminology to cPxD (eg, perhaps some of the movements we observe in dogs are truly chorea or athetosis, even though the classical appearance in humans is altered in dogs due to anatomic differences in distal joint motion). As we better understand the genetic influences of these diseases and their human correlates, we can make more reliable comparisons between species. Currently mutations and genetic tests are only available for a handful of canine movement disorders (Table 6).
TABLE 6.
Known genetic mutations for canine movement disorders
| Movement disorder | Subtype or breed | Mutation | Genetic test available? |
|---|---|---|---|
| Paroxysmal dyskinesias | Canine multiple system degeneration 46 | SERAC1 | Yes, for Kerry blue terriers and Chinese crested dogs and cats |
| Cavalier King Charles spaniel 52 , 53 | BCAN | Yes, for Cavalier King Charles spaniel | |
| Soft‐coated wheaten terrier 38 | PIGN | Yes, for Soft‐coated wheaten terriers | |
| Shetland sheepdog 39 | PCK2 | Yes, for Shetland sheepdogs | |
| Peripheral nerve hyperexcitability | Myotonia congenita 54 , 55 , 56 , 57 | CLCN‐1 | Yes, for miniature Schnauzer, Australian cattle dog, Jack Russell terrier and cats |
| Myokymia in Jack Russell terriers, Parson Russell terriers, and Smooth‐haired fox terriers 58 , 59 , 60 | KCNJ10 | Yes, for Jack Russell terriers, Parson Russell terriers, Russell terrier, Toy fox terrier, Smooth fox terrier, Tenterfield terrier, Belgian Malinois, and Chihuahua | |
| Myoclonus | Myoclonic epilepsy (LaFora's disease) in Miniature wire‐haired dachshunds and Beagles 61 , 62 | EPM2 | Yes, for miniature Wire‐haired Dachshunds and Beagles |
| Myoclonic epilepsy from neuronal ceroid lipofuscinosis 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 | ARSG, PPT1, TPP1/CLN2, CLN5, CLN6, CLN8, CTSD, ATP13A2, MFSD8 | Yes, for many breeds of dogs and cats | |
| Hyperekplexia (startle disease) in Irish Wolfhounds 75 | SLC6A5 | Yes, for Irish wolfhound |
7. CONCLUSIONS
This consensus statement represents a starting point for consistent reporting of terminology and clinical descriptions of canine movement disorders. As our knowledge evolves and we better understand the pathophysiology of individual syndromes, we anticipate that modifications to this information will be required over time. Further, as we identify additional mutations responsible for specific breed‐related movement disorders, we may be able to more accurately and specifically define the characteristics of the movements or disease, given form follows function. Over time as our knowledge base grows, we also anticipate development of classification schemes to group these disorders based on etiology, triggering events, and treatment responses. These consensus recommendations are a first step toward these long‐term goals.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Video S1 Video compilation of a soft‐coated Wheaten terrier and a Chinook dog exhibiting dyskinesia and dystonia. For the soft‐coated Wheaten terrier (start of video to time 0:28), note the sustained, abnormal posture of the body and limbs (dystonic posture, in this case manifesting as kyphosis). The dog continues to attempt to walk (co‐occurring voluntary movement) while the involuntary movements are occurring. At times there appears to be involuntary flexion and extension of the pelvic limbs (dyskinesia). For the Chinook dog (0:28 to 1:21), note the abnormal sustained posture of the limbs and head/neck (dystonic posture), and the irregular, involuntary movement of the limbs. A dystonic head tremor can be seen at time 1:02.
Video S2 Video of myokymia in a Jack Russell terrier. Note the vermicular nature of the muscle movements of the pelvic limb.
Video S3 Video of a dog with myotonia congenita. Note the generalized hypertrophy, and sustained contraction of the muscles, resulting in a stiff gait. As is typical with myotonia, the patients “warms” out of the myotonia and the gait normalizes with activity.
Video S4 Video compilation of neuromyotonia in the earlier and later stages of disease. The Bassett hound (start of video to time 0:50) shows earlier stages of neuromyotonia, and is exacerbated by stress. In this case the dog remains able to walk. In later stages of disease (time 0:50 to end of video) the muscle stiffness may cause collapse and difficulty walking. Note the persistent muscle stiffness and rippling appearance to the muscles in both patients.
ACKNOWLEDGMENT
No funding was received for this study. Video S1 courtesy of Dr Denny O'Brien and Dr Rebecca Packer. Video S2 courtesy of Dr Michael Reese. Video S3 courtesy of Dr Denny O'Brien. Video S4 courtesy of Dr Curtis Dewey and Dr Mark Lowrie. All videos used with permission.
Cerda‐Gonzalez S, Packer RA, Garosi L, et al. International veterinary canine dyskinesia task force ECVN consensus statement: Terminology and classification. J Vet Intern Med. 2021;35:1218–1230. 10.1111/jvim.16108
Consensus Statements of the European College of Veterinary Neurology (ECVN) provide the veterinary community with up‐to‐date information on the pathophysiology, diagnosis, and treatment of clinically important animal diseases. The ECVN Board oversees selection of relevant topics, identification of panel members for each topic with the expertise to draft the statements, and other aspects of assuring the integrity of the process. The statements are derived from evidence‐based medicine whenever possible and the panel offers interpretive comments when such evidence is inadequate or contradictory. A draft is prepared by the panel, followed by solicitation of input by the ECVN membership which may be incorporated into the statement. It is then submitted to the Journal of Veterinary Internal Medicine, where it is edited prior to publication. The authors are solely responsible for the content of the statements.
Sofia Cerda‐Gonzales and Rebecca A. Packer contributed equally to this work.
REFERENCES
- 1. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(suppl 3):2‐23. [DOI] [PubMed] [Google Scholar]
- 3. de Lahunta A, Glass E. Nonolfactory rhinencephalon: limbic system. In: de Lahunta A, Glass E, eds. Veterinary Neuroanatomy and Clinical Neurology. 3rd ed. St. Louis, MO: W.B. Saunders; 2009:448‐453. [Google Scholar]
- 4. Thomson C, Hahn C. Veterinary Neuroanatomy: A Clinical Approach. Edinburgh, UK: Saunders Elsevier; 2012. [Google Scholar]
- 5. Obeso JA, Rodriguez‐Oroz MC, Rodriguez M, et al. The basal ganglia and disorders of movement: pathophysiological mechanisms. News Physiol Sci. 2002;17:51‐55. [DOI] [PubMed] [Google Scholar]
- 6. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357‐381. [DOI] [PubMed] [Google Scholar]
- 7. Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133‐139. [DOI] [PubMed] [Google Scholar]
- 8. Tewari A, Fremont R, Khodakhah K. It's not just the basal ganglia: cerebellum as a target for dystonia therapeutics. Mov Disord. 2017;32:1537‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaji R, Bhatia K, Graybiel AM. Pathogenesis of dystonia: is it of cerebellar or basal ganglia origin? J Neurol Neurosurg Psychiatry. 2018;89:488‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kortman HG, Veldink JH, Drost G. Positive muscle phenomena—diagnosis, pathogenesis and associated disorders. Nat Rev Neurol. 2012;8:97‐107. [DOI] [PubMed] [Google Scholar]
- 11. Abdo WF, van de Warrenburg BP, Burn DJ, et al. The clinical approach to movement disorders. Nat Rev Neurol. 2010;6:29‐37. [DOI] [PubMed] [Google Scholar]
- 12. Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lowrie M, Garosi L. Classification of involuntary movements in dogs: tremors and twitches. Vet J. 2016;214:109‐116. [DOI] [PubMed] [Google Scholar]
- 14. de Carvalho M, Swash M. Origin of fasciculations in amyotrophic lateral sclerosis and benign fasciculation syndrome. JAMA Neurol. 2013;70:1562‐1565. [DOI] [PubMed] [Google Scholar]
- 15. Gutmann L, Libell D, Gutmann L. When is myokymia neuromyotonia? Muscle Nerve. 2001;24:151‐153. [DOI] [PubMed] [Google Scholar]
- 16. Walmsley GL, Smith PM, Herrtage ME, Jeffery ND. Facial myokymia in a puppy. Vet Rec. 2006;158:411‐412. [DOI] [PubMed] [Google Scholar]
- 17. Holland CT, Holland JT, Rozmanec M. Unilateral facial myokymia in a dog with an intracranial meningioma. Aust Vet J. 2010;88:357‐361. [DOI] [PubMed] [Google Scholar]
- 18. Vanhaesebrouck AE, Van Soens I, Poncelet L, et al. Clinical and electrophysiological characterization of myokymia and neuromyotonia in Jack Russell Terriers. J Vet Intern Med. 2010;24:882‐889. [DOI] [PubMed] [Google Scholar]
- 19. Bhatti SF, Vanhaesebrouck AE, Van Soens I, et al. Myokymia and neuromyotonia in 37 Jack Russell terriers. Vet J. 2011;189:284‐288. [DOI] [PubMed] [Google Scholar]
- 20. Reading MJ, McKerrell RE. Suspected myokymia in a Yorkshire terrier. Vet Rec. 1993;132:587‐588. [DOI] [PubMed] [Google Scholar]
- 21. Van Ham L, Bhatti S, Polis I, et al. ‘Continuous muscle fibre activity’ in six dogs with episodic myokymia, stiffness and collapse. Vet Rec. 2004;155:769‐774. [PubMed] [Google Scholar]
- 22. Saito M, Olby NJ, Obledo L, Gookin JL. Muscle cramps in two standard poodles with hypoadrenocorticism. J Am Anim Hosp Assoc. 2002;38:437‐443. [DOI] [PubMed] [Google Scholar]
- 23. de Lahunta A, Glass EN, Kent M. Classifying involuntary muscle contractions. Compend Contin Educ Vet. 2006;28:516‐529. [Google Scholar]
- 24. Vanhaesebrouck AE, Bhatti SF, Franklin RJ, et al. Myokymia and neuromyotonia in veterinary medicine: a comparison with peripheral nerve hyperexcitability syndrome in humans. Vet J. 2013;197:153‐162. [DOI] [PubMed] [Google Scholar]
- 25. Marsden CD, Hallet M, Fahn S. The nosology and pathophysiology of myoclonus. In: Marsden CD, Fahn S, eds. Movement Disorders. London, England: Butterworth Scientific; 1985:198‐248. [Google Scholar]
- 26. Lowrie M, Garosi L. Classification of involuntary movements in dogs: myoclonus and myotonia. J Vet Intern Med. 2017;31:979‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lowrie M, Garosi L. Classification of involuntary movements in dogs: paroxysmal dyskinesias. Vet J. 2017;220:65‐71. [DOI] [PubMed] [Google Scholar]
- 28. Vanhaesebrouck AE, Shelton GD, Garosi L, et al. A novel movement disorder in related male Labrador Retrievers characterized by extreme generalized muscular stiffness. J Vet Intern Med. 2011;25:1089‐1096. [DOI] [PubMed] [Google Scholar]
- 29. Packer RA, Patterson EE, Taylor JF, Coates JR, Schnabel RD, O'Brien DP. Characterization and mode of inheritance of a paroxysmal dyskinesia in Chinook dogs. J Vet Intern Med. 2010;24:1305‐1313. [DOI] [PubMed] [Google Scholar]
- 30. Herrtage ME, Palmer AC. Episodic falling in the cavalier King Charles spaniel. Vet Rec. 1983;112:458‐459. [DOI] [PubMed] [Google Scholar]
- 31. Black V, Garosi L, Lowrie M, Harvey RJ, Gale J. Phenotypic characterisation of canine epileptoid cramping syndrome in the Border terrier. J Small Anim Pract. 2014;55:102‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyers KM, Lund JE, Padgett G, Dickson WM. Hyperkinetic episodes in Scottish Terrier dogs. J Am Vet Med Assoc. 1969;155:129‐133. [PubMed] [Google Scholar]
- 33. Urkasemsin G, Olby NJ. Clinical characteristics of Scottie Cramp in 31 cases. J Small Anim Pract. 2015;56:276‐280. [DOI] [PubMed] [Google Scholar]
- 34. Lowrie M, Garosi L. Natural history of canine paroxysmal movement disorders in Labrador retrievers and Jack Russell terriers. Vet J. 2016;213:33‐37. [DOI] [PubMed] [Google Scholar]
- 35. Ramsey IK, Chandler KE, Franklin RJ. A movement disorder in boxer pups. Vet Rec. 1999;144:179‐180. [DOI] [PubMed] [Google Scholar]
- 36. Harcourt‐Brown T. Anticonvulsant responsive, episodic movement disorder in a German shorthaired pointer. J Small Anim Pract. 2008;49:405‐407. [DOI] [PubMed] [Google Scholar]
- 37. Polidoro D, Van Ham L, Santens P, et al. Phenotypic characterization of paroxysmal dyskinesia in Maltese dogs. J Vet Intern Med. 2020;34:1541‐1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kolicheski AL, Johnson GS, Mhlanga‐Mutangadura T, et al. A homozygous PIGN missense mutation in Soft‐Coated Wheaten Terriers with a canine paroxysmal dyskinesia. Neurogenetics. 2017;18:39‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nessler J, Hug P, Mandigers PJJ, et al. Mitochondrial PCK2 missense variant in Shetland Sheepdogs with paroxysmal exercise‐induced dyskinesia (PED). Genes. 2020;11(7):774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Risio L, Forman OP, Mellersh CS, et al. Paroxysmal dyskinesia in Norwich Terrier Dogs. Mov Disord Clin Pract. 2016;3:573‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richter A, Hamann M, Wissel J, et al. Dystonia and paroxysmal dyskinesias: under‐recognized movement disorders in domestic animals? A comparison with human dystonia/paroxysmal dyskinesias. Front Vet Sci. 2015;2:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morgante F, Klein C. Dystonia. Continuum. 2013;19:1225‐1241. [DOI] [PubMed] [Google Scholar]
- 43. Jankovic J, Hallett M, Okun M, Comella C, Fahn S. Dystonia: Phenomenology, classification, etiology, pathology, biochemistry, and genetics. Principles and Practice of Movement Disorders. Vol x. Philadelphia, PA: Churchill Livingstone/Elsevier; 2007:259‐292. [Google Scholar]
- 44. Suchowersky O, Comella C. Hyperkinetic Movement Disorders. Vol xiv. New York, NY: Humana Press; 2012:55‐83. [Google Scholar]
- 45. Pfeiffer RF. The phenotypic spectrum of Parkinson's disease. In: LeDoux MS, ed. Animal Models of Movement Disorders. London, England: Elsevier Academic Press; 2005:127‐137. [Google Scholar]
- 46. O'Brien DP, Johnson GS, Schnabel RD, et al. Genetic mapping of canine multiple system degeneration and ectodermal dysplasia loci. J Hered. 2005;96:727‐734. [DOI] [PubMed] [Google Scholar]
- 47. de Lahunta A, Averill DR Jr. Hereditary cerebellar cortical and extrapyramidal nuclear abiotrophy in Kerry Blue Terriers. J Am Vet Med Assoc. 1976;168:1119‐1124. [PubMed] [Google Scholar]
- 48. Montgomery DL, Storts RW. Hereditary striatonigral and cerebello‐olivary degeneration of the Kerry Blue Terrier. I. Gross and light microscopic central nervous system lesions. Vet Pathol. 1983;20:143‐159. [DOI] [PubMed] [Google Scholar]
- 49. Stedman TL. Stedman's Medical Dictionary for the Health Professions and Nursing, Illustrated. 6th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 50. Berendt M, Farquhar RG, Mandigers PJ, et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. 2015;11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jankovic J, Demirkiran M. Classification of paroxysmal dyskinesias and ataxias. Adv Neurol. 2002;89:387‐400. [PubMed] [Google Scholar]
- 52. Gill JL, Tsai KL, Krey C, et al. A canine BCAN microdeletion associated with episodic falling syndrome. Neurobiol Dis. 2012;45:130‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Forman OP, Penderis J, Hartley C, Hayward LJ, Ricketts SL, Mellersh CS. Parallel mapping and simultaneous sequencing reveals deletions in BCAN and FAM83H associated with discrete inherited disorders in a domestic dog breed. PLoS Genet. 2012;8:e1002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rhodes TH, Vite CH, Giger U, Patterson DF, Fahlke C, George AL. A missense mutation in canine C1C‐1 causes recessive myotonia congenita in the dog. FEBS Lett. 1999;456:54‐58. [DOI] [PubMed] [Google Scholar]
- 55. Finnigan DF, Hanna WJ, Poma R, et al. A novel mutation of the CLCN1 gene associated with myotonia hereditaria in an Australian cattle dog. J Vet Intern Med. 2007;21:458‐463. [DOI] [PubMed] [Google Scholar]
- 56. Lobetti RG. Myotonia congenita in a Jack Russell terrier. J S Afr Vet Assoc. 2009;80:106‐107. [DOI] [PubMed] [Google Scholar]
- 57. Bhalerao DP, Rajpurohit Y, Vite CH, Giger U. Detection of a genetic mutation for myotonia congenita among Miniature Schnauzers and identification of a common carrier ancestor. Am J Vet Res. 2002;63:1443‐1447. [DOI] [PubMed] [Google Scholar]
- 58. Gilliam D, O'Brien DP, Coates JR, et al. A homozygous KCNJ10 mutation in Jack Russell Terriers and related breeds with spinocerebellar ataxia with myokymia, seizures, or both. J Vet Intern Med. 2014;28:871‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rohdin C, Gilliam D, O'Leary CA, et al. A KCNJ10 mutation previously identified in the Russell group of terriers also occurs in Smooth‐Haired Fox Terriers with hereditary ataxia and in related breeds. Acta Vet Scand. 2015;57:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Van Poucke M, Stee K, Bhatti SF, et al. The novel homozygous KCNJ10 c.986T>C (p.(Leu329Pro)) variant is pathogenic for the SeSAME/EAST homologue in Malinois dogs. Eur J Hum Genet. 2017;25:222‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hajek I, Kettner F, Simerdova V, et al. NHLRC1 repeat expansion in two beagles with Lafora disease. J Small Anim Pract. 2016;57:650‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lohi H, Young EJ, Fitzmaurice SN, et al. Expanded repeat in canine epilepsy. Science. 2005;307:81. [DOI] [PubMed] [Google Scholar]
- 63. Kolicheski A, Johnson GS, O'Brien DP, et al. Australian cattle dogs with neuronal ceroid lipofuscinosis are homozygous for a CLN5 nonsense mutation previously identified in Border Collies. J Vet Intern Med. 2016;30:1149‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Awano T, Katz ML, O'Brien DP, et al. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile Dachshund with neuronal ceroid lipofuscinosis. Mol Genet Metab. 2006;89:254‐260. [DOI] [PubMed] [Google Scholar]
- 65. Awano T, Katz ML, O'Brien DP, et al. A mutation in the cathepsin D gene (CTSD) in American Bulldogs with neuronal ceroid lipofuscinosis. Mol Genet Metab. 2006;87:341‐348. [DOI] [PubMed] [Google Scholar]
- 66. Katz ML, Khan S, Awano T, Shahid SA, Siakotos AN, Johnson GS. A mutation in the CLN8 gene in English Setter dogs with neuronal ceroid‐lipofuscinosis. Biochem Biophys Res Commun. 2005;327:541‐547. [DOI] [PubMed] [Google Scholar]
- 67. Katz ML, Farias FH, Sanders DN, et al. A missense mutation in canine CLN6 in an Australian shepherd with neuronal ceroid lipofuscinosis. J Biomed Biotechnol. 2011;2011:198042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sanders DN, Farias FH, Johnson GS, et al. A mutation in canine PPT1 causes early onset neuronal ceroid lipofuscinosis in a Dachshund. Mol Genet Metab. 2010;100:349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Farias FH, Zeng R, Johnson GS, et al. A truncating mutation in ATP13A2 is responsible for adult‐onset neuronal ceroid lipofuscinosis in Tibetan terriers. Neurobiol Dis. 2011;42:468‐474. [DOI] [PubMed] [Google Scholar]
- 70. Abitbol M, Thibaud JL, Olby NJ, et al. A canine Arylsulfatase G (ARSG) mutation leading to a sulfatase deficiency is associated with neuronal ceroid lipofuscinosis. Proc Natl Acad Sci U S A. 2010;107:14775‐14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Melville SA, Wilson CL, Chiang CS, et al. A mutation in canine CLN5 causes neuronal ceroid lipofuscinosis in Border collie dogs. Genomics. 2005;86:287‐294. [DOI] [PubMed] [Google Scholar]
- 72. Wohlke A, Philipp U, Bock P, et al. A one base pair deletion in the canine ATP13A2 gene causes exon skipping and late‐onset neuronal ceroid lipofuscinosis in the Tibetan terrier. PLoS Genet. 2011;7:e1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guo J, O'Brien DP, Mhlanga‐Mutangadura T, et al. A rare homozygous MFSD8 single‐base‐pair deletion and frameshift in the whole genome sequence of a Chinese Crested dog with neuronal ceroid lipofuscinosis. BMC Vet Res. 2015;10:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Karli P, Oevermann A, Bauer A, Jagannathan V, Leeb T. MFSD8 single‐base pair deletion in a Chihuahua with neuronal ceroid lipofuscinosis. Anim Genet. 2016;47:631. [DOI] [PubMed] [Google Scholar]
- 75. Gill JL, Capper D, Vanbellinghen JF, et al. Startle disease in Irish wolfhounds associated with a microdeletion in the glycine transporter GlyT2 gene. Neurobiol Dis. 2011;43:184‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Video compilation of a soft‐coated Wheaten terrier and a Chinook dog exhibiting dyskinesia and dystonia. For the soft‐coated Wheaten terrier (start of video to time 0:28), note the sustained, abnormal posture of the body and limbs (dystonic posture, in this case manifesting as kyphosis). The dog continues to attempt to walk (co‐occurring voluntary movement) while the involuntary movements are occurring. At times there appears to be involuntary flexion and extension of the pelvic limbs (dyskinesia). For the Chinook dog (0:28 to 1:21), note the abnormal sustained posture of the limbs and head/neck (dystonic posture), and the irregular, involuntary movement of the limbs. A dystonic head tremor can be seen at time 1:02.
Video S2 Video of myokymia in a Jack Russell terrier. Note the vermicular nature of the muscle movements of the pelvic limb.
Video S3 Video of a dog with myotonia congenita. Note the generalized hypertrophy, and sustained contraction of the muscles, resulting in a stiff gait. As is typical with myotonia, the patients “warms” out of the myotonia and the gait normalizes with activity.
Video S4 Video compilation of neuromyotonia in the earlier and later stages of disease. The Bassett hound (start of video to time 0:50) shows earlier stages of neuromyotonia, and is exacerbated by stress. In this case the dog remains able to walk. In later stages of disease (time 0:50 to end of video) the muscle stiffness may cause collapse and difficulty walking. Note the persistent muscle stiffness and rippling appearance to the muscles in both patients.


