Abstract
Despite the development of a number of vaccines for COVID-19, there remains a need for prevention and treatment of the virus SARS-CoV-2 and the ensuing disease COVID-19. This report discusses the key elements of SARS-CoV-2 and COVID-19 that can be readily treated: viral entry, the immune system and inflammation, and the cytokine storm. It is shown that the essential nutrients zinc, ω-3 polyunsaturated fatty acids (PUFAs), vitamin D and magnesium provide the ideal combination for prevention and treatment of COVID-19: prevention of SARS-CoV-2 entry to host cells, prevention of proliferation of SARS-CoV-2, inhibition of excessive inflammation, improved control of the regulation of the immune system, inhibition of the cytokine storm, and reduction in the effects of acute respiratory distress syndrome (ARDS) and associated non-communicable diseases. It is emphasized that the non-communicable diseases associated with COVID-19 are inherently more prevalent in the elderly than the young, and that the maintenance of sufficiency of zinc, ω-3 PUFAs, vitamin D and magnesium is essential for the elderly to prevent the occurrence of non-communicable diseases such as diabetes, cardiovascular diseases, lung diseases and cancer. Annual checking of levels of these essential nutrients is recommended for those over 65 years of age, together with appropriate adjustments in their intake, with these services and supplies being at government cost. The cost:benefit ratio would be huge as the cost of the nutrients and the testing of their levels would be very small compared with the cost savings of specialists and hospitalization.
Keywords: Zinc, ω-3 PUFAs, Vitamin D, Magnesium, Essentiality, SARS-CoV-2, COVID-19, Diabetes, Cardiovascular diseases, Lung diseases, Cancer, Supplementation
Abbreviations
- 1α,25(OH)2D3
1α,25-dihydroxyvitamin D3, calcitriol
- 25(OH)D
25-hydroxyvitamin D3
- ACE
angiotensin converting enzyme
- ACE2
angiotensin converting enzyme 2
- ADAM17
disintegrin and metalloproteinase domain 17
- ALA
α-linolenic acid
- Ang
angiotensin
- ARDS
acute respiratory distress syndrome
- ATR1
AT1 receptor
- CCL2 (MCP-1)
monocyte chemotactic protein-1
- CCL3
(MIP-1α) macrophage inflammatory protein 1α
- c-Kit
stem cell factor receptor
- CRP
C-reactive protein
- CYP27B1
1α-hydroxylase
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- ERK1/2
extracellular signal-regulated kinase 1/2
- FGF
fibroblast growth factor
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HCQ
hydroxychloroquine
- HIF-1α
hypoxia-inducible factor 1-α
- IFN-γ
interferon-γ
- IL-x
interleukin x
- IL-1RN
IL-1 receptor antagonist protein
- IP-10
interferon-γ-inducible protein
- M1,2
macrophage type 1, (or 2)
- MMP-2,9
matrix metallopeptidase 2, (or 9)
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- P38 MAPK
p38 mitogen-activated protein kinases
- PDGF
platelet-derived growth factor
- PGE2
prostaglandin E2
- PUFA
polyunsaturated fatty acid
- RANTES
regulated upon activation, normal T cell expressed and presumably secreted
- RAS
renin-angiotensin system
- ROS
reactive oxygen species
- SAA
serum amyloid A
- SCF
stem cell factor
- TGF-β
transforming growth factor β
- Th
T helper
- TLR
toll-like receptor
- TMPRSS2
transmembrane serine protease 2
- TNF-α
tumour necrosis factor α
- Treg
regulatory T cell
- VDR
vitamin D receptor
- VEGF
vascular endothelial growth factor
1. Introduction
SARS-CoV is the original SARS that was virulent in the early 2000s; it is the virus that leads to the COVID-19 disease state. There is an immediate need for a prophylaxis and treatment for COVID-19. Even if the vaccines that are being distributed and further developed are successful in their efficacy and prevention of transference, there will always be a need for a prophylaxis and treatment for like viruses and comorbidities, particularly in poorer countries. It is therefore timely to present the advantages of the essential combination of zinc, ω-3 PUFAs, vitamin D and magnesium as these treatments are low in cost, extensive in their actions, and safe as they are naturally present in the human body.
The essential sufficiency of zinc, ω-3 PUFAs and vitamin D for prevention and treatment of cancers has been previously presented [1]. The prevention and treatment of COVID-19 and associated comorbidities is currently of the highest concern. Furthermore, the four principal non-communicable diseases that are most prevalent world-wide are diabetes, cardiovascular diseases, lung diseases and cancer [2]. The comorbidities discussed here in the context of COVID-19 include these non-communicable diseases as well as ageing and obesity. Zinc, ω-3 PUFAs, vitamin D and magnesium, although very different in their modes of action, have many similar end-effects. The commonality of zinc, ω-3 PUFAs, vitamin D and magnesium in inhibiting inflammatory situations, as well as their ability to correct immune dysfunction, together with the low cost and safety of these nutrients, makes them ideal as front-line prevention measures and adjuvants in treating COVID-19 and associated comorbidities, as well as the principal non-communicable diseases in non-COVID situations.
Extensive literature searches were made in preparation of this manuscript. PubMed was principally used with a wide range of search strings. Separate searches were made for key concepts together with each of the individual components: ie, zinc; (PUFAs OR DHA OR EPA); vitamin D; magnesium. A PubMed search was conducted using the following search string: (COVID-19 OR SARS-CoV-2 OR coronavirus) AND zinc AND (PUFA OR DHA OR EPA) AND “vitamin D″ AND magnesium. This search produced zero results suggesting that there have been virtually no studies examining the beneficial effects of supplementation of all four essential components together: zinc, ω-3 PUFAs, vitamin D and magnesium. Nevertheless, the four components are described or mentioned in a number of reviews of nutritional status and immune function and/or inflammation [eg, Refs. [[3], [4], [5], [6]]].
2. Zinc
Zinc is a vital cofactor for more than 2000 transcription factors and 300 enzymes in regulating cell differentiation and proliferation, as well as basic metabolic functions of cells [7]. Zinc deficiency is a world-wide problem with approximately 2 billion people subjected to zinc-deficient diets [7,8]. Zinc deficiency is not limited to the developing countries as it also exists in the industrialized world, mainly in the elderly [9,10]. In normal healthy adults, the plasma concentration of zinc is typically 14–23 μmol/L (0.9–1.5 μg/mL) [11].
Risk factors associated with Zn deficiency have been well reported in the literature [[12], [13], [14]]. Zinc deficiency can cause an imbalance in both the innate and adaptive immune systems, with severe deficiency leading to infections, skin disorders, gastrointestinal disorders, weight loss, growth retardation and male hypogonadism, amongst other symptoms [[14], [15], [16], [17]]. Low zinc levels have been found to affect the function of various types of immune cells, including macrophages, neutrophils, mast cells and dendritic cells [11,18]. Zinc is also essential for the development, differentiation and activation of T cells [19]. Zinc deficiency can therefore result in impaired production, activation and maturation of natural killer cells (cell-mediated innate immunity), T cells (cell-mediated adaptive immunity) and B cells (humoral adaptive immunity) [5].
Zinc deficiency, which is commonly reported in the elderly, lowers immune function, decreases resistance to invading pathogens and increases the risk of contracting pneumonia [20,21]. Zinc deficiency also occurs frequently in patients with cardiovascular disease, chronic pulmonary disease, diabetes or obesity [22,23].
Zinc exerts its anti-inflammatory activity by inhibiting activation and signalling of NF-κB, and through controlling regulatory T cell (Treg) functions [12]. Zinc supplementation causes pro-inflammatory Th17 cells to skew towards anti-inflammatory Treg cells [24]. Zinc supplementation inhibits NF-κB through expression of the A20 protein, resulting in suppression of TNF-α, IL-1β, IL-6, IL-8, IL-12, IL-18, IFN-γ, iNOS, COX-2, GM-CSF [12,25].
Macrophages, neutrophils, and T cells are activated through elevation of cytokines including IL-1, IL-6, and TNF-α, often leading to acute respiratory distress syndrome (ARDS) [26]. IL-6, IL-8 and TNF-α levels are elevated in elderly people who are zinc deficient, as well as in the obese [17,27], and zinc supplementation has been found to reduce these levels [10].
When levels of reactive oxygen species (ROS) are elevated, as is common in zinc deficiency, oxidative damage results. Zinc supplementation decreases ROS production and this has beneficial results, especially in the aged and in diabetes mellitus [13].
A number of comprehensive reviews of zinc and its involvement in ageing, COVID-type viruses and comorbidities have been presented [eg, Refs. [27,28]]. Zinc sufficiency is vital for reducing risk factors associated with COVID-19, such as obesity, diabetes, cardiovascular diseases, lung diseases and ageing [12,29]. Physiological supplementation of Zn in ageing and in age-related degenerative diseases corrects immune defects and reduces infection relapse [30].
One of the problems of zinc supplementation has been the variability of zinc bioavailability in cells. It has been found that increasing the intracellular levels of zinc using ionophores such as pyrithione can effectively decrease the replication of a variety of viruses, including the replication of SARS-CoV [31]. Unfortunately, pyrithione is not recommended for use internally, whereas it is efficacious and safe when used topically. Other proven zinc ionophores include chloroquine and hydroxychloroquine (HCQ) [12,32,33], disulfiram [33], quercetin and epigallocatechin-gallate [34], and zincophorin [35]. In addition, Rizzo [36] presented a sound rationale for ivermectin being an ionophore for zinc. A number of clinical studies are planned or underway that are based on HCQ and zinc, and ivermectin and zinc, together in some cases with an antibiotic such as azithromycin or doxycycline [37]. Interestingly, the HCQ studies are fundamentally based on testing whether zinc complements HCQ, and not whether HCQ complements zinc which would be expected if HCQ was recognized as an ionophore for zinc. A similar comment applies to ivermectin. The latest summary of ongoing studies at the time of preparing this manuscript was that of Pal et al. [38].
Zinc is also known for its capacity to modulate antiviral and antibacterial immunity [12]. The antibacterial properties of zinc are well demonstrated against S. pneumoniae [12]. Moreover, zinc has the capacity to reduce the risk of bacterial co-infection by improving lung function through mucociliary clearance and protecting lung barrier function.
Zinc inhibits SARS-CoV RNA polymerase, and thus its replication capacity [17]. Zinc has also been postulated as a stabilizer of cell membranes which could assist in blocking virus entry to cells [39]. In this context, zinc decreases activity of angiotensin converting enzyme 2 (ACE2) which is required for binding with SARS-CoV-2 for cell entry [12]. Wessels and co-workers [40] concluded that zinc has multiple functions in inhibiting viral entry to host cells and activity: prevention of fusion with the host membrane, inhibiting viral polymerase and ensuing replication, impairing protein translation and processing, blocking viral particle release, and destabilizing the viral envelope. It has been shown that zinc deficiency increases the leakage of the epithelium of the respiratory tract using an ex vivo model [41], in contrast to zinc supplementation which has been shown to improve lung integrity in a mouse model through decreased recruitment of neutrophils to the lungs [42].
According to the NIH, US National Library of Medicine, there are no formal studies evaluating zinc for COVID-19 management that have been completed and reported to date, although several trials are currently registered to test zinc as part of different regimens to treat COVID-19 [37] Nevertheless, zinc supplementation has been found to have a beneficial effect on COVID-19 patients [43,44]. A study of 47 COVID-19 patients showed that 57% of COVID-19 patients were zinc deficient. These zinc deficient patients developed more complications and had increased mortality than those with normal zinc levels [45].
Regarding the elderly, supplementation with 45 mg elemental zinc per day markedly reduced the incidence of infection in elderly subjects, ranging from 55 years to 87 years [10]. Consuming around 25–40 mg zinc per day is affordable, and less likely to induce human toxicity, as more than 200–400 mg per day of zinc consumption can induce adverse events [46].
3. ω-3 polyunsaturated fatty acids (PUFAs)
ω-3 PUFAs have not received the attention they deserve in the prevention and treatment of COVID-19 and associated comorbidities. ω-3 PUFAs have properties that are significantly different from those of zinc, vitamin D and magnesium, properties that are nevertheless ideally suited to prevention and treatment of COVID-19, obesity and diabetes, cardiovascular diseases, chronic pulmonary diseases and cancer, and improving immune function and anti-inflammatory effects in general ageing [1,[47], [48], [49]].
SARS-CoV and SARS-CoV-2 are very similar and they are both enveloped viruses that can lead to development of ARDS. ω-3 PUFAs, in particular eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been shown to inactivate enveloped viruses as well as to inhibit the proliferation of a range of microbial organisms [50].
The efficacy of α-linolenic acid (ALA) in treating inflammation and immune deficiency has been shown in a number of cases to be similar to that of DAH and EPA, although typically the potency is in the order DHA>EPA>ALA [eg, 49,51]. It has been reported that oil rich in ALA caused an immune modulation in cancer similar to that with fish oil, which was accompanied by a decrease in macrophage production of pro-inflammatory cytokines (eg, TNF-α and IL-6) [52].
ω-3 PUFAs have an important role in regulating macrophages as they modulate the production of cytokines and chemokines by macrophages; they change macrophage phagocytosis capacity and they convert macrophages from pro-inflammatory (M1 type macrophages) to anti-inflammatory (M2) type [53]. ω-3 PUFAs and their metabolites have a modulating effect on neutrophils as they affect neutrophil migration, phagocytic capacity and the production of ROS [53].
Weill and co-workers [51] described the action of PUFAs as having two phases in inhibiting inflammation: a promotion phase in which ω-6 PUFAs such as AA lead to the synthesis of pro-inflammatory leukotrienes and prostaglandins through the action of cyclooxygenases, lipoxygenases and cytochrome P450; and a resolution phase where ω-3 PUFAs are precursors to potent active mediators such as resolvins, maresins and protectins which inhibit the synthesis of pro-inflammatory cytokines through downregulation of the NF-κB pathway. Resolvins are sourced from EPA and DHA and protectins are sourced from DHA; they have anti-inflammatory effects by limiting leucocyte infiltration in infected tissues [51,54]. Maresins are sourced from DHA and they also resolve inflammation [51,55].
ω-3 PUFAs are notable for their influence on the properties of lipid rafts, which in turn perform an important role in the functioning of the outer leaflet of cellular membranes. ω-3 PUFAs regulate membrane properties such as membrane fluidity and signal transduction [1]. SARS-CoV has been shown to rely on lipid raft integrity for virus entry to host cells [56,57]. The entry receptor for the coronavirus, ACE2, is located in lipid rafts. The ACE2 receptor-mediated endocytosis is followed by activation of the spike protein in the viral envelope by the transmembrane serine protease 2 (TMPRSS2) which is located adjacent to the ACE2 receptor [51]. It has been shown that ω-3 PUFAs inhibit cellular entry through ACE2 and the enzymatic activity of TMPRSS2 [51,58]. The disruptive effect of ω-3 PUFAs on the integrity of lipid rafts has been described before [1], where ω-3 PUFAs were described as causing disruption to lipid rafts due to the very poor affinity of ω-3 PUFAs for cholesterol. It is therefore clear that ω-3 PUFAs have multiple inhibitory effects on viral entry into host cells.
There have been a number of clinical studies confirming the anti-inflammatory and immune response effects of ω-3 PUFA supplementation [4,5,[59], [60], [61]]. According to the NIH, US National Library of Medicine, there are no formal studies evaluating ω-3 PUFAs for COVID-19 management that have been completed and reported to date, although four trials are currently registered to test ω-3 PUFAs as part of different regimens [37].
4. Vitamin D
Vitamin D obtained from sunlight or dietary sources is catalysed by vitamin D-25-hydroxylase in the liver to 25-hydroxyvitamin D3 (25(OH)D), the major circulating form of vitamin D. 25(OH)D is biologically inert until it is hydroxylated by the enzyme 1α-hydroxylase (CYP27B1) in the kidney to the active form 1α,25-dihydroxyvitamin D3 (calcitriol, 1α,25(OH)2D3) [62].
Calcitriol has important immunoregulatory and anti-inflammatory effects that it exerts through interaction with the vitamin D receptor (VDR). The calcitriol/VDR complex can interact with different gene transcription factors that control inflammatory responses [63]. VDR and CYP27B1 are expressed in many types of immune cells, including lymphocytes, monocytes/macrophages, dendritic cells, T and B cells [64,65], and on pulmonary epithelial cells. These immune cells can convert 25(OH)D into biologically active calcitriol [63,66]. The calcitriol/VDR complex causes transcription of the antimicrobial peptides cathelicidins and defensins. Cathelicidins disrupt bacterial cell membranes as well as enveloped viruses such as SARS-CoV-2, while defensins promote chemotaxis of inflammatory cells through increased capillary permeability [65,67].
Synthesis of vitamin D in the skin is controlled by the season, the time of the exposure during the day and the latitude [68,69]. Vitamin D is poorly synthesized above (to the north) and below (to the south) of 35° latitude during winter months [70]. Lockdowns, implemented to minimize the spread of COVID-19, are therefore detrimental to vitamin D synthesis as people are prevented from going out from their homes and absorbing the sunshine, which has a cumulative effect in the winter months when COVID-19 is more prevalent. The Black and Asian populations produce less vitamin D as a result of a higher skin melanin content than those with white skin [71]. Excess exposure to sunlight is the major cause of skin cancer [72]. However, there is an increased incidence of skin cancer and other cancers in countries with low levels of sunlight compared with those countries with higher levels of sunlight throughout the year [73,74], supporting the proposition that sunlight is beneficial for synthesis of vitamin D and subsequent prevention of cancers. There have been a number of reports where low sun exposure has been shown to have a negative impact on a range of health issues [[75], [76], [77]]. The advantage of sun exposure in providing vitamin D needs to be sensibly balanced against the potential risk of skin cancer from excessive sun exposure [78].
In the early stages of acute inflammation, vitamin D inhibits the proliferation of Th1 and Th17 cells and their abnormal release of IFN-γ, TNF-α, IL-1, IL-2, IL12, IL-23 and IL-17, IL-21 [65]. During the resolution phase of inflammation, vitamin D-mediates differentiation of Th2 cells and release of their anti-inflammatory cytokines (IL-4 and IL-10), evading the organ damage that could be caused by an excessive immune response [65]. Vitamin D has powerful anti-inflammatory properties that play an important role in controlling immune function in pulmonary infection; eg, it inhibits the effects of TNF-α, it inhibits NF-κB activity in immune cells, it inhibits the activation of inflammasomes and hence release of IL-1β, and it decreases expression of IL-6, a major contributor to the so-called ‘cytokine storm’ [65,79].
The immune response acts in concert with the inflammatory response. The innate immune system acts as the first line of defence against invading pathogens such as viruses. Calcitriol enhances that defence by recruiting neutrophils, monocytes/macrophages and dendritic cells which kill and clear the viral pathogens, ultimately initiating the adaptive immune response. This response can be overactive resulting in the cytokine storm. Calcitriol inhibits this chronic immune response by downregulating the toll-like receptors (TLRs) that identify the viral pathogens initially, and inhibits the TNF-α/NF-κB and IFN-γ signalling pathways. Calcitriol shifts the T cell profile from the pro-inflammatory Th1 and Th17 forms to the anti-inflammatory Th2 and Treg forms, respectively [80]. Tregs provide a major defence against inflammation, releasing anti-inflammatory cytokines IL-10 and TGF-β. Treg levels are markedly decreased in severe COVID-19 disease, in contrast to high levels of Treg correlating with reduced levels of viral disease [81].
Natural killer cells are innate immune cells and they are known to possess strong antiviral activity as well as anticancer activity [82]. The count and activity of natural killer cells have been shown to be reduced below normal in COVID-19 patients, and vitamin D has been found to increase the activity of natural killer cells [82].
Although there is inconsistency in the data, it is apparent that vitamin D deficiency is influential in increasing risk of acute respiratory tract infections [83], particularly when considering the decrease in natural synthesis of vitamin D in winter-time when acute respiratory infections are most prevalent. Ali [84] conducted a study of COVID-19 cases and mortality in 20 European countries, finding that vitamin D status correlated negatively with COVID-19 cases but not with mortality. The effectiveness of vitamin D sufficiency in reducing risk of acute viral respiratory tract infections and pneumonia was also shown. Similar results were reported by Kara and co-workers [85], who also discussed the link between latitude, temperature and humidity and season on viral respiratory tract infections.
Allegra and co-workers [86] reported on the deficiency and supplementation of a range of vitamins including vitamin D, in particular in correlating hypovitaminosis with risk of contracting COVID-19 and associated mortality. They reported that there were positive and indeterminate results in their analysis of multiple studies. Vitamin D levels were particularly reduced in the ageing populations of Italy, Spain, and Switzerland, which were the most susceptible populations in relation to SARS-CoV-2 infection [87]. Additionally, Annweiler and co-workers [88] analysed a range of reports with the conclusion that inverse correlations were found between 25(OH)D levels in patients and COVID-19 incidence and mortality. Other reports have also covered the influence of vitamin D on outcomes of COVID-19 patients with the typical finding that vitamin D supplementation leads to an improved outcome for these patients and that vitamin D deficiency increases the risk and susceptibility for severe COVID-19 disease and mortality [69,84,87,[89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99]].
In another review, Lau and co-workers [100] found that vitamin D deficiency was highly prevalent in patients with severe COVID-19, which correlated in turn with obesity, male sex, advanced age, population concentration in northern climates, coagulopathy and immune dysfunction. A further meta-analysis found that vitamin D deficiency increased risk of severe infections and mortality of the critically ill [101]. Deficiency of vitamin D has been further claimed to increase the risk of contracting osteoporosis, cancer, diabetes, multiple sclerosis, hypertension, and inflammatory and immunological diseases [102]. Although vitamin D and the benefits of supplementation in preventing cancer have been discussed previously [1], it is of note that a number of researchers have demonstrated that the risk of cancer incidence and fatality is reduced with vitamin D supplementation [eg, Refs. [[103], [104], [105]]]. The mechanism of action of vitamin D in reducing cancer risk has also been addressed in a number of reviews [eg, Refs. [[106], [107], [108]]].
It has been specified that a reasonable level of 25(OH)D in serum is at least 30 ng/mL (75 nmol/L) [93,109], with a preference for 40–60 ng/mL (100–150 nmol/L) to ensure good health, particularly in the elderly [69,110].
In summary, vitamin D impedes the entry and replication of SARS-CoV-2, reduces the levels of pro-inflammatory cytokines, increases the levels of anti-inflammatory cytokines and increases the production of natural antimicrobial peptides [111].
5. Magnesium
Magnesium is an essential element in the optimal biological functioning of the human body. Magnesium is the second most abundant intracellular cation in the human body and it is fundamental for oxidative phosphorylation, glycolysis, DNA transcription, and protein synthesis [112]. Magnesium levels are not routinely analysed in clinical practice [113] which means there has been limited reporting of magnesium correlations for COVID-19 patients [113,114]. Despite this, there have been some reports of magnesium status being lower in severe COVID-19 cases than in less severe cases [[115], [116], [117]]. On the other hand, there have been a number of excellent reviews of magnesium and its essentiality in maintenance of proper immune function and control of oxidative stress and low-grade inflammation, particularly in the elderly [eg, Refs. [112,[118], [119], [120], [121], [122]]].
Magnesium is essential for the activation of vitamin D [122,123]. Magnesium and vitamin D are therefore both important for immune function and cellular stability and sufficiency of both is required to counteract the detrimental effects of COVID-19 development [122].
Deficiency of magnesium is common and it has been estimated that in a given population up to 30% may have a magnesium deficiency [122]. Magnesium is principally stored in bone (>50%) with only ∼1% in serum [124]. Magnesium homeostasis is maintained by absorption from the gastrointestinal tract, renal excretion and exchange from bone. Estimates of magnesium sufficiency are therefore dubious if reliant on serum analysis alone [118]. However, 0.75 mmol/L has been suggested as the serum level below which magnesium deficiency exists [125], and 0.85 mmol/L as the required level for magnesium sufficiency [118].
Magnesium has anti-inflammatory and anti-oxidative effects, as well as providing vasodilation and neuroprotection [120]. Magnesium suppresses NF-κB, expression of IL-6 and TNF-α, and levels of C-reactive protein (CRP) [6,121,126]. Magnesium therefore regulates the cardiovascular, digestive, neurological and respiratory systems, contributing significantly to maintenance of normal human health [120]. In this context, magnesium dietary intakes correlate negatively with cardiovascular disease, kidney disease and diabetes [118,127].
6. COVID-19
SARS-CoV-2 is an insidious virus that causes the disease COVID-19. There are many similarities between SARS-CoV-2 and the earlier coronavirus SARS-CoV. In order to present the overall essentiality of ensuring sufficiency of zinc, ω-3 PUFAs, vitamin D and magnesium, the key contributing stages of SARS-CoV-2 incursion and COVID-19 development will be discussed. These are viral entry, the involvement of the immune system and inflammation, and the subsequent cytokine storm that causes the eventual morbidity and mortality that is associated with COVID-19.
6.1. Viral entry
SARS-CoV-2 enters the host cells via the angiotensin-converting enzyme 2 (ACE2) receptor, in the same manner as SARS-CoV [128]. The spike protein of SARS-CoV-2 binds to ACE2, enabling endocytosis, which is followed by activation of the S protein in the viral envelope utilizing the transmembrane serine protease 2 (TMPRSS2), a membrane-bound enzyme located adjacent to the ACE2 receptor [51]. At the same time, the ADAM17 (disintegrin and metalloproteinase domain 17) ‘sheddase’ is activated by the SARS-CoV-2-ACE2 complex which in turn leads to ACE2 ectodomain shedding. Activation of the ADAM17 sheddase may also cause cleavage of TNF-α and IL-6 as well as other pro-inflammatory mediators [128]. It should be noted that the ACE2-binding affinity of the of S protein of SARS-CoV-2 is 10- to 20-fold higher than that of SARS-CoV [129], suggesting that SARS-CoV-2 is significantly more infectious than its predecessor SARS-CoV.
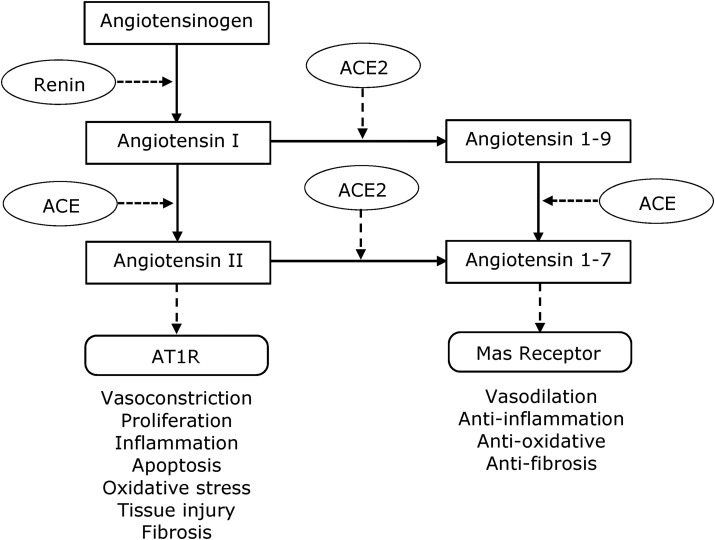
The balance of the renin-angiotensin system (RAS) is vital in controlling host-cell entry of viruses as well as associated comorbidities, as RAS regulates blood pressure. RAS is essentially a balance between ACE and ACE2, as illustrated in Fig. 1 . The ACE pathway requires conversion of angiotensin (Ang) I to Ang II and subsequent binding to the AT1 receptor (AT1R), which has dire consequences such as vasoconstriction, proliferation, inflammation, and apoptosis [91]. The alternative route involves conversion of Ang I and Ang II to angiotensin 1-9 and angiotensin 1-7, respectively, through the enzymatic action of ACE2. Angiotensin 1-9 is also converted to angiotensin 1-7 by ACE.
Fig. 1.
RAS pathways showing the balance between ACE and ACE2.
ACE2 is required for viral entry to host cells, but it is also desirable for conversion of Ang I and Ang II to angiotensins 1–9 and 1–7, respectively, leading to activation of the Mas receptor. Fig. 1 shows that this in turn will cause positive pathology in terms of vasodilation, and anti-inflammatory, anti-oxidative and anti-fibrosis effects [130].
There has been fairly extensive discussion about the contradictory actions of ACE2 in viral entry to host cells and its regulation of the RAS where modulation of RAS has a positive pathological effect. The ACE2 receptor is expressed in lung tissues, and a range of other tissues such as nose, heart, endothelium, kidney and intestine [131,132]. It has now been resolved that once the virus binds to ACE2, it effectively removes it from further action, promoting ACE activity which in turn leads to production of more Ang II. The removal of ACE2 from action therefore causes the virus to have a free run allowing it to proliferate, leading to enhanced morbidity.
The expression of ACE2 has been found to be lower in males than in females and lower in older adults than in young people, which could explain the higher incidence of deaths in elderly males with COVID-19 [130,133]. This category of patients has a worse prognosis when they are also implicated with comorbidities such as cardiovascular diseases, diabetes, hypertension, and obesity, all of which are stimulated by RAS [130]. It is therefore important to enhance the expression of ACE2 and its activity, and at the same time ensure that the entry of the virus to host cells is inhibited. This can be achieved by ensuring that sufficient levels of zinc, ω-3 PUFAs, vitamin D and magnesium are maintained at all times during prevention and treatment of COVID-19.
It should be noted that although there are no reported studies of the effect of zinc on ACE2 for host-cell entry, zinc does protect the human body from virus entry by improved mucociliary clearance of viruses as well as preserving tissue barriers [42]. It has been recognized that enhanced expression of ACE2 by calcitriol alleviates acute lung injury induced by SARS-CoV-2 [[134], [135], [136]]. Calcitriol also supresses renin activity and therefore reduces the generation of angiotensin II which causes pulmonary vasoconstriction [134]. As stated above, binding of ACE2 and cellular entry are inhibited by ω-3 PUFAs [51,58].
6.2. The immune system
The immune system provides two lines of defence: innate and adaptive immunity. Innate immunity is the first line of defence, reliant on mucosal barriers, monocytes, macrophages, neutrophils, eosinophils, and dendritic cells. Adaptive immunity is the process by which immunological memory to a specific antigen is created but more slowly than innate immunity. Dendritic cells also function as antigen-presenting cells, activating B and T lymphocytes of the adaptive immune response [53].
Mast cells are present in the submucosa of the nasal cavity and respiratory tract where they provide a protective barrier against microorganisms and they can be virus-activated [29]. When activated, mast cells initially release preformed inflammatory molecules such as histamine and proteases, while late activation activates the synthesis and release of pro-inflammatory IL-1 family members, including IL-1, IL-6, and IL-33 [137]. Mast cells therefore normally release a wide range of pro-inflammatory mediators. Vitamin D diverts the release characteristics of mast cells to produce and excrete IL-10 without inducing degranulation of pro-inflammatory mediators [138]. IL-10 is an important anti-inflammatory cytokine that inhibits the production of pro-inflammatory cytokines, such as IFN-γ, IL-2, IL-3, TNF-α, and GM-CSF [139].
Macrophages are fundamental to the innate immune system as they scavenge invading pathogens: they recognize invading pathogens through use of pathogen-associated molecular patterns which are in turn recognized by TLRs present on their surface. Macrophages then phagocytose the invading pathogen and at the same time secrete ROS and a large range of cytokines and chemokines to recruit and activate other types of immune cells from both the innate and the adaptive immune systems [53]. The most damaging cytokines released by macrophages when over-activated are IL-1β, IL-6 and TNF-α [140].
Eosinophils release pro-inflammatory mediators, including degranulated cationic proteins, synthesized eicosanoids, and cytokines [141]. Neutrophils are recruited to the initial site of inflammation where they also have a role in removing pathogens. Neutrophils can also interact with the adaptive immune system by promoting naïve T cells to transition into T helper 1 (Th1) cells [53].
T cells are thymus-derived lymphocytes. T cells can be classified into helper (Th) cells that regulate the function of other immune cells, and cytotoxic T cells that destroy virus-infected cells. Th cells differentiate into Th1, Th2, Th17 and Th22 cells. Th1 cells secrete IFN-γ; Th2 cells secrete IL-4; Th17 cells secrete IL-17A, IL17-F, IL-21, and IL-22; and Th22 cells secrete IL-22. Th1 and Th17 cells are pro-inflammatory, whereas Th2 cells are essentially anti-inflammatory [53]. Regulatory T cells (Tregs) suppress the activation of other immune cells such as Th1 cells, Th17 cells, B cells, macrophages or dendritic cells, through secretion of IL-10 and TGF-β [53].
The impact provided by the various contributing immune system cells on the SARS-CoV and SARS-CoV-2 viruses is given in Table 1 . It can be seen that the release of cytokines and chemokines is potentially huge, leading to the potential for production of the cytokine storm.
Table 1.
Mediators released/activated in cells in SARS and COVID-19.
| Cell Type | Mediators released/activated | References |
|---|---|---|
| Mast cells | Histamine, tryptase, NF-κB, IL-1α/β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-15, IL-16, IL-17, IL-18, IL-25, IL-33, TNF-α, IFN-γ, TGF-β, CCL2, CCL3, GM-CSF, VEGF, PDGF, SCF, PGE2, ROS, TLR2, c-Kit | [29,[142], [143], [144], [145], [146]] |
| Monocytes/macrophages | NF-κB, TNFα, IL-1α/β, IL-1RA, IL-6, IL-8, IL-10, IL-12, IFN-γ, TGF-β, ROS, TLR2, TLR4 | [6,110,[147], [148], [149], [150]] |
| Eosinophils | IL-1α, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-11, IL-12, IL-13, IL-16, IL-18, IL-25, TNF-α, IFN-γ, TGF-α/β, VEGF, GM-CSF | [145,[151], [152], [153], [154]] |
| Neutrophils | IL-1α/β, IL-1RA, IL-3, IL-4, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-16, IL-17, IL-18, IL-23, Th1/Th2, TGF-α/β, IFN-α, IFN-γ, TNF-α, G-CSG, GM-CSF, SCF, FGF, VEGF, CCL2, ROS, TLR2, TLR4 | [[155], [156], [157], [158]] |
| Dendritic cells | IL-6, IL-10, IL-12, TNF-α, CCL3, RANTES, IP-10, CCL2 | [110,142,159] |
| Th1 | IFN-γ, IL-1β, IL-2, IL-12, TNF-α | [110,148] |
| Th2 | TGF-β, IL-4, IL-5, IL-9, IL-10, IL-13 | [110,148] |
| Th17 | IL-17A, IL-17F, IL-21, IL-22 | [110,160] |
| Treg | IL-10, TGF-β | [53,110] |
Table 2 shows the key immune cells and regulators that contribute to immune function and inflammation together with the correcting effects of zinc, ω-3 PUFAs, vitamin D and magnesium on each of these immune cells and regulators when immune function and inflammation are over-activated.
Table 2.
Inhibition(↓)/activation(↑) of immune cells cells/regulators.
| Cell/regulator | Zinc | ω-3 PUFAs | Vitamin D | Magnesium |
|---|---|---|---|---|
| Mast cells | ↓ [161,162] | ↓ [144,163,164] | ↓ [138,[165], [166], [167]] | ↓ [168,169] |
| Monocytes | ↓ [170,171] | ↓ [172,173] | ↓ [147] | ↓ [174,175] |
| Macrophages | ↓ [176] | ↓ [177,178] | ↓ [179,180] | ↓ [175,181,182] |
| Neutrophils | ↓ [171,183,184] | ↓ [185,186] | ↓ [187,188] | ↓ [181,189] |
| Dendritic Cells | ↓ [190,191] | ↓ [192,193] | ↓ [63,194,195] | ↓ [196] |
| Eosinophils | ↓ [171,197,198] | ↓ [172,185,199] | ↓ [200,201] | ↓ [202] |
| Th1/Th2 ratio | ↓ [203,204] | ↓ [205,206] | ↓ [207] | ↓ [208] |
| Th17 | ↓ [24,[209], [210], [211]] | ↓ [[212], [213], [214]] | ↓ [215,216] | — |
| Treg | ↑ [24,211,217,218] | ↑ [213,[219], [220], [221]] | ↑ [[222], [223], [224]] | — |
| Inflammasome/caspase-1 | ↓ [225,226] | ↓ [[227], [228], [229]] | ↓ [79,[230], [231], [232]] | ↓ [233,234] |
| NF-κB | ↓ [12,17,25,235] | ↓ [132,236,237] | ↓ [79,[238], [239], [240]] | ↓ [121,174,241] |
— indicates no literature reference found.
6.3. Cytokine storm
The uncontrolled release of immune cells and excessive release of pro-inflammatory cytokines has been termed the ‘cytokine storm’. The cytokine storm normally presents as systemic inflammation, excessive oxidative stress and multiple organ failure [29,51], predominantly resulting in ARDS. The key to counteracting the cytokine storm lies in counteracting excessive inflammation. This can be largely addressed through maintenance of sufficiency of the essential nutrients zinc, ω-3 PUFAs, vitamin D and magnesium. Table 3 gives the key pro-inflammatory cytokines and other mediators involved in a cytokine storm, together with the inhibitory effects of zinc, ω-3 PUFAs, vitamin D and magnesium. It can be seen that these four nutrients are widely effective in inhibiting the key pro-inflammatory mediators of the cytokine storm.
Table 3.
Key pro-inflammatory mediators in a cytokine storm.
| Mechanisms | Effect of Zinc on mediator [Refs] | Effect of ω-3 PUFAs on mediator [Refs] | Effect of Vitamin D on mediator [Refs] | Effect of Magnesium on mediator [Refs] |
|---|---|---|---|---|
| TNF-α | ↓ [ [9,235]] | ↓ [177,[242], [243], [244]] | ↓ [[245], [246], [247], [248]] | ↓ [126,174,249] |
| IFN-γ | ↓ [ [217,218,250]] | ↓ [193,212,244] | ↓ [247,248,[251], [252], [253]] | ↓ [208,249] |
| IL-1β | ↓ [9,210,254] | ↓ [177,227,244,255,256] | ↓ [79,231,232,248,257] | ↓ [182,233,249,258,259] |
| IL-6 | ↓ [17,260] | ↓ [177,212,243,244,256] | ↓ [65,248,261,262] | ↓ [121,126,174,182,263] |
| IL-12 | ↓ [264] | ↓ [172,265] | ↓ [111,195,266,267] | – |
| IL-17 | ↓ [209,210,268] | ↓ [185,193,212,244] | ↓ [216,248,269,270] | – |
| IL-18 | ↓ [271] | ↓ [228] | ↓ [272] | – |
| IL-33 | ↓ [273] | ↓ [274] | ↓ [275] | – |
| CCL2 (MCP-1) | ↓ [9,171] | ↓ [[276], [277], [278], [279]] | ↓ [[280], [281], [282]] | ↓ [263,283] |
| CCL3 (MIP-1α) | ↓ [284] | ↓ [276,285] | ↓ [216,286] | – |
| C-reactive protein (CRP) | ↓ [[287], [288], [289]] | ↓ [[290], [291], [292]] | ↓ [93,293,294] | ↓ [121,126,295] |
| GM-CSF | ↓ [296] | ↓ [297,298] | ↓ [[299], [300], [301], [302]] | – |
| NF-κB | ↓ [12,25,226,235] | ↓ [132,236,237] | ↓ [79,[238], [239], [240]] | ↓ [121,174,241] |
— indicates no literature reference found.
↓ inhibits the mediator.
7. Ageing, obesity and non-communicable diseases
Deficiencies in zinc, ω-3 PUFAs, vitamin D and magnesium have been shown above to provide significant risk factors for severe COVID-19 disease as well as for pre-existing conditions such as ageing, obesity/diabetes, cardiovascular diseases, chronic respiratory diseases and cancer. All of these comorbidities are accompanied by systemic inflammation which likely impacts on the COVID-19 outcome [29].
Table 4 lists the immune cells and mediators that are released in COVID-19, the cytokine storm, ageing, obesity/diabetes and the principal non-communicable diseases. It can be seen that many of the mediators, particularly those that are key pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α and IFN-γ, are common to COVID-19, the cytokine storm (which in turn is part of COVID-19) and the listed comorbidities. The entry in Table 4 for chronic respiratory diseases has been taken to be the same as the cytokine storm, which is the principal force behind creation of ARDS.
Table 4.
Key cells and mediators associated with COVID-19, cytokine storm, ageing and comorbidities.
| Comorbidity/Activity | Cells/Mediators/Transcription factors | References |
|---|---|---|
| COVID-19 | Mast cells, neutrophils, eosinophils, monocytes, macrophages, dendritic cells, NF-κB, IL-1β, IL-1RA, IL-2, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-17, IL-18, IL-21, IL-22, IL-33, TNF-α, IFN-γ, GM-CSF, G-CSF, CCL2, CCL3, IP-10, Th1/Th2, PDGF, VEGF, FGF, CRP | [29,133,142,148,160,303] |
| Cytokine storm | IL-1β, IL-6, IL-7, IL-8, IL-9, IL-12, IL-17, IL-18, IL-33, TNF-α, IFN-γ, CCL2, CCL3, FGF, G-CSF, GM-CSF, IP-10, PDGF, VEGF, CRP | [29,142,155,160,304] |
| Ageing | IL-1, IL-1RN, IL-2, IL-6, IL-8, IL-12, IL-13, IL-18, CRP, IFN-γ, TGF-β, TNF-α, SAA | [305,306] |
| Obesity/diabetes | M2→M1, Th2→Th1, Treg→Th17, B cells, IL-1β, IL-6, IL-7, IL-22, IFN-γ, TNF-β, CCL2, TNF-α | [301,305] |
| Cardiovascular diseases | NF-κB, IL-1β, IL-2, IL-4, IL-6, IL-17, GM-CSF, MMP-2, MMP-9, CCL2, ERK1/2, P38 MAPK, TNF-α, IFN-γ, HIF-1α, TLR2, TLR4 | [302,309] |
| Chronic respiratory diseases | IL-1β, IL-6, IL-7, IL-8, IL-9, IL-12, IL-17, IL-18, IL-33, TNF-α, IFN-γ, CCL2, CCL3, FGF, G-CSF, GM-CSF, IP-10, PDGF, VEGF | [29,142,155,160,304] |
| Cancer | NF-κB, p53, COX-2/PGE2, TNFα, IL-1β, IL-6, IL-8, p27, PPARα,γ, GSK-3, EGFR, HER2, VEGF, Cyclin D1, c-Myc, PTEN, MDM2, HIPK2, A20, p21, TGF-β, PARP, caspases-3,7,8,9, Bcl-2, Bcl-xL, Bax, cytochrome c, ROS, iNOS, MMP9, HIF-1α, TLR4, | [1] |
7.1. Ageing
As the human body ages, there is a gradual decline in functioning of the innate and adaptive immune systems, designated immunosenescence, as well as an increase in the levels of pro-inflammatory cytokines IL-1β, IL-2, IL-6, IL-8, TNF-α and IFN-γ, as well as CRP [148,305,310]. There is also a decline in ACE2 expression, similarly to COVID-19 [148]. Ageing also produces excess ROS production which can initiate pro-inflammatory generation through activation of transcription factors such as NF-κB [148]. T-cell function has been found to become increasingly defective in the elderly, decreasing immune function [20].
Maintenance of healthy functioning of cells is of increasing importance as ageing progresses. Working against this, deficiencies in one or more of zinc, ω-3 PUFAs, vitamin D and magnesium will lead inevitably to a diminution in immune function and an increase in levels of inflammatory mediators [21,27,311]. Deficiencies of zinc, ω-3 PUFAs, vitamin D and magnesium increase with ageing, frequently contributing to age-related diseases such as diabetes, cardiovascular diseases and chronic pulmonary diseases [27].
Diet is very important for adequate intake of zinc, ω-3 PUFAs and magnesium as the importance and interest in quality of food diminishes with old-age, as well as the degree of absorption [312]. In addition, the exposure of the aged to sunlight becomes severely limited, leading to diminished vitamin D levels.
7.2. Obesity/diabetes
Obesity is related to the accumulation of pro-inflammatory cells in visceral adipose tissue, which can lead to insulin resistance and to diabetes mellitus [151]. Obesity is associated with low-grade inflammation, which in turn is associated with diminution of the innate and adaptive immune responses. Low-grade inflammation is linked to adipocyte hypoxia and dysfunction [307]. There is a significant release of pro-inflammatory cytokines (eg, IL-1β, IL-6, TNF-α) that activate in turn macrophages, T cells and B cells, creating an auto-regenerating loop [307,313]. Obesity is also associated with increased oxidative stress [314].
Zinc deficiency has been shown in a number of studies to be associated with obesity and diabetes [[315], [316], [317]]. Zinc is essential for the normal physiological processing of insulin and is therefore directly associated with diabetes [318].
The ω-6/ω-3 PUFA ratio has increase dramatically over the past 50 years and it has contributed to the increased proportion of the population who are obese [319]. It has been shown that this trend can be reversed by increasing the EPA and DHA intakes [319]. It has been recommended that fish oil emulsion supplement be administered to those who are obese and at risk of contracting COVID-19, due to the immune modulatory properties of EPA and DHA [320].
Obesity increases the risk of vitamin D deficiency, mainly due to the higher adiposity of the obese individual. Vitamin D is fat-soluble and is predominantly stored in the adipose tissues, leading to low levels of vitamin D in the circulation [321]. Low vitamin D levels have been reported consistently across age groups, ethnicity and geography [322,323]. Meta-analyses found that vitamin D deficiency correlated with increased obesity [321,324]. Vitamin D supplementation has been shown to reduce insulin resistance [325] and diabetes mellitus has been found to correlate with vitamin D deficiency in older adults [326].
There is a positive relationship between magnesium deficiency and obesity and chronic inflammation [327]. In turn, obesity is a major risk factor for chronic diseases which depend on chronic inflammation, such as diabetes, cardiovascular diseases and cancer [327].
7.3. Cardiovascular diseases
A large proportion of COVID-19 patients have risk factors associated with cardiovascular diseases [328]. The high levels of inflammation associated with COVID-19 can induce cardiovascular diseases [80,328]. Studies of COVID-19 individuals with underlying cardiovascular disease were at increased risk of severe disease and mortality [329].
Choi and co-workers [330] reviewed the literature covering zinc status and cardiovascular diseases. They found that zinc deficiency was associated with atherosclerosis, hypertension, myocardial infarction, atrial fibrillation and congestive heart failure. Similarly, Jurowski and co-workers [331] had reviewed the literature, reporting that zinc deficiency correlated with hypertension, atherosclerosis and heart failure. Further reports support the fact that zinc deficiency is associated with cardiovascular diseases [22,23].
The cardioprotective effects of n-3 PUFAs and their metabolites are ascribed mainly to their immunomodulatory properties. Emerging evidence demonstrates the ability of ω-3 PUFAs to reduce circulating levels of inflammatory chemokines, cytokines, and the pro-inflammatory metabolites derived from ω-6 PUFAs [332,333]. A number of studies have found that higher consumption of ω-3 PUFAs lowers the number of deaths related to cardiovascular disease [[334], [335], [336], [337]]. Darwesh and co-workers [338] presented a detailed report on the positive effects of ω-3 PUFAs in cardiovascular diseases, which included stabilization of atherosclerotic plaques, reducing the incidence of thrombus formation, enriching cellular membranes and altering the structure of lipid rafts and their function to benefit the treatment of cardiovascular diseases.
There is a strong correlation between obesity and vitamin D deficiency, as well as between obesity and cardiovascular disease. It would therefore be anticipated that there would be a benefit in supplemental vitamin D for obese patients at risk of cardiovascular disease [339]. A study of 137 elderly Brazilian patients found that 65% were vitamin D deficient and there was a strong association between vitamin D deficiency and the risk of heart failure [340]. A number of literature reviews have examined the association between vitamin D deficiency and the incidence of cardiovascular diseases, with the conclusion that vitamin D decreases inflammation and pro-inflammatory cytokines causing a strong association with cardiovascular diseases [308,341,342].
The anti-inflammatory and anti-oxidative effects of magnesium provide cardiovascular protection [119,120]. Qu and co-workers [127] provided a meta-analysis that showed an inverse correlation between magnesium serum concentrations and the risk of total cardiovascular events.
7.4. Lung diseases
Lung diseases include pneumonia, bronchitis and asthma. The most common lung disease associated with COVID-19 is acute respiratory distress syndrome (ARDS), promoted most often by the cytokine storm, and which is often lethal [51]. ARDS occurs in approximately 10% of COVID-19 patients [51].
Meydani and co-workers [20] found that nursing home elderly who were zinc deficient were more likely to contract pneumonia with its subsequent consequences. Further reports support the fact that zinc deficiency is associated with chronic pulmonary diseases [21,23]. Skalny and co-workers [12] deduced that zinc has the propensity to alleviate COVID-19 through its properties of reducing inflammation, improving mucociliary clearance and promoting antiviral and antibacterial immunity.
Weill and co-workers [51] discussed the properties of ω-3 PUFAs, which include interference of viral entry and replication and inhibition of inflammation, leading to improvement of the outcome of critically ill patients with ARDS. It was shown in a study where bronchoalveolar lavage fluid was added to A549 cells that by increasing the ratio of ω-3:ω-6 PUFAs, there was a decrease in levels of NF-κB, COX-2 and PGE2, and an increase in release of IL-10 and PPARγ [343].
It has been noted that there is a strong link between seasonality of low vitamin D levels and occurrence and prevalence of influenza in winter [80]. It has also been reported that a high percentage (>80%) of chronic obstructive pulmonary disease patients had low vitamin D levels [344]. The association between higher vitamin D levels and improved lung function has also been reported [[345], [346], [347]]. Moreover, it has been reported that vitamin D deficiency is associated with occurrence of respiratory disease and ensuing mortality [90,[347], [348], [349]].
The role of magnesium in lung function was discussed by de Baaij and co-workers [124], where magnesium was described as having three roles: a strong vasodilator and bronchodilator effect, regulation of the release of acetylcholine and histamine, and as an anti-inflammatory agent. Magnesium was therefore suggested as a useful treatment for asthma and chronic obstructive pulmonary disorder. Micke and co-workers [114] also discussed magnesium and lung function in some detail, with a similar analysis of the anticholinergic, antihistaminic and anti-inflammatory effects of magnesium.
7.5. Cancer
Cancer has been discussed in the context of essentiality of sufficiency of zinc, ω-3 PUFAs and vitamin D [1]. The opportunity of including magnesium as a further essential component in prevention and treatment of cancers is taken here, as magnesium is essential for the activation of vitamin D [122,123]. Magnesium, as discussed above, is also active in regulating the immune system and controlling untoward oxidative stress and inflammation [119,120] which are prevalent in the early development of cancers [350].
8. Discussion
COVID-19 and its virus SARS-CoV-2 have provided an ideal opportunity to reset the approach to prevention and treatment of non-communicable diseases, particularly those that occur predominantly in the aged. COVID-19 has been shown to be linked to comorbidities such as senescence occurring in the aged, obesity/diabetes which are more severe in the aged, and cardiovascular diseases and chronic pulmonary diseases which are more prevalent in the aged, as well as cancers. It is therefore opportune to examine carefully the prevention and treatment of COVID-19 and those diseases, with particular attention to those features and characteristics that are common to these diseases. The most outstanding common features are inflammation and overactivity of the innate and adaptive immune systems. Control of inflammation and the immune system are fundamentally dependent on sufficiency of the essential nutrients zinc, ω-3 PUFAs, vitamin D and magnesium.
This paper has been directed towards an appreciation of the benefits of having sufficiency of zinc, ω-3 PUFAs, vitamin D and magnesium. These four components are essential as they are natural to the normal functioning of cells and multiple other components of the human body. They are extremely safe when supplemented in a controlled manner. Control in the aged (eg, 65 years and older) can be maintained by annual analyses of their serum levels. This can be achieved with government support, as well as by government supplies of supplements where necessary. The cost of this service to those over 65 would be small compared with the potential savings in hospitalization and critical care costs. As an example, a German estimate of the effect of supplementing vitamin D alone on the cost savings of cancer alone in Germany showed a cost saving of approximately €254 million per year with a prevention of almost 30,000 deaths to cancer per year [351].
Zinc, ω-3 PUFAs, vitamin D and magnesium are pleiotropic as they allow, and in fact boost, the functioning of granulocytes such as mast cells, neutrophils and eosinophils, as well as monocytes/macrophages, dendritic cells, T cells and B cells in normal conditions and when there are minor invasions of pathogens such as minor viral and bacterial infections. In contrast, zinc, ω-3 PUFAs, vitamin D and magnesium all act to suppress hyperinflammation and major disruptions of the immune system that occur when there is a significant invasion by viral or bacterial pathogens such as SARS-CoV-2 or non-communicable diseases such as diabetes, cardiovascular disease or chronic pulmonary disease. In these situations, zinc, ω-3 PUFAs, vitamin D and magnesium have the ability to suppress excessive inflammation and dysregulation of the immune system. These nutrients are therefore essential in all aspects; when present in sufficiency they are directed towards ensuring good health for humans at all times and for all ages. This is not normally the case with non-natural drugs that are prescribed for treatment of particular pathological conditions.
Vaccines are rarely 100% in their prevention of transmission and their prevention of humans contracting the particular disease; there are potential problems with mutations and diminution of their effectiveness. It is of note that vaccines perform their function through the adaptive immune system, whilst zinc, ω-3 PUFAs, vitamin D and magnesium affect both the innate and adaptive immune systems. Supplementation of the four nutrients in treatment of COVID-19 is therefore desirable, especially if this supplementation is beneficial in preventing or treating non-communicable diseases or reducing the adverse effects of ageing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Story M.J. Zinc, ω-3 polyunsaturated fatty acids and vitamin D: an essential combination for prevention and treatment of cancers. Biochimie. 2021;181:100–1222. doi: 10.1016/j.biochi.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Global Status Report on Non-communicable Diseases,”. WHO; 2010. https://www.who.int/nmh/publications/ncd_report_full_en.pdf [Google Scholar]
- 3.Pecora F., Persico F., Argentiero A., Neglia C., Esposito S. The role of micronutrients in support of the immune response against viral infections. Nutrients. 2020;12(10) doi: 10.3390/nu12103198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabó Z., Marosvölgyi T., É Szabó, Bai P., Figler M., Verzár Z. The potential beneficial effect of EPA and DHA supplementation managing cytokine storm in coronavirus disease. Front. Physiol. 2020;11:752. doi: 10.3389/fphys.2020.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iddir M., Brito A., Dingeo G., Fernandez Del Campo S.S., Samouda H., La Frano M.R., et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mammadova-Bach E., Braun A. Zinc homeostasis in platelet-related diseases. Int. J. Mol. Sci. 2019;20(21):5258. doi: 10.3390/ijms20215258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad A.S. Zinc in human health: effect of zinc on immune cells. Mol. Med. 2008;14(5–6):353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao B., Prasad A.S., Beck F.W., Fitzgerald J.T., Snell D., Bao G.W., et al. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010;91(6):1634–1641. doi: 10.3945/ajcn.2009.28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad A.S., Beck F.W., Bao B., Fitzgerald J.T., Snell D.C., Steinberg J.D., et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007;85(3):837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 11.Gao H., Dai W., Zhao L., Min J., Wang F. The role of zinc and zinc homeostasis in macrophage function. J Immunol Res. 2018 doi: 10.1155/2018/6872621. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., et al. Zinc and respiratory tract infections: perspectives for COVID-19 (Review) Int. J. Mol. Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloubert V., Rink L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015;6(10):3195–3204. doi: 10.1039/c5fo00630a. [DOI] [PubMed] [Google Scholar]
- 14.Prasad A.S. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4(2):176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998;68(2 Suppl):447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 16.Haase H., Rink L. The immune system and the impact of zinc during aging. Immun. Ageing. 2009;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayor-Ibarguren A., Busca-Arenzana C., Robles-Marhuenda Á A hypothesis for the possible role of zinc in the immunological pathways related to COVID-19 infection. Front. Immunol. 2020;11:1736. doi: 10.3389/fimmu.2020.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haase H., Rink L. Zinc signals and immune function. Biofactors. 2014;40(1):27–40. doi: 10.1002/biof.1114. [DOI] [PubMed] [Google Scholar]
- 19.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meydani S.N., Barnett J.B., Dallal G.E., Fine B.C., Jacques P.F., Leka L.S., et al. Serum zinc and pneumonia in nursing home elderly. Am. J. Clin. Nutr. 2007;86(4):1167–1173. doi: 10.1093/ajcn/86.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnett J.B., Hamer D.H., Meydani S.N. Low zinc status: a new risk factor for pneumonia in the elderly? Nutr. Rev. 2010;68(1):30–37. doi: 10.1111/j.1753-4887.2009.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun L.A., Ou R., Kure C., Trang A., Rosenfeldt F. Prevalence of zinc deficiency in cardiac surgery patients. Heart Lung Circ. 2018;27(6):760–762. doi: 10.1016/j.hlc.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Derwand R., Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19? Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George M.M., Subramanian Vignesh K., Landero Figueroa J.A., Caruso J.A., Deepe G.S., Jr. Zinc induces dendritic cell tolerogenic phenotype and skews regulatory T cell-Th17 balance. J. Immunol. 2016;197(5):1864–1876. doi: 10.4049/jimmunol.1600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasad A.S., Bao B., Beck F.W., Sarkar F.H. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27(7–8):816–823. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 26.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arentz S., Yang G., Goldenberg J., Beardsley J., Myers S.P., Mertz D., et al. Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: a rapid review. Adv Integr Med. 2020;7(4):252–260. doi: 10.1016/j.aimed.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12(5):1466. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefanidou M., Maravelias C., Dona A., Spiliopoulou C. Zinc: a multipurpose trace element. Arch. Toxicol. 2006;80(1):1–9. doi: 10.1007/s00204-005-0009-5. [DOI] [PubMed] [Google Scholar]
- 31.te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajaiah R., Abhilasha K.V., Shekar M.A., Vogel S.N., Vishwanath B.S. Evaluation of mechanisms of action of re-purposed drugs for treatment of COVID-19. Cell. Immunol. 2020;358 doi: 10.1016/j.cellimm.2020.104240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doboszewska U., Wlaź P., Nowak G., Młyniec K. Targeting zinc metalloenzymes in coronavirus disease 2019. Br. J. Pharmacol. 2020;177(21):4887–4898. doi: 10.1111/bph.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabbagh-Bazarbachi H., Clergeaud G., Quesada I.M., Ortiz M., O'Sullivan C.K., Fernández-Larrea J.B. Zinc ionophore activity of quercetin and epigallocatechin-gallate: from Hepa 1-6 cells to a liposome model. J. Agric. Food Chem. 2014;62(32):8085–8093. doi: 10.1021/jf5014633. [DOI] [PubMed] [Google Scholar]
- 35.Brooks H.A., Gardner D., Poyser J.P., King T.J. The structure and absolute stereochemistry of zincophorin (antibiotic M144255): a monobasic carboxylic acid ionophore having a remarkable specificity for divalent cations. J. Antibiot. (Tokyo) 1984;37(11):1501–1504. doi: 10.7164/antibiotics.37.1501. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393(7):1153–1156. doi: 10.1007/s00210-020-01902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov. https://clinicaltrials.gov/ct2/home
- 38.Pal A., Squitti R., Picozza M., Pawar A., Rongioletti M., Dutta A.K., et al. Zinc and COVID-19: basis of current clinical trials. Biol Trace Elem Res Ahead of print. 2021 doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar A., Kubota Y., Chernov M., Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roscioli E., Jersmann H.P., Lester S., Badiei A., Fon A., Zalewski P., et al. Zinc deficiency as a codeterminant for airway epithelial barrier dysfunction in an ex vivo model of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:3503–3510. doi: 10.2147/COPD.S149589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wessels I., Pupke J.T., von Trotha K.T., Gombert A., Himmelsbach A., Fischer H.J., et al. Zinc supplementation ameliorates lung injury by reducing neutrophil recruitment and activity. Thorax. 2020;75(3):253–261. doi: 10.1136/thoraxjnl-2019-213357. [DOI] [PubMed] [Google Scholar]
- 43.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int. J. Infect. Dis. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlucci P.M., Ahuja T., Petrilli C., Rajagopalan H., Jones S., Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020;69(10):1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P., et al. COVID-19: poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razzaque M.S. COVID-19 pandemic: can maintaining optimal zinc balance enhance host resistance? Tohoku J. Exp. Med. 2020;251(3):175–181. doi: 10.1620/tjem.251.175. [DOI] [PubMed] [Google Scholar]
- 47.Simopoulos A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56(8):365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 48.Simopoulos A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 49.Blondeau N., Lipsky R.H., Bourourou M., Duncan M.W., Gorelick P.B., Marini A.M. Alpha-linolenic acid: an omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? BioMed Res. Int. 2015 doi: 10.1155/2015/519830. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Das U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Arch. Med. Res. 2020;51(3):282–286. doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weill P., Plissonneau C., Legrand P., Rioux V., Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275–280. doi: 10.1016/j.biochi.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schiessel D.L., Yamazaki R.K., Kryczyk M., Coelho I., Yamaguchi A.A., Pequito D.C., et al. α-Linolenic fatty acid supplementation decreases tumor growth and cachexia parameters in Walker 256 tumor-bearing rats. Nutr. Canc. 2015;67(5):839–846. doi: 10.1080/01635581.2015.1043021. [DOI] [PubMed] [Google Scholar]
- 53.Gutiérrez S., Svahn S.L., Johansson M.E. Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 2019;20(20):5028. doi: 10.3390/ijms20205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rius B., López-Vicario C., González-Périz A., Morán-Salvador E., García-Alonso V., Clária J., et al. Resolution of inflammation in obesity-induced liver disease. Front. Immunol. 2012;3:257. doi: 10.3389/fimmu.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y., Liu D.X., Tam J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008;369(2):344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li G.M., Li Y.G., Yamate M., Li S.M., Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microb. Infect. 2007;9(1):96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goc A., Niedzwiecki A., Rath M. Research Square Ahead of print; 2021. Polyunsaturated ω-3 Fatty Acids Inhibit ACE2-Controlled SARS-CoV-2 Binding and Cellular Entry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fritsche K. Fatty acids as modulators of the immune response. Annu. Rev. Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- 60.Vedin I., Cederholm T., Freund Levi Y., Basun H., Garlind A., et al. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am. J. Clin. Nutr. 2008;87(6):1616–1622. doi: 10.1093/ajcn/87.6.1616. [DOI] [PubMed] [Google Scholar]
- 61.Kiecolt-Glaser J.K., Belury M.A., Andridge R., Malarkey W.B., Hwang B.S., Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav. Immun. 2012;26(6):988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiang K.C., Chen T.C. Vitamin D for the prevention and treatment of pancreatic cancer. World J. Gastroenterol. 2009;15(27):3349–3354. doi: 10.3748/wjg.15.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barragan M., Good M., Kolls J.K. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7(9):8127–8151. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sassi F., Tamone C., D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11):1656. doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y., Baylink D.J., Chen C.S., Reeves M.E., Xiao J., Lacy C., et al. The importance of vitamin D metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020;18(1):322. doi: 10.1186/s12967-020-02488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohadian Moghadam S. A review on currently available potential therapeutic options for COVID-19. Int. J. Gen. Med. 2020;13:443–467. doi: 10.2147/IJGM.S263666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munshi R., Hussein M.H., Toraih E.A., Elshazli R.M., Jardak C., Sultana N., et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol Ahead of print. 2020 doi: 10.1002/jmv.26360. [DOI] [PubMed] [Google Scholar]
- 68.Chaabouni M., Feki W., Chaabouni K., Kammoun S. Vitamin D supplementation to prevent COVID-19 in patients with COPD: a research perspective. Adv Respir Med. 2020;88(4):364–365. doi: 10.5603/ARM.a2020.0101. [DOI] [PubMed] [Google Scholar]
- 69.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhodes J.M., Subramanian S., Laird E., Kenny R.A. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020;51(12):1434–1437. doi: 10.1111/apt.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mandal A.K.J., Baktash V., Hosack T., Missouris C.G. Vitamin D status and COVID-19 in older adults. Aging Clin. Exp. Res. 2020;32(11):2425–2426. doi: 10.1007/s40520-020-01716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dixon K.M., Tongkao-On W., Sequeira V.B., Carter S.E., Song E.J., Rybchyn M.S., Gordon-Thomson C., Mason R.S. Vitamin D and death by sunshine. Int. J. Mol. Sci. 2013;14(1):1964–1977. doi: 10.3390/ijms14011964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garland C.F., Garland F.C., Gorham E.D. The role of vitamin D in cancer prevention. Am. J. Publ. Health. 2006;96(2):252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Macdonald H.M. Contributions of sunlight and diet to vitamin D status. Calcif. Tissue Int. 2013;92(2):163–176. doi: 10.1007/s00223-012-9634-1. [DOI] [PubMed] [Google Scholar]
- 75.Dobnig H. A review of the health consequences of the vitamin D deficiency pandemic. J. Neurol. Sci. 2011;311(1–2):15–18. doi: 10.1016/j.jns.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 76.Hoel D.G., Berwick M., de Gruijl F.R., Holick M.F. The risks and benefits of sun exposure 2016. Dermatoendocrinol. 2016;8(1) doi: 10.1080/19381980.2016.1248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Rhee H., de Vries E., Coomans C., van de Velde P., Coebergh J.W. For better or for worse? A review of positive and negative effects of sun exposure. Canc. Res Front. 2016;2(2):156–183. [Google Scholar]
- 78.Green R.J., Samy G., Miqdady M.S., El-Hodhod M., Akinyinka O.O., Saleh G., et al. Vitamin D deficiency and insufficiency in Africa and the Middle East, despite year-round sunny days. S. Afr. Med. J. 2015;105(7):603–605. doi: 10.7196/samjnew.7785. [DOI] [PubMed] [Google Scholar]
- 79.Li H., Zhong X., Li W., Wang Q. Effects of 1,25-dihydroxyvitamin D3 on experimental periodontitis and AhR/NF-κB/NLRP3 inflammasome pathway in a mouse model. J. Appl. Oral Sci. 2019;27 doi: 10.1590/1678-7757-2018-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bilezikian J.P., Bikle D., Hewison M., Lazaretti-Castro M., Formenti A.M., Gupta A., et al. Mechanisms IN endocrinology: vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183(5):R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simonson W. Vitamin D and coronavirus. Geriatr. Nurs. 2020;41(4):496–497. doi: 10.1016/j.gerinurse.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Ani M., Elemam N.M., Hundt J.E., Maghazachi A.A. Drugs for multiple sclerosis activate natural kKiller cells: do they protect against COVID-19 infection? Infect. Drug Resist. 2020;13:3243–3254. doi: 10.2147/IDR.S269797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adams K.K., Baker W.L., Sobieraj D.M. Myth busters: dietary supplements and COVID-19. Ann. Pharmacother. 2020;54(8):820–826. doi: 10.1177/1060028020928052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13(10):1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kara M., Ekiz T., Ricci V., Kara Ö., Chang K.V., Özçakar L. Scientific Strabismus' or two related pandemics: coronavirus disease and vitamin D deficiency. Br. J. Nutr. 2020;124(7):736–741. doi: 10.1017/S0007114520001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Allegra A., Tonacci A., Pioggia G., Musolino C., Gangemi S. Vitamin deficiency as risk factor for SARS-CoV-2 infection: correlation with susceptibility and prognosis. Eur. Rev. Med. Pharmacol. Sci. 2020;24(18):9721–9738. doi: 10.26355/eurrev_202009_23064. [DOI] [PubMed] [Google Scholar]
- 87.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020;32(7):1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Annweiler C., Cao Z., Sabatier J.M. Point of view: should COVID-19 patients be supplemented with vitamin D? Maruritas. 2020;140:24–26. doi: 10.1016/j.maturitas.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baktash V., Hosack T., Patel N., Shah S., Kandiah P., Van Den Abbeele K., et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J Ahead of print. 2021 doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carpagnano G.E., Di Lecce V., Quaranta V.N., Zito A., Buonamico E., Capozza E., et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Invest. 2020;9:1–7. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hadizadeh F. Supplementation with vitamin D in the COVID-19 pandemic? Nutr. Rev. 2021;79(2):200–208. doi: 10.1093/nutrit/nuaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaufman H.W., Niles J.K., Kroll M.H., Bi C., Holick M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PloS One. 2020;15(9) doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maghbooli Z., Sahraian M.A., Ebrahimi M., Pazoki M., Kafan S., Tabriz H.M., et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PloS One. 2020;15(9) doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moozhipurath R.K., Kraft L., Skiera B. Evidence of protective role of Ultraviolet-B (UVB) radiation in reducing COVID-19 deaths. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-74825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weir E.K., Thenappan T., Bhargava M., Chen Y. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. 2020;20(4):e107–e108. doi: 10.7861/clinmed.2020-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zemb P., Bergman P., Camargo C.A., Jr., Cavalier E., Cormier C., Courbebaisse M., et al. Vitamin D deficiency and the COVID-19 pandemic. J Glob Antimicrob Resist. 2020;22:133–134. doi: 10.1016/j.jgar.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcalá Díaz J.F., López Miranda J., Bouillon R., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020;203 doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tarazona-Santabalbina F.J., Cuadra L., Cancio J.M., Carbonell F.R., Garrote J.M.P., Casas-Herrero Á., Martínez-Velilla N., Serra-Rexach J.A., Formiga F. Rev Esp Geriatr Gerontol Ahead of print; 2021. VitaminD Supplementation for the Prevention and Treatment of COVID-19: a Position Statement from the Spanish Society of Geriatrics and Gerontology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lau F.H., Majumder R., Torabi R., Saeg F., Hoffman R., Cirillo J.D., et al. MedRxiv preprint Ahead of print; 2021. Vitamin D Insufficiency Is Prevalent in Severe COVID-19. [Google Scholar]
- 101.de Haan K., Groeneveld A.B., de Geus H.R., Egal M., Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit. Care. 2014;18(6):660. doi: 10.1186/s13054-014-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Claro da Silva T., Hiller C., Gai Z., Kullak-Ublick G.A. Vitamin D3 transactivates the zinc and manganese transporter SLC30A10 via the Vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2016;163:77–87. doi: 10.1016/j.jsbmb.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 103.McDonnell S.L., Baggerly C.A., French C.B., Baggerly L.L., Garland C.F., Gorham E.D., et al. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs <20 ng/ml (150 vs 50 nmol/L): pooled analysis of two randomized trials and a prospective cohort. PloS One. 2018;13(6) doi: 10.1371/journal.pone.0199265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McCullough M.L., Zoltick E.S., Weinstein S.J., Fedirko V., Wang M., Cook N.R., et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J. Natl. Cancer Inst. 2019;111(2):158–169. doi: 10.1093/jnci/djy087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chandler P.D., Chen W.Y., Ajala O.N., Hazra A., Cook N., Bubes V., et al. Effect of vitamin D3 supplements on development of advanced cancer: a secondary analysis of the VITAL randomized clinical trial. JAMA Netw Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.25850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moukayed M., Grant W.B. The roles of UVB and vitamin D in reducing risk of cancer incidence and mortality: a review of the epidemiology, clinical trials, and mechanisms. Rev. Endocr. Metab. Disord. 2017;18(2):167–182. doi: 10.1007/s11154-017-9415-2. [DOI] [PubMed] [Google Scholar]
- 107.Ma Y., Johnson C.S., Trump D.L. Mechanistic insights of vitamin D anticancer effects. Vitam. Horm. 2016;100:395–431. doi: 10.1016/bs.vh.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 108.Abu El Maaty M.A., Wölfl S. Effects of 1,25(OH)₂D₃ on cancer cells and potential applications in combination with established and putative anti-cancer agents. Nutrients. 2017;9(1):87. doi: 10.3390/nu9010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.D'Avolio A., Avataneo V., Manca A., Cusato J., De Nicolò A., Lucchini R., et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Charoenngam N., Holick M.F. Immunologic Effects of vitamin D on human health and disease. Nutrients. 2020;12(7) doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kumar R., Rathi H., Haq A., Wimalawansa S.J., Sharma A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 2021;292 doi: 10.1016/j.virusres.2020.198235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dominguez L.J., Veronese N., Guerrero-Romero F., Barbagallo M. Magnesium in infectious diseases in older people. Nutrients. 2021;13(1):180. doi: 10.3390/nu13010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iotti S., Wolf F., Mazur A., Maier J.A. The COVID-19 pandemic: is there a role for magnesium? Hypotheses and perspectives. Magnes. Res. 2020;33(2):21–27. doi: 10.1684/mrh.2020.0465. [DOI] [PubMed] [Google Scholar]
- 114.Micke O., Vormann J., Kisters K. Magnesium and COVID-19 - some further comments - a commentary on Wallace TC. Combating COVID-19 and building immune resilience: a potential role for magnesium nutrition? J Am Coll Nutr Ahead of print. 2021 doi: 10.1080/07315724.2020.1816230. [DOI] [PubMed] [Google Scholar]
- 115.Alamdari N.M., Afaghi S., Rahimi F.S., Tarki F.E., Tavana S., Zali A., et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J. Exp. Med. 2020;252(1):73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 116.Quilliot D., Bonsack O., Jaussaud R., Mazur A. Dysmagnesemia in Covid-19 cohort patients: prevalence and associated factors. Magnes. Res. 2020;33(4):114–122. doi: 10.1684/mrh.2021.0476. [DOI] [PubMed] [Google Scholar]
- 117.Zeng H.L., Yang Q., Yuan P., Wang X., Cheng L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID-19. Faseb. J. 2021;35(3) doi: 10.1096/fj.202002346RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Costello R.B., Nielsen F. Interpreting magnesium status to enhance clinical care: key indicators. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20(6):504–511. doi: 10.1097/MCO.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nielsen F.H. Magnesium deficiency and increased inflammation: current perspectives. J. Inflamm. Res. 2018;11:25–34. doi: 10.2147/JIR.S136742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang C.F., Ding H., Jiao R.Q., Wu X.X., Kong L.D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wallace T.C. Combating COVID-19 and building immune resilience: a potential role for magnesium nutrition? J. Am. Coll. Nutr. 2020;39(8):685–693. doi: 10.1080/07315724.2020.1785971. [DOI] [PubMed] [Google Scholar]
- 122.DiNicolantonio J.J., O'Keefe J.H. Magnesium and vitamin D deficiency as a potential cause of immune dysfunction, cytokine storm and disseminated intravascular coagulation in covid-19 patients. Mo. Med. 2021;118(1):68–73. [PMC free article] [PubMed] [Google Scholar]
- 123.Dai Q., Zhu X., Manson J.E., Song Y., Li X., Franke A.A., et al. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am. J. Clin. Nutr. 2018;108(6):1249–1258. doi: 10.1093/ajcn/nqy274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Baaij J.H., Hoenderop J.G., Bindels R.J. Magnesium in man: implications for health and disease. Physiol. Rev. 2015;95(1):1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 125.Maier J.A., Castiglioni S., Locatelli L., Zocchi M., Mazur A. Magnesium and inflammation: advances and perspectives. Semin Cell Dev Biol Ahead of print. 2020 doi: 10.1016/j.semcdb.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 126.Chacko S.A., Song Y., Nathan L., Tinker L., de Boer I.H., Tylavsky F., et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care. 2010;33(2):304–310. doi: 10.2337/dc09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qu X., Jin F., Hao Y., Li H., Tang T., Wang H., et al. Magnesium and the risk of cardiovascular events: a meta-analysis of prospective cohort studies. PloS One. 2013;8(3) doi: 10.1371/journal.pone.0057720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zipeto D., Palmeira J.D.F., Argañaraz G.A., Argañaraz E.R. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martín Giménez V.M., Inserra F., Tajer C.D., Mariani J., Ferder L., Reiter R.J., et al. Lungs as target of COVID-19 infection: protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hathaway D., Pandav K., Patel M., Riva-Moscoso A., Singh B.M., Patel A., et al. Omega 3 fatty acids and COVID-19: a comprehensive review. Infect Chemother. 2020;52(4):478–495. doi: 10.3947/ic.2020.52.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aygun H. Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393(7):1157–1160. doi: 10.1007/s00210-020-01911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kumar D., Gupta P., Banerjee D. Letter: does vitamin D have a potential role against COVID-19? Aliment. Pharmacol. Ther. 2020;52(2):409–411. doi: 10.1111/apt.15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu J., Yang J., Chen J., Luo Q., Zhang Q., Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017;16(5):7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xiao D., Li X., Su X., Mu D., Qu Y. Could SARS-CoV-2-induced lung injury be attenuated by vitamin D? Int. J. Infect. Dis. 2020;102:196–202. doi: 10.1016/j.ijid.2020.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Biggs L., Yu C., Fedoric B., Lopez A.F., Galli S.J., Grimbaldeston M.A. Evidence that vitamin D(3) promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice. J. Exp. Med. 2010;207(3):455–463. doi: 10.1084/jem.20091725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Skrajnowska D., Bobrowska-Korczak B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients. 2019;11(10):2273. doi: 10.3390/nu11102273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mohan M., Cherian J.J., Sharma A. Exploring links between vitamin D deficiency and COVID-19. PLoS Pathog. 2020;16(9) doi: 10.1371/journal.ppat.1008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stone K.D., Prussin C., Metcalfe D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010;125(2 Suppl 2):S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kempuraj D., Selvakumar G.P., Ahmed M.E., Raikwar S.P., Thangavel R., Khan A., et al. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist. 2020;26(5–6):402–414. doi: 10.1177/1073858420941476. [DOI] [PubMed] [Google Scholar]
- 143.Graham A.C., Temple R.M., Obar J.J. Mast cells and influenza A virus: association with allergic responses and beyond. Front. Immunol. 2015;6:238. doi: 10.3389/fimmu.2015.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang X., Kulka M. n-3 Polyunsaturated fatty acids and mast cell activation. J. Leukoc. Biol. 2015;97(5):859–871. doi: 10.1189/jlb.2RU0814-388R. [DOI] [PubMed] [Google Scholar]
- 145.Mukai K., Tsai M., Saito H., Galli S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018;282(1):121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kawakami T., Ando T., Kimura M., Wilson B.S., Kawakami Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009;21(6):666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baeke F., Takiishi T., Korf H., Gysemans C., Mathieu C. Vitamin D: modulator of the immune system. Curr. Opin. Pharmacol. 2010;10(4):482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 148.Meftahi G.H., Jangravi Z., Sahraei H., Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of "inflame-aging. Inflamm. Res. 2020;69(9):825–839. doi: 10.1007/s00011-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jafarzadeh A., Chauhan P., Saha B., Jafarzadeh S., Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harihar1an A., Hakeem A.R., Radhakrishnan S., Reddy M.S., Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology. 2020;7:1–10. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wensveen F.M., Valentić S., Šestan M., Turk Wensveen T., Polić B. The "Big Bang" in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur. J. Immunol. 2015;45(9):2446–2456. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 152.Blanchard C., Rothenberg M.E. Biology of the eosinophil. Adv. Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hogan S.P., Rosenberg H.F., Moqbel R., Phipps S., Foster P.S., Lacy P., Kay A.B., Rothenberg M.E. Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy. 2008;38(5):709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 154.Davoine F., Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front. Immunol. 2014;5:570. doi: 10.3389/fimmu.2014.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pacha O., Sallman M.A., Evans S.E. COVID-19: a case for inhibiting IL-17? Nat. Rev. Immunol. 2020;20(6):345–346. doi: 10.1038/s41577-020-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aggarwal N., Korenbaum E., Mahadeva R., Immenschuh S., Grau V., Dinarello C.A., et al. α-Linoleic acid enhances the capacity of α-1 antitrypsin to inhibit lipopolysaccharide-induced IL-1β in human blood neutrophils. Mol. Med. 2016;22:680–693. doi: 10.2119/molmed.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 158.Tecchio C., Micheletti A., Cassatella M.A. Neutrophil-derived cytokines: facts beyond expression. Front. Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lau Y.L., Peiris J.S., Law H.K. Role of dendritic cells in SARS coronavirus infection. Hong Kong Med. J. 2012;18(Suppl 3):28–30. [PubMed] [Google Scholar]
- 160.Shibabaw T. Inflammatory Cytokine: IL-17A Signaling pathway in patients present with COVID-19 and current treatment strategy. J. Inflamm. Res. 2020;13:673–680. doi: 10.2147/JIR.S278335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marone G., Columbo M., de Paulis A., Cirillo R., Giugliano R., Condorelli M. Physiological concentrations of zinc inhibit the release of histamine from human basophils and lung mast cells. Agents Actions. 1986;18(1–2):103–106. doi: 10.1007/BF01987995. [DOI] [PubMed] [Google Scholar]
- 162.Feltis B.N., Elbaz A., Wright P.F., Mackay G.A., Turney T.W., Lopata A.L. Characterizing the inhibitory action of zinc oxide nanoparticles on allergic-type mast cell activation. Mol. Immunol. 2015;66(2):139–146. doi: 10.1016/j.molimm.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 163.Gueck T., Seidel A., Fuhrmann H. Effects of essential fatty acids on mediators of mast cells in culture. Prostaglandins Leukot. Essent. Fatty Acids. 2003;68(5):317–322. doi: 10.1016/s0952-3278(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 164.Park B.K., Park S., Park J.B., Park M.C., Min T.S., Jin M. Omega-3 fatty acids suppress Th2-associated cytokine gene expressions and GATA transcription factors in mast cells. J. Nutr. Biochem. 2013;24(5):868–876. doi: 10.1016/j.jnutbio.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 165.Yip K.H., Kolesnikoff N., Yu C., Hauschild N., Taing H., Biggs L., et al. Mech,anisms of vitamin D₃ metabolite repression of IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014;133(5):1356–1364. doi: 10.1016/j.jaci.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu Z.Q., Li X.X., Qiu S.Q., Yu Y., Li M.G., Yang L.T., et al. Vitamin D contributes to mast cell stabilization. Allergy. 2017;72(8):1184–1192. doi: 10.1111/all.13110. [DOI] [PubMed] [Google Scholar]
- 167.Zhao J.W., Ping J.D., Wang Y.F., Liu X.N., Li N., Hu Z.L., et al. Vitamin D suppress the production of vascular endothelial growth factor in mast cell by inhibiting PI3K/Akt/p38 MAPK/HIF-1α pathway in chronic spontaneous urticaria. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108444. [DOI] [PubMed] [Google Scholar]
- 168.Takemoto S., Yamamoto A., Tomonaga S., Funaba M., Matsui T. Magnesium deficiency induces the emergence of mast cells in the liver of rats. J. Nutr. Sci. Vitaminol. 2013;59(6):560–563. doi: 10.3177/jnsv.59.560. [DOI] [PubMed] [Google Scholar]
- 169.Ohbori K., Fujiwara M., Ohishi A., Nishida K., Uozumi Y., Nagasawa K. Prophylactic oral administration of magnesium ameliorates dextran sulfate sodium-induced colitis in mice through a decrease of colonic accumulation of P2X7 receptor-expressing mast cells. Biol. Pharm. Bull. 2017;40(7):1071–1077. doi: 10.1248/bpb.b17-00143. [DOI] [PubMed] [Google Scholar]
- 170.Haase H., Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20(3–4):579–585. doi: 10.1007/s10534-006-9029-8. [DOI] [PubMed] [Google Scholar]
- 171.Lu H., Xin Y., Tang Y., Shao G. Zinc suppressed the airway inflammation in asthmatic rats: effects of zinc on generation of eotaxin, MCP-1, IL-8, IL-4, and IFN-γ. Biol. Trace Elem. Res. 2012;150(1–3):314–321. doi: 10.1007/s12011-012-9493-7. [DOI] [PubMed] [Google Scholar]
- 172.Cho E., Park Y. Association between serum fatty acid composition and innate immune markers in healthy adults. Nutr Res Pract. 2016;10(2):182–187. doi: 10.4162/nrp.2016.10.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Snodgrass R.G., Huang S., Namgaladze D., Jandali O., Shao T., Sama S., et al. Docosahexaenoic acid and palmitic acid reciprocally modulate monocyte activation in part through endoplasmic reticulum stress. J. Nutr. Biochem. 2016;32:39–45. doi: 10.1016/j.jnutbio.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 174.Sugimoto J., Romani A.M., Valentin-Torres A.M., Luciano A.A., Ramirez Kitchen C.M., Funderburg N., et al. Magnesium decreases inflammatory cytokine production: a novel innate immunomodulatory mechanism. J. Immunol. 2012;188(12):6338–6346. doi: 10.4049/jimmunol.1101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun L., Li X., Xu M., Yang F., Wang W., Niu X. In vitro immunomodulation of magnesium on monocytic cell toward anti-inflammatory macrophages. Regen Biomater. 2020;7(4):391–401. doi: 10.1093/rb/rbaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aude-Garcia C., Dalzon B., Ravanat J.L., Collin-Faure V., Diemer H., Strub J.M., et al. A combined proteomic and targeted analysis unravels new toxic mechanisms for zinc oxide nanoparticles in macrophages. J Proteomics. 2016;134:174–185. doi: 10.1016/j.jprot.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 177.Zhao G., Etherton T.D., Martin K.R., Vanden Heuvel J.P., Gillies P.J., West S.G., et al. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem. Biophys. Res. Commun. 2005;336(3):909–917. doi: 10.1016/j.bbrc.2005.08.204. [DOI] [PubMed] [Google Scholar]
- 178.Rao Z., Zhang N., Xu N., Pan Y., Xiao M., Wu J., et al. 1,25-Dihydroxyvitamin D inhibits LPS-induced high-mobility group box 1 (HMGB1) secretion via targeting the NF-E2-related factor 2-hemeoxygenase-1-HMGB1 pathway in macrophages. Front. Immunol. 2017;8:1308. doi: 10.3389/fimmu.2017.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Helming L., Böse J., Ehrchen J., Schiebe S., Frahm T., Geffers R., et al. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106(13):4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 180.Karkeni E., Morin S.O., Bou Tayeh B., Goubard A., Josselin E., Castellano R., et al. Vitamin D controls tumor growth and CD8+ T cell infiltration in breast cancer. Front. Immunol. 2019;10:1307. doi: 10.3389/fimmu.2019.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Turner D.L., Ford W.R., Kidd E.J., Broadley K.J., Powell C. Effects of nebulised magnesium sulphate on inflammation and function of the Guinea-pig airway. Eur. J. Pharmacol. 2017;801:79–85. doi: 10.1016/j.ejphar.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 182.Hu T., Xu H., Wang C., Qin H., An Z. Magnesium enhances the chondrogenic differentiation of mesenchymal stem cells by inhibiting activated macrophage-induced inflammation. Sci. Rep. 2018;8(1):3406. doi: 10.1038/s41598-018-21783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Finamore A., Massimi M., Conti Devirgiliis L., Mengheri E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J. Nutr. 2008;138(9):1664–1670. doi: 10.1093/jn/138.9.1664. [DOI] [PubMed] [Google Scholar]
- 184.Sakakibara Y., Sato S., Kawashima Y., Someya Y., Shirato K., Tachiyashiki K., et al. Different recovery responses from dietary zinc-deficiency in the distribution of rat granulocytes. J. Nutr. Sci. Vitaminol. 2011;57(2):197–201. doi: 10.3177/jnsv.57.197. [DOI] [PubMed] [Google Scholar]
- 185.Yoshida S., Yasutomo K., Watanabe T. Treatment with DHA/EPA ameliorates atopic dermatitis-like skin disease by blocking LTB4 production. J. Med. Invest. 2016;63(3–4):187–191. doi: 10.2152/jmi.63.187. [DOI] [PubMed] [Google Scholar]
- 186.Chang Y.F., Hou Y.C., Pai M.H., Yeh S.L., Liu J.J. Effects of ω-3 polyunsaturated fatty acids on the homeostasis of CD4+ T cells and lung injury in mice with polymicrobial sepsis. J. Parenter. Enteral Nutr. 2017;41(5):805–814. doi: 10.1177/0148607115597670. [DOI] [PubMed] [Google Scholar]
- 187.Akbas E.M., Gungor A., Ozcicek A., Akbas N., Askin S., Polat M. Vitamin D and inflammation: evaluation with neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Arch. Med. Sci. 2016;12(4):721–727. doi: 10.5114/aoms.2015.50625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tang Y., Liu J., Yan Y., Fang H., Guo C., Xie R., et al. 1,25-dihydroxyvitamin-D3 promotes neutrophil apoptosis in periodontitis with type 2 diabetes mellitus patients via the p38/MAPK pathway. Medicine (Baltim.) 2018;97(52) doi: 10.1097/MD.0000000000013903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li W., Wu X., Yu J., Ma C., Zhuang P., Zeng J., Zhang J., Deng G., Wang Y. Magnesium sulfate attenuates lipopolysaccharides-induced acute lung injury in mice. Chin. J. Physiol. 2019;62(5):203–209. doi: 10.4103/CJP.CJP_48_19. [DOI] [PubMed] [Google Scholar]
- 190.Kitamura H., Morikawa H., Kamon H., Iguchi M., Hojyo S., Fukada T., et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat. Immunol. 2006;7(9):971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 191.Shumilina E., Xuan N.T., Schmid E., Bhavsar S.K., Szteyn K., Gu S., et al. Zinc induced apoptotic death of mouse dendritic cells. Apoptosis. 2010;15(10):1177–1186. doi: 10.1007/s10495-010-0520-x. [DOI] [PubMed] [Google Scholar]
- 192.Zeyda M., Säemann M.D., Stuhlmeier K.M., Mascher D.G., Nowotny P.N., Zlabinger G.J., et al. Association between serum fatty acid composition and innate immune markers in healthy adults. J. Biol. Chem. 2005;280(14):14293–14301. doi: 10.1074/jbc.M410000200. [DOI] [PubMed] [Google Scholar]
- 193.Kong W., Yen J.H., Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011;25(5):872–882. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vanherwegen A.S., Eelen G., Ferreira G.B., Ghesquière B., Cook D.P., Nikolic T., et al. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism. J. Steroid Biochem. Mol. Biol. 2019;187:134–145. doi: 10.1016/j.jsbmb.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 195.Piemonti L., Monti P., Sironi M., Fraticelli P., Leone B.E., Dal Cin E., et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000;164(9):4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 196.Libako P., Miller J., Nowacki W., Castiglioni S., Maier J.A., Mazur A. Extracellular Mg concentration and Ca blockers modulate the initial steps of the response of Th2 lymphocytes in co-culture with macrophages and dendritic cells. Eur. Cytokine Netw. 2015;26(1):1–9. doi: 10.1684/ecn.2015.0361. [DOI] [PubMed] [Google Scholar]
- 197.Richter M., Bonneau R., Girard M.A., Beaulieu C., Larivée P. Zinc status modulates bronchopulmonary eosinophil infiltration in a murine model of allergic inflammation. Chest. 2003;123(3 Suppl) doi: 10.1378/chest.123.3_suppl.446s. [DOI] [PubMed] [Google Scholar]
- 198.Lang C., Murgia C., Leong M., Tan L.W., Perozzi G., Knight D., et al. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;292(2):L577–L584. doi: 10.1152/ajplung.00280.2006. [DOI] [PubMed] [Google Scholar]
- 199.Tanigai T., Ueki S., Kihara J., Kamada R., Yamauchi Y., Sokal A., et al. Docosahexaenoic acid exerts anti-inflammatory action on human eosinophils through peroxisome proliferator-activated receptor-independent mechanisms. Int. Arch. Allergy Immunol. 2012;158(4):375–386. doi: 10.1159/000332965. [DOI] [PubMed] [Google Scholar]
- 200.Snyman J.R., de Sommers K., Steinmann M.A., Lizamore D.J. Effects of calcitriol on eosinophil activity and antibody responses in patients with schistosomiasis. Eur. J. Clin. Pharmacol. 1997;52(4):277–280. doi: 10.1007/s002280050289. [DOI] [PubMed] [Google Scholar]
- 201.Souto Filho Jtd, de Andrade A.S., Ribeiro F.M., Alves P.A.S., Simonini V.R.F. Impact of vitamin D deficiency on increased blood eosinophil counts. Hematol Oncol Stem Cell Ther. 2018;11(1):25–29. doi: 10.1016/j.hemonc.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 202.Hungerford G.F., Karson E.F. The eosinophilia of magnesium deficiency. Blood. 1960;16(5):1642–1650. [PubMed] [Google Scholar]
- 203.Prasad A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000;182(Suppl 1):S62–S68. doi: 10.1086/315916. [DOI] [PubMed] [Google Scholar]
- 204.Bao B., Thakur A., Li Y., Ahmad A., Azmi A.S., Banerjee S., et al. The immunological contribution of NF-κB within the tumor microenvironment: a potential protective role of zinc as an anti-tumor agent. Biochim. Biophys. Acta Rev. Canc. 2012;1825(2):160–172. doi: 10.1016/j.bbcan.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chang H.H., Chen C.S., Lin J.Y. Dietary perilla oil inhibits proinflammatory cytokine production in the bronchoalveolar lavage fluid of ovalbumin-challenged mice. Lipids. 2008;43(6):499–506. doi: 10.1007/s11745-008-3171-8. [DOI] [PubMed] [Google Scholar]
- 206.Mizota T., Fujita-Kambara C., Matsuya N., Hamasaki S., Fukudome T., Goto H., et al. Effect of dietary fatty acid composition on Th1/Th2 polarization in lymphocytes. J. Parenter. Enteral Nutr. 2009;33(4):390–396. doi: 10.1177/0148607108325252. [DOI] [PubMed] [Google Scholar]
- 207.Zhang P., Smith R., Chapkin R.S., McMurray D.N. Dietary (n-3) polyunsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. J. Nutr. 2005;135(7):1745–1751. doi: 10.1093/jn/135.7.1745. [DOI] [PubMed] [Google Scholar]
- 208.Chung H.S., Park C.S., Hong S.H., Lee S., Cho M.L., Her Y.M., et al. Effects of magnesium pretreatment on the levels of T helper cytokines and on the severity of reperfusion syndrome in patients undergoing living donor liver transplantation. Magnes. Res. 2013;26(2):46–55. doi: 10.1684/mrh.2013.0338. [DOI] [PubMed] [Google Scholar]
- 209.Kitabayashi C., Fukada T., Kanamoto M., Ohashi W., Hojyo S., Atsumi T., et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int. Immunol. 2010;22(5):375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- 210.Lee H., Kim B., Choi Y.H., Hwang Y., Kim D.H., Cho S., et al. Inhibition of interleukin-1β-mediated interleukin-1 receptor-associated kinase 4 phosphorylation by zinc leads to repression of memory T helper type 17 response in humans. Immunology. 2015;146(4):645–656. doi: 10.1111/imm.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rosenkranz E., Maywald M., Hilgers R.D., Brieger A., Clarner T., Kipp M., et al. Induction of regulatory T cells in Th1-/Th17-driven experimental autoimmune encephalomyelitis by zinc administration. J. Nutr. Biochem. 2016;29:116–123. doi: 10.1016/j.jnutbio.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 212.Monk J.M., Hou T.Y., Turk H.F., Weeks B., Wu C., McMurray D.N., et al. Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PloS One. 2012;7(11) doi: 10.1371/journal.pone.0049739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Han S.C., Koo D.H., Kang N.J., Yoon W.J., Kang G.J., Kang H.K., et al. Docosahexaenoic acid alleviates atopic dermatitis by generating Tregs and IL-10/TGF-β-modified macrophages via a TGF-β-dependent mechanism. J. Invest. Dermatol. 2015;135(6):1556–1564. doi: 10.1038/jid.2014.488. [DOI] [PubMed] [Google Scholar]
- 214.Huang C.H., Hou Y.C., Pai M.H., Yeh C.L., Yeh S.L. Dietary ω-6/ω-3 PUFA ratios affect the homeostasis of Th/Treg cells in mice with dextran sulfate sodium-induced colitis. J. Parenter. Enteral Nutr. 2017;41(4):647–656. doi: 10.1177/0148607116638493. [DOI] [PubMed] [Google Scholar]
- 215.Daniel C., Sartory N.A., Zahn N., Radeke H.H., Stein J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Therapeut. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 216.Tang J., Zhou R., Luger D., Zhu W., Silver P.B., Grajewski R.S., et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J. Immunol. 2009;182(8):4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rosenkranz E., Metz C.H., Maywald M., Hilgers R.D., Weßels I., Senff T., et al. Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol. Nutr. Food Res. 2016;60(3):661–671. doi: 10.1002/mnfr.201500524. [DOI] [PubMed] [Google Scholar]
- 218.Maywald M., Meurer S.K., Weiskirchen R., Rink L. Zinc supplementation augments TGF-β1-dependent regulatory T cell induction. Mol. Nutr. Food Res. 2017;61(3) doi: 10.1002/mnfr.201600493. [DOI] [PubMed] [Google Scholar]
- 219.Carlsson J.A., Wold A.E., Sandberg A.S., Östman S.M. The polyunsaturated fatty acids arachidonic acid and docosahexaenoic acid induce mouse dendritic cells maturation but reduce T-cell responses in vitro. PloS One. 2015;10(11) doi: 10.1371/journal.pone.0143741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lian M., Luo W., Sui Y., Li Z., Hua J. Dietary n-3 PUFA protects mice from Con A induced liver injury by modulating regulatory T cells and PPAR-γ expression. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0132741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Onodera T., Fukuhara A., Shin J., Hayakawa T., Otsuki M., Shimomura I. Eicosapentaenoic acid and 5-HEPE enhance macrophage-mediated Treg induction in mice. Sci. Rep. 2017;7(1):4560. doi: 10.1038/s41598-017-04474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Guillot X., Semerano L., Saidenberg-Kermanac'h N., Falgarone G., Boissier M.C. Vitamin D and inflammation. Joint Bone Spine. 2010;77(6):552–557. doi: 10.1016/j.jbspin.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 223.Gorman S., Geldenhuys S., Judge M., Weeden C.E., Waithman J., Hart P.H. Dietary vitamin D increases percentages and function of regulatory T cells in the skin-draining lymph nodes and suppresses dermal inflammation. J Immunol Res. 2016 doi: 10.1155/2016/1426503. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fisher S.A., Rahimzadeh M., Brierley C., Gration B., Doree C., Kimber C.E., et al. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: a systematic review. PloS One. 2019;14(9) doi: 10.1371/journal.pone.0222313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Summersgill H., England H., Lopez-Castejon G., Lawrence C.B., Luheshi N.M., Pahle J. Zinc depletion regulates the processing and secretion of IL-1β. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2013.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fan Y., Zhang X., Yang L., Wang J., Hu Y., Bian A., et al. Zinc inhibits high glucose-induced NLRP3 inflammasome activation in human peritoneal mesothelial cells. Mol. Med. Rep. 2017;16(4):5195–5202. doi: 10.3892/mmr.2017.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Williams-Bey Y., Boularan C., Vural A., Huang N.N., Hwang I.Y., Shan-Shi C., et al. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PloS One. 2014;9(6) doi: 10.1371/journal.pone.0097957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.De Boer A.A., Monk J.M., Liddle D.M., Hutchinson A.L., Power K.A., Ma D.W., et al. Fish-oil-derived n-3 polyunsaturated fatty acids reduce NLRP3 inflammasome activity and obesity-related inflammatory cross-talk between adipocytes and CD11b(+) macrophages. J. Nutr. Biochem. 2016;34:61–72. doi: 10.1016/j.jnutbio.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 229.Kumar N., Gupta G., Anilkumar K., Fatima N., Karnati R., Reddy G.V., et al. 15-Lipoxygenase metabolites of α-linolenic acid, [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci. Rep. 2016;6 doi: 10.1038/srep31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang J.H., Chen Y.P., Yang X., Li C.Q. Vitamin D3 levels and NLRP3 expression in murine models of obese asthma: association with asthma outcomes. Braz. J. Med. Biol. Res. 2017;51(1) doi: 10.1590/1414-431X20176841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lu L., Lu Q., Chen W., Li J., Li C., Zheng Z. Vitamin D 3 protects against diabetic retinopathy by inhibiting high-glucose-induced activation of the ROS/TXNIP/NLRP3 inflammasome pathway. J Diabet. Res. 2018 doi: 10.1155/2018/8193523. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dai Y., Zhang J., Xiang J., Li Y., Wu D., Xu J. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019;21 doi: 10.1016/j.redox.2018.101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chang C.Y., Shih H.J., Huang I.T., Tsai P.S., Chen K.Y., Huang C.J. Magnesium sulfate mitigates the progression of monocrotaline pulmonary hypertension in rats. Int. J. Mol. Sci. 2019;20(18):4622. doi: 10.3390/ijms20184622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li C., Chen M., He X., Ouyang D. A mini-review on ion fluxes that regulate NLRP3 inflammasome activation. Acta Biochim. Biophys. Sin. 2021;53(2):131–139. doi: 10.1093/abbs/gmaa155. [DOI] [PubMed] [Google Scholar]
- 235.von Bülow V., Dubben S., Engelhardt G., Hebel S., Plümäkers B., Heine H. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J. Immunol. 2007;179(6):4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- 236.Novak T.E., Babcock T.A., Jho D.H., Helton W.S., Espat N.J. NF-kappa B inhibition by omega -3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284(1):L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 237.Rogero M.M., Leão M.C., Santana T.M., Pimentel M.V.M.B., Carlini G.C.G., da Silveira T.F.F., et al. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic. Biol. Med. 2020;156:190–199. doi: 10.1016/j.freeradbiomed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yu X.P., Bellido T., Manolagas S.C. Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. U.S.A. 1995;92(24):10990–10994. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cohen-Lahav M., Shany S., Tobvin D., Chaimovitz C., Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol. Dial. Transplant. 2006;21(4):889–897. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 240.Wöbke T.K., Sorg B.L., Steinhilber D. Vitamin D in inflammatory diseases. Front. Physiol. 2014;5:244. doi: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Su N.Y., Peng T.C., Tsai P.S., Huang C.J. Phosphoinositide 3-kinase/Akt pathway is involved in mediating the anti-inflammation effects of magnesium sulfate. J. Surg. Res. 2013;185(2):726–732. doi: 10.1016/j.jss.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 242.Ren J., Chung S.H. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. J. Agric. Food Chem. 2007;55(13):5073–5080. doi: 10.1021/jf0702693. [DOI] [PubMed] [Google Scholar]
- 243.Xie N., Zhang W., Li J., Liang H., Zhou H., Duan W., et al. α-Linolenic acid intake attenuates myocardial ischemia/reperfusion injury through anti-inflammatory and anti-oxidative stress effects in diabetic but not normal rats. Arch. Med. Res. 2011;42(3):171–181. doi: 10.1016/j.arcmed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 244.Bi X., Li F., Liu S., Jin Y., Zhang X., Yang T., et al. ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J. Clin. Invest. 2017;127(5):1757–1771. doi: 10.1172/JCI87388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Choi M., Park H., Cho S., Lee M. Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high-intensity exercise in SD rats. Cytokine. 2013;63(1):27–35. doi: 10.1016/j.cyto.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 246.Bessler H., Djaldetti M. 1α,25-Dihydroxyvitamin D3 modulates the interaction between immune and colon cancer cells. Biomed. Pharmacother. 2012;66(6):428–432. doi: 10.1016/j.biopha.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 247.Lysandropoulos A.P., Jaquiéry E., Jilek S., Pantaleo G., Schluep M., Du Pasquier R.A. Vitamin D has a direct immunomodulatory effect on CD8+ T cells of patients with early multiple sclerosis and healthy control subjects. J. Neuroimmunol. 2011;233(1–2):240–244. doi: 10.1016/j.jneuroim.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 248.Lucisano S., Arena A., Stassi G., Iannello D., Montalto G., Romeo A., et al. Role of paricalcitol in modulating the immune response in patients with renal disease. Internet J. Endocrinol. 2015;2015 doi: 10.1155/2015/765364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Han F., Xu L., Huang Y., Chen T., Zhou T., Yang L. Magnesium sulphate can alleviate oxidative stress and reduce inflammatory cytokines in rat placenta of intrahepatic cholestasis of pregnancy model. Arch. Gynecol. Obstet. 2018;298(3):631–638. doi: 10.1007/s00404-018-4850-1. [DOI] [PubMed] [Google Scholar]
- 250.Schubert C., Guttek K., Grüngreiff K., Thielitz A., Bühling F., Reinhold A., et al. Oral zinc aspartate treats experimental autoimmune encephalomyelitis. Biometals. 2014;27(6):1249–1262. doi: 10.1007/s10534-014-9786-8. [DOI] [PubMed] [Google Scholar]
- 251.Cippitelli M., Santoni M. Vitamin D3 : a transcriptional modulator of the interferon- gene. Eur. J. Immunol. 1998;28:3017–3030. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 252.Sharifi A., Vahedi H., Nedjat S., Rafiei H., Hosseinzadeh-Attar M.J. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. APMIS. 2019;127(10):681–687. doi: 10.1111/apm.12982. [DOI] [PubMed] [Google Scholar]
- 253.Carvalho J.T.G., Schneider M., Cuppari L., Grabulosa C.C., T Aoike D., Q Redublo B.M., et al. Cholecalciferol decreases inflammation and improves vitamin D regulatory enzymes in lymphocytes in the uremic environment: a randomized controlled pilot trial. PloS One. 2017;12(6) doi: 10.1371/journal.pone.0179540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bao S., Liu M.J., Lee B., Besecker B., Lai J.P., Guttridge D.C., et al. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;298(6):L744–L754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tyagi A., Kumar U., Reddy S., Santosh V.S., Mohammed S.B., Ehtesham N.Z., et al. Attenuation of colonic inflammation by partial replacement of dietary linoleic acid with α-linolenic acid in a rat model of inflammatory bowel disease. Br. J. Nutr. 2012;108(9):1612–1622. doi: 10.1017/S0007114511007197. [DOI] [PubMed] [Google Scholar]
- 256.Liu Y.H., Li X.Y., Chen C.Y., Zhang H.M., Kang J.X. Omega-3 fatty acid intervention suppresses lipopolysaccharide-induced inflammation and weight loss in mice. Mar. Drugs. 2015;13(2):1026–1036. doi: 10.3390/md13021026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cui C., Xu P., Li G., Qiao Y., Han W., Geng C., et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. 2019;26 doi: 10.1016/j.redox.2019.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chang Y.Y., Kao M.C., Lin J.A., Chen T.Y., Cheng C.F., Wong C.S., Tzeng I.S., Huang C.J. Effects of MgSO4 on inhibiting Nod-like receptor protein 3 inflammasome involve decreasing intracellular calcium. J. Surg. Res. 2018;221:257–265. doi: 10.1016/j.jss.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 259.Ozen M., Xie H., Shin N., Al Yousif G., Clemens J., McLane M.W., et al. Magnesium sulfate inhibits inflammation through P2X7 receptors in human umbilical vein endothelial cells. Pediatr. Res. 2020;87(3):463–471. doi: 10.1038/s41390-019-0557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Uzzo R.G., Crispen P.L., Golovine K., Makhov P., Horwitz E.M., Kolenko V.M. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis. 2006;27(10):1980–1990. doi: 10.1093/carcin/bgl034. [DOI] [PubMed] [Google Scholar]
- 261.Orrù B., Szekeres-Bartho J., Bizzarri M., Spiga A.M., Unfer V. Inhibitory effects of Vitamin D on inflammation and IL-6 release. A further support for COVID-19 management? Eur. Rev. Med. Pharmacol. Sci. 2020;24(15):8187–8193. doi: 10.26355/eurrev_202008_22507. [DOI] [PubMed] [Google Scholar]
- 262.Silberstein M. Vitamin D: a simpler alternative to tocilizumab for trial in COVID-19? Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jiang J., Chen Q., Chen X., Li J., Li S., Yang B. Magnesium sulfate ameliorates sepsis-induced diaphragm dysfunction in rats via inhibiting HMGB1/TLR4/NF-κB pathway. Neuroreport. 2020;31(12):902–908. doi: 10.1097/WNR.0000000000001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rani V., Verma Y., Rana K., Rana S.V.S. Zinc oxide nanoparticles inhibit dimethylnitrosamine induced liver injury in rat. Chem. Biol. Interact. 2018;295:84–92. doi: 10.1016/j.cbi.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 265.Kong W., Yen J.H., Vassiliou E., Adhikary S., Toscano M.G., Ganea D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 2010;9:12. doi: 10.1186/1476-511X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Benson A.A., Toh J.A., Vernon N., Jariwala S.P. The role of vitamin D in the immunopathogenesis of allergic skin diseases. Allergy. 2012;67:296–301. doi: 10.1111/j.1398-9995.2011.02755.x. [DOI] [PubMed] [Google Scholar]
- 267.Martínez-Moreno J., Hernandez J.C., Urcuqui-Inchima S. Effect of high doses of vitamin D supplementation on dengue virus replication, Toll-like receptor expression, and cytokine profiles on dendritic cells. Mol. Cell. Biochem. 2020;464(1–2):169–180. doi: 10.1007/s11010-019-03658-w. [DOI] [PubMed] [Google Scholar]
- 268.Reda R., Abbas A.A., Mohammed M., El Fedawy S.F., Ghareeb H., El Kabarity R.H., et al. The interplay between zinc, vitamin D and, IL-17 in patients with chronic hepatitis C liver disease. J Immunol Res. 2015 doi: 10.1155/2015/846348. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cantorna M.T., Snyder L., Lin Y.D., Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7(4):3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Schardey J., Globig A.M., Janssen C., Hofmann M., Manegold P., Thimme R., et al. Vitamin D inhibits pro-inflammatory T cell function in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13(12):1546–1557. doi: 10.1093/ecco-jcc/jjz090. [DOI] [PubMed] [Google Scholar]
- 271.Muroi M., Tanamoto K. Zinc- and oxidative property-dependent degradation of pro-caspase-1 and NLRP3 by ziram in mouse macrophages. Toxicol. Lett. 2015;235(3):199–205. doi: 10.1016/j.toxlet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 272.Kong J., Grando S.A., Li Y.C. Regulation of IL-1 family cytokines IL-1alpha, IL-1 receptor antagonist, and IL-18 by 1,25-dihydroxyvitamin D3 in primary keratinocytes. J. Immunol. 2006;176(6):3780–3787. doi: 10.4049/jimmunol.176.6.3780. [DOI] [PubMed] [Google Scholar]
- 273.Yang F.X., Hou L., Wen W.L., Shen X.L., Feng N.Y., Ma R.X., et al. Role of zinc sulphate in immune regulation in Artemisia annua pollen-challenged P815 mastocytoma cells. Immunol. Invest. 2020;49(6):622–631. doi: 10.1080/08820139.2019.1694939. [DOI] [PubMed] [Google Scholar]
- 274.Fletcher P., Hamilton R.F., Buford M., Postma B., Pestka J.J., Holian A. Comparing docosahexaenoic acid as a prophylactic treatment for acute and chronic particle-exposed Balb/c mice. L Immunol. 2019;202(1 Suppl):117. 6. [Google Scholar]
- 275.Mohammadi-Kordkhayli M., Ahangar-Parvin R., Azizi S.V., Nemati M., Shamsizadeh A., Khaksari M., et al. Vitamin D modulates the expression of IL-27 and IL-33 in the central nervous system in experimental autoimmune encephalomyelitis (EAE) Iran J Immunol. 2015;12(1):35–49. [PubMed] [Google Scholar]
- 276.Monk J.M., Liddle D.M., Brown M.J., Zarepoor L., De Boer A.A., Ma D.W., et al. Anti-inflammatory and anti-chemotactic effects of dietary flaxseed oil on CD8(+) T cell/adipocyte-mediated cross-talk. Mol. Nutr. Food Res. 2016;60(3):621–630. doi: 10.1002/mnfr.201500541. [DOI] [PubMed] [Google Scholar]
- 277.Han H., Qiu F., Zhao H., Tang H., Li X., Shi D. Dietary flaxseed oil prevents Western-type diet-induced nonalcoholic fatty liver disease in apolipoprotein-E knockout mice. Oxid Med Cell Longev. 2017 doi: 10.1155/2017/3256241. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Song J., Jing Z., Hu W., Yu J., Cui X. α-Linolenic acid inhibits receptor activator of NF-κB ligand induced (RANKL-induced) osteoclastogenesis and prevents inflammatory bone loss via downregulation of nuclear factor-kappaB-inducible nitric oxide synthases (NF-κB-iNOS) signaling pathways. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2017;23:5056–5069. doi: 10.12659/MSM.904795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Spigoni V., Lombardi C., Cito M., Picconi A., Ridolfi V., Andreoli R., et al. N-3 PUFA increase bioavailability and function of endothelial progenitor cells. Food Func. 2014;5(8):1881–1890. doi: 10.1039/c3fo60641d. [DOI] [PubMed] [Google Scholar]
- 280.Zhang J., McCullough P.A., Tecson K.M. Vitamin D deficiency in association with endothelial dysfunction: implications for patients with COVID-19. Rev. Cardiovasc. Med. 2020;21(3):339–344. doi: 10.31083/j.rcm.2020.03.131. [DOI] [PubMed] [Google Scholar]
- 281.Thota C., Farmer T., Garfield R.E., Menon R., Al-Hendy A. Vitamin D elicits anti-inflammatory response, inhibits contractile-associated proteins, and modulates Toll-like receptors in human myometrial cells. Reprod. Sci. 2013;20(4):463–475. doi: 10.1177/1933719112459225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sanchez-Niño M.D., Bozic M., Córdoba-Lanús E., Valcheva P., Gracia O., Ibarz M., et al. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2012;302(6):F647–F657. doi: 10.1152/ajprenal.00090.2011. [DOI] [PubMed] [Google Scholar]
- 283.Beloosesky R., Khatib N., Ginsberg Y., Anabosy S., Shalom-Paz E., Dahis M., et al. Maternal magnesium sulfate fetal neuroprotective effects to the fetus: inhibition of neuronal nitric oxide synthase and nuclear factor kappa-light-chain-enhancer of activated B cells activation in a rodent model. Am. J. Obstet. Gynecol. 2016;215(3):382. doi: 10.1016/j.ajog.2016.03.032. e1-6. [DOI] [PubMed] [Google Scholar]
- 284.Slinko S., Piraino G., Hake P.W., Ledford J.R., O'Connor M., Lahni P., et al. Combined zinc supplementation with proinsulin C-peptide treatment decreases the inflammatory response and mortality in murine polymicrobial sepsis. Shock. 2014;41(4):292–300. doi: 10.1097/SHK.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lund A.S., Hasselbalch A.L., Gamborg M., Skogstrand K., Hougaard D.M., Heitmann B.L., et al. N-3 polyunsaturated fatty acids, body fat and inflammation. Obes Facts. 2013;6(4):369–379. doi: 10.1159/000354663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Boontanrart M., Hall S.D., Spanier J.A., Hayes C.E., Olson J.K. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J. Neuroimmunol. 2016;292:126–136. doi: 10.1016/j.jneuroim.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 287.Wang L.J., Wang M.Q., Hu R., Yang Y., Huang Y.S., Xian S.X., et al. Effect of zinc supplementation on maintenance hemodialysis patients: a systematic review and meta-analysis of 15 randomized controlled trials. BioMed Res. Int. 2017 doi: 10.1155/2017/1024769. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Khorsandi H., Nikpayam O., Yousefi R., Parandoosh M., Hosseinzadeh N., Saidpour A., et al. Zinc supplementation improves body weight management, inflammatory biomarkers and insulin resistance in individuals with obesity: a randomized, placebo-controlled, double-blind trial. Diabetol. Metab. Syndrome. 2019;11:101. doi: 10.1186/s13098-019-0497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hajji M., Khedher R., Mrad M., Bassem H.M., Rafrafi N., Chouchi S., et al. Effects of zinc supplementation on serum copper to zinc and CRP to albumin ratios in hemodialysis patients. J. Med. Biochem. 2021;40(2):193–198. doi: 10.5937/jomb0-26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Thota R.N., Rosato J.I., Burrows T.L., Dias C.B., Abbott K.A., Martins R.N., et al. Docosahexaenoic acid-rich fish oil supplementation reduces kinase associated with insulin resistance in overweight and obese midlife adults. Nutrients. 2020;12(6):1612. doi: 10.3390/nu12061612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kasemy Z.A., Hathout H.M., Omar Z.A., Samir M.A., Bahbah W.A. Effect of Omega-3 supplements on quality of life among children on dialysis: a prospective cohort study. Medicine (Baltim.) 2020;99(40) doi: 10.1097/MD.0000000000022240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.AbuMweis S., Abu Omran D., Al-Shami I., Jew S. The ratio of eicosapentaenoic acid to docosahexaenoic acid as a modulator for the cardio-metabolic effects of omega-3 supplements: a meta-regression of randomized clinical trials. Compl. Ther. Med. 2021;57 doi: 10.1016/j.ctim.2021.102662. [DOI] [PubMed] [Google Scholar]
- 293.Cheshmazar E., Hosseini A.F., Yazdani B., Razmpoosh E., Zarrati M. Effects of vitamin D supplementation on omentin-1 and spexin levels, inflammatory parameters, lipid profile, and anthropometric indices in obese and overweight adults with vitamin D deficiency under low-calorie diet. Evid Based Complement Alternat Med. 2020 doi: 10.1155/2020/3826237. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhao J.F., Li B.X., Zhang Q. Vitamin D improves levels of hormonal, oxidative stress and inflammatory parameters in polycystic ovary syndrome: a meta-analysis study. Ann. Palliat. Med. 2021;10(1):169–183. doi: 10.21037/apm-20-2201. [DOI] [PubMed] [Google Scholar]
- 295.Mazidi M., Rezaie P., Banach M. Effect of magnesium supplements on serum C-reactive protein: a systematic review and meta-analysis. Arch. Med. Sci. 2018;14(4):707–716. doi: 10.5114/aoms.2018.75719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Aster I., Barth L.M., Rink L., Wessels I. Alterations in membrane fluidity are involved in inhibition of GM-CSF-induced signaling in myeloid cells by zinc. J. Trace Elem. Med. Biol. 2019;54:214–220. doi: 10.1016/j.jtemb.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 297.Jensen K.N., Omarsdottir S.Y., Reinhardsdottir M.S., Hardardottir I., Freysdottir J. Docosahexaenoic acid modulates NK cell effects on neutrophils and their crosstalk. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ibrahim A., Mbodji K., Hassan A., Aziz M., Boukhettala N., Coëffier M., et al. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin. Nutr. 2011;30(5):678–687. doi: 10.1016/j.clnu.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 299.Yusupov E., Li-Ng M., Pollack S., Yeh J.K., Mikhail M., Aloia J.F. Vitamin D and serum cytokines in a randomized clinical trial. Internet J. Endocrinol. 2010;2010 doi: 10.1155/2010/305054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Konya V., Czarnewski P., Forkel M., Rao A., Kokkinou E., Villablanca E.J., et al. Vitamin D downregulates the IL-23 receptor pathway in human mucosal group 3 innate lymphoid cells. J. Allergy Clin. Immunol. 2018;141(1):279–292. doi: 10.1016/j.jaci.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 301.Towers T.L., Staeva T.P., Freedman L.P. A two-hit mechanism for vitamin D3-mediated transcriptional repression of the granulocyte-macrophage colony-stimulating factor gene: vitamin D receptor competes for DNA binding with NFAT1 and stabilizes c-Jun. Mol. Cell Biol. 1999;19(6):4191–4199. doi: 10.1128/mcb.19.6.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tobler A., Gasson J., Reichel H., Norman A.W., Koeffler H.P. Granulocyte-macrophage colony-stimulating factor. Sensitive and receptor-mediated regulation by 1,25-dihydroxyvitamin D3 in normal human peripheral blood lymphocytes. J. Clin. Invest. 1987;79(6):1700–1705. doi: 10.1172/JCI113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Fiorino S., Gallo C., Zippi M., Sabbatani S., Manfredi R., Moretti R., et al. Cytokine storm in aged people with CoV-2: possible role of vitamins as therapy or preventive strategy. Aging Clin. Exp. Res. 2020;32(10):2115–2131. doi: 10.1007/s40520-020-01669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Marazuela M., Giustina A., Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev. Endocr. Metab. Disord. 2020;21(4):495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rai V., Agrawal D.K. Role of vitamin D in cardiovascular diseases. Endocrinol Metab. Clin. N. Am. 2017;46(4):1039–1059. doi: 10.1016/j.ecl.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Williams J.W., Huang L.H., Randolph G.J. Cytokine circuits in cardiovascular disease. Immunity. 2019;50(4):941–954. doi: 10.1016/j.immuni.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Koelman L., Pivovarova-Ramich O., Pfeiffer A.F.H., Grune T., Aleksandrova K. Cytokines for evaluation of chronic inflammatory status in ageing research: reliability and phenotypic characterisation. Immun. Ageing. 2019;16:11. doi: 10.1186/s12979-019-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Janssen C.I., Kiliaan A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 312.Barbagallo M., Belvedere M., Dominguez L.J. Magnesium homeostasis and aging. Magnes. Res. 2009;22(4):235–246. doi: 10.1684/mrh.2009.0187. [DOI] [PubMed] [Google Scholar]
- 313.Morgante G., Troìa L., De Leo V. Coronavirus Disease 2019 (SARS-CoV-2) and polycystic ovarian disease: is there a higher risk for these women? J. Steroid Biochem. Mol. Biol. 2020;205 doi: 10.1016/j.jsbmb.2020.105770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wang E.W., Siu P.M., Pang M.Y., Woo J., Collins A.R., Benzie I.F.F. Vitamin D deficiency, oxidative stress and antioxidant status: only weak association seen in the absence of advanced age, obesity or pre-existing disease. Br. J. Nutr. 2017;118(1):11–16. doi: 10.1017/S000711451700188X. [DOI] [PubMed] [Google Scholar]
- 315.Olechnowicz J., Tinkov A., Skalny A., Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018;68(1):19–31. doi: 10.1007/s12576-017-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Fukunaka A., Fujitani Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int. J. Mol. Sci. 2018;19(2):476. doi: 10.3390/ijms19020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mossink J.P. Zinc as nutritional intervention and prevention measure for COVID-19 disease. BMJ Nutr Prev Health. 2020;3(1):111–117. doi: 10.1136/bmjnph-2020-000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Chabosseau P., Rutter G.A. Zinc and diabetes. Arch. Biochem. Biophys. 2016;611:79–85. doi: 10.1016/j.abb.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 319.Simopoulos A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Torrinhas R.S., Calder P.C., Waitzberg D.L. Response to Bistrian BR. Parenteral fish-oil emulsions in critically ill COVID-19 emulsions. J. Parenter. Enteral Nutr. 2020;44(7):1169–1170. doi: 10.1002/jpen.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rafiq S., Jeppesen P.B. Body mass index, vitamin D, and type 2 diabetes: a systematic review and meta-analysis. Nutrients. 2018;10(9):1182. doi: 10.3390/nu10091182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Walsh J.S., Bowles S., Evans A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017;24(6):389–394. doi: 10.1097/MED.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 323.Hyppönen E., Boucher B.J. Adiposity, vitamin D requirements, and clinical implications for obesity-related metabolic abnormalities. Nutr. Rev. 2018;76(9):678–692. doi: 10.1093/nutrit/nuy034. [DOI] [PubMed] [Google Scholar]
- 324.Yao Y., Zhu L., He L., Duan Y., Liang W., Nie Z., et al. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int. J. Clin. Exp. Med. 2015;8(9):14977–14984. [PMC free article] [PubMed] [Google Scholar]
- 325.Li X., Liu Y., Zheng Y., Wang P., Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018;10(3):375. doi: 10.3390/nu10030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mauss D., Jarczok M.N., Hoffmann K., Thomas G.N., Fischer J.E. Association of vitamin D levels with type 2 diabetes in older working adults. Int. J. Med. Sci. 2015;12(5):362–368. doi: 10.7150/ijms.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Nielsen F.H. Magnesium, inflammation, and obesity in chronic disease. Nutr. Rev. 2010;68(6):333–340. doi: 10.1111/j.1753-4887.2010.00293.x. [DOI] [PubMed] [Google Scholar]
- 328.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 329.Radenkovic D., Chawla S., Pirro M., Sahebkar A., Banach M. Cholesterol in relation to COVID-19: should we care about it? J. Clin. Med. 2020;9(6):1909. doi: 10.3390/jcm9061909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Choi S., Cui C., Luo Y., Kim S.H., Ko J.K., Huo X., et al. Selective inhibitory effects of zinc on cell proliferation in esophageal squamous cell carcinoma through Orai1. Faseb. J. 2018;32(1):404–416. doi: 10.1096/fj.201700227RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Jurowski K., Szewczyk B., Nowak G., Piekoszewski W. Biological consequences of zinc deficiency in the pathomechanisms of selected diseases. J. Biol. Inorg. Chem. 2014;19(7):1069–1079. doi: 10.1007/s00775-014-1139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Calder P.C. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem. Soc. Trans. 2017;45(5):1105–1115. doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- 333.Calder P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013;75(3):645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Darwesh A.M., Sosnowski D.K., Lee T.Y., Keshavarz-Bahaghighat H., Seubert J.M. Insights into the cardioprotective properties of n-3 PUFAs against ischemic heart disease via modulation of the innate immune system. Chem. Biol. Interact. 2019;308:20–44. doi: 10.1016/j.cbi.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 335.Kris-Etherton P.M., Harris W.S., Appel L.J., Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2003;23(2):e20–30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 336.Lee J.H., O'Keefe J.H., Lavie C.J., Harris W.S. Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat. Rev. Cardiol. 2009;6(12):753–758. doi: 10.1038/nrcardio.2009.188. [DOI] [PubMed] [Google Scholar]
- 337.Mozaffarian D., Lemaitre R.N., King I.B., Song X., Huang H., Sacks F.M., Rimm E.B., Wang M., Siscovick D.S. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann. Intern. Med. 2013;158(7):515–525. doi: 10.7326/0003-4819-158-7-201304020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Darwesh A.M., Bassiouni W., Sosnowski D.K., Seubert J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol. Ther. 2020;219 doi: 10.1016/j.pharmthera.2020.107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Paschou S.A., Kosmopoulos M., Nikas I.P., Spartalis M., Kassi E., Goulis D.G., et al. The impact of obesity on the association between vitamin D deficiency and cardiovascular disease. Nutrients. 2019;11(10):2458. doi: 10.3390/nu11102458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Porto C.M., Silva V.L., da Luz J.S.B., Filho B.M., da Silveira V.M. Association between vitamin D deficiency and heart failure risk in the elderly. ESC Heart Fail. 2018;5(1):63–74. doi: 10.1002/ehf2.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Hughes D.A., Norton R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009;158(1):20–25. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Liu L.C., Voors A.A., van Veldhuisen D.J., van der Veer E., Belonje A.M., Szymanski M.K., et al. Vitamin D status and outcomes in heart failure patients. Eur. J. Heart Fail. 2011;13(6):619–625. doi: 10.1093/eurjhf/hfr032. [DOI] [PubMed] [Google Scholar]
- 343.Cotogni P., Trombetta A., Muzio G., Maggiora M., Canuto R.A. The omega-3 fatty acid docosahexaenoic acid modulates inflammatory mediator release in human alveolar cells exposed to bronchoalveolar lavage fluid of ARDS patients. BioMed Res. Int. 2015 doi: 10.1155/2015/642520. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Mekov E., Slavova Y., Tsakova A., Genova M., Kostadinov D., Minchev D., et al. Vitamin D deficiency and insufficiency in hospitalized COPD patients. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0129080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Moghaddassi M., Pazoki M., Salimzadeh A., Ramim T., Alipour Z. Association of serum level of 25-hydroxy vitamin D deficiency and pulmonary function in healthy individuals. Sci. World J. 2018 doi: 10.1155/2018/3860921. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Mulrennan S., Knuiman M., Walsh J.P., Hui J., Hunter M., Divitini M., et al. Vitamin D and respiratory health in the busselton healthy ageing study. Respirology. 2018;23(6):576–582. doi: 10.1111/resp.13239. [DOI] [PubMed] [Google Scholar]
- 348.Brenner H., Holleczek B., Schöttker B. Vitamin D insufficiency and deficiency and mortality from respiratory diseases in a cohort of older adults: potential for limiting the death toll during and beyond the COVID-19 pandemic? Nutrients. 2020;12(8):2488. doi: 10.3390/nu12082488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ilyas M., Agussalim A., Megawati M., Massi N., Djaharuddin I., Bakri S., et al. Relationship between vitamin D level and serum TNF-α concentration on the severity of chronic obstructive pulmonary disease. Open Access Maced J Med Sci. 2019;7(14):2298–2304. doi: 10.3889/oamjms.2019.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Patterson W.L., 3rd, Georgel P.T. Breaking the cycle: the role of omega-3 polyunsaturated fatty acids in inflammation-driven cancers. Biochem. Cell. Biol. 2014;92(5):321–328. doi: 10.1139/bcb-2013-0127. [DOI] [PubMed] [Google Scholar]
- 351.Niedermaier T., Gredner T., Kuznia S., Schöttker B., Mons U., Brenner H. Vitamin D supplementation to the older adult population in Germany has the cost-saving potential of preventing almost 30 000 cancer deaths per year. Mol Oncol Ahead of print. 2021 doi: 10.1002/1878-0261.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]