Abstract
Objective
Cardiovascular risk factors (CVRFs) are associated with increased risk of cognitive decline, but little is known about how early adult CVRFs and those across the life course might influence late-life cognition. To test the hypothesis that CVRFs across the adult life course are associated with late-life cognitive changes, we pooled data from 4 prospective cohorts (n = 15,001, ages 18–95).
Methods
We imputed trajectories of body mass index (BMI), fasting glucose (FG), systolic blood pressure (SBP), and total cholesterol (TC) for older adults. We used linear mixed models to determine the association of early adult, midlife, and late-life CVRFs with late-life decline on global cognition (Modified Mini-Mental State Examination [3MS]) and processing speed (Digit Symbol Substitution Test [DSST]), adjusting for demographics, education, and cohort.
Results
Elevated BMI, FG, and SBP (but not TC) at each time period were associated with greater late-life decline. Early life CVRFs were associated with the greatest change, an approximate doubling of mean 10-year decline (an additional 3–4 points for 3MS or DSST). Late-life CVRFs were associated with declines in early late life (<80 years) but with gains in very late life (≥80 years). After adjusting for CVRF exposures at all time periods, the associations for early adult and late-life CVRFs persisted.
Conclusions
We found that imputed CVRFs across the life course, especially in early adulthood, were associated with greater late-life cognitive decline. Our results suggest that CVRF treatment in early adulthood could benefit late-life cognition, but that treatment in very late life may not be as helpful for these outcomes.
Cardiovascular risk factors (CVRFs), including hypertension, diabetes, and obesity, are among the most promising modifiable risk factors for prevention of cognitive aging and dementia.1–4 Not only are they associated with risk of vascular cognitive impairment and dementia but also with risk of Alzheimer disease and related dementias.5 Because CVRFs are common,6 the potential public health impact of reducing these risk factors is significant.3,7
Current research on delaying cognitive impairment emphasizes CVRF exposure during midlife,1–3 even though CVRFs may present earlier.6,8 This may be due to the lack of data on early-life CVRFs and cognitive decline within the same cohort. In addition, several observational studies have described differential CVRF associations with cognitive health in early vs very late life,9–11 finding positive associations between CVRFs and very late-life cognitive outcomes. Thus, a clear understanding of the role of CVRFs across the life course on cognitive outcomes is needed to more effectively target prevention efforts,12 particularly because CVRFs often go untreated.6,13
To address these challenges, we pooled data from 4 prospective cohorts spanning the adult life course and imputed CVRF levels in young adulthood and midlife for the 2 older cohorts. We then estimated the independent associations of CVRF exposures during early adult, midlife, and late-life periods with late-life cognitive outcomes in the older cohorts. We hypothesized that elevated CVRFs at early adulthood and midlife will be associated with greater cognitive decline, but late-life CVRFs may have a differential effect on cognitive change in early vs very late life.
Methods
Pooled Cohort and Data Sources
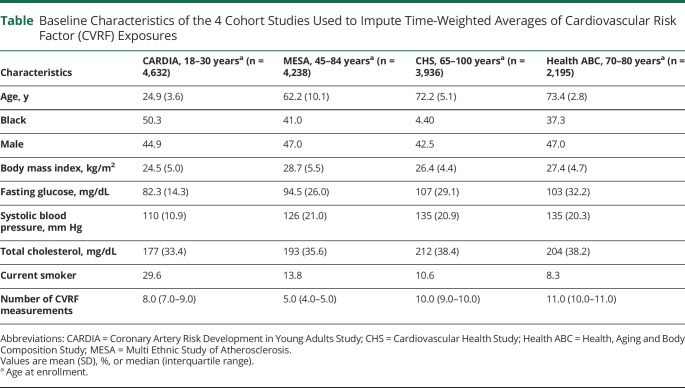
The 4 prospective cohorts were the Coronary Artery Risk Development in Young Adults study (CARDIA) of young to middle-aged adults, the Multi-Ethnic Study of Atherosclerosis (MESA) of middle-aged to older adults, the Cardiovascular Health Study (CHS), and the Health, Aging and Body Composition study (Health ABC) of older adults (table 1). Our pooled cohort included 15,001 White and Black adult participants ages 18–95 years old at enrollment, with at least 2 repeated measurements of each of the CVRFs, including 4,632 Black and White adults ages 18–30 at enrollment (1985–1986) and followed for 30 years from CARDIA, 4,238 Black and White adults ages 45–84 at enrollment (2000–2001) and followed for 10 years from MESA, 3,936 primarily White adults ages 65 and older at baseline (1989–1993) and followed for 10 years from CHS, and 2,195 Black and White adults ages 70–79 at enrollment (1997) and followed for 11 years from Health ABC. Details of the pooled cohort methodology are published elsewhere.14
Table 1.
Baseline Characteristics of the 4 Cohort Studies Used to Impute Time-Weighted Averages of Cardiovascular Risk Factor (CVRF) Exposures
Standard Protocol Approvals, Registrations, and Patient Consents
Our study was approved by local institutional review boards at Columbia University and the University of California San Francisco and the present analysis was approved by the publications & presentations committee of each cohort study. All participants provided written informed consent to participate in the parent studies.
CVRF Trajectories
The first step of the analysis used data from the 4 prospective cohorts to impute early adult and midlife CVRF trajectories in CHS and Health ABC participants.14 Informed by evidence from prior epidemiologic studies on risk factors for cognitive decline,1–4,15,16 we focused on body mass index (BMI), fasting glucose (FG), systolic blood pressure (SBP), and total cholesterol (TC). Using repeated measurements of each CVRF across the life course, we estimated person-specific CVRF trajectories using linear mixed models (LMMs) and calculated time-weighted average (TWAs) to summarize each CVRF in early adult (ages 20–49), midlife (ages 50–69), and late life (ages 70–89).14 Based on race and sex, smoking status, BMI, diabetes and hypertension, and diabetes, hypertensive, and lipid-lowering medication use were imputed sequentially and then used as time-dependent covariates in LMMs used to estimate the BMI, FG, SBP, and TC TWAs. More specifically, the imputation models used demographics including splines in age and birth year, a 4-level categorization of sex and race, as well as interactions of the age splines with sex/race, BMI, diabetes, and any history of relevant medication use. They also incorporated current information on smoking, current medication use, and cohort, as well as random intercepts and random age splines that are driven by the deviation of the observed CVRFs for each participant from the expected value determined by the fixed effects. The imputation procedure worked forwards, imputing in order the most distal to the most proximal factors. The full imputation methods are detailed in the supplementary Methods, available from Dryad (doi.org/10.7272/Q60000BJ).
Cognitive Outcomes
We examined change in Digit Symbol Substitution Test (DSST) and the Modified Mini-Mental State Examination (3MS), assessed annually in CHS and every 1 or 2 years in Health ABC (total number of 3MS assessments = 45,859; total number of DSST assessments = 42,353). DSST is a subtest of the Wechsler Adult Intelligence Scale and assesses processing speed with an element of executive functioning, with higher scores indicating better cognitive function.17 The 3MS is a test of global cognition assessing concentration, orientation, language, praxis, and immediate and delayed memory with a range of 0–100.18 3MS scores were slightly left skewed but analyzed without normalizing transformation, because large sample effects should ensure valid inferences, which we checked in sensitivity analyses using robust standard errors; DSST scores were approximately normal without transformation.
Association of CVRFs With Late-Life Cognitive Outcomes
We used additional LMMs with random intercepts and slopes adjusting for demographics, education, and cohort to assess the associations of the imputed CVRF trajectories estimated in the first step with repeated 3MS and DSST. The CVRF TWAs were categorized using standard cut points for analysis (BMI >30 kg/m2 vs ≤25 kg/m2, FG >125 mg/dL vs <100 mg/dL, SBP >140 mm Hg vs <120 mm Hg, TC >200 mg/dL vs < 160 mg/dL)19 and partially time-dependent; for example, both mid and late-life TWAs could differ across observations for a CHS participant contributing cognitive outcomes at ages 67, 69, 71, and 73. Specifically, the second-step LMMs were used to estimate the associations of individual CVRF TWAs with cognitive level at age 80 as well as with 10-year cognitive change, and in models for late-life CVRF exposure, 10-year cognitive change in early late life and very late life (before or after age 80), modeled by the interaction between the TWA category and a linear spline in age. Models were adjusted for age, race, sex, and education and examined the association of CVRF exposure at early adult, midlife, and late life, separately. In additional adjusted models, CVRF exposure at early adult, midlife, and late life were included in the same model. We also examined interactions of CVRF exposure with race and sex. Finally, to assess the potential for survival bias, which would require associations, possibly indirect, of both exposure and outcome with death or loss to follow-up,20 we examined the associations of cognitive function and CVRFs with attrition, adjusting for age, sex, race/ethnicity, and cohort. All analyses were implemented using Stata Version 16.1 (Stata LLP, College Station, TX).
Data Availability
Anonymized data from the cohort studies used in this analysis are available from each study's respective coordinating centers. Specific policies governing each study's data and the process to access data can be found online (CARDIA: cardia.dopm.uab.edu/; MESA: mesa-nhlbi.org/; CHS: chs-nhlbi.org/; Health ABC: healthabc.nia.nih.gov/).
Results
Characteristics of the 4 Prospective Cohorts Used to Impute CVRF Trajectories
The table shows the baseline characteristics of the 15,001 participants included in the 4 prospective cohorts spanning the adult life course used to estimate CVRF exposures across the life course. As expected, BMI, FG, SBP, and TC levels were higher in the older cohorts while current smoking was highest in the youngest cohort. The median number of CVRF measurements in each cohort ranged from 5 to 11.
Characteristics of the 2 Older Adult Cohorts
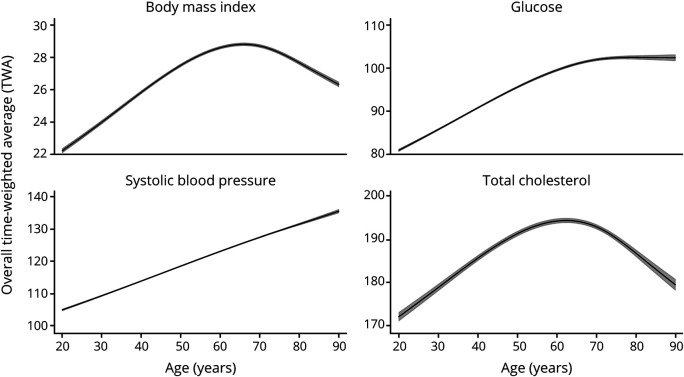
The mean age of the pooled cohort of older adults was 72.0 years (range 69.0–75.0, n = 6,073). In early adulthood, fewer than 5% of participants had elevated BMI, FG, or SBP, but almost 40% of participants had elevated cholesterol. Around 20% of participants had elevated BMI or SBP in midlife. Ten percent of participants had elevated FG in midlife, while over 68% had elevated cholesterol at midlife. In late life, 15% of participants had elevated BMI, 9% elevated FG, 24% elevated SBP, and 43% elevated cholesterol. Figure 1 shows the average trajectories of each CVRF across the life course. CVRF levels were low in early adulthood, increased into middle age, and in late life, SBP continued to increase steadily and FG leveled off, while BMI and TC levels started to decline.
Figure 1. Life Course Modeled Trajectories of Cardiovascular Risk Factors.
Trajectories of the overall average with 95% confidence intervals in gray shaded areas.
Baseline cognitive score (±SD) and overall 10-year change (±SE) were 90.0 ± 9.7 and −3.84 ± 0.16, respectively, for 3MS and 39.1 ± 13.7 and −6.37 ± 0.10 for DSST.
Association of CVRFs With Late-Life Cognitive Outcomes
Early Adult CVRF Exposure
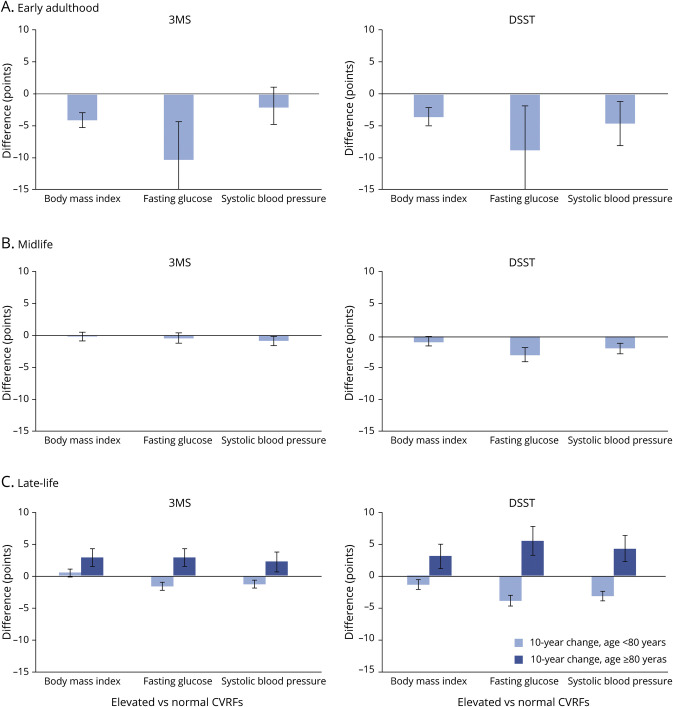
Early adult CVRFs were associated with greater cognitive decline in late life (figure 2A). Specifically, early adult elevated BMI (BMI >30 kg/m2), compared to the normal range, was associated with 3–4 points greater cognitive decline over 10 years in late life (10-year change in 3MS −4.10 points, 95% confidence interval [CI] [−5.24 to −2.97] and DSST −3.39 points, 95% CI [−4.75 to −2.02]). Similarly, early adult SBP (SBP >140 mm Hg) was associated with 4 points greater decline on DSST in late life (10-year change in DSST −4.44 points, 95% CI [−7.88 to −0.99]). Few participants had FG >125 mg/dL in early adulthood, but the results suggest elevated FG in early life was associated with greater late-life cognitive decline (10-year change in 3MS −10.31 points, 95% CI [−16.26 to −4.36] and DSST −8.66 points, 95% CI [−15.76 to −1.55]). Early adult elevated cholesterol (TC >200 mg/dL) was not a statistically significant risk factor for cognitive decline (p > 0.05 for all). Results were similar for early adult CVRF exposures even after adjusting for CVRF exposure at other time periods.
Figure 2. Difference in 10-Year Cognitive Change in Late Life for Elevated vs Normal CVRF Exposures in Early Adulthood, Midlife, and Late Life.
(A) Early adulthood. (B) Midlife. (C) Late life. 3MS = modified Mini-Mental State Examination; CVRF = cardiovascular risk factor; DSST = Digit Symbol Substitution Test.
Midlife CVRF Exposure
The association of midlife CVRFs with cognitive outcomes was less consistent (figure 2B). Elevated BMI and TC in midlife were not associated with cognitive change in late life (p > 0.05 for both). Elevated SBP, compared to SBP in the normal range, was associated with greater decline on 3MS and DSST (10-year change in 3MS −0.91 points, 95% CI [−1.61 to −0.21] and DSST −1.76 points, 95% CI [−2.60 to −0.92]), and elevated FG, compared to FG in the normal range, was associated with greater cognitive decline on DSST (10-year change in DSST −2.79 points, 95% CI [−3.78 to −1.80]). After adjusting for the same CVRF exposures in the early and late life, midlife CVRFs were not associated with cognitive change in late life (p > 0.05).
Late-Life CVRF Exposure
With the exception of cholesterol, the associations of late-life CVRF exposures differed for cognitive change in early vs very late life (figure 2C). Late-life elevated BMI compared to normal BMI was associated with minimal declines in early late life (10-year change in 3MS < age 80: 0.54 points, 95% CI [−0.07 to 1.14] and DSST −1.49 points, 95% CI [−2.28 to −0.70]) but with cognitive gains in very late life (10-year change in 3MS ≥ age 80: 3.12 points, 95% CI [1.62–4.62] and DSST 3.36 points, 95% CI [1.26–5.47]). Similarly, late-life elevated SBP compared to SBP in the normal range was associated with greater cognitive decline in early late life (10-year change in 3MS −1.40 points, 95% CI [−1.98 to −0.82] and DSST −3.51 points, 95% CI [−4.29 to −2.74]) but gains in very late life (3MS 2.36 points, 95% CI [0.68–4.03] and DSST 4.67 points, 95% CI [2.33–7.00]). Late-life elevated FG was also associated with cognitive decline in early late life (10-year change in 3MS −1.74 points, 95% CI [−2.45 to −1.02] and DSST −4.30 points, 95% CI [−5.23 to −3.36]) and gains in very late life (3MS 3.10 points, 95% CI [1.37–4.83] and DSST 6.05 points, 95% CI [3.63–8.47]). These results and patterns persisted after adjusting for CVRF exposure at the other time periods.
Interactions With Race and Sex
We did not observe significant interactions between CVRF exposures and race or sex.
Potential for Survival Bias
In our assessment of the potential for survival bias, we found that lower 3MS and particularly DSST scores were associated with increased risk of death or loss to follow-up, but the CVRF TWAs were not, after adjustment for age, race, sex, and cohort, suggesting that the associations we find between the CVRF TWAs and cognitive function are not meaningfully affected by survival bias.
Discussion
Overall, we found that across the adult life course, elevated CVRFs, including elevated BMI, FG, and SBP, but not TC, were associated with greater cognitive decline in late life. This was particularly true for the early adult period while late-life CVRF exposures were associated with cognitive decline in early late life but with gains in very late life. Our findings are striking and novel in demonstrating that CVRF exposures in early life are associated with late-life cognitive change, even after accounting for exposures in mid and late life.
We addressed the challenge of measuring CVRF exposures across the life course by pooling 4 cohorts of young, middle-aged, and older participants, then using information across cohorts to impute early and midlife exposures for the older cohorts where cognitive decline is common. The magnitude of the effect size of early life CVRFs on cognitive decline was very large, with about 80%–100% greater decline, and in some cases even more. These results suggest that early adulthood may be a critical time for the association of CVRFs on late-life cognitive outcomes. Some studies have demonstrated an association between early adult CVRFs and worse cognitive function in midlife and risk of late-life dementia, albeit in smaller cohorts with heterogenous age groups and often limited to 1 or 2 CVRFs.21–24 Our results are also consistent with those from a British cohort study, which demonstrated that a higher vascular risk score across the life course was associated with decreased total brain volume and increased white matter hyperintensity volume in late life, with the strongest associations for vascular risk at age 36, the youngest time point at which vascular risk was measured.25 Taken together, these studies along with ours suggest that CVRFs in young adulthood have an important downstream relationship with cognition many decades later. Young adulthood represents a time that may lend itself to intervention and an opportunity for widespread public health education regarding the importance of heart health to brain health.
A substantial literature has established the association between elevated midlife CVRFs and worse cognitive outcomes in late life.4,15,16,26–29 We observed associations between midlife FG and SBP and greater late-life cognitive decline in models that only included midlife CVRF exposure; however, these exposures were not associated with cognitive decline in late life after adjusting for CVRF exposures at other time periods. It is possible that those with elevated early adult CVRFs are an especially high-risk subset of those with elevated CVRFs in midlife.30–32 These results are unexpected and suggest that while midlife interventions almost surely remain critically important, the role of CVRFs likely begin even earlier in the life course, and there may be a wider window that should be targeted for prevention.
The relationship of elevated late-life CVRFs with late-life cognitive health remains unclear. There is evidence, although controversial, to support our finding of protective associations between very late-life CVRF exposures and cognitive outcomes, including in studies of centenarians.9,11,33 Some studies have reported similar findings for blood pressure/hypertension exposure, with increased cognitive decline in middle and early late life but less or no decline in very late life.10,34,35 The Longitudinal Aging Study Amsterdam followed older adults for an average of 9 years and observed less decline with CVRFs including hypertension among the oldest old compared to young old.36 Other studies have also reported no association between diabetes and increased risk of cognitive decline and impairment in late age.9,37,38
It is unclear whether the associations we observed between late-life CVRFs and late-life cognitive gains could be causal or whether these results reflect effect–cause or survival bias. In particular, effect–cause artifacts, in which cognitive decline drive CVRF exposure or control, could explain the association of higher BMI with very late-life cognitive gains if participants experiencing substantial cognitive decline lose interest in eating or experience metabolic changes that result in weight loss.39 Alternatively, survival bias could result if mortality and losses to follow-up are increased among participants with greater late-life CVRF exposure and very late-life cognitive loss; however, our failure to find interactions between late-life CVRF exposures and cognitive function is evidence against this bias. Nonetheless, recent trial results from SPRINT MIND support intensive control of systolic blood pressure in older adults as a strategy to lower risk of mild cognitive impairment and dementia. Although SPRINT MIND findings suggest that treatment in late life is protective of cognitive health,40,41 our results and data from other observational cohorts suggest that caution is needed in aggressive blood pressure treatment in very old adults.42–44 Indeed, a recent Atherosclerosis Risk in Communities study highlights the importance of longitudinal/life course perspective in considering risk factors. The study found that the pattern of hypertension/hypotension in midlife and late life was significantly associated with risk of dementia, suggesting that overtreatment in late life might exacerbate risk.45
The pathways associated with SBP, FG, and BMI and cognitive decline in late life are likely multifactorial.4,5,46,47 Elevated CVRFs may increase inflammation and oxidative stress, disrupt cerebral blood flow and endothelial function, increase arterial stiffness, as well as impair the blood–brain barrier. Such injuries could contribute to increased cerebral small vessel disease and white matter damage. Furthermore, increasing evidence has shown that CVRFs also interact with amyloidogenic pathways and are associated with increased amyloid burden.47–49 In our pooled cohort study, we did not observe a significant association between cholesterol and cognitive decline, but evidence for cholesterol as a risk factor for cognitive decline is less robust compared to SBP, FG, and BMI.50 The effects of cholesterol may have also been reduced due to the likely mixture of non–Alzheimer disease and mixed pathologies in in our cohorts.
Our study leverages innovative epidemiologic methods to explore the life course association between CVRFs and late-life cognitive outcomes, but with important limitations. Imputed early and midlife exposures are known to be biased towards average levels, probably attenuating their associations with cognitive decline.14 In addition, TWAs were based on longitudinal studies requiring many years of follow-up, so that selection bias may also be a concern. We were able to consider 2 cognitive domains—processing speed and global cognition (which includes memory)—and did not assess other domains. Our test of global cognition, the 3MS, has limitations related to floor and ceiling effects, although it is one of the most commonly used tests of cognitive functioning in large epidemiologic studies. Lastly, although our models adjusted for birth year and cohort, there may be some residual cohort effects that we were not able to control for. Strengths include large sample size across the life course, diverse cohorts of both Black and White adults, and the ability to adjust for exposure at other life stages.
This study contributes to the critical and expanding body of evidence emphasizing the need for a life course framework to better understand cardiovascular and modifiable risk factors for cognitive and brain health. The majority of research in this field, particularly with regards to interventions, has focused on midlife and late-life periods; however, our findings suggest that attention should be broadened to consider early adult cardiovascular health. Increasing trends in diabetes and obesity even in early adulthood coupled with higher levels of underdiagnosed and undertreated CVRFs in younger ages6 could have significant public health implications for cognitive health. Furthermore, with the growing focus on modifiable and multidomain interventions for older adults,3 more research is needed to determine the effects of late-life CVRFs and their treatment on cognition in very late life.
Glossary
- 3MS
modified Mini-Mental State Examination
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults Study
- CHS
Cardiovascular Health Study
- CI
confidence interval
- CVRF
cardiovascular risk factor
- DSST
Digit Symbol Substitution Test
- FG
fasting glucose
- Health ABC
Health, Aging and Body Composition Study
- LMM
linear mixed model
- MESA
Multi Ethnic Study of Atherosclerosis
- SBP
systolic blood pressure
- TC
total cholesterol
- TWA
time-weighted average
Appendix. Authors
Study Funding
This work was supported by grants from the NIH, National Institute on Aging (1RF1AG054443 and K01AG047273). CARDIA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by CARDIA for scientific content. MESA was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). A full list of participating MESA investigators and institutions can be found at mesa-nhlbi.org. The CHS is supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the NHLBI, with additional contribution from the National Institute of Neurologic Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The Health ABC study is supported by NIA contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, NIA grant R01-AG028050, and NINR grant R01-NR012459. Health ABC was funded in part by the Intramural Research Program of the NIH, NIA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
K. Yaffe serves on DSMBs for Eli Lilly and a National Institute on Aging–sponsored trial, is a board member of Alector, and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. E. Vittinghoff, T. Hoang, K. Matthews, S.H. Golden, and A. Zeki Al Hazzouri report no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Institute of Medicine. Cognitive Aging: Progress in Understanding and Opportunities for Action. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 2.National Academies of Sciences Engineering, and Medicine. Preventing Cognitive Decline and Dementia: A Way Forward. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 3.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 4.Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol 2015;12:267–277. [DOI] [PubMed] [Google Scholar]
- 5.Testai FD, Gorelick PB. Vascular cognitive impairment and Alzheimer disease: are these disorders linked to hypertension and other cardiovascular risk factors? In: Aiyagari V, Gorelick PB, eds. Hypertension and Stroke: Pathophysiology and Management. Humana Press, Springer International Publishing: 2016; 261–284. [Google Scholar]
- 6.Benjamin Emelia J, Muntner P, Alonso A, et al. Heart disease and stroke statistics: 2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 7.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy R, Lawlor DA, Kuh D. A life course approach to cardiovascular aging. Future Cardiol 2015;11:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deckers K, Kohler S, van Boxtel M, Verhey F, Brayne C, Fleming J. Lack of associations between modifiable risk factors and dementia in the very old: findings from the Cambridge City over-75s cohort study. Aging Ment Health 2018;22:1272–1278. [DOI] [PubMed] [Google Scholar]
- 10.Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus study. J Am Geriatr Soc 2012;60:2014–2019. [DOI] [PubMed] [Google Scholar]
- 11.Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 2018;14:653–666. [DOI] [PubMed] [Google Scholar]
- 13.Liu K, Colangelo LA, Daviglus ML, et al. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels? The Coronary Artery Risk Development in Young Adults (CARDIA) study and the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc 2015;4:e002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeki Al Hazzouri A, Vittinghoff E, Zhang Y, et al. Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course. Int J Epidemiol 2019;48:1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopman D, Boland L, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001;56:42–48. [DOI] [PubMed] [Google Scholar]
- 16.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–281. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D, Coalson DL, Raiford SE. WAIS-III: Wechsler Adult Intelligence Scale. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- 18.Teng E, Chui H. The Modified Mini-Mental State Examination (3MS). Can J Psychiatry 1987;41:114–121. [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 20.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004;15:615–625. [DOI] [PubMed] [Google Scholar]
- 21.Rovio SP, Pahkala K, Nevalainen J, et al. Cardiovascular risk factors from childhood and midlife cognitive performance: the Young Finns Study. J Am Coll Cardiol 2017;69:2279–2289. [DOI] [PubMed] [Google Scholar]
- 22.Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen-Manheim I, Sinnreich R, Doniger GM, Simon ES, Pinchas-Mizrachi R, Kark JD. Fasting plasma glucose in young adults free of diabetes is associated with cognitive function in midlife. Eur J Public Health 2017;28:496–503. [DOI] [PubMed] [Google Scholar]
- 24.Vu THT, Zhao L, Liu L, et al. Favorable cardiovascular health at young and middle ages and dementia in older age: the CHA study. J Am Heart Assoc 2019;8:e009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane CA, Barnes J, Nicholas JM, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurol 2019;18:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol 2014;71:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawlings AM, Sharrett AR, Schneider ALC, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 2014;161:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. JAMA 1995;274:1846–1851. [PubMed] [Google Scholar]
- 29.González HM, Tarraf W, Harrison K, et al. Midlife cardiovascular health and 20-year cognitive decline: atherosclerosis risk in communities study results. Alzheimers Dement 2018;14:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son JS, Choi S, Kim K, et al. Association of blood pressure classification in Korean young adults according to the 2017 American College of Cardiology/American Heart Association guidelines with subsequent cardiovascular disease events. JAMA 2018;320:1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano Y, Reis JP, Colangelo LA, et al. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with cardiovascular events later in life. JAMA 2018;320:1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Vittinghoff E, Pletcher MJ, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol 2019;74:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin P, Gondo Y, Arai Y, et al. Cardiovascular health and cognitive functioning among centenarians: a comparison between the Tokyo and Georgia centenarian studies. Int Psychogeriatr 2019;31:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Euser SM, Van Bemmel T, Schram MT, et al. The effect of age on the association between blood pressure and cognitive function later in life. J Am Geriatr Soc 2009;57:1232–1237. [DOI] [PubMed] [Google Scholar]
- 35.Corrada MM, Hayden KM, Paganini-Hill A, et al. Age of onset of hypertension and risk of dementia in the oldest-old: the 90+ study. Alzheimers Dement 2017;13:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legdeur N, Heymans MW, Comijs HC, Huisman M, Maier AB, Visser PJ. Age dependency of risk factors for cognitive decline. BMC Geriatr 2018;18:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Berg E, de Craen AJM, Biessels GJ, Gussekloo J, Westendorp RGJ. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia 2006;49:2015–2023. [DOI] [PubMed] [Google Scholar]
- 38.Roberts RO, Knopman DS, Przybelski SA, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014;82:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suemoto CK, Gilsanz P, Mayeda ER, Glymour MM. Body mass index and cognitive function: the potential for reverse causation. Int J Obes 2015;39:1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The SPRINT MIND Investigators for the SPRINT Research Group. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The SPRINT MIND Investigators for the SPRINT Research Group. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019;322:524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streit S, Poortvliet RKE, Gussekloo J. Lower blood pressure during antihypertensive treatment is associated with higher all-cause mortality and accelerated cognitive decline in the oldest-old: data from the Leiden 85-plus Study. Age Ageing 2018;47:545–550. [DOI] [PubMed] [Google Scholar]
- 43.Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res 2007;19:41–47. [DOI] [PubMed] [Google Scholar]
- 44.Gottesman RF. Should hypertension Be treated in late life to preserve cognitive function? Hypertension 2018;71:787–792. [DOI] [PubMed] [Google Scholar]
- 45.Walker KA, Sharrett AR, Wu A, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA 2019;322:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorelick PB, Furie KL, Iadecola C, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villeneuve S, Jagust WJ. Imaging vascular disease and amyloid in the aging brain: implications for treatment. J Prev Alzheimers Dis 2015;2:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gottesman RF, Schneider ALC, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vemuri P, Knopman DS, Lesnick TG, et al. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol 2017;74:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anstey KJ, Ashby-Mitchell K, Peters R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimers Dis 2017;56:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data from the cohort studies used in this analysis are available from each study's respective coordinating centers. Specific policies governing each study's data and the process to access data can be found online (CARDIA: cardia.dopm.uab.edu/; MESA: mesa-nhlbi.org/; CHS: chs-nhlbi.org/; Health ABC: healthabc.nia.nih.gov/).