Abstract
Digital breast tomosynthesis (DBT) has been widely adopted in breast imaging in both screening and diagnostic settings. The benefits of DBT are well established. Compared with two-dimensional digital mammography (DM), DBT preferentially increases detection of invasive cancers without increased detection of in-situ cancers, maximizing identification of biologically significant disease, while mitigating overdiagnosis. The higher sensitivity of DBT for architectural distortion allows increased diagnosis of invasive cancers overall and particularly improves the visibility of invasive lobular cancers. Implementation of DBT has decreased the number of recalls for false-positive findings at screening, contributing to improved specificity at diagnostic evaluation. Integration of DBT in diagnostic examinations has also resulted in an increased percentage of biopsies with positive results, improving diagnostic confidence. Although individual DBT examinations have a longer interpretation time compared with that for DM, DBT has streamlined the diagnostic workflow and minimized the need for short-term follow-up examinations, redistributing much-needed time resources to screening. Yet DBT has limitations. Although improvements in cancer detection and recall rates are seen for patients in a large spectrum of age groups and breast density categories, these benefits are minimal in women with extremely dense breast tissue, and the extent of these benefits may vary by practice environment and by geographic location. Although DBT allows detection of more invasive cancers than does DM, its incremental yield is lower than that of US and MRI. Current understanding of the biologic profile of DBT-detected cancers is limited. Whether DBT improves breast cancer–specific mortality remains a key question that requires further investigation.
Introduction
Since its initial approval by the U.S. Food and Drug Administration (FDA) in 2011, digital breast tomosynthesis (DBT) has been rapidly integrated into routine mammographic screening and has been shown to improve overall screening performance compared with digital mammography (DM) (1–3). The improved cancer detection and reduced number of screening recall examinations have also been shown to be preserved with the use of synthetic mammography (SM) instead of DM (4,5). Similarly, increased accuracy and reader confidence have been demonstrated with the use of DBT in the diagnostic setting (6,7).
Yet there remain challenges and unanswered questions as DBT becomes increasingly incorporated into clinical practice, and a more nuanced understanding of clinical outcomes is needed. For example, despite known improvements in screening benchmarks with DBT, the benefits of the modality vary throughout screening rounds and according to patient breast density, and are reduced in fatty and extremely dense breasts. Although women with fatty breasts benefit from a lower recall rate with DBT, for those with extremely dense breast tissue, the benefit is minimal (1). Although there is growing evidence that the benefits of DBT are sustainable over time, the magnitude of these benefits likely varies (1,8). DBT has allowed increased detection of small node-negative invasive cancers by mitigating tissue obscuration, but its long-term effect on mortality is currently unknown. In addition, the role of DBT must be evaluated in the context of supplemental screening modalities such as US and MRI in women who undergo multimodality screening. Finally, there are ongoing efforts to improve the efficiency, efficacy, safety, and quality assurance of DBT. Although vendors of imaging equipment have galvanized continued technical innovation to improve accuracy, reduce radiation dose, and diversify their systems, standardization and quality assurance have also become increasingly challenging and complex.
Therefore, the purpose of this article is to provide an update on the state of the field by examining current practice guidelines, discussing emerging data, considering vendor diversification, and exploring technical advances and clinical implications. A better understanding of the current status and progress of DBT will help inform our future practice in the best use of this important technology to optimize patient care.
Current Scope
DBT gained rapid early acceptance on the basis of retrospective data that consistently showed improved detection of cancer (15%–53%) and decreased recall (15%–37%) rates (Table 1) (9–18). Despite validation of these results in prospective trials, a current lack of long-term outcomes and randomized trials has precluded the endorsement of DBT as the standard of care for evaluation for breast cancer in some clinical guidelines (19). Nevertheless, DBT has been rapidly disseminated in the United States, in part supported by consumer confidence and demand. According to the Mammography Quality Standards Act (MQSA) National Statistics (20), 71.5% of all certified breast imaging facilities and 41.1% of all accredited mammography units in the United States have DBT capability as of August 2020. Dedicated Current Procedural Terminology (CPT) codes for DBT became available in 2015, simplifying the billing issues that plagued the early clinical adoption of DBT and paving the way for further integration (21). However, worldwide adoption of DBT has been relatively slow, often with cost-effectiveness as a primary concern, particularly in parts of the world where resources are limited (22). This uneven adoption highlights the unresolved question of whether an upgrade from conventional two-dimensional mammography to DBT provides an advantage sufficient enough to save more lives.
Table 1:
Screening Outcomes with Combined DBT and DM Compared with DM Alone in Representative Retrospective Studies Published in 2013–2016
| Study and Year | No. of Examinations | Cancer Detection Rate | Recall Rate | |||||
|---|---|---|---|---|---|---|---|---|
| DBT/DM | DM | DBT/DM* | DM* | PValue | DBT/DM† | DM† | PValue | |
| Rose, 2013 (9) | 9499 | 13 856 | 5.4 | 4.0 | .07 | 5.5 | 8.7 | <.001 |
| Haas, 2013(10) | 6100 | 7058 | 5.7 | 5.2 | .7 | 8.4 | 12 | <.01 |
| Greenberg, 2014(11) | 23 149 | 54 684 | 6.3 | 4.9 | .0056 | 13.6 | 16.2 | <.0001 |
| McCarthy, 2014(12) | 15 571 | 10 728 | 5.5 | 4.6 | .02 | 8.8 | 10.4 | <.001 |
| Friedewald, 2014 (13) | 173 663 | 281 187 | 5.4 | 4.2 | <.001 | 9.1 | 10.7 | <.001 |
| Durand, 2015 (14) | 8591 | 9364 | 5.9 | 5.7 | .88 | 7.8 | 12.3 | <.0001 |
| Lourenco, 2015 (15) | 12 921 | 12 577 | 5.4 | 4.6 | .44 | 6.4 | 9.3 | <.0001 |
| McDonald, 2015 (16) | 15 571 | 10 728 | 5.4 | 4.6 | .41 | 8.8 | 10.4 | <.001 |
| Sharpe, 2016 (17) | 5703 | 80 149 | 5.4 | 3.4 | .0001 | 6.1 | 7.5 | <.018 |
| Conant, 2016 (18) | 559 998 | 142 883 | 5.9 | 4.4 | .0026 | 8.7 | 10.4 | <.0001 |
| All | 830 766 | 623 214 | … | … | … | … | … | … |
Note.—Numbers in parentheses are reference numbers. Study populations may overlap in studies from the same institution.
Number per 1000 examinations.
Numbers are percentages.
Screening Outcomes and Guidelines
Cancer Detection Rates
The initial evidence for screening with DBT emerged in retrospective studies (9–18) performed during early adoption of the technology into clinical practice (Table 1). Although these studies have consistently shown an increase in detection of cancer, particularly invasive cancer, the majority of data are from first-round screening only, and questions of overestimation and sustainability of the advantage of cancer detection with DBT have been raised. Emerging longitudinal data from prospective studies (2,23) are shedding light on longer-term use, but results are mixed. Although studies (8,24–26) have shown a continued benefit from the use of DBT over consecutive rounds of screening, the extent of this gain varies, and the expected increase in cancer detection is not significant in some studies.
Neither of the two studies (8,25) with the longest consecutive use of DBT to date (each reporting on more than five rounds of annual screening) showed a statistically significant increase in cancer detection. One study (8) in which cancer detection rates with DBT were compared with those for DM showed six versus 5.1 cancers per 1000 examinations, respectively (P = .25), and the other (27) showed cancer detection rates of 5.24 versus 4.6 per 1000 examinations, respectively (P = .37). Nevertheless, sustained preferential detection of invasive cancers is demonstrated in most studies across age groups and breast density categories (23,27). Women who underwent their first DBT examination had a higher yield of cancer than did those who underwent at least one prior DBT examination (6.1 vs 4.4–5.7 per 1000 examinations, respectively) (P < .05) (25). Similarly, more nuanced differences were also observed in women who underwent screening with varying combinations of DBT and DM over time. In a large population-based study (28), cancer detection rates in women who underwent consecutive biennial screening with DM after DM, DBT after DM, and DBT after DBT were 4.6, 9.9, and 8.3 per 1000 examinations, respectively. Outcomes incorporating SM are discussed later as a part of technical considerations.
Mortality Benefit
The ultimate goal of screening is to reduce breast cancer mortality. Although we assume DBT, at minimum, shows a reduction in breast cancer mortality similar to that seen in the large randomized controlled trials (29–32) in which screening mammography was evaluated, whether it confers further mortality benefit through detection of additional cancers when compared with DM is less clear. Therefore, a better understanding of the types of cancers detected at DBT is essential. Because DBT simultaneously accentuates detection of masses and architectural distortions (more often true-positive results), and decreases recall of patients with asymmetries that are often areas of tissue summation (more often false-positive results), it enhances both the sensitivity and specificity of cancer detection (Fig 1) (15). DBT has not been shown to increase detection of in situ cancers, mitigating the potential issue of overdiagnosis (2,33).
Figure 1.
Screening-detected grade 1 node-negative estrogen receptor positive (ER+) , progesterone receptor positive (PR+) human epidermal growth factor receptor 2 negative (HER2−) breast cancer in a 44-year-old woman. (a) Two-dimensional mammogram shows that the cancer is obscured. (b) Mediolateral oblique projection in-plane DBT image clearly shows the cancer (arrow), with improved lesion conspicuity and depiction of tumor margins.
Although DBT has been consistently shown to allow preferential detection of invasive cancers, most studies have also shown that these cancers may tend to be more indolent. Early data have shown, to a varying extent, that DBT-detected cancers are often smaller (<1 cm) than DM-detected cancers (42.0%–50.0% vs 33.1%–41.6%, respectively; P < .001), of lower histologic grade (grade 1, 37.9%–43.5% vs 25.6%–26.1%, respectively; P < .001), and less likely to be node positive (11.2% vs 19.0%) (23, 24, 28,34). Cancers detected only with DBT, in particular, are more likely to be grade 1 tumors (DBT-only vs all cancers, respectively, 41.7% vs 12.1%; P < .001), of the luminal A–like subtype (45.8% vs 13.5%, respectively; P < .001), and associated with low proliferative indices (Ki-67 < 14%) (45.8% vs 17.0%, respectively; P< .001) (35). However, these findings have not been consistently reproduced in the more recent longitudinal studies (2,8,24), and the full prognostic implications of DBT require further study. Although improved depiction of small spiculated masses with DBT optimizes visualization of the size and shape of tumors that are typical of more indolent cancers, not all cancers or spiculated masses detected with DBT are indolent (36).
In a prospective study (2) of women undergoing consecutive biennial screening examinations, those who underwent prior DBT screening were found to have significantly fewer grade 1 (but not grade 2 or 3) invasive cancers (DM after DBT vs DM after DM; 0.5 vs 1.3 per 1000 women; P = .001). However, interval cancer rates did not vary between the two groups, which suggests that there are potentially important biologic differences between the screening-detected and interval cancers (2).
Without data from randomized controlled trials with long-term follow-up, the interval cancer rate serves as a surrogate measure of screening efficacy and is an indirect indicator of mortality benefit. True interval cancers are aggressive tumors with rapid growth that outpaces the frequency of screening. Interval cancers are known to have poor survival outcomes (37,38). Early experience with DBT led to anticipation of a curtailed interval cancer rate, given the significant increase in detection of invasive cancers. However, no change in the interval cancer rate has been reflected in published results (2,8,24,33,39,40) so far, although the available studies may have been statistically underpowered, and more robust data are likely needed (Table 2).
Table 2:
Interval Cancer Rates at DBT and DM Screening in Studies Published in 2018–2020
| Study and Year | Screening Interval | Rate of Interval Cancer (No.)* | |||
|---|---|---|---|---|---|
| No. of Rounds | Frequency | DBT | DM | PValue | |
| Skaane, 2018 (39) | 2 | Biennial | 2.1 | 2.0 | .73 |
| Houssami, 2018 (40) | 2 | Biennial | 1.23 | 1.6 | NA |
| Bahl, 2018 (25) | 3 | Annual | 1.1 | 1.1 | .84 |
| Hovda, 2020 (2) | 2 | Biennial | 2 | 1.5 | .12 |
| Conant, 2020 (8) | 5 | Annual | 0.6 | 0.9 | .30 |
| Bernardi, 2020 (33) | 1 | Biennial | 1.1 | 1.36 | NA |
Note.—Numbers in parentheses are reference numbers. NA = not available.
Per 1000 examinations.
Studies (2,39) performed specifically to evaluate interval cancers have also reported no difference in tumor size, grade, nodal status, or histopathologic characteristics between interval cancers found after DBT or DM screening examinations with negative results. These findings suggest potential modality-based limitations of mammography intrinsic to anatomic imaging (as opposed to functional imaging). However, further studies with longer-term follow-up are needed, and a small number of large randomized controlled trials are currently underway, including the much anticipated Tomosynthesis Mammographic Imaging Screening Trial (TMIST) (41–43). Results from TMIST, which will compare reduction in the incidence of advanced cancers in each arm of the study, will help us understand the biologic profiles of DBT- and DM-detected breast cancers and their long-term outcomes and hence will allow better understanding of the effect of DBT (42).
Multimodality Screening
US is currently the most common supplemental imaging modality used in women with dense breast tissue because of its wide availability. Mammographic sensitivity, including DBT sensitivity, is limited in women with dense breasts, particularly in those with extremely dense breast tissue (1,44). Mammographic density is also an independent risk factor for developing breast cancer; women with extremely dense breasts have been shown to have a four- to sixfold higher risk of developing breast cancer when compared with women with fatty breasts (45). Multiple studies have shown that supplemental US screening after mammography (DM) can increase the cancer detection rate by 3.2–4.3 per 1000 women with dense breasts (46–48).
The incremental gain of US has also been shown to be sustainable throughout multiple years of screening. For example, the large prospective multicentered American College of Radiology Imaging Network (ACRIN) 6666 trial (48) showed incremental cancer detection rates of 5.3, 3.7, and 3.7 per 1000 women in years 1, 2, and 3. Similarly, in a retrospective study (49) performed after the Connecticut breast density legislation came into effect, US showed incremental cancer detection rates of 4.0, 3.3, 3.1, and 3.3 per 1000 women in years 1–4. The additional cancers detected with US have also tended to be invasive, small (median size, 9–10 mm) (48,50), and node negative (86%–96%) (51,52). In the large randomized controlled Japan Strategic Anti-cancer Randomized Trial (J-START) (52), supplemental US screening not only improved sensitivity (91.1% vs 77.0%; P = .0004) and early cancer detection (71.3% vs 52.0% of stage 0 or 1 cancers; P = .0194) but also significantly lowered the interval cancer detection rate (0.05% vs 0.10%; P = .034).
However, if DBT is used, the added benefit of supplemental US is likely lower. The prospective Adjunct Screening with Tomosynthesis or Ultrasound in Women with Mammography-Negative Dense Breasts (ASTOUND) trial to evaluate the incremental cancer yield of DBT versus US in mammography showed that DBT allowed detection of more than one-half of the additional cancers found with US in women with dense breasts and DM results that were negative for cancer, with fewer incremental false-positive results (DBT vs US, incremental cancer detection rates: 2.83–4 vs 4.9–7.1 per 1000 women; P = .006–.015; incremental false-positive recall rate: 0.3% vs 1.0%, respectively; P < .001) (53,54).
The added cancer detection with US is accompanied by an increase in false-positive findings and lower positive predictive values for biopsy compared with mammography, although the false-positive rate tends to improve in subsequent rounds of screening. In the ACRIN 6666 trial, the absolute increase in the false-positive rate was 15.1% during the initial round of screening when US was added to mammography, which decreased to 7.4% in subsequent rounds because of the availability of prior US examinations (48). In the same study, the positive predictive value for lesions detected only with US (hereafter, US-only lesions) was 38.1%, compared with 11.7% for mammography (48).
In addition, handheld US is operator dependent, which is reflected in variable cancer detection rates reported with technologist-versus physician-performed handheld US (2.5 vs 5.3 per 1000 examinations, respectively) (47,48,51,55). Although automated breast US may help to address operator dependence and streamline imaging, challenges include incomplete coverage of larger breasts, longer interpretation time due to the increased number of images, and automated breast US recalls that require immediate handheld US examination for further assessment. For all of these reasons, automated breast US is currently not widely implemented, and screening data are less robust compared with those of handheld US.
Finally, despite increased cancer detection with supplemental US in addition to mammography, approximately 8% of cancers are not detected with US or mammography (48), and the cost-effectiveness of supplemental US remains uncertain (56).
Therefore, parallel investigation of breast MRI has been ongoing to bridge the gap in screening with mammography and US. Despite the decreased tissue masking and increased visibility with DBT, the incremental gain in the cancer detection rate when compared with DM has been modest (0.7–2.7 per 1000 examinations), and sensitivity for detection of biologically aggressive tumor subtypes is limited (30%–40%) (13, 57,58).
Dynamic contrast-enhanced MRI, on the other hand, has been shown to allow preferential detection of more invasive and higher-grade tumors when compared with mammography, owing to the high sensitivity that is intrinsic to functional imaging (59). In the ACRIN 6666 study, the sensitivity of MRI in addition to DM and US was 100%, compared with 76% with DM and US and 52% with DM alone. Although US performed in addition to DM allowed 3.7 additional cancers per 1000 examinations to be detected, MRI allowed 14.8 additional cancers per 1000 examinations to be identified, nine of which were only detected with MRI (48). The supplemental cancer yield of MRI in addition to DM is also maintained when DBT is used. In a study (60) evaluating the performance of screening MRI in women at higher-than-average risk of breast cancer, there was no significant difference between the supplemental cancer detection rate of MRI after negative DM versus that after DBT (11 vs 16 cancers per 1000 examinations, respectively; P = .23) . Multiple studies (61–64) have shown that if MRI is performed in addition to mammography, US does not provide further cancer yield but significantly increases false-positive results. Therefore, there has been growing interest in using MRI to screen women with dense breast tissue to better overcome the limitations of mammography.
In a recent prospective trial (65) in women with extremely dense breast tissue (n = 40 373; age range, 50–75 years), the incremental cancer detection rate of supplemental MRI was 16.5 per 1000 women, which resulted in a 50% reduction in the interval cancer rate.
To expand the role of MRI in screening, abbreviated MRI has been developed to curtail time and cost and increase the feasibility of wider use. In a multicenter study evaluating women with dense breasts who underwent both DBT and abbreviated MRI in two rounds of annual screening (1444 women with heterogeneously or extremely dense breasts; age range, 40–75 years), abbreviated MRI allowed detection of significantly more invasive cancers than did DBT (11.8 vs 4.8 invasive cancers per 1000 women, respectively; P = .002), with no interval cancers observed during follow-up. However, while abbreviated MRI has higher sensitivity than does DBT in dense breasts (95.7% vs 39.1%, respectively), DBT retains higher specificity than that of abbreviated MRI (97.4% vs 86.7%, respectively) (66). Finally, an alternative to MRI and another promising technique that is under investigation for supplemental imaging is contrast-enhanced mammography, which has been shown to have similar sensitivity and specificity when compared with MRI and has been integrated with DBT, with the potential to improve characterization of lesions (67–69).
Screening Environment
Screening outcomes with DBT vary widely according to the screening environment. A meta-analysis (70) of DBT outcomes across continents that included a large number of participants (n = 1 009 790) showed significantly higher pooled incremental cancer detection rates in European and Scandinavian studies than those in U.S. studies (2.4 vs 1.1 cancers per 1000 examinations, respectively; P < .001), and a significantly higher reduction in recall rates in U.S. studies compared with those in European or Scandinavian studies (−2.9% vs 0.5%, respectively; P < .001). This illustrates that the effect of a technology is dependent on the context of its clinical environment. DBT improves the cancer detection rate to a greater extent in Europe and Scandinavia than it does in the United States, in part because of typically biennial screening in Europe and Scandinavia as opposed to annual screening in the United States. DBT reduces recall rates to a greater extent in the United States, where the baseline recall rates are relatively high (pooled baseline recall rates of the United States vs Europe, 11.3% vs 3.5%) (70).
Further differences likely also exist, given population differences in breast cancer prevalence. In the screening environment of the United States, no differences were shown with adoption of DBT when comparing practice types. A large prospective study in the United States (26), in which 104 radiologists throughout 53 facilities participated as a part of the Breast Cancer Surveillance Consortium (BCSC), showed that DBT improved recall rates similarly for both academic breast radiologists and private practice radiologists who interpret breast examinations. Improvements in the recall rate may vary according to the degree of DBT integration. Although most studies in the United States at institutions with full DBT integration show significantly decreased screening recall rates (8,25), there is evidence that DBT may not lead to a significant change in the recall rate in hybrid practice environments where both DBT and DM are used (71,72).
DBT Screening Guidelines
Despite the benefits of improved cancer detection and decreased recall, DBT is largely considered to be optional in current breast cancer screening guidelines because of insufficient data supporting improvement in disease-specific mortality.
The nuances of screening with DBT are implicit in the opening statement of the most recent version of the National Comprehensive Cancer Network (NCCN) guidelines for breast cancer screening (73): “The NCCN panel emphasizes adopting strategies and research to reduce the harm of screening (false positives and overdiagnosis) rather than raising the age to initiate screening to potentially delay these issues. This includes newer imaging modalities that improve the detection of breast cancer with fewer recalls (eg, tomosynthesis). Research to better define the biology of breast cancer is needed so that lesions that are not destined to progress are either not treated or are treated less aggressively.” Accordingly, the NCCN guidelines recommend that DBT be considered at annual screening mammography in women of average risk for cancer beginning at the age of 40 years, and in annual screening among high-risk women starting at the age of 30 years in the appropriate clinical scenario (73).
The American College of Radiology (ACR) Appropriateness Criteria (74) similarly recommend either DM or DBT as appropriate for routine screening. Furthermore, the European Commission Initiative on Breast Cancer (ECIBC) guidelines for breast cancer screening (2020) also deem the use of DBT as conditional. The Commission stresses that integration of DBT must be considered in the context of organized screening programs, with cost as a mandatory consideration, because resources vary throughout Europe. Radiation dose is also an additional mandatory consideration and can be mitigated with SM. Accordingly, either DBT or DM is currently recommended in women at average risk of cancer, who are invited to biennial screening starting at the age of 45 years, regardless of mammographic density; and concurrent use of DBT and DM is not recommended to minimize radiation (75). These guidelines are echoed in recommendations from the European Society of Breast Imaging, on the basis of similar questions of clinically relevant reduction in the interval cancer rate and cost-benefit concerns that are currently insufficiently evaluated (76).
Diagnostic Outcomes and Guidelines
Reducing Additional Views
The improved lesion conspicuity and depiction of margins that DBT offers have not only allowed better detection of cancer at screening, but have also helped to streamline diagnostic evaluation. With DBT, the need for additional views has decreased for noncalcified findings, although magnification views are still required to fully assess microcalcifications. Authors of multiple studies (6,77,78) have found improved accuracy in evaluation of noncalcified breast lesions with DBT when compared with two-dimensional spot compression views. This has been shown both in screening recalls and evaluation of symptoms (77). In a large 15-reader study (79) in the diagnostic setting, DBT was found to improve overall accuracy when compared with DM, and improved individual performance for 14 of 15 radiologists (area under the curve, 0.927 vs 0.872, respectively; P = .008). In this study, DBT not only reduced the need for additional views (χ2 = 17.63; P < .001), but also improved sensitivity, specificity, and positive and negative predictive values when compared with DM (0.93, 0.75, 0.64, 0.96 vs 0.9, 0.56, 0.49, 0.92, respectively) (79). Further studies have also shown that the use of DBT in the diagnostic setting has led to a reduced need for US and biopsy, which further simplifies workup (79,80). Therefore, diagnostic examinations with DBT are more expedient, more accurate, and lower in radiation dose when compared with DM, which both benefits the patient and improves clinical outcomes and workflow.
Positive Predictive Value of Biopsy
The likelihood of malignancy in lesions recommended for biopsy is higher after DBT than after DM diagnostic assessment. Multiple studies have found that diagnostic DBT leads to an improved positive predictive value for biopsy while maintaining the cancer detection rate and improving patient selection for biopsy (81,82). In a prospective study (81) of women (n = 30 933) who were recalled for additional imaging in the U.K. National Health Services Breast Cancer Screening Program, the use of DBT in diagnostic evaluation of recalled women decreased the biopsy rate from 69% to 36% and increased the positive biopsy rate from 24.9% to 47.7% (P < .001) . Similarly, in a single-institution study in which the performance metrics of diagnostic examinations before and after integration of DBT (22 883 DM examinations vs 22 824 DBT and DM examinations), the DBT group was found to have a lower abnormal interpretation rate and higher positive predictive value (39.6% vs 33.8%, respectively; P < .001), with DBT examinations associated with a significantly higher proportional invasive cancer yield (83.7% vs 72.3%, respectively; P < .001) (82).
The increase in positive predictive value at diagnostic assessment is, in part, a result of a downstream shift in lesion distribution at diagnostic examinations because of changes in screening recall with DBT.
Some studies (7,15) have shown a relative decrease in Breast Imaging Reporting and Database System (BI-RADS) 3 classifications and a concomitant increase in BI-RADS 1 or 2 and 4 or 5 classifications with the use of DBT, while others have shown no significant change in the rate of BI-RADS 3 classifications (83,84). However, in all studies (7,15,83,84), the authors found a significant decrease in the number of patients for whom short-interval follow-up was recommended after DBT. Hence, another advantage of DBT is that fewer follow-up studies result from diagnostic examinations, freeing up resources and improving examination cost-effectiveness.
Architectural Distortion
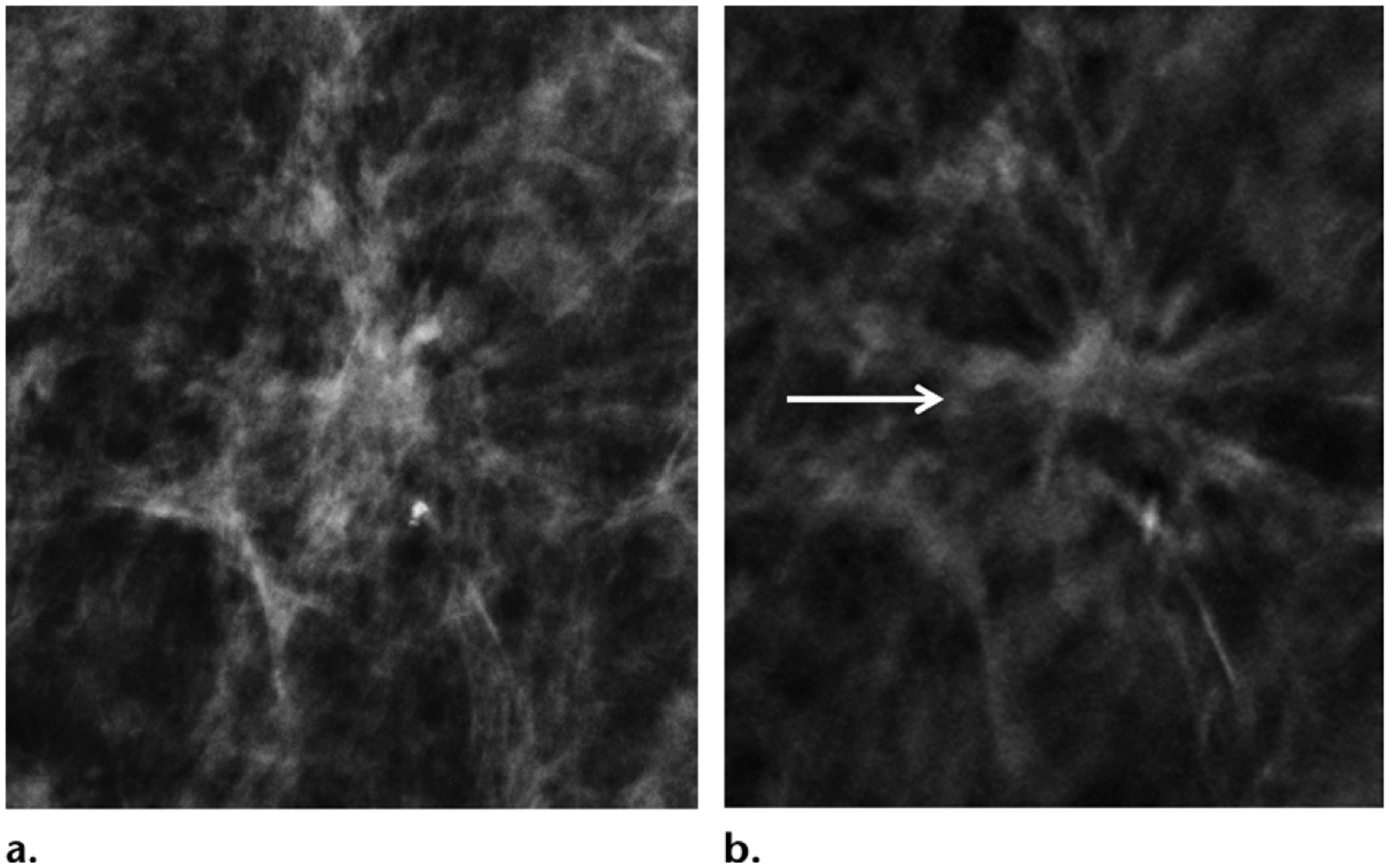
Architectural distortions are better visualized with DBT than with DM. Although DBT has decreased the overall recall rate, recall for architectural distortion has increased with the use of DBT (15). One study (84) showed that architectural distortion was approximately 50% more common with DBT than with DM (0.14% vs 0.07%, respectively; P < .001), thus increasing identification of both benign and malignant lesions. Unexplained architectural distortion in a patient without a history of surgery or trauma requires tissue diagnosis, because it is often associated with malignancy. However, multiple studies (84–86) have shown that not all architectural distortions are equal. For instance, architectural distortions detected only with DBT are less likely to be malignant than those also detected with DM (10.2% vs 43.4%, respectively; P < .001). Architectural distortions with US correlates are also more likely to be malignant than those without correlates (66.5% vs 29.2%, respectively; P < .001). However, architectural distortions detected only with DBT, with or without US correlates, still have substantial malignancy rates (12.1% vs 7.7%; P = .645) For these reasons, biopsy is usually appropriate for well-visualized architectural distortions, despite US findings. (86).
DBT detection of architectural distortions contributes to improved sensitivity for detection of invasive cancers, especially for invasive lobular cancers, which are historically elusive and often occult on DM images. Studies have shown that invasive lobular carcinomas are highly associated with architectural distortions (up to 87%) and are less likely to manifest as high-attenuating masses alone; therefore, they are well accentuated at DBT (87,88). Multiple studies (89–91) have shown a higher detection rate of invasive lobular carcinomas with DBT than that with DM. In addition, the literature shows higher accuracy in evaluation of the extent of the disease for invasive lobular carcinomas, which are frequently multifocal or multicentric and bilateral. On the other hand, DBT also contributes to increased detection and biopsy of nonmalignant architectural distortions, most commonly radial scars, which account for at least 33.2% of architectural distortions detected with DBT (84). The management of radial scars typically includes surgical excision because of a possibility of upgrading to malignancy at surgery, but surveillance may also be appropriate when factors such as sampling method, the presence of atypia, and radiologic-pathologic correlation are taken into consideration (92).
Workflow Efficiency
Efficiency is a common concern with DBT because of its increased interpretation time. DBT screening examinations have been shown to require 47% longer interpretation time than DM examinations (average minutes per study, 2.8 minutes vs 1.9 minutes, respectively, even for experienced readers) (93). Yet a concurrent increase in the number of screening examinations and a decrease in diagnostic examinations has also been observed with DBT (94). This redistribution of examinations is in keeping with the previously discussed decreased need for diagnostic evaluations due to decreased recall rates. Such a shift may result in an overall decrease in the number of more time-intensive practice-level diagnostic examinations, helping to balance out the longer interpretation times per case (93).
DBT Diagnostic Guidelines
DBT has been helpful in diagnostic assessment in practice settings with both full and partial integration of the modality for screening. For example, DBT has contributed to a significant increase in detection of architectural distortions, many of which are only visible with DBT (84). Screening recalls based on DM examinations in a hybrid practice also often benefit from DBT diagnostic workup with increased accuracy (95). Although DBT is not required for diagnostic evaluation of breast findings, it is a valuable tool when it is available. In fact, the 2020 European Commission Initiative on Breast Cancer (ECIBC) guidelines for screening recalls due to lesions suspected to be cancer in women at average risk suggest the use of DBT over that of DM, although DBT is not currently mandated for screening (75). This approach takes full advantage of the higher sensitivity and specificity associated with DBT but limits the scope of use to the diagnostic setting only, where the cancer yield is higher than that in the screening setting, and aims to optimize cost-effectiveness. In comparison, the current ACR Appropriateness Criteria (96) in the United States do not explicitly comment on use of DBT in the context of screening recall, but recommend either DM or DBT as appropriate for diagnostic evaluation in symptomatic women aged 30 years or older for common manifestations such as a palpable mass, nipple discharge, or focal noncyclical pain.
Technical Considerations
Synthetic Mammography
SM is derived from source projection images of tomosynthesis, which are rendered into a maximum intensity projection–like image to simulate two-dimensional digital mammography. The purpose of SM is to replace DM in the two-dimensional component of the DBT examination to curtail radiation exposure. This approach reduces the overall radiation dose by approximately one-half (97). In a recent study (98) in which the radiation dose per patient per screening examination in a real-life clinical workflow (two views per breast) was measured with the use of the Selenia Dimensions mammography system (Hologic), DBT and SM had a significantly lower entrance dose and mean glandular dose when compared with DM (entrance dose, 14.8 vs 21.8 mGy, respectively; P < .0001) (mean glandular dose, 3.84 vs 5.59 mGy, respectively; P < .0001) (98).
Overall clinical outcomes with SM have been promising, and improvement in cancer detection and reduction in recall rates with DBT are similar when DBT and DM are compared with DBT and SM (4,5). However, recent estimates also show that only approximately one-half of facilities with SM capability currently use DBT with SM without DM, suggesting persistent concerns about the technical limitations of SM (99).Compared with DM, SM has been shown to have significantly lower imaging resolution in phantom studies (SM vs DM, 5 vs 11 line pairs per millimeter) and may allow fine microcalcifications or small low-attenuation masses to be missed, despite better conspicuity of medium to large calcifications (100,101). This measurable loss of resolution has also been indirectly observed in the clinical setting, where decreased recall rates for microcalcifications and decreased detection of small-volume ductal carcinoma in situ have been reported, despite smaller reader studies that show otherwise (4,102–104). In particular, in a 5-year longitudinal study of DBT and DM in years 1–4 and DBT and SM in year 5, sensitivity with DBT was superior to that with DM in years 1–4 but decreased in year 5 to equal to that of DM (8). Whether this is a clinically significant loss is controversial. Some investigators (4,5) have argued that these findings may even be permissible in light of potential overdiagnosis, particularly in the larger context of overall maintained cancer detection and recall rates. It is possible that SM as a lower-resolution technique is nevertheless sufficient for adequate cancer depiction, and it is certainly worthwhile, given the substantial reduction in radiation. Further study with more robust data and longer-term follow-up is needed to determine if relevant disease may be missed with SM.
Vendor Diversification
The market share has been dominated by vendors who received early FDA approval, and the lion’s share of available clinical research outcomes of DBT are based on early systems in use. However, as of 2020, there are now four FDA-approved DBT systems in the United States. (Table 3). Although all vendors have achieved diagnostic quality imaging, each vendor has a unique technical approach, and systems vary in parameters including x-ray tube motion, filter material, detector material, pixel size, and pixel binning, as well as in reconstruction algorithms. Whether these differences result in clinical differences in cancer detection is unknown. However, in general, a wide-angle scan provides better depth resolution, and a narrow-angle scan improves in-plane resolution. On the other hand, wider angle scanning means that more projection images are acquired, which requires longer scanning time, rendering the examination more vulnerable to motion. In a phantom study (105) evaluating the performance of five DBT systems, although adequate geometric accuracy was achieved with all systems (within 0.5%), there were noticeable differences in technical image quality that were quantifiable by artifact extent, in-plane resolution, and the relative noise power spectrum among the DBT systems (Fig 2). These findings are consistent with visually perceivable differences in vendor-specific images in clinical practice (Figs 3–5). Although vendor diversification has promoted further integration of DBT technology, quality assurance and standardization have become more challenging yet increasingly necessary to ensure reproducible benefits in clinical practice. This is an active area of research, but DBT-specific quality assurance guidelines have been put in place by both the European Reference Organization for Quality Assured Breast Screening and Diagnostic Services (EUREF) and by the U.S. FDA Mammography Quality Standards Act (MQSA) (106,107).
Table 3:
FDA-approved DBT Systems in the United States
| Vendor | Tube Motion | Detector Angular Range | Scan Angle (degrees) | No. of Projections | Scan Time (sec) | Detector | Pixel Size (μm) | SM |
|---|---|---|---|---|---|---|---|---|
| Hologic | Continuous | Rotating (± 2.1°) | 15 | 15 | 4 | aSe | 140 (reg)/ 70 (HR) | C-view |
| GE | Step and shoot | Stationary | 25 | 9 | 10 | CsI-aSi | 100 | V-preview |
| Siemens | Continuous | Stationary | 50 | 25 | 22 | aSe | 85 | Insight-2D |
| Fuji | Continuous | Stationary | 15 (reg)/ 40 (HR) | 15 | 4 | aSe | 150 (reg)/ 100 (HR) | None |
Note.—aSe = amorphous selenium, CsI/aSi = cesium iodide/amorphous silicon, HR = high resolution, reg = regular.
Figure 2.
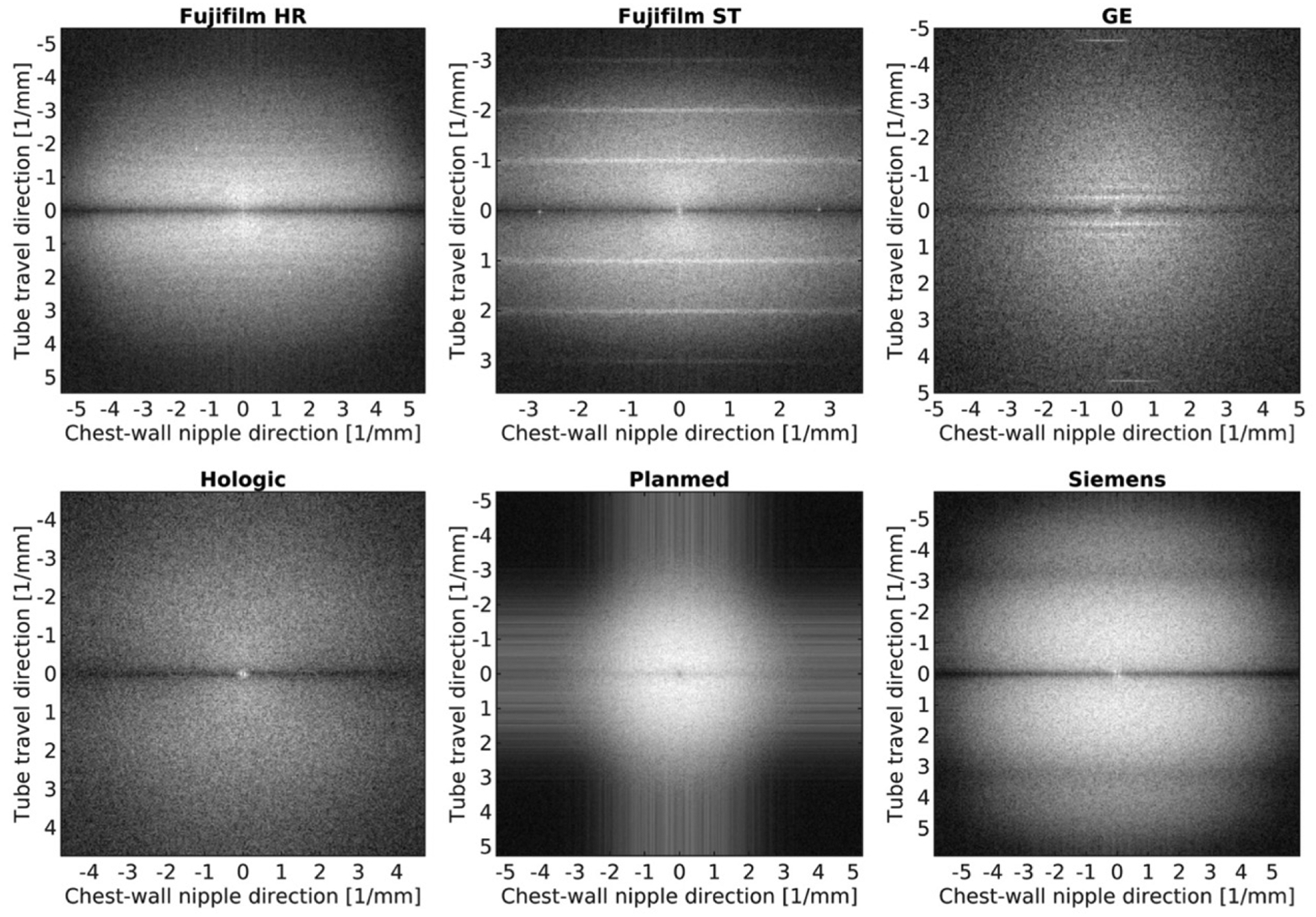
Images show distinct visual representations of the logarithm of two-dimensional noise power spectra calculated from the center of the reconstructed plane for each studied DBT system. (Reprinted, with permission, from reference 105.)
Figure 3.
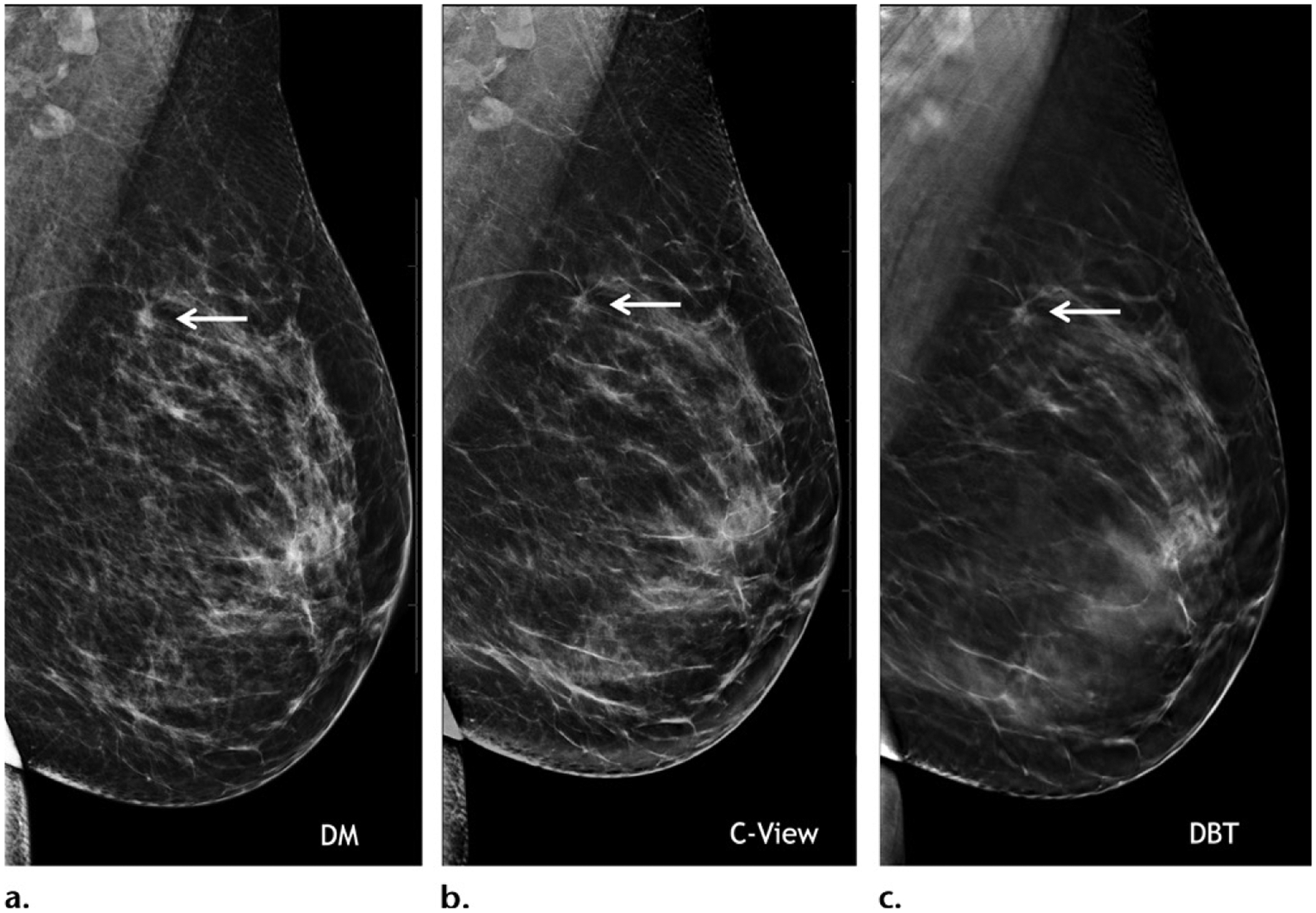
Images from the Hologic Dimensions DBT system, which was approved by the FDA in 2011. Mediolateral oblique two-dimensional mammogram (a), synthetic C-view image (b), and in-plane DBT image (c) of the left breast in a 40-year-old woman at baseline screening show diagnostic-quality images of a small spiculated mass (arrow) that was biopsy proven to be a grade 1 ER+/PR+/HER2− invasive ductal carcinoma.
Figure 5.

Images from the Siemens Mammomat Inspiration system, which was FDA approved in 2015. Mediolateral oblique DBT images at various depths through a normal left breast in a 50-year-old woman at a screening examination show diagnostic image quality.
Technical Innovations
DBT continues to advance with technical improvements. One of the intrinsic limitations of DBT is poor depth resolution due to limited angle sampling and nonisotropic imaging. For this reason, tumors may appear more conspicuous in one projection than in another (Fig 6). This may be improved with wide-angle tomography, which, when compared with narrow-angle tomography, not only has higher depth resolution, but also better blurring of out-of-plane structures, allowing sharper discrimination of masses. However, in-plane resolution is limited, and wide-angle DBT is inferior to narrow-angle DBT at depiction of calcifications.
Figure 6.
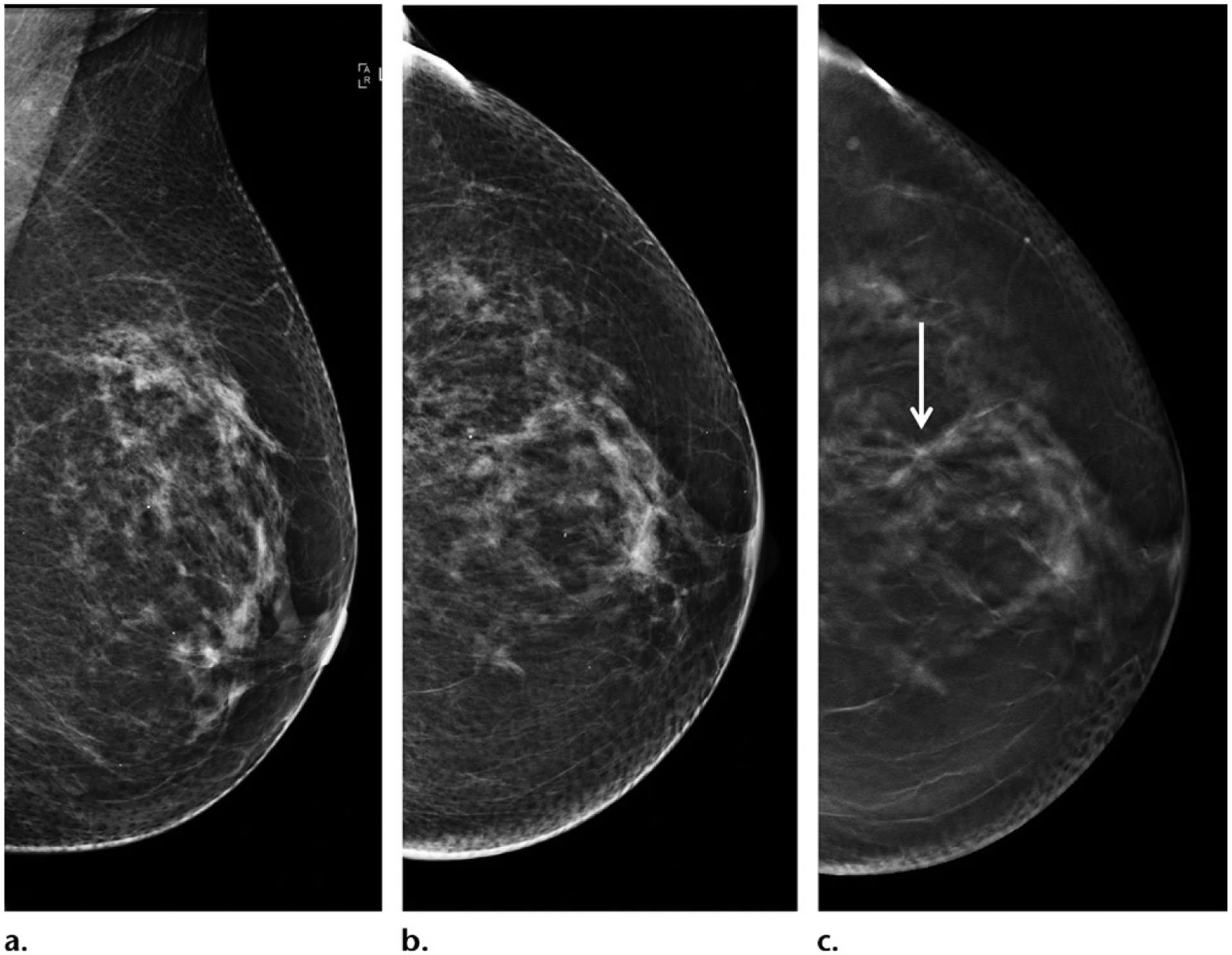
Small spiculated grade 1 node-negative ER+/PR+/HER2− invasive ductal carcinoma in a 62-year-old woman. (a, b) Mediolateral oblique (a) and craniocaudal (b) two-dimensional mammograms show that the tumor is occult. (c) In-plane craniocaudal DBT image shows that the tumor is visible (arrow), but it is occult on other DBT views (not shown). Note that cancers may be better seen or only seen in one projection at DBT because of nonisotropic imaging; therefore, findings suspected to be cancer on only one DBT view require complete workup.
Another approach to improving depth resolution is to improve overall image resolution, which is made possible by the recent development of high-resolution tomography with decreased pixel size from 140 μm to 70 μm. Interestingly, this may also mitigate the concern of loss of resolution with SM (Fig 7). SM derived from high-resolution tomography is expected to be able to show microcalcifications better than conventional SM does at the cost of a slightly higher dose of radiation. In addition, clinical data are needed for validation (Fig 8).
Figure 7.
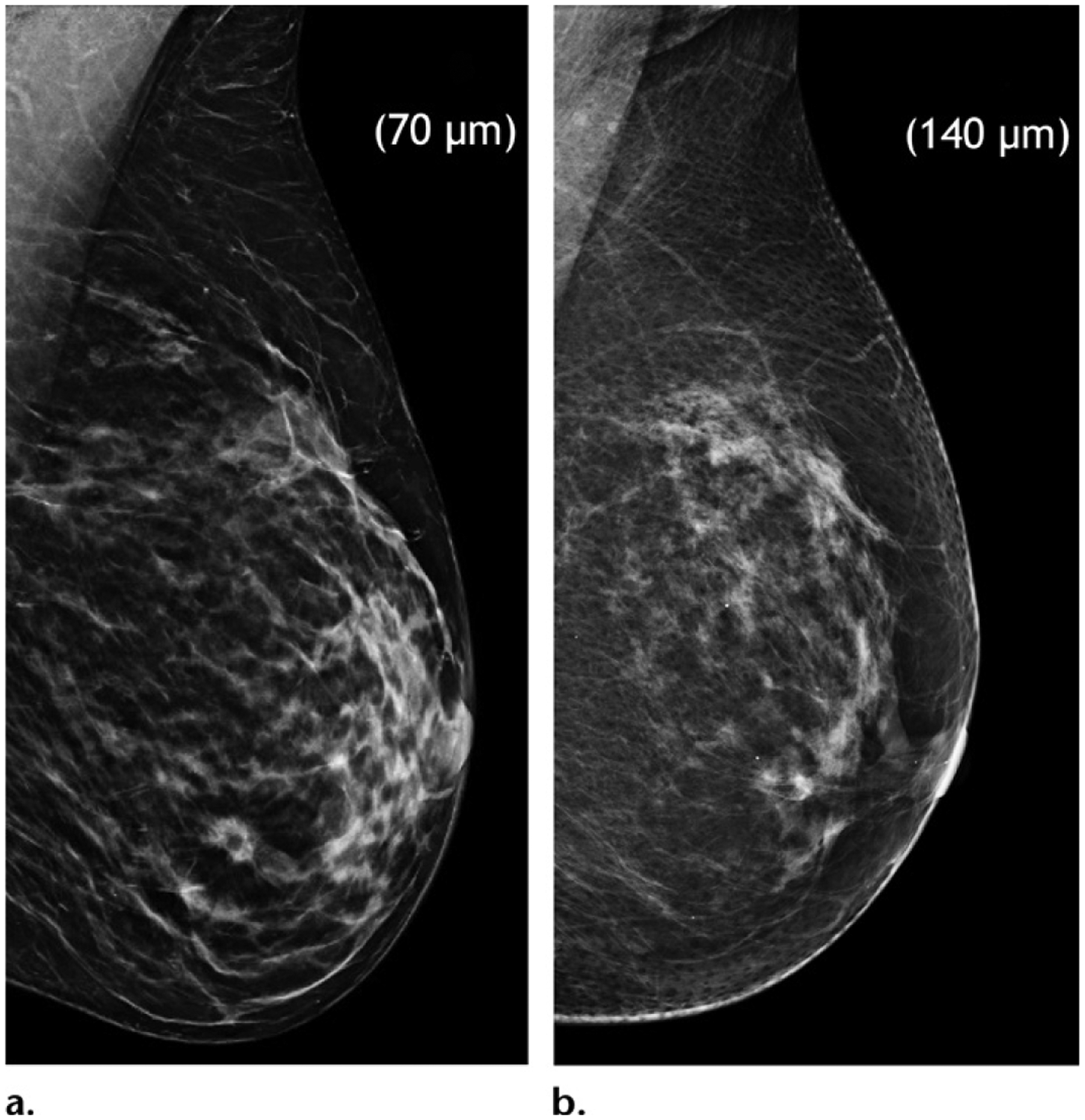
Juxtaposition of mediolateral oblique views of the left breast in two separate patients. High-resolution (70-μm pixel size) (a) and standard-resolution (140-μm pixel size) (b) SM images show that glandular details are seen to greater advantage on a than on b. High-resolution acquisition may compensate for the loss of resolution on SM images but is associated with a slightly higher dose of radiation.
Figure 8.
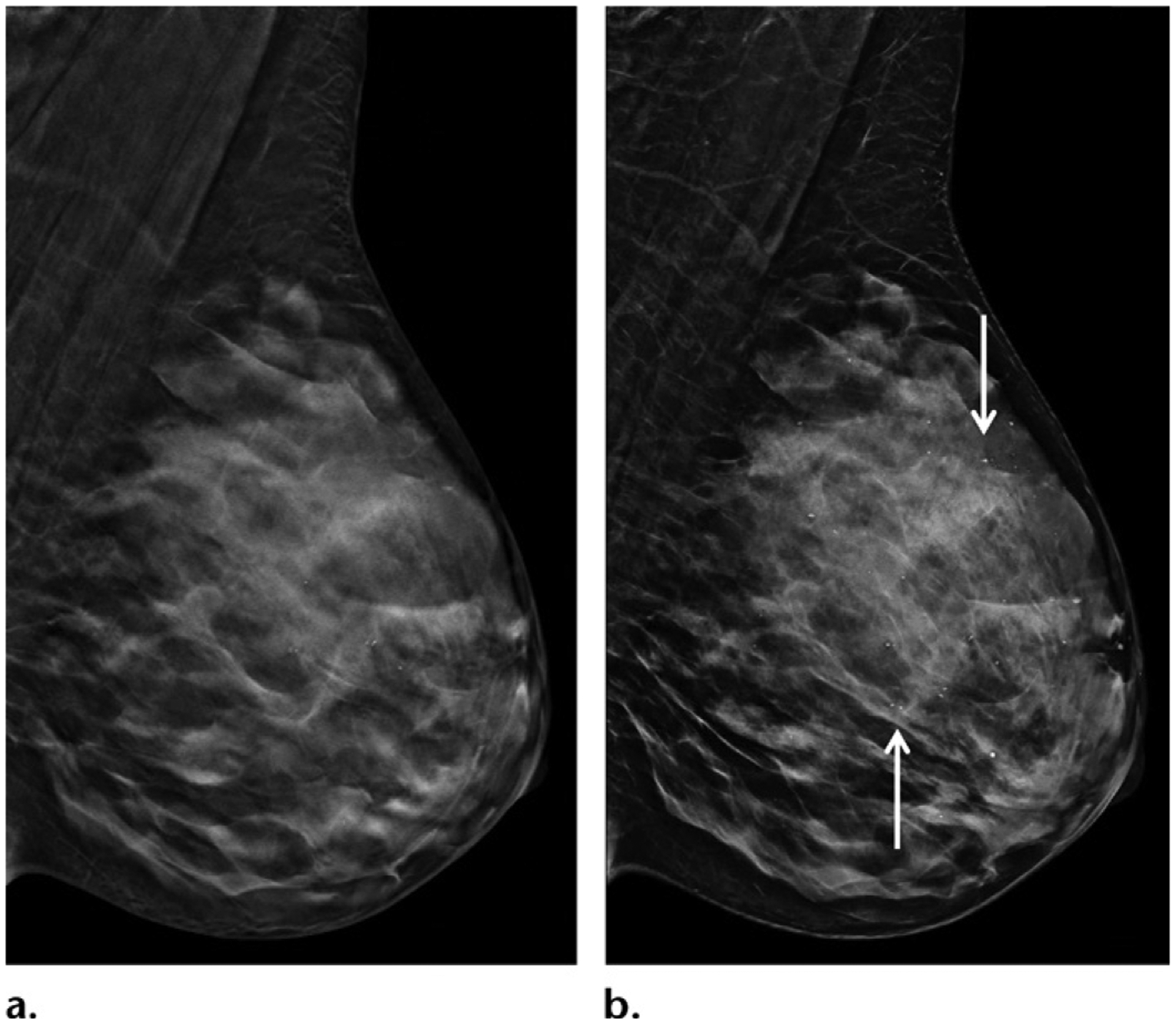
High-resolution DBT (a) and SM (b) images in mediolateral oblique projection of the left breast in a 45-year-old woman show excellent image quality because of improved resolution (70-μm pixel size). There are scattered punctate microcalcifications in the left breast, shown with great clarity and without significant artifacts on b (arrows).
The increased interpretation time of DBT may be addressed with slab viewing with adjustable thickness of the slab, allowing quicker coverage of the entire breast volume (Fig 9), although it is unknown how this approach may affect interpretative performance. Finally, artificial intelligence or deep learning–based computer-aided detection tools are now being used to improve both accuracy and efficiency, resulting in a decrease in reading time by 52.7% in one study (3). In addition to optimizing cancer detection, deep learning is also being investigated for topics such as quantification of breast density, radiomics analysis, risk stratification, and radiation reduction.
Figure 9.
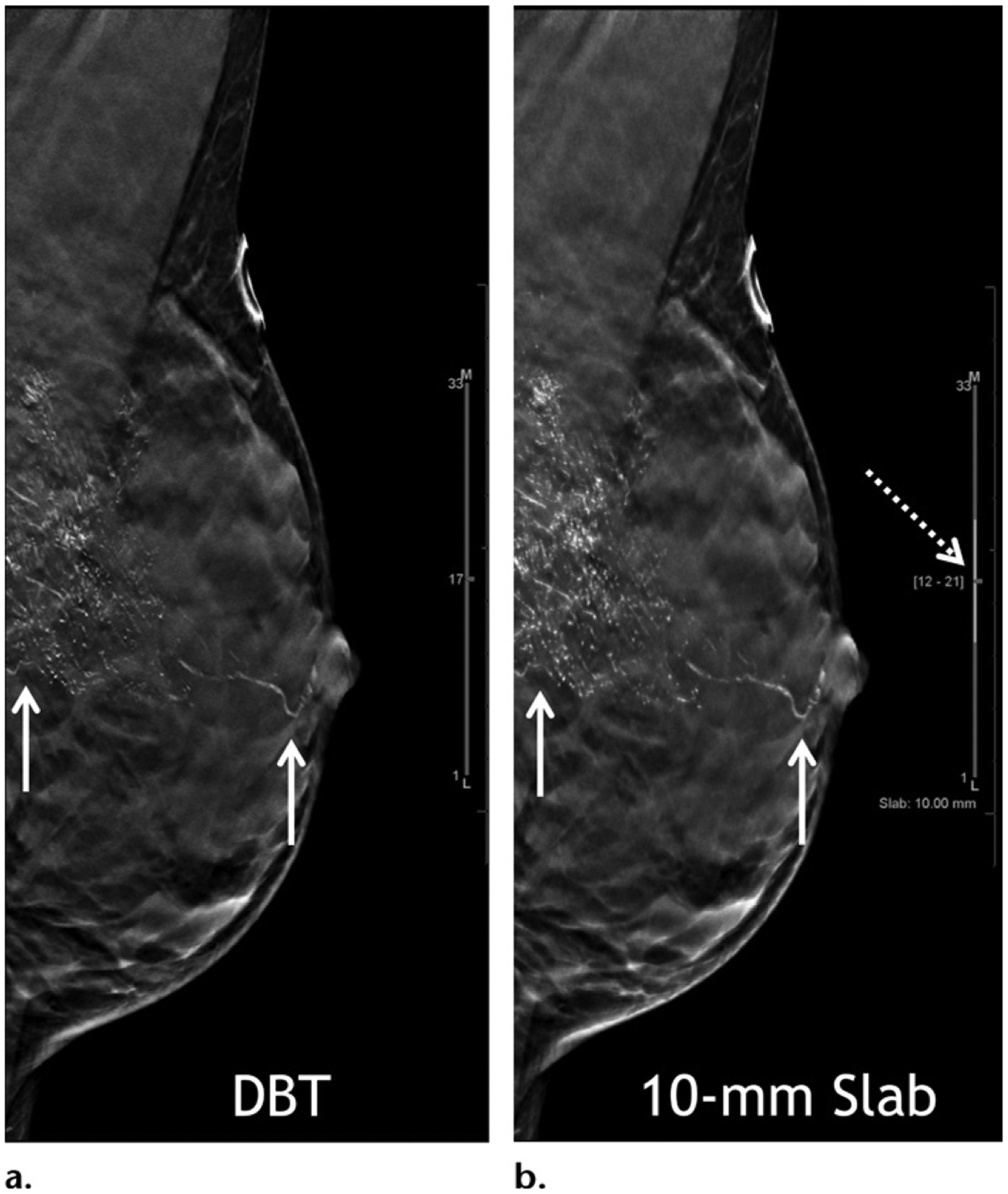
High-grade ductal carcinoma in situ at baseline screening in a 40-year-old woman. Mediolateral oblique in-plane DBT (a) and 10-mm slab (b) images show segmentally distributed fine linear branching calcifications in the left breast (solid arrows). Currently the standard section thickness for DBT reconstruction images is 1 mm. Slab viewing is possible (dashed arrow in b) with adjustable slab thickness to allow quicker review. In this patient, slab viewing also allows a maximum intensity projection–like capture of the overall extent of the abnormality. Stereotactic biopsy was performed and yielded a diagnosis of high-grade ductal carcinoma in situ.
DBT Lexicon
The DBT supplement to the 2013 ACR BI-RADS manual on mammography was published in 2019 to provide guidance on standardizing language and practice in the use of tomosynthesis in clinical care (108). Some terms are no longer deemed appropriate. For example, while tomosynthesis was frequently referred to as three-dimensional mammography, the term should no longer be used, because images are not truly three-dimensional owing to the z-axis being derived from planar data. The preferred terminology for tomosynthesis includes “DBT,” “tomosynthesis,” or “tomo.” Synthetic mammography should be referred to as “SM.” Finally, conventional two-dimensional (2D) full-field digital mammography may be referred to as either “DM” or “2D.” The document also provides guidance in interpretation. For global assessment of breast density, assessment should be made with standard DM or SM, but not with DBT sections. As with DM, classification of breast density should be based on the denser breast if the two breasts differ. For lesion location, standard nomenclature indicating the quadrant, clock-face location, depth, and distance from the nipple should be supplemented with specific in-plane section numbers to facilitate easy identification of abnormalities, particularly if they are only visible with DBT. Clarifications are also provided for certain lesion types in the context of DBT. Notably, an asymmetry is defined as a finding visible on only one mammographic (tomographic) projection. Recommendation is also made to carefully evaluate any circumscribed mass at DBT unless there are multiple bilateral circumscribed masses or long-term stability in the size and shape of masses over 2 years (Fig 10). Finally, full-field DM magnification views remain the standard for evaluation of microcalcifications.
Figure 10.
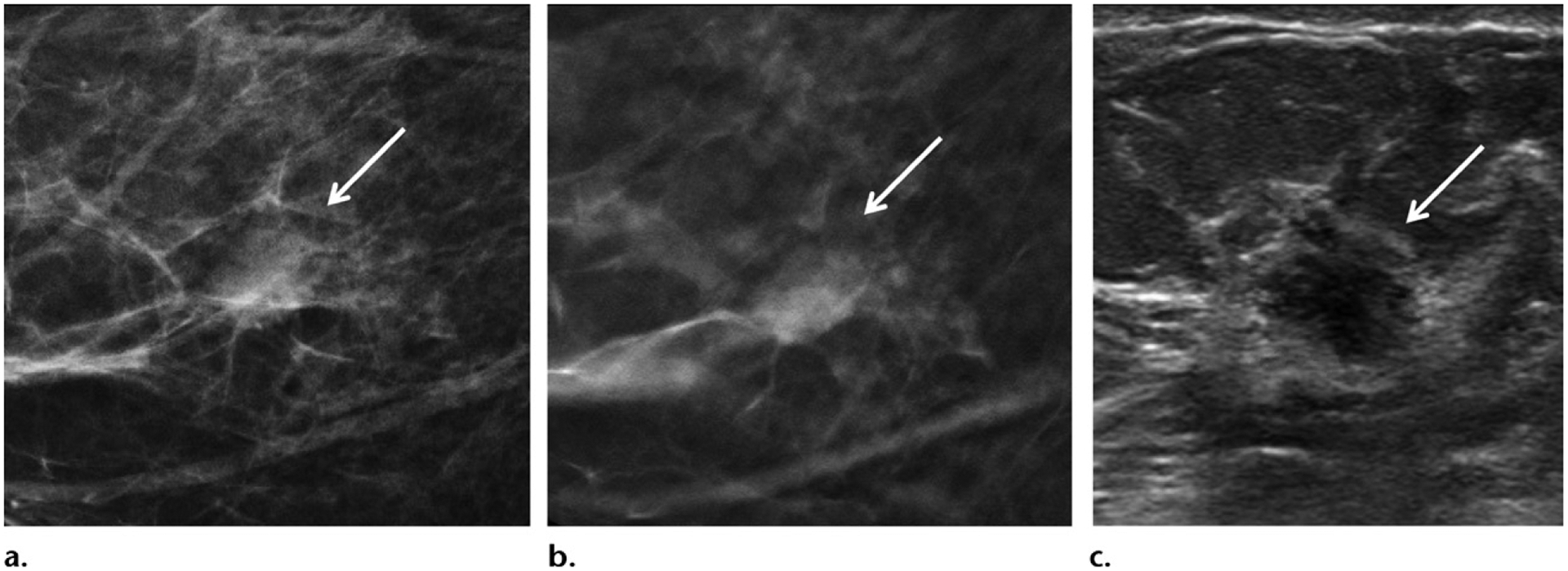
Invasive ductal carcinoma in a 56-year-old woman at screening mammography. (a, b) Craniocaudal projection two-dimensional mammogram (a) and in-plane DBT image (b) show scattered fibroglandular tissue and a circumscribed solitary right breast mass (arrow). (c) Subsequent US image shows a corresponding mass with irregular borders (arrow). Biopsy was performed and yielded a grade 2 ER+/PR+/HER2+ node-negative invasive ductal carcinoma.
Conclusion
DBT has shown sustainable improvement in detection of invasive cancer and recall rates, to varying extents, depending on the clinical environment. SM has allowed a significant decrease in radiation dose while maintaining the clinical benefits of DBT. Achieving greater accuracy at screening and diagnostic evaluation both increases cancer yield and decreases undue harm and anxiety associated with unnecessary recalls and biopsies. Although DBT increases detection of all invasive cancers, there is a trend toward detection of more indolent cancers, and long-term implications for breast cancer mortality are currently unknown. DBT is the latest technological advancement for mammography and is poised to become the standard of care in population-based screening. Further data are needed to better understand the full effect of DBT and to help define its place in the increasingly complex discipline of breast cancer care.
Figure 4.
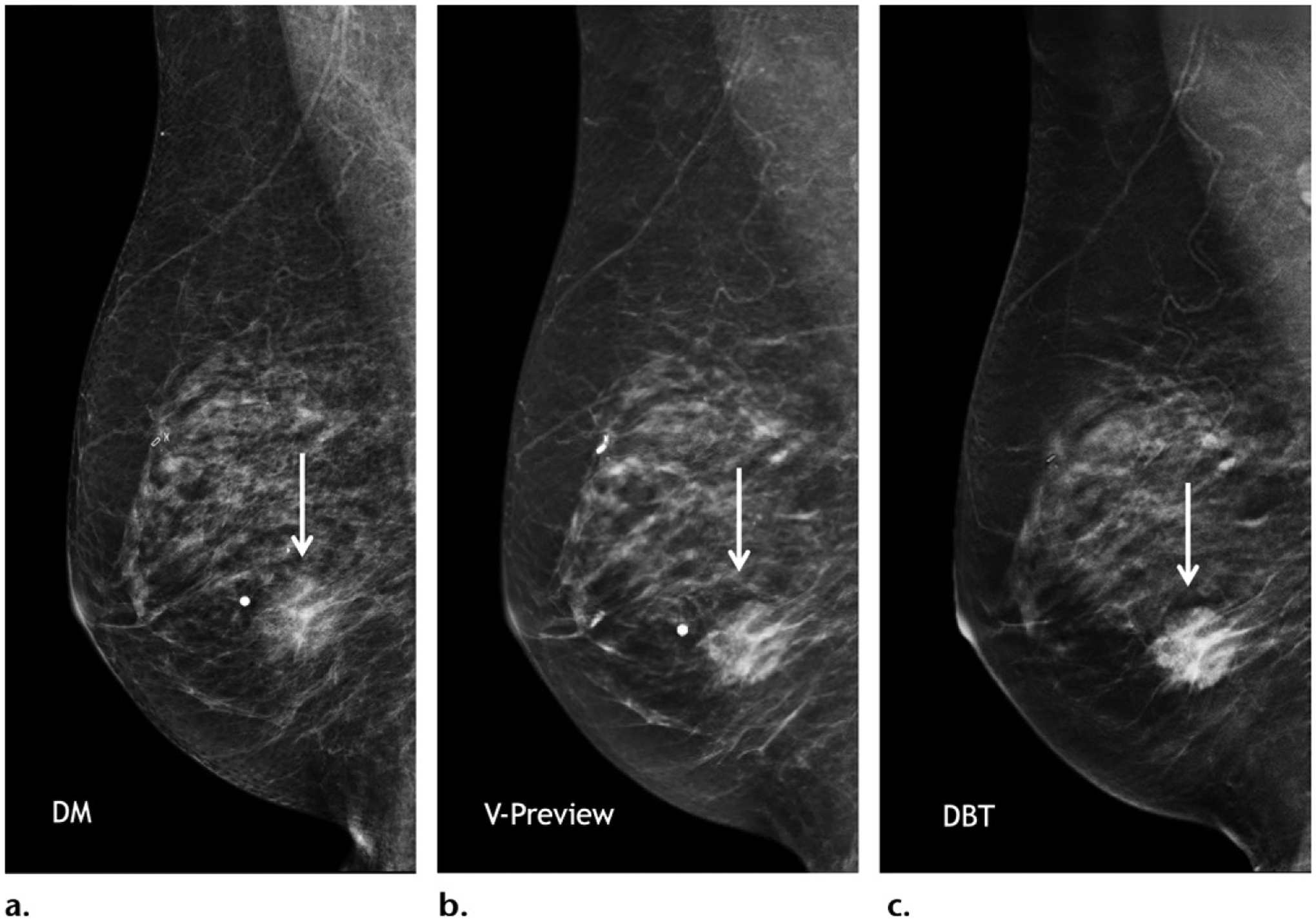
Images from the GE SenoClaire DBT system, which was approved by the FDA in 2014. Mediolateral oblique diagnostic-quality two-dimensional (a), synthetic V-preview (b), and DBT (c) images of the right breast in a 78-year-old woman at diagnostic imaging show an irregular mass in the inferior breast (arrow), which is consistent with a biopsy proven ER+/PR+/HER2− invasive lobular carcinoma.
SA-CME LEARNING OBJECTIVES.
After completing this journal-based SA-CME activity, participants will be able to:
Discuss clinical outcomes of digital breast tomosynthesis (DBT).
Explore DBT vendor diversification and clinical implications.
Describe technical advances and clinical utilities of DBT.
TEACHING POINTS.
The supplemental cancer yield of MRI in addition to DM is also maintained when DBT is used.
DBT improves the cancer detection rate to a greater extent in Europe and Scandinavia than it does in the United States, in part because of typically biennial screening in Europe and Scandinavia as opposed to annual screening in the United States. DBT reduces recall rates to a greater extent in the United States, where the baseline recall rates are relatively high.
Despite the benefits of improved cancer detection and decreased recall, DBT is largely considered to be optional in current breast cancer screening guidelines because of insufficient data supporting improvement in disease-specific mortality.
Architectural distortions detected only with DBT are less likely to be malignant than those also detected with DM (10.2% vs 43.4%, respectively; P < .001). Architectural distortions with US correlates are also more likely to be malignant than those without correlates (66.5% vs 29.2%, respectively; P < .001). However, architectural distortions detected only with DBT, with or without US correlates, still have substantial malignancy rates (12.1% vs 7.7%; P = .645) For these reasons, biopsy is usually appropriate for well-visualized architectural distortions, despite US findings.
A wide-angle scan provides better depth resolution, and a narrow-angle scan improves in-plane resolution.
Abbreviations:
- ACR
American College of Radiology
- ACRIN
American College of Radiology Imaging Network
- BI-RADS
Breast Imaging Reporting and Data System
- DBT
digital breast tomosynthesis
- DM
digital mammography
- ER
estrogen receptor
- FDA
Food and Drug Administration
- HER
human epidermal growth factor
- NCCN
National Comprehensive Cancer Network
- PR
progesterone receptor
- SM
synthetic mammography
Footnotes
Disclosures of Conflicts of Interest.—L.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: board membership for iCAD and Lunit, grants/grants pending from Siemens. Other activities: disclosed no relevant relationships.
References
- 1.Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open 2020;3(7):e2011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hovda T, Holen ÅS, Lång K, et al. Interval and consecutive round breast cancer after digital breast tomosynthesis and synthetic 2D mammography versus standard 2D digital mammography in BreastScreen Norway. Radiology 2020;294(2):256–264. [DOI] [PubMed] [Google Scholar]
- 3.Conant EF, Toledano AY, Periaswamy S, et al. Improving accuracy and efficiency with concurrent use of artificial intelligence for digital breast tomosynthesis. Radiol Artif Intell 2019;1(4):e180096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuckerman SP, Conant EF, Keller BM, et al. Implementation of synthesized two-dimensional mammography in a population-based digital breast tomosynthesis screening program. Radiology 2016;281(3):730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: The Oslo tomosynthesis screening trial. Radiology 2019;291(1):23–30. [DOI] [PubMed] [Google Scholar]
- 6.Zuley ML, Bandos AI, Ganott MA, et al. Digital breast tomosynthesis versus supplemental diagnostic mammographic views for evaluation of noncalcified breast lesions. Radiology 2013;266(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghu M, Durand MA, Andrejeva L, et al. Tomosynthesis in the diagnostic setting: Changing rates of BI-RADS final assessment over time. Radiology 2016;281(1):54–61. [DOI] [PubMed] [Google Scholar]
- 8.Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: Outcomes by screening year and round. Radiology 2020;295(2):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton R Jr. Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR Am J Roentgenol 2013;200(6):1401–1408. [DOI] [PubMed] [Google Scholar]
- 10.Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology 2013;269(3):694–700. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg JS, Javitt MC, Katzen J, Michael S, Holland AE. Clinical performance metrics of 3D digital breast tomosynthesis compared with 2D digital mammography for breast cancer screening in community practice. AJR Am J Roentgenol 2014;203(3):687–693. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy AM, Kontos D, Synnestvedt M, et al. Screening outcomes following implementation of digital breast tomosynthesis in a general-population screening program. J Natl Cancer Inst 2014;106(11):dju316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014;311(24):2499–2507. [DOI] [PubMed] [Google Scholar]
- 14.Durand MA, Haas BM, Yao X, et al. Early clinical experience with digital breast tomosynthesis for screening mammography. Radiology 2015;274(1):85–92. [DOI] [PubMed] [Google Scholar]
- 15.Lourenco AP, Barry-Brooks M, Baird GL, Tuttle A, Mainiero MB. Changes in recall type and patient treatment following implementation of screening digital breast tomosynthesis. Radiology 2015;274(2):337–342. [DOI] [PubMed] [Google Scholar]
- 16.McDonald ES, McCarthy AM, Akhtar AL, Synnestvedt MB, Schnall M, Conant EF. Baseline screening mammography: Performance of full-field digital mammography versus digital breast tomosynthesis. AJR Am J Roentgenol 2015;205(5):1143–1148. [DOI] [PubMed] [Google Scholar]
- 17.Sharpe RE Jr, Venkataraman S, Phillips J, et al. Increased cancer detection rate and variations in the recall rate resulting from implementation of 3D digital breast tomosynthesis into a population-based screening program. Radiology 2016;278(3):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conant EF, Beaber EF, Sprague BL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography compared to digital mammography alone: a cohort study within the PROSPR consortium. Breast Cancer Res Treat 2016;156(1):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siu AL; U.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164(4):279–296. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration MQSA Mammography Quality Standards Act National Statistics (as of August 2020). https://www.fda.gov/radiation-emitting-products/mqsainsights/mqsa-national-statistics. Accessed August 18, 2020.
- 21.Silva E 3rd. How to code tomosynthesis. J Am Coll Radiol 2015;12(1):15. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, Babb JS, Toth HK, Moy L, Heller SL. Digital breast tomosynthesis practice patterns following 2011 FDA approval: A survey of breast imaging radiologists. Acad Radiol 2017;24(8):947–953. [DOI] [PubMed] [Google Scholar]
- 23.Conant EF, Barlow WE, Herschorn SD, et al. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol 2019;5(5):635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahl M, Gaffney S, McCarthy AM, Lowry KP, Dang PA, Lehman CD. Breast cancer characteristics associated with 2D digital mammography versus digital breast tomosynthesis for screening-detected and interval cancers. Radiology 2018;287(1):49–57. [DOI] [PubMed] [Google Scholar]
- 25.Bahl M, Mercaldo S, Dang PA, McCarthy AM, Lowry KP, Lehman CD. Breast cancer screening with digital breast tomosynthesis: Are initial benefits sustained? Radiology 2020;295(3):529–539. [DOI] [PubMed] [Google Scholar]
- 26.Miglioretti DL, Abraham L, Lee CI, et al. Digital breast tomosynthesis: Radiologist learning curve. Radiology 2019;291(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahl M, Pinnamaneni N, Mercaldo S, McCarthy AM, Lehman CD. Digital 2D versus tomosynthesis screening mammography among women aged 65 and older in the United States. Radiology 2019;291(3):582–590. [DOI] [PubMed] [Google Scholar]
- 28.Hovda T, Brandal SHB, Sebuødegård S, et al. Screening outcome for consecutive examinations with digital breast tomosynthesis versus standard digital mammography in a population-based screening program. Eur Radiol 2019;29(12):6991–6999. [DOI] [PubMed] [Google Scholar]
- 29.Tabár L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260(3):658–663. [DOI] [PubMed] [Google Scholar]
- 30.Tabár L, Fagerberg CJ, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet 1985;1(8433):829–832. [DOI] [PubMed] [Google Scholar]
- 31.Tabár L, Vitak B, Chen HH, et al. The Swedish Two-County Trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am 2000;38(4):625–651. [DOI] [PubMed] [Google Scholar]
- 32.Moss SM, Wale C, Smith R, Evans A, Cuckle H, Duffy SW. Effect of mammographic screening from age 40 years on breast cancer mortality in the UK Age trial at 17 years’ follow-up: a randomised controlled trial. Lancet Oncol 2015;16(9):1123–1132. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi D, Gentilini MA, De Nisi M, et al. Effect of implementing digital breast tomosynthesis (DBT) instead of mammography on population screening outcomes including interval cancer rates: Results of the Trento DBT pilot evaluation. Breast 2020;50:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofvind S, Hovda T, Holen ÅS, et al. Digital breast tomosynthesis and synthetic 2D mammography versus digital mammography: Evaluation in a population-based screening program. Radiology 2018;287(3):787–794. [DOI] [PubMed] [Google Scholar]
- 35.Kim JY, Kang HJ, Shin JK, et al. Biologic profiles of invasive breast cancers detected only with digital breast tomosynthesis. AJR Am J Roentgenol 2017;209(6):1411–1418. [DOI] [PubMed] [Google Scholar]
- 36.Dang PA, Wang A, Senapati GM, et al. Comparing tumor characteristics and rates of breast cancers detected by screening digital breast tomosynthesis and full-field digital mammography. AJR Am J Roentgenol 2020;214(3):701–706. [DOI] [PubMed] [Google Scholar]
- 37.Houssami N, Hunter K. The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. NPJ Breast Cancer 2017;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilliland FD, Joste N, Stauber PM, et al. Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer Inst 2000;92(9):743–749. [DOI] [PubMed] [Google Scholar]
- 39.Skaane P, Sebuødegård S, Bandos AI, et al. Performance of breast cancer screening using digital breast tomosynthesis: results from the prospective population-based Oslo Tomosynthesis Screening Trial. Breast Cancer Res Treat 2018;169(3):489–496. [DOI] [PubMed] [Google Scholar]
- 40.Houssami N, Bernardi D, Caumo F, et al. Interval breast cancers in the ‘screening with tomosynthesis or standard mammography’ (STORM) population-based trial. Breast 2018;38:150–153. [DOI] [PubMed] [Google Scholar]
- 41.Pattacini P, Nitrosi A, Giorgi Rossi P, et al. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: The Reggio Emilia Tomosynthesis Randomized Trial. Radiology 2018;288(2):375–385. [DOI] [PubMed] [Google Scholar]
- 42.Pisano ED. Is tomosynthesis the future of breast cancer screening? Radiology 2018;287(1):47–48. [DOI] [PubMed] [Google Scholar]
- 43.Lee C, McCaskill-Stevens W. Tomosynthesis mammographic Imaging Screening Trial (TMIST): An invitation and opportunity for the National Medical Association Community to shape the future of precision screening for breast cancer. J Natl Med Assoc 2020;S0027(20):30121. [DOI] [PubMed] [Google Scholar]
- 44.Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016;315(16): 1784–1786. [DOI] [PubMed] [Google Scholar]
- 45.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15(6):1159–1169. [DOI] [PubMed] [Google Scholar]
- 46.Nothacker M, Duda V, Hahn M, et al. Early detection of breast cancer: benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer 2009;9:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corsetti V, Houssami N, Ferrari A, et al. Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost. Eur J Cancer 2008;44(4):539–544. [DOI] [PubMed] [Google Scholar]
- 48.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307(13):1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weigert JM. The Connecticut Experiment; The Third Installment: 4 Years of Screening Women with Dense Breasts with Bilateral Ultrasound. Breast J 2017;23(1):34–39. [DOI] [PubMed] [Google Scholar]
- 50.Buchberger W, Niehoff A, Obrist P, DeKoekkoek-Doll P, Dünser M. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR 2000;21(4):325–336. [DOI] [PubMed] [Google Scholar]
- 51.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008;299(18):2151–2163 [Published correction appears in JAMA 2010;303(15):1482.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet 2016;387(10016):341–348. [DOI] [PubMed] [Google Scholar]
- 53.Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: Interim report of a prospective comparative trial. J Clin Oncol 2016;34(16):1882–1888. [DOI] [PubMed] [Google Scholar]
- 54.Tagliafico AS, Mariscotti G, Valdora F, et al. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2). Eur J Cancer 2018;104:39–46. [DOI] [PubMed] [Google Scholar]
- 55.Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology 2014;272(1):12–27. [DOI] [PubMed] [Google Scholar]
- 56.Sprague BL, Stout NK, Schechter C, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med 2015;162(3):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013;14(7):583–589. [DOI] [PubMed] [Google Scholar]
- 58.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013;267(1):47–56. [DOI] [PubMed] [Google Scholar]
- 59.Sung JS, Stamler S, Brooks J, et al. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology 2016;280(3):716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roark AA, Dang PA, Niell BL, Halpern EF, Lehman CD. Performance of screening breast MRI after negative Full-Field Digital Mammography versus after negative Digital Breast Tomosynthesis in women at higher than average risk for breast cancer. AJR Am J Roentgenol 2019;212(2):271–279. [DOI] [PubMed] [Google Scholar]
- 61.Riedl CC, Luft N, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol 2015;33(10):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Zelst JCM, Mus RDM, Woldringh G, et al. Surveillance of women with the BRCA1 or BRCA2 mutation by using biannual automated breast US, MR imaging, and mammography. Radiology 2017;285(2):376–388. [DOI] [PubMed] [Google Scholar]
- 63.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351(5):427–437. [DOI] [PubMed] [Google Scholar]
- 64.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005;23(33):8469–8476. [DOI] [PubMed] [Google Scholar]
- 65.Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 2019;381(22):2091–2102. [DOI] [PubMed] [Google Scholar]
- 66.Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs Digital Breast Tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA 2020;323(8):746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewin J Comparison of contrast-enhanced mammography and contrast-enhanced breast MR imaging. Magn Reson Imaging Clin N Am 2018;26(2):259–263. [DOI] [PubMed] [Google Scholar]
- 68.Huang H, Scaduto DA, Liu C, et al. Comparison of contrast-enhanced digital mammography and contrast-enhanced digital breast tomosynthesis for lesion assessment. J Med Imaging (Bellingham) 2019;6(3):031407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chou CP, Lewin JM, Chiang CL, et al. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis--Comparison to contrast-enhanced breast MRI. Eur J Radiol 2015;84(12):2501–2508. [DOI] [PubMed] [Google Scholar]
- 70.Marinovich ML, Hunter KE, Macaskill P, Houssami N. Breast Cancer Screening Using Tomosynthesis or Mammography: A Meta-analysis of Cancer Detection and Recall. J Natl Cancer Inst 2018;110(9):942–949. [DOI] [PubMed] [Google Scholar]
- 71.Giess CS, Pourjabbar S, Ip IK, Lacson R, Alper E, Khorasani R. Comparing diagnostic performance of digital breast tomosynthesis and full-field digital mammography in a hybrid screening environment. AJR Am J Roentgenol 2017;209(4):929–934. [DOI] [PubMed] [Google Scholar]
- 72.Cochon LR, Giess CS, Khorasani R. Comparing Diagnostic Performance of Digital Breast Tomosynthesis and Full-Field Digital Mammography. J Am Coll Radiol 2020;17(8):999–1003. [DOI] [PubMed] [Google Scholar]
- 73.National Comprehensive Cancer Network (NCCN) Guidelines. Breast Cancer Screening and Diagnosis; Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf. Updated May 17, 2019. Accessed April 27, 2020.
- 74.Expert Panel on Breast Imaging; Mainiero MB, Moy L, et al. ACR Appropriateness Criteria® Breast Cancer Screening. J Am Coll Radiol 2017;14(11S):S383–S390. [DOI] [PubMed] [Google Scholar]
- 75.The European Commission Initiative on Breast Cancer (ECIBC) Guidelines for Breast Cancer Screening. https://healthcare-quality.jrc.ec.europa.eu/european-breast-cancerguidelines. Updated January 27, 2020. Accessed April 27, 2020.
- 76.Sardanelli F, Fallenberg EM, Clauser P, et al. Mammography: an update of the EUSOBI recommendations on information for women. Insights Imaging 2017;8(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waldherr C, Cerny P, Altermatt HJ, et al. Value of one-view breast tomosynthesis versus two-view mammography in diagnostic workup of women with clinical signs and symptoms and in women recalled from screening. AJR Am J Roentgenol 2013;200(1):226–231. [DOI] [PubMed] [Google Scholar]
- 78.Noroozian M, Hadjiiski L, Rahnama-Moghadam S, et al. Digital breast tomosynthesis is comparable to mammographic spot views for mass characterization. Radiology 2012;262(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mall S, Noakes J, Kossoff M, et al. Can digital breast tomosynthesis perform better than standard digital mammography work-up in breast cancer assessment clinic? Eur Radiol 2018;28(12):5182–5194. [DOI] [PubMed] [Google Scholar]
- 80.Hakim CM, Chough DM, Ganott MA, Sumkin JH, Zuley ML, Gur D. Digital breast tomosynthesis in the diagnostic environment: A subjective side-by-side review. AJR Am J Roentgenol 2010;195(2):W172–W176. [DOI] [PubMed] [Google Scholar]
- 81.Sharma N, McMahon M, Haigh I, Chen Y, Dall BJG. The potential impact of digital breast tomosynthesis on the benign biopsy rate in women recalled within the UK Breast Screening Programme. Radiology 2019;291(2):310–317. [DOI] [PubMed] [Google Scholar]
- 82.Bahl M, Mercaldo S, Vijapura CA, McCarthy AM, Lehman CD. Comparison of performance metrics with digital 2D versus tomosynthesis mammography in the diagnostic setting. Eur Radiol 2019;29(2):477–484. [DOI] [PubMed] [Google Scholar]
- 83.McDonald ES, McCarthy AM, Weinstein SP, Schnall MD, Conant EF. BI-RADS category 3 comparison: Probably benign category after recall from screening before and after implementation of digital breast tomosynthesis. Radiology 2017;285(3):778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography. AJR Am J Roentgenol 2017;209(5):1162–1167. [DOI] [PubMed] [Google Scholar]
- 85.Alshafeiy TI, Nguyen JV, Rochman CM, Nicholson BT, Patrie JT, Harvey JA. Outcome of architectural distortion detected only at breast tomosynthesis versus 2D mammography. Radiology 2018;288(1):38–46. [DOI] [PubMed] [Google Scholar]
- 86.Vijayaraghavan GR, Newburg A, Vedantham S. Positive Predictive Value of Tomosynthesis-guided Biopsies of Architectural Distortions Seen on Digital Breast Tomosynthesis and without an Ultrasound Correlate. J Clin Imaging Sci 2019;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grubstein A, Rapson Y, Morgenstern S, et al. Invasive lobular carcinoma of the breast: Appearance on digital breast tomosynthesis. Breast Care (Basel) 2016;11(5):359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chamming’s F, Kao E, Aldis A, et al. Imaging features and conspicuity of invasive lobular carcinomas on digital breast tomosynthesis. Br J Radiol 2017;90(1073):20170128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yun SJ, Ryu CW, Rhee SJ, Ryu JK, Oh JY. Benefit of adding digital breast tomosynthesis to digital mammography for breast cancer screening focused on cancer characteristics: a meta-analysis. Breast Cancer Res Treat 2017;164(3):557–569. [DOI] [PubMed] [Google Scholar]
- 90.Mariscotti G, Durando M, Houssami N, et al. Digital breast tomosynthesis as an adjunct to digital mammography for detecting and characterising invasive lobular cancers: a multi-reader study. Clin Radiol 2016;71(9):889–895. [DOI] [PubMed] [Google Scholar]
- 91.Garlaschi A, Calabrese M, Zaottini F, et al. Influence of Tumor Subtype, Radiological Sign and Prognostic Factors on Tumor Size Discrepancies Between Digital Breast Tomosynthesis and Final Histology. Cureus 2019;11(10):e6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Conlon N, D’Arcy C, Kaplan JB, et al. Radial scar at image-guided needle biopsy: Is excision necessary? Am J Surg Pathol 2015;39(6):779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dang PA, Freer PE, Humphrey KL, Halpern EF, Rafferty EA. Addition of tomosynthesis to conventional digital mammography: effect on image interpretation time of screening examinations. Radiology 2014;270(1):49–56. [DOI] [PubMed] [Google Scholar]
- 94.Lee CI, Zhu W, Onega TL, et al. The effect of digital breast tomosynthesis adoption on facility-level breast cancer screening volume. AJR Am J Roentgenol 2018;211(5):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peppard HR, Nicholson BE, Rochman CM, Merchant JK, Mayo RC 3rd, Harvey JA. Digital breast tomosynthesis in the diagnostic setting: Indications and clinical applications. RadioGraphics 2015;35(4):975–990. [DOI] [PubMed] [Google Scholar]
- 96.The American College of Radiology (ACR) Appropriateness Criteria. Breast cancer screening; Breast pain; Evaluation of nipple discharge; Palpable breast masses; https://acsearch.acr.org/list?_ga=2.52037290.248576019.1588076672-822975246.1588076672. Updated 2017–2018. Accessed April 28, 2020.
- 97.Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015;24(2):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi Y, Woo OH, Shin HS, Cho KR, Seo BK, Choi GY. Quantitative analysis of radiation dosage and image quality between digital breast tomosynthesis (DBT) with two-dimensional synthetic mammography and full-field digital mammography (FFDM). Clin Imaging 2019;55:12–17. [DOI] [PubMed] [Google Scholar]
- 99.Zuckerman SP, Sprague BL, Weaver DL, Herschorn SD, Conant EF. Survey results regarding uptake and impact of synthetic digital mammography with tomosynthesis in the screening setting. J Am Coll Radiol 2020;17(1 Pt A):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nelson JS, Wells JR, Baker JA, Samei E. How does c-view image quality compare with conventional 2D FFDM? Med Phys 2016;43(5):2538–2547. [DOI] [PubMed] [Google Scholar]
- 101.Petropoulos AE, Skiadopoulos SG, Karahaliou AN, Messaris GAT, Arikidis NS, Costaridou LI. Quantitative assessment of microcalcification cluster image quality in digital breast tomosynthesis, 2-dimensional and synthetic mammography. Med Biol Eng Comput 2020;58(1):187–209. [DOI] [PubMed] [Google Scholar]
- 102.Gilbert FJ, Tucker L, Gillan MG, et al. Accuracy of digital breast tomosynthesis for depicting breast cancer subgroups in a UK retrospective reading study (TOMMY Trial). Radiology 2015;277(3):697–706. [DOI] [PubMed] [Google Scholar]
- 103.Lai YC, Ray KM, Lee AY, et al. Microcalcifications detected at screening mammography: Synthetic mammography and digital breast tomosynthesis versus digital mammography. Radiology 2018;289(3):630–638. [DOI] [PubMed] [Google Scholar]
- 104.Dodelzon K, Simon K, Dou E, et al. Performance of 2D synthetic mammography versus digital mammography in the detection of microcalcifications at screening. AJR Am J Roentgenol 2020;214(6):1436–1444. [DOI] [PubMed] [Google Scholar]
- 105.Sundell VM, Jousi M, Hukkinen K, Blanco R, Mäkelä T, Kaasalainen T. A phantom study comparing technical image quality of five breast tomosynthesis systems. Phys Med 2019;63:122–130. [DOI] [PubMed] [Google Scholar]
- 106.EUREF - European Reference Organisation for Quality Assured Breast Screening and Diagnostic Services. Breast Tomosynthesis Quality Control Protocol (version 1.03); https://www.euref.org/european-guidelines/physico-technical-protocol. Updated March 2018. Accessed April 28, 2020.
- 107.U.S. FDA Mammography Quality Standards Act (MQSA) - Digital Breast Tomosynthesis (DBT) System requirements. https://www.fda.gov/radiation-emitting-products/facility-certification-and-inspection-mqsa/digital-breasttomosynthesis-dbt-system. Updated November 28, 2017. Accessed April 28, 2020
- 108.Lee CH, Destounis SV, Friedewald SM, Newell MS. Digital Breast Tomosynthesis (DBT) Guidance - A Supplement to ACR BI-RADS Mammography 2013. https://www.acr.org/-/media/ACR/Files/RADS/BI-RADS/BI-RADS-Digital-Breast-Tomosynthesis-Supplement.pdf. Updated 2019. Accessed April 28, 2020.


