Abstract
Background
We explored the outcome of convalescent plasma (CP) treatment in patients with moderate and severe coronavirus disease 2019 (COVID-19) and investigated variables for the design of further trials in Indonesia.
Methods
Hospitalised patients with moderate (n = 5) and severe (n = 5) COVID-19 were recruited and transfused with CP from donors who recovered from mild (n = 5), moderate (n = 5), or severe (n = 1) COVID-19. Neutralising antibodies (NAbs) to the virus were measured at the end of the study using a surrogate virus neutralisation test as an alternative to the plaque reduction assay. Clinical improvement was assessed based on the modified World Health Organization Research and Development Blueprint six-point scale, Brixia Chest-X-Ray scoring, and laboratory parameters. The study was registered at ClinicalTrials.gov (NCT04407208).
Findings
CP transfusion in three doses of 3 mL/kg of recipient body weight at 2-day intervals was well tolerated. Good clinical improvement was achieved in all patients with moderate disease and in two patients with severe disease. Most patients at baseline had detectable NAbs with median inhibition rates comparable to those of the donors (90·91% vs. 86·31%; p = 0·379). This could be due to the unavailability of pre-donation NAb testing and postponed CP administration that required communal consent.
Interpretation
This study highlights the safety of CP therapy. Although improvements were observed, we could not conclude that the outcomes were solely due to CP treatment. Further randomised controlled trials that cover different disease stages with pre-donation NAb measurements using locally applicable strategies are warranted.
Funding
The study was supported by PT Bio Farma, Indonesia.
Keywords: COVID-19, coronavirus, SARS-CoV-2, convalescent plasma, moderate, severe;LMIC, low- and middle-income countries, Indonesia
Research in context.
Evidence before this study
Convalescent plasma (CP) has been the subject of increasing expectation for treating coronavirus disease 2019 (COVID-19). Reports on CP transfusion have shown promising clinical improvements without serious adverse events. Most studies focused on reporting CP treatment in patients with severe COVID-19, but only a few addressed benefits on less severe disease. The vast majority of studies reporting COVID-19 infection and treatment have come from earlier affected countries with established health systems and research infrastructure, while very few are from low- and middle-income countries (LMICs). Nonetheless, CP therapy could be one of the few available options in LMICs where constraints may exist in the access to novel treatments, even once available. Clinical trials conducted in LMICs may differ in many respects from those in high-income countries.
Added value of this study
This study indicated the safety and potential benefit of CP therapy for patients with COVID-19. Measurement of NAb titres against SARS-CoV-2 in CP units before transfusion is key to ensure adequate antibody levels. As an alternative of the plaque reduction assay, a surrogate virus neutralization test was used to detect and quantify NAb titres. Lower NAb inhibition rates were found in CP donors who previously had mild COVID-19 compared to those with moderate or severe disease. Most CP-receiving patients had high NAb titres before CP transfusion. This could be due to the late timing of CP treatment that was given after endogenous antibodies had been synthesized. Simple tools such as the chest X-ray scoring system are helpful for assessing and monitoring lung involvement in COVID-19. In LMICs, community leaders need to be involved to educate how people view risks and benefits of research, thus affecting decision making to participate in the trial.
Implications of all the available evidence
The use of surrogate methods as alternatives for conventional neutralization tests is important in selecting potential donors in LMICs. Recruitment of recovered patients who had more severe disease and storage of plasma samples at -20oC for later NAb testing would be a solution for quick deployment of CP. Late administration of CP in the course of disease did not provide better benefits to the patients. Further well-designed randomized trials of CP treatment that cover the full spectrum of illness, from mild disease to complete recovery or death, are warranted. This study underscores the importance of community engagement and taking seriously the characteristics of local structures and values in LMICs to obtain supports for the trials.
Alt-text: Unlabelled box
1. Introduction
Following the first cases identified in Wuhan, China, in December 2019, the epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has rapidly spread worldwide [1]. The disease, named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO), was declared a global pandemic on March 11, 2020 [2]. While China and high-income countries (HICs) in the northern part of Europe and North America experienced major outbreaks in the first quarter of 2020, low- and middle-income countries (LMICs) in Latin America and the southern part of Asia began to see spikes in cases in early April 2020 [3,4].
Currently, no effective treatments are approved for COVID-19 [5]. Convalescent plasma (CP), a form of passive antibody immunotherapy, has been used to treat 1918 influenza, severe acute respiratory syndrome, pandemic 2009 influenza A (H1N1), avian influenza A (H5N1), Ebola, and other viral infections, and was associated with reduced mortality [6,7]. Initial case series of critically ill patients with COVID-19 treated with CP did not show serious adverse events amongst the participants, with some showing good improvements. However, most studies lacked adjustments for critical confounders, including co-treatments, baseline characteristics, disease severity, and timing of administration.
Since these initial studies, multiple trials have been initiated and reported. However, data from several larger studies, albeit supporting the safety of CP, did not show the overall efficacy of this treatment. A randomised control trial from China, which only included patients with severe or life-threatening COVID-19, was terminated early and was considered underpowered to define significant differences between the treatment and control groups [8]. Two studies from the Netherlands and Spain were also terminated prematurely―the Dutch study because interim analysis concluded the need for redesign of the trial [9] and the Spanish study because of inadequate patient enrolment [10]. In the Dutch study, most of the patients already had high neutralising antibody (NAb) titres at the time of inclusion, and no statistical differences in outcomes were observed between the treated and untreated groups [9,10].
Further studies have been published and assessed in several systematic reviews that remain uncertain about the safety and effectiveness of CP treatment for COVID-19 [11], [12], [13], [14]. Some studies, which were not conducted in a prospective manner, were non-randomised, or lacked untreated control arms, support the concept that CP should be administered before the disease is life-threatening to clear the virus more rapidly and to avoid further tissue damage [11,15]. Meanwhile, CP treatment has been increasingly adopted in clinical practice despite the regulatory approval in some countries that limit its use under an Emergency Use Authorization (EUA) law or in the context of clinical trials. The distribution of CP trials is centred in the countries most affected by COVID-19―predominantly in HICs [16], where large-scale procurement and stockpiling of CP has been undertaken.[17] In contrast, less than one-third of the trials are conducted in LMICs, with challenges pertaining to the provision of CP and limited capacity for pre-donation qualification [16,18].
Indonesia confirmed its first case on March 2, 2020. As of April 9, 2020, the pandemic had spread to 34 provinces in the country and continued to increase, eventually holding the highest number of cases in Southeast Asia on June 17, 2020 [19]. Due to the lack of treatment options for COVID-19, CP therapy was seen as a potential therapeutic strategy. As an LMIC with a geographically dispersed archipelago, access to healthcare remains a challenge in remote districts that could impact the adoption of CP deployment in Indonesia [17,20]. With the diverse social, cultural, and structural values of Indonesia, a well-designed trial protocol needs to be developed to ensure that the intervention and implementation are suitable in the local context [21]. We conducted this preliminary study to explore the effectiveness and safety of CP treatment in patients with moderate and severe COVID-19 and to investigate variables relevant for the design of further trials in Indonesia [22].
2. Methods
2.1. Study design and participants
The study was conducted at Gatot Soebroto Central Army Hospital (RSPAD) in collaboration with the Eijkman Institute for Molecular Biology and PT Bio Farma from May 1, 2020 to July 31, 2020. Ten hospitalised patients (male/female: 5/5) with COVID-19 with a median age of 56•6 (range: 42–75) years were enroled (Table 1). All the patients were diagnosed with COVID-19 using SARS-CoV-2 quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) on nasopharyngeal swab samples at admission. Written informed consent was obtained from each patient or their legal relatives. The study was approved by the Health Research Ethics Committee, Bethesda Hospital, Yogyakarta (No.77/KEPK-RSB/IV/2020) and registered at ClinicalTrials.gov (NCT04407208).
Table 1.
Characteristic of patients receiving convalescent plasma transfusion.
| Patient No | Sex | Age (year) | Clinical stage* | Days from symptom onset to hospital admission | Days from symptom onset to CP† therapy | Days from hospital admission to CP† therapy | Principal Symptoms | Comorbidity |
|---|---|---|---|---|---|---|---|---|
| 1• | F | 48 | Severe | 14 | 17 | 3 | Fever, cough, shortness of breath, nausea | None |
| 2• | M | 51 | Moderate | 9 | 17 | 8 | Fever, cough, shortness of breath | None |
| 3• | M | 58 | Severe | 5 | 17 | 12 | Fever, cough, shortness of breath | Hypertension |
| 4 | M | 52 | Moderate | 4 | 14 | 10 | Cough | Diabetes mellitus |
| 5 | F | 54 | Moderate | 6 | 14 | 8 | Fever, cough | None |
| 6 | M | 72 | Moderate | 4 | 14 | 10 | Fever, cough | Diabetes mellitus |
| 7 | F | 54 | Moderate | 6 | 28 | 22 | Fever, diarrhoea | None |
| 8 | M | 42 | Severe | 6 | 14 | 8 | Anosmia, ILI‡ | None |
| 9 | F | 60 | Severe | 2 | 6 | 4 | Fever, shortness of breath, ILI‡ | Diabetes mellitus |
| 10 | F | 75 | Severe | 2 | 28 | 26 | Shortness of breath, fever | None |
2.2. Procurement and administration of CP
Voluntary CP donors were recruited based on the following criteria: aged 18–60 years, previously diagnosed with COVID-19 by RT-PCR, discharged from hospital, and symptom-free for more than 14 days. All donors gave written consent for plasma donation through the plasmapheresis procedure and met the standard criteria for blood donation, including seronegativity for hepatitis B, hepatitis C, human immunodeficiency virus, and syphilis [21]. From each donor, 500–600 mL plasma was collected by the apheresis procedure using an MCS+ 9000 (Haemonetics, USA), which was aliquoted into four 100–150 mL plasma units and immediately stored at −20 °C.
SARS-CoV-2 NAbs in plasma were not measured before transfusion, as quantification assays were not available at the beginning of the study. An aliquot of plasma was kept at −20 °C for later investigation. CP transfusion was administered in three doses at 2-day intervals (approximately 3 mL/kg of recipient body weight) [23]. The transfusion was administered at 10–20 mL for the first 15 min, which was slowly increased and completed in 4 h. The recipient was closely monitored for 6 h after transfusion. The CP units were mostly from the same donors, although they could have been from different donors according to availability.
2.3. Definition of disease severity and assessment of outcomes
Clinical symptoms were recorded daily. Routine haematological tests were performed every 2–3 days, while biochemical, immunological, and lung imaging and SARS-CoV-2 RT-PCR was repeated every week. COVID-19 severity was defined according to the classification proposed by Siddiqi and Mehra and the WHO Interim Guidance (May 27, 2020) on Clinical Management of COVID-19 [24,25]. Mild grade (Stage I) was defined as disease with few symptoms (low fever, fatigue) and without pulmonary imaging (radiography or computed tomography [CT]) findings. Moderate grade (Stage II) was defined as disease with fever, respiratory symptoms (dry cough, chest distress, or shortness of breath after activities), and pulmonary imaging findings, subdivided into Stage IIa (without hypoxia) and Stage IIb (with hypoxia). Severe grade (Stage III) was defined as disease with a respiratory rate ≥30 breaths/min, oxygen saturation <90% or oxygenation index (PaO2/FiO2) ≤300 mmHg, and/or lung infiltrates >50% within 24–48 h. Critical grade was defined as respiratory failure, septic shock, and/or multiple organ dysfunction.
Organ dysfunction associated with COVID-19 was assessed using the Sequential Organ Failure Assessment (SOFA) scores for respiratory, coagulation, liver, cardiovascular, central nervous, and renal systems [26]. For outcome assessment, clinical improvement was scored based on a modified six-point ordinal scale recommended by the WHO Research and Development Blueprint Group [27]. Each patient was assigned a clinical status at baseline (day 0) and evaluated at days 7, 14, and 28. The six-point scale was as follows: 1, discharged alive; 2, hospitalised with no supplemental oxygen; 3, hospitalised with supplemental oxygen (not high-flow or non-invasive ventilation); 4, hospitalised with nasal high-flow supplemental oxygen, non-invasive mechanical ventilation, or both; 5, hospitalised with extracorporeal membrane oxygenation or invasive mechanical ventilation; and 6, death.
2.4. Detection of SARS-CoV-2 and measurement of NAbs
Nucleic acid was extracted from nasopharyngeal swabs in viral transport medium using a QIAamp Viral RNA Mini Kit (QIAGEN, Germany), and RT-PCR was performed using A*Star Fortitude Kit 2.0 (DxD Hub, Singapore) on a CFX96™ Real-Time PCR Detection System (Bio-Rad, USA), in accordance with the manufacturer's instructions. A cycle threshold (Ct) value of ≤40.0 was considered positive.
NAb against the receptor binding domain (RBD) of SARS-COV-2 spike protein was measured using the cPass™ surrogate virus neutralisation test (sVNT) (GenScript, USA) at the end of the study [28]. The assay was a competitive enzyme-linked immunosorbent assay between the RBD with human angiotensin converting enzyme-2,[28] which was amongst the first tests to obtain an EUA from the US Food and Drug Administration [29]. It was performed according to the manufacturer's instructions with slight modifications [30]. Briefly, samples were heat-inactivated at 56 °C for 30 min before the test, and incubated with an equal volume of horseradish peroxidase-conjugated RBD (HRP-RBD) at 37 °C for 30 min. The serum/HRP-RBD mix (100 μL) was then added to each well and incubated at 37 °C for 15 min. After removing unbound HRP-RBD by four washes, a chromogenic substrate provided in the kit was added, followed by incubation at 25 °C for 15 min. The colorimetric reaction was terminated by the addition of a stop solution. absorbence was measured at 450 nm. The reciprocal of the maximal dilution with 50% inhibition was counted as the NAb titre in the tested serum samples. An inhibition rate of ≥20% was considered positive for NAbs.
2.5. Radiological examination
Brixia Chest X-ray (CXR) scoring system according to Borghesi and Maroldi was used to monitor the progression of lung involvement [31]. This CXR scoring system includes two steps of image analysis. In the first step, each lung was divided into three zones on the frontal projection, marked by letters A, B, and C for the right lung, and D, E, and F for the left lung. The letters divide the lungs into three levels: upper level (A and D), above the inferior wall of the aortic arch; middle level (B and E), below the inferior wall of the aortic arch and above the inferior wall of the right inferior pulmonary vein (the hilar structures); and lower level (C and F), below the inferior wall of the right inferior pulmonary vein (the lung bases). A score (from 0 to 3) was assigned to each zone based on the detected lung abnormalities: 0, no lung abnormalities; 1, interstitial infiltrates; 2, interstitial and alveolar infiltrates (interstitial pre-dominance); and 3, interstitial and alveolar infiltrates (alveolar predominance). The overall CXR score is the sum of points from the six lung zones with a range from 0 to 18, followed by the partial score of each zone presented between square brackets.
2.6. Statistical analyses
Continuous variables were presented as medians and ranges. Categorical variables were expressed as counts and percentages and compared using the Chi-square or Fisher's exact test as appropriate. Tests were two-sided, and P values <0.05 were considered statistically significant. Statistical analyses were performed using the Statistical Program for Social Sciences (IBM SPSS version 22.0 for Windows; SPSS, IL, USA). Graphs were plotted using GraphPad Prism version 7•0.
2.7. Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
2.8. STROBE guidelines
The present study adheres to the STROBE reporting guidelines.
3. Results
3.1. Characteristics of patients and donors
The most common symptoms of patients at presentation were fever, non-productive cough, shortness of breath, anosmia, and diarrhoea. The patients were classified as moderate (n = 5) or severe (n = 5) COVID-19 [24,25], with five patients having comorbidities, including hypertension (n = 1) and diabetes mellitus (n = 4). The median time from onset of symptoms to hospital admission was 6 (range: 2–17) days, while the median time from symptom onset and hospital admission to CP transfusion was 16 (range: 6–28) days and 9 (range: 3–26) days, respectively (Table 1). Both groups of patients also received oral antivirals (oseltamivir and/or favipiravir and/or ritonavir/lopinavir) and supportive treatments according to the Indonesian National Guidelines [32]. Antibacterial therapy was administered to patients with suspected coinfection [25,32]. Corticosteroids (methylprednisolone or dexamethasone) were administered to severe patients under mechanical ventilation [33].
Eleven convalescent patients (male/female: 7/4) with a median age of 35 (range: 23–56) years who recovered from various stages (mild [n = 5], moderate [n = 5], and severe [n = 1]) of COVID-19 were enroled as donors (Table 2). None of the female donors were ever pregnant. The median time from symptom onset and hospital discharge to plasma collection was 22 (range: 23–56) days and 14 (range: 14–20) days, respectively.
Table 2.
Characteristics of convalescent plasma donors.
| No | Donor ID | Age (years) | Sex | Time from onset of symptom to donation (days) | Time from resolution of symptom to donation (days) | Clinical stage during illness‡ | % Inhibition of CP unit to SARS-CoV-2§ |
|---|---|---|---|---|---|---|---|
| 1 | TPT-D1011 | 56 | M | 28 | 14 | Moderate | −5.77 |
| 2 | TPT-D1013 | 33 | M | 23 | 14 | Moderate | −9.29 |
| 3 | TPT-D1018 | 23 | F | 21 | 14 | Mild | 63.41 |
| 4 | TPT-D1019 | 51 | F | 21 | 15 | Mild | 82.97 |
| 5 | TPT-D1020 | 35 | M | 20 | 14 | Mild | 78.08 |
| 6 | TPT-D1022 | 33 | M | 23 | 14 | Moderate | 90.76 |
| 7 | TPT-D1025 | 33 | F | 22 | 16 | Mild | 80.71 |
| 8 | TPT-D1026 | 47 | M | 22 | 14 | Moderate | 89.65 |
| 9 | TPT-D1029 | 42 | M | 41 | 20 | Severe | 91.40 |
| 10 | TPT-D1030 | 49 | M | 29 | 14 | Moderate | 94.53 |
| 11 | TPT-D2031 | 23 | F | 21 | 14 | Mild | 0.79 |
†CP, convalescent plasma; According to Siddiqi & Mehra24
§Cut-off ≥20%.28.
3.2. CP treatment, clinical response, respiratory function, and chest radiography findings
Symptoms in all patients, especially fever, cough, and chest pain, were largely alleviated within 1 to 3 days following the first unit of CP transfusion. Before CP treatment, four patients required mechanical ventilation and six required conventional nasal cannula oxygenation. In the first 2 weeks, four patients were still on mechanical ventilation; two of the four patients (Patients 3 and 9) who had comorbidities continued to deteriorate and passed at week 3.
There were clinical improvements as measured by the modified WHO six-point ordinal scale at 28 days of follow-up [27]; four patients were discharged from the hospital, two were treated without supplemental oxygenation, and one was treated with nasal oxygenation (Table 3). One patient (Patient 10) still required mechanical ventilation despite having improved clinical and laboratory parameters in the previous weeks. This patient had a positive bronchial lavage culture for Klebsiella pneumoniae.
Table 3.
Comparison of clinical characteristics and laboratory parameters of patients receiving convalescent plasma transfusion.
| Baseline | Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|---|
| (n = 10) | (n = 10) | (n = 10) | (n = 8) | (n = 8) | |
| Haemoglobin (g/dL) | 12·4 (9·7–1·5) | 12·2 (8·3– 1·45) | 11·5 (9·1–14·9) | 11·6 (10·5–15·0) | 11·4 (8·6–15·9) |
| Leucocyte (x106/mL) | 9·7 (4·1–23·8) | 9·0 (5·2–32·4) | 11·6 (7·5–53·5) | 11·9 (9·0–22·3) | 10·4 (5·4–12·5) |
| Neutrophil (x106/mL) | 81 (41–96) | 88 (65–96) | 91 (66–97) | 87 (62–95) | 72 (65–96) |
| Lymphocyte (x106/mL) | 13 (2–44) | 5 (2–28) | 5 (1–27) | 7 (3–30) | 16 (2–21) |
| NLR | 6·2 (0·9–48·0) | 22·8 (2·3–43·0) | 19·0 (2·4–93·0) | 13·5 (2·1–31·7) | 4·6 (3·1–48·0) |
| ≥3·3 | 8 (80%) | 6 (60%) | 7 (70%) | 6 (75%) | 1 (12·5%) |
| Thrombocyte (x106/mL) | 268 (112–384) | 293 (53–777) | 322 (108–721) | 358 (141–772) | 281 (123–332) |
| Prothrombin time (second) | 10·5 (9·7–12·0) | 11·2 (9·7–12·3) | 10·4 (9·6–11·4) | 10·4 (9·7–10·8) | 10·6 (9·9–13·8) |
| INR | 1·0 (0·9–1·1) | 1·0 (0·9–1·2) | 1·0 (0·9–1·1) | 0·9 (0·9–1·0) | 1·0 (0·9–1·2) |
| CRP (mg/L) | 67·6 (1·6–311·2) | 18·2 (0·5–152·0) | 8·6 (0·7–61·1) | 4·6 (0·5–16·3) | 30·7 (0·9–66·1) |
| ≥6 | 3 (30%) | 4 (40%) | 3 (30%) | 2 (25%) | 4 (50%) |
| D-Dimer (µg/mL) | 0·9 (0·2–10·1) | 0·4 (0·1–3·3) | 0·5 (0·1–3·4) | 0·3 (0·1–3·7) | 0·5 (0·2–6·0) |
| ≥0·5 | 6 (60%) | 3 (30%) | 6 (60%) | 3 (37·5%) | 5 (62·5%) |
| Procalcitonin (ng/mL) | 0·25 (0·03–5·61) | 0·20 (0·02–7·27) | 0·09 (0·05–10·16) | 0·06 (0·03–0·11) | 0·10 (0·02–6·04) |
| ≥0·5 | 1 (10%) | 3 (30%) | 2 (20%) | 1 (12·5%) | 2 (25%) |
| Interleukin-6 (ρg/mL) | 19·9 (1·5–79·1) | 1·7 (1·5–66·6) | 5·2 (1·5–125·6) | 1·5 (1·5–13·6) | 8·0 (1·5–82·9) |
| Aspartate aminotransferase (IU/L) | 25·5 (22–126) | 24 (11–69) | 29·5 (13–107) | 38 (14–229) | 22 (13–74) |
| Alanine aminotransferase (IU/L) | 37 (18–118) | 38 (18–136) | 45 (17–252) | 79 (14–730) | 54·5 (12–347) |
| Total Bilirubin (mg/dL) | 0·6 (0·3–1·5) | 0·4 (0·2–1·7) | 0·5(0·2–1·8) | 0·6 (0·2–1·2) | 0·5 (0·4–1·1) |
| Creatinine | 0·8 (0·6–1·4) | 0·8 (0·6–3·5) | 0·9 (0·5–4·9) | 0·7 (0·5–1·1) | 0·7 (0·65–1·6) |
| ≥1·5 mg/dL | |||||
| PaO2/FiO2 ratio | 276 (101–466) | 256 (113–499) | 203 (57–470) | 286 (170–404) | 357 (232–532) |
| ≤300 | 5 (50%) | 6 (60%) | 7 (70%) | 4 (50%) | 2 (25%) |
| SOFA Score | 2·0 (0·0–7·0) | 3·5 (0·0–9·0) | 3·5 (1·0–11·0) | 1·5 (0·0–7·0) | 1·0 (0·0–6·0) |
| ≥6 | 4 (40%) | 3 (30%) | 3 (30%) | 1 (13%) | 1 (13%) |
| Six-category scale: | |||||
| Discharged | 4 (50%) | ||||
| Hospitalization, no supplemental oxygen | 2 (25%) | ||||
| Hospitalization, requiring low-flow supplemental oxygen | 6 (60%) | 5 (50%) | 5 (50%) | 5 (50%) | 1 (12·5%) |
| Hospitalization, requiring HFNC/non-invasive ventilation | 1 (10%) | 1 (10%) | 1 (10%) | ||
| Hospitalization, requiring ECMO/invasive ventilation | 4 (40%) | 4 (40%) | 4 (40%) | 2 (20%) | 1 (12·5%) |
| Death | 2 (20%) | ||||
Data are median (range) or n (%); NLR, Neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; INR, International Normalized Ratio; SOFA, Sequential Organ Failure Assessment.
HFNC, high-flow nasal cannula; ECMO, Extracorporeal membrane oxygenation·.
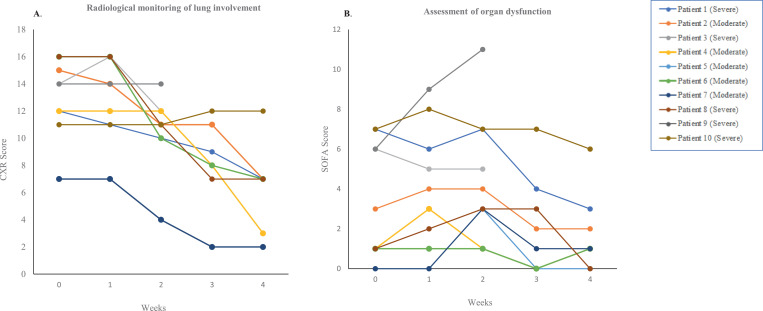
The overall median PaO2/FiO2 ratio was 276 (range: 101–466) mmHg before CP transfusion and fluctuated at weeks 1 and 2. At weeks 3 and 4, the PaO2/FiO2 ratio improved in eight patients, but remained low (<300) in the deceased patients. Chest radiography findings showed improvements in overall CXR scores in surviving patients, from a median of 14 (range: 7–16) at baseline to a median of 14 (range: 7–16), 11 (range: 4–14), 9 (range: 2–12), and 7 (range: 2–7) at weeks 1, 2, 3, and 4, respectively (Fig. 1a and Suppl. Table 3).
Fig. 1.
Radiological monitoring of lung involvement by CXR score (A) and assessment of organ dysfunction by SOFA score (B) in CP-receiving patients. SOFA, Sequential Organ Failure Assessment; CXR, Brixia Chest X-Ray. Horizontal axis represents the pre- (baseline) and post-transfusion weeks.
3.3. Laboratory findings
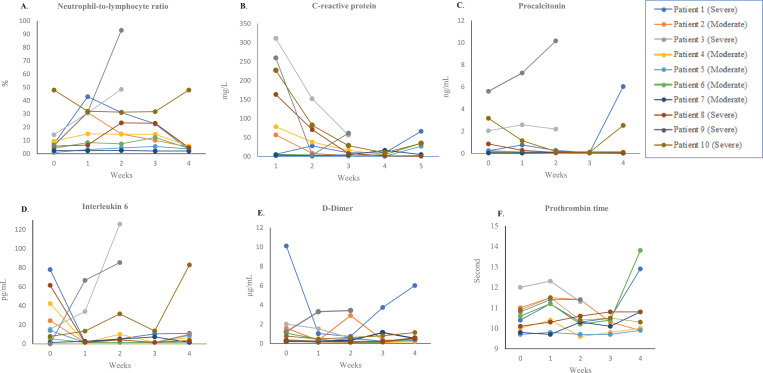
The neutrophil-to-lymphocyte ratio (NLR), a predictor of prognosis in COVID-19 [34], fluctuated between weeks 1 and 3 after CP treatment, but decreased at week 4 in surviving patients. C-reactive protein (CRP), an index for disease progression, significantly improved in most patients compared with baseline levels. A decrement tendency of other inflammatory parameters, interleukin (IL)−6 and procalcitonin, was documented in most surviving patients after CP therapy compared with the baseline levels (Fig. 2).
Fig. 2.
Changes of inflammatory (A-D) and coagulation (E-F) parameters in CP-receiving patients. Horizontal axis represents the pre- (baseline) and post-transfusion weeks.
A decline in d-dimer, a marker for inflammation and prothrombotic state, was also observed in most patients following CP transfusion, except for an obvious increase in Patient 1. However, other coagulation markers, including platelet count and prothrombin time, did not show significant changes in the majority of the patients (Fig. 2). Similarly, parameters indicative of organ dysfunction, including alanine aminotransferase, bilirubin, and creatinine, remained unchanged compared with their pre-treatment levels (Table 2), except for Patient 9, who experienced kidney failure. By clinical stage, better reduction tendencies of these markers were observed amongst patients with moderate disease compared with those with severe disease (Suppl. Fig. 1).
Measurement of the degree of organ dysfunction showed a decrease in the SOFA scores in most patients who survived by the end of the trial (Fig. 1B), except for Patients 3 and 9, who had SOFA scores of 5 and 11, respectively.
3.4. Changes in viral load and SARS-CoV-2 NAb
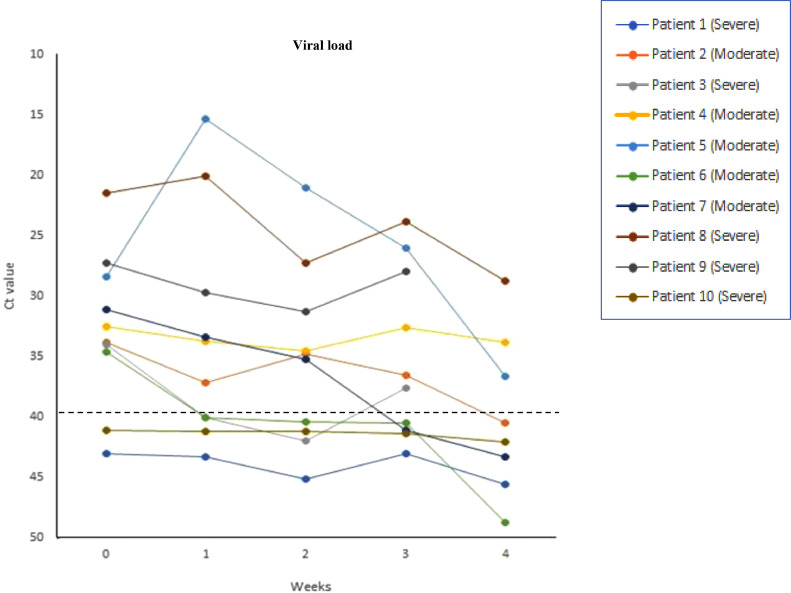
All patients had detectable viral nucleic acid levels upon admission. Before CP transfusion, the median Ct value was 33·2 (range: 21·5–43·1). It progressed to 35·5 (range: 15·4–43·3), 35.1 (range: 21·0–45·2), 37·1 (range: 23·8–43·1), and 40·2 (range: 28·8–49·8) at weeks 1, 2, 3, and 4 after the CP transfusion (Table 2 and Fig. 3), respectively. Five patients were negative for SARS-CoV-2 at the end of the trial, including the two patients (Patients 1 and 10) who were already negative at baseline. On the other hand, three patients remained positive for SARS-CoV-2 until the end of the trial.
Fig. 3.
Changes of viral load in nasopharyngeal swabs amongst CP-receiving patients. Horizontal axis represents the pre- (baseline) and post-transfusion weeks. Dashed lines indicate the detection threshold at 40. Ct, cycle threshold.
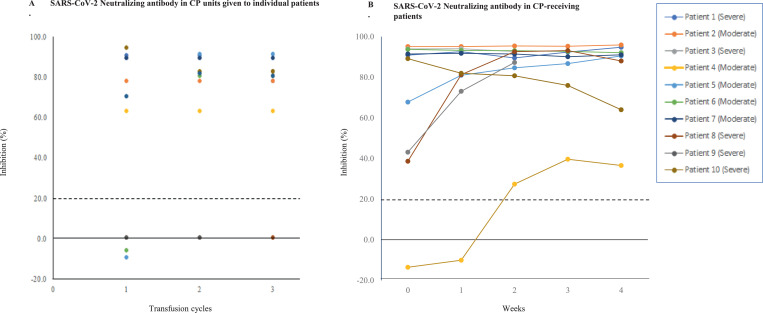
The NAb titres against SARS-CoV-2 in donors before transfusion and in CP-receiving patients could not be determined when the study began. Utilising a recently available sVNT [28], we were able to measure the antibody inhibition activity in the donors and patients. Figs. 4A and 4B illustrate the inhibition rates of NAbs in CP units administered to individual patients and in CP-receiving patients across the weeks of administration. amongst the donors, eight (72·7%) had detectable NAbs with a median inhibition rate of 86·1% (range: 63·41%–94·53%), while three (22·7%) had inhibition rates lower than the cut-off value (20%). Of the patients, nine had detectable NAbs before receiving CP with a median inhibition rate of 90·9% (range: 38·66%–95·13%) (Suppl. Table 1).
Fig. 4.
SARS-CoV-2 neutralizing antibody activity in CP units given to individual patients at transfusion cycles 1, 2, and 3 (A) and in CP-receiving patients (B). CP, convalescent plasma. Dashed lines indicate the inhibition cut-off at 20%.
Some patients had high NAb inhibition rates (>90%) even before the first CP administration: four patients (Patients 1, 2, 6, and 7) had CP transfusion after day 14, and one patient (Patient 3) had CP transfusion on day 5 after symptom onset (Table 1). Four patients (Patients 4, 5, 8, and 9) showed an accelerated increase in NAb inhibition following CP transfusion, including Patient 4, who passed away at week 3 (Suppl. Table 3). Delays in CP administration were mostly due to consent-related issues, particularly in three patients (Patients 3, 7, and 10) who required agreement from the community leaders. The NAb median inhibition rates in the patients at baseline and in the donors were comparable (90·91% [range: 38·66%–95·13%] vs. 86·31% [range: 78·08%–94·53%], respectively; p = 0·379).
3.5. Adverse effects
No noticeable adverse effects were observed with CP transfusion. Deaths occurred in two patients with severe COVID-19 who initially had comorbidities and experienced refractory multi-organ dysfunction.
4. Discussion
CP has been regarded with great expectations for treating COVID-19. However, it has not been approved as a standard of care for the disease. Adequate trials are necessary to definitively demonstrate its efficacy in determining the optimal product attributes and appropriate patient populations. Most studies reported CP treatment in patients with severe COVID-19 [8,[35], [36], [37], [38], [39]], although only a few addressed the possible impact on less severe disease [30,40].
To date, the vast majority of studies reporting COVID-19 infection and treatment have come from the earlier affected countries with established healthcare systems and better research infrastructure, while critical research questions specific to the needs of LMICs are difficult to answer [17,41]. A disproportionate impact is observed in the latter with their constraints for swift uptake of new technologies and medical supplies to deal with this pandemic [18]. In these countries, challenges prevail pertaining to the availability of resources for proper management and treatment of patients, such as reagents for assessing inflammation and radiological imaging for evaluating the disease course.
There are also questions regarding the capacity for donor mobilisation and CP procurement, not least of which are the pre-donation qualification and testing of NAbs against SARS-CoV-2 [16,18]. In light of these issues, we performed this study using existing facilities to evaluate the effectiveness of CP therapy in patients with moderate-to-severe COVID-19, and to identify factors relevant to the outcome measure set for the design of further trials in Indonesia [22]. To the best of our knowledge, this is a timely study from Indonesia to explore the scope where CP therapy could be one of the few available options, particularly in LMICs, where challenges may exist in the access to the benefits of scientific progress for the control and treatment of COVID-19 [18,42,43].
In this study, CP transfusion administered in three doses of 3 mL/kg of recipient body weight at 2-day intervals was well tolerated. The overall assessment showed improvement in clinical symptoms, CXR scores, and laboratory parameters. By 28 days after the first CP administration, the cumulative incidence of clinical improvement of the patients, as defined by either live discharge or at least a one-point reduction from baseline on the six-point ordinal scale, was 7 (70%), while that for no improvement and mortality was 1 (10%) and 2 (20%), respectively [44]. Based on disease severity, good improvements (discharged alive or without oxygen supplementation) were achieved in five (5/5) patients with moderate disease. amongst patients with severe disease, two (2/5) showed good improvement, one remained on mechanical ventilation, and two deteriorated and died. Our findings in patients with severe COVID-19 were not in accordance with the two previous initial studies on the improvement of clinical outcomes and reduction of viral load [35,36]. The difference could be related to two key factors: the NAb titre and treatment time point, which are discussed further below.
Although the pathogenesis of COVID-19 is yet to be understood, it has been generally recognised that the disease course is attributable to two distinctive but overlapping mechanisms: (a) damage induced by virus pathogenicity, and (b) exaggeration of the host immune system [24,45]. The first is marked by common clinical symptoms such as fever, cough, fatigue, myalgia, viremia, leucocytosis, lymphopenia, and reduced CD4+ and CD8+ T cells in the peripheral blood, while the second is by over-activation of the systemic immune system, with elevation of proinflammatory and coagulation parameters [24,46]. As a result, COVID-19 presents features of a multisystem disease with impairment of several organs [47].
Virus-specific NAb contained in CP serves as the main mechanism for accelerating the clearance of virus and preventing further entry into target cells [48]. CP treatment also provides further benefit, that is immunomodulation, by neutralisation of cytokines, complement, and autoantibodies associated with COVID-19 [48,49]. This current study showed decreased levels of NLR as well as inflammatory and coagulation markers, including CRP, IL-6, procalcitonin, and d-dimer, in most patients following CP transfusion. Serial evaluation of organ involvement showed decreased overall SOFA scores related to high mortality (≥6) [50], from 4 at baseline to 1 at week 4. When assessed based on disease stage, better improvement tendencies were observed in patients with moderate disease than in those with severe disease. This study also observed that patients with comorbidities had worse outcomes than those without comorbidities.
The titre of NAb to SARS-CoV-2 is an essential element in CP therapy. The plaque reduction neutralisation test (PRNT), which is the gold standard for conventional measurement of NAb, has been used for donor selection and treatment monitoring [35]. However, this test requires viable viruses, replication-competent cell lines, and well-trained personnel to perform the tedious and time-consuming procedure in biosafety level-3 laboratories. This test is not agreeable with high-throughput screening and requires long turnaround times (5–7 days) to obtain the result. Since the neutralisation test was not available when this study began, transfusions were administered based on ABO and Rhesus compatibility following the guidelines for donor recruitment from patients who recovered from COVID-19 [21,51]. The later availability of sVNT, which correlated well with PRNT [28], allowed us to measure the inhibition activity of NAb against the virus in CP donors’ and recipients’ repository samples.
Patients who recover from viral diseases may not have high titres of NAb. Of the 99 patients recovering from SARS, 87 had NAbs that declined with time [52]. Recent studies on COVID-19 reported that antibody titres to SARS-CoV-2 were positively correlated with disease severity [30,53,54]. In the current study, testing of CP repository using sVNT showed various levels of inhibitory activity against SARS-CoV-2 amongst the selected donors. There were three CP units without detectable NAbs that were administered to the patients; these samples were from convalescent patients who had mild COVID-19. This was also reported in other studies [9,40,46], which recommended the selection of convalescent patients who have had a more severe disease, particularly where NAb testing is not feasible.
Amongst the CP-receiving patients, nine had SARS-CoV-2 NAbs before the first transfusion, with five showing high (>90%) inhibitory activity. This could be due to the long intervals between the first CP administration and symptom onset, when the specific antibodies had been generated and reached peak levels in the third and fourth weeks of illness [55]. Several studies have shown that CP treatment is more effective when administered early in the disease process [35,[56], [57], [58]]. We observed one patient (Patient 4) who initially had negative inhibitory activity, but showed NAb titre following CP transfusion. The presence of NAb could be the result of either or both CP transfusion and endogenous antibody generation during the course of the disease [59]. However, the increasing inhibition rates were also observed in other patients (Patients 8 and 9) who received CP units without detectable NAbs. Taken together, the failure to benefit from this treatment in some patients in this study could be attributed to the late transfusion of CP. Prospective controlled cohort studies in the early stage of COVID-19 are needed to assess the benefit of this CP treatment in providing a good antibody titre to the patients.
Clearance of viral PCR results in respiratory specimens has been used to measure treatment outcomes in most CP studies [11,13,60]. Although all patients in this study were confirmed to have COVID-19 on admission, two patients showed negative RT-PCR results before the first CP transfusion. These patients received CP therapy 17 and 28 days after disease onset. The postponement of CP administration was due to delayed consent from the patients’ extended families, which was overcome after the involvement of local community leaders. It could be assumed that the endogenous NAbs to the virus, which are generated 10 to 15 days after the infection [30,59], had cleared the virus before CP was administered. The other eight patients were still RT-PCR-positive before receiving a CP transfusion. Overall, in these patients, a reduction in the median Ct value was observed along with the alleviation of clinical condition, chest imaging, and inflammatory parameters. Of note, three patients still showed viral shedding until the end of the trial. Previous studies reported that prolonged viral shedding could persist for a median of 20 days (up to 63 days) [60], which was associated with high initial viral load, severe illness at admission, corticosteroid treatment, or low NAb levels [38]. However, two (Patients 5 and 8) of the three patients had high NAb levels, and none received corticosteroid treatment. Nevertheless, our findings imply that Centers for Disease Control and Prevention strategies to discontinue transmission-based precautions and the WHO criteria for discharging patients from isolation regardless of disease severity should be implemented with caution, particularly in situations where there is a high risk of transmitting the virus to vulnerable groups of people and in locations where isolation is not feasible [61,62].
We acknowledge some limitations of this preliminary study. First, the number of patients studied was small, and no control group was included. Second, both groups of patients received antiviral treatment despite the uncertain effectiveness of the drugs [33]. Furthermore, corticosteroid treatment was also administered to severe patients. Thus, the possibility of additional contribution of these drugs to the patients’ improvement could not be ruled out [35,38,[63], [64], [65], [66]]. Third, convalescent donor testing for SARS-CoV-2 NAb could not be performed because the test for NAb determination was not available early in the study. Fourth, early CP treatment could not be administered to some patients because of the delay in obtaining consent that required agreements from their extended families, as in many LMICs, decision-making regarding medical interventions is conceived to be a communal process rather than an individualistic one [67].
There are systematic reviews and meta-analyses assessing studies on the effectiveness and safety of CP in the treatment of COVID-19 [11,13,15,59]. Most studies have focused on reporting all-cause mortality, rather than a wider spectrum of clinical outcomes [11,68]. To measure the benefit of CP therapy, studies are needed to analyse subgroups of patients to investigate the impacts of the treatment over the course of clinical illness, including non-mortal clinical outcomes [68]. In addition, they should not only represent the outcome from individual patients, but also reflect the capacity of the healthcare systems, from low- to high-income countries, to provide maximal benefit to the population [22,27].
Several lessons could be gained from this study for the design of clinical trials and deployment of CP, particularly in LMICs with resource-limited settings [18,42]: (a) whenever feasible, testing of donor's plasma for titres of anti-SARS-CoV-2 antibodies should be performed. This is becoming enabled as surrogate methods for conventional neutralisation tests are now available in some countries [14,30], including the tests recently approved for pre-donation qualification of COVID-19 CP 0.29 If NAb titres cannot be obtained in advance, consider storing a repository from the CP donation to determine NAb titres at a later date [28,69]; (b) take advantage of the availability of a population of convalescent donors to enable stockpiling and to create inventories of CP, which can be tested for NAbs before distributing the units for transfusion [6,17,40]; (c) early administration of CP in the course of disease [14,44,58]; (d) maximise utility of existing resources, as exemplified in this study in using CXR scoring to quantify and monitor lung involvement [31], where more advanced technologies such as CT are not always available or feasible. The WHO core measure outcome sets may be helpful to facilitate data pooling across the study [22]; (e) specifically for the consent process, perform community engagement and use appropriate channels to ensure that the concerns are considered when designing and implementing the intervention study [67,70].
In conclusion, this study highlights the safety of CP therapy. Although a better improvement tendency was observed amongst patients with moderate disease, we could not conclude that the outcomes were solely due to CP treatment. It is critically important to perform well-designed randomised clinical trials of CP therapy in LMICs that cover larger patient cohorts with different stages of COVID-19, highlighting the capacity for NAb testing and treatment delivery. Locally applicable research strategies, capacity building to execute the study, translating research into the local context, and the use of recognised communicating channels need to be implemented to obtain support for the trials.
Authors’ contributions
MSR, NS, and RW conceptualized the study, developed the proposal, performed sample collection, laboratory work and data management, analysis, and manuscript preparation. WYS participated in proposal development and manuscript preparation. FAY and EJ participated in laboratory work, result interpretation, and manuscript preparation. NN and NSB participated in project development, result interpretation, and manuscript preparation. DHM participated in data management, analysis, interpretation of results, overall supervision, and manuscript writing. All authors read and approved the final manuscript.
Data sharing statement
The data generated and analysed in this study are included in this article and its additional files. The original datasets used for this study are not publicly available due to the existing regulation, and only can be shared upon the approval of the directors of the participating institutions.
Funding
The study was supported by PT Bio Farma, Indonesia.
Declaration of Competing Interest
Marliana S. Rejeki reports a grant from PT Bio Farma to conduct the study. All other authors have nothing to report.
Acknowledgments
This study was funded by a non-restricted educational grant from PT Bio Farma, Bandung, Indonesia. The authors would like to extend their gratitude to Terawan Agus Putranto, Former Minister of Health, Republic of Indonesia, and Former Director of Gatot Soebroto Central Army Hospital; Rahman Roestan, Operation Director of PT Bio Farma; Albertus Budi Sulistya, Director of Gatot Soebroto Central Army Hospital; and Amin Soebandrio, Chairman of the Eijkman Institute and Molecular Biology, for invaluable supports to this study. Special thanks are given to R. Triono Soendoro, for consultation and guidance in ethics principles; all colleagues from Gatot Soebroto Hospital, in particular Agus Yunianto, Handrianto Setiajaya, Lukman Ma'ruf, Martina Lily Yana, Familia Bela Rahadiati, Ratna Andriyani, Jenie Erawati Muchti, and Dwi Novianingtyas, for assistance to execute this study; and all nurses and many others who volunteered their efforts and time. Sincere gratitude is expressed to all patients and donors who participated in this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100931.
Appendix. Supplementary materials
References
- 1.Zhu N., Zhang D., Wang W. A Novel Coronavirus from Patients with Pneumonia in China. N Engl J Med. 2019;382(8):727–733. doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Coronavirus disease (COVID-19) Pandemic 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 3.BBC News. Coronavirus pandemic: tracking the global outbreak. 2020. https://www.bbc.com/news/world-51235105 (accessed July 31 2020,).
- 4.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott HC. Pathophysiology, Transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020. [DOI] [PubMed]
- 5.NIH. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines (Updated: february 23, 2021). February 23, 2021. https://www.covid19treatmentguidelines.nih.gov/.
- 6.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L., Zhang W., Hu Y. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: a Randomized Clinical Trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gharbharan A., Jordans C.C.E., Geurtsvankessel C., et al. Convalescent Plasma for COVID-19. A randomized clinical trial. 2020. https://www.medrxiv.org/content/10.1101/2020.07.01.20139857v1.
- 10.Avendaño-Solà C., Ramos-Martínez A., Muñez-Rubio E., et al. Convalescent Plasma for COVID-19: a multicenter, randomized clinical trial. 2020. https://www.medrxiv.org/content/10.1101/2020.08.26.20182444v2.
- 11.Chai K.L., Valk S.J., Piechotta V. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;10 doi: 10.1002/14651858.CD013600.pub3. CD013600. [DOI] [PubMed] [Google Scholar]
- 12.Piechotta V., Chai K.L., Valk S.J. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;7 doi: 10.1002/14651858.CD013600.pub2. CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar S., Soni K.D., Khanna P. Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol. 2021;93(2):1111–1118. doi: 10.1002/jmv.26408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. Recommendations for Investigational COVID-19 Convalescent Plasma. February 11, 2021. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma.
- 15.Szako L., Farkas N., Kiss S. Convalescent plasma therapy for COVID-19 patients: a protocol of a prospective meta-analysis of randomized controlled trials. Trials. 2021;22(1):112. doi: 10.1186/s13063-021-05066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Focosi D., Anderson A.O., Tang J.W., Tuccori M. Convalescent Plasma Therapy for COVID-19: state of the Art. Clin Microbiol Rev. 2020;33(4) doi: 10.1128/CMR.00072-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloch E.M., Goel R., Montemayor C., Cohn C., Tobian A.A.R. Promoting access to COVID-19 convalescent plasma in low- and middle-income countries. Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.102957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloch E.M., Goel R., Wendel S. Guidance for the procurement of COVID-19 convalescent plasma: differences between high- and low-middle-income countries. Vox Sang. 2021;116:18–35. doi: 10.1111/vox.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Jakarta Post. Indonesia becomes country with most confirmed COVID-19 cases in Southeast Asia. June 17, 2020 2020. https://www.thejakartapost.com/news/2020/06/17/indonesia-becomes-country-with-most-confirmed-covid-19-cases-in-southeast-asia.html.
- 20.The World Bank. The World Bank in Indonesia (Updated: Oct 01, 2020). https://www.worldbank.org/en/country/indonesia/overview (accessed November 10 2020,).
- 21.Smid M.W., Thierry Burnouf T., 2 JEJ, et al. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma in low-and middle-income countries. Apr 2020 2020. https://isbtweb.org/fileadmin/user_upload/FINAL_Points_to_consider_in_the_preparation_of_COVID_convalescent_plasma_in_LMIC.pdf (accessed May 1 2020,). [DOI] [PMC free article] [PubMed]
- 22.WHO Working Group on the Clinical Characterisation Management of Covid-19 infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8) doi: 10.1016/S1473-3099(20)30483-7. e192-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloch E.M., Shoham S., Casadevall A. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Clinical management of COVID-19. 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19.
- 26.Jones A.E., Trzeciak S., Kline J.A. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649–1654. doi: 10.1097/CCM.0b013e31819def97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. WHO R&D Blueprintnovel Coronavirus COVID-19 Therapeutic Trial Synopsis. 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf (accessed Aug 20 2020).
- 28.Tan C.W., Chia W.N., Qin X. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. Convalescent plasma letter of authorization (Reissuance, February 4, 2021). 2021 (accessed Feb 12 2021).
- 30.Chen W., Zhang J., Qin X. SARS-CoV-2 neutralizing antibody levels are correlated with severity of COVID-19 pneumonia. Biomedicine & Pharmacotherapy. 2020 doi: 10.1016/j.biopha.2020.110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borghesi A., Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125(5):509–513. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dokter Perhimpunan, Indonesia Paru. Edisi 2. (Protocol management of COVID-19); Jakarta: 2020. Perhimpunan dokter spesialis kardiovaskular indonesia, perhimpunan dokter spesialis penyakit dalam indonesia, perhimpunan dokter anestesiologi dan terapi intensif indonesia, ikatan dokter anak indonesia. protokol tatalaksana COVID-19. [Google Scholar]
- 33.NIH. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Sep 1, 2020. https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 34.Cheng B., Hu J., Zuo X. Predictors of progression from moderate to severe coronavirus disease 2019: a retrospective cohort. Clin Microbiol Infect. 2020;26(10):1400–1405. doi: 10.1016/j.cmi.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan K., Liu B., Li C. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen C., Wang Z., Zhao F. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonovich V.A., Burgos Pratx L.D., Scibona P. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu K., Chen Y., Yuan J. Factors Associated With Prolonged Viral RNA Shedding in Patients with Coronavirus Disease 2019 (COVID-19) Clin Infect Dis. 2020;71(15):799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B., Liu S., Tan T. Treatment With Convalescent Plasma for Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Chest. 2020;158(1) doi: 10.1016/j.chest.2020.03.039. e9-e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maor Y., Cohen D., Paran N. Compassionate use of convalescent plasma for treatment of moderate and severe pneumonia in COVID-19 patients and association with IgG antibody levels in donated plasma. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worldometer. Coronavirus update. 2020. https://www.worldometers.info/coronavirus/(accessed April 18 2020,).
- 42.Pécoul B. How best to prevent and treat COVID-19 in resource-limited settings? Apr 3, 2020. https://dndi.org/viewpoints/2020/how-best-prevent-treat-covid19-resource-limited-settings-by-bernard-pecoul/.
- 43.Donders Y. The right to enjoy the benefits of scientific progress: in search of state obligations in relation to health. Med Health Care Philos. 2011;14(4):371–381. doi: 10.1007/s11019-011-9327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salazar E., Christensen P.A., Graviss E.A. Treatment of COVID-19 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality. Am J Pathol. 2020 doi: 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao W., Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30(5):367–369. doi: 10.1038/s41422-020-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robba C., Battaglini D., Pelosi P., Rocco P.R.M. Multiple organ dysfunction in SARS-CoV-2: mODS-CoV-2. Expert Rev Respir Med. 2020:1–4. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rojas M., Rodriguez Y., Monsalve D.M. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Xiao M., Zhang S. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO. Guidance on maintaining a safe and adequate blood supply during the coronavirus disease 2019 (COVID-19) pandemic and on the collection of COVID-19 convalescent plasma. Interim guidance - 10 July 2020. 2020. https://www.who.int/publications/i/item/maintaining-a-safe-and-adequate-blood-supply-during-the-pandemic-outbreak-of-coronavirus-disease-(covid-19) (accessed July 30 2020,).
- 52.Zhang J.S., Chen J.T., Liu Y.X. A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol. 2005;77(2):147–150. doi: 10.1002/jmv.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu W.T., Howell J.C., Ozturk T. Antibody Profiles According to Mild or Severe SARS-CoV-2 Infection. Emerg Infect Dis. 2020;26(12):2974–2978. doi: 10.3201/eid2612.203334. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li K., Huang B., Wu M. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat Commun. 2020;11(1):6044. doi: 10.1038/s41467-020-19943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 56.Ye M., Fu D., Ren Y. Treatment with convalescent plasma for COVID-19 patients in Wuhan. China. J Med Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joyner M.J., Senefeld J.W., Klassen S.A. medRxiv; 2020. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. [Google Scholar]
- 58.Libster R., Perez Marc G., Wappner D. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu F., Wang A., Liu M. Preprint with THE LANCET; 2020. Neutralizing antibody responses to SARS- CoV-2 in a COVID-19 recovered patient cohort and their implications.https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3566211 [Google Scholar]
- 60.Widders A., Broom A., Broom J. SARS-CoV-2: the viral shedding vs infectivity dilemma. Infect Dis Health. 2020;25(3):210–215. doi: 10.1016/j.idh.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.U.S. CDC. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (Interim guidance). Aug. 10, 2020 2020 (accessed Sep. 14 2020).
- 62.WHO. Criteria for releasing COVID-19 patients from isolation. Scientific Brief. 17 June 2020 2020. https://www.who.int/publications/i/item/criteria-for-releasing-covid-19-patients-from-isolation (accessed Sep 12 2020).
- 63.Li Q., Li W., Jin Y. Efficacy Evaluation of Early, Low-Dose, Short-Term Corticosteroids in Adults Hospitalized with Non-Severe COVID-19 Pneumonia: a Retrospective Cohort Study. Infect Dis Ther. 2020;9(4):823–836. doi: 10.1007/s40121-020-00332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Group R.C., Horby P., Lim W.S. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020 [Google Scholar]
- 65.Group Recovery Collaborative, P Horby, Lim W.S. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li T.Z., Cao Z.H., Chen Y. Duration of SARS-CoV-2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ekmekci P.E., Arda B. Interculturalism and Informed Consent: respecting Cultural Differences without Breaching Human Rights. Cultura (Iasi) 2017;14(2):159–172. [PMC free article] [PubMed] [Google Scholar]
- 68.Janiaud P., Axfors C., Schmitt A.M. Association of Convalescent Plasma Treatment With Clinical Outcomes in Patients With COVID-19: a Systematic Review and Meta-analysis. JAMA. 2021 doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agarwal A., Mukherjee A., Kumar G. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zulu J.M., Sandoy I.F., Moland K.M., Musonda P., Munsaka E., Blystad A. The challenge of community engagement and informed consent in rural Zambia: an example from a pilot study. BMC Med Ethics. 2019;20(1):45. doi: 10.1186/s12910-019-0382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.