Abstract
Background
Concomitant use of proton pump inhibitors (PPIs) with capecitabine was suggested to be associated with poor outcomes in gastrointestinal cancers. We analyzed the differential impact of PPI use on capecitabine and fluorouracil using the data set from the AXEPT trial, a phase III randomized trial that demonstrated the noninferiority of mXELIRI (modified XELIRI: capecitabine plus irinotecan) to FOLFIRI (leucovorin, fluorouracil, and irinotecan), either with or without bevacizumab in patients with metastatic colorectal cancer.
Patients and Methods
Out of the per‐protocol set (n = 620), patients with information on concomitant medications (n = 482) were included in this post hoc analysis. PPI use was defined as concomitant exposure of capecitabine and the use of any PPI for 20% or more of the study period. The treatment‐by‐PPI‐use interaction was examined after adjusting for stratification factors.
Results
Of the 482 patients, 49 (10.1%) used PPI. Among the PPI users, the mXELIRI group tended to have poorer overall survival compared with the FOLFIRI group. In contrast, among the nonusers, the overall survival of the mXELIRI group was significantly better than that of the FOLFIRI group. Similarly, a trend of worse progression‐free survival with mXELIRI compared with FOLFIRI was observed in PPI users but not in nonusers. Treatment‐by‐PPI‐use interaction was significant for overall survival and progression‐free survival.
Conclusion
The significant interaction between PPI use and the type of fluoropyrimidine in terms of overall and progression‐free survival suggests that fluorouracil could be a more favorable option than capecitabine for patients with metastatic colorectal cancer using PPIs.
Implications for Practice
This study showed a significant interaction between the use of proton pump inhibitors (PPIs) and the type of fluoropyrimidines. This interaction mainly comes from the positive impact of PPIs in the survival outcomes in the fluorouracil arm rather than a negative impact of PPIs in the capecitabine arm. The possible drug‐drug interaction shown in this study suggests that fluorouracil, rather than capecitabine, could be a more appropriate choice of fluoropyrimidine for patients who are taking PPIs in the treatment of metastatic colorectal cancer.
Keywords: Colorectal neoplasms, Proton pump inhibitors, Capecitabine, Fluorouracil
Short abstract
This article analyzes the differential effect of proton pump inhibitor use on capecitabine and fluorouracil using the data set from the AXEPT trial, which demonstrated the noninferiority of modified capecitabine plus irinotecan with FOLFIRI, either with or without bevacizumab in patients with metastatic colorectal cancer.
Introduction
Capecitabine is widely used as a treatment for many solid tumors as a convenient alternative to the infusion of fluorouracil [1]. In metastatic colorectal cancer (mCRC), capecitabine and fluorouracil have been used interchangeably as a monotherapy or in combination with bevacizumab or oxaliplatin [2, 3, 4]. With regard to the combination with irinotecan, the equivalence of capecitabine and fluorouracil in terms of safety and efficacy has long been questioned [5]. However, the recent phase III AXEPT trial demonstrated the noninferiority of capecitabine in combination with irinotecan (mXELIRI) in terms of overall survival compared with FOLFIRI (leucovorin, fluorouracil, and irinotecan), with or without bevacizumab as a second‐line treatment [6].
The degree of absorption of orally administered anticancer agents such as capecitabine in the gastrointestinal tract could significantly affect the pharmacokinetic profile of those drugs and thereby their safety and efficacy. The bioavailability of tyrosine kinase inhibitors is reduced with the administration of proton pump inhibitors (PPIs) because the solubility of the drugs is decreased in conditions of elevated intragastric pH [7]. However, whether the acidic environment of the stomach influences the absorption of capecitabine remains controversial. Although earlier studies have shown that neither intake of food nor antacids exerted a significant effect on the pharmacokinetics of capecitabine [8], more recent clinical investigations have suggested that PPIs could compromise the efficacy of capecitabine in gastrointestinal cancers [9, 10, 11]. However, the interaction between PPIs and capecitabine is yet to be demonstrated in a randomized trial comparing capecitabine with fluorouracil. If the absorption of capecitabine is decreased by PPIs to such an extent that the efficacy is significantly reduced, the comparability of capecitabine with fluorouracil would not be maintained in patients taking PPIs. As the subjects in the AXEPT trial were randomized to receive either capecitabine or fluorouracil, the data set of the AXEPT trial could be used to explore whether PPI use has a differential impact on capecitabine and fluorouracil in terms of the oncologic outcomes of mCRC.
Therefore, we used the data set from the AXEPT trial [6] to analyze whether there is an interaction between PPI use and the type of fluoropyrimidine (capecitabine vs. fluorouracil) in terms of efficacy and safety outcomes.
Materials and Methods
Patients
All patients in the per‐protocol population of the AXEPT trial with available information on concomitant medications were included in this post hoc analysis. Between December 2, 2013, and August 13, 2015, 650 patients were enrolled in the AXEPT trial from 98 hospitals in China, Japan, and South Korea. The cutoff date for the collection of survival data was July 28, 2017 [6]. The type and duration of concomitant medications were reviewed to determine the PPI use in each patient. PPI use was defined as the use of any PPI for 20% or more of the study period (from day 1 of cycle 1 to 30 days after the last dose), as defined in a previous study with a similar design with capecitabine [11]. The cutoff for defining PPI users was adapted from the authors’ former study on erlotinib [12]; albeit relatively lacking in empirical evidence, this cutoff was used in order to compensate for the considerable imbalance among the three countries in terms of the proportion of patients who took one or more doses of PPI (supplemental online Table 1). The proportion of PPI use versus non‐PPI use among the three countries was the most balanced when we defined the PPI use as PPI intake for 20% or more of the study treatment duration.
The AXEPT trial was conducted in accordance with the tenets of the Declaration of Helsinki and Good Clinical Practice. The study protocol was approved by the institutional review boards or independent ethics committees of all participating institutions, and written informed consent was obtained from all participants. This study was registered in ClinicalTrials.gov, number NCT01996306.
Post Hoc Analysis
The primary objective of the current study was to estimate the interaction between PPI exposure and treatment arm (capecitabine or fluorouracil) in terms of overall survival (OS), which was the primary endpoint of the AXEPT trial. The secondary objective was to estimate the same interaction for progression‐free survival (PFS), time to treatment failure (TTF), overall response rate (ORR), disease control rate (DCR), and grade 3–4 toxicities.
Statistical Analysis
The baseline characteristics are summarized as number (%) for categorical variables and median (interquartile range) for continuous variables. Differences between groups were analyzed using Pearson's chi‐squared test or Fisher's exact test for categorical variables, or Wilcoxon rank‐sum test for continuous variables.
Survival curves were plotted using the Kaplan‐Meier method. The hazard ratios (HRs) with 95% confidence intervals (CIs) for OS, PFS, and TTF were estimated using a stratified Cox proportional hazard model and the randomization stratification factors (i.e., country, performance status, number of metastatic sites, previous use of oxaliplatin treatment, and concurrent bevacizumab treatment). The ORR, DCR, odds ratios, and 95% CIs were calculated using stratified logistic regression models. Interactions between treatment allocation and the PPI exposure for individual endpoints were evaluated using the Cox proportional hazards regression and logistic regression models, respectively. Two‐sided values of p < .05 were considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Role of the Funding Source
The funder of the study was not involved in the study design, data analysis, and writing of the report. The corresponding author had full access to all the data used in the study and had the final responsibility for the decision to submit for publication.
Results
Patient Characteristics
Of the 620 patients in the per‐protocol population of the AXEPT trial, 482 had available information on concomitant medication. The patient demographics and disease characteristics of the post hoc study population were largely consistent with those of the intention‐to‐treat population (supplemental online Table 2). Among the 482 post hoc study population, 49 (10.1%; 7.9% of the total intention‐to‐treat population) patients were exposed to PPIs for 20% or more of the study period (Fig. 1). Patient characteristics including stratification factors and treatment arm allocation (FOLFIRI vs. mXELIRI) were balanced between patients who were exposed to PPIs (PPI users) and those who were not exposed (non‐PPI users) (Table 1).
Figure 1.
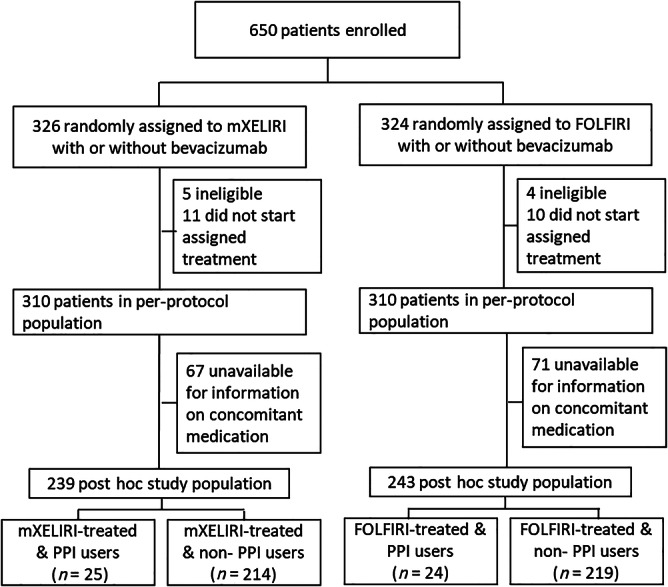
Trial profile and selection process of the post hoc study population.
Abbreviations: FOLFIRI, leucovorin, fluorouracil, and irinotecan; mXELIRI, modified capecitabine and plus irinotecan; PPI, proton pump inhibitor.
Table 1.
Patient characteristics according to the use of PPIs
| Characteristic | PPI use <20% (n = 433), n (%) | PPI use ≥20% (n = 49), n (%) | p value |
|---|---|---|---|
| Age (years) | .46 | ||
| <65 | 305 (70.4) | 32 (65.3) | |
| ≥65 | 128 (29.6) | 17 (34.7) | |
| Median (IQR) | 59 (51–66) | 60 (48–69) | .72 |
| Sex | .31 | ||
| Men | 262 (60.5) | 26 (53.1) | |
| Women | 171 (39.5) | 23 (46.9) | |
| Country | .37 | ||
| Japan | 103 (23.8) | 15 (30.6) | |
| South Korea | 203 (46.9) | 18 (36.7) | |
| China | 127 (29.3) | 16 (32.7) | |
| ECOG performance status | >.99 | ||
| 0−1 | 430 (99.3) | 49 (100.0) | |
| 2 | 3 (0.7) | 0 (0.0) | |
| Stage at diagnosis | .81 | ||
| Synchronous metastasis | 261 (60.3) | 31 (63.3) | |
| Metachronous metastasis | 170 (39.3) | 18 (36.7) | |
| Unknown | 2 (0.5) | 0 (0.0) | |
| Number of metastatic sites | .10 | ||
| 1 | 157 (36.3) | 12 (24.5) | |
| >1 | 276 (63.7) | 37 (75.5) | |
| Liver metastasis: Yes | 260 (60.0) | 28 (57.1) | .70 |
| Liver‐limited metastasis: Yes | 75 (17.3) | 6 (12.2) | .37 |
| Previous use of oxaliplatin: Yes | 426 (98.4) | 49 (100.0) | >.99 |
| Postoperative adjuvant chemotherapy: Yes | 112 (25.9) | 15 (30.6) | .48 |
| Previous use of anti‐EGFR antibody therapy: Yes | 58 (13.4) | 7 (14.3) | .86 |
| Concomitant bevacizumab in this study: Yes | 345 (79.7) | 38 (77.6) | .73 |
| Alkaline phosphatase | .12 | ||
| Normal | 266 (61.4) | 36 (73.5) | |
| Abnormal | 144 (33.3) | 13 (26.5) | |
| Unknown | 23 (5.3) | 0 (0.0) | |
| UGT1A1 polymorphism | .12 | ||
| Wild type | 217 (50.1) | 19 (38.8) | |
| Single heterozygote | 182 (42.0) | 28 (57.1) | |
| Double heterozygotes or homozygotes | 34 (7.9) | 2 (4.1) | |
| KRAS status | .29 | ||
| Wild type | 188 (43.4) | 19 (38.8) | |
| Mutant | 131 (30.3) | 12 (24.5) | |
| Unknown | 114 (26.3) | 18 (36.7) | |
| Treatment | .83 | ||
| FOLFIRI | 219 (50.6) | 24 (49.0) | |
| mXELIRI | 214 (49.4) | 25 (51.0) |
p value was calculated by chi‐squared test, Fisher's exact test, or Wilcoxon rank‐sum test.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; FOLFIRI, leucovorin, fluorouracil, and irinotecan; mXELIRI, modified capecitabine plus irinotecan; PPI, proton pump inhibitor; IQR, interquartile range; UGT1A1, UDP‐glucuronosyltransferase 1A1.
Interestingly, the duration of PPI use was strikingly different among the three countries. In China, 86% of the patients used PPIs at least once, but more than half of them used the PPIs for less than 10% of the study period. In Japan, whereas only 12.7% of patients used PPI, all of them used the PPIs for more than 50% of the study period (supplemental online Table 1). The pattern of PPI use in South Korea was in between those of the other two countries. The proportion of PPI users was balanced across the three countries.
OS According to PPI Exposure
In the overall post hoc study population (n = 482), the OS was representative of the main study outcome of the intention‐to‐treat population (HR, 0.86; 95% CI, 0.70–1.06; p = .15) [6]. Among the non‐PPI users, the OS was marginally better in the mXELIRI arm than in the FOLFIRI arm (16 vs. 15 months, respectively; HR, 0.80; 95% CI, 0.65–0.99; p = .044; Fig. 2A), whereas there was no statistically significant difference observed between the two arms among the PPI users (16 vs. 19 months, respectively; HR, 1.58; 95% CI, 0.85–2.94; p = .15; Fig. 2B). The interaction between PPI use and treatment arms (FOLFIRI vs. mXELIRI) was significant (p = .042 for interaction). Even after adjusting for stratification factors, the OS remained in favor of mXELIRI over FOLFIRI in non‐PPI users (HR, 0.76; 95% CI, 0.61–0.95; p = .016) but not in PPI users (HR, 1.83; 95% CI, 0.96–3.48; p = .064), and revealed a significant interaction (p = .012 for interaction; Table 2).
Figure 2.

Overall survival and progression‐free survival. Kaplan‐Meier curves showing overall survival according to treatment arm (FOLFIRI vs. mXELIRI) in non‐PPI users (A) and PPI users (B). Progression‐free survival in non‐PPI users (C) and PPI users (D).
Abbreviations: FOLFIRI, leucovorin, fluorouracil, and irinotecan; mXELIRI, capecitabine plus irinotecan; PPI, proton pump inhibitor.
Table 2.
Interaction of PPI with treatment in terms of study outcomes
| PPI and treatment | OS | PFS | TTF | Overall response | Disease control | Any grade 3/4 toxicities | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | p int | HR (95% CI) | p value | p int | HR (95% CI) | p value | p int | HR (95% CI) | p value | p int | HR (95% CI) | p value | p int | HR (95% CI) | p value | p int | |
| Univariate analysis | ||||||||||||||||||
| Non‐PPI user (n = 433) | .042 | .15 | .44 | .59 | .65 | |||||||||||||
| FOLFIRI | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||||||||||||
| mXELIRI |
0.80 (0.65–0.99) |
.044 |
0.92 (0.76–1.12) |
.42 |
0.88 (0.73–1.06) |
.18 |
1.47 (0.93–2.32) |
.10 |
1.44 (0.93–2.24) |
.10 |
0.51 (0.35–0.74) |
.0005 | ||||||
| PPI user (n = 49) | ||||||||||||||||||
| FOLFIRI | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||||||
| mXELIRI |
1.58 (0.85–2.94) |
.15 |
1.45 (0.81–2.60) |
.22 |
1.11 (0.63–1.96) |
.72 |
2.14 (0.59–7.68) |
.25 |
1.06 (0.29–3.88) |
.94 |
0.66 (0.22–2.05) |
.48 | ||||||
| Stratified analysis by the randomization factors | ||||||||||||||||||
| Non‐PPI user (n = 433) | .012 | .048 | .25 | .54 | .510 | .76 | ||||||||||||
| FOLFIRI | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||||||
| mXELIRI |
0.76 (0.61–0.95) |
.016 |
0.90 (0.73–1.10) |
.29 |
0.84 (0.69–1.03) |
.088 |
1.38 (0.85–2.23) |
.19 |
1.62 (0.99–2.63) |
.055 |
0.50 (0.33–0.74) |
.0006 | ||||||
| PPI user (n = 49) | ||||||||||||||||||
| FOLFIRI | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||||||
| mXELIRI |
1.83 (0.96–3.48) |
.064 |
1.73 (0.94–3.21) |
.080 |
1.21 (0.68–2.17) |
.52 |
2.17 (0.55–8.50) |
.27 |
0.98 (0.24–4.00) |
.98 |
0.60 (0.19–1.94) |
.40 | ||||||
Abbreviations: CI, confidence interval; FOLFIRI, leucovorin, fluorouracil, and irinotecan; HR, hazard ratio; mXELIRI, modified capecitabine plus irinotecan; OS, overall survival; p int, p value for interaction between treatment and PPI; PFS, progression‐free survival; PPI, proton pump inhibitor; TTF, time to treatment failure.
Secondary Outcomes
In univariate analysis, no significant interactions were found between PPI use and treatment arms in terms of PFS, TTF, ORR, DCR, and grade 3–4 toxicities (p > .05 for all interactions; Table 2). Meanwhile, the risk of grade 3–4 toxicities was significantly lower in the mXELIRI arm than in the FOLFIRI arm among the non‐PPI users, which is consistent with the results from the whole per‐protocol set [6].
Stratified analysis also showed no significant differences in PFS according to the regimens among the PPI users (HR, 1.73; 95% CI, 0.94–3.21; p = .080; Fig. 2C) and the non‐PPI users (HR, 0.90; 95% CI, 0.73–1.10; p = .29; Fig. 2D). The interaction between PPI use and treatment arms was marginally significant (p = .048 for interaction) for PFS in stratified analysis, whereas the interactions for other secondary outcomes (TTF, ORR, DCR, grade 3–4 toxicities) were not statistically significant (p > .05 for all interactions; Table 2).
PPI Versus No PPI
PPI use was not significantly associated with benefits in terms of OS and PFS in the overall post hoc study population as well as in each treatment arm in univariate analysis. In the FOLFIRI arm, however, a trend of a better OS with PPI use was observed (HR, 0.75; 95% CI, 0.46–1.22; p = .24; supplemental online Figs 1 and 2; Table 3); this tendency was more prominent in stratified analysis, which revealed a significantly better OS (HR, 0.5: 95% CI, 0.30–0.85; p = .011) and PFS (HR, 0.55; 95% CI, 0.33–0.91; p = .020) in PPI users. No significant difference was observed between the PPI users and nonusers in the mXELIRI arm (Table 3).
Table 3.
Association of PPI with survival outcomes in the whole study population and in each treatment arm
| PPI and treatment | OS, months | PFS, months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (95% CI) | Univariate analysis | Stratified analysis | Median (95% CI) | Univariate analysis | Stratified analysis | |||||
| uHR (95% CI) | p value | aHR | p value | uHR | p value | aHR | p value | |||
| All (n = 482) | ||||||||||
| Non‐PPI user | 16 (15–18) | 1 (Ref) | .80 | 1 (Ref) | .32 | 8 (7–9) | 1 (Ref) | .40 | 1 (Ref) | .11 |
| PPI user | 17 (13–21) | 1.04 (0.75–1.44) | 0.85 (0.61–1.18) | 10 (6–12) | 0.88 (0.64–1.19) | 0.77 (0.56–1.06) | ||||
| FOLFIRI (n = 243) | ||||||||||
| Non‐PPI user | 15 (13–17) | 1 (Ref) | .24 | 1 (Ref) | .011 | 7 (6–9) | 1 (Ref) | .12 | 1 (Ref) | .020 |
| PPI user | 19 (10–30) | 0.75 (0.46–1.22) | 0.50 (0.30–0.85) | 10 (5–16) | 0.70 (0.45–1.10) | 0.55 (0.33–0.91) | ||||
| mXELIRI (n = 239) | ||||||||||
| Non‐PPI user | 16 (15–20) | 1 (Ref) | .093 | 1 (Ref) | .22 | 8 (7–9) | 1 (Ref) | .62 | 1 (Ref) | .65 |
| PPI user | 16 (12–21) | 1.46 (0.94–2.26) | 1.32 (0.84–2.08) | 8 (5–12) | 1.11 (0.73–1.70) | 1.11 (0.71–1.74) | ||||
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; FOLFIRI, leucovorin, fluorouracil, and irinotecan; mXELIRI, modified capecitabine plus irinotecan; OS, overall survival; PFS, progression‐free survival; PPI, proton pump inhibitor; uHR, unadjusted hazard ratio.
Discussion
This post hoc analysis of the AXEPT trial demonstrated a significant interaction between PPI use and the type of fluoropyrimidine (fluorouracil vs. capecitabine) in terms of OS and PFS in patients with mCRC. The capecitabine‐containing regimen, mXELIRI, was associated with less benefit than FOLFIRI in PPI users, whereas the opposite phenomenon was found in nonusers, which suggests a potential drug‐drug interaction.
The increase in intragastric pH resulting from the use of antacids or PPIs affects the solubility and absorption of many oral drugs, and capecitabine is no exception. The results of several clinical studies support the idea that PPIs could hamper the absorption and distribution of capecitabine, and eventually influence the efficacy of the drug (supplemental online Table 3). An ad hoc analysis of the TRIO‐013/LOGiC trial, which tested capecitabine plus oxaliplatin (CapOx) with or without lapatinib in patients with metastatic gastroesophageal cancer, demonstrated a statistically significant deterioration of PFS and OS in patients who were administered PPIs [11]. Furthermore, a single‐center retrospective study reported worse recurrence‐free survival in PPI users than in nonusers among patients with early colorectal cancer who were given adjuvant chemotherapy with capecitabine monotherapy [10]. Another study compared the recurrence‐free survival between PPI users and nonusers with early colorectal cancer and suggested that the negative impact of PPI on the oncologic outcome is specific for capecitabine and not for intravenous fluorouracil; moreover, the risk of recurrence or death was significantly higher in PPI users than in nonusers among those treated with CapOx, whereas a trend of better outcomes in PPI users was noted in those given fluorouracil, leucovorin, and oxaliplatin (FOLFOX) [9].
In the current study, we focused on the difference between capecitabine and fluorouracil in PPI users compared with nonusers, because the AXEPT was a randomized trial that had comparability between patients treated with capecitabine and those treated with fluorouracil. Our results suggest that capecitabine may be associated with inferior OS and PFS compared with fluorouracil specifically in PPI users and not in nonusers. The interaction between study drugs (capecitabine vs. fluorouracil) and PPI use was statistically significant in terms of OS (p = .012) and PFS (p = .048). To the best of our knowledge, our study is the first to show a significant interaction between PPI exposure and drug treatment in patients with mCRC using a data set from a randomized trial comparing capecitabine with fluorouracil.
Unlike other studies, our current study did not show a significantly poorer outcome in PPI users compared with nonusers among capecitabine‐treated patients. The significant interaction shown in our study seems to highlight PPI‐associated benefits in the FOLFIRI arm rather than a possible detrimental effect of PPIs in the mXELIRI arm. Among FOLFIRI‐treated patients, PPI users showed a numerically longer median OS (19 vs. 15 months) and PFS (10 vs. 7 months) than did nonusers, with a significant difference in stratified analysis; in contrast, mXELIRI‐treated patients showed similar outcomes between PPI users and nonusers. A trend toward a beneficial effect of PPIs in fluorouracil‐treated patients was also observed in previous studies; as an example, Wong et al. reported that FOLFOX‐treated PPI recipients tended to have a longer 3‐year recurrence‐free survival compared with non‐PPI recipients (82.9% vs. 61.7%; adjusted HR, 0.51; p = .066) [9]. These findings may be due to the direct effect of PPIs on suppressing cancer growth [13] or increasing the chemosensitivity of cancer cells to fluorouracil [14]. This hypothesis is supported by a recent study, which showed that coadministration of pantoprazole with fluorouracil to colorectal cancer cell lines or xenografts resulted in further growth inhibition than fluorouracil alone; this study also showed improvements in OS and PFS from PPI in FOLFOX‐treated patients with mCRC, but not in those treated with CapOx [15]. The reduced benefit of PPIs with capecitabine than with fluorouracil could also be explained by reductions in drug absorption, because capecitabine is converted to fluorouracil after absorption.
Notwithstanding the consistent clinical study results including our own, the drug‐drug interaction between capecitabine and PPIs has yet to be supported by plausible pharmacokinetic evidence. Concomitant use of Maalox, an antacid containing magnesium hydroxide and aluminum hydroxide, did not significantly affect the extent and rate of absorption of capecitabine [16]. PPIs induce a stronger acid suppression than antacids, but the dissociation constant of capecitabine is 8.8, indicating that its absorption might be minimally affected in the elevated pH conditions (pH 3.5–4.9) induced by PPIs [7]. Therefore, drug‐drug interaction studies are warranted to understand the effect of PPIs on the pharmacokinetics of capecitabine.
Our current study showed an unexpected finding between the pattern of PPI use by patients with cancer in three Asian countries. All Japanese PPI users showed PPI usage for over 50% of the study period, whereas Chinese patients were more likely to show short‐term use (<20% of the study period). The pattern of PPI use in South Korea was partway between those observed in China and Japan. These discrepancies are likely to have been caused by different practices in antiemetic prophylaxis in each country. Although the National Comprehensive Cancer Network guideline for antiemesis recommends that PPIs or histamine receptor 2 antagonists could be used to prevent dyspepsia that mimics nausea before the administration of chemotherapeutic agents, the prescription patterns could be considerably different between countries [17]. We defined PPI users as patients who took PPIs for more than 20% of the study period by referring to the definition used in the ad hoc analysis of the TRIO‐013/LOGiC trial [11]. This cutoff, which was somewhat arbitrarily defined, was necessary to exclude patients who used PPI for brief periods from the “PPI users” because they were less likely to be influenced by the drug‐drug interaction. Indeed, the increases in intragastric pH after PPI use are quickly normalized within 12–14 hours because of the physiological generation of new proton pumps [18]. Furthermore, the proportion of short‐term users was not well balanced among the countries (supplemental online Table 1). Thus, the risk of bias from different prescription and management practices between nationalities would be unavoidable if short‐term users were to be grouped into the class of PPI users. In contrast, the proportion of long‐term users was well balanced among the three countries, which enabled adequate analysis of the data set after controlling for confounding variables.
There are several limitations to our study. First, the pharmacokinetic data of capecitabine and fluorouracil were lacking, which hindered the analysis of the association between PPI intake and the concentrations of capecitabine or fluorouracil. Second, we cannot exclude the possibility of bias stemming from missing data considering that a considerable proportion of the per‐protocol population (22.3%, 138/620) lacked information on concomitant medication and were therefore not included in the analysis. Third, the definition of PPI use followed the criteria used in a previous study [11]; however, the scientific rationale of the cutoff is weak. The association between the duration of PPI and the degree of pharmacokinetic and pharmacodynamic changes in capecitabine as well as other chemotherapeutic agents has rarely been investigated and warrants further targeted investigation using prospective study designs. Fourth, as only about 10% of the study population (49 out of 482) were classified as PPI users, this study does not have enough power to provide a strong conclusion on the drug‐drug interaction between PPI and capecitabine. This proportion is quite low compared with those reported in other studies in which 25% to 70% were PPI users; although the definition of PPI use was different among the studies (supplemental online Table 3) [9, 10, 11], the difference in the proportion of PPI users may be reflecting regional differences in the prescription pattern or disease characteristics of study populations. Studies using large‐scale, real‐world data on PPIs for patients receiving capecitabine are needed to fully elucidate the details of the drug‐drug interaction in diverse clinical settings.
Conclusion
We found that PPI use has a significant qualitative interaction with the type of fluoropyrimidine in terms of OS and PFS in patients with mCRC. Concomitant use of PPIs was associated with less benefit in terms of OS and PFS in the mXELIRI arm than in the FOLFIRI arm, suggesting that fluorouracil may be preferable to capecitabine in patients who are being treated with PPIs. Moreover, PPI use was associated with greater benefits in terms of OS and PFS in FOLFIRI‐treated patients and not in mXELIRI‐treated patients. We did not observe a significant antagonistic effect of PPIs on capecitabine‐treated patients, whereas the improved outcomes with PPI in fluorouracil‐treated patients could be the cause of the interaction. The drug‐drug interactions between PPIs and capecitabine or fluorouracil warrant further investigation.
Author Contributions
Conception/design: Sun Young Kim, Tae Won Kim
Provision of study material or patients: Satoshi Morita
Collection and/or assembly of data: Ji Sung Lee
Data analysis and interpretation: Sun Young Kim, Ji Sung Lee, Junho Kang
Manuscript writing: Sun Young Kim, Junho Kang
Final approval of manuscript: Sun Young Kim, Ji Sung Lee, Junho Kang, Satoshi Morita, Young Suk Park, Junichi Sakamoto, Kei Muro, Rui‐Hua Xu, Tae Won Kim
Disclosures
Sun Young Kim: Roche/Genentech (RF); Satoshi Morita: AstraZeneca KK, Bristol‐Myers Squibb, Chugai Pharmaceutical, Eisai, Eli Lilly & Co., Merck Sharp & Dohme, Nippon Boehringer Ingelheim Co. Ltd, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical (H), Nippon Boehringer Ingelheim Co. Ltd (RF); Junichi Sakamoto: Chugai Pharmaceutical, Nihon Karaku Co. Ltd (H), Olympus Medical Systems, Takeda (C/A); Kei Muro: Bayer, Chugai Pharmaceutical, Eli Lilly & Co., Merck Serono, Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical, Takeda, Yakult Honsha (H), Daiichi Sankyo, Gilead Sciences, Kyowa Hakko Kirin, Mediscience Planning, Merck Serono, Merck Sharp & Dohme, Ono Pharmaceutical, Parexel International, Pfizer, Sanofi, Shionogi, Solasia Pharmaceutical, Sumitomo Dainippon (RF); Rui‐Hua Xu: Merck KGaA, Roche (H); Tae Won Kim: AstraZeneca, Merck Serono, Sanofi (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Overall survival (A) and progression‐free survival (B) according to PPI use in the FOLFIRI arm
Table S1 Difference in the patterns of PPI use across the three Asian countries
Table S2. Characteristics of the intention‐to‐treat population and the post hoc study population
Table S3. Studies assessing the interaction between PPI and capecitabine
Acknowledgments
The AXEPT trial was sponsored by the Epidemiological and Clinical Research Information Network, the Asan Medical Center Academic Research Office, and the Sun Yat‐sen University Cancer Center and was funded by Chugai Pharmaceutical and F Hoffmann La Roche. We thank Dr. Joon Seo Lim from the Scientific Publications Team at Asan Medical Center for his assistance with the scientific editing of the manuscript. J.K. is currently affiliated with the Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, South Korea.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Koukourakis GV, Kouloulias V, Koukourakis MJ et al. Efficacy of the oral fluorouracil pro‐drug capecitabine in cancer treatment: A review. Molecules 2008;13:1897–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cassidy J, Clarke S, Diaz‐Rubio E et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first‐line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006–2012. [DOI] [PubMed] [Google Scholar]
- 3. Saltz LB, Clarke S, Diaz‐Rubio E et al. Bevacizumab in combination with oxaliplatin‐based chemotherapy as first‐line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 2008;26:2013–2019. [DOI] [PubMed] [Google Scholar]
- 4. Cunningham D, Lang I, Marcuello E et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open‐label, randomised phase 3 trial. Lancet Oncol 2013;14:1077–1085. [DOI] [PubMed] [Google Scholar]
- 5. Fuchs CS, Marshall J, Mitchell E et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first‐line treatment of metastatic colorectal cancer: Results from the BICC‐C study. J Clin Oncol 2007;25:4779–4786. [DOI] [PubMed] [Google Scholar]
- 6. Xu RH, Muro K, Morita S et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second‐line therapy for metastatic colorectal cancer (AXEPT): A multicentre, open‐label, randomised, non‐inferiority, phase 3 trial. Lancet Oncol 2018;19:660–671. [DOI] [PubMed] [Google Scholar]
- 7. Cheng V, Lemos M, Hunter N et al. Concomitant use of capecitabine and proton pump inhibitors ‐ is it safe? J Oncol Pharm Pract 2019;25:1705–1711. [DOI] [PubMed] [Google Scholar]
- 8. Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet 2001;40:85–104. [DOI] [PubMed] [Google Scholar]
- 9. Wong GG, Ha V, Chu MP et al. Effects of proton pump inhibitors on FOLFOX and CapeOx regimens in colorectal cancer. Clin Colorectal Cancer 2019;18:72–79. [DOI] [PubMed] [Google Scholar]
- 10. Sun J, Ilich AI, Kim CA et al. Concomitant administration of proton pump inhibitors and capecitabine is associated with increased recurrence risk in early stage colorectal cancer patients. Clin Colorectal Cancer 2016;15:257–263. [DOI] [PubMed] [Google Scholar]
- 11. Chu MP, Hecht JR, Slamon D et al. Association of proton pump inhibitors and capecitabine efficacy in advanced gastroesophageal cancer: Secondary analysis of the TRIO‐013/LOGiC randomized clinical trial. JAMA Oncol 2017;3:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu MP, Ghosh S, Chambers CR et al. Gastric acid suppression is associated with decreased erlotinib efficacy in non–small‐cell lung cancer. Clin Lung Cancer 2015;16:33–39. [DOI] [PubMed] [Google Scholar]
- 13. Zeng X, Liu L, Zheng M et al. Pantoprazole, an FDA‐approved proton‐pump inhibitor, suppresses colorectal cancer growth by targeting T‐cell‐originated protein kinase. Oncotarget 2016;7:22460–22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng S, Zheng Z, Feng L et al. Proton pump inhibitor pantoprazole inhibits the proliferation, self‐renewal and chemoresistance of gastric cancer stem cells via the EMT/β‐catenin pathways. Oncol Rep 2016;36:3207–3214. [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Liu C, Wang J et al. Proton pump inhibitors increase the chemosensitivity of patients with advanced colorectal cancer. Oncotarget 2017;8:58801–58808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reigner B, Clive S, Cassidy J et al. Influence of the antacid Maalox on the pharmacokinetics of capecitabine in cancer patients. Cancer Chemother Pharmacol 1999;43:309–315. [DOI] [PubMed] [Google Scholar]
- 17. Borner MM, Schoffski P, de Wit R et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: A randomised crossover trial in advanced colorectal cancer. Eur J Cancer 2002;38:349–358. [DOI] [PubMed] [Google Scholar]
- 18. van Leeuwen RWF, Jansman FGA, Hunfeld NG et al. Tyrosine kinase inhibitors and proton pump inhibitors: An evaluation of treatment options. Clin Pharmacokinet 2017;56:683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Overall survival (A) and progression‐free survival (B) according to PPI use in the FOLFIRI arm
Table S1 Difference in the patterns of PPI use across the three Asian countries
Table S2. Characteristics of the intention‐to‐treat population and the post hoc study population
Table S3. Studies assessing the interaction between PPI and capecitabine


