Abstract
SARS-CoV-2 infects host cells mainly through the interaction between the virus's Spike protein and the viral receptors namely Angiotensin-Converting Enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2). Both are highly expressed in the gastrointestinal tract, in the nasal and bronchial epithelium, as well as in the type II alveolar epithelial cells. The aim of this review is to report the evidences from the scientific literature on the pathophysiology and the available treatments for olfactory-gustatory disorders in patients with COVID-19. The mechanisms involved in these disorders are still unclear and studies on specific therapies are scarce. However, it has been hypothesized that a decrease in the sensitivity of the sensory neurons as well as the co-expression of ACE2 and TMPRSS2 in the alveolar epithelial cells are the main causes of olfactory-gustatory disorders. The possible mechanisms described in the literature for changes in taste perception in patients with COVID-19 include olfactory disorders and a competitive activity of COVID-19 on ACE2 receptors in the taste buds. In addition, SARS-CoV-2 can bind to sialic acid receptors in the taste buds. In general, evidences show that there is no specific treatment for olfactory-taste disorders induced by SARS-CoV-2, even though some treatments have been used and have shown some promising results, such as olfactory training, intranasal application of sodium citrate and vitamin A, as well as systemic use of omega-3 and zinc. Corticosteroids have also been used as a pharmacological approach to treat patients with olfactory dysfunction with some contradictory results. The knowledge of the mechanisms by which SARS-CoV-2 influences the sensory systems and how effective therapies can treat the loss of smell and taste will have important implications on the understanding and clinical management of olfactory-taste disorders.
Keywords: COVID-19, Smell, Taste, Anosmia, Ageusia, Therapeutics
1. Introduction
In the 1960s, human coronaviruses (HCoVs) were first identified in the nasal cavities of patients with cold (Ogimi et al., 2020). Coronaviruses (CoV) are a large family of viruses that cause diseases that range from a common cold to more serious disorders such as the Middle East Respiratory Syndrome (MERS-CoV) and the Severe Acute Respiratory Syndrome (SARS)–CoV (Cascella et al., 2020). SARS-CoV-2 belongs to the family of coronaviruses, which includes the pandemic MERS-CoV and SARS-CoV as well as the lesser known but more common endemic coronaviruses, HCoV-OC43, HCoV-HKU1, HCoV-229E and HCoV-NL63 (Cooper et al., 2020).
The disease caused by the new coronavirus was named coronavirus-19 disease (COVID-19) by the World Health Organization (WHO) in February 2020 (Keyhan et al., 2020). WHO has registered 126,697,603 confirmed cases of COVID-19 worldwide with 2,776,175 deaths in 223 countries as of March 29, 2021 (WHO, 2021).
COVID-19 is an infectious disease that varies from mild to more severe respiratory disorders, with common respiratory manifestations such as dry cough, fever, dyspnoea, arthralgia and malaise (Villalba et al., 2020). Less common symptoms such as nausea, vomiting, diarrhea and abdominal pain might occur (Song et al., 2020) and, in several countries, many infected people have reported taste and smell dysfunctions (Lovato et al., 2020).
An observational study with more than two million participants found that loss of smell and taste is more predictive than all other symptoms, including fatigue, fever or cough (Menni et al., 2020). A systematic review involving 6 studies and 1457 infected patients revealed that 60% had loss of smell and 56% had loss of taste, whose beginning of symptoms appeared before the common signs and symptoms of COVID-19 (Costa et al., 2020). The presence of anosmia or ageusia may be useful as a red flag for COVID-19. The loss of sense of smell or taste substantially increases the likelihood of COVID-19 infection. For instance, in a population in which 2% of the people have COVID-19, the loss of smell or taste would increase a persons' likelihood of having COVID-19 to 8% (Struyf et al., 2020).
Several evidences regarding potential treatments for post-viral morbidity have been reported, but few studies have exclusively investigated olfactory and gustatory loss following COVID-19 infection. Most of trials include a very small number of patients and the majority lacks blinding, randomisation and a control group. In addition, the rates of improvement after treatment are usually no greater than the reported rates of spontaneous improvement (Vaira et al., 2020b). The aim of this review is to clarify with evidence from the scientific literature the pathophysiology of olfactory-gustatory disorders from COVID-19 infection as well as to discuss potential therapies for anosmia and ageusia.
2. Pathophysiology of SARS-CoV-2 infection
SARS-CoV-2 enters the cell mainly through the interaction between the virus's Spike protein (protein S) and the Angiotensin-Converting Enzyme 2 (ACE2) receptor in the target cells (Walls et al., 2020; Zhou et al., 2020). In addition, the virus uses the transmembrane serine protease 2 (TMPRSS2) for the priming of protein S, implying the cleavage of the S1/S2 site. This process allows the fusion of viral and cellular membranes, a process conducted by the S2 subunit leading the virus to fuse with respiratory epithelia on the cell surface by binding to ACE2 (Hoffmann et al., 2020).
The dissemblance between SARS-CoV and SARS-CoV-2 in respect to their impacts on chemosensory systems may be related to biophysical differences as SARS-CoV-2 binds to ACE2 with greater affinity than does SARS-CoV (Walls et al., 2020). The pathogenesis of SARS-CoV-2 in most cases is similar to that of other respiratory viruses. Thus, most of the cases of COVID-19 reported in the literature are mild cases of upper respiratory tract infection. Studies that conducted virological analysis concluded that the greatest viral replication occurs in the upper respiratory tract on the fourth day of infection (Ylikoski et al., 2020).
Primary sites of SARS-CoV-2 infection are lungs and gastrointestinal tract, however. SARS-CoV-2 also affects multiple organ systems with major targets being the heart and kidneys (Trougakos et al., 2021). A systematic review involving 198 individual cases of COVID-19, in which pathological findings were reviewed, has revealed that in addition to the lungs, SARS-CoV-2 was detected in several other organs, including heart, liver, kidneys, gastrointestinal tract, spleen, lymph nodes, skin and placenta (Polak et al., 2020).
More recently, it was found that furin protease is also involved in the infection process as SARS-CoV-2 contains a site for furin cleavage in the S protein and that the cellular receptor neuropilin-1 (NRP1, binds to furin-cleaved substrates) potentiates SARS-CoV-2 infectivity also providing a pathway into the central nervous system (CNS). The two main infection pathways are the hematogenous and the neuronal, with the olfactory route (where nasal cell express high levels of ACE2), along with the lymphatic tissue and the cerebrospinal fluid play a significant role in SARS-CoV-2 neuroinvasion (Trougakos et al., 2021).
In humans, the mechanisms of possible viral transport via the olfactory nerve and subsequent spread in the CNS are poorly understood. SARS-CoV-2 seems to enter the CNS via the olfactory or trigeminal route. Initially, the CNS infection or inflammation could be relatively mild and cause olfactory damage (Ylikoski et al., 2020). The pathophysiology of anosmia associated with COVID-19 is still under debate and several mechanisms have been proposed. One hypothesis is that it can result from the obstruction of the olfactory clefts, thus preventing the activation of sensory neurons in the olfactory epithelium (Tham et al., 2020). Other mechanisms have been proposed to explain the loss of taste caused by SARS-CoV-2 such as the occupation of sialic acid receptors by the virus which results in the degradation of taste particles (Vaira et al., 2020a).(Figure 1).
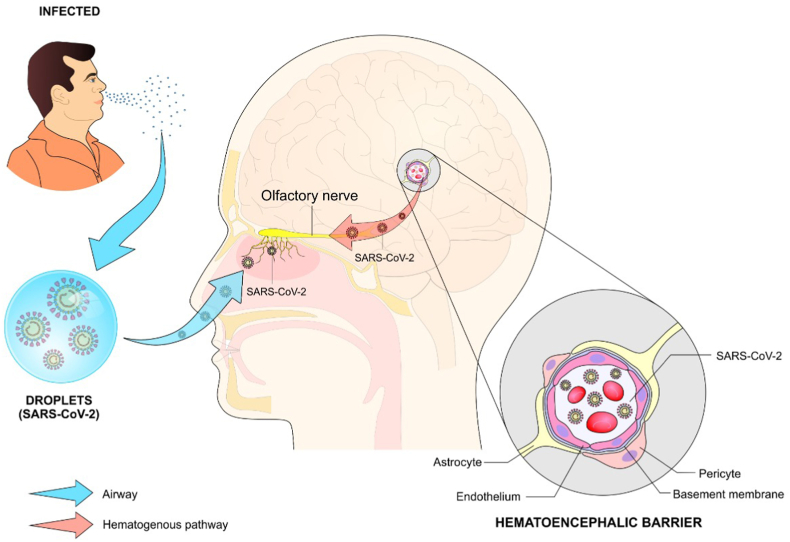
Figure 1.
Possible mechanisms involved in the olfactory dysfunction (anosmia) in patients with COVID-19. After contact with droplets containing viral particles from an infected patient, the virus penetrates the nasal cavity reaching the receptors for smell and the olfactory nerve, which characterizes the transneural pathway. On the other hand, the virus might reach these structures through the hematogenous route or by breaking the blood-brain barrier.
3. Effect of COVID-19 on smell
Some studies suggest that viruses such as rhinovirus, parainfluenza, Epstein Barr and SARS-CoV-2 can cause olfactory dysfunctions by mechanisms other than nasal obstruction, which may involve specific tropism of these viruses by structures of the sensory olfactory epithelium (Corbellini et al., 2020). Zayet and colleagues compared the clinical characteristics of COVID-19 with those of influenza and found a higher prevalence of anosmia (53 vs. 17%) and dysgeusia (49 vs. 20%) in patients with COVID-19 (Zayet et al., 2020).
COVID-19 has been associated with temporary olfactory loss in a large proportion of infected patients (Klopfenstein et al., 2020; Moein et al., 2020; Lechien et al., 2020; Costa et al., 2020). A study conducted by the Global Consortium for Chemosensory Research (GCCR) with 4039 participants from 41 different countries showed that 89% of the patients reported loss of smell at some degree (Parma et al., 2020). Similarly, Lechien et al. found that 81.6% of the infected patients reported total loss of smell while 18.4% reported partial loss. In addition, a total of 328 patients (24.5%) had not yet recovered their sense of smell even 60 days after the onset of the dysfunction (Lechien et al., 2021).
Boscolo-Rizzo et al. (2021) carried out a prospective study with 183 patients that tested positive for SARS-CoV-2 and had mild to moderate symptoms. The study investigated the prevalence of olfactory dysfunction during 6 months with 18% of the subjects describing altered sense of smell or taste. After 6 months, 85 (77.3%) subjects self-reported a complete resolution of these symptoms, whereas 22 (20.0%) reported a relative improvement and only 3 subjects (2.7%) informed that the symptoms had worsened or remained unchanged.
The importance of studies that demonstrate that patients affected by COVID-19 can persist with decreased sense of smell or total loss for 6 months after infection lies in the fact that potential treatments for their complete recovery need to be investigated. Two other studies reveal that some patients infected with SARS-CoV-2 reported persisting parosmia for 6 months after the onset of the disease (Hopkins et al., 2021; Berlich et al., 2021). The first study showed a prevalence of parosmia in 43% of the patients with an average duration of 2.5 months (Hopkins et al., 2021). In the second one, five patients (21.7%) out of twenty-three that had been diagnosed with hyposmia still suffered from impaired chemosensory function 6 months after initial diagnosis (Berlich et al., 2021).
The mechanisms involved in the olfactory dysfunctions in those with COVID-19 are still uncertain. One of the hypotheses is based on the ability of SARS-CoV-2 virus to cross the blood-brain barrier and enters the brain, or that the virus probably reaches the brain via a hematogenous route (Garg et al., 2020). On the other hand, the route of entry of the coronavirus into the brain might be through: 1- the olfactory nerves; 2- the cribriform plaque or 3- the peripheral trigeminal. The latter phenomenon can compromise the trigeminal and olfactory nerve resulting in dysosmia and dysgeusia (Natoli et al., 2020; Keyhan et al., 2020; Li, 2020). (Figure 2).
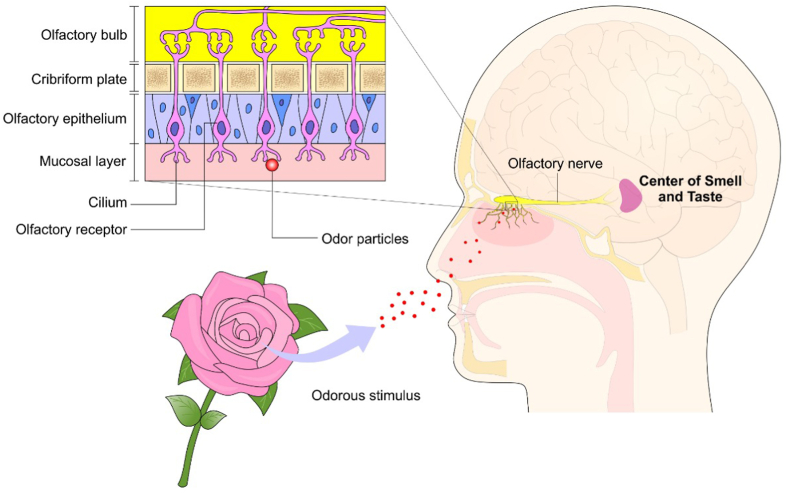
Figure 2.
Olfactory afferent pathway. The odorous particles enters the nasal cavity and directly stimulate the cilium of the olfactory receptors that connect the nerve fibers of the olfactory bulb, leading the stimulus to the center of smell and taste.
In addition, another possible mechanism is through a decrease in the sensitivity of the sensory neuron reflexes (Keyhan et al., 2020). However, four recently published studies (Brann et al., 2020; Chen et al., 2020; Fodoulian et al., 2020; Ziegler et al., 2020) investigated the cells in the olfactory epithelium that express ACE2 and other genes of viral entry and concluded that olfactory sensory neurons do not express ACE2. In contrast, the coexpression of ACE2 and TMPRSS2 has been observed in key support cells (including sustaining cells, Bowman's gland and microvillary cells) and in stem cells that repopulate the epithelium after damage (Brann et al., 2020).
There may be other ways in which COVID-19 induces anosmia. A study conducted by Cazzolla et al. showed that anosmia and ageusia happen simultaneously with the increase in the levels of interleukin-6 (IL-6), an important pro-inflammatory cytokine (Cazzolla et al., 2020). This hypothesis is supported by Tham et al. who stated that the inflammatory cytokine environment in the nasal cavity can potentially affect olfactory neuronal function, as in chronic rhinosinusitis (Tham et al., 2020). (Figure 3).
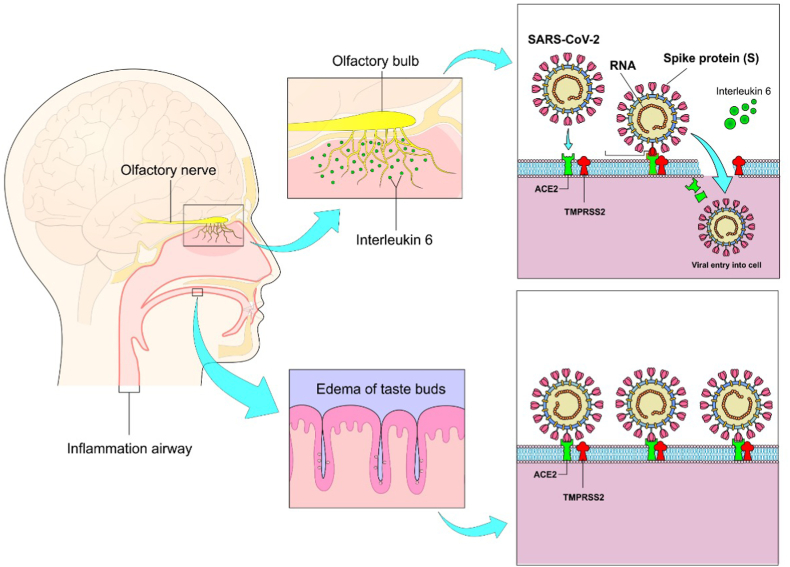
Figure 3.
Mechanisms involved in anosmia and ageusia: 1- Direct action of the virus and inflammatory reaction mediated by interleukin-6 in olfactory receptors resulting in impaired sense of smell (above). 2- Occupation of sialic acid receptors by the virus itself, accelerating the degradation of taste buds (below).
Some studies have reported inflammatory damage to the olfactory epithelium after SARS-CoV-2 infection. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31525-7/fulltextKirschenbaum et al. (2020) reported two cases of olfactory neuropathy in patients with severe acute SARS-CoV-2 infection, with one of the patients reporting anosmia. The authors concluded that it is uncertain whether the inflammatory neuropathy resulted from direct viral damage or was mediated by damage to supporting non-neural cells (Kirschenbaum et al., 2020). A case report has demonstrated significant disruption of the olfactory epithelium in the biopsy performed on a patient with persisting anosmia for more than three months after infection. Thus, they pointed out that histopathological findings suggest that failure of epithelial repair leads to thinning and loss of the olfactory dendrites and that disruption and desquamation of the olfactory epithelium is the underlying mechanism in COVID-19 related olfactory dysfunction (Vaira et al., 2020a).
4. Effects of COVID-19 on the taste
The study conducted by GCCR found that 76% of the participants reported loss of taste and 46% had reduced chemesthesia (detection of chemicals that evoke tingling and burning sensations), indicating that chemosensory impairment is not restricted to smell (Parma et al., 2020).
One possible reason for the acquired ageusia is that the ability to perceive flavors in patients with COVID-19 is affected by the concomitant presence of olfactory disorders, due to the intimate functioning between these two chemosensory systems (Small and Prescott, 2005). However, previous reports have shown that gustatory dysfunctions are always more frequent than olfactory ones and occur alone in 10.2–22.5% of patients (Vaira et al., 2020b; Yan et al., 2020a).
Another explanation for the acquired ageusia may be associated with a competitive activity of SARS-CoV-2 on ACE2 receptors in the taste buds (Vaira et al., 2020c; Tsuruoka et al., 2005). In fact, Xu and collaborators investigated the expression of ACE2 in the oral cavity and found that ACE2 receptors are expressed diffusely between the different locations of the oral cavity, being particularly greater in the tongue than in the oral and gingival tissues. These findings suggest that the mucosa of the oral cavity may be a route of potentially high risk of COVID-19 infection (Xu et al., 2020).
In Rhesus monkeys, salivary glands have been identified as primary targets for SARS-CoV-2 RNA. It is presumed, therefore, that the salivary glands in humans may present dysfunction with impaired salivary flow, both in quality and in quantity, being the process of dysgeusia an initial symptom in asymptomatic or oligosymptomatic patients with COVID-19 (Lozada-Nur et al., 2020).
In addition, SARS-CoV-2 can bind to sialic acid receptors, occupying sialic acid binding sites in the taste buds and accelerating the degradation of taste particles (Park et al., 2019; Milanetti et al., 2020; Vaira et al., 2020c). Another hypothesis is based on the inflammatory response pathway, which is mediated by the interaction of the virus with toll-like receptors, resulting in apoptotic processes with atypical taste bud renewal, therefore, leading to taste distortion and possible tissue hypoxia. (Lozada-Nur et al., 2020). (Figure 4).
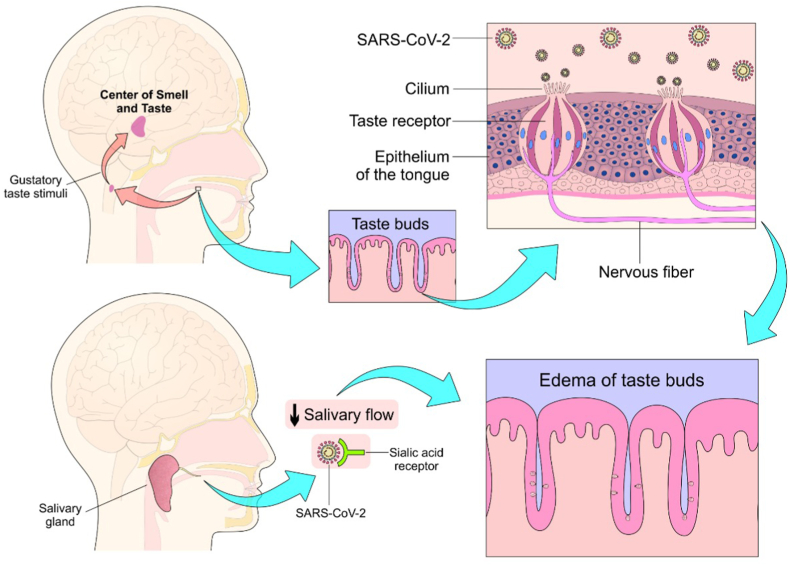
Figure 4.
Affection of the taste buds leading to ageusia. Viruses bind to sialic acid receptors located on the taste buds, causing inflammation and edema with further degradation and apoptosis of the taste receptors. In addition, it is believed that there is a reduction in salivary flow which may distort the sense of taste and/or ageusia.
5. Therapies for smell and taste disorders caused by COVID-19 infection
It has been reported that patients with olfactory and gustatory disorders may recover from 15 to 20 days after the onset of the disease (Tsuruoka, 2005), which means that those patients who are affected by olfactory disorders and spontaneously improve their oflatory condition may not need specific treatment. However, it may be necessary to consider some therapy when the smell and/or taste disorder persists for more than two weeks (Whitcroft and Hummel, 2020). The treatments based on evidence found in the literature are as follow:
5.1. Olfactory training
Although neuroplasticity plays a crucial role, the molecular and cellular mechanisms behind the beneficial effects of olfactory training still remain uncertain (Reichert and Schöpf, 2018). Despite this, olfactory training is considered the only current therapeutic alternative for post-viral olfactory loss that has a strong scientific base (Hummel et al., 2016). Meta-analyses have demonstrated that this training improves olfactory function after post-infectious olfactory disorder (PIOD) (Pekala et al., 2016; Sorokowska et al., 2017; Kattar et al., 2021). Phenyl ethyl alcohol, eucalyptol, citronella and eugenol are four of the six most significant odor qualities in the olfactory spectrum which have improved olfactory loss after training for 12 weeks or more (Addison et al., 2021).
A pioneering study of modified olfactory training (MOT) carried out by Altundag et al. was able to demonstrate better discrimination and odor identification in patients treated with the modified technique compared to the classic technique. In this study, four odors were used in the classic olfactory training for 12 weeks. A second set of odors (menthol, thyme, mandarin and jasmine) for another 12 weeks and, finally, by a third set of odors (green tea, bergamot, rosemary and gardenia) for the final period of 12 weeks (Altundag et al., 2015). In another study, the authors used the gradual dilution of odorous substances in a solution of alcohol and water, divided into groups, including citrus juices, rosemary, pepper, sage, neutral soaps, wine or vinegar, chocolate, coffee and camphoric substances. In addition, taste function was also assessed using coffee and more bitter substances with salt and sugar with the purpose of assessing whether there would be an improvement in smell and taste. The conclusion was that there was recovery in a relatively short time when applying this systematic sensory training (Petrocelli et al., 2020).
Whitcroft and Hummel recommend olfactory training with rose, lemon and eucalyptus for 20 s each, twice a day for at least 3 months as a treatment for persistent anosmia associated with COVID-19 (Whitcroft and Hummel, 2020). Walker and colleagues claim that despite the evidence being not sufficient, a systematic review supports the use of olfactory training for all patients with loss of smell, as it has confirmed its utility in recovering sensorineural loss of smell (Walker et al., 2020). Likewise, the British Rhinological Society (BRS) consensus and evidence-based review conducted by Hura et al. recommend this olfactory training for patients who complain of hyposmia or anosmia for more than 2 weeks (Hopkins et al., 2021b; Hura et al., 2020).
5.2. Caffeine
Hosseini and colleagues have reported that caffeine enhanced the sense of smell and taste in people with COVID-19. The lowest recovery rate was related to patients with comorbidities such as diabetes, hypertension and heart disease, while people who did not have underlying diseases recovered more quickly after consuming coffee. The effectiveness of coffee has been reported in 5–7 h for outpatients and between 2 and 4 days for patients with comorbidities (Hosseini et al., 2020).
The use of coffee as an olfactory rehabilitation strategy in anosmia has been adopted in the perspective of using different odors, such as herbs, ginger, mint, coffee and lemon colony to help the patient identifying the different odors as well as their intensity (Altundag et al., 2020).
In a case study, a patient was urged to undergo an olfactory training process that involved inhaling different containers containing coffee, cinnamon, cloves and lavender for 10 min each day from the 14th day of symptoms, in addition to the consumption of complex B vitamins. The results showed full olfaction recovery on the 30th day of treatment (Altin et al., 2020).
The use of caffeine for this purpose is based on its affinity for adenosine A2a receptors. Caffeine antagonizes adenosine A2a receptors in the olfactory bulb, which is one of the main regions affected by SARS-CoV-2. Stafford and Orgill (2020) describe previous studies that investigated the effects of caffeine in inhalation tests based on the classification of participants according to identified feelings and emotions. They did not identify a greater role in the olfactory function, with positive results (identification of an odorous threshold) for caffeine users, presenting more pronounced effects on more attention and vigilance. Such studies suggest that the beneficial effects of caffeine may be greater in older subjects, as well as in regular caffeine users. In this perspective, A2a adenosine receptors may participate in the process of deficit in odor function and may be temporarily reversed by the antagonism action of caffeine in these receptors. This is reflected in the increased transmission of neurotransmitters such as dopamine, norepinephrine and glutamate in brain areas responsible for cognitive function (Stafford e Orgill, 2020).
5.3. Corticosteroids
Corticosteroids have been used as a pharmacological approach to treat patients with olfactory dysfunction, especially upper respiratory infections such as chronic rhinosinusitis (Scangas and Bleier, 2017). Corticosteroids have also been used to eliminate inflammatory components in patients with post-infectious olfactory dysfunction - PIOD (Whitcroft and Hummel, 2019). However, although its use often improves smell sensation, this effect usually disappears after treatment ceases (Rebholz et al., 2020).
Although there is no conclusive data on the use of corticosteroids for post-COVID patients with PIOD, robust evidence is available that points to the effectiveness of these drugs in patients with severe respiratory distress syndrome who require mechanical ventilation, as their anti-inflammatory activity improves the cytokine storm and reduces inflammation in the respiratory system (Addison et al., 2021). A randomized clinical trial compared the use of nasal spray of the corticosteroid mometasone furoate with the use of olfactory training in 100 post-COVID patients with PIOD. The results showed that using mometasone furoate as nasal spray in post-COVID-19 patients with anosmia offered no superior benefits over the olfactory training, regarding smell scores, duration of anosmia and recovery rates (Abdelalim et al., 2021).
On the other hand, other studies have demonstrated some benefits with the use of corticosteroids in post-COVID patients with PIOD. A pilot study with 72 patients with COVID-19 reported that 27 (37.5%) of these patients showed persistent dysosmia. The authors investigated the efficacy and safety of oral corticosteroids together with olfactory training and concluded that the combination of corticosteroids with olfactory training is safe and can be beneficial for helping patients with persistent dysosmia to recover from olfactory loss due to infection by SARS-CoV-2 (Le Bon et al., 2021).
Vaira et al. (2021) conducted a multicentre randomized case-control study involving 18 patients with COVID-19 that reported anosmia or severe hyposmia for more than 30 days from which 9 received systemic prednisone and nasal irrigation with betamethasone, ambroxol and rhinazine for 15 days. The conclusion was that the association of drugs including corticosteroids could represent a useful specific therapy to reduce the prevalence of anosmia or severe hyposmia (Vaira et al., 2021).
The BRS consensus recommends the use of corticosteroids as nasal spray in patients with loss of smell for more than 2 weeks associated with nasal symptoms, but does not recommend the use of oral corticosteroids (Hopkins et al., 2021b). Hura et al. claim that insufficient evidence on the effectiveness of systemic corticosteroid therapy in addition to its risks and side effects might not justify its use in patients with PIOD. Even considering the minimal harm involved in the topical application of corticosteroids, the heterogeneous data available makes their use challenging (Hura et al., 2020).
Thus, it seems likely to conclude that patients infected with SARS-CoV-2 who have anosmia should avoid oral or topical corticosteroids as there is no robust evidence to demonstrate clear benefits over their potential risks (Addison et al., 2021).
5.4. Zinc sulfate
Previous reports suggest that zinc deficiency can cause dysfunction in smell and taste (Komai et al., 2000). It has been suggested that the drop in the nasal levels of zinc may induce transient anosmia due to the decreased activity of zinc-dependent carbonic anhydrase (CA) that is involved in the taste and smell perception. In addition, people with zinc deficiency at baseline may experience prolonged anosmia and a decrease in the type 1 interferon response (Equils et al., 2021). Thus, zinc intake positively regulates the production of alpha interferon, which improves its antiviral activity. Moreover, RNA polymerase activity is partially inhibited by zinc (te Velthuis et al., 2010).
Studies using zinc sulfate have not reported a statistically significant improvement in post-treatment olfactory function, especially in PIOD (Aiba et al., 1998; Mori et al., 1998; Addison et al., 2021). On the other hand, a clinical trial showed that daily intake of 20 mg of zinc sulfate for 5 months was able to reduce the morbidity of lower respiratory tract infection compared to placebo (Malik et al., 2014). Another clinical trial involving patients with COVID-19 who received zinc therapy showed a significantly less olfactory recovery time, indicating that there may be benefits in the zinc supplementation for anosmia therapy (Abdelmaksoud et al., 2021).
However, Hura et al. stated that there was a preponderance of risks over the benefits of using zinc sulfate for PIOD and that no study has shown improved olfactory outcomes after its use (Hura et al., 2020).
5.5. Vitamin A
Although the mechanism and degree of regeneration of the olfactory epithelium and the olfactory bulb in humans are not entirely clear, it is well known that certain signaling pathways are necessary. Retinoic acid (RA), which is a metabolite of vitamin A and a member of the thyroid hormone superfamily, is an important transcriptional regulator in tissue development and regeneration (Balmer and Blomhoff, 2002). Vitamin A can promote olfactory neurogenesis due to its ability to regenerate the olfactory neuroepithelium (Whitcroft e Hummel, 2019; Yan et al., 2020a; Addison et al., 2021).
A retrospective cohort study reported an improvement in smell function in patients with PIOD and post-traumatic olfactory loss in which 33% recovered after treatment with 10,000 IU of intranasal vitamin A compared to 23% of the control group. The administration of intranasal vitamin A in addition to olfactory training resulted in greater rates of improvement compared with olfactory training alone (Hummel et al., 2017). On the other hand, in a randomized, double-blind, placebo-controlled clinical trial that evaluated oral administration of vitamin A 10,000 IU per day for 3 months in patients with PIOD showed no statistically difference between the treatment and control groups (Reden et al., 2012). Due to this lack of difference in randomized controlled trials, Hura et al. do not recommend treating PIOD with vitamin A (Hura et al., 2020).
5.6. Alpha-lipoic acid (ALA)
Torabi and colleagues found higher levels of the proinflammatory cytokine TNF-α in the olfactory epithelium in patients with COVID-19, suggesting that direct inflammation of the olfactory epithelium may play a role in the acute olfactory loss (Torabi et al., 2020). According to previous reports, alpha-lipoic acid (ALA) may decrease ACE2 activity after SARS-CoV-2 replication and rmight reduce NADPH oxidase activity, leading to suppression of expression of inflammatory cytokines (Sayiner et al., 2020).
In a prospective uncontrolled study, 23 patients with PIOD were treated with 600 mg/day of ALA for an average of 4–5 months in which a moderate improvement in smell was observed in 61% of the participants (Hummel et al., 2002). However, its use may be associated with neurological side effects including headache, dizziness and confusion, which can be difficult to associate with its use due to similarities with COVID-19 manifestations (Vaira et al., 2020d). In the consensus of the British Rhinological Society (BRS) ALA is not recommended for a patient with loss of smell (LOS) as isolated symptom for more than 2 weeks or following resolution of any other COVID-19 symptoms (Hopkins et al., 2021b).
5.7. Intranasal sodium citrate
The cascade of olfactory receptor transduction can be modulated using intranasal sodium citrate (Whitcroft and Hummel, 2019; Yan et al., 2020a). Whitcroft and colleagues conducted a prospective placebo-controlled trial of sodium citrate versus sodium chloride treatment for patients with olfactory loss. The results showed improvement in olfactory threshold (Whitcroft et al., 2016). Another prospective study composed exclusively of patients with post-viral olfactory dysfunction (PVOD) showed significant improvement in the compound threshold and identification scores in patients treated with sodium citrate, but no change in odor or threshold identification compared to placebo (Whitcroft et al., 2017).
Philpott and colleagues compared a single application of 0.5 mL of 9% sodium citrate per nostril versus sterile water in a randomized clinical trial involving 55 patients in which they found a statistically significant improvement in olfactory function using olfactory thresholds for phenyl ethyl alcohol, 1-butanol and eucalyptol with thresholds measured up to 2 h after the intervention, showing an effect that lasts between 30 and 120 min after application. The sodium citrate solution administered to the nose binds to the free calcium ions in the nasal mucus, thereby reducing the available calcium in the mucosa (Philpott et al., 2017).
In all of the aforementioned studies, the sodium citrate spray was well tolerated with side effects that included transient rhinorrhea, sore throat and nasal obstruction. However, more robust data are needed with clinically relevant results in the long term, as the current evidence is not sufficient to justify the recommendation of this treatment (Hura et al., 2020).
5.8. Theophylline
A comprehensive study involving patients with loss of smell and taste has determined that the levels of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) in saliva (Henkin et al., 2007; Henkin and Velicu, 2009) and nasal mucus (Henkin and Velicu, 2008) were lower than those in healthy individuals. Such low levels of cAMP and cGMP seem to be responsible for the occurrence of hyposmia and hypogeusia in many of these patients (Henkin and Velicu, 2012a, 2012b). Theophylline is suggested to inhibit phosphodiesterase and increase secondary messengers, such as cAMP and cGMP, therefore assisting in the regeneration of the olfactory neuroepithelium (Henkin et al., 2009, 2011).
A study conducted by Henkin, Schultz and Minnick-Poppe showed that treatment with oral theophylline improved the acuity of taste and smell in 6 patients after 2–12 months of treatment (Henkin et al., 2012). However, the effectiveness of oral theophylline depends on the dose, requiring 200 mg–800 mg of the drug daily for periods of 4–18 months. Oral therapy is also limited due to its side effects (sleep and gastrointestinal disorders as well as tachycardia and anxiety) and interactions with other drugs (Henkin et al., 2009, 2013). On the other hand, improvement with the intranasal administration of this drug occurs more quickly and does not induce any systemic side effects (Henkin and Abdelmeguid, 2019). Besides acting on stem cells in the olfactory epithelium, the direct administration of drugs into the nose can also facilitate reaching the brain through bypassing the blood-brain barrier by direct entry through the cribriform plaque (Henkin, 2011). Thus, intranasal theophylline seems to influence the perception of smell and taste through a direct action on the brain (Henkin and Abdelmeguid, 2019). However, Hura et al. do not recommend its use as they point out the need for RCT, since the studies using theophylline did not include a control group (Hura et al., 2020).
5.9. Omega 3 Fatty Acids
The role of omega-3 on anti-inflammatory mechanisms has already been extensively investigated. Omega 3 Fatty Acids (OFAs) include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Hathaway et al., 2020). Omega-3 supplementation was found to be protective against olfactory loss during the recovery period after skull base surgery and therefore, may have potential in aiding recovery after post-viral olfactory loss, although this has not been formally tested in post-COVID-19 patients (Yan et al., 2020b; Vaira et al., 2020d).
Saedisomeolia et al. investigated the anti-inflammatory properties of DHA and EPA in airway epithelial cells infected with Rhinovirus. The investigators found that DHA significantly reduced the release of IL-6 and IP-10 from the cells infected with different strains of rhinovirus. This could be explained by the ability of OFAs in reducing inflammation by inhibiting arachidonic acid metabolism to eicosanoids and finally reducing pro-inflammatory cytokines (Saedisomeolia et al., 2009).
Studies have demonstrated that systemic OFAs have some beneficial activity in PIOD, as they may act through neuroregenerative or anti-inflammatory mechanisms, which seems to help the olfactory nerve to heal and may also serve as an adjuvant therapy in olfactory training (Whitcroft and Hummel, 2019, 2020; Yan et al., 2020b). In the consensus of BRS Omega-3 supplements are options for a patient with loss of smell for more than 2 weeks as an isolated symptom or following resolution of any other COVID-19 symptoms (Hopkins et al., 2021b).
6. Discussion
The prevalence of smell and taste dysfunctions in patients with SARS-CoV-2 infection is high. The pathophysiology of these disorders is multifactorial and few therapies have been shown to be effective in recovering smell and taste functions after COVID-19 infection. A possible explanation is that viral infection of the upper respiratory tract is among the most common causes of olfactory dysfunction, named as post-infectious olfactory dysfunction (PIOD) (Doty, 2019).
In addition, many studies have reported concomitant loss of smell and taste in patients infected during the pandemic (Bénézit et al., 2020; Giacomelli et al., 2020; Lechien et al., 2020). The overall prevalence of gustatory dysfunction is lower than olfactory dysfunction. One of the possible explanations for its lower prevalence is that the sensation of taste involves several senses, including smell, temperature and texture of the food. Each of these sensory components is stimulated separately to create a unique flavor. The ability to differentiate flavors depends on retronasal stimulation and, because it depends on separate stimuli, it becomes less frequent than anosmia. The main cause of the latter is the direct damage that SARS-CoV-2 causes in olfactory receptor neurons located in the olfactory epithelium (Kanjanaumporn et al., 2020; Butowt and Bartheld, 2020). This mechanism is supported by the abundant expression of the two entry proteins, ACE2 and TMPRSS2, in sustentacular cells in the olfactory epithelium (Butowt e Bartheld, 2020).
Previous reports have suggested that most patients will achieve smell and taste recovery up to 14 days after resolving the disease (Tanasa et al., 2020). The benefits of the available treatments for anosmia associated with SARS-CoV-2 remain unknown, although treatments aimed at post-COVID PIOD seem to be more effective (Whitcroft and Hummel., 2020). There is no therapeutic regimen for ageusia (Tanasa et al., 2020). However, a study found that the most prescribed treatments for infected patients with loss of smell and taste were nasal saline irrigations, nasal and oral corticosteroids, l-carnitine, trace elements and vitamins (Lechien et al., 2020).
The members of the Clinical Olfactory Working Group agree that the most recommended treatment for anosmia is the olfactory training (Addison et al., 2021). This treatment is based on a series of olfactory stimuli that triggers the regeneration of olfactory receptor neurons (Altundag et al., 2020). Previous reports have suggested that repeated short-term exposure to different odors may result in increased growth of olfactory receptor neurons and increased olfactory receptor expression (Youngentob e Kent, 1995; Hudson e Distel, 1998).
Another study showed that both anosmic and healthy control groups initially use the same three networks to process chemosensory input: the olfactory, the somatosensory and the integrative network. However, the sensitivity to detect odors increased significantly in the anosmic group after olfactory training, which also manifested itself in changes in the functional connections in the three networks. Therefore, the results of this study indicated that an olfactory training program can reorganize functional networks. Based on these findings, the high functional plasticity of the olfactory system may be the main reason for the effectiveness of the olfactory training (Kollndorfer et al., 2015).
Corticosteroids have a very important role in the management of patients with SARS-CoV-2 who need mechanical ventilation, especially for their anti-inflammatory activity (Carrillo-Larco and Altez-Fernandez, 2020). In addition, they can be effective in relieving the symptoms of anosmia and dysgeusia, as some studies argue that systemic corticosteroids can improve smell function in PIOD (Seo et al., 2009; Kanjanaumporn et al., 2020). Singh et al. revealed that the use of fluticasone and triamcinolone significantly enhanced the smell and taste. The study showed that olfactory and gustatory functions improved significantly in patients with COVID-19. For all cases of anosmia and dysgeusia who received nasal spray of fluticasone and oral triamcinolone, the recovery of sense of smell and taste occurred in one week (Singh et al., 2021). On the other hand, Abdelalim and colleagues suggested that the use of nasal spray of mometasone furoate in the treatment of post-COVID-19 anosmia was not superior when compared to olfactory training (Abdelalim et al., 2021). The interpretation of corticosteroids efficacy should be carefully evaluated because partial or complete spontaneous recovery occurs in about one-third of the patients within a year after infection (Kanjanaumporn et al., 2020; Daramola e Becker, 2015).
With regard to other therapeutic options, Panagiotopoulos et al. suggested that the intranasal application of sodium citrate might improve hyposmia by decreasing the levels of calcium in the mucus in the nose (Panagiotopoulos et al., 2005). Intranasal sodium citrate, due to its ability to sequester calcium ions, is believed to reduce mucosal free calcium with reduced negative feedback and increased sensitivity to odorants. The reduction in free calcium ions (Ca2+) is likely to increase the excitability of olfactory neurons, thereby improving the sense of smell (Addison et al., 2021).
In addition, studies indicate that theophylline, used by oral and intranasal routes, improves patients' sense of smell and taste, probably due to the inhibition of phosphodiesterase and increased levels of cAMP and cGMP, which may result in regeneration of the olfactory neuroepithelium (Henkin et al., 2009, 2011, 2012). Finally, some evidence on the positive effects of vitamin A and alpha lipoic acid (ALA) on improving smell and taste dysfunctions was reported in this current review. Possible mechanisms involved with sensory recovery after these treatments are the stimulation of the expression of nerve growth factors, substance P and neuropeptide Y by ALA and the regeneration of olfactory neuroepithelium by vitamin A (Addison et al., 2021).
7. Conclusion
The prevalence of olfactory and/or gustatory dysfunction is high in patients with COVID-19 in which the presence of anosmia and ageusia are symptoms associated with SARS-CoV-2 infection. Pathological mechanisms are multifactorial, but most likely as a result of primary infection of non-neuronal olfactory epithelial cell types leading to olfactory neuron injuries.
There is no specific therapeutic regime for ageusia and some of the treatments discussed in this review lack robust evidence and are not readily available for clinical use. Although some treatment options have shown some promise, such as olfactory training, oral or topical corticosteroids, vitamin A and sodium citrate. This review points to the need for randomized clinical trials to prove the effectiveness of these therapies in post-COVID PIOD.
CRediT authorship contribution statement
Francisca Idalina Neta: Literary research and writing development. Amélia Carolina Lopes Fernandes: Literary research and writing development. Adson José Martins Vale: Literary research, writing development and checking references. Francisco Irochima Pinheiro: Development of figures, fitting figures into the text and footing of figures. Ricardo Ney Cobucci: Survey of articles, development of writing and correction of all work. Eduardo Pereira de Azevedo: writing of the article in English. Fausto Pierdoná Guzen: Development of the topic, correction of the article, final analysis of the article and submission of the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdelalim A.A., Mohamady A.A., Elsayed R.A., Elawady M.A., Ghallab A.F. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am. J. Otolaryngol. 2021;42(2):102884. doi: 10.1016/j.amjoto.2020.102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmaksoud A.A., Ghweil A.A., Hassan M.H., Rashad A., Khodeary A., Aref Z.F., Sayed M., Elsamman M.K., Bazeed S. Olfactory disturbances as presenting manifestation among Egyptian patients with COVID-19: possible role of zinc. Biol. Trace Elem. Res. 2021:1–8. doi: 10.1007/s12011-020-02546-5. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison A.B., Wong B., Ahmed T., Macchi A., Konstantinidis I., Huart C., Frasnelli J., Fjaeldstad A.W., Ramakrishnan V.R., Rombaux P., Whitcroft K.L., Holbrook E.H., Poletti S.C., Hsieh J.W., Landis B.N., Boardman J., Welge-Lüssen A., Maru D., Hummel T., Philpott C.M. Clinical Olfactory Working Group consensus statement on the treatment of postinfectious olfactory dysfunction. J. Allergy Clin. Immunol. 2021 doi: 10.1016/j.jaci.2020.12.641. S0091-6749(21)00004-X. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Aiba T., Sugiura M., Mori J., Matsumoto K., Tomiyama K., Okuda F., Nakai Y. Effect of zinc sulfate on sensorineural olfactory disorder. Acta oto-laryngologica. Supplementum. 1998;538:202–204. doi: 10.1080/00016489850182936. [DOI] [PubMed] [Google Scholar]
- Altin F., Cingi C., Uzun T., Bal C. Olfactory and gustatory abnormalities in COVID-19 cases. Eur. Arch. Oto-Rhino-Laryngol. : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2020;277(10):2775–2781. doi: 10.1007/s00405-020-06155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altundag A., Cayonu M., Kayabasoglu G., Salihoglu M., Tekeli H., Saglam O., Hummel T. Modified olfactory training in patients with postinfectious olfactory loss. Laryngoscope. 2015;125(8):1763–1766. doi: 10.1002/lary.25245. [DOI] [PubMed] [Google Scholar]
- Altundag A., Saatci O., Sanli D., Duz O.A., Sanli A.N., Olmuscelik O., Temirbekov D., Kandemirli S.G., Karaaltin A.B. The temporal course of COVID-19 anosmia and relation to other clinical symptoms. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology. Head Neck Surg. 2020:1–7. doi: 10.1007/s00405-020-06496-5. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer J.E., Blomhoff R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002;43(11):1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- Bénézit F., Le Turnier P., Declerck C., Paillé C., Revest M., Dubée V., Tattevin P., Ran Covid Study Group Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. The Lancet. Infectious diseases. 2020;20(9):1014–1015. doi: 10.1016/S1473-3099(20)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertlich M., Stihl C., Lüsebrink E., et al. The course of subjective and objective chemosensory dysfunction in hospitalized patients with COVID-19: a 6-month follow-up. Eur. Arch. Oto-Rhino-Laryngol. 2021;1:1–7. doi: 10.1007/s00405-021-06796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P., Menegaldo A., Fabbris C., Spinato G., Borsetto D., Vaira L.A., Calvanese L., Pettorelli A., Sonego M., Frezza D., Bertolin A., Cestaro W., Rigoli R., D'Alessandro A., Tirelli G., Da Mosto M.C., Menini A., Polesel J., Hopkins C. Six-Month psychophysical evaluation of olfactory dysfunction in patients with COVID-19. Chem. Senses. 2021;46:bjab006. doi: 10.1093/chemse/bjab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann David H., Tsukahara Tatsuya, Weinreb Caleb, Lipovsek Marcela, Van den Berge Koen, Gong Boying, Chance Rebecca, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Science Advances. 2020;6(31) doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R., von Bartheld C.S. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2020 doi: 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Larco R.M., Altez-Fernandez C. Anosmia and dysgeusia in COVID-19: a systematic review. Wellcome open research. 2020;5:94. doi: 10.12688/wellcomeopenres.15917.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL: 2020. Features, evaluation, and treatment of coronavirus; p. 32150360. Oct 4. Jan. PMID. [PubMed] [Google Scholar]
- Cazzolla A.P., Lovero R., Lo Muzio L., Testa N.F., Schirinzi A., Palmieri G., Pozzessere P., Procacci V., Di Comite M., Ciavarella D., Pepe M., De Ruvo C., Crincoli V., Di Serio F., Santacroce L. Taste and smell disorders in COVID-19 patients: role of interleukin-6. ACS Chem. Neurosci. 2020;11(17):2774–2781. doi: 10.1021/acschemneuro.0c00447. [DOI] [PubMed] [Google Scholar]
- Chen M., Shen W., Rowan N.R., Kulaga H., Hillel A., Ramanathan M., Jr., Lane A.P. Elevated ACE2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. bioRxiv : the preprint server for biology. 2020;2020 doi: 10.1101/2020.05.08.084996. 05.08.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.W., Brann D.H., Farruggia M.C., Bhutani S., Pellegrino R., Tsukahara T., Weinreb C., Joseph P.V., Larson E.D., Parma V., et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020;107:219–233. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa K.V.T. da, Carnaúba A.T.L., Rocha K.W., Andrade K.C.L. de, Ferreira S.M.S., Menezes P. de L. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86:781–792. doi: 10.1016/j.bjorl.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daramola O.O., Becker S.S. An algorithmic approach to the evaluation and treatment of olfactory disorders. Curr. Opin. Otolaryngol. Head Neck Surg. 2015;23(1):8–14. doi: 10.1097/MOO.0000000000000118. [DOI] [PubMed] [Google Scholar]
- Doty R.L. Epidemiology of smell and taste dysfunction. Handb. Clin. Neurol. 2019;164:3–13. doi: 10.1016/B978-0-444-63855-7.00001-0. [DOI] [PubMed] [Google Scholar]
- Equils O., Lekaj K., Wu A., Fattani S., Liu G., Rink L. Intra-nasal zinc level relationship to COVID-19 anosmia and type 1 interferon response: a proposal. Laryngoscope Investigative Otolaryngology. 2021;6(1):21–24. doi: 10.1002/lio2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodoulian Leon, Tuberosa Joel, Rossier Daniel, Landis Basile N., Carleton Alan, Rodriguez e Ivan. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. bioRxiv. 2020;2020 doi: 10.1101/2020.03.31.013268. 03.31.013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R., Jain R., Sodani A., Chouksey D., Dosi R., Athale S., Goyal N., Rathi P., Singh H., Telang K. Neurological symptoms as initial manifestation of covid-19 - an observational study. Ann. Indian Acad. Neurol. 2020;23(4):482–486. doi: 10.4103/aian.AIAN_560_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., Antinori S., Galli M. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin. Infect. Dis. : an official publication of the Infectious Diseases Society of America. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway D., Pandav K., Patel M., Riva-Moscoso A., Singh B.M., Patel A., Min Z.C., Singh-Makkar S., Sana M.K., Sanchez-Dopazo R., Desir R., Fahem M., Manella S., Rodriguez I., Alvarez A., Abreu R. Omega 3 fatty acids and COVID-19: a comprehensive review. Infection & chemotherapy. 2020;52(4):478–495. doi: 10.3947/ic.2020.52.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin R. Intranasal delivery to the brain. Nat. Biotechnol. 2011;29:480. doi: 10.1038/nbt.1866. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Velicu I. cAMP and cGMP in nasal mucus related to severity of smell loss in patients with smell dysfunction. Clinical and investigative medicine. Med. Clin. Exp. 2008;31(2):E78–E84. doi: 10.25011/cim.v31i2.3367. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Velicu I. Decreased parotid salivary cyclic nucleotides related to smell loss severity in patients with taste and smell dysfunction. Metab., Clin. Exp. 2009;58(12):1717–1723. doi: 10.1016/j.metabol.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Velicu I. Etiological relationships of parotid saliva cyclic nucleotides in patients with taste and smell dysfunction. Arch. Oral Biol. 2012;57(6):670–677. doi: 10.1016/j.archoralbio.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Velicu I. Aetiological relationships of nasal mucus cyclic nucleotides in patients with taste and smell dysfunction. J. Clin. Pathol. 2012;65(5):447–451. doi: 10.1136/jclinpath-2012-200698. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Levy L.M., Fordyce A. Taste and smell function in chronic disease: a review of clinical and biochemical evaluations of taste and smell dysfunction in over 5000 patients at the Taste and Smell Clinic in Washington, DC. Am. J. Otolaryngol. 2013;34(5):477–489. doi: 10.1016/j.amjoto.2013.04.006. PubMed. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Schultz M., Minnick-Poppe L. Intranasal theophylline treatment of hyposmia and hypogeusia: a pilot study. Arch. Otolaryngol. Head Neck Surg. 2012;138(11):1064–1070. doi: 10.1001/2013.jamaoto.342. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Velicu I., Papathanassiu A. cAMP and cGMP in human parotid saliva: relationships to taste and smell dysfunction, gender, and age. Am. J. Med. Sci. 2007;334(6):431–440. doi: 10.1097/MAJ.0b013e3180de4d97. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Velicu I., Schmidt L. An open-label controlled trial of theophylline for treatment of patients with hyposmia. Am. J. Med. Sci. 2009;337(6):396–406. doi: 10.1097/MAJ.0b013e3181914a97. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Velicu I., Schmidt L. Relative resistance to oral theophylline treatment in patients with hyposmia manifested by decreased secretion of nasal mucus cyclic nucleotides. Am. J. Med. Sci. 2011;341(1):17–22. doi: 10.1097/MAJ.0b013e3181f1fdc8. [DOI] [PubMed] [Google Scholar]
- Henkin R., Abdelmeguid M. Improved smell and taste dysfunction with intranasal theophylline. Am J Otolaryngol Head Neck Surg. 2019;2(9):1070. 2019. [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. Epub 2020 Mar 5. PMID: 32142651; PMCID: PMC7102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C., Surda P., Vaira L.A., Lechien J.R., Safarian M., Saussez S., Kumar N. Six month follow-up of self-reported loss of smell during the COVID-19 pandemic. Rhinology. 2021;59(1):26–31. doi: 10.4193/Rhin20.544. [DOI] [PubMed] [Google Scholar]
- Hopkins C., Alanin M., Philpott C., Harries P., Whitcroft K., Qureishi A., Anari S., Ramakrishnan Y., Sama A., Davies E., Stew B., Gane S., Carrie S., Hathorn I., Bhalla R., Kelly C., Hill N., Boak D., Nirmal Kumar B. Management of new onset loss of sense of smell during the COVID-19 pandemic - BRS Consensus Guidelines. Clin. Otolaryngol. : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2021;46(1):16–22. doi: 10.1111/coa.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A., Mirmahdi E., Moghaddam M.A. A new strategy for treatment of Anosmia and Ageusia in COVID-19 patients. Integrat Respir Med. 2020;1(2):1–6. doi: 10.1051/irm/2020003. [DOI] [Google Scholar]
- Hudson R., Distel H. Induced peripheral sensitivity in the developing vertebrate olfactory system. Ann. N. Y. Acad. Sci. 1998;855:109–115. doi: 10.1111/j.1749-6632.1998.tb10552.x. [DOI] [PubMed] [Google Scholar]
- Hummel T., Heilmann S., Hüttenbriuk K.B. Lipoic acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope. 2002;112(11):2076–2080. doi: 10.1097/00005537-200211000-00031. [DOI] [PubMed] [Google Scholar]
- Hummel T., Whitcroft K.L., Andrews P., Altundag A., Cinghi C., Costanzo R.M., Damm M., Frasnelli J., Gudziol H., Gupta N., Haehner A., Holbrook E., Hong S.C., Hornung D., Hüttenbrink K.B., Kamel R., Kobayashi M., Konstantinidis I., Landis B.N., Leopold D.A., et al. Welge-Luessen A. Position paper on olfactory dysfunction. Rhinology. 2016;56(1):1–30. doi: 10.4193/Rhin16.248. [DOI] [PubMed] [Google Scholar]
- Hummel T., Whitcroft K.L., Rueter G., Haehner A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology. Head Neck Surg. 2017;274(7):2819–2825. doi: 10.1007/s00405-017-4576-x. [DOI] [PubMed] [Google Scholar]
- Hura N., Xie D.X., Choby G.W., Schlosser R.J., Orlov C.P., Seal S.M., Rowan N.R. Treatment of post-viral olfactory dysfunction: an evidence-based review with recommendations. International Forum of Allergy & Rhinology. 2020;10(9):1065–1086. doi: 10.1002/alr.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjanaumporn J., Aeumjaturapat S., Snidvongs K., Seresirikachorn K., Chusakul S. Smell and taste dysfunction in patients with SARS-CoV-2 infection: a review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac. J. Allergy Immunol. 2020;38(2):69–77. doi: 10.12932/AP-030520-0826. [DOI] [PubMed] [Google Scholar]
- Kattar N., Do T.M., Unis G.D., Migneron M.R., Thomas A.J., McCoul E.D. Olfactory training for postviral olfactory dysfunction: systematic review and meta-analysis. Otolaryngology--head and neck surgery. official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2021;164(2):244–254. doi: 10.1177/0194599820943550. [DOI] [PubMed] [Google Scholar]
- Keyhan S.O., Fallahi H.R., Cheshmi B. Dysosmia and dysgeusia due to the 2019 Novel Coronavirus; a hypothesis that needs further investigation. Maxillofac Plast Reconstr Surg. 2020;42:9. doi: 10.1186/s40902-020-00254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum Daniel, et al. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020;396(10245):166. doi: 10.1016/S0140-6736(20)31525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T., Kadiane-Oussou N.J., Toko L., Royer P.Y., Lepiller Q., Gendrin V., Zayet S. Features of anosmia in COVID-19. Med. Maladies Infect. 2020;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollndorfer K., Fischmeister F.P., Kowalczyk K., Hoche E., Mueller C.A., Trattnig S., Schöpf V. Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. NeuroImage. Clinical. 2015;9:401–410. doi: 10.1016/j.nicl.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai M., Goto T., Suzuki H., Takeda T., Furukawa Y. Zinc deficiency and taste dysfunction; contribution of carbonic anhydrase, a zinc-metalloenzyme, to normal taste sensation. Biofactors. 2000;12(1–4):65–70. doi: 10.1002/biof.5520120111. [DOI] [PubMed] [Google Scholar]
- Le Bon S.D., Konopnicki D., Pisarski N., Prunier L., Lechien J.R., Horoi M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology. Head Neck Surg. 2021:1–5. doi: 10.1007/s00405-020-06520-8. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L., Chekkoury-Idrissi Y., Hans S., Delgado I.L., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., Vaira L.A., De Riu G., Cammaroto G., Chekkoury-Idrissi Y., Circiu M., Distinguin L., Journe F., de Terwangne C., Machayekhi S., Barillari M.R., Calvo-Henriquez C., Hans S., Saussez S. Epidemiological, otolaryngological, olfactory and gustatory outcomes according to the severity of COVID-19: a study of 2579 patients. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology. Head Neck Surg. 2021:1–9. doi: 10.1007/s00405-020-06548-w. Advance online publication. [DOI] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science (New York, N.Y.) 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato A., de Filippis C., Marioni G. Upper airway symptoms in coronavirus disease 2019 (COVID-19) Am J Otolaryngol - Head Neck Med Surg. 2020;(41):1–2. doi: 10.1016/j.amjoto.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada-Nur F., Chainani-Wu N., Fortuna G., Sroussi H. Dysgeusia in COVID-19: possible mechanisms and implications. Oral surgery, oral medicine, oral pathology and oral radiology. 2020;130(3):344–346. doi: 10.1016/j.oooo.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A., Taneja D.K., Devasenapathy N., Rajeshwari K. Zinc supplementation for prevention of acute respiratory infections in infants: a randomized controlled trial. Indian Pediatr. 2014;51(10):780–784. doi: 10.1007/s13312-014-0503-z. [DOI] [PubMed] [Google Scholar]
- Menni C., Valdes A.M., Freidin M.B., Ganesh S., El-Sayed Moustafa J.S., Visconti A., Hysi P., Bowyer R.C.E., Mangino M., Falchi M., et al. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. medRxiv. 2020;2020 doi: 10.1101/2020.04.05.20048421. [DOI] [Google Scholar]
- Milanetti Edoardo, Miotto Mattia, Di Rienzo Lorenzo, Monti Michele, Gosti Giorgio, Ruocco e Giancarlo. In-Silico evidence for two receptors based strategy of SARS-CoV-2. bioRxiv, janeiro. 2020;3(24):6197. doi: 10.1101/2020.03.24.006197. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. International forum of allergy & rhinology. 2020;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori J., Aiba T., Sugiura M., Matsumoto K., Tomiyama K., Okuda F., Okigaki S., Nakai Y. Clinical study of olfactory disturbance. Acta oto-laryngologica. Supplementum. 1998;538:197–201. [PubMed] [Google Scholar]
- Natoli S., Oliveira V., Calabresi P., Maia L.F., Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur. J. Neurol. 2020;27(9):1764–1773. doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogimi C., Kim Y.J., Martin E.T., Huh H.J., Chiu C.-H., Englund J.A. What's new with the old coronaviruses? J Pediatric Infect Dis Soc. 2020;9:210–217. doi: 10.1093/jpids/piaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotopoulos G., Naxakis S., Papavasiliou A., Filipakis K., Papatheodorou G., Goumas P. Decreasing nasal mucus Ca++ improves hyposmia. Rhinology. 2005;43(2):130–134. [PubMed] [Google Scholar]
- Park Y.J., Walls A.C., Wang Z., et al. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat. Struct. Mol. Biol. 2019;26:1151–1157. doi: 10.1038/s41594-019-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V., Ohla K., Veldhuizen M.G., Niv M.Y., Kelly C.E., Bakke A.J., Cooper K.W., Bouysset C., Pirastu N., Dibattista M., Kaur R., Liuzza M.T., Pepino M.Y., Schöpf V., Pereda-Loth V., Olsson S.B., Gerkin R.C., Rohlfs Domínguez P., Albayay J., Farruggia M.C., et al. More than smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. Senses. 2020;45(7):609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala K., Chandra R.K., Turner J.H. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. International forum of allergy & rhinology. 2016;6(3):299–307. doi: 10.1002/alr.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocelli M., Ruggiero F., Baietti A.M., Pandolfi P., Salzano G., Salzano F.A., Lechien J.R., Saussez S., De Riu G., Vaira L.A. Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: the Bologna experience of 300 cases. J. Laryngol. Otol. 2020;134(7):571–576. doi: 10.1017/S0022215120001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott C.M., Erskine S.E., Clark A., Leeper A., Salam M., Sharma R., Murty G.E., Hummel T. A randomised controlled trial of sodium citrate spray for non-conductive olfactory disorders. Clin. Otolaryngol. : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2017;42(6):1295–1302. doi: 10.1111/coa.12878. [DOI] [PubMed] [Google Scholar]
- Polak S.B., Van Gool I.C., Cohen D., et al. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020;33:2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebholz H., Braun R.J., Ladage D., Knoll W., Kleber C., Hassel A.W. Loss of olfactory function-early indicator for covid-19, other viral infections and neurodegenerative disorders. Front. Neurol. 2020;11:569333. doi: 10.3389/fneur.2020.569333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reden J., Lill K., Zahnert T., Haehner A., Hummel T. Olfactory function in patients with postinfectious and posttraumatic smell disorders before and after treatment with vitamin A: a double-blind, placebo-controlled, randomized clinical trial. Laryngoscope. 2012;122(9):1906–1909. doi: 10.1002/lary.23405. [DOI] [PubMed] [Google Scholar]
- Reichert J.L., Schöpf V. Olfactory loss and regain: lessons for neuroplasticity. Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2018;24(1):22–35. doi: 10.1177/1073858417703910. [DOI] [PubMed] [Google Scholar]
- Saedisomeolia A., Wood L.G., Garg M.L., Gibson P.G., Wark P.A. Anti-inflammatory effects of long-chain n-3 PUFA in rhinovirus-infected cultured airway epithelial cells. Br. J. Nutr. 2009;101(4):533–540. doi: 10.1017/S0007114508025798. [DOI] [PubMed] [Google Scholar]
- Sayiner S., Sehirli A.O., Serakinci N. Alpha lipoic acid as a potential treatment for COVID-19 - a hypothesis. Curr. Top. Nutraceutical Res. 2020;19(2):172–175. doi: 10.37290/ctnr2641–452X.19:172–175. [DOI] [Google Scholar]
- Scangas G.A., Bleier B.S. Anosmia: differential diagnosis, evaluation, and management. American journal of rhinology & allergy. 2017;31(1):3–7. doi: 10.2500/ajra.2017.31.4403. [DOI] [PubMed] [Google Scholar]
- Seo B.S., Lee H.J., Mo J.H., Lee C.H., Rhee C.S., Kim J.W. Treatment of postviral olfactory loss with glucocorticoids, Ginkgo biloba, and mometasone nasal spray. Arch. Otolaryngol. Head Neck Surg. 2009;135(10):1000–1004. doi: 10.1001/archoto.2009.141. [DOI] [PubMed] [Google Scholar]
- Singh C.V., Jain S., Parveen S. The outcome of fluticasone nasal spray on anosmia and triamcinolone oral paste in dysgeusia in COVID-19 patients. Am. J. Otolaryngol. 2021;42(3):102892. doi: 10.1016/j.amjoto.2020.102892. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D.M., Prescott J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005;166(3–4):345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- Song Y., Liu P., Shi X.L., Chu Y.L., Zhang J., Xia J., Gao X.Z., Qu T., Wang M.Y. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;(69):1143–1144. doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- Sorokowska A., Drechsler E., Karwowski M., Hummel T. Effects of olfactory training: a meta-analysis. Rhinology. 2017;55(1):17–26. doi: 10.4193/Rhin16.195. [DOI] [PubMed] [Google Scholar]
- Stafford L.D., Orgill K. The effects of caffeine on olfactory function and mood: an exploratory study. Psychopharmacology. 2020;237(12):3511–3517. doi: 10.1007/s00213-020-05695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf T., Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Leeflang M.M., Spijker R., Hooft L., Emperador D., Dittrich S., Domen J., Horn S., Van den Bruel A., Cochrane Covid-19 Diagnostic Test Accuracy Group Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst. Rev. 2020;7(7):CD013665. doi: 10.1002/14651858.CD013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasa I.A., Manciuc C., Carauleanu A., Navolan D.B., Bohiltea R.E., Nemescu D. Anosmia and ageusia associated with coronavirus infection (COVID-19) - what is known? Experimental and therapeutic medicine. 2020;20(3):2344–2347. doi: 10.3892/etm.2020.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham A.C., Thein T.L., Lee C.S., Tan G., Manauis C.M., Siow J.K., Leo Y.S., Lim M.Y. Olfactory taste disorder as a presenting symptom of COVID-19: a large single-center Singapore study. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology. Head Neck Surg. 2020:1–10. doi: 10.1007/s00405-020-06455-0. Advance online publication. [DOI] [Google Scholar]
- Torabi A., Mohammadbagheri E., Akbari Dilmaghani N., Bayat A.-H., Fathi M., Vakili K., Alizadeh R., Rezaeimirghaed O., Hajiesmaeili M., Ramezani M., Simani L., Aliaghaei A. Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia. ACS Chem. Neurosci. 2020;11(13):1909–1913. doi: 10.1021/acschemneuro.0c00249. [DOI] [PubMed] [Google Scholar]
- Trougakos I.P., Stamatelopoulos K., Terpos E., et al. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021;28(9):1–18. doi: 10.1186/s12929-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka S., Wakaumi M., Araki N., Ioka T., Sugimoto K., Fujimura A. Comparative study of taste disturbance by losartan and perindopril in healthy volunteers. J. Clin. Pharmacol. 2005;45(11):1319–1323. doi: 10.1177/0091270005280445. [DOI] [PubMed] [Google Scholar]
- Vaira L.A., Hopkins C., Sandison A., Manca A., Machouchas N., Turilli D., Lechien J.R., Barillari M.R., Salzano G., Cossu A., Saussez S., De Riu G. Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia. J. Laryngol. Otol. 2020;134(12):1123–1127. doi: 10.1017/S0022215120002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(7):1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Salzano G., Fois A.G., Piombino P., De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. International forum of allergy & rhinology. 2020;10(9):1103–1104. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Hopkins C., Petrocelli M., Lechien J.R., Chiesa-Estomba C.M., Salzano G., Cucurullo M., Salzano F.A., Saussez S., Boscolo-Rizzo P., Biglioli F., De Riu G. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J. Laryngol. Otol. 2020;134(8):703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Hopkins C., Petrocelli M., Lechien J.R., Cutrupi S., Salzano G., Chiesa-Estomba C.M., Saussez S., De Riu G. Efficacy of corticosteroid therapy in the treatment of long- lasting olfactory disorders in COVID-19 patients. Rhinology. 2021;59(1):21–25. doi: 10.4193/Rhin20.515. [DOI] [PubMed] [Google Scholar]
- Villalba R., Santos S., Martinez M.J., Díaz M., Pevida M., Cemborain A., Casares C., Mirabet V. Analysis of impact on tissue activity during COVID-19 outbreak: a survey of 8 banks in Spain. Cell Tissue Bank. 2020;21(4):557–562. doi: 10.1007/s10561-020-09853-0. Epub 2020 Oct 15. PMID: 33063150; PMCID: PMC7561239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A., Pottinger G., Scott A., Hopkins C. Anosmia and loss of smell in the era of covid-19. BMJ (Clinical research ed.) 2020;370:m2808. doi: 10.1136/bmj.m2808. [DOI] [PubMed] [Google Scholar]
- Walls Alexandra C., Young-Jun Park M., Tortorici Alejandra, Wall Abigail, McGuire Andrew T., Veesler David. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcroft K.L., Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg. 2019 doi: 10.1001/jamaoto.2019.1728. 2019 Jul 18. Epub ahead of print. PMID: 31318413. [DOI] [PubMed] [Google Scholar]
- Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. J. Am. Med. Assoc. 2020;323(24):2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- Whitcroft K.L., Ezzat M., Cuevas M., Andrews P., Hummel T. The effect of intranasal sodium citrate on olfaction in post-infectious loss: results from a prospective, placebo-controlled trial in 49 patients. Clin. Otolaryngol. : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2017;42(3):557–563. doi: 10.1111/coa.12789. [DOI] [PubMed] [Google Scholar]
- Whitcroft K.L., Merkonidis C., Cuevas M., Haehner A., Philpott C., Hummel T. Intranasal sodium citrate solution improves olfaction in post-viral hyposmia. Rhinology. 2016;54(4):368–374. doi: 10.4193/Rhin16.054. [DOI] [PubMed] [Google Scholar]
- Who World Health organization. Coronavirus disease (COVID-19) [s.d.] https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Acessed in March 30th, 2021.
- Xu H., Zhong L., Deng J., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. International forum of allergy & rhinology. 2020;10(7):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Rathor A., Krook K., Ma Y., Rotella M.R., Dodd R.L., Hwang P.H., Nayak J.V., Oyesiku N.M., DelGaudio J.M., Levy J.M., Wise J., Wise S.K., Patel Z.M. Effect of omega-3 supplementation in patients with smell dysfunction following endoscopic sellar and parasellar tumor resection: a multicenter prospective randomized controlled trial. Neurosurgery. 2020;87(2):E91–E98. doi: 10.1093/neuros/nyz559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J., Markkanen M., Mäkitie A. Pathophysiology of the COVID-19 – entry to the CNS through the nose. Acta Otolaryngol. 2020;140(10):886–889. doi: 10.1080/00016489.2020.1773533. [DOI] [PubMed] [Google Scholar]
- Youngentob S.L., Kent P.F. Enhancement of odorant-induced mucosal activity patterns in rats trained on an odorant identification task. Brain Res. 1995;670(1):82–88. doi: 10.1016/0006-8993(94)01275-m. [DOI] [PubMed] [Google Scholar]
- Zayet S., Kadiane-Oussou N.J., Lepiller Q., Zahra H., Royer P.Y., Toko L., Gendrin V., Klopfenstein T. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microb. Infect. 2020;22(9):481–488. doi: 10.1016/j.micinf.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Fei, Ting Yu, Du Ronghui, Fan Guohui, Liu Ying, Liu Zhibo, Xiang Jie, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., 2nd, Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]