Abstract
The crisis of antibiotic resistance necessitates creative and innovative approaches, from chemical identification and analysis to the assessment of bioactivity. Plant natural products (NPs) represent a promising source of antibacterial lead compounds that could help fill the drug discovery pipeline in response to the growing antibiotic resistance crisis. The major strength of plant NPs lies in their rich and unique chemodiversity, their worldwide distribution and ease of access, their various antibacterial modes of action, and the proven clinical effectiveness of plant extracts from which they are isolated. While many studies have tried to summarize NPs with antibacterial activities, a comprehensive review with rigorous selection criteria has never been performed. In this work, the literature from 2012 to 2019 was systematically reviewed to highlight plant-derived compounds with antibacterial activity by focusing on their growth inhibitory activity. A total of 459 compounds were included in this review, of which 50.8% were phenolic derivatives, 26.6% were terpenoids, 5.7% were alkaloids, and 17% were classified as other metabolites. A selection of 183 compounds were further discussed regarding their antibacterial activity, biosynthesis, structure-activity relationship, mechanism of action, and their potential as antibiotics. Emerging trends in the field of antibacterial drug discovery from plants are also discussed. This review brings to the forefront key findings on the antibacterial potential of plant NPs for consideration in future antibiotic discovery and development efforts.
Graphical Abstract
1. Introduction
The ability to successfully treat infectious diseases is threatened due to the rise of antimicrobial resistance (AMR). According to the Centers for Disease Control and Prevention (CDC), about 2.9 million antibiotic-resistant infections occur in the United States each year, resulting in 35,900 deaths.1 The CDC lists sixteen bacteria and two fungi as urgent, serious or concerning threats, including Mycobacterium tuberculosis, of which there are extensively drug-resistant strains, resistant to two of four first-line antibiotics and at least one of the three second-line antibiotics.2
Several factors are involved in the rise of antibiotic resistance, especially the overuse and misuse of antibiotics in human and animal health and the lack of development of new antibiotics. The field of antibiotic discovery and development is in dire need of innovation in order to reinvigorate the pipeline that has not seen a new class of drugs discovered and approved by the FDA since the late 1980s.3 This status quo can be in large part explained by the economics of antibiotics post-approval, which has become so unfavorable to companies and investors that antibiotic start-ups and large pharmaceutical companies alike are unable to survive in the antibiotics development space.4
In the search for novel antibiotics, the screening of synthetic combinatorial compound libraries has failed to meet expectations and has consequently demonstrated the importance of exploring the biologically relevant chemical space.5,6 Compounds that occupy this space in chemistry are already able, unlike the majority of synthetic compounds, to interact with biological machinery and potentially act as drugs.7 Natural products (NPs) have a strong tendency to occupy this space, in part due to their core purpose of interacting with biological systems and their vast range of chemical and structural diversity that reach complexities above what many synthetic compound libraries possess.5
Historically, the discovery of penicillin by Alexander Fleming in the 1920s was made from a culture of the fungus Penicillium notatum.8 To date, of the 162 antibacterial agents approved by the U.S. Food and Drug Administration from 1981 to 2019, about 50% are from or derived from NPs.9 Almost all of these have a microbial source rather than a plant source in part due to former limited accessibility of plant natural products for drug discovery.10 The more recent improvement in accessibility of plant NPs is due to enhanced compatibility with high throughput screening (HTS) and advances in lead optimization, compound isolation, dereplication, and plant sample acquisition.5,7,11,12
Plants are particularly interesting as sources for antibiotic leads because they have developed complex defense mechanisms against microbes, such as the use of chemical defenses involving a wide range of structurally unique secondary metabolites.13 Plants also possess other distinctive features promising for drug discovery, including their rich chemodiversity across the approximately 374,000 species of plants worldwide,14 the proven clinical effectiveness of plant extracts in long-standing traditional medicinal practices across the world,15 their ease of access, and the potential for synergistic interactions between phytochemicals and other bioactive compounds.16
Ethnobotany is the scientific study of the interactions between humankind and the plant kingdom; it has also been defined as the science of survival.17 The field encompasses studies on how humans use plants for food, medicine, art, construction, music, ritual, and more. The history of medicine is rich in records of plants used to treat myriad ailments, including infectious diseases. Today, between 70–95% of people in the developing world continue to rely on plants for their primary pharmacopeia.18 According to the Medicinal Plant Names Services (MPNS), 28,187 species are used in medicine, representing nearly 7.5% of all plant life on Earth.19 Through focusing on those species already in use in traditional therapies for the treatment of infectious disease, a more targeted approach to identifying plant NPs with antibacterial properties can be achieved; this is known as the ethnobotanical approach to drug discovery.
Plant NPs can act as antibacterials through various modes of action. All antibiotics currently in the clinic work by inhibiting bacterial growth, either in a bactericidal or bacteriostatic fashion, and this is achieved by drug-mediated perturbation of an essential cellular process. One of the main drawbacks of growth inhibition is the inevitable selection for resistance due to the rapid evolution of the bacterial genome under selective pressure on essential cellular processes.20 Given the threat of antibiotic resistance, a growing body of research is focused on the development of drugs that target bacterial virulence, a non-essential cellular process. Antivirulence drug candidates target systems such as biofilm formation and quorum sensing, potentially exerting less selective pressure for resistance and less detrimental effects on commensal microbes.21 The potential of plant compounds to exert such antivirulence activities is covered in an exhaustive review by Silva et al.22 Also, of note, the engineering of natural products by synthetic chemistry was explored in depth by Rossiter et al.23
1.1. Scope and Terminology
We discuss the growth inhibitory activity of plants and define antimicrobials here as an agent that kills or limits the growth of microorganisms (bacteria, fungi, parasites, viruses). We specifically use the terms ‘antibacterial’ or ‘antibiotics’ when describing agents that kill or inhibit the growth of bacteria. We focused on plant-derived compounds with an emphasis on the minimum inhibitory concentration (MIC) value, which is commonly used as an indicator of antibacterial potency. The minimum inhibitory concentration (MIC) is defined as the lowest concentration of an antimicrobial agent that inhibits the visible growth of a microorganism in vitro.24 To provide a comprehensive analysis on the topic, we performed a thorough literature review by using electronic databases (Web of Science, PubMed, SciFinder) and specific keywords: “plant,” “inhibitory concentration,” and “antibacterial,” covering the period between January 1, 2012 to September 3, 2019. We then used rigorous inclusion and exclusion criteria to select plant-derived compounds with significant antibacterial activity defined as MICs ≤ 100 µg/mL. In this review, we refer to the antimicrobial activity of compounds with MICs ≤ 10 µg/mL as high and MICs of 11–100 µg/mL as moderate.
All plant names were checked for accuracy with The Plant List,25 and plant family assignments follow the Angiosperm Phylogeny Group IV guidance.26 All reported bacterial names were cross-checked for accuracy and updated in accordance with the List of Prokaryotic names with Standing in Nomenclature.27
Of the 459 plant-derived compounds extracted from 198 scientific articles included, we decided to focus on the 183 compounds with the best MIC values, representing compounds of interest for further study. Finally, we discuss recent technological developments in the field of chemistry and pharmacology useful for improving the drug discovery process from plant materials.
2. Plant-Derived Compounds with Antibacterial Properties
2.1. Overview
We identified 459 botanical antibacterial compounds with a total of 1,394 MIC values reported in µg/mL (Supporting Information File 1). These were isolated from 73 plant families, 152 plant genera, and 183 different species. Data were also deposited in the Shared Platform for Antibiotic Research and Knowledge (SPARK) with Pew Charitable Trusts, accessible at https://app.collaborativedrug.com/vaults/4724/protocols. Registration for a SPARK account is free and available at http://www.pewtrusts.org/spark-antibiotic-discovery.
2.1.1. Major Chemical Classes Investigated
Antibacterial plant NPs were categorized into four major chemical classes: alkaloids, phenolic derivatives, terpenoids, and other metabolites. Of the compounds tested, 50.8% (233/459) belong to the major class phenolic derivatives. Terpenoids compromise 26.6% (122/459) of the compounds, other metabolites account for 17% (79/459) of compounds, and alkaloids account for the fewest compounds at 5.7% (26/459). The antibacterial activity data on the most active 183 compounds is presented in Table 1.
Table 1.
Summary of antibacterial activity for select plant NPs. Compounds are presented with the stereochemistry and configurations determined from the original citation. However, for structures that underwent revisions or for which additional stereochemistry is necessary to differentiate it from other isomers, these clarifications and corrections are included in this table.
| N° | Structure | Compound | Bacteriaa | MIC (μg/mL) | Ref. |
|---|---|---|---|---|---|
| ALKALOIDS | |||||
| Quinolines | |||||
| 1 | 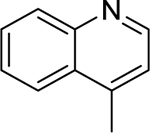 |
4-methylquinoline | Staphylococcus aureus | 12.5 | 28 |
| Listeria monocytogenes | 25 | ||||
| Bacillus cereus | 50 | ||||
| Salmonella enterica serotype Typhimurium | 75 | ||||
| Shigella sonnei | 100 | ||||
| 2 | 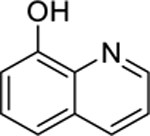 |
8-hydroxyquinoline | Staphylococcus aureus | 2 | 29 |
| Haemophilus influenzae | 8 | ||||
| 3 | evocarpine | Staphylococcus aureus | 8 | 30 | |
| Staphylococcus aureus (MRSA) | 8 | ||||
| Isoquinolines, Aporphines, and Phenanthrenes | |||||
| 4 | 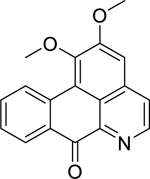 |
lysicamine | Listeria monocytogenes | 2.5 | 31 |
| Streptococcus pneumoniae | 2.5 | ||||
| Streptococcus agalactiae | 5 | ||||
| Klebsiella pneumoniae (ESBL-KP) | 10 | ||||
| Proteus vulgaris | 10 | ||||
| 5 | 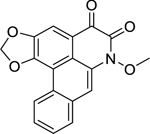 |
artabotrine | Bacillus cereus | 1.25 | 31 |
| Listeria monocytogenes | 1.25 | ||||
| Proteus vulgaris | 1.25 | ||||
| Staphylococcus sp. (ORCNS) | 1.25 | ||||
| Staphylococcus aureus | 2.5 | ||||
| Klebsiella pneumoniae (ESBL-KP) | 2.5 | ||||
| 6 | 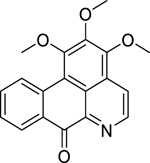 |
liridine | Bacillus subtilis | 0.625 | 31 |
| Listeria monocytogenes | 1.25 | ||||
| Staphylococcus sp. (OSCNS) | 1.25 | ||||
| Streptococcus agalactiae | 1.25 | ||||
| Klebsiella pneumoniae (ESBL-KP) | 2.5 | ||||
| 7 | 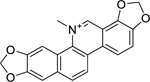 |
sanguinarine | Staphylococcus epidermidis | 0.5 | 32 |
| Staphylococcus aureus (MRSA) | 1 | ||||
| Enterococcus faecalis (Vancomycin-R) | 8 | ||||
| Escherichia coli | 4 | ||||
| Acinetobacter baumannii | 16 | ||||
| Klebsiella pneumoniae | 16 | ||||
| 8 | 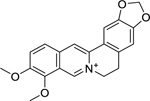 |
berberine | Brucella abortus | 1.56 | 33 |
| Prevotella intermedia | 3.8 | 34 | |||
| Fusobacterium nucleatum | 31.25 | ||||
| 9 | 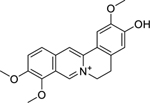 |
jatrorhizine | Brucella abortus | 0.78 | 33 |
| 10 | 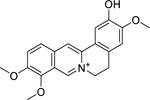 |
columbamine | Brucella abortus | 3.12 | 33 |
| 11 | 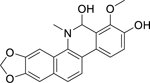 |
buesgenine | Escherichia coli | 4 | 35 |
| Klebsiella pneumoniae | 4 | ||||
| Enterobacter aerogenes | 16 | ||||
| Pseudomonas aeruginosa | 32 | ||||
| 12 | 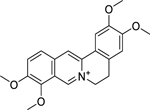 |
palmatine | Helicobacter pylori | 3.12 | 36 |
| Brucella abortus | 6.25 | 33 | |||
| Other Alkaloid Derivatives | |||||
| 13 | 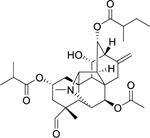 |
carmichaedine | Bacillus subtilis | 8 | 37 |
| PHENOLIC DERIVATIVES | |||||
| Chalcones | |||||
| 14 | 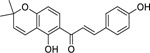 |
4-hydroxylonchocarpin | Staphylococcus aureus | 1 | 38 |
| 15 | isobavachalcone | Staphylococcus aureus | 2 | 38 | |
| 16 | 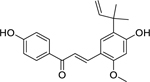 |
licochalcone A | Staphylococcus aureus | 1 | 39 |
| 17 | xanthoangelol | Staphylococcus aureus | 1.2 | 40 | |
| Enterococcus faecium | 1.2 | ||||
| Enterococcus faecalis | 2.5 | ||||
| 18 | 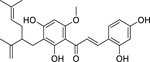 |
kuraridin | Staphylococcus aureus | 2 | 41 |
| Coumarins | |||||
| 19 | 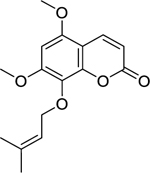 |
artanin | Staphylococcus aureus | 8 | 42 |
| Staphylococcus aureus (MRSA) | 8 | ||||
| 20 | 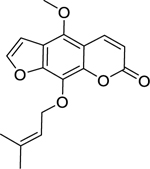 |
phellopterin | Staphylococcus aureus | 8 | 42 |
| Staphylococcus aureus (MRSA) | 16 | ||||
| 21 | 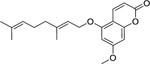 |
5-geranyloxy-7-methoxy-coumarin | Staphylococcus aureus | 8 | 42 |
| Staphylococcus aureus (MRSA) | 8 | ||||
| 22 | 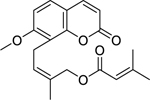 |
4′-senecioiloxyosthol | Bacillus subtilis | 5 | 43 |
| Flavonoids | |||||
| 23 | 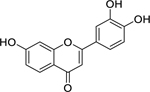 |
3′,4′,7-trihydroxyflavone | Providencia stuartii | 4 | 44 |
| Escherichia coli | 8 | ||||
| Klebsiella pneumoniae | 32 | ||||
| 24 | 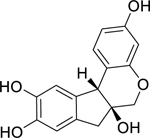 |
brazilin | Bacillus subtilis | 31.3 | 45 |
| Cutibacterium acnes | 15.6 | 46 | |||
| Staphylococcus epidermidis | 31.2 | ||||
| Staphylococcus aureus | 62.5 | ||||
| 25 | 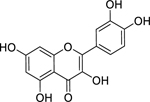 |
quercetin | Streptococcus pyogenes | 8 | 47 |
| Pseudomonas aeruginosa | 16 | ||||
| Staphylococcus aureus | 16 | ||||
| Aggregatibacter actinomycetemcomitans | 31.25 | 48 | |||
| Mycobacterium tuberculosis | 32 | 49 | |||
| Klebsiella pneumoniae | 32 | 47 | |||
| 26 | 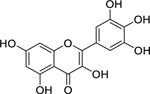 |
myricetin | Mycobacterium tuberculosis | 7.81 | 50 |
| 27 | 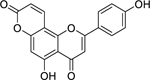 |
pseudarflavone A | Escherichia coli | 4 | 51 |
| Staphylococcus aureus | 8 | ||||
| Pseudomonas aeruginosa | 16 | ||||
| Klebsiella pneumoniae | 32 | ||||
| Enterococcus faecalis | 64 | ||||
| 28 | 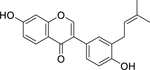 |
neobavaisoflavone | Enterococcus faecalis | 4 | 52 |
| Providencia stuartii | 4 | ||||
| Escherichia coli | 8 | 52,53 | |||
| Pseudomonas aeruginosa | 8 | ||||
| Klebsiella pneumoniae | 8 | ||||
| 29 | 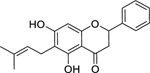 |
6-prenylpinocembrin | Escherichia coli | 4 | 51 |
| Klebsiella pneumoniae | 8 | ||||
| Staphylococcus aureus | 8 | ||||
| Enterococcus faecalis | 16 | ||||
| Pseudomonas aeruginosa | 32 | ||||
| 30 | 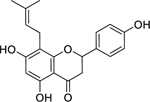 |
8-prenylnaringenin | Staphylococcus aureus | 12.5 | 54 |
| Staphylococcus epidermidis | 12.5 | ||||
| 31 | 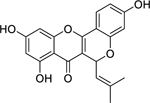 |
cyclocommunol | Staphylococcus aureus (MSSA) | 4 | 55 |
| Staphylococcus aureus (MRSA) | 8 | ||||
| 32 | 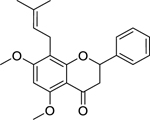 |
candidone | Enterococcus faecalis | 4 | 52 |
| Escherichia coli | 4 | ||||
| Klebsiella pneumoniae | 4 | ||||
| Enterobacter aerogenes | 16 | ||||
| 33 | 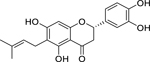 |
5,7,3’,4’-tetrahydroxy-6-(3’,3’-dimethylallyl)-flavanone | Staphylococcus aureus | 12.5 | 54 |
| Staphylococcus epidermidis | 50 | ||||
| 34 | 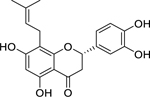 |
5,7,3’,4’-tetrahydroxy-8-(3’,3’-dimethylallyl)-flavanone | Staphylococcus aureus | 12.5 | 54 |
| Staphylococcus epidermidis | 25 | ||||
| 35 | 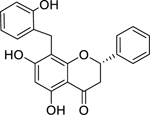 |
chamanetin | Staphylococcus aureus (MSSA) | 7.5 | 56 |
| Staphylococcus aureus (MRSA) | 15 | ||||
| Staphylococcus aureus (VISA) | 15 | ||||
| Enterococcus faecalis | 15 | ||||
| Bacillus subtilis | 15 | ||||
| 36 | 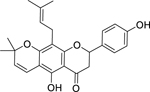 |
lupinifolin | Streptococcus mutans | 1 | 57 |
| Staphylococcus aureus | 8 | 58 | |||
| Bacillus cereus | 15.63 | 59 | |||
| 37 | 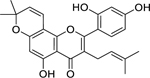 |
morusin | Staphylococcus aureus (MRSA) | 8 | 55 |
| Staphylococcus aureus (MSSA) | 16 | ||||
| 38 | 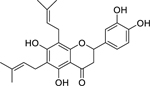 |
6–8-diprenyleriodictyol | Staphylococcus aureus | 0.5 | 38 |
| 39 | 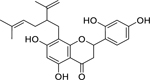 |
sophoraflavanone G | Staphylococcus aureus | 1 | 41 |
| 40 | 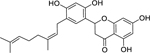 |
kuwanon E | Staphylococcus aureus (MSSA) | 4 | 55 |
| Staphylococcus aureus (MRSA) | 4 | ||||
| 41 | 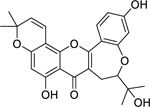 |
neocyclomorusin | Enterococcus faecalis | 4 | 52 |
| Klebsiella pneumoniae | 4 | ||||
| Enterobacter aerogenes | 8 | ||||
| Escherichia coli | 8 | ||||
| Providencia stuartii | 8 | ||||
| 42 | 6-geranyl-5,7,3′-trihydroxy-4′-methoxyisoflavone | Enterococcus faecium | 5.5 | 40 | |
| Enterococcus faecalis | 10.9 | ||||
| Staphylococcus aureus | 43.7 | ||||
| 43 | 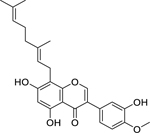 |
8-geranyl-5,7,3′-trihydroxy-4′-methoxyisoflavone | Enterococcus faecium | 10.9 | 40 |
| Enterococcus faecalis | 21.8 | ||||
| Staphylococcus aureus | 43.7 | ||||
| 44 |  |
luteolin-8-C-glucoside | Staphylococcus aureus | 4 | 60 |
| Shigella flexneri | 4 | ||||
| Vibrio cholerae | 16 | ||||
| 45 |  |
isoquercetin | Pseudomonas aeruginosa | 8 | 47 |
| Staphylococcus aureus | 16 | ||||
| 46 |  |
dichamanetin | Staphylococcus aureus (MRSA) | 1 | 56 |
| Staphylococcus aureus | 2 | ||||
| Staphylococcus aureus (VRSA) | 2 | ||||
| Bacillus subtilis | 4 | ||||
| Enterococcus faecalis (Vancomycin-R) | 7.5 | ||||
| 47 |  |
amentoflavone | Staphylococcus aureus | 4 | 61 |
| Enterococcus faecium | 8 | ||||
| Escherichia coli | 8 | ||||
| Pseudomonas aeruginosa | 8 | ||||
| Streptococcus mutans | 32 | ||||
| 48 | 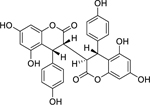 |
3″-epidiphysin | Staphylococcus aureus | 2.17 | 62 |
| Bacillus subtilis | 19.5 | ||||
| 49 | 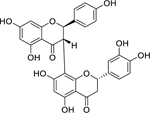 |
(2R,3S,2’’S)- 3’’’,4’,4’’’,5,5’’,7,7’’-heptahydroxy-3,8”-biflavanone | Staphylococcus aureus | 2 | 60 |
| Shigella flexneri | 4 | ||||
| Vibrio cholerae | 8 | ||||
| 50 | 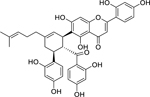 |
multicaulisin | Staphylococcus aureus (MRSA) | 2 | 63 |
| 51 | 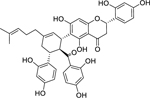 |
sanggenon G | Staphylococcus aureus (MRSA) | 4 | 63 |
| 52 | 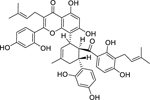 |
albanin G | Staphylococcus aureus (MRSA) | 4 | 63 |
| Lignans | |||||
| 53 | 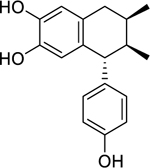 |
3′-demethoxy-6-O-demethylisoguaiacin | Enterococcus faecalis | 12.5 | 64 |
| Mycobacterium tuberculosis (MDR) | 12.5 | ||||
| Staphylococcus aureus (MRSA) | 12.5 | ||||
| Escherichia coli | 50 | ||||
| 54 | 4-epi-larreatricin | Enterococcus faecalis | 12.5 | 64 | |
| Mycobacterium tuberculosis (MDR) | 25 | ||||
| Mycobacterium tuberculosis | 50 | ||||
| 55 | 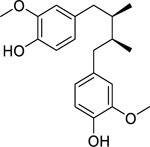 |
dihydroguaiaretic acid | Mycobacterium tuberculosis (MDR) | 12.5 | 64 |
| Mycobacterium tuberculosis | 50 | ||||
| Staphylococcus aureus (MRSA) | 50 | ||||
| Quinones and Related Compounds | |||||
| 56 | 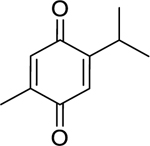 |
thymoquinone | Haemophilus influenzae | 8 | 29 |
| Staphylococcus aureus (MRSA) | 8 | 65 | |||
| Staphylococcus aureus | 16 | 29 | |||
| Streptococcus pneumoniae | 16 | ||||
| 57 | 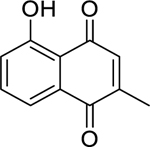 |
plumbagin | Mycobacterium tuberculosis (XDR) | 1.56 | 66 |
| Escherichia coli | 2 | 67 | |||
| Klebsiella pneumoniae | 2 | ||||
| Staphylococcus aureus (MRSA) | 2 | ||||
| Streptococcus pneumoniae | 5 | 68 | |||
| Staphylococcus aureus | 5 | 69 | |||
| 58 | 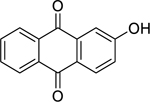 |
2-hydroxy-anthraquinone | Bacillus subtilis | 1.9 | 70 |
| Bacillus cereus | 62.5 | ||||
| 59 | 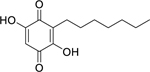 |
2,5-dihydroxy-3-heptyl-2,5-cyclohexadiene-1,4-dione | Enterobacter aerogenes | 4 | 67 |
| Escherichia coli | 4 | ||||
| Klebsiella pneumoniae | 4 | ||||
| Staphylococcus aureus | 4 | ||||
| 60 | 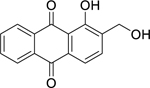 |
digiferruginol | Bacillus subtilis | 0.9 | 70 |
| 61 | 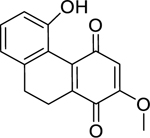 |
ephemeranthoquinone B | Bacillus subtilis | 1.1 | 71 |
| 62 | 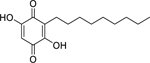 |
homoembelin | Staphylococcus aureus | 4 | 67 |
| Enterobacter aerogenes | 8 | ||||
| Pseudomonas aeruginosa | 16 | ||||
| Escherichia coli | 32 | ||||
| 63 | 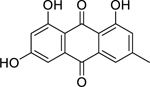 |
emodin | Staphylococcus aureus (MRSA) | 4 | 67 |
| Escherichia coli | 16 | ||||
| Staphylococcus aureus | 20 | 72 | |||
| Klebsiella pneumoniae | 32 | 67 | |||
| 64 | 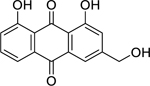 |
aloe-emodin | Staphylococcus aureus | 4 | 73 |
| Shigella flexneri | 16 | ||||
| Vibrio cholerae | 64 | ||||
| 65 | 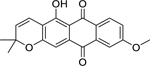 |
elastiquinone | Providencia stuartii | 4.9 | 74 |
| Pseudomonas aeruginosa | 4.9 | ||||
| Proteus vulgaris | 9.8 | ||||
| Staphylococcus aureus | 9.8 | ||||
| Escherichia coli | 19.5 | ||||
| 66 | 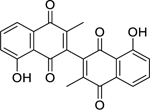 |
3,3′-biplumbagin | Mycobacterium tuberculosis (XDR) | 3.13 | 66 |
| Mycobacterium tuberculosis | 3.13 | ||||
| 67 | 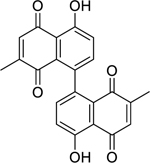 |
maritinone | Mycobacterium tuberculosis (XDR) | 3.13 | 66 |
| Mycobacterium tuberculosis | 3.13 | ||||
| 68 | 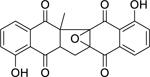 |
zeylanone epoxide | Mycobacterium tuberculosis (XDR) | 12.5 | 66 |
| Mycobacterium tuberculosis | 25 | ||||
| 69 | 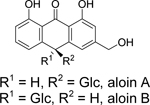 |
aloin A/B | Vibrio cholerae | 10 | 75 |
| Escherichia coli | 10 | 76 | |||
| Salmonella enterica serotype Typhi | 10 | ||||
| Shigella dysenteriae | 10 | ||||
| Staphylococcus aureus | 25 | ||||
| Bacillus subtilis | 50 | 75 | |||
| 70 | 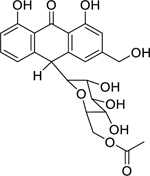 |
aloin-6’-O-acetate A/B | Bacillus subtilis | 10 | 75 |
| Salmonella enterica serotype Typhimurium | 10 | ||||
| Vibrio cholerae | 10 | ||||
| Bacillus pumilus | 25 | ||||
| Shigella dysenteriae | 25 | ||||
| Staphylococcus aureus | 25 | ||||
| 71 | 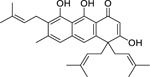 |
ferruginin A | Enterococcus faecalis | 4 | 77 |
| Escherichia coli | 4 | ||||
| Klebsiella pneumoniae | 4 | ||||
| Enterobacter aerogenes | 8 | ||||
| Xanthones | |||||
| 72 | 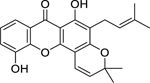 |
ananixanthone | Enterococcus faecalis | 2 | 78 |
| Staphylococcus aureus | 32 | ||||
| Pseudomonas aeruginosa | 64 | ||||
| Bacillus cereus | 64 | ||||
| 73 | 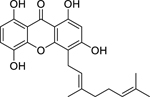 |
cheffouxanthone | Enterococcus faecalis | 8 | 78 |
| Bacillus cereus | 32 | ||||
| Escherichia coli | 64 | ||||
| Staphylococcus aureus | 64 | ||||
| 74 | 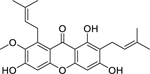 |
α-mangostin | Staphylococcus aureus | 4 | 79 |
| Staphylococcus saprophyticus | 8 | ||||
|
Leptospira
interrogans serovar Saigon |
100 | 80 | |||
| Leptospira interrogans serovar Javanica | 100 | ||||
| 75 | 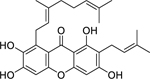 |
norcowanin | Staphylococcus aureus | 8 | 81 |
| Staphylococcus aureus (MRSA) | 16 | ||||
| Escherichia coli | 64 | ||||
| 76 | 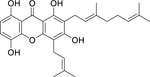 |
4-prenyl-2-(3,7-dimethyl-2,6-octadienyl)-1,3,5,8-tetrahydroxyxanthone | Mycobacterium tuberculosis | 8 | 82 |
| Enterobacter aerogenes | 64 | ||||
| Escherichia coli | 64 | ||||
| Providencia stuartii | 64 | ||||
| Pseudomonas aeruginosa | 64 | ||||
| 77 | 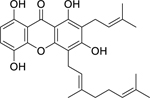 |
1,3,5,8-tetrahydroxy-2-(3-methybut-2-enyl)-4-(3,7-dimethyloct-2,6-dienyl) xanthone |
Enterococcus faecalis |
8 | 78 |
| Pseudomonas aeruginosa | 16 | ||||
| Escherichia coli | 32 | ||||
| Salmonella enterica serotype Typhimurium | 64 | ||||
| Staphylococcus aureus | 64 | ||||
| 78 | 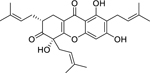 |
garciniacowone | Staphylococcus aureus (MRSA) | 2 | 81 |
| Staphylococcus aureus | 2 | ||||
| 79 | 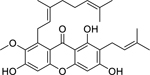 |
cowanin | Staphylococcus aureus (MRSA) | 4 | 81 |
| Staphylococcus aureus | 32 | ||||
| 80 | 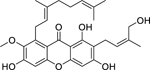 |
cowanol | Staphylococcus aureus (MRSA) | 2 | 81 |
| Staphylococcus aureus | 8 | ||||
| 81 | 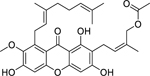 |
cowagarcinone E | Staphylococcus aureus (MRSA) | 8 | 81 |
| Other Phenolic Compounds | |||||
| 82 | 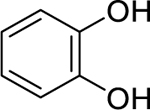 |
pyrocatechol | Aggregatibacter actinomycetemcomitans | 4.88 | 48 |
| 83 | 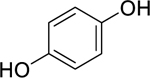 |
hydroquinone | Mycobacterium tuberculosis | 12.5 | 83 |
| Mycobacterium smegmatis | 50 | ||||
| Mycobacterium tuberculosis (XDR) | 50 | ||||
| 84 | 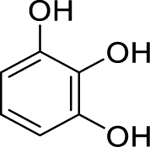 |
pyrogallol | Aggregatibacter actinomycetemcomitans | 2.4 | 48 |
| Streptococcus mitis | 9.76 | ||||
| 85 | 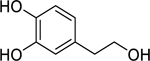 |
hydroxytyrosol | Staphylococcus aureus | 2 | 84 |
| 86 | 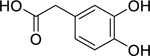 |
3,4-dihydroxyphenyl-acetic acid | Aggregatibacter actinomycetemcomitans | 4.88 | 48 |
| 87 | 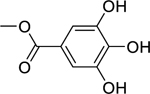 |
methyl gallate | Staphylococcus aureus | 7.8 |
85 86 85 |
| Vibrio cholerae | 64 | ||||
| Escherichia coli | 93 | ||||
| 88 | 4,5-(methylene-dioxy)-o-coumaroylputrescine | Staphylococcus aureus | 8 | 87 | |
| Streptococcus agalactiae | 8 | ||||
| Escherichia coli | 16 | ||||
| Pseudomonas aeruginosa | 16 | ||||
| 89 | 4,5-(methylene-dioxy)-o-coumaroyl-4’-N-methylputrescine | Staphylococcus aureus | 8 | 87 | |
| Streptococcus agalactiae | 8 | ||||
| Escherichia coli | 16 | ||||
| Pseudomonas aeruginosa | 16 | ||||
| 90 | 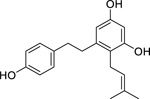 |
2-(3-methyl-2-butenyl)-3,5,4’-trihydroxy-bibenzyl | Staphylococcus epidermidis | 12.5 | 54 |
| Staphylococcus aureus | 50 | ||||
| 91 | 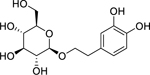 |
2-(3,4-dihydroxy)-phenyl-ethyl-O-β-D-glucopyranoside | Staphylococcus aureus | 8 | 84 |
| 92 | 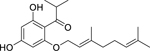 |
2-geranyloxy-1-(2-methylpropanoyl) phloroglucinol | Staphylococcus aureus | 3.91 | 88 |
| Staphylococcus aureus (MRSA) | 7.81 | ||||
| Staphylococcus epidermidis | 7.81 | ||||
| 93 | 3-geranyl-1-(2-methylpropanoyl) phloroglucinol | Staphylococcus aureus (MRSA) | 3.91 | 88 | |
| Staphylococcus aureus | 7.81 | ||||
| Staphylococcus epidermidis | 7.81 | ||||
| 94 | 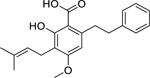 |
amorfrutin A | Staphylococcus aureus | 2.1 | 40 |
| Enterococcus faecalis | 8.5 | ||||
| Enterococcus faecium | 8.5 | ||||
| 95 | 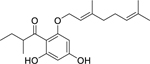 |
2-geranyloxy-1-(2-methylbutanoyl) phloroglucinol | Staphylococcus aureus | 3.91 | 88 |
| Staphylococcus epidermidis | 3.91 | ||||
| Staphylococcus aureus (MRSA) | 7.81 | ||||
| 96 | 3-geranyl-1-(2-methylbutanoyl) phloroglucinol | Staphylococcus aureus (MRSA) | 3.91 | 88 | |
| Staphylococcus epidermidis | 3.91 | ||||
| Staphylococcus aureus | 7.81 | ||||
| 97 | 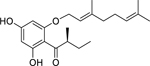 |
olympicin A | Staphylococcus aureus (MRSA) | 0.5 | 89 |
| Staphylococcus aureus | 1 | ||||
| Mycobacterium phlei | 4 | ||||
| Mycobacterium smegmatis | 4 | ||||
| Mycobacterium fortuitum | 8 | ||||
| 98 | 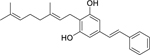 |
amorphastilbol | Staphylococcus aureus | 1.1 | 40 |
| Enterococcus faecalis | 2.2 | ||||
| Enterococcus faecium | 2.2 | ||||
| 99 | 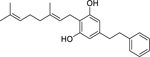 |
2-geranyl-5-(2-phenylethyl) resorcin | Staphylococcus aureus | 2.2 | 40 |
| Enterococcus faecalis | 4.4 | ||||
| Enterococcus faecium | 4.4 | ||||
| 100 | 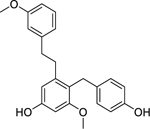 |
bulbocol | Staphylococcus aureus | 9 | 90 |
| Staphylococcus aureus (MRSA) | 37 | ||||
| 101 | 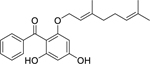 |
2-geranyloxy-4,6-dihydroxybenzo-phenone | Staphylococcus aureus | 1.95 | 88 |
| Staphylococcus aureus (MRSA) | 3.91 | ||||
| Staphylococcus epidermidis | 3.91 | ||||
| 102 | 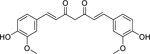 |
curcumin | Porphyromonas gingivalis | 7.81 | 48 |
| Fusobacterium nucleatum | 31.25 | ||||
| Streptococcus mitis | 62.5 | ||||
| Bacillus subtilis | 78 | 91 | |||
| 103 | 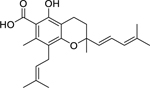 |
3,4-dihydro-5-hydroxy-2,7-dimethyl-8-(3”-methyl-2”-butenyl)-2-(4’-methyl-1’,3’-pentadienyl)-2H-1-benzopyran-6-carboxylic acid | Enterococcus faecalis | 4 | 92 |
| Staphylococcus aureus (MRSA) | 4 | ||||
| Staphylococcus aureus | 4 | ||||
| Staphylococcus epidermidis | 8 | ||||
| 104 | 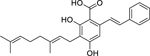 |
2-[(E)-styryl]-5-geranylresorcin-1-carboxylic acid | Staphylococcus aureus | 4.1 | 40 |
| Enterococcus faecalis | 9.8 | ||||
| Enterococcus faecium | 9.8 | ||||
| 105 | 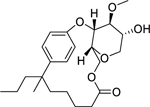 |
tetraceranoate | Mycobacterium smegmatis | 7.8 | 93 |
| 106 | 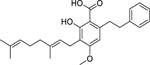 |
amorfrutin B | Enterococcus faecalis | 2.6 | 40 |
| Enterococcus faecium | 2.6 | ||||
| Staphylococcus aureus | 5.1 | ||||
| 107 | 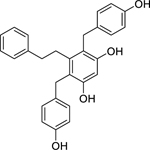 |
shancigusin B | Staphylococcus aureus | 3 | 90 |
| Staphylococcus aureus (MRSA) | 13 | ||||
| Bacillus subtilis | 26 | ||||
| 108 | 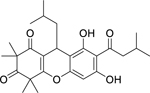 |
rhodomyrtone | Streptococcus mutans | 0.39 | 94 |
| Cutibacterium acnes | 0.5 | 95 | |||
| Staphylococcus aureus | 0.78 | 94 | |||
| 109 | 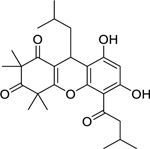 |
rhodomyrtosone B | Staphylococcus aureus | 0.62 | 96 |
| Staphylococcus aureus (MRSA) | 0.62 | ||||
| Bacillus cereus | 0.62 | ||||
| Cutibacterium acnes | 0.62 | ||||
| Enterococcus faecalis | 1.25 | ||||
| 110 | 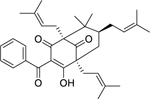 |
7-epi-clusianone | Enterococcus faecalis | 2 | 97 |
| Staphylococcus aureus | 2 | ||||
| 111 | 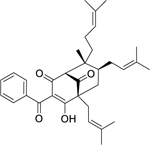 |
chamuangone | Streptococcus pyogenes | 7.8 | 98 |
| Helicobacter pylori | 15.6 | ||||
| Streptococcus viridans | 15.6 | ||||
| Bacillus subtilis | 31.2 | ||||
| Staphylococcus aureus | 31.2 | ||||
| 112 | 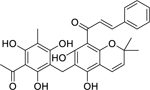 |
rottlerin | Enterococcus faecalis | 1 | 99 |
| Staphylococcus aureus (Norfloxacin-R) | 2 | ||||
| Staphylococcus aureus (MRSA) | 2 | ||||
| Bacillus subtilis | 4 | ||||
| Staphylococcus aureus | 4 | ||||
| 113 | 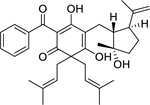 |
hypercalin B | Staphylococcus aureus (MRSA) | 2 | 100 |
| 114 | 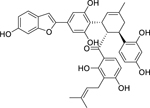 |
chalcomoracin | Staphylococcus aureus (MRSA) | 2 | 101 |
| Staphylococcus aureus | 4 | ||||
| 115 | 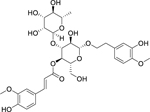 |
martynoside | Bacillus subtilis | 7.81 | 102 |
| Klebsiella pneumoniae | 31.2 | ||||
| TERPENOIDS | |||||
| Monoterpenoids | |||||
| 116 | 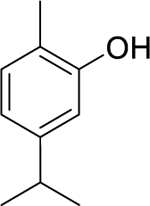 |
carvacrol | Micrococcus flavus | 2.5 | 103 |
| Bacillus cereus | 12.5 | ||||
| Staphylococcus aureus | 25 | ||||
| Escherichia coli | 50 | ||||
| Pseudomonas aeruginosa | 50 | ||||
| Salmonella enterica serotype Typhimurium | 50 | ||||
| 117 | 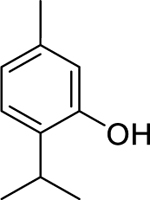 |
thymol | Bacillus cereus | 25 | 103 |
| Staphylococcus aureus | 25 | ||||
| Proteus mirabilis | 10 | ||||
| Staphylococcus epidermidis | 32 | 104 | |||
| Salmonella enteritidis | 32 | ||||
| Escherichia coli | 45 | 105 | |||
| 118 | linalool | Bacillus cereus | 10 | 106 | |
| Bacillus subtilis | 5 | ||||
| Escherichia coli | 5 | ||||
| Pseudomonas aeruginosa | 2.5 | ||||
| Salmonella enteritidis | 10 | ||||
| 119 | 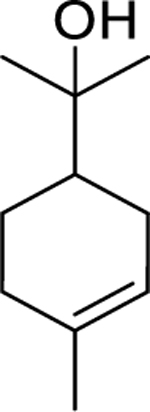 |
α-terpineol | Bacillus subtilis | 0.87 µL/mL | 107 |
| Staphylococcus epidermidis | 1.56 µL/mL | ||||
| Pseudomonas aeruginosa | 1.56 µL/mL | ||||
| Klebsiella pneumoniae | 1.56 µL/mL | ||||
| Staphylococcus aureus | 1.56 µL/mL | 108 | |||
| Escherichia coli | 55 | 105 | |||
| 120 | citronellol | Escherichia coli | 5 | 109 | |
| Sesquiterpenoids and Derivatives | |||||
| 121 | 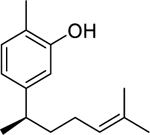 |
xanthorrhizol | Streptococcus mutans | 4.1 | 110 |
| Porphyromonas gingivalis | 6.8 | ||||
| 122 | 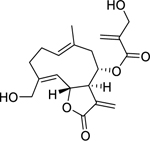 |
onopordopicrin | Bacillus subtilis | 86.2 | 111 |
| Vibrio fischeri | 2.2 | ||||
| 123 | 8,9-oxoisopropanyl-dshamirone | Staphylococcus aureus (MRSA) | 0.5 | 112 | |
| Staphylococcus aureus (Tetracycline-R) | 0.5 | ||||
| Diterpenoids | |||||
| 124 | 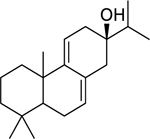 |
abieta-7,9(11)-dien-13-β-ol | Staphylococcus aureus | 0.98 | 113 |
| Bacillus cereus | 31.2 | ||||
| Enterococcus faecalis | 31.2 | ||||
| Salmonella enterica serotype Typhimurium | 62.5 | ||||
| 125 | 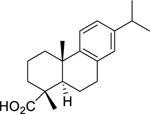 |
dehydroabietic acid | Actinomyces naeslundii | 12.5 | 114 |
| Cutibacterium acnes | 12.5 | ||||
| Porphyromonas gingivalis | 6.2 | ||||
| Bacteroides fragilis | 12.5 | ||||
| Prevotella intermedia | 12.5 | ||||
| Streptococcus mitis | 25 | 115 | |||
| 126 | 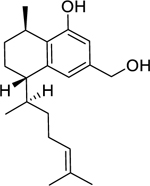 |
8,19-dihydroxyserrulat-14-ene | Bacillus subtilis | 3.1 | 116 |
| Staphylococcus aureus (MRSA) | 3.1 | ||||
| Streptococcus pneumoniae | 3.1 | ||||
| Moraxella catarrhalis | 3.1 | ||||
| 127 | 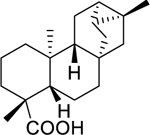 |
ent-trachyloban-19-oic acid | Streptococcus mutans | 8.9 | 110 |
| Porphyromonas gingivalis | 57.6 | ||||
| 128 | 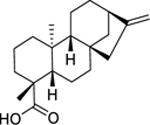 |
kaurenoic acid | Bacillus cereus | 12 | 117 |
| Staphylococcus epidermidis | 8 | ||||
| Streptococcus pneumoniae | 5 | ||||
| Cutibacterium acnes | 6.25 | 118 | |||
| Prevotella melaninogenica | 6.25 | ||||
| Porphyromonas gingivalis | 12.5 | ||||
| 129 | 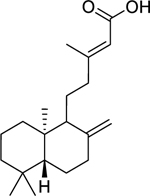 |
(-)-copalic acid | Staphylococcus epidermidis | 0.5 | 117 |
| Streptococcus pneumoniae | 2 | ||||
| Staphylococcus aureus (MRSA) | 15.6 | ||||
| 130 | 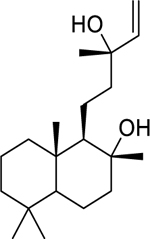 |
labd-14-ene-8,13-diol | Mycobacterium tuberculosis | 10.85 | 119 |
| 131 | 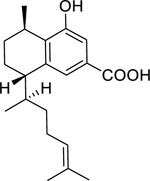 |
8-hydroxyserrulat-14-en-19-oic acid | Bacillus subtilis | 3.1 | 116 |
| Streptococcus pneumoniae | 6.2 | ||||
| Staphylococcus aureus (MRSA) | 12.5 | ||||
| Moraxella catarrhalis | 6.2 | ||||
| 132 | 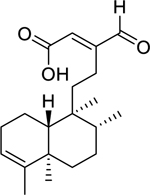 |
16-oxo-cleroda-3,13(14)-E-diene-15-oic acid | Staphylococcus aureus (MRSA) | 20 | 120 |
| Streptococcus mutans | 16 | ||||
| 133 | 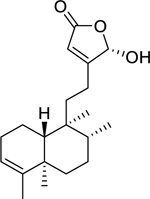 |
16α-hydroxycleroda-3,13(14)-Z-dien-15,16-olide | Staphylococcus aureus (MRSA) | 15.63 | 121,122 |
| 134 | 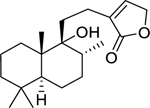 |
9-hydroxylabd-13-en-16,15-olide | Mycobacterium tuberculosis | 19.67 | 119 |
| 135 | 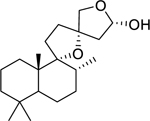 |
9,13:15,16-diepoxylabdan-15-ol | Mycobacterium tuberculosis | 14.88 | 119 |
| 136 | 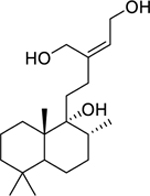 |
labd-13Z-ene-9,15,16-triol | Mycobacterium smegmatis | 30.57 | 119 |
| 137 | 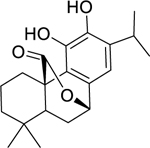 |
carnosol | Staphylococcus aureus (MRSA) | 15.6 | 123 |
| Enterococcus faecalis | 62.5 | ||||
| 138 | 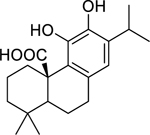 |
carnosic acid | Staphylococcus aureus (MRSA) | 7.8 | 123 |
| Enterococcus faecalis | 15.6 | ||||
| Staphylococcus aureus (MRSA) | 12 | 124 | |||
| 139 | 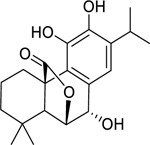 |
rosmanol | Staphylococcus aureus (MRSA) | 15.6 | 123 |
| Enterococcus faecalis | 62.5 | ||||
| 140 | 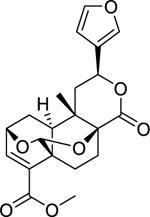 |
bafoudiosbulbin C | Escherichia coli | 16 | 125 |
| Enterobacter aerogenes | 64 | ||||
| Klebsiella pneumoniae | 64 | ||||
| Mycobacterium smegmatis | 8 | ||||
| Mycobacterium tuberculosis | 8 | ||||
| 141 | 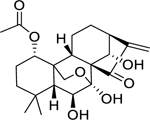 |
lasiodin | Bacillus cereus | 8 | 126 |
| Listeria monocytogenes | 16 | ||||
| Staphylococcus aureus | 16 | ||||
| Pseudomonas aeruginosa | 16 | ||||
| Nor-Triterpenes | |||||
| 142 | 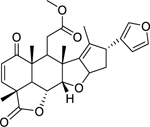 |
nimbolide | Staphylococcus aureus (MRSA) | 8 | 127 |
| Triterpenes and Saponins | |||||
| 143 | 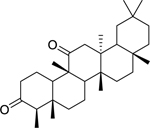 |
friedelane-3,11-dione | Mycobacterium tuberculosis | 3.9 | 50 |
| 144 | 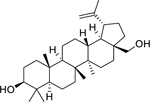 |
betulin | Mycobacterium aurum | 15 | 93 |
| Mycobacterium smegmatis | 15 | ||||
| 145 | 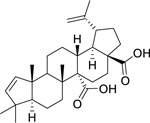 |
ceanothenic acid | Staphylococcus aureus | 8 | 128 |
| Enterococcus faecalis | 16 | ||||
| 146 | 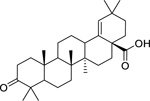 |
moronic acid | Bacillus subtilis | 1.52 | 129 |
| Staphylococcus aureus | 1.52 | ||||
| Streptococcus pyogenes | 1.52 | ||||
| 147 | 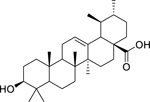 |
ursolic acid | Enterococcus faecalis | 1 | 130 |
| Listeria monocytogenes | 2 | ||||
| Mycobacterium tuberculosis | 10 | 83 | |||
| Mycobacterium tuberculosis (XDR) | 25 | ||||
| Klebsiella pneumoniae | 25 | 131 | |||
| Pseudomonas aeruginosa | 25 | ||||
| Vibrio cholerae | 100 | 132 | |||
| 148 | 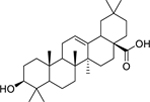 |
oleanolic acid | Enterococcus faecalis | 4 | 130 |
| Listeria monocytogenes | 8 | ||||
| Bacillus cereus | 16 | ||||
| Mycobacterium tuberculosis | 50 | ||||
| 149 | 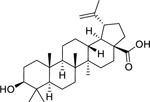 |
betulinic acid | Mycobacterium aurum | 15 | 93 |
| Mycobacterium smegmatis | 15 | ||||
| Staphylococcus aureus (MRSA) | 64 | 133 | |||
| 150 | 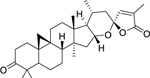 |
pseudolarolide B | Staphylococcus aureus | 25 | 134 |
| Escherichia coli | 6.3 | ||||
| 151 | 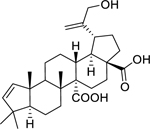 |
29-hydroxyceanothenic acid | Staphylococcus aureus | 4 | 128 |
|
Enterococcus faecalis |
16 | ||||
| 152 | 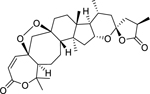 |
pseudolarolide Q | Staphylococcus aureus | 3.1 | 134 |
| Escherichia coli | 50 | ||||
| 153 | 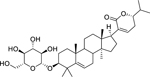 |
lanast-5-en-3β-D-glucopyranosyl-21 (24)-olide | Staphylococcus aureus | 2.4 | 135 |
| Bacillus subtilis | 4.8 | ||||
| Staphylococcus epidermidis | 4.8 | ||||
| Escherichia coli | 9.6 | ||||
| Klebsiella pneumoniae | 9.6 | ||||
| Pseudomonas aeruginosa | 9.6 | ||||
| 154 | 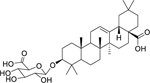 |
3-O-β-D-glucuronopyranosyl-oleanolic acid | Staphylococcus aureus | 8 | 136 |
| Shigella flexneri | 16 | ||||
| Escherichia coli | 32 | ||||
| 155 | 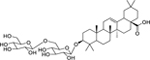 |
3-O-β-D-glucopyranosyl (1→6)-β-D-glucopyranosyl-oleanolic acid | Escherichia coli | 6.25 | 137 |
| Klebsiella pneumoniae | 6.25 | ||||
| Enterobacter aerogenes | 25 | ||||
| Salmonella enterica serotype Typhi | 100 | ||||
| 156 | 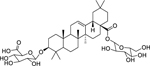 |
3-O-β-D-glucurono-pyranosyloleanolic acid 28-O-β-D-glucopyranosyl ester | Shigella flexneri | 16 | 136 |
| Staphylococcus aureus | 16 | ||||
| Escherichia coli | 32 | ||||
| Other Metabolites | |||||
| Aliphatic compounds | |||||
| 157 | 2E-undecenal | Salmonella enterica | 12.5 | 138 | |
| 158 | undecanol | Salmonella enterica | 12.5 | 138 | |
| 159 | 2E-dodecenal | Salmonella enterica | 6.25 | 138 | |
| 160 | dodecanol | Salmonella enterica | 6.25 | 138 | |
| 161 | 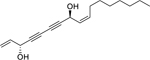 |
falcarindiol | Mycobacterium bovis | 5 | 139 |
| Mycobacterium tuberculosis | 20 | ||||
| 162 | n-dotriacont-9-one-13-ene | Bacillus subtilis | 9.6 | 135 | |
| Staphylococcus aureus | 19.2 | ||||
| Staphylococcus epidermidis | 19.2 | ||||
| Klebsiella pneumoniae | 19.2 | ||||
| Escherichia coli | 38.4 | ||||
| Pseudomonas aeruginosa | 38.4 | ||||
| Ceramides | |||||
| 163 | ficusoside B | Staphylococcus aureus | 4.9 | 74 | |
| Proteus vulgaris | 4.9 | ||||
| Providencia stuartii | 19.5 | ||||
| Pseudomonas aeruginosa | 39.1 | ||||
| Cyclic compounds | |||||
| 164 | 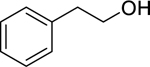 |
2-phenylethanol | Bacillus cereus | 5 | 106 |
| Lactobacillus plantarum | 10 | ||||
| Leuconostoc mesenteroides | 10 | ||||
| Staphylococcus aureus | 10 | ||||
| Salmonella enteritidis | 2.5 | ||||
| Shigella sonnei | 2.5 | ||||
| Pseudomonas aeruginosa | 5 | ||||
| 165 | 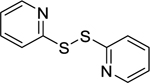 |
2,2’-dithiodipyridine | Streptococcus mutans | 2 | 140 |
| Streptococcus mitis | 4 | ||||
| 166 | 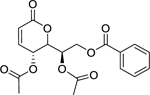 |
(-)-cleistenolide | Staphylococcus aureus (MRSA) | 7.5 | 56 |
| Enterococcus faecalis (Vancomycin-R) | 30 | ||||
| Staphylococcus aureus | 30 | ||||
| Staphylococcus aureus (VRSA) | 30 | ||||
| Glycosides | |||||
| 167 | 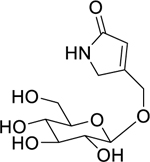 |
pinnatoside A | Escherichia coli | 1.562 | 137 |
| Enterobacter aerogenes | 3.125 | ||||
| Klebsiella pneumoniae | 3.125 | ||||
| Pseudomonas aeruginosa | 6.25 | ||||
| Salmonella enterica serotype Typhi | 12.5 | ||||
| 168 | 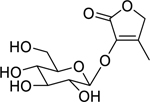 |
3-O-β-D-glucopyranosyloxy-4-methyl-2(5H)-furanone | Enterobacter aerogenes | 1.562 | 137 |
| Pseudomonas aeruginosa | 3.125 | ||||
| Klebsiella pneumoniae | 6.25 | ||||
| Salmonella enterica serotype Typhi | 25 | ||||
| Fatty Acids | |||||
| 169 | dodec-9,11-diynoic acid | Porphyromonas gingivalis | 1.2 | 141 | |
| Fusobacterium nucleatum | 9.6 | ||||
| 170 | (12E)-heptadec-12-en-8,10-diynoic acid | Porphyromonas gingivalis | 1.63 | 141 | |
| Fusobacterium nucleatum | 26 | ||||
| 171 | exocarpic acid | Streptococcus mutans | 13.7 | 141 | |
| Porphyromonas gingivalis | 0.86 | ||||
| Fusobacterium nucleatum | 3.4 | ||||
| 172 | 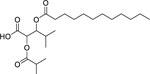 |
3-(dodecanoyloxy)-2-(isobutyryloxy)-4-methylpentanoic acid | Staphylococcus aureus | 3.12 | 142 |
| Bacillus subtilis | 6.25 | ||||
| Streptococcus pyogenes | 6.25 | ||||
| Enterococcus faecalis | 25 | ||||
| Organosulfurs and derivatives | |||||
| 173 | 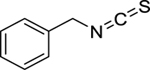 |
benzyl isothiocyanate | Enterococcus faecalis | 40 | 143 |
| Staphylococcus aureus | 40 | ||||
| Escherichia coli | 10 | ||||
| Pseudomonas aeruginosa | 20 | ||||
| Klebsiella pneumoniae | 40 | ||||
| Salmonella pullorum | 40 | ||||
| 174 | allicin | Burkholderia cenocepacia | 0.5 | 144 | |
| Burkholderia cepacia | 0.5 | ||||
| Burkholderia pyrrocinia | 0.5 | ||||
| 175 | 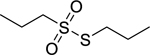 |
propyl-propane- thiosulfonate | Enterococcus faecalis | 4 | 145 |
| Streptococcus agalactiae | 4 | ||||
| Staphylococcus aureus | 8 | ||||
| Escherichia coli | 64 | ||||
| 176 | 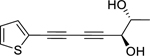 |
10,11-erythro-xanthopappin D | Staphylococcus aureus | 7.25 | 146 |
| Bacillus cereus | 15.5 | ||||
| Escherichia coli | 12.5 | ||||
| 177 |  |
10,11-threo-xanthopappin D | Bacillus subtilis | 7.25 | 146 |
| Bacillus cereus | 62.5 | ||||
| Escherichia coli | 62.5 | ||||
| Peptides | |||||
| 178 | Cys-Ala-Arg-Leu-Asn-Cys-Val-Pro-Lys-Gly-Thr-Ser-Gly-Asn-Thr-Glu-Thr-Cys-Pro-Cys-Tyr-Ala-Ser-Leu-His-Ser-Cys-Arg-Lys-Tyr-Gly | snakin-Z | Bacillus subtilis | 24.2 | 147 |
| Staphylococcus aureus | 28.8 | ||||
| Escherichia coli | 13.6 | ||||
| Klebsiella pneumoniae | 14.1 | ||||
| Steroids | |||||
| 179 | 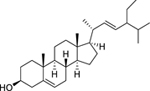 |
β-stigmasterol | Mycobacterium aurum | 15 | 93 |
| Mycobacterium smegmatis | 31 | ||||
| 180 | 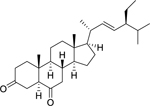 |
stigmast-22-ene-3,6-dione | Staphylococcus aureus | 10 | 148 |
| 181 | 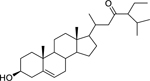 |
stigmast-5-en-3β-ol-23-one | Staphylococcus epidermidis | 9.6 | 135 |
| Bacillus subtilis | 19.2 | ||||
| Staphylococcus aureus | 19.2 | ||||
| Escherichia coli | 19.2 | ||||
| Klebsiella pneumoniae | 38.4 | ||||
| Pseudomonas aeruginosa | 38.4 | ||||
| 182 | 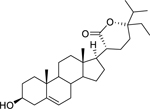 |
stigmast-5-en-3β-ol-21(24)-olide | Bacillus subtilis | 4.8 | 135 |
| Staphylococcus aureus | 4.8 | ||||
| Staphylococcus epidermidis | 9.6 | ||||
| Escherichia coli | 9.6 | ||||
| Klebsiella pneumoniae | 9.6 | ||||
| Pseudomonas aeruginosa | 19.2 | ||||
| 183 | 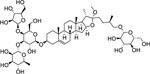 |
polyphyllin G | Bacillus subtilis | 6.2 | 149 |
| Enterococcus faecalis | 52.3 | ||||
| Staphylococcus aureus | 78 | ||||
| Salmonella enteritidis | 13.1 | ||||
| Proteus mirabilis | 26.2 | ||||
| Escherichia coli | 52.3 | ||||
Abbreviations: ESBL-KP: Extended-spectrum β-lactamase Klebsiella pneumoniae, MDR: Multi-drug resistant, MRSA: methicillin-resistant Staphylococcus aureus, MSSA: methicillin-sensitive Staphylococcus aureus, Norfloxacin-R: Norfloxacin-resistant, ORCNS: Oxacillin-resistant coagulase-negative staphylococci, OSCNS: Oxacillin-sensitive coagulase-negative staphylococci, Tetracycline-R: Tetracycline resistant, Vancomycin-R: Vancomycin-resistant, VISA: Vancomycin Intermediate Staphylococcus aureus, VRSA: Vancomycin Resistant Staphylococcus aureus, XDR: Extensively-drug resistant.
2.2. Alkaloids
Alkaloids are one of the largest groups of plant NPs, including more than 20,000 different molecules with a vast diversity of structures and routes to biosynthesis. Alkaloids are low-molecular-weight nitrogen-containing compounds and, due to the presence of a heterocyclic ring containing a nitrogen atom, are typically alkaline.150
Alkaloids are biosynthetically derived from amino acids such as phenylalanine, tyrosine, tryptophan, ornithine, and lysine. Building blocks from the acetate, shikimate, or deoxyxylulose phosphate pathways are also frequently incorporated into alkaloid structures. The biogenesis of alkaloids is used for their classification, as this is directly linked to their molecular skeleton; for example, the largest groups are indole alkaloids and isoquinoline alkaloids. Other relevant groups are tropane alkaloids, steroidal alkaloids, pyridine, and pyrrolizidine alkaloids. The botanical origin of the alkaloids is also used as a classification method, e.g., Papaver (opium) alkaloids, Cinchona alkaloids, Rauvolfia alkaloids, and others.151
Alkaloids are known for their numerous pharmacological effects. They impact different metabolic systems, and their mechanism of action (MOA) may be through enzymatic alterations affecting physiological processes. Such processes include inhibition of DNA synthesis and repair mechanisms by intercalating nucleic acids.152
2.2.1. Overview
Isoquinolines, aporphines, and phenanthrenes represent 46.1% of the alkaloid compounds reported in this review, while quinolines represent 26.9% of alkaloid compounds and indoles represent 11.5% of alkaloid antibacterial compounds. One piperidine compound was noted amongst the antibacterial alkaloids, representing 3.8% of the class (Figure 1). Interesting antibacterial activity (<10 ug/mL)was noted among the alkaloids discussed below.
Figure 1.
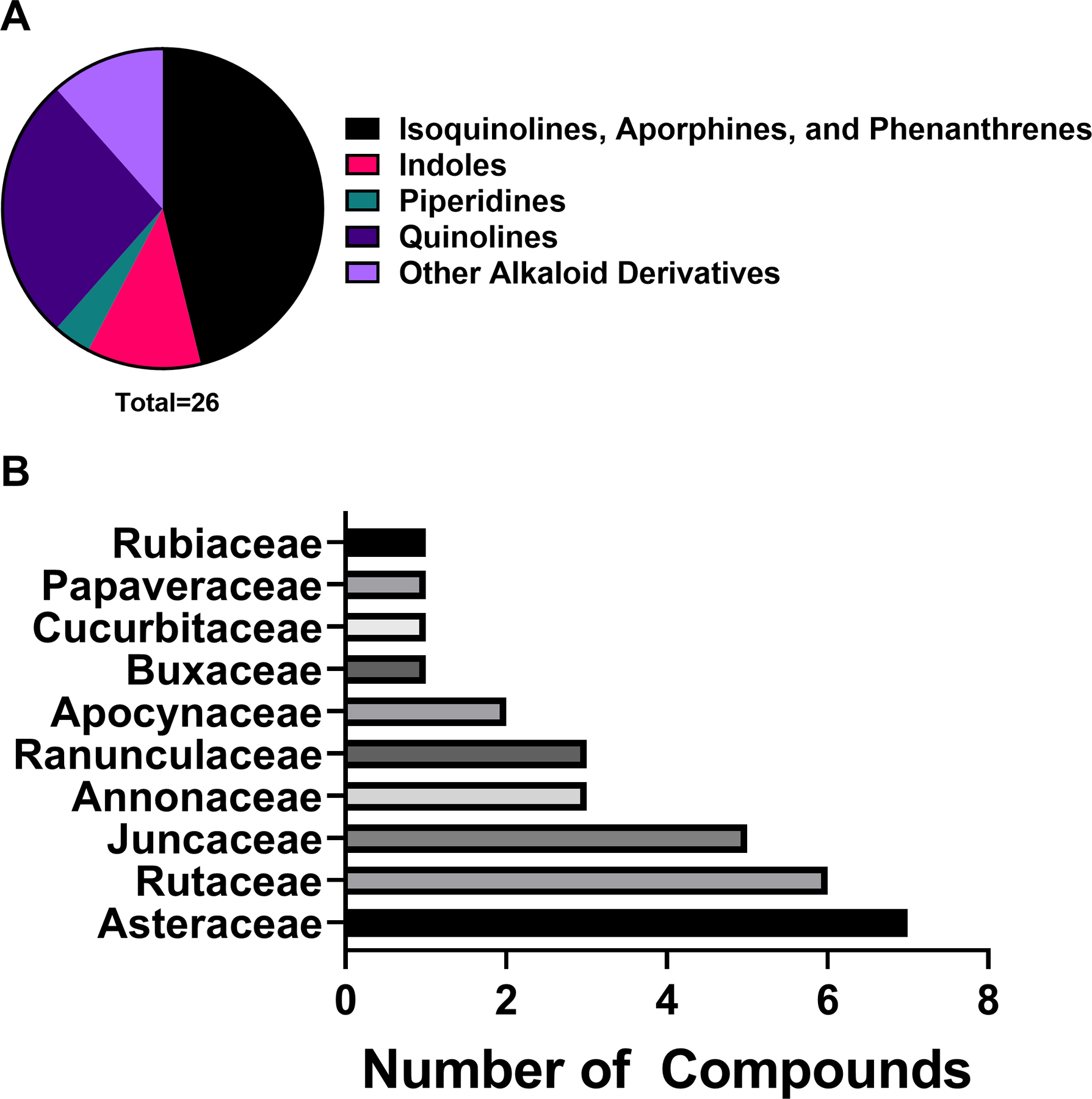
A) Chemical classes of alkaloids investigated for antibacterial activity and B) top ten plant families yielding antibacterial alkaloids under the study parameters.
2.2.2. Quinolines
Quinoline alkaloids are important nitrogen-containing heterocyclic aromatic compounds with a broad range of bioactivities, such as antitumor, antimalarial, antibacterial, antifungal, antiparasitic and insecticidal, antiviral, anti-inflammatory, and antiplatelet activities.153 Quinoline alkaloids occur mainly in the Rutaceae family and are biosynthetically derived from 3-hydroxyanthranilic acid, a metabolite formed from tryptophan through a sequence of enzymatic reactions. Specifically, the condensation reaction of 3-hydroxyanthranilic acid and malonyl-SCoA, followed by a cyclization, yields the quinoline alkaloids.154 Biological activities of hundreds of quinolines have been reported, many of which are promising in terms of their potential as antibacterial agents. Some of them are described below.
8-hydroxyquinoline (2) is a strong metal ion chelator and represents an excellent scaffold with a broad spectrum of pharmacological applications, which include antimicrobial properties. It is one of the oldest antibacterial agents with documented antiseptic uses dating back to 1895,155 and anti-infective uses in humans predating the age of modern antibiotics.156 Houdkova et al.29 investigated the growth inhibitory activity of 8-hydroxyquinoline against bacteria associated with respiratory system infections, with 8-hydroxyquinoline displaying high activity against S. aureus and Haemophilus influenzae and moderate activity against Streptococcus pneumoniae. Furthermore, 8-hydroxyquinoline derivatives have been identified as a major hit cluster against Mycobacterium tuberculosis, with more than 200 active analogues (concentration range from 0.1 to 50 μg/mL).157 With regards to the bacterial growth inhibitory effect, Anjaneyulu et al.158 proposed that the high lipophilicity of 8-hydroxyquinoline allows it to penetrate bacterial cell membranes in order to reach its target site of action. The charged 8-hydroxyquinoline metal complex can bind and block the metal-binding sites on bacterial enzymes, which gives rise to the antimicrobial effect; at the same time, the free ligand, having a strong chelating ability, can bind metallic cofactors of microbial enzymes thereby leading to the inhibition of bacterial enzymatic activity.158,159
Evocarpine (3), a quinolone alkaloid with a 13-carbon alkenyl chain substituent, was isolated from the fruits of Tetradium ruticarpum (A.Juss.) T.G.Hartley (Rutaceae); evocarpine demonstrated high activity against S. aureus and MRSA.30
Of the seven quinolines included in this review, only two (i.e., 8-hydroxyquinoline, evocarpine) showed a strong in vitro antibacterial activity (MIC ≤ 10 µg/mL) as defined by Kuete et al.160 In vitro experiments represent the first step towards the pharmacological validation of the anti-infective properties of compounds of interest. However, in vitro assays are less clinically relevant than in vivo tests, which must be considered as a second step in the validation process to ensure the safe and effective use of plant-derived compounds. Finally, clinical trials are the last step in the process of verifying or refuting the antibacterial activity.161 Here, only the antibacterial activity of 8-hydroxyquinoline and its derivatives have been further pursued. 8-hydroxyquinoline derivatives are perhaps the most promising antibacterial agents from the alkaloid class. Many 8-hydroxyquinoline derivatives have been developed, and some of them are already commercially available against bacterial disorders. For example, nitroxoline is used for the treatment of urinary tract infections in Europe and Asia.162 More recently, a quinoline-based compound (bedaquiline) was approved for the treatment of multi-drug resistant tuberculosis in the U.S.163 Future research should focus on developing safe and effective 8-hydroxyquinoline derivatives targeting multi-drug resistant bacteria, especially those considering as urgent threat by the CDC.1
2.2.3. Isoquinolines, Aporphines, and Phenanthrenes
Isoquinoline alkaloids constitute one of the largest groups of natural substances and are derived from phenylalanine and tyrosine; their skeleton includes an isoquinoline or a tetrahydroisoquinoline ring as a basic structural feature.164 This group of alkaloids is not structurally homogenous. Based on different degrees of oxygenation and intramolecular rearrangements, their distribution, and the presence of additional rings connected to the main system, they may be divided into eight subgroups.165 Isoquinolines are widely distributed among plants coming from the families Papaveraceae, Berberidaceae, Fumariaceae, Menispermaceae, Ranunculaceae, Rutaceae, and Annonaceae (in the dehydro forms). A few plant species which belong to the Magnoliaceae and Convolvulaceae are also rich in these alkaloids.165
Aporphine alkaloids are one subgroup of alkaloids and are characterized by the incorporation of a biphenyl system in their skeleton. They can be di-, tri-, tetra-, penta- and hexa-substituted derivatives where the substituents are hydroxyl, methoxyl, and methylenedioxy groups that can be situated over all four rings. Plants of the families Berberidaceae, Fumariaceae, Magnoliaceae, Papaveraceae, Ranunculaceae, and others are rich in these alkaloids.166 Furthermore, aporphine alkaloids have a close relationship with phenanthrene alkaloids. The phenanthrene alkaloids are derivatives of 1-(2-aminoethyl) phenanthrene, and although they do not contain a nitrogen heterocycle, they are considered alkaloids because they are derived from aporphines (by oxidative degradation), and they occur in the same plant families.167 Compounds belonging to this group showed the potential to be used as effective antibacterial agents and are described below.
Research by Tan et al.31 on a chloroform extract of Artabotrys crassifolius Hook.f. & Thomson (Annonaceae) bark led to the isolation of three aporphine alkaloids: lysicamine (4), artabotrine (5) and liridine (6). Lysicamine exhibited high activity against L. monocytogenes and S. pneumoniae, and S. agalactiae. Artabotrine displayed high activity against a broad array gram-positive bacteria, including B. cereus, L. monocytogenes, Staphylococcus sp., and S. aureus. They also found that liridine displayed high activity against Bacillus subtilis, L. monocytogenes, Staphylococcus sp., and Streptococcus agalactiae. All three compounds (liridine, lysicamine, and artabotrine) were highly active against extended-spectrum beta-lactamase-producing K. pneumoniae (ESBL-KP). Additionally, Proteus vulgaris growth was significantly inhibited by lysicamine and artabotrine.
Hamound et al.32 investigated the benzophenanthridine alkaloid sanguinarine (7), which can be isolated from several members of the Papaveraceae family, including Sanguinaria canadensis L., Macleaya cordata (Willd.) R.Br., and Eschscholzia californica Cham. The authors found that the antibacterial activity of sanguinarine was strongest against gram-positive bacteria, with high activity against Staphylococcus epidermidis and vancomycin-resistant Enterococcus faecalis. Additionally, sanguinarine inhibited the growth of gram-negative bacteria with high activity against Escherichia coli and moderate activity against Acinetobacter baumannii and Klebsiella pneumoniae.32 Furthermore, the authors observed synergistic activity against a panel of clinically relevant gram-positive and gram-negative strains with a drug cocktail consisting of sanguinarine, an antibiotic (streptomycin), and a chelating agent (ethylenediaminetetraacetic acid, EDTA).
Sanguinarine has been tested as an antibacterial in several clinical trials for the treatment of oral diseases such as gingivitis and periodontitis, but a number of studies found sanguinarine to have no significant benefit over the vehicle control.168 An extract of Sanguinaria canadensis, with sanguinarine as the active ingredient, was formulated into Viadent® toothpaste and mouthwash,169,170 which was later found to be associated with leukoplakia and was subsequently removed from Viadent® products.170,171 Due to its quaternary nitrogen, polycyclic and planar structure, sanguinarine can react with nucleophilic and anionic groups of amino acids in several biomolecules, receptors, enzymes and also exhibits strong DNA intercalating activity. Sanguinarine’s interaction with biological molecules and its DNA intercalating ability may be the cause of the negative effects associated with its use and lowers its appeal as a drug candidate.
Four quaternary benzylisoquinoline alkaloids, isolated from Berberis integerrima Bunge (Berberidaceae) roots, including berberine (8), jatrorhizine (9), columbamine (10), and palmatine (12) were investigated by Azimi et al.33 for growth inhibition against Brucella abortus. This study found these compounds to all have high activity against B. abortus, with jatrorhizine being the most effective. Another phytochemical study reported that palmatine, isolated from Tinospora sagittata Gagnep (Menispermaceae), showed a bactericidal effect and high growth inhibitory activity against Helicobacter pylori, both in vitro and in a murine model.36 In addition, Xie et al.34 evaluated the antibacterial efficacy of berberine against selected endodontic pathogens using a multispecies biofilm tooth model. They found berberine to have high activity against Prevotella intermedia and moderate activity against Fusobacterium nucleatum. Additionally, in a randomized controlled clinical trial of patients with diarrhea due to enterotoxigenic E. coli or Vibrio cholerae, berberine sulfate treatment was found to produce a significant reduction in stool volume.172
The antibacterial activity of berberine has been attributed to its ability to act as a hydrophobic cation that increases membrane permeability, and the positive charge on berberine facilitates its accumulation in bacterial cells, which enhances its antimicrobial activity.33,173 Berberine was also found to inhibit the bacterial cell division protein FtsZ, with FtsZ inhibition increasing with the addition of a 9-phenoxyalkyl substituent group at the C9 position of berberine.174 However, one of the major drawbacks of berberine is its ability to be extruded by bacterial efflux pumps (e.g., multidrug resistance pump NorA). Naturally occurring (e.g., 5′-methoxyhydnocarpin) and hybrid synthetic compounds (e.g., Berberine-INF55) have been reported to inhibit efflux pumps and thus improve the antibacterial activity of berberine.175,176 The bioavailability of berberine in vivo was reported to be less than 1%, and many studies have focused on developing derivatives to improve berberine’s bioavailability.177,178 Although berberine seems to be safe at clinical doses, a number of drug interactions have been reported with both antagonistic and synergistic effects.178 Berberine is a well-known drug and has promise as an antibacterial agent, but further work is needed to confirm its efficacy and improve its pharmacokinetic profile.
A SAR study examining quaternary protoberberine alkaloids revealed that growth inhibitory activity was more influenced by the type of the oxygen substituents on rings A, C, and D and especially the position of the oxygen functions on ring D.179 Azimi et al.33 also observed similar results; all of four isolated alkaloids showed potent antibacterial activity against B. abortus, but jatrorhizine and columbamine, with a free hydroxyl group on C-3 or C-2, showed stronger activity than berberine and palmatine, which have no free hydroxyl groups.33
Tankeo et al.35 isolated the benzophenanthridine alkaloid, buesgenine (11); it is one of the main active constituents of the roots of Zanthoxylum gilletii (De Wild.) P.G.Waterman (Rutaceae). The authors found buesgenine to have high activity against a panel of gram-negative bacteria, including multidrug-resistant (MDR) phenotypes. Buesgenine was found to have high activity against E. coli and K. pneumoniae, and moderate activity against Enterobacter aerogenes, P. aeruginosa and Providencia stuartii. Additionally, buesgenine was found to be nontoxic to mouse hepatocytes.180
Twelve isoquinolines, aporphines, and phenanthrenes are included in our review, of which nine (artabotrine, berberine, buesgenine, columbamine, jatrorrhizine, liridine, lysicamine, palmatine, sanguinarine) showed high in vitro antibacterial activity. Although promising as lead compounds, some of them present safety concerns (e.g., palmatine, sanguinarine), have pharmacokinetic issues (e.g., berberine and palmatine have a poor intestinal absorption due their interaction with p-glycoprotein, jatrorrhizine blood distribution is limited due to its binding with human serum albumin) or are poorly studied and need further pharmacological and toxicological investigations (e.g., artabotrine, buesgenine, columbamine, liridine, lysicamine).181–184 Despite its drawbacks, berberine is the most studied compound from this group, and more clinical research should be performed to evaluate its potential in the treatment of multi-drug resistant bacterial infections.
2.2.4. Other Alkaloid Derivatives
A phytochemical investigation by Yu et al.37 on the lateral roots of Aconitum carmichaelii Debeaux (Ranunculaceae) led to the isolation of a vakognavine-type C20-diterpenoid alkaloid, carmichaedine (13). The authors found carmichaedine to have high antibacterial activity against Bacillus subtilis. Of the three other alkaloids derivatives found in our review, carmichaedine is the only one exhibiting a high in vitro antibacterial activity. This compound was discovered in 2017,37 and no studies have yet investigated its efficacy in animal models or humans. Further research is needed to confirm its potential as an antibacterial agent.
2.3. Phenolic Derivatives
Phenolic compounds are a large class of plant secondary metabolites and are found across all plant families.185 These secondary metabolites are formed via the shikimic or phenylpropanoid pathways.186 The simplest phenolic, phenol, is an aromatic ring with a single hydroxyl group. Polyphenols consist of two or more of these phenolic units and show a wide diversity of structures. Phenolic compounds are associated with several plant processes, such as pigmentation, pollinator attraction, herbivory deterrence, and preventing UV tissue damage.187 This large class of compounds has equally diverse bioactivities. To better present this chemical class, it is further subdivided in the discussion below.
2.3.1. Overview
Flavonoids made up 31.6% of phenolic derivative antibacterial compounds reported in this review. The high ranking of “other phenolic compounds”, 28.6% of all phenolic derivatives, is likely due to the large diversity of phenolic acids, simple phenols and other phenolic phytochemicals included in this group. The next most relevant chemical class was the quinones and related compounds, at 15.3%. Anthocyanins were the least represented chemical class among phenolic derivatives, at 0.9% (Figure 2A).
Figure 2.
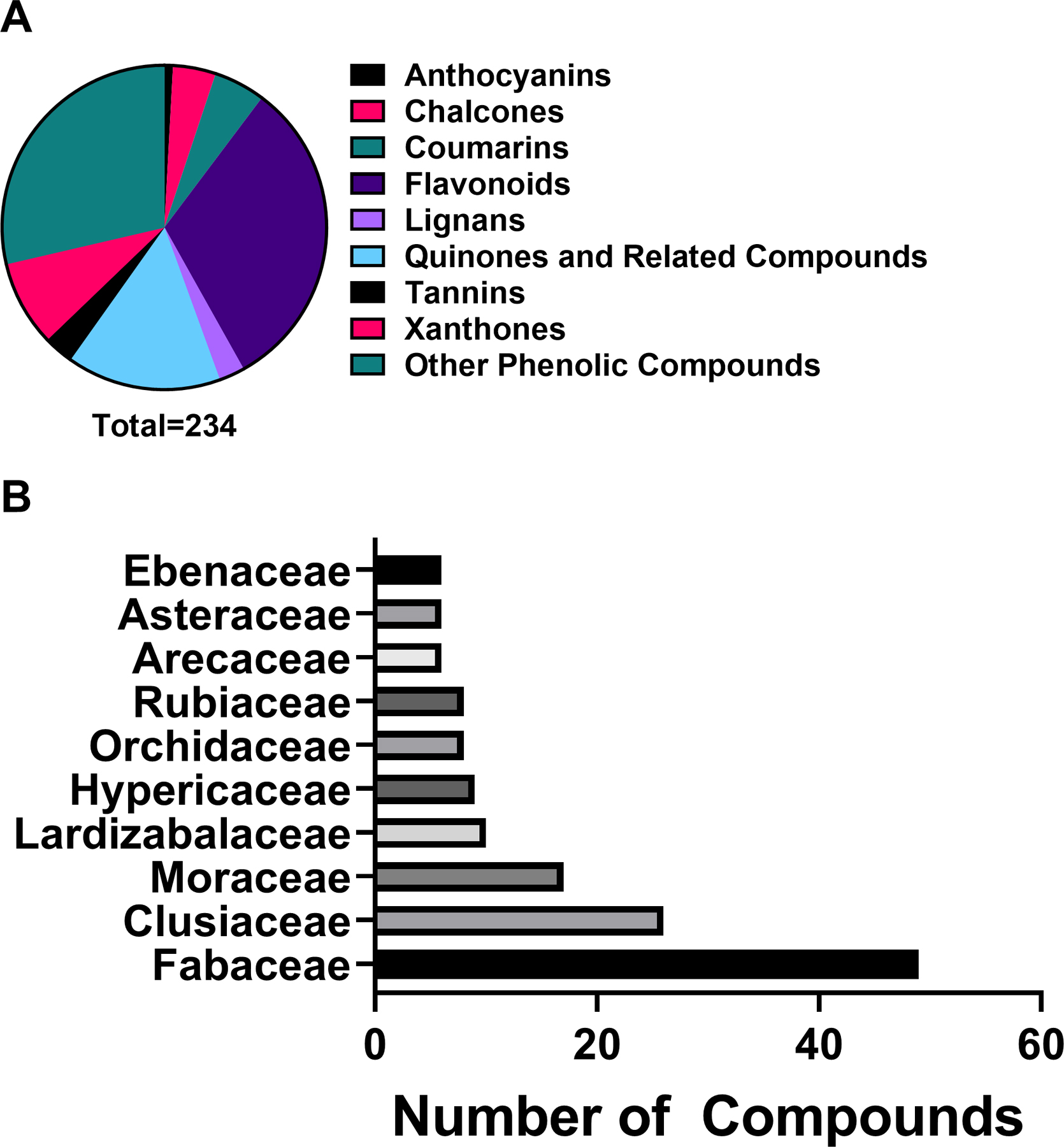
A) Chemical classes of phenolic derivatives investigated for antibacterial activity and B) top ten plant families yielding antibacterial phenolic derivatives.
The Fabaceae family had the most phenolic derivatives investigated in the studies reviewed (Figure 2B). Since Fabaceae is the third-largest botanical family,188 the number of available species for study may contribute to this high ranking. Fabaceae contains legumes, economically important food and cover crops, with an annual production of 77 million tons in 2014.189 However, most of the top 10 species studied in this family are not major food crops, suggesting that the Fabaceae produces an array of interesting phenolics across its genera.
2.3.2. Chalcones
Chalcones are found throughout nature and share a common chemical structure, a chalconoid or 1,3-diaryl-2-propen-1-one.190 Plants synthesize chalcones from a precursor produced by the shikimate pathway, cinnamoyl-coenzyme A (CoA) C6C3. This precursor is elongated by condensation of three acetate groups from malonyl-CoA catalyzed by chalcone synthase.191 There is no general mechanism of action (MOA) for chalcones; rather chalcones have a diverse set of biological targets for their anti-cancer, anti-inflammatory, neuroprotective and anti-microbial activities.192
The activity of 4-hydroxylonchocarpin (14) and isobavachalcone (15), isolated from the twigs of Dorstenia barteri Bureau (Moraceae) and found in the twigs of many other Dorstenia species,193 against MRSA and a susceptible S. aureus was investigated by Dzoyem et al.38 4-hydroxylonchocarpin and isobavachalcone were found to have high activity against S. aureus strains, including MRSA. The growth-inhibiting effects of 4-hydroxylonchocarpin and isobavachalcone are attributed to their ability to depolarize the cellular membrane and cause cell membrane damage. Additionally, toxicity studies using silkworms (Bombyx mori) found 4-hydroxylonchocarpin to be relatively safe with no signs of toxicity after 24 hours.38
Licochalcone A (16), isolated from the roots of Glycyrrhiza inflata Batalin (Fabaceae) and found in Glycyrrhiza glabra L.,194 was found to strongly inhibit the growth of S. aureus.39 Kinoshita et al. found that licochalcone A disrupts the bacterial respiratory chain by inhibiting NADH oxidase and effectively inhibited the growth of gram-positive bacteria.195 Concomitantly, the authors found that while licochalcone A did inhibit NADH oxidase in the outer membranes of gram-negative bacteria, no growth inhibitory effect was noted. This is likely due to the inability of licochalcone A to penetrate gram-negative bacterial cells. Licochalcone A was found to be well tolerated by human liver cells and African green monkey kidney cells, with a LD50 of 36.6 and 26.9 µg/mL, respectively.196 Currently, a clinical trial is studying the effects of licochalcone A in combination with decanediel, L-carnitine and salicylic acid against Acne vulgaris (Table 2).197
Table 2.
List of compounds (from Supporting Information File 1) in clinical trials for infectious disorders.198
| Compound names | Nº clinical trials related to infectious disorders | Indications |
|---|---|---|
| andrographolide | 3 | Acute tonsillitis. Acute bronchitis. Acute exacerbation of chronic bronchitis |
| berberine | 3 | Gastric Ulcer, Helicobacter pylori Infection, Gastritis |
| curcumin | 7 | Acute Pulpitis. Periodontitis. Positivity for Helicobacter pylori infection. HIV infections. Mucositis. |
| ellagic acid | 1 | HPV infection. |
| epigallocatechin gallate | 3 | Epstein-Barr virus reactivation. HIV infection. |
| eucalyptol | 7 | Cough due to infectious origin. Periodontitis. Gingivitis. |
| licochalcone A | 2 | Acne vulgaris. |
| quercetin | 3 | Chronic hepatitis C. Oral mucositis. Cystic fibrosis. |
| silymarin | 9 | Chronic hepatitis C. HIV infection. Tuberculosis |
| thymol | 6 | Periodontitis. Gingivitis. Ear infection. |
Xanthoangelol (17) was found to be most effective against Enterococcus faecium and S. aureus, with high activity against E. faecalis.40 MOA studies found that xanthoangelol treatment causes perforations in the membrane of gram-positive bacteria and a loss of membrane potential leading to cell death.199 This same study found xanthoangelol to be well tolerated with LD50 values of 21.5–58.8 µM against two human cell lines.199 Kuraridin (18) was found to have high activity against S. aureus41 and was well tolerated by human liver cells.200
Of the ten chalcones identified in our review, five (4-hydroxylonchocarpin, kuraridin, isobavachalcone, licochalcone A, xanthoangelol) showed high in vitro antibacterial activity, but none were examined either in vivo or in clinical trials. One of the main drawbacks of chalcones is their poor solubility leading to low oral bioavailability.201 Isobavachalcone has also been involved in drug-drug interactions202 and is subject to export via efflux pump mechanisms.203 However, the toxicity of chalcones is low, which could support their development as potential antibacterial agents.204 One chalcone-based compound, sofalcone, has already been approved in Japan for treating peptic ulcer, and this activity may partially be explained by its antibacterial effects against H. pylori.205 Overall, natural or synthetic chalcones are promising antibacterial lead compounds, and future trends include molecular hybridization, combination approaches, antibacterial mechanisms study, and improvement of physicochemical properties.206
2.3.3. Coumarins
Coumarins are found in over 30 different plant families and 150 plant species,207 and share a common structure of a benzene fused to an α-pyrone ring. They are found in the highest concentrations within fruits and flowers of plants and play a role in defense against herbivory and microbial infections.208 Plant biosynthesis of coumarins begins with the ortho-hydroxylation of cinnamic acid to produce 2-coumaric acid. The side chain undergoes a trans-cis isomerization from trans to the cis form, and enzyme-mediated lactone formation occurs to produce coumarin.191
Zuo et al.42 investigated the activity of coumarins isolated from the roots of Zanthoxylum nitidum (Roxb.) DC. (Rutaceae) against the growth of susceptible and drug-resistant strains of S. aureus. Of the coumarins isolated, artanin (19), phellopterin (20), and 5-geranyloxy-7-methoxy-coumarin (21) were found to have the highest level of activity. 5-geranyloxy-7-methoxy-coumarin and artanin were the most effective, with high activity against both S. aureus and MRSA. Similarly, phellopterin displayed high activity against a susceptible strain of S. aureus, but only moderate activity against MRSA.
Twelve coumarins were reported in our review, among which four exhibited high in vitro antibacterial activity: 4′-senecioiloxyosthol, 5-geranyloxy-7-methoxy-coumarin, artanin, and phellopterin. All of these NPs are poorly studied, and further investigations are needed to ensure their safety and explore their potential for applications in humans. Of note, one coumarin-based compound: novobiocin has been isolated from Streptomyces species, and is used as an antibiotic (Albamycin®) in the U.S.209
2.3.4. Flavonoids
Flavonoids are ubiquitous polyphenolic compounds in plants and are composed of two benzene rings connected by a pyran ring. Flavonoids are versatile plant NPs known to aid in attracting pollinators and fruit dispersers by imparting color and aroma to flowers and fruits. Within plants, flavonoids also have roles as responses to stressors, phytoalexins, antimicrobials, and signaling molecules.210 Flavonoid biosynthesis often begins with a chalcone as the precursor, after which it is enzyme-catalyzed into a variety of flavonoid derivatives.191 The biological activity of individual flavonoids depends on their structure, which can be quite varied. A general MOA for flavonoids revolves around their ability to complex with bacterial cell walls, with highly lipophilic flavonoids also disrupting bacterial membranes.211 Flavonoids have been found to have a wide range of therapeutic uses, such as anti-oxidant, anti-bacterial, antiviral, anti-inflammatory and anti-cancer applications.212
The growth inhibitory activity of two flavones, 3′,4′,7-trihydroxyflavone (23) and 6-prenylpinocembrin (29), isolated from the seeds of Myristica fragrans Houtt. (Myristicaceae), were investigated by Dzotam et al.44 3′,4′,7-trihydroxyflavone displayed high activity against P. stuartii and E. coli, with moderate activity against K. pneumoniae.44 The authors also demonstrated that 6-prenylpinocembrin displayed high activity against E. coli, K. pneumoniae, and S. aureus; with moderate activity against E. faecalis and P. aeruginosa.44 With regards to pharmacokinetics, Riyazuddin et al.213 demonstrated that 3′,4′,7-trihydroxyflavone, from an extract of Senna occidentalis (L.) Link (Fabaceae), was eliminated from rodents after 12 hours.213 A separate toxicity study found 6-prenylpinocembrin to be cytotoxic at 21.87 µg/mL against African green monkey kidney epithelial cells.214 While 6-prenylpinocembrin was highly active against a wider range of bacteria, the mammalian cytotoxicity of the compound lowers its appeal as a drug lead. In addition to being found in the Myristicaceae family, 6-prenylpinocembrin has also been found in members of the Fabaceae family, notably Eriosema robustum Baker.215
Quercetin (25) is a ubiquitous flavonoid found in a variety of fruits and vegetables, and it is commonly studied for its antioxidant and anticancer activity, among other applications.216 There is speculation that quercetin, and other flavonoids, contribute to the longevity and cardiovascular health associated with the Mediterranean diet.217 Biosynthesis of quercetin from glucose is accomplished by way of the shikimic acid pathway.217 As an antibacterial, quercetin has shown high activity in vitro against Streptococcus pyogenes and moderate activity against many bacteria including S. aureus, M. tuberculosis, P. aeruginosa, Aggregatibacter actinomycetemcomitans, and K. pneumoniae.47–49 A SAR study of quercetin and other flavonoids suggested that hydroxylation of the 2’ position of ring B of polyhydroxyflavones is associated with increased antibacterial activity; since quercetin is unsubstituted at this position, analogues of quercetin may be more potent.218 However, quercetin may have a larger role as a synergist with other antibacterials than as a single compound.219 For example, combinations of quercetin and ceftazidime synergistically inhibited growth of S. pyogenes (fractional inhibitory concentration index, FICI = 0.27) and S. aureus (FICI < 0.21), with quercetin acting by inhibiting β-lactamase and increasing the permeability of the cytoplasmic membrane.220,221
In addition to inhibition of bacterial growth, quercetin has been found to inhibit a variety of bacterial virulence factors, including quorum sensing in Chromobacterium violaceum and biofilm formation and exopolysaccharide (EPS) production in K. pneumoniae, P. aeruginosa, and Yersinia enterocolitica; in silico analysis suggests that quercetin may achieve this activity by binding to LasR, a receptor protein associated with bacterial quorum sensing.222 In general, the toxicity of quercetin is not a concern for humans: the average US diet includes approximately 1 g of flavonoids and 25–50 mg of quercetin per day, with high quercetin content in foods such as onions (284–486 mg/kg), kale (110 mg/kg), broccoli (30 mg/kg) and apples (21–72 mg/kg).217 Some studies have found mutagenicity in Salmonella in vitro and rats in vivo, but these results are not generalizable, and the International Agency for Research on Cancer has categorized quercetin as non-carcinogenic.216 To our knowledge, quercetin has not been tested in clinical trials for bacterial diseases, but there have been many trials of quercetin for other conditions; for example, a trial of 500 and 1000 mg/day quercetin supplements for upper respiratory tract infections found no effect overall but noted a reduction in the severity of upper respiratory tract infections for some demographics relative to the placebo treatment.223 Quercetin’s safety profile and its promising synergistic and antibacterial properties support its elevation as an antibacterial compound for further analogue development and lead optimization.
Myricetin (26) was isolated from the leaves of Triclisia gilletii (De Wild.) Staner (Menispermaceae) and investigated by Tiam et al.50 for its bioactivity. Myricetin is found in many plant species in the families Anacardiaceae, Myricaceae, Pinaceae, Polygonaceae, and Primulaceae, particularly in fruits.224 Myricetin was found to have high inhibitory growth activity against Mycobacterium tuberculosis.50 An in vitro study by Jayaraman et al.225 found that myricetin paired with sulfamethoxazole displayed synergistic growth inhibitory activity against three P. aeruginosa strains.
Pseudarflavone A (27), isolated from the whole plant Pseudarthria hookeri Wight & Arn. (Fabaceae). P. hookeri is traditionally used in Africa to treat pneumonia, coughing, and wounds,226 while also being used to treat gastrointestinal disorders, such as diarrhea and stomach pain.227 Dzoyem et al.51 found pseudarflavone A to have high activity against E. coli and S. aureus. This same study also found pseudarflavone A to have moderate activity against P. aeruginosa, K. pneumoniae, and E. faecalis.51 While the bacterial growth inhibitory properties of pseudarflavone A are promising, a cytotoxicity assay in this same study found pseudarflavone A to be cytotoxic against two cancer cell lines at low concentrations (3.59 µg/mL and 7.94 µg/mL).51 The low therapeutic index of pseudarflavone A against these mammalian cell lines warrants further cytotoxicity studies to determine the full safety profile of pseudarflavone A.
Mbaveng et al.52 examined the growth inhibitory activity of compounds from Cameroonian plants: the isoflavone neobavaisoflavone (28) isolated from the bark of Erythrina sigmoidea Hua (Fabaceae)52,53; the flavanone candidone (32) isolated from the rhizomes of Echinops giganteus A. Rich. (Asteraceae); and neocyclomorusin (41) isolated from the roots of Milicia excelsa (Welw.) C.C. Berg (Moraceae). The authors found that all three (28, 32, 41) compounds displayed high activity against E. faecalis, E. coli, and K. pneumoniae.52 Additionally, neobavaisoflavone and neocyclomorusin both displayed high activity against P. stuartii and P. aeruginosa.52 Amongst the three compounds, neocyclomorusin was the only one to be highly effective against E. aerogenes.52 From this same study, a SAR analysis52 found that the antibacterial activity of neobavaisoflavone was dependent on a prenyl group and α-β-unsaturated ketone; loss of either of these two functional groups reduced the antibacterial activity of neobavaisoflavone. Concomitantly, it was found that the presence of an α,β-unsaturated double bond in candidone and a cyclic prenyl moiety in the heterocyclic portion of neocyclomorusin increased their bioactivities. While a mechanism of action is not identified in the literature, the high lipophilicity of these compounds suggests that they are complexing with targets within the bacterial membrane and compromising its integrity.
Zuo et al.63 isolated the pyranoflavonoid cyclocommunol (31) and two prenylflavonoids: morusin (37) and kuwanon E (40), from the root bark of Morus alba L. (Moraceae). In the same study, the investigators also isolated two flavones: multicaulisin (50) and albanin G (52) and the flavanone sanggenon G (51). In Traditional Chinese Medicine, the root bark of M. alba is known as Sang-Bai-Pi and is used for treating shallow cuts, respiratory problems, and pulmonary disease.228 Ferraria et al.229 previously isolated and identified compounds multicaulisin and sanggenon G from M. alba in 2000, and multiple groups have isolated albanin G in the early 1980’s.230 Multicaulisin, albanin G, and sanggenon G were found to be highly active in inhibiting the growth of MRSA, with multicaulisin being the most effective.63 While the MOA for multicaulisin and sanggenon G is currently unknown, albanin G is believed to work against MRSA by increasing bacterial membrane permeability and lowering the proton motor force of ATP synthesis, leading to cell death.231
Cyclocommunol has also been found in other species from the Moraceae family, notably within the genus Artocarpus.232,233 The antibacterial activity of cyclocommunol was found to be high against a suspectable strain of S. aureus and a drug-resistant MRSA strain.55 This same study found cyclocommunol to be cytotoxic against human liver cells at 27.4 µg/mL. Kuwanon E has been found in other Morus species234 and displayed high growth inhibition activity against both methicillin-sensitive S. aureus and MRSA.55 The authors also found that morusin had high activity against MRSA, but only moderate activity against a susceptible S. aureus strain.55 Another study examining the metabolism of morusin, found that morusin is rapidly absorbed in the intestines of rats and is mainly metabolized through glucuronidation.235 It is believed that the phenyl group of kuwanon E and morusin, and the geranyl group of cyclocommunol, facilitates their antimicrobial effect.55 A separate study suggests that the antibacterial action of morusin is via attachment to the bacterial cell membrane, compromising its structural integrity leading to increased permeability and ultimately cell lysis.236
Pereira et al.56 isolated two bioactive flavanones, chamanetin (35) and dichamanetin (46) from the root and bark of Cleistochlamys kirkii (Benth.) Oliv. (Annonaceae), a plant traditionally used in Mozambique, Africa to treat wound infections and tuberculosis.56 Dichamanetin was found to effectively inhibit the growth of three drug-resistant bacterial strains: with high activity against MRSA, vancomycin-resistant S. aureus (VRSA) and vancomycin-resistant E. faecalis.56 According to Urgaonkar et al., dichamanetin inhibits the GTPase activity of FtsZ, a bacterial homologue for tubulin, leading to disruption of bacterial cell division.237 Dichamanetin has also been isolated from Piper sarmentosum Roxb. (Piperaceae)238.
Lupinifolin (36) is a flavanone isolated from the bark and wood of Albizia myriophylla Benth. (Fabaceae)57,59,239 and from the stems of Derris reticulata Craib (Fabaceae).58 Thai traditional remedies use A. myriophylla to treat dental carries.57,59,240 Lupinifolin was found to effectively inhibit the growth of various gram-positive bacteria; with high activity against Streptococcus mutans57 and Staphylococcus aureus58, and moderate activity against B. cereus.59 It was hypothesized that lupinifolin inhibits bacterial cell growth by permeating the bacterial cell wall and plasma membrane, which then causes leakage of the cytoplasmic membrane.239
Dzoyem et al.38 isolated the flavanone 6–8-diprenyleriodictyol (38) from the aerial parts of Dorstenia mannii Hook.f. (Moraceae). The authors found that 6–8-diprenyleriodictyol was highly active against S. aureus, displaying one of the lowest MICs of all the flavonoid compounds listed in this review. Plants from the genus Dorstenia have uses in African and South American traditional medicine for the treatment of snake bites and infectious diseases. Harborne et al.241 previously isolated 38 from the leaves of Vellozia coronata L.B.Sm. and Vellozia nanuzae L.B.Sm. & Ayensu (Velloziaceae).
Sophoraflavanone G (39) is a flavanone isolated from the roots of Sophora flavescens Aiton (Fabaceae) and other Sophora species.242,243 Biosynthesis of sophoraflavanone G is thought to occur in plastids and begin in the pathway for isopentenyl diphosphate (IPP) and 1-deoxy-D-xylulose-5-phosphate (DOXP),244 with the final 2’-hydroxlyation being catalyzed by cytochrome P450 monooxygenase at the endoplasmic reticulum.245 Sophoraflavanone G was reported by Chan et al.41 to have high activity against S. aureus. It was proposed that sophoraflavanone G binds directly to peptidoglycan in the cell wall of S. aureus, damaging and killing the bacterium.246 Additionally, prompted by cases of hepatoxicity from the ingestion of capsules containing Sophora flavescens extracts, a study by Yu and Cheng247 identified sophoraflavanone G as a hepatoxic compound. The hepatoxicity of sophoraflavanone G reduces its appeal as a lead antimicrobial compound.
A phytochemical study of Ludwigia leptocarpa (Nutt.) H. Hara (Onagraceae) was conducted by Mabou et al.60 and led to the isolation of two flavonoids, luteolin-8-C-glucoside (44) and (2R,3S,2’’S) 3’’’,4’,4’’’,5,5’’,7,7’’-heptahydroxy-3,8”-biflavanone (49). These flavonoids have been commonly found in edible plants and have been identified in at least twenty plant families worldwide.248 In regards to their antibacterial activity, (2R,3S,2’’S) 3’’’,4’,4’’’,5,5’’,7,7’’-heptahydroxy-3,8”-biflavanone and luteolin-8-C-glucoside were found to have high activity against S. aureus.60 This same study found that both of these flavonoids were highly active against Shigella flexneri and moderately active against V. cholerae. It is hypothesized that these compounds produce their antibacterial effect by forming complexes with extracellular and soluble bacterial proteins and also with components of the bacterial cell wall.211
Two studies noted the antibacterial effects of the flavonoid isoquercetin (45) isolated from the flowers of Trollius chinensis Bunge (Ranunculaceae)47 and the aerial parts of Aster yomena Makino (Asteraceae).249 In Korean traditional medicine, A. yomena is used to treat asthma and colds, and in traditional Chinese and Mongolian medicine, T. chinensis is used to treat viral and bacterial infections.249 Yun et al.249 found isoquercetin to have high activity against E. coli. A separate study found isoquercetin to have high activity against P. aeruginosa and moderate activity against S. aureus.47 Currently, there is one clinical trial underway for isoquercetin to investigate its ability to prevent blood clots in patients with small-cell lung cancer or colorectal cancer, but no trials are testing its antimicrobial properties.198 One possible area of future research is to investigate the antiviral properties of isoquercetin, as the plants containing it are used in traditional medicine to treat the symptoms of viral infections.
Amentoflavone (47), an ingredient commonly used in traditional Chinese medicine,250 was isolated from the whole plant of Selaginella tamariscina (P.Beauv.) Spring (Selaginellaceae) and investigated for its bioactivity by Hwang et al.61 The authors found amentoflavone to have a high level of growth inhibitory activity against S. aureus, E. faecium, E. coli, and P. aeruginosa. A cytotoxicity study by Srividhya et al.251 found amentoflavone to be toxic to human liver cells with an LD50 of 25.3 µg/mL. While amentoflavone is widely distributed in many plant families and over 120 different plant species,250 the in vitro hepatoxicity of amentoflavone warrants further studies into the drugs pharmacokinetic properties.
Chukwujekwu et al.62 isolated a neoflavonoid 3″-epidiphysin (48) from the aerial parts of Ormocarpum trichocarpum (Taub.) Engl. (Fabaceae), a plant traditionally used in parts of Africa as an emetic and in the treatment of tuberculosis, gastrointestinal disorders and strokes.62 The authors found that 3″-epidiphysin displayed high activity against S. aureus and moderate activity against B. subtilis.62
In our review, 74 flavonoids were reported, and 25 had high in vitro antibacterial activity. Of these, only three compounds (amentoflavone, myricetin, and quercetin) were evaluated with in vivo models of bacterial infections,252–255 and only two compounds (quercetin and isoquercetin) were evaluated in clinical trials, albeit not for their antibacterial activity. This indicates the lack of thorough investigation of flavonoid compounds for antibacterial activity. Pharmacokinetic and pharmacodynamic issues have also been noted for some highly active compounds such as a low bioavailability after oral administration (e.g., amentoflavone, quercetin, lupinifolin, morusin), a susceptibility to high temperature and certain conditions of pH (e.g., myricetin), and an induction effect on CYP3A4, potentially responsible for drug-drug interactions (e.g., neobavaisoflavone).202,256,257 Due to their high diversity, their presumable low toxicity, and a large number of antibacterial mechanisms of action, flavonoids can be considered as promising compounds, but further studies are needed to assess their antibacterial potential in animal models and humans.
2.3.5. Lignans
Plant biosynthesis of lignans begins via the shikimic pathway from cinnamic acid. Alcohol derivatives of cinnamic acid, such as coniferyl alcohol and p-coumaryl alcohol, dimerize to form lignans.191 Further modifications of these dimers produce a wide range of structurally different lignan types.
Three antimicrobial lignans were isolated from leaves of Larrea tridentata (Sesse & Moc. ex DC.) Coville (Zygophyllaceae): 3′-demethoxy-6-O-demethylisoguaiacin (53), 4-epi-larreatricin (54) and dihydroguaiaretic acid (55) by Favela-Hernandez et al.64 Mexican traditional medicine has used L. tridentata in remedies to treat urinary tract infections.258 The compound 3′-demethoxy-6-O-demethylisoguaiacin was found to have moderate activity against a drug resistant strain of M. tuberculosis and MRSA, while also having moderate activity against the susceptible strains of E. coli and E. faecalis.64 The authors also found 4-epi-larreatricin to have moderate activity against a MDR strain of M. tuberculosis and susceptible strains of M. tuberculosis and E. faecalis.64 Dihydroguaiaretic acid had moderate activity against a drug-resistant and susceptible strain of M. tuberculosis and MRSA.64 The MOA for 3′-demethoxy-6-O-demethylisoguaiacin against MRSA is thought to be through downregulation of a gene associated with a lipoprotein for releasing ATP-binding proteins, three genes associated with ABC transporters and another gene associated with a subfamily of transport proteins.259 Downregulation of these genes is believed to reduce MRSA’s ability to effectively remove 3′-demethoxy-6-O-demethylisoguaiacin from within the cell. Against M. tuberculosis, dihydroguaiaretic acid is thought to act upon an enzyme in the geraniol degradation pathway, the alpha subunit of coenzyme A transferase (CoAt-Mt). Inhibition of this enzyme leads to intracellular accumulation of 1- and 2-methylnaphthalene.260 These two intermediate products have previously been shown to be toxic to cyanobacteria261 and their accumulation may lead to toxicity in M. tuberculosis. The ability of 3′-demethoxy-6-O-demethylisoguaiacin to interfere with bacterial ABC transporters raises the appeal of this compound to be further optimized for potential use as an adjunct synergistic treatment with conventional antibiotics.
Six lignans were found in our literature search, but none of them showed high antibacterial activity (MIC ≤ 10 µg/mL). Overall, lignans are poorly studied for their antibacterial activity, and more effort should be dedicated to assessing their potential role as future antibacterial agents.
2.3.6. Quinones and Related Compounds
Quinones are aromatic rings with two ketone substitutions, and these metabolites are potentially derivable by oxidation of suitable phenolic compounds like catechols (1,2-dihydroxybenzenes), giving rise to ortho-quinones and quinols (1,4-dihydroxybenzenes) and yielding para-quinones. Consequently, quinones can be formed from phenolic systems generated by either the acetate or shikimate pathways.262
Quinones are characteristically highly reactive and known for providing a source of stable free radicals and forming irreversible complexes with nucleophilic amino acids, often leading to a loss of function of vital proteins in the microbial organism. Probable targets in the microbial cell could be surface-exposed adhesins, cell-wall peptides, and membrane-bound enzymes. Differences in cell wall structures between gram-positive bacteria and gram-negative bacteria can play an important role in the antibacterial activity and explain why some quinones can destroy the cell wall and cell membrane of bacteria. Quinones may also render substrates unavailable for the proliferation of the microorganism.211 We have selected quinones with potent antibacterial effects, described below.
Thymoquinone (56), a benzoquinone, is a biologically active compound from Nigella sativa L. (Ranunculaceae) seeds.263 The presence of this compound has also been confirmed within several genera of Lamiaceae, such as Monarda, and Cupressaceae, such as Juniperus.264 Thymoquinone exists in tautomeric forms, including the enol form, the keto form and mixtures, and it exhibits antibacterial activity, particularly against gram-positive cocci.265 The antibacterial activity of thymoquinone was reported to be high against MRSA65 and H. influenzae,29 with moderate activity against S. pneumoniae.29 Additionally, thymoquinone possesses selective antibacterial and resistance-modifying activity against oral bacteria. The oral strains of S. aureus, S. mutans, and S. salivarius were highly to moderately sensitive to thymoquinone.266 It also demonstrated synergistic effects in combination with antibacterial agents such as tetracycline and benzalkonium chloride.266 In vivo studies demonstrated that acute bacterial prostatitis induced by E. coli and P. aeruginosa could regress following administration of thymoquinone, improving the general histologic structure and downgraded the degree of inflammation in prostatic tissue.29,267
Uc-Cachon et al.268 obtained four naphthoquinones (57, 66, 67, 68) from a non-polar extract of Diospyros anisandra S.F. Blake (Ebanaceae). They found that plumbagin (57) and its dimers 3,3’-biplumbagin (66) and maritinone (67) had high antibacterial activity against sensitive and drug-resistant M. tuberculosis strains. Studies have suggested that naphthoquinones can interact with enzymes in the mycobacterial electron transport chain due to their structural similarity to the natural redox cycler in M. tuberculosis.269,270 Another proposed mechanism for this class of compounds is the inhibition of DNA gyrase in M. tuberculosis.271,272 It was also noted that 3,3’-biplumbagin and maritinone were not cytotoxic to mammalian cells and emerged as candidates for further development as novel anti-tuberculosis drugs for their potential against drug-resistant strains.66 A separate study by Omosa et al.67 reported on the antibacterial potential of plumbagin against a panel of sensitive and MDR gram-negative and gram-positive bacteria. Plumbagin displayed high activity against various E. coli, K. pneumoniae, and MRSA strains; moderate activity was observed for one MRSA strain.67 In addition, Cesari et al.68 and Nair et al.69 described the high and moderate antibacterial activity of plumbagin against S. pneumoniae and S. aureus.
The anthraquinones emodin (63) and aloe-emodin (64) are mainly reported in three plant families: Fabaceae (Cassia spp.), Polygonaceae (Rheum, Rumex, and Polygonum spp.), and Rhamnaceae (Rhamnus and Ventilago spp.). However, a comprehensive literature survey revealed that emodin had been identified in at least 17 plant families worldwide.273–275 Emodin is reported to have high activity against the growth of MRSA and moderate activity against E. coli and K. pneumoniae.67,72 Furthermore, the effects of emodin on virulence factors of S. mutans have been investigated, revealing that emodin significantly attenuated the growth, acid production, and insoluble glucan synthesis of S. mutans in vitro and suppressed the development of dental caries of rats in vivo.276 The MOA of bioactive anthraquinonoids derived from plants is still unknown but may be related to the destruction of the integrity of the bacterial cell wall and cell membrane, leading to loss of intracellular components.211
Antibacterial-guided fractionation of Senna alata (L.) Roxb. (Fabaceae) led to the isolation of aloe-emodin, which exhibited high activity against S. aureus and also moderately inhibited the growth of MDR S. flexneri and V. cholerae.73 Another study demonstrated that aloe-emodin exhibited no bactericidal activity against S. aureus but affected S. aureus biofilm formation in a dose-dependent manner.277 Wang et al.278 reported that aloe-emodin inhibited the arylamine N-acetyltransferase enzyme and growth of H. pylori cultures in a dose-dependent manner.
Yoshikawa et al.71 isolated the phenanthrendione ephemeranthoquinone B (61) from the roots of the orchid hybrid Cymbidium Great Flower Marie Laurencin (Orchidaceae). Ephemeranthoquinone B has been found in other members of the Orchidaceae family, such as the orchid hybrid Odontioda Marie Noel ‘Velan’ and Cymbidium finlaysonianum Lindl.279,280 Ephemeranthoquinone B had high activity against B. subtilis.71,279,280 A cytotoxicity study found ephemeranthoquinone B to be cytotoxic against human leukemia cells (HL-60);71 the cytotoxicity of ephemeranthoquinone B may hinder its development into an antibacterial drug.
Two C-glycosylated anthrones characterized as aloin A/B (69) and aloin-6’-O-acetate A/B (70) were isolated from leaves of Aloe trigonantha L.C. Leach (Asphodelaceae), an endemic Ethiopian plant. Aloin A/B and aloin-6’-O-acetate A/B possess broad antibacterial activities against several gram-negative and gram-positive bacteria. In particular, aloin A/B showed high activity against V. cholerae,75 E. coli, Salmonella enterica serotype Typhi, and Shigella dysenteriae.76 Additionally, aloin-6’-O-acetate A/B demonstrated high antibacterial activity against B. subtilis, S. enterica ser. Typhi and V. cholerae.75
A total of 36 quinones and related compounds were reported, and 17 exhibited high in vitro antibacterial activity. Of the latter, only three compounds (i.e., aloe-emodin, emodin, and thymoquinone) have been studied with in vivo models of bacterial infections,276,281–283 and two (emodin, thymoquinone) have been evaluated in clinical trials for other conditions than bacterial infections. While emodin has some toxicological issues (mainly nephrotoxicity and hepatotoxicity) that limits its therapeutic use,273 thymoquinone presents a good safety profile284 and could be a potential antibacterial lead. Further studies are needed to improve its bioavailability and to assess its antibacterial MOA with in vivo models.
2.3.7. Xanthones
Xanthones, represented as the C6-C1-C6 system, are secondary metabolites restricted in occurrence to only a few families of higher plants and some fungi and lichens. The majority of xanthones have been found in four families of higher plants, the Clusiaceae, Gentianaceae, Moraceae, and Polygalaceae.285 Xanthones can be classified based on their oxygenation, prenylation, and glucosylation patterns.286
Xanthones are biosynthesized from a mixed shikimate-acetate pathway. The main steps in their biosynthesis involve the condensation of shikimate and acetate moieties, which constitute a benzophenone intermediate followed by a regioselective, oxidative mediated intramolecular coupling to form the xanthone ring.287 Naturally occurring xanthones have emerged as an important class of organic compounds due to their remarkable pharmacological and biological activities. Xanthones with promising antibacterial activity are described below.
One of the more promising xanthones, α-mangostin (74), was isolated from Garcinia x mangostana L. (Clusiaceae).288 α-mangostin exhibited high activity against S. aureus and oxacillin-resistant Staphylococcus saprophyticus.79 It also exhibited moderate activity against Leptospira interrogans serovar Javanica and Leptospira interrogans serovar Saigon.80 The bactericidal activity of α-mangostin has been linked to the disruption of cytoplasmic membrane integrity leading to membrane breakdown and leakage of intracellular components. Isoprenyl groups from α-mangostin are directly involved in this activity as they trigger penetration into the hydrophobic region of the bacterial membrane.289 In addition to membrane perturbation, another study demonstrated α-mangostin downregulated genes involved in the fatty acid biosynthetic pathway, cell division, DNA replication, homologous recombination, mismatch repair, resistance development, biofilm, oxidative stress, and cellular stress responses in S. epidermidis.290 It was also hypothesized that α-mangostin could inhibit β-lactamase activity.79 Glycosides of α-mangostin were synthesized, and their antibacterial activity against gram-positive bacteria was largely improved by glycosylation at the C-3 position.291 A separate study found that the presence of hydroxyl groups at C-3 and C-6 positions are essential for the antibacterial activity against MRSA and vancomycin-resistant E. faecalis.292 Additionally, an ethanol extract of Garcinia x mangostana was found to promote wound healing and a reduction of MRSA colonies in vivo. This same study found α-mangostin to be ineffective in vivo. It was then hypothesized that other compounds such as β and γ-mangostin could act in synergy with α-mangostin to produce the antibacterial and wound-healing effect observed for the plant extract.293 Further in vivo studies should be performed to confirm the role of α-mangostin as an antibacterial agent.
Phytochemical investigation of Garcinia cowa Roxb. Ex Choisy (Clusiaceae) stem bark led to the isolation of a variety of xanthones, norcowanin (75), cowanin (79), cowanol (80), cowagarcinone E (81) and a rearranged triprenylxanthone, garciniacowone (78); these were evaluated for their antibacterial activity against gram-negative and gram-positive bacteria strains. Cowanol, garciniacowone, cowanin, and cowagarcinone E each displayed high activity against MRSA.81
Of the 20 xanthones found in our review, ten have high in vitro antibacterial activity. Of the latter, only one, α-mangostin, has been studied in an animal model of bacterial infections.293 While α-mangostin seems to have a good safety profile,294 its poor oral bioavailability295 and the lack of clear in vivo antibacterial efficacy necessitates a more thorough investigation of this compound.
2.3.8. Other Phenolic Compounds
Phenolic derivatives represent one of the most abundant groups of organic compounds in the plant kingdom with enormous structural variability. The diversity of structures is related to a variety of properties associated with specific roles in plants, hence their specific distribution. The site(s) and number of hydroxyl groups on the phenol group are thought to be related to their relative toxicity to microorganisms, with evidence that increased hydroxylation results in increased toxicity.296 In addition, some authors have found that more highly oxidized phenols are more inhibitory.297 The mechanisms thought to be responsible for phenolic toxicity to microorganisms include enzyme inhibition by the oxidized compounds, possibly through reaction with sulfhydryl groups or through more nonspecific interactions with the proteins.298 In addition to the groups described above, several other phenolic compounds are highlighted in this review.
Two phenols, pyrocatechol (82) and pyrogallol (84) a structural constituent of many polyphenols were screened against periodontopathic bacteria,48 and pyrogallol showed high antibacterial activity against A. actinomycetemcomitans and Streptococcus mitis. Similar results were obtained for pyrocatechol, which possessed high activity against A. actinomycetemcomitans.299 This study also reported the structure-function relationship against A. actinomycetemcomitans, the most susceptible of the tested strains, finding that increased hydroxylation of the benzene ring in phenolic acids and benzene alcohols is associated with an increase in antibacterial activity. Polyphenols containing a pyrogallol moiety in their chemical structure were more potent inhibitors of the growth of A. actinomycetemcomitans than compounds containing 1,2 dihydroxyphenyl (pyrocatechol) groups.299
Due to the localized hydroxyl groups, pyrogallol is capable of rapidly and robustly forming noncovalent interactions such as hydrogen bonds and hydrophobic interactions. Thus, a fundamental property of pyrogallol-containing molecules is strong affinity for a variety of proteins, peptides, DNA/RNA, and polysaccharides.300 Another feature is the oxidation of two hydroxyl groups adjacent to the reactive “quinone” form under physiological and weak basic conditions, leading to antioxidant effects. The quinone further reacts with amine and thiol groups, enabling the covalent modification of biomolecules via Michael addition or Schiff base formation. Additionally, two adjacent hydroxyl groups contribute to coordination bonds with transition metal ions, such as iron, copper, and others.301 Recent investigations have documented that the pyrogallol moiety exhibits a broad spectrum of pharmacological and health-promoting effects in various animal disease models. Baruah et al.302 reported that pyrogallol elicits a prooxidant action (e.g., generation of hydrogen peroxide, H2O2) that induces protein Hsp70 production to generate protective immunity.302
An investigation by Zeng et al.303 examined the main phenylethanol component present in the fruit and leaf of the olive (Olea europaea L., Oleaceae) and isolated hydroxytyrosol (85). Hydroxytyrosol has also been found in the stems of another Oleaceae species, Sargentodoxa cuneata (Oliv.) Rehd. et Wils.84 Hydroxytyrosol is found free, in acetate form, or as a part of more complex compounds such as oleacein, oleuropein, and verbascoside.304 Hydroxytyrosol showed high antibacterial activity against S. aureus.84
3,4-dihydroxyphenylacetic acid (86), also known as DOPAC or homoprotocatechuic acid, is a phenolic acid present in Thymus vulgaris L. (Lamiaceae) and Eucalyptus globulus Labill. (Myrtaceae).305,306 3,4-dihydroxyphenylacetic acid was found to have high activity inhibiting the growth of A. actinomycetemcomitans.48 Interestingly, 3,4-dihydroxyphenylacetic acid is largely studied as a metabolite of dopamine and a useful marker compound in mammalian physiology307; for example, rats experiencing a conditioned stress response were found to have increased levels of 3,4-dihydroxyphenylacetic acid in their brains.308 As such, 3,4-dihydroxyphenylacetic acid has been analyzed in several clinical trials as an outcome or marker, but not as an intervention.
Methyl gallate (87) is a phenolic compound found in a variety of plant species such as Sedum aizoon L. (Crassulaceae) and Terminalia chebula Retz. (Combretaceae).86,309 It is an ester of gallic acid and is biosynthesized from dehydroshikimate.310 Methyl gallate has high activity against the growth of S. aureus and moderate activity against V. cholerae and E. coli.86,309 In a mouse model of cholera, oral administration of non-toxic doses (23.80 to 95.23 mg/kg) of methyl gallate significantly decreased inflammation, V. cholerae colonization and fluid accumulation in the intestines.86 It has been proposed that the antibacterial activity of methyl gallate against V. cholerae is by its action against the bacterial membrane, reducing cytoplasmic pH, increasing membrane polarization, and decreasing ATP production.311 A SAR study of alkyl esters of gallic acid against Ralstonia solanacearum, a plant-pathogenic bacterium, found that methyl gallate has better antibacterial activity than gallic acid and that activity decreases as the length of the alkyl chain increases; however, it should be noted that different trends have been observed in other bacteria.312
4,5-(methylene-dioxy)-o-coumaroylputrescine (88) and 4,5-(methylene-dioxy)-o-coumaroyl-4’-N-methylputrescine (89) are phenylpropanoids isolated from the stems and bark of Drypetes staudtii (Pax) Hutch (Putranjivaceae), a plant traditionally used in Nigeria for wound treatment.87 A study by Grace et al.87 found 88 and 89 to have high activity against S. aureus and S. agalactiae and moderate activity against E. coli and P. aeruginosa.
A study by Zeng et al.84 reported on the phenylethanoid, 2-(3,4-dihydroxyphenyl) ethyl-O-β-D-glucopyranoside (91), that was isolated from the stems of Sargentodoxa cuneate (Oliv.) Rehder & E.H.Wilson (Lardizabalaceae). Compound 91 displayed high growth inhibition against S. aureus and significant inhibition of cell viability in two target cell lines, resulting in an antiproliferative rate of about 85% at the concentration of 64 μg/mL.84
Phloroglucinols (92, 93, 95, 96) were isolated from Hypericum species (Hypericaceae) and tested for inhibition of bacterial growth against a panel of clinically relevant pathogens.88 The results show that compounds 92, 93, 95, and 96 demonstrated high antibacterial activity against gram-positive bacteria. A benzoyl derivative, 2-geranyloxy-4,6-dihydroxybenzophenone (101), isolated from Hypericum densiflorum Pursh exhibited the most potent growth inhibition activity, in this series, with high activity against MRSA and S. epidermidis. They also found that compounds 92, 93, 95, 96, and 101 were inactive against gram-negative bacteria (E. coli, P. aeruginosa, A. baumannii) at the highest test concentration (125 μg/mL). Additionally, compounds 92, 93, 95, 96, and 101 displayed indistinguishable growth inhibitory effects against biofilm-producing and non-biofilm-producing S. epidermidis strains.88 Previous SAR studies with similar acylphloroglucinol compounds from Hypericum beanii N.Robson reported that compounds lacking the geranyl chain were two to fourfold less active against S. aureus, highlighting the importance of the geranyl structural feature.313
Detailed investigation of the secondary metabolites contained in a bioactive extract of dry fruits of Amorpha fruticosa (Fabaceae) resulted in the isolation of bibenzyl compounds amorfrutin A (94), amorphastilbol (98), 2-geranyl-5-(2-phenylethyl) resorcin (99), 2-[(E)-styryl]-5-geranylresorcin-1-carboxylic acid (104) and amorfrutin B (106). These were evaluated by Muharini et al.40 for their antibacterial activity against a panel of human pathogenic gram-positive bacteria. The geranylated bibenzyl derivatives 98, 99, and 106 showed high antibacterial activity against vancomycin-resistant E. faecalis and E. faecium. Likewise, the five bibenzyl metabolites (94, 98, 99, 104, and 106) exhibited high activity against S. aureus, with amorphastilbol displaying the most effectiveness. The authors also used SAR assessments of these geranylated bibenzyl derivatives and found that isoprenoid derived side chain length seems to be important for the antibacterial activity against E. faecalis and E. faecium strains. This was exemplified by the relatively weak activity of amorfrutin A, which only differs from amorfrutin B by an isoprene versus a geranyl side chain.40
The acylphloroglucinol olympicin A (97) was isolated from the aerial parts of the plant Hypericum olympicum L. cf. uniflorum (Hypericaceae) by Shiu et al.89 and investigated for its antibacterial activity. Olympicin A exhibited high antibacterial activity against MSSA and MRSA strains and was also highly active against a variety of Mycobacterium strains. The activity of olympicin A was higher than the control antibiotics against MDR strains. They also show that olympicin A was inactive against the gram-negative species that were included in this study. The authors suggest that this may be due to the impermeability of the compound to the outer membrane of the gram-negative bacteria or the different efflux pumps gram-negative bacteria express.89
Chemical investigation of Bletilla striata (Thunb.) Rchb.f. (Orchidaceae) rhizomes by Jiang et al.90 led to isolation of bibenzyl derivatives bulbocol (100) and shancigusin B (107). These compounds were evaluated for their antibacterial activities against gram-positive and gram-negative bacteria. Both shancigusin B and bulbocol exhibited high inhibitory activity against S. aureus. In addition, shancigusin B moderately inhibited the growth of MRSA and B. subtilis, while bulbocol had moderate activity against MRSA. Both compounds were inactive against gram-negative strains.90
Curcumin (102), a bis-α,β-unsaturated β-diketone was first isolated in 1815. It is one of the curcuminoids from Curcuma species, especially C. longa L. (Zingiberaceae), for which it represents 2 to 8% of the total content.314 Curcumin was effective against periodontal pathogens and demonstrated high growth inhibitory activity against Porphyromonas gingivalis and moderate activity against F. nucleatum and S. mitis.315 The antibacterial effect of curcumin could be due to its ability to inhibit bacterial cytokinesis by targeting the assembly dynamics of FtsZ in the Z-ring.316 Many in vivo studies demonstrated therapeutic effect of curcumin for bacterial infections, especially against H. pylori-induced gastric damage,317 and K. pneumoniae-induced lung infection,318 and on virulence factors produced by P. aeruginosa,319 on S. mutans adherence to tooth surfaces,320 and on a bacterial septicemia model with Vibrio vulnificus infection.321 Besides animal model studies, a total of 218 clinical trials mentioned the use of curcumin for a wide range of conditions.198 Among the completed clinical trials, two focused on the effect of curcumin on patients with H. pylori infection. These studies showed an improvement of dyspeptic symptoms and reduction of gastric inflammation serologic signs, but fail to demonstrate an effect on eradication of H. pylori.322,323 Another study demonstrated the effect of curcumin associated with lactoferrin on the reduction of respiratory tract infections in children, finding beneficial immunomodulatory effects.324 In combination with quercitin, Serenoa repens (W.Bartram) Small (Arecaceae), and Urtica dioica L. (Urticaceae), curcumin was able to improve the efficacy of prulofloxacin in patients with chronic bacterial prostatitis.325 Also, a recent study shows that topical application of 5% curcumin is as effective as 0.1% triamcinolone for treating recurrent aphthous stomatitis, an oral mucosal disease that may be caused by bacterial pathogens.326 Despite these interesting results, curcumin has low solubility in water and poor bioavailability, thus limiting its use as an antibacterial agent. To overcome these drawbacks, structural analogues have been synthetized and nanoparticle-based approaches have been developed.327,328 Further research on these new formulations are required to confirm their therapeutic values.
A prenylated benzopyran, 3,4-dihydro-5-hydroxy-2,7dimethyl-8-(3”-methyl-2”-butenyl)-2-(4’-methyl-1’,3’-pentadienyl)-2H-1-benzopyran 6-carboxylic acid (103), was isolated by Ruiz Mostacero et al.92 from Peperomia obtusifolia (L.) A. Dietr. (Piperaceae) aerial parts. Compound 103 displayed high antibacterial activity against S. aureus, three MRSA isolates, E. faecalis, and S. epidermidis. Based on the data obtained, the authors suggested that compound 103 may act on gram-positive bacteria by a different mechanism than β-lactams or glycopeptide antibiotics. In order to propose a possible MOA for 103, zeta potential experiments on S. aureus were conducted, and membrane damage was confirmed by fluorescent microscopy experiments. The results indicated that 103 was able to interact directly with bacterial membranes due to its amphipathic characteristics. The isoprenoid chains could interact with the phospholipid membrane, and the carboxylic acid moiety may possibly expose its negative charge to the interface, explaining the increase of the negative charge of both the S. aureus and liposomal surface.92
Tetraceranoate (105) was isolated from a stem bark extract of Tetracera potatoria Afzel. ex G.Don (Dilleniaceae). Tetraceranoate exhibited the high antibacterial activity against Mycobacterium smegmatis and was as effective as rifampicin, a first-line drug used for tuberculosis treatment. The cyclic structure of tetraceranoate and the presence of lipophilic domains may explain its anti-mycobacterial activity. Anti-mycobacterial activity is frequently enhanced by increased lipophilicity, which helps penetration through the highly lipophilic mycobacterial outer envelope and cell wall.329 Another hypothesis suggests that tetraceranoate may have the same mode of action as rifampicin: inhibiting bacterial DNA-dependent RNA polymerase.330
Rhodomyrtone (108), a member of the acylphloroglucinols isolated from Rhodomyrtus tomentosa (Aiton) Hassk. (Myrtaceae) leaves, displayed antibacterial activity against many bacterial pathogens.331 Studies conducted by Limsuwan et al. demonstrated the high activity of rhodomyrtone against oral microorganisms, including S. mutans and Staphylococcus aureus.94 Based on the excellent antibacterial activity of rhodomyrtone against gram-positive bacteria,332,333 Saising et al.95 investigated the efficacy of rhodomyrtone against C. acnes, finding a high level of growth inhibition with low toxicity on human skin cells.
Rhodomyrtosone B (109), an isomer of 108, was also isolated from the leaves of R. tomentosa and exhibited a high level of activity against gram-positive bacteria, including S. aureus, C. acnes, E. faecalis, S. epidermidis, and clinically relevant multidrug-resistant pathogens such as MRSA and vancomycin-resistant Enterococcus (VRE).96 This study also showed that rhodomyrtosone B had low mammalian cytotoxicity, and its MOA appeared to be associated with increased permeability into the bacterial cell membrane. In addition, compound rhodomyrtosone B profoundly attenuated skin ulcer formation in a murine model of MRSA infection with an efficacy comparable to or superior to the effects of vancomycin. These results suggested that rhodomyrtosone B might be a promising natural antibiotic to combat drug-resistant (MRSA and VRE) infections.96
Biloa Messi et al.97 isolated the polyisoprenylated benzophenone 7-epi-clusianone (110) from the hexane extract of the fruits of Garcinia preussii Engl. (Clusiaceae). The authors found that 7-epi-clusianone exhibited high antibacterial activity against S. aureus and E. faecalis. In previous investigations, 7-epi-clusianone has been shown to be a promising compound for the treatment of multidrug-resistant S. aureus infections.334 In addition, 7-epi-clusianone was found to affect the biofilm formation and acid tolerance of the cariogenic gram-positive bacteria S. mutans.335 The MOA of this compound is unknown; however, due to their specificity to gram-positive bacteria, the effect may be related to the characteristics of the cell envelope.97
The polycyclic polyprenylated acylphloroglucinol (PPAP), hypercalin B (113), was isolated from Hypericum acmosepalum N.Robson (Hypericaceae) and had high activity against the MDR S. aureus strain, SA-1199B.100,336 The majority of the over 400 known PPAPs are limited to the Hypericaceae family, with hypercalin B reported in only three genera.336–339 PPAPs, including hypercalin B, derive from a mixed mevalonate and polyketide biosynthetic pathway.
Kim et al.101 isolated a growth-inhibiting benzofuran, chalcomoracin (114), from the leaves of Morus alba L. (Moraceae)101, and it has also been found in Morus wittiorum Hand.-Mazz (Moraceae).340 Chalcomoracin was found to have high activity against both MRSA and MSSA.101 Chalcomoracin is believed to inhibit the growth of S. aureus by targeting the bacterial fatty acid system II. Specifically, chalcomoracin inhibits an isoform of enoyl-ACP reductase, FabI, a catalyst required for fatty acid synthesis.101
A phenylpropanoid glycoside identified as martynoside (115), was isolated from Boscia albitrunca (Burch.) Gilg & Benedict (Capparaceae) leaves.102 The same study found martynoside to have high antibacterial activity against B. subtilis and moderate activity against K. pneumoniae. Pendota et al. concluded that the antibacterial activity of martynoside provides rationale for the ethnomedicinal uses of the plant and suggested that this compound could be helpful in the management of eye infections.102
Of the 67 other phenolic compounds included in our review, 33 exhibited a high in vitro antibacterial activity. Of these, five compounds (curcumin, methyl gallate, pyrogallol, rhodomyrtone, rhodomyrtosone B) have demonstrated an in vivo antibacterial activity, two compounds (curcumin, hydroxytyrosol) have been investigated in clinical trials for conditions other than bacterial infections, and only one (curcumin) has been tested in clinical trials for bacterial infections (H. pylori infection).86,96,302,317–321,341–344 Rhodomyrtone and rhodomyrtosone B represent two promising antibacterial lead compounds, discovered in 2002 and 2008, respectively.345,346 Studies focusing on their potential for clinical applications (e.g., pharmacokinetic parameters, toxicity) are needed. On the other hand, hydroxytyrosol has been widely studied, but few studies have examined its potential as an antibacterial agent; further work should explore its efficacy and safety in animal models of infection. Overall, methyl gallate and curcumin exhibit the greatest potential as therapeutic agents for the treatment of bacterial infections in humans. They have demonstrated promising results in different in vivo models of bacterial infections, and they exhibit a good safety profile.347,348 Despite their great efficacy and safety, both compounds have some pharmacokinetic issues with low oral bioavailability and poor stability under physiological conditions. Thus, more research is needed to evaluate the antibacterial activity of synthetic analogues and pharmaceutical formulations with improved pharmacokinetics in animal models and humans.
2.4. Terpenoids
Formed by condensation of isoprene or isopentane units, terpenoids represent the largest chemical class of plant NPs and most are multicyclic structures that differ from one another on the basis of carbon skeleton and different functional groups 349 Plants form terpenoids via the mevalonate pathway from mevalonic acid or deoxyxylulose phosphate by the methylerythritol 4-phosphate pathway (MEP), also referred to as mevalonate independent pathway. These precursors form isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP).
2.4.1. Overview
Triterpenoids and saponins make up 33.6% of terpenoid antibacterial compounds documented in our literature search. Diterpenoids represented 30.3% of all terpenoid compounds. Monoterpenoids and sesquiterpenoids were seen less frequently, at 17.2% and 19% respectively (Figure 3).
Figure 3.
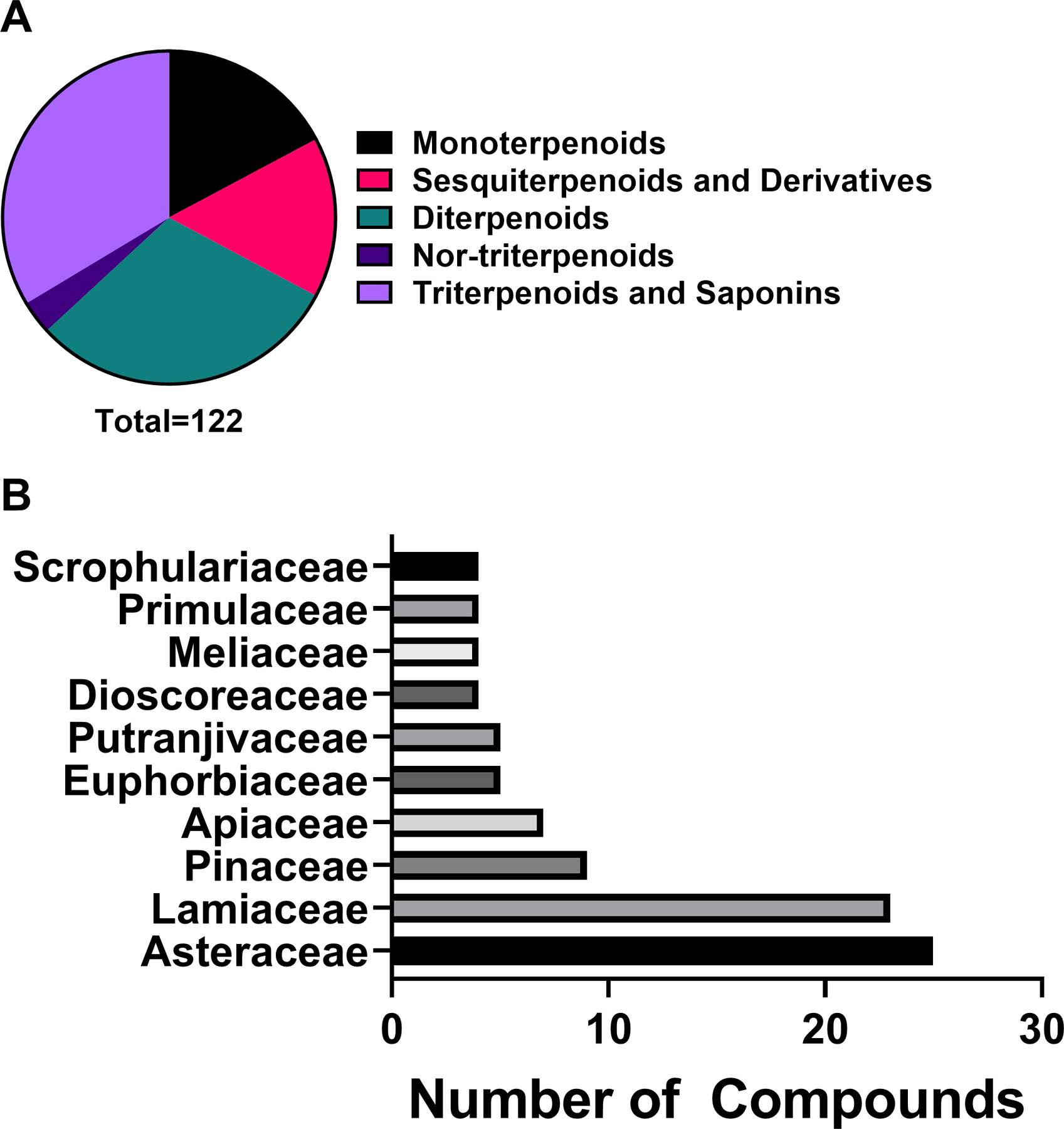
A) Chemical classes of terpenoids investigated for antibacterial activity and B) top ten plant families yielding antibacterial terpenoids.
2.4.2. Monoterpenoids
In the creation of monoterpenoids, the enzyme prenyl transferase converts the precursors into geranyl pyrophosphate (GPP). GPP ionizes to its isomers, linalyl diphosphate, and neryl diphosphate. These three isomers become the linear monoterpenoids and act as substrates for various cyclases which yield monoterpene ring systems.191
Carvacrol (116) is a monoterpenoid common to many aromatic plants. It is approved for human consumption, and it is often used as both a flavoring agent and for its anti-bacterial properties in many prepared foods.350 One study reported that carvacrol increases cell membrane permeability and depolarizes the membrane potential in L. monocytogenes; this leads to cell death.351 This study also found antibacterial synergy with nisin.
Thymol (117) is the phenolic isomer of carvacrol present mainly in essential oils (EOs) from plants of the Lamiaceae family. Thymol has shown moderate growth inhibitory effect on eleven different bacterial species, including Proteus mirabilis, B. cereus, M. flavus, S. aureus, S. enteritidis, and P. aeruginosa.103,352 Thymol induces the permeabilization and depolarization of the cytoplasmic membrane and thus disrupts the membrane integrity resulting in leakage of intracellular materials and cell death.353 Catheters impregnated with thymol (400 mg/mL) and chloroxylenol (150 mg/mL) have demonstrated in vitro antibacterial activity against uropathogens and in vivo antibacterial activity against Enterobacter cloacae, which suggests that thymol could be used to prevent catheter-associated urinary tract infection.354 A thymol-enriched bacterial cellulose hydrogel showed in vitro antibacterial activity against burn specific pathogens, as well as an improvement of wound healing in vivo and is proposed to be used as a delivery system for third-degree burns.355 A clinical trial evaluated the effectiveness of a topical lavender-thymol oil preparation in 60 postpartum women with episiotomy and showed a reduction in pain and improvement in dyspareunia for patients treated.356 Finally, a randomized clinical trial comparing the traditional Listerine® mouthwash with thymol and other EOs to an alcohol-free mouthwash Listerine Zero® with thymol showed that both products reduce plaque formation and biofilm thickness.357 Although thymol shows great potential as an antibacterial agent, its low bioavailability, along with its potential toxicity, limits its use.353 Further comprehensive studies should be performed to assess its safety in vivo.
Linalool (118) is a monoterpenoid with a tertiary alcohol group. This volatile oil was isolated from many plants, including Lavandula spp. (Lamiaceae), Cinnamomum camphora (L.) J.Presl (Lauraceae), and Cannabis sativa L. (Cannabaceae).358–360 Linalool isolated from Ligustrum compactum (Wall. ex G.Don) Hook.f. & Thomson ex Brandis (Oleaceae) flowers had high antibacterial activity against Salmonella enteritidis, B. cereus, B. subtilis, E. coli, P. aeruginosa, and S. sonnei.106 Alves et al. 361 isolated linalool from Coriandrum sativum (Apiaceae) and determined it had moderate antibacterial activity against A. baumannii. The antibacterial MOA of linalool against P. aeruginosa is multifaceted, like many other EOs; linalool changes cell morphology, destroys the cell wall, decreases membrane potential, interferes with cellular respiration, and causes nucleic acid leakage due to cell membrane damage. The culmination of these effects is cell death.362
The volatile monocyclic monoterpenoid alcohol α-terpineol (119) is one of 5 isomers of terpineol, which differ only in the position of the double bond. The compounds α-terpineol and terpinen-4-ol are the most common naturally-occurring terpineols.363 Additionally, α-terpineol is present in many aromatic plant substances, including orange juice364, and smells like lilac.363 Antibacterial activity of α-terpineol is high against B. subtilis, S. epidermidis, S. aureus, P. aeruginosa, and E. coli.365 The MOA of α-terpineol against S. aureus does not involve gross membrane damage, as is seen with other terpenoids and EOs. Rather, α-terpineol causes the loss of membrane-bound autolytic enzymes, which leads to cytoplasm leakage and an inability to osmoregulate. The combination of these effects over time induces cell lysis.365
Citronellol (120) usually occurs naturally as a mixture of enantiomers, (+)-citronellol and (-)-citronellol366. It was ineffective against S. aureus, but highly active against E. coli.109 The cell surface tension properties and surface charge of E. coli and S. aureus are altered when citronellol is applied, becoming more hydrophobic, having a less negative zeta potential and deteriorating membrane integrity.109
Twenty-one monoterpenoids were reported in our review, among which ten exhibited high in vitro antibacterial activity. All of them were found in EOs from plants and have received much attention for their significant antibacterial activity, with four compounds (α-terpineol, carvacrol, eucalyptol, and thymol) being investigated with in vivo models of bacterial infections,354,367–369 and six (α-pinene, carvacrol, eucalyptol, linalool, terpinen-4-ol, and thymol) tested in clinical trials (Table 2) related to infectious and inflammatory conditions (e.g., gingivitis, sore throat, episiotomy, acute bronchitis, blepharitis).198,356,370 Although highly active, these compounds present some drawbacks reducing their use as antibacterial agents, especially their unfavorable physico-chemical profile with high volatility leading to instability and a short half-life, a low water solubility leading to a poor bioavailability after oral administration, an unpleasant taste and smell, and potential toxicity due to poor knowledge concerning the optimal safe dose to be administered. Future research directions include improvement of physicochemical properties, the examination of acute and chronic toxicity as well as teratogenicity, development of standardized formulations with multiple compounds, and realization of clinical trials focused on multi-drug resistant bacterial infections.
2.4.3. Sesquiterpenoids and Derivatives
Sesquiterpenoids form via the mevalonate pathway from the addition of an IPP group to geranyl pyrophosphate (GPP) to form farnesyl diphosphate (FPP). FPP can form acyclic and cyclic sesquiterpenoids with varied stereochemistry.191 These compounds are usually less volatile than other terpenoids, and as they oxidize their aroma changes.371
The roots of Iostephane heterophylla (Cav.) Benth. (Asteraceae) yielded several compounds with antibacterial activity, including the bisabolane-type sesquiterpenoid xanthorrhizol (121) and the diterpenoid ent-trachyloban-19-oic acid (127)110. Compound 127 had high antibacterial activity against S. mutans, and also inhibited the formation of S. mutans biofilms. Xanthorrhizol and 127 were determined to have moderate to high antibacterial activity against P. gingivalis.110 The effectiveness of xanthorrhizol against gram-negative bacteria is improved when used in combination with polymyxin B nonapeptide, nisin, carvacrol (116), or thymol (117).372
Onopordopicrin (122), a germacranolide sesquiterpenoid lactone, was isolated from Onopordum acanthium L. (Asteraceae) and exhibited moderate activity against B. subtilis, Lactobacillus plantarum, Vibrio fischeri, and Xanthomonas euvesicatoria.111
The sesquiterpenoid 8,9-oxoisopropanyldshamirone (123) along with 27 other sesquiterpenoids were isolated from Ferula ferulioides (Steud.) Korovin (Apiaceae). It had high activity against both MRSA and tetracycline-resistant S. aureus; however, it was not active against other strains.112
Of the 19 sesquiterpenoids included in our review, only three (i.e., 8,9-oxoisopropanyldshamirone, onopordopicrin, xanthorrhizol) showed high in vitro antibacterial activity. However, none of them have been investigated either in animal models of bacterial infections or in clinical trials. Further research including in vivo pharmacological and toxicological studies are needed to assess the potential of sesquiterpenoids in the treatment of bacterial infections.
2.4.4. Diterpenoids
Diterpenoids form from the addition of isopentenyl diphosphate (IPP) to geranylgeranyl diphosphate (GGPP); cyclization, and Wagner-Meerwein rearrangements produce the diversity seen in this chemical class.191 One of the most ubiquitous diterpenoids is the acylic diterpene alcohol, phytol. It is a required sidechain in chlorophyll and is also necessary for the biosynthesis of vitamin E, tocopherol, and vitamin K, phylloquinol.373
Three abietane diterpenoids exhibited antibacterial activity: abieta-7,9(11)-dien-13-β-ol (124), dehydroabietic acid (125), and carnosic acid (138). A recent review of the antibacterial activity of these compounds and their relatives covers much of the existing knowledge on the MOA and SAR of abietane diterpenoids.374 Dehydroabietic acid is a defense metabolite in the resin of Pinus species (Pinaceae) and other conifers.114,375 Biosynthesis of dehydroabietic acid involves cyclization of copalyl diphosphate, followed by oxidation mediated by cytrochrome P450.375 From in vitro assays, dehydroabietic acid had high activity in P. gingivalis and moderate activity against Actinomyces naeslundii, C. acnes, S. mitis, Bacteroides fragilis, and P. intermedia.114,115 Dehydroabietic acid was less potent than the oleoresin from which it was isolated, suggesting that synergy among compounds in the oleoresin may be important for antibacterial activity.115
Carnosic acid (138)—sometimes classified as a polyphenol due to its catechol moiety—is found in the plant family Lamiaceae; it was first isolated from Salvia officinalis L., commonly known as sage, and is widely used and studied for its antibacterial and antioxidant properties.376 The biosynthesis of carnosic acid is not entirely clear, but several possible intermediates have been identified.376 A methyl ester of carnosic acid (20-methyl carnosate) is also found in Lamiaceae, specifically S. officinalis and Salvia lanigera Poir.123 An SAR study of carnosic acid and its methyl ester indicated the hydroxyl at C12 is needed for activity and that the methylation of C20 decreased the MIC against E. faecalis and MRSA.123 In another study with E. faecalis and S. aureus, the antibacterial MOA for carnosic acid was found to be inhibition of efflux pumps, suggesting possibilities for synergy with existing antibiotics.377 Several in vivo experiments have found carnosic acid to have an antioxidant effect, and rosemary extracts containing carnosic acid and carnosol (137) are approved in the European Union as antioxidant food additives.376 The wide availability of carnosic acid will facilitate further study of its antioxidant and antibacterial properties; cultivars of Rosmarinus officinalis (Lamiaceae) have been developed with up to 10% content of carnosic acid by weight in dried leaves.376
Serrulatane diterpenoids 8,19-dihydroxyserrulat-14-ene (126) and 8-hydroxyserrulat-14-en-19-oic acid (131) were isolated from the plant genus Eremophila, which is used in wound healing in Australian traditional medicine.116 Their in vitro antibacterial activity is generally against gram-positive bacteria; both compounds had high activity against B. subtilis, S. pneumoniae, MRSA, and the gram-negative bacterium Moraxella catarrhalis.116 However, they both exhibited cytotoxicity against Vero cells, indicating that toxicity may be a barrier to the use of these compounds as therapeutics.116 When tested in a mouse model of foreign body infection, 131 exhibited neither antibacterial activity nor toxicity, putatively due to binding to albumin.378 A patent application for the use of serrulatane diterpenoids in antimicrobial coatings on medical devices has been abandoned.379
Kaurenoic acid (128) and its enantiomer, ent-kaurenoic acid, are tetracyclic kaurane diterpenoids. They are composed of a perhydrophenantrene unit and cyclopentane ring. Like other diterpenoids, these are formed via the mevalonate pathway from geranylgeranyl pyrophosphate (GGPP).191 Under acidic conditions, GGPP can cyclize into two enantiomeric perhydronaphtalene bicyclic intermediates with stereocenters on C-5, C-9, and C-10; resulting in “normal” and ent- diterpenoids.380 This class of diterpenoids occurs in many plant families, including Asteraceae, Annonaceae, Apiaceae, Celastraceae, Chrysobalanaceae, Erythroxylaceae, Euphorbiaceae, Fabaceae, Lamiaceae, Jungermanniaceae, Rhizophoraceae, Rutaceae, and Velloziaceae.380 Kaurenoic acid is an intermediate for many other diterpenoids, including the gibberellins, a class of plant hormones involved in development.381,382 Many bioactivities have been attributed to kaurenoic acid, including anti-diabetic properties,383 genotoxicity against some tumor cells,384–386 antifungal activity against Trichophyton rubrum, T. metagrophytes and Epidermpphyton floccosum,387 and as a phagostimulant.388 It has also shown activity against the botanical gray mold, Botrytis cinerea.389 A 2007 review of the kaurane diterpenoids discusses their bioactivities and synthesis in detail.380 Kaurenoic acid isolated from Copaifera langsdorffii Desf. (Fabaceae) oleoresin had moderate to high antibacterial activity against B. cereus and S. epidermidis.390 Several bacteria linked to dental caries are inhibited by ent-kaurenoic acid, including S. mitis, S. mutans, Prevotella melaninogenica, P. gingivalis, and Prevotella nigrescens.118,391
The labdane diterpenoid copalic acid (129) is the primary biomarker of the plant genus Copaifera (Fabaceae) and a major constituent of the terpene-rich medicinal oleoresin of that genus.392 In in vitro assays, copalic acid had high activity against S. epidermidis and S. pneumoniae, and moderate activity against MRSA, demonstrating better activity against gram-positive bacteria than gram-negative bacteria.117 A methylated analogue of copalic acid exhibited MICs an order of magnitude higher than those of copalic acid, supporting the theory that diterpenoids with a single hydrogen bond donor group have more potent antibacterial activity than those with two such groups.393
The clerodane diterpenoid 16α-hydroxycleroda-3,13(14)Z-dien-15,16-olide (133), isolated from Polyalthia longifolia (Sonn.) Thwaites (Annonaceae) showed moderate antibacterial activity against several clinical isolates of MRSA.121,122 When used in combination with a β-lactam, oxacillin, tetracycline, daptomycin, and linezolid, synergistic interactions are observed in vitro, with the β-lactam showing a 10–80 fold reduction in the MIC depending on the strain tested.122 Similar synergistic activity was observed for several fluoroquinolones in vitro and for norfloxacin in vitro.121 A downregulation of major facilitator superfamily (MFS) and multidrug and toxin extrusion (MATE) efflux genes in the presence of 133 and norfloxacin demonstrates that 133 acts as an efflux pump inhibitor, allowing increased intercellular concentrations of the antibiotic.121
A total of 37 diterpenoids were reported in our review, among which 13 had high in vitro antibacterial activity. Only one compound (i.e., 8-hydroxyserrulat-14-en-19-oic acid) has been studied in animal model of bacterial infections but it did not demonstrate any antibacterial activity.378 None of these compounds have been tested in clinical trials. More research is needed to confirm the antibacterial activity of diterpenoids using in vivo models.
2.4.5. Nor-triterpenoids
Nimbolide (142) is a limonoid triterpenoid isolated from the leaves of Azadirachta indica A. Juss (Meliaceae). Nimbolide exhibited high activity against MRSA.127 A potential MOA for nimbolide against MRSA is thought to be targeting of the bacterial cell membrane, resulting in increased membrane permeability, the disintegration of the cell envelope, and bacterial cell lysis.127 However, a cytotoxicity assay found nimbolide to be cytotoxic to BeWo human choriocarcinoma cells, indicating a potential barrier to its utility as an antibacterial therapy.394 Due to its poor oral bioavailability and its cytotoxicity, the therapeutic application of nimbolide in bacterial infections seems to be limited.395
2.4.6. Triterpenoids and Saponins
While most terpenoids (mono-, di- and tetra-) are formed in the chloroplasts and use the methylerythritol 4-phosphate (MEP) pathway, the mevalonate pathway occurring in the cytosol forms triterpenoids, steroids, and some sesquiterpenoids. Indeed, the biosynthetic pathways for terpenoids (MEP and mevalonate) are compartmentalized, so that the chloroplast and cytosol represent two distinct locations for biosynthesis. Accordingly, triterpenoids are formed by converting the cytosolic pool of isopentenyl phosphate (IPP) to the C15 farnesyl pyrophosphate (FPP), then joining two FPP units tail-to-tail to yield the linear triterpene squalene. Squalene undergoes a cyclization via an intermediary 2,3-oxidosqualene, which through cyclized ring expansions and Wagner-Meerwein migrations yields triterpene alcohols or aldehydes including α- and β-amyrin and lupeol. Ursolic acid (147) contains the α-amyrin skeleton, β-amyrin is found in oleanolic acid (148), while betulinic acid (149) and betulin (144) are two oxidized versions of lupeol.191
Friedelane-3,11-dione (143) is a triterpenoid with high activity against clinically isolated, isoniazid resistant M. tuberculosis.50 It has been isolated from the stems of Drypetes molunduana Pax & K. Hoffm (Putranjivaceae)396 and leaves of Triclisia gilletii (De Wild.) Staner (Menispermaceae). The low polarity of 143 may explain its action against the hydrophobic cell wall of M. tuberculosis and validate its history as an anti-tubercular agent.50
Two norlupane triterpenoids, ceanothenic acid (145) and 29-hydroxyceanothenic acid (151), were isolated from the leaves of Alphitonia xerocarpus Baill (Rhamnaceae). Both showed moderate antibacterial activity against E. faecalis and high activity against S. aureus, as well as cytotoxicity against a human carcinoma cell line.397
Moronic acid (146) is a pentacyclic triterpenoid isolated from the aerial parts of Schinus lentiscifolius Marchand (Anacardiaceae). Moronic acid showed high growth inhibitory activity against B. subtilis, S. aureus and S. pyogenes. Some derivatives of moronic acid tested against these bacteria showed much higher MICs, indicating the importance of the carbonyl group for antibacterial activity.129
Ursolic acid (147) is an ursane-type pentacyclic triterpenoid isolated from various plant species including apple (Malus domestica Borkh., Rosaceae), coffee (Coffea arabica L., Rubiaceae), cranberry (Vaccinium macrocarpon Aiton, Ericaceae), guava (Psidium guajava L., Myrtaceae), marjoram (Origanum majorana L., Lamiaceae), olive (Olea europaea, Oleaceae) and thyme (Thymus vulgaris, Lamiaceae).398 Ursolic acid showed high growth inhibitory activity against E. faecalis, L. monocytogenes, and M. tuberculosis, and moderate activity against extremely drug-resistant (XDR) M. tuberculosis, K. pneumoniae, P. aeruginosa, and V. cholerae.83,131,132,399,400
Ursolic acid has been extensively studied against human pathogenic bacteria; however, its MOA is not yet fully elucidated. It relies on modulating microbial genes, interfering in the mycolic acid biosynthesis, inhibiting peptidoglycan turnover, damaging cell membrane integrity, inhibiting biofilm formation, inducing stress responses and cell autolysis.401–403 To improve its low water solubility and its antibacterial activities, chemical modifications were performed in the C-3, C-17, and C-28 positions. Acetylation of ursolic acid at the C-3 position afforded ursolic acetate, which exhibited higher antimycobacterial activity.404 Introducing two hydroxyl groups to ursolic acid at positions C-1 and C-2 together with a methyl ester group at C-17 position afforded methyl 1α,2β,3β-trihydroxy-urs-12-en-28-oate, which showed better growth inhibitory activity against S. aureus and B. subtilis.405 Incorporation of isopropyl or n-butyl chain onto the C-28 position led to derivatives able to reverse the resistance against multi-drug resistant E. coli by inhibiting efflux pumps.406 Other derivatives containing an aminoguanidine moiety at C-3 or at C-28 position led to better antibacterial activities against multi-drug resistant S. aureus.407,408 Finally, the incorporation of a carbazole moiety, as well as a N-(dimethylamino) propyl amide side chains at C-28 position had a beneficial effect on the antibacterial activity of ursolic acid.409
A mixture of ursolic acid and oleanolic acid (148) (3:1) was administered three times per week for 30 and 60 days to mice infected with M. tuberculosis and results showed a significant reduction of bacterial loads, improvement in lung histopathology and immunostimulatory effects.410 In a murine model of subcutaneous MRSA infection, a local administration of ursolic acid in combination with nafcillin every eight hours for 56 hours reduced the size of necrotic skin lesions and the production of the proinflammatory cytokine IL-1β.411 Although ursolic acid has been studied in some clinical trials—none focused on its effect on bacterial infectious disorders. Further studies should be performed to confirm the efficacy of ursolic acid and its derivatives as antibacterial agents.
Oleanolic acid (148) often occurs in combination with its isomer, ursolic acid (147), and has been isolated from more than 1,620 plant species, especially in plants belonging to the Oleaceae family.412 Oleanolic acid demonstrated moderate to high growth inhibitory activity against E. faecalis, L. monocytogenes, B. cereus, and M. tuberculosis.130,413 Derivatives of oleanolic acid with antibacterial activity have been isolated from a variety of plant species; this is the case of compound 156 (3-O-β-D-glucuronopyranosyloleanolic acid 28-O-β-D-glucopyranosyl ester) and compound 154 (3‑O‑β‑D‑glucuronopyranosyl‑oleanolic acid), both isolated from Melanthera elliptica O.Hoffm. Compounds 156 and 154 showed moderate activity against multidrug-resistant E. coli, S. flexneri and S. aureus.136 Compound 155 (3-O-β-D-glucopyranosyl(1→6)-β-D-glucopyranosyloleanolic acid) is another derivative of oleanolic acid and was isolated from Paullinia pinnata L. (Sapindaceae). Compound 155 showed moderate to high antibacterial activity against E. coli, K. pneumoniae, E. aerogenes, and S. enterica ser. Typhi.137 A set of oleanolic acid C-28 amide derivatives were synthesized and it was shown that a longer chain length improved antibacterial activity on gram-positive bacteria.414 Unlike ursolic acid, oleanolic acid inhibits peptidoglycan turnover, thus affecting the cell membrane of bacteria.415 Oleanolic acid also showed a synergistic effect in combination with conventional antibiotics against MRSA.416 Although oleanolic acid is one of the most investigated plant-derived compounds and has been approved as an OTC hepatoprotective drug in China,417 few in vivo studies and clinical trials have been undertaken to evaluate the use of oleanolic acid and its derivatives as antibacterial agents.
Pseudolarolide B (150), a cycloartane triterpenoid lactone and pseudolarolide Q (152), a triterpenoid cycloperoxide418, were isolated from the seeds of Pseudolarix amabilis (Nelson) Rehd (Pinaceae). Pseudolarolide Q and B exhibited high antibacterial activity against S. aureus and E. coli.134 Cytotoxicity studies of pseudolarolide B found toxicity against KB (nasopharyngeal), A-549 (lung), HCT-8 (colon) and P-388 (leukemia) tumor cell lines.419
Of the 41 triterpenoids and saponins included in our review, 12 showed high in vitro antibacterial activity. Of these, only two compounds (i.e., oleanolic acid and ursolic acid) have been studied in animal models of bacterial infections and tested in clinical trials for conditions other than infectious disorders.410,411,420 The development and therapeutic application of these two pentacyclic triterpenoids have been limited by their low oral bioavailability and their short half-life; thus many derivatives have been synthesized and tested using in vitro antibacterial assays. More in vivo studies are needed to explore their full potential as antibiotics.
2.5. Other Metabolites
Aliphatic compounds were the most abundant chemical class amongst other metabolites at 37.7%. Cyclic compounds and steroids were seen at the same rate, 14.3%. While lipids and organosulfurs and derivatives both occurred at 12%. Peptides and quinones and related compounds were the least represented chemical classes at 1.3%, each having a single compound in the subclass. (Figure 4).
Figure 4.
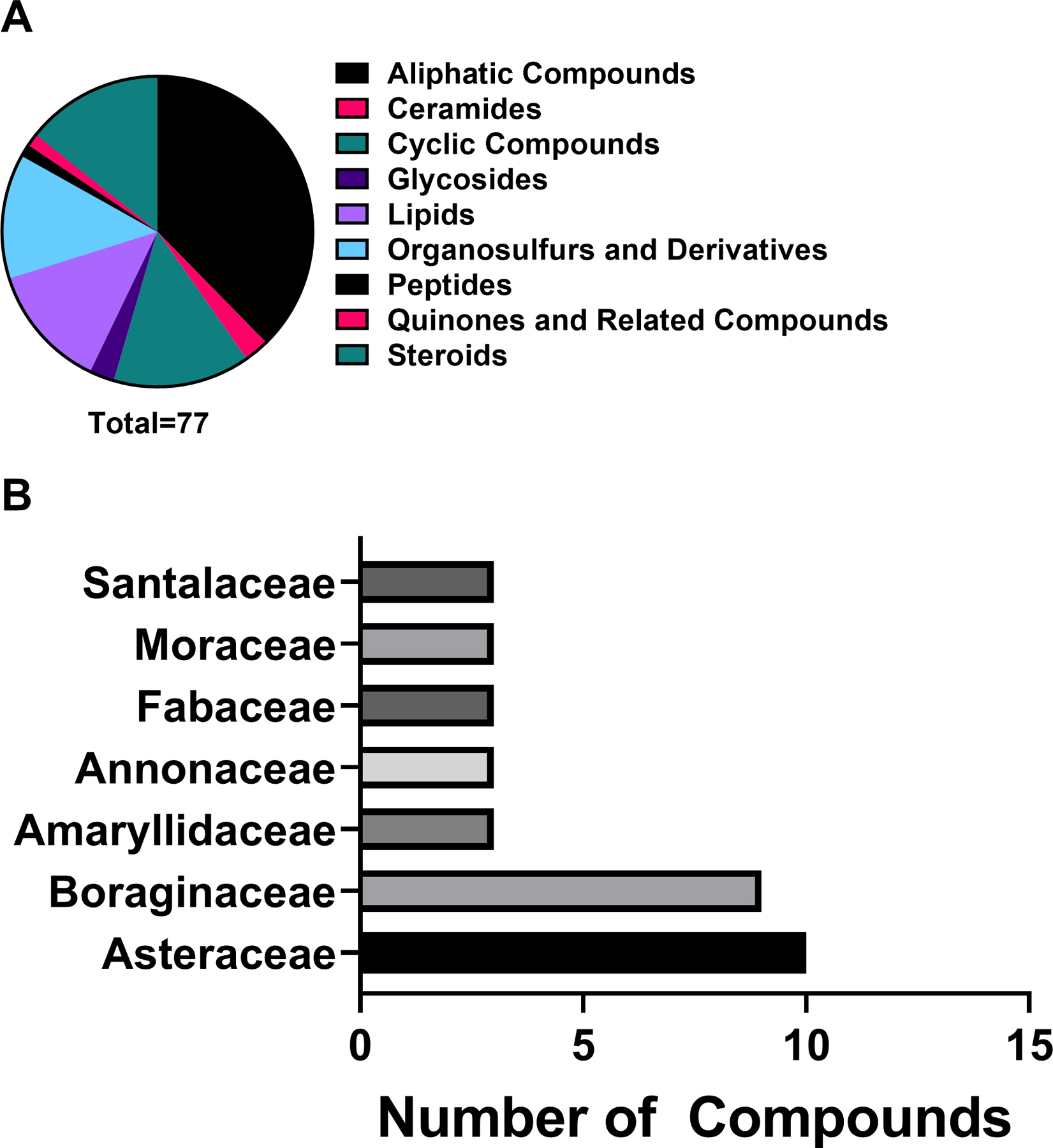
A) Chemical classes of other metabolites investigated for antibacterial activity and B) top seven plant families yielding other antibacterial metabolites. The remaining 20 plant families had less than three compounds each represented in the data.
2.5.1. Aliphatic Compounds
Aliphatic compounds are non-aromatic hydrocarbons. They are found as straight chains or branched compounds with a variety of single or double bonds throughout. These highly lipophilic compounds most likely exhibit their effect by compromising the bacterial membrane.
Fujita et al.138 examined the antibacterial components of EO from plants in the genus Polygonum (Polygonaceae)138 for activity against Salmonella, identifying 2E-undecenal (157), undecanol (158), 2E-dodecenal (159), and dodecanol (160), as having antibacterial properties. 2E-dodecenal has previously been found in the EO of Marrubium thessalum Boiss. & Heldr. (Lamiaceae).421 Dodecanol has also been found in the EO of Phlomoides laciniata (L.) Kamelin & Makhm. (Lamiaceae).422 2E-undecenal has been found in the EO of species within the genus Geranium.423 Undecanol was found in the EO of Senecio belgaumensis (Wight) C.B.Clarke (Asteraceae).424 The nonspecific MOA of these alkanals and alkanols is thought to occur by acting as a surfactant, denaturing integral proteins in the membrane and disturbing their normal function.138 All four of these inhibited S. enterica growth. One clinical trial examined the effects of a moisturizing hand wash solution containing dodecanol as one ingredient and compared differences in development of hand dermatitis to typical hand wash solutions.198
Guzman et al.139 investigated the roots of Levisticum officinale W.D.J.Koch (Apiaceae) and found the aliphatic compound falcarindiol (161) to have moderate to high activity against Mycobacterium tuberculosis and M. bovis. Falcarindiol is also found in many other members of the Apiaceae family.425–430 The biosynthesis of falcarindiol occurs from crepenynic acid in the crepenynate pathway.431 A cytotoxicity study by Dall’Acqua et al.432 found falcarindiol to be cytotoxic against a panel of cancer cell lines: promyelocytic leukemia, human fibrosarcoma, and human intestinal adenocarcinoma. A clinical study examined the bioavailability of dietary compounds found in carrots, including falcarindiol, and another clinical trial studied the effects of these dietary compounds in preventing cellular and DNA damage.198
Perianayagam et al.135 examined the roots of Trichodesma indicum (Boraginaceae), yielding the antibacterial aliphatic compound n-dotriacont-9-one-13-ene (162). T. indicum has been used in traditional medicine in India to treat many ailments, including dysentery and skin disease.135 Compound 162 was found to have a wide range of antibacterial activity against both gram-negative and gram-positive bacteria, with moderate activity against B. subtilis, S. aureus, S. epidermidis, K. pneumoniae, E. coli, and P. aeruginosa.
Twenty-nine aliphatic compounds were found in our review, and four (i.e., 2E-dodecenal, dodecanol, falcarindiol, n-dotriacont-9-one-13-ene) showed high in vitro antibacterial activity. Of these, falcarindiol is the only aliphatic compound studied in an in vivo model of bacterial infection.433 However, the toxicological profile of this compound is poorly studied, which limits our ability to draw any conclusions. More research is needed to assess the role of falcarindiol and other aliphatic compounds as antibacterial agents.
2.5.2. Ceramides
Ceramides are long-chain amino alcohols, or sphingosines, attached to a fatty acid. The synthesis of ceramides begins with the formation of an amide linkage, catalyzed by ceramide synthase, between a long-chain amino alcohol and fatty acyl-CoA.434
Teinkela et al.74 isolated ficusoside B (163) from the wood of Ficus elastica Roxb. ex Hornem. (Moraceae). Thus far, ficusoside B and a related compound, ficusoside, have only been found in F. elastica. Ficusoside B displayed high activity against S. aureus and P. vulgaris, and moderate activity against P. stuartii and P. aeruginosa.74 A cancer study found ficusoside B to have a weak cytotoxic effect against a panel of human cancer cell lines.435
Of the two ceramides found in our review, only ficusoside B exhibited high in vitro antibacterial activity. However, only one study focused on assessing its in vitro antibacterial activity. Further in vitro experiments should be performed to confirm these results.
2.5.3. Cyclic Compounds
Cyclic compounds are a large and diverse category of compounds defined by the presence of a ring. Cyclic compounds with significant antibacterial activity that do not belong to the previously discussed classes are described below.
Yu et al.106 isolated the cyclic compound 2-phenylethanol (164) from the flowers of Ligustrum compactum (Wall. ex G.Don) Hook.f. & Thomson ex Brandis (Oleaceae). It has also been found in roses (Rosa spp.) and strawberries (Fragaria spp.) in the Rosaceae family and contributes to their aromatic scent.436 The biosynthesis of 2-phenylethanol occurs from the conversion of L-phenylalanine to phenylacetaldehyde by the enzyme aromatic amino acid decarboxylases (AADC), which is then reduced by phenylacetaldehyde reductases (PAR) into 2-phenylethanol.437 The MOA for 2-phenylethanol is currently unknown. It displayed high activity against S. enteritidis, S. sonnei, B. cereus, P. aeruginosa, L. plantarum, Leuconostoc mesenteroides, and S. aureus.106 2-phenylethanol has been used in clinical trials as an odorant but has not been assessed for its antimicrobial properties in humans.198
Panyo et al.140 isolated an antibacterial dipyridyl disulfide, 2,2’-dithiodipyridine (165) from the stems of Ixora megalophylla Chamch. (Rubiaceae). It has previously been found in the genus Allium (Amaryllidaceae), which includes onion and garlic.438 2,2’-dithiodipyridine was found to have high activity against S. mutans and S. mitis.140
Of the 11 cyclic compounds included in our review, three compounds (2,2’-dithiodipyridine, (-)-cleistenolide, 2-phenylethanol) exhibited high in vitro antibacterial activity, but none of them have been studied in animal models or humans. More effort should be dedicated to assessing the therapeutic value of cyclic NPs in the treatment of bacterial infections.
2.5.4. Glycosides
The formation of glycosides occurs through the process of glycosylation. Glycosylation begins with the synthesis of a nucleoside diphosphosugar. Uridine diphosphoglucose (UDP-glucose) is often used for this process. The biosynthesis of UDP occurs from glucose 1-phosphate, uridine triphosphate, and the enzyme UTP-glucose 1-phosphate uridylyltransferase. What follows is an SN2 attack of UDP-glucose producing the respective O-β-D-glucoside.191
Lunga et al.137 identified two bacterial growth inhibiting glycosides, pinnatoside A (167) and 3-O-β-D-glucopyranosyloxy-4-methyl-2(5H)-furanone (168). Compound 168 and pinnatoside A were isolated from the stems of Paullinia pinnata (Sapindaceae). P. pinnata has been traditionally used to treat a wide range of bacterial infections, malaria, and erectile dysfunction.137 Compound 168 was found to have high activity against E. aerogenes, P. aeruginosa, and K. pneumoniae, and moderate activity against S. enterica ser. Typhi.137 Pinnatoside A was found to have high activity against E. coli, E. aerogenes, K. pneumoniae, and P. aeruginosa, and moderate activity against S. enterica ser. Typhi.137
The two glycosides identified in our review showed a high in vitro antibacterial activity. However, the lack of in vivo and clinical studies calls for a better investigation of these compounds.
2.5.5. Fatty Acids
Fatty acids are a large and diverse group of organic compounds that are characterized by their solubility in organic solvents and lack of solubility in water. Many endogenous lipids are bioactive and play key roles in physiological processes such as inflammation439.
Dodec-9,11-diynoic acid (169), (12E)-heptadec-12-en-8,10-diynoic acid (170), and exocarpic acid (171), are all fatty acids obtained from Thesium chinense Turcz. (Santalaceae).141 Exocarpic acid demonstrates high activity against F. nucleatum and P. gingivalis, and moderate activity against S. mutans. Compounds 169 and 170 have moderate to high activity against F. nucleatum and P. gingivalis.
3-(dodecanoyloxy)-2-(isobutyryloxy)-4-methylpentanoic acid (172) is another fatty acid isolated from the aerial parts of Sigesbeckia glabrescens (Makino) Makino (Asteraceae); it has moderate to high activity against B. subtilis, E. faecalis, S. aureus, and S. pyogenes.142 It is a lauryl ester with a laurate group, which may account for its antibacterial activity. A study on lauric acid and monolaurin, which also contain a laurate group, also indicated significant antimicrobial activity, particularly against gram-positive bacteria.440
Of the ten fatty acids included in our review, four compounds (discussed above) showed a high in vitro antibacterial activity. To date, none of them have been studied in animals or humans, and further research is needed to assess the potential of fatty acids from plants as antibacterial agents.
2.5.6. Organosulfurs and Derivatives
Organosulfurs are organic compounds characterized by the presence of a sulfur atom. Some of the best-known organosulfurs are the essential amino acids cysteine and methionine, antibacterials such as penicillin, the sulfonamides, and the chemical warfare agent sulfur mustard.
Diallyl thiosulfinate, or allicin (174), is a sulfur-containing compound produced when Allium sativum L. (Amaryllidaceae), garlic, is cut or damaged.441 It contains both carbon and sulfur stereochemistry, although it occurs naturally as a racemate. Allicin is present in most Allium spp. It was first isolated in 1944 by Cavallito and Bailey and shown to be the major antibacterial compound in garlic.442 However, garlic has a long history of medicinal uses before 1944. The Ebers papyrus, dated from the reign of the Egyptian Pharaoh Amenhotep I, circa 1534 BCE, describes 32 illnesses that can be treated with garlic.443 Interestingly, even though allicin represents up to 70% of the thiosulfinates present; it is not present in raw garlic. Rather, after the tissue is injured, alliin is formed by the enzymatic hydrolysis of alliinase and then spontaneously condenses into allicin.441 Allicin has shown a wide range of biological effects, including antifungal,444 antiparasitic,445 and antiviral446 activities. It exhibits high antibacterial activity against Burkholderia cenocepacia, B. cepacia and B. pyrrocinia.144 Burkholderia spp. can cause bacteremia, especially in young children,447 and is one of the major bacteria involved in cystic fibrosis along with S. aureus and P. aeruginosa.448 Allicin shows synergistic and adjuvant activity with conventional antibiotics such as oxacillin and cefazolin.449 Additionally, allicin is stable in simulated intestinal fluid,450 but was shown to have a relatively short half-life in aqueous solution at room temperature.451 However, the biological half-life of allicin was longer than its chemical decomposition half-life; indicating that even the degradation products of allicin are biologically active.451 The MOA for allicin was studied in red blood cells and synthetic membranes and was found to easily diffuse through the membrane and does not cause cell leakage or membrane fusion.452 In E. coli, allicin reacts with intercellular glutathione, reducing the availability of this antioxidant, and reduces overall thiol-containing molecules and inhibits key enzymes of cell metabolism by reacting with cysteine residues. The culmination of these activities is cell death.453
In vivo efficacy of allicin was evaluated in a rabbit model of prosthetic joint infection caused by S. epidermidis. Allicin inhibited biofilm formation and enhanced the bactericidal effect of vancomycin in this model, suggesting that allicin in combination with vancomycin may be useful for the treatment of prosthetic joint infection.454 In another in vivo study using a short-time hernia-repair rabbit model infected by S. aureus, a polypropylene mesh pretreated with a combination of allicin and chlorhexidine did not improve bacterial clearance compared to meshes treated with chlorhexidine alone, and the authors suggested that allicin could interfere with the necessary inflammatory process.455 A clinical study evaluated the possible role of allicin in the eradication of H. pylori. Sixty patients with H. pylori positive biopsies, randomized to two groups, received a standard antibiotic course either alone or in combination with allicin at 4,200 µg/day for 14 days. This treatment led to the eradication of H. pylori in 20 patients (66.6%) in the first group and 27 patients in the second group (90%), indicating that allicin may be effective in the treatment of H. pylori.456 Major drawbacks of allicin include its poor solubility, its sensitivity towards heat and alkaline, and its pungent odor. Therefore, the development of synthetic analogues and new delivery systems have recently been the main research foci for allicin.457,458
Propyl-propane-thiosulfonate145 (175) is another organosulfur compound commonly found in the Amaryllidaceae family. This compound is commonly used as an additive in animal feed to improve livestock health in place of antibiotic growth promoters.459 Propyl-propane-thiosulfonate was isolated from Allium spp. and shows high activity against E. faecalis, MRSA and S. agalactiae.145
Of the ten organosulfurs and derivatives found in our review, five (10,11-erythro-xanthopappin D, 10,11-threo-xanthopappin D, allicin, benzyl isothiocyanate, propyl-propane-thiosulfonate) showed high in vitro antibacterial activity. Allicin is the only compound from this group that was tested in animal models and clinical trials for the treatment of bacterial infections. Despite its great potential as an antibacterial agent, some concerns were raised regarding its pharmacokinetic (poor oral bioavailability, volatility, instability), organoleptic (pungent odor), and pharmacodynamic (herb-drug interactions) properties.460–462 Still, allicin represents one of the most promising antibacterial leads from the organosulfurs and derivatives, and further research should focus on improving its physicochemical properties and evaluating its toxicological profile.
2.5.7. Peptides
Peptides are short chains of amino acids, typically under 40 amino acid residues in length, linked via a peptide bond. Peptide biosynthesis occurs at ribosomes, where mRNA is translated into its corresponding amino acid sequence before being post-translationally modified.
A phytochemical study by Daneshmand et al.147 isolated the antibacterial peptide snakin-Z (178) from the fruit of Ziziphus jujuba Mill. (Rhamnaceae). Snakin-Z is a defensin-like cationic peptide. Plant defensin peptides typically lack antibacterial activity,463 but snakin-Z had moderate activity against B. subtilis, S. aureus, E. coli, and K. pneumoniae.147 The MOA for snakin-Z is currently unknown.
Only one peptide has been included in our review, but it exhibited moderate antibacterial activity. Overall, antibacterial peptides from plants represent an understudied group, with a small number of individuals discovered so far.464 Because it has been suggested that bacterial resistance might not occur, antibacterial peptides represent an interesting group to explore, and more effort should be made to identify them in plants.465 However, some limitations should also be considered such as their susceptibility to proteolytic degradation affecting their oral administration, a lack of information regarding their potential toxicity, and the high cost of peptide development and manufacturing.466
2.5.8. Steroids
Steroids are a group of lipids distinguished from other lipids by their quatracyclic structure. The most common endogenous steroid is cholesterol, which is a precursor to many biomolecules, including Vitamin D, testosterone, and estrogen. Most lipid hormones are steroid hormones, which are usually ketones or alcohols.
β-stigmasterol93 (179) is a sterol with moderate activity against M. aurum and M. smegmatis. β-stigmasterol was isolated from the stem bark of Tetracera potatoria (Dilleniaceae) and shows potential for tuberculosis,93 urinary tract infection, and typhoid fever treatments.467 Stigmast-22-ene-3,6-dione148 (180) is a steroid that was isolated from Salvinia auriculata Aubl. (Salviniaceae) that is moderately active against S. aureus.
Stigmast-5-en-3β-ol-23-one135 (181) and stigmast-5-en-3β-ol-21(24)-olide135 (182) are moderately active against B. subtilis, E. coli, K. pneumoniae, P. aeruginosa, S. aureus, and S. epidermidis. Both were isolated from the root of Trichodesma indicum (Boraginaceae). Compound 182 is a lipophilic aliphatic ester that may be important in microbial membrane disruption.468
Eleven steroids were found in our review, among which four (i.e., polyphyllin G, stigmast-22-ene-3,6-dione, stigmast-5-en-3β-ol-21(24)-olide, stigmast-5-en-3β-ol-23-one) exhibited high antibacterial activity. None of them have been studied in animal models highlighting the need to better assess their role as antibacterial agents.
2.6. Summary of Antibacterial Compounds
MIC values of 459 compounds included in this review (reported in µg/mL), were classified into four main chemical classes (phenolic derivatives, terpenoids, alkaloids, and other metabolites) and selected for further analysis. This represents a total of 1,394 MIC values, including 887 MIC values for gram-positive bacteria, and 507 MIC values for gram-negative bacteria. Alkaloids, phenolic derivatives, terpenoids, and other metabolites showed overall mean MIC values of 20.8, 32.3, 36,6, and 34.4 µg/mL, respectively. Alkaloids had significantly better overall growth inhibitory activity (P < 0.01 or better) than each of the other chemical classes (Figure 5A). For gram-positive bacteria, alkaloids also had the lowest mean MIC, 19.7 µg/mL, compared to 29.7 (no significant difference), 35.0 (P < 0.001), and 35.5 µg/mL (P < 0.01) for phenolic derivatives, terpenoids and other metabolites, respectively (Figure 5B). For gram-negative bacteria, alkaloids again showed the lowest mean MIC value, 23.2 µg/mL, while other metabolites ranked second (33.4 µg/mL), phenolic derivatives ranked third (37.0 µg/mL), and terpenoids ranked fourth (40.0 µg/mL) (Figure 5C). However, significant differences in the superior activity of alkaloids for both gram-positive and -negative bacteria were only noted in comparison to terpenoids (P < 0.05).
Figure 5.
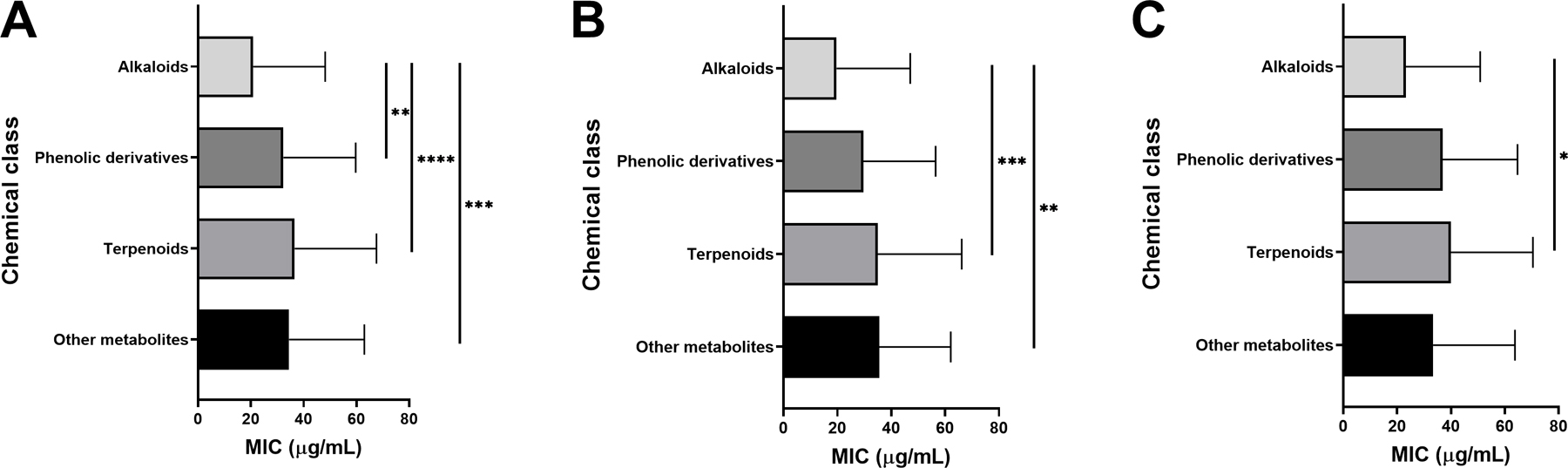
Mean and standard deviation of minimum inhibitory concentration (MIC) of compounds for each of the four chemical classes, with significant differences in MIC values (μg/mL) for A) all bacteria; B) gram-positive bacteria and C) gram-negative bacteria. P-values: *: P < 0.05, **: P < 0.01, *** P < 0.001, **** P < 0.0001.
Further examination of the MIC data was performed on the six most represented bacteria species (i.e. Staphylococcus aureus, Escherichia coli, drug-resistant strains of Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, and Klebsiella pneumoniae), but no major patterns emerged from this analysis. While not statistically significant, alkaloids had the lowest mean MIC against S. aureus, E. coli, and K. pneumoniae (Figure 6A, B and F). For drug-resistant strains of S. aureus, the phenolic derivatives have the lowest mean MIC (Figure 6C) and other metabolites for B. subtilis (Figure 6D).
Figure 6.
Minimum inhibitory concentration (MIC) of compounds reported by chemical class, with significant differences in MIC values for A) Staphylococcus aureus, B) Escherichia coli, C) all drug resistant strains of Staphylococcus aureus, D) Bacillus subtilis, E) Pseudomonas aeruginosa, and F) Klebsiella pneumoniae. No significant difference was observed between the chemical classes for each bacterium.
Another analysis was performed for the compounds with the lowest mean MICs tested against at least three different bacterial species. Overall, rhodomyrtone (108), allicin (174), and rhodomyrtosone B (109) had the lowest mean MICs with values of 0.56, 0.72, and 1.07 µg/mL, respectively. For gram-positive bacteria, rhodomyrtone (108), rhodomyrtosone B (109), and xanthoangelol (17) had the lowest mean MICs with values of 0.56, 1.07, and 1.63 µg/mL. For gram-negative bacteria, allicin (174), 2-phenylethanol (164), and pinnatoside A (167) had the lowest mean MICs with value of 0.72, 3.61 and 5.31 µg/mL. Further descriptive statistics on all compounds are reported in Table 3.
Table 3.
Descriptive statistics for the MIC data (µg/mL) on compounds discussed in this review with 3 or more MIC values reported.
| No. MIC Values | Mean MIC | Min MIC | Max MIC | Std. Deviation | |
|---|---|---|---|---|---|
| Alkaloids | |||||
| artabotrine (5) | 12 | 2.4 | 1.25 | 5 | 1.4 |
| liridine (6) | 9 | 2.8 | 0.625 | 10 | 3.0 |
| sanguinarine (7) | 10 | 6.0 | 0.5 | 16 | 6.0 |
| lysicamine (4) | 12 | 9.6 | 2.5 | 20 | 5.7 |
| berberine (8) | 3 | 12.2 | 1.56 | 31.25 | 16.5 |
| buesgenine (11) | 5 | 17.6 | 4 | 32 | 14.0 |
| palmatine (12) | 3 | 28.1 | 3.12 | 75 | 40.6 |
| 4-methylquinoline (1) | 5 | 52.5 | 12.5 | 100 | 35.8 |
| Phenolic derivatives | |||||
| rhodomyrtone (108) | 3 | 0.6 | 0.39 | 0.78 | 0.2 |
| rhodomyrtosone B (109) | 7 | 1.1 | 0.62 | 2.5 | 0.7 |
| xanthoangelol (17) | 3 | 1.6 | 1.2 | 2.5 | 0.8 |
| amorphastilbol (98) | 3 | 1.8 | 1.1 | 2.2 | 0.6 |
| rottlerin (112) | 5 | 2.6 | 1 | 4 | 1.3 |
| 2-geranyloxy-4,6-dihydroxybenzophenone (101) | 3 | 3.3 | 1.95 | 3.91 | 1.1 |
| dichamanetin (46) | 5 | 3.3 | 1 | 7.5 | 2.6 |
| amorfrutin B (106) | 3 | 3.4 | 2.6 | 5.1 | 1.4 |
| olympicin A (97) | 5 | 3.5 | 0.5 | 8 | 3.0 |
| 2-geranyl-5-(2-phenylethyl) resorcin (99) | 3 | 3.7 | 2.2 | 4.4 | 1.3 |
| (2R,3S,2’’S)- 3’’’,4’,4’’’,5,5’’,7,7’’-heptahydroxy-3,8”-biflavanone (49) | 3 | 4.7 | 2 | 8 | 3.1 |
| 3,4-dihydro-5-hydroxy-2,7dimethyl-8-(3”-methyl-2”-butenyl)-2-(4’-methyl-1’,3’-pentadienyl)-2H-1-benzopyran 6-carboxylic acid (103) | 4 | 5.0 | 4 | 8 | 2.0 |
| 2-geranyloxy-1-(2-methylbutanoyl) phloroglucinol (95) | 3 | 5.2 | 3.91 | 7.81 | 2.3 |
| 3-geranyl-1-(2-methylbutanoyl) phloroglucinol (96) | 3 | 5.2 | 3.91 | 7.81 | 2.3 |
| ferruginin A (71) | 5 | 5.6 | 4 | 8 | 2.2 |
| amorfrutin A (94) | 3 | 6.4 | 2.1 | 8.5 | 3.7 |
| 2-geranyloxy-1-(2-methylpropanoyl) phloroglucinol (92) | 3 | 6.5 | 3.91 | 7.81 | 2.3 |
| 3-geranyl-1-(2-methylpropanoyl) phloroglucinol (93) | 3 | 6.5 | 3.91 | 7.81 | 2.3 |
| lupinifolin (36) | 4 | 6.7 | 1 | 15.63 | 6.7 |
| 2-[(E)-styryl]-5-geranylresorcin-1-carboxylic acid (104) | 3 | 7.9 | 4.1 | 9.8 | 3.3 |
| luteolin-8-C-glucoside (44) | 3 | 8.0 | 4 | 16 | 6.9 |
| neobavaisoflavone (28) | 11 | 9.5 | 4 | 32 | 7.6 |
| elastiquinone (65) | 5 | 9.8 | 4.9 | 19.5 | 6.0 |
| 2,5-dihydroxy-3-heptyl-2,5-cyclohexadiene-1,4-dione (59) | 6 | 10.7 | 4 | 32 | 11.5 |
| neocyclomorusin (41) | 6 | 10.7 | 4 | 32 | 10.6 |
| isoquercetin (45) | 4 | 11.2 | 4.64 | 16 | 5.8 |
| plumbagin (57) | 10 | 11.3 | 1.56 | 64 | 19.4 |
| 4,5-(methylene-dioxy)-o-coumaroyl-4’-N-methylputrescine (89) | 4 | 12.0 | 8 | 16 | 4.6 |
| 4,5-(methylene-dioxy)-o-coumaroylputrescine (88) | 4 | 12.0 | 8 | 16 | 4.6 |
| amentoflavone (47) | 5 | 12.0 | 4 | 32 | 11.3 |
| thymoquinone (56) | 4 | 12.0 | 8 | 16 | 4.6 |
| chamanetin (35) | 5 | 13.5 | 7.5 | 15 | 3.4 |
| 6-prenylpinocembrin (29) | 5 | 13.6 | 4 | 32 | 11.2 |
| shancigusin B (107) | 3 | 14.0 | 3 | 26 | 11.5 |
| hydroquinone (83) | 8 | 15.6 | 12.5 | 25 | 5.8 |
| candidone (32) | 6 | 18.0 | 4 | 64 | 23.3 |
| emodin (63) | 4 | 18.0 | 4 | 32 | 11.5 |
| homoembelin (62) | 5 | 18.4 | 4 | 32 | 13.1 |
| 3′-demethoxy-6-O-demethylisoguaiacin (53) | 5 | 20.0 | 12.5 | 50 | 16.8 |
| 6-geranyl-5,7,3′-trihydroxy-4′-methoxyisoflavone (42) | 3 | 20.0 | 5.5 | 43.7 | 20.7 |
| chamuangone (111) | 6 | 22.1 | 7.8 | 31.2 | 10.4 |
| aloin A/B (69) | 20 | 22.8 | 10 | 50 | 13.7 |
| pseudarflavone A (27) | 5 | 24.8 | 4 | 64 | 24.4 |
| aloe-emodin (64) | 3 | 28.0 | 4 | 64 | 31.7 |
| 4-epi-larreatricin (54) | 3 | 29.2 | 12.5 | 50 | 19.1 |
| norcowanin (75) | 3 | 29.3 | 8 | 64 | 30.3 |
| 3′,4′,7-trihydroxyflavone (23) | 5 | 34.4 | 4 | 64 | 29.1 |
| brazilin (24) | 7 | 35.4 | 13.3 | 62.5 | 20.0 |
| 1,3,5,8-tetrahydroxy-2-(3-methylbut-2-enyl)-4-(3,7-dimethyloct-2,6-dienyl) xanthone (77) | 5 | 36.8 | 8 | 64 | 26.3 |
| curcumin (102) | 5 | 37.5 | 7.81 | 78 | 31.9 |
| dihydroguaiaretic acid (55) | 3 | 37.5 | 12.5 | 50 | 21.7 |
| apigenin | 10 | 38.1 | 15.62 | 64 | 20.1 |
| aloin-6’-O-acetate A/B (70) | 20 | 39.0 | 10 | 100 | 29.1 |
| ananixanthone (72) | 4 | 40.5 | 2 | 64 | 29.8 |
| cheffouxanthone (73) | 4 | 42.0 | 8 | 64 | 27.2 |
| ellagic acid | 7 | 44.2 | 16 | 64 | 19.6 |
| 4-prenyl-2-(3,7-dimethyl-2,6-octadienyl)-1,3,5,8-tetrahydroxyxanthone (76) | 5 | 52.8 | 8 | 64 | 25.0 |
| α-mangostin (74) | 4 | 53.0 | 4 | 100 | 54.3 |
| methyl gallate (87) | 4 | 57.2 | 7.8 | 93 | 35.7 |
| epigallocatechin gallate | 3 | 58.7 | 32 | 80 | 24.4 |
| quercetin (25) | 14 | 58.8 | 8 | 100 | 36.1 |
| silymarin | 3 | 60.0 | 60 | 60 | 0.0 |
| gallic acid | 5 | 100.0 | 100 | 100 | 0.0 |
| γ-mangostin | 3 | 100.0 | 100 | 100 | 0.0 |
| Terpenoids | |||||
| terpinen-4-ol | 3 | 2.1 | 1.562 | 3.125 | 0.9 |
| α-terpineol (119) | 11 | 6.4 | 0.78 | 55 | 16.1 |
| lanast-5-en-3β-D-glucopyranosyl-21 (24)-olide (153) | 6 | 6.8 | 2.4 | 9.6 | 3.2 |
| linalool (118) | 8 | 8.1 | 2 | 20 | 5.9 |
| 8,19-dihydroxyserrulat-14-ene (126) | 14 | 9.1 | 3.1 | 50 | 12.2 |
| α-pinene | 6 | 11.1 | 0.313 | 64 | 25.9 |
| carnosic acid (138) | 3 | 11.8 | 7.8 | 15.6 | 3.9 |
| (-) copalic acid (129) | 15 | 12.1 | 0.5 | 50 | 13.4 |
| lasiodin (141) | 5 | 14.4 | 8 | 16 | 3.6 |
| 3-O-β-D-glucuronopyranosyl-oleanolic acid (154) | 3 | 18.7 | 8 | 32 | 12.2 |
| kaurenoic acid (128) | 10 | 18.8 | 5 | 100 | 28.8 |
| ent-kaurenoic acid | 11 | 19.3 | 6.25 | 100 | 27.2 |
| oleanolic acid (148) | 4 | 19.5 | 4 | 50 | 20.9 |
| moronic acid (146) | 5 | 21.2 | 1.52 | 100 | 44.0 |
| 3-O-β-D-glucuronopyranosyloleanolic acid 28-O-β-D-glucopyranosyl ester (156) | 3 | 21.3 | 16 | 32 | 9.2 |
| ursolic acid (147) | 24 | 27.4 | 1 | 100 | 28.4 |
| abieta-7,9(11)-dien-13-β-ol (124) | 4 | 31.5 | 0.98 | 62.5 | 25.1 |
| bafoudiosbulbin C (140) | 5 | 32.0 | 8 | 64 | 29.4 |
| 8-hydroxyserrulat-14-en-19-oic acid (131) | 14 | 36.8 | 3.1 | 100 | 37.4 |
| dehydroabietic acid (125) | 17 | 37.1 | 6.2 | 100 | 33.1 |
| betulinic acid (149) | 4 | 39.5 | 15 | 64 | 28.3 |
| carvacrol (116) | 12 | 45.5 | 2.5 | 64 | 21.0 |
| 3-O-β-D-glucopyranosyl(1→6)-β-D-glucopyranosyloleanolic acid (155) | 5 | 47.5 | 6.25 | 100 | 48.5 |
| thymol (117) | 16 | 54.0 | 10 | 100 | 28.7 |
| andrographolide | 5 | 90.0 | 50 | 100 | 22.4 |
| Other metabolites | |||||
| allicin (174) | 9 | 0.7 | 0.5 | 1 | 0.3 |
| 2-phenylethanol (164) | 14 | 5.2 | 2.5 | 10 | 2.9 |
| pinnatoside A (167) | 5 | 5.3 | 1.562 | 12.5 | 4.4 |
| exocarpic acid (171) | 3 | 6.0 | 0.86 | 13.7 | 6.8 |
| 3-O-β-D-glucopyranosyloxy-4-methyl-2(5H)-furanone (168) | 5 | 7.5 | 1.562 | 25 | 10.0 |
| stigmast-5-en-3β-ol-21(24)-olide (182) | 6 | 9.6 | 4.8 | 19.2 | 5.3 |
| 10,11-erythro-xanthopappin D (176) | 3 | 11.8 | 7.25 | 15.5 | 4.2 |
| 3-(dodecanoyloxy)-2-(isobutyryloxy)-4-methylpentanoic acid (172) | 8 | 13.7 | 3.12 | 25 | 9.7 |
| ficusoside B (163) | 5 | 14.7 | 4.9 | 39.1 | 15.1 |
| propyl-propane-thiosulfonate (175) | 4 | 20.0 | 4 | 64 | 29.4 |
| snakin-Z (178) | 4 | 20.2 | 13.6 | 28.8 | 7.5 |
| stigmast-5-en-3β-ol-23-one (181) | 6 | 24.0 | 9.6 | 38.4 | 11.8 |
| n-dotriacont-9-one-13-ene (162) | 6 | 24.0 | 9.6 | 38.4 | 11.8 |
| (-)-cleistenolide (166) | 5 | 25.5 | 7.5 | 30 | 10.1 |
| benzyl isothiocyanate (173) | 6 | 31.7 | 10 | 40 | 13.3 |
| polyphyllin G (183) | 9 | 41.3 | 6.2 | 78 | 27.6 |
| 10,11-threo-xanthopappin D (177) | 3 | 44.1 | 7.25 | 62.5 | 31.9 |
Bacterial genera can be divided into three clusters based on the number of compounds that have been used to target them: (1) Staphylococcus was targeted by the most compounds by far, 289, (2) Bacillus and Escherichia were targeted by 110 and 112 compounds, respectively and (3) Mycobacterium, Streptococcus, Enterococcus, Pseudomonas and Klebsiella were targeted by 59–67 compounds each. All other bacterial genera were targeted by 45 compounds or fewer (Figure 7A). Of the 289 compounds used to target Staphylococcus species, 281 were specifically active against either antibiotic susceptible or resistant strains of S. aureus (Figure 7B).
Figure 7.
The most targeted A) bacterial genera and B) bacterial species by antibacterial plant compounds.
Analyzing the bacterial species on the number of compounds screened against them: antibiotic susceptible strains of Staphylococcus aureus was targeted by the most compounds, 242, while MDR strains of S. aureus were screened against 100 compounds, and Escherichia coli was targeted by 113 compounds. All other bacterial species were targeted by 86 compounds or fewer (Figure 7B).
Based on this analysis, alkaloids stand out as the most promising botanical NP antibacterial candidate based on the mean MIC value, especially considering they represent only 6% of all the compounds in this review and showed the lowest average MIC values against both gram-positive and gram-negative bacteria. It must be noted that if the total number of compounds with high antibacterial activity is considered, rather than the mean MIC values, the phenolic derivatives are more promising lead molecules. However, this is heavily biased, since phenolic derivatives represent 47% of the compounds screened. Lastly, since Staphylococcus species are so heavily represented as a test organism, any generalization about potential compound class activity relationships will be influenced by this single genus representing 62% of the reported antibacterial testing data.
3. Emerging Trends
In the area of plant NP drug discovery, much progress is being made in the generation and analysis of large chemical datasets. In parallel, our understanding of antibacterial activity is being broadened by assays for bacterial virulence inhibition and the study of synergy and other interactions between plant NPs. Together, these increasingly sophisticated chemical and pharmacological approaches are beginning to open up the complexities of ethnobotanical antimicrobials to useful scientific investigation. Perhaps more than any other space in drug development, antibiotic development is in dire need of innovation in order to reinvigorate a waning pipeline. Small molecules remain the primary focus of drug discovery for antibiotics. While it is essential to consider innovations beyond this realm, such as bacteriophage therapy and fecal microbiota transplant, we have focused on innovations connected to small molecule discovery and development for this review.
Ethnobotany is uniquely positioned to fill in a portion of the preclinical antibiotic innovation gap thanks to both: (1) its utilization of established traditional medicinal knowledge to hone in on promising plants and to (2) the unique and promising region of the biologically relevant chemical space occupied by plant NPs. At the same time, it must be noted that most studies cited in this review tend to use similar experimental approaches depending on whether extracts or purified compounds are under investigation. These approaches include classical bioassay-guided fractionation and structure elucidation by NMR after compound isolation and purification. In this section, we outline some emerging trends in drug discovery that are particularly well-suited for adoption into ethnobotanical approaches to antibiotic drug discovery. As the technological capacity of laboratories across the world grows, many of these trends will be adopted extensively and contribute to a streamlining of laboratory workflows.
3.1. Chemical Innovations
Plant NPs occupy a vast and largely untapped portion of the biologically relevant chemical space. Many chemical innovations for drug discovery can be explicitly applied to ethnobotanical drug discovery of antibiotics in order to advance the field.
3.1.1. Machine Learning
Machine learning (ML), a subset of artificial intelligence (AI), can be used to streamline decision-making by utilizing abundant, high-quality data to answer well-specified questions.469 Very recently, ML has been successfully utilized for the identification of a structurally divergent antibiotic lead, halicin, which has demonstrated potent broad-spectrum antibiotic activity in vitro and in mouse models of infection. The investigators first trained a directed-message passing deep neural network model on a group of 2,335 compounds binarized as hit or non-hit for E. coli growth inhibition. The investigators used the in silico model to screen >107 million compounds, and of the top hits, 99 were obtained and screened in vitro by the investigators, whose workflow ultimately yielded halicin.470
In this breakthrough example, ML was utilized in the drug screening stage to identify a small group of compounds with a high probability of antibiotic activity against E. coli. ML can be applied at all stages of drug discovery and can be based on any of a wide variety of data such as images, text, chemical structures, spectroscopic data, and omics data.469 AI has only in the last few years begun to see a practical application in drug discovery, much of this progress is due to advances in ML algorithms such as deep learning.470 The hope in the pharmaceutical industry is that the application of ML will increase drug development success rates and lower costs, and it is being utilized by most large biopharma companies.469,471
Academic research labs are also increasingly applying ML to drug discovery efforts, with several studies having been done on plant NPs specifically. A recent study reported the success of a deep recurrent neural network model in de novo molecule generation of new chemical entities that modulate the retinoid X receptor (RXR).472 This deep learning model was trained on the SMILES strings of a set 541,555 bioactive molecules from ChEMBL; by transfer learning, this model could generate new molecular structures based on template compounds. The investigators selected six known plant NP RXR ligands, and of the 1,000 SMILES strings generated, 201 were predicted as RXR agonists by self-organizing map-based prediction of drug equivalence relationships (SPiDER) target prediction software.473 Four compounds were chosen for synthesis and characterization in vitro for modulatory activity on the three RXR subtypes. Of the four, one demonstrated full agonistic activity on RXRα and RXRβ with low micromolar EC50 values. This early success, as well as the success of halicin should not, however, draw attention away from the progress yet to be made with ML. A recent review paper, for example, recommends the use of AI to correlate natural product 2D structure with biological function.474 The ability to predict biological function of a NP would greatly streamline bioassay-guided fractionation efforts, but as of now, only a foundation has been laid for the pursuit of this goal. Furthermore, not all AI approaches have enjoyed market success. For example, due to poor financial returns, IBM is stopping development and sales of its AI technology Watson for Drug Discovery, though Pfizer continues to use it for hypothesis generation.475
ML will occupy a growing role in plant NP antibiotic development as it possesses the potential to aid the emerging trends below in answering important questions. For a more in-depth discussion of how ML can be applied to drug discovery and development, refer to the review by Vamathevan et al.469
3.1.2. Molecular Networking and Dereplication
Since its introduction in 2012, molecular networking (MN) has emerged as a revolutionary tool in natural product isolation, streamlining the process immensely.476 This dereplication technique allows for rapid identification of compounds in complex mixtures by visualizing and organizing tandem MS/MS data sets and annotating them based on data sets of known compounds. As such, MN-based dereplication relies on the quality and availability of MS/MS data, for which the world’s largest repository and data analysis tool is Global Natural Products Social Molecular Networking (GNPS).477,478 Two ways to improve dereplication when relevant MS/MS data is unavailable are via in-house experimental MS/MS data and via in silico MS/MS data.476 The idea behind the former is that using natural product samples enriched for a particular chemical class generated in one’s lab, one can generate an MS/MS database on which a new dataset can be annotated for the identification of chemicals of that class and their derivatives. This strategy was pioneered by Fox Ramos et al. 479 and was used to isolate three new and three known monoterpene indole alkaloids from the bark of Geissospermum laeve (Vell.) Miers (Apocynacaeae) as well as dereplicate five known compounds previously undescribed in the genus. As for using in silico MS/MS data, the goal is to generate new databases to annotate on by using ML to generate MS/MS data of known compounds that lack experimental MS/MS data. The first in silico databases (ISDBs) were created by Allard et al. by using the ML-based tool CFM-ID v. 1.10 to generate MS/MS data for >220,000 compounds in the Dictionary of Natural Products and >170,000 compounds in the Universal Natural Products Database.480–484 Several studies have since used these ISDBs for successful targeted plant natural product isolations.485,486
MN allows other layers of information besides MS/MS to be mapped over networks, including analytical, taxonomical and biological details and this has been increasingly leveraged to inform NP prioritization.487–489 For example, one investigation by Olivon et al.488 used MN embedding both bioactivity and taxonomic data to identify potentially bioactive scaffolds from 292 Euphorbiaceae extracts from New Caledonia. The collection was screened against the oncogenic Wnt signaling pathway and chikungunya virus (CHIKV) replication. Crude extract activity levels in each assay were assigned a unique color tag and applied to the MN; as such, clusters of potentially bioactive metabolites became easily visualized. To better narrow down potential bioactive candidates and exclude ubiquitous compounds, extracts’ genus/species and plant part were also assigned a unique color tag which was applied to the MN. This way, four bioactive clusters related to the leaves of Bocquillonia nervosa Airy Shaw (Euphorbiaceae) were identified as being associated with high efficacy in both bioassays. Ultimately, the investigators’ workflow led to the isolation of a daphnane diterpene orthoester and four 12-deoxyphorbols with potent bioactivities. For an expanded discussion on natural product MN strategies and natural product prioritization, refer to reviews by Fox Ramos et al. and Wolfender et al.476,490
A tool of great interest that works well with dereplication protocols is the liquid micro junction surface sampling probe (LMJSS), which enables the in situ analysis of metabolites from a biological sample.491 The LMJSS works by depositing a solvent droplet on a sample such as a leaf; this droplet extracts the metabolites from the area it covers, and the microextract is injected into LC-MS. There are, in fact, many iterations of mass spectrometry imaging (MSI) that allow for such in situ analyses, such as DESI-MSI (desorption electrospray ionization), MALDI-MS (matrix assisted laser desorption ionization) and LAESI-MS (laser ablation electrospray ionization).492–495 The LMJSS distinguishes itself in that it uses chromatography while the others do not; it requires no sample preparation (as does LAESI), can sample any surface, and does not damage the sample. Additionally, the droplet probe can perform repeated sampling to concentrate microextractions by retaining a droplet on the top of the syringe. It cannot, however, take spatial measurements at high resolution due to the droplet size and so the other three techniques are superior at imaging. The droplet probe has been used to rapidly identify and differentiate acetogenin analogues and even isomers from the various organs of Asimina triloba (L.) Dunal (Annonaceae) through coupling to UPLC-PDA-HRMS/MS.496 In a later study also by the Oberlies group, the investigators successfully identified cytotoxic prenylated xanthones from an herbarium voucher specimen of Garcinia mangostana (Clusiaceae) without damaging the specimen.497 Although MS/MS data acquired from droplet probe experiments have yet to be used for prioritization of natural product isolation via MN, the possibility now exists of doing so without performing bulk extractions.
Although molecular networking is typically thought of as utilizing MS data, NMR spectroscopy is the other major analytical platform that yields highly specific fingerprints for a given compound.498 A new tool has been developed for molecular networking with NMR data, Metabolomics And Dereplication By Two-dimensional Experiments (MADByTE).499 MADByTE utilizes HSQC and TOCSY data to construct spin systems of a compound. Spin systems represent particular fragments of a compound; while MS/MS fragments also represent fragments, the two concepts are different. The spin systems are treated as features, and a sample is a collection of its features. If a compound is present in two samples, those samples will share features that correspond to it. From spin system features, MADByTE can generate a number of association networks and then output them as a graphML file for visualization in Cytoscape or Gephi. MADByTE is also capable of mapping bioactivity metadata over networks to visualize bioactivity profiles for sample prioritization. For MN, MADByTE does not use a centralized database of NMR spectra; rather, investigators can query against in-house experimental data.500 Other functions are currently being developed, including integration of MS data and the utilization of in silco databases for tentative structure elucidation.499
3.1.3. Metabolite Generation
The ability to efficiently produce desired natural products is of great importance to drug discovery. Typically, such production is done in bioreactors by reconstituting biosynthetic machinery in species such as E. coli and Saccharomyces cerevisiae. A challenge in this process, however, is that many biosynthetic pathways are not fully characterized. Genome mining is a strategy by which biosynthetic pathways are computationally predicted and prioritized for further studies such as natural product isolation.501 A number of bioinformatics tools exist for the identification of natural product biosynthetic pathways from sequenced DNA.502 Two examples are antiSMASH,503 a comprehensive pipeline used for bacterial and fungal genomes and plantiSMASH,504 an antiSMASH derivative that includes plant-specific cluster detection rules, co-expression analysis, and comparative genomic analysis. It is worth expanding on the latter two analyses. Co-expression analysis uses genes of known biosynthetic enzymes as bait and ranks other genes by correlation coefficient to the bait to identify candidate genes of biosynthetic enzymes. Rajniak et al.505 successfully used a combination of untargeted metabolomics and co-expression analysis to identify 4-hydroxyindole-3carbonyl nitrile (4-OH-ICN), a previously unknown Arabidopsis thaliana (L.) Heynh. (Brassicaceae) cyanogenic metabolite and elucidate its biosynthesis by using the CYP82C2 gene as bait. Comparative genomic analysis, on the other hand, uses phylogenetic profiling to find co-occurrence across genomes. CLIME (clustering by inferred models of evolution) is an algorithm that performs this analysis and it has been used to predict new members of a number of biosynthetic pathway based on shared inferred ancestry.506
While missing enzyme identification can be performed within the native species, similar enzymes in other organisms can be found via genome mining to construct an artificial pathway. Biosynthetic enzyme discovery studies often utilize genomic databases such as the Kyoto Encyclopedia of Genes and Genomes (KEGG) and the Medicinal Plant Genome Project, as well as transcriptomic databases such as the 1000 Plants (1KP) project.507–509 Luo et al.510 used the Medicinal Plant Genomics Resource, among other tools, to select six Cannabis (Cannabaceae) enzymes with potential geranylpyrophosphate:olivetolate geranyltransferase (GOT) activity, necessary for the biosynthesis of a precursor to several cannabinoids. Ultimately, the investigators constructed the complete biosynthesis of several major cannabinoids from galactose in S. cerevisiae.510,511 For a thorough discussion on computational tools for discovering and engineering natural product biosynthetic pathways, refer to the review by Ren et al.502
The ability to generate NP derivatives has also been an emerging trend in the field of plant NPs. After identifying and engineering a suitable host organism and planning and engineering a metabolic pathway, many routes are possible to generating novel NPs; these steps are elaborated on in a review by Cravens et al.512 Unnatural substrates can be fed to engineered microorganisms when the relevant biosynthetic enzymes are able to accommodate these structures for reactions.513–515 Additionally, biosynthetic enzymes can be replaced, added, or removed from a pathway to alter metabolite production.516–518 Finally, novel enzymes can be incorporated into a biosynthetic pathway, with protein engineering likely needed to facilitate substrate accommodation.519–521 While few studies have actually performed these feats, advancements in metabolic and protein engineering, discussed in dedicated review papers, will greatly enable the further pursuit of these strategies.512,522–525
Another system that can be thought of as a bioreactor for producing desired plant NPs are plants themselves. Cyber-agriculture is the growth of plants in contained environments under artificial climate control.526 It has been developed to address challenges in agriculture, including optimizing the flavor and nutrient content of edible plants and reducing waste and costs. A proof-of-principle study has shown that by combining cyber-agriculture with metabolomics phenotyping, or chemotyping and predictive ML, the flavor profile of common sweet basil (Ocimum basilicum var “Sweet”, Lamiaceae) could be optimized.527 Johnson et al. grew basil plants in a Food Server, which is a large, multi-tray, multi-rack hydroponic configuration of the Food Computer the size of a shipping crate. By experimenting with different growth conditions, or climate recipes, a condition was identified in which the production of volatile flavor-active compounds was increased. Possible future uses for cyber-agriculture include experimentation on different plants and the analysis of different conditions such as stressors to modulate the production of compounds of interest. This strategy will use ML to determine optimal recipes to contribute to the emerging field of ethnophytotechnology.528
3.1.4. Endophytic Fungi
The ability of endophytes to alter and contribute to their host plant’s chemistry is well known, though the intricacies of this phenomenon remained poorly understood until recently.529 Endophytes are endosymbiotic microorganisms that reside in internal plant tissues beneath the epidermal cell layer without causing disease.530,531 They have been shown to enhance host drought tolerance, growth, nutrient gain, and resilience to stressors and pests.532,533 Because of the chemistry they confer to plants, endophytes, mainly fungal and bacterial, have long been of great interest to drug discovery.534,535 Some fungal endophytes have even been shown to produce the same bioactive compounds as their hosts, which called more into question the extent to which plants alone are the sources of some botanical compounds.536–538 Other studies have demonstrated that the presence of endophytes in plants does, in fact, alter the in vitro bioactivity of their extracts.539,540 More recent ecological studies have explored in greater depth how endophytes interact with plants and play roles in their physiology. Khare et al. 541 discuss endophytism and the latest insights in the field. While much progress has been made in understanding the biology endophytes, Khare et al.541 call for the exploration of the vast majority of endophytes that remain untouched, deduction of the biochemistry and physiology of endophytes up to genomic and metabolomics levels and the creation of a database for endophytic microorganisms and their metabolites.
A recent review by Martiez-Klimova et al.542 covers antimicrobials isolated from endophytes between 2006–2016. Indeed, endophytes have evolved mechanisms to compete with other microbes inside plants and, as such, represent promising sources of antimicrobial compounds. Recent studies have capitalized on progress in the field and have discovered antibiotic compounds from endophytes. For example, Ibrahim et al. 543 describe their collections from wild and highbush blueberry plants of the novel endophytic fungus Xylaria ellisii, the genus of which is unique in its diverse production of secondary metabolites. By dereplicating extracts against the Dictionary of Natural Products, Antibase, and NORINE and performing comparative analysis against known fungal metabolites, 11 previously reported compounds were identified. Following this, eight new proline-containing cyclic nonribosomal pentapeptides were identified named ellisiiamides A–H; ellisiiamide A was active against E. coli (MIC = 100 µg/mL), a first report for this scaffold. Further investigation of plant endophytes, and especially those plants identified through the ethnobotanical approach as being used in traditional medicine for infections, holds great potential for future antibiotic discovery.
3.1.5. Structure Elucidation
The field of anisotropic NMR has recently seen significant advancements that greatly improve its accessibility to natural product chemists. Residual dipolar couplings (RDCs) and residual chemical shift anisotropies (RCSAs) are examples of anisotropic NMR data, the former reporting the relative angles between internuclear vectors, usually C–H bonds and the latter reporting the relative orientations of carbon chemical shift tensors.544–549 As such, these two anisotropic techniques complement each other and are particularly useful for structure elucidation in proton-deficient organic molecules. They have been used to unequivocally determine the 3D configurations of several complex natural products, the structures of which having previously been questioned.550 To obtain anisotropic NMR data, an anisotropic sample environment is needed, and this can be achieved by an alignment medium such as compressed or stretched polymeric gels. An anisotropic environment precludes fully random molecular rotation, which renders dipolar coupling (DC) and chemical shift anisotropy (CSA) unobservable; consequently, residual DC and CSA are observable. RDCs and RCSAs are measurable by eliminating the isotopic contribution to the interactions via a control under an alternative alignment. In a recent publication, Liu et al.551 describe the 2–3 day synthesis of two such gels and the sample setup as well as the 0.5–4 day experiments. Their results make the utilization of anisotropic NMR more accessible than ever to non-experts and hence more available for addressing complex structural assignment questions. Anisotropic NMR data can especially be used to aid in the structure elucidation of complex natural products by Computer-Assisted Structure Elucidation (CASE), which has less success determining the structures of proton-poor compounds.
CASE programs reduce the rate of error in natural product structure elucidation by generating all possible structures that agree with the input data, usually 2D COSY and HMBC data, and ranking them by probability.552 In the case that a CASE program generates multiple probable structures, further experimentation is done to select between the alternatives.553 To read about new developments in CASE methodology and future directions in the field, refer to the recent review by Burns et al.554 Most CASE programs do not solve for absolute configuration, but only for planar structures. As mentioned above, by combining RDC and RCSA data 3D structures can be unequivocally determined for complex natural products.550 To make use of these anisotropic NMR data, computational chemistry methods such as density functional theory (DFT) are used to generate 3D conformers based on probable 2D constitutions and 3D configurations. This strategy has already been implemented by the development of StereoFitter by Mestrelab, the first to our knowledge to take advantage of anisotropic NMR data to introduce 3D conformational and configurational analysis into CASE.555 Currently, StereoFitter accepts RDC, Nuclear Overhauser Effect (NOE), J-coupling, and chemical shift data to calculate 3D structure. Structure elucidation is often the rate-limiting step in natural products research.556 The ability to rapidly determine the 3D structure of a compound computationally based on NMR data promises to further streamline natural product drug discovery efforts.
A key emerging trend in structure elucidation is the continuation of the NMR Raw Data Initiative, begun in 2016, which aims to address the urgent need for public availability of raw NMR data.557 For further discussion on this need, the layers of the rationale behind it and action items for implementation, refer to the review by McAlpine et al.558 In brief, raw NMR data is necessary for: structure revisions, impurity detection and quantification, dereplication, enabling new methodologies such as the new analysis of published data by optimal processing of the free induction decay (FID), assigning signals from other nuclei such as 19F, building data repositories for purposes such as dereplication (via MADByTE, for example) and clinical applications such as magnetic resonance spectroscopy (MRS). While McAlpine et al. 558 outline several action items, the ultimate action item is the creation of a global and unified repository for raw NMR data.
Another emerging trend is the increased use of the cryo-electron microscopy (cryo-EM) technique microcrystal electron diffraction (microED), which has proven to be of astounding utility for the structure elucidation of small molecules, including NPs.559 With microED, the structure of a small molecule in nanocrystal form on an EM grid can be solved. A nanocrystal is a crystal thousands of times smaller and much easier to produce than the crystals X-ray diffraction requires, and nanocrystals often spontaneously occur in simple powders. Two breakthrough studies published back-to-back in late 2018 demonstrate that microED can in minutes obtain structures with atomic resolution, in many cases better than 1 Å, from pure powder samples, pure rotary evaporated samples and even heterogeneous mixtures by selecting single crystals off of the EM grid.560,561
3.2. Pharmacological Innovations
A number of advancing areas in pharmacology stand to guide plant NP antibiotic drug discovery. Given the complex formulations and diverse applications of plant-based anti-infectives in traditional medicine, knowledge of the interactions between compounds and consideration of a variety of biological mechanisms are necessary for a full understanding of the potential of ethnobotanical antibacterials.
3.2.1. Drug Delivery Systems
Innovative drug delivery systems are a developing area of research for antibacterial agents, including compounds derived from plants. The purpose of drug delivery systems is to lower the dose of a drug required for a therapeutic effect and to localize the drug to the site of action, minimizing side effects. In the case of wounds and other dermatological conditions, topical treatment is often used, allowing for localization of a drug and decreased risk of systemic toxicity.562,563 This approach is consistent with the historical medical tradition of using balms and poultices, often botanical.564 An emerging technology in topical treatments is the hydrogel, a moist polymeric mesh that provides a favorable environment for wound healing and can be impregnated with antibiotics.565 For systemic use of antibacterials, a variety of nanoscale systems are being investigated as delivery mechanisms.566 For example, the antibacterial flavone apigenin was found to have lowered MICs against both gram-positive and gram-negative bacteria when encapsulated in a liposome that allowed it to more easily enter cells.567 Beyond delivering a drug to the site of action, drug delivery systems can be used to facilitate drug administration. One example is microneedle patches, which have been developed to address the need for administering drugs with the ease of oral administration and the delivery efficacy of an injection.568 The continued development of precise drug delivery systems is crucial to the safe and effective use of plant-derived antibacterials.470
3.2.2. Synergy
The study of synergy and other interactions between plant compounds is also a growing research area in the field of ethnobotanical drug discovery.569,570 The evolutionary ecology of plants lends itself to the production of compounds that interact synergistically on bacterial targets; as sessile organisms, plants produce mixtures of secondary metabolites for defense and these metabolites often interact to produce a more potent effect.571
The main limitation in studying synergy is the inherent complexity that comes from studying two compounds together rather than one,16 a complexity that multiplies with the addition of more elements and is particularly daunting in the case of plant extracts, which can contain hundreds or thousands of potentially interacting compounds. Ethnobotanical drug discovery poses an even greater challenge, as combinations of diverse plant species are common among traditional formulations.569 Currently, there are very few methods developed for studying higher-order interactions, or interactions between more than two agents.572 Metabolomics approaches have recently arisen to systematically assess synergy in plant extracts, but with limitations on the complexity of the interactions that can be studied.16,573 Omics approaches may, therefore, be particularly useful in the search for compounds that synergize with existing antibiotics.
3.2.3. Alternative Microbial Targets
Much of the research in plant-derived antibacterials is spurred by the rise of resistance to existing antibiotics.574 Investigation of plant extracts may result in the discovery of antibiotics with novel mechanisms of action.575 Alternatively, existing antibiotics may be modified to bypass certain types of resistance; approaches for developing natural product antibiotics that are effective against resistant bacteria are discussed in a review by Rossiter et al.23
Given the threat of antibiotic resistance, a growing amount of research is focused on the development of drugs that can combat bacterial virulence by mechanisms such as biofilm or quorum sensing inhibition, resulting in less evolutionary pressure for resistance and less harm to the commensal microbiome.21 The role of plant NPs in these alternative antibacterial pathways is covered in a review by Silva et al.22
4. Conclusions and Future Perspectives
The antibacterial compounds reported herein represent dozens of unique scaffolds with abundant possibilities for derivatization for lead optimization. For antibacterial drug development, this is particularly true for some of the compounds with single-digit µg/mL MIC values and lower. Plant NPs that are poor candidates for lead optimization include those such as citronellol; they demonstrate potent activity in one or more bioassays but are likely not selective drugs due to their small molecular weight and a higher proportion of freely rotating bonds.576 The MIC values of NPs listed herein are predominantly reported for only one or a few bacterial strains and often without toxicological data against mammalian cells. Therefore, establishing the selectivity of these NPs remains a crucial next step for preliminary justification for further drug development.
There are other aspects of the current state of antibacterial discovery from plants that speak to its still being in its early stages, one being that plant NPs are still largely unexplored. There are approximately 374,000 species of plants in the world today,14 and 28,187 of them are estimated to be used by humans for medicine.19 The most common types of bioassays employed in laboratory studies of plant NPs are those that explore inhibition of bacterial growth, and the compounds reported for antibacterial activity herein were isolated from 183 plant species and 73 families. This reflects the minuscule proportion of plants that were studied at all for antibacterial activity and highlights the need for increased research volume and rigor, both of which can be aided with the adoption of new technologies emerging in the field.
Of the 459 compounds with reported antibacterial activity, phenolic derivatives (mainly flavonoids) represented about half of them, while terpenoids represented roughly one-quarter. Interestingly, Silva et al. 56 found antivirulence activity against bacteria to be similarly distributed among plant natural product classes. In their study, they found that phenolics made up 59% of the total antivirulence compounds reported, among which flavonoids were the most represented (~50%). In a review of plant NPs used as adjuvants to antibacterial drugs, Zacchino et al.577 showed that polyphenols were the most reported chemical class. The predominance of phenolics among antibacterial compounds from plants can be attributed to the unique features of this chemical class. Indeed, the number and positions of hydroxyl groups attached to the aromatic ring have been reported to be important for the antibacterial activity.578 Cushnie and Lamb579 reported that the antibacterial activity of flavonoids, in particular, could be mainly attributed to six structures out of 14: the flavones, chalcones, flavonols, flavan-3-ols, flavanones, and flavolans. Plants have developed the production of flavonoids for preventing much of the intercellular damage caused by free radicals and reactive oxygen species.210 Flavonoids are readily oxidized by reactive species, consequently quenching them;580 as such, many plant flavonoids are intrinsically reactive. Plant phenolic compounds also exhibit a wide range of MOAs, including cytoplasmic membrane damage, inhibition of nucleic acid synthesis, inhibition of energy metabolism, inhibition of cell wall synthesis, and efflux pump inhibition.578,579
In comparison to the other chemical classes, alkaloids demonstrated the lowest mean MIC value against reported bacteria. Alkaloid distribution is restricted in plants, with only 300 families producing these compounds.581 They are also known to be highly toxic in animals and to possess allelopathic effects on plants.582 A number of antibacterial drugs are alkaloids, including the antituberculosis medicine bedaquiline with its quinoline scaffold and the synthetic quinolones derived from quinine.581 Many alkaloids also fall well within the parameters for being considered drug-like by Lipinksi’s Rule of Five, and they have more skeletal structural and functional group diversity than other chemical classes.583 Cordell et al.583 noted that only 702 out of 21,120 known alkaloids have been evaluated in more than five bioassays and that many new alkaloid skeletons could be discovered from plant families that are already studied for alkaloids. Alkaloids thus represent a promising source of antibacterial compounds, and further research should be performed while considering their potential toxicity at an early stage of the drug discovery process.
Several chemical scaffolds are shared by a number of antibacterial plant natural products, and the relationship between their structures and reported MIC values makes clear the promising utility of more extensive SAR studies on lead optimization. For example, 2-hydroxy-anthraquinone has a structure very similar to that of digiferruginol: the latter has a methoxyl group instead of the hydroxyl group and an additional hydroxyl group on the same ring. Digiferruginol, reported in the same study as 2-hydroxy-anthraquinone, is approximately twice as potent at inhibiting B. subtilis growth. While modification of plant NPs by medicinal chemistry can yield derivatives with improved potency, it is also critical to assess subsequent changes in pharmacokinetics and toxicity. Such studies are largely yet to be undertaken in the field, and it is likely that they will be the next research frontier that plant NP chemists engage in order to bring candidate compounds through lead optimization. Derivatization is required in any case for patenting of NPs in the United States.576
Although many plant NPs present promising antibacterial activities, some also have drawbacks that limit their therapeutic use. For instance, many are poorly bioavailable when administered as single compound therapies. This is the case for a large portion of the most investigated plant compounds, such as allicin, berberine, curcumin, emodin, linalool, oleanolic acid, quercetin, and thymol. Other disadvantages associated with plant-derived compounds include a high volatility (e.g., linalool), low chemical stability (e.g., quercetin), pungent odor (e.g., allicin) and toxicity (e.g., sanguinarine). One solution is to improve their physicochemical properties through structural modifications; another is to load them onto drug delivery systems. The development of analogues by medicinal chemistry is key, particularly for performing SAR studies.
Despite NPs commonly violating Lipinski’s rules584 and often not meeting the solubility and permeability desired for druggable compounds, they occupy a vast area of chemical space that is unexplored, even in comparison to bioactive medicinal chemistry compounds.585 Therefore, NPs represent a large reservoir of understudied compounds with novel chemistry, from biological systems, to explore in the search for novel pharmaceuticals.
It is important to note that unlike the random sampling approaches common to the broader field of NP research (e.g., mining soil, water, terrestrial and marine organisms for novel chemical scaffolds), the ethnobotanical approach to drug discovery for infection control is uniquely targeted,586 relying on human knowledge and practice in the use of plant resources to treat infections, sometimes over periods spanning centuries or even millennia. Importantly, human interactions with nature can vary greatly between different cultural groups—even for communities living in the same environment with access to the same natural resources.587 Thus, further primary ethnobotanical field studies are also necessary to capture the full scope of human knowledge pertaining to medicinal plants across different environmental and cultural contexts. Lastly, the application of emerging technologies in drug discovery from plant NPs is likely to prove incredibly useful to fueling the antibiotic discovery pipeline in the future.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Disease (R21 AI136563 and R21 AT011105 to C.L.Q.), Emory University development funds to C.L.Q., and a graduate student fellowship from The Jones Center at Ichuaway to L.M. We thank Sarah Hanson, Apple Liu, Leah Scott, Emily Edwards, Kat Bagger, and Courtney Andrews for their assistance in the literature search.
List of Abbreviations
- 1KP
1000 Plants (initiative for plant gene sequencing)
- AADC
Aromatic Amino Acid Decarboxylases
- ABC transporters
ATP-binding cassette transporters
- AI
Artificial Intelligence
- AMR
Antimicrobial Resistance
- AntiSMASH
Platform for rapid genome-wide identification, annotation and analysis of secondary metabolite biosynthesis gene clusters
- ATP
Adenosine Triphosphate
- BCE
Before the Common Era
- BeWo
Human choriocarcinoma cell line
- Caco-2
Human epithelial colorectal adenocarcinoma cell line
- CASE
Computer-Assisted Structure Elucidation
- CDC
Centers for Disease Control
- CFM-ID
Competitive Fragmentation Modeling-ID
- ChEMBL
Curated chemical database of bioactive molecules with drug-like properties
- CHIKV
Chikungunya Virus
- CLIME
Clustering by Inferred Models of Evolution
- CoA
Coenzyme A
- COSY
Correlated Spectroscopy
- CoAt-Mt
coenzyme A transferase of M. tuberculosis
- Cryo-EM
Cryo-electron Microscopy
- CSA
Chemical Shift Anisotropy
- DC
Dipolar Coupling
- DESI
Desorption Electrospray Ionization
- DFT
Density Functional Theory
- DMAPP
dimethylallyl diphosphate
- DNA
Deoxyribonucleic Acid
- DOPAC
3,4-dihydroxyphenylacetic acid
- DOXP
1-deoxy-D-xylulose-5-phosphate
- EC50
Effective Concentration 50%
- EDTA
Ethylenediaminetetraacetic Acid
- EO
Essential Oil
- EPS
Exopolysaccharide
- ESBL-KP
extended-spectrum beta-lactamase-producing Klebsiella pneumoniae
- FDA
Food and Drug Administration
- FICI
Fractional Inhibitory Concentration Index
- FID
free induction decay
- FPP
farnesyl pyrophosphate
- FtsZ
Filamenting temperature-sensitive mutant Z
- GGPP
Geranylgeranyl Pyrophosphate
- GNPS
Global Natural Products Social Molecular Networking
- GOT
Geranylpyrophosphate:olivetolate Geranyltransferase
- GPP
Geranyl Pyrophosphate
- GTP
Guanosine Triphosphate
- GTPase
Guanosine Triphosphate Hydrolase
- HepG2
Human liver cancer cell line
- HIV
Human Immunodeficiency Virus
- HL-60
Human leukemia cell line
- HL-7702
Human liver cell line
- HMBC
Heteronuclear Multiple Bond Correlation
- HSQC
Heteronuclear Single Quantum Coherence Spectroscopy
- HTS
High Throughput Screening
- IPP
Isopentyl Pyrophosphate or Isopentyl Diphosphate
- ISDB
In Silico tandem mass spectrometry database
- ISM&H
Indian Systems of Medicine and Homoeopathy
- KB
human nasopharyngeal cell line
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LAESI
Laser ablation electrospray ionization
- LC-MS
Liquid Chromatography-Mass Spectrometry
- LD50
Lethal dose 50%
- MADByTE
Metabolomics And Dereplication By Two-dimensional Experiments
- MALDI
Matrix Assisted Laser Desorption Ionization
- MDR
Multidrug-resistant
- MEP
Methylerythritol 4-phosphate
- MFS
Major Facilitator Superfamily
- MIC
Minimum Inhibitory Concentration
- Micro-ED
Microcrystal Electron Diffraction
- ML
Machine Learning
- MN
Molecular Networking
- MOA
Mechanism of Action
- MPNS
Medicinal Plant Names Services
- MRS
Magnetic Resonance Spectroscopy
- MRSA
Methicillin-Resistant Staphylococcus aureus
- MSI
Mass Spectrometry Imaging
- MS/MS
Tandem mass spectrometry
- MSSA
Methicillin-Sensitive Staphylococcus aureus
- NADH
Reduced form of NAD (nicotinamide adenine dinucleotide)
- NMR
Nuclear Magnetic Resonance
- NOE
Nuclear Overhauser Effect
- NorA
Staphylococcus aureus multidrug efflux pump
- NORINE
Database platform of nonribosomal peptides
- NP
Natural Product
- ORCNS
Oxacillin-resistant Coagulase-negative Staphylococci
- OSCNS
Oxacillin-sensitive Coagulase-negative Staphylococci
- OTC
Over-the-Counter
- PAR
Phenylacetaldehyde Reductases
- plantiSMASH
Platform for plant secondary metabolite analysis
- PPAP
Polycyclic Polyprenylated Acylphloroglucinol
- RCSA
Residual Chemical Shift Anisotropy
- RDC
Residual Dipolar Couplings
- RNA
Ribonucleic Acid
- RXR
Retinoid X Receptor
- SAR
Structure-Activity Relationship
- SMILES
Simplified Molecular-Input Line-Entry System
- SN2
a reaction mechanism common in organic chemistry
- SPiDER
Self-organizing map-based prediction of drug equivalence relationships
- TOCSY
Total Correlated Spectroscopy
- UDP-glucose
Uridine diphosphoglucose
- UPLC-PDA-HRMS/MS
Ultra-Performance Liquid Chromatography–High-Resolution Tandem Mass Spectrometry
- US
United States
- UTP
Uridine Triphosphate
- UV
Ultraviolet
- VISA
Vancomycin-Intermediate Staphylococcus aureus
- VRE
Vancomycin-Resistant Enterococci
- VRSA
Vancomycin-Resistant Staphylococcus aureus
- XDR
Extensively Drug-Resistant
Biographies
Gina Porras, Ph.D. received her BSc in chemistry from the National University, Costa Rica in 2007. She obtained her Ph.D. from La Laguna University, Spain in 2013, where she studied the isolation and structural elucidation of biologically active secondary metabolites from marine invertebrates and associated microorganisms under the guidance of Dr. Mercedes Cueto and Dr. José Darias. She returned to Costa Rica and worked as Bioprospection Unit Coordinator at National Center for Biotechnological Innovations, CENAT-CONARE, Costa Rica until 2017. In 2018, she joined to Quave Research Group at Emory University as a postdoctoral fellow, where her current research involves the isolation and structure elucidation of bioactive natural products from plants.
François Chassagne, Pharm.D., Ph.D. graduated from a School of Pharmacy in Paris Descartes University (Paris, France) in 2008, and he holds a M.Sc. in Biodiversity, Ecology and Evolution from Paul Sabatier University (Toulouse, France) obtained in 2009. During his studies, he became passionate about mycology and botany, and thus decided to explore their medicinal potential in various research projects. His first ethnobotanical survey started in Vietnam as part of a research program with the Hanoi University of Pharmacy in 2010. Then, he carried out other ethnobotanical surveys in Cambodia and Lao PDR during his PhD program that he completed in 2017 under the guidance of Dr. Eric Deharo and Dr. Geneviève Bourdy. Curious to understand the pharmacology and chemistry behind these traditional uses, he undertook postdoctoral training with Dr. Guillaume Marti, an expert in metabolomics, and Dr Guillaume Cabanac, informatician in 2017. In 2018, he then joined the Quave Research Group at Emory University as a postdoctoral fellow where he studied the antimicrobial properties of plant extracts. In 2020, he was selected by the French Research Institute for Sustainable Development (IRD) to lead his own research group in the field of ethnopharmacology in France.
James T. Lyles, Ph.D. graduated from Rhodes College in 2001 with a B.S. in biochemistry. He earned his Ph.D. in plant sciences with Dr. Edward J. Kennelly in 2011 from The Graduate Center of the City University of New York’s joint program with the New York Botanical Garden. During this time, he studied natural products chemistry, pharmacognosy, botany, systematics, and field collection. He then developed a natural product research library consisting of botanicals, fungi, lichens, and seaweeds for The North Carolina Arboretum. While there, he also developed botanical authentication and adulteration testing for raw plant material and finished dietary supplements. Afterward, he undertook a post-doctoral fellowship at Emory University’s Center for the Study of Human Health. In 2018, he was hired as an Associate Academic Research Scientist in the Phytochemical Research Laboratory of Cassandra L. Quave at Emory University.
Lewis Marquez received his Bachelor of Science in Cell and Molecular Biology from California State University, Northridge in 2018. He is currently a doctoral student in the Molecular and Systems Pharmacology program at Emory University. In 2019, he accepted a fellowship through The Jones Center at Ichauway and began his research under Dr. Cassandra Quave and Dr. Kier Klepzig. His work focuses on isolating and identifying the bioactive small molecules within plants used in traditional medicine by Southeast Native American tribes for use against drug-resistant microbes.
Micah Dettweiler earned his B.S. in Biology from Emory University in 2017 and worked as a Research Specialist in the Quave Research Group. He is currently pursuing his PhD in Agronomy at the University of Florida. His interests include the historic use of plant medicines in wound healing and the role of synergy in the activity of complex plant extracts.
Akram M. Salam received his Bachelor of Science in Biochemistry in 2015 from the University of Rochester in Rochester, New York, where he did combinatorial chemistry research for HIV drug discovery under Dr. Benjamin L. Miller. He then matriculated into Emory University’s Molecular and Systems Pharmacology doctoral program and began research under Dr. Cassandra Quave on the discovery of plant natural products that inhibit quorum sensing in Staphylococcus aureus.
Tharanga Samarakoon, Ph.D. is Collections Manager of the Emory University Herbarium. She received her Bachelor of Science in Botany from the University of Peradeniya, Sri Lanka, in 2004. She completed her Ph.D. in Biology at the University of Southern Mississippi in 2015 with Dr. Mac Alford. Her graduate research was on a study of the phylogenetic relationships of the tropical family Samydaceae (former Flacourtiaceae) and the preparation of a monograph of the genus Casearia in south-central Asia. Her research interests are in plant systematics, including floristics, molecular phylogenetics, and phytogeography, particularly in southern and southeastern Asia.
Sarah Shabih is projected to graduate from Emory University in May 2021 with a Bachelor of Arts in Human Health. In 2019, she joined the Quave Research Group, where she undertakes research on the phytochemical makeup and antimicrobial activity of medicinal plants.
Darya Raschid Farrokhi is currently earning her Bachelor of Science in Biology at Emory University and is due to graduate in 2020. She is an undergraduate student that began her research with Dr. Quave in the Fall of 2019 after transitioning to Emory University from Oxford College of Emory University, where she earned her Associates of Arts. Her undergraduate research focuses on aiding in the isolation of fungal compounds with antibiofilm formation properties against a common human pathogen.
Cassandra Quave, Ph.D. is Curator of the Herbarium and Associate Professor of Dermatology and Human Health at Emory University, where she leads antibiotic drug discovery research initiatives and teaches courses on medicinal plants, microbiology, and pharmacology. Her research focuses on the documentation and biochemical analysis of plants used in the traditional medical treatment of infectious and inflammatory skin disease. She applies this unique approach to natural products drug discovery in her search for new antibiotics that target multidrug-resistant pathogens. She earned her B.S. in Biology and Anthropology from Emory University in 2000, her Ph.D. in Biology from Florida International University in 2008 in the laboratory of Dr. Bradley Bennett, and completed post-doctoral fellowships in Microbiology at the University of Arkansas for Medical Sciences (2009–2011) with Dr. Mark Smeltzer and Human Health at Emory University (2012) with Dr. Michelle Lampl. Her research has been supported by the National Institutes of Health, Fortune 100 industry contracts, and philanthropy. Dr. Quave has authored more than 100 scientific works and is the co-founder and CEO/CSO of PhytoTEK LLC, a drug discovery company dedicated to developing solutions from botanicals for the treatment of recalcitrant antibiotic-resistant infections. Quave is a Fellow of the Explorers Club, a past President of the Society for Economic Botany, a recipient of the Emory Williams Teaching Award and Charles Heiser, Jr. Mentor Award. She is the creator and host of Foodie Pharmacology, a podcast dedicated to exploring the links between food and medicine. Her research has been the subject of feature profiles in the New York Times Magazine, BBC Focus, National Geographic Magazine, Brigitte Magazin, National Geographic Channel, National Public Radio (NPR), and several major news outlets.
Footnotes
The authors declare no competing financial interest.
Supporting Information
Supporting Information File on antibacterial compounds: chemical class, compound name, SMILES, MICs, plant source, and references.
References
- (1).CDC “Antibiotic Resistance Threats in the United States, 2019 ” U.S. Department of Health and Human Services, 2019. [Google Scholar]
- (2).Hameed HMA; Islam MM; Chhotaray C; Wang C; Liu Y; Tan Y; Li X; Tan S; Delorme V; Yew WW et al. Molecular Targets Related Drug Resistance Mechanisms in MDR-, XDR-, and TDR-Mycobacterium tuberculosis Strains. Front. Cell Infect. Microbiol 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Durand GA; Raoult D; Dubourg G Antibiotic Discovery: History, Methods and Perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [DOI] [PubMed] [Google Scholar]
- (4).Årdal C; Balasegaram M; Laxminarayan R; McAdams D; Outterson K; Rex JH; Sumpradit N Antibiotic Development — Economic, Regulatory and Societal Challenges. Nat. Rev. Microbiol 2020, 18, 267–274. [DOI] [PubMed] [Google Scholar]
- (5).Harvey AL; Edrada-Ebel R; Quinn RJ The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov 2015, 14, 111–129. [DOI] [PubMed] [Google Scholar]
- (6).Payne DJ; Gwynn MN; Holmes DJ; Pompliano DL Drugs for Bad Bugs: Confronting the Challenges of Antibacterial Discovery. Nat. Rev. Drug Discov 2007, 6, 29–40. [DOI] [PubMed] [Google Scholar]
- (7).Shen B A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Singh SB; Barrett JF Empirical Antibacterial Drug Discovery—Foundation in Natural Products. Biochem. Pharmacol 2006, 71, 1006–1015. [DOI] [PubMed] [Google Scholar]
- (9).Newman DJ; Cragg GM Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod 2020, 83, 770–803. [DOI] [PubMed] [Google Scholar]
- (10).Newman DJ; Cragg GM Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod 2016, 79, 629–661. [DOI] [PubMed] [Google Scholar]
- (11).Szychowski J; Truchon J-F; Bennani YL Natural Products in Medicine: Transformational Outcome of Synthetic Chemistry. J. Med. Chem 2014, 57, 9292–9308. [DOI] [PubMed] [Google Scholar]
- (12).Maier ME Design and Synthesis of Analogues of Natural Products. Org. Biomol. Chem 2015, 13, 5302–5343. [DOI] [PubMed] [Google Scholar]
- (13).Zaynab M; Fatima M; Abbas S; Sharif Y; Umair M; Zafar MH; Bahadar K Role of Secondary Metabolites in Plant Defense Against Pathogens. Microb. Pathog 2018, 124, 198–202. [DOI] [PubMed] [Google Scholar]
- (14).Christenhusz M; Byng J The Number of Known Plant Species in the World and Its Annual Increase. Phytotaxa 2016, 261, 21–217. [Google Scholar]
- (15).Gurib-Fakim A Medicinal Plants: Traditions of Yesterday and Drugs of Tomorrow. Mol. Aspects Med 2006, 27, 1–93. [DOI] [PubMed] [Google Scholar]
- (16).Caesar LK; Cech NB Synergy and Antagonism in Natural Product Extracts: When 1+1 Does Not Equal 2. Nat. Prod. Rep 2019, 36, 869–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Prance GT Ethnobotany, The Science of Survival: A Declaration from Kaua’i. Econ. Bot 2007, 61, 1–2. [Google Scholar]
- (18).RBG; Willis KJ State of the World’s Plants, 2017; Royal Botanic Gardens, Kew: London, England, 2017. [Google Scholar]
- (19).MNPS. Medicinal Plant Names Services (MNPS) https://mpns.science.kew.org/mpns-portal/. 2020. Accessed on October 14, 2020.
- (20).Kolář M; Urbánek K; Látal T Antibiotic Selective Pressure and Development of Bacterial Resistance. Int. J. Antimicrob. Agents 2001, 17, 357–363. [DOI] [PubMed] [Google Scholar]
- (21).Johnson BK; Abramovitch RB Small Molecules That Sabotage Bacterial Virulence. Trends Pharmacol. Sci 2017, 38, 339–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Silva LN; Zimmer KR; Macedo AJ; Trentin DS Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev 2016, 116, 9162–9236. [DOI] [PubMed] [Google Scholar]
- (23).Rossiter SE; Fletcher MH; Wuest WM Natural Products as Platforms To Overcome Antibiotic Resistance. Chem. Rev 2017, 117, 12415–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).CLSI. Methods for Dilution Antimicrobial Susceptibiity Tests for Bacteria That Grow Aerobically; Approved Standard; CLSI document M07-A9; 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, 2012. [Google Scholar]
- (25).The Plant List http://www.theplantlist.org/, 2020. Accessed on October 14, 2020.
- (26).APG; Chase MW; Christenhusz MJM; Fay MF; Byng JW; Judd WS; Soltis DE; Mabberley DJ; Sennikov AN; Soltis PS et al. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc 2016, 181, 1–20. [Google Scholar]
- (27).Parte AC; Sardà Carbasse J; Meier-Kolthoff JP; Reimer LC; Göker M List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol 2020, DOI: 10.1099/ijsem.0.004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kim MG; Lee SE; Yang JY; Lee HS Antimicrobial Potentials of Active Component Isolated from Citrullus colocynthis Fruits and Structure-Activity Relationships of its Analogues against Foodborne Bacteria. J. Sci. Food Agric 2014, 94, 2529–2533. [DOI] [PubMed] [Google Scholar]
- (29).Houdkova M; Rondevaldova J; Doskocil I; Kokoska L Evaluation of Antibacterial Potential and Toxicity of Plant Volatile Compounds using New Broth Microdilution Volatilization Method and Modified MTT Assay. Fitoterapia 2017, 118, 56–62. [DOI] [PubMed] [Google Scholar]
- (30).Pan X; Bligh SW; Smith E Quinolone Alkaloids from Fructus Euodiae show Activity against Methicillin-Resistant Staphylococcus aureus. Phytother. Res 2014, 28, 305–307. [DOI] [PubMed] [Google Scholar]
- (31).Tan KK; Khoo TJ; Rajagopal M; Wiart C Antibacterial Alkaloids from Artabotrys crassifolius Hook.f. & Thomson. Nat. Prod. Res 2015, 29, 2346–2349. [DOI] [PubMed] [Google Scholar]
- (32).Hamoud R; Reichling J; Wink M Synergistic Antibacterial Activity of the Combination of the Alkaloid Aanguinarine with EDTA and the Antibiotic Streptomycin against Multidrug Resistant Bacteria. J. Pharm. Pharmacol 2015, 67, 264–273. [DOI] [PubMed] [Google Scholar]
- (33).Azimi G; Hakakian A; Ghanadian M; Joumaa A; Alamian S Bioassay-directed Isolation of Quaternary Benzylisoquinolines from Berberis integerrima with Bactericidal Activity against Brucella abortus. Res. Pharm. Sci 2018, 13, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Xie Q; Johnson BR; Wenckus CS; Fayad MI; Wu CD Efficacy of Berberine, an Antimicrobial Plant Alkaloid, as an Endodontic Irrigant Against a Mixed-Culture Biofilm in an In Vitro Tooth Model. J. Endod 2012, 38, 1114–1117. [DOI] [PubMed] [Google Scholar]
- (35).Tankeo SB; Damen F; Awouafack MD; Mpetga J; Tane P; Eloff JN; Kuete V Antibacterial Activities of the Methanol Extracts, Fractions and Compounds from Fagara tessmannii. J. Ethnopharmacol 2015, 169, 275–279. [DOI] [PubMed] [Google Scholar]
- (36).Rong Q; Xu M; Dong Q; Zhang Y; Li Y; Ye G; Zhao L In Vitro and In Vivo Bactericidal Activity of Tinospora sagittata (Oliv.) Gagnep. var. craveniana (S.Y.Hu) Lo and its Main Effective Component, Palmatine, against Porcine Helicobacter pylori. BMC Complement. Altern. Med 2016, 16, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Yu J; Yin TP; Wang JP; Mei RF; Cai L; Ding ZT A New C20-diterpenoid Alkaloid from the Lateral Roots of Aconitum carmichaeli. Nat. Prod. Res 2017, 31, 228–232. [DOI] [PubMed] [Google Scholar]
- (38).Dzoyem JP; Hamamoto H; Ngameni B; Ngadjui BT; Sekimizu K Antimicrobial Action Mechanism of Flavonoids from Dorstenia species. Drug Discov. Ther 2013, 7, 66–72. [PubMed] [Google Scholar]
- (39).Shen F; Tang X; Wang Y; Yang Z; Shi X; Wang C; Zhang Q; An Y; Cheng W; Jin K et al. Phenotype and Expression Profile Analysis of Staphylococcus aureus Biofilms and Planktonic Cells in Response to Licochalcone A. Appl. Microbiol. Biotechnol 2015, 99, 359–373. [DOI] [PubMed] [Google Scholar]
- (40).Muharini R; Diaz A; Ebrahim W; Mandi A; Kurtan T; Rehberg N; Kalscheuer R; Hartmann R; Orfali RS; Lin W et al. Antibacterial and Cytotoxic Phenolic Metabolites from the Fruits of Amorpha fruticosa. J. Nat. Prod 2017, 80, 169–180. [DOI] [PubMed] [Google Scholar]
- (41).Chan BC-L; Yu H; Wong C-W; Lui S-L; Jolivalt C; Ganem-Elbaz C; Paris J-M; Morleo B; Litaudon M; Lau CB-S et al. Quick Identification of Kuraridin, a Noncytotoxic anti-MRSA (Methicillin-Resistant Staphylococcus aureus) Agent from Sophora flavescens using High-Speed Counter-Current Chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2012, 880, 157–162. [DOI] [PubMed] [Google Scholar]
- (42).Zuo GY; Wang CJ; Han J; Li YQ; Wang GC Synergism of Coumarins from the Chinese Drug Zanthoxylum nitidum with Antibacterial Agents against Methicillin-Resistant Staphylococcus aureus (MRSA). Phytomedicine 2016, 23, 1814–1820. [DOI] [PubMed] [Google Scholar]
- (43).Tan N; Yazici-Tutunis S; Bilgin M; Tan E; Miski M Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos hulusii. Molecules 2017, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Dzotam JK; Simo IK; Bitchagno G; Celik I; Sandjo LP; Tane P; Kuete V In Vitro Antibacterial and Antibiotic Modifying Activity of Crude Extract, Fractions and 3’,4’,7-trihydroxyflavone from Myristica fragrans Houtt against MDR Gram-Negative Enteric Bacteria. BMC Complement. Altern. Med 2018, 18, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Nirmal NP; Panichayupakaranant P Antioxidant, Antibacterial, and Anti-inflammatory Activities of Standardized Brazilin-rich Caesalpinia sappan Extract. Pharm. Biol 2015, 53, 1339–1343. [DOI] [PubMed] [Google Scholar]
- (46).Nirmal NP; Panichayupakaranant P Anti-Propionibacterium acnes Assay-Guided Purification of Brazilin and Preparation of Brazilin Rich Extract from Caesalpinia sappan Heartwood. Pharm. Biol 2014, 52, 1204–1207. [DOI] [PubMed] [Google Scholar]
- (47).Yuan M; Shi DZ; Wang TY; Zheng SQ; Liu LJ; Sun ZX; Wang RF; Ding Y Transformation of Trollioside and Isoquercetin by Human Intestinal Flora In Vitro. Chin. J. Nat. Med 2016, 14, 220–226. [DOI] [PubMed] [Google Scholar]
- (48).Shahzad M; Millhouse E; Culshaw S; Edwards CA; Ramage G; Combet E Selected Dietary (Poly)phenols Inhibit Periodontal Pathogen Growth and Biofilm Formation. Food Funct 2015, 6, 719–729. [DOI] [PubMed] [Google Scholar]
- (49).Dey D; Ray R; Hazra B Antimicrobial Activity of Pomegranate Fruit Constituents against Drug-Resistant Mycobacterium tuberculosis and Beta-lactamase Producing Klebsiella pneumoniae. Pharm. Biol 2015, 53, 1474–1480. [DOI] [PubMed] [Google Scholar]
- (50).Tiam ER; Ngono Bikobo DS; Abouem AZA; Mbabi Nyemeck N 2nd; Moni Ndedi EDF; Betote Diboue PH; Nyegue MA; Atchade AT; Emmanuel Pegnyemb D; Bochet CG et al. Secondary Metabolites from Triclisia gilletii (De Wild) Staner (Menispermaceae) with Antimycobacterial Activity against Mycobacterium tuberculosis. Nat. Prod. Res 2019, 33, 642–650. [DOI] [PubMed] [Google Scholar]
- (51).Dzoyem JP; Tchamgoue J; Tchouankeu JC; Kouam SF; Choudhary MI; Bakowsky U Antibacterial Activity and Cytotoxicity of Flavonoids Compounds Isolated from Pseudarthria hookeri Wight & Arn. (Fabaceae). S. Afr. J. Bot 2018, 114, 100–103. [Google Scholar]
- (52).Mbaveng AT; Sandjo LP; Tankeo SB; Ndifor AR; Pantaleon A; Nagdjui BT; Kuete V Antibacterial Activity of Nineteen Selected Natural Products against Multi-Drug Resistant Gram-negative Phenotypes. SpringerPlus 2015, 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Djeussi DE; Noumedem JAK; Kuete V; Sandjo LP; Omosa LK; Bonaventure TN Antibacterial Activities of the Methanol Extracts and Compounds from Erythrina sigmoidea against Gram-Negative Multi-drug Resistant Phenotypes. BMC Complement. Altern. Med 2015, 15, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Zhou B; Wan CX Phenolic Constituents from the Aerial Parts of Glycyrrhiza inflata and their Antibacterial Activities. J. Asian Nat. Prod. Res 2015, 17, 256–261. [DOI] [PubMed] [Google Scholar]
- (55).Zuo GY; Yang CX; Han J; Li YQ; Wang GC Synergism of Prenylflavonoids from Morus alba Root Bark against Clinical MRSA Isolates. Phytomedicine 2018, 39, 93–99. [DOI] [PubMed] [Google Scholar]
- (56).Pereira F; Madureira AM; Sancha S; Mulhovo S; Luo X; Duarte A; Ferreira M-JU Cleistochlamys kirkii Chemical Constituents: Antibacterial Activity and Synergistic Effects against Resistant Staphylococcus aureus Strains. J. Ethnopharmacol 2016, 178, 180–187. [DOI] [PubMed] [Google Scholar]
- (57).Joycharat N; Thammavong S; Limsuwan S; Homlaead S; Voravuthikunchai SP; Yingyongnarongkul BE; Dej-Adisai S; Subhadhirasakul S Antibacterial Substances from Albizia myriophylla Wood Against Cariogenic Streptococcus mutans. Arch. Pharm. Res 2013, 36, 723–730. [DOI] [PubMed] [Google Scholar]
- (58).Yusook K; Weeranantanapan O; Hua Y; Kumkrai P; Chudapongse N Lupinifolin from Derris reticulata Possesses Bactericidal Activity on Staphylococcus aureus by Disrupting Bacterial Cell Membrane. J. Nat. Med 2017, 71, 357–366. [DOI] [PubMed] [Google Scholar]
- (59).Joycharat N; Boonma C; Thammavong S; Yingyongnarongkul BE; Limsuwan S; Voravuthikunchai SP Chemical Constituents and Biological Activities of Albizia myriophylla Wood. Pharm. Biol 2016, 54, 62–73. [DOI] [PubMed] [Google Scholar]
- (60).Mabou FD; Tamokou J.-d.-D.; Ngnokam D; Voutquenne-Nazabadioko L; Kuiate J-R; Bag PK Complex Secondary Metabolites from Ludwigia leptocarpa with Potent Antibacterial and Antioxidant Activities. Drug Discoveries Ther 2016, 10, 141–149. [DOI] [PubMed] [Google Scholar]
- (61).Hwang JH; Choi H; Woo E-R; Lee DG Antibacterial Effect of Amentoflavone and its Synergistic Effect with Antibiotics. J. Microbiol. Biotechnol 2013, 23, 953–958. [DOI] [PubMed] [Google Scholar]
- (62).Chukwujekwu JC; de Kock CA; Smith PJ; van Heerden FR; van Staden J Antiplasmodial and Antibacterial Activity of Compounds Isolated from Ormocarpum trichocarpum. Planta Med 2012, 78, 1857–1860. [DOI] [PubMed] [Google Scholar]
- (63).Zuo G-Y; Yang C-X; Ruan Z-J; Han J; Wang G-C Potent anti-MRSA Activity and Synergism with Aminoglycosides by Flavonoid Derivatives from the Root Barks of Morus alba, a Traditional Chinese Medicine. Med. Chem. Res 2019, 28, 1547–1556. [Google Scholar]
- (64).Favela-Hernandez JM; Garcia A; Garza-Gonzalez E; Rivas-Galindo VM; Camacho-Corona MR Antibacterial and Antimycobacterial Lignans and Flavonoids from Larrea tridentata. Phytother. Res 2012, 26, 1957–1960. [DOI] [PubMed] [Google Scholar]
- (65).Hariharan P; Paul-Satyaseela M; Gnanamani A In Vitro Profiling of Antimethicillin-Resistant Staphylococcus aureus Activity of Thymoquinone against Selected Type and Clinical Strains. Lett. Appl. Microbiol 2016, 62, 283–289. [DOI] [PubMed] [Google Scholar]
- (66).Uc-Cachon AH; Borges-Argaez R; Said-Fernandez S; Vargas-Villarreal J; Gonzalez-Salazar F; Mendez-Gonzalez M; Caceres-Farfan M; Molina-Salinas GM Naphthoquinones Isolated from Diospyros anisandra Exhibit Potent Activity against Pan-resistant First-line Drugs Mycobacterium tuberculosis Strains. Pulm. Pharmacol. Ther 2014, 27, 114–120. [DOI] [PubMed] [Google Scholar]
- (67).Omosa LK; Midiwo JO; Mbaveng AT; Tankeo SB; Seukep JA; Voukeng IK; Dzotam JK; Isemeki J; Derese S; Omolle RA et al. Antibacterial Activities and Structure-Activity Relationships of a Panel of 48 Compounds from Kenyan Plants against Multidrug Resistant Phenotypes. SpringerPlus 2016, 5, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Cesari I; Hoerle M; Simoes-Pires C; Grisoli P; Queiroz EF; Dacarro C; Marcourt L; Moundipa PF; Carrupt PA; Cuendet M et al. Anti-inflammatory, Antimicrobial and Antioxidant Activities of Diospyros bipindensis (Gurke) Extracts and its Main Constituents. J. Ethnopharmacol 2013, 146, 264–270. [DOI] [PubMed] [Google Scholar]
- (69).Nair SV; Baranwal G; Chatterjee M; Sachu A; Vasudevan AK; Bose C; Banerji A; Biswas R Antimicrobial Activity of Plumbagin, a Naturally Occurring Naphthoquinone from Plumbago rosea, against Staphylococcus aureus and Candida albicans. Int. J. Med. Microbiol 2016, 306, 237–248. [DOI] [PubMed] [Google Scholar]
- (70).Luo Y; Shen HY; Shen QX; Cao ZH; Zhang M; Long SY; Wang ZB; Tan JW A New Anthraquinone and a New Naphthoquinone from the Whole Plant of Spermacoce latifolia. J. Asian Nat. Prod. Res 2017, 19, 869–876. [DOI] [PubMed] [Google Scholar]
- (71).Yoshikawa K; Ito T; Iseki K; Baba C; Imagawa H; Yagi Y; Morita H; Asakawa Y; Kawano S; Hashimoto T Phenanthrene Derivatives from Cymbidium Great Flower Marie Laurencin and their Biological Activities. J. Nat. Prod 2012, 75, 605–609. [DOI] [PubMed] [Google Scholar]
- (72).Inoue Andrade F; Purgato GA; de Faria Maia T; Pais Siqueira R; Lima S; Diaz G; Diaz MA Chemical Constituents and an Alternative Medicinal Veterinary Herbal Soap Made from Senna macranthera. Evid. Based Complement. Alternat. Med 2015, 2015, 217598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Tatsimo SJN; Tamokou J.-d.-D.; Tsague VT; Lamshoft M; Sarkar P; Bag PK; Spiteller M Antibacterial-Guided Isolation of Constituents from Senna alata Leaves with a Particular Reference against Multi-Drug-Resistant Vibrio cholerae and Shigella flexneri. Int. J. Biol. Chem. Sci 2017, 11, 46–53. [Google Scholar]
- (74).Teinkela JEM; Noundou XS; Fannang S; Meyer F; Vardamides JC; Mpondo EM; Krause RWM; Azebaze AGB; Nguedia JCA In Vitro Antimicrobial Activity of the Methanol Extract and Compounds from the Wood of Ficus elastica Roxb. ex Hornem. Aerial Roots. S. Afr. J. Bot 2017, 111, 302–306. [Google Scholar]
- (75).Oumer A; Bisrat D; Mazumder A; Asres K A New Antimicrobial Anthrone from the Leaf Latex of Aloe trichosantha. Nat. Prod. Commun 2014, 9, 949–952. [PubMed] [Google Scholar]
- (76).Megeressa M; Bisrat D; Asres K; Mazumder A Structural Elucidation of Some Antimicrobial Constituents from the Leaf Latex of Aloe trigonantha L.C. Leach. BMC Complement. Altern. Med 2015, 15, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Tankeo SB; Damen F; Sandjo LP; Celik I; Tane P; Kuete V Antibacterial Activities of the Methanol Extracts, Fractions and Compounds from Harungana madagascariensis Lam. ex Poir. (Hypericaceae). J. Ethnopharmacol 2016, 190, 100–105. [DOI] [PubMed] [Google Scholar]
- (78).Fouotsa H; Lannang AM; Dzoyem JP; Tatsimo SJ; Neumann B; Mbazoa CD; Razakarivony AA; Nkengfack AE; Eloff JN; Sewald N Antibacterial and Antioxidant Xanthones and Benzophenone from Garcinia smeathmannii. Planta Med 2015, 81, 594–599. [DOI] [PubMed] [Google Scholar]
- (79).Phitaktim S; Chomnawang M; Sirichaiwetchakoon K; Dunkhunthod B; Hobbs G; Eumkeb G Synergism and the Mechanism of Action of the Combination of α-Mangostin Isolated from Garcinia mangostana L. and Oxacillin against an Oxacillin-Resistant Staphylococcus saprophyticus. BMC Microbiol 2016, 16, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Seesom W; Jaratrungtawee A; Suksamrarn S; Mekseepralard C; Ratananukul P; Sukhumsirichart W Antileptospiral Activity of Xanthones from Garcinia mangostana and Synergy of Gamma-mangostin with Penicillin G. BMC Complement. Altern. Med 2013, 13, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Siridechakorn I; Phakhodee W; Ritthiwigrom T; Promgool T; Deachathai S; Cheenpracha S; Prawat U; Laphookhieo S Antibacterial Dihydrobenzopyran and Xanthone Derivatives from Garcinia cowa Stem Barks. Fitoterapia 2012, 83, 1430–1434. [DOI] [PubMed] [Google Scholar]
- (82).Fouotsa H; Mbaveng AT; Mbazoa CD; Nkengfack AE; Farzana S; Iqbal CM; Meyer JJM; Lall N; Kuete V Antibacterial Constituents of Three Cameroonian Medicinal Plants: Garcinia nobilis, Oricia suaveolens and Balsamocitrus camerunensis. BMC Complement. Altern. Med 2013, 13, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Jyoti MA; Nam K-W; Jang WS; Kim Y-H; Kim S-K; Lee B-E; Song H-Y Antimycobacterial Activity of Methanolic Plant Extract of Artemisia capillaris Containing Ursolic Acid and Hydroquinone against Mycobacterium tuberculosis. J. Infect. Chemother 2016, 22, 200–208. [DOI] [PubMed] [Google Scholar]
- (84).Zeng X; Wang H; Gong Z; Huang J; Pei W; Wang X; Zhang J; Tang X Antimicrobial and Cytotoxic Phenolics and Phenolic Glycosides from Sargentodoxa cuneata. Fitoterapia 2015, 101, 153–161. [DOI] [PubMed] [Google Scholar]
- (85).Xu T; Wang Z; Lei T; Lv C; Wang J; Lu J New Flavonoid Glycosides from Sedum aizoon L. Fitoterapia 2015, 101, 125–132. [DOI] [PubMed] [Google Scholar]
- (86).Bag PK; Roy N; Acharyya S; Saha DR; Koley H; Sarkar P; Bhowmik P In Vivo Fluid Accumulation-Inhibitory, Anticolonization and Anti-inflammatory and In Vitro Biofilm-Inhibitory Activities of Methyl Gallate Isolated from Terminalia chebula against Fluoroquinolones Resistant Vibrio cholerae. Microb. Pathog 2019, 128, 41–46. [DOI] [PubMed] [Google Scholar]
- (87).Grace D; Khan MS; Friesen K; Ata A Antimicrobial Compounds from Drypetes staudtii. Chem. Biodivers 2016, 13, 913–917. [DOI] [PubMed] [Google Scholar]
- (88).Sarkisian SA; Janssen MJ; Matta H; Henry GE; Laplante KL; Rowley DC Inhibition of Bacterial Growth and Biofilm Production by Constituents from Hypericum spp. Phytother. Res 2012, 26, 1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Shiu WKP; Rahman MM; Curry J; Stapleton P; Zloh M; Malkinson JP; Gibbons S Antibacterial Acylphloroglucinols from Hypericum olympicum. J. Nat. Prod 2012, 75, 336–343. [DOI] [PubMed] [Google Scholar]
- (90).Jiang S; Wan K; Lou HY; Yi P; Zhang N; Zhou M; Song ZQ; Wang W; Wu MK; Pan WD Antibacterial Bibenzyl Derivatives from the Tubers of Bletilla striata. Phytochemistry 2019, 162, 216–223. [DOI] [PubMed] [Google Scholar]
- (91).Polaquini CR; Morao LG; Nazare AC; Torrezan GS; Dilarri G; Cavalca LB; Campos DL; Silva IC; Pereira JA; Scheffers DJ et al. Antibacterial Activity of 3,3’-dihydroxycurcumin (DHC) is Associated with Membrane Perturbation. Bioorg. Chem 2019, 90, 103031. [DOI] [PubMed] [Google Scholar]
- (92).Ruiz Mostacero N; Castelli MV; Cutro AC; Hollmann A; Batista JM Jr.; Furlan M; Valles J; Fulgueira CL; Lopez SN Antibacterial Activity of Prenylated Benzopyrans from Peperomia obtusifolia (Piperaceae). Nat. Prod. Res 2019, DOI: 10.1080/14786419.2019.1628751, 1–5. [DOI] [PubMed] [Google Scholar]
- (93).Fomogne-Fodjo MC; Ndinteh DT; Olivier DK; Kempgens P; van Vuuren S; Krause RW Secondary Metabolites from Tetracera potatoria Stem Bark with Anti-mycobacterial Activity. J. Ethnopharmacol 2017, 195, 238–245. [DOI] [PubMed] [Google Scholar]
- (94).Limsuwan S; Homlaead S; Watcharakul S; Chusri S; Moosigapong K; Saising J; Voravuthikunchai SP Inhibition of Microbial Adhesion to Plastic Surface and Human Buccal Epithelial Cells by Rhodomyrtus tomentosa Leaf Extract. Arch. Oral Biol 2014, 59, 1256–1265. [DOI] [PubMed] [Google Scholar]
- (95).Saising J; Voravuthikunchai SP Anti Propionibacterium acnes Activity of Rhodomyrtone, an Effective Compound from Rhodomyrtus tomentosa (Aiton) Hassk. Leaves. Anaerobe 2012, 18, 400–404. [DOI] [PubMed] [Google Scholar]
- (96).Zhao LY; Liu HX; Wang L; Xu ZF; Tan HB; Qiu SX Rhodomyrtosone B, a Membrane-targeting anti-MRSA Natural Acylgphloroglucinol from Rhodomyrtus tomentosa. J. Ethnopharmacol 2019, 228, 50–57. [DOI] [PubMed] [Google Scholar]
- (97).Biloa Messi B; Ho R; Meli Lannang A; Cressend D; Perron K; Nkengfack AE; Carrupt PA; Hostettmann K; Cuendet M Isolation and Biological Activity of Compounds from Garcinia preussii. Pharm. Biol 2014, 52, 706–711. [DOI] [PubMed] [Google Scholar]
- (98).Sakunpak A; Panichayupakaranant P Antibacterial Activity of Thai Edible Plants against Gastrointestinal Pathogenic Bacteria and Isolation of a New Broad Spectrum Antibacterial Polyisoprenylated Benzophenone, Chamuangone. Food Chem 2012, 130, 826–831. [Google Scholar]
- (99).Oyedemi BO; Shinde V; Shinde K; Kakalou D; Stapleton PD; Gibbons S Novel R-plasmid Conjugal Transfer Inhibitory and Antibacterial Activities of Phenolic Compounds from Mallotus philippensis (Lam.) Mull. Arg. J. Glob. Antimicrob. Resist 2016, 5, 15–21. [DOI] [PubMed] [Google Scholar]
- (100).Osman K; Evangelopoulos D; Basavannacharya C; Gupta A; McHugh TD; Bhakta S; Gibbons S An Antibacterial from Hypericum acmosepalum inhibits ATP-dependent MurE ligase from Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 2012, 39, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Kim YJ; Sohn MJ; Kim WG Chalcomoracin and Moracin C, New Inhibitors of Staphylococcus aureus Enoyl-acyl Carrier Protein Reductase from Morus alba. Biol. Pharm. Bull 2012, 35, 791–795. [DOI] [PubMed] [Google Scholar]
- (102).Pendota SC; Aderogba MA; Van Staden J In Vitro Antimicrobial Activity of Extracts and an Isolated Compound from Boscia albitrunca Leaves. S. Afr. J. Bot 2015, 96, 91–93. [Google Scholar]
- (103).Giweli A; Dzamic AM; Sokovic M; Ristic MS; Marin PD Antimicrobial and Antioxidant Activities of Essential Oils of Satureja thymbra Growing Wild in Libya. Molecules 2012, 17, 4836–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Miladi H; Zmantar T; Chaabouni Y; Fedhila K; Bakhrouf A; Mahdouani K; Chaieb K Antibacterial and Efflux Pump Inhibitors of Thymol and Carvacrol against Food-Borne Pathogens. Microb. Pathog 2016, 99, 95–100. [DOI] [PubMed] [Google Scholar]
- (105).Badawy MEI; Marei GIK; Rabea EI; Taktak NEM Antimicrobial and Antioxidant Activities of Hydrocarbon and Oxygenated Monoterpenes against some Foodborne Pathogens through In Vitro and In Silico Studies. Pestic. Biochem. Physiol 2019, 158, 185–200. [DOI] [PubMed] [Google Scholar]
- (106).Yu L; Ren JX; Nan HM; Liu BF Identification of Antibacterial and Antioxidant Constituents of the Essential Oils of Cynanchum chinense and Ligustrum compactum. Nat. Prod. Res 2015, 29, 1779–1782. [DOI] [PubMed] [Google Scholar]
- (107).Samadi N; Manayi A; Vazirian M; Samadi M; Zeinalzadeh Z; Saghari Z; Abadian N; Mozaffarian VO; Khanavi M Chemical Composition and Antimicrobial Activity of the Essential Oil of Anthemis altissima L. var. altissima. Nat. Prod. Res 2012, 26, 1931–1934. [DOI] [PubMed] [Google Scholar]
- (108).Li L; Li Z-W; Yin Z-Q; Wei Q; Jia R-Y; Zhou L-J; Xu J; Song X; Zhou Y; Du Y-H et al. Antibacterial Activity of Leaf Essential Oil and its Constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med 2014, 7, 1721–1727. [PMC free article] [PubMed] [Google Scholar]
- (109).Lopez-Romero JC; Gonzalez-Rios H; Borges A; Simoes M Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement. Alternat. Med 2015, 2015, 795435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Hernandez DM; Diaz-Ruiz G; Rivero-Cruz BE; Bye RA; Aguilar MI; Rivero-Cruz JF Ent-trachyloban-19-oic Acid isolated from Iostephane heterophylla as a Promising Antibacterial Agent against Streptococcus mutans Biofilms. Fitoterapia 2012, 83, 527–531. [DOI] [PubMed] [Google Scholar]
- (111).Moricz AM; Kruzselyi D; Alberti A; Darcsi A; Horvath G; Csontos P; Beni S; Ott PG Layer Chromatography-Bioassays Directed Screening and Identification of Antibacterial Compounds from Scotch thistle. J. Chromatogr. A 2017, 1524, 266–272. [DOI] [PubMed] [Google Scholar]
- (112).Liu T; Wang S; Xu L; Fu W; Gibbons S; Mu Q Sesquiterpenoids with Anti-MDR Staphylococcus aureus Activities from Ferula ferulioides. Chem. Biodivers 2015, 12, 599–614. [DOI] [PubMed] [Google Scholar]
- (113).Fernandez A; Rosa MF; Fernandez CMM; W, C. B.; Melo UZ; Siqueira VLD; Cortez DAG; Goncalves JE; Linde GA; Gazim ZC Antimicrobial and Antioxidant Activities of the Extract and Fractions of Tetradenia riparia (Hochst.) Codd (Lamiaceae) Leaves from Brazil. Curr. Microbiol 2017, 74, 1453–1460. [DOI] [PubMed] [Google Scholar]
- (114).Caetano da Silva SD; Mendes de Souza MG; Oliveira Cardoso MJ; da Silva Moraes T; Ambrosio SR; Sola Veneziani RC; Martins CH Antibacterial Activity of Pinus elliottii Against Anaerobic Bacteria Present in Primary Endodontic Infections. Anaerobe 2014, 30, 146–152. [DOI] [PubMed] [Google Scholar]
- (115).da Silva KR; Damasceno JL; Inacio MO; Abrao F; Ferreira NH; Tavares DC; Ambrosio SR; Veneziani RCS; Martins CHG Antibacterial and Cytotoxic Activities of Pinus tropicalis and Pinus elliottii Resins and of the Diterpene Dehydroabietic Acid Against Bacteria That Cause Dental Caries. Front. Microbiol 2019, 10, 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Anakok OF; Ndi CP; Barton MD; Griesser HJ; Semple SJ Antibacterial Spectrum and Cytotoxic Activities of Serrulatane Compounds from the Australian Medicinal Plant Eremophila neglecta. J. Appl. Microbiol 2012, 112, 197–204. [DOI] [PubMed] [Google Scholar]
- (117).Abrao F; de Araujo Costa LD; Alves JM; Senedese JM; de Castro PT; Ambrosio SR; Veneziani RC; Bastos JK; Tavares DC; Martins CH Copaifera langsdorffii Oleoresin and its Isolated Compounds: Antibacterial Effect and Antiproliferative Activity in Cancer Cell Lines. BMC Complement. Altern. Med 2015, 15, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Moreti DLC; Leandro LF; da Silva Moraes T; Moreira MR; Sola Veneziani RC; Ambrosio SR; Gomes BP; Martins CHG Mikania glomerata Sprengel Extract and its Major Compound Ent-Kaurenoic Acid Display Activity Against Bacteria Present in Endodontic Infections. Anaerobe 2017, 47, 201–208. [DOI] [PubMed] [Google Scholar]
- (119).Kulkarni RR; Shurpali K; Puranik VG; Sarkar D; Joshi SP Antimycobacterial Labdane Diterpenes from Leucas stelligera. J. Nat. Prod 2013, 76, 1836–1841. [DOI] [PubMed] [Google Scholar]
- (120).Khan AK; Khan IA; Ahmed A; Hussain M; Ali SA; Farooq AD; Faizi S Antibiofilm Potential of 16-oxo-cleroda-3, 13(14) E-diene-15 oic Acid and its Five New γ-Amino γ-Lactone Derivatives against Methicillin Resistant Staphylococcus aureus and Streptococcus mutans. Eur. J. Med. Chem 2017, 138, 480–490. [DOI] [PubMed] [Google Scholar]
- (121).Gupta VK; Tiwari N; Gupta P; Verma S; Pal A; Srivastava SK; Darokar MP A Clerodane Diterpene from Polyalthia longifolia as a Modifying Agent of the Resistance of Methicillin Resistant Staphylococcus aureus. Phytomedicine 2016, 23, 654–661. [DOI] [PubMed] [Google Scholar]
- (122).Gupta VK; Verma S; Pal A; Srivastava SK; Srivastava PK; Darokar MP In Vivo Efficacy and Synergistic Interaction of 16alpha-hydroxycleroda-3, 13 (14) Z-dien-15, 16-olide, a Clerodane Diterpene from Polyalthia longifolia against Methicillin-Resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol 2013, 97, 9121–9131. [DOI] [PubMed] [Google Scholar]
- (123).Chabán MF; Karagianni C; Joray MB; Toumpa D; Sola C; Crespo MI; Palacios SM; Athanassopoulos CM; Carpinella MC Antibacterial Effects of Extracts Obtained from Plants of Argentina: Bioguided Isolation of Compounds from the Anti-Infectious Medicinal Plant Lepechinia meyenii. J. Ethnopharmacol 2019, 239, 111930. [DOI] [PubMed] [Google Scholar]
- (124).Vazquez NM; Fiorilli G; Caceres Guido PA; Moreno S Carnosic Acid Acts Synergistically with Gentamicin in Killing Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Phytomedicine 2016, 23, 1337–1343. [DOI] [PubMed] [Google Scholar]
- (125).Kuete V; Betrandteponno R; Mbaveng AT; Tapondjou LA; Meyer JJ; Barboni L; Lall N Antibacterial Activities of the Extracts, Fractions and Compounds from Dioscorea bulbifera. BMC Complement. Altern. Med 2012, 12, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Lin L; Zhu D; Zou L; Yang B; Zhao M Antibacterial Activity-guided Purification and Identification of a Novel C-20 Oxygenated Ent-kaurane from Rabdosia serra (MAXIM.) HARA. Food Chem 2013, 139, 902–909. [DOI] [PubMed] [Google Scholar]
- (127).Sarkar P; Acharyya S; Banerjee A; Patra A; Thankamani K; Koley H; Bag PK Intracellular, Biofilm-Inhibitory and Membrane-Damaging Activities of Nimbolide Isolated from Azadirachta indica A. Juss (Meliaceae) against Meticillin-Resistant Staphylococcus aureus. J. Med. Microbiol 2016, 65, 1205–1214. [DOI] [PubMed] [Google Scholar]
- (128).Muhammad D; Lalun N; Bobichon H; Debar EL; Gangloff SC; Nour M; Voutquenne-Nazabadioko L Triterpenoids from the Leaves of Alphitonia xerocarpus Baill and their Biological Activity. Phytochemistry 2016, 129, 45–57. [DOI] [PubMed] [Google Scholar]
- (129).Gehrke IT; Neto AT; Pedroso M; Mostardeiro CP; Da Cruz IB; Silva UF; Ilha V; Dalcol II; Morel AF Antimicrobial Activity of Schinus lentiscifolius (Anacardiaceae). J. Ethnopharmacol 2013, 148, 486–491. [DOI] [PubMed] [Google Scholar]
- (130).Wang C-M; Chen H-T; Wu Z-Y; Jhan Y-L; Shyu C-L; Chou C-H Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules 2016, 21, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Srivastava G; Jain R; Vyas N; Mehta A; Kachhwaha S; Kotharim SL Antimicrobial Activity of the Methanolic Extract, Fractions and Isolated Compounds form Citrullus colocynthis (L.) Schrad. Int. J. Pharma. Bio. Sci 2013, 4, 825–833. [Google Scholar]
- (132).Manguro LOA; Lemmen P; Hao P; Wong K-C Triterpene Saponins of Maesa lanceolata stem wood. J. Asian Nat. Prod. Res 2012, 14, 987–1001. [DOI] [PubMed] [Google Scholar]
- (133).Chung PY; Chung LY; Navaratnam P Potential Targets by Pentacyclic Triterpenoids from Callicarpa farinosa against Methicillin-Resistant and Sensitive Staphylococcus aureus. Fitoterapia 2014, 94, 48–54. [DOI] [PubMed] [Google Scholar]
- (134).Guo H; Yao S; Yang X; Chen Y; Chen Y; Gong F; Tong J; Qian J; Zhang A; Cai X Oxidatively Rearranged Cycloartane Triterpenoids from the Seeds of Pseudolarix amabilis. Nat. Prod. Res 2018, 32, 1817–1823. [DOI] [PubMed] [Google Scholar]
- (135).Perianayagam JB; Sharma SK; Pillai KK; Pandurangan A; Kesavan D Evaluation of Antimicrobial Activity of Ethanol Extract and Compounds Isolated from Trichodesma indicum (Linn.) R. Br. Root. J. Ethnopharmacol 2012, 142, 283–286. [DOI] [PubMed] [Google Scholar]
- (136).Tagousop CN; Tamokou J.-d.-D.; Kengne IC; Ngnokam D; Voutquenne-Nazabadioko L Antimicrobial Activities of Saponins from Melanthera elliptica and their Synergistic Effects with Antibiotics against Pathogenic Phenotypes. Chem. Cent. J 2018, 12, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Lunga PK; Qin XJ; Yang XW; Kuiate JR; Du ZZ; Gatsing D A New Antimicrobial and Radical-Scavenging Glycoside from Paullinia pinnata var. cameroonensis. Nat. Prod. Res 2015, 29, 1688–1694. [DOI] [PubMed] [Google Scholar]
- (138).Fujita K; Chavasiri W; Kubo I Anti-Salmonella Activity of Volatile Compounds of Vietnam Coriander. Phytother. Res 2015, 29, 1081–1087. [DOI] [PubMed] [Google Scholar]
- (139).Guzman JD; Evangelopoulos D; Gupta A; Prieto JM; Gibbons S; Bhakta S Antimycobacterials from Lovage Root (Ligusticum officinale Koch). Phytother. Res 2013, 27, 993–998. [DOI] [PubMed] [Google Scholar]
- (140).Panyo J; Matsunami K; Panichayupakaranant P Bioassay-Guided Isolation and Evaluation of Antimicrobial Compounds from Ixora megalophylla against Some Oral Pathogens. Pharm. Biol 2016, 54, 1522–1527. [DOI] [PubMed] [Google Scholar]
- (141).Liu C; Li XT; Cheng RR; Han ZZ; Yang L; Song ZC; Wang ZT Anti-oral Common Pathogenic Bacterial Active Acetylenic Acids from Thesium chinense Turcz. J. Nat. Med 2018, 72, 433–438. [DOI] [PubMed] [Google Scholar]
- (142).Kim YS; Kim H; Jung E; Kim JH; Hwang W; Kang EJ; Lee S; Ha BJ; Lee J; Park D A Novel Antibacterial Compound from Siegesbeckia glabrescens. Molecules 2012, 17, 12469–12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).da Cruz RC; Denardi LB; Mossmann NJ; Piana M; Alves SH; de Campos MM Antimicrobial Activity and Chromatographic Analysis of Extracts from Tropaeolum pentaphyllum Lam. Tubers. Molecules 2016, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Wallock-Richards D; Doherty CJ; Doherty L; Clarke DJ; Place M; Govan JR; Campopiano DJ Garlic Revisited: Antimicrobial Activity of Allicin-Containing Garlic Extracts against Burkholderia cepacia Complex. PLoS One 2014, 9, e112726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Sorlozano-Puerto A; Albertuz-Crespo M; Lopez-Machado I; Ariza-Romero JJ; Banos-Arjona A; Exposito-Ruiz M; Gutierrez-Fernandez J In Vitro Antibacterial Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate Derived from Allium spp. against Gram-Negative and Gram-Positive Multidrug-Resistant Bacteria Isolated from Human Samples. Biomed. Res. Int 2018, 2018, 7861207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Zhang L; Chen CJ; Chen J; Zhao QQ; Li Y; Gao K Thiophene Acetylenes and Furanosesquiterpenes from Xanthopappus subacaulis and their Antibacterial Activities. Phytochemistry 2014, 106, 134–140. [DOI] [PubMed] [Google Scholar]
- (147).Daneshmand F; Zare-Zardini H; Ebrahimi L Investigation of the Antimicrobial Activities of Snakin-Z, a New Cationic Peptide Derived from Zizyphus jujuba Fruits. Nat. Prod. Res 2013, 27, 2292–2296. [DOI] [PubMed] [Google Scholar]
- (148).Lima S; Diaz G; Diaz MA Antibacterial Chemical Constituent and Antiseptic Herbal Soap from Salvinia auriculata Aubl. Evid. Based Complement. Alternat. Med 2013, 2013, 480509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Cai X; Guo L; Pei F; Chang X; Zhang R Polyphyllin G Exhibits Antimicrobial Activity and Exerts Anticancer Effects on Human Oral Cancer OECM-1 Cells by Triggering G2/M Cell Cycle Arrest by Inactivating cdc25C-cdc2. Arch. Biochem. Biophys 2018, 644, 93–99. [DOI] [PubMed] [Google Scholar]
- (150).Matsuura H; Fett-Neto A Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action, 2015.
- (151).Verpoorte R In Encyclopedia of Separation Science; Wilson ID, Ed.; Academic Press: Oxford, 2000, DOI: 10.1016/B0-12-226770-2/01421-6. [DOI] [Google Scholar]
- (152).Mithofer A; Boland W Plant Defense Against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol 2012, 63, 431–450. [DOI] [PubMed] [Google Scholar]
- (153).Diaz G Quinolines, Isoquinolines, Angustureine, and Congeneric Alkaloids — Occurrence, Chemistry, and Biological Activity, 2015.
- (154).Shang XF; Morris-Natschke SL; Liu YQ; Guo X; Xu XS; Goto M; Li JC; Yang GZ; Lee KH Biologically Active Quinoline and Quinazoline Alkaloids part I. Med. Res. Rev 2018, 38, 775–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Albert A; Magrath D The Choice of a Chelating Agent for Inactivating Trace Metals: II. Derivatives of Oxine (8-hydroxyquinoline). Biochem. J 1947, 41, 534–545. [PMC free article] [PubMed] [Google Scholar]
- (156).McElroy J The Treatment of Pulmonary Tuberculosis by Intravenous Injections of Chinosol with Formaldehyde. The Lancet 1910, 176, 1408–1409. [Google Scholar]
- (157).Ananthan S; Faaleolea ER; Goldman RC; Hobrath JV; Kwong CD; Laughon BE; Maddry JA; Mehta A; Rasmussen L; Reynolds RC et al. High-Throughput Screening for Inhibitors of Mycobacterium tuberculosis H37Rv. Tuberculosis 2009, 89, 334–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Anjaneyulu Y; Rao RP; Swamy RY; Eknath A; Rao KN In Vitro Antimicrobial-Activity Studies on the Mixed Ligand Complexes of Hg(II) with 8-Hydroxyquinoline and Salicylic Acids. J. Chem. Sci. (Bangalore) 1982, 91, 157–163. [Google Scholar]
- (159).Prachayasittikul V; Prachayasittikul S; Ruchirawat S; Prachayasittikul V 8-Hydroxyquinolines: A Review of their Metal Chelating Properties and Medicinal Applications. Drug Des. Devel. Ther 2013, 7, 1157–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Kuete V Potential of Cameroonian Plants and Derived Products Against Microbial Infections: A Review. Planta Med 2010, 76, 1479–1491. [DOI] [PubMed] [Google Scholar]
- (161).Cos P; Vlietinck AJ; Berghe DV; Maes L Anti-infective Potential of Natural Products: How to Develop a Stronger In Vitro ‘Proof-of-Concept’. J. Ethnopharmacol 2006, 106, 290–302. [DOI] [PubMed] [Google Scholar]
- (162).Cherdtrakulkiat R; Boonpangrak S; Sinthupoom N; Prachayasittikul S; Ruchirawat S; Prachayasittikul V Derivatives (Halogen, Nitro and Amino) of 8-hydroxyquinoline with Highly Potent Antimicrobial and Antioxidant Activities. Biochem. Biophys. Rep 2016, 6, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Fox GJ; Menzies D A Review of the Evidence for Using Bedaquiline (TMC207) to Treat Multi-Drug Resistant Tuberculosis. Infect. Dis. Ther 2013, 2, 123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Wu Y-C In Studies in Natural Products Chemistry, 2006; Vol. 33. [Google Scholar]
- (165).Kukula-Koch WA; Widelski J In Pharmacognosy; Badal S;Delgoda R, Eds.; Academic Press: Boston, 2017, DOI: 10.1016/B978-0-12-802104-0.00009-3. [DOI] [Google Scholar]
- (166).Natural Compounds. Alkaloids; Springer-Verlag New York, 2013. [Google Scholar]
- (167).Castedo L; Tojo G Chapter 3 Phenanthrene Alkaloids. The Alkaloids: Chemistry and Pharmacology 1990, 39, 99–138. [Google Scholar]
- (168).Polson AM; Garrett S; Stoller NH; Bandt CL; Hanes PJ; Killoy WJ; Southard GL; Duke SP; Bogle GC; Drisko CH et al. Multi-center Comparative Evaluation of Subgingivally Delivered Sanguinarine and Doxycycline in the Treatment of Periodontitis. II. Clinical results. J. Periodontol 1997, 68, 119–126. [DOI] [PubMed] [Google Scholar]
- (169).Munro IC; Delzell ES; Nestmann ER; Lynch BS Viadent Usage and Oral Leukoplakia: A Spurious Association. Regul. Toxicol. Pharmacol 1999, 30, 182–196. [DOI] [PubMed] [Google Scholar]
- (170).Vlachojannis C; Magora F; Chrubasik S Rise and Fall of Oral Health Products with Canadian Bloodroot Extract. Phytother. Res 2012, 26, 1423–1426. [DOI] [PubMed] [Google Scholar]
- (171).Damm DD; Curran A; White DK; Drummond JF Leukoplakia of the Maxillary Vestibule--An Association with Viadent? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 1999, 87, 61–66. [DOI] [PubMed] [Google Scholar]
- (172).Rabbani GH; Butler T; Knight J; Sanyal SC; Alam K Randomized Controlled Trial of Berberine Sulfate Therapy for Diarrhea Due to Enterotoxigenic Escherichia coli and Vibrio cholerae. J. Infect. Dis 1987, 155, 979–984. [DOI] [PubMed] [Google Scholar]
- (173).Bandyopadhyay S; Patra PH; Mahanti A; Mondal DK; Dandapat P; Bandyopadhyay S; Samanta I; Lodh C; Bera AK; Bhattacharyya D et al. Potential Antibacterial Activity of Berberine against Multi Drug Resistant Enterovirulent Escherichia coli Isolated from Yaks (Poephagus grunniens) with Haemorrhagic Diarrhoea. Asian Pac. J. Trop. Med 2013, 6, 315–319. [DOI] [PubMed] [Google Scholar]
- (174).Sun N; Chan F-Y; Lu Y-J; Neves MAC; Lui H-K; Wang Y; Chow K-Y; Chan K-F; Yan S-C; Leung Y-C et al. Rational Design of Berberine-Based FtsZ Inhibitors with Broad-Spectrum Antibacterial Activity. PLoS One 2014, 9, e97514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Stermitz FR; Lorenz P; Tawara JN; Zenewicz LA; Lewis K Synergy in a Medicinal Plant: Antimicrobial Action of Berberine Potentiated by 5′-Methoxyhydnocarpin, a Multidrug Pump Inhibitor. Proc. Natl. Acad. Sci. U. S. A 2000, 97, 1433–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Ball AR; Casadei G; Samosorn S; Bremner JB; Ausubel FM; Moy TI; Lewis K Conjugating Berberine to a Multidrug Resistance Pump Inhibitor Creates an Effective Antimicrobial. ACS Chem. Biol 2006, 1, 594–600. [DOI] [PubMed] [Google Scholar]
- (177).Gao W-W; Gopala L; Bheemanaboina RRY; Zhang G-B; Li S; Zhou C-H Discovery of 2-Aminothiazolyl Berberine Derivatives as Effectively Antibacterial Agents Toward Clinically Drug-Resistant Gram-negative Acinetobacter baumanii. Eur. J. Med. Chem 2018, 146, 15–37. [DOI] [PubMed] [Google Scholar]
- (178).Kumar A; Ekavali; Chopra K; Mukherjee M; Pottabathini R; Dhull DK Current Knowledge and Pharmacological Profile of Berberine: An Update. Eur. J. Pharmacol 2015, 761, 288–297. [DOI] [PubMed] [Google Scholar]
- (179).Iwasa K; Nishiyama Y; Ichimaru M; Moriyasu M; Kim H-S; Wataya Y; Yamori T; Takashi T; Lee D-U Structure-Activity Relationships of Quaternary Protoberberine Alkaloids Having an Antimalarial Activity. Eur. J. Med. Chem 1999, 34, 1077–1083. [Google Scholar]
- (180).Sandjo LP; Kuete V; Tchangna RS; Efferth T; Ngadjui BT Cytotoxic Benzophenanthridine and Furoquinoline Alkaloids from Zanthoxylum buesgenii (Rutaceae). Chem. Cent. J 2014, 8, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Pan G.-y.; Wang G-J; Liu X-D; Fawcett JP; Xie Y-Y The Involvement of P-Glycoprotein in Berberine Absorption. Pharmacology & Toxicology 2002, 91, 193–197. [DOI] [PubMed] [Google Scholar]
- (182).Mi R; Hu Y-J; Fan X-Y; Ouyang Y; Bai A-M Exploring the Site-Selective Binding of Jatrorrhizine to Human Serum Albumin: Spectroscopic and Molecular Modeling Approaches. Spectrochim. Acta A 2014, 117, 163–169. [DOI] [PubMed] [Google Scholar]
- (183).Long J; Song J; Zhong L; Liao Y; Liu L; Li X Palmatine: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Biochimie 2019, 162, 176–184. [DOI] [PubMed] [Google Scholar]
- (184).Singh N; Sharma B Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci 2018, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Aldred EM; Buck C; Vall K Chapter 21 - Phenols. Pharmacology 2009, DOI: 10.1016/B978-0-443-06898-0.00021-9, 149–166. [DOI] [Google Scholar]
- (186).de la Rosa LA; Moreno-Escamilla JO; Rodrigo-García J; Alvarez-Parrilla E In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia EM, Ed.; Woodhead Publishing, 2019, DOI: 10.1016/B978-0-12-813278-4.00012-9. [DOI] [Google Scholar]
- (187).Cheynier V Phenolic Compounds: From Plants to Foods. Phytochem. Rev 2012, 11, 153–177. [Google Scholar]
- (188).Käss E; Wink M Phylogenetic Relationships in the Papilionoideae (Family Leguminosae) Based on Nucleotide Sequences of cpDNA (rbcL) and ncDNA (ITS 1 and 2). Mol. Phylogenet. Evol 1997, 8, 65–88. [DOI] [PubMed] [Google Scholar]
- (189).The Global Economy of Pulses; Rawal V; Navarro DK, Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- (190).Zhuang C; Zhang W; Sheng C; Zhang W; Xing C; Miao Z Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev 2017, 117, 7762–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Dewick PM Medicinal Natural Products: A Biosynthetic Approach 3rd ed.; John Wiley & Sons: Chichester, England, 2009. [Google Scholar]
- (192).Zhou B; Xing C Diverse Molecular Targets for Chalcones with Varied Bioactivities. Med. Chem. (Los Angeles) 2015, 5, 388–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Kuete V; Noumedem JA; Nana F Chemistry and Pharmacology of 4-Hydroxylonchocarpin: A Review. Chin. J. Integr. Med 2013, 19, 475–480. [DOI] [PubMed] [Google Scholar]
- (194).Fu Y; Hsieh T.-c.; Guo J; Kunicki J; Lee MYWT; Darzynkiewicz Z; Wu JM Licochalcone-A, a Novel Flavonoid Isolated from Licorice Root (Glycyrrhiza glabra), Causes G2 and Late-G1 Arrests in Androgen-Independent PC-3 Prostate Cancer Cells. Biochem. Biophys. Res. Commun 2004, 322, 263–270. [DOI] [PubMed] [Google Scholar]
- (195).Haraguchi H; Tanimoto K; Tamura Y; Mizutani K; Kinoshita T Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125–129. [DOI] [PubMed] [Google Scholar]
- (196).Wu SC; Yang ZQ; Liu F; Peng WJ; Qu SQ; Li Q; Song XB; Zhu K; Shen JZ Antibacterial Effect and Mode of Action of Flavonoids From Licorice Against Methicillin-Resistant Staphylococcus aureus. Front. Microbiol 2019, 10, 2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Bethesda (MD): National Library of Medicine (US), 2019.
- (198).NLM. National Library of Medicine (US) Available from: http://clinicaltrials.gov. 2020. Accessed on March 31, 2020.
- (199).Meier D; Hernandez MV; van Geelen L; Muharini R; Proksch P; Bandow JE; Kalscheuer R The Plant-derived Chalcone Xanthoangelol Targets the Membrane of Gram-Positive Bacteria. Bioorg. Med. Chem 2019, 27, 115151. [DOI] [PubMed] [Google Scholar]
- (200).Sohn HY; Son KH; Kwon CS; Kwon GS; Kang SS Antimicrobial and Cytotoxic Activity of 18 Prenylated Flavonoids Isolated from Medicinal Plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [DOI] [PubMed] [Google Scholar]
- (201).Nielsen SF; Boesen T; Larsen M; Schønning K; Kromann H Antibacterial Chalcones––Bioisosteric Replacement of the 4′-hydroxy Group. Bioorg. Med. Chem 2004, 12, 3047–3054. [DOI] [PubMed] [Google Scholar]
- (202).Shi M; Cui Y; Liu C; Li C; Liu Z; Kang W -y. CYPs-Mediated Drug-Drug Interactions on Psoralidin, Isobavachalcone, Neobavaisoflavone and Daidzein in Rats Liver Microsomes. Food and Chemical Toxicology 2020, 136, 111027. [DOI] [PubMed] [Google Scholar]
- (203).Kuete V; Ngameni B; Tangmouo JG; Bolla J-M; Alibert-Franco S; Ngadjui BT; Pagès J-M Efflux Pumps Are Involved in the Defense of Gram-Negative Bacteria against the Natural Products Isobavachalcone and Diospyrone. Antimicrob. Agents Chemother 2010, 54, 1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Batovska DI; Todorova IT Trends in Utilization of the Pharmacological Potential of Chalcones. Curr. Clin. Pharmacol 2010, 5, 1–29. [DOI] [PubMed] [Google Scholar]
- (205).Isomoto H; Furusu H; Ohnita K; Wen CY; Inoue K; Kohno S Sofalcone, a Mucoprotective Agent, Increases the Cure Rate of Helicobacter pylori Infection When Combined with Rabeprazole, Amoxicillin and Clarithromycin. World J. Gastroenterol 2005, 11, 1629–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Dan W; Dai J Recent Developments of Chalcones as Potential Antibacterial Agents in Medicinal Chemistry. Eur. J. Med. Chem 2020, 187, 111980. [DOI] [PubMed] [Google Scholar]
- (207).Venugopala KN; Rashmi V; Odhav B Review on Natural Coumarin Lead Compounds for their Pharmacological Activity. Biomed. Res. Int 2013, 2013, 963248–963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Matos MJ; Santana L; Uriarte E; Abreu O; Molina Pérez E; Yordi E Coumarins — An Important Class of Phytochemicals 2015, DOI: 10.5772/59982, 113–140. [DOI] [Google Scholar]
- (209).Anderle C; Hennig S; Kammerer B; Li S-M; Wessjohann L; Gust B; Heide L Improved Mutasynthetic Approaches for the Production of Modified Aminocoumarin Antibiotics. Chemistry & Biology 2007, 14, 955–967. [DOI] [PubMed] [Google Scholar]
- (210).Panche AN; Diwan AD; Chandra SR Flavonoids: An Overview. J. Nutr. Sci 2016, 5, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Cowan MM Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev 1999, 12, 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Kumar S; Pandey AK Chemistry and Biological Activities of Flavonoids: An Overview. ScientificWorldJournal 2013, 2013, 162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Riyazuddin M; Husain A; Verma S; Katekar R; Garg R; Kumar S; Satish S; Maurya R; Narender T; Chattopadhyay N et al. Simultaneous Quantification of Five Biomarkers in Ethanolic Extract of Cassia occidentalis Linn. Stem Using Liquid Chromatography Tandem Mass Spectrometry: Application to its Pharmacokinetic Studies. RSC Adv 2020, 10, 4579–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Awouafack MD; McGaw LJ; Gottfried S; Mbouangouere R; Tane P; Spiteller M; Eloff JN Antimicrobial Activity and Cytotoxicity of the Ethanol Extract, Fractions and Eight Compounds Isolated from Eriosema robustum (Fabaceae). BMC Complement. Altern. Med 2013, 13, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Awouafack MD; Tane P; Eloff JN Two New Antioxidant Flavones from the Twigs of Eriosema robustum (Fabaceae). Phytochem. Lett 2013, 6, 62–66. [Google Scholar]
- (216).Okamoto T Safety of Quercetin for Clinical Application (Review). Int. J. Mol. Med 2005, 16, 275–278. [PubMed] [Google Scholar]
- (217).Formica JV; Regelson W Review of the Biology of Quercetin and Related Bioflavonoids. Food Chem. Toxicol 1995, 33, 1061–1080. [DOI] [PubMed] [Google Scholar]
- (218).Woznicka E; Kuzniar A; Nowak D; Nykiel E; Kopacz M; Gruszecka J; Golec K Comparative Study on the Antibacterial Activity of Some Flavonoids and their Sulfonic Derivatives. Acta Pol. Pharm 2013, 70, 567–571. [PubMed] [Google Scholar]
- (219).Siriwong S; Teethaisong Y; Thumanu K; Dunkhunthod B; Eumkeb G The Synergy and Mode of Action of Quercetin Plus Amoxicillin Against Amoxicillin-Resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol 2016, 17, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Siriwong S; Thumanu K; Hengpratom T; Eumkeb G Synergy and Mode of Action of Ceftazidime plus Quercetin or Luteolin on Streptococcus pyogenes. Evid. Based Complement. Alternat. Med 2015, 2015, 759459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Eumkeb G; Sakdarat S; Siriwong S Reversing Beta-Lactam Antibiotic Resistance of Staphylococcus aureus with Galangin from Alpinia officinarum Hance and Synergism with Ceftazidime. Phytomedicine 2010, 18, 40–45. [DOI] [PubMed] [Google Scholar]
- (222).Gopu V; Meena CK; Shetty PH Quercetin Influences Quorum Sensing in Food Borne Bacteria: In-Vitro and In-Silico Evidence. PLoS One 2015, 10, e0134684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (223).Heinz SA; Henson DA; Austin MD; Jin F; Nieman DC Quercetin Supplementation and Upper Respiratory Tract Infection: A Randomized Community Clinical Trial. Pharmacol. Res 2010, 62, 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Semwal DK; Semwal RB; Combrinck S; Viljoen A Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (225).Jayaraman P; Sakharkar MK; Lim CS; Tang TH; Sakharkar KR Activity and Interactions of Antibiotic and Phytochemical Combinations against Pseudomonas aeruginosa In Vitro. Int. J. Biol. Sci 2010, 6, 556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Yemele MD; Telefo PB; Lienou LL; Tagne SR; Fodouop CSP; Goka CS; Lemfack MC; Moundipa FP Ethnobotanical Survey of Medicinal Plants used for Pregnant Women׳s Health Conditions in Menoua division-West Cameroon. J. Ethnopharmacol 2015, 160, 14–31. [DOI] [PubMed] [Google Scholar]
- (227).Mugisha MK; Asiimwe S; Namutebi A; Borg-Karlson A-K; Kakudidi EK Ethnobotanical Study of Indigenous Knowledge on Medicinal and Nutritious Plants Used to Manage Opportunistic Infections Associated with HIV/AIDS in Western Uganda. J. Ethnopharmacol 2014, 155, 194–202. [DOI] [PubMed] [Google Scholar]
- (228).Wei H; Zhu J-J; Liu X-Q; Feng W-H; Wang Z-M; Yan L-H; Spencer J Review of Bioactive Compounds from Root Barks of Morus Plants (Sang-Bai-Pi) and their Pharmacological Effects. Cogent Chem 2016, 2. [Google Scholar]
- (229).Ferrari F; Delle Monache F; Suarez AI; Compagnone RS Multicaulisin, A New Diels–Alder Type Adduct from Morus multicaulis. Fitoterapia 2000, 71, 213–215. [DOI] [PubMed] [Google Scholar]
- (230).Nomura T; Fukai T; Narita T; Terada S; Uzawa J; Iitaka Y; Takasugi M; Ishikawa S.-i.; Nagao; Masamune T Confirmation of the Structures of Kuwanons G and H (Albanins F and G) by Partial Synthesis. Tetrahedron Lett 1981, 22, 2195–2198. [Google Scholar]
- (231).Wu SC; Han F; Song MR; Chen S; Li Q; Zhang Q; Zhu K; Shen JZ Natural Flavones from Morus alba against Methicillin-Resistant Staphylococcus aureus via Targeting the Proton Motive Force and Membrane Permeability. J. Agric. Food Chem 2019, 67, 10222–10234. [DOI] [PubMed] [Google Scholar]
- (232).Iverson CD; Zahid S; Li Y; Shoqafi AH; Ata A; Samarasekera R Glutathione S-transferase Inhibitory, Free Radical Scavenging, and Anti-Leishmanial Activities of Chemical Constituents of Artocarpus nobilis and Matricaria chamomilla. Phytochem. Lett 2010, 3, 207–211. [Google Scholar]
- (233).Hakim EH; Achmad SA; Juliawaty LD; Makmur L; Syah YM; Aimi N; Kitajima M; Takayama H; Ghisalberti EL Prenylated Flavonoids and Related Compounds of the Indonesian Artocarpus (Moraceae). J. Nat. Med 2006, 60, 161–184. [DOI] [PubMed] [Google Scholar]
- (234).Zoofishan Z; Kusz N; Csorba A; Toth G; Hajagos-Toth J; Kothencz A; Gaspar R; Hunyadi A Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra. Molecules 2019, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Hou C; Liu W; Liang Z; Han W; Li J; Ye L; Liu M; Cai Z; Zhao J; Chen Y et al. UGT-Mediated Metabolism Plays a Dominant Role in the Pharmacokinetic Behavior and the Disposition of Morusin In Vivo and In Vitro. J. Pharm. Biomed. Anal 2018, 154, 339–353. [DOI] [PubMed] [Google Scholar]
- (236).Pang D; Liao S; Wang W; Mu L; Li E; Shen W; Liu F; Zou Y Destruction of the Cell Membrane and Inhibition of Cell Phosphatidic Acid Biosynthesis in Staphylococcus aureus: An Explanation for the Antibacterial Mechanism of Morusin. Food Funct 2019, 10, 6438–6446. [DOI] [PubMed] [Google Scholar]
- (237).Urgaonkar S; La Pierre HS; Meir I; Lund H; RayChaudhuri D; Shaw JT Synthesis of Antimicrobial Natural Products Targeting FtsZ: (+/-)-dichamanetin and (+/-)-2’ ‘‘-hydroxy-5’ ‘-benzylisouvarinol-B. Org. Lett 2005, 7, 5609–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (238).Pan L; Matthew S; Lantvit DD; Zhang X; Ninh TN; Chai H; Carcache de Blanco EJ; Soejarto DD; Swanson SM; Kinghorn AD Bioassay-Guided Isolation of Constituents of Piper sarmentosum using a Mitochondrial Transmembrane Potential Assay. J. Nat. Prod 2011, 74, 2193–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (239).Limsuwan S; Moosigapong K; Jarukitsakul S; Joycharat N; Chusri S; Jaisamut P; Voravuthikunchai SP Lupinifolin from Albizia myriophylla Wood: A Study on its Antibacterial Mechanisms Against Cariogenic Streptococcus mutans. Arch. Oral Biol 2018, 93, 195–202. [DOI] [PubMed] [Google Scholar]
- (240).Joycharat N; Limsuwan S; Subhadhirasakul S; Voravuthikunchai SP; Pratumwan S; Madahin I; Nuankaew W; Promsawat A Anti-Streptococcus mutans Efficacy of Thai Herbal Formula used as a Remedy for Dental Caries. Pharm. Biol 2012, 50, 941–947. [DOI] [PubMed] [Google Scholar]
- (241).Harborne JB; Greenham J; Williams CA; Eagles J; Markham KR Ten Isoprenylated and C-methylated Flavonoids from the Leaves of three Vellozia species. Phytochemistry 1993, 34, 219–226. [Google Scholar]
- (242).Fakhimi A; Iranshahi M; Emami SA; Amin-Ar-Ramimeh E; Zarrini G; Shahverdi AR Sophoraflavanone G from Sophora pachycarpa Enhanced the Antibacterial Activity of Gentamycin against Staphylococcus aureus. Z. Naturforsch., C, J. Biosci 2006, 61, 769–772. [PubMed] [Google Scholar]
- (243).Tsuchiya H; Sato M; Iinuma M; Yokoyama J; Ohyama M; Tanaka T; Takase I; Namikawa I Inhibition of the Growth of Cariogenic Bacteria In Vitro by Plant Flavanones. Experientia 1994, 50, 846–849. [DOI] [PubMed] [Google Scholar]
- (244).Yamamoto H; Zhao P; Inoue K Origin of Two Isoprenoid Units in a Lavandulyl Moiety of Sophoraflavanone G from Sophora flavescens Cultured Cells. Phytochemistry 2002, 60, 263–267. [DOI] [PubMed] [Google Scholar]
- (245).Yamamoto H; Yatou A; Inoue K 8-Dimethylallylnaringenin 2′-hydroxylase, the Crucial Cytochrome P450 Mono-oxygenase for Lavandulylated Flavanone Formation in Sophora flavescens Cultured Cells. Phytochemistry 2001, 58, 671–676. [DOI] [PubMed] [Google Scholar]
- (246).Mun S-H; Joung D-K; Kim S-B; Park S-J; Seo Y-S; Gong R; Choi J-G; Shin D-W; Rho J-R; Kang O-H et al. The Mechanism of Antimicrobial Activity of Sophoraflavanone B Against Methicillin-Resistant Staphylococcus aureus. Foodborne Pathog. Dis 2014, 11, 234–239. [DOI] [PubMed] [Google Scholar]
- (247).Yu Q; Cheng N; Ni X Identifying 2 Prenylflavanones as Potential Hepatotoxic Compounds in the Ethanol Extract of Sophora flavescens. J. Food Sci 2013, 78, T1830–T1834. [DOI] [PubMed] [Google Scholar]
- (248).Miguel L-L Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev. Med. Chem 2009, 9, 31–59. [DOI] [PubMed] [Google Scholar]
- (249).Yun J; Woo ER; Lee DG Effect of Isoquercitrin on Membrane Dynamics and Apoptosis-like Death in Escherichia coli. Biochim. Biophys. Acta Biomembr 2018, 1860, 357–363. [DOI] [PubMed] [Google Scholar]
- (250).Yu S; Yan H; Zhang L; Shan M; Chen P; Ding A; Li SF A Review on the Phytochemistry, Pharmacology, and Pharmacokinetics of Amentoflavone, a Naturally-Occurring Biflavonoid. Molecules 2017, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (251).Srividhya M; Hridya H; Shanthi V; Ramanathan K Bioactive Amento Flavone Isolated from Cassia fistula L. Leaves Exhibits Therapeutic Efficacy. 3 Biotech 2017, 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (252).Shen X; Niu X; Li G; Deng X; Wang J Amentoflavone Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo. Appl. Environ. Microbiol 2018, 84, e01804–01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Silva LN; Da Hora GCA; Soares TA; Bojer MS; Ingmer H; Macedo AJ; Trentin DS Myricetin Protects Galleria mellonella against Staphylococcus aureus Infection and Inhibits Multiple Virulence Factors. Sci. Rep 2017, 7, 2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (254).Sahyon HA; Ramadan ENM; Mashaly MMA Synergistic Effect of Quercetin in Combination with Sulfamethoxazole as New Antibacterial Agent: In Vitro and In Vivo Study. Pharm. Chem. J 2019, 53, 803–813. [Google Scholar]
- (255).Törmäkangas L; Vuorela P; Saario E; Leinonen M; Saikku P; Vuorela H In Vivo Treatment of Acute Chlamydia pneumoniae Infection with the Flavonoids Quercetin and Luteolin and an Alkyl Gallate, Octyl Gallate, in a Mouse Model. Biochem. Pharmacol 2005, 70, 1222–1230. [DOI] [PubMed] [Google Scholar]
- (256).D’Andrea G Quercetin: A Flavonol with Multifaceted Therapeutic Applications? Fitoterapia 2015, 106, 256–271. [DOI] [PubMed] [Google Scholar]
- (257).Musika J; Chudapongse N Development of Lipid-Based Nanocarriers for Increasing Gastrointestinal Absorption of Lupinifolin. Planta Med 2020, 86, 364–372. [DOI] [PubMed] [Google Scholar]
- (258).Alonso-Castro AJ; Domínguez F; Maldonado-Miranda JJ; Castillo-Pérez LJ; Carranza-Álvarez C; Solano E; Isiordia-Espinoza MA; del Carmen Juárez-Vázquez M; Zapata-Morales JR; Argueta-Fuertes MA et al. Use of Medicinal Plants by Health Professionals in Mexico. J. Ethnopharmacol 2017, 198, 81–86. [DOI] [PubMed] [Google Scholar]
- (259).Favela-Hernández J; Clemente-Soto A; Balderas-Rentería I; Garza-González E; Camacho-Corona M Potential Mechanism of Action of 3′-Demethoxy-6-O-demethyl-isoguaiacin on Methicillin Resistant Staphylococcus aureus. Molecules 2015, 20, 12450–12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (260).Clemente-Soto AF; Balderas-Renteria I; Rivera G; Segura-Cabrera A; Garza-Gonzalez E; del Rayo Camacho-Corona M Potential Mechanism of Action of Meso-Dihydroguaiaretic Acid on Mycobacterium tuberculosis H37Rv. Molecules 2014, 19, 20170–20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (261).Cerniglia CE; Freeman JP; Althaus JR; van Baalen C Metabolism and Toxicity of 1- and 2-Methylnaphthalene and Their Derivatives in Cyanobacteria. Arch. Clin. Microbiol 1983, 136, 177–183. [Google Scholar]
- (262).Dewick PM Medicinal Natural Products: A Biosynthetic Approach; 2nd ed.; John Wiley & Sons: Chichester, England, 2009. [Google Scholar]
- (263).Ali BH; Blunden G Pharmacological and Toxicological Properties of Nigella sativa. Phytother. Res 2003, 17, 299–305. [DOI] [PubMed] [Google Scholar]
- (264).Taborsky J; Kunt M; Kloucek P; Lachman J; Zeleny V; Kokoska L Identification of Potential Sources of Thymoquinone and Related Compounds in Asteraceae, Cupressaceae, Lamiaceae, and Ranunculaceae Families. Open Chem 2012, 10, 1899–1906. [Google Scholar]
- (265).Chaieb K; Kouidhi B; Jrah H; Mahdouani K; Bakhrouf A Antibacterial Activity of Thymoquinone, an Active Principle of Nigella sativa and its Potency to Prevent Bacterial Biofilm Formation. BMC Complement. Altern. Med 2011, 11, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (266).Kouidhi B; Zmantar T; Jrah H; Souiden Y; Chaieb K; Mahdouani K; Bakhrouf A Antibacterial and Resistance-modifying Activities of Thymoquinone Against Oral Pathogens. Ann. Clin. Microbiol. Antimicrob 2011, 10, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).Rifaioglu MM; Nacar A; Yuksel R; Yonden Z; Karcioglu M; Zorba OU; Davarci I; Sefil NK Antioxidative and Anti-inflammatory Effect of Thymoquinone in an Acute Pseudomonas prostatitis Rat Model. Urol. Int 2013, 91, 474–481. [DOI] [PubMed] [Google Scholar]
- (268).Uc-Cachón AH; Molina-Salinas GM; Said-Fernández S; Méndez-González M; Cáceres-Farfán M; Borges-Argáez R A New Dimeric Naphthoquinone from Diospyros anisandra. Nat. Prod. Res 2013, 27, 1174–1178. [DOI] [PubMed] [Google Scholar]
- (269).Mathew R; Kruthiventi AK; Prasad JV; Kumar SP; Srinu G; Chatterji D Inhibition of Mycobacterial Growth by Plumbagin Derivatives. Chem. Biol. Drug Des 2010, 76, 34–42. [DOI] [PubMed] [Google Scholar]
- (270).van der Kooy F; Meyer JJM; Lall N Antimycobacterial Activity and Possible Mode of Action of Newly Isolated Neodiospyrin and other Naphthoquinones from Euclea natalensis. S. Afr. J. Bot 2006, 72, 349–352. [Google Scholar]
- (271).Bender RP; Ham A-JL; Osheroff N Quinone-Induced Enhancement of DNA Cleavage by Human Topoisomerase IIα: Adduction of Cysteine Residues 392 and 405. Biochemistry 2007, 46, 2856–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Karkare S; Chung TT; Collin F; Mitchenall LA; McKay AR; Greive SJ; Meyer JJ; Lall N; Maxwell A The Naphthoquinone Diospyrin is an Inhibitor of DNA Gyrase with a Novel Mechanism of Action. J. Biol. Chem 2013, 288, 5149–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (273).Dong X; Fu J; Yin X; Cao S; Li X; Lin L; Ni J Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res 2016, 30, 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (274).Izhaki I Emodin – A Secondary Metabolite with Multiple Ecological Functions in Higher Plants. New Phytol 2002, 155, 205–217. [Google Scholar]
- (275).Dong X; Zeng Y; Liu Y; You L; Yin X; Fu J; Ni J Aloe-Emodin: A Review of its Pharmacology, Toxicity, and Pharmacokinetics. Phytother. Res 2020, 34, 270–281. [DOI] [PubMed] [Google Scholar]
- (276).Xu JS; Cui Y; Liao XM; Tan XB; Cao X Effect of Emodin on the Cariogenic Properties of Streptococcus mutans and the Development of Caries in Rats. Exp. Ther. Med 2014, 8, 1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Xiang H; Cao F; Ming D; Zheng Y; Dong X; Zhong X; Mu D; Li B; Zhong L; Cao J et al. Aloe-emodin Inhibits Staphylococcus aureus Biofilms and Extracellular Protein Production at the Initial Adhesion Stage of Biofilm Development. Appl. Microbiol. Biotechnol 2017, 101, 6671–6681. [DOI] [PubMed] [Google Scholar]
- (278).Wang HH; Chung JG; Ho CC; Wu LT; Chang SH Aloe-modin Effects on Arylamine N-acetyltransferase Activity in the Bacterium Helicobacter pylori. Planta Med 1998, 64, 176–178. [DOI] [PubMed] [Google Scholar]
- (279).Masuda Y; Suzuki R; Sakagami H; Umemura N; Shirataki Y Novel Cytotoxic Phenanthrenequinone from Odontioda Marie Noel ‘Velano’. Chem. Pharm. Bull 2012, 60, 1216–1219. [DOI] [PubMed] [Google Scholar]
- (280).Lertnitikul N; Pattamadilok C; Chansriniyom C; Suttisri R A New Dihydrophenanthrene from Cymbidium finlaysonianum and Structure Revision of Cymbinodin-A. J. Asian Nat. Prod. Res 2020, 22, 83–90. [DOI] [PubMed] [Google Scholar]
- (281).Alkharfy KM; Al-Daghri NM; Al-Attas OS; Alokail MS The Protective Effect of Thymoquinone Against Sepsis Syndrome Morbidity and Mortality in Mice. Int. Immunopharmacol 2011, 11, 250–254. [DOI] [PubMed] [Google Scholar]
- (282).Yang J; Chen Z; Ching P; Shi Q; Li X An Integrated Microfluidic Platform for Evaluating In Vivo Antimicrobial Activity of Natural Compounds Using a Whole-Animal Infection Model. Lab Chip 2013, 13, 3373–3382. [DOI] [PubMed] [Google Scholar]
- (283).Jiang L; Yi T; Shen Z; Teng Z; Wang J Aloe-emodin Attenuates Staphylococcus aureus Pathogenicity by Interfering With the Oligomerization of α-Toxin. Front. Cell Infect. Microbiol 2019, 9, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (284).Mashayekhi-Sardoo H; Rezaee R; Karimi G An Overview of In Vivo Toxicological Profile of Thymoquinone. Toxin Rev 2020, 39, 115–122. [Google Scholar]
- (285).Peres V; Nagem TJ; de Oliveira FF Tetraoxygenated Naturally Occurring Xanthones. Phytochemistry 2000, 55, 683–710. [DOI] [PubMed] [Google Scholar]
- (286).Bisht V Naturally Occurring Xanthones: Chemistry and Biology. J. Appl. Chem 2013, 2013. [Google Scholar]
- (287).Peters S; Schmidt W; Beerhues L Regioselective Oxidative Phenol Couplings of 2,3′,4,6-tetrahydroxybenzophenone in Cell Cultures of Centaurium erythraea RAFN and Hypericum androsaemum L. Planta 1997, 204, 64–69. [Google Scholar]
- (288).Obolskiy D; Pischel I; Siriwatanametanon N; Heinrich M Garcinia mangostana L.: A Phytochemical and Pharmacological Review. Phytother. Res 2009, 23, 1047–1065. [DOI] [PubMed] [Google Scholar]
- (289).Koh J-J; Qiu S; Zou H; Lakshminarayanan R; Li J; Zhou X; Tang C; Saraswathi P; Verma C; Tan DTH et al. Rapid Bactericidal Action of Alpha-Mangostin against MRSA as an Outcome of Membrane Targeting. Biochim. Biophys. Acta Biomembr 2013, 1828, 834–844. [DOI] [PubMed] [Google Scholar]
- (290).Sivaranjani M; Leskinen K; Aravindraja C; Saavalainen P; Pandian SK; Skurnik M; Ravi AV Deciphering the Antibacterial Mode of Action of Alpha-Mangostin on Staphylococcus epidermidis RP62A Through an Integrated Transcriptomic and Proteomic Approach. Front. Microbiol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (291).Le TT; Pandey RP; Gurung RB; Dhakal D; Sohng JK Efficient Enzymatic Systems for Synthesis of Novel α-Mangostin Glycosides Exhibiting Antibacterial Activity against Gram-Positive Bacteria. Appl. Microbiol. Biotechnol 2014, 98, 8527–8538. [DOI] [PubMed] [Google Scholar]
- (292).Dharmaratne HRW; Sakagami Y; Piyasena KGP; Thevanesam V Antibacterial Activity of Xanthones from Garcinia mangostana (L.) and their Structure–Activity Relationship Studies. Nat. Prod. Res 2013, 27, 938–941. [DOI] [PubMed] [Google Scholar]
- (293).Tatiya-aphiradee N; Chatuphonprasert W; Jarukamjorn K In Vivo Antibacterial Activity of Garcinia mangostana Pericarp Extract against Methicillin-resistant Staphylococcus aureus in a Mouse Superficial Skin Infection Model. Pharm. Biol 2016, 54, 2606–2615. [DOI] [PubMed] [Google Scholar]
- (294).Ibrahim MY; Hashim NM; Mohan S; Abdulla MA; Abdelwahab SI; Arbab IA; Yahayu M; Ali LZ; Ishag OE α-Mangostin from Cratoxylum arborescens: An In Vitro and In Vivo Toxicological Evaluation. Arab. J. Chem 2015, 8, 129–137. [Google Scholar]
- (295).Li L; Brunner I; Han A-R; Hamburger M; Kinghorn AD; Frye R; Butterweck V Pharmacokinetics of α-mangostin in Rats after Intravenous and Oral Application. Molecular Nutrition & Food Research 2011, 55, S67–S74. [DOI] [PubMed] [Google Scholar]
- (296).Geissman TA Chapter X - Flavonoid Compounds, Tannins, Lignins and, Related Compounds. Compr. Biochem 1963, 9, 213–250. [Google Scholar]
- (297).Scalbert A Antimicrobial Properties of Tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar]
- (298).Mason TL; Wasserman BP Inactivation of Red Beet Beta Glucan Synthase by Native and Oxidized Phenolic Compounds. Phytochemistry 1987, 26, 2197–2202. [Google Scholar]
- (299).Shahzad M; Millhouse E; Culshaw S; Edwards CA; Ramage G; Combet E Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct 2015, 6, 719–729. [DOI] [PubMed] [Google Scholar]
- (300).McManus JP; Davis KG; Beart JE; Gaffney SH; Lilley TH; Haslam E Polyphenol Interactions. Part 1. Introduction; Some Observations on the Reversible Complexation of Polyphenols with Proteins and Polysaccharides. J. Chem. Soc. Perkin 2 1985, DOI: 10.1039/P29850001429, 1429–1438. [DOI] [Google Scholar]
- (301).Andjelković M; Van Camp J; De Meulenaer B; Depaemelaere G; Socaciu C; Verloo M; Verhe R Iron-Chelation Properties of Phenolic Acids Bearing Catechol and Galloyl Groups. Food Chem 2006, 98, 23–31. [Google Scholar]
- (302).Baruah K; Duy Phong HPP; Norouzitallab P; Defoirdt T; Bossier P The Gnotobiotic Brine Shrimp (Artemia franciscana) Model System Reveals that the Phenolic Compound Pyrogallol Protects against Infection Through its Prooxidant Activity. Free Radic. Biol. Med 2015, 89, 593–601. [DOI] [PubMed] [Google Scholar]
- (303).El SN; Karakaya S Olive Tree (Olea europaea) Leaves: Potential Beneficial Efects on Hman Halth. Nutr. Rev 2009, 67, 632–638. [DOI] [PubMed] [Google Scholar]
- (304).Vilaplana-Pérez C; Auñón D; García-Flores LA; Gil-Izquierdo A Hydroxytyrosol and Potential uses in Cardiovascular Diseases, Cancer, and AIDS. Front. Nutr 2014, 1, 18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (305).Santos SA; Freire CS; Domingues MR; Silvestre AJ; Pascoal Neto C Characterization of Phenolic Components in Polar Extracts of Eucalyptus globulus Labill. Bark by High-Performance Liquid Chromatography-Mass Spectrometry. J. Agric. Food Chem 2011, 59, 9386–9393. [DOI] [PubMed] [Google Scholar]
- (306).Martins N; Barros L; Santos-Buelga C; Silva S; Henriques M; Ferreira IC Decoction, Infusion and Hydroalcoholic Extract of Cultivated Thyme: Antioxidant and Antibacterial Activities, and Phenolic Characterisation. Food Chem 2015, 167, 131–137. [DOI] [PubMed] [Google Scholar]
- (307).Tsunoda M; Aoyama C; Nomura H; Toyoda T; Matsuki N; Funatsu T Simultaneous Determination of Dopamine and 3,4-Dihydroxyphenylacetic Acid in Mouse Striatum using Mixed-Mode Reversed-Phase and Cation-Exchange High-Performance Liquid Chromatography. J. Pharm. Biomed. Anal 2010, 51, 712–715. [DOI] [PubMed] [Google Scholar]
- (308).Deutch AY; Tam SY; Roth RH Footshock and Conditioned Stress Increase 3,4-dihydroxyphenylacetic acid (DOPAC) in the Ventral Tegmental Area but not Substantia Nigra. Brain Res 1985, 333, 143–146. [DOI] [PubMed] [Google Scholar]
- (309).Xu F; Cao S; Wang C; Wang K; Wei Y; Shao X; Wang H Antimicrobial Activity of Flavonoids from Sedum aizoon L. against Aeromonas in Culture Medium and in Frozen Pork. Food Sci. Nutr 2019, 7, 3224–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (310).Kamatham S; Kumar N; Gudipalli P Isolation and Characterization of Gallic Acid and Methyl Gallate from the Seed Coats of Givotia rottleriformis Griff. and their Anti-Proliferative Effect on Human Epidermoid Carcinoma A431 Cells. Toxicol. Rep 2015, 2, 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (311).Sanchez E; Heredia N; Camacho-Corona Mdel R; Garcia S Isolation, Characterization and Mode of Antimicrobial Action Against Vibrio cholerae of Methyl Gallate Isolated from Acacia farnesiana. J. Appl. Microbiol 2013, 115, 1307–1316. [DOI] [PubMed] [Google Scholar]
- (312).Ooshiro A; Kaji M; Katoh Y; Kawaide H; Natsume M Antibacterial Activity of Alkyl Gallates and Related Compounds against Ralstonia solanacearum. J. Pestic. Sci 2011, 36, 240–242. [Google Scholar]
- (313).Shiu WK; Gibbons S Anti-Staphylococcal Acylphloroglucinols from Hypericum beanii. Phytochemistry 2006, 67, 2568–2572. [DOI] [PubMed] [Google Scholar]
- (314).Sharma RA; Gescher AJ; Steward WP Curcumin: The Story So Far. Eur. J. Cancer 2005, 41, 1955–1968. [DOI] [PubMed] [Google Scholar]
- (315).Shahzad M; Millhouse E; Culshaw S; Edwards CA; Ramage G; Combet E Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food & function 2015, 6, 719–729. [DOI] [PubMed] [Google Scholar]
- (316).Rai D; Singh Jay K.; Roy N; Panda D Curcumin Inhibits FtsZ Assembly: An Attractive Mechanism for its Antibacterial Activity. Biochem. J 2008, 410, 147–155. [DOI] [PubMed] [Google Scholar]
- (317).De R; Kundu P; Swarnakar S; Ramamurthy T; Chowdhury A; Nair GB; Mukhopadhyay AK Antimicrobial Activity of Curcumin against Helicobacter pylori Isolates from India and during Infections in Mice. Antimicrob. Agents Chemother 2009, 53, 1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (318).Bansal S; Chhibber S Curcumin Alone and in Combination with Augmentin Protects against Pulmonary Inflammation and Acute Lung Injury Generated during Klebsiella pneumoniae B5055-Induced Lung Infection in BALB/c Mice. J. Med. Microbiol 2010, 59, 429–437. [DOI] [PubMed] [Google Scholar]
- (319).Rudrappa T; Bais HP Curcumin, a Known Phenolic from Curcuma longa, Attenuates the Virulence of Pseudomonas aeruginosa PAO1 in Whole Plant and Animal Pathogenicity Models. J. Agric. Food Chem 2008, 56, 1955–1962. [DOI] [PubMed] [Google Scholar]
- (320).Song J; Choi B; Jin EJ; Yoon Y; Choi KH Curcumin Suppresses Streptococcus mutans Adherence to Human Tooth Surfaces and Extracellular Matrix Proteins. Eur. J. Clin. Microbiol. Infect. Dis 2012, 31, 1347–1352. [DOI] [PubMed] [Google Scholar]
- (321).Na HS; Cha MH; Oh D-R; Cho C-W; Rhee JH; Kim YR Protective Mechanism of Curcumin against Vibrio vulnificus Infection. FEMS Immunol. Med. Microbiol 2011, 63, 355–362. [DOI] [PubMed] [Google Scholar]
- (322).Di Mario F; Cavallaro LG; Nouvenne A; Stefani N; Cavestro GM; Iori V; Maino M; Comparato G; Fanigliulo L; Morana E et al. A Curcumin-Based 1-Week Triple Therapy for Eradication of Helicobacter pylori Infection: Something to Learn From Failure? Helicobacter 2007, 12, 238–243. [DOI] [PubMed] [Google Scholar]
- (323).Koosirirat C; Linpisarn S; Changsom D; Chawansuntati K; Wipasa J Investigation of the Anti-Inflammatory Effect of Curcuma longa in Helicobacter pylori-Infected Patients. Int. Immunopharmacol 2010, 10, 815–818. [DOI] [PubMed] [Google Scholar]
- (324).Zuccotti GV; Trabattoni D; Morelli M; Borgonovo S; Schneider L; Clerici M Immune Modulation by Lactoferrin and Curcumin in Children with Recurrent Respiratory Infections. J. Biol. Regul. Homeost. Agents 2009, 23, 119–123. [PubMed] [Google Scholar]
- (325).Cai T; Mazzoli S; Bechi A; Addonisio P; Mondaini N; Pagliai RC; Bartoletti R Serenoa repens Associated with Urtica dioica (ProstaMEV®) and Curcumin and Quercitin (FlogMEV®) Extracts are able to Improve the Efficacy of Prulifloxacin in Bacterial Prostatitis Patients: Results from a Prospective Randomised Study. Int. J. Antimicrob. Agents 2009, 33, 549–553. [DOI] [PubMed] [Google Scholar]
- (326).Kia SJ; Mansourian A; Basirat M; Akhavan M; Mohtasham-Amiri Z; Moosavi M-S New Concentration of Curcumin Orabase in Recurrent Aphthous Stomatitis: A Randomized, Controlled Clinical Trial. J. Herb. Med 2020, 100336. [Google Scholar]
- (327).Praditya D; Kirchhoff L; Brüning J; Rachmawati H; Steinmann J; Steinmann E Anti-infective Properties of the Golden Spice Curcumin. Front. Microbiol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (328).Noureddin SA; El-Shishtawy RM; Al-Footy KO Curcumin Analogues and their Hybrid Molecules as Multifunctional Drugs. Eur. J. Med. Chem 2019, 182, 111631. [DOI] [PubMed] [Google Scholar]
- (329).Zitko J; Servusová B; Paterová P; Mandíková J; Kubíček V; Kučera R; Hrabcová V; Kuneš J; Soukup O; Dolezal M Synthesis, Antimycobacterial Activity and In Vitro Cytotoxicity of 5-Chloro-N-phenylpyrazine-2-carboxamides. Molecules 2013, 18, 14807–14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Floss HG; Yu TW Rifamycin-Mode of Action, Resistance, and Biosynthesis. Chem. Rev 2005, 105, 621–632. [DOI] [PubMed] [Google Scholar]
- (331).Limsuwan S; Hesseling-Meinders A; Voravuthikunchai SP; van Dijl JM; Kayser O Potential Antibiotic and Anti-Infective Effects of Rhodomyrtone from Rhodomyrtus tomentosa (Aiton) Hassk. on Streptococcus pyogenes as Revealed by Proteomics. Phytomedicine 2011, 18, 934–940. [DOI] [PubMed] [Google Scholar]
- (332).Limsuwan S; Trip EN; Kouwen TRHM; Piersma S; Hiranrat A; Mahabusarakam W; Voravuthikunchai SP; van Dijl JM; Kayser O Rhodomyrtone: A New Candidate as Natural Antibacterial Drug from Rhodomyrtus tomentosa. Phytomedicine 2009, 16, 645–651. [DOI] [PubMed] [Google Scholar]
- (333).Saising J; Hiranrat A; Mahabusarakam W; Ongsakul M; Voravuthikunchai S Rhodomyrtone from Rhodomyrtus tomentosa (Aiton) Hassk. as a Natural Antibiotic for Staphylococcal Cutaneous Infections. J. Health Sci 2008, 54, 589–595. [Google Scholar]
- (334).Xiao ZY; Mu Q; Shiu WK; Zeng YH; Gibbons S Polyisoprenylated Benzoylphloroglucinol Derivatives from Hypericum sampsonii. J. Nat. Prod 2007, 70, 1779–1782. [DOI] [PubMed] [Google Scholar]
- (335).Almeida LS; Murata RM; Yatsuda R; Dos Santos MH; Nagem TJ; Alencar SM; Koo H; Rosalen PL Antimicrobial Activity of Rheedia brasiliensis and 7-Epiclusianone against Streptococcus mutans. Phytomedicine 2008, 15, 886–891. [DOI] [PubMed] [Google Scholar]
- (336).Li Y-R; Xu W-J; Wei S-S; Lu W-J; Luo J; Kong L-Y Hyperbeanols F-Q, Diverse Monoterpenoid Polyprenylated Acylphloroglucinols from the Flowers of Hypericum beanii. Phytochemistry 2019, 159, 56–64. [DOI] [PubMed] [Google Scholar]
- (337).Xu F.-h.; Ding Y.-x.; Wang Q Chemical Constituents of Ethyl Acetate Fraction from Hypericum ascyron. Zhong Yao Cai 2016, 39, 322–325. [PubMed] [Google Scholar]
- (338).Chen X-Q; Li Y; Li K-Z; Peng L-Y; He J; Wang K; Pan Z-H; Cheng X; Li M-M; Zhao Q-S et al. Spirocyclic Acylphloroglucinol Derivatives from Hypericum beanii. Chem. Pharm. Bull 2011, 59, 1250–1253. [DOI] [PubMed] [Google Scholar]
- (339).Gronquist M; Bezzerides A; Attygalle A; Meinwald J; Eisner M; Eisner T Attractive and Defensive Functions of the Ultraviolet Pigments of a Flower (Hypericum calycinum). Proc. Natl. Acad. Sci. U. S. A 2001, 98, 13745–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (340).Tan YX; Yan RY; Wang HQ; Chen RY; Yu DQ Wittiorumins A - F, Antioxidant Diels-alder-type Adducts from Morus wittiorum. Planta Med 2009, 75, 249–255. [DOI] [PubMed] [Google Scholar]
- (341).Saeloh D; Tipmanee V; Jim KK; Dekker MP; Bitter W; Voravuthikunchai SP; Wenzel M; Hamoen LW The Novel Antibiotic Rhodomyrtone Traps Membrane Proteins in Vesicles with Increased Fluidity. PLoS Pathog 2018, 14, e1006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (342).Birhanu BT; Lee E-B; Park S-C Evaluation of the Pharmacokinetic-Pharmacodynamic Integration of Marbofloxacin in Combination with Methyl Gallate Against Salmonella Typhimurium in Rats. PLoS One 2020, 15, e0234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (343).Choi J-G; Mun S-H; Chahar HS; Bharaj P; Kang O-H; Kim S-G; Shin D-W; Kwon D-Y Methyl Gallate from Galla rhois Successfully Controls Clinical Isolates of Salmonella Infection in Both In Vitro and In Vivo Systems. PLoS One 2014, 9, e102697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (344).Reyes AWB; Kim DG; Simborio HLT; Hop HT; Arayan LT; Min W; Lee JJ; Chang HH; Kim S Methyl Gallate Limits Infection in Mice Challenged with Brucella abortus while Enhancing the Inflammatory Response. J. Appl. Microbiol 2016, 120, 552–559. [DOI] [PubMed] [Google Scholar]
- (345).Salni D; Sargent MV; Skelton BW; Soediro I; Sutisna M; White AH; Yulinah E Rhodomyrtone, an Antibotic from Rhodomyrtus tomentosa. Aust. J. Chem 2002, 55, 229–232. [Google Scholar]
- (346).Hiranrat A; Mahabusarakam W New Acylphloroglucinols from the Leaves of Rhodomyrtus tomentosa. Tetrahedron 2008, 64, 11193–11197. [Google Scholar]
- (347).Suganthy N; Muniasamy S; Archunan G Safety Assessment of Methanolic Extract of Terminalia chebula Fruit, Terminalia arjuna Bark and its Bioactive Constituent 7-Methyl Gallic Acid: In Vitro and In Vivo Studies. Regulatory Toxicology and Pharmacology 2018, 92, 347–357. [DOI] [PubMed] [Google Scholar]
- (348).Kotha RR; Luthria DL Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Gershenzon J; Dudareva N The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol 2007, 3, 408–414. [DOI] [PubMed] [Google Scholar]
- (350).Suntres ZE; Coccimiglio J; Alipour M The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr 2015, 55, 304–318. [DOI] [PubMed] [Google Scholar]
- (351).Churklam W; Chaturongakul S; Ngamwongsatit B; Aunpad R The Mechanisms of Action of Carvacrol and its Synergism with Nisin against Listeria monocytogenes on Sliced Bologna Sausage. Food Control 2020, 108, 106864. [Google Scholar]
- (352).Miladi H; Mili D; Ben SR; Bakhrouf A; Zouari S; Ammar E Antibiofilm Formation and Anti-Adhesive Property of three Mediterranean Essential Oils against a Foodborne Pathogen Salmonella strain. Microb. Pathog 2016, 93, 22–31. [DOI] [PubMed] [Google Scholar]
- (353).Marchese A; Orhan IE; Daglia M; Barbieri R; Di Lorenzo A; Nabavi SF; Gortzi O; Izadi M; Nabavi SM Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem 2016, 210, 402–414. [DOI] [PubMed] [Google Scholar]
- (354).Mansouri MD; Darouiche RO In-Vitro Activity and In-Vivo Efficacy of Catheters Impregnated with Chloroxylenol and Thymol Against Uropathogens. Clin. Microbiol. Infect 2008, 14, 190–192. [DOI] [PubMed] [Google Scholar]
- (355).Jiji S; Udhayakumar S; Rose C; Muralidharan C; Kadirvelu K Thymol Enriched Bacterial Cellulose Hydrogel as Effective Material for Third Degree Burn Wound Repair. Int. J. Biol. Macromol 2019, 122, 452–460. [DOI] [PubMed] [Google Scholar]
- (356).Marzouk T; Barakat R; Ragab A; Badria F; Badawy A Lavender-Thymol as a New Topical Aromatherapy Preparation for Episiotomy: A Randomised Clinical Trial. J. Obstet. Gynaecol 2015, 35, 472–475. [DOI] [PubMed] [Google Scholar]
- (357).Quintas V; Prada-López I; Carreira MJ; Suárez-Quintanilla D; Balsa-Castro C; Tomás I In Situ Antibacterial Activity of Essential Oils with and without Alcohol on Oral Biofilm: A Randomized Clinical Trial. Front. Microbiol 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (358).Che G; Pang H; Liao B; Zhang J; Liu J Analysis of the Volatile Components in Lavender from Supercritical CO2 Extraction by GC-MS. Se Pu 2005, 23, 322. [PubMed] [Google Scholar]
- (359).Bertoli A; Tozzi S; Pistelli L; Angelini LG Fibre Hemp Inflorescences: From Crop-residues to Essential Oil Production. Ind. Crops Prod 2010, 32, 329–337. [Google Scholar]
- (360).Chen J; Tang C; Zhang R; Ye S; Zhao Z; Huang Y; Xu X; Lan W; Yang D Metabolomics Analysis to Evaluate the Antibacterial Activity of the Essential Oil from the Leaves of Cinnamomum camphora (Linn.) Presl. J. Ethnopharmacol 2020, 253, 112652. [DOI] [PubMed] [Google Scholar]
- (361).Alves S; Duarte A; Sousa S; Domingues FC Study of the Major Essential Oil Compounds of Coriandrum sativum against Acinetobacter baumannii and the Effect of Linalool on Adhesion, Biofilms and Quorum Sensing. Biofouling 2016, 32, 155–165. [DOI] [PubMed] [Google Scholar]
- (362).Liu X; Cai J; Chen H; Zhong Q; Hou Y; Chen W; Chen W Antibacterial Activity and Mechanism of Linalool against Pseudomonas aeruginosa. Microb. Pathog 2020, 141, 103980. [DOI] [PubMed] [Google Scholar]
- (363).Khaleel C; Tabanca N; Buchbauer G α-Terpineol, a Natural Monoterpene: A Review of its Biological Properties. Open Chem 2018, 16, 349. [Google Scholar]
- (364).Held S; Schieberle P; Somoza V Characterization of α-Terpineol as an Anti-inflammatory Component of Orange Juice by In Vitro Studies Using Oral Buccal Cells. J. Agric. Food Chem 2007, 55, 8040–8046. [DOI] [PubMed] [Google Scholar]
- (365).Carson CF; Mee BJ; Riley TV Mechanism of Action of Melaleuca alternifolia (Tea Tree) Oil on Staphylococcus aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother 2002, 46, 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (366).Bauer K; Garbe D; Surburg H Flavors and Fragrances; 5th edition ed.; VCH Publishers: New York, NY, 1988. [Google Scholar]
- (367).Hassannejad N; Bahador A; Rudbari NH; Modarressi MH; Parivar K In Vivo Antibacterial Activity of Zataria multiflora Boiss Extract and Its Components, Carvacrol, and Thymol, Against Colistin-Resistant Acinetobacter baumannii in a Pneumonic BALB/c Mouse Model. J. Cell Biochem 2019, 120, 18640–18649. [DOI] [PubMed] [Google Scholar]
- (368).Trinh H-T; Lee I-A; Hyun Y-J; Kim D-H Artemisia princeps Pamp. Essential Oil and Its Constituents Eucalyptol and α-terpineol Ameliorate Bacterial Vaginosis and Vulvovaginal Candidiasis in Mice by Inhibiting Bacterial Growth and NF-κB Activation. Planta Med 2011, 77, 1996–2002. [DOI] [PubMed] [Google Scholar]
- (369).Hriouech S; Akhmouch AA; Mzabi A; Chefchaou H; Tanghort M; Oumokhtar B; Chami N; Remmal A The Antistaphylococcal Activity of Amoxicillin/Clavulanic Acid, Gentamicin, and 1,8-Cineole Alone or in Combination and Their Efficacy through a Rabbit Model of Methicillin-Resistant Staphylococcus aureus Osteomyelitis. Evid. Based Complement. Alternat. Med 2020, 2020, 4271017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (370).Matthys H; de Mey C; Carls C; Ryś A; Geib A; Wittig T Efficacy and Tolerability of Myrtol Standardized in Acute Bronchitis. Arzneimittelforschung 2000, 50, 700–711. [DOI] [PubMed] [Google Scholar]
- (371).Buckle J Chapter 3 - Basic Plant Taxonomy, Basic Essential Oil Chemistry, Extraction, Biosynthesis, and Analysis. Clinical Aromatherapy (Third Edition) 2015, DOI: 10.1016/B978-0-7020-5440-2.00003-6, 37–72. [DOI] [Google Scholar]
- (372).Kim MS; Kim H-R; Kim H; Choi S-K; Kim C-H; Hwang J-K; Park S-H Expansion of Antibacterial Spectrum of Xanthorrhizol Against Gram-negatives in Combination with PMBN and Food-Grade Antimicrobials. J. Microbiol 2019, 57, 405–412. [DOI] [PubMed] [Google Scholar]
- (373).Gutbrod K; Romer J; Dörmann P Phytol Metabolism in Plants. Prog. Lipid Res 2019, 74, 1–17. [DOI] [PubMed] [Google Scholar]
- (374).Neto I; Faustino C; Rijo P The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs, 2015.
- (375).Costa MS; Rego A; Ramos V; Afonso TB; Freitas S; Preto M; Lopes V; Vasconcelos V; Magalhaes C; Leao PN The Conifer Biomarkers Dehydroabietic and Abietic Acids are Widespread in Cyanobacteria. Sci. Rep 2016, 6, 23436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (376).Birtic S; Dussort P; Pierre FX; Bily AC; Roller M Carnosic Acid. Phytochemistry 2015, 115, 9–19. [DOI] [PubMed] [Google Scholar]
- (377).Ojeda-Sana AM; Repetto V; Moreno S Carnosic Acid is an Efflux Pumps Modulator by Dissipation of the Membrane Potential in Enterococcus faecalis and Staphylococcus aureus. World J. Microbiol. Biotechnol 2013, 29, 137–144. [DOI] [PubMed] [Google Scholar]
- (378).Nowakowska J; Griesser HJ; Textor M; Landmann R; Khanna N Antimicrobial Properties of 8-hydroxyserrulat-14-en-19-oic acid for Treatment of Implant-Associated Infections. Antimicrob. Agents Chemother 2013, 57, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (379).Griesser HJ; Semple SJ; Ys H Antimicrobial Surfaces 2010.
- (380).García PA; de Oliveira AB; Batista R Occurrence, Biological Activities and Synthesis of Kaurane Diterpenes and their Glycosides. Molecules 2007, 12, 455–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (381).Vieira HS; Takahashi JA; Pimenta LP; Boaventura MA Effects of Kaurane Diterpene Derivatives on Germination and Growth of Lactuca sativa Seedlings. Z. Naturforsch., C, J. Biosci 2005, 60, 72–78. [DOI] [PubMed] [Google Scholar]
- (382).Helliwell CA; Chandler PM; Poole A; Dennis ES; Peacock WJ The CYP88A Cytochrome P450, Kaurenoic Acid Oxidase, Catalyzes Three Steps of the Gibberellin Biosynthesis Pathway. Proc. Natl. Acad. Sci. U.S.A 2001, 98, 2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (383).Jung HJ; Jung HA; Kang SS; Lee JH; Cho YS; Moon KH; Choi JS Inhibitory Activity of Aralia continentalis Roots on Protein Tyrosine Phosphatase 1B and Rat Lens Aldose Reductase. Arch. Pharm. Res 2012, 35, 1771–1777. [DOI] [PubMed] [Google Scholar]
- (384).Cardoso P; Rocha C; Leal MF; Bahia MO; Alcantara D; Santos RAD; Goncalves NDS; Ambrosio SR; Cavalcanti BC; Moreira-Nunes CA et al. Effect of Diterpenoid Kaurenoic Acid on Genotoxicity and Cell Cycle Progression in Gastric Cancer Cell Lines. Biomed. Pharmacother 2017, 89, 772–780. [DOI] [PubMed] [Google Scholar]
- (385).Rocha S. M. M. d.; Cardoso P. C. d. S.; Bahia M. d. O.; Pessoa C. d. Ó.; Soares PC; Rocha S. M. d.; Burbano RMR; Rocha C. A. M. d. Effect of the Kaurenoic Acid on Genotoxicity and Cell Cycle Progression in Cervical Cancer Cells Lines. Toxicol. In Vitro 2019, 57, 126–131. [DOI] [PubMed] [Google Scholar]
- (386).Peria FM; Tiezzi DG; Tirapelli DP; Neto FS; Tirapelli CR; Ambrosio S; Oliveira HF; Tirapelli L Kaurenoic Acid Antitumor Activity in Breast Cancer Cells. J. Clin. Oncol 2010, 28, e13641–e13641. [Google Scholar]
- (387).Sartori MR; Pretto JB; Cruz AB; Bresciani LF; Yunes RA; Sortino M; Zacchino SA; Cechinel VF Antifungal Activity of Fractions and Two Pure Compounds of Flowers from Wedelia paludosa (Acmela brasiliensis) (Asteraceae). Pharmazie 2003, 58, 567–569. [PubMed] [Google Scholar]
- (388).Chirumamilla A; Knodel JJ; Charlet LD; Hulke BS; Foster SP; Ode PJ Ovipositional Preference and Larval Performance of the Banded Sunflower Moth (Lepidoptera: Tortricidae) and Its Larval Parasitoids on Resistant and Susceptible Lines of Sunflower (Asterales: Asteraceae). Environ. Entomol 2014, 43, 58–68. [DOI] [PubMed] [Google Scholar]
- (389).Cotoras M; Folch C; Mendoza L Characterization of the Antifungal Activity on Botrytis cinerea of the Natural Diterpenoids Kaurenoic Acid and 3β-Hydroxy-kaurenoic Acid. J. Agric. Food Chem 2004, 52, 2821–2826. [DOI] [PubMed] [Google Scholar]
- (390).Abrao F; de Araujo Costa LD; Alves JM; Senedese JM; de Castro PT; Ambrosio SR; Veneziani RC; Bastos JK; Tavares DC; Martins CH Copaifera langsdorffii oleoresin and its isolated compounds: antibacterial effect and antiproliferative activity in cancer cell lines. BMC Complement Altern Med 2015, 15, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (391).Moreira MR; Souza AB; Soares S; Bianchi TC; de Souza Eugenio D; Lemes DC; Martins CH; da Silva Moraes T; Tavares DC; Ferreira NH et al. ent-Kaurenoic Acid-Rich Extract from Mikania glomerata: In Vitro Activity against Bacteria Responsible for Dental Caries. Fitoterapia 2016, 112, 211–216. [DOI] [PubMed] [Google Scholar]
- (392).Souza AB; Moreira MR; Borges CH; Simao MR; Bastos JK; de Sousa JP; Ambrosio SR; Veneziani RC Development and Validation of a Rapid RP-HPLC Method for Analysis of (-)-Copalic Acid in Copaiba Oleoresin. Biomed. Chromatogr 2013, 27, 280–283. [DOI] [PubMed] [Google Scholar]
- (393).Souza AB; Martins CH; Souza MG; Furtado NA; Heleno VC; de Sousa JP; Rocha EM; Bastos JK; Cunha WR; Veneziani RC et al. Antimicrobial Activity of Terpenoids from Copaifera langsdorffii Desf. Against Cariogenic Bacteria. Phytother. Res 2011, 25, 215–220. [DOI] [PubMed] [Google Scholar]
- (394).Harish Kumar G; Chandra Mohan KV; Jagannadha Rao A; Nagini S Nimbolide a Limonoid from Azadirachta indica Inhibits Proliferation and Induces Apoptosis of Human Choriocarcinoma (BeWo) Cells. Invest. New Drugs 2009, 27, 246–252. [DOI] [PubMed] [Google Scholar]
- (395).Baira SM; Khurana A; Somagoni J; Srinivas R; Godugu C; Talluri MVNK First Report on the Pharmacokinetic Profile of Nimbolide, a Novel Anticancer Agent in Oral and Intravenous Administrated Rats by LC/MS Method. J. Chromatogr. B 2018, 1092, 191–198. [DOI] [PubMed] [Google Scholar]
- (396).Wandji J; Wansi JD; Fuendjiep V; Dagne E; Mulholland DA; Tillequin F; Fomum ZT; Sondengam BL; Nkeh BC; Njamen D Sesquiterpene Lactone and Friedelane Derivative from Drypetes molunduana. Phytochemistry 2000, 54, 811–815. [DOI] [PubMed] [Google Scholar]
- (397).Ajaib M; Hussain T; Farooq S; Ashiq M Analysis of Antimicrobial and Antioxidant Activities of Chenopodium ambrosioides: An Ethnomedicinal Plant. J. Chem 2016, DOI: 10.1155/2016/4827157, 4827157/4827151-4827157/4827111. [DOI] [Google Scholar]
- (398).Ikeda Y; Murakami A; Ohigashi H Ursolic Acid: An anti- and Pro-inflammatory Triterpenoid. Mol. Nutr. Food Res 2008, 52, 26–42. [DOI] [PubMed] [Google Scholar]
- (399).Jyoti MA; Zerin T; Kim T-H; Hwang T-S; Jang WS; Nam K-W; Song H-Y In Vitro Effect of Ursolic Acid on the Inhibition of Mycobacterium tuberculosis and its Cell Wall Mycolic Acid. Pulm. Pharmacol. Ther 2015, 33, 17–24. [DOI] [PubMed] [Google Scholar]
- (400).Wang C-M; Jhan Y-L; Tsai S-J; Chou C-H The Pleiotropic Antibacterial Mechanisms of Ursolic Acid against Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2016, 21, 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (401).Cargnin ST; Gnoatto SB Ursolic Acid from Apple Pomace and Traditional Plants: A Valuable Triterpenoid with Functional Properties. Food Chem 2017, 220, 477–489. [DOI] [PubMed] [Google Scholar]
- (402).Kashyap D; Tuli HS; Sharma AK Ursolic Acid (UA): A Metabolite with Promising Therapeutic Potential. Life Sci 2016, 146, 201–213. [DOI] [PubMed] [Google Scholar]
- (403).Qian W; Wang W; Zhang J; Wang T; Liu M; Yang M; Sun Z; Li X; Li Y Antimicrobial and Antibiofilm Activities of Ursolic Acid against Carbapenem-Resistant Klebsiella pneumoniae. J. Antibiot. (Tokyo) 2020, DOI: 10.1038/s41429-020-0285-6. [DOI] [PubMed] [Google Scholar]
- (404).Fadipe VO; Mongalo NI; Opoku AR; Dikhoba PM; Makhafola TJ Isolation of Anti-mycobacterial Compounds from Curtisia dentata (Burm.f.) C.A.Sm (Curtisiaceae). BMC Complement. Altern. Med 2017, 17, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (405).Huang L; Luo H; Li Q; Wang D; Zhang J; Hao X; Yang X Pentacyclic Triterpene Derivatives Possessing Polyhydroxyl Ring A Inhibit Gram-positive Bacteria Growth by Regulating Metabolism and Virulence Genes Expression. Eur. J. Med. Chem 2015, 95, 64–75. [DOI] [PubMed] [Google Scholar]
- (406).Dwivedi GR; Maurya A; Yadav DK; Khan F; Darokar MP; Srivastava SK Drug Resistance Reversal Potential of Ursolic Acid Derivatives against Nalidixic Acid- and Multidrug-resistant Escherichia coli. Chem. Biol. Drug Des 2015, 86, 272–283. [DOI] [PubMed] [Google Scholar]
- (407).Spivak AY; Khalitova RR; Nedopekina DA; Gubaidullin RR Antimicrobial Properties of Amine- and Guanidine-Functionalized Derivatives of Betulinic, Ursolic and Oleanolic Acids: Synthesis and Structure/Activity Evaluation. Steroids 2020, 154, 108530. [DOI] [PubMed] [Google Scholar]
- (408).Wu J; Ma S; Zhang T-Y; Wei Z-Y; Wang H-M; Guo F-Y; Zheng C-J; Piao H-R Synthesis and Biological Evaluation of Ursolic Acid Derivatives Containing an Aminoguanidine Moiety. Med. Chem. Res 2019, 28, 959–973. [Google Scholar]
- (409).Gu W; Hao Y; Zhang G; Wang S-F; Miao T-T; Zhang K-P Synthesis, In Vitro Antimicrobial and Cytotoxic Activities of New Carbazole Derivatives of Ursolic Acid. Bioorg. Med. Chem. Lett 2015, 25, 554–557. [DOI] [PubMed] [Google Scholar]
- (410).Jiménez-Arellanes A; Luna-Herrera J; Cornejo-Garrido J; López-García S; Castro-Mussot ME; Meckes-Fischer M; Mata-Espinosa D; Marquina B; Torres J; Hernández-Pando R Ursolic and Oleanolic Acids as Antimicrobial and Immunomodulatory Compounds for Tuberculosis Treatment. BMC Complement. Altern. Med 2013, 13, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (411).Catteau L; Reichmann NT; Olson J; Pinho MG; Nizet V; Van Bambeke F; Quetin-Leclercq J Synergy between Ursolic and Oleanolic Acids from Vitellaria paradoxa Leaf Extract and β-Lactams against Methicillin-Resistant Staphylococcus aureus: In Vitro and In Vivo Activity and Underlying Mechanisms. Molecules 2017, 22, 2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (412).Pollier J; Goossens A Oleanolic Acid. Phytochemistry 2012, 77, 10–15. [DOI] [PubMed] [Google Scholar]
- (413).Chien Y-C; Lin C-H; Chiang MY; Chang H-S; Liao C-H; Chen I-S; Peng C-F; Tsai I-L Secondary Metabolites from the Root of Ehretia longiflora and their Biological Activities. Phytochemistry 2012, 80, 50–57. [DOI] [PubMed] [Google Scholar]
- (414).Blanco-Cabra N; Vega-Granados K; Moya-Andérico L; Vukomanovic M; Parra A; Álvarez de Cienfuegos L; Torrents E Novel Oleanolic and Maslinic Acid Derivatives as a Promising Treatment against Bacterial Biofilm in Nosocomial Infections: An In Vitro and In Vivo Study. ACS Infect. Dis 2019, 5, 1581–1589. [DOI] [PubMed] [Google Scholar]
- (415).Kurek A; Grudniak AM; Szwed M; Klicka A; Samluk L; Wolska KI; Janiszowska W; Popowska M Oleanolic Acid and Ursolic Acid Affect Peptidoglycan Metabolism in Listeria monocytogenes. Antonie van Leeuwenhoek 2009, 97, 61. [DOI] [PubMed] [Google Scholar]
- (416).Catteau L; Zhu L; Van Bambeke F; Quetin-Leclercq J Natural and Hemi-synthetic Pentacyclic Triterpenes as Antimicrobials and Resistance Modifying Agents against Staphylococcus aureus: A Review. Phytochem. Lett 2018, 17, 1129–1163. [Google Scholar]
- (417).Lin C; Wen X; Sun H Oleanolic Acid Derivatives for Pharmaceutical Use: A Patent Review. Expert Opin. Ther. Pat 2016, 26, 643–655. [DOI] [PubMed] [Google Scholar]
- (418).Chiu P; Leung LT; Ko BC Pseudolaric Acids: Isolation, Bioactivity and Synthetic Studies. Nat. Prod. Rep 2010, 27, 1066–1083. [DOI] [PubMed] [Google Scholar]
- (419).Chen GF; Li ZL; Pan DJ; Tang CM; He X; Xu GY; Chen K; Lee KH The Isolation and Structural Elucidation of four Novel Triterpene Lactones, Pseudolarolides A, B, C, and D, from Pseudolarix kaempferi. J. Nat. Prod 1993, 56, 1114–1122. [DOI] [PubMed] [Google Scholar]
- (420).Hu Z; Gu Z; Sun M; Zhang K; Gao P; Yang Q; Yuan Y Ursolic Acid Improves Survival and Attenuates Lung Injury in Septic Rats Induced by Cecal Ligation and Puncture. J. Surg. Res 2015, 194, 528–536. [DOI] [PubMed] [Google Scholar]
- (421).Argyropoulou C; Skaltsa H Identification of Essential Oil Components of Marrubium thessalum Boiss. & Heldr., Growing Wild in Greece. Nat. Prod. Res 2012, 26, 593–599. [DOI] [PubMed] [Google Scholar]
- (422).Navaei MN; Mirza M Chemical Composition of the Oil of Eremostachys laciniata (L.) Bunge from Iran. Flavour Fragr. J 2006, 21, 645–646. [Google Scholar]
- (423).Ilic MD; Marcetic MD; Zlatkovic BK; Lakusic BS; Kovacevic NN; Drobac MM Chemical Composition of Volatiles of Eight Geranium L. Species from Vlasina Plateau (South Eastern Serbia). Chem. Biodivers 2020, 17, e1900544. [DOI] [PubMed] [Google Scholar]
- (424).Joshi RK GC/MS Analysis of the Essential Oil of Senecio belgaumensis Flowers. Nat. Prod. Commun 2011, 6, 1934578X1100600826. [PubMed] [Google Scholar]
- (425).Kern JR; Cardellina JH Native American Medicinal Plants. Falcarindiol and 3-O-Methylfalcarindiol From Osmorhiza occidentalis. J. Nat. Prod 1982, 45, 774–776. [Google Scholar]
- (426).Villegas M; Vargas D; Msonthi JD; Marston A; Hostettmann K Isolation of the Antifungal Compounds Falcarindiol and Sarisan from Heteromorpha trifoliata. Planta Med 1988, 54, 36–37. [DOI] [PubMed] [Google Scholar]
- (427).Miyazawa M; Shimamura H; Bhuva RC; Nakamura S -i.; Kameoka, H. Antimutagenic Activity of Falcarindiol from Peucedanum praeruptorum. J. Agric. Food Chem 1996, 44, 3444–3448. [Google Scholar]
- (428).Furumi K; Fujioka T; Fujii H; Okabe H; Nakano Y; Matsunaga H; Katano M; Mori M; Mihashi K Novel Antiproliferative Falcarindiol Furanocoumarin Ethers from the Root of Angelica japonica. Bioorg. Med. Chem. Lett 1998, 8, 93–96. [DOI] [PubMed] [Google Scholar]
- (429).Prior RM; Lundgaard NH; Light ME; Stafford GI; van Staden J; Jäger AK The Polyacetylene Falcarindiol with COX-1 Activity Isolated from Aegopodium podagraria L. J. Ethnopharmacol 2007, 113, 176–178. [DOI] [PubMed] [Google Scholar]
- (430).Yoshida J; Seino H; Ito Y; Nakano T; Satoh T; Ogane Y; Suwa S; Koshino H; Kimura K.-i. Inhibition of Glycogen Synthase Kinase-3β by Falcarindiol Isolated from Japanese Parsley (Oenanthe javanica). J. Agric. Food Chem 2013, 61, 7515–7521. [DOI] [PubMed] [Google Scholar]
- (431).Minto RE; Blacklock BJ Biosynthesis and Function of Polyacetylenes and Allied Natural Products. Prog. Lipid Res 2008, 47, 233–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (432).Dall’Acqua S; Viola G; Piacente S; Cappelletti EM; Innocenti G Cytotoxic Constituents of Roots of Chaerophyllum hirsutum. J. Nat. Prod 2004, 67, 1588–1590. [DOI] [PubMed] [Google Scholar]
- (433).Zhang P; Wu Q; Chen L; Duan K Virulence-Inhibiting Herbal Compound Falcarindiol Significantly Reduced Mortality in Mice Infected with Pseudomonas aeruginosa. Antibiotics (Basel, Switzerland) 2020, 9, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (434).Luttgeharm KD; Chen M; Mehra A; Cahoon RE; Markham JE; Cahoon EB Overexpression of Arabidopsis Ceramide Synthases Differentially Affects Growth, Sphingolipid Metabolism, Programmed Cell Death, and Mycotoxin Resistance. Plant Physiol 2015, 169, 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (435).Teinkela JE; Siwe Noundou X; Nguemfo EL; Meyer F; Djoukoue A; Van Antwerpen P; Ngouela S; Tsamo E; Mpondo Mpondo EA; Vardamides JC et al. Identification of Compounds with Anti-Proliferative Activity from the Wood of Ficus elastica Roxb. ex Hornem. Aerial Roots. Fitoterapia 2016, 112, 65–73. [DOI] [PubMed] [Google Scholar]
- (436).Hirata H; Ohnishi T; Ishida H; Tomida K; Sakai M; Hara M; Watanabe N Functional Characterization of Aromatic Amino Acid Aminotransferase Involved in 2-phenylethanol Biosynthesis in Isolated Rose Petal Protoplasts. J. Plant Physiol 2012, 169, 444–451. [DOI] [PubMed] [Google Scholar]
- (437).Sakai M; Hirata H; Sayama H; Sekiguchi K; Itano H; Asai T; Dohra H; Hara M; Watanabe N Production of 2-Phenylethanol in Roses as the Dominant Floral Scent Compound from L-phenylalanine by Two Key Enzymes, a PLP-Dependent Decarboxylase and a Phenylacetaldehyde Reductase. Biosci. Biotechnol. Biochem 2007, 71, 2408–2419. [DOI] [PubMed] [Google Scholar]
- (438).Kusterer J; Vogt A; Keusgen M Isolation and Identification of a New Cysteine Sulfoxide and Volatile Sulfur Compounds from Allium subgenus Melanocrommyum. J. Agric. Food Chem 2010, 58, 520–526. [DOI] [PubMed] [Google Scholar]
- (439).Chiurchiu V; Leuti A; Maccarrone M Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol 2018, 9, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (440).Dayrit FM The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc 2015, 92, 1–15. [Google Scholar]
- (441).Borlinghaus J; Albrecht F; Gruhlke MC; Nwachukwu ID; Slusarenko AJ Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (442).Cavallito CJ; Bailey JH Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action. J. Am. Chem. Soc 1944, 66, 1950–1951. [Google Scholar]
- (443).Petrovska BB; Cekovska S Extracts from the History and Medical Properties of Garlic. Pharmacogn. Rev 2010, 4, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (444).Parvu M; Mot CA; Parvu AE; Mircea C; Stoeber L; Rosca-Casian O; Tigu AB Allium sativum Extract Chemical Composition, Antioxidant Activity and Antifungal Effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa Causing Onychomycosis. Molecules 2019, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (445).Arguello-Garcia R; de la Vega-Arnaud M; Loredo-Rodriguez IJ; Mejia-Corona AM; Melgarejo-Trejo E; Espinoza-Contreras EA; Fonseca-Linan R; Gonzalez-Robles A; Perez-Hernandez N; Ortega-Pierres MG Activity of Thioallyl Compounds From Garlic Against Giardia duodenalis Trophozoites and in Experimental Giardiasis. Front. Cell Infect. Microbiol 2018, 8, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (446).Weber ND; Andersen DO; North JA; Murray BK; Lawson LD; Hughes BG In Vitro Virucidal effects of Allium sativum (Garlic) Extract and Compounds. Planta Med 1992, 58, 417–423. [DOI] [PubMed] [Google Scholar]
- (447).Abdallah M; Abdallah HA; Memish ZA Burkholderia cepacia Complex Outbreaks among Non-Cystic Fibrosis Patients in the Intensive Care Units: A Review of Adult and Pediatric Literature. Infez. Med 2018, 26, 299–307. [PubMed] [Google Scholar]
- (448).Blanchard AC; Waters VJ Microbiology of Cystic Fibrosis Airway Disease. Semin. Respir. Crit. Care Med 2019, 40, 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (449).Choo S; Chin VK; Wong EH; Madhavan P; Tay ST; Yong PVC; Chong PP Review: Antimicrobial Properties of Allicin Used Alone or in Combination with Other Medications. Folia Microbiol. (Praha) 2020, DOI: 10.1007/s12223-020-00786-5. [DOI] [PubMed] [Google Scholar]
- (450).Rahman MS Allicin and Other Functional Active Components in Garlic: Health Benefits and Bioavailability. Int. J. Food Prop 2007, 10, 245–268. [Google Scholar]
- (451).Fujisawa H; Suma K; Origuchi K; Kumagai H; Seki T; Ariga T Biological and Chemical Stability of Garlic-Derived Allicin. J. Agric. Food Chem 2008, 56, 4229–4235. [DOI] [PubMed] [Google Scholar]
- (452).Miron T; Rabinkov A; Mirelman D; Wilchek M; Weiner L The Mode of Action of Allicin: Its Ready Permeability Through Phospholipid Membranes may Contribute to its Biological Activity. Biochim. Biophys. Acta Biomembr 2000, 1463, 20–30. [DOI] [PubMed] [Google Scholar]
- (453).Muller A; Eller J; Albrecht F; Prochnow P; Kuhlmann K; Bandow JE; Slusarenko AJ; Leichert LI Allicin Induces Thiol Stress in Bacteria through S-Allylmercapto Modification of Protein Cysteines. J. Biol. Chem 2016, 291, 11477–11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (454).Zhai H; Pan J; Pang E; Bai B Lavage with Allicin in Combination with Vancomycin Inhibits Biofilm Formation by Staphylococcus epidermidis in a Rabbit Model of Prosthetic Joint Infection. PLoS One 2014, 9, e102760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (455).Pérez-Köhler B; García-Moreno F; Brune T; Pascual G; Bellón JM Preclinical Bioassay of a Polypropylene Mesh for Hernia Repair Pretreated with Antibacterial Solutions of Chlorhexidine and Allicin: An In Vivo Study. PLoS One 2015, 10, e0142768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (456).Koçkar C; Oztürk M; N, B. Helicobacter pylori Eradication With Beta Carotene, Ascorbic Acid and Allicin. Acta Medica (Hradec Kralove) 2001, 44, 97–100. [PubMed] [Google Scholar]
- (457).Leontiev R; Hohaus N; Jacob C; Gruhlke MCH; Slusarenko AJ A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep 2018, 8, 6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (458).Lu Q; Lu P-M; Piao J-H; Xu X-L; Chen J; Zhu L; Jiang J-G Preparation and Physicochemical Characteristics of an Allicin Nanoliposome and its Release Behavior. Food Sci. Technol 2014, 57, 686–695. [Google Scholar]
- (459).Abad P; Arroyo-Manzanares N; García-Campaña AM A Rapid and Simple UHPLC-ESI-MS/MS Method for the Screening of Propyl Propane Thiosulfonate, a New Additive for Animal Feed. Anal. Methods 2016, 8, 3730–3739. [Google Scholar]
- (460).Marchese A; Barbieri R; Sanches-Silva A; Daglia M; Nabavi SF; Jafari NJ; Izadi M; Ajami M; Nabavi SM Antifungal and Antibacterial Activities of Allicin: A Review. Trends Food Sci. Technol 2016, 52, 49–56. [Google Scholar]
- (461).Salehi B; Zucca P; Orhan IE; Azzini E; Adetunji CO; Mohammed SA; Banerjee SK; Sharopov F; Rigano D; Sharifi-Rad J et al. Allicin and Health: A Comprehensive Review. Trends Food Sci. Technol 2019, 86, 502–516. [Google Scholar]
- (462).Shaikh AS; Thomas AB; Chitlange SS Herb–drug Interaction Studies of Herbs used in Treatment of Cardiovascular Disorders—A Narrative Review of Preclinical and Clinical Studies. Phytother. Res 2020, 34, 1008–1026. [DOI] [PubMed] [Google Scholar]
- (463).Stotz HU; Thomson JG; Wang Y Plant Defensins: Defense, Development and Application. Plant Signal. Behav 2009, 4, 1010–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (464).Barbosa Pelegrini P; Del Sarto RP; Silva ON; Franco OL; Grossi-de-Sa MF Antibacterial Peptides From Plants: What They Are and How They Probably Work. Biochem. Res. Int 2011, 2011, 250349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (465).Boman HG Antibacterial Peptides: Basic Facts and Emerging Concepts. J. Intern. Med 2003, 254, 197–215. [DOI] [PubMed] [Google Scholar]
- (466).Jenssen H; Hamill P; Hancock REW Peptide Antimicrobial Agents. Clin. Microbiol. Rev 2006, 19, 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (467).Akoachere J-F, Suylika Y, Mbah A, Ayimele A, Assob JC, Fodouop S, Kodjio N, & Gatsing D In vitro Antimicrobial Activity of Agents from Spilanthes filicaulis and Laportea ovalifolia against Some Drug Resistant Bacteria. J. Pharm. Res. Int 2015, 6, 76–87. [Google Scholar]
- (468).Cowan MM Plant Products as Antimicrobial Agents. Clinical Microbiology Reviews 1999, 12, 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (469).Vamathevan J; Clark D; Czodrowski P; Dunham I; Ferran E; Lee G; Li B; Madabhushi A; Shah P; Spitzer M et al. Applications of Machine Learning in Drug Discovery and Development. Nat. Rev. Drug Discov 2019, 18, 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (470).Stokes JM; Yang K; Swanson K; Jin W; Cubillos-Ruiz A; Donghia NM; MacNair CR; French S; Carfrae LA; Bloom-Ackerman Z et al. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702.e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (471).Fleming N How Artificial Intelligence is Changing Drug Discovery. Nature 2018, 557, S55–s57. [DOI] [PubMed] [Google Scholar]
- (472).Merk D; Grisoni F; Friedrich L; Schneider G Tuning Artificial Intelligence on the De Novo Design of Natural-Product-Inspired Retinoid X Receptor Modulators. Commun. Chem 2018, 1, 68. [Google Scholar]
- (473).Reker D; Rodrigues T; Schneider P; Schneider G Identifying the Macromolecular Targets of De Novo-Designed Chemical Entities through Self-Organizing Map Consensus. Proc. Natl. Acad. Sci. U.S.A 2014, 111, 4067–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (474).Liu M; Karuso P; Feng Y; Kellenberger E; Liu F; Wang C; Quinn RJ Is it Time for Artificial Intelligence to Predict the Function of Natural Products Based on 2D-Structure. Medchemcomm 2019, 10, 1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (475).Ross C IBM Halting Sales of Watson AI Tool for Drug Discovery Amid Sluggish Growth. STAT News 2019.
- (476).Fox Ramos AE; Evanno L; Poupon E; Champy P; Beniddir MA Natural Products Targeting Strategies Involving Molecular Networking: Different Manners, One Goal. Nat. Prod. Rep 2019, 36, 960–980. [DOI] [PubMed] [Google Scholar]
- (477).Quinn RA; Nothias LF; Vining O; Meehan M; Esquenazi E; Dorrestein PC Molecular Networking As a Drug Discovery, Drug Metabolism, and Precision Medicine Strategy. Trends Pharmacol. Sci 2017, 38, 143–154. [DOI] [PubMed] [Google Scholar]
- (478).Wang M; Carver JJ; Phelan VV; Sanchez LM; Garg N; Peng Y; Nguyen DD; Watrous J; Kapono CA; Luzzatto-Knaan T et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol 2016, 34, 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (479).Fox Ramos AE; Alcover C; Evanno L; Maciuk A; Litaudon M; Duplais C; Bernadat G; Gallard J-F; Jullian J-C; Mouray E et al. Revisiting Previously Investigated Plants: A Molecular Networking-Based Study of Geissospermum laeve. J. Nat. Prod 2017, 80, 1007–1014. [DOI] [PubMed] [Google Scholar]
- (480).Allard P-M; Péresse T; Bisson J; Gindro K; Marcourt L; Pham VC; Roussi F; Litaudon M; Wolfender J-L Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem 2016, 88, 3317–3323. [DOI] [PubMed] [Google Scholar]
- (481).Allen F; Pon A; Wilson M; Greiner R; Wishart D CFM-ID: A Web Server for Annotation, Spectrum Prediction and Metabolite Identification from Tandem Mass Spectra. Nucleic Acids Res 2014, 42, W94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (482).Gu J; Gui Y; Chen L; Yuan G; Lu HZ; Xu X Use of Natural Products as Chemical Library for Drug Discovery and Network Pharmacology. PLoS One 2013, 8, e62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (483).DNP; Dictionary of Natural Products http://dnp.chemnetbase.com, 2017. Accessed on October 14, 2020.
- (484).Zani CL; Carroll AR Database for Rapid Dereplication of Known Natural Products Using Data from MS and Fast NMR Experiments. J. Nat. Prod 2017, 80, 1758–1766. [DOI] [PubMed] [Google Scholar]
- (485).Peresse T; Jezequel G; Allard PM; Pham VC; Huong DTM; Blanchard F; Bignon J; Levaique H; Wolfender JL; Litaudon M et al. Cytotoxic Prenylated Stilbenes Isolated from Macaranga tanarius. J. Nat. Prod 2017, 80, 2684–2691. [DOI] [PubMed] [Google Scholar]
- (486).Klein-Junior LC; Cretton S; Allard PM; Genta-Jouve G; Passos CS; Salton J; Bertelli P; Pupier M; Jeannerat D; Heyden YV et al. Targeted Isolation of Monoterpene Indole Alkaloids from Palicourea sessilis. J. Nat. Prod 2017, 80, 3032–3037. [DOI] [PubMed] [Google Scholar]
- (487).Naman CB; Rattan R; Nikoulina SE; Lee J; Miller BW; Moss NA; Armstrong L; Boudreau PD; Debonsi HM; Valeriote FA et al. Integrating Molecular Networking and Biological Assays To Target the Isolation of a Cytotoxic Cyclic Octapeptide, Samoamide A, from an American Samoan Marine Cyanobacterium. J. Nat. Prod 2017, 80, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (488).Olivon F; Allard PM; Koval A; Righi D; Genta-Jouve G; Neyts J; Apel C; Pannecouque C; Nothias LF; Cachet X et al. Bioactive Natural Products Prioritization Using Massive Multi-informational Molecular Networks. ACS Chem. Biol 2017, 12, 2644–2651. [DOI] [PubMed] [Google Scholar]
- (489).Olivon F; Apel C; Retailleau P; Allard PM; Wolfender JL; Touboul D; Roussi F; Litaudon M; Desrat S Searching for Original Natural Products by Molecular Networking: Detection, Isolation and Total Synthesis of Chloroaustralasines. Org. Chem. Front 2018, 5, 2171–2178. [Google Scholar]
- (490).Wolfender J-L; Litaudon M; Touboul D; Queiroz EF Innovative Omics-based Approaches for Prioritisation and Targeted Isolation of Natural Products – New Strategies for Drug Discovery. Nat. Prod. Rep 2019, 36, 855–868. [DOI] [PubMed] [Google Scholar]
- (491).Oberlies NH; Knowles SL; Amrine CSM; Kao D; Kertesz V; Raja HA Droplet Probe: Coupling Chromatography to the In Situ Evaluation of the Chemistry of Nature. Nat. Prod. Rep 2019, 36, 944–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (492).Wu C; Dill AL; Eberlin LS; Cooks RG; Ifa DR Mass Spectrometry Imaging Under Ambient Conditions. Mass Spectrom. Rev 2013, 32, 218–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (493).Huang MZ; Cheng SC; Cho YT; Shiea J Ambient Ionization Mass Spectrometry: A Tutorial. Anal. Chim. Acta 2011, 702, 1–15. [DOI] [PubMed] [Google Scholar]
- (494).Nemes P; Vertes A Laser Ablation Electrospray Ionization for Atmospheric Pressure, In Vivo, and Imaging Mass Spectrometry. Anal. Chem 2007, 79, 8098–8106. [DOI] [PubMed] [Google Scholar]
- (495).Jarmusch AK; Cooks RG Emerging Capabilities of Mass Spectrometry for Natural Products. Nat. Prod. Rep 2014, 31, 730–738. [DOI] [PubMed] [Google Scholar]
- (496).Sica VP; El-Elimat T; Oberlies NH In Situ Analysis of Asimina triloba (paw paw) Plant Tissues for Acetogenins via the Droplet-Liquid Microjunction-Surface Sampling Probe Coupled to UHPLC-PDA-HRMS/MS. Anal. Methods 2016, 8, 6143–6149. [Google Scholar]
- (497).Kao D; Henkin JM; Soejarto DD; Kinghorn AD; Oberlies NH Non-Destructive Chemical Analysis of a Garcinia mangostana L. (Mangosteen) Herbarium Voucher Specimen. Phytochem. Lett 2018, 28, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (498).Nagana Gowda GA; Raftery D Recent Advances in NMR-Based Metabolomics. Anal. Chem 2017, 89, 490–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (499).Egan J; Linington R Reinforcing Bioactivity Directed Discovery Using MADByTE (Metabolomics And Dereplication By Two-dimensional Experiments, 2019.
- (500).Egan JM; Santen J. v.; Linington RG MADByTE NMR https://www.madbyte.org/. 2020. Accessed on October 14, 2020.
- (501).Smanski MJ; Zhou H; Claesen J; Shen B; Fischbach MA; Voigt CA Synthetic Biology to Access and Expand Nature’s Chemical Diversity. Nat. Rev. Microbiol 2016, 14, 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (502).Ren H; Shi C; Zhao H Computational Tools for Discovering and Engineering Natural Product Biosynthetic Pathways. iScience 2020, 23, 100795–100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (503).Medema MH; Blin K; Cimermancic P; de Jager V; Zakrzewski P; Fischbach MA; Weber T; Takano E; Breitling R antiSMASH: Rapid Identification, Annotation and Analysis of Secondary Metabolite Biosynthesis Gene Clusters in Bacterial and Fungal Genome Sequences. Nucleic Acids Res 2011, 39, W339–W346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (504).Kautsar SA; Suarez Duran HG; Blin K; Osbourn A; Medema MH plantiSMASH: Automated Identification, Annotation and Expression Analysis of Plant Biosynthetic Gene Clusters. Nucleic Acids Res 2017, 45, W55–W63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (505).Rajniak J; Barco B; Clay NK; Sattely ES A New Cyanogenic Metabolite in Arabidopsis Required for Inducible Pathogen Defence. Nature 2015, 525, 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (506).Li Y; Calvo Sarah E.; Gutman R; Liu Jun S.; Mootha Vamsi K. Expansion of Biological Pathways Based on Evolutionary Inference. Cell 2014, 158, 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (507).Kanehisa M; Goto S KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 2000, 28, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (508).Chen S; Xiang L; Guo X; Li Q An Introduction to the Medicinal Plant Genome Project. Front. Med 2011, 5, 178–184. [DOI] [PubMed] [Google Scholar]
- (509).Matasci N; Hung LH; Yan Z; Carpenter EJ; Wickett NJ; Mirarab S; Nguyen N; Warnow T; Ayyampalayam S; Barker M et al. Data Access for the 1,000 Plants (1KP) Project. Gigascience 2014, 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (510).Luo X; Reiter MA; d’Espaux L; Wong J; Denby CM; Lechner A; Zhang Y; Grzybowski AT; Harth S; Lin W et al. Complete Biosynthesis of Cannabinoids and their Unnatural Analogues in Yeast. Nature 2019, 567, 123–126. [DOI] [PubMed] [Google Scholar]
- (511).Chappell J; DellaPenna D; O’Connor SE; Michigan State University; Vol. 2020. [Google Scholar]
- (512).Cravens A; Payne J; Smolke CD Synthetic Biology Strategies for Microbial Biosynthesis of Plant Natural Products. Nat. Commun 2019, 10, 2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (513).Li Y; Li S; Thodey K; Trenchard I; Cravens A; Smolke CD Complete Biosynthesis of Noscapine and Halogenated Alkaloids in Yeast. Proc. Natl. Acad. Sci. U.S.A 2018, 115, E3922–E3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (514).Hawkins KM; Smolke CD Production of Benzylisoquinoline alkaloids in Saccharomyces cerevisiae. Nat. Chem. Biol 2008, 4, 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (515).Valliere MA; Korman TP; Woodall NB; Khitrov GA; Taylor RE; Baker D; Bowie JU A Cell-Free Platform for the Prenylation of Natural Products and Application to Cannabinoid Production. Nat. Commun 2019, 10, 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (516).Li Y; Smolke CD Engineering Biosynthesis of the Anticancer Alkaloid Noscapine in Yeast. Nat. Commun 2016, 7, 12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (517).Beyraghdar Kashkooli A; van der Krol AR; Rabe P; Dickschat JS; Bouwmeester H Substrate Promiscuity of Enzymes from the Sesquiterpene Biosynthetic Pathways from Artemisia annua and Tanacetum parthenium Allows for Novel Combinatorial Sesquiterpene Production. Metab. Eng 2019, 54, 12–23. [DOI] [PubMed] [Google Scholar]
- (518).Sánchez C; Butovich IA; Braña AF; Rohr J; Méndez C; Salas JA The Biosynthetic Gene Cluster for the Antitumor Rebeccamycin: Characterization and Generation of Indolocarbazole Derivatives. Chem. Biol 2002, 9, 519–531. [DOI] [PubMed] [Google Scholar]
- (519).Fasan R; Chen MM; Crook NC; Arnold FH Engineered Alkane-Hydroxylating Cytochrome P450(BM3) Exhibiting Nativelike Catalytic Properties. Angew. Chem. Int. Ed. Engl 2007, 46, 8414–8418. [DOI] [PubMed] [Google Scholar]
- (520).Payne JT; Poor CB; Lewis JC Directed Evolution of RebH for Site-Selective Halogenation of Large Biologically Active Molecules. Angew. Chem. Int. Ed. Engl 2015, 54, 4226–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (521).Savile CK; Janey JM; Mundorff EC; Moore JC; Tam S; Jarvis WR; Colbeck JC; Krebber A; Fleitz FJ; Brands J et al. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309. [DOI] [PubMed] [Google Scholar]
- (522).Skellam E Strategies for Engineering Natural Product Biosynthesis in Fungi. Trends Biotechnol 2019, 37, 416–427. [DOI] [PubMed] [Google Scholar]
- (523).Jeffryes JG; Seaver SMD; Faria JP; Henry CS A Pathway for Every Product? Tools to Discover and Design Plant Metabolism. Plant Sci 2018, 273, 61–70. [DOI] [PubMed] [Google Scholar]
- (524).Pyne ME; Narcross L; Martin VJJ Engineering Plant Secondary Metabolism in Microbial Systems. Plant Physiol 2019, 179, 844–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (525).Lundy TA; Mori S; Thamban Chandrika N; Garneau-Tsodikova S Characterization of a Unique Interrupted Adenylation Domain That Can Catalyze Three Reactions. ACS Chem. Biol 2020, 15, 282–289. [DOI] [PubMed] [Google Scholar]
- (526).Harper C; Siller M OpenAG: A Globally Distributed Network of Food Computing. IEEE Pervasive Comput 2015, 14, 24–27. [Google Scholar]
- (527).Johnson AJ; Meyerson E; de la Parra J; Savas TL; Miikkulainen R; Harper CB Flavor-Cyber-Agriculture: Optimization of Plant Metabolites in an Open-Source Control Environment Through Surrogate Modeling. PLoS One 2019, 14, e0213918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (528).de la Parra J; Quave CL Ethnophytotechnology: Harnessing the Power of Ethnobotany with Biotechnology. Trends Biotechnol 2017, 35, 802–806. [DOI] [PubMed] [Google Scholar]
- (529).Egan JM; Kaur A; Raja HA; Kellogg JJ; Oberlies NH; Cech NB Antimicrobial Fungal Endophytes from the Botanical Medicine Goldenseal (Hydrastis canadensis). Phytochem. Lett 2016, 17, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (530).Schulz B; Boyle C In Microbial Root Endophytes; Schulz BJE;Boyle CJC;Sieber TN, Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2006, DOI: 10.1007/3-540-33526-9_1. [DOI] [Google Scholar]
- (531).Bacon CW; White J Microbial Endophytes; CRC press, 2000. [Google Scholar]
- (532).Bush LP; Wilkinson HH; Schardl CL Bioprotective Alkaloids of Grass-fungal Endophyte Symbioses. Plant Physiol 1997, 114, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (533).Nair DN; Padmavathy S Impact of Endophytic Microorganisms on Plants, Environment and Humans. ScientificWorldJournal 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (534).Strobel GA Endophytes as Sources of Bioactive Products. Microbes Infect 2003, 5, 535–544. [DOI] [PubMed] [Google Scholar]
- (535).Suryanarayanan T; Thirunavukkarasu N; Govindarajulu M; Sasse F; Jansen R; Murali T Fungal Endophytes and Bioprospecting. Fungal Biol. Rev 2009, 23, 9–19. [Google Scholar]
- (536).El-Elimat T; Raja HA; Graf TN; Faeth SH; Cech NB; Oberlies NH Flavonolignans from Aspergillus iizukae, a Fungal Endophyte of Milk Thistle (Silybum marianum). J. Nat. Prod 2014, 77, 193–199. [DOI] [PubMed] [Google Scholar]
- (537).Kusari S; Pandey SP; Spiteller M Untapped Mutualistic Paradigms Linking Host Plant and Endophytic Fungal Production of Similar Bioactive Secondary Metabolites. Phytochemistry 2013, 91, 81–87. [DOI] [PubMed] [Google Scholar]
- (538).Nisa H; Kamili AN; Nawchoo IA; Shafi S; Shameem N; Bandh SA Fungal Endophytes as Prolific Source of Phytochemicals and Other Bioactive Natural Products: A Review. Microb. Pathog 2015, 82, 50–59. [DOI] [PubMed] [Google Scholar]
- (539).Pugh ND; Jackson CR; Pasco DS Total Bacterial Load within Echinacea purpurea, Determined using a new PCR-based Quantification Method, is Correlated with LPS Levels and In Vitro Macrophage Activity. Planta Med 2013, 79, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (540).Todd DA; Gulledge TV; Britton ER; Oberhofer M; Leyte-Lugo M; Moody AN; Shymanovich T; Grubbs LF; Juzumaite M; Graf TN Ethanolic Echinacea purpurea Extracts Contain a Miixture of Cytokine-Suppressive and Cytokine-Inducing Compounds, Including some that Originate from Endophytic Bacteria. PLoS One 2015, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (541).Khare E; Mishra J; Arora NK Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (542).Martinez-Klimova E; Rodríguez-Peña K; Sánchez S Endophytes as Sources of Antibiotics. Biochem. Pharmacol 2017, 134, 1–17. [DOI] [PubMed] [Google Scholar]
- (543).Ibrahim A; Tanney JB; Fei F; Seifert KA; Cutler GC; Capretta A; Miller JD; Sumarah MW Metabolomic-guided Discovery of Cyclic Nonribosomal Peptides from Xylaria ellisii sp. nov., a Leaf and Stem Endophyte of Vaccinium angustifolium. Sci. Rep 2020, 10, 4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (544).Yan J; Kline AD; Mo H; Shapiro MJ; Zartler ER A Novel Method for the Determination of Stereochemistry in Six-Membered Chairlike Rings Using Residual Dipolar Couplings. J. Org. Chem 2003, 68, 1786–1795. [DOI] [PubMed] [Google Scholar]
- (545).Kummerlöwe G; Luy B Residual Dipolar Couplings for the Configurational and Conformational Analysis of Organic Molecules. Annu. Rep. NMR Spectrosc 2009, 68, 193–232. [Google Scholar]
- (546).Gil RR Constitutional, Configurational, and Conformational Analysis of Small Organic Molecules on the Basis of NMR Residual Dipolar Couplings. Angew. Chem. Int. Ed. Engl 2011, 50, 7222–7224. [DOI] [PubMed] [Google Scholar]
- (547).Hallwass F; Schmidt M; Sun H; Mazur A; Kummerlöwe G; Luy B; Navarro-Vázquez A; Griesinger C; Reinscheid UM Residual Chemical Shift Anisotropy (RCSA): A Tool for the Analysis of the Configuration of Small Molecules. Angew. Chem. Int. Ed. Engl 2011, 50, 9487–9490. [DOI] [PubMed] [Google Scholar]
- (548).Kummerlöwe G; Grage SL; Thiele CM; Kuprov I; Ulrich AS; Luy B Variable Angle NMR Spectroscopy and its Application to the Measurement of Residual Chemical Shift Anisotropy. J. Magn. Reson 2011, 209, 19–30. [DOI] [PubMed] [Google Scholar]
- (549).Nath N; Schmidt M; Gil RR; Williamson RT; Martin GE; Navarro-Vázquez A; Griesinger C; Liu Y Determination of Relative Configuration from Residual Chemical Shift Anisotropy. J. Am. Chem. Soc 2016, 138, 9548–9556. [DOI] [PubMed] [Google Scholar]
- (550).Liu Y; Sauri J; Mevers E; Peczuh MW; Hiemstra H; Clardy J; Martin GE; Williamson RT Unequivocal Determination of Complex Molecular Structures using Anisotropic NMR Measurements. Science 2017, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (551).Liu Y; Navarro-Vazquez A; Gil RR; Griesinger C; Martin GE; Williamson RT Application of Anisotropic NMR Parameters to the Confirmation of Molecular Structure. Nat. Protoc 2019, 14, 217–247. [DOI] [PubMed] [Google Scholar]
- (552).Elyashberg M; Williams A; Blinov K Contemporary Computer-Assisted Approaches to Molecular Structure Elucidation, 2011.
- (553).Elyashberg M; Williams AJ; Blinov K Structural Revisions of Natural products by Computer-Assisted Structure Elucidation (CASE) Systems. Nat. Prod. Rep 2010, 27, 1296–1328. [DOI] [PubMed] [Google Scholar]
- (554).Burns DC; Mazzola EP; Reynolds WF The role of Computer-Assisted Structure Elucidation (CASE) Programs in the Structure Elucidation of Complex Natural Products. Nat. Prod. Rep 2019, 36, 919–933. [DOI] [PubMed] [Google Scholar]
- (555).Mnova StereoFitter 2019, 2020. [Google Scholar]
- (556).Linington RG; Kubanek J; Luesch H New Methods for Isolation and Structure Determination of Natural Products. Nat. Prod. Rep 2019, 36, 942–943. [DOI] [PubMed] [Google Scholar]
- (557).Bisson J; Simmler C; Chen S-N; Friesen JB; Lankin DC; McAlpine JB; Pauli GF Dissemination of Original NMR Data Enhances Reproducibility and Integrity in Chemical Research. Nat. Prod. Rep 2016, 33, 1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (558).McAlpine JB; Chen SN; Kutateladze A; MacMillan JB; Appendino G; Barison A; Beniddir MA; Biavatti MW; Bluml S; Boufridi A et al. The Value of Universally Available Raw NMR Data for Transparency, Reproducibility, and Integrity in Natural Product Research. Nat. Prod. Rep 2019, 36, 35–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (559).Nannenga BL; Gonen T MicroED Opens a New Era for Biological Structure Determination. Curr. Opin. Struct. Biol 2016, 40, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (560).Jones CG; Martynowycz MW; Hattne J; Fulton TJ; Stoltz BM; Rodriguez JA; Nelson HM; Gonen T The CryoEM Method MicroED as a Powerful Tool for Small Molecule Structure Determination. ACS Cent. Sci 2018, 4, 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (561).Gruene T; Wennmacher JTC; Zaubitzer C; Holstein JJ; Heidler J; Fecteau-Lefebvre A; De Carlo S; Müller E; Goldie KN; Regeni I et al. Rapid Structure Determination of Microcrystalline Molecular Compounds Using Electron Diffraction. Angew. Chem. Int. Ed. Engl 2018, 57, 16313–16317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (562).Han G; Ceilley R Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther 2017, 34, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (563).Kosmadaki M; Katsambas A Topical Treatments for Acne. Clin. Dermatol 2017, 35, 173–178. [DOI] [PubMed] [Google Scholar]
- (564).Muhs A; Lyles JT; Parlet CP; Nelson K; Kavanaugh JS; Horswill AR; Quave CL Virulence Inhibitors from Brazilian Peppertree Block Quorum Sensing and Abate Dermonecrosis in Skin Infection Models. Sci. Rep 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (565).Nuutila K; Grolman J; Yang L; Broomhead M; Lipsitz S; Onderdonk A; Mooney D; Eriksson E Immediate Treatment of Burn Wounds with High Concentrations of Topical Antibiotics in an Alginate Hydrogel Using a Platform Wound Device. Adv. Wound Care 2019, DOI: 10.1089/wound.2019.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (566).Canaparo R; Foglietta F; Giuntini F; Della Pepa C; Dosio F; Serpe L Recent Developments in Antibacterial Therapy: Focus on Stimuli-Responsive Drug-Delivery Systems and Therapeutic Nanoparticles. Molecules 2019, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (567).Banerjee K; Banerjee S; Das S; Mandal M Probing the Potential of Apigenin Liposomes in Enhancing Bacterial Membrane Perturbation and Integrity Loss. J. Colloid Interface Sci 2015, 453, 48–59. [DOI] [PubMed] [Google Scholar]
- (568).Prausnitz MR Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu. Rev. Chem. Biomol. Eng 2017, 8, 177–200. [DOI] [PubMed] [Google Scholar]
- (569).van Vuuren S; Viljoen A Plant-based Antimicrobial Studies--Methods and Approaches to Study the Interaction Between Natural Products. Planta Med 2011, 77, 1168–1182. [DOI] [PubMed] [Google Scholar]
- (570).Dettweiler M; Marquez L; Bao M; Quave CL Quantifying Synergy in the Bioassay-guided Fractionation of Natural Product Extracts. PLoS One 2020, 15, e0235723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (571).Schmidt BM; Ribnicky DM; Lipsky PE; Raskin I Revisiting the Ancient Concept of Botanical Therapeutics. Nat. Chem. Biol 2007, 3, 360–366. [DOI] [PubMed] [Google Scholar]
- (572).Cokol M; Kuru N; Bicak E; Larkins-Ford J; Aldridge BB Efficient Measurement and Factorization of High-order Drug Interactions in Mycobacterium tuberculosis. Sci. Adv 2017, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (573).Inui T; Wang YH; Pro SM; Franzblau SG; Pauli GF Unbiased Evaluation of Bioactive Secondary Metabolites in Complex Matrices. Fitoterapia 2012, 83, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (574).Gibbons S Anti-Staphylococcal Plant Natural Products. Nat. Prod. Rep 2004, 21, 263–277. [DOI] [PubMed] [Google Scholar]
- (575).Cheesman MJ; Ilanko A; Blonk B; Cock IE Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev 2017, 11, 57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (576).Atanasov AG; Waltenberger B; Pferschy-Wenzig E-M; Linder T; Wawrosch C; Uhrin P; Temml V; Wang L; Schwaiger S; Heiss EH et al. Discovery and Resupply of Pharmacologically Active Plant-derived Natural Products: A review. Biotechnol. Adv 2015, 33, 1582–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (577).Zacchino SA; Butassi E; Liberto MD; Raimondi M; Postigo A; Sortino M Plant Phenolics and Terpenoids as Adjuvants of Antibacterial and Antifungal Drugs. Phytomedicine 2017, 37, 27–48. [DOI] [PubMed] [Google Scholar]
- (578).Rempe CS; Burris KP; Lenaghan SC; Stewart CN The Potential of Systems Biology to Discover Antibacterial Mechanisms of Plant Phenolics. Front. Microbiol 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (579).Cushnie TPT; Lamb AJ Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [DOI] [PubMed] [Google Scholar]
- (580).Nijveldt RJ; van Nood E; van Hoorn DEC; Boelens PG; van Norren K; van Leeuwen PAM Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. Am. J. Clin. Nutr 2001, 74, 418–425. [DOI] [PubMed] [Google Scholar]
- (581).Cushnie TPT; Cushnie B; Lamb AJ Alkaloids: An Overview of their Antibacterial, Antibiotic-Enhancing and Antivirulence Activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [DOI] [PubMed] [Google Scholar]
- (582).Goyal S In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat KG;Mérillon J-M, Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013, DOI: 10.1007/978-3-642-22144-6_98. [DOI] [Google Scholar]
- (583).Cordell GA; Quinn-Beattie ML; Farnsworth NR The Potential of Alkaloids in Drug Discovery. Phytother. Res 2001, 15, 183–205. [DOI] [PubMed] [Google Scholar]
- (584).Lipinski CA; Lombardo F; Dominy BW; Feeney PJ Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev 2001, 46, 3–26. [DOI] [PubMed] [Google Scholar]
- (585).Rosén J; Gottfries J; Muresan S; Backlund A; Oprea TI Novel Chemical Space Exploration via Natural Products. J. Med. Chem 2009, 52, 1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (586).Salam AM; Quave CL Opportunities for Plant Natural Products in Infection Control. Curr. Opin. Microbiol 2018, 45, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (587).Quave CL; Pieroni A A Reservoir of Ethnobotanical Knowledge Informs Resilient Food Security and Health Strategies in the Balkans. Nat. Plants 2015, 1, 14021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


