Abstract
This systematic review and meta-analysis was designed to determine the efficacy of mindfulness-based interventions (MBIs) in improving fatigue-related outcomes in adult cancer survivors. Randomized controlled trials (RCTs) were identified from PubMed, MEDLINE, PsycINFO, CINAHL, Web of Science, and EMBASE databases and reference lists of included studies. Separate random-effects meta-analyses were conducted for fatigue and vitality/vigor. Twenty-three studies reporting on 21 RCTs (N=2,239) met inclusion criteria. MBIs significantly reduced fatigue compared to controls at post-intervention (g=0.60, 95% CI [0.36, 0.83]) and first follow-up (g=0.42, 95% CI [0.20, 0.64]). Likewise, MBIs significantly improved vitality/vigor at post-intervention (g=0.39, 95% CI [0.25, 0.52]) and first follow-up (g=0.35, 95% CI [0.03, 0.67]). The evidence grade was low due to risk of bias, substantial heterogeneity, and publication bias among studies. MBIs show promise in improving fatigue and vitality/vigor in cancer survivors. More rigorous trials are needed to address current gaps in the evidence base.
Keywords: mindfulness, cancer, fatigue, vitality, vigor, randomized controlled trial, systematic review, meta-analysis
Graphical abstract
1. Introduction
Many of the estimated 43.8 million cancer survivors worldwide suffer from debilitating effects of cancer and its treatments [1]. These effects include both psychological (e.g., depression, anxiety) and physical symptoms (e.g., pain, fatigue) [2, 3]. Fatigue is one of the most prevalent and distressing symptoms reported by 25–99% of patients undergoing active cancer therapy [4, 5]. Moderate to severe levels of fatigue persist for 22–33% of survivors in the months and years following cancer treatment [6]. Fatigue profoundly interferes with survivors’ activities and mood [6, 7] and is often associated with other disruptive symptoms (e.g., sleep disturbance, pain, anxiety, depressive symptoms) [8, 9], attentional disturbance [10, 11], and impaired health-related quality of life [7, 12]. Fatigue is also associated with increased healthcare utilization [13], significant disability [13–15], and financial burden resulting from disability [7, 16–18].
One treatment for fatigue with rapid growth in popularity in the past two decades is mindfulness-based intervention (MBI). Through training in mindfulness meditation, individuals learn to focus attention on present-moment experiences with an attitude of open curiosity and acceptance, resulting in less reactivity to difficult internal experiences [19]. While Mindfulness-Based Stress Reduction (MBSR) was the first and arguably the most popular manualized MBI [20, 21], several other MBIs (e.g., Mindfulness-Based Cognitive Therapy [MBCT] [22], Mindfulness-Based Cancer Recovery [MBCR] [23], Mindfulness-Based Art Therapy [MBAT] [24], Mindful Awareness Practices [MAP] [25]) have been tested in cancer for psychological and physical symptoms, including fatigue [26].
Although MBIs are listed as evidence-based treatments for fatigue in clinical practice guidelines, the strength of the recommendations varies across guidelines. MBSR, for example, has the highest-level evidence (category 1) in the National Comprehensive Cancer Network guidelines [27] and lower level evidence in the American Society of Clinical Oncology [28, 29] and Canadian Association of Psychosocial Oncology [28, 29] guidelines (category 2A), with the Oncology Nursing Society designating MBSR as “likely to be effective” but with insufficient evidence to be “recommended for practice” [30]. These inconsistent recommendations reflect weaknesses in current analyses of available evidence. Although several recent meta-analyses of MBIs in cancer have included fatigue among the outcomes, most only examined breast cancer [31–35], several were exclusive to MBSR [31–33, 36], and none included all of the MBI types examined in the present review. Further, most meta-analyses did not: (1) examine the distinct constructs of fatigue and vitality/vigor separately [37], (2) systematically assess evidence quality, such as with the “Grading of Recommendations Assessment, Development and Evaluation” (GRADE) approach [38], or (3) analyze MBIs’ effects at follow-ups beyond post-intervention.
The primary aim of the current systematic review and meta-analysis was to evaluate the efficacy of MBIs on fatigue and vitality/vigor in adult cancer survivors to inform clinical practice guidelines. We included fatigue (measured by scales assessing tiredness, exhaustion, and need for rest) and vitality/vigor (measured by scales assessing energy and active levels of functioning), as they are common outcomes in fatigue trials in cancer; however, we analyzed the effects separately given that these are distinct constructs [37]. We compared the efficacy of MBIs with that of usual care/wait-list controls or active treatment controls at post-intervention and the first available follow-up, when applicable. The effects of potential moderators (e.g., gender, age, intervention type) were also examined. The GRADE approach was used to assess evidence quality. The present review is the largest and most inclusive meta-analysis to assess MBI’s impact on fatigue in cancer while providing a rigorous examination of study quality.
2. Materials and Methods
2.1. Search Strategy
This review followed the recommendations of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [39]. The protocol is registered in the PROSPERO database (CRD42020113022). A systematic literature search was conducted using PubMed [Cancer subset], MEDLINE, PsycINFO, CINAHL, Web of Science, and EMBASE databases from inception until December 2019. Each database was searched with a combination of keywords related to (a) cancer or neoplasm, (b) fatigue or vitality/vigor, and (c) mindfulness-based intervention (truncated keywords such as mindful* were used to capture full terms and phrases such as mindfulness-based therapy, mindfulness meditation, and Mindfulness-Based Stress Reduction). A complete list of search terms can be found in Table 1. Reference lists and forward citations of selected eligible articles were also examined to identify any studies that may have been missed in systematic database searches.
Table 1.
Operationalization of the search terms by topic.
| Topic | Search Terms |
|---|---|
| Cancer | Cancer, neoplasm |
| Fatigue | Fatigue, vitality, vigor, vigour |
| Mindfulness-based interventions | Mindful*, meditat*, MBSR, MBCT, MBCR |
Note:
represents truncations. Search terms within each category are combined with OR. Search terms between categories are combined with AND. Some terms were truncated.
2.2. Inclusion and Exclusion Criteria
Eligibility criteria were applied in three phases: (1) title screening, (2) abstract screening, and (3) full-text screening. Inclusion criteria included: (1) adult sample (≥18 years of age) of cancer survivors (with any type or stage of cancer; on active cancer treatment or post-treatment); (2) randomized controlled trials (RCTs) testing a mindfulness-based behavioral intervention (e.g., MBSR, MBCT) where the main intervention component was guided mindfulness meditation, (3) intervention outcomes of fatigue or vitality/vigor assessed at baseline and one or more times post-intervention with sufficient data to calculate an effect size (corresponding authors were contacted for needed data if not provided in the publication), and (4) peer-reviewed studies with results published in English. Studies were excluded if the MBI did not have mindfulness as the main component (e.g., Acceptance and Commitment Therapy; studies primarily focused on yoga).
2.3. Study Selection
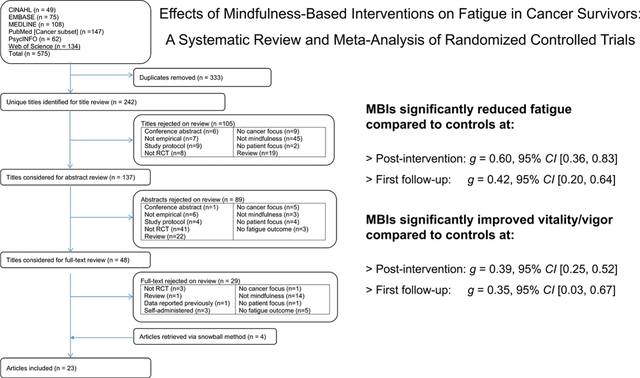
Figure 1 shows a flowchart of study selection. The second author (WLT) conducted the search. Two independent reviewers (SAJ and WLT) applied study eligibility criteria in three phases: (1) screened all titles and excluded articles that clearly did not have a focus on fatigue in cancer survivors or were not empirical, (2) screened selected abstracts and excluded those that clearly did not have a focus on MBIs, were not empirical, and/or did not assess fatigue or vitality/vigor, and (3) screened the full-text articles of the remaining citations. Disagreements were resolved by consensus.
Figure 1.
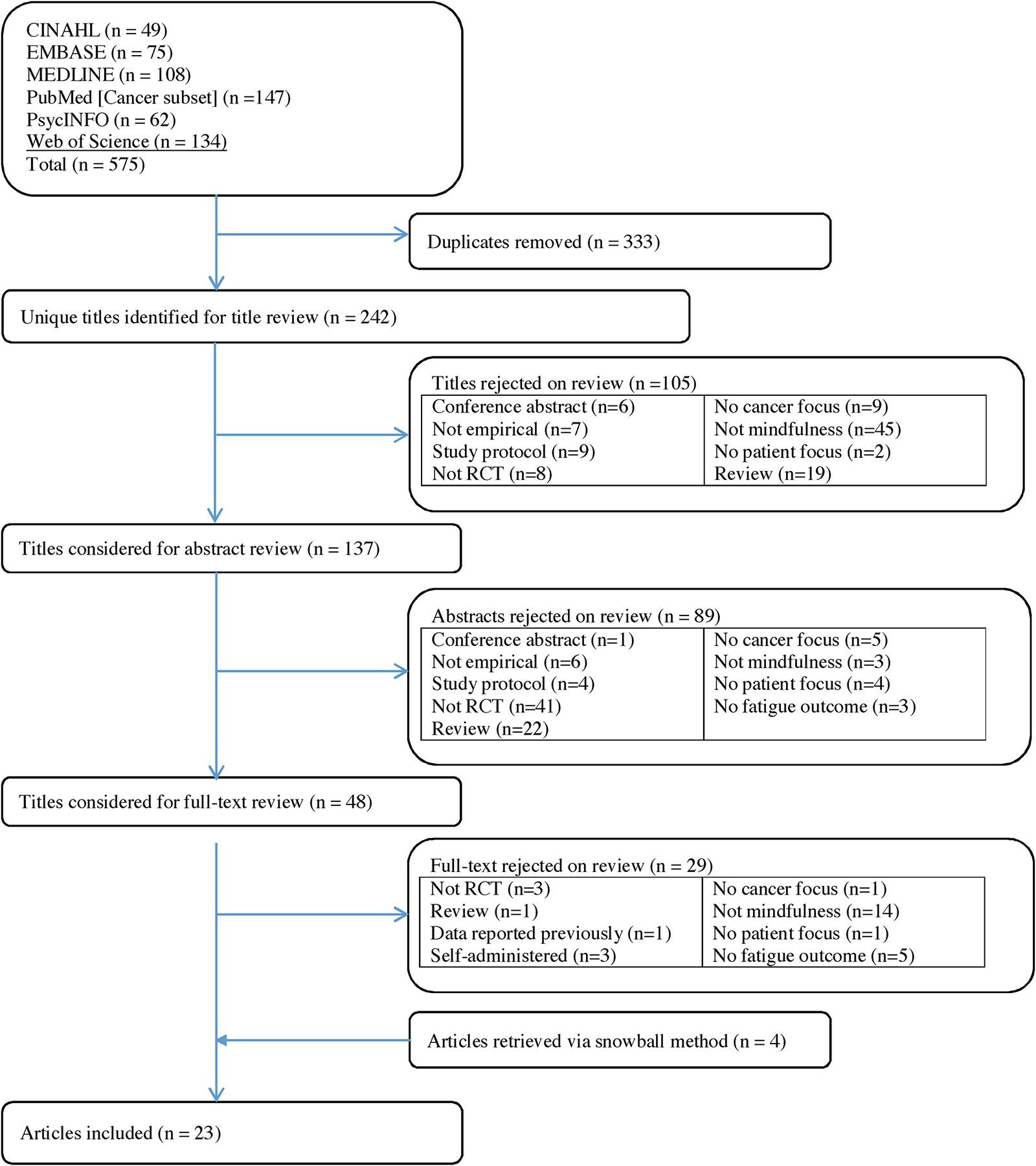
Systematic review flowchart.
2.4. Coding
A pair of reviewers from our team (SAJ, ES, PVS, JLC, TLT, MLS, MTF) individually extracted data from each paper using a standardized template created specifically for our review. Any disagreements were reconciled by consensus among the pairs with discrepancies resolved by judgment from the first author (SAJ). Data extracted included: authors, year of publication, sample size, treatment status, cancer type(s), cancer stage(s), intervention arms/details, study setting, outcomes, measures, eligibility criteria, baseline characteristics, assessment time points, and unadjusted means/SDs (effect sizes) for fatigue and/or vitality/vigor outcome(s) at each time point. Other extracted data included clinical trial registration, eligibility criteria based on clinically significant fatigue, mention of a theoretical framework underlying the intervention, interventionist qualifications, specification of mindfulness home practice assignments and completed practice time, documented assessment of MBI fidelity, and reporting on adverse effects of MBIs.
2.5. Risk of Bias Assessment
Risk of bias was assessed for the 23 included papers using the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0) [40]. Each included paper was independently reviewed by a pair of co-authors (SAJ, ES, PVS, JLC, TLT, MLS, MTF) for risk of bias, who then met in pairs to establish consensus for each risk of bias domain. Discrepancies were resolved by judgment from the first author (SAJ) in consultation with the senior author (KLR) when needed.
2.6. Quality of Evidence Assessment
The GRADE system was used to rate the overall quality of evidence of the meta-analytic results [38]. GRADE assessment goes beyond risk of bias, which addresses internal validity of the included studies, to instead reflect the general confidence in the overall effect size. GRADE uses a baseline rating of high for RCTs. This rating can be downgraded to moderate, low, or very low based on five assessment criteria: risk of bias, inconsistency of the results, indirectness, imprecision, and publication bias. The ratings were determined by two authors (SAJ and ES) who established consensus for ratings for each GRADE criterion.
2.7. Meta-analytic Method
Effect sizes were standardized weighted mean differences based on Hedges’s g, correcting for bias due to small sample sizes, for continuous measures of fatigue or vitality/vigor. Separate analyses were conducted for fatigue and vitality/vigor at post-intervention and first follow-up.
We calculated the standardized pre-post effect sizes using the formula d = (ΔT − ΔC)/SDP), where ΔT and ΔC are the mean pre-post change scores for the treatment and control conditions, and SDP is the pooled post-treatment standard deviation. This indicates the degree to which the intervention group changed compared to controls in standard deviation units.
Using Comprehensive Meta-Analysis software (Version 3.0), we corrected d for small sample sizes, resulting in Hedges’s g. According to Lipsey and Wilson [41], effect sizes from 0.00 to 0.32 are considered small, 0.33 to 0.55 are considered moderate, and 0.56 and above are considered large. Effect sizes were weighted by the inverse standard error and presented with 95% confidence intervals (CI). To obtain a summary statistic, effect sizes were pooled across studies using the inverse variance random-effects model [42]. When studies reported more than one relevant effect size for either fatigue or vitality/vigor, the average effect size was used so that only one result for either fatigue or vitality/vigor was included in the analyses per sample. A positive effect size value was chosen to represent the effect size in the hypothesized direction.
Heterogeneity of effect sizes was examined using Cochran’s Q and I2 statistics. A Q statistic of less than .10 was considered as evidence of significant heterogeneity. We described I2 values of 25%, 50%, and 75% as low, moderate, and high levels of heterogeneity, respectively. We examined potential publication bias using Begg’s funnel plots [43] with Duval and Tweedie’s trim-and-fill adjustment [44], as well as Egger’s test of asymmetry and Rosenthal’s fail-safe N. In addition, we conducted sensitivity analyses based on study quality.
We examined the effects of potential moderators, including participant gender composition (% female), mean age of the sample, type of MBI (MBSR vs. other), type of control condition (active control vs. non-active control [waitlist/usual care]), intervention dose (total number of intervention hours including retreat hours), drop-out rate, and time between post-intervention and first follow-up assessment (in months). Effects of potential moderators were examined with meta-regression analyses using a restricted maximum likelihood model. We examined each moderator independently to maximize the number of studies included in the analyses.
3. Results
3.1. Studies Included
The electronic database search identified 575 records. After excluding duplicates, 242 records were extracted for title and abstract screening. A total of 105 records were excluded based on title screening, with an additional 89 excluded based on abstract screening. Thus, 48 records were selected for full-text screening of which 29 records were excluded. We reviewed reference lists of the 19 included publications and found 4 additional eligible publications resulting in 23 eligible publications selected for meta-analyses. Of the 23 records examined for coding, 15 records included sufficient information for analyses. We contacted authors of the remaining 8 records and received sufficient data for all of these records. Overall, 23 records with sufficient information were included in effect size calculations [22–25, 45–63].
3.2. Study Characteristics
In total, 23 research papers describing results of 21 independent RCTs were included in the analyses. Seventeen of the included studies assessed fatigue, 11 assessed vitality/vigor, and 6 assessed both fatigue and vitality/vigor. The characteristics of the included studies are presented in Table 2. The included trials involved 2,239 cancer survivors, with a mean sample size of 106.6 (range: 24–322). The mean percentage of females across studies was 92.8% (range: 70.6–100%). The mean age of the samples was 53.4 (range: 42.8–58.0). The mean percentage of participants with breast cancer was 77.9% (range: 0–100%). Approximately half of the studies implemented original or adapted MBSR (K = 10, 47.6%). Most RCTs included a waitlist/usual care control (K = 15, 71.4%). The mean intervention dose was 18.9 hours (range: 9–36 hours). A total of 15 RCTs included one or more follow-up assessments beyond post-intervention, with a mean follow-up time of 3.1 months (range: 0.9–6.2 months).
Table 2.
Characteristics of the included studies
| Sample size | Mean age | Gender distribution [N (%) female] | Race/ Ethnicity [N (%)] | Intervention | Outcome assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Sample | Fatigue eligibility criterion | MBI | Control(s) | MBI | Control(s) | MBI | Control(s) | MBI | Control(s) | Origin | MBI | Control(s) | Follow-up time point(s) | Fatigue outcome (Measure) |
| Blaes, 2016 | Any cancer type (69.0% breast), stage I-III, ≤6 mos. post-curative tx | NA | 28 | 14 | 55 | 57 | 26 (92.9%) | 12 (85.7%) | NR | NR | United States | MBCR; 8 weekly 2.5-hour sessions + 8-hour retreat | Waitlist | Post-interv. 2 mos. post-interv. | Fatigue (FACT-F)b |
| Bower, 2015 | Breast cancer, stage 0-III dx at age ≤50, ≥3 mos. to ≤10 years post-tx (excl. hormone therapy) | NA | 39 | 32 | 46.1 | 47.7 | 39 (100.0%) | 32 (100.0%) | White: 29 (74.4%) Black: 1 (2.6%) Asian: 3 (7.7%) Other: 6 (15.4%) | White: 25 (78.1%) Black: 1 (3.1%) Asian: 5 (15.6%) Other: 1 (3.1%) | United States | MAPs; 6 weekly 2-hour sessions | Waitlist | Post-interv. 3 mos. post-interv. | Fatigue (FSI) |
| Bruggeman-Everts, 2017 | Any cancer type (47.3% breast), ≥3 mos. post-curative tx (excl. hormone therapy) | ≥35 CIS-FS | 55 | 62 (AAF); 50 (PE) | 51.36 | 56.45 (AAF); 56.54 (PE) | 39 (70.9%) | 44 (80.0%; AAF); 40 (80.0%; PE) | NR | NR | The Netherlands | Web-based eMBCT; 4 hours/week for 9 weeks | Home-based AAF; 3 hours/week for 9 weeks PE emails; 10 min./week for 9 weeks | Post-interv. 4 mos. post-interv. | Fatigue severity (CIS-FS)a,b |
| Carlson, 2016 | Breast cancer, stage 0-IV, ≥3 mos. post-tx (excl. hormone therapy), score ≥4 Distress Thermometer | NA | 134 | 118 | 55.12 | 54.14 | 134 (100.0%) | 118 (100.0%) | NR | NR | Canada | MBCR; 8 weekly 1.5-hour sessions + 6-hour retreat | SET; 12 weekly 1.5-hour sessions SMS;** 1-day minimal attention waitlist | Post-interv. 6 mos. post-interv. 12 mos. post-interv. | Fatigue (POMS)b Vigor (POMS)b |
| Cheng, 2019 | Any cancer type (65.4% breast), stage I-III, ≥1 mo. post-curative tx | NA | 25 | 27 | 54.04b | 53.74b | 23 (92.0%) | 24 (88.9%) | NR | NR | Taiwan | Unspecified Mindfulness interv. 12 weekly 2-hour sessions | Qigong; 12 weekly 2-hour sessions | Post-interv. 3 mos. post-interv. | Cancer Related Fatigue (CRF scale) |
| Dodds, 2015 | Breast cancer, stage I-IV, ≤10 years post-adj. systemic chemo | NA | 12* | 16* | 54.7 | 55.8 | 12 (100.0%) | 16 (100.0%) | White: 11 (91.7%) Other: 1 (8.3%) | White: 12 (75.0%) Other: 4 (25.0%) | United States | CBCT; 8 weekly 2-hour sessions + booster 1 mo. post-interv. | Waitlist | Post-interv. 1 mo. post-interv. | Vitality (SF-12) |
| Hoffman, 2012 | Breast cancer, stage 0-III, ≥2 mos. to ≤2 years post-tx (excl. hormone therapy) | NA | 103* | 111* | 49.0 | 50.1 | 103 (100.0%) | 111 (100.0%) | NR | NR | United Kingdom | Original MBSR; 8 weekly 2-hour sessions (session 1 and 8 were 2.25 hours) + 6-hour retreat | Waitlist | Post-interv. 1 mo. post-interv. | Fatigue (POMS) Vigor (POMS) |
| Jang, 2016 | Breast cancer, stage 0-III, <2 years post-curative tx | NA | 12 | 12 | 51.75 | 51.42 | 12 (100.0%) | 12 (100.0%) | NR | NR | South Korea | Adapted MBAT; 12 weekly 45-minute sessions | Waitlist | Post-interv. | Fatigue (QLQ-C30) |
| Johns, 2015 | Any cancer type (86% breast), stage I-IV, ≥3 mos. post-tx (excl. hormone therapy) | ≥4 FSI-SC | 18 | 17 | 58.8 | 55.7 | 17 (94.4%) | 16 (94.1%) | White: 15 (83.3%) Other: 3 (16.7%) | White: 13 (76.5%) Other: 4 (23.5%) | United States | Adapted MBSR; 7 weekly 2-hour sessions | Waitlist | Post-interv. 1 mo. post-interv. | Fatigue (FSI)a,b Vitality (SF-36)b |
| Johns, 2016 | Breast or colorectal cancer (85% breast), stage 0-III, ≥3 mos. to ≤5 years post-tx (excl. hormone therapy) | ≥4 FSI-SC | 35 | 36 | 56.9 | 56.4 | 33 (94.3%) | 31 (86.1%) | White: 27 (77.1%) Other: 8 (22.9%) | White: 23 (63.9%) Other: 13 (36.1%) | United States | Adapted MBSR; 8 weekly 2-hour sessions | PES; 8 weekly 2-hour sessions | Post-interv. 6 mos. post-interv. | Fatigue (FSI)a Vitality (SF-36) |
| Kenne Sarenmalm, 2017 | Breast cancer, post-adj. chemo and/or radiation | NA | 62 | 52 (S-MBSR); 52 (UC) | 57.2 | 57.2 (S-MBSR); 57.2 (UC) | 62 (100.0%) | 52 (100.0%; S-MBSR); 52 (100.0%; UC) | NR | NR | Sweden | Adapted MBSR; 8 weekly 2-hour sessions | Self-guided MBSR; 2 hours/week for 8 weeks UC | Post-interv. | Vitality (SF-36) |
| Lengacher, 2009 | Breast cancer, stage 0-III, ≤18 mos. post-curative tx (surgery + adj. radiation or chemo) | NA | 40* | 42* | 57.1 | 58.0 | 40 (100.0%) | 42 (100.0%) | White: 36 (87.8%) Black: 2 (4.9%) Hispanic: 6 (14.6%) Other: 3 (7.3%) | White: 34 (79.1%) Black: 8 (18.6%) Hispanic: 3 (7.0%) Other: 1 (2.3%) | United States | Adapted MBSR(BC); 6 weekly 2-hour sessions | Waitlist | Post-interv. | Vitality (SF-36)b |
| Lengacher, 2012 | Breast cancer, stage 0-III, ≤18 mos. post-curative tx (surgery + adj. radiation or chemo) | NA | 40* | 42* | 57.1 | 58.0 | 40 (100.0%) | 42 (100.0%) | White: 36 (87.8%) Black: 2 (4.9%) Hispanic: 6 (14.6%) Other: 3 (7.3%) | White: 34 (79.1%) Black: 8 (18.6%) Hispanic: 3 (7.0%) Other: 1 (2.3%) | United States | Adapted MBSR(BC); 6 weekly 2-hour sessions | Waitlist | Post-interv. | Fatigue symptom cluster (MDASI) |
| Lengacher, 2016 | Breast cancer, stage 0-III, ≥2 weeks to ≤2 years post-tx | NA | 167 | 155 | 56.5 | 57.6 | 167 (100.0%) | 155 (100.0%) | White: 112 (67.1%) Black: 21 (12.6%) Hispanic: 19 (11.4%) Other: 15 (9.0%) NR: 0 (0.0%) | White: 110 (71.0%) Black: 16 (10.3%) Hispanic: 14 (9.0%) Other: 13 (8.4%) NR: 2 (1.3%) | United States | Adapted MBSR(BC); 6 weekly 2-hour sessions | Waitlist | Post-interv. 1.5 mos. post-interv. | Fatigue (FSI) |
| Liu, 2019 | Differentiated thyroid cancer (DTC), stage I-IV (excl. metastasis to other organ), first upcoming radioactive iodine tx scheduled | NA | 49* | 53* | 43.32 | 42.38 | 34 (69.4%) | 38 (71.7%) | NR | NR | China | Adapted MBSR; 8 weekly 2.5-hour sessions | UC | Post-interv. 3 mos. post-interv. | Fatigue (QLQ-C30) |
| Monti, 2006 | Any female cancer type (46% breast), stage 0-IV, ≥4 mos. to ≤2 years post-dx | NA | 56 | 55 | 53.1 | 54.1 | 56 (100.0%) | 55 (100.0%) | White: 45 (80.4%) Black: 10 (17.9%) Asian: 1 (1.8%) Hispanic: 0 (0.0%) Other: 0 (0.0%) | White: 38 (69.1%) Black: 13 (23.6%) Asian: 1 (1.8%) Hispanic: 2 (3.6%) Other: 1 (1.8%) | United States | Original MBAT; 8 weekly 2.5-hour sessions | Waitlist | Post-interv. | Vitality (SF-36)b |
| Monti, 2013 | Breast cancer, stage 0-IV, ≥6 mos. to ≤3 years post-dx or post-recurrence | NA | 98 | 93 | 56.9 | 56.4 | 98 (100.0%) | 93 (100.0%) | White: 59 (60.2%) Black: 35 (35.7%) Other: 4 (4.1%) | White: 54 (58.1%) Black: 37 (38.8%) Other: 2 (2.2%) | United States | Original MBAT; 8 weekly 2.5-hour sessions | BCSG; 8 weekly 2.5-hour sessions | Post-interv. 6 mos. post-interv. | Vitality (SF-36)b |
| Rahmani, 2014 | Breast cancer, stage I-III, ≥1 mo. post-dx | NA | 12 | 12 (MCT); 12 (UC) | 43.25 | 44.92 (MCT); 44.08 (UC) | 12 (100.0%) | 12 (100.0%; MCT); 12 (100.0%; UC) | NR | NR | Iran | Adapted MBSR; 8 weekly 2-hour sessions | MCT; 8 weekly sessions UC | Post-interv. 2 mos. post-interv. | Fatigue (QLQ-C30) |
| Rahmani, 2015 | Breast cancer, stage I-III, ≥1 mo. post-dx | >4 FSS-P | 12 | 12 | 43.25 | 44.08 | 12 (100.0%) | 12 (100.0%) | NR | NR | Iran | Adapted MBSR; 8 weekly 2-hour sessions | UC | Post-interv. 2 mos. post-interv. | Fatigue severity (FSS-P)a |
| Speca, 2000 | Any cancer type (43% breast), stage I-IV | NA | 53* | 37* | 54.9 | 48.9 | 46 (86.8%) | 27 (73.0%) | NR | NR | Canada | Adapted MBSR; 7 weekly 1.5-hour sessions | Waitlist | Post-interv. | Fatigue (POMS) Vigor (POMS) |
| van der Lee, 2012 | Any cancer type (60% breast), ≥1 year post-curative tx | ≥35 CIS-FS | 59* | 24* | 53.1 | 49.4 | 51 (86.4%) | 19 (79.2%) | NR | NR | The Netherlands | Adapted MBCT; 8 weekly 2.5-hour sessions + 6-hour retreat + 2.5-hour booster 2 mos. post-interv. | Waitlist | Post-interv. 6 mos. post-interv. | Fatigue severity (CIS-FS)a |
| Witek-Janusek, 2019 | Breast cancer, stage 0-III, post-surgery | NA | 84* | 80* | 55 | 55.2 | 84 (100.0%) | 80 (100.0%) | White: 68 (81.0%) Black: 10 (11.9%) Asian: 1 (1.2%) Hispanic: 2 (2.4%) Other: 1 (1.2%) NR: 2 (2.4%) | White: 58 (72.5%) Black: 13 (16.3%) Asian: 1 (1.3%) Hispanic: 5 (6.3%) Other: 2 (2.5%) NR: 1 (1.3%) | United States | Original MBSR; 8 weekly 2.5-hour sessions + 6-hour retreat (after week 5) | ACC 8 weekly 2.5-hour sessions of education | Interv. midpoint Post-interv. 1 mo. post-interv. 6 mos. post-interv. | Fatigue (MFSI-SF) |
| Zernicke, 2014 | Any cancer type (33.9% breast), ≤3 years post-tx | NA | 30 | 32 | 58 | 58 | 22 (73.3%) | 23 (71.9%) | NR | NR | Canada | Online MBCR 8 weekly 2-hour sessions + 6-hour retreat (after week 6) | Waitlist | Post-interv. | Fatigue (POMS)b Vigor (POMS)b |
Note: AAF: Ambulant Activity Feedback; ACC: Active Control Condition; adj.: adjuvant; BC: breast cancer; BCSG: Breast Cancer Support Group; BL: baseline; CBCT: Cognitively-Based Compassion Training; chemo: chemotherapy; CIS-FS: Checklist Individual Strength-Fatigue Severity subscale; CRF scale: Cancer Related Fatigue scale; dx: diagnosis; eMBCT: web-based MBCT; excl.: excluding; FACT-F: Functional Assessment in Cancer Therapy-Fatigue; FSI: Fatigue Symptom Inventory; FSI-SC: Fatigue Symptom Inventory-Severity Composite; FSS-P: Fatigue Severity Scale - Persian; interv.: intervention; MAPs: Mindful Awareness Practices; MBAT: Mindfulness-Based Art Therapy; MBCR: Mindfulness-Based Cancer Recovery; MBCT: Mindfulness-Based Cognitive Therapy; MBI: mindfulness-based intervention; MBSR: Mindfulness-Based Stress Reduction; MCT: Metacognitive Therapy; MDASI: MD Anderson Symptom Inventory; MFSI-SF: Multidimensional Fatigue Scale Inventory-Short Form; min.: minutes; mo.: month; NA: not applicable; NR: not reported; PE: Psycho-educational; PES: Psycho-Educational Support; POMS: Profile of Mood States; QLQ-C30: Quality of Life Questionnaire-Core 30; SET: Supportive Expressive Therapy; SF-12: Medical Outcome Study-Short Form 12-Item Health Survey; SF-36: Medical Outcomes Study-Short Form 36-Item General Health Survey; SMS: Stress Management Seminar; tx: treatment; UC: Usual Care.
Number analyzed in outcome sample; full randomized sample N differs slightly.
SMS outcomes not assessed; all participants offered re-randomization to MBCR or SET after 12-week waitlist period.
Primary outcome(s) of the respective study.
Some of the data were not reported in study publications but were provided by the authors upon request.
3.3. MBI Study Quality
The characteristics of MBIs are presented in Table 3. Protocols of approximately half of the included studies were registered (K = 12, 52.2%) [23, 25, 45, 46, 51, 53–56, 62–64]. Only 5 studies (21.7%) screened participants for inclusion based on clinically significant fatigue [22, 46, 51, 60, 64], and only 5 studies [25, 45, 51, 56, 64] used a rigorous fatigue measure recommended for clinical trials [65, 66]. Monti and colleagues were the only researchers who described the theoretical framework underlying their MBI [24]. Nine studies (39.1%) reported that the interventionists who delivered the MBI had earned certification as a mindfulness teacher. Fewer than half of the included studies (K = 11, 47.8%) described the frequency and amount of mindfulness home practice suggested between class sessions [25, 45, 48, 49, 51, 54, 61, 63, 64], and this ranged from 5–45 minutes per day, 3–7 days per week. Most of these studies (K = 9, 81.8%) reported participants’ home practice time [25, 45, 48, 49, 51, 54, 61, 63, 64]. A minority of studies (K = 7, 30.4%) used MBI fidelity monitoring, with only one study reporting fidelity outcomes [64]. The large majority of included reports (K = 21, 91.3%) did not report on adverse effects of MBIs; however, the 2 studies that did report noted no adverse effects [51, 62].
Table 3.
Characteristics of mindfulness-based interventions
| Author, year | Mindfulness-based intervention | Guided by theory? | Intervention dose (hours) | Retreat | Assigned home practice | Mindfulness interventionist | Fidelity monitoring | Refusal rate | Retention rate (post-intervention) | Retention rate (follow-up) |
|---|---|---|---|---|---|---|---|---|---|---|
| Blaes, 2016 | MBCR | No | 28 | 8-hour retreat | 45 min./day, 7 days/week | MBSR-certified faculty with extensive MBCR training from Dr. Linda Carlson | NR | NR | 82.93% | 85.37% |
| Bower, 2015 | MAPs | No | 12 | NA | 5–20 min./day, 7 days/week | NR | NR | 45.70% | 92.86% | 84.29% |
| Bruggeman-Everts, 2017 | Web-based eMBCT | No | 36 | NA | NR | Psychologists (provided guidance remotely) | NR | 26.39% | 83.23% | 84.43% |
| Carlson, 2016 | MBCR | No | 18 | 6-hour retreat | NR | NR | NR | 15.90% | 65.48% | 51.59% |
| Cheng, 2019 | Unspecified Mindfulness interv. | No | 24 | NA | NR | Rehabilitation counselor | NR | NR | 100.00% | 100.00% |
| Dodds, 2015 | CBCT | No | 16 + Booster 1 mo. post-interv. | NA | 30 min./day, 3 days/week | CBCT-certified clinically-trained PhD social work researcher | 50% of class videos reviewed by CBCT supervisor; fidelity outcomes NR | 24.38% | 100.00% | 78.57% |
| Hoffman, 2012 | Original MBSR | No | 22.5 | 6-hour retreat | 40–45 min./day, 6–7 days/week | MBSR-certified clinician/researcher | NR | 17.29% | 96.26% | 92.06% |
| Jang, 2016 | Adapted MBAT | No | 9 | NA | NR | Doctoral-level therapist with degree in expressive arts therapy and >10 year’s experience in mental health medicine | NR | 10.00% | 95.83% | NA |
| Johns, 2015 | Adapted MBSR | No | 14 | NA | 20 min./day, 6 days/week | MBSR instructor with 6 years of MBSR teaching experience | NR | 30.19% | 100.00% | 100.00% |
| Johns, 2016 | Adapted MBSR | No | 16 | NA | NR | MBSR-trained physician and a doctoral-level clinical health psychologist with 9 and 3 years of MBSR teaching experience, respectively | 25% of audio-recorded sessions randomly reviewed; fidelity ratings were 85.1% for MBSR and 95.8% for PES | 33.04% | 97.18% | 95.77% |
| Kenne Sarenmalm, 2017 | Adapted MBSR | No | 16 | NA | 20 min./day, 6 days/week | MBSR-certified instructor | NR | NR | 93.79% | NA |
| Lengacher, 2009 | Adapted MBSR(BC) | No | 12 | NA | 15–45 min./day, 6 days/week | MBSR-certified psychologist | Independent assessor monitoring session consistency, quality, and timing of activities; fidelity outcomes NR | 54.50% | 97.62% | NA |
| Lengacher, 2012 | Adapted MBSR(BC) | No | 12 | NA | NR | MBSR-trained licensed clinical psychologist | NR | 54.50% | 97.62% | NA |
| Lengacher, 2016 | Adapted MBSR(BC) | No | 12 | NA | 15–45 min./day, 7 days/week | MBSR-trained clinical psychologist | Adherence to protocol evaluated using structured observational method; fidelity outcomes NR | 80.45% | 92.86% | 92.86% |
| Liu, 2019 | Adapted MBSR | No | 20 | NA | NR | MBSR-qualified psychologist | NR | NR | 90.83% | 85.00% |
| Monti, 2006 | Original MBAT | Yes; Self-regulation theory; Chapman’s neurodeve-lopmental approach to art therapy | 20 | NA | 30 min./day, 6 days/week | MBSR-certified, Master’s-level registered art therapist | NR | NR | 83.78% | NA |
| Monti, 2013 | Original MBAT | No | 20 | NA | NR | MBSR-certified mental health therapists | Psychiatrist co-investigator randomly performed fidelity checks | 3.46% | 76.96% | NR |
| Rahmani, 2014 | Adapted MBSR | No | 16 | NA | NR | MBSR-certified master clinical psychologists | NR | NR | 100.00% | 100.00% |
| Rahmani, 2015 | Adapted MBSR | No | 16 | NA | NR | Master clinical psychologists | NR | NR | 100.00% | 100.00% |
| Speca, 2000 | Adapted MBSR | No | 10.5 | NA | NR | NR | NR | NR | 82.57% | NA |
| van der Lee, 2012 | MBCT | No | 26 + 2.5-hour booster 2 mos. post-interv. | 6-hour retreat | 45 min./day, 6 days/week | MBSR-trained therapists with 16 and 8 years of MBCT teaching experience, respectively | NR | 23.66% | 89.00% | 81.00% |
| Witek-Janusek, 2019 | Original MBSR | No | 26 | 6-hour retreat | NR | MBSR-certified licensed clinical psychologist | Principal investigator (a PhD nurse) reviewed audio-recorded sessions for fidelity until consistency established; research assistant attended each ACC session to monitor content consistency | 20.19% | 83.54% | 79.27% |
| Zernicke, 2014 | Online MBCR | No | 22 | 6-hour retreat | 45 min./day, 7 days/week | Masters-level licensed clinician specializing in behavioral medicine with 15 years of online MBSR teaching experience | Principal investigator monitored online classes for treatment fidelity throughout the trial according to adapted face-to-face MBCR manual | 33.89% | 91.94% | NA |
Note: ACC: Active Control Condition; BC: breast cancer; CBCT: Cognitively-Based Compassion Training; eMBCT: web-based MBCT; interv.: intervention; MAPs: Mindful Awareness Practices; MBAT: Mindfulness-Based Art Therapy; MBCR: Mindfulness-Based Cancer Recovery; MBCT: Mindfulness-Based Cognitive Therapy; MBSR: Mindfulness-Based Stress Reduction; min.: minutes; mo.: month; NA: not applicable; NR: not reported; PES: Psycho-Educational Support.
3.4. Effect Sizes Post-Intervention
3.4.1. Effect Sizes.
A total of 18 publications describing 17 RCTs with independent effect sizes were included in the meta-analysis of change in fatigue from pre- to post-intervention (see Figure 2 for forest plots). The pooled effect size for improving fatigue was large and significant in favor of MBIs (Hedges’s g = 0.60, 95% CI [0.36, 0.83]; see Table 4 for results). A total of 11 studies were included in the meta-analysis of change in vitality/vigor from pre- to post-intervention. The pooled effect size for improving vitality/vigor was moderate and significant in favor of MBIs (Hedges’s g = 0.39, 95% CI [0.25, 0.52]).
Figure 2.
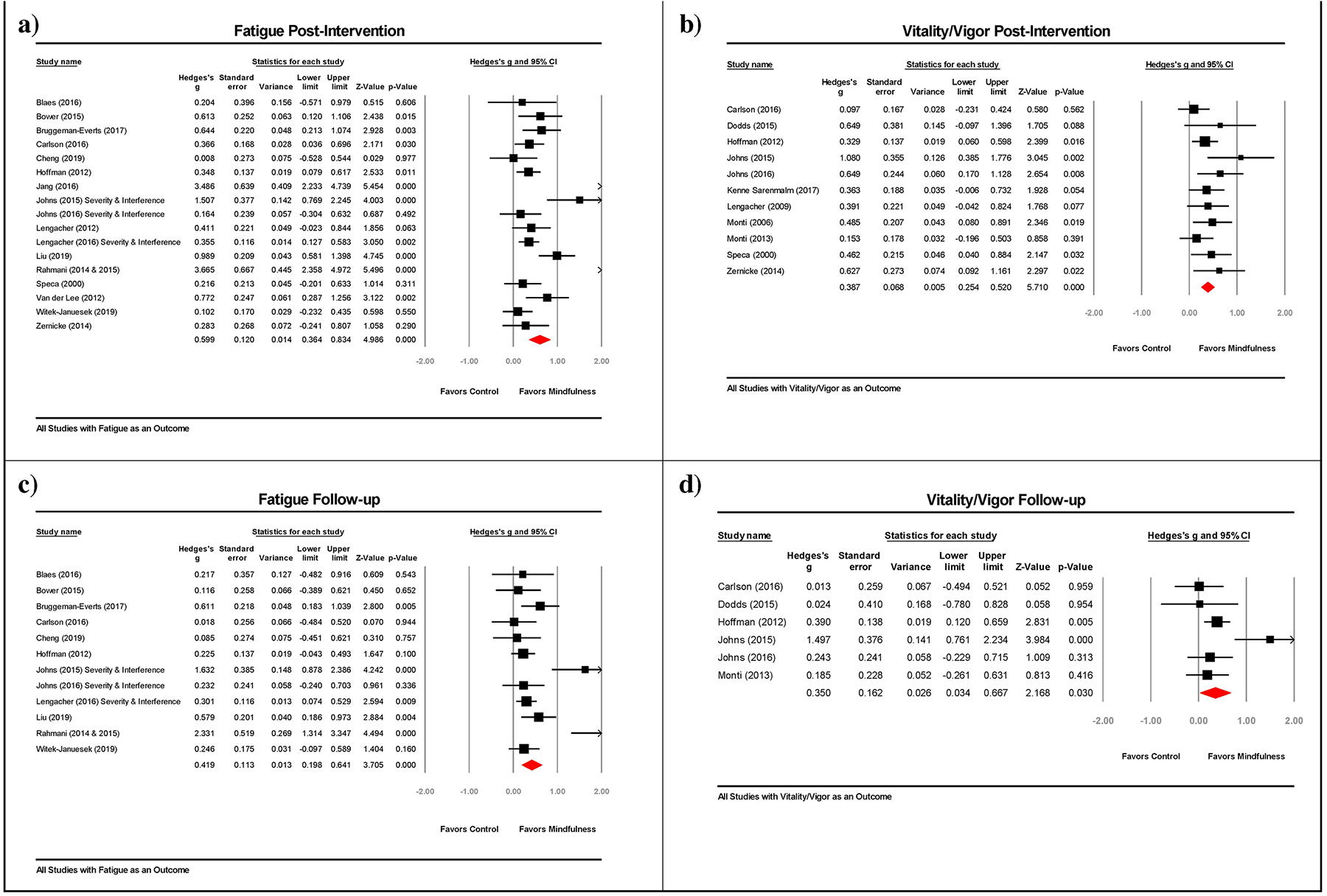
Forest plots for the intervention effect on (a) fatigue at post-intervention, (b) vitality/vigor at post-intervention, (c) fatigue at follow-up, and (d) vitality/vigor at follow-up.
Table 4.
Overall effect sizes for fatigue and vitality/vigor outcomes
| Sample Size | Effect Size Estimate | Heterogeneity | Publication Bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-intervention Outcome | K | n | Hedges’s g | 95% CI | p | Q | p | I2 | Fail-safe N | Criterion N | Egger’s test | p |
| Fatigue | 17 | 1,592 | 0.60 | [0.36, 0.83] | <0.001 | 74.99 | <0.001 | 78.66 | 436 | 95 | t(15) = 3.22 | 0.003 |
| Vitality/Vigor | 11 | 1,050 | 0.39 | [0.25, 0.52] | <0.001 | 11.46 | 0.323 | 12.74 | 112 | 65 | t(9) = 3.68 | 0.003 |
| First Follow-up Outcome | ||||||||||||
| Fatigue | 12 | 1,165 | 0.42 | [0.20, 0.64] | <0.001 | 33.60 | <0.001 | 67.27 | 120 | 70 | t(10) = 1.85 | 0.047 |
| Vitality/Vigor | 6 | 474 | 0.35 | [0.03, 0.67] | 0.030 | 12.42 | 0.029 | 59.75 | 14 | 40 | t(4) = 0.30 | 0.391 |
Note: K = number of studies. n = total number of participants included in the analysis. Q = Cochran’s (1954) Q-statistic of heterogeneity. I² = percentage of between-study variability. Fail-safe N = Rosenthal’s Fail-safe N. Criterion N = 5K+10.
3.4.2. Heterogeneity.
There was evidence of large heterogeneity between studies for changes in fatigue (Q = 74.99, p < 0.001, I2 = 78.66%). In contrast, no significant heterogeneity was detected for changes in vitality/vigor (Q = 11.46, p = 0.323, I2 = 12.74%).
3.4.3. Publication Bias.
For fatigue, a sensitivity analysis was conducted excluding two outlier effect sizes [50, 59, 60]. Omitting these studies resulted in a moderate effect in favor of MBIs (Hedges’s g = 0.43, 95% CI [0.28, 0.59]). For both fatigue and vitality/vigor, the fail-safe number exceeded the criterion for robustness of results. However, Egger’s regression tests showed evidence of asymmetry in the funnel plots for both outcomes (see Supplemental Figure 1 for funnel plots). Using the trim and fill method, one study was located in the funnel plot of effect sizes for fatigue. Adjusting the effect size for the missing study yielded a Hedges’s g of 0.53 (95% CI [0.27, 0.80]) for fatigue. Similarly, using the trim and fill method, four studies were located in the funnel plot of effect sizes for vitality/vigor. Adjusting the effect size for the missing studies yielded a Hedges’s g of 0.31 (95% CI [0.16, 0.46]) for vitality/vigor. Sensitivity analyses omitting studies with poor study quality [24, 45, 56, 58, 60] resulted in a Hedges’s g of 0.55 (95% CI [0.31, 0.78], K = 14) for fatigue and a Hedges’s g of 0.42 (95% CI [0.26, 0.58], K = 9) for vitality/vigor. Taken together, these results suggest that studies showing an advantage of MBI over controls for both fatigue and vitality/vigor were more likely to be published than studies favoring controls.
3.5. Effect Sizes at First Follow-up
3.5.1. Effect Sizes.
A total of 12 studies reported follow-up fatigue data (beyond post-intervention) for MBIs and controls, with an average follow-up period of 2.8 months beyond post-intervention (range: 0.9–6.0 months; see Figure 2 for forest plots). There was a moderate effect on fatigue in favor of MBIs at follow-up (Hedges’s g = 0.42, 95% CI [0.20, 0.64]; see Table 4). Six studies reported follow-up vitality/vigor data (beyond post-intervention) for MBIs and controls, with an average follow-up period of 3.5 months (range: 0.9–6.2 months). There was a moderate effect on vitality/vigor in favor of MBIs (Hedges’s g = 0.35, 95% CI [0.03, 0.67]).
3.5.2. Heterogeneity.
There was evidence of moderate heterogeneity between studies for changes in fatigue (Q = 33.60, p < 0.001, I2 = 67.27%) and vitality/vigor at follow-up (Q = 12.42, p = 0.029, I2 = 59.75%).
3.5.3. Publication Bias.
For fatigue, the fail-safe number exceeded the criterion for robustness of results. Even though Egger’s regression tests showed evidence of asymmetry in the funnel plot suggesting potential publication bias, the trim and fill method did not suggest any missing studies for fatigue at follow-up (see Supplemental Figure 1 for funnel plots). In contrast, the fail-safe number was below the criterion for robustness of results for vitality/vigor (see Table 4). Egger’s regression test, however, showed no evidence of asymmetry in the funnel plot. Moreover, the trim and fill method did not suggest any missing studies for vitality/vigor at follow-up. Sensitivity analyses omitting studies of poor quality [24, 45, 56, 58, 60] resulted in a Hedges’s g of 0.36 (95% CI [0.14, 0.58], K = 9) for fatigue and a Hedges’s g of 0.40 (95% CI [0.00, 0.80], K = 5) for vitality/vigor. Together, these results suggest the possibility of a publication bias for studies favoring MBI over control for both fatigue and vitality/vigor at follow-up.
3.6. Exploring Potential Moderators
Table 5 presents results of the meta-regression analyses. These results should be interpreted with caution as we had a limited number of studies in the meta-regression analyses. Thus, statistical power may have limited our ability to detect significant differences between subgroups [67].
Table 5.
Meta-regression analyses examining moderators of mindfulness intervention effects on fatigue and vitality/vigor outcomes
| Sample Size | Moderator Effect Size Estimate | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Moderator | Outcome | K | n | B | 95% CI | p | Q(w) | df | p |
| Gender | Fatigue Post-intervention | 17 | 1,592 | 0.01 | [−0.02, 0.05] | 0.472 | 72.44 | 15 | <0.001 |
| Vitality/Vigor Post-intervention | 11 | 1,050 | −0.01 | [−0.03, 0.00] | 0.107 | 8.87 | 9 | 0.450 | |
| Fatigue Follow-up | 12 | 1,165 | −0.01 | [−0.02, 0.00] | 0.077 | 30.48 | 10 | 0.001 | |
| Vitality/Vigor Follow-up | 6 | 474 | −0.04 | [−0.14, 0.06] | 0.418 | 11.78 | 4 | 0.019 | |
| Age | Fatigue Post-intervention | 17 | 1,592 | −0.08 | [−0.16, −0.01] | 0.035 | 63.66 | 15 | <0.001 |
| Vitality/Vigor Post-intervention | 11 | 1,050 | 0.01 | [−0.04, 0.06] | 0.607 | 11.22 | 9 | 0.261 | |
| Fatigue Follow-up | 12 | 1,165 | −0.03 | [−0.09, 0.03] | 0.341 | 32.35 | 10 | <0.001 | |
| Vitality/Vigor Follow-up | 6 | 474 | 0.03 | [−0.13, 0.18] | 0.727 | 12.40 | 4 | 0.015 | |
| Type of Mindfulness Intervention (MBSR vs. other) | Fatigue Post-intervention | 17 | 1,592 | −0.04 | [−0.85, 0.77] | 0.927 | 74.38 | 15 | <0.001 |
| Vitality/Vigor Post-intervention | 11 | 1,050 | −0.15 | [−0.39, 0.10] | 0.242 | 10.09 | 9 | 0.343 | |
| Fatigue Follow-up | 12 | 1,165 | −0.39 | [−0.96, 0.18] | 0.180 | 32.63 | 10 | <0.001 | |
| Vitality/Vigor Follow-up | 6 | 474 | −0.50 | [−1.15, 0.16] | 0.137 | 8.98 | 4 | 0.062 | |
| Type of Control Condition (Active vs. Non-active) | Fatigue Post-intervention | 17 | 1,592 | −0.63 | [−1.41, 0.16] | 0.116 | 69.46 | 15 | <0.001 |
| Vitality/Vigor Post-intervention | 11 | 1,050 | −0.22 | [−0.48, 0.04] | 0.096 | 8.69 | 9 | 0.466 | |
| Fatigue Follow-up | 12 | 1,165 | −0.36 | [−0.93, 0.20] | 0.207 | 32.62 | 10 | <0.001 | |
| Vitality/Vigor Follow-up | 6 | 474 | −0.44 | [−1.10, 0.22] | 0.194 | 9.45 | 4 | 0.051 | |
| Intervention Dose | Fatigue Post-intervention | 17 | 1,592 | −0.04 | [−0.09, 0.02] | 0.193 | 74.70 | 15 | <0.001 |
| Vitality/Vigor Post-intervention | 11 | 1,050 | −0.02 | [−0.05, 0.02] | 0.374 | 10.61 | 9 | 0.304 | |
| Fatigue Follow-up | 12 | 1,165 | −0.01 | [−0.05, 0.03] | 0.624 | 33.57 | 10 | <0.001 | |
| Vitality/Vigor Follow-up | 6 | 474 | −0.06 | [−0.20, 0.08] | 0.383 | 12.07 | 4 | 0.017 | |
| Drop-out Rate | Fatigue Post-intervention | 17 | 1,592 | −0.03 | [−0.07, 0.01] | 0.169 | 73.56 | 15 | <0.001 |
| Vitality/Vigor Post-intervention | 11 | 1,050 | −0.01 | [−0.02, −0.00] | 0.025 | 6.46 | 9 | 0.693 | |
| Fatigue Follow-up | 12 | 1,165 | −0.02 | [−0.04, 0.00] | 0.110 | 31.00 | 10 | 0.001 | |
| Vitality/Vigor Follow-up | 6 | 474 | −0.01 | [−0.03, −0.00] | 0.039 | 8.16 | 4 | 0.086 | |
| Refusal Rate | Fatigue Post-intervention | 12 | 1,290 | −0.01 | [−0.03, 0.01] | 0.320 | 40.30 | 10 | <0.001 |
| Vitality/Vigor Post-intervention | 8 | 751 | 0.01 | [−0.00, 0.02] | 0.075 | 7.76 | 6 | 0.256 | |
| Fatigue Follow-up | 8 | 1,045 | −0.00 | [−0.01, 0.01] | 0.978 | 16.19 | 6 | 0.013 | |
| Vitality/Vigor Follow-up | 6 | 474 | 0.02 | [−0.02, 0.06] | 0.353 | 11.01 | 4 | 0.026 | |
| Follow-up Time | Fatigue Follow-up | 12 | 1,165 | −0.09 | [−0.25, 0.08] | 0.312 | 33.34 | 10 | <0.001 |
| Vitality/Vigor Follow-up | 6 | 474 | −0.08 | [−0.21, 0.05] | 0.205 | 9.53 | 4 | 0.049 | |
Note: K = number of records included the analysis. n = total number of participants included in the analysis. Q(w) = the variance within group means. Age = the average age of the sample. Gender = percent female. Type of Mindfulness Intervention = MBSR vs other types of mindfulness interventions (reference = MBSR). Type of Control Condition = active vs. non-active control condition (reference = non-active control condition). Intervention Dose = total intervention hours. Drop-out Rate = drop-out rate at post-intervention/follow-up. Refusal Rate = total refusal rate. Follow-up Time = time between post-intervention and follow-up assessment in months.
3.6.1. Gender.
Gender composition of the sample (i.e., percent female) did not significantly moderate the intervention effects on fatigue or vitality/vigor at post-intervention or follow-up.
3.6.2. Age.
Age was a significant moderator of the intervention effect on fatigue at post-intervention. We found that for every 1-year increase in age, the intervention effect in favor of MBIs was weakened by 0.08 (b = −0.08, 95% CI [−0.16, −0.01]). However, age did not significantly moderate the intervention effects on fatigue at follow-up or on vitality/vigor at post-intervention or follow-up.
3.6.3. Type of MBI.
Type of MBI (i.e., MBSR vs. non-MBSR) did not significantly moderate the intervention effects on fatigue or vitality/vigor at post-intervention or follow-up.
3.6.4. Type of Control Condition.
Type of control condition (i.e., active vs. waitlist/usual care control) did not significantly moderate the intervention effects on fatigue or vitality/vigor at post-intervention or follow-up.
3.6.5. Intervention Dose.
Intervention dose did not significantly moderate the intervention effects on fatigue or vitality/vigor at post-intervention or follow-up.
3.6.6. Drop-out Rate.
Drop-out rates at post-intervention and follow-up did not significantly moderate the intervention effects on fatigue at post-intervention or follow-up. However, drop-out rates at post-intervention and follow-up significantly moderated intervention effects on vitality/vigor at post-intervention and follow-up; for every 1% increase in the drop-out rate, the intervention effect in favor of MBI was weakened by 0.01 at post-intervention and follow-up (b = −0.01, 95% CI [−0.02, −0.004], b = −0.01, 95% CI [−0.03, −0.001], respectively).
3.6.7. Follow-up Time.
Time between post-intervention and follow-up assessment in months did not significantly moderate the intervention effects on fatigue or vitality/vigor at follow-up.
3.7. Risk of Bias Assessment
As shown in Figure 3, most included RCTs were categorized as being at low risk of bias with respect to randomization sequence generation and incomplete outcome data (K = 14, 60.9% and K = 13, 56.5%, respectively). Allocation concealment and blinding of outcome assessment often went unreported (K = 15, 65.2% and K = 19, 82.6%, respectively) in the included studies. Risk of bias was high for blinding of participants/personnel in all included studies (K = 23, 100%). Likewise, the majority of studies (K = 13, 56.5%) were evaluated as being at high risk of bias in the domain of selective reporting.
Figure 3.
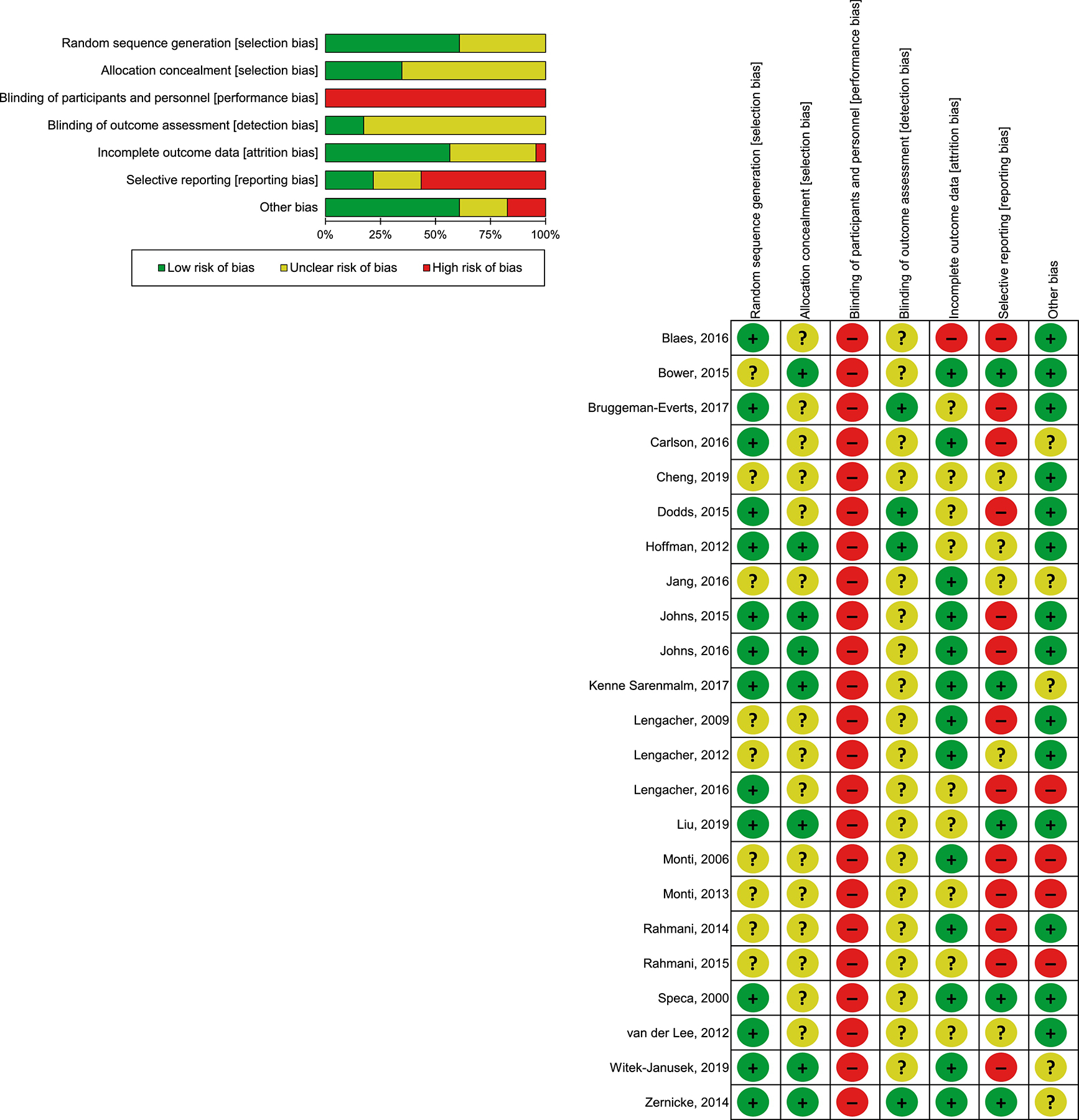
Risk of bias summary of authors’ judgments for each included study and risk of bias graph of authors’ judgments as percentages across all included studies.
3.8. Quality of Evidence
Based on GRADE [38], the quality of evidence for fatigue and vitality/vigor at post-intervention and follow-up were all rated as low, indicating a low level of confidence in the effect estimates. The level of evidence was downgraded to low primarily due to concerns regarding risk of bias and publication bias (see Supplementary Tables 1 and 2 for GRADE ratings).
4. Conclusions
The present meta-analysis provides an updated synthesis of the current evidence for MBIs targeting fatigue and vitality/vigor in cancer survivors and identifies research gaps that warrant attention. Results suggest that MBIs show promise in reducing fatigue and improving vitality/vigor at post-intervention and an average of 3–4 months later. Effects were large for fatigue at post-intervention and moderate at first follow-up. Effects were moderate for vitality/vigor at both time points. Given notable overall risk of bias, publication bias, and heterogeneity of findings, the quality of the evidence supporting these findings was low. Although current recommendations of MBIs for fatigue are mixed, overall, results support tentative recommendations for MBIs in clinical practice guidelines for fatigue in cancer survivors.
Our meta-analysis provides the most comprehensive and rigorous examination of the evidence regarding MBI for fatigue in cancer survivors. To date, nine meta-analyses of MBIs in cancer have included fatigue among the outcomes [31–36, 68–70]. Of these, only four were prospectively registered to support transparency and replication [31, 32, 68, 69]. Only two assessed the quality of the evidence (e.g., GRADE), and these meta-analyses only included five [32] and six [69] RCTs, respectively. The majority of the meta-analyses were breast cancer specific [31–35]. Finally, the meta-analyses included 2 to 14 studies compared to 21 independent studies in the present meta-analysis (17 assessing fatigue and 11 assessing vitality/vigor). The effect sizes of MBI for fatigue in published meta-analyses ranged from 0.28–0.89 at post-intervention (mean SMD = 0.57; median and mode = 0.50). Only three meta-analyses reported an effect beyond post-intervention [32, 34, 69]. Among these three, the effect sizes ranged from 0.19 to 0.40. Our effect sizes for fatigue were 0.60 at post-intervention and 0.42 at first follow-up, comparable to those found in other meta-analyses.
Across studies included in our review, participant characteristics were generally homogeneous (e.g., 92.8% female, 77.9% breast cancer, mean ages 42.8–58.0 years). This homogeneity may have contributed to largely null findings when examining possible moderators of MBI’s effects. Further, some studies failed to report relevant demographic, medical, and procedural variables, resulting in reduced statistical power for some of the moderation analyses. Inconsistent moderation effects of age and post-intervention drop-out rate were found. Age moderated the fatigue effect at post-intervention, with younger participants reporting a stronger effect from MBIs. However, this effect did not occur at first follow-up and was not found for vitality/vigor. MBI’s effect on vitality/vigor became weaker at both time points as the drop-out rate increased; however, this effect was not found for fatigue. It is possible that more symptomatic participants were more likely to drop out, thereby reducing intervention effects on vitality/vigor. The lack of a significant moderation effect for control condition type (i.e., active vs. waitlist/usual care control) on fatigue and vigor/vitality also warrants discussion. Several of the active controls offered minimal guidance on fatigue management to create an expectation of therapeutic benefit. This may explain why the magnitude of change in fatigue and vigor/vitality was similar across both control condition types.
Included RCTs had a number of strengths and weaknesses in their rigor. Strengths included low refusal rates (mean=30%) and high retention (mean=90% post-intervention and 86% at first follow-up). In addition, the sample size was ≥100 in nine trials. In terms of weaknesses, only five of the included studies [22, 46, 51, 60, 64] screened for fatigue as an inclusion criterion as recommended in existing guidelines [28]. Additionally, most studies (14/21, 66.7%) used a waitlist/usual care control group. Relative to active comparison groups, no-treatment controls often produce the largest effect size in favor of the experimental treatment [71]. The measurement of fatigue is another limitation, with some studies measuring fatigue severity, interference, or a combination of both. Notably, few of the included studies (5/21, 23.8%) [25, 45, 51, 56, 64] used a fatigue measure recommended for clinical trials based on strong psychometric properties and user-friendliness [65]. Most studies also did not report on the adherence of participants to MBI, adverse effects, or outcomes of fidelity monitoring that may allow further inferences to be made about study quality. Several aspects of trial procedures were not reported in sufficient detail to adequately assess risk of bias. The common lack of outcome assessor blinding is particularly problematic, given the self-reported nature of the outcomes. Finally, trials have mainly focused on breast cancer survivors, despite the ubiquity of fatigue across cancer types [72, 73].
Our findings point to a number of important directions for future research. Future MBI trials for fatigue should target those most in need by establishing a threshold of fatigue severity for eligibility as assessed by a rigorous measure of fatigue, perhaps coupled with an objective measure (e.g., fatigability). Additionally, MBI warrants testing in more diverse samples with respect to gender, race/ethnicity, age, and cancer type. Comparing MBIs to other behavioral interventions for fatigue (e.g., physical activity, cognitive-behavioral therapy) is another important future direction. Further studies are also needed to determine the long-term effectiveness of MBIs for fatigue in cancer. Only one of the included studies reported a follow-up assessment beyond 6 months post-intervention [23]. As our meta-analysis showed a decline in effect size during short-term follow-up, booster sessions warrant examination in future research. Testing theory-driven mechanisms that may explain the effect of MBIs on fatigue in cancer survivors is another priority for future research. Beyond clinical trials, future meta-analytic reviews of MBIs for fatigue in cancer could be strengthened by including both published and unpublished literature in this area. Our meta-analytic review may be affected by the file drawer problem (i.e., publication bias) because we only considered published articles for inclusion to ensure that studies had been peer-reviewed.
In conclusion, although MBIs show promise in the treatment of fatigue and improving vitality/vigor, further methodologically robust trials are required to definitively examine their long-term efficacy. Use of rigorous screening and outcome measures of fatigue across studies will strengthen the evidence base. Furthermore, comparisons of MBIs to other tested fatigue interventions are needed. Such research will ultimately reduce suffering and disability in the large population of cancer survivors.
Supplementary Material
Highlights.
Fatigue is a prevalent and disruptive symptom for many cancer survivors.
Mindfulness-based interventions (MBI) have been tested to reduce fatigue in cancer.
Meta-analyses tested the efficacy of MBIs in cancer for fatigue and vigor/vitality.
MBIs significantly improved fatigue and vigor/vitality compared to controls.
Evidence grade was low due to risk of bias, heterogeneity, and publication bias.
Vitae.
Shelley A. Johns is a board certified clinical health psychologist and associate professor at Indiana University School of Medicine where she conducts randomized controlled trials to improve symptom management in adults with cancer. Dr. Johns’ research primarily focuses on testing behavioral interventions to address fatigue, fear of recurrence, depression, and anxiety in cancer survivors.
Will L. Tarver is an assistant professor at The Ohio State University College of Medicine. Dr. Tarver’s research focuses on health information technologies and their potential to improve care coordination for people with cancer, including those who are part of underserved populations.
Ekin Secinti is a PhD candidate in the clinical psychology doctoral program at Indiana University – Purdue University Indianapolis (IUPUI). Her program of research broadly focuses on how medical patients and their family caregivers cope with symptoms. Her recent work has focused on social processes and acceptance-based coping in relation to mental health in adults with cancer and their family caregivers.
Catherine E. Mosher is an associate professor in the Department of Psychology at IUPUI, where she directs a behavioral oncology laboratory. Dr. Mosher’s primary research interests are in developing, evaluating, and disseminating psychosocial interventions for cancer patients and their family caregivers, as well as identifying predictors of health outcomes in these populations.
Patrick V. Stutz has been a clinical research specialist at Indiana University School of Medicine for three years assisting with research in behavioral oncology. A certified clinical research professional (CCRP) through the Society of Clinical Research Associates (SoCRA), he assists the first author in grant writing, study startup, recruitment, data collection, data cleaning, and manuscript preparation.
Jennifer L. Carnahan is a geriatrician and assistant professor at Indiana University School of Medicine. Dr. Carnahan’s research centers on improving care for adults with dementia in nursing homes, with emphasis on care transitions from skilled nursing facilities to home.
Tasneem L. Talib is a clinical research coordinator at Indiana University School of Nursing, where she assists with grant and manuscript writing. Dr. Talib is also an assistant teaching professor in the department of Educational Psychology at Ball State University, where she teaches courses in Human Growth and Development and Adolescent Psychology.
Mackenzie L. Shanahan is a PhD candidate in the clinical psychology doctoral program at IUPUI. She conducts research in the area of expectations, including hope and optimism, and clinical pain.
Micah T. Faidley is an alumnus of IUPUI Department of Psychology. During his summer internship at Regenstrief Institute, Inc., he worked closely with the first author to extract data from eligible studies in the present review.
Kelley M. Kidwell is an associate professor in the Department of Biostatistics at the University of Michigan School of Public Health. Dr. Kidwell’s research centers on the design and analysis of clinical trials, especially sequential multiple assignment randomized trials. She collaborates in many clinical areas and populations, including cancer, mental health, and rare diseases.
Kevin L. Rand is an associate professor in the IUPUI Department of Psychology, where he teaches and conducts research on personality, stress, and coping. Clinically, Dr. Rand applies existential psychotherapy principles in conjunction with cognitive-behavioral techniques to improve health outcomes for adults and adolescents with conditions like cancer and chronic pain.
Acknowledgments
The authors would like to thank Regenstrief Institute center coordinator Donna Burgett and research assistants Jacob Pell, Tayler Gowan, Jennifer Alwine, Maddie Wright, and Ana Danner for their contributions to this project. We also thank the authors of the included publications, especially the corresponding authors who graciously retrieved and provided additional data upon request.
Role of the funding source
This research was supported by the National Cancer Institute (K05CA175048: Johns, Mosher) and the Walther Cancer Foundation (0175: Johns). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or Walther Cancer Foundation. The funding organizations did not have a role in study design; collection, analysis or interpretation of data; writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare there are no conflicts of interest.
References
- [1].Jemal A, Torre L, Soerjomataram I, Bray F (Eds). The Cancer Atlas. Third Ed. Atlanta, GA: American Cancer Society, 2019. Retrieved on 6/8/20 from: www.cancer.org/canceratlas. [Google Scholar]
- [2].Harrington CB, Hansen JA, Moskowitz M, et al. It’s not over when it’s over: long-term symptoms in cancer survivors--a systematic review. Int J Psychiatry Med. 2010;40(2):163–81. [DOI] [PubMed] [Google Scholar]
- [3].American Cancer Society. (2019). Cancer Treatment & Survivorship Facts & Figures 2019–2021. Atlanta: American Cancer Society. [Google Scholar]
- [4].Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. European journal of cancer. 2002;38(1):27–43. [DOI] [PubMed] [Google Scholar]
- [5].Cleeland CS, Zhao F, Chang VT, et al. The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013;119(24):4333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014. February 1;120(3):425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. The oncologist. 2000;5(5):353–60. [DOI] [PubMed] [Google Scholar]
- [8].Kuhnt S, Ernst J, Singer S, et al. Fatigue in cancer survivors--prevalence and correlates. Onkologie. 2009. June;32(6):312–7. [DOI] [PubMed] [Google Scholar]
- [9].Minton O, Alexander S, Stone PC. Identification of factors associated with cancer related fatigue syndrome in disease-free breast cancer patients after completing primary treatment. Breast cancer research and treatment. 2012. November;136(2):513–20. [DOI] [PubMed] [Google Scholar]
- [10].Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care. 2012;2(3):231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Askren MK, Jung M, Berman MG, et al. Neuromarkers of fatigue and cognitive complaints following chemotherapy for breast cancer: a prospective fMRI investigation. Breast cancer research and treatment. 2014;147(2):445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ferreira KA, Kimura M, Teixeira MJ, et al. Impact of cancer-related symptom synergisms on health-related quality of life and performance status. J Pain Symptom Manage. 2008. June;35(6):604–16. [DOI] [PubMed] [Google Scholar]
- [13].Goldstein D, Bennett BK, Webber K, et al. Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol. 2012. May 20;30(15):1805–12. [DOI] [PubMed] [Google Scholar]
- [14].Blaney J, Lowe-Strong A, Rankin J, et al. The cancer rehabilitation journey: barriers to and facilitators of exercise among patients with cancer-related fatigue. Phys Ther. 2010. August;90(8):1135–47. [DOI] [PubMed] [Google Scholar]
- [15].Piper BF, Borneman T, Sun VC, et al. Cancer-related fatigue: role of oncology nurses in translating National Comprehensive Cancer Network assessment guidelines into practice. Clin J Oncol Nurs. 2008. October;12(5 Suppl):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Spelten ER, Verbeek JH, Uitterhoeve AL, et al. Cancer, fatigue and the return of patients to work-a prospective cohort study. Eur J Cancer. 2003. July;39(11):1562–7. [DOI] [PubMed] [Google Scholar]
- [17].Yabroff KR, Lawrence WF, Clauser S, et al. Burden of illness in cancer survivors: findings from a population-based national sample. Journal of the National Cancer Institute. 2004. September 1;96(17):1322–30. [DOI] [PubMed] [Google Scholar]
- [18].Buckwalter AE, Karnell LH, Smith RB, et al. Patient-reported factors associated with discontinuing employment following head and neck cancer treatment. Archives of otolaryngology--head & neck surgery. 2007. May;133(5):464–70. [DOI] [PubMed] [Google Scholar]
- [19].Kabat-Zinn J, University of Massachusetts Medical Center/Worcester. Stress Reduction Clinic. Full catastrophe living : using the wisdom of your body and mind to face stress, pain, and illness. New York, N.Y.: Delacorte Press; 1990. [Google Scholar]
- [20].Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatric clinics. 2017;40(4):739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shapero BG, Greenberg J, Pedrelli P, et al. Mindfulness-based interventions in psychiatry. Focus. 2018;16(1):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].van der Lee ML, Garssen B. Mindfulness-based cognitive therapy reduces chronic cancer-related fatigue: a treatment study. Psycho-oncology. 2012. March;21(3):264–72. [DOI] [PubMed] [Google Scholar]
- [23].Carlson LE, Tamagawa R, Stephen J, et al. Randomized-controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy among distressed breast cancer survivors (MINDSET): long-term follow-up results. Psycho-oncology. 2016. July;25(7):750–9. [DOI] [PubMed] [Google Scholar]
- [24].Monti DA, Peterson C, Kunkel EJ, et al. A randomized, controlled trial of mindfulness-based art therapy (MBAT) for women with cancer. Psycho-oncology. 2006. May;15(5):363–73. [DOI] [PubMed] [Google Scholar]
- [25].Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015. April 15;121(8):1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carlson LE. Mindfulness-based interventions for physical conditions: a narrative review evaluating levels of evidence. ISRN Psychiatry. 2012;2012:651583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue (version 1.2020). www.nccn.org [DOI] [PMC free article] [PubMed]
- [28].Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014. June 10;32(17):1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Howell D, Keshavarz H, Broadfield L, et al. A Pan Canadian Practice Guideline for Screening, Assessment, and Management of Cancer-Related Fatigue in Adults Version 2–2015. Toronto: Canadian Partnership Against Cancer (Cancer Journey Advisory Group) and the Canadian Association of Psychosocial Oncology. April 2015. [Google Scholar]
- [30].Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs. 2014;18 Suppl:38–58. [DOI] [PubMed] [Google Scholar]
- [31].Zhang Q, Zhao H, Zheng Y. Effectiveness of mindfulness-based stress reduction (MBSR) on symptom variables and health-related quality of life in breast cancer patients-a systematic review and meta-analysis. Support Care Cancer. 2019. March;27(3):771–81. [DOI] [PubMed] [Google Scholar]
- [32].Schell LK, Monsef I, Woeckel A, et al. Mindfulness-based stress reduction for women diagnosed with breast cancer. Cochrane Database of Systematic Reviews. 2019(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Castanhel FD, Liberali R. Mindfulness-Based Stress Reduction on breast cancer symptoms: systematic review and meta-analysis. Einstein (Sao Paulo). 2018. December 6;16(4):eRW4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Haller H, Winkler MM, Klose P, et al. Mindfulness-based interventions for women with breast cancer: an updated systematic review and meta-analysis. Acta Oncol. 2017. December;56(12):1665–76. [DOI] [PubMed] [Google Scholar]
- [35].Zhang J, Xu R, Wang B, et al. Effects of mindfulness-based therapy for patients with breast cancer: A systematic review and meta-analysis. Complement Ther Med. 2016. June;26:1–10. [DOI] [PubMed] [Google Scholar]
- [36].Xie C, Dong B, Wang L, et al. Mindfulness-based stress reduction can alleviate cancer-related fatigue: A meta-analysis. Journal of Psychosomatic Research. 2020;130:109916. [DOI] [PubMed] [Google Scholar]
- [37].Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychological Bulletin. 2008;134(5):700–41. [DOI] [PubMed] [Google Scholar]
- [38].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS medicine. 2009. July 21;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Higgins JPT, Green S. (2011). Cochrane handbook for systematic reviews of interventions Version 5.1.0: The Cochrane Collaboration; [Internet]. 2011. Available from: http://handbook.cochrane.org.
- [41].Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment. Confirmation from meta-analysis. Am Psychol. 1993. December;48(12):1181–209. [DOI] [PubMed] [Google Scholar]
- [42].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986. September;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- [43].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994. December;50(4):1088–101. [PubMed] [Google Scholar]
- [44].Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000. June;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- [45].Blaes AH, Fenner D, Bachanova V, et al. Mindfulness-based cancer recovery in survivors recovering from chemotherapy and radiation. J Commun Support Oncol. 2016;14(8):351–8. [Google Scholar]
- [46].Bruggeman-Everts FZ, Wolvers MDJ, van de Schoot R, et al. Effectiveness of Two Web-Based Interventions for Chronic Cancer-Related Fatigue Compared to an Active Control Condition: Results of the “Fitter na kanker” Randomized Controlled Trial. J Med Internet Res. 2017. October 19;19(10):e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cheng TC, Lee YH, Mar CL, et al. The Health Promoting Mindfulness or Qigong Educational Programs for Beneficial Lifestyle Changes of Cancer Survivors. J Cancer Educ. 2019. April 17. [DOI] [PubMed] [Google Scholar]
- [48].Dodds SE, Pace TW, Bell ML, et al. Feasibility of Cognitively-Based Compassion Training (CBCT) for breast cancer survivors: a randomized, wait list controlled pilot study. Support Care Cancer. 2015. December;23(12):3599–608. [DOI] [PubMed] [Google Scholar]
- [49].Hoffman CJ, Ersser SJ, Hopkinson JB, et al. Effectiveness of mindfulness-based stress reduction in mood, breast-and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. Journal of Clinical Oncology. 2012;30(12):1335–42. [DOI] [PubMed] [Google Scholar]
- [50].Jang SH, Kang SY, Lee HJ, et al. Beneficial Effect of Mindfulness-Based Art Therapy in Patients with Breast Cancer-A Randomized Controlled Trial. Explore (NY). 2016. Sep-Oct;12(5):333–40. [DOI] [PubMed] [Google Scholar]
- [51].Johns SA, Brown LF, Beck-Coon K, et al. Randomized controlled pilot study of mindfulness-based stress reduction for persistently fatigued cancer survivors. Psycho-Oncology. 2015;24(8):885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Johns SA, Von Ah D, Brown LF, et al. Randomized controlled pilot trial of mindfulness-based stress reduction for breast and colorectal cancer survivors: effects on cancer-related cognitive impairment. J Cancer Surviv. 2016. June;10(3):437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kenne Sarenmalm E, Mårtensson LB, Andersson BA, et al. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer medicine. 2017;6(5):1108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psycho-oncology. 2009. December;18(12):1261–72. [DOI] [PubMed] [Google Scholar]
- [55].Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. Journal of behavioral medicine. 2012. February;35(1):86–94. [DOI] [PubMed] [Google Scholar]
- [56].Lengacher CA, Reich RR, Paterson CL, et al. Examination of Broad Symptom Improvement Resulting From Mindfulness-Based Stress Reduction in Breast Cancer Survivors: A Randomized Controlled Trial. J Clin Oncol. 2016. August 20;34(24):2827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Liu T, Zhang W, Xiao S, et al. Mindfulness-based stress reduction in patients with differentiated thyroid cancer receiving radioactive iodine therapy: a randomized controlled trial. Cancer Management and Research. 2019;11:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Monti DA, Kash KM, Kunkel EJ, et al. Psychosocial benefits of a novel mindfulness intervention versus standard support in distressed women with breast cancer. Psycho-Oncology. 2013;22(11):2565–75. [DOI] [PubMed] [Google Scholar]
- [59].Rahmani S, Talepasand S, Ghanbary-Motlagh A. Comparison of effectiveness of the metacognition treatment and the mindfulness-based stress reduction treatment on global and specific life quality of women with breast cancer. Iran J Cancer Prev. 2014. Fall;7(4):184–96. [PMC free article] [PubMed] [Google Scholar]
- [60].Rahmani S, Talepasand S. The effect of group mindfulness - based stress reduction program and conscious yoga on the fatigue severity and global and specific life quality in women with breast cancer. Med J Islam Repub Iran. 2015;29:175. [PMC free article] [PubMed] [Google Scholar]
- [61].Speca M, Carlson LE, Goodey E, et al. A randomized, wait-list controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosomatic medicine. 2000;62(5):613–22. [DOI] [PubMed] [Google Scholar]
- [62].Janusek LW, Tell D, Mathews HL. Mindfulness based stress reduction provides psychological benefit and restores immune function of women newly diagnosed with breast cancer: A randomized trial with active control. Brain, behavior, and immunity. 2019;80:358–73. [DOI] [PubMed] [Google Scholar]
- [63].Zernicke KA, Campbell TS, Speca M, et al. A randomized wait-list controlled trial of feasibility and efficacy of an online mindfulness–based cancer recovery program: the etherapy for cancer applying mindfulness trial. Psychosomatic medicine. 2014;76(4):257–67. [DOI] [PubMed] [Google Scholar]
- [64].Johns SA, Brown LF, Beck-Coon K, et al. Randomized controlled pilot trial of mindfulness-based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Supportive Care in Cancer. 2016;24(10):4085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Agasi-Idenburg C, Velthuis M, Wittink H. Quality criteria and user-friendliness in self-reported questionnaires on cancer-related fatigue: a review. Journal of clinical epidemiology. [Review]. 2010. July;63(7):705–11. [DOI] [PubMed] [Google Scholar]
- [66].Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage. [GuidelineResearch Support, Non-U.S. Gov’t Review]. 2010. June;39(6):1086–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002. June 15;21(11):1559–73. [DOI] [PubMed] [Google Scholar]
- [68].Xunlin NG, Lau Y, Klainin-Yobas P. The effectiveness of mindfulness-based interventions among cancer patients and survivors: a systematic review and meta-analysis. Support Care Cancer. 2020. April;28(4):1563–78. [DOI] [PubMed] [Google Scholar]
- [69].Cillessen L, Johannsen M, Speckens AE, et al. Mindfulness-based interventions for psychological and physical health outcomes in cancer patients and survivors: A systematic review and meta-analysis of randomized controlled trials. Psycho-oncology. 2019;28(12):2257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Duong N, Davis H, Robinson PD, et al. Mind and body practices for fatigue reduction in patients with cancer and hematopoietic stem cell transplant recipients: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017. December;120:210–6. [DOI] [PubMed] [Google Scholar]
- [71].Mohr DC, Spring B, Freedland KE, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom. 2009;78(5):275–84. [DOI] [PubMed] [Google Scholar]
- [72].Jones JM, Howell D, Olson KL, et al. Prevalence of cancer-related fatigue in a population-based sample of colorectal, breast, and prostate cancer survivors. American Society of Clinical Oncology; 2012. [Google Scholar]
- [73].Ness S, Kokal J, Fee-Schroeder K, et al. , editors. Concerns Across the Survivorship Trajectory: Results From a Survey of Cancer Survivors. Oncology Nursing Forum; 2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


