Abstract
Background
There is debate whether body mass index is a good predictor of health outcomes because different tissues, namely skeletal muscle mass (SMM) and fat mass (FM), may be differentially associated with risk. We investigated the association of appendicular SMM (aSMM) and FM with fatal and nonfatal cardiovascular disease (CVD) and all‐cause mortality. We compared their prognostic value to that of body mass index.
Methods and Results
We studied 356 590 UK Biobank participants aged 40 to 69 years with bioimpedance analysis data for whole‐body FM and predicted limb muscle mass (to calculate aSMM). Associations between aSMM and FM with CVD and all‐cause mortality were examined using multivariable Cox proportional hazards models. Over 3 749 501 person‐years of follow‐up, there were 27 784 CVD events and 15 844 all‐cause deaths. In men, aSMM was positively associated with CVD incidence (hazard ratio [HR] per 1 SD 1.07; 95% CI, 1.06–1.09) and there was a curvilinear association in women. There were stronger positive associations between FM and CVD with HRs per SD of 1.20 (95% CI, 1.19–1.22) and 1.25 (95% CI, 1.23–1.27) in men and women respectively. Within FM tertiles, the associations between aSMM and CVD risk largely persisted. There were J‐shaped associations between aSMM and FM with all‐cause mortality in both sexes. Body mass index was modestly better at discriminating CVD risk.
Conclusions
FM showed a strong positive association with CVD risk. The relationship of aSMM with CVD risk differed between sexes, and potential mechanisms need further investigation. Body fat and SMM bioimpedance measurements were not superior to body mass index in predicting population‐level CVD incidence or all‐cause mortality.
Keywords: all‐cause mortality, cardiovascular disease, cohort study, fat mass, skeletal muscle mass
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Epidemiology, Risk Factors
Nonstandard Abbreviations and Acronyms
- aSMM
appendicular skeletal muscle mass
- BIA
bioimpedance analysis
- FM
fat mass
- SMM
skeletal muscle mass
Clinical Perspective
What Is New?
Reports of the relationship between muscle mass and cardiovascular disease (CVD) are inconsistent and have rarely been considered in the context of adiposity; analysis of body composition measured by bioimpedance in this large cohort of UK adults showed that fat mass showed strong positive associations with CVD events.
Appendicular skeletal muscle mass had a curvilinear association with CVD events in women and a positive association in men; the associations of appendicular skeletal muscle mass and fat mass with all‐cause mortality followed a J‐shape in both men and women.
Measurements of body fat and skeletal muscle mass were not superior to body mass index in predicting CVD events or mortality.
What Are the Clinical Implications?
Body mass index has been criticized as an inaccurate measure of health risks, but at a population level, more specific measurements of body composition, namely appendicular skeletal muscle mass and fat mass, were generally not more predictive of CVD events or mortality.
Although body mass index may be the simplest measurement to assess health risk, which is important from a public health perspective, some of this risk may not be attributable solely to adiposity, particularly if the association observed with appendicular skeletal muscle mass in men is confirmed.
Further research is needed to better understand the biological mechanisms and impact of different body tissue compartment on health outcomes.
The increasing prevalence of obesity is a significant public health concern because it is a known risk factor for several noncommunicable diseases, 1 , 2 , 3 , 4 , 5 estimated to account for 56 million deaths globally in 2017. 6 Evidence from prospective cohort studies 7 , 8 , 9 , 10 and meta‐analyses of such studies 2 , 11 , 12 has repeatedly shown a J‐ or U‐shaped relationship between body mass index (BMI), cardiovascular disease (CVD), and all‐cause mortality, even after efforts to account for confounding and reverse causality. 12 , 13 A potential explanation for this is that BMI does not distinguish between fat mass (FM) and skeletal muscle mass (SMM), 12 , 13 yet their contribution to the pathogenesis of disease is likely to be different.
A systematic literature review of the associations between body composition and CVD or mortality (Data S1, Tables S1 and S2) showed that the majority of studies found null or inverse associations with SMM, although a minority of studies reported positive or curvilinear associations. More studies have investigated the relationship of FM with these outcomes, with the majority of them reporting positive associations. Very few studies have investigated the combined impact of both types of tissues, yet weight change is associated with changes in both these body tissue compartments.
Dual energy X‐ray absorptiometry (DEXA) or magnetic resonance imaging techniques are considered to be reference methods for the measurement of body composition because of their precision and reliability. 14 However, these are often not feasible for large studies given they are expensive and not easily portable. 15 Bioimpedance analysis (BIA) is a noninvasive and practical method to assess FM and SMM in clinical practice and at scale in population‐based studies. 16 , 17 , 18 The UK Biobank uses a bioimpedance analyzer previously validated against DEXA in a mixed population of children and adults, and body composition estimates were found to be more accurate than those obtained from previous BIA estimates. 19 A recent validation study comparing BIA to DEXA in a subsample of the UK Biobank participants showed BIA to be a valid method for the assessment of appendicular skeletal muscle mass (aSMM) and FM. 20
In this study we aimed to use BIA‐derived aSMM and FM measurements to look at their associations with incident CVD and all‐cause mortality in the UK Biobank population. Furthermore, to investigate the prognostic value of these BIA‐derived measurements in comparison to more traditional measures such as BMI, grip strength, and waist circumference.
Methods
Data Availability Statement
Researchers can apply to use the UK Biobank resource and access the data used. No additional data are available.
Study Design and Participants
The UK Biobank recruited 502 664 participants aged 40 to 69 years between 2006 and 2010 (response rate 5.5%) via mailed invitations to the general public living within 25 miles of one of the 22 assessment centers in England, Scotland, and Wales. 21 , 22 At the baseline assessment clinic, participants completed a touch‐screen questionnaire and computer‐assisted interview, had physical measurements taken, and biological samples collected. 23 , 24 UK Biobank received ethical approval from the North West Multi‐centre Research Ethics Committee (REC reference: 11/NW/03820). All participants gave written informed consent before enrolment in the study, which was conducted in accordance with the principles of the Declaration of Helsinki.
Participants were excluded from analyses if they had prior CVD (defined later) or diseases that may affect body composition including fractures in the past year, respiratory diseases, musculoskeletal conditions, and some infectious diseases (n=116 679; Figure S1). 25 Participants were also excluded if they had missing data for the exposures (n=6751) or were not of a White race (n=22 644), because BIA estimates are derived from algorithms in White populations, which represent ≈95% of the UK Biobank sample. 26 , 27 , 28
Measurement of Exposures
Measures of body weight and body composition (muscle mass and fat mass) were derived from BIA in bare‐footed participants wearing light clothing using a Tanita BC418MA single frequency segmental body‐composition analyzer (Tanita, Tokyo, Japan) at the baseline assessment center visit. The aSMM (kg) was calculated as the sum of the predicted muscle mass from the 4 limbs. Whole body FM (kg) was also obtained from BIA. Standing height was measured using a Seca 202 scale (Seca, Hamburg, Germany). BMI was calculated by dividing weight (kg) by the squared height in meters.
Waist circumference (cm) was measured at the umbilicus using a tape measure. The mean grip strength (kg) of the left and right hands was taken once using a Jamar J00105 hydraulic hand dynamometer.
Ascertainment of Outcomes
Participants were followed via linkage to National Health Service hospital in‐patient data from hospital episode statistics in England, the Scottish Morbidity Records, and the Patient Episode Database for Wales. Patients were identified if they died of any cause or developed incident CVD, defined using International Statistical Classification of Diseases, Tenth Revision (ICD‐10) categories: coronary heart disease (I21–I24, 125.6, I42, I43, K49, K50, K75, K40–K46), congestive heart failure or cardiomyopathy (I50, I50.1, 150.9, I11.0, I13.0, I13.2), and total stroke (I60–I64). 29 Follow‐up was available until June 30, 2020, October 31, 2016, and February 29, 2016 for England, Scotland, and Wales respectively; and until July 31, 2020 for all‐cause mortality for all regions.
Statistical Analysis
Association of Skeletal Muscle Mass and Fat Mass With CVD and Mortality
First, age‐adjusted partial correlation coefficients between aSMM, FM, and height were calculated to examine the relationships between the body composition measurements and overall body size. As aSMM is highly correlated with FM and height, 30 aSMM was regressed on height and FM and the residuals from this model were divided into sex‐specific quintiles for the main analysis.
Multivariable Cox regression analyses with age as the underlying timescale were used to estimate hazard ratios (HRs) and 95% CIs for the associations of sex‐specific fifths of aSMM and fifths of FM as well as per 1 SD with incident CVD and all‐cause mortality. All analyses were sequentially adjusted for height and height2 (continuous), Townsend index of deprivation (quintiles), level of education (none, vocational qualifications, any degree, higher degree, other), smoking (never, previous, current), alcohol intake (none, <1 unit/week, 1–14 units/week, 14+ units/week), physical activity derived from metabolic equivalent of task scores (low, moderate, high), 31 and dietary factors (oily fish intake, saturated fat intake, fruit and vegetable intake [none, low, medium, high intake]), prior medical history (diabetes mellitus, cancer history >5 years ago, and menopausal status in women [binary for each]). We created a category for missing values for each of these covariates. Additionally, FM and aSMM were mutually adjusted for each other to assess the independent effects of each type of tissue. See Table S3 for the details on the derivation of these covariates.
The HRs and 95% CIs were computed using group‐specific variances 32 ; these reflect the uncertainty in the estimate of risk in each group (including the reference group), thereby allowing comparisons between any 2 quintiles independently of the reference group. Restricted cubic splines with 5 knots were also computed to visually explore nonlinear associations for continuous exposures, and departures from linearity were tested via the likelihood ratio statistic test used to evaluate if models with linear or categorical exposures were a better fit. 33 Five knots were specified to be consistent with the quintile analysis but also to provide enough flexibility to the model while also not being too many knots so that the model is oversensitive to the smallest fluctuations. 34 , 35 To correct for the measurement error that can arise from using a single baseline measurement to estimate long‐term exposure status (ie, regression dilution bias), 36 mean values of BIA measurements at resurvey (2012–2013) from 15 694 participants were used in 2 ways. First, the HR (95% CI) in the baseline‐defined groups of aSMM and FM were plotted against the mean resurvey values in those baseline‐defined groups (termed the “usual” value). Second, where there was evidence of a log‐linear relationship, regression dilution ratios were calculated using the MacMahon‐Peto method. 37 The log HRs (and theirSEs) per 1 SD of baseline aSMM and FM were then divided by the relevant regression dilution ratio to obtain HRs (and associated 95% CI) per 1 SD of usual aSMM and FM. 37
Sensitivity analyses were conducted to assess potential residual confounding or reverse causality so additional exclusions were made for events that occurred during the first 2 years of follow‐up to reduce the impact of reverse causality, for outliers, or for participants with BMI over 35 kg/m2 for whom BIA measurements may be less accurate. Additional adjustments were made for BMI (instead of FM in the SMM model and instead of SMM in the FM model) as well as for hypertension (diagnosed by doctor, taking medication, or blood pressure measurement), and blood cholesterol (defined as taking medication, plus levels of non‐high‐density lipoprotein cholesterol and triglycerides) to investigate if these are potential mediators of the associations. Finally, Cox regression models were conducted with BMI as the exposure as a “positive control” to confirm that the specified models would produce the same association that has been documented previously. 9
Associations of aSMM Within Tertiles of FM
To better assess the independent association of aSMM with the risk of disease, irrespective of its strong correlation with FM, we examined the sex‐specific associations of aSMM within subgroups of FM tertiles (subsequently referred to as "body composition groups," because we looked at low/moderate/high groups [tertiles] of aSMM within groups of FM). Multivariable Cox models adjusting for all covariates listed previously were used to assess the associations with CVD and all‐cause mortality using "moderate" aSMM as the reference category within each tertile of FM.
Prognostic Comparison of aSMM, FM, and Body Composition Groups With BMI, Waist Circumference, and Grip Strength
The relative importance of the various measures in prediction of CVD or mortality was assessed in several ways. First, where a linear association was present, the HRs associated with 1 usual SD change were compared for each measure. In order to assess the discriminatory ability of each measure with CVD and mortality Harrell's C‐statistic from the area under the receiver operating curve was computed. 38 Third, the Wald test χ2 statistic was used to compare a model with just confounders to a model with confounders plus the exposure of interest to explore how much of the variation in risk is explained by each exposure of interest, given confounders. 39
All analyses were conducted using Stata 15.0 for analyses and R 3.5.2 for graphs. Analyses used 2‐sided P values (α=0.05) without any correction for multiple testing.
Results
Study Participants
After exclusions, the final sample included 356 590 adults who were followed for a median of 10.5 years during which there were 27 784 CVD events and 15 844 deaths due to all causes. The mean age at recruitment was 56 (SD 8) years. Men had a higher aSMM (median 27.2 kg in men; and 18.3 kg in women), although the difference between the sexes was smaller for FM (median 21.8 kg in men, 26.3 kg in women; Table 1, Tables S4 and S5). There were strong partial correlations between aSMM and FM (men r=0.71, women r=0.78) and aSMM and height (men r=0.52, women r=0.44) but not between FM and height (r=men 0.14, women 0.15; Table S6).
Table 1.
Baseline Characteristics of the Study Population According to Appendicular Skeletal Muscle Mass and Fat Mass Quintiles in 356 590 UK Biobank Participants
| Men Appendicular Skeletal Muscle Mass Quintiles, Range (kg) | Men Fat Mass Quintiles, Range (kg) | Total | |||||
|---|---|---|---|---|---|---|---|
|
Q1 13.5 to ≤24.0 |
Q3 26.1 to ≤27.6 |
Q5 30.1 to ≤54.5 |
Q1 5.0 to ≤15.7 |
Q3 19.5 to ≤22.9 |
Q5 27.6 to ≤98.6 |
||
| Age at recruitment, y, mean (SD) | 61.0 (6.4) | 56.0 (7.8) | 51.7 (7.8) | 54.9 (8.3) | 56.5 (8.1) | 56.9 (7.8) | 56.2 (8.1) |
| aSMM, kg, mean (SD) | 24.6 (2.9) | 26.9 (2.9) | 30.5 (3.6) | 24.6 (2.7) | 26.7 (2.7) | 31.0 (3.6) | 27.2 (3.7) |
| FM, kg, mean (SD) | 22.4 (7.1) | 21.4 (7.4) | 22.2 (9.2) | 12.4 (2.4) | 21.0 (1.0) | 33.6 (6.2) | 21.8 (7.8) |
| BMI, kg/m2, mean (SD) | 26.0 (3.5) | 27.4 (3.6) | 29.7 (4.4) | 23.3 (1.8) | 27.1 (1.6) | 33.1 (3.5) | 27.6 (4.0) |
| Higher education, n (%) | 12 740 (39.7%) | 13 140 (40.9%) | 12 645 (39.3%) | 13 909 (43.1%) | 12 987 (40.6%) | 11 735 (36.7%) | 64 706 (40.2%) |
| Current smokers, n (%) | 3714 (11.6%) | 3573 (11.1%) | 3724 (11.6%) | 4366 (13.5%) | 3368 (10.5%) | 3286 (10.3%) | 18 005 (11.2%) |
| Low fruit and vegetable intake, n (%) | 14 779 (46.0%) | 13 977 (43.5%) | 13 367 (41.6%) | 13 624 (42.2%) | 14 191 (44.3%) | 14 349 (44.8%) | 70 241 (43.7%) |
| High saturated fat intake, n (%) | 11 706 (36.4%) | 11 647 (36.2%) | 11 778 (36.6%) | 10 457 (32.4%) | 11 699 (36.6%) | 12 933 (40.4%) | 58 554 (36.4%) |
| Low oily fish intake, n (%) | 10 919 (34.0%) | 11 342 (35.3%) | 11 988 (37.3%) | 11 197 (34.7%) | 11 518 (36.0%) | 11 661 (36.4%) | 57 348 (35.7%) |
| Heavy drinkers, n (%) | 19 948 (62.1%) | 19 841 (61.7%) | 18 666 (58.1%) | 17 562 (54.4%) | 20 297 (63.4%) | 19 695 (61.5%) | 97 948 (60.9%) |
| Low physical activity, n (%) | 7247 (22.6%) | 6300 (19.6%) | 5518 (17.2%) | 4580 (14.2%) | 6030 (18.8%) | 8757 (27.4%) | 31 636 (19.7%) |
| Hypertension, n (%) | 20 146 (62.7%) | 18 102 (56.3%) | 17 370 (54.0%) | 12 683 (39.3%) | 18 523 (57.9%) | 23 544 (73.5%) | 91 858 (57.1%) |
| Type 2 diabetes mellitus, n (%) | 1300 (4.1%) | 1156 (3.6%) | 1408 (4.4%) | 388 (1.2%) | 906 (2.8%) | 2883 (9.0%) | 6305 (3.9%) |
| Cancer history (>5 y ago), n (%) | 1176 (3.7%) | 816 (2.5%) | 652 (2.0%) | 780 (2.4%) | 856 (2.7%) | 889 (2.8%) | 4233 (2.6%) |
| Cholesterol medication, n (%) | 6204 (19.5%) | 4646 (14.6%) | 3842 (12.0%) | 2175 (6.8%) | 4664 (14.7%) | 7440 (23.4%) | 24 008 (15.0%) |
| Women Appendicular Skeletal Muscle MassQuintiles, Range (kg) | Women Fat Mass Quintiles, Range (kg) | Total | |||||
|---|---|---|---|---|---|---|---|
|
Q1 10.3 to ≤16.5 |
Q3 17.6 to ≤18.6 |
Q5 20.0 to ≤39.2 |
Q1 5.0 to ≤18.5 |
Q3 22.9 to ≤27.1 |
Q5 33.4 to ≤109.8 |
||
| Age at recruitment, y, mean (SD) | 58.9 (7.0) | 55.8 (7.9) | 53.2 (8.1) | 54.1 (8.1) | 56.6 (7.9) | 56.4 (7.8) | 55.9 (8.0) |
| aSMM, kg, mean (SD) | 17.0 (1.7) | 18.0 (1.8) | 20.3 (2.5) | 16.6 (1.5) | 17.9 (1.4) | 21.1 (2.3) | 18.3 (2.3) |
| FM, kg, mean (SD) | 27.7 (8.5) | 25.5 (8.8) | 27.0 (11.9) | 15.2 (2.6) | 24.9 (1.3) | 41.1 (7.4) | 26.3 (9.6) |
| BMI, mean (SD) | 25.9 (4.2) | 26.3 (4.4) | 28.5 (6.0) | 21.6 (1.7) | 25.9 (1.7) | 33.9 (4.3) | 26.7 (4.9) |
| Higher education, n (%) | 14 330 (36.6%) | 16 033 (40.9%) | 17 056 (43.6%) | 18 112 (45.5%) | 15 834 (39.8%) | 14 209 (36.4%) | 79 178 (40.4%) |
| Current smokers, n (%) | 3039 (7.8%) | 3231 (8.2%) | 3377 (8.6%) | 3792 (9.5%) | 3151 (7.9%) | 2898 (7.4%) | 16 069 (8.2%) |
| Low fruit and vegetable intake, n (%) | 12 608 (32.2%) | 11 874 (30.3%) | 10 910 (27.9%) | 12 125 (30.5%) | 11 756 (29.6%) | 12 234 (31.3%) | 59 246 (30.3%) |
| High saturated fat intake, n (%) | 11 699 (29.9%) | 11 252 (28.7%) | 11 212 (28.6%) | 10 415 (26.2%) | 11 615 (29.2%) | 12 439 (31.9%) | 57 055 (29.1%) |
| Low oily fish intake, n (%) | 12 200 (31.2%) | 12 619 (32.2%) | 13 137 (33.6%) | 12 971 (32.6%) | 12 669 (31.8%) | 13 082 (33.5%) | 63 668 (32.5%) |
| Heavy drinkers, n (%) | 12 955 (33.1%) | 13 025 (33.2%) | 12 318 (31.5%) | 13 562 (34.1%) | 13 525 (34.0%) | 10 970 (28.1%) | 64 272 (32.8%) |
| Low physical activity, n (%) | 10 117 (25.8%) | 8431 (21.5%) | 7837 (20.0%) | 6398 (16.1%) | 8288 (20.8%) | 12 160 (31.1%) | 43 345 (22.1%) |
| Hypertension, n (%) | 19 371 (49.5%) | 16 440 (42.0%) | 16 330 (41.7%) | 11 345 (28.5%) | 17 091 (43.0%) | 23 766 (60.9%) | 85 687 (43.8%) |
| Type 2 diabetes mellitus, n (%) | 635 (1.6%) | 669 (1.7%) | 1250 (3.2%) | 239 (0.6%) | 524 (1.3%) | 2046 (5.3%) | 4032 (2.1%) |
| Cancer history (>5 y ago), n (%) | 2540 (6.5%) | 2010 (5.1%) | 1845 (4.7%) | 1912 (4.8%) | 2278 (5.7%) | 2170 (5.6%) | 10 626 (5.4%) |
| Cholesterol medication, n (%) | 4294 (11.0%) | 3163 (8.1%) | 3080 (7.9%) | 1536 (3.9%) | 3349 (8.5%) | 5721 (14.8%) | 17 157 (8.8%) |
| Postmenopausal, n (%) | 28 352 (72.4%) | 23 352 (59.6%) | 17 594 (44.9%) | 21 266 (53.5%) | 24 686 (62.1%) | 22 973 (58.8%) | 116 065 (59.3%) |
χ2 test for trend was performed with P<0.05 for all characteristics across the aSMM and FM quintiles. All characteristics were determined at the baseline assessment clinic through touch‐screen questionnaires, interviews, and/or physical measurements. Higher education: college or university degree or professional qualifications. Low physical activity: <600 metabolic equivalent (MET)‐minutes per week. 31 Heavy alcohol drinker: >14 units of alcohol a week). 40 Hypertension: systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, was diagnosed by a doctor or were taking medication to lower blood pressure. Diabetes mellitus and cholesterol: taking medication for these conditions or diagnosed by a doctor. Cancer history: diagnosed with cancer >5 years ago (those with more recent cancer had been excluded). Low fruit and vegetable intake: the lowest consumption tertile (<21 portions per week). High saturated fat: the highest saturated fat tertile, based on portions per week of beef, lamb, pork, and whether they consumed animal‐ or plant‐based spreads. Low oily fish: lowest consumption tertile (<1 portion per week). aSMM indicates appendicular skeletal muscle mass; BMI, body mass index; and FM, fat mass.
Participants in the highest quintiles of aSMM and FM had similar diets (ie, high saturated fat intake, low oily fish intake, but similar fruit and vegetable intakes), and a higher percentage of participants had low physical activity and a higher prevalence of type 2 diabetes mellitus and hypertension. A higher percentage of participants in the highest FM quintile were taking medication for cholesterol but there were no differences across aSMM quintiles (Table 1, Tables S4 and S5).
Associations of Skeletal Muscle Mass and Fat Mass With Health Outcomes
There was a potential curvilinear association between aSMM and CVD in women (likelihood ratio test statistic of nonlinearity [df=4], P<0.001) with the nadir approximately at the median; this curvilinear shape was even more pronounced in the cubic spline analysis (Figure 1, Figure S2, Table S7). There was a positive linear association in men with an HR per 1 usual SD of 1.07 (95% CI, 1.06–1.09). FM showed much stronger positive log‐linear associations with the risk of CVD with HRs per 1 usual SD of 1.20 (95% CI, 1.19–1.22) in men and 1.25 (95% CI, 1.23–1.27) in women (Figure 1, Figure S2, Table S7). These associations were similar across CVD subtypes (nonfatal, fatal, coronary heart disease, congestive heart failure, and stroke) for both aSMM and FM (Figure S3). The associations of aSMM and FM with all‐cause mortality generally followed a J‐shape in both men and women, although the association with FM was more clear (Figure 2, Figure S2, Table S8).
Figure 1. Adjusted hazard ratios (HRs) of incident cardiovascular disease associated with appendicular skeletal muscle mass (aSMM) and fat mass (FM).
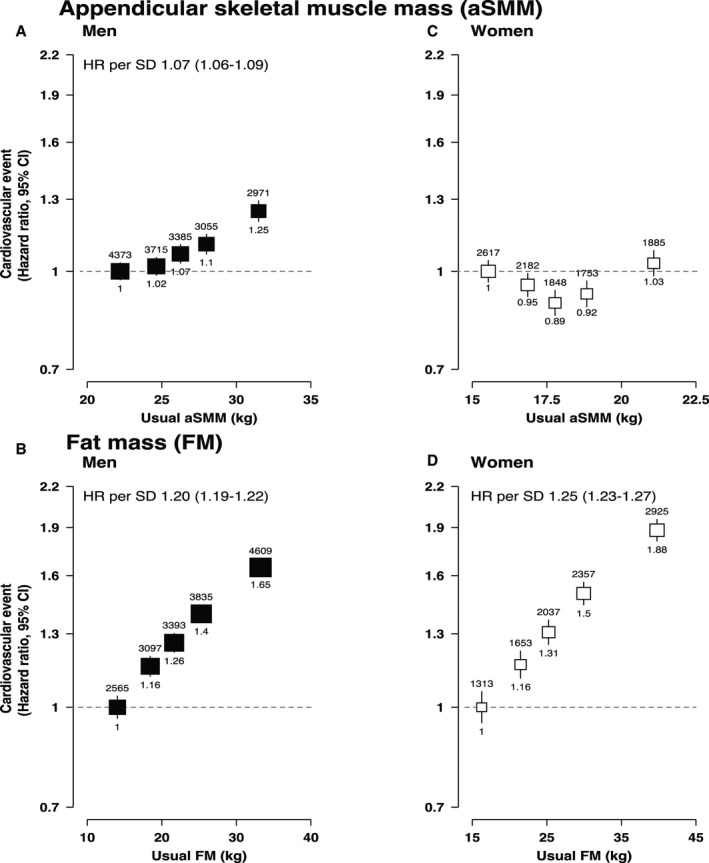
A, HRs of incident CVD associated with aSMM in men, 1 SD=5.66 kg. B, HRs of incident CVD associated with aSMM in women, 1 SD=1.45 kg. C, HRs of incident CVD associated with FM in men, 1 SD=6.75 kg. D, HRs of incident CVD associated with FM in women, 1 SD=8.28 kg. For all panels, likelihood ratio tests were used to estimate nonlinearity (aSMM in men, P=0.04; aSMM in women, P<0.001; FM in men, P=0.09; FM in women, P=0.09). Adjusted HRs and CIs obtained using the floated absolute risk method of Cox proportional hazards regression, number of cases shown above each estimate and HRs shown below. Adjusted for age (underlying timescale variable), height (as a continuous variable in FM and included by regression out of variation due to height for aSMM), Townsend index of deprivation, education, smoking status, alcohol intake, physical activity, oily fish intake, fruit and vegetable intake, saturated fat intake, diabetes mellitus, cancer history, menopause (women), and mutually adjusted for FM (in the aSMM models) and aSMM (in the FM models). HRs are plotted at the mean of the resurvey values for the baseline‐defined quintiles (“usual” values) to correct for measurement error. HRs per 1 SD given where there was no evidence of departure from linearity. CVD indicates cardiovascular disease.
Figure 2. Adjusted hazard ratios (HRs) of all‐cause mortality associated with appendicular skeletal muscle mass (ASMM) and fat mass (FM).
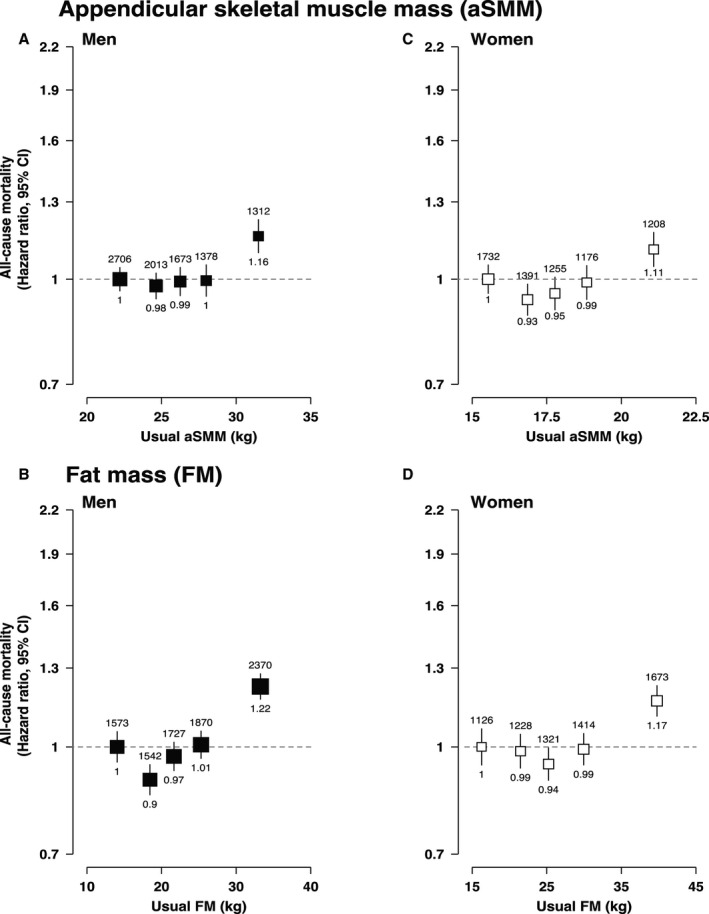
A, HRs of all‐cause mortality associated with aSMM in men, 1 SD=5.66 kg. B, HRs of all‐cause mortality associated with aSMM in women, 1 SD=1.45 kg. C, HRs of all‐cause mortality associated with FM in men, 1 SD=6.75 kg. D, HRs of all‐cause mortality associated with FM in women, 1 SD=8.28 kg. For all panels, likelihood ratio tests were used to estimate nonlinearity P values (aSMM in men, P=0.002; aSMM in women, P=0.008; FM in men, P<0.001; FM in women, P<0.001). Adjusted HRs and CIs obtained using the floated absolute risk method of Cox proportional hazards regression, number of cases shown above each estimate and HRs shown below. Adjusted for age (underlying timescale variable), height (as a continuous variable in FM and included by regression out of variation due to height for aSMM), Townsend index of deprivation, education, smoking status, alcohol intake, physical activity, oily fish intake, fruit and vegetable intake, saturated fat intake, diabetes mellitus, cancer history, menopause (women), and mutually adjusted for FM (in the aSMM models) and aSMM (in the FM models). HRs are plotted at the mean of the resurvey values for the baseline‐defined quintiles (“usual” values) to correct for measurement error.
These findings remained robust after sensitivity analyses (Tables S9 and S10). Exclusion of the first 2 years of follow‐up to reduce the risk of reverse causality, outliers, or participants with BMI >35 kg/m2 did not affect the associations.
Adjustment for hypertension and high blood cholesterol as mediators did not affect associations of aSMM with CVD or all‐cause mortality. These mediators explained ≈30% to 40% of the χ2 statistic in models of FM and CVD, although the association between FM and all‐cause mortality was affected less (Tables S11 and S12). However, adjustment for BMI (instead of SMM or FM in their respective models) removed the positive association between aSMM and CVD in men but did not change the association observed in women. Associations between FM and CVD and all‐cause mortality were largely attenuated after adjustment for BMI, with large % of the χ2 statistic explained in both men and women.
Analyses of body composition groups (aSMM tertiles within each FM tertile) showed positive linear associations between aSMM and incident CVD risk in men in all FM tertiles, whereas women still had curvilinear associations with incident CVD except those in the highest FM tertile (Figure 3). The associations of aSMM with all‐cause mortality within FM tertiles were broadly similar between men and women and to those observed in the main analysis, except for women in the middle tertile of FM, which showed a curvilinear association between aSMM and all‐cause mortality.
Figure 3. Adjusted hazard ratios (HRs) of cardiovascular disease and all‐cause mortality associated with appendicular skeletal muscle mass (aSMM) when participants are stratified into fat mass (FM) tertiles.
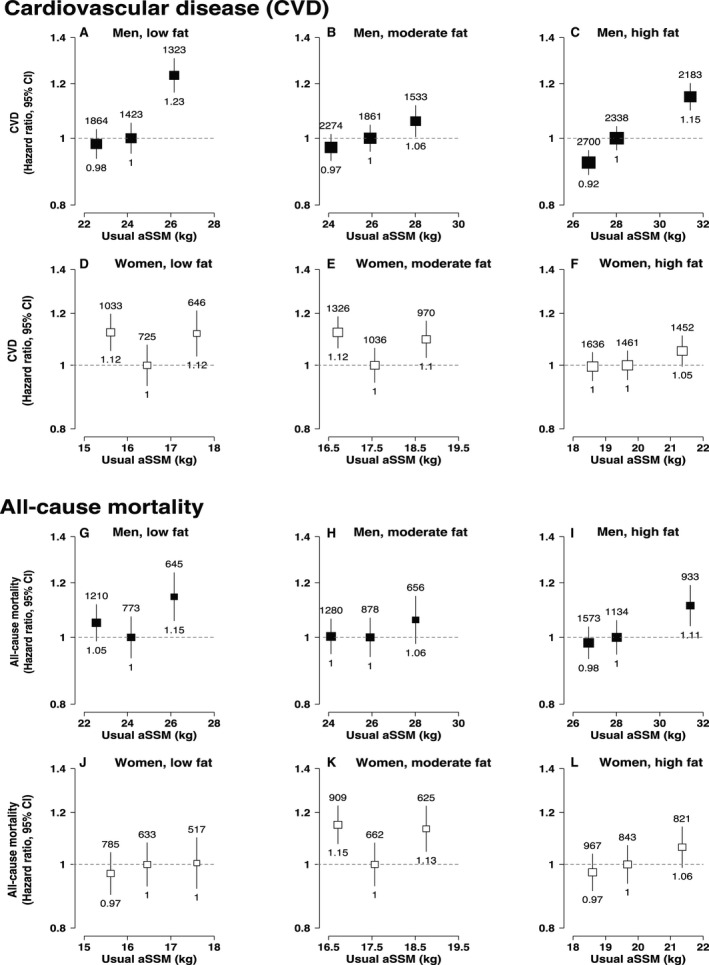
A, HRs of cardiovascular disease (CVD) associated with aSMM in low fat men. B, HRs of CVD associated with aSMM in moderate fat men. C, HRs of CVD associated with aSMM in high fat men. D, HRs of CVD associated with aSMM in low fat women. E, HRs of CVD associated with aSMM in moderate fat women. F, HRs of CVD associated with aSMM in high fat women. G, HRs of all‐cause mortality associated with aSMM in low fat men. H, HRs of all‐cause mortality associated with aSMM in moderate fat men. I, HRs of all‐cause mortality associated with aSMM in high fat men. J, HRs of all‐cause mortality associated with aSMM in low fat women. K, HRs of all‐cause mortality associated with aSMM in moderate fat women. L, HRs of all‐cause mortality associated with aSMM in high fat women. For all panels, adjusted hazard ratios (HR) and CIs obtained using Cox proportional hazards regression, number of cases shown above each estimate and HRs shown below. Adjusted for age (underlying timescale variable), height (included by regression out of variation due to height), Townsend index of deprivation, education, smoking status, alcohol intake, physical activity, oily fish intake, fruit and vegetable intake, saturated fat intake, diabetes mellitus, cancer history, menopause (women), and mutually adjusted for FM (in the aSMM models) and aSMM (in the FM models). HRs are plotted at the mean of the resurvey values for the baseline‐defined quintiles (“usual” values) to correct for measurement error.
Comparing the Prognostic Value of Body Composition Measures
For CVD risk, waist circumference and FM showed the strongest associations whereas aSMM and grip strength showed the weakest associations in both men and women. For all‐cause mortality, waist circumference and grip strength showed the strongest associations, whereas aSMM remained the weakest (Figure 4, Table 2). However, the discriminatory performance (Harrell's C‐statistics) for total CVD was slightly higher for BMI compared with all the other metrics in men (C=0.63; 95% CI, 0.63–0.64) and for BMI and waist circumference in women (C=0.45; 95% CI, 0.45–0.45). Similarly, for all‐cause mortality, the discriminatory performance was highest for BMI (C=0.62; 95% CI, 0.62–0.63) and waist circumference (C=0.61; 95% CI, 0.61–0.62) in men, whereas in women it was FM (C=0.63; 95% CI, 0.62–0.63) and aSMM (C=0.63; 95% CI, 0.62–0.63). The χ2 statistic was marginally higher in the BMI and waist circumference model in men and in the BMI and the combined aSMM/FM groups in women.
Figure 4. Independent effects of body mass index (BMI), fat mass (FM), waist circumference, appendicular skeletal muscle mass (aSMM), and grip strength on cardiovascular disease (CVD) subtypes and all‐cause mortality. Adjusted hazard ratios (HRs) per SD change.
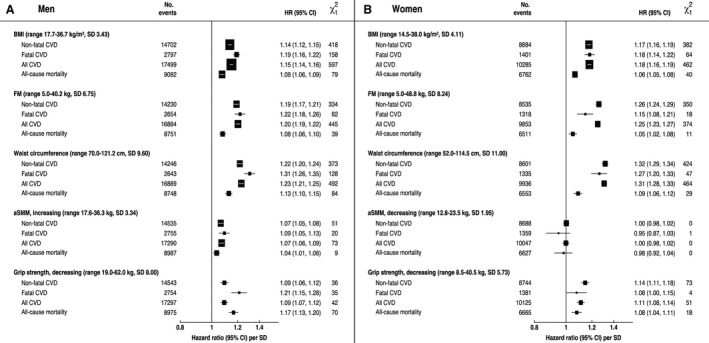
A, The independent effects of BMI, FM, waist circumference, aSMM and grip strength on CVD subtypes and all‐cause mortality in men. B, The independent effects of BMI, FM, waist circumference, aSMM, and grip strength on CVD subtypes and all‐cause mortality in women. Range excludes outliers. Adjusted hazard ratios (HR) and CIs obtained using Cox proportional hazard regression. Adjusted for age (underlying timescale variable), height (as a continuous variable in all models except aSMM where it was included by regression out of variation due to height for aSMM), Townsend index of deprivation, education, smoking status, alcohol intake, physical activity, oily fish intake, fruit and vegetable intake, saturated fat intake, diabetes mellitus, cancer history, menopause (women), and mutually adjusted for FM (in the aSMM models) and aSMM (in the FM models). HRs are corrected for regression dilution bias by the MacMahon‐Peto method.
Table 2.
The Discrimination Ability of Each Body Composition Measure for the Prediction of Cardiovascular Events and All‐Cause Mortality, as Calculated by Harrell's C‐Statistic From the Area Under the Receiver Operating Curve
| Men |
|
Women |
|
|||||
|---|---|---|---|---|---|---|---|---|
| HR Per SD (95% CI) | Harrell's C‐Statistic (95% CI) | HR Per SD (95% CI) | Harrell's C‐Statistic (95% CI) | |||||
| Cardiovascular disease | ||||||||
| BMI | 1.15 (1.14–1.16) | 0.63 (0.63–0.64) | 597 | 1.18 (1.16–1.19) | 0.45 (0.45–0.45) | 462 | ||
| FM | 1.20 (1.19–1.22) | 0.56 (0.55–0.56) | 445 | 1.25 (1.23–1.27) | 0.59 (0.58–0.59) | 374 | ||
| Waist circumference | 1.23 (1.21–1.25) | 0.61 (0.61–0.62) | 492 | 1.31 (1.28–1.33) | 0.45 (0.44–0.45) | 464 | ||
| aSMM | 1.07 (1.06–1.09) | 0.54 (0.54–0.54) | 73 | 1.00 (0.98–1.02) | 0.57 (0.56–0.57) | 0 | ||
| Decreasing grip strength | 1.09 (1.07–1.12) | 0.55 (0.54–0.55) | 42 | 1.11 (1.08–1.14) | 0.44 (0.43–0.44) | 51 | ||
| Body composition groups | … | 0.56 (0.55–0.56) | 504 | … | 0.45 (0.44–0.45) | 400 | ||
| All‐cause mortality | ||||||||
| BMI | 1.08 (1.06–1.09) | 0.62 (0.62–0.63) | 79 | 1.06 (1.05–1.08) | 0.61 (0.61–0.62) | 40 | ||
| FM | 1.08 (1.06–1.1) | 0.60 (0.59–0.60) | 39 | 1.05 (1.02–1.08) | 0.63 (0.62–0.63) | 11 | ||
| Waist circumference | 1.13 (1.1–1.15) | 0.61 (0.61–0.62) | 84 | 1.09 (1.06–1.12) | 0.61 (0.6–0.62) | 29 | ||
| aSMM | 1.04 (1.01–1.06) | 0.60 (0.59–0.60) | 9 | 0.98 (0.92–1.04) | 0.63 (0.62–0.63) | 0 | ||
| Decreasing grip strength | 1.17 (1.13–1.2) | 0.60 (0.60–0.61) | 70 | 1.08 (1.04–1.11) | 0.61 (0.60–0.61) | 18 | ||
| Body composition groups | … | 0.61 (0.61–0.62) | 83 | … | 0.61 (0.61–0.62) | 41 | ||
Harrell's C‐statistic and hazard ratios (HR) per SD change calculated from the fully‐adjusted model, which adjusted for: age (underlying timescale variable), height, Townsend index of deprivation, education, smoking status, alcohol intake, physical activity, oily fish intake, fruit and vegetable intake, saturated fat intake, diabetes mellitus, cancer history, menopause (women), and mutually adjusted for FM (in the aSMM models) and aSMM (in the FM models). HRs are corrected for regression dilution bias using the MacMahon‐Peto method. One SD of aSMM is 3.34 kg (men), 1.95 kg (women) and FM is 6.79 kg (men), 8.29 kg (women). The model for aSMM in men is for increasing aSMM; in women is for decreasing aSMM. Wald test statistic was used to compare a model with just confounders to a model with confounders plus the exposure of interest. aSMM indicates appendicular skeletal muscle mass; BMI, body mass index; and FM, fat mass.
Discussion
In this prospective study of 356 590 UK adults, FM had a strong positive log‐linear association with the risk of CVD in both sexes. There was also a positive log‐linear association with aSMM for men and a curvilinear association for women. The associations of aSMM and FM with all‐cause mortality followed a J‐shape in both men and women. Analysis of the association of aSMM within tertiles of FM supported these associations with CVD and all‐cause mortality. The discriminatory ability of BMI was similar to, or better than, more specific measures of body composition (aSMM and FM), waist circumference, or grip strength in relation to CVD events or all‐cause mortality.
Few previous studies have specifically examined the association between distinct body compartments with either incident CVD or mortality. In line with previous studies we consistently observed a positive association between FM and CVD. This is consistent with previous analyses from UK Biobank that found significant associations between body fat percentage, waist circumference, and waist‐to‐hip ratio on CVD outcomes 9 as well as meta‐analyses of prospective cohort studies assessing various adiposity measures. 3 , 41
The role of aSMM has been investigated in fewer studies, most of which used older populations with small sample sizes or a proxy for aSMM such as fat‐free mass. 42 , 43 , 44 , 45 , 46 Our rationale for using aSMM as opposed to whole body muscle or fat‐free mass is because this tissue is more likely to be modifiable by lifestyle factors such as physical activity than other components of fat‐free mass and it is less likely to be confounded by FM given that higher abdominal FM is often accompanied by greater muscle in the trunk region. 30 However, aSMM is a large contributor to whole body muscle and it is likely that participants would be classified in the same quintile regardless of the measure used. Although some studies have shown an inverse association between aSMM and CVD risk, our finding of a positive log‐linear association among men has been observed previously. The Aerobics Center Longitudinal Study found a similar pattern with fat‐free mass index measured by skinfold thicknesses as well as hydrostatic weighing (for which aSMM is the largest contributor) and had a comparably aged, predominantly male study population. 47 A plausible physiological mechanism linking higher aSMM with higher CVD risk may be a higher circulating blood volume, which increases cardiac output and increases systolic blood pressure and the risk of heart failure, a phenomenon previously described mainly among people with obesity. 48 , 49 , 50 A recent literature review has provided a more counterintuitive view of the role of lean mass on metabolic health, proposing the possibility of publication bias, especially if unexpected results were found. 51 Our analysis within tertiles of FM confirmed the increased risk of CVD with aSMM even among men with lower FM levels, reducing the possibility of residual confounding by FM although this cannot be completely ruled out. Our exploratory mediation analyses showed that the association between aSMM and CVD was no longer significant in men after adjusting for BMI. This implies that if aSMM increases, FM plus all the other body compartments have to decrease in order to hold BMI constant, such that changes in body composition that increase skeletal muscle while lowering total body fat, as expected with physical training, may not be associated with increased CVD risk. Furthermore, the large changes observed in the χ2 statistic after adjustment for BMI in the aSMM model suggest a large part of the association may be explained by confounding by BMI, especially among men.
Nevertheless, it is unusual to find a CVD risk factor with such different associations between sexes. 52 A potential explanation for this disparity could be because of differences in lifestyle factors between men and women classified as high aSMM within each FM tertile. For example, compared with women, a higher percentage of men in the same aSMM and FM tertile, reported poorer diets (low fruit and vegetable intake, high saturated fat intake), heavy drinking (over 14 units/week, National Health Service guidelines), or presented a higher prevalence of hypertension and cholesterol medication. Our findings are largely consistent with those reported from a recent study of 38 000 middle‐aged men that demonstrated a U‐shaped association between predicted lean mass and CVD death and mortality; however, these participants may have been healthier because they recruited health professionals rather than the general population. 53 Although we adjusted for several potential lifestyle confounders our study may still have residual confounding in relation to lifestyle factors.
Clinical and Public Health Implications
BMI has been criticized as an inaccurate measure of health risks, 54 , 55 but at a population level, more specific measurements of body composition, namely aSMM and FM, were generally not more predictive of CVD events or mortality; an observation that also been reported elsewhere. 47 , 56 , 57 The moderately improved prognostic value of BMI may reflect the combined effects of height, FM, and SMM that are each individually associated with CVD risk. 58 In addition, BMI has less measurement error than other measures that could contribute to its marginally stronger prognostic ability. 50 Waist circumference and other measures of central adiposity have been reported to better discriminate CVD risk in some studies, 59 although not superior to BMI in others 60 as happened in our study. However, waist circumference is particularly liable to observer error, 50 whereas measures of central adiposity do not indicate whole‐body adiposity, nor is there an equivalent measure for fat‐free mass. Grip strength is often used as a functional indicator of SMM; however, it includes a volitional component and the European Working Group of Sarcopenia in Older People recommends measuring the amount of SMM to assess risk. 61 However, the commonly used measure of the mid‐upper arm circumference is vulnerable to overestimation because it cannot distinguish between muscle fibers and intramuscular fat deposits. 61
However, although BMI may be the simplest measurement to assess health risk, which is important from a public health perspective, some of this risk may not be attributable solely to adiposity, particularly if the association observed with aSMM in men is confirmed, although further research is needed to better understand the biological mechanisms and impact of different body tissue compartment on health outcomes. It could therefore be beneficial to reframe BMI as a composite measure of risk. 47 , 58 In addition, at the individual level, additional measurements of CVD risk factors (eg, blood lipids, blood pressure) in addition to BMI or body composition are needed to classify individuals at risk and propose adequate treatments.
Strengths and Weaknesses of the Study
The strengths of this study include its large sample size, which reduces the risk of chance findings owing to random error, and the detailed measurements of the exposures, potential confounders, and outcomes from the hospital episode statistics follow‐up. Some participants had repeated measurements taken at resurvey, which allowed for the correction for random measurement error and consequent regression dilution bias. 36
Although BIA has many practical strengths in research and clinical settings, it is not as accurate as other methods that use physical properties of the body to measure composition, such as densitometry or DEXA or imaging methods such magnetic resonance imaging scans, and is vulnerable to estimation errors, especially at the extreme ranges of BMI or in people with conditions that affect water retention. 16 , 62 However, validation studies against DEXA show that it performs well in healthy individuals with a stable electrolyte and water balance. 20 Because algorithms to estimate body composition by BIA vary, it may be that our results can be replicated only using a Tanita BC‐418 MA segmental body composition analyzer. However, studies comparing different analyzers from this manufacturer or others have reported only small differences in % body fat (eg, equivalent to 0.7 kg of difference in FM), 63 suggesting that a participant would likely fall into the same quintile regardless of the method used.
Despite the large sample of participants studied here, one of the main limitations is that the UK Biobank presents a low response rate for the United Kingdom (5.5%); however, the associations in this study should still be valid and not affected by selection bias. 64 The participants were predominantly people of White race, which limits the generalizability of our findings. Despite the performance of BMI in this high‐income population of UK Biobank, there is emerging evidence that suggests that BMI is not an informative measure of risk for mortality in lean populations from low‐ and middle‐income countries. For example, research from a large cohort study of 0.5 million adults in India found little association between BMI and cardiac mortality. 65 It may be that BMI is a better indicator of risk in populations where fat mass is the dominant type of body tissue, supported in the current study by the largely equivalent associations of BMI and fat mass with the risk of CVD. Research has also suggested that the distribution of fat mass within equivalent levels of BMI may have distinct associations with the risk of cardiometabolic diseases in different ethnic groups. 66 Thus, although BMI may be the most effective tool for risk assessment within high‐income populations further work is needed to compare its prognostic ability with more detailed measures of body composition in diverse populations. Finally, as this is an observational study we cannot eliminate the possibility that residual confounding affected our results.
Conclusions
FM showed a strong positive association with CVD risk whereas SMM showed a positive log‐linear association with CVD risk in men but curvilinear in women. Although BMI has been criticized as an inaccurate measure of risk, more specific measurements of body composition did not demonstrate improved prognostic ability to detect the risk of CVD or all‐cause mortality.
Sources of Funding
This research was funded by the National Institute for Health Research (NIHR) School of Primary Care Research (SPCR) (Piernas and Jebb); NIHR Applied Research Collaboration Oxford (Piernas and Jebb), and the NIHR Biomedical Research Centre Oxford (Jebb, Carter, Lewington, and Bennett). Additional support was obtained from the Nuffield Department of Population Health (scholarship placement for Knowles), core grants to Clinical Trial Service Unit from the Medical Research Council (Carter and Lewington, Clinical Trial Service Unit A310) and the British Heart Foundation (Carter and Lewington, CH/1996001/9454). The views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care.
Disclosures
None.
Supporting information
Data S1
Tables S1–S12
Figures S1–S3
References 26 , 40 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134
Acknowledgments
We thank the UK Biobank participants. This research used data from the approved UK Biobank application 14990.
Author contributions: Carter, Piernas, and Jebb conceived and designed the research question. Knowles, Carter, and Piernas prepared the data for analysis, analyzed the data, and wrote the first draft of the article; and Jebb, Lewington, and Bennett provided input on data analysis and interpretation of results. All authors revised the article critically for important intellectual content and read and approved the final article. Piernas is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
(J Am Heart Assoc. 2021;10:e019337. DOI: 10.1161/JAHA.120.019337.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019337
For Sources of Funding and Disclosures, see page 12.
References
- 1. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case control study. Lancet. 2004;364:937–952. DOI: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 2. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R, MacMahon S, et al. Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. DOI: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Emerging Risk Factor Collaboration . Separate and combined associations of body‐mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. DOI: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet. 2008;371:569–578. DOI: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 5. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. DOI: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. IHME . GBD results tool, global health data exchange. 2019.
- 7. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26‐year follow‐up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. DOI: 10.1161/01.CIR.67.5.968. [DOI] [PubMed] [Google Scholar]
- 8. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard‐Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. DOI: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 9. Iliodromiti S, Celis‐Morales CA, Lyall DM, Anderson J, Gray SR, Mackay DF, Nelson SM, Welsh P, Pell JP, Gill JMR, et al. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J. 2018;39:1514–1520. DOI: 10.1093/eurheartj/ehy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wade KH, Carslake D, Sattar N, Davey Smith G, Timpson NJ. Obesity BMI and Mortality in UK Biobank: revised estimates using Mendelian randomization. Obesity. 2018;26:1796–1806. DOI: 10.1002/oby.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Romero‐Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez‐Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. DOI: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 12. Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, Cairns BJ, Huxley R, Jackson CL, Joshy G, et al. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. DOI: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories. JAMA. 2013;309:71–82. DOI: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–471. DOI: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 15. Kuriyan R. Body composition techniques. Indian J Med Res. 2018;148:648–658. DOI: 10.4103/ijmr.IJMR_1777_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franssen FME, Rutten EPA, Groenen MTJ, Vanfleteren LE, Wouters EFM, Spruit MA. New reference values for body composition by bioelectrical impedance analysis in the general population: results from the UK Biobank. J Am Med Dir Assoc. 2014;15:448.e1–448.e6. DOI: 10.1016/j.jamda.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 17. Uszko‐Lencer NHMK, Bothmer F, Van Pol PEJ, Schols AMWJ. Measuring body composition in chronic heart failure: a comparison of methods. Eur J Heart Fail. 2006;8:208–214. DOI: 10.1016/j.ejheart.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 18. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent‐Smith L, Melchior JC, Pirlich M, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr. 2004;23:1226–1243. DOI: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19. Pietrobelli A, Rubiano F, St‐Onge M‐P, Heymsfield SB. New bioimpedance analysis system: improved phenotyping with whole‐body analysis. Eur J Clin Nutr. 2004;58:1479–1484. DOI: 10.1038/sj.ejcn.1601993. [DOI] [PubMed] [Google Scholar]
- 20. Lee MM, Jebb SA, Oke J, Piernas C. Reference values for skeletal muscle mass and fat mass measured by bioelectrical impedance in 390 565 UK adults. J Cachexia Sarcopenia Muscle. 2020;11:487–496. DOI: 10.1002/jcsm.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collins R. What makes UK Biobank special? Lancet. 2012;379:1173–1174. DOI: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 22. Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, Gallacher J, Green J, Matthews P, Pell J, et al. UK Biobank: current status and what it means for epidemiology. Health Policy Technol. 2012;1:123–126. DOI: 10.1016/j.hlpt.2012.07.003. [DOI] [Google Scholar]
- 23. Biobank UK. Protocol for a large‐scale prospective epidemiological resource. 2007.
- 24. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:1–10. DOI: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalyani RR, Corriere M, Ferrucci L. Age‐related and disease‐related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–829. DOI: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abramowitz MK, Hall CB, Amodu A, Sharma D, Androga L, Hawkins M. Muscle mass, BMI, and mortality among adults in the United States: a population‐ based cohort study. PLoS One. 2018;13:1–16. DOI: 10.1371/journal.pone.0194697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baumgartner R, Koehler K, Gallagher D, Romero L, Heymsfield S, Ross R, Garry P, Lindeman R. Epidemiology of sarcopenia among the elderly in New México. Am J Epidemiol. 1998;147:755–763. DOI: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 28. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. DOI: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 29. WHO . International Statistical Classification of Diseases and Related Health Problems. 10th Revision ed. WHO; 2016. [Google Scholar]
- 30. Bosy‐Westphal A, Muller MJ. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease—there is need for a unified definition. Int J Obes. 2005;39:379–386. DOI: 10.1038/ijo.2014.161. [DOI] [PubMed] [Google Scholar]
- 31. IPAQ . IPAQ scoring protocol. 2005.
- 32. Plummer M. Improved estimated of floating absolute risk. Stat Med. 2004;23:93–104. DOI: 10.1002/sim.1485. [DOI] [PubMed] [Google Scholar]
- 33. Collett D. Modelling Survival Data in Medical Research. Boca Raton, FL: CRC Press; 2015. [Google Scholar]
- 34. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. DOI: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 35. Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure–response relationships. Stat Med. 2007;26:3735–3752. DOI: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 36. Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long‐term follow‐up of prospective studies. Am J Epidemiol. 1999;150:341–353. DOI: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 37. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. DOI: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 38. Altman D. Practical Statistics for Medical Research. London: Chapman & Hall; 1999. [Google Scholar]
- 39. Agresti A. Building and Extending Loglinear/Logit Models. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2002:357–408. [Google Scholar]
- 40. NHS . NHS alcohol units. 2018.
- 41. Dijk SBV, Takken T, Prinsen EC, Wittink H. Different anthropometric adiposity measures and their association with cardiovascular disease risk factors: a meta‐analysis. Neth Heart J. 2012;20:208–218. DOI: 10.1007/s12471-011-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population‐based sample of community‐dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7:290–298. DOI: 10.1002/jcsm.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, Guralnik JM, Ferrucci L. Skeletal muscle and mortality results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64:377–384. DOI: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Batsis JA, Mackenzie TA, Emeny RT, Lopez‐Jimenez F, Bartels SJ. Low lean mass with and without obesity, and mortality: results from the 1999–2004 National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2017;72:1445–1451. DOI: 10.1093/gerona/glx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spahillari A, Mukamal KJ, DeFilippi C, Kizer JR, Gottdiener JS, Djoussé L, Lyles MF, Bartz TM, Murthy VL, Shah RV. The association of lean and fat mass with all‐cause mortality in older adults: the Cardiovascular Health Study. Nutr Metab Cardiovasc Dis. 2016;26:1039–1047. DOI: 10.1016/j.numecd.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med. 2014;127:547–553. DOI: 10.1016/j.amjmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ortega FB, Sui X, Lavie CJ, Steven N. Body mass index, the most widely used but also widely criticized index: would a gold‐standard measure of total body fat be a better predictor of cardiovascular disease mortality? Mayo Clin Proc. 2017;91:443–455. DOI: 10.1016/j.mayocp.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. DOI: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 49. Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2015;56:391–400. DOI: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 50. Malden D, Lacey B, Emberson J, Karpe F, Allen N, Bennett D, Lewington S. Body fat distribution and systolic blood pressure in 10,000 adults with whole‐body imaging: UK Biobank and Oxford BioBank. Obesity. 2019;27:1200–1206. DOI: 10.1002/oby.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lagacé JC, Brochu M, Dionne IJ. A counterintuitive perspective for the role of fat‐free mass in metabolic health. J Cachexia Sarcopenia Muscle. 2020;11:343–347. DOI: 10.1002/jcsm.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Appelman Y, Rijn BBV, Monique E, Boersma E, Peters SAE. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241:211–218. DOI: 10.1016/j.atherosclerosis.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 53. Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC, Giovannucci EL. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. 2018;362:k2575. DOI: 10.1136/bmj.k2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. DOI: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 55. Pischon T. Commentary: use of the body mass index to assess the risk of health outcomes: time to say goodbye? Int J Epidemiol. 2010;39:528–529. DOI: 10.1093/ije/dyp388. [DOI] [PubMed] [Google Scholar]
- 56. Kuper H, Taylor A, Krishna KVR, Ben‐Shlomo Y, Gupta R, Kulkarni B, Prabhakaran D, Davey Smith G, Wells J, Ebrahim S, et al. Is vulnerability to cardiometabolic disease in Indians mediated by abdominal adiposity or higher body adiposity. BMC Public Health. 2014;14:1239. DOI: 10.1186/1471-2458-14-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Willett K, Jiang R, Lenart E, Spiegelman D, Willett W, Jiang RUI. Comparison of bioelectrical impedance and BMI in predicting obesity‐related medical conditions. Obesity. 2006;14:480–490. DOI: 10.1038/oby.2006.63. [DOI] [PubMed] [Google Scholar]
- 58. Wells JCK. Commentary: the paradox of body mass index in obesity assessment: not a good index of adiposity, but not a bad index of cardio‐metabolic risk. Int J Epidemiol. 2014;43:672–674. DOI: 10.1093/ije/dyu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee CMY, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta‐analysis. J Clin Epidemiol. 2008;61:646–653. DOI: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 60. Taylor AE, Ebrahim S, Ben‐Shlomo Y, Martin RM, Whincup PH, Yarnell JW, Wannamethee SG, Lawlor DA. Comparison of the associations of body mass index and measures of central adiposity and fat mass with coronary heart disease, diabetes, and all‐cause mortality: a study using data from 4 UK cohorts. Am J Clin Nutr. 2010;91:547–556. DOI: 10.3945/ajcn.2009.28757. [DOI] [PubMed] [Google Scholar]
- 61. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. DOI: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, Lilienthal Heitmann B, Kent‐Smith L, Melchior J‐C, Pirlich M, et al. Bioelectrical impedance analysis—part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–1453. DOI: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 63. Hemmingsson E, Udden J, Neovius M. No apparent progress in bioelectrical impedance accuracy: validation against metabolic risk and DXA. Obesity (Silver Spring). 2009;17:183–187. DOI: 10.1038/oby.2008.474. [DOI] [PubMed] [Google Scholar]
- 64. Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health‐related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. DOI: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gajalakshmi V, Lacey B, Kanimozhi V, Sherliker P, Peto R, Lewington S. Body‐mass index, blood pressure, and cause‐specific mortality in India: a prospective cohort study of 500 810 adults. Lancet Glob Health. 2018;6:e787–e794. DOI: 10.1016/S2214-109X(18)30267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abdullah N, Abdul Murad NA, Attia J, Oldmeadow C, Kamaruddin MA, Abd Jalal N, Ismail N, Jamal R, Scott RJ, Holliday EG. Differing contributions of classical risk factors to type 2 diabetes in multi‐ethnic Malaysian populations. Int J Environ Res Public Health. 2018;15:2813. DOI: 10.3390/ijerph15122813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Allison DB, Zhu SK, Plankey M, Faith MS, Heo M. Differential associations of body mass index and adiposity with all‐cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow‐up studies. Int J Obes. 2002;26:410–416. DOI: 10.1038/sj.ijo.0801925. [DOI] [PubMed] [Google Scholar]
- 68. Warren Andersen S, Shu X‐O, Gao Y‐T, Zhang X, Cai H, Yang G, Li H‐L, Xiang Y‐B, Zheng W. Prospective cohort study of central adiposity and risk of death in middle aged and elderly Chinese. PLoS One. 2015;10:e0138429. DOI: 10.1371/journal.pone.0138429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population‐based cohort study of older men. J Am Geriatr Soc. 2014;62:253–260. DOI: 10.1111/jgs.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bachettini NP, Bielemann RM, Barbosa‐Silva TG, Baptista Menezes AM, Tomasi E, Gonzalez MC. Sarcopenia as a mortality predictor in community‐dwelling older adults: a comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur J Clin Nutr. 2020;74:573–580. DOI: 10.1038/s41430-019-0508-8. [DOI] [PubMed] [Google Scholar]
- 71. Balogun S, Winzenberg T, Wills K, Scott D, Jones G, Aitken D, Callisaya ML. Prospective associations of low muscle mass and function with 10‐year falls risk, incident fracture and mortality in community‐dwelling older adults. J Nutr Health Aging. 2017;21:843–848. DOI: 10.1007/s12603-016-0843-6. [DOI] [PubMed] [Google Scholar]
- 72. Batsis JA, Mackenzie TA, Barre LK, Lopez‐Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007. DOI: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 73. Bea JW, Thomson CA, Wertheim BC, Nicholas JS, Ernst KC, Hu C, Jackson RD, Cauley JA, Lewis CE, Caan B, et al. Risk of mortality according to body mass index and body composition among postmenopausal women. Am J Epidemiol. 2015;182:585–596. DOI: 10.1093/aje/kwv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bigaard J, Frederiksen K, Tjønneland A, Thomsen BL, Overvad K, Heitmann BL, Sørensen TIA. Body fat and fat‐free mass and all‐cause mortality. Obes Res. 2004;12:1042–1049. DOI: 10.1038/oby.2004.131. [DOI] [PubMed] [Google Scholar]
- 75. Bigaard J, Frederiksen K, Tjønneland A, Thomsen BL, Overvad K, Heitmann BL, Sørensen TIA. Waist circumference and body composition in relation to all‐cause mortality in middle‐aged men and women. Int J Obes. 2005;29:778–784. DOI: 10.1038/sj.ijo.0802976. [DOI] [PubMed] [Google Scholar]
- 76. Boloukat RR, Ramezankhani A, Hasheminia M, Tasdighi E, Azizi F, Hadaegh F. Impact of blood pressure, cholesterol and glucose in the association between adiposity measures and coronary heart disease and stroke among Iranian population. Clin Nutr. 2018;37:2060–2067. DOI: 10.1016/j.clnu.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 77. Brown JC, Harhay MO, Harhay MN. Appendicular lean mass and mortality among prefrail and frail older adults. J Nutr Health Aging. 2017;21:5–8. DOI: 10.1007/s12603-016-0753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen Z, Iona A, Parish S, Chen Y, Guo Y, Bragg F, Yang L, Bian Z, Holmes MV, Lewington S, et al. Adiposity and risk of ischaemic and haemorrhagic stroke in 0·5 million Chinese men and women: a prospective cohort study. Lancet. 2018;6:e630–e640. DOI: 10.1016/S2214-109X(18)30216-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen G‐C, Arthur R, Iyengar NM, Kamensky V, Xue X, Wassertheil‐Smoller S, Allison MA, Shadyab AH, Wild RA, Sun Y, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J. 2019;40:2849–2855. DOI: 10.1093/eurheartj/ehz391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cl C, Lam KSL, Cheung BMY. Evaluation of cutpoints for low lean mass and slow gait speed in predicting death in the National Health and Nutrition Examination Survey 1999–2004. J Gerontol A Biol Sci Med Sci. 2016;71:90–95. DOI: 10.1093/gerona/glv112. [DOI] [PubMed] [Google Scholar]
- 81. Chin SO, Rhee SY, Chon S, Hwang Y‐C, Jeong I‐K, Oh S, Ahn KJ, Chung HY, Woo J‐T, Kim S‐W, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One. 2013;8:e60119. DOI: 10.1371/journal.pone.0060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chuang SY, Chang HY, Lee MS, Chia‐Yu Chen R, Pan WH. Skeletal muscle mass and risk of death in an elderly population. Nutr Metab Cardiovasc Dis. 2014;24:784–791. DOI: 10.1016/j.numecd.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 83. Chuang SY, Hsu YY, Chen RCY, Liu WL, Pan WH. Abdominal obesity and low skeletal muscle mass jointly predict total mortality and cardiovascular mortality in an elderly Asian population. J Gerontol A Biol Sci Med Sci. 2016;71:1049–1055. DOI: 10.1093/gerona/glv192. [DOI] [PubMed] [Google Scholar]
- 84. de Almeida Roediger M, de Fátima Nunes Marucci M, Quintiliano Scarpelli Dourado DA, de Oliveira C, Licio Ferreira Santos J, de Oliveira Duarte YA. Body composition changes and 10‐year mortality risk in older Brazilian adults: analysis of prospective data from the SABE study. J Nutr Health Aging. 2019;23:51–59. DOI: 10.1007/s12603-018-1118-1. [DOI] [PubMed] [Google Scholar]
- 85. de Santana FM, Domiciano DS, Gonçalves MA, Machado LG, Figueiredo CP, Lopes JB, Caparbo VF, Takayama L, Menezes PR, Pereira RM. Association of appendicular lean mass, and subcutaneous and visceral adipose tissue with mortality in older Brazilians: the São Paulo Ageing & Health Study. J Bone Miner Res. 2019;34:1264–1274. DOI: 10.1002/jbmr.3710. [DOI] [PubMed] [Google Scholar]
- 86. Dolan CM, Kraemer H, Browner W, Ensrud K, Kelsey JL. Associations between body composition, anthropometry, and mortality in women aged 65 years and older. Am J Public Health. 2007;97:913–918. DOI: 10.2105/AJPH.2005.084178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dong B, Peng Y, Wang Z, Adegbija O, Hu J, Ma J, Ma H. Joint association between body fat and its distribution with all‐cause mortality: a data linkage cohort study based on NHANES (1988–2011). PLoS One. 2018;13:e0193368. DOI: 10.1371/journal.pone.0193368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36:228–235. DOI: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 89. Gillum RF, Mussolino ME, Madans JH. Body fat distribution, obesity, overweight and stroke incidence in women and men: the NHANES I Epidemiologic Follow‐up Study. Int J Obes. 2001;25:628–638. DOI: 10.1038/sj.ijo.0801590. [DOI] [PubMed] [Google Scholar]
- 90. Gnatiuc L, Alegre‐Díaz J, Wade R, Ramirez‐Reyes R, Tapia‐Conyer R, Garcilazo‐Ávila A, Chiquete E, Gonzáles‐Carballo C, Solano‐Sanchez M, Clarke R, et al. General and abdominal adiposity and mortality in Mexico City: prospective study of 150000 adults. Ann Intern Med. 2019;171:397–405. DOI: 10.7326/M18-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Graf CE, Karsegard VL, Spoerri A, Makhlouf AM, Ho S, Herrmann FR, Genton L. Body composition and all‐cause mortality in subjects older than 65 y. Am J Clin Nutr. 2015;101:760–767. DOI: 10.3945/ajcn.114.102566. [DOI] [PubMed] [Google Scholar]
- 92. Han SS, Kim KW, Kim K‐I, Na KY, Chae D‐W, Kim S, Chin HJ. Lean mass index: a better predictor of mortality than body mass index in elderly Asians. J Am Geriatr Soc. 2010;58:312–317. DOI: 10.1111/j.1532-5415.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- 93. Heitmann BL, Erikson H, Bm E, Mikkelsen KL, Larsson B. Mortality associated with body fat, fat‐free mass and body mass index among 60‐year‐old Swedish men—a 22‐year follow‐up. The study of men born in 1913. Int J Obes. 2000;24:33–37. DOI: 10.1038/sj.ijo.0801082. [DOI] [PubMed] [Google Scholar]
- 94. Hirani V, Naganathan V, Blyth F, Le Couteur D, Seibel MJ, Waite LM, Handelsman DJ, Cumming RG. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community‐dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing. 2017;46:413–420. DOI: 10.1093/ageing/afw214. [DOI] [PubMed] [Google Scholar]
- 95. Hotchkiss JW, Davies CA, Leyland AH. Adiposity has differing associations with incident coronary heart disease and mortality in the Scottish population: cross‐sectional surveys with follow‐up. Int J Obes. 2013;37:732–739. DOI: 10.1038/ijo.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Howell CR, Mehta T, Ejima K, Ness KK, Cherrington A, Fontaine KR. Body composition and mortality in Mexican‐American adults: results from the National Health and Nutrition Examination Survey. Obesity. 2018;26:1372–1380. DOI: 10.1002/oby.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kahn HS, Bullard KM, Barker LE, Imperatore G. Differences between adiposity indicators for predicting all‐cause mortality in a representative sample of United States non‐elderly adults. PLoS One. 2012;7:e50428. DOI: 10.1371/journal.pone.0050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Katzmarzyk PT, Craig CL, Bouchard C. Adiposity, adipose tissue distribution and mortality rates in the Canada Fitness Survey follow‐up study. Int J Obes. 2002;26:1054–1059. DOI: 10.1038/sj.ijo.0802057. [DOI] [PubMed] [Google Scholar]
- 99. Kim Y, Wijndaele K, Lee D‐C, Sharp SJ, Wareham N, Brage S. Independent and joint associations of grip strength and adiposity with all‐cause and cardiovascular disease mortality in 403,199 adults: the UK Biobank study. Am J Clin Nutr. 2017;106:773–782. DOI: 10.3945/ajcn.117.156851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kizer JR, Biggs ML, Ix JH, Mukamal KJ, Zieman SJ, de Boer IH, Mozaffarian D, Barzilay JI, Strotmeyer ES, Luchsinger JA, et al. Original contribution measures of adiposity and future risk of ischemic stroke and coronary heart disease in older men and women. Am J Epidemiol. 2011;173:10–25. DOI: 10.1093/aje/kwq311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kouvari M, Panagiotakos DB, Chrysohoou C, Notara V, Georgousopoulou EN, Yannakoulia M, Tousoulis D, Pitsavos C, Pitsavos C; Investigators AaGs . A sex‐specific evaluation of predicted lean and fat mass composition and cardiovascular disease onset and progression: a combined analysis of the ATTICA and GREECS prospective epidemiological studies. Obes Res Clin Pract. 2019;13:469–477. DOI: 10.1016/j.orcp.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 102. Lee JSW, Auyeung TW, Kwok T, Li M, Leung J, Woo J. Survival benefit of abdominal adiposity: a 6‐year follow‐up study with dual X‐ray absorptiometry in 3,978 older adults. Age. 2012;34:597–608. DOI: 10.1007/s11357-011-9272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Levitan EB, Yang AZ, Wolk A, Mittleman MA. Adiposity and incidence of heart failure hospitalization and mortality a population‐based prospective study. Circ Heart Fail. 2009;2:202–208. DOI: 10.1161/CIRCHEARTFAILURE.108.794099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li R, Xia J, Zhang X, Gathirua‐Mwangi WG, Guo J, Li Y, McKenzie S; Song Y. Associations of muscle mass and strength with all‐cause mortality among US older adults. Med Sci Sports Exerc. 2018;50:458–467. DOI: 10.1249/MSS.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Myint PK, Kwok CS, Luben RN, Wareham NJ, Khaw KT. Body fat percentage, body mass index and waist‐to‐hip ratio as predictors of mortality and cardiovascular disease. Heart. 2014;100:1613–1619. DOI: 10.1136/heartjnl-2014-305816. [DOI] [PubMed] [Google Scholar]
- 106. Nalini M, Sharafkhah M, Poustchi H, Sepanlou SG, Pourshams A, Reza A. Comparing anthropometric indicators of visceral and general adiposity as determinants of overall and cardiovascular mortality. Arch Iran Med. 2019;22:301–309. [PMC free article] [PubMed] [Google Scholar]
- 107. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass is associated with mortality in the Health, Aging and Body Composition Study cohort. J Gerontol A Biol Sci Med Sci. 2006;61A:72–77. DOI: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 108. Ofstad AP, Sommer C, Birkeland KI, Bjørgaas MR, Gran JM, Gulseth HL, Johansen OE. Comparison of the associations between non‐traditional and traditional indices of adiposity and cardiovascular mortality: an observational study of one million person‐years of follow‐up. Int J Obes. 2019;43:1082–1092. DOI: 10.1038/s41366-019-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Otsuka R, Matsui Y, Tange C, Nishita Y, Tomida M, Ando F, Shimokata H, Arai H. What is the best adjustment of appendicular lean mass for predicting mortality or disability among Japanese community dwellers? BMC Geriatr. 2018;18:8. DOI: 10.1186/s12877-017-0699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Padwal R, Leslie WD, Lix LM, Majumdar SR. Relationship among body fat percentage, body mass index, and all‐cause mortality: a cohort study. Ann Intern Med. 2016;164:532–541. DOI: 10.7326/M15-1181. [DOI] [PubMed] [Google Scholar]
- 111. Park S, Ham JO, Lee BK. A positive association between stroke risk and sarcopenia in men aged 50 years, but not women: results from the Korean National Health and Nutrition Examination Survey 2008–2010. J Nutr Health Aging. 2014;18:806–812. DOI: 10.1007/s12603-014-0553-x. [DOI] [PubMed] [Google Scholar]
- 112. Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. 2018;8:16753. DOI: 10.1038/s41598-018-35073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Reis JP, MacEra CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity. 2009;17:1232–1239. DOI: 10.1038/oby.2008.664. [DOI] [PubMed] [Google Scholar]
- 114. Reis JP, Araneta MR, Wingard DL, Macera CA, Lindsay SP, Marshall SJ. Overall obesity and abdominal adiposity as predictors of mortality in US white and black adults. Ann Epidemiol. 2009;19:134–142. DOI: 10.1016/j.annepidem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 115. Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes. 2001;25:1047–1056. DOI: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 116. Sim M, Prince RL, Scott D, Daly RM, Duque G, Inderjeeth CA, Zhu K, Woodman RJ, Hodgson JM, Lewis JR. Sarcopenia definitions and their associations with mortality in older Australian women. J Am Med Dir Assoc. 2019;20:76–82. DOI: 10.1016/j.jamda.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 117. Simpson JA, MacInnis RJ, Peeters A, Hopper JL, Giles GG, English DR, Julie A. A comparison of adiposity measures as predictors of all‐cause mortality: the Melbourne Collaborative Cohort Study. Obesity. 2007;15:994–1003. DOI: 10.1038/oby.2007.622. [DOI] [PubMed] [Google Scholar]
- 118. Srikanthan P, Horwich TB, Tseng CH. Relation of muscle mass and fat mass to cardiovascular disease mortality. Am J Cardiol. 2016;117:1355–1360. DOI: 10.1016/j.amjcard.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 119. Stefan N, Kantartzis K, Häring H. Cardiorespiratory fitness, adiposity, and mortality. JAMA. 2008;299:1013–1014. DOI: 10.1001/jama.299.9.1013-b. [DOI] [PubMed] [Google Scholar]
- 120. Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Jooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. DOI: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tanne D, Medalie JH, Goldbourt U. Body fat distribution and long‐term risk of stroke mortality. Stroke. 2005;36:1021–1025. DOI: 10.1161/01.STR.0000162584.39366.1c. [DOI] [PubMed] [Google Scholar]
- 122. The Decode Study Group . Does the constellation of risk factors with and without abdominal adiposity associate with different cardiovascular mortality risk? Int J Obes. 2008;32:757–762. DOI: 10.1038/sj.ijo.0803797. [DOI] [PubMed] [Google Scholar]
- 123. Thomson CA, Garcia DO, Wertheim BC, Hingle MD, Bea JW, Zaslavsky O, Caire‐Juvera G, Rohan T, Vitolins MZ, Thompson PA, et al. Body shape, adiposity index, and mortality in postmenopausal women: findings from the Women’s Health Initiative Cynthia. Obesity. 2016;24:1061–1069. DOI: 10.1002/oby.21461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Toss F, Wiklund P, Nordstrom P, Nordstrom A. Body composition and mortality risk in later life. Age Ageing. 2012;41:677–681. DOI: 10.1093/ageing/afs087. [DOI] [PubMed] [Google Scholar]
- 125. Van Aller C, Lara J, Stephan BCM, Maria L, Heyms S, Katzmarzyk PT, Wells JCK, Prado CM, Siervo M. Sarcopenic obesity and overall mortality: results from the application of novel models of body composition phenotypes to the National Health and Nutrition Examination Survey 1999–2004. Clin Nutr. 2019;38:264–270. DOI: 10.1016/j.clnu.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 126. Wang A, Wu J, Zhou Y, Guo X, Luo Y, Wu S, Zhao X. Measures of adiposity and risk of stroke in China: a result from the Kailuan study. PLoS One. 2013;8:e61665. DOI: 10.1371/journal.pone.0061665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr. 2007;86:1339–1346. DOI: 10.1093/ajcn/86.5.1339. [DOI] [PubMed] [Google Scholar]
- 128. Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Papacosta O, Sattar N. The obesity paradox in men with coronary heart disease and heart failure: the role of muscle mass and leptin. Int J Cardiol. 2014;171:49–55. DOI: 10.1016/j.ijcard.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yang M, Jiang J, Zeng Y, Tang H. Sarcopenia for predicting mortality among elderly nursing home residents. Medicine. 2019;98:e14546. DOI: 10.1097/MD.0000000000014546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yuki A, Ando F, Otsuka R, Shimokata H. Sarcopenia based on the Asian Working Group for Sarcopenia criteria and all‐cause mortality risk in older Japanese adults. Geriatr Gerontol Int. 2017;17:1642–1647. DOI: 10.1111/ggi.12946. [DOI] [PubMed] [Google Scholar]
- 131. Zaslavsky O, Rillamas‐Sun E, Li W, Going S, Datta M, Snetselaar L, Zelber‐Sagi S. Association of dynamics in lean and fat mass measures with mortality in frail older women. J Nutr Health Aging. 2017;21:112–119. DOI: 10.1007/s12603-016-0730-1. [DOI] [PubMed] [Google Scholar]
- 132. Zhang X, Shu XO, Yang G, Li H, Cai H, Gao YT, Zheng W. Abdominal adiposity and mortality in Chinese women. Arch Intern Med. 2007;167:886–892. DOI: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]
- 133. Zhu S, Heo PM, Faith MS, Allison DB. Associations of body mass index and anthropometric indicators of fat mass and fat free mass with all‐cause mortality among women in the first and second National Health and Nutrition Examination Surveys follow‐up studies. Ann Epidemiol. 2003;13:286–293. DOI: 10.1016/S1047-2797(02)00417-9. [DOI] [PubMed] [Google Scholar]
- 134. Zong G, Zhang Z, Yang Q, Wu H, Hu FB, Sun Q. Total and regional adiposity measured by dual‐energy X‐ray absorptiometry and mortality in NHANES 1999–2006. Obesity. 2017;24:2414–2421. DOI: 10.1002/oby.21659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S12
Figures S1–S3
References 26 , 40 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134
Data Availability Statement
Researchers can apply to use the UK Biobank resource and access the data used. No additional data are available.


