Summary
Phagocytes form a family of immune cells that play a crucial role in tissue maintenance and help orchestrate the immune response. This family of cells can be separated by their nuclear morphology into mononuclear and polymorphonuclear phagocytes. The generation of these cells in the bone marrow, to the blood and finally into tissues is a tightly regulated process. Ensuring the adequate production of these cells and their timely removal is key for both the initiation and resolution of inflammation. Insight into the kinetic profiles of innate myeloid cells during steady state and pathology will permit the rational development of therapies to boost the production of these cells in times of need or reduce them when detrimental.
Keywords: homeostasis, inflammation, kinetics, mononuclear phagocyte, neutrophils
Patel et al., update our understanding regarding human phagocyte kinetics in health and disease.
Abbreviations
- CDP
common DC precursor
- cMoP
common monocyte progenitor
- DCs
dendritic cells
- E. coli
Escherichia coli
- E9·5
embryonic day 9·5
- EAE
experimental autoimmune encephalomyelitis
- EMP
erythromyeloid progenitors
- G‐CSF
granulocyte colony‐stimulating factor
- GMPs
granulocyte and macrophage progenitors
- GMPs
granulocyte–macrophage progenitors
- IRF8
interferon regulatory factor 8
- LPS
lipopolysaccharide
- MDP
monocyte and DC progenitor
- MPS
mononuclear phagocyte system
- NETs
neutrophil extracellular traps
- pre‐cDC
cDC precursor
- RES
reticuloendothelial system
- SatM
segregated nucleus‐containing atypical Ly6Clo monocyte
- VEGF‐α
vascular endothelial growth factor α
- WHIM
warts, hypogammaglobulinaemia, immunodeficiency and myelokathexis
- YSMP
yolk sac‐derived myeloid‐based progenitor
Introduction
Under healthy physiological conditions, the human body maintains a steady number of leucocytes by means of cell proliferation, differentiation, survival and cell death. The bone marrow is regarded as the primary haematopoietic factory for the production of the majority of adult immune cells. Following their egression into the circulation and tissues, these cells will fulfil their purpose and then eventually die, only to be replaced by newly produced younger cells thought to keep the immune system alert at all times. The importance of this fine balance can be appreciated in diseases such as haematological cancers where a substantial number of abnormal immune cells are present or, conversely, where a deficit of specific immune cell subsets consequently results in increased susceptibility to opportunistic infections. 1 , 2 , 3 , 4
During inflammation, this finely balanced sequence must quickly adapt to produce sufficient immune cells in order to combat injury or infection and replace those dysregulated by the inflammatory challenge. The cellular kinetics and regulation of the inflammatory response encompass several cell types including sensory C‐fibres, white blood cells, endothelial cells and fibroblasts. Here, we focus on the cellular kinetics of professional phagocytes, a group of innate myeloid immune cells (namely monocytes, dendritic cells, macrophages and neutrophils) specialized, but not limited to, their ability to phagocytose foreign bodies. Tissue macrophages are sentinels that reside within every tissue in an organism and act as first responders to an infectious agent. Depending on the tissue, macrophages are initially derived from progenitors within the yolk sac or fetal liver 5 , 6 , 7 , 8 and play an important role during tissue development and maintenance. 6 , 9 , 10 , 11 , 12 , 13 , 14 On the other hand, neutrophils are recognized for their mechanisms involved in pathogen clearance (e.g. reactive oxygen species production, degranulation of antimicrobial proteins, neutrophil extracellular traps (NETs)). Patients diagnosed with neutropenia are often more susceptible to infections, 15 , 16 which highlights the need for an adequate production of these cells. Monocytes also aid in the phagocytosis of pathogens and apoptotic cells, whereas dendritic cells (DCs) activate the adaptive immune response by migrating from the periphery to draining lymph nodes. The absence of monocytes and DC has been observed in patients bearing mutations in the transcription factor, interferon regulatory factor 8 (IRF8), as a result, these individuals are more predisposed to infections. 1
Inflammation is necessary to protect an organism from infectious agents, yet an overactive immune response can result in further tissue damage. This can be observed in chronic inflammatory diseases such as rheumatoid arthritis where a constant recruitment of monocytes to the synovial tissue has been observed in humans. 17 Therefore, though inflammation has been historically recognized as a salutary reaction, 18 ‘more is more’ is not often the case. Striking the fine balance of an essential yet limited inflammatory response may present as a potential therapeutic opportunity, highlighting the importance of understanding the kinetics of immune cells in health and disease.
Neutrophil kinetics
Neutrophils are an indispensable component of the innate immune system, if not the most important. Within the circulation, neutrophils constitute the largest proportion of human circulating leucocytes and estimated to be found at ~ 4·2 x109 cells per litre of blood although this can vary with age, ethnicity and sex. 19 , 20 Neutrophils are often the first white blood cell recruited to sites of injury, which is facilitated by both the upregulation and expression of membrane adhesion molecules on the endothelium and neutrophils. 21 , 22 Increased levels of neutrophil chemotactic factors such as IL‐8 (CXCL8) have been observed early on during models of human inflammation, which aids in the guidance of neutrophils to the site of inflammation. 23 , 24 The initial recognition of pathogens by tissue‐resident macrophages is also partly responsible for the recruitment of neutrophils 25 although other resident cells can also modulate their infiltration. 26 Recent studies in mice and humans have reported a role for vascular endothelial growth factor α (VEGF‐α) released from cDC1 in neutrophil recruitment in cutaneous infections. 27
The generation of neutrophils involves a series of maturation steps starting with granulocyte–macrophage progenitors (GMPs) in the bone marrow. These cells give rise to pre‐neutrophils, which subsequently develop into immature (band) and mature (segmented) neutrophils, which egress into the circulation. 28 , 29 , 30 CXCR4 is a key chemokine receptor involved in the retention of neutrophils within the bone marrow via interaction with CXCL12, whereas CXCR2 activation promotes the mobilization of neutrophils from the bone marrow into the blood. 31 , 32 , 33 The importance of the CXCR4 axis in the regulation of neutrophil egression can be highlighted in warts, hypogammaglobulinaemia, immunodeficiency and myelokathexis (WHIM) syndrome patients who primarily suffer from an autosomal, dominant, gain‐of‐function mutation in CXCR4. 15 , 16 Consequently, these patients suffer from defected neutrophil egression and thus circulating neutropenia, making them more vulnerable to infections.
Deuterium labelling acts in a non‐cytotoxic manner to label dividing cells in vivo. 34 , 35 Using this approach to monitor human neutrophil development, following the last proliferation in the bone marrow, the mean transit time before neutrophils enter the circulation has been estimated at 5·8 days. 19 Once within the circulation, these cells have an incredibly short half‐life of approximately 19 hours. The short half‐life of these cells may reflect their function. Whilst homeostatic functions have been assigned to neutrophils, 36 , 37 these cells are renowned for their defensive mechanisms against invading pathogens such as the production of reactive oxygen species, antimicrobial peptides and NETosis. 38 , 39 These mechanisms are not pathogen‐specific and can therefore result in collateral damage to host tissues, and consequently, it is thought these cells are likely programmed for a quick cell death in order to prevent excessive damage.
Once in the circulation, neutrophils can either be found within the circulating pool where they are readily accessible for blood sampling or be located in a marginating pool. Following stimuli including infection, trauma, the administration of adrenaline or an intense burst of exercise, this marginated pool can be mobilized into the circulating pool in humans. 40 , 41 It has been calculated that the marginating pool makes up approximately 50% of the total neutrophil pool. 19 Neutrophil transit within tissues refers to how quickly cells pass through the capillary beds. Within the spleen and bone marrow, it takes approximately 10 minutes for neutrophils to transit. 42 , 43 Therefore, the period of time cells spend within tissues may factor in determining the marginated fraction.
Neutrophilia is commonly associated with bacterial infections but can arise from other stimuli. In human models of local and systemic inflammation, neutrophilia accounts for the increase in the total white blood cell count at early time‐points. 23 , 44 , 45 , 46 , 47 Of note, left shift refers to the presence of an increased amount of immature neutrophils within the circulation. 48 This observation has been associated with cancer progression 28 and has more recently been observed in severe COVID‐19 patients, where these cells exhibit an immunosuppressive profile. 49 Elevated levels of systemic granulocyte colony‐stimulating factor (G‐CSF) during inflammation can lead to both the downregulation of CXCR4 50 and the increased levels of CXCR2 ligands, 51 which likely leads to an increase in neutrophil egression. Additionally, an increase in the number of neutrophil progenitors during inflammation can also contribute to the elevation of circulating neutrophils, 28 in addition to neutrophils from the marginating pool. During the resolution of inflammation, neutrophils are eventually cleared from tissues to restore tissue homeostasis. Neutrophil death can occur in various ways including apoptosis, necrosis, NETosis and autophagy. Efferocytosis refers to the phagocytosis of apoptotic cell bodies. The recognition of ‘eat me’ signals such as phospholipid phosphatidylserine on apoptotic cells facilitates the recognition by macrophages and monocyte‐derived cells to efferocytose apoptotic bodies. 14 , 52 Under steady physiological conditions, senescent neutrophils can also home back to bone marrow by upregulating CXCR4, where they will also be efferocytosed by resident macrophages. 53 , 54
These data demonstrate a significant amount of knowledge regarding neutrophils kinetics in both steady state and inflammation. The next step is to truly understand the kinetic changes within each compartment of the body, that is bone marrow, blood and tissue, in addition to the cues that skew the lifespan of these cells during pathology, which may allow for future targeting to promote an adequate response.
Macrophage kinetics
Tissue‐resident macrophages form a network of cells that reside throughout the organism. Macrophage nomenclature has evolved and is based on anatomical location or after the scientist who discovered the macrophage population, for example microglia in the brain, osteoclasts in the bone or Kupffer cells in the liver and Langerhans cells in the epidermis.
The idea that all macrophages arise from monocytes stemmed from studies as early as 1926 demonstrating the ability of monocytes to develop a macrophage phenotype both in vitro 55 and in vivo. 56 In 1969, Ralph van Furth and colleagues proposed the reticuloendothelial system (RES) was too broad a definition and coined the term the ‘Mononuclear Phagocyte System’ (MPS) including monocytes and macrophages. Furthermore, Van Furth and colleagues separated macrophages into two clear categories: ‘free’ or ‘fixed’ macrophages. When distinguishing the macrophages of the spleen, lymph nodes and bone marrow, ‘free’ macrophages were described as monocyte in origin, while the origin of the ‘fixed’ macrophages in these organs remained undetermined. 57 This insight into a division of labour of macrophage ontogeny is reflected at this time with the observation that macrophage development was noted within the yolk sac prior to bone marrow haematopoiesis. 58 , 59 These yolk sac macrophages appeared one day earlier than monocytes in mice and was one of the earliest observations – to the best of our knowledge – to report macrophage development independent of monocytes. 59 Furthermore, investigations into the kinetics of monocytes and macrophages by Ralph van Furth and Zan Cohen, using radioactive thymidine, demonstrated low labelling in peritoneal macrophages compared with blood monocytes. 60 Unaware at the time, these data supported the maintenance of macrophage populations independently of monocytes. More recently, the use of genetic reporter and fate‐mapping techniques has confirmed that tissue‐resident macrophages are embryonically (yolk sac and/or fetal liver) derived and can be maintained with little or significant monocyte input depending on the tissue of concern 5 , 6 , 7 , 8 , 61 , 62 , 63 (Figure 1).
Figure 1.
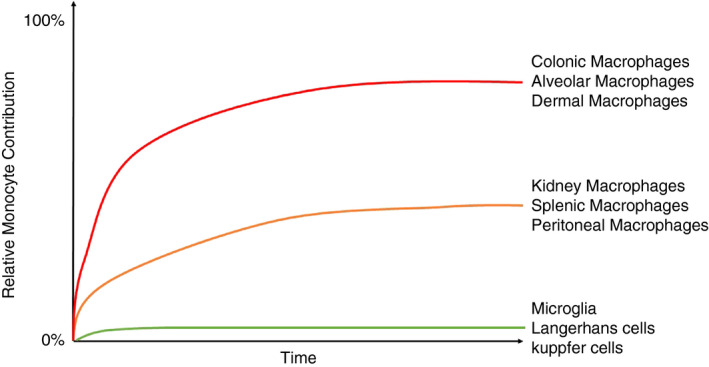
Tissue macrophage kinetics with age. Illustrative diagram demonstrating the relative and differential rates of blood monocyte contribution to tissue macrophage populations. Adapted from Liu et al. (2019).
Within the developing embryo, haematopoiesis occurs in a sequential manner, initially occurring within the yolk sac (primitive haematopoiesis) before transferring to the fetal liver until birth where haematopoiesis is ultimately transferred to the bone marrow (definitive haematopoiesis). 64 Primitive haematopoiesis begins in the yolk sac, where erythromyeloid progenitors (EMPs) give rise to erythrocytes, macrophages and mast cells. 6 , 65 A similar yolk sac‐derived myeloid‐based progenitor (YSMP) has been identified in humans, however lacks an erythroid gene signature. 66 Downstream of the EMP, a pre‐macrophage precursor has been identified in mice, which arises in the yolk sac before colonizing embryonic tissues around embryonic day 9·5 (E9·5) at the same time as organogenesis. 6 These pre‐macrophages are subjected to tissue‐specific signals, which help sculpt a tissue‐specific resident macrophage phenotype. 67 Recent studies on macrophage development in rodents have been corroborated in humans where an early monocyte‐independent primitive wave and later fetal liver monocyte‐derived wave have been described. 66
Following birth, resident macrophages can persist throughout life with or without monocyte input depending on the tissue compartment under steady state 8 , 62 , 63 (Figure 1). More recently, a novel fate‐mapping model utilizing the Ms4a3 gene demonstrated in a more specific quantitative manner the monocyte contribution to several tissue macrophage compartments. 63 In tissues such as the brain, skin and liver, monocytes do not replace the embryonically derived microglia, Langerhans cells nor Kupffer cells. Whilst this has been clearly demonstrated in mice, further clarity is required within the human setting. Interestingly, Langerhans cells of the skin have been shown to persist of donor origin – ten years following human hand allograft. 68 However, others have found that following bone marrow transplantation, the majority of Langerhans cells were donor‐derived within 3 months. 69 , 70 , 71 It is important to note chemotherapeutic or other pharmacological regimes could impact on these studies. The ontogeny of alveolar macrophages in humans has also been examined in humans where contrasting findings have again been observed. 72 , 73 , 74 An interesting study by Réu and colleagues took advantage of atmospheric 14C to estimate the turnover rate of human microglia. 75 Increases in atmospheric 14C in the 1950s due to nuclear bomb testing resulted in the increased presence of 14C within the atmosphere and subsequently DNA of newly formed cells, which in turn could provide insight into the age of a cell. 76 Réu and colleagues demonstrated that human microglia have a lifespan of approximately 4·2 years and renew at a rate of 28% per year. 75 Table 1 lists examples of studies where the longevity of macrophage populations has been examined in both murine models and the human setting.
Table 1.
Longevity of phagocytes. Summary of longevity of phagocyte populations in mouse and humans. Lifespans and half‐lives have been stated where available. TBC, to be confirmed
| Human | Mouse | |
|---|---|---|
| Blood neutrophil | Half‐life ~ 13–19 hours 19 | Half‐life ~ 12·5 hours 184 |
| Blood classical monocytes | Lifespan ~ 1·0 days 106 | Half‐life ~ 20 hours 8 |
| Blood intermediate monocytes | Lifespan ~ 4·3 days 106 | TBC |
| Blood non‐classical monocytes | Lifespan ~ 7·4 days 106 | Half‐life ~ 2·2 days 8 |
| Blood DC | TBC | TBC |
| Langerhans cell |
Donor‐derived cells from hand allograft transplant maintained up to 10 years 68 Langerhans cells are donor‐derived following bone marrow transplant after 3 months 69 , 70 |
Following bone marrow transplant, Langerhans cells remained of recipient origin up to 18 months 185 |
| Microglia | Lifespan ~ 4·2 years and median renewal rate of 28%/year 75 | Estimated ~ 96 days for entire microglia population to self‐renew 186 |
| Alveolar macrophage |
9·4 years post‐lung transplant ~ 73% of alveolar macrophage are recipient‐derived 73 3·5 years post‐lung transplantation > 87% of alveolar macrophages remain donor‐derived 72 |
8 months following bone marrow transplantation, negligible donor‐derived alveolar macrophages observed (1–5%) 187 |
| Liver mononuclear phagocyte | 11 years post‐liver transplant, donor‐derived CD14+ CD16‐ MNP observed 188 | TBC |
| Testicular macrophages | TBC | Long‐lived macrophages with slow turnover measured over 24 weeks 117 |
| Gut macrophages | Macrophages isolated from the duodenum–proximal jejunum are all recipient‐derived by 52 weeks following duodenal transplant 189 | Long‐lived macrophages observed up to 35 weeks within the submucosa and muscularis of ileum 190 |
| Cardiac macrophages | 8·8 years following heart transplant, <1% of CCR2‐ macrophages are recipient‐derived 191 | Cardiac macrophages gradually replaced with age 192 |
In the absence of tissue macrophages, a reduced infiltration of granulocytes has been observed during inflammation, 25 highlighting macrophages as one of the many cell types involved in the initiation of the immune response. Following tissue injury of infection, an interesting phenomenon known as the ‘macrophage disappearance reaction’ occurs, first described in 1963. 77 , 78 This observation has been noted by several groups during experimental peritonitis, where a reduced recovery of resident macrophages was reported. 79 , 80 , 81 This has also been extended to other tissues, such as the lung where a low number of alveolar macrophages are recovered following influenza challenge in mice 82 and the liver where a lower number of Kupffer cells are present during inflammation. 83 , 84 Zhang and colleagues recently showed that macrophages form clots and adhere to tissues accounting for the reduced recovery of these cells, which could be reversed by the use of anticoagulants. 81 Following the resolution of inflammation, the recovery of resident macrophage numbers may occur by proliferation, 85 repopulation by monocyte‐derived cells 8 , 63 , 79 , 86 , 87 , 88 or a combination of both. 89 , 90 As expected, exceptions to the macrophage disappearance paradigm have been observed in T helper cell type 2 immune response, where tissue‐resident macrophages proliferate to combat infection rather than depend on monocyte recruitment. 91
Monocyte kinetics
In contrast to macrophages, monocytes and DC are derived from bone marrow progenitors. Initially, the monocyte and DC progenitor (MDP) was demonstrated to lack neutrophil potential yet give rise to monocytes via the common monocyte progenitor (cMoP). 92 , 93 , 94 Although more recently, the cMoP has been proposed to descend from the granulocyte and macrophage progenitors (GMPs) bypassing the MDP stage in both mice 63 and humans. 95 In this study, the GMP and MDP were suggested to generate monocytes through two pathways, a GMP → cMoP → monocyte pathway and a MDP → monocyte pathway that lack a cMoP intermediate stage. 63 Commitment to monocyte development at the cMoP stage is dependent on the transcription factor IRF8. 96 This is consistent with the observation in patients bearing mutations in IRF8 who are also deficient of circulating monocytes. 1 Recently, a proliferative CXCR4hi CCR2lo transitional pre‐monocyte population was described in mice and humans within the bone marrow, which eventually downregulates CXCR4 and upregulates CCR2, resulting in the egression of mature classical monocytes into the circulation. 97 , 98 , 99
The kinetic profiles of circulating monocyte subsets have been examined in mice with the use of BrdU, where a sequential appearance of labelled classical monocytes and then non‐classical monocytes appears in the circulation. 8 This is owed to the fact that Ly6Chi classical monocytes convert into Ly6Clo non‐classical monocytes. 8 , 63 , 94 , 100 , 101 The half‐life of classical and non‐classical monocytes in mice is estimated at 20 hours and 2·2 days, respectively.
Early insights into the kinetics of human monocytes stemmed from studies nearly fifty years ago with the use of tritiated thymidine where the average monocyte lifespan was estimated at 4·25 days. 102 Deuterated glucose labelling studies proposed a half‐life of 2·2 days for CD14+ classical monocytes. 103 With regard to monocyte subsets, studies have demonstrated differences in the kinetic profiles of circulating CD14+ and CD16+ monocytes in patients following haematopoietic stem cell transplantation. 104 Using deuterium labelling, a sequential appearance of monocyte subsets has been observed within the circulation. 105 , 106 Using mathematical modelling, it was estimated classical monocytes are retained within the bone marrow for approximately 1·6–2 days between the last mitotic division and entry into the circulation 105 , 106 (Figure 2). These cells then circulate for approximately 1 day, whereas non‐classical monocytes circulate for a longer period of time (~7·5 days). 106 These results are akin to those observed in mice, 8 rats 107 and macaques 108 , 109 most likely due to a conserved developmental relationship between monocyte subsets.
Figure 2.
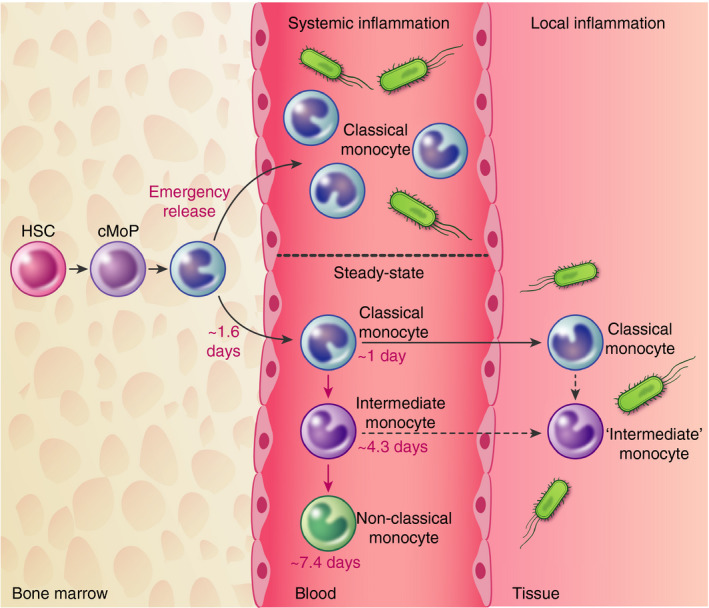
Overview of monocyte kinetics in health and inflammation. Under steady physiological conditions, classical monocytes arise from cMoP and reside within the bone marrow for approximately 1·6 days before being released into the circulation. Once within the circulation, these cells mature into intermediate and then non‐classical monocytes, which circulate for 4 and 7 days, respectively. During systemic inflammation, classical monocytes are rapidly released into the circulation. Following local injury, classical monocytes are initially recruited later followed an intermediate monocyte phenotype, although the origin of the cells is unknown (dashed lines). HSC, haematopoietic stem cell; cMoP, common monocyte progenitor.
Non‐classical monocytes reside within the circulation for a longer period of time possibly due to their function within the circulation where they are constantly monitoring the endothelium for damage. 110 , 111 These cells represent a terminally differentiated monocyte and may consequently even represent a ‘blood macrophage’. 8 Supplemental components encourage non‐classical monocyte survival include CX3CL1 112 and TNF. 113 On the other hand, classical monocytes are continuously recruited to repopulate tissue mononuclear phagocyte compartments, 61 , 63 , 104 , 114 , 115 , 116 , 117 which may explain their shorter circulating lifespan. Liu et al. demonstrate the rate at which monocytes replace tissue macrophages in mice is tissue‐specific, for example kidney macrophages are gradually replaced overtime but to a lesser extent than lung alveolar macrophages or dermal macrophages 63 (Figure 1). The determinants of these rates remain unknown, although the macrophage niche theory proposes a niche is filled by the competition between an embryonic macrophage and a monocyte‐derived cell. 118 Therefore, the differences between tissue microenvironments most likely dictate the rate of macrophage replacement.
In an inflammatory setting, a reduction in the number of circulating monocytes has been observed in both mice and humans, hours following intravenous endotoxin challenge. 44 , 97 , 106 , 119 , 120 The lung is a primary location where activated monocytes can marginate to following challenge. 97 , 121 Given the narrow diameter of lung capillaries, 122 changes in the morphology and size of monocytes following activation may result in hindrances and consequently an increased transit time. However, active retention of monocytes within the lung via CXCR4 retention has also been documented in mice. 97 Targeting margination in the lung may be of clinical relevance as this accumulation of monocytes can promote further lung injury. 121 Following temporary monocytopenia, monocytes can return from various sources. In mice and humans, the spleen has been implicated as a monocyte reservoir, which is deployed in response to specific inflammatory cues. 123 , 124 We and others have shown that bone marrow ‘immature’ classical monocytes are rapidly released into the circulation in response to bacterial 106 and sterile inflammation 124 (Figure 2). More recently, patients with severe COVID‐19 present with immature classical monocytes and neutrophils within the circulation, 49 suggesting this emergency release of monocytes is also apparent in a viral setting.
Intradermal challenges have allowed for the study of immune cell recruitment to the site of infection in response to various stimuli in humans. 23 , 125 , 126 In response to UV‐killed Escherichia coli (E. coli), classical monocytes are observed within the skin as early as 8 hours following challenge 23 (Figure 2). This observation is akin to mice, where Ly6Chi classical monocytes are typically the subset recruited 127 , 128 , 129 possibly via a CCR2‐dependent manner. 99 , 123 , 128 , 130 At later time‐points, a Ly6Clo ‘non‐classical’ phenotype is apparent, but it is thought that this is due to in situ conversion rather than a second wave of monocyte recruitment. 127 , 128 , 129 , 131 , 132 Similarly, in humans, CD16 expression increases over time 23 and may also represent maturation at the site of infection. While it may seem that classical monocytes are the prime effector subset responsible for inflammation and resolution, the use of Nr4a1‐deficient mice 133 , 134 has highlighted a role of non‐classical monocytes in various pathologies including tumour metastasis, 135 Alzheimer’s disease, 136 experimental autoimmune encephalomyelitis (EAE) 137 and vascular homeostasis. 111 The role of non‐classical monocytes has recently been extensively covered. 138 Of note, following the resolution of inflammation, it is thought that tissues return to baseline homeostasis. Whilst this is true at the symptomatic level, immunological processes have been observed to continue in mice where IFN‐γ triggers a second wave of monocyte recruitment creating an immune‐suppressed environment. 139 This may be of importance when considering secondary infections; therefore, targeting this second wave of monocytes may be of clinical relevance.
The question arises whether these recruited inflammatory monocytes engraft into the long‐lived macrophage pool. In models of peritonitis, monocyte‐derived cells persist up to 8 weeks, where their phenotype gradually changes into that of resident macrophages. 8 , 79 Similar observations have been extended to the liver 86 , 140 , 141 , 142 and lung. 87 , 88 , 143 It is possible that tissue residence could alter the longevity of monocytes although, in mouse models of experimental autoimmune encephalomyelitis, monocyte‐derived cells do not contribute to the resident microglia pool 144 yet contribute to pathology, 145 which possibly highlights the microglia as a unique population owed to their unique location within the blood–brain barrier. Whether monocyte‐derived cells exhibit the same function as their resident macrophage counterparts is of key importance. In a mouse model, the engraftment of monocyte‐derived cells into the lung was examined. After ten months, the graft cells showed a very similar transcriptome to alveolar macrophages and only exhibited a difference of 330 differentially expressed genes. 88 In a separate infection study, the replacement of alveolar macrophages with monocyte‐derived cells in response to herpesvirus resulted in protection against house dust mite‐induced asthma compared with mice without initial exposure to herpesvirus. 87 Similar findings have recently been documented, where initial exposure to influenza resulted in subsequent protection from Streptococcus pneumoniae due to the recruitment and engraftment of monocytes to the alveolar niche. 143 These studies demonstrate, in addition to ontogeny, the context in which monocytes are recruited and the type of stimuli may also shape the function of these cells.
Under pathological conditions, monocyte‐like populations have been observed, and YM1+ Ly6Chi monocytes are greatly expanded within the bone marrow, blood and spleen of mice following intravenous lipopolysaccharide (LPS) challenge where these cells exhibit immunoregulatory properties and aid in tissue repair (Ikeda et al., 2018). In the case of fibrosis, a segregated nucleus‐containing atypical Ly6Clo monocyte (SatM) has been documented, although they do not arise from the MDP differentiation route and do not arise from Ly6Chi progenitors (Satoh et al., 2017). Inflammation likely skews ‘healthy’ haematopoiesis; therefore, examining the kinetics of these cells under steady conditions will be the initial step and warrants the need to further investigate the development and kinetics of these cells under pathological conditions.
DC kinetics
A common school of thought has been monocytes are the immature precursor cells to macrophages and DC. However, the identification of the common DC precursor (CDP) that gives rise exclusively to pDC and cDC, but not monocytes, 93 , 146 , 147 challenged this view and established a DC‐dedicated lineage in both rodents and humans. Prior to the generation of cDC, CDP initially gives rise to a cDC precursor (pre‐cDC), 146 , 148 , 149 , 150 , 151 , 152 which can be skewed to pre‐cDC1 or pre‐cDC2 fate depending on the cues present. 96 , 150 , 151 , 153 , 154 , 155 In humans, pre‐cDC can be found within the circulation where they can further mature into cDC. 156 , 157 , 158 Whilst mouse pDCs are released as mature cells from the bone marrow and are thought to be derived mostly from lymphoid progenitors, 159 , 160 , 161 these observations are yet to be confirmed in humans.
DCs are renowned for their ability to stimulate naïve T cells and subsequently bridge the innate and adaptive immune response. Numerous DC subsets have been identified, each interacting with T cells in various ways. DCs are found at smaller numbers in comparison with other cell types, nevertheless a single DC can interact with up to 500 T cells per hour; 162 therefore, their low abundance should not undermine their functional relevance. Though two major cDC subsets are widely acknowledged (DC1 and DC2), further heterogeneity has recently been identified within the DC lineage. 156 , 157 , 163 , 164 , 165 , 166 , 167
BrdU labelling in mice demonstrated a rapid labelling of splenic DC, initially thought to be attributed to the rapid replenishment from circulating DC precursors. 168 However, splenic DCs are also proliferative; therefore, the labelling is likely to represent a combination of both in situ proliferation and blood derivation. 169 Parabiosis studies examined the decay of parabiont‐derived DC in the lymphoid and non‐lymphoid organs and demonstrated DCs are cleared within 10–14 days. 149 , 169 Taking into consideration, DC replenishment from blood precursors, division and cell death, Liu et al. calculated that lymphoid organ cDCs are replenished at a rate of 4,300 cells per hour. This rapid tissue replenishment is supported by the rapid turnover of blood cDC in macaques, where these cells were labelled prior to circulating monocytes. 109 In humans, donor dermal DCs have been identified as early as 18 days following allogeneic haematopoietic cell transplantation and by 56 days were 94% donor‐derived. 170 Similar studies have also demonstrated that human dermal DCs were replaced by donor origin within 40 days. 171 On the contrary, pDCs have a much slower turnover in comparison with cDC in mice 172 and macaques, 109 which is possibly owed to a bias towards cDC production over pDC in the bone marrow. 173 Furthermore, the lymphoid origins of pDC 160 , 161 may also account for the differences in kinetics between myeloid‐derived cDCs.
Akin to monocytes, a reduced number of circulating cDC and pDC have been reported during inflammation in both mice 174 , 175 , 176 and humans. 47 , 177 , 178 , 179 , 180 , 181 The fate of these DCs remains unknown, although DC death is thought to be a factor. 176 An elegant study by Pasquevich and colleagues demonstrated monopoiesis is favoured over DC production following bacterial infection in mice. 182 Following TLR4‐mediated inflammation, these mice had reduced numbers of CDP but elevated numbers of cMoP. It is possible the body increases the availability of monocytes to combat infection at the expense of DC; however, this leads to an immunosuppressive state. Interestingly, the rescue of DC from cell death 176 or increasing DC production via FLT3 ligand 183 reduced the inflammatory‐induced immunosuppression and improved survival in mice. Similarly in humans, the number of circulating DCs correlates with survival from secondary infections in sepsis patients. 177 , 178 Further exploration into DC kinetics in this setting could present a potential therapeutic target. However, given the diversity of dendritic cell subsets, it is first necessary to understand the foundational biology of these cells and their relationship to one another.
Conclusion
Phagocytes play a crucial role in facilitating the immune response, yet little is known about the tightly regulated processes governing the generation, maturation and disappearance of these cells, which allow them to fulfil their functions. Whilst macrophages are considered as a self‐maintaining population, it is clear this is not the case for all tissues. The question arises – what determines the longevity of macrophages? Why are the rates of monocyte replacement variable between tissues? And consequently, what does this mean functionally? Similarly, given the recent expansion of DC diversity in humans, our knowledge regarding the relationship of the cells to one another, in addition to their individual functions, remains limited. This review summarizes both our current understanding and highlights the gaps in our knowledge of one of the foundational aspects of phagocyte biology. By establishing the kinetics and turnover of these cells in addition to the regulatory mechanisms behind, this may allow for therapies to fine‐tune the immune response.
Acknowledgements
AP is supported by Fondation Gustave Roussy (including the Paediatric Campaign). F.G. is supported by Singapore Immunology Network (SIgN) core funding and Singapore National Research Foundation Senior Investigatorship (NRFI) NRF2016NRF‐NRFI001‐02 and is a European Molecular Biology Organization (EMBO) YIP awardee. SY is supported by the Israel Science Foundation Personal Grant (192/20).
Disclosures
No potential competing interest was reported by the authors.
Senior author: Florent Ginhoux & Simon Yona
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- 1. Hambleton S, Salem S, Bustamante J, Bigley V, Boisson‐Dupuis S, Azevedo J, et al. IRF8 mutations and human dendritic‐cell immunodeficiency. N Engl J Med. 2011; 365:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buckley RH. The multiple causes of human SCID. J Clin Invest. 2004; 114:1409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumrah R, Vignesh P, Patra P, Singh A, Anjani G, Saini P, et al. Genetics of severe combined immunodeficiency. Genes Dis. 2020; 7:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salzer E, Santos‐Valente E, Klaver S, Ban SA, Emminger W, Prengemann NK, et al. B‐cell deficiency and severe autoimmunity caused by deficiency of protein kinase C δ. Blood 2013; 121:3112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L, et al. Specification of tissue‐resident macrophages during organogenesis. Science (80‐. ). 2016; 353:aaf4238–aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schulz C, Perdiguero EG, Chorro L, Szabo‐Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science (80‐. ). 2012; 336:86–90. [DOI] [PubMed] [Google Scholar]
- 8. Yona S, Kim K‐W, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013; 38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011; 333:1456–8. [DOI] [PubMed] [Google Scholar]
- 10. Dai X‐M, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony‐stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 2002; 99:111–20. [DOI] [PubMed] [Google Scholar]
- 11. Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, et al. Rescue of the colony‐stimulating factor 1 (CSF‐1)‐nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF‐1 transgene and identification of sites of local CSF‐1 synthesis. Blood 2001; 98:74–84. [DOI] [PubMed] [Google Scholar]
- 12. Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, et al. Role for Spi‐C in the development of red pulp macrophages and splenic iron homeostasis. Nature 2009; 457:318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, Van Isterdael G, et al. Yolk Sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue‐resident macrophages. Immunity 2016; 44:755–68. [DOI] [PubMed] [Google Scholar]
- 14. Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM. Tissue‐resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity 2017; 47:913–927.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood 2005; 105:2449–57. [DOI] [PubMed] [Google Scholar]
- 16. Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003; 34:70–4. [DOI] [PubMed] [Google Scholar]
- 17. Thurlings RM, Wijbrandts CA, Bennink RJ, Dohmen SE, Voermans C, Wouters D, et al. Monocyte scintigraphy in rheumatoid arthritis: the dynamics of monocyte migration in immune‐mediated inflammatory disease. PLoS One 2009; 4:e7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunter J. 1794. A treatise on the blood, inflammation, and gun‐shot wounds Title. London: J. Richardson for G. Nicol. [Google Scholar]
- 19. Lahoz‐Beneytez J, Elemans M, Zhang Y, Ahmed R, Salam A, Block M, et al. Human neutrophil kinetics: Modeling of stable isotope labeling data supports short blood neutrophil half‐lives. Blood 2016; 127:3431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsieh MM, Everhart JE, Byrd‐Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: Age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007; 146:486–92. [DOI] [PubMed] [Google Scholar]
- 21. Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine‐induced endothelial cells via L‐selectin on the rolling Cells. J Exp Med. 1994; 180:1785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013; 13:159–75. [DOI] [PubMed] [Google Scholar]
- 23. Motwani MP, Flint JD, De Maeyer RP, Fullerton JN, Smith AM, Marks DJ, et al. Novel translational model of resolving inflammation triggered by UV‐killed E. coli. J Pathol Clin Res. 2016; 2:154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Oliveira S, Reyes‐Aldasoro CC, Candel S, Renshaw SA, Mulero V, Calado Â. Cxcl8 (IL‐8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J Immunol. 2013; 190:4349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cailhier JF, Partolina M, Vuthoori S, Wu S, Ko K, Watson S, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005; 174:2336–42. [DOI] [PubMed] [Google Scholar]
- 26. Kim ND, Luster AD. The role of tissue resident cells in neutrophil recruitment. Trends Immunol. 2015; 36:547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janela B, Patel AA, Lau MC, Goh CC, Msallam R, Kong WT, et al. A subset of type I conventional dendritic cells controls cutaneous bacterial infections through VEGFα‐mediated recruitment of neutrophils. Immunity. 2019; 50:1069–1083.e8. [DOI] [PubMed] [Google Scholar]
- 28. Evrard M, Kwok IWH, Chong SZ, Teng KWW, Becht E, Chen J, et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018; 48:364–379.e8. [DOI] [PubMed] [Google Scholar]
- 29. Kwok I, Becht E, Xia Y, Ng M, Teh YC, Tan L, et al. Combinatorial single‐cell analyses of granulocyte‐monocyte progenitor heterogeneity reveals an early uni‐potent neutrophil progenitor. Immunity. 2020; 53:303–318.e5. [DOI] [PubMed] [Google Scholar]
- 30. Dinh HQ, Eggert T, Meyer MA, Zhu YP, Olingy CE, Llewellyn R, et al. Coexpression of CD71 and CD117 identifies an early unipotent neutrophil progenitor population in human bone marrow. Immunity. 2020; 53:319–334.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010; 120:2423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, et al. Role of the CXCR4/SDF‐1 chemokine axis in circulating neutrophil homeostasis. Blood 2004; 104:565–71. [DOI] [PubMed] [Google Scholar]
- 33. Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, et al. Cxcr2 and Cxcl5 regulate the IL‐17/G‐CSF axis and neutrophil homeostasis in mice. J Clin Invest. 2012; 122:974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK. Measurement of cell proliferation by heavy water labeling. Nat Protoc. 2007; 2:3045–57. [DOI] [PubMed] [Google Scholar]
- 35. Macallan DC, Asquith B, Zhang Y, de Lara C, Ghattas H, Defoiche J, et al. Measurement of proliferation and disappearance of rapid turnover cell populations in human studies using deuterium‐labeled glucose. Nat Protoc. 2009; 4:1313–27. [DOI] [PubMed] [Google Scholar]
- 36. Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, et al. B cell‐helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012; 13:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casanova‐Acebes M, Pitaval C, Weiss LA, Nombela‐Arrieta C, Chèvre R, González N, Kunisaki Y, Zhang D, van Rooijen N , Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013; 153:1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lehman HK, Segal BH. The role of neutrophils in host defense and disease. J Allergy Clin Immunol. 2020; 145:1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005; 23:197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Athens JW, Haab OP, Raab SO, Mauer AM, Ashenbrucker H, Cartwright GE, et al. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest 1961; 40:989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neves PRDS, Tenório TRDS, Lins TA, Muniz MTC, Pithon‐Curi TC, Botero JP, et al. Acute effects of high‐ and low‐intensity exercise bouts on leukocyte counts. J Exerc Sci Fit. 2015; 13:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peters AM, Saverymuttu SH, Keshavarzian A, Bell RN, Lavender JP. Splenic pooling of granulocytes. Clin Sci. 1985; 68:283–9. [DOI] [PubMed] [Google Scholar]
- 43. Ussov WY, Aktolun C, Myers MJ, Jamar F, Peters AM. Granulocyte margination in bone marrow: Comparison with margination in the spleen and liver. Scand J Clin Lab Invest. 1995; 55:87–96. [DOI] [PubMed] [Google Scholar]
- 44. Tak T, van Groenendael R, Pickkers P, Koenderman L. Monocyte subsets are differentially lost from the circulation during acute inflammation induced by human experimental endotoxemia. J Innate Immun. 2017; 9:464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fullerton JN, Segre E, De Maeyer RPH, Maini AAN, Gilroy DW. Intravenous endotoxin challenge in healthy humans: an experimental platform to investigate and modulate systemic inflammation. J Vis Exp. 2016; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Basran A, Jabeen M, Bingle L, Stokes CA, Dockrell DH, Whyte MKB, et al. Roles of neutrophils in the regulation of the extent of human inflammation through delivery of IL‐1 and clearance of chemokines. J Leukoc Biol. 2013; 93:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jardine L, Wiscombe S, Reynolds G, McDonald D, Fuller A, Green K, et al. Lipopolysaccharide inhalation recruits monocytes and dendritic cell subsets to the alveolar airspace. Nat Commun. 2019; 10:1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Honda T, Uehara T, Matsumoto G, Arai S, Sugano M. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin Chim Acta 2016; 457:46–53. [DOI] [PubMed] [Google Scholar]
- 49. Silvin A, Chapuis N, Dunsmore G, Goubet AG, Dubuisson A, Derosa L, et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID‐19. Cell 2020; 182:1401–1418.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G‐CSF down‐regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006; 108:812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Köhler A, De Filippo K, Hasenberg M, Van Den Brandt C, Nye E, Hosking MP, et al. G‐CSF‐mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood. 2011; 117:4349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gordy C, Pua H, Sempowski GD, He YW. Regulation of steady‐state neutrophil homeostasis by macrophages. Blood. 2011; 117:618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008; 22:3111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martin C, Burdon PCE, Bridger G, Gutierrez‐Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003; 19:583–93. [DOI] [PubMed] [Google Scholar]
- 55. Carrel A, Ebeling AH. The fundamental properties of the fibroblast and the macrophage. J Exp Med. 1926; 44:285–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ebert RH, Florey HW. The extravascular development of the monocyte observed in vivo. Br J Exp Pathol. 1939; 20:342. [Google Scholar]
- 57. van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972; 46:845–52. [PMC free article] [PubMed] [Google Scholar]
- 58. Cline MJ, Moore MA. Embryonic origin of the mouse macrophage. Blood. 1972; 39:842–9. [PubMed] [Google Scholar]
- 59. Takahashi K. Differentiation, maturation, and proliferation macrophages in the mouse yolk sac : of and ultrastructural study. J Leukoc Biol. 1989; 96:87–96. [DOI] [PubMed] [Google Scholar]
- 60. van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968; 128:415–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and adult‐derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014; 40:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue‐resident macrophages self‐maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013; 38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Z, Gu Y, Chakarov S, Bleriot C, Kwok I, Chen X, et al. Fate mapping via Ms4a3‐expression history traces monocyte‐derived cells. Cell. 2019; 178:1509–1525.e19. [DOI] [PubMed] [Google Scholar]
- 64. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014; 14:392–404. [DOI] [PubMed] [Google Scholar]
- 65. Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999; 126:5073–84. [DOI] [PubMed] [Google Scholar]
- 66. Bian Z, Gong Y, Huang T, Lee CZW, Bian L, Bai Z, et al. Deciphering human macrophage development at single‐cell resolution. Nature. 2020; 1–6. [DOI] [PubMed] [Google Scholar]
- 67. Lavin Y, Winter D, Blecher‐Gonen R, David E, Keren‐Shaul H, Merad M, et al. Tissue‐resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014; 159:1312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kanitakis J, Morelon E, Petruzzo P, Badet L, Dubernard JM. Self‐renewal capacity of human epidermal Langerhans cells: Observations made on a composite tissue allograft. Exp Dermatol. 2011; 20:145–6. [DOI] [PubMed] [Google Scholar]
- 69. Mielcarek M, Kirkorian AY, Hackman RC, Price J, Storer BE, Wood BL, et al. Langerhans cell homeostasis and turnover after nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation. Transplantation. 2014; 98:563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Collin MP, Hart DNJ, Jackson GH, Cook G, Cavet J, Mackinnon S, et al. The fate of human Langerhans cells in hematopoietic stem cell transplantation. J Exp Med. 2006; 203:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Volc‐Platzer B, Stingl G, Wolff K, Hinterberg W, Schnedl W. Cytogenetic identification of allogeneic epidermal langerhans cells in a bone‐marrow–graft recipient. N Engl J Med. 1984; 310:1123–4. [DOI] [PubMed] [Google Scholar]
- 72. Nayak DK, Zhou F, Xu M, Huang J, Tsuji M, Hachem R, et al. Long‐term persistence of donor alveolar macrophages in human lung transplant recipients that influences donor‐specific immune responses. Am J Transplant. 2016; 16:2300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Byrne AJ, Powell JE, O’Sullivan BJ, Ogger PP, Hoffland A, Cook J, et al. Dynamics of human monocytes and airway macrophages during healthy aging and after transplant. J Exp Med. 2020; 217 3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science (80‐. ) 1976; 192:1016–8. [DOI] [PubMed] [Google Scholar]
- 75. Réu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 2017; 20:779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell. 2005; 122:133–43. [DOI] [PubMed] [Google Scholar]
- 77. Barth MW, Hendrzak JA, Melnicoff MJ, Morahan PS. Review of the macrophage disappearance reaction. J. Leukoc Biol. 1995; 57:361–7. [DOI] [PubMed] [Google Scholar]
- 78. Nelson DS. Reaction to antigens in vivo of the peritoneal macrophages of guinea‐pigs with delayed type hypersensitivity. Effects of anticoagulants and other drugs. Lancet. 1963; 282:175–6. [DOI] [PubMed] [Google Scholar]
- 79. Newson J, Stables M, Karra E, Arce‐Vargas F, Quezada S, Motwani M, et al. Resolution of acute inflammation bridges the gap between innate and adaptive immunity. Blood. 2014; 124:1748–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Davies LC, Rosas M, Jenkins SJ, Liao C‐T, Scurr MJ, Brombacher F, et al. Distinct bone marrow‐derived and tissue‐resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013; 4:1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang N, Czepielewski RS, Jarjour NN, Erlich EC, Esaulova E, Saunders BT, et al. Expression of factor V by resident macrophages boosts host defense in the peritoneal cavity. J Exp Med. 2019; 216:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lauder SN, Taylor PR, Clark SR, Evans RL, Hindley JP, Smart K, et al. Paracetamol reduces influenza‐induced immunopathology in a mouse model of infection without compromising virus clearance or the generation of protective immunity. Thorax 2011; 66:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zigmond E, Samia‐Grinberg S, Pasmanik‐Chor M, Brazowski E, Shibolet O, Halpern Z, et al. Infiltrating monocyte‐derived macrophages and resident Kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014; 193:344–53. [DOI] [PubMed] [Google Scholar]
- 84. Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly‐6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA 2012; 109:E3186–E3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol. 2011; 41:2155–64. [DOI] [PubMed] [Google Scholar]
- 86. Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver‐resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type‐2‐mediated tissue repair during bacterial infection. Immunity. 2015; 42:145–58. [DOI] [PubMed] [Google Scholar]
- 87. Machiels B, Dourcy M, Xiao X, Javaux J, Mesnil C, Sabatel C, et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat Immunol. 2017; 18:1310–20. [DOI] [PubMed] [Google Scholar]
- 88. Misharin AV, Morales‐Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie‐Pimentel AC, et al. Monocyte‐derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017; 214:2387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, et al. Bone marrow‐derived monocytes give rise to self‐renewing and fully differentiated Kupffer cells. Nat Commun. 2016; 7:10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bonnardel J, T’Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019; 51:638–654.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011; (80‐. ). 332:1284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bajaña S, Roach K, Turner S, Paul J, Kovats S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J Immunol. 2012; 189:3368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee J, Breton G, Oliveira TYK, Zhou YJ, Aljoufi A, Puhr S, et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med. 2015; 212:385–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hettinger J, Richards DM, Hansson J, Barra MM, Joschko A‐CC, Krijgsveld J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013; 14:821–30. [DOI] [PubMed] [Google Scholar]
- 95. Kawamura S, Onai N, Miya F, Sato T, Tsunoda T, Kurabayashi K, et al. Identification of a human clonogenic progenitor with strict monocyte differentiation potential: a counterpart of mouse cMoPs. Immunity. 2017; 46:835–848.e4. [DOI] [PubMed] [Google Scholar]
- 96. Sichien D, Scott CL, Martens L, Vanderkerken M, Van Gassen S, Plantinga M, et al. IRF8 Transcription factor controls survival and function of terminally differentiated conventional and plasmacytoid dendritic cells, respectively. Immunity. 2016; 45:626–40. [DOI] [PubMed] [Google Scholar]
- 97. Chong SZ, Evrard M, Devi S, Chen J, Lim JY, See P, et al. CXCR4 identifies transitional bone marrow premonocytes that replenish the mature monocyte pool for peripheral responses. J Exp Med. 2016; 213:2293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jung H, Mithal DS, Park JE, Miller RJ. Localized CCR2 activation in the bone marrow niche mobilizes monocytes by desensitizing CXCR4. PLoS One. 2015; 10:e0128387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006; 7:311–7. [DOI] [PubMed] [Google Scholar]
- 100. Mildner A, Schönheit J, Giladi A, David E, Lara‐Astiaso D, Lorenzo‐Vivas E, et al. Genomic characterization of murine monocytes reveals C/EBPβ transcription factor dependence of Ly6C − cells. Immunity. 2017; 46:849–862.e7. [DOI] [PubMed] [Google Scholar]
- 101. Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007; 204:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Whitelaw DM. Observations on human monocyte kinetics after pulse labelling. Cell Prolif. 1972; 5:311–7. [DOI] [PubMed] [Google Scholar]
- 103. Mohri H, Perelson AS, Tung K, Ribeiro RM, Ramratnam B, Markowitz M, et al. Increased turnover of T lymphocytes in HIV‐1 infection and its reduction by antiretroviral therapy. J Exp Med. 2001; 194:1277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E, et al. Human dermal CD14+ cells are a transient population of monocyte‐derived macrophages. Immunity. 2014; 41:465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tak T, Drylewicz J, Conemans L, de Boer RJ, Koenderman L, Borghans JAM, et al. Circulatory and maturation kinetics of human monocyte subsets in vivo. Blood. 2017; 130:1474–7. [DOI] [PubMed] [Google Scholar]
- 106. Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017; 214:1913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady‐state conditions. J Immunol. 2006; 176:4155–62. [DOI] [PubMed] [Google Scholar]
- 108. Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010; 6:e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sugimoto C, Hasegawa A, Saito Y, Fukuyo Y, Chiu KB, Cai Y, et al. Differentiation kinetics of blood monocytes and dendritic cells in macaques: insights to understanding human myeloid cell development. J Immunol. 2015; 195:1774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Auffray C, Fogg D, Garfa M, Elain G, Join‐Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007; 317:666–70. [DOI] [PubMed] [Google Scholar]
- 111. Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay‐Barrena G, et al. Nr4a1‐dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013; 153:362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Landsman L, Bar‐On L, Zernecke A, Kim K‐W, Krauthgamer R, Shagdarsuren E, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009; 113:963–72. [DOI] [PubMed] [Google Scholar]
- 113. Wolf Y, Shemer A, Polonsky M, Gross M, Mildner A, Yona S, et al. Autonomous TNF is critical for in vivo monocyte survival in steady state and inflammation. J Exp Med. 2017; 214:905–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bain CC, Bravo‐Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014; 15:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006; 176:3578–84. [DOI] [PubMed] [Google Scholar]
- 116. Kim K‐W, Williams JW, Wang Y‐T, Ivanov S, Gilfillan S, Colonna M, et al. MHC II + resident peritoneal and pleural macrophages rely on IRF4 for development from circulating monocytes. J Exp Med. 2016; 213:1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mossadegh‐Keller N, Gentek R, Gimenez G, Bigot S, Mailfert S, Sieweke MH. Developmental origin and maintenance of distinct testicular macrophage populations. J Exp Med. 2017; 214:2829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Guilliams M, Scott CL. Does niche competition determine the origin of tissue‐resident macrophages? Nat Rev Immunol. 2017; 17:451–60. [DOI] [PubMed] [Google Scholar]
- 119. Thaler B, Hohensinner PJ, Krychtiuk KA, Matzneller P, Koller L, Brekalo M, et al. Differential in vivo activation of monocyte subsets during low‐grade inflammation through experimental endotoxemia in humans. Sci Rep. 2016; 6:30162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shi C, Jia T, Mendez‐Ferrer S, Hohl TM, Serbina NV, Lipuma L, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll‐like receptor ligands. Immunity. 2011; 34:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. O’Dea KP, Wilson MR, Dokpesi JO, Wakabayashi K, Tatton L, van Rooijen N, et al. Mobilization and margination of bone marrow Gr‐1high monocytes during subclinical endotoxemia predisposes the lungs toward acute injury. J Immunol. 2009; 182:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Doerschuk CM, Beyers N, Coxson HO, Wiggs B, Hogg JC. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol. 1993; 74:3040–5. [DOI] [PubMed] [Google Scholar]
- 123. Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez‐Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science (80‐. ) 2009; 325:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. van der Laan AM, ter Horst EN, Delewi R, Begieneman MPV, Krijnen PAJ, Hirsch A, et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2014; 35:376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jenner W, Motwani M, Veighey K, Newson J, Audzevich T, Nicolaou A, et al. Characterisation of leukocytes in a human skin blister model of acute inflammation and resolution. PLoS One. 2014; 9:e89375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Eguíluz‐Gracia I, Bosco A, Dollner R, Melum GR, Lexberg MH, Jones AC, et al. Rapid recruitment of CD14 + monocytes in experimentally induced allergic rhinitis in human subjects. J Allergy Clin Immunol. 2016; 137:1872–1881.e12. [DOI] [PubMed] [Google Scholar]
- 127. Hilgendorf I, Gerhardt LMS, Tan TC, Winter C, Holderried TAW, Chousterman BG, et al. Ly‐6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014; 114:1611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6Chi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen‐presenting cells. Immunity. 2012; 37:1076–90. [DOI] [PubMed] [Google Scholar]
- 129. Dal‐Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CHY, Petri B, et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2 + monocytes at a site of sterile injury. J Exp Med. 2015; 212:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, et al. Critical roles for CCR2 and MCP‐3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007; 117:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Arnold L, Henry A, Poron F, Baba‐Amer Y, Van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007; 204:1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Avraham‐Davidi I, Yona S, Grunewald M, Landsman L, Cochain C, Silvestre JS, et al. On‐site education of VEGF‐recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J Exp Med. 2013; 210:2611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C− monocytes. Nat Immunol. 2011; 12:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Thomas GD, Hanna RN, Vasudevan NT, Hamers AA, Romanoski CE, McArdle S, et al. Deleting an Nr4a1 super‐enhancer subdomain ablates ly6clow monocytes while preserving macrophage gene function. Immunity. 2016; 45:975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, et al. Patrolling monocytes control tumor metastasis to the lung. Science (80‐. ) 2015; 350:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Michaud J‐P, Bellavance M‐A, Préfontaine P, Rivest S. real‐time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 2013; 5:646–53. [DOI] [PubMed] [Google Scholar]
- 137. Shaked I, Hanna RN, Shaked H, Chodaczek G, Nowyhed HN, Tweet G, et al. Transcription factor Nr4a1 couples sympathetic and inflammatory cues in CNS‐recruited macrophages to limit neuroinflammation. Nat Immunol. 2015; 16:1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Narasimhan PB, Marcovecchio P, Hamers AA, Hedrick CC. Nonclassical monocytes in health and disease. Annu Rev Immunol. 2019; 37:439–56. [DOI] [PubMed] [Google Scholar]
- 139. Newson J, Motwani MP, Kendall AC, Nicolaou A, Muccioli GG, Alhouayek M, et al. Inflammatory resolution triggers a prolonged phase of immune suppression through COX‐1/mPGES‐1‐derived prostaglandin E2. Cell Rep. 2017; 20:3162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Seidman JS, Troutman TD, Sakai M, Gola A, Spann NJ, Bennett H, et al. Niche‐specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity. 2020; 52:1057–1074.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Remmerie A, Martens L, Thoné T, Castoldi A, Seurinck R, Pavie B, et al. Osteopontin expression identifies a subset of recruited macrophages distinct from kupffer cells in the fatty liver. Immunity. 2020; 53:641–657.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tran S, Baba I, Poupel L, Dussaud S, Moreau M, Gélineau A, et al. Impaired Kupffer cell self‐renewal alters the liver response to lipid overload during non‐alcoholic steatohepatitis. Immunity. 2020; 53:627–640.e5. [DOI] [PubMed] [Google Scholar]
- 143. Aegerter H, Kulikauskaite J, Crotta S, Patel H, Kelly G, Hessel EM, et al. Influenza‐induced monocyte‐derived alveolar macrophages confer prolonged antibacterial protection. Nat Immunol. 2020; 21:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FMV. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011; 14:1142–50. [DOI] [PubMed] [Google Scholar]
- 145. Giladi A, Wagner LK, Li H, Dörr D, Medaglia C, Paul F, et al. Cxcl10 + monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat Immunol. 2020; 21:525–34. [DOI] [PubMed] [Google Scholar]
- 146. Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009; 324:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Onai N, Obata‐Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG Identification of clonogenic common Flt3+M‐CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007; 8:1207–16. [DOI] [PubMed] [Google Scholar]
- 148. Donnenberg VS, O’Connell PJ, Logar AJ, Zeevi A, Thomson AW, Donnenberg AD. Rare‐event analysis of circulating human dendritic cell subsets and their presumptive mouse counterparts. Transplantation 2001; 72:1946–51. [DOI] [PubMed] [Google Scholar]
- 149. Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, et al. The origin and development of nonlymphoid tissue CD103 + DCs. J Exp Med. 2009; 206:3115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Naik SH, Sathe P, Park H‐Y, Metcalf D, Proietto AI, Dakic A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007; 8:1217–26. [DOI] [PubMed] [Google Scholar]
- 151. Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, et al. Intrasplenic steady‐state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006; 7:663–71. [DOI] [PubMed] [Google Scholar]
- 152. O’Keeffe M, Hochrein H, Vremec D, Scott B, Hertzog P, Tatarczuch L, et al. Dendritic cell precursor populations of mouse blood: Identification of the murine homologues of human blood plasmacytoid pre‐DC2 and CD11c+ DC1 precursors. Blood. 2003; 101:1453–9. [DOI] [PubMed] [Google Scholar]
- 153. Cabeza‐Cabrerizo M, van Blijswijk J, Wienert S, Heim D, Jenkins RP, Chakravarty P, et al. Tissue clonality of dendritic cell subsets and emergency DCpoiesis revealed by multicolor fate mapping of DC progenitors. Sci Immunol. 2019; 4:eaaw1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Grajales‐Reyes GE, Iwata A, Albring J, Wu X, Tussiwand R, Wumesh K, et al. Batf3 maintains autoactivation of Irf8 for commitment of a CD8α+ conventional DC clonogenic progenitor. Nat Immunol. 2015; 16:708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Schlitzer A, Sivakamasundari V, Chen J, Bin Sumatoh HR, Schreuder J, Lum J, et al. Identification of cDC1‐ and cDC2‐committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol. 2015; 16:718–28. [DOI] [PubMed] [Google Scholar]
- 156. See P, Dutertre C‐A, Chen J, Günther P, McGovern N, Irac SE, et al. Mapping the human DC lineage through the integration of high‐dimensional techniques. Science. 2017; 356:eaag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Villani A‐C, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single‐cell RNA‐seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science (80‐. ). 2017; 356:eaah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Breton G, Lee J, Zhou YJ, Schreiber JJ, Keler T, Puhr S, et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J Exp Med. 2015; 212:401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Onai N, Kurabayashi K, Hosoi‐Amaike M, Toyama‐Sorimachi N, Matsushima K, Inaba K, et al. A clonogenic progenitor with prominent plasmacytoid dendritic cell developmental potential. Immunity. 2013; 38:943–57. [DOI] [PubMed] [Google Scholar]
- 160. Rodrigues PF, Alberti‐Servera L, Eremin A, Grajales‐Reyes GE, Ivanek R, Tussiwand R. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nat Immunol. 2018; 19:711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dress RJ, Dutertre CA, Giladi A, Schlitzer A, Low I, Shadan NB, et al. Plasmacytoid dendritic cells develop from Ly6D+ lymphoid progenitors distinct from the myeloid lineage. Nat Immunol. 2019; 20:852–64. [DOI] [PubMed] [Google Scholar]
- 162. Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003; 4:579–85. [DOI] [PubMed] [Google Scholar]
- 163. Dutertre C‐A, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, et al. Single‐cell analysis of human mononuclear phagocytes reveals subset‐defining markers and identifies circulating inflammatory dendritic cells. Immunity. 2019; 51:573–589.e8. [DOI] [PubMed] [Google Scholar]
- 164. Bourdely P, Anselmi G, Vaivode K, Ramos RN, Missolo‐Koussou Y, Hidalgo S, et al. Transcriptional and functional analysis of CD1c+ human dendritic cells identifies a CD163+ subset priming CD8+CD103+ T cells. Immunity. 2020; 53:335–352.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Cytlak U, Resteu A, Pagan S, Green K, Milne P, Maisuria S, et al. Differential IRF8 transcription factor requirement defines two pathways of dendritic cell development in humans. Immunity. 2020; 53:353–370.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bosteels C, Neyt K, Vanheerswynghels M, Hammad H, Guilliams M, Lambrecht BN, et al. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity. 2020; 52:1039–1056.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Brown CC, Gudjonson H, Pritykin Y, Leslie C, Pe’er D, and Rudensky AY. Transcriptional basis of mouse and human dendritic cell heterogeneity in brief single‐cell analyses of dendritic cells reveals new subsets with distinct pro‐and anti‐inflammatory potential. Cell. 2019; 179:846–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002; 100:1734–41. [PubMed] [Google Scholar]
- 169. Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007; 8:578–83. [DOI] [PubMed] [Google Scholar]
- 170. Auffermann‐Gretzinger S, Eger L, Bornhäuser M, Schäkel K, Oelschlaegel U, Schaich M, et al. Fast appearance of donor dendritic cells in human skin: Dynamics of skin and blood dendritic cells after allogeneic hematopoietic cell transplantation. Transplantation. 2006; 81:866–73. [DOI] [PubMed] [Google Scholar]
- 171. Haniffa M, Ginhoux F, Wang X, Bigley V, Abel M, Dimmick I, et al. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med. 2009; 206:371–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. O’Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, et al. Mouse plasmacytoid cells: long‐lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J Exp Med. 2002; 196:1307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Pelayo R, Hirose J, Huang J, Garrett KP, Delogu A, Busslinger M, et al. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005; 105:4407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ding Y, Chung C‐S, Newton S, Chen Y, Carlton S, Albina JE, et al. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock. 2004; 22:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Efron PA, Martins A, Minnich D, Tinsley K, Ungaro R, Bahjat FR, et al. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J Immunol. 2004; 173:3035–43. [DOI] [PubMed] [Google Scholar]
- 176. Gautier EL, Huby T, Saint‐Charles F, Ouzilleau B, Chapman MJ, Lesnik P. Enhanced dendritic cell survival attenuates lipopolysaccharide‐induced immunosuppression and increases resistance to lethal endotoxic shock. J Immunol. 2008; 180:6941–6. [DOI] [PubMed] [Google Scholar]
- 177. Grimaldi D, Louis S, Pène F, Sirgo G, Rousseau C, Claessens YE, et al. Profound and persistent decrease of circulating dendritic cells is associated with ICU‐acquired infection in patients with septic shock. Intensive Care Med. 2011; 37:1438–46. [DOI] [PubMed] [Google Scholar]
- 178. Guisset O, Dilhuydy M‐S, Thiébaut R, Lefèvre J, Camou F, Sarrat A, et al. Decrease in circulating dendritic cells predicts fatal outcome in septic shock. Intensive Care Med. 2007; 33:148–52. [DOI] [PubMed] [Google Scholar]
- 179. Poehlmann H, Schefold JC, Zuckermann‐Becker H, Volk H‐D, Meisel C. Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit Care. 2009; 13:R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Riccardi F, Della Porta MG, Rovati B, Casazza A, Radolovich D, De Amici M, et al. Flow cytometric analysis of peripheral blood dendritic cells in patients with severe sepsis. Cytom Part B Clin Cytom. 2011; 80B:14–21. [DOI] [PubMed] [Google Scholar]
- 181. Vittorakis S, Samitas K, Tousa S, Zervas E, Aggelakopoulou M, Semitekolou M, et al. Circulating conventional and plasmacytoid dendritic cell subsets display distinct kinetics during in vivo repeated allergen skin challenges in atopic subjects. Biomed Res Int. 2014; 2014:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Pasquevich KA, Bieber K, Günter M, Grauer M, Pötz O, Schleicher U, et al. Innate immune system favors emergency monopoiesis at the expense of DC‐differentiation to control systemic bacterial infection in mice. Eur J Immunol. 2015; 45:2821–33. [DOI] [PubMed] [Google Scholar]
- 183. Toliver‐Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by Fms‐like tyrosine kinase‐3 ligand increases the resistance of mice to a burn wound infection. J Immunol. 2005; 174:404–10. [DOI] [PubMed] [Google Scholar]
- 184. Pillay J, Den Braber I, Vrisekoop N, Kwast LM, De Boer RJ, Borghans JAM, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010; 116:625–7. [DOI] [PubMed] [Google Scholar]
- 185. Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady‐state conditions. Nat Immunol. 2002; 3:1135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Askew K, Li K, Olmos‐Alonso A, Garcia‐Moreno F, Liang Y, Richardson P, et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 2017; 18:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life‐span of alveolar macrophages. Am J Respir Cell Mol Biol. 2008; 38:380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Pallett LJ, Burton AR, Amin OE, Rodriguez‐Tajes S, Patel AA, Zakeri N, et al. Longevity and replenishment of human liver‐resident memory T cells and mononuclear phagocytes. J Exp Med. 2020; 217 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bujko A, Atlasy N, Landsverk OJB, Richter L, Yaqub S, Horneland R, et al. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J Exp Med. 2018; 215:441–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. De Schepper S, Verheijden S, Aguilera‐Lizarraga J, Viola MF, Boesmans W, Stakenborg N, et al. Self‐maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018; 175:400–415.e13. [DOI] [PubMed] [Google Scholar]
- 191. Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018; 24:1234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, et al. Progressive replacement of embryo‐derived cardiac macrophages with age. J Exp Med. 2014; 211:2151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


