Abstract
Background
Catheter infections remain one of the most persistent adverse events causing significant morbidity, economic impact and mortality. Several strategies have been proposed to reduce these infections including the use of catheters embedded with antibiotics and/or antiseptics. One reoccurring challenge is the fear that antimicrobial medical devices will induce resistance. The aim of this systematic review is to evaluate the evidence for induced antimicrobial resistance caused by exposure to antimicrobial medical devices.
Methods
Four electronic databases [MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Scopus] were screened for studies published between 1983 and 2019 regarding assessment of microbial resistance with use of medical devices containing chlorhexidine, minocycline, rifampicin or combinations thereof. Development of new resistance, selection for tolerant organisms and ‘no change in resistance’ were assessed.
Results
Forty-four publications, grouped by study type and stratified by drug assessed, were included for analyses. The majority of studies found no change in resistance after exposure to antimicrobial medical devices (13 in vitro, 2 in vivo, 20 clinical). Development of new resistance was commonly reported with the use of rifampicin as a single agent and only reported in one study assessing the minocycline/rifampicin combination (M/R); however, the increase in MIC was well below clinical relevance.
Conclusions
Emergence of new resistance to combinations of M/R, minocycline/rifampicin/chlorhexidine (M/R/CH) and chlorhexidine/silver sulfadiazine (CHXSS) was rare. No clinical trials confirmed its occurrence and some refuted it. The risk of development of new resistance to these antimicrobial combinations appears more fear-based than substantiated by clinical and experimental evidence but warrants continued surveillance.
Introduction
Central line-associated bloodstream infections (CLABSIs) remain one of the most persistent post-insertion adverse events causing significant morbidity as well as substantial economic impact and mortality.1 Central lines coated with antimicrobial agents were introduced to reduce the risk of CLABSIs.2 While using antimicrobial catheters can potentially reduce the risk of CLABSI, their use introduces other risks to patients.3,4 These include irritation and inflammatory responses to the antimicrobial agents, allergic reactions to the antimicrobial agents, breakthrough infections by virulent organisms against which the antimicrobial agents have limited effectiveness and the induction of antimicrobial resistance through prolonged exposure to the antimicrobial agents on the catheters. Allergic reactions by patients to the agents in the antimicrobial central lines (most commonly chlorhexidine) have been rare and doses of antimicrobial agents on catheters have been titrated to levels generally producing acceptably biocompatible responses following contact with the antimicrobial agents on the devices.5 Antimicrobial resistance is the result of organisms defensively adapting to exposure to subinhibitory or sublethal doses of antimicrobial agents and developing defensive mechanisms whereby the microbes are able to thwart and render ineffective the mechanisms of action of the antimicrobial agents.6 The consequence of antimicrobial resistance is that microbes may become more virulent and, when they cause infections, there may be fewer and potentially more toxic antimicrobial drugs that are available to treat the infections.
Two antimicrobial catheter treatments that are combinations of different agents have been widely studied and have been recommended by the CDC at the level of category 1A. One is a triple combination of two antiseptic agents and one antibiotic, specifically chlorhexidine/silver sulfadiazine (CHXSS), and the other is a combination of two antibiotics, specifically minocycline and rifampicin (M/R).7 Studies on the first-generation CHXSS catheter, performed at a time before rigorous hygienic insertion practices were widely adopted, demonstrated significant reduction in CLABSIs. Subsequent large prospective randomized clinical trials (RCTs) conducted following the adoption of modern insertion practices with a second-generation CHXSS catheter, having significantly higher chlorhexidine content, have repeatedly failed to significantly reduce CLABSIs.8–10 A peripherally inserted central catheter (PICC) containing chlorhexidine as a sole antimicrobial agent similarly failed to reduce CLABSIs in a randomized prospective trial when compared with a non-antimicrobial PICC.11 Several meta-analyses have reinforced the ineffectiveness of CHXSS catheters in preventing CLABSI.12 Nevertheless, chlorhexidine-based catheters remain widely used. In contrast, the M/R catheter has significantly reduced CLABSIs in multiple randomized prospective clinical trials and several meta-analyses have further reinforced the effectiveness of the M/R combination in reducing CLABSIs.12 The enhanced antimicrobial activity from combining chlorhexidine with M/R on catheters has been reported and the combination proposed for future use.13 We therefore focus in this systematic review on the evidence for induced antimicrobial resistance caused by exposure to catheters and other medical devices containing chlorhexidine, minocycline, rifampicin and combinations thereof because of the prevalence of their use and extensive history of published in vitro, in vivo and clinical studies.
Methods
Search strategy
Four electronic databases [MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Scopus] were searched for studies assessing resistance to rifampicin, minocycline or chlorhexidine that were published between 1983 and February 2019. Search terms were mapped to the MeSH headings (MEDLINE) and Emtree terms (Embase). The following search string was used: (rifampin OR rifampicin OR minocycline OR chlorhexidine) AND (central venous catheter OR catheters OR catheters, indwelling OR vascular access devices OR catheterization, central venous OR CVC OR catheterization, peripheral OR PICC OR bandages OR bandages, hydrocolloid OR biological dressings OR occlusive dressings OR prosthesis and implants OR dental implants OR penile prosthesis OR pacemaker, artificial OR pacemaker OR catheter dressing OR dressing OR penile implant OR disinfection OR wipes) AND (drug resistance OR drug resistance, microbial OR drug resistance, bacterial OR drug resistance, fungal OR resistance OR antibiotic resistance OR emerging antibiotic resistance).
Search results were first screened by title and abstract by two independent reviewers. Any disagreements were discussed by authors to an agreed-upon consensus. Resulting articles were read for full-text review and data abstraction. Relevant references cited in primary literature were also screened to be included in analyses. Manuscripts were excluded if they were in a language other than English, were conference abstracts, did not assess the drug combination of interest (minocycline, rifampicin, chlorhexidine or combination thereof), did not assess a medical device (i.e. systemic use of drug agents), did not directly assess development of resistance or were a descriptive case series. Literature reviews were also excluded from the primary assessment and will be summarized independently.
Data abstraction
All primary literature to be abstracted were first classified into in vitro, in vivo or clinical study types. Manuscripts with more than one study type had data extracted from each type. All data abstracted from each manuscript were recorded electronically in the data abstraction form (DAF). Data from the DAF were then organized by spreadsheet for assessment. Quality control was assessed periodically to ensure accurate transfer of abstracted data. Data, including (i) study objective; (ii) device assessed; (iii) drug assessed; (iv) method for drug attachment to device; (v) method for assessing resistance; (vi) results; and (vii) conclusions were collected from each study type. Data from in vitro studies also included organisms assessed (challenge organisms) and their antimicrobial resistance profiles. Data abstraction pertaining to in vivo animal models included species and number of animals tested as well as organisms assessed (challenge organisms) and their antimicrobial resistance profiles. Clinical studies included data abstraction for study design (retrospective, prospective, case–control or RCT) and objective, number of patients assessed, causative organisms being treated with the antimicrobial device and their resistance profiles.
Definitions
Based on authors’ conclusions regarding the potential for developing resistance after exposure to antimicrobial devices, each manuscript was categorized as follows:
No change in (antimicrobial) resistance
Selection for (antimicrobial agent-) tolerant strains (clinical studies only)
Development of new (antimicrobial) resistance
(Antimicrobial) resistance not assessed
‘No change in antimicrobial resistance’ was defined as no or inconsequential shift in MIC after use of antimicrobial devices. ‘Selection for antimicrobial agent-tolerant strains’ was defined as an increasing shift in MIC for the single agent or combination of agents; however, MIC remained below the threshold for clinical susceptibility (i.e. below CLSI cut-offs for resistance).14 ‘Development of new antimicrobial resistance’ was defined as a clinically consequential shift in MIC to concentrations above the CLSI resistance concentration cut-off for clinical susceptibility.
Quality assessment
To assess quality and bias in each manuscript, any critique to study design, methodology or conclusions based on presented data was also recorded in the DAF. Clinical studies were assessed for bias using the National Heart, Lung, and Blood Institute Study Quality Assessment Tools (2018).15 Clinical studies were stratified by study type (observational, case–control and controlled intervention trial) and then scored based on the questions examining various reporting measures. Observational studies were scored out of 12 points and had questions focused on study objective, population, exposures of interest and validated measurements of outcome. Case–control studies were scored out of 13 points and had questions focused on study objective, population, selection of cases and controls, exposures of interest and validated measurements of outcome. Clinical intervention trials were scored out of 14 points and had questions focused on study objective, randomization, blinding, interventions and adherence to intervention protocols, sample size and validated measurements of outcome.
For in vitro and in vivo studies, articles were assessed for bias using the Animal Research: Reporting in vivo experiments (ARRIVE) guidelines.16 While the ARRIVE guidelines were originally developed for in vivo studies, they have been adapted and assessed for quality criteria in in vitro studies.17In vitro studies were scored out of 19 and had questions pertaining to reporting of study objective and design, experimental procedure, use of appropriate controls, validated measurements of outcome and analyses. In vivo studies were scored out of 23 and had questions pertaining to reporting of study objective and design, experimental procedure, animal husbandry, exposures and controls, blinding, validated measurements of outcome and analyses.
Finally, all studies were graded with a score of A–D on methodology used for assessment of examining development of resistance. Articles were scored as follows:
Grade A if the article described AND referenced a validated model for assessing resistance such as CLSI or EUCAST methods for MIC or MBC.
Grade B if the article either described OR referenced a validated model for assessing resistance.
Grade C if the article assessed resistance with a method other than a microbiologically validated model (i.e. presence of resistance genes) or resistance was assessed only from the hospital record.
Grade D if the article did not describe or reference any method for resistance (i.e. only stated that resistance was assessed).
Results
Search strategy
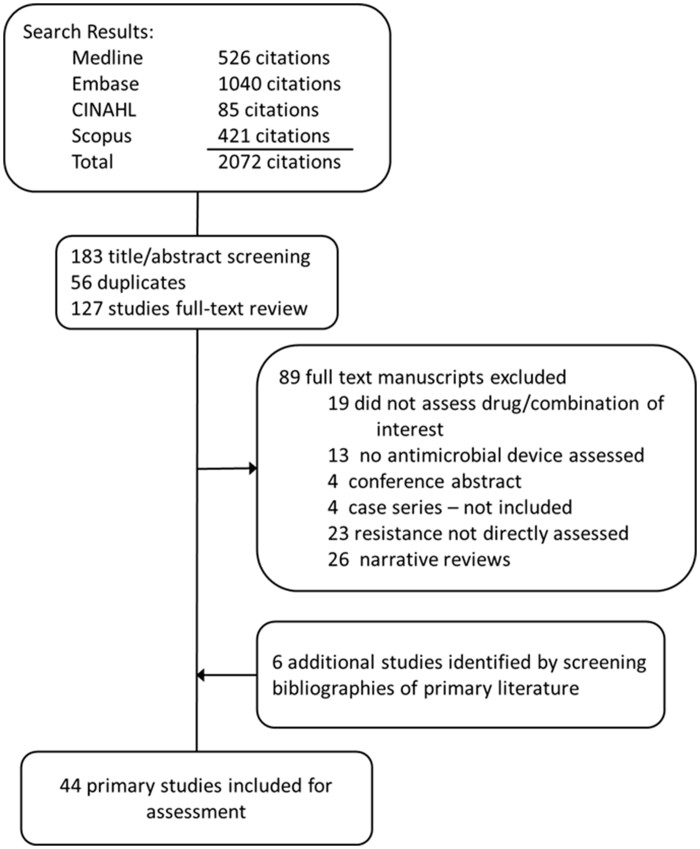
Searches in MEDLINE, Embase, CINAHL and Scopus identified 526, 1040, 85 and 421 studies, respectively, for a total 2072 citations. Title and abstract screening identified 183 studies, 66 of which were duplicates, resulting in a total of 127 studies for full-text review. During full-text review, 19 studies were excluded because they did not assess one of the target drugs or drug combination, 13 studies were excluded because no antimicrobial device was assessed, 4 were excluded because they were conference abstracts/proceedings that did not contain complete data for analyses, 4 studies were excluded because they were descriptive case series and 23 were excluded because they didn’t directly assess development of resistance. These studies typically reported whether organisms broke through with the use of an antimicrobial device, indicating that the antimicrobial device was not efficacious, not whether organisms developed resistance. Though CHXSS is a drug combination of chlorhexidine and silver sulfadiazine, which is not specifically our drug combination of interest (chlorhexidine, minocycline and rifampicin), it was included for assessing the potential for development of resistance against chlorhexidine combinations. Additionally, 26 manuscripts were identified as literature reviews and will be summarized independently. An additional six studies were identified by screening bibliographies of primary literature and were included in analyses. A total of 44 publications were included for qualitative assessment of the potential for developing new resistance after being exposed to antimicrobial medical devices (Figure 1).
Figure 1.
PRISMA flow chart for identification of primary literature included in review.
Study characteristics
Characteristics and results of all in vitro studies included are summarized in Table 1, in vivo studies in Table 2 and clinical studies in Table 3.
Table 1.
In vitro assessment of potential resistance to rifampicin, minocycline and/or chlorohexidine
| Citation | Drug | Device | Concentration(s) | Organisms assessed | Methodology; resistance measurement | Results | After contact with the device, which of the following does the study show: (i) no change in resistance; or (ii) development of new resistance |
|---|---|---|---|---|---|---|---|
| Rifampicin (n = 4) | |||||||
| Bayston et al., 200920 | R | peritoneal catheter | 0.2% | SE | Growth within ZOI during serial plate transfer; MIC Etest | MIC of SE increased from 0.008 to >32 mg/L after 9 days exposure to 0.2% R | development of new resistance |
| Berard et al., 201921 | R | vascular graft | 5000 mg/L | SE, MRSA, EC, CA | EUCAST; agar dilution, confirmed by sequencing rpoB gene | MIC of SE and SA increased to >512 mg/L after 7 days exposure to R; mutations of rpoB gene confirmed by sequencing | development of new resistance |
| Bergamini et al., 199619 | R | vascular graft | 4×, 64×, 100×, 1000× MIC | SE colonized on vascular graft | Biofilm eradication; R MIC by disc diffusion | R decreased biofilm concentration at 4 h but was ineffective at 18 and 42 h due to MIC increasing from 0.1 to >30 mg/L R | development of new resistance |
| Garrison et al., 199718 | R | uncoated vascular graft | aqueous R at 4×, 1000× MIC | SE | Biofilm eradication; MIC broth dilution | MIC of all recovered SE increased from baseline (0.1 mg/L) to >30 mg/L | development of new resistance |
| Chlorhexidine (n = 9) | |||||||
| Aarestrup et al., 200423 | CH | environmental (used for disinfection in food animals) | — | Salmonella (156), EC (202), SA (43), Staphylococcus hyicus (38), Enterococcus faecalis (52), Enterococcus faecium (78) | MIC agar dilution; development of resistance, defined as bimodal population | E. faecium showed high frequency of isolates at differing concentrations (0.5–1 versus 4–8 mg/L); all isolates formed one large population | no change in resistance |
| Apisarnthana rak et al., 201430 | CH | CH wipes | — | AN (100): pre-CH (50), post-CH (50) | MIC microbroth dilution | Increase in CH MIC after initiation CH baths; however, did not achieve threshold for new CH resistance | no change in resistance |
| Ekizoglu et al., 201624 | CH | — | MIC 0.125–512 mg/L; eradication 0.02%–4% | PS (22); AN (19); SM (13); KB (14); EB (15); MSSA (6); MRSA (15); EN (17) | MIC agar dilution, planktonic eradication; not conducted | Some organisms tested showed resistant MIC; however, CH at 4% was effective against all strains within 5 min of contact | no change in resistance (per Discussion section) |
| Johnson et al., 201325 | CH | — | — | MRSA: qacA/B+ (5), smr + (5), no plasmid (5) | MBC | All isolates tested had MBC <16 mg/L regardless of presence of resistance genes | no change in resistance |
| Martro et al., 200326 | CH | Hibiscrub | 4% | AN (9) from prior to, during and after outbreak | Planktonic eradication | 4% CH (Hibiscrub) eradicated all AN strains tested | no change in resistance |
| Modak et al., 199222 | CH, CHXSS | planktonic, associated with CHXSS CVC | — | SA, EC | MIC after 25 serial subinhibitory passages | 2-Fold increase in MIC for SA and EC tested against CH, SS and CHXSS | no change in resistance |
| Skovgaard et al., 201327 | CH | CH hand scrub | — | SE from hospital, community or historic (prior to hand scrub) | MIC/MBC by disc diffusion | No difference in CH susceptibility in hospital versus community versus historic; no selection for qacA/B genes in hospital versus community versus historic (no genes present) | no change in resistance |
| Suwantarat et al., 201428 | CH | CH cloths for skin antisepsis | 2% | EN (30), SA (20), CoNS (29), other G+ (2), AN (3), CB (1), EB (3), EC (5), KB (12), PS (11), other G− (10) | MIC/MBC by microbroth dilution | Patients with daily CH bathing were more likely to have an organism with reduced CH susceptibility (86% versus 64% P = 0.028) | development of new resistance |
| Wesgate et al., 201629 | CH | — | CH (0.00005%) | SA, EC | MIC measures of short and long exposure | No change in the CH susceptibility profile after short and long exposures to the CH | no change in resistance |
| M/R (n = 2) | |||||||
| Munson et al., 200432 | M/R | Spectrum CVC (Cook Critical Care) | — | SA, SE, EN, EC, PS | Organisms cultured from border of ZOI from M/R catheter; MIC disc diffusion | Colonies sampled from growth around ZOI failed to demonstrate emergence of resistance to M or R by disc diffusion | no change in resistance |
| Norton et al., 200131 | M/R | umbilical catheters | 11 mg M; 10.5 mg R | MRSA, MSSA, CoNS, CA, EN, AN, SM, PS, VRE | ZOI, biofilm colonization; disc diffusion | ZOI showed efficacy to all organisms tested except PS and CA; CoNS and SM in the study remained sensitive to M and R | no change in resistance |
| M/R/CH (n = 1) | |||||||
| Rosenblatt et al., 201933 | M/R/CH | CVC | — | SA, SE, VRE, KB, EC, EB, PS, AN, CA, CP | 20 serial passages through subinhibitory concentration; MIC microbroth dilution | All except one organism remained within 2-fold change in MIC after 20 passages; one strain of EB showed a 4-fold increase in MIC after 20 passages but after passages in broth (no M/R/CH) the MIC returned within the 2-fold limit | no change in resistance |
| Multiple drug combinations (n = 2) | |||||||
| Sampath et al., 200134 | CHXSS, M/R | CVC, CHXSS (Arrowguard Plus), M/R (Spectrum) | — | SE, EC | 20 serial passages through subinhibitory concentration; MIC tube dilution | CHXSS: 2-fold increase for SE, unchanged for EC; M/R: 8-fold increase for SE, 4-fold increase for EC | CHXSS: no change in resistance; M/R: development of new resistance |
| Tambe et al., 200135 | M, R, M/R, CHXSS | antimicrobials associated with CVC | — | SE | 20 serial passages through subinhibitory concentration; MIC tube dilution | M: NS increase in MIC; R: 25 000-fold increase in MIC; M/R: 10-fold increase for ATCC strain, 16-fold increase for clinical strain; CHXSS: NS increase in MIC | M, CHXSS: no change in resistance; R: development of new resistance; M/R: less development of resistance |
M, minocycline; R, rifampicin; CH, chlorhexidine; SS, silver sulfadiazine; ZOI, zone of inhibition; NS, not significant; MRSE, methicillin-resistant SE; EN, Enterococcus species; AN, Acinetobacter species; KB, Klebsiella pneumoniae; PS, Pseudomonas aeruginosa; SM, Stenotrophomonas maltophilia; EB, Enterobacter species; CB, Citrobacter species; CA, Candida albicans; CP, Candida parapsilosis G+, Gram-positive organisms; G−, Gram-negative organisms; —, data not presented in the manuscript being abstracted.
Table 2.
In vivo assessment of potential resistance to rifampicin, minocycline and/or chlorohexidine
| Citation | Drug | Device: how is drug attached; concentration | Animal model species (n); implant site; duration | Organism(s) assessed | Methodology; resistance measurement | Results | After contact with the device, which of the following does the study show: (i) no change in resistance; or (ii) development of new resistance |
|---|---|---|---|---|---|---|---|
| Rifampicin (n = 3) | |||||||
| Avramovic et al., 199136 | R | vascular graft; soaked in 100 mg aqueous R | sheep (20); carotid artery; 3 weeks | SA | Breakthrough ZOI; R antibiotic disc | All SA isolated from R-treated grafts remained sensitive | no change in resistance |
| Garrison et al., 199718 | R | uncoated vascular graft; aqueous 4× and 1000× MIC R infused in subcutaneous pocket | mouse (42); subcutaneous pocket; 4, 18 and 42 h | SE | Biofilm eradication; MIC broth dilution | MIC of recovered SE from high-dose R increased from BL (0.1 to >30 mg/L) | development of new resistance |
| Sardelic et al., 199537 | R | vascular graft; soaked in 1.2 mg/mL aqueous R | sheep (9); carotid artery; 3 weeks | MRSA | Breakthrough MIC; agar dilution | Breakthrough MRSA had same R MIC as starting inoculum | no change in resistance |
ZOI, zone of inhibition; BL, baseline.
Table 3.
Clinical assessment of potential resistance to rifampicin, minocycline and/or chlorohexidine
| Citation | Study type (n patients) | Device; drug | Design | Measure of resistance | Results | After contact with the device, which of the following does the study show: (i) no change in resistance; (ii) selection for tolerant strains; or (iii) development of new resistance |
|---|---|---|---|---|---|---|
| Rifampicin (n = 1) | ||||||
| Bandyk et al., 200140 | retrospective (27) | vascular graft soaked in aqueous R (45–60 mg/mL) | Patient with graft infections had graft replaced with R-soaked graft | not stated | Failure from MRSA infection and recurrent R-resistant SE infection in 2 patients; 18 patients remained infection free and survived | selection for tolerant strains; discussion: ‘need for continued evaluation to determine whether R grafts select for resistant G+’ |
| Chlorhexidine (n = 14) | ||||||
| Batra et al., 201041 | retrospective (4570: 2480 pre, 2090 post) | CH bathing; Hibitane, 1% CH dusting powder, Hibiscrub | Assessed for MRSA infection pre- and post-antiseptic protocol | MBC (CLSI) | Decrease in MRSA infections by endemic strain but increase in MRSA infection by outbreak strain after initiation of CH baths; all outbreak strains had qacA/B genes and 3-fold higher MBCs than endemic strain | selection for tolerant strains |
| Choudhury et al., 201746 | prospective (77: 43, CH dressing, 34 no CH dressing) | CH dressings at catheter insertion sites | Prevalence of smr or qacA/B from DNA from skin with CH dressing versus skin with no (or non-CH) dressing | assessment of smr or qacA/B genes by PCR | No significant difference in frequency of qacA/B and smr recovered from CH dressing versus no CH dressing; no evidence that CH increases frequency of CH-tolerance genes at catheter sites | no change in resistance |
| Chung et al., 201548 | prospective (3054: 1514 pre, 1540 post) | CH wipes | Assessment of AN infections pre- and post-CH bathing | MIC microbroth dilution | Prevalence of AN decreased from 25.8% to 18.2%; no difference in CH MIC between 42 AN in pre-CH bathing and 56 AN in post-CH bathing | no change in resistance |
| Ho et al., 201238 | case–control (156: 96 MRSA; 60 MSSA) | CHXSS CVC (Arrowguard blue) | Assessment of MIC and qacA/B or smr genes from MRSA and MSSA catheter-related infection | MIC agar dilution; prevalence of genes by PCR | Significantly more MRSA isolates containing qacA/B genes caused CHXSS-impregnated CRBSI; MIC stratified for CHXSS catheter not assessed | selection for more tolerant strains |
| Lee et al., 201139 | case–control (150: 75 case; 75 control) | CH wipes for decolonization | Assessed risk factors for persistence of MRSA carriage after decolonization | presence of resistance genes by PCR | Genotypic CH resistance alone did not predict persistent MRSA carriage | no change in resistance |
| Lowe et al., 201747 | prospective (4037: 2039 controls, 1998 CH) | CH wipes | MRSA and VRE infections in 7 month intervention of CH bathing versus non-medicated soap/water bathing | MIC by agar dilution; presence of resistance genes by PCR | In intervention group, one MRSA and one MSSA contained resistance genes though MIC was susceptible; one MRSA had resistant MIC (8 mg/L) but was not PCR positive | no change in resistance |
| Mendoza-Olazaran et al., 201449 | prospective (149: 80 prior, 69 CH bath) | CH wipes | AN infections in 6 months prior and 6 month intervention of CH bathing | MIC agar dilution | AN isolates in the CH bathing period showed a significant decrease in CH MIC likely due to change in clonality of infecting isolate | no change in resistance |
| Maki et al., 199761 | RCT (403: 195 controls, 208 CHXSS) | CHXSS CVC | Susceptibility to CHXSS CVC in organisms cultured from CHXSS versus control catheters | ZOI | None of isolates from infected catheters showed resistance to fresh CHXSS catheters by ZOI | no change in resistance |
| McNeil et al., 201642 | retrospective (247) | CH wipes | Assessed CH MIC, smr and qacA/B over time; increased CH bathing over time | MIC microbroth dilution; prevalence of genes by PCR | Increased prevalence of smr and qacA/B in SA infections over time; however, non-significant difference of CH MIC for isolates positive and negative for genes | no change in resistance |
| Schlett et al., 201458 | cluster randomized trial (30 209: 10 030 CH bathing) | CH wipes, Hibiclens | MIC, qacA/B from MRSA SSTI among standard bathing versus CH bathing | MIC microbroth dilution; prevalence of genes by PCR | No difference in MIC or prevalence qacA/B in CH bathing versus control | no change in resistance |
| Schuerer et al., 200753 | prospective (4630: 2079 pre, 2551 post) | CHXSS CVC | Assessment of CRBSI in control versus CHXSS | not conducted | No significant difference in rate of CRBSI or microbiological profile organisms cultured in control versus CHXSS | no change in resistance |
| Soma et al., 201250 | prospective (29 swabs: 4 no CH, 15 moderate CH, 10 heavy CH) | CH wipes | MIC, qacA/B among CoNS sampled from patients with no, moderate or heavy CH baths | MIC microbroth dilution; prevalence of genes by PCR | No significant difference in CH MIC in no versus moderate versus heavy CH samples. 11/17 CoNS had qacA/B | no change in resistance |
| Timsit et al., 200959 | RCT (1636: 819 controls, 817 CH sponge) | CH-impregnated sponges | Assessment of CRI, and MBC in CH sponge versus control | MBC | Reduction in CRI with use of CH sponge; no difference in MBC for control versus CH sponge | no change in resistance |
| Velazquez-Meza et al., 201751 | prospective (156: 61 pre, 52 during, 45 post) | CH wipes (2%) | MIC of SA isolates from pre-, during and post-CH bathing | MIC agar dilution | No isolates showed reduced susceptibility to CH in pre- versus during versus post-intervention | no change in resistance |
| M/R (n = 8) | ||||||
| Chatzinikolaou et al., 200354 | RCT (130: 66 M/R, 64 controls) | M/R CVC, haemodialysis | CRBSI; MIC of CoNS cultured from colonized catheters | MIC microbroth dilution | MIC of CoNS isolates from colonized catheters were similar in M/R versus control | no change in resistance |
| Chatzinikolaou et al., 200345 | retrospective (672: 212 M/R, 460 controls) | M/R CVC | CRBSI in BMT (M/R) versus leukaemia (no M/R) | MIC (CLSI) | All 67 SA and SE isolates were susceptible to M; one isolate (M/R) and 9 isolates (control) were resistant to R | no change in resistance |
| Gilbert et al., 201655 | RCT (1485: 502 controls, 486 M/R, 497 heparin) | M/R CVC | CRBSI in control versus M/R versus hep CVC | varied by centre (MIC) | 8/12 patients with MIC tested on BSI cultures were resistant to M, R or both: 3/5 control; 2/2 M/R; 3/5 heparin | no change in resistance |
| Hanna et al., 200456 | RCT (356: 182 M/R, 174 controls) | M/R CVC | MIC for organisms cultured from catheter and/or skin at insertion site | MIC microbroth dilution | Mean M and R MIC of SE from M/R catheter was lower than control catheters; no difference in MIC from skin cultures before insertion versus removal | no change in resistance |
| Raad et al., 199757 | RCT (298: 151 controls, 147 M/R) | M/R CVC | Colonization or CRBSI control versus M/R; ZOI with new M/R CVC from all organisms from colonized CVC; MIC (M, R) for SA, SE from M/R CVC versus insertion site | ZOI of new M/R CVC; MIC microbroth dilution | Significant reduction in colonization and CRBSI with M/R CVC; M/R CVC had activity by ZOI for all organisms isolated from patient CVC; SA or SE from M/R CVC and insertion site remained at ≤2 mg/L | no change in resistance |
| Ramos et al., 201143 | retrospective (4732 isolates: 2451 pre, 2281 post) | M/R CVC | Susceptibility of SA, SE to tetracycline and R prior to M/R (1999) and 7 years after use of M/R CVC (2006) | MIC (CLSI) | Decrease in percentage of SA and SE resistant to tetracycline or R after 7 years of M/R CVC use; all decreases were significant except R resistance in SA | no change in resistance |
| Turnbull et al., 201844 | retrospective (9703 isolates: 2818 pre, 6885 post) | M/R CVC | Susceptibilities of SA to tetracycline and R in ICU prior to and after use of M/R | not stated (reported at hospital) | Rate of R and tetracycline resistance unchanged prior to and after use of M/R CVC | no change in resistance |
| Wright et al., 200152 | prospective (40: 23 pre, 17 post) | M/R CVC | Susceptibilities of M and R in organisms cultured from CVC | MIC microbroth dilution | Pre: 3/12 SE isolates resistant to R; post: 8/8 SE isolates resistant to R; no other changes in susceptibility patterns | development of new resistance |
| Comparison of multiple drug combinations (n = 1) | ||||||
| Darouiche et al., 199960 | RCT (738: 356 M/R, 382 CHXSS) | M/R CVC, CHXSS CVC | Susceptibilities of M and R in organisms cultured from CVC or skin prior to insertion | MIC microbroth dilution | No differences in M or R MIC ranges for SE and EN cultured from M/R CVC, CHXSS CVC or skin | no change in resistance |
M, minocycline; R, rifampicin; CH, chlorhexidine; SS, silver sulfadiazine; MRSE, methicillin-resistant SE; EN, Enterococcus species; AN, Acinetobacter species; PS, Pseudomonas aeruginosa; SM, Stenotrophomonas maltophilia; EB, Enterobacter species; CB, Citrobacter species; CA, Candida albicans; G+, Gram-positive organisms; G-, Gram-negative organisms; CRBSI, catheter-related bloodstream infection; CRI, catheter-related infection; SSTI, skin and soft tissue infection; BMT, bone marrow transplant patients.
In vitro
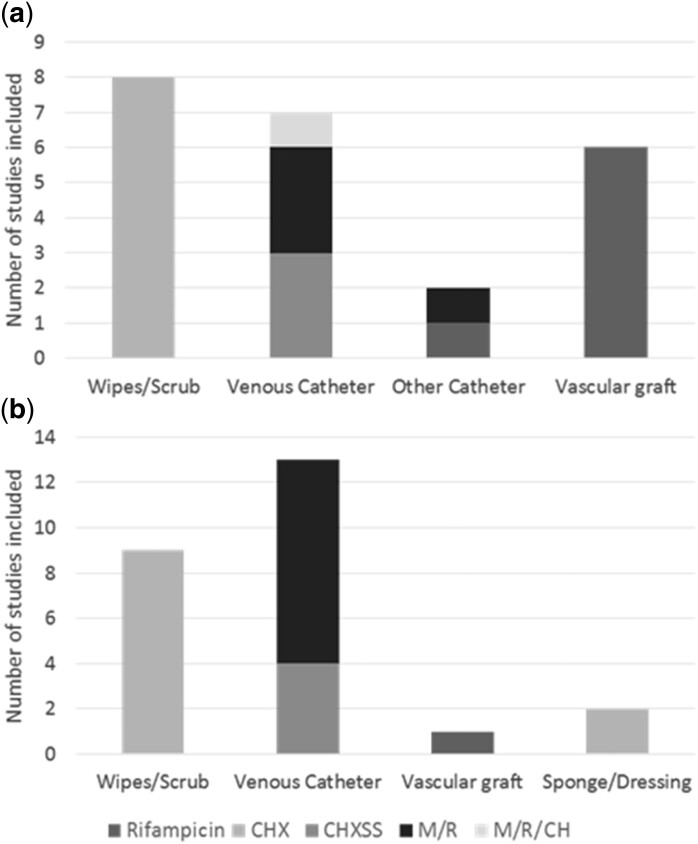
A total of 18 in vitro studies were included in the analyses (Table 1), including 4 studies assessing rifampicin alone,18–21 9 studies assessing chlorhexidine (CH or CHXSS),22–30 2 studies assessing M/R31,32 and 1 study assessing the triple combination of M/R/CH.33 Two studies assessed multiple drugs: Sampath et al.34 assessed the CHXSS and M/R combinations and Tambe et al.35 assessed minocycline, rifampicin, M/R and CHXSS. Three different devices (wipes/scrub,23–30 vascular grafts18,19,21 and catheters20,22,31–35) were assessed. Figure 2a depicts the device and drug combinations.
Figure 2.
In vitro, in vivo and clinical studies included in assessment by device and drug. Devices and drug/drug combinations for in vitro and in vivo studies (a) and clinical studies (b).
Authors reported no change in resistance in eight studies assessing exposure to devices containing chlorhexidine,22–27,29,30,34 two studies assessing M/R,31,32 one study that assessed minocycline alone35 and the one study that assessed M/R/CH.33 Development of new resistance was reported in five studies, the majority of which assessed rifampicin alone.18–21,35 Two studies concluded that resistance developed after exposure to M/R34,35 and one with exposure to chlorhexidine.28
In vivo
Three in vivo studies were included in the analyses (Table 2), all of which assessed rifampicin alone18,36,37 in vascular graft models. Two studies assessed rifampicin-soaked vascular grafts in a sheep model36,37 and one study assessed colonized vascular grafts with aqueous rifampicin in a subcutaneous mouse model.18 Figure 2a depicts the device and drug combinations studied.
Most in vivo studies with full-text review assessed efficacy of the antimicrobial device (breakthrough) and did not assess development of resistance after exposure to the device and thus were not included in the final analysis. Only three studies assessed resistance after exposure to a rifampicin vascular graft, two of which reported no change in resistance.36,37 Garrison et al.18 reported development of new resistance after implanting biofilm formed on an uncoated vascular graft into a subcutaneous pocket then exposing it to high-dose rifampicin infused into the pocket. MICs increased from 0.1 to >30 mg/L.18 This is consistent with other reports that de novo rifampicin resistance is more common with higher concentrations of organisms than those typically cultured from infected devices.
Clinical
In total, 24 clinical studies were included in the analyses (Table 3): 2 case–control studies,38,39 6 retrospective studies,40–45 8 prospective studies46–53 and 8 RCTs.54–61 Among the clinical studies, 15 assessed chlorhexidine in three different devices, 9 assessed wipes/scrub,39,41,42,48–51,58,62 4 assessed CHXSS central venous catheters (CVCs)38,53,60,61 and 2 assessed chlorhexidine in dressings or sponges.46,59 Nine studies assessed M/R-impregnated CVCs.43–45,52,54–57,60 Darouiche et al.60 assessed both CHXSS and M/R CVCs.60 Only one study assessed rifampicin alone in vascular grafts.40Figure 2b depicts all device and drug combinations studied.
The majority of studies found no change in resistance after exposure to either chlorhexidine (13 studies)39,42,46–51,53,58–61 or M/R CVCs (7 studies).43–45,54,55,57,63 Three studies reported selection for more tolerant strains in chlorhexidine wipes,41 CHXSS CVC38 and rifampicin alone.40 Wright et al.52 reported development of new resistance of Staphylococcus epidermidis (SE) to rifampicin after exposure to M/R catheters, with an increase in rifampicin resistance from 3/12 SE isolates recovered from catheters prior to implementation of M/R catheters to 8/8 SE isolates recovered from catheters after implementation of M/R catheters. No other changes in susceptibility patterns were reported.
Quality assessment
Quality assessments of each included manuscript are presented in Table 4. Out of the in vitro articles assessed, scores ranged from 13 to 18 (out of 19), indicating that the majority of studies have a low risk of bias. Ten out of 18 studies (55%) were graded ‘A’ for methodology, 7 of 18 (38.9%) were graded ‘B’ and only 1 study (5.5%) was graded ‘C’ for methodology. For the three in vivo studies assessed, scores ranged from 18 to 21 (out of 23). Two of the three studies (66.7%) were graded ‘A’ for methodology while one was graded ‘C’. In the assessment of clinical studies, observational studies scores ranged from 6 to 11 (out of 12), case–control studies scores from 11 to 13 (out of 13) and controlled intervention trials scores from 9 to 14 (out of 14). Six of the 24 clinical studies (25%) were graded ‘A’ for assessment of resistance methodology, 12 of 24 (50%) were graded ‘B’, 3 of 24 (12.5%) were graded ‘C’ and 3 of 24 (12.5%) were graded ‘D’.
Table 4.
Quality assessment
|
In vitro studies |
In vivo studies |
Clinical studies |
||||||
|---|---|---|---|---|---|---|---|---|
| citation | quality score | method score | citation | quality score | method score | citation | quality score | method score |
| Modak, 1992 | 14/19 | A | Avramovic, 1991 | 19/23 | C | Maki, 1997 | 11/14 | C |
| Bergamini, 1996 | 16/19 | B | Sardelic, 1995 | 18/23 | A | Raad, 1997 | 14/14 | B |
| Garrison, 1997 | 14/19 | A | Garrison, 1997 | 21/23 | A | Darouiche, 1999 | 11/14 | B |
| Sampath, 2001 | 14/19 | B | Bandyk, 2001 | 10/12 | D | |||
| Norton, 2001 | 13/19 | C | Wright, 2001 | 8/12 | B | |||
| Tambe, 2001 | 15/19 | B | Chatzinikolaou, 2003 | 11/12 | A | |||
| Martro, 2003 | 16/19 | B | Chatzinikolaou, 2003 | 13/14 | B | |||
| Aarestrup, 2004 | 18/19 | A | Hanna, 2004 | 12/14 | B | |||
| Munson, 2004 | 16/19 | A | Schuerer, 2007 | 9/12 | D | |||
| Bayston, 2009 | 17/19 | A | Timsit, 2009 | 9/14 | B | |||
| Skovgaard, 2013 | 16/19 | A | Batra, 2010 | 8/12 | A | |||
| Johnson, 2013 | 14/19 | B | Lee, 2011 | 11/13 | B | |||
| Apisarntharak, 2014 | 11/19 | B | Ramos, 2011 | 10/12 | A | |||
| Suwantarat, 2014 | 18/19 | A | Ho, 2012 | 12/13 | B | |||
| Wesgate, 2016 | 15/19 | A | Soma, 2012 | 8/12 | A | |||
| Ekizoglu, 2016 | 16/19 | B | Schlett, 2014 | 10/14 | A | |||
| Berard, 2019 | 16/19 | A | Mendoza-Olazaran, 2014 | 10/12 | A | |||
| Rosenblatt, 2019 | 13/19 | A | Chung, 2015 | 8/12 | B | |||
| McNeil, 2016 | 8/12 | B | ||||||
| Gilbert, 2016 | 12/14 | C | ||||||
| Lowe,2017 | 9/12 | B | ||||||
| Turnbull, 2018 | 6/12 | D | ||||||
| Velazquez-Meza, 2017 | 8/12 | B | ||||||
| Choudhury, 2017 | 8/12 | C | ||||||
Reviews
Of the literature reviews assessed, the majority discussed literature pertaining to other primary endpoints such as breakthrough after antimicrobial exposure, efficacy of antibiotics/biocides in infection prevention or mechanisms of potential resistance. Four reviews had varied conclusions on potential development of resistance with repeated or sublethal use of chlorhexidine.64–67 In two of the reviews, authors had no definitive conclusions, stating some studies assessed had found potential for chlorhexidine resistance while others did not and needed additional study.64,67 One of the reviews concluded that exposure to sublethal chlorhexidine increased the risk of resistance in Gram-negative organisms65 while another review reported no evidence of resistance with repeated chlorhexidine exposure.66 Three other reviews assessed the potential for antimicrobial resistance with the use of M/R and overwhelmingly concluded that the use of M/R devices is unlikely to cause resistance.68–70
Discussion
The focus of this review was evidence in the literature for the development of antimicrobial resistance as a consequence of the use of antimicrobial devices containing minocycline, rifampicin, chlorhexidine or combinations thereof. No trends in findings were seen based on which device was studied. There were trends based on the agents or combinations of agents in the devices so the discussion is structured accordingly. First, however, our analysis framework is described.
Development of new antimicrobial resistance versus development of antimicrobial tolerance versus selection of tolerant strains
For this review, development of new antimicrobial resistance to minocycline, rifampicin and chlorhexidine is taken as a complete loss of inhibitory or bactericidal effect at therapeutically achievable concentrations due to adaptive changes by organisms following exposure to minocycline, rifampicin and/or chlorhexidine. Organisms that were not susceptible to minocycline, rifampicin and chlorhexidine prior to de novo exposure could be classified as having pre-existing resistance; however, they would not have presented as having developed new antimicrobial resistance as a result of exposure to these agents (but rather as possessing an innate pre-existing absence of susceptibility). In contrast to newly developed antimicrobial resistance, we include development of antimicrobial tolerance as a milder form of reduced antimicrobial susceptibility following exposure to minocycline, rifampicin and/or chlorhexidine. Development of antimicrobial tolerance can be a result of organisms responding to the presence of antimicrobial agents by expressing similar adaptive genes that phenotypically alter the concentrations of antimicrobial agents required to be effective. In some cases there is a limit to the concentration of interfering molecules an organism can express and in others less efficient alternative pathway responses result in impairment but incomplete inactivation of the effects of the antimicrobial agents. Developed antimicrobial tolerance responses typically are seen as shifts in MICs of antimicrobial agents to higher MICs in order to be inhibitory or cidal but would still be within potentially therapeutically attainable concentrations for antimicrobial agents. In the extreme, if organisms lose susceptibility to minocycline, rifampicin and/or chlorhexidine at therapeutically achievable concentrations they would have crossed the threshold to have developed new antimicrobial resistance. Selection of tolerant strains from exposure to a device containing minocycline, rifampicin or chlorhexidine results when, as a consequence of prior exposure, organisms have different innate susceptibilities to particular antimicrobial agents or combinations. This is not an adaptive response but rather a separation of low-susceptibility strains from high-susceptibility ones based on their ability to tolerate the presence of threshold concentrations of minocycline, rifampicin, chlorhexidine or combinations thereof. Based on finite doses of antimicrobial agents on devices, tolerant organisms (higher MICs) will be able to survive and colonize antimicrobial devices before highly susceptible organisms (lower MICs) can. Differences in tolerance can explain why some organisms are able to break through and colonize antimicrobial devices before others are able to, as well as why organisms can preferentially colonize devices with one combination of antimicrobial agents versus a different combination. The selection of tolerant strains following exposure to an antimicrobial device is not indicative of development of antimicrobial tolerance nor indicative of development of new antimicrobial resistance unless, prior to exposure to the antimicrobial agents on the device, the organisms were susceptible to those agents.
Single antimicrobial agent device studies
Rifampicin alone
Four in vitro studies19,20,34,35 reported that devices containing rifampicin alone exhibited newly developed resistance following exposure. The culprit organism was SE in these studies and resistance was assessed through increases in MICs following exposure. Several in vivo studies assessed rifampicin devices; however, most did not assess development of new resistance. Two studies on soaked vascular grafts36,37 reported no changes in resistance for Staphylococcus aureus (SA) or MRSA but one study18 reported development of new resistance for SE. One human study40 evaluating vascular grafts soaked in rifampicin reported recurrent breakthrough infections in two patients with two rifampicin-resistant SE and with MRSA. These results were consistent with selection for rifampicin-resistant strains but it is not possible to determine whether exposure to the rifampicin grafts induced newly developed resistance or whether the rifampicin resistance was pre-existing.
Resistance to rifampicin has been reported by occurrence of single point mutations in the β-subchain of bacterial RNA polymerase.71 These point mutations so impair rifampicin binding and subsequent inactivation of bacterial RNA polymerase that the MIC increases to greater than the limit of testing (i.e. resistant). Point mutations have the greatest likelihood of spontaneously arising and being selected for when a bacterial population is in the presence of rifampicin alone since they involve changes to only a single amino acid. Consequently, development of new resistance has been repeatedly observed and use of rifampicin alone appears unwise. If other antimicrobial agents are present, they can continue to inhibit or kill bacteria even with favourable RNA polymerase mutations, so rifampicin-resistant bacterial populations might not be able to survive or propagate in the presence of rifampicin in combination with other antimicrobial agents.
Minocycline alone
One in vitro study35 reported no development of new resistance following repeated exposure to devices containing minocycline alone. No in vivo or human studies were conducted on devices containing minocycline alone. Minocycline resistance has been reported to occur through expression of efflux pumps and less commonly through expression of ribosomal protection proteins.72,73 In contrast to rifampicin, these types of resistance require acquisition of new genes and expression of new proteins, which are less probable than the occurrence of point mutations. Similarly, bacteria that do acquire minocycline efflux pump or ribosomal protection protein genes when in the presence of minocycline with other antimicrobial agents might not be able to survive and propagate in the presence of the combination because of the antimicrobial activity of the other agent.
Chlorhexidine alone
Eight in vitro studies22–25,27,29,30 assessing devices with chlorhexidine alone reported no change in resistance following exposure. One in vitro study28 reported development of new resistance. The experimental design assessed MICs of colonizing organisms following exposure to chlorhexidine wipes. The results of a significant increase in prevalence of reduced chlorhexidine susceptibility following chlorhexidine exposure are consistent with selection for more tolerant strains. Insufficient information was provided to determine whether the exposure induced new chlorhexidine resistance or whether the reduced susceptibility was pre-existing prior to the exposure. No in vivo studies with devices containing chlorhexidine alone assessed development of new resistance. Two human studies38,41 reported selection for chlorhexidine-tolerant strains following chlorhexidine exposure and nine studies39,42,46,48–51,58 reported no changes in resistance. Chlorhexidine resistance has been reported to be due to the presence of efflux pumps,74 requiring acquisition of new genes and expression of new proteins. Development of new chlorhexidine resistance appears to be rare but continued surveillance is warranted.
Antimicrobial combination device studies
M/R device studies
Two in vitro studies34,35 on combination M/R devices reported development of new resistance to SE and Escherichia coli (EC). The increases in MIC for the combination were modest (4–16-fold) and were much lower than increases in rifampicin MICs (25 000-fold) following exposure to devices containing rifampicin alone. In practical terms, in one of the studies35 the M/R MIC for one strain of SE was reported to increase from 0.02 to 0.25 mg/L following exhaustive sequential passaging at subinhibitory concentrations and for another strain it increased from 0.015 to 0.25 mg/L. These ultimate MICs remain below the CLSI thresholds for susceptibility for minocycline (less than or equal to 4 mg/L) and rifampicin (less than or equal to 1 mg/L);14 thus, while drift of MICs against the combination plausibly occurred, its clinical relevance was more theoretical (as a tolerance shift) than practical because the organisms remained within the therapeutic susceptible range and thus the MIC drifts reported for the SE strains were not clinically relevant. Similarly, the other study34 reported an MIC increase for SE from 0.02 to 0.31 mg/L and for EC from 0.25 to 1 mg/L. Again, the drift in MIC went from very susceptible to susceptible, reflecting increased antimicrobial tolerance; however, the clinical relevance was more theoretical than practical as the organisms remained susceptible and thus the tolerance shift was not clinically relevant, in that the organisms remained susceptible to the M/R combination. In contrast, two other in vitro studies31,32 reported no development of new resistance following exposure to devices containing the combination of M/R. More substantively, eight human studies43–45,54,55,57,60,63 reported no development of new M/R resistance following exposure to M/R devices. This includes two independent studies43,44 over multi-year periods that reported no changes in rifampicin or tetracycline resistance for SA isolates when compared over prolonged periods prior to and following implementation of the routine use of M/R devices. In contrast, one small human study52 reported the development of new SE resistance following implementation of the use of M/R catheters. In the period prior to use of the M/R devices, 3/12 infectious SE isolates were resistant to rifampicin, while following use of the M/R devices 8/8 SE isolates were rifampicin resistant. This study did not report development of new minocycline resistance or new resistance to the M/R combination and did not report results for MIC testing other than for rifampicin. In addition, since rifampicin-resistant isolates were present prior to use of the M/R device it is quite possible that the results reflect selection for more tolerant SE isolates rather than development of new resistance. The authors acknowledged that the clinical significance of their results was not clear.
It appears that development of new rifampicin resistance following exposure to devices containing the M/R combination might be theoretically possible for SE but was more a tolerance increase that was not clinically relevant because the culprit organisms remained susceptible to M/R combinations. Additionally, the development of new resistance against the M/R combination for SA was refuted by two independent studies. Since minocycline and rifampicin act with different mechanisms of action at entirely different places in microbial cells, the combination does appear to decrease the likelihood of development of newly resistant organisms, particularly over devices containing rifampicin alone, because two completely different resistance mechanisms would need to simultaneously emerge in the target cells.75,76
CHXSS device studies
Although silver sulfadiazine is not a component of the proposed M/R/CH catheter, studies on development of new resistance to CHXSS are included in this review to assess the potential for resistance to emerge against chlorhexidine combinations. No change in resistance was reported in three in vitro studies.22,34,35 No in vivo studies assessed development of new resistance following exposure to CHXSS. Three human studies reported no change in resistance.53,60,61 One human study38 reported selection of more tolerant MRSA strains following exposure to CHXSS; however, this finding was entirely based on genetic analysis (i.e. more isolates contained qacA/B efflux pump genes) and more clinically significant measurement of increases in MICs were not performed. It appears there is a theoretical potential for chlorhexidine resistance to develop but it has been unlikely and has not been clinically widespread or confirmed, particularly when chlorhexidine antimicrobial combinations have been present.
M/R/CH device studies
One in vitro study assessed the potential for development of resistance with repeated exposure to subinhibitory concentrations of M/R/CH.33 Organisms with high susceptibility to individual agents as well as low susceptibility to individual agents showed no development of resistance over 20 passages. Only one carbapenem-resistant Enterobacter showed a 4-fold increase in MIC during the 20 passages. While there was an increase in MIC, the increase was still well below clinical relevancy. Additionally, after the stressor (M/R/CH) was removed and the organism was passaged in broth alone, the MIC returned to baseline, indicating a phenotypic adaptation rather than development of new resistance. Further, for Gram-positive pathogens common to catheter infections, M/R/CH was highly effective. In the first few passages, subinhibitory concentrations were established for a few SA and SE; however, within two passages, the organisms died and passages could not continue. Based on this study and previous drug combination studies, with the addition of the third component (chlorhexidine) to M/R there is no evidence of the potential for new resistance developing after exposure.
Conclusions
A systematic literature review was undertaken to assess evidence for the development of new antimicrobial resistance from exposure to medical devices containing rifampicin, minocycline, chlorhexidine or their combinations. Strengths of this study include: a search strategy that was conducted in multiple databases; use of systematic criteria to select studies and abstract data from these studies, thus reducing potential for selection bias; and use of standardized metrics for assessing the quality of studies. Limitations include: while multiple databases were searched, we did not include conference abstracts or search grey literature for additional studies; manuscripts included were limited to English language; and variability in the quality of studies. While the majority of our studies were scored as ‘high quality’ there were a few studies that did not present detailed methods or criteria and were scored as ‘low quality’ and should not be compared with equal weight.
Resistance was most likely to emerge in devices containing rifampicin as a single antimicrobial agent because only a single point mutation in RNA polymerase was required for rifampicin resistance to emerge. Development of rifampicin resistance was seen in multiple studies. In contrast, development of new resistance to minocycline or chlorhexidine single-agent devices was much less likely because acquisition of genes and expression of new proteins was required for new resistance to develop. Clinically meaningful development of new resistance against minocycline or chlorhexidine in devices was not confirmed in human studies although slight drifts in MICs were observed.
Emergence of new resistance to double combinations of M/R or CHXSS, although theoretically possible, was rarer and no clinical trials confirmed its occurrence and some refuted it. Studies did demonstrate that selection of more tolerant isolates capable of colonizing devices occurred when testing was performed on these combinations, but the lower innate susceptibilities of the tolerant strains likely existed prior to the exposure. The risk of development of new resistance to these antimicrobial combinations appears more fear-based rather than substantiated by clinical and experimental evidence but warrants continued surveillance.
Supplementary Material
Acknowledgements
We acknowledge Y-Lan Truong for her help in editing and preparing the final manuscript for publication.
Funding
This work was partially supported by the Sociedad Española de Enfermedades Infecciosas y Microbiología Médica (Ayuda de la SEIMC).
Transparency declarations
Drs I. I. Raad and J. Rosenblatt are co-inventors of the minocycline, rifampicin, chlorhexidine combination technology which is owned by the University of Texas MD Anderson Cancer Center and has been licensed by Cook Medical LLC. All other authors: none to declare.
Supplementary data
The Reviewer report is available as Supplementary data at JAC-AMR Online.
References
- 1.CDC. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associated Bloodstream Infection). NHSN Device-associated Module 2017. https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf.
- 2. Lai NM, Chaiyakunapruk N, Lai NA. et al. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst Rev 2016; issue 3: CD007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schiffer CA, Mangu PB, Wade JC. et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013; 31: 1357–70. [DOI] [PubMed] [Google Scholar]
- 4. Cesaro S, Cavaliere M, Pegoraro A. et al. A comprehensive approach to the prevention of central venous catheter complications: results of 10-year prospective surveillance in pediatric hematology-oncology patients. Ann Hematol 2016; 95: 817–25. [DOI] [PubMed] [Google Scholar]
- 5. Sharp G, Green S, Rose M.. Chlorhexidine-induced anaphylaxis in surgical patients: a review of the literature. ANZ J Surg 2016; 86: 237–43. [DOI] [PubMed] [Google Scholar]
- 6. Holmes AH, Moore LS, Sundsfjord A. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387: 176–87. [DOI] [PubMed] [Google Scholar]
- 7. O’Grady NP, Alexander M, Burns LA. et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011; 52: e162–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brun-Buisson C, Doyon F, Sollet JP. et al. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med 2004; 30: 837–43. [DOI] [PubMed] [Google Scholar]
- 9. Rupp ME, Lisco SJ, Lipsett PA. et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med 2005; 143: 570–80. [DOI] [PubMed] [Google Scholar]
- 10. Ostendorf T, Meinhold A, Harter C. et al. Chlorhexidine and silver-sulfadiazine coated central venous catheters in haematological patients–a double-blind, randomised, prospective, controlled trial. Support Care Cancer 2005; 13: 993–1000. [DOI] [PubMed] [Google Scholar]
- 11. Storey S, Brown J, Foley A. et al. A comparative evaluation of antimicrobial coated versus nonantimicrobial coated peripherally inserted central catheters on associated outcomes: a randomized controlled trial. Am J Infect Control 2016; 44: 636–41. [DOI] [PubMed] [Google Scholar]
- 12. Viola GM, Rosenblatt J, Raad I.. Drug eluting antimicrobial vascular catheters: progress and promise. Adv Drug Deliver Rev 2017; 112: 35–47. [DOI] [PubMed] [Google Scholar]
- 13. Raad I, Mohamed JA, Reitzel RA. et al. Improved antibiotic-impregnated catheters with extended-spectrum activity against resistant bacteria and fungi. Antimicrob Agents Chemother 2012; 56: 935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Sixth Edition: M100. 2016. [Google Scholar]
- 15.National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 16. Kilkenny C, Browne W, Cuthill IC. et al. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 2010; 160: 1577–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lynch HN, Goodman JE, Tabony JA. et al. Systematic comparison of study quality criteria. Regul Toxicol Pharmacol 2016; 76: 187–98. [DOI] [PubMed] [Google Scholar]
- 18. Garrison JR Jr, Henke PK, Smith KR. et al. In vitro and in vivo effects of rifampin on Staphylococcus epidermidis graft infections. ASAIO J 1997; 43: 8–12. [PubMed] [Google Scholar]
- 19. Bergamini TM, McCurry TM, Bernard JD. et al. Antibiotic efficacy against Staphylococcus epidermidis adherent to vascular grafts. J Surg Res 1996; 60: 3–6. [DOI] [PubMed] [Google Scholar]
- 20. Bayston R, Fisher LE, Weber K.. An antimicrobial modified silicone peritoneal catheter with activity against both Gram-positive and Gram-negative bacteria. Biomaterials 2009; 30: 3167–73. [DOI] [PubMed] [Google Scholar]
- 21. Berard X, Puges M, Pinaquy JB. et al. In vitro evidence of improved antimicrobial efficacy of silver and triclosan containing vascular grafts compared with rifampicin soaked grafts. Eur J Vasc Endovasc Surg 2019; 57: 424–32 [DOI] [PubMed] [Google Scholar]
- 22. Modak SM, Sampath L.. Development and evaluation of a new polyurethane central venous antiseptic catheter: reducing central venous catheter infections. Complication Surg 1992; 11: 23–9. [Google Scholar]
- 23. Aarestrup FM, Hasman H.. Susceptibility of different bacterial species isolated from food animals to copper sulphate, zinc chloride and antimicrobial substances used for disinfection. Vet Microbiol 2004; 100: 83–9. [DOI] [PubMed] [Google Scholar]
- 24. Ekizoglu M, Sagiroglu M, Kilic E. et al. An investigation of the bactericidal activity of chlorhexidine digluconate against multidrug-resistant hospital isolates. Turk J Med Sci 2016; 46: 903–9. [DOI] [PubMed] [Google Scholar]
- 25. Johnson JG, Save EJ, Jimenez-Truque N. et al. Frequency of disinfectant resistance genes in pediatric strains of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2013; 34: 1326–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martro E, Hernandez A, Ariza J. et al. Assessment of Acinetobacter baumannii susceptibility to antiseptics and disinfectants. J Hosp Infect 2003; 55: 39–46. [DOI] [PubMed] [Google Scholar]
- 27. Skovgaard S, Larsen MH, Nielsen LN. et al. Recently introduced qacA/B genes in Staphylococcus epidermidis do not increase chlorhexidine MIC/MBC. J Antimicrob Chemother 2013; 68: 2226–33. [DOI] [PubMed] [Google Scholar]
- 28. Suwantarat N, Carroll KC, Tekle T. et al. High prevalence of reduced chlorhexidine susceptibility in organisms causing central line-associated bloodstream infections. Infect Control Hosp Epidemiol 2014; 35: 1183–6. [DOI] [PubMed] [Google Scholar]
- 29. Wesgate R, Grasha P, Maillard J-Y.. Use of a predictive protocol to measure the antimicrobial resistance risks associated with biocidal product usage. Am J Infect Control 2016; 44: 458–64. [DOI] [PubMed] [Google Scholar]
- 30. Apisarnthanarak A, Yang Hsu L, Lim TP. et al. Increase in chlorhexidine minimal inhibitory concentration of Acinetobacter baumannii clinical isolates after implementation of advanced source control. Infect Control Hosp Epidemiol 2014; 35: 98–9. [DOI] [PubMed] [Google Scholar]
- 31. Norton RE, Patole S, Whitehall J.. An in vitro study of the efficacy of rifampicin and minocycline coated umbilical venous catheters. Int J Antimicrob Agents 2001; 17: 237–40. [DOI] [PubMed] [Google Scholar]
- 32. Munson EL, Heard SO, Doern GV.. In vitro exposure of bacteria to antimicrobial impregnated-central venous catheters does not directly lead to the emergence of antimicrobial resistance. Chest 2004; 126: 1628–35. [DOI] [PubMed] [Google Scholar]
- 33. Rosenblatt J, Vargas-Cruz N, Reitzel RA. et al. Assessment of the potential for inducing resistance in multidrug resistant organisms from exposure to minocycline, rifampin and chlorhexidine used to treat intravascular devices. Antimicrob Agents Chemother 2019; 63:e00040–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sampath LA, Tambe SM, Modak SM.. In vitro and in vivo efficacy of catheters impregnated with antiseptics or antibiotics: evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infect Control Hosp Epidemiol 2001; 22: 640–6. [DOI] [PubMed] [Google Scholar]
- 35. Tambe SM, Sampath L, Modak SM.. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J Antimicrob Chemother 2001; 47: 589–98. [DOI] [PubMed] [Google Scholar]
- 36. Avramovic JR, Fletcher JP.. Rifampicin impregnation of a protein-sealed Dacron graft: an infection-resistant prosthetic vascular graft. ANZ J Surg 1991; 61: 436–40. [DOI] [PubMed] [Google Scholar]
- 37. Sardelic F, Ao PY, Fletcher JP.. Rifampicin impregnated Dacron grafts: no development of rifampicin resistance in an animal model. Eur J Vasc Endovasc 1995; 9: 314–8. [DOI] [PubMed] [Google Scholar]
- 38. Ho CM, Li CY, Ho MW. et al. High rate of qacA- and qacB-positive methicillin-resistant Staphylococcus aureus isolates from chlorhexidine-impregnated catheter-related bloodstream infections. Antimicrob Agents Chemother 2012; 56: 5693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee AS, MacEdo-Vinas M, François P. et al. Impact of combined low-level mupirocin and genotypic chlorhexidine resistance on persistent methicillin-resistant Staphylococcus aureus carriage after decolonization therapy: a case-control study. Clin Infect Dis 2011; 52: 1422–30. [DOI] [PubMed] [Google Scholar]
- 40. Bandyk DF, Novotney ML, Johnson BL. et al. Use of rifampin-soaked gelatin-sealed polyester grafts for in situ treatment of primary aortic and vascular prosthetic infections. J Surg Res 2001; 95: 44–9. [DOI] [PubMed] [Google Scholar]
- 41. Batra R, Cooper BS, Whiteley C. et al. Efficacy and limitation of a chlorhexidine-based decolonization strategy in preventing transmission of methicillin-resistant Staphylococcus aureus in an intensive care unit. Clin Infect Dis 2010; 50: 210–7. [DOI] [PubMed] [Google Scholar]
- 42. McNeil JC, Kok EY, Vallejo JG. et al. Clinical and molecular features of decreased chlorhexidine susceptibility among nosocomial Staphylococcus aureus isolates at Texas Children’s Hospital. Antimicrob Agents Chemother 2016; 60: 1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramos ER, Reitzel R, Jiang Y. et al. Clinical effectiveness and risk of emerging resistance associated with prolonged use of antibiotic-impregnated catheters: more than 0.5 million catheter days and 7 years of clinical experience. Crit Care Med 2011; 39: 245–51. [DOI] [PubMed] [Google Scholar]
- 44. Turnbull IR, Buckman SA, Horn CB. et al. Antibiotic-impregnated central venous catheters do not change antibiotic resistance patterns. Surg Infect 2018; 19: 40–7. [DOI] [PubMed] [Google Scholar]
- 45. Chatzinikolaou I, Hanna H, Graviss L. et al. Clinical experience with minocycline and rifampin-impregnated central venous catheters in bone marrow transplantation recipients: efficacy and low risk of developing staphylococcal resistance. Infect Control Hosp Epidemiol 2003; 24: 961–3. [DOI] [PubMed] [Google Scholar]
- 46. Choudhury MA, Sidjabat HE, Rathnayake IU. et al. Culture-independent detection of chlorhexidine resistance genes qacA/B and smr in bacterial DNA recovered from body sites treated with chlorhexidine-containing dressings. J Med Microbiol 2017; 66: 447–53. [DOI] [PubMed] [Google Scholar]
- 47. Lowe CF, Lloyd-Smith E, Sidhu B. et al. Reduction in hospital-associated methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus with daily chlorhexidine gluconate bathing for medical inpatients. Am J Infect Control 2017; 45: 255–9. [DOI] [PubMed] [Google Scholar]
- 48. Chung YK, Kim JS, Lee SS. et al. Effect of daily chlorhexidine bathing on acquisition of carbapenem-resistant Acinetobacter baumannii (CRAB) in the medical intensive care unit with CRAB endemicity. Am J Infect Control 2015; 43: 1171–7. [DOI] [PubMed] [Google Scholar]
- 49. Mendoza-Olazaran S, Camacho-Ortiz A, Martinez-Resendez MF. et al. Influence of whole-body washing of critically ill patients with chlorhexidine on Acinetobacter baumannii isolates. Am J Infect Control 2014; 42: 874–8. [DOI] [PubMed] [Google Scholar]
- 50. Soma VL, Qin X, Zhou C. et al. The effects of daily chlorhexidine bathing on cutaneous bacterial isolates: a pilot study. Infect Drug Resist 2012; 5: 75–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Velazquez-Meza ME, Mendoza-Olazaran S, Echaniz-Aviles G. et al. Chlorhexidine whole-body washing of patients reduces methicillin-resistant Staphylococcus aureus and has a direct effect on the distribution of the ST5-MRSA-II (New York/Japan) clone. J Med Microbiol 2017; 66: 721–8. [DOI] [PubMed] [Google Scholar]
- 52. Wright F, Heyland DK, Drover JW. et al. Antibiotic-coated central lines: do they work in the critical care setting? Clin Int Care 2001; 12: 21–8. [Google Scholar]
- 53. Schuerer DJ, Zack JE, Thomas J. et al. Effect of chlorhexidine/silver sulfadiazine-impregnated central venous catheters in an intensive care unit with a low blood stream infection rate after implementation of an educational program: a before-after trial. Surg Infect 2007; 8: 445–54. [DOI] [PubMed] [Google Scholar]
- 54. Chatzinikolaou I, Finkel K, Hanna H. et al. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am J Med 2003; 115: 352–7. [DOI] [PubMed] [Google Scholar]
- 55. Gilbert RE, Mok Q, Dwan K. et al. Impregnated central venous catheters for prevention of bloodstream infection in children (the CATCH trial): a randomised controlled trial. Lancet 2016; 387: 1732–42. [DOI] [PubMed] [Google Scholar]
- 56. Hanna H, Benjamin R, Chatzinikolaou I. et al. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: a prospective randomized clinical trial. J Clin Oncol 2004; 22: 3163–71. [DOI] [PubMed] [Google Scholar]
- 57. Raad I, Darouiche R, Dupuis J. et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med 1997; 127: 267–74. [DOI] [PubMed] [Google Scholar]
- 58. Schlett CD, Millar EV, Crawford KB. et al. Prevalence of chlorhexidine-resistant methicillin-resistant Staphylococcus aureus following prolonged exposure. Antimicrob Agents Chemother 2014; 58: 4404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Timsit JF, Schwebel C, Bouadma L. et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA 2009; 301: 1231–41. [DOI] [PubMed] [Google Scholar]
- 60. Darouiche RO, Raad I, Heard SO. et al. A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med 1999; 340: 1–8. [DOI] [PubMed] [Google Scholar]
- 61. Maki DG, Stolz SM, Wheeler S. et al. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter: a randomized, controlled trial. Ann Intern Med 1997; 127: 257–66. [DOI] [PubMed] [Google Scholar]
- 62. Boonyasiri A, Thaisiam P, Permpikul C. et al. Effectiveness of chlorhexidine wipes for the prevention of multidrug-resistant bacterial colonization and hospital-acquired infections in intensive care unit patients: a randomized trial in Thailand. Infect Control Hosp Epidemiol 2016; 37: 245–53. [DOI] [PubMed] [Google Scholar]
- 63. Hanna H, Afif C, Alakech B. et al. Central venous catheter-related bacteremia due to Gram-negative bacilli: significance of catheter removal in preventing relapse. Infect Control Hosp Epidemiol 2004; 25: 646–9. [DOI] [PubMed] [Google Scholar]
- 64. Denny J, Munro CL.. Chlorhexidine bathing effects on health-care-associated infections. Biol Res Nurs 2017; 19: 123–36. [DOI] [PubMed] [Google Scholar]
- 65. Kampf G. Acquired resistance to chlorhexidine - is it time to establish an ‘antiseptic stewardship’ initiative? J Hosp Infect 2016; 94: 213–27. [DOI] [PubMed] [Google Scholar]
- 66. Karki S, Cheng AC.. Impact of non-rinse skin cleansing with chlorhexidine gluconate on prevention of healthcare-associated infections and colonization with multi-resistant organisms: a systematic review. J Hosp Infect 2012; 82: 71–84. [DOI] [PubMed] [Google Scholar]
- 67. Maillard JY. Bacterial resistance to biocides in the healthcare environment: should it be of genuine concern? J Hosp Infect 2007; 65 Suppl 2: 60–72. [DOI] [PubMed] [Google Scholar]
- 68. Crnich CJ, Maki DG.. New technology for reducing infection and resistance in the ICU. J Crit Illness 2002; 17: 48–51. [Google Scholar]
- 69. Raad I, Hanna H.. Intravascular catheters impregnated with antimicrobial agents: a milestone in the prevention of bloodstream infections. Support Care Cancer 1999; 7: 386–90. [DOI] [PubMed] [Google Scholar]
- 70. Schierholz JM, Rump AFE, Pulverer G. et al. Anti-infective catheters: novel strategies to prevent nosocomial infections in oncology. Anticancer Res 1998; 18: 3629–38. [PubMed] [Google Scholar]
- 71. Furustrand Tafin U, Aubin GG, Eich G. et al. Occurrence and new mutations involved in rifampicin-resistant Propionibacterium acnes strains isolated from biofilm or device-related infections. Anaerobe 2015; 34: 116–9. [DOI] [PubMed] [Google Scholar]
- 72. Chopra I, Roberts M.. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Bio Rev 2001; 65: 232–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Draper MP, Weir S, Macone A. et al. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother 2014; 58: 1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wand ME, Bock LJ, Bonney LC. et al. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother 2017; 61: e01162–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Al-Hasan MN, Wilson JW, Lahr BD. et al. β-Lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by Gram-negative bacilli. Antimicrob Agents Chemother 2009; 53: 1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bonhoeffer S, Lipsitch M, Levin BR.. Evaluating treatment protocols to prevent antibiotic resistance. Proc Natl Acad Sci USA 1997; 94: 12106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.