Abstract
Purpose
Diabetes mellitus (DM) is a chronic non-communicable endocrine and metabolic disease that is thought to be the fastest emerging health challenge of the twenty-first century. Presently, 90% of diabetic population is handicapped with T2-DM, and the majority of pre-diabetes on the way to T2-DM progression. By keeping in view, a review article has been compiled to highlight the significance of value aided effective, low-cost, safe, and useful remedies that could easily be accessible to the global community in order to moderate the possibility of DM and related complications.
Methods
Literature search for this review was carried out using scientific databases including PubMed, EBSCO, Scopus, Web of science, and google scholar. Whilst, value aided articles were selected on the basis of their therapeutic potential, safety profile and outreach.
Results
Escalating research data validated that herbal remedies and physical activities significantly prevents hyperglycemia, hyperlipidemia, and other complications in people with T2-DM.
Conclusion
Globally, nearly half-billion individuals are living with diabetes. Therefore, it is urged to embrace herbal remedies and physical mediation in our daily routine in order to tackle such devastating disorder.
Keywords: Diabetes mellitus; Global public health; T2-DM; Kitchen herbs, herbal beverages; Physical therapies
Introduction
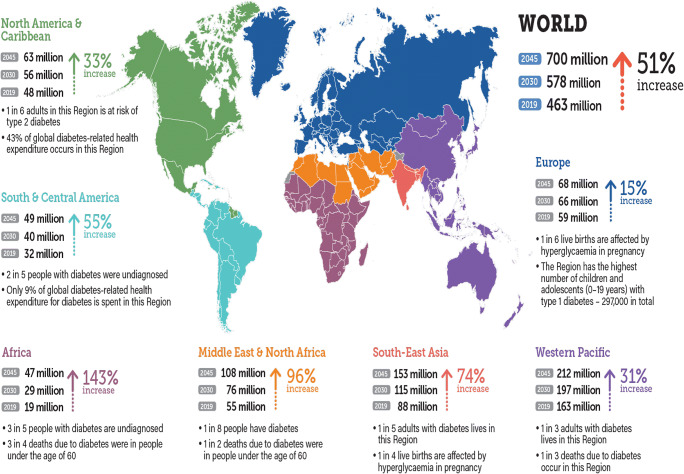
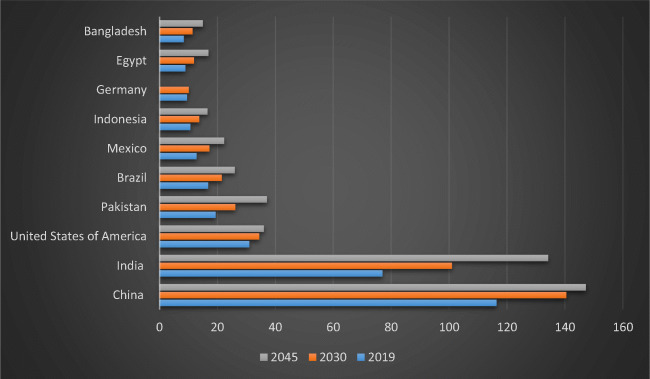
Diabetes mellitus (DM) is a chronic non-communicable endocrine and metabolic disease that is thought to be the fastest emerging health challenge of the twenty-first century. Over the past two decades, according to international diabetes federation (IDF) statistics, the number of diabetic patients had exponentially been increased from 151 million (adults aged 20–79) to 463 million (2019) and expected to be soared at 578 million in 2030 and 700 million by 2045, affecting almost all global populations as shown in Fig. 1 [1]. Whilst, incessant rise in DM cases unveils influence of multi-faceted factors such as socio-economic, demo-graphic, heredity, and environment [1, 2]. In the current scenario around Globe, 50.1% of people are handicapped with DM. In the next 25 years, it is expected that the prevalence of DM cases will more likely to be increased in the Asian and American populations (Fig. 2), imposing a significant impact on the wellbeing of individuals, their families, and financial prudence at large [3]. In this context, attention regarding alternative, safe and low-cost remedies to treat such devastating disease are of major concern to the wellbeing and health of people. By keeping in view, a review article has been compiled to highlight the significance of value aided effective, low-cost, safe, and useful remedies that could easily be accessible to the global community in order to moderate the possibility of DM and related complications.
Fig. 1.
Worldwide prevalence of diabetes mellitus in adult aged 20–79 years (2019, 2030 and 2045)
Fig. 2.
Prevalence of diabetes mellitus in various regions and territories (2019, 2030 and 2045). Asian countries including China, India, Pakistan, Indonesia and Bangladesh showing growing trends in a number of diabetic patients. Whilst, in United States of America (USA) number of diabetic patients shown to be stable with 34 million people by the end of 2045, falling to 4th position. Yet. Pakistan with high number will replace USA by 2045, moving to 3rd position. Nevertheless, Brazil and Mexico with tenfold increase in diabetic patients will remained be at 5th and 6th rank from 2019 to 2045
Diabetes mellitus
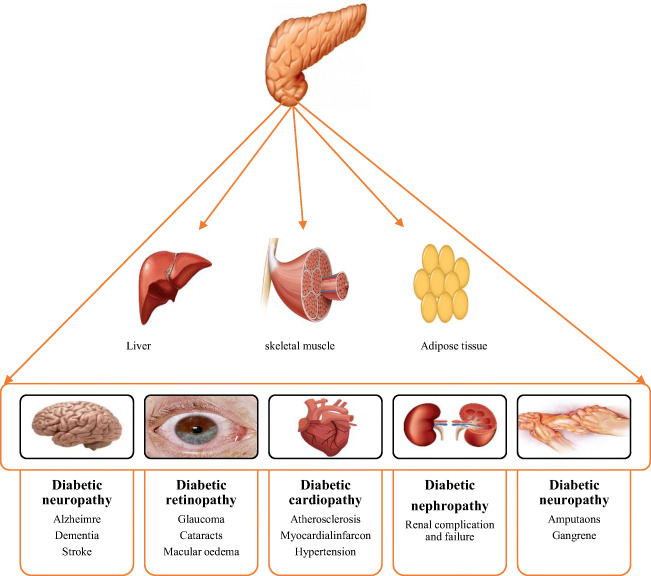
DM is characterized by improper, insufficient, or defective hormonal (insulin) secretion which affects protein, fat, and carbohydrate metabolism [4]. Pathophysiology of DM is interweaving with uneven blood glucose level, presenting with type-1(T1-DM) and type-2 (T2-DM). In T1-DM, individuals are more likely to be endured insulin deficiency. However, people with T2-DM underwent with insulin resistance, pancreatic β cell damage, deterioration in glucose uptake, and an upsurge in glucose level that is manifesting as hyperglycemia, and the condition is characterized as glucose-toxicity [2, 4, 5]. Escalating research evidence has shown that untreated glucose-toxicity can cause severe health consequences includes blindness, neuropathy, cardiovascular disorders, peripheral gangrene, and nephropathy as shown in Fig. 3, and might increase the risk of disability and mortality [1, 6]. Whilst in order to, treat DM several synthetic anti-diabetic medicines are available that provide short-term effects and also prompt adverse effects on the multiple natal systems of people with DM. Therefore effective and inexpensive anti-diabetic remedies that hold satisfactory effectiveness with no antagonistic effects are prerequisites. Prolific research studies have validated that since the prehistoric era, herbal (plants) remedy as to a primary DM management strategy has been used with low to no side effects. Mounting research data have demonstrated that herbal remedies are effective in controlling blood glucose level and insulin resistance. Moreover, herbal remedies could also improve β cells functionality, glucose absorption, glucagon-like peptide-1 (GLP-1) homeostasis and also have the ability to converse progression of pre-diabetes [7–9]. Due to such promising results, the World Health Organization (WHO) have also impelled the use of herbal remedies for the treatment of DM [10]. Correspondingly, physical activities, exercise, or fitness along with herbal remedies have also shown a significant impact on overall the health of DM patients [11].
Fig. 3.
Effect of diabetes mellitus on the various human organs
Literature search strategy
Relevant literature, articles, or references were retrieved from PubMed, EBSCO, Scopus, Web of science, and google scholar databases using a keyword such as “medicinal plants and diabetes”, “herbs and diabetes”, “condiments and diabetes,” “culinary herbs and diabetes”, “traditional medicinal plants for diabetes” “Physical activities”, “exercise”, “Yoga” or Tai chi” effects on the population with T2-DM. Published research articles in English and peer-reviewed journals were scrutinized on the basis of health benefits, safety profile, and quality of trial studies. Both randomized clinical trial and non-randomized clinical trial studies were included that were published between 2015 and 2020.
Result and discussion
Kitchen herbs
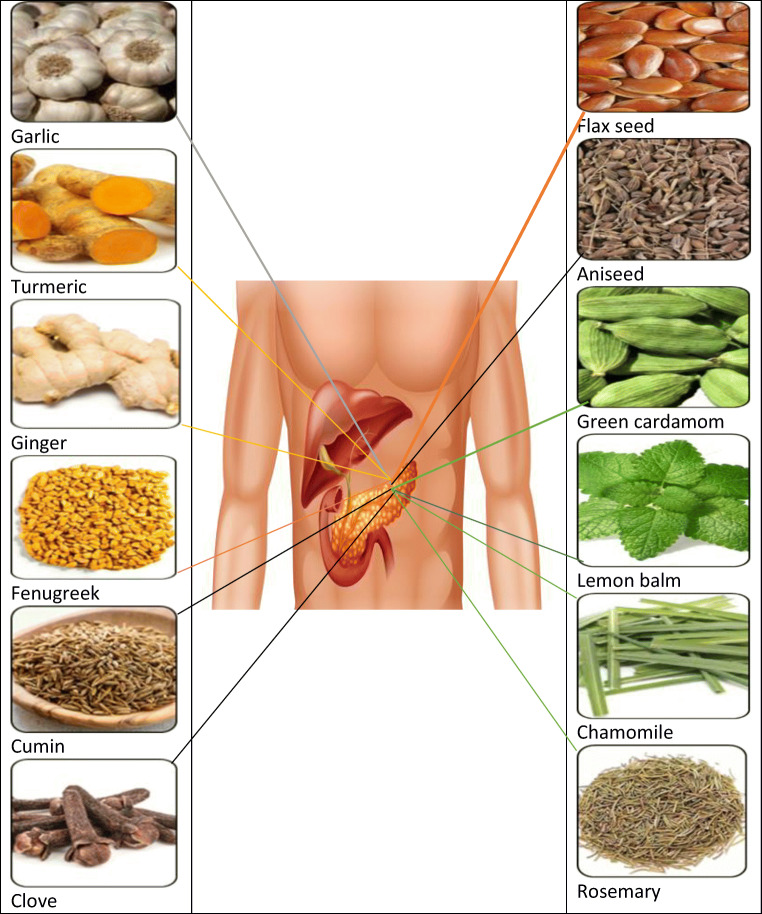
Around the globe, herbs, or plants have been used as a source of food and known as the kitchen or culinary herbs, available in fresh or dried form. Several scientific studies in the literature supporting that kitchen herb are extensively used as a traditional herbal medicine for the treatment of DM and its complications [8, 9]. Therefore kitchen herbs with promising anti-diabetic properties as shown in Fig. 4 are discussed in this section.
Fig. 4.
Effects of herbs on the T2-DM administration
Anise
Anise or aniseed is an aromatic plant which is commonly distributed in the Mediterranean region and also aboriginal to Iran, India and Turkey. It has been used as traditional medicine as it contains anethole which possess antioxidant, anti-microbial, anti-inflammatory, and anti-diabetic properties [12]. It was reported by Rajeshwari et al. [13] that 5 g aniseed uptake for the 2 months can decrease fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG) and also improve high-density lipoprotein cholesterol (HDL-C) in T2-DM patients. Likewise, Shobha and Andallu [14] demonstrated that aniseed extract oral supplementation induce anti-hyperglycemia, anti-oxidative scavenging and anti-hyperlipidemia properties. Their study data explored that 5 g aniseed oral supplementation can considerably reduce FBG, lipid profile, lipid peroxidation and protein oxidation (PO) in T2-DM patients, suggesting the possible use of aniseed for the DM administration [14].
Berry
Berry is a small, pulpy, and often edible fruit that shown to play a significant role in glucose homeostasis. For instance, mulberry (Morus alba and Morus nigra) contains a bioactive compound that significantly reduces blood glucose level, serum, hepatic-malonaldehyde level, and metabolic disorders [4]. Mounting research has revealed that mulberry leaves (Morus alba) could reduce postprandial plasma glucose (PPG) or after-meal blood glucose in people with impaired glucose tolerance [4, 15]. Similarly, growing pieces of evidence have explored that red raspberries’ contain valuable anti-diabetic properties that potentially maintain glucose metabolism via the adenosine monophosphate-activated protein kinase (AMPK) pathway and improves insulin sensitivity through the phosphatidylinositol 3 kinase signaling pathway [16, 17]. Current study data proposed that red raspberries’ can control post-meal glycemic profile in people who have obesity with prediabetes and insulin resistance. The authors’ findings suggest that red raspberries (250 g) intake in breakfast can reduce post-meal insulin and blood sugar or peak glucose concentrations with less insulin which might be associated with improved insulin sensitivity [17].
Cinnamon
Cinnamon spice derived from an innermost bark of the cinnamon tree is native to Sri Lanka, Burma, and India and also grown in South America and West Indies. It is considered super blood glucose regulating ingredient that maintains blood glucose level and DM-related complication. In a recent double-blind clinical trial, authors’ showed that 500 mg of cinnamon supplementation can bring promising changes in pre-diabetes glucose homeostasis compared to placebo [18]. Whilst, Kizilaslan, and colleagues [19] figured out that 3–6 g cinnamon intake significantly maintain the after-meal blood glucose level in healthy subjects. In another recent research, the author exhibited the effectiveness of cinnamon as a personalized remedy through Traditional Persian Medicine (TPM) syndrome differentiation or diagnostic model, based on aptitudes of either warm/cold, wet/dry syndromes (Mizaj) or reverse syndrome therapy. In this study, 140 T2-DM individuals according to the TPM, syndrome were divided into 2 clusters and received one gram of cinnamon dosage for the period of 90 days. Intervention outcomes of research data unveiled improved FBG, HBA1C, and insulin resistance in T2-DM individuals with cold or wet syndrome rather than hot or dry syndrome, suggesting cinnamon is more effective to cure T2-DM individuals with cold or wet syndrome [20]. The same researcher group through another clinical trial evaluated that 500 mg cinnamon capsules, twice a day for 90 days led to improve FBG, HBA1C, fasting insulin, insulin resistance, cholesterol, low-density lipoprotein cholesterol (LDL-C), HDL-C, and body mass index (BMI) in T2-DM patients with greater baseline BMI [21].
Clove
Cloves spice derived from the flower buds believed to work as a potential antidiabetic agent. Mohan et al. [22] in their current research shown that clove extract can potentially pull down pre-prandial glucose levels in healthy and pre-diabetes individuals. In order to confirm this hypothesis, 13 selected subjects were stratified into 2 groups as per their pre-prandial glucose criteria including ≤100 mg/dL (group1with 7 subjects) and 101 and 125 mg/dL (group 2 with 6 subjects) respectively. One month clove extract supplementation of 250 mg/day determined a significant cut in the pre-prandial glucose level in prediabetes groups, signifying clove promising role in maintaining healthy glucose metabolism and reducing the possibility of T2-DM and cardiovascular disorders in the vulnerable populations [22].
Cumin
Cumin spice possess magical anti-diabetic attributes. In 2019, Morovati and collaborates showed that cumin essential oil intake could improve anti-oxidative and inflammatory indices in patients with metabolic syndrome or insulin resistance. Trial participants aged between 18 and 60 years received 75 mg of cumin essential oil for the course of 2 months. After a due time period, noticeable improvement was observed in superoxide dismutase (SOD), total antioxidant capacity (TAC) and malondialdehyde (MDA) in comparison to placebo [23]. Another clinical trial demonstrated that 50–100 mg cumin essential oil supplementation could significantly reduce FBG, HBA1C, and inflammatory indices as Tumor necrosis factor (TNF-α) and high-sensitivity C-reactive protein (hs-CRP) in patients with T2-DM [24]. Samani Keihan et al. [25] observed that cumin essential oil dosage (25 mg/day for 12 weeks) profoundly decreases HBA1C, TG, blood glucose, leptin, and oxidized LDL (ox-LDL) in T2- DM patients.
Dill
Dill is used as a spice and traditional herbal medicine for the cure of several disorders including gastrointestinal disorders, metabolic disorders, cardiovascular disorders, and DM [26]. Haidari et al. [27] through their work explored promising effects of dill powder administration on the T2-DM patient’s glycemic, lipid, oxidative and gastrointestinal profiles. The authors’ stated that 3 g dill powder intake after each meal for the period of 2 months could attenuate FBG, LDL-C, TC, MDA, and insulin resistance in a population with T2-DM. In addition to this, HDL-C and TAC were also shown to increase whereas gastrointestinal or colonic motility disorder was significantly reduced with dill powder uptake. Similarly, dill tablet intake exhibited significantly anti-glycation and anti-oxidant activities in T2-DM patients, highlighting therapeutic effects of dill in DM management [28, 29].
Fenugreek
Fenugreek commonly used as a vegetable and condiment. In traditional medicine, it is used for several disorders including T2-DM administration. The latest investigation by Geberemeskel and his companion [30] revealed that fenugreek seed powder solution can potentially improve dyslipidemia profile in a population with T2-DM. Authors’ exhibited that 25 g of fenugreek seed powder solution intake twice a day for 30 days could substantially lower TC, TG, and LDL-C level in T2-DM patients with no adverse effects. Likewise, Ranade and Mudgalkar evaluated that 10 g/day of soaked (in hot water) fenugreek can potentially decrease FBG and HBA1C levels in T2-DM patients [31]. Moreover, 10 g fenugreek powder before meals reduce PPG, LDL-C, insulin resistance, and delays the onset of pre-diabetes to T2-DM thus provide safe and effective substitute therapy for T2-DM management [32].
Flax
Flaxseed is rich source of fiber that bears beneficial properties to delay gastric problems and might improve glycemic control. In a recent systematic review and meta-analysis, authors’ reported that eating whole flaxseed could significantly improve blood glucose level and insulin sensitivity [33]. In a recent clinical trial, Hasaniani et al. [34] investigated that flaxseed enriched yogurt can control glycemic and lipid profiles in T2-DM patients. They assigned participants to eat fat yogurt (200 g) containing 30 g flaxseed for a period of 2 months. After an assigned time period, HBA1C, TG, TC, systolic and diastolic blood pressure were significantly diminished in the intervention group as compared to the control one. In 2017, authors’ of a double-blind placebo-controlled trial noticed the effects of flaxseed omega-3 fatty acid on the diabetic nephropathy subjects. They observed that after 3 months supplementation, participants who ate 1000 mg/day flaxseed omega-3 fatty acid led to a substantial reduction in serum insulin homeostasis model of assessment-estimated B cell function (HOMA-B) and improvement in quantitative insulin sensitivity check index (QUICKI) [35]. Another clinical trial conducted on the metabolic syndrome subjects exhibited that flaxseed supplementation (30 g) can potentially reduce insulin resistance and FBG, indicating its potential role as an alternative therapy for DM administration [36].
Garlic
Garlic as spice and condiment is cultivated all over the world with remarkable health benefits as an anti-hyperlipidemic, anti-hypoglycemic, anti-hypertensive, anti-atherosclerotic, anti-oxidant, anti-inflammatory, and neuroprotective agent [37]. Hamal et al. [38] in a recent, randomized clinical trial determined the positive defensive effect of garlic on endothelial function, atherosclerotic plaque, or cardiovascular disease in people with T2-DM. They explored that 3-months intake of aged garlic extract (2400 mg daily) can reduce an arterial stiffness that in turn moderates the risk of coronary complications in T2-DM subjects [38]. Additionally, Libyan people with DM showed that 2 g fresh garlic intake before breakfast for 30 days can significantly reduce FBG and cholesterol [39]. Moreover, administration of garlic exhibited a noteworthy effect on the glycemic status via reducing FBG, 2-h postprandial glucose (2 h PG) and HBA1C in T2-DM patients with obesity [40].Taken together, garlic is the best adjuvant therapy for T2-DM, cardio and metabolic disorders.
Ginger
Like garlic, ginger is a super herb to cure DM and its complications. In recent systematic review and meta-analysis, authors’ indicated that long term intake of ginger can significantly improve HBA1C and could act as a principal protective remedy for the chronic disorders related to T2-DM [41]. In 2017, a double-blind placebo a controlled trial on T2-DM subjects revealed that intervention of 2 g of grounded ginger for the 10 weeks led to reducing FBG, TC, TG, LDL-C, HDL-C, and HBA1C [42]. Khandouzi et al. [43] double-blind, clinical trial comprised of 41, T2-DM patients who received 2 g ginger powder for the 3 months exhibited significantly improved FBG, HBA1C, MDA, apolipoprotein B, and apolipoprotein A-I suggesting its potential role in alleviating vulnerability of some of the DM related chronic complications.
Green cardamom
Green cardamom is a dietary spice with antioxidant, antidiabetic, and anti-inflammatory properties. In a recent study, authors’ evaluated that cardamom powder intake has significant effects on blood pressure and endothelial function in people with T2-DM. Eighty T2-DM people with obesity who received cardamom (3 g/day; 6 capsules) or placebo for the course of 10 weeks, showed effective changes in inflammatory markers (CD163, SIRT1, NO, ADMA), BMI, and waist circumferences (WC) [44]. Correspondingly, Aghasi et al. [45] demonstrated that 3 g green cardamom could improve the glycemic status, lipid profile, and oxidative stress biomarkers in T2-DM patients with obesity. Ten weeks intervention unveiled evident changes in HBA1C, FBG, insulin, lipid profile, oxidative stress biomarkers (TAC, SOD, GPx, MDA), BMI, and WC. These outcomes indicate that cardamom is an effective and safe therapy that could significantly control blood glucose, lipid levels, and oxidative stress in individuals with T2-DM.
Lemon balm
Lemon balm with a lemon-like aroma is cultivated in an eastern Mediterranean region, Asia, and all over the world [46]. Asadi and collaborates in their recent randomized controlled trial demonstrated that lemon balm capsules (700 mg/day for 3 months) can improve FBG, HBA1C, pancreatic β-cell activity, and lipid profile with no antagonistic effects in T2-DM patients [47]. In another clinical trial, the authors’ demonstrated effects of lemon balm on T2-DM patients with dyslipidemia. Nayebi et al. [46] showed that lemon balm capsules (500 mg/daily) intake for the period of 3 months can decrease TG serum levels from baseline and systolic and diastolic blood pressure in the T2-DM population with obesity.
Turmeric
Turmeric spice has significant value in traditional medicine due to curcumin bioactive compound which restores lipid profile and maintain glucose homeostasis in T2-DM population [48]. Jiménez et al. investigation articulate that 320 mg of curcumin intake for 2 months can abridge lipid peroxidation and improve antioxidant capacity (increased serum antioxidant Gpx and SOD) in T2-DM with chronic kidney complications [49]. Panahi et al. demonstrated that curcuminoids (1000 mg) and piperine (10 mg up to 3 months) administration can reduce serum TGL, HDL-C, and LDL-C in T2-DM patients with obesity [50]. The same group in another trial assessed that curcumin (500 mg/day) and piperine (5 mg/day) intake could significantly reduce serum glucose level, HBA1C, serum AST, serum ALT, C peptide, suggesting its potential role in reducing T2-DM related hepatic damage [51]. Furthermore, administration of curcumin (1500 mg) for the period of a 2 months exhibited moderate serum glucose levels, BMI, and WC [52], positing turmeric robust anti-diabetic potential.
Herbal beverage
Since long herbal beverages have been used in traditional medicine for healthy living and preventative health. In the current scenario, herbal beverages have been getting popularity among health conscious consumers due to life curing natural bioactive compounds that uphold a range of biological processes [53]. On the basis of accruing evidence, herbal beverage with promising outcomes are discussed in this section.
Chamomile
Chamomile around the globe is used as a supplementary food and herbal tea [54]. In 2018, Kaseb and colleagues through clinical trials find out that chamomile herbal tea has significant effects on the T2-DM patient’s glycemic status, lipid profile, and kidney function. In their trial selected T2-DM subjects consumed 200 ml/day of chamomile tea (10 g/100 mL boiling water) before lunch and dinner for 1 month. After a specific time period, T2-DM subjects experienced a notable decline in FBG, 2hPG, TC, LDL-C, and creatinine (Cr) respectively [55]. Similarly, Zemestani et al. [56] investigated that chamomile infusion (3 g/150 ml hot water) consumption thrice a day for up to 2 months can control glycemic and antioxidant status in people with T2-DM. Similarly, in another randomized controlled clinical trial T2-DM participants drank chamomile tea (3 cups (150 ml) after meals and experienced significantly reduced serum insulin, HBA1C, TC, TG, and LDL-C [57]. Villa Rodriguez et al. [58] shown that chamomile tea could manage glucose absorption and metabolism via impeding the activity of digestive enzymes connecting to sugar secretion and transport pathways.
Green tea
Green tea is popular herbal beverages around the globe due to innumerable health benefits. Increasing pieces of evidence have shown the multi-dimensional the implication of green tea intake that may reduce obesity, maintain blood sugar, stimulate glucose uptake, promote GLUT4 translocation and also, cure DM-related complications (nephropathy, cardiovascularpathy, neuropathy, retinopathy, and hepatopathy) [59]. Borges et al. [60] clinical trial showed that green tea polyphenols administration could reduce albuminuria in T2-DM patients with nephropathy. In addition to this, green tea extract (1120 mg) consumption for the period of 5 months shown to improved bone mineral content in DM subjects [61]. Ma et al. [62] clinical study on the diabetic retinopathy subjects concluded that regular and long-term consumption of green tea might be a novel therapy to prevent and reduce the risk of retinopathy in population with DM.
Rosemary
Rosemary used as a spice, natural preservative, ornamental, medicinal as well as an anti-diabetic plant with several health benefits. It has been demonstrated that rosemary tea (made up of 2 g in 1 l of water) intake for 3 months can decrease glycated hemoglobin, IR, pancreatic β-cell function, body mass, and waist in T2-DM patients [63]. In another study, 3 g rosemary powder supplementation for the period of 60 days showed substantial effects on FBG, HBA1C, and vitamin B12 in T2-DM subjects [64].
Physical activities
Physical activity refers to body movement and when it is planned and structured, it is named exercise. The exercise comprises of various categories including aerobic (walking, cycling, jogging, and swimming), balance, resistance, and flexibility (tai chi and yoga). Growing research has ascertained that regular exercise could control blood sugar levels, decrease the risk of cardiovascular disorders and also deter the development of T2-DM [11]. In addition, incorporation of 2–3 h (moderate-high) intensity exercise per week can improve BMI that is more likely to be associated with progression of T2-DM, and also reduce overall mortality risks in patients with T1-DM and T2-DM [65, 66]. In this section, most promising exercises is discussed that could be beneficial for managing T2-DM.
Tai chi
Tai chi is a low to moderate-intensity exercise. It is a Chinese traditional mind and body exercise that involves relaxed body movement and deep breathing. [67]. Due to its relaxed approach, it is considered to be suitable for elderly people and people of all ages with exceptionally low damage ratio and profuse health benefits [68]. Growing evidence has made it impeccable for the DM patients as it manages their neuroendocrine balance, control blood glucose, HBA1C, reduce stress and DM linked peripheral neuropathy [67, 68]. Chao et al. [66] meta-analysis summarized that Tai Chi exercise is an excellent therapy to cure T2- DM as it reduces FBG, 2 h after-meal blood glucose, and HBA1C. Whilst, Xia and colleagues through systematic review and meta-analysis of controlled trials found that Tai chi can control blood glucose and lipid levels. However, Tai Chi training lengths and styles result could exert variable effectiveness in patients with T2-DM [68].
Yoga
Like tai chi, the yoga approach has also been in practice for the management and prevention of T2-DM. It is an Indian mind-body exercise that covers physical, mental, and spiritual practices. It is safe, inexpensive, and can be practiced either indoors or outdoors by people of all ages including older adults and people with comorbidities [69, 70]. A recent systematic review on the influence of yoga practice on prediabetic, supported yoga as a complete preventing substitute approach for the patients of T2-DM as it rise insulin sensitivity, improves glucose tolerance and lipid metabolism. They concluded that the yoga approach potentially could ameliorate FBG, postprandial blood glucose, HBA1C, TG, TC, BW, LDL-C, systolic, and diastolic blood pressure in patients with T2-DM [70]. Due to such beneficial aspects of Yoga, lately, Chattopadhyay and colleagues have established a unique Yoga program consist of a booklet and video as a protective approach for the population with of risk of T2-DM progression [69].
Conclusion
In short, as we know that around the Globe, nearly half-billion individuals are living with diabetes so this is imperative to aware and educate people regarding effective low-no cost remedies that may aid to tackle such devastating disorder.
In this review, escalating research data have validated that kitchen herbs (shown in Table 1) and physical activities could significantly reduce risk of hyperglycemia, hyperlipidemia, and other complications in the population with T2-DM. Therefore, it is urged to embrace herbal remedies and physical mediation in our daily routine along with a healthy lifestyle.
Table 1.
Herbs with anti-diabetic potential
| Herb local name | Herb scientific name | Distribution | Anti-diabetic compound | Ref |
|---|---|---|---|---|
| Anise | Pimpinella anisum L. | Mediterranean region, south east Asia, Iran, and Turkey. | Anethole, flavonoids (flavonol, flavone, rutin, isoorientin, and isovitexin) | [12] |
| Berry (mulberry and red raspberries) |
Morus alba L. Rubus idaeus L. |
Asia, America, Europe, Africa. Europe and northern Asia and temperate regions |
Flavonoids (quercetin, kaempferol, and isoramnetin), Alkaloids. Phenolic compounds, (anthocyanins and ellagitannins), oxyresveratrol |
[13, 14] |
| Chamomile | Matricaria chamomilla L. | North Africa, Asia, North and South America, Australia, New Zealand, Germany, Hungary, France, Russia, Yugoslavia, Egypt Rome and Brazil | Apigenin, quercetin, patuletin luteolin, and glucosides | [15–18] |
| Cinnamon | Cinnamomum genus | Indonesia, Sri Lanka, Burma, India, china, South America, West Indies and Vietnam | Flavonoids | [19–21] |
| Cloves | Syzygium aromaticum L. | Asian and African countries | Eugenol, and acetyleugenol | [22] |
| Cumin | Cuminum cyminum L. | Mediterranean region, India, Iran Egypt, China, and Mexico | γ-terpinene-7-al and cumin aldehyde | [23–25] |
| Dill | Anethum graveolens L. |
Mediterranean region, Europe, Asia, Iran Canada, and America |
Terpenoids, saponins, polyphenols, tannins, alkaloids, and anthocyanin | [26–29] |
| Fenugreek | Trigonella foenum-graecum L. | Southeastern Europe, northern Africa, and western Asia | Trigonelline, diosgenin, galactomannan, and hydroxyisoleucine | [30–32] |
| Flaxseed | Linum usitatissimum L. | Egypt, Europe, South America and Asia | Omega-3 fatty acid and α-linolenic acid | [33, 34] |
| Garlic | Allium sativum L. | Asia, Iran, Russia spain and America | Diallyl trisulfide (DATS), and S-Allyl cysteine (SAC) | [35–38] |
| Ginger | Zingiber officinale Rosc. | South East Asia | Gingerol, paradol, shogaol, and zingerone | [39–41] |
| Green cardamom | Elettaria cardamomum L. | Africa, Asia and Australia | Quercetin, kaempferol, luteolin, and pelargonidin | [42, 43] |
| Green tea | Camellia sinensis (L.) Kuntze | China, Japan, Vietnam, and Thailand | Flavonoids, flavonols, polyphenols, theaflavins, tannins, and epigallocatechin gallate | [44–46] |
| Lemon balm | Melissa officinalis L. | Europe, Iran, and Central Asia, and America | Flavonols (flavonoids) | [47, 48] |
| Rosemary | Rosmarinus officinalis L. | Worldwide | Carnosic acid and rosmarinic acid | [49, 50] |
| Turmeric | Curcuma longa L. | Asian and South East Asia | Curcumin,turmerone and turmerin | [51–54] |
Source: International diabetes federation (2019)
Acknowledgments
Not applicable.
Author contributions
AS conceptualized, designed, analysed data, draft and reviewed manuscript.
Funding
No funding received for submitted manuscript.
Data availability
Not applicable/ No data sets were generated during the present study. The present review is based on the cited references.
Declaration
Conflict of interest
Author is declaring no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Worldwide toll of diabetes. Diabetesatlas.org. 2020. https://www.diabetesatlas.org/en/sections/worldwide-toll-of-diabetes.html. Accessed 7 Aug 2020.
- 2.Deyno S, Eneyew K, Seyfe S, Tuyiringire N, Peter EL, Muluye RA, Tolo CU, Ogwang PE. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: a meta-analysis and meta-regression. Diabetes Res Clin Pract. 2019;156:107815. doi: 10.1016/j.diabres.2019.107815. [DOI] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues EL, Marcelino G, Silva GT, Figueiredo PS, Garcez WS, Corsino J, Guimarães RCA, Freitas KC. Nutraceutical and medicinal potential of the Morus species in metabolic dysfunctions. Int J Mol Sci. 2019;20(2):301. doi: 10.3390/ijms20020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Balan P, Popovich DG. Review of ginseng anti-diabetic studies. Molecules. 2019;24(24):4501. doi: 10.3390/molecules24244501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, Groop PH, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JP, Ratner RE. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241–255. doi: 10.2337/db16-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaonkar VP, Hullatti K. Indian traditional medicinal plants as a source of potent anti-diabetic agents: a review. J Diabetes Metab Disord. 2020;19:1895–908. 10.1007/s40200-020-00628-8. [DOI] [PMC free article] [PubMed]
- 8.Mohammed A, Islam MS. Spice-derived bioactive ingredients: potential agents or food adjuvant in the management of diabetes mellitus. Front Pharmacol. 2018;9:893. doi: 10.3389/fphar.2018.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira ASP, Banegas-Luna AJ, Peña-García J, Pérez-Sánchez H, Apostolides Z. Evaluation of the anti-diabetic activity of some common herbs and spices: providing new insights with inverse virtual screening. Molecules. 2019;24(22):4030. doi: 10.3390/molecules24224030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, Ern LY, Ashraf NA, Kit SW, Yee TS, Pichika MR, Gorain B, Kesharwani P. An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J Tradit Complement Med. 2017;8(3):361–376. doi: 10.1016/j.jtcme.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun W, Shahrajabian MH, Cheng Q. Anise (Pimpinella anisum L.), a dominant spice and traditional medicinal herb for both food and medicinal purposes. Cogent Biol. 2019;5:1. doi: 10.1080/23312025.2019.1673688. [DOI] [Google Scholar]
- 13.Rajeshwari U, Shobha I, Andallu B. Comparison of aniseeds and coriander seeds for antidiabetic, hypolipidemic and antioxidant activities. Spatula DD. J Complement Med Drug Disc. 2011;1(1):9–16. doi: 10.5455/spatula.20110106123144. [DOI] [Google Scholar]
- 14.Shobha RI, Andallu B. Antioxidant, anti-diabetic and hypolipidemic effects of aniseeds (Pimpinella anisum L.): in vitro and in vivo studies. J Complement Med Alt Healthc. 2018;5(2). 10.19080/JCMAH.2018.05.555656.
- 15.Hwang SH, Li HM, Lim SS, Wang Z, Hong JS, Huang B. Evaluation of a standardized extract from Morus alba against α-Glucosidase inhibitory effect and postprandial Antihyperglycemic in patients with impaired glucose tolerance: a randomized double-blind clinical trial. Evid Based Complement Alternat Med. 2016;2016:8983232–8983210. doi: 10.1155/2016/8983232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing T, Kang Y, Xu X, Wang B, Du M, Zhu M-J. Raspberry supplementation improves insulin signaling and promotes brown-like adipocyte development in white adipose tissue of obese mice. Mol Nutr Food Res. 2018;62:1701035. doi: 10.1002/mnfr.201701035. [DOI] [PubMed] [Google Scholar]
- 17.Xiao D, Zhu L, Edirisinghe I, Fareed J, Brailovsky Y, Burton-Freeman B. Attenuation of Postmeal metabolic indices with red raspberries in individuals at risk for diabetes: a randomized controlled trial. Obesity. 2019;27(4):542–550. doi: 10.1002/oby.22406. [DOI] [PubMed] [Google Scholar]
- 18.Romeo GR, Lee J, Mulla CM, Noh Y, Holden C, Lee BC. Influence of cinnamon on glycemic control in individuals with prediabetes: a randomized controlled trial. J Endocr Soc. 2020:bvaa094. 10.1210/jendso/bvaa094. [DOI] [PMC free article] [PubMed]
- 19.Kizilaslan N, Erdem NZ. The effect of different amounts of cinnamon consumption on blood glucose in healthy adult individuals. I J Food Sci. 2019;2019:1–9. 10.1155/2019/4138534. [DOI] [PMC free article] [PubMed]
- 20.Zare R, Shams M, Heydari M, Najarzadeh A, Zarshenas M. Analysis of the efficacy of cinnamon for patients with diabetes mellitus type II based on traditional Persian medicine syndrome differentiation: a randomized controlled trial. Shiraz E-Med J. 2020;21(7):e95609. doi: 10.5812/semj.95609. [DOI] [Google Scholar]
- 21.Zare R, Nadjarzadeh A, Zarshenas MM, Shams M, Heydari M. Efficacy of cinnamon in patients with type II diabetes mellitus: a randomized controlled clinical trial. Clin Nutr. 2019;38(2):549–556. doi: 10.1016/j.clnu.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Mohan R, Jose S, Mulakkal J, Karpinsky-Semper D, Swick AG, Krishnakumar IM. Water-soluble polyphenol-rich clove extract lowers pre- and post-prandial blood glucose levels in healthy and prediabetic volunteers: an open label pilot study. BMC Complement Altern Med. 2019;19:99. doi: 10.1186/s12906-019-2507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morovati A, Pourghassem Gargari B, Sarbakhsh P, Azari H, Lotfi-Dizaji L. The effect of cumin supplementation on metabolic profiles in patients with metabolic syndrome: a randomized, triple blind, placebo-controlled clinical trial. Phytother Res. 2019;33(4):1182–1190. doi: 10.1002/ptr.6313. [DOI] [PubMed] [Google Scholar]
- 24.Jafari S, Sattari R, Ghavamzadeh S. Evaluation the effect of 50 and 100 mg doses of Cuminum cyminum essential oil on glycemic indices, insulin resistance and serum inflammatory factors on patients with diabetes type II: a double-blind randomized placebo-controlled clinical trial. J Tradit Complement Med. 2016;7:332–338. doi: 10.1016/j.jtcme.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samani Keihan G, Gharib MH, Momeni A, Hemati Z, Sedighin R. A comparison between the effect of Cuminum Cyminum and vitamin E on the level of Leptin, Paraoxonase 1, HbA1c and oxidized LDL in diabetic patients. Int J Mol Cell Med. 2016;5(4):229–235. [PMC free article] [PubMed] [Google Scholar]
- 26.Goodarzi MT, Khodadadi I, Tavilani H, Abbasi OE. The role of Anethum graveolens L. (Dill) in the management of diabetes. J Tropic Med. 2016:1098916. 10.1155/2016/1098916. [DOI] [PMC free article] [PubMed]
- 27.Haidari F, Zakerkish M, Borazjani F, Ahmadi Angali K, Amoochi FG. The effects of Anethum graveolens (dill) powder supplementation on clinical and metabolic status in patients with type 2 diabetes. Trials. 2020;21(1):483. doi: 10.1186/s13063-020-04401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sargolzari MS, Mansouri A, Shahdadi H, Masinaei Nezhad N, Poodineh MM. The effect of dill tablet on the level of fasting blood sugar in patients with type II diabetes. J Diabetes Nurs. 2017;5(2):86–94. [Google Scholar]
- 29.Oshaghi EA, Tavilani H, Khodadadi I, Goodarz MT. Dill tablet: a potential antioxidant and anti-diabetic medicine. Asian Pacific J Tropic Biomed. 2015;5:720–727. doi: 10.1016/j.apjtb.2015.06.012. [DOI] [Google Scholar]
- 30.Geberemeskel AG, Debebe GY, Nguse AN. Antidiabetic effect of Fenugreek seed powder solution (Trigonella foenum-graecum L.) on hyperlipidemia in diabetic patients. J Diabetis Res. 2019:8507453. 10.1155/2019/8507453. [DOI] [PMC free article] [PubMed]
- 31.Ranade M, Mudgalkar N. A simple dietary addition of fenugreek seed leads to the reduction in blood glucose levels: a parallel group, randomized single-blind trial. Ayu. 2017;38(1–2):24–27. doi: 10.4103/ayu.AYU_209_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaddam A, Galla C, Thummisetti S, Marikanty RK, Palanisamy UD, Rao PV. Role of fenugreek in the prevention of type 2 diabetes mellitus in prediabetes. J Diabetes Metab Disord. 2015;14:74. doi: 10.1186/s40200-015-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammadi-Sartang M, Sohrabi Z, Barati-Boldaji R, Raeisi-Dehkordi H, Mazloom Z. Flaxseed supplementation on glucose control and insulin sensitivity: a systematic review and meta-analysis of 25 randomized, placebo-controlled trials. Nutr Rev. 2018;76(2):125–139. doi: 10.1093/nutrit/nux052. [DOI] [PubMed] [Google Scholar]
- 34.Hasaniani N, Rahimlou M, Ramezani Ahmadi A, Mehdizadeh Khalifani A, Alizadeh M. The effect of flaxseed enriched yogurt on the glycemic status and cardiovascular risk factors in patients with type 2 diabetes mellitus: randomized, open-labeled, controlled study. Clin Nutr Res. 2019;8(4):284–295. doi: 10.7762/cnr.2019.8.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soleimani A, Taghizadeh M, Bahmani F, Badroj N, Asemi Z. Metabolic response to omega-3 fatty acid supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36(1):79–84. doi: 10.1016/j.clnu.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Yari Z, Rahimlou M, Poustchi H, Hekmatdoost A. Flaxseed supplementation in metabolic syndrome management: a pilot randomized, open-labeled, controlled study. Phytother Res. 2016;30:1339–1344. doi: 10.1002/ptr.5635. [DOI] [PubMed] [Google Scholar]
- 37.Ansary J, Forbes-Hernández TY, Gil E, Cianciosi D, Zhang J, Elexpuru-Zabaleta M, Simal-Gandara J, Giampieri F, Battino M. Potential health benefit of garlic based on human intervention studies: a brief overview. Antioxidants (Basel) 2020;9(7):619. doi: 10.3390/antiox9070619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamal S, Cherukuri L, Birudaraju D, Matsumoto S, Kinninger A, Chaganti BT, Flores F, Shaikh K, Roy SK, Budoff MJ. Short-term impact of aged garlic extract on endothelial function in diabetes: a randomized, double-blind, placebo-controlled trial. Exp Ther Med. 2020;19(2):1485–1489. doi: 10.3892/etm.2019.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banigesh IA, Hamad RA, Dihom AA, El-Mahdi MI. Reduction of cholesterol and fasting blood sugar levels by one month supplementation of fresh garlic in diabetic Libyan patients: a double blind, baseline controlled study. Libiyan Int Med Uni J. 2017;2(1). 10.21502/limuj.007.02.2017.
- 40.Shoshi JS, Akhter H. Effects of garlic (Allium sativum) on blood glucose level in type 2 diabetes mellitus patients treated with metformin. J Enam Med Coll. 2017;7(3):151–155. doi: 10.3329/jemc.v7i3.34075. [DOI] [Google Scholar]
- 41.Huang FY, Deng T, Meng LX, Ma XL. Dietary ginger as a traditional therapy for blood sugar control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Medicine. 2019;98(13):e15054. doi: 10.1097/MD.0000000000015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makhdoomi Arzati M, Mohammadzadeh Honarvar N, Saedisomeolia A, Anvari S, Effatpanah M, Makhdoomi Arzati R, Yekaninejad MS, Hashemi R, Djalali M. The effects of ginger on fasting blood sugar, hemoglobin A1c, and lipid profiles in patients with type 2 diabetes. Int J Endocrinol Metab. 2017;15(4):e57927. doi: 10.5812/ijem.57927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khandouzi N, Shidfar F, Rajab A, Rahideh T, Hosseini P, Mir TM. The effects of ginger on fasting blood sugar, hemoglobin A1C, apolipoprotein B, apolipoprotein a-I and malondialdehyde in type 2 diabetic patients. Iran J Pharm Res. 2015;14(1):131–140. [PMC free article] [PubMed] [Google Scholar]
- 44.Ghazi Zahedi S, Koohdani F, Qorbani M, Siassi F, Keshavarz A, Nasli-Esfahani E, Aghasi M, Khoshamal H, Sotoudeh G. The effects of green cardamom supplementation on blood pressure and endothelium function in type 2 diabetic patients: a study protocol for a randomized controlled clinical trial. Medicine. 2020;99(18):e11005. doi: 10.1097/MD.0000000000011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aghasi M, Ghazi-Zahedi S, Koohdani F, Siassi F, Nasli-Esfahani E, Keshavarz A, Qorbani M, Khoshamal H, Salari-Moghaddam A, Sotoudeh G. The effects of green cardamom supplementation on blood glucose, lipids profile, oxidative stress, sirtuin-1 and irisin in type 2 diabetic patients: a study protocol for a randomized placebo-controlled clinical trial. BMC Complement Altern Med. 2018;18(1):18. doi: 10.1186/s12906-017-2068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nayebi N, Esteghamati A, Meysamie A, Khalili N, Kamalinejad M, Emtiazy M, et al. The effects of a Melissa officinalis L. based product on metabolic parameters in patients with type 2 diabetes mellitus: a randomized double-blinded controlled clinical trial. J Complement Integr Med. 2019;16(3). 10.1515/jcim-2018-0088. [DOI] [PubMed]
- 47.Asadi A, Shidfar F, Safari M, Hosseini AF, Fallah Huseini H, Heidari I, Rajab A. Efficacy of Melissa officinalis L. (lemon balm) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a randomized, double-blind, clinical trial. Phytother Res. 2019;33(3):651–659. doi: 10.1002/ptr.6254. [DOI] [PubMed] [Google Scholar]
- 48.Rahimi HR, Mohammadpour AH, Dastani M, Jaafari MR, Abnous K, Ghayour Mobarhan M, Kazemi OR. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed. 2016;6:567–577. [PMC free article] [PubMed] [Google Scholar]
- 49.Jiménez-Osorio AS, García-Niño WR, González-Reyes S, Álvarez-Mejía AE, Guerra-León S, Salazar-Segovia J, Falcón I, Montes de Oca-Solano H, Madero M, Pedraza-Chaverri J. The effect of dietary supplementation with Curcumin on redox status and Nrf2 activation in patients with nondiabetic or diabetic Proteinuric chronic kidney disease: a pilot study. J Ren Nutr. 2016;26:237–244. doi: 10.1053/j.jrn.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 50.Panahi Y, Khalili N, Sahebi E, Namazi S, Reiner Ž, Majeed M, Sahebkar A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: a randomized controlled trial. Complement Ther Med. 2017;33:1–5. doi: 10.1016/j.ctim.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Panahi Y, Khalili N, Sahebi E, Namazi S, Simental-Mendía LE, Majeed M, Sahebkar A. Effects of Curcuminoids plus Piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled trial. Drug Res. 2018;68(7):403–409. doi: 10.1055/s-0044-101752. [DOI] [PubMed] [Google Scholar]
- 52.Hodaei H, Adibian M, Nikpayam O, Hedayati M, Sohrab G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: a randomized, double-blind clinical trial. Diabetol Metab Syndr. 2019;11:41. doi: 10.1186/s13098-019-0437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poswal FS, Russell G, Mackonochie M, MacLennan E, Adukwu EC, Rolfe V. Herbal teas and their health benefits: a scoping review. Plant Foods Hum Nutr. 2019;74(3):266–276. doi: 10.1007/s11130-019-00750-w. [DOI] [PubMed] [Google Scholar]
- 54.Kelleni MT. Chamomile tea potentials in prevention and amelioration of type 2 diabetes mellitus. J Diabetes Metab. 2016;7:2. doi: 10.4172/2155-6156.1000649. [DOI] [Google Scholar]
- 55.Kaseb F, Yazdanpanah Z, Biregani AN, Yazdanpanah Z. The effect of chamomile (Matricaria recutita L.) infusion on blood glucose, lipid profile and kidney function in Type 2 diabetic patients: a randomized clinical trial. Progress Nutr. 2018;1:110–118. doi: 10.23751/pn.v20i1-S.5884. [DOI] [Google Scholar]
- 56.Zemestani M, Rafraf M, Asghari-Jafarabadi M. Chamomile tea improves glycemic indices and antioxidants status in patients with type 2 diabetes mellitus. Nutrition. 2016;32(1):66–72. doi: 10.1016/j.nut.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Rafraf M, Zemestani M, Asghari-Jafarabadi M. Effectiveness of chamomile tea on glycemic control and serum lipid profile in patients with type 2 diabetes. J Endocrinol Investig. 2015;38:163–170. doi: 10.1007/s40618-014-0170-x. [DOI] [PubMed] [Google Scholar]
- 58.Villa-Rodriguez JA, Aydin E, Gauer JS, Pyner A, Williamson G, Kerimi A. Green and chamomile teas, but not acarbose, attenuate glucose and fructose transport via inhibition of GLUT2 and GLUT5. Mol Nutr Food Res. 2017;61(12). 10.1002/mnfr.201700566. [DOI] [PubMed]
- 59.Meng JM, Cao SY, Wei XL, Gan RY, Wang YF, Cai SX, Xu XY, Zhang PZ, Li HB. Effects and mechanisms of tea for the prevention and Management of Diabetes Mellitus and Diabetic Complications: an updated review. Antioxidants. 2019;8(6):170. doi: 10.3390/antiox8060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borges CM, Papadimitriou A, Duarte DA, Lopes de Faria JM, Lopes de Faria JB. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: a double-blind randomized clinical trial. Sci Rep. 2016;6:28282. doi: 10.1038/srep28282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Amorim LMN N, Vaz SR, Cesario G, ASG C, Botelho PB. Effect of green tea extract on bone mass and body composition in individuals with diabetes. J Funct Foods. 2018;40:589–594. doi: 10.1016/j.jff.2017.11.039. [DOI] [Google Scholar]
- 62.Ma Q, Chen D, Sun HP, Yan N, Xu Y, Pan CW. Regular Chinese green tea consumption is protective for diabetic retinopathy: a clinic-based case-control study. J Diabetes Res. 2015;231570:1–7. doi: 10.1155/2015/231570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quirarte-Báez SM, Zamora-Perez AL, Reyes-Estrada CA, et al. A shortened treatment with rosemary tea (Rosmarinus officinalis) instead of glucose in patients with diabetes mellitus type 2 (TSD) J Popul Ther Clin Pharmacol. 2019;26(4):e18–e28. doi: 10.15586/jptcp.v26i4.634. [DOI] [PubMed] [Google Scholar]
- 64.Shawabkeh JM, Jamal A. Effect of rosemary on fasting blood glucose, hemoglobin A1c and Vitamin B12 in healthy person and Type 2 diabetic patients taking glucomid or/and metformin. Nat J Physio, Pharm Pharmaco. 2018;8(1):87. [Google Scholar]
- 65.Herrera-Rangel AB, Aranda-Moreno C, Mantilla-Ochoa T, Zainos-Saucedo L, Jáuregui-Renaud K. Influence of the body mass index on the occurrence of falls in patients with type 2 diabetes mellitus. Obes Res Clin Pract. 2015;9(5):522–526. doi: 10.1016/j.orcp.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Chao M, Wang C, Dong X, Ding M. The effects of tai chi on type 2 diabetes mellitus: a meta-analysis. J Diabetes Res. 2018;2018:7350567–7350569. doi: 10.1155/2018/7350567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hermanns M, Haas BK, Rath L, Murley B, Arce-Esquivel AA, Ballard JE, Wang YT. Impact of tai chi on peripheral neuropathy revisited: a mixed-methods study. Gerontolo Geriatric Med. 2018;4:2333721418819532. doi: 10.1177/2333721418819532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia TW, Yang Y, Li WH, Tang ZH, Li ZR, Qiao LJ. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19(1):63. doi: 10.1186/s12906-019-2475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chattopadhyay K, Mishra P, Manjunath NK, Harris T, Hamer M, Greenfield SM, Wang H, Singh K, Lewis SA, Tandon N, Kinra S, Prabhakaran D. Development of a yoga program for Type-2 diabetes prevention (YOGA-DP) among high-risk people in India. Front Public Health. 2020;8:548674. doi: 10.3389/fpubh.2020.548674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramamoorthi R, Gahreman D, Skinner T, Moss S. The effect of yoga practice on glycemic control and other health parameters in the prediabetic state: a systematic review and meta-analysis. PLoS One. 2019;14(10):e0221067. doi: 10.1371/journal.pone.0221067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable/ No data sets were generated during the present study. The present review is based on the cited references.