Abstract
Early adversity influences brain development and emerging behavioral phenotypes relevant for psychiatric disorders. Understanding the effects of adversity before and after conception on brain development has implications for contextualizing current public health crises and pervasive health inequities. The use of functional magnetic resonance imaging (fMRI) to study the brain at rest has shifted understanding of brain functioning and organization in the earliest periods of life. Here we review applications of this technique to examine effects of early life stress (ELS) on neurodevelopment in infancy, and highlight targets for future research. Building on the foundation of existing work in this area will require tackling significant challenges, including greater inclusion of often marginalized segments of society, and conducting larger, properly powered studies.
Harnessing Advances in Neuroscience to Improve Prevention of Brain-Based Disorders and Address Public Health Challenges
Despite rapid scientific progress in understanding human brain functioning, global prevalence rates of mental health disorders exceed 10% across all geographic regions and account for more than 14% of years lived with disability (YLD) [1]. Recent trends identified in the United States do not suggest a more promising picture, as rates of suicidality have increased 33% over the last 20 years [2] to become the second leading cause of death among people 10–34 years old. [Centers for Disease Control and Prevention WISQARS: Leading causes of death reports, 1981–2016]i. Substance use disorders represent a major contributor to this global burden of disease, accounting for more than 5% of YLDs among people 15–49 years old, in a majority of global regions assessed [1]. The exceptionally high prevalence of opioid use disorders in the United States, described as ‘an era-defining epidemic’ [1], further speaks to the growing public health impact of brain-based disorders that have ironically and sadly paralleled increasing scientific insights into brain functioning. The challenges of identifying and providing effective treatments for psychiatric disorders [3,4] underlying public health crises, indicate a critical need to better understand and support healthy brain development.
A shift in the characterization and understanding of brain function has occurred over the past two decades. Investigators have identified a reliable, limited set of large-scale brain networks that interact to support early primary sensorimotor functions, along with higher order cognition. Resting state functional connectivity magnetic resonance imaging (rs-fcMRI), which measures correlated brain activity while subjects are at rest, has been an important tool for establishing and advancing this network based view of the brain [5,6]. This shift from more traditional research paradigms, that approach the brain as a set of unique regions, frequently defined by stimulus or task specific activation patterns, to characterizing it in terms of a set of intrinsically correlated dynamic systems [5], has revealed how brain functioning and organization varies between individuals [7,8], in relation to mental health conditions [9,10], and over the course of development [11].
One of the more promising related research trends highlights the early developmental emergence of these functional brain networks. This work, often using rs-fcMRI with infants, has provided compelling evidence for the rapid, early development of complex brain systems involved in future higher order cognitive functioning and psychopathologies. This work has also highlighted their vulnerabilities associated with exposure to ELS beginning in the prenatal period. Stress in this sense refers broadly to any form of adversity involving a threat to the homeostasis of an organism [12]. The findings regarding early brain network development in the context of ELS (including pre- and postnatal adversity) provide a natural target for early interventions to support healthy brain development and reduce the risk of subsequently emerging psychiatric disorders.
A 2015 paper from our group emphasized the potential of functional MRI research with infants to advance understanding of the influence of ELS on brain development and risk for psychiatric disorders [13]. We focused primarily on the future promise of this technique due to limited existing research. Without diminishing the contributions of other noninvasive neuroimaging techniques, such as electroencephalogram (EEG) and functional near-infrared spectroscopy (fNIRS), which have played critical and complementary roles in advancing understanding of neurodevelopmental susceptibility to ELS [14], we now take stock of recent progress in research using rs-fcMRI to examine effects of ELS on early brain development. We focus on rs-fcMRI due to its role in revealing novel information about human brain functioning and organization across the lifespan, and the advantage of consistent application across developmental stages and species due to the lack of task demands.
We begin with a brief overview of the application of rs-fcMRI to advance understanding of early developing functional brain architecture. Next, we provide a theoretical and mechanistic framework for interpreting research regarding effects of ELS on neurodevelopment, which includes an emphasis on the obligatory role of environmental input in early neurodevelopmental processes. This is followed by a review of key findings and relevant themes in research using rs-fcMRI to examine effects of ELS on early neurodevelopment. We then discuss methodological considerations for interpretation of the existing literature and for planning future research efforts. We conclude with a brief synthesis of current findings and relevant theoretical models, and a discussion of future research priorities, including identifying and testing mechanistic pathways, addressing the issue of reproducibility, and increasing representation of individuals facing high levels of adversity. We suggest that the current climate of social unrest, highlighting institutionalized racial violence, and widespread mistreatment of racial and ethnic minority groups, and those with limited economic resources, provides further impetus for research in this area regarding how various forms of adversity impact brain development and related health inequities.
Resting State Functional Connectivity MRI Provides Unique Insight into Early Neurodevelopmental Processes in Humans
Initial studies of correlated brain activity as assessed via rs-fcMRI, identified correlated activity in bilateral sensorimotor cortex [15]. This work, and much of the early literature, focused on using ‘seed’ brain regions, whose activity was correlated across all voxels (akin to pixels) of the brain to produce a connectivity map between that region and all other parts of the brain. Later, application of more data-driven approaches, based on the concept of independent components analysis [16], decomposed brain activity into set of spatial maps (i.e., components). This work was quickly followed by examination of connectivity measures using graph theory.
Graph theory defines a network as a collection of nodes (e.g., brain regions) connected by lines or edges (e.g., correlated brain activity). Within this framework, community structure refers to the appearance of densely connected groups of nodes with sparse connections between the groups [11]. Using graph theoretical analyses with rs-fcMRI revealed that the adult and child brain has several modules or communities, known as ‘resting-state networks’ (RSNs) [6,17]. These networks often include sensory/motor systems, the default-mode network (DMN), the dorsal attention network, the cingulo-opercular or salience module, the frontoparietal network, and the ventral attention network, amongst others (Figure 1). RSNs are highly reproducible across methods, datasets, and individuals [6,17–19], and have become an influential framework for interpreting functional and structural neuroimaging data. RSNs are also largely conserved across species [20], providing a bridge to translate mechanistic animal studies to humans.
Figure 1. Reproducible Resting State Network Topology at the Group and Individual Level.
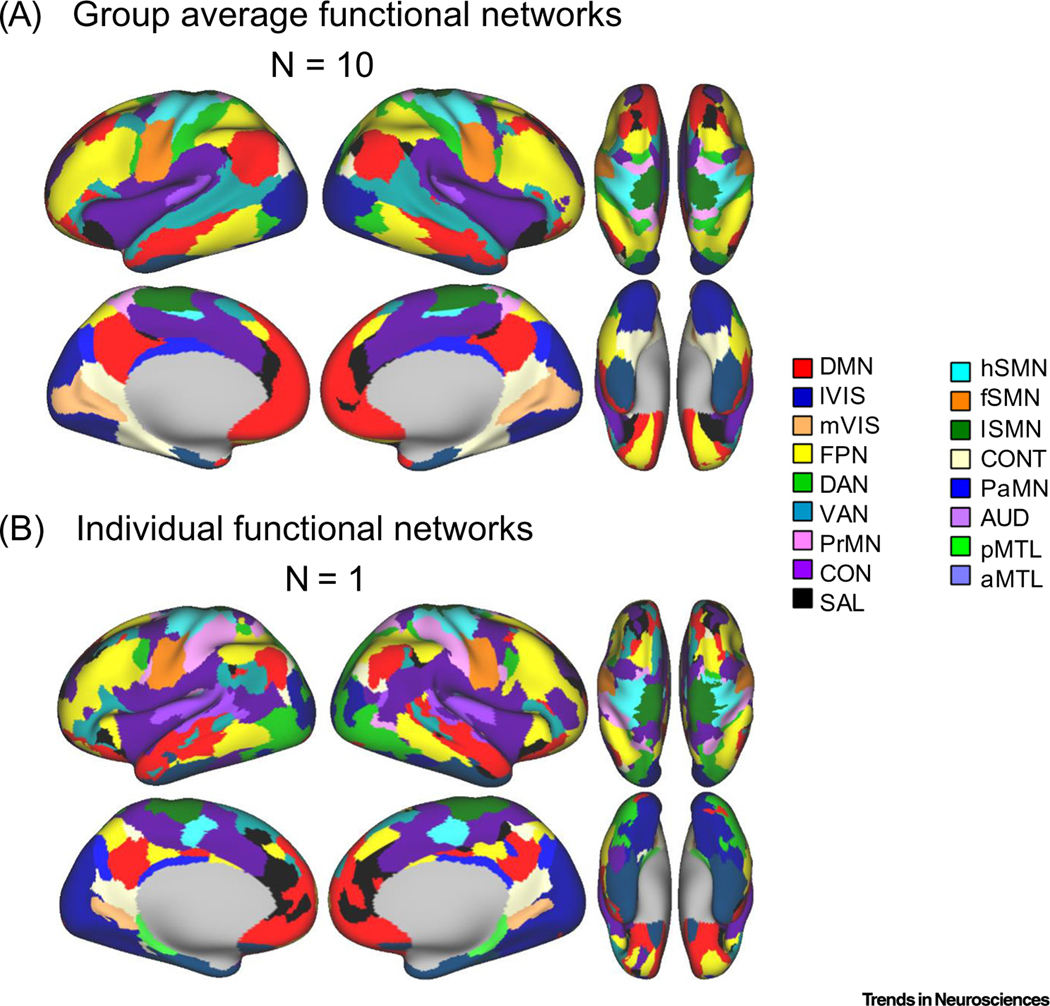
This figure, modified from [7], illustrates high resolution human functional neural network topology. Data used in these analyses were from the Midnight Scan Club, a study using a unique acquisition protocol involving extended sampling times for resting state functional magnetic resonance imaging (fMRI) data to facilitate reliable estimation of functional network topology at the individual level [7]. Community detection (Infomap) was utilized as a data driven way to identify highly interconnected functional systems across the brain. Panel (A) depicts an average whole brain vertex-to-vertex correlation matrix from ten highly sampled individuals, and (B) a highly sampled individual. Importantly, the individual networks highlight the vast heterogeneity of topologies within individuals, a characteristic that can be leveraged in a similar way to characterize individual network features in infants and toddlers for an array of scientific inquires. Networks identified in both images are labelled based on known functions in the literature. The list of networks included are the default mode network (DMN), the lateral and medial visual networks (lVIS and mVIS respectively), the frontal parietal network (FPN), the dorsal attention network (DAN), the ventral attention network (VAN), the premotor network (PrMN), the cingulo-opercular network (CON), the salience network (SAL), hand, face, and leg somatomotor networks (hSMN, fSMN, and lSMN respectively), the context network (CONT), the parietal memory network (PaMN), the auditory network (AUD), the posterior medial temporal network (pMTL), and the anterior medial temporal network (aMTL).
Several recent papers provide overviews of the early development of RSNs [11,21,22]. We therefore provide only a brief summary as a backdrop for understanding the potential influence of ELS. Interestingly, defining characteristics of adult brain organization, such as small worldness, involving a balance between long range connections and a high degree of local connectivity, are already identified in the neonatal period [23,24] and in preterm infants [25]. Sensory and motor RSNs, including somatomotor, primary auditory, and primary and secondary visual, are evident soon after birth [11,24]. Rs-fcMRI research with preterm infants [26] and a growing number of studies during the fetal period [27] have further investigated the emergence of these patterns of bilateral functional connectivity prior to birth or term equivalent age. Over the first 2 years of life, sensory and motor RSNs undergo refinement, and large scale RSNs, involving heteromodal association cortices and spanning greater anatomical distances, rapidly consolidate [11,13,28].
This work builds on an existing body of literature documenting the rapid progression of early neurodevelopmental processes beginning in utero, which sets the stage for both vulnerability and opportunity with regard to the potential influence of the environment (Box 1). However, critical questions remain and are actively under investigation. Research with rs-fcMRI in adults has recently pushed beyond examining network organization based on group averaging, to reveal novel individual-specific RSN maps (Figure 1), which correspond with structural, histological, and task-derived brain features [7,8]. This work now extends down to childhood [29], and efforts to identify the nature of individual networks in infancy are sorely needed. In addition, results of ongoing multisite efforts involving improved methods for acquisition, processing and analysis, larger sample sizes, and dense sampling across key early developmental periods [21,30], will be needed to update or confirm the current state of the literature. Nonetheless the research to date has shed light on the rapid and early emergence of complex functional brain architecture.
Box 1. Rapid Early Neurodevelopment Confers Vulnerability and Opportunity.
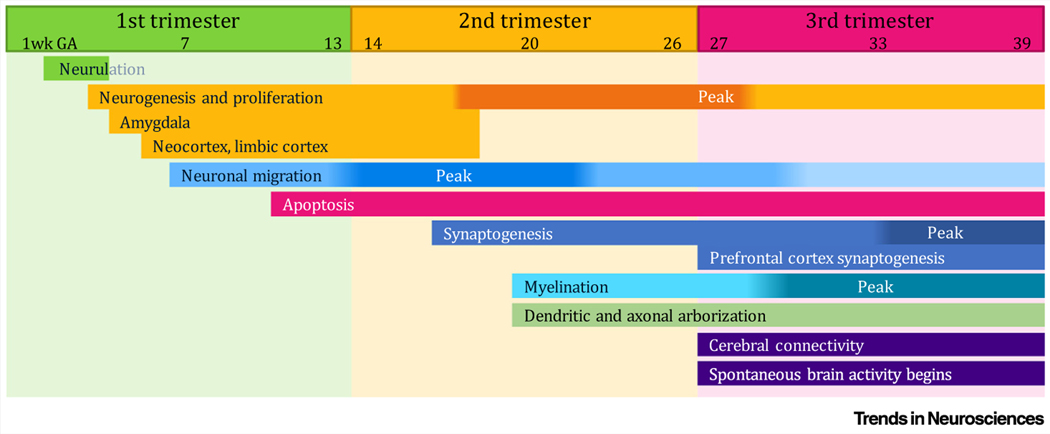
The field of the developmental origins of health and disease highlights the extent to which biological systems undergoing rapid changes, are particularly prone to both organizing and disorganizing influences [92]. The prenatal period through to early infancy represents a period of particularly concentrated change for the brain. During the prenatal period, the nervous system develops all the way from an initial basic organization of cells into a functioning and interconnected system capable of responding to a dynamic environment. During the first trimester, neuroepithelial cells differentiate into neural progenitors, followed by neurulation beginning at 2 to 3 weeks gestational age (GA), and subsequent neurogenesis and neuronal proliferation [93]. Beginning at 8 weeks GA, neuronal migration results in the formation of the subplate zone, which plays a critical role in guiding development of the cortex [94]. Subcortical brain regions, such as the amygdala, can be identified by 6-weeks GA with differentiation into the three known subnuclei occurring over the subsequent weeks [95] (Figure I, [96]).
Myelination, synaptogenesis, and dendritic and axonal arborization, happens rapidly during the second and third trimesters, providing the framework for communication between neurons and anatomically distant brain regions. Between the 8th and 24th week of GA, cortical circuits are organized [93], and by ~17 weeks the cingulum bundle connecting the frontal and parietal regions is visible [97]. In the transition from 2nd to 3rd trimester, long-distance corticocortical connections develop rapidly with maximal axonal growth and elongation evident from 21 to 43 weeks GA, and major structural pathways associated with rich club organization observed by 30 weeks GA [98]. The period right before birth, characterized by a rapid and dramatic increase in synaptic connections and cortical growth, is considered a critical period for the development of the cortical connectome (Figure I) [25].
Rapid brain growth and a complex array of neurodevelopmental processes continue during infancy and toddlerhood, as exemplified by a doubling in brain volume during the first year of life [99] and a fourfold increase by four years of age [100]. Development of communication between neurons is facilitated by rapid increases in synaptic density during the first year of life [101] and ongoing synaptic pruning as circuitry is refined [101,102]. This pace of early brain development, and the involvement of stress-sensitive biological mediators in guiding many aspects of neurodevelopment, sets the stage for environmental factors to play a significant role in shaping the brain.
Figure I. Timeline of Rapid Progression of Prenatal Neurodevelopmental Processes.
Brain development proceeds rapidly during the embryonic and fetal period. Stress-sensitive aspects of maternal–placental–fetal (MPF) biology play an obligatory role in guiding neurodevelopmental processes, providing ample opportunity for environmental conditions to influence the trajectory of development. Brain regions with high concentrations of glucocorticoid receptors and known roles in susceptibility for psychiatric disorders, including the amygdala, begin to develop during the first trimester of pregnancy. See also [96] for further details and relevant citations. Abbreviations: GA, Gestational age.
Framework and Mechanisms for Understanding Effects of ELS on the Developing Brain
Just as maternal and offspring genes play an obligatory role in the developing brain, environmental stimuli provide necessary input for neurodevelopment [31,32]. During embryonic and fetal brain development, stress-sensitive aspects of maternal–placental–fetal (MPF) biology play a role in guiding neurodevelopmental processes including neurogenesis, neuronal migration, growth of axons and dendrites, synaptogenesis, and initial myelination [31,32]. These include endocrine [e.g., cortisol and corticotropin-releasing hormone (CRH)], immune [e.g., interleukin-6 (IL-6) and other cytokines and chemokines], and oxidative stress mechanisms with capacity for sensing variation in environmental conditions (both preconception and during pregnancy), transducing this variation to the developing brain, and affecting neurodevelopmental processes [32]. Despite the obligatory role of these biological mediators, elevated levels in response to adverse environmental conditions can lead to alterations in the developing brain and increase risk for subsequent psychiatric disorders [32]. For example, a growing body of cross-species research has examined the role of heightened maternal inflammation during pregnancy, in influencing early neurodevelopment and emerging behavioral-risk phenotypes (Box 2) [33–41]. IL-6, a proinflammatory signaling protein or cytokine, which has been described as a sensor, transducer and effector of environmental influences on fetal brain development, due to its sensitivity to environmental influences, capacity to impact the more proximal fetal environment (placental tissue, amniotic fluid, and fetal brain), and known obligatory role in fetal neurodevelopmental processes (cellular survival, proliferation and differentiation, axonal growth, and synaptogenesis) [39,40], has been a frequent focus of this work. However, IL-6 is one of many mediators of inflammation with potential impacts on fetal brain development, and efforts to capture cumulative and interactive effects of an array of immune and endocrine factors are underway [34].
Box 2. Leveraging Cross Species Work to Advance Mechanistic Understanding.
Importantly, understanding of the effects of early life stress (ELS) on brain development draws heavily on animal models providing clear examples of phenomena that can be more challenging to capture and characterize in humans. Animal research provides a high level of control over the environmental variables of interest and confounding variables, and the ability for repeated assessment of brain development in the same animals across time. The use of resting state functional connectivity magnetic resonance imaging (rs-fcMRI), among other neuroimaging modalities, provides an important translational bridge between mechanistic animal model studies and larger human studies. Ongoing translation between human and animal model research aims to advance understanding of early brain development in the context of ELS in several ways, including a focus on hypothesized biological signaling pathways. As an example, heightened inflammation during pregnancy has been well studied in animal models by inducing maternal immune activation (MIA) through administering substances that model a viral infection [polyinosinic:polycytidylic acid (poly(I:C)] an agonist to the toll-like receptor-3 (TLR3), bacterial infection [lipopolysaccharide (LPS), an agonist to the TLR4], or administering bacteria such as streptococcus that commonly cause infection during pregnancy. Developmental exposure to MIA has been well documented to produce long-term alterations in brain development [103] and behaviors related to human mental health disorders such as anxiety [104].
This manipulation allows precise control of the timing of inflammation exposure, but may not mimic the chronic low grade inflammation that is associated with conditions such as depression and obesity in humans. In contrast, our recent and ongoing research includes examining systemic inflammation during pregnancy in non-human primates (NHPs) in the context of a diet manipulation allowing for variability which may be more relevant to these conditions [33]. Due to similarities between NHPs and humans in terms of brain structure, functioning, developmental timelines, and complex behaviors, this research provides a unique opportunity to use structural and functional MRI to examine analogous brain outcomes across species. Our recent work, provides an example of this potential by examining similar indicators of systemic inflammation during pregnancy and offspring brain outcomes across species [33,39,40].
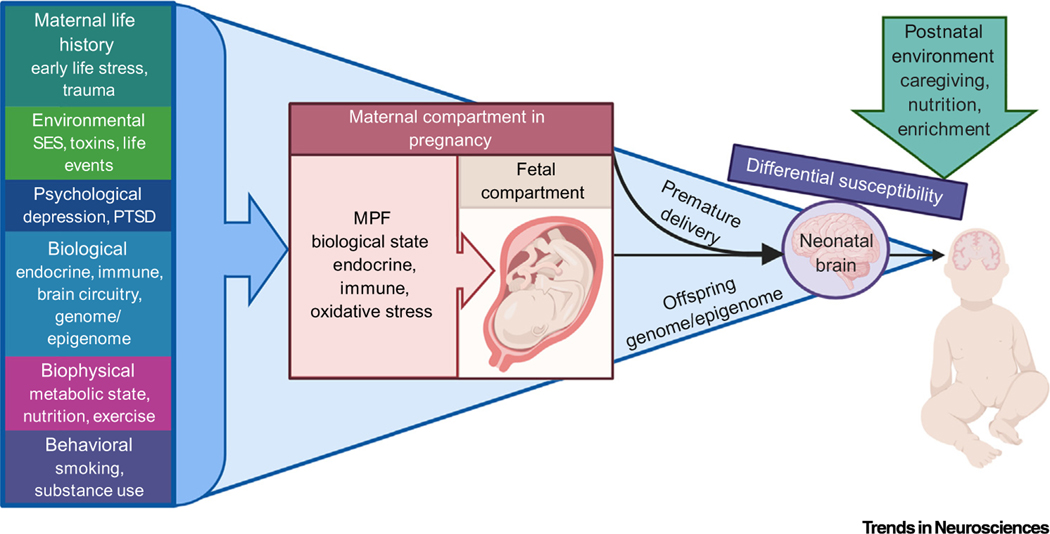
A large number of environmental factors occurring prior to conception and during pregnancy, such as maternal early life adversity, heightened psychological stress, high body mass index, poor nutrition, and substance use, have the potential to alter MPF stress biology (Figure 2) [32,42–44]. The wide array and frequent co-occurrence of these potential influences creates difficulty in disentangling them, and associated challenges for advancing scientific understanding of causal mechanisms and identifying potentially fruitful avenues for intervention. Animal models with more controlled experimental conditions can help in this regard (Box 2). To address this challenge in human research, we advocate an increasing focus on biological mechanisms, such that risk factors are not examined and conceptualized separately from hypothesized mechanistic pathways. This will ultimately involve a multivariate approach and larger sample sizes to allow for examining cumulative and interactive pathways at each step, from environmental conditions to MPF biology, and from MPF biology to developing brain circuitry (Figure 2).
Figure 2. Cumulative and Interactive Effects of the Preconceptional, Gestational, and Postnatal Environment on Early Neurodevelopment.
Diverse preconceptional and gestational sources of adversity have potential to influence embryonic and fetal neurodevelopmental processes through stress-sensitive aspects of maternal–placental–fetal (MPF) biology. These influences include aspects of maternal life history, current environment, psychological and biological state, physical health, and current behaviors. Variability in the maternal state and environment prior to and during gestation can be carried over into the postnatal environment (as indicated by the shaded blue area continuing across to the postnatal period), creating cumulative and interactive effects between the pre- and postnatal environment. Alterations in neurodevelopment due to prenatal exposures also have potential to alter sensitivity to postnatal environmental influences (i.e., differential susceptibility). Differential susceptibility is depicted as hinging on the fulcrum of the neonatal brain and being tipped in a specific direction by the postnatal environment to indicate that specific combinations of neurobiological phenotypes and environmental conditions will have implications for ongoing development. This is consistent with a view of early environmental influences as altering potential for resilience to, or risk for, psychiatric disorders in a non-deterministic manner, and in interaction with the future environment. Abbreviations: PTSD, Post-traumatic stress disorder; SES, socioeconomic status.
The obligatory role of environmental stimuli in postnatal brain development has been illustrated with animal models, demonstrating how sensory input during specific development windows facilitates cortical organization [45]. Given that environmental input represents a critical and time sensitive influence on postnatal brain development, ELS has been conceptualized in terms of deviation from expected environmental input. In the early postnatal environment, deviation from species typical caregiving is a well-studied form of ELS known to have lasting effects on neurodevelopment and increased risk for psychiatric disorders [46]. Due to the dependence of infants on caregivers for survival, alterations in caregiving also fit within the more general definition of stress as involving a threat to the homeostasis of an organism [12]. Research in humans and animal models has highlighted specific aspects of caregiving (including unpredictability, neglect, and threat) [47,48], that can result in alterations in offspring biology and subsequent behavior. Similar to the prenatal environment, these stress sensitive biological systems with potential to sculpt developing brain circuitry include endocrine factors (cortisol and CRH), inflammatory cytokines and chemokines, oxidative stress, and epigenetic alterations [47,48].
The extent to which various forms of postnatal ELS have negative implications for neurodevelopment and long-term health has been frequently considered. Allostatic load refers to the cumulative effects of stress, and is derived from the term allostasis, referring to the adaptive, acute response to and recovery from a stressor [49]. Building on this concept, developmental researchers derived the terms positive, tolerable, and toxic stress, to differentiate between homeostatic challenge as part of necessary environmental input for neurodevelopmental processes (positive), more significant challenge followed by recovery (tolerable), or more significant challenge, with limited recovery and long duration, or frequent repetition (toxic). [National Scientific Council on the Developing Child (2005) Excessive Stress Disrupts the Architecture of the Developing Brain: Working Paper No. 3.]ii
Regardless of the conceptual model, the influence of ELS on neurodevelopment is understood to involve alteration of susceptibility to psychiatric and other health conditions, as opposed to being deterministic [32]. This is consistent with a view of stress sensitivity in biological systems guiding neurodevelopment as an evolutionarily preserved mechanism, facilitating adaptation to a current or predicted environment [50], and involving an ongoing interaction between the individual and environment over time. This results in significant phenotypic variability between and within individuals over time. In the next section, we consider recent findings linking ELS with developing functional brain systems within this context.
Resting State fMRI Research with Infants Indicates Effects of Stress Exposure at Multiple Levels of Brain Organization
Using rs-fcMRI with infants allows for examining brain functioning within a more proximal timeframe of exposure to ELS, and with the necessary spatial resolution to examine brain circuitry of interest for mediating between stress exposure and subsequent risk for psychiatric disorders. As a prime example, study designs involving neuroimaging shortly after birth have increased capacity to differentiate between variation in brain functioning associated with pre- versus postnatal exposures. The amygdala has been a frequent focus of this work due to developing at an early embryonic stage, containing high levels of receptors for glucocorticoids (cortisol in humans), and the large existing body of stress related amygdala findings in animal models and human children and adults upon which to build [51].
Alterations in infant amygdala functional connectivity have now been documented in relation to several indicators of stress in the prenatal environment. For example, heightened maternal depressive symptoms during pregnancy have been associated with more pronounced negative connectivity between the neonatal mygdala and dorsal medial prefrontal cortex (dmPFC), a region later important for regulation of emotions [52]. Higher maternal depressive symptoms during pregnancy have also been associated with stronger amygdala coordinated functioning with the insula, ventromedial prefrontal cortex, and orbitofrontal cortex, among other regions involved in emotion processing and regulation, in 6-month-old infants, after adjusting for postpartum maternal depressive symptoms [53]. Preterm birth, which can result from elevations in prenatal stress exposure [54] and be considered a significant deviation in the expected early environment, has been associated with decreased strength of functional connectivity between the amygdala, insula, thalamus, cerebellum, and dorsal and medial prefrontal cortex (in preterm infants at term-equivalent age compared to full-term neonates) [55,56]. Discrepancies in the specific connections and direction of effects across studies are not surprising given the differences in study design, population, and analytic approach. However, these studies are hampered by a lack of evidence for how differences in amygdala functional connectivity relate to subsequent development.
Recent work directly examining aspects of MPF stress biology in relation to neonatal amygdala functional connectivity and subsequent behavioral phenotypes, may provide some increased specificity. Higher maternal cortisol levels during pregnancy have been associated with stronger amygdala functional connectivity to brain regions involved in sensory processing and integration, specifically in females [57]. These findings were consistent with prior research, with older children indicating a sex specific pathway from heightened maternal cortisol during pregnancy, to increased internalizing symptoms in females via alterations in the amygdala [57,58]. This work suggests a potential early pathway through which prenatal stress exposure may confer susceptibility to internalizing psychiatric disorders, which are more commonly observed in females [59]. Higher maternal systemic inflammation during pregnancy (as indicated by IL-6) has also been associated with stronger neonatal amygdala connectivity, although findings were not sex specific [39]. Heightened maternal inflammation was associated with increased amygdala connectivity to regions involved in salience detection (the anterior insula) and higher level sensory processing (the fusiform gyrus). Both larger amygdala volume and stronger amygdala to fusiform gyrus functional connectivity, mediated an effect of heightened maternal IL-6 on decreased child impulse control at 24-months of age. Future studies will be needed to replicate these effects, but the initial results indicate a possible influence of heightened MPF stress biology on emerging offspring behavioral risk phenotypes, via alterations in early amygdala coordinated functioning with regions involved in sensory processing and integration.
Despite substantial evidence for effects of ELS on early amygdala development, it is well recognized that impacts are not restricted to a single brain region or limited set of brain connections. For example, even alterations specifically within the amygdala can have a widespread impact on functional brain organization [60]. There is now also direct evidence for associations between pre- and postnatal ELS and alterations in large-scale brain systems beginning in the neonatal period. Preterm birth has been associated with an overall reduction in functional connectivity strength between anatomically distant brain regions, including reduced interhemispheric connectivity and connectivity within large scale RSNs [26,55,61]. Preterm birth has also been associated with reduced adult like global network architecture, including rich club organization [62]. Reduced strength of RSNs associated with preterm birth appears to be exacerbated by other factors, such as heightened maternal psychological distress during pregnancy [56] and the occurrence of painful procedures in the hospital following birth [63].
These findings highlight cumulative and interactive effects among frequently co-occurring sources of ELS, and indicate the utility of considering common biological pathways for effects of multiple sources of ELS. One such proposed pathway, heightened maternal inflammation during pregnancy, has been associated with alterations in neonatal brain functional connectivity within and between large scale brain systems [38,41]. These findings demonstrate consistency across different samples and analytic approaches with regard to effects on the developing salience network [38,41]. Alterations within and between connectivity of large scale brain systems linked to heightened maternal inflammation, further demonstrate significant overlap with brain regions implicated in working memory in adults, and a direct association between heightened maternal inflammation and poorer working memory at 24-months of age has been identified [38]. These results are of particular interest given common deficits in working memory among psychiatric disorders, linked to exposure to heightened inflammation in utero.
During the first year of life, common sources of ELS, such interparental conflict [64] and low socioeconomic status [65], have been associated with differences in connectivity of large scale RSNs, such as the DMN. These findings are not surprising given the rapid pace of DMN development over the first year of life [65]. However, in light of evidence for associations between prenatal stress and early functional brain system organization, future research efforts would benefit from consideration of the potential combined and interactive effects of the pre- and postnatal environment. Moreover, recent work highlighting the role of maternal preconceptional stress, such as childhood maltreatment, in effecting levels of maternal inflammation during pregnancy [42] and neonatal brain structure [66], indicates a need for assessment and consideration of maternal life history, in attempts to identify potential sources of ELS influencing early brain system development.
Viewed in the context of evidence for the early emergence of RSNs and core properties of global network topology, the last 5 years of research make a case that efforts to support healthy brain development and reduce the burden of psychiatric disorders should begin during the prenatal period or earlier. This is underscored by repeated findings that early developmental patterns of RSNs are associated with subsequently emerging cognitive skills and emotionality [67,68]. For example, research across two independent datasets identified stronger neonatal amygdala functional connectivity to the anterior insula and medial prefrontal cortex, in relation to subsequent emerging heightened negative emotionality [55,67,68], indicating the potential relevance of this early neural phenotype as a transdiagnostic risk factor for psychiatric disorders.
Methodological Considerations
Methodological considerations, particularly with regard to infant neuroimaging, have been recently reviewed elsewhere [11,21], but we would be remiss not to highlight several key issues. First, larger, longitudinal infant neuroimaging studies have not prioritized inclusion of infants exposed to high levels of ELS [30], or in-depth measurement of the environment beginning with preconception history [30,69]. Second, major advances in neuroimaging are typically designed first to work for adults [70], with infant researchers playing catch-up. This is highly problematic, as unique properties of the infant versus adult brain impact all stages of neuroimage acquisition and processing. Rapid brain development during infancy exacerbates this problem as unique processing challenges arise depending on specific age [21].
Promising developments for improving data quality and thereby reducing challenges with image processing include advances in handling motion during data acquisition for functional [71,72] and structural data [73]. Machine learning techniques combined with existing large infant neuroimaging datasets may also allow for generating missing or low-quality structural data [74]. Infants are scanned during natural sleep and long standing questions remain regarding how sleep, and different sleep stages, impact RSNs. Recent work using simultaneous EEG and fMRI with infants [75] indicates potential for beginning to address these questions.
Likely the most pressing challenge in infant neuroimaging relates to more recent trends in the neuroimaging literature, highlighting reproducibility failures. It is now well documented that brain-behavior associations or brain-wide association studies (BWAS) often fail to reproduce [76–79]. There are likely several reasons for this. Most effect sizes in BWAS studies are likely small [80–82], and thus many ‘true’ effects may only be confirmed with larger samples comprising thousands of participants [81]. Due to issues related to signal-to-noise ratio (SNR), methods distinctions [83], and sampling variability, small samples can lead to misleading, non-replicable findings [79]. The apparent robustness in predictive models and the larger effects in the current literature, likely reflect selective analyses and reporting (i.e., p-hacking) [84], and publication bias towards studies with positive effects [81,85,86]. Samples with N ~> 2000 may be required to see true effects with both functional and structural MRI in BWAS studies in infancy and childhood [79]. Under such a context, accelerating the increasing trend towards open science, data sharing, and data aggregation, in combination with plans for federally funded large scale projects in this area, such as the Healthy Brain and Child Development Study (HBCD)iii, will be required to address these challenges with infant imaging and BWAS studies.
Concluding Remarks
Recent rapid growth in research using infant rs-fcMRI to investigate effects of ELS on neurodevelopment has yielded valuable insights. Theoretical frameworks emphasizing the potential for adaptation and resilience, as well as risk, may be particularly useful in interpreting current findings and guiding future research. For example, the predictive adaptive response (PAR) hypothesis posits that during early periods of rapid development, organisms receive signals from the environment that induce biological changes with implications for adaptation to the future environment [50]. Interpreted within this framework, findings of heightened prenatal stress linked to larger infant amygdala volume and increased functional connectivity [39,57], may be adaptive to the extent that heightened vigilance and reactivity confer significant benefit in the future environment. However, the association is not expected to be deterministic or linear, such that a given PAR as manifested in a neural phenotype could contribute to a range of behavioral phenotypes and adaptive strategies, allowing for significant variability in the possible environments within which healthy outcomes can occur [50].
A more recent, related hypothesis, suggests that high levels of ELS serve as a cue for accelerated brain development at the expense of refinement [87]. Interestingly, some experiences closely tied to heightened prenatal stress and early postnatal adversity, such as preterm birth, appear to result in delayed development of functional brain systems [26,55,61,62]. However, limited studies examining trajectories of early brain development in relation to more subtle variation in prenatal stress (in the form of heightened maternal inflammation), suggest the possibility of an initial delay, followed by an accelerated trajectory of development [33,40].
Hypotheses regarding biological sensitivity to context [88] or differential susceptibility [89], posit that certain phenotypes, such as heightened negative emotionality [90], may confer greater sensitivity to environmental conditions, which is advantageous or disadvantageous depending on the environment. Considering these theoretical models together, accelerated neural development occurring in the context of ELS (stress acceleration) could be considered a form of PAR which involves diminished ongoing neural plasticity in response to subsequent environmental conditions (decreased sensitivity to context). All of these models highlight the importance of longitudinal studies with repeated in-depth measurement of both brain and environment due to ongoing interactive influences on susceptibility to health and disease (see Outstanding Questions).
Outstanding Questions.
What are the implications of different characteristics of ELS, including variability, chronicity and timing for early developing functional brain systems?
How do multiple, frequently co-occurring sources of adversity during pregnancy, including maternal psychological distress, substance use, and poor nutrition, interact to influence key biological mediators and developing functional brain organization?
What are the key protective factors for healthy brain development and do these vary significantly depending on specific constellations or characteristics of risk factors?
What environmental conditions and individual differences contribute to the phenomenon of accelerated neurodevelopment and what are the behavioral implications compared to delayed maturation?
What properties of early emerging functional brain architecture contribute to differential susceptibility to environmental conditions, and what factors influence the emergence of these properties?
How will consideration of individualized network architecture influence understanding of the effects of ELS on developing RSNs and global network topology?
What are the challenges, methodological, ethical, and legal, of including those at highest risk for exposure to severe ELS, including in utero substance exposure, into longitudinal infant MRI/fMRI research?
What are the potential consequences of not tailoring research efforts to ensure representation of those with particularly heightened stress exposure in infant neuroimaging research?
How can tools and knowledge supporting high quality infant neuroimaging data acquisition, processing, and analysis, be more widely and effectively disseminated?
Edward O. Wilson [91] described the intersection between academic disciplines as the space in which, ‘most real-world problems exist…the one in which fundamental analysis is most needed. Yet virtually no maps exist’. In order to harness recent advances in understanding human brain functioning, to improve capacity to support healthy early brain development, we face this intersection. The tools for studying early emerging functional brain systems are rapidly improving and there is a solid foundation of discovery upon which to build. We now encounter the challenge of balancing methodological rigor with increasing relevance to clinical applications and public health. This will require novel approaches to increase levels of collaboration and shared resources. Studies such as the Adolescent Brain Cognitive Development Study (ABCD)iv, Baby Connectome Project (BCP) [30], and Developing Human Connectome Project (dHCP) [69], demonstrate the possibility of successful, large scale, developmental neuroimaging studies. Moreover, just as periods of rapid change in biological systems confer both vulnerability and opportunity, societal upheaval in the context of the COVID-19 pandemic and the growing movement against racial injustice can be seen as fertile ground for gaining the attention of policy makers and the scientific community. Current events combined with the recent announcement of a second phase of the HBCD initiative, provide an opportunity for meaningful scientific progress characterized by methodological rigor and increasing relevance to supporting healthy early brain development for individuals facing the highest levels of adversity and associated disease burden.
Highlights.
The use of resting state functional connectivity MRI has contributed substantially to understanding of human brain functioning and organization, and revealed emerging large scale brain systems already in early infancy.
Recent research shows how early brain system development varies in relation to multiple sources of pre- and postnatal adversity and biological stress mediators.
Variability in early developing brain systems has implications for later emerging behaviors relevant for psychiatric disorders.
Recent methodological advances promise increased precision in examining individual differences in early emerging functional brain systems.
There is need for research examining mechanistic pathways for combined and interactive effects of multiple sources of early adversity on developing brain systems. This line of study is crucial for applying research findings to infants at highest risk of subsequent poor health outcomes.
Greater inclusion of individuals facing the highest levels of early adversity in developmental neuroimaging work represents a priority for future research, which will require significant investment and yield critical information to address public health challenges.
Acknowledgments
Support for this work was provided by R00 MH111805 (A.M.G.), R34DA050291 (A.M.G. & D.A.F.), R34DA050291-S2 (A.M.G. and D.A.F.), R01 MH096773 (D.A.F.), R01 MH115357 (D.A.F.), R01MH105538 (C.B.and D.A.F.).
Footnotes
Declaration of Interests
Dr Fair is a patent holder on the Framewise Integrated Real-Time Motion Monitoring (FIRMM) software. He is also a cofounder of Nous Imaging Inc. The other authors have no conflicts of interest to report in relation to this work.
Resources
References
- 1.James SL et al. (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedegaard H. et al. (2018) Suicide mortality in the United States, 1999–2017. NCHS Data Brief, Natl. Cent. Heal. Stat 330, 1–8 [PubMed] [Google Scholar]
- 3.Huhn M. et al. (2014) Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta-analyses. JAMA Psychiatry 71, 706–715 [DOI] [PubMed] [Google Scholar]
- 4.Le Cook B. et al. (2017) Trends in racial-ethnic disparities in access to mental health care, 2004–2012. Psychiatr. Serv 68, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raichle ME (2010) Two views of brain function. Trends Cogn. Sci 14, 180–190 [DOI] [PubMed] [Google Scholar]
- 6.Gordon EM et al. (2016) Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex 26, 288–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon EM et al. (2017) Precision functional mapping of individual human brains. Neuron 95, 791–807.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gratton C. et al. (2018) Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron 98, 439–452.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feczko E. et al. (2017) Subtyping cognitive profiles in Autism Spectrum Disorder using a random forest algorithm. Neuroimage 172, 674–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia CH et al. (2018) Linked dimensions of psychopathology and connectivity in functional brain networks. Nat. Commun 9, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayson DS and Fair DA (2017) Development of large-scale functional networks from birth to adulthood: a guide to the neuroimaging literature. Neuroimage 160, 15–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chrousos GP (2009) Stress and disorders of the stress system. Nat. Rev. Endocrinol 5, 374–381 [DOI] [PubMed] [Google Scholar]
- 13.Graham AM et al. (2015) The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev. Cogn. Neurosci 12, 12–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson CA (2017) Hazards to early development: the biological embedding of early life adversity. Neuron 96, 262–266 [DOI] [PubMed] [Google Scholar]
- 15.Biswal BB et al. (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med 34, 537–541 [DOI] [PubMed] [Google Scholar]
- 16.Beckmann CF and Smith SM (2004) Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 23, 137–152 [DOI] [PubMed] [Google Scholar]
- 17.Power JD et al. (2011) Functional network organization of the human brain. Neuron 72, 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolaidis A. et al. (2020) Bagging improves reproducibility of functional parcellation of the human brain. Neuroimage 214, 116678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H. et al. (2017) Large-scale sparse functional networks from resting state fMRI. Neuroimage 156, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu T. et al. (2019) Interindividual variability of functional connectivity in awake and anesthetized rhesus macaque mon-keys. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H. et al. (2019) Resting-state functional MRI studies on infant brains: a decade of gap-filling efforts. Neuroimage 185, 664–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keunen K. et al. (2017) The emergence of functional architecture during early brain development. Neuroimage 160, 2–14 [DOI] [PubMed] [Google Scholar]
- 23.De Asis-Cruz J. et al. (2015) Functional properties of resting state networks in healthy full-term newborns. Sci. Rep 5, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fransson P. et al. (2011) The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb. Cortex 21, 145–154 [DOI] [PubMed] [Google Scholar]
- 25.van den Heuvel MP et al. (2015) The neonatal connectome during preterm brain development. Cereb. Cortex 25, 3000–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smyser CD et al. (2010) Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex 20, 2852–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomason ME et al. (2018) Prenatal neural origins of infant motor development: associations between fetal brain and infant motor development. Dev. Psychopathol 30, 763–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao W. et al. (2014) Development of human brain cortical network architecture during infancy. Brain Struct. Funct 220, 1173–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui Z. et al. (2020) Individual variation in functional topography of association networks in youth. Neuron 106, 340–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howell BR et al. (2019) The UNC/UMN Baby Connectome Project (BCP): an overview of the study design and protocol development. Neuroimage 185, 891–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buss C. et al. (2012) Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Sci. Signal 5, pt7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Entringer S. et al. (2015) Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology 62, 366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez JSB et al. (2020) Maternal interleukin-6 is associated with macaque offspring amygdala development and behavior. Cereb. Cortex 30, 1573–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson JR et al. (2018) Maternal diet, metabolic state, and inflammatory response exert unique and long-lasting influences on offspring behavior in non-human primates. Front. Endocrinol. (Lausanne) 9, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gustafsson HC et al. (2018) Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain Behav. Immun 73, 470–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustafsson HC et al. (2020) Evaluation of maternal inflammation as a marker of future offspring ADHD symptoms: a prospective investigation. Brain Behav. Immun 89, 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gustafsson HC et al. (2019) Increased maternal prenatal adiposity, inflammation, and lower omega-3 fatty acid levels influence child negative affect. Front. Neurosci 13, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudolph MD et al. (2018) Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci 21, 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham AM et al. (2018) Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol. Psychiatry 83, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen JM et al. (2019) Maternal interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage 185, 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spann MN et al. (2018) Maternal immune activation during the third trimester is associated with neonatal functional connectivity of the salience network and fetal to toddler behavior. J. Neurosci 38, 2877–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moog NK et al. (2016) Maternal exposure to childhood trauma is associated during pregnancy with placental-fetal stress physiology. Biol. Psychiatry 79, 831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conradt E. et al. (2018) Early life stress and environmental influences on the neurodevelopment of children with prenatal opioid exposure. Neurobiol. Stress 9, 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jantzie LL et al. (2020) Prenatal opioid exposure: the next neonatal neuroinflammatory disease. Brain Behav. Immun 84, 45–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knudsen EI (2004) Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci 16, 1412–1425 [DOI] [PubMed] [Google Scholar]
- 46.Loman MM and Gunnar MR (2010) Early experience and the development of stress reactivity and regulation in children. Neurosci. Biobehav. Rev 34, 867–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y. and Baram TZ (2016) Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology 41, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLaughlin KA et al. (2014) Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev 47, 578–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEwen BS (1998) Protective and damaging effects of stress mediators. N. Engl. J. Med 338, 171–179 [DOI] [PubMed] [Google Scholar]
- 50.Gluckman PD et al. (2005) The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum. Dev 81, 51–59 [DOI] [PubMed] [Google Scholar]
- 51.VanTieghem MR and Tottenham N. (2018) Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr. Top. Behav. Neurosci 38, 117–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posner J. et al. (2016) Alterations in amygdala–prefrontal circuits in infants exposed to prenatal maternal depression. Transl. Psychiatry 6, e935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu A. et al. (2015) Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl. Psychiatry 5, e508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buss C. et al. (2009) The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am. J. Obstet. Gynecol 201, 398.e1–8 [DOI] [PubMed] [Google Scholar]
- 55.Rogers CE et al. (2017) Neonatal amygdala functional connectivity at rest in healthy and preterm infants and early internalizing symptoms. J. Am. Acad. Child Adolesc. Psychiatry 56, 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheinost D. et al. (2016) Prenatal stress alters amygdala functional connectivity in preterm neonates. NeuroImage Clin. 12, 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graham AM et al. (2019) Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatry 85, 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buss C. et al. (2012) Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci 109, E1312–E1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Altemus M. et al. (2014) Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol 35, 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grayson DS et al. (2016) The rhesus monkey connectome predicts disrupted functional networks resulting from pharma-cogenetic inactivation of the amygdala. Neuron 91, 453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doria V. et al. (2010) Emergence of resting state networks in the preterm human brain. Proc. Natl. Acad. Sci. U. S. A 107, 20015–20020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scheinost D. et al. (2016) Preterm birth alters neonatal, functional rich club organization. Brain Struct. Funct 221, 3211–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tortora D. et al. (2019) Early pain exposure influences functional brain connectivity in very preterm neonates. Front. Neurosci 13, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graham AM et al. (2015) Early life stress is associated with default system integrity and emotionality during infancy. J. Child Psychol. Psychiatry 56, 1212–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao W. et al. (2015) Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb. Cortex 25, 2919–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moog NK et al. (2018) Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biol. Psychiatry 83, 120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Graham AM et al. (2016) Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev. Cogn. Neurosci. 18, 12–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas E. et al. (2019) Newborn amygdala connectivity and early emerging fear. Dev. Cogn. Neurosci 37, 100604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Makropoulos A. et al. (2018) The developing human connectome project: a minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 173, 88–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glasser MF et al. (2013) The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dosenbach NUF et al. (2017) Real-time motion analytics during brain MRI improve data quality and reduce costs. Neuroimage 161, 80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fair DA et al. (2020) Correction of respiratory artifacts in MRI head motion estimates. Neuroimage 208, 116400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tisdall MD et al. (2016) Prospective motion correction with volumetric navigators (vNavs) reduces the bias and variance in brain morphometry induced by subject motion. Neuroimage 127, 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mazurowski MA et al. (2019) Deep learning in radiology: an overview of the concepts and a survey of the state of the art with focus on MRI. J. Magn. Reson. Imaging 49, 939–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arichi T. et al. (2017) Localization of spontaneous bursting neuronal activity in the preterm human brain with simultaneous EEG-fMRI. Elife 6, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boekel W. et al. (2015) A purely confirmatory replication study of structural brain-behavior correlations. Cortex 66, 115–133 [DOI] [PubMed] [Google Scholar]
- 77.Genon S. et al. (2017) Searching for behavior relating to grey matter volume in a priori defined right dorsal premotor regions: lessons learned. Neuroimage 157, 144–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kharabian Masouleh S. et al. (2019) Empirical examination of the replicability of associations between brain structure and psychological variables. Elife 8, e43464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marek S. et al. (2020) Towards Reproducible Brain-Wide Association Studies. bioRxiv Published online August 22, 2020. 10.1101/2020.08.21.257758 [DOI] [Google Scholar]
- 80.Button KS et al. (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci 14, 365–376 [DOI] [PubMed] [Google Scholar]
- 81.Smith SM and Nichols TE (2018) Statistical challenges in ‘Big Data’ Human neuroimaging. Neuron 97, 263–268 [DOI] [PubMed] [Google Scholar]
- 82.Steven Dick A. et al. (2020) Meaningful effects in the adolescent brain cognitive development study. bioRxiv Published online September 01, 2020. 10.1101/2020.09.01.276451 [DOI] [Google Scholar]
- 83.Poldrack RA et al. (2017) Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci 18, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munafò MR et al. (2017) A manifesto for reproducible science. Nat. Hum. Behav 1, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walum H. et al. (2016) Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol. Psychiatry 79, 251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sabuncu MR and Konukoglu E. (2014) Clinical prediction from structural brain MRI scans: a large-scale empirical study. Neuroinformatics 13, 31–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Callaghan BL and Tottenham N. (2016) The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci 7, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boyce WT and Ellis BJ (2005) Biological sensitivity to context: I. An evolutionary –developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol 17, 271–301 [DOI] [PubMed] [Google Scholar]
- 89.Belsky J. et al. (2007) For better and for worse: differential susceptibility to environmental influences. Psychol. Sci 16, 300–304 [Google Scholar]
- 90.Slagt M. et al. (2017) Children’s differential susceptibility to parenting: an experimental test of ‘for better and for worse. J. Exp. Child Psychol 154, 78–97 [DOI] [PubMed] [Google Scholar]
- 91.Wilson EO (1999) Consilience: The Unity of Knowledge (reprint edn), Vintage Books [Google Scholar]
- 92.Wadhwa PD et al. (2010) Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med 27, 358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tau GZ and Peterson BS (2010) Normal development of brain circuits. Neuropsychopharmacology 35, 147–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buss C. et al. (2012) The role of stress in brain development: the gestational environment’s long-term effects on the brain. Cerebrum 4 [PMC free article] [PubMed] [Google Scholar]
- 95.Humphrey T. (1968) The development of the human amygdala during early embryonic life. J. Comp. Neurol 132, 135–165 [DOI] [PubMed] [Google Scholar]
- 96.Marr M. (2020) Neurodevelopmental timing. Published online February 21, 2020. 10.5281/zenodo.3678836 [DOI]
- 97.Vasung L. et al. (2010) Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J. Anat 217, 400–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ball G. et al. (2014) Rich-club organization of the newborn human brain. Proc. Natl. Acad. Sci. U. S. A 111, 7456–7461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Knickmeyer RC et al. (2008) A structural MRI study of human brain development from birth to 2 years. J. Neurosci 28, 12176–12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Courchesne E. et al. (2000) Normal brain development and aging : quantitative analysis at in vivo MR imaging in healthy volunteers. Neuroradiology 216, 672–682 [DOI] [PubMed] [Google Scholar]
- 101.Huttenlocher PR and Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol 387, 167–178 [DOI] [PubMed] [Google Scholar]
- 102.Innocenti GM and Price DJ (2005) Exuberance in the development of cortical networks. Nat. Rev. Neurosci 6, 955–965 [DOI] [PubMed] [Google Scholar]
- 103.Estes ML and McAllister AK (2016) Maternal immune activation: implications for neuropsychiatric disorders. Science 353, 772–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Makinson R. et al. (2017) Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain Behav. Immun 66, 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]