Abstract
Given their sessile nature, plants have evolved sophisticated regulatory networks to confer developmental plasticity for adaptation to fluctuating environments. Epigenetic codes, like tri-methylation of histone H3 on Lys27 (H3K27me3), are evidenced to account for this evolutionary benefit. Polycomb repressive complex 2 (PRC2) and PRC1 implement and maintain the H3K27me3-mediated gene repression in most eukaryotic cells. Plants take advantage of this epigenetic machinery to reprogram gene expression in development and environmental adaption. Recent studies have uncovered a number of new players involved in the establishment, erasure, and regulation of H3K27me3 mark in plants, particularly highlighting new roles in plants’ responses to environmental cues. Here, we review current knowledge on PRC2-H3K27me3 dynamics occurring during plant growth and development, including its writers, erasers, and readers, as well as targeting mechanisms, and summarize the emerging roles of H3K27me3 mark in plant adaptation to environmental stresses.
Keywords: H3K27me3, epigenetics, environmental cues, polycomb repressive complex 2, polycomb repressive complex 1, demethylase, recruitment
1. Introduction
Plants are sessile organisms that live tightly associated with a changing environment. To cope with the unfavorable stimuli, e.g., extreme temperature, osmotic stress, nutrient deficiency, and biotic stress, plants thus increase their phenotypic plasticity by extensive genetic and epigenetic reprograming in order to adapt to the environmental challenges [1,2]. Epigenetic codes mainly include DNA methylation, histone modifications, and small RNAs, which alter the structure and accessibility of chromatin, therefore either activating or inhibiting gene expression in a heritable mechanism [2,3].
DNA methylation at the carbon-5 position of cytosine (5mC) is a well-understood epigenetic mark that is relevant to gene repression and genome stability, involved in diverse biological processes, as well as in response to environmental cues [4,5]. Histone modifications, including methylation, acetylation, phosphorylation, ubiquitination, sumoylation, biotinylation, glycosylation, and ADP-ribosylation, are also conserved epigenetic marks in eukaryotes [6]. Dynamics of histone modifications are maintained by enzymes that are able to catalyze and remove the marks, referred to as writers and erasers, respectively. These epigenetic codes are in turn recognized by reader proteins and give rise to biological outcomes [3,7]. Further, several types of methylation of lysine residues of histone H3 are widespread and of great importance in plants, like repressive di-methylation of Lys9 (H3K9me2) and tri-methylation of Lys27 (H3K27me3), and permissive tri-methylation of Lys4 (H3K4me3) and tri-methylation of Lys36 (H3K36me3) [8].
An increasing number of recent studies have revealed that epigenetic mechanisms are also implicated in plants’ responses to environmental stresses; in particular, the roles of DNA methylation have been extensively discussed in several reviews [3,4]. By contrast, functions of H3K27me3 in plants’ adaption to environmental cues have not been well documented. In this review, we will discuss molecular machinery of H3K27me3 from de novo synthesis, removal, and genomic reorganization and loading in plants, emphasizing the potential regulatory importance of H3K27me3 dynamics on adaptation to various environmental cues.
2. Writers and Erasers of H3K27me3 in Plants
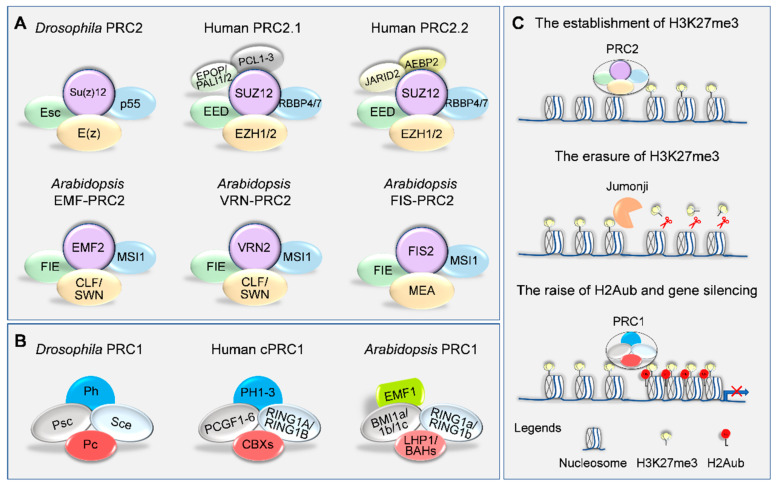
Basically, H3K27me3 is deposited on chromatin, covering thousands of genomic loci, including many developmental and stress-responsive genes in plants [9,10,11]. It is catalyzed by writer, polycomb repressive complex 2 (PRC2), via histone methyltransferases and related cofactors. PRC2 subunits were initially identified in Drosophila melanogaster and are well conserved across animal and plant kingdoms [12,13]. In Drosophila, PRC2 is composed of four core components, including the histone methyltransferase enhancer of zeste (E(z)), extra sex combs (Esc), suppressor of zeste 12 (Su(z)12), and the histone-binding nucleosome remodeling factor 55 kDa (Nurf55, also called p55) (Figure 1A) [14]. Specifically, E(z) belongs to the SET (Su(var)3-9; E(z); trithorax) domain family, responsible for tri-methylation on histone H3 at Lys27 [15], while Esc is a member of WD40 repeat family and possesses the β-propeller architecture that binds to the histone H3 tail [16]. Su(z)12 is a C2H2 zinc-finger protein with a VEFS (VRN2; EMF2; FIS2; Su(z)12) domain that interacts with E(z) and various cofactors [17]. Nurf55 is also a member of WD40 repeat-containing histone chaperone protein that can bridge PRC2 and chromatin together by directly binding to nucleosome, but is dispensable for PRC2-mediated gene silencing in vivo [18]. In comparison, mammalian core PRC2 subunits consist of enhancer of zeste homolog 2/1 (EZH2/1), embryonic ectoderm development (EED), suppressor of zeste 12 homolog (SUZ12), and retinoblastoma binding protein 4/7 (RBBP4/7) [19,20,21]. Associated with specific accessory proteins, two mutually exclusive subtype assemblies, PRC2.1 (PCL1–3, EPOP, and PALI1/2) and PRC2.2 (AEBP2 and JARID2), are formed and cooperate to mediate the deposition and maintenance of H3K27me3 via both synergistic and independent mechanisms (Figure 1A) [21,22,23].
Figure 1.
Core PRCs and their chromatin-modifying activities. (A,B) Compositions of the polycomb repressive complexes PRC2 (A) and PRC1 (B) in animals and plants, where Drosophila, human, and Arabidopsis were taken as representatives. (C) Schematic representation of the H3K27me3 deposition, erasure, and its roles in gene silencing. Acronyms and further details are explained in the text.
Unlike animals, the core PRC2 components undergo multiple duplications in plants [24,25]. In the case of Arabidopsis, three E(z) homologs (CURLY LEAF (CLF), SWINGER (SWN), and MEDEA (MEA)), three Su(z)12 homologs (EMBRYONIC FLOWER 2 (EMF2), VERNALIZATION 2 (VRN2), and FERTILIZATION INDEPENDENT SEED 2 (FIS2)), one Esc homolog (FERTILIZATION-INDEPENDENT ENDOSPERM (FIE)), and five Nurf55 homologs (MULTICOPY SUPRESSOR OF IRA 1–5 (MSI1–5)) are present [26,27]. Based on cell or tissue specificity, three distinct PRC2s are formed, referred to as the EMF-, VRN-, and FIS-PRC2 complexes (Figure 1A). The FIS-PRC2, harboring FIS2, MEA, FIE, and MSI1, is implicated in regulating megagametogenesis and endosperm development postfertilization [28,29,30,31,32,33]. Whereas the EMF-PRC2 (having EMF2, CLF/SWN, FIE, and MSI1) and VRN-PRC2 (including VRN2, CLF/SWN, FIE, and MSI1) control sporophyte development and the vegetative-to-reproductive phase transition [33,34,35,36,37,38,39].
In cereals, rice (Oryza sativa L.) and maize (Zea mays L.) both have two copies of EMF2-like genes (EMF2a and EMF2b) but no VRN2 or FIS2 homologs [40]. For E(z), two (OsCLF and OsiEZ1) and three (ZmMEZ1–3) homologs are present in rice and maize, respectively [40]. Intriguingly, a duplicated pair of Esc homologs are found in rice (OsFIE1 and OsFIE2) and maize (ZmFIE1 and ZmFIE2), in contrast to a single FIE in Arabidopsis [24,40]. At present, three and seven MSI homologs have been identified from rice and maize, respectively [24,25]. This scenario suggests gene duplication is a major evolutionary force shaping plant PRC2, most likely in a species- or genus-specific manner (Table 1). Nevertheless, the distinct PRC2 complexes and their functions in cereal growth and development remain to be elucidated [24,25,40,41,42,43,44].
Table 1.
PRC1 and PRC2 core complex components in animals and plants.
| Drosophila | Characteristic Domains | Human | Arabidopsis | Rice | Maize |
|---|---|---|---|---|---|
| Polycomb Repressive Complex 2 (PRC2) | |||||
| E(z) | SET, CXC and SANT | EZH1, EZH2 | CLF, SWN, MEA | OsCLF, OsiEZ1 | ZmMEZ1, ZmMEZ2, ZmMEZ3 |
| Esc | WD40 | EED | FIE | OsFIE1, OsFIE2 | ZmFIE1, ZmFIE2 |
| Su(z)12 | Zinc-finger and VEFS | SUZ12 | FIS2, VRN2, EMF2 | OsEMF2a, OsEMF2b | ZmEMF2a, ZmEMF2b |
| p55 | WD40 | RBBP4, RBBP7 | MSI1–5 | OsMSI1, OsMSI3, OsMSI4 | ZmMSI1a/b/c/d; ZmMSI3; ZmMSI4a/b |
| Polycomb Repressive Complex 1 (PRC1) | |||||
| Sce | RING | RING1A, RING1B | AtRING1A/B | OsRING1A/B | ZmRING1A/B/C/D |
| Psc | RING | PCGF1–6 | AtBMI1A/B/C | OsBMI1A/B/C | ZmBMI1A/B/C/D/E/F |
| Pc | CHROMO | CBX2/4/6/7/8 | – | – | – |
| Ph | SAM and Zinc-finger | PH1–3 | – | – | – |
| CHROMO | LHP1 | OsLHP1 | ZmLHP1 | ||
| EMF1 | – | – | |||
–, No homologues found; further acronyms and details are explained in the text.
To maintain H3K27me3 homeostasis, it is reasonable to have opposite players to remove the mark on targets (eraser). Indeed, Jumonji C (JmjC) domain-containing Fe(II)/α-ketoglutarate-dependent dioxygenases are able to demethylate tri-, di-, and mono-methylated lysine of histones, named as histone lysine demethylases (KDMs) [45,46]. Coupling to diverse histone methylation marks, the JmjC protein family has a complexity with an expanded gene number and varied domain architectures [46]. Like in animals, H3K27me3 is mainly demethylated by UBIQUITOUSLY TRANSCRIBED TETRATRICOPEPTIDE X (UTX/KDM6A) and JUMONJI D3 (JMJD3/KDM6B), which both contain a JmjC domain, with extra tetratricopeptide repeat (TPR) domains in UTX [47,48]. Interestingly, JUMONJI AND AT-RICH INTERACTION DOMAIN (ARID)-CONTAINING 2 (JARID2) contains JmjN, ARID, and JmjC domains and regulates the presence of the H3K27me3 mark by recruiting PRC2 complex to chromatin, but lacks any detectable histone demethylase activity [49,50].
UTX and JMJD3 are less conserved in plants, but up to five jumonji proteins have been identified as bona fide H3K27me3 demethylases in Arabidopsis, including EARLY FLOWERING 6 (ELF6/JMJ11), RELATIVE OF EARLY FLOWERING 6 (REF6/JMJ12), JMJ13, JMJ30, and JMJ32 [51]. They have largely non-overlapping chromatin-targeting machineries and biological functions. Besides a JmjC domain, ELF6 also contains a zinc-finger domain that could interact with transcription factor BRASSINAZOLE-RESISTANT1 (BZR1), thus targeting to specific loci involved in the photoperiod pathway [52,53]. Similarly, its relative, REF6, is also deposited at genomic loci via zinc-finger domain and acts as a FLOWERING LOCUS C (FLC) repressor, in turn counteracting the function of ELF6 [54,55,56,57]. Conversely, JMJ13 recognizes H3K27me3 through hydrogen bonding and hydrophobic interactions and acts as a temperature- and photoperiod-dependent flowering repressor [58]. Moreover, JMJ30 and JMJ32, the JmjC domain-only group proteins, could directly bind and demethylate H3K27me3 of the FLC locus in vitro and in vivo, and moderate precocious flowering at elevated temperatures [59].
So far, only one H3K27me3 demethylase, OsJMJ705, has been identified in rice. Its expression could be induced by stress signals and pathogen infection. In particular, it removes H3K27me3 of defense-related genes, but also takes part in meristem development and energy-generating pathways with the aid of WUSCHEL-RELATED HOMEBOX 11 (WOX11) [60,61]. In addition, a total of 19 JmjC domain-containing proteins have been identified in maize and all are responsive to heat stress [62]; however, their molecular and biological functions remain to be explored. Hence, it will be interesting and relevant to focus on functional dissection of JmjC like H3K27me3 erasers in plants’ responses to stresses.
3. PRC1-Mediated H3K27me3 Reading and Gene Silencing
The canonical hierarchical model proposed that PRC2-mediated H3K27me3 does not intrinsically impact the chromatin structure but serves as a docking site for PRC1 complex [63]. PRC1 in turn catalyzes the mono-ubiquitination of histone H2A (H2Aub), thus preventing the recruitment of nucleosome remodeling factors and subsequent gene silencing (Figure 1C) [63,64,65,66,67]. In line with this notion, genome-wide chromatin binding profiles demonstrated co-occupancy of PRC1 and PRC2 at many H3K27me3 deposition loci from animals to plants [9,67,68,69,70,71,72,73]. However, evidence was also shown that some PRC1/H2Aub targeting sites are completely independent of H3K27me3 deposition, especially in plants [71,74,75,76,77]. Notwithstanding, polycomb group (PcG) proteins are tightly associated with gene repression and various biological processes.
In Drosophila, PRC1 contains sex combs extra (Sce), posterior sex comb (Psc), polycomb (Pc), and polyhomeotic (Ph) (Figure 1B) [78]. Of note, Sce and Psc are really interesting new gene (RING) domain-containing proteins, exhibiting E3 ubiquitin ligase activity towards histone H2A [79,80]. Pc is characterized by a chromatin organization modifier (CHROMO) domain with affinity for H3K27me3 mark [81,82]. However, the precise molecular role of essential subunit Ph has not yet been well established [83]. A burst of duplication occurs in PRC1 subunits in mammalian species, which makes the combinations more complicated (Figure 1B; Table 1). Two Sce homologs (RING1A and RING1B) have been found [84], along with six homologs of Psc in humans, termed as the B LYMPHOMA Mo-MLV INSERTION REGION 1 (BMI1) subfamily (Bmi1/Pcgf4, Mel18/Pcgf2, Nspc1/Pcgf1, Pcgf3, Pcgf5, and MBLR/Pcgf6) [85]. For Pc, at least five members are present, known as Chromobox proteins (CBX2, CBX4, CBX6, CBX7, and CBX8), conferring distinct target selectivity to the PRC1 complex [86], in contrast to three Ph homologs, PH1, PH2, and PH3 [87]. Therefore, the specificity and functions of the distinct PRC1s in mammalian species remain to be addressed.
As no significant homologs of these mammalian components were found Just as there was a lack of evidence that PRC1 is significantly homologous to mammalian species, plant PRC1 had remained elusive for a long time until the RING-finger proteins (RING1A/B and BMI1A/B/C) were characterized in Arabidopsis [88,89]. Double mutants of either atring1a/b or atbmi1a/b phenocopies were found in severely compromised PRC2 mutants, such as fie, clf/swn or emf2/vrn2 mutants [90,91]. Indeed, these RING-finger proteins possess E3 ubiquitin ligase activity and are responsible for global genomic H2Aub in Arabidopsis [74,90,91,92,93,94]. Moreover, homologs of RING1 and BMI1 are widely present and conserved in the green lineage (Table 1) [95]. Unlike the canonical PRC1 in animals, plant PRC1 binding to H3K27me3 occurs through two distinct classes of proteins, LIKE HETEROCHROMATIN PROTEIN 1 (LHP1) and the N-terminal bromo adjacent homology (BAH) domain-containing family, which includes EARLY BOLTING IN SHORT DAYS (EBS) and its homolog SHORT LIFE (SHL), BAH DOMAIN-CONTAINING TRANSCRIPTIONAL REGULATOR 1 (BDT1), and ANTI-SILENCING 1 (ASI1)-IMMUNOPRECIPITATED PROTEIN 3 (AIPP3) [96]. Intriguingly, LHP1 was identified as a homolog of animal HP1, which contains the CHROMO domain involved in the formation of heterochromatin, but instead it functions as a Pc counterpart in plants [97,98]. In contrast, EBS and SHL are dual readers that recognize H3K27me3 and H3K4me2/3 through N-terminal BAH domain and C-terminal PHD domain, respectively [99,100,101]. Similarly, BDT1 and AIPP3 also use the BAH domain to bind to H3K27me3, facilitating the suppression of the expression of flowering genes, thus preventing flowering [102,103]. Therefore, LHP1, EBS, SHL, BDT1, and AIPP3 represent different readers of the H3K27me3 and are relevant for repression of PRC2 targets [9,97,98,99,100,101,102,103,104]. Remarkably, homologs of Drosophila Ph are lost in plants, instead EMF1 (phenocopying emf2) has been proposed to be a plant-specific PRC1 component that mediates chromatin compaction [68,105,106]. However, homologs of EMF1 appear only in dicotyledon species [95]. Phenotypes of emf1 mutants resemble those of EMF2-PRC2 mutants [105,106,107]. In support, genome-wide binding assay revealed that PRC1 components occupy a considerable number of genes also marked with H3K27me3 [71]. Moreover, EMF1 and LHP1 are co-purified with PRC2 components and required for H3K27me3 deposition [72,101,106,108,109]. These data strongly suggest that a PRC1-like complex indeed exists in plants as well, in spite of clear divergence in function compared with animals. Notwithstanding, the mechanism underlying PRC1- and PRC2-mediated gene silencing is still far from clear in plants.
4. Targeting Mechanisms of H3K27me3 Deposition on Genome
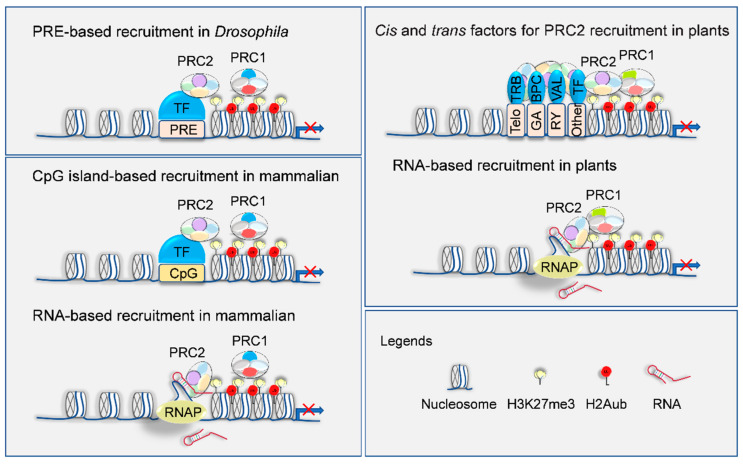
The majority of PcG proteins are ubiquitously expressed in both plants and animals. However, the H3K27me3 signature is precisely and dynamically decorated at specific chromatin loci, depending on cell/tissue type, developmental stage, and environmental cues. Considered that PRC2 itself does not have DNA affinity, other essential factors are needed to determine PRC2-bound loci and spatial–temporal specificity. Accumulating evidence over recent years has revealed that diverse players, like cis elements, transcription factors, RNAs, and other pre-existing epigenetic modifications, are implicated in recruitment of PRC2 to target loci. However, the underlying molecular mechanisms are still not fully understood.
In Drosophila, polycomb response elements (PREs) could be specifically recognized by transcription factors (e.g., pleiohomeotic (Pho), GAGA factor (GAF), and Zeste) that bridge PRC2 with chromatin (Figure 2) [110]. In mammalian species, CpG islands proximal to inactive gene promoters are highly occupied by PRC2 (Figure 2) [111]. However, the mechanism directing PRC2 recruitment to CpG islands remains to be explored. In parallel, it has been observed that non-coding RNA (ncRNA) and nascent RNA directly interact with PRC2 and facilitate gene repression, probably in a SUZ12-dependent manner (Figure 2) [108,109,112]. In addition, some pre-existing epigenetic marks, such as H3K4me3, di- or tri-methylation of histone H3K36 (H3K36me2/3) and 5mC in CpG islands, counteract PRC2 recruitment in local chromatin regions [113,114,115,116]. Notwithstanding, the above-characterized mechanisms appear insufficient to account for the widespread, and often tissue-specific, H3K27me3 signature in animals. Hence, other recruiters likely also contribute to shaping H3K27 methylation patterns.
Figure 2.
Targeting mechanisms of H3K27me3 deposition on a genome. Diagram shows PRE in the recruitment of PRC2 to target loci in Drosophila, CpG-island-, and RNA-based PRC2 recruitment in mammalian species, as well as cis- and trans-acting factors involved in PRC2 recruitment in plants. TF, trans-acting factor; RNAP, RNA polymerase. Details are explained in the text.
Recently, several PRE-like elements (e.g., GAGA motif, telobox motif, RY-repeat motif, and the repressive LEC2 element (RLE)) have been identified in plants (Figure 2; Table 2). For instance, GAGA motif can be recognized by BASIC PENTACYSTEINE (BPC) family members that directly interact with PcG proteins for H3K27me3 deposition [117,118,119,120,121,122], resembling the GA-repeat—GAF—module in animals [110]. Likewise, telobox motifs are bound by either TELOMERE REPEAT BINDING PROTEIN (TRB) factors or C2H2 zinc-finger family (C1-2iD subfamily) members that guide PRC2 to implement H3K27me3 deposition, which represents an evolutionarily ancient mechanism of telomeric repeats for PRC2-mediated H3K27me3 loading [120,123,124,125]. Moreover, the RY-repeat motif recruits the PRC2 complex through the intermediation of VIVIPAROUS1/ABI3-LIKE (VAL) transcription factors [126,127,128,129,130,131,132,133]. In agreement, mutants of either trb1/2/3, bpc1-1bpc2bpc4bpc6, or val1val2 in Arabidopsis genetically mimic the phenotypes of those strong PRC2 mutants; however, chromatin loci occupied by these proteins jointly overlap with around 60% of H3K27me3 targets [125,129,134,135]. Therefore, additional PcG protein interactors also participate in the recruitment of PRC2 to the targeted loci, such as transcription factors, chromatin remodelers, and PRC1- and PRC2-associated factors (Table 2) [38,52,120,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167]. Taken together, the canonical cis–trans interaction module establishes the global H3K27me3 profile and also determines the functional diversity and developmental specificity between distinct subsets of PRC2 complexes.
Table 2.
Cis- and trans-acting factors involved in PRC2 recruitment in plants.
| Trans-Acting Factor | Interacting PcG Protein | Cis-Acting Factor | Targeted Loci | Biological Function | Reference |
|---|---|---|---|---|---|
| Transcription factors | |||||
| BPC1/2/4/6 | SWN | N | ABI4 | Lateral root development | [119] |
| BPC class I subfamily | FIE | GAGA motif | N | N | [118,120] |
| BPC6 | LHP1, VRN2 | GAGA motif | Homeotic genes | Vegetative growth and flowering | [117] |
| BPC1/2/3 | FIE, MEA, FIS2, MSI1 | GAGA motif | FUSCA3 | Seed development | [122] |
| N | N | W-box, RY motif | FUSCA3 | Seed development | [131] |
| BBR/BPC | N | GAGA motif | N | Brassinosteroid signaling | [121] |
| TRB1/2/3 | CLF, SWN | Telobox | N | Vegetative growth and flowering | [123,124] |
| C2H2 ZnF family | FIE | Telobox | N | N | [120] |
| AP2 subfamily | FIE | PRE-like | N | N | [120] |
| VAL1/2 | CLF, SWN | RY repeat | N | Somatic embryonic calli | [132] |
| VAL1/2 | MSI1, LHP1 | N | FT, FLC | Flowering | [126,127] |
| VAL1/2 | CLF, MSI1, LHP1 | RY repeat | DOG1, AGL15 | Seed germination and vegetative growth | [128,129] |
| N | N | RLE | LEC2 | Embryo development | [164] |
| AS1/2 | CLF, FIE, EMF2, LHP1 | N | BP, KNAT2 | Leaf differentiation | [140,163] |
| PWO1 | CLF, SWN, MEA | N | N | Vegetative growth and flowering | [157] |
| PDP1/2/3 | MSI5 | N | FLC | Flowering | [138] |
| JAZ1/4/8/10/3/6/9 | EMF2, LHP | N | DYT1, AMS, MS1, JAZ1 | Jasmonate signaling | [151] |
| PPD1/2 | LHP1 | N | D3-type cyclins and HMGA | Lateral organ growth | [166] |
| NGR5 | LC2 | GCCGCC motif | D14, SPL14 | Tillering from nitrogen regulation | [165] |
| AG | LHP1 | Chromatin loop | WUS | Meristem maintenance and determinacy | [142] |
| KNU | FIE | N | WUS | Floral meristems determinacy | [150] |
| Di19 | MEA | N | RPS2 | Pathogen defense | [156] |
| RBR1 | FIE, MSI | N | MET1 | Female gametogenesis | [139,147,159] |
| OsRBR1/2 | OsMSI1 | N | N | Floral development | [160] |
| ESD7 | CLF, EMF2, MSI1 | N | FT, SOC1 | Vegetative growth and flowering | [134] |
| Chromatin remodelers | |||||
| BLI | CLF | N | Homeotic genes, FLC, FT | Vegetative growth and flowering | [144] |
| ICU11 | CLF, SWN, EMF2, MSI1, FIE | N | FLC | Flowering | [135] |
| ALP2 | MSI1 | N | N | Vegetative growth and flowering | [146] |
| EOL1 | CLF, SWN, LHP1 | N | N | H3K27me3 inheritance | [143] |
| OsCTF4 | OsCLF, OsLHP1, OsSWN | N | KRP1, KRP5 | Cell cycle and vegetative growth | [161] |
| ATX1 | CLF | N | AG | Vegetative development | [136] |
| TAF13 | MEA, SWN | N | PHE1, FUS3 and AtFH5 | Seed development | [162] |
| SVP | LHP1 | N | SEP3 | Floral patterning | [158] |
| ATRX | LHP1 | N | FLC | Flowering | [153] |
| PcG-associated proteins | |||||
| VIN3, VEL1, VRN5 | VRN2 | N | FLC | Flowering | [38] |
| OsVIL3 | OsVIL2 | N | OsLF | Rice flowering | [152] |
| OsVIL2 | OsEMF2b | N | OsLFL1, OsTB1 | Rice flowering | [141,154,155] |
| DFO1 | OsMSI1, OsiEZ1 | N | OsMADS58 | Floral organ identity | [145] |
| ncRNAs | |||||
| N | CLF | AG-incRNA4 | AG | Tissue specification | [169] |
| N | LHP1 | APOLO | PID | Auxin signaling | [170] |
| FCA | CLF | COOLAIR | FLC | Flowering | [171] |
| N | CLF | COLDAIR | FLC | Flowering | [168,172] |
| N | CLF | COLDWRAP | FLC | Flowering | [173] |
N, not detected or not applicable. References listed should be consulted for further details.
Although it is still controversial in regards to the molecular mechanism of the RNA-directed PRC2 recruitment in animals, several long ncRNAs have been shown to target the PRC2 complex and epigenetically silence transcription through a molecular interaction with defined loci in Arabidopsis (Figure 2; Table 2) [168,169,170,171,172,173]. Notably, the long ncRNAs COLD ASSISTED INTRONIC NONCODING RNA (COLDAIR), COOLAIR, and COLDWRAP could be induced by cold and repress transcription levels of floral repressor FLOWERING LOCUS C (FLC) via local loading of H3K27me3 [168,171,172,173]. It is likely that long ncRNAs function as a scaffold for the recruitment of chromatin-modifying factors in both plants and animals. There is a lack of any conservation of these ncRNAs; they are a general mechanism for mediating the deposition of H3K27me3 and gene silencing and by which machinery the ncRNAs recognize the chromatin are still enigmatic.
5. Emerging Roles of H3K27me3 in Plant Adaptation to Environmental Cues
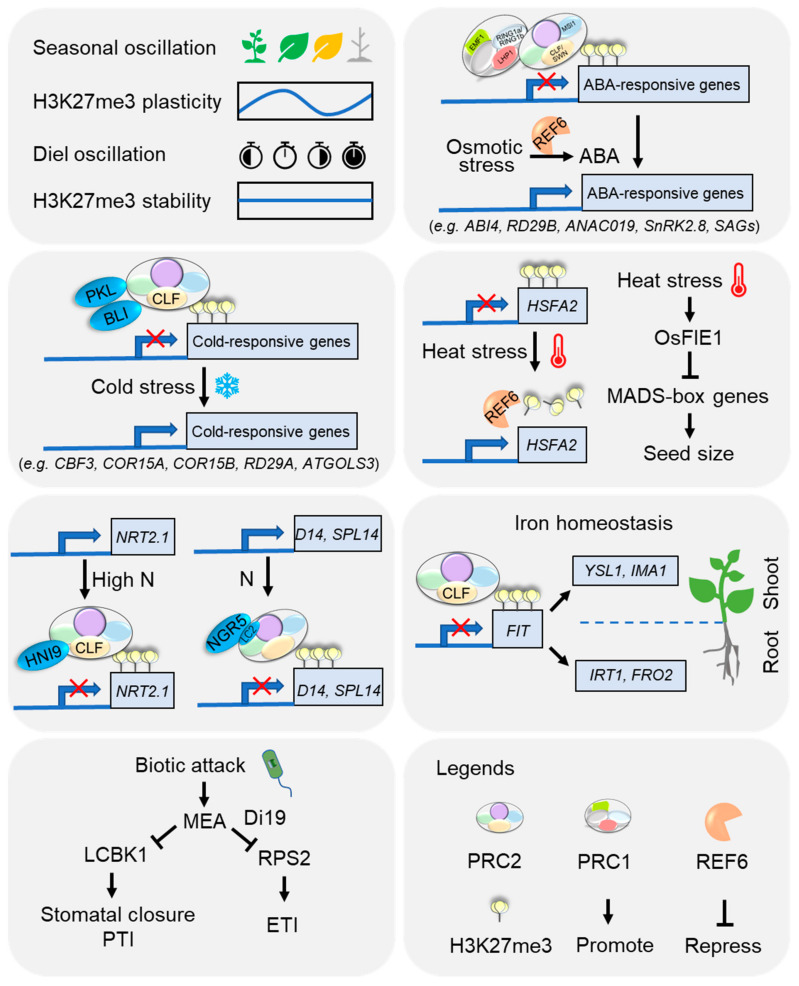
In parallel to their essential functions in the maintenance of cell identity and developmental phase transition in plants, PcG-protein-mediated H3K27me3 modifications are also involved in environmental stress response, either on a genome-wide scale or at specific loci [174,175,176,177,178,179,180]. Basically, changes in H3K27me3 levels are associated with transcriptional regulation of plants’ stress-responsive genes [181,182,183,184], suggesting that H3K27me3 dynamics are important in the regulation of plant adaption to environmental cues (Figure 3).
Figure 3.
Scheme summarizing current understanding on the roles of H3K27me3 in plant adaptation to environmental cues. Acronyms and further details are explained in the text.
5.1. Seasonal and Diel Oscillations
Seasonal and diel oscillations are two major environmental inputs into plant growth and development. Genome-wide chromatin immunoprecipitation sequencing (ChIP-seq) studies have demonstrated that H3K27me3 exhibits seasonal plasticity and diel stability, suggesting that H3K27me3 might act as a monitor to sense the fluctuating environmental stresses, in turn adjusting gene expression and regulation [185,186,187].
A prominent case is vernalization, a naturally occurring phenomenon that promotes flowering by a certain prolonged exposure to cold/low temperatures in winter. Specifically, PcG-protein-mediated histone modifications and epigenetic silencing of the main floral inhibitor FLC are the central hub in the control of vernalization, which is conserved in several plant species [188]. Studies regarding epigenetic regulation of the FLC locus have been thoroughly discussed in several reviews [175,189,190,191], and will not be discussed here.
5.2. Extreme Temperature
Temperature is a major environmental factor that greatly influences plant growth and development. Cold stress could increase chromatin accessibility by coordinating the bivalent H3K4me3 and H3K27me3 modifications, thereby activating cold-responsive gene expression in potatoes (Solanum tuberosum L.) [192]. In Arabidopsis, a CLF-interacting protein BLISTER (BLI) promotes the resistance to cold stress [181]. Moreover, the chromatin remodeler PICKLE (PKL) is required for proper chilling and freezing tolerance, through a CLF-related H3K27me3 pathway [193,194]. Indeed, PKL regulates the expression of many cold-responsive genes, including C-REPEAT BINDING FACTOR 3 (CBF3), RESPONSIVE TO DESICCATION 29A (RD29A), COLD-RESPONSIVE 15A (COR15A), and COR15B [194]. In line with this, cold exposure triggers a significant decrease in H3K27me3 levels of cold-responsive genes COR15A and GALACTINOL SYNTHASE 3 (ATGOLS3), and intriguingly the H3K27me3 amount could be stably maintained to normal growth conditions; hence, they likely serve as epigenetic memory markers for recent transcriptional activity in Arabidopsis [195].
Similarly, heat stress also seriously impairs the growth and production of plants. Conversely to cold, heat can erase the epigenetic marks established during vernalization in Arabidopsis [196]. Mechanically, the heat-induced HEAT SHOCK TRANSCRIPTION FACTOR A2 (HSFA2) directly activates the expression of H3K27me3 demethylase REF6 to reduce the H3K27me3 level at HSFA2 loci, which further enhances the expression HSFA2, thereby forming a REF6-HSFA2 regulatory loop orchestrating transgenerational thermomemory in Arabidopsis [184]. Whilst PRC2-mediated H3K27me3 modification controls the early endosperm development, it also accounts for the reduced seed size and yield under heat stress in both Arabidopsis and cereals [197]. Importantly, heat stress could misregulate OsFIE1 expression and alter the duration of syncytial stage endosperm development, whereas overexpression of OsFIE1 leads to reduced seed size as a result of precocious cellularization [198,199].
5.3. Nutrients
Nutrient availability from the diverse and variable environment is vital for plant growth and crop performance. Of which, nitrogen (N), one of the most important major mineral nutrients, is perceived and transported mainly by nitrate transporters (NRTs) [200]. NRT2.1, encoding a main component of the root high-affinity transport system for NO3−, is repressed under high N supply in a HIGH NITROGEN INSENSITIVE 9 (HNI9)-dependent manner, with increased levels of H3K27me3 at the NRT2.1 site in Arabidopsis [201]. In support of this, mutation of CLF leads to a loss of H3K27me3 at the NRT2.1 loci and enhanced N absorption [202]. Moreover, genome-wide analysis revealed that CLF-related H3K27me3 targets were significantly enriched in metabolic processes, in response to diverse stimuli, in nitrate transport and assimilation, as well as in mineral nutrition and secondary metabolism [202]. In addition, NITROGENMEDIATED TILLER GROWTH RESPONSE 5 (NGR5), an APETALA2-type transcription factor, is able to interact with a component of PRC2 and alter the genome-wide H3K27me3 levels in response to changes in nitrogen availability in rice [165].
Iron is an essential micronutrient but overdose can lead to toxicity. PRC2-mediated H3K27me3 modulates iron homeostasis in Arabidopsis as well. Notably, FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT), directly targeted by H3K27me3, is a master regulator of iron deficiency response [203]. Moreover, genome-wide analysis showed that CLF regulates the expression of FIT-dependent iron acquisition genes, like IRON-REGULATED TRANSPORTER 1 (IRT1) and FERRIC REDUCTASE OXIDASE 2 (FRO2) in roots, and also iron homeostasis genes (e.g., YELLOW STRIPE-LIKE 1 (YSL1), IRON MAN 1 (IMA1)) in shoots, thereby modulating iron translocation from roots to shoots; Consistently, clf mutants have been found to be more resistant to low-iron conditions than the wild type [203,204].
5.4. Osmotic Stress
Osmotic stresses, such as drought and salinity, are major factors restricting plant growth and development, survival and distribution, and are associated with the H3K27me3 mark [2]. Surprisingly, it has been reported that H3K27me3 is not responsible for the transcription reprogramming of stress-responsive genes under dehydration stress [177,205,206,207]. Notwithstanding, impairments of either MSI1 or LHP1 increase drought tolerance in Arabidopsis, probably through the abscisic acid (ABA) signaling pathway [182,208]. In contrast to drought, salt stress can greatly alter H3K27me3 patterns in different plant species [206,209,210,211]. In line with this, reduction of MSI1 expression or EMF1 activity can enhance salt tolerance, through upregulation of H3K27me3 targets, like ABA receptor genes and ABA-responsive genes [212,213].
As a key growth regulator, ABA plays pivotal roles in plant drought- and salt-stress responses [214]. Intriguingly, H3K27me3 demethylase REF6 has been shown to induce ABA biosynthesis in Arabidopsis seeds [215]. In line with removal of H3K27me3, a number of ABA-responsive genes (e.g., ABA-INSENSITIVE 4 (ABI4), RESPONSIVE TO DESSICATION 29B (RD29B), NAC DOMAIN CONTAINING PROTEIN 19 (ANAC019), ANAC055, SNF1-RELATED PROTEIN KINASE 2.8 (SnRK2.8)) and ABA-induced senescence-associated genes (SAGs) are induced and involved in stress responses [181,182,216,217,218]. By contrast, mutants of clf-50 swn-1, msi1-cs, atring1a atring1b, and lhp1 are all hypersensitive to ABA, which demonstrates the crucial roles of PcG proteins in attenuating ABA signaling [184,211,219,220].
5.5. Biotic Stress
In addition to abiotic stress, plants are also subjected to many biotic attacks from bacteria, fungi, oomycetes, viruses, and insects. Therefore, plants evolve a two-layered innate immune system consisting of the pattern-triggered immunity (PTI) and the effector-triggered immunity (ETI). PTI and ETI result in many overlapping downstream outputs, such as reactive oxygen species (ROS) burst, programmed cell death (PCD), transcriptional reprograming, and phytohormone signaling (e.g., salicylic acid (SA) and jasmonate acid (JA))[221]. Besides, genome-wide H3K27me3 atlas was observed in plant responses to infection by pathogens [219,220,222,223,224]. The expression of MEA could be induced by either pathogen inoculation or exogenous application of JA or SA. Indeed, MEA can suppress both PTI and ETI in Arabidopsis [156,183]. Specifically, MEA is able to interact with LONG-CHAIN BASE KINASE1 (LCBK1) and impair its function, which in turn results in a loss of pathogen-induced stomatal closure and PTI; meanwhile, MEA could be recruited by DROUGHT-INDUCED 19 (Di19) to implement H3K27me3 modification on the immune receptor RESISTANCE TO P. SYRINGAE2 (RPS2) loci, thereby repressing its expression and attenuating AvrRpt2 effector-mediated ETI [156,183]. Consistently, MEA-overexpressing transgenic plants are susceptible to fungal pathogens, bacterial pathogens, and Pst-AvrRpt2, whereas mea-6 mutant plants are more resistant to bacterial pathogens [156,183]. Moreover, loss of SWN caused a significantly increased hypersensitive response (HR) during the time course of AvrRpt2 induction, revealing a role of SWN in attenuating PCD [224]. In rice, the H3K27me3 demethylase, OsJMJ705, could be induced by stress signals and pathogen infection. Overexpression of OsJMJ705 derepresses H3K27me3-marked biotic stress-responsive genes and enhances rice resistance to the bacterial blight disease pathogen Xanthomonas oryzae pathovar oryzae, in contrast to reduced resistance of its mutant [60]. Altogether, these observations imply that PRC2-mediated H3K27me3 modification plays negative roles in plant pathogen defense, probably through blocking the phase transition from growth to senescence.
6. Conclusions and Perspectives
H3K27me3, a hallmark of gene silencing, plays prominent roles in cell identity control and developmental phase transition, both in animals and in plants. PRC2 complex, which is evolutionarily conserved across different lineages, catalyzes H3K27me3 modification, which is sequentially recognized by the PRC1 complex, thereafter raising the H2Aub mark and chromatin compaction. In contrast to PRC2, components and machinery of PRC1 appear less conserved between two aspects of animals and plants. Firstly, they utilize different kinds of readers to decode H3K27me3 mark; secondly, unlike the animal hierarchical model, plant PRC1 and PRC2 likely cooperate to exert H3K27me3 deposition and gene repression. Regarding erasing histone lysine methylation, Jumonji C domain proteins have context-dependent substrate specificity toward various histone lysine sites. Particularly, members of the H3K27me3-specific demethylase family identified in plants are relatively limited. Hence, great efforts should be made to address this question. Moreover, writers, erasers, and readers of H3K27me3 need to be functionally characterized in more plant species other than Arabidopsis, like monocot crops. Further investigations in this area promise to unravel conservation and functional diversification of H3K27me3 machinery during evolution of eukaryotic organisms.
In both plants and animals, cis and trans determinants are required for PRC2 recruitment and H3K27me3 deposition. In spite of the limited conservation, some common factors, like GAGA motif and telomeric repeats, do exist across kingdoms. This raises the question when and how eukaryotic cells obtain the ancestral cis-localized DNA sequence motif pathway for H3K27me3 loading. In parallel, trans-acting factors are also promising mediators for recruiting PRC2 through interaction with components of PRC2 and/or PRC1. Importantly, transcription factors play key roles in determining the specificity of H3K27me3 dynamics in different stages of growth and development, as well as responses to various environmental cues. Notwithstanding, we are still far from reaching a full understanding of these open questions. Therefore, research in this direction is expected to identify new regulators for H3K27me3-mediated gene repression, especially in stress responses. In addition, PRC2 can also methylate non-histone proteins. It will be fascinating to discriminate whether some of the interacting proteins are directly methylated by PRC2 independent of H3K27me3.
In genetic regulatory networks, epigenetic mechanisms are of great significance in fine-tuning gene expression in plants’ responses to environmental cues. However, reports about the roles of the H3K27me3 dynamics in plant environmental adaption are quite limited, except the vernalization pathway in Arabidopsis. Undoubtedly, recent advances in high-throughput sequencing technologies using small amounts of chromatin DNA, even at the single-cell level, will open new avenues for expanding our understanding of reprogramming of H3K27me3 in plant stress responses. As polycomb-mediated H3K27me3 regulation mostly plays a negative role in stress responses, it highlights a possible role of H3K27me3 in balancing plant growth and adaptation to stress. Nevertheless, more evidence is needed to ascertain this hypothesis. The in-depth knowledge on H3K27me3 regulation in plants promises to provide new candidates and methods for enhancing crop productivity under stressful environments.
Author Contributions
Writing-original draft preparation, Q.S.; conceptualization and writing-review and revision, G.W.; visualization, Q.S., Y.L. (Yisheng Lin) and Y.L. (Yingbo Li). All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the National Natural Sciences Foundation of China U1804235, 31771800 (to G.W.) and 32001562 (to Q.S.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mirouze M., Paszkowski J. Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 2011;14:267–274. doi: 10.1016/j.pbi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y.N., Zhu C., Jiang J., Zhang H., Zhu J.K., Duan C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020;62:563–580. doi: 10.1111/jipb.12901. [DOI] [PubMed] [Google Scholar]
- 3.Feng S., Jacobsen S.E. Epigenetic modifications in plants: An evolutionary perspective. Curr. Opin. Plant Biol. 2011;14:179–186. doi: 10.1016/j.pbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J., He Z. Small DNA Methylation, Big Player in Plant Abiotic Stress Responses and Memory. Front. Plant Sci. 2020;11:595603. doi: 10.3389/fpls.2020.595603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer P. Epigenetic variation and environmental change. J. Exp. Bot. 2015;66:3541–3548. doi: 10.1093/jxb/eru502. [DOI] [PubMed] [Google Scholar]
- 6.Liu C., Lu F., Cui X., Cao X. Histone Methylation in Higher Plants. Annu. Rev. Plant Biol. 2010;61:395–420. doi: 10.1146/annurev.arplant.043008.091939. [DOI] [PubMed] [Google Scholar]
- 7.Berr A., Shafiq S., Shen W.H. Histone modifications in transcriptional activation during plant development. Biochim. Biophys. Acta. 2011;1809:567–576. doi: 10.1016/j.bbagrm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Berger S.L. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 9.Turck F., Roudier F., Farrona S., Martin-Magniette M.L., Guillaume E., Buisine N., Gagnot S., Martienssen R.A., Coupland G., Colot V. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007;3:e86. doi: 10.1371/journal.pgen.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lafos M., Kroll P., Hohenstatt M.L., Thorpe F.L., Clarenz O., Schubert D. Dynamic regulation of H3K27 trimethylation during Arabidopsis differentiation. PLoS Genet. 2011;7:e1002040. doi: 10.1371/journal.pgen.1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Clarenz O., Cokus S., Bernatavichute Y.V., Pellegrini M., Goodrich J., Jacobsen S.E. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 2007;5:e129. doi: 10.1371/journal.pbio.0050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margueron R., Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanzuolo C., Orlando V. Memories from the polycomb group proteins. Annu. Rev. Genet. 2012;46:561–589. doi: 10.1146/annurev-genet-110711-155603. [DOI] [PubMed] [Google Scholar]
- 14.Simon J.A., Kingston R.E. Occupying Chromatin: Polycomb Mechanisms for Getting to Genomic Targets, Stopping Transcriptional Traffic, and Staying Put. Mol. Cell. 2013;49:808–824. doi: 10.1016/j.molcel.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czermin B., Melfi R., McCabe D., Seitz V., Imhof A., Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–196. doi: 10.1016/S0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 16.Suganuma T., Pattenden S.G., Workman J.L. Diverse functions of WD40 repeat proteins in histone recognition. Genes. Dev. 2008;22:1265–1268. doi: 10.1101/gad.1676208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S., Birve A., Rasmuson-Lestander A. In vivo analysis of Drosophila SU(Z)12 function. Mol. Genet. Genom. 2008;279:159–170. doi: 10.1007/s00438-007-0304-3. [DOI] [PubMed] [Google Scholar]
- 18.Wen P., Quan Z., Xi R. The biological function of the WD40 repeat-containing protein p55/Caf1 in Drosophila. Dev. Dyn. 2012;241:455–464. doi: 10.1002/dvdy.23730. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y., Zhao S., Wang G.G. Polycomb Gene Silencing Mechanisms: PRC2 Chromatin Targeting, H3K27me3 ’Readout’, and Phase Separation-Based Compaction. Trends Genet. 2021 doi: 10.1016/j.tig.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vann K.R., Kutateladze T.G. Architecture of PRC2 Holo Complexes. Trends Biochem. Sci. 2018;43:487–489. doi: 10.1016/j.tibs.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Mierlo G., Veenstra G.J.C., Vermeulen M., Marks H. The Complexity of PRC2 Subcomplexes. Trends Cell Biol. 2019;29:660–671. doi: 10.1016/j.tcb.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Youmans D.T., Gooding A.R., Dowell R.D., Cech T.R. Competition between PRC2.1 and 2.2 subcomplexes regulates PRC2 chromatin occupancy in human stem cells. Mol. Cell. 2021;81:488–501.e489. doi: 10.1016/j.molcel.2020.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healy E., Mucha M., Glancy E., Fitzpatrick D.J., Conway E., Neikes H.K., Monger C., Van Mierlo G., Baltissen M.P., Koseki Y., et al. PRC2.1 and PRC2.2 Synergize to Coordinate H3K27 Trimethylation. Mol. Cell. 2019;76:437–452.e436. doi: 10.1016/j.molcel.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y., Chen D.H., Liu B.Y., Shen W.H., Ruan Y. Conservation and diversification of polycomb repressive complex 2 (PRC2) proteins in the green lineage. Brief Funct. Genom. 2017;16:106–119. doi: 10.1093/bfgp/elw007. [DOI] [PubMed] [Google Scholar]
- 25.Butenko Y., Ohad N. Polycomb-group mediated epigenetic mechanisms through plant evolution. Biochim. Biophys. Acta. 2011;1809:395–406. doi: 10.1016/j.bbagrm.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Pien S., Grossniklaus U. Polycomb group and trithorax group proteins in Arabidopsis. Biochim. Biophys. Acta. 2007;1769:375–382. doi: 10.1016/j.bbaexp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Kim D.H., Sung S. Polycomb-mediated gene silencing in Arabidopsis thaliana. Mol. Cells. 2014;37:841–850. doi: 10.14348/molcells.2014.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiyosue T., Ohad N., Yadegari R., Hannon M., Dinneny J., Wells D., Katz A., Margossian L., Harada J.J., Goldberg R.B., et al. Control of fertilization-independent endosperm development by the MEDEA polycomb gene in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1999;96:4186–4191. doi: 10.1073/pnas.96.7.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhury A.M., Ming L., Miller C., Craig S., Dennis E.S., Peacock W.J. Fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 1997;94:4223–4228. doi: 10.1073/pnas.94.8.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guitton A.E., Page D.R., Chambrier P., Lionnet C., Faure J.E., Grossniklaus U., Berger F. Identification of new members of Fertilisation Independent Seed Polycomb Group pathway involved in the control of seed development in Arabidopsis thaliana. Development. 2004;131:2971–2981. doi: 10.1242/dev.01168. [DOI] [PubMed] [Google Scholar]
- 31.Grossniklaus U., Vielle-Calzada J.P., Hoeppner M.A., Gagliano W.B. Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science. 1998;280:446–450. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- 32.Ohad N., Margossian L., Hsu Y.C., Williams C., Repetti P., Fischer R.L. A mutation that allows endosperm development without fertilization. Proc. Natl. Acad. Sci. USA. 1996;93:5319–5324. doi: 10.1073/pnas.93.11.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G., Kohler C. Epigenetic processes in flowering plant reproduction. J Exp Bot. 2017;68:797–807. doi: 10.1093/jxb/erw486. [DOI] [PubMed] [Google Scholar]
- 34.Chanvivattana Y., Bishopp A., Schubert D., Stock C., Moon Y.H., Sung Z.R., Goodrich J. Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development. 2004;131:5263–5276. doi: 10.1242/dev.01400. [DOI] [PubMed] [Google Scholar]
- 35.Yang C.H., Chen L.J., Sung Z.R. Genetic regulation of shoot development in Arabidopsis: Role of the EMF genes. Dev. Biol. 1995;169:421–435. doi: 10.1006/dbio.1995.1158. [DOI] [PubMed] [Google Scholar]
- 36.Kim S.Y., Zhu T., Sung Z.R. Epigenetic regulation of gene programs by EMF1 and EMF2 in Arabidopsis. Plant Physiol. 2010;152:516–528. doi: 10.1104/pp.109.143495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gendall A.R., Levy Y.Y., Wilson A., Dean C. The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell. 2001;107:525–535. doi: 10.1016/S0092-8674(01)00573-6. [DOI] [PubMed] [Google Scholar]
- 38.De Lucia F., Crevillen P., Jones A.M., Greb T., Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc. Natl. Acad. Sci. USA. 2008;105:16831–16836. doi: 10.1073/pnas.0808687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood C.C., Robertson M., Tanner G., Peacock W.J., Dennis E.S., Helliwell C.A. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc. Natl. Acad. Sci. USA. 2006;103:14631–14636. doi: 10.1073/pnas.0606385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni J., Ma X., Feng Y., Tian Q., Wang Y., Xu N., Tang J., Wang G. Updating and interaction of polycomb repressive complex 2 components in maize (Zea mays) Planta. 2019;250:573–588. doi: 10.1007/s00425-019-03193-4. [DOI] [PubMed] [Google Scholar]
- 41.Cheng X., Pan M., Zhiguo E., Zhou Y., Niu B., Chen C. The maternally expressed polycomb group gene OsEMF2a is essential for endosperm cellularization and imprinting in rice. Plant Commun. 2021;2:100092. doi: 10.1016/j.xplc.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonosaki K., Ono A., Kunisada M., Nishino M., Nagata H., Sakamoto S., Kijima S.T., Furuumi H., Nonomura K.I., Sato Y., et al. Mutation of the imprinted gene OsEMF2a induces autonomous endosperm development and delayed cellularization in rice. Plant Cell. 2021;33:85–103. doi: 10.1093/plcell/koaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conrad L.J., Khanday I., Johnson C., Guiderdoni E., An G., Vijayraghavan U., Sundaresan V. The polycomb group gene EMF2B is essential for maintenance of floral meristem determinacy in rice. Plant J. 2014;80:883–894. doi: 10.1111/tpj.12688. [DOI] [PubMed] [Google Scholar]
- 44.Zhong J., Peng Z., Peng Q., Cai Q., Peng W., Chen M., Yao J. Regulation of plant height in rice by the Polycomb group genes OsEMF2b, OsFIE2 and OsCLF. Plant Sci. 2018;267:157–167. doi: 10.1016/j.plantsci.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Black J.C., Van Rechem C., Whetstine J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell. 2012;48:491–507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klose R.J., Kallin E.M., Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat. Rev. Genet. 2006;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 47.Hong S., Cho Y.W., Yu L.R., Yu H., Veenstra T.D., Ge K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc. Natl. Acad. Sci. USA. 2007;104:18439–18444. doi: 10.1073/pnas.0707292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agger K., Cloos P.A., Christensen J., Pasini D., Rose S., Rappsilber J., Issaeva I., Canaani E., Salcini A.E., Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 49.Li G., Margueron R., Ku M., Chambon P., Bernstein B.E., Reinberg D. Jarid2 and PRC2, partners in regulating gene expression. Genes. Dev. 2010;24:368–380. doi: 10.1101/gad.1886410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasini D., Cloos P.A., Walfridsson J., Olsson L., Bukowski J.P., Johansen J.V., Bak M., Tommerup N., Rappsilber J., Helin K. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature. 2010;464:306–310. doi: 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- 51.Crevillén P. Histone Demethylases as Counterbalance to H3K27me3 Silencing in Plants. iScience. 2020;23:101715. doi: 10.1016/j.isci.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z., Ou Y., Zhang Z., Li J., He Y. Brassinosteroid Signaling Recruits Histone 3 Lysine-27 Demethylation Activity to FLOWERING LOCUS C Chromatin to Inhibit the Floral Transition in Arabidopsis. Mol. Plant. 2018;11:1135–1146. doi: 10.1016/j.molp.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Yu X., Li L., Li L., Guo M., Chory J., Yin Y. Modulation of brassinosteroid-regulated gene expression by Jumonji domain-containing proteins ELF6 and REF6 in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2008;105:7618–7623. doi: 10.1073/pnas.0802254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C., Gu L., Gao L., Chen C., Wei C.Q., Qiu Q., Chien C.W., Wang S., Jiang L., Ai L.F., et al. Concerted genomic targeting of H3K27 demethylase REF6 and chromatin-remodeling ATPase BRM in Arabidopsis. Nat. Genet. 2016;48:687–693. doi: 10.1038/ng.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noh B., Lee S.H., Kim H.J., Yi G., Shin E.A., Lee M., Jung K.J., Doyle M.R., Amasino R.M., Noh Y.S. Divergent roles of a pair of homologous jumonji/zinc-finger-class transcription factor proteins in the regulation of Arabidopsis flowering time. Plant Cell. 2004;16:2601–2613. doi: 10.1105/tpc.104.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu F., Cui X., Zhang S., Jenuwein T., Cao X. Arabidopsis REF6 is a histone H3 lysine 27 demethylase. Nat. Genet. 2011;43:715–719. doi: 10.1038/ng.854. [DOI] [PubMed] [Google Scholar]
- 57.Tian Z., Li X., Li M., Wu W., Zhang M., Tang C., Li Z., Liu Y., Chen Z., Yang M., et al. Crystal structures of REF6 and its complex with DNA reveal diverse recognition mechanisms. Cell Discov. 2020;6:17. doi: 10.1038/s41421-020-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng S., Hu H., Ren H., Yang Z., Qiu Q., Qi W., Liu X., Chen X., Cui X., Li S., et al. The Arabidopsis H3K27me3 demethylase JUMONJI 13 is a temperature and photoperiod dependent flowering repressor. Nat. Commun. 2019;10:1303. doi: 10.1038/s41467-019-09310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gan E.S., Xu Y., Wong J.Y., Goh J.G., Sun B., Wee W.Y., Huang J., Ito T. Jumonji demethylases moderate precocious flowering at elevated temperature via regulation of FLC in Arabidopsis. Nat. Commun. 2014;5:5098. doi: 10.1038/ncomms6098. [DOI] [PubMed] [Google Scholar]
- 60.Li T., Chen X., Zhong X., Zhao Y., Liu X., Zhou S., Cheng S., Zhou D.X. Jumonji C domain protein JMJ705-mediated removal of histone H3 lysine 27 trimethylation is involved in defense-related gene activation in rice. Plant Cell. 2013;25:4725–4736. doi: 10.1105/tpc.113.118802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng S., Tan F., Lu Y., Liu X., Li T., Yuan W., Zhao Y., Zhou D.X. WOX11 recruits a histone H3K27me3 demethylase to promote gene expression during shoot development in rice. Nucleic Acids Res. 2018;46:2356–2369. doi: 10.1093/nar/gky017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian Y., Chen C., Jiang L., Zhang J., Ren Q. Genome-wide identification, classification and expression analysis of the JmjC domain-containing histone demethylase gene family in maize. BMC Genom. 2019;20:256. doi: 10.1186/s12864-019-5633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R.S., Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 64.Wani A.H., Boettiger A.N., Schorderet P., Ergun A., Münger C., Sadreyev R.I., Zhuang X., Kingston R.E., Francis N.J. Chromatin topology is coupled to Polycomb group protein subnuclear organization. Nat. Commun. 2016;7:10291. doi: 10.1038/ncomms10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eskeland R., Leeb M., Grimes G.R., Kress C., Boyle S., Sproul D., Gilbert N., Fan Y., Skoultchi A.I., Wutz A., et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol. Cell. 2010;38:452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehmann L., Ferrari R., Vashisht A.A., Wohlschlegel J.A., Kurdistani S.K., Carey M. Polycomb repressive complex 1 (PRC1) disassembles RNA polymerase II preinitiation complexes. J. Biol. Chem. 2012;287:35784–35794. doi: 10.1074/jbc.M112.397430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin X., Romero-Campero F.J., de Los Reyes P., Yan P., Yang J., Tian G., Yang X., Mo X., Zhao S., Calonje M., et al. H2AK121ub in Arabidopsis associates with a less accessible chromatin state at transcriptional regulation hotspots. Nat. Commun. 2021;12:315. doi: 10.1038/s41467-020-20614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz Y.B., Kahn T.G., Nix D.A., Li X.Y., Bourgon R., Biggin M., Pirrotta V. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat. Genet. 2006;38:700–705. doi: 10.1038/ng1817. [DOI] [PubMed] [Google Scholar]
- 69.Tolhuis B., de Wit E., Muijrers I., Teunissen H., Talhout W., van Steensel B., van Lohuizen M. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat. Genet. 2006;38:694–699. doi: 10.1038/ng1792. [DOI] [PubMed] [Google Scholar]
- 70.Ku M., Koche R.P., Rheinbay E., Mendenhall E.M., Endoh M., Mikkelsen T.S., Presser A., Nusbaum C., Xie X., Chi A.S., et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 2008;4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S.Y., Lee J., Eshed-Williams L., Zilberman D., Sung Z.R. EMF1 and PRC2 cooperate to repress key regulators of Arabidopsis development. PLoS Genet. 2012;8:e1002512. doi: 10.1371/journal.pgen.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blackledge N.P., Farcas A.M., Kondo T., King H.W., McGouran J.F., Hanssen L.L.P., Ito S., Cooper S., Kondo K., Koseki Y., et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014;157:1445–1459. doi: 10.1016/j.cell.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Y., Romero-Campero F.J., Gómez-Zambrano Á., Turck F., Calonje M. H2A monoubiquitination in Arabidopsis thaliana is generally independent of LHP1 and PRC2 activity. Genome Biol. 2017;18:69. doi: 10.1186/s13059-017-1197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang C., Bratzel F., Hohmann N., Koch M., Turck F., Calonje M. VAL- and AtBMI1-mediated H2Aub initiate the switch from embryonic to postgerminative growth in Arabidopsis. Curr. Biol. 2013;23:1324–1329. doi: 10.1016/j.cub.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 75.Kalb R., Latwiel S., Baymaz H.I., Jansen P.W., Müller C.W., Vermeulen M., Müller J. Histone H2A monoubiquitination promotes histone H3 methylation in Polycomb repression. Nat. Struct. Mol. Biol. 2014;21:569–571. doi: 10.1038/nsmb.2833. [DOI] [PubMed] [Google Scholar]
- 76.Tavares L., Dimitrova E., Oxley D., Webster J., Poot R., Demmers J., Bezstarosti K., Taylor S., Ura H., Koide H., et al. RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell. 2012;148:664–678. doi: 10.1016/j.cell.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kralemann L.E.M., Liu S., Trejo-Arellano M.S., Muñoz-Viana R., Köhler C., Hennig L. Removal of H2Aub1 by ubiquitin-specific proteases 12 and 13 is required for stable Polycomb-mediated gene repression in Arabidopsis. Genome Biol. 2020;21:144. doi: 10.1186/s13059-020-02062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shao Z., Raible F., Mollaaghababa R., Guyon J.R., Wu C.T., Bender W., Kingston R.E. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell. 1999;98:37–46. doi: 10.1016/S0092-8674(00)80604-2. [DOI] [PubMed] [Google Scholar]
- 79.Brunk B.P., Martin E.C., Adler P.N. Drosophila genes Posterior Sex Combs and Suppressor two of zeste encode proteins with homology to the murine bmi-1 oncogene. Nature. 1991;353:351–353. doi: 10.1038/353351a0. [DOI] [PubMed] [Google Scholar]
- 80.Gutiérrez L., Oktaba K., Scheuermann J.C., Gambetta M.C., Ly-Hartig N., Müller J. The role of the histone H2A ubiquitinase Sce in Polycomb repression. Development. 2012;139:117–127. doi: 10.1242/dev.074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Messmer S., Franke A., Paro R. Analysis of the functional role of the Polycomb chromo domain in Drosophila melanogaster. Genes. Dev. 1992;6:1241–1254. doi: 10.1101/gad.6.7.1241. [DOI] [PubMed] [Google Scholar]
- 82.Eissenberg J.C. Structural biology of the chromodomain: Form and function. Gene. 2012;496:69–78. doi: 10.1016/j.gene.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Narbonne K., Besse F., Brissard-Zahraoui J., Pret A.M., Busson D. polyhomeotic is required for somatic cell proliferation and differentiation during ovarian follicle formation in Drosophila. Development. 2004;131:1389–1400. doi: 10.1242/dev.01003. [DOI] [PubMed] [Google Scholar]
- 84.Schoorlemmer J., Marcos-Gutiérrez C., Were F., Martínez R., García E., Satijn D.P., Otte A.P., Vidal M. Ring1A is a transcriptional repressor that interacts with the Polycomb-M33 protein and is expressed at rhombomere boundaries in the mouse hindbrain. EMBO J. 1997;16:5930–5942. doi: 10.1093/emboj/16.19.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao Z., Zhang J., Bonasio R., Strino F., Sawai A., Parisi F., Kluger Y., Reinberg D. PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol. Cell. 2012;45:344–356. doi: 10.1016/j.molcel.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morey L., Pascual G., Cozzuto L., Roma G., Wutz A., Benitah S.A., Di Croce L. Nonoverlapping functions of the Polycomb group Cbx family of proteins in embryonic stem cells. Cell Stem. Cell. 2012;10:47–62. doi: 10.1016/j.stem.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Tonkin E., Hagan D.M., Li W., Strachan T. Identification and characterisation of novel mammalian homologues of Drosophila polyhomeoticpermits new insights into relationships between members of the polyhomeotic family. Hum. Genet. 2002;111:435–442. doi: 10.1007/s00439-002-0814-3. [DOI] [PubMed] [Google Scholar]
- 88.Xu L., Shen W.H. Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr. Biol. 2008;18:1966–1971. doi: 10.1016/j.cub.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 89.Sanchez-Pulido L., Devos D., Sung Z.R., Calonje M. RAWUL: A new ubiquitin-like domain in PRC1 ring finger proteins that unveils putative plant and worm PRC1 orthologs. BMC Genom. 2008;9:308. doi: 10.1186/1471-2164-9-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen D., Molitor A., Liu C., Shen W.H. The Arabidopsis PRC1-like ring-finger proteins are necessary for repression of embryonic traits during vegetative growth. Cell Res. 2010;20:1332–1344. doi: 10.1038/cr.2010.151. [DOI] [PubMed] [Google Scholar]
- 91.Bratzel F., López-Torrejón G., Koch M., Del Pozo J.C., Calonje M. Keeping cell identity in Arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr. Biol. 2010;20:1853–1859. doi: 10.1016/j.cub.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 92.Li W., Wang Z., Li J., Yang H., Cui S., Wang X., Ma L. Overexpression of AtBMI1C, a polycomb group protein gene, accelerates flowering in Arabidopsis. PLoS ONE. 2011;6:e21364. doi: 10.1371/journal.pone.0021364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bratzel F., Yang C., Angelova A., López-Torrejón G., Koch M., del Pozo J.C., Calonje M. Regulation of the new Arabidopsis imprinted gene AtBMI1C requires the interplay of different epigenetic mechanisms. Mol. Plant. 2012;5:260–269. doi: 10.1093/mp/ssr078. [DOI] [PubMed] [Google Scholar]
- 94.Merini W., Romero-Campero F.J., Gomez-Zambrano A., Zhou Y., Turck F., Calonje M. The Arabidopsis Polycomb Repressive Complex 1 (PRC1) Components AtBMI1A, B, and C Impact Gene Networks throughout All Stages of Plant Development. Plant Physiol. 2017;173:627–641. doi: 10.1104/pp.16.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang Y., Jiang L., Liu B.Y., Tan C.F., Chen D.H., Shen W.H., Ruan Y. Evolution and conservation of polycomb repressive complex 1 core components and putative associated factors in the green lineage. BMC Genom. 2019;20:533. doi: 10.1186/s12864-019-5905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krause K., Turck F. Plant H3K27me3 has finally found its readers. Nat. Genet. 2018;50:1206–1208. doi: 10.1038/s41588-018-0201-1. [DOI] [PubMed] [Google Scholar]
- 97.Kotake T., Takada S., Nakahigashi K., Ohto M., Goto K. Arabidopsis TERMINAL FLOWER 2 gene encodes a heterochromatin protein 1 homolog and represses both FLOWERING LOCUS T to regulate flowering time and several floral homeotic genes. Plant Cell Physiol. 2003;44:555–564. doi: 10.1093/pcp/pcg091. [DOI] [PubMed] [Google Scholar]
- 98.Gaudin V., Libault M., Pouteau S., Juul T., Zhao G., Lefebvre D., Grandjean O. Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development. 2001;128:4847–4858. doi: 10.1242/dev.128.23.4847. [DOI] [PubMed] [Google Scholar]
- 99.Yang Z., Qian S., Scheid R.N., Lu L., Chen X., Liu R., Du X., Lv X., Boersma M.D., Scalf M., et al. EBS is a bivalent histone reader that regulates floral phase transition in Arabidopsis. Nat. Genet. 2018;50:1247–1253. doi: 10.1038/s41588-018-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Z., Fu X., Wang Y., Liu R., He Y. Polycomb-mediated gene silencing by the BAH-EMF1 complex in plants. Nat. Genet. 2018;50:1254–1261. doi: 10.1038/s41588-018-0190-0. [DOI] [PubMed] [Google Scholar]
- 101.Qian S., Lv X., Scheid R.N., Lu L., Yang Z., Chen W., Liu R., Boersma M.D., Denu J.M., Zhong X., et al. Dual recognition of H3K4me3 and H3K27me3 by a plant histone reader SHL. Nat. Commun. 2018;9:2425. doi: 10.1038/s41467-018-04836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qian F., Zhao Q.Y., Zhang T.N., Li Y.L., Su Y.N., Li L., Sui J.H., Chen S., He X.J. A histone H3K27me3 reader cooperates with a family of PHD finger-containing proteins to regulate flowering time in Arabidopsis. J. Integr. Plant Biol. 2021 doi: 10.1111/jipb.13067. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y.Z., Yuan J., Zhang L., Chen C., Wang Y., Zhang G., Peng L., Xie S.S., Jiang J., Zhu J.K., et al. Coupling of H3K27me3 recognition with transcriptional repression through the BAH-PHD-CPL2 complex in Arabidopsis. Nat. Commun. 2020;11:6212. doi: 10.1038/s41467-020-20089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.López-González L., Mouriz A., Narro-Diego L., Bustos R., Martínez-Zapater J.M., Jarillo J.A., Piñeiro M. Chromatin-dependent repression of the Arabidopsis floral integrator genes involves plant specific PHD-containing proteins. Plant Cell. 2014;26:3922–3938. doi: 10.1105/tpc.114.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aubert D., Chen L., Moon Y.H., Martin D., Castle L.A., Yang C.H., Sung Z.R. EMF1, a novel protein involved in the control of shoot architecture and flowering in Arabidopsis. Plant Cell. 2001;13:1865–1875. doi: 10.1105/TPC.010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moon Y.H., Chen L., Pan R.L., Chang H.S., Zhu T., Maffeo D.M., Sung Z.R. EMF genes maintain vegetative development by repressing the flower program in Arabidopsis. Plant Cell. 2003;15:681–693. doi: 10.1105/tpc.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen L., Cheng J.C., Castle L., Sung Z.R. EMF genes regulate Arabidopsis inflorescence development. Plant Cell. 1997;9:2011–2024. doi: 10.1105/tpc.9.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beltran M., Yates C.M., Skalska L., Dawson M., Reis F.P., Viiri K., Fisher C.L., Sibley C.R., Foster B.M., Bartke T., et al. The interaction of PRC2 with RNA or chromatin is mutually antagonistic. Genome Res. 2016;26:896–907. doi: 10.1101/gr.197632.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao J., Sun B.K., Erwin J.A., Song J.J., Lee J.T. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kassis J.A., Brown J.L. Polycomb group response elements in Drosophila and vertebrates. Adv. Genet. 2013;81:83–118. doi: 10.1016/b978-0-12-407677-8.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laugesen A., Højfeldt J.W., Helin K. Molecular Mechanisms Directing PRC2 Recruitment and H3K27 Methylation. Mol. Cell. 2019;74:8–18. doi: 10.1016/j.molcel.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E., et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ballaré C., Lange M., Lapinaite A., Martin G.M., Morey L., Pascual G., Liefke R., Simon B., Shi Y., Gozani O., et al. Phf19 links methylated Lys36 of histone H3 to regulation of Polycomb activity. Nat. Struct. Mol. Biol. 2012;19:1257–1265. doi: 10.1038/nsmb.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cai L., Rothbart S.B., Lu R., Xu B., Chen W.-Y., Tripathy A., Rockowitz S., Zheng D., Patel D.J., Allis C.D., et al. An H3K36 Methylation-Engaging Tudor Motif of Polycomb-like Proteins Mediates PRC2 Complex Targeting. Mol. Cell. 2013;49:571–582. doi: 10.1016/j.molcel.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jermann P., Hoerner L., Burger L., Schübeler D. Short sequences can efficiently recruit histone H3 lysine 27 trimethylation in the absence of enhancer activity and DNA methylation. Proc. Natl. Acad. Sci. USA. 2014;111:E3415. doi: 10.1073/pnas.1400672111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lynch M.D., Smith A.J., De Gobbi M., Flenley M., Hughes J.R., Vernimmen D., Ayyub H., Sharpe J.A., Sloane-Stanley J.A., Sutherland L., et al. An interspecies analysis reveals a key role for unmethylated CpG dinucleotides in vertebrate Polycomb complex recruitment. EMBO J. 2012;31:317–329. doi: 10.1038/emboj.2011.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hecker A., Brand L.H., Peter S., Simoncello N., Kilian J., Harter K., Gaudin V., Wanke D. The Arabidopsis GAGA-Binding Factor BASIC PENTACYSTEINE6 Recruits the POLYCOMB-REPRESSIVE COMPLEX1 Component LIKE HETEROCHROMATIN PROTEIN1 to GAGA DNA Motifs. Plant Physiol. 2015;168:1013–1024. doi: 10.1104/pp.15.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deng W., Buzas D.M., Ying H., Robertson M., Taylor J., Peacock W.J., Dennis E.S., Helliwell C. Arabidopsis Polycomb Repressive Complex 2 binding sites contain putative GAGA factor binding motifs within coding regions of genes. BMC Genom. 2013;14:593. doi: 10.1186/1471-2164-14-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mu Y., Zou M., Sun X., He B., Xu X., Liu Y., Zhang L., Chi W. BASIC PENTACYSTEINE Proteins Repress ABSCISIC ACID INSENSITIVE4 Expression via Direct Recruitment of the Polycomb-Repressive Complex 2 in Arabidopsis Root Development. Plant Cell Physiol. 2017;58:607–621. doi: 10.1093/pcp/pcx006. [DOI] [PubMed] [Google Scholar]
- 120.Xiao J., Jin R., Yu X., Shen M., Wagner J.D., Pai A., Song C., Zhuang M., Klasfeld S., He C., et al. Cis and trans determinants of epigenetic silencing by Polycomb repressive complex 2 in Arabidopsis. Nat. Genet. 2017;49:1546–1552. doi: 10.1038/ng.3937. [DOI] [PubMed] [Google Scholar]
- 121.Theune M.L., Bloss U., Brand L.H., Ladwig F., Wanke D. Phylogenetic Analyses and GAGA-Motif Binding Studies of BBR/BPC Proteins Lend to Clues in GAGA-Motif Recognition and a Regulatory Role in Brassinosteroid Signaling. Front. Plant Sci. 2019;10:466. doi: 10.3389/fpls.2019.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu J., Mohamed D., Dowhanik S., Petrella R., Gregis V., Li J., Wu L., Gazzarrini S. Spatiotemporal Restriction of FUSCA3 Expression by Class I BPCs Promotes Ovule Development and Coordinates Embryo and Endosperm Growth. Plant Cell. 2020;32:1886–1904. doi: 10.1105/tpc.19.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Y., Hartwig B., James G.V., Schneeberger K., Turck F. Complementary Activities of TELOMERE REPEAT BINDING Proteins and Polycomb Group Complexes in Transcriptional Regulation of Target Genes. Plant Cell. 2016;28:87–101. doi: 10.1105/tpc.15.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou Y., Wang Y., Krause K., Yang T., Dongus J.A., Zhang Y., Turck F. Telobox motifs recruit CLF/SWN-PRC2 for H3K27me3 deposition via TRB factors in Arabidopsis. Nat. Genet. 2018;50:638–644. doi: 10.1038/s41588-018-0109-9. [DOI] [PubMed] [Google Scholar]
- 125.Marión R.M., Montero J.J., López de Silanes I., Graña-Castro O., Martínez P., Schoeftner S., Palacios-Fábrega J.A., Blasco M.A. TERRA regulate the transcriptional landscape of pluripotent cells through TRF1-dependent recruitment of PRC2. Elife. 2019;8 doi: 10.7554/eLife.44656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qüesta J.I., Song J., Geraldo N., An H., Dean C. Arabidopsis transcriptional repressor VAL1 triggers Polycomb silencing at FLC during vernalization. Science. 2016;353:485–488. doi: 10.1126/science.aaf7354. [DOI] [PubMed] [Google Scholar]
- 127.Jing Y., Guo Q., Lin R. The B3-Domain Transcription Factor VAL1 Regulates the Floral Transition by Repressing FLOWERING LOCUS T. Plant Physiol. 2019;181:236–248. doi: 10.1104/pp.19.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen N., Wang H., Abdelmageed H., Veerappan V., Tadege M., Allen R.D. HSI2/VAL1 and HSL1/VAL2 function redundantly to repress DOG1 expression in Arabidopsis seeds and seedlings. New Phytol. 2020;227:840–856. doi: 10.1111/nph.16559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen N., Veerappan V., Abdelmageed H., Kang M., Allen R.D. HSI2/VAL1 Silences AGL15 to Regulate the Developmental Transition from Seed Maturation to Vegetative Growth in Arabidopsis. Plant Cell. 2018;30:600–619. doi: 10.1105/tpc.17.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie Y., Zhang Y., Han J., Luo J., Li G., Huang J., Wu H., Tian Q., Zhu Q., Chen Y., et al. The Intronic cis Element SE1 Recruits trans-Acting Repressor Complexes to Repress the Expression of ELONGATED UPPERMOST INTERNODE1 in Rice. Mol. Plant. 2018;11:720–735. doi: 10.1016/j.molp.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 131.Roscoe T.J., Vaissayre V., Paszkiewicz G., Clavijo F., Kelemen Z., Michaud C., Lepiniec L.C., Dubreucq B., Zhou D.X., Devic M. Regulation of FUSCA3 Expression During Seed Development in Arabidopsis. Plant Cell Physiol. 2019;60:476–487. doi: 10.1093/pcp/pcy224. [DOI] [PubMed] [Google Scholar]
- 132.Yuan L., Song X., Zhang L., Yu Y., Liang Z., Lei Y., Ruan J., Tan B., Liu J., Li C. The transcriptional repressors VAL1 and VAL2 recruit PRC2 for genome-wide Polycomb silencing in Arabidopsis. Nucleic Acids Res. 2021;49:98–113. doi: 10.1093/nar/gkaa1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sasnauskas G., Kauneckaite K., Siksnys V. Structural basis of DNA target recognition by the B3 domain of Arabidopsis epigenome reader VAL1. Nucleic Acids Res. 2018;46:4316–4324. doi: 10.1093/nar/gky256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Derkacheva M., Steinbach Y., Wildhaber T., Mozgová I., Mahrez W., Nanni P., Bischof S., Gruissem W., Hennig L. Arabidopsis MSI1 connects LHP1 to PRC2 complexes. EMBO J. 2013;32:2073–2085. doi: 10.1038/emboj.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuwabara A., Gruissem W. Arabidopsis RETINOBLASTOMA-RELATED and Polycomb group proteins: Cooperation during plant cell differentiation and development. J. Exp. Bot. 2014;65:2667–2676. doi: 10.1093/jxb/eru069. [DOI] [PubMed] [Google Scholar]
- 136.Del Olmo I., López J.A., Vázquez J., Raynaud C., Piñeiro M., Jarillo J.A. Arabidopsis DNA polymerase ϵ recruits components of Polycomb repressor complex to mediate epigenetic gene silencing. Nucleic Acids Res. 2016;44:5597–5614. doi: 10.1093/nar/gkw156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bloomer R.H., Hutchison C.E., Bäurle I., Walker J., Fang X., Perera P., Velanis C.N., Gümüs S., Spanos C., Rappsilber J., et al. The Arabidopsis epigenetic regulator ICU11 as an accessory protein of Polycomb Repressive Complex 2. Proc. Natl. Acad. Sci. USA. 2020;117:16660–16666. doi: 10.1073/pnas.1920621117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saleh A., Al-Abdallat A., Ndamukong I., Alvarez-Venegas R., Avramova Z. The Arabidopsis homologs of trithorax (ATX1) and enhancer of zeste (CLF) establish ’bivalent chromatin marks’ at the silent AGAMOUS locus. Nucleic Acids Res. 2007;35:6290–6296. doi: 10.1093/nar/gkm464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou J.X., Liu Z.W., Li Y.Q., Li L., Wang B., Chen S., He X.J. Arabidopsis PWWP domain proteins mediate H3K27 trimethylation on FLC and regulate flowering time. J. Integr. Plant Biol. 2018;60:362–368. doi: 10.1111/jipb.12630. [DOI] [PubMed] [Google Scholar]
- 140.Lodha M., Marco C.F., Timmermans M.C. The ASYMMETRIC LEAVES complex maintains repression of KNOX homeobox genes via direct recruitment of Polycomb-repressive complex2. Genes. Dev. 2013;27:596–601. doi: 10.1101/gad.211425.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yoon J., Cho L.H., Lee S., Pasriga R., Tun W., Yang J., Yoon H., Jeong H.J., Jeon J.S., An G. Chromatin Interacting Factor OsVIL2 Is Required for Outgrowth of Axillary Buds in Rice. Mol. Cells. 2019;42:858–868. doi: 10.14348/molcells.2019.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo L., Cao X., Liu Y., Li J., Li Y., Li D., Zhang K., Gao C., Dong A., Liu X. A chromatin loop represses WUSCHEL expression in Arabidopsis. Plant J. 2018;94:1083–1097. doi: 10.1111/tpj.13921. [DOI] [PubMed] [Google Scholar]
- 143.Zhou Y., Tergemina E., Cui H., Förderer A., Hartwig B., Velikkakam James G., Schneeberger K., Turck F. Ctf4-related protein recruits LHP1-PRC2 to maintain H3K27me3 levels in dividing cells in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2017;114:4833–4838. doi: 10.1073/pnas.1620955114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schatlowski N., Stahl Y., Hohenstatt M.L., Goodrich J., Schubert D. The CURLY LEAF interacting protein BLISTER controls expression of polycomb-group target genes and cellular differentiation of Arabidopsis thaliana. Plant Cell. 2010;22:2291–2305. doi: 10.1105/tpc.109.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zheng M., Wang Y., Wang Y., Wang C., Ren Y., Lv J., Peng C., Wu T., Liu K., Zhao S., et al. DEFORMED FLORAL ORGAN1 (DFO1) regulates floral organ identity by epigenetically repressing the expression of OsMADS58 in rice (Oryza sativa) New Phytol. 2015;206:1476–1490. doi: 10.1111/nph.13318. [DOI] [PubMed] [Google Scholar]
- 146.Velanis C.N., Perera P., Thomson B., de Leau E., Liang S.C., Hartwig B., Förderer A., Thornton H., Arede P., Chen J., et al. The domesticated transposase ALP2 mediates formation of a novel Polycomb protein complex by direct interaction with MSI1, a core subunit of Polycomb Repressive Complex 2 (PRC2) PLoS Genet. 2020;16:e1008681. doi: 10.1371/journal.pgen.1008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Johnston A.J., Matveeva E., Kirioukhova O., Grossniklaus U., Gruissem W. A dynamic reciprocal RBR-PRC2 regulatory circuit controls Arabidopsis gametophyte development. Curr. Biol. 2008;18:1680–1686. doi: 10.1016/j.cub.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 148.Calonje M., Sanchez R., Chen L., Sung Z.R. EMBRYONIC FLOWER1 participates in polycomb group-mediated AG gene silencing in Arabidopsis. Plant Cell. 2008;20:277–291. doi: 10.1105/tpc.106.049957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zeng X., Gao Z., Jiang C., Yang Y., Liu R., He Y. HISTONE DEACETYLASE 9 Functions with Polycomb Silencing to Repress FLOWERING LOCUS C Expression. Plant Physiol. 2020;182:555–565. doi: 10.1104/pp.19.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun B., Zhou Y., Cai J., Shang E., Yamaguchi N., Xiao J., Looi L.S., Wee W.Y., Gao X., Wagner D., et al. Integration of Transcriptional Repression and Polycomb-Mediated Silencing of WUSCHEL in Floral Meristems. Plant Cell. 2019;31:1488–1505. doi: 10.1105/tpc.18.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Z., Luo X., Ou Y., Jiao H., Peng L., Fu X., Macho A.P., Liu R., He Y. JASMONATE-ZIM DOMAIN proteins engage Polycomb chromatin modifiers to modulate Jasmonate signaling in Arabidopsis. Mol. Plant. 2021 doi: 10.1016/j.molp.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 152.Wang J., Hu J., Qian Q., Xue H.W. LC2 and OsVIL2 promote rice flowering by photoperoid-induced epigenetic silencing of OsLF. Mol. Plant. 2013;6:514–527. doi: 10.1093/mp/sss096. [DOI] [PubMed] [Google Scholar]
- 153.Wang H., Jiang D., Axelsson E., Lorković Z.J., Montgomery S., Holec S., Pieters B., Al Temimi A.H.K., Mecinović J., Berger F. LHP1 Interacts with ATRX through Plant-Specific Domains at Specific Loci Targeted by PRC2. Mol. Plant. 2018;11:1038–1052. doi: 10.1016/j.molp.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 154.Yang J., Lee S., Hang R., Kim S.R., Lee Y.S., Cao X., Amasino R., An G. OsVIL2 functions with PRC2 to induce flowering by repressing OsLFL1 in rice. Plant J. 2013;73:566–578. doi: 10.1111/tpj.12057. [DOI] [PubMed] [Google Scholar]
- 155.Yoon H., Yang J., Liang W., Zhang D., An G. OsVIL2 Regulates Spikelet Development by Controlling Regulatory Genes in Oryza sativa. Front. Plant Sci. 2018;9:102. doi: 10.3389/fpls.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roy S., Gupta P., Rajabhoj M.P., Maruthachalam R., Nandi A.K. The Polycomb-Group Repressor MEDEA Attenuates Pathogen Defense. Plant Physiol. 2018;177:1728–1742. doi: 10.1104/pp.17.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hohenstatt M.L., Mikulski P., Komarynets O., Klose C., Kycia I., Jeltsch A., Farrona S., Schubert D. PWWP-DOMAIN INTERACTOR OF POLYCOMBS1 Interacts with Polycomb-Group Proteins and Histones and Regulates Arabidopsis Flowering and Development. Plant Cell. 2018;30:117–133. doi: 10.1105/tpc.17.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu C., Xi W., Shen L., Tan C., Yu H. Regulation of floral patterning by flowering time genes. Dev. Cell. 2009;16:711–722. doi: 10.1016/j.devcel.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 159.Jullien P.E., Mosquna A., Ingouff M., Sakata T., Ohad N., Berger F. Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol. 2008;6:e194. doi: 10.1371/journal.pbio.0060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Duan Y., Chen Y., Li W., Pan M., Qu X., Shi X., Cai Z., Liu H., Zhao F., Kong L., et al. RETINOBLASTOMA-RELATED Genes Specifically Control Inner Floral Organ Morphogenesis and Pollen Development in Rice. Plant Physiol. 2019;181:1600–1614. doi: 10.1104/pp.19.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang P., Zhu C., Geng Y., Wang Y., Yang Y., Liu Q., Guo W., Chachar S., Riaz A., Yan S., et al. Rice and Arabidopsis homologs of yeast CHROMOSOME TRANSMISSION FIDELITY PROTEIN 4 commonly interact with Polycomb complexes but exert divergent regulatory functions. Plant Cell. 2021 doi: 10.1093/plcell/koab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lindner M., Simonini S., Kooiker M., Gagliardini V., Somssich M., Hohenstatt M., Simon R., Grossniklaus U., Kater M.M. TAF13 interacts with PRC2 members and is essential for Arabidopsis seed development. Dev. Biol. 2013;379:28–37. doi: 10.1016/j.ydbio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 163.Li Z., Li B., Liu J., Guo Z., Liu Y., Li Y., Shen W.H., Huang Y., Huang H., Zhang Y., et al. Transcription factors AS1 and AS2 interact with LHP1 to repress KNOX genes in Arabidopsis. J. Integr. Plant Biol. 2016;58:959–970. doi: 10.1111/jipb.12485. [DOI] [PubMed] [Google Scholar]
- 164.Berger N., Dubreucq B., Roudier F., Dubos C., Lepiniec L. Transcriptional regulation of Arabidopsis LEAFY COTYLEDON2 involves RLE, a cis-element that regulates trimethylation of histone H3 at lysine-27. Plant Cell. 2011;23:4065–4078. doi: 10.1105/tpc.111.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu K., Wang S., Song W., Zhang J., Wang Y., Liu Q., Yu J., Ye Y., Li S., Chen J., et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science. 2020;367 doi: 10.1126/science.aaz2046. [DOI] [PubMed] [Google Scholar]
- 166.Zhu Y., Luo X., Liu X., Wu W., Cui X., He Y., Huang J. Arabidopsis PEAPODs function with LIKE HETEROCHROMATIN PROTEIN1 to regulate lateral organ growth. J. Integr. Plant Biol. 2020;62:812–831. doi: 10.1111/jipb.12841. [DOI] [PubMed] [Google Scholar]
- 167.Wang Y.Y., Jiang H., Wang G.F. PHERES1 Controls Endosperm Gene Imprinting and Seed Development. Trends Plant Sci. 2020;25:517–519. doi: 10.1016/j.tplants.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 168.Kim D.H., Xi Y., Sung S. Modular function of long noncoding RNA, COLDAIR, in the vernalization response. PLoS Genet. 2017;13:e1006939. doi: 10.1371/journal.pgen.1006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu H.W., Deng S., Xu H., Mao H.Z., Liu J., Niu Q.W., Wang H., Chua N.H. A noncoding RNA transcribed from the AGAMOUS (AG) second intron binds to CURLY LEAF and represses AG expression in leaves. New Phytol. 2018;219:1480–1491. doi: 10.1111/nph.15231. [DOI] [PubMed] [Google Scholar]
- 170.Ariel F., Jegu T., Latrasse D., Romero-Barrios N., Christ A., Benhamed M., Crespi M. Noncoding transcription by alternative RNA polymerases dynamically regulates an auxin-driven chromatin loop. Mol. Cell. 2014;55:383–396. doi: 10.1016/j.molcel.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 171.Tian Y., Zheng H., Zhang F., Wang S., Ji X., Xu C., He Y., Ding Y. PRC2 recruitment and H3K27me3 deposition at FLC require FCA binding of COOLAIR. Sci. Adv. 2019;5:eaau7246. doi: 10.1126/sciadv.aau7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Heo J.B., Sung S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science. 2011;331:76–79. doi: 10.1126/science.1197349. [DOI] [PubMed] [Google Scholar]
- 173.Kim D.H., Sung S. Vernalization-Triggered Intragenic Chromatin Loop Formation by Long Noncoding RNAs. Dev. Cell. 2017;40:302–312.e304. doi: 10.1016/j.devcel.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Molitor A.M., Latrasse D., Zytnicki M., Andrey P., Houba-Hérin N., Hachet M., Battail C., Del Prete S., Alberti A., Quesneville H., et al. The Arabidopsis hnRNP-Q Protein LIF2 and the PRC1 Subunit LHP1 Function in Concert to Regulate the Transcription of Stress-Responsive Genes. Plant Cell. 2016;28:2197–2211. doi: 10.1105/tpc.16.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baulcombe D.C., Dean C. Epigenetic regulation in plant responses to the environment. Cold Spring Harb Perspect Biol. 2014;6:a019471. doi: 10.1101/cshperspect.a019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sani E., Herzyk P., Perrella G., Colot V., Amtmann A. Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biol. 2013;14:R59. doi: 10.1186/gb-2013-14-6-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu N., Fromm M., Avramova Z. H3K27me3 and H3K4me3 chromatin environment at super-induced dehydration stress memory genes of Arabidopsis thaliana. Mol. Plant. 2014;7:502–513. doi: 10.1093/mp/ssu001. [DOI] [PubMed] [Google Scholar]
- 178.Payá-Milans M., Poza-Viejo L., Martín-Uriz P.S., Lara-Astiaso D., Wilkinson M.D., Crevillén P. Genome-wide analysis of the H3K27me3 epigenome and transcriptome in Brassica rapa. Gigascience. 2019;8 doi: 10.1093/gigascience/giz147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shu J., Chen C., Thapa R.K., Bian S., Nguyen V., Yu K., Yuan Z.C., Liu J., Kohalmi S.E., Li C., et al. Genome-wide occupancy of histone H3K27 methyltransferases CURLY LEAF and SWINGER in Arabidopsis seedlings. Plant Direct. 2019;3:e00100. doi: 10.1002/pld3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kleinmanns J.A., Schubert D. Polycomb and Trithorax group protein-mediated control of stress responses in plants. Biol. Chem. 2014;395:1291–1300. doi: 10.1515/hsz-2014-0197. [DOI] [PubMed] [Google Scholar]
- 181.Kleinmanns J.A., Schatlowski N., Heckmann D., Schubert D. BLISTER Regulates Polycomb-Target Genes, Represses Stress-Regulated Genes and Promotes Stress Responses in Arabidopsis thaliana. Front. Plant Sci. 2017;8:1530. doi: 10.3389/fpls.2017.01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ramirez-Prado J.S., Latrasse D., Rodriguez-Granados N.Y., Huang Y., Manza-Mianza D., Brik-Chaouche R., Jaouannet M., Citerne S., Bendahmane A., Hirt H., et al. The Polycomb protein LHP1 regulates Arabidopsis thaliana stress responses through the repression of the MYC2-dependent branch of immunity. Plant J. 2019;100:1118–1131. doi: 10.1111/tpj.14502. [DOI] [PubMed] [Google Scholar]
- 183.Gupta P., Roy S., Nandi A.K. MEDEA-interacting protein LONG-CHAIN BASE KINASE 1 promotes pattern-triggered immunity in Arabidopsis thaliana. Plant Mol. Biol. 2020;103:173–184. doi: 10.1007/s11103-020-00982-4. [DOI] [PubMed] [Google Scholar]
- 184.Liu J., Feng L., Gu X., Deng X., Qiu Q., Li Q., Zhang Y., Wang M., Deng Y., Wang E., et al. An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res. 2019;29:379–390. doi: 10.1038/s41422-019-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nishio H., Buzas D.M., Nagano A.J., Suzuki Y., Sugano S., Ito M., Morinaga S., Kudoh H. From the laboratory to the field: Assaying histone methylation at FLOWERING LOCUS C in naturally growing Arabidopsis halleri. Genes Genet. Syst. 2016;91:15–26. doi: 10.1266/ggs.15-00071. [DOI] [PubMed] [Google Scholar]
- 186.Nishio H., Buzas D.M., Nagano A.J., Iwayama K., Ushio M., Kudoh H. Repressive chromatin modification underpins the long-term expression trend of a perennial flowering gene in nature. Nat. Commun. 2020;11:2065. doi: 10.1038/s41467-020-15896-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nishio H., Nagano A.J., Ito T., Suzuki Y., Kudoh H. Seasonal plasticity and diel stability of H3K27me3 in natural fluctuating environments. Nat. Plants. 2020;6:1091–1097. doi: 10.1038/s41477-020-00757-1. [DOI] [PubMed] [Google Scholar]
- 188.Berry S., Dean C. Environmental perception and epigenetic memory: Mechanistic insight through FLC. Plant J. 2015;83:133–148. doi: 10.1111/tpj.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Menon G., Schulten A., Dean C., Howard M. Digital paradigm for Polycomb epigenetic switching and memory. Curr. Opin. Plant Biol. 2021;61:102012. doi: 10.1016/j.pbi.2021.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Whittaker C., Dean C. The FLC Locus: A Platform for Discoveries in Epigenetics and Adaptation. Annu. Rev. Cell Dev. Biol. 2017;33:555–575. doi: 10.1146/annurev-cellbio-100616-060546. [DOI] [PubMed] [Google Scholar]
- 191.Costa S., Dean C. Storing memories: The distinct phases of Polycomb-mediated silencing of Arabidopsis FLC. Biochem. Soc. Trans. 2019;47:1187–1196. doi: 10.1042/BST20190255. [DOI] [PubMed] [Google Scholar]
- 192.Zeng Z., Zhang W., Marand A.P., Zhu B., Buell C.R., Jiang J. Cold stress induces enhanced chromatin accessibility and bivalent histone modifications H3K4me3 and H3K27me3 of active genes in potato. Genome Biol. 2019;20:123. doi: 10.1186/s13059-019-1731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Carter B., Bishop B., Ho K.K., Huang R., Jia W., Zhang H., Pascuzzi P.E., Deal R.B., Ogas J. The Chromatin Remodelers PKL and PIE1 Act in an Epigenetic Pathway That Determines H3K27me3 Homeostasis in Arabidopsis. Plant Cell. 2018;30:1337–1352. doi: 10.1105/tpc.17.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang R., Hong Y., Ren Z., Tang K., Zhang H., Zhu J.-K., Zhao C. A Role for PICKLE in the Regulation of Cold and Salt Stress Tolerance in Arabidopsis. Front. Plant Sci. 2019;10:900. doi: 10.3389/fpls.2019.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kwon C.S., Lee D., Choi G., Chung W.I. Histone occupancy-dependent and -independent removal of H3K27 trimethylation at cold-responsive genes in Arabidopsis. Plant J. 2009;60:112–121. doi: 10.1111/j.1365-313X.2009.03938.x. [DOI] [PubMed] [Google Scholar]
- 196.Bouché F., Detry N., Périlleux C. Heat can erase epigenetic marks of vernalization in Arabidopsis. Plant Signal. Behav. 2015;10:e990799. doi: 10.4161/15592324.2014.990799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Huh J.H., Bauer M.J., Hsieh T.-F., Fischer R. Endosperm gene imprinting and seed development. Curr. Opin. Genet. Dev. 2007;17:480–485. doi: 10.1016/j.gde.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Folsom J.J., Begcy K., Hao X., Wang D., Walia H. Rice fertilization-Independent Endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant Physiol. 2014;165:238–248. doi: 10.1104/pp.113.232413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dhatt B.K., Paul P., Sandhu J., Hussain W., Irvin L., Zhu F., Adviento-Borbe M.A., Lorence A., Staswick P., Yu H., et al. Allelic variation in rice Fertilization Independent Endosperm 1 contributes to grain width under high night temperature stress. New Phytol. 2021;229:335–350. doi: 10.1111/nph.16897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Y.-Y., Cheng Y.-H., Chen K.-E., Tsay Y.-F. Nitrate Transport, Signaling, and Use Efficiency. Annu. Rev. Plant Biol. 2018;69:85–122. doi: 10.1146/annurev-arplant-042817-040056. [DOI] [PubMed] [Google Scholar]
- 201.Widiez T., El Kafafi E.S., Girin T., Berr A., Ruffel S., Krouk G., Vayssières A., Shen W.-H., Coruzzi G.M., Gojon A., et al. High nitrogen insensitive 9 (HNI9)-mediated systemic repression of root NO3- uptake is associated with changes in histone methylation. Proc. Natl. Acad. Sci. USA. 2011;108:13329–13334. doi: 10.1073/pnas.1017863108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bellegarde F., Herbert L., Séré D., Caillieux E., Boucherez J., Fizames C., Roudier F., Gojon A., Martin A. Polycomb Repressive Complex 2 attenuates the very high expression of the Arabidopsis gene NRT2.1. Sci. Rep. 2018;8:7905. doi: 10.1038/s41598-018-26349-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Park E.Y., Tsuyuki K.M., Hu F., Lee J., Jeong J. PRC2-Mediated H3K27me3 Contributes to Transcriptional Regulation of FIT-Dependent Iron Deficiency Response. Front. Plant Sci. 2019;10:627. doi: 10.3389/fpls.2019.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Park E.Y., Tsuyuki K.M., Parsons E.M., Jeong J. PRC2-mediated H3K27me3 modulates shoot iron homeostasis in Arabidopsis thaliana. Plant Signal. Behav. 2020;15:1784549. doi: 10.1080/15592324.2020.1784549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Widiez T., Symeonidi A., Luo C., Lam E., Lawton M., Rensing S.A. The chromatin landscape of the moss Physcomitrella patens and its dynamics during development and drought stress. Plant J. 2014;79:67–81. doi: 10.1111/tpj.12542. [DOI] [PubMed] [Google Scholar]
- 206.Liu N., Ding Y., Fromm M., Avramova Z. Different gene-specific mechanisms determine the ’revised-response’ memory transcription patterns of a subset of A. thaliana dehydration stress responding genes. Nucleic Acids Res. 2014;42:5556–5566. doi: 10.1093/nar/gku220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.González R.M., Ricardi M.M., Iusem N.D. Epigenetic marks in an adaptive water stress-responsive gene in tomato roots under normal and drought conditions. Epigenetics. 2013;8:864–872. doi: 10.4161/epi.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Alexandre C., Möller-Steinbach Y., Schönrock N., Gruissem W., Hennig L. Arabidopsis MSI1 is required for negative regulation of the response to drought stress. Mol. Plant. 2009;2:675–687. doi: 10.1093/mp/ssp012. [DOI] [PubMed] [Google Scholar]
- 209.Han B., Xu W., Ahmed N., Yu A., Wang Z., Liu A. Changes and Associations of Genomic Transcription and Histone Methylation with Salt Stress in Castor Bean. Plant Cell Physiol. 2020;61:1120–1133. doi: 10.1093/pcp/pcaa037. [DOI] [PubMed] [Google Scholar]
- 210.Sun L., Song G., Guo W., Wang W., Zhao H., Gao T., Lv Q., Yang X., Xu F., Dong Y., et al. Dynamic Changes in Genome-Wide Histone3 Lysine27 Trimethylation and Gene Expression of Soybean Roots in Response to Salt Stress. Front. Plant Sci. 2019;10:1031. doi: 10.3389/fpls.2019.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zheng D., Wang L., Chen L., Pan X., Lin K., Fang Y., Wang X.E., Zhang W. Salt-Responsive Genes are Differentially Regulated at the Chromatin Levels Between Seedlings and Roots in Rice. Plant Cell Physiol. 2019;60:1790–1803. doi: 10.1093/pcp/pcz095. [DOI] [PubMed] [Google Scholar]
- 212.Pu L., Liu M.S., Kim S.Y., Chen L.F., Fletcher J.C., Sung Z.R. EMBRYONIC FLOWER1 and ULTRAPETALA1 Act Antagonistically on Arabidopsis Development and Stress Response. Plant Physiol. 2013;162:812–830. doi: 10.1104/pp.112.213223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mehdi S., Derkacheva M., Ramström M., Kralemann L., Bergquist J., Hennig L. The WD40 Domain Protein MSI1 Functions in a Histone Deacetylase Complex to Fine-Tune Abscisic Acid Signaling. Plant Cell. 2016;28:42–54. doi: 10.1105/tpc.15.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhu J.K. Abiotic Stress Signaling and Responses in Plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen H., Tong J., Fu W., Liang Z., Ruan J., Yu Y., Song X., Yuan L., Xiao L., Liu J., et al. The H3K27me3 Demethylase RELATIVE OF EARLY FLOWERING6 Suppresses Seed Dormancy by Inducing Abscisic Acid Catabolism. Plant Physiol. 2020;184:1969–1978. doi: 10.1104/pp.20.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu J., Ichihashi Y., Suzuki T., Shibata A., Shirasu K., Yamaguchi N., Ito T. Abscisic acid-dependent histone demethylation during postgermination growth arrest in Arabidopsis. Plant Cell Environ. 2019;42:2198–2214. doi: 10.1111/pce.13547. [DOI] [PubMed] [Google Scholar]
- 217.Liu C., Cheng J., Zhuang Y., Ye L., Li Z., Wang Y., Qi M., Xu L., Zhang Y. Polycomb repressive complex 2 attenuates ABA-induced senescence in Arabidopsis. Plant J. 2019;97:368–377. doi: 10.1111/tpj.14125. [DOI] [PubMed] [Google Scholar]
- 218.Zhu Y., Hu X., Duan Y., Li S., Wang Y., Rehman A.U., He J., Zhang J., Hua D., Yang L., et al. The Arabidopsis Nodulin Homeobox Factor AtNDX Interacts with AtRING1A/B and Negatively Regulates Abscisic Acid Signaling. Plant Cell. 2020;32:703–721. doi: 10.1105/tpc.19.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Meller B., Kuźnicki D., Arasimowicz-Jelonek M., Deckert J., Floryszak-Wieczorek J. BABA-Primed Histone Modifications in Potato for Intergenerational Resistance to Phytophthora infestans. Front. Plant Sci. 2018;9:1228. doi: 10.3389/fpls.2018.01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Crespo-Salvador Ó., Escamilla-Aguilar M., López-Cruz J., López-Rodas G., González-Bosch C. Determination of histone epigenetic marks in Arabidopsis and tomato genes in the early response to Botrytis cinerea. Plant Cell Rep. 2018;37:153–166. doi: 10.1007/s00299-017-2218-9. [DOI] [PubMed] [Google Scholar]
- 221.Yuan M., Ngou B.P.M., Ding P., Xin X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021;62:102030. doi: 10.1016/j.pbi.2021.102030. [DOI] [PubMed] [Google Scholar]
- 222.Saripalli G., Singh K., Gautam T., Kumar S., Raghuvanshi S., Prasad P., Jain N., Sharma P.K., Balyan H.S., Gupta P.K. Genome-wide analysis of H3K4me3 and H3K27me3 modifications due to Lr28 for leaf rust resistance in bread wheat (Triticum aestivum) Plant Mol. Biol. 2020;104:113–136. doi: 10.1007/s11103-020-01029-4. [DOI] [PubMed] [Google Scholar]
- 223.Atighi M.R., Verstraeten B., De Meyer T., Kyndt T. Genome-wide shifts in histone modifications at early stage of rice infection with Meloidogyne graminicola. Mol. Plant Pathol. 2021;22:440–455. doi: 10.1111/mpp.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dvořák Tomaštíková E., Hafrén A., Trejo-Arellano M.S., Rasmussen S.R., Sato H., Santos-González J., Köhler C., Hennig L., Hofius D. Polycomb Repressive Complex 2 and KRYPTONITE regulate pathogen-induced programmed cell death in Arabidopsis. Plant Physiol. 2021 doi: 10.1093/plphys/kiab035. [DOI] [PMC free article] [PubMed] [Google Scholar]