Abstract
Aims/hypothesis
Homo Sapiens evolved under conditions of intermittent food availability and prolonged fasting between meals. Periods of fasting are important for recovery from meal-induced oxidative and metabolic stress, and tissue repair. Constant high energy-density food availability in present-day society contributes to the pathogenesis of chronic diseases, including diabetes and its complications, with intermittent fasting (IF) and energy restriction shown to improve metabolic health. We have previously demonstrated that IF prevents the development of diabetic retinopathy in a mouse model of type 2 diabetes (db/db); however the mechanisms of fasting-induced health benefits and fasting-induced risks for individuals with diabetes remain largely unknown. Sirtuin 1 (SIRT1), a nutrient-sensing deacetylase, is downregulated in diabetes. In this study, the effect of SIRT1 stimulation by IF, fasting mimicking cell culture conditions (FMC) or pharmacological treatment using SRT1720 was evaluated on systemic and retinal metabolism, systemic and retinal inflammation and vascular and bone marrow damage.
Methods
The effects of IF were modelled in vivo using db/db mice and in vitro using bovine retinal endothelial cells or rat retinal neuroglial/precursor R28 cell line serum starved for 24 h. mRNA expression was analysed by quantitative PCR (qPCR). SIRT1 activity was measured via histone deacetylase activity assay. NR1H3 (also known as Liver X receptor alpha (LXRα)) acetylation was measured via western blot analysis.
Results
IF increased Sirt1 mRNA expression in mouse liver and retina when compared with non-fasted animals. IF also increased SIRT1 activity eightfold in mouse retina while FMC increased SIRT1 activity and expression in retinal endothelial cells when compared with control. Sirt1 expression was also increased twofold in neuronal retina progenitor cells (R28) after FMC treatment. Moreover, FMC led to SIRT1-mediated LXRα deacetylation and subsequent 2.4-fold increase in activity, as measured by increased mRNA expression of the genes encoding ATP-binding cassette transporter (Abca1 and Abcg1). These changes were reduced when retinal endothelial cells expressing a constitutively acetylated LXRα mutant were tested. Increased SIRT1/LXR/ABC-mediated cholesterol export resulted in decreased retinal endothelial cell cholesterol levels. Direct activation of SIRT1 by SRT1720 in db/db mice led to a twofold reduction of diabetes-induced inflammation in the retina and improved diabetes-induced visual function impairment, as measured by electroretinogram and optokinetic response. In the bone marrow, there was prevention of diabetes-induced myeloidosis and decreased inflammatory cytokine expression.
Conclusions/interpretation
Taken together, activation of SIRT1 signalling by IF or through pharmacological activation represents an effective therapeutic strategy that provides a mechanistic link between the advantageous effects associated with fasting regimens and prevention of microvascular and bone marrow dysfunction in diabetes.
Keywords: ABCA1, ABCG1, Cholesterol, Deacetylation, Diabetic retinopathy, Endothelial cell, Intermittent fasting, LXR, RPE, SIRT1
Graphical Abstract
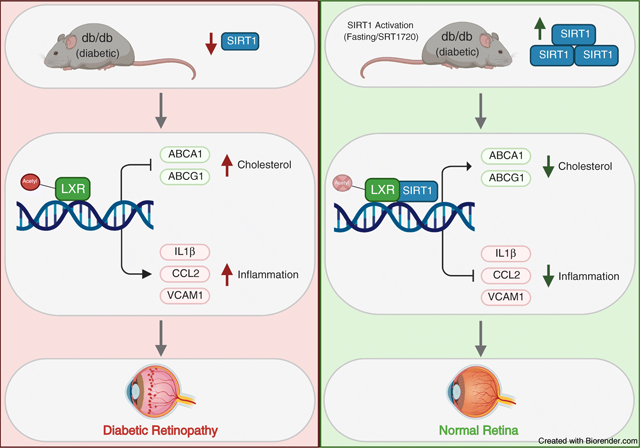

Introduction
In diabetes, the intricate balance between nutrient availability and utilisation is disturbed, leading to increased blood glucose levels, and dyslipidaemia characterised by increased plasma triacylglycerol and LDL-cholesterol levels and decreased HDL-cholesterol levels. High systemic nutrient availability is further exacerbated by the inability of tissues to access nutrients [1]. These changes result in macronutrient accumulation, proinflammatory and pro-apoptotic changes, and consequently vascular damage.
SIRT1, a member of the sirtuin family of NAD-dependent protein deacetylases, is a key metabolic regulator activated under low-nutrient states. Owing to the importance of SIRT1-mediated signalling in metabolism, deregulation of SIRT1 deacetylase activity results in chronic metabolic abnormalities. In diabetes, SIRT1 expression and activity are significantly decreased [2, 3], and activation of SIRT1 promotes insulin secretion, reduces insulin resistance and decreases body weight [4, 5]. Animal and in vitro studies have demonstrated the importance of SIRT1 regulation of inflammation [6, 7]. In the diabetic retina, increased DNA methylation of the SIRT1 promoter lead to transcriptional repression. Activation of SIRT1 is protective against diabetes-induced vascular and mitochondrial damage by inhibiting NF-κB [8], poly [ADP-ribose] polymerase (PARP-1) [9] and matrix-metallopeptidase 9 (MMP-9) [2] activation and decreasing histone acetylation of the DNA (cytosine-5)-methyltransferase 1 (DNMT1) promoter [10]. Importantly, diabetes-induced decreases in SIRT1 signalling result in dysregulated cholesterol metabolism and increased production of proinflammatory cytokines via decreased liver X receptor (LXR) signalling [11]. SIRT1 is critical to vascular health [12, 13]. SIRT1 levels fall with the development of macrovascular disease while maintaining SIRT1 levels prevents macrovascular damage [14]. Similarly, decreased SIRT1 levels have been implicated in the pathogenesis of microvascular complications [2].
Diabetic retinopathy is the most common microvascular complication [15], where high metabolic demands of the retina dictate complex regulatory pathways needed to meet retinal energy needs while preserving the delicate balance between nutrient availability and tightly regulated retinal lipid metabolism [16]. Normally, the retina maintains its cholesterol homeostasis by a tight control of cholesterol input/output [17]. Under normal conditions, cholesterol input in the retina includes local biosynthesis [18] [19] and uptake of the lipoprotein particles from the circulation [20–22] on the basal membrane of retinal pigment epithelium (RPE) cells.
Intermittent fasting (IF) was recently shown to have multiple health benefits and is now pursued as a metabolic strategy for management of obesity and other metabolic diseases, including diabetes. IF is not without considerable risks for individuals with diabetes [23–25]. There is insufficient clinical data regarding the safety of IF for diabetic patients; therefore having a pharmacological regimen that mimics fasting may have major clinical relevance. Fasting regimens and SIRT1 activation have been shown to increase longevity and delay onset of disease in yeast, fruit flies, mice and more recently, non-human primate animal models [26]. Fasting-induced SIRT1 activation has been linked to increased NAD+ levels, increased mitochondrial biogenesis and delayed senescence [27, 28]. The current study addresses the use of fasting and fasting mimicking treatments to activate SIRT1 signalling.
Methods
Animal studies
All animal procedures were in compliance with the NIH Guide for the care and use of laboratory animals, and with the Association for Research in Vision and Ophthalmology Statement for the use of animals in ophthalmic and vision research (IACUC #10917). For db/db mouse (strain B6.BKS(D)-Leprdb/J [stock #000697] purchased from Jackson Laboratory, USA) studies, male mice were treated with 100mg/kg body weight/day SRT1720 in their chow for 6 months after diabetes onset around 10 weeks of age [29]. Mice were considered diabetic after two consecutive daily measurements of blood glucose levels of 13.9 mmol/l or higher. db/db and db/m mice without treatment were analysed as control animals.
Cell culture and treatment
Bovine retinal endothelial cells (BRECs) were isolated from mycoplasma-free bovine tissue and validated according to a previously published protocol [11, 30]. BREC identity was authenticated morphologically and using anti-von Willebrand factor immunocytochemistry (Abcam; UK; ab6994, RRID:AB305689) and LDL uptake assay (Abcam; ab133127) [31]. All antibodies used in this study were diluted based on the manufactures guidelines. BRECs were cultured in 10% FBS BREC complete media with 1% antibiotic/antimycotic (AA) (ThermoFisher; USA; 15240062) [32, 33]. MCDB131 media was used to make BREC complete media (Sigma; USA; m8537) using mycoplasma-free reagents. Passages 4–8 were used for all experiments. In order to model a fasting environment in vitro, BRECs were cultured in 0% FBS for 24h (fasting mimicking cell culture conditions [FMC]). FMC media contained endothelial growth factors and 5 mmol/l glucose [32]. Base media only contained MCDB131 media (m8537). Cells were treated with 1 μg/ml N,N-dimethyl-3β-hydroxy-cholenamide (DMHCA; Avanti Polar Lipids; USA; 700125), 1μg/ml SRT1720 (Selleckchem; USA; S1129) or 10ng/ml diabetic relevant stimuli TNF-α (R&D Systems; USA; 210-TA020), for 24h. Cells were transfected using either Sirt1 siRNA or scrambled Sirt1 siRNA as control (Invitrogen; USA; 81801753) for 24h with the Human Coronary Artery Endothelial Cell Nucleofactor kit (Lonza; Switzerland; VPB-1001, S005). CBA-hLXR-alpha-q43 AAV2 vectors were custom made in the Department of Ophthalmology at the University of Florida, USA; referred to as Q432 throughout. The heterogeneous adherent rat retinal neuroglial/precursor R28 cell line was acquired from G. M. Seigel at the University at Buffalo, USA. R28 cells were cultured in DMEM+ media with 10% calf serum. Cells were treated with FMC for 24h. Cells used for all cell culture experiments were selected at random from different isolations. Experimental analysis was not conducted in a blinded matter.
Cell death assays
BRECs were cultured in FMC or basal media for 24–96h. Basal media was comprised of only MCB131 media without the addition of growth factors or supplements (m8537). This media was used as a positive control for cell death. Cell death was analysed via Trypan Blue exclusion assay [34] and annexin V (Abcam; ab14085). Cell death was calculated by dividing the number of dead cells by the total number of cells or by measuring intensity density of annexin V + cells, for Trypan Blue or annexin V assays, respectively. Apoptosis was thermally induced (50°C for 10min) as a positive control.
qPCR
RNA was isolated using RNeasy mini kit (Qiagen; USA; 74106) according to the manufacturer’s instructions. Gene-specific forward and reverse primers (Integrated DNA Technologies; USA) are shown in ESM Table 1 and were subjected to real-time quantitative PCR (qPCR) quantification using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems; USA). Inflammatory primers were chosen based on relevance to cell type. For whole retina, the macrophage and microglia contribution to the inflammatory phenotype makes IL-1β and CCL2 the relevant markers to investigate [35]. For endothelial cells, contribution of IL-1β and C-C motif chemokine ligand 2 (CCL2) to inflammation is minimal when compared with vascular cell adhesion protein 1 (VCAM1) [36, 37]. All reactions were performed in duplicate from at least three independent biological samples. Cyclophilin A was used as a control, and results were analysed using the comparative Ct method and Ct values were normalised to cyclophilin A levels. Data are shown as normalised relative to control levels or as non-normalised raw expression levels.
Western blot and immunoprecipitation
BRECs were cultured and treated as above. Lysates were processed as previously described [11]. To determine active LXRα levels, two identical aliquots of the cell lysate samples corresponding to 50µg of total protein were used for western blot detection using LXRα (Abcam; ab3585, RRID:AB_303930), and β-actin antibodies (Cell Signalling Technology; USA; Cat# 4970, RRID:AB_2223172); and for acetylation studies using LXRα antibody for immunoprecipitation (LSBio, USA; LS-C374316), and acetylated lysine #9441 (Cell Signalling Technology; RRID:AB_331805) antibody for immunoblotting. Complete immunoprecipitation was verified by the absence of LXR signal in the post-immunoprecipitation sample. Active LXR was calculated by subtracting acetylated LXR signal from total LXR signal in each sample. Total acetylated lysine was assayed using the immunoprecipitation kit Dynabeads Protein A (Invitrogen; 10006D). Blocking buffer for fluorescent western blotting MB-070 (Rockland; USA) was used for dilution of the antibodies. Dilution for secondary antibody was 1:1000. Blots were analysed using the LI-COR Odyssey (LICOR Biosciences; USA) imaging and quantification system.
HDAC assay
SIRT1 activity was measured using the histone deacetylase (HDAC) Kit (ABCAM; ab156064) according to the manufacturer’s instructions. Results are shown as fluorescence measurements at 355nm and 460nm excitation and emission, respectively over total protein. Samples were normalised to control.
Mitochondrial respiration measurements
Respiration of BRECs was measured using a microrespirometer as previously described [38]. Briefly, On the day of measurements, the chips were closed, mounted in a microrespirometer, and immediately perfused at a constant 10µl min−1 flow rate. Perfusion was controlled by a Harvard Apparatus syringe infusion pump. Cells were first perfused with 10% FBS media for 12 h, followed by perfusion with serum-free media for an additional 24 h. Respiratory activity was measured for 15 min in stationary medium, with a minimum of 15 min of re-perfusion between measurements. Total oxygen concentration was maintained above 200 µmol/l in all cases. Measurements in the presence of potassium cyanide (KCN,5 µmol l−1) were used to correct for non-mitochondrial oxygen consumption. Respiratory activity was calculated by linear fitting of the initial 5 min of steady oxygen consumption. The instrument was calibrated using air-equilibrated media as an aerobic standard and 1 mmol l−1 sodium dithionite in media as anaerobic standard.
Cholesterol measurement
In vivo
For lipid extraction, lipids from mouse retinas were extracted with methanol, chloroform, and water as previously described [39, 40].
For the analysis of free and total sterol content, sterols were analysed by high resolution/accurate mass LC-MS/MS using a Thermo Scientific LTQ-Orbitrap Velos LC-MS system as previously described [41]. Cholesterol identification was performed by comparison of retention time, exact mass, and MS/MS profile to an authentic standard purchased from Steraloids.
In vitro
Total cholesterol levels were measured using the Amplex Red Cholesterol Assay kit (Thermo Scientific; A12216) according to the manufacturer’s instructions. Briefly, cells were diluted in 1× reaction buffer and incubated with a working solution containing 300 µmol/l Amplex Red reagent. Fluorescence was measured at 590 nmol/l after 45 min at 37°C.
Immunohistochemical staining
Retinas were processed as previously described [42] using the following antibodies: primary monoclonal antibody for CD45 (R&D Systems; MAB114, RRID:AB_357485) with secondary Alexa Fluor 594 (A11007, Invitrogen, USA); primary ionised calcium binding adaptor molecule 1 (Iba-1; Fujifilm Cellular Dynamics; USA; 019–19741, RRID:AB_839504) with secondary Alexa Fluor 488 (A27023, Invitrogen USA) and NeuN (Millipore Sigma, USA; clone A60, Alexa Fluor 488 conjugated MAB377X) at 1:50; 1:500 and 1:100 dilutions, respectively. The antibodies were diluted in PBS with 1% normal goat serum (G9023 Sigma Aldrich, USA). Dilution for the secondary antibodies was 1:1000. The images were analysed in blinded manner.
Visual function studies
ERG studies
Electroretinogram (ERG) studies were performed as previously described with assessment of photopic and scotopic responses using UTAS Bigshot (LKC Technologies, USA) [43].
Optomotor response
The visual acuity of the mice was determined by use of optomotor response recordings using OptoMotry 1.7.7 (Cerebral Mechanics, Canada) [44, 45].
Statistical analyses
A Student’s two tailed t test was used for comparisons between two groups. One-way ANOVA, followed by the Tukey post hoc test, was used for multiple comparisons. All values are expressed as mean ± SEM. A value of p<0.05 was considered to be statistically significant. Statistical tests were performed using statistics software (GraphPad Software, USA).
Results
Fasting increases SIRT1 expression and activity
We determined the effect of IF for 48h on SIRT1 expression and activity in control mice. Sirt1 mRNA expression was significantly higher in the liver (Fig. 1a) with a tendency towards higher retinal Sirt1 expression (Fig. 1b) and eightfold increase in activity (Fig. 1c) in fasting animals compared with control non-fasting animals.
Fig. 1.
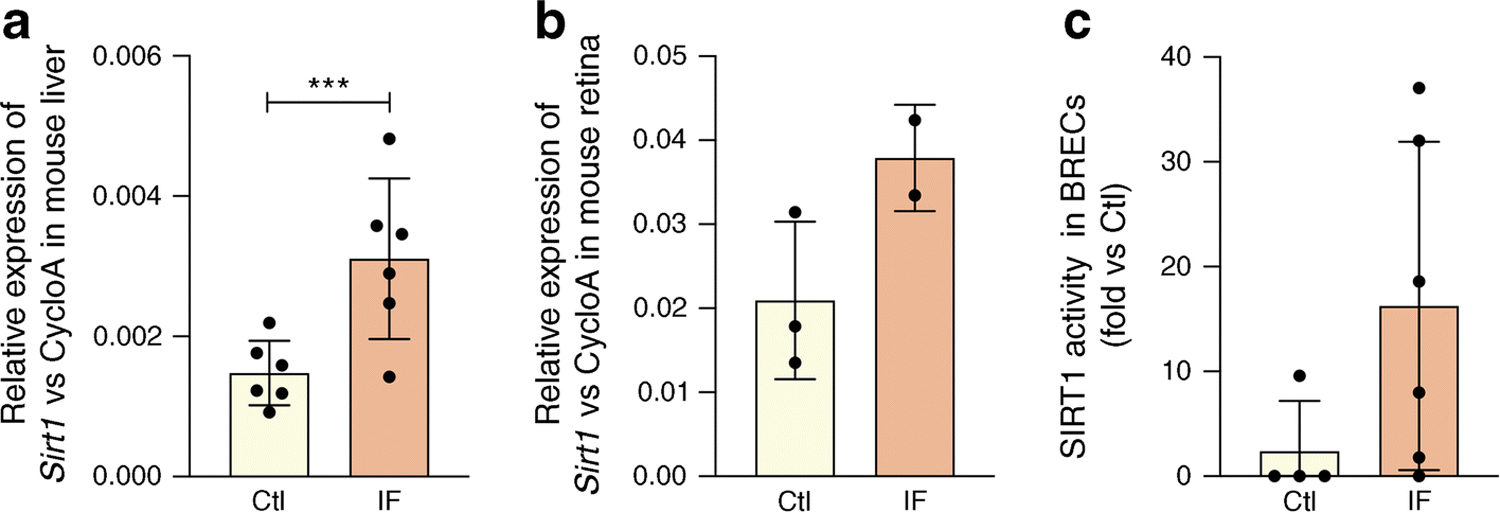
Fasting increases Sirt1 expression and activity. (a) Liver and (b) retinal Sirt1 mRNA expression is increased by IF in mice (48 h fast) when compared with non-fasting controls. (c) IF increases SIRT1 HDAC activity in mouse retinal tissue when compared with non-fasting controls, ***p<0.001. Data are represented as mean ± SEM. Ctl, control; CycloA, cyclophilin A; F355/F460, fluorescence at 355/460 nm
Fasting mimicking conditions activate SIRT1/LXR signalling in retinal vascular cells and neuronal cells
We next performed studies using BRECs and neuronal cell culture (R28) models. FMC (0% FBS) was used to model the low-nutrient, energy-reduced state of IF. FMC was also effective in activating SIRT1 HDAC and expression twofold in BRECs (Fig. 2a, b). Consequently, increased deacetylase activity resulted in 2.4-fold increased LXRα activity, as measured by expression of reverse cholesterol export (RCT) genes, ATP-binding cassette transporter A1 and G1 (Abca1 and Abcg1, respectively) (Fig. 2c). Treatment with FMC also decreased VCAM1 in BRECs (Fig. 2d).
Fig. 2.
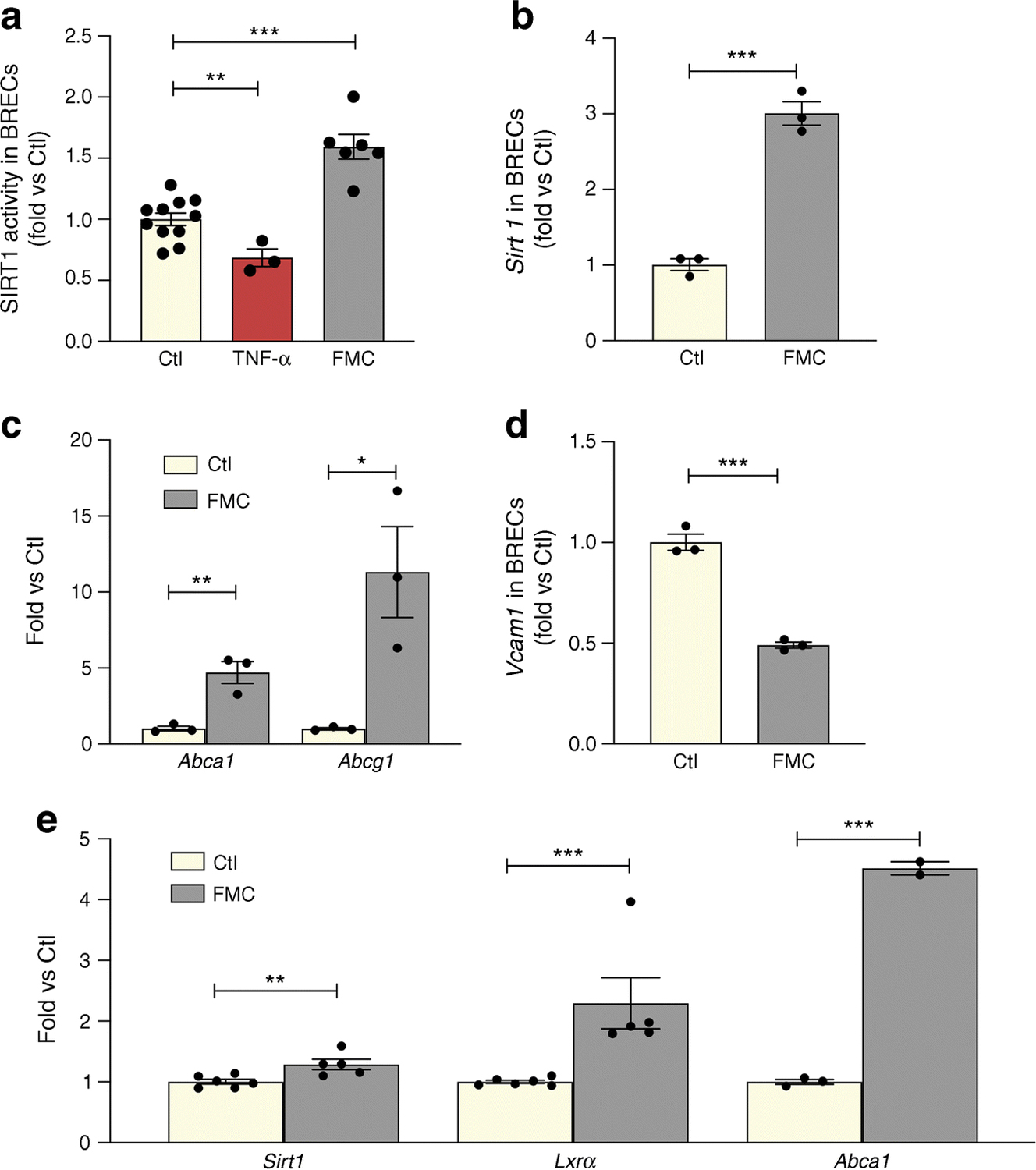
FMC activates the SIRT1/LXR signalling pathway in BRECs and neuronal (R28) cells. FMC (0% FBS, 24 h) in BRECs activates (a) SIRT1 HDAC activity, with TNF-α used as a positive control, (b) Sirt1 mRNA expression, and (c) increased expression of RCT genes (Abca1 and Abcg1). (d) Vcam1 is decreased after treatment with FMC conditions. (e) Treatment of R28 cells with FMC increases expression of Sirt1 and Lxrα mRNA, as well as LXRα activity, as measured by Abca1 mRNA expression *p<0.05, **p<0.01, ***p<0.001. Data are represented as mean ± SEM. Ctl, control
In order to assess the effect of FMC-induced SIRT1 activation in neuronal cells, SIRT1/LXRα activity was measured in R28 cells. Treatment with FMC significantly increased Sirt1 and Lxrα expression and increased LXRα activity, as measured by ABCA1 expression (Fig. 2e).
Active and total LXRα protein levels were significantly increased in BRECs treated with FMC (Fig. 3a–d). To determine if LXRα activation and control of cholesterol metabolism is through the direct deacetylation of LXRα by SIRT1, we used a constitutively acetylated lysine–glutamine K432Q LXRα mutant (Q432). BRECs infected with this mutant had a blunted response to SIRT1 activation by SRT1720 as determined by LXRα expression (Fig. 3e) and LXRα activity measured by Abca1 expression (Fig. 3f).
Fig. 3.
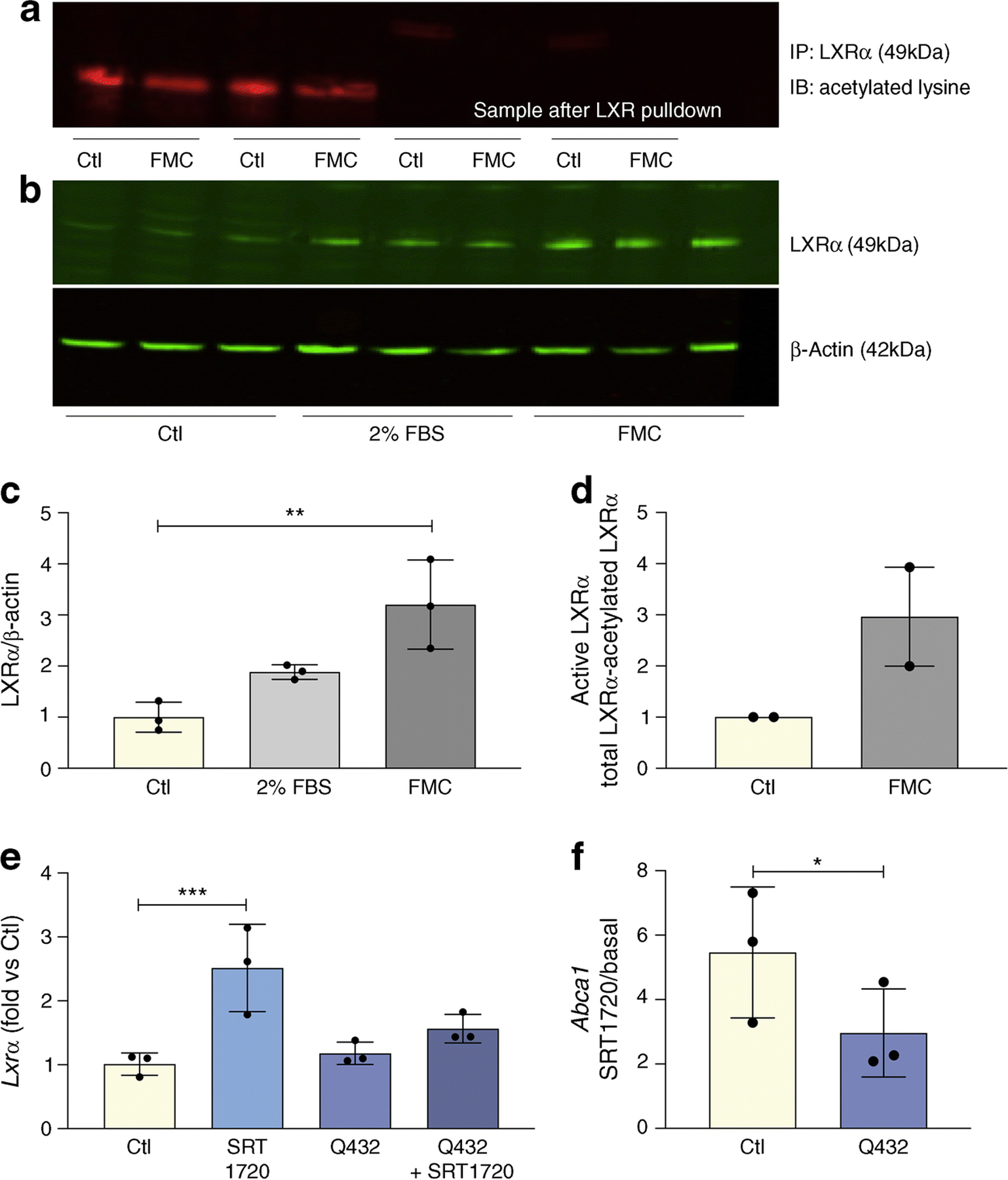
FMC increases LXRα activity via deacetylation. FMC (0% FBS, 24 h) in BRECs (a) decreases levels of non-active, deacetylated LXRα; and (b) increases total LXRα protein levels, quantification shown in (c). The data for active LXR quantified by subtracting acetylated LXRα in (a) from total LXRα in (b) is presented in (d) as mean ± SEM. BRECs were treated with SRT1720 (1 μmol/l) and/or infected with constitutively acetylated LXRα (Q432) for 24 h. Expression of Lxrα (e) or SIRT1-dependent activation of LXRα (f) presented as SRT1720-induced increase in Abca1 expression in BRECs (Ctl) and BRECs infected with Q432. Ratios determined from three independent experiments,*p<0.05, **p<0.01, ***p<0.001. Data are represented as mean ± SEM. Ctl, control; IB, immunoblot; IP, immunoprecipitation
As shown in Fig. 4a, BRECs were treated with TNF-α to recapitulate the diabetic milieu and this resulted in increased cholesterol levels. By contrast, FMC prevented TNF-α-induced cholesterol accumulation in BRECs (Fig. 4a). As expected, activation of LXRα via DMHCA, a steroidal LXR ligand, significantly reduced BREC cholesterol levels. Treatment with FMC, in combination with DMHCA administration, significantly augmented cholesterol export and lowered BREC cholesterol levels even further when compared with DMHCA treatment alone (Fig. 4b).
Fig. 4.
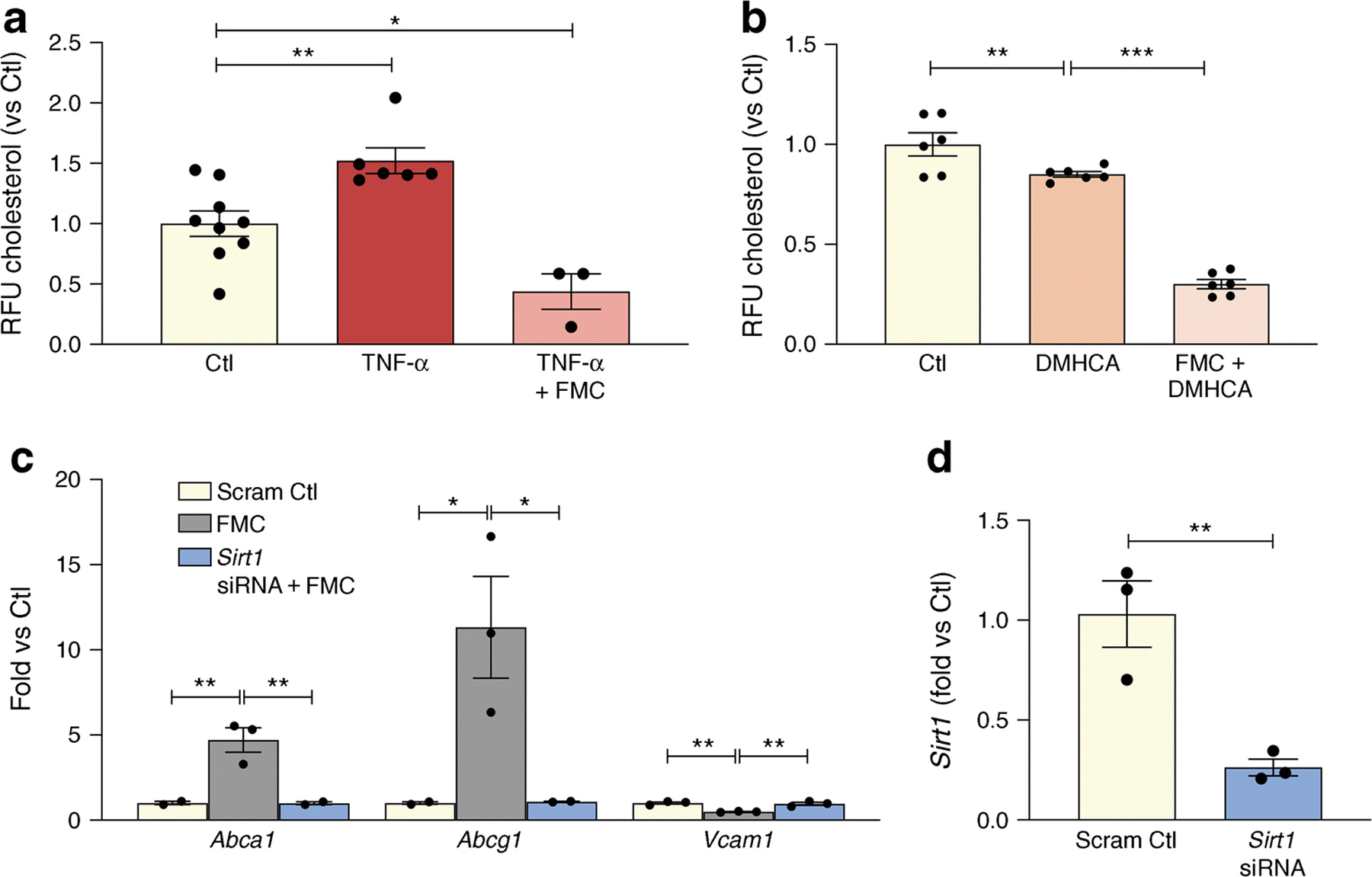
SIRT1 plays an important role in fasting mediated decrease of cholesterol levels in BRECs. (a) TNF-α treatment causes a significant increase in cholesterol levels while FMC (0% FBS, 24 h) prevents this TNF-α -induced increase. (b) This decrease is amplified by administration of the LXRα activator, DMHCA. (c) SIRT1 was reduced by exposure of the BRECs to Sirt1 siRNA. Administration of Sirt1 siRNA prevented serum-induced upregulation of Abca1 and Abcg1 and inhibited the downregulation of the proinflammatory gene, Vcam1. (d) Sirt1 knockdown efficiency. *p<0.05, **p<0.01, ***p<0.001. Data are represented as mean ± SEM. Ctl, control; CycloA, cyclophilin A, Scram Ctl; scrambled Sirt1 siRNA sequence used in 4c and 4d
In addition to augmenting cholesterol export, FMC in BRECs prevented the upregulation of the NF-κB-dependent proinflammatory gene, Vcam1 (Figure 2d). Importantly, data shown in Fig. 4c highlights the importance of SIRT1 signalling in regulating the FMC-induction of RCT and the anti-inflammatory responses in BRECs. Loss of SIRT1, via SIRT1-directed siRNA, prevents FMC-induced upregulation of ABCA1, ABCG1 and downregulation of VCAM1 (Fig. 4c).
Cell death and mitochondrial respiration are not affected by FMC
To ensure the safety of FMC, cell death and mitochondrial respiration were measured after 24h in FMC-treated BRECs. As shown in Fig. 5a, b and c, cell death, measured by Trypan Blue exclusion assay and annexin V, did not significantly differ between FMC-treated cells and BRECs cultured in control conditions (10% FBS-containing medium). The primary goal of these experiments was to access the effect of serum starvation on SIRT1/LXRα, and so the FMC treatment media contains growth factors and 5 mmol/l glucose, and the only component that is missing from this media is FBS. Notably, cells treated with basal media, without growth factors, glucose, or FBS, have increased cell death when compared with control or cells treated with FMC media (ESM Fig. 1). Moreover, there is no statistical difference between mitochondrial respiration in cells cultured in FMC or control conditions: FMC respiratory activity was 0.0555 ± 0.0058 μmol l–1 s–1 compared with 0.0507 ± 0.005 μmol l–1 s–1 in control (Fig. 5d).
Fig. 5.
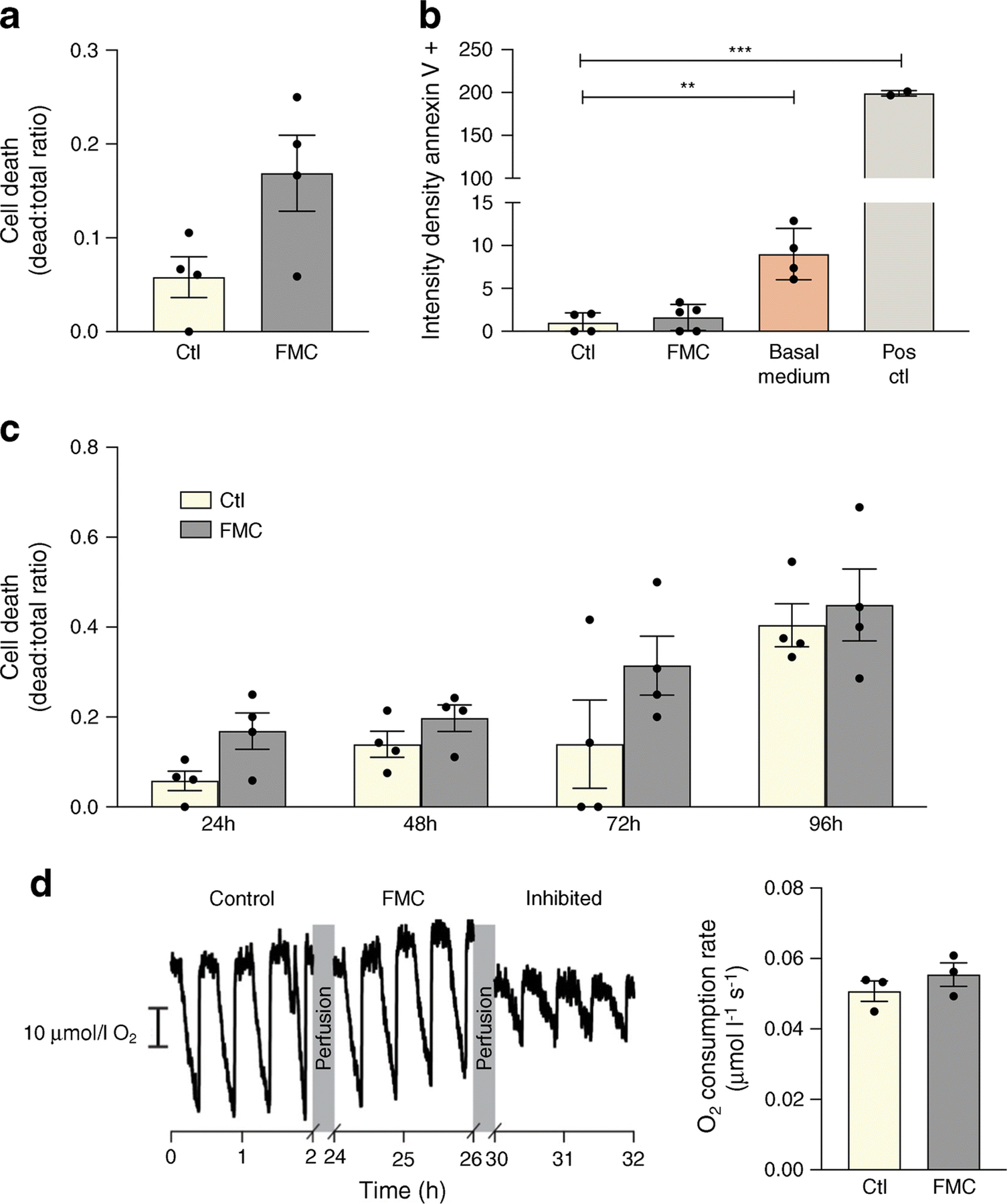
FMC does not significantly impact cell death or mitochondrial substrate-supported respiration in BRECs. (a, b) FMC (0% FBS, 24 h) does not significantly increase cell death. Basal media is comprised of only MCB131 media without the addition of growth factors or supplements (m8537). Positive control (Pos ctl): 50°C for 10 min. (c) Time course of cell death assay. (d) Respiratory activity of BRECs cultured in control or FMC media after 24 h, **p<0.01, ***p<0.001, Data are represented as mean ± SEM. Scale bar, 10 µmol/l O2. Ctl, control
Diabetes-induced retinal cholesterol accumulation and inflammation are prevented by SIRT1 activation
In order to investigate the effect of SIRT1 activation on diabetic retinopathy progression, control and db/db animals were fed chow containing the SIRT1 activator, SRT1720 for 6 months following the onset of diabetes. Diabetes significantly reduced SIRT1 retinal expression. Treatment of diabetic animals with SRT1720-containing chow significantly increased SIRT1 expression compared with retinas from diabetic mice fed control chow (Fig. 6a,b).
Fig. 6.
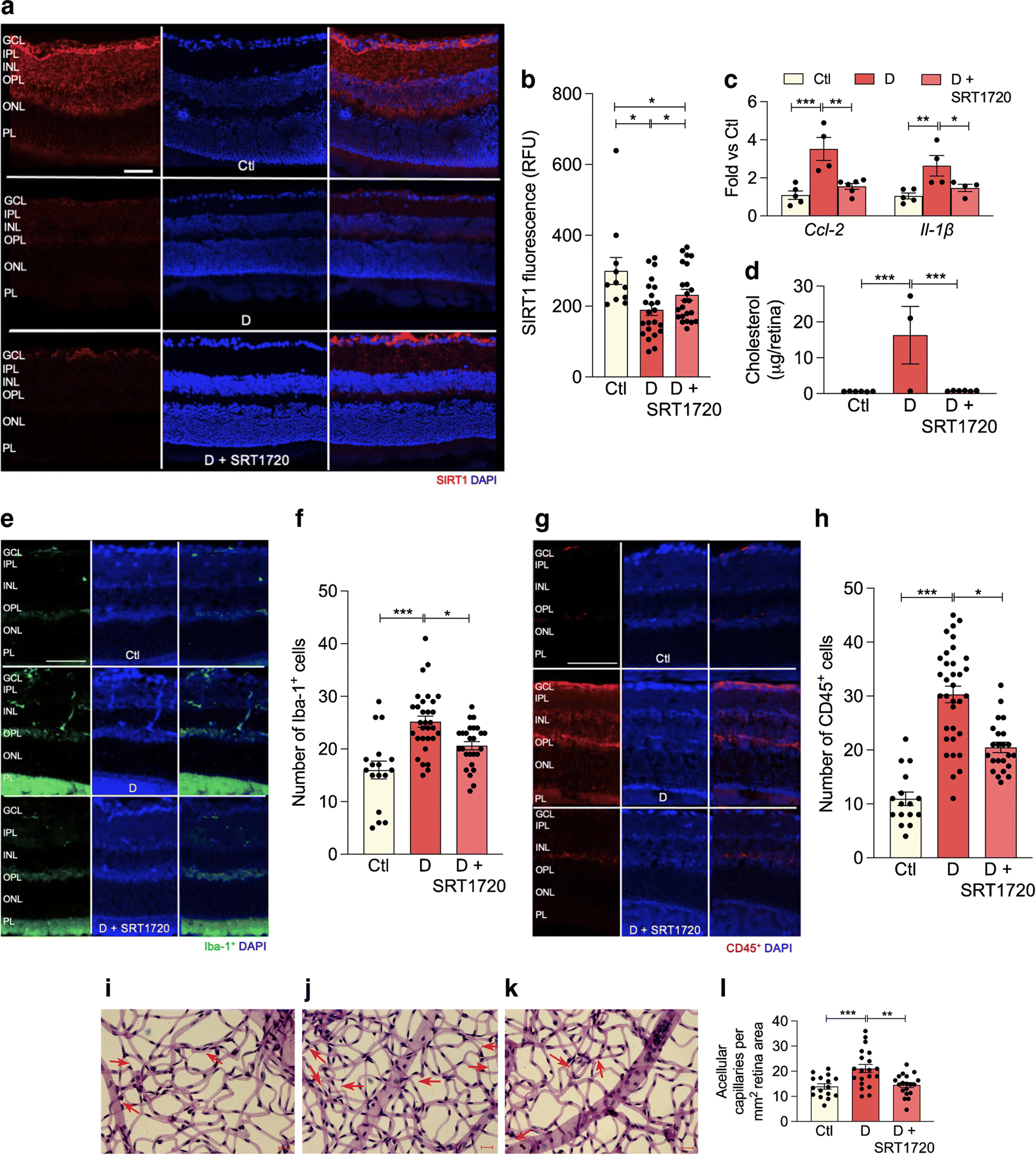
SRT1720 increases SIRT1 expression in retina of db/db mice with 6 month duration of diabetes. (a) Retinal sections from non-diabetic controls (top), diabetic mice fed normal chow (middle) or chow containing SRT1720 (bottom) were stained with anti-SIRT1 antibody (red) and DAPI was used to stain nuclei (blue). Quantification is shown in (b); several replicate samples were stained from n=4 mice. (c, d) Diabetes significantly increases proinflammatory markers Ccl-2, Il-1β mRNA expression (c) and significantly increases retinal cholesterol levels (mass spectrometry analysis) (d); the SIRT1 agonist SRT1720 restores Ccl-2 and Il-1β (c) and cholesterol (d), to non-diabetic levels; n=4–5 mice. (e–h) Diabetes increases, while SRT1720 normalises, the cell number of Iba-1+ (e, f) and CD45+ (g, h) cells in the retina, n=5 from 3 to 5 sections at 100 µm interval for each eye with a minimum of four images for section. (i–l) Acellular capillary formation (red arrows) was examined in non-diabetic animals (i), and in diabetic animals fed control chow (j) or chow containing SRT1720 (k). Diabetes significantly increases acellular capillary formation (j) while administration of the SIRT1 agonist prevents diabetes-induced acellular capillary formation (k). Quantification is shown in (l); n=5 from 4 to 5 images per mm2 retina area; *p<0.05, **p<0.01,***p<0.001. Data are represented as mean ± SEM. Scale bars, 20 µm. Ctl, control (db/m); D, diabetic (db/db); GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PL, photoreceptor layer. RFU, relative fluorescence units
As expected, diabetes resulted in elevated retinal cholesterol levels when compared with control retinas. Treatment with SRT1720 reduced diabetes-induced inflammation twofold, as measured by expression of Il-1β and Ccl-2 (Fig.6c), as well as a reduction in the number of Iba-1+ and CD45+ cells in the retina (Fig. 6e–h). SIRT1 activation, via SRT1720, also restored cholesterol levels in diabetic retinas to non-diabetic levels (Fig. 6d). As shown in Fig. 6i–l, the development of acellular capillaries was significantly increased in db/db mice (red arrows) while activation of SIRT1, via SRT1720 treatment, restored the number of acellular capillaries to non-diabetic levels.
The anti-inflammatory effect of SRT1720 treatment extended to the diabetic bone marrow. Typically, in chronic diabetes the bone marrow supernatant has elevated proinflammatory cytokines, which serve to skew haematopoiesis towards generation of increased myeloid cells [46]. SRT1720 treatment resulted in a reduction in the expression of the inflammatory cytokines Tnf-α and Ccl-2 in the bone marrow supernatant (Fig. 7a, b). The colony-forming capability of bone marrow-derived (BMD) cells from the SRT1720-treated diabetic cohort showed an increase in megakaryocyte–erythroid progenitors (MEPs) (Fig. 7c) compared with untreated diabetic animals. These findings in the bone marrow are similar to what we observed when db/db mice were treated with the LXRα agonist DMHCA [47]. Bone marrow circulating angiogenic cells (CACs) showed the typical reduction in the 6 month db/db mice compared with controls (Fig. 7d). Further examination of myeloid lineage in the bone marrow showed that there were no changes in common myeloid progenitors (CMP, ESM Fig. 2a) and an increase in granulocyte-macrophage progenitors (GMP, ESM Fig. 2b) in diabetes. SRT1720 treatment did not affect CMP and GMP levels (ESM Fig. 2a, b). Examination of the peripheral blood from the experimental cohorts demonstrated that diabetes resulted in the reduction of CACs in the peripheral blood but there was no increase in CACs in SRT1720-treated mice (Fig. 7e). db/db mice showed the typical increase in total circulating monocytes (Fig. 7f) with a decrease in classical (ESM Fig. 2c) and an increase in non-classical monocytes (ESM Fig. 2d) but no changes were observed in the SRT1720-treated diabetic mice. While systemic effects of SRT1720 were not readily apparent from changes in peripheral blood cells, in vitro studies of BMD macrophages were highly informative (Fig. 7g, h). When BMD macrophages from diabetic and control mice were polarised to M2 macrophages, a diabetes-induced decrease in the expression of Lxrα and the LXR-controlled target genes, Abca1 and Abcg1 was observed compared with controls. SRT1720 increased SIRT1 expression in M2 macrophages from diabetic mice leading to upregulation of LXRα activity as shown by an increase in Abcg1 expression (Fig. 7h).
Fig. 7.
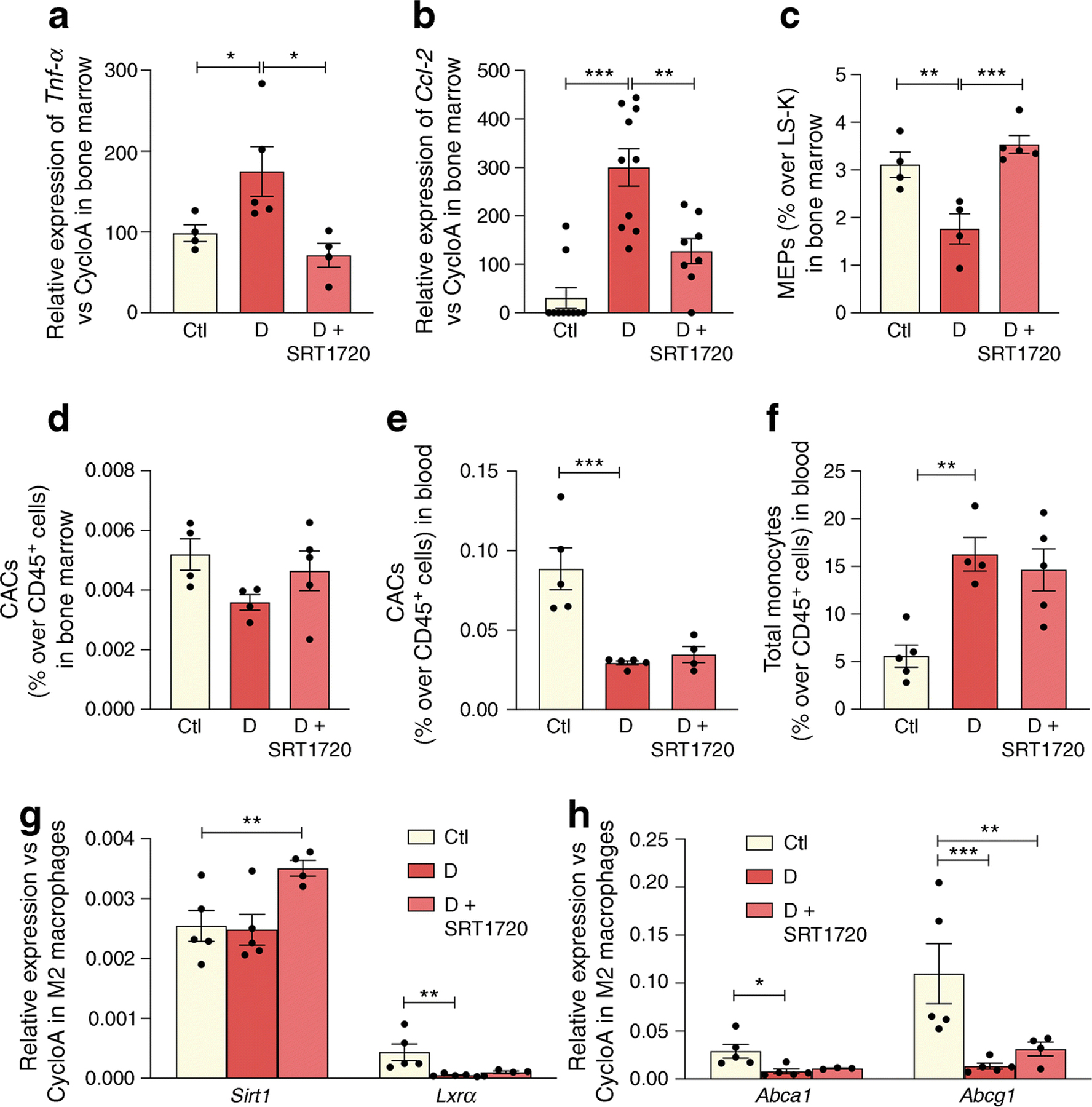
SRT1720 normalises inflammatory and reparative cell damage in the bone marrow and blood of db/db mice with 6 month duration of diabetes. (a, b) Tnf-α (n=4–5 mice) (a) and Ccl-2 (n=5 mice carried out in duplicate) (b) expression in the bone marrow of non-diabetic controls, and diabetic mice fed normal chow or chow containing SRT1720. (c, d) Diabetes significantly decreases the number of MEPs (c) and CACs (d) in the bone marrow, while SRT1720 restores MEP to non-diabetic levels, n=4–5. (e, f) In the blood, diabetes significantly decreases the number of reparative CACs (e) and increases the number of inflammatory circulating total monocytes (f); n=4–5. (g, h) In M2 macrophages, diabetes induces decrease in the expression of Lxrα and LXR-controlled target genes (Abca1 and Abcg1). SRT1720 increases Sirt1 expression in M2 macrophages (g), leading to upregulation of LXR activity as shown by an increase in Abcg1 expression (h); n=4–5; *p<0.05, **p<0.01, ***p<0.001. Data are represented as mean ± SEM. Ctl, control (db/m); CycloA, cyclophilin A; D, diabetic (db/db); LS-K, Lin−Sca−Kit+
SIRT1 activation prevents retinal vascular and neurodegeneration in vivo
Neuronal damage and loss (Fig. 8a) precede the microangiopathy of diabetic retinopathy. As expected, diabetes resulted in a loss of neurons as measured by NeuN+ immunohistochemistry (Fig. 8b). Administration of SRT1720 significantly preserved NeuN+ expression, predominately localised in the retinal ganglion cell layer (Fig. 8b).
Fig. 8.
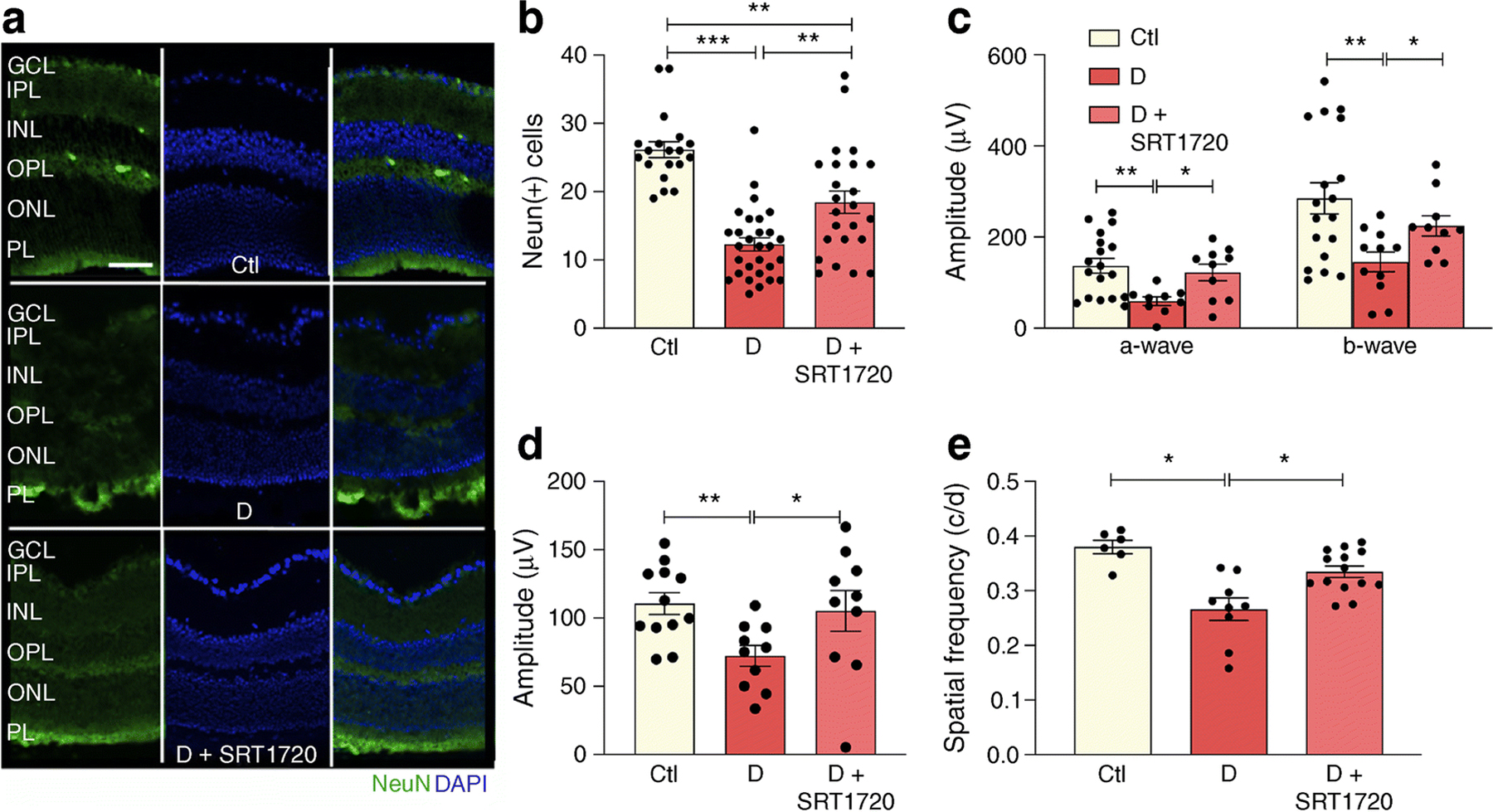
SRT1720 prevents diabetes-induced NeuN+ retinal decrease and improves visual and OKN response in db/db mice. (a, b) Chronic diabetes is associated with neuronal loss in the retina as demonstrated by reduced NeuN+ expression (green) in db/db mice when compared with non-diabetic db/m controls (a). Pharmacological SIRT1 activation using STR1720 in diabetic mice partly restores NeuN+ expression to control levels (a). Quantification shown in (b); n=4 from 3–5 sections at 100 µm interval for each eye with a minimum of four images per section; NeuN+ cells were quantified only in the GCL. (c) Analysis of the ERG showed diabetes-induced reduction of scotopic a- and b-waves; an increase and improvement in scotopic a- and b-wave was observed in diabetic mice treated with SRT1720 compared with diabetic mice on control chow. (d) An improvement in photopic b-wave was observed in diabetic mice treated with SRT1720 compared with diabetic mice on control chow; n=5–6 carried out from right and left eyes. (e) In diabetic mice, the OKN response is reduced compared with age-matched non-diabetic control mice. Diabetic mice treated with SRT1720 showed an improvement in visual acuity compared with diabetic mice on control chow; n=4–6 carried out from right and left eyes; *p<0.05, **p<0.01 ***p<0.001. Data are represented as mean ± SEM. Scale bar, 50 µm. Ctl, control (db/m); D, diabetic (db/db); GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PL, photoreceptor layer
SIRT1 activation improves visual function in db/db mice
The functional effect of SIRT1 activation in diabetes was assayed by measuring retinal and visual function of db/db mice fed control or SRT1720-containing chow. As expected, ERG of db/db mice showed decreased amplitude of scotopic a- and b-waves and lower photopic b-waves. ERG responses were restored to non-diabetic levels in SRT1720- treated diabetic mice (Fig. 8c,d). Visual response assayed using optokinetic (OKN) response measurements was significantly lower in diabetic mice when compared with control mice and SIRT1 activation similarly restored visual acuity to control levels (Fig. 8e).
Discussion
The beneficial effects of fasting in improving metabolic health, warding off chronic disease and increasing longevity have been established by several well-designed clinical trials; however, the data on IF in diabetic individuals remain limited [48, 49] and potential risks associated with IF in people with diabetes have to be carefully considered before it can become a viable treatment option. These include the risk of hypoglycaemia in patients on glucose-lowering medications (insulin, sulfonylureas) [24], and increased glucose level fluctuations between the feeding and fasting days [48].
This study provides one of the first mechanistic evaluations of the effect of SIRT1 activation by fasting, FMC or pharmacological approaches in the diabetic retina. Regulation of SIRT1 by nutrient availability in the liver is well known, however retinal SIRT1 regulation is less well understood, in part, due to the complex multicellular nature of the retina. As shown in this study, fasting-induced upregulation of SIRT1 was not evident in the whole retina, but was uncovered when cell-specific studies using BRECs and neuronal cells were performed. Importantly, the role of SIRT1 activation in normalising cholesterol metabolism in the diabetic retina and in bone marrow cells is demonstrated in this study. In health, the bone marrow has a perfect balance of immunomodulatory cells and vascular reparative populations. This balance is disturbed in chronic diabetes and can contribute to microvascular dysfunction as is seen in diabetic retinopathy [50–52]. In this study, we see a tendency toward improvement in the vascular reparative population, called circulating angiogenic cells (CACs). Cholesterol accumulation adversely impacts this population [53], which is exquisitely sensitive to the diabetic milieu [54]. High glucose adversely influences CAC function by reducing SIRT1/LXR [55, 56], while increasing SIRT1/LXR in CACs of diabetic origin corrects their dysfunction [57]. Systemic dyslipidaemia also impacts circulating monocytes to promote their activation to a highly proinflammatory state [58], which causes retinal damage. Thus, systemic and retinal SIRT1/LXR-mediated metabolic abnormalities simultaneously reduce vascular repair and also promote vascular damage by increasing inflammation.
Bone marrow pathology in the advanced stages also includes reduced erythropoiesis [59]. SRT1720 exhibited anti-inflammatory effects on the bone marrow supernatant and simultaneously increased the generation of megakaryocytes and erythrocytes. These findings suggest that SRT1720 has potential beneficial effects beyond the retina.
Using novel tools to examine long-term monitoring of mitochondrial function in BRECs, we demonstrated that, while providing the beneficial metabolic effects, FMC is devoid of detrimental effects on mitochondrial respiration and energy production. In addition to well described vascular effects, this study demonstrated the role of fasting-induced SIRT1 activation in the improvement of diabetes-induced neuronal damage, both in vivo and in vitro.
SRT1720 has been previously shown to increase lifespan and improve overall health in mice fed a high-fat diet as well as those fed a normal diet [60, 61]. Activation of SIRT1, via SRT1720, has been shown to significantly improve insulin sensitivity, lower glucose levels and increase mitochondrial and metabolic function [62]. SRT1720 was shown to activate SIRT1 with potencies 1,000-fold greater than resveratrol, another SIRT1 activator. Although administration of SRT1720 was successful in activating retinal SIRT1 levels in our studies and in diabetic individuals, not all studies support efficacy [63]
Moreover, there is contradictory evidence as to the pro-survival properties of SIRT1 activation. Zarse et al demonstrated the negative effects that SRT1720 has on C. elegans lifespan [64]. These confounding effects could be due to the governing role that SIRT1 plays in regulating circadian rhythm in peripheral tissues. Several studies have demonstrated SIRT1 activation of Bmal1 (also known as Arntl) and Clock genes, resulting in robust circadian restoration [65]. Thus, differences in timing and feeding patterns among different studies could result in varied physiological outcomes.
In addition to pharmacological activation of SIRT1, we show that FMC activates SIRT1/LXRα signalling in BRECs and neuronal cells (Fig. 2). Numerous studies highlight the beneficial effects IF and energy-restriction regimens on preventing diabetes-associated complications [42, 66, 67]. Recently, the beneficial effects of energy restriction were demonstrated by a clinical trial in which participants who maintained reduced energy intake for 2 years had a significant reduction in heart disease and diabetes prevalence [68]. The mechanism of action responsible for these beneficial effects, however, is still under active investigation.
High demand for cholesterol in the retina dictates its unique metabolism with local retinal cholesterol production coupled with import from the circulation. Under healthy conditions, cholesterol enters the retina via the outer blood–retinal barrier through LDL receptor-mediated processes. Rapid cholesterol turnover in the retina requires efficient cholesterol export. Retinal cells express several hydroxylases (CYP27A1, CYP46A1, CYP11A1) that metabolise cholesterol to more soluble oxysterols [35]. Unlike cholesterol, oxysterols can rapidly diffuse out of the retina for the delivery to the liver for generation of bile acids. Additionally, oxysterols produced by CYP27A1 and CYP46A1 activate LXRα, promoting LXRα-mediated retinal cholesterol export through activation of the RCT pathway via increased ABCA1 and ABCG1 expression in both inner (REC) and outer (RPE) retinal barrier. In the diabetic environment, both cholesterol uptake and removal from the retina are affected, resulting in elevated and dysregulated retinal cholesterol levels. The leaky retinal endothelial cells lead to increased cholesterol ingress, and the decrease in oxysterol levels and LXR activity result in cholesterol accumulation in the retina. In addition to the increase in RCT, activation of LXRα results in repression of NF-κB dependent proinflammatory gene upregulation in the retinal cells.
SIRT1 mediates a decrease in histone acetylation of the DNMT1 promoter. Interestingly, a feedback regulation of SIRT1 expression by DNMT1 through increased DNA methylation of the SIRT1 promoter resulting in SIRT1 transcriptional repression was demonstrated in the diabetic retina [10]. Figure 6 demonstrates the detrimental effects that type 2 diabetes has on retinal SIRT1 expression, cholesterol metabolism and inflammation. In diabetic mice, activation of SIRT1 via SRT1720 restores SIRT1 expression and cholesterol levels to normal. Additionally, diabetes-induced retinal inflammation is significantly reduced after SRT1720 treatment. It is important to note that SRT1720’s ability to increase SIRT1 expression in vitro and in vivo suggests a potential novel mechanism of action unique to the retina and retinal cells since other studies done in non-retinal tissues have not shown increases in SIRT1 expression after SRT1720 treatment [61]. Additionally, activation of SIRT1 has beneficial physiological effects as seen by the prevention of diabetic retinopathy-induced acellular capillary formation, a hallmark of diabetic retinopathy progression. Notably, in vivo activation of SIRT1 restores visual function in db/db mice as measured by ERG and OKN response.
In summary, this study examined the impact of the excess energy supply of type 2 diabetes on downregulation of SIRT1. Decreased LXRα signalling, a hallmark feature of type 2 diabetes, leads to dysregulated retinal cholesterol metabolism and increased production of proinflammatory cytokines. SIRT1 action, however, is not limited to LXRα signalling as activation of SIRT1 in the diabetic retina was protective against inflammation and mitochondrial damage by deacetylation and inhibition of NF-κB, PARP-1 and MMP-9 signalling [8, 9, 69]. Activation of SIRT1 supports normal mitochondrial respiration and energy production under FMC conditions without cell death and mitochondrial damage. Importantly, this study supports the growing evidence for the effective and advantageous effects of modulating time and quantity of nutrient intake and the need to expand strategies for individuals with diabetes that are safe and devoid of hypoglycaemic complications but remain metabolically robust.
Supplementary Material
Table 1.
Primer sequences
| Species | Name | Forward (5’ to 3’) | Reverse (5’ to 3’) | Accession ID |
|---|---|---|---|---|
| Bovine | SIRT1 | CCC TGA AAG TAA GAC CAG TAG C | GTG AGG CAA AGG TTC CCT ATT A | NM_001192980.3 |
| ABCA1 | CTC AGT GGG ATG GAT GGT AAA G | TGG CAA TCA GCA GTC TCT TC | NM_001024693.1 | |
| ABCG1 | AGA CCT GCC ATT TCC AGA AG | GAT GAG ACG CAG GGA GAT AAA G | NM_001205528.3 | |
| VCAM1 | TGCAGGGTTCCTAATGT GTATC | GTAAGAAAGCCCTAGAG ACCAAG | NM_174484.1 | |
| Cyclophilin A | GAGCACTGGAGAGAAA GGATTT | GACTTGCCACCAGTACC ATTAT | NM_178320.2 | |
| Rat (used For R28 cell culture studies) | SIRT1 | CATAGGTTAGGTGGCG AGTATG | GTTGGTGGCAACTCTGA TAAATG | NM_001372090.1 |
| ABCA1 | TTGGATTCGGCTGTGAG TATTT | GGACACTGAGGTGGTAA GATTG | NM_178095.2 | |
| LXRα | TGGCACTAAAGAGAGTC AAAGG | GGCTCTCCTGGCTAGTT TATTT | NM_031627.2 | |
| Cyclophilin A | TGGCAAGACCAGCAAG AA | CTCCTGAGCTACAGAAG GAATG | NM_017101.1 |
Acknowledgements
M. Gorbatyuk and T. W. Kraft (School of Optometry, University of Alabama at Birmingham, USA) provided UTAS Bigshot (LKC Technologies, USA) for ERG studies, and OptoMotry 1.7.7 (Cerebral Mechanics, Canada) for the visual acuity studies.
Funding
This study was supported by the National Institutes of Health Grants R01EY012601, R01EY028858, R01EY028037, R01EY025383 to MBG; T32HL134640-01 to MD; F32EY028426 to SSH; MICL02539, R01EY030766, R01EY016077 to JVB, R01EY028049 to DAP; NIH-5-R25-HL108864 to MS.
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- BMD
Bone marrow-derived
- BREC
Bovine retinal endothelial cell
- CAC
Circulating angiogenic cell
- CCL2
C-C motif chemokine ligand 2
- CMP
Common myeloid progenitors
- DMHCA
N,N-dimethyl-3β-hydroxy-cholenamide
- DNMT1
DNA (cytosine-5)-methyltransferase 1
- ERG
Electroretinogram
- FMC
Fasting mimicking cell culture conditions
- GMP
Granulocyte-macrophage progenitors
- HDAC
Histone deacetylase
- Iba-1
Ionised calcium binding adaptor molecule 1
- IF
Intermittent fasting
- LXR
Liver X receptor
- MEP
Megakaryocyte–erythroid progenitor
- MMP-9
Matrix-metallopeptidase 9
- OKN
Optokinetic
- PARP-1
poly [ADP-ribose] polymerase
- qPCR
Quantitative PCR
- RCT
Reverse cholesterol transport
- RPE
Retinal pigment epithelium
- SIRT1
Sirtuin 1
- VCAM1
Vascular cell adhesion protein 1
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Authors’ relationships and activities The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- [1].Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR (2017) Diabetic retinopathy: Breaking the barrier. Pathophysiology 24(4): 229–241. 10.1016/j.pathophys.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kowluru RA, Santos JM, Zhong Q (2014) Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Invest Ophthalmol Vis Sci 55(9): 5653–5660. 10.1167/iovs.14-14383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Karbasforooshan H, Karimi G (2018) The role of SIRT1 in diabetic retinopathy. Biomed Pharmacother 97: 190–194. 10.1016/j.biopha.2017.10.075 [DOI] [PubMed] [Google Scholar]
- [4].Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX (2011) Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. The Journal of clinical investigation 121(11): 4477–4490. 10.1172/JCI46243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baur JA, Pearson KJ, Price NL, et al. (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444(7117): 337–342. 10.1038/nature05354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yun JM, Chien A, Jialal I, Devaraj S (2012) Resveratrol up-regulates SIRT1 and inhibits cellular oxidative stress in the diabetic milieu: mechanistic insights. The Journal of nutritional biochemistry 23(7): 699–705. 10.1016/j.jnutbio.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zabolotny JM, Kim YB (2007) Silencing insulin resistance through SIRT1. Cell metabolism 6(4): 247–249. 10.1016/j.cmet.2007.09.004 [DOI] [PubMed] [Google Scholar]
- [8].Kowluru RA, Mishra M, Kumar B (2016) Diabetic retinopathy and transcriptional regulation of a small molecular weight G-Protein, Rac1. Exp Eye Res 147: 72–77. 10.1016/j.exer.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mishra M, Kowluru RA (2017) Role of PARP-1 as a novel transcriptional regulator of MMP-9 in diabetic retinopathy. Biochim Biophys Acta Mol Basis Dis 1863(7): 1761–1769. 10.1016/j.bbadis.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mishra M, Duraisamy AJ, Kowluru RA (2018) Sirt1: A Guardian of the Development of Diabetic Retinopathy. Diabetes 67(4): 745–754. 10.2337/db17-0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hammer SS, Beli E, Kady N, et al. (2017) The Mechanism of Diabetic Retinopathy Pathogenesis Unifying Key Lipid Regulators, Sirtuin 1 and Liver X Receptor. EBioMedicine 22: 181–190. 10.1016/j.ebiom.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mattagajasingh I, Kim CS, Naqvi A, et al. (2007) SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America 104(37): 14855–14860. 10.1073/pnas.0704329104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Potente M, Dimmeler S (2008) Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle 7(14): 2117–2122 [DOI] [PubMed] [Google Scholar]
- [14].Miranda MX, van Tits LJ, Lohmann C, et al. (2014) The Sirt1 activator SRT3025 provides atheroprotection in Apoe−/− mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. European heart journal. 10.1093/eurheartj/ehu095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Centers for Disease Control and Prevention (2020) National Diabetes Statistics Report. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed Sep 2020
- [16].Chang YC, Wu WC (2013) Dyslipidemia and diabetic retinopathy. Rev Diabet Stud 10(2–3): 121–132. 10.1900/RDS.2013.10.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fliesler SJ, Bretillon L (2010) The ins and outs of cholesterol in the vertebrate retina. J Lipid Res 51(12): 3399–3413. jlr.R010538 [pii] 10.1194/jlr.R010538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin JB, Mast N, Bederman IR, et al. (2016) Cholesterol in mouse retina originates primarily from in situ de novo biosynthesis. J Lipid Res 57(2): 258–264. 10.1194/jlr.M064469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zheng W, Reem RE, Omarova S, et al. (2012) Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS One 7(5): e37926. 10.1371/journal.pone.0037926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pikuleva IA, Curcio CA (2014) Cholesterol in the retina: the best is yet to come. Prog Retin Eye Res 41: 64–89. 10.1016/j.preteyeres.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tserentsoodol N, Sztein J, Campos M, et al. (2006) Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Molecular vision 12: 1306–1318 [PubMed] [Google Scholar]
- [22].Duncan KG, Hosseini K, Bailey KR, et al. (2009) Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. The British journal of ophthalmology 93(8): 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carter S, Clifton PM, Keogh JB (2018) Effect of Intermittent Compared With Continuous Energy Restricted Diet on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Noninferiority Trial. JAMA Netw Open 1(3): e180756. 10.1001/jamanetworkopen.2018.0756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grajower MM, Horne BD (2019) Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 11(4). 10.3390/nu11040873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harris L, Hamilton S, Azevedo LB, et al. (2018) Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep 16(2): 507–547. 10.11124/JBISRIR-2016-003248 [DOI] [PubMed] [Google Scholar]
- [26].Yuan Y, Cruzat VF, Newsholme P, Cheng J, Chen Y, Lu Y (2016) Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech Ageing Dev 155: 10–21. 10.1016/j.mad.2016.02.003 [DOI] [PubMed] [Google Scholar]
- [27].Hayashida S, Arimoto A, Kuramoto Y, et al. (2010) Fasting promotes the expression of SIRT1, an NAD+ -dependent protein deacetylase, via activation of PPARα in mice. Mol Cell Biochem 339(1–2): 285–292. 10.1007/s11010-010-0391-z [DOI] [PubMed] [Google Scholar]
- [28].Lee SH, Lee JH, Lee HY, Min KJ (2019) Sirtuin signaling in cellular senescence and aging. BMB Rep 52(1): 24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L (2000) The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism 49(1): 22–31. 10.1016/s0026-0495(00)90588-2 [DOI] [PubMed] [Google Scholar]
- [30].Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW (1998) Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 47(12): 1953–1959. 10.2337/diabetes.47.12.1953 [DOI] [PubMed] [Google Scholar]
- [31].Stewart EA, Samaranayake GJ, Browning AC, Hopkinson A, Amoaku WM (2011) Comparison of choroidal and retinal endothelial cells: characteristics and response to VEGF isoforms and anti-VEGF treatments. Exp Eye Res 93(5): 761–766. 10.1016/j.exer.2011.09.010 [DOI] [PubMed] [Google Scholar]
- [32].Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC Jr. (2002) Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem 80(4): 667–677. 10.1046/j.0022-3042.2001.00740.x [DOI] [PubMed] [Google Scholar]
- [33].Gardner TW, Lesher T, Khin S, Vu C, Barber AJ, Brennan WA Jr. (1996) Histamine reduces ZO-1 tight-junction protein expression in cultured retinal microvascular endothelial cells. Biochem J 320 (Pt 3): 717–721. 10.1042/bj3200717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Strober W (2015) Trypan Blue Exclusion Test of Cell Viability. Curr Protoc Immunol 111: A3.B.1–A3.B.3. 10.1002/0471142735.ima03bs111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Saadane A, Mast N, Trichonas G, et al. (2019) Retinal Vascular Abnormalities and Microglia Activation in Mice with Deficiency in Cytochrome P450 46A1-Mediated Cholesterol Removal. Am J Pathol 189(2): 405–425. 10.1016/j.ajpath.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hernandez C, Burgos R, Canton A, Garcia-Arumi J, Segura RM, Simo R (2001) Vitreous levels of vascular cell adhesion molecule and vascular endothelial growth factor in patients with proliferative diabetic retinopathy: a case-control study. Diabetes Care 24(3): 516–521. 10.2337/diacare.24.3.516 [DOI] [PubMed] [Google Scholar]
- [37].Capozzi ME, Hammer SS, McCollum GW, Penn JS (2016) Epoxygenated Fatty Acids Inhibit Retinal Vascular Inflammation. Sci Rep 6: 39211. 10.1038/srep39211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Levitsky Y, Pegouske DJ, Hammer SS et al. (2019) Micro-Respirometry of Whole Cells and Isolated Mitochondria. RSC Advances 9(57):33257–33267. doi: 10.1039/c9ra05289e [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lydic TA, Busik JV, Reid GE (2014) A monophasic extraction strategy for the simultaneous lipidome analysis of polar and nonpolar retina lipids. J Lipid Res 55(8): 1797–1809. 10.1194/jlr.D050302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McDonald JG, Thompson BM, McCrum EC, Russell DW (2007) Extraction and analysis of sterols in biological matrices by high performance liquid chromatography electrospray ionization mass spectrometry. Methods Enzymol 432: 145–170. 10.1016/S0076-6879(07)32006-5 [DOI] [PubMed] [Google Scholar]
- [41].Machacek M, Saunders H, Zhang Z, et al. (2019) Elevated O-GlcNAcylation enhances proinflammatory Th17 function by altering the intracellular lipid microenvironment. J Biol Chem 294(22): 8973–8990. 10.1074/jbc.RA119.008373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Beli E, Yan Y, Moldovan L, et al. (2018) Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes 67(9): 1867–1879. 10.2337/db18-0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Asare-Bediako B, Noothi SK, Li Calzi S, et al. (2020) Characterizing the Retinal Phenotype in the High-Fat Diet and Western Diet Mouse Models of Prediabetes. Cells 9(2). 10.3390/cells9020464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Prusky GT, Alam NM, Beekman S, Douglas RM (2004) Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci 45(12): 4611–4616. 10.1167/iovs.04-0541 [DOI] [PubMed] [Google Scholar]
- [45].Qi X, Pay SL, Yan Y, et al. (2017) Systemic Injection of RPE65-Programmed Bone Marrow-Derived Cells Prevents Progression of Chronic Retinal Degeneration. Mol Ther 25(4): 917–927. 10.1016/j.ymthe.2017.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hazra S, Jarajapu YP, Stepps V, et al. (2013) Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model. Diabetologia 56(3): 644–653. 10.1007/s00125-012-2781-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vieira CP, Fortmann SD, Hossain M, et al. (2020) Selective LXR agonist DMHCA corrects retinal and bone marrow dysfunction in type 2 diabetes. JCI Insight 5(13). 10.1172/jci.insight.137230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Horne BD, Grajower MM, Anderson JL (2020) Limited Evidence for the Health Effects and Safety of Intermittent Fasting Among Patients With Type 2 Diabetes. JAMA 10.1001/jama.2020.3908 [DOI] [PubMed] [Google Scholar]
- [49].Li C, Sadraie B, Steckhan N, et al. (2017) Effects of A One-week Fasting Therapy in Patients with Type-2 Diabetes Mellitus and Metabolic Syndrome - A Randomized Controlled Explorative Study. Exp Clin Endocrinol Diabetes 125(9): 618–624. 10.1055/s-0043-101700 [DOI] [PubMed] [Google Scholar]
- [50].Dimmeler S, Zeiher AM (2004) Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med (Berl) 82(10): 671–677. 10.1007/s00109-004-0580-x [DOI] [PubMed] [Google Scholar]
- [51].Jarajapu YP, Hazra S, Segal M, et al. (2014) Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLoS One 9(4): e93965. 10.1371/journal.pone.0093965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fadini GP, de Kreutzenberg S, Agostini C, et al. (2009) Low CD34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis 207(1): 213–219. 10.1016/j.atherosclerosis.2009.03.040 [DOI] [PubMed] [Google Scholar]
- [53].Tikhonenko M, Lydic TA, Opreanu M, et al. (2013) n-3 polyunsaturated fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS One 8(1): e55177. 10.1371/journal.pone.0055177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Busik JV, Tikhonenko M, Bhatwadekar A, et al. (2009) Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med 206(13): 2897–2906. 10.1084/jem.20090889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Balestrieri ML, Servillo L, Esposito A, et al. (2013) Poor glycaemic control in type 2 diabetes patients reduces endothelial progenitor cell number by influencing SIRT1 signalling via platelet-activating factor receptor activation. Diabetologia 56(1): 162–172. 10.1007/s00125-012-2749-0 [DOI] [PubMed] [Google Scholar]
- [56].Lemarie CA, Shbat L, Marchesi C, et al. (2011) Mthfr deficiency induces endothelial progenitor cell senescence via uncoupling of eNOS and downregulation of SIRT1. Am J Physiol Heart Circ Physiol 300(3): H745–753. 10.1152/ajpheart.00321.2010 [DOI] [PubMed] [Google Scholar]
- [57].Yuen DA, Zhang Y, Thai K, et al. (2012) Angiogenic dysfunction in bone marrow-derived early outgrowth cells from diabetic animals is attenuated by SIRT1 activation. Stem cells translational medicine 1(12): 921–926. 10.5966/sctm.2012-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sene A, Apte RS (2014) Eyeballing cholesterol efflux and macrophage function in disease pathogenesis. Trends Endocrinol Metab 25(3): 107–114. 10.1016/j.tem.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guo W, Zhou Q, Jia Y, Xu J (2019) Increased Levels of Glycated Hemoglobin A1c and Iron Deficiency Anemia: A Review. Med Sci Monit 25: 8371–8378. 10.12659/MSM.916719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Minor RK, Baur JA, Gomes AP, et al. (2011) SRT1720 improves survival and healthspan of obese mice. Sci Rep 1: 70. 10.1038/srep00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mitchell SJ, Martin-Montalvo A, Mercken EM, et al. (2014) The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep 6(5): 836–843. 10.1016/j.celrep.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Milne JC, Lambert PD, Schenk S, et al. (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450(7170): 712–716. 10.1038/nature06261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pacholec M, Bleasdale JE, Chrunyk B, et al. (2010) SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285(11): 8340–8351. 10.1074/jbc.M109.088682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zarse K, Schmeisser S, Birringer M, Falk E, Schmoll D, Ristow M (2010) Differential effects of resveratrol and SRT1720 on lifespan of adult Caenorhabditis elegans. Horm Metab Res 42(12): 837–839. 10.1055/s-0030-1265225 [DOI] [PubMed] [Google Scholar]
- [65].Belden WJ, Dunlap JC (2008) SIRT1 is a circadian deacetylase for core clock components. Cell 134(2): 212–214. 10.1016/j.cell.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Antoni R, Johnston KL, Collins AL, Robertson MD (2017) Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc 76(3): 361–368. 10.1017/S0029665116002986 [DOI] [PubMed] [Google Scholar]
- [67].Mattson MP, Longo VD, Harvie M (2017) Impact of intermittent fasting on health and disease processes. Ageing Res Rev 39: 46–58. 10.1016/j.arr.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kraus WE, Bhapkar M, Huffman KM, et al. (2019) 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol 7(9): 673–683. 10.1016/S2213-8587(19)30151-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mishra M, Flaga J, Kowluru RA (2016) Molecular Mechanism of Transcriptional Regulation of Matrix Metalloproteinase-9 in Diabetic Retinopathy. J Cell Physiol 231(8): 1709–1718. 10.1002/jcp.25268 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.


