Abstract
Regulation of sphingolipid metabolism plays a role in cellular homeostasis, and dysregulation of these pathways is involved in cancer progression. Previously, our reports identified ceramide as an anti-metastatic lipid. In the present study, we investigated the biochemical alterations in ceramide-centered metabolism of sphingolipids that were associated with metastatic potential. We established metastasis-prone sublines of SKOV3 ovarian cancer cells using an in vivo selection method. These cells showed decreases in ceramide levels and ceramide synthase (CerS) 2 expression. Moreover, CerS2 down regulation in ovarian cancer cells promoted metastasis in vivo and potentiated cell motility and invasiveness. Moreover, CerS2 knock-in suppressed the formation of lamellipodia required for cell motility in this cell line. In order to define specific roles of ceramide species in cell motility controlled by CerS2, the effect of exogenous long- and very long- chain ceramide species on the formation of lamellipodia was evaluated. Treatment with distinct ceramides increased cellular ceramides and had inhibitory effects on the formation of lamellipodia. Interestingly, blocking the recycling pathway of ceramides by a CerS inhibitor was ineffective in the suppression of exogenous C24:1-ceramide for the formation of lamellipodia. These results suggested that C24:1-ceramide, a CerS2 metabolite, predominantly suppresses the formation of lamellipodia without the requirement for deacylation/reacylation. Moreover, knock-down of neutral ceramidase suppressed the formation of lamellipodia concomitant with up-regulation of C24:1-ceramide. Collectively, the CerS2-C24:1-ceramide axis, which may be countered by neutral ceramidase, is suggested to limit cell motility and metastatic potential. These findings may provide insights that lead to further development of ceramide-based therapy and biomarkers for metastatic ovarian cancer.
Keywords: ceramide, sphingolipids, sphingolipid metabolism, cell motility, metastasis
Introduction
Ovarian cancer is the most lethal gynecologic malignancy in developed countries. Despite improvements in early detection and advances in cancer treatments, ovarian cancer frequently metastasizes, and metastasis remains a major challenge in the practical management of ovarian cancer (1-3). A better understanding of the molecular regulatory mechanisms of metastasis may help lead to the development of novel pharmacotherapeutics and biomarkers, improving the prognosis of ovarian cancer patients.
Cell motility and invasion are critical cell functions in cancer metastasis (4-6). A number of factors, such as growth factors, chemokines, and lysolipids, have been characterized as metastatic mediators, and the intracellular signals regulating cell motility have been partly elucidated. Phosphatidyl-3-kinase (PI3K) plays a key role in transducing cell motility-stimulating signals through tyrosine kinase receptors such as epidermal growth factor receptor and G-protein coupled-receptors for lysophosphatidic acid and chemokines (7-9). However, the regulatory mechanisms remain poorly understood.
Ceramide, the central molecule in sphingolipid metabolism, is a bioactive lipid that serves as a regulatory molecule in the anti-inflammatory response, apoptosis, programmed necrosis, autophagy, and cell motility of cancer cells (10-15). Recently, we found that ceramide is involved in the inhibitory regulation of ovarian cancer cell motility driven by class II PI3K β isoform (PI3KC2β) (8), and we proposed that ceramide serves as a metastasis suppressor in tumors (16). Biosynthesis of ceramide involves the de novo pathway, salvage pathway, and/or the sphingomyelinase (SMase) pathway with over 28 enzymes participating in ceramide metabolism (11, 13, 17). Alterations in the expressions of sphingolipid metabolizing enzymes may affect metastatic potential by down- or up-regulating cellular levels of ceramides.
In the present study, we established highly metastatic cells and studied genetic and biochemical alternations of these cells in comparison with the parental cells in vivo. These approaches identified an anti-metastatic ceramide-generating enzyme responsible for suppressing cell motility and regulating the metastatic potential of ovarian cancer.
Materials and Methods
Antibodies and reagents
C16-ceramide, C18-ceramide, C24-ceramide, and C24:1-ceramide were obtained from Matreya (Pleasant, PA, USA). Horseradish-peroxidase-conjugated antibodies for mouse (sc2005) and rabbit IgG (sc2004) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Human serum was obtained from Jackson ImmunoResearch (West Grove, PA, USA). RNAiMax, Lipofectamine2000, control siRNAs (Negative Control #1 and #2), and all siRNAs for human CerSs (CerS1 ID s194430, CerS2 ID s26788, CerS3 ID s47540, CerS4 ID s35896, CerS5 ID s40552, CerS6 ID s48447) and neutral ceramidase (CDase) (ID s232195) were obtained from Life Technologies (Carlsbad, CA, USA). Fumonisin B1, mouse anti-Flag antibody (M2 clone), tetramethylrhodamine isothiocyanate (TRITC)-conjugated phalloidin, and β-actin antibodies (A5441) were purchased from Sigma (St Louis, MO, USA). Hoechst 33342 was obtained from Dojindo (Kumamoto, Japan). TritonX-100, AlexaFluor488-conjugated anti-mouse IgG antibodies, SuperSignal West Dura Extended Duration Substrate and Halt Phosphatase Inhibitor Cocktail were purchased from Thermo Fisher Scientific (Rockford, IL, USA). Antibodies specific for CerS2 (ab176709) were purchased from Abcam (Cambridge, MA, USA). Human neutral CDase vectors (18) were kindly provided by Drs. Nozomu Okino and Makoto Ito (Kyushu University, Fukuoka, Japan).
Cell culture
Ovarian cancer SKOV3 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were authenticated by JCRB Cell Bank (Osaka, Japan). Cells were maintained at <80% confluence under standard incubator conditions (humidified atmosphere, 95% air, 5% CO2, 37°C). No mycoplasma contamination was observed in the cell lines.
Ethics statement
All animal studies were approved by the Institutional Animal Care and Use Committee of Tohoku University, Japan. The ID numbers are 2013MeDo-006 and 2015MeDo-001.
Establishment of metastasis-prone sublines of SKOV3 ovarian cancer cells by an in vivo selection method
SKOV3 cells (5 × 106 cells/mouse) were inoculated into the peritoneal cavity of five-week-old female nude mice (BALB/c; Charles River Japan). Two mice were sacrificed at 4 weeks after inoculation. The metastatic nodules (<1 mm, 2 nodules/mouse) were individually cut into small pieces and plated in a 35-mm dish in DMEM supplemented with 10% fetal bovine serum.
Human ovarian cancer cell xenograft studies
SKOV3 cells (5 × 106 cells/mouse) were transfected with 5 nM siRNAs for 24 h and then inoculated into the peritoneal cavity of five-week-old female nude mice (BALB/c; Charles River Japan) for the examination of metastatic potential. The mice were sacrificed at 4 weeks after inoculation, and the number and extent of overt metastases (>1 mm) were quantified. In cases in which cell inoculation failed, the mice were euthanized and excluded from the analysis. Moreover, mice with early death were also excluded from the analysis.
Library preparation and sequencing of RNAs
An Illumina TruSeq Stranded mRNA Sample Preparation Kit (Qiagen, Hilden, Germany) was used to generate (50 bp paired-end cDNA) sequencing libraries by following the manufacturer’s protocol. The libraries were loaded onto flow cell channels for sequencing on an Illumina HiSeq 2500 at Genome Network Analysis Support Facility, RIKEN CLST.
Immunoblotting
Cells were washed three times with ice-cold PBS supplemented with 10 mM EDTA and lysed using Laemmli buffer. Protein samples (10 μg) were subjected to SDS-PAGE (4–20% gradient gels) and electrophoretically transferred to nitrocellulose membranes (0.45 μm pore size; Bio-Rad, USA). Membranes were blocked with PBS/0.1% Tween 20 (PBS-T) containing 5% nonfat dried milk, washed with PBS-T, and incubated with primary antibodies for CerS2 (1:1,000 dilution), CerS6 (1:1,000 dilution), and β-actin (1:100,000 dilution) in PBS-T containing 5% bovine serum albumin. The blots were washed with PBS-T and incubated with secondary antibody conjugated with horseradish peroxidase in PBS-T containing 5% nonfat dried milk. Detection was performed using enhanced chemiluminescence reagent, and quantification of the chemiluminescent signals was performed with a digital imaging system.
Quantitative real-time PCR
Cells were washed with ice-cold PBS, and then RNAs were extracted using RNeasy mini kits (Qiagen, Hilden, Germany) according to the manufacturer’s manual. Reverse transcription of RNA (250 ng) was performed using the ReverTraAce® kit (TOYOBO, Osaka, Japan). Real-time PCR for CerS2 and neutral CDase genes was performed using the StepOne Plus™ Real-Time PCR System with TaqMan® Universal Master Mix II and a TaqMan® probe specific for TfR2 (Thermo Fisher Scientific).
Transfection with siRNAs or plasmid vectors
For siRNA transfection, SKOV3 cells (2 × 104) grown on glass-bottom dishes were transfected with siRNAs using RNAiMax transfection reagent. For transfection with plasmid vectors, cells (5 × 104)/dish were transfected with 2 μg plasmid vectors using Lipofectamine 2000.
Analysis of pseudopodia formation
SKOV3 cells growing on glass-bottom dishes were washed with PBS twice and fixed with 4% formaldehyde for 10 min. Fixed cells were then treated with 0.1% TritonX-100 for 10 min, followed by staining with Hoechst 33342 and TRITC-conjugated phalloidin for 5 min. For analysis of pseudopodia such as lamellipodia, samples were examined with confocal microscopy. Cells were counted as having formed lamellipodia if there was an increase in F-actin in the lamellipodia. The formation of lamellipodia was assessed by blinded quantification of fluorescence microscopy (each sample >200 cells). Two investigators independently assessed the formation of lamellipodia.
CerS2 knockout by a CRISPR-Cas9 system
SKOV3 cells (1 × 105 cells /well) were transfected with 2 μg Edit-R hCMV-mKate2-Cas9 (GE Health Care, Chicago, IL, USA) (19) in the absence (CRISPR control) or presence of 5 nM tracrRNAs and 5 nM CerS2-targetted crRNAs including three distinct sequences. After 24 h, mKate2-expressing cells were sorted by the cell sorter Aria II (BD) and plated into a 96-well plate (one cell/well) to acquire single clones for CRISPR control and CerS2 knockout (KO) cells. After 3 weeks of culture, CerS2 KO clones were identified by immunoblotting for CerS2. CerS2 KO and control clones were named as CerS2 KO 3-6 and CRISPR control C14, respectively. CerS2-targetted crRNA sequences were designed using the web tool CRISPR Design at http://crispr.mit.edu/. These sequences are as follows: sequence-1, UCG GUC UUC UAG AUC GGC CCG UUU UAG AGC UAU GCU GUU UUG; sequence-2, GGA ACC AAC GCU CUA CCU GGG UUU UAG AGC UAU GCU GUU UUG UGA CUC U; and sequence-3, UCC GAU UAC CUG CGU UUU AGA GCU AUG CUG UUU UG.
Trypan blue viability assay
Cells (CerS2 KO 3-6 and CRISPR control C14) were plated in 6-well plates and incubated for up to 96 h. Cells were then suspended in PBS. A 1:1 dilution of the cell suspension was made using 0.4% Trypan blue and loaded into a hemocytometer. Cells were immediately counted.
Cell migration assay
Cells (5 × 104 cells /well) were plated in the upper chambers of Transwell systems (8 μm pore); the lower chamber was filled with DMEM containing 10% fetal bovine serum. Cells were incubated for 6 h. The migratory cells attached to the lower surface were fixed with 100% methanol and then stained with 1% toluidine blue for 5 min. Cell imaging was performed by light microscopy. The migrating cells were quantitated by counting in optical microscopy images (at least three fields for each determination).
Invasion assay
Cells (5 × 104 cells /well) were plated onto the upper chambers of Transwell systems (8 μM pore) coated with 5% Matrigel (20 μl/well); the lower chamber was filled with DMEM containing 10% fetal bovine serum. Cells were incubated for 18 h. The invaded cells attached to the lower surface were fixed with 100% methanol and stained with 1% toluidine blue for 5 min. Cell imaging was performed by light microscopy. The invasiveness was quantitated by manual counting of cells in optical microscopy images (at least three fields for each determination).
Immunocytochemistry
Cells growing on 35-mm glass-bottom dishes were fixed for 10 min at room temperature with 4% formaldehyde in PBS and then washed with PBS. Cells were treated for 10 min with 0.1% TritonX-100, washed with PBS and blocked for 1 h with PBS containing 20% human serum. Cells were incubated with Flag antibodies (1:1,000 dilution) in PBS containing 20% human serum overnight. After washing with PBS, cells were incubated with AlexaFluor488-conjugated anti-mouse IgG antibodies (1:200 dilution) in PBS containing 20% human serum for 1 h. Furthermore, those cells were stained with TRITC-conjugated phalloidin and Hoechst 33342. Confocal laser microscopy was performed using an LSM780 confocal microscope (Carl Zeiss, Thornwood, NY, USA).
Lipid treatment
Long-chain or very long-chain ceramides (C16-, C18, C24-, and C24:1-ceramides) were dissolved in ethanol, generating 5 mM stocks. Cells (5 × 105 cells/100-mm dishes) were treated with ethanol or ceramides for 6 h. After treatment, cells were washed with ice-cold PBS containing 5 mM EDTA three times. Harvested cells were further washed with ice-cold PBS containing 5 mM EDTA and then the lipids were extracted.
Lipid measurement by liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Analysis of sphingolipids in lipid extracts was performed by LC-MS/MS as previously described (20, 21).
Statistical analysis
Data presented in bar graphs represent the mean ± SEM of independent experiments. Images are representative of at least three independent experiments. Sample sizes for relevant experiments were determined by power analyses conducted during experiment planning (β = 0.2, P = 0.05). Statistical analyses were performed using GraphPad Prism and Instat. Individual t tests were performed for significance assessment of the differences between treatments. A p-value less than 0.05 was considered as significant. *, p<0.05; **, p<0.01; ***, p<0.001
Results
Characterization of metastasis-prone cells
PI3KC2β has been implicated in promoting cell motility and cancer metastasis, which is inhibited by ceramide (16). Although a number of enzymes are involved in maintaining the cellular homeostasis of ceramide levels, the precise sphingolipid metabolizing enzymes that are responsible for ceramide regulation of metastasis are unknown. To identify metastasis-associated factors, we performed in vivo selection for metastasis-prone cells in a mouse model and characterized newly established metastasis-prone sublines of SKOV3 ovarian cancer cells regarding ceramide levels and enzyme expression.
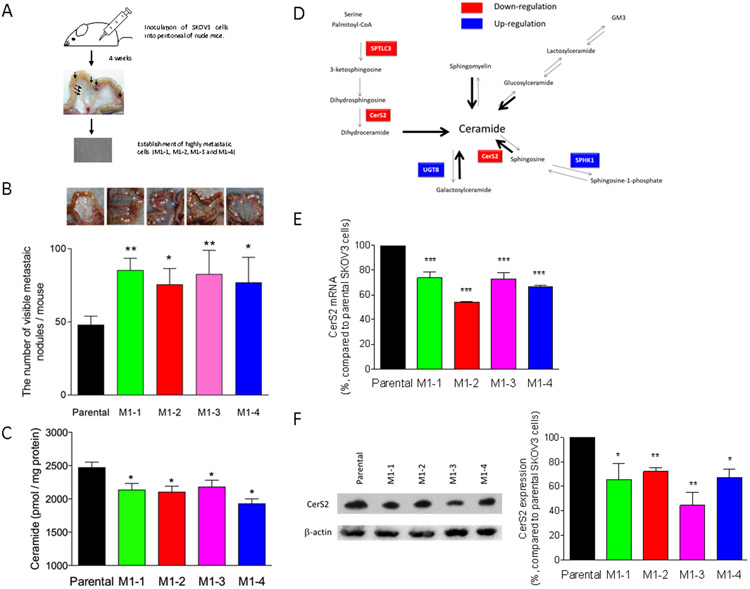
Parental SKOV3 ovarian cancer cells were inoculated into the peritoneal cavity of two nude mice (Figure 1A). Four weeks later, the cells were observed to form metastatic nodules throughout the interior of the mesentery. Four types of cells isolated from distinct metastatic nodules in the two mice were cultured, and we established four metastasis-prone sublines, termed M1-1, M1-2, M1-3, and M1-4. The sublines displayed more than 1.5 fold-increase in metastatic potential compared with parental SKOV3 cells (Figure 1B). We also found that cellular ceramide levels were significantly down-regulated in all metastasis-prone sublines compared with parental cells (Figure 1C). The profiles of individual ceramide species are shown in Supplementary data 1.
Figure 1. In vivo functional screening and characterization of metastasis-prone sublines.
(A) For in vivo functional screening, five million SKOV3 cells were inoculated into the peritoneal cavity of two nude mice. After 4 weeks, the mice were sacrificed and visible metastatic nodules (two metastatic nodules/mouse) were collected to establish daughter SKOV3 sublines from the nodules. Four sublines were established as metastasis-prone cells, named M1-1, M1-2, M1-3, and M1-4. (B) Parental SKOV3 or metastasis-prone cells (5 × 106 cells/mouse) were inoculated into the peritoneal cavity of nude mice (n = 5 or 6). The mice were sacrificed at 4 weeks after inoculation, and the number and extent of overt metastases (>1 mm) were quantified. (C) Lipids were extracted from parental SKOV3 or metastasis-prone cells and then ceramide contents were determined by mass spectrometry. (D) RNAs extracted from cells were submitted for RNAseq. Genes with altered expression in highly metastatic sublines are highlighted in the sphingolipid metabolic map. RNAs (E) or proteins (F) were extracted from cells and then quantitative real time PCR (n = 4) or immunoblotting using CerS antibody (n = 3) were performed, respectively.
To identify the key enzymes responsible for determining ceramide levels, we characterized the gene expression of the metastasis-prone cells relative to that of parental cells. RNAs were extracted from both cell lines and submitted for RNAseq. Among 36 genes encoding central sphingolipid metabolism-associated enzymes, expression of CerS2, ceramide transfer protein (CERT), SPTLC3 and LPP3 gene was significantly down-regulated in all metastasis-prone sublines, whereas UGT8 and SPHK1 genes were up-regulated (Figure 1D and Supplementary data 2).
Ceramide is synthesized by catalytic activities of CerS through the de novo or salvage pathways in the endoplasmic reticulum (ER) (22-24). Synthesized ceramides are transported via CERT to the Golgi, and metabolites are delivered to the plasma membranes. As CerS family proteins are rate-limiting enzymes for ceramide synthesis, CerS2 down-regulation may result in decreasing ceramide in metastasis-prone sublines.
Consistent with the RNAseq results, CerS2 protein and mRNA down-regulation was confirmed by immunoblotting with CerS2 antibody and quantitative real-time PCR (Figures 1E and 1F, Supplementary data 2).
Identification of CerS2 as an anti-metastatic gene in ovarian cancer cells.
To elucidate clinical significance of CerS2 mRNA expression in serous type ovarian cancer, an online analysis using ovarian cancer microarray datasets (Kaplan-Meier Plotter, http://www.kmplot.com) (25) was employed. Patients (p53 mutation-positive, serous type, stage 2-4) were split into two groups (with low and high gene expression). Overall survival periods and post-progression survival were plotted to generate Kaplan-Meier curves (Supplementary data 3). Though there was no significant difference in overall survival periods, the CerS2-high patient group showed longer post-progression survival than the CerS2-low group. Those results suggest a positive correlation of CerS2 mRNA expression in serous type ovarian cancer tissues with patient prognosis.
To experimentally evaluate the involvement of CerS2 down-regulation in promoting metastatic potential, the effects of CerS2 knock-down on ovarian cancer cell metastasis were examined. CerS2 knock-down by siRNA in parental SKOV3 cells (Figure 2A) significantly promoted cell metastasis in a xenograft model (Figure 2B), implicating CerS2 as a metastasis suppressor.
Figure 2. Inhibitory effects of CerS2 knock-down on the metastasis of ovarian cancer cells in a mouse model.
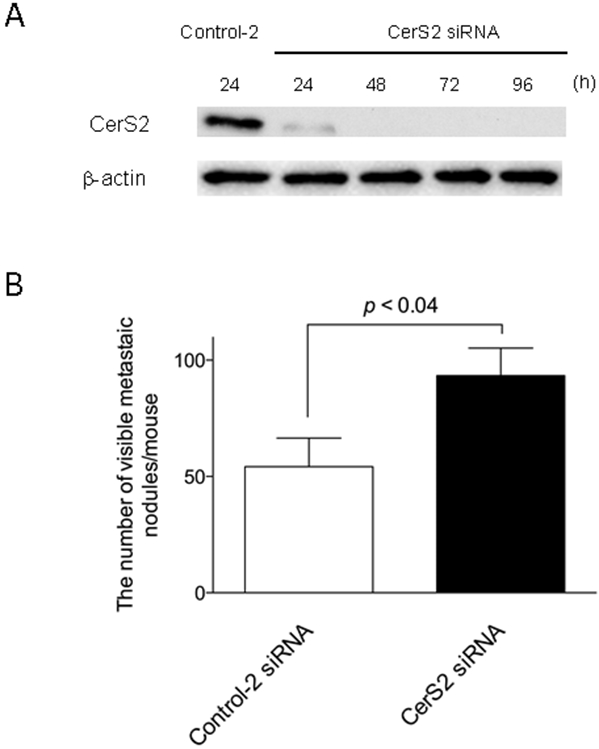
(A) SKOV3 cells were transfected with 5 nM control-2 or CerS2 siRNA for up to 96 h. Cell lysates were examined by immunoblotting using antibodies specific to β-actin or CerS2. (B) SKOV3 (5 × 106 cells / mouse) were transfected with 5 nM control-2 siRNAs (n = 11) or CerS2 siRNAs (n = 9) for 24 h and then inoculated into the peritoneal cavity of five-week-old female nude mice. Four weeks later, the mice were sacrificed and the number of metastatic nodules was determined.
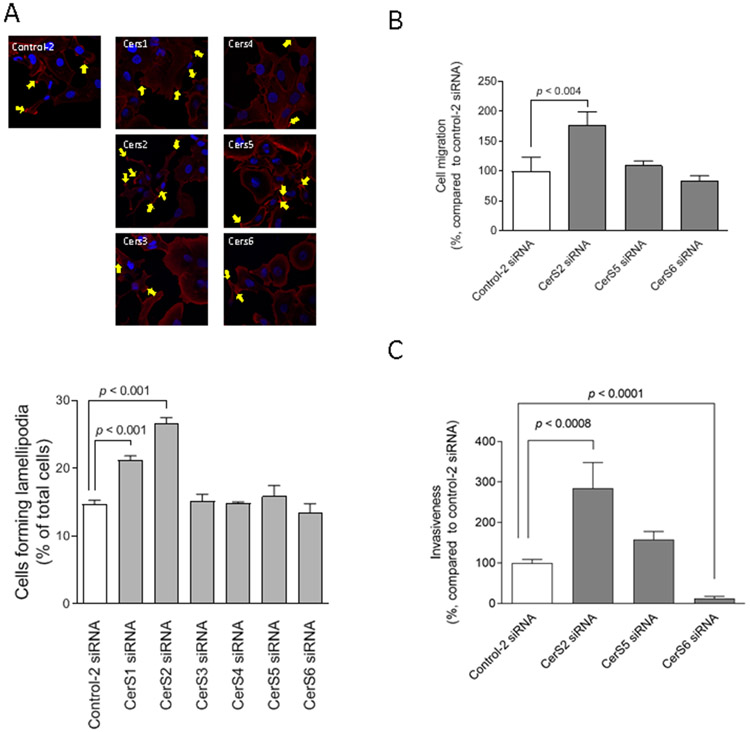
We next performed lamellipodia formation assay and Transwell cell migration assay to evaluate involvement of CerS2 in the regulation of cell motility. Among the CerS isoforms (23), CerS2 was revealed to play a predominant role in suppressing the formation of lamellipodia in experiments using cells transfected with CerS isoform siRNAs (Figures 3A). The knock-down effectiveness of siRNAs is shown in Supplementary data 4. CerS2 inhibition also significantly increased cell migration by 1.77-fold compared with the control siRNA (Figure 3B). Invasiveness was significantly increased in CerS2 knock-down cells (Figure 3C). These results suggest the involvement of CerS2 in suppressing the metastatic potential.
Figure 3. Predominant roles of CerS2 in the regulation of cell motility.
(A) SKOV3 cells (1 × 105 cells/35 mm glass-bottom dish) were transfected with 5 nM siRNAs for control-2 or distinct CerS isoforms for 48 h. Cells were fixed followed by staining with TRITC-conjugated phalloidin (red) and Hoechst 33342 (blue). The formation of lamellipodia was assessed as described in “Materials and Methods” and yellow arrows show lamellipodia. Imaging was performed by confocal microscopy, and representative images are shown. Quantified results (n = 3) are the percentages relative to cells treated with control-2 siRNAs. (B and C) SKOV3 cells were transfected with 5 nM siRNA for control-2, CerS2, CerS5, or CerS6 for 48 h and then examined in cell migration assay (B; n = 6) or cell invasion assay (C; n = 4 or 8) as described in “Materials and Methods.”.
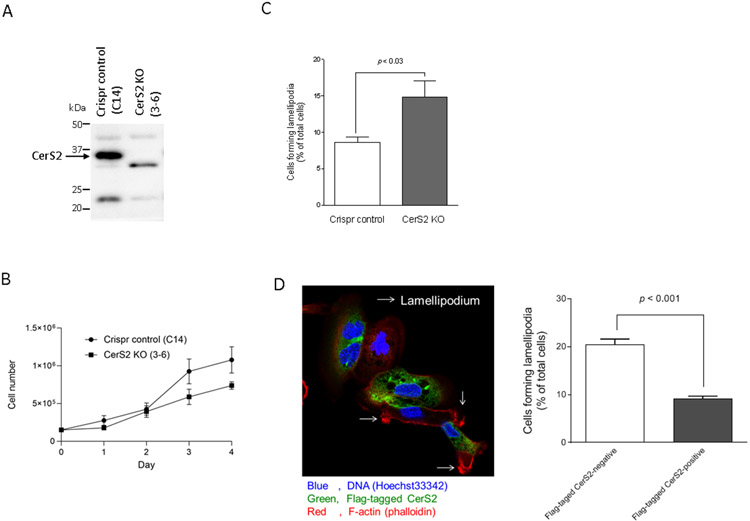
In addition to siRNA-mediated knock-down, we employed a CerS2 knockout approach to determine the specific involvement of CerS2 in the regulatory mechanisms of cell motility. We established CerS2 knockout SKOV3 cells (CerS2 KO (3-6)) using a CRISPR/Cas9 system (Figure 4A). CerS2 KO (3-6) cells grew as fast as that of Crispr control (C14) cells (Figure 4B). Importantly, CerS2 KO (3-6) cells showed an increased number of lamellipodia formation compared with control cells (Figure 4D). Moreover, we tested the effects of CerS2 knock-in on the formation of lamellipodia. Flag-tagged human CerS2 was overexpressed in CerS2 KO (3-6) cells, and then the lamellipodia formation was assessed. In Flag-tagged human CerS2 overexpression-negative or -positive cells, 20.5% or 9.1% of cells showed the lamellipodia-positive, respectively (Figures 4D). These results suggest that CerS2 limits cell motility as assessed by the formation of lamellipodia.
Figure 4. Effects of CerS2 knock-out and CerS2 knock-in on the formation of lamellipodia.
Cell lysates from CRISPR control (C14) and CerS KO (3-6) cells were examined by immunoblotting using antibodies specific to CerS2 (A). (B) CRISPR control (C14) and CerS KO (3-6) cells were plated on 6-well plates and incubated for up to 4 days. Cell numbers were determined by trypan blue staining (n = 6). (C) The formation of lamellipodia in CRISPR control (C14) and CerS KO (3-6) cells was assessed as described in “Materials and Methods” (n = 6). (D) CerS KO (3-6) cells were transfected with anti-Flag-tagged human CerS2 pCMVexSVneo vectors for 20 h. Cells were fixed followed by staining with Flag antibodies (Green), TRITC-conjugated phalloidin (red) and Hoechst 33342 (blue). Over 100 cells in each sample were assessed for negative- or positive-expression of Flag-tagged CerS2 and then the formation of lamellipodia was assessed as described in “Materials and Methods”. Data shown (mean ± standard error, n = 3) are the percentage of cells forming lamellipodia.
Roles of ceramide species in the formation of lamellipodia.
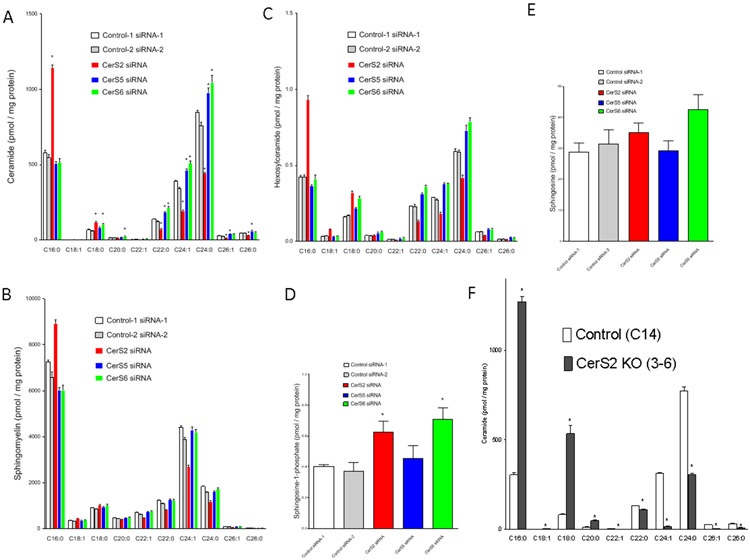
Recently, some studies have proposed specific roles for distinct ceramide species in cell pathobiology (9, 26, 27). We investigated the roles of ceramide species in the formation of lamellipodia. In CerSs-knocked down cells, the cellular sphingolipid profile for ceramides and their derivatives such as sphingomyelins and hexosylceramides were determined. Corresponding to biochemical characteristics of CerS as to acyl-CoA substrate specificity, CerS2 knock-down resulted in decreased very long-chain ceramides especially C24-ceramide and C24:1-ceramide and increased long chain ceramides such as C16-ceramide and C18-ceramide (Figure 5A). Acyl-chain composition of sphingomyelins (Figure 5B) and hexosyleramides (Figure 5C) also changed with trends similar to that of ceramides. Cellular contents of sphingosine-1-phosphate were significantly up-regulated in both CerS2- or CerS6-knocked down cells (Figure 5D), and neither knock-down of CerSs had no effects on sphingosine (Figure 5E). Moreover, CerS2 knockout down-regulated C24-ceramide and C24:1-ceramide and up-regulated C16-ceramide and C18-ceramide (Figure 5D).
Figure 5. Ceramide profiles in CerS2-downregulated cells.
(A-E) SKOV3 cells were transfected with 5 nM siRNA for control-2, CerS2, CerS5, or CerS6 for 48 h and then lipids were extracted. Lipid contents (ceramide, sphingomyelin, hexosylceramide, sphingosine-1-phosphate, and sphingosine) were determined by mass spectrometry (n = 4). (F) Lipids were extracted from CRISPR control (C14) and CerS KO (3-6) cells and then ceramide contents were determined by mass spectrometry (n = 3).
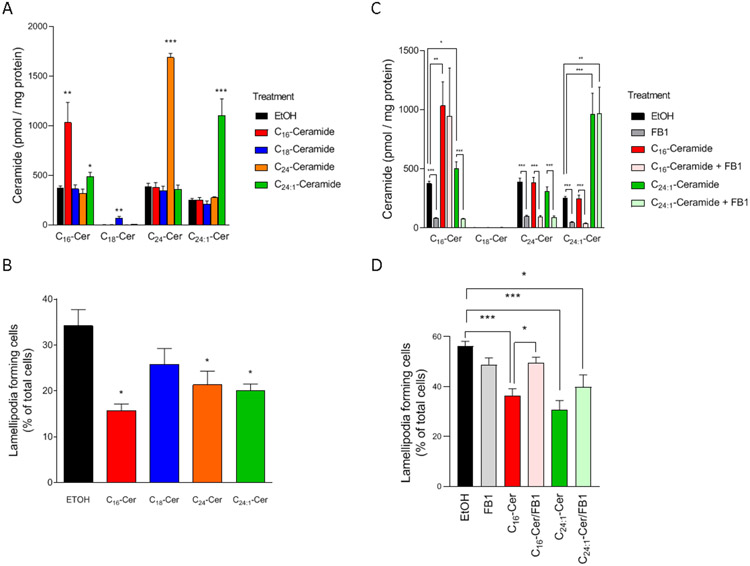
CerS2 inhibition by its gene knock-down or knockout resulted in: a) decreasing C24-ceramide and C24:1-ceramide, and b) increasing C16-ceramide and C18-ceramide (Figures 5A and 5D). Those results raised the question of whether specific ceramide species impacted the formation of lamellipodia. We examined the effects of individual ceramide species on the formation of lamellipodia. SKOV3 cells were treated with 5 μM natural ceramide species (C16-, C18-, C24-, and C24:1-ceramides) for 6 h, and then lamellipodia formation was assessed. As shown in Figure 6A, treatment with distinct ceramide species resulted in significant increases in its-counterpart ceramide species in cells, with little effects on the other major ceramide species although in C24:1-ceramide-treated cells, C16-ceramide was also moderately but significantly increased. Those results suggest exogenous very long-chain and long-chain ceramides are delivered to the cells and can serve as probes for the functions of individual ceramides. As shown in Figure 6B, ceramide species such as C16-ceramide, C24-ceramide, and C24:1-ceramide, significantly suppressed the formation of lamellipodia (Figure 6B).
Figure 6. Identification of ceramide species in regulating metastatic potential.
(A) SKOV3 cells (6 × 105 cells/10 cm dish) were treated with or without 5 μM ceramide species in the absence or presence of 100 μM fumonisin B1 (FB1) for 6 h. After lipid extraction, ceramide contents were determined (n=5-8). (B) SKOV3 cells (2 × 104 cells/35 mm glass-bottom dish) were treated with or without 5 μM ceramide species for 6 h. Cells were fixed followed by staining with TRITC-conjugated phalloidin and Hoechst 33342. The formation of lamellipodia was assessed as described in “Materials and Methods.” Data shown (mean ± standard error, n = 5) are the percentage of cells forming lamellipodia. (C, D) SKOV3 cells were treated with or without 5 μM ceramide species (C16-ceramide or C24:1-ceramide) in the absence or presence of 100 μM fumonisin B1 for 6 h. After lipid extraction, ceramide contents were determined by mass spectrometry (n = 6-10) (C). After cell fixation, cells were stained with TRITC-conjugated phalloidin and Hoechst 33342. The formation of lamellipodia was assessed as described in “Materials and Methods” (D). Data shown (n = 4) are the percentage of cells forming lamellipodia.
Exogenous short chain C6-ceramide has been shown to incorporate into cells undergo the recycling of its backbone sphingosine for forming sphingosine-1-phosphate or ceramide by the catalytic action of sphingosine kinase or ceramide synthase, respectively (13). In our previous studies using SKOV3 cells, C6-ceramide blocked the formation of lamellipodia, and inhibition of C6-ceramide recycling by fumonisin B1 (a ceramide synthase inhibitor) (28) partially restored the formation of lamellipodia (16). To assess the impact of exogenous ceramide recycling on the formation of lamellipodia, fumonisin B1 was employed to suppress the recycling of sphingosine backbone for forming ceramide. Inhibition of ceramide synthase by fumonisin B1 significantly decreased basal ceramide species (Figure 6C). In cells cotreated with fumonisin B1 and C24:1-ceramide, the increase in C16-ceramide was attenuated. Those results imply that fumonisin B1 blocked exogenous ceramide recycling.
In the presence of fumonisin B1, the inhibitory effects of C16-ceramide on the formation of lamellipodia were attenuated (Figure 6D). As its effects require diacylation/reacylation of exogenous C16-ceramide, these results argue that C16-ceramide per se is not inhibitory. Possibly, sphingosine backbone of C16-ceramide is recycled and converted to other ceramides that result in suppressing the formation of lamellipodia.
Importantly, the inhibitory effects of C24:1-ceramide remained in the presence of fumonisin B1 (Figure 6D). Those suggest that C24:1-ceramide per se serves as a suppressor in the formation of lamellipodia. However, the mechanisms by which other ceramide species including C18-ceramide and C24-ceramide had inhibitory effects on the cell motility remain fully undefined. Those results suggest that CerS2-C24:1-ceramide axis is inhibitory in the formation of lamellipodia.
Involvement of neutral CDase in the formation of lamellipodia
CerS2 predominantly forms C24-ceramide and C24:1-ceramide, which are also preferentially degraded by neutral CDase (29). The five human CDases are encoded by five different genes, and neutral CDase is encoded by ASAH2 (30-32). Previously, neutral CDase down-regulation was shown to increase C24-ceramide and C24:1-ceramide preferentially (29). Inhibition of neutral CDase by specific siRNA (Supplementary data 5A) significantly suppressed the formation of lamellipodia compared with controls (Figure 7A) and led to significant accumulation of C24:1-ceramide (Figure 7B). Reciprocally, enforced expression of neutral CDase (Supplementary data 5B) increased the formation of lamellipodia (Figure 7C). These results suggest that the CerS2-neutral CDase axis might regulate C24:1-ceramide, thereby modulating the formation of lamellipodia.
Figure 7. Involvement of neutral ceramidase in the regulation of cell motility.
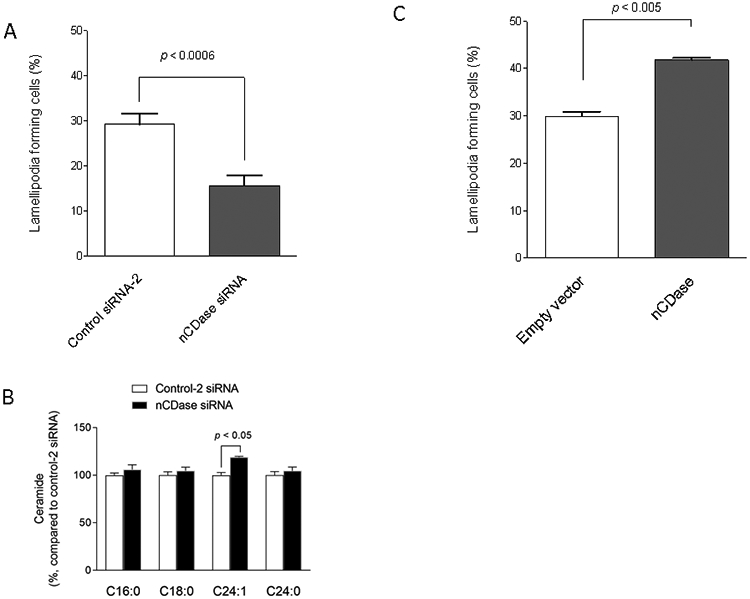
(A) SKOV3 cells were transfected with control-2 or neutral ceramidase (nCDase) siRNAs for 48 h. The formation of lamellipodia in the cells was assessed as described in “Materials and Methods” (n = 9 or 11). (B) Ceramide contents were determined (n = 3). (C) SKOV3 cells were transfected with 1 μg empty or human nCDase vectors for 24 h and then further cultured with G418-containing complete medium for 3 weeks, establishing empty vector or stably nCDase overexpressing cells, respectively. The formation of lamellipodia in the cells was assessed as described in “Materials and Methods” (n = 3).
Discussion
In this study, an in vivo selection-based approach identified CerS2 as a key regulatory enzyme that determines cellular ceramide as well as ceramide-mediated motility and metastatic potential. Previously, we have proposed that ceramide is an anti-metastatic lipid (16). We demonstrated the inhibitory effects of exogenous unnatural short chain ceramide on PI3KC2β-mediated in vivo metastasis and in vitro cell motility as assessed by lamellipodia formation in multiple ovarian and breast cancer cell lines (16). The present study newly implicates the CerS2-C24:1-ceramide pathway in limiting the formation of lamellipodia and the associated metastatic potential in ovarian cancer cells.
Currently, there is little information about the clinical significance of CerS2 in cancer progression. Fan et al. showed that higher CerS2 expression in patients with breast cancer was associated with fewer lymph node metastases (33). In contrast, Sheng et al. showed an association of high expression of CerS2 with unfavorable prognosis in patients with ovarian cancer (34). Epithelial ovarian cancer is histologically classified into endometrioid, mucinous, clear cell, and serous subtypes. Among these histological subtypes, the serous subtype is genetically unstable and highly metastatic (35). In our online analysis of the database, low level of CerS-2 mRNA expression was indicative of a poor prognosis in patients with p53-mutated serous subtype ovarian cancer (Supplementary data 3). In light of SKOV3 known as a serous subtype, p53-mutant cell line (36), our experimental findings are thought to associate with our clinical findings in cancer progression. Therefore, our current findings provide specific insights into understanding the cellular and hence clinical significance of CerS2 in metastatic ovarian cancer.
Metastasis is a highly inefficient process (37). In our metastasis model, only a few hundred metastatic nodules developed in the mesentery of mouse, even though 5 million ovarian cancer cells were inoculated into the peritoneal cavity (Figure 1). Metastasis in our mouse model also seems to be inefficient, thereby conversely enabling our functional screening to be highly effective for isolating metastasis-prone cancer cells. Our in vivo metastatic cell selection and subsequent molecular characterization of metastasis-prone sublines along with genetic approaches successfully identified CerS2 that limits metastatic potential in ovarian cancer cells.
Previously, we have demonstrated that PI3KC2β is predominantly responsible for the formation of lamellipodia in SKOV3 cells and that short chain C6-ceramide limits the formation of lamellipodia by interacting with the catalytic domain of PI3KC2β (16). The molecular recognition between C24:1-ceramide and PI3KC2β is under investigation. Those studies are believed to gain a better understanding of the regulatory system of cell motility and cancer metastasis. Moreover, intensive studies are also required for defining if C18-ceramide and C24-ceramide are inhibitory in lamellipodia formation.
CerS2 knock-down potentiated the migration of ovarian cancer cells along with C16-ceramide up-regulation and C24:1-ceramide down-regulation (Figures 3B and 5A). Since efficient migration on adhesive surfaces are believed to involve the protrusion of lamellipodial actin networks (38), the decreases of C24:1-ceramide with CerS2 down-regulation were probably associated with promoting cell migration. Additionally, sphingosine-1-phosphate known as a stimulatory lipid in migration (39-41) needs to be considered, because CerS2 down-regulation led to sphingosine-1-phosphate increases (Figure 5D).
Invasiveness also plays a key role in promoting metastatic potential. Previous studies demonstrated that CerS2 suppressed tumor invasiveness by inhibiting the vacuolar-H+-ATPase (V-ATPase) proton pump that acidifies the tumor microenvironment and promotes cancer progression (42). Mechanistically, CerS2 was shown to interact with the c subunit of the V-ATPase and inhibit its function (43), probably independently of the CerS2-ceramide axis. CerS2 appears to thus control cancer metastasis via its direct interaction with other cellular factors and/or regulation of its metabolic product ceramide.
Shi et al. (44) showed that CerS6, which predominantly forms C16-ceramide, is stimulatory in cell migration and invasiveness of serous type ovarian cancer cells. The results as to invasiveness, not migration, are consistent with ours (Figure 3C). Very recently, Canals et al. demonstrated that acute formation of plasma membrane ceramides by bacterial SMase was stimulatory in the migration of HeLa cells (21). It should be noted that in this context, C16-ceramide is a major ceramide species, although this study did not focus on defining the function of specific molecular species of ceramide but focused on the action of ‘ceramides’ in one defined compartment (the plasma membrane). Consistent with these studies invoking a distinct action of C16-ceramide, CerS6 down-regulation was shown to reduce plasma membrane fluidity and prevent cell migration of breast cancer cells. To begin to reconcile these differences, the Many Ceramides hypothesis needs to be considered: “individual ceramide molecular species are regulated by specific biochemical pathways in distinct subcellular compartments and execute distinct functions” (Yusuf A. Hannun and Lina M. Obeid, 2011, Journal of Biological Chemistry, 286, 27855-27862) (26). Indeed, extensive studies have uncovered specific biological roles of ceramide species including C16-ceramide (27, 45-48), C18-ceramide (49), and C24-ceramide (9). Therefore, this study discloses a function for C24:1 ceramide that is quite distinct from that of C16-ceramide, consistent with the Many Ceramides hypothesis.
Our results showed that neutral CDase is also involved in the formation of lamellipodia by regulating ceramide metabolism. CerS2 and neutral CDase activities have opposite effects on the formation of lamellipodia by modulating the levels of specific ceramide species; both enzymes metabolize ceramides directly and maintain sphingolipid homeostasis. This reciprocal action of CerS2 and neutral CDase might play an important role in the regulation of cell motility and metastatic potential of cancer cells.
In light of the results showing that ceramide is an anti-metastatic lipid, down-regulation of ceramide may be a contributing factor to cancer progression. Hence, ceramide-based therapies have been developed and tested in a preclinical study of multiple types of cancer (12, 14, 50-54). Multiple laboratories have been employing nanotechnologies to improve the therapeutic efficacy of ceramides (50, 52, 55). The therapeutic efficacy of ceramide nanoliposomes for cancer progression has been validated in multiple preclinical studies, and published preclinical studies have supported an FDA phase 1 first-in-man-dose escalation study (51, 56). In our preclinical studies, ceramide nanoliposomes suppressed metastasis in a SKOV3 ovarian cancer cell xenograft model (16, 57). The present study demonstrating that downregulation of CerS2-C24:1-ceramide axis is responsible for potentiating metastatic potential may support further development of ceramide-based therapy for metastatic ovarian cancer.
In conclusion, the CerS2-C24:1-ceramide axis is a determinant that limits ovarian cancer metastasis. Thus, the current findings provide insight into ovarian cancer biology and metastasis and may lead to further development of biomarkers and cancer therapy.
Supplementary Material
Acknowledgments
We thank the laboratory members of the Biomedical Research Unit of Tohoku University Hospital and Department of Obstetrics and Gynecology (Tohoku University, Sendai, Japan) for critical discussion. We also thank Dr. Hideo Ogiso and Janet Allopenna for the lipid measurement by LC-MS/MS. This study was supported in part by JSPS KAKENHI Grants (16K11125 to K.K.) and National Institutes of Health grants (CA218678 to Y.A.H.). We thank Gabrielle White Wolf, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Abbreviations:
- CerS
ceramide synthase
- CERT
ceramide transfer protein
- CDase
ceramidase
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- PIP4K2
phosphatidylinositol 5-phosphate 4-kinase type-2
- PI3K
phosphatidyl-3-kinase
- PI3KC2β
class II PI3K β
- SMase
sphingomyelinase
- TRITC
tetramethylrhodamine isothiocyanate
- V-ATPase
vacuolar-H+-ATPase
Footnotes
Disclosure of Potential Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Lengyel E (2010) Ovarian cancer development and metastasis. Am J Pathol 177, 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bast RC Jr., Hennessy B, and Mills GB (2009) The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 9, 415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naora H, and Montell DJ (2005) Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer 5, 355–366 [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki D, Kurisu S, and Takenawa T (2005) Regulation of cancer cell motility through actin reorganization. Cancer science 96, 379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson MF, and Sahai E (2009) The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis 26, 273–287 [DOI] [PubMed] [Google Scholar]
- 6.Wells A, Grahovac J, Wheeler S, Ma B, and Lauffenburger D (2013) Targeting tumor cell motility as a strategy against invasion and metastasis. Trends in pharmacological sciences 34, 283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maffucci T, Cooke FT, Foster FM, Traer CJ, Fry MJ, and Falasca M (2005) Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. The Journal of cell biology 169, 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulluni F, De Santis MC, Margaria JP, Martini M, and Hirsch E (2019) Class II PI3K Functions in Cell Biology and Disease. Trends Cell Biol 29, 339–359 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang H, Chen T, Wang H, Liang X, Zhang Y, Duan J, Qian S, Qiao K, Zhang L, Liu Y, and Wang J (2020) C24 -Ceramide Drives Gallbladder Cancer Progression through Directly Targeting PIP4K2C to Facilitate mTOR Signaling Activation. Hepatology [DOI] [PubMed] [Google Scholar]
- 10.Hannun YA (1996) Functions of ceramide in coordinating cellular responses to stress. Science 274, 1855–1859 [DOI] [PubMed] [Google Scholar]
- 11.Ogretmen B, and Hannun YA (2004) Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 4, 604–616 [DOI] [PubMed] [Google Scholar]
- 12.Morad SA, and Cabot MC (2013) Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 13, 51–65 [DOI] [PubMed] [Google Scholar]
- 13.Kitatani K, Idkowiak-Baldys J, and Hannun YA (2008) The sphingolipid salvage pathway in ceramide metabolism and signaling. Cellular signalling 20, 1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kester M, and Kolesnick R (2003) Sphingolipids as therapeutics. Pharmacological research : the official journal of the Italian Pharmacological Society 47, 365–371 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Matsuda M, Yaegashi N, Nabe T, and Kitatani K (2020) Regulation of Necroptosis by Phospholipids and Sphingolipids. Cells 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitatani K, Usui T, Sriraman SK, Toyoshima M, Ishibashi M, Shigeta S, Nagase S, Sakamoto M, Ogiso H, Okazaki T, Hannun YA, Torchilin VP, and Yaegashi N (2016) Ceramide limits phosphatidylinositol-3-kinase C2beta-controlled cell motility in ovarian cancer: potential of ceramide as a metastasis-suppressor lipid. Oncogene 35, 2801–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaji T, and Hanada K (2015) Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic 16, 101–122 [DOI] [PubMed] [Google Scholar]
- 18.Hwang YH, Tani M, Nakagawa T, Okino N, and Ito M (2005) Subcellular localization of human neutral ceramidase expressed in HEK293 cells. Biochem Biophys Res Commun 331, 37–42 [DOI] [PubMed] [Google Scholar]
- 19.Sivan G, Ormanoglu P, Buehler EC, Martin SE, and Moss B (2015) Identification of Restriction Factors by Human Genome-Wide RNA Interference Screening of Viral Host Range Mutants Exemplified by Discovery of SAMD9 and WDR6 as Inhibitors of the Vaccinia Virus K1L-C7L- Mutant. MBio 6, e01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogiso H, Taniguchi M, Araya S, Aoki S, Wardhani LO, Yamashita Y, Ueda Y, and Okazaki T (2014) Comparative Analysis of Biological Sphingolipids with Glycerophospholipids and Diacylglycerol by LC-MS/MS. Metabolites 4, 98–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canals D, Salamone S, Santacreu BJ, Nemeth E, Aguilar D, Hernandez-Corbacho MJ, Adada M, Staquicini DI, Arap W, Pasqualini R, Haley J, Obeid LM, and Hannun YA (2020) Ceramide launches an acute anti-adhesion pro-migration cell signaling program in response to chemotherapy. FASEB J 34, 7610–7630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen TD, Hannun YA, and Obeid LM (2012) Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J 441, 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiban J, Tidhar R, and Futerman AH (2010) Ceramide synthases: roles in cell physiology and signaling. Adv Exp Med Biol 688, 60–71 [DOI] [PubMed] [Google Scholar]
- 24.Mizutani Y, Mitsutake S, Tsuji K, Kihara A, and Igarashi Y (2009) Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie 91, 784–790 [DOI] [PubMed] [Google Scholar]
- 25.Nagy A, Lanczky A, Menyhart O, and Gyorffy B (2018) Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep 8, 9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannun YA, and Obeid LM (2011) Many ceramides. J Biol Chem 286, 27855–27862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmond V, Dufour F, Poiroux G, Shoji K, Malleter M, Fouque A, Tauzin S, Rimokh R, Sergent O, Penna A, Dupuy A, Levade T, Theret N, Micheau O, Segui B, and Legembre P (2015) Downregulation of ceramide synthase-6 during epithelial-to-mesenchymal transition reduces plasma membrane fluidity and cancer cell motility. Oncogene 34, 996–1005 [DOI] [PubMed] [Google Scholar]
- 28.Merrill AH Jr., van Echten G, Wang E, and Sandhoff K (1993) Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J Biol Chem 268, 27299–27306 [PubMed] [Google Scholar]
- 29.Wu BX, Zeidan YH, and Hannun YA (2009) Downregulation of neutral ceramidase by gemcitabine: Implications for cell cycle regulation. Biochim Biophys Acta 1791, 730–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito M, Okino N, and Tani M (2014) New insight into the structure, reaction mechanism, and biological functions of neutral ceramidase. Biochim Biophys Acta 1841, 682–691 [DOI] [PubMed] [Google Scholar]
- 31.Coant N, and Hannun YA (2019) Neutral ceramidase: Advances in mechanisms, cell regulation, and roles in cancer. Adv Biol Regul 71, 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coant N, Sakamoto W, Mao C, and Hannun YA (2017) Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul 63, 122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan S, Niu Y, Tan N, Wu Z, Wang Y, You H, Ke R, Song J, Shen Q, Wang W, Yao G, Shu H, Lin H, Yao M, Zhang Z, Gu J, and Qin W (2013) LASS2 enhances chemosensitivity of breast cancer by counteracting acidic tumor microenvironment through inhibiting activity of V-ATPase proton pump. Oncogene 32, 1682–1690 [DOI] [PubMed] [Google Scholar]
- 34.Sheng N, Wang Y, Xie Y, Chen S, Lu J, Zhang Z, Li M, Shan Q, Wu D, Zheng G, Zheng Y, and Fan S (2019) High expression of LASS2 is associated with unfavorable prognosis in patients with ovarian cancer. J Cell Physiol 234, 13001–13013 [DOI] [PubMed] [Google Scholar]
- 35.Lengyel E (2010) Ovarian cancer development and metastasis. Am J Pathol 177, 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaginuma Y, and Westphal H (1992) Abnormal structure and expression of the p53 gene in human ovarian carcinoma cell lines. Cancer Res 52, 4196–4199 [PubMed] [Google Scholar]
- 37.Valastyan S, and Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dimchev G, Amiri B, Humphries AC, Schaks M, Dimchev V, Stradal TEB, Faix J, Krause M, Way M, Falcke M, and Rottner K (2020) Lamellipodin tunes cell migration by stabilizing protrusions and promoting adhesion formation. J Cell Sci 133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takabe K, and Spiegel S (2014) Export of sphingosine-1-phosphate and cancer progression. J Lipid Res 55, 1839–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anelli V, Gault CR, Snider AJ, and Obeid LM (2010) Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J 24, 2727–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stepanovska B, and Huwiler A (2020) Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res 154, 104170. [DOI] [PubMed] [Google Scholar]
- 42.Fan SH, Wang YY, Lu J, Zheng YL, Wu DM, Zhang ZF, Shan Q, Hu B, Li MQ, and Cheng W (2015) CERS2 suppresses tumor cell invasion and is associated with decreased V-ATPase and MMP-2/MMP-9 activities in breast cancer. J Cell Biochem 116, 502–513 [DOI] [PubMed] [Google Scholar]
- 43.Pan H, Qin WX, Huo KK, Wan DF, Yu Y, Xu ZG, Hu QD, Gu KT, Zhou XM, Jiang HQ, Zhang PP, Huang Y, Li YY, and Gu JR (2001) Cloning, mapping, and characterization of a human homologue of the yeast longevity assurance gene LAG1. Genomics 77, 58–64 [DOI] [PubMed] [Google Scholar]
- 44.Shi Y, Zhou C, Lu H, Cui X, Li J, Jiang S, Zhang H, and Zhang R (2020) Ceramide synthase 6 predicts poor prognosis and activates the AKT/mTOR/4EBP1 pathway in high-grade serous ovarian cancer. Am J Transl Res 12, 5924–5939 [PMC free article] [PubMed] [Google Scholar]
- 45.Stiban J, and Perera M (2015) Very long chain ceramides interfere with C16-ceramide-induced channel formation: A plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim Biophys Acta 1848, 561–567 [DOI] [PubMed] [Google Scholar]
- 46.Lu P, White-Gilbertson S, Nganga R, Kester M, and Voelkel-Johnson C (2019) Expression of the SNAI2 transcriptional repressor is regulated by C16-ceramide. Cancer biology & therapy 20, 922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim MH, Park JW, Lee EJ, Kim S, Shin SH, Ahn JH, Jung Y, Park I, and Park WJ (2018) C16-ceramide and sphingosine 1-phosphate/S1PR2 have opposite effects on cell growth through mTOR signaling pathway regulation. Oncol Rep 40, 2977–2987 [DOI] [PubMed] [Google Scholar]
- 48.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, and Ogretmen B (2010) Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J 24, 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Senkal CE, Ponnusamy S, Rossi MJ, Bialewski J, Sinha D, Jiang JC, Jazwinski SM, Hannun YA, and Ogretmen B (2007) Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther 6, 712–722 [DOI] [PubMed] [Google Scholar]
- 50.Koshkaryev A, Piroyan A, and Torchilin VP (2012) Increased apoptosis in cancer cells in vitro and in vivo by ceramides in transferrin-modified liposomes. Cancer biology & therapy 13, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kester M, Bassler J, Fox TE, Carter CJ, Davidson JA, and Parette MR (2015) Preclinical development of a C6-ceramide NanoLiposome, a novel sphingolipid therapeutic. Biological chemistry 396, 737–747 [DOI] [PubMed] [Google Scholar]
- 52.Watters RJ, Kester M, Tran MA, Loughran TP Jr., and Liu X (2012) Development and use of ceramide nanoliposomes in cancer. Methods in enzymology 508, 89–108 [DOI] [PubMed] [Google Scholar]
- 53.Ogretmen B (2018) Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 18, 33–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barth BM, Cabot MC, and Kester M (2011) Ceramide-based therapeutics for the treatment of cancer. Anticancer Agents Med Chem 11, 911–919 [DOI] [PubMed] [Google Scholar]
- 55.van Vlerken LE, Duan Z, Seiden MV, and Amiji MM (2007) Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res 67, 4843–4850 [DOI] [PubMed] [Google Scholar]
- 56.Tagaram HR, Divittore NA, Barth BM, Kaiser JM, Avella D, Kimchi ET, Jiang Y, Isom HC, Kester M, and Staveley-O’Carroll KF (2011) Nanoliposomal ceramide prevents in vivo growth of hepatocellular carcinoma. Gut 60, 695–701 [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Kitatani K, Toyoshima M, Ishibashi M, Usui T, Minato J, Egiz M, Shigeta S, Fox T, Deering T, Kester M, and Yaegashi N (2018) Ceramide Nanoliposomes as a MLKL-Dependent, Necroptosis-Inducing, Chemotherapeutic Reagent in Ovarian Cancer. Mol Cancer Ther 17, 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.