Abstract
Coronavirus disease 2019 (COVID‐19), caused by coronavirus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has caused extensive disruption and mortality since its recent emergence. Concomitantly, there has been a race to understand the virus and its pathophysiology. The clinical manifestations of COVID‐19 are manifold and not restricted to the respiratory tract. Extrapulmonary manifestations involving the gastrointestinal tract, hepatobiliary system, cardiovascular and renal systems have been widely reported. However, the pathophysiology of many of these manifestations is controversial with questionable support for direct viral invasion and an abundance of alternative explanations such as pre‐existing medical conditions and critical illness. Prior research on SARS‐Co‐V and NL63 was rapidly leveraged to identify angiotensin‐converting enzyme 2 (ACE2) receptor as the key cell surface receptor for SARS‐CoV‐2. The distribution of ACE2 has been used as a starting point for estimating vulnerability of various tissue types to SARS‐CoV‐2 infection. Sophisticated organoid and animal models have been used to demonstrate such infectivity of extrapulmonary tissues in vitro, but the clinical relevance of these findings remains uncertain. Clinical autopsy studies are typically small and inevitably biased towards patients with severe COVID‐19 and prolonged hospitalization. Technical issues such as delay between time of death and autopsy, use of inappropriate antibodies for paraffin‐embedded tissue sections and misinterpretation of cellular structures as virus particles on electron micrograph images are additional problems encountered in the extant literature. Given that SARS‐CoV‐2 is likely to circulate permanently in human populations, there is no doubt that further work is required to clarify the pathobiology of COVID‐19.
Keywords: COVID‐19, pathophysiology, SARS‐CoV‐2, transmission
INTRODUCTION
The spread of coronavirus disease 2019 (COVID‐19) across the world has led to an explosion of publications related to COVID‐19. Over 65% of these publications were however not based on original data (i.e., viewpoints, editorials, perspectives or expert opinion), with original studies (14.9%), case reports (9.3%) and research letters (10%) comprising the remainder. 1 Sixty percent of published articles have been posted on preprint servers, which have the advantage of easy access, easy feedback and fast dissemination, 2 but this increase in publication has also been associated with increased numbers of articles retracted. Of the top 50 cited publications, there are two related to the clinicopathological aspects of this review—the detection of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in different specimens and the lung pathophysiology of fatal COVID‐19. 3 , 4
The intention of this review is to summarize and consolidate the clinical and pathological changes seen in COVID‐19; however, one should be mindful that most publications have dealt with hospitalized patients. This is important because this population as a whole has varied admission rates depending on regional, societal, seasonal and political factors, and thus much of what is reported in the medical literature is but the tip of the clinical COVID‐19 iceberg.
Another challenge with performing a review is that most of the accessed articles in December to February 2021 were published in a timeframe based on data collated and obtained from the first ‘wave’ of the pandemic. Since the emergence of the ‘UK’, ‘South African’ or ‘Indian’ variants of SARS‐CoV‐2, it remains to be seen to what extent the putative organ dissemination and pathophysiology of the original strain reviewed in most of these publications will be seen in 2021.
PORTALS OF ENTRY
Nasal and oral
The seasonal coronaviruses that are ubiquitous in the general population are associated with upper respiratory tract and nasal symptoms, so it is not surprising that this anatomical site is one of the main portals of entry of coronavirus into the body; however, one of the features that distinguishes COVID‐19 from other seasonal coronaviruses has been the relative lack of typical nasal symptoms, such as rhinitis and sneezing, but in contrast to SARS and Middle East respiratory syndrome (MERS) infection, there is a high frequency of anosmia, implying involvement of the olfactory epithelium. 5
The viral dynamics of COVID‐19 in the nasal mucosa will be detailed elsewhere 6 but in general the infected individual can be asymptomatic for up to 5 days after infection, with a high viral load and infectivity in this period. There is a peak at days 5–7 post onset of symptoms. 7 After day 15, the probability of culturing live virus in severe and critically ill or immunocompromised patients is less than 5%, but there may be prolonged shedding in individuals who are of older age, and, or, have medical comorbidities, immunosuppressive conditions, severe disease, delayed hospitalization and are managed with steroids. 8
The high viral load in the pre‐symptomatic phase is in contrast to SARS, and has presented one of the major challenges in reducing and mitigating transmission of viruses from individual to individual. The high frequency of anosmia indicates that viral replication involves the olfactory mucosa, and as this anatomical site is more posterior in the nose, it means that rapid antigen testing will need a more intrusive sampling than just swabbing the anterior nasal cavity. Viral loads are higher in nasal swabs than throat swabs. 9 However, certain nasal pathology such as polyps or deviated nasal septum can lead to a false‐negative result, thereby creating an additional challenge for pathologists. The main surface receptor mediating SARS‐CoV‐2 cell entry is angiotensin‐converting enzyme 2 (ACE2) and the serine protease transmembrane protease, serine 2 (TMPRSS2) is important for priming the viral spike protein. As such, expression of ACE2 and TMPRSS2 in various tissues is often taken as evidence that particular organs can potentially support SARS‐CoV‐2 replication. In an unsolicited review, 10 a high expression of ACE2 and TMPRSS2 was detected in the nasal epithelial cells, and also appeared to be in high expression on the tongue. In the oral cavity, there is a paucity of definitive clinical symptoms with taste alterations, blisters, ulcers and Kawasaki disease reported. 11 A large number of studies have proposed the salivary glands could be potential reservoirs for SARS‐CoV‐2 based on immunohistochemical detection of ACE2 12 , 13 , 14 , 15 , 16 , 17 or the presence of sialadenitis in early stages of COVID‐19 infection. 15 Presently, there are no data to indicate that virus replication can be detected in this site, and ex vivo studies of human explants did not show infection of minor salivary glands. 18
Within the olfactory mucosa, there has been much attention focused on the sustentacular cells as a target for viral entry. 19 ACE2 has been identified on the motile cilia of the airway epithelial cells, 20 but the use of angiotensin‐converting enzyme inhibitors or receptor blockers did not increase susceptibility to infection in humans. The direct infection of the epithelium by the virus has been blamed for the loss of smell, 21 and in 25% of patients this anosmia may fail to resolve. 9 In the laboratory setting, despite a large concentration of viral antigen in the olfactory mucosa of hamsters, 22 there is no involvement of the olfactory nerve as a potential route of transmission to the central nervous system. In hACE2 transgenic mice infected with SARS, there was involvement of the olfactory bulb, but this was not seen in infection with SARS‐CoV‐2, even though infection of the sustentacular cells was documented.
Ocular
The importance of the eye as a site for viral entry, spread to the upper respiratory tract via the lacrimal duct or transmission to other individuals has been the subject of a number of well‐written and comprehensive reviews. 23 , 24 , 25 , 26 Ocular involvement of other respiratory viruses, including the seasonal coronaviruses has been well documented, 27 but there have been conflicting results on whether the conjunctiva or other ocular tissues express ACE2. 28 , 29 , 30 Patients with clinical COVID‐19 have presented with folliculitis, keratoconjunctivitis, ocular pain and discharge, 31 but these are thought to reflect a publication bias. Ex vivo conjunctival tissues were reported to be susceptible to infection 18 ; however, it was commented in a reply to this article that the study was an artificial laboratory setting which may not exist in the in vivo setting. Sampling of tear fluid for virus is reportedly unreliable. 26 The conclusion from most of these reviews is that the ocular route of entry is low, with insufficient evidence to provide a conclusive statement on this portal. Despite this low potential risk, professional bodies such as the American Academy of Ophthalmology advise the use of goggles and face shields. 27 In fact, the use of face shields has become widespread clinically.
Respiratory
At the beginning of the COVID outbreak in 2020, the main concern was how severe this emerging disease was. In particular, would this outbreak have the same manifestation and pathology to that seen in two of the three recently described coronavirus infections in the past 20 years—SARS and MERS, or follow a similar pattern to the third emerging coronavirus NL63, with mild respiratory disease? Initially, it was thought that infection was limited to the respiratory tract, but with the rapidly increasing number of cases, it became evident that this was a respiratory virus with multi‐system complications (Figure 1) As lung biopsies were rarely performed in the SARS outbreak, the respiratory pathology was based on post‐mortem/autopsy material. Three chronological histological patterns were thus identified: an exudative phase characterized by hyaline membrane formation and pulmonary oedema, followed by a proliferative phase of Type II pneumocyte hyperplasia, with or without giant cell formation, then an organizing phase of fibrosis and vascular proliferation, together with squamous metaplasia in the conducting airways. 32 As the standard treatment in many institutions in the SARS outbreak was the use of steroids to reduce the ‘cytokine storm’, patients with prolonged disease had secondary bacterial or fungal infection.
FIGURE 1.
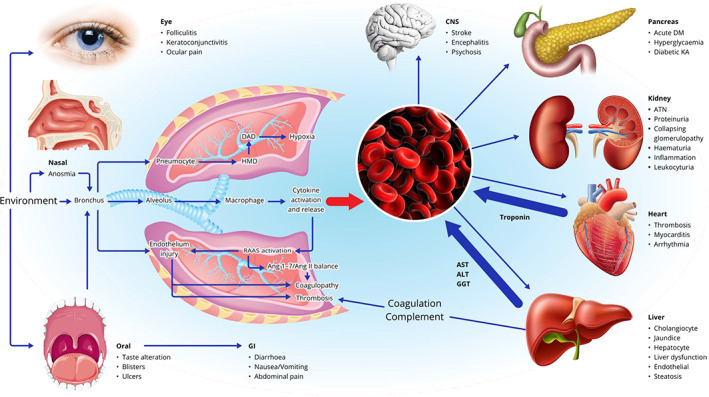
Simplified schematic of proposed pathological changes in severe acute respiratory syndrome coronavirus 2 infection. The three main portals of entry into the respiratory tract are through the eye, nasal cavity and oral route, with the latter also leading to infection of the gastrointestinal tract. In the respiratory tract, infection of pneumocytes leads to exudation of fibrinogen and hyaline membrane formation, followed by diffuse alveolar damage with hypoxia. Stimulation of macrophages and bronchiolar epithelial damage causes cytokine release into the alveolar spaces and into the blood. Either virus infection of endothelium or cytokine release activates the renin–angiotensin–aldosterone system, producing a pro‐thrombotic tendency, with the formation of thrombi, mainly in the pulmonary vasculature. Either viraemia or cytokinaemia in the systemic circulation damages the brain, pancreas, kidneys, heart and liver producing a number of organ‐specific changes, in addition to the increased thrombotic tendency. The multi‐system damage is manifest by elevated troponin and liver enzymes in the blood, and the release of factors aggravates the pro‐thrombotic tendency
Once antibodies to SARS‐CoV nucleoprotein were available, these demonstrated antigen in the first 14 days, but little evidence of positive staining after that, indicating that the proliferative phase and organizing phase were secondary to viral‐induced pneumocyte damage. 33 Review of the respiratory pathology caused by H1N1, H2N2, H7N9 and H5N1 influenza viruses showed that there were similar morphological changes in the lung in these conditions to that seen in SARS. Thus, it was proposed that there were no unique morphological changes seen in SARS infection compared to severe influenza, and that what was seen was a similar pattern of cellular damage followed by tissue repair. 34
The first reports of COVID lung pathology appeared in February and March 2020 in two publications involving seven patients, 35 but these were based on limited autopsy material. These reports confirmed that the early changes were very similar to SARS, with the presence of hyaline membranes in the alveolar spaces with a variable degree of oedema present.
As the pandemic spread to involve more countries and the long‐term temporal course of disease was better appreciated, there were seven autopsy publications in April 2020, and 12 more in May, in total involving 97 patients, where the early changes of oedema and diffuse alveolar damage progressed to an organizing pneumonia and repair within the lung. 35 However, in this period, two features emerged which would be different from that reported in SARS. The first was that, owing to the lessons learned from the SARS outbreak, there was less use of steroid treatment, and so secondary opportunistic infection was not as prevalent. The second, and probably more important feature from a clinical point of view, was a reporting of the presence of fibrin and organizing thrombi in the vessels in the pulmonary vasculature, 36 raising the possibility that there was damage to the pulmonary endothelium, with the pulmonary thromboemboli detected in a number of post‐mortem and antemortem cases. 37 , 38 , 39 This thromboembolism was noted in the SARS cases, but was not as pronounced as that seen in COVID infection, possibly because of the limited numbers of fatalities with autopsies performed in SARS. 35 Once antibodies that were reliable for formalin‐fixed tissues became available, studies performed by the US Centers for Disease Control and Prevention 40 and National Institutes of Health 41 showed positive staining in alveolar epithelium, hyaline membranes and epithelial cells in the conducting airways. Although co‐localization of viral antigen with the endothelial marker CD31 has been reported, detection of virus in endothelial cells has been challenging, with occasional reports showing co‐localization of viral antigen with endothelial markers, but in these positive areas there is no thrombosis or vascular damage noted. 42 , 43
The lung pathophysiology model which was developed after SARS proposed that Type I pneumocytes would become infected and damaged, leading to a loss of the integrity of the basal layer, fibrin exudation and hyaline membrane formation. The Type II pneumocytes would then differentiate to replace the Type I pneumocytes. Since that time, there has been the emergence of new concepts of lung repair and regeneration, where even though the Type II pneumocytes may replace damaged Type I pneumocytes, there exists a population of Krt‐5‐positive basal cells which may also replace the Type I pneumocytes. 44 , 45 If the SARS paradigm is relevant for COVID‐19, then the initiating event would therefore be demonstration of viral antigen in Type I pneumocytes. Unfortunately, antibodies that are definitive for Type I pneumocytes rather than Type II pneumocytes (such as caveolin) have not been routinely used in the autopsy studies for COVID infection, and thus many publications have based tropism on cellular morphology combined with immunohistochemistry (IHC)/ISH‐positive cells. One publication showed co‐localization of SARS‐CoV‐2 antigen with Thyroid transcription factor 1 (a marker of Type II pneumocytes and club cells) and also in macrophages of the lung, in p63‐positive basal cells and ciliated cells, but not MUC5AC‐positive mucus‐secreting cells. 46 In this study, there was no report of endothelial cell tropism. Occasional reports of intracytoplasmic viral‐like inclusions are mentioned, 47 but these tend to be the exception rather than the rule. Giant cells have also been reported, 48 but these can also be seen in non‐COVID‐associated diffuse alveolar damage. There have also been the occasional reports of the presence of the ‘viral type particles’ in bronchiolar epithelial cells and alveolar epithelial cells. 49
It should also be recognized that most autopsy studies have failed to distinguish whether the tissue was from people who died with COVID‐19, or of COVID‐19, or whether the clinical and pathological changes are secondary to intensive care management. Goligher et al. posed this clinical question on whether severe COVID was a typical or atypical form of ARDS, 50 and concluded that in classical ARDS the hypoxia was a result from atelectasis and consolidation, with an increased physiological shunt fraction, but that COVID‐19 was different in that the hypoxaemia was out of proportion to the lung parameters and was more vasocentric in keeping with the pro‐thrombotic tendency. Their suggestion was that apart from more anticoagulation there should not be other major changes in management and the goal should be to avoid unsafe lung stress and strain.
On the pathological findings to investigate if the changes seen in the lung were secondary to intensive care management, an early report of COVID‐19 patients who died without hospital admission showed diffuse alveolar damage with hyaline membrane formation, Type II hyperplasia and interstitial lymphocytic inflammation. 51 Intriguingly, of the nine cases studied, there was no evidence of microthrombi formation and no myocarditis was seen. This thromboembolic tendency was seen in another clinical study that showed 18 of 370 patients admitted with COVID had computed tomography (CT) evidence of thrombosis. 52 A review of most of the autopsy publications shows a confirmation bias of histological changes of diffuse alveolar damage but more attention should be given to images which show areas of positive antigen, but no significant other morphological changes, 46 , 53 suggesting that not all virus infection of the functional lung unit leads to massive damage and that the cytokine storm is only seen in a minor proportion of cases. 54 The problems with understanding human lung pathophysiology and COVID‐19 are illustrated in two cases of fatal COVID‐19 in which post‐mortem was performed. In the first case involving a patient with cardiovascular morbidities who died after 3 days, typical features of hyaline membrane disease are identified (Figure 2A), and yet IHC for virus was negative (Figure 2B). In a second case (Figure 2C–F), occurring in a patient who died after 10 days, there is antigen in macrophages and desquamated epithelial cells, but not in endothelial cells but as this is autopsy tissue accurately determining if the antigen is in Type 1 or Type 2 cells is not possible. In most countries, there is a delay of 3–10 days from death until autopsy, during which tissue breakdown occurs, thus accurate understanding of the nature of disease in future will probably rely on laboratory animal models, which may not faithfully replicate human pathophysiology.
FIGURE 2.
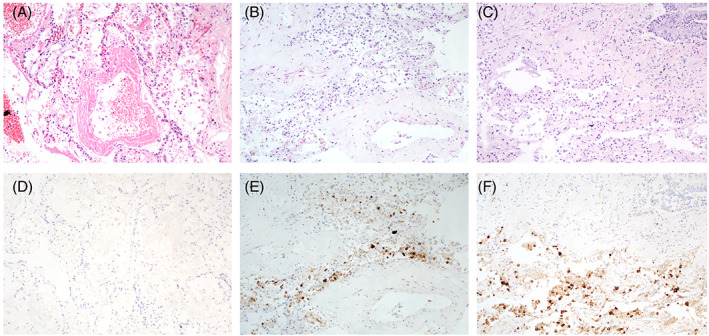
(A–C) Haematoxylin and eosin‐stained sections of two fatal cases of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and (D–F) corresponding immunohistochemistry using a polyclonal rabbit antibody to SARS‐co‐V N‐protein. Magnification ×100
Gastrointestinal
The potential role of the gastrointestinal tract as a route of replication and transmission was proposed soon after there was evidence of viral spread from China to other parts of the world. 55 , 56 This hypothesis was initially based on historical evidence of the finding of virus in intestinal samples during the 2003 SARS outbreak, 57 as well as the documented spread of the virus through wastewater systems in the Amoy Gardens housing complex in Hong Kong, leading to a large community outbreak (reviewed in 2006 58 ). The MERS outbreak in 2012 documented one quarter of patients having gastrointestinal symptoms, and viral RNA was detected in the gastrointestinal tract. 59
In the early reports of clinical presentation in COVID‐19, the symptoms were, as expected, non‐specific with diarrhoea, nausea and vomiting reported. 60 The gastrointestinal symptoms were more frequent in patients after hospitalization, rather than on admission. 61
The presence of ACE2 has been detected in the oesophageal epithelium and enterocytes of the ileum and colon, 62 , 63 and intriguingly the colonic organoid system developed in the rat model showed upregulation of ACE2 in organoids derived from hypertensive rats. 63 Of note, ACE2 has been detected in the intestines of cats and tigers, in which sporadic reported cases of infection have occurred, 64 leading to concern that these animals may be reservoirs for virus mutation. 65 Even though human ex vivo explants have been used to study tropism, 18 gastrointestinal explants have not been successful, and as a result some centres are using organoid cultures derived from human pluripotent stem cells (hPSC). Organoid systems act as multicellular composites that faithfully replicate the characteristics of the native epithelium. The first report of this system from the United States 66 showed multiple colonic cell types, including enterocytes that were susceptible to infection with expression of ACE2 detected.
CT abnormalities in the abdomen have been identified in 18.1% of patients admitted with COVID‐19, with fluid‐filled colon and pericolic stranding reported as the most common features 67 ; however, these appearances did not correlate with abdominal symptoms. There have been isolated case reports of colonic ischaemic change, 68 , 69 in line with the increased thromboembolic tendency seen in more severe cases of COVID‐19.
As with other systems reviewed here, the challenge has been to detect actual virus replication in human gastrointestinal tissues, and unfortunately the hard data supporting this are lacking. There have been two case reports documenting the presence of virus in human tissues. The first, from Germany, 70 was from a 43‐year old male who 6 weeks after admission for COVID‐19, developed large bowel obstruction requiring resection, in which electron microscopy reported isolated viral particles, but this was not confirmed by ISH, IHC or PCR, and the isolated particles appeared to have a hollow core. A second report from a patient with colonic carcinoma, 71 who was positive for viral RNA, also claimed to find viral particles; however, close examination shows that these isolated particles are 150 nm in diameter, larger than the spectrum of coronavirus particles seen in other studies, and most likely represent other structures.
Despite the lack of definitive sites of replication of SARS‐CoV‐2 in the gastrointestinal site there has been a great deal of interest in the detection of viral genetic material in waste water from a number of countries and regions to screen for virus prevalence in the community setting. This has been recently reviewed in a number of multi‐regional publications. 72 , 73 , 74 , 75 From these studies, it can be concluded that the viral load is extremely variable in waste water treatment plants depending on stages of the outbreak, and not surprisingly that viral RNA does not imply infectiousness. This finding of RNA in waste water is important as it has been proposed that in regions where there is poor hand hygiene, open defecation, squat toilets and lack of water sealing U‐traps, the possibility of environmental transmission from an infected individual to another should be considered. 76
DISSEMINATION AND TRANSMISSION
Viraemia
One of the crucial clinical questions asked about COVID‐19 is whether this is a condition limited to the respiratory and gastrointestinal sites, or whether there is dissemination to other organs. In the 2003 SARS outbreak, there were two publications which found evidence of viral RNA in the blood, 77 , 78 and one publication demonstrating viral RNA in 33% of patients with MERS‐CoV infection 79 ; however, there has been no published information on the other human coronaviruses. Although there has been interest in exploring the multi‐organ tropism of this virus, 80 in view of the time frame of organs sampled from 20 to 81 days after infection correlation with damage due to direct virus interaction needs to be examined more closely.
With clinical COVID‐19, there have been a number of publications on viral RNA in the blood with the first publication using data from the early 2020 Wuhan outbreak. 81 , 82 Since then, more extensive publications from Spain, 83 , 84 Germany, 85 , 86 United States 87 , 88 , 89 and Norway 90 have been published using outpatients, hospital ward patients and critically ill patients. The positive detection rate is between 28% and 32% of hospitalized patients; however, those in intensive care have rates up to 78%. Only a few studies have looked at the temporal changes, 88 , 91 , 92 and it appears the peak of viral load is in the first 10 days with plasma levels declining after that. It is noteworthy that this decline is regardless of outcome. 92 One early study found that the RNA level correlated with IL6 levels, 82 and other inflammatory chemokine levels. 83 A review of the presence of viral RNA in the blood 93 commented that in only two of the 23 published studies was there an attempt to grow virus in culture (i.e., to distinguish RNAaemia from true viraemia) and both of these failed to grow virus. Another study 92 involving 71 patients stated that additional studies were needed to confirm that the plasma ‘viraemia’ actually represented infectious virions. This distinction is important not only in understanding the clinical spectrum of disease, but also whether virus can be transmitted by blood, as a number of centres have been using convalescent plasma for the management of severe disease. It was also noted in the review of RNAaemia that in most studies reporting positive RNA, the level was close to the limit of detection with a cycle threshold (Ct) value on quantitative PCR greater than 30 in most cases. The results of virus culture in blood have been recently summarized in a preprint review, 94 with no publications demonstrating virus growth from PCR‐positive blood samples. Thus, the conclusions from another review of virus tropism 95 have concluded that there was no firm evidence that there could be virus infection of other organs via the bloodstream, and in view of the correlation of viral RNA with severe disease, the RNA in the blood could be a manifestation of viral RNA released from damaged epithelial cells.
Cardiovascular system
Initially, the clinical spectrum of COVID‐19 was thought to be a respiratory disease, similar to SARS‐CoV; however, as the pandemic evolved, multi‐organ involvement was seen and the heart was no exception. Initial autopsy studies from the United States, 96 , 97 , 98 , 99 Germany 100 , 101 , 102 and later other countries such as Netherlands, 103 UK, 104 Belgium, 105 Italy, 106 Switzerland, 107 Austria 108 and China 109 have shown that in patients who have died with COVID‐19 there have been a range of cardiac findings including lymphocytic myocarditis, pericardial damage, dilated cardiomyopathy, hypertrophic cardiomyopathy, hypertensive cardiomyopathy, 96 septal myocardial infarction, 97 perivascular lymphocytic inflammation, 110 right ventricular damage 105 and even amyloidosis. 99 A number of studies have highlighted the presence of a prothrombotic state, characterized by the identification of thrombo‐emboli in a number of organs, 104 including non‐occlusive fibrin microthrombi. 99
Although these autopsy series would initially indicate that clinical COVID‐19 can have widespread cardiac involvement, a number of review publications published 10 months after the outbreak have pointed out a number of challenges with these reports. 111 The first is a disconnect between autopsy studies and clinical myocarditis, pointing out that the diagnosis of clinical myocarditis is a challenge, requiring a number of distinct and distinguishing investigations including ECG, non‐invasive technologies like echocardiography and cardiac MRI. Although a definitive diagnosis of myocarditis needs endomyocardial biopsy (EMB), there have actually been very few published studies in which EMBs have been performed. 112 Even if EMB is performed, the sensitivity is between 10% and 22%, because all autopsy data have demonstrated that myocardial involvement is patchy and there is significant interobserver variability. 113
The second problem with autopsy data is that mortality from COVID‐19 is increased in patients with cardiovascular diseases such as heart failure, diabetes, hypertension and atherosclerosis. Therefore, whether the histological findings in the heart can be attributed to direct viral damage, or pre‐existing disease has to be carefully detailed. A review of cardiovascular pathology 108 came to the conclusion that even though 7.2% of patients had histological evidence of myocarditis, most of these cases would likely not be functionally significant, and reduced the true prevalence to less than 2%, suggesting that the patients with autopsy were dying ‘with’ myocarditis rather than ‘of’ myocarditis. A similar conclusion was reached by another group of researchers in a review article 111 indicating that distinct European Society of Cardiology criteria should be used before a diagnosis of myocarditis was made The pathogenesis of myocardial dysfunction is probably multifactorial (see below).
In patients who died from SARS, autopsy data showed evidence of an increased thrombotic tendency, 33 and many studies from the COVID‐19 patients have shown that patients admitted to hospital had increased tendency towards thrombosis, including deep vein thrombosis, pulmonary embolism 100 and microthrombi 98 , 102 despite anticoagulation. 97 These thromboembolic features were seen in multiple organs 104 and have resulted in many authors proposing the term endothelial dysfunction. This is taken to imply a change of the endothelium from an anti‐thrombotic state to a pro‐thrombotic one, with a consequent increase in vascular permeability and a change to a large number of biomarkers including prothrombin time, C‐reactive protein, ferritin, IL6 and plasma creatinine.
The mechanisms for this endothelial dysfunctional have been extensively reviewed, 114 and have been attributed to interference with the renin–angiotensin–aldosterone system (RAAS), oxidative damage, cytokine storm, immune damage, disseminated intravascular coagulation and even by a recently described process called ferroptosis, 110 an iron‐dependent form of regulated cell death where there is a loss of glutathione peroxidase activity leading to excessive peroxidation of polyunsaturated fatty acids.
In most models of the mechanisms of endothelial dysfunction, the initiating trigger has been proposed to be direct viral infection of myocytes or endothelial cells, causing cellular damage, disturbances of intercellular junctions and exposure of the subendothelial collagen leading to the prothrombotic tendency. It has been previously acknowledged using IHC that endothelial cells express ACE2 (the SARS‐CoV‐2 receptor), and thus should be a likely target for viral entry and replication; however, the data supporting this mechanism are by no means conclusive. Distribution of ACE2 was investigated after the SARS 2003 outbreak by a number of publications using ACE2 antibodies, with the distribution found in many organs, 115 thus implying that SARS could be a systemic disease; however, the in situ hybridization studies done in the fatal SARS patients found no evidence of signal in endothelial cells. A number of structural proteins apart from ACE2 have been proposed to serve as viral attachment factors such as CD147 (BSG) which is expressed on the basal surface of the endothelium in culture in one study, 116 but this has not been confirmed by another study, 117 with a further publication showing that ACE2 decreases with age, but BSG actually increased. 118
Most review articles on whether there is direct endothelial infection cite two articles in which ‘virus‐like particles’ were identified in endothelial cells using electron microscopy. 119 , 120 These two articles have been quickly followed by a number or short communications challenging these findings, 121 , 122 attributing these ultrastructural changes to rough endoplasmic reticulum, ribosomes, clathrin‐coated vesicles, or multi‐vesicles—mimics of virus particles. 123 In a laboratory setting, human microvascular endothelial cells showed a low level of viral replication, 124 with no electron microscopy performed. Unfortunately, another human primary lung microvascular endothelial cell line was not able to replicate this finding. 125 RNA‐seq studies on vascular arterial venous and microvascular beds also failed to find ACE2, although pericytes were found to be positive. 126 Similarly, a report of finding virus within endothelial cells in the skin from a series of paediatric patients presenting with chilblains 127 was challenged in a number of correspondence replies 128 , 129 , 130 regarding the veracity of the virus particles in the endothelium.
If finding virus in endothelial cells had been challenging, finding virus in the myocardium and thus attributing the myocarditis to direct viral damage have been in general unrewarding. Despite many autopsy findings of PCR‐positive results in the myocardium, in no cases has virus been able to be cultured from the myocardium, and in only a few cases have possible single viral particles been identified, 131 with no groups of viruses or replication complexes observed. 132 The recent report of fulminant myocarditis with positive IHC 133 is confounded by the fact that the N‐protein antibody used is not considered reliable in paraffin‐embedded tissue. 134
It should also be noted that small animal and primate studies have also failed to detect viral antigen or show significant myocardial damage. 22 , 135 The negative IHC, and EM findings despite a lymphocytic myocarditis is not unique to SARS‐Co‐2 infection, and is more likely to be immune mediated (rather than direct viral damage). It should also be mentioned that this immune‐mediated damage has been documented in other virus‐associated myocarditis and has been attributed to an exaggerated immune response, with increased levels of serum cytokines and TNFα detected. 136 This is supported by evidence that cardiac troponin is higher in patients with more severe infection with non‐ischaemic cardiomyopathy with limited evidence of direct infection. 137
It therefore appears that the cardiac damage is not a direct viral damage, 138 nevertheless the recognition of myocarditis is important as it leads to an increased risk of in‐hospital mortality with adverse long‐term clinical outcome, with current management being supportive unless cardiogenic shock occurs.
Liver
There are a number of excellent reviews on liver involvement in clinical COVID‐19. 139 , 140 , 141 , 142 As with other organs system reviews, these are biased towards an inpatient cohort, often with severe disease, so the extent by which there may be hepatic involvement in mild disease has not been properly studied. Liver involvement can be subdivided into damage to hepatocytes, leading to changes in biochemical processes and coagulation, changes to the cholangiocytes leading to jaundice and changes to the hepatic sinusoids resulting from an endotheliitis.
It has been postulated that hepatocyte damage may be drug induced, direct viral damage or resulting from endotheliitis, coagulopathy, with damage to cholangiocytes by the cytokine storm, and drug‐induced liver injury. 139 The extent of biochemical liver dysfunction increases with the severity of disease in patients and is associated with older age and male sex, 143 and the liver injury correlates with the severity of pulmonary disease. 144
Many of the patients who develop severe disease have coagulopathy; therefore, it has been difficult to determine pathological changes in the inpatient setting using liver biopsy because of the risk of bleeding. However, one study which did perform liver biopsies found steatosis, Kupffer cell activation, luminal thrombosis and portal fibrosis. 145
From a virological point of view, a major question (similar to other organs) has been whether the damage seen in clinical disease is due to direct viral infection or whether the liver is an ‘innocent bystander’. 146 In studies done on macaques by the Erasmus group, there was no evidence of extrapulmonary virus spread 135 ; conversely, a number of studies have commented that ACE2 is expressed in the liver, 147 and this expression can be increased by activation of the RAAS. 148 In vitro, liver cancer cell lines appear susceptible to infection, and indeed a HuH7 has been used as a positive control for a number of virus replication studies. Furthermore, use of liver organoids that have been derived from human pluripotential stem cells are able to support virus replication. 149
A number of autopsy studies detailing the liver pathology in COVID‐19 have been reported in the United States, 142 , 150 Belgium 103 , 146 and Netherlands, 103 in which a common feature is steatosis (often microvesicular), platelet fibrin microthrombi, lobular inflammation, Zone 3 haemorrhage and ischaemic type hepatic necrosis. The challenge with these finding is that hepatic pathologies such as steatosis can be seen in patients who have a high risk of developing severe disease, that is, diabetes, obesity and patients with cardiovascular disease. One report showed interesting ‘atypical basophilic sinusoidal structures’ in which detailed IHC was not able to elucidate the nature of these structures. 142
Immunohistochemical analysis 150 and electron microscopy 151 for antigen and virus, respectively, have claimed to find positive staining; however, subsequent correspondence by Philips et al. 152 challenged these findings and proposed that these particles were not of viral origin and could represent cholesterol crystals. Most reviews to date have come to the conclusion that apart from the prothrombotic tendency which is seen in severe disease, possibly by endothelial dysfunction, most of the changes are non‐specific and secondary to factors such as hypoxaemia, drug induced, ischaemia or result from systemic inflammation. 139 , 140 , 141 , 142 , 153
Kidney and urine
One of the first series of articles investigating the renal involvement in clinical COVID‐19 was a multicentre US analysis from February to May 2020 involving 3993 hospitalized patients with clinical COVID. 154 Of these patients, 46% developed acute kidney injury (AKI) and 19% of this cohort required dialysis. The main renal manifestations were proteinuria (84%), haematuria (81%) and leukocyturia (60%). The frequency of this AKI varies significantly in a number of review articles from 0.5% to 80%, with factors such as geography, race/ethnicity and sex accounting for this variation. 155 Similar to other organ systems reviewed, the AKI correlated with disease severity and morbidity. 156 Other review articles indicated that the main pathology was acute renal tubular damage. 157 Similar to other organs discussed in this review paper, there has been much discussion on whether the changes are due to direct viral damage or secondary to hypoxaemia and hypercoagulability. 158
As people who contract COVID‐19 and require hospitalization often have disturbed coagulopathy, renal biopsies have not been routinely performed except for other conditions. 159 , 160 These biopsies have shown AKI with podocytopathy and collapsing glomerulopathy (a form of focal segmental glomerulosclerosis). The first large multicentre renal biopsy study was from the United States. 161 This confirmed the presence of acute tubular injury and collapsing glomerulopathy, but in keeping with disturbed coagulopathy seen in many inpatients reported endothelial injury and a thrombotic microangiopathy. The AKI was predictive of multi‐organ dysfunction. 162 As ACE2 is expressed in the renal tubules, it was expected that this region would be a site of viral damage; however, it was reported that though the tubules might express ACE2, there was a lack of expression of TMPRSS2. 163
The first autopsy study detailing the renal findings was from China which described finding virus‐like particles in six of 2626 cases with positive IHC, 164 although others have described the renal findings as non‐specific. 165 Another article by Farkash et al. also reported finding virus‐like particles, 166 but the EM conclusions of these articles was challenged by two consecutive letters, which proposed that the structures were either endocytic vesicles coated with clathrin or multivesicular bodies (MVBs), as the putative viral particles lacked electron‐dense nucleocapsid material. 167 , 168 Calomeni et al. furthermore reported that when they examined 10 renal biopsies from the pre‐COVID era they were able to identify the same MVBs, 169 and a subsequent review of the challenges of identifying coronaviruses indicated that confirmation of these ‘virus‐like structures’ needed immunoelectron microscopy. 170 Puelles et al. 171 also came to the same conclusion and also commented that for the cases of PCR‐positive renal material obtained at autopsy, the RNA levels were low, and that the increased numbers of endocytic vesicles could be seen in patients with proteinuria. There have been a number of publications using IHC to investigate viral antigen in the tubules, most have found no positive staining, 172 , 173 and in the ones finding some positive findings, the antibodies used (against the spike protein or nucleoprotein) were not reliable in formalin‐fixed tissues. 134 , 174 , 175
If there was supposed to be widespread replication of virus in the kidney, especially the tubules, then one would expect to find a high frequency and detection of virus or RNA in the urine. In one study from China using droplet digital PCR of the urine, 5.41% of patients tested positive, 176 with no detection found in 74 recovered patients. 177 Other studies have varied from region to region 95 , 177 , 178 , 179 with review studies finding overall rates of less than 10%, correlating with severe disease status, but similar to the viraemia studies, in quite a few cases this was a low concentration with a high cT values. 180 Only one case from China has reported positive virus culture from urine, and in another series from South Korea ‘contagious’ virus was found in two of 247 samples. Not surprisingly, this was reported as being at a very low level. The general conclusion on the urine therefore is that a positive finding is rare, and this tends not to be associated with renal disease. 181
Pancreas
COVID‐19 has been implicated as a cause of acute pancreatitis in several case reports, but a causal link is yet to be established. 182 , 183 , 184 In a retrospective observational study of a large inpatient population in New York, 32 of 11,883 (0.27%) patients hospitalized with COVID‐19 had acute pancreatitis according to the Revised Atlanta Classification. 185 Furthermore, pancreatitis in COVID‐19 patients was less likely to be attributable to other causes such as gallstones or alcohol, increasing the possibility of a causal link between SARS‐CoV‐2 infection and pancreatitis. 185 Hyperglycaemia, insulin‐dependent diabetes mellitus and diabetic ketoacidosis have been reported as complications of SARS‐CoV‐2 infection. 186 , 187 , 188 Whether this is due to infection‐induced islet cell dysfunction is debatable as acute stress responses, glucocorticoid treatment and underlying poorly controlled diabetes are all plausible explanations for these clinical observations.
Autopsy series have reported a spectrum of involvement including microscopic inflammation, focal pancreatitis and necrotic‐haemorrhagic pancreatitis in some deceased COVID‐19 patients. 104 , 189 SARS‐CoV‐2 RNA has been detected in pancreatic tissue from COVID‐19 deceased patients, albeit at lower viral loads than respiratory tissue. 190 , 191 Virus‐infected cell clusters (identified by immunohistochemical staining) were mostly in the exocrine compartment in one study, but with proximity to the islets of Langerhans. Viral RNA has also been documented in the endothelium of pancreatic blood vessels. 190 Both exocrine (acinar and ductal cells) and endocrine cell populations in the pancreas have been reported to express ACE2 and TMPRSS2, although there may be significant inter‐individual variations in distribution of these entry factors within the various pancreatic cell populations. 191 , 192 Endocrine cells and hPSC‐derived β‐cells are susceptible to SARS‐CoV‐2 infection in vitro . 11 , 13 , 149 , 191 Interestingly, recent evidence suggests that SARS‐CoV‐2 infection might decrease insulin‐positivity in β‐cells driving them to degranulation and dedifferentiation in patients with severe COVID‐19. 191
CONCLUSION
At the time of writing, just over 1 year has passed since a novel coronavirus entered the community in China. From there, it rapidly spread throughout the world. Even though a number of vaccines have been developed in a short period of time, with initiation of mass population vaccination, it is acknowledged that the virus will not be eradicated and most likely will become one of the circulating seasonal viruses. SARS‐CoV‐2 infection will continue to cause human disease, and despite the large number of clinical cases and excess mortality, to date, owing to the limited number of autopsies and restricted availability of clinical samples, it is disappointing that the progress made in vaccinology has not been matched by progress in understanding many aspects of the pathobiology of this disease. 193 Unlike the first wave of the pandemic in which healthcare settings were overwhelmed with untreated cases and deaths occurring in early stages of the disease resulting in most of the publications cited in this review, with worldwide vaccination strategies in effect in 2021, plus improved testing and therapeutics, there will be a reduced ability to understand the natural, untreated course of disease and many aspects of this disease in terms of pathophysiology will continue to be a mystery.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Biographies
Siddharth Sridhar is a Clinical Assistant Professor at the Department of Microbiology of The University of Hong Kong. His research interest is in emerging infectious diseases. He led the team characterizing rat hepatitis E as an important zoonotic pathogen. He has also been involved in several studies on COVID‐19 evaluating diagnostic tests, animal models and population seroprevalence.
John Nicholls is a Clinical Professor in Pathology at the University of Hong Kong. He has been researching viral pathogenesis for over 30 years and was part of the team which characterized the SARS coronavirus in 2003. He established a human respiratory explant system for risk assessment of emerging viruses in 2007 which is now the longest established system. For the past year, he has been researching tropism of SARS‐CoV‐2 in different organs and tissues.
Sridhar S, Nicholls J. Pathophysiology of infection with SARS‐CoV‐2—What is known and what remains a mystery . Respirology. 2021;26:652–665. 10.1111/resp.14091
REFERENCES
- 1. Raynaud M, Zhang H, Louis K, Goutaudier V, Wang J, Dubourg Q, et al. COVID‐19‐related medical research: a meta‐research and critical appraisal. BMC Med Res Methodol. 2021;21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oikonomidi T, Boutron I, Pierre O, Cabanac G, Ravaud P, COVID‐19 NMA Consortium . Changes in evidence for studies assessing interventions for COVID‐19 reported in preprints: meta‐research study. BMC Med. 2020;18:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323:1843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desai M, Oppenheimer J. The importance of considering olfactory dysfunction during the COVID‐19 pandemic and in clinical practice. J Allergy Clin Immunol Pract. 2021;9:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwok KO, Huang Y, Tsoi MTF, Tang A, Wong SYS, Wei WI, et al. Epidemiology, clinical spectrum, viral kinetics and impact of COVID‐19 in the Asia‐Pacific region. Respirology. 2021;26:322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tirupathi R, Ramparas TR, Wadhwa G, Areti S, Kaur J, Salim S, et al. Viral dynamics in the upper respiratory tract (URT) of SARS‐CoV‐2. Infez Med. 2020;28:486–99. [PubMed] [Google Scholar]
- 8. Cardenas G, Torres‐Garcia D, Cervantes‐Torres J, Rosales‐Mendoza S, Fleury A, Fragoso G, et al. Role of systemic and nasal glucocorticoid treatment in the regulation of the inflammatory response in patients with SARS‐Cov‐2 infection. Arch Med Res. 2021;52:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis ME, Yan CH. Coronavirus disease‐19 and rhinology/facial plastics. Otolaryngol Clin North Am. 2020;53:1139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao Y, Wang H, Liu Z. Expression of ACE2 in airways: implication for COVID‐19 risk and disease management in patients with chronic inflammatory respiratory diseases. Clin Exp Allergy. 2020;50:1313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Capocasale G, Nocini R, Faccioni P, Donadello D, Bertossi D, Albanese M, et al. How to deal with coronavirus disease 2019: a comprehensive narrative review about oral involvement of the disease. Clin Exp Dent Res. 2021;7:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soldatova L, Rassekh CH, Baloch ZW, Jalaly JB, Sedora‐Roman NI, Loevner LL, et al. Salivary gland disease in the era of COVID‐19 pandemic. Head Neck. 2020;42:1339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song J, Li Y, Huang X, Chen Z, Li Y, Liu C, et al. Systematic analysis of ACE2 and TMPRSS2 expression in salivary glands reveals underlying transmission mechanism caused by SARS‐CoV‐2. J Med Virol. 2020;92:2556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lechien JR, Chetrit A, Chekkoury‐Idrissi Y, Distinguin L, Circiu M, Saussez S, et al. Parotitis‐like symptoms associated with COVID‐19, France, march‐April 2020. Emerg Infect Dis. 2020;26:2270–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chern A, Famuyide AO, Moonis G, Lalwani AK. Sialadenitis: a possible early manifestation of COVID‐19. Laryngoscope. 2020;130:2595–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Usami Y, Hirose K, Okumura M, Toyosawa S, Sakai T. Brief communication: immunohistochemical detection of ACE2 in human salivary gland. Oral Sci Int. 2020. Sep 28:10.1002/osi2.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soares CD, Mosqueda‐Taylor A, Hernandez‐Guerrero JC, de Carvalho MGF, de Almeida OP. Immunohistochemical expression of angiotensin‐converting enzyme 2 in minor salivary glands during SARS‐CoV‐2 infection. J Med Virol. 2021;93:1905–6. [DOI] [PubMed] [Google Scholar]
- 18. Hui KPY, Cheung MC, Perera R, Ng KC, Bui CHT, Ho JCW, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS‐CoV‐2 in human respiratory tract and conjunctiva: an analysis in ex‐vivo and in‐vitro cultures. Lancet Respir Med. 2020;8:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butowt R, von Bartheld CS. Anosmia in COVID‐19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2020;1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee IT, Nakayama T, Wu CT, Goltsev Y, Jiang S, Gall PA, et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat Commun. 2020;11:5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glezer I, Bruni‐Cardoso A, Schechtman D, Malnic B. Viral infection and smell loss: the case of COVID‐19. J Neurochem. 2021;157(4):930–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, et al. Pathogenesis and transmission of SARS‐CoV‐2 in golden hamsters. Nature. 2020;583:834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernabei F, Versura P, Rossini G, Re MC. There is a role in detection of SARS‐CoV‐2 in conjunctiva and tears: a comprehensive review. New Microbiol. 2020;43:149–55. [PubMed] [Google Scholar]
- 24. Lange C, Wolf J, Auw‐Haedrich C, Schlecht A, Boneva S, Lapp T, et al. What is the importance of the conjunctiva as a potential transmission pathway for SARS‐CoV‐2 infections? Ophthalmologe. 2020;117:626–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guemes‐Villahoz N, Burgos‐Blasco B, Vidal‐Villegas B, Garcia‐Feijoo J, Arriola‐Villalobos P, Martinez‐de‐la‐Casa JM, et al. Novel insights into the transmission of SARS‐CoV‐2 through the ocular surface and its detection in tears and conjunctival secretions: a review. Adv Ther. 2020;37:4086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abdul‐Kadir MA, Lim LT. Human coronaviruses: ophthalmic manifestations. BMJ Open Ophthalmol. 2020;5:e000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al‐Sharif E, Strianese D, AlMadhi NH, D'Aponte A, dell'Omo R , Di Benedetto R, et al. Ocular tropism of coronavirus (CoVs): a comparison of the interaction between the animal‐to‐human transmitted coronaviruses (SARS‐CoV‐1, SARS‐CoV‐2, MERS‐CoV, CoV‐229E, NL63, OC43, HKU1) and the eye. Int Ophthalmol. 2021;41:349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lange C, Wolf J, Auw‐Haedrich C, Schlecht A, Boneva S, Lapp T, et al. Expression of the COVID‐19 receptor ACE2 in the human conjunctiva. J Med Virol. 2020;92:2081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS‐CoV‐2 infection. Ocul Surf. 2020;18:537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roehrich H, Yuan C, Hou JH. Immunohistochemical study of SARS‐CoV‐2 viral entry factors in the cornea and ocular surface. Cornea. 2020;39:1556–62. [DOI] [PubMed] [Google Scholar]
- 31. Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID‐19). Can J Ophthalmol. 2020;55:e125–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nicholls JM, Butany J, Poon LL, Chan KH, Beh SL, Poutanen S, et al. Time course and cellular localization of SARS‐CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nicholls JM, Peiris JS. Avian influenza: update on pathogenesis and laboratory diagnosis. Respirology. 2008;13 (Suppl 1):S14–8. [DOI] [PubMed] [Google Scholar]
- 35. Calabrese F, Pezzuto F, Fortarezza F, Hofman P, Kern I, Panizo A, et al. Pulmonary pathology and COVID‐19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch. 2020;477:359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID‐19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang H, Wang CY, Zhou P, Yue H, Du R. Histopathologic changes and SARS‐CoV‐2 immunostaining in the lung of a patient with COVID‐19. Ann Intern Med. 2020;173:324. [DOI] [PubMed] [Google Scholar]
- 38. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pernazza A, Mancini M, Rullo E, Bassi M, De Giacomo T, Rocca CD, et al. Early histologic findings of pulmonary SARS‐CoV‐2 infection detected in a surgical specimen. Virchows Arch. 2020;477:743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, et al. Pathology and pathogenesis of SARS‐CoV‐2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020;26:2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sauter JL, Baine MK, Butnor KJ, Buonocore DJ, Chang JC, Jungbluth AA, et al. Insights into pathogenesis of fatal COVID‐19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology. 2020;77:915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller SE. Visualization of SARS‐CoV‐2 in the lung. N Engl J Med. 2020;383:2689. [DOI] [PubMed] [Google Scholar]
- 43. Ackermann M, Mentzer SJ, Jonigk D. Visualization of SARS‐CoV‐2 in the lung. Reply. N Engl J Med. 2020;383:2689–90. [DOI] [PubMed] [Google Scholar]
- 44. Mason RJ. Thoughts on the alveolar phase of COVID‐19. Am J Physiol Lung Cell Mol Physiol. 2020;319:L115–L20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schaefer IM, Padera RF, Solomon IH, Kanjilal S, Hammer MM, Hornick JL, et al. In situ detection of SARS‐CoV‐2 in lungs and airways of patients with COVID‐19. Mod Pathol. 2020;33:2104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zeng Z, Xu L, Xie XY, Yan HL, Xie BJ, Xu WZ, et al. Pulmonary pathology of early‐phase COVID‐19 pneumonia in a patient with a benign lung lesion. Histopathology. 2020;77:823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deshmukh V, Motwani R, Kumar A, Kumari C, Raza K. Histopathological observations in COVID‐19: a systematic review. J Clin Pathol. 2021;74:76–83. [DOI] [PubMed] [Google Scholar]
- 49. Yao XH, He ZC, Li TY, Zhang HR, Wang Y, Mou H, et al. Pathological evidence for residual SARS‐CoV‐2 in pulmonary tissues of a ready‐for‐discharge patient. Cell Res. 2020;30:541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goligher EC, Ranieri VM, Slutsky AS. Is severe COVID‐19 pneumonia a typical or atypical form of ARDS? And does it matter? Intensive Care Med. 2021;47:83–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Youd E, Moore L. COVID‐19 autopsy in people who died in community settings: the first series. J Clin Pathol. 2020;73:840–4. [DOI] [PubMed] [Google Scholar]
- 52. Vlachou M, Drebes A, Candilio L, Weeraman D, Mir N, Murch N, et al. Pulmonary thrombosis in Covid‐19: before, during and after hospital admission. J Thromb Thrombolysis. 2021;51:978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Menter T, Tzankov A. Investigations of pathologists as a key to understanding coronavirus disease 2019. Pathobiology. 2021;88:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mudd PA, Crawford JC, Turner JS, Souquette A, Reynolds D, Bender D, et al. Distinct inflammatory profiles distinguish COVID‐19 from influenza with limited contributions from cytokine storm. Sci Adv. 2020;6:eabe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal‐oral transmission of SARS‐CoV‐2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cipriano M, Ruberti E, Giacalone A. Gastrointestinal infection could be new focus for coronavirus diagnosis. Cureus. 2020;12:e7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, et al. Enteric involvement of severe acute respiratory syndrome‐associated coronavirus infection. Gastroenterology. 2003;125:1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McKinney KR, Gong YY, Lewis TG. Environmental transmission of SARS at Amoy Gardens. J Environ Health. 2006;68:26–30; quiz 51–2. [PubMed] [Google Scholar]
- 59. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, Al‐Rabiah FA, Al‐Hajjar S, Al‐Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nie K, Yang YY, Deng MZ, Wang XY. Gastrointestinal insights during the COVID‐19 epidemic. World J Clin Cases. 2020;8:3934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS‐CoV‐2 infection. Gut. 2020;69:997–1001. [DOI] [PubMed] [Google Scholar]
- 62. Barbosa da Luz B, de Oliveira NMT, Franca Dos Santos IW, Paza LZ, Braga L, Platner FDS, et al. An overview of the gut side of the SARS‐CoV‐2 infection. Intest Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li J, Stevens BR, Richards EM, Raizada MK. SARS‐CoV‐2 receptor ACE2 (angiotensin‐converting enzyme 2) is upregulated in colonic organoids from hypertensive rats. Hypertension. 2020;76:e26–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. do Vale B, Lopes AP, Fontes MDC, Silvestre M, Cardoso L, Coelho AC. Bats, pangolins, minks and other animals – villains or victims of SARS‐CoV‐2? Vet Res Commun. 2021;45:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Elaswad A, Fawzy M, Basiouni S, Shehata AA. Mutational spectra of SARS‐CoV‐2 isolated from animals. PeerJ. 2020;8:e10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Han Y, Duan X, Yang L, Nilsson‐Payant BE, Wang P, Duan F, et al. Identification of SARS‐CoV‐2 inhibitors using lung and colonic organoids. Nature. 2021;589:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tirumani SH, Rahnemai‐Azar AA, Pierce JD, Parikh KD, Martin SS, Gilkeson R, et al. Are asymptomatic gastrointestinal findings on imaging more common in COVID‐19 infection? Study to determine frequency of abdominal findings of COVID‐19 infection in patients with and without abdominal symptoms and in patients with chest‐only CT scans. Abdom Radiol (NY). 2021. Jan 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singh B, Mechineni A, Kaur P, Ajdir N, Maroules M, Shamoon F, et al. Acute intestinal ischemia in a patient with COVID‐19 infection. Korean J Gastroenterol. 2020;76:164–6. [DOI] [PubMed] [Google Scholar]
- 69. Almeida Vargas A, Valenti V, Sanchez Justicia C, Martinez Regueira F, Marti Cruchaga P, Lujan Colas J, et al. Severe colon ischemia in patients with severe coronavirus‐19 (COVID‐19). Rev Esp Enferm Dig. 2020;112:784–7. [DOI] [PubMed] [Google Scholar]
- 70. Stahl K, Brasen JH, Hoeper MM, David S. Direct evidence of SARS‐CoV‐2 in gut endothelium. Intensive Care Med. 2020;46:2081–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Qian Q, Fan L, Liu W, Li J, Yue J, Wang M, et al. Direct evidence of active SARS‐CoV‐2 replication in the intestine. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Perisetti A, Goyal H, Gajendran M, Boregowda U, Mann R, Sharma N. Prevalence, mechanisms, and implications of gastrointestinal symptoms in COVID‐19. Front Med (Lausanne). 2020;7:588711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mohapatra S, Menon NG, Mohapatra G, Pisharody L, Pattnaik A, Menon NG, et al. The novel SARS‐CoV‐2 pandemic: possible environmental transmission, detection, persistence and fate during wastewater and water treatment. Sci Total Environ. 2021;765:142746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jones DL, Baluja MQ, Graham DW, Corbishley A, McDonald JE, Malham SK, et al. Shedding of SARS‐CoV‐2 in feces and urine and its potential role in person‐to‐person transmission and the environment‐based spread of COVID‐19. Sci Total Environ. 2020;749:141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Foladori P, Cutrupi F, Segata N, Manara S, Pinto F, Malpei F, et al. SARS‐CoV‐2 from faeces to wastewater treatment: what do we know? A review. Sci Total Environ. 2020;743:140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sun S, Han J. Open defecation and squat toilets, an overlooked risk of fecal transmission of COVID‐19 and other pathogens in developing communities. Environ Chem Lett. 2020. Nov 29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Grant PR, Garson JA, Tedder RS, Chan PK, Tam JS, Sung JJ. Detection of SARS coronavirus in plasma by real‐time RT‐PCR. N Engl J Med. 2003;349:2468–9. [DOI] [PubMed] [Google Scholar]
- 78. Zhou YM, Yang RQ, Tao SC, Li Z, Zhang Q, Gao HF, et al. The design and application of DNA chips for early detection of SARS‐CoV from clinical samples. J Clin Virol. 2005;33:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Corman VM, Albarrak AM, Omrani AS, Albarrak MM, Farah ME, Almasri M, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, et al. Multi‐organ proteomic landscape of COVID‐19 autopsies. Cell. 2021;184:775–91.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xu K, Chen Y, Yuan J, Yi P, Ding C, Wu W, et al. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID‐19). Clin Infect Dis. 2020;71:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bermejo‐Martin JF, Gonzalez‐Rivera M, Almansa R, Micheloud D, Tedim AP, Dominguez‐Gil M, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID‐19. Crit Care. 2020;24:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Berastegui‐Cabrera J, Salto‐Alejandre S, Valerio M, Perez‐Palacios P, Revillas FAL, Abelenda‐Alonso G, et al. SARS‐CoV‐2 RNAemia is associated with severe chronic underlying diseases but not with nasopharyngeal viral load. J Infect. 2021;82:e38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schlesinger T, Weissbrich B, Wedekink F, Notz Q, Herrmann J, Krone M, et al. Biodistribution and serologic response in SARS‐CoV‐2 induced ARDS: a cohort study. PLoS One. 2020;15:e0242917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Strasser EF, Steininger PA, Korn K, Achenbach S, Tenbusch M, Cunningham S, et al. Validation of a SARS‐CoV‐2 RNA RT‐PCR assay for high‐throughput testing in blood of COVID‐19 convalescent plasma donors and patients. Transfusion. 2021;61:368–74. [DOI] [PubMed] [Google Scholar]
- 87. Christensen J, Kumar D, Moinuddin I, Bryson A, Kashi Z, Kimball P, et al. Coronavirus disease 2019 viremia, serologies, and clinical course in a case series of transplant recipients. Transplant Proc. 2020;52:2637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hogan CA, Stevens BA, Sahoo MK, Huang C, Garamani N, Gombar S, et al. High frequency of SARS‐CoV‐2 RNAemia and association with severe disease. Clin Infect Dis. 2021;72:e291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pham TD, Huang C, Wirz OF, Roltgen K, Sahoo MK, Layon A, et al. SARS‐CoV‐2 RNAemia in a healthy blood donor 40 days after respiratory illness resolution. Ann Intern Med. 2020;173:853–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Prebensen C, Myhre PL, Jonassen C, Rangberg A, Blomfeldt A, Svensson M, et al. SARS‐CoV‐2 RNA in plasma is associated with ICU admission and mortality in patients hospitalized with COVID‐19. Clin Infect Dis. 2020. Sep 5:ciaa1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xu D, Zhou F, Sun W, Chen L, Lan L, Li H, et al. Relationship between serum SARS‐CoV‐2 nucleic acid (RNAemia) and organ damage in COVID‐19 patients: a cohort study. Clin Infect Dis. 2020. July 28:ciaa1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, et al. SARS‐CoV‐2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Leblanc JF, Germain M, Delage G, O'Brien S, Drews SJ, Lewin A. Risk of transmission of severe acute respiratory syndrome coronavirus 2 by transfusion: a literature review. Transfusion. 2020;60:3046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jefferson T, Spencer EA, Brassey J, Heneghan C. Viral cultures for COVID‐19 infectious potential assessment – a systematic review. Clin Infect Dis. 2020. Dec 3:ciaa1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Trypsteen W, Van Cleemput J, Snippenberg WV, Gerlo S, Vandekerckhove L. On the whereabouts of SARS‐CoV‐2 in the human body: a systematic review. PLoS Pathog. 2020;16:e1009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID‐19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet‐fibrin thrombi characterize multi‐organ thrombosis at autopsy in COVID‐19: a case series. EClinicalMedicine. 2020;24:100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bois MC, Boire NA, Layman AJ, Aubry MC, Alexander MP, Roden AC, et al. COVID‐19‐associated nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143:230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wichmann D. Autopsy findings and venous thromboembolism in patients with COVID‐19. Ann Intern Med. 2020;173:1030. [DOI] [PubMed] [Google Scholar]
- 101. Lindner D, Fitzek A, Brauninger H, Aleshcheva G, Edler C, Meissner K, et al. Association of cardiac infection with SARS‐CoV‐2 in confirmed COVID‐19 autopsy cases. JAMA Cardiol. 2020;5:1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, et al. Immunothrombotic dysregulation in COVID‐19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schurink B, Roos E, Radonic T, Barbe E, Bouman CSC, de Boer HH, et al. Viral presence and immunopathology in patients with lethal COVID‐19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID‐19: a post‐mortem study. Lancet Microbe. 2020;1:e245–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, et al. Pathological features of COVID‐19‐associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bussani R, Schneider E, Zentilin L, Collesi C, Ali H, Braga L, et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID‐19 pathology. EBioMedicine. 2020;61:103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Grosse C, Grosse A, Salzer HJF, Dunser MW, Motz R, Langer R. Analysis of cardiopulmonary findings in COVID‐19 fatalities: high incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020;49:107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Halushka MK, Vander Heide RS. Myocarditis is rare in COVID‐19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang X, Tu Y, Huang B, Li Y, Li Y, Zhang S, et al. Pulmonary vascular endothelial injury and acute pulmonary hypertension caused by COVID‐19: the fundamental cause of refractory hypoxemia? Cardiovasc Diagn Ther. 2020;10:892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jacobs W, Lammens M, Kerckhofs A, Voets E, Van San E, Van Coillie S, et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID‐19): autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020;7:3772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ozieranski K, Tyminska A, Jonik S, Marcolongo R, Baritussio A, Grabowski M, et al. Clinically suspected myocarditis in the course of severe acute respiratory syndrome novel coronavirus‐2 infection: fact or fiction? J Card Fail. 2021;27:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liu J, Deswal A, Khalid U. COVID‐19 myocarditis and long‐term heart failure sequelae. Curr Opin Cardiol. 2021;36:234–40. [DOI] [PubMed] [Google Scholar]
- 113. Agdamag ACC, Edmiston JB, Charpentier V, Chowdhury M, Fraser M, Maharaj VR, et al. Update on COVID‐19 myocarditis. Medicina (Kaunas). 2020;56:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Loo J, Spittle DA, Newnham M. COVID‐19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76:412–20. [DOI] [PubMed] [Google Scholar]
- 115. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, et al. CD147‐spike protein is a novel route for SARS‐CoV‐2 infection to host cells. Signal Transduct Target Ther. 2020;5:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shilts J, Crozier TWM, Greenwood EJD, Lehner PJ, Wright GJ. No evidence for basigin/CD147 as a direct SARS‐CoV‐2 spike binding receptor. Sci Rep. 2021;11:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ahmetaj‐Shala B, Vaja R, Atanur SS, George PM, Kirkby NS, Mitchell JA. Cardiorenal tissues express SARS‐CoV‐2 entry genes and basigin (BSG/CD147) increases with age in endothelial cells. JACC Basic Transl Sci. 2020;5:1111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR. Electron microscopy of SARS‐CoV‐2: a challenging task. Lancet. 2020;395:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Scholkmann F, Nicholls J. Pulmonary vascular pathology in Covid‐19. N Engl J Med. 2020;383:887–8. [DOI] [PubMed] [Google Scholar]
- 123. Akilesh S, Nicosia RF, Alpers CE, Tretiakova M, Hsiang TY, Gale M Jr, et al. Characterizing viral infection by electron microscopy: lessons from the coronavirus disease 2019 pandemic. Am J Pathol. 2021;191:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bernard I, Limonta D, Mahal LK, Hobman TC. Endothelium infection and dysregulation by SARS‐CoV‐2: evidence and caveats in COVID‐19. Viruses. 2020;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH 3rd, et al. SARS‐CoV‐2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–46.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. McCracken IR, Saginc G, He L, Huseynov A, Daniels A, Fletcher S, et al. Lack of evidence of ACE2 expression and replicative infection by SARSCoV‐2 in human endothelial cells. Circulation. 2021;143:865–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Colmenero I, Santonja C, Alonso‐Riano M, Noguera‐Morel L, Hernandez‐Martin A, Andina D, et al. SARS‐CoV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Colmenero I, Santonja C, Alonso‐Riano M, Andina D, Rodriguez Peralto JL, Requena L, et al. SARS‐CoV‐2 has not been detected directly by electron microscopy in the endothelium of chilblain lesions: reply from authors. Br J Dermatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Colmenero I, Santonja C, Alonso‐Riano M, Andina D, Rodriguez Peralto JL, Requena L, et al. SARS‐CoV‐2 has not been detected directly by electron microscopy in the endothelium of chilblain lesions: reply from the authors. Br J Dermatol. 2021;184:186–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Colmenero I, Santonja C, Alonso‐Riano M, Andina D, Rodriguez‐Peralto JL, Requena L, et al. Chilblains and COVID‐19: why SARS‐CoV‐2 endothelial infection is questioned. Reply from the authors. Br J Dermatol. 2020;183:1153–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Albert CL, Carmona‐Rubio AE, Weiss AJ, Procop GG, Starling RC, Rodriguez ER. The enemy within: sudden‐onset reversible cardiogenic shock with biopsy‐proven cardiac myocyte infection by severe acute respiratory syndrome coronavirus 2. Circulation. 2020;142:1865–70. [DOI] [PubMed] [Google Scholar]
- 132. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gauchotte G, Venard V, Segondy M, Cadoz C, Esposito‐Fava A, Barraud D, et al. SARS‐Cov‐2 fulminant myocarditis: an autopsy and histopathological case study. Int J Leg Med. 2021;135:577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Best Rocha A, Stroberg E, Barton LM, Duval EJ, Mukhopadhyay S, Yarid N, et al. Detection of SARS‐CoV‐2 in formalin‐fixed paraffin‐embedded tissue sections using commercially available reagents. Lab Invest. 2020;100:1485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, et al. Comparative pathogenesis of COVID‐19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sellers SA, Hagan RS, Hayden FG, Fischer WA 2nd. The hidden burden of influenza: a review of the extra‐pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11:372–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Imazio M, Klingel K, Kindermann I, Brucato A, De Rosa FG, Adler Y, et al. COVID‐19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106:1127–31. [DOI] [PubMed] [Google Scholar]
- 138. Mansueto G, Niola M, Napoli C. Can COVID 2019 induce a specific cardiovascular damage or it exacerbates pre‐existing cardiovascular diseases? Pathol Res Pract. 2020;216:153086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nardo AD, Schneeweiss‐Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID‐19. Liver Int. 2021;41:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Huang C, Li Q, Xu W, Chen L. Molecular and cellular mechanisms of liver dysfunction in COVID‐19. Discov Med. 2020;30:107–12. [PubMed] [Google Scholar]
- 141. Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: an A‐Z literature review. Dig Liver Dis. 2021;53:146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Elsoukkary SS, Mostyka M, Dillard A, Berman DR, Ma LX, Chadburn A, et al. Autopsy findings in 32 patients with COVID‐19: a single‐institution experience. Pathobiology. 2021;88:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Saini RK, Saini N, Ram S, Soni SL, Suri V, Malhotra P, et al. COVID‐19 associated variations in liver function parameters: a retrospective study. Postgrad Med J. 2020. Nov 12:postgradmedj‐2020‐138930 [DOI] [PubMed] [Google Scholar]
- 144. Kaneko S, Kurosaki M, Nagata K, Taki R, Ueda K, Hanada S, et al. Liver injury with COVID‐19 based on gastrointestinal symptoms and pneumonia severity. PLoS One. 2020;15:e0241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Schmit G, Lelotte J, Vanhaebost J, Horsmans Y, Van Bockstal M, Baldin P. The liver in COVID‐19‐related death: protagonist or innocent bystander? Pathobiology. 2021;88:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lei HY, Ding YH, Nie K, Dong YM, Xu JH, Yang ML, et al. Potential effects of SARS‐CoV‐2 on the gastrointestinal tract and liver. Biomed Pharmacother. 2021;133:111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Galanopoulos M, Gkeros F, Doukatas A, Karianakis G, Pontas C, Tsoukalas N, et al. COVID‐19 pandemic: pathophysiology and manifestations from the gastrointestinal tract. World J Gastroenterol. 2020;26:4579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yang L, Han Y, Nilsson‐Payant BE, Gupta V, Wang P, Duan X, et al. A human pluripotent stem cell‐based platform to study SARS‐CoV‐2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–36.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhao CL, Rapkiewicz A, Maghsoodi‐Deerwester M, Gupta M, Cao W, Palaia T, et al. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID‐19). Hum Pathol. 2020;109:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol. 2020;73:807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Philips CA, Ahamed R, Augustine P. SARS‐CoV‐2 related liver impairment – perception may not be the reality. J Hepatol. 2020;73:991–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, et al. COVID‐19‐associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. AKI in hospitalized patients with COVID‐19. J Am Soc Nephrol. 2021;32:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD. Pathophysiology and pathology of acute kidney injury in patients with COVID‐19. Adv Chronic Kidney Dis. 2020;27:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zheng X, Zhao Y, Yang L. Acute kidney injury in COVID‐19: the Chinese experience. Semin Nephrol. 2020;40:430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Satturwar S, Fowkes M, Farver C, Wilson AM, Eccher A, Girolami I, et al. Postmortem findings associated with SARS‐CoV‐2: systematic review and meta‐analysis. Am J Surg Pathol. 2021;45:587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Stasi A, Castellano G, Ranieri E, Infante B, Stallone G, Gesualdo L, et al. SARS‐CoV‐2 and viral Sepsis: immune dysfunction and implications in kidney failure. J Clin Med. 2020;9:4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Huang Y, Li XJ, Li YQ, Dai W, Shao T, Liu WY, et al. Clinical and pathological findings of SARS‐CoV‐2 infection and concurrent IgA nephropathy: a case report. BMC Nephrol. 2020;21:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shetty AA, Tawhari I, Safar‐Boueri L, Seif N, Alahmadi A, Gargiulo R, et al. COVID‐19‐associated glomerular disease. J Am Soc Nephrol. 2021;32:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Akilesh S, Nast CC, Yamashita M, Henriksen K, Charu V, Troxell ML, et al. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID‐19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis. 2021;77:82–93.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chueh TI, Zheng CM, Hou YC, Lu KC. Novel evidence of acute kidney injury in COVID‐19. J Clin Med. 2020;9:3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Parmar MS. Acute kidney injury associated with COVID‐19 – cumulative evidence and rationale supporting against direct kidney injury (infection). Nephrology (Carlton). 2021;26:239–47. [DOI] [PubMed] [Google Scholar]
- 164. Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney Int. 2020;98:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Remmelink M, De Mendonca R, D'Haene N, De Clercq S, Verocq C, Lebrun L, et al. Unspecific post‐mortem findings despite multiorgan viral spread in COVID‐19 patients. Crit Care. 2020;24:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS‐CoV‐2. J Am Soc Nephrol. 2020;31:1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Miller SE, Brealey JK. Visualization of putative coronavirus in kidney. Kidney Int. 2020;98:231–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Delsante M, Rossi GM, Gandolfini I, Bagnasco SM, Rosenberg AZ. Kidney involvement in COVID‐19: need for better definitions. J Am Soc Nephrol. 2020;31:2224–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Calomeni E, Satoskar A, Ayoub I, Brodsky S, Rovin BH, Nadasdy T. Multivesicular bodies mimicking SARS‐CoV‐2 in patients without COVID‐19. Kidney Int. 2020;98:233–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Neil D, Moran L, Horsfield C, Curtis E, Swann O, Barclay W, et al. Ultrastructure of cell trafficking pathways and coronavirus: how to recognise the wolf amongst the sheep. J Pathol. 2020;252:346–57. [DOI] [PubMed] [Google Scholar]
- 171. Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med. 2020;383:590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. Kidney biopsy findings in patients with COVID‐19. J Am Soc Nephrol. 2020;31:1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Brook OR, Piper KG, Mercado NB, Gebre MS, Barouch DH, Busman‐Sahay K, Starke CE, Estes JD, Martinot AJ, Wrijil L, Ducat S, Hecht JL. Feasibility and safety of ultrasound‐guided minimally invasive autopsy in COVID‐19 patients. Abdominal Radiology. 2021;46(3):1263–1271. 10.1007/s00261-020-02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID‐19 infections in Washington State: a case series. Lancet. 2020;396:320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Massoth LR, Desai N, Szabolcs A, Harris CK, Neyaz A, Crotty R, et al. Comparison of RNA in situ hybridization and immunohistochemistry techniques for the detection and localization of SARS‐CoV‐2 in human tissues. Am J Surg Pathol. 2021;45:14–24. [DOI] [PubMed] [Google Scholar]
- 176. Li L, Tan C, Zeng J, Luo C, Hu S, Peng Y, et al. Analysis of viral load in different specimen types and serum antibody levels of COVID‐19 patients. J Transl Med. 2021;19:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Jin XD, Li Y, Song YS, Yang ZZ, Wang P, Wei TT, et al. Progress in research on the detection of the novel coronavirus in human samples of different groups. Eur Rev Med Pharmacol Sci. 2020;24:10879–84. [DOI] [PubMed] [Google Scholar]
- 178. de Jong FC, GeurtsvanKessel CH, Molenkamp R, Bangma CH, Zuiverloon TCM. Sewage surveillance system using urological wastewater: Key to COVID‐19 monitoring?. Urologic Oncology: Seminars and Original Investigations. 2020. 10.1016/j.urolonc.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Roshandel MR, Nateqi M, Lak R, Aavani P, Sari Motlagh R, F Shariat S, et al. Diagnostic and methodological evaluation of studies on the urinary shedding of SARS‐CoV‐2, compared to stool and serum: a systematic review and meta‐analysis. Cell Mol Biol (Noisy‐le‐Grand). 2020;66:148–56. [PubMed] [Google Scholar]
- 180. Medema G, Been F, Heijnen L, Petterson S. Implementation of environmental surveillance for SARS‐CoV‐2 virus to support public health decisions: opportunities and challenges. Curr Opin Environ Sci Health. 2020;17:49–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Frithiof R, Bergqvist A, Jarhult JD, Lipcsey M, Hultstrom M. Presence of SARS‐CoV‐2 in urine is rare and not associated with acute kidney injury in critically ill COVID‐19 patients. Crit Care. 2020;24:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. de‐Madaria E , Capurso G. COVID‐19 and acute pancreatitis: examining the causality. Nat Rev Gastroenterol Hepatol. 2021;18:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H. COVID‐19 presenting as acute pancreatitis. Pancreatology. 2020;20:1026–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Anand ER, Major C, Pickering O, Nelson M. Acute pancreatitis in a COVID‐19 patient. Br J Surg. 2020;107:e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Inamdar S, Benias PC, Liu Y, Sejpal DV, Satapathy SK, Trindade AJ, et al. Prevalence, risk factors, and outcomes of hospitalized patients with coronavirus disease 2019 presenting as acute pancreatitis. Gastroenterology. 2020;159:2226–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hollstein T, Schulte DM, Schulz J, Gluck A, Ziegler AG, Bonifacio E, et al. Autoantibody‐negative insulin‐dependent diabetes mellitus after SARS‐CoV‐2 infection: a case report. Nat Metab. 2020;2:1021–4. [DOI] [PubMed] [Google Scholar]
- 187. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kamrath C, Monkemoller K, Biester T, Rohrer TR, Warncke K, Hammersen J, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID‐19 pandemic in Germany. JAMA. 2020;324:801–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center, clinicopathologic case series. Ann Intern Med. 2020;173:350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Bhatnagar J, Gary J, Reagan‐Steiner S, Estetter LB, Tong S, Tao Y, et al. Evidence of SARS‐CoV‐2 replication and tropism in the lungs, airways and vascular endothelium of patients with fatal COVID‐19: an autopsy case‐series. J Infect Dis. 2021;223:752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Muller JA, Gross R, Conzelmann C, Kruger J, Merle U, Steinhart J, et al. SARS‐CoV‐2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149–65. [DOI] [PubMed] [Google Scholar]
- 192. Coate KC, Cha J, Shrestha S, Wang W, Goncalves LM, Almaca J, et al. SARS‐CoV‐2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in beta cells. Cell Metab. 2020;32:1028–40.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Mansueto G. COVID‐19: brief check through the pathologist's eye (autopsy archive). Pathol Res Pract. 2020;216:153195. [DOI] [PMC free article] [PubMed] [Google Scholar]


