Abstract
Dopamine, the main catecholamine neurotransmitter in the brain, is predominately produced in the basal ganglia and released to various brain regions including the frontal cortex, midbrain and brainstem. Dopamine’s effects are widespread and include modulation of a number of voluntary and innate behaviors. Vigilant regulation and modulation of dopamine levels throughout the brain is imperative for proper execution of motor behaviors, in particular speech and other types of vocalizations. While dopamine’s role in motor circuitry is widely accepted, its unique function in normal and abnormal speech production is not fully understood. In this perspective, we first review the role of dopaminergic circuits in vocal production. We then discuss and propose the conceivable involvement of astrocytes, the numerous star-shaped glia cells of the brain, in the dopaminergic network modulating normal and abnormal vocal productions.
Keywords: astrocytes, basal ganglia, dopamine, glia, speech, vocalization
1. Introduction
Speech production is one of the most complex motor behaviors, requiring precise coordination of activities between multiple functional areas in the brain and more than 100 muscles in the body, including laryngeal, supralaryngeal, and respiratory muscles. The complete brain circuitry involved in vocal production is not fully identified; however, it has been established that both archetypal brain regions [i.e., nucleus ambiguous (NA), periaqueductal gray (PAG), and striatum] as well as highly developed areas [i.e., laryngeal motor cortex (LMC) and supplementary motor area (SMA)] are critically involved in the motor control and modulation of vocalizations (i.e. vocal production) (Alm, 2004; Jarvis, 2019a; Uwe Jürgens, 2002; Simonyan, 2014). Moreover, orofacial precision for articulation, synchronization of breathing behaviors with speech output, and higher level motor controls are critically involved and synchronized in successful generation of fluent speech (Jarvis, 2019a; Uwe Jürgens, 2002; Sommer, Koch, Paulus, Weiller, & Büchel, 2002). Better understanding the brain motor circuits controlling vocal communication may help identify pathophysiology of speech motor disorders.
Similar to other complex motor behaviors, elaborative circuitry and numerous neurotransmitters are involved in conducting the intricacies of vocal production circuits. It was proposed that vocalization is dependent on circuits stemming from the basal ganglia, a telencephalic brain region integral for initiation and maintenance of motor behavior, to multiple brain areas including the cerebral cortex, midbrain and the brainstem (Figure 1) (Creese & Iversen, 1975; Scheel-Krüger & Randrup, 1967). Moreover, studies in experimental animal models, including rodents, songbirds, and non-human primates (NHPs) (Arriaga, Zhou, & Jarvis, 2012; Ciucci, Vinney, Wahoske, & Connor, 2010; Jarvis, 2019a; Uwe Jürgens, 2002), suggest that dopamine, a neurotransmitter intimately involved in motor behaviors, plays a central role in vocal production circuits of the brain (Dastur, McGregor, & Brown, 1999; de Lanerolle & Youngren, 1978; Jenner et al., 1984; Rose, Nomoto, Jenner, & Marsden, 1989; Sasaki, Sotnikova, Gainetdinov, & Jarvis, 2006). These models, in conjunctions with human imaging studies, have been imperative for defining the dopaminergic circuitry controlling the vocal production network (Fuertinger, Zinn, Sharan, Hamzei-Sichani, & Simonyan, 2018; Simonyan & Fuertinger, 2015; Simonyan, Herscovitch, & Horwitz, 2013).
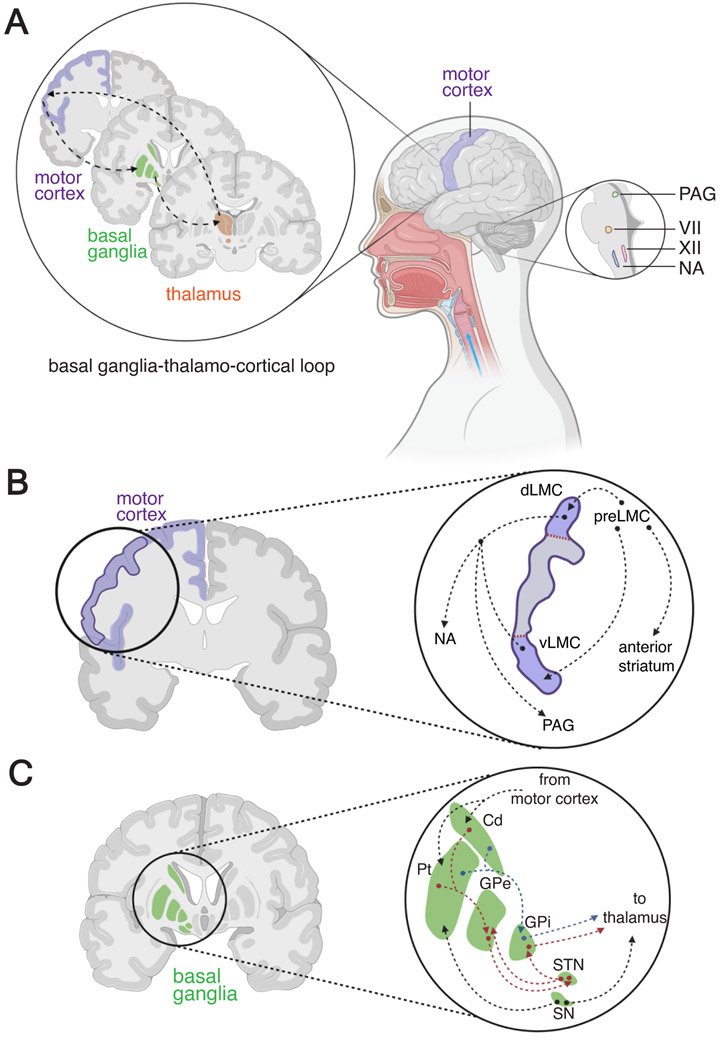
Figure 1 ∣. Basal ganglia and motor control of speech production circuits.
(A) The basal ganglia thalamo-cortical loop that connects the motor circuits of the basal ganglia to the laryngeal motor cortex through a relay station in the thalamus is important for speech production. The higher order brain structures, directly or indirectly [via periaquedutal gray (PAG)], modulate activities of orofacial and laryngeal motor neurons of facial (VII), hypoglossal (XII) nuclei and nucleus ambiguus (NA) located in the brainstem. (B) The laryngeal motor cortex (LMC) is located towards the ventro-lateral portion of primary motor cortex, and divided into dorsal (dLMC) and ventral (vLMC) regions. Both portions of LMC are involved in the vocal production circuits. Neurons in the preLMC sends direct connections to dLMC and vLMC as well as to the anterior striatum. dLMC and vLMC neurons send projections to PAG and NA . (C) The Basal ganglia region includes the striatum (caudate and putamen), globus pallidus [external (GPe) and internal (GPi)], subthalamic nucleus (STN) and substantial nigra (SN). The intricate connectivity between these regions allows for proper execution of motor behaviors. Information from motor cortex is inputted to the striatum which in conjunction with dopaminergic inputs from the SN, sends information to two parallel pathways in the basal ganglia. In the first pathway (red arrows), the GPe received inhibitory signals from the striatum and relays inhibitory signals to the subthalamic nucleus. The subthalamic nucleus then send an excitatory signal to the GPi which in turn sends an output signal to the thalamus. In the second, parallel pathway (blue arrows), the striatum ends a direct inhibitory signal to the GPi which directly sends an output signal to the thalamus. By integrating inhibitory and excitatory signals, these two pathways can delicately modulate motor behaviors control, including speech production. Abbreviations: Cd – caudate, dLMC – dorsal laryngeal motor cortex, GPe – globus pallidus external, GPi – globus pallidus internal, NA – nucleus ambiguus, PAG – periaqueductal gray, preLMC – premotor laryngeal motor cortex, Pt – putamen, SN – Substantia Nigra, STN – sub thalamic nucleus, vLMC – ventral laryngeal motor cortex, VII – facial nucleus, XII – hypoglossal nucleus.
Similar to other communication systems, both production and perception of vocalization are critical aspects of vocal communication. Vocalization control circuits integrate a myriad of sensory functions and information from various brain regions, notably the auditory cortex (Eliades & Wang, 2003; Moore & Woolley, 2019), somatosensory cortex, cerebellum, and thalamus (Riecker, Kassubek, Gröschel, Grodd, & Ackermann, 2006). In doing so, communication can become a conversation through combining new information, articulating thoughts, producing meaningful speech and incorporating realtime feedback to correct mistakes (Chow & Chang, 2017). Although acoustic feedback control of speech is critical for vocal production, we do not discuss audio-motor interactions here, as it is reviewed comprehensively elsewhere (Poeppel & Assaneo, 2020). Moreover, it is critical to note a distinction between innate and learned vocalizations. While vocal communications with conspecifics in some species, such as humans and songbirds, is mainly learned, other species, such as rodents, mostly use innate vocalizations to communicate (Jarvis, 2007, 2019a; Petkov & Jarvis, 2012; Shu et al., 2005). This distinction is important when considering the brain regions involved in the vocalization circuits (Arriaga et al., 2012). In addition, it has been shown recently that astrocytes are critically involved in the control of complex motor behaviors (Morquette et al., 2015; Sheikhbahaei, Turovsky, et al., 2018) and can modulate dopaminergic neural circuits (Corkrum et al., 2020; Khan, Koulen, Rubinstein, Grandy, & Goldman-Rakic, 2001; Xin et al., 2019). In this perspective, we focus on vocal production circuits in the brain and the role of dopamine in regulation of these circuits. We then discuss the role of dopamine and the basal ganglia in normal and abnormal vocal production. Lastly, we propose a possible mechanism for involvement of astrocytes in the dopaminergic modulation of vocalization. A disruption to the proposed mechanism might lead to speech disorders, including stuttering disorders.
2. Vocal production circuits
2.1. Physiological considerations in vocalization pathways
Breathing is vital for voice production. The normal breathing cycle consists of three behaviorally distinct sequential phases: inspiration, post-inspiration, and expiration (Del Negro, Funk, & Feldman, 2018; Richter & Smith, 2014). Breathing and vocalization are highly coordinated, as vocalization is restricted to periods during post-inspiration and expiration. Therefore, speech production requires a precise coordination of laryngeal muscle action with activities of brain circuits controlling orofacial and respiratory movements. In addition, proprioceptive input from mechanoreceptors and chemoreceptors in the larynx, orofacial region, and respiratory system that affect rhythm or pattern of breathing may provide feedback controls and might affect vocal production (Andalman, Foerster, & Fee, 2011; Dutschmann & Herbert, 2006; Forster, 2003; Uwe Jürgens, 2002; Mörschel & Dutschmann, 2009; Mulkey et al., 2004; O’Regan & Majcherczyk, 1982; Sheikhbahaei, Turovsky, et al., 2018; Sheikhbahaei, Gourine, & Smith, 2017; Sheikhbahaei & Smith, 2017; Teppema & Dahan, 2010).
2.2. Involvement of the Basal Ganglia
It has been shown that the basal ganglia and its main neurotransmitter, dopamine, have an important role in speech production (U Jürgens, 2009; Uwe Jürgens, 2002; Kristina Simonyan et al., 2013; Kristina Simonyan, Horwitz, & Jarvis, 2012). The conventional model suggests that neurons from the LMC project to the striatum (putamen and caudate nucleus) which itself sends projections to the NA, the brainstem motor nucleus that enervates laryngeal muscles (Uwe Jürgens, 2002; Kristina Simonyan & Jürgens, 2003; Kristina Simonyan, Ostuni, Ludlow, & Horwitz, 2009). In addition to NA, the motor nuclei of the trigeminal, facial (VII), and hypoglossal (XII) are also involved in vocal production (Uwe Jürgens, 2002) (Figure 1).
In addition to the established model, an "anterior pathway," including the anterior SMA, and vocal premotor cortex, namely premotor laryngeal motor cortex (preLMC) and inferior frontal gyrus (Broca's area), are also suggested to be involved in speech production (Jarvis, 2019b). Brain regions in this pathway send converging projections to the anterior striatum (Figure 1B). This information is integrated into the basal ganglia thalamo-cortical loop, a network that connects the motor circuits of the basal ganglia to the higher order processing centers in the cortex through the relay station in the thalamus. Specifically, the ventral tegmental area (VTA) as well as the substantia nigra pars compacta (SN) have been found in NHPs to send direct projections to LMC (Jürgens, 1982; K Simonyan & Jürgens, 2005). For speech motor learning, the anterior striatum sends projections to the anterior thalamus which relays back to the preLMC (Jarvis, 2019a).
As discussed above, various regions of the basal ganglia are involved in vocal production, however, the anterior striatum and the subthalamic nucleus receive direct dopaminergic input from the SNc and VTA (Hornykiewicz, 1966; Rice, Patel, & Cragg, 2011) and may have a direct role in speech production. In this setting, the striatum is proposed to have specific roles in speech initiation and vocal production and cessation of vocalizations (Price, 2010), the subthalamic nucleus may also be involved in initiation of speech production (Hebb, Darvas, & Miller, 2012).
3. Dopamine
Dopamine is one of the main catecholamine neurotransmitters in the central nervous system (CNS) (Hornykiewicz, 1966). The level of dopamine in the basal ganglia is well regulated via various mechanisms involves neurons and astrocytes (Adrover et al., 2020; Björklund & Dunnett, 2007; Rice et al., 2011; Y Smith & Kieval, 2000; Vaughan & Foster, 2013). Hypoactivity or hyperactivity of dopamine lead to a general inhibition or disinhibition of motor behaviors, respectively, that manifest in a range of motor control disorders, such as Parkinson’s disease, amyotrophic lateral sclerosis (ALS), Tourette’s Syndrome, and developmental stuttering (Alm, 2004; Bromberg-Martin, Matsumoto, & Hikosaka, 2010; Crocker, 1997; Groenewegen, 2003; Laverty, 1978).
3.1. Dopamine synthesis
Catecholamines are synthesized through a number of steps with tyrosine hydroxylase catalyzing the rate limiting step of this process, converting tyrosine to Levodopa (L-DOPA). L-DOPA is then decarboxylated by amino acid decarboxylase to form an early form of dopamine (Kaushik, Gorin, & Vali, 2007; Vaughan & Foster, 2013). This predopamine transmitter is packaged into a vesicle along with other enzymes to continue dopamine synthesis inside the transmission vesicles in pre-synaptic neurons (Daubner, Le, & Wang, 2011; Hornykiewicz, 1966; Kaushik et al., 2007; Sourkes, 1971). Upon neuronal activation, dopamine is released into the synaptic cleft where it can bind to receptors on post-synaptic cell membranes. The excess dopamine is subsequently recycled back to the pre-synaptic neuron through the dopamine transporter (DAT) or is broken down by monoamine oxidase (MAO) and/or catechol-O-methyltransferase (COMT). This synthesis-release-reuptake process controls and regulates physiological dopaminergic responses to bodily demands (Blakely & Bauman, 2000; Rice et al., 2011; Vaughan & Foster, 2013).
3.2. Dopamine functions
Dopamine is involved in activities of many brain circuits including those that control movement (Creese & Iversen, 1974; Hornykiewicz, 1973), cognition (Brozoski, Brown, Rosvold, & Goldman, 1979; Joseph, Frith, & Waddington, 1979; Nakajima et al., 2013), emotion (de Lanerolle & Youngren, 1978; Goschke & Bolte, 2014; Laverty, 1978), reward (Bromberg-Martin et al., 2010; Christie & Crow, 1971; Schultz, 2007; Shugart et al., 2004; Vaughan & Foster, 2013), and others. Interestingly, although dopamine is involved in a variety of behaviors, the majority of dopamine in the brain is released by a subpopulation of neurons in the SNc and VTA that provide the neurotransmitter dopamine to the rest of the brain (Björklund & Dunnett, 2007; Fürtinger, Zinn, & Simonyan, 2014; Fuxe, 1965; Hornykiewicz, 1966; Lewis & Sesack, 1997; Rommelfanger & Wichmann, 2010; Simonyan et al., 2012).
4. Dopamine in vocalization circuits
The structure and function of the vocal production circuits and the involvement of dopamine in this network has been studied in several animal models , including chickens, songbirds, rodents, and NHPs. As previously noted and in comparison to the innate vocalizations in chicken and rodents, speech and song in human and songbirds, respectively, are considerered as learned vocalizations. In young chicks, where trill calls can be evaluated, injection of apomorphine hydrochloride, a dopaminergic receptors agonist, elicited a significantly higher rate of trill calls (de Lanerolle & Youngren, 1978) elucidating the possible role that dopamine is modulating chick vocal production. Songbirds are another animal model that has been used extensively to look at vocalization and in particular, vocal learning (Chakraborty et al., 2017; A. N. Chen & Meliza, 2020; Jarvis, 2019a; Kubikova et al., 2014). In vivo microdialysis (see also Appendix A for more details) of dopamine in awake, behaving songbirds has established that singing induces an increase in dopamine in area X, a nucleus located within the equivalent to the human striatum and similarly receives dopaminergic input from VTA and SNc, (Sasaki et al., 2006). Lesion studies in area X lead to increased syllable repetitions and a stuttering-like vocalization phenotype (Kubikova et al., 2014). Moreover, overexpression of N-methyl-D-aspartate (NMDA) subtype 2B glutamate receptor (NR2B) gene in lateral magnocellular nucleus of the anterior nidopallium (LMAN), a cortical region that has direct connections to area X, also induced changes in pitch and rhythmicity of vocalizations (i.e., stuttering-like vocalization) (Chakraborty et al., 2017). These studies help define a prominent role for both the basal ganglia and cortical-basal ganglia circuits in songbird vocalization.
In rodent pups, injection of either an agonists of dopamine receptor D1 (D1R) or dopamine receptor D3 (D3R) reduced the frequency of ultrasonic vocalizations (Dastur et al., 1999), further suggesting a critical role for dopamine in the vocalization pathway of rodents too. These studies suggest that dopamine’s effects within the basal ganglia and cerebral cortex dictate the ability to produce a vocalization. Dopamine may also have the capability to create proper pitch and fluidity of vocalization (Bragoni et al., 2000; Goberman & Blomgren, 2003; Uwe Jürgens, 2002; Lan et al., 2009; Poeppel & Assaneo, 2020; Simonyan et al., 2012). In particular, dopamine released in the striatum, may directly modulate neural activities of the speech motor regions in the basal ganglia and primary motor cortex (Crocker, 1997; Salamone, 1992). Similar to many motor behaviors, the basal ganglia-thalamo-cortical connections are also involved in speech production. The difference arises from where the dopamine release occurs; and for vocalization circuits, this termination occurs in the LMC (Boë, Fagot, Perrier, & Schwartz, 2017; Kumar, Croxson, & Simonyan, 2016; Mor, Simonyan, & Blitzer, 2018; Simonyan et al., 2012).
Clinical studies in humans further evaluated the involvement of dopamine in vocalization and speech (Bragoni et al., 2000; Goberman & Blomgren, 2003). The left hemisphere of the human brain is well-known for its particular involvement in speech production (Fuertinger et al., 2018; Jackson, 1915; Loring et al., 1990; Vigneau et al., 2006). Recently, combinations of positron emission tomography (PET) with dopamine receptors radioligands, functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI) and/or diffusion-weighed MRI (Fuertinger et al., 2018; Simonyan et al., 2013) were used to show that during speech production, release of dopamine from the SNc and VTA was lateralized to the left ventromedial striatum (Fuertinger et al., 2018; Simonyan et al., 2013) and LMC (Fuertinger et al., 2018).
Evaluating the dopaminergic circuits in various animal models is crucial to understand how this system works and to gain insight on the role of dopamine in human brain. It is imperative that there be an understanding of similarities and differences between animal models and humans such that the information can be properly taken into account when treating human patients. An investigation of the basal level of plasma dopamine in various species, including rodents and humans, shed light on the fact that the resting dopamine values vary greatly between humans and rats (Bühler, da Prada, Haefely, & Picotti, 1978). Not only are the basal levels different, but the mechanisms of action to breakdown dopamine seem to differ as well. Whereas primates (including humans and NHPs) use MAO-A predominately, rodents seem to use MAO-B (Garrick & Murphy, 1980). However, while these differences exist, commonalities between species are apparent too. Most species seem to use the various dopamine receptors similarly (Bäckman et al., 2011; Seamans & Yang, 2004; Zahrt, Taylor, Mathew, & Arnsten, 1997) and commonalities in dopamine release into neural networks, particularly in motor control and reward circuits (Chini & Hanganu-Opatz, 2020; Grimm, Balsters, & Zerbi, 2020; Peters, Liu, & Komiyama, 2017; Walton & Bouret, 2019), have allowed investigators to confidently use various animal models to understand the dopaminergic circuits at large.
In addition to physiological functions, the role of dopamine in the control of speech production circuits can be further understood through its role in the pathophysiology of disorders that affect the control of motor circuits, such as Parkinson’s disease and developmental stuttering disorder (Alm, 2004; Elgueta et al., 2017; Goberman & Blomgren, 2003; Yoland Smith & Villalba, 2008; Tani & Sakai, 2011). A precise control of dopamine release creates an intricate balance in the brain and modulation of dopamine release is associated with impairments in vocalization, such that a decrease in dopamine affects vocalization in Parkinson’s disease, whereas an increase in dopamine level is associated with childhood-onset fluency disorder (also known as stuttering). In patients with Parkinson’s disease, in which dopaminergic neurons in the striatum prominently degenerate, changes in vocalization quality, including a declined loudness, decrease in a range of vocal pitch, and a lack of expression during speaking are reported (Ciucci et al., 2010; Goberman & Blomgren, 2003; Walsh & Smith, 2011). Interestingly, Parkinson’s patients treated with dopamine receptor antagonists report alterations in vocal production that reverse once the patients stops taking the medication (Newton-John, 1988; Warren & Thompson, 1998).
5. Dopamine in childhood-onset fluency disorder (stuttering)
Childhood-onset fluency disorder is a neurodevelopmental disorder (Ludlow & Loucks, 2003; Mawson, Radford, & Jacob, 2016; A. Smith & Weber, 2016; Watkins, Smith, Davis, & Howell, 2008) that affects normal fluency of speech which is not appropriate for individual’s age (American Psychiatric Association, 2013). Although the etiology of stuttering is not fully understood, many lines of evidence suggest that defects in dopaminergic circuits in the basal ganglia may be involved in the development of stuttering disorders in a subgroup of people who stutter; some adults who stutter may have three-times higher level of dopamine precursors in the basal ganglia (Wu et al., 1995, 1997).
This excess dopamine hypothesis of stuttering is supported by recent clinical and imaging studies (Alm, 2004; Chang & Guenther, 2019; G A Maguire, Riley, Franklin, & Gottschalk, 2000; Gerald A. Maguire et al., 2004; Gerald A Maguire et al., 2019; Gerald A Maguire, Nguyen, Simonson, & Kurz, 2020; Gerald A. Maguire, Yeh, & Ito, 2012; Gerald A. Maguire, Yoo, & SheikhBahaei, 2021). Because of this unique relationship, dopaminergic agents have been used as a potential therapy in clinical trials for patients with stuttering disorders. Although dopamine receptor D2 (D2R) blockers (such as risperidone, olanzapine, and haloperidol) reduce stuttering symptoms significantly, their broader effects on modulation of serotonin (5-HT), acetylcholine, and glutamate receptors, have limited their usage. Recently, Ecopipam, a selective D1R antagonist, and lurasidone, antagonist of D2, 5HT2a and partial agonist at 5HT1a, have been proposed as novel therapies for stuttering disorders (Charoensook & Maguire, 2017; Gerald A Maguire et al., 2019, 2020). Both clinical studies in humans and experiments on animal model further the importance of dopaminergic signaling in vocal production circuits of the basal ganglia.
6. Astrocytes, dopamine and stuttering
Astrocytes, the numerous star-shaped glial cells of the brain, play a myriad of roles including regulation of extracellular concentration of ions and neurotransmitters, regulation of the blood-brain barrier, and have been proposed to regulate neuronal excitability and synaptic plasticity (Abbott, Rönnbäck, & Hansson, 2006; Alfonso Araque et al., 2014; Haydon & Carmignoto, 2006). Physiology of glial cells is managed by changes in their intracellular Ca2+ concentration ([Ca2+]i) in a way that increases in [Ca2+]i triggers release of gliotransmitters (such as ATP/adenosine, D-serine, glutamate and others) (Heinrich, Andó, Túri, Rózsa, & Sperlágh, 2012; Marina et al., 2018; Martineau et al., 2013; Sheikhbahaei, Turovsky, et al., 2018).
Recent studies have suggested that in addition to their neuro-regulatory effects, astrocytes can directly modulate motor circuits in vivo and impact complex behaviors (Sheikhbahaei, Turovsky, et al., 2018). While the exact role of astrocytes in motor circuitry remains elusive, key features of astrocytes provide evidence for their importance. For instance, changes of [Ca2+]i in astrocytes in response to neuronal activity not only triggers gliotransmitter release but also may modulate neuronal activity (A Araque, Carmignoto, & Haydon, 2001; Alfonso Araque et al., 2014; Haydon & Carmignoto, 2006; Marina et al., 2018; Pascual et al., 2005; Santello, Calì, & Bezzi, 2012). Accumulating evidence for this glia-induced modulation of complex behaviors is illustrated in many circuits. For instance, in locomotor circuits where it is postulated that rhythmic releases of [Ca2+]i modulate the neural circuit of locomotion (Christensen, Petersen, & Perrier, 2013; Hegyi et al., 2018; Hegyi, Kis, Holló, Ledent, & Antal, 2009), in mastication circuitry where astrocytes seem to be involved in the regulation of extracellular Ca2+ by release of s100ß (Morquette et al., 2015), and in respiratory circuitry where [Ca2+]i-dependent release of ATP from brainstem astrocytes regulate breathing rhythms (Sheikhbahaei, Turovsky, et al., 2018), respiratory responses to hypoxia (low inspired oxygen) (Angelova et al., 2015; Rajani et al., 2018; Sheikhbahaei, Turovsky, et al., 2018), hypercapnia (high inspired carbon dioxide), and determine exercise capacity (Sheikhbahaei, Turovsky, et al., 2018).
One of the main functions of astrocytes throughout the brain is to maintain a level of homeostasis. One way in which astrocytes accomplish this goal is through the regulation of extracellular neurotransmitter concentrations (Barres, 2008; Clarke & Barres, 2013). As previously mentioned, dopamine has a number of reuptake mechanisms to regulate the levels of extracellular dopamine, and in addition to neurons, astrocytes also express DAT (which recycles the dopamine back to the cell), and COMT/MAO (which break dopamine down into metabolites) (Asanuma, Miyazaki, Murakami, Diaz-Corrales, & Ogawa, 2014; Hansson & Sellström, 1983; Karakaya, Kipp, & Beyer, 2007; Takeda, Inazu, & Matsumiya, 2002). Astrocytes from the rodent cortex have been found to be sensitive to various dopamine concentrations (Requardt et al., 2012; Vaarmann, Gandhi, & Abramov, 2010). It was shown that over 80% of cortical astrocytes took up and metabolized dopamine using COMT and MAO (Hansson & Sellström, 1983; Pelton, Kimelberg, Shipherd, & Bourke, 1981; Takeda et al., 2002). On the other hand, astrocytes from striatum and midbrain express DAT and recycle dopamine through this transporter and break it down into its precursor to be reused (Asanuma et al., 2014; Karakaya et al., 2007).
Astrocytes are heterogenous and may play a variety of modulatory roles depending on their regional location in the brain (Chai et al., 2017; Sheikhbahaei, Morris, et al., 2018; Zhang & Barres, 2010). Recent comparative genomic, structural, and physiological studies have provided clear evidence for circuit-dependent specialization of astrocytes and their functional diversity (Chai et al., 2017; Doyle et al., 2008; Khakh, 2019). In dopaminergic circuits, dopamine-induced calcium transients in astrocytes is independent of neuronal activity (Fischer, Scheffler, & Lohr, 2020). Recently it was shown that even a moderate increase of astroglial resting [Ca2+]i can translate into a considerable increase of gliotransmitter release (King et al., 2020). Therefore, dopamine can induce release of gliotransmitters in a Ca2+-dependent manner. This intimate relationship with dopamine seems to also extend throughout the ventral midbrain dopaminergic circuit where astrocytes have critical functions in the SNc, VTA (Xin et al., 2019) and NAc (Corkrum et al., 2020), and possibly in other regions. In these regions and in response to dopamine and in particular, D2R modulation, astrocytes show a change in [Ca2+]i activity which may lead to a release of gliotransmitters (such as ATP), (Corkrum et al., 2020; Khan et al., 2001; Xin et al., 2019) suggesting that astrocytes may be regulating the dopaminergic circuits in a similar fashion to other motor circuits (Figure 2A).
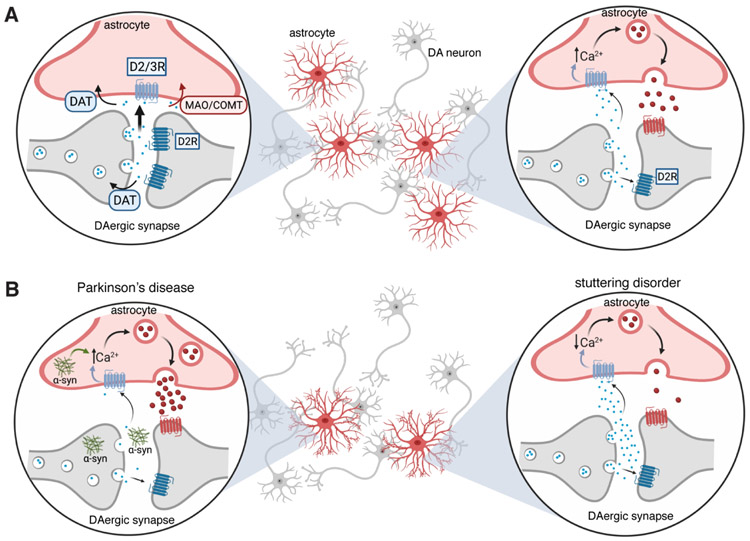
Figure 2 ∣. Astrocytes in dopaminergic circuits of basal ganglia.
(A) (left) Presynaptic dopaminergic neuron releases dopamine (DA) into synaptic cleft, in which, DA binds to the postsynaptic DA receptor (such as D2R) and is also recycled back into the presynaptic neuron by DAT. However, some dopamine transmitter may also be recycled back through astrocytes potentially through DAT and/or COMT to recycle or break down dopamine, respectively. (right) Following the release of dopamine from the presynaptic terminal, dopamine may also bind to the D2R on astrocytes and lead to an increase in the levels of intracellular calcium ([Ca2+]i), which in turn facilitates the release of gliotransmitters (such as ATP/adenosine and others). The released gliotransmitters affect neurons in the region. (B) (left) Dopaminergic neuronal synapse in a Parkinson’s diseased brain where DA degeneration is prominent and less DA is released into the synapse. In addition to release of DA is release of aggregate alpha-synculein (α-syn). DA and aggregated alpha-synculein are taken up by astrocytes, which causes a dysregulation of [Ca2+]i and an increased release of adenosine. (right) Presynaptic dopamine release increases in the case of developmental stuttering disorder which accumulates in the synapse where it can bind to the postsynaptic receptors, be taken back up by the presynaptic neuron or by the nearby astrocyte. In the proposed model, this increase in DA causes a decrease in [Ca2+]i activity and a reduction in release of gliotransmitters. Abbreviations: [Ca2+]i – intracellular calcium concentrations, COMT - catechol-O-methyltransferase, D2R – dopamine receptor D2, D3R – dopamine receptor D3, DA - dopamine, DAT – dopamine transporter, MAO - monoamine oxidase.
In addition to these physiological roles, astrocytes are proposed to be involved in the pathogenesis of numerous motor disorders, including Parkinson’s disease (Booth, Hirst, & Wade-Martins, 2017; P.-C. Chen et al., 2009; Cressatti et al., 2019; Rappold & Tieu, 2010; Yun et al., 2018), amyotrophic lateral sclerosis (Haidet-Phillips et al., 2011; Meyer et al., 2014; Nagai et al., 2007; Yamanaka & Komine, 2018), and Tourette’s Syndrome (de Leeuw et al., 2015; van Passel, Schlooz, Lamers, Lemmens, & Rotteveel, 2001). In Parkinson’s disease, for instance, one of the main pathological features is aggregation of protein alpha-synuclein (α-syn). α-syn will aggregate in the dopaminergic neurons but will also be released into the synapse and taken up by nearby astrocytes (Cressatti et al., 2019; Lee et al., 2011). The lack of available dopamine and the presence of this aggregated protein cause an increase in [Ca2+]i activity in astrocytes (Sonninen et al., 2020) which may lead to an increase in gliotransmitter release, in particular, adenosine. Adenosine will in turn affect the nearby neurons and cause motor symptoms seen in Parkinson’s disease (Hosford et al., 2020; Rappold & Tieu, 2010; Sonninen et al., 2020) (Figure 2B).
Considering the anatomical and functional relationship between dopamine, the motor circuits, and astrocytes, it is plausible that astrocytes can have a significant role in pathogenesis of other disorder that affects motor patterns, such as developmental stuttering. Interestingly, in the newly established mouse models of stuttering (Barnes et al., 2016; Han et al., 2019) (see Appendix B for details), the number of astrocytes in the corpus collosum was decreased (Han et al., 2019). Although the role of astrocytes in the pathogenesis of stuttering is not defined yet, a recent study suggests that the effects of D2R blockers in increasing speech fluency in patients who stutter are mainly due to an increase of astrocytes’ metabolism in the striatum (Gerald A. Maguire et al., 2021). According to this hypothesis, hypoactivity of striatal astrocytes due to excess dopamine concentration in the dopaminergic circuits of the basal ganglia could be one of the mechanisms that might lead to stuttering behavior (Figure 2B). We propose that similar to what is reported in the VTA (Xin et al., 2019), excess dopamine decreases the [Ca2+]i activity of striatal astrocytes. In this model, lower [Ca2+]i in astrocytes leads to less gliotransmitter release and implabance activity of stiatal neurons. Interestingly, it has been shown recently that decreases in [Ca2+]i in striatal astrocytes is linked to repetitive behaviors in rodents (Yu et al., 2018). More experiments are required to idendify the main gliotransmitter released from astrocytes that may affect vocal production circuits, and in general, to further elucidate how astrocytes regulate striatal neurons, dopaminergic circuits, and vocalization behaviors.
7. Summary
In summary, dopamine projection from the basal ganglia to a variety of brain regions involved in motor functions, implicates its importance in voluntary and involuntary complex movements, especially in speech and other types of vocalizations. Recent studies illustrating dopaminergic release in vocal production regions further elucidates the strong possibility that dopamine plays an important role in vocal production. Further, recent data are uncovering the involvement of astrocytes in physiology of motor circuit activities and pathophysiology of motor disorders. This information, together with the fact that astrocytes have a close-knit relationship with the dopaminergic circuit strongly suggest that these glia cells have a critical role in modulating vocal production circuitry. Involvement of astroglial cells in these circuits will provide additional therapeutic target for disorders that affect motor control of speech production, such as stuttering disorder.
High lights:
Dopamine’s role in regulation of motor circuits is accepted, but its function in speech production is not fully understood.
In this perspective, we reviewed the modulatory role of dopamine in vocal production circuits of the basal ganglia.
We proposed a modulatory role for astrocytes residing in the dopaminergic circuits of the basal ganglia in vocal productions.
Acknowledgements:
We thank Mitchell Bishop for constructive comments on the previous version of the manuscript, and BioRender (Biorender.com) for figures. This work was supported by the Intramural Research Program (IRP) of the NIH, NINDS and NIMH (SSB) (ZIA NS009420-01), and the philanthropic funding provided to UC Riverside from RG Kirkup (GAM).
Appendix A ∣. Approaches to quantify dopamine in vivo
Four general approaches have evolved to quantify dopamine or its dynamics in the brain of humans and animals in vivo. They are governed by distinctively different principles, with contrasting advantages and disadvantages.
Microdialysis.
The most common approach to measure tonic, extracellular dopamine at nanomolar concentrations is microdialysis coupled with analytical separations methods (Benveniste & Hüttemeier, 1990; Sasaki et al., 2006). Microdialysis is an invasive sampling technique based on diffusion of small molecules (< 20 kDa) through a semi-permeable membrane and into a perfusing fluid (artificial cerebral spinal fluid for neural samples) (Benveniste & Hüttemeier, 1990; Bucher & Wightman, 2015; Darvesh et al., 2011; Stenken & Patton, n.d.). The collected dialysate is then analyzed, typically with high performance liquid chromatography (HPLC) or capillary electrophoresis (CE) (Jaquins-Gerstl & Michael, 2015; Lu, Peters, & Michael, 1998; Shou, Ferrario, Schultz, Robinson, & Kennedy, 2006). Tonic concentrations of dopamine as small as nanomolar can be detected by microdialysis, with temporal resolution in order of minute, (Heien et al., 2005; Lu et al., 1998; Shou et al., 2006). Microdialysis probes are ≥ 200 μm in diameter, and therefore spatial resolution and tissue damage are matters of concern (Cenci, Kalén, Mandel, & Björklund, 1992; Jaquins-Gerstl & Michael, 2009; Justice, 1993; Parsons & Justice, 1992). Tissue damage can elucidate immune response leading to ineffective sampling (Jaquins-Gerstl & Michael, 2009; Mitala et al., 2008).
Electrochemical methods.
The most common techniques used to measure dopamine transients are amperometry (Dugast, Suaud-Chagny, & Gonon, 1994; Gulley, Larson, & Zahniser, 2007; D. J. Michael & Wightman, 1999; Willuhn, Wanat, Clark, & Phillips, 2010) and fast scan cyclic voltammetry (FSCV) (Bucher & Wightman, 2015; Heien, Johnson, & Wightman, 2004; Venton & Cao, 2020; Wightman et al., 1988). Dopamine undergoes oxidation at the surface of an active electrode, generating a current with an amplitude proportional to dopamine concentration. FSCV has been a gold standard for monitoring naturally occurring or stimulated changes in dopamine concentration by repeatedly applying a triangular potential waveform at high scan rates (> 100 V/s) to a carbon fiber electrode (5 – 40 μm in diameter and 50 – 200 μm in length), protruding from a sealed glass coating and inserted into tissue. Thus, high spatial resolution with minimal tissue damage at the tip is possible (Abdalla et al., 2020; Adrover et al., 2020; Nguyen & Venton, 2015; Roberts, Lugo-Morales, Loziuk, & Sombers, 2013). FSCV can detect sub-micromolar changes in dopamine, at sub-second time scales (Arbuthnott & Wickens, 2007; Roberts et al., 2013; Robinson, Hermans, Seipel, & Wightman, 2008; Robinson, Venton, Heien, & Wightman, 2003), and can be used to complement microdialysis. The high scan rate leads to a large charging current that must be subtracted to obtain the current from dopamine (Bucher & Wightman, 2015; A. C. Michael & Borland, 2007), leading to challenges with detection of basal levels (Bucher & Wightman, 2015; Roberts & Sombers, 2018; Rodeberg, Sandberg, Johnson, Phillips, & Wightman, 2017). Amperometry, which does not suffer from large charging currents can measure basal levels, but it does not provide much information about the identity of the detected substance and therefore selectivity to dopamine is a major challenge unless the probe is inserted into areas known to include measurable amounts of dopamine (Ganesana, Lee, Wang, & Venton, 2017).
Genetically encoded fluorescent detectors:
Changes in concentration of dopamine can be tracked by coupling the binding–induced conformational changes in dopamine receptors to changes in the fluorescence intensity of circularly permuted green fluorescent protein (cpGFP) (Patriarchi et al., 2018). Dopamine chemical sensors such as dLight1 (an intensity-based genetically encoded dopamine indicator which was developed from D1R) (Patriarchi et al., 2018) or GRABDA (genetically encoded GPCR-activation-based-dopamine which was constructed from D2R) (Sun et al., 2018) indicate physiological or behaviorally relevant dopamine transients with high spatiotemporal optical resolution and provide high selectivity and sensitivity for dopamine dynamics (Patriarchi et al., 2018; Sun et al., 2018). Both dLight1 and GRABDA demonstrate similar performance, however, GRABDA has a narrower range of binding affinities (10 – 130 nM vs. 300 nM – 1.6 μM in dLight1) (Patriarchi et al., 2018; Sun et al., 2018). The second-generation GRABDA has a 2–3-fold higher range for measuring dopamine dynamics (Sun et al., 2020). Other methods, such as Tango assays, in which the release of endogenous dopamine in vivo is reported as changes in the concentration, could be useful for circuit mapping, but they require longer signal amplification times (in order of hours) (Barnea et al., 2008; Inagaki et al., 2012). These methods are the latest trend in detection of endogenous dopamine in deep brain structures; however, they require implantation of optical probes (> 100 μm) that cause tissue damage and are incapable of measuring basal dopamine levels.
Positron emission tomography (PET).
This non-invasive imaging technique has been used to assess dopamine systems in humans and animals (Drevets et al., 2001; Pal et al., 2001; Schiffer, Alexoff, Shea, Logan, & Dewey, 2005; Volkow, Fowler, Wang, Baler, & Telang, 2009). This method commonly uses intravenously-administered radiolabeled molecules, such as dopamine receptor ligands or precursors of dopamine, that decay and emit positrons, which upon encountering electrons in tissue create gamma rays that are detected by the PET camera. Ligands available for dopamine studies include [11C]raclopride for striatal dopamine receptors (Jonasson et al., 2014) and [11C]fallypride for those in amygdala and media temporal lobe (Badgaiyan, Fischman, & Alpert, 2009). These ligands are displaced by endogenously released dopamine (Ganesana et al., 2017; Volkow et al., 1996). The local dopamine concentration is correlated to the quantity of displaced ligand (Volkow et al., 1996). It is challenging to identify optimal ligands that compete with small molecules like dopamine (Volkow et al., 1996) that will bind to certain receptors and also avoid non-specific binding, which adds noise and lowers sensitivity (Badgaiyan, 2014). Studies of task-induced or drug-induced changes in dopamine concentration have achieved a temporal resolution of 30 – 60 s and a spatial resolution of millimeters (Ganesana et al., 2017; Volkow et al., 1996).
Appendix B ∣. Rodent models of speech disorders
Childhood-onset fluency disorder (stuttering) is a neurodevelopmental disorder (American Psychiatric Association, 2013) that affects more than 1 % of US adult populations (Yairi & Ambrose, 2013). It is now accepted that genetic substrates play a major role in pathophysiology of stuttering (Yairi & Ambrose, 2013). While a number of genes have been found to be important for speech (such as FOXP2, CNTNAP2, GNPTAB, GNPTG, NAGPA, AP4E1), three genes in particular, FOXP2, GNPTAB, and CNTNAP2, have been studied extensively.
FOXP2.
FOXP2 gene, located on chromosome 7q31, encodes a transcription factor with a forkhead and DNA binding domain (Lai, Fisher, Hurst, Vargha-Khadem, & Monaco, 2001; Shu et al., 2005). As a transcription factor, its activity in binding to DNA affects downstream genes, some of which (for example CNTNAP2) have also been implicated in speech disorders (Newbury & Monaco, 2010). A mutation in FOXP2 and/or its translated protein reduces DNA binding and nuclear localization (Fujita et al., 2008) and causes speech deficits in humans as well as in rodents [such as reduced ultrasonic vocalizations and symptoms of developmental verbal dyspraxia (Castellucci, McGinley, & McCormick, 2016; Chabout et al., 2016; Fujita et al., 2008; Lai et al., 2001; Shu et al., 2005)]. In particular, the Foxp2 mutant mouse was found to produce simpler sequences of ultrasonic vocalizations (USVs) and was less able to switch to more complex sequences when compared to wild type mice (Castellucci et al., 2016; Chabout et al., 2016). Thus, using this mutant mouse model, it has been suggested that a mutation in Foxp2 in mice is similar to difficult in sequencing phonemes and syllable syntax to make complex words in humans (Chabout et al., 2016). Interestingly, FOXP2 is highly conserved among species, such that the human FOXP2 protein only varies from the mouse homolog by three amino acids (Shu et al., 2005). Additionally, although FOXP2 expresses in a variety of regions including the basal ganglia, cortex and thalamus (Fujita et al., 2008; Shu et al., 2005), the expression is lower in the cortex (mostly restricted to layer VI) than in the striatum. In the thalamus, FOXP2 expression is higher in the ascending sensory nuclei. There are no known cases of humans with a complete deletion of FOXP2 mutation (Chabout et al., 2016). Similarly, mice with homozygous knockout of Foxp2 are not viable. These animals have severe motor and respiratory impairments, inaudible vocalizations and die in 3 – 4 weeks (Enard et al., 2009; French et al., 2007; Fujita et al., 2008; Shu et al., 2005). Therefore, multiple heterozygous knockout mouse models with varying degrees of phenotypic changes have been generated (Enard et al., 2009; French et al., 2007; French & Fisher, 2014; Shu et al., 2005). In general, the heterozygous mouse models have fewer ultrasonic vocalizations and some motor impairments. A knock-in model of the Foxp2 mutation has also been illustrated to show a decrease in ultrasonic vocalization and an increase in motor and respiratory impairments (Castellucci et al., 2016).
CNTNAP2
Contactin-associated protein-like 2 gene is located on chromosome 7q35 (Petrin et al., 2010; Rodenas-Cuadrado, Ho, & Vernes, 2014). The gene codes for CASPR2 protein which is part of the neurexin superfamily (Peñagarikano et al., 2011; Peñagarikano & Geschwind, 2012). Interestingly, in the common fruit fly (Drosophila Melanogaster), the homolog of this gene codes for neurexin IV and is intimately involved in the neuron-glia interactions necessary for myelination of axons in the cortex (Peñagarikano & Geschwind, 2012). CNTNAP2 has been found to be implicated in a variety of neurological disorders including schizophrenia and Tourette’s syndrome (Rodenas-Cuadrado et al., 2014), however, it is most well-known for its prominent role in neurodevelopmental disorders such as Autism Spectrum Disorder (ASD), epilepsy, and language impairment disorders (Peñagarikano et al., 2011; Peñagarikano & Geschwind, 2012; Rodenas-Cuadrado et al., 2014; Selimbeyoglu et al., 2017). A Cntnap2 knockout mouse model shows similarities to human features of ASD and is created to evaluate behavioral and pathological features of ASD (Brunner et al., 2015; Peñagarikano et al., 2011; Peñagarikano & Geschwind, 2012; Selimbeyoglu et al., 2017). The interesting overlap between the Cntnap2 knock out mouse model used to study neurodevelopmental disorders and speech impairments is further elucidated through a chromosomal relationship. I Interestingly, intron 1 of CNTNAP2 gene contains a binding spot for FOXP2 (mentioned above) (Peñagarikano & Geschwind, 2012). Thus, when CNTNAP2 is mutated, and a region containing intron 1 is deleting from the gene, aside from neurodevelopmental delays, language impairments, and in particular stuttering is apparent.
GNPTAB.
GNPTAB codes for the N-acetylglucosamine-1-phosphate transferase alpha and beta subunits, an enzyme involved in the intracellular trafficking pathway (Kazemi, Estiar, Fazilaty, & Sakhinia, 2018; Newbury & Monaco, 2010; Raza et al., 2016). Four coding mutations in GNPTAB located in chromosome 14q that are linked to stuttering disorders have been identified so far (Kang et al., 2010; Kazemi et al., 2018; Raza et al., 2016). Along with this gene, a number of other genes closely related to GNPTAB and its function (i.e., GNPTG, NAGPA, AP4E1) have also been implicated in developmental stuttering.
The original transgenic mouse model of stuttering incorporated a Gnptab Glu1200Lys mutation (Barnes et al., 2016). Generated mice were on all accounts normal except for a deficit in the fluency of ultrasonic vocalizations (Barnes et al., 2016; Han et al., 2019). While this mutation seems to recapitulate the deficits seen in human stuttering disorders, other mutations in this gene have been tested too and a Ser321Gly mutation created a similar decrease in pup’s vocalizations (Han et al., 2019). In these mice, the number of astrocytes in the corpus callosum was lower, which suggests that astrocytes might play a significant role for proper vocalization (Han et al., 2019).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: GA Maguire reports the following conflicts: Research Grants: Teva, Emalex, Otsuka, Intracellular. Consulting Fees: Sunovion, Otsuka, Teva, Neurocrine, Eisai, Takeda, Janssen.
References
- Abbott NJ, Rönnbäck L, & Hansson E (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews. Neuroscience, 7(1), 41–53. doi: 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- Abdalla A, West A, Jin Y, Saylor RA, Qiang B, Peña E, … Hashemi P (2020). Fast serotonin voltammetry as a versatile tool for mapping dynamic tissue architecture: I. Responses at carbon fibers describe local tissue physiology. Journal of Neurochemistry, 153(1), 33–50. doi: 10.1111/jnc.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrover MF, Shin JH, Quiroz C, Ferré S, Lemos JC, & Alvarez VA (2020). Prefrontal Cortex-Driven Dopamine Signals in the Striatum Show Unique Spatial and Pharmacological Properties. The Journal of Neuroscience, 40(39), 7510–7522. doi: 10.1523/JNEUROSCI.1327-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm PA (2004). Stuttering and the basal ganglia circuits: a critical review of possible relations. Journal of Communication Disorders, 37(4), 325–369. doi: 10.1016/j.jcomdis.2004.03.001 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic And Statistical Manual Of Mental Disorders, 5th Edition: Dsm-5 (5th ed., p. 991). Washington, D.C: American Psychiatric Publishing. [Google Scholar]
- Andalman AS, Foerster JN, & Fee MS (2011). Control of vocal and respiratory patterns in birdsong: dissection of forebrain and brainstem mechanisms using temperature. Plos One, 6(9), e25461. doi: 10.1371/journal.pone.0025461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, … Gourine AV (2015). Functional oxygen sensitivity of astrocytes. The Journal of Neuroscience, 35(29), 10460–10473. doi: 10.1523/JNEUROSCI.0045-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, & Haydon PG (2001). Dynamic signaling between astrocytes and neurons. Annual Review of Physiology, 63, 795–813. doi: 10.1146/annurev.physiol.63.1.795 [DOI] [PubMed] [Google Scholar]
- Araque Alfonso, Carmignoto G, Haydon PG, Oliet SHR, Robitaille R, & Volterra A (2014). Gliotransmitters travel in time and space. Neuron, 81(4), 728–739. doi: 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott GW, & Wickens J (2007). Space, time and dopamine. Trends in Neurosciences, 30(2), 62–69. doi: 10.1016/j.tins.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Arriaga G, Zhou EP, & Jarvis ED (2012). Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds. Plos One, 7(10), e46610. doi: 10.1371/journal.pone.0046610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Murakami S, Diaz-Corrales FJ, & Ogawa N (2014). Striatal astrocytes act as a reservoir for L-DOPA. Plos One, 9(9), e106362. doi: 10.1371/journal.pone.0106362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Karlsson S, Fischer H, Karlsson P, Brehmer Y, Rieckmann A, … Nyberg L (2011). Dopamine D(1) receptors and age differences in brain activation during working memory. Neurobiology of Aging, 32(10), 1849–1856. doi: 10.1016/j.neurobiolaging.2009.10.018 [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD (2014). Imaging dopamine neurotransmission in live human brain. In Progress in brain research (Vol. 211, pp. 165–182). Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, & Alpert NM (2009). Dopamine release during human emotional processing. Neuroimage, 47(4), 2041–2045. doi: 10.1016/j.neuroimage.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, … Lee KJ (2008). The genetic design of signaling cascades to record receptor activation. Proceedings of the National Academy of Sciences of the United States of America, 105(1), 64–69. doi: 10.1073/pnas.0710487105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TD, Wozniak DF, Gutierrez J, Han T-U, Drayna D, & Holy TE (2016). A Mutation Associated with Stuttering Alters Mouse Pup Ultrasonic Vocalizations. Current Biology. doi: 10.1016/j.cub.2016.02.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA (2008). The mystery and magic of glia: a perspective on their roles in health and disease. Neuron, 60(3), 430–440. doi: 10.1016/j.neuron.2008.10.013 [DOI] [PubMed] [Google Scholar]
- Benveniste H, & Hüttemeier PC (1990). Microdialysis—Theory and application. Progress in Neurobiology, 35(3), 195–215. doi: 10.1016/0301-0082(90)90027-E [DOI] [PubMed] [Google Scholar]
- Björklund A, & Dunnett SB (2007). Dopamine neuron systems in the brain: an update. Trends in Neurosciences, 30(5), 194–202. doi: 10.1016/j.tins.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Blakely RD, & Bauman AL (2000). Biogenic amine transporters: regulation in flux. Current Opinion in Neurobiology, 10(3), 328–336. doi: 10.1016/S0959-4388(00)00088-X [DOI] [PubMed] [Google Scholar]
- Boë L-J, Fagot J, Perrier P, & Schwartz J-L (Eds.). (2017). Origins of Human Language: Continuities and Discontinuities with Nonhuman Primates. Peter Lang D. doi: 10.3726/b12405 [DOI] [Google Scholar]
- Booth HDE, Hirst WD, & Wade-Martins R (2017). The role of astrocyte dysfunction in parkinson’s disease pathogenesis. Trends in Neurosciences, 40(6), 358–370. doi: 10.1016/j.tins.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragoni M, Altieri M, Di Piero V, Padovani A, Mostardini C, & Lenzi GL (2000). Bromocriptine and speech therapy in non-fluent chronic aphasia after stroke. Neurological Sciences, 21(1), 19–22. doi: 10.1007/s100720070114 [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, & Hikosaka O (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron, 68(5), 815–834. doi: 10.1016/j.neuron.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, & Goldman PS (1979). Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science, 205(4409), 929–932. doi: 10.1126/science.112679 [DOI] [PubMed] [Google Scholar]
- Brunner D, Kabitzke P, He D, Cox K, Thiede L, Hanania T, … Biemans B (2015). Comprehensive analysis of the 16p11.2 deletion and null cntnap2 mouse models of autism spectrum disorder. Plos One, 10(8), e0134572. doi: 10.1371/journal.pone.0134572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher ES, & Wightman RM (2015). Electrochemical analysis of neurotransmitters. Annual Review of Analytical Chemistry (Palo Alto, Calif.), 8, 239–261. doi: 10.1146/annurev-anchem-071114-040426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler HU, da Prada M, Haefely W, & Picotti GB (1978). Plasma adrenaline, noradrenaline and dopamine in man and different animal species. The Journal of Physiology, 276, 311–320. doi: 10.1113/jphysiol.1978.sp012235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci GA, McGinley MJ, & McCormick DA (2016). Knockout of Foxp2 disrupts vocal development in mice. Scientific Reports, 6, 23305. doi: 10.1038/srep23305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Kalén P, Mandel RJ, & Björklund A (1992). Regional differences in the regulation of dopamine and noradrenaline release in medial frontal cortex, nucleus accumbens and caudate-putamen: a microdialysis study in the rat. Brain Research, 581(2), 217–228. doi: 10.1016/0006-8993(92)90711-h [DOI] [PubMed] [Google Scholar]
- Chabout J, Sarkar A, Patel SR, Radden T, Dunson DB, Fisher SE, & Jarvis ED (2016). A foxp2 mutation implicated in human speech deficits alters sequencing of ultrasonic vocalizations in adult male mice. Frontiers in Behavioral Neuroscience, 10, 197. doi: 10.3389/fnbeh.2016.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, … Khakh BS (2017). Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron, 95(3), 531–549.e9. doi: 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M, Chen L-F, Fridel EE, Klein ME, Senft RA, Sarkar A, & Jarvis ED (2017). Overexpression of human NR2B receptor subunit in LMAN causes stuttering and song sequence changes in adult zebra finches. Scientific Reports, 7(1), 942. doi: 10.1038/s41598-017-00519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, & Guenther FH (2019). Involvement of the Cortico-Basal Ganglia-Thalamocortical Loop in Developmental Stuttering. Frontiers in Psychology, 10, 3088. doi: 10.3389/fpsyg.2019.03088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoensook J, & Maguire GA (2017). A case series on the effectiveness of lurasidone in patients with stuttering. Annals of Clinical Psychiatry, 29(3), 191–194. [PubMed] [Google Scholar]
- Chen AN, & Meliza CD (2020). Experience- and Sex-Dependent Intrinsic Plasticity in the Zebra Finch Auditory Cortex during Song Memorization. The Journal of Neuroscience, 40(10), 2047–2055. doi: 10.1523/JNEUROSCI.2137-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-C, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, & Johnson JA (2009). Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proceedings of the National Academy of Sciences of the United States of America, 106(8), 2933–2938. doi: 10.1073/pnas.0813361106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini M, & Hanganu-Opatz IL (2020). Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends in Neurosciences. doi: 10.1016/j.tins.2020.10.017 [DOI] [PubMed] [Google Scholar]
- Chow HM, & Chang S-E (2017). White matter developmental trajectories associated with persistence and recovery of childhood stuttering. Human Brain Mapping, 38(7), 3345–3359. doi: 10.1002/hbm.23590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen RK, Petersen AV, & Perrier J-F (2013). How do glial cells contribute to motor control? Current Pharmaceutical Design, 19(24), 4385–4399. doi: 10.2174/13816128113199990384 [DOI] [PubMed] [Google Scholar]
- Christie JE, & Crow TJ (1971). Possible role of dopamine-containing neurones in the behavioural effects of cocaine. British Journal of Pharmacology, 42(4), 643P–645P. [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Vinney L, Wahoske EJ, & Connor NP (2010). A translational approach to vocalization deficits and neural recovery after behavioral treatment in Parkinson disease. Journal of Communication Disorders, 43(4), 319–326. doi: 10.1016/j.jcomdis.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, & Barres BA (2013). Emerging roles of astrocytes in neural circuit development. Nature Reviews. Neuroscience, 14(5), 311–321. doi: 10.1038/nrn3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkrum M, Covelo A, Lines J, Bellocchio L, Pisansky M, Loke K, … Araque A (2020). Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron, 105(6), 1036–1047.e5. doi: 10.1016/j.neuron.2019.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, & Iversen SD (1974). The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia, 39(4), 345–357. doi: 10.1007/BF00422974 [DOI] [PubMed] [Google Scholar]
- Creese I, & Iversen SD (1975). The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Research, 83(3), 419–436. doi: 10.1016/0006-8993(75)90834-3 [DOI] [PubMed] [Google Scholar]
- Cressatti M, Song W, Turk AZ, Garabed LR, Benchaya JA, Galindez C, … Schipper HM (2019). Glial HMOX1 expression promotes central and peripheral α-synuclein dysregulation and pathogenicity in parkinsonian mice. Glia, 67(9), 1730–1744. doi: 10.1002/glia.23645 [DOI] [PubMed] [Google Scholar]
- Crocker AD (1997). The regulation of motor control: an evaluation of the role of dopamine receptors in the substantia nigra. Reviews in the Neurosciences, 8(1), 55–76. doi: 10.1515/revneuro.1997.8.1.55 [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Carroll RT, Geldenhuys WJ, Gudelsky GA, Klein J, Meshul CK, & Van der Schyf CJ (2011). In vivo brain microdialysis: advances in neuropsychopharmacology and drug discovery. Expert Opinion on Drug Discovery, 6(2), 109–127. doi: 10.1517/17460441.2011.547189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastur FN, McGregor IS, & Brown RE (1999). Dopaminergic modulation of rat pup ultrasonic vocalizations. European Journal of Pharmacology, 382(2), 53–67. doi: 10.1016/s0014-2999(99)00590-7 [DOI] [PubMed] [Google Scholar]
- Daubner SC, Le T, & Wang S (2011). Tyrosine hydroxylase and regulation of dopamine synthesis. Archives of Biochemistry and Biophysics, 508(1), 1–12. doi: 10.1016/j.abb.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle NC, & Youngren OM (1978). Chick vocalization and emotional behavior influenced by apomorphine. Journal of Comparative and Physiological Psychology, 92(3), 416–430. doi: 10.1037/h0077486 [DOI] [PubMed] [Google Scholar]
- de Leeuw C, Goudriaan A, Smit AB, Yu D, Mathews CA, Scharf JM, … Posthuma. (2015). Involvement of astrocyte metabolic coupling in Tourette syndrome pathogenesis. European Journal of Human Genetics, 23(11), 1519–1522. doi: 10.1038/ejhg.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Funk GD, & Feldman JL (2018). Breathing matters. Nature Reviews. Neuroscience, 19(6), 351–367. doi: 10.1038/s41583-018-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, … Heintz N (2008). Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell, 135(4), 749–762. doi: 10.1016/j.cell.2008.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, … Mathis CA (2001). Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry, 49(2), 81–96. doi: 10.1016/s0006-3223(00)01038-6 [DOI] [PubMed] [Google Scholar]
- Dugast C, Suaud-Chagny MF, & Gonon F (1994). Continuousin vivo monitoring of evoked dopamine release in the rat nucleus accumbens by amperometry. Neuroscience, 62(3), 647–654. doi: 10.1016/0306-4522(94)90466-9 [DOI] [PubMed] [Google Scholar]
- Dutschmann M, & Herbert H (2006). The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. The European Journal of Neuroscience, 24(4), 1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x [DOI] [PubMed] [Google Scholar]
- Elgueta D, Aymerich MS, Contreras F, Montoya A, Celorrio M, Rojo-Bustamante E, … Pacheco R (2017). Pharmacologic antagonism of dopamine receptor D3 attenuates neurodegeneration and motor impairment in a mouse model of Parkinson’s disease. Neuropharmacology, 113(Pt A), 110–123. doi: 10.1016/j.neuropharm.2016.09.028 [DOI] [PubMed] [Google Scholar]
- Eliades SJ, & Wang X (2003). Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. Journal of Neurophysiology, 89(4), 2194–2207. doi: 10.1152/jn.00627.2002 [DOI] [PubMed] [Google Scholar]
- Enard W, Gehre S, Hammerschmidt K, Hölter SM, Blass T, Somel M, … Pääbo S (2009). A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell, 137(5), 961–971. doi: 10.1016/j.cell.2009.03.041 [DOI] [PubMed] [Google Scholar]
- Fischer T, Scheffler P, & Lohr C (2020). Dopamine-induced calcium signaling in olfactory bulb astrocytes. Scientific Reports, 10(1), 631. doi: 10.1038/s41598-020-57462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV (2003). Plasticity in Respiratory Motor Control. J Appl Physiol. [Google Scholar]
- French CA, & Fisher SE (2014). What can mice tell us about Foxp2 function? Current Opinion in Neurobiology, 28, 72–79. doi: 10.1016/j.conb.2014.07.003 [DOI] [PubMed] [Google Scholar]
- French CA, Groszer M, Preece C, Coupe A-M, Rajewsky K, & Fisher SE (2007). Generation of mice with a conditional Foxp2 null allele. Genesis, 45(7), 440–446. doi: 10.1002/dvg.20305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertinger S, Zinn JC, Sharan AD, Hamzei-Sichani F, & Simonyan K (2018). Dopamine drives left-hemispheric lateralization of neural networks during human speech. The Journal of Comparative Neurology, 526(5), 920–931. doi: 10.1002/cne.24375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Tanabe Y, Shiota A, Ueda M, Suwa K, Momoi MY, & Momoi T (2008). Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proceedings of the National Academy of Sciences of the United States of America, 105(8), 3117–3122. doi: 10.1073/pnas.0712298105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürtinger S, Zinn JC, & Simonyan K (2014). A neural population model incorporating dopaminergic neurotransmission during complex voluntary behaviors. PLoS Computational Biology, 10(11), e1003924. doi: 10.1371/journal.pcbi.1003924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K (1965). Evidence for the existence of monoamine neurons in the central nervous system. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie, 65(4), 573–596. doi: 10.1007/BF00337069 [DOI] [PubMed] [Google Scholar]
- Ganesana M, Lee ST, Wang Y, & Venton BJ (2017). Analytical techniques in neuroscience: recent advances in imaging, separation, and electrochemical methods. Analytical Chemistry, 89(1), 314–341. doi: 10.1021/acs.analchem.6b04278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick NA, & Murphy DL (1980). Species differences in the deamination of dopamine and other substrates for monoamine oxidase in brain. Psychopharmacology, 72(1), 27–33. doi: 10.1007/BF00433804 [DOI] [PubMed] [Google Scholar]
- Goberman AM, & Blomgren M (2003). Parkinsonian speech disfluencies: effects of L-dopa-related fluctuations. Journal of Fluency Disorders, 28(1), 55–10. doi: 10.1016/S0094-730X(03)00005-6 [DOI] [PubMed] [Google Scholar]
- Goschke T, & Bolte A (2014). Emotional modulation of control dilemmas: the role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia, 62, 403–423. doi: 10.1016/j.neuropsychologia.2014.07.015 [DOI] [PubMed] [Google Scholar]
- Grimm C, Balsters JH, & Zerbi V (2020). Shedding light on social reward circuitry: (un)common blueprints in humans and rodents. The Neuroscientist, 1073858420923552. doi: 10.1177/1073858420923552 [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ (2003). The basal ganglia and motor control. Neural Plasticity, 70(1–2), 107–120. doi: 10.1155/NP.2003.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Larson GA, & Zahniser NR (2007). Using High-Speed Chronoamperometry with Local Dopamine Application to Assess Dopamine Transporter Function. In Michael AC & Borland LM (Eds.), Electrochemical Methods for Neuroscience. Boca Raton (FL): CRC Press/Taylor & Francis. doi: 10.1201/9781420005868.ch6 [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, … Kaspar BK (2011). Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nature Biotechnology, 29(9), 824–828. doi: 10.1038/nbt.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T-U, Root J, Reyes LD, Huchinson EB, du Hoffmann J, Lee W-S, … Drayna D (2019). Human GNPTAB stuttering mutations engineered into mice cause vocalization deficits and astrocyte pathology in the corpus callosum. Proceedings of the National Academy of Sciences of the United States of America, 116(35), 17515–17524. doi: 10.1073/pnas.1901480116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E, & Sellström A (1983). MAO COMT, and GABA-T activities in primary astroglial cultures. Journal of Neurochemistry, 40(1), 220–225. doi: 10.1111/j.1471-4159.1983.tb12674.x [DOI] [PubMed] [Google Scholar]
- Haydon PG, & Carmignoto G (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiological Reviews, 86(3), 1009–1031. doi: 10.1152/physrev.00049.2005 [DOI] [PubMed] [Google Scholar]
- Hebb AO, Darvas F, & Miller KJ (2012). Transient and state modulation of beta power in human subthalamic nucleus during speech production and finger movement. Neuroscience, 202, 218–233. doi: 10.1016/j.neuroscience.2011.11.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi Z, Kis G, Holló K, Ledent C, & Antal M (2009). Neuronal and glial localization of the cannabinoid-1 receptor in the superficial spinal dorsal horn of the rodent spinal cord. The European Journal of Neuroscience, 30(2), 251–262. doi: 10.1111/j.1460-9568.2009.06816.x [DOI] [PubMed] [Google Scholar]
- Hegyi Z, Oláh T, Kőszeghy Á, Piscitelli F, Holló K, Pál B, … Antal M (2018). CB1 receptor activation induces intracellular Ca2+ mobilization and 2-arachidonoylglycerol release in rodent spinal cord astrocytes. Scientific Reports, 8(1), 10562. doi: 10.1038/s41598-018-28763-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Johnson MA, & Wightman RM (2004). Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Analytical Chemistry, 76(19), 5697–5704. doi: 10.1021/ac0491509 [DOI] [PubMed] [Google Scholar]
- Heien MLAV, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Wassum KM, & Wightman RM (2005). Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proceedings of the National Academy of Sciences of the United States of America, 102(29), 10023–10028. doi: 10.1073/pnas.0504657102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich A, Andó RD, Túri G, Rózsa B, & Sperlágh B (2012). K+ depolarization evokes ATP, adenosine and glutamate release from glia in rat hippocampus: a microelectrode biosensor study. British Journal of Pharmacology, 167(5), 1003–1020. doi: 10.1111/j.1476-5381.2012.01932.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O (1966). Dopamine (3-hydroxytyramine) and brain function. Pharmacological Reviews, 18(2), 925–964. [PubMed] [Google Scholar]
- Hornykiewicz O (1973). Dopamine in the basal ganglia. Its role and therapeutic implications (including the clinical use of L-DOPA). British Medical Bulletin, 29(2), 172–178. doi: 10.1093/oxfordjournals.bmb.a070990 [DOI] [PubMed] [Google Scholar]
- Hosford PS, Ninkina N, Buchman VL, Smith JC, Marina N, & SheikhBahaei S (2020). Synuclein Deficiency Results in Age-Related Respiratory and Cardiovascular Dysfunctions in Mice. Brain Sciences, 10(9). doi: 10.3390/brainsci10090583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Ben-Tabou de-Leon S, Wong AM, Jagadish S, Ishimoto H, Barnea G, … Anderson DJ (2012). Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell, 148(3), 583–595. doi: 10.1016/j.cell.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson H (1915). On the nature of the duality of the brain. Brain, 38, 80–103. doi: 10.1093/brain/38.1-2.80 [DOI] [Google Scholar]
- Jaquins-Gerstl A, & Michael AC (2009). Comparison of the brain penetration injury associated with microdialysis and voltammetry. Journal of Neuroscience Methods, 183(2), 127–135. doi: 10.1016/j.jneumeth.2009.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquins-Gerstl A, & Michael AC (2015). A review of the effects of FSCV and microdialysis measurements on dopamine release in the surrounding tissue. The Analyst, 140(11), 3696–3708. doi: 10.1039/c4an02065k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED (2007). Neural systems for vocal learning in birds and humans: a synopsis. Journal of Ornithology / DO-G, 148(1), 35–44. doi: 10.1007/s10336-007-0243-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED (2019a). Evolution of vocal learning and spoken language. Science. [DOI] [PubMed] [Google Scholar]
- Jarvis ED (2019b). Evolution of vocal learning and spoken language. Science, 366(6461), 50–54. doi: 10.1126/science.aax0287 [DOI] [PubMed] [Google Scholar]
- Jenner P, Rupniak NM, Rose S, Kelly E, Kilpatrick G, Lees A, & Marsden CD (1984). 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the common marmoset. Neuroscience Letters, 50(1–3), 85–90. doi: 10.1016/0304-3940(84)90467-1 [DOI] [PubMed] [Google Scholar]
- Jonasson LS, Axelsson J, Riklund K, Braver TS, Ögren M, Bäckman L, & Nyberg L (2014). Dopamine release in nucleus accumbens during rewarded task switching measured by [11C]raclopride. Neuroimage, 99, 357–364. doi: 10.1016/j.neuroimage.2014.05.047 [DOI] [PubMed] [Google Scholar]
- Joseph MH, Frith CD, & Waddington JL (1979). Dopaminergic mechanisms and cognitive deficit in schizophrenia. A neurobiological model. Psychopharmacology, 63(3), 273–280. doi: 10.1007/BF00433561 [DOI] [PubMed] [Google Scholar]
- Jürgens U (2009). The neural control of vocalization in mammals: a review. Journal of Voice, 23(1), 1–10. doi: 10.1016/j.jvoice.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Jürgens Uwe. (2002). Neural pathways underlying vocal control. Neuroscience and Biobehavioral Reviews, 26(2), 235–258. doi: 10.1016/s0149-7634(01)00068-9 [DOI] [PubMed] [Google Scholar]
- Justice JB (1993). Quantitative microdialysis of neurotransmitters. Journal of Neuroscience Methods, 48(3), 263–276. doi: 10.1016/0165-0270(93)90097-b [DOI] [PubMed] [Google Scholar]
- Kang C, Riazuddin S, Mundorff J, Krasnewich D, Friedman P, Mullikin JC, & Drayna D (2010). Mutations in the lysosomal enzyme-targeting pathway and persistent stuttering. The New England Journal of Medicine, 362(8), 677–685. doi: 10.1056/NEJMoa0902630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaya S, Kipp M, & Beyer C (2007). Oestrogen regulates the expression and function of dopamine transporters in astrocytes of the nigrostriatal system. Journal of Neuroendocrinology, 19(9), 682–690. doi: 10.1111/j.1365-2826.2007.01575.x [DOI] [PubMed] [Google Scholar]
- Kaushik P, Gorin F, & Vali S (2007). Dynamics of tyrosine hydroxylase mediated regulation of dopamine synthesis. Journal of Computational Neuroscience, 22(2), 147–160. doi: 10.1007/s10827-006-0004-8 [DOI] [PubMed] [Google Scholar]
- Kazemi N, Estiar MA, Fazilaty H, & Sakhinia E (2018). Variants in GNPTAB, GNPTG and NAGPA genes are associated with stutterers. Gene, 647, 93–100. doi: 10.1016/j.gene.2017.12.054 [DOI] [PubMed] [Google Scholar]
- Khakh BS (2019). Astrocyte-Neuron Interactions in the Striatum: Insights on Identity, Form, and Function. Trends in Neurosciences, 42(9), 617–630. doi: 10.1016/j.tins.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Koulen P, Rubinstein M, Grandy DK, & Goldman-Rakic PS (2001). An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 98(4), 1964–1969. doi: 10.1073/pnas.98.4.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CM, Bohmbach K, Minge D, Delekate A, Zheng K, Reynolds J, … Henneberger C (2020). Local resting ca2+ controls the scale of astroglial ca2+ signals. Cell Reports, 30(10), 3466–3477.e4. doi: 10.1016/j.celrep.2020.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Bosikova E, Cvikova M, Lukacova K, Scharff C, & Jarvis ED (2014). Basal ganglia function, stuttering, sequencing, and repair in adult songbirds. Scientific Reports, 4, 6590. doi: 10.1038/srep06590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Croxson PL, & Simonyan K (2016). Structural organization of the laryngeal motor cortical network and its implication for evolution of speech production. The Journal of Neuroscience, 36(15), 4170–4181. doi: 10.1523/JNEUROSCI.3914-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, & Monaco AP (2001). A forkhead-domain gene is mutated in a severe speech and language disorder. Nature, 413(6855), 519–523. doi: 10.1038/35097076 [DOI] [PubMed] [Google Scholar]
- Lan J, Song M, Pan C, Zhuang G, Wang Y, Ma W, … Wang W (2009). Association between dopaminergic genes (SLC6A3 and DRD2) and stuttering among Han Chinese. Journal of Human Genetics, 54(8), 457–460. doi: 10.1038/jhg.2009.60 [DOI] [PubMed] [Google Scholar]
- Laverty R (1978). Catecholamines: role in health and disease. Drugs, 16(5), 418–440. doi: 10.2165/00003495-197816050-00003 [DOI] [PubMed] [Google Scholar]
- Lee H-J, Baek SM, Ho D-H, Suk J-E, Cho E-D, & Lee S-J (2011). Dopamine promotes formation and secretion of non-fibrillar alpha-synuclein oligomers. Experimental & Molecular Medicine, 43(4), 216–222. doi: 10.3858/emm.2011.43.4.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, & Sesack SR (1997). Chapter VI Dopamine systems in the primate brain. In The primate nervous system, part I (Vol. 13, pp. 263–375). Elsevier. doi: 10.1016/S0924-8196(97)80008-5 [DOI] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, Murro AM, Smith JR, Flanigin HF, … King DW (1990). Cerebral language lateralization: evidence from intracarotid amobarbital testing. Neuropsychologia, 28(8), 831–838. doi: 10.1016/0028-3932(90)90007-b [DOI] [PubMed] [Google Scholar]
- Lu Y, Peters JL, & Michael AC (1998). Direct comparison of the response of voltammetry and microdialysis to electrically evoked release of striatal dopamine. Journal of Neurochemistry, 70(2), 584–593. doi: 10.1046/j.1471-4159.1998.70020584.x [DOI] [PubMed] [Google Scholar]
- Ludlow CL, & Loucks T (2003). Stuttering: a dynamic motor control disorder. Journal of Fluency Disorders, 28(4), 273–295. doi: 10.1016/j.jfludis.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Maguire GA, Riley GD, Franklin DL, & Gottschalk LA (2000). Risperidone for the treatment of stuttering. Journal of Clinical Psychopharmacology, 20(4), 479–482. doi: 10.1097/00004714-200008000-00013 [DOI] [PubMed] [Google Scholar]
- Maguire Gerald A, LaSalle L, Hoffmeyer D, Nelson M, Lochhead JD, Davis K, … Yaruss JS (2019). Ecopipam as a pharmacologic treatment of stuttering. Annals of Clinical Psychiatry, 31(3), 164–168. [PubMed] [Google Scholar]
- Maguire Gerald A, Nguyen DL, Simonson KC, & Kurz TL (2020). The pharmacologic treatment of stuttering and its neuropharmacologic basis. Frontiers in Neuroscience, 14, 158. doi: 10.3389/fnins.2020.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire Gerald A., Riley GD, Franklin DL, Maguire ME, Nguyen CT, & Brojeni PH (2004). Olanzapine in the Treatment of Developmental Stuttering: A Double-Blind, Placebo-Controlled Trial. Annals of Clinical Psychiatry, 16(2), 63–67. doi: 10.1080/10401230490452834 [DOI] [PubMed] [Google Scholar]
- Maguire Gerald A., Yeh CY, & Ito BS (2012). Overview of the diagnosis and treatment of stuttering. Journal of Experimental & Clinical Medicine, 4(2), 92–97. doi: 10.1016/j.jecm.2012.02.001 [DOI] [Google Scholar]
- Maguire Gerald A., Yoo BR, & SheikhBahaei S (2021). Investigation of risperidone treatment associated with enhanced brain activity in patients who stutter. Frontiers in Neuroscience, 15. doi: 10.3389/fnins.2021.598949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Turovsky E, Christie IN, Hosford PS, Hadjihambi A, Korsak A, … Gourine AV (2018). Brain metabolic sensing and metabolic signaling at the level of an astrocyte. Glia, 66(6), 1185–1199. doi: 10.1002/glia.23283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau M, Shi T, Puyal J, Knolhoff AM, Dulong J, Gasnier B, … Mothet J-P (2013). Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission. The Journal of Neuroscience, 33(8), 3413–3423. doi: 10.1523/JNEUROSCI.3497-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawson AR, Radford NT, & Jacob B (2016). Toward a theory of stuttering. European Neurology, 76(5–6), 244–251. doi: 10.1159/000452215 [DOI] [PubMed] [Google Scholar]
- Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S, … Kaspar BK (2014). Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proceedings of the National Academy of Sciences of the United States of America, 111(2), 829–832. doi: 10.1073/pnas.1314085111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael AC, & Borland LM (Eds.). (2007). Electrochemical Methods for Neuroscience. Boca Raton (FL): CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Michael DJ, & Wightman RM (1999). Electrochemical monitoring of biogenic amine neurotransmission in real time. Journal of Pharmaceutical and Biomedical Analysis, 19(1–2), 33–46. doi: 10.1016/S0731-7085(98)00145-9 [DOI] [PubMed] [Google Scholar]
- Mitala CM, Wang Y, Borland LM, Jung M, Shand S, Watkins S, … Michael AC (2008). Impact of microdialysis probes on vasculature and dopamine in the rat striatum: a combined fluorescence and voltammetric study. Journal of Neuroscience Methods, 174(2), 177–185. doi: 10.1016/j.jneumeth.2008.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JM, & Woolley SMN (2019). Emergent tuning for learned vocalizations in auditory cortex. Nature Neuroscience, 22(9), 1469–1476. doi: 10.1038/s41593-019-0458-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N, Simonyan K, & Blitzer A (2018). Central voice production and pathophysiology of spasmodic dysphonia. The Laryngoscope, 128(1), 177–183. doi: 10.1002/lary.26655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquette P, Verdier D, Kadala A, Féthière J, Philippe AG, Robitaille R, & Kolta A (2015). An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nature Neuroscience, 18(6), 844–854. doi: 10.1038/nn.4013 [DOI] [PubMed] [Google Scholar]
- Mörschel M, & Dutschmann M (2009). Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1529), 2517–2526. doi: 10.1098/rstb.2009.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, & Guyenet PG (2004). Respiratory control by ventral surface chemoreceptor neurons in rats. Nature Neuroscience, 7(12), 1360–1369. doi: 10.1038/nn1357 [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, & Przedborski S (2007). Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nature Neuroscience, 10(5), 615–622. doi: 10.1038/nn1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, … Graff-Guerrero A (2013). The potential role of dopamine D3 receptor neurotransmission in cognition. European Neuropsychopharmacology, 23(8), 799–813. doi: 10.1016/j.euroneuro.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, & Monaco AP (2010). Genetic advances in the study of speech and language disorders. Neuron, 68(2), 309–320. doi: 10.1016/j.neuron.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-John H (1988). Acute upper airway obstruction due to supraglottic dystonia induced by a neuroleptic. BMJ (Clinical Research Ed.), 297(6654), 964–965. doi: 10.1136/bmj.297.6654.964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, & Venton BJ (2015). Fast-scan Cyclic Voltammetry for the Characterization of Rapid Adenosine Release. Computational and Structural Biotechnology Journal, 13, 47–54. doi: 10.1016/j.csbj.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan RG, & Majcherczyk S (1982). Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. The Journal of Experimental Biology, 100, 23–40. [DOI] [PubMed] [Google Scholar]
- Pal PK, Wszolek ZK, Uitti R, Markopoulou K, Calne SM, Stoessl AJ, & Calne DB (2001). Positron emission tomography of dopamine pathways in familial Parkinsonian syndromes. Parkinsonism & Related Disorders, 8(1), 51–56. doi: 10.1016/s1353-8020(01)00008-6 [DOI] [PubMed] [Google Scholar]
- Parsons LH, & Justice JB (1992). Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. Journal of Neurochemistry, 58(1), 212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul J-Y, … Haydon PG (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science, 310(5745), 113–116. doi: 10.1126/science.1116916 [DOI] [PubMed] [Google Scholar]
- Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong W-H, … Tian L (2018). Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science, 360(6396). doi: 10.1126/science.aat4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton EW, Kimelberg HK, Shipherd SV, & Bourke RS (1981). Dopamine and norepinephrine uptake and metabolism by astroglial cells in culture. Life Sciences, 28(14), 1655–1663. doi: 10.1016/0024-3205(81)90322-2 [DOI] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, … Geschwind DH (2011). Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell, 147(1), 235–246. doi: 10.1016/j.cell.2011.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, & Geschwind DH (2012). What does CNTNAP2 reveal about autism spectrum disorder? Trends in Molecular Medicine, 18(3), 156–163. doi: 10.1016/j.molmed.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AJ, Liu H, & Komiyama T (2017). Learning in the rodent motor cortex. Annual Review of Neuroscience, 40, 77–97. doi: 10.1146/annurev-neuro-072116-031407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, & Jarvis ED (2012). Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Frontiers in Evolutionary Neuroscience, 4, 12. doi: 10.3389/fnevo.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrin AL, Giacheti CM, Maximino LP, Abramides DVM, Zanchetta S, Rossi NF, … Murray JC (2010). Identification of a microdeletion at the 7q33-q35 disrupting the CNTNAP2 gene in a Brazilian stuttering case. American Journal of Medical Genetics. Part A, 152A(12), 3164–3172. doi: 10.1002/ajmg.a.33749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D, & Assaneo MF (2020). Speech rhythms and their neural foundations. Nature Reviews. Neuroscience, 21(6), 322–334. doi: 10.1038/s41583-020-0304-4 [DOI] [PubMed] [Google Scholar]
- Price CJ (2010). The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences, 1191, 62–88. doi: 10.1111/j.1749-6632.2010.05444.x [DOI] [PubMed] [Google Scholar]
- Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD, … Funk GD (2018). Release of ATP by pre-Bötzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca2+ -dependent P2Y1 receptor mechanism. J Physiol, 596(15), 3245–3269. doi: 10.1113/JP274727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold PM, & Tieu K (2010). Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics, 7(4), 413–423. doi: 10.1016/j.nurt.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza MH, Domingues CEF, Webster R, Sainz E, Paris E, Rahn R, … Drayna D (2016). Mucolipidosis types II and III and non-syndromic stuttering are associated with different variants in the same genes. European Journal of Human Genetics, 24(4), 529–534. doi: 10.1038/ejhg.2015.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requardt RP, Hirrlinger PG, Wilhelm F, Winkler U, Besser S, & Hirrlinger J (2012). Ca2+ signals of astrocytes are modulated by the NAD+/NADH redox state. Journal of Neurochemistry, 120(6), 1014–1025. doi: 10.1111/j.1471-4159.2012.07645.x [DOI] [PubMed] [Google Scholar]
- Rice ME, Patel JC, & Cragg SJ (2011). Dopamine release in the basal ganglia. Neuroscience, 198, 112–137. doi: 10.1016/j.neuroscience.2011.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, & Smith JC (2014). Respiratory rhythm generation in vivo. Physiology, 29(1), 58–71. doi: 10.1152/physiol.00035.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Kassubek J, Gröschel K, Grodd W, & Ackermann H (2006). The cerebral control of speech tempo: opposite relationship between speaking rate and BOLD signal changes at striatal and cerebellar structures. Neuroimage, 29(1), 46–53. doi: 10.1016/j.neuroimage.2005.03.046 [DOI] [PubMed] [Google Scholar]
- Roberts JG, Lugo-Morales LZ, Loziuk PL, & Sombers LA (2013). Real-time chemical measurements of dopamine release in the brain. Methods in Molecular Biology, 964, 275–294. doi: 10.1007/978-1-62703-251-3_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JG, & Sombers LA (2018). Fast-Scan Cyclic Voltammetry: Chemical Sensing in the Brain and Beyond. Analytical Chemistry, 90(1), 490–504. doi: 10.1021/acs.analchem.7b04732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Hermans A, Seipel AT, & Wightman RM (2008). Monitoring rapid chemical communication in the brain. Chemical Reviews, 108(7), 2554–2584. doi: 10.1021/cr068081q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien MLAV, & Wightman RM (2003). Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clinical Chemistry, 49(10), 1763–1773. doi: 10.1373/49.10.1763 [DOI] [PubMed] [Google Scholar]
- Rodeberg NT, Sandberg SG, Johnson JA, Phillips PEM, & Wightman RM (2017). Hitchhiker’s Guide to Voltammetry: Acute and Chronic Electrodes for in Vivo Fast-Scan Cyclic Voltammetry. ACS Chemical Neuroscience, 8(2), 221–234. doi: 10.1021/acschemneuro.6b00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas-Cuadrado P, Ho J, & Vernes SC (2014). Shining a light on CNTNAP2: complex functions to complex disorders. European Journal of Human Genetics, 22(2), 171–178. doi: 10.1038/ejhg.2013.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, & Wichmann T (2010). Extrastriatal dopaminergic circuits of the Basal Ganglia. Frontiers in Neuroanatomy, 4, 139. doi: 10.3389/fnana.2010.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Nomoto M, Jenner P, & Marsden CD (1989). Transient depletion of nucleus accumbens dopamine content may contribute to initial akinesia induced by MPTP in common marmosets. Biochemical Pharmacology, 38(21), 3677–3681. doi: 10.1016/0006-2952(89)90572-8 [DOI] [PubMed] [Google Scholar]
- Salamone JD (1992). Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology, 107(2–3), 160–174. doi: 10.1007/BF02245133 [DOI] [PubMed] [Google Scholar]
- Santello M, Calì C, & Bezzi P (2012). Gliotransmission and the tripartite synapse. Advances in Experimental Medicine and Biology, 970, 307–331. doi: 10.1007/978-3-7091-0932-8_14 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, & Jarvis ED (2006). Social context-dependent singing-regulated dopamine. The Journal of Neuroscience, 26(35), 9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel-Krüger J, & Randrup A (1967). Stereotype hyperactive behaviour produced by dopamine in the absence of noradrenaline. Life Sciences, 6(13), 1389–1398. doi: 10.1016/0024-3205(67)90186-5 [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Alexoff DL, Shea C, Logan J, & Dewey SL (2005). Development of a simultaneous PET/microdialysis method to identify the optimal dose of 11C-raclopride for small animal imaging. Journal of Neuroscience Methods, 144(1), 25–34. doi: 10.1016/j.jneumeth.2004.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (2007). Multiple dopamine functions at different time courses. Annual Review of Neuroscience, 30, 259–288. doi: 10.1146/annurev.neuro.28.061604.135722 [DOI] [PubMed] [Google Scholar]
- Seamans JK, & Yang CR (2004). The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in Neurobiology, 74(1), 1–58. doi: 10.1016/j.pneurobio.2004.05.006 [DOI] [PubMed] [Google Scholar]
- Selimbeyoglu A, Kim CK, Inoue M, Lee SY, Hong ASO, Kauvar I, … Deisseroth K (2017). Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Science Translational Medicine, 9(401). doi: 10.1126/scitranslmed.aah6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhbahaei S, Gourine AV, & Smith JC (2017). Respiratory rhythm irregularity after carotid body denervation in rats. Respiratory Physiology & Neurobiology, 246, 92–97. doi: 10.1016/j.resp.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhbahaei S, Morris B, Collina J, Anjum S, Znati S, Gamarra J, … Smith JC (2018). Morphometric analysis of astrocytes in brainstem respiratory regions. The Journal of Comparative Neurology, 526(13), 2032–2047. doi: 10.1002/cne.24472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhbahaei S, & Smith JC (2017). Breathing to inspire and arouse. Science, 355(6332), 1370–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, Liu B, … Gourine AV (2018). Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nature Communications, 9(1), 370. doi: 10.1038/s41467-017-02723-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou M, Ferrario CR, Schultz KN, Robinson TE, & Kennedy RT (2006). Monitoring dopamine in vivo by microdialysis sampling and on-line CE-laser-induced fluorescence. Analytical Chemistry, 78(19), 6717–6725. doi: 10.1021/ac0608218 [DOI] [PubMed] [Google Scholar]
- Shu W, Cho JY, Jiang Y, zhang M, Weisz D, Elder GA, … Buxbaum JD (2005). Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9643–9648. doi: 10.1073/pnas.0503739102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugart YY, Mundorff J, Kilshaw J, Doheny K, Doan B, Wanyee J, … Drayna D (2004). Results of a genome-wide linkage scan for stuttering. American Journal of Medical Genetics. Part A, 124A(2), 133–135. doi: 10.1002/ajmg.a.20347 [DOI] [PubMed] [Google Scholar]
- Simonyan K (2014). The laryngeal motor cortex: its organization and connectivity. Current Opinion in Neurobiology, 28, 15–21. doi: 10.1016/j.conb.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, & Fuertinger S (2015). Speech networks at rest and in action: interactions between functional brain networks controlling speech production. Journal of Neurophysiology, 113(7), 2967–2978. doi: 10.1152/jn.00964.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Herscovitch P, & Horwitz B (2013). Speech-induced striatal dopamine release is left lateralized and coupled to functional striatal circuits in healthy humans: a combined PET, fMRI and DTI study. Neuroimage, 70, 21–32. doi: 10.1016/j.neuroimage.2012.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Horwitz B, & Jarvis ED (2012). Dopamine regulation of human speech and bird song: a critical review. Brain and Language, 122(3), 142–150. doi: 10.1016/j.bandl.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, & Jürgens U (2003). Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Research, 974(1–2), 43–59. doi: 10.1016/s0006-8993(03)02548-4 [DOI] [PubMed] [Google Scholar]
- Smith A, & Weber C (2016). Childhood stuttering: where are we and where are we going? Seminars in Speech and Language, 37(4), 291–297. doi: 10.1055/s-0036-1587703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, & Kieval JZ (2000). Anatomy of the dopamine system in the basal ganglia. Trends in Neurosciences, 23(10 Suppl), S28–33. doi: 10.1016/s1471-1931(00)00023-9 [DOI] [PubMed] [Google Scholar]
- Smith Yoland, & Villalba R (2008). Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Movement Disorders, 23 Suppl 3, S534–47. doi: 10.1002/mds.22027 [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, & Büchel C (2002). Disconnection of speech-relevant brain areas in persistent developmental stuttering. The Lancet, 360(9330), 380–383. doi: 10.1016/S0140-6736(02)09610-1 [DOI] [PubMed] [Google Scholar]
- Sonninen T-M, Hämäläinen RH, Koskuvi M, Oksanen M, Shakirzyanova A, Wojciechowski Š, … Lehtonen S (2020). Metabolic alterations in Parkinson’s disease astrocytes. Scientific Reports, 10(1), 14474. doi: 10.1038/s41598-020-71329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourkes TL (1971). Actions of levodopa and dopamine in the central nervous system. The Journal of the American Medical Association, 218(13), 1909. doi: 10.1001/jama.1971.03190260025006 [DOI] [PubMed] [Google Scholar]
- Stenken JA, & Patton SL (n.d.). Microdialysis flux considerations (Vol. 2, pp. 337–374). World Scientific Publishing Co. Pte. Ltd. [Google Scholar]
- Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, … Li Y (2018). A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell, 174(2), 481–496.e19. doi: 10.1016/j.cell.2018.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, … Li Y (2020). Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nature Methods. doi: 10.1038/s41592-020-00981-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Inazu M, & Matsumiya T (2002). Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn-Schmiedeberg’s Archives of Pharmacology, 366(6), 620–623. doi: 10.1007/s00210-002-0640-0 [DOI] [PubMed] [Google Scholar]
- Tani T, & Sakai Y (2011). Analysis of five cases with neurogenic stuttering following brain injury in the basal ganglia. Journal of Fluency Disorders, 36(1), 1–16. doi: 10.1016/j.jfludis.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Teppema LJ, & Dahan A (2010). The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiological Reviews, 90(2), 675–754. doi: 10.1152/physrev.00012.2009 [DOI] [PubMed] [Google Scholar]
- Vaarmann A, Gandhi S, & Abramov AY (2010). Dopamine induces Ca2+ signaling in astrocytes through reactive oxygen species generated by monoamine oxidase. The Journal of Biological Chemistry, 285(32), 25018–25023. doi: 10.1074/jbc.M110.111450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Passel R, Schlooz WA, Lamers KJ, Lemmens WA, & Rotteveel JJ (2001). S100B protein, glia and Gilles de la Tourette syndrome. European Journal of Paediatric Neurology, 5(1), 15–19. doi: 10.1053/ejpn.2001.0399 [DOI] [PubMed] [Google Scholar]
- Vaughan RA, & Foster JD (2013). Mechanisms of dopamine transporter regulation in normal and disease states. Trends in Pharmacological Sciences, 34(9), 489–496. doi: 10.1016/j.tips.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, & Cao Q (2020). Fundamentals of fast-scan cyclic voltammetry for dopamine detection. The Analyst, 145(4), 1158–1168. doi: 10.1039/c9an01586h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, … Tzourio-Mazoyer N (2006). Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage, 30(4), 1414–1432. doi: 10.1016/j.neuroimage.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, Logan J, Wang GJ, Ding YS, & Dewey S (1996). PET evaluation of the dopamine system of the human brain. Journal of Nuclear Medicine, 37(7), 1242–1256. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, & Telang F (2009). Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology, 56 Suppl 1, 3–8. doi: 10.1016/j.neuropharm.2008.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, & Smith A (2011). Linguistic Complexity, Speech Production, and Comprehension in Parkinson’s Disease: Behavioral and Physiological Indices. Journal of Speech, Language, and Hearing Research, 54(3), 787. doi: 10.1044/1092-4388(2010/09-0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, & Bouret S (2019). What Is the Relationship between Dopamine and Effort? Trends in Neurosciences, 42(2), 79–91. doi: 10.1016/j.tins.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J, & Thompson P (1998). Drug-induced supraglottic dystonia and spasmodic dysphonia. Movement Disorders, 13(6), 978–979. doi: 10.1002/mds.870130623 [DOI] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, & Howell P (2008). Structural and functional abnormalities of the motor system in developmental stuttering. Brain: A Journal of Neurology, 131(Pt 1), 50–59. doi: 10.1093/brain/awm241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, & May LJ (1988). Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience, 25(2), 513–523. doi: 10.1016/0306-4522(88)90255-2 [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, & Phillips PEM (2010). Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. In Behavioral neuroscience of drug addiction (pp. 29–71). Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Maguire G, Riley G, Fallon J, LaCasse L, Chin S, … Lottenberg S (1995). A positron emission tomography [18F]deoxyglucose study of developmental stuttering. Neuroreport, 6(3), 501–505. doi: 10.1097/00001756-199502000-00024 [DOI] [PubMed] [Google Scholar]
- Wu JC, Maguire G, Riley G, Lee A, Keator D, Tang C, … Najafi A (1997). Increased dopamine activity associated with stuttering. Neuroreport, 8(3), 767–770. doi: 10.1097/00001756-199702100-00037 [DOI] [PubMed] [Google Scholar]
- Xin W, Schuebel KE, Jair K-W, Cimbro R, De Biase LM, Goldman D, & Bonci A (2019). Ventral midbrain astrocytes display unique physiological features and sensitivity to dopamine D2 receptor signaling. Neuropsychopharmacology, 44(2), 344–355. doi: 10.1038/s41386-018-0151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E, & Ambrose N (2013). Epidemiology of stuttering: 21st century advances. Journal of Fluency Disorders, 38(2), 66–87. doi: 10.1016/j.jfludis.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, & Komine O (2018). The multi-dimensional roles of astrocytes in ALS. Neuroscience Research, 126, 31–38. doi: 10.1016/j.neures.2017.09.011 [DOI] [PubMed] [Google Scholar]
- Yu X, Taylor AMW, Nagai J, Golshani P, Evans CJ, Coppola G, & Khakh BS (2018). Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron, 99(6), 1170–1187.e9. doi: 10.1016/j.neuron.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SP, Kam T-I, Panicker N, Kim S, Oh Y, Park J-S, … Ko HS (2018). Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nature Medicine, 24(7), 931–938. doi: 10.1038/s41591-018-0051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, & Arnsten AF (1997). Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. The Journal of Neuroscience, 17(21), 8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, & Barres BA (2010). Astrocyte heterogeneity: an underappreciated topic in neurobiology. Current Opinion in Neurobiology, 20(5), 588–594. doi: 10.1016/j.conb.2010.06.005 [DOI] [PubMed] [Google Scholar]