Abstract
Periodontitis is the sixth-most prevalent disease in the world and the first cause for tooth loss in adults. With focus shifted to the inflammatory/immune response in the pathogenesis of periodontitis, there is a critical need to evaluate host modulatory agents. Synthetic and biological disease-modifying antirheumatic drugs are a cornerstone for the treatment of inflammatory diseases. Recent prospective cohort studies showed that synthetic disease-modifying antirheumatic drugs improved periodontal clinical parameters following nonsurgical periodontal treatment in patients with rheumatoid arthritis. Treatment with recombinant humanized monoclonal antibodies against CD20 (rituximab) and IL-6 receptor (tocilizumab), the latter also in clinical trials for the treatment of COVID-19 pneumonia, resulted in decreased periodontal inflammation and improved periodontal status. Studies on the effect of TNF-α inhibitors in patients with periodontitis yielded inconsistent results. Recent data suggest that probiotics provide anti-inflammatory clinical benefit, as do nutritional supplements, such as n-3 fatty acids, when combined with periodontal therapy. Probiotics reduce the production of proinflammatory cytokines/chemokines by suppressing NF-κB pathways and promote the accumulation of T regulatory cells. Statins, like aspirin, have been shown to exhibit anti-inflammatory and bone-preserving actions by upregulating production of Specialized Proresolving Mediators (SPMs). Currently, there is insufficient scientific support for the topical delivery of statins or bisphosphonates as adjuncts to periodontal therapy. Here, we present a critical review of the most recent host modulatory agents applied in humans and the key immune pathways that they target. Emerging evidence from novel drug candidates, including SPMs and complement inhibitors as previously studied in animal models and currently in human clinical trials, suggests future availability of adjunctive therapeutic strategies for the management of periodontitis.
Keywords: cytokines, inflammation, periodontitis, therapeutics, immune system, bone resorption
Introduction
Periodontitis is associated with the presence of a dysbiotic microbial community in a susceptible host (Hajishengallis et al. 2020). Although bacteria are required in disease pathogenesis, patients are not equally susceptible and do not respond similarly to treatment; it is ultimately the host inflammatory response to the microbial challenge that primarily drives immune cell–mediated self-degradation of periodontal tissues resulting in eventual tooth loss (Van Dyke 2020). Nonsurgical periodontal therapy with or without antimicrobials mechanically removes etiologic dental biofilm and remains our standard of care; however, targeting only microbes does not achieve favorable outcomes in all periodontal patients (Preshaw 2018). There is an unmet need to complement current therapeutic approaches with strategies that will modulate destructive aspects of the host inflammatory response to achieve better long-term clinical outcomes. The adjunctive use of host modulatory agents can have a positive impact on the progression of periodontal disease, especially in susceptible patients who develop a chronic (hyper)inflammatory response against the microbiome associated with genetic, systemic, or environmental factors (Van Dyke 2020) and for whom conventional therapeutic approaches are not effective.
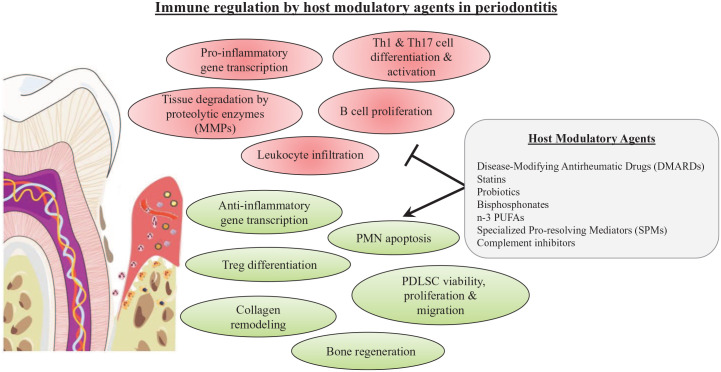
Periodontal cases often present with common systemic comorbidities requiring pharmaceutical management, including rheumatoid arthritis (RA) and dyslipidemia (Jung et al. 2018; Petit et al. 2019). Studies of such patients also taking immunoregulatory drugs have offered the possibility to observe the impact of these agents on periodontitis. Additionally, recent evidence has provided a better understanding of the molecular events driving periodontal inflammation and its resolution and has paved the way for the design of novel therapeutic candidates (e.g., specialized proresolving mediators [SPMs] and complement inhibitors) as adjuncts to periodontal treatment (Hajishengallis et al. 2020; Fig. 1).
Figure 1.
Immune regulation by host modulatory agents in periodontitis. The host immune/inflammatory response to the microbial challenge triggers the self-degradation of periodontal tissues and drives the pathogenic process in periodontitis. Recent preclinical and human trials revealed promising host modulatory agents in periodontitis that inhibit destructive aspects of inflammation and upregulate anti-inflammatory and regenerative mechanisms promoting a favorable environment for the return to periodontal tissue homeostasis. MMP, metalloproteinase; PDLSC, periodontal ligament stem cell; PMN, polymorphonuclear neutrophil; n-3 PUFA, omega-3 polyunsaturated fatty acid.
Here, we present a critical review of host modulatory agents that have been applied in patients with periodontitis and the key immune pathways that they target (Fig. 2), except for tetracyclines, which have been extensively described in other recent reviews (Preshaw 2018; Golub and Lee 2020). In addition, we touch on the future therapeutic potential provided by novel drug candidates previously studied in animal models and currently in human clinical trials.
Figure 2.
Cellular targets of host modulation agents in periodontitis. Conventional synthetic and biological disease-modifying antirheumatic drugs (DMARDs) are currently being prescribed for the treatment of rheumatoid arthritis. Biological DMARDs inhibit the expression of key proinflammatory markers in periodontitis, such as IL-17 and IL-6, and suppress the proliferation of CD4+ T cells. Rituximab is a monoclonal antibody targeting CD20 that results in the depletion of B cells. Bisphosphonates disrupt osteoclastic activity, inhibit bone degradation, and promote osteoblastic activity. Omega-3 (n-3) polyunsaturated fatty acids (PUFAs) regulate fatty acid metabolites of inflammatory cells, suppress proinflammatory gene expression by inhibiting proinflammatory pathways, and decrease the production of inflammatory mediators. n-3 PUFAs are the substrates for the generation of specialized proresolving lipid mediators (SPMs), including resolvins, maresins, and protectins, which orchestrate the active resolution of inflammation. Statins exert their anti-inflammatory and bone-preserving actions by modulating the ERK, MAPK, PI3-Akt, and NF-κB pathways. They also induce the upregulation of SPMs, namely 15-epi-LXA4 and 13-series resolvins, via the LOX-5 and COX-2 enzymatic pathways, respectively, especially when combined with aspirin. Complement inhibitors such as Cp40 suppress osteoclastogenesis and bone loss and decrease IL-17 and the RANKL/OPG ratio in nonhuman primates. Probiotics reduce the production of proinflammatory cytokines by inhibiting the NF-kB pathway and promote the accumulation of Tregs by inhibiting histone deacetylases. SPMs downregulate cytokines/chemokines and promote bone regeneration. D-resolvins antagonize IL-17, induce the expression of the endothelial anti-inflammatory marker Del-1 through the GSK-3b-C/EBPb pathway, and decrease CD4+ cells. SPMs restore viability, proliferation, and migration of human PDLSCs and enhance differentiation of cementoblasts/osteoblasts. IL, interleukin; PDLSC, periodontal ligament stem cell.
Disease-Modifying Antirheumatic Drugs
Periodontitis and RA share many similarities in pathophysiology and clinical progression, and periodontal inflammation has been associated with an increased risk of developing RA (Preshaw 2018). The 2 pathologies may be partially linked by the presence of periodontal pathogens (i.e., Porphyromonas gingivalis) that promote protein citrullination and generation of anticitrullinated protein antibodies, a known triggering factor for RA progression (Corrêa et al. 2019). Conversely, RA-induced systemic inflammation can bolster local inflammatory responses in the periodontium and has been associated with increased abundance of periodontopathogenic subgingival species (Corrêa et al. 2019).
The current first-line treatment of RA aims to suppress inflammation to limit disease activity or induce remission as reflected by downregulation of systemic markers associated with RA activity (e.g., DAS28-CRP and DAS28-ESR; Jung et al. 2018). Over the last 2 decades, targeted disease-modifying antirheumatic drugs (DMARDs) have been shown to efficiently modify disease progression and significantly decrease and/or delay joint deformity (Äyräväinen et al. 2017). The similar proinflammatory and tissue-damaging networks and cellular activities involved in RA and periodontitis have raised the question of DMARDs’ impact on periodontal inflammation (Table 1).
Table 1.
Therapeutic Impact of DMARDs on Periodontitis.
| Host Modulatory Agent | Type of Study | Follow-up | Clinical Outcomes | Subclinical Outcomes | Authors and Year |
|---|---|---|---|---|---|
| Conventional synthetic DMARDs | |||||
| Cs DMARDs (methotrexate, leflunomide, hydroxychloroquine, sulfasalazine) or anti-TNF-α therapy and synthetic DMARDs | Cross-sectional | Not available | Methotrexate + leflunomide exhibited significantly higher percentage of interproximal sites (31.6%) with clinical attachment loss ≥4 mm vs. methotrexate + other conventional DMARD (10%) or leflunomide + other conventional DMARD (13.3%). | Treatment with >1 DMARD was associated with the presence of Porphyromonas gingivalis. | Romero-Sanchez et al. (2017) |
| Cs DMARDs (methotrexate, leflunomide, hydroxychloroquine, sulfasalazine) or biological DMARDs (TNF-α inhibitors, IL-1 inhibitor, anti-B-cell agent) combined with methotrexate | Prospective cohort | 16 mo | 80% of early untreated RA and 85% of chronic RA groups had periodontitis vs. 40% of controls at baseline. No statistical difference in periodontal parameters in intra- and intergroup analyses. | Patients with early RA had higher presence of P. gingivalis at follow-up. | Äyräväinen et al. (2017) |
| Cs DMARDs (methotrexate, hydroxychloroquine, sulfasalazine) | Prospective cohort | 4 wk after SRP | Additional PPD reduction of 0.8 mm (RA group) vs. 0.6 mm (control group). Additional CAL gain of 1.1 mm (RA group) vs. 0.7 mm (control group). Higher PPD reduction was associated with combined synthetic DMARDs over methotrexate as monotherapy. | No significant difference of RA activity (DAS28-ESR) among groups of synthetic DMARDs. | Jung et al. (2018) |
| Biological DMARDs | |||||
| TNF-α inhibitors (infliximab, adalimumab, etanercept) | Prospective cohort | 6 mo | Significant improvement in % sites with BOP, PPD reduction (−1.49 ± 0.22 mm), and CAL gain (0.5 ± 0.26 mm) vs. baseline. | Significant reduction in RA activity (DAS28), inflammatory (ESR, CRP), and serologic (ACPAs) parameters. | Codrina et al. (2017) |
| TNF-α inhibitors (etanercept, infliximab, adalimumab, golimumab) or IL-6 receptor inhibitors (tocilizumab) | Prospective cohort | 6 mo | Significant improvements in GI, BOP, and PPD reduction in both groups vs. baseline. Significant additional CAL gain of 0.1 mm vs. baseline only for tocilizumab-treated group. Greater GI decrease in tocilizumab-treated group. | TNF inhibitors and tocilizumab were associated with significant decrease in DAS28-CRP, serum levels of anti-CCP antibodies, rheumatoid factor, and MMP-3. | Kobayashi et al. (2015) |
| TNF-α inhibitors (infliximab, etanercept, and adalimumab) with synthetic DMARDs (methotrexate, hydroxychloroquine, leflunomide, sulfasalazine) | Prospective cohort | 6 wk after SRP | Significant improvements in PPD reduction, CAL gain, BOP, PI, and GI were observed for all groups receiving periodontal therapy/SRP alone; significant reduction in PPD (0.2 mm), BOP, and GI vs. baseline/anti-TNF-α + SRP; significant reduction in PPD (0.4 mm), BOP, and GI and significant CAL gain (0.3 mm) vs. baseline. | Significant reduction in ESR for the groups receiving anti-TNF-α therapy over those receiving only synthetic DMARDs. No significant decrease in DAS28 for all groups. | Ortiz et al. (2009) |
| TNF-α inhibitor (infliximab) | Cross-sectional | Not available | Patients receiving infliximab had higher modified GI and papillary GI but no significant differences in PPD and CAL vs. patients not receiving infliximab. | No significant differences were found for DAS28 between the groups. | Pers et al. (2008) |
| TNF-α inhibitor (infliximab) | Prospective cohort | 9 mo | Patients receiving infliximab with methotrexate had higher MGI and PGI but higher CAL gain (6.27 ± 0.97 vs. 5.22 ± 1.05 mm) vs. baseline. | Not investigated | Pers et al. (2008) |
| TNF-α inhibitor (adalimumab, infliximab) | Prospective cohort | 30 d | Significant increase in GI vs. baseline. No significant changes for BOP, PPD, and CAL vs. baseline. | Significant decrease in GCF volume, GCF levels of IL-1β and IL-8, salivary IL-8 and MCP-1 levels vs. baseline. Decrease in CRP, ESR, and DAS28 vs. baseline. | Üstün et al. (2013) |
| Methotrexate, leflunomide, TNF-α inhibitors, IL-6 antagonists, rituximab | Cross-sectional | Not available | Significantly higher BOP and PBI in patients receiving methotrexate + TNF-α inhibitors vs. patients receiving leflunomide. Higher BOP in patients receiving methotrexate + TNF-α inhibitors vs. patients receiving methotrexate and rituximab. | Prevalence of P. gingivalis and Treponema denticola was associated with medication group | Ziebolz et al. (2018) |
| JAK inhibitor (baricitinib) | Prospective cohort | 6 mo | Significant reduction in GI, PPD (0.8 mm), and % sites with PPD ≥4 mm (3.5%). | Significant decrease in RA activity (DAS28-CRP), inflammation (CRP-ESR), and serologic markers (ACPA, RF) vs. baseline | Ancuţa et al. (2020) |
| Anti-B lymphocyte immunotherapy (rituximab) | Cross sectional | Not available | Patients receiving rituximab had significantly lower marginal GI, PPD (Δ = 0.6 mm), and clinical attachment loss (Δ = 0.3 mm) than patients not receiving rituximab. | Not investigated | Coat et al. (2015) |
| Anti-B lymphocyte immunotherapy (rituximab) | Prospective cohort | 6 mo (subgroup assessed up to 4 y) | No significant changes in MGI, PBI, and PI after 6 mo of rituximab. Significantly higher PPD reduction of 0.2 mm after rituximab vs. baseline. Significant CAL gain of 0.3 mm after rituximab vs. baseline. Tendency for further improvements in periodontal parameters from 6 mo to 4 y of rituximab treatment. | Not investigated | Coat et al. (2015) |
| IL-23 and IL-12 inhibitor (ustekinumab) | Case report | 1 y | Refractory severe periodontitis associated with leukocyte adhesion deficiency was resolved. Decrease in BOP after 3 wk of treatment with ustekinumab. | Gingival expression of IL-23 and IL-17 became undetectable after 3 wk of treatment with ustekinumab. | Moutsopoulos et al. (2017) |
ACPA, anti–citrullinated protein antibody; anti-CCP, anti–cyclic citrullinated peptides; BOP, bleeding on probing; CAL, clinical attachment level; CRP, C-reactive protein; Cs, conventional synthetic; DAS28, disease activity score 28; DMARD, disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; GCF, gingival crevicular fluid; GI, gingival index; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; MGI, marginal gingival index; MMP-3, metalloproteinase 3; PBI, papillary bleeding index; PGI, papillary gingival index; PI, plaque index; PPD, probing pocket depth; RA, rheumatoid arthritis; RF, rheumatoid factor; SRP, scaling and root planing.
Conventional Synthetic DMARDs
Conventional synthetic DMARDs (csDMARDs) are combined with biologics to increase their efficacy (Äyräväinen et al. 2017; see Appendix for details). Methotrexate, one of the oldest csDMARDs, decreases the de novo synthesis of purines and pyrimidines and increases adenosine, leading to reduced cell proliferation, concentration of leukocytes, and proinflammatory cytokines (Ziebolz et al. 2018). However, methotrexate with leflunomide was associated with higher clinical attachment loss in patients with established RA (Romero-Sanchez et al. 2017). A recent prospective study evaluating the effect of csDMARDs on periodontal inflammation in patients with RA without additional periodontal therapy reported no significant differences in the periodontal parameters (Äyräväinen et al. 2017); however, the majority of patients had no more than one site with probing pocket depth (PPD) ≥6 mm and ≤15% of sites with bleeding on probing (BOP) at baseline, suggesting low periodontal inflammation. In contrast, a prospective study investigating adjunctive csDMARDs such as methotrexate, hydroxychloroquine, and sulfasalazine on the response to nonsurgical periodontal treatment reported an additional benefit, but the group using methotrexate as a monotherapy presented with inferior PPD reduction in comparison with the group receiving a combination of csDMARDs (Jung et al. 2018).
Biological DMARDs
TNF-α Inhibitors
Overexpression of TNF-α has been directly linked to the development of several autoimmune diseases, such as RA (Zamri and de Vries 2020). Infliximab (chimeric monoclonal antibody [mAb] to TNF-α) and etanercept (a soluble form of TNF receptor) block TNF binding and inhibit its downstream proinflammatory signaling (Hajishengallis et al. 2020). Several in vivo and in vitro studies have documented the inhibition of osteoclast formation and consequent bone resorption (Zamri and de Vries 2020). Given the well-described immunomodulatory role of TNF-α in periodontitis, several studies have investigated the impact of TNF-α inhibitors in periodontal disease (Preshaw 2018). Thus far, the results have been inconsistent.
A small body of evidence demonstrated significant benefit of TNF-α inhibitors on periodontal clinical parameters when delivered alone or in combination with periodontal treatment in patients with RA, while other studies described aggravated gingival inflammation with higher BOP (Pers et al. 2008; Ortiz et al. 2009; Üstün et al. 2013; Kobayashi et al. 2015; Codrina et al. 2017). In this vein, increased BOP was found in patients with RA receiving methotrexate with TNF-α inhibitors as compared with those receiving methotrexate and rituximab in a cross-sectional study (Ziebolz et al. 2018). A recent systematic review investigated how the duration of anti-TNF therapy influenced periodontal clinical parameters. The authors reported a trend for additional clinical benefit when these therapeutic agents were administered for periods of 6 wk to 6 mo, whereas >6 mo was associated with higher gingival index and BOP values. This suggests decreased compliance or secondary loss of response possibly related to development of antidrug antibodies, a frequent finding during treatment with biological DMARDs (Zamri and de Vries 2020).
IL6-Receptor Inhibitors
IL-6 is another key cytokine that regulates the inflammatory/immune response and bone metabolism in the pathogenesis of periodontitis and RA (Kobayashi et al. 2015). Tocilizumab is a recombinant humanized mAb shown to inhibit IL-6 signaling by binding to the IL-6 receptor (Preshaw 2018). Treatment with tocilizumab without additional periodontal treatment and/or oral hygiene instructions resulted in a small statistically significant improvement in PPD and clinical attachment level (CAL) after 6 mo. In addition, percentage of sites with BOP, PPD ≥4 mm, and CAL ≥4 mm decreased significantly after 6 mo of tocilizumab (Kobayashi et al. 2015). Interestingly, the group receiving tocilizumab demonstrated higher reduction of the gingival index as compared with the group treated with TNF-α inhibitors.
Janus Kinase Inhibitors
Since redundant cytokine networks operate across multiple signaling pathways to result in inflammatory tissue damage in periodontitis and RA, the question of whether blocking a single cytokine will exhibit any measurable clinical impact has been raised (Hajishengallis et al. 2020). The inhibition of intracellular enzymes, such as receptor-associated kinases, represents a novel way to simultaneously inhibit multiple cytokines. Janus kinase inhibitors (tofacitinib, baritinicib) reversibly inhibit janus kinase–dependent cytokine signaling and were approved by the Food and Drug Administration for the management of RA (Ancuța et al. 2020). In a recent prospective longitudinal study, 24 wk of therapy with baricitinib without any additional periodontal therapy and/or oral hygiene instructions resulted in a significant reduction in PPD, percentage of sites with PPD ≥4 mm, and sites with BOP (Ancuța et al. 2020). The improvement in clinical parameters was accompanied by reduction in systemic inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate (Ancuța et al. 2020).
Anti–B Lymphocyte Immunotherapy
Treatment with rituximab, an anti-CD20 mAb that promotes the selective depletion of CD20+ B cells, is used in B-cell malignancies and nonmalignant immune-related diseases such as RA (Coat et al. 2015). Despite minimal presence in healthy gingiva, B cells infiltrate and dominate sites of progressive chronic periodontal inflammation, where they directly stimulate osteoclasts by generating IL-17, IL-6, and RANKL (Coat et al. 2015). Patients who received B-cell blockade with rituximab for other diseases presented with less severe periodontitis after 6 mo (Coat et al. 2015). Since almost all participants received rituximab after one or more failures with anti-TNF-α immunotherapy to treat their medical problem, the effect of the latter on the clinical outcome could not be excluded.
Anti-IL-23/IL-17 Inhibitors
In contrast to periodontitis, IL-17 release by Th17 cells has not been linked to osteoclastogenesis and consequent inflammatory bone loss in established RA. In fact, IL-17 inhibition in patients with moderate to severe RA lacks robust therapeutic efficacy (Hajishengallis et al. 2020). However, this is not the case for psoriasis and psoriatic arthritis, where these therapies are commonly administered with clear clinical benefit. Ustekinumab is a monoclonal antibody that targets the IL-12/IL-23 p40 subunit and inhibits IL-12 and IL-23 activity, including the downstream production of IL-17 (Moutsopoulos et al. 2017). A recent case report described the benefit of ustekinumab in the resolution of refractory periodontal inflammation associated with leukocyte adhesion deficiency. Three weeks after administration of ustekinumab, BOP decreased, and the expression levels of IL-23 and IL-17 in gingiva were almost undetectable (Moutsopoulos et al. 2017).
To date, most data on the impact of these synthetic and biologic immunomodulators on periodontitis are small-scale cross-sectional clinical studies describing the periodontal status in patients undergoing treatment for RA. Prolonged use of synthetic and biological DMARDs is associated with side effects, including hepatotoxicity, higher risk for malignancy, infection, heart failure, and cutaneous reactions, putting the risk-benefit ratio into question for use in healthy individuals with periodontitis (Äyräväinen et al. 2017; Zamri and de Vries 2020).
Adjunctive Therapies for Periodontitis: Host Modulation Therapeutic Agents
Statins
Statins have been prescribed for decades to manage hyperlipidemia for the prevention of cardiovascular diseases. Statins inhibit HMG-CoA reductase (3-hydroxy-3-methylglutaryl coenzyme A reductase), hampering cholesterol biosynthesis. They are known to possess pleiotropic anti-inflammatory and osteostimulating properties (Petit et al. 2019), inhibiting activation of GTPases, enhancing alkaline phosphatase activity, and increasing the expression of bone morphogenetic protein 2 and vascular endothelial growth factor, which consequently induce proliferation and differentiation of osteoblasts promoting bone formation (see Appendix for details).
Current evidence suggests adjunctive clinical benefit with local, not systemic, delivery of statins (Table 2). Topical application of atorvastatin, simvastatin, and rosuvastatin prepared in gels improved nonsurgical treatment of deep pockets associated with intrabony defects (Pradeep et al. 2016; Priyanka et al. 2017). A systematic review reported significant PPD reduction and CAL gain with radiographic improvements at 6 mo (Bertl et al. 2017). An additional recent systematic review showed significant PPD reduction at 6 and 9 mo with locally delivered statins combined with nonsurgical therapy (Donos et al. 2020). A few studies have investigated statin gel and platelet-rich fibrin (PRF) combined in infrabony defects and open flap debridement with improvement in residual radiographic defects as compared with open flap debridement and PRF alone (Bertl et al. 2017). In addition, limited evidence suggests PPD reduction and relative horizontal and vertical CAL gain as an adjunction to nonsurgically treated mandibular class II furcation defects (Garg and Pradeep 2017). However, surgically treated class II furcation defects with 1.2% rosuvastatin gel, autologous PRF, and porous hydroxyapatite bone graft were not significantly better than with hydroxyapatite/PRF alone (Pradeep et al. 2016). Systemically administered statins and periodontal treatment were beneficial in preclinical animal studies; however, results from human clinical trials do not confirm this outcome (Bertl et al. 2017; Bertl et al. 2018). Heterogeneity among studies, with different types and doses of statins as well as lower concentrations within periodontal tissues when administered systemically, limits interpretation of data. Safety is also a concern with chronically administered statins (Bertl et al. 2017).
Table 2.
Host Modulation Therapeutic Agents in Patients with Periodontitis.
| Host Modulatory Agent | Type of Study | Follow-up | Clinical Outcomes | Subclinical Outcomes | Authors and Year |
|---|---|---|---|---|---|
| Statins | |||||
| RSV or ATV | RCT | 9 mo | Significantly higher PPD reduction for RSV gel (4.6 mm) and ATV gel (3.4 mm) than with placebo gel (1.9 mm) as adjuncts to SRP at 9-mo follow-up. Significantly higher CAL gain for RSV gel (4.3 mm) and ATV gel (3.3 mm) vs. placebo gel (1.9 mm) as adjuncts to SRP at 9-mo follow-up. Significantly higher intrabony defect depth reduction for RSV gel (4.3 mm) and ATV gel (3.3 mm) vs. placebo gel (0.1 mm). Significantly greater improvements in PPD reduction, CAL gain, and defect depth reduction with RSV gel than with ATV gel from baseline to 6 mo and from 6 to 9 mo. | Not investigated | Pradeep et al. (2016) |
| SMV | RCT | 6 mo | Significantly greater PPD reduction of 2.6 mm for SMV gel with SRP over placebo gel with SRP at 6 mo. Greater percentage of bone fill for SMV gel over placebo gel (34.01%) versus (2.62%). | Not investigated | Priyanka et al.2017) |
| RSV or ATV | RCT | 9 mo | Additional PPD reduction (1.2 mm) and additional gain in relative vertical CAL (0.6 mm), relative horizontal CAL (0.5 mm), and 7.6% additional defect depth reduction in the group with 1.2% RSV gel with SRP vs. group with 1.2% ATV with SRP. | Not investigated | Garg and Pradeep 2017) |
| SMV, RSV, ATV | Systematic review with meta-analysis | 3, 6, and 9 mo | Significant PPD reduction: weighted mean difference of 0.76 mm (at 3 mo) and 1.51 mm (at 6 mo) for local statins over placebo. Significant CAL gain: weighted mean difference of 1.17 mm (at 3 mo) and 1.84 mm (at 6 mo) for local statins over placebo. Significant residual radiographic depth reduction: weighted mean difference of 0.38 mm for OFD + PRF + local statins over OFD + PRF. | No significant decrease in IL-1α concentration in GCF and IL-6 expression in periodontal tissues for local statins combined with periodontal treatment. | Bertl et al. (2017) |
| SMV, RSV, ATV | Systematic review with meta-analysis | 6 and 9 mo | Significant PPD reduction: Mean difference of 1.83 mm (at 3 mo) and 2.25 mm (at 6 mo) for local statins over placebo combined with SRP in infrabony defects. | Not investigated | Donos et al. (2020) |
| Probiotics | |||||
| Lactobacillus brevis CD2 | RCT | 14 d | No significant changes in BOP in the probiotic group. Significant increase in BOP at days 10 and 14 vs. baseline in the placebo group. | Significant increase in nitric oxide levels in GCF only in the placebo group vs. baseline. | Lee et al. (2015) |
| Lactobacillus plantarum, L. brevis, and Pediococcus acidilactici | RCT | 6 wk | Significantly higher reduction in the number of sites with higher GI scores (GI = 3) in the probiotic group combined with mechanical plaque removal for treatment of gingivitis. | Significant reduction in Tannerella forsythia only in the probiotics group. | Montero et al. (2017) |
| Bifido-bacterium animalis subsp. lactis | RCT | 90 d | Significantly higher PPD reduction for moderate (0.3 mm) and deep (0.9 mm) pockets in the probiotics group over placebo group as adjuncts to SRP. Significantly higher CAL gain for moderate (0.5 mm) and deep (1.5 mm) pockets in the probiotics group over placebo group as adjuncts to SRP. | Significantly higher levels of IL-1β (at 30 and 90 d) and IL-8 (30 d) in the placebo group vs. the probiotics group as adjuncts to SRP. Significantly higher levels of IL-10 (at 30 d) in the probiotics group vs. baseline. | Invernici et al. (2018) |
| Lactobacillus reuteri | RCT | 24 wk | Additional reduction of 0.3 mm in PPD in the probiotics group vs. placebo as adjuncts to SRP. Significantly fewer sites in need for surgery in the probiotics group vs. placebo as adjuncts to SRP. | No significant differences on the levels of Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans between groups | Laleman et al. (2020) |
| Probiotics (different strains) | Systematic review with meta-analysis | >6 mo | No significant benefit in PPD reduction (0.38 mm) for probiotics vs. placebo as adjuncts to SRP at 6 mo follow-up. | Not investigated | Donos et al. (2020) |
| Probiotics (different strains) | Systematic review with meta-analysis | >3 mo | Significant benefit for probiotics vs. placebo in CAL gain at 3 mo (0.18 mm), 6 mo (0.3 mm), and 9 mo (0.9 mm). Significant improvement in PPD reduction for probiotics vs. placebo at 3 mo (0.2 mm) and 12 mo (1.2 mm). | No significant changes in the reduction of P. gingivalis, T. forsythia, P. intermedia, and F. nucleatum associated with probiotics use. | Ho et al. (2020) |
| Bisphosphonates | |||||
| Alendronate | RCT | 6 mo | Additional PPD reduction of 2.3 mm, CAL gain of 2.4 mm, and vertical defect fill for 1% alendronate gel vs. placebo gel as adjuncts to SRP. | Not investigated | Sharma and Pradeep 2012) |
| Alendronate | RCT | 6 mo | Additional PPD reduction, CAL gain, and radiographic bone fill in intrabony defects for 1% alendronate gel vs. placebo gel as adjuncts to OFD. | Not investigated | Carvalho Dutra et al. (2019) |
| Alendronate | RCT | 9 mo | Additional PPD reduction of 0.8 mm, CAL gain of 1.0 mm, and 8% vertical defect fill in intrabony defects for 1% alendronate gel combined with OFD vs. PRF and OFD. Additional PPD reduction of 1.7 mm, CAL gain of 2.1 mm, and 47% vertical defect fill in intrabony defects for 1% alendronate gel vs. OFD alone. | Not investigated | Kanoriya et al. (2016) |
| Alendronate | RCT | 9 mo | Additional PPD reduction and CAL gain of 0.7 mm and 6% vertical defect fill in mandibular degree II furcation defects for 1% alendronate gel combined with OFD vs. PRF and OFD. Additional PPD reduction of 2.0 mm, CAL gain of 1.8 mm, and 45% vertical defect fill in intrabony defects for 1% alendronate gel vs. OFD alone. | Not investigated | Kanoriya et al. (2017) |
| Alendronate | RCT | 9 mo | Additional PPD reduction of 0.8 mm and 10% vertical defect fill in intrabony defects for 1% alendronate gel vs. 1.2% ATV gel as adjuncts to SRP. Additional PPD reduction of 2.8 mm, CAL gain of 2.0 mm, and 43% vertical defect fill in intrabony defects for 1% alendronate gel vs. placebo gel as adjuncts to SRP. | Not investigated | Pradeep et al. (2017) |
| Alendronate | Systematic review with meta-analysis | 6 to 9 mo | PPD reduction: mean difference of 2.15 ± 0.4 mm for alendronate gels and SRP in infrabony defects vs. SRP and placebo gels. | Not investigated | Donos et al. (2020) |
| n-3 PUFAs | |||||
| n-3 PUFAs and ASA | RCT | 6 mo | Additional PPD reduction of 0.6 mm and CAL gain of 0.7 mm for n-3 PUFAs and ASA vs. placebo capsules as adjuncts to SRP. | Significant reduction of salivary RANKL and MMP-8 for n-3 PUFAs and ASA vs. placebo capsules as adjuncts to SRP. | El-Sharkawy et al. (2010) |
| n-3 PUFAs and ASA | RCT | 6 mo | Additional PPD reduction of 0.7 mm and CAL gain of 0.4 mm for n-3 PUFAs and ASA vs. placebo capsules as adjuncts to OFD and DFDBA. | Significant reduction of IL-1β in GCF for n-3 PUFAs and ASA vs. placebo capsules as adjuncts to OFD and DFDBA. | Elkhouli (2011) |
| n-3 PUFAs and ASA | RCT | 3 mo | Additional PPD reduction of 0.17 mm and higher reduction in number of sites with PPD ≥5 mm for DHA and ASA vs. placebo capsules and ASA. | Significant reduction of CRP and IL-1β in GCF but not IL-6 for DHA and ASA vs. placebo capsules and ASA. | Naqvi et al. (2014) |
| n-3 PUFAs and ASA | RCT | 6 mo | No additional clinical benefit for PPD reduction and CAL gain for low-dose n-3 PUFAs vs. placebo capsules as adjuncts to SRP. | Significant reduction of salivary TNF-α for low dose n-3 PUFAs. | Keskiner et al. (2017) |
| n-3 PUFAs | RCT | 6 mo | Additional PPD reduction of 1.0 mm and CAL gain of 0.8 mm for n-3 PUFAs vs. placebo capsules as adjuncts to SRP in women who are postmenopausal with periodontitis. | Significantly higher superoxide dismutase activity in GCF for n-3 PUFA. | Elgendy and Kazem (2018) |
| n-3 PUFAs and ASA | RCT | 6 mo | Additional CAL gain of 0.5 mm for moderate and 1.0 mm for deep pockets for n-3 PUFAs and ASA after SRP vs. placebo capsules and SRP in diabetic patients. | Significant reduction in hemoglobin A1c for n-3 PUFAs and ASA after SRP in diabetic patients. | Castro Dos Santos et al. (2020) |
ASA, aspirin; ATV, atorvastatin; BOP, bleeding on probing; CAL, clinical attachment level; DFDBA, demineralized freeze-dried bone allograft; DHA, docosahexaenoic acid; GCF, gingival crevicular fluid; GI, gingival index; IL, interleukin; MMP-8, metalloproteinase 8; n-3, omega-3; OFD, open flap debridement; PPD, probing pocket depth; PRF, platelet-rich fibrin; PUFA, polyunsaturated fatty acid; RCT, randomized controlled trial; RSV, rosuvastatin; SMV, simvastatin; SRP, scaling and root planing.
In summary, the overall data supporting the use of statins are not strong. Systemically administered statins show no consistent improvements in human trials. The data supporting the topical application of statins in periodontal defects originate mainly from one research group and have not been independently reproduced.
Probiotics
Emerging insights from research suggest that probiotics are potentially effective prophylactics or therapeutic candidates for various inflammatory diseases. Apart from their benefit related to microbiome modulation and pathogen suppression, probiotics may regulate the expression of immune-related genes, inhibit inflammatory pathways (e.g., NF-kB signaling pathway), promote activity of T-regulatory cells, enhance protection of host cells against physiologic stress, and improve epithelial barrier function (Suez et al. 2019; Hajishengallis et al. 2020). A number of human clinical trials have investigated the efficacy of Lactobacillus- and Bifidobacterium genera–based probiotics as adjuncts to scaling and root planing (SRP; Table 2). Lactobacillus brevis CD2 appeared to improve clinical gingival inflammation in experimental gingivitis (Lee et al. 2015): the host modulatory effect of this strain relies on enzymes that compete with arginase and nitric oxide synthase for the substrate arginine, thereby reducing generation of the potent proinflammatory mediator nitric oxide in gingival crevicular fluid (Lee et al. 2015). Probiotics and mechanical plaque removal in gingivitis demonstrate larger reduction in sites with high gingival index scores (Montero et al. 2017). Recent randomized controlled trials of probiotic lozenges/tablets with SRP in periodontitis reported significant PPD and CAL improvements when analyzed at the site level, especially for moderate and deep pockets (Invernici et al. 2018; Laleman et al. 2020). A recent systematic review reported significant benefit of probiotics in conjunction with SRP, with greater median PPD reduction and CAL gain at 3 and 12 mo (Ho et al. 2020). Conversely, other randomized controlled trials with a follow-up of 6 mo did not confirm any clear clinical benefit of probiotics as adjuncts to SRP (Donos et al. 2020).
The conflicting results may stem from disparity of studied dose, duration and strains of probiotics, differences in baseline severity of periodontal disease, and quality of SRP and follow-up. In addition, studies with small sample sizes testing unrelated microorganisms with different vehicles of administration were grouped in the scope of meta-analyses, thereby skewing results (Appendix). Of note, while regulation of cytokines such as IL-10, IL-1β, and IL-8 and host protective proteins such as human beta defensin 3, TLR4, and CD-4 is reported, most clinical studies attribute additional improvement to decreases in periopathogens rather than host modulation (Invernici et al. 2018; Invernici et al. 2020).
No adverse effects of probiotics were reported in a recent systematic review (Ho et al. 2020). However, their use has been associated with a higher risk of infection and/or morbidity (bacteremia and fungemia) in patients who are critically ill, hospitalized, or immunocompromised (Suez et al. 2019). The clinical efficacy, safety, and host modulatory potential of probiotics or synbiotics (Appendix) should be further established through larger-scale, longer clinical trials.
Bisphosphonates
Bisphosphonates disrupt osteoclastic activity, inhibit bone destruction, and promote osteoblastic activity, and they are used extensively in the management of osteoporosis and bone-resorptive pathologies (Preshaw 2018). Their use in periodontitis is questionable. Systemic bisphosphonates (oral or parenteral) as adjuncts to SRP were not better than SRP alone, and their efficacy was questioned in larger clinical trials (Preshaw 2018). A longitudinal study showed that orally administered bisphosphonates in older adults did not protect against bone loss (Helmi et al. 2019). Considering the severity of their main side effect, medication-related osteonecrosis of the jaw, oral and systemic use of bisphosphonates as an adjunct therapy is not indicated.
Reports of topical application of a bisphosphonate in combination with periodontal therapy showed that local delivery of 1% alendronate (ALN) gel (10 mg/mL) with SRP improved PPD, CAL, and bone fill of intrabony defects (Sharma and Pradeep 2012). Similar benefits were shown with the topical use of 1% ALN gel with surgical treatment of intrabony defects (Carvalho Dutra et al. 2019). Combination of PRF and 1% ALN gel further improved reduction of PPD and increase in CAL in intrabony defects (Kanoriya et al. 2016) and mandibular class II furcation defects (Kanoriya et al. 2017) as compared with PRF alone. Local delivery of 1% ALN gel combined with SRP was more effective in intrabony defects than 1.2% atorvastatin gel with SRP (Pradeep et al. 2017). Although no adverse effects have been reported with the topical application of ALN in periodontal treatment, the trend for benefit in periodontal clinical parameters needs to be interpreted with caution; the studies originate from one group and have not been independently reproduced. Further confirmation from studies in other clinical centers is required.
Dietary Omega-3 Fatty Acid Supplementation
Dietary supplementation with omega-3 (n-3) polyunsaturated fatty acids (PUFAs) is a therapeutic strategy for chronic inflammatory diseases, including RA, cardiovascular disease (Hu et al. 2019), diabetes, and cancer (Calder 2020), as well as treatment for COVID-19. A recent systematic review showed that n-3 PUFAs are associated with lower inflammatory biomarkers among diabetic and cardiovascular cases. n-3 PUFAs regulate inflammation through a variety of mechanisms. Eicosapentaenoic acid and docosahexaenoic acid are derived from marine dietary sources, while linolenic acid in found in vegetables. Fish oil, krill, and algal oils are commonly used as n-3 PUFA supplements (Vors et al. 2020). The dosing, time course, and formulations of n-3 PUFAs in clinical studies vary and remain ill-defined. However, it is evident that the anti-inflammatory actions are dose dependent and higher doses (2.0-6.0 g/d) exhibit greater efficacy (Hu et al. 2019; Calder 2020). The precise mechanisms underlying the protective actions of n-3 PUFAs are not fully understood (Souza et al. 2020).
Eicosapentaenoic acid and docosahexaenoic acid are the substrates for SPMs, including resolvins, maresins, and protectins, that orchestrate the active resolution of inflammation (Norris et al. 2018). Recent human randomized controlled trials confirm that n-3 PUFA supplementation increases plasma levels of SPMs (Norris et al. 2018). n-3 PUFAs change fatty acid metabolites of inflammatory cells, downregulate proinflammatory gene expression (e.g., NF-κB, NLPR3 inflammasome), and reduce the production of inflammatory mediators, including cytokines, chemokines, and adhesion molecules (Calder 2020). SPMs reprogram the host immune responses by shifting the leukocyte profile to a less inflammatory and more proresolving phenotype and increase the phagocytic activity of peripheral blood monocytes (Souza et al. 2020). n-3 PUFAs also suppress osteoclast differentiation and decrease alveolar bone loss in animal models of periodontitis. Recent meta-analyses of human clinical trials show that supplementation with n-3 PUFAs reduces systemic markers of chronic inflammation in plasma, including CRP, IL-6, TNF-α, and adiponectin (Vors et al. 2020).
A lower ratio of precursors of proresolution to proinflammatory lipid mediators in the gingival crevicular fluid of patients with aggressive periodontitis (Elabdeen et al. 2013) supports a mechanistic rationale for incorporating n-3 PUFA supplementation as a clinical strategy for periodontitis. The beneficial actions of dietary eicosapentaenoic acid and docosahexaenoic acid have been reported in several human clinical studies of periodontitis (El-Sharkawy et al. 2010; Naqvi et al. 2010; Elkhouli 2011; Naqvi et al. 2014; Elgendy and Kazem 2018; Castro Dos Santos et al. 2020; Table 2; see Appendix for details). Monotherapy with 2,000 mg of daily docosahexaenoic acid with 81-mg aspirin (ASA) significantly improved moderate periodontitis and gingival inflammation when compared with placebo with low-dose ASA (Naqvi et al. 2014). The combined daily use of n-3 PUFA and ASA for 2 mo as an adjunct to SRP promoted clinical and immunologic benefits in patients with periodontitis and type 2 diabetes (Castro Dos Santos et al. 2020). However, whether n-3 PUFA supplementation without low-dose ASA improves periodontal outcomes has not been well investigated. Dietary supplementation of low-dose n-3 PUFAs with SRP resulted in significant reduction of TNF-α levels in saliva of patients with periodontitis without any additional improvement in clinical periodontal parameters (Keskiner et al. 2017). Potential side effects of n-3 PUFAs and ASA include increased bleeding time, allergy to fish or ASA, diarrhea, and steatorrhea.
Novel Drug Candidates for Periodontitis
Specialized Proresolving Mediators
Nonresolving inflammation constitutes the common denominator in many chronic pathologies, including periodontitis (Van Dyke 2020). An imbalance between the proinflammatory and proresolving host response plays a significant role in the establishment and progression of periodontal inflammation (Balta et al. 2017). SPMs include a large family of lipid mediators (lipoxins, resolvins, protectins, and maresins) enzymatically derived from n-3 and n-6 PUFAs that collectively regulate the inflammatory process without immunosuppression. SPMs act as receptor agonists that initiate proresolving pathways, thereby promoting the termination of inflammation to facilitate restoration of tissue homeostasis (Norris et al. 2018; Van Dyke 2020).
Evidence provided in animal models demonstrates that topical application of SPMs reverses inflammatory gene expression, decreases inflammatory infiltrate, and promotes a host-mediated shift in the subgingival microflora reversing dysbiosis. Resolvins prevent endothelial transmigration of neutrophils in the gingiva. Systemic (intraperitoneal) administration of SPMs reduces circulating monocytes and increases the proportion of M2-like healing macrophages that exhibit enhanced phagocytosis of bacteria and apoptotic neutrophils (Mizraji et al. 2018). SPMs also modulate the adaptive immune response by downregulating major histocompatibility complex class II expression, inhibiting dendritic cell maturation and antigen presentation, with a consequent reduction in CD4+ T-cell activation in ligature-induced periodontitis (Mizraji et al. 2018). SPMs prevent alveolar bone resorption and regenerate lost bone due to periodontal inflammation. Several studies have elucidated their direct immunoregulatory actions on human periodontal ligament stem cells (Albuquerque-Souza et al. 2020) and bone cell differentiation and activation (El Kholy et al. 2018; Appendix).
The well-described protective actions and impact of SPMs in targeting resolution of inflammation without immunosuppression in several animal models of periodontitis (Van Dyke 2020) make them ideal future drug candidates. Studies in human periodontitis are underway.
Complement Inhibitors
The complement system is an integral part of innate immunity consisting of fluid-phase and cell surface–bound proteins (Mastellos et al. 2019). Complement plays a major role in effectively containing microbial infections via tight regulation of the inflammatory response (Mastellos et al. 2019). Complement dysregulation or excessive activation can be a key pathogenic driver in ocular and neurodegenerative disorders, cancer, periodontitis (Mastellos et al. 2019), and acute respiratory distress syndrome in patients with COVID-19 (Skendros et al. 2020).
Higher levels of complement activation in gingival tissues and gingival crevicular fluid (Hajishengallis et al. 2020) and saliva (Grande et al. 2020) of patients with periodontitis establish a clear role of complement in the pathogenesis of periodontitis. The central component of complement, C3, amplifies recruitment and phagocytosis by inflammatory leukocytes and is a promising therapeutic target for periodontitis (Skendros et al. 2020).
Intragingival injections of Cp40, an improved analog of the compstatin family of C3 inhibitors, was tested in ligature-induced periodontitis in young as well as old nonhuman primates with naturally occurring periodontitis (Hajishengallis et al. 2020). Cp40 suppressed osteoclastogenesis and bone loss and decreased gingival crevicular fluid IL-17 and the RANKL/OPG ratio (Maekawa et al. 2016; Bostanci et al. 2018). Since monkeys display clinical, microbiological, and immunohistologic features of periodontitis similar to those of humans, these candidate complement inhibitors are likely to be beneficial in humans and merit further clinical investigation (Mastellos et al. 2019; Hajishengallis et al. 2020). Cp40 formed the basis for AMY-101, a novel C3 inhibitor peptide that is currently in human trials for complement-mediated diseases, including periodontitis (Mastellos et al. 2019; Hajishengallis et al. 2020; ClinicalTrials.gov NCT03694444).
Conclusions
The contribution of a dysregulated exaggerated host immune/inflammatory response in periodontitis is clear. Current anti-infective therapies of periodontitis that target bacterial plaque have limited success; the focus needs to shift toward host modulatory agents that will promote the resolution of inflammation and the restoration of tissue homeostasis (Van Dyke 2020), especially in susceptible patients with high risk for periodontitis. Currently, there are insufficient data to suggest that the topical application of statin or ALN gels as adjuncts to SRP or surgical therapy may improve periodontal clinical parameters and increase radiographic bone fill in intrabony defects. Although no definitive conclusions can be drawn regarding the use of DMARDs in periodontitis, the data suggest a trend for additional clinical benefit. Since DMARDs are associated with significant unwanted side effects, more studies of larger size are necessary to determine the type, dose, and most effective duration of DMARD prescription. Supplementation with probiotics or n-3 PUFAs and ASA seems to be a promising adjunctive therapy for periodontitis. However, further longitudinal studies with larger samples are required to draw definitive conclusions about their therapeutic impact. Current preclinical evidence of SPMs and complement inhibitors supports their high potential to revolutionize treatment of periodontitis in the near future.
Author Contributions
M.G. Balta, E. Papathanasiou, contributed to conception, design, data acquisition, and interpretation, drafted and critically revised the manuscript; I.J. Blix, T.E. Van Dyke, contributed to conception, design, data acquisition, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_0022034521995157 for Host Modulation and Treatment of Periodontal Disease by M.G. Balta, E. Papathanasiou, I.J. Blix and T.E. Van Dyke in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported in part by US Public Health Service grant K08DE027119 to E. Papathanasiou and R01DE025020 to T.E. Van Dyke from the National Institute of Dental and Craniofacial Research.
ORCID iDs: M.G. Balta  https://orcid.org/0000-0002-5831-1253
https://orcid.org/0000-0002-5831-1253
E. Papathanasiou  https://orcid.org/0000-0002-6425-4777
https://orcid.org/0000-0002-6425-4777
T.E. Van Dyke  https://orcid.org/0000-0003-0856-3396
https://orcid.org/0000-0003-0856-3396
References
- Albuquerque-Souza E, Schulte F, Chen T, Hardt M, Hasturk H, Van Dyke TE, Holzhausen M, Kantarci A. 2020. Maresin-1 and resolvin E1 promote regenerative properties of periodontal ligament stem cells under inflammatory conditions. Front Immunol. 11:585530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancuța C, Pomîrleanu C, Mihailov C, Chirieac R, Ancuța E, Iordache C, Bran C, Țănculescu O. 2020. Efficacy of baricitinib on periodontal inflammation in patients with rheumatoid arthritis. Joint Bone Spine. 87(3):235–239. [DOI] [PubMed] [Google Scholar]
- Äyräväinen L, Leirisalo-Repo M, Kuuliala A, Ahola K, Koivuniemi R, Meurman JH, Heikkinen AM. 2017. Periodontitis in early and chronic rheumatoid arthritis: a prospective follow-up study in finnish population. BMJ Open. 7(1):e011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balta MG, Loos BG, Nicu EA. 2017. Emerging concepts in the resolution of periodontal inflammation: a role for resolvin E1. Front Immunol. 8:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl K, Parllaku A, Pandis N, Buhlin K, Klinge B, Stavropoulos A. 2017. The effect of local and systemic statin use as an adjunct to non-surgical and surgical periodontal therapy—a systematic review and meta-analysis. J Dent. 67:18–28. [DOI] [PubMed] [Google Scholar]
- Bertl K, Steiner I, Pandis N, Buhlin K, Klinge B, Stavropoulos A. 2018. Statins in nonsurgical and surgical periodontal therapy: a systematic review and meta-analysis of preclinical in vivo trials. J Periodontal Res. 53(3):267–287. [DOI] [PubMed] [Google Scholar]
- Bostanci N, Bao K, Li X, Maekawa T, Grossmann J, Panse C, Briones RA, Resuello RRG, Tuplano JV, Garcia CAG, et al. 2018. Gingival exudatome dynamics implicate inhibition of the alternative complement pathway in the protective action of the C3 inhibitor Cp40 in nonhuman primate periodontitis. J Proteome Res. 17(9):3153–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. 2020. N-3 PUFA and inflammation: from membrane to nucleus and from bench to bedside. Proc Nutr Soc. 79(4):404–416. [DOI] [PubMed] [Google Scholar]
- Carvalho Dutra B, Oliveira A, Oliveira PAD, Miranda Cota LO, Silveira JO, Costa FO. 2019. Effects of topical application of 1% sodium alendronate gel in the surgical treatment of periodontal intrabony defects: a 6-month randomized controlled clinical trial. J Periodontol. 90(10):1079–1087. [DOI] [PubMed] [Google Scholar]
- Castro Dos Santos NC, Andere N, Araujo CF, de Marco AC, Kantarci A, Van Dyke TE, Santamaria MP. 2020. Omega-3 PUFA and aspirin as adjuncts to periodontal debridement in patients with periodontitis and type 2 diabetes mellitus: randomized clinical trial. J Periodontol. 91(10):1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coat J, Demoersman J, Beuzit S, Cornec D, Devauchelle-Pensec V, Saraux A, Pers JO. 2015. Anti-B lymphocyte immunotherapy is associated with improvement of periodontal status in subjects with rheumatoid arthritis. J Clin Periodontol. 42(9):817–823. [DOI] [PubMed] [Google Scholar]
- Codrina A, Eugen A, Ridica C, Antohe M, Cristina I. 2017. Anti-tumor necrosis factor alpha therapy and periodontal inflammation in rheumatoid arthritis a clinical and biochemical approach. Rev de Chim. 68(2):369–372. [Google Scholar]
- Corrêa JD, Fernandes GR, Calderaro DC, Mendonça SMS, Silva JM, Albiero ML, Cunha FQ, Xiao E, Ferreira GA, Teixeira AL, et al. 2019. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci Rep. 9(1):8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donos N, Calciolari E, Brusselaers N, Goldoni M, Bostanci N, Belibasakis GN. 2020. The adjunctive use of host modulators in non-surgical periodontal therapy: a systematic review of randomized, placebo-controlled clinical studies. J Clin Periodontol. 47 Suppl 22:199–238. [DOI] [PubMed] [Google Scholar]
- El Kholy K, Freire M, Chen T, Van Dyke TE. 2018. Resolvin E1 promotes bone preservation under inflammatory conditions. Front Immunol. 9:1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy H, Aboelsaad N, Eliwa M, Darweesh M, Alshahat M, Kantarci A, Hasturk H, Van Dyke TE. 2010. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 fatty acids and low-dose aspirin. J Periodontol. 81(11):1635–1643. [DOI] [PubMed] [Google Scholar]
- Elabdeen HR, Mustafa M, Szklenar M, Ruhl R, Ali R, Bolstad AI. 2013. Ratio of pro-resolving and pro-inflammatory lipid mediator precursors as potential markers for aggressive periodontitis. PLoS One. 8(8):e70838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgendy EA, Kazem HH. 2018. Effect of omega-3 fatty acids on chronic periodontitis patients in postmenopausal women: a randomised controlled clinical study. Oral Health Prev Dent. 16(4):327–332. [DOI] [PubMed] [Google Scholar]
- Elkhouli AM. 2011. The efficacy of host response modulation therapy (omega-3 plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study). J Periodontal Res. 46(2):261–268. [DOI] [PubMed] [Google Scholar]
- Garg S, Pradeep AR. 2017. 1.2% rosuvastatin and 1.2% atorvastatin gel local drug delivery and redelivery in the treatment of class II furcation defects: a randomized controlled clinical trial. J Periodontol. 88(3):259–265. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM. 2020. Periodontal therapeutics: current host-modulation agents and future directions. Periodontol 2000. 82(1):186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande MA, Belstrom D, Damgaard C, Holmstrup P, Thangaraj SS, Nielsen CH, Palarasah Y. 2020. Complement split product C3c in saliva as biomarker for periodontitis and response to periodontal treatment. J Periodontal Res. 56(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Chavakis T, Lambris JD. 2020. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000. 84(1):14–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmi M, AlOsaimy S, Goodson JM, Hasturk H, Natto ZS. 2019. Annual alveolar bone loss in older adults taking oral bisphosphonate: a retrospective cohort study. BMC Oral Health. 19(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Acharya A, Sidharthan S, Li KY, Leung WK, McGrath C, Pelekos G. 2020. A systematic review and meta-analysis of clinical, immunological, and microbiological shift in periodontitis after nonsurgical periodontal therapy with adjunctive use of probiotics. J Evid Based Dent Pract. 20(1):101397. [DOI] [PubMed] [Google Scholar]
- Hu Y, Hu FB, Manson JE. 2019. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 8(19):e013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernici MM, Furlaneto FAC, Salvador SL, Ouwehand AC, Salminen S, Mantziari A, Vinderola G, Ervolino E, Santana SI, Silva PHF, et al. 2020. Bifidobacterium animalis subsp lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS One. 15(9):e0238425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernici MM, Salvador SL, Silva PHF, Soares MSM, Casarin R, Palioto DB, Souza SLS, Taba M, Jr, Novaes AB, Jr, Furlaneto FAC, et al. 2018. Effects of bifidobacterium probiotic on the treatment of chronic periodontitis: a randomized clinical trial. J Clin Periodontol. 45(10):1198–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung GU, Han JY, Hwang KG, Park CJ, Stathopoulou PG, Fiorellini JP. 2018. Effects of conventional synthetic disease-modifying antirheumatic drugs on response to periodontal treatment in patients with rheumatoid arthritis. Biomed Res Int. 2018:1465402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoriya D, Pradeep AR, Garg V, Singhal S. 2017. Mandibular degree II furcation defects treatment with platelet-rich fibrin and 1% alendronate gel combination: a randomized controlled clinical trial. J Periodontol. 88(3):250–258. [DOI] [PubMed] [Google Scholar]
- Kanoriya D, Pradeep AR, Singhal S, Garg V, Guruprasad CN. 2016. Synergistic approach using platelet-rich fibrin and 1% alendronate for intrabony defect treatment in chronic periodontitis: a randomized clinical trial. J Periodontol. 87(12):1427–1435. [DOI] [PubMed] [Google Scholar]
- Keskiner I, Saygun I, Bal V, Serdar M, Kantarci A. 2017. Dietary supplementation with low-dose omega-3 fatty acids reduces salivary tumor necrosis factor-alpha levels in patients with chronic periodontitis: a randomized controlled clinical study. J Periodontal Res. 52(4):695–703. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ito S, Kobayashi D, Kojima A, Shimada A, Narita I, Murasawa A, Nakazono K, Yoshie H. 2015. Interleukin-6 receptor inhibitor tocilizumab ameliorates periodontal inflammation in patients with rheumatoid arthritis and periodontitis as well as tumor necrosis factor inhibitors. Clin Exp Dent Res. 1(2):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laleman I, Pauwels M, Quirynen M, Teughels W. 2020. A dual-strain lactobacilli reuteri probiotic improves the treatment of residual pockets: a randomized controlled clinical trial. J Clin Periodontol. 47(1):43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Kim SJ, Ko SH, Ouwehand AC, Ma DS. 2015. Modulation of the host response by probiotic lactobacillus brevis CD2 in experimental gingivitis. Oral Dis. 21(6):705–712. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Briones RA, Resuello RR, Tuplano JV, Hajishengallis E, Kajikawa T, Koutsogiannaki S, Garcia CA, Ricklin D, Lambris JD, et al. 2016. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. J Clin Periodontol. 43(3):238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastellos DC, Reis ES, Lambris JD. 2019. Editorial: therapeutic modulation of the complement system: clinical indications and emerging drug leads. Front Immunol. 10:3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizraji G, Heyman O, Van Dyke TE, Wilensky A. 2018. Resolvin D2 restrains Th1 immunity and prevents alveolar bone loss in murine periodontitis. Front Immunol. 9:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero E, Iniesta M, Rodrigo M, Marín MJ, Figuero E, Herrera D, Sanz M. 2017. Clinical and microbiological effects of the adjunctive use of probiotics in the treatment of gingivitis: a randomized controlled clinical trial. J Clin Periodontol. 44(7):708–716. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos NM, Zerbe CS, Wild T, Dutzan N, Brenchley L, DiPasquale G, Uzel G, Axelrod KC, Lisco A, Notarangelo LD, et al. 2017. Interleukin-12 and interleukin-23 blockade in leukocyte adhesion deficiency type 1. N Engl J Med. 376(12):1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi AZ, Buettner C, Phillips RS, Davis RB, Mukamal KJ. 2010. N-3 fatty acids and periodontitis in us adults. J Am Diet Assoc. 110(11):1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi AZ, Hasturk H, Mu L, Phillips RS, Davis RB, Halem S, Campos H, Goodson JM, Van Dyke TE, Mukamal KJ. 2014. Docosahexaenoic acid and periodontitis in adults: a randomized controlled trial. J Dent Res. 93(8):767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris PC, Skulas-Ray AC, Riley I, Richter CK, Kris-Etherton PM, Jensen GL, Serhan CN, Maddipati KR. 2018. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation. Sci Rep. 8(1):18050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz P, Bissada NF, Palomo L, Han YW, Al-Zahrani MS, Panneerselvam A, Askari A. 2009. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 80(4):535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pers JO, Saraux A, Pierre R, Youinou P. 2008. Anti-TNF-alpha immunotherapy is associated with increased gingival inflammation without clinical attachment loss in subjects with rheumatoid arthritis. J Periodontol. 79(9):1645–1651. [DOI] [PubMed] [Google Scholar]
- Petit C, Batool F, Bugueno IM, Schwinté P, Benkirane-Jessel N, Huck O. 2019. Contribution of statins towards periodontal treatment: a review. Mediators Inflamm. 2019:6367402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep AR, Garg V, Kanoriya D, Singhal S. 2016. 1.2% rosuvastatin versus 1.2% atorvastatin gel local drug delivery and redelivery in treatment of intrabony defects in chronic periodontitis: a randomized placebo-controlled clinical trial. J Periodontol. 87(7):756–762. [DOI] [PubMed] [Google Scholar]
- Pradeep AR, Kanoriya D, Singhal S, Garg V, Manohar B, Chatterjee A. 2017. Comparative evaluation of subgingivally delivered 1% alendronate versus 1.2% atorvastatin gel in treatment of chronic periodontitis: a randomized placebo-controlled clinical trial. J Investig Clin Dent. 8(3):e12215. [DOI] [PubMed] [Google Scholar]
- Preshaw PM. 2018. Host modulation therapy with anti-inflammatory agents. Periodontol 2000. 76(1):131–149. [DOI] [PubMed] [Google Scholar]
- Priyanka N, Abhilash A, Saquib S, Malgaonkar N, Kudyar N, Gupta A, Kalra N, Pradeep AR. 2017. Clinical efficacy of subgingivally delivered 1.2 mg simvastatin in the treatment of patients with aggressive periodontitis: a randomized controlled clinical trial. Int J Periodontics Restorative Dent. 37(2):e135–e141. [DOI] [PubMed] [Google Scholar]
- Romero-Sanchez C, Rodríguez C, Santos-Moreno P, Mesa AM, Lafaurie GI, Giraldo QS, De-Avila J, Castillo DM, Duran M, Chalem PC, et al. 2017. Is the treatment with biological or non-biological DMARDS a modifier of periodontal condition in patients with rheumatoid arthritis? Curr Rheumatol Rev. 13(2):139–151. [DOI] [PubMed] [Google Scholar]
- Sharma A, Pradeep AR. 2012. Clinical efficacy of 1% alendronate gel as a local drug delivery system in the treatment of chronic periodontitis: a randomized, controlled clinical trial. J Periodontol. 83(1):11–18. [DOI] [PubMed] [Google Scholar]
- Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou E, Tsironidou V, Tsigalou C, et al. 2020. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 130(11):6151–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza PR, Marques RM, Gomez EA, Colas RA, De Matteis R, Zak A, Patel M, Collier DJ, Dalli J. 2020. Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators concentrations and reprogram host immune responses: a randomized double-blind placebo-controlled study. Circ Res. 126(1):75–90. [DOI] [PubMed] [Google Scholar]
- Suez J, Zmora N, Segal E, Elinav E. 2019. The pros, cons, and many unknowns of probiotics. Nature Medicine. 25(5):716–729. [DOI] [PubMed] [Google Scholar]
- Üstün K, Erciyas K, Kısacık B, Sezer U, Pehlivan Y, Öztuzcu S, Gündoğar H, Onat AM. 2013. Host modulation in rheumatoid arthritis patients with TNF blockers significantly decreases biochemical parameters in periodontitis. Inflammation. 36(5):1171–1177. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE. 2020. Shifting the paradigm from inhibitors of inflammation to resolvers of inflammation in periodontitis. J Periodontol. 91 Suppl 1:S19–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vors C, Allaire J, Mejia SB, Khan TA, Sievenpiper JL, Lamarche B. 2020. Comparing the effects of docosahexaenoic and eicosapentaenoic acids on inflammation markers using pairwise and network meta-analyses of randomized controlled trials. Adv Nutr [epub ahead of print 12 Aug 2020] in press. doi: 10.1093/advances/nmaa086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamri F, de Vries TJ. 2020. Use of tnf inhibitors in rheumatoid arthritis and implications for the periodontal status: for the benefit of both? Front Immunol. 11:591365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebolz D, Rupprecht A, Schmickler J, Bothmann L, Krämer J, Patschan D, Müller GA, Mausberg RF, Schmidt J, Schmalz G, et al. 2018. Association of different immunosuppressive medications with periodontal condition in patients with rheumatoid arthritis: results from a cross-sectional study. J Periodontol. 89(11):1310–1317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_0022034521995157 for Host Modulation and Treatment of Periodontal Disease by M.G. Balta, E. Papathanasiou, I.J. Blix and T.E. Van Dyke in Journal of Dental Research