Abstract
Alcoholism is a persistent worldwide problem associated with long-lasting impairments to decision-making processes. Some aspects of dysfunction are thought to reflect alcohol-induced changes to relevant brain areas such as the orbitofrontal cortex (OFC). In this review, we will examine how chronic alcohol exposure alters OFC function to potentially contribute to maladaptive decision-making, and explore experimental behavioral approaches that may be better suited to test whether alcohol dependence disrupts OFC’s function. We argue that although past works suggest impairments in aspects of OFC function, more information may be gained by specifically targeting tasks to the broader function of OFC as put forth by the recent hypothesis of OFC as a “cognitive map” of task space. Overall, we suggest that such a focus could provide a better understanding of how OFC function changes in alcohol dependence, and could inform better assessment tools and treatment options for clinicians working with this population.
Keywords: Orbitofrontal cortex, alcohol dependence, decision-making, cognitive map, goal-directed
The Orbitofrontal Cortex
Deficits in decision-making are seen in alcohol dependence and use disorders, and likely contribute to continuing alcohol use and relapse. However, the basic behavioral and neural mechanisms underlying this dysfunctional decision-making are not altogether clear. A growing body of evidence suggests the observed dysfunction may in part reflect alcohol-induced changes to the orbitofrontal cortex (OFC), a region integral to decision-making. Here we review the current state of OFC’s role in supporting decision-making and provide an overview of the existing evidence for disrupted OFC function in alcohol dependence. Based on the recent cognitive map hypothesis of OFC function, we propose that alcohol dependence may affect OFC’s contribution to inference-based decision making (Figure 1). As prolonged exposure to alcohol alters OFC function, conceptualizing and examining OFC according to the cognitive map hypothesis may be more informative and increase our understanding of how alcohol dependence disrupts OFC-dependent decision-making. Overall, we argue that an increased understanding of how alcohol dependence disrupts OFC function in the context of the most up-to-date theories is crucial to better inform potential therapeutic approaches in the clinical treatment of alcohol use disorder.
Figure 1.
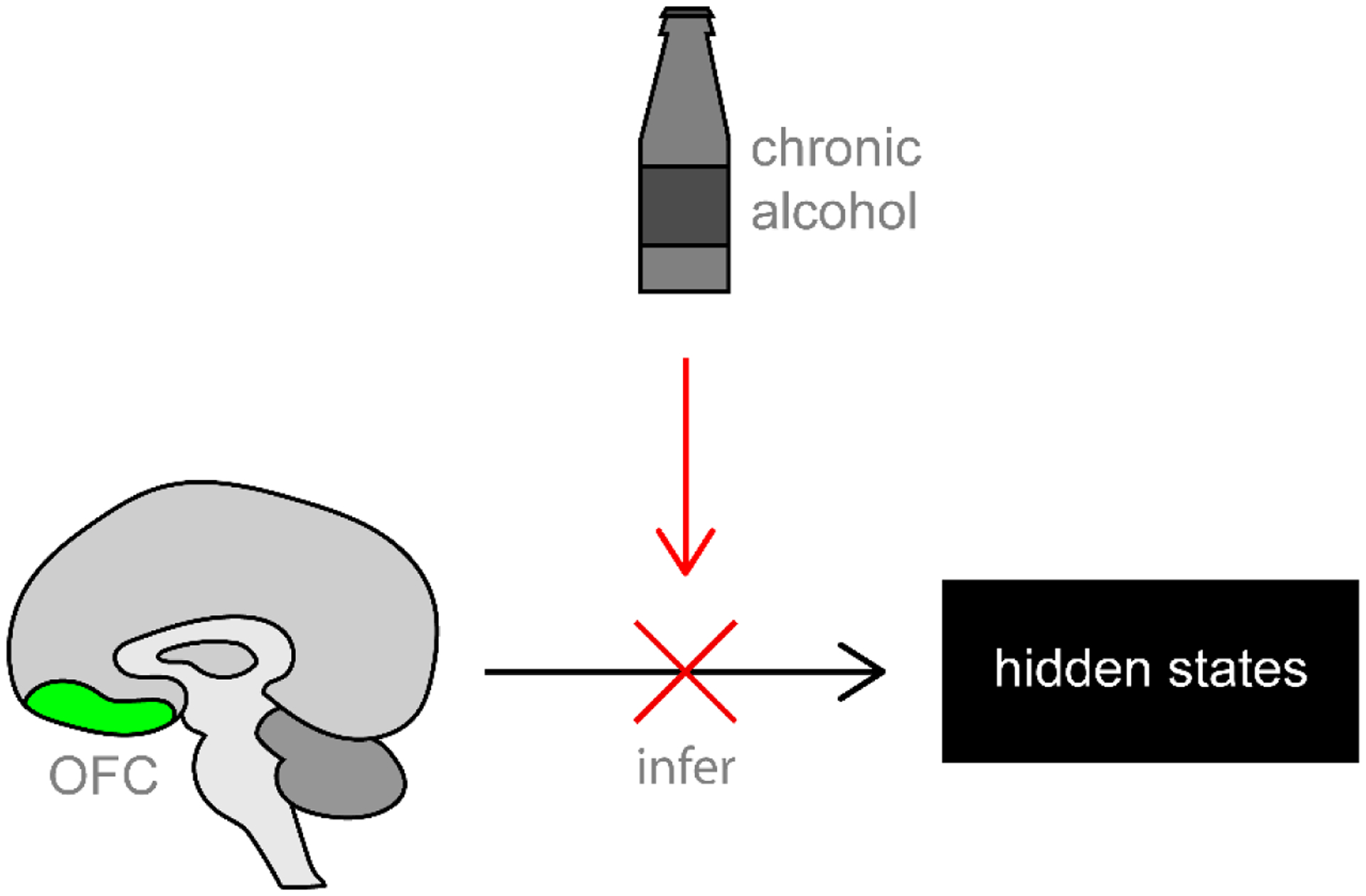
A recent hypothesis conceptualizes the orbitofrontal cortex (OFC) as providing a “cognitive map” of task space. In line with this hypothesis, alcohol dependence may alter decision-making by disrupting OFC function critical for inferring hidden task states. We argue that research using tasks targeted to this broader function will provide better understanding of how OFC changes in alcohol dependence, and could better inform future clinical practice for alcohol dependent populations.
OFC Function
In general, the cortical and subcortical connections of OFC suggest the area is well-positioned to contribute to ongoing decision-making by integrating sensory information with learning and motivational processes (for an in-depth review of OFC anatomy, see Kringelbach and Rolls, 2004). Recent works showing similar patterns of afferent and efferent connectivity across rodents and primates support this general function of OFC and suggest reasonable homology between species (Heilbronner et al., 2016; Hunnicutt et al., 2016). However, OFC’s contribution to decision-making has long been debated, leading to numerous theories over time (for review, see Stalnaker et al., 2015). One of the earlier dominant theories of OFC function was response inhibition, often operationalized in reversal learning tasks where subjects should alter behavior following the reversal of a previously-learned association between a reward and a cue or response. Indeed, OFC lesions often impair performance on reversal learning tasks (e.g., Dias et al., 1996a; Jones and Mishkin, 1972; Rolls et al., 1994; Teitelbaum, 1964), with the interpretation that OFC-lesioned subjects were unable to inhibit the now-incorrect response (Dias et al., 1996a; Jones and Mishkin, 1972). Another classic task impaired by OFC damage is reinforcer devaluation, where an outcome (e.g., food) is devalued with selective satiation or paired illness. In Pavlovian and instrumental tasks, intact animals decrease responding for a devalued outcome, whereas animals with OFC disruption often fail to diminish behavior to the same degree or at all (Bradfield et al., 2015; Gallagher et al., 1999; Gourley et al., 2016; Gremel and Costa, 2013; Izquierdo et al., 2004; Pickens et al., 2005; Rhodes and Murray, 2013; Rudebeck et al., 2013), but not always (see Ostlund and Balleine, 2007; Parkes et al., 2018 for two-choice instrumental tasks where outcome devaluation was not affected). While these deficits alone do not provide a complete picture of OFC contributions, they do provide a framework with which to build broader theories of OFC function.
One recent hypothesis conceptualizes OFC function as a “cognitive map” of task space. According to this proposal, OFC abstractly represents the information relevant for a given decision as the current task state—essentially, the current “location” in a task (Rich and Wallis, 2016; Schuck et al., 2016; Wilson et al., 2014; Zhou et al., 2019). This hypothesis accounts for OFC integrating rich sensory and value information to build a representation of the associative structure of a task, and could explain OFC’s contribution to decision-making as a comparison of different decision-making values at each task state (Schuck et al., 2016). The OFC cognitive map hypothesis therefore aligns with broader theories that prefrontal cortex flexibly represents current relevant information (Duncan 2001). Importantly, OFC’s contribution to such flexible representations is thought to be most critical for inferred, partially unobservable task properties (Wilson et al., 2014). For example, in reinforcer devaluation procedures, OFC may be important for informing behavior related to a devalued reward when that reward cannot be sampled, meaning that its reduced value is perceptually unavailable and must be inferred from memory. This process is distinguishable from working memory, thought to involve other prefrontal areas such as the medial prefrontal cortex (Liu et al., 2014), as unobservable information may be recalled only when relevant for task performance and is not necessarily actively retained in memory. Other examples of hidden task properties include associative task structure (e.g., in reversal learning tasks, where the task structure may change without warning), or reward value informed by motivational state (e.g., in incentive learning tasks, where hunger state can indirectly alter the value of reward outcomes). In contrast, observable task properties include perceptually available information such as cues in the environment, reward value attained through direct consumption, and other information gained through sensory experience. Thus, though OFC is thought to represent both observable and unobservable information, its crucial contribution to decision-making involves the latter (Wilson et al., 2014).
The cognitive map hypothesis accounts for behaviors thought to involve OFC. For example, in a reversal learning task, an animal with intact OFC function might construct a map of task space that integrates the relationships between actions and their outcomes, flexibly updating these relationships when contingencies are reversed. In this framework, for example, poorer reversal learning in animals with OFC damage suggests impaired updating of task structure after contingency reversal, where the new contingency is not apparent from observable task features and must be inferred (Bradfield et al., 2015). This hypothesis also accounts for other recent theories of OFC function, such as the proposal that OFC provides flexible encoding of cue-elicited outcome expectancy (e.g., Schoenbaum and Roesch, 2005), and even represents valueless sensory associations (Sadacca et al., 2018). For instance, neurophysiological work suggests that OFC can represent outcome sensory properties that are unrelated to value (McDannald et al., 2014), as well as more complex schemas integrating value with the context and position of objects (Farovik et al., 2015). These findings emphasize that OFC represents task features beyond just outcome value, consistent with a map of overall task structure. Thus, though work is ongoing, the cognitive map hypothesis appears to be a promising framework to investigate OFC and its dysfunction in diseases such as addiction. Though some abnormalities may pre-exist pathology, accruing evidence suggests addictive drugs produce deficits in behavior by directly altering OFC function (e.g., DePoy et al., 2014; Renteria et al., 2018). We will next review evidence for changes to OFC in alcohol dependence.
Orbitofrontal Cortex in Human Alcohol Dependence
OFC Structural and Functional Changes
Chronic alcohol use in humans has repeatedly been associated with structural changes to OFC. Studies of patients with alcohol use disorder report lower volume in OFC (e.g., Demirakca et al., 2011; Durazzo et al., 2011; Wang et al., 2016), with differences between patients and controls continuing even months into sustained abstinence (Laakso et al., 2002). Differences in OFC volume may also indicate disorder severity. Compared to active heavy drinkers, abstinent recovering patients had greater white and grey matter volume in OFC (O’Neill et al., 2001), and a more recent study found greater volume in the left lateral OFC of abstinent recovering patients compared to even light drinkers (Cardenas et al., 2011). Additional evidence suggests lower OFC volume correlates with relapse risk and relapse drinking behavior (Cardenas et al., 2011; Durazzo et al., 2011). Furthermore, OFC volume can increase over abstinence and correlates positively with abstinence duration (Demirakca et al., 2011; O’Neill et al., 2001).
Structural changes to OFC extend beyond reductions in volume. A diffusion tensor MRI study revealed decreased fractional anisotropy in the right OFC of abstinent alcoholics, suggesting white matter damage (Harris et al., 2008). In postmortem OFC, alcoholics had about 25% lower neural and glial density than controls, which also correlated negatively with duration of alcohol use disorder (Miguel-Hidalgo et al., 2006). These results are consistent with observed increases in neuroinflammation and neurodegeneration in OFC of postmortem human alcoholic brains and likely reflect the long-term neurotoxic effects of chronic alcohol use (Crews et al., 2013; Qin and Crews, 2012; Vetreno et al., 2013). Together, these studies support that chronic alcohol use is associated with extensive structural changes to OFC.
Given these structural changes, it is not surprising that alterations to OFC function have also been reported with chronic alcohol use. These functional changes observed at rest (i.e., independent of any behavioral task) generally suggest persistent OFC hypoactivity. Two studies reported reduced glucose metabolism in the OFC of abstinent alcoholics compared to controls that lasted weeks to months after detoxification (Volkow et al., 1994; Volkow et al., 1997), and the same group also found lower regional cerebral blood flow in the left OFC of 10-day abstinent alcoholics compared to healthy controls (Catafau et al., 1999). Combined with data suggesting chronic alcohol may be associated with OFC changes in inhibitory neurotransmission (Jin et al., 2012; Volkow et al., 1993; Volkow et al., 1997; Volkow and Fowler, 2000), increased opioid release (Mitchell et al., 2012), and other changes in neuromodulator relationships (e.g., Hommer et al., 1997; Volkow et al., 2007), these results indicate baseline alterations to OFC function and point to a role for OFC in behavioral changes associated with chronic alcohol use.
OFC Dysfunction in Decision-Making Behavior
Given the changes to baseline OFC structure and function, it seems likely that chronic high consumption of alcohol could change how OFC is recruited during behavior. Individuals with alcohol use disorder do often perform worse in behaviors thought to involve the OFC. For instance, impaired reversal learning performance of alcohol dependent patients has been seen in go/nogo tasks (Fillmore and Rush, 2006; Vanes et al., 2014) as well as reflexive behaviors (Fortier et al., 2008; Fortier et al., 2009). Other tasks requiring inhibition of a response, classically thought to involve OFC, show similar deficits in alcohol dependent patients (for meta-analyses, see Smith et al., 2014; Stavro et al., 2013). In a go/nogo task, alcoholics made significantly more errors than controls in both go and nogo conditions (Kamarajan et al., 2005). One interesting study examined the effect of multiple alcohol detoxifications in an incentive conflict task, where two stimuli individually produced reward but together were unrewarded or punished (Duka et al., 2011). The task recruited OFC in healthy controls, but the authors were unable to perform any correlations in the patient group because performance on the task was so poor, particularly in alcoholics with multiple detoxifications (Duka et al., 2011). These results suggest that normal OFC function, important for integrating the inferred relationship between two normally-rewarded cues into task structure, was greatly impaired in alcoholics.
As well as showing deficits in behaviors classically considered OFC-dependent, people with alcohol use disorder exhibit differences in OFC activity during reward-related decision-making. In a delay discounting task, abstinent alcoholics chose disadvantageous immediate rewards more frequently and exhibited decreased OFC activity during decision-making compared to controls (Boettiger et al., 2007). In addition, OFC activity correlated positively with tendency to choose the beneficial delayed reward across groups, suggesting that OFC hypoactivation in alcoholic subjects could contribute to poorer decision-making (Boettiger et al., 2007). In a rewarded card-guessing task with fixed outcomes, the lateral OFC was less active in abstinent alcoholics compared to controls in response to monetary rewards (Forbes et al., 2014). These results support the notion that OFC dysfunction may contribute to maladaptive decision-making following chronic alcohol use, particularly when reward value is involved.
It is also important to note that behavioral deficits in alcoholic subjects often overlap with those in patients with OFC damage. Severe deficits in reversal learning are widely demonstrated in OFC lesion patients, similar to people with alcohol use disorder (Camille et al., 2011; Fellows and Farah, 2003; Fellows, 2011; Hornak et al., 2004; Rahman et al., 1999; Rolls et al., 1994; Tsuchida et al., 2010). Another area of clear overlap is in the Iowa Gambling Task (IGT), where participants must learn through trial and error which deck of cards provides the best reward/penalty ratio (Bechara et al., 1994). Patients with damage to ventromedial prefrontal cortex, a broader area that overlaps with OFC, show severe impairment in the IGT compared to controls (Bechara et al., 1994; Bechara et al., 1997; Bechara et al., 1999). Similarly, alcoholics also perform poorly on the IGT relative to controls (Bechara et al., 2001; Brevers et al., 2014; Cantrell et al., 2008; Dom et al., 2006; Fein et al., 2004; Le Berre et al., 2014; Miranda et al., 2009; Noël et al., 2007). In light of these data, it appears that OFC dysfunction may underlie the impaired ability of alcoholics to retrieve and infer previous information—such as experience with value—to influence future decision-making behavior.
OFC Dysfunction in Alcohol Cues
Decision-making is often influenced by predictive environmental cues, and this process is commonly disrupted in alcohol dependence, especially for stimuli with a conditioned association with alcohol. In contrast to decreased OFC activation in other decision-making tasks, cue reactivity studies of alcoholics commonly find OFC hyperactivation to pictures of alcoholic versus non-alcoholic beverages (Ernst et al., 2012; Hermann et al., 2006; Myrick et al., 2008; Reinhard et al., 2015; Tapert et al., 2003; Wrase et al., 2002). One study intriguingly found patients with alcohol use disorder were more likely to approach alcohol cues and showed greater OFC activation when approaching alcohol cues compared to controls (Ernst et al., 2012). Another found similarly increased OFC activation to alcohol versus juice taste cues, and that this response correlated positively with clinical measures of alcohol dependence (Claus et al., 2011).
OFC cue reactivity may have clinical relevance for craving and relapse risk. In non-treatment seeking alcoholics, exposure to alcohol cues elicited increased OFC activity and self-reported craving compared to social drinkers (Myrick et al., 2004; Myrick et al., 2008). In abstinent alcoholics undergoing treatment, alcohol cue-induced OFC activity related to later risk of relapse (Reinhard et al., 2015). OFC alcohol cue reactivity may even predict the transition to heavy drinking and alcohol problems (Dager et al., 2014), suggesting that some OFC differences could either predate alcohol use or emerge in early stages of alcohol use. Supporting possible clinical relevance of OFC cue reactivity, varenicline, a nicotinic acetylcholine receptor agonist, reduced both cue-induced OFC activity and alcohol craving in non-treatment-seeking alcoholics (Schacht et al., 2014). That said, other drugs have been shown to reduce cue-induced craving without necessarily affecting OFC (Myrick et al., 2008), suggesting OFC’s role needs to be considered in the broader circuit controlling cue-related behaviors.
It is important to note that OFC hyperactivation is not a universal finding of alcohol cue reactivity studies. One study puzzlingly shows the opposite, finding reduced OFC activation in response to alcohol cues (Seo et al., 2013), though some have cautioned these findings may reflect some aspect of the experimental stress condition used in the study or the authors’ method of delineating OFC (Moorman, 2018). Other studies do not report any cue-induced activity in OFC (George et al., 2001; Ihssen et al., 2010; Lingford-Hughes et al., 2006; Schneider et al., 2001; Tapert et al., 2004). The reason for this discrepancy is uncertain, but previous reviews (e.g., Dom et al., 2005) have offered some suggestions. Briefly, there may be sex differences in cue-induced OFC activity, treatment status and abstinence may reduce cue reactivity in general, activation may differ for cues of different modalities, and older fMRI studies may be particularly susceptible to technical limitations in measuring OFC due to artifacts related to the air-tissue interface of the sinuses (for greater discussion, see Dom et al., 2005). Though there remains convincing evidence for OFC involvement in aspects of alcohol cue behavior, these results emphasize that the exact parameters are unclear. In addition, the behavioral and neural mechanisms underlying OFC dysfunction in chronic alcohol use are not entirely understood, and whether dysfunction was pre-existing or produced by alcohol exposure is often not established in human research. To begin answering these questions, we next move to animal models.
Orbitofrontal Cortex in Nonhuman Animal Chronic Alcohol Exposure
Findings from Nonhuman Primates
Nonhuman primate models of chronic alcohol use can offer excellent translational relevance due to established homologies between human and nonhuman primate OFC (Mackey and Petrides, 2010; Petrides and Pandya, 1994). Nonhuman primates will drink alcohol to high levels voluntarily, with a subpopulation of “heavy drinkers” displaying high intake, escalated consumption, and behavioral inflexibility (Carlson et al., 2011; Grant et al., 2008; Vivian et al., 2001). Monkeys also display changes to brain structure under chronic alcohol that are similar to humans. One study found similar cortical volume loss in high-drinking monkeys that was proportional to intake; however, there was no statistical relationship between drinking and frontal cortex volume (Kroenke et al., 2014). Other studies have found evidence of functional changes to OFC in chronic high-drinking monkeys, including a reduction in the excitability of OFC neurons (Nimitvilai et al., 2017b) that parallels lower baseline OFC neural activity in human alcoholics (Catafau et al., 1999; Volkow et al., 1994; Volkow et al., 1997). Chronic alcohol use in monkeys was also associated with decreased expression of GABAA receptor subunit mRNA in the OFC (Hemby et al., 2006), aligning with similar results in humans (Jin et al., 2012) and decreased GABAergic function more generally in the OFC of human chronic alcohol users (Volkow et al., 1993; Volkow et al., 1997). Other studies support OFC glutamate receptor dysregulation in monkeys with chronic alcohol intake (Acosta et al., 2010; Acosta et al., 2012), providing further evidence for disruption to baseline OFC function and potentially mirroring altered prefrontal cortex glutamatergic neurotransmission in human alcoholism (Davis and Wu, 2001; Krystal et al., 2003; Tsai, 1998). Monkeys with OFC lesions also show similar behavioral deficits as humans with OFC dysfunction. These deficits include impaired performance in reversal learning tasks (Dias et al., 1996a; Dias et al., 1996b; Izquierdo et al., 2004) and in instrumental reinforcer devaluation, with impairment lasting even 19 months after surgery (e.g., Izquierdo et al., 2004; Rhodes and Murray, 2013). In summary, though work on nonhuman primate OFC in chronic alcohol use is relatively limited, the findings overall correspond with results from human research.
OFC Structural and Functional Changes in Rodent Alcohol Models
Rodent models offer excellent manipulability of genetic and neural factors. However, as reliable drinking behavior to the level of physical dependence is often difficult to obtain in rodents, involuntary models of alcohol exposure are commonly used for examining the effects of alcohol dependence. Chronic intermittent ethanol (CIE) vapor exposure and intragastric (IG) administration have been used to reliably produce chronically elevated blood alcohol concentrations (BACs), physical dependence, and withdrawal symptomology (Becker, 2000). Indeed, work using these models has demonstrated changes to OFC that converge with human research in a number of areas.
Structural changes to OFC have been identified using both CIE and IG models. Seven days after CIE, one study found reduced spine density in the lateral OFC of CIE mice compared to controls exposed only to air (McGuier et al., 2015), consistent with changes to dendritic morphology in humans such as reduced spine density and arborization in the frontal cortex of alcoholic postmortem brain tissue (Ferrer et al., 1986; Harper and Corbett, 1990). However, two previous studies examining lateral OFC spine density three days after CIE found no effect of alcohol vapor exposure (DePoy et al., 2013; Holmes et al., 2012), suggesting that the underlying mechanisms are likely complex and highly dependent on time since withdrawal, among other factors. Rodents treated with IG alcohol show evidence of neuroinflammation and degeneration in the OFC (Corso et al., 1998; Crews et al., 2013; Vetreno et al., 2013), similar to changes in OFC of human alcoholic postmortem brain tissue (Crews et al., 2013; Qin and Crews, 2012; Vetreno et al., 2013).
Rodent models have also revealed functional changes to OFC. A prominent finding in this area is altered OFC neuron excitability, though the direction of change is inconsistent. Previous electrophysiological work from our lab identified decreased excitability of OFC neurons in CIE-exposed mice (Renteria et al., 2018). These results are consistent with past work showing reduced OFC excitability in high-drinking nonhuman primates (Nimitvilai et al., 2017b) and lower baseline OFC activity in human alcoholics (Catafau et al., 1999; Volkow et al., 1994; Volkow et al., 1997). However, the same group that found reduced OFC excitability in macaques has also consistently reported opposite results in CIE mice, finding increased excitability in both males and females (Nimitvilai et al., 2016; Nimitvilai et al., 2017a; Nimitvilai et al., 2018a; Nimitvilai et al., 2018b). Though these discrepancies are not yet fully understood, the underlying effect of altered OFC excitability remains. Additional work points to other adaptations within OFC that may occur with chronic alcohol. A study found alterations to glutamatergic transmission in the medial OFC of CIE mice, including upregulation of NMDA receptor subunit expression and increased NMDA receptor-mediated currents (Radke et al., 2017), likely reflecting long-term neuroadaptations. In addition, mice exposed to IG alcohol show reduced D4 dopamine receptors in OFC, indicating changes to dopamine function reminiscent of altered dopamine response in alcoholic humans (Volkow et al., 2007).
Communication between OFC and other brain areas is also altered in rodent chronic alcohol models. Our lab has previously reported that mice exposed to CIE had decreased neurotransmission between OFC and the dorsal medial striatum, another area necessary for goal-directed control (Renteria et al., 2018). Similar results were found in a study using fMRI to examine resting state connectivity in the brains of adult rats exposed to intermittent IG alcohol during adolescence. Broad decreased functional connectivity was found between OFC and a number of other brain areas, including the dorsal striatum, nucleus accumbens, and infralimbic prefrontal cortex (Broadwater et al., 2018). OFC connectivity is also disrupted in alcohol dependent humans, where past work has shown lower resting state functional connectivity between OFC and hippocampal gyrus (Wang et al., 2016), and altered dopamine activity that is suggestive of reduced prefrontal control over dopaminergic mesolimbic pathways (Volkow et al., 2007).
Additional work has emphasized altered monoamine circuit contribution following chronic alcohol. Human alcoholics showed reduced OFC glucose utilization following administration of a mixed serotonin agonist/antagonist (Hommer et al., 1997). In CIE mice, monoamine application to OFC slices did not produce the normal inhibition of lateral OFC firing (Nimitvilai et al., 2017a; Nimitvilai et al., 2018a). In summary, chronic alcohol affects OFC function across a variety of measures, suggesting potential disrupted or compensatory mechanisms that might underlie behavioral deficits associated with alcohol. Further, these studies frequently overlap with OFC functional changes in human alcoholics, supporting involuntary methods of alcohol administration such as IG and CIE as valid models of chronic alcohol exposure in rodents.
OFC Dysfunction in Rodent Behavioral Models
Structural and functional changes to brain areas such as the OFC are associated with altered behavior in rodent alcohol models. These changes frequently overlap with those seen in humans with both alcohol dependence and lesions to OFC. For instance, poorer performance in reversal learning tasks has been demonstrated across various animal models of alcohol exposure. Using the CIE model, one study found that reversal learning was impaired in mice up to ten days after CIE (Badanich et al., 2011), although a separate group found CIE mice made fewer errors in later sessions of reversal learning (DePoy et al., 2013). A number of IG studies have demonstrated selective deficits in reversal learning, particularly using a binge model of exposure in adolescent rodents (Coleman et al., 2011; Coleman et al., 2014; Fernandez et al., 2017; Obernier et al., 2002). Furthermore, longer periods of BAC elevation are associated with poorer reversal learning (Badanich et al., 2016; McMurray et al., 2014). Thus, it appears that chronic high levels of intoxication and physical dependence may be critical for producing alcohol-related behavioral deficits in animal models that overlap with poor reversal learning performance in humans with OFC lesions or alcohol use disorder.
Other tasks indicate similarly impaired performance. Rats exposed to CIE exhibit poorer behavioral flexibility in operant (Gass et al., 2014; Trantham-Davidson et al., 2014) and attentional set-shifting tasks (Hu et al., 2015; Kroener et al., 2012; Rodberg et al., 2017). In reward-related decision-making, rodents exposed to chronic alcohol typically display greater preference for risky behavior, an effect particularly well demonstrated in adolescents (Boutros et al., 2015; Clark et al., 2012; McMurray et al., 2014; Nasrallah et al., 2009; Nasrallah et al., 2011; Schindler et al., 2014). Interestingly, one study found that increased risk preference was associated with blunted response in OFC specifically for reward outcomes, suggesting disruption to reward-related OFC activity (McMurray et al., 2016).
Importantly, one OFC-dependent task affected by alcohol is instrumental outcome devaluation. In a chronic drinking model, rats trained to lever press for alcohol were insensitive to alcohol devaluation after 8 weeks of training (Corbit et al., 2012; Corbit et al., 2014). Impaired outcome devaluation was also found in alcohol-dependent CIE mice trained to self-administer alcohol (Lopez et al., 2014; Renteria et al., 2020). Furthermore, prior CIE exposure left even food reward seeking in mice insensitive to outcome devaluation (Renteria et al., 2018). These findings echo impairments in devaluation produced by OFC chemical lesions, chemogenetic inhibition of OFC projection neurons, and knockdown of brain-derived neurotrophic factor in OFC (Bradfield et al., 2015; Gourley et al., 2016; Gremel and Costa, 2013), suggesting that alcohol dependence itself may affect OFC-based devaluation through alterations to OFC function.
Indeed, recent work has begun to uncover OFC neural mechanisms underlying behavioral deficits associated with alcohol dependence. Renteria et al. (2018) found that decreased sensitivity to outcome devaluation in CIE mice was associated with decreased OFC excitability as well as decreased synaptic transmission from OFC to the direct pathway in dorsal medial striatum, a circuit implicated in goal-directed behavior. CIE-induced behavioral deficits were rescued with OFC chemogenetic activation (Renteria et al., 2018), aligning with past work showing goal-directed behavior is strengthened with OFC activation and disrupted by OFC inhibition (Gremel and Costa, 2013). Another group found that lateral OFC lesions and OFC chemogenetic inhibition increased alcohol consumption in CIE mice, an effect that continued even after the addition of bitter quinine to devalue alcohol (den Hartog et al., 2016). Together, these studies support the hypothesis that OFC disruption contributes to the loss of goal-directed control in chronic alcohol exposure.
OFC Dysfunction in Rodent Alcohol Cue Behavior
Although human research often reports cue-induced OFC activation in chronic alcohol users, the mechanisms behind this cue reactivity are not well understood. Just a handful of rodent studies have investigated OFC involvement in alcohol cues, generally echoing findings of OFC overactivation in human alcoholics. For example, exposure to contextual, light, and olfactory cues associated with alcohol in reinstatement paradigms increases Fos expression in OFC (Bianchi et al., 2018; Jupp et al., 2011). Further, inactivation of OFC disrupted the reinstatement of alcohol seeking after exposure to alcohol cues (Bianchi et al., 2018). These studies parallel human work by suggesting OFC hyperactivity to alcohol cues that perhaps induce craving and alcohol seeking, overall supporting increased OFC-mediated cue control over alcohol behavior. However, more work is needed to tease apart how cue-related information engages the OFC to produce hyperactivity, and to better understand the role OFC plays in cue control over alcohol-related behaviors.
Alcohol Dependence and the Orbitofrontal Cortex Cognitive Map
OFC as a Cognitive Map of Task Space
The picture emerging from the literature so far is one of extensive changes to OFC structure, function, and dependent behaviors in chronic alcohol exposure across species. However, it is not yet clear how alcohol dependence alters OFC function and behaviors within the framework of the recent “cognitive map” hypothesis of OFC function. Future work in both humans and non-human research subjects could provide better understanding by explicitly examining the partially unobservable states thought to be OFC’s crucial contribution to representations of task structure. To accomplish this, an appropriate task would incorporate properties that cannot be perceived through direct sensory experience. For instance, in rodent reversal learning procedures, observable task properties may include cue lights, instrumental levers, and other directly perceivable contextual information in the training environment. In contrast, an unpredictable and unsignaled change in task associative structure is an unobservable task property (not experienced sensorially), and the ability to alter behavior after such a change is crucial for performance in reversal learning. Disrupted ability to infer the change in associative structure, for example produced by alcohol-induced changes to OFC function, could lead to deficits in task performance (Figure 2).
Figure 2.
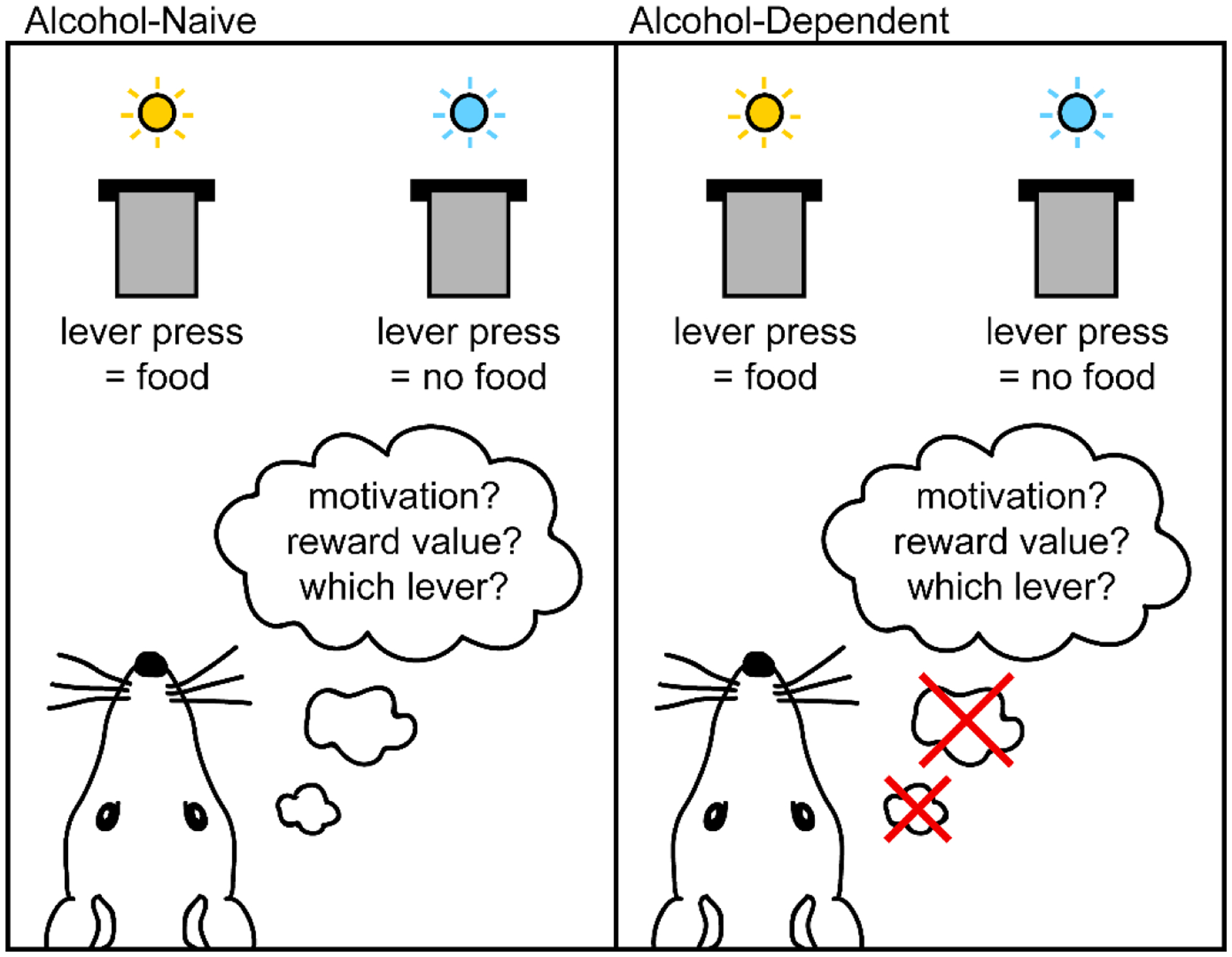
Example of task properties in reversal learning. In this scenario, reward delivery had previously occurred when the right lever was pressed, but has just switched unpredictably such that reward delivery now only occurs when the left lever is pressed. Properties in the task are distinguishable as observable (available through sensory experience) and unobservable (not available through sensory experience). Here, observable properties include both levers and cue lights, and other sensory information in the environment such as visual, auditory, or olfactory contextual cues. Unobservable properties include motivation to perform the task, expected reward value, and representation of task associative structure. Performance on reversal learning tasks relies on such unobservable properties, in particular the ability to infer the change in task structure following reversal of a previously-learned association. We propose that alcohol dependence may disrupt performance in tasks like reversal learning by altering the function of the orbitofrontal cortex, which is thought to be crucial in representing unobservable task properties.
In an example of an OFC task designed for human participants, Schuck et al. (2016) examined OFC function by utilizing both observable and unobservable task features. The task involved judging the age (young or old) of just one object within an image of two superimposed objects, and participants had to recall information from previous trials to know which object to judge. Thus, the observable task properties were the ages and identities of the images in the current trial, but task performance relied on unobservable task properties such as the knowledge of which object and age were judged in the previous trial. The authors found that unobservable task states could be decoded from OFC neuroimaging data, and that decoding accuracy was related to task performance (Schuck et al., 2016). This task could certainly be used to examine how alcohol dependence alters OFC function in humans. However, it is important to highlight that within experimental psychology there already exist well-validated, experimentally-defined tasks used in both humans and nonhumans that incorporate hidden states and therefore have the potential to reveal deficits in OFC function within the context of the cognitive map hypothesis. We suggest three such tasks next.
Tasks to Examine Alcohol Dependence Effects on OFC
Performance following outcome devaluation, already demonstrated to be OFC-dependent, can be used to examine inferred value control over both Pavlovian and instrumentally controlled behavior. That is, devaluing an outcome can reduce the amount of conditioned responding supported by a cue (Colwill and Motzkin, 1994; Holland, 1990), as well as reducing the amount of actions made toward procuring the outcome (Adams and Dickinson, 1981; Colwill and Rescorla, 1985). Additionally, though outcome devaluation is commonly examined in rodents, the task is also suited to primate research and has been demonstrated to involve OFC in both macaques (West et al., 2011) and humans (Gottfried et al., 2003; Valentin et al., 2007).
Outcome devaluation of the cue- or action-paired outcome is often performed in one of two ways: with sensory-specific satiation, or with repeated aversive pairings of the outcome with illness (see Box 1A). Following devaluation procedures, either conditioned responding or instrumental responding is assessed in subsequent extinction tests. Subjects with intact outcome devaluation demonstrate lower conditioned responding or reduce the frequency of actions made to get the outcome, respectively. In both tasks, subjects cannot directly experience the now-devalued outcome during testing; instead, they must infer this perceptually unavailable change in outcome value from memory to appropriately alter their behavior. In the framework of the cognitive map hypothesis, a rodent performing an outcome devaluation task might for example construct a map of task space that integrates lever pressing for food (an action-outcome contingency) with the reduced value of the food after devaluation (an inferred outcome property), resulting in decreased lever pressing. Multiple labs have reported an insensitivity to outcome devaluation of rodent instrumental responding following chronic alcohol consumption (Corbit et al., 2012) or following induction of alcohol dependence (Lopez et al., 2014; Renteria et al., 2018). In addition, alcohol-related contextual cues can produce an immediate and selective loss of goal-directed control in rats (Ostlund et al., 2010). However, in contrast, outcome devaluation of Pavlovian alcohol-related conditioned responses has been more elusive.
Box 1. OFC-dependent tasks.
The basic outcome devaluation procedure is illustrated in A. The procedure involves an initial stimulus-outcome (S–O) or action-outcome (A–C)) training period, followed by devaluation of the outcome with either selective satiation (e.g., Gremel & Costa, 2013) or paired sickness (e.g., Gallagher et al., 1999). Final testing involves comparing responses (the trained action, A, or the conditioned response, CFR) for a valued outcome versus a devalued outcome. Previous work has demonstrated that animals with OFC disruption fail to reduce responding for the devalued outcome, showing similar levels of responding as for a non-devalued outcome (see A for simulated data; Gremel and Costa, 2013; Gourley et al., 2016; Gallagher et al., 1999). B. Pavlovian-to-instrumental transfer (PIT) introduces Pavlovian (S–O) and instrumental (A–O) associations in separate training periods. In the subsequent test session, the instrumental action is measured in the presence of the conditioned stimulus. with PIT demonstrated via an increase in A during S (compared to A during no S). Previous work has shown that post-training OFC lesions (Ostlund and Balleine. 2007) and inactivation of basolateral amygdala terminals in OFC (Lichtenberg et al., 2017) can reduce or eliminate transfer in the PIT test (see B for simulated data). C. The incentive learning procedure trains an instrumental chain in which completion of the first action requirement (A1) produces a second action (A2) that when made earns the reward outcome. After training this instrumental sequence, a shift in motivational state is achieved by changing food restriction state (e.g., from hungry to not hungry). Importantly, subjects only update the value of the outcome if subsequently re-exposed to the outcome in the new motivational state. The incentive learning test then compares responses for shifted animals vs non-shifted animals. Previous work suggests that OFC is critical for altering behavior following the shift in motivational state (Baltz et al., 2018; Malvaez et al.. 2019; see C for simulated data). Though the tasks discussed above incorporate hidden states and have been shown to involve OFC, it is unclear how alcohol-induced changes to OFC might affect their performance.
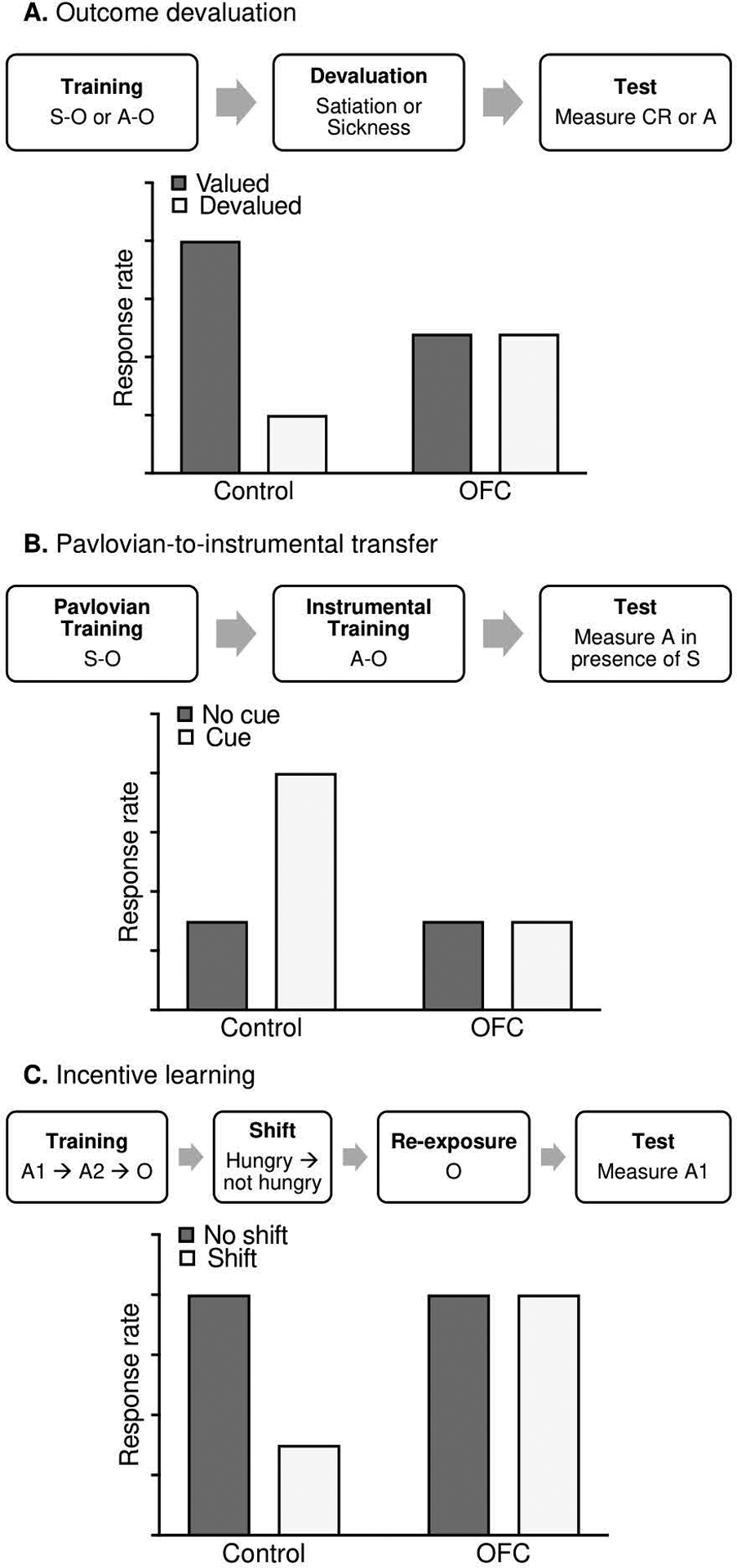
Another behavioral task that may better capture OFC function is Pavlovian-to-instrumental transfer (PIT; see Box 1B). Broadly, PIT is the process by which previously conditioned Pavlovian cues influence instrumental behavior. Described as an interaction between Pavlovian and instrumental associative learning processes (Holmes et al., 2010), PIT has been demonstrated across species, including in humans (e.g., Garbusow et al., 2016; Lehner et al., 2017; Talmi et al., 2008). A basic PIT procedure involves a period of training a Pavlovian association (e.g., auditory tone predicts outcome delivery), a separate period of training an instrumental contingency (e.g., lever press now produces the same outcome), and a test session in which the instrumental action is available and the Pavlovian cues are presented intermittently but no rewards are delivered. If transfer occurs, the frequency of instrumental behaviors changes during cue presentation (e.g., lever presses are energized during the Pavlovian cue), even though the Pavlovian cue and instrumental action were never previously associated. This means that PIT explicitly includes partially unobservable states, important for investigating OFC function, in two ways. First, the reward outcome is not delivered at any point during the test, meaning its value and sensory characteristics are perceptually unavailable; second, connections between the Pavlovian cue, instrumental behavior, and any overlap in their outcome representations must be inferred, as the PIT test represents the first and only time Pavlovian cues are presented during instrumental behavior. Indeed, previous work indicates post-training OFC lesions (Ostlund and Balleine, 2007) and chemogenetic inactivation of isolated basolateral amygdala terminals located in OFC (Lichtenberg et al., 2017) can disrupt transfer in the PIT test, when partially unobservable states become critical.
It is unclear how alcohol might disrupt OFC function as measured with PIT. Clues come from research in humans. One study found stronger PIT in abstinent human alcoholics compared to controls (Garbusow et al., 2016), with alcoholics showing potentiated instrumental behavior when predictive cues were present. Importantly, this was observed for non-drug Pavlovian cues, suggesting that chronic alcohol may induce long-lasting functional changes to neural circuits that support PIT processing. However, the authors limited their fMRI analyses in this study to the nucleus accumbens, so the contribution of other brain areas such as OFC is unknown (Garbusow et al., 2016). Future work could specifically investigate the impact of chronic alcohol exposure on OFC function using PIT, focusing in particular on any differences in OFC function during the PIT test, when partially unobservable states become an integral part of the task structure.
Another task demonstrated to engage OFC function is incentive learning (see Box 1C), which underlies the ability to use motivational states to assign value to our actions (Balleine and Dickinson, 1991; Balleine, 1992; Balleine et al., 1995; Balleine et al., 2005; Corbit and Balleine, 2003; Wassum et al., 2009). In rodents, an incentive learning task usually involves completing a lever press requirement on one lever, which then releases a second lever that, when pressed, triggers food delivery. Following a shift in motivational state (e.g., from hungry to full), intact animals only reduce lever pressing on the first lever in the sequence, and do so only if allowed prior experience of the outcome in the new motivational state. This task involves an excellent example of partially unobservable states, as the updated incentive value of the outcome must be inferred from memory during testing, when no rewards are delivered. In other words, animals unable to encode or retrieve the updated outcome value will not change their behavior even after a shift in motivational state. An additional benefit of this task is that the change in motivational state can be bi-directional, with incentive learning demonstrable following both increases as well as decreases in motivational state.
Recent work suggests OFC is critical for incentive learning processes, both at the point of encoding the updated value (Baltz et al., 2018; Malvaez et al., 2019) and when retrieving the updated value from memory to control responding (Malvaez et al., 2019). However, it is currently unknown whether alcohol-induced deficits in incentive learning are largely driven by changes to OFC function. Future work could investigate alcohol’s effect on OFC function using the incentive learning task, focusing in particular on the inferred change in incentive value that drives behavior after a shift in motivational state.
Together, the tasks discussed above and illustrated in Box 1 could provide a better understanding of how alcohol affects OFC function. The use of experimentally defined behaviors that critically depend on OFC function offers an excellent way to examine the neural mechanisms underlying behavioral deficits in task structures that include unobservable states.
Summary
A wealth of past work indicates that aspects of OFC structure, function, and dependent behaviors are disrupted in alcohol dependence. However, it remains unclear how alcohol affects OFC function as understood in the most recent theories of OFC’s role in decision-making. We suggest future research could better investigate the impact of long-term alcohol on OFC by using tools that incorporate partially unobservable task states, which are better suited to capture OFC’s complex representation of task structure. Finally, advancing work in this area has important clinical implications, as insights into alcohol-induced changes to OFC could help identify methods for assessing and potentially even treating OFC-dependent decision-making deficits in patients with alcohol use disorder.
Acknowledgments
Work supported by: NIH R01 AA026077 awarded to C.M.G.
Footnotes
Conflict of Interest. The authors have no conflicts of interest to declare.
References
- Acosta G, Hasenkamp W, Daunais JB, Friedman DP, Grant KA, Hemby SE (2010) Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys. Brain Research 1318:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta G, Friedman DP, Grant KA, Hemby SE (2012) Alternative splicing of AMPA subunits in prefrontal cortical fields of cynomolgus monkeys following chronic ethanol self-administration. Frontiers in Psychiatry 2(72). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CD, Dickinson A (1981) Instrumental responding following reinforcer devaluation. The Quarterly Journal of Experimental Psychology Section B 33(2b):109–121. [Google Scholar]
- Badanich KA, Becker HC, Woodward JJ (2011) Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behavioral Neuroscience 125(6):879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Fakih ME, Gurina TS, Roy EK, Hoffman JL, Uruena-Agnes AR, Kirstein CL (2016) Reversal learning and experimenter-administered chronic intermittent ethanol exposure in male rats. Psychopharmacology 233(19–20):3615–3626. [DOI] [PubMed] [Google Scholar]
- Balleine B (1992) Instrumental performance following a shift in primary motivation depends on incentive learning. Journal of Experimental Psychology: Animal Behavior Processes 18(3):236. [PubMed] [Google Scholar]
- Balleine B, Dickinson A (1991) Instrumental performance following reinforcer devaluation depends upon incentive learning. The Quarterly Journal of Experimental Psychology 43(3):279–296. [Google Scholar]
- Balleine BW, Garner C, Gonzalez F, Dickinson A (1995) Motivational control of heterogeneous instrumental chains. Journal of Experimental Psychology: Animal Behavior Processes 21(3):203. [Google Scholar]
- Balleine BW, Paredes-Olay C, Dickinson A (2005) Effects of outcome devaluation on the performance of a heterogeneous instrumental chain. International Journal of Comparative Psychology 18(4). [Google Scholar]
- Baltz ET, Yalcinbas EA, Renteria R, Gremel CM (2018) Orbital frontal cortex updates state-induced value change for decision-making. Elife 7:e35988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50(1–3):7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP (1999) Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of neuroscience 19(13):5473–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR (1997) Deciding advantageously before knowing the advantageous strategy. Science 275(5304):1293–1295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE (2001) Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39(4):376–389. [DOI] [PubMed] [Google Scholar]
- Becker HC (2000) Animal models of alcohol withdrawal. Alcohol Research & Health, 24(2):105–105. [PMC free article] [PubMed] [Google Scholar]
- Bianchi PC, de Oliveira PEC, Palombo P, Leão RM, Cogo-Moreira H, da Silva Planeta C, Cruz FC (2018) Functional inactivation of the orbitofrontal cortex disrupts context-induced reinstatement of alcohol seeking in rats. Drug and Alcohol Dependence 186:102–112. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’Esposito M, Fields HL (2007) Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158Val/Val genotype. Journal of Neuroscience 27(52):14383–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Liu W, Crews FT, Markou A (2015) Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. International Journal of Neuropsychopharmacology 18(2):pyu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield LA, Dezfouli A, van Holstein M, Chieng B, Balleine BW (2015) Medial orbitofrontal cortex mediates outcome retrieval in partially observable task situations. Neuron 88(6):1268–1280. [DOI] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Cleeremans A, Kornreich C, Verbanck P, Noël X (2014) Impaired decision-making under risk in individuals with alcohol dependence. Alcoholism: Clinical and Experimental Research 38(7):1924–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Lee SH, Yu Y, Zhu H, Crews FT, Robinson DL, Shih YYI (2018) Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addiction Biology 23(2):810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camille N, Tsuchida A, Fellows LK (2011) Double dissociation of stimulus-value and action-value learning in humans with orbitofrontal or anterior cingulate cortex damage. Journal of Neuroscience 31(42):15048–15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell H, Finn PR, Rickert ME, Lucas J (2008) Decision making in alcohol dependence: insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcoholism: Clinical and Experimental Research 32(8):1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ (2011) Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biological Psychiatry 70(6):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson VCC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA (2011) Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36(12):2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catafau AM, Etcheberrigaray A, Perez de los Cobos J, Estorch M, Guardia J, Flotats A, Berna L, Mari C, Casas M, Carrio I (1999) Regional cerebral blood flow changes in chronic alcoholic patients induced by naltrexone challenge during detoxification. The Journal of Nuclear Medicine 40(1):19–24. [PubMed] [Google Scholar]
- Claus ED, Ewing SWF, Filbey FM, Sabbineni A, Hutchison KE (2011) Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 36(10):2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Nasrallah NA, Hart AS, Collins AL, Bernstein IL, Phillips PE (2012) Altered risk-based decision making following adolescent alcohol use results from an imbalance in reinforcement learning in rats. PLoS One 7(5):e37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr, He J, Lee J, Styner M, Crews FT (2011) Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism: Clinical and Experimental Research 35(4): 671–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr, Liu W, Oguz I, Styner M, Crews FT (2014) Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacology Biochemistry and Behavior 116:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Motzkin DK (1994) Encoding of the unconditioned stimulus in Pavlovian conditioning. Animal Learning & Behavior 22(4):384–394. [Google Scholar]
- Colwill RM, Rescorla RA (1985) Postconditioning devaluation of a reinforcer affects instrumental responding. Journal of Experimental Psychology: Animal Behavior Processes, 11(1):120. [PubMed] [Google Scholar]
- Corbit LH, Balleine BW (2003) Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. Journal of Experimental Psychology: Animal Behavior Processes 29(2):99. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH (2012) Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry 72(5):389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH (2014) Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Frontiers in Behavioral Neuroscience 8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso TD, Mostafa HM, Collins MA, Neafsey EJ (1998) Brain neuronal degeneration caused by episodic alcohol intoxication in rats: effects of nimodipine, 6, 7-dinitro-quinoxaline-2, 3-dione, and MK-801. Alcoholism: Clinical and Experimental Research 22(1):217–224. [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J (2013) High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological Psychiatry 73(7):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio-Kelly RE, Austad CS, Raskin SA, Tennen H, Wood RM, Fallahi CR (2014) Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction 109(4):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KM, Wu JY (2001) Role of glutamatergic and GABAergic systems in alcoholism. Journal of Biomedical Science 8(1):7–19. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kämmerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K (2011) Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcoholism: Clinical and Experimental Research 35(9):1678–1685. [DOI] [PubMed] [Google Scholar]
- den Hartog C, Zamudio-Bulcock P, Nimitvilai S, Gilstrap M, Eaton B, Fedarovich H, Motts A, Woodward JJ (2016) Inactivation of the lateral orbitofrontal cortex increases drinking in ethanol-dependent but not non-dependent mice. Neuropharmacology 107:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saskida LM, Kunos G, Lovinger DM (2013) Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proceedings of the National Academy of Sciences 110(36):14783–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy LM, Perszyk RE, Zimmermann KS, Koleske AJ, Gourley SL (2014) Adolescent cocaine exposure simplifies orbitofrontal cortical dendritic arbors. Frontiers in Pharmacology 5:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC (1996a) Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380(6569):69. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC (1996b) Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behavioral Neuroscience 110(5):872. [DOI] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, Van Den Brink W, Sabbe B (2006) Decision-making deficits in alcohol-dependent patients with and without comorbid personality disorder. Alcoholism: Clinical and Experimental Research 30(10):1670–1677. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe BGCC, Hulstijn W, Van Den Brink W (2005) Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. The British Journal of Psychiatry 187(3):209–220. [DOI] [PubMed] [Google Scholar]
- Duncan J (2001) An adaptive coding model of neural function in prefrontal cortex. Nature Reviews Neuroscience 2(11):820–829. [DOI] [PubMed] [Google Scholar]
- Duka T, Trick L, Nikolaou K, Gray MA, Kempton MJ, Williams H, Critchley HD, Stephens DN (2011) Unique brain areas associated with abstinence control are damaged in multiply detoxified alcoholics. Biological Psychiatry 70(6):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ (2011) Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcoholism: Clinical and Experimental Research 35(6):1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst LH, Plichta MM, Dresler T, Zesewitz AK, Tupak SV, Haeussinger FB, Fischer M, Polak T, Fallgatter AJ, Ehlis AC (2012) Prefrontal correlates of approach preferences for alcohol stimuli in alcohol dependence. Addiction Biology 19(3):497–508. [DOI] [PubMed] [Google Scholar]
- Farovik A, Place RJ, McKenzie S, Porter B, Munro CE, Eichenbaum H (2015) Orbitofrontal cortex encodes memories within value-based schemas and represents contexts that guide memory retrieval. Journal of Neuroscience 35(21):8333–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P (2004) Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcoholism: Clinical and Experimental Research 28(10):1487–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK (2011) Orbitofrontal contributions to value-based decision making: evidence from humans with frontal lobe damage. Annals of the New York Academy of Sciences 1239(1):51–58. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ (2003). Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain 126(8):1830–1837. [DOI] [PubMed] [Google Scholar]
- Fernandez GM, Lew BJ, Vedder LC, Savage LM (2017) Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience 348:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Fábregues I, Rairiz J, Galofré E (1986) Decreased numbers of dendritic spines on cortical pyramidal neurons in human chronic alcoholism. Neuroscience Letters, 69(1):115–119. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR (2006) Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. Journal of Psychopharmacology 20(1):24–32. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R (2014) Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PloS One 9(5):e94640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier C, Maksimovskiy A, Venne J, LaFleche G, McGlinchey R (2009) Silent trace eliminates differential eyeblink learning in abstinent alcoholics. International Journal of Environmental Research and Public Health 6(7):2007–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Steffen EM, LaFleche G, Venne JR, Disterhoft JF, McGlinchey RE (2008) Delay discrimination and reversal eyeblink classical conditioning in abstinent chronic alcoholics. Neuropsychology 22(2):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G (1999) Orbitofrontal cortex and representation of incentive value in associative learning. Journal of Neuroscience 19(15):6610–6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbusow M, Schad DJ, Sebold M, Friedel E, Bernhardt N, Koch SP, Steinacher B, Kathmann N, Geurts DE, Sommer C, Müller DK (2016) Pavlovian-to-instrumental transfer effects in the nucleus accumbens relate to relapse in alcohol dependence. Addiction Biology, 21(3):719–731. [DOI] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ (2014) Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 39(11):2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ (2001) Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry 58(4):345–352. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ (2003) Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301(5636):1104–1107. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Zimmermann KS, Allen AG, Taylor JR (2016) The medial orbitofrontal cortex regulates sensitivity to outcome value. Journal of Neuroscience 36(16):4600–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW (2008) Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcoholism: Clinical and Experimental Research 32(10):1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM (2013) Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nature Communications 4:2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, Papadimitriou GM, Makris N, Oscar-Berman M (2008) Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcoholism: Clinical and Experimental Research 32(6):1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Corbett D (1990) Changes in the basal dendrites of cortical pyramidal cells from alcoholic patients--a quantitative Golgi study. Journal of Neurology, Neurosurgery & Psychiatry 53(10):856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN (2016) Circuit-based corticostriatal homologies between rat and primate. Biological Psychiatry 80(7):509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, O’connor JA, Acosta G, Floyd D, Anderson N, McCool BA, Friedman D, Grant KA (2006) Ethanol-Induced Regulation of GABAA Subunit mRNAs in Prefrontal Fields of Cynomolgus Monkeys. Alcoholism: Clinical and Experimental Research 30(12):1978–1985. [DOI] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Mann K, Heinz A (2006) Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcoholism: Clinical and Experimental Research 30(8):1349–1354. [DOI] [PubMed] [Google Scholar]
- Holland PC (1990) Event representation in Pavlovian conditioning: Image and action. Cognition 37(1–2):105–131. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL (2012) Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nature Neuroscience 15(10):1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes NM, Marchand AR, Coutureau E (2010) Pavlovian to instrumental transfer: A neurobehavioural perspective. Neuroscience and Biobehavioral Reviews 34(8):1277–1295. [DOI] [PubMed] [Google Scholar]
- Hommer D, Andreasen P, Rio D, Williams W, Ruttimann U, Momenan R, Zametkin A, Rawlings R, Linnoila M (1997) Effects of m-chlorophenylpiperazine on regional brain glucose utilization: a positron emission tomographic comparison of alcoholic and control subjects. Journal of Neuroscience 17(8):2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, O’doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE (2004) Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience 16(3):463–478. [DOI] [PubMed] [Google Scholar]
- Hu W, Morris B, Carrasco A, Kroener S (2015) Effects of Acamprosate on Attentional Set-Shifting and Cellular Function in the Prefrontal Cortex of Chronic Alcohol-Exposed Mice. Alcoholism: Clinical and Experimental Research 39(6):953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ, Zhong H, Mao T (2016) A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife 5:e19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DE (2010) Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cerebral Cortex 21(6):1408–1415. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA (2004) Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. Journal of Neuroscience 24(34):7540–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Bazov I, Kononenko O, Korpi ER, Bakalkin G, Birnir B (2012) Selective changes of GABAA channel subunit mRNAs in the hippocampus and orbitofrontal cortex but not in prefrontal cortex of human alcoholics. Frontiers in Cellular Neuroscience 5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M (1972) Limbic lesions and the problem of stimulus—reinforcement associations. Experimental Neurology 36(2):362–377. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ (2011) Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin1 receptors. British Journal of Pharmacology 162(4):880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H (2005) Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biological Psychology 69(3):353–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET (2004) The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology Progress in Neurobiology 72(5):341–372. [DOI] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ (2012) Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PloS One 7(5):e37541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CD, Rohlfing T, Park B, Sullivan EV, Pfefferbaum A, Grant KA (2014) Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI study. Neuropsychopharmacology 39(4):823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC (2003) N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacology & Therapeutics 99(1):79–94. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J (2002) Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatry Research: Neuroimaging 114(2):95–102. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Rauchs G, La Joie R, Mezenge F, Boudehent C, Vabret F, Segobin S, Viader F, Allain P, Eustache F, Pitel AL (2014) Impaired decision-making and brain shrinkage in alcoholism. European Psychiatry 29(3):125–133. [DOI] [PubMed] [Google Scholar]
- Lehner R, Balsters JH, Herger A, Hare TA, Wenderoth N (2017) Monetary, food, and social rewards induce similar Pavlovian-to-instrumental transfer effects. Frontiers in Behavioral Neuroscience 10(247). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg NT, Pennington ZT, Holley SM, Greenfield VY, Cepeda C, Levine MS, Wassum KM (2017) Basolateral amygdala to orbitofrontal cortex projections enable cue-triggered reward expectations. Journal of Neuroscience 37(35):8374–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Daglish MR, Stevenson BJ, Feeney A, Pandit SA, Wilson SJ, Myles J, Grasby PM, Nutt DJ (2006) Imaging alcohol cue exposure in alcohol dependence using a PET 15O-H2O paradigm: results from a pilot study. Addiction Biology 11(1):107–115. [DOI] [PubMed] [Google Scholar]
- Liu D, Gu X, Zhu J, Zhang X, Han Z, Yan W, Cheng Q, Hao J, Fan H, Hou R, Chen Z, Chen Y, Li CT (2014) Medial prefrontal activity during delay period contributes to learning of a working memory task. Science 346(6208):458–463. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC, Chandler LJ (2014) Repeated episodes of chronic intermittent ethanol promote insensitivity to devaluation of the reinforcing effect of ethanol. Alcohol 48(7):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Petrides M (2010) Quantitative demonstration of comparable architectonic areas within the ventromedial and lateral orbital frontal cortex in the human and the macaque monkey brains. European Journal of Neuroscience 32(11):1940–1950. [DOI] [PubMed] [Google Scholar]
- Malvaez M, Shieh C, Murphy MD, Greenfield VY, Wassum KM (2019) Distinct cortical–amygdala projections drive reward value encoding and retrieval. Nature Neuroscience 22(5):762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA, Esber GR, Wegener MA, Wied HM, Liu TL, Stalnaker TA, Jones JL, Trageser J, Schoenbaum G (2014) Orbitofrontal neurons acquire responses to ‘valueless’ Pavlovian cues during unblocking. Elife 3:e02653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuier NS, Padula AE, Lopez MF, Woodward JJ, Mulholland PJ (2015) Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol 49(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Amodeo LR, Roitman JD (2014) Effects of voluntary alcohol intake on risk preference and behavioral flexibility during rat adolescence. PLoS One 9(7):e100697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Amodeo LR, Roitman JD (2016) Consequences of adolescent ethanol consumption on risk preference and orbitofrontal cortex encoding of reward. Neuropsychopharmacology 41(5):1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G (2006) Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcoholism: Clinical and Experimental Research 30(11):1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R Jr, MacKillop J, Meyerson LA, Justus A, Lovallo WR (2009) Influence of antisocial and psychopathic traits on decision-making biases in alcoholics. Alcoholism: Clinical and Experimental Research 33(5):817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, O’neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL (2012) Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Science Translational Medicine 4(116):116ra6–116ra6. [DOI] [PubMed] [Google Scholar]
- Moorman DE (2018) The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Progress in Neuro-Psychopharmacology and Biological Psychiatry 87:85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS (2004) Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology 29(2):393. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K (2008) Effect of naltrexone and ondansetron on alcohol cue–induced activation of the ventral striatum in alcohol-dependent people. Archives of General Psychiatry 65(4):466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL (2011) Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proceedings of the National Academy of Sciences 108(13):5466–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TW, Bernstein IL (2009) Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proceedings of the National Academy of Sciences 106(41):17600–17604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ (2016) Chronic intermittent ethanol exposure enhances the excitability and synaptic plasticity of lateral orbitofrontal cortex neurons and induces a tolerance to the acute inhibitory actions of ethanol. Neuropsychopharmacology 41(4):1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ (2017a) Ethanol dependence abolishes monoamine and GIRK (Kir3) channel inhibition of orbitofrontal cortex excitability. Neuropsychopharmacology 42(9):1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Woodward JJ (2018a) Effects of monoamines on the intrinsic excitability of lateral orbitofrontal cortex neurons in alcohol-dependent and non-dependent female mice. Neuropharmacology 137:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Woodward JJ (2018b) Sex-dependent differences in ethanol inhibition of mouse lateral orbitofrontal cortex neurons. Addiction Biology 25(1):e12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Uys JD, Woodward JJ, Randall PK, Ball LE, Williams RW, Jones BC, Lu L, Grant KA, Mulholland PJ (2017b) Orbitofrontal neuroadaptations and cross-species synaptic biomarkers in heavy-drinking macaques. Journal of Neuroscience 37(13):3646–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël X, Bechara A, Dan B, Hanak C, Verbanck P (2007) Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology 21(6):778. [DOI] [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT (2002) Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology Biochemistry and Behavior 72(3):521–532. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ (2001) Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcoholism: Clinical and Experimental Research 25(11):1673–1682. [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW (2007) Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. Journal of Neuroscience 27(18):4819–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT, Balleine BW (2010) Alcohol-paired contextual cues produce an immediate and selective loss of goal-directed action in rats. Frontiers in Integrative Neuroscience 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL, Ravassard PM, Cerpa JC, Wolff M, Ferreira G, Coutureau E (2018) Insular and ventrolateral orbitofrontal cortices differentially contribute to goal-directed behavior in rodents. Cerebral Cortex 28(7):2313–2325. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN (1994) Comparative architectonic analysis of the human and the macaque frontal cortex, in Handbook of Neuropsychology (Grafman J, Boller F eds), Vol. 9, pp 17–58. Elsevier, Amsterdam. [Google Scholar]
- Pickens CL, Saddoris MP, Gallagher M, Holland PC (2005) Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behavioral Neuroscience 119(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Crews FT (2012) NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. Journal of Neuroinflammation 9(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, Holmes A (2017) Chronic EtOH effects on putative measures of compulsive behavior in mice. Addiction Biology 22(2):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW (1999) Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain 122(8):1469–1493. [DOI] [PubMed] [Google Scholar]
- Reinhard I, Lemenager T, Fauth-Bühler M, Hermann D, Hoffmann S, Heinz A, Kiefer F, Smolka MN, Wellek S, Mann K, Vollstädt-Klein S (2015) A comparison of region-of-interest measures for extracting whole brain data using survival analysis in alcoholism as an example. Journal of Neuroscience Methods 242:58–64. [DOI] [PubMed] [Google Scholar]
- Renteria R, Baltz ET, Gremel CM (2018) Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nature Communications 9(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Cazares C, Gremel CM (2020) Habitual ethanol seeking and licking microstructure of enhanced ethanol self-administration in ethanol dependent mice. Alcoholism: Clinical and Experimental Research 44(4):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SE, Murray EA (2013) Differential effects of amygdala, orbital prefrontal cortex, and prelimbic cortex lesions on goal-directed behavior in rhesus macaques. Journal of Neuroscience 33(8):3380–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Wallis JD (2016) Decoding subjective decisions from orbitofrontal cortex. Nature Neuroscience 19(7):973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodberg EM, den Hartog CR, Anderson RI, Becker HC, Moorman DE, Vazey EM (2017) Stress facilitates the development of cognitive dysfunction after chronic ethanol exposure. Alcoholism: Clinical and Experimental Research 41(9):1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J (1994) Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery & Psychiatry 57(12):1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA (2013) Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience 16(8):1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca BF, Wied HM, Lopatina N, Saini GK, Nemirovsky D, Schoenbaum G (2018) Orbitofrontal neurons signal sensory associations underlying model-based inference in a sensory preconditioning task. eLife 7:e30373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H (2014) Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology 231(18):3799–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT, Clark JJ (2014) Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcoholism: Clinical and Experimental Research 38(6):1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Hönig K, Maier W, Gaebel W (2001) Subcortical correlates of craving in recently abstinent alcoholic patients. American Journal of Psychiatry 158(7):1075–1083. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M (2005) Orbitofrontal cortex, associative learning, and expectancies. Neuron 47(5):633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck NW, Cai MB, Wilson RC, Niv Y (2016) Human orbitofrontal cortex represents a cognitive map of state space. Neuron 91(6):1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R (2013) Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry 70(7):727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM (2014) Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug and Alcohol Dependence 145:1–33. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G (2015) What the orbitofrontal cortex does not do. Nature Neuroscience 18(5):620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S (2013) Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addiction Biology 18(2):203–213. [DOI] [PubMed] [Google Scholar]
- Talmi D, Seymour B, Dayan P, Dolan RJ (2008) Human Pavlovian-instrumental transfer. Journal of Neuroscience 28(2):360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA (2004) fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addictive Behaviors 29(1):33–50. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA (2003) Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry 60(7):727–735. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H (1964) A comparison of effects of orbitofrontal and hippocampal lesions upon discrimination learning and reversal in the cat. Experimental Neurology 9(6):452–462. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ (2014) Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. Journal of Neuroscience 34(10):3706–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G (1998) Glutamatergic neurotransmission in alcoholism. Journal of Biomedical Science 5(5):309–320. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK (2010) Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. Journal of Neuroscience 30(50):16868–16875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin VV, Dickinson A, O’Doherty JP (2007) Determining the neural substrates of goal-directed learning in the human brain. Journal of Neuroscience 27(15):4019–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanes LD, van Holst RJ, Jansen JM, van den Brink W, Oosterlaan J, Goudriaan AE (2014) Contingency learning in alcohol dependence and pathological gambling: learning and unlearning reward contingencies. Alcoholism: Clinical and Experimental Research 38(6):1602–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Qin L, Crews FT (2013) Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of Disease 59:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA (2001) Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcoholism: Clinical and Experimental Research 25(8):1087–1097. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS (2000) Addiction, a disease of brain activation in alcoholics. European Psychiatry 17(5):287–291. [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, Wolf AP (1994) Recovery of brain glucose metabolism in detoxified alcoholics. The American Journal of Psychiatry 151:2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Wolf AP, Pappas N, Biegon A, Dewey SL (1993) Decreased cerebral response to inhibitory neurotransmission in alcoholics. The American Journal of Psychiatry 150(3). [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Overall JE, Hitzemann R, Fowler JS, Pappas N, Frecska E, Piscani K (1997) Regional brain metabolic response to lorazepam in alcoholics during early and late alcohol detoxification. Alcoholism: Clinical and Experimental Research 21(7):1278–1284. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C (2007) Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. Journal of Neuroscience 27(46):12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fan Y, Dong Y, Ma M, Ma Y, Dong Y, Niu Y, Jiang Y, Wang H, Wang Z, Wu L (2016) Alterations in brain structure and functional connectivity in alcohol dependent patients and possible association with impulsivity. PloS One 11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, Balleine BW (2009) Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proceedings of the National Academy of Sciences 106(30):12512–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, DesJardin JT, Gale K, Malkova L (2011) Transient inactivationi of orbitofrontal cortex blocks reinforcer devaluation in macaques. Journal of Neuroscience 21(42):15128–15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, Niv Y (2014) Orbitofrontal cortex as a cognitive map of task space. Neuron 81(2):267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Grüsser SM, Klein S, Diener C, Hermann D, Flor HEEA, Mann K, Braus DF, Heinz A (2002) Development of alcohol-associated cues and cue-induced brain activation in alcoholics. European Psychiatry 17(5):287–291. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gardner MP, Stalnaker TA, Ramus SJ, Wikenheiser AM, Niv Y, Schoenbaum G (2019) Rat orbitofrontal ensemble activity contains multiplexed but dissociable representations of value and task structure in an odor sequence task. Current Biology 29(6):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]


