Abstract
The role of oxytocin (OT) in close relationships is complex, as both positive and negative associations have been found between OT and relationship processes. Also, with most research focusing on the effects of exogenous OT administration on communication and couple behaviors, our knowledge about the association between endogenous OT and couple dynamics remains limited. This study is the first to assess the link between peripheral OT levels and observed communication behaviors during sexual and nonsexual conflict discussions in romantic relationships. A sample of 126 young, heterosexual couples (Mean age = 23.3, SD = 2.4; average relationship duration = 1.9 years, SD = 0.9) participated in videotaped sexual and nonsexual couple conflict discussions of 7 minutes each. Communication behaviors were coded using an adaptation of the Specific Affect Coding System (SPAFF) and the System for Coding Interactions and Family Functioning (SCIFF). Blood samples were collected prior to the couple discussions, during a separate lab visit, and OT plasma levels were determined using enzyme-linked immunosorbent assays (ELISA). Plasma OT levels were positively associated with validating behaviors during sexual discussions in both women (r = +.24, p = .008) and men (r = +.18, p = .052). No significant associations were found between OT levels and validating behaviors during nonsexual discussions and between OT and affectionate and negative behaviors during either sexual or nonsexual discussions. Analyses revealed significant associations between OT levels and one’s own validating behaviors during sexual discussions (b = 47.82, t(201.16) = 3.81, p < .001) and one’s partner’s (b = 32.12, t(216.35) = 2.62, p = .009). The results highlight the biobehavioral aspects of couples’ sexual communication and may contribute to a better understanding of the processes involved in individual and relational well-being. This study is the first to report an association between peripheral OT levels and validating behaviors during sexual communication, indicating neurophysiological involvement in dyadic sexual communication patterns.
Keywords: Oxytocin, sexual communication, couples
Introduction
Relationship conflict and distress negatively impact physiological functioning and health and are risk factors for depression (Proulx et al., 2007). Although the literature on the association between relationship satisfaction and physical and mental health continues to grow (Fincham et al., 2018), the physiological and neuroendocrine mechanisms underlying such associations are still not fully understood (Schneiderman et al., 2014). Oxytocin (OT)’s role in the promotion of prosocial and affiliative behaviors has increasingly gained attention (Carter, 1992, 1998; Carter, 2014; Crockford et al., 2014). The current study aims to further our understanding of OT in romantic relationships and is the first to assess the link between peripheral OT levels and observed communication behaviors during sexual and nonsexual conflict discussions in a sample of young, heterosexual couples.
Oxytocin and Romantic Relationships
OT is involved in a wide range of relational, reproductive, and sexual processes (Veening et al., 2015). In the context of romantic relationships, studies have pointed at OT’s role in relationship formation (Carter, 1998), prosocial and affiliative behavior (Carter, 2014), orgasm (Carter, 1992) and trust, support, and threat reduction (Crockford et al., 2014). Neural correlates of the capacity for the formation of romantic attachment have been associated with OT receptors (Carter et al., 2020) and bidirectional influences have been found between physiological, including hormonal, and behavioral processes in romantic partners (Schneiderman et al., 2014). Additionally, evidence is emerging for the importance of OT pathways in the adaptive consequences and benefits of human attachment formation (Carter, 2017b). OT levels are higher in partnered individuals during the early stages of romantic attachment, suggesting an important role of OT during these first stages of couple formation (Schneiderman et al., 2012). Although factors modulating the association between relationship quality and plasma OT remain unclear (Smith et al., 2013), evidence implicates OT’s pivotal role in social behavior (Gouin et al., 2010), including couple interactions (Algoe et al., 2017; Schneiderman et al., 2014).
Oxytocin and Couple Interactions
Most research on OT involves the measurement of endogenous levels in individuals (Ebner et al., 2019; Plasencia et al., 2019) or the administration of exogenous OT and assessment of its effects on couple communication and dynamics (Jarnecke et al., 2018). In comparison, research on endogenous OT, measured peripherally in saliva or blood, and couple processes and interaction behaviors is limited. Nevertheless, peripheral levels of OT have been associated with positive communication behaviors, affiliation, and social support in romantic relationships (Gouin et al., 2010; Light et al., 2005; Schneiderman et al., 2012). Moreover, a link has been found between levels of naturally occurring OT and subjective psychological responses to expressed gratitude of one’s partner, implying OT’s role in promoting bonds between human adult romantic partners and its ability to serve as ‘rose-colored glasses’ (Algoe et al., 2017). Others, however, have reported a link between OT and post-conflict anxiety as well as reduced forgiveness (Tabak et al., 2011). These contrasting findings illustrate the complexity of OT and contrast the ubiquitous ‘love story’ to which OT is often reduced, with authors stressing that consideration of context is important (Bartz et al., 2011). Indeed, OT seems to be associated with different and potentially contrasting effects, depending on situational and individual-based factors (Bartz et al., 2011).
Sexual Communication
In the study of the effects of romantic relationships on health and well-being, couple interaction and communication processes are considered of central importance (Kiecolt-Glaser et al., 2010; Robles et al., 2014). Supportive behaviors have stress-relieving qualities (Holt-Lunstad et al., 2008), whereas hostile behaviors have been associated with increased blood pressure and altered endocrine and immune functioning (Broadwell & Light, 1999; Kiecolt-Glaser et al., 1997). More generally, positive and negative communication behaviors are differentially associated with relationship quality and outcomes in longitudinal research (Gottman & Krokoff, 1989).
Although the scope of studies examining the role of OT in romantic relationships and couple communication processes is expanding (Algoe et al., 2017), most studies rely on self-report measures of sexual communication and, to date, no studies have examined association between OT and observed sexual communication. Defined as the process of discussing sexual preferences and sharing and responding to requests of change in the sexual relationship (Simon & Gagnon, 1986), sexual communication has been a focus of empirical research for many years with studies highlighting its unique contribution to sexual and overall relationship satisfaction (Frederick et al., 2017; Montesi et al., 2010). In light of past work, it can be argued that in romantic relationships, nonsexual and sexual communication provide unique contributions to promoting couple’s relational well-being (Mark & Jozkowski, 2013; Rehman et al., 2011; Rehman et al., 2017).
The Current Study
The goal of the current study was to investigate OT’s associations with communication behaviors during both sexual and nonsexual couple discussions. Consistent with the notion that OT plays a role in pair bonding and romantic attachment, we focused on the initial stages of romantic relationships in young, heterosexual couples. Using hormonal, observational, and questionnaire-based methodologies, we assessed characteristics of both partners. Consistent with previous findings, we expected to find an association between peripheral OT and prosocial communication behaviors between partners. Based on the intimate nature of sexual communication and its association with greater displays of interpersonal warmth compared to nonsexual communication (Rehman et al., 2017), we tentatively predicted stronger associations between OT levels and communication behaviors during sexual discussions.
Methods
Participants
The sample consisted of 126 heterosexual couples (N = 252) who are part of an ongoing longitudinal study. Data were taken from the first wave of data collection. The study was advertised using posters and flyers, and advertisements were placed online (e.g., Facebook) and in local newspapers. The following inclusion criteria were used: (a) between ages 18 to 30 years; (b) in a committed, heterosexual relationship for at most three years; (c) cohabitating, or spending at least four nights a week together, for no more than two years; (d) able to speak and read Dutch. Individuals were excluded if they had cohabited or been married before, if they had children or were pregnant, and if they were being treated for sexual dysfunctions. All study measures and procedures were reviewed and approved by the university’s research ethics board.
Questionnaires
Demographics and Sexual History Questionnaire (DSHQ) (Janssen et al., 2013).
This questionnaire covers general demographic characteristics (e.g., age, income, education), relationship and sexual variables (e.g., relationship duration, previous partner), and general physical and mental health.
Areas of Change Questionnaire (ACQ) (Margolin et al., 1983).
The ACQ was used to determine topics for the two conflict discussions (sexual and nonsexual). On a 7-point Likert-type scale ranging from 1 (much more) to 7 (much less), with 4 representing no desired change, participants were asked to rate how much they would like their spouses to change in various areas (e.g., expressing emotions; meeting up with friends or family). Responses were recoded such that higher numbers represent greater desired change. Similar to a prior pilot study (Rehman et al., 2011), we modified the ACQ to include a total of nine sexual topics (e.g., pay more/less attention to one’s sexual needs, show more/less interest in sex, be more/less experimental). In addition, a question was added to assess the importance of each topic to the participant on a scale from 1 (not at all) to 10 (very important), as well as another question which asked participants to indicate whether or not they would feel comfortable discussing this topic in our study.
Oxytocin Assays
After written informed consent was obtained during a first lab visit, blood was drawn from the antecubital vein of participants into pre-chilled 6mL vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA), spray-died to the walls of the tube (K2). Per participant, two tubes were taken and inverted five times. They were immediately kept ice-chilled before being centrifuged at 4°C at 1000 × g for 15 min at the Rega Institute for Medical Research, KU Leuven (Belgium). Blood plasma was aliquoted and stored at −70°C.
Plasma aliquots were shipped on dry ice to The Kinsey Institute Resource Center at Indiana University (USA) for analysis. Samples were thawed at room temperature immediately before the assays. Plasma OT was measured using an enzyme-linked immunosorbent assay (ELISA) kit purchased from Enzo Life Sciences, Inc. (Farmingdale, New York), according to the manufacturer’s instructions. This approach is consistent with previous research (Ebner et al., 2019; Plasencia et al., 2019). The plasma was diluted in assay buffer (at a ratio of 1:8 for the OT assay) to give results reliably within the linear portion of the standard curve. The ELISA kit has been reported by the manufacturer to be highly sensitive (minimal detection rate = 15.6 picogram per milliliter (pg/ml) for OT) with very little antibody cross-reactivity for other neuropeptides. All samples were run at once and the inter- and intra-assay coefficients of variation were less than 7.7 and 8.4 respectively.
In the present study, as in earlier research from our group, unextracted samples were used for analysis. Whether extracted or non-extracted samples lead to different results or whether extracted samples may hamper results has been extensively discussed in a recent study by (MacLean et al., 2019). McCullough et al. (2013) suggested a single approach should be adopted as the standard in the field, arguing that valid levels of OT can only be determined in unextracted samples. However, as reviewed in MacLean et al. (2019), and as indicated by studies using other methods including mass spectrometry, it is premature to accept any single approach as a gold standard.
Discrepancies between methods are not necessarily an indicator that “some methods are valid whereas others are not”. In fact, McCullough et al.’s (2013) conclusions regarding the usefulness in predicting behavior based on blood levels of OT taken from unextracted samples are contradicted by more recent research. For example, two recent studies show that unextracted samples are more likely to show associations with behavior (Chu et al., 2020; Saxbe et al., 2019). Our experience with assays in unextracted plasma indicates reliable and replicable relationships between peripheral measures of OT and behavior, even in analyses done several years apart (see for example, Lancaster et al., 2015).
Sexual and Nonsexual Couple Communication1
During a second lab visit,2 couples were asked to engage in a sexual and a nonsexual conflict discussion, each lasting 7 minutes. The topics were presented in counterbalanced order. Topic selection for the sexual and nonsexual discussions was based on both partners’ individual responses to the ACQ (described above). The researchers compared both partners’ responses and selected the topics that scored highest on (discrepancy in) desired change and on topic importance. The researchers consulted with each partner individually, and if one or both partners were reluctant to discuss the selected topic, the topic with the second highest rating was offered as an alternative. After topic selection, both partners were re-united in the observation room, where wall-mounted video cameras captured the couple interactions.
Observational data were coded using two previously developed coding systems: the Specific Affect Coding System (SPAFF) (Gottman & Krokoff, 1989) and the System for Coding Interactions and Family Functioning (SCIFF) (Lindahl & Malik, 2001). In addition to the codes that were adapted from these coding systems, we included two new codes, one related to annoyance, irritability, or frustration, and one related to lightness, resulting in a total of four negative and five positive codes. The four negative dimensions were: (a) contemptuous behaviors; (b) domineering and belligerent behaviors; (c) annoyance, irritability, and frustration; and (d) overall level of conflict. The five positive dimensions were: (a) affection; (b) understanding and validation; (c) collaboration; (d) interest in and seeking out partner’s perspective; and (e) lightness.
Five observers underwent training using observational data from past studies of couples’ conflict communication, before behaviors of participants of the current study were coded. Each coding category was rated on a scale of 1 (none) to 10 (a great deal) after the coder had observed the entire interaction. Coders were instructed to pay attention to both verbal and nonverbal aspects of the behavior belonging to a specific category. A total of 504 interactions were coded (126 couples x 2 partners x 2 discussions). A random selection of 56 discussions (11% of total number of discussions) was coded by all five coders and was used to examine interrater reliability. The single-measure ICC scores for the 9 codes ranged from .61 to .89, suggesting good overall interrater reliability (Cicchetti, 1994).
Statistical Analysis
All analyses were conducted using SPSS 25.0 (SPSS Inc., Chicago, IL, USA). An exploratory factor analysis was used to examine the factor structure of our coded communication behavior variables. Differences between women and men in plasma OT was tested with t-tests and Pearson’s correlations examined associations between OT and couple communication behaviors during sexual and nonsexual discussions. We utilized the Actor-Partner Interdependence Model (APIM) (Kenny et al., 2006) to test the statistical effects of both partners’ communication behaviors on OT levels, controlling for dyadic data dependency.
Results
Sample Characteristics
The average age of the participants was 23.3 years (SD = 2.4; women: 22.7 years, SD = 2.2; men: 23.9 years, SD = 2.4). Relationship length at time of participation was on average 1.9 years (SD = 0.9). A total of 119 (94%) couples lived together and the remaining 7 couples spent on average 4.6 nights per week together. The majority of participants (98%) were White. Also, most (96%) of women used some kind of hormonal contraceptives (63% oral contraceptives; 24% intrauterine device; 9% vaginal ring), and the mean number of days since their last menstrual cycle was 12.75 (SD = 8.75). No associations were found between the time since last menstrual cycle and any of the hormonal and behavioral variables. Table 1 provides an overview of key demographics.
Table 1.
Demographics
| Variable | Women (N = 126) | Men (N = 126) |
|---|---|---|
| Age, years | 22.71 (±2.23) | 23.88 (±2.43) |
| Race, White | 124 (98%) | 124 (98%) |
| Job status | ||
| Full-time job | 37 (29%) | 58 (46%) |
| Other (e.g., part-time; temporary job) | 23 (18%) | 21 (17%) |
| Unemployed | 24 (19%) | 20 (16%) |
| Student | 42 (33%) | 27 (21%) |
Factor Analysis of Communication Behaviors
A principal axis factor analysis (FA) with oblique rotation (direct oblimin) was conducted on the 9 codes that had high or excellent ICCs. Three factors had eigenvalues over Kaiser’s criterion of 1 and in combination explained 74% of the variance. This was supported by visual inspection of the scree plot, which revealed an inflexion that also justified retaining three factors. Factor 1 represents Negative behaviors (level of conflict; contemptuous behaviors; domineering and belligerence; and annoyance and frustration), Factor 2 represents Affectionate behaviors (lightness; collaboration; and affection), and Factor 3 represents Validating behaviors (understanding and validation; and interest in and seeking out partner’s perspective). To test the consistency and robustness of this factor solution, we performed additional factor analyses, separately for men and women and for the two discussion topics, which all resulted in the same three factor structure. Internal consistency of the three factors ranged from acceptable to good for both nonsexual discussions (Negative behaviors: αWomen = .88, αMen = .84; Affectionate behaviors: αWomen = .79, αMen = .77; Validating behaviors: αWomen = .76, αMen = .72) and sexual discussions (Negative behaviors: αWomen = .87, αMen = .74; Affectionate behaviors: αWomen = .78, αMen = .77; Validating behaviors: αWomen = .71, αMen = .75). Factor correlations are presented in Table 2.
Table 2.
Factor Correlation Matrix for the Communication Behaviors
| 1 | 2 | 3 | |
|---|---|---|---|
| 1. Negative Behaviors | - | ||
| 2. Affectionate Behaviors | −.43** | - | |
| 3. Validating Behaviors | −.39** | .49** | - |
Note. N= 252.
p ≤ .01.
Oxytocin and Communication Behaviors
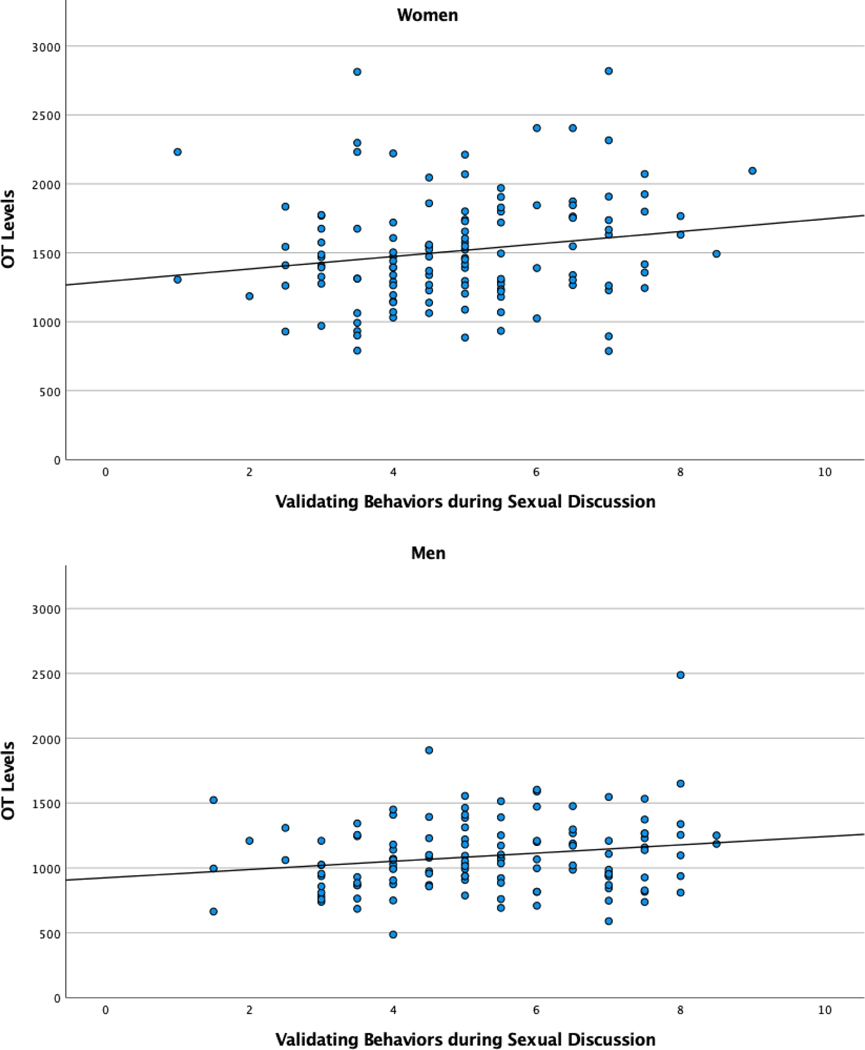
Plasma OT levels are presented in Table 3. OT levels were significantly higher in women than in men, t(125) = 11.21, p < .001. During nonsexual discussions, women expressed more negative behaviors than men (t(124) = 2.69, p = .008). No significant differences between men and women were found for affectionate (t(125) = −.020, p = .984) and validating behaviors (t(125) = −.21, p = .832) during nonsexual discussions. During sexual discussions, women also expressed significantly more negative behaviors (t(124) = 2.10, p = .038). Again, no significant differences were found for affectionate (t(124) = −1.28, p = .203) and validating behaviors (t(124) = −1.80, p = .075). For both women and men, OT levels were significantly correlated with validating behaviors during sexual discussions (see Table 4). Visual inspection of the scatterplots (Figure 1) suggests that the correlations between OT levels and validating behaviors during sexual discussions could have been affected by outliers. A total of four (three female and one male) multivariate outliers were identified using the computation of Mahalanobis Distances (p < .001). Correlations between OT levels and validating behaviors during sexual discussion, after excluding these subjects, were r = +.24, p = .008, in women, and r = +.18, p = .052, in men. We found no significant correlations between plasma OT levels and validating behaviors during nonsexual discussions. Also, no significant correlations were found between OT and negative behaviors and OT and affectionate behaviors during either sexual or nonsexual discussions.
Table 3.
Descriptives for Predictor and Outcome Variables
| Variable | Gender | M | SD | α | t | Range Potential | Range Actual |
|---|---|---|---|---|---|---|---|
| Oxytocin (pg/mL) | F | 1514.48 | 394.19 | - | 11.21** | - | 787.90 −2819.00 |
| M | 1094.06 | 282.17 | 487.00 −2488.00 | ||||
| Negative behaviors during nonsexual discussion | F | 1.85 | 1.27 | .88 | 2.69** | 1–10 | 1.00–6.25 |
| M | 1.55 | 1.11 | .84 | 1.00–8.00 | |||
| Negative behaviors during sexual discussion | F | 1.49 | 1.00 | .87 | 2.10* | 1–10 | 1.00–6.25 |
| M | 1.33 | .72 | .74 | 1.00–5.50 | |||
| Affectionate behaviors during nonsexual discussion | F | 4.68 | 1.61 | .79 | −.02 | 1–10 | 1.00–9.00 |
| M | 4.68 | 1.43 | .77 | 1.33–7.67 | |||
| Affectionate behaviors during sexual discussion | F | 4.86 | 1.44 | .78 | −1.28 | 1–10 | 1.00–8.00 |
| M | 5.03 | 1.46 | .77 | 1.33–8.00 | |||
| Validating behaviors during nonsexual discussion | F | 4.63 | 1.80 | .76 | −.21 | 1–10 | 1.50–9.50 |
| M | 4.67 | 1.60 | .72 | 1.00–8.50 | |||
| Validating behaviors during sexual discussion | F | 4.91 | 1.58 | .71 | −1.80 | 1–10 | 1.00–9.00 |
| M | 5.28 | 1.70 | .75 | 1.50–8.50 |
p ≤ .05
p ≤. 01.
Table 4.
Correlations Between Predictor and Outcome Variables
| Study variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Oxytocin | .26** | .01 | .07 | −.05 | −.05 | .05 | .18* |
| 2. Negative behaviors during nonsexual discussion | −.09 | .46** | .45** | −.50** | −.38** | −.41** | −.20* |
| 3. Negative behaviors during sexual discussion | −.10 | .56** | .56** | −.24** | −.46** | −.23** | −.31** |
| 4. Affectionate behaviors during nonsexual discussion | .08 | −.44** | −.35** | .51** | .52** | .56** | .12 |
| 5. Affectionate behaviors during sexual discussion | −.06 | −.41** | −.44** | .45** | .45** | .34** | .36** |
| 6. Validating behaviors during nonsexual discussion | .10 | −.33** | −.30** | .45** | .25** | .08 | .27** |
| 7. Validating behaviors during sexual discussion | .19* | −.24** | −.27** | .24** | .45** | .20* | −.02 |
Note. Correlations for women appear above the diagonal; correlations for men appear below the diagonal. Correlations along the diagonals are between dyad members.
p ≤ .05
p ≤ .01.
Figure 1.
Scatterplots for the correlation between oxytocin levels and validating behaviors during sexual discussions in women and men.
To make full use of the dyadic nature of our data, we explored the associations between OT and validating communication behaviors during sexual discussions using APIM (Kenny et al., 2006).3 APIM provides estimates for both actor effects (the effect Partner A’s predictor variable on Partner A’s outcome variable) and partner effects (effect of a Partner A’s predictor variable on Partner B’s outcome variable) and is widely implemented for dealing with interdependence between couple members. We found a significant positive correlation between both partners’ OT levels (r = .27, p = .003), indicating nonindependence of the data, providing further support for the use of APIM.
We tested an APIM model in which validating communication behavior during sexual discussions was included as predictor and OT as outcome variable. The APIM model had good model fit (Χ2 (4) = 98.29, p < .001) and the deviance test for distinguishability was significant (p < .001), indicating that we should conduct analyses separately based on self-reported gender/sex. The results revealed significant main actor effects for both women and men (see Table 5), indicating that women and men who expressed more validating behaviors during sexual discussions had higher plasma OT levels. Similarly, our results revealed significant partner effects, meaning higher levels of validating behaviors were associated with higher levels of OT in one’s partner.4 We did find a significant difference in mean OT levels for women and men, while controlling for validating behaviors during sexual discussions, with women having higher levels of plasma OT than men. We did not find significant interactions with gender, meaning there were no significant differences for actor or partner effects between women and men in our sample.5
Table 5.
APIM Parameter Estimates for Oxytocin levels
| Predictor | b | SE | β | t |
|---|---|---|---|---|
| Actor Validating behaviors during Sexual discussions | 47.82*** | 12.54 | .20*** | 3.81 |
| Partner Validating behaviors during Sexual discussions | 32.12** | 12.24 | .13** | 2.62 |
Note. b = unstandardized regression coefficient, SE = standard error of unstandardized regression coefficient, beta= standardized regression coefficient. df = 201.16 for actor effects and df = 216.35 for partner effects.
p ≤ .01,
p ≤ .001.
Discussion
Plasma OT levels were significantly and positively associated with validating behaviors during sexual discussions in both women and men. We did not find such an association for nonsexual discussions, nor did we find any association between OT and affective and negative communication behaviors, during either sexual or nonsexual discussions. Previous studies found behaviors such as affectionate touch and interpersonal focus to be associated with OT levels in romantic partners, pointing at the possibility of a bio-behavioral feedback loop, whereby higher levels of reciprocity and touch may increase involvement in the relationship at physiological, behavioral, and representational levels (Schneiderman et al., 2012). Consistent with Feldman’s (2012) bio-behavioral synchrony model, we found associations between partners’ hormones and behavior, in that both the individual’s and the partner’s OT levels were associated with validating behaviors. According to the bio-behavioral synchrony model, bond formation is characterized by neurohormonal and behavioral attunement between partners as well as by mutual influences between the physiology of one partner and the behavior of the other. In our sample, we found higher OT levels in women and men who exhibit more validating behaviors during sexual discussions themselves (actor effects) or whose partners exhibit more validating behaviors during these discussions (partner effects).
These findings indicate that the effects of neurohormonal processes on bonding are not limited to the level of the individual but are also impacted by the partner’s behavior. This is in line with findings by Schneiderman et al. (2014), who expressed the value of moving beyond the individual and to include the dyad as the unit of analysis for a “fuller biobehavioral matrix” focusing on all possible interactions between hormones and behavior. Though hormones can shape relational behavior, the reverse should be considered as well, with relational behaviors potentially shaping hormone levels. Additionally, research suggests that OT can facilitate the transformation of anxiety and avoidance into approach and positive emotional states (Carter, 1998). As couples tend to report higher levels of anxiety before sexual conflict discussions (Rehman et al., 2017) and sexual topics generally tend to be avoided (Peplau, 2003), our findings are consistent with the possibility that OT may be part of a process that helps transform avoidance and associated behaviors into active engagement and communication with one’s partner, in which validation may serve as a particularly effective form of approach behavior.
In our study, women had significantly higher plasma OT levels than men. This is consistent with findings of Plasencia and colleagues (2019), but in contrast with Weisman et al. (2013) who found higher plasma OT levels in men than in women. These differences may possibly be explained by differences in sample composition. While both previous studies focused on individuals rather than couples, Weisman et al. (2013) also included women who were breastfeeding at the time of the study. Interestingly, we used a similar assay kit to that used by Plasencia et al. (2019), which differed from Weisman et al. (2013), who used a OTELISA kit by Assay-Design (MI, USA).
As described in the methods section, ELISA kits purchased from Enzo Life Sciences, Inc. (Farmingdale, New York) were used to measure plasma OT levels, and, noteworthily, we found high levels of plasma OT in our sample. The present study was based on the version of the Enzo Life Sciences kits available in 2019. This kit yields levels of OT that are much higher than those seen previously using an assay system from Enzo Life Sciences. Unfortunately, the earlier Enzo Life Sciences kit and its antibodies are no longer available for comparison. However, in another ongoing study, using unextracted samples, and comparing between the currently available Enzo Life Sciences kit and an assay kit from Arbor Assays (Ann Arbor, MI) we also obtained much higher levels of OT with the Enzo Life Sciences kit. It should be noted that the relationship between behavior and plasma OT was stronger in data from studies yielding higher levels of OT, as is the case in Chu, et al., (2020) and Saxbe, et al., (2019).
Studies using enzyme-based (or radio-immunoassays) different assay methods routinely give different values. The sources of this variation remain poorly understood, and might be due to extraction (which discards significant amounts of OT from plasma) (MacLean, et al., 2019), but also may be due to differences in the antibodies in these kits. Quantitative comparisons across studies using different assay kits and antibodies are not recommended, and in our experience within-study associations between plasma OT and behavior are often significant regardless of the procedures employed (extraction or not, or different assay kits).
However, different antibodies used in these assays are only one of several possible sources of between-study variation (MacLean et al., 2019). Consistent with animal studies (Cho et al., 1999), higher levels of OT at the beginning of romantic relationships suggest its involvement in processes of partner attachment in humans, and substantially higher plasma levels of OT have been found in couples compared to non-attached singles (Schneiderman et al., 2014). Additionally, though some findings report stability of structural aspects of OT-expressing neurons (e.g., number and size) over time (Ishunina & Swaab, 1999), previous research has also found that central OT release decreases with age (Plasencia et al., 2019).
We did not find significant correlations between OT levels and affectionate behaviors during sexual or nonsexual discussions. Previous research has found associations between OT and positive couple behaviors, including relationship-enhancing attribution (Gouin et al., 2010), partner hugs (Light et al., 2005), and interactive reciprocity (Schneiderman et al., 2012). Considering our factor ‘affectionate behaviors’ represented a combination of affection, collaboration, and lightness, it could be that this dimension reflects a more generally positive ambience and conversation context between the two partners, rather than more specific, individual expressions or communication behaviors. Consistent with this interpretation, the dimension of validating behaviors consisted of codes capturing more active expressions and communication behaviors. For example, the code ‘interested in and seeking out partner perspective’ captured the extent to which a partner expressed interest in the other partner’s thoughts, feelings, and his/her perspective. Similarly, ‘understanding and validation’ captured expressions of understanding and acceptance of one’s partner’s views, feelings, and behaviors. These constructs seem to more closely resemble OT’s behavioral effects as reflecting “the capacity to be close to and sensitive to others” (Carter, 2014).
Additionally, Gouin et al.’s (2010) code for positive communication behaviors involved an aggregation of behaviors, such as acceptance and relationship-enhancing attribution, that come closer to validating than affectionate behaviors as operationalized in our study. A similar observation can be made about Schneiderman et al.’s (2012) construct of interactive reciprocity, which included codes close to our construct of validating behaviors (e.g., dyadic reciprocity, interpersonal focus, and matching of emotional state). Hence, we believe our findings are consistent with those of previous studies, and point to the usefulness of fine-grained view regarding which positive behaviors can be of interest in the study of OT.
We did not find any associations between OT levels and communication behaviors observed during nonsexual conflict discussions. This seems to be in contrast with previous research (Schneiderman et al., 2014), in which it was found that individuals whose partners had higher OT showed greater empathy during conflict discussion. No actor effects for OT were found in this study but, perhaps more importantly, the authors used a conflict interaction paradigm in which no distinction is made between sexual and nonsexual areas of disagreement (Gottman, 1979). In a similar fashion, our results may contradict other findings (Schneiderman et al., 2012), showing a link between OT and couples’ interactive reciprocity. However, in the present study couples were asked to engage in conflict discussions, rather than discuss a shared positive experience.
Overall, our results indicate that OT levels are specifically associated with validating behaviors expressed during sexual discussions. Past empirical and theoretical work has consistently emphasized the importance and unique contribution of sexual communication to relational outcomes (Frederick et al., 2017; Mark & Jozkowski, 2013; Montesi et al., 2010; Rehman et al., 2011). It could be argued that sexual issues touch upon aspects of the self and the relationship that are particularly sensitive and salient, an observation that is consistent with clinical observations and reflected in couples’ therapy, where intimacy and sexuality-related concerns and problems are among the most prevalent (Peplau, 2003). Since this study was not based on a clinical sample, speculation about implications for clinical practice should be done with restraint. However, our results hint at the potential qualities and relevance of the neurophysiological dimensions of sexual communication, which could be explored further and in more depth in future research, which could include clinical samples.
Strengths and Limitations
Although the number and scope of studies examining the role of OT in couple interactions is increasing (Algoe et al., 2017; Smith et al., 2013), and although OT is gaining ground as subject of study in the areas of human sexuality and reproductive behavior (Veening et al., 2015), studies have not directly examined the association of OT with sexual communication. Additionally, the study of sexual communication using observational measures remains greatly underrepresented in the literature, with the vast majority of studies relying on self-report measures. Another strength of the current study is that we recruited both members of a dyad, which gave us the opportunity to investigate how one partner’s behavior influences the other’s outcomes. Through this dyadic approach, we believe we can develop a more comprehensive understanding of the ways in which partners influence and are influenced by each other. The specificity of the association between OT levels and sexual communication found in the current study is intriguing and points to the need for additional research that replicates this finding and extends it by investigating which aspects of sexual communication influence OT levels. Specifying the biobehavioral processes underpinning romantic attachment may illuminate the contribution of such relationships to both individual and relational well-being.
Several limitations of our study should be considered as well. First, as is common in human research, we did not measure OT at the central level but in the periphery. Whereas animal studies paved the way for research on OT in human romantic attachment, a direct assessment of central OT levels via brain neurochemistry in humans would be invasive. Although the link between peripheral and central OT levels remains a topic of debate (Macdonald & Feifel, 2013), increasing anatomical and functional evidence does suggest the two are connected (Carson et al., 2015). Due to these factors and the nature of our study design, this study does not allow for any conclusions about underlying neural correlates and mechanisms through which validating sexual communication behaviors might influence, or be influenced by, OT. Prior research has revealed a high density of OT receptors in brain systems associated with attachment formation, such as the substantia nigra, the globus pallidus, and thalamus (Acevedo et al., 2012), suggesting a neurological basis for OT’s role in romantic bonding. In addition, in both healthy controls and individuals diagnosed with neurodevelopmental disorders (e.g., schizophrenia), peripheral peptide concentrations have been associated with central nervous system activity (Carter et al., 2020). Though our findings point at a neurophysiological involvement in sexual communication patterns of romantic partners in the early stages of their relationship, further research is needed to establish the mechanisms underlying these associations.
Another limitation involves the fact that our study did not include measurement of vasopressin (AVP), a neuropeptide that shares functional overlap with OT in socioemotional and cognitive processes in humans and animals (Carter, 2017a; Plasencia et al., 2019). Such assessment would be highly informative and future studies could benefit from examining the two neuropeptides together. Also, we focused on a community sample of heterosexual couples, and the inclusion and exclusion criteria used (in terms of children, sexual function, etc.) ensured a minimal influence of variables that were beyond the scope of our research. Future studies could include more heterogeneous and diverse samples, in terms of ethnicity and education as well as relationship duration, sexual function, and sexual orientation.
We did not find any associations between menstrual cycle phase and plasma OT levels in our sample. Previous research has revealed plasma OT fluctuations over the course of the menstrual cycle (Salonia et al., 2005). It should be noted, however, that a more recent study found no association between OT levels and contraception use or menstrual cycle (Weisman et al., 2013). In addition, the influence on OT levels of exogenous hormone use, such as hormonal contraceptives, and the effects of menstrual cycle variation are not always replicated (Light et al., 2005; Schneiderman et al., 2014; Tabak et al., 2011). Hence, evidence suggests that controlling for contraceptive use and menstrual cycle variation may not have affected our results significantly.
A final limitation is that the design of our study was correlational in nature and we measured plasma OT only once, prior to the couple discussions. This approach assumes a stability in OT levels, which is supported by findings of some previous studies showing high intra-individual stability of peripheral OT levels over a period of 6 months in individuals (Weisman et al., 2013) and couples (Schneiderman et al., 2012). Additionally, Smith et al. (2013) found no changes in plasma OT levels measured repeatedly, following positive and negative couple interactions. It should be noted, however, that studies have revealed variations in OT levels in response to specific situations or contexts such as breast feeding, sexual activity, exercise, affiliative touch, and stress (Carter et al., 2020; Ellis et al., 2021; Jong et al., 2015; MacLean et al., 2017; White-Traut et al., 2009) and have highlighted the association between changes in OT levels and behavioral variables (McClung et al., 2018). Additionally, the question remains whether single measurements of baseline OT concentrations in plasma or saliva are a reliable trait marker of the physiology of the OT system in humans (Martins et al., 2020), contradicting the previous studies reporting within-individual stability cited above. However, our study, which included 252 participants, supports the approach used in previous studies exploring associations with endogenous OT, and which tend to be based on sample sizes of less than 100 participants (Torres et al., 2018). To further substantiate our findings, future research could benefit from repeated measurements as they would allow for an exploration of causal relationships between our variables of interest. This approach would also allow for the further exploration of the stability of OT levels within individuals, and the question of whether validating behaviors during sexual discussions are better predicted by such trait-like dimensions of OT or by more reactive, adaptive OT release triggered by contextual demand (e.g., anxiety or threat associated with sexual communication).
In our study, we measured peripheral OT using blood samples as a proxy for central OT levels (Carson et al., 2015; Feldman et al., 2011; Taylor et al., 2010). It should be noted that the associations between peptide levels in saliva, blood, and the brain are still not well-understood (Quintana et al., 2018) and OT levels in saliva have been described as superior to plasma OT levels in indexing concentrations in the cerebrospinal fluid (Martin et al., 2018). However, in a recent study, MacLean et al. (2019) concluded that discrepancies do not automatically reflect errors or invalid measurements, stating that “we should consider the possibility that different measures capture different components of the biological story that OT has to tell” (p. 9). Additionally, studies have found no increase in salivary OT after administration of exogenous OT, raising the question of how OT reaches saliva if not through blood (Martins et al., 2020; Quintana et al., 2018). Hence, we believe that plasma OT levels provide a valid operationalization to explore our current research question, yielding results that are consistent with findings from prior studies associating peripheral plasma OT levels with positive communication behaviors (Gouin et al., 2010; Light et al., 2005; Schneiderman et al., 2012). Since this is the first study to look at peripheral OT levels and sexual and nonsexual couple communication, we believe our results are promising and lay a foundation for more research on associated processes and underlying mechanisms.
Conclusion
This is the first study to examine the association between endogenous OT and couple communication behaviors during sexual and nonsexual conflict discussions. Our findings revealed a positive association, in both partners, between plasma OT levels and validating behaviors during sexual discussions. Plasma OT levels were not associated with negative and affectionate behaviors during either sexual or nonsexual discussions in our sample of young, heterosexual couples. Our data suggest neurophysiological involvement in the sexual communication patterns of couples and point at the need for further research on the neurohormonal basis of sexual communication.
Highlights.
Oxytocin plays a role in relationship formation and prosocial/affiliative behaviors
Couple communication is considered of central importance to health and well-being
Nonsexual and sexual communication provide unique contributions to well-being
Oxytocin is associated with observed validating behaviors during sexual discussions
There is a need for further research on the neurohormonal basis of sexual communication
Acknowledgements
This research was supported by grants from the Research Foundation - Flanders (FWO; G0C8216N; PI: Erick Janssen) and KU Leuven/University of Leuven (C14/16/076; PI: Erick Janssen). The assay methods were refined with support from NIH P01 HD 075750 (CSC; PI: Sue Carter). The authors wish to thank Daisy Mechelmans and Linde Bastanie, Annelien Cordonnier, Ans Cornelissen, Merel de Bie, Victor Guillaume Antoine Honée, Laura Michiels for their help with data collection, and Maarten Jackers, Zita Leenaerts, Sanne Nieuwenhuis and Kelly van den Heuvel for their help with coding. The authors are grateful to the research teams of the Rega Institute for Medical Research, KU Leuven (Belgium) for assistance in processing the blood samples, and would specifically like to thank Patrick Matthys for granting access to his laboratory facilities.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
The data from the present study were obtained as part of a larger project examining the affective and behavioral characteristics of sexuality in romantic relationships. For more detailed information on the procedures and the coding of the conflict discussions, see (Roels et al., 2020)
Average time between first and second lab visit was 16 days (M = 16.02, SD = 23.50).
The four outliers described above were excluded from all further analyses.
Rerunning the analyses, while controlling for relationship duration, did not change the pattern of results: No significant main (b = −15.31, t(119.69) = −.64, p = .521) or interaction effects (relationship duration x validating behaviors) were found for the sexual discussions (Actor: b = −7.86, t(223.13) = −.55, p = .586; Partner: b = −40.12, t(210.33) = −2.82, p = .067).
To explore the possible impact of time between blood sampling and observational task on these findings, we reran the analyses controlling for the time between the two lab visits. The covariate was grand-mean centered prior to analyses, using means across both partners. Controlling for time did not change the pattern of results. For this reason, we present findings from the more parsimonious models that did not include time as a covariate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo BP, Aron A, Fisher HE, & Brown LL (2012). Neural correlates of long-term intense romantic love. Soc Cogn Affect Neurosci, 7(2), 145–159. doi: 10.1093/scan/nsq092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algoe SB, Kurtz LE, & Grewen K. (2017). Oxytocin and Social Bonds: The Role of Oxytocin in Perceptions of Romantic Partners’ Bonding Behavior. Psychol Sci, 28(12), 1763–1772. doi: 10.1177/0956797617716922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, & Ochsner KN (2011). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences, 15(7), 301–309. doi: 10.1016/j.tics.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Broadwell SD, & Light KC (1999). Family support and cardiovascular responses in married couples during conflict and other interactions. Int J Behav Med, 6(1), 40–63. doi: 10.1207/s15327558ijbm0601_4 [DOI] [PubMed] [Google Scholar]
- Carson DS, Berquist SW, Trujillo TH, Garner JP, Hannah SL, Hyde SA, . . . Parker KJ (2015). Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol Psychiatry, 20(9), 1085–1090. doi: 10.1038/mp.2014.132 [DOI] [PubMed] [Google Scholar]
- Carter CS (1992). Oxytocin and sexual behavior. Neuroscience and Biobehavioral Reviews, 16(2), 131–144. [DOI] [PubMed] [Google Scholar]
- Carter CS (1998). Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology, 23(8), 779–818. [DOI] [PubMed] [Google Scholar]
- Carter CS (2014). Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol, 65, 17–39. doi: 10.1146/annurev-psych-010213-115110 [DOI] [PubMed] [Google Scholar]
- Carter CS (2017a). The Oxytocin-Vasopressin Pathway in the Context of Love and Fear. Front Endocrinol (Lausanne), 8, 356. doi: 10.3389/fendo.2017.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2017b). The Role of Oxytocin and Vasopressin in Attachment. Psychodyn Psychiatry, 45(4), 499–517. doi: 10.1521/pdps.2017.45.4.499 [DOI] [PubMed] [Google Scholar]
- Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, . . . Kingsbury MA (2020). Is Oxytocin “Nature’s Medicine”? Pharmacol Rev, 72(4), 829–861. doi: 10.1124/pr.120.019398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, & Carter CS (1999). The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav Neurosci, 113(5), 1071–1079. doi: 10.1037//0735-7044.113.5.1071 [DOI] [PubMed] [Google Scholar]
- Chu C, Hammock EAD, & Joiner TE (2020). Unextracted plasma oxytocin levels decrease following in-laboratory social exclusion in young adults with a suicide attempt history. J Psychiatr Res, 121, 173–181. doi: 10.1016/j.jpsychires.2019.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti DV (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment, 6(4), 284–290. [Google Scholar]
- Crockford C, Deschner T, Ziegler TE, & Wittig RM (2014). Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Front Behav Neurosci, 8, 68. doi: 10.3389/fnbeh.2014.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Lin T, Muradoglu M, Weir DH, Plasencia GM, Lillard TS, . . . Connelly JJ (2019). Associations between oxytocin receptor gene (OXTR) methylation, plasma oxytocin, and attachment across adulthood. Int J Psychophysiol, 136, 22–32. doi: 10.1016/j.ijpsycho.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Horn AJ, Sue Carter C, van Ijzendoorn MH, & Bakermans-Kranenburg MJ (2021). Developmental programming of oxytocin through variation in early-life stress: Four meta-analyses and a theoretical reinterpretation. Clinical Psychology Review, 86. doi: 10.1016/j.cpr.2021.101985 [DOI] [PubMed] [Google Scholar]
- Feldman R. (2012). Oxytocin and social affiliation in humans. Horm Behav, 61(3), 380–391. doi: 10.1016/j.yhbeh.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, & Zagoory-Sharon O. (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev Sci, 14(4), 752–761. doi: 10.1111/j.1467-7687.2010.01021.x [DOI] [PubMed] [Google Scholar]
- Fincham FD, Rogge R, & Beach SRH (2018). Relationship satisfaction. In Vangelisti AL & Perlman D. (Eds.), The Cambridge handbook of personal relationships (pp. 422–436): Cambridge University Press. [Google Scholar]
- Frederick DA, Lever J, Gillespie BJ, & Garcia JR (2017). What Keeps Passion Alive? Sexual Satisfaction Is Associated With Sexual Communication, Mood Setting, Sexual Variety, Oral Sex, Orgasm, and Sex Frequency in a National US Study. Journal of sex research, 54(2), 186–201. doi: 10.1080/00224499.2015.1137854 [DOI] [PubMed] [Google Scholar]
- Gottman JM (1979). Marital interaction: Experimental investigations. New York NY: Academic Press. [Google Scholar]
- Gottman JM, & Krokoff LJ (1989). Marital interaction and satisfaction: A longitudinal view. Journal of Consulting & Clinical Psychology, 57(1), 47–52. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, . . . Kiecolt-Glaser JK (2010). Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology, 35(7), 1082–1090. doi: 10.1016/j.psyneuen.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, & Light KC (2008). Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med, 70(9), 976–985. doi: 10.1097/PSY.0b013e318187aef7 [DOI] [PubMed] [Google Scholar]
- Ishunina TA, & Swaab DF (1999). Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J Clin Endocrinol Metab, 84(12), 4637–4644. doi: 10.1210/jcem.84.12.6187 [DOI] [PubMed] [Google Scholar]
- Janssen E, Macapagal KR, & Mustanski B. (2013). Individual differences in the effects of mood on sexuality: the revised Mood and Sexuality Questionnaire (MSQ-R). J Sex Res, 50(7), 676–687. doi: 10.1080/00224499.2012.684251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnecke AM, Barden E, Back SE, Brady KT, & Flanagan JC (2018). Intimate partner violence moderates the association between oxytocin and reactivity to dyadic conflict among couples. Psychiatry Res, 270, 404–411. doi: 10.1016/j.psychres.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong TR, Menon R, Bludau A, Grund T, Biermeier V, Klampfl SM, . . . Neumann ID (2015). Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology, 62, 381–388. doi: 10.1016/j.psyneuen.2015.08.027 [DOI] [PubMed] [Google Scholar]
- Kenny DA, Kashy DA, & Cook WL (2006). Dyadic data analysis. New York: Guilford Press. [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmith M, Kim C, & Malarkey WB (1997). Marital conflict in older adults: endocrinological and immunological correlates. Psychosom Med, 59(4), 339–349. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, & Hantsoo L. (2010). Close relationships, inflammation, and health. Neurosci Biobehav Rev, 35(1), 33–38. doi: 10.1016/j.neubiorev.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster K, Carter CS, Pournajafi-Nazarloo H, Karaoli T, Lillard TS, Jack A, . . . Connelly JJ (2015). Plasma oxytocin explains individual differences in neural substrates of social perception. Front Hum Neurosci, 9, 132. doi: 10.3389/fnhum.2015.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KC, Grewen KM, & Amico JA (2005). More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol Psychol, 69(1), 5–21. doi: 10.1016/j.biopsycho.2004.11.002 [DOI] [PubMed] [Google Scholar]
- Lindahl KM, & Malik NM (2001). The System for Coding Interactions and Family Functioning. Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Macdonald K, & Feifel D. (2013). Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci, 7, 35. doi: 10.3389/fnins.2013.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Gesquiere LR, Gruen ME, Sherman BL, Martin WL, & Carter CS (2017). Endogenous Oxytocin, Vasopressin, and Aggression in Domestic Dogs. Front Psychol, 8, 1613. doi: 10.3389/fpsyg.2017.01613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, & Carter CS (2019). Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology, 107, 225–231. doi: 10.1016/j.psyneuen.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin G, Talovic S, & Weinstein CD (1983). Areas of change questionnaire: A practical approach to marital assessment. Journal of Consulting & Clinical Psychology, 51(6), 920–931. [Google Scholar]
- Mark KP, & Jozkowski KN (2013). The mediating role of sexual and nonsexual communication between relationship and sexual satisfaction in a sample of college-age heterosexual couples. J Sex Marital Ther, 39(5), 410–427. doi: 10.1080/0092623X.2011.644652 [DOI] [PubMed] [Google Scholar]
- Martin J, Kagerbauer SM, Gempt J, Podtschaske A, Hapfelmeier A, & Schneider G. (2018). Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. J Neuroendocrinol, e12596. doi: 10.1111/jne.12596 [DOI] [PubMed] [Google Scholar]
- Martins D, Gabay AS, Mehta M, & Paloyelis Y. (2020). Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans. Elife, 9. doi: 10.7554/eLife.62456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JS, Triki Z, Clement F, Bangerter A, & Bshary R. (2018). Endogenous oxytocin predicts helping and conversation as a function of group membership. Proc Biol Sci, 285(1882). doi: 10.1098/rspb.2018.0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, & Mendez AJ (2013). Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neuroscience and Biobehavioral Reviews, 37(8), 1485–1492. doi: 10.1016/j.neubiorev.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Montesi JL, Fauber RL, Gordon EA, & Heimberg RG (2010). The specific importance of communication about sex to couple’s sexual and overall relationship satisfaction. Journal of Social and Personal Relationships, 28, 591–609. [Google Scholar]
- Peplau LA (2003). Human sexuality how do men and women differ? Current Directions in Psychological Science, 12, 37–40. [Google Scholar]
- Plasencia G, Luedicke JM, Nazarloo HP, Carter CS, & Ebner NC (2019). Plasma oxytocin and vasopressin levels in young and older men and women: Functional relationships with attachment and cognition. Psychoneuroendocrinology, 110, 104419. doi: 10.1016/j.psyneuen.2019.104419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx CM, Helms HM, & Buehler C. (2007). Marital quality and personal well-being: A meta-analysis. Journal of Marriage and Family, 69(3), 576–593. doi:DOI 10.1111/j.1741-3737.2007.00393.x [DOI] [Google Scholar]
- Quintana DS, Westlye LT, Smerud KT, Mahmoud RA, Andreassen OA, & Djupesland PG (2018). Saliva oxytocin measures do not reflect peripheral plasma concentrations after intranasal oxytocin administration in men. Horm Behav, 102, 85–92. doi: 10.1016/j.yhbeh.2018.05.004 [DOI] [PubMed] [Google Scholar]
- Rehman US, Janssen E, Newhouse S, Heiman J, Holtzworth-Munroe A, Fallis E, & Rafaeli E. (2011). Marital satisfaction and communication behaviors during sexual and nonsexual conflict discussions in newlywed couples: a pilot study. J Sex Marital Ther, 37(2), 94–103. doi: 10.1080/0092623X.2011.547352 [DOI] [PubMed] [Google Scholar]
- Rehman US, Lizdek I, Fallis EE, Sutherland S, & Goodnight JA (2017). How Is Sexual Communication Different from Nonsexual Communication? A Moment-by-Moment Analysis of Discussions Between Romantic Partners. Arch Sex Behav, 46(8), 2339–2352. doi: 10.1007/s10508-017-1006-5 [DOI] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, & McGinn MM (2014). Marital quality and health: a meta-analytic review. Psychol Bull, 140(1), 140–187. doi: 10.1037/a0031859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels R, Rehman US, Goodnight JA, & Janssen E. (2020). Couple communication behaviors during sexual and nonsexual discussions and their association with relationship satisfaction. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, . . . Montorsi F. (2005). Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav, 47(2), 164–169. doi: 10.1016/j.yhbeh.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Saxbe D, Khaled M, Horton KT, & Mendez AJ (2019). Maternal prenatal plasma oxytocin is positively associated with prenatal psychological symptoms, but method of immunoassay extraction may affect results. Biol Psychol, 147, 107718. doi: 10.1016/j.biopsycho.2019.107718 [DOI] [PubMed] [Google Scholar]
- Schneiderman I, Kanat-Maymon Y, Zagoory-Sharon O, & Feldman R. (2014). Mutual influences between partners’ hormones shape conflict dialog and relationship duration at the initiation of romantic love. Soc Neurosci, 9(4). [DOI] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF, & Feldman R. (2012). Oxytocin during the initial stages of romantic attachment: relations to couples’ interactive reciprocity. Psychoneuroendocrinology, 37(8), 1277–1285. doi: 10.1016/j.psyneuen.2011.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon W, & Gagnon JH (1986). Sexual scripts: permanence and change. Arch Sex Behav, 15(2), 97–120. doi: 10.1007/bf01542219 [DOI] [PubMed] [Google Scholar]
- Smith TW, Uchino BN, MacKenzie J, Hicks AM, Campo RA, Reblin M, . . . Light KC (2013). Effects of couple interactions and relationship quality on plasma oxytocin and cardiovascular reactivity: empirical findings and methodological considerations. Int J Psychophysiol, 88(3), 271–281. doi: 10.1016/j.ijpsycho.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, McCullough ME, Szeto A, Mendez AJ, & McCabe PM (2011). Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology, 36(1), 115–122. doi: 10.1016/j.psyneuen.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, & Seeman TE (2010). Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol Sci, 21(1), 3–7. doi: 10.1177/0956797609356507 [DOI] [PubMed] [Google Scholar]
- Torres N, Martins D, Santos AJ, Prata D, & Verissimo M. (2018). How do hypothalamic nonapeptides shape youth’s sociality? A systematic review on oxytocin, vasopressin and human socio-emotional development. Neurosci Biobehav Rev, 90, 309–331. doi: 10.1016/j.neubiorev.2018.05.004 [DOI] [PubMed] [Google Scholar]
- Veening JG, de Jong TR, Waldinger MD, Korte SM, & Olivier B. (2015). The role of oxytocin in male and female reproductive behavior. Eur J Pharmacol, 753, 209–228. doi: 10.1016/j.ejphar.2014.07.045 [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, & Feldman R. (2013). Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology, 38(5), 694–701. doi: 10.1016/j.psyneuen.2012.08.011 [DOI] [PubMed] [Google Scholar]
- White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, & Carter CS (2009). Detection of salivary oxytocin levels in lactating women. Dev Psychobiol, 51(4), 367–373. doi: 10.1002/dev.20376 [DOI] [PMC free article] [PubMed] [Google Scholar]